Epithelial Haven and Autophagy Breakout in Gonococci Infection
- Departamento de Genética, Ecologia e Evolução, Instituto de Ciencias Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
The World Health Organization (WHO) has estimated that in 2016, there were 87 million new cases of gonorrhea. Gonorrhea is caused by the sexually transmitted human-exclusive agent Neisseria gonorrhoeae, a Gram-negative diplococcus that causes cervicitis in females and urethritis in males and may lead to more severe complications. Currently, there is no vaccine against N. gonorrhoeae. Its resistance to antibiotics has been increasing in the past few years, reducing the range of treatment options. N. gonorrhoeae requires a surface protein/receptor (Opa proteins, porin, Type IV pili, LOS) to adhere to and invade epithelial cells. During invasion and transcytosis, N. gonorrhoeae is targeted by the autophagy pathway, a cellular maintenance process which balances sources of energy at critical times by degrading damaged organelles and macromolecules in the lysosome. Autophagy is an important host defense mechanism which targets invading pathogens. Based on transmission electron microscopy (TEM) analysis, the intracellular bacteria occupy the autophagosome, a double-membraned vesicle that is formed around molecules or microorganisms during macroautophagy and fuses with lysosomes for degradation. Most of the gonococci end up in autolysosomes for degradation, but a subpopulation of the intracellular bacteria inhibits the maturation of the autophagosome and its fusion with lysosomes by activating mTORC1 (a known suppressor of the autophagy signaling), thus escaping autophagic elimination. This mini review focuses on the cellular features of N. gonorrhoeae during epithelial cell invasion, with a particular focus on how N. gonorrhoeae evades the autophagy pathway.
Introduction
Neisseria gonorrhoeae, also known as gonococcus, is the causative agent of gonorrhea, a sexually transmitted infection that occurs exclusively in humans. In 2016, The World Health Organization (WHO) estimated that 86.9 million new cases of gonorrhea occurred globally. In 2017, the WHO included N. gonorrhoeae in its list of bacteria for which new antibiotics are urgently needed1. N. gonorrhoeae is a major global public health concern due to its increasing resistance to antibiotics, which leads to the possibility of untreatable gonorrhea infections (World Health Organization [WHO], 2017; Rowley et al., 2019). N. gonorrhoeae is a Gram-negative diplococcus that usually infects urogenital epithelia, but it is also able to infect rectal, pharynx, and conjunctival mucosa (Britigan et al., 1985).
At the sites of gonococci colonization, the activation of the innate immune response causes the symptoms of gonorrhea, including discomfort in the affected area and purulent urethral or cervical discharge. Acute gonorrhea results in an intensely inflammatory exudate, which contains macrophages, exfoliated epithelial cells, and polymorphonuclear neutrophils (Hook, 2012). Many studies have shown that asymptomatic infections are common in both men and women, but are more prevalent in women than in men (Muzny et al., 2017). This may be due to the relative ease in diagnosing symptoms in men, as the purulent exudate causes painful urination in men. Symptoms in women are mostly unnoticed and/or non-specific and are often mistaken for symptoms of bacterial vaginosis, hormonal alterations, or normal vaginal secretions (Grimley et al., 2006; Quillin and Seifert, 2018). Untreated gonorrhea may result in pelvic inflammatory disease, infertility, ectopic pregnancies, or neonatal blindness as a consequence of vertical transmission. In addition, untreated gonorrhea can lead to gonococcal dissemination and enhanced transmission of HIV (Masi and Eisenstein, 1981; Sandstrom, 1987; Little, 2006).
Adherence and Invasion
Neisseria gonorrhoeae adheres to urogenital tract by attaching to surface structures as Type IV pili (Tfp) (Pearce and Buchanan, 1978), opacity (Opa) proteins, LOS, or outer membrane protein porin (PorB) (Stern et al., 1986; van Putten and Paul, 1995). Type IV pili (Tfp) mediate initial cellular adherence, its retraction brings the bacteria closer to the epithelial cell surface and activates Ca2+ flux, PI3K/Akt, and the ERK/MAP kinase pathways (Ayala et al., 2005; Lee et al., 2005). The Opa family of proteins includes two classes: the Opa50 protein, which binds to surface heparan sulfate proteoglycan (HSPG) receptors; and Opa51-60, which bind to carcinoembryonic antigen-related cellular adhesion molecules (CEACAMs) and mediate the complex interactions between the gonococci and epithelial cells or phagocytes after Tfp adhesion (van Putten and Paul, 1995). After adhesion, N. gonorrhoeae replicates in microcolonies, which are collections of bacteria formed from a few diplococci after the initial adhesion on epithelial cells, competes with the local microbiota, and is able to invade and disseminate by transmigrating across the epithelial cell monolayer (Quillin and Seifert, 2018). Gonococcal microcolonies can move and promote interaction between bacterial cells, helping them to deal with environmental pressures. In addition, microcolonies play a role in gonococci-host interactions (Higashi et al., 2007).
