WO2018003924A1 - Alkynylpyridine-substituted amide compound and pest control agent - Google Patents
Alkynylpyridine-substituted amide compound and pest control agent Download PDFInfo
- Publication number
- WO2018003924A1 WO2018003924A1 PCT/JP2017/023955 JP2017023955W WO2018003924A1 WO 2018003924 A1 WO2018003924 A1 WO 2018003924A1 JP 2017023955 W JP2017023955 W JP 2017023955W WO 2018003924 A1 WO2018003924 A1 WO 2018003924A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- atom
- hydrogen atom
- methyl
- haloalkyl
- Prior art date
Links
- 0 CC(C(*N)=C(C)C(C*)=*)=C(C)II Chemical compound CC(C(*N)=C(C)C(C*)=*)=C(C)II 0.000 description 5
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/40—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/48—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with two nitrogen atoms as the only ring hetero atoms
- A01N43/56—1,2-Diazoles; Hydrogenated 1,2-diazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/443—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with oxygen as a ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4436—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a heterocyclic ring having sulfur as a ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/444—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring heteroatom, e.g. amrinone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4965—Non-condensed pyrazines
- A61K31/497—Non-condensed pyrazines containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/10—Antimycotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/61—Halogen atoms or nitro radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D405/00—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom
- C07D405/02—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings
- C07D405/12—Heterocyclic compounds containing both one or more hetero rings having oxygen atoms as the only ring hetero atoms, and one or more rings having nitrogen as the only ring hetero atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D411/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen and sulfur atoms as the only ring hetero atoms
- C07D411/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen and sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D411/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen and sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D413/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D413/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings
- C07D413/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings
- C07D417/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
Definitions
- the present invention relates to a novel alkynylpyridine-substituted amide compound, an N-oxide thereof, or a salt thereof, and a pest control agent containing these compounds as active ingredients.
- alkynylpyridine-substituted amide compounds for example, N- [2- [3-chloro-5- (2-phenylethynyl) pyridin-2-yl] -1-methylethyl] -3-difluoromethyl-1- Methyl-1H-pyrazole-4-carboxamide exhibits pesticidal activity (see Patent Document 1), N- [2- [3-chloro-5- (2-phenylethynyl) pyridin-2-yl]- 2- (Isopropoxyimino) ethyl] -2- (trifluoromethyl) benzamide, N- [2- [3-chloro-5- [2- [4- (tert-butyl) phenyl] ethynyl] pyridine-2- Yl] -2- (isopropoxyimino) ethyl] -2- (trifluoromethyl) benzamide, N- [2- [3-chloro-5-
- Infections and infestations of pests such as pathogens and parasites can occur when the host is a plant such as cereals, fruit trees, vegetables, ornamental plants, etc. Cause serious economic losses not only to producers but also to consumers. Therefore, effective control of these pests is a very important issue in order to achieve efficient and stable crop production.
- the host is an animal such as a companion animal, pet animal, livestock, poultry, etc., for the purpose of maintaining the health of the target animal, and when the target animal is livestock, poultry, etc. Effective control of these pests is also an important issue for the purpose of stably producing safe food and high-quality living materials such as wool, feathers and leather.
- a novel alkynylpyridine-substituted amide compound represented by the following formula (I) has excellent pest control activity, particularly antifungal / nematicidal activity.
- the present invention has been completed by finding an extremely useful compound that exhibits insect activity and has almost no adverse effect on non-target organisms such as plants, mammals, fish, useful insects and natural enemies. That is, the present invention relates to the following [1] to [86].
- X 1 represents a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 3 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 1 -C 3 alkylthio, C 1- represents C 3 alkylsulfinyl, C 1 ⁇ C 3 alkylsulfonyl, C 1 ⁇ C 3 haloalkylthio or C 1 ⁇ C 3 haloalkylsulfonyl, X 2 represents a hydrogen atom or a halogen atom, provided that when G 1 is a structure represented by G 1 -10 and X 1 represents dihalomethyl, X 2 represents a hydrogen atom; X 3 represents a hydrogen atom or C 1 -C 4 alkyl, Y 1 represents a hydrogen atom, a halogen atom, nitro, C 1 -C 4 alkyl, C 1 -C 3 hal
- R 3 represents a hydrogen atom, C 1 -C 6 alkyl or C 1 -C 6 haloalkyl
- R 4 represents a hydrogen atom or C 1 -C 6 alkyl
- R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring together with the carbon atom to which R 3 and R 4 are bonded
- R 5 is a hydrogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, (C 1 -C 2 ) alkyl substituted by R 8 , C 3 -C 6 cycloalkyl, C 2 -C 4 alkenyl C 3 -C 4 alkynyl, —OH, C 1 -C 4 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 haloalkylthio, —C (O) R 9 or C 1 -C 4 alkoxycarbonyl
- R 6 represents C 3 -
- R 1 represents a fluorine atom, C 1 -C 6 alkyl or C 1 -C 6 alkoxy
- R 2 represents a fluorine atom or C 1 -C 6 alkyl
- R 3 represents C 1 -C 6 alkyl or C 1-
- R 6 is a hydrogen atom, a halogen atom, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl
- Z is a halogen atom, cyano, nitro, —SF 5 , C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, cyano (C 1 -C 4 ) alkyl, C 1 -C 4 alkoxy (C 1 -C 4 ) Alkyl, C 1 -C 4 haloalkoxy (C 1 -C 4 ) alkyl, C 1 -C 4 alkylthio (C 1 -C 4 ) alkyl, C 1 -C 4 alkylsulfinyl (C 1 -C 4 ) alkyl, C 1 -C 4 alkylsulfonyl (C 1 -C 4 ) alkyl, C 1 -C 4 haloalkylthio (C 1 -C 4 ) alkyl, C 1 -C 4 haloalkylsulfinyl (C 1 -C 4 ) alkyl, C 1 -C 4
- Z a represents a halogen atom, cyano, methyl, difluoromethyl, trifluoromethyl, cyclopropyl, methoxy, or trifluoromethylsulfonyl, and when n is 2 or more, each Z a may be the same as each other Or they may be different from each other,
- R 7 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl
- R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 , C 1 -C 4 alkylthio, C 1 -C 4 alkoxycarbonyl, —C (O) NH 2 or —C (S) NH 2
- R 9 represents C 1 -C 4 alkyl, C 1 -C 4 alkoxymethyl, C 3 -C 4 cycloalkyl or C 2 -C 4 alkenyl
- R 10 represents cyano, —OR 15 , —S (O)
- R 16 is C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy (C 1 -C 2 ) alkyl, C 3 -C 4 alkenyl, C 3 -C 4 haloalkenyl, C 3 -C 4 alkynyl, C 3 -C 4 haloalkynyl, phenyl or phenyl substituted by (Z) m
- R 17 and R 18 are each independently a hydrogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy (C 1 -C 2 ) alkyl, C 3 -C 4 alkenyl, Represents C 3 -C 4 alkynyl, phenyl or phenyl substituted by (Z) m ,
- R 17 and R 18 may be combined to form a C 2 -C 5 alkylene chain to form a 3- to 6-
- R 19 represents C 1 -C 4 alkyl, and when p is 2, each R 19 may be the same as or different from each other; m represents 1, 2, 3, 4 or 5; n represents 0, 1, 2, 3 or 4; p represents 0, 1 or 2; r represents 0, 1 or 2. ]
- G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -9, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented
- X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl, difluoromethylsulfonyl or trifluoromethylsulfonyl
- X 2 represents a hydrogen atom, a fluorine atom or a chlorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom.
- X 3 represents methyl
- Y 1 represents a halogen atom, methyl, trifluoromethyl or methoxy
- Y 2 represents a hydrogen atom or methyl
- Y 3 represents a hydrogen atom
- R 1 represents a hydrogen atom, a fluorine atom, C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, benzyl, (Z) m- substituted phenylmethyl, cyclopropyl, C 1 -C 3 alkoxy, C 1- C 3 haloalkoxy, C 3 -C 4 alkenyloxy, C 3 -C 4 alkynyloxy, cyanomethoxy, benzyloxy, phenylmethoxy substituted by (Z) m , C 1 -C 3 alkylthio or C 1 -C 3 Represents haloalkylthio
- R 2 represents a hydrogen atom, a fluorine atom or C 1 -C 3
- Two Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —, —OCH 2 O—, —CH 2 CH 2 S (O) r —, —CH 2 CH 2.
- the hydrogen atom bonded to each carbon atom forming the ring may be optionally substituted by a halogen atom, cyano, methyl, difluoromethyl, trifluoromethyl or methoxy
- Z a represents a halogen atom, methyl or trifluoromethyl
- R 7 represents methyl or ethyl
- R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 , C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2
- R 9 represents C 1 -C 4 alkyl or C 3 -C 4 cycloalkyl
- R 10 represents —OR 15 or —S (O) r R 16 ;
- R 11 represents a hydrogen atom or methyl
- R 12 represents methyl or ethyl
- R 13 represents C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 3 -
- G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
- X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl
- X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
- Y 1 represents a halogen atom or trifluoromethyl, Y 2 represents a hydrogen atom,
- R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy
- R 2 represents a
- G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -7, G 1 -8, represents a structure represented by G 1 -10
- Y 1 represents a halogen atom
- Y 2 represents a hydrogen atom
- R 1 represents a hydrogen atom, a fluorine atom, methyl, methoxy, ethoxy, trifluoroethoxy, allyloxy, propargyloxy, benzyloxy, methylthio
- R 2 represents a hydrogen atom, a fluorine atom or methyl
- R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded
- R 3 represents a hydrogen atom or methyl
- R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , C 2 -C 4 alkenyl,
- a 5-membered ring or a 6-membered ring may be formed together with the carbon atom to which each Z is bonded.
- the hydrogen atom bonded to the atom may optionally be substituted by a halogen atom, methyl or trifluoromethyl, R 7 represents methyl, R 9 represents C 1 -C 4 alkyl, R 14 represents C 1 -C 4 alkyl, m represents 1, 2 or 3; n represents 0, 1 or 2;
- G 1 represents a structure represented by G 1 -1, G 1 -2, G 1 -3, G 1 -4 or G 1 -10;
- X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
- X 2 represents a hydrogen atom
- Y 1 represents a halogen atom
- R 1 represents a hydrogen atom, a fluorine atom or methyl;
- R 5 represents a hydrogen atom,
- R 6 represents phenyl substituted by (Z) m ,
- Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, triflu
- the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom or a trif May be optionally substituted by Oromechiru
- m represents 1, 2 or 3
- n 0, [4]
- G 1 represents a structure represented by G 1 -1, G 1 -2, G 1 -3, G 1 -4 or G 1 -10;
- X 1 represents a halogen atom, difluoromethyl or trifluoromethyl,
- X 2 represents a hydrogen atom,
- Y 1 represents a halogen atom,
- R 1 represents a hydrogen atom, a fluorine atom or methyl;
- R 2 represents a hydrogen atom, a fluorine atom or methyl,
- R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
- R 4 represents a hydrogen atom,
- R 5 represents a hydrogen atom, ethyl, cyanomethyl, propynyl or —C (O) R 9 ;
- R 6 represents phenyl substituted by (Z) m , Z represents a halogen atom, trifluoromethyl,
- the hydrogen atom bonded to each carbon atom forming may be optionally substituted with a halogen atom, R 9 represents methyl, m represents 1, 2 or 3;
- R 9 represents methyl, m represents 1, 2 or 3;
- G 1 represents a structure represented by G 1 -2 or G 1 -10;
- R 1 represents a hydrogen atom or methyl;
- R 2 represents a hydrogen atom or methyl,
- R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
- R 3 represents a hydrogen atom or methyl,
- R 5 represents a hydrogen atom,
- Z represents a halogen atom, m represents 1;
- [6] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [6].
- G 1 represents the structure represented by G 1 -1
- X 1 represents a halogen atom, nitro, methyl, trifluoromethyl or methylsulfonyl
- X 2 represents a hydrogen atom
- G 1 represents the structure represented by G 1 -1, X 1 and X 2 represent a hydrogen atom, The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
- G 1 represents a structure represented by G 1 -1
- X 1 represents a halogen atom or trifluoromethyl
- G 1 represents the structure represented by G 1 -1
- X 1 represents a halogen atom, methyl or trifluoromethyl
- X 2 represents a hydrogen atom or a fluorine atom
- G 1 represents a structure represented by G 1 -2 or G 1 -3
- X 1 represents a halogen atom, methyl, difluoromethyl, trifluoromethyl, methylthio, methylsulfonyl or difluoromethylsulfonyl
- G 1 represents the structure represented by G 1 -2
- X 1 represents methoxy or trifluoromethylsulfonyl
- G 1 represents the structure represented by G 1 -4, X 1 represents a halogen atom or trifluoromethyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
- G 1 represents a structure represented by G 1 -4, X 1 represents methyl or difluoromethyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
- G 1 represents a structure represented by G 1 -5 or G 1 -6
- X 1 represents difluoromethyl or trifluoromethyl
- X 3 represents a hydrogen atom or methyl
- G 1 represents a structure represented by G 1 -7 or G 1 -8
- X 1 represents a halogen atom, methyl or trifluoromethyl
- G 1 represents a structure represented by G 1 -7 or G 1 -8, X 1 represents difluoromethyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
- G 1 represents the structure represented by G 1 -9
- X 1 represents difluoromethyl or trifluoromethyl
- R 7 represents methyl
- G 1 represents a structure represented by G 1 -10;
- X 1 represents difluoromethyl or trifluoromethyl,
- X 2 represents a hydrogen atom,
- R 7 represents methyl,
- G 1 represents a structure represented by G 1 -10
- X 1 represents methyl, ethyl or trifluoromethyl
- X 2 represents a fluorine atom or a chlorine atom
- R 7 represents methyl
- G 1 represents a structure represented by G 1 -11 or G 1 -12
- X 1 represents difluoromethyl or trifluoromethyl
- X 3 represents a hydrogen atom or methyl
- G 1 represents a structure represented by G 1 -13
- X 1 represents difluoromethyl or trifluoromethyl
- X 3 represents methyl
- G 1 represents the structure represented by G 1 -14, X 1 represents difluoromethyl or trifluoromethyl, X 3 represents methyl R 7 represents methyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
- G 1 represents the structure represented by G 1 -15, X 1 represents difluoromethyl or trifluoromethyl, R 7 represents methyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
- G 1 represents a structure represented by G 1 -16, X 1 represents methyl or trifluoromethyl, r represents 0, 1 or 2;
- Y 1 represents a halogen atom
- Y 2 and Y 3 are hydrogen atoms
- Y 1 represents methyl, trifluoromethyl or methoxy
- Y 2 and Y 3 are simultaneously hydrogen atoms
- Y 1 represents a halogen atom or methyl
- Y 2 represents methyl
- Y 3 represents a hydrogen atom
- R 1 represents C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, benzyl and phenylmethyl substituted by (Z) m
- R 2 , R 3 and R 4 represent a hydrogen atom
- Z represents a halogen atom, cyano, nitro, methyl, trifluoromethyl, methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio or trifluoromethylsulfonyl.
- R 1 represents C 1 -C 3 alkyl
- R 2 represents a hydrogen atom
- R 3 represents methyl
- R 4 represents a hydrogen atom
- R 1 represents C 1 -C 3 alkyl
- R 2 represents a fluorine atom and methyl
- R 3 and R 4 represent a hydrogen atom
- R 1 represents C 1 -C 3 alkyl
- R 2 represents a fluorine atom and methyl
- R 3 represents methyl
- R 4 represents a hydrogen atom
- R 1 represents phenylmethyl or cyclopropyl substituted by (Z) m ;
- R 2 , R 3 and R 4 represent a hydrogen atom,
- R 4 represents a hydrogen atom,
- Z represents a halogen atom,
- m represents 1 or 2
- each Z may be the same as or different from each other.
- the alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
- R 1 is phenyl substituted by C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 3 -C 4 alkenyloxy, C 3 -C 4 alkynyloxy, cyanomethoxy, benzyloxy, (Z) m Represents methoxy, C 1 -C 3 alkylthio or C 1 -C 3 haloalkylthio, R 2 represents a hydrogen atom, R 3 represents a hydrogen atom and methyl, R 4 represents a hydrogen atom, Z represents a halogen atom, cyano, nitro, methyl, trifluoromethyl, methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio or trifluoromethylsulfonyl.
- R 1 represents a fluorine atom, methyl, methoxy, ethoxy, trifluoroethoxy, allyloxy, propargyloxy, benzyloxy or methylthio;
- R 2 represents a hydrogen atom,
- R 3 represents a hydrogen atom and methyl,
- R 4 represents a hydrogen atom,
- the alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
- R 1 and R 2 each independently represents a fluorine atom or methyl
- R 3 and R 4 represent a hydrogen atom
- R 1 represents a fluorine atom or methyl
- R 2 represents a fluorine atom
- R 3 represents methyl or ethyl
- R 4 represents a hydrogen atom or methyl
- R 1 and R 2 represent a hydrogen atom
- R 3 represents a hydrogen atom or methyl
- R 4 represents a hydrogen atom
- R 1 and R 2 represent a hydrogen atom
- R 3 represents C 1 -C 3 alkyl or C 1 -C 3 haloalkyl
- R 4 represents a hydrogen atom or C 1 -C 3 alkyl
- R 1 and R 2 represent a hydrogen atom
- R 3 and R 4 represent methyl
- R 3 and R 4 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are attached
- the alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
- R 1 and R 2 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are attached;
- R 3 and R 4 represent a hydrogen atom, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
- R 1 and R 2 together form a C 1 -C 2 alkylidene or C 1 -C 2 haloalkylidene;
- R 3 and R 4 represent a hydrogen atom, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
- R 5 represents a hydrogen atom, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 5 represents C 1 -C 4 alkyl, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 5 represents (C 1 -C 2 ) alkyl substituted by R 8 , R 8 represents cyano, C 3 -C 6 cycloalkyl or —OR 14 ; R 14 represents C 1 -C 4 alkyl, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 5 represents (C 1 -C 2 ) alkyl substituted by R 8
- R 8 represents —OR 14 , C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2
- R 14 represents C 2 -C 4 haloalkyl, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 5 represents cyclopropyl, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 5 represents C 2 -C 4 alkenyl or C 3 -C 4 alkynyl, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 5 represents C 1 -C 4 haloalkylthio, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 5 represents —C (O) R 9 or C 1 -C 4 alkoxycarbonyl
- R 9 represents C 1 -C 4 alkyl
- R 14 represents C 1 -C 4 alkyl
- R 5 represents -C (O) R 9 ;
- R 9 represents C 3 -C 4 cycloalkyl, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 5 represents C 3 -C 6 cycloalkyl, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 5 represents C 1 -C 4 alkoxy, The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
- R 6 represents C 1 -C 4 alkyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents C 3 -C 6 cycloalkyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents (C 1 -C 4 ) alkyl optionally substituted by R 10 ;
- R 10 represents -OR 15 ;
- R 15 represents a hydrogen atom, methyl, ethyl or C 1 -C 2 haloalkyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents tri (C 1 -C 4 alkyl) silyl; The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents phenyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
- Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ⁇ C 4 alkylsulfonyl, C 1 ⁇ C 4 haloalkylthio, C 1 ⁇ C 4 haloalkylsulfinyl, C 1 ⁇ C 4 haloalkylsulfonyl, D-31, D-32 or D-34,
- each Z may be the same as or different from each other.
- R 6 is, represents phenyl substituted by (Z) m
- Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio
- trifluoro M represents methylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34, and when m represents 2 or 3, each Z may be the same as or different from each other.
- Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio, trifluoro Represents methylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34, [62] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [62].
- G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
- X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl
- X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
- Y 1 represents a halogen atom or trifluoromethyl, Y 2 represents a hydrogen atom,
- R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy
- R 2 represents a
- the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or tri- Optionally substituted by fluoromethyl, [62]
- G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
- X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl
- X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
- Y 1 represents a halogen atom or trifluoromethyl, Y 2 represents a hydrogen atom,
- R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy
- R 2 represents a
- Z is a halogen atom, methyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, C 1 -C 4 alkylsulfonyl, C 1 -C 4 haloalkylthio, C 1 -C 4 Haloalkylsulfinyl, C 1 -C 4 haloalkylsulfonyl, D-31 to D-35 or D-37, and when m represents 2, 3, 4 or 5, each Z may be the same as each other Or when two Zs are adjacent to each other, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —.
- the hydrogen atom bonded to may be optionally substituted by a halogen atom, cyano, methyl, difluoromethyl, trifluoromethyl or methoxy
- Z a represents a halogen atom, methyl or trifluoromethyl
- R 13 represents C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 3 -C 4 cycloalkylmethyl or C 3 -C 4 cycloalkyl
- m represents 1, 2, 3, 4 or 5
- n represents 0 or 1
- the alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents D-1, D-2, D-6, D-8, D-23, D-24, D-25 or D-26;
- Z represents a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy or C 1 -C 4 alkylsulfonyl, and n is 2, 3 or 4
- the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trimethyl.
- R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl
- n represents 0, 1, 2, 3 or 4;
- R 6 represents D-1, D-2, D-6, D-8 or D-23
- Z represents a halogen atom, C 1 -C 4 alkyl or trifluoromethyl, and when n represents 2, each Z may be the same as or different from each other
- the alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents a hydrogen atom, The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents a halogen atom or C 1 -C 4 haloalkyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents (C 1 -C 4 ) alkyl optionally substituted by R 10 ;
- R 10 represents -S (O) r R 16 ;
- R 16 represents methyl, ethyl or C 1 -C 2 haloalkyl, r represents 0, 1 or 2;
- the alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 6 represents C 3 -C 6 halocycloalkyl, The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
- R 1 represents a fluorine atom
- R 2 represents a fluorine atom
- R 3 represents methyl
- R 4 represents a hydrogen atom
- R 6 represents C 1 -C 4 haloalkyl or C 3 -C 6 cycloalkyl
- a hydrogen atom bonded to each carbon atom forming the ring is arbitrarily selected by a halogen atom, methyl or trifluoromethyl. May be replaced with R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl, m represents 1, 2 or 3; n represents 0, 1, 2 or 3. ]
- a pesticidal composition comprising as an active ingredient at least one selected from the group consisting of the alkynylpyridine-substituted amide compounds according to any one of [1] to [75], their N-oxides and their salts .
- [78] [1] to [75], wherein the alkynylpyridine-substituted amide compound according to any one of [1] to [75], an N-oxide thereof, and a salt thereof as an active ingredient contain at least one selected from the group consisting of Fungal agent or parasite control agent composition.
- the compound of the present invention represented by the formula (I) and a pest control agent containing the compound as an active ingredient are excellent control for pests in the fields of agriculture, horticulture and livestock / hygiene, especially fungi and linear animals. It exerts its effect and exerts a sufficient control effect against those pests that have acquired resistance to existing drugs. Furthermore, there is almost no adverse effect on non-target organisms such as plants, mammals, fish, useful insects and natural enemies, low persistence and light environmental impact. Therefore, the present invention can provide a useful novel pest control agent.
- an optically active substance resulting from the presence of one or more asymmetric carbon atoms may exist depending on the substituent.
- the present invention encompasses all optically active forms or racemates.
- geometric isomers of E-form and Z-form may exist depending on the substituent, but the present invention is not limited to these E-form, Z-form or E-form. And a mixture containing the Z-form in an arbitrary ratio.
- halogen atom examples include a fluorine atom, a chlorine atom, a bromine atom, and an iodine atom.
- the notation “halo” also represents these halogen atoms.
- n- is “normal”
- i- is “iso”
- sec- is “secondary”
- tert- is “tarsha”.
- Lee means each.
- C a -C b alkyl represents a straight or branched hydrocarbon having a to b carbon atoms, for example, methyl, ethyl, n-propyl, i-propyl, n- Specific examples include butyl, i-butyl, sec-butyl, tert-butyl, pentyl, 1-ethylpropyl, 2,2-dimethylpropyl, hexyl, etc., each selected within the specified number of carbon atoms. .
- C a -C b haloalkyl refers to a linear or branched hydrocarbon having a to b carbon atoms in which a hydrogen atom bonded to a carbon atom is optionally substituted with a halogen atom.
- the halogen atoms when being substituted by two or more halogen atoms, the halogen atoms may be the same as or different from each other.
- C a -C b cycloalkyl represents a cyclic hydrocarbon having a carbon number of a to b, and may form a monocyclic or complex ring structure of 3 to 10 members. I can do it. Each ring may be optionally substituted with alkyl within the range of the specified number of carbon atoms. Specific examples include, for example, cyclopropyl, cyclobutyl, 1-methylcyclopropyl, 2-methylcyclopropyl, cyclopentyl, 2,2-dimethylcyclopropyl, 1-methylcyclobutyl, cyclohexyl and the like. The range is selected.
- C a -C b halocycloalkyl represents a cyclic hydrocarbon having a to b carbon atoms in which a hydrogen atom bonded to a carbon atom is optionally substituted by a halogen atom, A monocyclic or complex ring structure from a ring to a 10-membered ring can be formed.
- Each ring may be optionally substituted with alkyl within the range of the specified number of carbon atoms, and the substitution with a halogen atom may be a ring structure portion, a side chain portion, or It may be both, and when it is substituted by two or more halogen atoms, the halogen atoms may be the same as or different from each other.
- C a -C b alkenyl refers to an unsaturated hydrocarbon having a linear or branched chain of a to b carbon atoms and having at least one double bond in the molecule.
- Specific examples include -methyl-3-butenyl and the like, each selected within a range of the specified number of carbon atoms.
- C a -C b haloalkenyl is a linear or branched chain having a carbon number of a to b in which a hydrogen atom bonded to a carbon atom is optionally substituted with a halogen atom, and It represents an unsaturated hydrocarbon having at least one double bond in the molecule. At this time, when substituted by two or more halogen atoms, these halogen atoms may be the same as or different from each other.
- C a -C b cycloalkenyl refers to a cyclic unsaturated hydrocarbon having at least one double bond and having from 3 to 10 members. Monocyclic or complex ring structures up to the ring can be formed. Each ring may be optionally substituted with alkyl within the range of the specified number of carbon atoms, and the double bond may be either endo- or exo-. Specific examples include 1-cyclopentenyl, 2-cyclopentenyl, 1-cyclohexenyl, 2-cyclohexenyl, bicyclo [2.2.1] -5-hepten-2-yl, and the like. It is selected in the range of the number of atoms.
- C a -C b halocycloalkenyl is a cyclic structure comprising at least one carbon atom in which a hydrogen atom bonded to a carbon atom is optionally substituted by a halogen atom, and at least one in the molecule. It represents an unsaturated hydrocarbon having at least two double bonds, and can form a monocyclic or complex ring structure of 3 to 10 members. Each ring may be optionally substituted with alkyl within the range of the specified number of carbon atoms, and the double bond may be either endo- or exo-. At this time, when substituted by two or more halogen atoms, these halogen atoms may be the same as or different from each other. For example, 2-chloro-1-cyclobutenyl, 2-bromo-1-cyclopentenyl and the like are listed as specific examples, and each is selected within the range of the designated number of carbon atoms.
- C a -C b alkynyl represents an unsaturated hydrocarbon having a linear or branched chain of a to b carbon atoms and having at least one triple bond in the molecule.
- Specific examples include methyl-1-pentynyl, 4-methyl-1-pentynyl, 3,3-dimethyl-1-butynyl and the like, each selected in the range of the designated number of carbon atoms.
- C a ⁇ C b haloalkynyl represents a hydrogen atom bonded to carbon atom is optionally substituted by halogen atom, carbon atoms in a ⁇ b number than consisting linear or branched, and An unsaturated hydrocarbon having at least one triple bond in the molecule. At this time, when substituted by two or more halogen atoms, these halogen atoms may be the same as or different from each other.
- C a -C b alkoxy represents alkyl-O— as defined above consisting of a to b carbon atoms, eg methoxy, ethoxy, n-propyloxy, i-propyloxy, n Specific examples include -butyloxy, i-butyloxy, sec-butyloxy, tert-butyloxy, pentyloxy, hexyloxy, and the like, each selected within the range of the specified number of carbon atoms.
- C a -C b haloalkoxy represents haloalkyl-O— as defined above consisting of a to b carbon atoms, eg difluoromethoxy, trifluoromethoxy, chlorodifluoromethoxy, bromodifluoromethoxy.
- C a -C b alkenyloxy represents alkenyl-O— having the above-mentioned meanings consisting of a to b carbon atoms, for example 2-propenyloxy, 2-butenyloxy, 1-methyl-2 Specific examples include -propenyloxy, 2-methyl-2-propenyloxy, 3-methyl-2-butenyloxy, 1,1-dimethyl-2-propenyloxy, etc., selected in the range of each designated number of carbon atoms Is done.
- C a -C b haloalkenyloxy represents haloalkenyl-O— as defined above, consisting of a to b carbon atoms, for example 2-fluoro-2-propenyloxy, 2-chloro -2-propenyloxy, 3-chloro-2-propenyloxy, 3,3-difluoro-2-propenyloxy, 2,3-dichloro-2-propenyloxy, 3,3-dichloro-2-propenyloxy, 2, Specific examples include 3,3-trifluoro-2-propenyloxy and the like, each selected within the range of the designated number of carbon atoms.
- C a -C b alkynyloxy represents alkynyl-O— as defined above consisting of a to b carbon atoms, for example 2-propynyloxy, 2-butynyloxy, 1-methyl-2 Specific examples include -propynyloxy, 1,1-dimethyl-2-propynyloxy, etc., each selected within the specified number of carbon atoms.
- C a -C b haloalkynyloxy represents haloalkynyl-O— as defined above consisting of a to b carbon atoms, eg 3-chloro-2-propynyloxy, 3-bromo Specific examples include -2-propynyloxy, 3-iodo-2-propynyloxy, and the like, and each is selected within the specified number of carbon atoms.
- C a -C b alkylthio represents alkyl-S— as defined above, comprising a to b carbon atoms, for example methylthio, ethylthio, n-propylthio, i-propylthio, n-butylthio. Specific examples include i-butylthio, sec-butylthio, tert-butylthio and the like, each selected within the range of the designated number of carbon atoms.
- C a -C b alkylsulfinyl represents alkyl-S (O) — as defined above, comprising a to b carbon atoms, for example methylsulfinyl, ethylsulfinyl, n-propylsulfinyl, Specific examples include i-propylsulfinyl and the like, each selected within the range of the specified number of carbon atoms.
- C a -C b alkylsulfonyl represents alkyl-SO 2 — having the above-mentioned meanings consisting of a to b carbon atoms, such as methylsulfonyl, ethylsulfonyl, n-propylsulfonyl, i- Specific examples include propylsulfonyl and the like, and each is selected within the range of the designated number of carbon atoms.
- C a -C b haloalkylthio represents haloalkyl-S—, as defined above, comprising a to b carbon atoms, for example difluoromethylthio, trifluoromethylthio, chlorodifluoromethylthio, trichloromethylthio, Bromodifluoromethylthio, 2,2,2-trifluoroethylthio, 1,1,2,2-tetrafluoroethylthio, 2-chloro-1,1,2-trifluoroethylthio, pentafluoroethylthio, 1, Specific examples include 1,2,3,3,3-hexafluoropropylthio, heptafluoropropylthio, 1,2,2,2-tetrafluoro-1- (trifluoromethyl) ethylthio, nonafluorobutylthio and the like. Selected within a range of each specified number of carbon atoms.
- C a -C b haloalkylsulfinyl represents the above-mentioned haloalkyl-S (O) — having from a to b carbon atoms, such as difluoromethylsulfinyl, trifluoromethylsulfinyl and the like. As an example, each selected range of carbon atoms is selected.
- the expression “C a -C b haloalkylsulfonyl” represents the above-mentioned haloalkyl-SO 2 — having a carbon number of a to b, for example, difluoromethylsulfonyl, trifluoromethylsulfonyl and the like. Each of which is selected for each specified number of carbon atoms.
- di (C a ⁇ C b alkyl) amino are both hydrogen atoms, the alkyl being the meaning of a good number of carbon atoms be the same or different from each other, each consisting of a ⁇ b Pieces
- a substituted amino is represented, for example, dimethylamino, ethyl (methyl) amino, diethylamino, di (n-propyl) amino and the like are selected as specific examples, each selected in the range of the specified number of carbon atoms.
- C a -C b alkoxyamino represents alkyl-ONH— as defined above consisting of a to b carbon atoms, eg methoxyamino, ethoxyamino, n-propyloxyamino, i- Specific examples include propyloxyamino, n-butyloxyamino, i-butyloxyamino, sec-butyloxyamino, tert-butyloxyamino, and the like, each selected within the range of the designated number of carbon atoms.
- tri (C a -C b alkyl) silyl represents silyl substituted by alkyl having the above meaning consisting of a to b carbon atoms which may be the same or different from each other. Specific examples include trimethylsilyl, triethylsilyl, tri (n-propyl) silyl, ethyldimethylsilyl, n-propyldimethylsilyl, n-butyldimethylsilyl, i-butyldimethylsilyl, tert-butyldimethylsilyl, and the like. Each selected range of carbon atoms is selected.
- C a -C b alkylcarbonyl represents alkyl-C (O) — as defined above, comprising a to b carbon atoms, eg acetyl, propionyl, butyryl, isobutyryl, valeryl, isovaleryl Specific examples include 2-methylbutanoyl, pivaloyl and the like, each selected within the range of the specified number of carbon atoms.
- C a -C b cycloalkylcarbonyl represents cycloalkyl-C (O) — which has the above-mentioned meaning consisting of a to b carbon atoms, such as cyclopropylcarbonyl, cyclobutylcarbonyl, 1 Specific examples include -methylcyclopropylcarbonyl, 2-methylcyclopropylcarbonyl, cyclopentylcarbonyl, 2,2-dimethylcyclopropylcarbonyl, cyclohexylcarbonyl and the like, each selected within the range of the number of carbon atoms specified.
- C a -C b alkoxycarbonyl represents alkyl-OC (O) — which has the above-mentioned meaning consisting of a to b carbon atoms, such as methoxycarbonyl, ethoxycarbonyl, n-propyloxycarbonyl. Specific examples include i-propyloxycarbonyl, n-butoxycarbonyl, i-butoxycarbonyl, tert-butoxycarbonyl and the like, each selected within the range of the designated number of carbon atoms.
- R 8 (C a ⁇ C b ) alkyl by any R 8, meaning the number of carbon atoms in which the hydrogen atoms bonded to carbon atoms are substituted is made of a ⁇ b Pieces Each selected from the range of each designated number of carbon atoms. At this time, when two or more substituents R 8 on (C a -C b ) alkyl are present, each R 8 may be the same as or different from each other.
- (C a -C b ) alkyl optionally substituted with R 10 refers to the number of carbon atoms in which a hydrogen atom bonded to a carbon atom is optionally substituted with any R 10 from a to b. Each of which is selected within the range of the specified number of carbon atoms. At this time, when there are two or more substituents R 10 on each (C a -C b ) alkyl, each R 10 may be the same as or different from each other.
- C a -C b cycloalkyl (C d -C e ) cycloalkyl, hydroxy (C d -C e ) cycloalkyl”, “C a -C b alkoxy (C d -C e ) cycloalkyl” or phenyl ( C d -C e ) cycloalkyl is represented by any C a -C b cycloalkyl, hydroxyl group, any C a -C b alkoxy, or phenyl, as defined above, to a carbon atom, respectively.
- the cycloalkyl having the above-mentioned meaning consisting of d to e carbon atoms substituted with bonded hydrogen atoms is represented and selected in the range of each designated number of carbon atoms.
- the expression “C a -C b alkylidene” represents a straight or branched hydrocarbon having a carbon number of a to b and bonded by a double bond, such as methylidene, ethylidene, propylidene, 1
- Specific examples include -methylethylidene, butylidene, 1-methylpropylidene, pentylidene, 1-methylbutylidene, 1-ethylethylidene, hexylidene, and the like, and each is selected within the range of the designated number of carbon atoms.
- C a -C b haloalkylidene is a linear or branched chain comprising a to b carbon atoms, in which a hydrogen atom bonded to a carbon atom is optionally substituted with a halogen atom, Represents a hydrocarbon bonded by a double bond.
- the halogen atoms may be the same as or different from each other.
- C a -C b alkoxy (C d -C e ) alkylidene is the number of carbon atoms in which a hydrogen atom bonded to a carbon atom is substituted by any C a -C b alkoxy having the above-mentioned meaning, respectively.
- cyano (C a -C b ) alkoxy cyano
- phenyl Or (Z) represents an alkoxy having the above meaning consisting of a to b carbon atoms in which a hydrogen atom bonded to a carbon atom is substituted by phenyl substituted by m , each of the designated number of carbon atoms Selected by range.
- R 1 and R 2 together form a C 2 -C 5 alkylene chain, where a 3- to 6-membered ring may be formed with the carbon atom to which R 1 and R 2 are attached
- the alkylene chain may contain 1 to 2 oxygen atoms, sulfur atoms or nitrogen atoms, and examples thereof include, for example, cyclopropane, oxirane, thiirane, aziridine, cyclobutane, oxetane, thietane, azetidine, cyclopentane, Rings such as oxolane, thiolane, pyrrolidine, dioxolane, dithiolane, cyclohexane, tetrahydropyran, tetrahydrothiopyran, piperidine, 1,3-dioxane, 1,3-dithiane, and the like, each selected within the specified number of atoms
- the alkylene chain may contain 1 to 2 oxygen atoms, sulfur
- R 17 and R 18 together form a C 2 -C 5 alkylene chain to form a 3- to 6-membered ring with the nitrogen atom to which R 17 and R 18 are bonded.
- the alkylene chain may contain one oxygen atom or sulfur atom.
- Specific examples of the notation include, for example, aziridine, azetidine, pyrrolidine, oxazolidine, thiazolidine, piperidine, 2H-3,4,5,6-tetrahydro- Examples include rings such as 1,3-oxazine, morpholine, 2H-3,4,5,6-tetrahydro-1,3-thiazine, thiomorpholine, and the like, and each is selected within the range of the specified number of atoms.
- a preferred range of the substituent of G 1 include G 1 -I ⁇ each group G 1 -xviii below. That is, G 1 -I: G 1 -1 [where X 1 represents a halogen atom, nitro, methyl, trifluoromethyl, or methylsulfonyl, and X 2 represents a hydrogen atom. ]. G 1 -II: G 1 -1 [where X 1 and X 2 represent a fluorine atom. ].
- G 1 -III G 1 -2 and G 1 -3 [wherein X 1 represents a halogen atom, methyl, difluoromethyl, trifluoromethyl, methylthio, methylsulfonyl or difluoromethylsulfonyl. ].
- G 1 -IV G 1 -4 [where X 1 represents a halogen atom or trifluoromethyl. ].
- G 1 -V G 1 -7 and G 1 -8 [wherein X 1 represents a halogen atom, methyl or trifluoromethyl. ].
- G 1 -VI G 1 -10 [where X 1 represents difluoromethyl or trifluoromethyl, X 2 represents a hydrogen atom, and R 7 represents methyl. ].
- G 1 -VII G 1 -14 [wherein X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents methyl. ].
- G 1 -VIII G 1 -1 [where X 1 represents a halogen atom or trifluoromethyl, and X 2 represents a fluorine atom or a chlorine atom. ].
- G 1 -IX G 1 -2 and G 1 -3 [wherein X 1 represents methoxy or trifluoromethylsulfonyl. ].
- G 1 -X G 1 -4 [where X 1 represents methyl or difluoromethyl. ].
- G 1 -XI G 1 -5 and G 1 -6 [wherein X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents a hydrogen atom or methyl. ].
- G 1 -XII G 1 -7 and G 1 -8 [where X 1 represents difluoromethyl. ].
- G 1 -XIV G 1 -10 [where X 1 represents methyl, ethyl or trifluoromethyl, X 2 represents a fluorine atom or a chlorine atom, and R 7 represents methyl. ].
- G 1 -XV G 1 -11 and G 1 -12 [where X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents a hydrogen atom or methyl. ].
- G 1 -XVI G 1 -13 [where X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents methyl. ].
- G 1 -XVII G 1 -15 [where X 1 represents difluoromethyl or trifluoromethyl, and R 7 represents methyl. ].
- G 1 -XVIII G 1 -16 [wherein X 1 represents methyl or trifluoromethyl, and r represents 0, 1 or 2. ].
- examples of combinations of preferable ranges of substituents represented by Y 1 , Y 2 and Y 3 include the following groups YI to Y-III. That, YI: Y 1 is a halogen atom, and Y 2 and Y 3 are simultaneously hydrogen atom.
- Y-III Y 1 is a halogen atom or methyl, Y 2 is methyl, and Y 3 is a hydrogen atom.
- YI and Y-II are more preferable as a combination of substituents represented by Y 1 , Y 2 and Y 3 , and YI is particularly preferable.
- examples of combinations of preferred ranges of substituents represented by R 1 , R 2 , R 3 and R 4 include the following groups of RI to R-XIV. That is, RI: R 1 and R 2 are hydrogen atoms, R 3 is a hydrogen atom or methyl, and R 4 is a hydrogen atom.
- R-II phenylmethyl wherein R 1 is substituted by (Z) m [wherein Z represents a halogen atom, m represents 1 or 2, and when m represents 2, each Z is They may be the same or different from each other. ] And cyclopropyl, and R 2 , R 3 and R 4 are hydrogen atoms.
- R-III R 1 is a fluorine atom, methyl, methoxy, ethoxy, trifluoroethoxy, allyloxy, propargyloxy, benzyloxy or methylthio, R 2 is a hydrogen atom, R 3 is a hydrogen atom or methyl, and R 4 is a hydrogen atom .
- R-IV R 1 and R 2 are each independently a fluorine atom or methyl, and R 3 and R 4 are hydrogen atoms.
- RV R 1 is a fluorine atom or methyl
- R 2 is a fluorine atom
- R 3 is methyl or ethyl
- R 4 is a hydrogen atom or methyl.
- R-VI R 1 and R 2 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded, and R 3 and R 4 are hydrogen atoms.
- R-VII R 1 and R 2 together form C 1 -C 2 alkylidene or C 1 -C 2 haloalkylidene, and R 3 and R 4 are hydrogen atoms.
- R-VIII R 1 and R 2 are hydrogen atoms, R 3 is C 1 -C 3 alkyl or C 1 -C 3 haloalkyl, and R 4 is hydrogen atom or C 1 -C 3 alkyl.
- R-IX a carbon atom to which R 3 and R 4 are bonded by R 1 and R 2 being a hydrogen atom and R 3 and R 4 being methyl or R 3 and R 4 being combined to form an ethylene chain Together with the cyclopropyl ring.
- R 1 is C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, benzyl or phenylmethyl substituted by (Z) m [where Z is a halogen atom, cyano, nitro, methyl, trifluoromethyl, Methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio or trifluoromethylsulfonyl; Each Z may be the same as or different from each other. ], And R 2, R 3 and R 4 are hydrogen atoms.
- R-XI R 1 is replaced by C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 3 -C 4 alkenyloxy, C 3 -C 4 alkynyloxy, cyanomethoxy, benzyloxy, (Z) m
- Z is a halogen atom, cyano, nitro, methyl, trifluoromethyl, methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio
- m represents 1 or 2
- each Z may be the same as or different from each other.
- R 2 is a hydrogen atom
- R-XII R 1 is C 1 -C 3 alkyl, R 2 is a hydrogen atom, R 3 is methyl, and R 4 is a hydrogen atom.
- R-XIII R 1 is C 1 -C 3 alkyl, R 2 is a fluorine atom or methyl, and R 3 and R 4 are hydrogen atoms.
- R-XIV R 1 is C 1 -C 3 alkyl, R 2 is a fluorine atom or methyl, R 3 is methyl, and R 4 is a hydrogen atom.
- RI to R-VII, R-VIII and R-XI are more preferable, and further, RI, R -III to RV, R-VI and R-VII are particularly preferred.
- examples of the preferred range of the substituent represented by R 5 include the following groups of R 5 -I to R 5 -XI. That is, R 5 -I: a hydrogen atom. R 5 -II: C 1 -C 4 alkyl. R 5 -III: (C 1 -C 2 ) alkyl substituted by R 8 [wherein R 8 represents cyano, C 3 -C 6 cycloalkyl or —OR 14 and R 14 represents C 1 -C 4 Represents alkyl. ].
- R 5 -IV cyclopropyl.
- R 5 -V C 2 -C 4 alkenyl or C 3 -C 4 alkynyl.
- R 5 -VI C 1 -C 4 haloalkylthio.
- R 5 -VII —C (O) R 9 [wherein R 9 represents C 1 -C 4 alkyl. Or C 1 -C 4 alkoxycarbonyl.
- R 5 -VIII (C 1 -C 2 ) alkyl substituted by R 8 [where R 8 is —OR 14 or C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2 represents R 14 represents C 2 -C 4 haloalkyl. ].
- R 5 -IX C 3 -C 6 cycloalkyl.
- R 5 -X C 1 -C 4 alkoxy.
- R 5 -XI —C (O) R 9 wherein R 9 represents C 3 -C 4 cycloalkyl. ].
- R 5 -I ⁇ R 5 -VIII and R 5 -XI as range of the substituent of R 5, further, R 5 -I ⁇ R 5 -III and R 5 -V ⁇ R 5 -VII is particularly preferred.
- examples of the preferred range of the substituent represented by R 6 include the following groups of R 6 -II to R 6 -XV. That is, R 6 -I: C 1 -C 4 alkyl. R 6 -II: C 3 -C 6 cycloalkyl. R 6 -III: optionally substituted by R 10 (C 1 ⁇ C 4 ) alkyl [wherein, R 10 represents -OR 15, R 15 is a hydrogen atom, methyl, ethyl or C 1 ⁇ C 2 haloalkyl Represents. ].
- R 6 -IV Tri (C 1 -C 4 alkyl) silyl.
- R 6 -V —CH ⁇ NOR 12 [wherein R 12 represents methyl or ethyl. ].
- R 6 -VI phenyl.
- R 6 -VII (Z) phenyl substituted by m [where Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 Haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio, trifluoromethylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34, when m represents 2 or 3,
- the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trifluoromethyl.
- R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl, m represents 1, 2 or 3 and n represents 0. ].
- R 6 -IX a hydrogen atom.
- R 6 -X a halogen atom or C 1 -C 4 haloalkyl.
- R 6 -XI optionally substituted by R 10 (C 1 ⁇ C 4 ) alkyl [wherein, R 10 represents -S (O) r R 16, R 16 is methyl, ethyl or C 1 ⁇ C Represents 2 haloalkyl, r represents 0, 1 or 2; ].
- R 6 -XII C 3 ⁇ C 6 halocycloalkyl.
- R 6 -XIV (Z) phenyl substituted by m [wherein Z is a halogen atom, methyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, C 1- C 4 alkylsulfonyl, represents a C 1 ⁇ C 4 haloalkylthio, C 1 ⁇ C 4 haloalkylsulfinyl, C 1 ⁇ C 4 haloalkylsulfonyl, D-31 ⁇ D-35 or D-37, m is 2, 3, 4 Or 5 may be the same as or different from each other, and when two Zs are adjacent, the two adjacent Zs are —CH 2 CH 2.
- the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, cyano.
- Za represents a halogen atom, methyl or trifluoromethyl
- R 13 represents C 1 -C 4 alkyl
- C 1 -C 4 represents haloalkyl, C 3 -C 4 cycloalkylmethyl or C 3 -C 4 cycloalkyl
- m represents 1, 2, 3, 4 or 5 and n represents 0 or 1; ].
- R 6 -XV D-1, D-2, D-6, D-8, D-23, D-24, D-25 or D-26
- a 6-membered ring may be formed together with the atoms, in which case the hydrogen atom bonded to each carbon atom forming the ring may be optionally substituted with a halogen atom, methyl or trifluoromethyl, and R 13 is Represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl, and n represents 0, 1, 2, 3 or 4.
- Each group which shows the preferable range of each substituent in this invention can be combined arbitrarily, respectively, Each represents the preferable range of this invention compound.
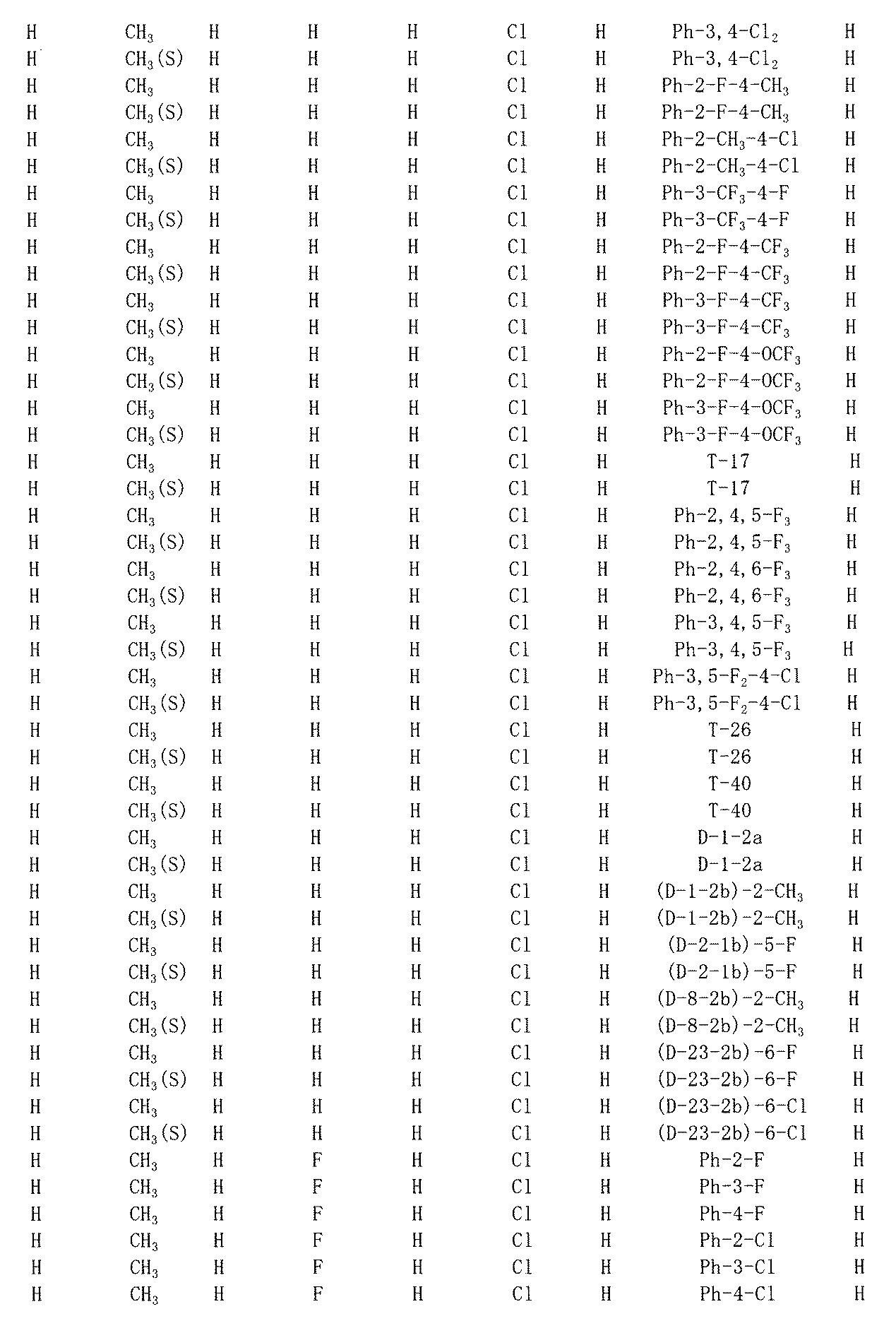
- Examples of preferable ranges of combinations of G 1 , R 1 to R 4 (indicated by RI to R-XIV) and R 6 of the compound represented by the formula (I) include, for example, combinations shown in the following Table 1. Is mentioned. However, the combinations in Table 1 are for illustrative purposes, and the compounds represented by Formula (I) are not limited to these.
- examples of the acid addition salt include hydrohalic acid salts such as hydrofluoric acid, hydrochloric acid, hydrobromic acid, hydroiodic acid, nitric acid, sulfuric acid, Salts of inorganic acids such as phosphoric acid, chloric acid, perchloric acid, salts of sulfonic acids such as methanesulfonic acid, ethanesulfonic acid, trifluoromethanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, formic acid, acetic acid, propionic acid , Salts of carboxylic acids such as trifluoroacetic acid, fumaric acid, tartaric acid, succinic acid, maleic acid, malic acid, succinic acid, benzoic acid, mandelic acid, ascorbic acid, lactic acid, gluconic acid, citric acid, glutamic acid, aspartic acid, etc.
- Examples include amino acid salts such as hydrofluoric acid
- examples of the metal salt include alkali metal salts such as lithium, sodium and potassium, alkaline earth metal salts such as calcium, barium and magnesium, and aluminum salts. It is done.
- the term “pest control agent” means a fungicide and a parasite control agent that are intended to control harmful pathogens and parasites that infect or infest plants or animals. It means fungicides and nematicides in the field of horticulture, or animal antifungals and parasite control agents.
- pathogenic bacteria means microorganisms that cause plant diseases and animal infectious diseases. Specific examples include the following microorganisms, but specific examples of microorganisms are not limited to these. It is not something.
- Taphrina spp. (Taphrina deformans, T. pruni, etc.), Pneumocystis spp., Geotrichum spp., Candida spp. (Candida albicans, C. sorbosa, etc.), Pichia spp. (Eg, Pichia kluyveri, etc.), Capnodiumgospps, Fnouma spp. ., Hypocapnodiumppspp., Cercospora spp. (Cercospora apii, C. asparagi, C. beticola, C. capsici, C. carotae, C. kaki, C. kikuchii, C.
- Cercosporidium spp., Clppspor. Cercosporidium spp., Clppspor. (Cladosporium colocasiae, C. cucumerinum, C. variabile, etc.), Davidiella spp., Didymosporium spp., Heterosporium spp. (Heterosporium allii, etc.), Mycosphaerella spp. M. fragariae, M. graminicola, M. nawae, M. pinodes, M. pomi, M. zingiberis, etc., Mycovellosiella spp. (Mycovellosiella fulva, M. nattrassii, etc.), Paracercospora spp.
- Botryosphaeria spp. Dothiorella spp., Fusicoccum spp., Guignardia spp., Lasiodiplodia spp. (Lasiodiplodia theobromae, etc.), Macrophoma spp., Macrophomina spp., Neofusicoccum spp., Phyllosticta umizpp , Leptosphaerulina spp., Aspergillus spp., Penicillium spp. (Penicillium digitatum, P. italicum, P.
- Trichophyton spp. Trichophyton mentagrophytes, T. plasmrubrum.
- Blumeria graminis f. Sp. Hordei, B. g. F. Sp. Tritici, etc. Erysiphe spp. (Erysiphe betae, E. cichoracearum, E. c. Var. Cichoracearum, E. heraclei, E. pisi, etc.), Golovinomyces spp. (Golovinomyces cichoracearum var. Latisporus etc.), Leveillula spp.
- Ciborinia spp. Grovesinia spp., Monilia mumecola, Monilinia spp. , M. fructigena, M. laxa, M. mali, M. vaccinii-corymbos i), Sclerotinia spp. (Sclerotinia borealis, S. homoeocarpa, S. minor, S. sclerotiorum, etc.), Valdensia spp. (Valdensia heterodoxa, etc.), Claviceps spp. (Claviceps sorghi, C.
- lactucae lactucae, F. o. f. sp. lagenariae, F. o. f. sp. lycopersici, F. o. f. sp. melongenae, F. o. f. sp. melonis, F. o. f. sp. nelumbinicola, F. o. f. sp. niveum, F. o. f. sp. radicis-lycopersici, F. o. f. sp. raphani, F. o. f. sp. spinaciae, F. sporotrichioides, F. solani, F. s. f.
- Septobasidium spp. (Septobasidium bogoriense, S. tanakae, etc.), Helicobasidium spp. (Helicobasidium longisporum, etc.), Coleosporium spp. (Coleosporium plectranthi, etc.), Cronartium spp. ), Physopella spp. (Physopella ampelopsidis etc.), Kuehneola spp. (Kuehneola japonica etc.), Phragmidium spp. (Phragmidium fusiforme, P. mucronatum, P. rosae-multiflorae etc.), Gymnosporangium spo.
- Puccinia spp. (Puccinia allii, P. brachypodii var. Poae-nemoralis, P. coronata, P. c. Var. Coronata, P. cynodontis, P. graminis, P. g. Subsp. Graminicola, P. hordei, P. horiana, P. kuehnii, P. melanocephala, P. recondita, P. striiformis var. Striiformis, P. tanaceti var. Tanaceti, P. tokyensis, P. zoysiae, etc.), Uromyces phasezuki, U.
- vexille ia, strom, strom spp.
- Tala caries, T. controversa, T. laevis, etc. Itersonilia spp. (Itersonilia perplexans, etc.), Cryptococcus spp., Bovista spp. (Bovista dermoxantha, etc.), Lycoperdon spp. (Lycoperdon pertis, etc.) , Conocybe spp. (Conocybe apala, etc.), Marasmius spp. (Marasmius oreades, etc.), Armillaria spp., Hellotium spp., Lepista spp.
- Chipidiomycota fungi such as Olpidium spp.
- Blastocladiomycota fungi such as Physoderma spp. Choanephora spp., Choanephoroidea cucurbitae, Mucor spp. (Mucor fragilis, etc.), Rhizopus spp. (Rhizopus arrhizus, R. chinensis, R. oryzae, R. stolonifer var. Stolonifer, etc.)
- Cercozoa protists such as Plasmodiophora spp. (Plasmodiophora brassicae, etc.), Spongospora subterranea f. Sp.
- Aphanomyces spp. (Aphanomyces cochlioides, A. raphani, etc.), Albugo spp. (Albugo macrospora, A. wasabiae, etc.), Bremia spp. (Bremia lactucae, etc.), Hyaloperonospora spp., Peronosclerospora spp., Peronospora spp. wasabi, P. chrysanthemi-coronarii, P. destructor, P. farinosa f. sp. spinaciae, P. manshurica, P. parasitica, P. sparsa, etc., Plasmopara spp.
- Pseudoperonospora spp. Pseudoperonospora cubensis, etc.
- Sclerophthora spp. Phytophthora spp. (Phytophthora cactorum, P. capsici, P. citricola, P. citrophthora, P. cryptogea, P. fragariae, P. infestans, P. infestans, P. nicotianae, P. palmivora, P. porri, P. sojae, P. syringae, P. vignae f. Sp.
- Pythium spp. Pythium afertile, P. aphanidermatum, P. apleroticum, P. aristosporum, P. arrhenomanes, P. buismaniae, P. debaryanum, P. graminicola, P. horinouchiense, P. irregulare, P. iwayamai, P. myrioty lum, P. okanoganense, P. paddicum, P. paroecandrum, P. periplocum, P. spinosum, P. sulcatum, P. sylvaticum, P. ultimum var. ultimum, P. vanterpoolii, P.
- Heteromony vexans, P. volutum, etc. Heteromonyphyta oomycetes (Oomycetes).
- Actinobacter gram-positive bacteria such as Clavibacter spp. (Clavibacter michiganensis subsp. Michiganensis etc.), Curtobacterium spp., Leifsonia spp. (Leifsonia xyli subsp. Xyli etc.), Streptomyces spp. (Streptomyces ipomoeae etc.)
- Firmicutes gram-positive fungi such as Clostridium sp.
- Tenericutes gram-positive fungi such as Phytoplasma. Rhizobium spp. (Rhizobium radiobacter, etc.), Acetobacter spp., Burkholderia spp. (Burkholderia andropogonis, B. cepacia, B. gladioli, B. glumae, B. plantarii, etc.), Acidovorax spp. (Acidovorax avenae subA. subsp. citrulli, A. konjaci, etc.), Herbaspirillum spp., Ralstonia spp.
- Xanthomonas spp. Xanthomonas albilineans, X. arboricola pv. pruni, X. axonopodis pv. vitians, p. campestris campestris, X. c. pv. cucurbitae, X. c. pv. glycines, X. c. pv. mangiferaeindicae, X. c. pv. nigromaculans, X. c. pv. vesicatoria, X. citri subsp. citri, X. oryzae pv.
- Pseudomonas spp. Pseudomonas cichorii, P. fluorescens, P. marginalis, P. m. Pv. Marginalis, P. savastanoi pv. Glycinea, P. syringae, P. s. Pv. Actinidiae , P. s. Pv. Eriobotryae, P. s. Pv. Helianthi, P. s. Pv. Lachrymans, P. s. Pv. Maculicola, P. s. Pv. Mori, P. s. Pv.
- plant diseases and animal infections caused by infection and growth of these pathogenic bacteria include the following plant diseases and animal infections, but are not limited thereto.
- Plant diseases Leaf curl (Taphrina deformans), Plum pockets (Taphrina pruni), Asparagus brown spot Leaf spot (Cercospora asparagi), sugar beet brown spot Cercospora leaf spot (Cercospora beticola), pepper spot Frogeye leaf spot (Cercospora capsici), oyster horn spot leaf disease Angular leaf spot (Cercospora kaki), soybean purpura purple stain (Cercospora kikuchii), groundnut brown leaf spot (Mycosphaerella arachidis), sweet cherry brown spot disease (Cylindrosporium leaf spot) Mycosphaerella cerasella, Blumeriella jaapii), wheat leaf blight, Speckled leaf blotch (Mycosphaerella graminicola), oyster lobster circular leaf spot (Mycosphaerella nawae), pea brown spot Mycosphaerella blight (Mycosphaerella pinodes), myaf spot Mycos
- cucumber powdery mildew Powdery mildew (Erysiphe betae, Leveillula taurica, Oidium sp., Podosphaera xanthii), eggplant udon Powdery mildew (Erysiphe cichoracearum, Leveillula taurica, Sphaerotheca fuliginea), carrots, parsley powdery mildew Powdery mildew (Erysiphe heraclei), pea powdery powdery mildew (Erysiphe pisi), tomato powdery mildew Poillery mildew Oidium neolycopersici, Oidium sp.), Pepper powdery mildew (Leveillula taurica), pumpkin powdery powdery mildew (Oidium sp., Podosphaera xanthii), powdery mildew (Oidium sp.), oyster powdery mildew Powdery mildew (Phyllactinia kaki
- Fig rust Rust (Phakopsora nishidana), soybean rust Rust (Phakopsorachypachyrhizi), rose rust Rust (Kuehneolanejaponica, hPhragmidiumragfusiforme, P. mucronatum, cronP.
- Rosae-multiflorae Rosae-multiflorae
- pear Red Star Rust Gamnosporangiumdayamadae
- Rust of Rubiaceae Rust Puccinia allii
- Chrysanthemum Rust Puccinia horiana
- Wheat Red Rust BrownBrrust Puccinia recondita
- Chrysanthemum Rust Puccinia tanaceti var.tanaceti
- Broad bean rust Rust Uromyces viciae-fabae var.
- Rhizopus rot Rhizopus stolonifer var. Stolonifer
- Clubroot Pierroot
- Sugar beet root disease Aphanomyces root rot (Aphanomyces cochlioides), white rust (Albugo macrospora) of Brassicaceae, lettuce downy mildew (Bremia lactucae), downy mildew (Peronospora chrysanthemi-coronarii), onion, Downy mildew (Peronospora destructor), spinach downy mildew (Peronospora farinosa f. Sp.
- Tomato Scab Bacterial canker (Clavibacter michiganensis subsp. Michiganensis), Potato Scab Scab (Streptomyces spp.), Crown gall (Rhizobium radiobacter), sorghum streak bacterial disease Bacterial stripe (Burkholderia andropogonis), onion rot Soft rot (Burkholderia cepacia, Pseudomonas marginalis pv. Marginalis, Erwinia rhapontici), rice blast Bacterial grain rot (Burkholderia gladioli, B. glumae), Bacterial fruit blotch (Acidovorax avenae subsp.
- Bacterial leaf blight (Acidovorax konjaci), Bacterial wilt (Ralrumonia soace) , Bacterial shot hole (Xanthomonas arboricola pv. Pruni, Pseudomonas syringae pv. Syringae, Brenneria nigrifluens), peach black spot Bacterial leaf spot (Xanthomonas arboricola pv. Pruni) vitians), cruciferous black rot (Xanthomonas campestris pv. campestris), soybean leaf burning Bacterial pustule (Xanthomonas ca mpestris pv.
- Bacterial canker Pseudomonas syringae pv. actinidiae
- loquat cancer canker Pseudomonas syringae pv. eriobotryae
- Bacterial spot Pseudomonas syringae pv.
- leek soft rot Bacterial soft rot (Dickeya sp., Pectobacterium carotovorum), Rosaceae Fire blight (Erwinia amylovora), konjac rot Soft rot (Pectobacterium carotovorum), soft rot Bacterial soft rot (Pectobacterium carotovorum).
- Animal infections Pneumocystis pneumonia (Pneumocystis jirovecii), Candidiasis (Candida albicans), Aspergillosis (Aspergillus fumigatus), Trichophytosis (Microsporum canis, M. gypseum, T. phytorum. verrucosum), Histoplasmosis (Histoplasma capsulatum), Cryptococcosis (Cryptococcus neoformans).
- parasite means plant parasitic linear animals parasitic on plants, animal parasitic linear animals parasitic on animals, bald animals, flat animals, protozoa, etc. Include, but are not limited to, the following parasites:
- Giant kidney worm (Dioctophyma renale), ringed capillary worm Thread worms (Capillaria annulata), torsion capillary nematode Cropworm (Capillaria contorta), liver capillary nematode Capillary liver worm (Capillaria hepatica), penetrating capillary nematode (Capillaria) perforans), Philippine Capillaria philippinensis, Capillaria suis, Caterpillar Whipworm (Trichuris discolor), Whipworm Whipworm (Trichuris ovis), Pig whipworm Pig Whipworm (Trichuris suis), human Enoplida nematodes such as the whipworm Human whipworm (Trichuris trichiura), the dog whipworm Dog whipworm (Trichuris vulpis), and the Trichinella pork worm (Trichinella spiralis).
- Nipple worm Nematode Intestinal threadworm (Strongyloides papillosus), Cat feline worm (Strongyloides planiceps), Pig worm nematode Pig threadworm (Strongyloides ransomi), Human dung beetle Threadworm (Strongyloides stercoralis), Micronema spp. Rhabditida nematode.
- Rice white nematode (Aphelenchoides besseyi), strawberry nematode, Strawberry foliar nematode (Aphelenchoides fragariae), chrysanthemum foliar nematode (Aphelenchoides ritzemabosi), pine wood nematode (Bineurus) Aphelenchida) Nematode.
- Potato cyst nematode (Globodera pallida), Potato cyst nematode (Globodera rostochiensis), Wheat cyst nematode (Heterodera avenae), Soybean cyst nematode cyst nematode (Heterodera schachtii), clover cyst nematode Clover cyst nematode (Heterodera trifolii), arenaria root nematode Peanut root-knot nematode (Meloidogyne arenaria), red root nematode Northern root-knot nematode (Meloidogyne incognita), Java root-knot nematode (Meloidogyne javanica), Apple root-knot nematode (Meloidogyne mali), Southern nematode sale nematode Cof fee root-lesion nematode (Pratylenchus coffeae), sawt
- Oxyurida nematodes such as human worm Pinworm (Enterobius vermicularis), horse worm Equine pinworm (Oxyuris equi), rabbit worm Rabbit pinworm (Passalurus ambiguus), Pig roundworm (Ascaris suum), Horse roundworm Horse roundworm (Parascaris equorum), Dog roundworm Dog roundworm (Toxascaris leonina), Dog roundworm Dog intestinal roundworm (Toxocara canis), Cat roundworm Feline roundworm (Toxocara cati), Cattle roundworm Large cattle roundworm (Toxocara vitulorum), Anisakis spp., Pseudoterranova spp., chicken caecal caecal worm (Heterakis gallinarum), chicken roundworm (Ascaridia galli) and other roundworms (Ascaridida) Nematode.
- human worm Pinworm Enterobius vermicularis
- Horse worm Equine pinworm Oxyuris
- Medinaworm Guinea worm (Dracunculus medinensis), Dolores jaw-and-mouth beetle (Gnathostoma doloresi), Spine-and-mouth-mouth beetle (Gnathostoma hispidum), Japanese jaw-and-mouth beetle (Gnathostoma nipponicum), Spiny-mouthed mouth-worm , Dog stomachworm Dog stomach worm (Physaloptera canis), cat stomachworm Cat stomach worm (Physaloptera felidis, P.
- Craniopods such as Moniliformis moniliformis and Giant thorny-headed worm (Macracanthorhynchus hirudinaceus). Artificial leaves such as Fish tapeworm (Diphyllobothrium latum), Diphyllobothrium nihonkaiense, Manson tapeworm (Spirometra erinaceieuropaei), Diplogonoporus grandis Eye (Pseudophyllidea) tapeworm.
- Wire worms (Mesocestoides lineatus), striped worms Chicken tapeworm (Raillietina cesticillus), spiny cleft worm Fowl tapeworm (Raillietina echinobothrida), square tapeworm Chicken tapeworm (Raillietina tetragona), canine tapeworm (Taenia hydatigena), Canine tapeworm (Taenia multiceps), sheep worm Sheep measles (Taenia ovis), beetle tapeworm Dog tapeworm (Taenia pisiformis), striped beetle Beef tapeworm (Taenia saginata), striped worm Tapeworm (Taenia serialis) ), Rodentworm Pork tapeworm (Taenia solium), caterpillar Feline tapeworm (Taenia taeniaeformis), single tapeworm Hydatid tapeworm (Echinococcus granulosus), multi-budworm, small fox tape
- Echinostoma cinetorchis Echinostoma hortense, Giant liver fluke (Fasciola gigantica), Liver common liver fluke (Fasciola hepatica), Fasciolopsis buski, Flat belly twin Echinostomida flukes such as Homalogaster paloniae.
- Amphimers (Amphimerus spp.), Liver fluke, Chinese liver fluke (Clonorchis sinensis), Cat liver fluke Cat liver fluke (Opisthorchis felineus), Thai liver fluke Southeast Aasian liver fluke (Opisthorchis viverrini), Pseudomust .), Metrokis genus (Metorchis spp.), Parametrochis genus (Parametorchis spp.), Heteromorphic flukes Intestinal fluke (Heterophyes heterophyes), Yokokawa flukes (Metagonimus yokokawai), foregut flukes (Pygidiopsis summa), etc. Opisthorchiida).
- Amoeba such as Entamoeba histolytica (E. invadens). Futago Babesia (Babesia bigemina), Cattle Babesia bovis, Big Horse Babesia caballi, Dog Babesia canis, Cat Babesia felis, Gibson dog Babesia gibsoni, Large Piroplasma (Babesia ovata) ), Cytauxzoon felis, tropical piroplasmosis Tyleria (Theileria annulata), pseudocoastal fever Tyreria (Theileria mutans), small piroplasma (Theileria orientalis), east coast fever Tyrelia (Theileria parva), etc. Piroplasmida sporozoites.
- Haemoproteus mansoni chicken leucocytozone (Leucocytozoon caulleryi), Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, Plasmodium ovale Haemosporida spores such as protozoa (Plasmodium vivax).
- Vestibuliferida ciliates such as large colonic barantidium coli.
- Trichomonadida flagellates such as Histomonas meleagridis, Pentatrichomonas hominis, Trichomonas tenax.
- Vaccinonadida flagellates such as Giardia intestinalis, Giardia muris, turkey hexamita (Hexamita meleagridis), Hexamita parva.
- Leishmania donovani Leishmania infantum, Leishmania major, Leishmania tropica
- Trypanosoma brucei gambiense Trypanosoma brucei cruise
- Trypanosoma brucei rhodesi Kinetoplastid flagellates such as Trypanosoma cruzi, Trypanosoma equiperdum, and Trypanosoma evansi.
- Plant in this specification refers to cereals and fruit trees / vegetables cultivated as human food, feed crops such as livestock and poultry, appreciation plants that love their form and shape, or planting in parks, streets, etc. It means a vascular plant (Tracheophyta), and specific examples include, but are not limited to, the following plants.
- Pine Plants plants belonging to Pinaceae such as Japanese red pine Pine (Pinus densiflora), European red pine Scots Pine (Pinus sylvestris), Japanese black pine Black Pine (Pinus thunbergii).
- Pepper (Piper nigrum) such as Pepperaceae (Piperaceae), Avocado Avocado (Persea americana) and other magnoliaceae (Lauraceae) etc., magnoliids, Konjac (Amorphophallus konjac), taro edode (Colocasia esculenta) and other taro (Araceae), Chinese yam (Dioscorea batatas), yam Japanese yam (Dioscorea japonica) and other yam (Dioscoreacesum var) porrum), Onion Onion (Allium cepa), Rakkyo Rakkyo (Allium chinense), Leek Welsh onion (Allium fistulosum), Garlic Garlic (Allium sativum), Chives Chives (Allium schoenoprasum), Asatsuchi Chive (Allium schoenofosum var) Japanese leek Oriental garlic (Allium tuberosum), Allium
- Lotus root such as Lotus root (Nelumbo nucifera) (Nelumbonaceae), groundnut Peanut (Arachis hypogaea), chickpea Chickpea (Cicer arietinum), lentil Lentil (Lens culinaris), pea Pea (Pisum sativum) , Soybean Soybean (Glycine max), common bean Common bean (Phaseolus vulgaris), adzuki bean (Vigna angularis) adzuki bean, cowpea (Vigna unguiculata), legaceae (Fabaceae), aceae such as Hop Hop (Humulus lupulus) ), Fig Fig Tree (Ficus carica), Mulberry (Moraceae) such as Mulberry (Morus spp.), Mameaceae (Rhamnaceae) such as jujuba (Rhamnaceae), Strawberry Strawberry (Fragaria), Rose Rose (Rosa) s
- Makuwa cucumber Cucumber (Cucumis sativus), Cucubitneaestren (Cucurbitaceest, Japanese chestnut) )
- Citrus junos Citrus junos
- lemon Lemon Cisi
- Natsumikan Cisikan
- Currus natsudaidai gray Fruit Grapefruit
- Orange Orange Cisi
- Kabosu Kabosu Cisi sphaerocarpa
- Sudachi Sudachi Citrus sudachi
- Ponkan Mandarin Orange Cisi tangerina
- Satsuma Cisi unshiu
- Citrus unshiu Cirus unshiu
- Citrus unshiu Citrus unshiu
- Citrus unshiu Citrus unshiu
- Citrus unshiu Citrus unshiu
- Citrus (Rutaceae) Citrus subfamily (Aurantioideae), Horseradish Horseradish (Armoracia rusticana), Mustard (Brassica juncea), Takana Takana (Brassica juncea var.
- Frutescens Sage Common Sage (Salvia officinalis), Thyme (Thymus spp.) (Lamiaceae), Sesame (Pedaliaceae) such as sesame Sesame (Sesamum indicum), Oleaceae such as olive Olive (Olea europaea), Convolvulaceae such as Sweet potato (Ipomoea batatas), Tomato (Convolvulaceae) Solanum lycopersicum), Eggplant Eggplant (Solanum melongena), Potato Potato (Solanum tuberosum), Pepper chili pepper (Capsicum annuum), Bell Bell pepper (Capsicum annuum var.
- animal in this specification means humans, companion animals, pets, livestock, poultry, and vertebrates (Vertebrata) such as research and laboratory animals. Specific examples include the following animals: However, it is not limited to only these.
- Capuchin such as Capuchin monkey Tuftedecapuchin (Cebus apella), Crab-eating macaque (Macaca fascicularis), Rhesus macaque (Macaca mulatta), etc.
- Homonidae such as Homo ⁇ ⁇ sapiens, Rabidae (Leporidae) such as European Europerabbit (Oryctolagus cuniculus), Chinchilla chinchilla (Chinchilla lanigera), Guinea origus ) Guinea pigs (Caviidae), Golden hamsters Golden hamster (Mesocricetus auratus), Djungarian hamster (Phodopus sungorus), Mongolian gerbils , House mice (Mus musculus), black rats (Rattus rattus), etc.
- Alpaca Vicugna pacos
- Camelidae such as Llama (Lama glama), Wild boar (Suidae) such as pig Pig (Sus scrofa domesticus), Reindeer (Rangifer tarandus), Red deer Deer (Cervidae) such as elaphus, Yak Yak (Bos grunniens), Cattle Cattle (Bos taurus), Asian Buffalo Water ⁇ ⁇ ⁇ buffalo (Bubalus arnee), Goat Goat (Capra hircus), Sheep Sheep (Ovis aries) (Bovidae), Cats such as Cat (Felis silvestris catus), Felidae, Dogu Dog (Canis lupus familiaris), Red Fox Canidae such as Vulpes vulpes), European Mink European mink (Mustela lutreola), American Mink American mink (Mustela vison), Ferret Ferre
- Veiled chameleon (Chamaeleo calyptratus), etc.
- Chameleonidae (Chamaeleonidae), Green iguana Green iguana (Iguana iguana), Green anole Carolinaa anole (Anolis carolinensis), etc.
- Iguanidae Nile monitor Lizards such as the Lizard Lizard Water ⁇ monitor (Varanus salvator), etc.
- Constrictor (Boa constrictor), etc.
- Boidae (Boidae), Indian python Indian python (Python molurus), Japanese snake Reticulated python (Python reticulatus), etc.
- Carp (Cyprinus carpio), Goldfish (Carassius auratus auratus), Zebrafish Zebrafish (Danio rerio), etc.
- piranha Pieris Archasus tshawytscha
- Atlantic salmon Atlantic Salmon (Salmo) salar)
- Brown trout Brown trout (Salmo trutta) salmonid (Salmonidae)
- Pseudanthias squamipinnis Que Longtooth grouper (Epinephelus bruneus), Mahata ConvictCongrouper (Epinephelus septemfasciatus), etc.
- Greater amberjack (Seriola dumerili), Yellowtail amberjack (Seriola quinqueradiata), etc. Carangidae, Red sea bream (Pagrus major), etc., Thai family (Sparidae), Nile tilapia Nile tilapia (Oreochromis niloticus), Scalare Cichlidae (Cichlidae) such as Angelfish (Pterophyllum ⁇ scalare), Sababridae (Scombridae) such as Pacific Bluefin unatuna (Thunnus orientalis), Bonefish (Tetraodontidae) such as Japanese pufferfish (Takifugu rubripes) Actinopterygii).
- useful insects in this specification means insects that are useful for human life by using their products, or for improving the efficiency of agricultural work such as use for pollination of fruit trees and vegetables.
- Japanese bees Japanese honeybee (Apis cerana japonica), Western bees Western honey bee (Apis mellifera), Bumblebees Bumblebee (Bombus consobrinus wittenburgi, B. diversus diversus, B. hypocrita terporis, B. (Osmia cornifrons), silkworm Silkworm (Bombyx ⁇ mori) and the like, but are not limited thereto.
- natural enemy in the present specification means an organism that kills or suppresses the growth of a specific species of organisms, particularly a specific species that harms agricultural crops, by predation or parasitism.
- the following organisms may be mentioned, but are not limited to these.
- Sasakawa leaf scallop (Dacnusa sasakawai), leaf squirrel (Dacnusa sibirica), coleman hornet (Aphidius colemani), corn bee (Apanteles glomeratus), Braconida, pod (Aphelinus asychis), cotton wasp (Aphelinus gossypii), cross-horned bee (Aphelinus maculatus), wasp (Aphelinus varipes), oncary bee (Encarsia formosa), Eretmoceruscer mundus, etc.
- the compound of the present invention represented by the formula (I) can be produced, for example, by the following method. Manufacturing method A (In Formula (I) and Formula (II), Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 , R 5 and R 6 represent the same meaning as described above.
- a compound represented by the formula (II) or a salt thereof for example, hydrochloride, hydrobromide, trifluoroacetate, p-toluenesulfonate, etc.
- a compound represented by the formula (III) If necessary, benzene, toluene, dichloromethane, chloroform, 1,2-dichloroethane, diethyl ether, tert-butyl methyl ether, tetrahydrofuran, 1,4-dioxane, ethyl acetate, N, N-dimethylformamide, N, N-dimethylacetamide , Acetonitrile, water or a mixture of any two or more of them as a solvent, and if necessary, 1 to 3 molar equivalents of sodium carbonate with respect to 1 molar equivalent of the compound represented by formula (II), Potassium carbonate, sodium hydrogen carbonate, sodium acetate, triethylamine, ethyldi
- R 5 represents Except for hydrogen atom, —OH, C 1 -C 4 alkoxy and C 1 -C 4 haloalkoxy, the same meaning as described above
- J 2 represents chlorine atom, bromine atom, iodine atom, C 1 -C 4 alkylcarbonyloxy ( For example, pivaloyloxy etc., C 1 -C 4 alkyl sulfonate (eg methanesulfonyloxy etc.), C 1 -C 4 haloalkyl sulfonate (eg trifluoromethanesulfonyloxy etc.), aryl sulfonate (eg benzenesulfonyloxy, p- Represents a good leaving group such as tol
- G 1 , Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 and R 5 represent the same meaning as described above, J 3 represents a chlorine atom, a bromine atom, Represents a good leaving group such as an iodine atom or a C 1 -C 4 haloalkylsulfonate (eg, trifluoromethanesulfonyloxy, etc.)
- the compound represented by the formula (V) is a general Sonogashira coupling described in, for example, European Journal of Organic Chemistry [Eur. J. Org. Chem.] 2015, 2015, 4389.
- a substituted acetylene represented by the formula for example, in accordance with International Patent Application Publication (WO 2005/094822), etc., in the presence of tetrabutylammonium fluoride under the reaction conditions of Sonogashira coupling, or
- the compound represented by the formula (V) can be prepared by reacting the compound represented by the formula (VI) [wherein R is a compound under the general Negishi coupling reaction conditions described in Heterocycles 1997, 46, 209, etc. 6 represents the same meaning as described above, and Q represents -ZnCl, -ZnBr, -ZnI, or the like.
- the compound of the present invention represented by the formula (I) can be obtained by reacting with a substituted acetylene represented by the formula:
- Some of the compounds represented by the formula (V) used here are, for example, International Patent Application Publication (WO 2005/014545 Publication), International Patent Application Publication (WO 2013/064461 Publication), International Patent Application Publication ( WO 2013/064521) and the like, and other compounds can be synthesized in the same manner as known compounds.
- Some of the compounds represented by formula (VI) are known compounds, and some of them are available as commercial products.
- other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
- the reaction mixture after completion of the reaction is subjected to normal post-treatment such as direct concentration, or dissolution in an organic solvent, concentration after washing with water, or addition to ice water, extraction after organic solvent extraction,
- the target alkynylpyridine-substituted amide compound can be obtained.
- impurities can be separated and purified by any purification method such as recrystallization, column chromatograph, thin layer chromatograph, liquid chromatographic fractionation and the like.
- the compound represented by the formula (II) used in the production method A can be synthesized, for example, as shown in the reaction formulas 1 to 10.
- Reaction formula 1 (In formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above.
- formula (VIII) Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 have the same meaning as above, in formula (VI), R 6 has the same meaning as above, Q represents a hydrogen atom, trimethylsilyl, etc.
- formula (IX) Y 1 , Y 2 , Y 3 , R 1 , R 2 and R 6 have the same meanings as above, and Y 1 , Y 2 , Y 3 , R 1 , R 2 and R 6 have the same meanings as described above in formula (IIa). .
- the compound represented by the formula (VIII) can be synthesized by reacting with di-tert-butyl dicarbonate.
- the compound represented by the formula (VIII) is replaced with the compound represented by the formula (VI), for example, Organic and Biomolecular Chemistry [Org. Biomol. Chem.] 2002, 12, 185 pages, journal. Of Medicinal Chemistry [J. Med. Chem.] 2009, Vol. 52, p. 3563, etc., by reacting using the Sonogashira coupling reaction conditions, the compound represented by the formula (IX) Can be synthesized.
- R 3 , R 4 and R 5 are hydrogen atoms by reacting with hydrochloric acid, hydrobromic acid, trifluoroacetic acid or the like according to the method described in JP
- a compound represented by the formula (IIa) or a salt thereof for example, hydrochloride, hydrobromide, trifluoroacetate, etc.
- Reaction formula 2 (In formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above.
- R 3 is C 1 -C 4 alkyl or C 1. Represents a C 4 haloalkyl, J 4 represents a chlorine atom, a bromine atom or an iodine atom, and in formula (XI), R 3 represents a C 1 to C 4 alkyl or a C 1 to C 4 haloalkyl, Formula (XII)
- Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 and J 3 represent the same meaning as above, and in formula (IIb), Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 and R 6 have the same meaning as above.
- the compound represented by the formula (VII) can be prepared by, for example, Bioorganic & Medicinal Chemistry Letters [Bioorganic & Med. Chem. & Lett.] 2009, 19, 1488 and 1492, 2011, 21; In accordance with the method described on page 1434, etc., after reacting with a known Grignard reactant represented by formula (X) or reacting with a known lithium reactant represented by formula (XI), hydrogen
- the compound represented by the formula (XII) can be synthesized by reduction using sodium borohydride or the like.
- the compound represented by the formula (VII) is reacted with a methyllithium reagent in the presence of cerium trichloride according to the method described in Synthesis 2006, page 4143, etc., or Synlett 2007
- the compound represented by the formula (XV) can be synthesized by reacting with a methyl Grignard reagent in the presence of titanium (IV) tetraisopropoxide according to the method described on page 652, etc.
- R 3 and R 4 in the formula (II) are methyl, and R 5 is a hydrogen atom.
- a compound represented by the formula (IIc) or a salt thereof for example, hydrochloride, hydrobromide, trifluoroacetate, etc.
- Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 and R 6 have the same meaning as above, wherein R 5 is C 1 -C 4 haloalkylthio, —C (O) R 9 and represent the same meaning as C 1 ⁇ C 4 alkoxy the non carbonyl in.
- formula (IIe) Y 1, Y 2, Y 3, R 1, R 2, R 3, R 5 and R 6 are as defined above Represents meaning.
- the compound represented by the formula (VII) is converted into a compound represented by the formula (X) according to the method described in Chemical and Pharmaceutical Bulletin [Chem. Pharm. Bull.] 1986, 34, 4653, etc.
- R 3 and J 4 represent the same meaning as described above. And then hydrolyzed after reaction with a known Grignard reagent represented by the following formula, for example, The Journal of Organic Chemistry [J. Org. Chem.] 1981, 46, 4600, etc.
- the compound represented by the formula (XXI) can be synthesized by reacting with diisobutylaluminum hydride according to the method described, followed by hydrolysis.
- the compound represented by the formula (XXI) is replaced with a substituted acetylene represented by the formula (VI), for example, Tetrahedron Letters 2012, 53, 4117, Tetrahedron 2005. 61, 2697, etc.
- the compound represented by the formula (XXII) can be synthesized by reaction using the Sonogashira coupling reaction conditions described in the above.
- the compound represented by the formula (XXVI) is reduced with zinc-hydrochloric acid or the like according to the method described in, for example, Synlett 2014, 25, 2891, etc. Can be synthesized.
- the compound represented by the formula (XXVII) thus obtained is reacted in the same manner as in the reaction formula 1, so that R 1 , R 2 , R 4 and R 5 are hydrogen atoms in the formula (II).
- a compound represented by the formula (IIf) or a salt thereof for example, hydrochloride, hydrobromide, trifluoroacetate, etc.
- Some of the compounds represented by the formula (XXIV) used here are known compounds, and some of them are available as commercial products.
- other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
- Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as described above.
- W represents an oxygen atom, a sulfur atom or —NHO—
- R a represents C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C 6 alkenyl, C 3 -C 6 alkynyl, (Z) m- substituted phenyl (C 1 -C 4 ) alkyl, etc.
- a compound represented by the formula (XXXI) can be synthesized by reacting according to the method described.
- the compound represented by the formula (XXXI) is converted into a method described in, for example, Bulletin of the Chemical Society of Japan [Bull. Chem. Soc. Jpn.] 2014, Vol. 87, 127.
- the compound represented by the formula (XXXII) can be synthesized by reduction using zinc-acetic acid or the like according to the above.
- R 1 is —WR a in Formula (II)
- R 2 , R 4 and R A compound represented by the formula (IIg) in which 5 is a hydrogen atom or a salt thereof (for example, hydrochloride, hydrobromide, trifluoroacetate, etc.) can be obtained.
- Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as described above.
- Y 1 , Y 2 , Y 3 , R 3 and J 3 are In formula (VI), R 6 represents the same meaning as described above, and Q represents a hydrogen atom, trimethylsilyl, —ZnCl, —ZnBr, —ZnI, etc.
- Q represents a hydrogen atom, trimethylsilyl, —ZnCl, —ZnBr, —ZnI, etc.
- Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meanings as described above, in formula (XXXVIII), R b represents ethyl, 2-methoxyethyl, etc.
- Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meaning as described above, and Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meaning as described above in formula (IIh).
- the compound represented by the formula (XXXV) is halogenated according to the method described in, for example, International Patent Application Publication (WO 2014/010737), and then reacted with potassium phthalimide to represent the compound represented by the formula (XXXVI). Can be synthesized.
- the formula A compound represented by the formula (XXXIX) can be synthesized by reacting with a known fluorinating agent represented by (XXXVIII).
- the compound represented by the formula (XXXIX) thus obtained is converted to toluene, dichloromethane, chloroform, methanol, ethanol, tetrahydrofuran, 1,4-dioxane, water, or any ratio of two or more thereof if necessary. 1 to 4 molar equivalents of a methylamine aqueous solution, a hydrazine aqueous solution or 1 to 4 molar equivalents of the compound represented by the formula (XXXIX) under an inert gas atmosphere such as nitrogen or argon if necessary.
- R 1 represents a fluorine atom
- R 2 , R 4 and R 5 are hydrogen atoms.
- a compound represented by the formula (IIh) can be obtained.
- R c represents a hydrogen atom, a halogen atom, or a C 1 -C 5 alkyl. , C 1 -C 5 haloalkyl or C 1 -C 4 alkoxy, etc.
- J 4 has the same meaning as described above, wherein Y 1 , Y 2 , Y 3 , R 3 , R 6 and R c represents the same meaning as above, and Y 1 , Y 2 , Y 3 , R 3 , R 6 and R c represent the same meaning as described above in formula (IIi).
- a compound represented by the formula (XXXVII), which is a synthetic intermediate of Reaction Formula 8, can be obtained by using, for example, Angewante Chemie International Edition [Angew. Chemie, Int. Ed.] 2011, 50, 2593, Organic Letters. [Organic. Lett.] 2007, 9, 5219, The Journal of Organic Chemistry [J. Org. Chem.] 1993, 58, 6509, Tetrahedron 2010, 66 Vol. 3499, European Journal of Organic Chemistry [Eur. J. Org.
- the compound represented by the formula (XLI) thus obtained is reacted with a methylamine aqueous solution, a hydrazine aqueous solution, hydrazine monohydrate, or the like in the same manner as in the reaction formula 8 to obtain R in the formula (II).
- 1 and R 2 together form C 1 -C 6 alkylidene, C 1 -C 6 haloalkylidene or C 1 -C 4 alkoxy (C 1 -C 2 ) alkylidene, and R 4 and R 5 are hydrogen
- a compound represented by the formula (IIi) which is an atom can be obtained.
- Some of the compounds represented by the formula (XL) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
- the compound represented by the formula (XLIV) is reacted with potassium phthalimide according to the method described in, for example, International Patent Application Publication (WO 2006/136821), etc. Then, for example, by reacting with a known fluorinating agent represented by the formula (XXXVIII) according to the method described in International Patent Application Publication (WO (2009/123855) etc., the formula (XLV) Can be synthesized.
- the compound represented by the formula (XLVI) thus obtained is reacted with the substituted acetylene represented by the formula (VI) in the same manner as in Production Method C to obtain the compound represented by the formula (XLVI).
- Can be synthesized by reacting the compound represented by the formula (XLVI) with an aqueous methylamine solution, an aqueous hydrazine solution or hydrazine monohydrate in the same manner as in the reaction formula 8, R 2 in the formula (II) is a fluorine atom, A compound represented by the formula (IIj) in which R 3 , R 4 and R 5 are hydrogen atoms can be obtained.
- reaction formula 11 The compounds represented by the formula (VIII) used in the reaction formulas 1 to 5 can be synthesized, for example, as the following reaction formulas 11 to 15. Reaction formula 11
- the compound represented by the formula (VIIa) in which R 1 and R 2 are hydrogen atoms in the formula (VII) can be synthesized by heat decarboxylation according to the method of Some of the compounds represented by the formula (XLVII) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
- Reaction formula 12 (In Formula (VIIa), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above.
- J 3 represents the same meaning as described above, and R 1 represents C 1 to C 6.
- R 1 represents C 1 to C 6.
- formula (VIIb) Y 1 , Y 2 , Y 3 , R 1 and J 3 Represents the same meaning as above.
- the compound represented by the formula (VIIa) is converted to a known formula (L) according to the method described in, for example, Journal of Heterocyclic Chemistry [J. Heterocyclic Chem.] 1987, Vol. 24, page 1061.
- a compound represented by the formula (VIIb) in which R 2 is a hydrogen atom in the formula (VII) can be synthesized.
- Reaction formula 13 (In Formula (XLVII), Y 1 , Y 2 , Y 3 , J 3 and J 5 represent the same meaning as described above.
- R 1 represents the same meaning as described above except for a halogen atom.
- (VIIb) Y 1 , Y 2 , Y 3 , R 1 and J 3 represent the same meaning as described above.
- a compound represented by the formula (XLVII) can be synthesized by using, for example, Journal of Heterocyclic Chem. [J. Heterocyclic Chem.] 1987, 24, 1061, The Journal of Organic Chemistry [J. Org. Chem.] 2005, 70, 10186, etc., by reacting with a compound represented by the formula (LI) according to the method described in the formula (VII), wherein R 2 is a hydrogen atom (VIIb ) Can also be synthesized.
- Reaction formula 14 (In Formula (VIIb), Y 1 , Y 2 , Y 3 , R 1 and J 3 represent the same meaning as described above.
- J 3 represents the same meaning as described above, and R 2 represents C 1.
- R 2 represents C 1.
- Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above.
- the compound represented by the formula (VIIb) is converted to a known formula according to the method described in, for example, European Journal of Medicinal Chemistry [Eur. J. Med. Chem.] 2004, 39, 993. By reacting with the compound represented by (LII), the compound represented by formula (VII) can be synthesized.
- Reaction formula 15 (In Formula (VIIa), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above.
- Formula (VIIc) Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above. .
- the compound represented by the formula (VIIa) is tert-butyllithium.
- Reaction formula 16 (In Formula (VIIa), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above.
- J 5 represents the same meaning as described above.
- Y 1 , Y 2 , Y 3 and J 3 have the same meaning as above.
- the compound represented by the formula (VIIa) is converted to a known formula (LIII) according to the method described in, for example, Journal of Medicinal Chemistry [J. Med. Chem.] 2010, Vol. 53, page 6003.
- R 1 and R 2 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded.
- a compound represented by the formula (VIId) can be synthesized.
- each of the production intermediates that will be the raw material compounds of production method A to production method C can be obtained by performing a general post-treatment.
- each production intermediate produced by these methods can be used as it is in the subsequent step without isolation and purification.
- alkynylpyridine-substituted amide compound represented by the formula (I) included in the present invention that can be produced using these methods include the following structural formulas [I] -1 to [I] -50: The compound represented by these is mentioned. However, the compounds represented by the structural formulas [I] -1 to [I] -50 are for illustrative purposes, and the alkynylpyridine-substituted amide compounds included in the present invention are limited to these compounds. is not.
- Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 , R 5 and R 6 are represented in the structural formulas [I] -1 to [I] -50.
- Examples of specific combinations of substituents include, for example, combinations shown in Table 2. However, the combinations in Table 2 are for illustrative purposes, and Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 , of the alkynylpyridine-substituted amide compounds included in the present invention, Specific combinations of substituents represented by R 5 and R 6 are not limited to these.
- Et represents ethyl
- n-Pr is normal propyl
- i-Pr and Pr-i are isopropyl
- c-Pr and Pr-c are cyclopropyl
- Bu- s is sec-butyl
- c-Bu is cyclobutyl
- t-Bu and Bu-t are tert-butyl
- Pen is pentyl
- c-Pen is cyclopentyl
- c-Hex is cyclohexyl
- Ph is phenyl 1-Naph represents 1-naphthyl
- 2-Naph represents 2-naphthyl
- the aromatic heterocycles represented by D-1-1a to D-26-1c each represent the following structure
- the number representing the substitution position of the substituent (Z) n corresponds to the position of the number described in the above structural formula.
- (D-23-2b) -6-F” The description represents “6-fluoropyridin-3-yl”
- the aromatic heterocycles represented by D-31-a to D-37-c each represent the following structure,
- T-1 to T-78 each represent the following structure.
- the compounds of the present invention include plant diseases and mammals (Mammalia) that occur in vascular plants (Tracheophyta) such as pine (Pinales), magnolias, monocots, monocots (eudicots), etc.
- Pathogens of vertebrate (Vertebrata) infections such as birds (Aves), reptiles (Reptilia), and teleosts (Actinopterygii), plant parasitic or animal parasitic linear animals, bald animals, flat animals, protozoa, etc. Can remove the pests.
- Plant pests include Ascomycota fungi, Basidiomycota fungi, Chitridiomycota fungi, Blastocladiomycota fungi, Mucoromycotina fungi, Cercozoa fungi Cercozoa Protists, Heteromonyphyta Oomycetes, Actinobacteria gram-positive fungi, Tenericutes gram-positive fungi, Proteobacteria gram-negative fungi and leaf lines Aphelenchida nematodes, Tylenchida nematodes, etc., among which the compounds of the present invention are phytopathogenic fungi belonging to the Ascomycota and Basidiomycota, leaf nematodes It exhibits excellent control effects at low concentrations against plant parasitic nematodes belonging to the order of the nematode.
- Animal pests include Ascomycota fungi, Basidiomycota fungi, Actinobacteria gram-positive fungi, Firmicutes gram-positive fungi, Tenericutes gram-positive fungi , Proteobacteria gram-negative fungi and Enoplida nematodes, Rhabditida nematodes, Strongylida nematodes, Ascaridida nematodes, rotate nematodes Spirurida nematodes, bald worms, Pseudophyllidea tapeworms, Cyclophyllidea tapeworms, Strigeidida fluke, Echinostomida flute Plachyorchiida, Opisthorchiida, Amoeba, Piroplasmida, Haemosporida, Eucoccidiorida, Eucoccidiorida , Vestibuliferida ciliates, Trichomonadidae
- the compounds of the present invention are also effective against pests that have developed resistance to existing fungicides and nematicides. Furthermore, the compound of the present invention has extremely useful features that have almost no adverse effect on non-target organisms such as mammals, fish, crustaceans, natural enemies and useful insects. When using the compound of the present invention, it is usually mixed with a suitable solid carrier or liquid carrier, and if desired, a surfactant, penetrant, spreading agent, thickener, antifreezing agent, binder, anti-caking agent.
- a water-soluble package such as a water-soluble capsule and a bag of a water-soluble film.
- solid carrier examples include quartz, calcite, gypsum, dolomite, chalk, kaolinite, pyrophyllite, sericite, halosite, metahalosite, kibushi clay, glazed clay, porcelain stone, nostirite, and allophane.
- Natural minerals such as calcined clay, perlite, shirasu balloon, vermiculite, attapulgus clay, calcined diatomaceous earth Baked products; inorganic salts such as magnesium carbonate, calcium carbonate, sodium carbonate, sodium bicarbonate, ammonium sulfate, sodium sulfate, magnesium sulfate, diammonium hydrogen phosphate, ammonium dihydrogen phosphate, potassium chloride; glucose, fructose, sucrose, Sugars such as lactose; starch, Polysaccharides such as powdered cellulose and dextrin; Organic substances such as urea, urea derivatives, benzoic acid and benzoic acid salts; Plants such as wood flour, cork flour, corn cobs, walnut shells, tobacco stems, etc.
- liquid carrier such as xylene, alkyl (C 9 or C 10, etc.) benzene, phenylxylylethane, alkyl (C 1 or C 3, etc.) aromatic hydrocarbons such as naphthalene; machine oil, normal paraffin, isoparaffin, Aliphatic hydrocarbons such as naphthene; mixtures of aromatic and aliphatic hydrocarbons such as kerosene; alcohols such as ethanol, isopropanol, cyclohexanol, phenoxyethanol, and benzyl alcohol; ethylene glycol, propylene glycol, diethylene glycol, hexylene glycol Polyhydric alcohols such as polyethylene glycol and polypropylene glycol; propyl cellosolve, butyl cellosolve, phenyl cellosolve, propylene glycol monomethyl ether, propylene glycol Ethers such as monoethyl ether, propylene glycol monopropyl ether, propylene glyco
- solid and liquid carriers may be used alone or in combination of two or more.
- the surfactant include polyoxyethylene alkyl ether, polyoxyethylene alkyl (mono or di) phenyl ether, polyoxyethylene (mono, di or tri) styryl phenyl ether, polyoxyethylene polyoxypropylene block copolymer, polyoxyethylene Ethylene fatty acid (mono or di) ester, sorbitan fatty acid ester, polyoxyethylene sorbitan fatty acid ester, castor oil ethylene oxide adduct, acetylene glycol, acetylene alcohol, ethylene oxide adduct of acetylene glycol, ethylene oxide adduct of acetylene alcohol, alkyl Nonionic surfactants such as glycosides; alkyl sulfate salts, alkylbenzene sulfonates, lignin sulfonates, alkyls Sulfosuccinate, naphthalen
- the content of these surfactants is not particularly limited, but is usually preferably in the range of 0.05 to 20 parts by weight, more preferably 0.1 to 20 parts by weight with respect to 100 parts by weight of the preparation of the present invention. desirable. These surfactants may be used alone or in combination of two or more.
- the application amount of the compound of the present invention varies depending on the application scene, application time, application method, cultivated crop, etc., but the amount of active ingredient is usually preferably 0.005 to 50 kg per hectare (ha), 0.01 to 5 kg is more preferable.
- an effective amount of the compound of the present invention may be administered orally or parenterally with a pharmaceutical additive.
- parenteral administration include injection (intramuscular, subcutaneous, intravenous, intraperitoneal), transdermal administration (immersion, spray, bathing, washing, pouring-on, spotting-on ), Dusting), or nasal administration.
- the compounds of the present invention can also be administered by molded products using strips, plates, bands, collars, ear marks, limb bands, labeling devices and the like.
- the compound of the present invention can be in any dosage form suitable for the administration route.
- Arbitrary forms to be prepared include solid preparations such as powders, granules, wettable powders, pellets, tablets, large pills, capsules, molded products containing active compounds; injection solutions, oral solutions, skin Liquid preparations used above or in body cavities; solution preparations such as pour-on, spot-on, flowable, and emulsion; semi-solid preparations such as ointments and gels.
- the solid preparation can be used mainly for oral administration or diluted with water for transdermal administration or environmental treatment.
- Solid preparations can be prepared by adding the active compound, if necessary, with adjuncts, mixing with appropriate excipients, and converting to the desired shape.
- the excipient include inorganic substances such as carbonate, hydrogen carbonate, phosphate, aluminum oxide, silica, and clay, and organic substances such as sugar, cellulose, pulverized grains, and starch.
- Injection solutions can be administered intravenously, intramuscularly and subcutaneously.
- Injection solutions dissolve the active compound in a suitable solvent and, if necessary, solubilizers, acids, bases, buffer salts, antioxidants. It can be prepared by adding additives such as a protective agent.
- Solvents include water, ethanol, butanol, benzyl alcohol, glycerin, propylene glycol, polyethylene glycol, N-methylpyrrolidone and mixtures thereof, physiologically acceptable vegetable oils, synthetic oils suitable for injection, and the like.
- solubilizer include polyvinylpyrrolidone, polyoxyethylated castor oil, polyoxyethylated sorbitan ester and the like.
- the protective agent include benzyl alcohol, trichlorobutanol, p-hydroxybenzoic acid ester, n-butanol and the like.
- Oral solutions can be administered directly or diluted. Moreover, it can prepare similarly to the liquid for injection. Flowables, emulsions and the like can be administered directly or diluted transdermally or by environmental treatment. Solutions for use on the skin can be administered by dripping, spreading, rubbing, spraying, spraying or applying by dipping (dipping, bathing or washing). These solutions can be prepared in the same manner as injection solutions.
- Drops and drop preparations can be prepared by dissolving, suspending or emulsifying the active ingredient in suitable skin-compatible solvents or solvent mixtures. If necessary, auxiliary agents such as surfactants, colorants, absorption promoting substances, antioxidants, light stabilizers, and adhesives may be added.
- Solvents include water, alkanol, glycol, polyethylene glycol, polypropylene glycol, glycerin, benzyl alcohol, phenylethanol, phenoxyethanol, ethyl acetate, butyl acetate, benzyl benzoate, dipropylene glycol monomethyl ether, diethylene glycol monobutyl ether, acetone, methyl ethyl ketone, Aromatic and / or aliphatic hydrocarbons, vegetable or synthetic oils, DMF, liquid paraffin, light liquid paraffin, silicone, dimethylacetamide, N-methylpyrrolidone or 2,2-dimethyl-4-oxy-methylene-1,3- And dioxolane.
- Examples of the absorption promoting substance include DMSO, isopropyl myristate, dipropylene glycol pelargonate, silicone oil, aliphatic ester, triglyceride, fatty alcohol and the like.
- Examples of the antioxidant include sulfite, metabisulfite, ascorbic acid, butylhydroxytoluene, butylhydroxyanisole, tocopherol and the like.
- the emulsion can be administered orally, transdermally or as an injection.
- the active ingredient is dissolved in a hydrophobic phase or a hydrophilic phase, and this is further added with a suitable emulsifier, if necessary, a colorant, an absorption promoting substance, a protective agent, an antioxidant, a light-shielding agent, a thickening substance, etc.
- a suitable emulsifier if necessary, a colorant, an absorption promoting substance, a protective agent, an antioxidant, a light-shielding agent, a thickening substance, etc.
- a suitable emulsifier if necessary, a colorant, an absorption promoting substance, a protective agent, an antioxidant, a light-shielding agent, a thickening substance, etc.
- a suitable emulsifier if necessary, a colorant, an absorption promoting substance, a protective agent, an antioxidant, a light-shielding agent, a thickening substance, etc.
- hydrophobic phase paraffin oil, silicone oil, sesame oil, almond oil, castor oil, synthetic triglyceride, ethyl stearate, di-n-butylyl adipate, hexyl laurate, dipropylene glycol pelargonate, branched chain -Like short chain fatty acid and a saturated fatty acid having a chain length of C 16 to C 18 , isopropyl myristate, isopropyl palmitate, caprylic / capric acid ester of a saturated fatty alcohol having a chain length of C 12 to C 18 , isopropyl stearate Oleyl oleate, decyl oleate, ethyl oleate, ethyl lactate, waxy fatty acid ester, dibutyl phthalate, diisopropyl adipate, isotridecyl alcohol, 2-octyldodecanol, cet
- hydrophilic phase examples include water, propylene glycol, glycerin, sorbitol and the like.
- Emulsifiers include nonionic interfaces such as polyoxyethylated castor oil, polyoxyethylated sorbitan monoolefin acid, sorbitan monostearate, glyceryl monostearate, polyoxyethyl stearate, alkylphenol polyglycol ether, etc.
- amphoteric surfactants such as disodium N-lauryl- ⁇ -iminodipropionate and lecithin
- anions such as sodium lauryl sulfate, fatty alcohol sulfate ether, monoethanolamine salt of mono / dialkyl polyglycol orthophosphate
- cationic surfactants such as cetyltrimethylammonium chloride.
- adjuvants include carboxymethyl cellulose, methyl cellulose, polyacrylate, alginate, gelatin, gum arabic, polyvinyl pyrrolidone, polyvinyl alcohol, methyl vinyl ether, maleic anhydride copolymer, polyethylene glycol, wax, colloidal silica, etc. It is done.
- Semi-solid preparations can be administered by application on the skin or spreading or introduction into body cavities. Gels can be prepared by adding sufficient thickener to a solution prepared as described above for an injectable solution to produce a clear material having an ointment-like consistency.
- composition examples of the present invention are not limited to these.
- “parts” means parts by weight.
- Compound of the present invention 0.1 to 80 parts Solid carrier 5 to 98.9 parts
- Surfactant 1 to 10 parts Others 0 to 5 parts Others include, for example, anti-caking agent, decomposition inhibitor and the like.
- Compounds of the present invention 0.1 to 30 parts Organic solvent 45 to 95 parts Surfactant 4.9 to 30 parts Water 0 to 50 parts Others 0 to 10 parts Others include, for example, spreading agents and decomposition inhibitors.
- wettable powder Compound No. 1-019 of the present invention 20 parts Pyrophyllite 74 parts Solpol 5039 4 parts (trade name, mixture of nonionic surfactant and anionic surfactant: Toho Chemical Industry (Made by company) Carplex # 80D 2 parts (trade name, synthetic hydrous silicic acid: manufactured by Shionogi & Co.) The above is uniformly mixed and ground to obtain a wettable powder.
- Emulsion Invention Compound No. 7-006 4 parts DBE 11 parts (trade name, dimethyl adipate, dimethyl glutarate, dimethyl succinate mixture: manufactured by INVISTA) Diisobutyl adipate 30 parts N-methylpyrrolidone 5 parts Soprophor BSU 14 parts (trade name, nonionic surfactant: manufactured by Rhodia) 6 parts of rhodacal 70BC (trade name, anionic surfactant: manufactured by Rhodia) Propylene glycol 10 parts Water 20 parts or more are mixed uniformly to make an emulsion.
- Granule wettable powder Compound No. 9-004 of the present invention 75 parts Hytenol NE-15 5 parts (trade name, anionic surfactant: manufactured by Daiichi Kogyo Seiyaku Co., Ltd.) Vanillex N 10 parts (trade name, anionic surfactant: manufactured by Nippon Paper Industries Co., Ltd.) Carplex # 80D 10 parts (trade name, synthetic hydrous silicic acid: manufactured by Shionogi & Co.) After uniformly mixing and pulverizing the above, a small amount of water is added, stirred and mixed, granulated with an extrusion granulator, and dried to obtain a granulated wettable powder.
- Granule Compound No. 3-064 of the present invention 5 parts Bentonite 50 parts Talc 45 parts And dried into granules.
- composition Example 8 Powder Compound of the present invention No.3-059 3 parts Carplex # 80D 0.5 part (trade name, synthetic hydrous silicic acid: manufactured by Shionogi & Co.) Kaolinite 95 parts Diisopropyl phosphate 1.5 parts The above is uniformly mixed and ground to obtain a powder.
- wettable powder preparation Compound No. 1-016 of the present invention 25 parts sodium diisobutylnaphthalenesulfonate 1 part calcium n-dodecylbenzenesulfonate 10 parts alkylaryl polyglycol ether 12 parts naphthalenesulfonic acid formalin condensate Sodium salt 3 parts Emulsion type silicone 1 part Silicon dioxide 3 parts Kaolin 45 parts
- an effective amount of the compound of the present invention can be used alone as an active ingredient. It may be mixed with seed nematicides, insecticides, acaricides, plant growth regulators, herbicides, synergists, fertilizers, soil conditioners, etc. at the time of formulation or spraying. Further, when the compound of the present invention is used as a parasite control agent, an effective amount of the compound of the present invention can be administered alone as an active ingredient. It can also be mixed with an agent or the like at the time of preparation or administration.
- the control spectrum can be expanded by the additive / synergistic action of the mixed agents, Effects such as improvement of pest control effect, reduction of control cost by reducing the amount of applied medicine, and sustained control effect over a longer period can be expected.
- mixing with other types of fungicides, nematicides, antibacterial agents and anthelmintic agents with different mechanisms of action is an extremely effective control method from the viewpoint of preventing the acquisition of drug resistance by pests. .
- a combination of a plurality of known fungicides, known nematodes, known insecticides, known acaricides, known antibacterial agents or known anthelmintic agents is also possible.
- Examples of types of fungicides, nematicides, insecticides, acaricides, anthelmintics or antibacterials used in combination with the compounds of the present invention include, for example, The Pesticide Manual 16th edition, 2012, etc. Examples include the compounds described. Specific examples of common names are as follows, but the general names are not necessarily limited to these.
- Fungicide A: Nucleic acid biosynthesis inhibitors benalaxyl, benalaxyl-M (benalaxyl-M), furaxyl (furalaxyl), metalaxyl, metalaxyl-M, ofurace, oxadixyl, Bupirimate, ethirimol, Hymexazol.
- amino acid and protein biosynthesis inhibitors cyprodinil, mepanipyrim, pyrimethanil, Blasticidin-S, Kasugamycin, E; drugs that act on the signal transduction system proquinazid, quinoxyfen, Fenpiclonil, fludioxonil, Clozolinate, iprodione, procymidone, vinclozolin.
- F Lipid synthesis and cell membrane formation inhibitor Edifenphos, iprobenfos, isoprothiolane, pyrazophos, Biphenyl, chloroneb, dicloran, etridiazole, quintozene, tecnazene, tolclofos-methyl, Propamocarb hydrochloride, Bacillus subtilis (Strain: D747, FZB24, GBO3, HAI0404, MBI600, QST713, Y1336, etc.).
- G sterol biosynthesis inhibitor azaconazole, abitertanol, bromuconazole, cyproconazole, difenoconazole, dinicoazole, dinicoazole-M (diniconazole-M), Epoxiconazole, etaconazole, fenarimol, fenbuconazole, fluquinconazole, flusilazole, flutriafol, hexaconazole, Imazalil, imibenconazole, ipconazole, ipfentrifluconazole, mefentrifluconazole, metconazole (metconazole) azole), microbutanil, nuarimol, oxpoconazole fumarate, pefurazoate, penconazole, prochloraz, propiconazole, prothioconazole (Prothioconazole), pyrifenox, pyrisoxazole, simeconazole, tebucon
- H cell wall synthesis inhibitor validamycin, Polyoxins, polyoxins-D (polyoxorim), Benthiavalicarb-isopropyl, dimethomorph, flumorph, iprovalicarb, mandipropamid, pyrimorph, valifenalate,
- I Melanin synthesis inhibitor phthalide, pyroquilon, tricyclazole, Carpropamid, diclocymet, fenoxanil.
- P plant resistance inducer acibenzolar-S-methyl, Diclobentiazox, probenazole, Isotianil, tiadinil, Laminarin.
- M drugs with multiple action points Bordeaux mixture, cheshunt mixture, basic carbonate, copper carbonate, copper hydroxide, copper naphthenate , Copper oleate, copper oxychloride, copper sulfate, copper sulfate, basic, oxine copper, lime sulfur polysulfide, sulfur, amobam, ferbam, mancozeb, maneb, maneb, metiram, polycarbamate, propineb, thiram, ziram (Ziram), captan, folpet, chlorothalonil, dichlofluanid, torruf Enid (tolylfluanid), guazatine (guazatine), iminoctadine - Arubeshiru salt (iminoctadine-albesilate), iminoctadine acetate (iminoctadine-triacetate), Anirajin (anilazine), dithianon (dithianon), Kinomechioneto (chinomethionat), Furu
- Insecticides and acaricides A: Acetylcholinesterase (AChE) inhibitor Alanicarb, aldicarb, bendiocarb, benfuracarb, butofcarboxim, carbaryl, carbofuran, carbofuran Fan (carbosulfan), ethiofencarb, fenobucarb, formetanate, furathiocarb, isoprocarb, methiocarb, methomyl, pirimicarb, thiodicarb Thiofanox, triazamate, Acephate, azamethiphos, azinphos-ethyl, azinphos-methyl, chlorethoxyfos, chlorfenvinphos, chlormephos, chlorpyrifos ( chlorpyrifos, chlorpyrifos-methyl, cyanophos, diazinon, dichlorvos, dimethoate, dimethylvinphos, disulfoton, EPN, fenitro
- B GABAergic chloride channel antagonist, endosulfan, alpha-endosulfan, Ethiprole, fipronil, flufiprole, pyriprole, Afoxolaner, fluralaner, lotilaner, sarolaner.
- C Sodium channel modulators acrinathrin, allethrin, benfluthrin, bifenthrin, kappa-bifenthrin, bioallethrin, bioresmethrin, chloropralethrin ), Cycloprothrin, cyfluthrin, beta-cyfluthrin, cyhalothrin, gamma-cyhalothrin, lambda-cyhalothrin, cypermethrin ), Alpha-cypermethrin, beta-cypermethrin, zeta-cypermethrin, cyphenothrin, deltamethrin Empenthrin, esfenvalerate, etofenprox, fenpropathrin, fenvalerate, flucytrinate, flumethrin, fluvalinate, Tau-fluvalinate, halfenprox, heptaflu
- D Nicotinic acetylcholine receptor (nAChR) agonists acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, thiacloprid, thiamethoxam, thiamethoxam Sulfoxaflor, Flupyradifurone.
- E Nicotinic acetylcholine receptor (nAChR) allosteric modulators spinetoram, spinosad.
- F chloride ion channel activator abamectin, emamectin-benzoate, lepimectin, milbemectin.
- G juvenile hormone analog mesoprene, Phenoxycarb, Pyriproxyfen.
- H Hemiptera selective feeding inhibitor pymetrozine, Flonicamid.
- I mite growth inhibitor clofentezine, hexythiazox, Etoxazole.
- J Bacillus thuringiensis, subsp.israelensis, subsp.aizawai, subsp.kurstaki, subsp.tenebrionis, etc.
- K mitochondrial ATP synthase inhibitor diafenthiuron, Azocyclotin, fenbutatin oxide, Propargite.
- L oxidative phosphorylation uncoupler, chlorfenapyr
- M nicotinic acetylcholine receptor (nAChR) channel blocker bensultap, cartap, thiocyclam.
- nAChR nicotinic acetylcholine receptor
- N chitin biosynthesis inhibitor (type 0) Bistrifluron, chlorfluazuron, diflubenzuron, flucycloxuron, flufenoxuron, hexaflumuron, lufenuron, novaluron ), Noviflumuron, teflubenzuron, triflumuron.
- O Chitin biosynthesis inhibitor (type 1) Buprofezin.
- P molting inhibitor (fly insect) Cyromazine Q; molting hormone (ecdysone) receptor agonists chromafenozide, halofenozide, methoxyfenozide, tebufenozide.
- R Octopamine receptor agonist amitraz
- S Mitochondrial electron transport system complex III inhibitor hydramethylnon, Acequinocyl, Fluacrypyrim.
- T Mitochondrial electron transport complex I inhibitor fenazaquin, fenpyroximate, pyrimidifen, pyridaben, tebufenpyrad, tolfenpyrad, tolfenpyrad, Rotenone.
- U voltage-gated sodium channel blocker indoxacarb (indoxacarb), indoxacarb-MP (indoxacarb-MP), Metaflumizone.
- V acetyl CoA carboxylase inhibitor spirodiclofen, spiromesifen, spirotetramat.
- W Mitochondrial electron transport system complex II inhibitor cyenopyrafen, cyflumetofen.
- X ryanodine receptor modulators chlorantraniliprole, cyantraniliprole, cyclaniliprole, cyhalodiamide, flubendiamide, tetraniliprole.
- UN unknown mechanism of action and other drugs azadirachtin, benzoximate, bifenazate, bromopropylate, dicohol, pyridalyl, pyrifluquinazon, Acynonapyr, afidopyropen, benzpyrimoxan, broflanilide, dicloromezotiaz, flometoquin, fluhexafon, fluxametamide, fluxametamide , Triflumezopyrim, ME5382 (test name), NC-515 (test name) and ZDI2501 (test name).
- Nematicides cadusafos, dichlofenthion, ethoprophos, fenamiphos, fluensulfone, fluazaindolizine, fosthiazate, fosthiazate, fostiatan, fothiatan (Imicyafos), isamidofos, isazofos, methyl bromide, methyl isothiocyanate, oxamyl, sodium azide, tioxazafen, BYI- 1921 (study name) and MAI-08015 (study name).
- Anthelmintic acriflavine, albendazole, atovaguone, azithromycin, bithionol, bromfenofos, cambendazole, carnidazole, chloroquine chloroquine, clazuril, clindamycin hydrochloride, clorsulon, closantel, coumaphos, cymiazol, dichlorophen, dichlorophen, diethylcarbamazine, diminazen (Diminazene), disophenol, dithiazanine iodide, doxycycline hydrochloride, doramectin, emodepside, eprinomecti (eprinomecti) n), febantel, fenbendazole, flubendazole, furazolidone, glycalpyramide, imidocarb, ivermectin, levamisole, mebendazole ( mebendazole, mef
- Antibacterial agents amoxicillin, ampicillin, bethoxazin, bithionol, bronopol, cefapirin, cefazolin, cefquinome, cefquinome, cefurthiofluce (Chlortetracycline), clavulanic acid, danofloxacin, difloxacin, dinitrmide, enrofloxacin, florfenicol, lincomycin, lomefloxacin lomefloxacin, marbofloxacin, miloxacin, mimycin, nitrapyrin, norfloxacin, octhilinone, off Ofloxacin, orbifloxacin, oxolinic acid, oxytetracycline, penicillin, streptomycin, thiamphenicol, tiamulin fumarate , Tilmicosin phosphate, tyrosin phosphate, tylosin phosphate, tulathromycin
- Step 1 Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroacetonitrile 2- (5-Bromo-3-chloropyridin-2-yl) acetonitrile 2.00 g of tetrahydrofuran To the 40 ml solution, 16 ml of a 1.3M tetrahydrofuran solution of lithium bis (trimethylsilyl) amide was added dropwise with stirring at ⁇ 78 ° C. After completion of the dropwise addition, the mixture was stirred at the same temperature for 30 minutes.
- Step 2 Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethylamine 2- (5-Bromo-3-chloropyridin-2-yl) -2 under nitrogen atmosphere , 2-Difluoroacetonitrile (810 mg) in dichloromethane (20 ml) was added dropwise with stirring at -78 ° C., and 9 ml of a 1.0 M hexane solution of diisobutylaluminum hydride was added dropwise.
- Step 3 Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridine-
- 2-yl) -2,2-difluoroethylamine (607 mg) and triethylamine (271 mg) in dichloromethane (5 ml) was added di-tert-butyl dicarbonate (584 mg), and the mixture was stirred at room temperature for 2 hours.
- Step 4 Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl N- [2 -(5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethyl] carbamic acid-tert-butyl 1.50 g of N, N-dimethylformamide in 4 ml of solution was added 1.64 g of triethylamine, 1- Ethinyl-4-fluorobenzene 631 mg, copper (I) iodide 231 mg, and dichlorobis (triphenylphosphine) palladium (II) 283 mg were added, and the mixture was stirred at room temperature for 2 hours under a nitrogen atmosphere.
- Step 5 Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine N- [2- [3-Chloro-5-[( 4-Fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl 1.40 g of dichloromethane in 5 ml was added with 3 ml of trifluoroacetic acid and stirred at room temperature for 1 hour. did.
- Step 6 Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) benzamide 2 -[3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine 90 mg in 2 ml of ethyl acetate, 2 ml of 10 wt% aqueous potassium carbonate solution and 2- (tri 67 mg of fluoromethyl) benzoyl chloride was added and stirred at room temperature for 1 hour.
- reaction mixture was then added dropwise to a solution of 5.0 g of 1- (5-bromo-3-chloropyridin-2-yl) ethane-1-one in 10 ml of dimethyl sulfoxide under ice-cooling. And stirring was continued for another 15 hours. After completion of the reaction, 20 ml of water was added to the reaction mixture and extracted with ethyl acetate (30 ml ⁇ 1). The obtained organic layer was washed with water (30 ml ⁇ 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure.
- Step 2 Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-hydroxypropyl] phthalimide 5-Bromo-3-chloro-2- (2-methyloxiran-2-yl )
- pyridine a solution of 5.70 g of pyridine in 40 ml of dimethyl sulfoxide
- 4.48 g of potassium phthalimide was added and stirred at 100 ° C. for 6 hours.
- the reaction mixture was allowed to cool to room temperature, added with 30 ml of water, and extracted with ethyl acetate (20 ml ⁇ 2).
- Step 3 Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -2-fluoropropyl] phthalimide N- [2- (5-Bromo-3-chloropyridine] under ice-cooling and stirring -2-yl) -2-hydroxypropyl] phthalimide 2.62 g of bis (2-methoxyethyl) aminosulfur trifluoride was added dropwise to a solution of 3.90 g of dichloromethane in 20 ml of dichloromethane, and stirred at 50 ° C. for 12 hours. did.
- reaction mixture was allowed to cool to room temperature, 30 ml of saturated aqueous sodium hydrogen carbonate solution was added, and the mixture was extracted with dichloromethane (20 ml ⁇ 2). The organic layers were combined and washed with water (40 ml ⁇ 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was washed with 10 ml of diisopropyl ether to obtain 3.50 g of the desired product as pale yellow crystals.
- Step 4 Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropyl] phthalimide N- [2- (5-Bromo-3 -Chloropyridin-2-yl) -2-fluoropropyl] phthalimide in 4 ml of N, N-dimethylformamide in a solution of 762 mg of triethylamine, 452 mg of 1-ethynyl-4-fluorobenzene, 143 mg of copper (I) iodide and 176 mg of dichlorobis (triphenylphosphine) palladium (II) was added, and the mixture was stirred at 60 ° C.
- Step 5 Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropylamine N- [2- [3-Chloro-5-[(4 -Fluorine) ethynyl] pyridin-2-yl] -2-fluoropropyl] phthalimide
- a solution of 1.2 g of ethanol in 4 ml of ethanol was added 432 mg of hydrazine monohydrate and stirred at 80 ° C. for 5 hours.
- Step 6 Preparation of 2-chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropyl] nicotinamide 2 under ice-cooling and stirring -To a solution of 100 mg of [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropylamine and 50 mg of triethylamine in 1 ml of dichloromethane, 63 mg of 2-chloronicotinyl chloride was added dropwise. After the dropping, stirring was continued for 1 hour at room temperature.
- Step 1 Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethylamine 2- (5-Bromo-3) prepared in Step 1 of Synthesis Example 1 -Chloropyridin-2-yl) -2,2-difluoroacetonitrile 1.0 ml of a diethyl ether solution (5 ml) was added dropwise with ice-cooling and stirring with 3.1 ml of a 35 wt% diethyl ether solution of methylmagnesium bromide. And stirred for 1 hour.
- Step 2 Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl 2- (5-bromo-3 -Chloropyridin-2-yl) -2,2-difluoro-1-methylethylamine
- dichloromethane 895 mg of di-tert-butyl dicarbonate and 566 mg of triethylamine, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with dichloromethane (10 ml ⁇ 1).
- Step 3 Preparation of N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl N- [2 -(5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl 1.3 g of N, N-dimethylformamide in 3 ml of triethylamine 1.
- Step 4 Preparation of 2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethylamine N- [2- [3-Chloro-5- (cyclo To a solution of 750 mg of propylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl in 5 ml of dichloromethane was added 2.5 ml of trifluoroacetic acid, and the mixture was stirred at room temperature for 1 hour. did.
- Step 5 N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -3- (trifluoromethyl) thiophene-2-
- carboxamide 2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethylamine 150 mg of N, N-dimethylformamide in 3 ml solution, 62 mg of triethylamine, N , N-dimethyl-4-aminopyridine 5 mg, 3- (trifluoromethyl) thiophene-2-carboxylic acid 120 mg and 1- (3-dimethylaminopropyl) -3-ethylcarbodiimide hydrochloride 212 mg were added at room temperature.
- Step 1 Preparation of 1- (5-bromo-3-chloropyridin-2-yl) -1,1-difluoropropan-2-one 2- (5-Bromo-3) prepared in Step 1 of Synthesis Example 1 -Chloropyridin-2-yl) -2,2-difluoroacetonitrile 1.0 ml of a diethyl ether solution (5 ml) was added dropwise with ice-cooling and stirring with 3.1 ml of a 35 wt% diethyl ether solution of methylmagnesium bromide. And stirred for 1 hour.
- Step 2 Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropylamine 1- (5-Bromo-3 -Chloropyridin-2-yl) -1,1-difluoropropan-2-one
- methanol-acetic acid 10: 1
- cyclopropylamine was added to stirred at 90 ° C. for 1 hour. did.
- the reaction mixture was allowed to cool to room temperature, 381 mg of picoline borane complex was added, and stirring was further continued at 90 ° C. for 2 hours.
- Step 3 Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl) benzamide
- N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropylamine 112 mg of triethylamine in 2 ml of dichloromethane.
- 2- (trifluoromethyl) benzoyl chloride was added dropwise and stirred at 40 ° C. for 5 hours.
- reaction mixture was allowed to cool to room temperature, 2 ml of water was added, and the mixture was extracted with dichloromethane (10 ml ⁇ 1). The obtained organic layer was washed with water (10 ml ⁇ 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 130 mg of the desired product as a colorless resinous substance.
- Step 4 N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl )
- N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl) benzamide 100 mg
- N-dimethylformamide was added 61 mg of triethylamine, 26 mg of cyclopropylacetylene, 11 mg of copper (I) iodide and 14 mg of dichlorobis (triphenylphosphine) palladium (II).
- Step 1 Preparation of 2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethylamine 2- (5-bromo prepared in Step 1 of Synthesis Example 1
- 2 ml of 3M diethyl ether solution of methylmagnesium bromide was added dropwise with stirring under ice cooling, and the mixture was stirred at the same temperature for 30 minutes. did.
- Step 2 Production of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide 2- ( 5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethylamine was added to a mixed solution of 534 mg of ethyl acetate in 5 ml and potassium carbonate 828 mg in 5 ml of water under ice-cooling and stirring. , 417 mg of 2- (trifluoromethyl) benzoyl chloride was added dropwise and stirred at room temperature for 1 hour.
- Step 3 N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide
- Step 1 Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) ethyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridin-2- under nitrogen atmosphere I) A solution of 1.00 g of acetonitrile in 10 ml of dichloromethane was added dropwise over 12 minutes with 12 ml of a 1.0 M hexane solution of diisobutylaluminum hydride while stirring at -40 ° C. After completion of the dropwise addition, the mixture was stirred at the same temperature for 1 hour. .
- reaction mixture was then warmed to room temperature and added dropwise to 50 ml of a saturated aqueous solution of potassium sodium tartrate (Rochelle salt) under ice-cooling and stirring. After completion of the dropwise addition, stirring was continued for another hour at room temperature. After completion of the reaction, the reaction mixture was extracted with dichloromethane (50 ml ⁇ 2), and the organic layers were combined and dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure.
- dichloromethane 50 ml ⁇ 2
- Step 2 Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethyl] carbamic acid-tert-butyl N- [2- (5-Bromo- 3-chloropyridin-2-yl) ethyl] carbamic acid-tert-butyl in a solution of 1.0 g of N, N-dimethylformamide in 1 ml of triethylamine 455 mg, 1-ethynyl-4-fluorobenzene 540 mg, copper (I) iodide 114 mg And dichlorobis (triphenylphosphine) palladium (II) (209 mg) were added, and the mixture was stirred at 80 ° C.
- Step 3 Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethylamine N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] [Pyridin-2-yl] ethyl] carbamic acid-tert-butyl
- Step 4 Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethyl] -2- (trifluoromethyl) benzamide 2- [3-Chloro- To a solution of 200 mg of 5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethylamine and 110 mg of triethylamine in 2 ml of dichloromethane was added dropwise 182 mg of 2- (trifluoromethyl) benzoyl chloride with ice-cooling and stirring. The stirring was continued for 1 hour at room temperature.
- Step 2 Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-hydroxyethyl] phthalimide 2-Bromo-1- (5-bromo-3-chloropyridin-2-yl ) 1.17 g of potassium phthalimide was added to 2 ml of dimethyl sulfoxide in 1.90 g of ethanol, and the mixture was stirred at 60 ° C. for 1 hour. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 15 ml of water was added, and the mixture was extracted with ethyl acetate (15 ml ⁇ 1).
- Step 3 Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-fluoroethyl] phthalimide N- [2- (5-Bromo-3-chloropyridin-2-yl) Bis (2-methoxyethyl) aminosulfur trifluoride (808 mg) was added dropwise to a solution of -2-mg-hydroxyethyl] phthalimide (930 mg) in dichloromethane (3 ml), and the mixture was stirred at 60 ° C. for 3 hours.
- reaction mixture was then allowed to cool to room temperature, 540 mg of bis (2-methoxyethyl) aminosulfur trifluoride was added, and the mixture was stirred again at 60 ° C. for 1 hour. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 15 ml of saturated aqueous sodium hydrogen carbonate solution was added, and the mixture was extracted with ethyl acetate (15 ml ⁇ 1). The organic layer was washed with water (15 ml x 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure.
- Step 4 Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] phthalimide N- [2- (5-bromo-3 -Chloropyridin-2-yl) -2-fluoroethyl] phthalimide in a solution of 700 mg of N, N-dimethylformamide in 276 mg of triethylamine, 285 mg of 1-ethynyl-4-fluorobenzene, 69 mg of copper (I) iodide and dichlorobis (tri Phenylphosphine) palladium (II) 64 mg was added, and the mixture was stirred at 80 ° C.
- Step 5 Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethylamine N- [2- [3-Chloro-5-[(4- To a solution of 580 mg of fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] phthalimide in 3 ml of ethanol was added 110 mg of hydrazine monohydrate, and the mixture was stirred at room temperature for 2 hours.
- Step 6 Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] -2- (trifluoromethyl) benzamide 2- [ 2-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethylamine (100 mg) and triethylamine (51 mg) in dichloromethane (2 ml) were stirred with ice cooling and 2- (trifluoromethyl) benzoyl chloride.
- Step 1 Preparation of 2- (5-bromo-3-chloropyridin-2-yl) propanenitrile 2- (5-Bromo-3-chloropyridin-2-yl) acetonitrile 1.50 g of tetrahydrofuran solution in 20 ml Under stirring at 5 ° C., 7 ml of a 1.0M solution of potassium tert-butoxide in tetrahydrofuran was added and stirred at room temperature for 2 hours. Subsequently, 0.97 g of iodomethane was added to the reaction mixture, and stirring was continued for another hour at room temperature.
- Step 2 Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -1-propanamine
- 2- (5-Bromo-3-chloropyridin-2-yl) propanenitrile under nitrogen atmosphere 16 ml of a 1.0 M hexane solution of diisobutylaluminum hydride was added dropwise over 20 minutes to a solution of 38 g of dichloromethane in 20 ml at ⁇ 40 ° C., and the mixture was stirred at the same temperature for 1 hour. The reaction mixture was then warmed to room temperature and added dropwise to 50 ml of a saturated aqueous solution of potassium sodium tartrate (Rochelle salt) under ice-cooling and stirring.
- Rochelle salt potassium sodium tartrate
- Step 3 Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) propyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridin-2-yl) -1 -To a solution of 1.04 g of propanamine and 0.63 g of triethylamine in 10 ml of dichloromethane, 1.08 g of di-tert-butyl dicarbonate was added and stirred at room temperature for 1 hour.
- Step 4 Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] propyl] carbamic acid-tert-butyl N- [2- (5-Bromo- 3-chloropyridin-2-yl) propyl] carbamic acid-tert-butyl in a solution of 1.0 g of N, N-dimethylformamide in 1 ml of triethylamine 434 mg, 1-ethynyl-4-fluorobenzene 447 mg, copper (I) iodide 109 mg And 100 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at 80 ° C.
- Step 5 Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine N- [2- [3-Chloro-5-[(4- 1 ml of trifluoroacetic acid was added to 1 ml of a solution of 480 mg of fluorophenyl) ethynyl] pyridin-2-yl] propyl] carbamic acid-tert-butyl and stirred at room temperature for 1 hour.
- the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml ⁇ 2). The organic layers were combined and washed with water (10 ml ⁇ 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 380 mg of a crude target product as a brown resinous substance. The obtained target product was directly used in the next step without further purification.
- Step 6 Preparation of 2-chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] propyl] nicotinamide 2- [3-chloro-5- [ To a solution of 120 mg of (4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine and 63 mg of triethylamine in 2 ml of dichloromethane was added 88 mg of 2-chloronicotinyl chloride with stirring under ice cooling. For 1 hour.
- Step 1 Preparation of 2- (5-bromo-3-chloropyridin-2-yl) -2-methylpropanenitrile 2- (5-Bromo-3-chloropyridin-2-yl) acetonitrile 1.50 g of tetrahydrofuran 20 ml To the solution, 13.6 ml of 1.0M tetrahydrofuran solution of potassium tert-butoxide was added with stirring at ⁇ 5 ° C., and stirred at the same temperature for 10 minutes. Next, 1.94 g of iodomethane was added to the reaction mixture, and stirring was continued for another hour at room temperature.
- Step 2 Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2-methyl-1-propanamine 2- (5-Bromo-3-chloropyridin-2-yl) under nitrogen atmosphere
- a solution of 1.61 g of -2-methylpropanenitrile in 15 ml of dichloromethane 18.5 ml of a 1.0 M hexane solution of diisobutylaluminum hydride was added dropwise over 20 minutes with stirring at -40 ° C. After completion of dropping, the mixture was stirred at the same temperature for 15 minutes. After completion of the reaction, 30 ml of water was added dropwise to the reaction mixture, stirred for 30 minutes at the same temperature and then warmed to room temperature.
- Step 3 Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-methylpropyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridin-2- I) 2-methyl-1-propanamine (1.54 g) and triethylamine (1.60 ml) in dichloromethane (15 ml) were added with di-tert-butyl dicarbonate (1.50 g) and stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with ethyl acetate (20 ml ⁇ 2).
- Step 4 Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] carbamic acid-tert-butyl N- [2- ( 5-Bromo-3-chloropyridin-2-yl) -2-methylpropyl] carbamic acid-tert-butyl in a solution of 1.0 g of N, N-dimethylformamide in 1 ml of triethylamine 419 mg, 1-ethynyl-4-fluorobenzene 431 mg Then, 105 mg of copper (I) iodide and 97 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at 80 ° C.
- Step 5 Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine N- [2- [3-Chloro-5-[(4- To a solution of 1.0 g of fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] carbamic acid-tert-butyl in 5 ml of dichloromethane was added 3 ml of trifluoroacetic acid and stirred at room temperature for 1 hour.
- Step 6 Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] -2- (trifluoromethyl) benzamide 2- [ To a solution of 260 mg of 3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine and 130 mg of triethylamine in 2 ml of dichloromethane was added 2- (trifluoromethyl) benzoyl chloride under ice-cooling and stirring. 215 mg was added, and after completion of the dropwise addition, the mixture was stirred at room temperature for 1 hour.
- Step 1 Preparation of N-[[1- (5-Bromo-3-chloropyridin-2-yl) cyclopropyl] methyl] carbamic acid-tert-butyl [1- (5-Bromo-3-chloropyridine-2
- a solution of 462 mg of -yl) cyclopropyl] methylamine and 269 mg of triethylamine in 5 ml of dichloromethane was added 578 mg of di-tert-butyl dicarbonate, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with dichloromethane (10 ml ⁇ 2).
- Step 2 Preparation of N-[[1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] carbamic acid-tert-butyl N-[[1- (5-Bromo-3-chloropyridin-2-yl) cyclopropyl] methyl] carbamic acid-tert-butyl 450 mg of N, N-dimethylformamide in 3 ml solution of triethylamine 380 mg, 1-ethynyl-4-fluorobenzene 224 mg, iodine Copper (I) 71 mg and dichlorobis (triphenylphosphine) palladium (II) 87 mg were added, and the mixture was stirred at 60 ° C.
- Step 3 Preparation of [1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methylamine N-[[1- [3-Chloro-5-[( To a solution of 4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] carbamic acid-tert-butyl in 6 ml of dichloromethane was added 2 ml of trifluoroacetic acid with stirring under ice cooling, and then at room temperature for 1 hour. Stirring was continued.
- Step 4 Preparation of N-[[1- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] -2- (trifluoromethyl) benzamide [1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methylamine (110 mg) and triethylamine (56 mg) in dichloromethane (2 ml) was stirred under ice-cooling and 2- (trifluoromethyl) benzoyl chloride. 99 mg was added dropwise.
- Step 2 Preparation of 5-bromo-3-chloro-2- (2-nitropropyl) pyridine 5-Bromo-3-chloro-2- (2-nitro-1-propenyl) pyridine (156 mg) in methanol (1.5 ml) While stirring on ice, 85 mg of sodium borohydride was added and stirred at room temperature for 6 hours. After completion of the reaction, 5 ml of water was added to the reaction mixture and extracted with ethyl acetate (10 ml ⁇ 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 120 mg of a crude target product as a yellow resinous substance.
- Step 3 Preparation of 2- (5-bromo-3-chloropyridin-2-yl) -1-methylethylamine 120 mg of 5-bromo-3-chloro-2- (2-nitropropyl) pyridine was dissolved in methanol-water (2 1) It melt
- the residue was dissolved in 10 ml of diethyl ether and extracted with 10 ml of 1N aqueous hydrochloric acid.
- a 1N aqueous sodium hydroxide solution was added to the aqueous layer until pH 14 was reached, followed by extraction with ethyl acetate (30 ml ⁇ 2).
- the organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 86 mg of the crude desired product as a yellow resinous substance.
- the obtained target product was directly used in the next step without further purification.
- Step 4 Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -1-methylethyl] carbamic acid-tert-butyl 2- (5-bromo-3-chloropyridin-2- Yl)
- 2- (5-bromo-3-chloropyridin-2- Yl)
- Step 5 Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] carbamic acid-tert-butyl N- [2- ( 5-bromo-3-chloropyridin-2-yl) -1-methylethyl] carbamic acid-tert-butyl 350 mg of N, N-dimethylformamide in 1 ml solution of triethylamine 152 mg, 1-ethynyl-4-fluorobenzene 156 mg, iodine Copper (I) 38 mg and dichlorobis (triphenylphosphine) palladium (II) 56 mg were added, and the mixture was stirred at 80 ° C.
- Step 6 Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethylamine N- [2- [3-Chloro-5-[(4- To a solution of fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] carbamic acid-tert-butyl 260 mg in dichloromethane 2 ml was added 1 ml of trifluoroacetic acid under ice-cooling, and then stirred at room temperature for 1 hour. Continued.
- the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml ⁇ 2). The organic layers were combined, washed with water (10 ml ⁇ 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 250 mg of the crude target product as a brown resinous substance. . The obtained target product was directly used in the next step without further purification.
- Step 7 Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] -2- (trifluoromethyl) benzamide 2- [ 2-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethylamine 80 mg and triethylamine 57 mg in 2 ml of dichloromethane was stirred under ice-cooling and 2- (trifluoromethyl) benzoyl chloride. 87 mg was added dropwise. After completion of dropping, the mixture was stirred at room temperature for 1 hour.
- Step 1 Preparation of 5-bromo-3-chloro-2- (1-methoxy-2-nitropropyl) pyridine 5-Bromo-3-chloro-2- (2-nitro prepared in Step 1 of Synthesis Example 11
- a 10 ml toluene solution of 760 mg of -1-propenyl) pyridine 2.1 g of a 28 wt% sodium methoxide methanol solution was diluted with 3 ml of methanol and added dropwise with stirring under ice cooling. Stir for 30 minutes at the same temperature. After completion of the reaction, 1.5 ml of acetic acid and 10 ml of water were added to the reaction mixture and extracted with dichloromethane (30 ml ⁇ 1).
- the organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure.
- the residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (3:97) to obtain 683 mg of the desired product as a red oily substance.
- Step 2 Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2-methoxy-1-methylethylamine 5-Bromo-3-chloro-2- (1-methoxy-2-nitropropyl) 340 mg of pyridine was dissolved in 4 ml of a methanol-water (1: 1) mixed solvent, 350 mg of ammonium chloride and 182 mg of reduced iron were added, and the mixture was stirred at room temperature for 30 minutes and then at 65 ° C. for 1.5 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, insoluble materials were removed by Celite filtration, and then the solvent was distilled off under reduced pressure.
- the residue was dissolved in 30 ml of diethyl ether and extracted with 20 ml of a 1% by weight aqueous hydrochloric acid solution.
- the aqueous layer was made basic by adding 20 ml of a saturated aqueous solution of sodium bicarbonate, and then extracted with 100 ml of ethyl acetate.
- the organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 200 mg of the crude desired product as a brown oily substance.
- the obtained target product was directly used in the next step without further purification.
- Step 3 Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-methoxy-1-methylethyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloro
- a solution of 190 mg of pyridin-2-yl) -2-methoxy-1-methylethylamine and 80 mg of pyridine in 2 ml of dichloromethane was added 178 mg of di-tert-butyl dicarbonate and stirred at room temperature for 30 minutes. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with dichloromethane (10 ml ⁇ 2).
- Step 4 Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] carbamic acid-tert-butyl [2- (5-Bromo-3-chloropyridin-2-yl) -2-methoxy-1-methylethyl] carbamic acid-tert-butyl 272 mg of N, N-dimethylformamide 1 ml in a solution of 109 mg of triethylamine, 1-ethynyl 109 mg of -4-fluorobenzene, 27 mg of copper (I) iodide and 25 mg of dichlorobis (triphenylphosphine) palladium (II) were added and stirred at 80 ° C.
- Step 5 Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethylamine N- [2- [3-Chloro-5- To a solution of 220 mg of [(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] carbamic acid-tert-butyl in 2 ml of dichloromethane was added 1 ml of trifluoroacetic acid under ice-cooling and stirring. And stirred at room temperature for 1 hour.
- the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml ⁇ 2). The organic layers were combined, washed with water (10 ml ⁇ 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 167 mg of the crude target product as a dark brown resinous substance. It was. The obtained target product was directly used in the next step without further purification.
- Step 2 Preparation of 2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethylamine N under ice-cooling and stirring -[2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl 830 mg 2 ml of trifluoroacetic acid was added to a 6 ml solution of dichloromethane and stirred at room temperature for 1 hour.
- Step 1 Preparation of N- [2- [3-Chloro-5-[(trimethylsilyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl Synthesis Example 1 Step 3 N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethyl] carbamic acid-tert-butyl 1.86 g of N-N-dimethylformamide in 15 ml of 1.52 g, 0.59 g of trimethylsilylacetylene, 285 mg of copper (I) iodide and 350 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at room temperature for 1 hour in a nitrogen atmosphere.
- Step 2 Preparation of N- [2- [3-chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl N- [2- [3-Chloro-5-[(trimethylsilyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl 480 mg, triethylamine 375 mg, 2-fluoro-5-iodo To a solution of 303 mg of pyridine, 71 mg of copper (I) iodide and 87 mg of dichlorobis (triphenylphosphine) palladium (II) in 6 ml of N, N-dimethylformamide was added 1.3 ml of a 1.0 M tetrahydrofuran solution of te
- the mixture was stirred at room temperature for 16 hours under a nitrogen atmosphere. After completion of the reaction, 10 ml of a saturated aqueous ammonium chloride solution and 10 ml of ethyl acetate were added to the reaction mixture, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (10 ml ⁇ 2). The organic layers were combined, washed with 20 ml of saturated aqueous ammonium chloride solution, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure.
- Step 3 Preparation of 2- [3-chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine N- [2- under ice-cooling stirring [3-Chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl 324 mg in dichloromethane 3 ml solution with trifluoroacetic acid 1 ml And stirred at room temperature for 1 hour.
- the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml ⁇ 2). The organic layers were combined and washed with water (10 ml ⁇ 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 253 mg of the crude desired product as yellow crystals. The obtained target product was directly used in the next step without further purification.
- Step 4 N- [2- [3-Chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl)
- 2- (trifluoromethyl) benzoyl chloride was added dropwise.
- Step 1 Preparation of 1-[(5-bromo-3-chloropyridin-2-yl) methyl] cyclopropanamine 2.38 g of 2- (5-bromo-3-chloropyridin-2-yl) acetonitrile and titanium ( IV) To a solution of 3.21 g of tetraisopropoxide in 20 ml of diethyl ether was added dropwise 6.90 ml of 3M ethylmagnesium bromide diethyl ether solution with stirring under ice cooling, and the mixture was stirred at room temperature for 1 hour.
- Step 2 Preparation of N- [1-[(5-bromo-3-chloropyridin-2-yl) methyl] cyclopropyl] -2- (trifluoromethyl) benzamide 1-[(5-Bromo-3-chloro To a solution of 2.50 g of pyridin-2-yl) methyl] cyclopropanamine and 2.08 g of triethylamine in 10 ml of dichloromethane, 2.04 g of 2- (trifluoromethyl) benzoyl chloride was added dropwise with stirring under ice-cooling. Stir for hours. After completion of the reaction, 5 ml of water was added to the reaction mixture and extracted with chloroform (5 ml ⁇ 2).
- Step 3 Preparation of N- [1-[[3-Chloro-5-[(4-chlorophenyl) ethynyl] pyridin-2-yl] methyl] cyclopropyl] -2- (trifluoromethyl) benzamide N- [1 -[(5-Bromo-3-chloropyridin-2-yl) methyl] cyclopropyl] -2- (trifluoromethyl) benzamide in a solution of 270 mg of N, N-dimethylformamide in 3 ml of cesium carbonate, 409 mg of copper iodide (I 12 mg, 14 mg of palladium (II) acetate, 36 mg of 4,5-bis (diphenylphosphino) -9,9-dimethylxanthene and 102 mg of 4-chloro-1-ethynylbenzene were added at 70 ° C.
- This invention compound can be manufactured according to the said manufacturing method and an Example.
- Examples of alkynylpyridine-substituted amide compounds produced in the same manner as in Synthesis Examples 1 to 16 are included in Tables 3 to 17, and examples of production intermediates are shown in Tables 18 to As shown in Table 20, the alkynylpyridine-substituted amide compounds and their production intermediates encompassed by the present invention are not limited to these.
- Et is ethyl
- c-Pr and Pr-c are cyclopropyl
- Bu-t is tert-butyl
- Pen is pentyl
- Ph is phenyl
- 1-Naph is 1-naphthyl
- 2-Naph represents 2-naphthyl
- the aromatic heterocycles represented by D-1-2a to D-25-3a each represent the following structure
- This invention compound can be manufactured according to the said manufacturing method and an Example.
- alkynylpyridine-substituted amide compounds included in the present invention produced in the same manner as in Synthesis Examples 1 to 14 are shown in Tables 3 to 17, and examples of their production intermediates are shown in Tables 18 to As shown in Table 20, the alkynylpyridine-substituted amide compounds and their production intermediates encompassed by the present invention are not limited to these.
- Et is ethyl
- c-Pr is cyclopropyl
- Bu-t is tert-butyl
- Pen is pentyl
- Ph is phenyl
- 1-Naph is 1-naphthyl
- 2-Naph is Each represents 2-naphthyl
- the aromatic heterocycles represented by D-1-2a to D-25-3a each represent the following structure
- the number representing the substitution position of the substituent (Z) n corresponds to the position of the number described in the above structural formula.
