- 1Victor Babes University of Medicine and Pharmacy, Timisoara, Romania
- 2Department of Biomedical Sciences, College of Medicine and Biological Science, University of Suceava, Suceava, Romania
- 3Integrated Center for Research, Development and Innovation for Advanced Materials, Nanotechnologies, Manufacturing and Control Distributed Systems (MANSiD), University of Suceava, Suceava, Romania
- 4Suceava Emergency Clinical County Hospital, Suceava, Romania
- 5Institute of Cardiovascular Science, Hemostasis Research Unit, University College London (UCL), London, United Kingdom
- 6Department of Computer, Electronics and Automation, University of Suceava, Suceava, Romania
- 7Department of Clinical Pharmacy, USC Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences, University of Southern California, Los Angeles, CA, United States
- 8Department of Quantitative and Computational Biology, USC Dornsife College of Letters, Arts and Sciences, University of Southern California (USC), Los Angeles, CA, United States
- 9Department of Basic Medical Sciences, Western University of Health Sciences, College of Osteopathic Medicine, Pomona, CA, United States
The coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 has been defined as the greatest global health and socioeconomic crisis of modern times. While most people recover after being infected with the virus, a significant proportion of them continue to experience health issues weeks, months and even years after acute infection with SARS-CoV-2. This persistence of clinical symptoms in infected individuals for at least three months after the onset of the disease or the emergence of new symptoms lasting more than two months, without any other explanation and alternative diagnosis have been named long COVID, long-haul COVID, post-COVID-19 conditions, chronic COVID, or post-acute sequelae of SARS-CoV-2 (PASC). Long COVID has been characterized as a constellation of symptoms and disorders that vary widely in their manifestations. Further, the mechanisms underlying long COVID are not fully understood, which hamper efficient treatment options. This review describes predictors and the most common symptoms related to long COVID’s effects on the central and peripheral nervous system and other organs and tissues. Furthermore, the transcriptional markers, molecular signaling pathways and risk factors for long COVID, such as sex, age, pre-existing condition, hospitalization during acute phase of COVID-19, vaccination, and lifestyle are presented. Finally, recommendations for patient rehabilitation and disease management, as well as alternative therapeutical approaches to long COVID sequelae are discussed. Understanding the complexity of this disease, its symptoms across multiple organ systems and overlapping pathologies and its possible mechanisms are paramount in developing diagnostic tools and treatments.
1 Introduction
COVID-19 pandemic has caused an unprecedented worldwide health and socioeconomic crisis leading to more than 768 million cases of viral infections, of which approximately 7 million deaths and 13,490,832,730 vaccine doses administered globally (1). The pandemic was caused by SARS-CoV-2, severe acute respiratory syndrome coronavirus-2 which infects the host by invading cells via ACE2–angiotensin-converting enzyme 2 (2). Although the respiratory tract is the site of entry and infection of SARS-CoV-2, COVID-19 is a complex disease, affecting the cardiovascular, renal, hematological, gastrointestinal, and central nervous systems, and can present a wide severity spectrum, from asymptomatic to severe, moderate or mild symptoms. The occurrence of acute COVID-19 last from 1-2 weeks in mild cases and up to 12 weeks for the most severe ones, based on factors such as age, symptoms, comorbidities, vaccination status, access to treatment and medical services (3). More than half of the infected individuals are presented with persistent symptoms even longer than four weeks after the onset of first clinical signs, a condition defined as post-acute sequelae of COVID-19 (PASC). The presence of clinical symptoms in infected individuals that continue at least three months after the onset of disease or with new symptoms that last for more than two months with no other explanation and that cannot be associated with other existing pathologies has been defined as long COVID-19 (L-C19) (4). The National Institute for Health and Care Excellence (NICE) classifies L-C19 in two categories: 1) “ongoing symptomatic C19” with symptoms that persist from 4 to 12 weeks and 2) “post C19” with persisting symptoms beyond 12 weeks after disease onset. Several other terms have been used based on the length and persistence of symptoms to define L-C19, such as “post-acute sequelae of SARS-CoV-2 infection”, “persistent C19 symptoms”, “post C19 syndrome” (PCS), “long haulers”, or “post C19 manifestations” (5, 6) (Table 1).
In L-C19, the virus is no longer present in the nasal cavity (7), however, viral protein and/or RNA has been detected in the reproductive and cardiovascular system, brain, muscles, eyes, olfactory mucosa, lymph nodes, appendix, breast, hepatic and lung tissue, plasma, intestinal microbiome, and urine (8, 9).
2 Long COVID-19 symptoms and predictors
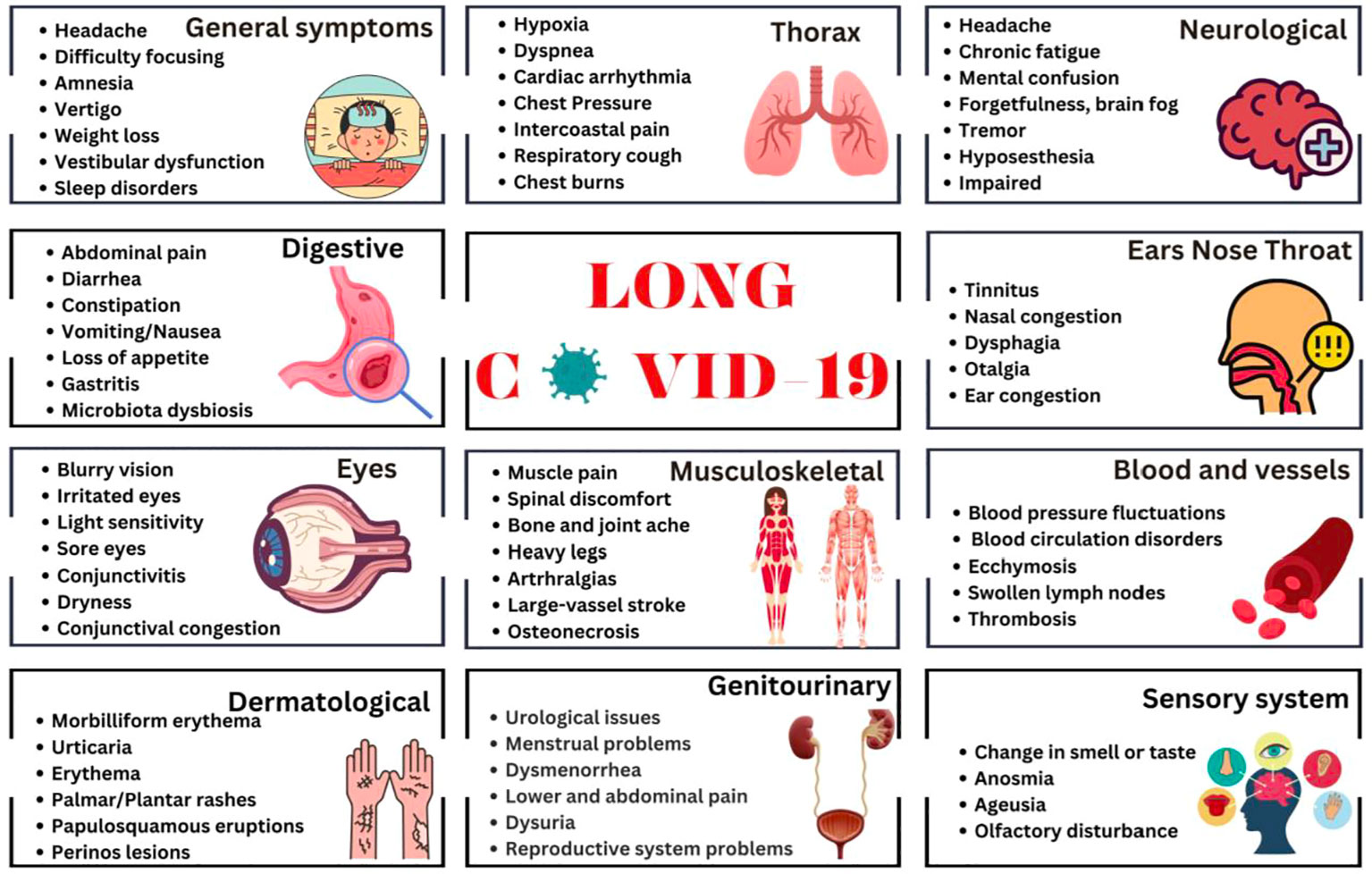
Given the wide spectrum of the L-C19 clinical symptomatology, establishing with certainty the syndrome, clinical manifestations, pathogenic factors, or its time framework, had proved challenging. The most common and representative symptoms of L-C19 include fatigue or muscle weakness, malaise, dyspnea, headache, dizziness or “brain fog”, depression, irritability, frustration, insomnia, and many other neurological disorders (10, 11). Other symptoms are related to cardiac, digestive, respiratory, reproduction, or dermatologic disorders. A recent meta-analysis study showed that the five most relevant physiological signs are fatigue, headache, deficit of attention, hair loss and dyspnea, followed by skin rashes, palpitations, and diarrhea (12), with recurrent spikes of fever as common symptom, but higher than observed after common infections, such as Epstein-Barr virus or influenza (13). The clinical presentation and symptomatology of L-C19 is similar with that of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) (14), a known complicated illness with 4-6 months of fatigue and exhaustion, reduced daily activity and post-exertional malaise (15). Other common symptoms may include myalgia, muscle weakness, headache, sleep disorders, neurocognitive and psychiatric manifestations, anorexia or autonomic manifestations (orthostatic intolerance, cardiovascular, respiratory, gastro-intestinal, or gastro-urinary) (16) Compared with influenza, sequelae of L-C19 were higher in terms of anxiety and mood disorders, insomnia, and dementia (17). There have been over 200 symptoms associated with L-C19, with the most representative being depicted in Figure 1.
Due to the over-production of pro-inflammatory cytokines, C19 is not a usual viral pneumonia, but rather one with major consequences on the central and peripheral nervous system, cardiovascular, respiratory, urinary, immune system or on metabolic functions (11). For example, Østergaard et al. using scanning microscopy reported the presence of SARS-CoV-2 particles in the endothelium of lung, heart, kidney, brain or skin of C19 patients, with capillary changes and inflammation (18). It appears that L-C19 manifestation does not usually depend on the severity of the acute COVID-19 illness. In a 14-month study de Miranda et al. showed that most patients (75.4%) who experienced L-C19 had moderate infections and only 33.1% had been severe (19). Similar results were observed by Sugyiama et al. who reported that 49.5% from the patients identified with L-C19 were mild cases (2). Unfortunately, due to the multiple symptomatology and its undefined nature, it is difficult to detect L-C19 through laboratory findings. Thus, guidelines and regulations would be of great benefit in identifying L-C19. To this end, Roth & Gadebusch-Bondio proposed, in addition to conventional measures, presentation of cases, symptoms, and side effects through mass media platforms that are easily accessible globally (20). This could facilitate a more rapid self-identification of L-C19 symptoms, thus enhancing the possibility for treatment in a much shorter time.
Approximately half of the individuals infected at some point with SARS-CoV-2 developed L-C19. Exhaustion, cognitive dysfunction, myalgia, shortness of breath, chest pain or muscle aches were observed in most of these patients. Most of them confirmed that L-C19 affected self-care (50%), mental health (64%), and overall work (75%) (21). The greater risk of developing L-C19 was associated with the number of initial symptoms, with five or more being strongly correlated with persistent symptoms. In addition, hypertension was the most significant comorbidity associated with the development of L-C19, followed by diabetes, smoking, chronic cardiovascular or lung disease, and chronic kidney failure (22, 23). L-C19 has been more frequently encountered in individuals who have been reinfected with COVID-19 (24).
