Fungalpedia – Note 108 Oxydothis
Oxydothis Penz. & Sacc.
Citation when using this entry: Manawasinghe et al., in prep – Fungalpedia, palm fungi, genera, and terminology. Mycosphere.
Index Fungorum: IF3661, Facesoffungi: FoF05091, MycoBank 3661, GenBank 63191, Fig 1.
Oxydothis was introduced by Penzig & Saccardo (1897) from Cibodas, Java, Indonesia. Oxydothis grisea (= O. nigricans) is the type species (Penzig & Saccardo 1897). These species are characterized by ascomatal orientation, which is often horizontal, unitunicate asci which are fusiform or filiform with a J+ (rarely J-), wedge-shaped or discoid, subapical ring and ascospores which are 1-septate with spine-like or rounded ends (Hyde 1994). Following Oxydothis taxa develop two types of ascomata (Fröhlich & Hyde 2000), The first type develops on the host surface singly or in clusters. They are dark and ellipsoidal raised areas on the host surface and have eccentric ostioles. The second type develops below a raised sheet of host epidermis and is usually not darkened. The asci of Oxydothis are most similar to those of Diatrypaceae species (Wang & Hyde 1999). Therefore, the taxonomic position of this genus has gone through several changes. Penzig & Saccardo (1897) placed Oxydothis in Amphisphaeriaceae, Xylariales, Sordariomycetes. However, Oxydothis was transferred to Hyponectriaceae (Barr 1990, Hawksworth et al. 1995), then Clypeosphaeriaceae (Kang et al. 1998), and Pseudomassariaceae (Jeewon et al. 2003). Based on morphology and phylogeny, previous studies treated Oxydothis as genus incertae sedis in Xylariales (Maharachchikumbura et al. 2015). Konta et al. (2016) introduced Oxydothidaceae and placed Oxydothis in this new family. Oxydothis species are mostly reported on leaves or petioles of palms or leaves of Pandanus species (Hyde 1993). They are mostly reported as saprobic (Konta et al. 2016). However, O. parasitica and O. oraniopsis have been associated with palm leaf spots (Fröhlich & Hyde 2000), while Taylor (1988) and Konta et al. (2016) reported their potential role as endophytes . The current taxonomic placement of Oxydothis is Oxydothidaceae, Sordariomycetidae (Wijayawardene et al. 2022).
Type species: Oxydothis grisea Penz. & Sacc.,
Synonyms: = Merrilliopeltis Hennings, Hedwigia 47: 261. 1908.
= Plagiothecium Schrantz, Bull. Soc. Myc. France 76: 335. 1960.
= Plagiolagynion Schrantz, Bull. Soc. Myc. France 78: 218. 1962.
Other accepted species: see Species Fungorum – search Oxydothis
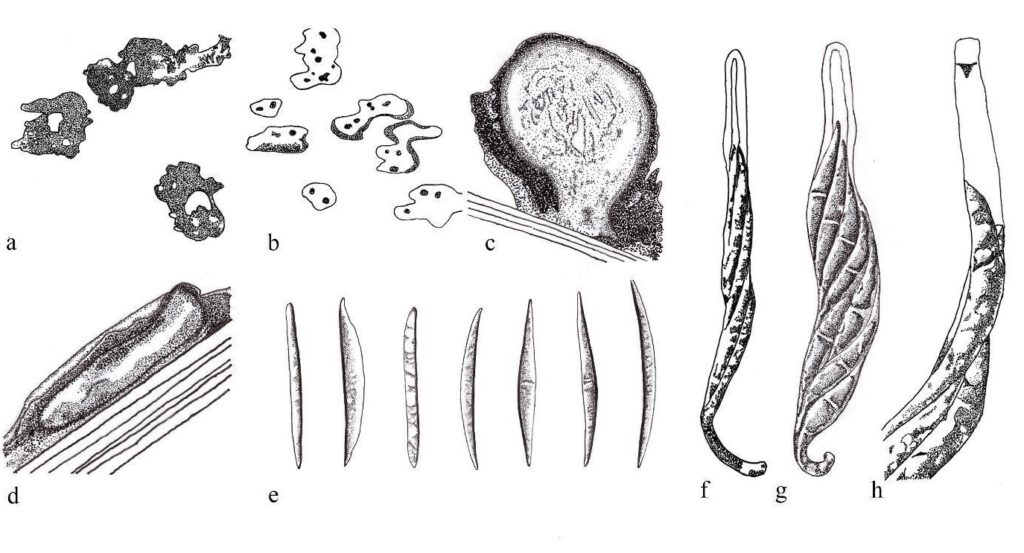
Figure 1 – Oxydothis spp. redrawn from Hyde (1993, 1994) and Konta et al. (2016). Appearance of ascomata of O. froehlichii on the host surface. b Ascomata of O. metroxylonis on the host substrate. c Section through ascoma of O. poliothea. d Section through ascoma of O. grisea. e Ascospores of O. maquilingian, O. hoehnelii, O. licualae, O. nypicola, O. maculosa, O. aequalis and O. frondicola. f Ascus of O. metroxylonis. g Ascus of O. palmicola. h J+ reaction of apical ring in Melzer’s reagent of O. metroxylonis. Scale bars:
References
Barr ME. 1990 – Prodromus to nonlichenized, Pyrenomycetous members of Class Hymenoascomycetes. Mycotaxon 39, 43–104.
Fröhlich J, Hyde KD. 2000 – Palm Microfungi. Fungal Diversity Research Series 3, 1–393.
Hyde KD. 1994 – Fungi from palms. XIII. The genus Oxydothis, a revision. Sydowia 46, 265–314.
Entry by
Ishara S Manawasinghe, Innovative Institute for Plant Health, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, P.R. China, Yinru Xiong, Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand, Innovative Institute for Plant Health, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, P.R. China
(Edited by Kevin D. Hyde & Eric H.C. McKenzie)