Jennwenomyces navicularis (R.F. Castañeda & Heredia) Goh & C.H. Kuo, comb. nov. (Figs. 2, 3, 4, and 5).
MycoBank number: MB 835027; Index Fungorum number: IF 835027; Facesoffungi number: FoF;
≡ Belemnospora navicularis R.F. Castañeda & Heredia, Cryptog. Mycol. 21: 221 (2000)
Colonies on natural substratum effuse, velvety, golden brown. Mycelium partly immersed in the substratum and part- ly superficial, composed of branched, septate, pale brown to brown, 3–5-μm-wide hyphae, often forming aerial hyphae. Conidiophores are borne as lateral branches from aerial hy- phae or rarely arising directly from the immersed substratum, solitary, unbranched, cylindrical, (63–)70–86.5(–146)-μm long, 3.5–4.5(–5.5)-μm wide, uniform in width, straight or rarely slightly flexuous, 3–7-septate, pale brown to brown, paler towards the apex, with regular multiple terminal percurrent extensions. Conidiogenous cells holoblastic, monoblastic, integrated, terminal, percurrently extending and annellate at the apex. Conidia acrogenous, solitary, dry, se- ceding schizolytically, straight or sometimes very slightly bent, navicular or obclavate to cylindro-clavate, obconic at the base which is (1.5–)2.0(–2.5)-μm wide, obtusely conic or rounded at the apex, smooth-walled when viewed under the light microscope, finely verruculose when viewed under the scanning electron microscopy (SEM), 4–5(–6)-euseptate, (27–)33–51 × (5–)6–8.5(–9) μm, pale olivaceous to olivaceous brown, versicolored, with the end cells paler. Teleomorph unknown.
Cultural characteristics: Conidia germinated readily but colony is slow growing, with a growth rate of 0.5 mm/day. Colonies on PDA reached 18 mm diameter in 6 weeks at 20 °C. On PDA plates, colonies attained 4.7 cm diameter in 100 days at 20 °C, effuse, funiculose to cottony, golden brown, with a narrow, white fimbriate margin and somewhat cottony brown aerial mycelium, reverse side similar in appearance, darker at the center (Fig. 2). Mycelium consists of smooth, branched, septate hyphae. Hyphae hyaline or pale olivaceous, (2.5–)3.0(–3.5) μm wide. Chlamydospores were formed in large quantity in culture, terminal and intercalary on maturing hyphae, unicellular, borne singly or sometimes in chains on older hyphae, sometimes detached and becoming solitary, pale brown, thick-walled, globose (7.5–11 μm). Conidial production lacking on PDA, Malt Extract Agar (MEA), Sach’s Agar (SA), and V8 agar.
Specimens examined: Taiwan, Chiayi County: Fanlu Township, Dahu Village (23.481697–120.621649, 778 m a.s.l.), on a decaying culm of Miscanthus floridulus (Poaceae) submerged in a freshwater stream, leg. Chiao- Chih Chien & Chang-Hsin Kuo, 13 May 2016, NCYU-JW1 (TNM: F0033449); BCRC: FU30873; sequences: ITS = MT224911 and MT224912, the two sequences were derived from two individual conidia (single-spore cultures); LSU = MT224910. ibid. (23.476216–120.642116, 575 m a.s.l.), ibid. 5 Feb. 2017, NCYU-H1-1 (TNM: F0033450); BCRC: FU30872; sequences: ITS = MT224913, MT224914, the two sequences were derived from two individual conidia (single- spore cultures); LSU = MT224909.
Notes: We have been trying to procure the type specimen of Belemnospora navicularis from Mexico but in vain. The current transfer of this taxon to Jennwenomyces is, however, based on morphological and molecular data of the two Taiwanese collections, with reference to the original description and illustration of the Mexican collection given by Castañeda-Ruiz and Heredia (2000). Jennwenomyces navicularis is, therefore, represented by these three collections, which are literally three strains of the same fungus. A morphological comparison of these three strains is given in Table 3. There exist intraspecific variations among the three strains. Although the conidial size and septation of these strains overlap, the Mexican strain has conidiophores, which are comparatively more robust (100–188 × 8–10 μm). The strain NCYU-JW1 is more similar to the Mexican strain in conidial morphology than the strain NCYU-H1-1, with statistical support (Table 3). The Mexican strain was reported to have an association with the setae of an unidentified fungus occurring on decaying branches of the tree fern Cyathea (Castañeda-Ruiz and Heredia 2000). No such mycophilic association was observed in the two Taiwanese collections. The conidia of J. navicularis (NCYU-H1-1) are finely verruculose as visualized under the SEM (Fig. 5). Such roughened wall texture is however invisible under the light microscope.