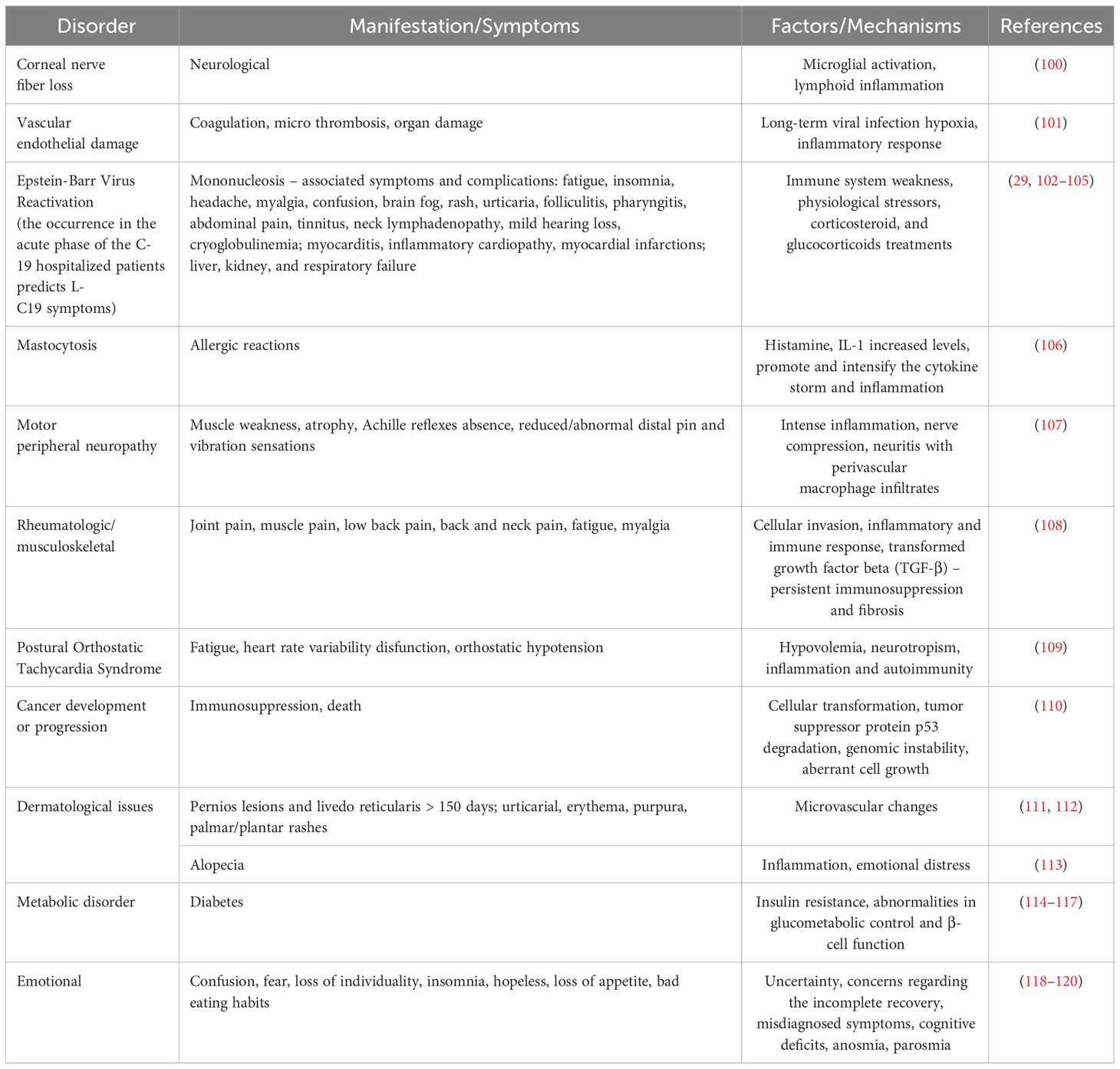
Clinical predictors of C19 involved increased levels of troponin, high white blood cell counts, blood sugar level, elevated cytokine response (tumor necrosis factor-alpha, interferon-gamma, C-C motif chemokine ligand 5, interleukin 6, IL-8, and IL-18) and altered gut microbiota. To establish L-C19 diagnosis, recommendations include electrocardiogram and transthoracic echocardiogram, as well as laboratory tests for CRP, troponin-T, pro-inflammatory markers (TNF-α, C-C motif chemokine ligand 5, IL-6, IL-8, IL-18, and interferon-gamma) levels. Moreover, several studies have identified a hypercoagulable state in L-C19 patients (25–27), with D-dimer levels being monitored (28) while other studies showed that reactivation of latent viruses or chronic inflammation led to L-C19. Thus, individuals with L-C19 symptoms presented chronic inflammatory and autoimmune conditions due to high level of monocytes and low levels of circulating cDC1, a type 1 dendritic cell with a role in immunity and viral infection (29). Several main mechanisms of L-C19 and its effects are presented in Figure 2.
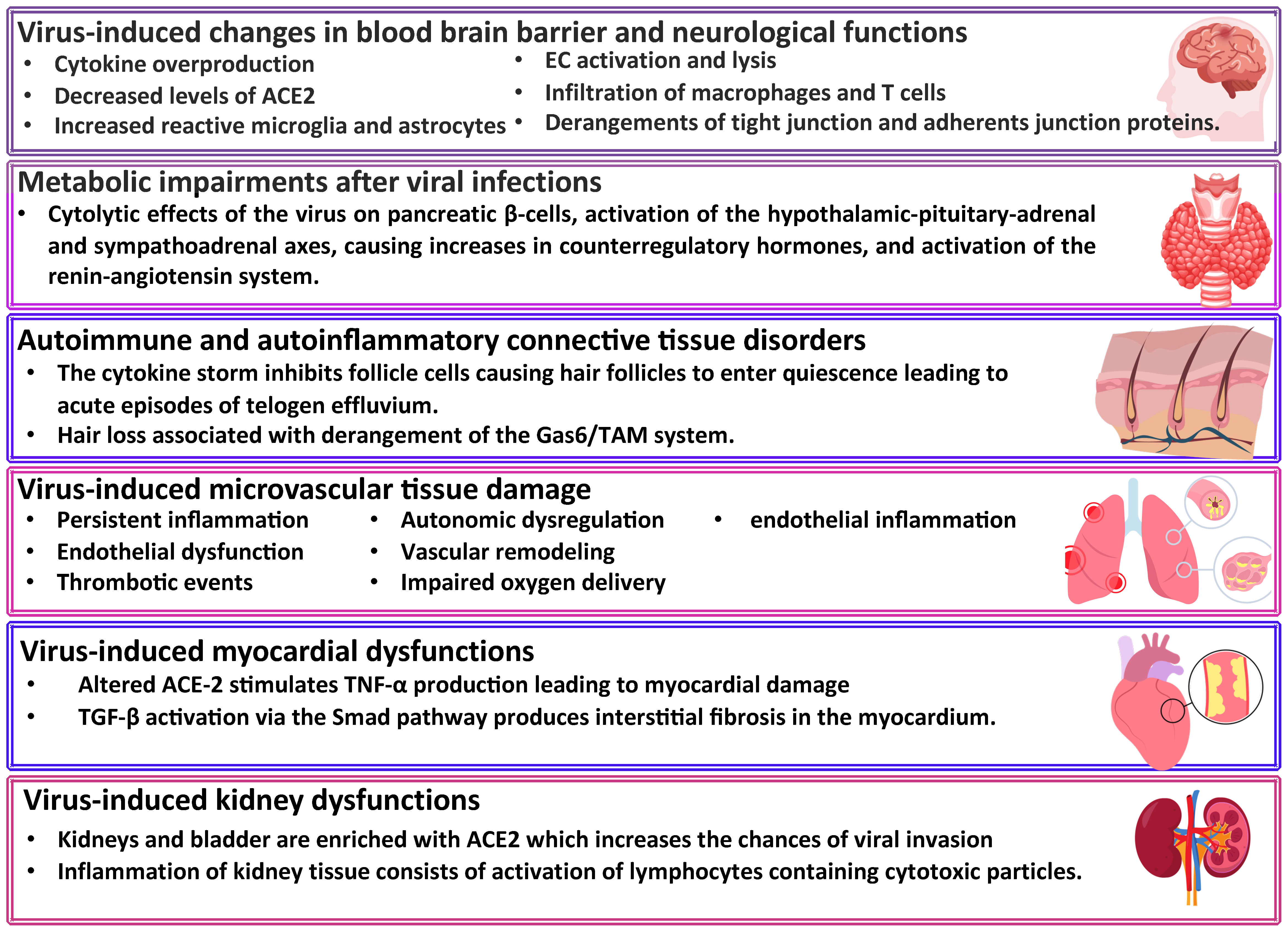
Figure 2 Long COVID-19 sequelae. ACE2, angiotensin converting enzyme-2; Gas6, gamma-carboxyglutamic acid protein; TAM, Transient abnormal myelopoiesis; TNF-α, tumor necrosis factor-alpha; TGF, transforming growth factor.
3 Central nervous system clinical manifestations
3.1 Chronic fatigue
Multiple studies have shown that chronic fatigue is the underlying symptom in L-C19 (30–35). This led to significant interest in understanding the mechanisms and develop appropriate treatment strategies. It is known that chronic fatigue syndrome and depression are immune, oxidative and nitrosative stress-related disorders. These are significantly correlated with increased levels of inflammatory mediators such as C-reactive protein, increased aldehyde formation due to oxidative damage, increased nitric oxide production and hyper nityrosylation and low antioxidant levels (zinc, selenium, glutathione SH groups, glutathione peroxidase, total antioxidant capacity) (36). Several studies have suggested that certain vaccines such as those produced by Astra Zeneca and Pfizer may trigger the neuropsychiatric symptoms, especially somatic symptoms of Hamilton Depression and Anxiety. They concluded that these vaccines may be associated with some L-C19 symptoms such as anxiety, depression, fatigue, autoimmune response, and increased production of spike protein (36). Since fatigue is a general characteristic of L-C19, Twomey et al. suggested that measurement of fatigue level should be considered and a fatigue scale (such as FACIT-F) should be applied, especially because a validated treatment for L-C19 fatigue is not known and exercise therapy is generally not beneficial (37). L-C19 fatigue was strongly linked with comorbidities such as high blood pressure, high cholesterol, rheumatoid arthritis, diabetes, previous blood clots, cardiovascular diseases, auto immune disease. Elevated levels of anti-nuclear/extractable-nuclear antibodies are strongly correlated with higher level of TNF-α and are linked to fatigue in various diseases including chronic fatigue syndrome and rheumatoid arthritis (38). Negative psychological and social factors have also been associated with chronic fatigue (39, 40). To establish the true effects of L-C19’s chronic fatigue, it is thus necessary to identify and eliminate other common causes of chronic fatigue, such as anemia, hyperglycemia, thyroid disorders, dehydration or diabetes (41).
3.2 Brain fog, concentration, forgetfulness
Cognitive dysfunction is a main L-C19 symptom occurring in approximately 70% of patients (42). The most representative symptoms are concentration difficulties, brain fog, forgetfulness, semantic disfluency, or tip-of-the-tongue (ToT) word-finding problems. According to Guo et al., chronic fatigue and neurological symptoms observed in the first three weeks of illness can be a predictive factor for cognitive disorders (43). Most of these clinical manifestations are identified in patients who experienced severe C-19 illness and were hospitalized (42). Although brain fog has been reported after ordinary upper respiratory tract infections, its prevalence is significantly higher after C19, with 17.8% prevalence 2 months following acute C19 (44, 45). Using Montreal Cognitive Assessment (MoCA) score, Alemano et al. showed that more than 80% of patients with severe C-19 developed cognitive deficits later (memory, executive function, and language). It is interesting that patients who underwent sedation and ventilation presented fewer cognitive disorders, an effect attributed to reduction of stress by sedation and/or increased oxygenation due to ventilation (46). Several mechanisms have been proposed in these neurological disorders that include neuroinflammation, aberrant immune response between SARS-CoV-2 and body antigens, residual virus particle, metabolic brain disorders, and activation of peripheral trigeminal nerve roots (47). Other factors are structural or volumetric vascularization disorders, with high blood pressure or high cholesterol. Unfortunately, these comorbidities and C19’s cognitive disorders are strongly associated with loss of grey matter within the temporal lobe which, along with reduced memory performance, may be an increased risk for neurodegeneration or dementia (48). This hypothesis was confirmed by Hugon et al. who observed abnormal hypometabolic area after cerebral FDG PET scans of L-C19’ patients. Thus, the abnormal neurological functions may be attributed to deficient brain connections between the anterior and posterior cingulate cortex. The cingulate cortex is involved in memory, emotions, depression, or action decision, and its hypometabolism have been observed in neurological and psychiatric diseases, such as Alzheimer, depression or internet gaming disorder (49). The neurocognitive and neuropsychiatric deficits change CNS immune and glial cells and the negative effect on neuronal pathophysiology is mainly due to myelin homeostasis and plasticity disturbance, hippocampal neurogenesis impairment with neurotoxic astrocyte reactivity (50). The differences in white matter were observed only three months after acute phase and completely recovered after 10 months. After 3 months, a self-recovery mechanism of neuroplasticity was observed (51). These effects were mainly observed in females, patients with respiratory problems or those who needed ICU interventions (52).
Some of the medications received in C-19 acute infection may also affect the neurological system of patients as well. Thus, the neurological side effects of drugs such as lopinavir-ritonavir and corticosteroids should be considered when assessing L-C19 effects (28). More recent studies suggested that brain fog in L-C19 patients may be caused by the presence of blood clots in the cerebral or pulmonary circulation (53) and were associated with increased serum ferritin levels during C19 hospitalization (45). Since cognitive disorders are difficult to manage and treat, many patients with cognitive impairments, require long-term psychological support and treatment. Unfortunately, cognitive dysfunction led to reduced work capacity, prolonged time of normal activities, difficult work ability, mental exertion, stress and loss of employment, thus having a severe impact on the quality of life (54).
3.3 Headache
It is one of the earliest and most common symptoms of L-C19 and it is usually accompanied by hyposmia, fatigue, dyspnea, myalgia, and cough. It is influenced by C-19 acute phase severity and the use of analgesics, genetic predisposition to migraine due to trigeminovascular system activation, and systemic immune response at viral infection (55). Without a specific clinical phenotype, the headache topography is predominantly bilateral, with frontal or periocular presence, and the pain is felt like a tension-type headache, usually without any symptoms, or migraine-type headache, accompanied by vomiting or nausea (56). The presence of headache in C-19 acute phase have been considered a positive prognosis, being associated with lower severity, lower mortality, and lower need of ICU interventions (57). Some symptoms, such as headaches, continue to persist to a significant extent in the long term. For example, 16.5% of patients continue to experience headaches after 60 days from the start of the disease, of which 8.4% reported significant headaches even after 180 days post COVID-19 (58, 59). Thus, symptoms such as headaches can persist to a significant extent even months after the onset of the disease, highlighting the importance of a holistic approach and continued research to better understand the long-term health impact of this disease.
