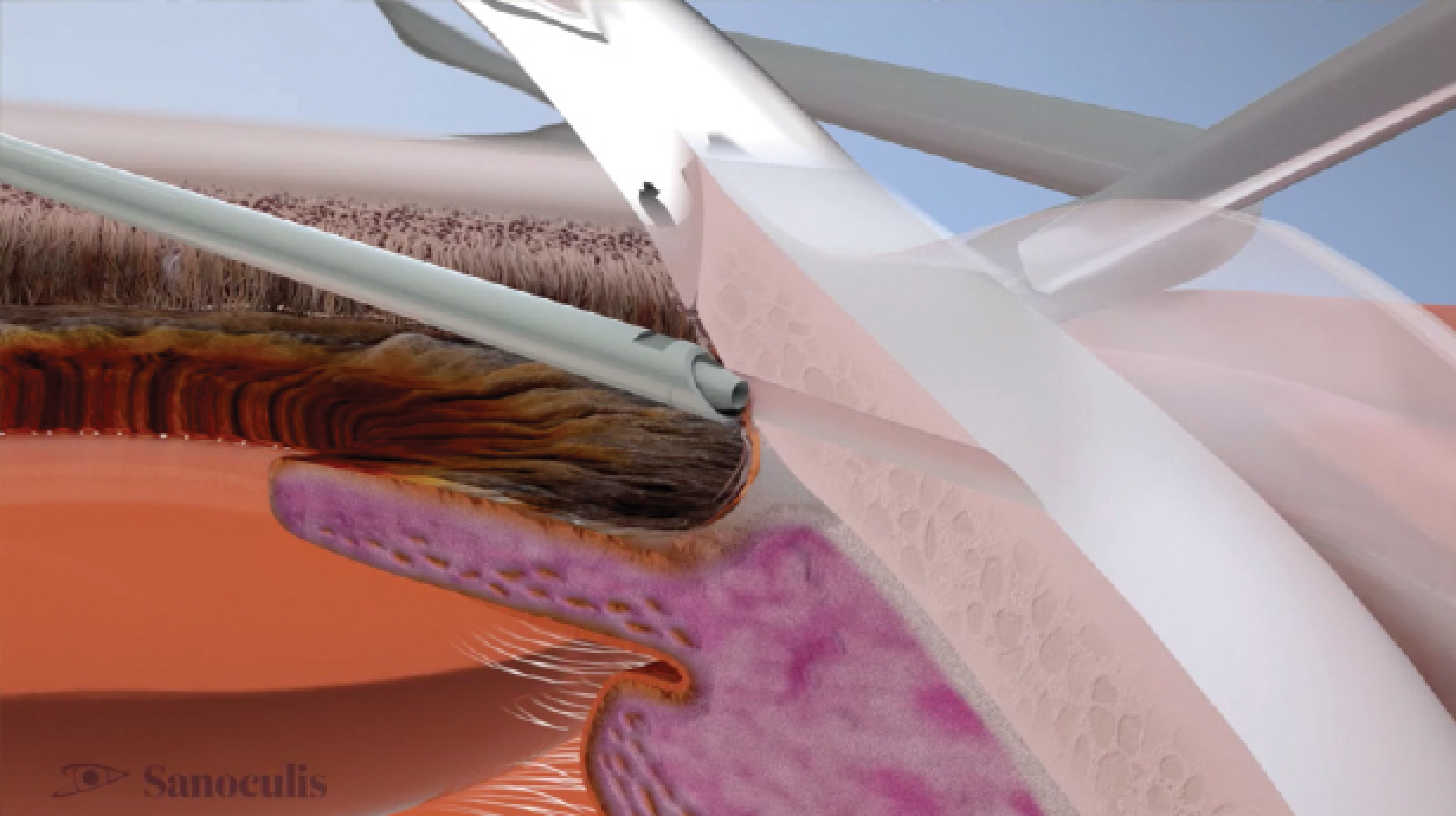
Minimally invasive micro sclerostomy, or MIMS (Sanoculis), is a stentless procedure used to create a sclerocorneal drainage channel for the reduction of IOP. MIMS is performed with a surgical system composed of a multiuse control touchscreen and footpedal and a disposable handpiece unit (Figure), either as a standalone procedure or in combination with cataract surgery. The procedure has received the CE Mark but is not yet approved by the FDA.
