Management Implications
Ventenata dubia (ventenata) has expanded within North America from an early report in northern Idaho in 1956 to the northeastern and northwestern Canadian provinces and at least 6 additional western states. The North American distribution is within the northern and southern latitudes of its Eastern Hemisphere distribution. The distribution of V. dubia likely will continue to expand, so land managers should be vigilant to discover new infestations in states adjacent to those currently infested. Examination of herbaria and written reports established that V. dubia is well distributed but infrequently found from Spain to the Caspian Sea. There may be one or more organisms limiting the frequency of occurrence in the native range, suggesting biological control holds some promise. Biological control of V. dubia would expand the control options for a well-rounded integrated pest management approach.
Introduction
Ventenata [Ventenata dubia (Leers) Coss.] is a relatively new invader in the inland Pacific Northwest (PNW) of North America. It was first reported in northern Idaho (Kootenai County) in 1956 (Northam and Callihan Reference Northam and Callihan1994). Before the year 2000 it was not reported as weedy. In recent years it has become a serious invader of pastures, Conservation Reserve Program fields, and hayfields (Mackey Reference Mackey2014; Wallace et al. Reference Wallace, Pavek and Prather2015). Perennial grass foliar cover increased 10% to 20% when V. dubia was controlled, suggesting the annual grass reduced productivity of perennial grasses (Wallace et al. Reference Wallace, Pavek and Prather2015). Timothy (Phleum pratense L.) hay production declined from 4,540 kg ha−1 to 2,270 kg ha−1 when V. dubia was not controlled (Mackey Reference Mackey2014). The negative impacts of V. dubia have been recognized in the West, as indicated by its addition to state noxious weed lists in Nevada, Oregon, Utah, Washington, and Wyoming.
Natural enemies likely limit the abundance and distribution of V. dubia in its native range, as is suggested by its listing as rare or endangered in some areas of Europe (Bicknell Reference Bicknell1896; Frey and Paszko Reference Frey and Paszko1998; Merce et al. Reference Merce, Oarcea and Adrian2007). Arthropods may be involved in regulating abundance and distribution of V. dubia, yet the species has relatively high silica content (DiTomaso et al. Reference DiTomaso, Kyser, Oneto, Wilson, Orloff, Anderson, Wright, Roncoroni, Miller, Prather, Ransom, Beck, Duncan, Wilson and Mann2013), and silica can be a deterrent to herbivory (Keeping and Kvedaras Reference Keeping and Kvedaras2008). While plant pathogens have been introduced to North America for classical biological control (Trujillo Reference Trujillo2005), to date no pathogen of grasses has been intentionally introduced, and if one were proposed, it would receive extra scrutiny, as so many of our agricultural crops are grasses. Currently, pathogen use against grass weeds in the United States has been limited to use of native fungi, generally applied as bioherbicides on targeted species and requiring regular applications to maintain control of the target species (Chandramohan and Charudattan Reference Chandramohan and Charudattan2001).
Biogeography is important for understanding risk of invasion (Pheloung et al. Reference Pheloung, Williams and Halloy1999). Gaining an understanding of the latitudinal range of V. dubia’s distribution in its native range would increase our understanding of risk of additional invasive spread within North America. In addition, collecting distributional data provides parameters for exploration for biological control agents. We investigated the distribution of V. dubia across its suspected native range to assist in discovery of biological control agents and to provide insight into risk of invasion. We also conducted surveys of V. dubia populations for evidence of pathogenic expression in the PNW and searched the literature for documentation of fungi that have been observed on V. dubia.
Materials and Methods
Plants were collected to look for signs and symptoms of disease within the introduced range in the PNW. In 2014, samples of V. dubia were collected across a broad geographic area with a minimum of 6 individual plants per site, with each plant separately bagged, from 41 sites ranging from Pend Oreille County, WA, in the north, south to Grant County, OR, and east to Elmore County, ID, with one sample from Gallatin County, MT. In addition, roughly 1,000 V. dubia plants near the campus of the University of Idaho (Moscow, ID) were observed for pathogens each year in early June from 2016 to 2018. We visually examined these plants for Septoria ventenatae Sandu, which causes necrotic leaf lesions in V. dubia (Radulescu et al. Reference Radulescu, Negru and Docea1973), but found no evidence of these lesions. These lesions are very similar to the lesions that other species of Septoria cause on other plant hosts. If we had found suspected pathogens, we would have induced sporulation on specimens by moist incubation and then assessed the spores morphologically, and cultures would have been sequenced to identify the pathogen. Tilletia species replace the seeds of their hosts with their own spores (Denchev and Denchev Reference Denchev and Denchev2018; Scholz and Scholz Reference Scholz and Scholz1988). We surveyed for this disease, commonly called smut or bunt, searching for dark seeds filled with a powdery mass, but failed to find any evidence of its presence on V. dubia. We also looked for rust, although no rust has ever been reported on V. dubia. We found no evidence of rust on V. dubia plants examined near the University of Idaho campus.