3.4 Depression and anxiety
Depression and anxiety are found in over 50% of patients with L-C19, especially due to hospitalization or cognitive disorders (60). Even if these symptoms were not identified in the first phase, they are usually found in patients with certain difficulties, being part of the long-term symptoms category. The first psychiatric sequelae were noticed 14-90 days after C-19 and the estimated probability to develop new sequelae after 90 days was 5-8%. The anxiety disorders are shown to be more prevalent than mood disorders (53). Some authors suggested that depression and anxiety may be a result of disease severity and trauma of hospitalization rather than of the viral infection (61). Due to individuality of each patient, presentation can vary which makes the treatment challenging (62) thus early diagnosis and close follow-up is critical. Unfortunately, depression and anxiety are difficult to identify since they are intrinsically related to patients’ overall health and life quality. When comparing COVID infected patients with or without symptoms of depression and anxiety, imaging tests via MRI showed atrophy of the brain areas responsible for processing emotions and memory. Thus, it is clear that the anxiety and depression caused by this virus have significant long-term consequences (63).
3.5 Sleep disorders
Sleep disorders and fatigue are the most persistent symptoms that affect the daily life of L-C19 patients (64). More than 40% of patients experienced insomnia, however, the symptoms gradually improved after 3 months. When tested with wearable health devices, L-C19 patients reported decreased total sleep and light or deep sleep time leading to related health issues (65). In some patients, sleep disturbances were present even after 12-month post C-19, with negative impact on patients’ quality of life. This, along with fatigue, altered respiratory functions, stomach burn, or abdominal pain add to the significant burden of L-C19 patients (66, 67).
3.6 Cognitive disorders
Most patients seem to return to normal life after infection with C-19, but many remain with mild symptoms that are often attributed to other daily factors such as fatigue, memory problems, and loss of attention, without realizing that these symptoms may be long-term symptoms caused by C-19 infection (68). Research shows that 1 in 8 patients may receive a neurological diagnosis after C-19, even after 6 months post SARS-2 infection (69). Several biomarkers have been used to differentiate between LC-19 and other causes of cognitive disorders symptomatology; however, research thus far is relatively scarce. One promising study analyzed fibrinogen and D-dimer compared to C-Reactive Protein (CRP) levels in more than 1830 hospitalized patients and found positive correlations between cognitive impairment and COVID-19 infection (70). Subjective as well as objective cognitive deficits were analyzed, considering occupational effects. Using canonical correlation (CCA) methodology to assess associations between sets of variables, it was shown that increased fibrinogen was correlated with both objective as well as subjective cognitive deficits at 6 and 12 post COVID (70). Fibrinogen has a direct effect on blood clotting, but it also fulfils site-binding functions that could affect axon binding or even have effects that degrade the β-amyloid protein (71, 72). High levels of D-dimer were associated with subjective cognitive difficulties and occupational effects (69). An increased level of D-dimer has been associated with a risk of pulmonary thromboembolism, which may be related to poor oxygenation of the brain, with direct implications for cognitive impairment (73). Determination of cognitive impairments after C-19 infection is a complex process and requires identification of factors and mechanisms of action of different biomarkers, including correlation with brain imaging.
4 Peripheral nervous system and other organs clinical manifestations
4.1 Anosmia/ageusia or altered smell and taste
Smell and taste dysfunctions are the most prevalent symptoms in L-C19, after fatigue (74, 75). Anosmia was more frequent present in patients with mild C-19 forms and less reported in patients with severe C-19, eye, nose and throat complaints. Initial studies demonstrated that, in mild C-19 cases, patients might have had stronger local immunity and the virus replicated in the mucosa (76). Persistent olfactory dysfunction has been associated with poorer emotion recognition in patients who experienced mild C-19 (77). The persistence of anosmia or ageusia in L-C19 patients has been attributed to injuries in the olfactory neuronal pathways or persistence of neuroepithelium inflammation (47). However, in patients with previous mild SARS-CoV-19 infection, the 3-year prevalence and recovery rate of COVID-19-related alteration in sense of smell or taste was 5% and 92%, respectively. In patients experiencing chemosensory dysfunction still 2 years after COVID-19, a delayed complete or partial recovery has been observed even after 3 years, while some patients continue to have unchanged persistent chemosensory alteration (78). Gene expression profiling of olfactory epithelium of patients with persistent olfactory symptoms showed changes in the expression levels of miRNAs involved in neural development and immune response. Further, overexpression of metallothioneins, in response to increased inflammation of the olfactory epithelium may results in decreased zinc levels and subsequent loss of smell and taste (79). L-C19 chemosensory disfunction significantly reduces quality of life, with negative implications in mental health: anxiety, depression, loss of appetite, weight changes, even work and study difficulties or social and interpersonal limitations (80).
4.2 Renal system clinical manifestations
Among LC-19 sequelae related to the renal system were acute kidney injury or renal failure, mainly due to high abundance of pf ACE2 expression in kidneys, with declined glomerular filtration rate or kidney infarction, mainly due to thromboembolism. The prognosis is associated with significant risks of mortality or morbidity. Acute kidney injury was observed mainly in non-survivors and in 1% of survivors, with renal function restauration. Therefore, investigation tools should include early recognition of kidney functional impairments or injury through urine analysis, glomerular filtration rate, ultrasound scanning or renal biopsy (81–83).
4.3 Gastrointestinal clinical manifestations
The main gastrointestinal (GI) symptoms shown to persist even six months after C-19 acute phase are constipation, diarrhea, abdominal pain, nausea/vomiting, and heartburn. Patients who reported L-C19 anxiety or sadness were more prone to present GI symptoms (55% vs. 14%) and conversely, GI symptoms lead to anxiety/sadness (84). The possible mechanisms involve the abundance in ACE2 and furin expression, fecal-oral transmission, lymphocytic infiltrations into intestinal tissues’ lamina propria, intestinal dysbiosis, or high cytokine levels (81). Inflammation and intestinal metabolites dysfunction are influenced by nutrition, diet, malnutrition, old age or diabetes and obesity (85). Further, gut microbiota dysbiosis, GI peripheral tissue damage and altered immune status have been reported six months after the infection (86). The investigation tools should include colorectal transit observations, CT scan, defecography and swallowing studies (81).
4.4 Cardiovascular and respiratory clinical manifestations
Approximately half of patients with L-C19 reported incomplete recovery, with shortness of breath and other respiratory problems (37, 87), that included chest pain, cough, or sputum production (88). The breathing discomfort has been attributed to chronic changes in breathing pattern and persisting inflammation in lungs or mediastinal lymph nodes, as well as association of metabolic abnormalities with lung sequelae. These pulmonary vascular network abnormalities have been the cause for pulmonary hypertension and damaged respiratory reflexes due to intrathoracic receptors destruction (47). L-C19 patients developed endothelial dysfunction which, even if it had progressively improved after 6 months, the risk for thrombotic or cardiovascular events remained high. Usually, endothelial dysfunctions develop in the acute phase of the disease and were responsible for atherosclerosis. These symptoms persisted even 6-months after C-19 in 58% of patients. There was a negative correlation between IL-6 levels and brachial artery flow-mediated dilation, an effect that was improved by treatment with tocilizumab, an IL-6 inhibitor. Patients hospitalized in a medical ward recovered faster than those hospitalized in ICU (89, 90). The presence of microclots associated with insoluble inflammatory molecules, antibodies and immunoglobulins were present not only in the acute phase of C-19, but also played a critical role in the development of L-C19 symptoms and other auto-immune pathologies. For example, increased level of galectin-3-binding protein (responsible for cancer development, progression and metastasis) inflammatory markers (IFN-α, IFN-β, IFN-γ, TNF-α), trombospondin-1(increased in tumors and associated with thrombosis), α-1-acid glycoprotein-2 (associated with demyelinating diseases), and reduced levels of long palate, lung and nasal epithelium carcinoma-associated protein 1, lactotransferrin, adiponectin and α-1-acid glycoprotein-1 may be the result of immunosuppression similar to that of sepsis. Evidence suggested that if these microclots resolved in the first phase of the disease, hypoxia could be avoided. This is an important finding, especially because hypoxia led to irreversible tissue damage and can be life threatening for patients with diabetes 2 or cardiovascular co-morbidities (91). For example, fibrin amyloid microclots blocked capillaries and impeded O2 to be transported to the tissues, leading to thrombotic events, myocardial infarction, strokes, kidney disfunction, or neurological disorders. Thus, their removal could be a potential therapeutic strategy which may allow the body the possibility to self-repair (92, 93). The presence of ‘amyloid’ microclots and platelet hyperactivation has prompted some research groups to propose a triple anticoagulant therapy as a potential treatment of L-C19, though this needs to be further assessed (27, 92). Furthermore, studies showed an increase in thrombin generation in patients with L-C19 (26, 94), in line with the presence of a hypercoagulable state in these patients. Persistently raised D-dimers and sustained inflammation has also been identified in convalescing C-19 patients who were not hospitalized during acute infection (24, 95, 96). More recently, a study using microfluidic strategies to reflect the physiological conditions of the vasculature, revealed that patients suffering from L-C19 have higher thrombogenicity under flow, with increased platelet capture and larger thrombi compared to matched healthy controls. These patients had been suffering of L-C19 for an average of 23 months, suggesting that this emerging disease might cause long-term thrombogenicity. These findings were correlated with levels of Von Willebrand Factor (VWF) or ADAMTS13 activity, involved in arterial thrombotic pathologies (26). An increased VWF concentrations and decreased ADAMTS13 activity were correlated with a higher risk for myocardial infections (97). Indeed, patients with L-C19 were also found to have an increased VWF : ADAMTS13 ratio, above 1.5, which was associated with impaired exercise capacity (98, 99).
Comorbidities such as high blood pressure, high cholesterol, rheumatoid arthritis, or cardiovascular diseases can lead to respiratory system symptoms. NICE recommends that breathlessness should be auto identified through simple tolerance test exercises and blood oxygen levels with pulse oximeter. For example, Mayo Clinic suggests that smoking, pollutants and extreme temperature may contribute to L-C19 pulmonary symptoms’ persistence (39). WHO rehabilitation guidelines include precautions regarding gradual return of daily activities of L-C19 patients and recommends that physical exercise must be adapted in order to prevent fatigue and patients’ activity must be in accordance with their symptoms (98). Other L-C19 symptoms are presented in Table 2.
5 Transcriptional markers for long COVID-19
In addition to clinical symptoms and alteration of biochemical markers’ levels, long COVID affects several regulatory processes in human body, in eluding changes at the transcriptional level. This comes from the fact that, the virus hijacks the host cell transcriptional/translational machinery during acute infection to produce large amounts of viral proteins and RNA, while shutting down host messenger RNA translation (8). For instance, persistent alterations in the blood transcriptome 446 genes displayed significant differential expression in individuals referred to a long COVID clinic compared to those not referred, at 24 weeks post-infection. No such differences were observed at earlier timepoints, suggesting that while many individuals note resolution in transcriptional dysregulation approximately 6 months post-infection, this is not the case for those with long COVID symptoms (121). Furthermore, pathway analysis of patient groups revealed four transcriptome groups. First, enriched with Th1-like signatures in CD4+ T cells, M1-like pro-inflammatory signatures in monocytes, cytotoxic effector signatures in CD8+, T cells and NK cells, and memory signatures in B cells. Second, enriched for Th2-like CD4+ T cell signatures, M2-like (anti-inflammatory) monocyte signatures, and a plasma B cell signature. Third, a transitional immune status between types 1 and type 2. Fourth, a naive group, with naive-like T and B cell signatures, and resting NK cell signatures (122).
Transcriptional modifications may lead to disease progression, as transcriptomic analysis of bronchoalveolar lavage from severe COVID-19 patients showed reduced IFN-responsive genes response, compared to blood. Also, downregulation of interferon stimulated genes such as MX1, IFITM1, and IFIT2 were observed in critical COVID-19 cases, as well as undetected messenger RNA levels of IFN-β in blood (123). Nevertheless, transcriptional modifications may also lead to delays in recovery, as ongoing perturbations on a transcriptional level were observed for up to 6 months after infection, with PASC patients showing a distinct profile, such as upregulation of the alarmins S100A8 and HMGB1, mediators and markers of innate immune activation (124). Also, increased expression of acute inflammatory markers was observed 9 weeks after acute phase, also positively correlated with COVID-19 severity (125).
SARS-CoV-2-induced transcriptional modifications may be involved in inflammation and, possibly, processes like thrombosis. For example, transcriptomic analysis of platelets from SARS-CoV-2 patients revealed increased transcription of IL-6, tumor necrosis factor TNF-α, blood coagulation, and hemostasis, possibly leading to thrombosis (123). This behavior could suggest mild thrombocytopenia, which usually implies fatigue, that is common in long COVID, but also increases in S100B, a marker of neurological damage, which is linked to the neurological symptoms observed in long COVID (126). Tregs (regulatory T cells), a subset of CD4+ T cells displayed elevated transcriptional signatures inducing correspondent high levels of proinflammatory molecules, which may lead to reduced antiviral T cell responses in acute COVID-19, while also promoting inflammation (127).
The role of Tregs in the context of Long COVID, particularly regarding transcriptional markers, represents an area of active investigation with emerging insights. Tregs, known for their immunosuppressive and immunoregulatory properties, play a significant role in the prognosis of COVID-19. Patients with COVID-19 are reported to have fewer Tregs compared to the general population, leading to diminished inflammatory inhibition and an increased likelihood of respiratory failure and long COVID development (128). Further, Tregs exert control over both adaptive and innate immune responses through various mechanisms. They produce cytokines such as TGF-beta, IL-10, and IL-35, which inhibit T cells, leading to suppressed actions of Th1, Th2, and Th17 type T cells. Tregs also directly affect B cells and inhibit macrophages, further highlighting their comprehensive role in modulating the immune response. An imbalance in Treg numbers can have deleterious effects by limiting antiviral effects of effector T cells and contributing to an excessively stimulated immune response in severely infected patients (128).