Matsushima described and illustrated Sporidesmium coprophilum Matsush., which was collected from hare dung (Matsushima 1975) and decaying palm stems (Matsushima 1980, 1993). Conidia of S. coprophilum are 3–5- euseptate, with paler end cells; fusiform-cylindrical with a broadly rounded apex and obconic base; and thus resemble those of J. navicularis. However, conidiophores in S. coprophilum are not terminally annellate, and are shorter (70–90 μm) and narrower (3–4 μm) when compared with those of J. navicularis. In their account of Sporidesmium species and related genera, Wu and Zhuang (2005) considered S. coprophilum as a distinct species and suggested to retain it in Sporidesmium.
Table 3 Comparison of Taiwanese and Mexican collections of Jennwenomyces navicularis
| Specimen No | NCYU-JW1 (TNM: F0033449) | NCYU-H1-1 (TNM: F0033450) | 862-2 XAL (MUCL: 41734) |
| Collection locality | Dahu Village, Taiwan | Dahu Village, Taiwan | Veracruz, Huatusco, Mexico |
| Conidiophores | Borne as side branches
from aerial hyphae, 63–108 × 3.5–4.5 μm |
Arising directly from substratum, 82.5–146 × 4.5–5.5 μm | Arising directly from substratum, 100–188 × 8–10 μm |
| Conidial sizeθ | 33–51 × 6–8.5 μm
(X̄ = 41 × 7.5 μm, n = 30) a |
27–48 × 5.5–9 μm (X̄ = 36.5 × 7.5
μm, n = 90) b |
29–38(–45) × 5–7 μm (X̄ = 38 × 7 μm, n = 9) a |
| Mean L/W ra- tioϕ | 5.6 ± 0.15 μm A | 5.0 ± 0.07 μm B | 5.5 ± 0.15 μm A |
| Conidial shape | Navicular, fusiform, obclavate | Cylindrical, cylindro-ellipsoidal | Navicular, broadly fusiform |
| Apex of conidia | Conical | Bluntly conical or rounded | Conical |
| No. of conidial septa | (4–)5(–6) | 4 | 4(–5) |
| Natural substrata | Submerged decaying culms
of Miscanthus floridulus |
Submerged decaying culms of Miscanthus floridulus | Decaying branch of Cyathea (a tree fern) |
| Reference | This paper | This pape | Castañeda-Ruiz and Heredia 2000 |
θ Mean conidial sizes with the same letter in superscript are not significantly different from each other according to the LSD test (P = 0.05)
ϕ Mean length/width (L/W) ratios with the same letter in superscript are not significantly different from each other according to the LSD test (P = 0.05)