- (D-23-2b) -6-F” The description represents “6-fluoropyridin-3-yl”
- the aromatic heterocycles represented by D-31-a to D-34-a each represent the following structure:
- T-15 to T-78 each represent the following structure.
- the description such as 1-015 (-) and 1-015 (+) in the column of compound number represents that the specific optical rotation is an optical isomer of (-) and (+), respectively.
- the notation of (R) and (S) in the column of substituent R 3 indicates the ratio of (R) -isomer or (S) -isomer in the mixing ratio of optical isomers of carbon atoms to which R 3 is bonded. Represents 90% or more
- “* 1” in the melting point column means that the property of the compound was oily or resinous.
- Solpol registered trademark
- Solvesso registered trademark
- ExxonMobil 1: 5: 2
- This chemical solution was diluted to a predetermined concentration and used in Test Examples 1 to 8 below.
- test drug solution B The compound of the present invention was dissolved in dimethyl sulfoxide to prepare a 1% strength by weight solution. This chemical solution was diluted to a predetermined concentration and used in the following Test Examples 9 to 13 and a comparative test.
- Test Example 1 Cucumber powdery mildew prevention effect test Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and the test drug solution A of the present compound was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml sprayed per pot. After air drying, the cucumber was placed in an air-conditioned greenhouse (20 ° C.) and sprayed with a conidial spore suspension of cucumber powdery mildew (Erysiphe polygoni, Synonym; Erysiphe betae). After 9 days at the same temperature, the proportion of the lesions formed in the inoculated leaves was measured, and the control value was calculated according to the following formula. In addition, the test was performed by 2 continuous systems.
- Control value [1- (Sickness area ratio of treated area / Uncovered lesion area ratio)] ⁇ 100
- the following compounds showed a control value of 70% or more.
- Compound of the present invention No.1-002,1-005 to 1-007,1-011,1-012,1-016,1-019,1-022,1-023,1-031,1-038, 1-042,1-044 to 1-046,1-048 to 1-052,1-059 to 1-063,1-066 to 1-068,1-069 * , 1-070,1-073,1 -074,1-086,1-090,1-091,1-104,1-105,1-108,1-109,1-114,1-116,1-121,1-124,1-142 , 1-144,1-147,1-148,2-002,2-008,2-011,2-016,2-018,2-054 to 2-056,2-058,2-059,2 -067,2-084,2-086,3-002 * , 3-010,3-012,3-014,
- Test example 2 Cucumber gray mold prevention effect test (spore inoculation) Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and at the cotyledon stage, the test drug solution A of the compound of the present invention was diluted with distilled water to a concentration of 500 ppm and sprayed with 5 ml per pot. After air drying, the treated leaves were cut out and placed in a plastic container. The conidial spore suspension of cucumber gray mold fungus (Botrytis cinerea) and the dissolved PDA medium were mixed at a ratio of 1: 1, and 30 ⁇ l of each of the treated leaves was inoculated dropwise. After inoculation, after placing it at 20 ° C.
- the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1.
- the test was performed by 2 continuous systems. As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
- Test example 3 Cucumber gray mold prevention effect test (mycelia inoculation) Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and at the cotyledon stage, the test drug solution A of the compound of the present invention was diluted with distilled water to a concentration of 500 ppm and sprayed with 5 ml per pot. One day later, the pot was placed in a plastic container and inoculated into a cucumber cotyledon treated with a drug with a bacterial agar piece (diameter 5 mm) of cucumber gray mold (Botrytis cinerea) previously cultured in PDA medium. After inoculation, the plastic container was covered with vinyl and humidified, and placed at 20 ° C.
- Test Example 4 Cucumber Nucleus Disease Prevention Effect Test Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and the test drug solution A of the compound of the present invention was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml sprayed per pot. After air-drying, the pot was placed in a plastic container and inoculated into a cucumber cotyledon treated with a drug, a garnish containing agar (Sclerotinia sclerotiorum) -containing agar pieces (diameter 5 mm) previously cultured in a PDA medium.
- agar Sterotinia sclerotiorum
- the plastic container After inoculation, the plastic container is covered with plastic, humidified, and left at 20 ° C for 2 days. Then, the proportion of the lesions formed in the inoculated leaves is measured, and the control value is calculated from the same formula as in Test Example 1. did. In addition, the test was performed by 2 continuous systems. As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
- Test Example 5 Cucumber Anthrax Preventive Effect Test Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and test chemical solution A of the compound of the present invention was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml spraying treatment was performed. One day later, a conidial spore suspension of cucumber anthracnose fungus (Colletotrichum lagenarium, Synonym; Colletotrichum orbiculare) was spray-inoculated and placed in an inoculation box at a temperature of 25 ° C. and a humidity of 100% RH for 2 days.
- Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and test chemical solution A of the compound of the present invention was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml spraying treatment was performed. One day later, a
- Test Example 6 Wheat powdery mildew prevention effect test 1.3 Test chemical solution A of the compound of the present invention was added to distilled water in a 90 cm 3 plastic pot planted with 1.3 leaves of wheat (variety: Norin 61), and the concentration was 500 ppm. And 5 ml sprayed per pot. One day after spraying, the pot was placed in an air-conditioned greenhouse (20 ° C.), and wheat was inoculated with conidia of wheat powdery mildew (Blumeria graminis f. Sp. Tritici). Thereafter, it was held for 7 days, the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
- Test Example 7 Wheat Blight Prevention Effect Test 1.3 Distilled water was added to test chemical solution A of the present invention in a 90 cm 3 plastic pot planted with 3 leaf stage wheat (variety: Haruyutaka) to a concentration of 500 ppm. Then, 5 ml per pot was sprayed. One day after spraying, a conidial spore suspension of wheat blight fungus (Phaeosphaeria nodorum, Synonym; Septoria nodorum) was spray-inoculated on the wheat and placed in an inoculation box at a temperature of 20 ° C. and a humidity of 100% RH for 2 days. Thereafter, it was placed in an air-conditioned greenhouse (20 ° C.) and held for 6 days.
- a conidial spore suspension of wheat blight fungus (Phaeosphaeria nodorum, Synonym; Septoria nodorum) was spray-inoculated on the wheat and placed in an inoculation box at a temperature of
- the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same calculation formula as in Test Example 1.
- the test was performed by 2 continuous systems. As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
- Test Example 8 Wheat Red Rust Prevention Effect Test 1.3 Into a 90 cm 3 plastic pot planted with 3 leaf stage wheat (variety: Norin 61), distilled water was added to the test chemical solution A of the present invention to a concentration of 500 ppm. Diluted and sprayed 5 ml per pot. One day after spraying, the wheat spore suspension of Puccinia recondita was spray-inoculated on the wheat and placed in an inoculation box at a temperature of 20 ° C. and a humidity of 100% RH for one day. Thereafter, it was placed in an air-conditioned greenhouse (20 ° C.) and held for 8 days.
- the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same calculation formula as in Test Example 1. In addition, the test was performed by 2 continuous systems. As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
- Compound of the present invention No. 3-053, 3-059, 3-083, 3-172, 4-004, 8-005, 9-003, 9-004, 9-009, 9-014, 9-016, 9-018,9-020.
- Test Example 10 Insecticidal test against sweet potato nematode After dispensing 60 ⁇ l of potato dextrose 1% agar medium into a 96-well plate, each well was given sterile water (10 eggs / 3 ⁇ l) containing eggs of sweet potato nematode (Meloidogyne incognita). 30 ⁇ l per dose was added. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 ⁇ l per well, and allowed to stand at 25 ° C. under dark conditions.
- Efficacy (%) [(number of untreated eggs treated + number of inactive larvae) / number of untreated group active larvae] x 100 As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
- Test Example 11 Control Effect Test on Sweet Potato Nematode Cellulose-planted spinach seedlings (about 2 weeks after germination) filled with 10 g of soil per cell were added 100 ppm of test chemical solution B of the compound of the present invention with distilled water. Dilute to concentration and treat 1 ml per strain. One hour after the treatment, water containing 2 L larvae of Meloidogyne incognita (2 L larvae: 100/1 ml) was inoculated to the strain, 1 ml per cell. Then, it hold
- Test Example 12 Insecticidal test for torsion stomachworms After dispensing 60 ⁇ l of potato dextrose 1% agar medium to a 96-well plate, sterile water (10 eggs / 3 ⁇ l) containing eggs of torsion stomachworms (Haemonchus contortus), 30 ⁇ l was added per well. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 ⁇ l per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were measured, and the efficiency (%) for the untreated group was calculated from the same calculation formula as in Test Example 10. As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
- Test Example 13 Insecticidal test for soybean cyst nematodes After dispensing 60 ⁇ l of 1% agar medium to a 96-well plate, sterile water (10 eggs / 3 ⁇ l) containing soybean cyst nematode (Heterodera glycines) eggs was added to each well. 30 ⁇ l was added. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 ⁇ l per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were measured, and the efficiency (%) for the untreated group was calculated from the same calculation formula as in Test Example 10. As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
- Test Example 14 Insecticidal test against torsion stomachworm (comparison test) After dispensing 60 ⁇ l of potato dextrose 1% agar medium to a 96-well plate, sterile water (10 eggs / 3 ⁇ l) containing eggs of Haemonchus contortus was added in an amount of 30 ⁇ l per well. From this, the test drug solution B of the compound of the present invention and the comparative compound was diluted to each predetermined concentration by adding distilled water, added 10 ⁇ l per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were measured, and the efficiency (%) for the untreated group was calculated from the same calculation formula as in Test Example 10.
- Tables 22 and 23 show the efficiency (%) of each test compound at each predetermined concentration.
- Table 22 ⁇ Concentration (ppm) Test compound 10 3 0.3 0.1 0.03 ⁇ Compound of the present invention No.2-052 100 100 70 0 0 Comparative compound A 30 0 0 0 0 Compound of the present invention No.2-054 100 100 90 80 0 Comparative compound B 100 0 0 0 0 ⁇ Comparative compound A: WO2014-173921, compound X.8 Comparative compound B: WO2014-173921, compound X.7
- the alkynylpyridine-substituted amide compound according to the present invention exhibits excellent pest control activity, particularly bactericidal and nematicidal activity, and has almost no adverse effect on non-target organisms such as mammals, fish and useful insects. Is a very useful compound.
- Japanese Patent Application No. 2016-129158 filed on June 29, 2016, Japanese Patent Application No. 2016-197920 filed on October 6, 2016, Japanese Patent Application filed on January 17, 2017
- the specification, claims, and abstract of Japanese Patent Application No. 2017-084394 filed on April 21, 2017, and Japanese Patent Application No. 2017-084394 are incorporated herein by reference. It is incorporated as a disclosure.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Environmental Sciences (AREA)
- Agronomy & Crop Science (AREA)
- Pest Control & Pesticides (AREA)
- Plant Pathology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Dentistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Pyridine Compounds (AREA)
Abstract
Provided is a novel pest control agent, especially a bactericide or a nematicide.
An alkynylpyridine-substituted amide compound represented by formula (I) or a salt thereof; and a pest control agent which contains the compound or the salt.
(In the formulae, G1 represents a structure represented by G1-1, G1-3, G1-7 or the like; X1 represents a halogen atom, a trifluoromethyl group, a methylsulfonyl group or the like; X2 represents a hydrogen atom, a fluorine atom or the like; Y1 represents a halogen atom or the like; each of Y2 and Y3 represents a hydrogen atom or the like; R1 represents a hydrogen atom, a fluorine atom, a methyl group, a methoxy group or the like, and R2 represents a hydrogen atom, a fluorine atom, a methyl group or the like, or alternatively, R1 and R2 combine with each other to form an ethylene chain, thereby forming a cyclopropyl ring together with carbon atoms to which R1 and R2 are bonded; R3 represents a hydrogen atom, a methyl group or the like; R4 represents a hydrogen atom or the like; R5 represents a hydrogen atom or the like; R6 represents a phenyl group substituted by (Z)m, or the like; Z represents a halogen atom, a C1-C4 alkyl group, a C1-C4 haloalkyl group, a C1-C4 alkoxy group, a C1-C4 haloalkoxy group or the like; and m represents 1, 2, 3 or the like.)
Description
本発明は、新規なアルキニルピリジン置換アミド化合物、そのN-オキシド、又はそれらの塩、およびこれらの化合物を有効成分として含有する有害生物防除剤に関する。
The present invention relates to a novel alkynylpyridine-substituted amide compound, an N-oxide thereof, or a salt thereof, and a pest control agent containing these compounds as active ingredients.
従来、アルキニルピリジン置換アミド化合物に関しては、例えば、N-[2-[3-クロロ-5-(2-フェニルエチニル)ピリジン-2-イル]-1-メチルエチル]-3-ジフルオロメチル-1-メチル-1H-ピラゾール-4-カルボキサミドが有害生物防除活性を示すこと(特許文献1参照。)、N-[2-[3-クロロ-5-(2-フェニルエチニル)ピリジン-2-イル]-2-(イソプロポキシイミノ)エチル]-2-(トリフルオロメチル)ベンズアミド、N-[2-[3-クロロ-5-[2-[4-(tert-ブチル)フェニル]エチニル]ピリジン-2-イル]-2-(イソプロポキシイミノ)エチル]-2-(トリフルオロメチル)ベンズアミド、N-[2-[3-クロロ-5-[2-(2-ピリジル)エチニル]ピリジン-2-イル]-2-(イソプロポキシイミノ)エチル]-2-(トリフルオロメチル)ベンズアミド、N-[2-[3-クロロ-5-[2-(3-ピリジル)エチニル]ピリジン-2-イル]-2-(イソプロポキシイミノ)エチル]-2-(トリフルオロメチル)ベンズアミド又はN-[2-[3-クロロ-5-[2-(4-ピリジル)エチニル]ピリジン-2-イル]-2-(イソプロポキシイミノ)エチル]-2-(トリフルオロメチル)ベンズアミドが有害生物防除活性を示すこと(特許文献2及び3参照。)、N-[[1-[3-クロロ-5-(3,3,3-トリフルオロ-1-プロピニル)ピリジン-2-イル]シクロプロピル]メチル]-3-フルオロピリジン-2-カルボキサミド、N-[[1-[3-クロロ-5-(3,3,3-トリフルオロ-1-プロピニル)ピリジン-2-イル]シクロプロピル]メチル]-3-クロロピリジン-2-カルボキサミド、N-[[1-[3-クロロ-5-(3,3,3-トリフルオロ-1-プロピニル)ピリジン-2-イル]シクロプロピル]メチル]-3-メチルピリジン-2-カルボキサミド又はN-[[1-[3-クロロ-5-(3,3,3-トリフルオロ-1-プロピニル)ピリジン-2-イル]シクロプロピル]メチル]-3-(トリフルオロメチル)ピリジン-2-カルボキサミドが殺線虫活性を示すこと(特許文献4参照。)が知られている。
Conventionally, regarding alkynylpyridine-substituted amide compounds, for example, N- [2- [3-chloro-5- (2-phenylethynyl) pyridin-2-yl] -1-methylethyl] -3-difluoromethyl-1- Methyl-1H-pyrazole-4-carboxamide exhibits pesticidal activity (see Patent Document 1), N- [2- [3-chloro-5- (2-phenylethynyl) pyridin-2-yl]- 2- (Isopropoxyimino) ethyl] -2- (trifluoromethyl) benzamide, N- [2- [3-chloro-5- [2- [4- (tert-butyl) phenyl] ethynyl] pyridine-2- Yl] -2- (isopropoxyimino) ethyl] -2- (trifluoromethyl) benzamide, N- [2- [3-chloro-5- [2- (2-pyridyl) ethynyl] pyridin-2-yl] -2- (Isopropoxyimino) ethyl] 2- (trifluoromethyl) benzamide, N- [2- [3-chloro-5- [2- (3-pyridyl) ethynyl] pyridin-2-yl] -2- (isopropoxyimino) ethyl] -2- (Trifluoromethyl) benzamide or N- [2- [3-chloro-5- [2- (4-pyridyl) ethynyl] pyridin-2-yl] -2- (isopropoxyimino) ethyl] -2- (tri Fluoromethyl) benzamide exhibits pesticidal activity (see Patent Documents 2 and 3), N-[[1- [3-chloro-5- (3,3,3-trifluoro-1-propynyl) pyridine -2-yl] cyclopropyl] methyl] -3-fluoropyridine-2-carboxamide, N-[[1- [3-chloro-5- (3,3,3-trifluoro-1-propynyl) pyridine-2 -Yl] cyclopropyl] methyl] -3-chloropyridine-2-cal Xamide, N-[[1- [3-chloro-5- (3,3,3-trifluoro-1-propynyl) pyridin-2-yl] cyclopropyl] methyl] -3-methylpyridine-2-carboxamide or N-[[1- [3-Chloro-5- (3,3,3-trifluoro-1-propynyl) pyridin-2-yl] cyclopropyl] methyl] -3- (trifluoromethyl) pyridine-2- It is known that carboxamide exhibits nematicidal activity (see Patent Document 4).
しかしながら、本発明に係るアルキニルピリジン置換アミド化合物に関しては何ら開示した文献はこれまで見当たらず、また、それらの有害生物防除剤としての有用性は知られていない。
However, there are no documents disclosed so far regarding the alkynylpyridine-substituted amide compounds according to the present invention, and their usefulness as pest control agents is not known.
病原菌や寄生虫といった有害生物の感染や寄生は、宿主が穀類、果樹、野菜、鑑賞植物等の植物である場合、農作物の品質の低下、収量の大幅な低下、さらに、場合によっては植物の枯死といった深刻な被害を生じ、生産者のみならず、消費者に対しても多大な経済的損失を与える。故に、それら有害生物の有効な防除は効率的・安定的な農作物の生産を達成するために、極めて重要な課題である。また、宿主が伴侶動物・愛玩動物や家畜・家禽等の動物である場合には、対象となる動物の健康を維持する目的で、さらに、対象となる動物が家畜・家禽等である場合には、安全な食料や高品質な羊毛・羽毛・皮革等の生活資材を安定して生産するという目的からもそれら有害生物の有効な防除は重要な課題である。このような観点から、従来、病原菌や寄生虫の防除を目的とする有害生物防除剤の開発が進み、多数の有効な薬剤が今日まで実用に供されてきた。
しかしながら、こうした薬剤の長年にわたる使用により、近年、病原菌や寄生虫が薬剤抵抗性を獲得し、従来用いられてきた既存の有害生物防除剤による防除が困難となる場面が増えてきている。また、既存の有害生物防除剤の一部のものは毒性が高く、或いはあるものは環境中に長期間残留することにより、生態系を攪乱するという問題も顕在化しつつある。このような状況下、病原菌や寄生虫に対する優れた防除活性を有するのみならず、低毒性且つ低残留性等の高度な防除特性を併せ持つ新規な有害生物防除剤並びに有効な防除方法の開発が常に期待されている。 Infections and infestations of pests such as pathogens and parasites can occur when the host is a plant such as cereals, fruit trees, vegetables, ornamental plants, etc. Cause serious economic losses not only to producers but also to consumers. Therefore, effective control of these pests is a very important issue in order to achieve efficient and stable crop production. In addition, when the host is an animal such as a companion animal, pet animal, livestock, poultry, etc., for the purpose of maintaining the health of the target animal, and when the target animal is livestock, poultry, etc. Effective control of these pests is also an important issue for the purpose of stably producing safe food and high-quality living materials such as wool, feathers and leather. From this point of view, the development of pest control agents for the purpose of controlling pathogenic bacteria and parasites has progressed, and many effective agents have been put to practical use until now.
However, due to the long-term use of such drugs, in recent years, pathogens and parasites have acquired drug resistance, making it difficult to control with existing pest control agents that have been used. In addition, some existing pest control agents are highly toxic, or some of them remain in the environment for a long time, and the problem of disturbing the ecosystem is becoming apparent. Under such circumstances, development of novel pesticides and effective control methods not only having excellent control activity against pathogenic bacteria and parasites but also having advanced control characteristics such as low toxicity and low persistence has always been made. Expected.
しかしながら、こうした薬剤の長年にわたる使用により、近年、病原菌や寄生虫が薬剤抵抗性を獲得し、従来用いられてきた既存の有害生物防除剤による防除が困難となる場面が増えてきている。また、既存の有害生物防除剤の一部のものは毒性が高く、或いはあるものは環境中に長期間残留することにより、生態系を攪乱するという問題も顕在化しつつある。このような状況下、病原菌や寄生虫に対する優れた防除活性を有するのみならず、低毒性且つ低残留性等の高度な防除特性を併せ持つ新規な有害生物防除剤並びに有効な防除方法の開発が常に期待されている。 Infections and infestations of pests such as pathogens and parasites can occur when the host is a plant such as cereals, fruit trees, vegetables, ornamental plants, etc. Cause serious economic losses not only to producers but also to consumers. Therefore, effective control of these pests is a very important issue in order to achieve efficient and stable crop production. In addition, when the host is an animal such as a companion animal, pet animal, livestock, poultry, etc., for the purpose of maintaining the health of the target animal, and when the target animal is livestock, poultry, etc. Effective control of these pests is also an important issue for the purpose of stably producing safe food and high-quality living materials such as wool, feathers and leather. From this point of view, the development of pest control agents for the purpose of controlling pathogenic bacteria and parasites has progressed, and many effective agents have been put to practical use until now.
However, due to the long-term use of such drugs, in recent years, pathogens and parasites have acquired drug resistance, making it difficult to control with existing pest control agents that have been used. In addition, some existing pest control agents are highly toxic, or some of them remain in the environment for a long time, and the problem of disturbing the ecosystem is becoming apparent. Under such circumstances, development of novel pesticides and effective control methods not only having excellent control activity against pathogenic bacteria and parasites but also having advanced control characteristics such as low toxicity and low persistence has always been made. Expected.
本発明者らは、上記の課題解決を目標に鋭意研究を重ねた結果、下記式(I)で表される新規なアルキニルピリジン置換アミド化合物が優れた有害生物防除活性、特に抗真菌・殺線虫活性を示し、且つ、植物やホ乳動物、魚類、有用昆虫及び天敵等の非標的生物に対してほとんど悪影響の無い、極めて有用な化合物であることを見い出し、本発明を完成した。
すなわち、本発明は下記〔1〕~〔86〕に関するものである。 As a result of intensive research aimed at solving the above-mentioned problems, the present inventors have found that a novel alkynylpyridine-substituted amide compound represented by the following formula (I) has excellent pest control activity, particularly antifungal / nematicidal activity. The present invention has been completed by finding an extremely useful compound that exhibits insect activity and has almost no adverse effect on non-target organisms such as plants, mammals, fish, useful insects and natural enemies.
That is, the present invention relates to the following [1] to [86].
すなわち、本発明は下記〔1〕~〔86〕に関するものである。 As a result of intensive research aimed at solving the above-mentioned problems, the present inventors have found that a novel alkynylpyridine-substituted amide compound represented by the following formula (I) has excellent pest control activity, particularly antifungal / nematicidal activity. The present invention has been completed by finding an extremely useful compound that exhibits insect activity and has almost no adverse effect on non-target organisms such as plants, mammals, fish, useful insects and natural enemies.
That is, the present invention relates to the following [1] to [86].
〔1〕
式(I)で表されるアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
[式中、G1は、G1-1~G1-16で表される構造を表し、
[1]
An alkynylpyridine-substituted amide compound represented by the formula (I), an N-oxide thereof or a salt thereof.
Wherein, G 1 represents a structure represented by G 1 -1 ~ G 1 -16,
式(I)で表されるアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
An alkynylpyridine-substituted amide compound represented by the formula (I), an N-oxide thereof or a salt thereof.
X1は、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C3ハロアルキル、C1~C3アルコキシ、C1~C3ハロアルコキシ、C1~C3アルキルチオ、C1~C3アルキルスルフィニル、C1~C3アルキルスルホニル、C1~C3ハロアルキルチオ又はC1~C3ハロアルキルスルホニルを表し、
X2は、水素原子又はハロゲン原子を表し、ただし、G1がG1-10で表される構造であり、且つX1がジハロメチルを表す場合には、X2は水素原子を表し、
X3は、水素原子又はC1~C4アルキルを表し、
Y1は、水素原子、ハロゲン原子、ニトロ、C1~C4アルキル、C1~C3ハロアルキル、C1~C3アルコキシ、C1~C3ハロアルコキシ又はC1~C3アルキルチオを表し、
Y2及びY3は、各々独立して水素原子、ハロゲン原子又はメチルを表し、
R1は、水素原子、ハロゲン原子、シアノ、C1~C6アルキル、C1~C6ハロアルキル、フェニル(C1~C4)アルキル、(Z)mによって置換されたフェニル(C1~C4)アルキル、C3~C6シクロアルキル、C2~C6アルケニル、C2~C6アルキニル、C1~C6アルコキシ、C1~C6ハロアルコキシ、C3~C6アルケニルオキシ、C3~C6ハロアルケニルオキシ、C3~C6アルキニルオキシ、C3~C6ハロアルキニルオキシ、シアノ(C1~C4)アルコキシ、フェニル(C1~C4)アルコキシ、(Z)mによって置換されたフェニル(C1~C4)アルコキシ、C1~C6アルキルチオ、C1~C6ハロアルキルチオ、C1~C6アルコキシアミノ、-C(O)NH2又は-C(S)NH2を表し、
R2は、水素原子、ハロゲン原子又はC1~C6アルキルを表し、
ここで、R1とR2とは一緒になってC2~C5アルキレン鎖を形成することにより、R1及びR2が結合する炭素原子と共に3~6員環を形成してもよく、このとき前記アルキレン鎖は酸素原子、硫黄原子又は窒素原子を1~2個含んでもよく、且つC1~C4アルキル、C1~C4ハロアルキル、オキソ又はチオキソによって任意に置換されていてもよいことを表すか、R1とR2とが一緒になってC1~C6アルキリデン、C1~C6ハロアルキリデン又はC1~C4アルコキシ(C1~C2)アルキリデンを形成することを表し、
R3は、水素原子、C1~C6アルキル又はC1~C6ハロアルキルを表し、
R4は、水素原子又はC1~C6アルキルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成してもよく、
R5は、水素原子、C1~C4アルキル、C1~C4ハロアルキル、R8によって置換された(C1~C2)アルキル、C3~C6シクロアルキル、C2~C4アルケニル、C3~C4アルキニル、-OH、C1~C4アルコキシ、C1~C4ハロアルコキシ、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、C3~C6シクロアルキル(C3~C6)シクロアルキル、フェニル(C3~C6)シクロアルキル、(Z)mによって置換されたフェニル又はD-1~D-29を表し、
R1がフッ素原子、C1~C6アルキル又はC1~C6アルコキシを表し、R2がフッ素原子又はC1~C6アルキルを表し、R3がC1~C6アルキル又はC1~C6ハロアルキルを表し、且つR4が水素原子又はC1~C6アルキルを表す場合には、R6は水素原子、ハロゲン原子、C1~C6アルキル、C1~C6ハロアルキル、R10によって任意に置換された(C1~C4)アルキル、C3~C10シクロアルキル、C3~C10ハロシクロアルキル、ヒドロキシ(C3~C6)シクロアルキル、C1~C4アルコキシ(C3~C6)シクロアルキル、C4~C10シクロアルケニル、C4~C10ハロシクロアルケニル、トリ(C1~C4アルキル)シリル、フェニルジメチルシリル、-C(R11)=NOR12又はフェニルを表してもよく、
D-1~D-29は、それぞれ下記の構造式で表される芳香族複素環を表し、 X 1 represents a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 3 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 1 -C 3 alkylthio, C 1- represents C 3 alkylsulfinyl, C 1 ~ C 3 alkylsulfonyl, C 1 ~ C 3 haloalkylthio or C 1 ~ C 3 haloalkylsulfonyl,
X 2 represents a hydrogen atom or a halogen atom, provided that when G 1 is a structure represented by G 1 -10 and X 1 represents dihalomethyl, X 2 represents a hydrogen atom;
X 3 represents a hydrogen atom or C 1 -C 4 alkyl,
Y 1 represents a hydrogen atom, a halogen atom, nitro, C 1 -C 4 alkyl, C 1 -C 3 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy or C 1 -C 3 alkylthio,
Y 2 and Y 3 each independently represent a hydrogen atom, a halogen atom or methyl,
R 1 is a hydrogen atom, a halogen atom, cyano, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, phenyl (C 1 -C 4 ) alkyl, (Z) m- substituted phenyl (C 1 -C 4 ) alkyl, C 3 -C 6 cycloalkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 3 -C 6 alkenyloxy, C 3 to C 6 haloalkenyloxy, C 3 to C 6 alkynyloxy, C 3 to C 6 haloalkynyloxy, cyano (C 1 to C 4 ) alkoxy, phenyl (C 1 to C 4 ) alkoxy, (Z) m Substituted phenyl (C 1 -C 4 ) alkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkoxyamino, -C (O) NH 2 or -C (S) NH 2
R 2 represents a hydrogen atom, a halogen atom or C 1 -C 6 alkyl,
Here, R 1 and R 2 may form a C 2 -C 5 alkylene chain together to form a 3- to 6-membered ring with the carbon atom to which R 1 and R 2 are bonded, in this case the alkylene chain an oxygen atom, a sulfur atom or a nitrogen atom may be 1 to 2 comprise, and C 1 ~ C 4 alkyl, C 1 ~ C 4 haloalkyl, may be optionally substituted by oxo or thioxo Or that R 1 and R 2 together form C 1 -C 6 alkylidene, C 1 -C 6 haloalkylidene or C 1 -C 4 alkoxy (C 1 -C 2 ) alkylidene. Represent,
R 3 represents a hydrogen atom, C 1 -C 6 alkyl or C 1 -C 6 haloalkyl,
R 4 represents a hydrogen atom or C 1 -C 6 alkyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring together with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, (C 1 -C 2 ) alkyl substituted by R 8 , C 3 -C 6 cycloalkyl, C 2 -C 4 alkenyl C 3 -C 4 alkynyl, —OH, C 1 -C 4 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 haloalkylthio, —C (O) R 9 or C 1 -C 4 alkoxycarbonyl Represent,
R 6 represents C 3 -C 6 cycloalkyl (C 3 -C 6 ) cycloalkyl, phenyl (C 3 -C 6 ) cycloalkyl, (Z) phenyl substituted by m , or D-1 through D-29. Represent,
R 1 represents a fluorine atom, C 1 -C 6 alkyl or C 1 -C 6 alkoxy, R 2 represents a fluorine atom or C 1 -C 6 alkyl, and R 3 represents C 1 -C 6 alkyl or C 1- When C 6 haloalkyl is represented and R 4 represents a hydrogen atom or C 1 -C 6 alkyl, R 6 is a hydrogen atom, a halogen atom, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, R 10 (C 1 -C 4 ) alkyl, C 3 -C 10 cycloalkyl, C 3 -C 10 halocycloalkyl, hydroxy (C 3 -C 6 ) cycloalkyl, C 1 -C 4 alkoxy ( C 3 -C 6 ) cycloalkyl, C 4 -C 10 cycloalkenyl, C 4 -C 10 halocycloalkenyl, tri (C 1 -C 4 alkyl) silyl, phenyldimethylsilyl, —C (R 11 ) = NOR 12 Or it may represent phenyl,
D-1 to D-29 each represent an aromatic heterocycle represented by the following structural formula;
X2は、水素原子又はハロゲン原子を表し、ただし、G1がG1-10で表される構造であり、且つX1がジハロメチルを表す場合には、X2は水素原子を表し、
X3は、水素原子又はC1~C4アルキルを表し、
Y1は、水素原子、ハロゲン原子、ニトロ、C1~C4アルキル、C1~C3ハロアルキル、C1~C3アルコキシ、C1~C3ハロアルコキシ又はC1~C3アルキルチオを表し、
Y2及びY3は、各々独立して水素原子、ハロゲン原子又はメチルを表し、
R1は、水素原子、ハロゲン原子、シアノ、C1~C6アルキル、C1~C6ハロアルキル、フェニル(C1~C4)アルキル、(Z)mによって置換されたフェニル(C1~C4)アルキル、C3~C6シクロアルキル、C2~C6アルケニル、C2~C6アルキニル、C1~C6アルコキシ、C1~C6ハロアルコキシ、C3~C6アルケニルオキシ、C3~C6ハロアルケニルオキシ、C3~C6アルキニルオキシ、C3~C6ハロアルキニルオキシ、シアノ(C1~C4)アルコキシ、フェニル(C1~C4)アルコキシ、(Z)mによって置換されたフェニル(C1~C4)アルコキシ、C1~C6アルキルチオ、C1~C6ハロアルキルチオ、C1~C6アルコキシアミノ、-C(O)NH2又は-C(S)NH2を表し、
R2は、水素原子、ハロゲン原子又はC1~C6アルキルを表し、
ここで、R1とR2とは一緒になってC2~C5アルキレン鎖を形成することにより、R1及びR2が結合する炭素原子と共に3~6員環を形成してもよく、このとき前記アルキレン鎖は酸素原子、硫黄原子又は窒素原子を1~2個含んでもよく、且つC1~C4アルキル、C1~C4ハロアルキル、オキソ又はチオキソによって任意に置換されていてもよいことを表すか、R1とR2とが一緒になってC1~C6アルキリデン、C1~C6ハロアルキリデン又はC1~C4アルコキシ(C1~C2)アルキリデンを形成することを表し、
R3は、水素原子、C1~C6アルキル又はC1~C6ハロアルキルを表し、
R4は、水素原子又はC1~C6アルキルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成してもよく、
R5は、水素原子、C1~C4アルキル、C1~C4ハロアルキル、R8によって置換された(C1~C2)アルキル、C3~C6シクロアルキル、C2~C4アルケニル、C3~C4アルキニル、-OH、C1~C4アルコキシ、C1~C4ハロアルコキシ、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、C3~C6シクロアルキル(C3~C6)シクロアルキル、フェニル(C3~C6)シクロアルキル、(Z)mによって置換されたフェニル又はD-1~D-29を表し、
R1がフッ素原子、C1~C6アルキル又はC1~C6アルコキシを表し、R2がフッ素原子又はC1~C6アルキルを表し、R3がC1~C6アルキル又はC1~C6ハロアルキルを表し、且つR4が水素原子又はC1~C6アルキルを表す場合には、R6は水素原子、ハロゲン原子、C1~C6アルキル、C1~C6ハロアルキル、R10によって任意に置換された(C1~C4)アルキル、C3~C10シクロアルキル、C3~C10ハロシクロアルキル、ヒドロキシ(C3~C6)シクロアルキル、C1~C4アルコキシ(C3~C6)シクロアルキル、C4~C10シクロアルケニル、C4~C10ハロシクロアルケニル、トリ(C1~C4アルキル)シリル、フェニルジメチルシリル、-C(R11)=NOR12又はフェニルを表してもよく、
D-1~D-29は、それぞれ下記の構造式で表される芳香族複素環を表し、 X 1 represents a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 3 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 1 -C 3 alkylthio, C 1- represents C 3 alkylsulfinyl, C 1 ~ C 3 alkylsulfonyl, C 1 ~ C 3 haloalkylthio or C 1 ~ C 3 haloalkylsulfonyl,
X 2 represents a hydrogen atom or a halogen atom, provided that when G 1 is a structure represented by G 1 -10 and X 1 represents dihalomethyl, X 2 represents a hydrogen atom;
X 3 represents a hydrogen atom or C 1 -C 4 alkyl,
Y 1 represents a hydrogen atom, a halogen atom, nitro, C 1 -C 4 alkyl, C 1 -C 3 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy or C 1 -C 3 alkylthio,
Y 2 and Y 3 each independently represent a hydrogen atom, a halogen atom or methyl,
R 1 is a hydrogen atom, a halogen atom, cyano, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, phenyl (C 1 -C 4 ) alkyl, (Z) m- substituted phenyl (C 1 -C 4 ) alkyl, C 3 -C 6 cycloalkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 3 -C 6 alkenyloxy, C 3 to C 6 haloalkenyloxy, C 3 to C 6 alkynyloxy, C 3 to C 6 haloalkynyloxy, cyano (C 1 to C 4 ) alkoxy, phenyl (C 1 to C 4 ) alkoxy, (Z) m Substituted phenyl (C 1 -C 4 ) alkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkoxyamino, -C (O) NH 2 or -C (S) NH 2
R 2 represents a hydrogen atom, a halogen atom or C 1 -C 6 alkyl,
Here, R 1 and R 2 may form a C 2 -C 5 alkylene chain together to form a 3- to 6-membered ring with the carbon atom to which R 1 and R 2 are bonded, in this case the alkylene chain an oxygen atom, a sulfur atom or a nitrogen atom may be 1 to 2 comprise, and C 1 ~ C 4 alkyl, C 1 ~ C 4 haloalkyl, may be optionally substituted by oxo or thioxo Or that R 1 and R 2 together form C 1 -C 6 alkylidene, C 1 -C 6 haloalkylidene or C 1 -C 4 alkoxy (C 1 -C 2 ) alkylidene. Represent,
R 3 represents a hydrogen atom, C 1 -C 6 alkyl or C 1 -C 6 haloalkyl,
R 4 represents a hydrogen atom or C 1 -C 6 alkyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring together with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, (C 1 -C 2 ) alkyl substituted by R 8 , C 3 -C 6 cycloalkyl, C 2 -C 4 alkenyl C 3 -C 4 alkynyl, —OH, C 1 -C 4 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 haloalkylthio, —C (O) R 9 or C 1 -C 4 alkoxycarbonyl Represent,
R 6 represents C 3 -C 6 cycloalkyl (C 3 -C 6 ) cycloalkyl, phenyl (C 3 -C 6 ) cycloalkyl, (Z) phenyl substituted by m , or D-1 through D-29. Represent,
R 1 represents a fluorine atom, C 1 -C 6 alkyl or C 1 -C 6 alkoxy, R 2 represents a fluorine atom or C 1 -C 6 alkyl, and R 3 represents C 1 -C 6 alkyl or C 1- When C 6 haloalkyl is represented and R 4 represents a hydrogen atom or C 1 -C 6 alkyl, R 6 is a hydrogen atom, a halogen atom, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, R 10 (C 1 -C 4 ) alkyl, C 3 -C 10 cycloalkyl, C 3 -C 10 halocycloalkyl, hydroxy (C 3 -C 6 ) cycloalkyl, C 1 -C 4 alkoxy ( C 3 -C 6 ) cycloalkyl, C 4 -C 10 cycloalkenyl, C 4 -C 10 halocycloalkenyl, tri (C 1 -C 4 alkyl) silyl, phenyldimethylsilyl, —C (R 11 ) = NOR 12 Or it may represent phenyl,
D-1 to D-29 each represent an aromatic heterocycle represented by the following structural formula;
Zは、ハロゲン原子、シアノ、ニトロ、-SF5、C1~C4アルキル、C1~C4ハロアルキル、シアノ(C1~C4)アルキル、C1~C4アルコキシ(C1~C4)アルキル、C1~C4ハロアルコキシ(C1~C4)アルキル、C1~C4アルキルチオ(C1~C4)アルキル、C1~C4アルキルスルフィニル(C1~C4)アルキル、C1~C4アルキルスルホニル(C1~C4)アルキル、C1~C4ハロアルキルチオ(C1~C4)アルキル、C1~C4ハロアルキルスルフィニル(C1~C4)アルキル、C1~C4ハロアルキルスルホニル(C1~C4)アルキル、C3~C4シクロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、ジ(C1~C4アルキル)アミノ、フェニル又はD-30~D-38を表し、m又はnが2以上の場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-CH2OCH2-、-OCH2O-、-CH2CH2S(O)r-、-CH2S(O)rCH2-、-SCH2S-、-CH2CH2CH2CH2-、-CH2CH2CH2O-、-CH2CH2OCH2-、-CH2OCH2O-、-OCH2CH2O-、-CH2CH2CH2S(O)r-、-OCH2CH2S-、-SCH2CH2S-、-OCH=CH-、-SCH=CH-、-SO2CH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-SO2N=CH-、-N(R13)N=CH-、-ON=N-、-SN=N-、-N(R13)N=N-、=NOCH=、=NSCH=、=NN(R13)CH=、=NON=、=NSN=、=NN(R13)N=、-CH=CHCH=CH-、-N=CHCH=CH-、-CH=NCH=CH-、-N=NCH=CH-、-N=CHN=CH-、-N=CHCH=N-、-CH=NN=CH-又は-N=NCH=N-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、シアノ、C1~C4アルキル、C1~C4ハロアルキル、C1~C4アルコキシ、C1~C4ハロアルコキシ又はC1~C4ハロアルキルチオによって任意に置換されていてもよく、
D-30~D-38は、それぞれ下記の構造式で表される芳香族複素環を表し、 Z is a halogen atom, cyano, nitro, —SF 5 , C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, cyano (C 1 -C 4 ) alkyl, C 1 -C 4 alkoxy (C 1 -C 4 ) Alkyl, C 1 -C 4 haloalkoxy (C 1 -C 4 ) alkyl, C 1 -C 4 alkylthio (C 1 -C 4 ) alkyl, C 1 -C 4 alkylsulfinyl (C 1 -C 4 ) alkyl, C 1 -C 4 alkylsulfonyl (C 1 -C 4 ) alkyl, C 1 -C 4 haloalkylthio (C 1 -C 4 ) alkyl, C 1 -C 4 haloalkylsulfinyl (C 1 -C 4 ) alkyl, C 1 -C 4 haloalkylsulfonyl (C 1 -C 4 ) alkyl, C 3 -C 4 cycloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkyl Sulfinyl, C 1 -C 4 alkylsulfonyl, C 1 -C 4 haloal Represents thio, C 1 -C 4 haloalkylsulfinyl, C 1 -C 4 haloalkylsulfonyl, di (C 1 -C 4 alkyl) amino, phenyl or D-30-D-38, when m or n is 2 or more Each Z may be the same as or different from each other. Further, when two Zs are adjacent to each other, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —, —OCH 2 O—, —CH 2 CH 2 S (O) r —, —CH 2 S (O) r CH 2 —, —SCH 2 S—, — CH 2 CH 2 CH 2 CH 2- , -CH 2 CH 2 CH 2 O-, -CH 2 CH 2 OCH 2- , -CH 2 OCH 2 O-, -OCH 2 CH 2 O-, -CH 2 CH 2 CH 2 S (O) r - , - OCH 2 CH 2 S -, - SCH 2 CH 2 S -, - OCH = CH -, - SCH = CH -, - SO 2 CH = CH -, - N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -SO 2 N = CH-,- N (R 13 ) N = CH-, -ON = N-, -SN = N-, -N (R 13 ) N = N-, = NOCH =, = NSCH =, = NN (R 13 ) CH =, = NON =, = NSN =, = NN (R 13 ) N =, -CH = CHCH = CH-, -N = CHCH = CH-, -CH = NCH = CH-, -N = NCH = CH-, -N By forming = CHN = CH-, -N = CHCH = N-, -CH = NN = CH- or -N = NCH = N-, together with the carbon atom to which each Z is bonded, a 5-membered ring or 6-membered ring A hydrogen atom bonded to each carbon atom forming the ring may be a halogen atom, cyano, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy Optionally substituted by C 1 -C 4 haloalkoxy or C 1 -C 4 haloalkylthio,
D-30 to D-38 each represents an aromatic heterocycle represented by the following structural formula,
D-30~D-38は、それぞれ下記の構造式で表される芳香族複素環を表し、 Z is a halogen atom, cyano, nitro, —SF 5 , C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, cyano (C 1 -C 4 ) alkyl, C 1 -C 4 alkoxy (C 1 -C 4 ) Alkyl, C 1 -C 4 haloalkoxy (C 1 -C 4 ) alkyl, C 1 -C 4 alkylthio (C 1 -C 4 ) alkyl, C 1 -C 4 alkylsulfinyl (C 1 -C 4 ) alkyl, C 1 -C 4 alkylsulfonyl (C 1 -C 4 ) alkyl, C 1 -C 4 haloalkylthio (C 1 -C 4 ) alkyl, C 1 -C 4 haloalkylsulfinyl (C 1 -C 4 ) alkyl, C 1 -C 4 haloalkylsulfonyl (C 1 -C 4 ) alkyl, C 3 -C 4 cycloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkyl Sulfinyl, C 1 -C 4 alkylsulfonyl, C 1 -C 4 haloal Represents thio, C 1 -C 4 haloalkylsulfinyl, C 1 -C 4 haloalkylsulfonyl, di (C 1 -C 4 alkyl) amino, phenyl or D-30-D-38, when m or n is 2 or more Each Z may be the same as or different from each other. Further, when two Zs are adjacent to each other, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —, —OCH 2 O—, —CH 2 CH 2 S (O) r —, —CH 2 S (O) r CH 2 —, —SCH 2 S—, — CH 2 CH 2 CH 2 CH 2- , -CH 2 CH 2 CH 2 O-, -CH 2 CH 2 OCH 2- , -CH 2 OCH 2 O-, -OCH 2 CH 2 O-, -CH 2 CH 2 CH 2 S (O) r - , - OCH 2 CH 2 S -, - SCH 2 CH 2 S -, - OCH = CH -, - SCH = CH -, - SO 2 CH = CH -, - N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -SO 2 N = CH-,- N (R 13 ) N = CH-, -ON = N-, -SN = N-, -N (R 13 ) N = N-, = NOCH =, = NSCH =, = NN (R 13 ) CH =, = NON =, = NSN =, = NN (R 13 ) N =, -CH = CHCH = CH-, -N = CHCH = CH-, -CH = NCH = CH-, -N = NCH = CH-, -N By forming = CHN = CH-, -N = CHCH = N-, -CH = NN = CH- or -N = NCH = N-, together with the carbon atom to which each Z is bonded, a 5-membered ring or 6-membered ring A hydrogen atom bonded to each carbon atom forming the ring may be a halogen atom, cyano, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy Optionally substituted by C 1 -C 4 haloalkoxy or C 1 -C 4 haloalkylthio,
D-30 to D-38 each represents an aromatic heterocycle represented by the following structural formula,
R7は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14、C1~C4アルキルチオ、C1~C4アルコキシカルボニル、-C(O)NH2又は-C(S)NH2を表し、
R9は、C1~C4アルキル、C1~C4アルコキシメチル、C3~C4シクロアルキル又はC2~C4アルケニルを表し、
R10は、シアノ、-OR15、-S(O)rR16又は-N(R18)R17を表し、
R11は、水素原子又はC1~C4アルキルを表し、
R12は、C1~C4アルキルを表し、
R13は、C1~C4アルキル、C1~C4ハロアルキル、C3~C4シクロアルキルメチル、C3~C4ハロシクロアルキルメチル、C3~C4シクロアルキル又はC3~C4ハロシクロアルキルを表し、
R14は、C1~C4アルキル、C2~C4ハロアルキル、C1~C4アルキルカルボニル、C3~C6シクロアルキルカルボニル又はC1~C4アルコキシカルボニルを表し、
R15は、水素原子、C1~C4アルキル、C1~C4ハロアルキル、C1~C4アルコキシ(C1~C2)アルキル、E-1、E-2、C3~C4アルケニル、C3~C4ハロアルケニル、C3~C4アルキニル、C3~C4ハロアルキニル、フェニル又は(Z)mによって置換されたフェニルを表し、
E-1及びE-2は、それぞれ下記の構造式で表される飽和複素環を表し、
R 7 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 , C 1 -C 4 alkylthio, C 1 -C 4 alkoxycarbonyl, —C (O) NH 2 or —C (S) NH 2 ,
R 9 represents C 1 -C 4 alkyl, C 1 -C 4 alkoxymethyl, C 3 -C 4 cycloalkyl or C 2 -C 4 alkenyl,
R 10 represents cyano, —OR 15 , —S (O) r R 16 or —N (R 18 ) R 17 ,
R 11 represents a hydrogen atom or C 1 -C 4 alkyl,
R 12 represents C 1 -C 4 alkyl;
R 13 is C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 3 -C 4 cycloalkylmethyl, C 3 -C 4 halocycloalkylmethyl, C 3 -C 4 cycloalkyl or C 3 -C 4 Represents halocycloalkyl,
R 14 represents C 1 -C 4 alkyl, C 2 -C 4 haloalkyl, C 1 -C 4 alkylcarbonyl, C 3 -C 6 cycloalkylcarbonyl or C 1 -C 4 alkoxycarbonyl,
R 15 is a hydrogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy (C 1 -C 2 ) alkyl, E-1, E-2, C 3 -C 4 alkenyl C 3 -C 4 haloalkenyl, C 3 -C 4 alkynyl, C 3 -C 4 haloalkynyl, phenyl or phenyl substituted by (Z) m
E-1 and E-2 each represent a saturated heterocyclic ring represented by the following structural formula,
R17及びR18は、各々独立して水素原子、C1~C4アルキル、C1~C4ハロアルキル、C1~C4アルコキシ(C1~C2)アルキル、C3~C4アルケニル、C3~C4アルキニル、フェニル又は(Z)mによって置換されたフェニルを表すか、
或いは、R17とR18とが一緒になってC2~C5アルキレン鎖を形成することにより、R17及びR18が結合する窒素原子と共に3~6員環を形成してもよく、このとき前記アルキレン鎖は酸素原子又は硫黄原子を1個含んでもよく、且つC1~C4アルキル、C1~C4ハロアルキル、オキソ又はチオキソによって任意に置換されていてもよく、
R19は、C1~C4アルキルを表し、pが2の場合には、各々のR19は互いに同一であっても、または互いに相異なっていてもよく、
mは、1、2、3、4又は5を表し、
nは、0、1、2、3又は4を表し、
pは、0、1又は2を表し、
rは、0、1又は2を表す。]
R 17 and R 18 are each independently a hydrogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy (C 1 -C 2 ) alkyl, C 3 -C 4 alkenyl, Represents C 3 -C 4 alkynyl, phenyl or phenyl substituted by (Z) m ,
Alternatively, R 17 and R 18 may be combined to form a C 2 -C 5 alkylene chain to form a 3- to 6-membered ring with the nitrogen atom to which R 17 and R 18 are bonded. when the alkylene chain may contain one oxygen atom or sulfur atom, and C 1 ~ C 4 alkyl, C 1 ~ C 4 haloalkyl, may be optionally substituted by oxo or thioxo,
R 19 represents C 1 -C 4 alkyl, and when p is 2, each R 19 may be the same as or different from each other;
m represents 1, 2, 3, 4 or 5;
n represents 0, 1, 2, 3 or 4;
p represents 0, 1 or 2;
r represents 0, 1 or 2. ]
〔2〕
G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-9、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル、ジフルオロメチルスルホニル又はトリフルオロメチルスルホニルを表し、
X2は、水素原子、フッ素原子又は塩素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
X3は、メチルを表し、
Y1は、ハロゲン原子、メチル、トリフルオロメチル又はメトキシを表し、
Y2は、水素原子又はメチルを表し、
Y3は、水素原子を表し、
R1は、水素原子、フッ素原子、C1~C3アルキル、C1~C3ハロアルキル、ベンジル、(Z)mによって置換されたフェニルメチル、シクロプロピル、C1~C3アルコキシ、C1~C3ハロアルコキシ、C3~C4アルケニルオキシ、C3~C4アルキニルオキシ、シアノメトキシ、ベンジルオキシ、(Z)mによって置換されたフェニルメトキシ、C1~C3アルキルチオ又はC1~C3ハロアルキルチオを表し、
R2は、水素原子、フッ素原子又はC1~C3アルキルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、R1とR2とは一緒になってC1~C2アルキリデン又はC1~C2ハロアルキリデンを形成することを表してもよく、
R3は、水素原子、C1~C3アルキル又はC1~C3ハロアルキルを表し、
R4は、水素原子又はC1~C3アルキルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、C3~C6シクロアルキル、C2~C4アルケニル、C3~C4アルキニル、C1~C4アルコキシ、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23、D-24、D-25又はD-26を表し、
さらに、R1がフッ素原子又はC1~C3アルキル表し、R2がフッ素原子又はメチルを表し、R3がメチル又はエチルを表し、且つR4が水素原子又はメチルを表す場合には、R6は水素原子、ハロゲン原子、C1~C4アルキル、C1~C4ハロアルキル、R10によって任意に置換された(C1~C4)アルキル、C3~C6シクロアルキル、C3~C6ハロシクロアルキル、ヒドロキシ(C3~C6)シクロアルキル、C1~C2アルコキシ(C3~C6)シクロアルキル、C4~C6シクロアルケニル、トリ(C1~C4アルキル)シリル、-C(R11)=NOR12又はフェニルを表してもよく、
Zは、ハロゲン原子、シアノ、ニトロ、-SF5、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31~D-35又はD-37を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-CH2OCH2-、-OCH2O-、-CH2CH2S(O)r-、-CH2CH2CH2CH2-、-CH2CH2CH2O-、-CH2OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-SN=N-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=、-CH=CHCH=CH-、-N=CHCH=CH-、-CH=NCH=CH-、-N=NCH=CH-、-N=CHN=CH-、-N=CHCH=N-、-CH=NN=CH-又は-N=NCH=N-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、シアノ、メチル、ジフルオロメチル、トリフルオロメチル又はメトキシによって任意に置換されていてもよく、
Zaは、ハロゲン原子、メチル又はトリフルオロメチルを表し、
R7は、メチル又はエチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14、C1~C4アルキルチオ、-C(O)NH2又は-C(S)NH2を表し、
R9は、C1~C4アルキル又はC3~C4シクロアルキルを表し、
R10は、-OR15又は-S(O)rR16を表し、
R11は、水素原子又はメチルを表し、
R12は、メチル又はエチルを表し、
R13は、C1~C4アルキル、C1~C4ハロアルキル、C3~C4シクロアルキルメチル又はC3~C4シクロアルキルを表し、
R14は、C1~C4アルキル又はC2~C4ハロアルキルを表し、
R15は、水素原子、メチル、エチル又はC1~C2ハロアルキルを表し、
R16は、メチル、エチル又はC1~C2ハロアルキルを表す、
〔1〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [2]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -9, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl, difluoromethylsulfonyl or trifluoromethylsulfonyl,
X 2 represents a hydrogen atom, a fluorine atom or a chlorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom. Represent,
X 3 represents methyl,
Y 1 represents a halogen atom, methyl, trifluoromethyl or methoxy;
Y 2 represents a hydrogen atom or methyl,
Y 3 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, benzyl, (Z) m- substituted phenylmethyl, cyclopropyl, C 1 -C 3 alkoxy, C 1- C 3 haloalkoxy, C 3 -C 4 alkenyloxy, C 3 -C 4 alkynyloxy, cyanomethoxy, benzyloxy, phenylmethoxy substituted by (Z) m , C 1 -C 3 alkylthio or C 1 -C 3 Represents haloalkylthio,
R 2 represents a hydrogen atom, a fluorine atom or C 1 -C 3 alkyl,
Here, by forming the ethylene chain together R 1 and R 2, may represent that together with the carbon atoms to which R 1 and R 2 are attached form a cyclopropyl ring, R 1 and R the 2 may represent the formation of a C 1 ~ C 2 alkylidene or C 1 ~ C 2 Haroarukiriden together,
R 3 represents a hydrogen atom, C 1 -C 3 alkyl or C 1 -C 3 haloalkyl,
R 4 represents a hydrogen atom or C 1 -C 3 alkyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl, C 3 -C 6 cycloalkyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl substituted by R 8 , C 1 -C 4 alkoxy, C 1 -C 4 haloalkylthio, —C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 6 represents phenyl, D-1, D-2, D-6, D-8, D-23, D-24, D-25 or D-26 substituted by (Z) m ;
Furthermore, when R 1 represents a fluorine atom or C 1 -C 3 alkyl, R 2 represents a fluorine atom or methyl, R 3 represents methyl or ethyl, and R 4 represents a hydrogen atom or methyl, R 6 is a hydrogen atom, a halogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, (C 1 -C 4 ) alkyl optionally substituted by R 10 , C 3 -C 6 cycloalkyl, C 3- C 6 halocycloalkyl, hydroxy (C 3 -C 6 ) cycloalkyl, C 1 -C 2 alkoxy (C 3 -C 6 ) cycloalkyl, C 4 -C 6 cycloalkenyl, tri (C 1 -C 4 alkyl) May represent silyl, -C (R 11 ) = NOR 12 or phenyl;
Z is a halogen atom, cyano, nitro, —SF 5 , C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 ~ C 4 alkylsulfinyl, C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31 ~ D-35 or D- 37, when m or n represents 2 or more, each Z may be the same as or different from each other. Further, when two Zs are adjacent to each other, two Zs are adjacent to each other. Two Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —, —OCH 2 O—, —CH 2 CH 2 S (O) r —, —CH 2 CH 2. CH 2 CH 2- , -CH 2 CH 2 CH 2 O-, -CH 2 OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) C H = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -SN = N-, -N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN =, -CH = CHCH = CH-, -N = CHCH = CH-, -CH = NCH = CH-, -N = NCH = CH-, -N = CHN = CH By forming-, -N = CHCH = N-, -CH = NN = CH- or -N = NCH = N-, a 5-membered or 6-membered ring is formed with the carbon atom to which each Z is bonded. In this case, the hydrogen atom bonded to each carbon atom forming the ring may be optionally substituted by a halogen atom, cyano, methyl, difluoromethyl, trifluoromethyl or methoxy,
Z a represents a halogen atom, methyl or trifluoromethyl,
R 7 represents methyl or ethyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 , C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2
R 9 represents C 1 -C 4 alkyl or C 3 -C 4 cycloalkyl,
R 10 represents —OR 15 or —S (O) r R 16 ;
R 11 represents a hydrogen atom or methyl,
R 12 represents methyl or ethyl,
R 13 represents C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 3 -C 4 cycloalkylmethyl or C 3 -C 4 cycloalkyl,
R 14 represents C 1 -C 4 alkyl or C 2 -C 4 haloalkyl,
R 15 represents a hydrogen atom, methyl, ethyl or C 1 -C 2 haloalkyl,
R 16 represents methyl, ethyl or C 1 -C 2 haloalkyl,
The alkynylpyridine-substituted amide compound according to [1], an N-oxide thereof or a salt thereof.
G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-9、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル、ジフルオロメチルスルホニル又はトリフルオロメチルスルホニルを表し、
X2は、水素原子、フッ素原子又は塩素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
X3は、メチルを表し、
Y1は、ハロゲン原子、メチル、トリフルオロメチル又はメトキシを表し、
Y2は、水素原子又はメチルを表し、
Y3は、水素原子を表し、
R1は、水素原子、フッ素原子、C1~C3アルキル、C1~C3ハロアルキル、ベンジル、(Z)mによって置換されたフェニルメチル、シクロプロピル、C1~C3アルコキシ、C1~C3ハロアルコキシ、C3~C4アルケニルオキシ、C3~C4アルキニルオキシ、シアノメトキシ、ベンジルオキシ、(Z)mによって置換されたフェニルメトキシ、C1~C3アルキルチオ又はC1~C3ハロアルキルチオを表し、
R2は、水素原子、フッ素原子又はC1~C3アルキルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、R1とR2とは一緒になってC1~C2アルキリデン又はC1~C2ハロアルキリデンを形成することを表してもよく、
R3は、水素原子、C1~C3アルキル又はC1~C3ハロアルキルを表し、
R4は、水素原子又はC1~C3アルキルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、C3~C6シクロアルキル、C2~C4アルケニル、C3~C4アルキニル、C1~C4アルコキシ、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23、D-24、D-25又はD-26を表し、
さらに、R1がフッ素原子又はC1~C3アルキル表し、R2がフッ素原子又はメチルを表し、R3がメチル又はエチルを表し、且つR4が水素原子又はメチルを表す場合には、R6は水素原子、ハロゲン原子、C1~C4アルキル、C1~C4ハロアルキル、R10によって任意に置換された(C1~C4)アルキル、C3~C6シクロアルキル、C3~C6ハロシクロアルキル、ヒドロキシ(C3~C6)シクロアルキル、C1~C2アルコキシ(C3~C6)シクロアルキル、C4~C6シクロアルケニル、トリ(C1~C4アルキル)シリル、-C(R11)=NOR12又はフェニルを表してもよく、
Zは、ハロゲン原子、シアノ、ニトロ、-SF5、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31~D-35又はD-37を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-CH2OCH2-、-OCH2O-、-CH2CH2S(O)r-、-CH2CH2CH2CH2-、-CH2CH2CH2O-、-CH2OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-SN=N-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=、-CH=CHCH=CH-、-N=CHCH=CH-、-CH=NCH=CH-、-N=NCH=CH-、-N=CHN=CH-、-N=CHCH=N-、-CH=NN=CH-又は-N=NCH=N-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、シアノ、メチル、ジフルオロメチル、トリフルオロメチル又はメトキシによって任意に置換されていてもよく、
Zaは、ハロゲン原子、メチル又はトリフルオロメチルを表し、
R7は、メチル又はエチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14、C1~C4アルキルチオ、-C(O)NH2又は-C(S)NH2を表し、
R9は、C1~C4アルキル又はC3~C4シクロアルキルを表し、
R10は、-OR15又は-S(O)rR16を表し、
R11は、水素原子又はメチルを表し、
R12は、メチル又はエチルを表し、
R13は、C1~C4アルキル、C1~C4ハロアルキル、C3~C4シクロアルキルメチル又はC3~C4シクロアルキルを表し、
R14は、C1~C4アルキル又はC2~C4ハロアルキルを表し、
R15は、水素原子、メチル、エチル又はC1~C2ハロアルキルを表し、
R16は、メチル、エチル又はC1~C2ハロアルキルを表す、
〔1〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [2]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -9, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl, difluoromethylsulfonyl or trifluoromethylsulfonyl,
X 2 represents a hydrogen atom, a fluorine atom or a chlorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom. Represent,
X 3 represents methyl,
Y 1 represents a halogen atom, methyl, trifluoromethyl or methoxy;
Y 2 represents a hydrogen atom or methyl,
Y 3 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, benzyl, (Z) m- substituted phenylmethyl, cyclopropyl, C 1 -C 3 alkoxy, C 1- C 3 haloalkoxy, C 3 -C 4 alkenyloxy, C 3 -C 4 alkynyloxy, cyanomethoxy, benzyloxy, phenylmethoxy substituted by (Z) m , C 1 -C 3 alkylthio or C 1 -C 3 Represents haloalkylthio,
R 2 represents a hydrogen atom, a fluorine atom or C 1 -C 3 alkyl,
Here, by forming the ethylene chain together R 1 and R 2, may represent that together with the carbon atoms to which R 1 and R 2 are attached form a cyclopropyl ring, R 1 and R the 2 may represent the formation of a C 1 ~ C 2 alkylidene or C 1 ~ C 2 Haroarukiriden together,
R 3 represents a hydrogen atom, C 1 -C 3 alkyl or C 1 -C 3 haloalkyl,
R 4 represents a hydrogen atom or C 1 -C 3 alkyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl, C 3 -C 6 cycloalkyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl substituted by R 8 , C 1 -C 4 alkoxy, C 1 -C 4 haloalkylthio, —C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 6 represents phenyl, D-1, D-2, D-6, D-8, D-23, D-24, D-25 or D-26 substituted by (Z) m ;
Furthermore, when R 1 represents a fluorine atom or C 1 -C 3 alkyl, R 2 represents a fluorine atom or methyl, R 3 represents methyl or ethyl, and R 4 represents a hydrogen atom or methyl, R 6 is a hydrogen atom, a halogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, (C 1 -C 4 ) alkyl optionally substituted by R 10 , C 3 -C 6 cycloalkyl, C 3- C 6 halocycloalkyl, hydroxy (C 3 -C 6 ) cycloalkyl, C 1 -C 2 alkoxy (C 3 -C 6 ) cycloalkyl, C 4 -C 6 cycloalkenyl, tri (C 1 -C 4 alkyl) May represent silyl, -C (R 11 ) = NOR 12 or phenyl;
Z is a halogen atom, cyano, nitro, —SF 5 , C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 ~ C 4 alkylsulfinyl, C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31 ~ D-35 or D- 37, when m or n represents 2 or more, each Z may be the same as or different from each other. Further, when two Zs are adjacent to each other, two Zs are adjacent to each other. Two Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —, —OCH 2 O—, —CH 2 CH 2 S (O) r —, —CH 2 CH 2. CH 2 CH 2- , -CH 2 CH 2 CH 2 O-, -CH 2 OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) C H = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -SN = N-, -N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN =, -CH = CHCH = CH-, -N = CHCH = CH-, -CH = NCH = CH-, -N = NCH = CH-, -N = CHN = CH By forming-, -N = CHCH = N-, -CH = NN = CH- or -N = NCH = N-, a 5-membered or 6-membered ring is formed with the carbon atom to which each Z is bonded. In this case, the hydrogen atom bonded to each carbon atom forming the ring may be optionally substituted by a halogen atom, cyano, methyl, difluoromethyl, trifluoromethyl or methoxy,
Z a represents a halogen atom, methyl or trifluoromethyl,
R 7 represents methyl or ethyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 , C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2
R 9 represents C 1 -C 4 alkyl or C 3 -C 4 cycloalkyl,
R 10 represents —OR 15 or —S (O) r R 16 ;
R 11 represents a hydrogen atom or methyl,
R 12 represents methyl or ethyl,
R 13 represents C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 3 -C 4 cycloalkylmethyl or C 3 -C 4 cycloalkyl,
R 14 represents C 1 -C 4 alkyl or C 2 -C 4 haloalkyl,
R 15 represents a hydrogen atom, methyl, ethyl or C 1 -C 2 haloalkyl,
R 16 represents methyl, ethyl or C 1 -C 2 haloalkyl,
The alkynylpyridine-substituted amide compound according to [1], an N-oxide thereof or a salt thereof.
〔3〕
G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表し、
X2は、水素原子又はフッ素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
Y1は、ハロゲン原子又はトリフルオロメチルを表し、
Y2は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とが一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、シクロプロピル、C2~C4アルケニル、C3~C4アルキニル、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23又はD-25を表し、
さらに、R1がフッ素原子を表し、R2がフッ素原子を表し、R3がメチルを表し、且つR4が水素原子又はメチルを表す場合には、R6はC1~C4ハロアルキル又はC3~C6シクロアルキルを表してもよく、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R7は、メチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14又はC1~C4アルキルチオを表し、
R9は、C1~C4アルキルを表し、
R13は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
R14は、C1~C4アルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
〔2〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [3]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
Y 1 represents a halogen atom or trifluoromethyl,
Y 2 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , cyclopropyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl, C 1 -C 4 haloalkylthio, -C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
Further, when R 1 represents a fluorine atom, R 2 represents a fluorine atom, R 3 represents methyl, and R 4 represents a hydrogen atom or methyl, R 6 represents C 1 -C 4 haloalkyl or C May represent 3 to C 6 cycloalkyl,
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -CH 2 CH 2 O-, -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-,- N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH = CHCH = CH- May form a 5-membered ring or a 6-membered ring together with the carbon atom to which each Z is bonded, in which case the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trifluoromethyl Optionally substituted by
R 7 represents methyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 or C 1 -C 4 alkylthio;
R 9 represents C 1 -C 4 alkyl,
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
R 14 represents C 1 -C 4 alkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
The alkynylpyridine-substituted amide compound according to [2], its N-oxide or a salt thereof.
G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表し、
X2は、水素原子又はフッ素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
Y1は、ハロゲン原子又はトリフルオロメチルを表し、
Y2は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とが一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、シクロプロピル、C2~C4アルケニル、C3~C4アルキニル、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23又はD-25を表し、
さらに、R1がフッ素原子を表し、R2がフッ素原子を表し、R3がメチルを表し、且つR4が水素原子又はメチルを表す場合には、R6はC1~C4ハロアルキル又はC3~C6シクロアルキルを表してもよく、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R7は、メチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14又はC1~C4アルキルチオを表し、
R9は、C1~C4アルキルを表し、
R13は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
R14は、C1~C4アルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
〔2〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [3]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
Y 1 represents a halogen atom or trifluoromethyl,
Y 2 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , cyclopropyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl, C 1 -C 4 haloalkylthio, -C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
Further, when R 1 represents a fluorine atom, R 2 represents a fluorine atom, R 3 represents methyl, and R 4 represents a hydrogen atom or methyl, R 6 represents C 1 -C 4 haloalkyl or C May represent 3 to C 6 cycloalkyl,
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -CH 2 CH 2 O-, -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-,- N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH = CHCH = CH- May form a 5-membered ring or a 6-membered ring together with the carbon atom to which each Z is bonded, in which case the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trifluoromethyl Optionally substituted by
R 7 represents methyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 or C 1 -C 4 alkylthio;
R 9 represents C 1 -C 4 alkyl,
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
R 14 represents C 1 -C 4 alkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
The alkynylpyridine-substituted amide compound according to [2], its N-oxide or a salt thereof.
〔4〕
G1は、G1-1、G1-2、G1-3、G1-4、G1-7、G1-8、G1-10で表される構造を表し、
Y1は、ハロゲン原子を表し、
Y2は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル、メトキシ、エトキシ、トリフルオロエトキシ、アリルオキシ、プロパルギルオキシ、ベンジルオキシ、メチルチオを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子又はメチルを表し、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、C2~C4アルケニル、C3~C4アルキニル、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23、D-25又はD-26を表し、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-OCH=N-、-SCH=N-又は-CH=CH-CH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R7は、メチルを表し、
R9は、C1~C4アルキルを表し、
R14は、C1~C4アルキルを表し、
mは、1、2又は3を表し、
nは、0、1又は2を表す、
〔2〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [4]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -7, G 1 -8, represents a structure represented by G 1 -10,
Y 1 represents a halogen atom,
Y 2 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl, methoxy, ethoxy, trifluoroethoxy, allyloxy, propargyloxy, benzyloxy, methylthio,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom or methyl,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , C 2 -C 4 alkenyl, C 3 -C 4 alkynyl, C 1 -C 4 haloalkylthio , -C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23, D-25 or D-26;
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -OCH = N-, -SCH = N- or -CH = CH By forming —CH═CH—, a 5-membered ring or a 6-membered ring may be formed together with the carbon atom to which each Z is bonded. The hydrogen atom bonded to the atom may optionally be substituted by a halogen atom, methyl or trifluoromethyl,
R 7 represents methyl,
R 9 represents C 1 -C 4 alkyl,
R 14 represents C 1 -C 4 alkyl,
m represents 1, 2 or 3;
n represents 0, 1 or 2;
The alkynylpyridine-substituted amide compound according to [2], its N-oxide or a salt thereof.
G1は、G1-1、G1-2、G1-3、G1-4、G1-7、G1-8、G1-10で表される構造を表し、
Y1は、ハロゲン原子を表し、
Y2は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル、メトキシ、エトキシ、トリフルオロエトキシ、アリルオキシ、プロパルギルオキシ、ベンジルオキシ、メチルチオを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子又はメチルを表し、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、C2~C4アルケニル、C3~C4アルキニル、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23、D-25又はD-26を表し、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-OCH=N-、-SCH=N-又は-CH=CH-CH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R7は、メチルを表し、
R9は、C1~C4アルキルを表し、
R14は、C1~C4アルキルを表し、
mは、1、2又は3を表し、
nは、0、1又は2を表す、
〔2〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [4]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -7, G 1 -8, represents a structure represented by G 1 -10,
Y 1 represents a halogen atom,
Y 2 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl, methoxy, ethoxy, trifluoroethoxy, allyloxy, propargyloxy, benzyloxy, methylthio,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom or methyl,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , C 2 -C 4 alkenyl, C 3 -C 4 alkynyl, C 1 -C 4 haloalkylthio , -C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23, D-25 or D-26;
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -OCH = N-, -SCH = N- or -CH = CH By forming —CH═CH—, a 5-membered ring or a 6-membered ring may be formed together with the carbon atom to which each Z is bonded. The hydrogen atom bonded to the atom may optionally be substituted by a halogen atom, methyl or trifluoromethyl,
R 7 represents methyl,
R 9 represents C 1 -C 4 alkyl,
R 14 represents C 1 -C 4 alkyl,
m represents 1, 2 or 3;
n represents 0, 1 or 2;
The alkynylpyridine-substituted amide compound according to [2], its N-oxide or a salt thereof.
〔5〕
G1は、G1-1、G1-2、G1-3、G1-4又はG1-10で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表し、
X2は、水素原子を表し、
Y1は、ハロゲン原子を表し、
R1は、水素原子、フッ素原子又はメチルを表し、
R5は、水素原子を表し、
R6は、(Z)mによって置換されたフェニルを表し、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、トリフルオロメチル、C1~C6アルコキシ、C1~C4ハロアルコキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、トリフルオロメチルチオ、トリフルオロメチルスルフィニル、トリフルオロメチルスルホニル、D-31、D-32又はD-34を表し、mが2又は3を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-OCH=N-、-SCH=N-又は-CH=CH-CH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子又はトリフルオロメチルによって任意に置換されていてもよく、mは1、2又は3を表し、nは0を表す、
〔4〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [5]
G 1 represents a structure represented by G 1 -1, G 1 -2, G 1 -3, G 1 -4 or G 1 -10;
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
X 2 represents a hydrogen atom,
Y 1 represents a halogen atom,
R 1 represents a hydrogen atom, a fluorine atom or methyl;
R 5 represents a hydrogen atom,
R 6 represents phenyl substituted by (Z) m ,
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio, trifluoro M represents methylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34, and when m represents 2 or 3, each Z may be the same as or different from each other. Well, in addition, when two Zs are adjacent, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —OCH 2 O—, —OCH 2 CH 2 O—, —OCH═CH—, — Form SCH = CH-, -OCH = N-, -SCH = N- or -CH = CH-CH = CH- to form a 5-membered or 6-membered ring with the carbon atom to which each Z binds In this case, the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom or a trif May be optionally substituted by Oromechiru, m represents 1, 2 or 3, n represents 0,
[4] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [4].
G1は、G1-1、G1-2、G1-3、G1-4又はG1-10で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表し、
X2は、水素原子を表し、
Y1は、ハロゲン原子を表し、
R1は、水素原子、フッ素原子又はメチルを表し、
R5は、水素原子を表し、
R6は、(Z)mによって置換されたフェニルを表し、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、トリフルオロメチル、C1~C6アルコキシ、C1~C4ハロアルコキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、トリフルオロメチルチオ、トリフルオロメチルスルフィニル、トリフルオロメチルスルホニル、D-31、D-32又はD-34を表し、mが2又は3を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-OCH=N-、-SCH=N-又は-CH=CH-CH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子又はトリフルオロメチルによって任意に置換されていてもよく、mは1、2又は3を表し、nは0を表す、
〔4〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [5]
G 1 represents a structure represented by G 1 -1, G 1 -2, G 1 -3, G 1 -4 or G 1 -10;
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
X 2 represents a hydrogen atom,
Y 1 represents a halogen atom,
R 1 represents a hydrogen atom, a fluorine atom or methyl;
R 5 represents a hydrogen atom,
R 6 represents phenyl substituted by (Z) m ,
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio, trifluoro M represents methylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34, and when m represents 2 or 3, each Z may be the same as or different from each other. Well, in addition, when two Zs are adjacent, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —OCH 2 O—, —OCH 2 CH 2 O—, —OCH═CH—, — Form SCH = CH-, -OCH = N-, -SCH = N- or -CH = CH-CH = CH- to form a 5-membered or 6-membered ring with the carbon atom to which each Z binds In this case, the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom or a trif May be optionally substituted by Oromechiru, m represents 1, 2 or 3, n represents 0,
[4] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [4].
〔6〕
G1は、G1-1、G1-2、G1-3、G1-4又はG1-10で表される構造を表し、
X1は、ハロゲン原子、ジフルオロメチル又はトリフルオロメチルを表し、
X2は、水素原子を表し、
Y1は、ハロゲン原子を表し、
R1は、水素原子、フッ素原子又はメチルを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R4は、水素原子を表し、
R5は、水素原子、エチル、シアノメチル、プロピニル又は-C(O)R9を表し、
R6は、(Z)mによって置換されたフェニルを表し、
Zは、ハロゲン原子、トリフルオロメチル、又はトリフルオロメトキシを表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-OCH2O-を形成することにより、それぞれのZが結合する炭素原子と共に5員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子によって任意に置換されていてもよく、
R9は、メチルを表し、
mは、1、2又は3を表す、
〔3〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [6]
G 1 represents a structure represented by G 1 -1, G 1 -2, G 1 -3, G 1 -4 or G 1 -10;
X 1 represents a halogen atom, difluoromethyl or trifluoromethyl,
X 2 represents a hydrogen atom,
Y 1 represents a halogen atom,
R 1 represents a hydrogen atom, a fluorine atom or methyl;
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 4 represents a hydrogen atom,
R 5 represents a hydrogen atom, ethyl, cyanomethyl, propynyl or —C (O) R 9 ;
R 6 represents phenyl substituted by (Z) m ,
Z represents a halogen atom, trifluoromethyl, or trifluoromethoxy, and when m or n represents 2 or more, each Z may be the same as or different from each other; When two Zs are adjacent to each other, the two adjacent Zs may form a 5-membered ring with the carbon atom to which each Z is bonded by forming —OCH 2 O—. The hydrogen atom bonded to each carbon atom forming may be optionally substituted with a halogen atom,
R 9 represents methyl,
m represents 1, 2 or 3;
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [3].
G1は、G1-1、G1-2、G1-3、G1-4又はG1-10で表される構造を表し、
X1は、ハロゲン原子、ジフルオロメチル又はトリフルオロメチルを表し、
X2は、水素原子を表し、
Y1は、ハロゲン原子を表し、
R1は、水素原子、フッ素原子又はメチルを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R4は、水素原子を表し、
R5は、水素原子、エチル、シアノメチル、プロピニル又は-C(O)R9を表し、
R6は、(Z)mによって置換されたフェニルを表し、
Zは、ハロゲン原子、トリフルオロメチル、又はトリフルオロメトキシを表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-OCH2O-を形成することにより、それぞれのZが結合する炭素原子と共に5員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子によって任意に置換されていてもよく、
R9は、メチルを表し、
mは、1、2又は3を表す、
〔3〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [6]
G 1 represents a structure represented by G 1 -1, G 1 -2, G 1 -3, G 1 -4 or G 1 -10;
X 1 represents a halogen atom, difluoromethyl or trifluoromethyl,
X 2 represents a hydrogen atom,
Y 1 represents a halogen atom,
R 1 represents a hydrogen atom, a fluorine atom or methyl;
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 4 represents a hydrogen atom,
R 5 represents a hydrogen atom, ethyl, cyanomethyl, propynyl or —C (O) R 9 ;
R 6 represents phenyl substituted by (Z) m ,
Z represents a halogen atom, trifluoromethyl, or trifluoromethoxy, and when m or n represents 2 or more, each Z may be the same as or different from each other; When two Zs are adjacent to each other, the two adjacent Zs may form a 5-membered ring with the carbon atom to which each Z is bonded by forming —OCH 2 O—. The hydrogen atom bonded to each carbon atom forming may be optionally substituted with a halogen atom,
R 9 represents methyl,
m represents 1, 2 or 3;
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [3].
〔7〕
G1は、G1-2又はG1-10で表される構造を表し、
R1は、水素原子又はメチルを表し、
R2は、水素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子又はメチルを表し、
R5は、水素原子を表し、
Zは、ハロゲン原子を表し、
mは、1を表す、
〔6〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [7]
G 1 represents a structure represented by G 1 -2 or G 1 -10;
R 1 represents a hydrogen atom or methyl;
R 2 represents a hydrogen atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom or methyl,
R 5 represents a hydrogen atom,
Z represents a halogen atom,
m represents 1;
[6] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [6].
G1は、G1-2又はG1-10で表される構造を表し、
R1は、水素原子又はメチルを表し、
R2は、水素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子又はメチルを表し、
R5は、水素原子を表し、
Zは、ハロゲン原子を表し、
mは、1を表す、
〔6〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [7]
G 1 represents a structure represented by G 1 -2 or G 1 -10;
R 1 represents a hydrogen atom or methyl;
R 2 represents a hydrogen atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom or methyl,
R 5 represents a hydrogen atom,
Z represents a halogen atom,
m represents 1;
[6] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [6].
〔8〕
G1は、G1-1で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、トリフルオロメチル又はメチルスルホニルを表し、
X2は、水素原子を表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [8]
G 1 represents the structure represented by G 1 -1,
X 1 represents a halogen atom, nitro, methyl, trifluoromethyl or methylsulfonyl,
X 2 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-1で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、トリフルオロメチル又はメチルスルホニルを表し、
X2は、水素原子を表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [8]
G 1 represents the structure represented by G 1 -1,
X 1 represents a halogen atom, nitro, methyl, trifluoromethyl or methylsulfonyl,
X 2 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔9〕
G1は、G1-1で表される構造を表し、
X1及びX2は、水素原子を表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [9]
G 1 represents the structure represented by G 1 -1,
X 1 and X 2 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-1で表される構造を表し、
X1及びX2は、水素原子を表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [9]
G 1 represents the structure represented by G 1 -1,
X 1 and X 2 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔10〕
G1は、G1-1で表される構造を表し、
X1は、ハロゲン原子又はトリフルオロメチルを表し、
X2は、フッ素原子又は塩素原子を表す
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [10]
G 1 represents a structure represented by G 1 -1,
X 1 represents a halogen atom or trifluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], wherein X 2 represents a fluorine atom or a chlorine atom, an N-oxide thereof or a salt thereof.
G1は、G1-1で表される構造を表し、
X1は、ハロゲン原子又はトリフルオロメチルを表し、
X2は、フッ素原子又は塩素原子を表す
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [10]
G 1 represents a structure represented by G 1 -1,
X 1 represents a halogen atom or trifluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], wherein X 2 represents a fluorine atom or a chlorine atom, an N-oxide thereof or a salt thereof.
〔11〕
G1は、G1-1で表される構造を表し、
X1は、ハロゲン原子、メチル又はトリフルオロメチルを表し、
X2は、水素原子又はフッ素原子を表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [11]
G 1 represents the structure represented by G 1 -1,
X 1 represents a halogen atom, methyl or trifluoromethyl,
X 2 represents a hydrogen atom or a fluorine atom,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-1で表される構造を表し、
X1は、ハロゲン原子、メチル又はトリフルオロメチルを表し、
X2は、水素原子又はフッ素原子を表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [11]
G 1 represents the structure represented by G 1 -1,
X 1 represents a halogen atom, methyl or trifluoromethyl,
X 2 represents a hydrogen atom or a fluorine atom,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔12〕
G1は、G1-2又はG1-3で表される構造を表し、
X1は、ハロゲン原子、メチル、ジフルオロメチル、トリフルオロメチル、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [12]
G 1 represents a structure represented by G 1 -2 or G 1 -3,
X 1 represents a halogen atom, methyl, difluoromethyl, trifluoromethyl, methylthio, methylsulfonyl or difluoromethylsulfonyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
G1は、G1-2又はG1-3で表される構造を表し、
X1は、ハロゲン原子、メチル、ジフルオロメチル、トリフルオロメチル、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [12]
G 1 represents a structure represented by G 1 -2 or G 1 -3,
X 1 represents a halogen atom, methyl, difluoromethyl, trifluoromethyl, methylthio, methylsulfonyl or difluoromethylsulfonyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
〔13〕
G1は、G1-2で表される構造を表し、
X1は、メトキシ又はトリフルオロメチルスルホニルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [13]
G 1 represents the structure represented by G 1 -2,
X 1 represents methoxy or trifluoromethylsulfonyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
G1は、G1-2で表される構造を表し、
X1は、メトキシ又はトリフルオロメチルスルホニルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [13]
G 1 represents the structure represented by G 1 -2,
X 1 represents methoxy or trifluoromethylsulfonyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
〔14〕
G1は、G1-4で表される構造を表し、
X1は、ハロゲン原子又はトリフルオロメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [14]
G 1 represents the structure represented by G 1 -4,
X 1 represents a halogen atom or trifluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-4で表される構造を表し、
X1は、ハロゲン原子又はトリフルオロメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [14]
G 1 represents the structure represented by G 1 -4,
X 1 represents a halogen atom or trifluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔15〕
G1は、G1-4で表される構造を表し、
X1は、メチル又はジフルオロメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [15]
G 1 represents a structure represented by G 1 -4,
X 1 represents methyl or difluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-4で表される構造を表し、
X1は、メチル又はジフルオロメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [15]
G 1 represents a structure represented by G 1 -4,
X 1 represents methyl or difluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔16〕
G1は、G1-5又はG1-6で表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
X3は、水素原子又はメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [16]
G 1 represents a structure represented by G 1 -5 or G 1 -6,
X 1 represents difluoromethyl or trifluoromethyl,
X 3 represents a hydrogen atom or methyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-5又はG1-6で表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
X3は、水素原子又はメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [16]
G 1 represents a structure represented by G 1 -5 or G 1 -6,
X 1 represents difluoromethyl or trifluoromethyl,
X 3 represents a hydrogen atom or methyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔17〕
G1は、G1-7又はG1-8で表される構造を表し、
X1は、ハロゲン原子、メチル又はトリフルオロメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [17]
G 1 represents a structure represented by G 1 -7 or G 1 -8,
X 1 represents a halogen atom, methyl or trifluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-7又はG1-8で表される構造を表し、
X1は、ハロゲン原子、メチル又はトリフルオロメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [17]
G 1 represents a structure represented by G 1 -7 or G 1 -8,
X 1 represents a halogen atom, methyl or trifluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔18〕
G1は、G1-7又はG1-8で表される構造を表し、
X1は、ジフルオロメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [18]
G 1 represents a structure represented by G 1 -7 or G 1 -8,
X 1 represents difluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-7又はG1-8で表される構造を表し、
X1は、ジフルオロメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [18]
G 1 represents a structure represented by G 1 -7 or G 1 -8,
X 1 represents difluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔19〕
G1は、G1-9で表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
R7は、メチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [19]
G 1 represents the structure represented by G 1 -9,
X 1 represents difluoromethyl or trifluoromethyl,
R 7 represents methyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-9で表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
R7は、メチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [19]
G 1 represents the structure represented by G 1 -9,
X 1 represents difluoromethyl or trifluoromethyl,
R 7 represents methyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔20〕
G1は、G1-10で表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
X2は、水素原子を表し、
R7は、メチルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [20]
G 1 represents a structure represented by G 1 -10;
X 1 represents difluoromethyl or trifluoromethyl,
X 2 represents a hydrogen atom,
R 7 represents methyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
G1は、G1-10で表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
X2は、水素原子を表し、
R7は、メチルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [20]
G 1 represents a structure represented by G 1 -10;
X 1 represents difluoromethyl or trifluoromethyl,
X 2 represents a hydrogen atom,
R 7 represents methyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
〔21〕
G1は、G1-10で表される構造を表し、
X1は、メチル、エチル又はトリフルオロメチルを表し、
X2は、フッ素原子又は塩素原子を表し、
R7はメチルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [21]
G 1 represents a structure represented by G 1 -10,
X 1 represents methyl, ethyl or trifluoromethyl,
X 2 represents a fluorine atom or a chlorine atom,
R 7 represents methyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
G1は、G1-10で表される構造を表し、
X1は、メチル、エチル又はトリフルオロメチルを表し、
X2は、フッ素原子又は塩素原子を表し、
R7はメチルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [21]
G 1 represents a structure represented by G 1 -10,
X 1 represents methyl, ethyl or trifluoromethyl,
X 2 represents a fluorine atom or a chlorine atom,
R 7 represents methyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
〔22〕
G1は、G1-11又G1-12はで表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
X3は、水素原子又はメチルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [22]
G 1 represents a structure represented by G 1 -11 or G 1 -12,
X 1 represents difluoromethyl or trifluoromethyl,
X 3 represents a hydrogen atom or methyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
G1は、G1-11又G1-12はで表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
X3は、水素原子又はメチルを表す、
〔1〕~〔7〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [22]
G 1 represents a structure represented by G 1 -11 or G 1 -12,
X 1 represents difluoromethyl or trifluoromethyl,
X 3 represents a hydrogen atom or methyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [7].
〔23〕
G1は、G1-13はで表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
X3は、メチルを表す、
〔1〕、〔4〕又は〔5〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [23]
G 1 represents a structure represented by G 1 -13,
X 1 represents difluoromethyl or trifluoromethyl,
X 3 represents methyl,
[1] An alkynylpyridine-substituted amide compound, an N-oxide thereof or a salt thereof according to any one of [4] and [5].
G1は、G1-13はで表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
X3は、メチルを表す、
〔1〕、〔4〕又は〔5〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [23]
G 1 represents a structure represented by G 1 -13,
X 1 represents difluoromethyl or trifluoromethyl,
X 3 represents methyl,
[1] An alkynylpyridine-substituted amide compound, an N-oxide thereof or a salt thereof according to any one of [4] and [5].
〔24〕
G1は、G1-14で表される構造を表し、
X1はジフルオロメチル又はトリフルオロメチルを表し、
X3はメチルを表す
R7はメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [24]
G 1 represents the structure represented by G 1 -14,
X 1 represents difluoromethyl or trifluoromethyl,
X 3 represents methyl R 7 represents methyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-14で表される構造を表し、
X1はジフルオロメチル又はトリフルオロメチルを表し、
X3はメチルを表す
R7はメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [24]
G 1 represents the structure represented by G 1 -14,
X 1 represents difluoromethyl or trifluoromethyl,
X 3 represents methyl R 7 represents methyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔25〕
G1は、G1-15で表される構造を表し、
X1はジフルオロメチル又はトリフルオロメチルを表し、
R7はメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [25]
G 1 represents the structure represented by G 1 -15,
X 1 represents difluoromethyl or trifluoromethyl,
R 7 represents methyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-15で表される構造を表し、
X1はジフルオロメチル又はトリフルオロメチルを表し、
R7はメチルを表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [25]
G 1 represents the structure represented by G 1 -15,
X 1 represents difluoromethyl or trifluoromethyl,
R 7 represents methyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔26〕
G1は、G1-16で表される構造を表し、
X1はメチル又はトリフルオロメチルを表し、
rは0、1又は2を表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [26]
G 1 represents a structure represented by G 1 -16,
X 1 represents methyl or trifluoromethyl,
r represents 0, 1 or 2;
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
G1は、G1-16で表される構造を表し、
X1はメチル又はトリフルオロメチルを表し、
rは0、1又は2を表す、
〔1〕~〔6〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [26]
G 1 represents a structure represented by G 1 -16,
X 1 represents methyl or trifluoromethyl,
r represents 0, 1 or 2;
The alkynylpyridine-substituted amide compound according to any one of [1] to [6], its N-oxide or a salt thereof.
〔27〕
Y1は、ハロゲン原子を表し、
Y2及びY3が水素原子である、
〔1〕~〔26〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [27]
Y 1 represents a halogen atom,
Y 2 and Y 3 are hydrogen atoms,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [26].
Y1は、ハロゲン原子を表し、
Y2及びY3が水素原子である、
〔1〕~〔26〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [27]
Y 1 represents a halogen atom,
Y 2 and Y 3 are hydrogen atoms,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [26].
〔28〕
Y1は、メチル、トリフルオロメチル又はメトキシを表し、
Y2及びY3が同時に水素原子である、
〔1〕~〔26〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [28]
Y 1 represents methyl, trifluoromethyl or methoxy,
Y 2 and Y 3 are simultaneously hydrogen atoms,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [26].
Y1は、メチル、トリフルオロメチル又はメトキシを表し、
Y2及びY3が同時に水素原子である、
〔1〕~〔26〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [28]
Y 1 represents methyl, trifluoromethyl or methoxy,
Y 2 and Y 3 are simultaneously hydrogen atoms,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [26].
〔29〕
Y1は、ハロゲン原子又はメチルを表し、
Y2は、メチルを表し、
Y3は、水素原子を表す、
〔1〕~〔26〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [29]
Y 1 represents a halogen atom or methyl;
Y 2 represents methyl,
Y 3 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [26].
Y1は、ハロゲン原子又はメチルを表し、
Y2は、メチルを表し、
Y3は、水素原子を表す、
〔1〕~〔26〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [29]
Y 1 represents a halogen atom or methyl;
Y 2 represents methyl,
Y 3 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [26].
〔30〕
R1が、C1~C3アルキル、C1~C3ハロアルキル、ベンジル及び(Z)mによって置換されたフェニルメチルを表し、
R2、R3及びR4が水素原子を表し、
Zは、ハロゲン原子、シアノ、ニトロ、メチル、トリフルオロメチル、メトキシ、ジフルオロメトキシ、トリフルオロメトキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、ジフルオロメチルチオ、ジフルオロメチルスルホニル、トリフルオロメチルチオ又はトリフルオロメチルスルホニルを表し、mは1又は2を表し、mが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよい、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [30]
R 1 represents C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, benzyl and phenylmethyl substituted by (Z) m ,
R 2 , R 3 and R 4 represent a hydrogen atom,
Z represents a halogen atom, cyano, nitro, methyl, trifluoromethyl, methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio or trifluoromethylsulfonyl. , M represents 1 or 2, and when m represents 2, each Z may be the same as or different from each other.
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1が、C1~C3アルキル、C1~C3ハロアルキル、ベンジル及び(Z)mによって置換されたフェニルメチルを表し、
R2、R3及びR4が水素原子を表し、
Zは、ハロゲン原子、シアノ、ニトロ、メチル、トリフルオロメチル、メトキシ、ジフルオロメトキシ、トリフルオロメトキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、ジフルオロメチルチオ、ジフルオロメチルスルホニル、トリフルオロメチルチオ又はトリフルオロメチルスルホニルを表し、mは1又は2を表し、mが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよい、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [30]
R 1 represents C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, benzyl and phenylmethyl substituted by (Z) m ,
R 2 , R 3 and R 4 represent a hydrogen atom,
Z represents a halogen atom, cyano, nitro, methyl, trifluoromethyl, methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio or trifluoromethylsulfonyl. , M represents 1 or 2, and when m represents 2, each Z may be the same as or different from each other.
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔31〕
R1が、C1~C3アルキルを表し、
R2が、水素原子を表し、
R3が、メチルを表し、
R4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [31]
R 1 represents C 1 -C 3 alkyl;
R 2 represents a hydrogen atom,
R 3 represents methyl,
R 4 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1が、C1~C3アルキルを表し、
R2が、水素原子を表し、
R3が、メチルを表し、
R4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [31]
R 1 represents C 1 -C 3 alkyl;
R 2 represents a hydrogen atom,
R 3 represents methyl,
R 4 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔32〕
R1が、C1~C3アルキルを表し、
R2が、フッ素原子及びメチルを表し、
R3及びR4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [32]
R 1 represents C 1 -C 3 alkyl;
R 2 represents a fluorine atom and methyl;
R 3 and R 4 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1が、C1~C3アルキルを表し、
R2が、フッ素原子及びメチルを表し、
R3及びR4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [32]
R 1 represents C 1 -C 3 alkyl;
R 2 represents a fluorine atom and methyl;
R 3 and R 4 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔33〕
R1が、C1~C3アルキルを表し、
R2が、フッ素原子及びメチルを表し、
R3が、メチルを表し、
R4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [33]
R 1 represents C 1 -C 3 alkyl;
R 2 represents a fluorine atom and methyl;
R 3 represents methyl,
R 4 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1が、C1~C3アルキルを表し、
R2が、フッ素原子及びメチルを表し、
R3が、メチルを表し、
R4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [33]
R 1 represents C 1 -C 3 alkyl;
R 2 represents a fluorine atom and methyl;
R 3 represents methyl,
R 4 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔34〕
R1は、(Z)mによって置換されたフェニルメチル又はシクロプロピルを表し、
R2、R3及びR4は水素原子を表し、
R4は、水素原子を表す、
Zはハロゲン原子を表し、mは1又は2を表し、mが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよい、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [34]
R 1 represents phenylmethyl or cyclopropyl substituted by (Z) m ;
R 2 , R 3 and R 4 represent a hydrogen atom,
R 4 represents a hydrogen atom,
Z represents a halogen atom, m represents 1 or 2, and when m represents 2, each Z may be the same as or different from each other.
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1は、(Z)mによって置換されたフェニルメチル又はシクロプロピルを表し、
R2、R3及びR4は水素原子を表し、
R4は、水素原子を表す、
Zはハロゲン原子を表し、mは1又は2を表し、mが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよい、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [34]
R 1 represents phenylmethyl or cyclopropyl substituted by (Z) m ;
R 2 , R 3 and R 4 represent a hydrogen atom,
R 4 represents a hydrogen atom,
Z represents a halogen atom, m represents 1 or 2, and when m represents 2, each Z may be the same as or different from each other.
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔35〕
R1が、C1~C3アルコキシ、C1~C3ハロアルコキシ、C3~C4アルケニルオキシ、C3~C4アルキニルオキシ、シアノメトキシ、ベンジルオキシ、(Z)mによって置換されたフェニルメトキシ、C1~C3アルキルチオ又はC1~C3ハロアルキルチオを表し、
R2が、水素原子を表し、
R3が、水素原子及びメチルを表し、
R4が、水素原子を表し、
Zは、ハロゲン原子、シアノ、ニトロ、メチル、トリフルオロメチル、メトキシ、ジフルオロメトキシ、トリフルオロメトキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、ジフルオロメチルチオ、ジフルオロメチルスルホニル、トリフルオロメチルチオ又はトリフルオロメチルスルホニルを表し、mは1又は2を表し、mが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよい、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [35]
R 1 is phenyl substituted by C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 3 -C 4 alkenyloxy, C 3 -C 4 alkynyloxy, cyanomethoxy, benzyloxy, (Z) m Represents methoxy, C 1 -C 3 alkylthio or C 1 -C 3 haloalkylthio,
R 2 represents a hydrogen atom,
R 3 represents a hydrogen atom and methyl,
R 4 represents a hydrogen atom,
Z represents a halogen atom, cyano, nitro, methyl, trifluoromethyl, methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio or trifluoromethylsulfonyl. , M represents 1 or 2, and when m represents 2, each Z may be the same as or different from each other.
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1が、C1~C3アルコキシ、C1~C3ハロアルコキシ、C3~C4アルケニルオキシ、C3~C4アルキニルオキシ、シアノメトキシ、ベンジルオキシ、(Z)mによって置換されたフェニルメトキシ、C1~C3アルキルチオ又はC1~C3ハロアルキルチオを表し、
R2が、水素原子を表し、
R3が、水素原子及びメチルを表し、
R4が、水素原子を表し、
Zは、ハロゲン原子、シアノ、ニトロ、メチル、トリフルオロメチル、メトキシ、ジフルオロメトキシ、トリフルオロメトキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、ジフルオロメチルチオ、ジフルオロメチルスルホニル、トリフルオロメチルチオ又はトリフルオロメチルスルホニルを表し、mは1又は2を表し、mが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよい、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [35]
R 1 is phenyl substituted by C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 3 -C 4 alkenyloxy, C 3 -C 4 alkynyloxy, cyanomethoxy, benzyloxy, (Z) m Represents methoxy, C 1 -C 3 alkylthio or C 1 -C 3 haloalkylthio,
R 2 represents a hydrogen atom,
R 3 represents a hydrogen atom and methyl,
R 4 represents a hydrogen atom,
Z represents a halogen atom, cyano, nitro, methyl, trifluoromethyl, methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio or trifluoromethylsulfonyl. , M represents 1 or 2, and when m represents 2, each Z may be the same as or different from each other.
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔36〕
R1は、フッ素原子、メチル、メトキシ、エトキシ、トリフルオロエトキシ、アリルオキシ、プロパルギルオキシ、ベンジルオキシ又はメチルチオを表し、
R2が、水素原子を表し、
R3が、水素原子及びメチルを表し、
R4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [36]
R 1 represents a fluorine atom, methyl, methoxy, ethoxy, trifluoroethoxy, allyloxy, propargyloxy, benzyloxy or methylthio;
R 2 represents a hydrogen atom,
R 3 represents a hydrogen atom and methyl,
R 4 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1は、フッ素原子、メチル、メトキシ、エトキシ、トリフルオロエトキシ、アリルオキシ、プロパルギルオキシ、ベンジルオキシ又はメチルチオを表し、
R2が、水素原子を表し、
R3が、水素原子及びメチルを表し、
R4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [36]
R 1 represents a fluorine atom, methyl, methoxy, ethoxy, trifluoroethoxy, allyloxy, propargyloxy, benzyloxy or methylthio;
R 2 represents a hydrogen atom,
R 3 represents a hydrogen atom and methyl,
R 4 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔37〕
R1及びR2が各々独立してフッ素原子又はメチルを表し、
R3及びR4が水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [37]
R 1 and R 2 each independently represents a fluorine atom or methyl,
R 3 and R 4 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1及びR2が各々独立してフッ素原子又はメチルを表し、
R3及びR4が水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [37]
R 1 and R 2 each independently represents a fluorine atom or methyl,
R 3 and R 4 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔38〕
R1が、フッ素原子又はメチルを表し、
R2が、フッ素原子を表し、
R3が、メチル又はエチルを表し、
R4が、水素原子又はメチルを表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [38]
R 1 represents a fluorine atom or methyl;
R 2 represents a fluorine atom,
R 3 represents methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1が、フッ素原子又はメチルを表し、
R2が、フッ素原子を表し、
R3が、メチル又はエチルを表し、
R4が、水素原子又はメチルを表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [38]
R 1 represents a fluorine atom or methyl;
R 2 represents a fluorine atom,
R 3 represents methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔39〕
R1及びR2が水素原子を表し、
R3は、水素原子又はメチルを表し、
R4は、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [39]
R 1 and R 2 represent a hydrogen atom,
R 3 represents a hydrogen atom or methyl,
R 4 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1及びR2が水素原子を表し、
R3は、水素原子又はメチルを表し、
R4は、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [39]
R 1 and R 2 represent a hydrogen atom,
R 3 represents a hydrogen atom or methyl,
R 4 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔40〕
R1及びR2が、水素原子を表し、
R3が、C1~C3アルキル又はC1~C3ハロアルキルを表し、
R4が、水素原子又はC1~C3アルキルを表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [40]
R 1 and R 2 represent a hydrogen atom,
R 3 represents C 1 -C 3 alkyl or C 1 -C 3 haloalkyl,
R 4 represents a hydrogen atom or C 1 -C 3 alkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1及びR2が、水素原子を表し、
R3が、C1~C3アルキル又はC1~C3ハロアルキルを表し、
R4が、水素原子又はC1~C3アルキルを表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [40]
R 1 and R 2 represent a hydrogen atom,
R 3 represents C 1 -C 3 alkyl or C 1 -C 3 haloalkyl,
R 4 represents a hydrogen atom or C 1 -C 3 alkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔41〕
R1及びR2が、水素原子を表し、
R3及びR4がメチルを表し、又はR3及びR4が一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成する、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [41]
R 1 and R 2 represent a hydrogen atom,
R 3 and R 4 represent methyl, or R 3 and R 4 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are attached,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1及びR2が、水素原子を表し、
R3及びR4がメチルを表し、又はR3及びR4が一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成する、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [41]
R 1 and R 2 represent a hydrogen atom,
R 3 and R 4 represent methyl, or R 3 and R 4 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are attached,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔42〕
R1とR2とが一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成し、
R3及びR4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [42]
R 1 and R 2 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are attached;
R 3 and R 4 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1とR2とが一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成し、
R3及びR4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [42]
R 1 and R 2 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are attached;
R 3 and R 4 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔43〕
R1とR2とが一緒になってC1~C2アルキリデン又はC1~C2ハロアルキリデンを形成し、
R3及びR4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [43]
R 1 and R 2 together form a C 1 -C 2 alkylidene or C 1 -C 2 haloalkylidene;
R 3 and R 4 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1とR2とが一緒になってC1~C2アルキリデン又はC1~C2ハロアルキリデンを形成し、
R3及びR4が、水素原子を表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [43]
R 1 and R 2 together form a C 1 -C 2 alkylidene or C 1 -C 2 haloalkylidene;
R 3 and R 4 represent a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔44〕
R5が、水素原子を表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [44]
R 5 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
R5が、水素原子を表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [44]
R 5 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
〔45〕
R5が、C1~C4アルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [45]
R 5 represents C 1 -C 4 alkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
R5が、C1~C4アルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [45]
R 5 represents C 1 -C 4 alkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
〔46〕
R5が、R8によって置換された(C1~C2)アルキルを表し、
R8が、シアノ、C3~C6シクロアルキル又は-OR14を表し、
R14が、C1~C4アルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [46]
R 5 represents (C 1 -C 2 ) alkyl substituted by R 8 ,
R 8 represents cyano, C 3 -C 6 cycloalkyl or —OR 14 ;
R 14 represents C 1 -C 4 alkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
R5が、R8によって置換された(C1~C2)アルキルを表し、
R8が、シアノ、C3~C6シクロアルキル又は-OR14を表し、
R14が、C1~C4アルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [46]
R 5 represents (C 1 -C 2 ) alkyl substituted by R 8 ,
R 8 represents cyano, C 3 -C 6 cycloalkyl or —OR 14 ;
R 14 represents C 1 -C 4 alkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
〔47〕
R5が、R8によって置換された(C1~C2)アルキルを表し、
R8が、-OR14、C1~C4アルキルチオ、-C(O)NH2又は-C(S)NH2を表し、
R14が、C2~C4ハロアルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [47]
R 5 represents (C 1 -C 2 ) alkyl substituted by R 8 ,
R 8 represents —OR 14 , C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2 ;
R 14 represents C 2 -C 4 haloalkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
R5が、R8によって置換された(C1~C2)アルキルを表し、
R8が、-OR14、C1~C4アルキルチオ、-C(O)NH2又は-C(S)NH2を表し、
R14が、C2~C4ハロアルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [47]
R 5 represents (C 1 -C 2 ) alkyl substituted by R 8 ,
R 8 represents —OR 14 , C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2 ;
R 14 represents C 2 -C 4 haloalkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
〔48〕
R5が、シクロプロピルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [48]
R 5 represents cyclopropyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
R5が、シクロプロピルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [48]
R 5 represents cyclopropyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
〔49〕
R5が、C2~C4アルケニル又はC3~C4アルキニルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [49]
R 5 represents C 2 -C 4 alkenyl or C 3 -C 4 alkynyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
R5が、C2~C4アルケニル又はC3~C4アルキニルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [49]
R 5 represents C 2 -C 4 alkenyl or C 3 -C 4 alkynyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
〔50〕
R5が、C1~C4ハロアルキルチオを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [50]
R 5 represents C 1 -C 4 haloalkylthio,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
R5が、C1~C4ハロアルキルチオを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [50]
R 5 represents C 1 -C 4 haloalkylthio,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
〔51〕
R5が、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R9が、C1~C4アルキルを表し、
R14が、C1~C4アルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [51]
R 5 represents —C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 9 represents C 1 -C 4 alkyl,
R 14 represents C 1 -C 4 alkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
R5が、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R9が、C1~C4アルキルを表し、
R14が、C1~C4アルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [51]
R 5 represents —C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 9 represents C 1 -C 4 alkyl,
R 14 represents C 1 -C 4 alkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
〔52〕
R5が、-C(O)R9を表し、
R9が、C3~C4シクロアルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔53〕
R5が、C3~C6シクロアルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [52]
R 5 represents -C (O) R 9 ;
R 9 represents C 3 -C 4 cycloalkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
[53]
R 5 represents C 3 -C 6 cycloalkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
R5が、-C(O)R9を表し、
R9が、C3~C4シクロアルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔53〕
R5が、C3~C6シクロアルキルを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [52]
R 5 represents -C (O) R 9 ;
R 9 represents C 3 -C 4 cycloalkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
[53]
R 5 represents C 3 -C 6 cycloalkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
〔54〕
R5が、C1~C4アルコキシを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔55〕
R6が、C1~C4アルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [54]
R 5 represents C 1 -C 4 alkoxy,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
[55]
R 6 represents C 1 -C 4 alkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R5が、C1~C4アルコキシを表す、
〔1〕~〔43〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔55〕
R6が、C1~C4アルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [54]
R 5 represents C 1 -C 4 alkoxy,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [43].
[55]
R 6 represents C 1 -C 4 alkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔56〕
R6が、C3~C6シクロアルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔57〕
R6が、R10によって任意に置換された(C1~C4)アルキルを表し、
R10は、-OR15を表し、
R15は水素原子、メチル、エチル又はC1~C2ハロアルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [56]
R 6 represents C 3 -C 6 cycloalkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
[57]
R 6 represents (C 1 -C 4 ) alkyl optionally substituted by R 10 ;
R 10 represents -OR 15 ;
R 15 represents a hydrogen atom, methyl, ethyl or C 1 -C 2 haloalkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R6が、C3~C6シクロアルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔57〕
R6が、R10によって任意に置換された(C1~C4)アルキルを表し、
R10は、-OR15を表し、
R15は水素原子、メチル、エチル又はC1~C2ハロアルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [56]
R 6 represents C 3 -C 6 cycloalkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
[57]
R 6 represents (C 1 -C 4 ) alkyl optionally substituted by R 10 ;
R 10 represents -OR 15 ;
R 15 represents a hydrogen atom, methyl, ethyl or C 1 -C 2 haloalkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔58〕
R6が、トリ(C1~C4アルキル)シリルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔59〕
R6が、-CH=NOR12を表し、
R12は、メチル又はエチルを表し、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [58]
R 6 represents tri (C 1 -C 4 alkyl) silyl;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
[59]
R 6 represents -CH = NOR 12 ;
R 12 represents methyl or ethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R6が、トリ(C1~C4アルキル)シリルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔59〕
R6が、-CH=NOR12を表し、
R12は、メチル又はエチルを表し、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [58]
R 6 represents tri (C 1 -C 4 alkyl) silyl;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
[59]
R 6 represents -CH = NOR 12 ;
R 12 represents methyl or ethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔60〕
R6が、フェニルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [60]
R 6 represents phenyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R6が、フェニルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [60]
R 6 represents phenyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔61〕
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23又はD-25を表し、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R13は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
〔1〕~〔8〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [61]
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -CH 2 CH 2 O-, -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-,- N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH = CHCH = CH- May form a 5-membered ring or a 6-membered ring together with the carbon atom to which each Z is bonded, in which case the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trifluoromethyl Optionally substituted by
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
The alkynylpyridine-substituted amide compound according to any one of [1] to [8], an N-oxide thereof, or a salt thereof.
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23又はD-25を表し、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R13は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
〔1〕~〔8〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [61]
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -CH 2 CH 2 O-, -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-,- N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH = CHCH = CH- May form a 5-membered ring or a 6-membered ring together with the carbon atom to which each Z is bonded, in which case the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trifluoromethyl Optionally substituted by
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
The alkynylpyridine-substituted amide compound according to any one of [1] to [8], an N-oxide thereof, or a salt thereof.
〔62〕
R6が、(Z)mによって置換されたフェニルを表し、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、トリフルオロメチル、C1~C6アルコキシ、C1~C4ハロアルコキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、トリフルオロメチルチオ、トリフルオロメチルスルフィニル、トリフルオロメチルスルホニル、D-31、D-32又はD-34を表し、mが2又は3を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R13はC1~C4アルキル又はC1~C4ハロアルキルを表し、
mは1、2又は3を表し、
nは0を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [62]
R 6 is, represents phenyl substituted by (Z) m,
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio, trifluoro M represents methylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34, and when m represents 2 or 3, each Z may be the same as or different from each other. Well, in addition, when two Zs are adjacent, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —OCH 2 O—, —OCH 2 CH 2 O—. , -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH By forming = CHCH = CH-, a 5-membered ring with the carbon atom to which each Z is attached May form a 6-membered ring, this time, the hydrogen atoms bonded to each carbon atom forming the ring may be optionally substituted by a halogen atom, methyl or trifluoromethyl,
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
m represents 1, 2 or 3;
n represents 0,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R6が、(Z)mによって置換されたフェニルを表し、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、トリフルオロメチル、C1~C6アルコキシ、C1~C4ハロアルコキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、トリフルオロメチルチオ、トリフルオロメチルスルフィニル、トリフルオロメチルスルホニル、D-31、D-32又はD-34を表し、mが2又は3を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R13はC1~C4アルキル又はC1~C4ハロアルキルを表し、
mは1、2又は3を表し、
nは0を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [62]
R 6 is, represents phenyl substituted by (Z) m,
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio, trifluoro M represents methylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34, and when m represents 2 or 3, each Z may be the same as or different from each other. Well, in addition, when two Zs are adjacent, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —OCH 2 O—, —OCH 2 CH 2 O—. , -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH By forming = CHCH = CH-, a 5-membered ring with the carbon atom to which each Z is attached May form a 6-membered ring, this time, the hydrogen atoms bonded to each carbon atom forming the ring may be optionally substituted by a halogen atom, methyl or trifluoromethyl,
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
m represents 1, 2 or 3;
n represents 0,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔63〕
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、トリフルオロメチル、C1~C6アルコキシ、C1~C4ハロアルコキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、トリフルオロメチルチオ、トリフルオロメチルスルフィニル、トリフルオロメチルスルホニル、D-31、D-32又はD-34を表す、
〔62〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [63]
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio, trifluoro Represents methylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34,
[62] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [62].
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、トリフルオロメチル、C1~C6アルコキシ、C1~C4ハロアルコキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、トリフルオロメチルチオ、トリフルオロメチルスルフィニル、トリフルオロメチルスルホニル、D-31、D-32又はD-34を表す、
〔62〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [63]
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio, trifluoro Represents methylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34,
[62] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [62].
〔64〕
G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表し、
X2は、水素原子又はフッ素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
Y1は、ハロゲン原子又はトリフルオロメチルを表し、
Y2は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、シクロプロピル、C2~C4アルケニル、C3~C4アルキニル、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R7は、メチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14又はC1~C4アルキルチオを表し、
R9は、C1~C4アルキルを表し、
R14は、C1~C4アルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
〔63〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [64]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
Y 1 represents a halogen atom or trifluoromethyl,
Y 2 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , cyclopropyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl, C 1 -C 4 haloalkylthio, -C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 7 represents methyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 or C 1 -C 4 alkylthio;
R 9 represents C 1 -C 4 alkyl,
R 14 represents C 1 -C 4 alkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
[63] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [63].
G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表し、
X2は、水素原子又はフッ素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
Y1は、ハロゲン原子又はトリフルオロメチルを表し、
Y2は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、シクロプロピル、C2~C4アルケニル、C3~C4アルキニル、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R7は、メチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14又はC1~C4アルキルチオを表し、
R9は、C1~C4アルキルを表し、
R14は、C1~C4アルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
〔63〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [64]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
Y 1 represents a halogen atom or trifluoromethyl,
Y 2 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , cyclopropyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl, C 1 -C 4 haloalkylthio, -C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 7 represents methyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 or C 1 -C 4 alkylthio;
R 9 represents C 1 -C 4 alkyl,
R 14 represents C 1 -C 4 alkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
[63] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [63].
〔65〕
2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成し、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよい、
〔62〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [65]
When two Z's are adjacent, the two adjacent Z's are -CH 2 CH 2 CH 2- , -CH 2 CH 2 O-, -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-,- SN = CH-, -N (R 13 ) N = CH-, -N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH = CHCH = CH To form a 5-membered or 6-membered ring together with the carbon atom to which each Z is bonded. At this time, the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or tri- Optionally substituted by fluoromethyl,
[62] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [62].
2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成し、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよい、
〔62〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [65]
When two Z's are adjacent, the two adjacent Z's are -CH 2 CH 2 CH 2- , -CH 2 CH 2 O-, -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-,- SN = CH-, -N (R 13 ) N = CH-, -N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH = CHCH = CH To form a 5-membered or 6-membered ring together with the carbon atom to which each Z is bonded. At this time, the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or tri- Optionally substituted by fluoromethyl,
[62] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [62].
〔66〕
G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表し、
X2は、水素原子又はフッ素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
Y1は、ハロゲン原子又はトリフルオロメチルを表し、
Y2は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とが一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とが一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、シクロプロピル、C2~C4アルケニル、C3~C4アルキニル、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R7は、メチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14又はC1~C4アルキルチオを表し、
R9は、C1~C4アルキルを表し、
R14は、C1~C4アルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
〔64〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [66]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
Y 1 represents a halogen atom or trifluoromethyl,
Y 2 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring together with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , cyclopropyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl, C 1 -C 4 haloalkylthio, -C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 7 represents methyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 or C 1 -C 4 alkylthio;
R 9 represents C 1 -C 4 alkyl,
R 14 represents C 1 -C 4 alkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
[64] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [64].
G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表し、
X2は、水素原子又はフッ素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
Y1は、ハロゲン原子又はトリフルオロメチルを表し、
Y2は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とが一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とが一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、シクロプロピル、C2~C4アルケニル、C3~C4アルキニル、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R7は、メチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14又はC1~C4アルキルチオを表し、
R9は、C1~C4アルキルを表し、
R14は、C1~C4アルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
〔64〕に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [66]
G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
Y 1 represents a halogen atom or trifluoromethyl,
Y 2 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring together with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , cyclopropyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl, C 1 -C 4 haloalkylthio, -C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 7 represents methyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 or C 1 -C 4 alkylthio;
R 9 represents C 1 -C 4 alkyl,
R 14 represents C 1 -C 4 alkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
[64] The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to [64].
〔67〕
Zはハロゲン原子、メチル、C1~C4ハロアルキル、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31~D-35又はD-37を表し、mが2、3、4又は5を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-CH2OCH2-、-OCH2O-、-CH2CH2S(O)r-、-CH2CH2CH2CH2-、-CH2CH2CH2O-、-CH2OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-SN=N-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=、-CH=CHCH=CH-、-N=CHCH=CH-、-CH=NCH=CH-、-N=NCH=CH-、-N=CHN=CH-、-N=CHCH=N-、-CH=NN=CH-又は-N=NCH=N-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、シアノ、メチル、ジフルオロメチル、トリフルオロメチル又はメトキシによって任意に置換されていてもよく、
Zaはハロゲン原子、メチル又はトリフルオロメチルを表し、
R13はC1~C4アルキル、C1~C4ハロアルキル、C3~C4シクロアルキルメチル又はC3~C4シクロアルキルを表し、
mは1、2、3、4又は5を表し、
nは0又は1を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [67]
Z is a halogen atom, methyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, C 1 -C 4 alkylsulfonyl, C 1 -C 4 haloalkylthio, C 1 -C 4 Haloalkylsulfinyl, C 1 -C 4 haloalkylsulfonyl, D-31 to D-35 or D-37, and when m represents 2, 3, 4 or 5, each Z may be the same as each other Or when two Zs are adjacent to each other, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —. , —OCH 2 O—, —CH 2 CH 2 S (O) r —, —CH 2 CH 2 CH 2 CH 2 —, —CH 2 CH 2 CH 2 O—, —CH 2 OCH 2 O—, —OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N -, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -SN = N-, -N (R 13 ) N = N-, = NN (R 13 ) CH = , = NON =, = NSN =, -CH = CHCH = CH-, -N = CHCH = CH-, -CH = NCH = CH-, -N = NCH = CH-, -N = CHN = CH-, -N = CHCH = N-, -CH = NN = By forming CH- or -N = NCH = N-, a 5-membered ring or a 6-membered ring may be formed together with the carbon atom to which each Z is bonded. At this time, each carbon atom forming the ring The hydrogen atom bonded to may be optionally substituted by a halogen atom, cyano, methyl, difluoromethyl, trifluoromethyl or methoxy,
Z a represents a halogen atom, methyl or trifluoromethyl,
R 13 represents C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 3 -C 4 cycloalkylmethyl or C 3 -C 4 cycloalkyl,
m represents 1, 2, 3, 4 or 5;
n represents 0 or 1;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
Zはハロゲン原子、メチル、C1~C4ハロアルキル、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31~D-35又はD-37を表し、mが2、3、4又は5を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-CH2OCH2-、-OCH2O-、-CH2CH2S(O)r-、-CH2CH2CH2CH2-、-CH2CH2CH2O-、-CH2OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-SN=N-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=、-CH=CHCH=CH-、-N=CHCH=CH-、-CH=NCH=CH-、-N=NCH=CH-、-N=CHN=CH-、-N=CHCH=N-、-CH=NN=CH-又は-N=NCH=N-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、シアノ、メチル、ジフルオロメチル、トリフルオロメチル又はメトキシによって任意に置換されていてもよく、
Zaはハロゲン原子、メチル又はトリフルオロメチルを表し、
R13はC1~C4アルキル、C1~C4ハロアルキル、C3~C4シクロアルキルメチル又はC3~C4シクロアルキルを表し、
mは1、2、3、4又は5を表し、
nは0又は1を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [67]
Z is a halogen atom, methyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, C 1 -C 4 alkylsulfonyl, C 1 -C 4 haloalkylthio, C 1 -C 4 Haloalkylsulfinyl, C 1 -C 4 haloalkylsulfonyl, D-31 to D-35 or D-37, and when m represents 2, 3, 4 or 5, each Z may be the same as each other Or when two Zs are adjacent to each other, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —. , —OCH 2 O—, —CH 2 CH 2 S (O) r —, —CH 2 CH 2 CH 2 CH 2 —, —CH 2 CH 2 CH 2 O—, —CH 2 OCH 2 O—, —OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N -, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -SN = N-, -N (R 13 ) N = N-, = NN (R 13 ) CH = , = NON =, = NSN =, -CH = CHCH = CH-, -N = CHCH = CH-, -CH = NCH = CH-, -N = NCH = CH-, -N = CHN = CH-, -N = CHCH = N-, -CH = NN = By forming CH- or -N = NCH = N-, a 5-membered ring or a 6-membered ring may be formed together with the carbon atom to which each Z is bonded. At this time, each carbon atom forming the ring The hydrogen atom bonded to may be optionally substituted by a halogen atom, cyano, methyl, difluoromethyl, trifluoromethyl or methoxy,
Z a represents a halogen atom, methyl or trifluoromethyl,
R 13 represents C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 3 -C 4 cycloalkylmethyl or C 3 -C 4 cycloalkyl,
m represents 1, 2, 3, 4 or 5;
n represents 0 or 1;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔68〕
R6が、D-1、D-2、D-6、D-8、D-23、D-24、D-25又はD-26を表し、
Zは、Zはハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C4アルコキシ又はC1~C4アルキルスルホニルを表し、nが2、3又は4を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH=CH-CH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、R13はC1~C4アルキル又はC1~C4ハロアルキルを表し、nは0、1、2、3又は4を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [68]
R 6 represents D-1, D-2, D-6, D-8, D-23, D-24, D-25 or D-26;
Z represents a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy or C 1 -C 4 alkylsulfonyl, and n is 2, 3 or 4 Each Z may be the same as or different from each other, and when two Zs are adjacent, the two adjacent Zs are -CH = CH-CH = By forming CH-, a 6-membered ring may be formed together with the carbon atom to which each Z is bonded. At this time, the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trimethyl. Optionally substituted by fluoromethyl, R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl, n represents 0, 1, 2, 3 or 4;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R6が、D-1、D-2、D-6、D-8、D-23、D-24、D-25又はD-26を表し、
Zは、Zはハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C4アルコキシ又はC1~C4アルキルスルホニルを表し、nが2、3又は4を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH=CH-CH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、R13はC1~C4アルキル又はC1~C4ハロアルキルを表し、nは0、1、2、3又は4を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [68]
R 6 represents D-1, D-2, D-6, D-8, D-23, D-24, D-25 or D-26;
Z represents a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy or C 1 -C 4 alkylsulfonyl, and n is 2, 3 or 4 Each Z may be the same as or different from each other, and when two Zs are adjacent, the two adjacent Zs are -CH = CH-CH = By forming CH-, a 6-membered ring may be formed together with the carbon atom to which each Z is bonded. At this time, the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trimethyl. Optionally substituted by fluoromethyl, R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl, n represents 0, 1, 2, 3 or 4;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔69〕
R6が、D-1、D-2、D-6、D-8又はD-23を表し、
Zは、Zはハロゲン原子、C1~C4アルキル又はトリフルオロメチルを表し、nが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に6員環を形成してもよく、
R13はC1~C4アルキルを表し、
nは0、1、2又は3を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [69]
R 6 represents D-1, D-2, D-6, D-8 or D-23,
Z represents a halogen atom, C 1 -C 4 alkyl or trifluoromethyl, and when n represents 2, each Z may be the same as or different from each other; When two Zs are adjacent to each other, the two adjacent Zs may form a 6-membered ring with the carbon atom to which each Z is bonded by forming -CH = CHCH = CH-
R 13 represents C 1 -C 4 alkyl;
n represents 0, 1, 2 or 3;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R6が、D-1、D-2、D-6、D-8又はD-23を表し、
Zは、Zはハロゲン原子、C1~C4アルキル又はトリフルオロメチルを表し、nが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に6員環を形成してもよく、
R13はC1~C4アルキルを表し、
nは0、1、2又は3を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [69]
R 6 represents D-1, D-2, D-6, D-8 or D-23,
Z represents a halogen atom, C 1 -C 4 alkyl or trifluoromethyl, and when n represents 2, each Z may be the same as or different from each other; When two Zs are adjacent to each other, the two adjacent Zs may form a 6-membered ring with the carbon atom to which each Z is bonded by forming -CH = CHCH = CH-
R 13 represents C 1 -C 4 alkyl;
n represents 0, 1, 2 or 3;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔70〕
R6が、水素原子を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔71〕
R6が、ハロゲン原子又はC1~C4ハロアルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [70]
R 6 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
[71]
R 6 represents a halogen atom or C 1 -C 4 haloalkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R6が、水素原子を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔71〕
R6が、ハロゲン原子又はC1~C4ハロアルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [70]
R 6 represents a hydrogen atom,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
[71]
R 6 represents a halogen atom or C 1 -C 4 haloalkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔72〕
R6が、R10によって任意に置換された(C1~C4)アルキルを表し、
R10は-S(O)rR16を表し、
R16はメチル、エチル又はC1~C2ハロアルキルを表し、
rは0、1又は2を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [72]
R 6 represents (C 1 -C 4 ) alkyl optionally substituted by R 10 ;
R 10 represents -S (O) r R 16 ;
R 16 represents methyl, ethyl or C 1 -C 2 haloalkyl,
r represents 0, 1 or 2;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R6が、R10によって任意に置換された(C1~C4)アルキルを表し、
R10は-S(O)rR16を表し、
R16はメチル、エチル又はC1~C2ハロアルキルを表し、
rは0、1又は2を表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [72]
R 6 represents (C 1 -C 4 ) alkyl optionally substituted by R 10 ;
R 10 represents -S (O) r R 16 ;
R 16 represents methyl, ethyl or C 1 -C 2 haloalkyl,
r represents 0, 1 or 2;
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔73〕
R6が、C3~C6ハロシクロアルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔74〕
R6が、-C(R11)=NOR12を表し、
R11はメチルを表し、
R12はメチル又はエチルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [73]
R 6 represents C 3 -C 6 halocycloalkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
[74]
R 6 represents -C (R 11 ) = NOR 12 ;
R 11 represents methyl,
R 12 represents methyl or ethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
R6が、C3~C6ハロシクロアルキルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
〔74〕
R6が、-C(R11)=NOR12を表し、
R11はメチルを表し、
R12はメチル又はエチルを表す、
〔1〕~〔54〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [73]
R 6 represents C 3 -C 6 halocycloalkyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
[74]
R 6 represents -C (R 11 ) = NOR 12 ;
R 11 represents methyl,
R 12 represents methyl or ethyl,
The alkynylpyridine-substituted amide compound according to any one of [1] to [54], its N-oxide or a salt thereof.
〔75〕
R1がフッ素原子を表し、
R2がフッ素原子を表し、
R3がメチルを表し、
R4が水素原子を表し、
R6は、C1~C4ハロアルキル又はC3~C6シクロアルキルを表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [75]
R 1 represents a fluorine atom,
R 2 represents a fluorine atom,
R 3 represents methyl,
R 4 represents a hydrogen atom,
R 6 represents C 1 -C 4 haloalkyl or C 3 -C 6 cycloalkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
R1がフッ素原子を表し、
R2がフッ素原子を表し、
R3がメチルを表し、
R4が水素原子を表し、
R6は、C1~C4ハロアルキル又はC3~C6シクロアルキルを表す、
〔1〕~〔29〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 [75]
R 1 represents a fluorine atom,
R 2 represents a fluorine atom,
R 3 represents methyl,
R 4 represents a hydrogen atom,
R 6 represents C 1 -C 4 haloalkyl or C 3 -C 6 cycloalkyl,
The alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [29].
〔76〕
下記式(II)で表される、〔1〕~〔75〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩の製造中間体。
[式中、Y1は、ハロゲン原子又はトリフルオロメチルを表し、
Y2は、水素原子を表し、
Y3は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル又はシクロプロピルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23又はD-25を表し、
さらに、R1がフッ素原子を表し、R2がフッ素原子を表し、R3がメチルを表し、且つR4が水素原子又はメチルを表す場合には、R6はC1~C4ハロアルキル、C3~C6シクロアルキル又はトリ(C1~C4アルキル)シリルを表してもよく、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R13は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す。] [76]
An intermediate for producing an alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [75] represented by the following formula (II):
[Wherein Y 1 represents a halogen atom or trifluoromethyl,
Y 2 represents a hydrogen atom,
Y 3 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 represents a hydrogen atom, C 1 -C 4 alkyl or cyclopropyl,
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
Further, when R 1 represents a fluorine atom, R 2 represents a fluorine atom, R 3 represents methyl, and R 4 represents a hydrogen atom or methyl, R 6 represents C 1 -C 4 haloalkyl, C May represent 3 to C 6 cycloalkyl or tri (C 1 to C 4 alkyl) silyl;
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N- , -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -N (R 13 ) N = N -, = = NN (R 13 ) CH, = NON =, = NSN = or by forming a -CH = CHCH = CH-, it A 5-membered ring or a 6-membered ring may be formed together with the carbon atom to which Z is bonded. In this case, a hydrogen atom bonded to each carbon atom forming the ring is arbitrarily selected by a halogen atom, methyl or trifluoromethyl. May be replaced with
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3. ]
下記式(II)で表される、〔1〕~〔75〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩の製造中間体。
Y2は、水素原子を表し、
Y3は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル又はシクロプロピルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23又はD-25を表し、
さらに、R1がフッ素原子を表し、R2がフッ素原子を表し、R3がメチルを表し、且つR4が水素原子又はメチルを表す場合には、R6はC1~C4ハロアルキル、C3~C6シクロアルキル又はトリ(C1~C4アルキル)シリルを表してもよく、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R13は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す。] [76]
An intermediate for producing an alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of [1] to [75] represented by the following formula (II):
Y 2 represents a hydrogen atom,
Y 3 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 represents a hydrogen atom, C 1 -C 4 alkyl or cyclopropyl,
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
Further, when R 1 represents a fluorine atom, R 2 represents a fluorine atom, R 3 represents methyl, and R 4 represents a hydrogen atom or methyl, R 6 represents C 1 -C 4 haloalkyl, C May represent 3 to C 6 cycloalkyl or tri (C 1 to C 4 alkyl) silyl;
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N- , -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -N (R 13 ) N = N -, = = NN (R 13 ) CH, = NON =, = NSN = or by forming a -CH = CHCH = CH-, it A 5-membered ring or a 6-membered ring may be formed together with the carbon atom to which Z is bonded. In this case, a hydrogen atom bonded to each carbon atom forming the ring is arbitrarily selected by a halogen atom, methyl or trifluoromethyl. May be replaced with
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3. ]
〔77〕
〔1〕~〔75〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシドおよびそれらの塩からなる群より選ばれる1種以上を有効成分として含有する有害生物防除剤組成物。
〔78〕
〔1〕~〔75〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシドおよびそれらの塩からなる群より選ばれる1種以上を有効成分として含有する哺乳動物又は鳥類の抗真菌剤又は寄生虫防除剤組成物。 [77]
A pesticidal composition comprising as an active ingredient at least one selected from the group consisting of the alkynylpyridine-substituted amide compounds according to any one of [1] to [75], their N-oxides and their salts .
[78]
[1] to [75], wherein the alkynylpyridine-substituted amide compound according to any one of [1] to [75], an N-oxide thereof, and a salt thereof as an active ingredient contain at least one selected from the group consisting of Fungal agent or parasite control agent composition.
〔1〕~〔75〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシドおよびそれらの塩からなる群より選ばれる1種以上を有効成分として含有する有害生物防除剤組成物。
〔78〕
〔1〕~〔75〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシドおよびそれらの塩からなる群より選ばれる1種以上を有効成分として含有する哺乳動物又は鳥類の抗真菌剤又は寄生虫防除剤組成物。 [77]
A pesticidal composition comprising as an active ingredient at least one selected from the group consisting of the alkynylpyridine-substituted amide compounds according to any one of [1] to [75], their N-oxides and their salts .
[78]
[1] to [75], wherein the alkynylpyridine-substituted amide compound according to any one of [1] to [75], an N-oxide thereof, and a salt thereof as an active ingredient contain at least one selected from the group consisting of Fungal agent or parasite control agent composition.
〔79〕
哺乳動物又は鳥類に経口投与するための〔78〕に記載の抗真菌剤又は寄生虫防除剤組成物。
〔80〕
哺乳動物又は鳥類に非経口投与するための〔78〕に記載の抗真菌剤又は寄生虫防除剤組成物。 [79]
The antifungal agent or parasite control composition according to [78] for oral administration to mammals or birds.
[80]
The antifungal agent or parasite control agent composition according to [78] for parenteral administration to mammals or birds.
哺乳動物又は鳥類に経口投与するための〔78〕に記載の抗真菌剤又は寄生虫防除剤組成物。
〔80〕
哺乳動物又は鳥類に非経口投与するための〔78〕に記載の抗真菌剤又は寄生虫防除剤組成物。 [79]
The antifungal agent or parasite control composition according to [78] for oral administration to mammals or birds.
[80]
The antifungal agent or parasite control agent composition according to [78] for parenteral administration to mammals or birds.
〔81〕
哺乳動物又は鳥類に非経口投与する方法が、注射による投与である〔80〕に記載の抗真菌剤又は寄生虫防除剤組成物。
〔82〕
哺乳動物又は鳥類に非経口投与する方法が、経皮投与である〔80〕に記載の抗真菌剤又は寄生虫防除剤組成物。 [81]
The antifungal agent or parasite control agent composition according to [80], wherein the method of parenteral administration to mammals or birds is administration by injection.
[82]
The antifungal agent or parasite control agent composition according to [80], wherein the parenteral administration method to mammals or birds is transdermal administration.
哺乳動物又は鳥類に非経口投与する方法が、注射による投与である〔80〕に記載の抗真菌剤又は寄生虫防除剤組成物。
〔82〕
哺乳動物又は鳥類に非経口投与する方法が、経皮投与である〔80〕に記載の抗真菌剤又は寄生虫防除剤組成物。 [81]
The antifungal agent or parasite control agent composition according to [80], wherein the method of parenteral administration to mammals or birds is administration by injection.
[82]
The antifungal agent or parasite control agent composition according to [80], wherein the parenteral administration method to mammals or birds is transdermal administration.
〔83〕
〔1〕~〔75〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシドおよびそれらの塩からなる群より選ばれる1種以上を有効成分として含有する農園芸用殺菌剤又は殺線虫剤組成物。 [83]
An agricultural and horticultural fungicide containing, as an active ingredient, one or more selected from the group consisting of the alkynylpyridine-substituted amide compound according to any one of [1] to [75], an N-oxide thereof, and a salt thereof, Nematicide composition.
〔1〕~〔75〕のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシドおよびそれらの塩からなる群より選ばれる1種以上を有効成分として含有する農園芸用殺菌剤又は殺線虫剤組成物。 [83]
An agricultural and horticultural fungicide containing, as an active ingredient, one or more selected from the group consisting of the alkynylpyridine-substituted amide compound according to any one of [1] to [75], an N-oxide thereof, and a salt thereof, Nematicide composition.
〔84〕
植物に茎葉散布するための〔83〕に記載の農園芸用殺菌剤又は殺線虫剤組成物。
〔85〕
植物が生育する土壌を処理するための〔83〕に記載の農園芸用殺菌剤又は殺線虫剤組成物。 [84]
The agricultural and horticultural fungicide or nematicide composition according to [83] for spraying foliage on plants.
[85]
The agricultural and horticultural fungicide or nematicide composition according to [83] for treating soil in which plants grow.
植物に茎葉散布するための〔83〕に記載の農園芸用殺菌剤又は殺線虫剤組成物。
〔85〕
植物が生育する土壌を処理するための〔83〕に記載の農園芸用殺菌剤又は殺線虫剤組成物。 [84]
The agricultural and horticultural fungicide or nematicide composition according to [83] for spraying foliage on plants.
[85]
The agricultural and horticultural fungicide or nematicide composition according to [83] for treating soil in which plants grow.
〔86〕
植物の種子、塊根又は根茎を処理するための〔83〕に記載の農園芸用殺菌剤又は殺線虫剤組成物。 [86]
The agricultural and horticultural fungicide or nematicide composition according to [83] for treating plant seeds, tuberous roots or rhizomes.
植物の種子、塊根又は根茎を処理するための〔83〕に記載の農園芸用殺菌剤又は殺線虫剤組成物。 [86]
The agricultural and horticultural fungicide or nematicide composition according to [83] for treating plant seeds, tuberous roots or rhizomes.
式(I)で表される本発明化合物及び該化合物を有効成分として含有する有害生物防除剤は農園芸分野又は畜産・衛生分野等における有害生物、特に真菌類及び線形動物に対して優れた防除効果を発揮し、既存の薬剤に対して抵抗性を獲得したそれらの有害生物に対しても十分な防除効果を発揮する。さらに、植物やホ乳動物、魚類、有用昆虫及び天敵等の非標的生物に対してほとんど悪影響を及ぼさず、低残留性で環境に対する負荷も軽い。
従って、本発明は有用な新規有害生物防除剤を提供することができる。 The compound of the present invention represented by the formula (I) and a pest control agent containing the compound as an active ingredient are excellent control for pests in the fields of agriculture, horticulture and livestock / hygiene, especially fungi and linear animals. It exerts its effect and exerts a sufficient control effect against those pests that have acquired resistance to existing drugs. Furthermore, there is almost no adverse effect on non-target organisms such as plants, mammals, fish, useful insects and natural enemies, low persistence and light environmental impact.
Therefore, the present invention can provide a useful novel pest control agent.
従って、本発明は有用な新規有害生物防除剤を提供することができる。 The compound of the present invention represented by the formula (I) and a pest control agent containing the compound as an active ingredient are excellent control for pests in the fields of agriculture, horticulture and livestock / hygiene, especially fungi and linear animals. It exerts its effect and exerts a sufficient control effect against those pests that have acquired resistance to existing drugs. Furthermore, there is almost no adverse effect on non-target organisms such as plants, mammals, fish, useful insects and natural enemies, low persistence and light environmental impact.
Therefore, the present invention can provide a useful novel pest control agent.
本発明に包含される式(I)で表されるアルキニルピリジン置換アミド化合物においては、置換基によっては1個又は2個以上の不斉炭素原子の存在に起因する光学活性体が存在する場合があるが、本発明は全ての光学活性体又はラセミ体を包含する。また、本発明に包含される化合物においては、置換基によってはE-体及びZ-体の幾何異性体が存在する場合があるが、本発明はこれらE-体、Z-体又はE-体及びZ-体を任意の割合で含む混合物を包含するものである。
In the alkynylpyridine-substituted amide compound represented by the formula (I) included in the present invention, an optically active substance resulting from the presence of one or more asymmetric carbon atoms may exist depending on the substituent. However, the present invention encompasses all optically active forms or racemates. Further, in the compounds included in the present invention, there are cases where geometric isomers of E-form and Z-form may exist depending on the substituent, but the present invention is not limited to these E-form, Z-form or E-form. And a mixture containing the Z-form in an arbitrary ratio.
本明細書において、以下の用語又は表現は、それぞれ、以下の意味又は用法で使用される。
ハロゲン原子としては、フッ素原子、塩素原子、臭素原子及びヨウ素原子が挙げられる。尚、本明細書中「ハロ」の表記もこれらのハロゲン原子を表す。
置換基の具体的な説明において、以下「n-」との表記は「ノルマル」を、「i-」は「イソ」を、「sec-」は「セカンダリー」を、「tert-」は「ターシャリー」を各々意味する。 In this specification, the following terms or expressions are respectively used in the following meanings or usages.
Examples of the halogen atom include a fluorine atom, a chlorine atom, a bromine atom, and an iodine atom. In the present specification, the notation “halo” also represents these halogen atoms.
In the specific description of the substituent, the notation “n-” is “normal”, “i-” is “iso”, “sec-” is “secondary”, and “tert-” is “tarsha”. "Lee" means each.
ハロゲン原子としては、フッ素原子、塩素原子、臭素原子及びヨウ素原子が挙げられる。尚、本明細書中「ハロ」の表記もこれらのハロゲン原子を表す。
置換基の具体的な説明において、以下「n-」との表記は「ノルマル」を、「i-」は「イソ」を、「sec-」は「セカンダリー」を、「tert-」は「ターシャリー」を各々意味する。 In this specification, the following terms or expressions are respectively used in the following meanings or usages.
Examples of the halogen atom include a fluorine atom, a chlorine atom, a bromine atom, and an iodine atom. In the present specification, the notation “halo” also represents these halogen atoms.
In the specific description of the substituent, the notation “n-” is “normal”, “i-” is “iso”, “sec-” is “secondary”, and “tert-” is “tarsha”. "Lee" means each.
「Ca~Cbアルキル」の表記は、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状の炭化水素を表し、例えばメチル、エチル、n-プロピル、i-プロピル、n-ブチル、i-ブチル、sec-ブチル、tert-ブチル、ペンチル、1-エチルプロピル、2,2-ジメチルプロピル、ヘキシル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The expression “C a -C b alkyl” represents a straight or branched hydrocarbon having a to b carbon atoms, for example, methyl, ethyl, n-propyl, i-propyl, n- Specific examples include butyl, i-butyl, sec-butyl, tert-butyl, pentyl, 1-ethylpropyl, 2,2-dimethylpropyl, hexyl, etc., each selected within the specified number of carbon atoms. .
「Ca~Cbハロアルキル」の表記は、炭素原子に結合した水素原子がハロゲン原子によって任意に置換された、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状の炭化水素を表し、このとき、2個以上のハロゲン原子によって置換されている場合、それらのハロゲン原子は互いに同一でも、または互いに相異なっていてもよい。例えばフルオロメチル、クロロメチル、ブロモメチル、ヨードメチル、ジフルオロメチル、ジクロロメチル、トリフルオロメチル、クロロジフルオロメチル、トリクロロメチル、ブロモジフルオロメチル、1-フルオロエチル、2-フルオロエチル、2-クロロエチル、2-ブロモエチル、1,1-ジフルオロエチル、2,2-ジフルオロエチル、2,2,2-トリフルオロエチル、2-クロロ-2,2-ジフルオロエチル、2,2,2-トリクロロエチル、2-ブロモ-2,2-ジフルオロエチル、1,1,2,2-テトラフルオロエチル、2-クロロ-1,1,2-トリフルオロエチル、ペンタフルオロエチル、2,2-ジフルオロプロピル、3,3,3-トリフルオロプロピル、3-ブロモ-3,3-ジフルオロプロピル、2,2,3,3-テトラフルオロプロピル、2,2,3,3,3-ペンタフルオロプロピル、1,1,2,3,3,3-ヘキサフルオロプロピル、ヘプタフルオロプロピル、2,2,2-トリフルオロ-1-メチルエチル、2,2,2-トリフルオロ-1-(トリフルオロメチル)エチル、1,2,2,2-テトラフルオロ-1-(トリフルオロメチル)エチル、2,2,2-トリフルオロ-1,1-ジメチルエチル、2,2,3,4,4,4-ヘキサフルオロブチル、2,2,3,3,4,4,4-ヘプタフルオロブチル、ノナフルオロブチル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The expression “C a -C b haloalkyl” refers to a linear or branched hydrocarbon having a to b carbon atoms in which a hydrogen atom bonded to a carbon atom is optionally substituted with a halogen atom. In this case, when being substituted by two or more halogen atoms, the halogen atoms may be the same as or different from each other. For example, fluoromethyl, chloromethyl, bromomethyl, iodomethyl, difluoromethyl, dichloromethyl, trifluoromethyl, chlorodifluoromethyl, trichloromethyl, bromodifluoromethyl, 1-fluoroethyl, 2-fluoroethyl, 2-chloroethyl, 2-bromoethyl, 1,1-difluoroethyl, 2,2-difluoroethyl, 2,2,2-trifluoroethyl, 2-chloro-2,2-difluoroethyl, 2,2,2-trichloroethyl, 2-bromo-2, 2-difluoroethyl, 1,1,2,2-tetrafluoroethyl, 2-chloro-1,1,2-trifluoroethyl, pentafluoroethyl, 2,2-difluoropropyl, 3,3,3-trifluoro Propyl, 3-bromo-3,3-difluoropropyl, 2,2,3,3-tetrafluoropropyl, 2,2,3,3,3-pentafluoropropyl Lopyl, 1,1,2,3,3,3-hexafluoropropyl, heptafluoropropyl, 2,2,2-trifluoro-1-methylethyl, 2,2,2-trifluoro-1- (trifluoro Methyl) ethyl, 1,2,2,2-tetrafluoro-1- (trifluoromethyl) ethyl, 2,2,2-trifluoro-1,1-dimethylethyl, 2,2,3,4,4, Specific examples include 4-hexafluorobutyl, 2,2,3,3,4,4,4-heptafluorobutyl, nonafluorobutyl, and the like, and each is selected within the range of the designated number of carbon atoms.
「Ca~Cbシクロアルキル」の表記は、炭素原子数がa~b個よりなる環状の炭化水素を表し、3員環から10員環までの単環又は複合環構造を形成することが出来る。また、各々の環は指定の炭素原子数の範囲でアルキルによって任意に置換されていてもよい。例えばシクロプロピル、シクロブチル、1-メチルシクロプロピル、2-メチルシクロプロピル、シクロペンチル、2,2-ジメチルシクロプロピル、1-メチルシクロブチル、シクロヘキシル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The expression “C a -C b cycloalkyl” represents a cyclic hydrocarbon having a carbon number of a to b, and may form a monocyclic or complex ring structure of 3 to 10 members. I can do it. Each ring may be optionally substituted with alkyl within the range of the specified number of carbon atoms. Specific examples include, for example, cyclopropyl, cyclobutyl, 1-methylcyclopropyl, 2-methylcyclopropyl, cyclopentyl, 2,2-dimethylcyclopropyl, 1-methylcyclobutyl, cyclohexyl and the like. The range is selected.
「Ca~Cbハロシクロアルキル」の表記は、炭素原子に結合した水素原子がハロゲン原子によって任意に置換された、炭素原子数がa~b個よりなる環状の炭化水素を表し、3員環から10員環までの単環又は複合環構造を形成することが出来る。また、各々の環は指定の炭素原子数の範囲でアルキルによって任意に置換されていてもよく、ハロゲン原子による置換は環構造部分であっても、側鎖部分であっても、又ははそれらの両方であってもよく、さらに、2個以上のハロゲン原子によって置換されている場合、それらのハロゲン原子は互いに同一でも、若しくは互いに相異なっていてもよい。例えば1-フルオロシクロプロピル、1-クロロシクロプロピル、2-クロロシクロプロピル、2,2-ジフルオロシクロプロピル、2,2-ジクロロシクロプロピル、2,2-ジブロモシクロプロピル、2,2-ジフルオロ-1-メチルシクロプロピル、2,2-ジクロロ-1-メチルシクロプロピル、2,2-ジブロモ-1-メチルシクロプロピル、3-フルオロシクロブチル、2,2,3,3-テトラフルオロシクロブチル、1-フルオロシクロペンチル、1-クロロ-2,2-ジメチルシクロプロピル、1-フルオロシクロヘキシル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The notation “C a -C b halocycloalkyl” represents a cyclic hydrocarbon having a to b carbon atoms in which a hydrogen atom bonded to a carbon atom is optionally substituted by a halogen atom, A monocyclic or complex ring structure from a ring to a 10-membered ring can be formed. Each ring may be optionally substituted with alkyl within the range of the specified number of carbon atoms, and the substitution with a halogen atom may be a ring structure portion, a side chain portion, or It may be both, and when it is substituted by two or more halogen atoms, the halogen atoms may be the same as or different from each other. For example, 1-fluorocyclopropyl, 1-chlorocyclopropyl, 2-chlorocyclopropyl, 2,2-difluorocyclopropyl, 2,2-dichlorocyclopropyl, 2,2-dibromocyclopropyl, 2,2-difluoro-1 2-methylcyclopropyl, 2,2-dichloro-1-methylcyclopropyl, 2,2-dibromo-1-methylcyclopropyl, 3-fluorocyclobutyl, 2,2,3,3-tetrafluorocyclobutyl, 1- Specific examples include fluorocyclopentyl, 1-chloro-2,2-dimethylcyclopropyl, 1-fluorocyclohexyl and the like, each selected within the range of the specified number of carbon atoms.
「Ca~Cbアルケニル」の表記は、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状で、且つ分子内に少なくとも1個以上の二重結合を有する不飽和炭化水素を表し、例えばビニル、1-プロペニル、2-プロペニル、1-メチルエテニル、1-ブテニル、2-ブテニル、1-メチル-1-プロペニル、2-メチル-1-プロペニル、2-メチル-2-プロペニル、3-メチル-3-ブテニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The expression “C a -C b alkenyl” refers to an unsaturated hydrocarbon having a linear or branched chain of a to b carbon atoms and having at least one double bond in the molecule. For example, vinyl, 1-propenyl, 2-propenyl, 1-methylethenyl, 1-butenyl, 2-butenyl, 1-methyl-1-propenyl, 2-methyl-1-propenyl, 2-methyl-2-propenyl, 3 Specific examples include -methyl-3-butenyl and the like, each selected within a range of the specified number of carbon atoms.
「Ca~Cbハロアルケニル」の表記は、炭素原子に結合した水素原子がハロゲン原子によって任意に置換された、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状で、且つ分子内に少なくとも1個以上の二重結合を有する不飽和炭化水素を表す。このとき、2個以上のハロゲン原子によって置換されている場合、それらのハロゲン原子は互いに同一でも、又は互いに相異なっていてもよい。例えば2-フルオロビニル、2-クロロビニル、1,2-ジクロロビニル、2,2-ジクロロビニル、2,2-ジブロモビニル、2-フルオロ-2-プロペニル、2-クロロ-2-プロペニル、3-クロロ-2-プロペニル、3,3-ジフルオロ-2-プロペニル、2,3-ジクロロ-2-プロペニル、3,3-ジクロロ-2-プロペニル、2,3,3-トリフルオロ-2-プロペニル、2,3,3-トリクロロ-2-プロペニル、1-(トリフルオロメチル)エテニル、4,4-ジフルオロ-3-ブテニル、3,4,4-トリフルオロ-3-ブテニル、2,4,4,4-テトラフルオロ-2-ブテニル、3-クロロ-4,4,4-トリフルオロ-2-ブテニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The notation “C a -C b haloalkenyl” is a linear or branched chain having a carbon number of a to b in which a hydrogen atom bonded to a carbon atom is optionally substituted with a halogen atom, and It represents an unsaturated hydrocarbon having at least one double bond in the molecule. At this time, when substituted by two or more halogen atoms, these halogen atoms may be the same as or different from each other. For example, 2-fluorovinyl, 2-chlorovinyl, 1,2-dichlorovinyl, 2,2-dichlorovinyl, 2,2-dibromovinyl, 2-fluoro-2-propenyl, 2-chloro-2-propenyl, 3- Chloro-2-propenyl, 3,3-difluoro-2-propenyl, 2,3-dichloro-2-propenyl, 3,3-dichloro-2-propenyl, 2,3,3-trifluoro-2-propenyl, 2, , 3,3-trichloro-2-propenyl, 1- (trifluoromethyl) ethenyl, 4,4-difluoro-3-butenyl, 3,4,4-trifluoro-3-butenyl, 2,4,4,4 Specific examples include -tetrafluoro-2-butenyl, 3-chloro-4,4,4-trifluoro-2-butenyl, etc., each selected within the range of the specified number of carbon atoms.