In a longitudinal analysis, immunological, inflammatory, and metabolic data were collected from patients to generate a composite signature predictive of systemic recovery. The study found intrapatient covariation of innate immune cell numbers, levels of kynurenine and lipid metabolites, and other molecular and cellular parameters that predicted recovery, mortality, and post-acute sequelae of SARS-CoV-2 infection. Notably, patients with persisting inflammation, a characteristic of the recovery group in this study, had reduced Treg cell counts (129).
A further longitudinal transcriptome analysis, which emphasized robust T cell immunity during recovery from COVID-19 showed that, during recovery, there was a significant downregulation of humoral immunity and type I interferon response, alongside upregulation in genes involved in T cell activation and differentiation. These findings support the role of T cell immunity, including the function of Tregs, in the immune protection against COVID-19 (130). Also, a scoping review that focused on Treg dynamics in convalescent COVID-19 patients showed that while Treg populations can reconstitute during recovery, there is an observed dysregulation in the Treg compartment that can persist for months. This dysregulation may be linked to the immune system-associated sequelae observed in Long COVID patients (131). Therefore, the evidence suggests that Treg dysregulation, as reflected in altered transcriptional markers, plays a crucial role in the pathophysiology of Long COVID. These findings underscore the importance of further research to elucidate the complex interplay between Tregs and other immune components in Long COVID, which could potentially inform targeted therapeutic strategies.
The importance of transcriptome analysis for PASC assessment is underlined by the fact that a transcriptome-wide investigation showed that processes leading to PASC already start during hospitalization for acute COVID-19 and divergent etiologies for different sets of symptoms were identified, depending on the antibody response to SARS-CoV-2 spike protein. This points to the fact that study designs capturing only the post-acute phase may not take into account valuable explanations for pathogenesis of PASC (132). Furthermore, variable recovery rates in the transcriptome of COVID-19 convalescents were observed, as some convalescents return to baseline transcriptome within 24 weeks after infection, but not those with long COVID, suggesting persistent transcriptional dysregulation. This persistence of dysregulation might explain continued symptoms like fatigue post COVID-19 (126).
6 Risk factors for long COVID-19
At the onset of SARS-CoV-2 pandemic, heath care providers were caught off guard and, racing against time to identify solutions to limit the spread of the virus and treat C-19. Early studies identified several risk factors that influence the severity of C-19 such as age, gender, co-morbidities, persistent lesions, time of hospitalization, treatment, or lifestyle. When the spread was contained and more effective treatments and vaccines became available, the heath care communities were faced with another challenge which was that over half of the population that had C-19 showed symptoms of L-C19. Due to the long period of manifestation, this led to the exacerbation of existing diseases, triggering of new ones, but also to major changes in the quality of life. Thus, the recognition, diagnosis, and management of L-C19 became critical. Similar to the acute phase of C-19, L-C19 also depends on a series of risk factors such as sex, age, co-morbidities, severity of C-19, hospitalization and its duration, C-19 sequelae, the treatment applied in the acute phase, vaccination, as well as lifestyle.
6.1 Sex
All studies indicate that regardless of the severity of C-19, women are more likely to develop L-C19. Although the precise mechanisms for sex differences are not completely elucidated, the faster immune response of women, which protect them from initial infection and severity might be one important factor. Unfortunately, this makes females more vulnerable to prolonged autoimmune related diseases. Other sex differences include different sex hormones, high exposure and high psychological stress occupation. Furthermore, women have been shown to be diagnosed later than men (133) and are more likely to develop L-19 cognitive disorders, anosmia, dysgeusia, respiratory and rheumatological sequelae (134–137). On the other hand, men are more exposed to renal sequelae and endothelium disfunction (138–140). Other findings suggest that women had lower mortality and lower levels of inflammation than men (141) which may be due to the immunomodulatory effects of estradiol in females and higher antiplatelet and vasodilatory activity (142, 143). Conversely, men have higher risk of inflammation or tissue damage due to higher amount of cytokines and chemokines like IL-2, TNFα, IL-7, IL10, IL-18, CCL14, or CCL23 (144). Furthermore, it has been shown that production of IL-6 inflammatory marker is lower after viral infection in women which has been associated with a higher risk of developing L-C19 symptoms (145). In a meta-analysis study, Notarte et al. showed that females presented a higher risk of developing L-C19 symptoms such as dyspnea, fatigue, breathlessness, chest pain, palpitations, depression, sleep disorders, hair loss, ocular problems, and GI-related problems than men (146).
In pregnant women, C19 had more serious consequences in the acute phase, with premature births and even deaths among both mothers and newborns. In terms of the long-term effects of the virus on pregnant women, research shows a similarity between the symptoms of the general population and pregnant women (147, 148). For example, in their study conducted on 99 pregnant women, Zhou et al. demonstrated that 74.75% exhibited at least one symptom of L-C19 (presumably referring to a condition related to COVID-19), with the most common symptoms being fatigue, myalgia, and anosmia/ageusia (148). The same symptoms, to which difficulty in concentration and hair loss were added, have also been described by Vásconez-González et al. Most symptoms were initiated with infection (42.4%) and 3-5 weeks after infection (35.5%), which lasted between 3 and 6 months (21.2%), more than two months (18.6%) or between 4 and 8 weeks (17.8%) (149). Antenatal depression (25.2%) and antenatal anxiety (27.9%) were the most common symptoms observed in post-epidemic period among pregnant women. The prevalence of these symptoms were lower than those during pandemic, but higher than those from pre COVID period. The factors that most influenced the physiological state were psychiatric treatment history, psychological counseling before pregnancy, age, education, access to information, financial instability, trimester, pregnancy complications, number of hospital stays and poor marital relationship. Financial security and high levels of social support have led to a reduction in anxiety and depression among pregnant women (150–152).
6.2 Age
Although C-19 is less common in children than adults, L-C19 and multisystem inflammatory syndrome (MIS-C) are long-term consequences observed in asymptomatic patients (153, 154). Like adults, adolescent girls are also more prone to L-C19 than boys (155, 156). The main L-C19 symptoms reported in children and adolescents were neuropsychiatric: mood, fatigue, sleep disorder, headache, cognition, dizziness, neurological abnormalities (pins, tremor), balance problems; cardiorespiratory: respiratory symptoms, sputum/nasal congestion. orthostatic intolerance, exercise intolerance, chest pain, rhinorrhea, cough, sore throat, chest tightness, variation in heart rate, palpitations; dermatologic/teguments: hyperhidrosis, dermatologic (dry skin, rashes, hives), hair loss; gastrointestinal: abdominal pain, constipation, diarrhea, vomiting/nausea; and other: loss of appetite, altered smell, body weight variations, myalgia, altered smell, otalgia, ophthalmologic (conjunctivitis, dry eyes), fever, changes in menstruation, urinary symptoms, dysphagia, speech disturbances (157–159).
The quality of life of children and adolescents was not as impacted by L-C19 as that of adults. A small proportion reported feeling scared and worried and experienced a lack of friendship. School absence due to ill period affected their general wellbeing and it was a disturbance factor for both children and parents (160, 161). Buonsenso et al. reported that L-C19 greatly influenced the quality of life of children that included energy level (83.3%), mood (58.8%), sleep (56.3%), appetite (49.6%) and lack of concentration (60.6%). The authors concluded that most children had worse activity level than before infection and almost half of them experienced recovery from L-C19 symptoms’ cycles with 25% of them presenting constant symptoms) (162). The treatment strategy for these children should be multidisciplinary and the conventional approaches should be accompanied by changes in dietary habits (163). It is recommended that every affected child to be monitored by a primary care pediatrician four months after C-19 acute phase to check the presence of symptoms and development of new ones. The presence of L-C19 symptoms must be immediately considered and in absence of any doubt, the visit should be rescheduled after three months (164). Children with neuropsychiatric disorders must be followed up by mental health experts, pediatricians and must be supported by family and friends (165). An important issue that should be addressed is that during the pandemic, many children without C-19 experienced similar L-C19 symptoms with those infected: headaches, fatigue, sleep disturbance, and concentration difficulties. Several studies showed that almost all symptoms reported by SARS-CoV-2 infected children were also present in patients with negative test (145, 166).
6.3 Pre-existing conditions
L-C19 symptoms are more prevalent in individuals with underlying diseases, such as diabetes, obesity, cardiovascular or neuropsychiatric related disorders (167). Functional impairments of one or more organs due to C-19 acute phase was also strongly associated with L-C19 symptoms. Thus, coagulation issues, reactivation of some existing viruses (i.e., herpesviruses), dysfunctional nerve signaling, or autoimmunity are considered potential contributors of C-19 sequelae (168, 169). Asthma (39, 170), hyperthyroidism (171), hyperglycemia and dyslipidemia (172) have all been considered high risks for L-C19 development at old age patients. These pathologies degrade ACE2, leading to increase activation of the ACE2 receptors, virus entry and persistence of inflammatory cytokine storm or oxidative stress (145).
6.4 C-19 patients’ hospitalization
Many L-C19 sequelae have been observed after C-19 patients’ hospitalization. At 6-months after hospitalization, 90% of patients presented anxiety, depression, and sleep disorders (173), and the most prevalent symptoms reported at 12 months after discharge were anxiety, dyspnea, and fatigue (174). Evidence shows that individuals hospitalized for C-19 were more predisposed to develop anxiety than those hospitalized for other causes. Furthermore, hospitalized older patients were more predisposed to memory loss or confusion (175). The symptoms attributed to hospitalization are strongly correlated with other factors or comorbidities. For example, women with obesity were more predisposed to severe L-C19 at one year after discharging due to hormonal, pro-inflammatory and metabolic state (176).
When comparing hospitalized with non-hospitalized patients, O’Mahoney et al. reported that the five most common symptoms of hospitalized patients were fatigue (28.4%), pain/discomfort (27.9%), sleep difficulties (23.5%), breathlessness (22.6%), and limited regular activity (22.3%). Other symptoms included changes in lung structure/function, ground glass opacification, fibrotic changes, and reticular patterns. The non-hospitalized patients presented similar symptoms, however, with less incidence: fatigue (34.8%), breathless (20.4%), muscle pain/myalgia (17.0%), sleep disorders (15.3%), and loss of smell (12.7%) (177). Other symptoms observed in non-hospitalized patients were pneumonia, chest pain, palpitation, diabetes mellitus, and alopecia (178).
6.5 Reinfection
Reinfection was defined as the presence of new C-19 symptoms that occur more than 90 days after the previous diagnosis of confirmed SARS-CoV-2 infection (179). The severity of reinfection depends on the severity of the initial episode and is strongly correlated with genetic factors particularly related to the innate immune response and pathogenicity of the specific variant. Reinfections increased the development of L-C19, but were less identified in mild or asymptomatic patients, children and adolescents (180, 181). The most vulnerable to reinfections were healthcare workers who have been predisposed to C-19 infection since the beginning of the pandemic. The most frequent reported symptoms of L-C19 were asthenia (14.2%), cough (8.9%), myalgia (3.0%), dyspnea (2.0%), anosmia (1.8%), concentration deficit (1.5%), and headache (1.4%). When there were two symptoms, fatigue was always one of them. Other symptoms were related to gastroenteric and pulmonary dysfunctions. The most persistent symptoms were those related to neurological (23.3%) and psychological symptoms (18.2%), even after 61 days since the first negative swab (182). Reinfections of healthcare workers led to mild L-C19 symptoms, since most were younger than 65 (179).
6.6 SARS-CoV-2 strain/variant
In addition to host factors such as immunity status, comorbidities, vaccination status etc., Long COVID is also influenced by the virus, including strain/variant (180). Therefore, it is important to distinguish between long COVID infections with the wild type virus and subsequent variants. For instance, a significant decrease in cardiac symptoms, such as chest pain and palpitations were observed during Variant of Concern (VOC) infections periods, compared with wild-type virus infections. This decrease was sufficiently robust in adjusted statistical models, suggesting that this change occurred as a result of different infecting variants. However, the broad spectrum of symptoms belonging to either musculoskeletal or cardiorespiratory symptom clusters was similar in both wild-type and VOC infections, though more studies with higher number of observations/patients are needed to confirm the effects (183). Other studies reported differences in clinical symptoms between viral variants. For instance, most severe symptoms were observed in wild-type, during early waves of COVID, when most symptoms affected upper respiratory and central neurological systems, while anosmia, abdominal symptoms and a vast array of other symptoms were observed in alpha and delta variant infections (184). Similar findings point to the fact that wildtype SARS-CoV-2 infections had the highest post COVID condition (PCC) risks, with 6.44 times higher than subsequent variants. It has been suggested that previous natural infections, rather than vaccination, reduced the PCC risk, implying that natural immunity does not wane and is efficient for protection, including post COVID conditions. Another explanation is the possibility that individuals who did not develop PCC after the first infection, have specific traits that lower the risk of developing the condition after subsequent infections (185). The later variants, such as Omicron or Epsilon, were associated with reduced risk of developing long COVID (186). The fact that initial infections, due to more virulent strains such as the original Wuhan strain and the Alpha variant, are associated with a higher risk of long COVID may be due to an erratic and overwhelmed immune response following SARS-CoV-2. Thus, the possibility of developing severe symptoms is more likely. Also, the immunity status developed due to previous infections could play a role. However, factors such as vaccination, subsequent infection waves or social influences, such as post-traumatic stress disorder, physical inactivity, and lack of exercise during lockdowns may have confounded reports of emotional or cognitive symptoms, which may not be a true reflection of post-C OVID conditions (187). Notwithstanding, there is a general consensus that large cohort studies are needed to better discriminate between the factors leading to post COVID occurrence.