The gonococci initiate cross-talk with host cells using multiple surface molecules, resulting in activation of signaling pathways and changes in gene expression in the host cells and in the gonococci themselves (Stein et al., 2015). Interactions between CEACAMs and Opa proteins can induce phagocytosis, triggering the engulfment of the bacteria into the epithelial cells and neutrophils (Fox et al., 2014).
Neisseria gonorrhoeae facilitates its invasion into host cells by modulating the activity and distribution of host epidermal growth factor receptor (EGFR), which is a signaling receptor that pathogens can manipulate for their survival in host cells (Zhang et al., 2004). The gonococcal microcolonies recruit EGFR to the site of microcolony adherence. The kinase activity of EGFR is necessary for gonococcal invasion into epithelial cells. The gonococci activate EGFR by inducing the production of EGFR ligands. This suggests that microcolonies are important for invasion of N. gonorrhoeae into epithelial cells. Studies have shown that EGFR kinase inhibition reduces gonococcal invasion, further indicating an important role for EGFR in gonococci invasion (Swanson et al., 2011).
Apicella et al. (1996) analyzed urethral exudates from men infected with N. gonorrhoeae and observed that bacteria were clustered within vacuoles upon invasion. However, they also found bacteria in the cytosol without evidence of a surrounding vacuole. Harvey et al. (1997) studied primary human urethral epithelial cell cultures infected with N. gonorrhoeae and showed that after the invasion, the gonococci appeared to reside and multiply within vacuoles close to the apical layers of the epithelial cells and were later released from the epithelial cell monolayer either in membrane-bound vacuoles or after rupturing the infected cells (Mosleh et al., 1997). Gonococcal infection of the urethral epithelium modulates host anti-apoptotic factors, thereby promoting bacterial survival within the epithelial tissue (Binnicker et al., 2003).
What Is Autophagy?
Autophagy is a cellular mechanism that can be upregulated in response to stress conditions and lack of nutrients. It is a pathway that delivers organelles and cytoplasmic proteins to the lysosome for degradation (Yang et al., 2005).
Mammalian cells have three types of autophagy: microautophagy, in which the lysosome captures the molecules by invagination of the lysosomal membrane; chaperone-mediated autophagy (CMA), in which chaperone proteins identify molecules that contain a particular pentapeptide motif and transport them directly to lysosomes; and macroautophagy, referred to in this text as autophagy, which involves the formation of cytosolic vesicles to transport the molecules, including damaged organelles or pathogens, to the lysosome for degradation. Upon activation of autophagy, a membrane structure known as a phagophore forms and expands, eventually closing to form a double-membrane vesicle called autophagosome (Parzych and Klionsky, 2014).
Autophagosomes fuse with lysosomes (autolysosomes), and the sequestered cargo is digested. The initial formation of the autophagosomes requires the activation of the unc-51-like kinase 1 (ULK1) complex. Then, ULK activates the Vps34 (class III phosphatidylinositol 3-kinase) complex, which comprises Vps34, associated to Beclin 1, VPS15, and ATG14L, triggering vesicle nucleation. The subsequent steps involve the ATG12 and the LC3 (microtubule-associated light chain 3) conjugation systems. Both systems promote the elongation of the isolation membrane (Joubert et al., 2009; Rubinsztein et al., 2012).