This article reviews the surgical steps of standalone MIMS and gives an overview of some experience and evidence reported to date for this new filtration procedure.
PREOPERATIVE STEPS OF STANDALONE MIMS
One week before surgery. One week before surgery, some surgeons begin steroid eye drops, instilled two to four times per day to with the aim to quiet the eye and prevent subconjunctival bleeding.
Just before surgery. Approximately 15 to 30 minutes before the start of surgery, one drop of Iopidine (apraclonidine 0.1%, Alcon) is instilled to constrict blood vessels and prevent subconjunctival bleeding. Next, some surgeons instill pilocarpine 2% to stretch the iris, make maximum room for the device in the angle, and constrict the pupil with the hope to maintain maximum protection of the lens.
INTRAOPERATIVE STEPS of STANDALONE MIMS
Topical anesthesia is used for MIMS. A topical antiseptic solution (eg, povidone-iodine 5%) is administered. After prepping and draping according to protocols, a wide-open Lieberman lid speculum is placed (preferably nasally). The three subsequent primary components of the procedure are as follows: (1) an injection of mitomycin C (MMC), (2) a subconjunctival injection of an OVD, and (3) MIMS.
No. 1: MMC Injection. A 30-gauge needle attached to a 1 cc syringe with MMC solution 0.3 mg/mL or 0.2 mg/mL (final concentration after dilution in lidocaine 2% solution) is prepared. Next, a subconjunctival injection of 0.1 mL of MMC mixture (20–30 μg of MMC) is performed in the superonasal quadrant, penetrating temporally 5 mm from the limbus and advancing the needle superonasally. MMC is spread with a sterile-tip applicator to the area where aqueous will be shunted through the sclerotomy while care is taken to protect the limbus and minimize the risk of an ischemic bleb. The ocular surface is then washed with 5 to 10 cc of balanced salt solution, and apraclonidine (Iopidine 0.1%, Alcon) is applied.
No. 2: Subconjunctival OVD Injection. A subconjunctival injection of 0.1 to 0.2 mL of an OVD is performed with a 30-gauge bent needle or a 27-gauge Atkinson peribulbar needle at the microtrephine’s intended exit location, close to the limbus. This step can be performed before or after the main incision is created and the anterior chamber is filled.
No. 3: MIMS. For the MIMS procedure, a temporal stab incision is created with a 1.5-mm surgical blade. OVD is injected into the anterior chamber until it is completely filled and fully formed. Next, the MIMS system is tested by observing it under the microscope and pressing the footpedal once. The microtrephine should come out of the protective sleeve, rotate clockwise to protrude 2.7 mm, and then rotate counterclockwise back into the sleeve. After this test, the MIMS surgical device is inserted via the stab incision and advanced gently above the iris plane toward the superonasal quadrant of the angle until the mark on the device tip can be seen at the edge of the limbus while leaning against the eye wall. Moderate counterpressure is then applied at the corneal limbus on both sides of the device sleeve with open forceps.
The MIMS footpedal is pressed to create the drainage channel, and there is a pause until the controller’s signal is heard. When the motion is complete, the MIMS instrument is withdrawn. The average cylinder of scleral tissue removed is typically 90 µm in diameter and 1,200 µm long.
BSS is injected into the anterior chamber, and OVD is removed. Sanoculis recommends removing approximately 50% of the OVD from phakic eyes and 60% to 70% of the OVD from pseudophakic eyes. Next, enlargement of the filtration bleb is typically observed. The eye is left with a fully formed anterior chamber via BSS and OVD. The paracentesis is closed by stromal hydration, the lid speculum is released, and the drape is removed. A topical steroid-antibiotic solution is administered.