6.7 Vaccination
Several studies have shown that more than half of the vaccinated individuals have fewer symptoms than those who were not immunized by vaccination (188, 189). The most notable differences were observed in fatigue, brain fog, myalgia, and shortness of breath. It seems that these differences were strongly correlated with vaccine type. In their study on 812 L-C19 vaccinated participants, Strain et al. showed that the average symptoms score was greatly improved after vaccination with Astra Zeneca/Oxford vaccine being more efficient only for the fever. The mRNA Moderna vaccine was the best in reducing fatigue, brain-fog, myalgia, gastro-intestinal symptoms and autonomic dysfunction of L-C19. The average symptoms improvement score was 22.6% after Astra Zeneca/Oxford vaccine, 24.4% after the Pfizer/BioNTech vaccine, and 31% after Moderna vaccine. The authors concluded that mRNA Moderna and Pfizer vaccines presented more advantages compared to the modified adenoviral vector vaccine from Astra Zeneca (190). A similar pattern was observed by Notarte et al. in their meta-analysis of the effect of vaccination against L-C10 symptomatology. In addition, the latter study reported that two doses of vaccine were more effective to reduce the risk of L-C19 than a single dose, which was in line with other studies (191, 192). The time of vaccination was also very important, suggesting that individuals who were vaccinated a month before getting infected had reduced risk of experiencing L-C19 symptoms (193). By contrast, Ayoubkhani et al. showed that L-C19 symptoms decreased after the second dose of vaccine, regardless of the type and the time of immunization. If after the first dose, the loss of smell and taste, and trouble sleeping were reduced, after the second dose, the main decrease were observed for fatigue and headache (194). The incidence rate for L-C19 was lowest in individuals vaccinated with the third dose and the symptoms were more persistent in infection with delta and omicron SARS-CoV-2 variant (195–197). Norway national health care programs suggested that vaccination should be considered especially for non-hospitalized patients who experienced mild C-19 because they did not completely recover even eight months after infection (198). Similar results have been reported by Yelin et al. who concluded that almost 60% of individuals with mild or moderate C-19 experienced L-C19 symptoms, with difficulties in returning to the previous way of life (199). Irrespective of the type of vaccine and number of doses, the immunized patients presented an overall improvement in L-C19 symptoms, especially fatigue, breathless or insomnia, quality of life and wellbeing (200, 201).
6.6 Lifestyle
The lifestyle before the acute C-19 phase is also important in the development of symptoms associated with L-C19. For example, L-C19 symptoms were common in smokers and in individuals who worked from home and used car or public transportation instead of walking or cycling (202). This was also confirmed by Wright et al. who showed that physical activity improved mental health and improved quality of life, although it increased fatigue. These results are in line with NICE recommendations that limits graded exercise therapy due to the possibility of symptoms worsening (203). Air pollution has also been associated with L-C19 symptoms’ persistence with patients’ exposure to pollutants being strongly correlated with increased levels of inflammatory cytokines and proteins. Furthermore, the virulence was higher in air polluted areas, with adverse effects on respiratory diseases (204).
7 Management and countries approach to long COVID-19 management and treatment
During the C-19 pandemic, many governments have established strategies and took mitigation actions to reduce and stop the pandemic such as quarantine, social isolation, or confinement. This led to reduction in physical activity, with a negative impact on nutrition behavior and resultant composition of gut microbiota, with major implications in the severity of C19 (3). The burden of COVID as well as available treatments, resources and research have revealed significant disparities between affected communities with different income. However, the evidence on developing long COVID based on income and socio demographic factors is scarce. Determining the number of patients from those infected who later experience persistent symptoms is extremely difficult although it is estimated that between 10% and 45% experience long COVID (205). Therefore, there is an urgent need to identify and address global inequities in access to testing, surveillance, vaccinations and treatment. Very few studies have been conducted in low-income countries, such as Africa. The inadequate medical system, the challenge of following-up patients with L-C19, the lack of multidisciplinary services for patient rehabilitation, insufficient grants for research, and even inadequate funds for treatments and vaccines are significant problems that require comprehensive strategies (206). In general, individuals from middle and low-income countries who have developed L-C19 presented similar symptoms. For example, in African populations, fatigue was the most common symptom (26-56%), followed by confusion or lack of concentration (12-68%) and dyspnea (12-38%). Risk factors for L-C19 development were female sex, comorbidities, such as chronic illness, obesity, hypertension, hyperlipidemia, diabetes mellitus, and cardiovascular diseases, or lack of vaccines (207). Patients from low incomes countries have limited access to treatments, vaccines, and proper nutrition thus ensuring access for all individuals to quality medical services, rehabilitation, and disease management is critical (208).
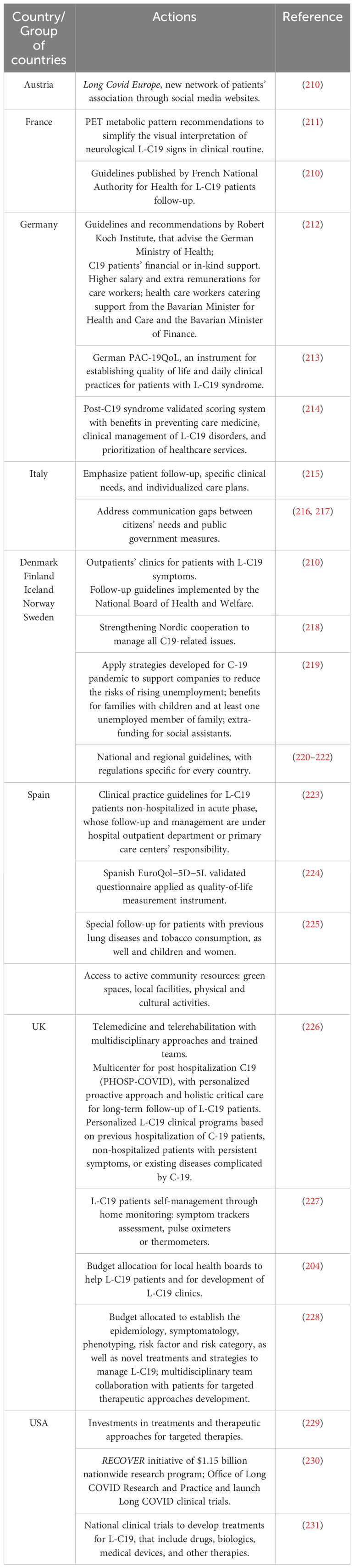
As early as March 2021, the WHO Regional Office for Europe developed protocols and guidelines for long COVID with several countries following suit on developing their own strategies. For example, Denmark was the first country that published national guidelines for managing the effects of L-C19 (November 2020, revised March 2021). On February 2021, France came up with its own guidelines, focusing on treatment and prevention of L-C19. This included rehabilitation centers for patients with severe problems and physiotherapy delivered through outpatient units for those with less severe symptoms. The Netherlands employed online strategies for patients and their representatives. For instance, “Long-form Corona Line” is a platform were L-C19 patients share their experiences with other patients and access medical experts; “Support around Corona” is a platform specialized in psychotraumatology, which offers support for mental health issues that is available to the public and health professionals. On the other hand, Georgia’s rehabilitation program focused on L-C19 patients with pulmonary symptoms, who received long-term treatment in centers (more than 21 days). Other measures included patient education, training and support for physicians. Poland was the first country that applied WHO’s recommendations regarding physiotherapy for L-C19 patients. Portugal developed a national guideline for L-C19 management, with focus on multidisciplinary management in primary care. In Italy, the National Institute of Health and academic centers developed and implemented multidisciplinary approaches that involved clinical, psychological and neurological factors. Austria developed multi-centers that followed-up patients with severe L-C19 symptoms in their first, third and sixth month after discharge. In Kazakhstan, the rehabilitation of L-C19 patients is provided mainly in hospitals, particularly for those with severe manifestations of L-C19 symptoms that include immune deficiency conditions, cognitive disorders, progression of atherosclerosis and exacerbation of hypertension (209). Table 3 depicts several examples of global good practices that may be used as models for recognizing, diagnosing, and managing L-C19.
8 Therapeutical approaches for long COVID-19
Prospective interventions studies on L-C19 include rehabilitation programs (on-site or online) for cognitive disorders, targeted drug therapies (specific drugs or microbiota modulating drugs), metabolic modulators, immunomodulatory therapies, antifibrotic and anticoagulation therapies (232, 233). Supplementation with vitamins such as B2, E, C and antioxidants have also been recommended and may represent a potential therapeutic strategy for neurorehabilitation. Evidenced obtained thus far concluded that some pharmaceutical treatments such as antidepressants have no effect, and non-pharmaceutical procedures, such as cognitive-behavior therapy, graded-exercise-related therapies, rehabilitation, or acupuncture showed inconsistent results. Since L-C19 affects multi organ systems, a multidisciplinary approach is required for its management and treatment. Hence, for a better understanding of the pathology and therapeutic approaches, the early initiation of treatment is crucial, and the strategies must be the result of teamwork with different expertise, such as neurologists, psychiatrists, psychologists, physiotherapists, occupational therapists, geriatric, respiratory, cardiovascular, endocrine, renal, hematologic, or autoimmune disease specialists (174, 234, 235). Other potential therapeutic approaches for L-C19 are presented in Table 4.
Irrespective of the proposed therapeutic method, it is essential that individuals suffering from L-C19 be included in rehabilitation programs to identify physical, emotional, cognitive and social treatable traits. The issue may be even more pressing for those who, after 6 months of L-C19, not only continue to experience significant symptoms but also to identify new ones (247).
Pharmacological treatment approaches include treatment with β-blockers, fludrocortisone, midodrine, increase in salt and fluid intake, intravenously administered salt, compression stockings for postural orthostatic tachycardia syndrome (POTS) and even for myalgic encephalomyelitis or chronic fatigue syndrome treatment (248). Administration of intravenous immunoglobulin can be considered for immune dysfunction and a new drug, BC007 who neutralizes G protein-coupled receptor autoantibodies has shown promising results for autoimmunity that occur in L-C19 (249). Administration of nirmatrelvir-ritonavir has been used for patients with persistent SARS COV-2, since some studies suggest that the persistence of SARS-CoV-2 in tissues and especially in the intestinal microbiome and virome may be involved in the pathogenesis of L-C19 (250). The use of valacyclovir, valganciclovir, famciclovir for reactivation of infections with Epstein–Barr virus, cytomegalovirus and varicella zoster virus may help prevent the neurological manifestations of L-C19, because reactivated herpesviruses are also associated with ME/CFS (251). Anticoagulants can prevent abnormal clotting and can be useful in L-C19, with some studies recommending even triple anticoagulant therapy (100, 252). Apheresis can also help to remove microvascular blood clotting and has been shown to reduce autoantibodies in ME/CFS, but high costs limit its use (253). Finally, Coenzyme Q10 and d-ribose have been shown to have a beneficial role in asthenia and neurological symptoms found in MEC/CFS (254).
9 Imaging investigations in long COVID-19
Imaging studies have been used to evaluate lung diseases both in detecting the degree of damage in the acute phase and in the evaluation of post-COVID sequelae. Computer tomography (CT) scans are the standard method for accurately estimating the lung area affected by the infection. Imaging analyses have been less used in investigating the long-term effects of C-19. L-C19 causes damage to other organs such as the kidneys, heart, and brain, thus using MRI examinations of COVID patients showed multiorgan abnormalities in 61% of patients vs. 14% in non-infected controls that were seen in the lungs, kidneys, and brain. At five months after discharge, a significant number of patients had lung damage in excess of 5%. These findings underscore the need for imaging surveillance of patients in addition to blood tests or detailed medical history evaluation (255).
10 Current and future research opportunities
L-C19 management involves physical rehabilitation which should begin in the acute phase because early mobilization could reduce hospitalization and functional outcomes; management of pre-existing comorbidities that can avoid clinical deterioration, readmission of patients and avoid other pathologies and symptoms development; mental health support, or social services support. The management of patients include patient-reported outcomes for long-term follow-up care and for early detection of possible adverse events and the use of digital technologies, such as telemedicine - videoconferences for patients’ follow-up and reinfection risk elimination, digital therapeutics approach, especially for non-pharmaceutical interventions (e.g., breathing exercises), electronic control of body parameters such as temperature, oxygen saturation, blood pressure, that can be measured with wearable devices and the data can be analyzed with artificial intelligence (256, 257). In their Delphi study, Nurek et al. suggested that recommendations for the recognition, diagnosis, and management of L-C19 should meet several criteria related to i) clinic organization (cross-specialty doctors, multi-specialized medical team, individualized investigations, patients’ equitability regardless of the comorbid mental health difficulties), ii) diagnosis of disorders (specific guidelines, early-stage symptoms’ identification), and iii) management (patients’ follow-up and support, encouraging the report of new signs and symptoms) (258). Healthcare and research workers training and education, public communication campaigns and research funding should be one of the main goals of L-C19 management (259).