Location of geographic records was accomplished using the search engines Google Books and Google Scholar and the key words “Ventenata dubia” and its synonyms “Avena dubia,” “Ventenata avenacea,” “Avena tenuis,” and “Gaudinia tenuis.” We also searched regional monographs across the Eastern Hemisphere that contained V. dubia through libraries at University of Idaho, Washington State University (Pullman, WA), and collections of USDA-ARS Plant Introduction in Pullman, WA. Scientists who have published materials that included V. dubia were contacted, as were herbaria located in areas where V. dubia has been reported to occur.
Results and Discussion
Our disease surveys did not reveal any pathogens of V. dubia in the PNW. Environmental conditions during our 2014 surveys and our 2016 to 2018 observations near the University of Idaho campus were conducive to disease expression: other annual grass species did exhibit symptoms of pathogen infection. For example, spikelets of downy brome (Bromus tectorum L.) frequently were affected by a smut. Ventenata dubia has been identified as a host for the barley yellow dwarf virus in the PNW but without any expression of symptoms (Ingwell and Bosque‐Pérez Reference Ingwell and Bosque‐Pérez2015).
Classical biological control of V. dubia might be attempted should a pathogen be found that limits plant abundance in its native range. Pathogens could contribute to its lack of abundance, as suggested by the listing of V. dubia as rare or endangered in some areas of Europe. For example, it is rare in the following areas: between Coldirodi and San Romolo, Italy (Bicknell Reference Bicknell1896), Poland (Frey and Paszko Reference Frey and Paszko1998), the Codru-Moma Mountains in Romania (Merce et al. Reference Merce, Oarcea and Adrian2007), and Andalusia, Spain (M Vizoso, personal communication). Ventenata dubia is on a red list and is an endangered species in the state of Hesse in Germany (Uebeler et al. Reference Uebeler, Ehmke, Nawrath, König and Wittig2008), and it is an endangered species in Slovakia (Dúbravková and Jaroslav Reference Dúbravková and Jaroslav2012; Turis et al. Reference Turis, Kliment, Ferakova, Dite, Elias, Hrivnák, Košťál, Šuvada, Mráz and Bernátová2014; P Eliášjun, personal communication). It is extremely rare and critically threatened in the Czech Republic (Danihelka et al. Reference Danihelka, Chrtek and Kaplan2012; K Zdeněk, personal communication), and it is critically endangered in the Nature Park Papuk, Slavonia, eastern Croatia (Pandža Reference Pandža2010). Other factors may also limit its abundance in these areas.
Plant pathogens have been used for biological control and are becoming of increasing importance in biocontrol of invasive plants (Morin et al. Reference Morin, Evans and Sheppard2006). Species of rusts are highly specific to the plant species they infect (Kolmer et al. Reference Kolmer, Ordonez and Groth2009), and that specificity makes it unlikely that non-target plants would be affected. One example of a rust used as a biological control agent is Puccinia chondrillina Bubak for control of rush skeletonweed (Chondrilla juncea L.), introduced to both North America and Australia (Lee Reference Lee1986). Globally, there are records of just three species of fungal pathogens of V. dubia: Septoria ventenatae has been reported on V. dubia in Romania (Farr and Rossman n.d.; Radulescu et al. Reference Radulescu, Negru and Docea1973), Tilletia fusca Ellis & Everh. in Germany (Farr and Rossman n.d.; Scholz and Scholz Reference Scholz and Scholz1988), and Tilletia elizabethae T. Denchev & Denchev in Slovakia (Denchev and Denchev Reference Denchev and Denchev2018). No rusts have been reported for V. dubia.
Occurrence records of V. dubia, available from the Global Biodiversity Information Facility (GBIF) (Figure 1), were entirely from Europe; no occurrences were reported from North Africa. Because V. dubia was collected in Algeria by Cosson in the mid-19th century (Cosson and de Maisonneuve Reference Cosson and de Maisonneuve1854), it was clear that the GBIF data did not include those records. We thus sought other records from North Africa. Cosson had found the species in the Djebel Herfah and Djebel Tougour Mountains of Algeria, and based his new name for this species (Ventenata dubia rather than Avena dubia) on these collections. We found a second record from Mont de Bellezm from Algeria more than a century later (Quezel and Santa Reference Quezel and Santa1962). Recently, V. dubia was also reported in Morocco (T Seipel, Montana State University, personal communication). Apart from the three records from Algeria and Morocco, we could find no other records from North Africa. In fact, Salima Benhouhou (École Nationale Supérieure Agronomique, Algeria) stated that V. dubia is currently rare in Algeria. We found no evidence of V. dubia in other countries of North Africa (Egypt, Libya, or Tunisia).