「Ca~Cbシクロアルケニル」の表記は、炭素原子数がa~b個よりなる環状の、且つ少なくとも1個以上の二重結合を有する不飽和炭化水素を表し、3員環から10員環までの単環又は複合環構造を形成することが出来る。また、各々の環は指定の炭素原子数の範囲でアルキルによって任意に置換されていてもよく、さらに、二重結合はendo-又はexo-のどちらの形式であってもよい。例えば1-シクロペンテニル、2-シクロペンテニル、1-シクロヘキセニル、2-シクロヘキセニル、ビシクロ[2.2.1]-5-ヘプテン-2-イル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The expression “C a -C b cycloalkenyl” refers to a cyclic unsaturated hydrocarbon having at least one double bond and having from 3 to 10 members. Monocyclic or complex ring structures up to the ring can be formed. Each ring may be optionally substituted with alkyl within the range of the specified number of carbon atoms, and the double bond may be either endo- or exo-. Specific examples include 1-cyclopentenyl, 2-cyclopentenyl, 1-cyclohexenyl, 2-cyclohexenyl, bicyclo [2.2.1] -5-hepten-2-yl, and the like. It is selected in the range of the number of atoms.
「Ca~Cbハロシクロアルケニル」の表記は、炭素原子に結合した水素原子がハロゲン原子によって任意に置換された炭素原子数がa~b個よりなる環状の、且つ分子内に少なくとも1個個以上の二重結合を有する不飽和炭化水素を表し、3員環から10員環までの単環又は複合環構造を形成することが出来る。また、各々の環は指定の炭素原子数の範囲でアルキルによって任意に置換されていてもよく、さらに、二重結合はendo-又はexo-のどちらの形式であってもよい。このとき、2個以上のハロゲン原子によって置換されている場合、それらのハロゲン原子は互いに同一でも、又は互いに相異なっていてもよい。例えば2-クロロ-1-シクロブテニル、2-ブロモ-1-シクロペンテニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The notation “C a -C b halocycloalkenyl” is a cyclic structure comprising at least one carbon atom in which a hydrogen atom bonded to a carbon atom is optionally substituted by a halogen atom, and at least one in the molecule. It represents an unsaturated hydrocarbon having at least two double bonds, and can form a monocyclic or complex ring structure of 3 to 10 members. Each ring may be optionally substituted with alkyl within the range of the specified number of carbon atoms, and the double bond may be either endo- or exo-. At this time, when substituted by two or more halogen atoms, these halogen atoms may be the same as or different from each other. For example, 2-chloro-1-cyclobutenyl, 2-bromo-1-cyclopentenyl and the like are listed as specific examples, and each is selected within the range of the designated number of carbon atoms.
「Ca~Cbアルキニル」の表記は、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状で、且つ分子内に少なくとも1個以上の三重結合を有する不飽和炭化水素を表し、例えばエチニル、1-プロピニル、2-プロピニル、1-ブチニル、2-ブチニル、3-ブチニル、1-メチル-2-プロピニル、1-ペンチニル、2-ペンチニル、1-ヘキシニル、3-ヘキシニル、3-メチル-1-ペンチニル、4-メチル-1-ペンチニル、3,3-ジメチル-1-ブチニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
「Ca~Cbハロアルキニル」の表記は、炭素原子に結合した水素原子がハロゲン原子によって任意に置換された、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状で、且つ分子内に少なくとも1個以上の三重結合を有する不飽和炭化水素を表す。このとき、2個以上のハロゲン原子によって置換されている場合、それらのハロゲン原子は互いに同一でも、又は互いに相異なっていても良い。例えば2-クロロエチニル、2-ブロモエチニル、2-ヨードエチニル、3-フルオロ-1-プロピニル、3-クロロ-1-プロピニル、3-クロロ-2-プロピニル、3-ブロモ-1-プロピニル、3-ブロモ-2-プロピニル、3-ヨード-2-プロピニル、3,3-ジフルオロ-1-プロピニル、3,3,3-トリフルオロ-1-プロピニル、3-ブロモ-1-ブチニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The expression “C a -C b alkynyl” represents an unsaturated hydrocarbon having a linear or branched chain of a to b carbon atoms and having at least one triple bond in the molecule. For example, ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-methyl-2-propynyl, 1-pentynyl, 2-pentynyl, 1-hexynyl, 3-hexynyl, 3- Specific examples include methyl-1-pentynyl, 4-methyl-1-pentynyl, 3,3-dimethyl-1-butynyl and the like, each selected in the range of the designated number of carbon atoms.
Notation "C a ~ C b haloalkynyl" represents a hydrogen atom bonded to carbon atom is optionally substituted by halogen atom, carbon atoms in a ~ b number than consisting linear or branched, and An unsaturated hydrocarbon having at least one triple bond in the molecule. At this time, when substituted by two or more halogen atoms, these halogen atoms may be the same as or different from each other. For example, 2-chloroethynyl, 2-bromoethynyl, 2-iodoethynyl, 3-fluoro-1-propynyl, 3-chloro-1-propynyl, 3-chloro-2-propynyl, 3-bromo-1-propynyl, 3- Specific examples include bromo-2-propynyl, 3-iodo-2-propynyl, 3,3-difluoro-1-propynyl, 3,3,3-trifluoro-1-propynyl, and 3-bromo-1-butynyl. Selected within a range of each specified number of carbon atoms.
「Ca~Cbハロアルキニル」の表記は、炭素原子に結合した水素原子がハロゲン原子によって任意に置換された、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状で、且つ分子内に少なくとも1個以上の三重結合を有する不飽和炭化水素を表す。このとき、2個以上のハロゲン原子によって置換されている場合、それらのハロゲン原子は互いに同一でも、又は互いに相異なっていても良い。例えば2-クロロエチニル、2-ブロモエチニル、2-ヨードエチニル、3-フルオロ-1-プロピニル、3-クロロ-1-プロピニル、3-クロロ-2-プロピニル、3-ブロモ-1-プロピニル、3-ブロモ-2-プロピニル、3-ヨード-2-プロピニル、3,3-ジフルオロ-1-プロピニル、3,3,3-トリフルオロ-1-プロピニル、3-ブロモ-1-ブチニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The expression “C a -C b alkynyl” represents an unsaturated hydrocarbon having a linear or branched chain of a to b carbon atoms and having at least one triple bond in the molecule. For example, ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1-methyl-2-propynyl, 1-pentynyl, 2-pentynyl, 1-hexynyl, 3-hexynyl, 3- Specific examples include methyl-1-pentynyl, 4-methyl-1-pentynyl, 3,3-dimethyl-1-butynyl and the like, each selected in the range of the designated number of carbon atoms.
Notation "C a ~ C b haloalkynyl" represents a hydrogen atom bonded to carbon atom is optionally substituted by halogen atom, carbon atoms in a ~ b number than consisting linear or branched, and An unsaturated hydrocarbon having at least one triple bond in the molecule. At this time, when substituted by two or more halogen atoms, these halogen atoms may be the same as or different from each other. For example, 2-chloroethynyl, 2-bromoethynyl, 2-iodoethynyl, 3-fluoro-1-propynyl, 3-chloro-1-propynyl, 3-chloro-2-propynyl, 3-bromo-1-propynyl, 3- Specific examples include bromo-2-propynyl, 3-iodo-2-propynyl, 3,3-difluoro-1-propynyl, 3,3,3-trifluoro-1-propynyl, and 3-bromo-1-butynyl. Selected within a range of each specified number of carbon atoms.
「Ca~Cbアルコキシ」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルキル-O-を表し、例えばメトキシ、エトキシ、n-プロピルオキシ、i-プロピルオキシ、n-ブチルオキシ、i-ブチルオキシ、sec-ブチルオキシ、tert-ブチルオキシ、ペンチルオキシ、ヘキシルオキシ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The notation “C a -C b alkoxy” represents alkyl-O— as defined above consisting of a to b carbon atoms, eg methoxy, ethoxy, n-propyloxy, i-propyloxy, n Specific examples include -butyloxy, i-butyloxy, sec-butyloxy, tert-butyloxy, pentyloxy, hexyloxy, and the like, each selected within the range of the specified number of carbon atoms.
「Ca~Cbハロアルコキシ」の表記は、炭素原子数がa~b個よりなる前記の意味であるハロアルキル-O-を表し、例えばジフルオロメトキシ、トリフルオロメトキシ、クロロジフルオロメトキシ、ブロモジフルオロメトキシ、2-フルオロエトキシ、2-クロロエトキシ、2,2,2-トリフルオロエトキシ、1,1,2,2,-テトラフルオロエトキシ、2-クロロ-1,1,2-トリフルオロエトキシ、1,1,2,3,3,3-ヘキサフルオロプロピルオキシ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The notation “C a -C b haloalkoxy” represents haloalkyl-O— as defined above consisting of a to b carbon atoms, eg difluoromethoxy, trifluoromethoxy, chlorodifluoromethoxy, bromodifluoromethoxy. 2-fluoroethoxy, 2-chloroethoxy, 2,2,2-trifluoroethoxy, 1,1,2,2, -tetrafluoroethoxy, 2-chloro-1,1,2-trifluoroethoxy, 1, Specific examples include 1,2,3,3,3-hexafluoropropyloxy and the like, and each is selected within the range of the designated number of carbon atoms.
「Ca~Cbアルケニルオキシ」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルケニル-O-を表し、例えば2-プロペニルオキシ、2-ブテニルオキシ、1-メチル-2-プロペニルオキシ、2-メチル-2-プロペニルオキシ、3-メチル-2-ブテニルオキシ、1,1-ジメチル-2-プロペニルオキシ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
「Ca~Cbハロアルケニルオキシ」の表記は、炭素原子数がa~b個よりなる前記の意味であるハロアルケニル-O-を表し、例えば2-フルオロ-2-プロペニルオキシ、2-クロロ-2-プロペニルオキシ、3-クロロ-2-プロペニルオキシ、3,3-ジフルオロ-2-プロペニルオキシ、2,3-ジクロロ-2-プロペニルオキシ、3,3-ジクロロ-2-プロペニルオキシ、2,3,3-トリフルオロ-2-プロペニルオキシ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The expression “C a -C b alkenyloxy” represents alkenyl-O— having the above-mentioned meanings consisting of a to b carbon atoms, for example 2-propenyloxy, 2-butenyloxy, 1-methyl-2 Specific examples include -propenyloxy, 2-methyl-2-propenyloxy, 3-methyl-2-butenyloxy, 1,1-dimethyl-2-propenyloxy, etc., selected in the range of each designated number of carbon atoms Is done.
The notation “C a -C b haloalkenyloxy” represents haloalkenyl-O— as defined above, consisting of a to b carbon atoms, for example 2-fluoro-2-propenyloxy, 2-chloro -2-propenyloxy, 3-chloro-2-propenyloxy, 3,3-difluoro-2-propenyloxy, 2,3-dichloro-2-propenyloxy, 3,3-dichloro-2-propenyloxy, 2, Specific examples include 3,3-trifluoro-2-propenyloxy and the like, each selected within the range of the designated number of carbon atoms.
「Ca~Cbハロアルケニルオキシ」の表記は、炭素原子数がa~b個よりなる前記の意味であるハロアルケニル-O-を表し、例えば2-フルオロ-2-プロペニルオキシ、2-クロロ-2-プロペニルオキシ、3-クロロ-2-プロペニルオキシ、3,3-ジフルオロ-2-プロペニルオキシ、2,3-ジクロロ-2-プロペニルオキシ、3,3-ジクロロ-2-プロペニルオキシ、2,3,3-トリフルオロ-2-プロペニルオキシ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The expression “C a -C b alkenyloxy” represents alkenyl-O— having the above-mentioned meanings consisting of a to b carbon atoms, for example 2-propenyloxy, 2-butenyloxy, 1-methyl-2 Specific examples include -propenyloxy, 2-methyl-2-propenyloxy, 3-methyl-2-butenyloxy, 1,1-dimethyl-2-propenyloxy, etc., selected in the range of each designated number of carbon atoms Is done.
The notation “C a -C b haloalkenyloxy” represents haloalkenyl-O— as defined above, consisting of a to b carbon atoms, for example 2-fluoro-2-propenyloxy, 2-chloro -2-propenyloxy, 3-chloro-2-propenyloxy, 3,3-difluoro-2-propenyloxy, 2,3-dichloro-2-propenyloxy, 3,3-dichloro-2-propenyloxy, 2, Specific examples include 3,3-trifluoro-2-propenyloxy and the like, each selected within the range of the designated number of carbon atoms.
「Ca~Cbアルキニルオキシ」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルキニル-O-を表し、例えば2-プロピニルオキシ、2-ブチニルオキシ、1-メチル-2-プロピニルオキシ、1,1-ジメチル-2-プロピニルオキシ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
「Ca~Cbハロアルキニルオキシ」の表記は、炭素原子数がa~b個よりなる前記の意味であるハロアルキニル-O-を表し、例えば3-クロロ-2-プロピニルオキシ、3-ブロモ-2-プロピニルオキシ、3-ヨード-2-プロピニルオキシ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b alkynyloxy” represents alkynyl-O— as defined above consisting of a to b carbon atoms, for example 2-propynyloxy, 2-butynyloxy, 1-methyl-2 Specific examples include -propynyloxy, 1,1-dimethyl-2-propynyloxy, etc., each selected within the specified number of carbon atoms.
The notation “C a -C b haloalkynyloxy” represents haloalkynyl-O— as defined above consisting of a to b carbon atoms, eg 3-chloro-2-propynyloxy, 3-bromo Specific examples include -2-propynyloxy, 3-iodo-2-propynyloxy, and the like, and each is selected within the specified number of carbon atoms.
「Ca~Cbハロアルキニルオキシ」の表記は、炭素原子数がa~b個よりなる前記の意味であるハロアルキニル-O-を表し、例えば3-クロロ-2-プロピニルオキシ、3-ブロモ-2-プロピニルオキシ、3-ヨード-2-プロピニルオキシ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b alkynyloxy” represents alkynyl-O— as defined above consisting of a to b carbon atoms, for example 2-propynyloxy, 2-butynyloxy, 1-methyl-2 Specific examples include -propynyloxy, 1,1-dimethyl-2-propynyloxy, etc., each selected within the specified number of carbon atoms.
The notation “C a -C b haloalkynyloxy” represents haloalkynyl-O— as defined above consisting of a to b carbon atoms, eg 3-chloro-2-propynyloxy, 3-bromo Specific examples include -2-propynyloxy, 3-iodo-2-propynyloxy, and the like, and each is selected within the specified number of carbon atoms.
「Ca~Cbアルキルチオ」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルキル-S-を表し、例えばメチルチオ、エチルチオ、n-プロピルチオ、i-プロピルチオ、n-ブチルチオ、i-ブチルチオ、sec-ブチルチオ、tert-ブチルチオ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The notation “C a -C b alkylthio” represents alkyl-S— as defined above, comprising a to b carbon atoms, for example methylthio, ethylthio, n-propylthio, i-propylthio, n-butylthio. Specific examples include i-butylthio, sec-butylthio, tert-butylthio and the like, each selected within the range of the designated number of carbon atoms.
「Ca~Cbアルキルスルフィニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルキル-S(O)-を表し、例えばメチルスルフィニル、エチルスルフィニル、n-プロピルスルフィニル、i-プロピルスルフィニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
「Ca~Cbアルキルスルホニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルキル-SO2-を表し、例えばメチルスルホニル、エチルスルホニル、n-プロピルスルホニル、i-プロピルスルホニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b alkylsulfinyl” represents alkyl-S (O) — as defined above, comprising a to b carbon atoms, for example methylsulfinyl, ethylsulfinyl, n-propylsulfinyl, Specific examples include i-propylsulfinyl and the like, each selected within the range of the specified number of carbon atoms.
The expression “C a -C b alkylsulfonyl” represents alkyl-SO 2 — having the above-mentioned meanings consisting of a to b carbon atoms, such as methylsulfonyl, ethylsulfonyl, n-propylsulfonyl, i- Specific examples include propylsulfonyl and the like, and each is selected within the range of the designated number of carbon atoms.
「Ca~Cbアルキルスルホニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルキル-SO2-を表し、例えばメチルスルホニル、エチルスルホニル、n-プロピルスルホニル、i-プロピルスルホニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b alkylsulfinyl” represents alkyl-S (O) — as defined above, comprising a to b carbon atoms, for example methylsulfinyl, ethylsulfinyl, n-propylsulfinyl, Specific examples include i-propylsulfinyl and the like, each selected within the range of the specified number of carbon atoms.
The expression “C a -C b alkylsulfonyl” represents alkyl-SO 2 — having the above-mentioned meanings consisting of a to b carbon atoms, such as methylsulfonyl, ethylsulfonyl, n-propylsulfonyl, i- Specific examples include propylsulfonyl and the like, and each is selected within the range of the designated number of carbon atoms.
「Ca~Cbハロアルキルチオ」の表記は、炭素原子数がa~b個よりなる前記の意味であるハロアルキル-S-を表し、例えばジフルオロメチルチオ、トリフルオロメチルチオ、クロロジフルオロメチルチオ、トリクロロメチルチオ、ブロモジフルオロメチルチオ、2,2,2-トリフルオロエチルチオ、1,1,2,2-テトラフルオロエチルチオ、2-クロロ-1,1,2-トリフルオロエチルチオ、ペンタフルオロエチルチオ、1,1,2,3,3,3-ヘキサフルオロプロピルチオ、ヘプタフルオロプロピルチオ、1,2,2,2-テトラフルオロ-1-(トリフルオロメチル)エチルチオ、ノナフルオロブチルチオ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The notation “C a -C b haloalkylthio” represents haloalkyl-S—, as defined above, comprising a to b carbon atoms, for example difluoromethylthio, trifluoromethylthio, chlorodifluoromethylthio, trichloromethylthio, Bromodifluoromethylthio, 2,2,2-trifluoroethylthio, 1,1,2,2-tetrafluoroethylthio, 2-chloro-1,1,2-trifluoroethylthio, pentafluoroethylthio, 1, Specific examples include 1,2,3,3,3-hexafluoropropylthio, heptafluoropropylthio, 1,2,2,2-tetrafluoro-1- (trifluoromethyl) ethylthio, nonafluorobutylthio and the like. Selected within a range of each specified number of carbon atoms.
「Ca~Cbハロアルキルスルフィニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるハロアルキル-S(O)-を表し、例えばジフルオロメチルスルフィニル、トリフルオロメチルスルフィニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
「Ca~Cbハロアルキルスルホニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるハロアルキル-SO2-を表し、例えばジフルオロメチルスルホニル、トリフルオロメチルスルホニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The expression “C a -C b haloalkylsulfinyl” represents the above-mentioned haloalkyl-S (O) — having from a to b carbon atoms, such as difluoromethylsulfinyl, trifluoromethylsulfinyl and the like. As an example, each selected range of carbon atoms is selected.
The expression “C a -C b haloalkylsulfonyl” represents the above-mentioned haloalkyl-SO 2 — having a carbon number of a to b, for example, difluoromethylsulfonyl, trifluoromethylsulfonyl and the like. Each of which is selected for each specified number of carbon atoms.
「Ca~Cbハロアルキルスルホニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるハロアルキル-SO2-を表し、例えばジフルオロメチルスルホニル、トリフルオロメチルスルホニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The expression “C a -C b haloalkylsulfinyl” represents the above-mentioned haloalkyl-S (O) — having from a to b carbon atoms, such as difluoromethylsulfinyl, trifluoromethylsulfinyl and the like. As an example, each selected range of carbon atoms is selected.
The expression “C a -C b haloalkylsulfonyl” represents the above-mentioned haloalkyl-SO 2 — having a carbon number of a to b, for example, difluoromethylsulfonyl, trifluoromethylsulfonyl and the like. Each of which is selected for each specified number of carbon atoms.
「ジ(Ca~Cbアルキル)アミノ」の表記は、水素原子が両方とも、それぞれ同一でも又は互いに相異なっていてもよい炭素原子数がa~b個よりなる前記の意味であるアルキルによって置換されたアミノを表し、例えばジメチルアミノ、エチル(メチル)アミノ、ジエチルアミノ、ジ(n-プロピル)アミノ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
Notation "di (C a ~ C b alkyl) amino" are both hydrogen atoms, the alkyl being the meaning of a good number of carbon atoms be the same or different from each other, each consisting of a ~ b Pieces A substituted amino is represented, for example, dimethylamino, ethyl (methyl) amino, diethylamino, di (n-propyl) amino and the like are selected as specific examples, each selected in the range of the specified number of carbon atoms.
「Ca~Cbアルコキシアミノ」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルキル-ONH-を表し、例えばメトキシアミノ、エトキシアミノ、n-プロピルオキシアミノ、i-プロピルオキシアミノ、n-ブチルオキシアミノ、i-ブチルオキシアミノ、sec-ブチルオキシアミノ、tert-ブチルオキシアミノ等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
「トリ(Ca~Cbアルキル)シリル」の表記は、それぞれ同一でも又は互いに相異なっていてもよい炭素原子数がa~b個よりなる前記の意味であるアルキルによって置換されたシリルを表し、例えばトリメチルシリル、トリエチルシリル、トリ(n-プロピル)シリル、エチルジメチルシリル、n-プロピルジメチルシリル、n-ブチルジメチルシリル、i-ブチルジメチルシリル、tert-ブチルジメチルシリル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b alkoxyamino” represents alkyl-ONH— as defined above consisting of a to b carbon atoms, eg methoxyamino, ethoxyamino, n-propyloxyamino, i- Specific examples include propyloxyamino, n-butyloxyamino, i-butyloxyamino, sec-butyloxyamino, tert-butyloxyamino, and the like, each selected within the range of the designated number of carbon atoms.
The notation “tri (C a -C b alkyl) silyl” represents silyl substituted by alkyl having the above meaning consisting of a to b carbon atoms which may be the same or different from each other. Specific examples include trimethylsilyl, triethylsilyl, tri (n-propyl) silyl, ethyldimethylsilyl, n-propyldimethylsilyl, n-butyldimethylsilyl, i-butyldimethylsilyl, tert-butyldimethylsilyl, and the like. Each selected range of carbon atoms is selected.
「トリ(Ca~Cbアルキル)シリル」の表記は、それぞれ同一でも又は互いに相異なっていてもよい炭素原子数がa~b個よりなる前記の意味であるアルキルによって置換されたシリルを表し、例えばトリメチルシリル、トリエチルシリル、トリ(n-プロピル)シリル、エチルジメチルシリル、n-プロピルジメチルシリル、n-ブチルジメチルシリル、i-ブチルジメチルシリル、tert-ブチルジメチルシリル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b alkoxyamino” represents alkyl-ONH— as defined above consisting of a to b carbon atoms, eg methoxyamino, ethoxyamino, n-propyloxyamino, i- Specific examples include propyloxyamino, n-butyloxyamino, i-butyloxyamino, sec-butyloxyamino, tert-butyloxyamino, and the like, each selected within the range of the designated number of carbon atoms.
The notation “tri (C a -C b alkyl) silyl” represents silyl substituted by alkyl having the above meaning consisting of a to b carbon atoms which may be the same or different from each other. Specific examples include trimethylsilyl, triethylsilyl, tri (n-propyl) silyl, ethyldimethylsilyl, n-propyldimethylsilyl, n-butyldimethylsilyl, i-butyldimethylsilyl, tert-butyldimethylsilyl, and the like. Each selected range of carbon atoms is selected.
「Ca~Cbアルキルカルボニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルキル-C(O)-を表し、例えばアセチル、プロピオニル、ブチリル、イソブチリル、バレリル、イソバレリル、2-メチルブタノイル、ピバロイル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
「Ca~Cbシクロアルキルカルボニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるシクロアルキル-C(O)-を表し、例えばシクロプロピルカルボニル、シクロブチルカルボニル、1-メチルシクロプロピルカルボニル、2-メチルシクロプロピルカルボニル、シクロペンチルカルボニル、2,2-ジメチルシクロプロピルカルボニル、シクロヘキシルカルボニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b alkylcarbonyl” represents alkyl-C (O) — as defined above, comprising a to b carbon atoms, eg acetyl, propionyl, butyryl, isobutyryl, valeryl, isovaleryl Specific examples include 2-methylbutanoyl, pivaloyl and the like, each selected within the range of the specified number of carbon atoms.
The notation “C a -C b cycloalkylcarbonyl” represents cycloalkyl-C (O) — which has the above-mentioned meaning consisting of a to b carbon atoms, such as cyclopropylcarbonyl, cyclobutylcarbonyl, 1 Specific examples include -methylcyclopropylcarbonyl, 2-methylcyclopropylcarbonyl, cyclopentylcarbonyl, 2,2-dimethylcyclopropylcarbonyl, cyclohexylcarbonyl and the like, each selected within the range of the number of carbon atoms specified.
「Ca~Cbシクロアルキルカルボニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるシクロアルキル-C(O)-を表し、例えばシクロプロピルカルボニル、シクロブチルカルボニル、1-メチルシクロプロピルカルボニル、2-メチルシクロプロピルカルボニル、シクロペンチルカルボニル、2,2-ジメチルシクロプロピルカルボニル、シクロヘキシルカルボニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b alkylcarbonyl” represents alkyl-C (O) — as defined above, comprising a to b carbon atoms, eg acetyl, propionyl, butyryl, isobutyryl, valeryl, isovaleryl Specific examples include 2-methylbutanoyl, pivaloyl and the like, each selected within the range of the specified number of carbon atoms.
The notation “C a -C b cycloalkylcarbonyl” represents cycloalkyl-C (O) — which has the above-mentioned meaning consisting of a to b carbon atoms, such as cyclopropylcarbonyl, cyclobutylcarbonyl, 1 Specific examples include -methylcyclopropylcarbonyl, 2-methylcyclopropylcarbonyl, cyclopentylcarbonyl, 2,2-dimethylcyclopropylcarbonyl, cyclohexylcarbonyl and the like, each selected within the range of the number of carbon atoms specified.
「Ca~Cbアルコキシカルボニル」の表記は、炭素原子数がa~b個よりなる前記の意味であるアルキル-O-C(O)-を表し、例えばメトキシカルボニル、エトキシカルボニル、 n-プロピルオキシカルボニル、i-プロピルオキシカルボニル、n-ブトキシカルボニル、i-ブトキシカルボニル、tert-ブトキシカルボニル等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
The expression “C a -C b alkoxycarbonyl” represents alkyl-OC (O) — which has the above-mentioned meaning consisting of a to b carbon atoms, such as methoxycarbonyl, ethoxycarbonyl, n-propyloxycarbonyl. Specific examples include i-propyloxycarbonyl, n-butoxycarbonyl, i-butoxycarbonyl, tert-butoxycarbonyl and the like, each selected within the range of the designated number of carbon atoms.
「シアノ(Cd~Ce)アルキル」、「Ca~Cbアルコキシメチル」、「Ca~Cbアルコキシ(Cd~Ce)アルキル」、「Ca~Cbハロアルコキシ(Cd~Ce)アルキル」、「Ca~Cbアルキルチオ(Cd~Ce)アルキル」、「Ca~Cbアルキルスルフィニル(Cd~Ce)アルキル」、「Ca~Cbアルキルスルホニル(Cd~Ce)アルキル」、「Ca~Cbハロアルキルチオ(Cd~Ce)アルキル」、「Ca~Cbハロアルキルスルフィニル(Cd~Ce)アルキル」、「Ca~Cbハロアルキルスルホニル(Cd~Ce)アルキル」、「フェニル(Cd~Ce)アルキル」又は「(Z)mによって置換されたフェニル(Cd~Ce)アルキル」の表記は、それぞれ前記の意味である任意のシアノ、Ca~Cbアルコキシ、Ca~Cbハロアルコキシ、Ca~Cbアルキルチオ、Ca~Cbアルキルスルフィニル、Ca~Cbアルキルスルホニル、Ca~Cbハロアルキルチオ、Ca~Cbハロアルキルスルフィニル、Ca~Cbハロアルキルスルホニル、フェニル又は(Z)mによって置換されたフェニルによって、炭素原子に結合した水素原子が任意に置換されたメチル又は炭素原子数がd~e個よりなる前記の意味であるアルキルを表し、各々の指定の炭素原子数の範囲で選択される。
“Cyano (C d -C e ) alkyl”, “C a -C b alkoxymethyl”, “C a -C b alkoxy (C d -C e ) alkyl”, “C a -C b haloalkoxy (C d )” -C e ) alkyl "," C a -C b alkylthio (C d -C e ) alkyl "," C a -C b alkylsulfinyl (C d -C e ) alkyl "," C a -C b alkylsulfonyl " (C d -C e ) alkyl ”,“ C a -C b haloalkylthio (C d -C e ) alkyl ”,“ C a -C b haloalkylsulfinyl (C d -C e ) alkyl ”,“ C a- C b haloalkylsulfonyl (C d ~ C e) alkyl ", notation" phenyl (C d ~ C e) alkyl "or" (Z) phenyl (C d ~ C e) alkyl substituted by m ', respectively Any cyano as defined above, C a -C b alkoxy, C a -C b haloalkoxy, C a -C b alkylthio, C a ~ C b alkylsulfinyl, C a ~ C b alkylsulfonyl, C a ~ C b haloalkylthio, C a ~ C b haloalkylsulfinyl, C a ~ C b haloalkylsulfonyl, by phenyl or (Z) m A hydrogen atom bonded to a carbon atom by a substituted phenyl, optionally substituted methyl or alkyl as defined above comprising d to e carbon atoms, each in the range of the specified number of carbon atoms Selected.
「R8によって置換された(Ca~Cb)アルキル」の表記は、任意のR8によって、炭素原子に結合した水素原子が置換された炭素原子数がa~b個よりなる前記の意味であるアルキルを表し、各々の指定の炭素原子数の範囲で選択される。このとき、(Ca~Cb)アルキル上の置換基R8が2個以上存在するとき、それぞれのR8は互いに同一でも、または互いに相異なっていてもよい。
「R10によって任意に置換された(Ca~Cb)アルキル」の表記は、任意のR10によって、炭素原子に結合した水素原子が任意に置換された炭素原子数がa~b個よりなる前記の意味であるアルキルを表し、各々の指定の炭素原子数の範囲で選択される。このとき、それぞれの(Ca~Cb)アルキル上の置換基R10が2個以上存在するとき、それぞれのR10は互いに同一でも、または互いに相異なっていてもよい。 Notation "substituted by R 8 (C a ~ C b ) alkyl", by any R 8, meaning the number of carbon atoms in which the hydrogen atoms bonded to carbon atoms are substituted is made of a ~ b Pieces Each selected from the range of each designated number of carbon atoms. At this time, when two or more substituents R 8 on (C a -C b ) alkyl are present, each R 8 may be the same as or different from each other.
The notation “(C a -C b ) alkyl optionally substituted with R 10 ” refers to the number of carbon atoms in which a hydrogen atom bonded to a carbon atom is optionally substituted with any R 10 from a to b. Each of which is selected within the range of the specified number of carbon atoms. At this time, when there are two or more substituents R 10 on each (C a -C b ) alkyl, each R 10 may be the same as or different from each other.
「R10によって任意に置換された(Ca~Cb)アルキル」の表記は、任意のR10によって、炭素原子に結合した水素原子が任意に置換された炭素原子数がa~b個よりなる前記の意味であるアルキルを表し、各々の指定の炭素原子数の範囲で選択される。このとき、それぞれの(Ca~Cb)アルキル上の置換基R10が2個以上存在するとき、それぞれのR10は互いに同一でも、または互いに相異なっていてもよい。 Notation "substituted by R 8 (C a ~ C b ) alkyl", by any R 8, meaning the number of carbon atoms in which the hydrogen atoms bonded to carbon atoms are substituted is made of a ~ b Pieces Each selected from the range of each designated number of carbon atoms. At this time, when two or more substituents R 8 on (C a -C b ) alkyl are present, each R 8 may be the same as or different from each other.
The notation “(C a -C b ) alkyl optionally substituted with R 10 ” refers to the number of carbon atoms in which a hydrogen atom bonded to a carbon atom is optionally substituted with any R 10 from a to b. Each of which is selected within the range of the specified number of carbon atoms. At this time, when there are two or more substituents R 10 on each (C a -C b ) alkyl, each R 10 may be the same as or different from each other.
「Ca~Cbシクロアルキル(Cd~Ce)シクロアルキル、ヒドロキシ(Cd~Ce)シクロアルキル」、「Ca~Cbアルコキシ(Cd~Ce)シクロアルキル」又はフェニル(Cd~Ce)シクロアルキルの表記は、それぞれ前記の意味である任意のCa~Cbシクロアルキル、水酸基、前記の意味である任意のCa~Cbアルコキシ又はフェニルによって、炭素原子に結合した水素原子が置換された炭素原子数がd~e個よりなる前記の意味であるシクロアルキルを表し、各々の指定の炭素原子数の範囲で選択される。
「Ca~Cbアルキリデン」の表記は、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状で、二重結合によって結合した炭化水素を表し、例えばメチリデン、エチリデン、プロピリデン、1-メチルエチリデン、ブチリデン、1-メチルプロピリデン、ペンチリデン、1-メチルブチリデン、1-エチルエチリデン、ヘキシリデン等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 “C a -C b cycloalkyl (C d -C e ) cycloalkyl, hydroxy (C d -C e ) cycloalkyl”, “C a -C b alkoxy (C d -C e ) cycloalkyl” or phenyl ( C d -C e ) cycloalkyl is represented by any C a -C b cycloalkyl, hydroxyl group, any C a -C b alkoxy, or phenyl, as defined above, to a carbon atom, respectively. The cycloalkyl having the above-mentioned meaning consisting of d to e carbon atoms substituted with bonded hydrogen atoms is represented and selected in the range of each designated number of carbon atoms.
The expression “C a -C b alkylidene” represents a straight or branched hydrocarbon having a carbon number of a to b and bonded by a double bond, such as methylidene, ethylidene, propylidene, 1 Specific examples include -methylethylidene, butylidene, 1-methylpropylidene, pentylidene, 1-methylbutylidene, 1-ethylethylidene, hexylidene, and the like, and each is selected within the range of the designated number of carbon atoms.
「Ca~Cbアルキリデン」の表記は、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状で、二重結合によって結合した炭化水素を表し、例えばメチリデン、エチリデン、プロピリデン、1-メチルエチリデン、ブチリデン、1-メチルプロピリデン、ペンチリデン、1-メチルブチリデン、1-エチルエチリデン、ヘキシリデン等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。 “C a -C b cycloalkyl (C d -C e ) cycloalkyl, hydroxy (C d -C e ) cycloalkyl”, “C a -C b alkoxy (C d -C e ) cycloalkyl” or phenyl ( C d -C e ) cycloalkyl is represented by any C a -C b cycloalkyl, hydroxyl group, any C a -C b alkoxy, or phenyl, as defined above, to a carbon atom, respectively. The cycloalkyl having the above-mentioned meaning consisting of d to e carbon atoms substituted with bonded hydrogen atoms is represented and selected in the range of each designated number of carbon atoms.
The expression “C a -C b alkylidene” represents a straight or branched hydrocarbon having a carbon number of a to b and bonded by a double bond, such as methylidene, ethylidene, propylidene, 1 Specific examples include -methylethylidene, butylidene, 1-methylpropylidene, pentylidene, 1-methylbutylidene, 1-ethylethylidene, hexylidene, and the like, and each is selected within the range of the designated number of carbon atoms.
「Ca~Cbハロアルキリデン」の表記は、炭素原子に結合した水素原子が、ハロゲン原子によって任意に置換された、炭素原子数がa~b個よりなる直鎖状又は分岐鎖状で、二重結合によって結合した炭化水素を表す。このとき、2個以上のハロゲン原子によって置換されている場合、それらのハロゲン原子は互いに同一でも、または互いに相異なっていてもよい。例えばフルオロメチリデン、クロロメチリデン、ジフルオロメチリデン、ジクロロメチリデン、2,2,2-トリフルオロエチリデン等が具体例として挙げられ、各々の指定の炭素原子数の範囲で選択される。
「Ca~Cbアルコキシ(Cd~Ce)アルキリデン」の表記は、それぞれ前記の意味である任意のCa~Cbアルコキシによって、炭素原子に結合した水素原子が置換された炭素原子数がd~e個よりなる前記の意味であるアルキリデンを表し、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b haloalkylidene” is a linear or branched chain comprising a to b carbon atoms, in which a hydrogen atom bonded to a carbon atom is optionally substituted with a halogen atom, Represents a hydrocarbon bonded by a double bond. At this time, when substituted with two or more halogen atoms, the halogen atoms may be the same as or different from each other. Specific examples include, for example, fluoromethylidene, chloromethylidene, difluoromethylidene, dichloromethylidene, 2,2,2-trifluoroethylidene, and the like, and each is selected within the range of the designated number of carbon atoms.
The notation “C a -C b alkoxy (C d -C e ) alkylidene” is the number of carbon atoms in which a hydrogen atom bonded to a carbon atom is substituted by any C a -C b alkoxy having the above-mentioned meaning, respectively. Represents an alkylidene having the above meaning consisting of d to e, and is selected within each specified number of carbon atoms.
「Ca~Cbアルコキシ(Cd~Ce)アルキリデン」の表記は、それぞれ前記の意味である任意のCa~Cbアルコキシによって、炭素原子に結合した水素原子が置換された炭素原子数がd~e個よりなる前記の意味であるアルキリデンを表し、各々の指定の炭素原子数の範囲で選択される。 The notation “C a -C b haloalkylidene” is a linear or branched chain comprising a to b carbon atoms, in which a hydrogen atom bonded to a carbon atom is optionally substituted with a halogen atom, Represents a hydrocarbon bonded by a double bond. At this time, when substituted with two or more halogen atoms, the halogen atoms may be the same as or different from each other. Specific examples include, for example, fluoromethylidene, chloromethylidene, difluoromethylidene, dichloromethylidene, 2,2,2-trifluoroethylidene, and the like, and each is selected within the range of the designated number of carbon atoms.
The notation “C a -C b alkoxy (C d -C e ) alkylidene” is the number of carbon atoms in which a hydrogen atom bonded to a carbon atom is substituted by any C a -C b alkoxy having the above-mentioned meaning, respectively. Represents an alkylidene having the above meaning consisting of d to e, and is selected within each specified number of carbon atoms.
「シアノ(Ca~Cb)アルコキシ」、「フェニル(Ca~Cb)アルコキシ」又は「(Z)mによって置換されたフェニル(Ca~Cb)アルコキシ」の表記は、シアノ、フェニル又は(Z)mによって置換されたフェニルによって、炭素原子に結合した水素原子が置換された炭素原子数がa~b個よりなる前記の意味であるアルコキシを表し、各々の指定の炭素原子数の範囲で選択される。
「R1とR2とは一緒になってC2~C5アルキレン鎖を形成し、ここでR1及びR2が結合する炭素原子と共に3~6員環を形成してもよく、このとき前記アルキレン鎖は酸素原子、硫黄原子又は窒素原子を1~2個含んでもよく、」の表記の具体例として、例えばシクロプロパン、オキシラン、チイラン、アジリジン、シクロブタン、オキセタン、チエタン、アゼチジン、シクロペンタン、オキソラン、チオラン、ピロリジン、ジオキソラン、ジチオラン、シクロヘキサン、テトラヒドロピラン、テトラヒドロチオピラン、ピペリジン、1,3-ジオキサン、1,3-ジチアン等の環が挙げられ、各々の指定の原子数の範囲で選択される。 The expressions “cyano (C a -C b ) alkoxy”, “phenyl (C a -C b ) alkoxy” or “(Z) m- substituted phenyl (C a -C b ) alkoxy” are cyano, phenyl Or (Z) represents an alkoxy having the above meaning consisting of a to b carbon atoms in which a hydrogen atom bonded to a carbon atom is substituted by phenyl substituted by m , each of the designated number of carbon atoms Selected by range.
“R 1 and R 2 together form a C 2 -C 5 alkylene chain, where a 3- to 6-membered ring may be formed with the carbon atom to which R 1 and R 2 are attached, The alkylene chain may contain 1 to 2 oxygen atoms, sulfur atoms or nitrogen atoms, and examples thereof include, for example, cyclopropane, oxirane, thiirane, aziridine, cyclobutane, oxetane, thietane, azetidine, cyclopentane, Rings such as oxolane, thiolane, pyrrolidine, dioxolane, dithiolane, cyclohexane, tetrahydropyran, tetrahydrothiopyran, piperidine, 1,3-dioxane, 1,3-dithiane, and the like, each selected within the specified number of atoms The
「R1とR2とは一緒になってC2~C5アルキレン鎖を形成し、ここでR1及びR2が結合する炭素原子と共に3~6員環を形成してもよく、このとき前記アルキレン鎖は酸素原子、硫黄原子又は窒素原子を1~2個含んでもよく、」の表記の具体例として、例えばシクロプロパン、オキシラン、チイラン、アジリジン、シクロブタン、オキセタン、チエタン、アゼチジン、シクロペンタン、オキソラン、チオラン、ピロリジン、ジオキソラン、ジチオラン、シクロヘキサン、テトラヒドロピラン、テトラヒドロチオピラン、ピペリジン、1,3-ジオキサン、1,3-ジチアン等の環が挙げられ、各々の指定の原子数の範囲で選択される。 The expressions “cyano (C a -C b ) alkoxy”, “phenyl (C a -C b ) alkoxy” or “(Z) m- substituted phenyl (C a -C b ) alkoxy” are cyano, phenyl Or (Z) represents an alkoxy having the above meaning consisting of a to b carbon atoms in which a hydrogen atom bonded to a carbon atom is substituted by phenyl substituted by m , each of the designated number of carbon atoms Selected by range.
“R 1 and R 2 together form a C 2 -C 5 alkylene chain, where a 3- to 6-membered ring may be formed with the carbon atom to which R 1 and R 2 are attached, The alkylene chain may contain 1 to 2 oxygen atoms, sulfur atoms or nitrogen atoms, and examples thereof include, for example, cyclopropane, oxirane, thiirane, aziridine, cyclobutane, oxetane, thietane, azetidine, cyclopentane, Rings such as oxolane, thiolane, pyrrolidine, dioxolane, dithiolane, cyclohexane, tetrahydropyran, tetrahydrothiopyran, piperidine, 1,3-dioxane, 1,3-dithiane, and the like, each selected within the specified number of atoms The
「R17とR18とが一緒になってC2~C5アルキレン鎖を形成することにより、R17及びR18が結合する窒素原子と共に3~6員環を形成してもよく、このとき前記アルキレン鎖は酸素原子又は硫黄原子を1個含んでもよく、」の表記の具体例としては、例えばアジリジン、アゼチジン、ピロリジン、オキサゾリジン、チアゾリジン、ピペリジン、2H-3,4,5,6-テトラヒドロ-1,3-オキサジン、モルホリン、2H-3,4,5,6-テトラヒドロ-1,3-チアジン、チオモルホリン等の環が挙げられ、各々の指定の原子数の範囲で選択される。
“R 17 and R 18 together form a C 2 -C 5 alkylene chain to form a 3- to 6-membered ring with the nitrogen atom to which R 17 and R 18 are bonded. The alkylene chain may contain one oxygen atom or sulfur atom. Specific examples of the notation include, for example, aziridine, azetidine, pyrrolidine, oxazolidine, thiazolidine, piperidine, 2H-3,4,5,6-tetrahydro- Examples include rings such as 1,3-oxazine, morpholine, 2H-3,4,5,6-tetrahydro-1,3-thiazine, thiomorpholine, and the like, and each is selected within the range of the specified number of atoms.
本発明において、G1で表される置換基の好ましい範囲として、例えば下記のG1-I~G1-XVIIIの各群が挙げられる。
すなわち、G1-I:G1-1[ここで、X1はハロゲン原子、ニトロ、メチル、トリフルオロメチル又はメチルスルホニルを表し、X2は水素原子を表す。]。
G1-II:G1-1[ここで、X1及びX2はフッ素原子を表す。]。
G1-III:G1-2及びG1-3[ここで、X1はハロゲン原子、メチル、ジフルオロメチル、トリフルオロメチル、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表す。]。 In the present invention, a preferred range of the substituent of G 1, for example, include G 1 -I ~ each group G 1 -xviii below.
That is, G 1 -I: G 1 -1 [where X 1 represents a halogen atom, nitro, methyl, trifluoromethyl, or methylsulfonyl, and X 2 represents a hydrogen atom. ].
G 1 -II: G 1 -1 [where X 1 and X 2 represent a fluorine atom. ].
G 1 -III: G 1 -2 and G 1 -3 [wherein X 1 represents a halogen atom, methyl, difluoromethyl, trifluoromethyl, methylthio, methylsulfonyl or difluoromethylsulfonyl. ].
すなわち、G1-I:G1-1[ここで、X1はハロゲン原子、ニトロ、メチル、トリフルオロメチル又はメチルスルホニルを表し、X2は水素原子を表す。]。
G1-II:G1-1[ここで、X1及びX2はフッ素原子を表す。]。
G1-III:G1-2及びG1-3[ここで、X1はハロゲン原子、メチル、ジフルオロメチル、トリフルオロメチル、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表す。]。 In the present invention, a preferred range of the substituent of G 1, for example, include G 1 -I ~ each group G 1 -xviii below.
That is, G 1 -I: G 1 -1 [where X 1 represents a halogen atom, nitro, methyl, trifluoromethyl, or methylsulfonyl, and X 2 represents a hydrogen atom. ].
G 1 -II: G 1 -1 [where X 1 and X 2 represent a fluorine atom. ].
G 1 -III: G 1 -2 and G 1 -3 [wherein X 1 represents a halogen atom, methyl, difluoromethyl, trifluoromethyl, methylthio, methylsulfonyl or difluoromethylsulfonyl. ].
G1-IV:G1-4[ここで、X1はハロゲン原子又はトリフルオロメチルを表す。]。
G1-V:G1-7及びG1-8[ここで、X1はハロゲン原子、メチル又はトリフルオロメチルを表す。]。
G1-VI:G1-10[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X2は水素原子を表し、R7はメチルを表す。]。
G1-VII:G1-14[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X3はメチルを表す。]。
G1-VIII:G1-1[ここで、X1はハロゲン原子又はトリフルオロメチルを表し、X2はフッ素原子又は塩素原子を表す。]。
G1-IX:G1-2及びG1-3[ここで、X1はメトキシ又はトリフルオロメチルスルホニルを表す。]。 G 1 -IV: G 1 -4 [where X 1 represents a halogen atom or trifluoromethyl. ].
G 1 -V: G 1 -7 and G 1 -8 [wherein X 1 represents a halogen atom, methyl or trifluoromethyl. ].
G 1 -VI: G 1 -10 [where X 1 represents difluoromethyl or trifluoromethyl, X 2 represents a hydrogen atom, and R 7 represents methyl. ].
G 1 -VII: G 1 -14 [wherein X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents methyl. ].
G 1 -VIII: G 1 -1 [where X 1 represents a halogen atom or trifluoromethyl, and X 2 represents a fluorine atom or a chlorine atom. ].
G 1 -IX: G 1 -2 and G 1 -3 [wherein X 1 represents methoxy or trifluoromethylsulfonyl. ].
G1-V:G1-7及びG1-8[ここで、X1はハロゲン原子、メチル又はトリフルオロメチルを表す。]。
G1-VI:G1-10[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X2は水素原子を表し、R7はメチルを表す。]。
G1-VII:G1-14[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X3はメチルを表す。]。
G1-VIII:G1-1[ここで、X1はハロゲン原子又はトリフルオロメチルを表し、X2はフッ素原子又は塩素原子を表す。]。
G1-IX:G1-2及びG1-3[ここで、X1はメトキシ又はトリフルオロメチルスルホニルを表す。]。 G 1 -IV: G 1 -4 [where X 1 represents a halogen atom or trifluoromethyl. ].
G 1 -V: G 1 -7 and G 1 -8 [wherein X 1 represents a halogen atom, methyl or trifluoromethyl. ].
G 1 -VI: G 1 -10 [where X 1 represents difluoromethyl or trifluoromethyl, X 2 represents a hydrogen atom, and R 7 represents methyl. ].
G 1 -VII: G 1 -14 [wherein X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents methyl. ].
G 1 -VIII: G 1 -1 [where X 1 represents a halogen atom or trifluoromethyl, and X 2 represents a fluorine atom or a chlorine atom. ].
G 1 -IX: G 1 -2 and G 1 -3 [wherein X 1 represents methoxy or trifluoromethylsulfonyl. ].
G1-X:G1-4[ここで、X1はメチル又はジフルオロメチルを表す。]。
G1-XI:G1-5及びG1-6[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X3は水素原子又はメチルを表す。]。
G1-XII:G1-7及びG1-8[ここで、X1はジフルオロメチルを表す。]。
G1-XIII:G1-9[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、R7はメチルを表す。]。 G 1 -X: G 1 -4 [where X 1 represents methyl or difluoromethyl. ].
G 1 -XI: G 1 -5 and G 1 -6 [wherein X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents a hydrogen atom or methyl. ].
G 1 -XII: G 1 -7 and G 1 -8 [where X 1 represents difluoromethyl. ].
G 1 -XIII: G 1 -9 [wherein X 1 represents difluoromethyl or trifluoromethyl, and R 7 represents methyl. ].
G1-XI:G1-5及びG1-6[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X3は水素原子又はメチルを表す。]。
G1-XII:G1-7及びG1-8[ここで、X1はジフルオロメチルを表す。]。
G1-XIII:G1-9[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、R7はメチルを表す。]。 G 1 -X: G 1 -4 [where X 1 represents methyl or difluoromethyl. ].
G 1 -XI: G 1 -5 and G 1 -6 [wherein X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents a hydrogen atom or methyl. ].
G 1 -XII: G 1 -7 and G 1 -8 [where X 1 represents difluoromethyl. ].
G 1 -XIII: G 1 -9 [wherein X 1 represents difluoromethyl or trifluoromethyl, and R 7 represents methyl. ].
G1-XIV:G1-10[ここで、X1はメチル、エチル又はトリフルオロメチルを表し、X2はフッ素原子又は塩素原子を表し、R7はメチルを表す。]。
G1-XV:G1-11及びG1-12[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X3は水素原子又はメチルを表す。]。
G1-XVI:G1-13[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X3はメチルを表す。]。
G1-XVII:G1-15[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、R7はメチルを表す。]。
G1-XVIII:G1-16[ここで、X1はメチル又はトリフルオロメチルを表し、rは0、1又は2を表す。]。 G 1 -XIV: G 1 -10 [where X 1 represents methyl, ethyl or trifluoromethyl, X 2 represents a fluorine atom or a chlorine atom, and R 7 represents methyl. ].
G 1 -XV: G 1 -11 and G 1 -12 [where X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents a hydrogen atom or methyl. ].
G 1 -XVI: G 1 -13 [where X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents methyl. ].
G 1 -XVII: G 1 -15 [where X 1 represents difluoromethyl or trifluoromethyl, and R 7 represents methyl. ].
G 1 -XVIII: G 1 -16 [wherein X 1 represents methyl or trifluoromethyl, and r represents 0, 1 or 2. ].
G1-XV:G1-11及びG1-12[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X3は水素原子又はメチルを表す。]。
G1-XVI:G1-13[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、X3はメチルを表す。]。
G1-XVII:G1-15[ここで、X1はジフルオロメチル又はトリフルオロメチルを表し、R7はメチルを表す。]。
G1-XVIII:G1-16[ここで、X1はメチル又はトリフルオロメチルを表し、rは0、1又は2を表す。]。 G 1 -XIV: G 1 -10 [where X 1 represents methyl, ethyl or trifluoromethyl, X 2 represents a fluorine atom or a chlorine atom, and R 7 represents methyl. ].
G 1 -XV: G 1 -11 and G 1 -12 [where X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents a hydrogen atom or methyl. ].
G 1 -XVI: G 1 -13 [where X 1 represents difluoromethyl or trifluoromethyl, and X 3 represents methyl. ].
G 1 -XVII: G 1 -15 [where X 1 represents difluoromethyl or trifluoromethyl, and R 7 represents methyl. ].
G 1 -XVIII: G 1 -16 [wherein X 1 represents methyl or trifluoromethyl, and r represents 0, 1 or 2. ].
これらのうち、G1で表される置換基の範囲としてはG1-I~G1-VII、G1-XI、G1-XIII、G1-XVII又はG1-XVIIIがより好ましく、さらに、G1-I~G1-VIIが特に好ましい。
本発明において、Y1、Y2及びY3で表される置換基の好ましい範囲の組み合わせとして、例えば下記Y-I~Y-IIIの各群が挙げられる。
すなわち、Y-I:Y1がハロゲン原子、且つY2及びY3が同時に水素原子。
Y-II:Y1がメチル、トリフルオロメチル又はメトキシ、且つY2及びY3が同時に水素原子。 Of these, G 1 -I ~ G 1 -VII as range of the substituent of G 1, G 1 -XI, G 1 -XIII, more preferably G 1 -XVII or G 1 -xviii, further G 1 -I to G 1 -VII are particularly preferred.
In the present invention, examples of combinations of preferable ranges of substituents represented by Y 1 , Y 2 and Y 3 include the following groups YI to Y-III.
That, YI: Y 1 is a halogen atom, and Y 2 and Y 3 are simultaneously hydrogen atom.
Y-II: Y 1 is methyl, trifluoromethyl or methoxy, and Y 2 and Y 3 are simultaneously hydrogen atoms.
本発明において、Y1、Y2及びY3で表される置換基の好ましい範囲の組み合わせとして、例えば下記Y-I~Y-IIIの各群が挙げられる。
すなわち、Y-I:Y1がハロゲン原子、且つY2及びY3が同時に水素原子。
Y-II:Y1がメチル、トリフルオロメチル又はメトキシ、且つY2及びY3が同時に水素原子。 Of these, G 1 -I ~ G 1 -VII as range of the substituent of G 1, G 1 -XI, G 1 -XIII, more preferably G 1 -XVII or G 1 -xviii, further G 1 -I to G 1 -VII are particularly preferred.
In the present invention, examples of combinations of preferable ranges of substituents represented by Y 1 , Y 2 and Y 3 include the following groups YI to Y-III.
That, YI: Y 1 is a halogen atom, and Y 2 and Y 3 are simultaneously hydrogen atom.
Y-II: Y 1 is methyl, trifluoromethyl or methoxy, and Y 2 and Y 3 are simultaneously hydrogen atoms.
Y-III:Y1がハロゲン原子又はメチル、Y2がメチル、且つY3が水素原子。
これらのうち、Y1、Y2及びY3で表される置換基の組み合わせとしてはY-I及びY-IIがより好ましく、さらに、Y-Iが特に好ましい。
本発明において、R1、R2、R3及びR4で表される置換基の好ましい範囲の組み合わせとして、例えば下記R-I~R-XIVの各群が挙げられる。
すなわち、R-I:R1及びR2が水素原子、R3が水素原子又はメチル、且つR4が水素原子。 Y-III: Y 1 is a halogen atom or methyl, Y 2 is methyl, and Y 3 is a hydrogen atom.
Among these, YI and Y-II are more preferable as a combination of substituents represented by Y 1 , Y 2 and Y 3 , and YI is particularly preferable.
In the present invention, examples of combinations of preferred ranges of substituents represented by R 1 , R 2 , R 3 and R 4 include the following groups of RI to R-XIV.
That is, RI: R 1 and R 2 are hydrogen atoms, R 3 is a hydrogen atom or methyl, and R 4 is a hydrogen atom.
これらのうち、Y1、Y2及びY3で表される置換基の組み合わせとしてはY-I及びY-IIがより好ましく、さらに、Y-Iが特に好ましい。
本発明において、R1、R2、R3及びR4で表される置換基の好ましい範囲の組み合わせとして、例えば下記R-I~R-XIVの各群が挙げられる。
すなわち、R-I:R1及びR2が水素原子、R3が水素原子又はメチル、且つR4が水素原子。 Y-III: Y 1 is a halogen atom or methyl, Y 2 is methyl, and Y 3 is a hydrogen atom.
Among these, YI and Y-II are more preferable as a combination of substituents represented by Y 1 , Y 2 and Y 3 , and YI is particularly preferable.
In the present invention, examples of combinations of preferred ranges of substituents represented by R 1 , R 2 , R 3 and R 4 include the following groups of RI to R-XIV.
That is, RI: R 1 and R 2 are hydrogen atoms, R 3 is a hydrogen atom or methyl, and R 4 is a hydrogen atom.
R-II:R1が(Z)mによって置換されたフェニルメチル[ここで、Zはハロゲン原子を表し、mは1又は2を表し、mが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよい。]及びシクロプロピル、且つR2、R3及びR4が水素原子。
R-III:R1がフッ素原子、メチル、メトキシ、エトキシ、トリフルオロエトキシ、アリルオキシ、プロパルギルオキシ、ベンジルオキシ又はメチルチオ、R2が水素原子、R3が水素原子又はメチル、且つR4が水素原子。
R-IV:R1及びR2が各々独立してフッ素原子又はメチル、且つR3及びR4が水素原子。
R-V:R1がフッ素原子又はメチル、R2がフッ素原子、R3がメチル又はエチル、且つR4が水素原子又はメチル。 R-II: phenylmethyl wherein R 1 is substituted by (Z) m [wherein Z represents a halogen atom, m represents 1 or 2, and when m represents 2, each Z is They may be the same or different from each other. ] And cyclopropyl, and R 2 , R 3 and R 4 are hydrogen atoms.
R-III: R 1 is a fluorine atom, methyl, methoxy, ethoxy, trifluoroethoxy, allyloxy, propargyloxy, benzyloxy or methylthio, R 2 is a hydrogen atom, R 3 is a hydrogen atom or methyl, and R 4 is a hydrogen atom .
R-IV: R 1 and R 2 are each independently a fluorine atom or methyl, and R 3 and R 4 are hydrogen atoms.
RV: R 1 is a fluorine atom or methyl, R 2 is a fluorine atom, R 3 is methyl or ethyl, and R 4 is a hydrogen atom or methyl.
R-III:R1がフッ素原子、メチル、メトキシ、エトキシ、トリフルオロエトキシ、アリルオキシ、プロパルギルオキシ、ベンジルオキシ又はメチルチオ、R2が水素原子、R3が水素原子又はメチル、且つR4が水素原子。
R-IV:R1及びR2が各々独立してフッ素原子又はメチル、且つR3及びR4が水素原子。
R-V:R1がフッ素原子又はメチル、R2がフッ素原子、R3がメチル又はエチル、且つR4が水素原子又はメチル。 R-II: phenylmethyl wherein R 1 is substituted by (Z) m [wherein Z represents a halogen atom, m represents 1 or 2, and when m represents 2, each Z is They may be the same or different from each other. ] And cyclopropyl, and R 2 , R 3 and R 4 are hydrogen atoms.
R-III: R 1 is a fluorine atom, methyl, methoxy, ethoxy, trifluoroethoxy, allyloxy, propargyloxy, benzyloxy or methylthio, R 2 is a hydrogen atom, R 3 is a hydrogen atom or methyl, and R 4 is a hydrogen atom .
R-IV: R 1 and R 2 are each independently a fluorine atom or methyl, and R 3 and R 4 are hydrogen atoms.
RV: R 1 is a fluorine atom or methyl, R 2 is a fluorine atom, R 3 is methyl or ethyl, and R 4 is a hydrogen atom or methyl.
R-VI:R1とR2とが一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成し、且つR3及びR4が水素原子。
R-VII:R1とR2とが一緒になってC1~C2アルキリデン又はC1~C2ハロアルキリデンを形成し、且つR3及びR4が水素原子。
R-VIII:R1及びR2が水素原子、R3がC1~C3アルキル又はC1~C3ハロアルキル、且つR4が水素原子又はC1~C3アルキル。
R-IX:R1及びR2が水素原子、且つR3及びR4がメチル又はR3及びR4が一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成。 R-VI: R 1 and R 2 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded, and R 3 and R 4 are hydrogen atoms. .
R-VII: R 1 and R 2 together form C 1 -C 2 alkylidene or C 1 -C 2 haloalkylidene, and R 3 and R 4 are hydrogen atoms.
R-VIII: R 1 and R 2 are hydrogen atoms, R 3 is C 1 -C 3 alkyl or C 1 -C 3 haloalkyl, and R 4 is hydrogen atom or C 1 -C 3 alkyl.
R-IX: a carbon atom to which R 3 and R 4 are bonded by R 1 and R 2 being a hydrogen atom and R 3 and R 4 being methyl or R 3 and R 4 being combined to form an ethylene chain Together with the cyclopropyl ring.
R-VII:R1とR2とが一緒になってC1~C2アルキリデン又はC1~C2ハロアルキリデンを形成し、且つR3及びR4が水素原子。
R-VIII:R1及びR2が水素原子、R3がC1~C3アルキル又はC1~C3ハロアルキル、且つR4が水素原子又はC1~C3アルキル。
R-IX:R1及びR2が水素原子、且つR3及びR4がメチル又はR3及びR4が一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成。 R-VI: R 1 and R 2 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded, and R 3 and R 4 are hydrogen atoms. .
R-VII: R 1 and R 2 together form C 1 -C 2 alkylidene or C 1 -C 2 haloalkylidene, and R 3 and R 4 are hydrogen atoms.
R-VIII: R 1 and R 2 are hydrogen atoms, R 3 is C 1 -C 3 alkyl or C 1 -C 3 haloalkyl, and R 4 is hydrogen atom or C 1 -C 3 alkyl.
R-IX: a carbon atom to which R 3 and R 4 are bonded by R 1 and R 2 being a hydrogen atom and R 3 and R 4 being methyl or R 3 and R 4 being combined to form an ethylene chain Together with the cyclopropyl ring.
R-X:R1がC1~C3アルキル、C1~C3ハロアルキル、ベンジル又は (Z)mによって置換されたフェニルメチル[ここで、Zはハロゲン原子、シアノ、ニトロ、メチル、トリフルオロメチル、メトキシ、ジフルオロメトキシ、トリフルオロメトキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、ジフルオロメチルチオ、ジフルオロメチルスルホニル、トリフルオロメチルチオ又はトリフルオロメチルスルホニルを表し、mは1又は2を表し、mが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよい。]、且つR2、R3及びR4が水素原子。
RX: R 1 is C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, benzyl or phenylmethyl substituted by (Z) m [where Z is a halogen atom, cyano, nitro, methyl, trifluoromethyl, Methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio or trifluoromethylsulfonyl; Each Z may be the same as or different from each other. ], And R 2, R 3 and R 4 are hydrogen atoms.
R-XI:R1がC1~C3アルコキシ、C1~C3ハロアルコキシ、C3~C4アルケニルオキシ、C3~C4アルキニルオキシ、シアノメトキシ、ベンジルオキシ、(Z)mによって置換されたフェニルメトキシ[ここで、Zはハロゲン原子、シアノ、ニトロ、メチル、トリフルオロメチル、メトキシ、ジフルオロメトキシ、トリフルオロメトキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、ジフルオロメチルチオ、ジフルオロメチルスルホニル、トリフルオロメチルチオ又はトリフルオロメチルスルホニルを表し、mは1又は2を表し、mが2を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよい。]、C1~C3アルキルチオ又はC1~C3ハロアルキルチオ、R2が水素原子、R3が水素原子又はメチル、且つR4が水素原子。
R-XI: R 1 is replaced by C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 3 -C 4 alkenyloxy, C 3 -C 4 alkynyloxy, cyanomethoxy, benzyloxy, (Z) m [Wherein Z is a halogen atom, cyano, nitro, methyl, trifluoromethyl, methoxy, difluoromethoxy, trifluoromethoxy, methylthio, methylsulfinyl, methylsulfonyl, difluoromethylthio, difluoromethylsulfonyl, trifluoromethylthio] Alternatively, it represents trifluoromethylsulfonyl, m represents 1 or 2, and when m represents 2, each Z may be the same as or different from each other. ], C 1 -C 3 alkylthio or C 1 -C 3 haloalkylthio, R 2 is a hydrogen atom, R 3 is a hydrogen atom or methyl, and R 4 is a hydrogen atom.
R-XII:R1がC1~C3アルキル、R2が水素原子、R3がメチル、且つR4が水素原子。
R-XIII:R1がC1~C3アルキル、R2がフッ素原子又はメチル、且つR3及びR4が水素原子。
R-XIV:R1がC1~C3アルキル、R2がフッ素原子又はメチル、R3がメチル、且つR4が水素原子。 R-XII: R 1 is C 1 -C 3 alkyl, R 2 is a hydrogen atom, R 3 is methyl, and R 4 is a hydrogen atom.
R-XIII: R 1 is C 1 -C 3 alkyl, R 2 is a fluorine atom or methyl, and R 3 and R 4 are hydrogen atoms.
R-XIV: R 1 is C 1 -C 3 alkyl, R 2 is a fluorine atom or methyl, R 3 is methyl, and R 4 is a hydrogen atom.
R-XIII:R1がC1~C3アルキル、R2がフッ素原子又はメチル、且つR3及びR4が水素原子。
R-XIV:R1がC1~C3アルキル、R2がフッ素原子又はメチル、R3がメチル、且つR4が水素原子。 R-XII: R 1 is C 1 -C 3 alkyl, R 2 is a hydrogen atom, R 3 is methyl, and R 4 is a hydrogen atom.
R-XIII: R 1 is C 1 -C 3 alkyl, R 2 is a fluorine atom or methyl, and R 3 and R 4 are hydrogen atoms.
R-XIV: R 1 is C 1 -C 3 alkyl, R 2 is a fluorine atom or methyl, R 3 is methyl, and R 4 is a hydrogen atom.
これらのうち、R1、R2、R3及びR4で表される置換基の範囲の組み合わせとしてとしてはR-I~R-VII、R-VIII及びR-XIがより好ましく、さらに、R-I、R-III~R-V、R-VI及びR-VIIが特に好ましい。
Among these, as combinations of the ranges of substituents represented by R 1 , R 2 , R 3 and R 4 , RI to R-VII, R-VIII and R-XI are more preferable, and further, RI, R -III to RV, R-VI and R-VII are particularly preferred.
本発明に包含される化合物において、R5で表される置換基の好ましい範囲として、例えば下記のR5-I~R5-XIの各群が挙げられる。
すなわち、R5-I:水素原子。
R5-II:C1~C4アルキル。
R5-III:R8によって置換された(C1~C2)アルキル[ここで、R8はシアノ、C3~C6シクロアルキル又は-OR14を表し、R14はC1~C4アルキルを表す。]。
R5-IV:シクロプロピル。
R5-V:C2~C4アルケニル又はC3~C4アルキニル。
R5-VI:C1~C4ハロアルキルチオ。
R5-VII:-C(O)R9[ここで、R9はC1~C4アルキルを表す。]又はC1~C4アルコキシカルボニル。 In the compounds included in the present invention, examples of the preferred range of the substituent represented by R 5 include the following groups of R 5 -I to R 5 -XI.
That is, R 5 -I: a hydrogen atom.
R 5 -II: C 1 -C 4 alkyl.
R 5 -III: (C 1 -C 2 ) alkyl substituted by R 8 [wherein R 8 represents cyano, C 3 -C 6 cycloalkyl or —OR 14 and R 14 represents C 1 -C 4 Represents alkyl. ].
R 5 -IV: cyclopropyl.
R 5 -V: C 2 -C 4 alkenyl or C 3 -C 4 alkynyl.
R 5 -VI: C 1 -C 4 haloalkylthio.
R 5 -VII: —C (O) R 9 [wherein R 9 represents C 1 -C 4 alkyl. Or C 1 -C 4 alkoxycarbonyl.
すなわち、R5-I:水素原子。
R5-II:C1~C4アルキル。
R5-III:R8によって置換された(C1~C2)アルキル[ここで、R8はシアノ、C3~C6シクロアルキル又は-OR14を表し、R14はC1~C4アルキルを表す。]。
R5-IV:シクロプロピル。
R5-V:C2~C4アルケニル又はC3~C4アルキニル。
R5-VI:C1~C4ハロアルキルチオ。
R5-VII:-C(O)R9[ここで、R9はC1~C4アルキルを表す。]又はC1~C4アルコキシカルボニル。 In the compounds included in the present invention, examples of the preferred range of the substituent represented by R 5 include the following groups of R 5 -I to R 5 -XI.
That is, R 5 -I: a hydrogen atom.
R 5 -II: C 1 -C 4 alkyl.
R 5 -III: (C 1 -C 2 ) alkyl substituted by R 8 [wherein R 8 represents cyano, C 3 -C 6 cycloalkyl or —OR 14 and R 14 represents C 1 -C 4 Represents alkyl. ].
R 5 -IV: cyclopropyl.
R 5 -V: C 2 -C 4 alkenyl or C 3 -C 4 alkynyl.
R 5 -VI: C 1 -C 4 haloalkylthio.
R 5 -VII: —C (O) R 9 [wherein R 9 represents C 1 -C 4 alkyl. Or C 1 -C 4 alkoxycarbonyl.
R5-VIII:R8によって置換された(C1~C2)アルキル[ここで、R8は-OR14又はC1~C4アルキルチオ、-C(O)NH2又は-C(S)NH2を表し、R14はC2~C4ハロアルキルを表す。]。
R5-IX:C3~C6シクロアルキル。
R5-X:C1~C4アルコキシ。
R5-XI:-C(O)R9[ここで、R9はC3~C4シクロアルキルを表す。]。
これらのうち、R5で表される置換基の範囲としてはR5-I~R5-VIII及びR5-XIがより好ましく、さらに、R5-I~R5-III及びR5-V~R5-VIIが特に好ましい。 R 5 -VIII: (C 1 -C 2 ) alkyl substituted by R 8 [where R 8 is —OR 14 or C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2 represents R 14 represents C 2 -C 4 haloalkyl. ].
R 5 -IX: C 3 -C 6 cycloalkyl.
R 5 -X: C 1 -C 4 alkoxy.
R 5 -XI: —C (O) R 9 wherein R 9 represents C 3 -C 4 cycloalkyl. ].
Of these, more preferred R 5 -I ~ R 5 -VIII and R 5 -XI as range of the substituent of R 5, further, R 5 -I ~ R 5 -III and R 5 -V ~ R 5 -VII is particularly preferred.
R5-IX:C3~C6シクロアルキル。
R5-X:C1~C4アルコキシ。
R5-XI:-C(O)R9[ここで、R9はC3~C4シクロアルキルを表す。]。
これらのうち、R5で表される置換基の範囲としてはR5-I~R5-VIII及びR5-XIがより好ましく、さらに、R5-I~R5-III及びR5-V~R5-VIIが特に好ましい。 R 5 -VIII: (C 1 -C 2 ) alkyl substituted by R 8 [where R 8 is —OR 14 or C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2 represents R 14 represents C 2 -C 4 haloalkyl. ].
R 5 -IX: C 3 -C 6 cycloalkyl.
R 5 -X: C 1 -C 4 alkoxy.
R 5 -XI: —C (O) R 9 wherein R 9 represents C 3 -C 4 cycloalkyl. ].
Of these, more preferred R 5 -I ~ R 5 -VIII and R 5 -XI as range of the substituent of R 5, further, R 5 -I ~ R 5 -III and R 5 -V ~ R 5 -VII is particularly preferred.
本発明において、R6で表される置換基の好ましい範囲として、例えば下記のR6-II~R6-XVの各群が挙げられる。
すなわち、R6-I:C1~C4アルキル。
R6-II:C3~C6シクロアルキル。
R6-III:R10によって任意に置換された(C1~C4)アルキル[ここで、R10は-OR15を表し、R15は水素原子、メチル、エチル又はC1~C2ハロアルキルを表す。]。
R6-IV:トリ(C1~C4アルキル)シリル。
R6-V:-CH=NOR12[ここで、R12はメチル又はエチルを表す。]。
R6-VI:フェニル。 In the present invention, examples of the preferred range of the substituent represented by R 6 include the following groups of R 6 -II to R 6 -XV.
That is, R 6 -I: C 1 -C 4 alkyl.
R 6 -II: C 3 -C 6 cycloalkyl.
R 6 -III: optionally substituted by R 10 (C 1 ~ C 4 ) alkyl [wherein, R 10 represents -OR 15, R 15 is a hydrogen atom, methyl, ethyl or C 1 ~ C 2 haloalkyl Represents. ].
R 6 -IV: Tri (C 1 -C 4 alkyl) silyl.
R 6 -V: —CH═NOR 12 [wherein R 12 represents methyl or ethyl. ].
R 6 -VI: phenyl.
すなわち、R6-I:C1~C4アルキル。
R6-II:C3~C6シクロアルキル。
R6-III:R10によって任意に置換された(C1~C4)アルキル[ここで、R10は-OR15を表し、R15は水素原子、メチル、エチル又はC1~C2ハロアルキルを表す。]。
R6-IV:トリ(C1~C4アルキル)シリル。
R6-V:-CH=NOR12[ここで、R12はメチル又はエチルを表す。]。
R6-VI:フェニル。 In the present invention, examples of the preferred range of the substituent represented by R 6 include the following groups of R 6 -II to R 6 -XV.
That is, R 6 -I: C 1 -C 4 alkyl.
R 6 -II: C 3 -C 6 cycloalkyl.
R 6 -III: optionally substituted by R 10 (C 1 ~ C 4 ) alkyl [wherein, R 10 represents -OR 15, R 15 is a hydrogen atom, methyl, ethyl or C 1 ~ C 2 haloalkyl Represents. ].
R 6 -IV: Tri (C 1 -C 4 alkyl) silyl.
R 6 -V: —CH═NOR 12 [wherein R 12 represents methyl or ethyl. ].
R 6 -VI: phenyl.
R6-VII:(Z)mによって置換されたフェニル[ここで、Zはハロゲン原子、シアノ、ニトロ、C1~C4アルキル、トリフルオロメチル、C1~C6アルコキシ、C1~C4ハロアルコキシ、メチルチオ、メチルスルフィニル、メチルスルホニル、トリフルオロメチルチオ、トリフルオロメチルスルフィニル、トリフルオロメチルスルホニル、D-31、D-32又はD-34を表し、mが2又は3を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、R13はC1~C4アルキル又はC1~C4ハロアルキルを表し、mは1、2又は3を表し、nは0を表す。]。
R 6 -VII: (Z) phenyl substituted by m [where Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, trifluoromethyl, C 1 -C 6 alkoxy, C 1 -C 4 Haloalkoxy, methylthio, methylsulfinyl, methylsulfonyl, trifluoromethylthio, trifluoromethylsulfinyl, trifluoromethylsulfonyl, D-31, D-32 or D-34, when m represents 2 or 3, Each Z may be the same as or different from each other, and when two Zs are adjacent to each other, the two adjacent Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O-, -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -N (R 13 ) N = N-, = NN (R 13) CH =, = NON =, = NSN = or -CH = CHCH = CH- to form a In addition, a 5-membered ring or a 6-membered ring may be formed together with the carbon atom to which each Z is bonded. In this case, the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trifluoromethyl. Optionally substituted by R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl, m represents 1, 2 or 3 and n represents 0. ].
R6-VIII:D-1、D-2、D-6、D-8又はD-23[ここで、Zはハロゲン原子、C1~C4アルキル又はトリフルオロメチルを表し、nは0、1、2又は3を表し、nが2又は3を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に6員環を形成してもよく、R13はC1~C4アルキルを表す。]。
R6-IX:水素原子。
R6-X:ハロゲン原子又はC1~C4ハロアルキル。
R6-XI:R10によって任意に置換された(C1~C4)アルキル[ここで、R10は-S(O)rR16を表し、R16はメチル、エチル又はC1~C2ハロアルキルを表し、rは0、1又は2を表す。]。
R6-XII:C3~C6ハロシクロアルキル。
R6-XIII:-C(R11)=NOR12[ここで、R11はメチルを表し、R12はメチル又はエチルを表す。]。 R 6 -VIII: D-1, D-2, D-6, D-8 or D-23 [wherein Z represents a halogen atom, C 1 -C 4 alkyl or trifluoromethyl, n is 0, 1 represents 1, 2 or 3, and when n represents 2 or 3, each Z may be the same as or different from each other, and when two Zs are adjacent to each other, Two adjacent Z's may form -CH = CHCH = CH- to form a 6-membered ring with the carbon atom to which each Z is bonded, and R 13 represents C 1 -C 4 alkyl. ].
R 6 -IX: a hydrogen atom.
R 6 -X: a halogen atom or C 1 -C 4 haloalkyl.
R 6 -XI: optionally substituted by R 10 (C 1 ~ C 4 ) alkyl [wherein, R 10 represents -S (O) r R 16, R 16 is methyl, ethyl or C 1 ~ C Represents 2 haloalkyl, r represents 0, 1 or 2; ].
R 6 -XII: C 3 ~ C 6 halocycloalkyl.
R 6 -XIII: —C (R 11 ) = NOR 12 [wherein R 11 represents methyl and R 12 represents methyl or ethyl. ].
R6-IX:水素原子。
R6-X:ハロゲン原子又はC1~C4ハロアルキル。
R6-XI:R10によって任意に置換された(C1~C4)アルキル[ここで、R10は-S(O)rR16を表し、R16はメチル、エチル又はC1~C2ハロアルキルを表し、rは0、1又は2を表す。]。
R6-XII:C3~C6ハロシクロアルキル。
R6-XIII:-C(R11)=NOR12[ここで、R11はメチルを表し、R12はメチル又はエチルを表す。]。 R 6 -VIII: D-1, D-2, D-6, D-8 or D-23 [wherein Z represents a halogen atom, C 1 -C 4 alkyl or trifluoromethyl, n is 0, 1 represents 1, 2 or 3, and when n represents 2 or 3, each Z may be the same as or different from each other, and when two Zs are adjacent to each other, Two adjacent Z's may form -CH = CHCH = CH- to form a 6-membered ring with the carbon atom to which each Z is bonded, and R 13 represents C 1 -C 4 alkyl. ].
R 6 -IX: a hydrogen atom.
R 6 -X: a halogen atom or C 1 -C 4 haloalkyl.
R 6 -XI: optionally substituted by R 10 (C 1 ~ C 4 ) alkyl [wherein, R 10 represents -S (O) r R 16, R 16 is methyl, ethyl or C 1 ~ C Represents 2 haloalkyl, r represents 0, 1 or 2; ].
R 6 -XII: C 3 ~ C 6 halocycloalkyl.
R 6 -XIII: —C (R 11 ) = NOR 12 [wherein R 11 represents methyl and R 12 represents methyl or ethyl. ].
R6-XIV:(Z)mによって置換されたフェニル[ここで、Zはハロゲン原子、メチル、C1~C4ハロアルキル、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31~D-35又はD-37を表し、mが2、3、4又は5を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-CH2OCH2-、-OCH2O-、-CH2CH2S(O)r-、-CH2CH2CH2CH2-、-CH2CH2CH2O-、-CH2OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-SN=N-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=、-CH=CHCH=CH-、-N=CHCH=CH-、-CH=NCH=CH-、-N=NCH=CH-、-N=CHN=CH-、-N=CHCH=N-、-CH=NN=CH-又は-N=NCH=N-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、シアノ、メチル、ジフルオロメチル、トリフルオロメチル又はメトキシによって任意に置換されていてもよく、Zaはハロゲン原子、メチル又はトリフルオロメチルを表し、R13はC1~C4アルキル、C1~C4ハロアルキル、C3~C4シクロアルキルメチル又はC3~C4シクロアルキルを表し、mは1、2、3、4又は5を表し、nは0又は1を表す。]。
R 6 -XIV: (Z) phenyl substituted by m [wherein Z is a halogen atom, methyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, C 1- C 4 alkylsulfonyl, represents a C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31 ~ D-35 or D-37, m is 2, 3, 4 Or 5 may be the same as or different from each other, and when two Zs are adjacent, the two adjacent Zs are —CH 2 CH 2. CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —, —OCH 2 O—, —CH 2 CH 2 S (O) r —, —CH 2 CH 2 CH 2 CH 2 —, —CH 2 CH 2 CH 2 O—, —CH 2 OCH 2 O—, —OCH 2 CH 2 O—, —OCH═CH—, —SCH═CH—, —N (R 13 ) CH═CH—, —OCH═ N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = C H-, -SN = N-, -N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN =, -CH = CHCH = CH-, -N = CHCH = CH-, -CH = NCH = CH-, -N = NCH = CH-, -N = CHN = CH-, -N = CHCH = N-, -CH = NN = CH- or -N = NCH = N- May form a 5-membered ring or a 6-membered ring together with the carbon atom to which each Z is bonded. At this time, the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, cyano. Optionally substituted by methyl, difluoromethyl, trifluoromethyl or methoxy, Za represents a halogen atom, methyl or trifluoromethyl, R 13 represents C 1 -C 4 alkyl, C 1 -C 4 Represents haloalkyl, C 3 -C 4 cycloalkylmethyl or C 3 -C 4 cycloalkyl, m represents 1, 2, 3, 4 or 5 and n represents 0 or 1; ].
R6-XV:D-1、D-2、D-6、D-8、D-23、D-24、D-25又はD-26[ここで、Zはハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C4アルコキシ又はC1~C4アルキルスルホニルを表し、nが2、3又は4を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、R13はC1~C4アルキル又はC1~C4ハロアルキルを表し、nは0、1、2、3又は4を表す。]。
R 6 -XV: D-1, D-2, D-6, D-8, D-23, D-24, D-25 or D-26 [where Z is a halogen atom, cyano, nitro, C 1 to C 4 alkyl, C 1 to C 4 haloalkyl, C 1 to C 4 alkoxy or C 1 to C 4 alkylsulfonyl, and when n represents 2, 3 or 4, each Z is the same May be or different from each other, and when two Zs are adjacent, the two adjacent Zs form -CH = CHCH = CH- so that the carbons to which each Z binds A 6-membered ring may be formed together with the atoms, in which case the hydrogen atom bonded to each carbon atom forming the ring may be optionally substituted with a halogen atom, methyl or trifluoromethyl, and R 13 is Represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl, and n represents 0, 1, 2, 3 or 4. ].
これらのうち、R6で表される置換基の範囲としてはR6-I~R6-VIII、R6-X、R6-XII、R6-XIV又はR6-XVがより好ましく、さらに、R6-I~R6-VIIIが特に好ましい。
これらの本発明における各置換基の好ましい範囲を示す各群はそれぞれ任意に組み合わせることができ、それぞれ本発明化合物の好ましい範囲を表す。
式(I)で表される化合物のG1、R1~R4(R-I~R-XIVで表記)及びR6についての好ましい範囲の組み合わせの例としては、例えば以下の第1表に示す組み合わせが挙げられる。但し、第1表の組み合わせは例示のためのものであって、式(I)で表される化合物はこれらのみに限定されるものではない。 Of these, R R 6 -I ~ R 6 as the range of the substituent of 6 -VIII, R 6 -X, R 6 -XII, R 6 -XIV or R 6 -XV more preferably, further R 6 -I to R 6 -VIII are particularly preferred.
Each group which shows the preferable range of each substituent in this invention can be combined arbitrarily, respectively, Each represents the preferable range of this invention compound.
Examples of preferable ranges of combinations of G 1 , R 1 to R 4 (indicated by RI to R-XIV) and R 6 of the compound represented by the formula (I) include, for example, combinations shown in the following Table 1. Is mentioned. However, the combinations in Table 1 are for illustrative purposes, and the compounds represented by Formula (I) are not limited to these.
これらの本発明における各置換基の好ましい範囲を示す各群はそれぞれ任意に組み合わせることができ、それぞれ本発明化合物の好ましい範囲を表す。
式(I)で表される化合物のG1、R1~R4(R-I~R-XIVで表記)及びR6についての好ましい範囲の組み合わせの例としては、例えば以下の第1表に示す組み合わせが挙げられる。但し、第1表の組み合わせは例示のためのものであって、式(I)で表される化合物はこれらのみに限定されるものではない。 Of these, R R 6 -I ~ R 6 as the range of the substituent of 6 -VIII, R 6 -X, R 6 -XII, R 6 -XIV or R 6 -XV more preferably, further R 6 -I to R 6 -VIII are particularly preferred.
Each group which shows the preferable range of each substituent in this invention can be combined arbitrarily, respectively, Each represents the preferable range of this invention compound.
Examples of preferable ranges of combinations of G 1 , R 1 to R 4 (indicated by RI to R-XIV) and R 6 of the compound represented by the formula (I) include, for example, combinations shown in the following Table 1. Is mentioned. However, the combinations in Table 1 are for illustrative purposes, and the compounds represented by Formula (I) are not limited to these.
式(I)で表される化合物のうちで、酸付加塩としては、例えば、フッ化水素酸、塩酸、臭化水素酸、沃化水素酸等のハロゲン化水素酸の塩、硝酸、硫酸、燐酸、塩素酸、過塩素酸等の無機酸の塩、メタンスルホン酸、エタンスルホン酸、トリフルオロメタンスルホン酸、ベンゼンスルホン酸、p-トルエンスルホン酸等のスルホン酸の塩、ギ酸、酢酸、プロピオン酸、トリフルオロ酢酸、フマール酸、酒石酸、蓚酸、マレイン酸、リンゴ酸、コハク酸、安息香酸、マンデル酸、アスコルビン酸、乳酸、グルコン酸、クエン酸等のカルボン酸の塩又はグルタミン酸、アスパラギン酸等のアミノ酸の塩等が挙げられる。
Among the compounds represented by the formula (I), examples of the acid addition salt include hydrohalic acid salts such as hydrofluoric acid, hydrochloric acid, hydrobromic acid, hydroiodic acid, nitric acid, sulfuric acid, Salts of inorganic acids such as phosphoric acid, chloric acid, perchloric acid, salts of sulfonic acids such as methanesulfonic acid, ethanesulfonic acid, trifluoromethanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid, formic acid, acetic acid, propionic acid , Salts of carboxylic acids such as trifluoroacetic acid, fumaric acid, tartaric acid, succinic acid, maleic acid, malic acid, succinic acid, benzoic acid, mandelic acid, ascorbic acid, lactic acid, gluconic acid, citric acid, glutamic acid, aspartic acid, etc. Examples include amino acid salts.
式(I)で表される化合物のうちで、金属塩としては、例えば、リチウム、ナトリウム、カリウムといったアルカリ金属の塩、カルシウム、バリウム、マグネシウムといったアルカリ土類金属の塩又はアルミニウムの塩等が挙げられる。
本明細書における「有害生物防除剤」とは、植物又は動物に感染・寄生する有害な病原菌及び寄生虫を防除対象とした殺菌剤及び寄生虫防除剤を意味し、より具体的には、農園芸分野における殺菌剤及び殺線虫剤、或いは動物の抗真菌剤及び寄生虫防除剤を意味する。 Among the compounds represented by the formula (I), examples of the metal salt include alkali metal salts such as lithium, sodium and potassium, alkaline earth metal salts such as calcium, barium and magnesium, and aluminum salts. It is done.
As used herein, the term “pest control agent” means a fungicide and a parasite control agent that are intended to control harmful pathogens and parasites that infect or infest plants or animals. It means fungicides and nematicides in the field of horticulture, or animal antifungals and parasite control agents.
本明細書における「有害生物防除剤」とは、植物又は動物に感染・寄生する有害な病原菌及び寄生虫を防除対象とした殺菌剤及び寄生虫防除剤を意味し、より具体的には、農園芸分野における殺菌剤及び殺線虫剤、或いは動物の抗真菌剤及び寄生虫防除剤を意味する。 Among the compounds represented by the formula (I), examples of the metal salt include alkali metal salts such as lithium, sodium and potassium, alkaline earth metal salts such as calcium, barium and magnesium, and aluminum salts. It is done.
As used herein, the term “pest control agent” means a fungicide and a parasite control agent that are intended to control harmful pathogens and parasites that infect or infest plants or animals. It means fungicides and nematicides in the field of horticulture, or animal antifungals and parasite control agents.
本明細書における「病原菌」とは、植物の病害及び動物の感染症の病原となる微生物を意味し、具体的に例えば、以下の微生物が挙げられるが、微生物の具体例はこれらのみに限定されるものではない。
As used herein, the term “pathogenic bacteria” means microorganisms that cause plant diseases and animal infectious diseases. Specific examples include the following microorganisms, but specific examples of microorganisms are not limited to these. It is not something.
Taphrina spp.(Taphrina deformans、T. pruni等)、Pneumocystis spp.、Geotrichum spp.、Candida spp.(Candida albicans、C. sorbosa等)、Pichia spp.(例えばPichia kluyveri等)、Capnodium spp.、Fumago spp.、Hypocapnodium spp.、Cercospora spp.(Cercospora apii、C. asparagi、C. beticola、C. capsici、C. carotae、C. kaki、C. kikuchii、C. zonata等)、Cercosporidium spp.、Cladosporium spp.(Cladosporium colocasiae、C. cucumerinum、C. variabile等)、Davidiella spp.、Didymosporium spp.、Heterosporium spp.(Heterosporium allii等)、Mycosphaerella spp.(Mycosphaerella arachidis、M. berkeleyi、M. cerasella、M. fijiensis、M. fragariae、M. graminicola、M. nawae、M. pinodes、M. pomi、M. zingiberis等)、Mycovellosiella spp.(Mycovellosiella fulva、M. nattrassii等)、Paracercospora spp.(Paracercospora egenula等)、Phaeoisariopsis spp.、Phaeoramularia spp.、Pseudocercospora spp.(Pseudocercospora abelmoschi、P. fuligena、P. vitis等)、Pseudocercosporella spp.(Pseudocercosporella capsellae等)、Ramichloridium spp.、Ramularia spp.、Septogloeum spp.、Septoria spp.(Septoria albopunctata、S. apiicola、S. chrysanthemella、S. helianthi、S. obesa等)、Sphaerulina spp.、Aureobasidium spp.、Kabatiella spp.、Plowrightia spp.、Stigmina spp.、Elsinoe spp.(Elsinoe ampelina、E. araliae、E. fawcettii等)、Sphaceloma spp.(Sphaceloma caricae等)、Ascochyta spp.(Ascochyta pisi等)、Corynespora spp.(Corynespora cassiicola等)、Leptosphaeria spp.(Leptosphaeria coniothyrium、L. maculans等)、Saccharicola spp.、Phaeosphaeria spp.、Ophiosphaerella spp.、Setophoma spp.、Helminthosporium spp.、Alternaria spp.(Alternaria alternata、A. brassicae、A. brassicicola、A. citri、A. dauci、A. helianthi、A. japonica、A. kikuchiana、A. mali、A. panax、A. porri、A. radicina、A. solani等)、Bipolaris spp.(Bipolaris sorghicola等)、Cochliobolus spp.(Cochliobolus heterostrophus、C. lunatus、C. miyabeanus等)、Curvularia spp.(Curvularia geniculata、C. verruculosa等)、Drechslera spp.、Pleospora spp.(Pleospora herbarum等)、Pyrenophora spp.(Pyrenophora graminea、P. teres等)、Setosphaeria spp.(Setosphaeria turcica等)、Stemphylium spp.(Stemphylium botryosum、S. lycopersici、S. solani、S. vesicarium等)、Fusicladium spp.、Venturia spp.(Venturia carpophila、V. Inaequalis、V. nashicola、V. pirina等)、Didymella spp.(Didymella bryoniae、D. fabae等)、Hendersonia spp.、Phoma spp.(Phoma erratica var. mikan、P. exigua var. exigua、P. wasabiae等)、Pyrenochaeta spp.(Pyrenochaeta lycopersici等)、Stagonospora spp.(Stagonospora sacchari等)、Botryosphaeria spp.(Botryosphaeria berengeriana f. sp. piricola、B. dothidea等)、Dothiorella spp.、Fusicoccum spp.、Guignardia spp.、Lasiodiplodia spp.(Lasiodiplodia theobromae等)、Macrophoma spp.、Macrophomina spp.、Neofusicoccum spp.、Phyllosticta spp. (Phyllosticta zingiberis等)、Schizothyrium spp.(Schizothyrium pomi等)、Acrospermum spp.、Leptosphaerulina spp.、Aspergillus spp.、Penicillium spp.(Penicillium digitatum、P. italicum、P. sclerotigenum等)、Microsporum spp.、Trichophyton spp.(Trichophyton mentagrophytes、T. rubrum等)、Histoplasma spp.、Blumeria spp.(Blumeria graminis f. sp. hordei、B. g. f. sp. tritici等)、Erysiphe spp.(Erysiphe betae、E. cichoracearum、E. c. var. cichoracearum、E. heraclei、E. pisi等)、Golovinomyces spp.(Golovinomyces cichoracearum var. latisporus等)、Leveillula spp.(Leveillula taurica等)、Microsphaera spp.、Oidium spp.(Oidium neolycopersici等)、Phyllactinia spp.(Phyllactinia kakicola、P. mali、P. moricola等)、Podosphaera spp.(Podosphaera fusca、P. leucotricha、P. pannosa、P. tridactyla var. tridactyla、P. xanthii等)、Sphaerotheca spp.(Sphaerotheca aphanis var. aphanis、S. fuliginea等)、Uncinula spp.(Uncinula necator、U. n. var. necator等)、Uncinuliella spp.(Uncinuliella simulans var. simulans、U. s. var. tandae等)、Blumeriella spp.(Blumeriella jaapii等)、Cylindrosporium spp.、Diplocarpon spp.(Diplocarpon mali、D. mespili、D. rosae等)、Gloeosporium spp.(Gloeosporium minus等)、Marssonina spp.、Tapesia spp.(Tapesia acuformis、T. yallundae等)、Lachnum spp.、Scleromitrula spp.、Botryotinia spp.(Botryotinia fuckeliana等)、Botrytis spp.(Botrytis allii、B. byssoidea、B. cinerea、B. elliptica、B. fabae、B. squamosa等)、Ciborinia spp.、Grovesinia spp.、Monilia mumecola、Monilinia spp.(Monilinia fructicola、M. fructigena、M. laxa、M. mali、M. vaccinii-corymbosi等)、Sclerotinia spp.(Sclerotinia borealis、S. homoeocarpa、S. minor、S. sclerotiorum等)、Valdensia spp.(Valdensia heterodoxa等)、Claviceps spp.(Claviceps sorghi、C. sorghicola等)、Epichloe spp.、Ephelis japonica、Villosiclava virens、Hypomyces spp.(Hypomyces solani f. sp. mori、H. s. f. sp. pisi等)、Trichoderma spp.(Trichoderma viride等)、Calonectria spp.(Calonectria ilicicola等)、Candelospora spp.、Cylindrocarpon spp.、Cylindrocladium spp.、Fusarium spp.(Fusarium arthrosporioides、F. crookwellense、F. culmorum、F. cuneirostrum、F. oxysporum、F. o. f. sp. adzukicola、F. o. f. sp. allii、F. o. f. sp. asparagi、F. o. f. sp. batatas、F. o. f. sp. cepae、F. o. f. sp. colocasiae、F. o. f. sp. conglutinans、F. o. f. sp. cubense、F. o. f. sp. cucumerinum、F. o. f. sp. fabae、F. o. f. sp. fragariae、F. o. f. sp. lactucae、F. o. f. sp. lagenariae、F. o. f. sp. lycopersici、F. o. f. sp. melongenae、F. o. f. sp. melonis、F. o. f. sp. nelumbinicola、F. o. f. sp. niveum、F. o. f. sp. radicis-lycopersici、F. o. f. sp. raphani、F. o. f. sp. spinaciae、F. sporotrichioides、F. solani、F. s. f. sp. cucurbitae、F. s. f. sp. eumartii、F. s. f. sp. pisi、F. s. f. sp. radicicola等)、Gibberella spp.(Gibberella avenacea、G. baccata、G. fujikuroi、G. zeae等)、Haematonectria spp.、Nectria spp.、Ophionectria spp.、Caldariomyces spp.、Myrothecium spp.、Trichothecium spp.、Verticillium spp.(Verticillium albo-atrum、V. dahliae、V. longisporum等)、Ceratocystis spp.(Ceratocystis ficicola, C. fimbriata等)、Thielaviopsis spp.(Thielaviopsis basicola等)、Adisciso spp.、Monochaetia spp.、Pestalotia spp.(Pestalotia eriobotrifolia等)、Pestalotiopsis spp.(Pestalotiopsis funerea、P. longiseta、P. neglecta、P. theae等)、Physalospora spp.、Nemania spp.、Nodulisporium spp.、Rosellinia spp.(Rosellinia necatrix等)、Monographella spp.(Monographella nivalis等)、Ophiostoma spp.、Cryphonectria spp.(Cryphonectria parasitica等)、Diaporthe spp.(Diaporthe citri、D. kyushuensis、D. nomurai、D. tanakae等)、Diaporthopsis spp.、Phomopsis spp.(Phomopsis asparagi、P. fukushii、P. obscurans、P. vexans等)、Cryptosporella spp.、Discula spp.(Discula theae-sinensis等)、Gnomonia spp.、Coniella spp.、Coryneum spp.、Greeneria spp.、Melanconis spp.、Cytospora spp.、Leucostoma spp.、Valsa spp.(Valsa ceratosperma等)、Tubakia spp.、Monosporascus spp.、Clasterosporium spp.、Gaeumannomyces spp.(Gaeumannomyces graminis等)、Magnaporthe spp.(Magnaporthe grisea等)、Pyricularia spp.(Pyricularia zingiberis等)、Monilochaetes infuscans、Colletotrichum spp.(Colletotrichum acutatum、C. capsici、C. cereale、C. destructivum、C. fragariae、C. lindemuthianum、C. nigrum、C. orbiculare、C. spinaciae等)、Glomerella spp.(Glomerella cingulata等)、Khuskia oryzae、Phyllachora spp.(Phyllachora pomigena等)、Ellisembia spp.、Briosia spp.、Cephalosporium spp.(Cephalosporium gramineum等)、Epicoccum spp.、Gloeocercospora sorghi、Mycocentrospora spp.、Peltaster spp.(Peltaster fructicola等)、Phaeocytostroma spp.、Phialophora spp.(Phialophora gregata等)、Pseudophloeosporella dioscoreae、Pseudoseptoria spp.、Rhynchosporium spp.(Rhynchosporium secalis等)、Sarocladium spp.、Coleophoma spp.、Helicoceras oryzae等の子嚢菌門(Ascomycota)菌類。
Taphrina spp. (Taphrina deformans, T. pruni, etc.), Pneumocystis spp., Geotrichum spp., Candida spp. (Candida albicans, C. sorbosa, etc.), Pichia spp. (Eg, Pichia kluyveri, etc.), Capnodiumgospps, Fnouma spp. ., Hypocapnodiumppspp., Cercospora spp. (Cercospora apii, C. asparagi, C. beticola, C. capsici, C. carotae, C. kaki, C. kikuchii, C. zonata, etc.), Cercosporidium spp., Clppspor. (Cladosporium colocasiae, C. cucumerinum, C. variabile, etc.), Davidiella spp., Didymosporium spp., Heterosporium spp. (Heterosporium allii, etc.), Mycosphaerella spp. M. fragariae, M. graminicola, M. nawae, M. pinodes, M. pomi, M. zingiberis, etc., Mycovellosiella spp. (Mycovellosiella fulva, M. nattrassii, etc.), Paracercospora spp. (Paracercospora egenis) ., Phaeoramularia spp., Pseudocercospora spp. (Pseu docercospora abelmoschi, P. fuligena, P. vitis, etc.), Pseudocercosporella spp. (Pseudocercosporella capsellae, etc.), Ramichloridium spp. Helianthi, S. obesa, etc.), Sphaerulina spp., Aureobasidiumsispp., Kabatiella spp., Plowrightia spp., Stigminatigspp., Elsinoe spp. (Elsinoe ampelina, E. araliae, E. fawcphatioma) Sphaceloma caricae), Ascochytachyspp. (Ascochyta pisi etc.), Corynespora spp. (Corynespora cassiicola etc.), Leptosphaeria spp. (Leptosphaeria coniothyrium, L. maculans, etc.) ., Helminthosporiumppspp., Alternaria spp. (Alternaria alternata, A. brassicae, A. brassicicola, A. citri, A. dauci, A. helianthi, A. japonica, A. kikuchiana, A. panax, A. panax, A . Porri, A. radicina, A. s olani etc.), Bipolaris spp. (Bipolaris sorghicola etc.), Cochliobolusbolspp. (Cochliobolus heterostrophus, C. lunatus, C. miyabeanus etc.), Curvularia spp. (Curvularia geniculata, C. verruculosa etc.), Dreporasle (Pleospora herbarum, etc.), Pyrenophora spp. (Pyrenophora graminea, P. teres, etc.), Setosphaeria spp. (Setosphaeria turcica, etc.), Stemphylium spp. spp., Venturia spp. (Venturia carpophila, V. Inaequalis, V. nashicola, V. pirina, etc.), Didymella spp. (Didymella bryoniae, D. fabae, etc.), Hendersonia spp., Phoma srpkan (Phoma errakan , P. exigua var. Exigua, P. wasabiae etc.), Pyrenochaeta spp. (Pyrenochaeta lycopersici etc.), Stagonospora spp. (Stagonospora sacchari etc.), Botryosphaeria spp. Dothiorella spp., Fusicoccum spp., Guignardia spp., Lasiodiplodia spp. (Lasiodiplodia theobromae, etc.), Macrophoma spp., Macrophomina spp., Neofusicoccum spp., Phyllosticta umizpp , Leptosphaerulina spp., Aspergillus spp., Penicillium spp. (Penicillium digitatum, P. italicum, P. sclerotigenum, etc.), Microsporum spp., Trichophyton spp. (Trichophyton mentagrophytes, T. plasmrubrum. (Blumeria graminis f. Sp. Hordei, B. g. F. Sp. Tritici, etc.), Erysiphe spp. (Erysiphe betae, E. cichoracearum, E. c. Var. Cichoracearum, E. heraclei, E. pisi, etc.), Golovinomyces spp. (Golovinomyces cichoracearum var. Latisporus etc.), Leveillula spp. (Leveillula taurica etc.), Microsphaera spp., Oidium spp. , Po dosphaera spp. (Podosphaera fusca, P. leucotricha, P. pannosa, P. tridactyla var. tridactyla, P. xanthii, etc.), Sphaerotheca spp. (Sphaerotheca aphanis var. , U. n. Var. Necator, etc.), Uncinuliella spp. (Uncinuliella simulans var. Simulans, U. s. Var. Tandae, etc.), Blumeriella spp. (Blumeriella jaapii, etc.), Cylindrosporium spp., Diplocarpon spon. , D. mespili, D. rosae, etc.), Gloeosporium spp. (Gloeosporium minus, etc.), Marssonina spp., Tapesia spp. (Tapesia acuformis, T. yallundae, etc.), Lachnum spp., Scleromitrula nia, Botry, Botry, Botry, fuckeliana, etc.), Botrytis spp. (Botrytis allii, B. byssoidea, B. cinerea, B. elliptica, B. fabae, B. squamosa, etc.), Ciborinia spp., Grovesinia spp., Monilia mumecola, Monilinia spp. , M. fructigena, M. laxa, M. mali, M. vaccinii-corymbos i), Sclerotinia spp. (Sclerotinia borealis, S. homoeocarpa, S. minor, S. sclerotiorum, etc.), Valdensia spp. (Valdensia heterodoxa, etc.), Claviceps spp. (Claviceps sorghi, C. spichloe, etc.) , Ephelis japonica, Villosiclava virens, Hypomyces spp. (Hypomyces solani f. Sp. Mori, H. s. F. Sp. Pisi etc.), Trichoderma spp. (Trichoderma viride etc.), Calonectria spp. (Calonectria spili spp., Cylindrocarpon spp., Cylindrocladium spp., Fusarium spp. (Fusarium arthrosporioides, F. crookwellense, F. culmorum, F. cuneirostrum, F. oxysporum, F. o. f. sp. adzukicola, F. adzukicolaf. sp. allii, F. o. f. sp. asparagi, F. o. f. sp. batatas, F. o. f. sp. cepae, F. o. f. sp. colocasiae, F. o. f. sp. conglutinans, F. o. f. sp. cubense, F. o. f. sp. cucumerinum, F. o. f. sp. fabae, F. o. f. sp. fragariae, F. o. f. sp. lactucae, F. o. f. sp. lagenariae, F. o. f. sp. lycopersici, F. o. f. sp. melongenae, F. o. f. sp. melonis, F. o. f. sp. nelumbinicola, F. o. f. sp. niveum, F. o. f. sp. radicis-lycopersici, F. o. f. sp. raphani, F. o. f. sp. spinaciae, F. sporotrichioides, F. solani, F. s. f. sp. cucurbitae, F. s. f. sp. eumartii, F. s. f. sp. pisi, F. s. f. sp. radicicola, etc.), Gibberella spp. (Gibberella avenacea, G. baccata, G. fujikuroi, G Zeae etc.), Haematonectria spp., Nectria spp., Ophionectria spp., Caldariomyces spp., Myrothecium spp., Trichothecium spp., Verticillium spp. (Verticillium albo-atrum, V. dahliae, rum C (Ceratocystis ficicola, C. fimbriata, etc.), Thielaviopsis spp. (Thielaviopsis basicola, etc.), Adisciso spp., Monochaetia spp., Pestalotia spp. neglecta P. theae), Physalospora spp., Nemania spp., Nodulisporium spp., Rosellinia spp. spp. (Diaporthe citri, D. kyushuensis, D. nomurai, D. tanakae, etc.), Diaporthopsis spp., Phomopsis spp. (Phomopsis asparagi, P. fukushii, P. obscurans, P. vexans, etc.), Cryptosporella spp. spp. (Discula theae-sinensis etc.), Gnomonia spp., Coniella spp., Coryneum spp., Greeneria spp., Melanconis spp., Cytospora spp., Leucostoma spp., Valsa spp. , Monosporacus spp., Clasterosporium spp., Gaeumannomyces spp. (Gaeumannomyces graminis, etc.), Magnaporthe spp. (Magnaporthe grisea, etc.), Pyricularia spp. C. cereale, C. destructivum, C. fragariae, C. lindemuthianum, C. nigrum, C. orbiculare, C. spinaciae, etc.), Glomerella spp. (Glomerella cingulata etc.), Khuskia oryzae, Phyllachorallachach (Phyllachorallachp) , Ellisembia spp., Briosia spp., Cephalosporium spp. (Cephalosporium 等 gramineum etc.), Epicocumcum spp., Gloeocercospora sorg., Mycocentrospora spp. , Pseudophloeosporella coredioscoreae, Pseudoseptoria spp., Rhynchosporium spp. (Rhynchosporium secalis, etc.), Sarocladium spp., Coleophoma spp., Helicoceras oryzae, etc. Ascomycota fungi
Septobasidium spp.(Septobasidium bogoriense、S. tanakae等)、Helicobasidium spp.(Helicobasidium longisporum等)、Coleosporium spp.(Coleosporium plectranthi等)、Cronartium spp.、Phakopsora spp.(Phakopsora artemisiae、P. nishidana、P. pachyrhizi等)、Physopella spp.(Physopella ampelopsidis等)、Kuehneola spp.(Kuehneola japonica等)、Phragmidium spp.(Phragmidium fusiforme、P. mucronatum、P. rosae-multiflorae等)、Gymnosporangium spp.(Gymnosporangium asiaticum、G. yamadae等)、Puccinia spp.(Puccinia allii、P. brachypodii var. poae-nemoralis、P. coronata、P. c. var. coronata、P. cynodontis、P. graminis、P. g. subsp. graminicola、P. hordei、P. horiana、P. kuehnii、P. melanocephala、P. recondita、P. striiformis var. striiformis、P. tanaceti var. tanaceti、P. tokyensis、P. zoysiae等)、Uromyces spp.(Uromyces phaseoli var. azukicola、U. p. var. phaseoli、Uromyces viciae-fabae var. viciae-fabae等)、Naohidemyces vaccinii、Nyssopsora spp.、Leucotelium spp.、Tranzschelia spp.(Tranzschelia discolor等)、Aecidium spp.、Blastospora spp.(Blastospora smilacis等)、Uredo spp.、Sphacelotheca spp.、Urocystis spp.、Sporisorium spp.(Sporisorium scitamineum等)、Ustilago spp.(Ustilago maydis、U. nuda等)、Entyloma spp.、Exobasidium spp.(Exobasidium reticulatum、E. vexans等)、Microstroma spp.、Tilletia spp.(Tilletia caries、T. controversa、T. laevis等)、Itersonilia spp.(Itersonilia perplexans等)、Cryptococcus spp.、Bovista spp.(Bovista dermoxantha等)、Lycoperdon spp.(Lycoperdon curtisii、L. perlatum等)、Conocybe spp.(Conocybe apala等)、Marasmius spp.(Marasmius oreades等)、Armillaria spp.、Helotium spp.、Lepista spp.(Lepista subnuda等)、Sclerotium spp.(Sclerotium cepivorum等)、Typhula spp.(Typhula incarnata、T. ishikariensis var. ishikariensis等)、Athelia spp.(Athelia rolfsii等)、Ceratobasidium spp.(Ceratobasidium cornigerum等)、Ceratorhiza spp.、Rhizoctonia spp.(Rhizoctonia solani等)、Thanatephorus spp.(Thanatephorus cucumeris等)、Laetisaria spp.、Waitea spp.、Fomitiporia spp.、Ganoderma spp.、Chondrostereum purpureum、Phanerochaete spp.等の担子菌門(Basidiomycota)菌類。
Septobasidium spp. (Septobasidium bogoriense, S. tanakae, etc.), Helicobasidium spp. (Helicobasidium longisporum, etc.), Coleosporium spp. (Coleosporium plectranthi, etc.), Cronartium spp. ), Physopella spp. (Physopella ampelopsidis etc.), Kuehneola spp. (Kuehneola japonica etc.), Phragmidium spp. (Phragmidium fusiforme, P. mucronatum, P. rosae-multiflorae etc.), Gymnosporangium spo. ), Puccinia spp. (Puccinia allii, P. brachypodii var. Poae-nemoralis, P. coronata, P. c. Var. Coronata, P. cynodontis, P. graminis, P. g. Subsp. Graminicola, P. hordei, P. horiana, P. kuehnii, P. melanocephala, P. recondita, P. striiformis var. Striiformis, P. tanaceti var. Tanaceti, P. tokyensis, P. zoysiae, etc.), Uromyces phasezuki, U. p. Var. Phaseoli, Uromyces viciae-fabae var. viciae-fabae), Naohidemyces vaccinii, Nyssopsora spp., Leucotelium spp., Tranzschelia spp. (Tranzschelia discolor etc.), Aecidium spp. ., Urocystis spp., Sporisorium spp. (Sporisorium scitamineum, etc.), Ustilago spp. (Ustilago maydis, U. nuda, etc.), Entyloma spp., Exobasidium spp. (Exobasidium reticulatum, E. vexille ia, strom, strom) spp. (Tilletia caries, T. controversa, T. laevis, etc.), Itersonilia spp. (Itersonilia perplexans, etc.), Cryptococcus spp., Bovista spp. (Bovista dermoxantha, etc.), Lycoperdon spp. (Lycoperdon pertis, etc.) , Conocybe spp. (Conocybe apala, etc.), Marasmius spp. (Marasmius oreades, etc.), Armillaria spp., Hellotium spp., Lepista spp. (Lepista subnuda, etc.), Sclerotium spp. (Sclerotium cepivoulay, pp, etc.) incarnata, T. ishika riensis var. ishikariensis etc.), Athelia spp. (Athelia rolfsii etc.), Ceratobasidium spp. (Ceratobasidium cornigerum etc.), Ceratorhiza spp. Basidiomycota fungi such as Waitea spp., Fomitiporia spp., Ganoderma spp., Chondrostereum purpureum, Phanerochaete spp.
Olpidium spp.等のツボカビ門(Chitridiomycota)菌類。
Physoderma spp.等のコウマクノウキン門(Blastocladiomycota)菌類。
Choanephora spp.、Choanephoroidea cucurbitae、Mucor spp.(Mucor fragilis等)、Rhizopus spp.(Rhizopus arrhizus、R. chinensis、R. oryzae、R. stolonifer var. stolonifer等)等のケカビ亜門(Mucoromycotina)菌類。
Plasmodiophora spp.(Plasmodiophora brassicae等)、Spongospora subterranea f. sp. subterranea等のケルコゾア門(Cercozoa)原生生物。
Aphanomyces spp.(Aphanomyces cochlioides、A. raphani等)、Albugo spp.(Albugo macrospora、A. wasabiae等)、Bremia spp.(Bremia lactucae等)、Hyaloperonospora spp.、Peronosclerospora spp.、Peronospora spp.(Peronospora alliariae-wasabi、P. chrysanthemi-coronarii、P. destructor、P. farinosa f. sp. spinaciae、P. manshurica、P. parasitica、P. sparsa等)、Plasmopara spp.(Plasmopara halstedii、P. nivea、P. viticola等)、Pseudoperonospora spp.(Pseudoperonospora cubensis等)、Sclerophthora spp.、Phytophthora spp.(Phytophthora cactorum、P. capsici、P. citricola、P. citrophthora、P. cryptogea、P. fragariae、P. infestans、P. melonis、P. nicotianae、P. palmivora、P. porri、P. sojae、P. syringae、P. vignae f. sp. adzukicola等)、Pythium spp.(Pythium afertile、P. aphanidermatum、P. apleroticum、P. aristosporum、P. arrhenomanes、P. buismaniae、P. debaryanum、P. graminicola、P. horinouchiense、P. irregulare、P. iwayamai、P. myriotylum、P. okanoganense、P. paddicum、P. paroecandrum、P. periplocum、P. spinosum、P. sulcatum、P. sylvaticum、P. ultimum var. ultimum、P. vanterpoolii、P. vexans、P. volutum等)等の不等毛植物門(Heterokontophyta)卵菌類(Oomycetes)。
Clavibacter spp.(Clavibacter michiganensis subsp. michiganensis等)、Curtobacterium spp.、Leifsonia spp.(Leifsonia xyli subsp. xyli等)、Streptomyces spp.(Streptomyces ipomoeae等)等の放線菌門(Actinobacteria)グラム陽性菌類。 Chipidiomycota fungi such as Olpidium spp.
Blastocladiomycota fungi such as Physoderma spp.
Choanephora spp., Choanephoroidea cucurbitae, Mucor spp. (Mucor fragilis, etc.), Rhizopus spp. (Rhizopus arrhizus, R. chinensis, R. oryzae, R. stolonifer var. Stolonifer, etc.)
Cercozoa protists such as Plasmodiophora spp. (Plasmodiophora brassicae, etc.), Spongospora subterranea f. Sp. Subterranea, etc.
Aphanomyces spp. (Aphanomyces cochlioides, A. raphani, etc.), Albugo spp. (Albugo macrospora, A. wasabiae, etc.), Bremia spp. (Bremia lactucae, etc.), Hyaloperonospora spp., Peronosclerospora spp., Peronospora spp. wasabi, P. chrysanthemi-coronarii, P. destructor, P. farinosa f. sp. spinaciae, P. manshurica, P. parasitica, P. sparsa, etc., Plasmopara spp. (Plasmopara halstedii, P. nivea, P. viticola, etc.) ), Pseudoperonospora spp. (Pseudoperonospora cubensis, etc.), Sclerophthora spp., Phytophthora spp. (Phytophthora cactorum, P. capsici, P. citricola, P. citrophthora, P. cryptogea, P. fragariae, P. infestans, P. infestans, P. nicotianae, P. palmivora, P. porri, P. sojae, P. syringae, P. vignae f. Sp. Adzukicola, etc.), Pythium spp. (Pythium afertile, P. aphanidermatum, P. apleroticum, P. aristosporum, P. arrhenomanes, P. buismaniae, P. debaryanum, P. graminicola, P. horinouchiense, P. irregulare, P. iwayamai, P. myrioty lum, P. okanoganense, P. paddicum, P. paroecandrum, P. periplocum, P. spinosum, P. sulcatum, P. sylvaticum, P. ultimum var. ultimum, P. vanterpoolii, P. vexans, P. volutum, etc.) Heterokontophyta oomycetes (Oomycetes).
Actinobacter gram-positive bacteria such as Clavibacter spp. (Clavibacter michiganensis subsp. Michiganensis etc.), Curtobacterium spp., Leifsonia spp. (Leifsonia xyli subsp. Xyli etc.), Streptomyces spp. (Streptomyces ipomoeae etc.)
Physoderma spp.等のコウマクノウキン門(Blastocladiomycota)菌類。
Choanephora spp.、Choanephoroidea cucurbitae、Mucor spp.(Mucor fragilis等)、Rhizopus spp.(Rhizopus arrhizus、R. chinensis、R. oryzae、R. stolonifer var. stolonifer等)等のケカビ亜門(Mucoromycotina)菌類。
Plasmodiophora spp.(Plasmodiophora brassicae等)、Spongospora subterranea f. sp. subterranea等のケルコゾア門(Cercozoa)原生生物。
Aphanomyces spp.(Aphanomyces cochlioides、A. raphani等)、Albugo spp.(Albugo macrospora、A. wasabiae等)、Bremia spp.(Bremia lactucae等)、Hyaloperonospora spp.、Peronosclerospora spp.、Peronospora spp.(Peronospora alliariae-wasabi、P. chrysanthemi-coronarii、P. destructor、P. farinosa f. sp. spinaciae、P. manshurica、P. parasitica、P. sparsa等)、Plasmopara spp.(Plasmopara halstedii、P. nivea、P. viticola等)、Pseudoperonospora spp.(Pseudoperonospora cubensis等)、Sclerophthora spp.、Phytophthora spp.(Phytophthora cactorum、P. capsici、P. citricola、P. citrophthora、P. cryptogea、P. fragariae、P. infestans、P. melonis、P. nicotianae、P. palmivora、P. porri、P. sojae、P. syringae、P. vignae f. sp. adzukicola等)、Pythium spp.(Pythium afertile、P. aphanidermatum、P. apleroticum、P. aristosporum、P. arrhenomanes、P. buismaniae、P. debaryanum、P. graminicola、P. horinouchiense、P. irregulare、P. iwayamai、P. myriotylum、P. okanoganense、P. paddicum、P. paroecandrum、P. periplocum、P. spinosum、P. sulcatum、P. sylvaticum、P. ultimum var. ultimum、P. vanterpoolii、P. vexans、P. volutum等)等の不等毛植物門(Heterokontophyta)卵菌類(Oomycetes)。
Clavibacter spp.(Clavibacter michiganensis subsp. michiganensis等)、Curtobacterium spp.、Leifsonia spp.(Leifsonia xyli subsp. xyli等)、Streptomyces spp.(Streptomyces ipomoeae等)等の放線菌門(Actinobacteria)グラム陽性菌類。 Chipidiomycota fungi such as Olpidium spp.
Blastocladiomycota fungi such as Physoderma spp.
Choanephora spp., Choanephoroidea cucurbitae, Mucor spp. (Mucor fragilis, etc.), Rhizopus spp. (Rhizopus arrhizus, R. chinensis, R. oryzae, R. stolonifer var. Stolonifer, etc.)
Cercozoa protists such as Plasmodiophora spp. (Plasmodiophora brassicae, etc.), Spongospora subterranea f. Sp. Subterranea, etc.
Aphanomyces spp. (Aphanomyces cochlioides, A. raphani, etc.), Albugo spp. (Albugo macrospora, A. wasabiae, etc.), Bremia spp. (Bremia lactucae, etc.), Hyaloperonospora spp., Peronosclerospora spp., Peronospora spp. wasabi, P. chrysanthemi-coronarii, P. destructor, P. farinosa f. sp. spinaciae, P. manshurica, P. parasitica, P. sparsa, etc., Plasmopara spp. (Plasmopara halstedii, P. nivea, P. viticola, etc.) ), Pseudoperonospora spp. (Pseudoperonospora cubensis, etc.), Sclerophthora spp., Phytophthora spp. (Phytophthora cactorum, P. capsici, P. citricola, P. citrophthora, P. cryptogea, P. fragariae, P. infestans, P. infestans, P. nicotianae, P. palmivora, P. porri, P. sojae, P. syringae, P. vignae f. Sp. Adzukicola, etc.), Pythium spp. (Pythium afertile, P. aphanidermatum, P. apleroticum, P. aristosporum, P. arrhenomanes, P. buismaniae, P. debaryanum, P. graminicola, P. horinouchiense, P. irregulare, P. iwayamai, P. myrioty lum, P. okanoganense, P. paddicum, P. paroecandrum, P. periplocum, P. spinosum, P. sulcatum, P. sylvaticum, P. ultimum var. ultimum, P. vanterpoolii, P. vexans, P. volutum, etc.) Heterokontophyta oomycetes (Oomycetes).
Actinobacter gram-positive bacteria such as Clavibacter spp. (Clavibacter michiganensis subsp. Michiganensis etc.), Curtobacterium spp., Leifsonia spp. (Leifsonia xyli subsp. Xyli etc.), Streptomyces spp. (Streptomyces ipomoeae etc.)
Clostridium sp.等のフィルミクテス門(Firmicutes)グラム陽性菌類。
Phytoplasma等のテネリクテス門(Tenericutes)グラム陽性菌類。
Rhizobium spp.(Rhizobium radiobacter等)、Acetobacter spp.、Burkholderia spp.(Burkholderia andropogonis、B. cepacia、B. gladioli、B. glumae、B. plantarii等)、Acidovorax spp.(Acidovorax avenae subsp. avenae、A. a. subsp. citrulli、A. konjaci等)、Herbaspirillum spp.、Ralstonia spp.(Ralstonia solanacearum等)、Xanthomonas spp.(Xanthomonas albilineans、X. arboricola pv. pruni、X. axonopodis pv. vitians、X. campestris pv. campestris、X. c. pv. cucurbitae、X. c. pv. glycines、X. c. pv. mangiferaeindicae、X. c. pv. nigromaculans、X. c. pv. vesicatoria、X. citri subsp. citri、X. oryzae pv. oryzae等)、Pseudomonas spp.(Pseudomonas cichorii、P. fluorescens、P. marginalis、P. m. pv. marginalis、P. savastanoi pv. glycinea、P. syringae、P. s. pv. actinidiae、P. s. pv. eriobotryae、P. s. pv. helianthi、P. s. pv. lachrymans、P. s. pv. maculicola、P. s. pv. mori、P. s. pv. morsprunorum、P. s. pv. spinaciae、P. s. pv. syringae、P. s. pv. theae、P. viridiflava等)、Rhizobacter spp.、Brenneria spp.(Brenneria nigrifluens等)、Dickeya spp.(Dickeya dianthicola、D. zeae等)、Erwinia spp.(Erwinia amylovora、E. rhapontici等)、Pantoea spp.、Pectobacterium spp.(Pectobacterium atrosepticum、P. carotovorum、P. wasabiae等)等のプロテオバクテリア門(Proteobacteria)グラム陰性菌類。 Firmicutes gram-positive fungi such as Clostridium sp.
Tenericutes gram-positive fungi such as Phytoplasma.
Rhizobium spp. (Rhizobium radiobacter, etc.), Acetobacter spp., Burkholderia spp. (Burkholderia andropogonis, B. cepacia, B. gladioli, B. glumae, B. plantarii, etc.), Acidovorax spp. (Acidovorax avenae subA. subsp. citrulli, A. konjaci, etc.), Herbaspirillum spp., Ralstonia spp. (Ralstonia solanacearum, etc.), Xanthomonas spp. (Xanthomonas albilineans, X. arboricola pv. pruni, X. axonopodis pv. vitians, p. campestris) campestris, X. c. pv. cucurbitae, X. c. pv. glycines, X. c. pv. mangiferaeindicae, X. c. pv. nigromaculans, X. c. pv. vesicatoria, X. citri subsp. citri, X. oryzae pv. Oryzae etc.), Pseudomonas spp. (Pseudomonas cichorii, P. fluorescens, P. marginalis, P. m. Pv. Marginalis, P. savastanoi pv. Glycinea, P. syringae, P. s. Pv. Actinidiae , P. s. Pv. Eriobotryae, P. s. Pv. Helianthi, P. s. Pv. Lachrymans, P. s. Pv. Maculicola, P. s. Pv. Mori, P. s. Pv. Morsprunorum, P s.pv.spinaciae, P.s.pv.syringae, P.s.pv.the ae, P. viridiflava, etc.), Rhizobacter spp., Brenneria spp. (Brenneria nigrifluens, etc.), Dickeya spp. (Dickeya dianthicola, D. zeae, etc.), Erwinia spp. (Erwinia amylovora, E. rhapontici, etc.), Pantoea sp. Proteobacteria gram-negative fungi such as Pectobacterium spp. (Pectobacterium atrosepticum, P. carotovorum, P. wasabiae, etc.).
Phytoplasma等のテネリクテス門(Tenericutes)グラム陽性菌類。
Rhizobium spp.(Rhizobium radiobacter等)、Acetobacter spp.、Burkholderia spp.(Burkholderia andropogonis、B. cepacia、B. gladioli、B. glumae、B. plantarii等)、Acidovorax spp.(Acidovorax avenae subsp. avenae、A. a. subsp. citrulli、A. konjaci等)、Herbaspirillum spp.、Ralstonia spp.(Ralstonia solanacearum等)、Xanthomonas spp.(Xanthomonas albilineans、X. arboricola pv. pruni、X. axonopodis pv. vitians、X. campestris pv. campestris、X. c. pv. cucurbitae、X. c. pv. glycines、X. c. pv. mangiferaeindicae、X. c. pv. nigromaculans、X. c. pv. vesicatoria、X. citri subsp. citri、X. oryzae pv. oryzae等)、Pseudomonas spp.(Pseudomonas cichorii、P. fluorescens、P. marginalis、P. m. pv. marginalis、P. savastanoi pv. glycinea、P. syringae、P. s. pv. actinidiae、P. s. pv. eriobotryae、P. s. pv. helianthi、P. s. pv. lachrymans、P. s. pv. maculicola、P. s. pv. mori、P. s. pv. morsprunorum、P. s. pv. spinaciae、P. s. pv. syringae、P. s. pv. theae、P. viridiflava等)、Rhizobacter spp.、Brenneria spp.(Brenneria nigrifluens等)、Dickeya spp.(Dickeya dianthicola、D. zeae等)、Erwinia spp.(Erwinia amylovora、E. rhapontici等)、Pantoea spp.、Pectobacterium spp.(Pectobacterium atrosepticum、P. carotovorum、P. wasabiae等)等のプロテオバクテリア門(Proteobacteria)グラム陰性菌類。 Firmicutes gram-positive fungi such as Clostridium sp.
Tenericutes gram-positive fungi such as Phytoplasma.
Rhizobium spp. (Rhizobium radiobacter, etc.), Acetobacter spp., Burkholderia spp. (Burkholderia andropogonis, B. cepacia, B. gladioli, B. glumae, B. plantarii, etc.), Acidovorax spp. (Acidovorax avenae subA. subsp. citrulli, A. konjaci, etc.), Herbaspirillum spp., Ralstonia spp. (Ralstonia solanacearum, etc.), Xanthomonas spp. (Xanthomonas albilineans, X. arboricola pv. pruni, X. axonopodis pv. vitians, p. campestris) campestris, X. c. pv. cucurbitae, X. c. pv. glycines, X. c. pv. mangiferaeindicae, X. c. pv. nigromaculans, X. c. pv. vesicatoria, X. citri subsp. citri, X. oryzae pv. Oryzae etc.), Pseudomonas spp. (Pseudomonas cichorii, P. fluorescens, P. marginalis, P. m. Pv. Marginalis, P. savastanoi pv. Glycinea, P. syringae, P. s. Pv. Actinidiae , P. s. Pv. Eriobotryae, P. s. Pv. Helianthi, P. s. Pv. Lachrymans, P. s. Pv. Maculicola, P. s. Pv. Mori, P. s. Pv. Morsprunorum, P s.pv.spinaciae, P.s.pv.syringae, P.s.pv.the ae, P. viridiflava, etc.), Rhizobacter spp., Brenneria spp. (Brenneria nigrifluens, etc.), Dickeya spp. (Dickeya dianthicola, D. zeae, etc.), Erwinia spp. (Erwinia amylovora, E. rhapontici, etc.), Pantoea sp. Proteobacteria gram-negative fungi such as Pectobacterium spp. (Pectobacterium atrosepticum, P. carotovorum, P. wasabiae, etc.).
これら病原菌の感染・増殖によって引き起こされる植物病害、動物感染症の具体例としては、以下の植物病害、動物感染症が挙げられるが、これらのみに限定されるものではない。
Specific examples of plant diseases and animal infections caused by infection and growth of these pathogenic bacteria include the following plant diseases and animal infections, but are not limited thereto.
植物病害:
モモ縮葉病Leaf curl(Taphrina deformans)、スモモふくろ実病Plum pockets(Taphrina pruni)、アスパラガス褐斑病Leaf spot(Cercospora asparagi)、テンサイ褐斑病Cercospora leaf spot(Cercospora beticola)、ピーマン斑点病Frogeye leaf spot(Cercospora capsici)、カキ角斑落葉病Angular leaf spot(Cercospora kaki)、ダイズ紫斑病Purple stain(Cercospora kikuchii)、ラッカセイ褐斑病Brown Leaf spot(Mycosphaerella arachidis)、オウトウ褐色せん孔病Cylindrosporium leaf spot(Mycosphaerella cerasella、Blumeriella jaapii)、コムギ葉枯病Speckled leaf blotch(Mycosphaerella graminicola)、カキ円星落葉病Circular leaf spot(Mycosphaerella nawae)、エンドウ褐紋病Mycosphaerella blight(Mycosphaerella pinodes)、ミョウガ葉枯病Leaf spot(Mycosphaerella zingiberis)、トマト葉かび病Leaf mold(Mycovellosiella fulva)、ナスすすかび病Leaf mold(Mycovellosiella nattrassii)、トマトすすかび病Cercospora leaf mold(Pseudocercospora fuligena)、ブドウ褐斑病Isariopsis leaf spot(Pseudocercospora vitis)、ハクサイ白斑病Leaf spot(Pseudocercosporella capsellae)、キク黒斑病Leaf spot(Septoria chrysanthemella)、キク褐斑病Leaf blight(Septoria obesa)、ブドウ黒とう病Anthracnose(Elsinoe ampelina)、タラノキそうか病Spot anthracnose(Elsinoe araliae)、カンキツそうか病Scab(Elsinoe fawcettii)、エンドウ褐斑病Leaf spot(Ascochyta pisi)、キュウリ褐斑病Corynespora leaf spot(Corynespora cassiicola)、バラ枝枯病Stem canker(Leptosphaeria coniothyrium)、バラ黒斑病Leaf spot(Alternaria alternata)、ニンジン黒葉枯病Leaf blight(Alternaria dauci)、ナシ黒斑病Black spot(Alternaria kikuchiana)、リンゴ斑点落葉病Alternaria blotch(Alternaria mali)、ネギ黒斑病Alternaria leaf spot(Alternaria porri)、ソルガム紫斑点病Target spot(Bipolaris sorghicola)、トウモロコシごま葉枯病Southern leaf blight(Cochliobolus heterostrophus)、イネごま葉枯病Brown spot(Cochliobolus miyabeanus)、ニンニク葉枯病Tip blight(Pleospora herbarum)、オオムギ斑葉病Stripe(Pyrenophora graminea)、オオムギ網斑病Net blotch(Pyrenophora teres)、ソルガムすす紋病Leaf blight(Setosphaeria turcica)、トウモロコシすす紋病Northern leaf blight(Setosphaeria turcica)、アスパラガス斑点病Leaf spot(Stemphylium botryosum)、バラ科サクラ亜科の黒星病Scab(Venturia carpophila)、リンゴ黒星病Scab(Venturia Inaequalis)、ナシ黒星病Scab(Venturia nashicola)、ウリ科のつる枯病Gummy stem blight(Didymella bryoniae)、ゴボウ黒斑病Leaf spot(Phoma exigua var. exigua)、ワサビ墨入病Streak(Phoma wasabiae)、バラ科ナシ亜科の輪紋病Ring rot(Botryosphaeria berengeriana f. sp. piricola)、キウイフルーツ果実軟腐病Soft rot(Botryosphaeria dothidea, Lasiodiplodia theobromae, Diaporthe sp.)、カンキツ緑かび病Common green mold(Penicillium digitatum)、カンキツ青かび病Blue mold(Penicillium italicum)、オオムギうどんこ病Powdery mildew(Blumeria graminis f. sp. hordei)、コムギうどんこ病Powdery mildew(Blumeria graminis f. sp. tritici)、キュウリうどんこ病Powdery mildew(Erysiphe betae, Leveillula taurica, Oidium sp., Podosphaera xanthii)、ナスうどんこ病Powdery mildew(Erysiphe cichoracearum, Leveillula taurica, Sphaerotheca fuliginea)、ニンジン、パセリのうどんこ病Powdery mildew(Erysiphe heraclei)、エンドウうどんこ病Powdery mildew(Erysiphe pisi)、トマトうどんこ病Powdery mildew(Leveillula taurica, Oidium neolycopersici, Oidium sp.)、ピーマンうどんこ病Powdery mildew(Leveillula taurica)、カボチャうどんこ病Powdery mildew(Oidium sp., Podosphaera xanthii)、ニガウリうどんこ病Powdery mildew(Oidium sp.)、カキうどんこ病Powdery mildew(Phyllactinia kakicola)、ゴボウうどんこ病Powdery mildew(Podosphaera fusca)、リンゴうどんこ病Powdery mildew(Podosphaera leucotricha)、バラうどんこ病Powdery mildew(Podosphaera pannosa, Uncinuliella simulans var. simulans, U. s. var. tandae)、ズッキーニ、マクワウリのうどんこ病Powdery mildew(Podosphaera xanthii)、イチゴうどんこ病Powdery mildew(Sphaerotheca aphanis var. aphanis)、スイカ、メロンのうどんこ病Powdery mildew(Sphaerotheca fuliginea)、ブドウうどんこ病Powdery mildew(Uncinula necator, U. n. var. necator)、リンゴ褐斑病Blotch(Diplocarpon mali)、バラ黒星病Black spot(Diplocarpon rosae)、タマネギ灰色腐敗病Gray mold neck rot(Botrytis allii)、灰色かび病Gray mold、Botrytis blight(Botrytis cinerea)、ニラ白斑葉枯病Leaf blight(Botrytis cinerea, B. byssoidea, B. squamosa)、ソラマメ赤色斑点病Chocolate spot(Botrytis cinerea, B. elliptica, B. fabae)、バラ科の灰星病Brown rot(Monilinia fructicola, M. fructigena, M. laxa)、リンゴモニリア病Blossom blight(Monilinia mali)、シバダラースポット病Dollar spot(Sclerotinia homoeocarpa)、菌核病Cottony rot、Sclerotinia rot、Stem rot(Sclerotinia sclerotiorum)、稲こうじ病False smut(Villosiclava virens)、ダイズ黒根腐病Root necrosis(Calonectria ilicicola)、コムギ赤かび病Fusarium blight(Fusarium crookwellense, F. culmorum, Gibberella avenacea, G. zeae, Monographella nivalis)、オオムギ赤かび病Fusarium blight(Fusarium culmorum, Gibberella avenacea, G. zeae)、コンニャク乾腐病Dry rot(Fusarium oxysporum, F. solani f. sp. radicicola)、ヤマノイモ褐色腐敗病Brown rot(Fusarium oxysporum, F. solani f. sp. pisi, F. s. f. sp. radicicola)、アズキ萎凋病Fusarium wilt(Fusarium oxysporum f. sp. adzukicola)、ラッキョウ乾腐病Fusarium basal rot(Fusarium oxysporum f. sp. allii, F. solani f. sp. radicicola)、サツマイモつる割病Stem rot(Fusarium oxysporum f. sp. batatas, F. solani)、サトイモ乾腐病Dry rot(Fusarium oxysporum f. sp. colocasiae)、キャベツ、コマツナの萎黄病Yellows(Fusarium oxysporum f. sp. conglutinans)、バナナパナマ病Panama disease(Fusarium oxysporum f. sp. cubense)、イチゴ萎黄病Fusarium wilt(Fusarium oxysporum f. sp. fragariae)、レタス根腐病Root rot(Fusarium oxysporum f. sp. lactucae)、スイカつる割病Fusarium wilt(Fusarium oxysporum f. sp. lagenariae, F. o. f. sp. niveum)、トマト萎凋病Fusarium wilt(Fusarium oxysporum f. sp. lycopersici)、メロンつる割病Fusarium wilt(Fusarium oxysporum f. sp. melonis)、ダイコン萎黄病Yellows(Fusarium oxysporum f. sp. raphani)、ホウレンソウ萎凋病Fusarium wilt(Fusarium oxysporum f. sp. spinaciae)、イネばか苗病“Bakanae”disease(Gibberella fujikuroi)、ダイコンバーティシリウム黒点病Verticillium black spot(Verticillium albo-atrum, V. dahliae)、トマト、ナス、フキの半身萎凋病Verticillium wilt(Verticillium dahliae)、イチジク株枯病Ceratocystis canker(Ceratocystis ficicola)、サツマイモ黒斑病Black rot(Ceratocystis fimbriata)、チャ輪斑病Gray blight(Pestalotiopsis longiseta, P. theae)、クリ胴枯病Endothia canker(Cryphonectria parasitica)、カンキツ黒点病Melanose(Diaporthe citri)、アスパラガス茎枯病Stem blight(Phomopsis asparagi)、ナシ胴枯病Phomopsis canker(Phomopsis fukushii)、ナス褐紋病Brown spot(Phomopsis vexans)、チャ炭疽病Anthracnose(Discula theae-sinensis)、リンゴ腐らん病Valsa canker(Valsa ceratosperma)、イネいもち病Blast(Magnaporthe grisea)、イチゴ炭疽病Crown rot(Colletotrichum acutatum, C. fragariae, Glomerella cingulata)、リンゴ炭疽病Bitter rot(Colletotrichum acutatum, Glomerella cingulata)、オウトウ炭疽病Anthracnose(Colletotrichum acutatum, Glomerella cingulata)、スモモ炭疽病Anthracnose(Colletotrichum acutatum)、ブドウ晩腐病Ripe rot(Colletotrichum acutatum, Glomerella cingulata)、シュンギク炭疽病Anthracnose(Colletotrichum acutatum)、インゲンマメ炭疽病Anthracnose(Colletotrichum lindemuthianum)、ウリ科の炭疽病Anthracnose(Colletotrichum orbiculare)、ヤマノイモ炭疽病Anthracnose(Glomerella cingulata)、クリ炭疽病Anthracnose(Glomerella cingulata)、カキ炭疽病Anthracnose(Glomerella cingulata)、アズキ落葉病Brown stem rot(Phialophora gregata)、ナガイモ葉渋病Leaf spot(Pseudophloeosporella dioscoreae)、オオムギ雲形病Scald(Rhynchosporium secalis)。 Plant diseases:
Leaf curl (Taphrina deformans), Plum pockets (Taphrina pruni), Asparagus brown spot Leaf spot (Cercospora asparagi), sugar beet brown spot Cercospora leaf spot (Cercospora beticola), pepper spot Frogeye leaf spot (Cercospora capsici), oyster horn spot leaf disease Angular leaf spot (Cercospora kaki), soybean purpura purple stain (Cercospora kikuchii), groundnut brown leaf spot (Mycosphaerella arachidis), sweet cherry brown spot disease (Cylindrosporium leaf spot) Mycosphaerella cerasella, Blumeriella jaapii), wheat leaf blight, Speckled leaf blotch (Mycosphaerella graminicola), oyster lobster circular leaf spot (Mycosphaerella nawae), pea brown spot Mycosphaerella blight (Mycosphaerella pinodes), myaf spot Mycosphaerella zingiberis), tomato leaf mold Leaf mold (Mycovellosiella fulva), eggplant soot mold Leaf mold (Mycovellosiella natt rassii), tomato scab fungus Cercospora leaf mold (Pseudocercospora fuligena), grape brown spot Isariopsis leaf spot (Pseudocercospora vitis), Chinese cabbage leaf spot Leaf spot (Pseudocercosporella capsellae), chrysanthemum black spot Leaf spot (Septoria chrysant brown) Leaf blight (Septoria obesa), grape black scab Anthracnose (Elsinoe ampelina), peanut scab Spot anthracnose (Elsinoe araliae), citrus scab Scab (Elsinoe fawcettii), pea brown spot Leaf spot (Ascochyta pisi) , Cucumber brown spot Corynespora leaf spot (Corynespora cassiicola), rose blight Stem canker (Leptosphaeria coniothyrium), rose black spot Leaf spot (Alternaria alternata), carrot black leaf blight Leaf blight (Alternaria dauci), pear black spot Disease Black spot (Alternaria kikuchiana), Apple spot leaf fall Alternaria blotch (Alternaria mali), Green onion black spot Alternaria leaf spot (Alternaria porri), Sorghum Purple spot disease (Bipolaris sorghicola), maize sesame leaf blight Southern leaf blight (Cochliobolus heterostrophus), rice sesame leaf blight Brown spot (Cochliobolus miyabeanus), garlic leaf blight Tip blight (Pleospora herbarum), barley leaf blight Stripe (Pyrenophora graminea), Barley net spot disease Net blotch (Pyrenophora teres), Sorghum soot disease Leaf blight (Setosphaeria turcica), Corn soot disease Northern leaf blight (Setosphaeria turcica), Asparagus spot disease leaf spot (Stemphylium botryosum) Scab (Venturia carpophila), apple scab Scab (Venturia Inaequalis), pear scab Scab (Venturia nashicola), cucurbit vine blight Gummy stem blight (Didymella bryoniae), burdock black spot Disease leaf spot (Phoma exigua var. Exigua), Wasabi ink-filled disease Streak (Phoma wasabiae), Ring rot (Botryosphaeria berengeriana f. Sp.) piricola), soft rot (Botryosphaeria dothidea, Lasiodiplodia theobromae, Diaporthe sp.), citrus green mold (Penicillium digitatum), citrus blue mold (Penicillium italicum), barley powdery mildew Powdery mildew (Blumeria graminis f. sp. hordei), wheat powdery mildew Powdery mildew (Blumeria graminis f. sp. tritici), cucumber powdery mildew Powdery mildew (Erysiphe betae, Leveillula taurica, Oidium sp., Podosphaera xanthii), eggplant udon Powdery mildew (Erysiphe cichoracearum, Leveillula taurica, Sphaerotheca fuliginea), carrots, parsley powdery mildew Powdery mildew (Erysiphe heraclei), pea powdery powdery mildew (Erysiphe pisi), tomato powdery mildew Poillery mildew Oidium neolycopersici, Oidium sp.), Pepper powdery mildew (Leveillula taurica), pumpkin powdery powdery mildew (Oidium sp., Podosphaera xanthii), powdery mildew (Oidium sp.), oyster powdery mildew Powdery mildew (Phyllactinia kakicola), burdock powdery mildew (Podosphaera fusca), apple powdery mildew (Podosphaera leucotricha), powdery mildew (Podosphaera pannosa, Uncinuliella simulans var. Simulans, U. s. Var. Tandae), powdery mildew (podosphaera xanthii), strawberry wdery mildew (Sphaerotheca aphanis var. Aphanis), watermelon, melon powdery mildew (Sphaerotheca fuliginea), grape powdery mildew (Uncinula necator, U. n. Var. Necator), apple brown spot Blotch (Diplocarpon mali) Black spot (Diplocarpon rosae), onion gray rot, Gray mold neck rot (Botrytis allii), gray mold, Botrytis blight (Botrytis cinerea), Leaf blight (Botrytis cinerea, B. byssoidea, B. squamosa), Chocolate spot (Botrytis cinerea, B. elliptica, B. fabae), Brown rot (Monilinia fructicola) , M. fructigena, M. laxa), apple moniliosis Blossom blight (Monilinia mali), Shivadara spot disease, Dollar spot (Sclerotinia homoeocarpa), mycorrhizal disease Cottony rot, Sclerotinia rot, Stem rot (Sclerotinia sclerotiorum), rice koji False smut (Villosiclava virens), soybean black root rot Root necrosis (Calonectria ilicicola), wheat red mold Fusarium blight (Fusarium crookwellense, F. culmorum, Gibberella avenacea, G. zeae, Monographella nivalis), barley red mold blight Fus Fusarium culmorum, Gibberella avenacea, G. zeae), dry rot of konjac Dry rot (Fusarium oxysporum, F. solani f. Sp. Radicicola), brown rot (Fusarium oxysporum, F. solani f. Sp. Pisi, F. sf s p. radicicola), Fusarium wilt (Fusarium oxysporum f. sp. adzukicola), dry rot of Fusarium basal rot (Fusarium oxysporum f. sp. allii, F. solani f. sp. radicicola), sweet potato vine split disease Stem rot (Fusarium oxysporum f. Sp. Batatas, F. solani), dry rot dry rot (Fusarium oxysporum f. Sp. Colocasiae), cabbage, yellow rot of Komatsuna Yellows (Fusarium oxysporum f. Sp. Conglutinans), banana Panama disease (Fusarium oxysporum f. Sp. Cubense), Fusarium wilt (Fusarium oxysporum f. Sp. Fragariae), lettuce root rot (Fusarium oxysporum f. Sp. Lactucae), Fusarium citrus disease wilt (Fusarium oxysporum f. sp. lagenariae, F. of sp. niveum), tomato wilt Fusarium wilt (Fusarium oxysporum f. sp. lycopersici), melon creeper Fusarium wilt (Fusarium oxysporum f. sp. melonis) Dwarf Yellows (Fusarium oxysporum f. Sp. Raphani Spinach wilt Fusarium wilt (Fusarium oxysporum f. Sp. Spinaciae), rice seedling disease “Bakanae” disease (Gibberella fujikuroi), diverticium black spot (Verticillium albo-atrum, V. dahliae), tomato Verticillium wilt (Verticillium dahliae), fig strain blight Ceratocystis canker (Ceratocystis ficicola), sweet potato black spot rot (Ceratocystis fimbriata), tea ring spot gray blight (Pestalotiopsis longaeta, Pestalotiopsis longaeta, ), Chestnut blight Endothia canker (Cryphonectria parasitica), citrus black spot Melanose (Diaporthe citri), asparagus stem blight Stem blight (Phomopsis asparagi), pear blight Phomopsis canker (Phomopsis fukushii), eggplant brown spot disease Brown spot (Phomopsis vexans), tea anthracnose Anthracnose (Discula theae-sinensis), apple rot Valsa canker (Valsa ceratosperma), rice blast Blast (Magnapo) rthe grisea), strawberry anthracnose Crown rot (Colletotrichum acutatum, C. fragariae, Glomerella cingulata), apple anthracnose Bitter rot (Colletotrichum acutatum, Glomerella cingulata), sweet potato anthracnose (Colletotrichum acutatum, Glomerellathracnose) (Colletotrichum acutatum), Grape late rot Ripe rot (Colletotrichum acutatum, Glomerella cingulata), Anthracnose (Colletotrichum acutatum), Anthracnose (Colletotrichum lindemuthianum), Anthracnose (Colletotrichum lindemuthianum) Diseases Anthracnose (Glomerella cingulata), chestnut anthracnose Anthracnose (Glomerella cingulata), oyster anthracnose Anthracnose (Glomerella cingulata), azuki leaf defoliation Brown stem rot (Phialophora gregata), Chinese yam leaf astringency Leaf spot (Pseudophoseo Scald (Rhynchosporium secalis).