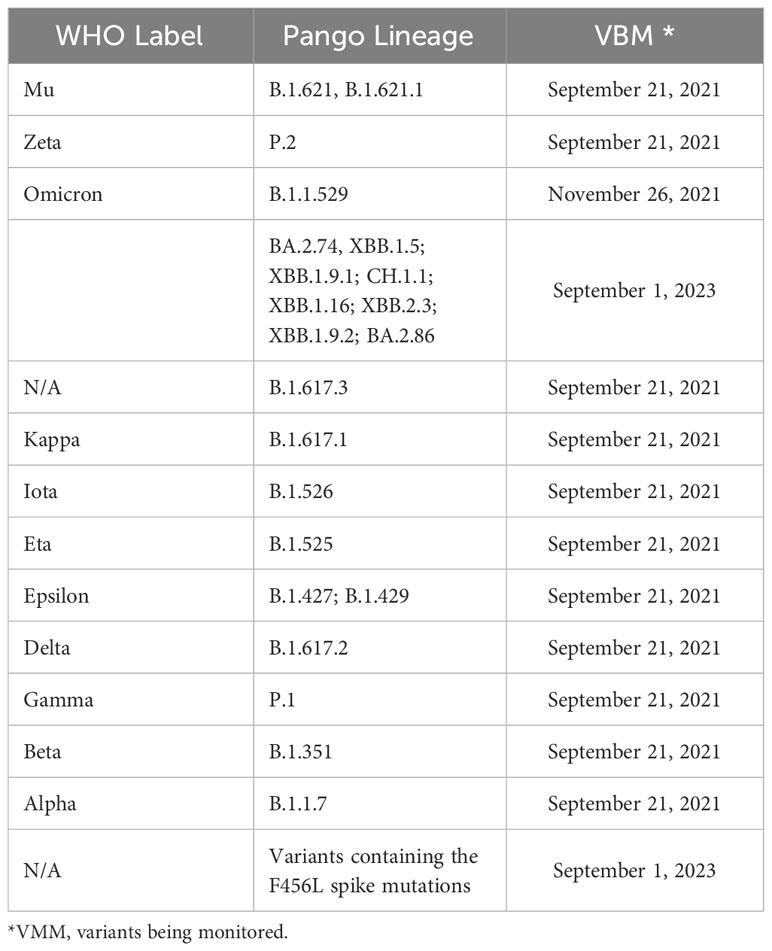
Current priorities for research should include: i) determining the L-C19 sequelae and symptoms in early stage of development; ii) identifying the pathophysiological mechanisms that contribute to L-C19 development; iii) evaluating the long-term impact for patients with other pre-existing co-morbidities, hospitalized or with ICU interventions in acute C-19 phase; iv) establishing strategies and regulations for L-C-19 recovery; v) identifying therapeutic solutions, innovations in novel fast-acting therapies and drugs, vi) evaluating the role of vaccination; vii) understanding the impact of L-C19 on patients’ daily life, management and healthcare costs (260). These strategies should be corroborated with emerging viral mutations that have been reported (Table 5).
Whether the newly emerging variants are likely to cause more serious effects is not yet clear, predicting their long-term effects is also challenging. Thus, devising novel strategies such as examination of SARS-CoV-2 spike protein epistatic networks may be useful in forecasting viral haplotypes with high transmissibility (263). Notwithstanding, the COVID-19 pandemic taught us that, uncertainty is specific to such viruses that undergo multiple mutations within a short period of time, thus it is incumbent on the research community to decipher the mechanisms of action and to predict as best as possible the likely consequences (264).
Author contributions
RG: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. IS: Data curation, Writing – original draft. AL: Data curation, Writing – original draft. OS: Data curation, Writing – original draft. RF: Conceptualization, Writing – original draft. AC-B: Data curation, Writing – original draft. MD: Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing. SM: Data curation, Funding acquisition, Project administration, Writing – review & editing. MC: Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant of the Ministry of Research, Innovation and Digitization, under the Romania’s National Recovery and Resilience Plan funded by EU Next Generation EU program, project “Artificial intelligence-powered personalized health and genomics libraries for the analysis of long-term effects in COVID-19 patients (AI-PHGL-COVID)” number 760073/23.05.2023, code 285/30.11.2022, within Pillar III, Component C9, Investment 8 and by a grant of the Ministry of Research, Innovation and Digitization, CNCS, UEFISCDI, project number PN-III-Pl-1.1-PD-2021-0273, within PNCDI III.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1344086/full#supplementary-material
References
1. WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online at: https://covid19.who.int/ (Accessed Jul. 29, 2023).
2. Sugiyama A, Miwata K, Kitahara Y, Okimoto M, Abe K, Bunthen E, et al. Long COVID occurrence in COVID-19 survivors. Sci Rep. (2022) 12. doi: 10.1038/s41598-022-10051-z
3. Rodriguez-Sanchez I, Rodriguez-Mañas L, Laosa O. Long COVID-19: the need for an interdisciplinary approach. Clin Geriatr Med. (2022) 38:533–44. doi: 10.1016/J.CGER.2022.03.005
4. Post COVID-19 condition (Long COVID). Available online at: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (Accessed Jul. 29, 2023).
5. Nittas V, Gao M, West E, Ballouz T, Menges D, Hanson SW, et al. Long COVID through a public health lens: an umbrella review. Public Health Rev. (2022) 43:1–10. doi: 10.3389/PHRS.2022.1604501
6. Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. (2021) 9:129. doi: 10.1016/S2213-2600(21)00031-X
7. Raveendran AV. Long COVID-19: Challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab Syndrome: Clin Res Rev. (2021) 15:145–6. doi: 10.1016/J.DSX.2020.12.025
8. Mantovani A, Concetta Morrone M, Patrono C, Santoro MG, Schiaffino S, Remuzzi G, et al. Long Covid: where we stand and challenges ahead. Cell Death Differ. (2022) 29:1891–900. doi: 10.1038/S41418-022-01052-6
9. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/S41579-022-00846-2
10. Rando HM, Bennett TD, Byrd JB, Bramante C, Callahan TJ, Chute CG, et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv. (2021). doi: 10.1101/2021.03.20.21253896
11. Fernández-De-las-peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining post-COVID symptoms (Post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. (2021) 18:1–9. doi: 10.3390/IJERPH18052621
12. Khazaal S, Harb J, Rima M, Annweiler C, Wu Y, Cao Z, et al. The pathophysiology of long COVID throughout the renin-angiotensin system. Molecules. (2022) 27(9):2903. doi: 10.3390/MOLECULES27092903
13. Blomberg B, Greve-Isdahl Mohn K, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. (2021) 27:1607–13. doi: 10.1038/S41591-021-01433-3
14. Byrne EA. Understanding Long Covid: Nosology, social attitudes and stigma. Brain Behav Immun. (2022) 99:17–24. doi: 10.1016/J.BBI.2021.09.012
15. Vernon SD, Hartle M, Sullivan K, Bell J, Abbaszadeh S, Unutmaz D, et al. Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Work. (2023) 74:1179–86. doi: 10.3233/WOR-220581
16. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-A systemic review and comparison of clinical presentation and symptomatology. Med (Kaunas). (2021) 57(5):418. doi: 10.3390/MEDICINA57050418
17. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PloS Med. (2021) 18:e1003773. doi: 10.1371/JOURNAL.PMED.1003773
18. Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep. (2021) 9(3):e14726. doi: 10.14814/PHY2.14726
19. de Miranda DA, Gomes S, Filgueiras PS, Corsini CA, Almeida NB, Silva RA, et al. Long COVID-19 syndrome: a 14-months longitudinal study during the two first epidemic peaks in Southeast Brazil. Trans R Soc Trop Med Hyg. (2022) 116:1007–14. doi: 10.1093/TRSTMH/TRAC030
20. Roth PH, Gadebusch-Bondio M. The contested meaning of ‘long COVID’ - Patients, doctors, and the politics of subjective evidence. Soc Sci Med. (2022) 292:1–8. doi: 10.1016/J.SOCSCIMED.2021.114619
21. Ziauddeen N, Gurdasani D, O’Hara ME, Hastie C, Roderick P, Yao G, et al. Characteristics and impact of Long Covid: Findings from an online survey. PloS One. (2022) 17(3):e0264331. doi: 10.1371/JOURNAL.PONE.0264331
22. Chan Sui Ko A, Candellier A, Mercier M, Joseph C, Schmit JL, Lanoix JP, et al. Number of initial symptoms is more related to long COVID-19 than acute severity of infection: a prospective cohort of hospitalized patients. Int J Infect Dis. (2022) 118:220–3. doi: 10.1016/J.IJID.2022.03.006
23. Mendelson M, Nel J, Blumberg L, Madhi SA, Dryden M, Stevens W, et al. Long-COVID: An evolving problem with an extensive impact. South Afr Med J. (2020) 111:10–2. doi: 10.7196/SAMJ.2020.V111I11.15433
24. Kostka K, Roel E, Trinh NTH, Mercadé-Besora N, Delmestri A, Mateu L, et al. The burden of post-acute COVID-19 symptoms in a multinational network cohort analysis. Nat Commun. (2023) 14:7449. doi: 10.1038/s41467-023-42726-0
25. Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. (2021) 19:2546–53. doi: 10.1111/JTH.15490
26. Townsend L, Fogarty H, Dyer A, Martin-Loeches I, Bannan C, Nadarajan P, et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. (2021) 19:1064–70. doi: 10.1111/JTH.15267
27. Constantinescu-Bercu A, Kessler A, de Groot R, Dragunaite B, Heightman M, Hillman T, et al. Analysis of thrombogenicity under flow reveals new insights into the prothrombotic state of patients with post-COVID syndrome. J Thromb Hemostasis. (2023) 21:94–100. doi: 10.1016/J.JTHA.2022.10.013
28. Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. (2021) 20. doi: 10.1186/S12933-021-01359-7
29. Joshee S, Vatti N, Chang C. Long-term effects of COVID-19. Mayo Clin Proc. (2022) 97:579–99. doi: 10.1016/J.MAYOCP.2021.12.017
30. Kelin J, Wood J, Jaycox JR, Dhodapkar RM, Lu P, Gehlhausen JR, et al. Distinguishing features of long COVID identified through immune profiling. Nature. (2023) 623:139–48. doi: 10.1038/s41586-023-06651-y
31. Liang L, Yang B, Jiang N, Fu W, He X, Zhou Y, et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. (2020) 35(47):e418. doi: 10.3346/JKMS.2020.35.E418
32. Bungenberg J, Humkamp K, Hohenfeld C, Rust MI, Ermis U, Dreher M, et al. Long COVID-19: Objectifying most self-reported neurological symptoms. Ann Clin Transl Neurol. (2022) 9:141–54. doi: 10.1002/ACN3.51496
33. Van Kessel SAM, Olde Hartman TC, Lucassen PLBJ, Van Jaarsveld CHM. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract. (2022) 39:159–67. doi: 10.1093/FAMPRA/CMAB076
34. Bansal R, Gubbi S, Koch CA. COVID-19 and chronic fatigue syndrome: An endocrine perspective. J Clin Transl Endocrinol. (2022) 27:100284. doi: 10.1016/j.jcte.2021.100284
35. Salari N, Khodayari Y, Hosseinian-Far A, Zarei H, Rasoulpoor S, Akbari H, et al. Global prevalence of chronic fatigue syndrome among long COVID-19 patients: A systematic review and meta-analysis. Biopsychosoc Med. (2022) 16:21. doi: 10.1186/s13030-022-00250-5
36. Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, Almulla AF, Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol Psychiatry. (2022) 28:564–78. doi: 10.1038/s41380-022-01836-9
37. Twomey R, Demars J, Franklin K, Nicole Culos-Reed S, Weatherald J, Wrightson JG. Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys Ther. (2022) 102(4):pzac005. doi: 10.1093/PTJ/PZAC005
38. Son K, Jamil R, Chowdhury A, Mukherjee M, Venegas C, Miyasaki K, et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J. (2023) 61:2200970. doi: 10.1183/13993003.00970-2022
39. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. (2021) 374:n1648. doi: 10.1136/BMJ.N1648
40. Righi E, Mirandola M, Mazzaferri F, Dossi G, Razzaboni E Zaffagnini E, et al. Determinants of persistence of symptoms and impact on physical and mental wellbeing in Long COVID: A prospective cohort study. J Infect. (2022) 84:566–72. doi: 10.1016/J.JINF.2022.02.003
41. Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: an overview,” Diabetes & Metabolic syndrome. Clin Res Rev. (2021) 15:869–75. doi: 10.1016/J.DSX.2021.04.007
42. Wiech M, Chroscicki P, Swatler J, Stepnik D, De Biasi S, Hampel M, et al. Remodeling of T cell dynamics during long COVID is dependent on severity of SARS-coV-2 infection. Front Immunol. (2022) 13:886431/BIBTEX. doi: 10.3389/fimmu.2022.886431
43. Guo P, Ballesteros AB, Yeung SP, Liu R, Saha A, Curtis L, et al. COVCOG 2: cognitive and memory deficits in long COVID: A second publication from the COVID and cognition study. Front Aging Neurosci. (2022) 14:804937/BIBTEX. doi: 10.3389/fnagi.2022.804937
44. Morioka S, Tsuzuki S, Maruki T, Terada M, Miyazato Y, Kutsuna S, et al. Epidemiology of post-COVID conditions beyond 1 year: a cross-sectional study. Public Health. (2023) 216:39–44. doi: 10.1016/J.PUHE.2023.01.008
45. Ishikura T, Nakano T, Kitano T, Tokuda T, Sumi-Akamaru H, Naka T. Serum ferritin level during hospitalization is associated with Brain Fog after COVID-19. Sci Rep. (2023) 13:1–8. doi: 10.1038/s41598-023-40011-0
46. Alemanno F, Houdayer E, Parma A, Spina A, Del Forno A, Scatolini A, et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: A COVID-rehabilitation unit experience. PloS One. (2021) 16:e0246590. doi: 10.1371/JOURNAL.PONE.0246590
47. Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med. (2022) 54:1473–87. doi: 10.1080/07853890.2022.2076901
48. de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S. The chronic neuropsychiatric sequelae of COVID-19: The need for a prospective study of viral impact on brain functioning. Alzheimer’s Dementia. (2021) 17:1056–65. doi: 10.1002/ALZ.12255
49. Hugon J, Msika EF, Queneau M, Farid K, Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. (2022) 269:44–6. doi: 10.1007/s00415-021-10655-x