An important regulator of autophagy is the target of rapamycin (TOR), which inhibits multiple autophagy-promoting proteins via phosphorylation. TOR is a phosphatidylinositol-related kinase involved in regulatory pathways that control the responses to nutrients and energy metabolism changes. In mammalian cells, mTOR nucleates two structurally and functionally different complexes termed mTORC2, which regulates cytoskeleton organization and cell survival, and mTORC1, which is essential to sense and respond to intracellular and extracellular nutrients, amino acids, growth factors, energy, and oxygen levels. In the presence of stimuli, mTORC1 phosphorylates the ULK1 complex, inhibiting autophagy. On the contrary, when mTORC1 is inactive, ULK1 is released and autophagy is initiated (Yang et al., 2005; Huang and Brumell, 2014; Rabanal-Ruiz et al., 2017).
Although autophagy can be induced to control infection upon intracellular pathogen invasion, many pathogens have developed strategies to avoid or subvert autophagy for their own benefit. Bacteria are targets of selective autophagy, a process known as xenophagy. Xenophagy is a mechanism that targets and removes pathogens after cellular invasion (Bauckman et al., 2015; Escoll et al., 2016). It can be induced upon bacterial infection and involves the formation of autophagosomes, which target bacteria and transport them to lysosomes for degradation. Some bacteria can inhibit autophagy signaling pathways, avoid autophagy recognition, inhibit fusion of the autophagosome with the lysosome, or escape targeting by interfering with the autophagy machinery (Gomes and Dikic, 2014; Bauckman et al., 2015).
CD46 acts as an immunomodulatory protein and plays a role in autophagy signaling. CD46 is a glycoprotein expressed on the surface of every nucleated human cell, and it has isoforms that contain one of two short cytoplasmic tails (cyt), cyt-1 or cyt-2, the most abundant CD46 isoform (Meiffren et al., 2010). CD46 is a cellular receptor for several pathogens, including measles virus, human herpes virus 6, adenovírus B and D, group A Streptococcus (GAS), and Neisseria bacteria (Cattaneo, 2004).
Joubert et al. (2009) demonstrated that CD46 is connected to autophagy. They found an interaction between the scaffold protein GOPC and cyt-1. GOPC contains two coiled-coil domains (CC) and a PDZ domain, and it interacts with cyt-1 through the PDZ domain. GOPC is reported to interact with Beclin-1, (an important molecule in autophagy induction, part of the Vps34 complex) through CC domains. The CD46-cyt-1/GOPC interaction is associated with the autophagosome formation complex Vps34/Beclin-1, recruiting this complex to initiate autophagy (Joubert et al., 2009).
Autophagy Induction and Escape
Very little is known about N. gonorrhoeae’s interaction with autophagy and its impact on intracellular survival, and recent studies have demonstrated that autophagy does affect the survival of intracellular gonococci. As a consequence of cellular invasion, N. gonorrhoeae is targeted by the autophagy pathway: N. gonorrhoeae was found within double-membrane autophagic structures by transmission electron microscopy (TEM), suggesting that the gonococcus ended up in autophagosomes (Lu et al., 2019).
Kim et al. (2019) reported that N. gonorrhoeae (MS11 strain and only piliated and Opa non-expressing bacteria) infection led to autophagosome formation and activation of autophagy in the endocervical cell lines ME180 and Hec1B, induced through CD46-cyt1/GOPC in host cells. The gonococcus interacts with CD46-cyt1 via the Type IV pilus (Tfp), recruiting CD46-cyt1 at the site of infection (Figure 1). Thus, N. gonorrhoeae stimulates matrix metalloproteinases, which are host extracellular sheddases that cleave the CD46-cyt1 ectodomain. After the cleavage of the CD46-cyt1 ectodomain, the presenilin/γ-secretase complex, a host membrane protease complex that modifies type I transmembrane protein function and signaling, cleaves the transmembrane domain, resulting in the release of the cytoplasmic domain. Consequently, this complex gradually reduces the pool of intracellular CD46-cyt1, which decreases the ability of infected cells to initiate autophagy (Weyand et al., 2010).