モモ縮葉病Leaf curl(Taphrina deformans)、スモモふくろ実病Plum pockets(Taphrina pruni)、アスパラガス褐斑病Leaf spot(Cercospora asparagi)、テンサイ褐斑病Cercospora leaf spot(Cercospora beticola)、ピーマン斑点病Frogeye leaf spot(Cercospora capsici)、カキ角斑落葉病Angular leaf spot(Cercospora kaki)、ダイズ紫斑病Purple stain(Cercospora kikuchii)、ラッカセイ褐斑病Brown Leaf spot(Mycosphaerella arachidis)、オウトウ褐色せん孔病Cylindrosporium leaf spot(Mycosphaerella cerasella、Blumeriella jaapii)、コムギ葉枯病Speckled leaf blotch(Mycosphaerella graminicola)、カキ円星落葉病Circular leaf spot(Mycosphaerella nawae)、エンドウ褐紋病Mycosphaerella blight(Mycosphaerella pinodes)、ミョウガ葉枯病Leaf spot(Mycosphaerella zingiberis)、トマト葉かび病Leaf mold(Mycovellosiella fulva)、ナスすすかび病Leaf mold(Mycovellosiella nattrassii)、トマトすすかび病Cercospora leaf mold(Pseudocercospora fuligena)、ブドウ褐斑病Isariopsis leaf spot(Pseudocercospora vitis)、ハクサイ白斑病Leaf spot(Pseudocercosporella capsellae)、キク黒斑病Leaf spot(Septoria chrysanthemella)、キク褐斑病Leaf blight(Septoria obesa)、ブドウ黒とう病Anthracnose(Elsinoe ampelina)、タラノキそうか病Spot anthracnose(Elsinoe araliae)、カンキツそうか病Scab(Elsinoe fawcettii)、エンドウ褐斑病Leaf spot(Ascochyta pisi)、キュウリ褐斑病Corynespora leaf spot(Corynespora cassiicola)、バラ枝枯病Stem canker(Leptosphaeria coniothyrium)、バラ黒斑病Leaf spot(Alternaria alternata)、ニンジン黒葉枯病Leaf blight(Alternaria dauci)、ナシ黒斑病Black spot(Alternaria kikuchiana)、リンゴ斑点落葉病Alternaria blotch(Alternaria mali)、ネギ黒斑病Alternaria leaf spot(Alternaria porri)、ソルガム紫斑点病Target spot(Bipolaris sorghicola)、トウモロコシごま葉枯病Southern leaf blight(Cochliobolus heterostrophus)、イネごま葉枯病Brown spot(Cochliobolus miyabeanus)、ニンニク葉枯病Tip blight(Pleospora herbarum)、オオムギ斑葉病Stripe(Pyrenophora graminea)、オオムギ網斑病Net blotch(Pyrenophora teres)、ソルガムすす紋病Leaf blight(Setosphaeria turcica)、トウモロコシすす紋病Northern leaf blight(Setosphaeria turcica)、アスパラガス斑点病Leaf spot(Stemphylium botryosum)、バラ科サクラ亜科の黒星病Scab(Venturia carpophila)、リンゴ黒星病Scab(Venturia Inaequalis)、ナシ黒星病Scab(Venturia nashicola)、ウリ科のつる枯病Gummy stem blight(Didymella bryoniae)、ゴボウ黒斑病Leaf spot(Phoma exigua var. exigua)、ワサビ墨入病Streak(Phoma wasabiae)、バラ科ナシ亜科の輪紋病Ring rot(Botryosphaeria berengeriana f. sp. piricola)、キウイフルーツ果実軟腐病Soft rot(Botryosphaeria dothidea, Lasiodiplodia theobromae, Diaporthe sp.)、カンキツ緑かび病Common green mold(Penicillium digitatum)、カンキツ青かび病Blue mold(Penicillium italicum)、オオムギうどんこ病Powdery mildew(Blumeria graminis f. sp. hordei)、コムギうどんこ病Powdery mildew(Blumeria graminis f. sp. tritici)、キュウリうどんこ病Powdery mildew(Erysiphe betae, Leveillula taurica, Oidium sp., Podosphaera xanthii)、ナスうどんこ病Powdery mildew(Erysiphe cichoracearum, Leveillula taurica, Sphaerotheca fuliginea)、ニンジン、パセリのうどんこ病Powdery mildew(Erysiphe heraclei)、エンドウうどんこ病Powdery mildew(Erysiphe pisi)、トマトうどんこ病Powdery mildew(Leveillula taurica, Oidium neolycopersici, Oidium sp.)、ピーマンうどんこ病Powdery mildew(Leveillula taurica)、カボチャうどんこ病Powdery mildew(Oidium sp., Podosphaera xanthii)、ニガウリうどんこ病Powdery mildew(Oidium sp.)、カキうどんこ病Powdery mildew(Phyllactinia kakicola)、ゴボウうどんこ病Powdery mildew(Podosphaera fusca)、リンゴうどんこ病Powdery mildew(Podosphaera leucotricha)、バラうどんこ病Powdery mildew(Podosphaera pannosa, Uncinuliella simulans var. simulans, U. s. var. tandae)、ズッキーニ、マクワウリのうどんこ病Powdery mildew(Podosphaera xanthii)、イチゴうどんこ病Powdery mildew(Sphaerotheca aphanis var. aphanis)、スイカ、メロンのうどんこ病Powdery mildew(Sphaerotheca fuliginea)、ブドウうどんこ病Powdery mildew(Uncinula necator, U. n. var. necator)、リンゴ褐斑病Blotch(Diplocarpon mali)、バラ黒星病Black spot(Diplocarpon rosae)、タマネギ灰色腐敗病Gray mold neck rot(Botrytis allii)、灰色かび病Gray mold、Botrytis blight(Botrytis cinerea)、ニラ白斑葉枯病Leaf blight(Botrytis cinerea, B. byssoidea, B. squamosa)、ソラマメ赤色斑点病Chocolate spot(Botrytis cinerea, B. elliptica, B. fabae)、バラ科の灰星病Brown rot(Monilinia fructicola, M. fructigena, M. laxa)、リンゴモニリア病Blossom blight(Monilinia mali)、シバダラースポット病Dollar spot(Sclerotinia homoeocarpa)、菌核病Cottony rot、Sclerotinia rot、Stem rot(Sclerotinia sclerotiorum)、稲こうじ病False smut(Villosiclava virens)、ダイズ黒根腐病Root necrosis(Calonectria ilicicola)、コムギ赤かび病Fusarium blight(Fusarium crookwellense, F. culmorum, Gibberella avenacea, G. zeae, Monographella nivalis)、オオムギ赤かび病Fusarium blight(Fusarium culmorum, Gibberella avenacea, G. zeae)、コンニャク乾腐病Dry rot(Fusarium oxysporum, F. solani f. sp. radicicola)、ヤマノイモ褐色腐敗病Brown rot(Fusarium oxysporum, F. solani f. sp. pisi, F. s. f. sp. radicicola)、アズキ萎凋病Fusarium wilt(Fusarium oxysporum f. sp. adzukicola)、ラッキョウ乾腐病Fusarium basal rot(Fusarium oxysporum f. sp. allii, F. solani f. sp. radicicola)、サツマイモつる割病Stem rot(Fusarium oxysporum f. sp. batatas, F. solani)、サトイモ乾腐病Dry rot(Fusarium oxysporum f. sp. colocasiae)、キャベツ、コマツナの萎黄病Yellows(Fusarium oxysporum f. sp. conglutinans)、バナナパナマ病Panama disease(Fusarium oxysporum f. sp. cubense)、イチゴ萎黄病Fusarium wilt(Fusarium oxysporum f. sp. fragariae)、レタス根腐病Root rot(Fusarium oxysporum f. sp. lactucae)、スイカつる割病Fusarium wilt(Fusarium oxysporum f. sp. lagenariae, F. o. f. sp. niveum)、トマト萎凋病Fusarium wilt(Fusarium oxysporum f. sp. lycopersici)、メロンつる割病Fusarium wilt(Fusarium oxysporum f. sp. melonis)、ダイコン萎黄病Yellows(Fusarium oxysporum f. sp. raphani)、ホウレンソウ萎凋病Fusarium wilt(Fusarium oxysporum f. sp. spinaciae)、イネばか苗病“Bakanae”disease(Gibberella fujikuroi)、ダイコンバーティシリウム黒点病Verticillium black spot(Verticillium albo-atrum, V. dahliae)、トマト、ナス、フキの半身萎凋病Verticillium wilt(Verticillium dahliae)、イチジク株枯病Ceratocystis canker(Ceratocystis ficicola)、サツマイモ黒斑病Black rot(Ceratocystis fimbriata)、チャ輪斑病Gray blight(Pestalotiopsis longiseta, P. theae)、クリ胴枯病Endothia canker(Cryphonectria parasitica)、カンキツ黒点病Melanose(Diaporthe citri)、アスパラガス茎枯病Stem blight(Phomopsis asparagi)、ナシ胴枯病Phomopsis canker(Phomopsis fukushii)、ナス褐紋病Brown spot(Phomopsis vexans)、チャ炭疽病Anthracnose(Discula theae-sinensis)、リンゴ腐らん病Valsa canker(Valsa ceratosperma)、イネいもち病Blast(Magnaporthe grisea)、イチゴ炭疽病Crown rot(Colletotrichum acutatum, C. fragariae, Glomerella cingulata)、リンゴ炭疽病Bitter rot(Colletotrichum acutatum, Glomerella cingulata)、オウトウ炭疽病Anthracnose(Colletotrichum acutatum, Glomerella cingulata)、スモモ炭疽病Anthracnose(Colletotrichum acutatum)、ブドウ晩腐病Ripe rot(Colletotrichum acutatum, Glomerella cingulata)、シュンギク炭疽病Anthracnose(Colletotrichum acutatum)、インゲンマメ炭疽病Anthracnose(Colletotrichum lindemuthianum)、ウリ科の炭疽病Anthracnose(Colletotrichum orbiculare)、ヤマノイモ炭疽病Anthracnose(Glomerella cingulata)、クリ炭疽病Anthracnose(Glomerella cingulata)、カキ炭疽病Anthracnose(Glomerella cingulata)、アズキ落葉病Brown stem rot(Phialophora gregata)、ナガイモ葉渋病Leaf spot(Pseudophloeosporella dioscoreae)、オオムギ雲形病Scald(Rhynchosporium secalis)。 Plant diseases:
Leaf curl (Taphrina deformans), Plum pockets (Taphrina pruni), Asparagus brown spot Leaf spot (Cercospora asparagi), sugar beet brown spot Cercospora leaf spot (Cercospora beticola), pepper spot Frogeye leaf spot (Cercospora capsici), oyster horn spot leaf disease Angular leaf spot (Cercospora kaki), soybean purpura purple stain (Cercospora kikuchii), groundnut brown leaf spot (Mycosphaerella arachidis), sweet cherry brown spot disease (Cylindrosporium leaf spot) Mycosphaerella cerasella, Blumeriella jaapii), wheat leaf blight, Speckled leaf blotch (Mycosphaerella graminicola), oyster lobster circular leaf spot (Mycosphaerella nawae), pea brown spot Mycosphaerella blight (Mycosphaerella pinodes), myaf spot Mycosphaerella zingiberis), tomato leaf mold Leaf mold (Mycovellosiella fulva), eggplant soot mold Leaf mold (Mycovellosiella natt rassii), tomato scab fungus Cercospora leaf mold (Pseudocercospora fuligena), grape brown spot Isariopsis leaf spot (Pseudocercospora vitis), Chinese cabbage leaf spot Leaf spot (Pseudocercosporella capsellae), chrysanthemum black spot Leaf spot (Septoria chrysant brown) Leaf blight (Septoria obesa), grape black scab Anthracnose (Elsinoe ampelina), peanut scab Spot anthracnose (Elsinoe araliae), citrus scab Scab (Elsinoe fawcettii), pea brown spot Leaf spot (Ascochyta pisi) , Cucumber brown spot Corynespora leaf spot (Corynespora cassiicola), rose blight Stem canker (Leptosphaeria coniothyrium), rose black spot Leaf spot (Alternaria alternata), carrot black leaf blight Leaf blight (Alternaria dauci), pear black spot Disease Black spot (Alternaria kikuchiana), Apple spot leaf fall Alternaria blotch (Alternaria mali), Green onion black spot Alternaria leaf spot (Alternaria porri), Sorghum Purple spot disease (Bipolaris sorghicola), maize sesame leaf blight Southern leaf blight (Cochliobolus heterostrophus), rice sesame leaf blight Brown spot (Cochliobolus miyabeanus), garlic leaf blight Tip blight (Pleospora herbarum), barley leaf blight Stripe (Pyrenophora graminea), Barley net spot disease Net blotch (Pyrenophora teres), Sorghum soot disease Leaf blight (Setosphaeria turcica), Corn soot disease Northern leaf blight (Setosphaeria turcica), Asparagus spot disease leaf spot (Stemphylium botryosum) Scab (Venturia carpophila), apple scab Scab (Venturia Inaequalis), pear scab Scab (Venturia nashicola), cucurbit vine blight Gummy stem blight (Didymella bryoniae), burdock black spot Disease leaf spot (Phoma exigua var. Exigua), Wasabi ink-filled disease Streak (Phoma wasabiae), Ring rot (Botryosphaeria berengeriana f. Sp.) piricola), soft rot (Botryosphaeria dothidea, Lasiodiplodia theobromae, Diaporthe sp.), citrus green mold (Penicillium digitatum), citrus blue mold (Penicillium italicum), barley powdery mildew Powdery mildew (Blumeria graminis f. sp. hordei), wheat powdery mildew Powdery mildew (Blumeria graminis f. sp. tritici), cucumber powdery mildew Powdery mildew (Erysiphe betae, Leveillula taurica, Oidium sp., Podosphaera xanthii), eggplant udon Powdery mildew (Erysiphe cichoracearum, Leveillula taurica, Sphaerotheca fuliginea), carrots, parsley powdery mildew Powdery mildew (Erysiphe heraclei), pea powdery powdery mildew (Erysiphe pisi), tomato powdery mildew Poillery mildew Oidium neolycopersici, Oidium sp.), Pepper powdery mildew (Leveillula taurica), pumpkin powdery powdery mildew (Oidium sp., Podosphaera xanthii), powdery mildew (Oidium sp.), oyster powdery mildew Powdery mildew (Phyllactinia kakicola), burdock powdery mildew (Podosphaera fusca), apple powdery mildew (Podosphaera leucotricha), powdery mildew (Podosphaera pannosa, Uncinuliella simulans var. Simulans, U. s. Var. Tandae), powdery mildew (podosphaera xanthii), strawberry wdery mildew (Sphaerotheca aphanis var. Aphanis), watermelon, melon powdery mildew (Sphaerotheca fuliginea), grape powdery mildew (Uncinula necator, U. n. Var. Necator), apple brown spot Blotch (Diplocarpon mali) Black spot (Diplocarpon rosae), onion gray rot, Gray mold neck rot (Botrytis allii), gray mold, Botrytis blight (Botrytis cinerea), Leaf blight (Botrytis cinerea, B. byssoidea, B. squamosa), Chocolate spot (Botrytis cinerea, B. elliptica, B. fabae), Brown rot (Monilinia fructicola) , M. fructigena, M. laxa), apple moniliosis Blossom blight (Monilinia mali), Shivadara spot disease, Dollar spot (Sclerotinia homoeocarpa), mycorrhizal disease Cottony rot, Sclerotinia rot, Stem rot (Sclerotinia sclerotiorum), rice koji False smut (Villosiclava virens), soybean black root rot Root necrosis (Calonectria ilicicola), wheat red mold Fusarium blight (Fusarium crookwellense, F. culmorum, Gibberella avenacea, G. zeae, Monographella nivalis), barley red mold blight Fus Fusarium culmorum, Gibberella avenacea, G. zeae), dry rot of konjac Dry rot (Fusarium oxysporum, F. solani f. Sp. Radicicola), brown rot (Fusarium oxysporum, F. solani f. Sp. Pisi, F. sf s p. radicicola), Fusarium wilt (Fusarium oxysporum f. sp. adzukicola), dry rot of Fusarium basal rot (Fusarium oxysporum f. sp. allii, F. solani f. sp. radicicola), sweet potato vine split disease Stem rot (Fusarium oxysporum f. Sp. Batatas, F. solani), dry rot dry rot (Fusarium oxysporum f. Sp. Colocasiae), cabbage, yellow rot of Komatsuna Yellows (Fusarium oxysporum f. Sp. Conglutinans), banana Panama disease (Fusarium oxysporum f. Sp. Cubense), Fusarium wilt (Fusarium oxysporum f. Sp. Fragariae), lettuce root rot (Fusarium oxysporum f. Sp. Lactucae), Fusarium citrus disease wilt (Fusarium oxysporum f. sp. lagenariae, F. of sp. niveum), tomato wilt Fusarium wilt (Fusarium oxysporum f. sp. lycopersici), melon creeper Fusarium wilt (Fusarium oxysporum f. sp. melonis) Dwarf Yellows (Fusarium oxysporum f. Sp. Raphani Spinach wilt Fusarium wilt (Fusarium oxysporum f. Sp. Spinaciae), rice seedling disease “Bakanae” disease (Gibberella fujikuroi), diverticium black spot (Verticillium albo-atrum, V. dahliae), tomato Verticillium wilt (Verticillium dahliae), fig strain blight Ceratocystis canker (Ceratocystis ficicola), sweet potato black spot rot (Ceratocystis fimbriata), tea ring spot gray blight (Pestalotiopsis longaeta, Pestalotiopsis longaeta, ), Chestnut blight Endothia canker (Cryphonectria parasitica), citrus black spot Melanose (Diaporthe citri), asparagus stem blight Stem blight (Phomopsis asparagi), pear blight Phomopsis canker (Phomopsis fukushii), eggplant brown spot disease Brown spot (Phomopsis vexans), tea anthracnose Anthracnose (Discula theae-sinensis), apple rot Valsa canker (Valsa ceratosperma), rice blast Blast (Magnapo) rthe grisea), strawberry anthracnose Crown rot (Colletotrichum acutatum, C. fragariae, Glomerella cingulata), apple anthracnose Bitter rot (Colletotrichum acutatum, Glomerella cingulata), sweet potato anthracnose (Colletotrichum acutatum, Glomerellathracnose) (Colletotrichum acutatum), Grape late rot Ripe rot (Colletotrichum acutatum, Glomerella cingulata), Anthracnose (Colletotrichum acutatum), Anthracnose (Colletotrichum lindemuthianum), Anthracnose (Colletotrichum lindemuthianum) Diseases Anthracnose (Glomerella cingulata), chestnut anthracnose Anthracnose (Glomerella cingulata), oyster anthracnose Anthracnose (Glomerella cingulata), azuki leaf defoliation Brown stem rot (Phialophora gregata), Chinese yam leaf astringency Leaf spot (Pseudophoseo Scald (Rhynchosporium secalis).
イチジクさび病Rust(Phakopsora nishidana)、ダイズさび病Rust(Phakopsora pachyrhizi)、バラさび病Rust(Kuehneola japonica, Phragmidium fusiforme, P. mucronatum, P. rosae-multiflorae)、ナシ赤星病Rust(Gymnosporangium asiaticum)、リンゴ赤星病Rust(Gymnosporangium yamadae)、ネギ科のさび病Rust(Puccinia allii)、キク白さび病Rust(Puccinia horiana)、コムギ赤さび病Brown rust(Puccinia recondita)、キク黒さび病Rust(Puccinia tanaceti var. tanaceti)、ソラマメさび病Rust(Uromyces viciae-fabae var. viciae-fabae)、サトウキビ黒穂病Smut(Sporisorium scitamineum)、トウモロコシ黒穂病Smut(Ustilago maydis)、オオムギ裸黒穂病Loose smut(Ustilago nuda)、チャ網もち病Net blister blight(Exobasidium reticulatum)、チャもち病Blister blight(Exobasidium vexans)、白絹病Stem rot、Southern blight(Athelia rolfsii)、キク立枯病Root and stem rot(Ceratobasidium cornigerum, Rhizoctonia solani)、ショウガ紋枯病(Rhizoctonia solani)、キャベツ苗立枯病Damping-off(Rhizoctonia solani)、ミツバ立枯病Damping-off(Rhizoctonia solani)、レタスすそ枯病Bottom rot(Rhizoctonia solani)、シバ葉腐病Brown patch、Large patch(Rhizoctonia solani)、イネ紋枯病Sheath blight(Thanatephorus cucumeris)、テンサイ根腐病Root rot・葉腐病Leaf blight(Thanatephorus cucumeris)。
Fig rust Rust (Phakopsora nishidana), soybean rust Rust (Phakopsorachypachyrhizi), rose rust Rust (Kuehneolanejaponica, hPhragmidiumragfusiforme, P. mucronatum, cronP. Rosae-multiflorae), pear Red Star Rust (Gymnosporangiumdayamadae), Rust of Rubiaceae Rust (Puccinia allii), Chrysanthemum Rust (Puccinia horiana), Wheat Red Rust BrownBrrust (Puccinia recondita), Chrysanthemum Rust (Puccinia tanaceti var.tanaceti) ), Broad bean rust Rust (Uromyces viciae-fabae var. Viciae-fabae), sugarcane smut Smut (Sporisorium scitamineum), corn smut Smut (Ustilago maydis), barley bare smut Loose smut (Ustilago nuda) Diseases Net blister blight (Exobasidium reticulatum), tea blast Blister blight (Exobasidium vexans), white silkworm Stem rot, Southern blight (Athelia rolfsii), ki Root and stem rot (Ceratobasidium cornigerum, Rhizoctonia solani), ginger rot (Rhizoctonia solani), cabbage seedling damping Damping-off (Rhizoctonia solani), honey beetle Damping-off (Rhizoctonia solani) Lettuce bottom blight Bottom rot (Rhizoctonia solani), Shiba leaf rot Brown patch, Large patch (Rhizoctonia solani), rice blight, Heath blight (Thanatephorus cucumeris), sugar beet root rot Root rot and leaf rot Leafaf blight (Thanatephorus) cucumeris).
イチジク黒かび病Rhizopus rot(Rhizopus stolonifer var. stolonifer)、
アブラナ科根こぶ病Clubroot(Plasmodiophora brassicae)、
テンサイ黒根病Aphanomyces root rot(Aphanomyces cochlioides)、アブラナ科の白さび病White rust(Albugo macrospora)、レタスべと病Downy mildew(Bremia lactucae)、シュンギクべと病Downy mildew(Peronospora chrysanthemi-coronarii)、タマネギ、ネギのべと病Downy mildew(Peronospora destructor)、ホウレンソウべと病Downy mildew(Peronospora farinosa f. sp. spinaciae)、ダイズべと病Downy mildew(Peronospora manshurica)、アブラナ科のべと病Downy mildew(Peronospora parasitica)、バラべと病Downy mildew(Peronospora sparsa)、ヒマワリべと病Downy mildew(Plasmopara halstedii)、ミツバべと病Downy mildew(Plasmopara nivea)、ブドウべと病Downy mildew(Plasmopara viticola)、ウリ科のべと病Downy mildew(Pseudoperonospora cubensis)、タラノキ立枯疫病Phytophthora root rot(Phytophthora cactorum)、スイカ褐色腐敗病Brown rot(Phytophthora capsici)、カボチャ疫病Phytophthora rot(Phytophthora capsici)、ピーマン疫病Phytophthora blight(Phytophthora capsici)、スイカ疫病Phytophthora rot(Phytophthora cryptogea)、トマト、ジャガイモの疫病Late blight(Phytophthora infestans)、イチジク疫病White powdery rot(Phytophthora palmivora)、ネギ科の白色疫病Leaf blight(Phytophthora porri)、ダイズ茎疫病Phytophthora root and stem rot(Phytophthora sojae)、アズキ茎疫病Phytophthora stem rot(Phytophthora vignae f. sp. adzukicola)、ホウレンソウ立枯病Damping-off(Pythium aphanidermatum, P. myriotylum, P. paroecandrum, P. ultimum var. ultimum)、コンニャク根腐病Root rot(Pythium aristosporum)、トウモロコシ根腐病Browning root rot(Pythium arrhenomanes, P. graminicola)、キャベツ苗立枯病Damping-off(Pythium buismaniae, P. myriotylum)、ミョウガ根茎腐敗病Root rot(Pythium myriotylum)、ショウガ根茎腐敗病Root rot(Pythium myriotylum, P. ultimum var. ultimum)、ニンジンしみ腐病Brown blotted root rot(Pythium sulcatum)。 Rhizopus rot (Rhizopus stolonifer var. Stolonifer),
Clubroot (Plasmodiophora brassicae),
Sugar beet root disease Aphanomyces root rot (Aphanomyces cochlioides), white rust (Albugo macrospora) of Brassicaceae, lettuce downy mildew (Bremia lactucae), downy mildew (Peronospora chrysanthemi-coronarii), onion, Downy mildew (Peronospora destructor), spinach downy mildew (Peronospora farinosa f. Sp. Spinaciae), downy mildew (Peronospora manshurica), downy mildew (Peronospora parasitica) ), Downy mildew (Peronospora sparsa), downy mildew (Plasmopara halstedii), downy mildew (Plasmopara nivea), downy mildew (Plasmopara viticola), downy mildew And Downy mildew (Pseudoperonospora cubensis), Phylophthora root rot (Phytophthora cactorum), watermelon brown rot (Phytophthora capsici), Phytophthora rot (Phytophthora capsici), Pepper plague Phytophthora blight (Phytophthora capsici), Watermelon plague Phytophthora rot (Phytophthora cryptogea), Tomato, Potato plague Late blight (Phytophthora infestans) Leaf blight (Phytophthora porri), soybean stem plague Phytophthora root and stem rot (Phytophthora sojae), azuki bean stem disease Phytophthora stem rot (Phytophthora vignae f. Sp. Adzukicola), spinach blight , P. myriotylum, P. paroecandrum, P. ultimum var. Ultimum), konjac root rot Root rot (Pythium aristosporum), corn root rot Browning root rot (Pythium arrhenomanes, P. graminicola), cabbage seedling damping -off (Pythium buismaniae, P. myriotylum), Root rot (Pythium myriotylum), Ginger rhizome rot Roo t rot (Pythium myriotylum, P. ultimum var. ultimum), carrot stain rot Brown blotted root rot (Pythium sulcatum).
アブラナ科根こぶ病Clubroot(Plasmodiophora brassicae)、
テンサイ黒根病Aphanomyces root rot(Aphanomyces cochlioides)、アブラナ科の白さび病White rust(Albugo macrospora)、レタスべと病Downy mildew(Bremia lactucae)、シュンギクべと病Downy mildew(Peronospora chrysanthemi-coronarii)、タマネギ、ネギのべと病Downy mildew(Peronospora destructor)、ホウレンソウべと病Downy mildew(Peronospora farinosa f. sp. spinaciae)、ダイズべと病Downy mildew(Peronospora manshurica)、アブラナ科のべと病Downy mildew(Peronospora parasitica)、バラべと病Downy mildew(Peronospora sparsa)、ヒマワリべと病Downy mildew(Plasmopara halstedii)、ミツバべと病Downy mildew(Plasmopara nivea)、ブドウべと病Downy mildew(Plasmopara viticola)、ウリ科のべと病Downy mildew(Pseudoperonospora cubensis)、タラノキ立枯疫病Phytophthora root rot(Phytophthora cactorum)、スイカ褐色腐敗病Brown rot(Phytophthora capsici)、カボチャ疫病Phytophthora rot(Phytophthora capsici)、ピーマン疫病Phytophthora blight(Phytophthora capsici)、スイカ疫病Phytophthora rot(Phytophthora cryptogea)、トマト、ジャガイモの疫病Late blight(Phytophthora infestans)、イチジク疫病White powdery rot(Phytophthora palmivora)、ネギ科の白色疫病Leaf blight(Phytophthora porri)、ダイズ茎疫病Phytophthora root and stem rot(Phytophthora sojae)、アズキ茎疫病Phytophthora stem rot(Phytophthora vignae f. sp. adzukicola)、ホウレンソウ立枯病Damping-off(Pythium aphanidermatum, P. myriotylum, P. paroecandrum, P. ultimum var. ultimum)、コンニャク根腐病Root rot(Pythium aristosporum)、トウモロコシ根腐病Browning root rot(Pythium arrhenomanes, P. graminicola)、キャベツ苗立枯病Damping-off(Pythium buismaniae, P. myriotylum)、ミョウガ根茎腐敗病Root rot(Pythium myriotylum)、ショウガ根茎腐敗病Root rot(Pythium myriotylum, P. ultimum var. ultimum)、ニンジンしみ腐病Brown blotted root rot(Pythium sulcatum)。 Rhizopus rot (Rhizopus stolonifer var. Stolonifer),
Clubroot (Plasmodiophora brassicae),
Sugar beet root disease Aphanomyces root rot (Aphanomyces cochlioides), white rust (Albugo macrospora) of Brassicaceae, lettuce downy mildew (Bremia lactucae), downy mildew (Peronospora chrysanthemi-coronarii), onion, Downy mildew (Peronospora destructor), spinach downy mildew (Peronospora farinosa f. Sp. Spinaciae), downy mildew (Peronospora manshurica), downy mildew (Peronospora parasitica) ), Downy mildew (Peronospora sparsa), downy mildew (Plasmopara halstedii), downy mildew (Plasmopara nivea), downy mildew (Plasmopara viticola), downy mildew And Downy mildew (Pseudoperonospora cubensis), Phylophthora root rot (Phytophthora cactorum), watermelon brown rot (Phytophthora capsici), Phytophthora rot (Phytophthora capsici), Pepper plague Phytophthora blight (Phytophthora capsici), Watermelon plague Phytophthora rot (Phytophthora cryptogea), Tomato, Potato plague Late blight (Phytophthora infestans) Leaf blight (Phytophthora porri), soybean stem plague Phytophthora root and stem rot (Phytophthora sojae), azuki bean stem disease Phytophthora stem rot (Phytophthora vignae f. Sp. Adzukicola), spinach blight , P. myriotylum, P. paroecandrum, P. ultimum var. Ultimum), konjac root rot Root rot (Pythium aristosporum), corn root rot Browning root rot (Pythium arrhenomanes, P. graminicola), cabbage seedling damping -off (Pythium buismaniae, P. myriotylum), Root rot (Pythium myriotylum), Ginger rhizome rot Roo t rot (Pythium myriotylum, P. ultimum var. ultimum), carrot stain rot Brown blotted root rot (Pythium sulcatum).
トマトかいよう病Bacterial canker(Clavibacter michiganensis subsp. michiganensis)、ジャガイモそうか病Scab(Streptomyces spp.)、
バラ根頭がんしゅ病Crown gall(Rhizobium radiobacter)、ソルガム条斑細菌病Bacterial stripe(Burkholderia andropogonis)、タマネギ腐敗病Soft rot(Burkholderia cepacia, Pseudomonas marginalis pv. marginalis, Erwinia rhapontici)、イネもみ枯細菌病Bacterial grain rot(Burkholderia gladioli, B. glumae)、スイカ果実汚斑細菌病Bacterial fruit blotch(Acidovorax avenae subsp. citrulli)、コンニャク葉枯病Bacterial leaf blight(Acidovorax konjaci)、青枯病Bacterial wilt(Ralstonia solanacearum)、モモせん孔細菌病Bacterial shot hole(Xanthomonas arboricola pv. pruni, Pseudomonas syringae pv. syringae, Brenneria nigrifluens)、スモモ黒斑病Bacterial leaf spot(Xanthomonas arboricola pv. pruni)、レタス斑点細菌病Bacterial spot(Xanthomonas axonopodis pv. vitians)、アブラナ科の黒腐病Black rot(Xanthomonas campestris pv. campestris)、ダイズ葉焼病Bacterial pustule(Xanthomonas campestris pv. glycines)、ゴボウ黒斑細菌病Bacterial spot(Xanthomonas campestris pv. nigromaculans)、ピーマン斑点細菌病Bacterial spot(Xanthomonas campestris pv. vesicatoria)、カンキツかいよう病Citrus canker(Xanthomonas citri subsp. citri)、ニンニク春腐病(Pseudomonas cichorii, P. marginalis pv. marginalis, Erwinia sp.)、レタス腐敗病Bacterial rot(Pseudomonas cichorii, P. marginalis pv. marginalis, P. viridiflava)、キウイフルーツ花腐細菌病Bacterial blossom blight(Pseudomonas marginalis pv. marginalis, P. syringae pv. syringae, P. viridiflava)、キウイフルーツかいよう病Bacterial canker(Pseudomonas syringae pv. actinidiae)、ビワがんしゅ病Canker(Pseudomonas syringae pv. eriobotryae)、ウリ科の斑点細菌病Bacterial spot(Pseudomonas syringae pv. lachrymans)、アブラナ科の黒斑細菌病Bacterial black spot(Pseudomonas syringae pv. maculicola)、ウメかいよう病Bacterial canker(Pseudomonas syringae pv. morsprunorum, Erwinia sp.)、チャ赤焼病Bacterial shoot blight(Pseudomonas syringae pv. theae)、ネギ軟腐病Bacterial soft rot(Dickeya sp., Pectobacterium carotovorum)、バラ科ナシ亜科の火傷病Fire blight(Erwinia amylovora)、コンニャク腐敗病Soft rot(Pectobacterium carotovorum)、軟腐病Bacterial soft rot(Pectobacterium carotovorum)。 Tomato Scab Bacterial canker (Clavibacter michiganensis subsp. Michiganensis), Potato Scab Scab (Streptomyces spp.),
Crown gall (Rhizobium radiobacter), sorghum streak bacterial disease Bacterial stripe (Burkholderia andropogonis), onion rot Soft rot (Burkholderia cepacia, Pseudomonas marginalis pv. Marginalis, Erwinia rhapontici), rice blast Bacterial grain rot (Burkholderia gladioli, B. glumae), Bacterial fruit blotch (Acidovorax avenae subsp. Citrulli), Bacterial leaf blight (Acidovorax konjaci), Bacterial wilt (Ralrumonia soace) , Bacterial shot hole (Xanthomonas arboricola pv. Pruni, Pseudomonas syringae pv. Syringae, Brenneria nigrifluens), peach black spot Bacterial leaf spot (Xanthomonas arboricola pv. Pruni) vitians), cruciferous black rot (Xanthomonas campestris pv. campestris), soybean leaf burning Bacterial pustule (Xanthomonas ca mpestris pv. glycines), burdock black spot Bacterial spot (Xanthomonas campestris pv. nigromaculans), bell pepper spot Bacterial spot (Xanthomonas campestris pv. vesicatoria), citrus canker (Xanthomonas citri subsp. citri), spring Rot (Pseudomonas cichorii, P. marginalis pv. Marginalis, Erwinia sp.), Lettuce rot Bacterial rot (Pseudomonas cichorii, P. marginalis pv. Marginalis, P. viridiflava), kiwi fruit bacteriomycosis Bacterial blossom blight (Pseudomonmon marginalis pv. marginalis, P. syringae pv. syringae, P. viridiflava), kiwifruit scab Bacterial canker (Pseudomonas syringae pv. actinidiae), loquat cancer canker (Pseudomonas syringae pv. eriobotryae) Bacterial spot (Pseudomonas syringae pv. Lachrymans), Bacterial black spot (Pseudomonas syringae pv. Maculicola), Japanese scab B acterial canker (Pseudomonas syringae pv. morsprunorum, Erwinia sp.), tea red burn Bacterial shoot blight (Pseudomonas syringae pv. theae), leek soft rot Bacterial soft rot (Dickeya sp., Pectobacterium carotovorum), Rosaceae Fire blight (Erwinia amylovora), konjac rot Soft rot (Pectobacterium carotovorum), soft rot Bacterial soft rot (Pectobacterium carotovorum).
バラ根頭がんしゅ病Crown gall(Rhizobium radiobacter)、ソルガム条斑細菌病Bacterial stripe(Burkholderia andropogonis)、タマネギ腐敗病Soft rot(Burkholderia cepacia, Pseudomonas marginalis pv. marginalis, Erwinia rhapontici)、イネもみ枯細菌病Bacterial grain rot(Burkholderia gladioli, B. glumae)、スイカ果実汚斑細菌病Bacterial fruit blotch(Acidovorax avenae subsp. citrulli)、コンニャク葉枯病Bacterial leaf blight(Acidovorax konjaci)、青枯病Bacterial wilt(Ralstonia solanacearum)、モモせん孔細菌病Bacterial shot hole(Xanthomonas arboricola pv. pruni, Pseudomonas syringae pv. syringae, Brenneria nigrifluens)、スモモ黒斑病Bacterial leaf spot(Xanthomonas arboricola pv. pruni)、レタス斑点細菌病Bacterial spot(Xanthomonas axonopodis pv. vitians)、アブラナ科の黒腐病Black rot(Xanthomonas campestris pv. campestris)、ダイズ葉焼病Bacterial pustule(Xanthomonas campestris pv. glycines)、ゴボウ黒斑細菌病Bacterial spot(Xanthomonas campestris pv. nigromaculans)、ピーマン斑点細菌病Bacterial spot(Xanthomonas campestris pv. vesicatoria)、カンキツかいよう病Citrus canker(Xanthomonas citri subsp. citri)、ニンニク春腐病(Pseudomonas cichorii, P. marginalis pv. marginalis, Erwinia sp.)、レタス腐敗病Bacterial rot(Pseudomonas cichorii, P. marginalis pv. marginalis, P. viridiflava)、キウイフルーツ花腐細菌病Bacterial blossom blight(Pseudomonas marginalis pv. marginalis, P. syringae pv. syringae, P. viridiflava)、キウイフルーツかいよう病Bacterial canker(Pseudomonas syringae pv. actinidiae)、ビワがんしゅ病Canker(Pseudomonas syringae pv. eriobotryae)、ウリ科の斑点細菌病Bacterial spot(Pseudomonas syringae pv. lachrymans)、アブラナ科の黒斑細菌病Bacterial black spot(Pseudomonas syringae pv. maculicola)、ウメかいよう病Bacterial canker(Pseudomonas syringae pv. morsprunorum, Erwinia sp.)、チャ赤焼病Bacterial shoot blight(Pseudomonas syringae pv. theae)、ネギ軟腐病Bacterial soft rot(Dickeya sp., Pectobacterium carotovorum)、バラ科ナシ亜科の火傷病Fire blight(Erwinia amylovora)、コンニャク腐敗病Soft rot(Pectobacterium carotovorum)、軟腐病Bacterial soft rot(Pectobacterium carotovorum)。 Tomato Scab Bacterial canker (Clavibacter michiganensis subsp. Michiganensis), Potato Scab Scab (Streptomyces spp.),
Crown gall (Rhizobium radiobacter), sorghum streak bacterial disease Bacterial stripe (Burkholderia andropogonis), onion rot Soft rot (Burkholderia cepacia, Pseudomonas marginalis pv. Marginalis, Erwinia rhapontici), rice blast Bacterial grain rot (Burkholderia gladioli, B. glumae), Bacterial fruit blotch (Acidovorax avenae subsp. Citrulli), Bacterial leaf blight (Acidovorax konjaci), Bacterial wilt (Ralrumonia soace) , Bacterial shot hole (Xanthomonas arboricola pv. Pruni, Pseudomonas syringae pv. Syringae, Brenneria nigrifluens), peach black spot Bacterial leaf spot (Xanthomonas arboricola pv. Pruni) vitians), cruciferous black rot (Xanthomonas campestris pv. campestris), soybean leaf burning Bacterial pustule (Xanthomonas ca mpestris pv. glycines), burdock black spot Bacterial spot (Xanthomonas campestris pv. nigromaculans), bell pepper spot Bacterial spot (Xanthomonas campestris pv. vesicatoria), citrus canker (Xanthomonas citri subsp. citri), spring Rot (Pseudomonas cichorii, P. marginalis pv. Marginalis, Erwinia sp.), Lettuce rot Bacterial rot (Pseudomonas cichorii, P. marginalis pv. Marginalis, P. viridiflava), kiwi fruit bacteriomycosis Bacterial blossom blight (Pseudomonmon marginalis pv. marginalis, P. syringae pv. syringae, P. viridiflava), kiwifruit scab Bacterial canker (Pseudomonas syringae pv. actinidiae), loquat cancer canker (Pseudomonas syringae pv. eriobotryae) Bacterial spot (Pseudomonas syringae pv. Lachrymans), Bacterial black spot (Pseudomonas syringae pv. Maculicola), Japanese scab B acterial canker (Pseudomonas syringae pv. morsprunorum, Erwinia sp.), tea red burn Bacterial shoot blight (Pseudomonas syringae pv. theae), leek soft rot Bacterial soft rot (Dickeya sp., Pectobacterium carotovorum), Rosaceae Fire blight (Erwinia amylovora), konjac rot Soft rot (Pectobacterium carotovorum), soft rot Bacterial soft rot (Pectobacterium carotovorum).
動物感染症:
ニューモシスチス肺炎Pneumocystis pneumonia(Pneumocystis jirovecii)、カンジダ症Candidiasis(Candida albicans)、アスペルギルス症Aspergillosis(Aspergillus fumigatus)、白癬菌症Trichophytosis(Microsporum canis、M. gypseum、Trichophyton mentagrophytes、T. rubrum、T. tonsurans、T. verrucosum)、ヒストプラズマ症Histoplasmosis(Histoplasma capsulatum)、クリプトコッカス症Cryptococcosis(Cryptococcus neoformans)。 Animal infections:
Pneumocystis pneumonia (Pneumocystis jirovecii), Candidiasis (Candida albicans), Aspergillosis (Aspergillus fumigatus), Trichophytosis (Microsporum canis, M. gypseum, T. phytorum. verrucosum), Histoplasmosis (Histoplasma capsulatum), Cryptococcosis (Cryptococcus neoformans).
ニューモシスチス肺炎Pneumocystis pneumonia(Pneumocystis jirovecii)、カンジダ症Candidiasis(Candida albicans)、アスペルギルス症Aspergillosis(Aspergillus fumigatus)、白癬菌症Trichophytosis(Microsporum canis、M. gypseum、Trichophyton mentagrophytes、T. rubrum、T. tonsurans、T. verrucosum)、ヒストプラズマ症Histoplasmosis(Histoplasma capsulatum)、クリプトコッカス症Cryptococcosis(Cryptococcus neoformans)。 Animal infections:
Pneumocystis pneumonia (Pneumocystis jirovecii), Candidiasis (Candida albicans), Aspergillosis (Aspergillus fumigatus), Trichophytosis (Microsporum canis, M. gypseum, T. phytorum. verrucosum), Histoplasmosis (Histoplasma capsulatum), Cryptococcosis (Cryptococcus neoformans).
本明細書における「寄生虫」とは、植物に寄生する植物寄生性の線形動物、動物に寄生する動物寄生性の線形動物、鉤頭動物、扁形動物及び原生動物等を意味し、具体例としては、以下の寄生虫が挙げられるが、これらのみに限定されるものではない。
As used herein, the term “parasite” means plant parasitic linear animals parasitic on plants, animal parasitic linear animals parasitic on animals, bald animals, flat animals, protozoa, etc. Include, but are not limited to, the following parasites:
腎虫Giant kidney worm(Dioctophyma renale)、有環毛細線虫Thread worms(Capillaria annulata)、捻転毛細線虫Cropworm(Capillaria contorta)、肝毛細線虫Capillary liver worm(Capillaria hepatica)、穿通毛細線虫(Capillaria perforans)、フィリピン毛細線虫(Capillaria philippinensis)、豚毛細線虫(Capillaria suis)、牛鞭虫Whipworm(Trichuris discolor)、羊鞭虫Whipworm(Trichuris ovis)、豚鞭虫Pig whipworm(Trichuris suis)、ヒト鞭虫Human whipworm(Trichuris trichiura)、犬鞭虫Dog whipworm(Trichuris vulpis)、旋毛虫Pork worm(Trichinella spiralis)等のエノプルス目(Enoplida)線虫。
乳頭糞線虫Intestinal threadworm(Strongyloides papillosus)、猫糞線虫(Strongyloides planiceps)、豚糞線虫Pig threadworm(Strongyloides ransomi)、ヒト糞線虫Threadworm(Strongyloides stercoralis)、ミクロネマ属(Micronema spp.)等の桿線虫目(Rhabditida)線虫。 Giant kidney worm (Dioctophyma renale), ringed capillary worm Thread worms (Capillaria annulata), torsion capillary nematode Cropworm (Capillaria contorta), liver capillary nematode Capillary liver worm (Capillaria hepatica), penetrating capillary nematode (Capillaria) perforans), Philippine Capillaria philippinensis, Capillaria suis, Caterpillar Whipworm (Trichuris discolor), Whipworm Whipworm (Trichuris ovis), Pig whipworm Pig Whipworm (Trichuris suis), human Enoplida nematodes such as the whipworm Human whipworm (Trichuris trichiura), the dog whipworm Dog whipworm (Trichuris vulpis), and the Trichinella pork worm (Trichinella spiralis).
桿 such as Nipple worm Nematode Intestinal threadworm (Strongyloides papillosus), Cat feline worm (Strongyloides planiceps), Pig worm nematode Pig threadworm (Strongyloides ransomi), Human dung beetle Threadworm (Strongyloides stercoralis), Micronema spp. Rhabditida nematode.
乳頭糞線虫Intestinal threadworm(Strongyloides papillosus)、猫糞線虫(Strongyloides planiceps)、豚糞線虫Pig threadworm(Strongyloides ransomi)、ヒト糞線虫Threadworm(Strongyloides stercoralis)、ミクロネマ属(Micronema spp.)等の桿線虫目(Rhabditida)線虫。 Giant kidney worm (Dioctophyma renale), ringed capillary worm Thread worms (Capillaria annulata), torsion capillary nematode Cropworm (Capillaria contorta), liver capillary nematode Capillary liver worm (Capillaria hepatica), penetrating capillary nematode (Capillaria) perforans), Philippine Capillaria philippinensis, Capillaria suis, Caterpillar Whipworm (Trichuris discolor), Whipworm Whipworm (Trichuris ovis), Pig whipworm Pig Whipworm (Trichuris suis), human Enoplida nematodes such as the whipworm Human whipworm (Trichuris trichiura), the dog whipworm Dog whipworm (Trichuris vulpis), and the Trichinella pork worm (Trichinella spiralis).
桿 such as Nipple worm Nematode Intestinal threadworm (Strongyloides papillosus), Cat feline worm (Strongyloides planiceps), Pig worm nematode Pig threadworm (Strongyloides ransomi), Human dung beetle Threadworm (Strongyloides stercoralis), Micronema spp. Rhabditida nematode.
ブラジル鉤虫Hookworm(Ancylostoma braziliense)、犬鉤虫Dog hookworm(Ancylostoma caninum)、ズビニ鉤虫Old World hookworm(Ancylostoma duodenale)、猫鉤虫Cat hookworm(Ancylostoma tubaeforme)、狭頭鉤虫The Northern hookworm of dogs(Uncinaria stenocephala)、牛鉤虫Cattle hookworm(Bunostomum phlebotomum)、羊鉤虫Small ruminant hookworm(Bunostomum trigonocephalum)、アメリカ鉤虫New World hookworm(Necator americanus)、シアトストーマム属(Cyathostomum spp.)、シリコシクラス属(Cylicocyclus spp.)、シリコドントフォラス属(Cylicodontophorus spp.)、シリコステファナス属(Cylicostephanus spp.)、ロバ円虫(Strongylus asini)、無歯円虫(Strongylus edentatus)、馬円虫Blood worm(Strongylus equinus)、普通円虫Blood worm(Strongylus vulgaris)、羊縮小線虫Large-mouthed bowel worm(Chabertia ovina)、インド腸結節虫Nodular worm(Oesophagostomum brevicaudatum)、コロンビア腸結節虫Nodule worm(Oesophagostomum columbianum)、豚腸結節虫Nodule worm(Oesophagostomum dentatum)、アメリカ腸結節虫Nodular worm(Oesophagostomum georgianum)、腸結節虫Nodular worm(Oesophagostomum maplestonei)、豚盲結虫Nodular worm(Oesophagostomum quadrispinulatum)、牛腸結節虫Nodular worm(Oesophagostomum radiatum)、山羊腸結節虫Nodular worm(Oesophagostomum venulosum)、スクリジャビン開嘴虫(Syngamus skrjabinomorpha)、鶏開嘴虫Gapeworm(Syngamus trachea)、豚腎虫Swine kidney worm(Stephanurus dentatus)、クーペリアCattle bankrupt worm(Cooperia oncophora)、紅色毛様線虫Red stomach worm(Hyostrongylus rubidus)、皺胃毛様線虫Stomach hair worm(Trichostrongylus axei)、蛇状毛様線虫(Trichostrongylus colubriformis)、東洋毛様線虫Oriental trichostrongylus(Trichostrongylus orientalis)、捻転胃虫Red stomach worm(Haemonchus contortus)、牛捻転胃虫Cattle stomach worm(Mecistocirrus digitatus)、オステルターグ胃虫Brown stomach worm(Ostertagia ostertagi)、糸状肺虫Common lungworm(Dictyocaulus filaria)、牛肺虫Bovine lungworm(Dictyocaulus viviparus)、細頸毛円虫Thin-necked intestinal worm(Nematodirus filicollis)、豚肺虫Swine lungworm(Metastrongylus elongatus)、犬肺虫Lungworm(Filaroides hirthi)、肺毛細線虫Lungworm(Crenosoma aerophila)、キツネ肺虫Fox lungworm(Crenosoma vulpis)、広東住血線虫Rat lung worm(Angiostrongylus cantonensis)、住血線虫French heartworm(Angiostrongylus vasorum)、プロトストロンギラス属(Protostrongylus spp.)等の円虫目(Strongylida)線虫。
Brazilian worm Hookworm (Ancylostoma braziliense), Dog 鉤 worm Dog hookworm (Ancylostoma caninum), Zubini worm Old 鉤 World hookworm (Ancylostoma duodenale), Cat worm Cat hookworm (Ancylostoma tubaeforme), Narrow head worm The Northern tenocscounUns Caterpillar Cattle hookworm (Bunostomum phlebotomum), sheep moth beetle Small ruminant hookworm (Bunostomum trigonocephalum), American helminth New World hookworm (Necator americannus), Cytothostom genus (Cyathostomum spp.), Silico cycos class spp.), Cylicostephanus spp., Donkeys (Strongylus asini), edentulous (Strongylus edentatus), Horse worms Blood worm (Strongylus equinus), Common worms Blood worm (Strongylus vulgaris) , Large-mouthed bowel worm (Chabertia ovina), Indian intestinal nodule Nodular worm (Oesophagostomum brevicaudatum), Colombian intestinal tuberculosis Nodule worm (Oesophagostomum columbianum), swine intestinal tuberculosis Nodule worm (Oesophagostomum dentatum), American intestinal tuberculosis Nodular worm (Oesophagostomum georgianum), intestinal tuberculosis Nodular worm (Oesophagostomum quadrispinulatum), Nodular worm (Oesophagostomum radiatum), Nodular worm (Oesophagostomum venulosum), Scrijabin worm (Syngamus skrjabinogamus pe) , Swine nematode Swine kidney worm (Stephanurus dentatus), couperia Cattle bankrupt worm (Cooperia oncophora), red-haired nematode Red stomach worm (Hyostrongylus rubidus), stomach-like nematode Stomach hair worm (Trichostrongylus axei) Ciliate nematode (Trichostrongylus colubriformis), Oriental ciliate nematode Oriental trichostrongylus (Trichost rongylus orientalis), torsion stomachworm Red stomach worm (Haemonchus contortus), cow torsion stomachworm Cattle stomach worm (Mecistocirrus digitatus), Ostertag stomachworm Brown stomach worm (Ostertagia ostertagi), filamentous lungworm Common lungworm filaria, lung Insect Bovine lungworm (Dictyocaulus viviparus), thin-necked roundworm Thin-necked intestinal worm (Nematodirus filicollis), swine pneumoniae Swine lungworm (Metastrongylus elongatus), canine lungworm Lungworm (Filaroides hirthi), pulmonary ciliate Lomawormrenosa ), Fox lungworm Fox lungworm (Crenosoma vulpis), Cantonese blood nematode Rat lung worm (Angiostrongylus cantonensis), Schistosoma nematode French heartworm (Angiostrongylus vasorum), Protostrongylus spp. (Strongylida) Nematode.
イネシンガレセンチュウRice white tip nematode(Aphelenchoides besseyi)、イチゴセンチュウStrawberry foliar nematode(Aphelenchoides fragariae)、ハガレセンチュウChrysanthemum foliar nematode(Aphelenchoides ritzemabosi)、マツノザイセンチュウPine wood nematode(Bursaphelenchus xylophilus)等の葉線虫目(Aphelenchida)線虫。
ジャガイモシロシストセンチュウWhite potato cyst nematode(Globodera pallida)、ジャガイモシストセンチュウPotato cyst nematode(Globodera rostochiensis)、ムギシストセンチュウCereal cyst nematode(Heterodera avenae)、ダイズシストセンチュウSoybean cyst nematode(Heterodera glycines)、テンサイシストセンチュウSugarbeet cyst nematode(Heterodera schachtii)、クローバシストセンチュウClover cyst nematode(Heterodera trifolii)、アレナリアネコブセンチュウPeanut root-knot nematode(Meloidogyne arenaria)、キタネコブセンチュウNorthern root-knot nematode(Meloidogyne hapla)、サツマイモネコブセンチュウSouthern root-knot nematode(Meloidogyne incognita)、ジャワネコブセンチュウJavanese root-knot nematode(Meloidogyne javanica)、リンゴネコブセンチュウApple root-knot nematode(Meloidogyne mali)、ミナミネグサレセンチュウCoffee root-lesion nematode(Pratylenchus coffeae)、ノコギリネグサレセンチュウ(Pratylenchus drenatus)、チャネグサレセンチュウTea root-lesion nematode(Pratylenchus loosi)、ムギネグサレセンチュウCalifornia root-lesion nematode(Pratylenchus neglectus)、キタネグサレセンチュウCobb's root-lesion nematode(Pratylenchus penetrans)、クルミネグサレセンチュウWalnut root-lesion nematode(Pratylenchus vulnus)、カンキツネモグリセンチュウCitrus burrowing nematode(Radopholus citrophilus)、バナナネモグリセンチュウBanana burrowing nematode(Radopholus similis)等のハリセンチュウ目(Tylenchida)線虫。 Rice white nematode (Aphelenchoides besseyi), strawberry nematode, Strawberry foliar nematode (Aphelenchoides fragariae), chrysanthemum foliar nematode (Aphelenchoides ritzemabosi), pine wood nematode (Bineurus) Aphelenchida) Nematode.
Potato cyst nematode (Globodera pallida), Potato cyst nematode (Globodera rostochiensis), Wheat cyst nematode (Heterodera avenae), Soybean cyst nematode cyst nematode (Heterodera schachtii), clover cyst nematode Clover cyst nematode (Heterodera trifolii), arenaria root nematode Peanut root-knot nematode (Meloidogyne arenaria), red root nematode Northern root-knot nematode (Meloidogyne incognita), Java root-knot nematode (Meloidogyne javanica), Apple root-knot nematode (Meloidogyne mali), Southern nematode sale nematode Cof fee root-lesion nematode (Pratylenchus coffeae), sawtooth nematode (Pratylenchus drenatus), chanegusaresenchu Tea root-lesion nematode (Pratylenchus loosi), wheat root nematode California root-lesion nematode (bb) -lesion nematode (Pratylenchus penetrans), Walnut root-lesion nematode (Pratylenchus vulnus), Citrus burrowing nematode (Radopholus citrophilus), Banana burrowing nesimde (Radopholus) (Tylenchida) Nematode.
ジャガイモシロシストセンチュウWhite potato cyst nematode(Globodera pallida)、ジャガイモシストセンチュウPotato cyst nematode(Globodera rostochiensis)、ムギシストセンチュウCereal cyst nematode(Heterodera avenae)、ダイズシストセンチュウSoybean cyst nematode(Heterodera glycines)、テンサイシストセンチュウSugarbeet cyst nematode(Heterodera schachtii)、クローバシストセンチュウClover cyst nematode(Heterodera trifolii)、アレナリアネコブセンチュウPeanut root-knot nematode(Meloidogyne arenaria)、キタネコブセンチュウNorthern root-knot nematode(Meloidogyne hapla)、サツマイモネコブセンチュウSouthern root-knot nematode(Meloidogyne incognita)、ジャワネコブセンチュウJavanese root-knot nematode(Meloidogyne javanica)、リンゴネコブセンチュウApple root-knot nematode(Meloidogyne mali)、ミナミネグサレセンチュウCoffee root-lesion nematode(Pratylenchus coffeae)、ノコギリネグサレセンチュウ(Pratylenchus drenatus)、チャネグサレセンチュウTea root-lesion nematode(Pratylenchus loosi)、ムギネグサレセンチュウCalifornia root-lesion nematode(Pratylenchus neglectus)、キタネグサレセンチュウCobb's root-lesion nematode(Pratylenchus penetrans)、クルミネグサレセンチュウWalnut root-lesion nematode(Pratylenchus vulnus)、カンキツネモグリセンチュウCitrus burrowing nematode(Radopholus citrophilus)、バナナネモグリセンチュウBanana burrowing nematode(Radopholus similis)等のハリセンチュウ目(Tylenchida)線虫。 Rice white nematode (Aphelenchoides besseyi), strawberry nematode, Strawberry foliar nematode (Aphelenchoides fragariae), chrysanthemum foliar nematode (Aphelenchoides ritzemabosi), pine wood nematode (Bineurus) Aphelenchida) Nematode.
Potato cyst nematode (Globodera pallida), Potato cyst nematode (Globodera rostochiensis), Wheat cyst nematode (Heterodera avenae), Soybean cyst nematode cyst nematode (Heterodera schachtii), clover cyst nematode Clover cyst nematode (Heterodera trifolii), arenaria root nematode Peanut root-knot nematode (Meloidogyne arenaria), red root nematode Northern root-knot nematode (Meloidogyne incognita), Java root-knot nematode (Meloidogyne javanica), Apple root-knot nematode (Meloidogyne mali), Southern nematode sale nematode Cof fee root-lesion nematode (Pratylenchus coffeae), sawtooth nematode (Pratylenchus drenatus), chanegusaresenchu Tea root-lesion nematode (Pratylenchus loosi), wheat root nematode California root-lesion nematode (bb) -lesion nematode (Pratylenchus penetrans), Walnut root-lesion nematode (Pratylenchus vulnus), Citrus burrowing nematode (Radopholus citrophilus), Banana burrowing nesimde (Radopholus) (Tylenchida) Nematode.
ヒト蟯虫Pinworm(Enterobius vermicularis)、馬蟯虫Equine pinworm(Oxyuris equi)、ウサギ蟯虫Rabbit pinworm(Passalurus ambiguus)等の蟯虫目(Oxyurida)線虫、
豚回虫Pig roundworm(Ascaris suum)、馬回虫Horse roundworm(Parascaris equorum)、犬小回虫Dog roundworm(Toxascaris leonina)、犬回虫Dog intestinal roundworm(Toxocara canis)、猫回虫Feline roundworm(Toxocara cati)、牛回虫Large cattle roundworm(Toxocara vitulorum)、アニサキス属(Anisakis spp.)、シュードテラノーバ属(Pseudoterranova spp.)、鶏盲腸虫Caecal worm(Heterakis gallinarum)、鶏回虫Chicken roundworm(Ascaridia galli)等の回虫目(Ascaridida)線虫。 Oxyurida nematodes such as human worm Pinworm (Enterobius vermicularis), horse worm Equine pinworm (Oxyuris equi), rabbit worm Rabbit pinworm (Passalurus ambiguus),
Pig roundworm (Ascaris suum), Horse roundworm Horse roundworm (Parascaris equorum), Dog roundworm Dog roundworm (Toxascaris leonina), Dog roundworm Dog intestinal roundworm (Toxocara canis), Cat roundworm Feline roundworm (Toxocara cati), Cattle roundworm Large cattle roundworm (Toxocara vitulorum), Anisakis spp., Pseudoterranova spp., chicken caecal caecal worm (Heterakis gallinarum), chicken roundworm (Ascaridia galli) and other roundworms (Ascaridida) Nematode.
豚回虫Pig roundworm(Ascaris suum)、馬回虫Horse roundworm(Parascaris equorum)、犬小回虫Dog roundworm(Toxascaris leonina)、犬回虫Dog intestinal roundworm(Toxocara canis)、猫回虫Feline roundworm(Toxocara cati)、牛回虫Large cattle roundworm(Toxocara vitulorum)、アニサキス属(Anisakis spp.)、シュードテラノーバ属(Pseudoterranova spp.)、鶏盲腸虫Caecal worm(Heterakis gallinarum)、鶏回虫Chicken roundworm(Ascaridia galli)等の回虫目(Ascaridida)線虫。 Oxyurida nematodes such as human worm Pinworm (Enterobius vermicularis), horse worm Equine pinworm (Oxyuris equi), rabbit worm Rabbit pinworm (Passalurus ambiguus),
Pig roundworm (Ascaris suum), Horse roundworm Horse roundworm (Parascaris equorum), Dog roundworm Dog roundworm (Toxascaris leonina), Dog roundworm Dog intestinal roundworm (Toxocara canis), Cat roundworm Feline roundworm (Toxocara cati), Cattle roundworm Large cattle roundworm (Toxocara vitulorum), Anisakis spp., Pseudoterranova spp., chicken caecal caecal worm (Heterakis gallinarum), chicken roundworm (Ascaridia galli) and other roundworms (Ascaridida) Nematode.
メジナ虫Guinea worm(Dracunculus medinensis)、ドロレス顎口虫(Gnathostoma doloresi)、剛棘顎口虫(Gnathostoma hispidum)、日本顎口虫(Gnathostoma nipponicum)、有棘顎口虫Reddish‐coloured worm(Gnathostoma spinigerum)、犬胃虫Dog stomach worm(Physaloptera canis)、猫胃虫Cat stomach worm(Physaloptera felidis, P. praeputialis)、ラーラ胃虫Feline/canine stomach worm(Physaloptera rara)、東洋眼虫Eye worm(Thelazia callipaeda)、ロデシア眼虫Bovine eyeworm(Thelazia rhodesi)、大口馬胃虫Large mouth stomach worm(Draschia megastoma)、小口胃虫Equine stomach worm(Habronema microstoma)、ハエ胃虫Stomach worm(Habronema muscae)、美麗食道虫Gullet worm(Gongylonema pulchrum)、類円豚胃虫Thick stomach worm(Ascarops strongylina)、牛パラフィラリアParafilaria(Parafilaria bovicola)、多乳頭糸状虫(Parafilaria multipapillosa)、沖縄糸状虫(Stephanofilaria okinawaensis)、バンクロフト糸状虫Bancroft filaria(Wuchereria bancrofti)、マレー糸状虫(Brugia malayi)、頸部糸状虫Neck threadworm(Onchocerca cervicalis)、ギブソン糸状虫(Onchocerca gibsoni)、咽頭糸状虫Cattle filarial worm(Onchocerca gutturosa)、回旋糸状虫(Onchocerca volvulus)、指状糸状虫Bovine filarial worm(Setaria digitata)、馬糸状虫Peritoneal worm(Setaria equina)、唇乳頭糸状虫(Setaria labiatopapillosa)、マーシャル糸状虫(Setaria marshalli)、犬糸状虫Dog heartworm(Dirofilaria immitis)、ロア糸状虫African eye worm(Loa loa)等の旋尾線虫目(Spirurida)線虫。
Medinaworm Guinea worm (Dracunculus medinensis), Dolores jaw-and-mouth beetle (Gnathostoma doloresi), Spine-and-mouth-mouth beetle (Gnathostoma hispidum), Japanese jaw-and-mouth beetle (Gnathostoma nipponicum), Spiny-mouthed mouth-worm , Dog stomachworm Dog stomach worm (Physaloptera canis), cat stomachworm Cat stomach worm (Physaloptera felidis, P. praeputialis), lala stomachworm Feline / canine stomach worm (Physaloptera rara), oriental eyeworm EyeEworm (Thelazia callipaeda) Rhodesian eyeworm Bovine eyeworm (Thelazia rhodesi), large mouth horse stomach insect Large mouth stomach worm (Draschia megastoma), small mouth stomach insect Equine stomach worm (Habronema microstoma), fly stomach insect Stomach worm (Habronema muscae), lettuce worm worm Gullet Gongylonema pulchrum), horoscope pork stomachworm Thick stomach worm (Ascarops strongylina), cow parafilaria Parafilaria (Parafilaria bovicola), multi-papillary fungus (Parafilaria multipapillo) sa), Okinawa fungi (Stephanofilariaofokinawaensis), Bancroft バ ン ク filaria (Wuchereria bancrofti), Malay fungus (Brugia malayi), Neck fungus Neck threadworm (Onchocerca cervicalis), Gibson fungi (Onchocerca gibs) Filamentous Cattle filarial worm (Onchocerca gutturosa), convolvulus worm (Onchocerca volvulus), finger filamentous worm Bovine filarial worm (Setaria digitata), equine worm, Peritoneal worm (Setaria equina), papillary larvae (Setaria labiatopapillosa) Spirurida nematodes such as Setaria marshalli, Dog 犬 wormworm Dirofilaria immitis, and Loir 糸 eye worm (Loa loa).
鎖状鉤頭虫(Moniliformis moniliformis)、大鉤頭虫Giant thorny-headed worm(Macracanthorhynchus hirudinaceus)等の鉤頭虫類。
広節裂頭条虫Fish tapeworm(Diphyllobothrium latum)、日本海裂頭条虫(Diphyllobothrium nihonkaiense)、マンソン裂頭条虫Manson tapeworm(Spirometra erinaceieuropaei)、大複殖門条虫(Diplogonoporus grandis)等の擬葉目(Pseudophyllidea)条虫。 Craniopods such as Moniliformis moniliformis and Giant thorny-headed worm (Macracanthorhynchus hirudinaceus).
Artificial leaves such as Fish tapeworm (Diphyllobothrium latum), Diphyllobothrium nihonkaiense, Manson tapeworm (Spirometra erinaceieuropaei), Diplogonoporus grandis Eye (Pseudophyllidea) tapeworm.
広節裂頭条虫Fish tapeworm(Diphyllobothrium latum)、日本海裂頭条虫(Diphyllobothrium nihonkaiense)、マンソン裂頭条虫Manson tapeworm(Spirometra erinaceieuropaei)、大複殖門条虫(Diplogonoporus grandis)等の擬葉目(Pseudophyllidea)条虫。 Craniopods such as Moniliformis moniliformis and Giant thorny-headed worm (Macracanthorhynchus hirudinaceus).
Artificial leaves such as Fish tapeworm (Diphyllobothrium latum), Diphyllobothrium nihonkaiense, Manson tapeworm (Spirometra erinaceieuropaei), Diplogonoporus grandis Eye (Pseudophyllidea) tapeworm.
有線条虫(Mesocestoides lineatus)、有輪条虫Chicken tapeworm(Raillietina cesticillus)、棘溝条虫Fowl tapeworm(Raillietina echinobothrida)、方形条虫Chicken tapeworm(Raillietina tetragona)、胞状条虫Canine tapeworm(Taenia hydatigena)、多頭条虫Canine tapeworm(Taenia multiceps)、羊条虫Sheep measles(Taenia ovis)、豆状条虫Dog tapeworm(Taenia pisiformis)、無鉤条虫Beef tapeworm(Taenia saginata)、連節条虫Tapeworm(Taenia serialis)、有鉤条虫Pork tapeworm(Taenia solium)、猫条虫Feline tapeworm(Taenia taeniaeformis)、単包条虫Hydatid tapeworm(Echinococcus granulosus)、多包条虫Small fox tapeworm(Echinococcus multilocularis)、ヤマネコ包条虫(Echinococcus oligarthrus)、フォーゲル包条虫(Echinococcus vogeli)、縮小条虫Rat tapeworm(Hymenolepis diminuta)、小型条虫Dwarf tapeworm(Hymenolepis nana)、瓜実条虫Double-pored dog tapeworm(Dipylidium caninum)、楔状条虫(Amoebotaenia sphenoides)、漏斗状条虫(Choanotaenia infundibulum)、ウズラ条虫(Metroliasthes coturnix)、大条虫Equine tapeworm(Anoplocephala magna)、葉状条虫Cecal tapeworm(Anoplocephala perfoliata)、乳頭条虫Dwarf equine tapeworm(Paranoplocephala mamillana)、ベネデン条虫Common tapeworm(Moniezia benedeni)、拡張条虫Sheep tapeworm(Moniezia expansa)、スティレシア属(Stilesia spp.)等の円葉目(Cyclophyllidea)条虫。
壷型吸虫(Pharyngostomum cordatum)、ビルハルツ住血吸虫Blood fluke(Schistosoma haematobium)、日本住血吸虫Blood fluke(Schistosoma japonicum)、マンソン住血吸虫Blood fluke(Schistosoma mansoni)等の有壁吸虫目(Strigeidida)吸虫。 Wire worms (Mesocestoides lineatus), striped worms Chicken tapeworm (Raillietina cesticillus), spiny cleft worm Fowl tapeworm (Raillietina echinobothrida), square tapeworm Chicken tapeworm (Raillietina tetragona), canine tapeworm (Taenia hydatigena), Canine tapeworm (Taenia multiceps), sheep worm Sheep measles (Taenia ovis), beetle tapeworm Dog tapeworm (Taenia pisiformis), striped beetle Beef tapeworm (Taenia saginata), striped worm Tapeworm (Taenia serialis) ), Rodentworm Pork tapeworm (Taenia solium), caterpillar Feline tapeworm (Taenia taeniaeformis), single tapeworm Hydatid tapeworm (Echinococcus granulosus), multi-budworm, small fox tapeworm (Echinococcus multilocularis), cat (Echinococcus oligarthrus), Vogel's crustacean (Echinococcus vogeli), reduced tapeworm Rat tapeworm (Hymenolepis diminuta), small tapeworm Dwarf tapeworm (Hymenolepis nana), crustacea double-pored dog tapeworm (Dipylidium ca ninum), cuneate tapeworms (Amoebotaenia sphenoides), funnel-like crustaceans (Choanotaenia infundibulum), quail crustaceans (Metroliasthes coturnix), crustacea Equine tapeworm (Anoplocephala magna), phytophyte crustacean Cecal tapeworm (Anoplocephala perfoliata), papillae Cyclophyllidea worms such as insect Dwarf equine tapeworm (Paranoplocephala mamillana), Benedenta worm Common tapeworm (Moniezia benedeni), extended tapeworm Sheep tapeworm (Moniezia expansa), genus Stilesia spp.
Stripeidida, such as Pharyngostomum cordatum, Blood fluke (Schistosoma haematobium), Blood fluke (Schistosoma japonicum), and Schistosoma mansoni.
壷型吸虫(Pharyngostomum cordatum)、ビルハルツ住血吸虫Blood fluke(Schistosoma haematobium)、日本住血吸虫Blood fluke(Schistosoma japonicum)、マンソン住血吸虫Blood fluke(Schistosoma mansoni)等の有壁吸虫目(Strigeidida)吸虫。 Wire worms (Mesocestoides lineatus), striped worms Chicken tapeworm (Raillietina cesticillus), spiny cleft worm Fowl tapeworm (Raillietina echinobothrida), square tapeworm Chicken tapeworm (Raillietina tetragona), canine tapeworm (Taenia hydatigena), Canine tapeworm (Taenia multiceps), sheep worm Sheep measles (Taenia ovis), beetle tapeworm Dog tapeworm (Taenia pisiformis), striped beetle Beef tapeworm (Taenia saginata), striped worm Tapeworm (Taenia serialis) ), Rodentworm Pork tapeworm (Taenia solium), caterpillar Feline tapeworm (Taenia taeniaeformis), single tapeworm Hydatid tapeworm (Echinococcus granulosus), multi-budworm, small fox tapeworm (Echinococcus multilocularis), cat (Echinococcus oligarthrus), Vogel's crustacean (Echinococcus vogeli), reduced tapeworm Rat tapeworm (Hymenolepis diminuta), small tapeworm Dwarf tapeworm (Hymenolepis nana), crustacea double-pored dog tapeworm (Dipylidium ca ninum), cuneate tapeworms (Amoebotaenia sphenoides), funnel-like crustaceans (Choanotaenia infundibulum), quail crustaceans (Metroliasthes coturnix), crustacea Equine tapeworm (Anoplocephala magna), phytophyte crustacean Cecal tapeworm (Anoplocephala perfoliata), papillae Cyclophyllidea worms such as insect Dwarf equine tapeworm (Paranoplocephala mamillana), Benedenta worm Common tapeworm (Moniezia benedeni), extended tapeworm Sheep tapeworm (Moniezia expansa), genus Stilesia spp.
Stripeidida, such as Pharyngostomum cordatum, Blood fluke (Schistosoma haematobium), Blood fluke (Schistosoma japonicum), and Schistosoma mansoni.
移睾棘口吸虫(Echinostoma cinetorchis)、浅田棘口吸虫(Echinostoma hortense)、巨大肝蛭Giant liver fluke(Fasciola gigantica)、肝蛭Common liver fluke(Fasciola hepatica)、肥大吸虫(Fasciolopsis buski)、平腹双口吸虫(Homalogaster paloniae)等の棘口吸虫目(Echinostomida)吸虫。
大陸槍型吸虫(Dicrocoelium chinensis)、槍型吸虫Lancet liver fluke(Dicrocoelium dendriticum)、アフリカ槍型吸虫African lancet fluke(Dicrocoelium hospes)、小型膵蛭(Eurytrema coelomaticum)、膵蛭Pancreatic fluke(Eurytrema pancreaticum)、宮崎肺吸虫(Paragonimus miyazakii)、大平肺吸虫(Paragonimus ohirai)、ウエステルマン肺吸虫Lung fluke(Paragonimus westermani)等の斜睾吸虫目(Plagiorchiida)吸虫。 Echinostoma cinetorchis, Echinostoma hortense, Giant liver fluke (Fasciola gigantica), Liver common liver fluke (Fasciola hepatica), Fasciolopsis buski, Flat belly twin Echinostomida flukes such as Homalogaster paloniae.
Continental flukes (Dicrocoelium chinensis), moth-type flukes Lancet liver fluke (Dicrocoelium dendriticum), African moth-type flukes African lancet fluke (Dicrocoelium hospes), small pancreatic folds (Eurytrema coelomaticum), pancreatic folds Pancreatic fluke (Eurytrema pancreaticum) Plumiorchiida flukes, such as Paragonimus miyazakii, Paragonimus ohirai, Lester fluke (Paragonimus westermani), etc.
大陸槍型吸虫(Dicrocoelium chinensis)、槍型吸虫Lancet liver fluke(Dicrocoelium dendriticum)、アフリカ槍型吸虫African lancet fluke(Dicrocoelium hospes)、小型膵蛭(Eurytrema coelomaticum)、膵蛭Pancreatic fluke(Eurytrema pancreaticum)、宮崎肺吸虫(Paragonimus miyazakii)、大平肺吸虫(Paragonimus ohirai)、ウエステルマン肺吸虫Lung fluke(Paragonimus westermani)等の斜睾吸虫目(Plagiorchiida)吸虫。 Echinostoma cinetorchis, Echinostoma hortense, Giant liver fluke (Fasciola gigantica), Liver common liver fluke (Fasciola hepatica), Fasciolopsis buski, Flat belly twin Echinostomida flukes such as Homalogaster paloniae.
Continental flukes (Dicrocoelium chinensis), moth-type flukes Lancet liver fluke (Dicrocoelium dendriticum), African moth-type flukes African lancet fluke (Dicrocoelium hospes), small pancreatic folds (Eurytrema coelomaticum), pancreatic folds Pancreatic fluke (Eurytrema pancreaticum) Plumiorchiida flukes, such as Paragonimus miyazakii, Paragonimus ohirai, Lester fluke (Paragonimus westermani), etc.
アンフィメルス属(Amphimerus spp.)、肝吸虫Chinese liver fluke(Clonorchis sinensis)、猫肝吸虫Cat liver fluke(Opisthorchis felineus)、タイ肝吸虫Southeast Aasian liver fluke(Opisthorchis viverrini)、シュードアンフィストーマム属(Pseudamphistomum spp.)、メトロキス属(Metorchis spp.)、パラメトロキス属(Parametorchis spp.)、異形吸虫Intestinal fluke(Heterophyes heterophyes)、横川吸虫(Metagonimus yokokawai)、前腸異形吸虫(Pygidiopsis summa)等の後睾吸虫目(Opisthorchiida)吸虫。
Amphimers (Amphimerus spp.), Liver fluke, Chinese liver fluke (Clonorchis sinensis), Cat liver fluke Cat liver fluke (Opisthorchis felineus), Thai liver fluke Southeast Aasian liver fluke (Opisthorchis viverrini), Pseudomust .), Metrokis genus (Metorchis spp.), Parametrochis genus (Parametorchis spp.), Heteromorphic flukes Intestinal fluke (Heterophyes heterophyes), Yokokawa flukes (Metagonimus yokokawai), foregut flukes (Pygidiopsis summa), etc. Opisthorchiida).
赤痢アメーバ(Entamoeba histolytica, E. invadens)等のアメーバ類。
フタゴバベシア(Babesia bigemina)、牛バベシア(Babesia bovis)、大形馬バベシア(Babesia caballi)、犬バベシア(Babesia canis)、猫バベシア(Babesia felis)、ギブソン犬バベシア(Babesia gibsoni)、大型ピロプラズマ(Babesia ovata)、サイタウクスゾーン・フェリス(Cytauxzoon felis)、熱帯ピロプラズマ病タイレリア(Theileria annulata)、仮性沿岸熱タイレリア(Theileria mutans)、小型ピロプラズマ(Theileria orientalis)、東沿岸熱タイレリア(Theileria parva)等のピロプラズマ目(Piroplasmida)胞子虫類。 Amoeba such as Entamoeba histolytica (E. invadens).
Futago Babesia (Babesia bigemina), Cattle Babesia bovis, Big Horse Babesia caballi, Dog Babesia canis, Cat Babesia felis, Gibson dog Babesia gibsoni, Large Piroplasma (Babesia ovata) ), Cytauxzoon felis, tropical piroplasmosis Tyleria (Theileria annulata), pseudocoastal fever Tyreria (Theileria mutans), small piroplasma (Theileria orientalis), east coast fever Tyrelia (Theileria parva), etc. Piroplasmida sporozoites.
フタゴバベシア(Babesia bigemina)、牛バベシア(Babesia bovis)、大形馬バベシア(Babesia caballi)、犬バベシア(Babesia canis)、猫バベシア(Babesia felis)、ギブソン犬バベシア(Babesia gibsoni)、大型ピロプラズマ(Babesia ovata)、サイタウクスゾーン・フェリス(Cytauxzoon felis)、熱帯ピロプラズマ病タイレリア(Theileria annulata)、仮性沿岸熱タイレリア(Theileria mutans)、小型ピロプラズマ(Theileria orientalis)、東沿岸熱タイレリア(Theileria parva)等のピロプラズマ目(Piroplasmida)胞子虫類。 Amoeba such as Entamoeba histolytica (E. invadens).
Futago Babesia (Babesia bigemina), Cattle Babesia bovis, Big Horse Babesia caballi, Dog Babesia canis, Cat Babesia felis, Gibson dog Babesia gibsoni, Large Piroplasma (Babesia ovata) ), Cytauxzoon felis, tropical piroplasmosis Tyleria (Theileria annulata), pseudocoastal fever Tyreria (Theileria mutans), small piroplasma (Theileria orientalis), east coast fever Tyrelia (Theileria parva), etc. Piroplasmida sporozoites.
ヘモプロテウス・マンソニ(Haemoproteus mansoni)、鶏ロイコチトゾーン(Leucocytozoon caulleryi)、熱帯熱マラリア原虫(Plasmodium falciparum)、四日熱マラリア原虫(Plasmodium malariae)、卵形マラリア原虫(Plasmodium ovale)、三日熱マラリア原虫(Plasmodium vivax)等の住血胞子虫目(Haemosporida)胞子虫類。
カリオスポラ属(Caryospora spp.)、エイメリア・アセルブリナ(Eimeria acervulina)、エイメリア・ボビス(Eimeria bovis)、エイメリア・ブルネッチ(Eimeria brunetti)、エイメリア・マクシマ(Eimeria maxima)、エイメリア・ネカトリクス(Eimeria necatrix)、エイメリア・オビノイダリス(Eimeria ovinoidalis)、ウサギ肝コクシジウム(Eimeria stiedae)、鶏盲腸コクシジウム(Eimeria tenella)、犬イソスポーラ(Isospora canis)、猫イソスポーラ(Isospora felis)、豚イソスポーラ(Isospora suis)、ティゼリア・アレニ(Tyzzeria alleni)、ティゼリア・アンセリス(Tyzzeria anseris)、ティゼリア・パーニシオサ(Tyzzeria perniciosa)、ウェニョネラ・アナティス(Wenyonella anatis)、ウェニョネラ・ガガリ(Wenyonella gagari)、犬クリプトスポリジウム(Cryptosporidium canis)、猫クリプトスポリジウム(Cryptosporidium felis)、ヒトクリプトスポリジウム(Cryptosporidium hominis)、シチメンチョウクリプトスポリジウム(Cryptosporidium meleagridis)、ネズミクリプトスポリジウム(Cryptosporidium muris)、小形クリプトスポリジウム(Cryptosporidium parvum)、サルコシスチス・カニス(Sarcocystis canis)、クルーズ肉胞子虫(Sarcocystis cruzi)、サルコシスチス・フェリス(Sarcocystis felis)、ヒト肉胞子虫(Sarcocystis hominis)、サルコシスチス・ミーシェリアナ(Sarcocystis miescheriana)、サルコシスチス・ニューロナ(Sarcocystis neurona)、サルコシスチス・テネラ(Sarcocystis tenella)、サルコシスチス・オバリス(Sarcocystis ovalis)、トキソプラズマ(Toxoplasma gondii)、犬ヘパトゾーン(Hepatozoon canis)、猫ヘパトゾーン(Hepatozoon felis)等の真コクシジウム目(Eucoccidiorida)胞子虫類。 Haemoproteus mansoni, chicken leucocytozone (Leucocytozoon caulleryi), Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, Plasmodium ovale Haemosporida spores such as protozoa (Plasmodium vivax).
Caryospora spp., Eimeria acervulina, Eimeria bovis, Eimeria brunetti, Eimeria maxima, Eimeria necatrix, Eimeria necatrix Eimeria ovinoidalis, rabbit liver coccidium (Eimeria stiedae), chicken cecal coccidium (Eimeria tenella), dog isospora canis, feline isospora (Isospora felis), porcine isospora suis (Isospora suis), Tizeria aleni , Tyzzeria anseris, Tyzzeria perniciosa, Wenyonella anatis, Wenyonella gagari, Cryptosporidium canis, cat crypt Cryptosporidium felis, Cryptosporidium hominis, turkey Cryptosporidium meleagridis, Cryptosporidium muris, small Cryptosporidium parvco, sarcosis (Sarcocystis cruzi), Sarcocystis felis, Sarcocystis hominis, Sarcocystis miescheriana, Sarcocystis neurona, Sarcocystis colostis tenis S tenis Sarcocystis ovalis), Toxoplasma gondii, dog hepatozoon (Hepatozoon canis), cat hepatozoon (Hepatozoon felis), etc. cidiorida).
カリオスポラ属(Caryospora spp.)、エイメリア・アセルブリナ(Eimeria acervulina)、エイメリア・ボビス(Eimeria bovis)、エイメリア・ブルネッチ(Eimeria brunetti)、エイメリア・マクシマ(Eimeria maxima)、エイメリア・ネカトリクス(Eimeria necatrix)、エイメリア・オビノイダリス(Eimeria ovinoidalis)、ウサギ肝コクシジウム(Eimeria stiedae)、鶏盲腸コクシジウム(Eimeria tenella)、犬イソスポーラ(Isospora canis)、猫イソスポーラ(Isospora felis)、豚イソスポーラ(Isospora suis)、ティゼリア・アレニ(Tyzzeria alleni)、ティゼリア・アンセリス(Tyzzeria anseris)、ティゼリア・パーニシオサ(Tyzzeria perniciosa)、ウェニョネラ・アナティス(Wenyonella anatis)、ウェニョネラ・ガガリ(Wenyonella gagari)、犬クリプトスポリジウム(Cryptosporidium canis)、猫クリプトスポリジウム(Cryptosporidium felis)、ヒトクリプトスポリジウム(Cryptosporidium hominis)、シチメンチョウクリプトスポリジウム(Cryptosporidium meleagridis)、ネズミクリプトスポリジウム(Cryptosporidium muris)、小形クリプトスポリジウム(Cryptosporidium parvum)、サルコシスチス・カニス(Sarcocystis canis)、クルーズ肉胞子虫(Sarcocystis cruzi)、サルコシスチス・フェリス(Sarcocystis felis)、ヒト肉胞子虫(Sarcocystis hominis)、サルコシスチス・ミーシェリアナ(Sarcocystis miescheriana)、サルコシスチス・ニューロナ(Sarcocystis neurona)、サルコシスチス・テネラ(Sarcocystis tenella)、サルコシスチス・オバリス(Sarcocystis ovalis)、トキソプラズマ(Toxoplasma gondii)、犬ヘパトゾーン(Hepatozoon canis)、猫ヘパトゾーン(Hepatozoon felis)等の真コクシジウム目(Eucoccidiorida)胞子虫類。 Haemoproteus mansoni, chicken leucocytozone (Leucocytozoon caulleryi), Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, Plasmodium ovale Haemosporida spores such as protozoa (Plasmodium vivax).
Caryospora spp., Eimeria acervulina, Eimeria bovis, Eimeria brunetti, Eimeria maxima, Eimeria necatrix, Eimeria necatrix Eimeria ovinoidalis, rabbit liver coccidium (Eimeria stiedae), chicken cecal coccidium (Eimeria tenella), dog isospora canis, feline isospora (Isospora felis), porcine isospora suis (Isospora suis), Tizeria aleni , Tyzzeria anseris, Tyzzeria perniciosa, Wenyonella anatis, Wenyonella gagari, Cryptosporidium canis, cat crypt Cryptosporidium felis, Cryptosporidium hominis, turkey Cryptosporidium meleagridis, Cryptosporidium muris, small Cryptosporidium parvco, sarcosis (Sarcocystis cruzi), Sarcocystis felis, Sarcocystis hominis, Sarcocystis miescheriana, Sarcocystis neurona, Sarcocystis colostis tenis S tenis Sarcocystis ovalis), Toxoplasma gondii, dog hepatozoon (Hepatozoon canis), cat hepatozoon (Hepatozoon felis), etc. cidiorida).
大腸バランチジウム(Balantidium coli)等の前庭目(Vestibuliferida)繊毛虫類。
ヒストモナス(Histomanas meleagridis)、腸トリコモナス(Pentatrichomonas hominis)、口腔トリコモナス(Trichomonas tenax)等のトリコモナス目(Trichomonadida)鞭毛虫類。
ランブル鞭毛虫(Giardia intestinalis)、ジアルディア・ムリス(Giardia muris)、シチメンチョウヘキサミタ(Hexamita meleagridis)、ヘキサミタ・パルバ(Hexamita parva)等のディプロモナス目(Diplomonadida)鞭毛虫類。
ドノバンリーシュマニア(Leishmania donovani)、幼児リーシュマニア(Leishmania infantum)、大形リーシュマニア(Leishmania major)、熱帯リーシュマニア(Leishmania tropica)、ガンビアトリパノソーマ(Trypanosoma brucei gambiense)、ローデシアトリパノソーマ(Trypanosoma brucei rhodesiense)、クルーズトリパノソーマ(Trypanosoma cruzi)、媾疫トリパノソーマ(Trypanosoma equiperdum)、エバンストリパノソーマ(Trypanosoma evansi)等のキネトプラスト目(Kinetoplastida)鞭毛虫類。 Vestibuliferida ciliates such as large colonic barantidium coli.
Trichomonadida flagellates such as Histomonas meleagridis, Pentatrichomonas hominis, Trichomonas tenax.
Diplomonadida flagellates such as Giardia intestinalis, Giardia muris, turkey hexamita (Hexamita meleagridis), Hexamita parva.
Leishmania donovani, Leishmania infantum, Leishmania major, Leishmania tropica, Trypanosoma brucei gambiense, Trypanosoma brucei cruise, Trypanosoma brucei rhodesi Kinetoplastid flagellates such as Trypanosoma cruzi, Trypanosoma equiperdum, and Trypanosoma evansi.
ヒストモナス(Histomanas meleagridis)、腸トリコモナス(Pentatrichomonas hominis)、口腔トリコモナス(Trichomonas tenax)等のトリコモナス目(Trichomonadida)鞭毛虫類。
ランブル鞭毛虫(Giardia intestinalis)、ジアルディア・ムリス(Giardia muris)、シチメンチョウヘキサミタ(Hexamita meleagridis)、ヘキサミタ・パルバ(Hexamita parva)等のディプロモナス目(Diplomonadida)鞭毛虫類。
ドノバンリーシュマニア(Leishmania donovani)、幼児リーシュマニア(Leishmania infantum)、大形リーシュマニア(Leishmania major)、熱帯リーシュマニア(Leishmania tropica)、ガンビアトリパノソーマ(Trypanosoma brucei gambiense)、ローデシアトリパノソーマ(Trypanosoma brucei rhodesiense)、クルーズトリパノソーマ(Trypanosoma cruzi)、媾疫トリパノソーマ(Trypanosoma equiperdum)、エバンストリパノソーマ(Trypanosoma evansi)等のキネトプラスト目(Kinetoplastida)鞭毛虫類。 Vestibuliferida ciliates such as large colonic barantidium coli.
Trichomonadida flagellates such as Histomonas meleagridis, Pentatrichomonas hominis, Trichomonas tenax.
Diplomonadida flagellates such as Giardia intestinalis, Giardia muris, turkey hexamita (Hexamita meleagridis), Hexamita parva.
Leishmania donovani, Leishmania infantum, Leishmania major, Leishmania tropica, Trypanosoma brucei gambiense, Trypanosoma brucei cruise, Trypanosoma brucei rhodesi Kinetoplastid flagellates such as Trypanosoma cruzi, Trypanosoma equiperdum, and Trypanosoma evansi.
本明細書における「植物」とは、ヒトの食料として栽培される穀類や果樹・野菜、家畜・家禽等の飼料作物、その姿や形を愛でる鑑賞植物、或いは公園・街路等の植栽等の維管束植物(Tracheophyta)を意味し、具体例としては、以下の植物が挙げられるが、これらのみに限定されるものではない。
“Plant” in this specification refers to cereals and fruit trees / vegetables cultivated as human food, feed crops such as livestock and poultry, appreciation plants that love their form and shape, or planting in parks, streets, etc. It means a vascular plant (Tracheophyta), and specific examples include, but are not limited to, the following plants.
アカマツJapanese Red Pine(Pinus densiflora)、ヨーロッパアカマツScots Pine(Pinus sylvestris)、クロマツJapanese Black Pine(Pinus thunbergii)等のマツ科(Pinaceae)等に属するマツ目(Pinales)植物。
Pine (Pinales) plants belonging to Pinaceae such as Japanese red pine Pine (Pinus densiflora), European red pine Scots Pine (Pinus sylvestris), Japanese black pine Black Pine (Pinus thunbergii).
コショウPepper(Piper nigrum)等のコショウ科(Piperaceae)、アボカドAvocado(Persea americana)等のクスノキ科(Lauraceae)等に属するモクレン類(magnoliids)、
コンニャクKonjac(Amorphophallus konjac)、サトイモEddoe(Colocasia esculenta)等のサトイモ科(Araceae)、ナガイモChinese yam(Dioscorea batatas)、ヤマノイモJapanese yam(Dioscorea japonica)等のヤマノイモ科(Dioscoreaceae)、リーキLeek(Allium ampeloprasum var. porrum)、タマネギOnion(Allium cepa)、ラッキョウRakkyo(Allium chinense)、ネギWelsh onion(Allium fistulosum)、ニンニクGarlic(Allium sativum)、チャイブChives(Allium schoenoprasum)、アサツキChive(Allium schoenoprasum var. foliosum)、ニラOriental garlic(Allium tuberosum)、ワケギScallion(Allium x wakegi)等のネギ科(Alliaceae)、アスパラガスAsparagus(Asparagus officinalis)等のクサスギカズラ科(Asparagaceae)、ココヤシCoconut palm(Cocos nucifera)、ギニアアブラヤシOil palm(Elaeis guineensis)等のヤシ科(Arecaceae)アレカヤシ亜科(Arecoideae)、ナツメヤシDate palm(Phoenix dactylifera)等のヤシ科(Arecaceae)タリポットヤシ亜科(Coryphoideae)、パイナップルPineapple(Ananas comosus)等のパイナップル科(Bromeliaceae)、イネRice(Oryza sativa)等のイネ科(Poaceae)エールハルタ亜科(Ehrhartoideae)、ベントグラスBent grass(Agrostis spp.)、ブルーグラスBlue grass(Poa spp.)、オオムギBarley(Hordeum vulgare)、コムギWheat(Triticum aestivum, T. durum)、ライムギRye(Secale cereale)等のイネ科(Poaceae)イチゴツナギ亜科(Pooideae)、ギョウギシバBermuda grass(Cynodon dactylon)、シバGrass(Zoysia spp.)等のイネ科(Poaceae)ヒゲシバ亜科(Chloridoideae)、サトウキビSugarcane(Saccharum officinarum)、ソルガムSorgum(Sorghum bicolor)、トウモロコシCorn(Zea mays)等のイネ科(Poaceae)キビ亜科(Panicoideae)、バナナBanana(Musa spp.)等のバショウ科(Musaceae)、ミョウガMyoga(Zingiber mioga)、ショウガGinger(Zingiber officinale)等のショウガ科(Zingiberaceae)等に属する単子葉類(monocots)。 Pepper (Piper nigrum) such as Pepperaceae (Piperaceae), Avocado Avocado (Persea americana) and other magnoliaceae (Lauraceae) etc., magnoliids,
Konjac (Amorphophallus konjac), taro edode (Colocasia esculenta) and other taro (Araceae), Chinese yam (Dioscorea batatas), yam Japanese yam (Dioscorea japonica) and other yam (Dioscoreacesum var) porrum), Onion Onion (Allium cepa), Rakkyo Rakkyo (Allium chinense), Leek Welsh onion (Allium fistulosum), Garlic Garlic (Allium sativum), Chives Chives (Allium schoenoprasum), Asatsuchi Chive (Allium schoenofosum var) Japanese leek Oriental garlic (Allium tuberosum), Alliumaceae such as Scallion (Allium x wakegi), Asparagus Asparagus (Asparagus officinalis), etc. (Elaeis guineensis) and other palms (Arecaceae) arecaidea (Arecoideae), na Date palm (Phoenix dactylifera) and other palms (Arecaceae) Talipot subfamily (Coryphoideae), pineapples Pineapple (Ananas comosus) and other pineapples (Bromeliaceae), rice Rice (Poryae sativa) and other poaceae Family (Ehrhartoideae), bentgrass Bent grass (Agrostis spp.), Blue grass (Poa spp.), Barley Barley (Hordeum vulgare), wheat Wheat (Triticum aestivum, T. durum), rye Rye (Secale cereale), etc. Poaceae Strawberry subfamily (Pooideae), Googyushiba Bermuda grass (Cynodon dactylon), Shiba Grass (Zoysia spp.), Etc. Sorgum (Sorghum bicolor), Corn Corn (Zea mays), etc. Gramineae (Poaceae) Millet subfamily (Panicoideae), Banana Banana (Musa spp.), Etc. C family (Musaceae), Zingiber mioga Myoga (Zingiber mioga), ginger Ginger (Zingiber officinale) ginger family (Zingiberaceae) monocots that belong to such as (monocots).
コンニャクKonjac(Amorphophallus konjac)、サトイモEddoe(Colocasia esculenta)等のサトイモ科(Araceae)、ナガイモChinese yam(Dioscorea batatas)、ヤマノイモJapanese yam(Dioscorea japonica)等のヤマノイモ科(Dioscoreaceae)、リーキLeek(Allium ampeloprasum var. porrum)、タマネギOnion(Allium cepa)、ラッキョウRakkyo(Allium chinense)、ネギWelsh onion(Allium fistulosum)、ニンニクGarlic(Allium sativum)、チャイブChives(Allium schoenoprasum)、アサツキChive(Allium schoenoprasum var. foliosum)、ニラOriental garlic(Allium tuberosum)、ワケギScallion(Allium x wakegi)等のネギ科(Alliaceae)、アスパラガスAsparagus(Asparagus officinalis)等のクサスギカズラ科(Asparagaceae)、ココヤシCoconut palm(Cocos nucifera)、ギニアアブラヤシOil palm(Elaeis guineensis)等のヤシ科(Arecaceae)アレカヤシ亜科(Arecoideae)、ナツメヤシDate palm(Phoenix dactylifera)等のヤシ科(Arecaceae)タリポットヤシ亜科(Coryphoideae)、パイナップルPineapple(Ananas comosus)等のパイナップル科(Bromeliaceae)、イネRice(Oryza sativa)等のイネ科(Poaceae)エールハルタ亜科(Ehrhartoideae)、ベントグラスBent grass(Agrostis spp.)、ブルーグラスBlue grass(Poa spp.)、オオムギBarley(Hordeum vulgare)、コムギWheat(Triticum aestivum, T. durum)、ライムギRye(Secale cereale)等のイネ科(Poaceae)イチゴツナギ亜科(Pooideae)、ギョウギシバBermuda grass(Cynodon dactylon)、シバGrass(Zoysia spp.)等のイネ科(Poaceae)ヒゲシバ亜科(Chloridoideae)、サトウキビSugarcane(Saccharum officinarum)、ソルガムSorgum(Sorghum bicolor)、トウモロコシCorn(Zea mays)等のイネ科(Poaceae)キビ亜科(Panicoideae)、バナナBanana(Musa spp.)等のバショウ科(Musaceae)、ミョウガMyoga(Zingiber mioga)、ショウガGinger(Zingiber officinale)等のショウガ科(Zingiberaceae)等に属する単子葉類(monocots)。 Pepper (Piper nigrum) such as Pepperaceae (Piperaceae), Avocado Avocado (Persea americana) and other magnoliaceae (Lauraceae) etc., magnoliids,
Konjac (Amorphophallus konjac), taro edode (Colocasia esculenta) and other taro (Araceae), Chinese yam (Dioscorea batatas), yam Japanese yam (Dioscorea japonica) and other yam (Dioscoreacesum var) porrum), Onion Onion (Allium cepa), Rakkyo Rakkyo (Allium chinense), Leek Welsh onion (Allium fistulosum), Garlic Garlic (Allium sativum), Chives Chives (Allium schoenoprasum), Asatsuchi Chive (Allium schoenofosum var) Japanese leek Oriental garlic (Allium tuberosum), Alliumaceae such as Scallion (Allium x wakegi), Asparagus Asparagus (Asparagus officinalis), etc. (Elaeis guineensis) and other palms (Arecaceae) arecaidea (Arecoideae), na Date palm (Phoenix dactylifera) and other palms (Arecaceae) Talipot subfamily (Coryphoideae), pineapples Pineapple (Ananas comosus) and other pineapples (Bromeliaceae), rice Rice (Poryae sativa) and other poaceae Family (Ehrhartoideae), bentgrass Bent grass (Agrostis spp.), Blue grass (Poa spp.), Barley Barley (Hordeum vulgare), wheat Wheat (Triticum aestivum, T. durum), rye Rye (Secale cereale), etc. Poaceae Strawberry subfamily (Pooideae), Googyushiba Bermuda grass (Cynodon dactylon), Shiba Grass (Zoysia spp.), Etc. Sorgum (Sorghum bicolor), Corn Corn (Zea mays), etc. Gramineae (Poaceae) Millet subfamily (Panicoideae), Banana Banana (Musa spp.), Etc. C family (Musaceae), Zingiber mioga Myoga (Zingiber mioga), ginger Ginger (Zingiber officinale) ginger family (Zingiberaceae) monocots that belong to such as (monocots).
レンコンLotus root(Nelumbo nucifera)等のハス科(Nelumbonaceae)、ラッカセイPeanut(Arachis hypogaea)、ヒヨコマメChickpea(Cicer arietinum)、ヒラマメLentil(Lens culinaris)、エンドウPea(Pisum sativum)、ソラマメBroad bean(Vicia faba)、ダイズSoybean(Glycine max)、インゲンマメCommon bean(Phaseolus vulgaris)、アズキAdzuki bean(Vigna angularis)、ササゲCowpea(Vigna unguiculata)等のマメ科(Fabaceae)、ホップHop(Humulus lupulus)等のアサ科(Cannabaceae)、イチジクFig Tree(Ficus carica)、クワMulberry(Morus spp.)等のクワ科(Moraceae)、ナツメCommon jujube(Ziziphus jujuba)等のクロウメモドキ科(Rhamnaceae)、イチゴStrawberry(Fragaria)、バラRose(Rosa spp.)等のバラ科(Rosaceae)バラ亜科(Rosoideae)、ビワJapanese loquat(Eriobotrya japonica)、リンゴApple(Malus pumila)、セイヨウナシEuropean Pear(Pyrus communis)、ナシNashi Pear(Pyrus pyrifolia var. culta)等のバラ科(Rosaceae)ナシ亜科(Maloideae)、モモPeach(Amygdalus persica)、アンズApricot(Prunus armeniaca)、オウトウCherry(Prunus avium)、プルーンPrune(Prunus domestica)、アーモンドAlmond(Prunus dulcis)、ウメJapanese Apricot(Prunus mume)、スモモJapanese Plum(Prunus salicina)、オオシマザクラ(Cerasus speciosa)、ソメイヨシノ(Cerasus x yedoensis‘Somei-yoshino’)等のバラ科(Rosaceae)サクラ亜科(Prunoideae)、トウガンWinter melon(Benincasa hispida)、スイカWatermelon(Citrullus lanatus)、ユウガオBottle gourd(Lagenaria siceraria var. hispida)、ヘチマLuffa(Luffa cylindrica)、カボチャPumpkin(Cucurbita spp.)、ズッキーニZucchini(Cucurbita pepo)、ニガウリBitter melon(Momordica charantia var. pavel)、メロンMuskmelon(Cucumis melo)、シロウリOriental pickling melon(Cucumis melo var. conomon)、マクワウリOriental melon(Cucumis melo var. makuwa)、キュウリCucumber(Cucumis sativus)等のウリ科(Cucurbitaceae)、クリJapanese Chestnut(Castanea crenata)等のブナ科(Fagaceae)、クルミWalnut(Juglans spp.)等のクルミ科(Juglandaceae)、カシューナッツCashew(Anacardium occidentale)、マンゴーMango(Mangifera indica)、ピスタチオPistachio(Pistacia vera)等のウルシ科(Anacardiaceae)、サンショウJapanese pepper(Zanthoxylum piperitum)等のミカン科(Rutaceae)ヘンルーダ亜科(Rutoideae)、ダイダイBitter orange(Citrus aurantium)、ライムLime(Citrus aurantifolia)、ハッサクHassaku orange(Citrus hassaku)、ユズYuzu(Citrus junos)、レモンLemon(Citrus limon)、ナツミカンNatsumikan(Citrus natsudaidai)、グレープフルーツGrapefruit(Citrus x paradisi)、オレンジOrange(Citrus sinensis)、カボスKabosu(Citrus sphaerocarpa)、スダチSudachi(Citrus sudachi)、ポンカンMandarin Orange(Citrus tangerina)、ウンシュウミカンSatsuma(Citrus unshiu)、キンカンKumquat(Fortunella spp.)等のミカン科(Rutaceae)ミカン亜科(Aurantioideae)、セイヨウワサビHorseradish(Armoracia rusticana)、カラシナMustard(Brassica juncea)、タカナTakana(Brassica juncea var. integrifolia)、セイヨウアブラナRapeseed(Brassica napus)、カリフラワーCauliflower(Brassica oleracea var. botrytis)、キャベツCabbage(Brassica oleracea var. capitata)、メキャベツBrussels sprout(Brassica oleracea var. gemmifera)、ブロッコリーBroccoli(Brassica oleracea var. italica)、チンゲンサイGreen pak choi(Brassica rapa var. chinensis)、ノザワナNozawana(Brassica rapa var. hakabura)、アブラナNapa cabbage(Brassica rapa var. nippo-oleifera)、ミズナPotherb Mustard(Brassica rapa var. nipposinica)、ハクサイNapa cabbage(Brassica rapa var. pekinensis)、コマツナTurnip leaf(Brassica rapa var. perviridis)、カブTurnip(Brassica rapa var. rapa)、ルッコラGarden rocket(Eruca vesicaria)、ダイコンDaikon(Raphanus sativus var. longipinnatus)、ワサビWasabi(Wasabia japonica)等のアブラナ科(Brassicaceae)、パパイアPapaya(Carica papaya)等のパパイア科(Caricaceae)、オクラOkra(Abelmoschus esculentus)、ワタCotton plant(Gossypium spp.)、カカオCacao(Theobroma cacao)等のアオイ科(Malvaceae)、ブドウGrape(Vitis spp.)等のブドウ科(Vitaceae)、テンサイSugar beet(Beta vulgaris ssp. vulgaris var. altissima)、テーブルビートTable beet(Beta vulgaris ssp. vulgaris var. vulgaris)、ホウレンソウSpinach(Spinacia oleracea)等のヒユ科(Amaranthaceae)、ソバBuckweat(Fagopyrum esculentum)等のタデ科(Polygonaceae)、カキKaki Persimmon(Diospyros kaki)等のカキノキ科(Ebenaceae)、チャTea plant(Camellia sinensis)等のツバキ科(Theaceae)、キウイフルーツKiwifruit(Actinidia deliciosa, A. chinensis)等のマタタビ科(Actinidiaceae)、ブルーベリーBlueberry(Vaccinium spp.)、クランベリーCranberry(Vaccinium spp.)等のツツジ科(Ericaceae)、コーヒーノキCoffee plants(Coffea spp.)等のアカネ科(Rubiaceae)、レモンバームLemon balm(Melissa officinalis)、ミントMint(Mentha spp.)、バジルBasil(Ocimum basilicum)、シソShiso(Perilla frutescens var. crispa)、エゴマ(Perilla frutescens var. frutescens)、セージCommon Sage(Salvia officinalis)、タイムThyme(Thymus spp.)等のシソ科(Lamiaceae)、ゴマSesame(Sesamum indicum)等のゴマ科(Pedaliaceae)、オリーブOlive(Olea europaea)等のモクセイ科(Oleaceae)、サツマイモSweet potato(Ipomoea batatas)等のヒルガオ科(Convolvulaceae)、トマトTomato(Solanum lycopersicum)、ナスEggplant(Solanum melongena)、ジャガイモPotato(Solanum tuberosum)、トウガラシChili pepper(Capsicum annuum)、ピーマンBell pepper(Capsicum annuum var. 'grossum')、タバコTobacco(Nicotiana tabacum)等のナス科(Solanaceae)、セロリCelery(Apium graveolens var. dulce)、コリアンダーCoriander(Coriandrum sativum)、ミツバJapanese honeywort(Cryptotaenia Canadensis subsp. japonica)、ニンジンCarrot(Daucus carota subsp. sativus)、パセリParsley(Petroselium crispum)、イタリアンパセリItalian parsley(Petroselinum neapolitanum)等のセリ科(Apiaceae)、ウドUdo(Aralia cordata)、タラノキ(Aralia elata)等のウコギ科(Araliaceae)、アーティチョークArtichoke(Cynara scolymus)等のキク科(Asteraceae)アザミ亜科(Carduoideae)、キクニガナChicory(Cichorium intybus)、レタスLettuce(Lactuca sativa)等のキク科(Asteraceae)タンポポ亜科(Asteraceae)、キクFlorists’ daisy(Dendranthema grandiflorum)、シュンギクCrown daisy(Glebionis coronaria)、ヒマワリSunflower(Helianthus annuus)、フキFuki(Petasites japonicus)、ゴボウBurdock(Arctium lappa)等のキク科(Asteraceae)キク亜科(Asteraceae)等に属する真正双子葉類(eudicots)。
Lotus root such as Lotus root (Nelumbo nucifera) (Nelumbonaceae), groundnut Peanut (Arachis hypogaea), chickpea Chickpea (Cicer arietinum), lentil Lentil (Lens culinaris), pea Pea (Pisum sativum) , Soybean Soybean (Glycine max), common bean Common bean (Phaseolus vulgaris), adzuki bean (Vigna angularis) adzuki bean, cowpea (Vigna unguiculata), legaceae (Fabaceae), aceae such as Hop Hop (Humulus lupulus) ), Fig Fig Tree (Ficus carica), Mulberry (Moraceae) such as Mulberry (Morus spp.), Mameaceae (Rhamnaceae) such as jujuba (Rhamnaceae), Strawberry Strawberry (Fragaria), Rose Rose (Rosa) spp.) and other roses (Rosaceae), rose subfamily (Rosoideae), loquat Japanese loquat (Eriobotrya japonica), apple Apple (Malus pumila), sei Roses (Rosaceae), Pears (Maloideae), Peach Peach (Amygdalus persica), Apricots (Prunus armeniaca), Prunus armeniaca, Prunus armeniaca, Prunus armeniaca, Prunus armeniaca avium), prune Prune (Prunus domestica), almond Almond (Prunus dulcis), Japanese plum Apricot (Prunus mume), plum Japanese Plum (Prunus salicina), Oshima cherry (Cerasus speciosa), Yoshino cherry (yoshiru) Rosaceae, Rosaceae, Prunoideae, Togan, Winter melon (Benincasa hispida), Watermelon Watermelon (Citrullus lanatus), Yuugao Bottle gourd (Lagenaria siceraria var. Hispida), Loofah Cucurbita spp.), Zucchini (Cucurbita pepo), bitter melon Bitter melon (Momordica charantia var. Pavel), me Ron Muskmelon (Cucumis melo), sardine OrientalOrpickling melon (Cucumis melo var. Conomon), cucumber Oriental melon (Cucumis melo var. Makuwa), cucumber Cucumber (Cucumis sativus), Cucubitneaestren (Cucurbitaceest, Japanese chestnut) ) Such as beech (Fagaceae), walnut Walnut (Juglans spp.) And other walnuts (Juglandaceae), cashew nut Cashew (Anacardium occidentale), mango Mango (Mangifera indica), pistachio Pistachio (Pistacia vera), etc. ), Japanese salmon Japanese peper (Zanthoxylum piperitum), etc. Citrus junos), lemon Lemon (Citrus limon), Natsumikan (Citrus natsudaidai), gray Fruit Grapefruit (Citrus x paradisi), Orange Orange (Citrus sinensis), Kabosu Kabosu (Citrus sphaerocarpa), Sudachi Sudachi (Citrus sudachi), Ponkan Mandarin Orange (Citrus tangerina), Satsuma (Citrus unshiu) (Citrus unshiu) .) Citrus (Rutaceae) Citrus subfamily (Aurantioideae), Horseradish Horseradish (Armoracia rusticana), Mustard (Brassica juncea), Takana Takana (Brassica juncea var. Integrifolia), Brassica rapeseed (Brassica) Cauliflower (Brassica oleracea var. ), Nozawana (Brassica) rapa var. hakabura), rape Napa cabbage (Brassica rapa var. nippo-oleifera), Mizuna Potherb Mustard (Brassica rapa var. nipposinica), Chinese cabbage Napa cabbage (Brassica rapa var. pekinenica), leaf ), Turnip (Brassica rapa var. Rapa), Arugula GardenGrocket (Eruca vesicaria), Daikon Daikon (Raphanus sativus var. Longipinnatus), Wasabi (Wasabia japonica) Grapeaceae (Malvaceae) such as Papayaceae (Caricaceae), Okra Okra (Abelmoschus esculentus), Cotton Cotton plant (Gossypium spp.), Cacao (Theobroma cacao), Grape (Vitis spp.) Vitaceae), sugar beet Sugar beet (Beta vulgaris ssp. Vulgaris var. Altissima), table beat Table beet (Beta vulgaris ssp. Vulgaris var. Vulga ris), spinach Spinach (Spinacia oleracea), etc. Amaranthaceae, buckwheat Buckweat (Fagopyrum esculentum), etc. (Polygonaceae), oysters Kaki Persimmon (Diospyros kaki), oysters (Ebenaceae), tea Camellia sinensis) and other camelliaceae (Theaceae), Kiwifruit Kiwifruit (Actinidia deliciosa, A.sischinensis) and other crickets (Actinidiaceae), blueberry Blueberry (Vaccinium spp.), Cranberry Cranberry (Vaccinium spp.) And other azaleas Ericaceae), Rubiaceae such as Coffee (plants (Coffea spp.), Lemon balm (Melissa officinalis), Mint Mint (Mentha spp.), Basil Basil (Ocimum basilicum), Perilla Shiso (Perilla frutescris s) ), Sesame (Perilla frutescens var. Frutescens), Sage Common Sage (Salvia officinalis), Thyme (Thymus spp.) (Lamiaceae), Sesame (Pedaliaceae) such as sesame Sesame (Sesamum indicum), Oleaceae such as olive Olive (Olea europaea), Convolvulaceae such as Sweet potato (Ipomoea batatas), Tomato (Convolvulaceae) Solanum lycopersicum), Eggplant Eggplant (Solanum melongena), Potato Potato (Solanum tuberosum), Pepper Chili pepper (Capsicum annuum), Bell Bell pepper (Capsicum annuum var. 'Grossum'), Tobacco family of Tobacco (cum) Solanaceae), Celery Celery (Apium graveolens var. Apiaceae such as Italian parsley (Petroselinum neapolitanum), Udo Udo (Aralia co) rdata), Araliaceae such as Aralia elata, Artichoke (Cynara scolymus) such as Asteraceae, Carduoideae, Chicory (Cichorium intybus), Lettuce (Lactucaat) Asteraceae, Asteraceae, Chrysanthemum Florists' daisy (Dendranthema grandiflorum), Shungiku Crown daisy (Glebionis coronaria), Sunflower Sunflower (Helianthus annuus), Fuki (Petasites japcturus) Authentic dicots (eudicots) belonging to Asteraceae and Asteraceae.
本明細書における「動物」とは、ヒト又は伴侶動物・愛玩動物や家畜・家禽、さらには研究・実験動物等の脊椎動物(Vertebrata)を意味し、具体例としては、以下の動物が挙げられるが、これらのみに限定されるものではない。
The term “animal” in this specification means humans, companion animals, pets, livestock, poultry, and vertebrates (Vertebrata) such as research and laboratory animals. Specific examples include the following animals: However, it is not limited to only these.
フサオマキザルTufted capuchin(Cebus apella)等のオマキザル科(Cebidae)、カニクイザルCrab-eating macaque(Macaca fascicularis)、アカゲザルRhesus macaque(Macaca mulatta)等のオナガザル科(Cercopithecidae)、チンパンジーChimpanzee(Pan troglodytes)、ヒトHuman(Homo sapiens)等のヒト科(Hominidae)、アナウサギEuropean rabbit(Oryctolagus cuniculus)等のウサギ科(Leporidae)、チンチラLong-tailed chinchilla(Chinchilla lanigera)等のチンチラ科(Chinchillidae)、モルモットGuinea pig(Cavia porcellus)等のテンジクネズミ科(Caviidae)、ゴールデンハムスターGolden hamster(Mesocricetus auratus)、ヒメキヌゲネズミDjungarian hamster(Phodopus sungorus)、モンゴルキヌゲネズミChinese hamster(Cricetulus griseus)等のキヌゲネズミ科(Cricetidae)、スナネズミMongolian gerbil(Meriones unguiculatus)、ハツカネズミHouse mouse(Mus musculus)、クマネズミBlack rat(Rattus rattus)等のネズミ科(Muridae)、シマリスChipmunk(Tamias sibiricus)等のリス科(Sciuridae)、ヒトコブラクダDromedary(Camelus dromedarius)、フタコブラクダBactrian camel(Camelus bactrianus)、アルパカAlpaca(Vicugna pacos)、リャマLlama(Lama glama)等のラクダ科(Camelidae)、ブタPig(Sus scrofa domesticus)等のイノシシ科(Suidae)、トナカイReindeer(Rangifer tarandus)、アカシカRed deer(Cervus elaphus)等のシカ科(Cervidae)、ヤクYak(Bos grunniens)、ウシCattle(Bos taurus)、アジアスイギュウWater buffalo(Bubalus arnee)、ヤギGoat(Capra hircus)、ヒツジSheep(Ovis aries)等のウシ科(Bovidae)、イエネコCat(Felis silvestris catus)等のネコ科(Felidae)、イエイヌDog(Canis lupus familiaris)、アカギツネRed fox(Vulpes vulpes)等のイヌ科(Canidae)、ヨーロッパミンクEuropean mink(Mustela lutreola)、アメリカミンクAmerican mink(Mustela vison)、フェレットFerret(Mustela putorius furo)等のイタチ科(Mustelidae)、ロバDonkey(Equus asinus)、ウマHorse(Equus caballus)等のウマ科(Equidae)、アカカンガルーRed kangaroo(Macropus rufus)等のカンガルー科(Macropodidae)等に属する哺乳類(Mammalia)。
Capuchin (Cebidae) such as Capuchin monkey Tuftedecapuchin (Cebus apella), Crab-eating macaque (Macaca fascicularis), Rhesus macaque (Macaca mulatta), etc. (Homonidae) such as Homo ヒ ト sapiens, Rabidae (Leporidae) such as European Europerabbit (Oryctolagus cuniculus), Chinchilla chinchilla (Chinchilla lanigera), Guinea origus ) Guinea pigs (Caviidae), Golden hamsters Golden hamster (Mesocricetus auratus), Djungarian hamster (Phodopus sungorus), Mongolian gerbils , House mice (Mus musculus), black rats (Rattus rattus), etc. ), Alpaca (Vicugna pacos), Camelidae such as Llama (Lama glama), Wild boar (Suidae) such as pig Pig (Sus scrofa domesticus), Reindeer (Rangifer tarandus), Red deer Deer (Cervidae) such as elaphus, Yak Yak (Bos grunniens), Cattle Cattle (Bos taurus), Asian Buffalo Water ア ジ ア buffalo (Bubalus arnee), Goat Goat (Capra hircus), Sheep Sheep (Ovis aries) (Bovidae), Cats such as Cat (Felis silvestris catus), Felidae, Dogu Dog (Canis lupus familiaris), Red Fox Canidae such as Vulpes vulpes), European Mink European mink (Mustela lutreola), American Mink American mink (Mustela vison), Ferret Ferret (Mustela putorius furo) etc. Weasel (Mustelidae), Donkey Donnus (Equus asi) Mammals belonging to equine family (Equidae) such as horse Horse (Equus caballus), kangaroo family (Macropodidae) such as red kangaroo (Macropus rufus), etc.
ダチョウOstrich(Struthio camelus)等のダチョウ科(Struthionidae)、アメリカレアAmerican rhea(Rhea americana)等のレア科(Rheidae)、エミューEmu(Dromaius novaehollandiae)等のエミュー科(Dromaiidae)、ライチョウPtarmigan(Lagopus muta)、シチメンチョウWild turkey(Meleagris gallopavo)、ウズラJapanese quail(Coturnix japonica)、ニワトリChicken(Gallus gallus domesticus)、コウライキジCommon pheasant(Phasianus colchicus)、キンケイGolden pheasant(Chrysolophus pictus)、インドクジャクIndian peafowl(Pavo cristatus)等のキジ科(Phasianidae)、ホロホロチョウHelmeted guineafowl(Numida meleagris)等のホロホロチョウ科(Numididae)、マガモMallard(Anas platyrhynchos)、アヒルDomesticated duck(Anas platyrhynchos var.domesticus)、カルガモSpot-billed duck(Anas poecilorhyncha)、ハイイロガンGreylag goose(Anser anser)、サカツラガンSwan goose(Anser cygnoides)、オオハクチョウWhooper swan(Cygnus cygnus)、コブハクチョウMute swan(Cygnus olor)等のカモ科(Anatidae)、カワラバトRock dove(Columba livia)、キジバトOriental turtle dove(Streptopelia orientalis)、コキジバトEuropean turtle dove(Streptopelia turtur)等のハト科(Columbidae)、キバタンSulphur-crested cockatoo(Cacatua galerita)、モモイロインコGalah(Eolophus roseicapilla)、オカメインコCockatiel(Nymphicus hollandicus)等のオウム科(Cacatuidae)、コザクラインコRosy-faced lovebird(Agapornis roseicollis)、ルリコンゴウインコBlue-and-yellow macaw(Ara ararauna)、コンゴウインコScarlet Macaw(Ara macao)、セキセイインコBudgerigar(Melopsittacus undulatus)、ヨウムAfrican grey parrot(Psittacus erithacus)等のインコ科(Psittacidae)、キュウカンチョウCommon hill myna(Gracula religiosa)等のムクドリ科(Sturnidae)、ベニスズメRed avadavat(Amandava amandava)、キンカチョウZebra finch(Taeniopygia guttata)、ジュウシマツBengalese finch(Lonchura striata var. domestica)、ブンチョウJava sparrow(Padda oryzivora)等のカエデチョウ科(Estrildidae)、カナリアDomestic canary(Serinus canaria domestica)、ゴシキヒワEuropean goldfinch(Carduelis carduelis)等のアトリ科(Fringillidae)等に属する鳥類(Aves)。
Ostriches (Struthio camelus) and other ostriches (Struthionidae), American rare American rhea (Rhea americana) and others , Turkey Wild turkey (Meleagris gallopavo), quail Japanese quail (Coturnix japonica), chicken Chiken (Gallus gallus domesticus), common pheasant Common pheasant (Phasianus colchicus), golden pheasant (Chrysolavo cucu) Pheasants (Phasianidae), guinea butterflies Helmeted guineafowl (Numida meleagris), etc. ), Greylag goose (Anser anser), Swan goose (Anser cygnoides), Whooper swan (Cygnus cygnus), Mute Swan (Cygnus olor), etc. orientalis), Pigeons such as European turtle turtle dove (Streptopelia turtur), Columbidae, Kibatan Sulphur-crested cockatoo (Cacatua galerita), Great white parakeets Galah (Eolophus roseicapilla), Cockaticat (Nic phi) Rosy-faced lovebird (Agapornis roseicollis), Blue-and-yellow macaw (Ara ararauna), Macaw Scarlet Macaw (Ara macao), Budgerigar (Melopsittacus undulatus), African greytta parrot Department (Psittacidae), Kyu Common hill hill myna (Gracula religiosa), etc. Birds (Aves) belonging to the family Atriidae (Eringriidae), the canary domestic canary (Serinus canaria domestica), the goldfinch (Fringillidae) such as European goldfinch (Carduelis carduelis) and the like.
エボシカメレオンVeiled chameleon(Chamaeleo calyptratus)等のカメレオン科(Chamaeleonidae)、グリーンイグアナGreen iguana(Iguana iguana)、グリーンアノールCarolina anole(Anolis carolinensis)等のイグアナ科(Iguanidae)、ナイルオオトカゲNile monitor(Varanus niloticus)、ミズオオトカゲWater monitor(Varanus salvator)等のオオトカゲ科(Varanidae)、オマキトカゲSolomon islands skink(Corucia zebrata)等のトカゲ科(Scincidae)、スジオナメラBeauty rat snake(Elaphe taeniura)等のナミヘビ科(Colubridae)、アカオボアBoa constrictor(Boa constrictor)等のボア科(Boidae)、インドニシキヘビIndian python(Python molurus)、アミメニシキヘビReticulated python(Python reticulatus)等のニシキヘビ科(Pythonidae)、カミツキガメCommon snapping turtle(Chelydra serpentina)等のカミツキガメ科(Chelydridae)、キスイガメDiamondback terrapin(Malaclemys terrapin)、アカミミガメPond slider(Trachemys scripta)等のヌマガメ科(Emydidae)、ニホンイシガメJapanese pond turtle(Mauremys japonica)等のイシガメ科(Geoemydidae)、ヨツユビリクガメCentral Asian tortoise(Agrionemys horsfieldii)等のリクガメ科(Testudinidae)、スッポンSoft-shelled turtle(Pelodiscus sinensis)等のスッポン科(Trionychidae)、アメリカアリゲーターAmerican alligator(Alligator mississippiensis)、クロカイマンBlack caiman(Melanosuchus niger)等のアリゲータ科(Alligatoridae)、シャムワニSiamese crocodile(Crocodylus siamensis)等のクロコダイル科(Crocodylidae)等に属する爬虫類(Reptilia)。
Veiled chameleon (Chamaeleo calyptratus), etc. Chameleonidae (Chamaeleonidae), Green iguana Green iguana (Iguana iguana), Green anole Carolinaa anole (Anolis carolinensis), etc. Iguanidae, Nile monitor Lizards such as the Lizard Lizard Water 、 monitor (Varanus salvator), etc. Constrictor (Boa constrictor), etc. Boidae (Boidae), Indian python Indian python (Python molurus), Japanese snake Reticulated python (Python reticulatus), etc. (Ch elydridae), turtle turtles Diamondback terrapin (Malaclemys terrapin), red turtles Pond slider (Trachemys scripta) and other turtles (Emydidae), Japanese turtles Alligatoridae (Testudinidae), Soft-shelled turtle (Pelodiscus sinensis), etc. Reptiles (Reptilia) belonging to Crocodylidae (Crocodylidae) such as Siamese crocodile (Crocodylus siamensis).
コイCarp(Cyprinus carpio)、キンギョGoldfish(Carassius auratus auratus)、ゼブラフィッシュZebrafish(Danio rerio)等のコイ科(Cyprinidae)、クーリーローチKuhli loach(Pangio kuhlii)等のドジョウ科(Cobitidae)、ピラニア・ナッテリーRed piranha(Pygocentrus nattereri)、ネオンテトラNeon tetra(Paracheirodon innesi)等のカラシン科(Characidae)、シナノユキマスMaraena whitefish(Coregonus lavaretus maraena)、ギンザケCoho salmon(Oncorhynchus kisutsh)、ニジマスRainbow trout(Oncorhynchus mykiss)、マスノスケChinook salmon(Oncorhynchus tshawytscha)、タイセイヨウサケAtlantic salmon(Salmo salar)、ブラウントラウトBrown trout(Salmo trutta)等のサケ科(Salmonidae)、タイリクスズキSpotted sea bass(Lateolabrax maculatus)等のスズキ科(Percichthyidae)、キンギョハナダイSea goldie(Pseudanthias squamipinnis)、クエLongtooth grouper(Epinephelus bruneus)、マハタConvict grouper(Epinephelus septemfasciatus)等のハタ科(Serranidae)、ブルーギルBluegill(Lepomis macrochirus)等のサンフィッシュ科(Centrarchidae)、シマアジWhite trevally(Pseudocaranx dentex)、カンパチGreater amberjack(Seriola dumerili)、ブリJapanese amberjack(Seriola quinqueradiata)等のアジ科(Carangidae)、マダイRed sea bream(Pagrus major)等のタイ科(Sparidae)、ナイルティラピアNile tilapia(Oreochromis niloticus)、スカラレ・エンゼルAngelfish(Pterophyllum scalare)等のシクリッド科(Cichlidae)、クロマグロPacific bluefin tuna(Thunnus orientalis)等のサバ科(Scombridae)、トラフグJapanese pufferfish(Takifugu rubripes)等のフグ科(Tetraodontidae)等に属する真骨魚類(Actinopterygii)。
Carp (Cyprinus carpio), Goldfish (Carassius auratus auratus), Zebrafish Zebrafish (Danio rerio), etc. piranha (Pygocentrus nattereri), Neon Tetra (Paracheirodon innesi), etc. Oncorhynchus tshawytscha), Atlantic salmon Atlantic Salmon (Salmo) salar), Brown trout Brown trout (Salmo trutta) salmonid (Salmonidae); Pseudanthias squamipinnis), Que Longtooth grouper (Epinephelus bruneus), Mahata ConvictCongrouper (Epinephelus septemfasciatus), etc. Greater amberjack (Seriola dumerili), Yellowtail amberjack (Seriola quinqueradiata), etc. Carangidae, Red sea bream (Pagrus major), etc., Thai family (Sparidae), Nile tilapia Nile tilapia (Oreochromis niloticus), Scalare Cichlidae (Cichlidae) such as Angelfish (Pterophyllum バ scalare), Sababridae (Scombridae) such as Pacific Bluefin unatuna (Thunnus orientalis), Bonefish (Tetraodontidae) such as Japanese pufferfish (Takifugu rubripes) Actinopterygii).
本明細書における「有用昆虫」とは、その生産物を利用することで人間の生活に役立てたり、果樹・野菜の受粉に用いる等の農作業の効率化等に役立つ昆虫を意味し、具体例としては、ニホンミツバチJapanese honeybee(Apis cerana japonica)、セイヨウミツバチWestern honey bee(Apis mellifera)、マルハナバチBumblebee(Bombus consobrinus wittenburgi、B. diversus diversus、B. hypocrita hypocrita、B. ignitus、B. terrestris)、マメコバチHornfaced bee(Osmia cornifrons)、カイコSilkworm(Bombyx mori)等が挙げられるが、これらに限定されるものではない。
The term “useful insects” in this specification means insects that are useful for human life by using their products, or for improving the efficiency of agricultural work such as use for pollination of fruit trees and vegetables. Japanese bees Japanese honeybee (Apis cerana japonica), Western bees Western honey bee (Apis mellifera), Bumblebees Bumblebee (Bombus consobrinus wittenburgi, B. diversus diversus, B. hypocrita terporis, B. (Osmia cornifrons), silkworm Silkworm (Bombyx 等 mori) and the like, but are not limited thereto.
本明細書における「天敵」とは、捕食や寄生によって特定の種の生物、特に農作物を加害する特定の種の生物を死に至らしめる又はその繁殖を抑制する生物を意味し、具体例としては、以下の生物が挙げられるが、これらのみに限定されるものではない。
The term “natural enemy” in the present specification means an organism that kills or suppresses the growth of a specific species of organisms, particularly a specific species that harms agricultural crops, by predation or parasitism. The following organisms may be mentioned, but are not limited to these.
ササカワハモグリコマユバチ(Dacnusa sasakawai)、ハモグリコマユバチ(Dacnusa sibirica)、コレマンアブラバチ(Aphidius colemani)、アオムシコマユバチ(Apanteles glomeratus)等のコマユバチ科(Braconidae)、キアシアブラコバチ(Aphelinus albipodus)、チャバラアブラコバチ(Aphelinus asychis)、ワタアブラコバチ(Aphelinus gossypii)、クロスジアブラコバチ(Aphelinus maculatus)、アブラコバチ(Aphelinus varipes)、オンシツツヤコバチ(Encarsia formosa)、サバクツヤコバチ(Eretmocerus eremicus)、チチュウカイツヤコバチ(Eretmocerus mundus)等のツヤコバチ科(Aphelinidae)及びハモグリヤドリヒメコバチ(Chrysocharis pentheus)、ハモグリミドリヒメコバチ(Neochrysocharis formosa)、イサエアヒメコバチ(Diglyphus isaea)、カンムリヒメコバチ(Hemiptarsenus varicornis)等のヒメコバチ科(Eulophidae)等に属する寄生蜂Parasitic wasp;ショクガタマバエAphidophagous gall midge(Aphidoletes aphidimyza);ナナホシテントウSeven-spot ladybird(Coccinella septempunctata);ナミテントウAsian lady beetle(Harmonia axyridis);ヒメカメノコテントウPredatory beetle(Propylea japonica);コヒメハナカメムシ(Orius minutus)、ツヤヒメハナカメムシ(Orius nagaii)、ナミヒメハナカメムシ(Orius sauteri)、タイリクヒメハナカメムシMinute pirate bug(Orius strigicollis)等のハナカメムシ科(Anthocoridae)に属する捕食性カメムシAnthocorid predatory bug;クロヒョウタンカスミカメ(Pilophorus typicus)、タバコカスミカメ(Nesidiocoris tenuis)等のカスミカメムシ科(Miridae)に属する捕食性カメムシPredatory mirid;アリガタシマアザミウマ(Franklinothrips vespiformis)等のシマアザミウマ科(Aeolothripidae)に属する捕食性アザミウマPredatory thrips;フタモンクサカゲロウ(Dichochrysa formosanus)、ヤマトクサカゲロウ(Chrysoperla nipponensis)等のクサカゲロウ科(Chrysopidae)に属するクサカゲロウGreen lacewing;ミヤコカブリダニ(Neoseiulus californicus)、ククメリスカブリダニ(Amblyseius cucumeris)、デジェネランスカブリダニ(Amblyseius degenerans)、スワルスキーカブリダニ(Amblyseius swirskii)、チリカブリダニ(Phytoseiulus persimilis)等のカブリダニ科(Phytoseiidae)に属するカブリダニPredatory mite;キクヅキコモリグモWolf spider(Pardosa pseudoannulata);ハナグモCrab spider(Misumenops tricuspidatus)。
Sasakawa leaf scallop (Dacnusa sasakawai), leaf squirrel (Dacnusa sibirica), coleman hornet (Aphidius colemani), corn bee (Apanteles glomeratus), Braconida, pod (Aphelinus asychis), cotton wasp (Aphelinus gossypii), cross-horned bee (Aphelinus maculatus), wasp (Aphelinus varipes), oncary bee (Encarsia formosa), Eretmoceruscer mundus, etc. (Aphelinidae) Parasitic wasp belonging to the order of Eulophidae, etc .; Aphidophagous gall midge (Aphidoletes aphidimyza); Seven-spot ladybird (Coccinella septempunctata) beetle (Propylea japonica); Koheihanamemushi (Orius minutus); Predatory mirid Predatory mirid Predatory mirid pi Predatory thrips belonging to Aeolothripidae (Aeolothripidae); Chrysopidae (Chrysoperid forus) Predatory mite Predatory ); Spider Crab spider (Misumenops tricuspidatus).
式(I)で表される本発明化合物は、例えば以下の方法により製造することが出来る。
製造法A
(式(I)及び式(II)中、Y1, Y2, Y3, R1, R2, R3, R4, R5及びR6は前記と同じ意味を表す。式(I)及び式(III)中、G1は前記と同じ意味を表す。式(III)中、J1は塩素原子、臭素原子、C1~C4アルキルカルボニルオキシ(例えば、ピバロイルオキシ)、C1~C4アルコキシカルボニルオキシ(例えば、イソブチルオキシカルボニルオキシ)又はアゾリル(例えば、イミダゾール-1-イル)等を表す。)
式(II)で表される化合物又はその塩(例えば塩酸塩、臭化水素酸塩、トリフルオロ酢酸塩、p-トルエンスルホン酸塩等)と、式(III)で表される化合物とを、必要ならばベンゼン、トルエン、ジクロロメタン、クロロホルム、1,2-ジクロロエタン、ジエチルエーテル、tert-ブチルメチルエーテル、テトラヒドロフラン、1,4-ジオキサン、酢酸エチル、N,N-ジメチルホルムアミド、N,N-ジメチルアセトアミド、アセトニトリル、水又はそれらの2種類以上の任意の割合の混合物等を溶媒として用い、必要ならば式(II)で表される化合物1モル当量に対して、1~3モル当量の炭酸ナトリウム、炭酸カリウム、炭酸水素ナトリウム、酢酸ナトリウム、トリエチルアミン、エチルジイソプロピルアミン、N-メチルモルホリン、ピリジン、4-(ジメチルアミノ)ピリジン等の塩基存在下、0℃~反応混合物の還流温度の温度範囲で30分~24時間反応させることにより、式(I)で表される本発明化合物を得ることができる。 The compound of the present invention represented by the formula (I) can be produced, for example, by the following method.
Manufacturing method A
(In Formula (I) and Formula (II), Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 , R 5 and R 6 represent the same meaning as described above. Formula (I) In formula (III), G 1 represents the same meaning as described above, wherein J 1 represents chlorine atom, bromine atom, C 1 -C 4 alkylcarbonyloxy (eg, pivaloyloxy), C 1 -C 4 represents alkoxycarbonyloxy (eg, isobutyloxycarbonyloxy) or azolyl (eg, imidazol-1-yl).
A compound represented by the formula (II) or a salt thereof (for example, hydrochloride, hydrobromide, trifluoroacetate, p-toluenesulfonate, etc.) and a compound represented by the formula (III) If necessary, benzene, toluene, dichloromethane, chloroform, 1,2-dichloroethane, diethyl ether, tert-butyl methyl ether, tetrahydrofuran, 1,4-dioxane, ethyl acetate, N, N-dimethylformamide, N, N-dimethylacetamide , Acetonitrile, water or a mixture of any two or more of them as a solvent, and if necessary, 1 to 3 molar equivalents of sodium carbonate with respect to 1 molar equivalent of the compound represented by formula (II), Potassium carbonate, sodium hydrogen carbonate, sodium acetate, triethylamine, ethyldiisopropylamine, N-methylmorpholine, pyridine, 4- (dimethyl The compound of the present invention represented by the formula (I) can be obtained by reacting in the presence of a base such as amino) pyridine at a temperature ranging from 0 ° C. to the reflux temperature of the reaction mixture for 30 minutes to 24 hours.
製造法A
式(II)で表される化合物又はその塩(例えば塩酸塩、臭化水素酸塩、トリフルオロ酢酸塩、p-トルエンスルホン酸塩等)と、式(III)で表される化合物とを、必要ならばベンゼン、トルエン、ジクロロメタン、クロロホルム、1,2-ジクロロエタン、ジエチルエーテル、tert-ブチルメチルエーテル、テトラヒドロフラン、1,4-ジオキサン、酢酸エチル、N,N-ジメチルホルムアミド、N,N-ジメチルアセトアミド、アセトニトリル、水又はそれらの2種類以上の任意の割合の混合物等を溶媒として用い、必要ならば式(II)で表される化合物1モル当量に対して、1~3モル当量の炭酸ナトリウム、炭酸カリウム、炭酸水素ナトリウム、酢酸ナトリウム、トリエチルアミン、エチルジイソプロピルアミン、N-メチルモルホリン、ピリジン、4-(ジメチルアミノ)ピリジン等の塩基存在下、0℃~反応混合物の還流温度の温度範囲で30分~24時間反応させることにより、式(I)で表される本発明化合物を得ることができる。 The compound of the present invention represented by the formula (I) can be produced, for example, by the following method.
Manufacturing method A
A compound represented by the formula (II) or a salt thereof (for example, hydrochloride, hydrobromide, trifluoroacetate, p-toluenesulfonate, etc.) and a compound represented by the formula (III) If necessary, benzene, toluene, dichloromethane, chloroform, 1,2-dichloroethane, diethyl ether, tert-butyl methyl ether, tetrahydrofuran, 1,4-dioxane, ethyl acetate, N, N-dimethylformamide, N, N-dimethylacetamide , Acetonitrile, water or a mixture of any two or more of them as a solvent, and if necessary, 1 to 3 molar equivalents of sodium carbonate with respect to 1 molar equivalent of the compound represented by formula (II), Potassium carbonate, sodium hydrogen carbonate, sodium acetate, triethylamine, ethyldiisopropylamine, N-methylmorpholine, pyridine, 4- (dimethyl The compound of the present invention represented by the formula (I) can be obtained by reacting in the presence of a base such as amino) pyridine at a temperature ranging from 0 ° C. to the reflux temperature of the reaction mixture for 30 minutes to 24 hours.
ここで用いられる式(III)で表される化合物の或るものは公知化合物であり、一部は市販品として入手できる。また、それ以外のものも、文献記載の公知の方法、例えばジャーナル・オブ・メディシナル・ケミストリー[J. Med. Chem.]1991年、34巻、1630頁等に記載の方法に準じて、対応する公知のカルボン酸を、塩化チオニル、五塩化リン又は塩化オキザリル等のハロゲン化剤と反応させる方法、テトラヘドロン・レターズ[Tetrahedron Lett.]2003年、44巻、4819頁、ジャーナル・オブ・メディシナル・ケミストリー[J. Med. Chem.]1991年、34巻、222頁等に記載の方法に準じて、対応する公知のカルボン酸と、塩化ピバロイル又はクロルギ酸イソブチル等の有機酸ハロゲン化物とを、必要ならば塩基の存在下、反応させる方法、又は、ザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]1989年、54巻、5620頁等に記載の、対応する公知のカルボン酸と、カルボニルジイミダゾール又はスルホニルジイミダゾール等とを反応させる方法等を用いて合成することができる。
Some of the compounds represented by the formula (III) used here are known compounds, and some of them are available as commercial products. In addition, other methods correspond to known methods described in the literature, for example, the method described in Journal of Medicinal Chemistry [J. Med. Chem.] 1991, 34, 1630, etc. A method of reacting a known carboxylic acid with a halogenating agent such as thionyl chloride, phosphorus pentachloride or oxalyl chloride, Tetrahedron Lett. 2003, 44, 4819, Journal of Medicinal Chemistry. [J. Med. Chem.] According to the method described in 1991, 34, 222, etc., a corresponding known carboxylic acid and an organic acid halide such as pivaloyl chloride or isobutyl chloroformate, if necessary. In the presence of a base, a method of reaction, or The Journal of Organic Chemistry [J. Org. Chem.] 1989, 5 Winding, according to 5620 pages, etc., and the corresponding known carboxylic acids, can be synthesized using a method in which reacting a carbonyldiimidazole or sulfonyl diimidazole, etc..
製造法B
(式(Ia)中、G1, Y1, Y2, Y3, R1, R2, R3, R4及びR6は前記と同じ意味を表す。式(IV)中、R5は水素原子、-OH、C1~C4アルコキシ及びC1~C4ハロアルコキシ以外の前記と同じ意味を表し、J2は塩素原子、臭素原子、ヨウ素原子、C1~C4アルキルカルボニルオキシ(例えば、ピバロイルオキシ等)、C1~C4アルキルスルホネート(例えば、メタンスルホニルオキシ等)、C1~C4ハロアルキルスルホネート(例えば、トリフルオロメタンスルホニルオキシ等)、アリールスルホネート(例えば、ベンゼンスルホニルオキシ、p-トルエンスルホニルオキシ等)又はアゾリル(例えば、イミダゾール-1-イル等)等の良好な脱離基を表す。式(I)中、G1, Y1, Y2, Y3, R1, R2, R3, R4, R5及びR6は上記と同じ意味を表す。)
Manufacturing method B
(In formula (Ia), G 1 , Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 and R 6 represent the same meaning as described above. In formula (IV), R 5 represents Except for hydrogen atom, —OH, C 1 -C 4 alkoxy and C 1 -C 4 haloalkoxy, the same meaning as described above, J 2 represents chlorine atom, bromine atom, iodine atom, C 1 -C 4 alkylcarbonyloxy ( For example, pivaloyloxy etc., C 1 -C 4 alkyl sulfonate (eg methanesulfonyloxy etc.), C 1 -C 4 haloalkyl sulfonate (eg trifluoromethanesulfonyloxy etc.), aryl sulfonate (eg benzenesulfonyloxy, p- Represents a good leaving group such as toluenesulfonyloxy or the like, or azolyl (for example, imidazol-1-yl, etc.) In formula (I), G 1 , Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 , R 5 and R 6 are the same as above Represents meaning.)
式(I)においてR5が水素原子である式(Ia)で表される本発明化合物1モル当量と、式(Ia)で表される化合物1モル当量に対して、1~10モル当量の式(IV)で表される化合物とを、必要ならばtert-ブチルメチルエーテル、テトラヒドロフラン、1,4-ジオキサン、アセトニトリル又はN, N-ジメチルホルムアミド等の極性溶媒を用い、必要ならば式(Ia)で表される化合物1モル当量に対して1~3モル当量の水素化ナトリウム、カリウム tert-ブトキシド、水酸化カリウム、炭酸カリウム、トリエチルアミン又はピリジン等の塩基存在下、0~90℃の温度範囲で10分~24時間反応させることにより、式(I)で表される本発明化合物を得ることができる。
1 to 10 molar equivalents of the present compound represented by the formula (Ia) in which R 5 is a hydrogen atom in the formula (I) and 1 molar equivalent of the compound represented by the formula (Ia) The compound represented by the formula (IV) is used, if necessary, with a polar solvent such as tert-butyl methyl ether, tetrahydrofuran, 1,4-dioxane, acetonitrile or N, N-dimethylformamide, and if necessary, the formula (Ia In the presence of a base such as sodium hydride, potassium tert-butoxide, potassium hydroxide, potassium carbonate, triethylamine or pyridine, in a temperature range of 0 to 90 ° C. The compound of the present invention represented by the formula (I) can be obtained by reacting for 10 minutes to 24 hours.
ここで用いられる式(IV)で表される化合物の或るものは公知化合物であり、一部は市販品として入手できる。また、それ以外のものも、文献記載の一般的な合成方法、例えばケミカル・アンド・ファーマシューティカル・ブレティン [Chem. Pharm. Bull.] 1986年、34巻、540頁及び2001年、49巻、1102頁、ジャーナル・オブ・ジ・アメリカン・ケミカル・ソサイエティー[J. Am. Chem. Soc.]1964年、86巻、4383頁、ザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]1983年、48巻、5280頁、オーガニック・シンセシス[Org. Synth.]1988年、コレクティブボリューム6巻、101頁、シンレット[Synlett]2005年、2847頁、シンセシス[Synthesis]1990年、1159頁、日本国公開特許公報(JP H05-125017号公報)、欧州特許公報(EP 0,051,273号公報)、英国特許公報(GB 2,161,802号公報)等に記載の方法に準じて合成することができる。
Some of the compounds represented by formula (IV) used here are known compounds, and some of them are available as commercial products. Other than the above, general synthesis methods described in the literature, for example, Chemical and Pharmaceutical Bulletin [Chem. Pharm. Bull.] 1986, 34, 540 and 2001, 49, 1102, Journal of the American Chemical Society [J. Am. Chem. Soc.] 1964, 86, 4383, The Journal of Organic Chemistry [J. Org. Chem.] 1983, 48, 5280, Organic Synthesis [Org. Synth.] 1988, Collective Volume 6, 101, Synlett 2005, 2847, Synthesis 1990, 1159, Japan Nationally published patent publication (JP H05-125017), European patent publication (EP 0,051,273 publication), British patent publication (GB 2,161,802 publication), etc. It can be synthesized according to the method described.
製造法C
(式(V)中、G1, Y1, Y2, Y3, R1, R2, R3, R4及びR5は前記と同じ意味を表し、J3は塩素原子、臭素原子、ヨウ素原子又はC1~C4ハロアルキルスルホネート(例えば、トリフルオロメタンスルホニルオキシ等)等の良好な脱離基を表す。)
式(V)で表される化合物を、例えばヨーロピアン・ジャーナル・オブ・オーガニック・ケミストリー[Eur. J. Org. Chem.]2015年、2015巻、4389頁等に記載の一般的な薗頭カップリングの反応条件を用い、式(VI)[式中、R6は前記と同じ意味を表し、Qは水素原子を表す。]で表される置換アセチレンと反応させるか、式(V)で表される化合物と式(VI)[式中、R6は前記と同じ意味を表し、Qはトリメチルシリル等を表す。]で表される置換アセチレンとを、例えば国際特許出願公報(WO 2005/094822号公報)等に準じて、フッ化テトラブチルアンモニウム共存下、薗頭カップリングの反応条件下反応させるか、或いは、式(V)で表される化合物を、例えばヘテロサイクルズ[Heterocycles]1997年、46巻、209頁等に記載の一般的な根岸カップリングの反応条件下、式(VI)[式中、R6は前記と同じ意味を表し、Qは-ZnCl、-ZnBr又は-ZnI等を表す。]で表される置換アセチレンと反応させることにより、式(I)で表される本発明化合物を得ることができる。 Manufacturing method C
(In the formula (V), G 1 , Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 and R 5 represent the same meaning as described above, J 3 represents a chlorine atom, a bromine atom, Represents a good leaving group such as an iodine atom or a C 1 -C 4 haloalkylsulfonate (eg, trifluoromethanesulfonyloxy, etc.)
The compound represented by the formula (V) is a general Sonogashira coupling described in, for example, European Journal of Organic Chemistry [Eur. J. Org. Chem.] 2015, 2015, 4389. In the formula (VI) [wherein R 6 represents the same meaning as described above, and Q represents a hydrogen atom. Or a compound represented by the formula (V) and the formula (VI) [wherein R 6 represents the same meaning as described above, and Q represents trimethylsilyl or the like. Or a substituted acetylene represented by the formula, for example, in accordance with International Patent Application Publication (WO 2005/094822), etc., in the presence of tetrabutylammonium fluoride under the reaction conditions of Sonogashira coupling, or The compound represented by the formula (V) can be prepared by reacting the compound represented by the formula (VI) [wherein R is a compound under the general Negishi coupling reaction conditions described in Heterocycles 1997, 46, 209, etc. 6 represents the same meaning as described above, and Q represents -ZnCl, -ZnBr, -ZnI, or the like. The compound of the present invention represented by the formula (I) can be obtained by reacting with a substituted acetylene represented by the formula:
式(V)で表される化合物を、例えばヨーロピアン・ジャーナル・オブ・オーガニック・ケミストリー[Eur. J. Org. Chem.]2015年、2015巻、4389頁等に記載の一般的な薗頭カップリングの反応条件を用い、式(VI)[式中、R6は前記と同じ意味を表し、Qは水素原子を表す。]で表される置換アセチレンと反応させるか、式(V)で表される化合物と式(VI)[式中、R6は前記と同じ意味を表し、Qはトリメチルシリル等を表す。]で表される置換アセチレンとを、例えば国際特許出願公報(WO 2005/094822号公報)等に準じて、フッ化テトラブチルアンモニウム共存下、薗頭カップリングの反応条件下反応させるか、或いは、式(V)で表される化合物を、例えばヘテロサイクルズ[Heterocycles]1997年、46巻、209頁等に記載の一般的な根岸カップリングの反応条件下、式(VI)[式中、R6は前記と同じ意味を表し、Qは-ZnCl、-ZnBr又は-ZnI等を表す。]で表される置換アセチレンと反応させることにより、式(I)で表される本発明化合物を得ることができる。 Manufacturing method C
The compound represented by the formula (V) is a general Sonogashira coupling described in, for example, European Journal of Organic Chemistry [Eur. J. Org. Chem.] 2015, 2015, 4389. In the formula (VI) [wherein R 6 represents the same meaning as described above, and Q represents a hydrogen atom. Or a compound represented by the formula (V) and the formula (VI) [wherein R 6 represents the same meaning as described above, and Q represents trimethylsilyl or the like. Or a substituted acetylene represented by the formula, for example, in accordance with International Patent Application Publication (WO 2005/094822), etc., in the presence of tetrabutylammonium fluoride under the reaction conditions of Sonogashira coupling, or The compound represented by the formula (V) can be prepared by reacting the compound represented by the formula (VI) [wherein R is a compound under the general Negishi coupling reaction conditions described in Heterocycles 1997, 46, 209, etc. 6 represents the same meaning as described above, and Q represents -ZnCl, -ZnBr, -ZnI, or the like. The compound of the present invention represented by the formula (I) can be obtained by reacting with a substituted acetylene represented by the formula:
ここで用いられる式(V)で表される化合物の或ものは、例えば国際特許出願公報(WO 2005/014545号公報)、国際特許出願公報(WO 2013/064461号公報)、国際特許出願公報(WO 2013/064521号公報)等記載の公知化合物であり、また、それ以外のものも公知化合物と同様にして合成することができる。
また、式(VI)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。 Some of the compounds represented by the formula (V) used here are, for example, International Patent Application Publication (WO 2005/014545 Publication), International Patent Application Publication (WO 2013/064461 Publication), International Patent Application Publication ( WO 2013/064521) and the like, and other compounds can be synthesized in the same manner as known compounds.
Some of the compounds represented by formula (VI) are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
また、式(VI)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。 Some of the compounds represented by the formula (V) used here are, for example, International Patent Application Publication (WO 2005/014545 Publication), International Patent Application Publication (WO 2013/064461 Publication), International Patent Application Publication ( WO 2013/064521) and the like, and other compounds can be synthesized in the same manner as known compounds.
Some of the compounds represented by formula (VI) are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
製造法A~製造法Cにおいて、反応終了後の反応混合物は、直接濃縮、又は有機溶媒に溶解し、水洗後濃縮、又は氷水に投入、有機溶媒抽出後濃縮といった、通常の後処理を行ない、目的のアルキニルピリジン置換アミド化合物を得ることができる。また、精製の必要が生じたときには、再結晶、カラムクロマトグラフ、薄層クロマトグラフ、液体クロマトグラフ分取等の、任意の精製方法によって不純物を分離し、精製することができる。
製造法Aで用いられる式(II)で表される化合物は、例えば反応式1~反応式10のようにして合成することができる。 In production method A to production method C, the reaction mixture after completion of the reaction is subjected to normal post-treatment such as direct concentration, or dissolution in an organic solvent, concentration after washing with water, or addition to ice water, extraction after organic solvent extraction, The target alkynylpyridine-substituted amide compound can be obtained. Further, when the necessity for purification arises, impurities can be separated and purified by any purification method such as recrystallization, column chromatograph, thin layer chromatograph, liquid chromatographic fractionation and the like.
The compound represented by the formula (II) used in the production method A can be synthesized, for example, as shown in the reaction formulas 1 to 10.
製造法Aで用いられる式(II)で表される化合物は、例えば反応式1~反応式10のようにして合成することができる。 In production method A to production method C, the reaction mixture after completion of the reaction is subjected to normal post-treatment such as direct concentration, or dissolution in an organic solvent, concentration after washing with water, or addition to ice water, extraction after organic solvent extraction, The target alkynylpyridine-substituted amide compound can be obtained. Further, when the necessity for purification arises, impurities can be separated and purified by any purification method such as recrystallization, column chromatograph, thin layer chromatograph, liquid chromatographic fractionation and the like.