50. Monje M, Iwasaki A. The neurobiology of long COVID. Neuron. (2022) 110:3484–96. doi: 10.1016/J.NEURON.2022.10.006
51. Tian T, Wu J, Chen T, Li J, Yan S, Zhou Y, et al. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight. (2022) 7(4):e155827. doi: 10.1172/JCI.INSIGHT.155827
52. Asadi-Pooya AA, Akbari A, Emami A, Lotfi M, Rostamihosseinkhani M, Nemati H, et al. Long COVID syndrome-associated brain fog. J Med Virol. (2022) 94:979–84. doi: 10.1002/JMV.27404
53. Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. (2021) 8:130–40. doi: 10.1016/S2215-0366(20)30462-4
54. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. (2021) 38. doi: 10.1016/j.eclinm.2021.101019
55. Tana C, Bentivegna E, Cho SJ, Harriott AM, García-Azorín D, Labastida-Ramirez A, et al. Long COVID headache. J Headache Pain. (2022) 23:1–12. doi: 10.1186/s10194-022-01450-8
56. Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P. Toward a better understanding of persistent headache after mild COVID-19: Three migraine-like yet distinct scenarios. Headache. (2021) 61:1277–80. doi: 10.1111/HEAD.14197
57. Trigo J, García-Azorín D, Planchuelo-Gómez A, Martínez-Pías E, Talavera B, Hernández-Pérez I, et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: a retrospective cohort study. J Headache Pain. (2020) 21. doi: 10.1186/S10194-020-01165-8
58. Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. EClinicalMedicine. (2021), 100899. doi: 10.1016/j.eclinm.2021.100899
59. Fernández-de-las-Peñas C, Navarro-Santana M, Gómez-Mayordomo V, Cuadrado ML, García-Azorín D, Arendt-Nielsen L, et al. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: A meta-analysis of the current literature. Eur J Neurol. (2021) 28:3820–5. doi: 10.1111/ene.15040
60. Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al. Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalization for COVID-19. Thorax. (2021) 76:396–8. doi: 10.1136/THORAXJNL-2020-215818
61. Caspersen IH, Magnus P, Trogstad L. Excess risk and clusters of symptoms after COVID-19 in a large Norwegian cohort. Eur J Epidemiol. (2022) 37:539–48. doi: 10.1007/S10654-022-00847-8
62. Pinzon RT, Wijaya VO, Al Jody A, Nunsio PN, Buana RB. Persistent neurological manifestations in long COVID-19 syndrome: A systematic review and meta-analysis. J Infect Public Health. (2022) 15:856–69. doi: 10.1016/J.JIPH.2022.06.013
63. Da Costa BA, Silva L, Mendes M, Karmann I, Campos B, Nogueira M, et al. Anxiety and depression are associated with limbic atrophy and severe disruption of brain functional connectivity after mild COVID-19 infection (S21.007). J Alzheimer’s Dis (2023) 96(4):1711–20. doi: 10.3233/JAD-230632
64. Efstathiou V, Stefanou MI, Demetriou M, Siafakas N, Makris M, Tsivgoulis G, et al. Long COVID and neuropsychiatric manifestations (Review). Exp Ther Med. (2022) 23(5):363. doi: 10.3892/ETM.2022.11290
65. Mekhael M, Lim CH, El Hajjar AH, Noujaim C, Pottle C, Makan N, et al. Studying the effect of long COVID-19 infection on sleep quality using wearable health devices: observational study. J Med Internet Res. (2022) 24(7):e38000. doi: 10.2196/38000
66. Fischer A, Zhang L, Elbéji A, Wilmes P, Oustric P, Staub T, et al. Long COVID symptomatology after 12 months and its impact on quality of life according to initial coronavirus disease 2019 disease severity. Open Forum Infect Dis. (2022) 9(8):ofac397. doi: 10.1093/OFID/OFAC397
67. Seeβle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): A prospective cohort study. Clin Infect Dis. (2022) 74:1191–8. doi: 10.1093/CID/CIAB611
68. Li Z, Zhang Z, Zhang Z, Wang Z, Li H. Cognitive impairment after long COVID-19: current evidence and perspectives. Front Neurol. (2023) 14:1239182/BIBTEX. doi: 10.3389/fneur.2023.1239182
69. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. (2021) 8:416–27. doi: 10.1016/S2215-0366(21)00084-5
70. Taquet M, Skorniewska Z, Hampshire A, Chalmers JD, Ho LP, Horsley A, et al. Acute blood biomarker profiles predict cognitive deficits 6 and 12 months after COVID-19 hospitalization. Nat Med. (2023) 29:2498–508. doi: 10.1038/s41591-023-02525-y
71. Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RA, et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. (2022) 22:639–49. doi: 10.1038/s41577-022-00762-9
72. Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. (2018) 19:283–301. doi: 10.1038/nrn.2018.13
73. Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicenter prospective observational study. Lancet Respir Med. (2020) 8:1201–8. doi: 10.1016/S2213-2600(20)30370-2
74. Petersen MS, Kristiansen MF, Hanusson KD, Foldbo BM, Danielsen ME, Steig BA, et al. Prevalence of long COVID in a national cohort: longitudinal measures from disease onset until 8 months’ follow-up. Int J Infect Dis. (2022) 122:437. doi: 10.1016/J.IJID.2022.06.031
75. Deer RR, Rock MA, Vasilevsky N, Carmody L, Rando H, Anzalone AJ, et al. Characterizing long COVID: deep phenotype of a complex condition. EBioMedicine. (2021) 74. doi: 10.1016/J.EBIOM.2021.103722
76. Najafloo R, Majidi J, Asghari A, Aleemardani M, Kamrava SK, Simorgh S, et al. Mechanism of anosmia caused by symptoms of COVID-19 and emerging treatments. ACS Chem Neurosci. (2021) 12:3795–805. doi: 10.1021/ACSCHEMNEURO.1C00477
77. Jung S, Allali G, Benzakour L, Nuber-Champier A, Thomasson M, Jacot de Alcântara I, et al. Long COVID neuropsychological deficits after severe, moderate, or mild infection. Clin Trans Neurosci. (2022) 6:9. doi: 10.3390/CTN6020009
78. Boscolo-Rizzo P, Spinato G, Hopkins C, Marzolino R, Cavicchia A, Zucchini S, et al. Evaluating long-term smell or taste dysfunction in mildly symptomatic COVID-19 patients: a 3-year follow-up study. Eur Arch Otorhinolaryngol. (2023) 280:5625–30. doi: 10.1007/s00405-023-08227-y
79. Lupi L, Bordin A, Sales G, Colaianni D, Vitiello A, Biscontin Av , et al. Persistent and transient olfactory deficits in COVID-19 are associated to inflammation and zinc homeostasis. Front Immunol. (2023) 14:1148595. doi: 10.3389/fimmu.2023.1148595
80. Vaira LA, Gessa C, Deiana G, Salzano G, Maglitto F, Lechien JRv, et al. The effects of persistent olfactory and gustatory dysfunctions on quality of life in long-COVID-19 patients. Life. (2022) 12:141. doi: 10.3390/LIFE12020141
81. Yan Z, Yang M, Lai CL. Long COVID-19 syndrome: A comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines. (2021) 9(8):966. doi: 10.3390/BIOMEDICINES9080966
82. Yende S, Parikh CR. Long COVID and kidney disease. Nat Rev Nephrol. (2021) 17:792–3. doi: 10.1038/s41581-021-00487-3
83. Silver SA, Beaubien-Souligny W, Shah PS, Harel S, Blum D, Kishibe T, et al. The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: A systematic review and meta-analysis. Kidney Med. (2021) 3:83–98.e1. doi: 10.1016/j.xkme.2020.11.008
84. Blackett JW, Wainberg M, Elkind MSV, Freedberg DE. Potential long coronavirus disease 2019 gastrointestinal symptoms 6 months after coronavirus infection are associated with mental health symptoms. Gastroenterology. (2022) 162:648–650.e2. doi: 10.1053/J.GASTRO.2021.10.040
85. Andrade BS, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, dos Santos Freitas A, et al. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. (2021) 13(4):700. doi: 10.3390/V13040700
86. Galán M, Vigón L, Fuertes D, Murciano-Antón MACasado-Fernández G, Domínguez-Mateos S, et al. Persistent overactive cytotoxic immune response in a spanish cohort of individuals with long-COVID: identification of diagnostic biomarkers. Front Immunol. (2022) 13:848886/BIBTEX. doi: 10.3389/fimmu.2022.848886
87. Tate W, Walker M, Sweetman E, Helliwell A, Peppercorn K, Edgar C, et al. Molecular mechanisms of neuroinflammation in ME/CFS and long COVID to sustain disease and promote relapses. Front Neurol. (2022) 13:877772/BIBTEX. doi: 10.3389/fneur.2022.877772
88. Hastie CE, Lowe DJ, McAuley A, Winter AJ, Mills NL, Black C, et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun. (2022) 13. doi: 10.1038/S41467-022-33415-5
89. Healey Q, Sheikh A, Daines L, Vasileiou E. Symptoms and signs of long COVID: A rapid review and meta-analysis. J Glob Health. (2022) 12:5014. doi: 10.7189/JOGH.12.05014
90. Hadi HAR, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. (2005) 1:183.
91. Kruger A, Vlok M, Turner S, Venter C, Laubscher GJ, Kell DB, et al. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol. (2022) 21(1):190. doi: 10.1186/S12933-022-01623-4
92. Kell DB, Laubscher GJ, Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. (2022) 479:537–59. doi: 10.1042/BCJ20220016
93. Leng A, Shah M, Ahmad SA, Premraj L, Wildi K, Bassi GL, et al. Pathogenesis underlying neurological manifestations of long COVID syndrome and potential therapeutics. Cells. (2023) 12(5):816. doi: 10.3390/CELLS12050816
94. von Meijenfeldt FA, Havervall S, Adelmeijer J, Lundström A, Magnusson M, Mackman N, et al. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv. (2021) 5:756–9. doi: 10.1182/BLOODADVANCES.2020003968
95. Willems LH, Nagy M, Ten Cate H, Spronk H, Groh LA, et al. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb Res. (2022) 209:106–14. doi: 10.1016/J.THROMRES.2021.11.027
96. Mancini I, Baronciani L, Artoni A, Colpani P, Biganzoli M, Cozzi G, et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost. (2021) 19:513–21. doi: 10.1111/JTH.15191
97. Prasannan N, Heightman M, Hillman T, Wall E, Bell R, Kessler A, et al. Impaired exercise capacity in post-COVID-19 syndrome: the role of VWF-ADAMTS13 axis. Blood Adv. (2022) 6:4041–8. doi: 10.1182/BLOODADVANCES.2021006944
98. Besnier F, Bérubé B, Malo J, Gagnon C, Grégoire Ca Juneau M, et al. Cardiopulmonary rehabilitation in long-COVID-19 patients with persistent breathlessness and fatigue: the COVID-rehab study. Int J Environ Res Public Health. (2022) 19(7):4133. doi: 10.3390/IJERPH19074133
99. Bitirgen G, Korkmaz C, Zamani A, Ozkagnici A, Zengin N, Ponirakis G, et al. Corneal confocal microscopy identifies corneal nerve fiber loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol. (2022) 106:1635–41. doi: 10.1136/BJOPHTHALMOL-2021-319450
100. Wang C, Yu C, Jing H, Wu X, Novakovic VA, Xie R, et al. Long COVID: the nature of thrombotic sequelae determines the necessity of early anticoagulation. Front Cell Infect Microbiol. (2022) 12:861703/BIBTEX. doi: 10.3389/fcimb.2022.861703
101. Naendrup JH, Garcia Borrega J, Eichenauer DA, Shimabukuro-Vornhagen A, Kochanek M, Böll B. Reactivation of EBV and CMV in severe COVID-19—Epiphenomena or trigger of hyperinflammation in need of treatment? A large case series of critically ill patients. J Intensive Care Med. (2022) 37:1152. doi: 10.1177/08850666211053990
102. Manoharan S, Ying LY. Epstein Barr Virus Reactivation during COVID-19 Hospitalization Significantly Increased Mortality/Death in SARS-CoV-2(+)/EBV(+) than SARS-CoV-2(+)/EBV(–) Patients: A Comparative Meta-Analysis. Int J Clin Pract. (2023) 2023. doi: 10.1155/2023/1068000
103. Rohrhofer J, Graninger M, Lettenmaier L, Schweighardt J, Gentile SA, Koidl L, et al. Association between Epstein-Barr-Virus reactivation and development of Long-COVID fatigue. Allergy. (2023) 78:297–9. doi: 10.1111/ALL.15471
104. Kasl SV, Evans AS, Niederman JC. Psychosocial risk factors in the developmental of infectious mononucleosis. Psychosom Med. (1979) 41:445–66. doi: 10.1097/00006842-197910000-00002
105. Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis. (2021) 112:217–26. doi: 10.1016/j.ijid.2021.09.043
106. Oaklander AL, Mills AJ, Kelley M, Toran LS, Smith B, Dalakas MC, et al. Peripheral Neuropathy Evaluations of Patients With Prolonged Long COVID,”. Neurology. Neuroimmunology & Neuroinflammation (2022) 9(3):. doi: 10.1212/NXI.0000000000001146