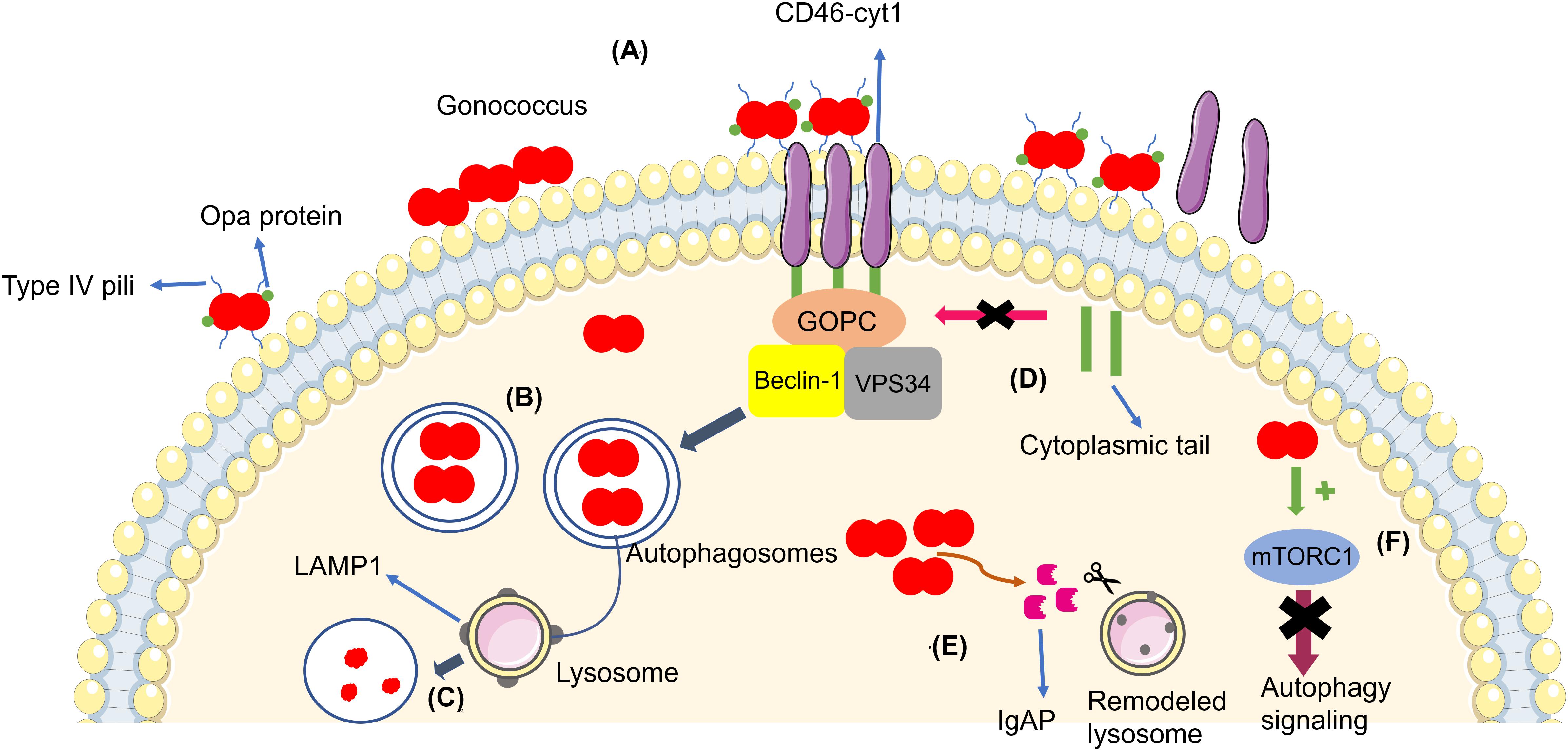
Figure 1. (A) Gonococci (in red) adhere to the cellular membrane and form a microcolony, triggering the autophagy pathway through CD46-cyt1/GOPC, which recruits the Beclin-1/Vps34 complex. (B) Bacteria become enveloped in the autophagosome (blue arrow). (C) The autophagosome fuses with the lysosome in order to kill the bacteria. (D) Gonococci evade the autophagy pathway by inducing the cleavage of the CD46-cyt ectodomain via metalloproteinases and by inducing the cleavage and release of the cytoplasmic tail via presenilin/y-secretase, decreasing intracellular CD46-cyt1 and the cell’s ability to initiate autophagy (pink arrow). (E) Bacteria secrete IgAP, (orange arrow) which cleaves LAMP1, resulting in remodeling of the lysosomal membranes and prevention of autophagosome/lysosome fusion, therefore increasing the survival of the gonococci. (F) Gonococci activate mTORC1 (green arrow), therefore suppressing autophagy signaling (purple arrow).