The compound represented by the formula (II) used in the production method A can be synthesized, for example, as shown in the reaction formulas 1 to 10.
反応式1
(式(VII)中、Y1, Y2, Y3, R1, R2及びJ3は前記と同じ意味を表す。式(VIII)中、Y1, Y2, Y3, R1, R2及びJ3は上記と同じ意味を表す。式(VI)中、R6は前記と同じ意味を表し、Qは水素原子、トリメチルシリル等を表す。式(IX)中、Y1, Y2, Y3, R1, R2及びR6は上記と同じ意味を表す。式(IIa)中、Y1, Y2, Y3, R1, R2及びR6は上記と同じ意味を表す。)
Reaction formula 1
(In formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above. In formula (VIII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 have the same meaning as above, in formula (VI), R 6 has the same meaning as above, Q represents a hydrogen atom, trimethylsilyl, etc. In formula (IX), Y 1 , Y 2 , Y 3 , R 1 , R 2 and R 6 have the same meanings as above, and Y 1 , Y 2 , Y 3 , R 1 , R 2 and R 6 have the same meanings as described above in formula (IIa). .)
式(VII)で表される化合物を、例えばバイオオーガニック・アンド・メディシナル・ケミストリー・レターズ[Bioorganic & Med. Chem. Lett.]2012年、22巻、6108頁等に記載の方法に準じて、ジイソブチルアルミニウムヒドリド等の還元剤を用いて還元した後、二炭酸ジ-tert-ブチルと反応させることにより式(VIII)で表される化合物を合成することができる。
According to the method described in, for example, Bioorganic & Medicinal Chemistry Letters [Bioorganic & Med. Chem. Lett.] 2012, Vol. 22, p. 6108, etc. After reduction using a reducing agent such as aluminum hydride, the compound represented by the formula (VIII) can be synthesized by reacting with di-tert-butyl dicarbonate.
次いで、式(VIII)で表される化合物を式(VI)で表される化合物と、例えばオーガニック・アンド・バイオモレキュラー・ケミストリー[Org. Biomol. Chem.]2002年、12巻、185頁、ジャーナル・オブ・メディシナル・ケミストリー[J. Med. Chem.]2009年、52巻、3563頁等に記載の薗頭カップリングの反応条件を用いて反応させることにより式(IX)で表される化合物を合成することができる。
Next, the compound represented by the formula (VIII) is replaced with the compound represented by the formula (VI), for example, Organic and Biomolecular Chemistry [Org. Biomol. Chem.] 2002, 12, 185 pages, journal. Of Medicinal Chemistry [J. Med. Chem.] 2009, Vol. 52, p. 3563, etc., by reacting using the Sonogashira coupling reaction conditions, the compound represented by the formula (IX) Can be synthesized.
このようにして得られた式(IX)で表される化合物を、例えばヨーロッパ特許公報(EP 1,574,511号公報)、国際特許出願公報(WO 2008/021927号公報)、国際特許出願公報(WO 2010/075200号公報)等に記載の方法に準じて塩酸、臭化水素酸、トリフルオロ酢酸等と反応させて脱保護することにより、式(II)においてR3, R4及びR5が水素原子である式(IIa)で表される化合物又はその塩(例えば塩酸塩、臭化水素酸塩、トリフルオロ酢酸塩等)を得ることができる。
The compounds represented by the formula (IX) thus obtained are, for example, European Patent Publication (EP 1,574,511 Publication), International Patent Application Publication (WO 2008/021927 Publication), International Patent Application Publication (WO 2010 / In the formula (II), R 3 , R 4 and R 5 are hydrogen atoms by reacting with hydrochloric acid, hydrobromic acid, trifluoroacetic acid or the like according to the method described in JP A compound represented by the formula (IIa) or a salt thereof (for example, hydrochloride, hydrobromide, trifluoroacetate, etc.) can be obtained.
反応式2
(式(VII)中、Y1, Y2, Y3, R1, R2及びJ3は前記と同じ意味を表す。式(X)中、R3はC1~C4アルキル又はC1~C4ハロアルキルを表し、J4は塩素原子、臭素原子又はヨウ素原子を表す。式(XI)中、R3はC1~C4アルキル又はC1~C4ハロアルキルを表す。式(XII)中、Y1, Y2, Y3, R1, R2, R3及びJ3は上記と同じ意味を表す。式(IIb)中、Y1, Y2, Y3, R1, R2, R3及びR6は上記と同じ意味を表す。)
Reaction formula 2
(In formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above. In formula (X), R 3 is C 1 -C 4 alkyl or C 1. Represents a C 4 haloalkyl, J 4 represents a chlorine atom, a bromine atom or an iodine atom, and in formula (XI), R 3 represents a C 1 to C 4 alkyl or a C 1 to C 4 haloalkyl, Formula (XII) In the formula, Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 and J 3 represent the same meaning as above, and in formula (IIb), Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 and R 6 have the same meaning as above.)
式(VII)で表される化合物を、例えばバイオオーガニック・アンド・メディシナル・ケミストリー・レターズ[Bioorganic & Med. Chem. Lett.]2009年、19巻、1488頁及び1492頁、2011年、21巻、1434頁等に記載の方法に準じて、式(X)で表される公知のグリニャール反応剤と反応させるか、又は式(XI)で表される公知のリチウム反応剤と反応させた後、水素化ホウ素ナトリウム等を用いて還元することにより式(XII)で表される化合物を合成することができる。
The compound represented by the formula (VII) can be prepared by, for example, Bioorganic & Medicinal Chemistry Letters [Bioorganic & Med. Chem. & Lett.] 2009, 19, 1488 and 1492, 2011, 21; In accordance with the method described on page 1434, etc., after reacting with a known Grignard reactant represented by formula (X) or reacting with a known lithium reactant represented by formula (XI), hydrogen The compound represented by the formula (XII) can be synthesized by reduction using sodium borohydride or the like.
このようにして得られた式(XII)で表される化合物を反応式1と同様にして反応させることにより、式(II)においてR4及びR5が水素原子である式(IIb)で表される化合物又はその塩(例えば塩酸塩、臭化水素酸塩、トリフルオロ酢酸塩等)を得ることができる。
By reacting the compound represented by the formula (XII) thus obtained in the same manner as in the reaction formula 1, it is represented by the formula (IIb) in which R 4 and R 5 are hydrogen atoms in the formula (II). Or a salt thereof (for example, hydrochloride, hydrobromide, trifluoroacetate, etc.) can be obtained.
(式(VII)中、Y1, Y2, Y3, R1, R2及びJ3は前記と同じ意味を表す。式(XV)中、Y1, Y2, Y3, R1, R2及びJ3は上記と同じ意味を表す。式(IIc)中、Y1, Y2, Y3, R1, R2及びR6は上記と同じ意味を表す。)
式(VII)で表される化合物を、例えばシンセシス[Synthesis]2006年、4143頁等に記載の方法に準じて三塩化セリウム存在下メチルリチウム反応剤と反応させるか、又はシンレット[Synlett]2007年、652頁等に記載の方法に準じてチタニウム(IV)テトライソプロポキシド存在下メチルグリニャール反応剤と反応させることにより、式(XV)で表される化合物を合成することができる。 (In Formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above. In Formula (XV), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as above, and Y 1 , Y 2 , Y 3 , R 1 , R 2 and R 6 represent the same meaning as described above in formula (IIc).
The compound represented by the formula (VII) is reacted with a methyllithium reagent in the presence of cerium trichloride according to the method described in Synthesis 2006, page 4143, etc., or Synlett 2007 The compound represented by the formula (XV) can be synthesized by reacting with a methyl Grignard reagent in the presence of titanium (IV) tetraisopropoxide according to the method described on page 652, etc.
式(VII)で表される化合物を、例えばシンセシス[Synthesis]2006年、4143頁等に記載の方法に準じて三塩化セリウム存在下メチルリチウム反応剤と反応させるか、又はシンレット[Synlett]2007年、652頁等に記載の方法に準じてチタニウム(IV)テトライソプロポキシド存在下メチルグリニャール反応剤と反応させることにより、式(XV)で表される化合物を合成することができる。 (In Formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above. In Formula (XV), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as above, and Y 1 , Y 2 , Y 3 , R 1 , R 2 and R 6 represent the same meaning as described above in formula (IIc).
The compound represented by the formula (VII) is reacted with a methyllithium reagent in the presence of cerium trichloride according to the method described in Synthesis 2006, page 4143, etc., or Synlett 2007 The compound represented by the formula (XV) can be synthesized by reacting with a methyl Grignard reagent in the presence of titanium (IV) tetraisopropoxide according to the method described on page 652, etc.
このようにして得られた式(XV)で表される化合物を反応式1と同様にして反応させることにより、式(II)においてR3及びR4がメチルであり、R5が水素原子である式(IIc)で表される化合物又はその塩(例えば塩酸塩、臭化水素酸塩、トリフルオロ酢酸塩等)を得ることができる。
By reacting the compound represented by the formula (XV) thus obtained in the same manner as in the reaction formula 1, R 3 and R 4 in the formula (II) are methyl, and R 5 is a hydrogen atom. A compound represented by the formula (IIc) or a salt thereof (for example, hydrochloride, hydrobromide, trifluoroacetate, etc.) can be obtained.
(式(VII)中、Y1, Y2, Y3, R1, R2及びJ3は前記と同じ意味を表す。式(XVIII)中、Y1, Y2, Y3, R1, R2及びJ3は上記と同じ意味を表す。式(IId)中、Y1, Y2, Y3, R1, R2及びR6は上記と同じ意味を表す。)
式(VII)で表される化合物を、例えばケミカル・コミュニケーションズ[Chem. Commun.]2001年、1792頁等に記載の方法に準じてチタニウム(IV)テトライソプロポキシド及び三フッ化ホウ素ジエチルエーテル錯体存在下エチルグリニャール反応剤と反応させることにより、式(XVIII)で表される化合物を合成することができる。 (In formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above. In formula (XVIII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as above, and Y 1 , Y 2 , Y 3 , R 1 , R 2 and R 6 represent the same meaning as described above in formula (IId).
The compound represented by the formula (VII) is converted into a titanium (IV) tetraisopropoxide and boron trifluoride diethyl ether complex according to the method described in, for example, Chemical Communications [Chem. Commun.] 2001, page 1792. A compound represented by the formula (XVIII) can be synthesized by reacting with an ethyl Grignard reagent in the presence.
式(VII)で表される化合物を、例えばケミカル・コミュニケーションズ[Chem. Commun.]2001年、1792頁等に記載の方法に準じてチタニウム(IV)テトライソプロポキシド及び三フッ化ホウ素ジエチルエーテル錯体存在下エチルグリニャール反応剤と反応させることにより、式(XVIII)で表される化合物を合成することができる。 (In formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above. In formula (XVIII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as above, and Y 1 , Y 2 , Y 3 , R 1 , R 2 and R 6 represent the same meaning as described above in formula (IId).
The compound represented by the formula (VII) is converted into a titanium (IV) tetraisopropoxide and boron trifluoride diethyl ether complex according to the method described in, for example, Chemical Communications [Chem. Commun.] 2001, page 1792. A compound represented by the formula (XVIII) can be synthesized by reacting with an ethyl Grignard reagent in the presence.
このようにして得られた式(XVIII)で表される化合物を反応式1と同様にして反応させることにより、式(II)においてR3及びR4が一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成し、R5が水素原子である式(IId)で表される化合物又はその塩(例えば塩酸塩、臭化水素酸塩、トリフルオロ酢酸塩等)を得ることができる。
By reacting the compound represented by the formula (XVIII) thus obtained in the same manner as in Reaction Scheme 1, R 3 and R 4 together in Formula (II) form an ethylene chain. To form a cyclopropyl ring together with the carbon atom to which R 3 and R 4 are bonded, and a compound represented by the formula (IId) wherein R 5 is a hydrogen atom or a salt thereof (eg, hydrochloride, hydrobromide, Trifluoroacetate, etc.) can be obtained.
(式(VII)中、Y1, Y2, Y3, R1, R2及びJ3は前記と同じ意味を表す。式(XXI)中、Y1, Y2, Y3, R1, R2, R3及びJ3は上記と同じ意味を表す。式(VI)中、R6は前記と同じ意味を表し、Qは水素原子、トリメチルシリル等を表す。式(XXII)中、Y1, Y2, Y3, R1, R2, R3及びR6は上記と同じ意味を表す。式(XXIII)中、R5はC1~C4ハロアルキルチオ、-C(O)R9及びC1~C4アルコキシカルボニル以外の前記と同じ意味を表す。式(IIe)中、Y1, Y2, Y3, R1, R2, R3, R5及びR6は上記と同じ意味を表す。)
式(VII)で表される化合物を、例えばケミカル・アンド・ファーマシューティカル・ブレティン [Chem. Pharm. Bull.] 1986年、34巻、4653頁等に記載の方法に準じて式(X)[式中、R3及びJ4は前記と同じ意味を表す。]で表される公知のグリニャール反応剤と反応させた後加水分解するか、又は、例えばザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]1981年、46巻、4600頁等に記載の方法に準じてジイソブチルアルミニウムヒドリドと反応させた後加水分解することにより、式(XXI)で表される化合物を合成することができる。 (In formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above. In formula (XXI), Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 and J 3 represent the same meaning as above, in formula (VI), R 6 represents the same meaning as described above, Q represents a hydrogen atom, trimethylsilyl, etc. In formula (XXII), Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 and R 6 have the same meaning as above, wherein R 5 is C 1 -C 4 haloalkylthio, —C (O) R 9 and represent the same meaning as C 1 ~ C 4 alkoxy the non carbonyl in. formula (IIe), Y 1, Y 2, Y 3, R 1, R 2, R 3, R 5 and R 6 are as defined above Represents meaning.)
The compound represented by the formula (VII) is converted into a compound represented by the formula (X) according to the method described in Chemical and Pharmaceutical Bulletin [Chem. Pharm. Bull.] 1986, 34, 4653, etc. In the formula, R 3 and J 4 represent the same meaning as described above. And then hydrolyzed after reaction with a known Grignard reagent represented by the following formula, for example, The Journal of Organic Chemistry [J. Org. Chem.] 1981, 46, 4600, etc. The compound represented by the formula (XXI) can be synthesized by reacting with diisobutylaluminum hydride according to the method described, followed by hydrolysis.
式(VII)で表される化合物を、例えばケミカル・アンド・ファーマシューティカル・ブレティン [Chem. Pharm. Bull.] 1986年、34巻、4653頁等に記載の方法に準じて式(X)[式中、R3及びJ4は前記と同じ意味を表す。]で表される公知のグリニャール反応剤と反応させた後加水分解するか、又は、例えばザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]1981年、46巻、4600頁等に記載の方法に準じてジイソブチルアルミニウムヒドリドと反応させた後加水分解することにより、式(XXI)で表される化合物を合成することができる。 (In formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above. In formula (XXI), Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 and J 3 represent the same meaning as above, in formula (VI), R 6 represents the same meaning as described above, Q represents a hydrogen atom, trimethylsilyl, etc. In formula (XXII), Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 and R 6 have the same meaning as above, wherein R 5 is C 1 -C 4 haloalkylthio, —C (O) R 9 and represent the same meaning as C 1 ~ C 4 alkoxy the non carbonyl in. formula (IIe), Y 1, Y 2, Y 3, R 1, R 2, R 3, R 5 and R 6 are as defined above Represents meaning.)
The compound represented by the formula (VII) is converted into a compound represented by the formula (X) according to the method described in Chemical and Pharmaceutical Bulletin [Chem. Pharm. Bull.] 1986, 34, 4653, etc. In the formula, R 3 and J 4 represent the same meaning as described above. And then hydrolyzed after reaction with a known Grignard reagent represented by the following formula, for example, The Journal of Organic Chemistry [J. Org. Chem.] 1981, 46, 4600, etc. The compound represented by the formula (XXI) can be synthesized by reacting with diisobutylaluminum hydride according to the method described, followed by hydrolysis.
次いで、式(XXI)で表される化合物を式(VI)で表される置換アセチレンと、例えばテトラヘドロン・レターズ[Tetrahedron Lett.]2012年、53巻、4117頁、テトラヘドロン[Tetrahedron]2005年、61巻、2697頁等に記載の薗頭カップリングの反応条件を用いて反応させることにより、式(XXII)で表される化合物を合成することができる。
Subsequently, the compound represented by the formula (XXI) is replaced with a substituted acetylene represented by the formula (VI), for example, Tetrahedron Letters 2012, 53, 4117, Tetrahedron 2005. 61, 2697, etc., the compound represented by the formula (XXII) can be synthesized by reaction using the Sonogashira coupling reaction conditions described in the above.
このようにして得られた式(XXII)で表される化合物を、例えばヨーロピアン・ジャーナル・オブ・メディシナル・ケミストリー[Eur. J. Med. Chem.]2009年、44巻、4862頁等に記載の方法に準じてシアノ水素化ホウ素ナトリウム等の還元剤存在下、式(XXIII)で表される公知のアミン又はそれらの塩(例えば塩酸塩、酢酸塩等)と反応させることにより、式(II)においてR4が水素原子である式(IIe)で表される化合物を得ることができる。
The compound represented by the formula (XXII) thus obtained is described in, for example, European Journal of Medicinal Chemistry [Eur. J. Med. Chem.] 2009, 44, 4862, etc. According to the method, by reacting with a known amine represented by the formula (XXIII) or a salt thereof (for example, hydrochloride, acetate, etc.) in the presence of a reducing agent such as sodium cyanoborohydride, the formula (II) A compound represented by the formula (IIe) in which R 4 is a hydrogen atom can be obtained.
(式(XXIV)中、Y1, Y2, Y3及びJ3は前記と同じ意味を表す。式(XXV)中、R3は前記と同じ意味を表す。式(XXVI)中、Y1, Y2, Y3, R3及びJ3は上記と同じ意味を表す。式(XXVII)中、Y1, Y2, Y3, R3及びJ3は上記と同じ意味を表す。式(IIf)中、Y1, Y2, Y3, R3及びR6は上記と同じ意味を表す。)
式(XXIV)で表される化合物を、例えばジャーナル・オブ・ザ・ケミカル・ソサイエティー・パーキン・トランスアクションズ、1[J. Chem. Soc. Perkin Trans. 1]1979年、643頁等に記載の方法に準じて式(XXV)で表される公知のニトロアルカン誘導体と反応させることにより式(XXVI)で表される化合物を合成することができる。 (In Formula (XXIV), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above. In Formula (XXV), R 3 represents the same meaning as described above. In Formula (XXVI), Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as above, and Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as described above in formula (XXVII). IIf), Y 1 , Y 2 , Y 3 , R 3 and R 6 represent the same meaning as above.
The compound represented by the formula (XXIV) can be obtained by, for example, the method described in Journal of the Chemical Society Perkin Transactions, 1 [J. Chem. Soc. Perkin Trans. 1] 1979, page 643, etc. According to the above, the compound represented by the formula (XXVI) can be synthesized by reacting with a known nitroalkane derivative represented by the formula (XXV).
式(XXIV)で表される化合物を、例えばジャーナル・オブ・ザ・ケミカル・ソサイエティー・パーキン・トランスアクションズ、1[J. Chem. Soc. Perkin Trans. 1]1979年、643頁等に記載の方法に準じて式(XXV)で表される公知のニトロアルカン誘導体と反応させることにより式(XXVI)で表される化合物を合成することができる。 (In Formula (XXIV), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above. In Formula (XXV), R 3 represents the same meaning as described above. In Formula (XXVI), Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as above, and Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as described above in formula (XXVII). IIf), Y 1 , Y 2 , Y 3 , R 3 and R 6 represent the same meaning as above.
The compound represented by the formula (XXIV) can be obtained by, for example, the method described in Journal of the Chemical Society Perkin Transactions, 1 [J. Chem. Soc. Perkin Trans. 1] 1979, page 643, etc. According to the above, the compound represented by the formula (XXVI) can be synthesized by reacting with a known nitroalkane derivative represented by the formula (XXV).
次いで、式(XXVI)で表される化合物を、例えばシンレット[Synlett]2014年、25巻、2891頁等に記載の方法に準じて亜鉛-塩酸等を用いて還元することにより、式(XXVII)で表される化合物を合成することができる。
Next, the compound represented by the formula (XXVI) is reduced with zinc-hydrochloric acid or the like according to the method described in, for example, Synlett 2014, 25, 2891, etc. Can be synthesized.
このようにして得られた式(XXVII)で表される化合物を反応式1と同様にして反応させることにより、式(II)においてR1, R2, R4及びR5が水素原子である式(IIf)で表される化合物又はその塩(例えば塩酸塩、臭化水素酸塩、トリフルオロ酢酸塩等)を得ることができる。
ここで用いられる式(XXIV)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。 The compound represented by the formula (XXVII) thus obtained is reacted in the same manner as in the reaction formula 1, so that R 1 , R 2 , R 4 and R 5 are hydrogen atoms in the formula (II). A compound represented by the formula (IIf) or a salt thereof (for example, hydrochloride, hydrobromide, trifluoroacetate, etc.) can be obtained.
Some of the compounds represented by the formula (XXIV) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
ここで用いられる式(XXIV)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。 The compound represented by the formula (XXVII) thus obtained is reacted in the same manner as in the reaction formula 1, so that R 1 , R 2 , R 4 and R 5 are hydrogen atoms in the formula (II). A compound represented by the formula (IIf) or a salt thereof (for example, hydrochloride, hydrobromide, trifluoroacetate, etc.) can be obtained.
Some of the compounds represented by the formula (XXIV) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
(式(XXVI)中、Y1, Y2, Y3, R3及びJ3は前記と同じ意味を表す。式式(XXX)中、Wは酸素原子、硫黄原子又は-NHO-を表し、RaはC1~C6アルキル、C1~C6ハロアルキル、C3~C6アルケニル、C3~C6アルキニル、(Z)mによって置換されたフェニル(C1~C4)アルキル等を表す。式(XXXI)中、Y1, Y2, Y3, W, Ra, R3及びJ3は上記と同じ意味を表す。式(XXXII)中、Y1, Y2, Y3, W, Ra, R3及びJ3は上記と同じ意味を表す。式(IIg)中、Y1, Y2, Y3, W, Ra, R3及びR6は上記と同じ意味を表す。)
反応式6の合成中間体である式(XXVI)で表される化合物と式(XXX)で表される公知のアルコール類、チオール類又はアルコキシアミン類とを、例えばテトラヘドロン[Tetrahedron]2012年、68巻、1521頁、テトラヘドロン・レターズ[Tetrahedron Lett.]2008年、49巻、1244頁、ヨーロピアン・ジャーナル・オブ・オーガニック・ケミストリー[Eur. J. Org. Chem.]2010年、5482頁等に記載の方法に準じて反応させることにより式(XXXI)で表される化合物を合成することができる。 (In Formula (XXVI), Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as described above. In Formula (XXX), W represents an oxygen atom, a sulfur atom or —NHO—, R a represents C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C 6 alkenyl, C 3 -C 6 alkynyl, (Z) m- substituted phenyl (C 1 -C 4 ) alkyl, etc. In formula (XXXI), Y 1 , Y 2 , Y 3 , W, R a , R 3 and J 3 represent the same meaning as above, and in formula (XXXII), Y 1 , Y 2 , Y 3 , W, R a , R 3 and J 3 represent the same meaning as above, and Y 1 , Y 2 , Y 3 , W, R a , R 3 and R 6 represent the same meaning as described above in formula (IIg). .)
A compound represented by formula (XXVI), which is a synthetic intermediate of reaction formula 6, and known alcohols, thiols or alkoxyamines represented by formula (XXX), for example, tetrahedron (Tetrahedron) 2012, 68, 1521, Tetrahedron Lett. 2008, 49, 1244, European Journal of Organic Chemistry [Eur. J. Org. Chem.] 2010, 5482, etc. A compound represented by the formula (XXXI) can be synthesized by reacting according to the method described.
反応式6の合成中間体である式(XXVI)で表される化合物と式(XXX)で表される公知のアルコール類、チオール類又はアルコキシアミン類とを、例えばテトラヘドロン[Tetrahedron]2012年、68巻、1521頁、テトラヘドロン・レターズ[Tetrahedron Lett.]2008年、49巻、1244頁、ヨーロピアン・ジャーナル・オブ・オーガニック・ケミストリー[Eur. J. Org. Chem.]2010年、5482頁等に記載の方法に準じて反応させることにより式(XXXI)で表される化合物を合成することができる。 (In Formula (XXVI), Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as described above. In Formula (XXX), W represents an oxygen atom, a sulfur atom or —NHO—, R a represents C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C 6 alkenyl, C 3 -C 6 alkynyl, (Z) m- substituted phenyl (C 1 -C 4 ) alkyl, etc. In formula (XXXI), Y 1 , Y 2 , Y 3 , W, R a , R 3 and J 3 represent the same meaning as above, and in formula (XXXII), Y 1 , Y 2 , Y 3 , W, R a , R 3 and J 3 represent the same meaning as above, and Y 1 , Y 2 , Y 3 , W, R a , R 3 and R 6 represent the same meaning as described above in formula (IIg). .)
A compound represented by formula (XXVI), which is a synthetic intermediate of reaction formula 6, and known alcohols, thiols or alkoxyamines represented by formula (XXX), for example, tetrahedron (Tetrahedron) 2012, 68, 1521, Tetrahedron Lett. 2008, 49, 1244, European Journal of Organic Chemistry [Eur. J. Org. Chem.] 2010, 5482, etc. A compound represented by the formula (XXXI) can be synthesized by reacting according to the method described.
次いで、式(XXXI)で表される化合物を、例えばブレティン・オブ・ザ・ケミカル・ソサイエティー・オブ・ジャパン[Bull. Chem. Soc. Jpn.]2014年、87巻、127頁等に記載の方法に準じて亜鉛-酢酸等を用いて還元することにより、式(XXXII)で表される化合物を合成することができる。
Subsequently, the compound represented by the formula (XXXI) is converted into a method described in, for example, Bulletin of the Chemical Society of Japan [Bull. Chem. Soc. Jpn.] 2014, Vol. 87, 127. The compound represented by the formula (XXXII) can be synthesized by reduction using zinc-acetic acid or the like according to the above.
このようにして得られた式(XXXII)で表される化合物を反応式1と同様にして反応させることにより、式(II)においてR1が-W-Raであり、R2, R4及びR5が水素原子である式(IIg)で表される化合物又はその塩(例えば塩酸塩、臭化水素酸塩、トリフルオロ酢酸塩等)を得ることができる。
By reacting the compound represented by the formula (XXXII) thus obtained in the same manner as in Reaction Scheme 1, R 1 is —WR a in Formula (II), and R 2 , R 4 and R A compound represented by the formula (IIg) in which 5 is a hydrogen atom or a salt thereof (for example, hydrochloride, hydrobromide, trifluoroacetate, etc.) can be obtained.
(式(XXXV)中、Y1, Y2, Y3, R3及びJ3は前記と同じ意味を表す。式(XXXVI)中、Y1, Y2, Y3, R3及びJ3は上記と同じ意味を表す。式(VI)中、R6は前記と同じ意味を表し、Qは水素原子、トリメチルシリル、-ZnCl、-ZnBr又は-ZnI等を表す。式(XXXVII)中、Y1, Y2, Y3, R3及びR6は上記と同じ意味を表す。式(XXXVIII)中、Rbはエチル、2-メトキシエチル等を表す。式(XXXIX)中、Y1, Y2, Y3, R3及びR6は上記と同じ意味を表す。式(IIh)中、Y1, Y2, Y3, R3及びR6は上記と同じ意味を表す。)
式(XXXV)で表される化合物を、例えば国際特許出願公報(WO 2014/010737号公報)等に記載の方法に準じてハロゲン化した後フタルイミドカリウムと反応させることにより、式(XXXVI)で表される化合物を合成することができる。 (In Formula (XXXV), Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as described above. In Formula (XXXVI), Y 1 , Y 2 , Y 3 , R 3 and J 3 are In formula (VI), R 6 represents the same meaning as described above, and Q represents a hydrogen atom, trimethylsilyl, —ZnCl, —ZnBr, —ZnI, etc. In formula (XXXVII), Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meanings as described above, in formula (XXXVIII), R b represents ethyl, 2-methoxyethyl, etc. In formula (XXXIX), Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meaning as described above, and Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meaning as described above in formula (IIh).
The compound represented by the formula (XXXV) is halogenated according to the method described in, for example, International Patent Application Publication (WO 2014/010737), and then reacted with potassium phthalimide to represent the compound represented by the formula (XXXVI). Can be synthesized.
式(XXXV)で表される化合物を、例えば国際特許出願公報(WO 2014/010737号公報)等に記載の方法に準じてハロゲン化した後フタルイミドカリウムと反応させることにより、式(XXXVI)で表される化合物を合成することができる。 (In Formula (XXXV), Y 1 , Y 2 , Y 3 , R 3 and J 3 represent the same meaning as described above. In Formula (XXXVI), Y 1 , Y 2 , Y 3 , R 3 and J 3 are In formula (VI), R 6 represents the same meaning as described above, and Q represents a hydrogen atom, trimethylsilyl, —ZnCl, —ZnBr, —ZnI, etc. In formula (XXXVII), Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meanings as described above, in formula (XXXVIII), R b represents ethyl, 2-methoxyethyl, etc. In formula (XXXIX), Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meaning as described above, and Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meaning as described above in formula (IIh).
The compound represented by the formula (XXXV) is halogenated according to the method described in, for example, International Patent Application Publication (WO 2014/010737), and then reacted with potassium phthalimide to represent the compound represented by the formula (XXXVI). Can be synthesized.
このようにして得られた式(XXXVI)で表される化合物を、式(VI)で表される置換アセチレンと製造法Cと同様にして反応させることにより、式(XXXVII)で表される化合物を合成することができる。
The compound represented by the formula (XXXVII) obtained by reacting the compound represented by the formula (XXXVI) thus obtained with the substituted acetylene represented by the formula (VI) in the same manner as in Production Method C. Can be synthesized.
次いで、式(XXXVII)で表される化合物を、例えば国際特許出願公報(WO 2013/003740号公報)等に記載の方法に準じて水素化ホウ素ナトリウム等の還元剤を用いて還元した後、式(XXXVIII)で表される公知のフッ素化剤と反応させることにより、式(XXXIX)で表される化合物を合成することができる。
Subsequently, after reducing the compound represented by the formula (XXXVII) using a reducing agent such as sodium borohydride according to the method described in, for example, International Patent Application Publication (WO 2013/003740), the formula A compound represented by the formula (XXXIX) can be synthesized by reacting with a known fluorinating agent represented by (XXXVIII).
このようにして得られた式(XXXIX)で表される化合物を、必要ならばトルエン、ジクロロメタン、クロロホルム、メタノール、エタノール、テトラヒドロフラン、1,4-ジオキサン、水又はそれらの2種類以上の任意の割合の混合物等を溶媒として用い、必要ならば窒素、アルゴン等の不活性ガス雰囲気下、式(XXXIX)で表される化合物1モル当量に対して1~4モル当量のメチルアミン水溶液、ヒドラジン水溶液又はヒドラジン一水和物と室温~反応混合物の還流温度の温度範囲で1~24時間反応させることにより、式(II)においてR1がフッ素原子を表し、R2, R4及びR5が水素原子である式(IIh)で表される化合物を得ることができる。
The compound represented by the formula (XXXIX) thus obtained is converted to toluene, dichloromethane, chloroform, methanol, ethanol, tetrahydrofuran, 1,4-dioxane, water, or any ratio of two or more thereof if necessary. 1 to 4 molar equivalents of a methylamine aqueous solution, a hydrazine aqueous solution or 1 to 4 molar equivalents of the compound represented by the formula (XXXIX) under an inert gas atmosphere such as nitrogen or argon if necessary. By reacting with hydrazine monohydrate in the temperature range from room temperature to the reflux temperature of the reaction mixture for 1 to 24 hours, in formula (II), R 1 represents a fluorine atom, and R 2 , R 4 and R 5 are hydrogen atoms. A compound represented by the formula (IIh) can be obtained.
ここで用いられる式(XXXV)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。
Some of the compounds represented by the formula (XXXV) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
(式(XXXVII)中、Y1, Y2, Y3, R3及びR6は前記と同じ意味を表す。式(XL)中、Rcは水素原子、ハロゲン原子、C1~C5アルキル、C1~C5ハロアルキル又はC1~C4アルコキシ等を表し、J4は前記と同じ意味を表す。式(XLI)中、Y1, Y2, Y3, R3, R6及びRcは上記と同じ意味を表す。式(IIi)中、Y1, Y2, Y3, R3, R6及びRcは上記と同じ意味を表す。)
反応式8の合成中間体である式(XXXVII)で表される化合物を、例えばアンゲヴァンテ・ヘミー・インターナショナル・エディション[Angew. Chemie, Int. Ed.]2011年、50巻、2593頁、オーガニック・レターズ[Organic. Lett.]2007年、9巻、5219頁、ザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]1993年、58巻、6509頁、テトラヘドロン[Tetrahedron]2010年、66巻、3499頁、ヨーロピアン・ジャーナル・オブ・オーガニック・ケミストリー[Eur. J. Org. Chem.]2007年、266頁等に記載の方法に準じて、例えばカリウム tert-ブトキシド、ナトリウムヘキサメチルジシラジド、リチウムジイソプロピルアミド、アルキルリチウム等の強塩基存在下、式(XL)で表される化合物と反応させることにより、式(XLI)で表される化合物を合成することができる。 (In the formula (XXXVII), Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meaning as described above. In the formula (XL), R c represents a hydrogen atom, a halogen atom, or a C 1 -C 5 alkyl. , C 1 -C 5 haloalkyl or C 1 -C 4 alkoxy, etc., and J 4 has the same meaning as described above, wherein Y 1 , Y 2 , Y 3 , R 3 , R 6 and R c represents the same meaning as above, and Y 1 , Y 2 , Y 3 , R 3 , R 6 and R c represent the same meaning as described above in formula (IIi).
A compound represented by the formula (XXXVII), which is a synthetic intermediate of Reaction Formula 8, can be obtained by using, for example, Angewante Chemie International Edition [Angew. Chemie, Int. Ed.] 2011, 50, 2593, Organic Letters. [Organic. Lett.] 2007, 9, 5219, The Journal of Organic Chemistry [J. Org. Chem.] 1993, 58, 6509, Tetrahedron 2010, 66 Vol. 3499, European Journal of Organic Chemistry [Eur. J. Org. Chem.] 2007, page 266 etc., for example, potassium tert-butoxide, sodium hexamethyldisilazide By reacting with a compound represented by the formula (XL) in the presence of a strong base such as lithium diisopropylamide or alkyllithium It is possible to synthesize a compound of formula (XLI).
反応式8の合成中間体である式(XXXVII)で表される化合物を、例えばアンゲヴァンテ・ヘミー・インターナショナル・エディション[Angew. Chemie, Int. Ed.]2011年、50巻、2593頁、オーガニック・レターズ[Organic. Lett.]2007年、9巻、5219頁、ザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]1993年、58巻、6509頁、テトラヘドロン[Tetrahedron]2010年、66巻、3499頁、ヨーロピアン・ジャーナル・オブ・オーガニック・ケミストリー[Eur. J. Org. Chem.]2007年、266頁等に記載の方法に準じて、例えばカリウム tert-ブトキシド、ナトリウムヘキサメチルジシラジド、リチウムジイソプロピルアミド、アルキルリチウム等の強塩基存在下、式(XL)で表される化合物と反応させることにより、式(XLI)で表される化合物を合成することができる。 (In the formula (XXXVII), Y 1 , Y 2 , Y 3 , R 3 and R 6 have the same meaning as described above. In the formula (XL), R c represents a hydrogen atom, a halogen atom, or a C 1 -C 5 alkyl. , C 1 -C 5 haloalkyl or C 1 -C 4 alkoxy, etc., and J 4 has the same meaning as described above, wherein Y 1 , Y 2 , Y 3 , R 3 , R 6 and R c represents the same meaning as above, and Y 1 , Y 2 , Y 3 , R 3 , R 6 and R c represent the same meaning as described above in formula (IIi).
A compound represented by the formula (XXXVII), which is a synthetic intermediate of Reaction Formula 8, can be obtained by using, for example, Angewante Chemie International Edition [Angew. Chemie, Int. Ed.] 2011, 50, 2593, Organic Letters. [Organic. Lett.] 2007, 9, 5219, The Journal of Organic Chemistry [J. Org. Chem.] 1993, 58, 6509, Tetrahedron 2010, 66 Vol. 3499, European Journal of Organic Chemistry [Eur. J. Org. Chem.] 2007, page 266 etc., for example, potassium tert-butoxide, sodium hexamethyldisilazide By reacting with a compound represented by the formula (XL) in the presence of a strong base such as lithium diisopropylamide or alkyllithium It is possible to synthesize a compound of formula (XLI).
このようにして得られた式(XLI)で表される化合物を、反応式8と同様にしてメチルアミン水溶液、ヒドラジン水溶液又はヒドラジン一水和物等と反応させることにより、式(II)においてR1とR2とが一緒になってC1~C6アルキリデン、C1~C6ハロアルキリデン又はC1~C4アルコキシ(C1~C2)アルキリデンを形成し、R4及びR5が水素原子である式(IIi)で表される化合物を得ることができる。
ここで用いられる式(XL)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。 The compound represented by the formula (XLI) thus obtained is reacted with a methylamine aqueous solution, a hydrazine aqueous solution, hydrazine monohydrate, or the like in the same manner as in the reaction formula 8 to obtain R in the formula (II). 1 and R 2 together form C 1 -C 6 alkylidene, C 1 -C 6 haloalkylidene or C 1 -C 4 alkoxy (C 1 -C 2 ) alkylidene, and R 4 and R 5 are hydrogen A compound represented by the formula (IIi) which is an atom can be obtained.
Some of the compounds represented by the formula (XL) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
ここで用いられる式(XL)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。 The compound represented by the formula (XLI) thus obtained is reacted with a methylamine aqueous solution, a hydrazine aqueous solution, hydrazine monohydrate, or the like in the same manner as in the reaction formula 8 to obtain R in the formula (II). 1 and R 2 together form C 1 -C 6 alkylidene, C 1 -C 6 haloalkylidene or C 1 -C 4 alkoxy (C 1 -C 2 ) alkylidene, and R 4 and R 5 are hydrogen A compound represented by the formula (IIi) which is an atom can be obtained.
Some of the compounds represented by the formula (XL) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
(式(XLII)中、Y1, Y2, Y3及びJ3は前記と同じ意味を表し、R1はC1~C6アルキル等を表す。式(XLIII)中、Y1, Y2, Y3, R1及びJ3は上記と同じ意味を表す。式(XLIV)中、Y1, Y2, Y3, R1及びJ3は上記と同じ意味を表す。式(XXXVIII)中、Rbは前記と同じ意味を表す。式(XLV)中、Y1, Y2, Y3, R1及びJ3は上記と同じ意味を表す。式、式(VI)中、R6は前記と同じ意味を表し、Qは水素原子、トリメチルシリル、-ZnCl、-ZnBr又は-ZnI等を表す。式(XLVI)中、Y1, Y2, Y3, R1及びR6は上記と同じ意味を表す。式(IIj)中、Y1, Y2, Y3, R1及びR6は上記と同じ意味を表す。)
式(XLII)で表される化合物を、例えばシンセティック・コミュニケーションズ[Synth. Commun.]2003年、33巻、2135頁等に記載の方法に準じてトリメチルスルホキソニウムヨージド等と反応させることにより、式(XLIII)で表される化合物を合成することができる。 (In formula (XLII), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above, and R 1 represents C 1 -C 6 alkyl, etc. In formula (XLIII), Y 1 , Y 2 , Y 3 , R 1 and J 3 have the same meaning as above, and in formula (XLIV), Y 1 , Y 2 , Y 3 , R 1 and J 3 have the same meaning as above, in formula (XXXVIII) , R b represents the same meaning as described above, wherein Y 1 , Y 2 , Y 3 , R 1 and J 3 represent the same meaning as described above, wherein R 6 represents the formula, formula (VI), In the formula (XLVI), Y 1 , Y 2 , Y 3 , R 1 and R 6 are the same as above, and Q represents a hydrogen atom, trimethylsilyl, —ZnCl, —ZnBr, —ZnI, etc. In formula (IIj), Y 1 , Y 2 , Y 3 , R 1 and R 6 represent the same meaning as described above.)
By reacting the compound represented by the formula (XLII) with trimethylsulfoxonium iodide or the like according to the method described in, for example, Synthetic Communications [Synth. Commun.] 2003, 33, 2135, etc., A compound represented by the formula (XLIII) can be synthesized.
式(XLII)で表される化合物を、例えばシンセティック・コミュニケーションズ[Synth. Commun.]2003年、33巻、2135頁等に記載の方法に準じてトリメチルスルホキソニウムヨージド等と反応させることにより、式(XLIII)で表される化合物を合成することができる。 (In formula (XLII), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above, and R 1 represents C 1 -C 6 alkyl, etc. In formula (XLIII), Y 1 , Y 2 , Y 3 , R 1 and J 3 have the same meaning as above, and in formula (XLIV), Y 1 , Y 2 , Y 3 , R 1 and J 3 have the same meaning as above, in formula (XXXVIII) , R b represents the same meaning as described above, wherein Y 1 , Y 2 , Y 3 , R 1 and J 3 represent the same meaning as described above, wherein R 6 represents the formula, formula (VI), In the formula (XLVI), Y 1 , Y 2 , Y 3 , R 1 and R 6 are the same as above, and Q represents a hydrogen atom, trimethylsilyl, —ZnCl, —ZnBr, —ZnI, etc. In formula (IIj), Y 1 , Y 2 , Y 3 , R 1 and R 6 represent the same meaning as described above.)
By reacting the compound represented by the formula (XLII) with trimethylsulfoxonium iodide or the like according to the method described in, for example, Synthetic Communications [Synth. Commun.] 2003, 33, 2135, etc., A compound represented by the formula (XLIII) can be synthesized.
次いで、式(XLIII)で表される化合物を、例えば国際特許出願公報(WO 2006/136821号公報)等に記載の方法に準じてフタルイミドカリウムと反応させることにより式(XLIV)で表される化合物とした後、例えば国際特許出願公報(WO 2009/123855号公報)等に記載の方法に準じて式(XXXVIII)で表される公知のフッ素化剤と反応させることにより、式(XLV)で表される化合物を合成することができる。
Next, the compound represented by the formula (XLIV) is reacted with potassium phthalimide according to the method described in, for example, International Patent Application Publication (WO 2006/136821), etc. Then, for example, by reacting with a known fluorinating agent represented by the formula (XXXVIII) according to the method described in International Patent Application Publication (WO (2009/123855) etc., the formula (XLV) Can be synthesized.
このようにして得られた式(XLV)で表される化合物を、式(VI)で表される置換アセチレンと製造法Cと同様にして反応させることにより、式(XLVI)で表される化合物を合成することができる。
次いで、式(XLVI)で表される化合物を反応式8と同様にしてメチルアミン水溶液、ヒドラジン水溶液又はヒドラジン一水和物と反応させることにより、式(II)においてR2がフッ素原子であり、R3, R4及びR5が水素原子である式(IIj)で表される化合物を得ることができる。 The compound represented by the formula (XLVI) thus obtained is reacted with the substituted acetylene represented by the formula (VI) in the same manner as in Production Method C to obtain the compound represented by the formula (XLVI). Can be synthesized.
Next, by reacting the compound represented by the formula (XLVI) with an aqueous methylamine solution, an aqueous hydrazine solution or hydrazine monohydrate in the same manner as in the reaction formula 8, R 2 in the formula (II) is a fluorine atom, A compound represented by the formula (IIj) in which R 3 , R 4 and R 5 are hydrogen atoms can be obtained.
次いで、式(XLVI)で表される化合物を反応式8と同様にしてメチルアミン水溶液、ヒドラジン水溶液又はヒドラジン一水和物と反応させることにより、式(II)においてR2がフッ素原子であり、R3, R4及びR5が水素原子である式(IIj)で表される化合物を得ることができる。 The compound represented by the formula (XLVI) thus obtained is reacted with the substituted acetylene represented by the formula (VI) in the same manner as in Production Method C to obtain the compound represented by the formula (XLVI). Can be synthesized.
Next, by reacting the compound represented by the formula (XLVI) with an aqueous methylamine solution, an aqueous hydrazine solution or hydrazine monohydrate in the same manner as in the reaction formula 8, R 2 in the formula (II) is a fluorine atom, A compound represented by the formula (IIj) in which R 3 , R 4 and R 5 are hydrogen atoms can be obtained.
ここで用いられる式(XLII)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて容易に合成することができる。
Some of the compounds represented by the formula (XLII) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be easily synthesized according to general synthesis methods described in literatures concerning known compounds.
反応式1~反応式5で用いられる式(VIII)で表される化合物は、例えば次の反応式11~反応式15のようにして合成することができる。
反応式11
The compounds represented by the formula (VIII) used in the reaction formulas 1 to 5 can be synthesized, for example, as the following reaction formulas 11 to 15.
Reaction formula 11
反応式11
Reaction formula 11
(式(XLVII)中、Y1, Y2, Y3及びJ3は前記と同じ意味を表し、J5はハロゲン原子を表す。式(XLVIII)中、RdはC1~C4アルキルを表す。式(XLIX)中、Y1, Y2, Y3, J3及びRdは上記と同じ意味を表す。式(VIIa)中、Y1, Y2, Y3及びJ3は上記と同じ意味を表す。)
式(XLVII)で表される化合物を、例えばザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]2008年、73巻、1643頁、バイオオーガニック・アンド・メディシナル・ケミストリー・レターズ[Bioorganic & Med. Chem. Lett.]2009年、19巻、4484頁等に記載の方法に準じて公知の式(XLVIII)で表されるシアノ酢酸エステルと反応させることにより、式(XLIX)で表される化合物を合成することができる。
次いで、式(XLIX)で表される化合物を、例えばジャーナル・オブ・メディシナル・ケミストリー[J. Med. Chem.]2005年、48巻、2167頁、シンセシス[Synthesis]2010年、3332頁等に記載の方法に準じて加熱脱炭酸することにより、式(VII)においてR1及びR2が水素原子である式(VIIa)で表される化合物を合成することができる。
ここで用いられる式(XLVII)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。 (In formula (XLVII), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above, J 5 represents a halogen atom. In formula (XLVIII), R d represents C 1 -C 4 alkyl. In formula (XLIX), Y 1 , Y 2 , Y 3 , J 3 and R d have the same meaning as above, and in formula (VIIa), Y 1 , Y 2 , Y 3 and J 3 are It represents the same meaning.)
The compound represented by the formula (XLVII) can be synthesized by, for example, The Journal of Organic Chemistry [J. Org. Chem.] 2008, 73, 1643, Bioorganic and Medicinal Chemistry Letters [Bioorganic. & Med. Chem. Lett.] In accordance with the method described in 2009, Vol. 19, p. 4484, etc., by reacting with a known cyanoacetate represented by the formula (XLVIII), it is represented by the formula (XLIX). Can be synthesized.
Next, the compound represented by the formula (XLIX) is described in, for example, Journal of Medicinal Chemistry [J. Med. Chem.] 2005, 48, 2167, Synthesis 2010, 3332, etc. The compound represented by the formula (VIIa) in which R 1 and R 2 are hydrogen atoms in the formula (VII) can be synthesized by heat decarboxylation according to the method of
Some of the compounds represented by the formula (XLVII) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
式(XLVII)で表される化合物を、例えばザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]2008年、73巻、1643頁、バイオオーガニック・アンド・メディシナル・ケミストリー・レターズ[Bioorganic & Med. Chem. Lett.]2009年、19巻、4484頁等に記載の方法に準じて公知の式(XLVIII)で表されるシアノ酢酸エステルと反応させることにより、式(XLIX)で表される化合物を合成することができる。
次いで、式(XLIX)で表される化合物を、例えばジャーナル・オブ・メディシナル・ケミストリー[J. Med. Chem.]2005年、48巻、2167頁、シンセシス[Synthesis]2010年、3332頁等に記載の方法に準じて加熱脱炭酸することにより、式(VII)においてR1及びR2が水素原子である式(VIIa)で表される化合物を合成することができる。
ここで用いられる式(XLVII)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。 (In formula (XLVII), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above, J 5 represents a halogen atom. In formula (XLVIII), R d represents C 1 -C 4 alkyl. In formula (XLIX), Y 1 , Y 2 , Y 3 , J 3 and R d have the same meaning as above, and in formula (VIIa), Y 1 , Y 2 , Y 3 and J 3 are It represents the same meaning.)
The compound represented by the formula (XLVII) can be synthesized by, for example, The Journal of Organic Chemistry [J. Org. Chem.] 2008, 73, 1643, Bioorganic and Medicinal Chemistry Letters [Bioorganic. & Med. Chem. Lett.] In accordance with the method described in 2009, Vol. 19, p. 4484, etc., by reacting with a known cyanoacetate represented by the formula (XLVIII), it is represented by the formula (XLIX). Can be synthesized.
Next, the compound represented by the formula (XLIX) is described in, for example, Journal of Medicinal Chemistry [J. Med. Chem.] 2005, 48, 2167, Synthesis 2010, 3332, etc. The compound represented by the formula (VIIa) in which R 1 and R 2 are hydrogen atoms in the formula (VII) can be synthesized by heat decarboxylation according to the method of
Some of the compounds represented by the formula (XLVII) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
反応式12
(式(VIIa)中、Y1, Y2, Y3及びJ3は前記と同じ意味を表す。式(L)中、J3は前記と同じ意味を表し、R1はC1~C6アルキル、C1~C6ハロアルキル、(Z)mによって置換されたフェニル(C1~C4)アルキル等を表す。式(VIIb)中、Y1, Y2, Y3, R1及びJ3は上記と同じ意味を表す。)
Reaction formula 12
(In Formula (VIIa), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above. In Formula (L), J 3 represents the same meaning as described above, and R 1 represents C 1 to C 6. Represents alkyl, C 1 -C 6 haloalkyl, phenyl (C 1 -C 4 ) alkyl substituted by (Z) m , etc. In formula (VIIb), Y 1 , Y 2 , Y 3 , R 1 and J 3 Represents the same meaning as above.)
式(VIIa)で表される化合物を、例えばジャーナル・オブ・ヘテロサイクリック・ケミストリー[J. Heterocyclic Chem.]1987年、24巻、1061頁等に記載の方法に準じて公知の式(L)で表される化合物と反応させることにより、式(VII)においてR2が水素原子である式(VIIb)で表される化合物を合成することができる。
The compound represented by the formula (VIIa) is converted to a known formula (L) according to the method described in, for example, Journal of Heterocyclic Chemistry [J. Heterocyclic Chem.] 1987, Vol. 24, page 1061. By reacting with a compound represented by the formula (VII), a compound represented by the formula (VIIb) in which R 2 is a hydrogen atom in the formula (VII) can be synthesized.
反応式13
(式(XLVII)中、Y1, Y2, Y3, J3及びJ5は前記と同じ意味を表す。式(LI)中、R1はハロゲン原子以外の前記と同じ意味を表す。式(VIIb)中、Y1, Y2, Y3, R1及びJ3は上記と同じ意味を表す。)
式(XLVII)で表される化合物を、例えばジャーナル・オブ・ヘテロサイクリック・ケミストリー[J. Heterocyclic Chem.]1987年、24巻、1061頁、ザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]2005年、70巻、10186頁等に記載の方法に準じて式(LI)で表される化合物と反応させることにより、式(VII)においてR2が水素原子である式(VIIb)で表される化合物を合成することもできる。 Reaction formula 13
(In Formula (XLVII), Y 1 , Y 2 , Y 3 , J 3 and J 5 represent the same meaning as described above. In Formula (LI), R 1 represents the same meaning as described above except for a halogen atom. In (VIIb), Y 1 , Y 2 , Y 3 , R 1 and J 3 represent the same meaning as described above.)
A compound represented by the formula (XLVII) can be synthesized by using, for example, Journal of Heterocyclic Chem. [J. Heterocyclic Chem.] 1987, 24, 1061, The Journal of Organic Chemistry [J. Org. Chem.] 2005, 70, 10186, etc., by reacting with a compound represented by the formula (LI) according to the method described in the formula (VII), wherein R 2 is a hydrogen atom (VIIb ) Can also be synthesized.
式(XLVII)で表される化合物を、例えばジャーナル・オブ・ヘテロサイクリック・ケミストリー[J. Heterocyclic Chem.]1987年、24巻、1061頁、ザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]2005年、70巻、10186頁等に記載の方法に準じて式(LI)で表される化合物と反応させることにより、式(VII)においてR2が水素原子である式(VIIb)で表される化合物を合成することもできる。 Reaction formula 13
A compound represented by the formula (XLVII) can be synthesized by using, for example, Journal of Heterocyclic Chem. [J. Heterocyclic Chem.] 1987, 24, 1061, The Journal of Organic Chemistry [J. Org. Chem.] 2005, 70, 10186, etc., by reacting with a compound represented by the formula (LI) according to the method described in the formula (VII), wherein R 2 is a hydrogen atom (VIIb ) Can also be synthesized.
ここで用いられる式(LI)で表される化合物の或ものは公知化合物であり、一部は市販品としても入手できる。また、それ以外のものも公知化合物に関する文献記載の一般的な合成方法に準じて合成することができる。
Some of the compounds represented by the formula (LI) used here are known compounds, and some of them are available as commercial products. In addition, other compounds can be synthesized according to a general synthesis method described in literatures concerning known compounds.
反応式14
(式(VIIb)中、Y1, Y2, Y3, R1及びJ3は前記と同じ意味を表す。式(LII)中、J3は前記と同じ意味を表し、R2はC1~C6アルキル、C1~C6ハロアルキル等を表す。式(VII)中、Y1, Y2, Y3, R1, R2及びJ3は上記と同じ意味を表す。)
式(VIIb)で表される化合物を、例えばヨーロピアン・ジャーナル・オブ・メディシナル・ケミストリー[Eur. J. Med. Chem.]2004年、39巻、993頁等に記載の方法に準じて公知の式(LII)で表される化合物と反応させることにより、式(VII)で表される化合物を合成することができる。 Reaction formula 14
(In Formula (VIIb), Y 1 , Y 2 , Y 3 , R 1 and J 3 represent the same meaning as described above. In Formula (LII), J 3 represents the same meaning as described above, and R 2 represents C 1. Represents -C 6 alkyl, C 1 -C 6 haloalkyl, etc. In formula (VII), Y 1 , Y 2 , Y 3 , R 1 , R 2 and J 3 represent the same meaning as described above.
The compound represented by the formula (VIIb) is converted to a known formula according to the method described in, for example, European Journal of Medicinal Chemistry [Eur. J. Med. Chem.] 2004, 39, 993. By reacting with the compound represented by (LII), the compound represented by formula (VII) can be synthesized.
式(VIIb)で表される化合物を、例えばヨーロピアン・ジャーナル・オブ・メディシナル・ケミストリー[Eur. J. Med. Chem.]2004年、39巻、993頁等に記載の方法に準じて公知の式(LII)で表される化合物と反応させることにより、式(VII)で表される化合物を合成することができる。 Reaction formula 14
The compound represented by the formula (VIIb) is converted to a known formula according to the method described in, for example, European Journal of Medicinal Chemistry [Eur. J. Med. Chem.] 2004, 39, 993. By reacting with the compound represented by (LII), the compound represented by formula (VII) can be synthesized.
反応式15
(式(VIIa)中、Y1, Y2, Y3及びJ3は前記と同じ意味を表す。式(VIIc)中、Y1, Y2, Y3及びJ3は上記と同じ意味を表す。)
式(VIIa)で表される化合物を、例えばザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]1998年、63巻、8052頁等に記載の方法に準じて、tert-ブチルリチウム等の強塩基存在下、N-フルオロベンゼンスルホン酸イミド等のフッ素化剤と反応させることにより、式(VII)においてR1及びR2がフッ素原子である式(VIIc)で表される化合物を合成することができる。 Reaction formula 15
(In Formula (VIIa), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above. In Formula (VIIc), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above. .)
According to the method described in, for example, The Journal of Organic Chemistry [J. Org. Chem.] 1998, 63, 8052, etc., the compound represented by the formula (VIIa) is tert-butyllithium. By reacting with a fluorinating agent such as N-fluorobenzenesulfonic acid imide in the presence of a strong base such as the compound of formula (VIIc) wherein R 1 and R 2 are fluorine atoms Can be synthesized.
式(VIIa)で表される化合物を、例えばザ・ジャーナル・オブ・オーガニック・ケミストリー[J. Org. Chem.]1998年、63巻、8052頁等に記載の方法に準じて、tert-ブチルリチウム等の強塩基存在下、N-フルオロベンゼンスルホン酸イミド等のフッ素化剤と反応させることにより、式(VII)においてR1及びR2がフッ素原子である式(VIIc)で表される化合物を合成することができる。 Reaction formula 15
According to the method described in, for example, The Journal of Organic Chemistry [J. Org. Chem.] 1998, 63, 8052, etc., the compound represented by the formula (VIIa) is tert-butyllithium. By reacting with a fluorinating agent such as N-fluorobenzenesulfonic acid imide in the presence of a strong base such as the compound of formula (VIIc) wherein R 1 and R 2 are fluorine atoms Can be synthesized.
反応式16
(式(VIIa)中、Y1, Y2, Y3及びJ3は前記と同じ意味を表す。式(LIII)中、J5は前記と同じ意味を表す。式(VIId)中、Y1, Y2, Y3及びJ3は上記と同じ意味を表す。)
式(VIIa)で表される化合物を、例えばジャーナル・オブ・メディシナル・ケミストリー[J. Med. Chem.]2010年、53巻、6003頁等に記載の方法に準じて公知の式(LIII)で表される化合物と反応させることにより、式(VII)においてR1とR2とが一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成する式(VIId)で表される化合物を合成することができる。 Reaction formula 16
(In Formula (VIIa), Y 1 , Y 2 , Y 3 and J 3 represent the same meaning as described above. In Formula (LIII), J 5 represents the same meaning as described above. In Formula (VIId), Y 1 , Y 2 , Y 3 and J 3 have the same meaning as above.)
The compound represented by the formula (VIIa) is converted to a known formula (LIII) according to the method described in, for example, Journal of Medicinal Chemistry [J. Med. Chem.] 2010, Vol. 53, page 6003. By reacting with the compound represented by formula (VII), R 1 and R 2 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded. A compound represented by the formula (VIId) can be synthesized.
式(VIIa)で表される化合物を、例えばジャーナル・オブ・メディシナル・ケミストリー[J. Med. Chem.]2010年、53巻、6003頁等に記載の方法に準じて公知の式(LIII)で表される化合物と反応させることにより、式(VII)においてR1とR2とが一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成する式(VIId)で表される化合物を合成することができる。 Reaction formula 16
The compound represented by the formula (VIIa) is converted to a known formula (LIII) according to the method described in, for example, Journal of Medicinal Chemistry [J. Med. Chem.] 2010, Vol. 53, page 6003. By reacting with the compound represented by formula (VII), R 1 and R 2 together form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded. A compound represented by the formula (VIId) can be synthesized.
上記の各反応においては、反応終了後、通常の後処理を行なうことにより、製造法A~製造法Cの原料化合物となる各々の製造中間体を得ることができる。
また、これらの方法により製造された各々の製造中間体は、単離・精製することなく、それぞれそのまま次工程の反応に用いることもできる。 In each of the above reactions, after the completion of the reaction, each of the production intermediates that will be the raw material compounds of production method A to production method C can be obtained by performing a general post-treatment.
In addition, each production intermediate produced by these methods can be used as it is in the subsequent step without isolation and purification.
また、これらの方法により製造された各々の製造中間体は、単離・精製することなく、それぞれそのまま次工程の反応に用いることもできる。 In each of the above reactions, after the completion of the reaction, each of the production intermediates that will be the raw material compounds of production method A to production method C can be obtained by performing a general post-treatment.
In addition, each production intermediate produced by these methods can be used as it is in the subsequent step without isolation and purification.
これらの方法を用いて製造できる本発明に包含される式(I)で表されるアルキニルピリジン置換アミド化合物としては、具体的に例えば下記の[I]-1~[I]-50の構造式で表される化合物が挙げられる。但し、[I]-1~[I]-50の構造式で表される化合物は例示のためのものであって、本発明に包含されるアルキニルピリジン置換アミド化合物はこれらのみに限定されるものではない。
Specific examples of the alkynylpyridine-substituted amide compound represented by the formula (I) included in the present invention that can be produced using these methods include the following structural formulas [I] -1 to [I] -50: The compound represented by these is mentioned. However, the compounds represented by the structural formulas [I] -1 to [I] -50 are for illustrative purposes, and the alkynylpyridine-substituted amide compounds included in the present invention are limited to these compounds. is not.
ここで、[I]-1~[I]-50の構造式に於いてY1、Y2、Y3、R1、R2、R3、R4、R5及びR6で表される置換基の具体的な組み合わせの例としては、例えば第2表に示す組み合わせが挙げられる。但し、第2表の組み合わせは例示のためのものであって、本発明に包含されるアルキニルピリジン置換アミド化合物のY1、Y2、Y3、R1、R2、R3、R4、R5及びR6で表される置換基の具体的な組み合わせはこれらのみに限定されるものではない。
Here, Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 , R 5 and R 6 are represented in the structural formulas [I] -1 to [I] -50. Examples of specific combinations of substituents include, for example, combinations shown in Table 2. However, the combinations in Table 2 are for illustrative purposes, and Y 1 , Y 2 , Y 3 , R 1 , R 2 , R 3 , R 4 , of the alkynylpyridine-substituted amide compounds included in the present invention, Specific combinations of substituents represented by R 5 and R 6 are not limited to these.
尚、表中、Etとの記載はエチルを表し、以下同様にn-Prはノルマルプロピルを、i-Pr及びPr-iはイソプロピルを、c-Pr及びPr-cはシクロプロピルを、Bu-sはsec-ブチルを、c-Buはシクロブチルを、t-Bu及びBu-tはtert-ブチルを、Penはペンチルを、c-Penはシクロペンチルを、c-Hexはシクロヘキシルを、Phはフェニルを、1-Naphは1-ナフチルを、2-Naphは2-ナフチルをそれぞれ表し、
表中、D-1-1a~D-26-1cで表される芳香族複素環は、それぞれ下記の構造を表し、 In the table, Et represents ethyl, and n-Pr is normal propyl, i-Pr and Pr-i are isopropyl, c-Pr and Pr-c are cyclopropyl, Bu- s is sec-butyl, c-Bu is cyclobutyl, t-Bu and Bu-t are tert-butyl, Pen is pentyl, c-Pen is cyclopentyl, c-Hex is cyclohexyl, Ph is phenyl 1-Naph represents 1-naphthyl, 2-Naph represents 2-naphthyl,
In the table, the aromatic heterocycles represented by D-1-1a to D-26-1c each represent the following structure,
表中、D-1-1a~D-26-1cで表される芳香族複素環は、それぞれ下記の構造を表し、 In the table, Et represents ethyl, and n-Pr is normal propyl, i-Pr and Pr-i are isopropyl, c-Pr and Pr-c are cyclopropyl, Bu- s is sec-butyl, c-Bu is cyclobutyl, t-Bu and Bu-t are tert-butyl, Pen is pentyl, c-Pen is cyclopentyl, c-Hex is cyclohexyl, Ph is phenyl 1-Naph represents 1-naphthyl, 2-Naph represents 2-naphthyl,
In the table, the aromatic heterocycles represented by D-1-1a to D-26-1c each represent the following structure,
置換基(Z)nの置換位置を表す番号は、上記の構造式において記された番号の位置に対応するものであり、例えば、表中、「(D-23-2b)-6-F」との記載は「6-フルオロピリジン-3-イル」を表し、
表中、D-31-a~D-37-cで表される芳香族複素環は、それぞれ下記の構造を表し、 The number representing the substitution position of the substituent (Z) n corresponds to the position of the number described in the above structural formula. For example, in the table, “(D-23-2b) -6-F” The description represents “6-fluoropyridin-3-yl”,
In the table, the aromatic heterocycles represented by D-31-a to D-37-c each represent the following structure,
表中、D-31-a~D-37-cで表される芳香族複素環は、それぞれ下記の構造を表し、 The number representing the substitution position of the substituent (Z) n corresponds to the position of the number described in the above structural formula. For example, in the table, “(D-23-2b) -6-F” The description represents “6-fluoropyridin-3-yl”,
In the table, the aromatic heterocycles represented by D-31-a to D-37-c each represent the following structure,
また、表中、置換基R1及び置換基R3の欄における(R)及び(S)の表記は、R1又はR3が結合する炭素原子の光学異性体の混合比において、(R)-体又は(S)-体の比が90%以上であることを表し、
表中、置換基R1及びR2の欄における(E)及び(Z)の記載は、置換基R1とR2とが一緒になって形成するアルキリデンの幾何異性体の混合比において、それぞれ(E)-体又は(Z)-体の比が90%以上であることを表す。 Further, in the table, the notation (R) and (S) in the column of the substituents R 1 and substituent R 3, the mixing ratio of the optical isomers of the carbon atoms of R 1 or R 3 is attached, (R) -Represents that the ratio of -body or (S) -body is 90% or more,
In the table, the description of the column of the substituents R 1 and R 2 (E) and (Z), the mixing ratio of the geometric isomers of alkylidene and substituents R 1 and R 2 together form, respectively The ratio of (E) -isomer or (Z) -isomer is 90% or more.
表中、置換基R1及びR2の欄における(E)及び(Z)の記載は、置換基R1とR2とが一緒になって形成するアルキリデンの幾何異性体の混合比において、それぞれ(E)-体又は(Z)-体の比が90%以上であることを表す。 Further, in the table, the notation (R) and (S) in the column of the substituents R 1 and substituent R 3, the mixing ratio of the optical isomers of the carbon atoms of R 1 or R 3 is attached, (R) -Represents that the ratio of -body or (S) -body is 90% or more,
In the table, the description of the column of the substituents R 1 and R 2 (E) and (Z), the mixing ratio of the geometric isomers of alkylidene and substituents R 1 and R 2 together form, respectively The ratio of (E) -isomer or (Z) -isomer is 90% or more.
本発明化合物は、マツ目(Pinales)、モクレン類(magnoliids)、単子葉類(monocots)、真正双子葉類(eudicots)等の維管束植物(Tracheophyta)に発生する植物病害及び哺乳類(Mammalia)、鳥類(Aves)、爬虫類(Reptilia)、真骨魚類(Actinopterygii)等の脊椎動物(Vertebrata)感染症の病原菌、さらに植物寄生性又は動物寄生性の線形動物、鉤頭動物、扁形動物及び原生動物等の有害生物を駆除できる。
植物の有害生物としては、子嚢菌門(Ascomycota)菌類、担子菌門(Basidiomycota)菌類、ツボカビ門(Chitridiomycota)菌類、コウマクノウキン門(Blastocladiomycota)菌類、ケカビ亜門(Mucoromycotina)菌類、ケルコゾア門(Cercozoa)原生生物、不等毛植物門(Heterokontophyta)卵菌類(Oomycetes)、放線菌門(Actinobacteria)グラム陽性菌類、テネリクテス門(Tenericutes)グラム陽性菌類、プロテオバクテリア門(Proteobacteria)グラム陰性菌類及び葉線虫目(Aphelenchida)線虫、ハリセンチュウ目(Tylenchida)線虫等が挙げられるが、本発明化合物は、これらのうち特に子嚢菌門及び担子菌門に属する植物病原性真菌類、葉線虫目及びハリセンチュウ目に属する植物寄生性線虫類に対して低濃度で優れた防除効果を発揮する。 The compounds of the present invention include plant diseases and mammals (Mammalia) that occur in vascular plants (Tracheophyta) such as pine (Pinales), magnolias, monocots, monocots (eudicots), etc. Pathogens of vertebrate (Vertebrata) infections such as birds (Aves), reptiles (Reptilia), and teleosts (Actinopterygii), plant parasitic or animal parasitic linear animals, bald animals, flat animals, protozoa, etc. Can remove the pests.
Plant pests include Ascomycota fungi, Basidiomycota fungi, Chitridiomycota fungi, Blastocladiomycota fungi, Mucoromycotina fungi, Cercozoa fungi Cercozoa Protists, Heterokontophyta Oomycetes, Actinobacteria gram-positive fungi, Tenericutes gram-positive fungi, Proteobacteria gram-negative fungi and leaf lines Aphelenchida nematodes, Tylenchida nematodes, etc., among which the compounds of the present invention are phytopathogenic fungi belonging to the Ascomycota and Basidiomycota, leaf nematodes It exhibits excellent control effects at low concentrations against plant parasitic nematodes belonging to the order of the nematode.
植物の有害生物としては、子嚢菌門(Ascomycota)菌類、担子菌門(Basidiomycota)菌類、ツボカビ門(Chitridiomycota)菌類、コウマクノウキン門(Blastocladiomycota)菌類、ケカビ亜門(Mucoromycotina)菌類、ケルコゾア門(Cercozoa)原生生物、不等毛植物門(Heterokontophyta)卵菌類(Oomycetes)、放線菌門(Actinobacteria)グラム陽性菌類、テネリクテス門(Tenericutes)グラム陽性菌類、プロテオバクテリア門(Proteobacteria)グラム陰性菌類及び葉線虫目(Aphelenchida)線虫、ハリセンチュウ目(Tylenchida)線虫等が挙げられるが、本発明化合物は、これらのうち特に子嚢菌門及び担子菌門に属する植物病原性真菌類、葉線虫目及びハリセンチュウ目に属する植物寄生性線虫類に対して低濃度で優れた防除効果を発揮する。 The compounds of the present invention include plant diseases and mammals (Mammalia) that occur in vascular plants (Tracheophyta) such as pine (Pinales), magnolias, monocots, monocots (eudicots), etc. Pathogens of vertebrate (Vertebrata) infections such as birds (Aves), reptiles (Reptilia), and teleosts (Actinopterygii), plant parasitic or animal parasitic linear animals, bald animals, flat animals, protozoa, etc. Can remove the pests.
Plant pests include Ascomycota fungi, Basidiomycota fungi, Chitridiomycota fungi, Blastocladiomycota fungi, Mucoromycotina fungi, Cercozoa fungi Cercozoa Protists, Heterokontophyta Oomycetes, Actinobacteria gram-positive fungi, Tenericutes gram-positive fungi, Proteobacteria gram-negative fungi and leaf lines Aphelenchida nematodes, Tylenchida nematodes, etc., among which the compounds of the present invention are phytopathogenic fungi belonging to the Ascomycota and Basidiomycota, leaf nematodes It exhibits excellent control effects at low concentrations against plant parasitic nematodes belonging to the order of the nematode.
動物の有害生物としては、子嚢菌門(Ascomycota)菌類、担子菌門(Basidiomycota)菌類、放線菌門(Actinobacteria)グラム陽性菌類、フィルミクテス門(Firmicutes)グラム陽性菌類、テネリクテス門(Tenericutes)グラム陽性菌類、プロテオバクテリア門(Proteobacteria)グラム陰性菌類及びエノプルス目(Enoplida)線虫、桿線虫目(Rhabditida)線虫、円虫目(Strongylida)線虫、回虫目(Ascaridida)線虫、旋尾線虫目(Spirurida)線虫、鉤頭虫類、擬葉目(Pseudophyllidea)条虫、円葉目(Cyclophyllidea)条虫、有壁吸虫目(Strigeidida)吸虫、棘口吸虫目(Echinostomida)吸虫、斜睾吸虫目(Plagiorchiida)吸虫、後睾吸虫目(Opisthorchiida)吸虫、アメーバ類、ピロプラズマ目(Piroplasmida)胞子虫類、住血胞子虫目(Haemosporida)胞子虫類、真コクシジウム目(Eucoccidiorida)胞子虫類、前庭目(Vestibuliferida)繊毛虫類、トリコモナス目(Trichomonadida)鞭毛虫類、ディプロモナス目(Diplomonadida)鞭毛虫類、キネトプラスト目(Kinetoplastida)鞭毛虫類等が挙げられるが、本発明化合物は、これらのうち特にオマキザル科(Cebidae)、オナガザル科(Cercopithecidae)、ヒト科(Hominidae)、ウサギ科(Leporidae)、チンチラ科(Chinchillidae)、テンジクネズミ科(Caviidae)、キヌゲネズミ科(Cricetidae)、ネズミ科(Muridae)、リス科(Sciuridae)、ラクダ科(Camelidae)、イノシシ科(Suidae)、シカ科(Cervidae)、ウシ科(Bovidae)、ネコ科(Felidae)、イヌ科(Canidae)、イタチ科(Mustelidae)、ウマ科(Equidae)、カンガルー科(Macropodidae)等に属する哺乳類(Mammalia)の寄生虫、とりわけイノシシ科、ウシ科、ネコ科、イヌ科及びウマ科哺乳動物に寄生するエノプルス目、桿線虫目、円虫目、葉線虫目、ハリセンチュウ目、回虫目、旋尾線虫目に属する動物寄生性線虫の駆除に優れた効果を示す。
Animal pests include Ascomycota fungi, Basidiomycota fungi, Actinobacteria gram-positive fungi, Firmicutes gram-positive fungi, Tenericutes gram-positive fungi , Proteobacteria gram-negative fungi and Enoplida nematodes, Rhabditida nematodes, Strongylida nematodes, Ascaridida nematodes, rotate nematodes Spirurida nematodes, bald worms, Pseudophyllidea tapeworms, Cyclophyllidea tapeworms, Strigeidida fluke, Echinostomida flute Plachyorchiida, Opisthorchiida, Amoeba, Piroplasmida, Haemosporida, Eucoccidiorida, Eucoccidiorida , Vestibuliferida ciliates, Trichomonadidae flagellates, Diplomonadida flagellates, Kinetoplastida flagellates, etc., the compounds of the present invention are: Of these, the capuchin family (Cebidae), the rhesus family (Cercopithecidae), the human family (Hominidae), the rabbit family (Leporidae), the chinchilla family (Chinchillidae), the guinea pig family (Caviidae), the mouse family (Cricetidae), the mouse family (Cricetidae) Muridae), Squiridae, Camelidae, Wild Boar (Suidae), Deer (Cervidae), Bovine (Bovidae), Felidae, Canidae, Mustelidae , Mammaria parasites belonging to Equidae, Macropodidae, etc., especially wild boar, bovine, feline, canine and equine mammals Enopurusu eyes parasitic, show 桿線 insect eyes, circle insect eyes, the leaves wilt eyes, Hari nematode eyes, roundworm eyes, an excellent effect for combating animal parasitic nematodes belonging to the 旋尾 nematodes eyes.
また、本発明化合物は、既存の殺菌剤・殺線虫剤に対して抵抗性の発達した有害生物に対しても有効である。さらに、本発明化合物はホ乳類、魚類、甲殻類、天敵類及び有用昆虫等の非標的生物に対してはほとんど悪影響の無い極めて有用な特長を有している。
本発明化合物を使用するにあたっては、通常適当な固体担体又は液体担体と混合し、更に所望により界面活性剤、浸透剤、展着剤、増粘剤、凍結防止剤、結合剤、固結防止剤、崩壊剤、消泡剤、防腐剤および分解防止剤等を添加して、液剤(soluble concentrate)、乳剤(emulsifiable concentrate)、水和剤(wettable powder)、水溶剤(water soluble powder)、顆粒水和剤(water dispersible granule)、顆粒水溶剤(water soluble granule)、懸濁剤(suspension concentrate)、乳濁剤(concentrated emulsion)、サスポエマルジョン(suspoemulsion)、マイクロエマルジョン(microemulsion)、粉剤(dustable powder)、粒剤(granule)、錠剤(tablet)および乳化性ゲル剤(emulsifiable gel)等任意の剤型の製剤にて実用に供することができる。また、省力化および安全性向上の観点から、上記任意の剤型の製剤を、水溶性カプセルおよび水溶性フィルムの袋等の水溶性包装体に封入して供することもできる。 The compounds of the present invention are also effective against pests that have developed resistance to existing fungicides and nematicides. Furthermore, the compound of the present invention has extremely useful features that have almost no adverse effect on non-target organisms such as mammals, fish, crustaceans, natural enemies and useful insects.
When using the compound of the present invention, it is usually mixed with a suitable solid carrier or liquid carrier, and if desired, a surfactant, penetrant, spreading agent, thickener, antifreezing agent, binder, anti-caking agent. , Disintegrating agents, antifoaming agents, preservatives and anti-degradation agents, etc., and soluble concentrate, emulsion (emulsifiable concentrate), wettable powder, water-soluble powder, granular water Water dispersible granule, water soluble granule, suspension concentrate, concentrated emulsion, suspoemulsion, microemulsion, dustable powder ), Granule, tablet, and emulsifiable gel, and can be put to practical use. Further, from the viewpoint of labor saving and safety improvement, the preparations of any of the above dosage forms can be provided by being enclosed in a water-soluble package such as a water-soluble capsule and a bag of a water-soluble film.
本発明化合物を使用するにあたっては、通常適当な固体担体又は液体担体と混合し、更に所望により界面活性剤、浸透剤、展着剤、増粘剤、凍結防止剤、結合剤、固結防止剤、崩壊剤、消泡剤、防腐剤および分解防止剤等を添加して、液剤(soluble concentrate)、乳剤(emulsifiable concentrate)、水和剤(wettable powder)、水溶剤(water soluble powder)、顆粒水和剤(water dispersible granule)、顆粒水溶剤(water soluble granule)、懸濁剤(suspension concentrate)、乳濁剤(concentrated emulsion)、サスポエマルジョン(suspoemulsion)、マイクロエマルジョン(microemulsion)、粉剤(dustable powder)、粒剤(granule)、錠剤(tablet)および乳化性ゲル剤(emulsifiable gel)等任意の剤型の製剤にて実用に供することができる。また、省力化および安全性向上の観点から、上記任意の剤型の製剤を、水溶性カプセルおよび水溶性フィルムの袋等の水溶性包装体に封入して供することもできる。 The compounds of the present invention are also effective against pests that have developed resistance to existing fungicides and nematicides. Furthermore, the compound of the present invention has extremely useful features that have almost no adverse effect on non-target organisms such as mammals, fish, crustaceans, natural enemies and useful insects.
When using the compound of the present invention, it is usually mixed with a suitable solid carrier or liquid carrier, and if desired, a surfactant, penetrant, spreading agent, thickener, antifreezing agent, binder, anti-caking agent. , Disintegrating agents, antifoaming agents, preservatives and anti-degradation agents, etc., and soluble concentrate, emulsion (emulsifiable concentrate), wettable powder, water-soluble powder, granular water Water dispersible granule, water soluble granule, suspension concentrate, concentrated emulsion, suspoemulsion, microemulsion, dustable powder ), Granule, tablet, and emulsifiable gel, and can be put to practical use. Further, from the viewpoint of labor saving and safety improvement, the preparations of any of the above dosage forms can be provided by being enclosed in a water-soluble package such as a water-soluble capsule and a bag of a water-soluble film.
固体担体としては、例えば石英、方解石、海泡石、ドロマイト、チョーク、カオリナイト、パイロフィライト、セリサイト、ハロサイト、メタハロサイト、木節粘土、蛙目粘土、陶石、ジークライト、アロフェン、シラス、きら、タルク、ベントナイト、活性白土、酸性白土、軽石、アタパルジャイト、ゼオライト、珪藻土等の天然鉱物質;焼成クレー、パーライト、シラスバルーン、バーミキュライト、アタパルガスクレー、焼成珪藻土等の天然鉱物質の焼成品;炭酸マグネシウム、炭酸カルシウム、炭酸ナトリウム、炭酸水素ナトリウム、硫酸アンモニウム、硫酸ナトリウム、硫酸マグネシウム、リン酸水素二アンモニウム、リン酸二水素アンモニウム、塩化カリウム等の無機塩類;ブドウ糖、果糖、しょ糖、乳糖などの糖類;澱粉、粉末セルロース、デキストリン等の多糖類;尿素、尿素誘導体、安息香酸、安息香酸の塩等の有機物;木粉、コルク粉、トウモロコシ穂軸、クルミ殻、タバコ茎等の植物類;フライアッシュ、ホワイトカーボン(含水合成シリカ、無水合成シリカ、含水合成シリケート等)、肥料等が挙げられる。
Examples of the solid carrier include quartz, calcite, gypsum, dolomite, chalk, kaolinite, pyrophyllite, sericite, halosite, metahalosite, kibushi clay, glazed clay, porcelain stone, siegrite, and allophane. , Shirasu, Kira, Talc, Bentonite, Activated clay, Acid clay, Pumice, Attapulgite, Zeolite, Diatomaceous earth, etc .; Natural minerals such as calcined clay, perlite, shirasu balloon, vermiculite, attapulgus clay, calcined diatomaceous earth Baked products; inorganic salts such as magnesium carbonate, calcium carbonate, sodium carbonate, sodium bicarbonate, ammonium sulfate, sodium sulfate, magnesium sulfate, diammonium hydrogen phosphate, ammonium dihydrogen phosphate, potassium chloride; glucose, fructose, sucrose, Sugars such as lactose; starch, Polysaccharides such as powdered cellulose and dextrin; Organic substances such as urea, urea derivatives, benzoic acid and benzoic acid salts; Plants such as wood flour, cork flour, corn cobs, walnut shells, tobacco stems; fly ash, white carbon (Hydrous synthetic silica, anhydrous synthetic silica, hydrous synthetic silicate, etc.), fertilizers and the like.
液体担体としては、例えばキシレン、アルキル(C9またはC10等)ベンゼン、フェニルキシリルエタン、アルキル(C1またはC3等)ナフタレン等の芳香族炭化水素類;マシン油、ノルマルパラフィン、イソパラフィン、ナフテン等の脂肪族炭化水素類;ケロシン等の芳香族炭化水素と脂肪族炭化水素の混合物;エタノール、イソプロパノール、シクロヘキサノール、フェノキシエタノール、ベンジルアルコール等のアルコール;エチレングリコール、プロピレングリコール、ジエチレングリコール、ヘキシレングリコール、ポリエチレングリコール、ポリプロピレングリコール等の多価アルコール;プロピルセロソルブ、ブチルセロソルブ、フェニルセロソルブ、プロピレングリコールモノメチルエーテル、プロピレングリコールモノエチルエーテル、プロピレングリコールモノプロピルエーテル、プロピレングリコールモノブチルエーテル、プロピレングリコールモノフェニルエーテル等のエーテル;アセトフェノン、シクロヘキサノン、γ-ブチロラクトン等のケトン;脂肪酸メチルエステル、コハク酸ジアルキルエステル、グルタミン酸ジアルキルエステル、アジピン酸ジアルキルエステル、フタル酸ジアルキルエステル等のエステル;N-アルキル(C1、C8またはC12等)ピロリドン等の酸アミド;大豆油、アマニ油、ナタネ油、ヤシ油、綿実油およびヒマシ油等の油脂;ジメチルスルホキシド、水等が挙げられる。
Examples of the liquid carrier, such as xylene, alkyl (C 9 or C 10, etc.) benzene, phenylxylylethane, alkyl (C 1 or C 3, etc.) aromatic hydrocarbons such as naphthalene; machine oil, normal paraffin, isoparaffin, Aliphatic hydrocarbons such as naphthene; mixtures of aromatic and aliphatic hydrocarbons such as kerosene; alcohols such as ethanol, isopropanol, cyclohexanol, phenoxyethanol, and benzyl alcohol; ethylene glycol, propylene glycol, diethylene glycol, hexylene glycol Polyhydric alcohols such as polyethylene glycol and polypropylene glycol; propyl cellosolve, butyl cellosolve, phenyl cellosolve, propylene glycol monomethyl ether, propylene glycol Ethers such as monoethyl ether, propylene glycol monopropyl ether, propylene glycol monobutyl ether, propylene glycol monophenyl ether; ketones such as acetophenone, cyclohexanone and γ-butyrolactone; fatty acid methyl esters, succinic acid dialkyl esters, glutamic acid dialkyl esters, adipic acid Esters such as dialkyl esters and dialkyl phthalates; Acid amides such as N-alkyl (C 1 , C 8 or C 12 ) pyrrolidone; Fats and oils such as soybean oil, linseed oil, rapeseed oil, coconut oil, cottonseed oil and castor oil Dimethyl sulfoxide, water and the like.
これら固体および液体担体は、単独で用いても2種以上を併用してもよい。
界面活性剤としては、例えばポリオキシエチレンアルキルエーテル、ポリオキシエチレンアルキル(モノまたはジ)フェニルエーテル、ポリオキシエチレン(モノ、ジまたはトリ)スチリルフェニルエーテル、ポリオキシエチレンポリオキシプロピレンブロックコポリマー、ポリオキシエチレン脂肪酸(モノまたはジ)エステル、ソルビタン脂肪酸エステル、ポリオキシエチレンソルビタン脂肪酸エステル、ヒマシ油エチレンオキサイド付加物、アセチレングリコール、アセチレンアルコール、アセチレングリコールのエチレンオキサイド付加物、アセチレンアルコールのエチレンオキサイド付加物、アルキルグリコシド等のノニオン性界面活性剤;アルキル硫酸エステル塩、アルキルベンゼンスルホン酸塩、リグニンスルホン酸塩、アルキルスルホコハク酸塩、ナフタレンスルホン酸塩、アルキルナフタレンスルホン酸塩、ナフタレンスルホン酸のホルマリン縮合物の塩、アルキルナフタレンスルホン酸のホルマリン縮合物の塩、ポリオキシエチレンアルキルエーテル硫酸または燐酸エステル塩、ポリオキシエチレン(モノまたはジ)アルキルフェニルエーテル硫酸または燐酸エステル塩、ポリオキシエチレン(モノ、ジまたはトリ)スチリルフェニルエーテル硫酸または燐酸エステル塩、ポリカルボン酸塩(例えば、ポリアクリル酸塩、ポリマレイン酸塩、マレイン酸とオレフィンとの共重合物等)、ポリスチレンスルホン酸塩等のアニオン性界面活性剤;アルキルアミン塩、アルキル4級アンモニウム塩等のカチオン性界面活性剤;アミノ酸型、ベタイン型等の両性界面活性剤;シリコーン系界面活性剤、フッ素系界面活性剤等が挙げられる。
これら界面活性剤の含有量は、特に限定されるものではないが、本発明の製剤100重量部に対し、通常0.05~20重量部の範囲が望ましく、0.1~20重量部がより望ましい。また、これら界面活性剤は、単独で用いても2種以上を併用してもよい。 These solid and liquid carriers may be used alone or in combination of two or more.
Examples of the surfactant include polyoxyethylene alkyl ether, polyoxyethylene alkyl (mono or di) phenyl ether, polyoxyethylene (mono, di or tri) styryl phenyl ether, polyoxyethylene polyoxypropylene block copolymer, polyoxyethylene Ethylene fatty acid (mono or di) ester, sorbitan fatty acid ester, polyoxyethylene sorbitan fatty acid ester, castor oil ethylene oxide adduct, acetylene glycol, acetylene alcohol, ethylene oxide adduct of acetylene glycol, ethylene oxide adduct of acetylene alcohol, alkyl Nonionic surfactants such as glycosides; alkyl sulfate salts, alkylbenzene sulfonates, lignin sulfonates, alkyls Sulfosuccinate, naphthalene sulfonate, alkyl naphthalene sulfonate, salt of formalin condensate of naphthalene sulfonic acid, salt of formalin condensate of alkyl naphthalene sulfonic acid, polyoxyethylene alkyl ether sulfate or phosphate ester salt, polyoxyethylene (Mono or di) alkyl phenyl ether sulfate or phosphate ester salt, polyoxyethylene (mono, di or tri) styryl phenyl ether sulfate or phosphate ester salt, polycarboxylate (eg, polyacrylate, polymaleate, malee) Copolymer of acid and olefin), anionic surfactant such as polystyrene sulfonate; cationic surfactant such as alkylamine salt and alkyl quaternary ammonium salt; amphoteric surfactant such as amino acid type and betaine type ; Silicone surfactants, fluorine-based surfactants, and the like.
The content of these surfactants is not particularly limited, but is usually preferably in the range of 0.05 to 20 parts by weight, more preferably 0.1 to 20 parts by weight with respect to 100 parts by weight of the preparation of the present invention. desirable. These surfactants may be used alone or in combination of two or more.
界面活性剤としては、例えばポリオキシエチレンアルキルエーテル、ポリオキシエチレンアルキル(モノまたはジ)フェニルエーテル、ポリオキシエチレン(モノ、ジまたはトリ)スチリルフェニルエーテル、ポリオキシエチレンポリオキシプロピレンブロックコポリマー、ポリオキシエチレン脂肪酸(モノまたはジ)エステル、ソルビタン脂肪酸エステル、ポリオキシエチレンソルビタン脂肪酸エステル、ヒマシ油エチレンオキサイド付加物、アセチレングリコール、アセチレンアルコール、アセチレングリコールのエチレンオキサイド付加物、アセチレンアルコールのエチレンオキサイド付加物、アルキルグリコシド等のノニオン性界面活性剤;アルキル硫酸エステル塩、アルキルベンゼンスルホン酸塩、リグニンスルホン酸塩、アルキルスルホコハク酸塩、ナフタレンスルホン酸塩、アルキルナフタレンスルホン酸塩、ナフタレンスルホン酸のホルマリン縮合物の塩、アルキルナフタレンスルホン酸のホルマリン縮合物の塩、ポリオキシエチレンアルキルエーテル硫酸または燐酸エステル塩、ポリオキシエチレン(モノまたはジ)アルキルフェニルエーテル硫酸または燐酸エステル塩、ポリオキシエチレン(モノ、ジまたはトリ)スチリルフェニルエーテル硫酸または燐酸エステル塩、ポリカルボン酸塩(例えば、ポリアクリル酸塩、ポリマレイン酸塩、マレイン酸とオレフィンとの共重合物等)、ポリスチレンスルホン酸塩等のアニオン性界面活性剤;アルキルアミン塩、アルキル4級アンモニウム塩等のカチオン性界面活性剤;アミノ酸型、ベタイン型等の両性界面活性剤;シリコーン系界面活性剤、フッ素系界面活性剤等が挙げられる。
これら界面活性剤の含有量は、特に限定されるものではないが、本発明の製剤100重量部に対し、通常0.05~20重量部の範囲が望ましく、0.1~20重量部がより望ましい。また、これら界面活性剤は、単独で用いても2種以上を併用してもよい。 These solid and liquid carriers may be used alone or in combination of two or more.
Examples of the surfactant include polyoxyethylene alkyl ether, polyoxyethylene alkyl (mono or di) phenyl ether, polyoxyethylene (mono, di or tri) styryl phenyl ether, polyoxyethylene polyoxypropylene block copolymer, polyoxyethylene Ethylene fatty acid (mono or di) ester, sorbitan fatty acid ester, polyoxyethylene sorbitan fatty acid ester, castor oil ethylene oxide adduct, acetylene glycol, acetylene alcohol, ethylene oxide adduct of acetylene glycol, ethylene oxide adduct of acetylene alcohol, alkyl Nonionic surfactants such as glycosides; alkyl sulfate salts, alkylbenzene sulfonates, lignin sulfonates, alkyls Sulfosuccinate, naphthalene sulfonate, alkyl naphthalene sulfonate, salt of formalin condensate of naphthalene sulfonic acid, salt of formalin condensate of alkyl naphthalene sulfonic acid, polyoxyethylene alkyl ether sulfate or phosphate ester salt, polyoxyethylene (Mono or di) alkyl phenyl ether sulfate or phosphate ester salt, polyoxyethylene (mono, di or tri) styryl phenyl ether sulfate or phosphate ester salt, polycarboxylate (eg, polyacrylate, polymaleate, malee) Copolymer of acid and olefin), anionic surfactant such as polystyrene sulfonate; cationic surfactant such as alkylamine salt and alkyl quaternary ammonium salt; amphoteric surfactant such as amino acid type and betaine type ; Silicone surfactants, fluorine-based surfactants, and the like.
The content of these surfactants is not particularly limited, but is usually preferably in the range of 0.05 to 20 parts by weight, more preferably 0.1 to 20 parts by weight with respect to 100 parts by weight of the preparation of the present invention. desirable. These surfactants may be used alone or in combination of two or more.
本発明化合物の施用薬量は適用場面、施用時期、施用方法、栽培作物等により差異は有るが、通常は、有効成分量としてヘクタール(ha)当たり0.005~50kgが好ましく、0.01~5kgがより好ましい。
The application amount of the compound of the present invention varies depending on the application scene, application time, application method, cultivated crop, etc., but the amount of active ingredient is usually preferably 0.005 to 50 kg per hectare (ha), 0.01 to 5 kg is more preferable.
一方、家畜・家禽及び愛玩動物としての哺乳動物及び鳥類の寄生虫の防除に本発明化合物を使用するにあたっては、有効量の本発明化合物を製剤用添加物とともに経口投与又は非経口投与することができる。ここで、非経口投与の具体例としては、注射(筋肉内、皮下、静脈内、腹腔内)、経皮投与(浸漬、スプレー、入浴、洗浄、滴下(pouring-on)、スポッティング(spotting-on)、ダスティング(dusting))、又は経鼻投与などが挙げられる。
On the other hand, when the compound of the present invention is used for controlling parasites of mammals and birds as domestic animals, poultry and pets, an effective amount of the compound of the present invention may be administered orally or parenterally with a pharmaceutical additive. it can. Here, specific examples of parenteral administration include injection (intramuscular, subcutaneous, intravenous, intraperitoneal), transdermal administration (immersion, spray, bathing, washing, pouring-on, spotting-on ), Dusting), or nasal administration.
本発明化合物はまた、細片、プレート、バンド、カラー、イヤー・マーク(ear mark)、リム(limb)・バンド、標識装置などを用いた成形製品により投与することができる。投与にあたっては本発明化合物を投与経路に適した任意の剤型とすることができる。
調製される任意の剤型としては、粉剤、粒剤、水和剤、ペレット、錠剤、大丸薬、カプセル剤、活性化合物を含む成形製品などの固体調製物;注射用液剤、経口用液剤、皮膚上または体腔中に用いる液剤;滴下(Pour-on)剤、点下(Spot-on)剤、フロアブル剤、乳剤などの溶液調製物;軟膏剤、ゲルなどの半固体調製物などが挙げられる。 The compounds of the present invention can also be administered by molded products using strips, plates, bands, collars, ear marks, limb bands, labeling devices and the like. In administration, the compound of the present invention can be in any dosage form suitable for the administration route.
Arbitrary forms to be prepared include solid preparations such as powders, granules, wettable powders, pellets, tablets, large pills, capsules, molded products containing active compounds; injection solutions, oral solutions, skin Liquid preparations used above or in body cavities; solution preparations such as pour-on, spot-on, flowable, and emulsion; semi-solid preparations such as ointments and gels.
調製される任意の剤型としては、粉剤、粒剤、水和剤、ペレット、錠剤、大丸薬、カプセル剤、活性化合物を含む成形製品などの固体調製物;注射用液剤、経口用液剤、皮膚上または体腔中に用いる液剤;滴下(Pour-on)剤、点下(Spot-on)剤、フロアブル剤、乳剤などの溶液調製物;軟膏剤、ゲルなどの半固体調製物などが挙げられる。 The compounds of the present invention can also be administered by molded products using strips, plates, bands, collars, ear marks, limb bands, labeling devices and the like. In administration, the compound of the present invention can be in any dosage form suitable for the administration route.
Arbitrary forms to be prepared include solid preparations such as powders, granules, wettable powders, pellets, tablets, large pills, capsules, molded products containing active compounds; injection solutions, oral solutions, skin Liquid preparations used above or in body cavities; solution preparations such as pour-on, spot-on, flowable, and emulsion; semi-solid preparations such as ointments and gels.
固体調製物は、主に経口投与あるいは水などで希釈して経皮投与にあるいは環境処理にて用いることができる。固体調製物は、活性化合物を必要ならば補助剤を加えて適当な賦形剤と共に混合し、そして所望の形状に変えることにより調製できる。
賦形剤としては、例えば炭酸塩、炭酸水素塩、リン酸塩、酸化アルミニウム、シリカ、粘土などの無機物質、糖、セルロース、粉砕された穀物、澱粉などの有機物質が挙げられる。 The solid preparation can be used mainly for oral administration or diluted with water for transdermal administration or environmental treatment. Solid preparations can be prepared by adding the active compound, if necessary, with adjuncts, mixing with appropriate excipients, and converting to the desired shape.
Examples of the excipient include inorganic substances such as carbonate, hydrogen carbonate, phosphate, aluminum oxide, silica, and clay, and organic substances such as sugar, cellulose, pulverized grains, and starch.
賦形剤としては、例えば炭酸塩、炭酸水素塩、リン酸塩、酸化アルミニウム、シリカ、粘土などの無機物質、糖、セルロース、粉砕された穀物、澱粉などの有機物質が挙げられる。 The solid preparation can be used mainly for oral administration or diluted with water for transdermal administration or environmental treatment. Solid preparations can be prepared by adding the active compound, if necessary, with adjuncts, mixing with appropriate excipients, and converting to the desired shape.
Examples of the excipient include inorganic substances such as carbonate, hydrogen carbonate, phosphate, aluminum oxide, silica, and clay, and organic substances such as sugar, cellulose, pulverized grains, and starch.
注射用液剤は、静脈内、筋肉内および皮下に投与できる、注射用液剤は、活性化合物を適当な溶媒に溶解させ、そして必要ならば可溶化剤、酸、塩基、緩衝用塩、酸化防止剤、保護剤などの添加剤を加えることにより調製できる。
Injection solutions can be administered intravenously, intramuscularly and subcutaneously. Injection solutions dissolve the active compound in a suitable solvent and, if necessary, solubilizers, acids, bases, buffer salts, antioxidants. It can be prepared by adding additives such as a protective agent.
溶媒としては、水、エタノール、ブタノール、ベンジルアルコール、グリセリン、プロピレングリコール、ポリエチレングリコール、N-メチルピロリドン並びにこれらの混合物、生理学的に許容しうる植物油、注射に適する合成油などが挙げられる。
可溶化剤としては、ポリビニルピロリドン、ポリオキシエチル化されたヒマシ油、ポリオキシエチル化されたソルビタンエステルなどが挙げられる。
保護剤としては、ベンジルアルコール、トリクロロブタノール、p-ヒドロキシ安息香酸エステル、n-ブタノールなどが挙げられる。 Solvents include water, ethanol, butanol, benzyl alcohol, glycerin, propylene glycol, polyethylene glycol, N-methylpyrrolidone and mixtures thereof, physiologically acceptable vegetable oils, synthetic oils suitable for injection, and the like.
Examples of the solubilizer include polyvinylpyrrolidone, polyoxyethylated castor oil, polyoxyethylated sorbitan ester and the like.
Examples of the protective agent include benzyl alcohol, trichlorobutanol, p-hydroxybenzoic acid ester, n-butanol and the like.
可溶化剤としては、ポリビニルピロリドン、ポリオキシエチル化されたヒマシ油、ポリオキシエチル化されたソルビタンエステルなどが挙げられる。
保護剤としては、ベンジルアルコール、トリクロロブタノール、p-ヒドロキシ安息香酸エステル、n-ブタノールなどが挙げられる。 Solvents include water, ethanol, butanol, benzyl alcohol, glycerin, propylene glycol, polyethylene glycol, N-methylpyrrolidone and mixtures thereof, physiologically acceptable vegetable oils, synthetic oils suitable for injection, and the like.
Examples of the solubilizer include polyvinylpyrrolidone, polyoxyethylated castor oil, polyoxyethylated sorbitan ester and the like.
Examples of the protective agent include benzyl alcohol, trichlorobutanol, p-hydroxybenzoic acid ester, n-butanol and the like.
経口液剤は直接または希釈して投与することができる。また、注射用液剤と同様に調製することができる。
フロアブル剤、乳剤などは直接または希釈して経皮的に、または環境処理にて投与できる。
皮膚上で用いる液剤は、滴下し、広げ、すり込み、噴霧し、散布するか、または浸漬(浸漬、入浴または洗浄)により塗布することにより投与できる。これらの液剤は注射用液剤と同様に調製できる。 Oral solutions can be administered directly or diluted. Moreover, it can prepare similarly to the liquid for injection.
Flowables, emulsions and the like can be administered directly or diluted transdermally or by environmental treatment.
Solutions for use on the skin can be administered by dripping, spreading, rubbing, spraying, spraying or applying by dipping (dipping, bathing or washing). These solutions can be prepared in the same manner as injection solutions.
フロアブル剤、乳剤などは直接または希釈して経皮的に、または環境処理にて投与できる。
皮膚上で用いる液剤は、滴下し、広げ、すり込み、噴霧し、散布するか、または浸漬(浸漬、入浴または洗浄)により塗布することにより投与できる。これらの液剤は注射用液剤と同様に調製できる。 Oral solutions can be administered directly or diluted. Moreover, it can prepare similarly to the liquid for injection.
Flowables, emulsions and the like can be administered directly or diluted transdermally or by environmental treatment.
Solutions for use on the skin can be administered by dripping, spreading, rubbing, spraying, spraying or applying by dipping (dipping, bathing or washing). These solutions can be prepared in the same manner as injection solutions.
滴下(Pour-on)剤および点下(Spot-on)剤は皮膚の限定された場所に滴下するか、または噴霧し、これにより活性化合物を皮膚に浸漬させそして全身的に作用させることができる。滴下剤および点下剤は、有効成分を適当な皮膚適合性溶媒または溶媒混合物に溶解するか、懸濁させるかまたは乳化することにより調製できる。必要ならば、界面活性剤、着色剤、吸収促進物質、酸化防止剤、光安定剤、接着剤などの補助剤を加えてもよい。
溶媒としては、水、アルカノール、グリコール、ポリエチレングリコール、ポリプロピレングリコール、グリセリン、ベンジルアルコール、フェニルエタノール、フェノキシエタノール、酢酸エチル、酢酸ブチル、安息香酸ベンジル、ジプロピレングリコールモノメチルエーテル、ジエチレングリコールモノブチルエーテル、アセトン、メチルエチルケトン、芳香族および/または脂肪族炭化水素、植物または合成油、DMF、流動パラフィン、軽質流動パラフィン、シリコーン、ジメチルアセトアミド、N-メチルピロリドンまたは2,2-ジメチル-4-オキシ-メチレン-1,3-ジオキソラン等が挙げられる。 Pour-on and spot-on agents can be dripped or sprayed onto a limited area of the skin, so that the active compound can be immersed in the skin and act systemically . Drops and drop preparations can be prepared by dissolving, suspending or emulsifying the active ingredient in suitable skin-compatible solvents or solvent mixtures. If necessary, auxiliary agents such as surfactants, colorants, absorption promoting substances, antioxidants, light stabilizers, and adhesives may be added.
Solvents include water, alkanol, glycol, polyethylene glycol, polypropylene glycol, glycerin, benzyl alcohol, phenylethanol, phenoxyethanol, ethyl acetate, butyl acetate, benzyl benzoate, dipropylene glycol monomethyl ether, diethylene glycol monobutyl ether, acetone, methyl ethyl ketone, Aromatic and / or aliphatic hydrocarbons, vegetable or synthetic oils, DMF, liquid paraffin, light liquid paraffin, silicone, dimethylacetamide, N-methylpyrrolidone or 2,2-dimethyl-4-oxy-methylene-1,3- And dioxolane.
溶媒としては、水、アルカノール、グリコール、ポリエチレングリコール、ポリプロピレングリコール、グリセリン、ベンジルアルコール、フェニルエタノール、フェノキシエタノール、酢酸エチル、酢酸ブチル、安息香酸ベンジル、ジプロピレングリコールモノメチルエーテル、ジエチレングリコールモノブチルエーテル、アセトン、メチルエチルケトン、芳香族および/または脂肪族炭化水素、植物または合成油、DMF、流動パラフィン、軽質流動パラフィン、シリコーン、ジメチルアセトアミド、N-メチルピロリドンまたは2,2-ジメチル-4-オキシ-メチレン-1,3-ジオキソラン等が挙げられる。 Pour-on and spot-on agents can be dripped or sprayed onto a limited area of the skin, so that the active compound can be immersed in the skin and act systemically . Drops and drop preparations can be prepared by dissolving, suspending or emulsifying the active ingredient in suitable skin-compatible solvents or solvent mixtures. If necessary, auxiliary agents such as surfactants, colorants, absorption promoting substances, antioxidants, light stabilizers, and adhesives may be added.
Solvents include water, alkanol, glycol, polyethylene glycol, polypropylene glycol, glycerin, benzyl alcohol, phenylethanol, phenoxyethanol, ethyl acetate, butyl acetate, benzyl benzoate, dipropylene glycol monomethyl ether, diethylene glycol monobutyl ether, acetone, methyl ethyl ketone, Aromatic and / or aliphatic hydrocarbons, vegetable or synthetic oils, DMF, liquid paraffin, light liquid paraffin, silicone, dimethylacetamide, N-methylpyrrolidone or 2,2-dimethyl-4-oxy-methylene-1,3- And dioxolane.
吸収促進物質としては、DMSO、ミリスチン酸イソプロピル、ペラルゴン酸ジプロピレングリコール、シリコーン油、脂肪族エステル、トリグリセリド、脂肪アルコール等が挙げられる。
酸化防止剤としては、亜硫酸塩、メタ重亜硫酸塩、アスコルビン酸、ブチルヒドロキシトルエン、ブチルヒドロキシアニソール、トコフェロール等が挙げられる。
乳剤は、経口投与、経皮投与または注射として投与できる。乳剤は、有効成分を疎水性相または親水性相に溶解させ、このものを適当な乳化剤により、必要ならばさらに着色剤、吸収促進物質、保護剤、酸化防止剤、遮光剤、増粘物質などの補助剤と共に他の相の溶媒と均質化することにより調製できる。 Examples of the absorption promoting substance include DMSO, isopropyl myristate, dipropylene glycol pelargonate, silicone oil, aliphatic ester, triglyceride, fatty alcohol and the like.
Examples of the antioxidant include sulfite, metabisulfite, ascorbic acid, butylhydroxytoluene, butylhydroxyanisole, tocopherol and the like.
The emulsion can be administered orally, transdermally or as an injection. In the emulsion, the active ingredient is dissolved in a hydrophobic phase or a hydrophilic phase, and this is further added with a suitable emulsifier, if necessary, a colorant, an absorption promoting substance, a protective agent, an antioxidant, a light-shielding agent, a thickening substance, etc. Can be prepared by homogenizing with other phase solvents together with other auxiliary agents.
酸化防止剤としては、亜硫酸塩、メタ重亜硫酸塩、アスコルビン酸、ブチルヒドロキシトルエン、ブチルヒドロキシアニソール、トコフェロール等が挙げられる。
乳剤は、経口投与、経皮投与または注射として投与できる。乳剤は、有効成分を疎水性相または親水性相に溶解させ、このものを適当な乳化剤により、必要ならばさらに着色剤、吸収促進物質、保護剤、酸化防止剤、遮光剤、増粘物質などの補助剤と共に他の相の溶媒と均質化することにより調製できる。 Examples of the absorption promoting substance include DMSO, isopropyl myristate, dipropylene glycol pelargonate, silicone oil, aliphatic ester, triglyceride, fatty alcohol and the like.
Examples of the antioxidant include sulfite, metabisulfite, ascorbic acid, butylhydroxytoluene, butylhydroxyanisole, tocopherol and the like.
The emulsion can be administered orally, transdermally or as an injection. In the emulsion, the active ingredient is dissolved in a hydrophobic phase or a hydrophilic phase, and this is further added with a suitable emulsifier, if necessary, a colorant, an absorption promoting substance, a protective agent, an antioxidant, a light-shielding agent, a thickening substance, etc. Can be prepared by homogenizing with other phase solvents together with other auxiliary agents.
疎水性相(油)としては、パラフィン油、シリコーン油、ゴマ油、アーモンド油、ヒマシ油、合成トリグリセリド、ステアリン酸エチル、アジピン酸ジーn-ブチリル、ラウリル酸ヘキシル、ペラルゴン酸ジプロピレングリコール、分枝鎖状の短鎖長脂肪酸と鎖長C16~C18の飽和脂肪酸とのエステル、ミリスチン酸イソプロピル、パルミチン酸イソプロピル、鎖長C12~C18の飽和脂肪アルコールのカプリル/カプリン酸エステル、ステアリン酸イソプロピル、オレイン酸オレイル、オレイン酸デシル、オレイン酸エチル、乳酸エチル、ワックス状脂肪酸エステル、フタル酸ジブチル、アジピン酸ジイソプロピル、イソトリデシルアルコール、2-オクチルドデカノール、セチルステアリルアルコール、オレイルアルコール等が挙げられる。
As hydrophobic phase (oil), paraffin oil, silicone oil, sesame oil, almond oil, castor oil, synthetic triglyceride, ethyl stearate, di-n-butylyl adipate, hexyl laurate, dipropylene glycol pelargonate, branched chain -Like short chain fatty acid and a saturated fatty acid having a chain length of C 16 to C 18 , isopropyl myristate, isopropyl palmitate, caprylic / capric acid ester of a saturated fatty alcohol having a chain length of C 12 to C 18 , isopropyl stearate Oleyl oleate, decyl oleate, ethyl oleate, ethyl lactate, waxy fatty acid ester, dibutyl phthalate, diisopropyl adipate, isotridecyl alcohol, 2-octyldodecanol, cetyl stearyl alcohol, oleyl alcohol, etc. And the like.
親水性相としては、水、プロピレングリコール、グリセリン、ソルビトール等が挙げられる。
乳化剤としては、ポリオキシエチル化されたヒマシ油、ポリオキシエチル化されたモノオレフィン酸ソルビタン、モノステアリン酸ソルビタン、モノステアリン酸グリセリン、ステアリン酸ポリオキシエチル、アルキルフェノールポリグリコールエーテルなどの非イオン性界面活性剤;N-ラウリル-β-イミノジプロピオン酸二ナトリウム、レシチンなどの両性界面活性剤;ラウリル硫酸ナトリウム、脂肪アルコール硫酸エーテル、モノ/ジアルキルポリグリコールオルトリン酸エステルのモノエタノールアミン塩などの陰イオン性界面活性剤;塩化セチルトリメチルアンモニウムなどの陽イオン性界面活性剤などが挙げられる。 Examples of the hydrophilic phase include water, propylene glycol, glycerin, sorbitol and the like.
Emulsifiers include nonionic interfaces such as polyoxyethylated castor oil, polyoxyethylated sorbitan monoolefin acid, sorbitan monostearate, glyceryl monostearate, polyoxyethyl stearate, alkylphenol polyglycol ether, etc. Activators; amphoteric surfactants such as disodium N-lauryl-β-iminodipropionate and lecithin; anions such as sodium lauryl sulfate, fatty alcohol sulfate ether, monoethanolamine salt of mono / dialkyl polyglycol orthophosphate Surfactants; cationic surfactants such as cetyltrimethylammonium chloride.
乳化剤としては、ポリオキシエチル化されたヒマシ油、ポリオキシエチル化されたモノオレフィン酸ソルビタン、モノステアリン酸ソルビタン、モノステアリン酸グリセリン、ステアリン酸ポリオキシエチル、アルキルフェノールポリグリコールエーテルなどの非イオン性界面活性剤;N-ラウリル-β-イミノジプロピオン酸二ナトリウム、レシチンなどの両性界面活性剤;ラウリル硫酸ナトリウム、脂肪アルコール硫酸エーテル、モノ/ジアルキルポリグリコールオルトリン酸エステルのモノエタノールアミン塩などの陰イオン性界面活性剤;塩化セチルトリメチルアンモニウムなどの陽イオン性界面活性剤などが挙げられる。 Examples of the hydrophilic phase include water, propylene glycol, glycerin, sorbitol and the like.
Emulsifiers include nonionic interfaces such as polyoxyethylated castor oil, polyoxyethylated sorbitan monoolefin acid, sorbitan monostearate, glyceryl monostearate, polyoxyethyl stearate, alkylphenol polyglycol ether, etc. Activators; amphoteric surfactants such as disodium N-lauryl-β-iminodipropionate and lecithin; anions such as sodium lauryl sulfate, fatty alcohol sulfate ether, monoethanolamine salt of mono / dialkyl polyglycol orthophosphate Surfactants; cationic surfactants such as cetyltrimethylammonium chloride.
他の補助剤としては、カルボキシメチルセルロース、メチルセルロース、ポリアクリレート、アルギネート、ゼラチン、アラビアゴム、ポリビニルピロリドン、ポリビニルアルコール、メチルビニルエーテル、無水マレイン酸の共重合体、ポリエチレングリコール、ワックス、コロイド状シリカ等が挙げられる。
半固体調製物は皮膚上に塗布するか、もしくは広げるか、または体腔中に導入することにより投与できる。ゲルは注射用液剤について上記したように調製した溶液に、軟膏状の粘稠性を有する透明な物質を生じさせるに十分なシックナーを加えることにより調製できる。 Other adjuvants include carboxymethyl cellulose, methyl cellulose, polyacrylate, alginate, gelatin, gum arabic, polyvinyl pyrrolidone, polyvinyl alcohol, methyl vinyl ether, maleic anhydride copolymer, polyethylene glycol, wax, colloidal silica, etc. It is done.
Semi-solid preparations can be administered by application on the skin or spreading or introduction into body cavities. Gels can be prepared by adding sufficient thickener to a solution prepared as described above for an injectable solution to produce a clear material having an ointment-like consistency.
半固体調製物は皮膚上に塗布するか、もしくは広げるか、または体腔中に導入することにより投与できる。ゲルは注射用液剤について上記したように調製した溶液に、軟膏状の粘稠性を有する透明な物質を生じさせるに十分なシックナーを加えることにより調製できる。 Other adjuvants include carboxymethyl cellulose, methyl cellulose, polyacrylate, alginate, gelatin, gum arabic, polyvinyl pyrrolidone, polyvinyl alcohol, methyl vinyl ether, maleic anhydride copolymer, polyethylene glycol, wax, colloidal silica, etc. It is done.
Semi-solid preparations can be administered by application on the skin or spreading or introduction into body cavities. Gels can be prepared by adding sufficient thickener to a solution prepared as described above for an injectable solution to produce a clear material having an ointment-like consistency.
以下に本発明化合物を用いる場合の製剤の配合例を示す。但し本発明の配合例は、これらのみに限定されるものではない。なお、以下の配合例において「部」は重量部を意味する。
〔水和剤〕
本発明化合物 0.1~80部
固体担体 5~98.9部
界面活性剤 1~10部
その他 0~5部
その他として、例えば固結防止剤、分解防止剤等が挙げられる。
〔乳 剤〕
本発明化合物 0.1~30部
有機溶剤 45~95部
界面活性剤 4.9~30部
水 0~50部
その他 0~10部
その他として、例えば展着剤、分解防止剤等が挙げられる。 The formulation example of the case where this invention compound is used is shown below. However, the formulation examples of the present invention are not limited to these. In the following formulation examples, “parts” means parts by weight.
[Wettable powder]
Compound of the present invention 0.1 to 80 parts Solid carrier 5 to 98.9 parts Surfactant 1 to 10 parts Others 0 to 5 parts Others include, for example, anti-caking agent, decomposition inhibitor and the like.
[Milk]
Compounds of the present invention 0.1 to 30 parts Organic solvent 45 to 95 parts Surfactant 4.9 to 30 parts Water 0 to 50 parts Others 0 to 10 parts Others include, for example, spreading agents and decomposition inhibitors.
〔水和剤〕
本発明化合物 0.1~80部
固体担体 5~98.9部
界面活性剤 1~10部
その他 0~5部
その他として、例えば固結防止剤、分解防止剤等が挙げられる。
〔乳 剤〕
本発明化合物 0.1~30部
有機溶剤 45~95部
界面活性剤 4.9~30部
水 0~50部
その他 0~10部
その他として、例えば展着剤、分解防止剤等が挙げられる。 The formulation example of the case where this invention compound is used is shown below. However, the formulation examples of the present invention are not limited to these. In the following formulation examples, “parts” means parts by weight.
[Wettable powder]
Compound of the present invention 0.1 to 80 parts Solid carrier 5 to 98.9 parts Surfactant 1 to 10 parts Others 0 to 5 parts Others include, for example, anti-caking agent, decomposition inhibitor and the like.
[Milk]
Compounds of the present invention 0.1 to 30 parts Organic solvent 45 to 95 parts Surfactant 4.9 to 30 parts Water 0 to 50 parts Others 0 to 10 parts Others include, for example, spreading agents and decomposition inhibitors.
〔懸濁剤〕
本発明化合物 0.1~70部
液体担体 15~98.89部
界面活性剤 1~12部
その他 0.01~30部
その他として、例えば凍結防止剤、増粘剤等が挙げられる。 [Suspension]
Compound of the present invention 0.1 to 70 parts Liquid carrier 15 to 98.89 parts Surfactant 1 to 12 parts Others 0.01 to 30 parts Others include, for example, antifreezing agents and thickeners.
本発明化合物 0.1~70部
液体担体 15~98.89部
界面活性剤 1~12部
その他 0.01~30部
その他として、例えば凍結防止剤、増粘剤等が挙げられる。 [Suspension]
Compound of the present invention 0.1 to 70 parts Liquid carrier 15 to 98.89 parts Surfactant 1 to 12 parts Others 0.01 to 30 parts Others include, for example, antifreezing agents and thickeners.
〔顆粒水和剤〕
本発明化合物 0.1~90部
固体担体 0~98.9部
界面活性剤 1~20部
その他 0~10部
その他として、例えば結合剤、分解防止剤等が挙げられる。
〔液 剤〕
本発明化合物 0.01~70部
液体担体 20~99.99部
その他 0~10部
その他として、例えば凍結防止剤、展着剤等が挙げられる。 [Granule wettable powder]
Compound of the present invention 0.1 to 90 parts Solid carrier 0 to 98.9 parts Surfactant 1 to 20 parts Others 0 to 10 parts Others include, for example, binders, decomposition inhibitors and the like.
(Liquid)
Compound of the present invention 0.01 to 70 parts Liquid carrier 20 to 99.99 parts Others 0 to 10 parts Others include, for example, antifreezing agents and spreading agents.
本発明化合物 0.1~90部
固体担体 0~98.9部
界面活性剤 1~20部
その他 0~10部
その他として、例えば結合剤、分解防止剤等が挙げられる。
〔液 剤〕
本発明化合物 0.01~70部
液体担体 20~99.99部
その他 0~10部
その他として、例えば凍結防止剤、展着剤等が挙げられる。 [Granule wettable powder]
Compound of the present invention 0.1 to 90 parts Solid carrier 0 to 98.9 parts Surfactant 1 to 20 parts Others 0 to 10 parts Others include, for example, binders, decomposition inhibitors and the like.
(Liquid)
Compound of the present invention 0.01 to 70 parts Liquid carrier 20 to 99.99 parts Others 0 to 10 parts Others include, for example, antifreezing agents and spreading agents.
〔粒 剤〕
本発明化合物 0.01~80部
固体担体 10~99.99部
その他 0~10部
その他として、例えば結合剤、分解防止剤等が挙げられる。
〔粉 剤〕
本発明化合物 0.01~30部
固体担体 65~99.99部
その他 0~5部
その他として、例えばドリフト防止剤、分解防止剤等が挙げられる。 [Granule]
Compound of the present invention 0.01 to 80 parts Solid carrier 10 to 99.99 parts Others 0 to 10 parts Others include, for example, binders, decomposition inhibitors and the like.
[Powder]
Compound of the present invention 0.01 to 30 parts Solid support 65 to 99.99 parts Others 0 to 5 parts Others include, for example, drift inhibitors and decomposition inhibitors.
本発明化合物 0.01~80部
固体担体 10~99.99部
その他 0~10部
その他として、例えば結合剤、分解防止剤等が挙げられる。
〔粉 剤〕
本発明化合物 0.01~30部
固体担体 65~99.99部
その他 0~5部
その他として、例えばドリフト防止剤、分解防止剤等が挙げられる。 [Granule]
Compound of the present invention 0.01 to 80 parts Solid carrier 10 to 99.99 parts Others 0 to 10 parts Others include, for example, binders, decomposition inhibitors and the like.
[Powder]
Compound of the present invention 0.01 to 30 parts Solid support 65 to 99.99 parts Others 0 to 5 parts Others include, for example, drift inhibitors and decomposition inhibitors.
次に、本発明化合物を有効成分とする製剤例をより具体的に示すが、本発明はこれらに限定されるものではない。
尚、以下の配合例において、「部」は重量部を意味する。 Next, although the formulation example which uses this invention compound as an active ingredient is shown more concretely, this invention is not limited to these.
In the following formulation examples, “parts” means parts by weight.
尚、以下の配合例において、「部」は重量部を意味する。 Next, although the formulation example which uses this invention compound as an active ingredient is shown more concretely, this invention is not limited to these.
In the following formulation examples, “parts” means parts by weight.
〔配合例1〕水和剤
本発明化合物No.1-019 20部
パイロフィライト 74部
ソルポール5039 4部
(商品名、非イオン性界面活性剤とアニオン性界面活性剤との混合物:東邦化学工業社製)
カープレックス#80D 2部
(商品名、合成含水珪酸:塩野義製薬社製)
以上を均一に混合粉砕して水和剤とする。 [Formulation Example 1] wettable powder Compound No. 1-019 of the present invention 20 parts Pyrophyllite 74 parts Solpol 5039 4 parts (trade name, mixture of nonionic surfactant and anionic surfactant: Toho Chemical Industry (Made by company)
Carplex # 80D 2 parts (trade name, synthetic hydrous silicic acid: manufactured by Shionogi & Co.)
The above is uniformly mixed and ground to obtain a wettable powder.
本発明化合物No.1-019 20部
パイロフィライト 74部
ソルポール5039 4部
(商品名、非イオン性界面活性剤とアニオン性界面活性剤との混合物:東邦化学工業社製)
カープレックス#80D 2部
(商品名、合成含水珪酸:塩野義製薬社製)
以上を均一に混合粉砕して水和剤とする。 [Formulation Example 1] wettable powder Compound No. 1-019 of the present invention 20 parts Pyrophyllite 74 parts Solpol 5039 4 parts (trade name, mixture of nonionic surfactant and anionic surfactant: Toho Chemical Industry (Made by company)
Carplex # 80D 2 parts (trade name, synthetic hydrous silicic acid: manufactured by Shionogi & Co.)
The above is uniformly mixed and ground to obtain a wettable powder.
〔配合例2〕乳 剤
本発明化合物No.3-012 5部
キシレン 75部
N-メチルピロリドン 15部
ソルポール2680 5部
(商品名、非イオン性界面活性剤とアニオン性界面活性剤との混合物:東邦化学工業社製)
以上を均一に混合して乳剤とする。
〔配合例3〕乳 剤
本発明化合物No.3-053 4部
DBE 36部
(商品名、アジピン酸ジメチル、グルタル酸ジメチル、コハク酸ジメチルの混合物:インビスタ(INVISTA)社製)
アジピン酸ジイソブチル 30部
N-メチルピロリドン 10部
ソプロフォールBSU 14部
(商品名、非イオン性界面活性剤:ローディア(Rhodia)社製)
ローダカル70BC 6部
(商品名、アニオン性界面活性剤:ローディア(Rhodia)社製)
以上を均一に混合して乳剤とする。 [Formulation Example 2] Milk Compound of the present invention No.3-012 5 parts Xylene 75 parts N-methylpyrrolidone 15 parts Solpol 2680 5 parts (trade name, mixture of nonionic surfactant and anionic surfactant: (Toho Chemical Industries)
The above is uniformly mixed to obtain an emulsion.
[Formulation Example 3] Emulsion Invention Compound No. 3-053 4 parts DBE 36 parts (trade name, dimethyl adipate, dimethyl glutarate, dimethyl succinate mixture: manufactured by INVISTA)
Diisobutyl adipate 30 parts N-methylpyrrolidone 10 parts Soprophor BSU 14 parts (trade name, nonionic surfactant: manufactured by Rhodia)
6 parts of rhodacal 70BC (trade name, anionic surfactant: manufactured by Rhodia)
The above is uniformly mixed to obtain an emulsion.
本発明化合物No.3-012 5部
キシレン 75部
N-メチルピロリドン 15部
ソルポール2680 5部
(商品名、非イオン性界面活性剤とアニオン性界面活性剤との混合物:東邦化学工業社製)
以上を均一に混合して乳剤とする。
〔配合例3〕乳 剤
本発明化合物No.3-053 4部
DBE 36部
(商品名、アジピン酸ジメチル、グルタル酸ジメチル、コハク酸ジメチルの混合物:インビスタ(INVISTA)社製)
アジピン酸ジイソブチル 30部
N-メチルピロリドン 10部
ソプロフォールBSU 14部
(商品名、非イオン性界面活性剤:ローディア(Rhodia)社製)
ローダカル70BC 6部
(商品名、アニオン性界面活性剤:ローディア(Rhodia)社製)
以上を均一に混合して乳剤とする。 [Formulation Example 2] Milk Compound of the present invention No.3-012 5 parts Xylene 75 parts N-methylpyrrolidone 15 parts Solpol 2680 5 parts (trade name, mixture of nonionic surfactant and anionic surfactant: (Toho Chemical Industries)
The above is uniformly mixed to obtain an emulsion.
[Formulation Example 3] Emulsion Invention Compound No. 3-053 4 parts DBE 36 parts (trade name, dimethyl adipate, dimethyl glutarate, dimethyl succinate mixture: manufactured by INVISTA)
Diisobutyl adipate 30 parts N-methylpyrrolidone 10 parts Soprophor BSU 14 parts (trade name, nonionic surfactant: manufactured by Rhodia)
6 parts of rhodacal 70BC (trade name, anionic surfactant: manufactured by Rhodia)
The above is uniformly mixed to obtain an emulsion.
〔配合例4〕乳 剤
本発明化合物No.7-006 4部
DBE 11部
(商品名、アジピン酸ジメチル、グルタル酸ジメチル、コハク酸ジメチルの混合物:インビスタ(INVISTA)社製)
アジピン酸ジイソブチル 30部
N-メチルピロリドン 5部
ソプロフォールBSU 14部
(商品名、非イオン性界面活性剤:ローディア(Rhodia)社製)
ローダカル70BC 6部
(商品名、アニオン性界面活性剤:ローディア(Rhodia)社製)
プロピレングリコール 10部
水 20部
以上を均一に混合して乳剤とする。 [Composition Example 4] Emulsion Invention Compound No. 7-006 4 parts DBE 11 parts (trade name, dimethyl adipate, dimethyl glutarate, dimethyl succinate mixture: manufactured by INVISTA)
Diisobutyl adipate 30 parts N-methylpyrrolidone 5 parts Soprophor BSU 14 parts (trade name, nonionic surfactant: manufactured by Rhodia)
6 parts of rhodacal 70BC (trade name, anionic surfactant: manufactured by Rhodia)
Propylene glycol 10 parts Water 20 parts or more are mixed uniformly to make an emulsion.
本発明化合物No.7-006 4部
DBE 11部
(商品名、アジピン酸ジメチル、グルタル酸ジメチル、コハク酸ジメチルの混合物:インビスタ(INVISTA)社製)
アジピン酸ジイソブチル 30部
N-メチルピロリドン 5部
ソプロフォールBSU 14部
(商品名、非イオン性界面活性剤:ローディア(Rhodia)社製)
ローダカル70BC 6部
(商品名、アニオン性界面活性剤:ローディア(Rhodia)社製)
プロピレングリコール 10部
水 20部
以上を均一に混合して乳剤とする。 [Composition Example 4] Emulsion Invention Compound No. 7-006 4 parts DBE 11 parts (trade name, dimethyl adipate, dimethyl glutarate, dimethyl succinate mixture: manufactured by INVISTA)
Diisobutyl adipate 30 parts N-methylpyrrolidone 5 parts Soprophor BSU 14 parts (trade name, nonionic surfactant: manufactured by Rhodia)
6 parts of rhodacal 70BC (trade name, anionic surfactant: manufactured by Rhodia)
Propylene glycol 10 parts Water 20 parts or more are mixed uniformly to make an emulsion.
〔配合例5〕懸濁剤
本発明化合物No.9-003 25部
アグリゾールS-710 10部
(商品名、非イオン性界面活性剤:花王社製)
ルノックス1000C 0.5部
(商品名、アニオン性界面活性剤:東邦化学工業社製)
キサンタンガム 0.2部
水 64.3部
以上を均一に混合した後、湿式粉砕して懸濁剤とする。 [Formulation Example 5] Suspending Agent Compound No. 9-003 25 parts Agrisol S-710 10 parts (trade name, nonionic surfactant: manufactured by Kao Corporation)
0.5 parts of LUNOX 1000C (trade name, anionic surfactant: manufactured by Toho Chemical Industry Co., Ltd.)
Xanthan gum 0.2 parts water 64.3 parts After uniformly mixing the above, wet pulverize to make a suspension.
本発明化合物No.9-003 25部
アグリゾールS-710 10部
(商品名、非イオン性界面活性剤:花王社製)
ルノックス1000C 0.5部
(商品名、アニオン性界面活性剤:東邦化学工業社製)
キサンタンガム 0.2部
水 64.3部
以上を均一に混合した後、湿式粉砕して懸濁剤とする。 [Formulation Example 5] Suspending Agent Compound No. 9-003 25 parts Agrisol S-710 10 parts (trade name, nonionic surfactant: manufactured by Kao Corporation)
0.5 parts of LUNOX 1000C (trade name, anionic surfactant: manufactured by Toho Chemical Industry Co., Ltd.)
Xanthan gum 0.2 parts water 64.3 parts After uniformly mixing the above, wet pulverize to make a suspension.
〔配合例6〕顆粒水和剤
本発明化合物No.9-004 75部
ハイテノールNE-15 5部
(商品名、アニオン性界面活性剤:第一工業製薬社製)
バニレックスN 10部
(商品名、アニオン性界面活性剤:日本製紙社製)
カープレックス#80D 10部
(商品名、合成含水珪酸:塩野義製薬社製)
以上を均一に混合粉砕した後、少量の水を加えて攪拌混合し、押出式造粒機で造粒し、乾燥して顆粒水和剤とする。
〔配合例7〕粒 剤
本発明化合物No.3-064 5部
ベントナイト 50部
タルク 45部
以上を均一に混合粉砕した後、少量の水を加えて攪拌混合し、押出式造粒機で造粒し、乾燥して粒剤とする。 [Formulation Example 6] Granule wettable powder Compound No. 9-004 of the present invention 75 parts Hytenol NE-15 5 parts (trade name, anionic surfactant: manufactured by Daiichi Kogyo Seiyaku Co., Ltd.)
Vanillex N 10 parts (trade name, anionic surfactant: manufactured by Nippon Paper Industries Co., Ltd.)
Carplex # 80D 10 parts (trade name, synthetic hydrous silicic acid: manufactured by Shionogi & Co.)
After uniformly mixing and pulverizing the above, a small amount of water is added, stirred and mixed, granulated with an extrusion granulator, and dried to obtain a granulated wettable powder.
[Formulation Example 7] Granule Compound No. 3-064 of the present invention 5 parts Bentonite 50 parts Talc 45 parts And dried into granules.
本発明化合物No.9-004 75部
ハイテノールNE-15 5部
(商品名、アニオン性界面活性剤:第一工業製薬社製)
バニレックスN 10部
(商品名、アニオン性界面活性剤:日本製紙社製)
カープレックス#80D 10部
(商品名、合成含水珪酸:塩野義製薬社製)
以上を均一に混合粉砕した後、少量の水を加えて攪拌混合し、押出式造粒機で造粒し、乾燥して顆粒水和剤とする。
〔配合例7〕粒 剤
本発明化合物No.3-064 5部
ベントナイト 50部
タルク 45部
以上を均一に混合粉砕した後、少量の水を加えて攪拌混合し、押出式造粒機で造粒し、乾燥して粒剤とする。 [Formulation Example 6] Granule wettable powder Compound No. 9-004 of the present invention 75 parts Hytenol NE-15 5 parts (trade name, anionic surfactant: manufactured by Daiichi Kogyo Seiyaku Co., Ltd.)
Vanillex N 10 parts (trade name, anionic surfactant: manufactured by Nippon Paper Industries Co., Ltd.)
Carplex # 80D 10 parts (trade name, synthetic hydrous silicic acid: manufactured by Shionogi & Co.)
After uniformly mixing and pulverizing the above, a small amount of water is added, stirred and mixed, granulated with an extrusion granulator, and dried to obtain a granulated wettable powder.
[Formulation Example 7] Granule Compound No. 3-064 of the present invention 5 parts Bentonite 50 parts Talc 45 parts And dried into granules.
〔配合例8〕粉 剤
本発明化合物No.3-059 3部
カープレックス#80D 0.5部
(商品名、合成含水珪酸:塩野義製薬社製)
カオリナイト 95部
リン酸ジイソプロピル 1.5部
以上を均一に混合粉砕して粉剤とする。 [Composition Example 8] Powder Compound of the present invention No.3-059 3 parts Carplex # 80D 0.5 part (trade name, synthetic hydrous silicic acid: manufactured by Shionogi & Co.)
Kaolinite 95 parts Diisopropyl phosphate 1.5 parts The above is uniformly mixed and ground to obtain a powder.
本発明化合物No.3-059 3部
カープレックス#80D 0.5部
(商品名、合成含水珪酸:塩野義製薬社製)
カオリナイト 95部
リン酸ジイソプロピル 1.5部
以上を均一に混合粉砕して粉剤とする。 [Composition Example 8] Powder Compound of the present invention No.3-059 3 parts Carplex # 80D 0.5 part (trade name, synthetic hydrous silicic acid: manufactured by Shionogi & Co.)
Kaolinite 95 parts Diisopropyl phosphate 1.5 parts The above is uniformly mixed and ground to obtain a powder.
使用に際しては、上記の各製剤を水で1~20000倍に希釈して、有効成分が1ヘクタール(ha)当たり0.005~50kgになるように散布する。
When using, dilute each of the above preparations 1 to 20000 times with water and spray it so that the active ingredient is 0.005 to 50 kg per hectare (ha).
〔配合例9〕水和剤調製物
本発明化合物No.1-016 25部
ジイソブチルナフタレンスルホン酸ナトリウム 1部
n-ドデシルベンゼンスルホン酸カルシウム 10部
アルキルアリール ポリグリコールエーテル 12部
ナフタレンスルホン酸ホルマリン縮合物のナトリウム塩 3部
エマルジョン型シリコーン 1部
二酸化ケイ素 3部
カオリン 45部 [Formulation Example 9] wettable powder preparation Compound No. 1-016 of the present invention 25 parts sodium diisobutylnaphthalenesulfonate 1 part calcium n-dodecylbenzenesulfonate 10 parts alkylaryl polyglycol ether 12 parts naphthalenesulfonic acid formalin condensate Sodium salt 3 parts Emulsion type silicone 1 part Silicon dioxide 3 parts Kaolin 45 parts
本発明化合物No.1-016 25部
ジイソブチルナフタレンスルホン酸ナトリウム 1部
n-ドデシルベンゼンスルホン酸カルシウム 10部
アルキルアリール ポリグリコールエーテル 12部
ナフタレンスルホン酸ホルマリン縮合物のナトリウム塩 3部
エマルジョン型シリコーン 1部
二酸化ケイ素 3部
カオリン 45部 [Formulation Example 9] wettable powder preparation Compound No. 1-016 of the present invention 25 parts sodium diisobutylnaphthalenesulfonate 1 part calcium n-dodecylbenzenesulfonate 10 parts alkylaryl polyglycol ether 12 parts naphthalenesulfonic acid formalin condensate Sodium salt 3 parts Emulsion type silicone 1 part Silicon dioxide 3 parts Kaolin 45 parts
〔配合例10〕水溶性濃厚剤調製物
本発明化合物No.1-049 20部
ポリオキシエチレンラウリルエーテル 3部
ジオクチルスルホコハク酸ナトリウム 3.5部
ジメチルスルホキシド 37部
2-プロパノール 36.5部
〔配合例11〕噴霧用液剤
本発明化合物No.1-048 2部
ジメチルスルホキシド 10部
2-プロパノール 35部
アセトン 53部 [Formulation Example 10] Preparation of water-soluble thickener Compound No. 1-049 of the present invention 20 parts Polyoxyethylene lauryl ether 3 parts Sodium dioctyl sulfosuccinate 3.5 parts Dimethyl sulfoxide 37 parts 2-propanol 36.5 parts 11] Solution for spraying Compound No. 1-048 of the present invention 2 parts Dimethyl sulfoxide 10 parts 2-propanol 35 parts Acetone 53 parts
本発明化合物No.1-049 20部
ポリオキシエチレンラウリルエーテル 3部
ジオクチルスルホコハク酸ナトリウム 3.5部
ジメチルスルホキシド 37部
2-プロパノール 36.5部
〔配合例11〕噴霧用液剤
本発明化合物No.1-048 2部
ジメチルスルホキシド 10部
2-プロパノール 35部
アセトン 53部 [Formulation Example 10] Preparation of water-soluble thickener Compound No. 1-049 of the present invention 20 parts Polyoxyethylene lauryl ether 3 parts Sodium dioctyl sulfosuccinate 3.5 parts Dimethyl sulfoxide 37 parts 2-propanol 36.5 parts 11] Solution for spraying Compound No. 1-048 of the present invention 2 parts Dimethyl sulfoxide 10 parts 2-propanol 35 parts Acetone 53 parts
〔配合例12〕経皮投与用液剤
本発明化合物No.1-088 5部
ヘキシレングリコール 50部
イソプロパノール 45部
〔配合例13〕経皮投与用液剤
本発明化合物No.3-090 5部
プロピレングリコールモノメチルエーテル 50部
ジプロピレングリコール 45部 [Formulation 12] Solution for transdermal administration Compound No. 1-088 5 parts Hexylene glycol 50 parts Isopropanol 45 parts [Formulation 13] Solution for transdermal administration Compound No. 3-090 5 parts propylene glycol Monomethyl ether 50 parts Dipropylene glycol 45 parts
本発明化合物No.1-088 5部
ヘキシレングリコール 50部
イソプロパノール 45部
〔配合例13〕経皮投与用液剤
本発明化合物No.3-090 5部
プロピレングリコールモノメチルエーテル 50部
ジプロピレングリコール 45部 [Formulation 12] Solution for transdermal administration Compound No. 1-088 5 parts Hexylene glycol 50 parts Isopropanol 45 parts [Formulation 13] Solution for transdermal administration Compound No. 3-090 5 parts propylene glycol Monomethyl ether 50 parts Dipropylene glycol 45 parts
〔配合例14〕経皮投与(滴下)用液剤
本発明化合物No.1-087 2部
軽質流動パラフィン 98部
〔配合例15〕経皮投与(滴下)用液剤
本発明化合物No.3-055 2部
軽質流動パラフィン 58部
オリーブ油 30部
ODO-H 9部
信越シリコーン(商品名、シリコーン:信越化学工業社製) 1部 [Formulation 14] Solution for transdermal administration (dropping) The present compound No. 1-087 2 parts Light liquid paraffin 98 parts [Formulation 15] Solution for transdermal administration (dropping) The present compound No. 3-055 2 Part light liquid paraffin 58 parts Olive oil 30 parts ODO-H 9 parts Shin-Etsu Silicone (trade name, silicone: manufactured by Shin-Etsu Chemical Co., Ltd.) 1 part
本発明化合物No.1-087 2部
軽質流動パラフィン 98部
〔配合例15〕経皮投与(滴下)用液剤
本発明化合物No.3-055 2部
軽質流動パラフィン 58部
オリーブ油 30部
ODO-H 9部
信越シリコーン(商品名、シリコーン:信越化学工業社製) 1部 [Formulation 14] Solution for transdermal administration (dropping) The present compound No. 1-087 2 parts Light liquid paraffin 98 parts [Formulation 15] Solution for transdermal administration (dropping) The present compound No. 3-055 2 Part light liquid paraffin 58 parts Olive oil 30 parts ODO-H 9 parts Shin-Etsu Silicone (trade name, silicone: manufactured by Shin-Etsu Chemical Co., Ltd.) 1 part
本発明化合物を農園芸用殺菌剤及び線虫防除剤として使用する場合には、有効量の本発明化合物を活性成分として単独で用いることもできるが、必要に応じて他種の殺菌剤、他種の殺線虫剤、殺虫剤、殺ダニ剤、植物生長調節剤、除草剤、共力剤、肥料、土壌改良剤等と製剤時又は散布時に混合施用しても良い。
また、本発明化合物を寄生虫防除剤として使用する場合には、有効量の本発明化合物を活性成分として単独で投与することもできるが、必要に応じて他種の抗菌剤、他種の駆虫剤等と製剤時又は投与時に混合して投与することもできる。 When the compound of the present invention is used as an agricultural and horticultural fungicide and nematode control agent, an effective amount of the compound of the present invention can be used alone as an active ingredient. It may be mixed with seed nematicides, insecticides, acaricides, plant growth regulators, herbicides, synergists, fertilizers, soil conditioners, etc. at the time of formulation or spraying.
Further, when the compound of the present invention is used as a parasite control agent, an effective amount of the compound of the present invention can be administered alone as an active ingredient. It can also be mixed with an agent or the like at the time of preparation or administration.
また、本発明化合物を寄生虫防除剤として使用する場合には、有効量の本発明化合物を活性成分として単独で投与することもできるが、必要に応じて他種の抗菌剤、他種の駆虫剤等と製剤時又は投与時に混合して投与することもできる。 When the compound of the present invention is used as an agricultural and horticultural fungicide and nematode control agent, an effective amount of the compound of the present invention can be used alone as an active ingredient. It may be mixed with seed nematicides, insecticides, acaricides, plant growth regulators, herbicides, synergists, fertilizers, soil conditioners, etc. at the time of formulation or spraying.
Further, when the compound of the present invention is used as a parasite control agent, an effective amount of the compound of the present invention can be administered alone as an active ingredient. It can also be mixed with an agent or the like at the time of preparation or administration.
特に他種の殺菌剤、他種の殺線虫剤、他種の抗菌剤或いは他種の駆虫剤等と混合施用することにより、混合した薬剤相互の相加・相乗作用による防除スペクトラムの拡大、有害生物防除効果の向上、施用薬量の低減による防除コストの軽減、さらには、より長期間にわたる防除効果の持続等の効果が期待できる。特に、作用機作の異なる他種の殺菌剤、殺線虫剤、抗菌剤、駆虫剤と混合して施用することは、有害生物の薬剤抵抗性獲得を防ぐ観点から極めて有効な防除方法である。この際、同時に複数の公知殺菌剤、公知線虫剤、公知殺虫剤、公知殺ダニ剤、公知抗菌剤或いは公知駆虫剤との組み合わせも可能である。
In particular, by applying mixed with other types of fungicides, other types of nematicides, other types of antibacterial agents or other types of anthelmintic agents, etc., the control spectrum can be expanded by the additive / synergistic action of the mixed agents, Effects such as improvement of pest control effect, reduction of control cost by reducing the amount of applied medicine, and sustained control effect over a longer period can be expected. In particular, mixing with other types of fungicides, nematicides, antibacterial agents and anthelmintic agents with different mechanisms of action is an extremely effective control method from the viewpoint of preventing the acquisition of drug resistance by pests. . At this time, a combination of a plurality of known fungicides, known nematodes, known insecticides, known acaricides, known antibacterial agents or known anthelmintic agents is also possible.
本発明化合物と混合使用する殺菌剤、殺線虫剤、殺虫剤、殺ダニ剤、駆虫剤或いは抗菌剤の種類としては、例えばザ・ペスティサイド・マニュアル(The Pesticide Manual)16版、2012年等に記載されている化合物等が挙げられる。具体的にその一般名を例示すれば次の通りであるが、必ずしもこれらのみに限定されるものではない。
Examples of types of fungicides, nematicides, insecticides, acaricides, anthelmintics or antibacterials used in combination with the compounds of the present invention include, for example, The Pesticide Manual 16th edition, 2012, etc. Examples include the compounds described. Specific examples of common names are as follows, but the general names are not necessarily limited to these.
殺菌剤:
A;核酸生合成阻害剤
ベナラキシル(benalaxyl)、ベナラキシル-M(benalaxyl-M)、フララキシル(furalaxyl)、メタラキシル(metalaxyl)、メタラキシル-M(metalaxyl-M)、オフラセ(ofurace)、オキサジキシル(oxadixyl)、
ブピリメート(bupirimate)、エチリモール(ethirimol)、
ヒメキサゾール(hymexazol)。
B;有糸分裂及び細胞分裂阻害剤
ベノミル(benomyl)、カルベンダジム(carbendazim)、フベリダゾール(fuberidazole)、チアベンダゾール(thiabendazole)、チオファネート-メチル(thiophanate-methyl)、
ジエトフェンカルブ(diethofencarb)、
エタボキサム(ethaboxam)、ゾキサミド(zoxamide)、
ペンシクロン(pencycuron)、
フルオピコリド(fluopicolide)。 Fungicide:
A: Nucleic acid biosynthesis inhibitors benalaxyl, benalaxyl-M (benalaxyl-M), furaxyl (furalaxyl), metalaxyl, metalaxyl-M, ofurace, oxadixyl,
Bupirimate, ethirimol,
Hymexazol.
B: Mitotic and mitotic inhibitors benomyl, carbendazim, fuberidazole, thiabendazole, thiophanate-methyl,
Dietofencarb,
Ethaboxam, zoxamide,
Pencicuron (pencycuron),
Fluopicolide.
A;核酸生合成阻害剤
ベナラキシル(benalaxyl)、ベナラキシル-M(benalaxyl-M)、フララキシル(furalaxyl)、メタラキシル(metalaxyl)、メタラキシル-M(metalaxyl-M)、オフラセ(ofurace)、オキサジキシル(oxadixyl)、
ブピリメート(bupirimate)、エチリモール(ethirimol)、
ヒメキサゾール(hymexazol)。
B;有糸分裂及び細胞分裂阻害剤
ベノミル(benomyl)、カルベンダジム(carbendazim)、フベリダゾール(fuberidazole)、チアベンダゾール(thiabendazole)、チオファネート-メチル(thiophanate-methyl)、
ジエトフェンカルブ(diethofencarb)、
エタボキサム(ethaboxam)、ゾキサミド(zoxamide)、
ペンシクロン(pencycuron)、
フルオピコリド(fluopicolide)。 Fungicide:
A: Nucleic acid biosynthesis inhibitors benalaxyl, benalaxyl-M (benalaxyl-M), furaxyl (furalaxyl), metalaxyl, metalaxyl-M, ofurace, oxadixyl,
Bupirimate, ethirimol,
Hymexazol.
B: Mitotic and mitotic inhibitors benomyl, carbendazim, fuberidazole, thiabendazole, thiophanate-methyl,
Dietofencarb,
Ethaboxam, zoxamide,
Pencicuron (pencycuron),
Fluopicolide.
C;呼吸阻害剤
ジフルメトリム(diflumetorim)、
ベノダニル(benodanil)、ベンゾビンジフルピル(benzovindiflupyr)、ビキサフェン(bixafen)、ボスカリド(boscalid)、カルボキシン(carboxin)、フェンフラム(fenfuram)、フルインダピル(fluindapyr)、フルオピラム(fluopyram)、フルトラニル(flutolanil)、フルキサピロキサド(fluxapyroxad)、フラメトピル(furametpyr)、イソフェタミド(isofetamid)、イソフルシプラム(isoflucypram)、イソピラザム(isopyrazam)、メプロニル(mepronil)、オキシカルボキシン(oxycarboxin)、ペンフルフェン(penflufen)、ペンチオピラド(penthiopyrad)、ピジフルメトフェン(pydiflumetofen)、ピラジフルミド(pyraziflumid)、セダキサン(sedaxane)、チフルザミド(thifluzamide)、
アゾキシストロビン(azoxystrobin)、クモキシストロビン(coumoxystrobin)、ジモキシストロビン(dimoxystrobin)、エネストロビン(enestrobin)、エノキサストロビン(enoxastrobin)、ファモキサドン(famoxadone)、フェナミドン(fenamidone)、フェナミンストロビン(fenaminstrobin)、フルフェノキシストロビン(flufenoxystrobin)、フルオキサストロビン(fluoxastrobin)、クレソキシム-メチル(kresoxim-methyl)、マンデストロビン(mandestrobin)、メトミノストロビン(metominostrobin)、オリサストロビン(orysastrobin)、ピコキシストロビン(picoxystrobin)、ピラクロストロビン(pyraclostrobin)、ピラメトストロビン(pyrametostrobin)、ピラオキシストロビン(pyraoxystrobin)、ピリベンカルブメチル(pyribencarb-methyl)、ピリミノストロビン(pyriminostrobin)、トリクロピリカルブ(triclopyricab)、トリフロキシストロビン(trifloxystrobin)、
アミスルブロム(amisulbrom)、シアゾファミド(cyazofamid)、
ジノカップ(dinocap)、フルアジナム(fluazinam)、メプチルジノカップ(meptyldinocap)、
フェンチン(fentin)、トリブチル錫オキシド(tributyltin oxide)、
シルチオファム(silthiofam)、
アメトクトラジン(ametoctradin)、
フェンピコキサミド(fenpicoxamid)。 C: Respiratory inhibitor diflumetorim,
Benodanil, benzovindiflupyr, bixafen, boscalid, carboxin, fenfuram, fluindapyr, fluopyram, flutolanil, flutolanil Xapiroxad (fluxapyroxad), furametpyr, isofetamid, isoflucypram, isoplucam, isoprazam, mepronil, oxycarboxin, penflufen, pyrad , Pidiflumetofen, pyraziflumid, sedaxane, thifluzamide,
Azoxystrobin, coumoxystrobin, dimoxystrobin, enestrobin, enoxastrobin, famoxadone, fenamidone, phenaminestrobin ( fenaminstrobin, flufenoxystrobin, fluoxastrobin, kresoxim-methyl, mandestrobin, metminostrobin, orysastrobin, picoxist Robin (picoxystrobin), pyraclostrobin, pyrametostrobin, pyraoxystrobin, pyribencarb-methyl, pyriminostrobin, triclopyrical Triflopyricab, trifloxystrobin,
Amisulbrom, cyazofamid,
Dinocap, fluazinam, meptyldinocap,
Fentin, tributyltin oxide,
Silthiofam,
Ametoctradin,
Fenpicoxamid.