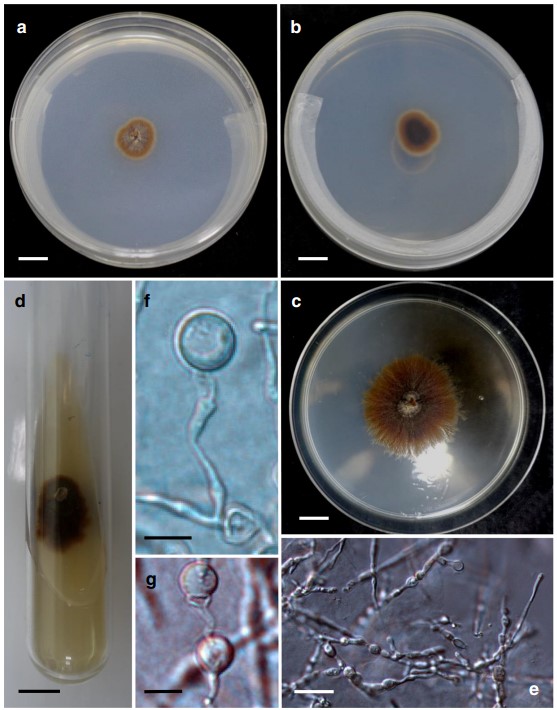
Fig. 2 Jennwenomyces navicularis (NCYU-JW1, TNM: F0033449). a–d Colonies on PDA. a 14-day old, surface view. b 14-day old, reversed side. c, d 30-day old, surface view. e Mycelium showing developing chlamydospores. f, g Chlamydospores. Scale bars a–d = 1 cm; e = 20 μm; f, g = 10 μm
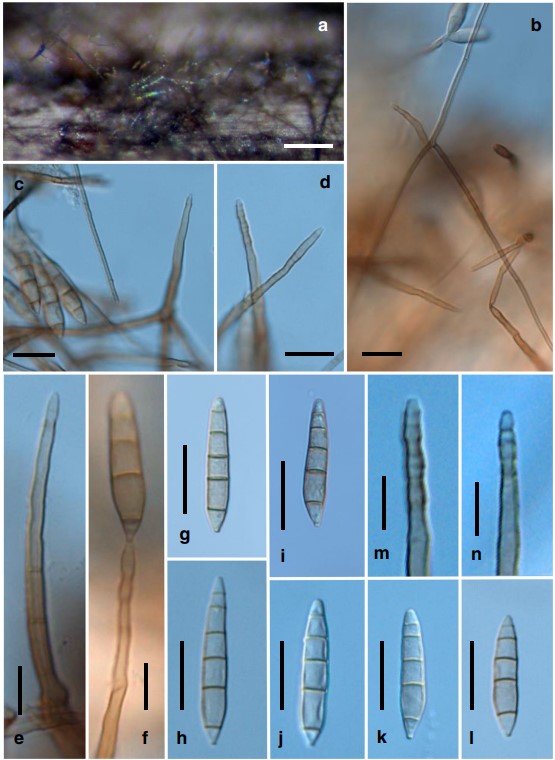
Fig. 3 Jennwenomyces navicularis (NCYU-JW1, TNM: F0033449). a Colonies on the natural substratum. b, c Aerial hyphae, conidiophores, and conidia. d, e Conidiophores with apices showing percurrent extensions. f Conidiophore showing percurrent extensions and a developing conidium. g–l Conidia. m, n Apices of conidiophores showing percurrent extensions. Scale bars a = 200 μm; b–d, g–l = 20 μm; e, f, m, n = 10 μm
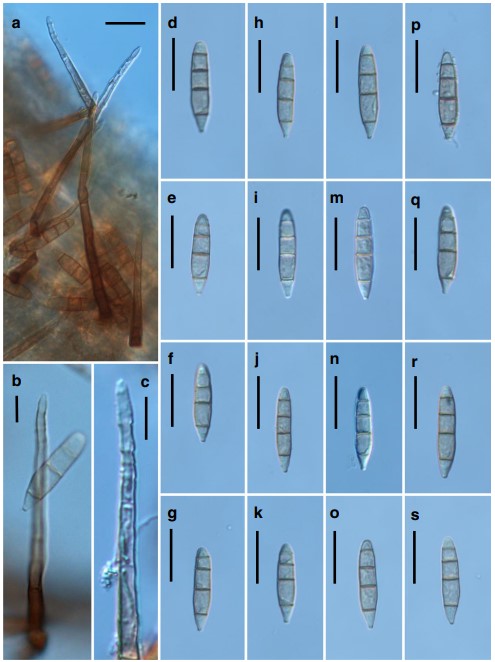
Fig. 4 Jennwenomyces navicularis (NCYU-H1-1, TNM: F0033450). a Conidiophores and conidia. b Conidiophore with apex showing percurrent extensions and a conidium. c Apex of conidiophore showing percurrent extensions. d–s Conidia. Scale bars a = 20 μm; b, c = 10 μm; d–s = 20 μm
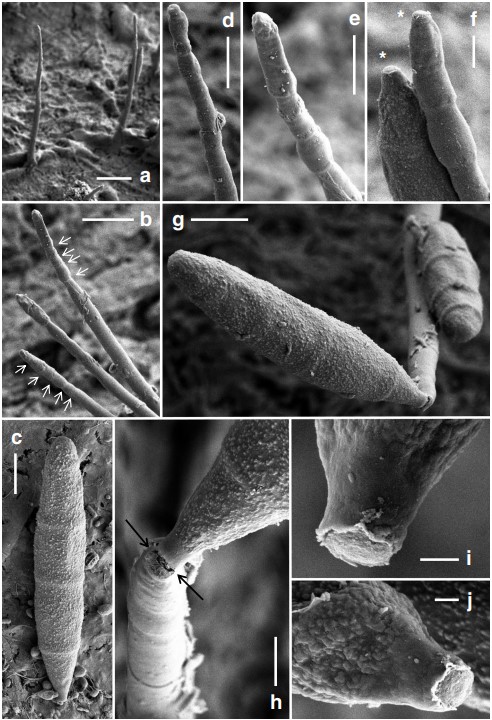
Fig. 5 Jennwenomyces navicularis (NCYU-H1-1, TNM: F0033450). Scanning electron micrographs. a Conidiophores on the natural substratum. b Detail of conidiophore. Arrows indicate apical percurrent extensions. c Conidium with four septa, obconic base, rounded apex, and finely verruculose wall. d, e Apical portion of conidiophores with percurrent extensions. f Apex of a conidiophore showing percurrent extensions and the basal portion of a conidium.
Asterisks near the tip of the conidiophore and at the hilum of the conidium respectively denote the result of schizolytic conidial secession. g A conidiophore with two conidia attaching to it. Note that the wall of the conidia is finely verruculose. h Apical portion of a conidiophore with a conidium in the process of schizolytic secession (arrowed). i, j Basal portion of two conidia showing a flushed hilum resulting from schizolytic conidial secession. Scale bars a = 20 μm; b = 10 μm; c–e, g =5 μm; f, h = 2 μm; i, j = 0.5 μm