Autophagosome maturation and fusion with the lysosome is inhibited by N. gonorrhoeae. Studies have shown that Legionella pneumophila (Choy et al., 2012; Arasaki and Tagaya, 2017) and Mycobacterium tuberculosis (Vergne et al., 2005) are also able to inhibit autophagosome maturation. Additionally, N. gonorrhoeae secrets IgAP, a protein which cleaves the major lysosomal membrane protein LAMP1. IgAP cleaves LAMP1 gradually, remodels lysosomes, and blocks lysosome/autophagosome fusion. This dual interference in the autophagy pathway promotes the survival of N. gonorrhoeae until the later stages of infection (Kim et al., 2019).
Lu et al. (2019) quantified intracellular and extracellular bacteria (American Type Culture Collection 49226) in order to evaluate the escape of gonococci from autophagy in HeLa cells. After initial gonococci invasion, the authors added gentamicin in the culture medium to eliminate extracellular bacteria, showing that the extracellular bacteria in the later stages of infection are the subpopulation of the intracellular N. gonorrhoeae that survived autophagy degradation and underwent exocytosis, the process through bacteria are transported inside vesicles to extracellular environment. When N. gonorrhoeae invades cells, many gonococci end up in autophagosomes for elimination, showing that the autophagy pathway affects gonococcus survival in the early stages of invasion. However, a subpopulation of bacteria evades the autophagy pathway (Lu et al., 2019). In the same study, they found that N. gonorrhoeae also activates the autophagy repressor mTORC1 during the intracellular stages, resulting in suppression of autophagy signaling and thus subverting autophagy for its own benefit (Figure 1). This strategy is also used by other intracellular pathogens, including Legionella sp., Shigella flexneri, and Salmonella typhimurium (Ogawa et al., 2005; Choy et al., 2012; Tattoli et al., 2012). In addition, N. gonorrhoeae also inhibited autophagosome maturation and autophagolysosome formation in a way independent of mTORC1 activation through a mechanism that remains unclear (Lu et al., 2019).
In order to grow inside epithelial cells, N. gonorrhoeae uses host sources to acquire iron, resulting in interruption of iron homeostasis in the cells and liberation of bioavailable iron from ferritin storage compartments. This supports the intracellular growth of N. gonorrhoeae in the late stages of infection, since autophagy mediates ferritin degradation. Therefore, the autophagic flux in the early stages of invasion may increase iron availability in the cell, resulting in the growth of intracellular N. gonorrhoeae in the later stages of infection (Bonnah et al., 2000; Larson et al., 2004; Dowdle et al., 2014).
Transcytosis is the transit across cellular epithelium monolayers into the subepithelial space by a bacterium and usually requires endocytic recycling and vesicular transport systems. The epithelial invasion of N. gonorrhoeae and its transcytosis is related to disseminated gonococcal infection and results in complications. The importance of epithelial cell invasion and transcytosis in uncomplicated infections is not clear and needs further investigation (Quillin and Seifert, 2018).
Conclusion
Neisseria gonorrhoeae is already resistant to most antibiotics, which makes the treatment of this disease difficult. In addition, the lack of immunologic memory due to surface antigenic variation complicates the development of an efficient vaccine. Consequently, new studies related to the survival, proliferation, and permanence strategies used by N. gonorrhoeae are important. However, studies on the pathogenesis of the bacteria are challenging because N. gonorrhoeae is an exclusive human pathogen, which means that it needs specific human proteins to interact and nutrients to grow. As a consequence, animal or culture models that mimic human tissue are needed to better study the pathogenesis of N. gonorrhoeae. Some studies have developed such models, although they still have limitations and may not represent all human conditions.
The recent studies of autophagy and N. gonorrhoeae infection show that in the early stages of invasion, bacteria survival is impaired by the autophagy pathway. However, in the later stages of infection, some gonococci are capable of subverting autophagy signaling and maintaining the infection. Consequently, targeting specific bacterial proteins related to autophagy inhibition could be another strategy to control the infection. The development of drugs that affect the bacterial-host interactions and not only the bacteria itself would be promising given that it would allow the host innate immune system to respond to the infection upon autophagy reactivation. We can propose, for instance, the development of drug or antibody to antagonize IgAP, which would prevent the blockage of lysosome/autophagosome fusion. Nonetheless, more studies are needed to better understand the interactions between N. gonorrhoeae and host autophagy.