ジフルメトリム(diflumetorim)、
ベノダニル(benodanil)、ベンゾビンジフルピル(benzovindiflupyr)、ビキサフェン(bixafen)、ボスカリド(boscalid)、カルボキシン(carboxin)、フェンフラム(fenfuram)、フルインダピル(fluindapyr)、フルオピラム(fluopyram)、フルトラニル(flutolanil)、フルキサピロキサド(fluxapyroxad)、フラメトピル(furametpyr)、イソフェタミド(isofetamid)、イソフルシプラム(isoflucypram)、イソピラザム(isopyrazam)、メプロニル(mepronil)、オキシカルボキシン(oxycarboxin)、ペンフルフェン(penflufen)、ペンチオピラド(penthiopyrad)、ピジフルメトフェン(pydiflumetofen)、ピラジフルミド(pyraziflumid)、セダキサン(sedaxane)、チフルザミド(thifluzamide)、
アゾキシストロビン(azoxystrobin)、クモキシストロビン(coumoxystrobin)、ジモキシストロビン(dimoxystrobin)、エネストロビン(enestrobin)、エノキサストロビン(enoxastrobin)、ファモキサドン(famoxadone)、フェナミドン(fenamidone)、フェナミンストロビン(fenaminstrobin)、フルフェノキシストロビン(flufenoxystrobin)、フルオキサストロビン(fluoxastrobin)、クレソキシム-メチル(kresoxim-methyl)、マンデストロビン(mandestrobin)、メトミノストロビン(metominostrobin)、オリサストロビン(orysastrobin)、ピコキシストロビン(picoxystrobin)、ピラクロストロビン(pyraclostrobin)、ピラメトストロビン(pyrametostrobin)、ピラオキシストロビン(pyraoxystrobin)、ピリベンカルブメチル(pyribencarb-methyl)、ピリミノストロビン(pyriminostrobin)、トリクロピリカルブ(triclopyricab)、トリフロキシストロビン(trifloxystrobin)、
アミスルブロム(amisulbrom)、シアゾファミド(cyazofamid)、
ジノカップ(dinocap)、フルアジナム(fluazinam)、メプチルジノカップ(meptyldinocap)、
フェンチン(fentin)、トリブチル錫オキシド(tributyltin oxide)、
シルチオファム(silthiofam)、
アメトクトラジン(ametoctradin)、
フェンピコキサミド(fenpicoxamid)。 C: Respiratory inhibitor diflumetorim,
Benodanil, benzovindiflupyr, bixafen, boscalid, carboxin, fenfuram, fluindapyr, fluopyram, flutolanil, flutolanil Xapiroxad (fluxapyroxad), furametpyr, isofetamid, isoflucypram, isoplucam, isoprazam, mepronil, oxycarboxin, penflufen, pyrad , Pidiflumetofen, pyraziflumid, sedaxane, thifluzamide,
Azoxystrobin, coumoxystrobin, dimoxystrobin, enestrobin, enoxastrobin, famoxadone, fenamidone, phenaminestrobin ( fenaminstrobin, flufenoxystrobin, fluoxastrobin, kresoxim-methyl, mandestrobin, metminostrobin, orysastrobin, picoxist Robin (picoxystrobin), pyraclostrobin, pyrametostrobin, pyraoxystrobin, pyribencarb-methyl, pyriminostrobin, triclopyrical Triflopyricab, trifloxystrobin,
Amisulbrom, cyazofamid,
Dinocap, fluazinam, meptyldinocap,
Fentin, tributyltin oxide,
Silthiofam,
Ametoctradin,
Fenpicoxamid.
D;アミノ酸及びタンパク質生合成阻害剤
シプロジニル(cyprodinil)、メパニピリム(mepanipyrim)、ピリメタニル(pyrimethanil)、
ブラストサイジン-S(blasticidin-S)、
カスガマイシン(kasugamycin)、
E;シグナル伝達系に作用する薬剤
プロキナジド(proquinazid)、キノキシフェン(quinoxyfen)、
フェンピクロニル(fenpiclonil)、フルジオキソニル(fludioxonil)、
クロゾリネート(chlozolinate)、イプロジオン(iprodione)、プロシミドン(procymidone)、ビンクロゾリン(vinclozolin)。 D; amino acid and protein biosynthesis inhibitors cyprodinil, mepanipyrim, pyrimethanil,
Blasticidin-S,
Kasugamycin,
E; drugs that act on the signal transduction system proquinazid, quinoxyfen,
Fenpiclonil, fludioxonil,
Clozolinate, iprodione, procymidone, vinclozolin.
シプロジニル(cyprodinil)、メパニピリム(mepanipyrim)、ピリメタニル(pyrimethanil)、
ブラストサイジン-S(blasticidin-S)、
カスガマイシン(kasugamycin)、
E;シグナル伝達系に作用する薬剤
プロキナジド(proquinazid)、キノキシフェン(quinoxyfen)、
フェンピクロニル(fenpiclonil)、フルジオキソニル(fludioxonil)、
クロゾリネート(chlozolinate)、イプロジオン(iprodione)、プロシミドン(procymidone)、ビンクロゾリン(vinclozolin)。 D; amino acid and protein biosynthesis inhibitors cyprodinil, mepanipyrim, pyrimethanil,
Blasticidin-S,
Kasugamycin,
E; drugs that act on the signal transduction system proquinazid, quinoxyfen,
Fenpiclonil, fludioxonil,
Clozolinate, iprodione, procymidone, vinclozolin.
F;脂質合成及び細胞膜形成阻害剤
エジフェンホス(edifenphos)、イプロベンホス(iprobenfos)、イソプロチオラン(isoprothiolane)、ピラゾホス(pyrazophos)、
ビフェニル(biphenyl)、クロロネブ(chloroneb)、ジクロラン(dicloran)、エトリジアゾール(etridiazole)、キントゼン(quintozene)、テクナゼン(tecnazene)、トルクロホス-メチル(tolclofos-methyl)、
プロパモカルブ塩酸塩(propamocarb hydrochloride)、
バチルス ズブチリス(Bacillus subtilis, Strain:D747, FZB24, GBO3, HAI0404, MBI600, QST713, Y1336等)。 F: Lipid synthesis and cell membrane formation inhibitor Edifenphos, iprobenfos, isoprothiolane, pyrazophos,
Biphenyl, chloroneb, dicloran, etridiazole, quintozene, tecnazene, tolclofos-methyl,
Propamocarb hydrochloride,
Bacillus subtilis (Strain: D747, FZB24, GBO3, HAI0404, MBI600, QST713, Y1336, etc.).
エジフェンホス(edifenphos)、イプロベンホス(iprobenfos)、イソプロチオラン(isoprothiolane)、ピラゾホス(pyrazophos)、
ビフェニル(biphenyl)、クロロネブ(chloroneb)、ジクロラン(dicloran)、エトリジアゾール(etridiazole)、キントゼン(quintozene)、テクナゼン(tecnazene)、トルクロホス-メチル(tolclofos-methyl)、
プロパモカルブ塩酸塩(propamocarb hydrochloride)、
バチルス ズブチリス(Bacillus subtilis, Strain:D747, FZB24, GBO3, HAI0404, MBI600, QST713, Y1336等)。 F: Lipid synthesis and cell membrane formation inhibitor Edifenphos, iprobenfos, isoprothiolane, pyrazophos,
Biphenyl, chloroneb, dicloran, etridiazole, quintozene, tecnazene, tolclofos-methyl,
Propamocarb hydrochloride,
Bacillus subtilis (Strain: D747, FZB24, GBO3, HAI0404, MBI600, QST713, Y1336, etc.).
G;ステロール生合成阻害剤
アザコナゾール(azaconazole)、ビテルタノール(bitertanol)、ブロムコナゾール(bromuconazole)、シプロコナゾール(cyproconazole)、ジフェノコナゾール(difenoconazole)、ジニコナゾール(diniconazole)、ジニコナゾール-M(diniconazole-M)、エポキシコナゾール(epoxiconazole)、エタコナゾール(etaconazole)、フェナリモル(fenarimol)、フェンブコナゾール(fenbuconazole)、フルキンコナゾール(fluquinconazole)、フルシラゾール(flusilazole)、フルトリアホール(flutriafol)、ヘキサコナゾール(hexaconazole)、イマザリル(imazalil)、イミベンコナゾール(imibenconazole)、イプコナゾール(ipconazole)、イプフェントリフルコナゾール(ipfentrifluconazole)、メフェントリフルコナゾール(mefentrifluconazole)、メトコナゾール(metconazole)、ミクロブタニル(myclobutanil)、ヌアリモール(nuarimol)、オキスポコナゾールフマル酸塩(oxpoconazole fumarate)、ペフラゾエート(pefurazoate)、ペンコナゾール(penconazole)、プロクロラズ(prochloraz)、プロピコナゾール(propiconazole)、プロチオコナゾール(prothioconazole)、ピリフェノックス(pyrifenox)、ピリソキサゾール(pyrisoxazole)、シメコナゾール(simeconazole)、テブコナゾール(tebuconazole)、テトラコナゾール(tetraconazole)、トリアジメホン(triadimefon)、トリアジメノール(triadimenol)、トリフルミゾール(triflumizole)、トリホリン(triforine)、トリチコナゾール(triticonazole)、
アルジモルフ(aldimorph)、ドデモルフ酢酸塩(dodemorph-acetate)、フェンプロピジン(fenpropidin)、フェンプロピモルフ(fenpropimorph)、ピペラリン(piperalin)、スピロキサミン(spiroxamine)、トリデモルフ(tridemorph)、
フェンヘキサミド(fenhexamid)、フェンピラザミン(fenpyrazamine)。 G: sterol biosynthesis inhibitor azaconazole, abitertanol, bromuconazole, cyproconazole, difenoconazole, dinicoazole, dinicoazole-M (diniconazole-M), Epoxiconazole, etaconazole, fenarimol, fenbuconazole, fluquinconazole, flusilazole, flutriafol, hexaconazole, Imazalil, imibenconazole, ipconazole, ipfentrifluconazole, mefentrifluconazole, metconazole (metconazole) azole), microbutanil, nuarimol, oxpoconazole fumarate, pefurazoate, penconazole, prochloraz, propiconazole, prothioconazole (Prothioconazole), pyrifenox, pyrisoxazole, simeconazole, tebuconazole, tetraconazole, triadimefon, triadimenol, triflumizole ), Triforine, triticonazole,
Aldimorph, dodemorph-acetate, fenpropidin, fenpropimorph, piperalin, spiroxamine, tridemorph,
Fenhexamid, fenpyrazamine.
アザコナゾール(azaconazole)、ビテルタノール(bitertanol)、ブロムコナゾール(bromuconazole)、シプロコナゾール(cyproconazole)、ジフェノコナゾール(difenoconazole)、ジニコナゾール(diniconazole)、ジニコナゾール-M(diniconazole-M)、エポキシコナゾール(epoxiconazole)、エタコナゾール(etaconazole)、フェナリモル(fenarimol)、フェンブコナゾール(fenbuconazole)、フルキンコナゾール(fluquinconazole)、フルシラゾール(flusilazole)、フルトリアホール(flutriafol)、ヘキサコナゾール(hexaconazole)、イマザリル(imazalil)、イミベンコナゾール(imibenconazole)、イプコナゾール(ipconazole)、イプフェントリフルコナゾール(ipfentrifluconazole)、メフェントリフルコナゾール(mefentrifluconazole)、メトコナゾール(metconazole)、ミクロブタニル(myclobutanil)、ヌアリモール(nuarimol)、オキスポコナゾールフマル酸塩(oxpoconazole fumarate)、ペフラゾエート(pefurazoate)、ペンコナゾール(penconazole)、プロクロラズ(prochloraz)、プロピコナゾール(propiconazole)、プロチオコナゾール(prothioconazole)、ピリフェノックス(pyrifenox)、ピリソキサゾール(pyrisoxazole)、シメコナゾール(simeconazole)、テブコナゾール(tebuconazole)、テトラコナゾール(tetraconazole)、トリアジメホン(triadimefon)、トリアジメノール(triadimenol)、トリフルミゾール(triflumizole)、トリホリン(triforine)、トリチコナゾール(triticonazole)、
アルジモルフ(aldimorph)、ドデモルフ酢酸塩(dodemorph-acetate)、フェンプロピジン(fenpropidin)、フェンプロピモルフ(fenpropimorph)、ピペラリン(piperalin)、スピロキサミン(spiroxamine)、トリデモルフ(tridemorph)、
フェンヘキサミド(fenhexamid)、フェンピラザミン(fenpyrazamine)。 G: sterol biosynthesis inhibitor azaconazole, abitertanol, bromuconazole, cyproconazole, difenoconazole, dinicoazole, dinicoazole-M (diniconazole-M), Epoxiconazole, etaconazole, fenarimol, fenbuconazole, fluquinconazole, flusilazole, flutriafol, hexaconazole, Imazalil, imibenconazole, ipconazole, ipfentrifluconazole, mefentrifluconazole, metconazole (metconazole) azole), microbutanil, nuarimol, oxpoconazole fumarate, pefurazoate, penconazole, prochloraz, propiconazole, prothioconazole (Prothioconazole), pyrifenox, pyrisoxazole, simeconazole, tebuconazole, tetraconazole, triadimefon, triadimenol, triflumizole ), Triforine, triticonazole,
Aldimorph, dodemorph-acetate, fenpropidin, fenpropimorph, piperalin, spiroxamine, tridemorph,
Fenhexamid, fenpyrazamine.
H;細胞壁合成阻害剤
バリダマイシン(validamycin)、
ポリオキシン(polyoxins)、ポリオキシン-D(polyoxorim)、
ベンチアバリカルブ-イソプロピル(benthiavalicarb-isopropyl)、ジメトモルフ(dimethomorph)、フルモルフ(flumorph)、イプロバリカルブ(iprovalicarb)、マンジプロパミド(mandipropamid)、ピリモルフ(pyrimorph)、バリフェナレート(valifenalate)、
I;メラニン合成阻害剤
フサライド(phthalide)、ピロキロン(pyroquilon)、トリシクラゾール(tricyclazole)、
カルプロパミド(carpropamid)、ジクロシメット(diclocymet)、フェノキサニル(fenoxanil)。 H; cell wall synthesis inhibitor validamycin,
Polyoxins, polyoxins-D (polyoxorim),
Benthiavalicarb-isopropyl, dimethomorph, flumorph, iprovalicarb, mandipropamid, pyrimorph, valifenalate,
I: Melanin synthesis inhibitor phthalide, pyroquilon, tricyclazole,
Carpropamid, diclocymet, fenoxanil.
バリダマイシン(validamycin)、
ポリオキシン(polyoxins)、ポリオキシン-D(polyoxorim)、
ベンチアバリカルブ-イソプロピル(benthiavalicarb-isopropyl)、ジメトモルフ(dimethomorph)、フルモルフ(flumorph)、イプロバリカルブ(iprovalicarb)、マンジプロパミド(mandipropamid)、ピリモルフ(pyrimorph)、バリフェナレート(valifenalate)、
I;メラニン合成阻害剤
フサライド(phthalide)、ピロキロン(pyroquilon)、トリシクラゾール(tricyclazole)、
カルプロパミド(carpropamid)、ジクロシメット(diclocymet)、フェノキサニル(fenoxanil)。 H; cell wall synthesis inhibitor validamycin,
Polyoxins, polyoxins-D (polyoxorim),
Benthiavalicarb-isopropyl, dimethomorph, flumorph, iprovalicarb, mandipropamid, pyrimorph, valifenalate,
I: Melanin synthesis inhibitor phthalide, pyroquilon, tricyclazole,
Carpropamid, diclocymet, fenoxanil.
P;植物の抵抗性誘導剤
アシベンゾラル-S-メチル(acibenzolar-S-methyl)、
ジクロベンチアゾックス(dichlobentiazox)、プロベナゾール(probenazole)、
イソチアニル(isotianil)、チアジニル(tiadinil)、
ラミナリン(laminarin)。 P: plant resistance inducer acibenzolar-S-methyl,
Diclobentiazox, probenazole,
Isotianil, tiadinil,
Laminarin.
アシベンゾラル-S-メチル(acibenzolar-S-methyl)、
ジクロベンチアゾックス(dichlobentiazox)、プロベナゾール(probenazole)、
イソチアニル(isotianil)、チアジニル(tiadinil)、
ラミナリン(laminarin)。 P: plant resistance inducer acibenzolar-S-methyl,
Diclobentiazox, probenazole,
Isotianil, tiadinil,
Laminarin.
M;多作用点の薬剤
ボルドー液(bordeaux mixture)、チェシュントミクスチャ(cheshunt mixture)、塩基性炭酸銅(copper carbonate, basic)、水酸化第二銅(copper hydroxide)、カッパーナフテネート(copper naphthenate)、カッパーオレエート(copper oleate)、塩基性塩化銅(copper oxychloride)、硫酸銅(copper sulfate)、塩基性硫酸銅(copper sulfate, basic)、オキシキノリン銅(oxine copper)、石灰硫黄合剤(calcium polysulfide)、硫黄(sulfur)、アンバム(amobam)、ファーバム(ferbam)、マンコゼブ(mancozeb)、マンネブ(maneb)、メチラム(metiram)、ポリカーバメート(polycarbamate)、プロピネブ(propineb)、チウラム(thiram)、ジラム(ziram)、キャプタン(captan)、フォルペット(folpet)、クロロタロニル(chlorothalonil)、ジクロフルアニド(dichlofluanid)、トリルフルアニド(tolylfluanid)、グアザチン(guazatine)、イミノクタジン-アルベシル酸塩(iminoctadine-albesilate)、イミノクタジン酢酸塩(iminoctadine-triacetate)、アニラジン(anilazine)、ジチアノン(dithianon)、キノメチオネート(chinomethionat)、フルオルイミド(fluoroimide)。 M: drugs with multiple action points Bordeaux mixture, cheshunt mixture, basic carbonate, copper carbonate, copper hydroxide, copper naphthenate , Copper oleate, copper oxychloride, copper sulfate, copper sulfate, basic, oxine copper, lime sulfur polysulfide, sulfur, amobam, ferbam, mancozeb, maneb, maneb, metiram, polycarbamate, propineb, thiram, ziram (Ziram), captan, folpet, chlorothalonil, dichlofluanid, torruf Enid (tolylfluanid), guazatine (guazatine), iminoctadine - Arubeshiru salt (iminoctadine-albesilate), iminoctadine acetate (iminoctadine-triacetate), Anirajin (anilazine), dithianon (dithianon), Kinomechioneto (chinomethionat), Furuoruimido (fluoroimide).
ボルドー液(bordeaux mixture)、チェシュントミクスチャ(cheshunt mixture)、塩基性炭酸銅(copper carbonate, basic)、水酸化第二銅(copper hydroxide)、カッパーナフテネート(copper naphthenate)、カッパーオレエート(copper oleate)、塩基性塩化銅(copper oxychloride)、硫酸銅(copper sulfate)、塩基性硫酸銅(copper sulfate, basic)、オキシキノリン銅(oxine copper)、石灰硫黄合剤(calcium polysulfide)、硫黄(sulfur)、アンバム(amobam)、ファーバム(ferbam)、マンコゼブ(mancozeb)、マンネブ(maneb)、メチラム(metiram)、ポリカーバメート(polycarbamate)、プロピネブ(propineb)、チウラム(thiram)、ジラム(ziram)、キャプタン(captan)、フォルペット(folpet)、クロロタロニル(chlorothalonil)、ジクロフルアニド(dichlofluanid)、トリルフルアニド(tolylfluanid)、グアザチン(guazatine)、イミノクタジン-アルベシル酸塩(iminoctadine-albesilate)、イミノクタジン酢酸塩(iminoctadine-triacetate)、アニラジン(anilazine)、ジチアノン(dithianon)、キノメチオネート(chinomethionat)、フルオルイミド(fluoroimide)。 M: drugs with multiple action points Bordeaux mixture, cheshunt mixture, basic carbonate, copper carbonate, copper hydroxide, copper naphthenate , Copper oleate, copper oxychloride, copper sulfate, copper sulfate, basic, oxine copper, lime sulfur polysulfide, sulfur, amobam, ferbam, mancozeb, maneb, maneb, metiram, polycarbamate, propineb, thiram, ziram (Ziram), captan, folpet, chlorothalonil, dichlofluanid, torruf Enid (tolylfluanid), guazatine (guazatine), iminoctadine - Arubeshiru salt (iminoctadine-albesilate), iminoctadine acetate (iminoctadine-triacetate), Anirajin (anilazine), dithianon (dithianon), Kinomechioneto (chinomethionat), Furuoruimido (fluoroimide).
UN;作用機作不明及びその他の薬剤
シフルフェナミド(cyflufenamid)、シモキサニル(cymoxanil)、ジクロメジン(diclomezine)、ジピメチトロン(dipymetitrone)、ドジン(dodine)、フェリムゾン(ferimzone)、フルスルファミド(flusulfamide)、フルチアニル(flutianil)、ホセチル-アルミニウム(fosetyl-aluminium)、メトラフェノン(metrafenone)、オキサチアピプロリン(oxathiapiprolin)、ピカルブトラゾックス(picarbutrazox)、ピリオフェノン(pyriofenone)、キノフメリン(quinofumelin)、テブフロキン(tebufloquin)、トルプロカルブ(tolprocarb)、トリアゾキシド(triazoxide)、炭酸水素カリウム(potassium hydrogen carbonate)、炭酸水素ナトリウム(sodium hydrogen carbonate)、シイタケ菌糸体抽出物、シイタケ子実体抽出物、BCF-082(試験名)、ZF-9646(試験名)。 UN; unknown mechanism of action and other drugs Cyflufenamid, cymoxanil, diclomezine, dipymetitrone, dodine, ferimzone, flusulfamide, flutianil, flutianil Fosetyl-aluminium, metrafenone, oxathiapiprolin, picarbutrazox, pyriofenone, quinofumelin, tebufloquin, tolprocarb, tolprocarb Triazoxide, potassium hydrogen carbonate, sodium hydrogen carbonate, shiitake mycelium extract, shiitake fruiting body extract, BCF-082 (test name), ZF-9 46 (test name).
シフルフェナミド(cyflufenamid)、シモキサニル(cymoxanil)、ジクロメジン(diclomezine)、ジピメチトロン(dipymetitrone)、ドジン(dodine)、フェリムゾン(ferimzone)、フルスルファミド(flusulfamide)、フルチアニル(flutianil)、ホセチル-アルミニウム(fosetyl-aluminium)、メトラフェノン(metrafenone)、オキサチアピプロリン(oxathiapiprolin)、ピカルブトラゾックス(picarbutrazox)、ピリオフェノン(pyriofenone)、キノフメリン(quinofumelin)、テブフロキン(tebufloquin)、トルプロカルブ(tolprocarb)、トリアゾキシド(triazoxide)、炭酸水素カリウム(potassium hydrogen carbonate)、炭酸水素ナトリウム(sodium hydrogen carbonate)、シイタケ菌糸体抽出物、シイタケ子実体抽出物、BCF-082(試験名)、ZF-9646(試験名)。 UN; unknown mechanism of action and other drugs Cyflufenamid, cymoxanil, diclomezine, dipymetitrone, dodine, ferimzone, flusulfamide, flutianil, flutianil Fosetyl-aluminium, metrafenone, oxathiapiprolin, picarbutrazox, pyriofenone, quinofumelin, tebufloquin, tolprocarb, tolprocarb Triazoxide, potassium hydrogen carbonate, sodium hydrogen carbonate, shiitake mycelium extract, shiitake fruiting body extract, BCF-082 (test name), ZF-9 46 (test name).
殺虫・殺ダニ剤:
A;アセチルコリンエステラーゼ(AChE)阻害剤
アラニカルブ(alanycarb)、アルジカルブ(aldicarb)、ベンダイオカルブ(bendiocarb)、ベンフラカルブ(benfuracarb)、ブトカルボキシム(butocarboxim)、カルバリル(carbaryl)、カルボフラン(carbofuran)、カルボスルファン(carbosulfan)、エチオフェンカルブ(ethiofencarb)、フェノブカルブ(fenobucarb)、ホルメタネート(formetanate)、フラチオカルブ(furathiocarb)、イソプロカルブ(isoprocarb)、メチオカルブ(methiocarb)、メソミル(methomyl)、ピリミカーブ(pirimicarb)、チオジカルブ(thiodicarb)、チオファノックス(thiofanox)、トリアザメート(triazamate)、
アセフェート(acephate)、アザメチホス(azamethiphos)、アジンホス-エチル(azinphos-ethyl)、アジンホス-メチル(azinphos-methyl)、クロルエトキシホス(chlorethoxyfos)、クロルフェンビンホス(chlorfenvinphos)、クロルメホス(chlormephos)、クロルピリホス(chlorpyrifos)、クロルピリホス-メチル(chlorpyrifos-methyl)、シアノホス(cyanophos)、ダイアジノン(diazinon)、ジクロルボス(dichlorvos)、ジメトエート(dimethoate)、ジメチルビンホス(dimethylvinphos)、ジスルフォトン(disulfoton)、イーピーエヌ(EPN)、フェニトロチオン(fenitrothion)、フェンチオン(fenthion)、イソキサチオン(isoxathion)、マラチオン(malathion)、メタミドホス(methamidophos)、メチダチオン(methidathion)、オメトエート(omethoate)、オキシジメトン-メチル(oxydemeton-methyl)、パラチオン-メチル(parathion-methyl)、フェントエート(phenthoate)、ホレート(phorate)、ホサロン(phosalone)、ホスメット(phosmet)、ホキシム(phoxim)、ピリミホス-メチル(pirimiphos-methyl)、プロフェノホス(profenofos)、プロチオホス(prothiofos)、ピラクロホス(pyraclofos)、スルホテップ(sulfotep)、テルブホス(terbufos)、テトラクロロビンホス(tetrachlorvinphos)、チオメトン(thiometon)、トリクロルホン(trichlorfon)。 Insecticides and acaricides:
A: Acetylcholinesterase (AChE) inhibitor Alanicarb, aldicarb, bendiocarb, benfuracarb, butofcarboxim, carbaryl, carbofuran, carbofuran Fan (carbosulfan), ethiofencarb, fenobucarb, formetanate, furathiocarb, isoprocarb, methiocarb, methomyl, pirimicarb, thiodicarb Thiofanox, triazamate,
Acephate, azamethiphos, azinphos-ethyl, azinphos-methyl, chlorethoxyfos, chlorfenvinphos, chlormephos, chlorpyrifos ( chlorpyrifos, chlorpyrifos-methyl, cyanophos, diazinon, dichlorvos, dimethoate, dimethylvinphos, disulfoton, EPN, fenitrothion (Fenitrothion), fenthion, isoxathion, malathion, methamidophos, methidathion, omethoate, oxydemeton-methy l), parathion-methyl, phenthoate, phorate, phosalone, phosmet, phoxim, pirimiphos-methyl, profenofos , Prothiofos, pyraclofos, sulfopep, terbufos, tetrachlorvinphos, thiometon, trichlorfon.
A;アセチルコリンエステラーゼ(AChE)阻害剤
アラニカルブ(alanycarb)、アルジカルブ(aldicarb)、ベンダイオカルブ(bendiocarb)、ベンフラカルブ(benfuracarb)、ブトカルボキシム(butocarboxim)、カルバリル(carbaryl)、カルボフラン(carbofuran)、カルボスルファン(carbosulfan)、エチオフェンカルブ(ethiofencarb)、フェノブカルブ(fenobucarb)、ホルメタネート(formetanate)、フラチオカルブ(furathiocarb)、イソプロカルブ(isoprocarb)、メチオカルブ(methiocarb)、メソミル(methomyl)、ピリミカーブ(pirimicarb)、チオジカルブ(thiodicarb)、チオファノックス(thiofanox)、トリアザメート(triazamate)、
アセフェート(acephate)、アザメチホス(azamethiphos)、アジンホス-エチル(azinphos-ethyl)、アジンホス-メチル(azinphos-methyl)、クロルエトキシホス(chlorethoxyfos)、クロルフェンビンホス(chlorfenvinphos)、クロルメホス(chlormephos)、クロルピリホス(chlorpyrifos)、クロルピリホス-メチル(chlorpyrifos-methyl)、シアノホス(cyanophos)、ダイアジノン(diazinon)、ジクロルボス(dichlorvos)、ジメトエート(dimethoate)、ジメチルビンホス(dimethylvinphos)、ジスルフォトン(disulfoton)、イーピーエヌ(EPN)、フェニトロチオン(fenitrothion)、フェンチオン(fenthion)、イソキサチオン(isoxathion)、マラチオン(malathion)、メタミドホス(methamidophos)、メチダチオン(methidathion)、オメトエート(omethoate)、オキシジメトン-メチル(oxydemeton-methyl)、パラチオン-メチル(parathion-methyl)、フェントエート(phenthoate)、ホレート(phorate)、ホサロン(phosalone)、ホスメット(phosmet)、ホキシム(phoxim)、ピリミホス-メチル(pirimiphos-methyl)、プロフェノホス(profenofos)、プロチオホス(prothiofos)、ピラクロホス(pyraclofos)、スルホテップ(sulfotep)、テルブホス(terbufos)、テトラクロロビンホス(tetrachlorvinphos)、チオメトン(thiometon)、トリクロルホン(trichlorfon)。 Insecticides and acaricides:
A: Acetylcholinesterase (AChE) inhibitor Alanicarb, aldicarb, bendiocarb, benfuracarb, butofcarboxim, carbaryl, carbofuran, carbofuran Fan (carbosulfan), ethiofencarb, fenobucarb, formetanate, furathiocarb, isoprocarb, methiocarb, methomyl, pirimicarb, thiodicarb Thiofanox, triazamate,
Acephate, azamethiphos, azinphos-ethyl, azinphos-methyl, chlorethoxyfos, chlorfenvinphos, chlormephos, chlorpyrifos ( chlorpyrifos, chlorpyrifos-methyl, cyanophos, diazinon, dichlorvos, dimethoate, dimethylvinphos, disulfoton, EPN, fenitrothion (Fenitrothion), fenthion, isoxathion, malathion, methamidophos, methidathion, omethoate, oxydemeton-methy l), parathion-methyl, phenthoate, phorate, phosalone, phosmet, phoxim, pirimiphos-methyl, profenofos , Prothiofos, pyraclofos, sulfopep, terbufos, tetrachlorvinphos, thiometon, trichlorfon.
B;GABA作動性塩素イオンチャネルアンタゴニスト
エンドスルファン(endosulfan)、アルファ-エンドスルファン(alpha-endosulfan)、
エチプロール(ethiprole)、フィプロニル(fipronil)、フルフィプロール(flufiprole)、ピリプロール(pyriprole)、
アフォキソラネル(afoxolaner)、フルララネル(fluralaner)、ロチラネル(lotilaner)、サロラネル(sarolaner)。
C;ナトリウムチャネルモジュレーター
アクリナトリン(acrinathrin)、アレスリン(allethrin)、ベンフルトリン(benfluthrin)、ビフェントリン(bifenthrin)、カッパ-ビフェントリン(kappa-bifenthrin)、ビオアレスリン(bioallethrin)、ビオレスメトリン(bioresmethrin)、クロルプラレトリン(chloroprallethrin)、シクロプロトリン(cycloprothrin)、シフルトリン(cyfluthrin)、ベータ-シフルトリン(beta-cyfluthrin)、シハロトリン(cyhalothrin)、ガンマ-シハロトリン(gamma-cyhalothrin)、ラムダ-シハロトリン(lambda-cyhalothrin)、シペルメトリン(cypermethrin)、アルファ-シペルメトリン(alpha-cypermethrin)、ベータ-シペルメトリン(beta-cypermethrin)、ゼタ-シペルメトリン(zeta-cypermethrin)、シフェノトリン(cyphenothrin)、デルタメトリン(deltamethrin)、エンペントリン(empenthrin)、エスフェンバレレート(esfenvalerate)、エトフェンプロックス(etofenprox)、フェンプロパトリン(fenpropathrin)、フェンバレレート(fenvalerate)、フルシトリネート(flucythrinate)、フルメトリン(flumethrin)、フルバリネート(fluvalinate)、タウ-フルバリネート(tau-fluvalinate)、ハルフェンプロックス(halfenprox)、ヘプタフルトリン(heptafluthrin)、イミプロトリン(imiprothrin)、メペルフルトリン(meperfluthrin)、メトフルトリン(metofluthrin)、イプシロン-メトフルトリン(epsilon-metofluthrin)、モムフルオロトリン(momfluorothrin)、イプシロン-モムフルオロトリン(epsilon-momfluorothrin)、ペルメトリン(permethrin)、フェノトリン(phenothrin)、ピレトリン(pyrethrins)、レスメトリン(resmethrin)、シラフルオフェン(silafluofen)、テフルトリン(tefluthrin)、カッパ-テフルトリン(kappa-tefluthrin)、テトラメスリン(tetramethrin)、d-T-80-フタルスリン(d-tetramethrin)、テトラメチルフルスリン(tetramethylfluthrin)、トラロメトリン(tralomethrin)、トランスフルトリン(transfluthrin)、
メトキシクロル(methoxychlor)。 B: GABAergic chloride channel antagonist, endosulfan, alpha-endosulfan,
Ethiprole, fipronil, flufiprole, pyriprole,
Afoxolaner, fluralaner, lotilaner, sarolaner.
C: Sodium channel modulators acrinathrin, allethrin, benfluthrin, bifenthrin, kappa-bifenthrin, bioallethrin, bioresmethrin, chloropralethrin ), Cycloprothrin, cyfluthrin, beta-cyfluthrin, cyhalothrin, gamma-cyhalothrin, lambda-cyhalothrin, cypermethrin ), Alpha-cypermethrin, beta-cypermethrin, zeta-cypermethrin, cyphenothrin, deltamethrin Empenthrin, esfenvalerate, etofenprox, fenpropathrin, fenvalerate, flucytrinate, flumethrin, fluvalinate, Tau-fluvalinate, halfenprox, heptafluthrin, imiprothrin, meperfluthrin, metofluthrin, epsilon-metofluthrin, epsilon-metoflurin Thrin (momfluorothrin), epsilon-momfluorothrin, permethrin, phenothrin, pyrethrin, resmethrin, silafluofe (Silafluofen), tefluthrin, kappa-tefluthrin, tetramethrin, dT-80-phthalthrin (d-tetramethrin), tetramethylfluthrin, tralomethrin, trans Flutrin, transfluthrin,
Methoxychlor.
エンドスルファン(endosulfan)、アルファ-エンドスルファン(alpha-endosulfan)、
エチプロール(ethiprole)、フィプロニル(fipronil)、フルフィプロール(flufiprole)、ピリプロール(pyriprole)、
アフォキソラネル(afoxolaner)、フルララネル(fluralaner)、ロチラネル(lotilaner)、サロラネル(sarolaner)。
C;ナトリウムチャネルモジュレーター
アクリナトリン(acrinathrin)、アレスリン(allethrin)、ベンフルトリン(benfluthrin)、ビフェントリン(bifenthrin)、カッパ-ビフェントリン(kappa-bifenthrin)、ビオアレスリン(bioallethrin)、ビオレスメトリン(bioresmethrin)、クロルプラレトリン(chloroprallethrin)、シクロプロトリン(cycloprothrin)、シフルトリン(cyfluthrin)、ベータ-シフルトリン(beta-cyfluthrin)、シハロトリン(cyhalothrin)、ガンマ-シハロトリン(gamma-cyhalothrin)、ラムダ-シハロトリン(lambda-cyhalothrin)、シペルメトリン(cypermethrin)、アルファ-シペルメトリン(alpha-cypermethrin)、ベータ-シペルメトリン(beta-cypermethrin)、ゼタ-シペルメトリン(zeta-cypermethrin)、シフェノトリン(cyphenothrin)、デルタメトリン(deltamethrin)、エンペントリン(empenthrin)、エスフェンバレレート(esfenvalerate)、エトフェンプロックス(etofenprox)、フェンプロパトリン(fenpropathrin)、フェンバレレート(fenvalerate)、フルシトリネート(flucythrinate)、フルメトリン(flumethrin)、フルバリネート(fluvalinate)、タウ-フルバリネート(tau-fluvalinate)、ハルフェンプロックス(halfenprox)、ヘプタフルトリン(heptafluthrin)、イミプロトリン(imiprothrin)、メペルフルトリン(meperfluthrin)、メトフルトリン(metofluthrin)、イプシロン-メトフルトリン(epsilon-metofluthrin)、モムフルオロトリン(momfluorothrin)、イプシロン-モムフルオロトリン(epsilon-momfluorothrin)、ペルメトリン(permethrin)、フェノトリン(phenothrin)、ピレトリン(pyrethrins)、レスメトリン(resmethrin)、シラフルオフェン(silafluofen)、テフルトリン(tefluthrin)、カッパ-テフルトリン(kappa-tefluthrin)、テトラメスリン(tetramethrin)、d-T-80-フタルスリン(d-tetramethrin)、テトラメチルフルスリン(tetramethylfluthrin)、トラロメトリン(tralomethrin)、トランスフルトリン(transfluthrin)、
メトキシクロル(methoxychlor)。 B: GABAergic chloride channel antagonist, endosulfan, alpha-endosulfan,
Ethiprole, fipronil, flufiprole, pyriprole,
Afoxolaner, fluralaner, lotilaner, sarolaner.
C: Sodium channel modulators acrinathrin, allethrin, benfluthrin, bifenthrin, kappa-bifenthrin, bioallethrin, bioresmethrin, chloropralethrin ), Cycloprothrin, cyfluthrin, beta-cyfluthrin, cyhalothrin, gamma-cyhalothrin, lambda-cyhalothrin, cypermethrin ), Alpha-cypermethrin, beta-cypermethrin, zeta-cypermethrin, cyphenothrin, deltamethrin Empenthrin, esfenvalerate, etofenprox, fenpropathrin, fenvalerate, flucytrinate, flumethrin, fluvalinate, Tau-fluvalinate, halfenprox, heptafluthrin, imiprothrin, meperfluthrin, metofluthrin, epsilon-metofluthrin, epsilon-metoflurin Thrin (momfluorothrin), epsilon-momfluorothrin, permethrin, phenothrin, pyrethrin, resmethrin, silafluofe (Silafluofen), tefluthrin, kappa-tefluthrin, tetramethrin, dT-80-phthalthrin (d-tetramethrin), tetramethylfluthrin, tralomethrin, trans Flutrin, transfluthrin,
Methoxychlor.
D;ニコチン性アセチルコリン受容体(nAChR)アゴニスト
アセタミプリド(acetamiprid)、クロチアニジン(clothianidin)、ジノテフラン(dinotefuran)、イミダクロプリド(imidacloprid)、ニテンピラム(nitenpyram)、チアクロプリド(thiacloprid)、チアメトキサム(thiamethoxam)、
スルホキサフロール(sulfoxaflor)、
フルピラジフロン(flupyradifurone)。
E;ニコチン性アセチルコリン受容体(nAChR)アロステリックモジュレーター
スピネトラム(spinetoram)、スピノサド(spinosad)。
F;塩素イオンチャネルアクチベーター
アバメクチン(abamectin)、エマメクチンベンゾエート(emamectin-benzoate)、レピメクチン(lepimectin)、ミルベメクチン(milbemectin)。
G;幼若ホルモン類似剤
メソプレン(methoprene)、
フェノキシカルブ(fenoxycarb)、
ピリプロキシフェン(pyriproxyfen)。 D: Nicotinic acetylcholine receptor (nAChR) agonists acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, thiacloprid, thiamethoxam, thiamethoxam
Sulfoxaflor,
Flupyradifurone.
E; Nicotinic acetylcholine receptor (nAChR) allosteric modulators spinetoram, spinosad.
F: chloride ion channel activator abamectin, emamectin-benzoate, lepimectin, milbemectin.
G; juvenile hormone analog mesoprene,
Phenoxycarb,
Pyriproxyfen.
アセタミプリド(acetamiprid)、クロチアニジン(clothianidin)、ジノテフラン(dinotefuran)、イミダクロプリド(imidacloprid)、ニテンピラム(nitenpyram)、チアクロプリド(thiacloprid)、チアメトキサム(thiamethoxam)、
スルホキサフロール(sulfoxaflor)、
フルピラジフロン(flupyradifurone)。
E;ニコチン性アセチルコリン受容体(nAChR)アロステリックモジュレーター
スピネトラム(spinetoram)、スピノサド(spinosad)。
F;塩素イオンチャネルアクチベーター
アバメクチン(abamectin)、エマメクチンベンゾエート(emamectin-benzoate)、レピメクチン(lepimectin)、ミルベメクチン(milbemectin)。
G;幼若ホルモン類似剤
メソプレン(methoprene)、
フェノキシカルブ(fenoxycarb)、
ピリプロキシフェン(pyriproxyfen)。 D: Nicotinic acetylcholine receptor (nAChR) agonists acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, thiacloprid, thiamethoxam, thiamethoxam
Sulfoxaflor,
Flupyradifurone.
E; Nicotinic acetylcholine receptor (nAChR) allosteric modulators spinetoram, spinosad.
F: chloride ion channel activator abamectin, emamectin-benzoate, lepimectin, milbemectin.
G; juvenile hormone analog mesoprene,
Phenoxycarb,
Pyriproxyfen.
H;半翅目選択的摂食阻害剤
ピメトロジン(pymetrozine)、
フロニカミド(flonicamid)。
I;ダニ類成長阻害剤
クロフェンテジン(clofentezine)、ヘキシチアゾクス(hexythiazox)、
エトキサゾール(etoxazole)。
J;微生物由来昆虫中腸内膜破壊剤
バチルスチューリンギエンシス(bacillus thuringiensis, subsp.israelensis, subsp.aizawai, subsp.kurstaki, subsp.tenebrionis等)
K;ミトコンドリアATP合成酵素阻害剤
ジアフェンチウロン(diafenthiuron)、
アゾシクロチン(azocyclotin)、フェンブタチン-オキシド(fenbutatin oxide)、
プロパルギット(propargite)。
L;酸化的リン酸化脱共役剤
クロルフェナピル(chlorfenapyr)、
M;ニコチン性アセチルコリン受容体(nAChR)チャネルブロッカー
ベンスルタップ(bensultap)、カルタップ(cartap)、チオシクラム(thiocyclam)。 H; Hemiptera selective feeding inhibitor pymetrozine,
Flonicamid.
I: mite growth inhibitor clofentezine, hexythiazox,
Etoxazole.
J: Bacillus thuringiensis, subsp.israelensis, subsp.aizawai, subsp.kurstaki, subsp.tenebrionis, etc.
K; mitochondrial ATP synthase inhibitor diafenthiuron,
Azocyclotin, fenbutatin oxide,
Propargite.
L: oxidative phosphorylation uncoupler, chlorfenapyr,
M: nicotinic acetylcholine receptor (nAChR) channel blocker bensultap, cartap, thiocyclam.
ピメトロジン(pymetrozine)、
フロニカミド(flonicamid)。
I;ダニ類成長阻害剤
クロフェンテジン(clofentezine)、ヘキシチアゾクス(hexythiazox)、
エトキサゾール(etoxazole)。
J;微生物由来昆虫中腸内膜破壊剤
バチルスチューリンギエンシス(bacillus thuringiensis, subsp.israelensis, subsp.aizawai, subsp.kurstaki, subsp.tenebrionis等)
K;ミトコンドリアATP合成酵素阻害剤
ジアフェンチウロン(diafenthiuron)、
アゾシクロチン(azocyclotin)、フェンブタチン-オキシド(fenbutatin oxide)、
プロパルギット(propargite)。
L;酸化的リン酸化脱共役剤
クロルフェナピル(chlorfenapyr)、
M;ニコチン性アセチルコリン受容体(nAChR)チャネルブロッカー
ベンスルタップ(bensultap)、カルタップ(cartap)、チオシクラム(thiocyclam)。 H; Hemiptera selective feeding inhibitor pymetrozine,
Flonicamid.
I: mite growth inhibitor clofentezine, hexythiazox,
Etoxazole.
J: Bacillus thuringiensis, subsp.israelensis, subsp.aizawai, subsp.kurstaki, subsp.tenebrionis, etc.
K; mitochondrial ATP synthase inhibitor diafenthiuron,
Azocyclotin, fenbutatin oxide,
Propargite.
L: oxidative phosphorylation uncoupler, chlorfenapyr,
M: nicotinic acetylcholine receptor (nAChR) channel blocker bensultap, cartap, thiocyclam.
N;キチン生合成阻害剤(タイプ0)
ビストリフルロン(bistrifluron)、クロルフルアズロン(chlorfluazuron)、ジフルベンズロン(diflubenzuron)、フルシクロクスロン(flucycloxuron)、フルフェノクスロン(flufenoxuron)、ヘキサフルムロン(hexaflumuron)、ルフェヌロン(lufenuron)、ノバルロン(novaluron)、ノビフルムロン(noviflumuron)、テフルベンズロン(teflubenzuron)、トリフルムロン(triflumuron)。
O;キチン生合成阻害剤(タイプ1)
ブプロフェジン(buprofezin)。 N: chitin biosynthesis inhibitor (type 0)
Bistrifluron, chlorfluazuron, diflubenzuron, flucycloxuron, flufenoxuron, hexaflumuron, lufenuron, novaluron ), Noviflumuron, teflubenzuron, triflumuron.
O: Chitin biosynthesis inhibitor (type 1)
Buprofezin.
ビストリフルロン(bistrifluron)、クロルフルアズロン(chlorfluazuron)、ジフルベンズロン(diflubenzuron)、フルシクロクスロン(flucycloxuron)、フルフェノクスロン(flufenoxuron)、ヘキサフルムロン(hexaflumuron)、ルフェヌロン(lufenuron)、ノバルロン(novaluron)、ノビフルムロン(noviflumuron)、テフルベンズロン(teflubenzuron)、トリフルムロン(triflumuron)。
O;キチン生合成阻害剤(タイプ1)
ブプロフェジン(buprofezin)。 N: chitin biosynthesis inhibitor (type 0)
Bistrifluron, chlorfluazuron, diflubenzuron, flucycloxuron, flufenoxuron, hexaflumuron, lufenuron, novaluron ), Noviflumuron, teflubenzuron, triflumuron.
O: Chitin biosynthesis inhibitor (type 1)
Buprofezin.
P;脱皮阻害剤(ハエ目昆虫)
シロマジン(cyromazine)
Q;脱皮ホルモン(エクダイソン)受容体アゴニスト
クロマフェノジド(chromafenozide)、ハロフェノジド(halofenozide)、メトキシフェノジド(methoxyfenozide)、テブフェノジド(tebufenozide)。
R;オクトパミン受容体アゴニスト
アミトラズ(amitraz)
S;ミトコンドリア電子伝達系複合体III阻害剤
ヒドラメチルノン(hydramethylnon)、
アセキノシル(acequinocyl)、
フルアクリピリム(fluacrypyrim)。 P: molting inhibitor (fly insect)
Cyromazine
Q; molting hormone (ecdysone) receptor agonists chromafenozide, halofenozide, methoxyfenozide, tebufenozide.
R: Octopamine receptor agonist amitraz
S: Mitochondrial electron transport system complex III inhibitor hydramethylnon,
Acequinocyl,
Fluacrypyrim.
シロマジン(cyromazine)
Q;脱皮ホルモン(エクダイソン)受容体アゴニスト
クロマフェノジド(chromafenozide)、ハロフェノジド(halofenozide)、メトキシフェノジド(methoxyfenozide)、テブフェノジド(tebufenozide)。
R;オクトパミン受容体アゴニスト
アミトラズ(amitraz)
S;ミトコンドリア電子伝達系複合体III阻害剤
ヒドラメチルノン(hydramethylnon)、
アセキノシル(acequinocyl)、
フルアクリピリム(fluacrypyrim)。 P: molting inhibitor (fly insect)
Cyromazine
Q; molting hormone (ecdysone) receptor agonists chromafenozide, halofenozide, methoxyfenozide, tebufenozide.
R: Octopamine receptor agonist amitraz
S: Mitochondrial electron transport system complex III inhibitor hydramethylnon,
Acequinocyl,
Fluacrypyrim.
T;ミトコンドリア電子伝達系複合体I阻害剤
フェナザキン(fenazaquin)、フェンピロキシメート(fenpyroximate)、ピリミジフェン(pyrimidifen)、ピリダベン(pyridaben)、テブフェンピラド(tebufenpyrad)、トルフェンピラド(tolfenpyrad)、
ロテノン(rotenone)。
U;電位依存性ナトリウムチャネルブロッカー
インドキサカルブ(indoxacarb)、インドキサカルブ-MP(indoxacarb-MP)、
メタフルミゾン(metaflumizone)。
V;アセチルCoAカルボキシラーゼ阻害剤
スピロジクロフェン(spirodiclofen)、スピロメシフェン(spiromesifen)、スピロテトラマト(spirotetramat)。 T: Mitochondrial electron transport complex I inhibitor fenazaquin, fenpyroximate, pyrimidifen, pyridaben, tebufenpyrad, tolfenpyrad, tolfenpyrad,
Rotenone.
U; voltage-gated sodium channel blocker indoxacarb (indoxacarb), indoxacarb-MP (indoxacarb-MP),
Metaflumizone.
V; acetyl CoA carboxylase inhibitor spirodiclofen, spiromesifen, spirotetramat.
フェナザキン(fenazaquin)、フェンピロキシメート(fenpyroximate)、ピリミジフェン(pyrimidifen)、ピリダベン(pyridaben)、テブフェンピラド(tebufenpyrad)、トルフェンピラド(tolfenpyrad)、
ロテノン(rotenone)。
U;電位依存性ナトリウムチャネルブロッカー
インドキサカルブ(indoxacarb)、インドキサカルブ-MP(indoxacarb-MP)、
メタフルミゾン(metaflumizone)。
V;アセチルCoAカルボキシラーゼ阻害剤
スピロジクロフェン(spirodiclofen)、スピロメシフェン(spiromesifen)、スピロテトラマト(spirotetramat)。 T: Mitochondrial electron transport complex I inhibitor fenazaquin, fenpyroximate, pyrimidifen, pyridaben, tebufenpyrad, tolfenpyrad, tolfenpyrad,
Rotenone.
U; voltage-gated sodium channel blocker indoxacarb (indoxacarb), indoxacarb-MP (indoxacarb-MP),
Metaflumizone.
V; acetyl CoA carboxylase inhibitor spirodiclofen, spiromesifen, spirotetramat.
W;ミトコンドリア電子伝達系複合体II阻害剤
シエノピラフェン(cyenopyrafen)、シフルメトフェン(cyflumetofen)。
X;リアノジン受容体モジュレーター
クロルアントラニリプロール(chlorantraniliprole)、シアントラニリプロール(cyantraniliprole)、シクラニリプロール(cyclaniliprole)、シハロジアミド(cyhalodiamide)、フルベンジアミド(flubendiamide)、テトラニリプロール(tetraniliprole)。
UN;作用機作不明及びその他の薬剤
アザジラクチン(azadirachtin)、ベンゾキシメート(benzoximate)、ビフェナゼート(bifenazate)、ブロモプロピレート(bromopropylate)、ジコホール(dicofol)、ピリダリル(pyridalyl)、ピリフルキナゾン(pyrifluquinazon)、
アシノナピル(acynonapyr)、アフィドピロペン(afidopyropen)、ベンズピリモキサン(benzpyrimoxan)、ブロフラニリド(broflanilide)、ジクロロメゾチアズ(dicloromezotiaz)、フロメトキン(flometoquin)、フルヘキサホン(fluhexafon)、フルキサメタミド(fluxametamide)、ピフルブミド(pyflubumide)、トリフルメゾピリム(triflumezopyrim)、ME5382(試験名)、NC-515(試験名)及びZDI2501(試験名)。 W: Mitochondrial electron transport system complex II inhibitor cyenopyrafen, cyflumetofen.
X: ryanodine receptor modulators chlorantraniliprole, cyantraniliprole, cyclaniliprole, cyhalodiamide, flubendiamide, tetraniliprole.
UN; unknown mechanism of action and other drugs azadirachtin, benzoximate, bifenazate, bromopropylate, dicohol, pyridalyl, pyrifluquinazon,
Acynonapyr, afidopyropen, benzpyrimoxan, broflanilide, dicloromezotiaz, flometoquin, fluhexafon, fluxametamide, fluxametamide , Triflumezopyrim, ME5382 (test name), NC-515 (test name) and ZDI2501 (test name).
シエノピラフェン(cyenopyrafen)、シフルメトフェン(cyflumetofen)。
X;リアノジン受容体モジュレーター
クロルアントラニリプロール(chlorantraniliprole)、シアントラニリプロール(cyantraniliprole)、シクラニリプロール(cyclaniliprole)、シハロジアミド(cyhalodiamide)、フルベンジアミド(flubendiamide)、テトラニリプロール(tetraniliprole)。
UN;作用機作不明及びその他の薬剤
アザジラクチン(azadirachtin)、ベンゾキシメート(benzoximate)、ビフェナゼート(bifenazate)、ブロモプロピレート(bromopropylate)、ジコホール(dicofol)、ピリダリル(pyridalyl)、ピリフルキナゾン(pyrifluquinazon)、
アシノナピル(acynonapyr)、アフィドピロペン(afidopyropen)、ベンズピリモキサン(benzpyrimoxan)、ブロフラニリド(broflanilide)、ジクロロメゾチアズ(dicloromezotiaz)、フロメトキン(flometoquin)、フルヘキサホン(fluhexafon)、フルキサメタミド(fluxametamide)、ピフルブミド(pyflubumide)、トリフルメゾピリム(triflumezopyrim)、ME5382(試験名)、NC-515(試験名)及びZDI2501(試験名)。 W: Mitochondrial electron transport system complex II inhibitor cyenopyrafen, cyflumetofen.
X: ryanodine receptor modulators chlorantraniliprole, cyantraniliprole, cyclaniliprole, cyhalodiamide, flubendiamide, tetraniliprole.
UN; unknown mechanism of action and other drugs azadirachtin, benzoximate, bifenazate, bromopropylate, dicohol, pyridalyl, pyrifluquinazon,
Acynonapyr, afidopyropen, benzpyrimoxan, broflanilide, dicloromezotiaz, flometoquin, fluhexafon, fluxametamide, fluxametamide , Triflumezopyrim, ME5382 (test name), NC-515 (test name) and ZDI2501 (test name).
殺線虫剤:カズサホス(cadusafos)、ジクロフェンチオン(dichlofenthion)、エトプロホス(ethoprophos)、フェナミホス(fenamiphos)、フルエンスルフォン(fluensulfone)、フルアザインドリジン(fluazaindolizine)、ホスチアゼート(fosthiazate)、フォスチエタン(fosthietan)、イミシアホス(imicyafos)、イサミドホス(isamidofos)、イサゾホス (isazofos)、臭化メチル(methyl bromide)、メチルイソチオシアネート(methyl isothiocyanate)、オキサミル(oxamyl)、アジ化ナトリウム(sodium azide)、チオキサザフェン(tioxazafen)、BYI-1921(試験名)及びMAI-08015(試験名)。
Nematicides: cadusafos, dichlofenthion, ethoprophos, fenamiphos, fluensulfone, fluazaindolizine, fosthiazate, fosthiazate, fostiatan, fothiatan (Imicyafos), isamidofos, isazofos, methyl bromide, methyl isothiocyanate, oxamyl, sodium azide, tioxazafen, BYI- 1921 (study name) and MAI-08015 (study name).
駆虫剤:アクリフラビン(acriflavine)、アルベンダゾール(albendazole)、アトバコン(atovaguone)、アジスロマイシン(azithromycin)、ビチオノール(bithionol)、ブロムフェノホス(bromofenofos)、カンベンダゾール(cambendazole)、カルニダゾール(carnidazole)、クロロキン(chloroquine)、クラズリル(clazuril)、クリンダマイシン(clindamycin hydrochloride)、クロルスロン(clorsulon)、クロサンテル(closantel)、クマホス(coumaphos)、シミアゾル(cymiazol)、ジクロロフェン(dichlorophen)、ジエチルカルバマジン(diethylcarbamazine)、ジミナゼン(diminazene)、ジソフェノール(disophenol)、ヨウ化ジチアザニン(dithiazanine iodide)、ドキシサイクリン(doxycycline hydrochloride)、ドラメクチン(doramectin)、エモデプシド(emodepside)、エプリノメクチン(eprinomectin)、フェバンテル(febantel)、フェンベンダゾール(fenbendazole)、フルベンダゾール(flubendazole)、フラゾリドン(furazolidone)、グリカルピラミド(glycalpyramide)、イミドカルブ(imidocarb)、イベルメクチン(ivermectin)、レバミゾール(levamisole)、メベンダゾール(mebendazole)、メフロキン(mefloquine)、メラルソミン二塩酸塩(melarsamine hydrochloride)、メトロニダゾール(metronidazole)、メチリジン(metyridine)、ミルベマイシンオキシム(milbemycin oxime)、モネパンテル(monepantel)、酒石酸モランテル(morantel tartrate)、モキシデクチン(moxidectin)、ナイカルバジン(nicarbazin)、ニクロサミド(niclosamide)、ニトロスカネート(nitroscanate)、ニトロキシニル(nitroxynil)、オムファロチン(omphalotin)、パモ酸オキサンテル(oxantel pamoate)、酒石酸オキサンテル(oxantel tartrate)、オクスフェンダゾール(oxfendazolee)、オキシベンダゾール(oxibendazole)、オキシクロザニド(oxyclozanide)、パマキン(pamaquine)、フェノチアジン(phenothiazine)、アジピン酸ピペラジン(piperazine adipate)、クエン酸ピペラジン(piperazine citrate)、リン酸ピペラジン(piperazine phosphate)、PNU-97333(paraherquamide A)、PNU-141962(2-deoxyparaherquamide)、プラジクアンテル(praziquantel)、プリマキン(primaquine)、プロペタムホス(propetamphos)、プロポクスル(propoxur)、パモ酸ピランテル(pyrantel pamoate)、ピリメタミン(pyrimethamine)、サントニン(santonin)、セラメクチン(selamectin)、スルファジメトキシン(sulfadimethoxine)、スルファドキシン(sulfadoxine)、スルファメラジン(sulfamerazine)、スルファモノメトキシン(sulfamonomethoxine)、スルファモイルダプソン(sulfamoildapsone)、チアベンダゾール(thiabendazole)、チニダゾール(tinidazole)、トルトラズリル(toltrazuril)、トリブロムサラン(tribromsalan)、トリクラベンダゾール(triclabendazole)。
抗真菌剤:クリムバゾール(climbazole)、ケトコナゾール(ketoconazole)及びミコナゾール硝酸塩(miconazole nitrate)。 Anthelmintic: acriflavine, albendazole, atovaguone, azithromycin, bithionol, bromfenofos, cambendazole, carnidazole, chloroquine chloroquine, clazuril, clindamycin hydrochloride, clorsulon, closantel, coumaphos, cymiazol, dichlorophen, dichlorophen, diethylcarbamazine, diminazen (Diminazene), disophenol, dithiazanine iodide, doxycycline hydrochloride, doramectin, emodepside, eprinomecti (eprinomecti) n), febantel, fenbendazole, flubendazole, furazolidone, glycalpyramide, imidocarb, ivermectin, levamisole, mebendazole ( mebendazole, mefloquine, melarsamine dihydrochloride (melarsamine hydrochloride), metronidazole, metyridine, milbemycin oxime, monepantel, morantel tartrate, moxidectin , Nicarbazin, niclosamide, nitroscanate, nitroxynil, omphalotin, oxantel pamoate, tartar Oxantel tartrate, oxfendazolee, oxibendazole, oxyclozanide, pamaquine, phenothiazine, piperazine adipate, piperazine citrate , Piperazine phosphate, PNU-97333 (paraherquamide A), PNU-141962 (2-deoxyparaherquamide), praziquantel, primaquine, propetamphos, propoxur, pirantel pamoate pyrantel pamoate, pyrimethamine, santonin, selamectin, sulfadimethoxine, sulfadoxine, sulfamerazine, sulphamerazine Fan monomethoxy emissions (sulfamonomethoxine), sulfamoyl dapsone (sulfamoildapsone), thiabendazole (thiabendazole), tinidazole (tinidazole), toltrazuril (toltrazuril), tri bromine Saran (tribromsalan), triclabendazole (triclabendazole).
Antifungal agents: climbazole, ketoconazole and miconazole nitrate.
抗真菌剤:クリムバゾール(climbazole)、ケトコナゾール(ketoconazole)及びミコナゾール硝酸塩(miconazole nitrate)。 Anthelmintic: acriflavine, albendazole, atovaguone, azithromycin, bithionol, bromfenofos, cambendazole, carnidazole, chloroquine chloroquine, clazuril, clindamycin hydrochloride, clorsulon, closantel, coumaphos, cymiazol, dichlorophen, dichlorophen, diethylcarbamazine, diminazen (Diminazene), disophenol, dithiazanine iodide, doxycycline hydrochloride, doramectin, emodepside, eprinomecti (eprinomecti) n), febantel, fenbendazole, flubendazole, furazolidone, glycalpyramide, imidocarb, ivermectin, levamisole, mebendazole ( mebendazole, mefloquine, melarsamine dihydrochloride (melarsamine hydrochloride), metronidazole, metyridine, milbemycin oxime, monepantel, morantel tartrate, moxidectin , Nicarbazin, niclosamide, nitroscanate, nitroxynil, omphalotin, oxantel pamoate, tartar Oxantel tartrate, oxfendazolee, oxibendazole, oxyclozanide, pamaquine, phenothiazine, piperazine adipate, piperazine citrate , Piperazine phosphate, PNU-97333 (paraherquamide A), PNU-141962 (2-deoxyparaherquamide), praziquantel, primaquine, propetamphos, propoxur, pirantel pamoate pyrantel pamoate, pyrimethamine, santonin, selamectin, sulfadimethoxine, sulfadoxine, sulfamerazine, sulphamerazine Fan monomethoxy emissions (sulfamonomethoxine), sulfamoyl dapsone (sulfamoildapsone), thiabendazole (thiabendazole), tinidazole (tinidazole), toltrazuril (toltrazuril), tri bromine Saran (tribromsalan), triclabendazole (triclabendazole).
Antifungal agents: climbazole, ketoconazole and miconazole nitrate.
抗菌剤:アモキシシリン(amoxicillin)、アンピシリン(ampicillin)、ベトキサジン(bethoxazin)、ビチオノール(bithionol)、ブロノポール(bronopol)、セファピリン(cefapirin)、セファゾリン(cefazolin)、セフキノム(cefquinome)、セフチオフル(ceftiofur)、クロルテトラサイクリン(chlortetracycline)、クラブラン酸(clavulanic acid)、ダノフロキサシン(danofloxacin)、ジフロキサシン(difloxacin)、ジニトルミド(dinitolmide)、エンロフロキサシン(enrofloxacin)、フロルフェニコール(florfenicol)、リンコマイシン(lincomycin)、ロメフロキサシン(lomefloxacin)、マルボフロキサシン(marbofloxacin)、ミロキサシン(miloxacin)、ミロサマイシン(mirosamycin)、ニトラピリン(nitrapyrin)、ノルフロキサシン(norfloxacin)、オクチリノン(octhilinone)、オフロキサシン(ofloxacin)、オルビフロキサシン(orbifloxacin)、オキソリニック酸(oxolinic acid)、オキシテトラサイクリン(oxytetracycline)、ペニシリン(penicillin)、ストレプトマイシン(streptomycin)、チアンフェニコール(thiamphenicol)、フマル酸チアムリン(tiamulin fumarate)、リン酸チルミコシン(tilmicosin phosphate)、酢酸イソ吉草酸タイロシン(acetylisovaleryltylosin)、リン酸タイロシン(tylosin phosphate)、ツラスロマイシン(tulathromycin)、バルネムリン(valnemulin)、貝殻焼成カルシウム(酸化カルシウム)、タラロマイセス属菌、トリコデルマ属菌、コニオチリウム属菌。
Antibacterial agents: amoxicillin, ampicillin, bethoxazin, bithionol, bronopol, cefapirin, cefazolin, cefquinome, cefquinome, cefurthiofluce (Chlortetracycline), clavulanic acid, danofloxacin, difloxacin, dinitrmide, enrofloxacin, florfenicol, lincomycin, lomefloxacin lomefloxacin, marbofloxacin, miloxacin, mirosamycin, nitrapyrin, norfloxacin, octhilinone, off Ofloxacin, orbifloxacin, oxolinic acid, oxytetracycline, penicillin, streptomycin, thiamphenicol, tiamulin fumarate , Tilmicosin phosphate, tyrosin phosphate, tylosin phosphate, tulathromycin, valnemulin, shell calcined calcium (calcium oxide), taralomyces, Trichoderma spp., Coniotylium spp.
以下に本発明化合物の合成例、試験例を実施例として具体的に述べることで、本発明をさらに詳しく説明するが、本発明はこれらによって限定されるものではない。
Hereinafter, the present invention will be described in more detail by specifically describing the synthesis examples and test examples of the compounds of the present invention as examples, but the present invention is not limited thereto.
[合成例]
合成例1
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-004)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル2.00gのテトラヒドロフラン40ml溶液に、-78℃にて攪拌下、リチウムビス(トリメチルシリル)アミドの1.3Mテトラヒドロフラン溶液16mlを滴下し、滴下終了後、同温度にて30分間攪拌した。次いで、この反応混合物にN-フルオロベンゼンスルホンイミド6.00gのテトラヒドロフラン25ml溶液を滴下し、滴下終了後、同温度にてさらに3時間攪拌を継続した。反応完結後、反応混合物に水50mlを加えヘキサンにて抽出(50mlx3)した。有機層を併せ水洗(50mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物を、シリカゲルカラムクロマトグラフィー{酢酸エチル-ヘキサン[0:100~5:95(体積比、以下同様である。)のグラジエント]}にて精製し、目的物0.81gを淡黄色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.66 (d, J=2.1Hz, 1H), 8.08 (d, J=2.1Hz, 1H)。 [Synthesis example]
Synthesis example 1
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) benzamide (Compound No. 1 of the present invention) 1-004).
Step 1: Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroacetonitrile 2- (5-Bromo-3-chloropyridin-2-yl) acetonitrile 2.00 g of tetrahydrofuran To the 40 ml solution, 16 ml of a 1.3M tetrahydrofuran solution of lithium bis (trimethylsilyl) amide was added dropwise with stirring at −78 ° C. After completion of the dropwise addition, the mixture was stirred at the same temperature for 30 minutes. Next, 25 ml of a tetrahydrofuran solution containing 6.00 g of N-fluorobenzenesulfonimide was added dropwise to the reaction mixture. After completion of the dropwise addition, stirring was continued for 3 hours at the same temperature. After completion of the reaction, 50 ml of water was added to the reaction mixture and extracted with hexane (50 ml × 3). The organic layers were combined and washed with water (50 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography {ethyl acetate-hexane [gradient of 0: 100 to 5:95 (volume ratio, the same applies hereinafter)]} to obtain 0.81 g of the desired product as a pale yellow oily substance. Got as.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.66 (d, J = 2.1 Hz, 1H), 8.08 (d, J = 2.1 Hz, 1H).
合成例1
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-004)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル2.00gのテトラヒドロフラン40ml溶液に、-78℃にて攪拌下、リチウムビス(トリメチルシリル)アミドの1.3Mテトラヒドロフラン溶液16mlを滴下し、滴下終了後、同温度にて30分間攪拌した。次いで、この反応混合物にN-フルオロベンゼンスルホンイミド6.00gのテトラヒドロフラン25ml溶液を滴下し、滴下終了後、同温度にてさらに3時間攪拌を継続した。反応完結後、反応混合物に水50mlを加えヘキサンにて抽出(50mlx3)した。有機層を併せ水洗(50mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物を、シリカゲルカラムクロマトグラフィー{酢酸エチル-ヘキサン[0:100~5:95(体積比、以下同様である。)のグラジエント]}にて精製し、目的物0.81gを淡黄色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.66 (d, J=2.1Hz, 1H), 8.08 (d, J=2.1Hz, 1H)。 [Synthesis example]
Synthesis example 1
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) benzamide (Compound No. 1 of the present invention) 1-004).
Step 1: Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroacetonitrile 2- (5-Bromo-3-chloropyridin-2-yl) acetonitrile 2.00 g of tetrahydrofuran To the 40 ml solution, 16 ml of a 1.3M tetrahydrofuran solution of lithium bis (trimethylsilyl) amide was added dropwise with stirring at −78 ° C. After completion of the dropwise addition, the mixture was stirred at the same temperature for 30 minutes. Next, 25 ml of a tetrahydrofuran solution containing 6.00 g of N-fluorobenzenesulfonimide was added dropwise to the reaction mixture. After completion of the dropwise addition, stirring was continued for 3 hours at the same temperature. After completion of the reaction, 50 ml of water was added to the reaction mixture and extracted with hexane (50 ml × 3). The organic layers were combined and washed with water (50 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography {ethyl acetate-hexane [gradient of 0: 100 to 5:95 (volume ratio, the same applies hereinafter)]} to obtain 0.81 g of the desired product as a pale yellow oily substance. Got as.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.66 (d, J = 2.1 Hz, 1H), 8.08 (d, J = 2.1 Hz, 1H).
工程2;2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロエチルアミンの製造
窒素雰囲気下の2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリル810mgのジクロロメタン20ml溶液に、-78℃にて攪拌下、ジイソブチルアルミニウムヒドリドの1.0Mヘキサン溶液9mlを滴下し、滴下終了後、同温度にて2時間攪拌した。次いで、反応混合物に酒石酸カリウムナトリウム(ロッシェル塩)飽和水溶液20ml及びジクロロメタン20mlを添加し、室温にてさらに2時間攪拌を継続した。反応完結後、反応混合物に水30mlを加え有機層を分取し、得られた有機層を飽和ロッシェル塩水溶液(20mlx1)、次いで水(20mlx2)にて洗浄した後、減圧下にて溶媒を留去、粗製の目的物607mgを褐色油状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.56 (d, J=2.1Hz, 1H), 8.00 (d, J=2.1Hz, 1H), 3.49 (t, J=13.8Hz, 2H)。 Step 2: Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethylamine 2- (5-Bromo-3-chloropyridin-2-yl) -2 under nitrogen atmosphere , 2-Difluoroacetonitrile (810 mg) in dichloromethane (20 ml) was added dropwise with stirring at -78 ° C., and 9 ml of a 1.0 M hexane solution of diisobutylaluminum hydride was added dropwise. Then, 20 ml of a saturated aqueous solution of potassium sodium tartrate (Rochelle salt) and 20 ml of dichloromethane were added to the reaction mixture, and stirring was continued for another 2 hours at room temperature. After completion of the reaction, 30 ml of water was added to the reaction mixture, and the organic layer was separated. The obtained organic layer was washed with a saturated Rochelle salt aqueous solution (20 ml × 1) and then with water (20 ml × 2), and then the solvent was distilled off under reduced pressure. Lastly, 607 mg of the crude target product was obtained as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.56 (d, J = 2.1 Hz, 1H), 8.00 (d, J = 2.1 Hz, 1H), 3.49 (t, J = 13.8 Hz, 2H) .
窒素雰囲気下の2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリル810mgのジクロロメタン20ml溶液に、-78℃にて攪拌下、ジイソブチルアルミニウムヒドリドの1.0Mヘキサン溶液9mlを滴下し、滴下終了後、同温度にて2時間攪拌した。次いで、反応混合物に酒石酸カリウムナトリウム(ロッシェル塩)飽和水溶液20ml及びジクロロメタン20mlを添加し、室温にてさらに2時間攪拌を継続した。反応完結後、反応混合物に水30mlを加え有機層を分取し、得られた有機層を飽和ロッシェル塩水溶液(20mlx1)、次いで水(20mlx2)にて洗浄した後、減圧下にて溶媒を留去、粗製の目的物607mgを褐色油状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.56 (d, J=2.1Hz, 1H), 8.00 (d, J=2.1Hz, 1H), 3.49 (t, J=13.8Hz, 2H)。 Step 2: Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethylamine 2- (5-Bromo-3-chloropyridin-2-yl) -2 under nitrogen atmosphere , 2-Difluoroacetonitrile (810 mg) in dichloromethane (20 ml) was added dropwise with stirring at -78 ° C., and 9 ml of a 1.0 M hexane solution of diisobutylaluminum hydride was added dropwise. Then, 20 ml of a saturated aqueous solution of potassium sodium tartrate (Rochelle salt) and 20 ml of dichloromethane were added to the reaction mixture, and stirring was continued for another 2 hours at room temperature. After completion of the reaction, 30 ml of water was added to the reaction mixture, and the organic layer was separated. The obtained organic layer was washed with a saturated Rochelle salt aqueous solution (20 ml × 1) and then with water (20 ml × 2), and then the solvent was distilled off under reduced pressure. Lastly, 607 mg of the crude target product was obtained as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.56 (d, J = 2.1 Hz, 1H), 8.00 (d, J = 2.1 Hz, 1H), 3.49 (t, J = 13.8 Hz, 2H) .
工程3;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロエチル]カルバミド酸-tert-ブチルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロエチルアミン607mg及びトリエチルアミン271mgの、ジクロロメタン5ml溶液に、二炭酸ジ-tert-ブチル584mgを添加し、室温にて2時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(0:10~1:9のグラジエント)]にて精製し、目的物430mgを淡黄色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55 (d, J=2.1Hz, 1H), 8.00 (d, J=2.1Hz, 1H), 5.05 (bs, 1H), 4.0-4.2 (m, 2H), 1.40 (s, 9H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridine- To a solution of 2-yl) -2,2-difluoroethylamine (607 mg) and triethylamine (271 mg) in dichloromethane (5 ml) was added di-tert-butyl dicarbonate (584 mg), and the mixture was stirred at room temperature for 2 hours. After completion of the reaction, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography [ethyl acetate-hexane (0:10 to 1: 9 gradient)] to give 430 mg of the desired product as a pale yellow oil. Obtained as material.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55 (d, J = 2.1 Hz, 1H), 8.00 (d, J = 2.1 Hz, 1H), 5.05 (bs, 1H), 4.0-4.2 ( m, 2H), 1.40 (s, 9H).
2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロエチルアミン607mg及びトリエチルアミン271mgの、ジクロロメタン5ml溶液に、二炭酸ジ-tert-ブチル584mgを添加し、室温にて2時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(0:10~1:9のグラジエント)]にて精製し、目的物430mgを淡黄色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55 (d, J=2.1Hz, 1H), 8.00 (d, J=2.1Hz, 1H), 5.05 (bs, 1H), 4.0-4.2 (m, 2H), 1.40 (s, 9H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridine- To a solution of 2-yl) -2,2-difluoroethylamine (607 mg) and triethylamine (271 mg) in dichloromethane (5 ml) was added di-tert-butyl dicarbonate (584 mg), and the mixture was stirred at room temperature for 2 hours. After completion of the reaction, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography [ethyl acetate-hexane (0:10 to 1: 9 gradient)] to give 430 mg of the desired product as a pale yellow oil. Obtained as material.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55 (d, J = 2.1 Hz, 1H), 8.00 (d, J = 2.1 Hz, 1H), 5.05 (bs, 1H), 4.0-4.2 ( m, 2H), 1.40 (s, 9H).
工程4;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチルの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル1.50gのN,N-ジメチルホルムアミド4ml溶液に、トリエチルアミン1.64g、1-エチニル-4-フルオロベンゼン631mg、ヨウ化銅(I)231mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)283mgを添加し、窒素雰囲気下、室温にて2時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液15mlを添加し、酢酸エチルにて抽出(20mlx1)した。得られた有機層を水洗(10mlx2)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.40gを白色結晶として得た。
融点107.0~108.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.57 (d, J=1.5Hz, 1H), 7.92 (d, J=1.5Hz, 1H), 7.5-7.6 (m, 2H), 7.05-7.15 (m, 2H), 5.10 (bs, 1H), 4.0-4.2 (m, 2H), 1.41 (s, 9H)。 Step 4: Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl N- [2 -(5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethyl] carbamic acid-tert-butyl 1.50 g of N, N-dimethylformamide in 4 ml of solution was added 1.64 g of triethylamine, 1- Ethinyl-4-fluorobenzene 631 mg, copper (I) iodide 231 mg, and dichlorobis (triphenylphosphine) palladium (II) 283 mg were added, and the mixture was stirred at room temperature for 2 hours under a nitrogen atmosphere. After completion of the reaction, 15 ml of saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (20 ml × 1). The obtained organic layer was washed with water (10 ml × 2), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 2: 8) to obtain 1.40 g of the desired product as white crystals.
Melting point: 107.0-108.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.57 (d, J = 1.5 Hz, 1H), 7.92 (d, J = 1.5 Hz, 1H), 7.5-7.6 (m, 2H), 7.05- 7.15 (m, 2H), 5.10 (bs, 1H), 4.0-4.2 (m, 2H), 1.41 (s, 9H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル1.50gのN,N-ジメチルホルムアミド4ml溶液に、トリエチルアミン1.64g、1-エチニル-4-フルオロベンゼン631mg、ヨウ化銅(I)231mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)283mgを添加し、窒素雰囲気下、室温にて2時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液15mlを添加し、酢酸エチルにて抽出(20mlx1)した。得られた有機層を水洗(10mlx2)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.40gを白色結晶として得た。
融点107.0~108.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.57 (d, J=1.5Hz, 1H), 7.92 (d, J=1.5Hz, 1H), 7.5-7.6 (m, 2H), 7.05-7.15 (m, 2H), 5.10 (bs, 1H), 4.0-4.2 (m, 2H), 1.41 (s, 9H)。 Step 4: Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl N- [2 -(5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethyl] carbamic acid-tert-butyl 1.50 g of N, N-dimethylformamide in 4 ml of solution was added 1.64 g of triethylamine, 1- Ethinyl-4-fluorobenzene 631 mg, copper (I) iodide 231 mg, and dichlorobis (triphenylphosphine) palladium (II) 283 mg were added, and the mixture was stirred at room temperature for 2 hours under a nitrogen atmosphere. After completion of the reaction, 15 ml of saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (20 ml × 1). The obtained organic layer was washed with water (10 ml × 2), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 2: 8) to obtain 1.40 g of the desired product as white crystals.
Melting point: 107.0-108.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.57 (d, J = 1.5 Hz, 1H), 7.92 (d, J = 1.5 Hz, 1H), 7.5-7.6 (m, 2H), 7.05- 7.15 (m, 2H), 5.10 (bs, 1H), 4.0-4.2 (m, 2H), 1.41 (s, 9H).
工程5;2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチルアミンの製造
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル1.40gのジクロロメタン5ml溶液に、トリフルオロ酢酸3mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸カリウム水溶液10mlを加えて、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.01gを褐色油状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.59 (d, J=1.5Hz, 1H), 7.91 (d, J=1.5Hz, 1H), 7.5-7.6 (m, 2H), 7.0-7.15 (m, 2H), 3.4-3.6 (m, 2H)。 Step 5: Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine N- [2- [3-Chloro-5-[( 4-Fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl 1.40 g of dichloromethane in 5 ml was added with 3 ml of trifluoroacetic acid and stirred at room temperature for 1 hour. did. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous potassium carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and then the solvent was distilled off under reduced pressure to obtain 1.01 g of the crude desired product as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.59 (d, J = 1.5 Hz, 1H), 7.91 (d, J = 1.5 Hz, 1H), 7.5-7.6 (m, 2H), 7.0- 7.15 (m, 2H), 3.4-3.6 (m, 2H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル1.40gのジクロロメタン5ml溶液に、トリフルオロ酢酸3mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸カリウム水溶液10mlを加えて、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.01gを褐色油状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.59 (d, J=1.5Hz, 1H), 7.91 (d, J=1.5Hz, 1H), 7.5-7.6 (m, 2H), 7.0-7.15 (m, 2H), 3.4-3.6 (m, 2H)。 Step 5: Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine N- [2- [3-Chloro-5-[( 4-Fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl 1.40 g of dichloromethane in 5 ml was added with 3 ml of trifluoroacetic acid and stirred at room temperature for 1 hour. did. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous potassium carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and then the solvent was distilled off under reduced pressure to obtain 1.01 g of the crude desired product as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.59 (d, J = 1.5 Hz, 1H), 7.91 (d, J = 1.5 Hz, 1H), 7.5-7.6 (m, 2H), 7.0- 7.15 (m, 2H), 3.4-3.6 (m, 2H).
工程6;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-2-(トリフルオロメチル)ベンズアミドの製造
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチルアミン90mgの酢酸エチル2ml溶液に、10重量%炭酸カリウム水溶液2ml及び2-(トリフルオロメチル)ベンゾイルクロリド67mgを添加し、室温にて1時間攪拌した。反応完結後、反応混合物に水10ml添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(0:10~2:8のグラジエント)]にて精製し、目的物105mgを白色結晶として得た。
融点164.0~166.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.54 (d, J=1.8Hz, 1H), 7.95 (d, J=1.8Hz, 1H), 7.45-7.7 (m, 6H), 7.0-7.15 (m, 2H), 6.45 (bs, 1H), 4.4-4.55 (m, 2H)。 Step 6: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) benzamide 2 -[3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine 90 mg in 2 ml of ethyl acetate, 2 ml of 10 wt% aqueous potassium carbonate solution and 2- (tri 67 mg of fluoromethyl) benzoyl chloride was added and stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (gradient from 0:10 to 2: 8)] to obtain 105 mg of the desired product as white crystals.
Melting point: 164.0-166.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.54 (d, J = 1.8 Hz, 1H), 7.95 (d, J = 1.8 Hz, 1H), 7.45-7.7 (m, 6H), 7.0- 7.15 (m, 2H), 6.45 (bs, 1H), 4.4-4.55 (m, 2H).
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチルアミン90mgの酢酸エチル2ml溶液に、10重量%炭酸カリウム水溶液2ml及び2-(トリフルオロメチル)ベンゾイルクロリド67mgを添加し、室温にて1時間攪拌した。反応完結後、反応混合物に水10ml添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(0:10~2:8のグラジエント)]にて精製し、目的物105mgを白色結晶として得た。
融点164.0~166.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.54 (d, J=1.8Hz, 1H), 7.95 (d, J=1.8Hz, 1H), 7.45-7.7 (m, 6H), 7.0-7.15 (m, 2H), 6.45 (bs, 1H), 4.4-4.55 (m, 2H)。 Step 6: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) benzamide 2 -[3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine 90 mg in 2 ml of ethyl acetate, 2 ml of 10 wt% aqueous potassium carbonate solution and 2- (tri 67 mg of fluoromethyl) benzoyl chloride was added and stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (gradient from 0:10 to 2: 8)] to obtain 105 mg of the desired product as white crystals.
Melting point: 164.0-166.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.54 (d, J = 1.8 Hz, 1H), 7.95 (d, J = 1.8 Hz, 1H), 7.45-7.7 (m, 6H), 7.0- 7.15 (m, 2H), 6.45 (bs, 1H), 4.4-4.55 (m, 2H).
合成例2
2-クロロ-N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロプロピル]ニコチンアミド(本発明化合物No.3-010)。
工程1;5-ブロモ-3-クロロ-2-(2-メチルオキシラン-2-イル)ピリジンの製造
トリメチルスルホキソニムヨージド5.2gのジメチルスルホキシド15ml溶液に、氷冷攪拌下、55重量%油性水素化ナトリウム1.1gを添加し、同温度にて30分間攪拌した。次いで、この反応混合物を、氷冷攪拌下、1-(5-ブロモ-3-クロロピリジン-2-イル)エタン-1-オン5.0gのジメチルスルホキシド10ml溶液に滴下し、滴下終了後、室温にてさらに15時間攪拌を継続した。反応完結後、反応混合物に水20mlを加え、酢酸エチルにて抽出(30mlx1)した。得られた有機層を水洗(30mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(0:10~3:7のグラジエント)]にて精製し、目的物5.7gを褐色樹脂状物質として得た。
1H NMR (DMSO-d6, Me4Si, 300MHz) δ8.66 (d, J=1.8Hz, 1H), 8.36 (d, J=1.8Hz, 1H), 3.03 (d, J=5.2Hz, 1H), 2.92 (d, J=5.2Hz, 1H), 1.58 (s, 3H)。 Synthesis example 2
2-Chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropyl] nicotinamide (the present compound No. 3-010).
Step 1: Preparation of 5-bromo-3-chloro-2- (2-methyloxiran-2-yl) pyridine 55% by weight of a solution of 5.2 g of trimethylsulfoxonium iodide in 15 ml of dimethylsulfoxide under ice-cooling and stirring 1.1 g of oily sodium hydride was added and stirred at the same temperature for 30 minutes. The reaction mixture was then added dropwise to a solution of 5.0 g of 1- (5-bromo-3-chloropyridin-2-yl) ethane-1-one in 10 ml of dimethyl sulfoxide under ice-cooling. And stirring was continued for another 15 hours. After completion of the reaction, 20 ml of water was added to the reaction mixture and extracted with ethyl acetate (30 ml × 1). The obtained organic layer was washed with water (30 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (0:10 to 3: 7 gradient)] to obtain 5.7 g of the desired product as a brown resinous substance.
1 H NMR (DMSO-d 6 , Me 4 Si, 300 MHz) δ8.66 (d, J = 1.8 Hz, 1H), 8.36 (d, J = 1.8 Hz, 1H), 3.03 (d, J = 5.2 Hz, 1H), 2.92 (d, J = 5.2Hz, 1H), 1.58 (s, 3H).
2-クロロ-N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロプロピル]ニコチンアミド(本発明化合物No.3-010)。
工程1;5-ブロモ-3-クロロ-2-(2-メチルオキシラン-2-イル)ピリジンの製造
トリメチルスルホキソニムヨージド5.2gのジメチルスルホキシド15ml溶液に、氷冷攪拌下、55重量%油性水素化ナトリウム1.1gを添加し、同温度にて30分間攪拌した。次いで、この反応混合物を、氷冷攪拌下、1-(5-ブロモ-3-クロロピリジン-2-イル)エタン-1-オン5.0gのジメチルスルホキシド10ml溶液に滴下し、滴下終了後、室温にてさらに15時間攪拌を継続した。反応完結後、反応混合物に水20mlを加え、酢酸エチルにて抽出(30mlx1)した。得られた有機層を水洗(30mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(0:10~3:7のグラジエント)]にて精製し、目的物5.7gを褐色樹脂状物質として得た。
1H NMR (DMSO-d6, Me4Si, 300MHz) δ8.66 (d, J=1.8Hz, 1H), 8.36 (d, J=1.8Hz, 1H), 3.03 (d, J=5.2Hz, 1H), 2.92 (d, J=5.2Hz, 1H), 1.58 (s, 3H)。 Synthesis example 2
2-Chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropyl] nicotinamide (the present compound No. 3-010).
Step 1: Preparation of 5-bromo-3-chloro-2- (2-methyloxiran-2-yl) pyridine 55% by weight of a solution of 5.2 g of trimethylsulfoxonium iodide in 15 ml of dimethylsulfoxide under ice-cooling and stirring 1.1 g of oily sodium hydride was added and stirred at the same temperature for 30 minutes. The reaction mixture was then added dropwise to a solution of 5.0 g of 1- (5-bromo-3-chloropyridin-2-yl) ethane-1-one in 10 ml of dimethyl sulfoxide under ice-cooling. And stirring was continued for another 15 hours. After completion of the reaction, 20 ml of water was added to the reaction mixture and extracted with ethyl acetate (30 ml × 1). The obtained organic layer was washed with water (30 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (0:10 to 3: 7 gradient)] to obtain 5.7 g of the desired product as a brown resinous substance.
1 H NMR (DMSO-d 6 , Me 4 Si, 300 MHz) δ8.66 (d, J = 1.8 Hz, 1H), 8.36 (d, J = 1.8 Hz, 1H), 3.03 (d, J = 5.2 Hz, 1H), 2.92 (d, J = 5.2Hz, 1H), 1.58 (s, 3H).
工程2;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-ヒドロキシプロピル]フタルイミドの製造
5-ブロモ-3-クロロ-2-(2-メチルオキシラン-2-イル)ピリジン5.70gのジメチルスルホキシド40ml溶液にフタルイミドカリウム4.48gを添加し、100℃にて6時間攪拌した。反応完結後、反応混合物を室温まで放冷し、水30mlを加え、酢酸エチルにて抽出(20mlx2)した。有機層を併せて水洗(40mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物3.90gを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.3-8.4 (m, 1H), 7.9-7.95 (m, 1H), 7.7-7.8 (m, 2H), 7.65-7.7 (m, 2H), 5.64 (s, 1H), 4.31 (d, J=14.0Hz, 1H), 4.21 (d, J=14.0Hz, 1H), 1.80 (s, 3H)。 Step 2: Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-hydroxypropyl] phthalimide 5-Bromo-3-chloro-2- (2-methyloxiran-2-yl ) To a solution of 5.70 g of pyridine in 40 ml of dimethyl sulfoxide, 4.48 g of potassium phthalimide was added and stirred at 100 ° C. for 6 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, added with 30 ml of water, and extracted with ethyl acetate (20 ml × 2). The organic layers were combined and washed with water (40 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 3.90 g of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.3-8.4 (m, 1H), 7.9-7.95 (m, 1H), 7.7-7.8 (m, 2H), 7.65-7.7 (m, 2H) , 5.64 (s, 1H), 4.31 (d, J = 14.0Hz, 1H), 4.21 (d, J = 14.0Hz, 1H), 1.80 (s, 3H).
5-ブロモ-3-クロロ-2-(2-メチルオキシラン-2-イル)ピリジン5.70gのジメチルスルホキシド40ml溶液にフタルイミドカリウム4.48gを添加し、100℃にて6時間攪拌した。反応完結後、反応混合物を室温まで放冷し、水30mlを加え、酢酸エチルにて抽出(20mlx2)した。有機層を併せて水洗(40mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物3.90gを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.3-8.4 (m, 1H), 7.9-7.95 (m, 1H), 7.7-7.8 (m, 2H), 7.65-7.7 (m, 2H), 5.64 (s, 1H), 4.31 (d, J=14.0Hz, 1H), 4.21 (d, J=14.0Hz, 1H), 1.80 (s, 3H)。 Step 2: Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-hydroxypropyl] phthalimide 5-Bromo-3-chloro-2- (2-methyloxiran-2-yl ) To a solution of 5.70 g of pyridine in 40 ml of dimethyl sulfoxide, 4.48 g of potassium phthalimide was added and stirred at 100 ° C. for 6 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, added with 30 ml of water, and extracted with ethyl acetate (20 ml × 2). The organic layers were combined and washed with water (40 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 3.90 g of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.3-8.4 (m, 1H), 7.9-7.95 (m, 1H), 7.7-7.8 (m, 2H), 7.65-7.7 (m, 2H) , 5.64 (s, 1H), 4.31 (d, J = 14.0Hz, 1H), 4.21 (d, J = 14.0Hz, 1H), 1.80 (s, 3H).
工程3;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-フルオロプロピル]フタルイミドの製造
氷冷攪拌下のN-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-ヒドロキシプロピル]フタルイミド3.90gのジクロロメタン20ml溶液に、ビス(2-メトキシエチル)アミノサルファートリフルオリド2.62gを滴下し、滴下終了後、50℃にて12時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和炭酸水素ナトリウム水溶液30mlを加え、ジクロロメタンにて抽出(20mlx2)した。有機層を併せて水洗(40mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をジイソプロピルエーテル10mlにて洗浄し、目的物3.50gを淡黄色結晶として得た。
融点134.0~136.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.55 (m, 1H), 7.85-7.9 (m, 1H), 7.8-7.85 (m, 2H), 7.7-7.8 (m, 2H), 4.3-4.55 (m, 2H), 1.82 (d, J=22.4Hz, 3H)。 Step 3; Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -2-fluoropropyl] phthalimide N- [2- (5-Bromo-3-chloropyridine] under ice-cooling and stirring -2-yl) -2-hydroxypropyl] phthalimide 2.62 g of bis (2-methoxyethyl) aminosulfur trifluoride was added dropwise to a solution of 3.90 g of dichloromethane in 20 ml of dichloromethane, and stirred at 50 ° C. for 12 hours. did. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 30 ml of saturated aqueous sodium hydrogen carbonate solution was added, and the mixture was extracted with dichloromethane (20 ml × 2). The organic layers were combined and washed with water (40 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was washed with 10 ml of diisopropyl ether to obtain 3.50 g of the desired product as pale yellow crystals.
Melting point: 134.0 to 136.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.55 (m, 1H), 7.85-7.9 (m, 1H), 7.8-7.85 (m, 2H), 7.7-7.8 (m, 2H) , 4.3-4.55 (m, 2H), 1.82 (d, J = 22.4Hz, 3H).
氷冷攪拌下のN-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-ヒドロキシプロピル]フタルイミド3.90gのジクロロメタン20ml溶液に、ビス(2-メトキシエチル)アミノサルファートリフルオリド2.62gを滴下し、滴下終了後、50℃にて12時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和炭酸水素ナトリウム水溶液30mlを加え、ジクロロメタンにて抽出(20mlx2)した。有機層を併せて水洗(40mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をジイソプロピルエーテル10mlにて洗浄し、目的物3.50gを淡黄色結晶として得た。
融点134.0~136.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.55 (m, 1H), 7.85-7.9 (m, 1H), 7.8-7.85 (m, 2H), 7.7-7.8 (m, 2H), 4.3-4.55 (m, 2H), 1.82 (d, J=22.4Hz, 3H)。 Step 3; Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -2-fluoropropyl] phthalimide N- [2- (5-Bromo-3-chloropyridine] under ice-cooling and stirring -2-yl) -2-hydroxypropyl] phthalimide 2.62 g of bis (2-methoxyethyl) aminosulfur trifluoride was added dropwise to a solution of 3.90 g of dichloromethane in 20 ml of dichloromethane, and stirred at 50 ° C. for 12 hours. did. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 30 ml of saturated aqueous sodium hydrogen carbonate solution was added, and the mixture was extracted with dichloromethane (20 ml × 2). The organic layers were combined and washed with water (40 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was washed with 10 ml of diisopropyl ether to obtain 3.50 g of the desired product as pale yellow crystals.
Melting point: 134.0 to 136.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.55 (m, 1H), 7.85-7.9 (m, 1H), 7.8-7.85 (m, 2H), 7.7-7.8 (m, 2H) , 4.3-4.55 (m, 2H), 1.82 (d, J = 22.4Hz, 3H).
工程4;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロプロピル]フタルイミドの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-フルオロプロピル]フタルイミド1.0gのN,N-ジメチルホルムアミド4ml溶液に、トリエチルアミン762mg、1-エチニル-4-フルオロベンゼン452mg、ヨウ化銅(I)143mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)176mgを添加し、窒素雰囲気下、60℃にて5時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(5:95~10:90のグラジエント)]にて精製し、目的物1.2gを褐色結晶として得た。
融点153.0~155.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.55 (m, 1H), 7.8-7.9 (m, 3H), 7.7-7.75 (m, 2H), 7.45-7.6 (m, 2H), 7.0-7.15 (m, 2H), 4.3-4.6 (m, 2H), 1.86 (d, J=23.0Hz, 3H)。 Step 4: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropyl] phthalimide N- [2- (5-Bromo-3 -Chloropyridin-2-yl) -2-fluoropropyl] phthalimide in 4 ml of N, N-dimethylformamide in a solution of 762 mg of triethylamine, 452 mg of 1-ethynyl-4-fluorobenzene, 143 mg of copper (I) iodide and 176 mg of dichlorobis (triphenylphosphine) palladium (II) was added, and the mixture was stirred at 60 ° C. for 5 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (gradient 5:95 to 10:90)] to obtain 1.2 g of the desired product as brown crystals.
Melting point: 153.0-155.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.55 (m, 1H), 7.8-7.9 (m, 3H), 7.7-7.75 (m, 2H), 7.45-7.6 (m, 2H) , 7.0-7.15 (m, 2H), 4.3-4.6 (m, 2H), 1.86 (d, J = 23.0Hz, 3H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-フルオロプロピル]フタルイミド1.0gのN,N-ジメチルホルムアミド4ml溶液に、トリエチルアミン762mg、1-エチニル-4-フルオロベンゼン452mg、ヨウ化銅(I)143mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)176mgを添加し、窒素雰囲気下、60℃にて5時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(5:95~10:90のグラジエント)]にて精製し、目的物1.2gを褐色結晶として得た。
融点153.0~155.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.55 (m, 1H), 7.8-7.9 (m, 3H), 7.7-7.75 (m, 2H), 7.45-7.6 (m, 2H), 7.0-7.15 (m, 2H), 4.3-4.6 (m, 2H), 1.86 (d, J=23.0Hz, 3H)。 Step 4: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropyl] phthalimide N- [2- (5-Bromo-3 -Chloropyridin-2-yl) -2-fluoropropyl] phthalimide in 4 ml of N, N-dimethylformamide in a solution of 762 mg of triethylamine, 452 mg of 1-ethynyl-4-fluorobenzene, 143 mg of copper (I) iodide and 176 mg of dichlorobis (triphenylphosphine) palladium (II) was added, and the mixture was stirred at 60 ° C. for 5 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (gradient 5:95 to 10:90)] to obtain 1.2 g of the desired product as brown crystals.
Melting point: 153.0-155.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.55 (m, 1H), 7.8-7.9 (m, 3H), 7.7-7.75 (m, 2H), 7.45-7.6 (m, 2H) , 7.0-7.15 (m, 2H), 4.3-4.6 (m, 2H), 1.86 (d, J = 23.0Hz, 3H).
工程5;2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロプロピルアミンの製造
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロプロピル]フタルイミド1.2gのエタノール4ml溶液に、ヒドラジン一水和物432mgを添加し、80℃にて5時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液5mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せて水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(5:5~9:1のグラジエント)]にて精製し、目的物323mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.75 (m, 1H), 7.75-8.05 (m, 1H), 7.45-7.75 (m, 2H), 7.0-7.3 (m, 2H), 3.05-3.8 (m, 2H), 1.74 (d, J=23.0Hz, 3H), 1.05-1.45 (m, 2H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropylamine N- [2- [3-Chloro-5-[(4 -Fluorine) ethynyl] pyridin-2-yl] -2-fluoropropyl] phthalimide To a solution of 1.2 g of ethanol in 4 ml of ethanol was added 432 mg of hydrazine monohydrate and stirred at 80 ° C. for 5 hours. After completion of the reaction, the solvent was distilled off under reduced pressure, 5 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (5: 5 to 9: 1 gradient)] to obtain 323 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.75 (m, 1H), 7.75-8.05 (m, 1H), 7.45-7.75 (m, 2H), 7.0-7.3 (m, 2H) , 3.05-3.8 (m, 2H), 1.74 (d, J = 23.0Hz, 3H), 1.05-1.45 (m, 2H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロプロピル]フタルイミド1.2gのエタノール4ml溶液に、ヒドラジン一水和物432mgを添加し、80℃にて5時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液5mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せて水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(5:5~9:1のグラジエント)]にて精製し、目的物323mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.75 (m, 1H), 7.75-8.05 (m, 1H), 7.45-7.75 (m, 2H), 7.0-7.3 (m, 2H), 3.05-3.8 (m, 2H), 1.74 (d, J=23.0Hz, 3H), 1.05-1.45 (m, 2H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropylamine N- [2- [3-Chloro-5-[(4 -Fluorine) ethynyl] pyridin-2-yl] -2-fluoropropyl] phthalimide To a solution of 1.2 g of ethanol in 4 ml of ethanol was added 432 mg of hydrazine monohydrate and stirred at 80 ° C. for 5 hours. After completion of the reaction, the solvent was distilled off under reduced pressure, 5 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (5: 5 to 9: 1 gradient)] to obtain 323 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.75 (m, 1H), 7.75-8.05 (m, 1H), 7.45-7.75 (m, 2H), 7.0-7.3 (m, 2H) , 3.05-3.8 (m, 2H), 1.74 (d, J = 23.0Hz, 3H), 1.05-1.45 (m, 2H).
工程6;2-クロロ-N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロプロピル]ニコチンアミドの製造
氷冷攪拌下の2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロプロピルアミン100mg及びトリエチルアミン50mgの、ジクロロメタン1ml溶液に、2-クロロニコチニルクロリド63mgを滴下し、滴下終了後、室温にて1時間攪拌を継続した。反応完結後、反応混合物に水2ml添加して、ジクロロメタンにて抽出(5mlx1)した。得られた有機層を水洗(5mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(2:8~5:5のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物69mgを白色結晶として得た。
融点149.0~151.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.54 (d, J=1.7Hz, 1H), 8.4-8.45 (m, 1H), 8.05-8.1 (m, 1H), 7.86 (d, J=1.7Hz, 1H), 7.45-7.6 (m, 2H), 7.3-7.35 (m, 1H), 7.2-7.25 (m, 1H), 7.05-7.15 (m, 2H), 4.15-4.4 (m, 2H), 1.90 (d, J=21.0Hz, 3H)。 Step 6; Preparation of 2-chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropyl] nicotinamide 2 under ice-cooling and stirring -To a solution of 100 mg of [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropylamine and 50 mg of triethylamine in 1 ml of dichloromethane, 63 mg of 2-chloronicotinyl chloride was added dropwise. After the dropping, stirring was continued for 1 hour at room temperature. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The obtained organic layer was washed with water (5 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (2: 8 to 5: 5 gradient) to obtain 69 mg of the desired product as white crystals.
Melting point: 149.0-151.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.54 (d, J = 1.7 Hz, 1H), 8.4-8.45 (m, 1H), 8.05-8.1 (m, 1H), 7.86 (d, J = 1.7Hz, 1H), 7.45-7.6 (m, 2H), 7.3-7.35 (m, 1H), 7.2-7.25 (m, 1H), 7.05-7.15 (m, 2H), 4.15-4.4 (m, 2H ), 1.90 (d, J = 21.0Hz, 3H).
氷冷攪拌下の2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロプロピルアミン100mg及びトリエチルアミン50mgの、ジクロロメタン1ml溶液に、2-クロロニコチニルクロリド63mgを滴下し、滴下終了後、室温にて1時間攪拌を継続した。反応完結後、反応混合物に水2ml添加して、ジクロロメタンにて抽出(5mlx1)した。得られた有機層を水洗(5mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(2:8~5:5のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物69mgを白色結晶として得た。
融点149.0~151.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.54 (d, J=1.7Hz, 1H), 8.4-8.45 (m, 1H), 8.05-8.1 (m, 1H), 7.86 (d, J=1.7Hz, 1H), 7.45-7.6 (m, 2H), 7.3-7.35 (m, 1H), 7.2-7.25 (m, 1H), 7.05-7.15 (m, 2H), 4.15-4.4 (m, 2H), 1.90 (d, J=21.0Hz, 3H)。 Step 6; Preparation of 2-chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropyl] nicotinamide 2 under ice-cooling and stirring -To a solution of 100 mg of [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoropropylamine and 50 mg of triethylamine in 1 ml of dichloromethane, 63 mg of 2-chloronicotinyl chloride was added dropwise. After the dropping, stirring was continued for 1 hour at room temperature. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The obtained organic layer was washed with water (5 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (2: 8 to 5: 5 gradient) to obtain 69 mg of the desired product as white crystals.
Melting point: 149.0-151.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.54 (d, J = 1.7 Hz, 1H), 8.4-8.45 (m, 1H), 8.05-8.1 (m, 1H), 7.86 (d, J = 1.7Hz, 1H), 7.45-7.6 (m, 2H), 7.3-7.35 (m, 1H), 7.2-7.25 (m, 1H), 7.05-7.15 (m, 2H), 4.15-4.4 (m, 2H ), 1.90 (d, J = 21.0Hz, 3H).
合成例3
N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]-3-(トリフルオロメチル)チオフェン-2-カルボキサミド(本発明化合物No.7-006)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチルアミンの製造
合成例1の工程1にて製造した2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリル1.0gのジエチルエーテル5ml溶液に、氷冷攪拌下、メチルマグネシウムブロミドの35重量%ジエチルエーテル溶液3.1mlを滴下し、同温度にて1時間攪拌した。次いで、この反応混合物に、氷冷攪拌下、メタノール15mlを添加した後水素化ホウ素ナトリウム423mgを添加し、同温度にてさらに1時間攪拌を継続した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和塩化アンモニウム水溶液10mlを加え、酢酸エチルにて抽出(15mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.0gを褐色樹脂状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.65 (m, 1H), 7.95-8.0 (m, 1H), 3.7-3.95 (m, 1H), 1.5-1.6 (m, 2H), 1.23 (d, J=6.7Hz, 3H)。 Synthesis example 3
N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -3- (trifluoromethyl) thiophene-2-carboxamide (this Invention compound No. 7-006).
Step 1; Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethylamine 2- (5-Bromo-3) prepared in Step 1 of Synthesis Example 1 -Chloropyridin-2-yl) -2,2-difluoroacetonitrile 1.0 ml of a diethyl ether solution (5 ml) was added dropwise with ice-cooling and stirring with 3.1 ml of a 35 wt% diethyl ether solution of methylmagnesium bromide. And stirred for 1 hour. Next, 15 ml of methanol was added to the reaction mixture with stirring under ice cooling, followed by 423 mg of sodium borohydride, and stirring was continued at the same temperature for another hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous ammonium chloride solution was added to the residue, and the mixture was extracted with ethyl acetate (15 ml × 2). The organic layers were combined and washed with water (20 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 1.0 g of the crude target product as a brown resinous substance. . This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.65 (m, 1H), 7.95-8.0 (m, 1H), 3.7-3.95 (m, 1H), 1.5-1.6 (m, 2H) , 1.23 (d, J = 6.7Hz, 3H).
N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]-3-(トリフルオロメチル)チオフェン-2-カルボキサミド(本発明化合物No.7-006)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチルアミンの製造
合成例1の工程1にて製造した2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリル1.0gのジエチルエーテル5ml溶液に、氷冷攪拌下、メチルマグネシウムブロミドの35重量%ジエチルエーテル溶液3.1mlを滴下し、同温度にて1時間攪拌した。次いで、この反応混合物に、氷冷攪拌下、メタノール15mlを添加した後水素化ホウ素ナトリウム423mgを添加し、同温度にてさらに1時間攪拌を継続した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和塩化アンモニウム水溶液10mlを加え、酢酸エチルにて抽出(15mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.0gを褐色樹脂状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.65 (m, 1H), 7.95-8.0 (m, 1H), 3.7-3.95 (m, 1H), 1.5-1.6 (m, 2H), 1.23 (d, J=6.7Hz, 3H)。 Synthesis example 3
N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -3- (trifluoromethyl) thiophene-2-carboxamide (this Invention compound No. 7-006).
Step 1; Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethylamine 2- (5-Bromo-3) prepared in Step 1 of Synthesis Example 1 -Chloropyridin-2-yl) -2,2-difluoroacetonitrile 1.0 ml of a diethyl ether solution (5 ml) was added dropwise with ice-cooling and stirring with 3.1 ml of a 35 wt% diethyl ether solution of methylmagnesium bromide. And stirred for 1 hour. Next, 15 ml of methanol was added to the reaction mixture with stirring under ice cooling, followed by 423 mg of sodium borohydride, and stirring was continued at the same temperature for another hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous ammonium chloride solution was added to the residue, and the mixture was extracted with ethyl acetate (15 ml × 2). The organic layers were combined and washed with water (20 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 1.0 g of the crude target product as a brown resinous substance. . This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.65 (m, 1H), 7.95-8.0 (m, 1H), 3.7-3.95 (m, 1H), 1.5-1.6 (m, 2H) , 1.23 (d, J = 6.7Hz, 3H).
工程2;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチルアミン1.0gのジクロロメタン5mlに、二炭酸ジ-tert-ブチル895mg及びトリエチルアミン566mgを添加し、室温にて1時間攪拌した。反応完結後、反応混合物に水10mlを加え、ジクロロメタンにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物1.3gを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.6 (m, 1H), 7.95-8.0 (m, 1H), 4.5-4.9 (m, 1H), 1.35 (d, J=7.0Hz, 3H), 1.29 (bs, 9H)。 Step 2: Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl 2- (5-bromo-3 -Chloropyridin-2-yl) -2,2-difluoro-1-methylethylamine To 1.0 g of dichloromethane was added 895 mg of di-tert-butyl dicarbonate and 566 mg of triethylamine, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with dichloromethane (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 1.3 g of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.6 (m, 1H), 7.95-8.0 (m, 1H), 4.5-4.9 (m, 1H), 1.35 (d, J = 7.0Hz , 3H), 1.29 (bs, 9H).
2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチルアミン1.0gのジクロロメタン5mlに、二炭酸ジ-tert-ブチル895mg及びトリエチルアミン566mgを添加し、室温にて1時間攪拌した。反応完結後、反応混合物に水10mlを加え、ジクロロメタンにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物1.3gを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.6 (m, 1H), 7.95-8.0 (m, 1H), 4.5-4.9 (m, 1H), 1.35 (d, J=7.0Hz, 3H), 1.29 (bs, 9H)。 Step 2: Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl 2- (5-bromo-3 -Chloropyridin-2-yl) -2,2-difluoro-1-methylethylamine To 1.0 g of dichloromethane was added 895 mg of di-tert-butyl dicarbonate and 566 mg of triethylamine, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with dichloromethane (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 1.3 g of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.6 (m, 1H), 7.95-8.0 (m, 1H), 4.5-4.9 (m, 1H), 1.35 (d, J = 7.0Hz , 3H), 1.29 (bs, 9H).
工程3;N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチルの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチル1.3gのN,N-ジメチルホルムアミド3ml溶液に、トリエチルアミン1.1g、シクロプロピルアセチレン493mg、ヨウ化銅(I)213mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)262mgを添加し、窒素雰囲気下、室温にて18時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物750mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.4-8.45 (m, 1H), 7.7-7.75 (m, 1H), 7.35-7.4 (m, 1H), 4.6-4.8 (m, 1H), 1.4-1.5 (m, 1H), 1.33 (d, J=6.7Hz, 3H), 1.30 (s, 9H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H)。 Step 3; Preparation of N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl N- [2 -(5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl 1.3 g of N, N-dimethylformamide in 3 ml of triethylamine 1. 1 g, 493 mg of cyclopropylacetylene, 213 mg of copper (I) iodide and 262 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at room temperature for 18 hours under a nitrogen atmosphere. After completion of the reaction, 10 ml of a saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 750 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.4-8.45 (m, 1H), 7.7-7.75 (m, 1H), 7.35-7.4 (m, 1H), 4.6-4.8 (m, 1H) , 1.4-1.5 (m, 1H), 1.33 (d, J = 6.7Hz, 3H), 1.30 (s, 9H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチル1.3gのN,N-ジメチルホルムアミド3ml溶液に、トリエチルアミン1.1g、シクロプロピルアセチレン493mg、ヨウ化銅(I)213mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)262mgを添加し、窒素雰囲気下、室温にて18時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物750mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.4-8.45 (m, 1H), 7.7-7.75 (m, 1H), 7.35-7.4 (m, 1H), 4.6-4.8 (m, 1H), 1.4-1.5 (m, 1H), 1.33 (d, J=6.7Hz, 3H), 1.30 (s, 9H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H)。 Step 3; Preparation of N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl N- [2 -(5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl 1.3 g of N, N-dimethylformamide in 3 ml of triethylamine 1. 1 g, 493 mg of cyclopropylacetylene, 213 mg of copper (I) iodide and 262 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at room temperature for 18 hours under a nitrogen atmosphere. After completion of the reaction, 10 ml of a saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 750 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.4-8.45 (m, 1H), 7.7-7.75 (m, 1H), 7.35-7.4 (m, 1H), 4.6-4.8 (m, 1H) , 1.4-1.5 (m, 1H), 1.33 (d, J = 6.7Hz, 3H), 1.30 (s, 9H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H).
工程4;2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチルアミンの製造
N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチル750mgのジクロロメタン5ml溶液に、トリフルオロ酢酸2.5mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物660mgを褐色樹脂状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.46 (d, J=1.5Hz, 1H), 7.74 (d, J=1.5Hz, 1H), 3.7-3.9 (m, 1H), 1.5-1.7 (m, 2H), 1.4-1.55 (m, 1H), 1.21 (d, J=6.7Hz, 3H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H)。 Step 4; Preparation of 2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethylamine N- [2- [3-Chloro-5- (cyclo To a solution of 750 mg of propylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl in 5 ml of dichloromethane was added 2.5 ml of trifluoroacetic acid, and the mixture was stirred at room temperature for 1 hour. did. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 660 mg of a crude desired product as a brown resinous substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.46 (d, J = 1.5 Hz, 1H), 7.74 (d, J = 1.5 Hz, 1H), 3.7-3.9 (m, 1H), 1.5- 1.7 (m, 2H), 1.4-1.55 (m, 1H), 1.21 (d, J = 6.7Hz, 3H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H).
N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチル750mgのジクロロメタン5ml溶液に、トリフルオロ酢酸2.5mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物660mgを褐色樹脂状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.46 (d, J=1.5Hz, 1H), 7.74 (d, J=1.5Hz, 1H), 3.7-3.9 (m, 1H), 1.5-1.7 (m, 2H), 1.4-1.55 (m, 1H), 1.21 (d, J=6.7Hz, 3H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H)。 Step 4; Preparation of 2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethylamine N- [2- [3-Chloro-5- (cyclo To a solution of 750 mg of propylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl in 5 ml of dichloromethane was added 2.5 ml of trifluoroacetic acid, and the mixture was stirred at room temperature for 1 hour. did. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 660 mg of a crude desired product as a brown resinous substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.46 (d, J = 1.5 Hz, 1H), 7.74 (d, J = 1.5 Hz, 1H), 3.7-3.9 (m, 1H), 1.5- 1.7 (m, 2H), 1.4-1.55 (m, 1H), 1.21 (d, J = 6.7Hz, 3H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H).
工程5;N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]-3-(トリフルオロメチル)チオフェン-2-カルボキサミドの製造
2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチルアミン150mgのN,N-ジメチルホルムアミド3ml溶液に、トリエチルアミン62mg、N,N-ジメチル-4-アミノピリジン5mg、3-(トリフルオロメチル)チオフェン-2-カルボン酸120mg及び1-(3-ジメチルアミノプロピル)-3-エチルカルボジイミド塩酸塩212mgを添加し、室温にて16時間攪拌した。反応完結後、反応混合物に水10ml添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(3:7~5:5のグラジエント)]にて精製し、目的物23mgを淡黄色結晶として得た。
融点98.0~101.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.41 (d, J=1.7Hz, 1H), 7.76 (d, J=1.7Hz, 1H), 7.4-7.5 (m, 1H), 7.35-7.4 (m, 1H), 6.85-7.05 (m, 1H), 5.1-5.3 (m, 1H), 1.5-1.55 (m, 1H), 1.45 (d, J=6.8Hz, 3H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H)。 Step 5: N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -3- (trifluoromethyl) thiophene-2- Preparation of carboxamide 2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethylamine 150 mg of N, N-dimethylformamide in 3 ml solution, 62 mg of triethylamine, N , N-dimethyl-4-aminopyridine 5 mg, 3- (trifluoromethyl) thiophene-2-carboxylic acid 120 mg and 1- (3-dimethylaminopropyl) -3-ethylcarbodiimide hydrochloride 212 mg were added at room temperature. Stir for 16 hours. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (3: 7 to 5: 5 gradient)] to obtain 23 mg of the desired product as pale yellow crystals.
Melting point 98.0-101.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.41 (d, J = 1.7 Hz, 1H), 7.76 (d, J = 1.7 Hz, 1H), 7.4-7.5 (m, 1H), 7.35- 7.4 (m, 1H), 6.85-7.05 (m, 1H), 5.1-5.3 (m, 1H), 1.5-1.55 (m, 1H), 1.45 (d, J = 6.8Hz, 3H), 0.9-1.0 ( m, 2H), 0.8-0.9 (m, 2H).
2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチルアミン150mgのN,N-ジメチルホルムアミド3ml溶液に、トリエチルアミン62mg、N,N-ジメチル-4-アミノピリジン5mg、3-(トリフルオロメチル)チオフェン-2-カルボン酸120mg及び1-(3-ジメチルアミノプロピル)-3-エチルカルボジイミド塩酸塩212mgを添加し、室温にて16時間攪拌した。反応完結後、反応混合物に水10ml添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(3:7~5:5のグラジエント)]にて精製し、目的物23mgを淡黄色結晶として得た。
融点98.0~101.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.41 (d, J=1.7Hz, 1H), 7.76 (d, J=1.7Hz, 1H), 7.4-7.5 (m, 1H), 7.35-7.4 (m, 1H), 6.85-7.05 (m, 1H), 5.1-5.3 (m, 1H), 1.5-1.55 (m, 1H), 1.45 (d, J=6.8Hz, 3H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H)。 Step 5: N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -3- (trifluoromethyl) thiophene-2- Preparation of carboxamide 2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethylamine 150 mg of N, N-dimethylformamide in 3 ml solution, 62 mg of triethylamine, N , N-dimethyl-4-aminopyridine 5 mg, 3- (trifluoromethyl) thiophene-2-carboxylic acid 120 mg and 1- (3-dimethylaminopropyl) -3-ethylcarbodiimide hydrochloride 212 mg were added at room temperature. Stir for 16 hours. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), dehydrated and dried over saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (3: 7 to 5: 5 gradient)] to obtain 23 mg of the desired product as pale yellow crystals.
Melting point 98.0-101.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.41 (d, J = 1.7 Hz, 1H), 7.76 (d, J = 1.7 Hz, 1H), 7.4-7.5 (m, 1H), 7.35- 7.4 (m, 1H), 6.85-7.05 (m, 1H), 5.1-5.3 (m, 1H), 1.5-1.55 (m, 1H), 1.45 (d, J = 6.8Hz, 3H), 0.9-1.0 ( m, 2H), 0.8-0.9 (m, 2H).
合成例4
N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]-N-シクロプロピル-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.15-001)。
工程1;1-(5-ブロモ-3-クロロピリジン-2-イル)-1,1-ジフルオロプロパン-2-オンの製造
合成例1の工程1にて製造した2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリル1.0gのジエチルエーテル5ml溶液に、氷冷攪拌下、メチルマグネシウムブロミドの35重量%ジエチルエーテル溶液3.1mlを滴下し、同温度にて1時間攪拌した。次いで、この反応混合物を、氷冷攪拌下、4M塩酸水溶液20mlに滴下し、滴下終了後、同温度にてさらに1時間攪拌を継続した。反応完結後、反応混合物を酢酸エチルにて抽出(15mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物920mgを褐色油状物質として得た。このものは更なる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55 (d, J=1.8Hz, 1H), 8.01 (d, J=1.8Hz, 1H), 2.47 (s, 3H)。 Synthesis example 4
N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl) benzamide ( Compound No. 15-001 of the present invention.
Step 1; Preparation of 1- (5-bromo-3-chloropyridin-2-yl) -1,1-difluoropropan-2-one 2- (5-Bromo-3) prepared in Step 1 of Synthesis Example 1 -Chloropyridin-2-yl) -2,2-difluoroacetonitrile 1.0 ml of a diethyl ether solution (5 ml) was added dropwise with ice-cooling and stirring with 3.1 ml of a 35 wt% diethyl ether solution of methylmagnesium bromide. And stirred for 1 hour. The reaction mixture was then added dropwise to 20 ml of 4M aqueous hydrochloric acid with stirring under ice cooling, and stirring was continued for an additional hour at the same temperature after completion of the dropwise addition. After completion of the reaction, the reaction mixture was extracted with ethyl acetate (15 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 920 mg of the crude desired product as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3, Me 4 Si, 300MHz) δ8.55 (d, J = 1.8Hz, 1H), 8.01 (d, J = 1.8Hz, 1H), 2.47 (s, 3H).
N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]-N-シクロプロピル-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.15-001)。
工程1;1-(5-ブロモ-3-クロロピリジン-2-イル)-1,1-ジフルオロプロパン-2-オンの製造
合成例1の工程1にて製造した2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリル1.0gのジエチルエーテル5ml溶液に、氷冷攪拌下、メチルマグネシウムブロミドの35重量%ジエチルエーテル溶液3.1mlを滴下し、同温度にて1時間攪拌した。次いで、この反応混合物を、氷冷攪拌下、4M塩酸水溶液20mlに滴下し、滴下終了後、同温度にてさらに1時間攪拌を継続した。反応完結後、反応混合物を酢酸エチルにて抽出(15mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物920mgを褐色油状物質として得た。このものは更なる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55 (d, J=1.8Hz, 1H), 8.01 (d, J=1.8Hz, 1H), 2.47 (s, 3H)。 Synthesis example 4
N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl) benzamide ( Compound No. 15-001 of the present invention.
Step 1; Preparation of 1- (5-bromo-3-chloropyridin-2-yl) -1,1-difluoropropan-2-one 2- (5-Bromo-3) prepared in Step 1 of Synthesis Example 1 -Chloropyridin-2-yl) -2,2-difluoroacetonitrile 1.0 ml of a diethyl ether solution (5 ml) was added dropwise with ice-cooling and stirring with 3.1 ml of a 35 wt% diethyl ether solution of methylmagnesium bromide. And stirred for 1 hour. The reaction mixture was then added dropwise to 20 ml of 4M aqueous hydrochloric acid with stirring under ice cooling, and stirring was continued for an additional hour at the same temperature after completion of the dropwise addition. After completion of the reaction, the reaction mixture was extracted with ethyl acetate (15 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 920 mg of the crude desired product as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3, Me 4 Si, 300MHz) δ8.55 (d, J = 1.8Hz, 1H), 8.01 (d, J = 1.8Hz, 1H), 2.47 (s, 3H).
工程2;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]-N-シクロプロピルアミンの製造
1-(5-ブロモ-3-クロロピリジン-2-イル)-1,1-ジフルオロプロパン-2-オン920mgのメタノール-酢酸(10:1)2.2ml溶液に、シクロプロピルアミン203mgを添加し、90℃にて1時間攪拌した。次いで、この反応混合物を、室温まで放冷後、ピコリンボラン錯体381mgを添加し、90℃にてさらに2時間攪拌を継続した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを添加し、酢酸エチルにて抽出(20mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物830mgを褐色油状物質として得た。このものは更なる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.6 (m, 1H), 7.9-8.0 (m, 1H), 3.6-3.85 (m, 1H), 2.15-2.3 (m, 1H), 1.65-1.85 (m, 1H), 1.29 (d, J=7.4Hz, 3H), 0.05-0.55 (m, 4H)。 Step 2: Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropylamine 1- (5-Bromo-3 -Chloropyridin-2-yl) -1,1-difluoropropan-2-one To a solution of 920 mg of methanol-acetic acid (10: 1) in 2.2 ml was added 203 mg of cyclopropylamine and stirred at 90 ° C. for 1 hour. did. Next, the reaction mixture was allowed to cool to room temperature, 381 mg of picoline borane complex was added, and stirring was further continued at 90 ° C. for 2 hours. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (20 ml × 2). The organic layers were combined and washed with water (20 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 830 mg of the crude desired product as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.6 (m, 1H), 7.9-8.0 (m, 1H), 3.6-3.85 (m, 1H), 2.15-2.3 (m, 1H) , 1.65-1.85 (m, 1H), 1.29 (d, J = 7.4Hz, 3H), 0.05-0.55 (m, 4H).
1-(5-ブロモ-3-クロロピリジン-2-イル)-1,1-ジフルオロプロパン-2-オン920mgのメタノール-酢酸(10:1)2.2ml溶液に、シクロプロピルアミン203mgを添加し、90℃にて1時間攪拌した。次いで、この反応混合物を、室温まで放冷後、ピコリンボラン錯体381mgを添加し、90℃にてさらに2時間攪拌を継続した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを添加し、酢酸エチルにて抽出(20mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物830mgを褐色油状物質として得た。このものは更なる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.6 (m, 1H), 7.9-8.0 (m, 1H), 3.6-3.85 (m, 1H), 2.15-2.3 (m, 1H), 1.65-1.85 (m, 1H), 1.29 (d, J=7.4Hz, 3H), 0.05-0.55 (m, 4H)。 Step 2: Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropylamine 1- (5-Bromo-3 -Chloropyridin-2-yl) -1,1-difluoropropan-2-one To a solution of 920 mg of methanol-acetic acid (10: 1) in 2.2 ml was added 203 mg of cyclopropylamine and stirred at 90 ° C. for 1 hour. did. Next, the reaction mixture was allowed to cool to room temperature, 381 mg of picoline borane complex was added, and stirring was further continued at 90 ° C. for 2 hours. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (20 ml × 2). The organic layers were combined and washed with water (20 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 830 mg of the crude desired product as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.6 (m, 1H), 7.9-8.0 (m, 1H), 3.6-3.85 (m, 1H), 2.15-2.3 (m, 1H) , 1.65-1.85 (m, 1H), 1.29 (d, J = 7.4Hz, 3H), 0.05-0.55 (m, 4H).
工程3;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]-N-シクロプロピル-2-(トリフルオロメチル)ベンズアミドの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]-N-シクロプロピルアミン300mg及びトリエチルアミン112mgの、ジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド211mgを滴下し、40℃にて5時間攪拌した。反応完結後、反応混合物を室温まで放冷、水2mlを加え、ジクロロメタンにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物130mgを無色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.7 (m, 1H), 7.9-8.0 (m, 1H), 7.55-7.75 (m, 2H), 7.35-7.55 (m, 2H), 5.8-6.1 (m, 1H), 2.4-2.65 (m, 1H), 1.56 (d, J=7.0Hz, 3H), 0.45-0.8 (m, 3H), 0.3-0.45 (m, 1H)。 Step 3: Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl) benzamide To a solution of 300 mg of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropylamine and 112 mg of triethylamine in 2 ml of dichloromethane, With stirring, 211 mg of 2- (trifluoromethyl) benzoyl chloride was added dropwise and stirred at 40 ° C. for 5 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 2 ml of water was added, and the mixture was extracted with dichloromethane (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 130 mg of the desired product as a colorless resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.7 (m, 1H), 7.9-8.0 (m, 1H), 7.55-7.75 (m, 2H), 7.35-7.55 (m, 2H) , 5.8-6.1 (m, 1H), 2.4-2.65 (m, 1H), 1.56 (d, J = 7.0Hz, 3H), 0.45-0.8 (m, 3H), 0.3-0.45 (m, 1H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]-N-シクロプロピルアミン300mg及びトリエチルアミン112mgの、ジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド211mgを滴下し、40℃にて5時間攪拌した。反応完結後、反応混合物を室温まで放冷、水2mlを加え、ジクロロメタンにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物130mgを無色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.7 (m, 1H), 7.9-8.0 (m, 1H), 7.55-7.75 (m, 2H), 7.35-7.55 (m, 2H), 5.8-6.1 (m, 1H), 2.4-2.65 (m, 1H), 1.56 (d, J=7.0Hz, 3H), 0.45-0.8 (m, 3H), 0.3-0.45 (m, 1H)。 Step 3: Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl) benzamide To a solution of 300 mg of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropylamine and 112 mg of triethylamine in 2 ml of dichloromethane, With stirring, 211 mg of 2- (trifluoromethyl) benzoyl chloride was added dropwise and stirred at 40 ° C. for 5 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 2 ml of water was added, and the mixture was extracted with dichloromethane (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 130 mg of the desired product as a colorless resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.7 (m, 1H), 7.9-8.0 (m, 1H), 7.55-7.75 (m, 2H), 7.35-7.55 (m, 2H) , 5.8-6.1 (m, 1H), 2.4-2.65 (m, 1H), 1.56 (d, J = 7.0Hz, 3H), 0.45-0.8 (m, 3H), 0.3-0.45 (m, 1H).
工程4;N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]-N-シクロプロピル-2-(トリフルオロメチル)ベンズアミドの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]-N-シクロプロピル-2-(トリフルオロメチル)ベンズアミド100mgのN,N-ジメチルホルムアミド1ml溶液に、トリエチルアミン61mg、シクロプロピルアセチレン26mg、ヨウ化銅(I)11mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)14mgを添加し、窒素雰囲気下、室温にて5時間攪拌した。反応完結後、反応混合物に、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物79mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.55 (m, 1H), 7.73 (d, J=1.7Hz, 1H), 7.6-7.65 (m, 1H), 7.35-7.5 (m, 3H), 5.9-6.1 (m, 1H), 2.45-2.6 (m, 1H), 1.55 (d, J=9.0Hz, 3H), 1.4-1.5 (m, 1H), 0.9-1.05 (m, 2H), 0.8-0.9 (m, 2H), 0.6-0.7 (m, 2H), 0.25-0.4 (m, 2H)。 Step 4: N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl ) Preparation of benzamide N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl) benzamide 100 mg To a 1 ml solution of N, N-dimethylformamide was added 61 mg of triethylamine, 26 mg of cyclopropylacetylene, 11 mg of copper (I) iodide and 14 mg of dichlorobis (triphenylphosphine) palladium (II). Stir for hours. After completion of the reaction, 10 ml of a saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 79 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.55 (m, 1H), 7.73 (d, J = 1.7Hz, 1H), 7.6-7.65 (m, 1H), 7.35-7.5 (m , 3H), 5.9-6.1 (m, 1H), 2.45-2.6 (m, 1H), 1.55 (d, J = 9.0Hz, 3H), 1.4-1.5 (m, 1H), 0.9-1.05 (m, 2H ), 0.8-0.9 (m, 2H), 0.6-0.7 (m, 2H), 0.25-0.4 (m, 2H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]-N-シクロプロピル-2-(トリフルオロメチル)ベンズアミド100mgのN,N-ジメチルホルムアミド1ml溶液に、トリエチルアミン61mg、シクロプロピルアセチレン26mg、ヨウ化銅(I)11mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)14mgを添加し、窒素雰囲気下、室温にて5時間攪拌した。反応完結後、反応混合物に、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。得られた有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物79mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.55 (m, 1H), 7.73 (d, J=1.7Hz, 1H), 7.6-7.65 (m, 1H), 7.35-7.5 (m, 3H), 5.9-6.1 (m, 1H), 2.45-2.6 (m, 1H), 1.55 (d, J=9.0Hz, 3H), 1.4-1.5 (m, 1H), 0.9-1.05 (m, 2H), 0.8-0.9 (m, 2H), 0.6-0.7 (m, 2H), 0.25-0.4 (m, 2H)。 Step 4: N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl ) Preparation of benzamide N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] -N-cyclopropyl-2- (trifluoromethyl) benzamide 100 mg To a 1 ml solution of N, N-dimethylformamide was added 61 mg of triethylamine, 26 mg of cyclopropylacetylene, 11 mg of copper (I) iodide and 14 mg of dichlorobis (triphenylphosphine) palladium (II). Stir for hours. After completion of the reaction, 10 ml of a saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (10 ml × 1). The obtained organic layer was washed with water (10 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 79 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45-8.55 (m, 1H), 7.73 (d, J = 1.7Hz, 1H), 7.6-7.65 (m, 1H), 7.35-7.5 (m , 3H), 5.9-6.1 (m, 1H), 2.45-2.6 (m, 1H), 1.55 (d, J = 9.0Hz, 3H), 1.4-1.5 (m, 1H), 0.9-1.05 (m, 2H ), 0.8-0.9 (m, 2H), 0.6-0.7 (m, 2H), 0.25-0.4 (m, 2H).
合成例5
N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1,1-ジメチルエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-017)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1,1-ジメチルエチルアミンの製造
合成例1の工程1にて製造した2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリル423mgのジエチルエーテル3ml溶液に、氷冷攪拌下、メチルマグネシウムブロミドの3Mジエチルエーテル溶液2mlを滴下し、同温度にて30分間攪拌した。次いで、この反応混合物に、氷冷攪拌下、チタニウム(IV)テトライソプロポキシド568mgを添加し、室温にてさらに4時間攪拌を継続した。反応完結後、反応混合物に、水酸化ナトリウム300mgの水10ml溶液を加え、ジエチルエーテルにて抽出(10mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物534mgを褐色油状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.60 (d, J=1.8Hz, 1H), 7.98 (d, J=1.8Hz, 1H), 1.29 (s, 6H)。 Synthesis example 5
N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide (Compound of the present invention) No.1-017).
Step 1; Preparation of 2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethylamine 2- (5-bromo prepared in Step 1 of Synthesis Example 1 To 3 ml of diethyl ether in 423 mg of -3-chloropyridin-2-yl) -2,2-difluoroacetonitrile, 2 ml of 3M diethyl ether solution of methylmagnesium bromide was added dropwise with stirring under ice cooling, and the mixture was stirred at the same temperature for 30 minutes. did. Next, 568 mg of titanium (IV) tetraisopropoxide was added to the reaction mixture under ice-cooling and stirring was continued at room temperature for another 4 hours. After completion of the reaction, a solution of 300 mg of sodium hydroxide in 10 ml of water was added to the reaction mixture, and extracted with diethyl ether (10 ml × 2). The organic layers were combined and washed with water (20 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 534 mg of the crude desired product as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.60 (d, J = 1.8 Hz, 1H), 7.98 (d, J = 1.8 Hz, 1H), 1.29 (s, 6H).
N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1,1-ジメチルエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-017)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1,1-ジメチルエチルアミンの製造
合成例1の工程1にて製造した2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロアセトニトリル423mgのジエチルエーテル3ml溶液に、氷冷攪拌下、メチルマグネシウムブロミドの3Mジエチルエーテル溶液2mlを滴下し、同温度にて30分間攪拌した。次いで、この反応混合物に、氷冷攪拌下、チタニウム(IV)テトライソプロポキシド568mgを添加し、室温にてさらに4時間攪拌を継続した。反応完結後、反応混合物に、水酸化ナトリウム300mgの水10ml溶液を加え、ジエチルエーテルにて抽出(10mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物534mgを褐色油状物質として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.60 (d, J=1.8Hz, 1H), 7.98 (d, J=1.8Hz, 1H), 1.29 (s, 6H)。 Synthesis example 5
N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide (Compound of the present invention) No.1-017).
Step 1; Preparation of 2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethylamine 2- (5-bromo prepared in Step 1 of Synthesis Example 1 To 3 ml of diethyl ether in 423 mg of -3-chloropyridin-2-yl) -2,2-difluoroacetonitrile, 2 ml of 3M diethyl ether solution of methylmagnesium bromide was added dropwise with stirring under ice cooling, and the mixture was stirred at the same temperature for 30 minutes. did. Next, 568 mg of titanium (IV) tetraisopropoxide was added to the reaction mixture under ice-cooling and stirring was continued at room temperature for another 4 hours. After completion of the reaction, a solution of 300 mg of sodium hydroxide in 10 ml of water was added to the reaction mixture, and extracted with diethyl ether (10 ml × 2). The organic layers were combined and washed with water (20 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 534 mg of the crude desired product as a brown oily substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.60 (d, J = 1.8 Hz, 1H), 7.98 (d, J = 1.8 Hz, 1H), 1.29 (s, 6H).
工程2;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1,1-ジメチルエチル]-2-(トリフルオロメチル)ベンズアミドの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1,1-ジメチルエチルアミン534mgの酢酸エチル5ml溶液、及び炭酸カリウム828mgの水5ml溶液の混合溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド417mgを滴下、室温にて1時間攪拌した。反応完結後、有機層を分取して水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(5:95~15:85のグラジエント)]にて精製し、目的物340mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.50 (d, J=2.1Hz, 1H), 8.00 (d, J=2.1Hz, 1H), 7.4-7.7 (m, 4H), 6.85 (s, 1H), 1.54 (s, 6H)。 Step 2: Production of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide 2- ( 5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethylamine was added to a mixed solution of 534 mg of ethyl acetate in 5 ml and potassium carbonate 828 mg in 5 ml of water under ice-cooling and stirring. , 417 mg of 2- (trifluoromethyl) benzoyl chloride was added dropwise and stirred at room temperature for 1 hour. After completion of the reaction, the organic layer was separated, washed with water (10 ml × 1), dehydrated and dried with saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (5:95 to 15:85 gradient)] to obtain 340 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.50 (d, J = 2.1 Hz, 1H), 8.00 (d, J = 2.1 Hz, 1H), 7.4-7.7 (m, 4H), 6.85 ( s, 1H), 1.54 (s, 6H).
2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1,1-ジメチルエチルアミン534mgの酢酸エチル5ml溶液、及び炭酸カリウム828mgの水5ml溶液の混合溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド417mgを滴下、室温にて1時間攪拌した。反応完結後、有機層を分取して水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(5:95~15:85のグラジエント)]にて精製し、目的物340mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.50 (d, J=2.1Hz, 1H), 8.00 (d, J=2.1Hz, 1H), 7.4-7.7 (m, 4H), 6.85 (s, 1H), 1.54 (s, 6H)。 Step 2: Production of N- [2- (5-bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide 2- ( 5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethylamine was added to a mixed solution of 534 mg of ethyl acetate in 5 ml and potassium carbonate 828 mg in 5 ml of water under ice-cooling and stirring. , 417 mg of 2- (trifluoromethyl) benzoyl chloride was added dropwise and stirred at room temperature for 1 hour. After completion of the reaction, the organic layer was separated, washed with water (10 ml × 1), dehydrated and dried with saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (5:95 to 15:85 gradient)] to obtain 340 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.50 (d, J = 2.1 Hz, 1H), 8.00 (d, J = 2.1 Hz, 1H), 7.4-7.7 (m, 4H), 6.85 ( s, 1H), 1.54 (s, 6H).
工程3;N-[2-[3-クロロ-5-(シクロプロピルエチニル)ピリジン-2-イル]-2,2-ジフルオロ-1,1-ジメチルエチル]-2-(トリフルオロメチル)ベンズアミドの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1,1-ジメチルエチル]-2-(トリフルオロメチル)ベンズアミド170mgのジメチルスルホキシド1ml溶液に、トリエチルアミン146mg、シクロプロピルアセチレン31mg、ヨウ化銅(I)20mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)25mgを添加し、窒素雰囲気下、室温にて2時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液5mlを添加し、酢酸エチルにて抽出(5mlx1)した。得られた有機層を水洗(5mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物41mgを無色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.35 (d, J=1.5Hz, 1H), 7.76 (d, J=1.5Hz, 1H), 7.4-7.7 (m, 4H), 7.13 (bs, 1H), 1.73 (s, 6H), 1.4-1.55 (m, 1H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H)。 Step 3; N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide Preparation N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide in a solution of 170 mg of dimethyl sulfoxide in 1 ml 146 mg of triethylamine, 31 mg of cyclopropylacetylene, 20 mg of copper (I) iodide and 25 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at room temperature for 2 hours under a nitrogen atmosphere. After completion of the reaction, 5 ml of saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (5 ml × 1). The obtained organic layer was washed with water (5 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 41 mg of the desired product as a colorless resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.35 (d, J = 1.5 Hz, 1H), 7.76 (d, J = 1.5 Hz, 1H), 7.4-7.7 (m, 4H), 7.13 ( bs, 1H), 1.73 (s, 6H), 1.4-1.55 (m, 1H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1,1-ジメチルエチル]-2-(トリフルオロメチル)ベンズアミド170mgのジメチルスルホキシド1ml溶液に、トリエチルアミン146mg、シクロプロピルアセチレン31mg、ヨウ化銅(I)20mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)25mgを添加し、窒素雰囲気下、室温にて2時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液5mlを添加し、酢酸エチルにて抽出(5mlx1)した。得られた有機層を水洗(5mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムにて脱水・乾燥後、減圧下にて溶媒を留去した。残留物をシリカゲルカラムクロマトグラフィー[酢酸エチル-ヘキサン(1:9~3:7のグラジエント)]にて精製し、目的物41mgを無色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.35 (d, J=1.5Hz, 1H), 7.76 (d, J=1.5Hz, 1H), 7.4-7.7 (m, 4H), 7.13 (bs, 1H), 1.73 (s, 6H), 1.4-1.55 (m, 1H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H)。 Step 3; N- [2- [3-Chloro-5- (cyclopropylethynyl) pyridin-2-yl] -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide Preparation N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1,1-dimethylethyl] -2- (trifluoromethyl) benzamide in a solution of 170 mg of dimethyl sulfoxide in 1 ml 146 mg of triethylamine, 31 mg of cyclopropylacetylene, 20 mg of copper (I) iodide and 25 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at room temperature for 2 hours under a nitrogen atmosphere. After completion of the reaction, 5 ml of saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (5 ml × 1). The obtained organic layer was washed with water (5 ml × 1), dehydrated and dried over saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography [ethyl acetate-hexane (1: 9 to 3: 7 gradient)] to obtain 41 mg of the desired product as a colorless resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.35 (d, J = 1.5 Hz, 1H), 7.76 (d, J = 1.5 Hz, 1H), 7.4-7.7 (m, 4H), 7.13 ( bs, 1H), 1.73 (s, 6H), 1.4-1.55 (m, 1H), 0.9-1.0 (m, 2H), 0.8-0.9 (m, 2H).
合成例6
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]エチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-019)。
工程1;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)エチル]カルバミド酸-tert-ブチルの製造
窒素雰囲気下の2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル1.00gのジクロロメタン10ml溶液に、-40℃にて攪拌下、ジイソブチルアルミニウムヒドリドの1.0Mヘキサン溶液12mlを30分かけて滴下し、滴下終了後、同温度にて1時間攪拌した。次いで、反応混合物を室温まで昇温し、氷冷攪拌下の酒石酸カリウムナトリウム(ロッシェル塩)飽和水溶液50mlに滴下した。滴下終了後、室温にてさらに1時間攪拌を継続した。反応完結後、反応混合物をジクロロメタンにて抽出(50mlx2)、有機層を併せ飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。 Synthesis Example 6
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethyl] -2- (trifluoromethyl) benzamide (the present compound No. 1-019).
Step 1; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) ethyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridin-2- under nitrogen atmosphere I) A solution of 1.00 g of acetonitrile in 10 ml of dichloromethane was added dropwise over 12 minutes with 12 ml of a 1.0 M hexane solution of diisobutylaluminum hydride while stirring at -40 ° C. After completion of the dropwise addition, the mixture was stirred at the same temperature for 1 hour. . The reaction mixture was then warmed to room temperature and added dropwise to 50 ml of a saturated aqueous solution of potassium sodium tartrate (Rochelle salt) under ice-cooling and stirring. After completion of the dropwise addition, stirring was continued for another hour at room temperature. After completion of the reaction, the reaction mixture was extracted with dichloromethane (50 ml × 2), and the organic layers were combined and dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure.
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]エチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-019)。
工程1;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)エチル]カルバミド酸-tert-ブチルの製造
窒素雰囲気下の2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル1.00gのジクロロメタン10ml溶液に、-40℃にて攪拌下、ジイソブチルアルミニウムヒドリドの1.0Mヘキサン溶液12mlを30分かけて滴下し、滴下終了後、同温度にて1時間攪拌した。次いで、反応混合物を室温まで昇温し、氷冷攪拌下の酒石酸カリウムナトリウム(ロッシェル塩)飽和水溶液50mlに滴下した。滴下終了後、室温にてさらに1時間攪拌を継続した。反応完結後、反応混合物をジクロロメタンにて抽出(50mlx2)、有機層を併せ飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。 Synthesis Example 6
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethyl] -2- (trifluoromethyl) benzamide (the present compound No. 1-019).
Step 1; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) ethyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridin-2- under nitrogen atmosphere I) A solution of 1.00 g of acetonitrile in 10 ml of dichloromethane was added dropwise over 12 minutes with 12 ml of a 1.0 M hexane solution of diisobutylaluminum hydride while stirring at -40 ° C. After completion of the dropwise addition, the mixture was stirred at the same temperature for 1 hour. . The reaction mixture was then warmed to room temperature and added dropwise to 50 ml of a saturated aqueous solution of potassium sodium tartrate (Rochelle salt) under ice-cooling and stirring. After completion of the dropwise addition, stirring was continued for another hour at room temperature. After completion of the reaction, the reaction mixture was extracted with dichloromethane (50 ml × 2), and the organic layers were combined and dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure.
残留物をジクロロメタン20mlに溶解し、二炭酸ジ-tert-ブチル1.41g及びトリエチルアミン0.66gを添加、室温にて1時間攪拌した。反応完結後、反応混合物に水20mlを加えクロロホルムにて抽出(20mlx1)し、有機層を水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~5:5のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物615mgを淡黄色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.49 (d, J=1.8Hz, 1H), 7.82 (d, J=1.8Hz, 1H), 5.08 (bs, 1H), 3.5-3.65 (m, 2H), 3.06 (t, J=6.0Hz, 2H), 1.42 (s, 9H)。 The residue was dissolved in 20 ml of dichloromethane, 1.41 g of di-tert-butyl dicarbonate and 0.66 g of triethylamine were added, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 20 ml of water was added to the reaction mixture and the mixture was extracted with chloroform (20 ml × 1). The organic layer was washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine, then anhydrous sodium sulfate, Was distilled off. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 5: 5 gradient) to obtain 615 mg of the desired product as a pale yellow oily substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.49 (d, J = 1.8 Hz, 1H), 7.82 (d, J = 1.8 Hz, 1H), 5.08 (bs, 1H), 3.5-3.65 ( m, 2H), 3.06 (t, J = 6.0 Hz, 2H), 1.42 (s, 9H).
1H NMR (CDCl3, Me4Si, 300MHz) δ8.49 (d, J=1.8Hz, 1H), 7.82 (d, J=1.8Hz, 1H), 5.08 (bs, 1H), 3.5-3.65 (m, 2H), 3.06 (t, J=6.0Hz, 2H), 1.42 (s, 9H)。 The residue was dissolved in 20 ml of dichloromethane, 1.41 g of di-tert-butyl dicarbonate and 0.66 g of triethylamine were added, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 20 ml of water was added to the reaction mixture and the mixture was extracted with chloroform (20 ml × 1). The organic layer was washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine, then anhydrous sodium sulfate, Was distilled off. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 5: 5 gradient) to obtain 615 mg of the desired product as a pale yellow oily substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.49 (d, J = 1.8 Hz, 1H), 7.82 (d, J = 1.8 Hz, 1H), 5.08 (bs, 1H), 3.5-3.65 ( m, 2H), 3.06 (t, J = 6.0 Hz, 2H), 1.42 (s, 9H).
工程2;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]エチル]カルバミド酸-tert-ブチルの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)エチル]カルバミド酸-tert-ブチル1.0gのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン455mg、1-エチニル-4-フルオロベンゼン540mg、ヨウ化銅(I)114mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)209mgを添加し、窒素雰囲気下、80℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(5mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.1gを褐色結晶として得た。
融点109.0~111.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.54 (d, J=1.7Hz, 1H), 7.76 (d, J=1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.15 (m, 2H), 5.13 (bs, 1H), 3.55-3.7 (m, 2H), 3.12 (t, J=6.1Hz, 2H), 1.42 (s, 9H)。 Step 2; Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethyl] carbamic acid-tert-butyl N- [2- (5-Bromo- 3-chloropyridin-2-yl) ethyl] carbamic acid-tert-butyl in a solution of 1.0 g of N, N-dimethylformamide in 1 ml of triethylamine 455 mg, 1-ethynyl-4-fluorobenzene 540 mg, copper (I) iodide 114 mg And dichlorobis (triphenylphosphine) palladium (II) (209 mg) were added, and the mixture was stirred at 80 ° C. for 2 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (5 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (0:10 to 1: 9 gradient) to obtain 1.1 g of the objective product as brown crystals.
Melting point: 109.0-111.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.54 (d, J = 1.7 Hz, 1H), 7.76 (d, J = 1.7 Hz, 1H), 7.45-7.55 (m, 2H), 7.0- 7.15 (m, 2H), 5.13 (bs, 1H), 3.55-3.7 (m, 2H), 3.12 (t, J = 6.1Hz, 2H), 1.42 (s, 9H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)エチル]カルバミド酸-tert-ブチル1.0gのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン455mg、1-エチニル-4-フルオロベンゼン540mg、ヨウ化銅(I)114mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)209mgを添加し、窒素雰囲気下、80℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(5mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.1gを褐色結晶として得た。
融点109.0~111.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.54 (d, J=1.7Hz, 1H), 7.76 (d, J=1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.15 (m, 2H), 5.13 (bs, 1H), 3.55-3.7 (m, 2H), 3.12 (t, J=6.1Hz, 2H), 1.42 (s, 9H)。 Step 2; Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethyl] carbamic acid-tert-butyl N- [2- (5-Bromo- 3-chloropyridin-2-yl) ethyl] carbamic acid-tert-butyl in a solution of 1.0 g of N, N-dimethylformamide in 1 ml of triethylamine 455 mg, 1-ethynyl-4-fluorobenzene 540 mg, copper (I) iodide 114 mg And dichlorobis (triphenylphosphine) palladium (II) (209 mg) were added, and the mixture was stirred at 80 ° C. for 2 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (5 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (0:10 to 1: 9 gradient) to obtain 1.1 g of the objective product as brown crystals.
Melting point: 109.0-111.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.54 (d, J = 1.7 Hz, 1H), 7.76 (d, J = 1.7 Hz, 1H), 7.45-7.55 (m, 2H), 7.0- 7.15 (m, 2H), 5.13 (bs, 1H), 3.55-3.7 (m, 2H), 3.12 (t, J = 6.1Hz, 2H), 1.42 (s, 9H).
工程3;2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]エチルアミンの製造
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]エチル]カルバミド酸-tert-ブチル1.1gのジクロロメタン2ml溶液にトリフルオロ酢酸2mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物710mgを暗褐色結晶として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
融点116.0~119.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.5-8.6 (m, 1H), 7.75-7.8 (m, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H), 3.1-3.35 (m, 2H), 2.3-2.45 (m, 2H)。 Step 3; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethylamine N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] [Pyridin-2-yl] ethyl] carbamic acid-tert-butyl To a solution of 1.1 g of dichloromethane in 2 ml of dichloromethane was added 2 ml of trifluoroacetic acid and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 710 mg of the crude desired product as dark brown crystals. The obtained target product was directly used in the next step without further purification.
Melting point 116.0-119.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.5-8.6 (m, 1H), 7.75-7.8 (m, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H) , 3.1-3.35 (m, 2H), 2.3-2.45 (m, 2H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]エチル]カルバミド酸-tert-ブチル1.1gのジクロロメタン2ml溶液にトリフルオロ酢酸2mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物710mgを暗褐色結晶として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
融点116.0~119.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.5-8.6 (m, 1H), 7.75-7.8 (m, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H), 3.1-3.35 (m, 2H), 2.3-2.45 (m, 2H)。 Step 3; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethylamine N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] [Pyridin-2-yl] ethyl] carbamic acid-tert-butyl To a solution of 1.1 g of dichloromethane in 2 ml of dichloromethane was added 2 ml of trifluoroacetic acid and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 710 mg of the crude desired product as dark brown crystals. The obtained target product was directly used in the next step without further purification.
Melting point 116.0-119.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.5-8.6 (m, 1H), 7.75-7.8 (m, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H) , 3.1-3.35 (m, 2H), 2.3-2.45 (m, 2H).
工程4;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]エチル]-2-(トリフルオロメチル)ベンズアミドの製造
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]エチルアミン200mg及びトリエチルアミン110mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド182mgを滴下し、滴下終了後、室温にて1時間攪拌を継続した。反応完結後、反応混合物に水2mlを添加し、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物91mgを黄色結晶として得た。
融点133.0~135.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.49 (d, J=1.7Hz, 1H), 7.79 (d, J=1.7Hz, 1H), 7.45-7.7 (m, 6H), 7.0-7.1 (m, 2H), 6.65-6.8 (m, 1H), 0.95-4.05 (m, 2H), 3.24 (t, J=6.1Hz, 2H)。 Step 4: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethyl] -2- (trifluoromethyl) benzamide 2- [3-Chloro- To a solution of 200 mg of 5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethylamine and 110 mg of triethylamine in 2 ml of dichloromethane was added dropwise 182 mg of 2- (trifluoromethyl) benzoyl chloride with ice-cooling and stirring. The stirring was continued for 1 hour at room temperature. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 91 mg of the objective product as yellow crystals.