INDICATED POPULATION
According to the company, the characteristics of the population for whom MIMS is indicated are as follows:
- Primary open-angle glaucoma ([POAG] including pseudoexfoliation and pigmentary glaucoma);
- Chronic angle-closure glaucoma only if pseudophakic or undergoing combined surgery;
- Uncontrolled glaucoma (IOP ≥ 21 mm Hg on maximum tolerated medical therapy), phakic or pseudophakic;
- Shaffer grade 3 or higher (wide-open angle) in the quadrant intended for surgery; and
- Mobile and elastic conjunctiva in the quadrant intended for surgery.
If a patient has a cataract, MIMS combined with cataract surgery is indicated.
EXPERIENCE AND EVIDENCE
MIMS has been performed in Israel, India, Armenia, and throughout Europe.
Outcomes in Armenia. Reported outcomes from Armenia have been positive to date. In a study of 120 eyes with OAG, 100 standalone MIMS procedures and 20 combined MIMS-phacoemulsification procedures were performed (data on file with Sanoculis). Mean patient age was 69 ±10.1 years. Complete success was defined as an IOP of 21 mm Hg or lower and IOP reduction from baseline of 20% or more on no hypotensive medications, and qualified success was defined as an IOP of 21 mm Hg or lower and IOP reduction of 20% or more on the same number of or fewer hypotensive medications.
Scleral tunnels were achieved in all cases. No device malfunctions, intraoperative complications, or serious adverse events were reported. Iris plugging of the sclerostomy site and early IOP spikes were the most common adverse events. At 52 weeks, mean IOP change from baseline was 38% (P < .001, n = 95), for an average reduction in IOP of 10.5 mm Hg (95% CI, -11.7, -9.3), with no statistically significant differences between the standalone MIMS and MIMS-phacoemulsification groups. The number of antiglaucoma medications was reduced by 85%, from 1.8 ±0.8 to 0.27 ±0.7. Qualified and complete successes were achieved in 72 (75.8%) and 62 (65.3%) of the 95 patients at 52 weeks, respectively.
The investigators concluded, “[the]MIMS procedure is relatively efficacious and safe for OAG patients with an uncontrolled IOP on medication and may be considered in glaucomatous patients who need surgical intervention.”
Outcomes in India. Geffen et al recently conducted a prospective, open-label, single-arm study of patients with OAG who were candidates for a filtration procedure.1 Mean patient age was 63.94 ±6.33 years, and all surgeries were performed by Amar Agarwal, MS, FRCS, FRCOphth. After MMC application, ab interno MIMS was performed alone or in combination with phacoemulsification. A total of 21 eyes underwent MIMS and phacoemulsification, and 10 eyes underwent standalone MIMS. Scleral tunnels were achieved in all cases.
No device malfunctions, intraoperative complications, or serious adverse events were reported by Geffen et al. Five (16.12%) patients presented with iris clogging 1 to 24 weeks after the procedure. Two underwent laser treatment, and three required trabeculectomy.
Mean IOP change from baseline at 24 weeks was 47.4% (31.2–16.4 mm Hg, P < .0001). The mean difference was -14.8 mm Hg (95% CI, -17.6, -11.9), with no statistically significant differences between groups. The number of antiglaucoma medications was reduced by 58%, from 1.16 ±0.97 to 0.5 ±0.76. Qualified success was achieved in 21 eyes (84%), 17 eyes (74%), and 13 eyes (93%) after 12, 24, and 52 weeks, respectively. Complete success was achieved in 17 eyes (68%), 13 eyes (57%), and eight eyes (57%) after 12, 24, and 52 weeks, respectively.
THE FUTURE
MIMS is a promising technology and technique that is minimally invasive, involves no implant, requires the use of MMC, and forms a bleb. The initial results are encouraging, and the FDA investigational process is underway. The procedure is currently being used and marketed in Europe and Israel. Real-world experience and published data will illuminate the path forward for MIMS. Additionally, as with all procedures, as more surgeons from around the world adopt MIMS, the technique itself, pre- and postoperative management, and methods of MMC application will continue to evolve and improve.
1. Geffen N, Kumar DA, Barayev E, et al. Minimally invasive micro sclerostomy (MIMS) procedure: a novel glaucoma filtration procedure. J Glaucoma. 2022;31(3):191-200.