Author Contributions
AM, MC, BG, and DB conceived and wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank the following funding sources: FAPEMIG (PPM-00604-16), CNPq (404182/2016-0), and CAPES. DB is a recipient of CNPq fellowship. The authors also thank BioMed Proofreading for English language editing of this manuscript.
Footnotes
References
Apicella, M. A., Ketterer, M., Lee, F. K. N., Zhou, D., Rice, P. A., and Blake, M. S. (1996). The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J. Infect. 173, 636–646. doi: 10.1093/infdis/173.3.636
Arasaki, K., and Tagaya, M. (2017). Legionella blocks autophagy by cleaving STX17 (syntaxin 17). Autophagic Punctum. 13, 2008–2009. doi: 10.1080/15548627.2017.1371395
Ayala, P., Wilbur, J. S., Wetzler, L. M., Tainer, J. A., Snyder, A., and So, M. (2005). The pilus and porin of Neisseria gonorrhoeae cooperatively induce Ca(2+) transients in infected epithelial cells. Cell Microbiol. 7, 1736–1748. doi: 10.1111/j.1462-5822.2005.00586.x
Bauckman, K. A., Owusu-Boaitey, N., and Mysorekar, I. U. (2015). Selective autophagy: xenophagy. Methods 75, 120–127. doi: 10.1016/j.ymeth.2014.12.005
Binnicker, M. J., Williams, R. D., and Apicella, M. A. (2003). Infection of human urethral epithelium with Neisseria gonorhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cell Microbiol. 5, 549–560. doi: 10.1046/j.1462-5822.2003.00300.x
Bonnah, R. A., Lee, S. W., Vasquez, B. L., Enns, C. A., and So, M. (2000). Alteration of epithelial cell transferrin-iron homeostasis by Neisseria meningitidis and Neisseria gonorrhoeae. Cell Microbiol. 2, 207–218. doi: 10.1046/j.1462-5822.2000.00042.x
Britigan, B. E., Cohen, M. S., and Sparling, P. F. (1985). Gonococcal infection: a model of molecular pathogenesis. N. Engl. J. Med. 312, 1683–1694. doi: 10.1056/NEJM198506273122606
Cattaneo, R. (2004). Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’. Magnet. J. Virol. 78, 4385–4388. doi: 10.1128/jvi.78.9.4385-4388.2004
Choy, A., Dancourt, J., Mugo, B., O’Connor, T. J., Isberg, R. R., Melia, T. J., et al. (2012). The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 338:6110. doi: 10.1126/science.1227026
Dowdle, W. E., Nyfeler, B., Nagel, J., Elling, R. A., Liu, S., Triantafellow, E., et al. (2014). Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 16, 1069–1079. doi: 10.1038/ncb3053
Escoll, P., Rolando, M., and Buchrieser, C. (2016). Modulation of host autophagy during bacterial infection: sabotaging host munitions for pathogen nutrition. Front. Immunol. 7:81. doi: 10.3389/fimmu.2016.00081
Fox, D. A., Larsson, P., Lo, R. H., Kroncke, B. M., Kasson, P. M., and Columbus, L. (2014). Structure of the Neisserial outer membrane protein Opa60: loop flexibility essential to receptor recognition and bacterial engulfment. J. Am. Chem. Soc. 136, 9938–9946. doi: 10.1021/ja503093y
Gomes, L. C., and Dikic, I. (2014). Autophagy in antimicrobial immunity. Mol. Cell 54, 224–233. doi: 10.1016/j.molcel.2014.03.009
Grimley, D. M., Annang, L., Lewis, I., Smith, R. W., Aban, I., Hooks, T., et al. (2006). Sexually transmitted infections among urban shelter clients. Sex Transm. Dis. 33, 666–669. doi: 10.1097/01.olq.0000223285.18331.4d
Harvey, H. A., Ketterer, M. R., Preston, A., Lubaroff, D., Williams, R., and Apicella, M. A. (1997). Ultrastructural analysis of primary human urethral epithelial cell cultures infected with Neisseria gonorrhoeae. Infect. Immun. 65, 2420–2427. doi: 10.1128/iai.65.6.2420-2427.1997