Melting point 133.0 to 135.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.49 (d, J = 1.7 Hz, 1H), 7.79 (d, J = 1.7 Hz, 1H), 7.45-7.7 (m, 6H), 7.0- 7.1 (m, 2H), 6.65-6.8 (m, 1H), 0.95-4.05 (m, 2H), 3.24 (t, J = 6.1Hz, 2H).
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]エチルアミン200mg及びトリエチルアミン110mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド182mgを滴下し、滴下終了後、室温にて1時間攪拌を継続した。反応完結後、反応混合物に水2mlを添加し、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物91mgを黄色結晶として得た。
融点133.0~135.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.49 (d, J=1.7Hz, 1H), 7.79 (d, J=1.7Hz, 1H), 7.45-7.7 (m, 6H), 7.0-7.1 (m, 2H), 6.65-6.8 (m, 1H), 0.95-4.05 (m, 2H), 3.24 (t, J=6.1Hz, 2H)。 Step 4: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethyl] -2- (trifluoromethyl) benzamide 2- [3-Chloro- To a solution of 200 mg of 5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] ethylamine and 110 mg of triethylamine in 2 ml of dichloromethane was added dropwise 182 mg of 2- (trifluoromethyl) benzoyl chloride with ice-cooling and stirring. The stirring was continued for 1 hour at room temperature. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 91 mg of the objective product as yellow crystals.
Melting point 133.0 to 135.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.49 (d, J = 1.7 Hz, 1H), 7.79 (d, J = 1.7 Hz, 1H), 7.45-7.7 (m, 6H), 7.0- 7.1 (m, 2H), 6.65-6.8 (m, 1H), 0.95-4.05 (m, 2H), 3.24 (t, J = 6.1Hz, 2H).
合成例7
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-020)。
工程1;2-ブロモ-1-(5-ブロモ-3-クロロピリジン-2-イル)エタノールの製造
2-ブロモ-1-(5-ブロモ-3-クロロピリジン-2-イル)エタノン2.0gのメタノール10ml溶液に、氷冷攪拌下、水素化ホウ素ナトリウム266mgを添加し、同温度にて1時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液20mlを添加し酢酸エチルにて抽出(25mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.9gを褐色油状物質として得た。得られた目的物は更なる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.58 (d, J=1.7Hz, 1H), 7.90 (d, J=1.7Hz, 1H), 5.2-5.3 (m, 1H), 4.36 (bs, 1H), 3.6-3.9 (m, 1H), 3.15-3.3 (m, 1H)。 Synthesis example 7
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] -2- (trifluoromethyl) benzamide (Compound No. 1- 020).
Step 1: Preparation of 2-bromo-1- (5-bromo-3-chloropyridin-2-yl) ethanol 2-Bromo-1- (5-bromo-3-chloropyridin-2-yl) ethanone 2.0 g 266 mg of sodium borohydride was added to a 10 ml methanol solution under ice-cooling and the mixture was stirred at the same temperature for 1 hour. After completion of the reaction, 20 ml of a saturated aqueous ammonium chloride solution was added to the reaction mixture and extracted with ethyl acetate (25 ml × 2). The organic layers were combined and washed with water (20 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 1.9 g of a crude desired product as a brown oily substance. . The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.58 (d, J = 1.7 Hz, 1H), 7.90 (d, J = 1.7 Hz, 1H), 5.2-5.3 (m, 1H), 4.36 ( bs, 1H), 3.6-3.9 (m, 1H), 3.15-3.3 (m, 1H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-020)。
工程1;2-ブロモ-1-(5-ブロモ-3-クロロピリジン-2-イル)エタノールの製造
2-ブロモ-1-(5-ブロモ-3-クロロピリジン-2-イル)エタノン2.0gのメタノール10ml溶液に、氷冷攪拌下、水素化ホウ素ナトリウム266mgを添加し、同温度にて1時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液20mlを添加し酢酸エチルにて抽出(25mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.9gを褐色油状物質として得た。得られた目的物は更なる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.58 (d, J=1.7Hz, 1H), 7.90 (d, J=1.7Hz, 1H), 5.2-5.3 (m, 1H), 4.36 (bs, 1H), 3.6-3.9 (m, 1H), 3.15-3.3 (m, 1H)。 Synthesis example 7
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] -2- (trifluoromethyl) benzamide (Compound No. 1- 020).
Step 1: Preparation of 2-bromo-1- (5-bromo-3-chloropyridin-2-yl) ethanol 2-Bromo-1- (5-bromo-3-chloropyridin-2-yl) ethanone 2.0 g 266 mg of sodium borohydride was added to a 10 ml methanol solution under ice-cooling and the mixture was stirred at the same temperature for 1 hour. After completion of the reaction, 20 ml of a saturated aqueous ammonium chloride solution was added to the reaction mixture and extracted with ethyl acetate (25 ml × 2). The organic layers were combined and washed with water (20 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 1.9 g of a crude desired product as a brown oily substance. . The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.58 (d, J = 1.7 Hz, 1H), 7.90 (d, J = 1.7 Hz, 1H), 5.2-5.3 (m, 1H), 4.36 ( bs, 1H), 3.6-3.9 (m, 1H), 3.15-3.3 (m, 1H).
工程2;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-ヒドロキシエチル]フタルイミドの製造
2-ブロモ-1-(5-ブロモ-3-クロロピリジン-2-イル)エタノール1.90gのジメチルスルホキシド2ml溶液にフタルイミドカリウム1.17gを添加し、60℃にて1時間攪拌した。反応完結後、反応混合物を室温まで放冷し、水15mlを加え酢酸エチルにて抽出(15mlx1)した。有機層を水洗(15mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物0.93gを暗褐色結晶として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
融点146.0~149.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.52 (d, J=2.1Hz, 1H), 7.88 (d, J=2.1Hz, 1H), 7.8-7.85 (m, 2H), 7.65-7.8 (m, 2H), 5.3-5.5 (m, 1H), 3.95-4.2 (m, 2H), 1.55-1.8 (m, 1H)。 Step 2: Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-hydroxyethyl] phthalimide 2-Bromo-1- (5-bromo-3-chloropyridin-2-yl ) 1.17 g of potassium phthalimide was added to 2 ml of dimethyl sulfoxide in 1.90 g of ethanol, and the mixture was stirred at 60 ° C. for 1 hour. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 15 ml of water was added, and the mixture was extracted with ethyl acetate (15 ml × 1). The organic layer was washed with water (15 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 0.93 g of the crude desired product as dark brown crystals. This was directly used in the next step without further purification.
Melting point: 146.0-149.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.52 (d, J = 2.1 Hz, 1H), 7.88 (d, J = 2.1 Hz, 1H), 7.8-7.85 (m, 2H), 7.65- 7.8 (m, 2H), 5.3-5.5 (m, 1H), 3.95-4.2 (m, 2H), 1.55-1.8 (m, 1H).
2-ブロモ-1-(5-ブロモ-3-クロロピリジン-2-イル)エタノール1.90gのジメチルスルホキシド2ml溶液にフタルイミドカリウム1.17gを添加し、60℃にて1時間攪拌した。反応完結後、反応混合物を室温まで放冷し、水15mlを加え酢酸エチルにて抽出(15mlx1)した。有機層を水洗(15mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物0.93gを暗褐色結晶として得た。このものはさらなる精製を行うことなく、そのまま次の工程に用いた。
融点146.0~149.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.52 (d, J=2.1Hz, 1H), 7.88 (d, J=2.1Hz, 1H), 7.8-7.85 (m, 2H), 7.65-7.8 (m, 2H), 5.3-5.5 (m, 1H), 3.95-4.2 (m, 2H), 1.55-1.8 (m, 1H)。 Step 2: Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-hydroxyethyl] phthalimide 2-Bromo-1- (5-bromo-3-chloropyridin-2-yl ) 1.17 g of potassium phthalimide was added to 2 ml of dimethyl sulfoxide in 1.90 g of ethanol, and the mixture was stirred at 60 ° C. for 1 hour. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 15 ml of water was added, and the mixture was extracted with ethyl acetate (15 ml × 1). The organic layer was washed with water (15 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 0.93 g of the crude desired product as dark brown crystals. This was directly used in the next step without further purification.
Melting point: 146.0-149.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.52 (d, J = 2.1 Hz, 1H), 7.88 (d, J = 2.1 Hz, 1H), 7.8-7.85 (m, 2H), 7.65- 7.8 (m, 2H), 5.3-5.5 (m, 1H), 3.95-4.2 (m, 2H), 1.55-1.8 (m, 1H).
工程3;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-フルオロエチル]フタルイミドの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-ヒドロキシエチル]フタルイミド930mgのジクロロメタン3ml溶液にビス(2-メトキシエチル)アミノサルファートリフルオリド808mgを滴下し、滴下終了後、60℃にて3時間攪拌した。次いで反応混合物を室温まで放冷し、ビス(2-メトキシエチル)アミノサルファートリフルオリド540mgを追加し、再び60℃にて1時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和炭酸水素ナトリウム水溶液15mlを加え、酢酸エチルにて抽出(15mlx1)した。有機層を水洗(15mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物700mgを黄色結晶として得た。
融点153.0~154.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.6-8.65 (m, 1H), 7.9-7.95 (m, 1H), 7.85-7.9 (m, 2H), 7.7-7.8 (m, 2H), 6.17 (ddd, J=47.0, 8.5, 4.1Hz, 1H), 4.55-4.7 (m, 1H), 4.0-4.2 (m, 1H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-fluoroethyl] phthalimide N- [2- (5-Bromo-3-chloropyridin-2-yl) Bis (2-methoxyethyl) aminosulfur trifluoride (808 mg) was added dropwise to a solution of -2-mg-hydroxyethyl] phthalimide (930 mg) in dichloromethane (3 ml), and the mixture was stirred at 60 ° C. for 3 hours. The reaction mixture was then allowed to cool to room temperature, 540 mg of bis (2-methoxyethyl) aminosulfur trifluoride was added, and the mixture was stirred again at 60 ° C. for 1 hour. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 15 ml of saturated aqueous sodium hydrogen carbonate solution was added, and the mixture was extracted with ethyl acetate (15 ml × 1). The organic layer was washed with water (15 ml x 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9-2: 8 gradient) to obtain 700 mg of the objective product as yellow crystals.
Melting point: 153.0-154.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.6-8.65 (m, 1H), 7.9-7.95 (m, 1H), 7.85-7.9 (m, 2H), 7.7-7.8 (m, 2H) 6.17 (ddd, J = 47.0, 8.5, 4.1 Hz, 1H), 4.55-4.7 (m, 1H), 4.0-4.2 (m, 1H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-ヒドロキシエチル]フタルイミド930mgのジクロロメタン3ml溶液にビス(2-メトキシエチル)アミノサルファートリフルオリド808mgを滴下し、滴下終了後、60℃にて3時間攪拌した。次いで反応混合物を室温まで放冷し、ビス(2-メトキシエチル)アミノサルファートリフルオリド540mgを追加し、再び60℃にて1時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和炭酸水素ナトリウム水溶液15mlを加え、酢酸エチルにて抽出(15mlx1)した。有機層を水洗(15mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物700mgを黄色結晶として得た。
融点153.0~154.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.6-8.65 (m, 1H), 7.9-7.95 (m, 1H), 7.85-7.9 (m, 2H), 7.7-7.8 (m, 2H), 6.17 (ddd, J=47.0, 8.5, 4.1Hz, 1H), 4.55-4.7 (m, 1H), 4.0-4.2 (m, 1H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-fluoroethyl] phthalimide N- [2- (5-Bromo-3-chloropyridin-2-yl) Bis (2-methoxyethyl) aminosulfur trifluoride (808 mg) was added dropwise to a solution of -2-mg-hydroxyethyl] phthalimide (930 mg) in dichloromethane (3 ml), and the mixture was stirred at 60 ° C. for 3 hours. The reaction mixture was then allowed to cool to room temperature, 540 mg of bis (2-methoxyethyl) aminosulfur trifluoride was added, and the mixture was stirred again at 60 ° C. for 1 hour. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 15 ml of saturated aqueous sodium hydrogen carbonate solution was added, and the mixture was extracted with ethyl acetate (15 ml × 1). The organic layer was washed with water (15 ml x 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9-2: 8 gradient) to obtain 700 mg of the objective product as yellow crystals.
Melting point: 153.0-154.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.6-8.65 (m, 1H), 7.9-7.95 (m, 1H), 7.85-7.9 (m, 2H), 7.7-7.8 (m, 2H) 6.17 (ddd, J = 47.0, 8.5, 4.1 Hz, 1H), 4.55-4.7 (m, 1H), 4.0-4.2 (m, 1H).
工程4;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロエチル]フタルイミドの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-フルオロエチル]フタルイミド700mgのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン276mg、1-エチニル-4-フルオロベンゼン285mg、ヨウ化銅(I)69mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)64mgを添加し、窒素雰囲気下、80℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物580mgを褐色結晶として得た。
融点184.0~186.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.65-8.7 (m, 1H), 7.8-7.95 (m, 3H), 7.7-7.8 (m, 2H), 7.5-7.6 (m, 2H), 7.0-7.15 (m, 2H), 6.22 (ddd, J=48.7, 8.5, 4.1Hz, 1H), 4.55-4.75 (m, 1H), 4.05-4.25 (m, 1H)。 Step 4: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] phthalimide N- [2- (5-bromo-3 -Chloropyridin-2-yl) -2-fluoroethyl] phthalimide in a solution of 700 mg of N, N-dimethylformamide in 276 mg of triethylamine, 285 mg of 1-ethynyl-4-fluorobenzene, 69 mg of copper (I) iodide and dichlorobis (tri Phenylphosphine) palladium (II) 64 mg was added, and the mixture was stirred at 80 ° C. for 2 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 580 mg of the desired product as brown crystals.
Melting point: 184.0-186.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.65-8.7 (m, 1H), 7.8-7.95 (m, 3H), 7.7-7.8 (m, 2H), 7.5-7.6 (m, 2H) , 7.0-7.15 (m, 2H), 6.22 (ddd, J = 48.7, 8.5, 4.1Hz, 1H), 4.55-4.75 (m, 1H), 4.05-4.25 (m, 1H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-フルオロエチル]フタルイミド700mgのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン276mg、1-エチニル-4-フルオロベンゼン285mg、ヨウ化銅(I)69mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)64mgを添加し、窒素雰囲気下、80℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物580mgを褐色結晶として得た。
融点184.0~186.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.65-8.7 (m, 1H), 7.8-7.95 (m, 3H), 7.7-7.8 (m, 2H), 7.5-7.6 (m, 2H), 7.0-7.15 (m, 2H), 6.22 (ddd, J=48.7, 8.5, 4.1Hz, 1H), 4.55-4.75 (m, 1H), 4.05-4.25 (m, 1H)。 Step 4: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] phthalimide N- [2- (5-bromo-3 -Chloropyridin-2-yl) -2-fluoroethyl] phthalimide in a solution of 700 mg of N, N-dimethylformamide in 276 mg of triethylamine, 285 mg of 1-ethynyl-4-fluorobenzene, 69 mg of copper (I) iodide and dichlorobis (tri Phenylphosphine) palladium (II) 64 mg was added, and the mixture was stirred at 80 ° C. for 2 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 580 mg of the desired product as brown crystals.
Melting point: 184.0-186.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.65-8.7 (m, 1H), 7.8-7.95 (m, 3H), 7.7-7.8 (m, 2H), 7.5-7.6 (m, 2H) , 7.0-7.15 (m, 2H), 6.22 (ddd, J = 48.7, 8.5, 4.1Hz, 1H), 4.55-4.75 (m, 1H), 4.05-4.25 (m, 1H).
工程5;2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロエチルアミンの製造
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロエチル]フタルイミド580mgのエタノール3ml溶液にヒドラジン一水和物110mgを添加し、室温にて2時間攪拌した。反応完結後、反応混合物に飽和炭酸水素ナトリウム水溶液5mlを加え、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物300mgを黄色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.65-8.7 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.6 (m, 2H), 7.0-7.15 (m, 2H), 5.85 (ddd, J=47.8, 7.4, 3.7Hz, 1H), 3.1-3.45 (m, 2H), 1.57 (bs, 2H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethylamine N- [2- [3-Chloro-5-[(4- To a solution of 580 mg of fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] phthalimide in 3 ml of ethanol was added 110 mg of hydrazine monohydrate, and the mixture was stirred at room temperature for 2 hours. After completion of the reaction, 5 ml of saturated aqueous sodium hydrogen carbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 300 mg of the crude target product as a yellow resinous substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.65-8.7 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.6 (m, 2H), 7.0-7.15 (m, 2H) , 5.85 (ddd, J = 47.8, 7.4, 3.7Hz, 1H), 3.1-3.45 (m, 2H), 1.57 (bs, 2H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロエチル]フタルイミド580mgのエタノール3ml溶液にヒドラジン一水和物110mgを添加し、室温にて2時間攪拌した。反応完結後、反応混合物に飽和炭酸水素ナトリウム水溶液5mlを加え、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物300mgを黄色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.65-8.7 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.6 (m, 2H), 7.0-7.15 (m, 2H), 5.85 (ddd, J=47.8, 7.4, 3.7Hz, 1H), 3.1-3.45 (m, 2H), 1.57 (bs, 2H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethylamine N- [2- [3-Chloro-5-[(4- To a solution of 580 mg of fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] phthalimide in 3 ml of ethanol was added 110 mg of hydrazine monohydrate, and the mixture was stirred at room temperature for 2 hours. After completion of the reaction, 5 ml of saturated aqueous sodium hydrogen carbonate solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 300 mg of the crude target product as a yellow resinous substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.65-8.7 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.6 (m, 2H), 7.0-7.15 (m, 2H) , 5.85 (ddd, J = 47.8, 7.4, 3.7Hz, 1H), 3.1-3.45 (m, 2H), 1.57 (bs, 2H).
工程6;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロエチル]-2-(トリフルオロメチル)ベンズアミドの製造
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロエチルアミン100mg及びトリエチルアミン51mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド86mgを滴下し、滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2mlを加えジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物49mgを淡黄色結晶として得た。
融点141.0~143.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.64 (d, J=1.7Hz, 1H), 8.47 (dd, J=4.8, 1.7Hz, 1H), 8.12 (dd, J=8.2, 2.0Hz, 1H), 7.85-7.9 (m, 1H), 7.5-7.6 (m, 2H), 7.3-7.4 (m, 2H), 7.15-7.2 (m, 1H), 7.05-7.1 (m, 2H), 6.13 (ddd, J=46.7, 6.5, 4.1Hz, 1H), 4.1-4.45 (m, 2H)。 Step 6; Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] -2- (trifluoromethyl) benzamide 2- [ 2-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethylamine (100 mg) and triethylamine (51 mg) in dichloromethane (2 ml) were stirred with ice cooling and 2- (trifluoromethyl) benzoyl chloride. 86 mg was added dropwise, and after completion of the dropwise addition, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 49 mg of the objective product as pale yellow crystals.
Melting point: 141.0-143.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.64 (d, J = 1.7 Hz, 1H), 8.47 (dd, J = 4.8, 1.7 Hz, 1H), 8.12 (dd, J = 8.2, 2.0 Hz, 1H), 7.85-7.9 (m, 1H), 7.5-7.6 (m, 2H), 7.3-7.4 (m, 2H), 7.15-7.2 (m, 1H), 7.05-7.1 (m, 2H), 6.13 (ddd, J = 46.7, 6.5, 4.1Hz, 1H), 4.1-4.45 (m, 2H).
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-フルオロエチルアミン100mg及びトリエチルアミン51mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド86mgを滴下し、滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2mlを加えジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物49mgを淡黄色結晶として得た。
融点141.0~143.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.64 (d, J=1.7Hz, 1H), 8.47 (dd, J=4.8, 1.7Hz, 1H), 8.12 (dd, J=8.2, 2.0Hz, 1H), 7.85-7.9 (m, 1H), 7.5-7.6 (m, 2H), 7.3-7.4 (m, 2H), 7.15-7.2 (m, 1H), 7.05-7.1 (m, 2H), 6.13 (ddd, J=46.7, 6.5, 4.1Hz, 1H), 4.1-4.45 (m, 2H)。 Step 6; Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethyl] -2- (trifluoromethyl) benzamide 2- [ 2-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-fluoroethylamine (100 mg) and triethylamine (51 mg) in dichloromethane (2 ml) were stirred with ice cooling and 2- (trifluoromethyl) benzoyl chloride. 86 mg was added dropwise, and after completion of the dropwise addition, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 49 mg of the objective product as pale yellow crystals.
Melting point: 141.0-143.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.64 (d, J = 1.7 Hz, 1H), 8.47 (dd, J = 4.8, 1.7 Hz, 1H), 8.12 (dd, J = 8.2, 2.0 Hz, 1H), 7.85-7.9 (m, 1H), 7.5-7.6 (m, 2H), 7.3-7.4 (m, 2H), 7.15-7.2 (m, 1H), 7.05-7.1 (m, 2H), 6.13 (ddd, J = 46.7, 6.5, 4.1Hz, 1H), 4.1-4.45 (m, 2H).
合成例8
2-クロロ-N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]プロピル]ニコチンアミド(本発明化合物No.3-050)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)プロパンニトリルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル1.50gのテトラヒドロフラン20ml溶液に、-5℃にて攪拌下、カリウムtert-ブトキシドの1.0Mテトラヒドロフラン溶液7mlを添加、室温にて2時間攪拌した。次いで、この反応混合物にヨードメタン0.97gを添加し、室温にてさらに1時間攪拌を継続した。反応完結後、反応混合物に水30mlを加え酢酸エチルにて抽出(25mlx2)し、有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(5:95~15:85のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.38gを無色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.58 (d, J=2.1Hz, 1H), 7.88 (d, J=2.1Hz, 1H), 4.43 (q, J=7.2Hz, 1H), 1.67 (d, J=7.2Hz, 3H)。 Synthesis example 8
2-Chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] propyl] nicotinamide (the present compound No. 3-050).
Step 1; Preparation of 2- (5-bromo-3-chloropyridin-2-yl) propanenitrile 2- (5-Bromo-3-chloropyridin-2-yl) acetonitrile 1.50 g of tetrahydrofuran solution in 20 ml Under stirring at 5 ° C., 7 ml of a 1.0M solution of potassium tert-butoxide in tetrahydrofuran was added and stirred at room temperature for 2 hours. Subsequently, 0.97 g of iodomethane was added to the reaction mixture, and stirring was continued for another hour at room temperature. After completion of the reaction, 30 ml of water was added to the reaction mixture, and the mixture was extracted with ethyl acetate (25 ml × 2). The organic layers were combined and washed with water (20 ml × 1). The solvent was distilled off. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (5:95 to 15:85 gradient) to obtain 1.38 g of the desired product as a colorless oil.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.58 (d, J = 2.1 Hz, 1H), 7.88 (d, J = 2.1 Hz, 1H), 4.43 (q, J = 7.2 Hz, 1H) , 1.67 (d, J = 7.2Hz, 3H).
2-クロロ-N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]プロピル]ニコチンアミド(本発明化合物No.3-050)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)プロパンニトリルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル1.50gのテトラヒドロフラン20ml溶液に、-5℃にて攪拌下、カリウムtert-ブトキシドの1.0Mテトラヒドロフラン溶液7mlを添加、室温にて2時間攪拌した。次いで、この反応混合物にヨードメタン0.97gを添加し、室温にてさらに1時間攪拌を継続した。反応完結後、反応混合物に水30mlを加え酢酸エチルにて抽出(25mlx2)し、有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(5:95~15:85のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.38gを無色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.58 (d, J=2.1Hz, 1H), 7.88 (d, J=2.1Hz, 1H), 4.43 (q, J=7.2Hz, 1H), 1.67 (d, J=7.2Hz, 3H)。 Synthesis example 8
2-Chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] propyl] nicotinamide (the present compound No. 3-050).
Step 1; Preparation of 2- (5-bromo-3-chloropyridin-2-yl) propanenitrile 2- (5-Bromo-3-chloropyridin-2-yl) acetonitrile 1.50 g of tetrahydrofuran solution in 20 ml Under stirring at 5 ° C., 7 ml of a 1.0M solution of potassium tert-butoxide in tetrahydrofuran was added and stirred at room temperature for 2 hours. Subsequently, 0.97 g of iodomethane was added to the reaction mixture, and stirring was continued for another hour at room temperature. After completion of the reaction, 30 ml of water was added to the reaction mixture, and the mixture was extracted with ethyl acetate (25 ml × 2). The organic layers were combined and washed with water (20 ml × 1). The solvent was distilled off. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (5:95 to 15:85 gradient) to obtain 1.38 g of the desired product as a colorless oil.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.58 (d, J = 2.1 Hz, 1H), 7.88 (d, J = 2.1 Hz, 1H), 4.43 (q, J = 7.2 Hz, 1H) , 1.67 (d, J = 7.2Hz, 3H).
工程2;2-(5-ブロモ-3-クロロピリジン-2-イル)-1-プロパンアミンの製造
窒素雰囲気下の2-(5-ブロモ-3-クロロピリジン-2-イル)プロパンニトリル1.38gのジクロロメタン20ml溶液に、-40℃にて攪拌下、ジイソブチルアルミニウムヒドリドの1.0Mヘキサン溶液16mlを20分かけて滴下し、滴下終了後、同温度にて1時間攪拌した。次いで、反応混合物を室温まで昇温し、氷冷攪拌下の酒石酸カリウムナトリウム(ロッシェル塩)飽和水溶液50mlに滴下した。滴下終了後、室温にてさらに1時間攪拌を継続した。反応完結後、反応混合物をジクロロメタンにて抽出(50mlx2)し、有機層を併せ水洗(20mlx1)後、減圧下にて溶媒を留去した。残留物を酢酸エチル10mlに溶解し、1N塩酸水溶液10mlにて抽出した。水層に10重量%水酸化ナトリウム水溶液を添加してpH14とした後、酢酸エチル30mlにて抽出した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.04gを赤褐色油状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.50 (d, J=2.1Hz, 1H), 7.80 (d, J=2.1Hz, 1H), 3.35-3.45 (m, 1H), 3.05-3.15 (m, 1H), 2.85-2.95 (m, 1H), 1.20 (d, J=6.6Hz, 3H)。 Step 2; Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -1-propanamine 2- (5-Bromo-3-chloropyridin-2-yl) propanenitrile under nitrogen atmosphere 16 ml of a 1.0 M hexane solution of diisobutylaluminum hydride was added dropwise over 20 minutes to a solution of 38 g of dichloromethane in 20 ml at −40 ° C., and the mixture was stirred at the same temperature for 1 hour. The reaction mixture was then warmed to room temperature and added dropwise to 50 ml of a saturated aqueous solution of potassium sodium tartrate (Rochelle salt) under ice-cooling and stirring. After completion of the dropwise addition, stirring was continued for another hour at room temperature. After completion of the reaction, the reaction mixture was extracted with dichloromethane (50 ml × 2), the organic layers were combined and washed with water (20 ml × 1), and then the solvent was distilled off under reduced pressure. The residue was dissolved in 10 ml of ethyl acetate and extracted with 10 ml of 1N aqueous hydrochloric acid. A 10% by weight aqueous sodium hydroxide solution was added to the aqueous layer to adjust the pH to 14, followed by extraction with 30 ml of ethyl acetate. The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 1.04 g of the crude desired product as a reddish brown oily substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.50 (d, J = 2.1 Hz, 1H), 7.80 (d, J = 2.1 Hz, 1H), 3.35-3.45 (m, 1H), 3.05- 3.15 (m, 1H), 2.85-2.95 (m, 1H), 1.20 (d, J = 6.6Hz, 3H).
窒素雰囲気下の2-(5-ブロモ-3-クロロピリジン-2-イル)プロパンニトリル1.38gのジクロロメタン20ml溶液に、-40℃にて攪拌下、ジイソブチルアルミニウムヒドリドの1.0Mヘキサン溶液16mlを20分かけて滴下し、滴下終了後、同温度にて1時間攪拌した。次いで、反応混合物を室温まで昇温し、氷冷攪拌下の酒石酸カリウムナトリウム(ロッシェル塩)飽和水溶液50mlに滴下した。滴下終了後、室温にてさらに1時間攪拌を継続した。反応完結後、反応混合物をジクロロメタンにて抽出(50mlx2)し、有機層を併せ水洗(20mlx1)後、減圧下にて溶媒を留去した。残留物を酢酸エチル10mlに溶解し、1N塩酸水溶液10mlにて抽出した。水層に10重量%水酸化ナトリウム水溶液を添加してpH14とした後、酢酸エチル30mlにて抽出した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.04gを赤褐色油状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.50 (d, J=2.1Hz, 1H), 7.80 (d, J=2.1Hz, 1H), 3.35-3.45 (m, 1H), 3.05-3.15 (m, 1H), 2.85-2.95 (m, 1H), 1.20 (d, J=6.6Hz, 3H)。 Step 2; Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -1-propanamine 2- (5-Bromo-3-chloropyridin-2-yl) propanenitrile under nitrogen atmosphere 16 ml of a 1.0 M hexane solution of diisobutylaluminum hydride was added dropwise over 20 minutes to a solution of 38 g of dichloromethane in 20 ml at −40 ° C., and the mixture was stirred at the same temperature for 1 hour. The reaction mixture was then warmed to room temperature and added dropwise to 50 ml of a saturated aqueous solution of potassium sodium tartrate (Rochelle salt) under ice-cooling and stirring. After completion of the dropwise addition, stirring was continued for another hour at room temperature. After completion of the reaction, the reaction mixture was extracted with dichloromethane (50 ml × 2), the organic layers were combined and washed with water (20 ml × 1), and then the solvent was distilled off under reduced pressure. The residue was dissolved in 10 ml of ethyl acetate and extracted with 10 ml of 1N aqueous hydrochloric acid. A 10% by weight aqueous sodium hydroxide solution was added to the aqueous layer to adjust the pH to 14, followed by extraction with 30 ml of ethyl acetate. The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 1.04 g of the crude desired product as a reddish brown oily substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.50 (d, J = 2.1 Hz, 1H), 7.80 (d, J = 2.1 Hz, 1H), 3.35-3.45 (m, 1H), 3.05- 3.15 (m, 1H), 2.85-2.95 (m, 1H), 1.20 (d, J = 6.6Hz, 3H).
工程3;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)プロピル]カルバミド酸-tert-ブチルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)-1-プロパンアミン1.04g及びトリエチルアミン0.63gのジクロロメタン10ml溶液に二炭酸ジ-tert-ブチル1.08gを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物を酢酸エチル-ヘキサン(5:95~15:85のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.05gを無色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.51 (d, J=1.8Hz, 1H), 7.82 (d, J=1.8Hz, 1H), 4.86 (bs, 1H), 3.55-3.7 (m, 1H), 3.4-3.5 (m, 2H), 1.41 (s, 9H), 1.21 (d, J=7.2Hz, 3H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) propyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridin-2-yl) -1 -To a solution of 1.04 g of propanamine and 0.63 g of triethylamine in 10 ml of dichloromethane, 1.08 g of di-tert-butyl dicarbonate was added and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (5:95 to 15:85 gradient) to obtain 1.05 g of the desired product. Was obtained as a colorless oil.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.51 (d, J = 1.8Hz, 1H), 7.82 (d, J = 1.8Hz, 1H), 4.86 (bs, 1H), 3.55-3.7 ( m, 1H), 3.4-3.5 (m, 2H), 1.41 (s, 9H), 1.21 (d, J = 7.2Hz, 3H).
2-(5-ブロモ-3-クロロピリジン-2-イル)-1-プロパンアミン1.04g及びトリエチルアミン0.63gのジクロロメタン10ml溶液に二炭酸ジ-tert-ブチル1.08gを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物を酢酸エチル-ヘキサン(5:95~15:85のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.05gを無色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.51 (d, J=1.8Hz, 1H), 7.82 (d, J=1.8Hz, 1H), 4.86 (bs, 1H), 3.55-3.7 (m, 1H), 3.4-3.5 (m, 2H), 1.41 (s, 9H), 1.21 (d, J=7.2Hz, 3H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) propyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridin-2-yl) -1 -To a solution of 1.04 g of propanamine and 0.63 g of triethylamine in 10 ml of dichloromethane, 1.08 g of di-tert-butyl dicarbonate was added and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (5:95 to 15:85 gradient) to obtain 1.05 g of the desired product. Was obtained as a colorless oil.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.51 (d, J = 1.8Hz, 1H), 7.82 (d, J = 1.8Hz, 1H), 4.86 (bs, 1H), 3.55-3.7 ( m, 1H), 3.4-3.5 (m, 2H), 1.41 (s, 9H), 1.21 (d, J = 7.2Hz, 3H).
工程4;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]プロピル]カルバミド酸-tert-ブチルの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)プロピル]カルバミド酸-tert-ブチル1.0gのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン434mg、1-エチニル-4-フルオロベンゼン447mg、ヨウ化銅(I)109mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)100mgを添加し、窒素雰囲気下、80℃にて12時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物480mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.56 (d, J=1.8Hz, 1H), 7.96 (d, J=1.8Hz, 1H), 7.45-7.6 (m, 2H), 6.95-7.2 (m, 2H), 4.91 (bs, 1H), 3.6-3.75 (m, 1H), 3.50 (t, J=6.3Hz, 2H), 1.41 (s, 9H), 1.24 (d, J=6.7Hz, 3H)。 Step 4: Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] propyl] carbamic acid-tert-butyl N- [2- (5-Bromo- 3-chloropyridin-2-yl) propyl] carbamic acid-tert-butyl in a solution of 1.0 g of N, N-dimethylformamide in 1 ml of triethylamine 434 mg, 1-ethynyl-4-fluorobenzene 447 mg, copper (I) iodide 109 mg And 100 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at 80 ° C. for 12 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 2: 8 gradient) to obtain 480 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.56 (d, J = 1.8Hz, 1H), 7.96 (d, J = 1.8Hz, 1H), 7.45-7.6 (m, 2H), 6.95- 7.2 (m, 2H), 4.91 (bs, 1H), 3.6-3.75 (m, 1H), 3.50 (t, J = 6.3Hz, 2H), 1.41 (s, 9H), 1.24 (d, J = 6.7Hz , 3H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)プロピル]カルバミド酸-tert-ブチル1.0gのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン434mg、1-エチニル-4-フルオロベンゼン447mg、ヨウ化銅(I)109mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)100mgを添加し、窒素雰囲気下、80℃にて12時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物480mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.56 (d, J=1.8Hz, 1H), 7.96 (d, J=1.8Hz, 1H), 7.45-7.6 (m, 2H), 6.95-7.2 (m, 2H), 4.91 (bs, 1H), 3.6-3.75 (m, 1H), 3.50 (t, J=6.3Hz, 2H), 1.41 (s, 9H), 1.24 (d, J=6.7Hz, 3H)。 Step 4: Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] propyl] carbamic acid-tert-butyl N- [2- (5-Bromo- 3-chloropyridin-2-yl) propyl] carbamic acid-tert-butyl in a solution of 1.0 g of N, N-dimethylformamide in 1 ml of triethylamine 434 mg, 1-ethynyl-4-fluorobenzene 447 mg, copper (I) iodide 109 mg And 100 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at 80 ° C. for 12 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 2: 8 gradient) to obtain 480 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.56 (d, J = 1.8Hz, 1H), 7.96 (d, J = 1.8Hz, 1H), 7.45-7.6 (m, 2H), 6.95- 7.2 (m, 2H), 4.91 (bs, 1H), 3.6-3.75 (m, 1H), 3.50 (t, J = 6.3Hz, 2H), 1.41 (s, 9H), 1.24 (d, J = 6.7Hz , 3H).
工程5;2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-プロパンアミンの製造
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]プロピル]カルバミド酸-tert-ブチル480mgのジクロロメタン1ml溶液にトリフルオロ酢酸1mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物380mgを褐色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55 (d, J=1.7Hz, 1H), 7.79 (d, J=1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.15 (m, 2H), 3.55-3.7 (m, 1H), 3.2-3.35 (m, 1H), 3.05-3.2 (m, 1H), 2.59 (s, 2H), 1.31 (d, J=7.2Hz, 3H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine N- [2- [3-Chloro-5-[(4- 1 ml of trifluoroacetic acid was added to 1 ml of a solution of 480 mg of fluorophenyl) ethynyl] pyridin-2-yl] propyl] carbamic acid-tert-butyl and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 380 mg of a crude target product as a brown resinous substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55 (d, J = 1.7 Hz, 1H), 7.79 (d, J = 1.7 Hz, 1H), 7.45-7.55 (m, 2H), 7.0- 7.15 (m, 2H), 3.55-3.7 (m, 1H), 3.2-3.35 (m, 1H), 3.05-3.2 (m, 1H), 2.59 (s, 2H), 1.31 (d, J = 7.2Hz, 3H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]プロピル]カルバミド酸-tert-ブチル480mgのジクロロメタン1ml溶液にトリフルオロ酢酸1mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物380mgを褐色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55 (d, J=1.7Hz, 1H), 7.79 (d, J=1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.15 (m, 2H), 3.55-3.7 (m, 1H), 3.2-3.35 (m, 1H), 3.05-3.2 (m, 1H), 2.59 (s, 2H), 1.31 (d, J=7.2Hz, 3H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine N- [2- [3-Chloro-5-[(4- 1 ml of trifluoroacetic acid was added to 1 ml of a solution of 480 mg of fluorophenyl) ethynyl] pyridin-2-yl] propyl] carbamic acid-tert-butyl and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 380 mg of a crude target product as a brown resinous substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55 (d, J = 1.7 Hz, 1H), 7.79 (d, J = 1.7 Hz, 1H), 7.45-7.55 (m, 2H), 7.0- 7.15 (m, 2H), 3.55-3.7 (m, 1H), 3.2-3.35 (m, 1H), 3.05-3.2 (m, 1H), 2.59 (s, 2H), 1.31 (d, J = 7.2Hz, 3H).
工程6;2-クロロ-N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]プロピル]ニコチンアミドの製造
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-プロパンアミン120mg及びトリエチルアミン63mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-クロロニコチニルクロリド88mgを添加し、滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2mlを加え、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物74mgを褐色結晶として得た。
融点129.0~130.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.56 (d, J=1.7Hz, 1H), 8.42 (dd, J=4.8, 2.0Hz, 1H), 8.09 (dd, J=7.5, 2.0Hz, 1H), 7.79 (d, J=1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.25-7.35 (m, 2H), 7.0-7.1 (m, 2H), 3.8-4.0 (m, 3H), 1.34 (d, J=6.5Hz, 3H)。 Step 6; Preparation of 2-chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] propyl] nicotinamide 2- [3-chloro-5- [ To a solution of 120 mg of (4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine and 63 mg of triethylamine in 2 ml of dichloromethane was added 88 mg of 2-chloronicotinyl chloride with stirring under ice cooling. For 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 74 mg of the objective compound as brown crystals.
Melting point: 129.0-133.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.56 (d, J = 1.7 Hz, 1H), 8.42 (dd, J = 4.8, 2.0 Hz, 1H), 8.09 (dd, J = 7.5, 2.0 Hz, 1H), 7.79 (d, J = 1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.25-7.35 (m, 2H), 7.0-7.1 (m, 2H), 3.8-4.0 (m, 3H), 1.34 (d, J = 6.5Hz, 3H).
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-プロパンアミン120mg及びトリエチルアミン63mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-クロロニコチニルクロリド88mgを添加し、滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2mlを加え、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物74mgを褐色結晶として得た。
融点129.0~130.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.56 (d, J=1.7Hz, 1H), 8.42 (dd, J=4.8, 2.0Hz, 1H), 8.09 (dd, J=7.5, 2.0Hz, 1H), 7.79 (d, J=1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.25-7.35 (m, 2H), 7.0-7.1 (m, 2H), 3.8-4.0 (m, 3H), 1.34 (d, J=6.5Hz, 3H)。 Step 6; Preparation of 2-chloro-N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] propyl] nicotinamide 2- [3-chloro-5- [ To a solution of 120 mg of (4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine and 63 mg of triethylamine in 2 ml of dichloromethane was added 88 mg of 2-chloronicotinyl chloride with stirring under ice cooling. For 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 74 mg of the objective compound as brown crystals.
Melting point: 129.0-133.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.56 (d, J = 1.7 Hz, 1H), 8.42 (dd, J = 4.8, 2.0 Hz, 1H), 8.09 (dd, J = 7.5, 2.0 Hz, 1H), 7.79 (d, J = 1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.25-7.35 (m, 2H), 7.0-7.1 (m, 2H), 3.8-4.0 (m, 3H), 1.34 (d, J = 6.5Hz, 3H).
合成例9
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メチルプロピル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-059)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチルプロパンニトリルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル1.50gのテトラヒドロフラン20ml溶液に、-5℃にて攪拌下、カリウムtert-ブトキシドの1.0Mテトラヒドロフラン溶液13.6mlを添加、同温度にて10分間攪拌した。次いで、この反応混合物にヨードメタン1.94gを添加し、室温にてさらに1時間攪拌を継続した。反応完結後、反応混合物に水30mlを加え酢酸エチルにて抽出(25mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.61gを無色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45 (d, J=2.0Hz, 1H), 7.84 (d, J=2.0Hz, 1H), 1.76 (s, 6H)。 Synthesis Example 9
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] -2- (trifluoromethyl) benzamide (Compound No.1- 059).
Step 1: Preparation of 2- (5-bromo-3-chloropyridin-2-yl) -2-methylpropanenitrile 2- (5-Bromo-3-chloropyridin-2-yl) acetonitrile 1.50 g of tetrahydrofuran 20 ml To the solution, 13.6 ml of 1.0M tetrahydrofuran solution of potassium tert-butoxide was added with stirring at −5 ° C., and stirred at the same temperature for 10 minutes. Next, 1.94 g of iodomethane was added to the reaction mixture, and stirring was continued for another hour at room temperature. After completion of the reaction, 30 ml of water was added to the reaction mixture and extracted with ethyl acetate (25 ml × 2). The organic layers were combined, washed with water (20 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 1.61 g of the objective product as a colorless oil.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45 (d, J = 2.0 Hz, 1H), 7.84 (d, J = 2.0 Hz, 1H), 1.76 (s, 6H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メチルプロピル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-059)。
工程1;2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチルプロパンニトリルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル1.50gのテトラヒドロフラン20ml溶液に、-5℃にて攪拌下、カリウムtert-ブトキシドの1.0Mテトラヒドロフラン溶液13.6mlを添加、同温度にて10分間攪拌した。次いで、この反応混合物にヨードメタン1.94gを添加し、室温にてさらに1時間攪拌を継続した。反応完結後、反応混合物に水30mlを加え酢酸エチルにて抽出(25mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.61gを無色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45 (d, J=2.0Hz, 1H), 7.84 (d, J=2.0Hz, 1H), 1.76 (s, 6H)。 Synthesis Example 9
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] -2- (trifluoromethyl) benzamide (Compound No.1- 059).
Step 1: Preparation of 2- (5-bromo-3-chloropyridin-2-yl) -2-methylpropanenitrile 2- (5-Bromo-3-chloropyridin-2-yl) acetonitrile 1.50 g of tetrahydrofuran 20 ml To the solution, 13.6 ml of 1.0M tetrahydrofuran solution of potassium tert-butoxide was added with stirring at −5 ° C., and stirred at the same temperature for 10 minutes. Next, 1.94 g of iodomethane was added to the reaction mixture, and stirring was continued for another hour at room temperature. After completion of the reaction, 30 ml of water was added to the reaction mixture and extracted with ethyl acetate (25 ml × 2). The organic layers were combined, washed with water (20 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 1.61 g of the objective product as a colorless oil.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45 (d, J = 2.0 Hz, 1H), 7.84 (d, J = 2.0 Hz, 1H), 1.76 (s, 6H).
工程2;2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチル-1-プロパンアミンの製造
窒素雰囲気下の2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチルプロパンニトリル1.61gのジクロロメタン15ml溶液に、-40℃にて攪拌下、ジイソブチルアルミニウムヒドリドの1.0Mヘキサン溶液18.5mlを20分かけて滴下した。滴下終了後、同温度にて15分間攪拌した。反応完結後、反応混合物に水30mlを滴下し、同温度にて30分間攪拌した後に室温まで昇温し、ジクロロメタン15ml及び酒石酸カリウムナトリウム(ロッシェル塩)6.70gを添加し、室温にてさらに3時間攪拌を継続した。有機層を分取し、水層はジクロロメタンにて抽出(50mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.54gを褐色油状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.44 (d, J=2.1Hz, 1H), 7.74 (d, J=2.1Hz, 1H), 3.90 (bs, 2H), 3.10 (s, 2H), 1.44 (s, 6H)。 Step 2: Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2-methyl-1-propanamine 2- (5-Bromo-3-chloropyridin-2-yl) under nitrogen atmosphere To a solution of 1.61 g of -2-methylpropanenitrile in 15 ml of dichloromethane, 18.5 ml of a 1.0 M hexane solution of diisobutylaluminum hydride was added dropwise over 20 minutes with stirring at -40 ° C. After completion of dropping, the mixture was stirred at the same temperature for 15 minutes. After completion of the reaction, 30 ml of water was added dropwise to the reaction mixture, stirred for 30 minutes at the same temperature and then warmed to room temperature. 15 ml of dichloromethane and 6.70 g of potassium sodium tartrate (Rochelle salt) were added, and further 3 Stirring was continued for an hour. The organic layer was separated, and the aqueous layer was extracted with dichloromethane (50 ml × 2). The organic layers were combined and washed with water (20 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 1.54 g of the crude desired product as a brown oily substance. . The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.44 (d, J = 2.1 Hz, 1H), 7.74 (d, J = 2.1 Hz, 1H), 3.90 (bs, 2H), 3.10 (s, 2H), 1.44 (s, 6H).
窒素雰囲気下の2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチルプロパンニトリル1.61gのジクロロメタン15ml溶液に、-40℃にて攪拌下、ジイソブチルアルミニウムヒドリドの1.0Mヘキサン溶液18.5mlを20分かけて滴下した。滴下終了後、同温度にて15分間攪拌した。反応完結後、反応混合物に水30mlを滴下し、同温度にて30分間攪拌した後に室温まで昇温し、ジクロロメタン15ml及び酒石酸カリウムナトリウム(ロッシェル塩)6.70gを添加し、室温にてさらに3時間攪拌を継続した。有機層を分取し、水層はジクロロメタンにて抽出(50mlx2)した。有機層を併せ水洗(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物1.54gを褐色油状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.44 (d, J=2.1Hz, 1H), 7.74 (d, J=2.1Hz, 1H), 3.90 (bs, 2H), 3.10 (s, 2H), 1.44 (s, 6H)。 Step 2: Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2-methyl-1-propanamine 2- (5-Bromo-3-chloropyridin-2-yl) under nitrogen atmosphere To a solution of 1.61 g of -2-methylpropanenitrile in 15 ml of dichloromethane, 18.5 ml of a 1.0 M hexane solution of diisobutylaluminum hydride was added dropwise over 20 minutes with stirring at -40 ° C. After completion of dropping, the mixture was stirred at the same temperature for 15 minutes. After completion of the reaction, 30 ml of water was added dropwise to the reaction mixture, stirred for 30 minutes at the same temperature and then warmed to room temperature. 15 ml of dichloromethane and 6.70 g of potassium sodium tartrate (Rochelle salt) were added, and further 3 Stirring was continued for an hour. The organic layer was separated, and the aqueous layer was extracted with dichloromethane (50 ml × 2). The organic layers were combined and washed with water (20 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 1.54 g of the crude desired product as a brown oily substance. . The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.44 (d, J = 2.1 Hz, 1H), 7.74 (d, J = 2.1 Hz, 1H), 3.90 (bs, 2H), 3.10 (s, 2H), 1.44 (s, 6H).
工程3;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチルプロピル]カルバミド酸-tert-ブチルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチル-1-プロパンアミン1.54g及びトリエチルアミン1.60mlのジクロロメタン15ml溶液に二炭酸ジ-tert-ブチル1.50gを添加し、室温にて1時間攪拌した。反応完結後、反応混合物に水10mlを加え、酢酸エチルにて抽出(20mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物911mgを黄色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45 (d, J=2.0Hz, 1H), 7.80 (d, J=2.0Hz, 1H), 5.41 (bs, 1H), 3.54 (d, J=6.5Hz, 2H), 1.47 (s, 6H), 1.42 (s, 9H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-methylpropyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridin-2- I) 2-methyl-1-propanamine (1.54 g) and triethylamine (1.60 ml) in dichloromethane (15 ml) were added with di-tert-butyl dicarbonate (1.50 g) and stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with ethyl acetate (20 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 3: 7) to obtain 911 mg of the desired product as a yellow oily substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45 (d, J = 2.0 Hz, 1H), 7.80 (d, J = 2.0 Hz, 1H), 5.41 (bs, 1H), 3.54 (d, J = 6.5Hz, 2H), 1.47 (s, 6H), 1.42 (s, 9H).
2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチル-1-プロパンアミン1.54g及びトリエチルアミン1.60mlのジクロロメタン15ml溶液に二炭酸ジ-tert-ブチル1.50gを添加し、室温にて1時間攪拌した。反応完結後、反応混合物に水10mlを加え、酢酸エチルにて抽出(20mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物911mgを黄色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45 (d, J=2.0Hz, 1H), 7.80 (d, J=2.0Hz, 1H), 5.41 (bs, 1H), 3.54 (d, J=6.5Hz, 2H), 1.47 (s, 6H), 1.42 (s, 9H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-methylpropyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloropyridin-2- I) 2-methyl-1-propanamine (1.54 g) and triethylamine (1.60 ml) in dichloromethane (15 ml) were added with di-tert-butyl dicarbonate (1.50 g) and stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with ethyl acetate (20 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 3: 7) to obtain 911 mg of the desired product as a yellow oily substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.45 (d, J = 2.0 Hz, 1H), 7.80 (d, J = 2.0 Hz, 1H), 5.41 (bs, 1H), 3.54 (d, J = 6.5Hz, 2H), 1.47 (s, 6H), 1.42 (s, 9H).
工程4;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メチルプロピル]カルバミド酸-tert-ブチルの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチルプロピル]カルバミド酸-tert-ブチル1.0gのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン419mg、1-エチニル-4-フルオロベンゼン431mg、ヨウ化銅(I)105mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)97mgを添加し、窒素雰囲気下、80℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.0gを褐色結晶として得た。
融点70.0~73.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.51 (d, J=1.7Hz, 1H), 7.75 (d, J=1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.15 (m, 2H), 5.44 (bs, 1H), 3.55 (d, J=6.8Hz, 2H), 1.48 (s, 6H), 1.42 (s, 9H)。 Step 4: Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] carbamic acid-tert-butyl N- [2- ( 5-Bromo-3-chloropyridin-2-yl) -2-methylpropyl] carbamic acid-tert-butyl in a solution of 1.0 g of N, N-dimethylformamide in 1 ml of triethylamine 419 mg, 1-ethynyl-4-fluorobenzene 431 mg Then, 105 mg of copper (I) iodide and 97 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at 80 ° C. for 2 hours in a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 1.0 g of the objective product as brown crystals.
Melting point: 70.0-73.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.51 (d, J = 1.7 Hz, 1H), 7.75 (d, J = 1.7 Hz, 1H), 7.45-7.55 (m, 2H), 7.0- 7.15 (m, 2H), 5.44 (bs, 1H), 3.55 (d, J = 6.8Hz, 2H), 1.48 (s, 6H), 1.42 (s, 9H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メチルプロピル]カルバミド酸-tert-ブチル1.0gのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン419mg、1-エチニル-4-フルオロベンゼン431mg、ヨウ化銅(I)105mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)97mgを添加し、窒素雰囲気下、80℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.0gを褐色結晶として得た。
融点70.0~73.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.51 (d, J=1.7Hz, 1H), 7.75 (d, J=1.7Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.15 (m, 2H), 5.44 (bs, 1H), 3.55 (d, J=6.8Hz, 2H), 1.48 (s, 6H), 1.42 (s, 9H)。 Step 4: Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] carbamic acid-tert-butyl N- [2- ( 5-Bromo-3-chloropyridin-2-yl) -2-methylpropyl] carbamic acid-tert-butyl in a solution of 1.0 g of N, N-dimethylformamide in 1 ml of triethylamine 419 mg, 1-ethynyl-4-fluorobenzene 431 mg Then, 105 mg of copper (I) iodide and 97 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at 80 ° C. for 2 hours in a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 1.0 g of the objective product as brown crystals.
Melting point: 70.0-73.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.51 (d, J = 1.7 Hz, 1H), 7.75 (d, J = 1.7 Hz, 1H), 7.45-7.55 (m, 2H), 7.0- 7.15 (m, 2H), 5.44 (bs, 1H), 3.55 (d, J = 6.8Hz, 2H), 1.48 (s, 6H), 1.42 (s, 9H).
工程5;2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-プロパンアミンの製造
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メチルプロピル]カルバミド酸-tert-ブチル1.0gのジクロロメタン5ml溶液にトリフルオロ酢酸3mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物780mgを褐色結晶として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
融点60.0~62.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.51 (d, J=2.0Hz, 1H), 7.79 (d, J=2.0Hz, 1H), 7.45-7.6 (m, 2H), 7.0-7.15 (m, 2H), 3.26 (s, 2H), 1.57 (s, 6H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine N- [2- [3-Chloro-5-[(4- To a solution of 1.0 g of fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] carbamic acid-tert-butyl in 5 ml of dichloromethane was added 3 ml of trifluoroacetic acid and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 780 mg of a crude desired product as brown crystals. The obtained target product was directly used in the next step without further purification.
Melting point: 60.0-62.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.51 (d, J = 2.0 Hz, 1H), 7.79 (d, J = 2.0 Hz, 1H), 7.45-7.6 (m, 2H), 7.0- 7.15 (m, 2H), 3.26 (s, 2H), 1.57 (s, 6H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メチルプロピル]カルバミド酸-tert-ブチル1.0gのジクロロメタン5ml溶液にトリフルオロ酢酸3mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物780mgを褐色結晶として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
融点60.0~62.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.51 (d, J=2.0Hz, 1H), 7.79 (d, J=2.0Hz, 1H), 7.45-7.6 (m, 2H), 7.0-7.15 (m, 2H), 3.26 (s, 2H), 1.57 (s, 6H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine N- [2- [3-Chloro-5-[(4- To a solution of 1.0 g of fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] carbamic acid-tert-butyl in 5 ml of dichloromethane was added 3 ml of trifluoroacetic acid and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 780 mg of a crude desired product as brown crystals. The obtained target product was directly used in the next step without further purification.
Melting point: 60.0-62.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.51 (d, J = 2.0 Hz, 1H), 7.79 (d, J = 2.0 Hz, 1H), 7.45-7.6 (m, 2H), 7.0- 7.15 (m, 2H), 3.26 (s, 2H), 1.57 (s, 6H).
工程6;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メチルプロピル]-2-(トリフルオロメチル)ベンズアミドの製造
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-プロパンアミン260mg及びトリエチルアミン130mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド215mgを添加し、滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2mlを添加しジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物218mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.44 (d, J=2.0Hz, 1H), 7.77 (d, J=2.0Hz, 1H), 7.65-7.75 (m, 2H), 7.45-7.6 (m, 4H), 6.95-7.15 (m, 3H), 3.89 (d, J=6.5Hz, 2H), 1.58 (s, 6H)。 Step 6: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] -2- (trifluoromethyl) benzamide 2- [ To a solution of 260 mg of 3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine and 130 mg of triethylamine in 2 ml of dichloromethane was added 2- (trifluoromethyl) benzoyl chloride under ice-cooling and stirring. 215 mg was added, and after completion of the dropwise addition, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 218 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.44 (d, J = 2.0 Hz, 1H), 7.77 (d, J = 2.0 Hz, 1H), 7.65-7.75 (m, 2H), 7.45- 7.6 (m, 4H), 6.95-7.15 (m, 3H), 3.89 (d, J = 6.5Hz, 2H), 1.58 (s, 6H).
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-プロパンアミン260mg及びトリエチルアミン130mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド215mgを添加し、滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2mlを添加しジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物218mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.44 (d, J=2.0Hz, 1H), 7.77 (d, J=2.0Hz, 1H), 7.65-7.75 (m, 2H), 7.45-7.6 (m, 4H), 6.95-7.15 (m, 3H), 3.89 (d, J=6.5Hz, 2H), 1.58 (s, 6H)。 Step 6: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methylpropyl] -2- (trifluoromethyl) benzamide 2- [ To a solution of 260 mg of 3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-propanamine and 130 mg of triethylamine in 2 ml of dichloromethane was added 2- (trifluoromethyl) benzoyl chloride under ice-cooling and stirring. 215 mg was added, and after completion of the dropwise addition, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 218 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.44 (d, J = 2.0 Hz, 1H), 7.77 (d, J = 2.0 Hz, 1H), 7.65-7.75 (m, 2H), 7.45- 7.6 (m, 4H), 6.95-7.15 (m, 3H), 3.89 (d, J = 6.5Hz, 2H), 1.58 (s, 6H).
合成例10
N-[[1-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]シクロプロピル]メチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-060)。
工程1;N-[[1-(5-ブロモ-3-クロロピリジン-2-イル)シクロプロピル]メチル]カルバミド酸-tert-ブチルの製造
[1-(5-ブロモ-3-クロロピリジン-2-イル)シクロプロピル]メチルアミン462mg及びトリエチルアミン269mgのジクロロメタン5ml溶液に二炭酸ジ-tert-ブチル578mgを添加し、室温にて1時間攪拌した。反応完結後、反応混合物に水10mlを加えジクロロメタンにて抽出(10mlx2)した。有機層を併せ飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物498mgを白色結晶として得た。
融点81.0~82.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.47 (d, J=2.1Hz, 1H), 7.83 (d, J=2.1Hz, 1H), 4.78 (bs, 1H), 3.38 (d, J=6.0Hz, 2H), 1.33 (s, 9H), 0.9-1.05 (m, 4H)。 Synthesis Example 10
N-[[1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] -2- (trifluoromethyl) benzamide (Compound No.1- 060).
Step 1; Preparation of N-[[1- (5-Bromo-3-chloropyridin-2-yl) cyclopropyl] methyl] carbamic acid-tert-butyl [1- (5-Bromo-3-chloropyridine-2 To a solution of 462 mg of -yl) cyclopropyl] methylamine and 269 mg of triethylamine in 5 ml of dichloromethane was added 578 mg of di-tert-butyl dicarbonate, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with dichloromethane (10 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 498 mg of the desired product as white crystals.
Melting point: 81.0-82.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.47 (d, J = 2.1 Hz, 1H), 7.83 (d, J = 2.1 Hz, 1H), 4.78 (bs, 1H), 3.38 (d, J = 6.0Hz, 2H), 1.33 (s, 9H), 0.9-1.05 (m, 4H).
N-[[1-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]シクロプロピル]メチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-060)。
工程1;N-[[1-(5-ブロモ-3-クロロピリジン-2-イル)シクロプロピル]メチル]カルバミド酸-tert-ブチルの製造
[1-(5-ブロモ-3-クロロピリジン-2-イル)シクロプロピル]メチルアミン462mg及びトリエチルアミン269mgのジクロロメタン5ml溶液に二炭酸ジ-tert-ブチル578mgを添加し、室温にて1時間攪拌した。反応完結後、反応混合物に水10mlを加えジクロロメタンにて抽出(10mlx2)した。有機層を併せ飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物498mgを白色結晶として得た。
融点81.0~82.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.47 (d, J=2.1Hz, 1H), 7.83 (d, J=2.1Hz, 1H), 4.78 (bs, 1H), 3.38 (d, J=6.0Hz, 2H), 1.33 (s, 9H), 0.9-1.05 (m, 4H)。 Synthesis Example 10
N-[[1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] -2- (trifluoromethyl) benzamide (Compound No.1- 060).
Step 1; Preparation of N-[[1- (5-Bromo-3-chloropyridin-2-yl) cyclopropyl] methyl] carbamic acid-tert-butyl [1- (5-Bromo-3-chloropyridine-2 To a solution of 462 mg of -yl) cyclopropyl] methylamine and 269 mg of triethylamine in 5 ml of dichloromethane was added 578 mg of di-tert-butyl dicarbonate, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with dichloromethane (10 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 498 mg of the desired product as white crystals.
Melting point: 81.0-82.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.47 (d, J = 2.1 Hz, 1H), 7.83 (d, J = 2.1 Hz, 1H), 4.78 (bs, 1H), 3.38 (d, J = 6.0Hz, 2H), 1.33 (s, 9H), 0.9-1.05 (m, 4H).
工程2;N-[[1-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]シクロプロピル]メチル]カルバミド酸-tert-ブチルの製造
N-[[1-(5-ブロモ-3-クロロピリジン-2-イル)シクロプロピル]メチル]カルバミド酸-tert-ブチル450mgのN,N-ジメチルホルムアミド3ml溶液にトリエチルアミン380mg、1-エチニル-4-フルオロベンゼン224mg、ヨウ化銅(I)71mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)87mgを添加し、窒素雰囲気下、60℃にて3時間攪拌した。反応完結後、反応混合物を室温まで放冷し、酢酸エチル10ml及び飽和塩化アンモニウム水溶液10mlを添加し、有機層を分取し、水層は酢酸エチルにて抽出(10mlx2)した。有機層を併せて飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:100~15:85のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物407mgを褐色結晶として得た。
融点103.0~104.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.52 (d, J=1.8Hz, 1H), 7.77 (d, J=1.8Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H), 4.83 (bs, 1H), 3.41 (d, J=5.7Hz, 2H), 1.34 (s, 9H), 0.95-1.05 (m, 4H)。 Step 2; Preparation of N-[[1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] carbamic acid-tert-butyl N-[[1- (5-Bromo-3-chloropyridin-2-yl) cyclopropyl] methyl] carbamic acid-tert-butyl 450 mg of N, N-dimethylformamide in 3 ml solution of triethylamine 380 mg, 1-ethynyl-4-fluorobenzene 224 mg, iodine Copper (I) 71 mg and dichlorobis (triphenylphosphine) palladium (II) 87 mg were added, and the mixture was stirred at 60 ° C. for 3 hours in a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of ethyl acetate and 10 ml of saturated aqueous ammonium chloride solution were added, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient of 0: 100 to 15:85) to obtain 407 mg of the objective product as brown crystals.
Melting point: 103.0-104.0 ° C
1 H NMR (CDCl 3, Me 4 Si, 300MHz) δ8.52 (d, J = 1.8Hz, 1H), 7.77 (d, J = 1.8Hz, 1H), 7.45-7.55 (m, 2H), 7.0- 7.1 (m, 2H), 4.83 (bs, 1H), 3.41 (d, J = 5.7Hz, 2H), 1.34 (s, 9H), 0.95-1.05 (m, 4H).
N-[[1-(5-ブロモ-3-クロロピリジン-2-イル)シクロプロピル]メチル]カルバミド酸-tert-ブチル450mgのN,N-ジメチルホルムアミド3ml溶液にトリエチルアミン380mg、1-エチニル-4-フルオロベンゼン224mg、ヨウ化銅(I)71mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)87mgを添加し、窒素雰囲気下、60℃にて3時間攪拌した。反応完結後、反応混合物を室温まで放冷し、酢酸エチル10ml及び飽和塩化アンモニウム水溶液10mlを添加し、有機層を分取し、水層は酢酸エチルにて抽出(10mlx2)した。有機層を併せて飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:100~15:85のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物407mgを褐色結晶として得た。
融点103.0~104.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.52 (d, J=1.8Hz, 1H), 7.77 (d, J=1.8Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H), 4.83 (bs, 1H), 3.41 (d, J=5.7Hz, 2H), 1.34 (s, 9H), 0.95-1.05 (m, 4H)。 Step 2; Preparation of N-[[1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] carbamic acid-tert-butyl N-[[1- (5-Bromo-3-chloropyridin-2-yl) cyclopropyl] methyl] carbamic acid-tert-butyl 450 mg of N, N-dimethylformamide in 3 ml solution of triethylamine 380 mg, 1-ethynyl-4-fluorobenzene 224 mg, iodine Copper (I) 71 mg and dichlorobis (triphenylphosphine) palladium (II) 87 mg were added, and the mixture was stirred at 60 ° C. for 3 hours in a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of ethyl acetate and 10 ml of saturated aqueous ammonium chloride solution were added, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient of 0: 100 to 15:85) to obtain 407 mg of the objective product as brown crystals.
Melting point: 103.0-104.0 ° C
1 H NMR (CDCl 3, Me 4 Si, 300MHz) δ8.52 (d, J = 1.8Hz, 1H), 7.77 (d, J = 1.8Hz, 1H), 7.45-7.55 (m, 2H), 7.0- 7.1 (m, 2H), 4.83 (bs, 1H), 3.41 (d, J = 5.7Hz, 2H), 1.34 (s, 9H), 0.95-1.05 (m, 4H).
工程3;[1-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]シクロプロピル]メチルアミンの製造
N-[[1-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]シクロプロピル]メチル]カルバミド酸-tert-ブチル400mgのジクロロメタン6ml溶液に、氷冷攪拌下、トリフルオロ酢酸2mlを添加し、次いで室温にて1時間攪拌を継続した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せて水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物337mgを褐色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.6 (m, 1H), 7.75-7.8 (m, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H), 2.75-3.25 (m, 2H), 1.47 (bs, 2H), 0.95-1.05 (m, 4H)。 Step 3; Preparation of [1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methylamine N-[[1- [3-Chloro-5-[( To a solution of 4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] carbamic acid-tert-butyl in 6 ml of dichloromethane was added 2 ml of trifluoroacetic acid with stirring under ice cooling, and then at room temperature for 1 hour. Stirring was continued. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 337 mg of a crude target product as a brown resinous substance. . The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.6 (m, 1H), 7.75-7.8 (m, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H) , 2.75-3.25 (m, 2H), 1.47 (bs, 2H), 0.95-1.05 (m, 4H).
N-[[1-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]シクロプロピル]メチル]カルバミド酸-tert-ブチル400mgのジクロロメタン6ml溶液に、氷冷攪拌下、トリフルオロ酢酸2mlを添加し、次いで室温にて1時間攪拌を継続した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せて水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物337mgを褐色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.6 (m, 1H), 7.75-7.8 (m, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H), 2.75-3.25 (m, 2H), 1.47 (bs, 2H), 0.95-1.05 (m, 4H)。 Step 3; Preparation of [1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methylamine N-[[1- [3-Chloro-5-[( To a solution of 4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] carbamic acid-tert-butyl in 6 ml of dichloromethane was added 2 ml of trifluoroacetic acid with stirring under ice cooling, and then at room temperature for 1 hour. Stirring was continued. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 337 mg of a crude target product as a brown resinous substance. . The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.6 (m, 1H), 7.75-7.8 (m, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H) , 2.75-3.25 (m, 2H), 1.47 (bs, 2H), 0.95-1.05 (m, 4H).
工程4;N-[[1-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]シクロプロピル]メチル]-2-(トリフルオロメチル)ベンズアミドの製造
[1-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]シクロプロピル]メチルアミン110mg及びトリエチルアミン56mgのジクロロメタン2mlに、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド99mgを滴下した。滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2ml添加し、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物をジイソプロピルエーテル10mlにて洗浄し、目的物107mgを褐色結晶として得た。
融点148.0~150.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.49 (d, J=1.8Hz, 1H), 7.81 (d, J=1.8Hz, 1H), 7.65-7.7 (m, 2H), 7.45-7.6 (m, 3H), 7.4-7.45 (m, 1H), 7.0-7.1 (m, 2H), 6.19 (bs, 1H), 3.73 (d, J=5.1Hz, 2H), 1.05-1.15 (m, 4H)。 Step 4: Preparation of N-[[1- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] -2- (trifluoromethyl) benzamide [1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methylamine (110 mg) and triethylamine (56 mg) in dichloromethane (2 ml) was stirred under ice-cooling and 2- (trifluoromethyl) benzoyl chloride. 99 mg was added dropwise. After completion of dropping, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was washed with 10 ml of diisopropyl ether to obtain 107 mg of the desired product as brown crystals.
Melting point: 148.0-155.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.49 (d, J = 1.8 Hz, 1H), 7.81 (d, J = 1.8 Hz, 1H), 7.65-7.7 (m, 2H), 7.45- 7.6 (m, 3H), 7.4-7.45 (m, 1H), 7.0-7.1 (m, 2H), 6.19 (bs, 1H), 3.73 (d, J = 5.1Hz, 2H), 1.05-1.15 (m, 4H).
[1-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]シクロプロピル]メチルアミン110mg及びトリエチルアミン56mgのジクロロメタン2mlに、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド99mgを滴下した。滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2ml添加し、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物をジイソプロピルエーテル10mlにて洗浄し、目的物107mgを褐色結晶として得た。
融点148.0~150.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.49 (d, J=1.8Hz, 1H), 7.81 (d, J=1.8Hz, 1H), 7.65-7.7 (m, 2H), 7.45-7.6 (m, 3H), 7.4-7.45 (m, 1H), 7.0-7.1 (m, 2H), 6.19 (bs, 1H), 3.73 (d, J=5.1Hz, 2H), 1.05-1.15 (m, 4H)。 Step 4: Preparation of N-[[1- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methyl] -2- (trifluoromethyl) benzamide [1- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] cyclopropyl] methylamine (110 mg) and triethylamine (56 mg) in dichloromethane (2 ml) was stirred under ice-cooling and 2- (trifluoromethyl) benzoyl chloride. 99 mg was added dropwise. After completion of dropping, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was washed with 10 ml of diisopropyl ether to obtain 107 mg of the desired product as brown crystals.
Melting point: 148.0-155.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.49 (d, J = 1.8 Hz, 1H), 7.81 (d, J = 1.8 Hz, 1H), 7.65-7.7 (m, 2H), 7.45- 7.6 (m, 3H), 7.4-7.45 (m, 1H), 7.0-7.1 (m, 2H), 6.19 (bs, 1H), 3.73 (d, J = 5.1Hz, 2H), 1.05-1.15 (m, 4H).
合成例11
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-メチルエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-061)。
工程1;5-ブロモ-3-クロロ-2-(2-ニトロ-1-プロペニル)ピリジンの製造
5-ブロモ-3-クロロピコリルアルデヒド2.06g及び1-ブチルアミン1.00gのトルエン10ml溶液を、ディーンスターク管を用いて共沸脱水しながら、2時間過熱還流させた。次いで、減圧下にて溶媒を留去し、残留物に酢酸10ml及びニトロエタン1.05gを添加し、100℃にて40分間攪拌した。反応完結後、反応混合物を室温まで放冷し、水30mlを加えて酢酸エチルにて抽出(50mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(3:97)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物0.76gを褐色結晶として得た。
融点44.0~50.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.65 (d, J=1.8Hz, 1H), 8.25 (s, 1H), 7.96 (d, J=1.8Hz, 1H), 2.67 and 2.66 (s, 3H)。 Synthesis Example 11
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] -2- (trifluoromethyl) benzamide (Compound No.1- 061).
Step 1: Preparation of 5-bromo-3-chloro-2- (2-nitro-1-propenyl) pyridine A solution of 2.06 g of 5-bromo-3-chloropicolylaldehyde and 1.00 g of 1-butylamine in 10 ml of toluene. While being azeotropically dehydrated using a Dean-Stark tube, the mixture was heated to reflux for 2 hours. Subsequently, the solvent was distilled off under reduced pressure, 10 ml of acetic acid and 1.05 g of nitroethane were added to the residue, and the mixture was stirred at 100 ° C. for 40 minutes. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 30 ml of water was added, and the mixture was extracted with ethyl acetate (50 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (3:97) to obtain 0.76 g of the objective product as brown crystals.
Melting point: 44.0-50.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.65 (d, J = 1.8Hz, 1H), 8.25 (s, 1H), 7.96 (d, J = 1.8Hz, 1H), 2.67 and 2.66 ( s, 3H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-メチルエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-061)。
工程1;5-ブロモ-3-クロロ-2-(2-ニトロ-1-プロペニル)ピリジンの製造
5-ブロモ-3-クロロピコリルアルデヒド2.06g及び1-ブチルアミン1.00gのトルエン10ml溶液を、ディーンスターク管を用いて共沸脱水しながら、2時間過熱還流させた。次いで、減圧下にて溶媒を留去し、残留物に酢酸10ml及びニトロエタン1.05gを添加し、100℃にて40分間攪拌した。反応完結後、反応混合物を室温まで放冷し、水30mlを加えて酢酸エチルにて抽出(50mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(3:97)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物0.76gを褐色結晶として得た。
融点44.0~50.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.65 (d, J=1.8Hz, 1H), 8.25 (s, 1H), 7.96 (d, J=1.8Hz, 1H), 2.67 and 2.66 (s, 3H)。 Synthesis Example 11
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] -2- (trifluoromethyl) benzamide (Compound No.1- 061).
Step 1: Preparation of 5-bromo-3-chloro-2- (2-nitro-1-propenyl) pyridine A solution of 2.06 g of 5-bromo-3-chloropicolylaldehyde and 1.00 g of 1-butylamine in 10 ml of toluene. While being azeotropically dehydrated using a Dean-Stark tube, the mixture was heated to reflux for 2 hours. Subsequently, the solvent was distilled off under reduced pressure, 10 ml of acetic acid and 1.05 g of nitroethane were added to the residue, and the mixture was stirred at 100 ° C. for 40 minutes. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 30 ml of water was added, and the mixture was extracted with ethyl acetate (50 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (3:97) to obtain 0.76 g of the objective product as brown crystals.
Melting point: 44.0-50.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.65 (d, J = 1.8Hz, 1H), 8.25 (s, 1H), 7.96 (d, J = 1.8Hz, 1H), 2.67 and 2.66 ( s, 3H).
工程2;5-ブロモ-3-クロロ-2-(2-ニトロプロピル)ピリジンの製造
5-ブロモ-3-クロロ-2-(2-ニトロ-1-プロペニル)ピリジン156mgのメタノール1.5ml溶液に、氷冷攪拌下、水素化ホウ素ナトリウム85mgを添加し室温にて6時間攪拌した。反応完結後、反応混合物に水5mlを添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥、減圧下にて溶媒を留去し、粗製の目的物120mgを黄色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.38 (d, J=2.1Hz, 1H), 7.76 (d, J=2.1Hz, 1H), 5.1-5.25 (m, 1H), 3.62 (dd, J=16.5, 8.6Hz, 1H), 3.11 (dd, J=16.5, 5.2Hz, 1H), 1.599 (d, J=7.0Hz, 3H)。 Step 2; Preparation of 5-bromo-3-chloro-2- (2-nitropropyl) pyridine 5-Bromo-3-chloro-2- (2-nitro-1-propenyl) pyridine (156 mg) in methanol (1.5 ml) While stirring on ice, 85 mg of sodium borohydride was added and stirred at room temperature for 6 hours. After completion of the reaction, 5 ml of water was added to the reaction mixture and extracted with ethyl acetate (10 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 120 mg of a crude target product as a yellow resinous substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.38 (d, J = 2.1 Hz, 1H), 7.76 (d, J = 2.1 Hz, 1H), 5.1-5.25 (m, 1H), 3.62 ( dd, J = 16.5, 8.6Hz, 1H), 3.11 (dd, J = 16.5, 5.2Hz, 1H), 1.599 (d, J = 7.0Hz, 3H).
5-ブロモ-3-クロロ-2-(2-ニトロ-1-プロペニル)ピリジン156mgのメタノール1.5ml溶液に、氷冷攪拌下、水素化ホウ素ナトリウム85mgを添加し室温にて6時間攪拌した。反応完結後、反応混合物に水5mlを添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥、減圧下にて溶媒を留去し、粗製の目的物120mgを黄色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.38 (d, J=2.1Hz, 1H), 7.76 (d, J=2.1Hz, 1H), 5.1-5.25 (m, 1H), 3.62 (dd, J=16.5, 8.6Hz, 1H), 3.11 (dd, J=16.5, 5.2Hz, 1H), 1.599 (d, J=7.0Hz, 3H)。 Step 2; Preparation of 5-bromo-3-chloro-2- (2-nitropropyl) pyridine 5-Bromo-3-chloro-2- (2-nitro-1-propenyl) pyridine (156 mg) in methanol (1.5 ml) While stirring on ice, 85 mg of sodium borohydride was added and stirred at room temperature for 6 hours. After completion of the reaction, 5 ml of water was added to the reaction mixture and extracted with ethyl acetate (10 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 120 mg of a crude target product as a yellow resinous substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.38 (d, J = 2.1 Hz, 1H), 7.76 (d, J = 2.1 Hz, 1H), 5.1-5.25 (m, 1H), 3.62 ( dd, J = 16.5, 8.6Hz, 1H), 3.11 (dd, J = 16.5, 5.2Hz, 1H), 1.599 (d, J = 7.0Hz, 3H).
工程3;2-(5-ブロモ-3-クロロピリジン-2-イル)-1-メチルエチルアミンの製造
5-ブロモ-3-クロロ-2-(2-ニトロプロピル)ピリジン120mgをメタノール-水(2:1)混合溶媒3mlに溶解し、塩化アンモニウム138mg及び還元鉄72mgを添加し、室75℃にて16時間攪拌した。反応完結後、反応混合物を室温まで放冷し、セライト濾過にて不溶物を除去した後、減圧下にて溶媒を留去した。残留物をジエチルエーテル10mlに溶解し、1N塩酸水溶液10mlにて抽出した。水層に1N水酸化ナトリウム水溶液をpH14となるまで添加した後、酢酸エチルにて抽出(30mlx2)した。有機層を併せ、飽和食塩水次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物86mgを黄色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.50 (d, J=2.1Hz, 1H), 7.82 (d, J=2.1Hz, 1H), 3.4-3.55 (m, 1H), 2.98 (dd, J=14.4, 4.9Hz, 1H), 2.85 (dd, J=14.2, 8.1Hz, 1H), 1.76 (bs, 2H), 1.18 (d, J=6.4Hz, 3H)。 Step 3; Preparation of 2- (5-bromo-3-chloropyridin-2-yl) -1-methylethylamine 120 mg of 5-bromo-3-chloro-2- (2-nitropropyl) pyridine was dissolved in methanol-water (2 1) It melt | dissolved in 3 ml of mixed solvents, 138 mg of ammonium chloride and 72 mg of reduced iron were added, and it stirred at room 75 degreeC for 16 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, insoluble materials were removed by Celite filtration, and then the solvent was distilled off under reduced pressure. The residue was dissolved in 10 ml of diethyl ether and extracted with 10 ml of 1N aqueous hydrochloric acid. A 1N aqueous sodium hydroxide solution was added to the aqueous layer until pH 14 was reached, followed by extraction with ethyl acetate (30 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 86 mg of the crude desired product as a yellow resinous substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.50 (d, J = 2.1 Hz, 1H), 7.82 (d, J = 2.1 Hz, 1H), 3.4-3.55 (m, 1H), 2.98 ( dd, J = 14.4, 4.9Hz, 1H), 2.85 (dd, J = 14.2, 8.1Hz, 1H), 1.76 (bs, 2H), 1.18 (d, J = 6.4Hz, 3H).
5-ブロモ-3-クロロ-2-(2-ニトロプロピル)ピリジン120mgをメタノール-水(2:1)混合溶媒3mlに溶解し、塩化アンモニウム138mg及び還元鉄72mgを添加し、室75℃にて16時間攪拌した。反応完結後、反応混合物を室温まで放冷し、セライト濾過にて不溶物を除去した後、減圧下にて溶媒を留去した。残留物をジエチルエーテル10mlに溶解し、1N塩酸水溶液10mlにて抽出した。水層に1N水酸化ナトリウム水溶液をpH14となるまで添加した後、酢酸エチルにて抽出(30mlx2)した。有機層を併せ、飽和食塩水次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物86mgを黄色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.50 (d, J=2.1Hz, 1H), 7.82 (d, J=2.1Hz, 1H), 3.4-3.55 (m, 1H), 2.98 (dd, J=14.4, 4.9Hz, 1H), 2.85 (dd, J=14.2, 8.1Hz, 1H), 1.76 (bs, 2H), 1.18 (d, J=6.4Hz, 3H)。 Step 3; Preparation of 2- (5-bromo-3-chloropyridin-2-yl) -1-methylethylamine 120 mg of 5-bromo-3-chloro-2- (2-nitropropyl) pyridine was dissolved in methanol-water (2 1) It melt | dissolved in 3 ml of mixed solvents, 138 mg of ammonium chloride and 72 mg of reduced iron were added, and it stirred at room 75 degreeC for 16 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, insoluble materials were removed by Celite filtration, and then the solvent was distilled off under reduced pressure. The residue was dissolved in 10 ml of diethyl ether and extracted with 10 ml of 1N aqueous hydrochloric acid. A 1N aqueous sodium hydroxide solution was added to the aqueous layer until pH 14 was reached, followed by extraction with ethyl acetate (30 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 86 mg of the crude desired product as a yellow resinous substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.50 (d, J = 2.1 Hz, 1H), 7.82 (d, J = 2.1 Hz, 1H), 3.4-3.55 (m, 1H), 2.98 ( dd, J = 14.4, 4.9Hz, 1H), 2.85 (dd, J = 14.2, 8.1Hz, 1H), 1.76 (bs, 2H), 1.18 (d, J = 6.4Hz, 3H).
工程4;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-1-メチルエチル]カルバミド酸-tert-ブチルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)-1-メチルエチルアミン86mg及びトリエチルアミン42mgのジクロロメタン1ml溶液に二炭酸ジ-tert-ブチル91mgを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物を酢酸エチル-ヘキサン(2:8~4:6のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物74mgを黄色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.48 (d, J=2.0Hz, 1H), 7.81 (d, J=1.7Hz, 1H), 4.92 (bs, 1H), 4.0-4.3 (m, 1H), 2.9-3.2 (m, 2H), 1.36 (s, 9H), 1.21 (d, J=6.5Hz, 3H)。 Step 4; Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -1-methylethyl] carbamic acid-tert-butyl 2- (5-bromo-3-chloropyridin-2- Yl) To a solution of 86 mg of 1-methylethylamine and 42 mg of triethylamine in 1 ml of dichloromethane was added 91 mg of di-tert-butyl dicarbonate, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (2: 8 to 4: 6 gradient). Obtained as a resinous material.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.48 (d, J = 2.0 Hz, 1H), 7.81 (d, J = 1.7 Hz, 1H), 4.92 (bs, 1H), 4.0-4.3 ( m, 1H), 2.9-3.2 (m, 2H), 1.36 (s, 9H), 1.21 (d, J = 6.5Hz, 3H).
2-(5-ブロモ-3-クロロピリジン-2-イル)-1-メチルエチルアミン86mg及びトリエチルアミン42mgのジクロロメタン1ml溶液に二炭酸ジ-tert-ブチル91mgを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物を酢酸エチル-ヘキサン(2:8~4:6のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物74mgを黄色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.48 (d, J=2.0Hz, 1H), 7.81 (d, J=1.7Hz, 1H), 4.92 (bs, 1H), 4.0-4.3 (m, 1H), 2.9-3.2 (m, 2H), 1.36 (s, 9H), 1.21 (d, J=6.5Hz, 3H)。 Step 4; Preparation of N- [2- (5-bromo-3-chloropyridin-2-yl) -1-methylethyl] carbamic acid-tert-butyl 2- (5-bromo-3-chloropyridin-2- Yl) To a solution of 86 mg of 1-methylethylamine and 42 mg of triethylamine in 1 ml of dichloromethane was added 91 mg of di-tert-butyl dicarbonate, and the mixture was stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (2: 8 to 4: 6 gradient). Obtained as a resinous material.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.48 (d, J = 2.0 Hz, 1H), 7.81 (d, J = 1.7 Hz, 1H), 4.92 (bs, 1H), 4.0-4.3 ( m, 1H), 2.9-3.2 (m, 2H), 1.36 (s, 9H), 1.21 (d, J = 6.5Hz, 3H).
工程5;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-メチルエチル]カルバミド酸-tert-ブチルの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-1-メチルエチル]カルバミド酸-tert-ブチル350mgのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン152mg、1-エチニル-4-フルオロベンゼン156mg、ヨウ化銅(I)38mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)56mgを添加し、窒素雰囲気下、80℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物260mgを褐色結晶として得た。
融点117.0~119.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.54 (d, J=1.7Hz, 1H), 7.76 (d, J=1.7Hz, 1H), 7.5-7.55 (m, 2H), 7.1-7.0 (m, 2H), 4.85-5.05 (m, 1H), 4.1-4.3 (m, 1H), 3.09 (d, J=6.8Hz, 2H), 1.37 (s, 9H), 1.21 (d, J=6.5Hz, 3H)。 Step 5; Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] carbamic acid-tert-butyl N- [2- ( 5-bromo-3-chloropyridin-2-yl) -1-methylethyl] carbamic acid-tert-butyl 350 mg of N, N-dimethylformamide in 1 ml solution of triethylamine 152 mg, 1-ethynyl-4-fluorobenzene 156 mg, iodine Copper (I) 38 mg and dichlorobis (triphenylphosphine) palladium (II) 56 mg were added, and the mixture was stirred at 80 ° C. for 2 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 260 mg of the desired product as brown crystals.
Melting point 117.0 to 119.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.54 (d, J = 1.7 Hz, 1H), 7.76 (d, J = 1.7 Hz, 1H), 7.5-7.55 (m, 2H), 7.1- 7.0 (m, 2H), 4.85-5.05 (m, 1H), 4.1-4.3 (m, 1H), 3.09 (d, J = 6.8Hz, 2H), 1.37 (s, 9H), 1.21 (d, J = 6.5Hz, 3H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-1-メチルエチル]カルバミド酸-tert-ブチル350mgのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン152mg、1-エチニル-4-フルオロベンゼン156mg、ヨウ化銅(I)38mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)56mgを添加し、窒素雰囲気下、80℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物260mgを褐色結晶として得た。
融点117.0~119.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.54 (d, J=1.7Hz, 1H), 7.76 (d, J=1.7Hz, 1H), 7.5-7.55 (m, 2H), 7.1-7.0 (m, 2H), 4.85-5.05 (m, 1H), 4.1-4.3 (m, 1H), 3.09 (d, J=6.8Hz, 2H), 1.37 (s, 9H), 1.21 (d, J=6.5Hz, 3H)。 Step 5; Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] carbamic acid-tert-butyl N- [2- ( 5-bromo-3-chloropyridin-2-yl) -1-methylethyl] carbamic acid-tert-butyl 350 mg of N, N-dimethylformamide in 1 ml solution of triethylamine 152 mg, 1-ethynyl-4-fluorobenzene 156 mg, iodine Copper (I) 38 mg and dichlorobis (triphenylphosphine) palladium (II) 56 mg were added, and the mixture was stirred at 80 ° C. for 2 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 260 mg of the desired product as brown crystals.
Melting point 117.0 to 119.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.54 (d, J = 1.7 Hz, 1H), 7.76 (d, J = 1.7 Hz, 1H), 7.5-7.55 (m, 2H), 7.1- 7.0 (m, 2H), 4.85-5.05 (m, 1H), 4.1-4.3 (m, 1H), 3.09 (d, J = 6.8Hz, 2H), 1.37 (s, 9H), 1.21 (d, J = 6.5Hz, 3H).
工程6;2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-メチルエチルアミンの製造
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-メチルエチル]カルバミド酸-tert-ブチル260mgのジクロロメタン2ml溶液に、氷冷攪拌下、トリフルオロ酢酸1mlを添加し、次いで室温にて1時間攪拌を継続した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ、水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物250mgを褐色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.5 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.6 (m, 2H), 7.0-7.1 (m, 2H), 3.55-3.95 (m, 1H), 3.26 (d, J=8.5Hz, 2H), 1.65-1.85 (m, 2H), 1.51 (d, J=6.5Hz, 3H)。 Step 6; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethylamine N- [2- [3-Chloro-5-[(4- To a solution of fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] carbamic acid-tert-butyl 260 mg in dichloromethane 2 ml was added 1 ml of trifluoroacetic acid under ice-cooling, and then stirred at room temperature for 1 hour. Continued. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 250 mg of the crude target product as a brown resinous substance. . The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3, Me 4 Si, 300MHz) δ8.45-8.5 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.6 (m, 2H), 7.0-7.1 (m, 2H) , 3.55-3.95 (m, 1H), 3.26 (d, J = 8.5Hz, 2H), 1.65-1.85 (m, 2H), 1.51 (d, J = 6.5Hz, 3H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-メチルエチル]カルバミド酸-tert-ブチル260mgのジクロロメタン2ml溶液に、氷冷攪拌下、トリフルオロ酢酸1mlを添加し、次いで室温にて1時間攪拌を継続した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ、水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物250mgを褐色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.45-8.5 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.6 (m, 2H), 7.0-7.1 (m, 2H), 3.55-3.95 (m, 1H), 3.26 (d, J=8.5Hz, 2H), 1.65-1.85 (m, 2H), 1.51 (d, J=6.5Hz, 3H)。 Step 6; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethylamine N- [2- [3-Chloro-5-[(4- To a solution of fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] carbamic acid-tert-butyl 260 mg in dichloromethane 2 ml was added 1 ml of trifluoroacetic acid under ice-cooling, and then stirred at room temperature for 1 hour. Continued. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 250 mg of the crude target product as a brown resinous substance. . The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3, Me 4 Si, 300MHz) δ8.45-8.5 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.6 (m, 2H), 7.0-7.1 (m, 2H) , 3.55-3.95 (m, 1H), 3.26 (d, J = 8.5Hz, 2H), 1.65-1.85 (m, 2H), 1.51 (d, J = 6.5Hz, 3H).
工程7;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-メチルエチル]-2-(トリフルオロメチル)ベンズアミドの製造
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-メチルエチルアミン80mg及びトリエチルアミン57mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド87mgを滴下した。滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2ml添加し、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物72mgを淡黄色結晶として得た。
融点139.0~141.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.49 (d, J=1.8Hz, 1H), 7.80 (d, J=1.8Hz, 1H), 7.6-7.7 (m, 1H), 7.4-7.65 (m, 6H), 7.0-7.1 (m, 2H), 6.7-6.8 (m, 1H), 3.15-3.3 (m, 2H), 1.33 (d, J=6.7Hz, 3H)。 Step 7: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] -2- (trifluoromethyl) benzamide 2- [ 2-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethylamine 80 mg and triethylamine 57 mg in 2 ml of dichloromethane was stirred under ice-cooling and 2- (trifluoromethyl) benzoyl chloride. 87 mg was added dropwise. After completion of dropping, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 72 mg of the objective product as pale yellow crystals.
Melting point: 139.0-141.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.49 (d, J = 1.8 Hz, 1H), 7.80 (d, J = 1.8 Hz, 1H), 7.6-7.7 (m, 1H), 7.4- 7.65 (m, 6H), 7.0-7.1 (m, 2H), 6.7-6.8 (m, 1H), 3.15-3.3 (m, 2H), 1.33 (d, J = 6.7Hz, 3H).
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-1-メチルエチルアミン80mg及びトリエチルアミン57mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド87mgを滴下した。滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2ml添加し、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物72mgを淡黄色結晶として得た。
融点139.0~141.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.49 (d, J=1.8Hz, 1H), 7.80 (d, J=1.8Hz, 1H), 7.6-7.7 (m, 1H), 7.4-7.65 (m, 6H), 7.0-7.1 (m, 2H), 6.7-6.8 (m, 1H), 3.15-3.3 (m, 2H), 1.33 (d, J=6.7Hz, 3H)。 Step 7: Preparation of N- [2- [3-chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethyl] -2- (trifluoromethyl) benzamide 2- [ 2-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -1-methylethylamine 80 mg and triethylamine 57 mg in 2 ml of dichloromethane was stirred under ice-cooling and 2- (trifluoromethyl) benzoyl chloride. 87 mg was added dropwise. After completion of dropping, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 72 mg of the objective product as pale yellow crystals.
Melting point: 139.0-141.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.49 (d, J = 1.8 Hz, 1H), 7.80 (d, J = 1.8 Hz, 1H), 7.6-7.7 (m, 1H), 7.4- 7.65 (m, 6H), 7.0-7.1 (m, 2H), 6.7-6.8 (m, 1H), 3.15-3.3 (m, 2H), 1.33 (d, J = 6.7Hz, 3H).
合成例12
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メトキシ-1-メチルエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-066)。 Synthesis Example 12
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] -2- (trifluoromethyl) benzamide (compound of the present invention) No.1-066).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メトキシ-1-メチルエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-066)。 Synthesis Example 12
N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] -2- (trifluoromethyl) benzamide (compound of the present invention) No.1-066).
工程1;5-ブロモ-3-クロロ-2-(1-メトキシ-2-ニトロプロピル)ピリジンの製造
合成例11の工程1にて製造した5-ブロモ-3-クロロ-2-(2-ニトロ-1-プロペニル)ピリジン760mgのトルエン10ml溶液に、氷冷攪拌下、28重量%ナトリウムメトキシドメタノール溶液2.1gをメタノール3mlにて希釈して滴下した。同温度にて30分間攪拌した。反応完結後、反応混合物に酢酸1.5ml及び水10mlを添加し、ジクロロメタンにて抽出(30mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(3:97)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物683mgを赤色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.67 and 8.64 (d, J=2.1Hz, 1H), 7.95 and 7.91 (d, J=2.1Hz, 1H), 5.35 (d, J=6.3Hz, 1H) and 5.30 (d, J=9.6Hz, 1H), 5.1-5.25 (m, 1H) and 5.04 (qui, J=6.6Hz, 1H), 3.36 and 3.27 (s, 3H), 1.69 and 1.29 (d, J=6.9Hz, 3H)。 Step 1: Preparation of 5-bromo-3-chloro-2- (1-methoxy-2-nitropropyl) pyridine 5-Bromo-3-chloro-2- (2-nitro prepared in Step 1 of Synthesis Example 11 To a 10 ml toluene solution of 760 mg of -1-propenyl) pyridine, 2.1 g of a 28 wt% sodium methoxide methanol solution was diluted with 3 ml of methanol and added dropwise with stirring under ice cooling. Stir for 30 minutes at the same temperature. After completion of the reaction, 1.5 ml of acetic acid and 10 ml of water were added to the reaction mixture and extracted with dichloromethane (30 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (3:97) to obtain 683 mg of the desired product as a red oily substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.67 and 8.64 (d, J = 2.1 Hz, 1H), 7.95 and 7.91 (d, J = 2.1 Hz, 1H), 5.35 (d, J = 6.3 Hz, 1H) and 5.30 (d, J = 9.6Hz, 1H), 5.1-5.25 (m, 1H) and 5.04 (qui, J = 6.6Hz, 1H), 3.36 and 3.27 (s, 3H), 1.69 and 1.29 (d, J = 6.9Hz, 3H).
合成例11の工程1にて製造した5-ブロモ-3-クロロ-2-(2-ニトロ-1-プロペニル)ピリジン760mgのトルエン10ml溶液に、氷冷攪拌下、28重量%ナトリウムメトキシドメタノール溶液2.1gをメタノール3mlにて希釈して滴下した。同温度にて30分間攪拌した。反応完結後、反応混合物に酢酸1.5ml及び水10mlを添加し、ジクロロメタンにて抽出(30mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(3:97)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物683mgを赤色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.67 and 8.64 (d, J=2.1Hz, 1H), 7.95 and 7.91 (d, J=2.1Hz, 1H), 5.35 (d, J=6.3Hz, 1H) and 5.30 (d, J=9.6Hz, 1H), 5.1-5.25 (m, 1H) and 5.04 (qui, J=6.6Hz, 1H), 3.36 and 3.27 (s, 3H), 1.69 and 1.29 (d, J=6.9Hz, 3H)。 Step 1: Preparation of 5-bromo-3-chloro-2- (1-methoxy-2-nitropropyl) pyridine 5-Bromo-3-chloro-2- (2-nitro prepared in Step 1 of Synthesis Example 11 To a 10 ml toluene solution of 760 mg of -1-propenyl) pyridine, 2.1 g of a 28 wt% sodium methoxide methanol solution was diluted with 3 ml of methanol and added dropwise with stirring under ice cooling. Stir for 30 minutes at the same temperature. After completion of the reaction, 1.5 ml of acetic acid and 10 ml of water were added to the reaction mixture and extracted with dichloromethane (30 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (3:97) to obtain 683 mg of the desired product as a red oily substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.67 and 8.64 (d, J = 2.1 Hz, 1H), 7.95 and 7.91 (d, J = 2.1 Hz, 1H), 5.35 (d, J = 6.3 Hz, 1H) and 5.30 (d, J = 9.6Hz, 1H), 5.1-5.25 (m, 1H) and 5.04 (qui, J = 6.6Hz, 1H), 3.36 and 3.27 (s, 3H), 1.69 and 1.29 (d, J = 6.9Hz, 3H).
工程2;2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メトキシ-1-メチルエチルアミンの製造
5-ブロモ-3-クロロ-2-(1-メトキシ-2-ニトロプロピル)ピリジン340mgをメタノール-水(1:1)混合溶媒4mlに溶解し、塩化アンモニウム350mg及び還元鉄182mgを添加し、室温にて30分、次いで、65℃にて1.5時間攪拌した。反応完結後、反応混合物を室温まで放冷し、セライト濾過にて不溶物を除去した後、減圧下にて溶媒を留去した。残留物をジエチルエーテル30mlに溶解し、1重量%塩酸水溶液20mlにて抽出した。水層に飽和炭酸水素ナトリウム水溶液20mlを添加して塩基性とした後、酢酸エチル100mlにて抽出した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物200mgを褐色油状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.66 and 8.65 (d, J=2.1Hz, 1H), 7.88 and 7.87 (d, J=2.1Hz, 1H), 4.58 (d, J=5.7Hz, 1H) and 4.46 (d, J=7.5Hz, 1H), 3.30 and 3.27 (s, 3H), 3.25-3.45 (m, 1H), 1.12 and 0.93 (d, J=6.6Hz, 3H)。 Step 2; Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2-methoxy-1-methylethylamine 5-Bromo-3-chloro-2- (1-methoxy-2-nitropropyl) 340 mg of pyridine was dissolved in 4 ml of a methanol-water (1: 1) mixed solvent, 350 mg of ammonium chloride and 182 mg of reduced iron were added, and the mixture was stirred at room temperature for 30 minutes and then at 65 ° C. for 1.5 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, insoluble materials were removed by Celite filtration, and then the solvent was distilled off under reduced pressure. The residue was dissolved in 30 ml of diethyl ether and extracted with 20 ml of a 1% by weight aqueous hydrochloric acid solution. The aqueous layer was made basic by adding 20 ml of a saturated aqueous solution of sodium bicarbonate, and then extracted with 100 ml of ethyl acetate. The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 200 mg of the crude desired product as a brown oily substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.66 and 8.65 (d, J = 2.1 Hz, 1H), 7.88 and 7.87 (d, J = 2.1 Hz, 1H), 4.58 (d, J = 5.7 Hz, 1H) and 4.46 (d, J = 7.5Hz, 1H), 3.30 and 3.27 (s, 3H), 3.25-3.45 (m, 1H), 1.12 and 0.93 (d, J = 6.6Hz, 3H).
5-ブロモ-3-クロロ-2-(1-メトキシ-2-ニトロプロピル)ピリジン340mgをメタノール-水(1:1)混合溶媒4mlに溶解し、塩化アンモニウム350mg及び還元鉄182mgを添加し、室温にて30分、次いで、65℃にて1.5時間攪拌した。反応完結後、反応混合物を室温まで放冷し、セライト濾過にて不溶物を除去した後、減圧下にて溶媒を留去した。残留物をジエチルエーテル30mlに溶解し、1重量%塩酸水溶液20mlにて抽出した。水層に飽和炭酸水素ナトリウム水溶液20mlを添加して塩基性とした後、酢酸エチル100mlにて抽出した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物200mgを褐色油状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.66 and 8.65 (d, J=2.1Hz, 1H), 7.88 and 7.87 (d, J=2.1Hz, 1H), 4.58 (d, J=5.7Hz, 1H) and 4.46 (d, J=7.5Hz, 1H), 3.30 and 3.27 (s, 3H), 3.25-3.45 (m, 1H), 1.12 and 0.93 (d, J=6.6Hz, 3H)。 Step 2; Preparation of 2- (5-Bromo-3-chloropyridin-2-yl) -2-methoxy-1-methylethylamine 5-Bromo-3-chloro-2- (1-methoxy-2-nitropropyl) 340 mg of pyridine was dissolved in 4 ml of a methanol-water (1: 1) mixed solvent, 350 mg of ammonium chloride and 182 mg of reduced iron were added, and the mixture was stirred at room temperature for 30 minutes and then at 65 ° C. for 1.5 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, insoluble materials were removed by Celite filtration, and then the solvent was distilled off under reduced pressure. The residue was dissolved in 30 ml of diethyl ether and extracted with 20 ml of a 1% by weight aqueous hydrochloric acid solution. The aqueous layer was made basic by adding 20 ml of a saturated aqueous solution of sodium bicarbonate, and then extracted with 100 ml of ethyl acetate. The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 200 mg of the crude desired product as a brown oily substance. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.66 and 8.65 (d, J = 2.1 Hz, 1H), 7.88 and 7.87 (d, J = 2.1 Hz, 1H), 4.58 (d, J = 5.7 Hz, 1H) and 4.46 (d, J = 7.5Hz, 1H), 3.30 and 3.27 (s, 3H), 3.25-3.45 (m, 1H), 1.12 and 0.93 (d, J = 6.6Hz, 3H).
工程3;N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メトキシ-1-メチルエチル]カルバミド酸-tert-ブチルの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メトキシ-1-メチルエチルアミン190mg及びピリジン80mgのジクロロメタン2ml溶液に二炭酸ジ-tert-ブチル178mgを添加し、室温にて30分間攪拌した。反応完結後、反応混合物に水10mlを加え、ジクロロメタンにて抽出(10mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(2:98~20:80のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物200mgを無色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.65 and 8.59 (d, J=1.8Hz, 1H), 7.87 and 7.85 (d, J=1.8Hz, 1H), 5.31 and 4.85 (d, J=8.1Hz, 1H), 4.75 (d, J=4.2Hz, 1H) and 4.67 (d, J=2.4Hz, 1H), 4.0-4.3 (m, 1H), 3.53 (s, 3H), 1.42 and 1.26 (s, 9H), 1.32 and 1.05 (d, J=6.6Hz, 3H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-methoxy-1-methylethyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloro To a solution of 190 mg of pyridin-2-yl) -2-methoxy-1-methylethylamine and 80 mg of pyridine in 2 ml of dichloromethane was added 178 mg of di-tert-butyl dicarbonate and stirred at room temperature for 30 minutes. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with dichloromethane (10 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (2: 98-20: 80 gradient) to obtain 200 mg of the objective product as a colorless oil.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.65 and 8.59 (d, J = 1.8Hz, 1H), 7.87 and 7.85 (d, J = 1.8Hz, 1H), 5.31 and 4.85 (d, J = 8.1Hz, 1H), 4.75 (d, J = 4.2Hz, 1H) and 4.67 (d, J = 2.4Hz, 1H), 4.0-4.3 (m, 1H), 3.53 (s, 3H), 1.42 and 1.26 (s, 9H), 1.32 and 1.05 (d, J = 6.6 Hz, 3H).
2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メトキシ-1-メチルエチルアミン190mg及びピリジン80mgのジクロロメタン2ml溶液に二炭酸ジ-tert-ブチル178mgを添加し、室温にて30分間攪拌した。反応完結後、反応混合物に水10mlを加え、ジクロロメタンにて抽出(10mlx2)した。有機層を併せ、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(2:98~20:80のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物200mgを無色油状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.65 and 8.59 (d, J=1.8Hz, 1H), 7.87 and 7.85 (d, J=1.8Hz, 1H), 5.31 and 4.85 (d, J=8.1Hz, 1H), 4.75 (d, J=4.2Hz, 1H) and 4.67 (d, J=2.4Hz, 1H), 4.0-4.3 (m, 1H), 3.53 (s, 3H), 1.42 and 1.26 (s, 9H), 1.32 and 1.05 (d, J=6.6Hz, 3H)。 Step 3; Preparation of N- [2- (5-Bromo-3-chloropyridin-2-yl) -2-methoxy-1-methylethyl] carbamic acid-tert-butyl 2- (5-Bromo-3-chloro To a solution of 190 mg of pyridin-2-yl) -2-methoxy-1-methylethylamine and 80 mg of pyridine in 2 ml of dichloromethane was added 178 mg of di-tert-butyl dicarbonate and stirred at room temperature for 30 minutes. After completion of the reaction, 10 ml of water was added to the reaction mixture and extracted with dichloromethane (10 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (2: 98-20: 80 gradient) to obtain 200 mg of the objective product as a colorless oil.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.65 and 8.59 (d, J = 1.8Hz, 1H), 7.87 and 7.85 (d, J = 1.8Hz, 1H), 5.31 and 4.85 (d, J = 8.1Hz, 1H), 4.75 (d, J = 4.2Hz, 1H) and 4.67 (d, J = 2.4Hz, 1H), 4.0-4.3 (m, 1H), 3.53 (s, 3H), 1.42 and 1.26 (s, 9H), 1.32 and 1.05 (d, J = 6.6 Hz, 3H).
工程4;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メトキシ-1-メチルエチル]カルバミド酸-tert-ブチルの製造
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メトキシ-1-メチルエチル]カルバミド酸-tert-ブチル272mgのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン109mg、1-エチニル-4-フルオロベンゼン109mg、ヨウ化銅(I)27mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)25mgを添加し、窒素雰囲気下、80℃にて2時間攪拌し、次いでジクロロビス(トリフェニルホスフィン)パラジウム(II)42mgを追加し、さらに1時間攪拌を継続した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物220mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.69 and 8.64 (d, J=2.0Hz, 1H), 7.80 and 7.78 (d, J=2.0Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H), 5.35-5.5 and 4.85-4.95 (m, 1H), 4.75-4.85 and 4.15-4.3 (m, 1H), 3.36 (s, 3H), 1.43 and 1.29 (s, 9H), 1.32 and 1.06 (d, J=6.8Hz, 3H)。 Step 4; Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] carbamic acid-tert-butyl [2- (5-Bromo-3-chloropyridin-2-yl) -2-methoxy-1-methylethyl] carbamic acid-tert-butyl 272 mg of N, N-dimethylformamide 1 ml in a solution of 109 mg of triethylamine, 1-ethynyl 109 mg of -4-fluorobenzene, 27 mg of copper (I) iodide and 25 mg of dichlorobis (triphenylphosphine) palladium (II) were added and stirred at 80 ° C. for 2 hours under a nitrogen atmosphere, and then dichlorobis (triphenylphosphine). An additional 42 mg of palladium (II) was added and stirring was continued for an additional hour. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 220 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.69 and 8.64 (d, J = 2.0 Hz, 1H), 7.80 and 7.78 (d, J = 2.0 Hz, 1H), 7.45-7.55 (m, 2H ), 7.0-7.1 (m, 2H), 5.35-5.5 and 4.85-4.95 (m, 1H), 4.75-4.85 and 4.15-4.3 (m, 1H), 3.36 (s, 3H), 1.43 and 1.29 (s, 9H), 1.32 and 1.06 (d, J = 6.8 Hz, 3H).
N-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2-メトキシ-1-メチルエチル]カルバミド酸-tert-ブチル272mgのN,N-ジメチルホルムアミド1ml溶液にトリエチルアミン109mg、1-エチニル-4-フルオロベンゼン109mg、ヨウ化銅(I)27mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)25mgを添加し、窒素雰囲気下、80℃にて2時間攪拌し、次いでジクロロビス(トリフェニルホスフィン)パラジウム(II)42mgを追加し、さらに1時間攪拌を継続した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~1:9のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物220mgを褐色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.69 and 8.64 (d, J=2.0Hz, 1H), 7.80 and 7.78 (d, J=2.0Hz, 1H), 7.45-7.55 (m, 2H), 7.0-7.1 (m, 2H), 5.35-5.5 and 4.85-4.95 (m, 1H), 4.75-4.85 and 4.15-4.3 (m, 1H), 3.36 (s, 3H), 1.43 and 1.29 (s, 9H), 1.32 and 1.06 (d, J=6.8Hz, 3H)。 Step 4; Preparation of N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] carbamic acid-tert-butyl [2- (5-Bromo-3-chloropyridin-2-yl) -2-methoxy-1-methylethyl] carbamic acid-tert-butyl 272 mg of N, N-dimethylformamide 1 ml in a solution of 109 mg of triethylamine, 1-ethynyl 109 mg of -4-fluorobenzene, 27 mg of copper (I) iodide and 25 mg of dichlorobis (triphenylphosphine) palladium (II) were added and stirred at 80 ° C. for 2 hours under a nitrogen atmosphere, and then dichlorobis (triphenylphosphine). An additional 42 mg of palladium (II) was added and stirring was continued for an additional hour. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate (10 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 1: 9) to obtain 220 mg of the desired product as a brown resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.69 and 8.64 (d, J = 2.0 Hz, 1H), 7.80 and 7.78 (d, J = 2.0 Hz, 1H), 7.45-7.55 (m, 2H ), 7.0-7.1 (m, 2H), 5.35-5.5 and 4.85-4.95 (m, 1H), 4.75-4.85 and 4.15-4.3 (m, 1H), 3.36 (s, 3H), 1.43 and 1.29 (s, 9H), 1.32 and 1.06 (d, J = 6.8 Hz, 3H).
工程5;2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メトキシ-1-メチルエチルアミンの製造
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メトキシ-1-メチルエチル]カルバミド酸-tert-ブチル220mgのジクロロメタン2ml溶液に、氷冷攪拌下、トリフルオロ酢酸1mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去、残留物に飽和炭酸水素ナトリウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ、水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物167mgを暗褐色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.7-8.75 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.55 (m, 2H), 7.0-7.1 (m, 2H), 4.66 and 4.52 (d, J=5.5Hz, 1H), 3.35-3.55 (m, 1H), 3.32 and 3.29 (s, 3H), 1.84 and 1.68 (bs, 2H), 1.14 and 0.97 (d, J=6.5Hz, 3H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethylamine N- [2- [3-Chloro-5- To a solution of 220 mg of [(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] carbamic acid-tert-butyl in 2 ml of dichloromethane was added 1 ml of trifluoroacetic acid under ice-cooling and stirring. And stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 167 mg of the crude target product as a dark brown resinous substance. It was. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.7-8.75 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.55 (m, 2H), 7.0-7.1 (m, 2H) , 4.66 and 4.52 (d, J = 5.5Hz, 1H), 3.35-3.55 (m, 1H), 3.32 and 3.29 (s, 3H), 1.84 and 1.68 (bs, 2H), 1.14 and 0.97 (d, J = 6.5Hz, 3H).
N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メトキシ-1-メチルエチル]カルバミド酸-tert-ブチル220mgのジクロロメタン2ml溶液に、氷冷攪拌下、トリフルオロ酢酸1mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去、残留物に飽和炭酸水素ナトリウム水溶液10mlを添加し、酢酸エチルにて抽出(10mlx2)した。有機層を併せ、水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物167mgを暗褐色樹脂状物質として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.7-8.75 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.55 (m, 2H), 7.0-7.1 (m, 2H), 4.66 and 4.52 (d, J=5.5Hz, 1H), 3.35-3.55 (m, 1H), 3.32 and 3.29 (s, 3H), 1.84 and 1.68 (bs, 2H), 1.14 and 0.97 (d, J=6.5Hz, 3H)。 Step 5; Preparation of 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethylamine N- [2- [3-Chloro-5- To a solution of 220 mg of [(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] carbamic acid-tert-butyl in 2 ml of dichloromethane was added 1 ml of trifluoroacetic acid under ice-cooling and stirring. And stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 167 mg of the crude target product as a dark brown resinous substance. It was. The obtained target product was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.7-8.75 (m, 1H), 7.8-7.85 (m, 1H), 7.5-7.55 (m, 2H), 7.0-7.1 (m, 2H) , 4.66 and 4.52 (d, J = 5.5Hz, 1H), 3.35-3.55 (m, 1H), 3.32 and 3.29 (s, 3H), 1.84 and 1.68 (bs, 2H), 1.14 and 0.97 (d, J = 6.5Hz, 3H).
工程6;N-[2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メトキシ-1-メチルエチル]-2-(トリフルオロメチル)ベンズアミドの製造
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メトキシ-1-メチルエチルアミン80mg及びトリエチルアミン38mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド63mgを滴下し、滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2ml添加し、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物125mgを無色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.64 and 8.62 (d, J=1.8Hz, 1H), 7.85 and 7.83 (d, J=1.8Hz, 1H), 7.45-7.75 (m, 6H), 7.3-7.4 and 7.15-7.25 (m, 1H), 7.0-7.1 (m, 2H), 4.96 and 4.83 (d, J=4.0Hz, 1H), 4.75-4.9 and 4.55-4.7 (m, 1H), 3.41 and 3.38 (s, 3H), 1.48 and 1.13 (d, J=6.7Hz, 3H)。 Step 6; N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] -2- (trifluoromethyl) benzamide Preparation 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethylamine (80 mg) and triethylamine (38 mg) in dichloromethane (2 ml) were stirred under ice-cooling with 2 -(Trifluoromethyl) benzoyl chloride (63 mg) was added dropwise, and after completion of the addition, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain the desired product (125 mg) as a colorless resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.64 and 8.62 (d, J = 1.8Hz, 1H), 7.85 and 7.83 (d, J = 1.8Hz, 1H), 7.45-7.75 (m, 6H ), 7.3-7.4 and 7.15-7.25 (m, 1H), 7.0-7.1 (m, 2H), 4.96 and 4.83 (d, J = 4.0Hz, 1H), 4.75-4.9 and 4.55-4.7 (m, 1H) , 3.41 and 3.38 (s, 3H), 1.48 and 1.13 (d, J = 6.7Hz, 3H).
2-[3-クロロ-5-[(4-フルオロフェニル)エチニル]ピリジン-2-イル]-2-メトキシ-1-メチルエチルアミン80mg及びトリエチルアミン38mgのジクロロメタン2ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド63mgを滴下し、滴下終了後、室温にて1時間攪拌した。反応完結後、反応混合物に水2ml添加し、ジクロロメタンにて抽出(5mlx1)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物125mgを無色樹脂状物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.64 and 8.62 (d, J=1.8Hz, 1H), 7.85 and 7.83 (d, J=1.8Hz, 1H), 7.45-7.75 (m, 6H), 7.3-7.4 and 7.15-7.25 (m, 1H), 7.0-7.1 (m, 2H), 4.96 and 4.83 (d, J=4.0Hz, 1H), 4.75-4.9 and 4.55-4.7 (m, 1H), 3.41 and 3.38 (s, 3H), 1.48 and 1.13 (d, J=6.7Hz, 3H)。 Step 6; N- [2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethyl] -2- (trifluoromethyl) benzamide Preparation 2- [3-Chloro-5-[(4-fluorophenyl) ethynyl] pyridin-2-yl] -2-methoxy-1-methylethylamine (80 mg) and triethylamine (38 mg) in dichloromethane (2 ml) were stirred under ice-cooling with 2 -(Trifluoromethyl) benzoyl chloride (63 mg) was added dropwise, and after completion of the addition, the mixture was stirred at room temperature for 1 hour. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with dichloromethane (5 ml × 1). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain the desired product (125 mg) as a colorless resinous substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.64 and 8.62 (d, J = 1.8Hz, 1H), 7.85 and 7.83 (d, J = 1.8Hz, 1H), 7.45-7.75 (m, 6H ), 7.3-7.4 and 7.15-7.25 (m, 1H), 7.0-7.1 (m, 2H), 4.96 and 4.83 (d, J = 4.0Hz, 1H), 4.75-4.9 and 4.55-4.7 (m, 1H) , 3.41 and 3.38 (s, 3H), 1.48 and 1.13 (d, J = 6.7Hz, 3H).
合成例13
2-クロロ-N-[2-[3-クロロ-5-[(4-クロロ-2-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]ニコチンアミド(本発明化合物No.3-089)。 Synthesis Example 13
2-chloro-N- [2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethyl] nicotinamide ( Compound No. 3-089 of the present invention.
2-クロロ-N-[2-[3-クロロ-5-[(4-クロロ-2-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]ニコチンアミド(本発明化合物No.3-089)。 Synthesis Example 13
2-chloro-N- [2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethyl] nicotinamide ( Compound No. 3-089 of the present invention.
工程1;N-[2-[3-クロロ-5-[(4-クロロ-2-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチルの製造
合成例3の工程2にて製造したN-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチル1.23g、トリエチルアミン969mg、[(4-クロロ-2-フルオロフェニル)エチニル]トリメチルシラン832mg、ヨウ化銅(I)182mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)224mgのN,N-ジメチルホルムアミド6ml溶液にフッ化テトラブチルアンモニウムの1.0Mテトラヒドロフラン溶液3.8mlを添加し、窒素雰囲気下、70℃にて1.5時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10ml及び酢酸エチル10mlを添加し、有機層を分取し、水層は酢酸エチルにて抽出(10mlx2)した。有機層を併せ、飽和塩化アンモニウム水溶液にて洗浄(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:100~15:85のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物851mgを褐色結晶として得た。
融点127.0~129.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.63 (d, J=1.8Hz, 1H), 7.92 (d, J=1.8Hz, 1H), 7.47 (dd, J=8.4, 7.2Hz, 1H), 7.1-7.2 (m, 2H), 4.7-4.9 (m, 2H), 1.37 (d, J=6.6Hz, 3H), 1.31 (s, 9H)。 Step 1; N- [2- [3-Chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert -Butyl Production N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] carbamic acid-tert- produced in Step 2 of Synthesis Example 3 1.23 g of butyl, 969 mg of triethylamine, 832 mg of [(4-chloro-2-fluorophenyl) ethynyl] trimethylsilane, 182 mg of copper (I) iodide and 224 mg of dichlorobis (triphenylphosphine) palladium (II) To a 6 ml solution of formamide, 3.8 ml of a 1.0 M tetrahydrofuran solution of tetrabutylammonium fluoride was added and stirred at 70 ° C. for 1.5 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution and 10 ml of ethyl acetate were added, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with a saturated aqueous ammonium chloride solution (20 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0: 100 to 15:85) to obtain 851 mg of the desired product as brown crystals.
Melting point: 127.0-129.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.63 (d, J = 1.8 Hz, 1H), 7.92 (d, J = 1.8 Hz, 1H), 7.47 (dd, J = 8.4, 7.2 Hz, 1H), 7.1-7.2 (m, 2H), 4.7-4.9 (m, 2H), 1.37 (d, J = 6.6Hz, 3H), 1.31 (s, 9H).
合成例3の工程2にて製造したN-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチル1.23g、トリエチルアミン969mg、[(4-クロロ-2-フルオロフェニル)エチニル]トリメチルシラン832mg、ヨウ化銅(I)182mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)224mgのN,N-ジメチルホルムアミド6ml溶液にフッ化テトラブチルアンモニウムの1.0Mテトラヒドロフラン溶液3.8mlを添加し、窒素雰囲気下、70℃にて1.5時間攪拌した。反応完結後、反応混合物を室温まで放冷し、飽和塩化アンモニウム水溶液10ml及び酢酸エチル10mlを添加し、有機層を分取し、水層は酢酸エチルにて抽出(10mlx2)した。有機層を併せ、飽和塩化アンモニウム水溶液にて洗浄(20mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:100~15:85のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物851mgを褐色結晶として得た。
融点127.0~129.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.63 (d, J=1.8Hz, 1H), 7.92 (d, J=1.8Hz, 1H), 7.47 (dd, J=8.4, 7.2Hz, 1H), 7.1-7.2 (m, 2H), 4.7-4.9 (m, 2H), 1.37 (d, J=6.6Hz, 3H), 1.31 (s, 9H)。 Step 1; N- [2- [3-Chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert -Butyl Production N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoro-1-methylethyl] carbamic acid-tert- produced in Step 2 of Synthesis Example 3 1.23 g of butyl, 969 mg of triethylamine, 832 mg of [(4-chloro-2-fluorophenyl) ethynyl] trimethylsilane, 182 mg of copper (I) iodide and 224 mg of dichlorobis (triphenylphosphine) palladium (II) To a 6 ml solution of formamide, 3.8 ml of a 1.0 M tetrahydrofuran solution of tetrabutylammonium fluoride was added and stirred at 70 ° C. for 1.5 hours under a nitrogen atmosphere. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 10 ml of saturated aqueous ammonium chloride solution and 10 ml of ethyl acetate were added, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with a saturated aqueous ammonium chloride solution (20 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0: 100 to 15:85) to obtain 851 mg of the desired product as brown crystals.
Melting point: 127.0-129.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.63 (d, J = 1.8 Hz, 1H), 7.92 (d, J = 1.8 Hz, 1H), 7.47 (dd, J = 8.4, 7.2 Hz, 1H), 7.1-7.2 (m, 2H), 4.7-4.9 (m, 2H), 1.37 (d, J = 6.6Hz, 3H), 1.31 (s, 9H).
工程2;2-[3-クロロ-5-[(4-クロロ-2-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチルアミンの製造
氷冷攪拌下のN-[2-[3-クロロ-5-[(4-クロロ-2-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチル830mgのジクロロメタン6ml溶液にトリフルオロ酢酸2mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せ、水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物653mgを褐色結晶として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
融点69.0~71.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.6-8.65 (m, 1H), 7.9-7.95 (m, 1H), 7.47 (dd, J=8.4, 7.2Hz, 1H), 7.15-7.2 (m, 2H), 3.8-3.95 (m, 1H), 1.58 (bs, 2H), 1.25 (d, J=6.9Hz, 3H)。 Step 2; Preparation of 2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethylamine N under ice-cooling and stirring -[2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl 830 mg 2 ml of trifluoroacetic acid was added to a 6 ml solution of dichloromethane and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 653 mg of the crude desired product as brown crystals. The obtained target product was directly used in the next step without further purification.
Melting point: 69.0-71.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.6-8.65 (m, 1H), 7.9-7.95 (m, 1H), 7.47 (dd, J = 8.4, 7.2Hz, 1H), 7.15-7.2 (m, 2H), 3.8-3.95 (m, 1H), 1.58 (bs, 2H), 1.25 (d, J = 6.9Hz, 3H).
氷冷攪拌下のN-[2-[3-クロロ-5-[(4-クロロ-2-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]カルバミド酸-tert-ブチル830mgのジクロロメタン6ml溶液にトリフルオロ酢酸2mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加え、酢酸エチルにて抽出(10mlx2)した。有機層を併せ、水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物653mgを褐色結晶として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
融点69.0~71.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.6-8.65 (m, 1H), 7.9-7.95 (m, 1H), 7.47 (dd, J=8.4, 7.2Hz, 1H), 7.15-7.2 (m, 2H), 3.8-3.95 (m, 1H), 1.58 (bs, 2H), 1.25 (d, J=6.9Hz, 3H)。 Step 2; Preparation of 2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethylamine N under ice-cooling and stirring -[2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethyl] carbamic acid-tert-butyl 830 mg 2 ml of trifluoroacetic acid was added to a 6 ml solution of dichloromethane and stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 653 mg of the crude desired product as brown crystals. The obtained target product was directly used in the next step without further purification.
Melting point: 69.0-71.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.6-8.65 (m, 1H), 7.9-7.95 (m, 1H), 7.47 (dd, J = 8.4, 7.2Hz, 1H), 7.15-7.2 (m, 2H), 3.8-3.95 (m, 1H), 1.58 (bs, 2H), 1.25 (d, J = 6.9Hz, 3H).
工程3;2-クロロ-N-[2-[3-クロロ-5-[(4-クロロ-2-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチル]ニコチンアミドの製造
2-[3-クロロ-5-[(4-クロロ-2-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチルアミン131mgのN,N-ジメチルホルムアミド2ml溶液にトリエチルアミン111mg、N,N-ジメチル-4-アミノピリジン9mg、2-クロロニコチン酸69mg及びО-(ベンゾトリアゾール-1-イル)-N,N,N',N'-テトラメチルウロニウムテトラフルオロボラート176mgを添加し、室温にて16時間攪拌した。反応完結後、反応混合物に水2mlを添加し、酢酸エチルにて抽出(5mlx2)した。有機層を併せ、水洗(5mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~4:6のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物111mgを白色結晶として得た。
融点141.0~147.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.61 (d, J=1.8Hz, 1H), 8.45 (dd, J=4.8, 2.1Hz, 1H), 7.95-8.0 (m, 2H), 7.46 (dd, J=8.4, 7.8Hz, 1H), 7.32 (dd, J=7.5, 4.8Hz, 1H), 7.15-7.2 (m, 2H), 7.04 (d, J=9.9Hz, 1H), 5.3-5.4 (m, 1H), 1.52 (d, J=6.9Hz, 3H)。 Step 3; 2-chloro-N- [2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethyl] Preparation of Nicotinamide 2- [3-Chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethylamine 131 mg of N, N-dimethyl To a solution of formamide 2 ml, triethylamine 111 mg, N, N-dimethyl-4-aminopyridine 9 mg, 2-chloronicotinic acid 69 mg and O- (benzotriazol-1-yl) -N, N, N ′, N′-tetramethyluro 176 mg of nitrotetrafluoroborate was added and stirred at room temperature for 16 hours. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with ethyl acetate (5 ml × 2). The organic layers were combined, washed with water (5 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 4: 6 gradient) to obtain 111 mg of the objective product as white crystals.
Melting point: 141.0-147.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.61 (d, J = 1.8 Hz, 1H), 8.45 (dd, J = 4.8, 2.1 Hz, 1H), 7.95-8.0 (m, 2H), 7.46 (dd, J = 8.4, 7.8Hz, 1H), 7.32 (dd, J = 7.5, 4.8Hz, 1H), 7.15-7.2 (m, 2H), 7.04 (d, J = 9.9Hz, 1H), 5.3 -5.4 (m, 1H), 1.52 (d, J = 6.9Hz, 3H).
2-[3-クロロ-5-[(4-クロロ-2-フルオロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロ-1-メチルエチルアミン131mgのN,N-ジメチルホルムアミド2ml溶液にトリエチルアミン111mg、N,N-ジメチル-4-アミノピリジン9mg、2-クロロニコチン酸69mg及びО-(ベンゾトリアゾール-1-イル)-N,N,N',N'-テトラメチルウロニウムテトラフルオロボラート176mgを添加し、室温にて16時間攪拌した。反応完結後、反応混合物に水2mlを添加し、酢酸エチルにて抽出(5mlx2)した。有機層を併せ、水洗(5mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~4:6のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物111mgを白色結晶として得た。
融点141.0~147.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.61 (d, J=1.8Hz, 1H), 8.45 (dd, J=4.8, 2.1Hz, 1H), 7.95-8.0 (m, 2H), 7.46 (dd, J=8.4, 7.8Hz, 1H), 7.32 (dd, J=7.5, 4.8Hz, 1H), 7.15-7.2 (m, 2H), 7.04 (d, J=9.9Hz, 1H), 5.3-5.4 (m, 1H), 1.52 (d, J=6.9Hz, 3H)。 Step 3; 2-chloro-N- [2- [3-chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethyl] Preparation of Nicotinamide 2- [3-Chloro-5-[(4-chloro-2-fluorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoro-1-methylethylamine 131 mg of N, N-dimethyl To a solution of formamide 2 ml, triethylamine 111 mg, N, N-dimethyl-4-aminopyridine 9 mg, 2-chloronicotinic acid 69 mg and O- (benzotriazol-1-yl) -N, N, N ′, N′-tetramethyluro 176 mg of nitrotetrafluoroborate was added and stirred at room temperature for 16 hours. After completion of the reaction, 2 ml of water was added to the reaction mixture and extracted with ethyl acetate (5 ml × 2). The organic layers were combined, washed with water (5 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 4: 6 gradient) to obtain 111 mg of the objective product as white crystals.
Melting point: 141.0-147.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.61 (d, J = 1.8 Hz, 1H), 8.45 (dd, J = 4.8, 2.1 Hz, 1H), 7.95-8.0 (m, 2H), 7.46 (dd, J = 8.4, 7.8Hz, 1H), 7.32 (dd, J = 7.5, 4.8Hz, 1H), 7.15-7.2 (m, 2H), 7.04 (d, J = 9.9Hz, 1H), 5.3 -5.4 (m, 1H), 1.52 (d, J = 6.9Hz, 3H).
合成例14
N-[2-[3-クロロ-5-[(6-フルオロピリジン-3-イル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-011)。
工程1;N-[2-[3-クロロ-5-[(トリメチルシリル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチルの製造
合成例1の工程3にて製造したN-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル1.86gのN,N-ジメチルホルムアミド15ml溶液にトリエチルアミン1.52g、トリメチルシリルアセチレン0.59g、ヨウ化銅(I)285mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)350mgを添加し、窒素雰囲気下、室温にて1時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液20ml及び酢酸エチル20mlを添加し、有機層を分取し、水層は酢酸エチルにて抽出(10mlx2)した。有機層を併せて、飽和塩化アンモニウム水溶液20mlにて洗浄後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.44gを黄色結晶として得た。
融点93.0~97.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.50 (d, J=1.5Hz, 1H), 7.85 (d, J=1.5Hz, 1H), 5.08 (bs, 1H), 4.07 (td, J=12.9, 6.6Hz, 2H), 1.41 (s, 9H), 0.27 (s, 9H)。 Synthesis Example 14
N- [2- [3-Chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) benzamide (this Invention compound No. 1-011).
Step 1; Preparation of N- [2- [3-Chloro-5-[(trimethylsilyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl Synthesis Example 1 Step 3 N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethyl] carbamic acid-tert-butyl 1.86 g of N-N-dimethylformamide in 15 ml of 1.52 g, 0.59 g of trimethylsilylacetylene, 285 mg of copper (I) iodide and 350 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at room temperature for 1 hour in a nitrogen atmosphere. After completion of the reaction, 20 ml of saturated aqueous ammonium chloride solution and 20 ml of ethyl acetate were added to the reaction mixture, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with 20 ml of saturated aqueous ammonium chloride solution, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 2: 8) to obtain 1.44 g of the objective product as yellow crystals.
Melting point: 93.0-97.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.50 (d, J = 1.5Hz, 1H), 7.85 (d, J = 1.5Hz, 1H), 5.08 (bs, 1H), 4.07 (td, J = 12.9, 6.6Hz, 2H), 1.41 (s, 9H), 0.27 (s, 9H).
N-[2-[3-クロロ-5-[(6-フルオロピリジン-3-イル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-011)。
工程1;N-[2-[3-クロロ-5-[(トリメチルシリル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチルの製造
合成例1の工程3にて製造したN-[2-(5-ブロモ-3-クロロピリジン-2-イル)-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル1.86gのN,N-ジメチルホルムアミド15ml溶液にトリエチルアミン1.52g、トリメチルシリルアセチレン0.59g、ヨウ化銅(I)285mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)350mgを添加し、窒素雰囲気下、室温にて1時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液20ml及び酢酸エチル20mlを添加し、有機層を分取し、水層は酢酸エチルにて抽出(10mlx2)した。有機層を併せて、飽和塩化アンモニウム水溶液20mlにて洗浄後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物1.44gを黄色結晶として得た。
融点93.0~97.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.50 (d, J=1.5Hz, 1H), 7.85 (d, J=1.5Hz, 1H), 5.08 (bs, 1H), 4.07 (td, J=12.9, 6.6Hz, 2H), 1.41 (s, 9H), 0.27 (s, 9H)。 Synthesis Example 14
N- [2- [3-Chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) benzamide (this Invention compound No. 1-011).
Step 1; Preparation of N- [2- [3-Chloro-5-[(trimethylsilyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl Synthesis Example 1 Step 3 N- [2- (5-Bromo-3-chloropyridin-2-yl) -2,2-difluoroethyl] carbamic acid-tert-butyl 1.86 g of N-N-dimethylformamide in 15 ml of 1.52 g, 0.59 g of trimethylsilylacetylene, 285 mg of copper (I) iodide and 350 mg of dichlorobis (triphenylphosphine) palladium (II) were added, and the mixture was stirred at room temperature for 1 hour in a nitrogen atmosphere. After completion of the reaction, 20 ml of saturated aqueous ammonium chloride solution and 20 ml of ethyl acetate were added to the reaction mixture, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with 20 ml of saturated aqueous ammonium chloride solution, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 2: 8) to obtain 1.44 g of the objective product as yellow crystals.
Melting point: 93.0-97.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.50 (d, J = 1.5Hz, 1H), 7.85 (d, J = 1.5Hz, 1H), 5.08 (bs, 1H), 4.07 (td, J = 12.9, 6.6Hz, 2H), 1.41 (s, 9H), 0.27 (s, 9H).
工程2;N-[2-[3-クロロ-5-[(6-フルオロピリジン-3-イル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチルの製造
N-[2-[3-クロロ-5-[(トリメチルシリル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル480mg、トリエチルアミン375mg、2-フルオロ-5-ヨードピリジン303mg、ヨウ化銅(I)71mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)87mgのN,N-ジメチルホルムアミド6ml溶液にフッ化テトラブチルアンモニウムの1.0Mテトラヒドロフラン溶液1.3mlを添加し、窒素雰囲気下、室温にて16時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液10ml及び酢酸エチル10mlを添加し有機層を分取し、水層は酢酸エチルにて抽出(10mlx2)した。有機層を併せ、飽和塩化アンモニウム水溶液20mlにて洗浄後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物324mgを無色樹脂上物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.65 (m, 1H), 8.4-8.5 (m, 1H), 7.9-8.0 (m, 2H), 6.95-7.05 (m, 1H), 5.05-5.2 (m, 1H), 4.0-4.2 (m, 2H), 1.41 (s, 9H)。 Step 2; Preparation of N- [2- [3-chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl N- [2- [3-Chloro-5-[(trimethylsilyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl 480 mg, triethylamine 375 mg, 2-fluoro-5-iodo To a solution of 303 mg of pyridine, 71 mg of copper (I) iodide and 87 mg of dichlorobis (triphenylphosphine) palladium (II) in 6 ml of N, N-dimethylformamide was added 1.3 ml of a 1.0 M tetrahydrofuran solution of tetrabutylammonium fluoride. The mixture was stirred at room temperature for 16 hours under a nitrogen atmosphere. After completion of the reaction, 10 ml of a saturated aqueous ammonium chloride solution and 10 ml of ethyl acetate were added to the reaction mixture, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with 20 ml of saturated aqueous ammonium chloride solution, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 2: 8) to obtain 324 mg of the desired product as a colorless resin substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.65 (m, 1H), 8.4-8.5 (m, 1H), 7.9-8.0 (m, 2H), 6.95-7.05 (m, 1H) , 5.05-5.2 (m, 1H), 4.0-4.2 (m, 2H), 1.41 (s, 9H).
N-[2-[3-クロロ-5-[(トリメチルシリル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル480mg、トリエチルアミン375mg、2-フルオロ-5-ヨードピリジン303mg、ヨウ化銅(I)71mg及びジクロロビス(トリフェニルホスフィン)パラジウム(II)87mgのN,N-ジメチルホルムアミド6ml溶液にフッ化テトラブチルアンモニウムの1.0Mテトラヒドロフラン溶液1.3mlを添加し、窒素雰囲気下、室温にて16時間攪拌した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液10ml及び酢酸エチル10mlを添加し有機層を分取し、水層は酢酸エチルにて抽出(10mlx2)した。有機層を併せ、飽和塩化アンモニウム水溶液20mlにて洗浄後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(0:10~2:8のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物324mgを無色樹脂上物質として得た。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.65 (m, 1H), 8.4-8.5 (m, 1H), 7.9-8.0 (m, 2H), 6.95-7.05 (m, 1H), 5.05-5.2 (m, 1H), 4.0-4.2 (m, 2H), 1.41 (s, 9H)。 Step 2; Preparation of N- [2- [3-chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl N- [2- [3-Chloro-5-[(trimethylsilyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl 480 mg, triethylamine 375 mg, 2-fluoro-5-iodo To a solution of 303 mg of pyridine, 71 mg of copper (I) iodide and 87 mg of dichlorobis (triphenylphosphine) palladium (II) in 6 ml of N, N-dimethylformamide was added 1.3 ml of a 1.0 M tetrahydrofuran solution of tetrabutylammonium fluoride. The mixture was stirred at room temperature for 16 hours under a nitrogen atmosphere. After completion of the reaction, 10 ml of a saturated aqueous ammonium chloride solution and 10 ml of ethyl acetate were added to the reaction mixture, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (10 ml × 2). The organic layers were combined, washed with 20 ml of saturated aqueous ammonium chloride solution, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (gradient from 0:10 to 2: 8) to obtain 324 mg of the desired product as a colorless resin substance.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.65 (m, 1H), 8.4-8.5 (m, 1H), 7.9-8.0 (m, 2H), 6.95-7.05 (m, 1H) , 5.05-5.2 (m, 1H), 4.0-4.2 (m, 2H), 1.41 (s, 9H).
工程3;2-[3-クロロ-5-[(6-フルオロピリジン-3-イル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチルアミンの製造
氷冷攪拌下のN-[2-[3-クロロ-5-[(6-フルオロピリジン-3-イル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル324mgのジクロロメタン3ml溶液にトリフルオロ酢酸1mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加えて酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物253mgを黄色結晶として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
融点109.0~111.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.61 (d, J=1.2Hz, 1H), 8.43 (d, J=2.4Hz, 1H), 7.9-8.0 (m, 2H), 7.00 (dd, J=8.4, 3.0Hz, 1H), 3.52 (t, J=14.1Hz, 2H), 1.58 (bs, 2H)。 Step 3; Preparation of 2- [3-chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine N- [2- under ice-cooling stirring [3-Chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl 324 mg in dichloromethane 3 ml solution with trifluoroacetic acid 1 ml And stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 253 mg of the crude desired product as yellow crystals. The obtained target product was directly used in the next step without further purification.
Melting point: 109.0-111.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.61 (d, J = 1.2 Hz, 1H), 8.43 (d, J = 2.4 Hz, 1H), 7.9-8.0 (m, 2H), 7.00 ( dd, J = 8.4, 3.0 Hz, 1H), 3.52 (t, J = 14.1 Hz, 2H), 1.58 (bs, 2H).
氷冷攪拌下のN-[2-[3-クロロ-5-[(6-フルオロピリジン-3-イル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]カルバミド酸-tert-ブチル324mgのジクロロメタン3ml溶液にトリフルオロ酢酸1mlを添加し、室温にて1時間攪拌した。反応完結後、減圧下にて溶媒を留去し、残留物に飽和炭酸水素ナトリウム水溶液10mlを加えて酢酸エチルにて抽出(10mlx2)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物253mgを黄色結晶として得た。得られた目的物はさらなる精製を行うことなく、そのまま次の工程に用いた。
融点109.0~111.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.61 (d, J=1.2Hz, 1H), 8.43 (d, J=2.4Hz, 1H), 7.9-8.0 (m, 2H), 7.00 (dd, J=8.4, 3.0Hz, 1H), 3.52 (t, J=14.1Hz, 2H), 1.58 (bs, 2H)。 Step 3; Preparation of 2- [3-chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine N- [2- under ice-cooling stirring [3-Chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] carbamic acid-tert-butyl 324 mg in dichloromethane 3 ml solution with trifluoroacetic acid 1 ml And stirred at room temperature for 1 hour. After completion of the reaction, the solvent was distilled off under reduced pressure, 10 ml of a saturated aqueous sodium hydrogen carbonate solution was added to the residue, and the mixture was extracted with ethyl acetate (10 ml × 2). The organic layers were combined and washed with water (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 253 mg of the crude desired product as yellow crystals. The obtained target product was directly used in the next step without further purification.
Melting point: 109.0-111.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.61 (d, J = 1.2 Hz, 1H), 8.43 (d, J = 2.4 Hz, 1H), 7.9-8.0 (m, 2H), 7.00 ( dd, J = 8.4, 3.0 Hz, 1H), 3.52 (t, J = 14.1 Hz, 2H), 1.58 (bs, 2H).
工程4;N-[2-[3-クロロ-5-[(6-フルオロピリジン-3-イル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-2-(トリフルオロメチル)ベンズアミドの製造
氷冷攪拌下の2-[3-クロロ-5-[(6-フルオロピリジン-3-イル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチルアミン119mg及びトリチルアミン77mgのジクロロメタン2ml溶液に2-(トリフルオロメチル)ベンゾイルクロリド88mgを滴下した。滴下終了後、室温にて16時間攪拌した。反応完結後、反応混合物に水2mlを添加し有機層を分取し、水層はジクロロメタンにて抽出(5mlx1)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留した固体をジイソプロピルエーテル10mlにて洗浄し、目的物148mgを白色結晶として得た。
融点155.0~157.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.6 (m, 1H), 8.4-8.45 (m, 1H), 7.97 (d, J=1.8Hz, 1H), 7.93 (ddd, J=8.7, 7.5, 2.4Hz, 1H), 7.5-7.7 (m, 4H), 6.99 (ddd, J=8.7, 3.3, 0.9Hz, 1H), 6.42 (t, J=6.6Hz, 1H), 4.47 (td, J=13.5, 6.6Hz, 2H)。 Step 4; N- [2- [3-Chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) Preparation of benzamide Dichloromethane of 2- [3-chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine and 77 mg of tritylamine under ice-cooling stirring To a 2 ml solution, 88 mg of 2- (trifluoromethyl) benzoyl chloride was added dropwise. After completion of dropping, the mixture was stirred at room temperature for 16 hours. After completion of the reaction, 2 ml of water was added to the reaction mixture, the organic layer was separated, and the aqueous layer was extracted with dichloromethane (5 ml × 1). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The remaining solid was washed with 10 ml of diisopropyl ether to obtain 148 mg of the desired product as white crystals.
Melting point 155.0-157.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.6 (m, 1H), 8.4-8.45 (m, 1H), 7.97 (d, J = 1.8Hz, 1H), 7.93 (ddd, J = 8.7, 7.5, 2.4Hz, 1H), 7.5-7.7 (m, 4H), 6.99 (ddd, J = 8.7, 3.3, 0.9Hz, 1H), 6.42 (t, J = 6.6Hz, 1H), 4.47 ( td, J = 13.5, 6.6Hz, 2H).
氷冷攪拌下の2-[3-クロロ-5-[(6-フルオロピリジン-3-イル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチルアミン119mg及びトリチルアミン77mgのジクロロメタン2ml溶液に2-(トリフルオロメチル)ベンゾイルクロリド88mgを滴下した。滴下終了後、室温にて16時間攪拌した。反応完結後、反応混合物に水2mlを添加し有機層を分取し、水層はジクロロメタンにて抽出(5mlx1)した。有機層を併せ水洗(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去した。残留した固体をジイソプロピルエーテル10mlにて洗浄し、目的物148mgを白色結晶として得た。
融点155.0~157.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55-8.6 (m, 1H), 8.4-8.45 (m, 1H), 7.97 (d, J=1.8Hz, 1H), 7.93 (ddd, J=8.7, 7.5, 2.4Hz, 1H), 7.5-7.7 (m, 4H), 6.99 (ddd, J=8.7, 3.3, 0.9Hz, 1H), 6.42 (t, J=6.6Hz, 1H), 4.47 (td, J=13.5, 6.6Hz, 2H)。 Step 4; N- [2- [3-Chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) Preparation of benzamide Dichloromethane of 2- [3-chloro-5-[(6-fluoropyridin-3-yl) ethynyl] pyridin-2-yl] -2,2-difluoroethylamine and 77 mg of tritylamine under ice-cooling stirring To a 2 ml solution, 88 mg of 2- (trifluoromethyl) benzoyl chloride was added dropwise. After completion of dropping, the mixture was stirred at room temperature for 16 hours. After completion of the reaction, 2 ml of water was added to the reaction mixture, the organic layer was separated, and the aqueous layer was extracted with dichloromethane (5 ml × 1). The organic layers were combined, washed with water (10 ml × 1), dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The remaining solid was washed with 10 ml of diisopropyl ether to obtain 148 mg of the desired product as white crystals.
Melting point 155.0-157.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55-8.6 (m, 1H), 8.4-8.45 (m, 1H), 7.97 (d, J = 1.8Hz, 1H), 7.93 (ddd, J = 8.7, 7.5, 2.4Hz, 1H), 7.5-7.7 (m, 4H), 6.99 (ddd, J = 8.7, 3.3, 0.9Hz, 1H), 6.42 (t, J = 6.6Hz, 1H), 4.47 ( td, J = 13.5, 6.6Hz, 2H).
合成例15
N-[2-[3-クロロ-5-[(4-クロロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-N-エチル-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.15-002)。
合成例1と同様にして製造したN-[2-[3-クロロ-5-[(4-クロロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-023)150mgのテトラヒドロフラン1ml溶液に、氷冷攪拌下、55%油性水素化ナトリウム26mgを添加して、窒素雰囲気下、同温度にて30分間攪拌した。水素ガスの発生が止んだ後、ヨードエタン92mgのテトラヒドロフラン0.5ml溶液を添加し、室温にて2時間攪拌した。次いで55%油性水素化ナトリウム26mg及びヨードエタン92mgのテトラヒドロフラン0.5ml溶液を追加、室温にてさらに16時間、攪拌を継続した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液2mlを添加し酢酸エチルにて抽出(5mlx2)した。有機層を併せて、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(5:95~30:70のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物105mgを黄色結晶として得た。
融点100.0~103.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.62 and 8.48 (d, J=1.8Hz, 1H), 7.90 and 7.81 (d, J=1.8Hz, 1H), 7.65-7.7 (m, 1H), 7.3-7.6 (m, 7H), 3.9-4.9 (m, 2H), 3.35-3.5 (m, 2H), 1.30 and 1.11 (t, J=6.9Hz, 3H)。 Synthesis Example 15
N- [2- [3-Chloro-5-[(4-chlorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -N-ethyl-2- (trifluoromethyl) benzamide (invention Compound No. 15-002).
N- [2- [3-Chloro-5-[(4-chlorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) prepared in the same manner as in Synthesis Example 1. ) 26 mg of 55% oily sodium hydride was added to 1 ml of tetrahydrofuran solution of 150 mg of benzamide (present compound No. 1-023) under ice-cooling and stirred at the same temperature for 30 minutes under nitrogen atmosphere. After the evolution of hydrogen gas ceased, a solution of 92 mg of iodoethane in 0.5 ml of tetrahydrofuran was added and stirred at room temperature for 2 hours. Subsequently, a solution of 55% oily sodium hydride (26 mg) and iodoethane (92 mg) in 0.5 ml of tetrahydrofuran was added, and stirring was continued at room temperature for another 16 hours. After completion of the reaction, 2 ml of a saturated aqueous ammonium chloride solution was added to the reaction mixture and extracted with ethyl acetate (5 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (5: 95-30: 70 gradient) to obtain 105 mg of the objective product as yellow crystals.
Melting point: 100.0-103.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.62 and 8.48 (d, J = 1.8Hz, 1H), 7.90 and 7.81 (d, J = 1.8Hz, 1H), 7.65-7.7 (m, 1H ), 7.3-7.6 (m, 7H), 3.9-4.9 (m, 2H), 3.35-3.5 (m, 2H), 1.30 and 1.11 (t, J = 6.9Hz, 3H).
N-[2-[3-クロロ-5-[(4-クロロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-N-エチル-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.15-002)。
合成例1と同様にして製造したN-[2-[3-クロロ-5-[(4-クロロフェニル)エチニル]ピリジン-2-イル]-2,2-ジフルオロエチル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-023)150mgのテトラヒドロフラン1ml溶液に、氷冷攪拌下、55%油性水素化ナトリウム26mgを添加して、窒素雰囲気下、同温度にて30分間攪拌した。水素ガスの発生が止んだ後、ヨードエタン92mgのテトラヒドロフラン0.5ml溶液を添加し、室温にて2時間攪拌した。次いで55%油性水素化ナトリウム26mg及びヨードエタン92mgのテトラヒドロフラン0.5ml溶液を追加、室温にてさらに16時間、攪拌を継続した。反応完結後、反応混合物に飽和塩化アンモニウム水溶液2mlを添加し酢酸エチルにて抽出(5mlx2)した。有機層を併せて、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(5:95~30:70のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物105mgを黄色結晶として得た。
融点100.0~103.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.62 and 8.48 (d, J=1.8Hz, 1H), 7.90 and 7.81 (d, J=1.8Hz, 1H), 7.65-7.7 (m, 1H), 7.3-7.6 (m, 7H), 3.9-4.9 (m, 2H), 3.35-3.5 (m, 2H), 1.30 and 1.11 (t, J=6.9Hz, 3H)。 Synthesis Example 15
N- [2- [3-Chloro-5-[(4-chlorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -N-ethyl-2- (trifluoromethyl) benzamide (invention Compound No. 15-002).
N- [2- [3-Chloro-5-[(4-chlorophenyl) ethynyl] pyridin-2-yl] -2,2-difluoroethyl] -2- (trifluoromethyl) prepared in the same manner as in Synthesis Example 1. ) 26 mg of 55% oily sodium hydride was added to 1 ml of tetrahydrofuran solution of 150 mg of benzamide (present compound No. 1-023) under ice-cooling and stirred at the same temperature for 30 minutes under nitrogen atmosphere. After the evolution of hydrogen gas ceased, a solution of 92 mg of iodoethane in 0.5 ml of tetrahydrofuran was added and stirred at room temperature for 2 hours. Subsequently, a solution of 55% oily sodium hydride (26 mg) and iodoethane (92 mg) in 0.5 ml of tetrahydrofuran was added, and stirring was continued at room temperature for another 16 hours. After completion of the reaction, 2 ml of a saturated aqueous ammonium chloride solution was added to the reaction mixture and extracted with ethyl acetate (5 ml × 2). The organic layers were combined, dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (5: 95-30: 70 gradient) to obtain 105 mg of the objective product as yellow crystals.
Melting point: 100.0-103.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.62 and 8.48 (d, J = 1.8Hz, 1H), 7.90 and 7.81 (d, J = 1.8Hz, 1H), 7.65-7.7 (m, 1H ), 7.3-7.6 (m, 7H), 3.9-4.9 (m, 2H), 3.35-3.5 (m, 2H), 1.30 and 1.11 (t, J = 6.9Hz, 3H).