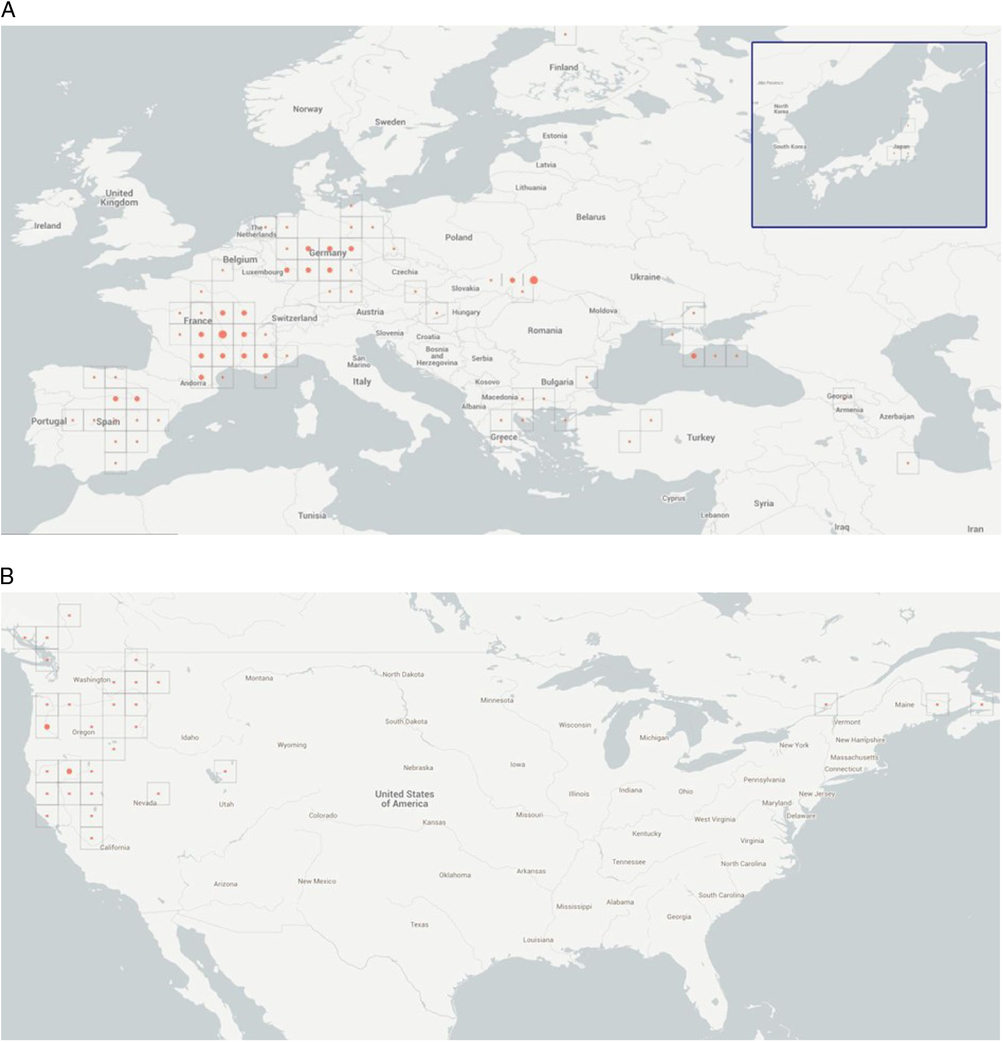
Figure 1. (A) Eastern Hemisphere locations of Ventenata dubia range from Spain to the Caspian Sea and from Norway to Finland. Two locations are in Japan. The three symbols are circles denoting number of records: small circle indicates fewer than 10 records; medium circle indicates 10 to 20 records; and large circle indicates more than 20 records. (B) In the Western Hemisphere, the new range locations span British Columbia, Canada, to California, USA, and from Washington, USA, to Nova Scotia, Canada (GBIF 2015).
Occurrence records of V. dubia available from GBIF indicate that the species is widespread across Europe (Figure 1; Supplementary Table S1). Ventenata dubia has been found in the following European countries: Austria, Bulgaria, Croatia, the Czech Republic, France, Germany, Greece, Hungary, Italy, Macedonia, Moldova, Montenegro, the Netherlands, Poland, Romania, Serbia, Slovakia, Spain, the Ukraine, the United Kingdom, and Finland. Ventenata dubia was especially common in Spain, France, and Germany (Supplementary Table S1). Ventenata dubia is also present in southwest Asia in Azerbaijan, Iran, Russia, and Turkey. There are no records of V. dubia in the Middle Eastern countries: Saudi Arabia, Kuwait, Iraq, Syria, and Israel (Supplementary Table S1).
Ventenata dubia is without pathogens that we have been able to detect in the PNW of North America. Globally, there are so few records in European literature or personal communications that it is not meaningful to draw any conclusions about pathogen release. There may be pathogens that limit V. dubia in its native range that have not been reported, and exploration for potential biological control agents should focus on those areas where it has been frequently reported but is also considered limited in its distribution.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2019.24
Acknowledgments
MA would like to thank the Princess Nora Bint AbdulRahman University, and the Government of Saudi Arabia for financial support. We also wish to thank Sara Ashiglar for her contributions to the disease surveys. No conflicts of interest have been declared.