Higashi, D. L., Lee, S. W., Snyder, A., Weyand, N. J., Bakke, A., and So, M. (2007). Dynamics of Neisseria gonorrhoeae attachment: microcolony development, cortical plaque formation, and cytoprotection. Infect. Immun. 75, 4743–4753. doi: 10.1128/IAI.00687-07
Hook, E. W. III (2012). Gender differences in risk for sexually transmitted diseases. Am. J. Med. Sci. 343, 10–11. doi: 10.1097/MAJ.0b013e31823ea276
Huang, J., and Brumell, J. H. (2014). Bacteria-autophagy interplay: a battle for survival. Nat. Rev. Microbiol. 12, 101–114. doi: 10.1038/nrmicro3160
Joubert, P. E., Meiffren, G., Grégoire, I. P., Pontini, G., Richetta, C., Flacher, M., et al. (2009). Autophagy induction by the pathogen receptor CD46. Cell Host Microbe 6, 354–366. doi: 10.1016/j.chom.2009.09.006
Kim, W. J., Mai, A., Weyand, N. J., Rendón, M. A., Van Doorslaer, K., and So, M. (2019). Neisseria gonorrhoeae evades autophagic killing by downregulating CD46-cyt1 and remodeling lysosomes. PLoS Pathog. 15:e1007495. doi: 10.1371/journal.ppat.1007495
Larson, J. A., Howie, H. L., and So, M. (2004). Neisseria meningitidis accelerates ferritin degradation in host epithelial cells to yield an essential iron source. Mol. Microbiol. 53, 807–820. doi: 10.1111/j.1365-2958.2004.04169.x
Lee, S. W., Higashi, D. L., Snyder, A., Merz, A. J., Potter, L., and So, M. (2005). PilT is required for PI(3,4,5)P3-mediated crosstalk between Neisseria gonorrhoeae and epithelial cells. Cell Microbiol. 7, 1271–1284. doi: 10.1111/j.1462-5822.2005.00551.x
Little, J. W. (2006). Gonorrhea: update. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 101, 137–143. doi: 10.1016/j.tripleo.2005.05.077
Lu, P., Wang, S., Lu, Y., Neculai, D., Sun, Q., and Van Der Veen, S. (2019). A subpopulation of intracellular Neisseria gonorrhoeae escapes autophagy-mediated killing inside epithelial cells. J. Infect. 219, 133–144. doi: 10.1093/infdis/jiy237
Masi, A. T., and Eisenstein, B. I. (1981). Disseminated gonococcal infection (DGI) and gonococcal arthritis (GCA): II. Clinical manifestations, diagnosis, complications, treatment, and prevention. Semin. Arthritis Rheum. 10, 173–197. doi: 10.1016/s0049-0172(81)80002-9
Meiffren, G., Joubert, P. E., Grégoire, I. P., Codogno, P., Rabourdin-Combe, C., and Faure, M. (2010). Pathogen recognition by the cell surface receptor CD46 induces autophagy. Autophagy 6, 299–300. doi: 10.4161/auto.6.2.11132
Mosleh, I. M., Boxberger, H. J., Sessler, M. J., and Meyer, T. F. (1997). Experimental infection of native human ureteral tissue with Neisseria gonorrhoeae: adhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect. Immun. 65, 3391–3398. doi: 10.1128/iai.65.8.3391-3398.1997
Muzny, C. A., Harbison, H. S., Austin, E. L., Schwebke, J. R., Van Der Pol, B., and Hook, E. W. III (2017). Sexually transmitted infection risk among women is not fully explained by partner numbers. South Med. J. 110, 161–167. doi: 10.14423/SMJ.0000000000000621
Ogawa, M., Yoshimori, T., Suzuki, T., Sagara, H., Mizushima, N., and Sasakawa, C. (2005). Escape of Intracellular Shigella from Autophagy. Science 307:5710. doi: 10.1126/science.1106036
Parzych, K. R., and Klionsky, D. J. (2014). An overview of autophagy: morphology, mechanism, and regulation. Antioxid. Redox Sign. 20, 460–473. doi: 10.1089/ars.2013.5371
Pearce, W. A., and Buchanan, T. M. (1978). Attachment role of gonococcal pili. Optimum conditions and quantitation of adherence of isolated pili to human cells in vitro. J. Clin. Invest. 61, 931–943. doi: 10.1172/JCI109018