合成例16
N-[1-[[3-クロロ-5-[(4-クロロフェニル)エチニル]ピリジン-2-イル]メチル]シクロプロピル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-160)。
工程1;1-[(5-ブロモ-3-クロロピリジン-2-イル)メチル]シクロプロパンアミンの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル2.38g及びチタニウム(IV)テトライソプロポキシド3.21gのジエチルエーテル20ml溶液に、氷冷攪拌下、3Mエチルマグネシウムブロミドジエチルエーテル溶液6.90mlを滴下し、室温にて1時間攪拌した。次いで、この反応混合物に三フッ化ホウ素ジエチルエーテル錯体2.92gを滴下し、同温度にてさらに2時間攪拌を継続した。反応完結後、反応混合物に氷冷攪拌下10%水酸化ナトリウム水溶液15ml及び酢酸エチル15mlを加え30分間攪拌、セライト濾過後、有機層を分取した。飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物2.50gを褐色樹脂状物質として得た。このものは更なる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55 (d, J=1.8Hz, 1H), 7.84 (d, J=1.8Hz, 1H), 3.04 (s, 2H), 1.9-2.0 (m, 2H), 0.6-0.65 (m, 4H)。 Synthesis Example 16
N- [1-[[3-Chloro-5-[(4-chlorophenyl) ethynyl] pyridin-2-yl] methyl] cyclopropyl] -2- (trifluoromethyl) benzamide (Compound No. 1-160 of the present invention) ).
Step 1: Preparation of 1-[(5-bromo-3-chloropyridin-2-yl) methyl] cyclopropanamine 2.38 g of 2- (5-bromo-3-chloropyridin-2-yl) acetonitrile and titanium ( IV) To a solution of 3.21 g of tetraisopropoxide in 20 ml of diethyl ether was added dropwise 6.90 ml of 3M ethylmagnesium bromide diethyl ether solution with stirring under ice cooling, and the mixture was stirred at room temperature for 1 hour. Subsequently, 2.92 g of boron trifluoride diethyl ether complex was added dropwise to the reaction mixture, and stirring was continued for another 2 hours at the same temperature. After completion of the reaction, 15 ml of 10% aqueous sodium hydroxide and 15 ml of ethyl acetate were added to the reaction mixture with ice-cooling and stirring, and the mixture was stirred for 30 minutes and filtered through celite, and the organic layer was separated. After dehydration and drying in the order of saturated saline and then anhydrous sodium sulfate, the solvent was distilled off under reduced pressure to obtain 2.50 g of a crude desired product as a brown resinous substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55 (d, J = 1.8 Hz, 1H), 7.84 (d, J = 1.8 Hz, 1H), 3.04 (s, 2H), 1.9-2.0 ( m, 2H), 0.6-0.65 (m, 4H).
N-[1-[[3-クロロ-5-[(4-クロロフェニル)エチニル]ピリジン-2-イル]メチル]シクロプロピル]-2-(トリフルオロメチル)ベンズアミド(本発明化合物No.1-160)。
工程1;1-[(5-ブロモ-3-クロロピリジン-2-イル)メチル]シクロプロパンアミンの製造
2-(5-ブロモ-3-クロロピリジン-2-イル)アセトニトリル2.38g及びチタニウム(IV)テトライソプロポキシド3.21gのジエチルエーテル20ml溶液に、氷冷攪拌下、3Mエチルマグネシウムブロミドジエチルエーテル溶液6.90mlを滴下し、室温にて1時間攪拌した。次いで、この反応混合物に三フッ化ホウ素ジエチルエーテル錯体2.92gを滴下し、同温度にてさらに2時間攪拌を継続した。反応完結後、反応混合物に氷冷攪拌下10%水酸化ナトリウム水溶液15ml及び酢酸エチル15mlを加え30分間攪拌、セライト濾過後、有機層を分取した。飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥後、減圧下にて溶媒を留去し、粗製の目的物2.50gを褐色樹脂状物質として得た。このものは更なる精製を行うことなく、そのまま次の工程に用いた。
1H NMR (CDCl3, Me4Si, 300MHz) δ8.55 (d, J=1.8Hz, 1H), 7.84 (d, J=1.8Hz, 1H), 3.04 (s, 2H), 1.9-2.0 (m, 2H), 0.6-0.65 (m, 4H)。 Synthesis Example 16
N- [1-[[3-Chloro-5-[(4-chlorophenyl) ethynyl] pyridin-2-yl] methyl] cyclopropyl] -2- (trifluoromethyl) benzamide (Compound No. 1-160 of the present invention) ).
Step 1: Preparation of 1-[(5-bromo-3-chloropyridin-2-yl) methyl] cyclopropanamine 2.38 g of 2- (5-bromo-3-chloropyridin-2-yl) acetonitrile and titanium ( IV) To a solution of 3.21 g of tetraisopropoxide in 20 ml of diethyl ether was added dropwise 6.90 ml of 3M ethylmagnesium bromide diethyl ether solution with stirring under ice cooling, and the mixture was stirred at room temperature for 1 hour. Subsequently, 2.92 g of boron trifluoride diethyl ether complex was added dropwise to the reaction mixture, and stirring was continued for another 2 hours at the same temperature. After completion of the reaction, 15 ml of 10% aqueous sodium hydroxide and 15 ml of ethyl acetate were added to the reaction mixture with ice-cooling and stirring, and the mixture was stirred for 30 minutes and filtered through celite, and the organic layer was separated. After dehydration and drying in the order of saturated saline and then anhydrous sodium sulfate, the solvent was distilled off under reduced pressure to obtain 2.50 g of a crude desired product as a brown resinous substance. This was directly used in the next step without further purification.
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.55 (d, J = 1.8 Hz, 1H), 7.84 (d, J = 1.8 Hz, 1H), 3.04 (s, 2H), 1.9-2.0 ( m, 2H), 0.6-0.65 (m, 4H).
工程2;N-[1-[(5-ブロモ-3-クロロピリジン-2-イル)メチル]シクロプロピル]-2-(トリフルオロメチル)ベンズアミドの製造
1-[(5-ブロモ-3-クロロピリジン-2-イル)メチル]シクロプロパンアミン2.50g及びトリエチルアミン2.08gのジクロロメタン10ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド2.04gを滴下し、室温にて1時間攪拌した。反応完結後、反応混合物に水5mlを加えクロロホルムにて抽出(5mlx2)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(5:95~30:70のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物584mgを黄色結晶として得た。
融点151.0~152.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.48 (d, J=1.8Hz, 1H), 7.83 (d, J=1.8Hz, 1H), 7.45-7.65 (m, 4H), 6.56 (s, 1H), 3.28 (s, 2H), 0.9-1.1 (m, 4H)。 Step 2: Preparation of N- [1-[(5-bromo-3-chloropyridin-2-yl) methyl] cyclopropyl] -2- (trifluoromethyl) benzamide 1-[(5-Bromo-3-chloro To a solution of 2.50 g of pyridin-2-yl) methyl] cyclopropanamine and 2.08 g of triethylamine in 10 ml of dichloromethane, 2.04 g of 2- (trifluoromethyl) benzoyl chloride was added dropwise with stirring under ice-cooling. Stir for hours. After completion of the reaction, 5 ml of water was added to the reaction mixture and extracted with chloroform (5 ml × 2). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (5: 95-30: 70 gradient) to obtain 584 mg of the objective product as yellow crystals.
Melting point 151.0-152.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.48 (d, J = 1.8Hz, 1H), 7.83 (d, J = 1.8Hz, 1H), 7.45-7.65 (m, 4H), 6.56 ( s, 1H), 3.28 (s, 2H), 0.9-1.1 (m, 4H).
1-[(5-ブロモ-3-クロロピリジン-2-イル)メチル]シクロプロパンアミン2.50g及びトリエチルアミン2.08gのジクロロメタン10ml溶液に、氷冷攪拌下、2-(トリフルオロメチル)ベンゾイルクロリド2.04gを滴下し、室温にて1時間攪拌した。反応完結後、反応混合物に水5mlを加えクロロホルムにて抽出(5mlx2)した。有機層を飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(5:95~30:70のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物584mgを黄色結晶として得た。
融点151.0~152.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.48 (d, J=1.8Hz, 1H), 7.83 (d, J=1.8Hz, 1H), 7.45-7.65 (m, 4H), 6.56 (s, 1H), 3.28 (s, 2H), 0.9-1.1 (m, 4H)。 Step 2: Preparation of N- [1-[(5-bromo-3-chloropyridin-2-yl) methyl] cyclopropyl] -2- (trifluoromethyl) benzamide 1-[(5-Bromo-3-chloro To a solution of 2.50 g of pyridin-2-yl) methyl] cyclopropanamine and 2.08 g of triethylamine in 10 ml of dichloromethane, 2.04 g of 2- (trifluoromethyl) benzoyl chloride was added dropwise with stirring under ice-cooling. Stir for hours. After completion of the reaction, 5 ml of water was added to the reaction mixture and extracted with chloroform (5 ml × 2). The organic layer was dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (5: 95-30: 70 gradient) to obtain 584 mg of the objective product as yellow crystals.
Melting point 151.0-152.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ 8.48 (d, J = 1.8Hz, 1H), 7.83 (d, J = 1.8Hz, 1H), 7.45-7.65 (m, 4H), 6.56 ( s, 1H), 3.28 (s, 2H), 0.9-1.1 (m, 4H).
工程3;N-[1-[[3-クロロ-5-[(4-クロロフェニル)エチニル]ピリジン-2-イル]メチル]シクロプロピル]-2-(トリフルオロメチル)ベンズアミドの製造
N-[1-[(5-ブロモ-3-クロロピリジン-2-イル)メチル]シクロプロピル]-2-(トリフルオロメチル)ベンズアミド270mgのN,N-ジメチルホルムアミド3ml溶液に炭酸セシウム409mg、ヨウ化銅(I)12mg、酢酸パラジウム(II)14mg、4,5-ビス(ジフェニルホスフィノ)-9,9-ジメチルキサンテン36mg及び4-クロロ-1-エチニルベンゼン102mgを添加し、窒素雰囲気下、70℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し酢酸エチル5ml及び水5mlを添加して有機層を分取、水層は酢酸エチルにて抽出(5mlx2)した。有機層を併せ飽和塩化アンモニウム水溶液にて洗浄(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物230mgを褐色結晶として得た。
融点161.0~162.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.53 (d, J=1.8Hz, 1H), 7.78 (d, J=1.8Hz, 1H), 7.6-7.65 (m, 1H), 7.4-7.55 (m, 5H), 7.3-7.35 (m, 2H), 6.62 (s, 1H), 3.35 (s, 2H), 0.95-1.1 (m, 4H)。 Step 3; Preparation of N- [1-[[3-Chloro-5-[(4-chlorophenyl) ethynyl] pyridin-2-yl] methyl] cyclopropyl] -2- (trifluoromethyl) benzamide N- [1 -[(5-Bromo-3-chloropyridin-2-yl) methyl] cyclopropyl] -2- (trifluoromethyl) benzamide in a solution of 270 mg of N, N-dimethylformamide in 3 ml of cesium carbonate, 409 mg of copper iodide (I 12 mg, 14 mg of palladium (II) acetate, 36 mg of 4,5-bis (diphenylphosphino) -9,9-dimethylxanthene and 102 mg of 4-chloro-1-ethynylbenzene were added at 70 ° C. under a nitrogen atmosphere. Stir for 2 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 5 ml of ethyl acetate and 5 ml of water were added, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (5 ml × 2). The organic layers were combined and washed with a saturated aqueous ammonium chloride solution (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 230 mg of the objective compound as brown crystals.
Melting point: 161.0-162.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.53 (d, J = 1.8 Hz, 1H), 7.78 (d, J = 1.8 Hz, 1H), 7.6-7.65 (m, 1H), 7.4- 7.55 (m, 5H), 7.3-7.35 (m, 2H), 6.62 (s, 1H), 3.35 (s, 2H), 0.95-1.1 (m, 4H).
N-[1-[(5-ブロモ-3-クロロピリジン-2-イル)メチル]シクロプロピル]-2-(トリフルオロメチル)ベンズアミド270mgのN,N-ジメチルホルムアミド3ml溶液に炭酸セシウム409mg、ヨウ化銅(I)12mg、酢酸パラジウム(II)14mg、4,5-ビス(ジフェニルホスフィノ)-9,9-ジメチルキサンテン36mg及び4-クロロ-1-エチニルベンゼン102mgを添加し、窒素雰囲気下、70℃にて2時間攪拌した。反応完結後、反応混合物を室温まで放冷し酢酸エチル5ml及び水5mlを添加して有機層を分取、水層は酢酸エチルにて抽出(5mlx2)した。有機層を併せ飽和塩化アンモニウム水溶液にて洗浄(10mlx1)後、飽和食塩水、次いで無水硫酸ナトリウムの順で脱水・乾燥、減圧下にて溶媒を留去した。残留物を酢酸エチル-ヘキサン(1:9~3:7のグラジエント)にて溶出するシリカゲルカラムクロマトグラフィーにて精製し、目的物230mgを褐色結晶として得た。
融点161.0~162.0℃
1H NMR (CDCl3, Me4Si, 300MHz) δ8.53 (d, J=1.8Hz, 1H), 7.78 (d, J=1.8Hz, 1H), 7.6-7.65 (m, 1H), 7.4-7.55 (m, 5H), 7.3-7.35 (m, 2H), 6.62 (s, 1H), 3.35 (s, 2H), 0.95-1.1 (m, 4H)。 Step 3; Preparation of N- [1-[[3-Chloro-5-[(4-chlorophenyl) ethynyl] pyridin-2-yl] methyl] cyclopropyl] -2- (trifluoromethyl) benzamide N- [1 -[(5-Bromo-3-chloropyridin-2-yl) methyl] cyclopropyl] -2- (trifluoromethyl) benzamide in a solution of 270 mg of N, N-dimethylformamide in 3 ml of cesium carbonate, 409 mg of copper iodide (I 12 mg, 14 mg of palladium (II) acetate, 36 mg of 4,5-bis (diphenylphosphino) -9,9-dimethylxanthene and 102 mg of 4-chloro-1-ethynylbenzene were added at 70 ° C. under a nitrogen atmosphere. Stir for 2 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature, 5 ml of ethyl acetate and 5 ml of water were added, the organic layer was separated, and the aqueous layer was extracted with ethyl acetate (5 ml × 2). The organic layers were combined and washed with a saturated aqueous ammonium chloride solution (10 ml × 1), then dehydrated and dried in the order of saturated brine and then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1: 9 to 3: 7 gradient) to obtain 230 mg of the objective compound as brown crystals.
Melting point: 161.0-162.0 ° C
1 H NMR (CDCl 3 , Me 4 Si, 300 MHz) δ8.53 (d, J = 1.8 Hz, 1H), 7.78 (d, J = 1.8 Hz, 1H), 7.6-7.65 (m, 1H), 7.4- 7.55 (m, 5H), 7.3-7.35 (m, 2H), 6.62 (s, 1H), 3.35 (s, 2H), 0.95-1.1 (m, 4H).
本発明化合物は、前記製造法及び実施例に準じて製造することができる。合成例1~合成例16と同様に製造した本発明に包含されるアルキニルピリジン置換アミド化合物の例を第3表~第17表に、さらに、それらの製造中間体の例を第18表~第20表に示すが、本発明に包含されるアルキニルピリジン置換アミド化合物及びそれらの製造中間体はこれらのみに限定されるものではない。
尚、表中、Etはエチルを、c-Pr及びPr-cはシクロプロピルを、Bu-tはtert-ブチルを、Penはペンチルを、Phはフェニルを、1-Naphは1-ナフチルを、2-Naphは2-ナフチルをそれぞれ表し、
表中、D-1-2a~D-25-3aで表される芳香族複素環は、それぞれ下記の構造を表し、
本発明化合物は、前記製造法及び実施例に準じて製造することができる。合成例1~合成例14と同様に製造した本発明に包含されるアルキニルピリジン置換アミド化合物の例を第3表~第17表に、さらに、それらの製造中間体の例を第18表~第20表に示すが、本発明に包含されるアルキニルピリジン置換アミド化合物及びそれらの製造中間体はこれらのみに限定されるものではない。
尚、表中、Etはエチルを、c-Prはシクロプロピルを、Bu-tはtert-ブチルを、Penはペンチルを、Phはフェニルを、1-Naphは1-ナフチルを、2-Naphは2-ナフチルをそれぞれ表し、
表中、D-1-2a~D-25-3aで表される芳香族複素環は、それぞれ下記の構造を表し、 This invention compound can be manufactured according to the said manufacturing method and an Example. Examples of alkynylpyridine-substituted amide compounds produced in the same manner as in Synthesis Examples 1 to 16 are included in Tables 3 to 17, and examples of production intermediates are shown in Tables 18 to As shown in Table 20, the alkynylpyridine-substituted amide compounds and their production intermediates encompassed by the present invention are not limited to these.
In the table, Et is ethyl, c-Pr and Pr-c are cyclopropyl, Bu-t is tert-butyl, Pen is pentyl, Ph is phenyl, 1-Naph is 1-naphthyl, 2-Naph represents 2-naphthyl,
In the table, the aromatic heterocycles represented by D-1-2a to D-25-3a each represent the following structure,
This invention compound can be manufactured according to the said manufacturing method and an Example. Examples of alkynylpyridine-substituted amide compounds included in the present invention produced in the same manner as in Synthesis Examples 1 to 14 are shown in Tables 3 to 17, and examples of their production intermediates are shown in Tables 18 to As shown in Table 20, the alkynylpyridine-substituted amide compounds and their production intermediates encompassed by the present invention are not limited to these.
In the table, Et is ethyl, c-Pr is cyclopropyl, Bu-t is tert-butyl, Pen is pentyl, Ph is phenyl, 1-Naph is 1-naphthyl, 2-Naph is Each represents 2-naphthyl,
In the table, the aromatic heterocycles represented by D-1-2a to D-25-3a each represent the following structure,
尚、表中、Etはエチルを、c-Pr及びPr-cはシクロプロピルを、Bu-tはtert-ブチルを、Penはペンチルを、Phはフェニルを、1-Naphは1-ナフチルを、2-Naphは2-ナフチルをそれぞれ表し、
表中、D-1-2a~D-25-3aで表される芳香族複素環は、それぞれ下記の構造を表し、
本発明化合物は、前記製造法及び実施例に準じて製造することができる。合成例1~合成例14と同様に製造した本発明に包含されるアルキニルピリジン置換アミド化合物の例を第3表~第17表に、さらに、それらの製造中間体の例を第18表~第20表に示すが、本発明に包含されるアルキニルピリジン置換アミド化合物及びそれらの製造中間体はこれらのみに限定されるものではない。
尚、表中、Etはエチルを、c-Prはシクロプロピルを、Bu-tはtert-ブチルを、Penはペンチルを、Phはフェニルを、1-Naphは1-ナフチルを、2-Naphは2-ナフチルをそれぞれ表し、
表中、D-1-2a~D-25-3aで表される芳香族複素環は、それぞれ下記の構造を表し、 This invention compound can be manufactured according to the said manufacturing method and an Example. Examples of alkynylpyridine-substituted amide compounds produced in the same manner as in Synthesis Examples 1 to 16 are included in Tables 3 to 17, and examples of production intermediates are shown in Tables 18 to As shown in Table 20, the alkynylpyridine-substituted amide compounds and their production intermediates encompassed by the present invention are not limited to these.
In the table, Et is ethyl, c-Pr and Pr-c are cyclopropyl, Bu-t is tert-butyl, Pen is pentyl, Ph is phenyl, 1-Naph is 1-naphthyl, 2-Naph represents 2-naphthyl,
In the table, the aromatic heterocycles represented by D-1-2a to D-25-3a each represent the following structure,
This invention compound can be manufactured according to the said manufacturing method and an Example. Examples of alkynylpyridine-substituted amide compounds included in the present invention produced in the same manner as in Synthesis Examples 1 to 14 are shown in Tables 3 to 17, and examples of their production intermediates are shown in Tables 18 to As shown in Table 20, the alkynylpyridine-substituted amide compounds and their production intermediates encompassed by the present invention are not limited to these.
In the table, Et is ethyl, c-Pr is cyclopropyl, Bu-t is tert-butyl, Pen is pentyl, Ph is phenyl, 1-Naph is 1-naphthyl, 2-Naph is Each represents 2-naphthyl,
In the table, the aromatic heterocycles represented by D-1-2a to D-25-3a each represent the following structure,
置換基(Z)nの置換位置を表す番号は、上記の構造式において記された番号の位置に対応するものであり、例えば、表中、「(D-23-2b)-6-F」との記載は「6-フルオロピリジン-3-イル」を表し、
表中、D-31-a~D-34-aで表される芳香族複素環は、それぞれ下記の構造を表し、
The number representing the substitution position of the substituent (Z) n corresponds to the position of the number described in the above structural formula. For example, in the table, “(D-23-2b) -6-F” The description represents “6-fluoropyridin-3-yl”,
In the table, the aromatic heterocycles represented by D-31-a to D-34-a each represent the following structure:
また、表中、化合物番号の欄における1-015(-)及び1-015(+)等の記載は、それぞれ比旋光度が(-)及び(+)の光学異性体であることを表し、
表中、置換基R3の欄における(R)及び(S)の表記は、R3が結合する炭素原子の光学異性体の混合比において、(R)-体又は(S)-体の比が90%以上であることを表し、
表中、融点の欄における「*1」との記載は化合物の性状が油状又は樹脂状であったことを意味する。 Further, in the table, the description such as 1-015 (-) and 1-015 (+) in the column of compound number represents that the specific optical rotation is an optical isomer of (-) and (+), respectively.
In the table, the notation of (R) and (S) in the column of substituent R 3 indicates the ratio of (R) -isomer or (S) -isomer in the mixing ratio of optical isomers of carbon atoms to which R 3 is bonded. Represents 90% or more,
In the table, “* 1” in the melting point column means that the property of the compound was oily or resinous.
表中、置換基R3の欄における(R)及び(S)の表記は、R3が結合する炭素原子の光学異性体の混合比において、(R)-体又は(S)-体の比が90%以上であることを表し、
表中、融点の欄における「*1」との記載は化合物の性状が油状又は樹脂状であったことを意味する。 Further, in the table, the description such as 1-015 (-) and 1-015 (+) in the column of compound number represents that the specific optical rotation is an optical isomer of (-) and (+), respectively.
In the table, the notation of (R) and (S) in the column of substituent R 3 indicates the ratio of (R) -isomer or (S) -isomer in the mixing ratio of optical isomers of carbon atoms to which R 3 is bonded. Represents 90% or more,
In the table, “* 1” in the melting point column means that the property of the compound was oily or resinous.
第3表~第20表の化合物のうち、表中に融点の記載のない化合物のスペクトルデータを第21表に示す。
Of the compounds in Tables 3 to 20, spectrum data of compounds having no melting point in the table are shown in Table 21.
[試験例]
次に、本発明化合物の有害生物防除剤としての有用性について、以下の試験例により具体的に説明するが、本発明はこれらのみに限定されるものではない。
試験薬液Aの調製;本発明化合物を乳化白試料(ソルポール(登録商標)3005XL(東邦化学工業社製):N-メチルピロリドン:ソルベッソ(登録商標)200(エクソンモービル製)=1:5:2混合物)中に溶解し、20重量%濃度の乳剤を調製した。この薬液を所定の濃度に希釈し、以下の試験例1~8にて供試した。
試験薬液Bの調製;本発明化合物をジメチルスルホキシド中に溶解し、1重量%濃度の溶液を調製した。この薬液を所定の濃度に希釈し、以下の試験例9~試験例13及び比較試験にて供試した。 [Test example]
Next, the usefulness of the compound of the present invention as a pest control agent will be specifically described by the following test examples, but the present invention is not limited to these.
Preparation of test drug solution A; emulsified white sample of the compound of the present invention (Solpol (registered trademark) 3005XL (manufactured by Toho Chemical Industry Co., Ltd.): N-methylpyrrolidone: Solvesso (registered trademark) 200 (manufactured by ExxonMobil) = 1: 5: 2 To give a 20% strength by weight emulsion. This chemical solution was diluted to a predetermined concentration and used in Test Examples 1 to 8 below.
Preparation of test drug solution B: The compound of the present invention was dissolved in dimethyl sulfoxide to prepare a 1% strength by weight solution. This chemical solution was diluted to a predetermined concentration and used in the following Test Examples 9 to 13 and a comparative test.
次に、本発明化合物の有害生物防除剤としての有用性について、以下の試験例により具体的に説明するが、本発明はこれらのみに限定されるものではない。
試験薬液Aの調製;本発明化合物を乳化白試料(ソルポール(登録商標)3005XL(東邦化学工業社製):N-メチルピロリドン:ソルベッソ(登録商標)200(エクソンモービル製)=1:5:2混合物)中に溶解し、20重量%濃度の乳剤を調製した。この薬液を所定の濃度に希釈し、以下の試験例1~8にて供試した。
試験薬液Bの調製;本発明化合物をジメチルスルホキシド中に溶解し、1重量%濃度の溶液を調製した。この薬液を所定の濃度に希釈し、以下の試験例9~試験例13及び比較試験にて供試した。 [Test example]
Next, the usefulness of the compound of the present invention as a pest control agent will be specifically described by the following test examples, but the present invention is not limited to these.
Preparation of test drug solution A; emulsified white sample of the compound of the present invention (Solpol (registered trademark) 3005XL (manufactured by Toho Chemical Industry Co., Ltd.): N-methylpyrrolidone: Solvesso (registered trademark) 200 (manufactured by ExxonMobil) = 1: 5: 2 To give a 20% strength by weight emulsion. This chemical solution was diluted to a predetermined concentration and used in Test Examples 1 to 8 below.
Preparation of test drug solution B: The compound of the present invention was dissolved in dimethyl sulfoxide to prepare a 1% strength by weight solution. This chemical solution was diluted to a predetermined concentration and used in the following Test Examples 9 to 13 and a comparative test.
試験例1 キュウリうどんこ病予防効果試験
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。風乾後、キュウリを空調温室(20℃)に置き、キュウリうどんこ病菌(Erysiphe polygoni、Synonym;Erysiphe betae)の分生胞子懸濁液を噴霧接種した。同温度にて9日間置いた後、形成された病斑の接種葉に占める割合を測定し、下記の式に従い、防除価を算出した。尚、試験は2連制で行なった。 Test Example 1 Cucumber powdery mildew prevention effect test Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and the test drug solution A of the present compound was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml sprayed per pot. After air drying, the cucumber was placed in an air-conditioned greenhouse (20 ° C.) and sprayed with a conidial spore suspension of cucumber powdery mildew (Erysiphe polygoni, Synonym; Erysiphe betae). After 9 days at the same temperature, the proportion of the lesions formed in the inoculated leaves was measured, and the control value was calculated according to the following formula. In addition, the test was performed by 2 continuous systems.
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。風乾後、キュウリを空調温室(20℃)に置き、キュウリうどんこ病菌(Erysiphe polygoni、Synonym;Erysiphe betae)の分生胞子懸濁液を噴霧接種した。同温度にて9日間置いた後、形成された病斑の接種葉に占める割合を測定し、下記の式に従い、防除価を算出した。尚、試験は2連制で行なった。 Test Example 1 Cucumber powdery mildew prevention effect test Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and the test drug solution A of the present compound was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml sprayed per pot. After air drying, the cucumber was placed in an air-conditioned greenhouse (20 ° C.) and sprayed with a conidial spore suspension of cucumber powdery mildew (Erysiphe polygoni, Synonym; Erysiphe betae). After 9 days at the same temperature, the proportion of the lesions formed in the inoculated leaves was measured, and the control value was calculated according to the following formula. In addition, the test was performed by 2 continuous systems.
防除価=〔1-(処理区病斑面積率/無処理区病斑面積率)〕×100
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-002,1-005~1-007,1-011,1-012,1-016,1-019,1-022,1-023,1-031,1-038,1-042,1-044~1-046,1-048~1-052,1-059~1-063,1-066~1-068,1-069*,1-070,1-073,1-074,1-086,1-090,1-091,1-104,1-105,1-108,1-109,1-114,1-116,1-121,1-124,1-142,1-144,1-147,1-148,2-002,2-008,2-011,2-016,2-018,2-054~2-056,2-058,2-059,2-067,2-084,2-086,3-002*,3-010,3-012,3-014,3-025,3-036,3-037,3-043,3-051,3-053,3-059,3-062~3-067,3-084,3-085,3-088,3-089,3-095,3-104,3-128,3-135,3-142,3-146~3-148,3-150,3-156,4-008,4-009,7-004,7-005*,7-006*,7-007,7-009,7-010,7-012,7-013,7-019,7-021,8-001*,8-002,8-004,8-005,9-002~9-006,9-008~9-011,9-014,9-018,9-019,11-001,12-001,15-001。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Control value = [1- (Sickness area ratio of treated area / Uncovered lesion area ratio)] × 100
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-002,1-005 to 1-007,1-011,1-012,1-016,1-019,1-022,1-023,1-031,1-038, 1-042,1-044 to 1-046,1-048 to 1-052,1-059 to 1-063,1-066 to 1-068,1-069 * , 1-070,1-073,1 -074,1-086,1-090,1-091,1-104,1-105,1-108,1-109,1-114,1-116,1-121,1-124,1-142 , 1-144,1-147,1-148,2-002,2-008,2-011,2-016,2-018,2-054 to 2-056,2-058,2-059,2 -067,2-084,2-086,3-002 * , 3-010,3-012,3-014,3-025,3-036,3-037,3-043,3-051,3- 053,3-059,3-062 to 3-067,3-084,3-085,3-088,3-089,3-095,3-104,3-128,3-135,3-142, 3-146 ~ 3-148,3-150,3-156,4-008,4-009,7-004,7-005 * , 7-006 * , 7-007,7-009,7-010, 7-012,7-013,7-019,7-021,8-001 * , 8-002,8-004,8-005,9-002 to 9-006,9-008 to 9-011,9 -014,9-018,9-019,11-001,12-001,15-001.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-002,1-005~1-007,1-011,1-012,1-016,1-019,1-022,1-023,1-031,1-038,1-042,1-044~1-046,1-048~1-052,1-059~1-063,1-066~1-068,1-069*,1-070,1-073,1-074,1-086,1-090,1-091,1-104,1-105,1-108,1-109,1-114,1-116,1-121,1-124,1-142,1-144,1-147,1-148,2-002,2-008,2-011,2-016,2-018,2-054~2-056,2-058,2-059,2-067,2-084,2-086,3-002*,3-010,3-012,3-014,3-025,3-036,3-037,3-043,3-051,3-053,3-059,3-062~3-067,3-084,3-085,3-088,3-089,3-095,3-104,3-128,3-135,3-142,3-146~3-148,3-150,3-156,4-008,4-009,7-004,7-005*,7-006*,7-007,7-009,7-010,7-012,7-013,7-019,7-021,8-001*,8-002,8-004,8-005,9-002~9-006,9-008~9-011,9-014,9-018,9-019,11-001,12-001,15-001。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Control value = [1- (Sickness area ratio of treated area / Uncovered lesion area ratio)] × 100
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-002,1-005 to 1-007,1-011,1-012,1-016,1-019,1-022,1-023,1-031,1-038, 1-042,1-044 to 1-046,1-048 to 1-052,1-059 to 1-063,1-066 to 1-068,1-069 * , 1-070,1-073,1 -074,1-086,1-090,1-091,1-104,1-105,1-108,1-109,1-114,1-116,1-121,1-124,1-142 , 1-144,1-147,1-148,2-002,2-008,2-011,2-016,2-018,2-054 to 2-056,2-058,2-059,2 -067,2-084,2-086,3-002 * , 3-010,3-012,3-014,3-025,3-036,3-037,3-043,3-051,3- 053,3-059,3-062 to 3-067,3-084,3-085,3-088,3-089,3-095,3-104,3-128,3-135,3-142, 3-146 ~ 3-148,3-150,3-156,4-008,4-009,7-004,7-005 * , 7-006 * , 7-007,7-009,7-010, 7-012,7-013,7-019,7-021,8-001 * , 8-002,8-004,8-005,9-002 to 9-006,9-008 to 9-011,9 -014,9-018,9-019,11-001,12-001,15-001.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
試験例2 キュウリ灰色かび病予防効果試験(胞子接種)
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。風乾後、処理葉を切り取り、プラスチック容器に入れた。キュウリ灰色かび病菌(Botrytis cinerea)の分生胞子懸濁液と溶解させたPDA培地を1:1の割合で混合し、上記の処理葉に30μlずつ滴下接種した。接種後、20℃、多湿下に3日間置いた後、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-002~1-004,1-006,1-007,1-009,1-011,1-012,1-015,1-016,1-019,1-023,1-027~1-031,1-033,1-034,1-036~1-038,1-042,1-045~1-052,1-054~1-057,1-061~1-063,1-066~1-068,1-069*,1-070,1-071,1-073~1-075,1-083,1-086~1-088,1-090,1-091,1-093,1-094,1-097,1-104~1-106,1-108~1-113,1-115~1-117,1-119~1-121,1-123~1-125,1-128,1-136,1-137,1-140,1-142~1-144,1-146~1-149,2-002,2-007,2-008,2-010,2-011,2-016~2-020,2-031~2-033,2-044,2-045,2-055~2-067,2-069~2-072,2-081~2-088,3-002*,3-003,3-004,3-007,3-009~3-012,3-014,3-015,3-018,3-021,3-023,3-025,3-026,3-029~3-033,3-035~3-045,3-047,3-049,3-050,3-053~3-055,3-058,3-059,3-062~3-068,3-070,3-071,3-074,3-076,3-078,3-084,3-085,3-088~3-096,3-098~3-107,3-109~3-111,3-117,3-118,3-121~3-124,3-126,3-128~3-142,3-144~3-153,3-155~3-157,3-164~3-167,3-170~3-172,3-174~3-181,3-188,4-002~4-009,6-001,7-001,7-004,7-006*,7-007,7-010,7-012,7-013,7-018,7-019,7-021,7-022,8-001*,8-002,8-003*,8-004,8-005,9-001*,9-002~9-020,12-004,14-038。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test example 2 Cucumber gray mold prevention effect test (spore inoculation)
Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and at the cotyledon stage, the test drug solution A of the compound of the present invention was diluted with distilled water to a concentration of 500 ppm and sprayed with 5 ml per pot. After air drying, the treated leaves were cut out and placed in a plastic container. The conidial spore suspension of cucumber gray mold fungus (Botrytis cinerea) and the dissolved PDA medium were mixed at a ratio of 1: 1, and 30 μl of each of the treated leaves was inoculated dropwise. After inoculation, after placing it at 20 ° C. under high humidity for 3 days, the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-002 to 1-004,1-006,1-007,1-009,1-011,1-012,1-015,1-016,1-019,1-023, 1-027 ~ 1-031,1-033,1-034,1-036 ~ 1-038,1-042,1-045 ~ 1-052,1-054 ~ 1-057,1-061 ~ 1- 063,1-066 ~ 1-068,1-069 * , 1-070,1-071,1-073 ~ 1-075,1-083,1-086 ~ 1-088,1-090,1-091 , 1-093,1-094,1-097,1-104 to 1-106,1-108 to 1-113,1-115 to 1-117,1-119 to 1-121,1-123 to 1 -125,1-128,1-136,1-137,1-140,1-142 to 1-144,1-146 to 1-149,2-002,2-007,2-008,2-010 , 2-011,2-016 to 2-020,2-031 to 2-033,2-044,2-045,2-055 to 2-067,2-069 to 2-072,2-081 to 2 -088,3-002 * , 3-003,3-004,3-007,3-009 to 3-012,3-014,3-015,3-018,3-021,3-023,3- 025,3-026,3-029 ~ 3-033,3-035 ~ 3-045,3-047,3-049,3-050,3-053 ~ 3-055,3-058,3-059, 3-062 ~ 3-068,3-070,3-071,3-074,3-076,3-078,3-084,3-085,3-088 ~ 3-096,3-098 ~ 3- 107, 3-109 to 3-111, 3-117, 3-118, 3-121 to 3-124, 3-126, 3-128 to 3-142, 3-144 to 3-153, 3-155 3-157, 3-164 to 3-167, 3-170 to 3-172, 3-174 to 3-181, 3-188, 4-002 to 4-009, 6-001, 7-001, 7- 004,7-006 * , 7-007,7-010,7-012,7-013,7-018,7-019,7-021,7-022,8-001 * , 8-002, 8-003 * , 8-004, 8-005, 9-001 * , 9-002 to 9-020, 12-004, 14-038.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。風乾後、処理葉を切り取り、プラスチック容器に入れた。キュウリ灰色かび病菌(Botrytis cinerea)の分生胞子懸濁液と溶解させたPDA培地を1:1の割合で混合し、上記の処理葉に30μlずつ滴下接種した。接種後、20℃、多湿下に3日間置いた後、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-002~1-004,1-006,1-007,1-009,1-011,1-012,1-015,1-016,1-019,1-023,1-027~1-031,1-033,1-034,1-036~1-038,1-042,1-045~1-052,1-054~1-057,1-061~1-063,1-066~1-068,1-069*,1-070,1-071,1-073~1-075,1-083,1-086~1-088,1-090,1-091,1-093,1-094,1-097,1-104~1-106,1-108~1-113,1-115~1-117,1-119~1-121,1-123~1-125,1-128,1-136,1-137,1-140,1-142~1-144,1-146~1-149,2-002,2-007,2-008,2-010,2-011,2-016~2-020,2-031~2-033,2-044,2-045,2-055~2-067,2-069~2-072,2-081~2-088,3-002*,3-003,3-004,3-007,3-009~3-012,3-014,3-015,3-018,3-021,3-023,3-025,3-026,3-029~3-033,3-035~3-045,3-047,3-049,3-050,3-053~3-055,3-058,3-059,3-062~3-068,3-070,3-071,3-074,3-076,3-078,3-084,3-085,3-088~3-096,3-098~3-107,3-109~3-111,3-117,3-118,3-121~3-124,3-126,3-128~3-142,3-144~3-153,3-155~3-157,3-164~3-167,3-170~3-172,3-174~3-181,3-188,4-002~4-009,6-001,7-001,7-004,7-006*,7-007,7-010,7-012,7-013,7-018,7-019,7-021,7-022,8-001*,8-002,8-003*,8-004,8-005,9-001*,9-002~9-020,12-004,14-038。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test example 2 Cucumber gray mold prevention effect test (spore inoculation)
Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and at the cotyledon stage, the test drug solution A of the compound of the present invention was diluted with distilled water to a concentration of 500 ppm and sprayed with 5 ml per pot. After air drying, the treated leaves were cut out and placed in a plastic container. The conidial spore suspension of cucumber gray mold fungus (Botrytis cinerea) and the dissolved PDA medium were mixed at a ratio of 1: 1, and 30 μl of each of the treated leaves was inoculated dropwise. After inoculation, after placing it at 20 ° C. under high humidity for 3 days, the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-002 to 1-004,1-006,1-007,1-009,1-011,1-012,1-015,1-016,1-019,1-023, 1-027 ~ 1-031,1-033,1-034,1-036 ~ 1-038,1-042,1-045 ~ 1-052,1-054 ~ 1-057,1-061 ~ 1- 063,1-066 ~ 1-068,1-069 * , 1-070,1-071,1-073 ~ 1-075,1-083,1-086 ~ 1-088,1-090,1-091 , 1-093,1-094,1-097,1-104 to 1-106,1-108 to 1-113,1-115 to 1-117,1-119 to 1-121,1-123 to 1 -125,1-128,1-136,1-137,1-140,1-142 to 1-144,1-146 to 1-149,2-002,2-007,2-008,2-010 , 2-011,2-016 to 2-020,2-031 to 2-033,2-044,2-045,2-055 to 2-067,2-069 to 2-072,2-081 to 2 -088,3-002 * , 3-003,3-004,3-007,3-009 to 3-012,3-014,3-015,3-018,3-021,3-023,3- 025,3-026,3-029 ~ 3-033,3-035 ~ 3-045,3-047,3-049,3-050,3-053 ~ 3-055,3-058,3-059, 3-062 ~ 3-068,3-070,3-071,3-074,3-076,3-078,3-084,3-085,3-088 ~ 3-096,3-098 ~ 3- 107, 3-109 to 3-111, 3-117, 3-118, 3-121 to 3-124, 3-126, 3-128 to 3-142, 3-144 to 3-153, 3-155 3-157, 3-164 to 3-167, 3-170 to 3-172, 3-174 to 3-181, 3-188, 4-002 to 4-009, 6-001, 7-001, 7- 004,7-006 * , 7-007,7-010,7-012,7-013,7-018,7-019,7-021,7-022,8-001 * , 8-002, 8-003 * , 8-004, 8-005, 9-001 * , 9-002 to 9-020, 12-004, 14-038.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
試験例3 キュウリ灰色かび病予防効果試験(菌糸接種)
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。1日後、ポットをプラスチックコンテナーに入れ、予めPDA培地で培養したキュウリ灰色かび病菌(Botrytis cinerea)の含菌寒天片(直径5mm)を薬剤処理したキュウリの子葉に接種した。接種後、プラスチックコンテナーをビニールで覆って加湿し、20℃にて2日間置いた。その後、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-002,1-003,1-006,1-007,1-009,1-012,1-015,1-016,1-017*,1-019,1-038,1-042,1-044~1-046,1-049~1-052,1-062,1-063,1-067,1-068,1-070,1-071,1-073,1-074,1-076,1-081,1-089,1-090,1-091,1-094,1-097,1-105,1-106,1-108,1-109,1-116,1-117,1-124,1-128,1-143,1-144,1-147,1-148,2-002,2-007,2-016,2-018~2-020,2-056~2-059,2-062,2-065~2-067,2-081,2-082,2-084,2-086~2-088,3-003,3-004,3-006,3-007,3-010~3-012,3-014,3-025,3-026,3-030,3-031,3-035~3-038,3-040~3-044,3-053,3-054,3-058,3-059,3-062~3-067,3-070,3-074,3-084,3-085,3-088,3-089,3-091,3-093~3-095,3-102~3-104,3-106,3-117,3-118,3-121,3-130,3-134,3-135,3-140~3-142,3-146,3-147,3-150,3-156,3-167,3-172,3-175,3-179,3-181,4-003~4-009,6-001,7-004,7-006*,7-010,7-012,7-015,7-021,7-022,8-001*,8-002,8-003*,8-004,8-005,9-001*,9-002~9-007,9-009~9-011,9-014,9-016,9-018~9-020,12-001。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test example 3 Cucumber gray mold prevention effect test (mycelia inoculation)
Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and at the cotyledon stage, the test drug solution A of the compound of the present invention was diluted with distilled water to a concentration of 500 ppm and sprayed with 5 ml per pot. One day later, the pot was placed in a plastic container and inoculated into a cucumber cotyledon treated with a drug with a bacterial agar piece (diameter 5 mm) of cucumber gray mold (Botrytis cinerea) previously cultured in PDA medium. After inoculation, the plastic container was covered with vinyl and humidified, and placed at 20 ° C. for 2 days. Thereafter, the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-002,1-003,1-006,1-007,1-009,1-012,1-015,1-016,1-017 * , 1-019,1-038 , 1-042,1-044 ~ 1-046,1-049 ~ 1-052,1-062,1-063,1-067,1-068,1-070,1-071,1-073,1 -074,1-076,1-081,1-089,1-090,1-091,1-094,1-097,1-105,1-106,1-108,1-109,1-116 , 1-117,1-124,1-128,1-143,1-144,1-147,1-148,2-002,2-007,2-016,2-018 to 2-020,2 -056 to 2-059,2-062,2-065 to 2-067,2-081,2-082,2-084,2-086 to 2-088,3-003,3-004,3-006 , 3-007,3-010 to 3-012,3-014,3-025,3-026,3-030,3-031,3-035 to 3-038,3-040 to 3-044,3 -053,3-054,3-058,3-059,3-062 to 3-067,3-070,3-074,3-084,3-085,3-088,3-089,3-091 , 3-093 to 3-095,3-102 to 3-104,3-106,3-117,3-118,3-121,3-130,3-134,3-135,3-140 to 3 -142,3-146,3-147,3-150,3-156,3-167,3-1172,3-175,3-179,3-181,4-003 to 4-009,6-001 , 7-004,7-006 * , 7-010,7-012,7-015,7-021,7-022,8-001 * , 8-002,8-003 * , 8-004,8- 005,9-001 * , 9-002 to 9-007,9-009 to 9-011,9-014,9-016,9-018 to 9-020,12-001.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。1日後、ポットをプラスチックコンテナーに入れ、予めPDA培地で培養したキュウリ灰色かび病菌(Botrytis cinerea)の含菌寒天片(直径5mm)を薬剤処理したキュウリの子葉に接種した。接種後、プラスチックコンテナーをビニールで覆って加湿し、20℃にて2日間置いた。その後、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-002,1-003,1-006,1-007,1-009,1-012,1-015,1-016,1-017*,1-019,1-038,1-042,1-044~1-046,1-049~1-052,1-062,1-063,1-067,1-068,1-070,1-071,1-073,1-074,1-076,1-081,1-089,1-090,1-091,1-094,1-097,1-105,1-106,1-108,1-109,1-116,1-117,1-124,1-128,1-143,1-144,1-147,1-148,2-002,2-007,2-016,2-018~2-020,2-056~2-059,2-062,2-065~2-067,2-081,2-082,2-084,2-086~2-088,3-003,3-004,3-006,3-007,3-010~3-012,3-014,3-025,3-026,3-030,3-031,3-035~3-038,3-040~3-044,3-053,3-054,3-058,3-059,3-062~3-067,3-070,3-074,3-084,3-085,3-088,3-089,3-091,3-093~3-095,3-102~3-104,3-106,3-117,3-118,3-121,3-130,3-134,3-135,3-140~3-142,3-146,3-147,3-150,3-156,3-167,3-172,3-175,3-179,3-181,4-003~4-009,6-001,7-004,7-006*,7-010,7-012,7-015,7-021,7-022,8-001*,8-002,8-003*,8-004,8-005,9-001*,9-002~9-007,9-009~9-011,9-014,9-016,9-018~9-020,12-001。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test example 3 Cucumber gray mold prevention effect test (mycelia inoculation)
Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and at the cotyledon stage, the test drug solution A of the compound of the present invention was diluted with distilled water to a concentration of 500 ppm and sprayed with 5 ml per pot. One day later, the pot was placed in a plastic container and inoculated into a cucumber cotyledon treated with a drug with a bacterial agar piece (diameter 5 mm) of cucumber gray mold (Botrytis cinerea) previously cultured in PDA medium. After inoculation, the plastic container was covered with vinyl and humidified, and placed at 20 ° C. for 2 days. Thereafter, the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-002,1-003,1-006,1-007,1-009,1-012,1-015,1-016,1-017 * , 1-019,1-038 , 1-042,1-044 ~ 1-046,1-049 ~ 1-052,1-062,1-063,1-067,1-068,1-070,1-071,1-073,1 -074,1-076,1-081,1-089,1-090,1-091,1-094,1-097,1-105,1-106,1-108,1-109,1-116 , 1-117,1-124,1-128,1-143,1-144,1-147,1-148,2-002,2-007,2-016,2-018 to 2-020,2 -056 to 2-059,2-062,2-065 to 2-067,2-081,2-082,2-084,2-086 to 2-088,3-003,3-004,3-006 , 3-007,3-010 to 3-012,3-014,3-025,3-026,3-030,3-031,3-035 to 3-038,3-040 to 3-044,3 -053,3-054,3-058,3-059,3-062 to 3-067,3-070,3-074,3-084,3-085,3-088,3-089,3-091 , 3-093 to 3-095,3-102 to 3-104,3-106,3-117,3-118,3-121,3-130,3-134,3-135,3-140 to 3 -142,3-146,3-147,3-150,3-156,3-167,3-1172,3-175,3-179,3-181,4-003 to 4-009,6-001 , 7-004,7-006 * , 7-010,7-012,7-015,7-021,7-022,8-001 * , 8-002,8-003 * , 8-004,8- 005,9-001 * , 9-002 to 9-007,9-009 to 9-011,9-014,9-016,9-018 to 9-020,12-001.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
試験例4 キュウリ菌核病予防効果試験
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。風乾後、ポットをプラスチックコンテナーに入れ、予めPDA培地で培養したキュウリ菌核病(Sclerotinia sclerotiorum)の含菌寒天片(直径5mm)を薬剤処理したキュウリの子葉に接種した。接種後、プラスチックコンテナーをビニールで覆い加湿し、20℃にて2日間置いた後、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-001~1-007,1-009~1-012,1-015,1-016,1-017*,1-019,1-021~1-023,1-025~1-031,1-033,1-034,1-036~1-038,1-042~1-057,1-059~1-063,1-066~1-068,1-069*,1-070~1-083,1-086~1-095,1-097,1-099,1-102~1-106,1-108~1-126,1-128,1-131,1-133~1-137,1-139,1-140,1-142~1-150,1-152,1-153,2-002,2-007~2-012,2-014,2-016~2-020,2-022,2-025,2-027,2-028,2-031~2-033,2-035,2-037,2-038,2-040,2-042~2-048,2-050,2-054~2-067,2-069~2-072,2-075~2-077,2-081~2-088,2-090~2-092,3-001,3-002*,3-003,3-004,3-006,3-007,3-009~3-012,3-014,3-015,3-018~3-033,3-035~3-050,3-052~3-056,3-058,3-059,3-061~3-072,3-074~3-076,3-078,3-079,3-084~3-086,3-088~3-096,3-098~3-107,3-109~3-113,3-116~3-119,3-121~3-158,3-160,3-161,3-164~3-167,3-170~3-181,3-184~3-186,3-188,4-001*,4-002~4-009,5-001,6-001~6-003,7-001,7-003,7-004,7-006*,7-007,7-009~7-016,7-018,7-019,7-021,7-022,8-001*,8-002,8-003*,8-004,8-005,9-001*,9-002~9-020,12-001,12-002,12-004~12-007,13-001,14-002,14-007,14-009,14-012,14-032,14-037,14-038,14-041,15-001。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test Example 4 Cucumber Nucleus Disease Prevention Effect Test Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and the test drug solution A of the compound of the present invention was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml sprayed per pot. After air-drying, the pot was placed in a plastic container and inoculated into a cucumber cotyledon treated with a drug, a garnish containing agar (Sclerotinia sclerotiorum) -containing agar pieces (diameter 5 mm) previously cultured in a PDA medium. After inoculation, the plastic container is covered with plastic, humidified, and left at 20 ° C for 2 days. Then, the proportion of the lesions formed in the inoculated leaves is measured, and the control value is calculated from the same formula as in Test Example 1. did. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compounds of the present invention: No.1-001 to 1-007,1-009 to 1-012,1-015,1-016,1-017 * , 1-019,1-021 to 1-023,1-025 ~ 1-031,1-033,1-034,1-036 ~ 1-038,1-042 ~ 1-057,1-059 ~ 1-063,1-066 ~ 1-068,1-069 * , 1-070 ~ 1-083,1-086 ~ 1-095,1-097,1-099,1-102 ~ 1-106,1-108 ~ 1-126,1-128,1-131,1- 133-1-137,1-139,1-140,1-142-1-150,1-152,1-153,2-002,2-007-2-012,2-014,2-016- 2-020,2-022,2-025,2-027,2-028,2-031 ~ 2-033,2-035,2-037,2-038,2-040,2-042 ~ 2- 048,2-050,2-054 to 2-067,2-069 to 2-072,2-075 to 2-077,2-081 to 2-088,2-090 to 2-092,3-001, 3-002 * , 3-003,3-004,3-006,3-007,3-009 to 3-012,3-014,3-015,3-018 to 3-033,3-035 to 3 -050,3-052 to 3-056,3-058,3-059,3-061 to 3-072,3-074 to 3-076,3-078,3-079,3-084 to 3-086 , 3-088 to 3-096,3-098 to 3-107,3-109 to 3-113,3-116 to 3-119,3-121 to 3-158,3-160,3-161,3 -164 to 3-167,3-170 to 3-181,3-184 to 3-186,3-1188,4-001 * , 4-002 to 4-009,5-001,6-001 to 6- 003,7-001,7-003,7-004,7-006 * , 7-007,7-009 to 7-016,7-018,7-019,7-021,7-022,8-001 * , 8-002,8-003 * , 8-004,8-005,9-001 * , 9-002 to 9-020, 12-001,12-002,12-004 to 12-007,13-001,14-002,14-007,14-009,14-012,14-032,14-037,14-038,14- 041,15-001.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。風乾後、ポットをプラスチックコンテナーに入れ、予めPDA培地で培養したキュウリ菌核病(Sclerotinia sclerotiorum)の含菌寒天片(直径5mm)を薬剤処理したキュウリの子葉に接種した。接種後、プラスチックコンテナーをビニールで覆い加湿し、20℃にて2日間置いた後、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-001~1-007,1-009~1-012,1-015,1-016,1-017*,1-019,1-021~1-023,1-025~1-031,1-033,1-034,1-036~1-038,1-042~1-057,1-059~1-063,1-066~1-068,1-069*,1-070~1-083,1-086~1-095,1-097,1-099,1-102~1-106,1-108~1-126,1-128,1-131,1-133~1-137,1-139,1-140,1-142~1-150,1-152,1-153,2-002,2-007~2-012,2-014,2-016~2-020,2-022,2-025,2-027,2-028,2-031~2-033,2-035,2-037,2-038,2-040,2-042~2-048,2-050,2-054~2-067,2-069~2-072,2-075~2-077,2-081~2-088,2-090~2-092,3-001,3-002*,3-003,3-004,3-006,3-007,3-009~3-012,3-014,3-015,3-018~3-033,3-035~3-050,3-052~3-056,3-058,3-059,3-061~3-072,3-074~3-076,3-078,3-079,3-084~3-086,3-088~3-096,3-098~3-107,3-109~3-113,3-116~3-119,3-121~3-158,3-160,3-161,3-164~3-167,3-170~3-181,3-184~3-186,3-188,4-001*,4-002~4-009,5-001,6-001~6-003,7-001,7-003,7-004,7-006*,7-007,7-009~7-016,7-018,7-019,7-021,7-022,8-001*,8-002,8-003*,8-004,8-005,9-001*,9-002~9-020,12-001,12-002,12-004~12-007,13-001,14-002,14-007,14-009,14-012,14-032,14-037,14-038,14-041,15-001。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test Example 4 Cucumber Nucleus Disease Prevention Effect Test Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and the test drug solution A of the compound of the present invention was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml sprayed per pot. After air-drying, the pot was placed in a plastic container and inoculated into a cucumber cotyledon treated with a drug, a garnish containing agar (Sclerotinia sclerotiorum) -containing agar pieces (diameter 5 mm) previously cultured in a PDA medium. After inoculation, the plastic container is covered with plastic, humidified, and left at 20 ° C for 2 days. Then, the proportion of the lesions formed in the inoculated leaves is measured, and the control value is calculated from the same formula as in Test Example 1. did. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compounds of the present invention: No.1-001 to 1-007,1-009 to 1-012,1-015,1-016,1-017 * , 1-019,1-021 to 1-023,1-025 ~ 1-031,1-033,1-034,1-036 ~ 1-038,1-042 ~ 1-057,1-059 ~ 1-063,1-066 ~ 1-068,1-069 * , 1-070 ~ 1-083,1-086 ~ 1-095,1-097,1-099,1-102 ~ 1-106,1-108 ~ 1-126,1-128,1-131,1- 133-1-137,1-139,1-140,1-142-1-150,1-152,1-153,2-002,2-007-2-012,2-014,2-016- 2-020,2-022,2-025,2-027,2-028,2-031 ~ 2-033,2-035,2-037,2-038,2-040,2-042 ~ 2- 048,2-050,2-054 to 2-067,2-069 to 2-072,2-075 to 2-077,2-081 to 2-088,2-090 to 2-092,3-001, 3-002 * , 3-003,3-004,3-006,3-007,3-009 to 3-012,3-014,3-015,3-018 to 3-033,3-035 to 3 -050,3-052 to 3-056,3-058,3-059,3-061 to 3-072,3-074 to 3-076,3-078,3-079,3-084 to 3-086 , 3-088 to 3-096,3-098 to 3-107,3-109 to 3-113,3-116 to 3-119,3-121 to 3-158,3-160,3-161,3 -164 to 3-167,3-170 to 3-181,3-184 to 3-186,3-1188,4-001 * , 4-002 to 4-009,5-001,6-001 to 6- 003,7-001,7-003,7-004,7-006 * , 7-007,7-009 to 7-016,7-018,7-019,7-021,7-022,8-001 * , 8-002,8-003 * , 8-004,8-005,9-001 * , 9-002 to 9-020, 12-001,12-002,12-004 to 12-007,13-001,14-002,14-007,14-009,14-012,14-032,14-037,14-038,14- 041,15-001.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
試験例5 キュウリ炭疽病予防効果試験
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。1日後、キュウリ炭疽病菌(Colletotrichum lagenarium、Synonym;Colletotrichum orbiculare)の分生胞子懸濁液を噴霧接種し、温度25℃、湿度100%RHの接種箱内に2日間置いた。その後、空調温室(23℃)に置き、同温度にて7日間置いた。その後、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-054,1-066,1-067,1-070,1-084,1-090~1-092,1-109,1-113,1-115,1-129,1-138,1-139,1-145,2-006,2-007,2-011,2-032,2-058,2-059,2-072,2-073,2-080,3-002*,3-024,3-030,3-048,3-053,3-059,3-065,3-081,3-083,3-095,3-117,3-130,3-134,3-135,3-138~3-140,3-142,3-143,3-170,3-172,3-174,4-002,4-004,4-006,4-007,7-013,7-018,8-004,8-005,9-005,9-009,9-014,9-018,12-006,13-003,14-007,14-011,14-027,15-007。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test Example 5 Cucumber Anthrax Preventive Effect Test Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and test chemical solution A of the compound of the present invention was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml spraying treatment was performed. One day later, a conidial spore suspension of cucumber anthracnose fungus (Colletotrichum lagenarium, Synonym; Colletotrichum orbiculare) was spray-inoculated and placed in an inoculation box at a temperature of 25 ° C. and a humidity of 100% RH for 2 days. Thereafter, it was placed in an air-conditioned greenhouse (23 ° C.) and placed at the same temperature for 7 days. Thereafter, the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compounds of the present invention: No. 1-054, 1-066, 1-067, 1-070, 1-084, 1-090 to 1-092, 1-109, 1-113, 1-115, 1-129, 1-138,1-139,1-145,2-006,2-007,2-011,2-032,2-058,2-059,2-072,2-073,2-080,3- 002 * , 3-024,3-030,3-048,3-053,3-059,3-065,3-081,3-083,3-095,3-117,3-130,3-134 , 3-135, 3-138 to 3-140, 3-142, 3-143, 3-170, 3-172, 3-174, 4-002, 4-004, 4-006, 4-007, 7 -013,7-018,8-004,8-005,9-005,9-009,9-014,9-018,12-006,13-003,14-007,14-011,14-027 15-007.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
90cm3のプラスチックポットにキュウリ(品種:相模半白)を植え、子葉期に本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。1日後、キュウリ炭疽病菌(Colletotrichum lagenarium、Synonym;Colletotrichum orbiculare)の分生胞子懸濁液を噴霧接種し、温度25℃、湿度100%RHの接種箱内に2日間置いた。その後、空調温室(23℃)に置き、同温度にて7日間置いた。その後、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-054,1-066,1-067,1-070,1-084,1-090~1-092,1-109,1-113,1-115,1-129,1-138,1-139,1-145,2-006,2-007,2-011,2-032,2-058,2-059,2-072,2-073,2-080,3-002*,3-024,3-030,3-048,3-053,3-059,3-065,3-081,3-083,3-095,3-117,3-130,3-134,3-135,3-138~3-140,3-142,3-143,3-170,3-172,3-174,4-002,4-004,4-006,4-007,7-013,7-018,8-004,8-005,9-005,9-009,9-014,9-018,12-006,13-003,14-007,14-011,14-027,15-007。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test Example 5 Cucumber Anthrax Preventive Effect Test Cucumber (variety: Sagamihanjiro) was planted in a 90 cm 3 plastic pot, and test chemical solution A of the compound of the present invention was diluted to a concentration of 500 ppm by adding distilled water at the cotyledon stage. 5 ml spraying treatment was performed. One day later, a conidial spore suspension of cucumber anthracnose fungus (Colletotrichum lagenarium, Synonym; Colletotrichum orbiculare) was spray-inoculated and placed in an inoculation box at a temperature of 25 ° C. and a humidity of 100% RH for 2 days. Thereafter, it was placed in an air-conditioned greenhouse (23 ° C.) and placed at the same temperature for 7 days. Thereafter, the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compounds of the present invention: No. 1-054, 1-066, 1-067, 1-070, 1-084, 1-090 to 1-092, 1-109, 1-113, 1-115, 1-129, 1-138,1-139,1-145,2-006,2-007,2-011,2-032,2-058,2-059,2-072,2-073,2-080,3- 002 * , 3-024,3-030,3-048,3-053,3-059,3-065,3-081,3-083,3-095,3-117,3-130,3-134 , 3-135, 3-138 to 3-140, 3-142, 3-143, 3-170, 3-172, 3-174, 4-002, 4-004, 4-006, 4-007, 7 -013,7-018,8-004,8-005,9-005,9-009,9-014,9-018,12-006,13-003,14-007,14-011,14-027 15-007.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
試験例6 コムギうどんこ病予防効果試験
1.3葉期のコムギ(品種:農林61号)を植えた90cm3のプラスチックポットに、本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。散布1日後、空調温室(20℃)にポットを置き、コムギうどんこ病菌(Blumeria graminis f. sp. tritici)の分生胞子をコムギに接種した。その後7日間保持し、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-001,1-002,1-004~1-007,1-009,1-011,1-013,1-015,1-016,1-017*,1-019,1-021~1-023,1-025,1-027,1-028,1-030,1-031,1-034,1-036~1-039,1-042,1-044~1-054,1-056~1-063,1-066~1-068,1-069*,1-070~1-074,1-076,1-079~1-081,1-086,1-087,1-089,1-091,1-093~1-097,1-099,1-104~1-106,1-108~1-112,1-114~1-117,1-119~1-125,1-131,1-135~1-137,1-140,1-143~1-149,2-002,2-003,2-005,2-007~2-011,2-015~2-020,2-031,2-032,2-042,2-044,2-045,2-054~2-063,2-065~2-067,2-069~2-071,2-082,2-084~2-088,3-002*,3-003,3-004,3-006,3-007,3-010~3-012,3-014,3-020,3-025,3-026,3-028,3-030,3-031,3-035~3-043,3-045,3-049,3-050,3-052~3-055,3-058,3-059,3-062~3-068,3-070,3-074,3-075,3-078,3-084,3-085,3-088,3-089,3-091~3-095,3-104,3-106,3-107,3-109,3-117,3-126,3-128,3-130~3-133,3-135~3-138,3-140~3-143,3-146~3-148,3-150,3-151,3-153,3-156,3-157,3-167,3-169,3-172,3-177~3-181,4-001*,4-002~4-004,4-006~4-009,6-002,7-001,7-004,7-006*,7-007,7-009,7-010,7-012~7-015,7-018,7-019,7-021,7-022,8-002,8-003*,8-004,8-005,9-002~9-006,9-008~9-012,9-014~9-020,12-001,12-002,14-009,14-013,14-014,14-023,14-032,15-001,15-007。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test Example 6 Wheat powdery mildew prevention effect test 1.3 Test chemical solution A of the compound of the present invention was added to distilled water in a 90 cm 3 plastic pot planted with 1.3 leaves of wheat (variety: Norin 61), and the concentration was 500 ppm. And 5 ml sprayed per pot. One day after spraying, the pot was placed in an air-conditioned greenhouse (20 ° C.), and wheat was inoculated with conidia of wheat powdery mildew (Blumeria graminis f. Sp. Tritici). Thereafter, it was held for 7 days, the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-001,1-002,1-004 to 1-007,1-009,1-011,1-013,1-015,1-016,1-017 * , 1-019 , 1-021 ~ 1-023,1-025,1-027,1-028,1-030,1-031,1-034,1-036 ~ 1-039,1-042,1-044 ~ 1 -054,1-056 to 1-063,1-066 to 1-068,1-069 * , 1-070 to 1-074,1-076,1-079 to 1-081,1-086,1- 087,1-089,1-091,1-093 ~ 1-097,1-099,1-104 ~ 1-106,1-108 ~ 1-112,1-114 ~ 1-117,1-119 ~ 1-125,1-131,1-135 to 1-137,1-140,1-143 to 1-149,2-002,2-003,2-005,2-007 to 2-011,2- 015 ~ 2-020,2-031,2-032,2-042,2-044,2-045,2-054 ~ 2-063,2-065 ~ 2-067,2-069 ~ 2-071, 2-082,2-084 to 2-088,3-002 * , 3-003,3-004,3-006,3-007,3-010 to 3-012,3-014,3-020,3 -025,3-026,3-028,3-030,3-031,3-035-3-043,3-045,3-049,3-050,3-052-3-055,3-058 , 3-059,3-062 to 3-068,3-070,3-074,3-075,3-078,3-084,3-085,3-088,3-089,3-091 to 3 -095,3-104,3-106,3-107,3-109,3-117,3-126,3-128,3-130 to 3-133,3-135 to 3-138,3-1140 ~ 3-143,3-146 ~ 3-148,3-150,3-151,3-153,3-156,3-157,3-167,3-169,3-172,3-1177 ~ 3 -181,4-001 * , 4-002 to 4-004,4-006 to 4-009,6-002,7-001,7-004,7-006 * , 7-007,7-009,7 -0 10,7-012 to 7-015,7-018,7-019,7-021,7-022,8-002,8-003 * , 8-004,8-005,9-002 to 9-006 , 9-008 ~ 9-012,9-014 ~ 9-020,12-001,12-002,14-009,14-013,14-014,14-023,14-032,15-001,15 -007.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
1.3葉期のコムギ(品種:農林61号)を植えた90cm3のプラスチックポットに、本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。散布1日後、空調温室(20℃)にポットを置き、コムギうどんこ病菌(Blumeria graminis f. sp. tritici)の分生胞子をコムギに接種した。その後7日間保持し、形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-001,1-002,1-004~1-007,1-009,1-011,1-013,1-015,1-016,1-017*,1-019,1-021~1-023,1-025,1-027,1-028,1-030,1-031,1-034,1-036~1-039,1-042,1-044~1-054,1-056~1-063,1-066~1-068,1-069*,1-070~1-074,1-076,1-079~1-081,1-086,1-087,1-089,1-091,1-093~1-097,1-099,1-104~1-106,1-108~1-112,1-114~1-117,1-119~1-125,1-131,1-135~1-137,1-140,1-143~1-149,2-002,2-003,2-005,2-007~2-011,2-015~2-020,2-031,2-032,2-042,2-044,2-045,2-054~2-063,2-065~2-067,2-069~2-071,2-082,2-084~2-088,3-002*,3-003,3-004,3-006,3-007,3-010~3-012,3-014,3-020,3-025,3-026,3-028,3-030,3-031,3-035~3-043,3-045,3-049,3-050,3-052~3-055,3-058,3-059,3-062~3-068,3-070,3-074,3-075,3-078,3-084,3-085,3-088,3-089,3-091~3-095,3-104,3-106,3-107,3-109,3-117,3-126,3-128,3-130~3-133,3-135~3-138,3-140~3-143,3-146~3-148,3-150,3-151,3-153,3-156,3-157,3-167,3-169,3-172,3-177~3-181,4-001*,4-002~4-004,4-006~4-009,6-002,7-001,7-004,7-006*,7-007,7-009,7-010,7-012~7-015,7-018,7-019,7-021,7-022,8-002,8-003*,8-004,8-005,9-002~9-006,9-008~9-012,9-014~9-020,12-001,12-002,14-009,14-013,14-014,14-023,14-032,15-001,15-007。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test Example 6 Wheat powdery mildew prevention effect test 1.3 Test chemical solution A of the compound of the present invention was added to distilled water in a 90 cm 3 plastic pot planted with 1.3 leaves of wheat (variety: Norin 61), and the concentration was 500 ppm. And 5 ml sprayed per pot. One day after spraying, the pot was placed in an air-conditioned greenhouse (20 ° C.), and wheat was inoculated with conidia of wheat powdery mildew (Blumeria graminis f. Sp. Tritici). Thereafter, it was held for 7 days, the ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-001,1-002,1-004 to 1-007,1-009,1-011,1-013,1-015,1-016,1-017 * , 1-019 , 1-021 ~ 1-023,1-025,1-027,1-028,1-030,1-031,1-034,1-036 ~ 1-039,1-042,1-044 ~ 1 -054,1-056 to 1-063,1-066 to 1-068,1-069 * , 1-070 to 1-074,1-076,1-079 to 1-081,1-086,1- 087,1-089,1-091,1-093 ~ 1-097,1-099,1-104 ~ 1-106,1-108 ~ 1-112,1-114 ~ 1-117,1-119 ~ 1-125,1-131,1-135 to 1-137,1-140,1-143 to 1-149,2-002,2-003,2-005,2-007 to 2-011,2- 015 ~ 2-020,2-031,2-032,2-042,2-044,2-045,2-054 ~ 2-063,2-065 ~ 2-067,2-069 ~ 2-071, 2-082,2-084 to 2-088,3-002 * , 3-003,3-004,3-006,3-007,3-010 to 3-012,3-014,3-020,3 -025,3-026,3-028,3-030,3-031,3-035-3-043,3-045,3-049,3-050,3-052-3-055,3-058 , 3-059,3-062 to 3-068,3-070,3-074,3-075,3-078,3-084,3-085,3-088,3-089,3-091 to 3 -095,3-104,3-106,3-107,3-109,3-117,3-126,3-128,3-130 to 3-133,3-135 to 3-138,3-1140 ~ 3-143,3-146 ~ 3-148,3-150,3-151,3-153,3-156,3-157,3-167,3-169,3-172,3-1177 ~ 3 -181,4-001 * , 4-002 to 4-004,4-006 to 4-009,6-002,7-001,7-004,7-006 * , 7-007,7-009,7 -0 10,7-012 to 7-015,7-018,7-019,7-021,7-022,8-002,8-003 * , 8-004,8-005,9-002 to 9-006 , 9-008 ~ 9-012,9-014 ~ 9-020,12-001,12-002,14-009,14-013,14-014,14-023,14-032,15-001,15 -007.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
試験例7 コムギふ枯病予防効果試験
1.3葉期のコムギ(品種:ハルユタカ)を植えた90cm3のプラスチックポットに、本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。散布1日後、コムギふ枯病菌(Phaeosphaeria nodorum、Synonym;Septoria nodorum)の分生胞子懸濁液をコムギに噴霧接種し、温度20℃、湿度100%RHの接種箱内に2日間入れた。その後、空調温室(20℃)に置き、6日間保持した。形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-002,1-004,1-006,1-007,1-009,1-010,1-012,1-015,1-017*,1-031,1-042,1-045,1-049~1-052,1-057,1-060,1-062,1-063,1-066,1-073,1-074,1-087,1-124,1-144,1-146,1-148,1-149,2-002,2-007,2-015,2-016,2-018,2-020,2-056,2-085,3-002*,3-006,3-010~3-012,3-014,3-025,3-026,3-036,3-040~3-044,3-051,3-053,3-054,3-059,3-062,3-070,3-084,3-085,3-088,3-089,3-095,3-135,3-138,3-156,3-170,3-181,7-001,7-006*,7-010,7-012,7-013,8-003*,8-004,8-005,9-001*,9-002~9-006,9-008~9-010,9-014,9-018,9-020,14-003,14-009,15-001。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test Example 7 Wheat Blight Prevention Effect Test 1.3 Distilled water was added to test chemical solution A of the present invention in a 90 cm 3 plastic pot planted with 3 leaf stage wheat (variety: Haruyutaka) to a concentration of 500 ppm. Then, 5 ml per pot was sprayed. One day after spraying, a conidial spore suspension of wheat blight fungus (Phaeosphaeria nodorum, Synonym; Septoria nodorum) was spray-inoculated on the wheat and placed in an inoculation box at a temperature of 20 ° C. and a humidity of 100% RH for 2 days. Thereafter, it was placed in an air-conditioned greenhouse (20 ° C.) and held for 6 days. The ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same calculation formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-002,1-004,1-006,1-007,1-009,1-010,1-012,1-015,1-017 * , 1-031,1-042 , 1-045,1-049 to 1-052,1-057,1-060,1-062,1-063,1-066,1-073,1-074,1-087,1-124,1 -144,1-146,1-148,1-149,2-002,2-007,2-015,2-016,2-018,2-020,2-056,2-085,3-002 * , 3-006,3-010 to 3-012,3-014,3-025,3-026,3-036,3-040 to 3-044,3-051,3-053,3-054, 3-059,3-062,3-070,3-084,3-085,3-088,3-089,3-095,3-135,3-138,3-156,3-170,3- 181,7-001,7-006 * , 7-010,7-012,7-013,8-003 * , 8-004,8-005,9-001 * , 9-002 to 9-006,9 -008 to 9-010,9-014,9-018,9-020,14-003,14-009,15-001.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
1.3葉期のコムギ(品種:ハルユタカ)を植えた90cm3のプラスチックポットに、本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。散布1日後、コムギふ枯病菌(Phaeosphaeria nodorum、Synonym;Septoria nodorum)の分生胞子懸濁液をコムギに噴霧接種し、温度20℃、湿度100%RHの接種箱内に2日間入れた。その後、空調温室(20℃)に置き、6日間保持した。形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.1-002,1-004,1-006,1-007,1-009,1-010,1-012,1-015,1-017*,1-031,1-042,1-045,1-049~1-052,1-057,1-060,1-062,1-063,1-066,1-073,1-074,1-087,1-124,1-144,1-146,1-148,1-149,2-002,2-007,2-015,2-016,2-018,2-020,2-056,2-085,3-002*,3-006,3-010~3-012,3-014,3-025,3-026,3-036,3-040~3-044,3-051,3-053,3-054,3-059,3-062,3-070,3-084,3-085,3-088,3-089,3-095,3-135,3-138,3-156,3-170,3-181,7-001,7-006*,7-010,7-012,7-013,8-003*,8-004,8-005,9-001*,9-002~9-006,9-008~9-010,9-014,9-018,9-020,14-003,14-009,15-001。
尚、上記*印は100ppm濃度の薬液を用いて試験を実施したことを表す。 Test Example 7 Wheat Blight Prevention Effect Test 1.3 Distilled water was added to test chemical solution A of the present invention in a 90 cm 3 plastic pot planted with 3 leaf stage wheat (variety: Haruyutaka) to a concentration of 500 ppm. Then, 5 ml per pot was sprayed. One day after spraying, a conidial spore suspension of wheat blight fungus (Phaeosphaeria nodorum, Synonym; Septoria nodorum) was spray-inoculated on the wheat and placed in an inoculation box at a temperature of 20 ° C. and a humidity of 100% RH for 2 days. Thereafter, it was placed in an air-conditioned greenhouse (20 ° C.) and held for 6 days. The ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same calculation formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No.1-002,1-004,1-006,1-007,1-009,1-010,1-012,1-015,1-017 * , 1-031,1-042 , 1-045,1-049 to 1-052,1-057,1-060,1-062,1-063,1-066,1-073,1-074,1-087,1-124,1 -144,1-146,1-148,1-149,2-002,2-007,2-015,2-016,2-018,2-020,2-056,2-085,3-002 * , 3-006,3-010 to 3-012,3-014,3-025,3-026,3-036,3-040 to 3-044,3-051,3-053,3-054, 3-059,3-062,3-070,3-084,3-085,3-088,3-089,3-095,3-135,3-138,3-156,3-170,3- 181,7-001,7-006 * , 7-010,7-012,7-013,8-003 * , 8-004,8-005,9-001 * , 9-002 to 9-006,9 -008 to 9-010,9-014,9-018,9-020,14-003,14-009,15-001.
In addition, the said * mark represents having implemented the test using a 100 ppm concentration chemical | medical solution.
試験例8 コムギ赤さび病予防効果試験
1.3葉期のコムギ(品種:農林61号)を植えた90cm3のプラスチックポットに、本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。散布1日後、コムギ赤さび病菌(Puccinia recondita)の胞子懸濁液をコムギに噴霧接種し、温度20℃、湿度100%RHの接種箱内に1日間入れた。その後、空調温室(20℃)に置き、8日間保持した。形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.3-053,3-059,3-083,3-172,4-004,8-005,9-003,9-004,9-009,9-014,9-016,9-018,9-020。 Test Example 8 Wheat Red Rust Prevention Effect Test 1.3 Into a 90 cm 3 plastic pot planted with 3 leaf stage wheat (variety: Norin 61), distilled water was added to the test chemical solution A of the present invention to a concentration of 500 ppm. Diluted and sprayed 5 ml per pot. One day after spraying, the wheat spore suspension of Puccinia recondita was spray-inoculated on the wheat and placed in an inoculation box at a temperature of 20 ° C. and a humidity of 100% RH for one day. Thereafter, it was placed in an air-conditioned greenhouse (20 ° C.) and held for 8 days. The ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same calculation formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No. 3-053, 3-059, 3-083, 3-172, 4-004, 8-005, 9-003, 9-004, 9-009, 9-014, 9-016, 9-018,9-020.
1.3葉期のコムギ(品種:農林61号)を植えた90cm3のプラスチックポットに、本発明化合物の試験薬液Aを、蒸留水を加えて500ppm濃度に希釈し、ポット当たり5ml散布処理した。散布1日後、コムギ赤さび病菌(Puccinia recondita)の胞子懸濁液をコムギに噴霧接種し、温度20℃、湿度100%RHの接種箱内に1日間入れた。その後、空調温室(20℃)に置き、8日間保持した。形成された病斑の接種葉に占める割合を測定し、試験例1と同様の計算式から防除価を算出した。尚、試験は2連制で行なった。
その結果、供試した化合物の内、下記の化合物が70%以上の防除価を示した。
本発明化合物:No.3-053,3-059,3-083,3-172,4-004,8-005,9-003,9-004,9-009,9-014,9-016,9-018,9-020。 Test Example 8 Wheat Red Rust Prevention Effect Test 1.3 Into a 90 cm 3 plastic pot planted with 3 leaf stage wheat (variety: Norin 61), distilled water was added to the test chemical solution A of the present invention to a concentration of 500 ppm. Diluted and sprayed 5 ml per pot. One day after spraying, the wheat spore suspension of Puccinia recondita was spray-inoculated on the wheat and placed in an inoculation box at a temperature of 20 ° C. and a humidity of 100% RH for one day. Thereafter, it was placed in an air-conditioned greenhouse (20 ° C.) and held for 8 days. The ratio of the formed lesions to the inoculated leaves was measured, and the control value was calculated from the same calculation formula as in Test Example 1. In addition, the test was performed by 2 continuous systems.
As a result, among the tested compounds, the following compounds showed a control value of 70% or more.
Compound of the present invention: No. 3-053, 3-059, 3-083, 3-172, 4-004, 8-005, 9-003, 9-004, 9-009, 9-014, 9-016, 9-018,9-020.
試験例9 黒かび病菌に対する抗菌活性試験
96ウェルプレートにポテト・デキストロース 1%寒天培地を60μlずつ分注した後、黒かび病菌(Aspergillus niger)の胞子を含む滅菌水(胞子10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加2日後の菌叢面積率(%)を判定し、無処理区に対するefficacy(%)を下式により算出した。
efficacy(%)=〔1-(処理区菌叢面積率/無処理区菌叢面積率)〕×100
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 9 Antibacterial activity test against Aspergillus oryzae After dispensing 60 μl of potato dextrose 1% agar to a 96-well plate, sterile water containing 10 Aspergillus niger spores (10 spores / 3 μl) was added. 30 μl was added per well. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The bacterial flora area ratio (%) 2 days after the addition of the drug was determined, and the efficiency (%) for the untreated area was calculated by the following formula.
Efficacy (%) = [1− (area ratio of treated flora / area area of untreated flora)] × 100
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
96ウェルプレートにポテト・デキストロース 1%寒天培地を60μlずつ分注した後、黒かび病菌(Aspergillus niger)の胞子を含む滅菌水(胞子10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加2日後の菌叢面積率(%)を判定し、無処理区に対するefficacy(%)を下式により算出した。
efficacy(%)=〔1-(処理区菌叢面積率/無処理区菌叢面積率)〕×100
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 9 Antibacterial activity test against Aspergillus oryzae After dispensing 60 μl of potato dextrose 1% agar to a 96-well plate, sterile water containing 10 Aspergillus niger spores (10 spores / 3 μl) was added. 30 μl was added per well. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The bacterial flora area ratio (%) 2 days after the addition of the drug was determined, and the efficiency (%) for the untreated area was calculated by the following formula.
Efficacy (%) = [1− (area ratio of treated flora / area area of untreated flora)] × 100
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
本発明化合物:No.1-002,1-003,1-006,1-007,1-009,1-011~1-017,1-019,1-020,1-022,1-023,1-025~1-028,1-031,1-033~1-035,1-037~1-039,1-042,1-045~1-063,1-065~1-071,1-073,1-074,1-076,1-079~1-081,1-083,1-086~1-098,1-100,1-104~1-109,1-112~1-125,1-127,1-128,1-132,1-134,1-136,1-137,1-141~1-149,2-001,2-002,2-005,2-007~2-012,2-014~2-023,2-025,2-027~2-029,2-031,2-032,2-034,2-038,2-040,2-044,2-045,2-047,2-049~2-051,2-053~2-070,2-078,2-081~2-088,2-092,3-001~3-004,3-006,3-007,3-009~3-018,3-021~3-023,3-026~3-033,3-035~3-045,3-047~3-071,3-073~3-078,3-081~3-107,3-109~3-113,3-115~3-118,3-121,3-123,3-125~3-131,3-133~3-157,3-167,3-172~3-181,3-185,4-001~4-009,5-001,6-001,6-002,7-002~7-004,7-006,7-007,7-010,7-012,7-013,7-017~7-022,8-001~8-005,9-001,9-003~9-013,9-015~9-020,12-001,12-004,12-005,13-001,13-002,14-002,14-009,14-048,14-049。
Compound of the present invention: No.1-002,1-003,1-006,1-007,1-009,1-011 to 1-017,1-019,1-020,1-022,1-023, 1-025 ~ 1-028,1-031,1-033 ~ 1-035,1-037 ~ 1-039,1-042,1-045 ~ 1-063,1-065 ~ 1-071,1- 073,1-074,1-076,1-079 ~ 1-081,1-083,1-086 ~ 1-098,1-100,1-104 ~ 1-109,1-112 ~ 1-125, 1-127,1-128,1-132,1-134,1-136,1-137,1-141-1-149,2-001,2-002,2-005,2-007-2 012,2-014 ~ 2-023,2-025,2-027 ~ 2-029,2-031,2-032,2-034,2-038,2-040,2-044,2-045, 2-047,2-049 to 2-051,2-053 to 2-070,2-078,2-081 to 2-088,2-092,3-001 to 3-004,3-006,3- 007,3-009 to 3-018,3-021 to 3-023,3-026 to 3-033,3-035 to 3-045,3-047 to 3-071,3-073 to 3-078, 3-081 to 3-107, 3-109 to 3-113, 3-115 to 3-118, 3-121, 3-123, 3-125 to 3-131, 3-133 to 3-157, 3- 167,3-172 to 3-181,3-185,4-001 to 4-009,5-001,6-001,6-002,7-002 to 7-004,7-006,7-007, 7-010,7-012,7-013,7-017-7-022,8-001-8-005,9-001,9-003-9-013,9-015-9-020,12- 001,12-004,12-005,13-001,13-002,14-002,14-009,14-048,14-049.
試験例10 サツマイモネコブセンチュウに対する殺虫試験
96ウェルプレートにポテト・デキストロース 1%寒天培地を60μlずつ分注した後、サツマイモネコブセンチュウ(Meloidogyne incognita)の卵を含む滅菌水(卵10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加4日後の未孵化卵数及び不活動幼虫数を計測し、無処理区に対するefficacy(%)を下式により算出した。
efficacy(%)=〔(処理区未孵化卵数+不活動幼虫数)/無処理区活動幼虫数〕×100
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 10 Insecticidal test against sweet potato nematode After dispensing 60 μl of potato dextrose 1% agar medium into a 96-well plate, each well was given sterile water (10 eggs / 3 μl) containing eggs of sweet potato nematode (Meloidogyne incognita). 30 μl per dose was added. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were counted, and the efficiency (%) for the untreated group was calculated by the following equation.
Efficacy (%) = [(number of untreated eggs treated + number of inactive larvae) / number of untreated group active larvae] x 100
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
96ウェルプレートにポテト・デキストロース 1%寒天培地を60μlずつ分注した後、サツマイモネコブセンチュウ(Meloidogyne incognita)の卵を含む滅菌水(卵10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加4日後の未孵化卵数及び不活動幼虫数を計測し、無処理区に対するefficacy(%)を下式により算出した。
efficacy(%)=〔(処理区未孵化卵数+不活動幼虫数)/無処理区活動幼虫数〕×100
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 10 Insecticidal test against sweet potato nematode After dispensing 60 μl of potato dextrose 1% agar medium into a 96-well plate, each well was given sterile water (10 eggs / 3 μl) containing eggs of sweet potato nematode (Meloidogyne incognita). 30 μl per dose was added. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were counted, and the efficiency (%) for the untreated group was calculated by the following equation.
Efficacy (%) = [(number of untreated eggs treated + number of inactive larvae) / number of untreated group active larvae] x 100
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
本発明化合物:No.1-002~1-004,1-006,1-007,1-009,1-011,1-013~1-023,1-025~1-031,1-034~1-039,1-042,1-044~1-056,1-058~1-063,1-065,1-066,1-068~1-076,1-080,1-086,1-087,1-089~1-092,1-094~1-100,1-103~1-112,1-114~1-119,1-121~1-126,1-128,1-132,1-134~1-138,1-140~1-149,1-152~1-155,1-159,2-001~2-003,2-005,2-007~2-011,2-013,2-014,2-016~2-019,2-021,2-022,2-025,2-027~2-032,2-041~2-045,2-047,2-050,2-051,2-054,2-055,2-057~2-060,2-062~2-071,2-076,2-077,2-082~2-088,2-090,2-092,3-001~3-004,3-006,3-007,3-009~3-015,3-018,3-020~3-026,3-028,3-029,3-031~3-033,3-035~3-048,3-050~3-059,3-061,3-062,3-064~3-071,3-073~3-078,3-084,3-086~3-089,3-091~3-096,3-098~3-102,3-104~3-107,3-109~3-114,3-116~3-119,3-122,3-124~3-131,3-134~3-150,3-153~3-158,3-164~3-167,3-170,3-172~3-181,3-185,3-186,3-190,4-004,4-007,4-009,4-013,5-001,6-001,7-001~7-010,7-012,7-013,7-017,7-018,7-020~7-022,8-001~8-004,9-003,14-001~14-003,14-007~14-014,14-016~14-025,14-028,14-030,14-032~14-042,14-046,14-048,14-049。
Compounds of the present invention: No.1-002 to 1-004,1-006,1-007,1-009,1-011,1-013 to 1-023,1-025 to 1-031,1-034 to 1-039,1-042,1-044 to 1-056,1-058 to 1-063,1-065,1-066,1-068 to 1-076,1-080,1-086,1- 087,1-089 to 1-092,1-094 to 1-100,1-103 to 1-112,1-114 to 1-119,1-121 to 1-126,1-128,1-132, 1-134 to 1-138,1-140 to 1-149,1-152 to 1-155,1-159,2-001 to 2-003,2-005,2-007 to 2-011,2- 013,2-014,2-016 ~ 2-019,2-021,2-022,2-025,2-027 ~ 2-032,2-041 ~ 2-045,2-047,2-050, 2-051,2-054,2-055,2-057 to 2-060,2-062 to 2-071,2-076,2-077,2-082 to 2-088,2-090,2- 092,3-001 to 3-004,3-006,3-007,3-009 to 3-015,3-018,3-020 to 3-026,3-028,3-029,3-031 to 3-033,3-035-3-048,3-050-3-059,3-061,3-062,3-064-3-071,3-073-3-078,3-084,3- 086 to 3-089, 3-091 to 3-096, 3-098 to 3-102, 3-104 to 3-107, 3-109 to 3-114, 3-116 to 3-119, 3-122, 3-124 to 3-131, 3-134 to 3-150, 3-153 to 3-158, 3-164 to 3-167, 3-170, 3-172 to 3-181, 3-185, 3- 186,3-190,4-004,4-007,4-009,4-013,5-001,6-001,7-001 to 7-010,7-012,7-013,7-017, 7-018,7-020-7-022,8-001-8-004,9-003,14-001-14-003,14-007-14-014,14 -016 ~ 14-025,14-028,14-030,14-032 ~ 14-042,14-046,14-048,14-049.
試験例11 サツマイモネコブセンチュウに対する防除効果試験
1セルあたり10gの土壌を充填したセルトレイ植えのホウセンカ苗(発芽後約2週間)の株元に、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、1株当たり1mlずつ処理した。処理1時間後にサツマイモネコブセンチュウ(Meloidogyne incognita)の2L幼虫を含む水(2L幼虫100頭/1ml)を、各セル当たり1ml、株元に接種した。その後、温室内で3週間保持し、根部に形成された根こぶの着生程度を下記の発病指数及び発病度に従って判定し、無処理区に対するefficacy(%)を下式により算出した。
〈発病指数〉0:こぶが認められない。 1:根系一部にこぶが認められる。
2:根系の全体にこぶが認められる。3:大きなこぶが認められる。
4:根系全体に大きなこぶが認められる。
〔発病度〕=〔Σ(発病指数×指数別発病株数)/(4×調査株数)〕×100
efficacy(%)=〔1-(処理区発病度/無処理区発病度)〕×100
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 11 Control Effect Test on Sweet Potato Nematode Cellulose-planted spinach seedlings (about 2 weeks after germination) filled with 10 g of soil per cell were added 100 ppm of test chemical solution B of the compound of the present invention with distilled water. Dilute to concentration and treat 1 ml per strain. One hour after the treatment, water containing 2 L larvae of Meloidogyne incognita (2 L larvae: 100/1 ml) was inoculated to the strain, 1 ml per cell. Then, it hold | maintained for 3 weeks in a greenhouse, the extent of the formation of the nodule formed in the root part was determined according to the following disease index and disease severity, and efficiency (%) with respect to an untreated area was computed by the following formula.
<Disease index> 0: No humps are observed. 1: A hump is recognized by a part of root system.
2: A hump is recognized in the whole root system. 3: A large hump is recognized.
4: A large hump is recognized in the whole root system.
[Disease severity] = [Σ (attack index × number of diseased strains by index) / (4 × number of strains)] × 100
Efficacy (%) = [1- (treatment area incidence / no treatment area incidence)] × 100
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
1セルあたり10gの土壌を充填したセルトレイ植えのホウセンカ苗(発芽後約2週間)の株元に、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、1株当たり1mlずつ処理した。処理1時間後にサツマイモネコブセンチュウ(Meloidogyne incognita)の2L幼虫を含む水(2L幼虫100頭/1ml)を、各セル当たり1ml、株元に接種した。その後、温室内で3週間保持し、根部に形成された根こぶの着生程度を下記の発病指数及び発病度に従って判定し、無処理区に対するefficacy(%)を下式により算出した。
〈発病指数〉0:こぶが認められない。 1:根系一部にこぶが認められる。
2:根系の全体にこぶが認められる。3:大きなこぶが認められる。
4:根系全体に大きなこぶが認められる。
〔発病度〕=〔Σ(発病指数×指数別発病株数)/(4×調査株数)〕×100
efficacy(%)=〔1-(処理区発病度/無処理区発病度)〕×100
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 11 Control Effect Test on Sweet Potato Nematode Cellulose-planted spinach seedlings (about 2 weeks after germination) filled with 10 g of soil per cell were added 100 ppm of test chemical solution B of the compound of the present invention with distilled water. Dilute to concentration and treat 1 ml per strain. One hour after the treatment, water containing 2 L larvae of Meloidogyne incognita (2 L larvae: 100/1 ml) was inoculated to the strain, 1 ml per cell. Then, it hold | maintained for 3 weeks in a greenhouse, the extent of the formation of the nodule formed in the root part was determined according to the following disease index and disease severity, and efficiency (%) with respect to an untreated area was computed by the following formula.
<Disease index> 0: No humps are observed. 1: A hump is recognized by a part of root system.
2: A hump is recognized in the whole root system. 3: A large hump is recognized.
4: A large hump is recognized in the whole root system.
[Disease severity] = [Σ (attack index × number of diseased strains by index) / (4 × number of strains)] × 100
Efficacy (%) = [1- (treatment area incidence / no treatment area incidence)] × 100
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
本発明化合物:No.1-002~1-004,1-006,1-007,1-009,1-013~1-017,1-019~1-023,1-025,1-027,1-028,1-031,1-034~1-036,1-038,1-044~1-047,1-049~1-052,1-056,1-058,1-060,1-062,1-095,1-096,1-098~1-103,1-114~1-118,1-125,2-001,2-002,2-021~2-024,2-027,2-028,2-031,2-032,2-034,2-037,2-039,2-041,2-055,2-059~2-064,3-002~3-004,3-006,3-007,3-009~3-014,3-016,3-017,3-020,3-021,3-024~3-029,3-031,3-036~3-039,3-041~3-043,3-048,3-050~3-052,3-054,3-061,3-071,3-096,3-098~3-100,3-104~3-106,3-108,3-110,3-111,3-114,3-116~3-118,3-120~3-122,3-124,3-125,3-139~3-148,4-001,4-003,4-007,4-009,6-001,7-001~7-004,7-006,7-008,7-010,7-012,7-018~7-020,7-022,8-001~8-005,9-003,12-002,14-002,14-003,14-005,14-007,14-008~14-014,14-016~14-020,14-022,14-024,14-030,14-032,14-035~14-039,14-046。
Compounds of the present invention: No.1-002 to 1-004,1-006,1-007,1-009,1-013 to 1-017,1-019 to 1-023,1-025,1-027, 1-028,1-031,1-034 ~ 1-036,1-038,1-044 ~ 1-047,1-049 ~ 1-052,1-056,1-058,1-060,1- 062,1-095,1-096,1-098 to 1-103,1-114 to 1-118,1-125,2-001,2-002,2-021 to 2-024,2-027, 2-028,2-031,2-032,2-034,2-037,2-039,2-041,2-055,2-059 to 2-064,3-002 to 3-004,3- 006,3-007,3-009 to 3-014,3-016,3-017,3-020,3-021,3-024 to 3-029,3-031,3-036 to 3-039, 3-041 to 3-043,3-048,3-050 to 3-052,3-054,3-061,3-071,3-096,3-098 to 3-100,3-104 to 3- 106,3-108,3-110,3-111,3-114,3-116 to 3-118,3-120 to 3-122,3-124,3-125,3-139 to 3-148, 4-001,4-003,4-007,4-009,6-001,7-001 to 7-004,7-006,7-008,7-010,7-012,7-018 to 7- 020,7-022,8-001 to 8-005,9-003,12-002,14-002,14-003,14-005,14-007,14-008 to 14-014,14-016 ~ 14-020,14-022,14-024,14-030,14-032,14-035-14-039,14-046.
試験例12 捻転胃虫に対する殺虫試験
96ウェルプレートにポテト・デキストロース 1%寒天培地を60μlずつ分注した後、捻転胃虫(Haemonchus contortus)の卵を含む滅菌水(卵10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加4日後の未孵化卵数および不活動幼虫数を計測し、試験例10と同様の計算式から無処理区に対するefficacy(%)を算出した。
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 12 Insecticidal test for torsion stomachworms After dispensing 60 μl of potato dextrose 1% agar medium to a 96-well plate, sterile water (10 eggs / 3 μl) containing eggs of torsion stomachworms (Haemonchus contortus), 30 μl was added per well. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were measured, and the efficiency (%) for the untreated group was calculated from the same calculation formula as in Test Example 10.
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
96ウェルプレートにポテト・デキストロース 1%寒天培地を60μlずつ分注した後、捻転胃虫(Haemonchus contortus)の卵を含む滅菌水(卵10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加4日後の未孵化卵数および不活動幼虫数を計測し、試験例10と同様の計算式から無処理区に対するefficacy(%)を算出した。
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 12 Insecticidal test for torsion stomachworms After dispensing 60 μl of potato dextrose 1% agar medium to a 96-well plate, sterile water (10 eggs / 3 μl) containing eggs of torsion stomachworms (Haemonchus contortus), 30 μl was added per well. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were measured, and the efficiency (%) for the untreated group was calculated from the same calculation formula as in Test Example 10.
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
本発明化合物:No.1-001~1-010,1-012~1-015,1-015(+),1-015(-),1-016~1-081,1-083~1-134,1-136~1-150,1-152~1-155,1-157~1-159,2-001~2-066,2-068~2-072,2-074~2-081,2-083~2-095,3-001~3-027,3-029~3-079,3-083~3-159,3-161~3-165,3-167~3-182,3-184~3-186,3-188~3-190,4-001,4-002,4-004~4-009,4-011,4-013,5-001,6-001,7-001~7-022,8-001~8-005,9-001~9-003,9-005~9-016,9-018~9-020,11-001,12-003~12-005,14-001~14-028,14-030~14-053,15-001~15-010。
Compounds of the present invention: No.1-001 to 1-010, 1-012 to 1-015, 1-015 (+), 1-015 (-), 1-016 to 1-081, 1-083 to 1- 134,1-136 to 1-150,1-152 to 1-155,1-157 to 1-159,2-001 to 2-066,2-068 to 2-072,2-074 to 2-081, 2-083 to 2-095, 3-001 to 3-027, 3-029 to 3-079, 3-083 to 3-159, 3-161 to 3-165, 3-167 to 3-182,3- 184 to 3-186, 3-188 to 3-190, 4-001, 4-002, 4-004 to 4-009, 4-011, 4-013, 5-001, 6-001, 7-001 ... 7-022,8-001 to 8-005,9-001 to 9-003,9-005 to 9-016,9-018 to 9-020,11-001,12-003 to 12-005,14- 001-14-028,14-030-14-053,15-001-15-010.
試験例13 ダイズシストセンチュウに対する殺虫試験
96ウェルプレートに1%寒天培地を60μlずつ分注した後、ダイズシストセンチュウ(Heterodera glycines)の卵を含む滅菌水(卵10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加4日後の未孵化卵数および不活動幼虫数を計測し、試験例10と同様の計算式から無処理区に対するefficacy(%)を算出した。
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 13 Insecticidal test for soybean cyst nematodes After dispensing 60 μl of 1% agar medium to a 96-well plate, sterile water (10 eggs / 3 μl) containing soybean cyst nematode (Heterodera glycines) eggs was added to each well. 30 μl was added. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were measured, and the efficiency (%) for the untreated group was calculated from the same calculation formula as in Test Example 10.
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
96ウェルプレートに1%寒天培地を60μlずつ分注した後、ダイズシストセンチュウ(Heterodera glycines)の卵を含む滅菌水(卵10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物の試験薬液Bを、蒸留水を加えて100ppm濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加4日後の未孵化卵数および不活動幼虫数を計測し、試験例10と同様の計算式から無処理区に対するefficacy(%)を算出した。
その結果、供試した化合物の内、下記の化合物が50%以上のefficacy(%)を示した。 Test Example 13 Insecticidal test for soybean cyst nematodes After dispensing 60 μl of 1% agar medium to a 96-well plate, sterile water (10 eggs / 3 μl) containing soybean cyst nematode (Heterodera glycines) eggs was added to each well. 30 μl was added. From this, the test chemical solution B of the compound of the present invention was diluted with distilled water to a concentration of 100 ppm, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were measured, and the efficiency (%) for the untreated group was calculated from the same calculation formula as in Test Example 10.
As a result, among the tested compounds, the following compounds showed an efficiency (%) of 50% or more.
本発明化合物:No.1-002,1-053,1-069~1-072,1-074,1-091,1-092,1-094~1-112,1-114~1-116,1-118,1-119,1-121,1-123~1-125,1-127,1-128,1-132,1-134~1-137,1-140~1-147,1-149,1-150,1-152~1-155,1-159,2-010,2-011,2-016,2-021~2-023,2-025,2-027~2-035,2-037,2-039,2-040,2-042~2-050,2-054~2-071,2-076,2-078,2-081~2-088,2-090~2-092,3-002,3-044,3-066~3-068,3-070,3-071,3-076,3-077,3-087,3-088,3-095,3-096,3-098,3-100~3-102,3-104~3-107,3-110~3-114,3-116~3-122,3-124,3-126,3-128~3-131,3-133~3-158,3-161,3-164,3-167,3-172~3-181,3-185,3-186,3-189,4-007~4-009,4-011,4-013,7-018~7-021,8-004,8-005,9-011,9-020,14-002,14-040,15-013。
Compound of the present invention: No.1-002,1-053,1-069 to 1-072,1-074,1-091,1-092,1-094 to 1-112,1-114 to 1-116, 1-118,1-119,1-121,1-123 to 1-125,1-127,1-128,1-132,1-134 to 1-137,1-140 to 1-147,1- 149,1-150,1-152 to 1-155,1-159,2-010,2-011,2-016,2-021 to 2-023,2-025,2-027 to 2-035, 2-037,2-039,2-040,2-042 to 2-050,2-054 to 2-071,2-076,2-078,2-081 to 2-088,2-090 to 2- 092,3-002,3-044,3-066 to 3-068,3-070,3-071,3-076,3-077,3-087,3-088,3-095,3-096, 3-098, 3-100 to 3-102, 3-104 to 3-107, 3-110 to 3-114, 3-116 to 3-122, 3-124, 3-126, 3-128 to 3- 131,3-133 to 3-158,3-161,3-164,3-167,3-172 to 3-181,3-185,3-186,3-189,4-007 to 4-009, 4-011,4-013,7-018 to 7-021,8-004,8-005,9-011,9-020,14-002,14-040,15-013.
試験例14 捻転胃虫に対する殺虫試験(比較試験)
96ウェルプレートにポテト・デキストロース 1%寒天培地を60μlずつ分注した後、捻転胃虫(Haemonchus contortus)の卵を含む滅菌水(卵10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物及び比較化合物の試験薬液Bを、蒸留水を加えて各々の所定濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加4日後の未孵化卵数および不活動幼虫数を計測し、試験例10と同様の計算式から無処理区に対するefficacy(%)を算出した。 Test Example 14 Insecticidal test against torsion stomachworm (comparison test)
After dispensing 60 μl of potato dextrose 1% agar medium to a 96-well plate, sterile water (10 eggs / 3 μl) containing eggs of Haemonchus contortus was added in an amount of 30 μl per well. From this, the test drug solution B of the compound of the present invention and the comparative compound was diluted to each predetermined concentration by adding distilled water, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were measured, and the efficiency (%) for the untreated group was calculated from the same calculation formula as in Test Example 10.
96ウェルプレートにポテト・デキストロース 1%寒天培地を60μlずつ分注した後、捻転胃虫(Haemonchus contortus)の卵を含む滅菌水(卵10個/3μl)を、各ウェル当たり30μlずつ加えた。この上から、本発明化合物及び比較化合物の試験薬液Bを、蒸留水を加えて各々の所定濃度に希釈し、各ウェル当たり10μlずつ添加し、暗黒条件下、25℃にて静置した。薬剤添加4日後の未孵化卵数および不活動幼虫数を計測し、試験例10と同様の計算式から無処理区に対するefficacy(%)を算出した。 Test Example 14 Insecticidal test against torsion stomachworm (comparison test)
After dispensing 60 μl of potato dextrose 1% agar medium to a 96-well plate, sterile water (10 eggs / 3 μl) containing eggs of Haemonchus contortus was added in an amount of 30 μl per well. From this, the test drug solution B of the compound of the present invention and the comparative compound was diluted to each predetermined concentration by adding distilled water, added 10 μl per well, and allowed to stand at 25 ° C. under dark conditions. The number of unhatched eggs and the number of inactive larvae 4 days after the addition of the drug were measured, and the efficiency (%) for the untreated group was calculated from the same calculation formula as in Test Example 10.
各供試化合物の各所定濃度におけるefficacy(%)を第22表及び第23表に示す。
第22表
――――――――――――――――――――――――――――――――
濃度(ppm)
供試化合物 10 3 0.3 0.1 0.03
――――――――――――――――――――――――――――――――
本発明化合物 No.2-052 100 100 70 0 0
比較化合物 A 30 0 0 0 0
本発明化合物 No.2-054 100 100 90 80 0
比較化合物 B 100 0 0 0 0
――――――――――――――――――――――――――――――――
比較化合物 A:国際公開第2014-173921号明細書、化合物 X.8
比較化合物 B:国際公開第2014-173921号明細書、化合物 X.7
Tables 22 and 23 show the efficiency (%) of each test compound at each predetermined concentration.
Table 22 ――――――――――――――――――――――――――――――――
Concentration (ppm)
Test compound 10 3 0.3 0.1 0.03
――――――――――――――――――――――――――――――――
Compound of the present invention No.2-052 100 100 70 0 0
Comparative compound A 30 0 0 0 0
Compound of the present invention No.2-054 100 100 90 80 0
Comparative compound B 100 0 0 0 0
――――――――――――――――――――――――――――――――
Comparative compound A: WO2014-173921, compound X.8
Comparative compound B: WO2014-173921, compound X.7
第22表
――――――――――――――――――――――――――――――――
濃度(ppm)
供試化合物 10 3 0.3 0.1 0.03
――――――――――――――――――――――――――――――――
本発明化合物 No.2-052 100 100 70 0 0
比較化合物 A 30 0 0 0 0
本発明化合物 No.2-054 100 100 90 80 0
比較化合物 B 100 0 0 0 0
――――――――――――――――――――――――――――――――
比較化合物 A:国際公開第2014-173921号明細書、化合物 X.8
Table 22 ――――――――――――――――――――――――――――――――
Concentration (ppm)
Test compound 10 3 0.3 0.1 0.03
――――――――――――――――――――――――――――――――
Compound of the present invention No.2-052 100 100 70 0 0
Comparative compound A 30 0 0 0 0
Compound of the present invention No.2-054 100 100 90 80 0
Comparative compound B 100 0 0 0 0
――――――――――――――――――――――――――――――――
Comparative compound A: WO2014-173921, compound X.8
第23表
――――――――――――――――――――――――――――――――――
濃度(ppm)
供試化合物 10 5 2.5 1.3 0.6 0.3
――――――――――――――――――――――――――――――――――
本発明化合物 No.9-005 100 90 100 100 100 0
本発明化合物 No.9-006 100 90 100 100 90 0
比較化合物 C 100 80 0 0 0 0
――――――――――――――――――――――――――――――――――
比較化合物 C:国際公開第2015-125824号明細書、化合物 8-027
Table 23 ――――――――――――――――――――――――――――――――――
Concentration (ppm)
Test compound 10 5 2.5 1.3 0.6 0.3
――――――――――――――――――――――――――――――――――
Compound No. 9-005 100 90 100 100 100 0
Compound No. 9-006 100 90 100 100 90 0
Comparative compound C 100 80 0 0 0 0
――――――――――――――――――――――――――――――――――
Comparative compound C: WO2015-125824, compound 8-027
――――――――――――――――――――――――――――――――――
濃度(ppm)
供試化合物 10 5 2.5 1.3 0.6 0.3
――――――――――――――――――――――――――――――――――
本発明化合物 No.9-005 100 90 100 100 100 0
本発明化合物 No.9-006 100 90 100 100 90 0
比較化合物 C 100 80 0 0 0 0
――――――――――――――――――――――――――――――――――
比較化合物 C:国際公開第2015-125824号明細書、化合物 8-027
Concentration (ppm)
Test compound 10 5 2.5 1.3 0.6 0.3
――――――――――――――――――――――――――――――――――
Compound No. 9-005 100 90 100 100 100 0
Compound No. 9-006 100 90 100 100 90 0
Comparative compound C 100 80 0 0 0 0
――――――――――――――――――――――――――――――――――
Comparative compound C: WO2015-125824, compound 8-027
本発明に係るアルキニルピリジン置換アミド化合物は、優れた有害生物防除活性、特に殺菌・殺線虫活性を示し、且つ、ホ乳動物、魚類及び有用昆虫等の非標的生物に対してほとんど悪影響の無い、極めて有用な化合物である。
なお、2016年6月29日に出願された日本特許出願2016-129158号、2016年10月6日に出願された日本特許出願2016-197920号、2017年1月17日に出願された日本特許出願2017-006154号、及び2017年4月21日に出願された日本特許出願2017-084394号の明細書、特許請求の範囲、及び要約書の全内容をここに引用し、本発明の明細書の開示として、取り入れるものである。 The alkynylpyridine-substituted amide compound according to the present invention exhibits excellent pest control activity, particularly bactericidal and nematicidal activity, and has almost no adverse effect on non-target organisms such as mammals, fish and useful insects. Is a very useful compound.
Japanese Patent Application No. 2016-129158 filed on June 29, 2016, Japanese Patent Application No. 2016-197920 filed on October 6, 2016, Japanese Patent Application filed on January 17, 2017 The specification, claims, and abstract of Japanese Patent Application No. 2017-084394 filed on April 21, 2017, and Japanese Patent Application No. 2017-084394, are incorporated herein by reference. It is incorporated as a disclosure.
なお、2016年6月29日に出願された日本特許出願2016-129158号、2016年10月6日に出願された日本特許出願2016-197920号、2017年1月17日に出願された日本特許出願2017-006154号、及び2017年4月21日に出願された日本特許出願2017-084394号の明細書、特許請求の範囲、及び要約書の全内容をここに引用し、本発明の明細書の開示として、取り入れるものである。 The alkynylpyridine-substituted amide compound according to the present invention exhibits excellent pest control activity, particularly bactericidal and nematicidal activity, and has almost no adverse effect on non-target organisms such as mammals, fish and useful insects. Is a very useful compound.
Japanese Patent Application No. 2016-129158 filed on June 29, 2016, Japanese Patent Application No. 2016-197920 filed on October 6, 2016, Japanese Patent Application filed on January 17, 2017 The specification, claims, and abstract of Japanese Patent Application No. 2017-084394 filed on April 21, 2017, and Japanese Patent Application No. 2017-084394, are incorporated herein by reference. It is incorporated as a disclosure.
Claims (21)
- 式(I)で表されるアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。
X2は、水素原子又はハロゲン原子を表し、ただし、G1がG1-10で表される構造であり、且つX1がジハロメチルを表す場合には、X2は水素原子を表し、
X3は、水素原子又はC1~C4アルキルを表し、
Y1は、水素原子、ハロゲン原子、ニトロ、C1~C4アルキル、C1~C3ハロアルキル、C1~C3アルコキシ、C1~C3ハロアルコキシ又はC1~C3アルキルチオを表し、
Y2及びY3は、各々独立して水素原子、ハロゲン原子又はメチルを表し、
R1は、水素原子、ハロゲン原子、シアノ、C1~C6アルキル、C1~C6ハロアルキル、フェニル(C1~C4)アルキル、(Z)mによって置換されたフェニル(C1~C4)アルキル、C3~C6シクロアルキル、C2~C6アルケニル、C2~C6アルキニル、C1~C6アルコキシ、C1~C6ハロアルコキシ、C3~C6アルケニルオキシ、C3~C6ハロアルケニルオキシ、C3~C6アルキニルオキシ、C3~C6ハロアルキニルオキシ、シアノ(C1~C4)アルコキシ、フェニル(C1~C4)アルコキシ、(Z)mによって置換されたフェニル(C1~C4)アルコキシ、C1~C6アルキルチオ、C1~C6ハロアルキルチオ、C1~C6アルコキシアミノ、-C(O)NH2又は-C(S)NH2を表し、
R2は、水素原子、ハロゲン原子又はC1~C6アルキルを表し、
ここで、R1とR2とは一緒になってC2~C5アルキレン鎖を形成することにより、R1及びR2が結合する炭素原子と共に3~6員環を形成してもよく、このとき前記アルキレン鎖は酸素原子、硫黄原子又は窒素原子を1~2個含んでもよく、且つC1~C4アルキル、C1~C4ハロアルキル、オキソ又はチオキソによって任意に置換されていてもよいことを表すか、R1とR2とが一緒になってC1~C6アルキリデン、C1~C6ハロアルキリデン又はC1~C4アルコキシ(C1~C2)アルキリデンを形成することを表し、
R3は、水素原子、C1~C6アルキル又はC1~C6ハロアルキルを表し、
R4は、水素原子又はC1~C6アルキルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成してもよく、
R5は、水素原子、C1~C4アルキル、C1~C4ハロアルキル、R8によって置換された(C1~C2)アルキル、C3~C6シクロアルキル、C2~C4アルケニル、C3~C4アルキニル、-OH、C1~C4アルコキシ、C1~C4ハロアルコキシ、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、C3~C6シクロアルキル(C3~C6)シクロアルキル、フェニル(C3~C6)シクロアルキル、(Z)mによって置換されたフェニル又はD-1~D-29を表し、
R1がフッ素原子、C1~C6アルキル又はC1~C6アルコキシを表し、R2がフッ素原子又はC1~C6アルキルを表し、R3がC1~C6アルキル又はC1~C6ハロアルキルを表し、且つR4が水素原子又はC1~C6アルキルを表す場合には、R6は水素原子、ハロゲン原子、C1~C6アルキル、C1~C6ハロアルキル、R10によって任意に置換された(C1~C4)アルキル、C3~C10シクロアルキル、C3~C10ハロシクロアルキル、ヒドロキシ(C3~C6)シクロアルキル、C1~C4アルコキシ(C3~C6)シクロアルキル、C4~C10シクロアルケニル、C4~C10ハロシクロアルケニル、トリ(C1~C4アルキル)シリル、フェニルジメチルシリル、-C(R11)=NOR12又はフェニルを表してもよく、
D-1~D-29は、それぞれ下記の構造式で表される芳香族複素環を表し、
D-30~D-38は、それぞれ下記の構造式で表される芳香族複素環を表し、
R7は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14、C1~C4アルキルチオ、C1~C4アルコキシカルボニル、-C(O)NH2又は-C(S)NH2を表し、
R9は、C1~C4アルキル、C1~C4アルコキシメチル、C3~C4シクロアルキル又はC2~C4アルケニルを表し、
R10は、シアノ、-OR15、-S(O)rR16又は-N(R18)R17を表し、
R11は、水素原子又はC1~C4アルキルを表し、
R12は、C1~C4アルキルを表し、
R13は、C1~C4アルキル、C1~C4ハロアルキル、C3~C4シクロアルキルメチル、C3~C4ハロシクロアルキルメチル、C3~C4シクロアルキル又はC3~C4ハロシクロアルキルを表し、
R14は、C1~C4アルキル、C2~C4ハロアルキル、C1~C4アルキルカルボニル、C3~C6シクロアルキルカルボニル又はC1~C4アルコキシカルボニルを表し、
R15は、水素原子、C1~C4アルキル、C1~C4ハロアルキル、C1~C4アルコキシ(C1~C2)アルキル、E-1、E-2、C3~C4アルケニル、C3~C4ハロアルケニル、C3~C4アルキニル、C3~C4ハロアルキニル、フェニル又は(Z)mによって置換されたフェニルを表し、
E-1及びE-2は、それぞれ下記の構造式で表される飽和複素環を表し、
R17及びR18は、各々独立して水素原子、C1~C4アルキル、C1~C4ハロアルキル、C1~C4アルコキシ(C1~C2)アルキル、C3~C4アルケニル、C3~C4アルキニル、フェニル又は(Z)mによって置換されたフェニルを表すか、
或いは、R17とR18とが一緒になってC2~C5アルキレン鎖を形成することにより、R17及びR18が結合する窒素原子と共に3~6員環を形成してもよく、このとき前記アルキレン鎖は酸素原子又は硫黄原子を1個含んでもよく、且つC1~C4アルキル、C1~C4ハロアルキル、オキソ又はチオキソによって任意に置換されていてもよく、
R19は、C1~C4アルキルを表し、pが2を表す場合には、各々のR19は互いに同一であっても、または互いに相異なっていてもよく、
mは、1、2、3、4又は5を表し、
nは、0、1、2、3又は4を表し、
pは、0、1又は2を表し、
rは、0、1又は2を表す。] An alkynylpyridine-substituted amide compound represented by the formula (I), an N-oxide thereof or a salt thereof.
X 2 represents a hydrogen atom or a halogen atom, provided that when G 1 is a structure represented by G 1 -10 and X 1 represents dihalomethyl, X 2 represents a hydrogen atom;
X 3 represents a hydrogen atom or C 1 -C 4 alkyl,
Y 1 represents a hydrogen atom, a halogen atom, nitro, C 1 -C 4 alkyl, C 1 -C 3 haloalkyl, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy or C 1 -C 3 alkylthio,
Y 2 and Y 3 each independently represent a hydrogen atom, a halogen atom or methyl,
R 1 is a hydrogen atom, a halogen atom, cyano, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, phenyl (C 1 -C 4 ) alkyl, (Z) m- substituted phenyl (C 1 -C 4 ) alkyl, C 3 -C 6 cycloalkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, C 1 -C 6 alkoxy, C 1 -C 6 haloalkoxy, C 3 -C 6 alkenyloxy, C 3 to C 6 haloalkenyloxy, C 3 to C 6 alkynyloxy, C 3 to C 6 haloalkynyloxy, cyano (C 1 to C 4 ) alkoxy, phenyl (C 1 to C 4 ) alkoxy, (Z) m Substituted phenyl (C 1 -C 4 ) alkoxy, C 1 -C 6 alkylthio, C 1 -C 6 haloalkylthio, C 1 -C 6 alkoxyamino, -C (O) NH 2 or -C (S) NH 2
R 2 represents a hydrogen atom, a halogen atom or C 1 -C 6 alkyl,
Here, R 1 and R 2 may form a C 2 -C 5 alkylene chain together to form a 3- to 6-membered ring with the carbon atom to which R 1 and R 2 are bonded, in this case the alkylene chain an oxygen atom, a sulfur atom or a nitrogen atom may be 1 to 2 comprise, and C 1 ~ C 4 alkyl, C 1 ~ C 4 haloalkyl, may be optionally substituted by oxo or thioxo Or that R 1 and R 2 together form C 1 -C 6 alkylidene, C 1 -C 6 haloalkylidene or C 1 -C 4 alkoxy (C 1 -C 2 ) alkylidene. Represent,
R 3 represents a hydrogen atom, C 1 -C 6 alkyl or C 1 -C 6 haloalkyl,
R 4 represents a hydrogen atom or C 1 -C 6 alkyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring together with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, (C 1 -C 2 ) alkyl substituted by R 8 , C 3 -C 6 cycloalkyl, C 2 -C 4 alkenyl C 3 -C 4 alkynyl, —OH, C 1 -C 4 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 haloalkylthio, —C (O) R 9 or C 1 -C 4 alkoxycarbonyl Represent,
R 6 represents C 3 -C 6 cycloalkyl (C 3 -C 6 ) cycloalkyl, phenyl (C 3 -C 6 ) cycloalkyl, (Z) phenyl substituted by m , or D-1 through D-29. Represent,
R 1 represents a fluorine atom, C 1 -C 6 alkyl or C 1 -C 6 alkoxy, R 2 represents a fluorine atom or C 1 -C 6 alkyl, and R 3 represents C 1 -C 6 alkyl or C 1- When C 6 haloalkyl is represented and R 4 represents a hydrogen atom or C 1 -C 6 alkyl, R 6 is a hydrogen atom, a halogen atom, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, R 10 (C 1 -C 4 ) alkyl, C 3 -C 10 cycloalkyl, C 3 -C 10 halocycloalkyl, hydroxy (C 3 -C 6 ) cycloalkyl, C 1 -C 4 alkoxy ( C 3 -C 6 ) cycloalkyl, C 4 -C 10 cycloalkenyl, C 4 -C 10 halocycloalkenyl, tri (C 1 -C 4 alkyl) silyl, phenyldimethylsilyl, —C (R 11 ) = NOR 12 Or it may represent phenyl,
D-1 to D-29 each represent an aromatic heterocycle represented by the following structural formula;
D-30 to D-38 each represents an aromatic heterocycle represented by the following structural formula,
R 7 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 , C 1 -C 4 alkylthio, C 1 -C 4 alkoxycarbonyl, —C (O) NH 2 or —C (S) NH 2 ,
R 9 represents C 1 -C 4 alkyl, C 1 -C 4 alkoxymethyl, C 3 -C 4 cycloalkyl or C 2 -C 4 alkenyl,
R 10 represents cyano, —OR 15 , —S (O) r R 16 or —N (R 18 ) R 17 ,
R 11 represents a hydrogen atom or C 1 -C 4 alkyl,
R 12 represents C 1 -C 4 alkyl;
R 13 is C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 3 -C 4 cycloalkylmethyl, C 3 -C 4 halocycloalkylmethyl, C 3 -C 4 cycloalkyl or C 3 -C 4 Represents halocycloalkyl,
R 14 represents C 1 -C 4 alkyl, C 2 -C 4 haloalkyl, C 1 -C 4 alkylcarbonyl, C 3 -C 6 cycloalkylcarbonyl or C 1 -C 4 alkoxycarbonyl,
R 15 is a hydrogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy (C 1 -C 2 ) alkyl, E-1, E-2, C 3 -C 4 alkenyl C 3 -C 4 haloalkenyl, C 3 -C 4 alkynyl, C 3 -C 4 haloalkynyl, phenyl or phenyl substituted by (Z) m
E-1 and E-2 each represent a saturated heterocyclic ring represented by the following structural formula,
R 17 and R 18 are each independently a hydrogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 4 alkoxy (C 1 -C 2 ) alkyl, C 3 -C 4 alkenyl, Represents C 3 -C 4 alkynyl, phenyl or phenyl substituted by (Z) m ,
Alternatively, R 17 and R 18 may be combined to form a C 2 -C 5 alkylene chain to form a 3- to 6-membered ring with the nitrogen atom to which R 17 and R 18 are bonded. when the alkylene chain may contain one oxygen atom or sulfur atom, and C 1 ~ C 4 alkyl, C 1 ~ C 4 haloalkyl, may be optionally substituted by oxo or thioxo,
R 19 represents C 1 -C 4 alkyl, and when p represents 2, each R 19 may be the same as or different from each other,
m represents 1, 2, 3, 4 or 5;
n represents 0, 1, 2, 3 or 4;
p represents 0, 1 or 2;
r represents 0, 1 or 2. ] - G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-9、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル、ジフルオロメチルスルホニル又はトリフルオロメチルスルホニルを表し、
X2は、水素原子、フッ素原子又は塩素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
X3は、メチルを表し、
Y1は、ハロゲン原子、メチル、トリフルオロメチル又はメトキシを表し、
Y2は、水素原子又はメチルを表し、
Y3は、水素原子を表し、
R1は、水素原子、フッ素原子、C1~C3アルキル、C1~C3ハロアルキル、ベンジル、(Z)mによって置換されたフェニルメチル、シクロプロピル、C1~C3アルコキシ、C1~C3ハロアルコキシ、C3~C4アルケニルオキシ、C3~C4アルキニルオキシ、シアノメトキシ、ベンジルオキシ、(Z)mによって置換されたフェニルメトキシ、C1~C3アルキルチオ又はC1~C3ハロアルキルチオを表し、
R2は、水素原子、フッ素原子又はC1~C3アルキルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、R1とR2とは一緒になってC1~C2アルキリデン又はC1~C2ハロアルキリデンを形成することを表してもよく、
R3は、水素原子、C1~C3アルキル又はC1~C3ハロアルキルを表し、
R4は、水素原子又はC1~C3アルキルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、C3~C6シクロアルキル、C2~C4アルケニル、C3~C4アルキニル、C1~C4アルコキシ、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23、D-24、D-25又はD-26を表し、
さらに、R1がフッ素原子又はC1~C3アルキル表し、R2がフッ素原子又はメチルを表し、R3がメチル又はエチルを表し、且つR4が水素原子又はメチルを表す場合には、R6は水素原子、ハロゲン原子、C1~C4アルキル、C1~C4ハロアルキル、R10によって任意に置換された(C1~C4)アルキル、C3~C6シクロアルキル、C3~C6ハロシクロアルキル、ヒドロキシ(C3~C6)シクロアルキル、C1~C2アルコキシ(C3~C6)シクロアルキル、C4~C6シクロアルケニル、トリ(C1~C4アルキル)シリル、-C(R11)=NOR12又はフェニルを表してもよく、
Zは、ハロゲン原子、シアノ、ニトロ、-SF5、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31~D-35又はD-37を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-CH2OCH2-、-OCH2O-、-CH2CH2S(O)r-、-CH2CH2CH2CH2-、-CH2CH2CH2O-、-CH2OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-SN=N-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=、-CH=CHCH=CH-、-N=CHCH=CH-、-CH=NCH=CH-、-N=NCH=CH-、-N=CHN=CH-、-N=CHCH=N-、-CH=NN=CH-又は-N=NCH=N-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、シアノ、メチル、ジフルオロメチル、トリフルオロメチル又はメトキシによって任意に置換されていてもよく、
Zaは、ハロゲン原子、メチル又はトリフルオロメチルを表し、
R7は、メチル又はエチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14、C1~C4アルキルチオ、-C(O)NH2又は-C(S)NH2を表し、
R9は、C1~C4アルキル又はC3~C4シクロアルキルを表し、
R10は、-OR15又は-S(O)rR16を表し、
R11は、水素原子又はメチルを表し、
R12は、メチル又はエチルを表し、
R13は、C1~C4アルキル、C1~C4ハロアルキル、C3~C4シクロアルキルメチル又はC3~C4シクロアルキルを表し、
R14は、C1~C4アルキル又はC2~C4ハロアルキルを表し、
R15は、水素原子、メチル、エチル又はC1~C2ハロアルキルを表し、
R16は、メチル、エチル又はC1~C2ハロアルキルを表す、
請求項1に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -9, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl, difluoromethylsulfonyl or trifluoromethylsulfonyl,
X 2 represents a hydrogen atom, a fluorine atom or a chlorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom. Represent,
X 3 represents methyl,
Y 1 represents a halogen atom, methyl, trifluoromethyl or methoxy;
Y 2 represents a hydrogen atom or methyl,
Y 3 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, benzyl, (Z) m- substituted phenylmethyl, cyclopropyl, C 1 -C 3 alkoxy, C 1- C 3 haloalkoxy, C 3 -C 4 alkenyloxy, C 3 -C 4 alkynyloxy, cyanomethoxy, benzyloxy, phenylmethoxy substituted by (Z) m , C 1 -C 3 alkylthio or C 1 -C 3 Represents haloalkylthio,
R 2 represents a hydrogen atom, a fluorine atom or C 1 -C 3 alkyl,
Here, by forming the ethylene chain together R 1 and R 2, may represent that together with the carbon atoms to which R 1 and R 2 are attached form a cyclopropyl ring, R 1 and R the 2 may represent the formation of a C 1 ~ C 2 alkylidene or C 1 ~ C 2 Haroarukiriden together,
R 3 represents a hydrogen atom, C 1 -C 3 alkyl or C 1 -C 3 haloalkyl,
R 4 represents a hydrogen atom or C 1 -C 3 alkyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl, C 3 -C 6 cycloalkyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl substituted by R 8 , C 1 -C 4 alkoxy, C 1 -C 4 haloalkylthio, —C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 6 represents phenyl, D-1, D-2, D-6, D-8, D-23, D-24, D-25 or D-26 substituted by (Z) m ;
Furthermore, when R 1 represents a fluorine atom or C 1 -C 3 alkyl, R 2 represents a fluorine atom or methyl, R 3 represents methyl or ethyl, and R 4 represents a hydrogen atom or methyl, R 6 is a hydrogen atom, a halogen atom, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, (C 1 -C 4 ) alkyl optionally substituted by R 10 , C 3 -C 6 cycloalkyl, C 3- C 6 halocycloalkyl, hydroxy (C 3 -C 6 ) cycloalkyl, C 1 -C 2 alkoxy (C 3 -C 6 ) cycloalkyl, C 4 -C 6 cycloalkenyl, tri (C 1 -C 4 alkyl) May represent silyl, -C (R 11 ) = NOR 12 or phenyl;
Z is a halogen atom, cyano, nitro, —SF 5 , C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 ~ C 4 alkylsulfinyl, C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31 ~ D-35 or D- 37, when m or n represents 2 or more, each Z may be the same as or different from each other. Further, when two Zs are adjacent to each other, two Zs are adjacent to each other. Two Zs are —CH 2 CH 2 CH 2 —, —CH 2 CH 2 O—, —CH 2 OCH 2 —, —OCH 2 O—, —CH 2 CH 2 S (O) r —, —CH 2 CH 2. CH 2 CH 2- , -CH 2 CH 2 CH 2 O-, -CH 2 OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) C H = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -SN = N-, -N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN =, -CH = CHCH = CH-, -N = CHCH = CH-, -CH = NCH = CH-, -N = NCH = CH-, -N = CHN = CH By forming-, -N = CHCH = N-, -CH = NN = CH- or -N = NCH = N-, a 5-membered or 6-membered ring is formed with the carbon atom to which each Z is bonded. In this case, the hydrogen atom bonded to each carbon atom forming the ring may be optionally substituted by a halogen atom, cyano, methyl, difluoromethyl, trifluoromethyl or methoxy,
Z a represents a halogen atom, methyl or trifluoromethyl,
R 7 represents methyl or ethyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 , C 1 -C 4 alkylthio, —C (O) NH 2 or —C (S) NH 2
R 9 represents C 1 -C 4 alkyl or C 3 -C 4 cycloalkyl,
R 10 represents —OR 15 or —S (O) r R 16 ;
R 11 represents a hydrogen atom or methyl,
R 12 represents methyl or ethyl,
R 13 represents C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 3 -C 4 cycloalkylmethyl or C 3 -C 4 cycloalkyl,
R 14 represents C 1 -C 4 alkyl or C 2 -C 4 haloalkyl,
R 15 represents a hydrogen atom, methyl, ethyl or C 1 -C 2 haloalkyl,
R 16 represents methyl, ethyl or C 1 -C 2 haloalkyl,
The alkynylpyridine-substituted amide compound according to claim 1, its N-oxide or a salt thereof. - G1は、G1-1、G1-2、G1-3、G1-4、G1-5、G1-6、G1-7、G1-8、G1-10、G1-11、G1-12、G1-14又はG1-16で表される構造を表し、
X1は、ハロゲン原子、ニトロ、メチル、ジフルオロメチル、トリフルオロメチル、メトキシ、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表し、
X2は、水素原子又はフッ素原子を表し、ただし、G1がG1-10で表される構造を表し、且つX1がジフルオロメチルを表す場合には、X2は水素原子を表し、
Y1は、ハロゲン原子又はトリフルオロメチルを表し、
Y2は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル、R8によって置換された(C1~C2)アルキル、シクロプロピル、C2~C4アルケニル、C3~C4アルキニル、C1~C4ハロアルキルチオ、-C(O)R9又はC1~C4アルコキシカルボニルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23又はD-25を表し、
さらに、R1がフッ素原子を表し、R2がフッ素原子を表し、R3がメチルを表し、且つR4が水素原子又はメチルを表す場合には、R6はC1~C4ハロアルキル又はC3~C6シクロアルキルを表してもよく、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R7は、メチルを表し、
R8は、シアノ、C3~C6シクロアルキル、-OR14又はC1~C4アルキルチオを表し、
R9は、C1~C4アルキルを表し、
R13は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
R14は、C1~C4アルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
請求項2に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 G 1 is, G 1 -1, G 1 -2 , G 1 -3, G 1 -4, G 1 -5, G 1 -6, G 1 -7, G 1 -8, G 1 -10, G 1 -11, G 1 -12, G 1 -14 or G 1 -16 represents a structure represented,
X 1 represents a halogen atom, nitro, methyl, difluoromethyl, trifluoromethyl, methoxy, methylthio, methylsulfonyl or difluoromethylsulfonyl,
X 2 represents a hydrogen atom or a fluorine atom, provided that when G 1 represents a structure represented by G 1 -10 and X 1 represents difluoromethyl, X 2 represents a hydrogen atom;
Y 1 represents a halogen atom or trifluoromethyl,
Y 2 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 is a hydrogen atom, C 1 -C 4 alkyl, (C 1 -C 2 ) alkyl substituted by R 8 , cyclopropyl, C 2 -C 4 alkenyl, C 3 -C 4 alkynyl, C 1 -C 4 haloalkylthio, -C (O) R 9 or C 1 -C 4 alkoxycarbonyl,
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
Further, when R 1 represents a fluorine atom, R 2 represents a fluorine atom, R 3 represents methyl, and R 4 represents a hydrogen atom or methyl, R 6 represents C 1 -C 4 haloalkyl or C May represent 3 to C 6 cycloalkyl,
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -CH 2 CH 2 O-, -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-,- N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH = CHCH = CH- May form a 5-membered ring or a 6-membered ring together with the carbon atom to which each Z is bonded, in which case the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trifluoromethyl Optionally substituted by
R 7 represents methyl,
R 8 represents cyano, C 3 -C 6 cycloalkyl, —OR 14 or C 1 -C 4 alkylthio;
R 9 represents C 1 -C 4 alkyl,
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
R 14 represents C 1 -C 4 alkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
The alkynylpyridine-substituted amide compound according to claim 2, its N-oxide or a salt thereof. - G1は、G1-1で表される構造を表し、
X1は、ハロゲン原子、メチル又はトリフルオロメチルを表し、
X2は、水素原子又はフッ素原子を表す、
請求項1~3のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 G 1 represents the structure represented by G 1 -1,
X 1 represents a halogen atom, methyl or trifluoromethyl,
X 2 represents a hydrogen atom or a fluorine atom,
The alkynylpyridine-substituted amide compound according to any one of claims 1 to 3, its N-oxide or a salt thereof. - G1は、G1-2又はG1-3で表される構造を表し、
X1は、ハロゲン原子、メチル、ジフルオロメチル、トリフルオロメチル、メチルチオ、メチルスルホニル又はジフルオロメチルスルホニルを表す、
請求項1~3のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 G 1 represents a structure represented by G 1 -2 or G 1 -3,
X 1 represents a halogen atom, methyl, difluoromethyl, trifluoromethyl, methylthio, methylsulfonyl or difluoromethylsulfonyl,
The alkynylpyridine-substituted amide compound according to any one of claims 1 to 3, its N-oxide or a salt thereof. - G1は、G1-4で表される構造を表し、
X1は、ハロゲン原子又はトリフルオロメチルを表す、
請求項1~3のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 G 1 represents the structure represented by G 1 -4,
X 1 represents a halogen atom or trifluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of claims 1 to 3, its N-oxide or a salt thereof. - G1は、G1-7又はG1-8で表される構造を表し、
X1は、ハロゲン原子、メチル又はトリフルオロメチルを表す、
請求項1~3のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 G 1 represents a structure represented by G 1 -7 or G 1 -8,
X 1 represents a halogen atom, methyl or trifluoromethyl,
The alkynylpyridine-substituted amide compound according to any one of claims 1 to 3, its N-oxide or a salt thereof. - G1は、G1-10で表される構造を表し、
X1は、ジフルオロメチル又はトリフルオロメチルを表し、
X2は、水素原子を表し、
R7は、メチルを表す、
請求項1~3のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 G 1 represents a structure represented by G 1 -10;
X 1 represents difluoromethyl or trifluoromethyl,
X 2 represents a hydrogen atom,
R 7 represents methyl,
The alkynylpyridine-substituted amide compound according to any one of claims 1 to 3, its N-oxide or a salt thereof. - R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23又はD-25を表し、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-CH2CH2O-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R13は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す、
請求項1~8のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -CH 2 CH 2 O-, -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N-, -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-,- N (R 13 ) N = N-, = NN (R 13 ) CH =, = NON =, = NSN = or -CH = CHCH = CH- May form a 5-membered ring or a 6-membered ring together with the carbon atom to which each Z is bonded, in which case the hydrogen atom bonded to each carbon atom forming the ring is a halogen atom, methyl or trifluoromethyl Optionally substituted by
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3;
The alkynylpyridine-substituted amide compound according to any one of claims 1 to 8, its N-oxide or a salt thereof. - R1がフッ素原子を表し、
R2がフッ素原子を表し、
R3がメチルを表し、
R4が水素原子を表し、
R6は、C1~C4ハロアルキル又はC3~C6シクロアルキルを表す、
請求項1~8のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩。 R 1 represents a fluorine atom,
R 2 represents a fluorine atom,
R 3 represents methyl,
R 4 represents a hydrogen atom,
R 6 represents C 1 -C 4 haloalkyl or C 3 -C 6 cycloalkyl,
The alkynylpyridine-substituted amide compound according to any one of claims 1 to 8, its N-oxide or a salt thereof. - 下記式(II)で表される、請求項1~10のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシド又はそれらの塩の製造中間体。
Y2は、水素原子を表し、
Y3は、水素原子を表し、
R1は、水素原子、フッ素原子、メチル又はメトキシを表し、
R2は、水素原子、フッ素原子又はメチルを表し、
ここで、R1とR2とは一緒になってエチレン鎖を形成することにより、R1及びR2が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R3は、水素原子、メチル又はエチルを表し、
R4は、水素原子又はメチルを表し、
ここで、R3とR4とは一緒になってエチレン鎖を形成することにより、R3及びR4が結合する炭素原子と共にシクロプロピル環を形成することを表してもよく、
R5は、水素原子、C1~C4アルキル又はシクロプロピルを表し、
R6は、(Z)mによって置換されたフェニル、D-1、D-2、D-6、D-8、D-23又はD-25を表し、
さらに、R1がフッ素原子を表し、R2がフッ素原子を表し、R3がメチルを表し、且つR4が水素原子又はメチルを表す場合には、R6はC1~C4ハロアルキル、C3~C6シクロアルキル又はトリ(C1~C4アルキル)シリルを表してもよく、
Zは、ハロゲン原子、シアノ、ニトロ、C1~C4アルキル、C1~C4ハロアルキル、C1~C6アルコキシ、C1~C4ハロアルコキシ、C1~C4アルキルチオ、C1~C4アルキルスルフィニル、C1~C4アルキルスルホニル、C1~C4ハロアルキルチオ、C1~C4ハロアルキルスルフィニル、C1~C4ハロアルキルスルホニル、D-31、D-32又はD-34を表し、m又はnが2以上を表す場合には、各々のZは互いに同一であっても又は互いに相異なっていてもよく、さらに、2つのZが隣接する場合には、隣接する2つのZは-CH2CH2CH2-、-OCH2O-、-OCH2CH2O-、-OCH=CH-、-SCH=CH-、-N(R13)CH=CH-、-OCH=N-、-SCH=N-、-N(R13)CH=N-、-ON=CH-、-SN=CH-、-N(R13)N=CH-、-N(R13)N=N-、=NN(R13)CH=、=NON=、=NSN=又は-CH=CHCH=CH-を形成することにより、それぞれのZが結合する炭素原子と共に5員環又は6員環を形成してもよく、このとき、環を形成する各々の炭素原子に結合した水素原子はハロゲン原子、メチル又はトリフルオロメチルによって任意に置換されていてもよく、
R13は、C1~C4アルキル又はC1~C4ハロアルキルを表し、
mは、1、2又は3を表し、
nは、0、1、2又は3を表す。] The production intermediate of an alkynylpyridine-substituted amide compound, its N-oxide or a salt thereof according to any one of claims 1 to 10, represented by the following formula (II):
Y 2 represents a hydrogen atom,
Y 3 represents a hydrogen atom,
R 1 represents a hydrogen atom, a fluorine atom, methyl or methoxy,
R 2 represents a hydrogen atom, a fluorine atom or methyl,
Here, R 1 and R 2 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 1 and R 2 are bonded,
R 3 represents a hydrogen atom, methyl or ethyl,
R 4 represents a hydrogen atom or methyl,
Here, R 3 and R 4 may form an ethylene chain to form a cyclopropyl ring with the carbon atom to which R 3 and R 4 are bonded,
R 5 represents a hydrogen atom, C 1 -C 4 alkyl or cyclopropyl,
R 6 represents (Z) m substituted phenyl, D-1, D-2, D-6, D-8, D-23 or D-25;
Further, when R 1 represents a fluorine atom, R 2 represents a fluorine atom, R 3 represents methyl, and R 4 represents a hydrogen atom or methyl, R 6 represents C 1 -C 4 haloalkyl, C May represent 3 to C 6 cycloalkyl or tri (C 1 to C 4 alkyl) silyl;
Z is a halogen atom, cyano, nitro, C 1 -C 4 alkyl, C 1 -C 4 haloalkyl, C 1 -C 6 alkoxy, C 1 -C 4 haloalkoxy, C 1 -C 4 alkylthio, C 1 -C 4 alkylsulfinyl, represents C 1 ~ C 4 alkylsulfonyl, C 1 ~ C 4 haloalkylthio, C 1 ~ C 4 haloalkylsulfinyl, C 1 ~ C 4 haloalkylsulfonyl, D-31, D-32 or D-34, When m or n represents 2 or more, each Z may be the same as or different from each other. Furthermore, when two Zs are adjacent to each other, the two adjacent Zs are − CH 2 CH 2 CH 2- , -OCH 2 O-, -OCH 2 CH 2 O-, -OCH = CH-, -SCH = CH-, -N (R 13 ) CH = CH-, -OCH = N- , -SCH = N-, -N (R 13 ) CH = N-, -ON = CH-, -SN = CH-, -N (R 13 ) N = CH-, -N (R 13 ) N = N -, = = NN (R 13 ) CH, = NON =, = NSN = or by forming a -CH = CHCH = CH-, it A 5-membered ring or a 6-membered ring may be formed together with the carbon atom to which Z is bonded. In this case, a hydrogen atom bonded to each carbon atom forming the ring is arbitrarily selected by a halogen atom, methyl or trifluoromethyl. May be replaced with
R 13 represents C 1 -C 4 alkyl or C 1 -C 4 haloalkyl,
m represents 1, 2 or 3;
n represents 0, 1, 2 or 3. ] - 請求項1~10のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシドおよびそれらの塩からなる群より選ばれる1種以上を有効成分として含有する有害生物防除剤組成物。 A pesticidal composition comprising as an active ingredient at least one selected from the group consisting of an alkynylpyridine-substituted amide compound according to any one of claims 1 to 10, its N-oxide, and a salt thereof.
- 請求項1~10のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシドおよびそれらの塩からなる群より選ばれる1種以上を有効成分として含有する哺乳動物又は鳥類の抗真菌剤又は寄生虫防除剤組成物。 An antifungal agent for mammals or birds comprising as an active ingredient at least one selected from the group consisting of alkynylpyridine-substituted amide compounds according to any one of claims 1 to 10, their N-oxides and their salts Or a parasite control agent composition.
- 哺乳動物又は鳥類に経口投与するための請求項13に記載の抗真菌剤又は寄生虫防除剤組成物。 14. The antifungal or parasite control composition according to claim 13 for oral administration to mammals or birds.
- 哺乳動物又は鳥類に非経口投与するための請求項13に記載の抗真菌剤又は寄生虫防除剤組成物。 The antifungal agent or parasite control agent composition according to claim 13 for parenteral administration to mammals or birds.
- 哺乳動物又は鳥類に非経口投与する方法が、注射による投与である請求項15に記載の抗真菌剤又は寄生虫防除剤組成物。 The antifungal agent or parasite control agent composition according to claim 15, wherein the parenteral administration to mammals or birds is administration by injection.
- 哺乳動物又は鳥類に非経口投与する方法が、経皮投与である請求項15に記載の抗真菌剤又は寄生虫防除剤組成物。 The antifungal or parasite control composition according to claim 15, wherein the method of parenteral administration to mammals or birds is transdermal administration.
- 請求項1~10のいずれか1項に記載のアルキニルピリジン置換アミド化合物、そのN-オキシドおよびそれらの塩からなる群より選ばれる1種以上を有効成分として含有する農園芸用殺菌剤又は殺線虫剤組成物。 An agricultural or horticultural fungicide or killing agent containing as an active ingredient at least one selected from the group consisting of alkynylpyridine-substituted amide compounds according to any one of claims 1 to 10, their N-oxides and their salts Insecticide composition.
- 植物に茎葉散布するための請求項18に記載の農園芸用殺菌剤又は殺線虫剤組成物。 The agricultural and horticultural fungicide or nematicide composition according to claim 18 for spraying foliage on plants.
- 植物が生育する土壌を処理するための請求項18に記載の農園芸用殺菌剤又は殺線虫剤組成物。 The agricultural or horticultural fungicide or nematicide composition according to claim 18 for treating soil in which plants grow.
- 植物の種子、塊根又は根茎を処理するための請求項18に記載の農園芸用殺菌剤又は殺線虫剤組成物。 The agricultural or horticultural fungicide or nematicide composition according to claim 18 for treating plant seeds, tuberous roots or rhizomes.
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016129158 | 2016-06-29 | ||
| JP2016-129158 | 2016-06-29 | ||
| JP2016-197920 | 2016-10-06 | ||
| JP2016197920 | 2016-10-06 | ||
| JP2017006154 | 2017-01-17 | ||
| JP2017-006154 | 2017-01-17 | ||
| JP2017-084394 | 2017-04-21 | ||
| JP2017084394A JP2019142778A (en) | 2016-06-29 | 2017-04-21 | Alkynyl pyridine substituted amide compound and pest control agent |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018003924A1 true WO2018003924A1 (en) | 2018-01-04 |
Family
ID=60785439
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/023955 WO2018003924A1 (en) | 2016-06-29 | 2017-06-29 | Alkynylpyridine-substituted amide compound and pest control agent |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2018003924A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10029986B2 (en) * | 2014-02-18 | 2018-07-24 | Nissan Chemical Industries, Ltd. | Alkynyl pyridine-substituted amide compound and pesticide |
| US20190382372A1 (en) * | 2018-06-15 | 2019-12-19 | Nissan Chemical Corporation | Method for producing 5-alkynyl pyridine compound |
| WO2020002593A1 (en) | 2018-06-29 | 2020-01-02 | Intervet International B.V. | Compound for use against helminthic infection |
| US10710977B2 (en) | 2017-02-08 | 2020-07-14 | Nissan Chemical Corporation | Method for producing geometrical isomer of oximino compound |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009539791A (en) * | 2006-06-08 | 2009-11-19 | シンジェンタ パーティシペーションズ アクチェンゲゼルシャフト | N- (1-alkyl-2-phenylethyl) -carboxamide derivatives and methods for using them as fungicides |
| JP2013541513A (en) * | 2010-09-02 | 2013-11-14 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Novel compounds, pharmaceutical compositions and uses thereof |
| WO2014173921A1 (en) * | 2013-04-26 | 2014-10-30 | Syngenta Participations Ag | Carboxamides as pesticidal compounds |
| WO2015125824A1 (en) * | 2014-02-18 | 2015-08-27 | 日産化学工業株式会社 | Alkynylpyridine-substituted amide compound and noxious organism control agent |
| JP2017036275A (en) * | 2015-08-10 | 2017-02-16 | 日産化学工業株式会社 | Alkynyl pyridine substituted amide compound and pest control agent |
| JP2017039722A (en) * | 2015-08-19 | 2017-02-23 | 日産化学工業株式会社 | Alkynyl pyridine substituted pyrazole amide compound and pest control agent |
-
2017
- 2017-06-29 WO PCT/JP2017/023955 patent/WO2018003924A1/en active Application Filing
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009539791A (en) * | 2006-06-08 | 2009-11-19 | シンジェンタ パーティシペーションズ アクチェンゲゼルシャフト | N- (1-alkyl-2-phenylethyl) -carboxamide derivatives and methods for using them as fungicides |
| JP2013541513A (en) * | 2010-09-02 | 2013-11-14 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | Novel compounds, pharmaceutical compositions and uses thereof |
| WO2014173921A1 (en) * | 2013-04-26 | 2014-10-30 | Syngenta Participations Ag | Carboxamides as pesticidal compounds |
| WO2015125824A1 (en) * | 2014-02-18 | 2015-08-27 | 日産化学工業株式会社 | Alkynylpyridine-substituted amide compound and noxious organism control agent |
| JP2017036275A (en) * | 2015-08-10 | 2017-02-16 | 日産化学工業株式会社 | Alkynyl pyridine substituted amide compound and pest control agent |
| JP2017039722A (en) * | 2015-08-19 | 2017-02-23 | 日産化学工業株式会社 | Alkynyl pyridine substituted pyrazole amide compound and pest control agent |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10029986B2 (en) * | 2014-02-18 | 2018-07-24 | Nissan Chemical Industries, Ltd. | Alkynyl pyridine-substituted amide compound and pesticide |
| US10710977B2 (en) | 2017-02-08 | 2020-07-14 | Nissan Chemical Corporation | Method for producing geometrical isomer of oximino compound |
| US20190382372A1 (en) * | 2018-06-15 | 2019-12-19 | Nissan Chemical Corporation | Method for producing 5-alkynyl pyridine compound |
| US10730852B2 (en) * | 2018-06-15 | 2020-08-04 | Nissan Chemical Corporation | Method for producing 5-alkynyl pyridine compound |
| WO2020002593A1 (en) | 2018-06-29 | 2020-01-02 | Intervet International B.V. | Compound for use against helminthic infection |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6330928B2 (en) | Oxime-substituted amide compounds and pest control agents | |
| US10321683B2 (en) | Insecticidal, miticidal, nematicidal, molluscicidal, microbicidal, or bactericidal composition and method for controlling pest | |
| US10029986B2 (en) | Alkynyl pyridine-substituted amide compound and pesticide | |
| TWI670254B (en) | Terpene substituted guanamine compounds and pest control agents | |
| JP2014114246A (en) | Insecticidal, acarid killing, nematode killing, mollusk killing, disinfection or bacterium killing composition and pest control method of pest | |
| WO2018003924A1 (en) | Alkynylpyridine-substituted amide compound and pest control agent | |
| JP2014037382A (en) | Insecticidal, miticide, nematicide, molluscicide, germicide and bactericide composition, and method of controlling pest | |
| JP2017039722A (en) | Alkynyl pyridine substituted pyrazole amide compound and pest control agent | |
| JP2020186245A (en) | Oxime substituted amide compound and pest control agent | |
| JP2017036275A (en) | Alkynyl pyridine substituted amide compound and pest control agent | |
| JP2015155399A (en) | Oxime substituted pyrazole amide compound and pest controlling agent | |
| WO2018124088A1 (en) | Alkylphenyl-substituted amide compound and pest control agent | |
| JP2020203897A (en) | Oxime-substituted amide compounds and pest control agents | |
| JP2019112344A (en) | Alkynylpyridine-substituted amide compound and noxious organism control agent | |
| JP2015214535A (en) | N-alkenyl-substituted amide compound and noxious organism control agent | |
| JP2019151593A (en) | Phenyl acetylene-substituted amide compound and pest control agent | |
| JP2019142778A (en) | Alkynyl pyridine substituted amide compound and pest control agent | |
| JP2020029402A (en) | Alkylphenyl-substituted amide compound and pest control agent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17820278 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17820278 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: JP |