Quillin, S. J., and Seifert, H. S. (2018). Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 16, 226–240. doi: 10.1038/nrmicro.2017.169
Rabanal-Ruiz, Y., Otten, E. G., and Korolchuk, V. I. (2017). MTORC1 as the main gateway to autophagy. Essays Biochem. 61, 565–584. doi: 10.1042/EBC20170027
Rowley, J., Hoorn, S. V., Korenromp, E., Low, N., Unemo, M., Abu-Raddad, L. J., et al. (2019). Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull. World Health Organ. 97, 548–562. doi: 10.2471/BLT.18.228486
Rubinsztein, D. C., Shpilka, T., and Elazar, Z. (2012). Mechanisms of autophagosome biogenesis. Curr. Biol. 22, R29–R34. doi: 10.1016/j.cub.2011.11.034
Sandstrom, I. (1987). Etiology and diagnosis of neonatal conjunctivitis. Acta. Paediatrica. 79, 221–227. doi: 10.1111/j.1651-2227.1987.tb10451.x
Stein, D. C., LeVan, A., Hardy, B., Wang, L. C., Zimmerman, L., and Song, W. (2015). Expression of opacity proteins interferes with the transmigration of Neisseria gonorrhoeae across polarized epithelial cells. PLoS One 10:e013434. doi: 10.1371/journal.pone.013434
Stern, A., Brown, M., Nickel, P., and Meyer, T. F. (1986). Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47, 61–71. doi: 10.1016/0092-8674(86)90366-1
Swanson, K. V., Griffiss, J. M., Edwards, V. L., Stein, D. C., and Song, W. (2011). Neisseria gonorrhoeae-induced transactivation of EGFR enhances gonococcal invasion. Cell. Microbiol. 13, 1078–1790. doi: 10.1111/j.1462-5822.2011.01603.x
Tattoli, I., Philpott, D. J., and Girardin, S. E. (2012). The bacterial and cellular determinants controlling the recruitment of mTOR to the Salmonella-containing vacuole. Biol. Open 1, 1215–1225. doi: 10.1242/bio.20122840
van Putten, J. P., and Paul, S. M. (1995). Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 14, 2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x
Vergne, I., Chua, J., Lee, H. H., Lucas, M., Belisle, J., and Deretic, V. (2005). Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 102, 4033–4038. doi: 10.1073/pnas.0409716102
Weyand, N. J., Calton, C. M., Higashi, D. L., Kanack, K. J., and So, M. (2010). Presenilin/gamma-secretase cleaves CD46 in response to Neisseria infection. J. Immunol. 184, 694–701. doi: 10.4049/jimmunol.0900522
World Health Organization [WHO] (2017). WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Avaiable online at: https://www.who.int/medicines/news/bacteria-antibiotics-needed/en/ (accessed April 20, 2020).
Yang, Y. P., Liang, Z. Q., Gu, Z. L., and Qin, Z. H. (2005). Molecular mechanism and regulation of autophagy. Acta Pharmacol. Sin. 26, 1421–1434. doi: 10.1111/j.1745-7254.2005.00235.x
Keywords: N. gonorrhoeae, autophagy, epithelial cell, intracellular pathogen, epithelial cell invasion
Citation: Mendes AC, Ciccone M, Gazolla B and Bahia D (2020) Epithelial Haven and Autophagy Breakout in Gonococci Infection. Front. Cell Dev. Biol. 8:439. doi: 10.3389/fcell.2020.00439
Received: 06 February 2020; Accepted: 11 May 2020;
Published: 09 June 2020.
Edited by:
Si-Yang Huang, Yangzhou University, ChinaReviewed by:
Chihiro Aikawa, Kyoto University, JapanRavi Manjithaya, Jawaharlal Nehru Centre for Advanced Scientific Research, India
Copyright © 2020 Mendes, Ciccone, Gazolla and Bahia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diana Bahia, dianabahia@hotmail.com
†These authors share first authorship
 Ana Clara Mendes
Ana Clara Mendes Marcone Ciccone†
Marcone Ciccone†  Bruna Gazolla
Bruna Gazolla Diana Bahia
Diana Bahia