107. Karaarslan F, Güneri FD, Kardeş S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheumatol. (2022) 41:289–96. doi: 10.1007/s10067-021-05942-x
108. Chadda KR, Blakey EE, Huang CLH, Jeevaratnam K. Long COVID-19 and postural orthostatic tachycardia syndromeIs dysautonomia to be blamed? Front Cardiovasc Med. (2022) 9:860198/BIBTEX. doi: 10.3389/fcvm.2022.860198
109. Saini G, Aneja R. Cancer as a prospective sequela of long COVID-19. Bioessays. (2021) 43(6):2000331. doi: 10.1002/BIES.202000331
110. Sapkota HR, Nune A. Long COVID from rheumatology perspective - a narrative review. Clin Rheumatol. (2022) 41:337–48. doi: 10.1007/S10067-021-06001-1
111. McMahon DE, Gallman AE, Hruza GJ, Rosenbach M, Lipoff J, Desai SR, et al. Long COVID in the skin: a registry analysis of COVID-19 dermatological duration. Lancet Infect Dis. (2021) 21:313–4. doi: 10.1016/S1473-3099(20)30986-5
112. Wong-Chew RM, Rodríguez Valdez CA, Lomelin-Gascon J, Morales-Juárez L, Rodríguez de la Cerda ML, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. (2022) 9:1–17. doi: 10.1177/20499361211069264
113. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. (2022) 10:311–21. doi: 10.1016/S2213-8587(22)00044-4
114. Raveendran AV, Misra A. Post COVID-19 syndrome (‘Long COVID’) and diabetes: challenges in diagnosis and management. Diabetes Metab Syndrome: Clin Res Rev. (2021) 15:102235. doi: 10.1016/J.DSX.2021.102235
115. Rezel-Potts E, Douiri A, Sun X, Chowienczyk PJ, Shah AM, Gulliford MC. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PloS Med. (2022) 19(7):e1004052. doi: 10.1371/JOURNAL.PMED.1004052
116. Harding JL, Oviedo SA, Ali MK, Ofotokun I, Gander JC, Patel SA, et al. The bidirectional association between diabetes and long-COVID-19 - A systematic review. Diabetes Res Clin Pract. (2023) 195. doi: 10.1016/J.DIABRES.2022.110202
117. MacPherson K, Cooper K, Harbour J, Mahal D, Miller C, Nairn M. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. (2022) 12:e050979. doi: 10.1136/BMJOPEN-2021-050979
118. Orrù G, Bertelloni D, Diolaiuti F, Mucci F, Di Giuseppe M, Biella M, et al. Long-COVID syndrome? A study on the persistence of neurological, psychological and physiological symptoms. Healthcare (Basel). (2021) 9(5):575. doi: 10.3390/HEALTHCARE9050575
119. Watson DLB, Campbell M, Hopkins C, Smith B, Kelly C, Deary V. Altered smell and taste: Anosmia, parosmia and the impact of long Covid-19. PloS One. (2021) 16(9):e0256998. doi: 10.1371/JOURNAL.PONE.0256998
120. Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mulè G, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. (2022) 28:611.e9–611.e16. doi: 10.1016/J.CMI.2021.11.002
121. Ryan FJ, Hope CM, Masavuli MG, Lynn MA, Mekonnen ZA, Yeow AE, et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. (2022) 20:26. doi: 10.1186/s12916-021-02228-6
122. Su Y, Yuan D, Chen DG, Ng R, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. (2022) 185(5):881–895.e20. doi: 10.1016/j.cell.2022.01.014
123. Arish M, Qian W, Narasimhan H, Siu J. COVID-19 immunopathology: From acute diseases to chronic sequelae. J Med Virol. (2023) 95:e28122. doi: 10.1002/jmv.28122
124. Nicolai N, Kaiser R, Stark K. Thromboinflammation in long COVID—the elusive key to postinfection sequelae? J Thromb Haemost. (2023) 21:2020–31. doi: 10.1016/j.jtha.2023.04.039
125. Chun HI, Coutavas E, Pine AB, Lee AI, Yu VL, Shallow MK, et al. Immunofibrotic drivers of impaired lung function in postacute sequelae of SARS-CoV-2 infection. JCI Insight. (2021) 6:e148476. doi: 10.1172/jci.insight.148476
126. Tsuhakara T, Brann D, Datta SR. Mechanisms of SARS-coV-2-associated anosmia. Physiol Rev. (2023) 103:2759–66. doi: 10.1152/physrev.00012.2023
127. Galván-Peña S, Leon J, Chowdhary K, Michelson DA, Vijaykumar B, Yang L, et al. Profound Treg perturbations correlate with COVID-19 severity. PNAS. (2021) 118:e2111315118. doi: 10.1073/pnas.211131511
128. Dhawan M, Rabaan AA, Alwarthan S, Alhajri M, Halwani MA, Alshengeti A, et al. Regulatory T cells (Tregs) and COVID-19: unveiling the mechanisms, and therapeutic potentialities with a special focus on long COVID. Vaccines. (2023) 11:699. doi: 10.3390/vaccines11030699
129. Cron RQ. Immunologic prediction of long COVID. Nat Immunol. (2023) 24:207–8. doi: 10.1038/s41590-022-01396-8
130. Zheng HY, Xu M, Yang CX, Tian RR, Zhang M, Li JJ, et al. Longitudinal transcriptome analyses show robust T cell immunity during recovery from COVID-19. Sig Transduct Target Ther. (2020) 5(1):294. doi: 10.1038/s41392-020-00457-4
131. Haunhorst S, Bloch W, Javelle F, Krüger K, Baumgart S, Drube S, et al. A scoping review of regulatory T cell dynamics in convalescent COVID-19 patients - indications for their potential involvement in the development of Long COVID? Front Immunol. (2022) 13:1070994. doi: 10.3389/fimmu.2022.1070994
132. Thompson RC, Simons NW, Wilkins L, Cheng E, Del Valle DM, Hoffman Ge , et al. Acute COVID-19 gene-expression profiles show multiple etiologies of long-term sequelae. medRxi. (2021). doi: 10.1101/2021.10.04.21264434
133. Asadi-Pooya AA, Akbari A, Emami A, Lotfi M, Rostamihosseinkhani M, Nemati H, et al. Risk factors associated with long COVID syndrome: A retrospective study. Iran J Med Sci. (2021) 46:428–36. doi: 10.30476/IJMS.2021.92080.2326
134. Jacob L, Koyanagi A, Smith L, Tanislav C, Konrad M, van der Beck S, et al. Prevalence of, and factors associated with, long-term COVID-19 sick leave in working-age patients followed in general practices in Germany. Int J Infect Dis. (2021) 109:203–8. doi: 10.1016/J.IJID.2021.06.063
135. Sigfrid L, Drake TM, Pauley E, Jesudason EC, Olliaro P, Lim WS, et al. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterization Protocol. Lancet regional Health. (2021) 8. doi: 10.1016/J.LANEPE.2021.100186
136. Michelutti M, Furlanis G, Stella AB, Bellavita G, Frezza N, Torresin G, et al. Sex-dependent characteristics of Neuro-Long-COVID: Data from a dedicated neurology ambulatory service. J Neurol Sci. (2022) 441. doi: 10.1016/J.JNS.2022.120355
137. Sylvester SV, Rusu R, Chan B, Bellows M, O’Keefe C, Nicholson S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Curr Med Res Opin. (2022) 38:1391–9. doi: 10.1080/03007995.2022.2081454
138. Lemogne C, Gouraud C, Pitron V, Ranque B. Why the hypothesis of psychological mechanisms in long COVID is worth considering. J Psychosom Res. (2023) 165. doi: 10.1016/J.JPSYCHORES.2022.111135
139. Charfeddine S, Hadj Amor HI, Jdidi J, Torjmen S, Kraiem S, Hammami R, et al. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-endCOV study. Front Cardiovasc Med. (2021) 8:745758/BIBTEX. doi: 10.3389/fcvm.2021.745758
140. Westerlind E, Palstam A, Sunnerhagen KS, Persson HC. Patterns and predictors of sick leave after Covid-19 and long Covid in a national Swedish cohort. BMC Public Health. (2021) 21. doi: 10.1186/S12889-021-11013-2
141. Stewart S, Newson L, Briggs TA, Grammatopoulos D, Young L, Gill P. Long COVID risk - a signal to address sex hormones and women’s health,” The Lancet regional health. Europe. (2021) 11. doi: 10.1016/J.LANEPE.2021.100242
142. Ortona E, Malorni W. Long COVID: to investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur Respir J. (2022) 59. doi: 10.1183/13993003.02245-2021
143. Bucciarelli V, Nasi M, Bianco F, Seferovic J, Ivkovic V, Gallina S, et al. Depression pandemic and cardiovascular risk in the COVID-19 era and long COVID syndrome: Gender makes a difference. Trends Cardiovasc Med. (2022) 32:12–7. doi: 10.1016/J.TCM.2021.09.009
144. Fernández-De-las-peñas C, Martín-Guerrero JD, Pellicer-Valero OJ, Navarro-Pardo E, Gómez-Mayordomo V, Cuadrado ML, et al. Female sex is a risk factor associated with long-term post-COVID related-symptoms but not with COVID-19 symptoms: the LONG-COVID-EXP-CM multicenter study. J Clin Med. (2022) 11(2):413. doi: 10.3390/JCM11020413
145. Noval Rivas M, Porritt RA, Cheng MH, Bahar I, Arditi M. Multisystem inflammatory syndrome in children and long COVID: the SARS-coV-2 viral superantigen hypothesis. Front Immunol. (2022) 13:941009/BIBTEX. doi: 10.3389/fimmu.2022.941009
146. Notarte KI, Santos de Oliveira MH, Peligro PJ, Velasco JV, Macaranas I, Ver AT, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-coV-2 infection for long COVID-19: A systematic review and meta-analysis. J Clin Med. (2022) 11(24):7314. doi: 10.3390/JCM11247314
147. Kandemir H, Bülbül GA, Kirtiş E, Güney S, Sanhal CY, Mendilcioğlu II, et al. Evaluation of long-COVID symptoms in women infected with SARS-CoV-2 during pregnancy. Int J Gynaecol Obstet. (2023) 164:148–56. doi: 10.1002/ijgo.14972
148. Hui Y, Guoqiang S, Fei T, Min P, Ying G, Jing P, et al. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J Infect. (2020) 81:40–4. doi: 10.1016/j.jinf.2020.04.003
149. Vasconez-Gonzales J, Fernandez-Naranjo R, Izquierdo-Condoy JS, Delgado-Moreira K, Cordovez S, Tello-De-la-Torre A, et al. Comparative analysis of long-term self-reported COVID-19 symptoms among pregnant women. J Infect Public Heal. (2023) 16:430–40. doi: 10.1016/j.jiph.2023.01.012
150. Yang H, Pan Y, Chen W, Yang X, Liu B, Yuan N, et al. Prevalence of and relevant factors for depression and anxiety symptoms among pregnant women on the eastern seaboard of China in the post-COVID-19 era: a cross-sectional study. BMC Psychiatry. (2023) 23:564. doi: 10.1186/s12888-023-05059-2
151. Stepowicz A, Wencka B, Bieńkiewicz J, Horzelski W, Grzesiak M, et al. Stress and anxiety levels in pregnant and post-partum women during the COVID-19 pandemic. Int J Environ Res Public Health. (2020) 17:9450. doi: 10.3390/ijerph17249450
152. Wu F, Lin W, Liu P, Zhang M, Huang S, Chen C, et al. Prevalence and contributory factors of anxiety and depression among pregnant women in the post-pandemic era of COVID-19 in Shenzhen, China. J Affect. Disord. (2021) 291:243–51. doi: 10.1016/j.jad.2021.05.014
153. Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. (2021) 40:e482. doi: 10.1097/INF.0000000000003328
154. Haddad A, Janda A, Renk H, Stich M, Frieh P, Kaier K, et al. Long COVID symptoms in exposed and infected children, adolescents and their parents one year after SARS-CoV-2 infection: A prospective observational cohort study. EBioMedicine. (2022) 84. doi: 10.1016/J.EBIOM.2022.104245
155. Kikkenborg Berg S, Palm P, Nygaard U, Bundgaard H, Schmidt Petersen MN, Rosenkilde s, et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. (2022) 6:614–23. doi: 10.1016/S2352-4642(22)00154-7
156. Lopez-Leon S, Wegman-Ostrosky T, Ayuzo del Valle NC, Perelman C, Sepulveda R, Rebolledo Pa, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. (2022) 12. doi: 10.1038/S41598-022-13495-5
157. Li J, Zhou Y, Ma J, Zhang Q, Shao J, Liang S, et al. The long-term health outcomes, pathophysiological mechanisms and multidisciplinary management of long COVID. Sig Transduct Target Ther. (2023) 8:416. doi: 10.1038/s41392-023-01640-z
158. Fainardi V, Meoli A, Chiopris G, Motta M, Skenderaj K, Grandinetti R, et al. Long COVID in children and adolescents. Life (Basel). (2022) 12(2):285. doi: 10.3390/LIFE12020285
159. Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur J Pediatr. (2022) 181:1597–607. doi: 10.1007/s00431-021-04345-z
160. Kikkenborg Berg S, Nielsen SD, Nygaard U, Bundgaard H, Palm P, Rotvig C, et al. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. (2022) 6:240–8. doi: 10.1016/S2352-4642(22)00004-9
161. Stephenson T, Allin B, Nugawela MD, Rojas N, Dalrymple E, Pereira SP, et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child. (2022) 107:674–80. doi: 10.1136/ARCHDISCHILD-2021-323624
162. Buonsenso D, Pujol FE, Munblit D, Pata D, McFarland S, Simpson FK. Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: a survey of 510 children. Future Microbiol. (2022) 17:577–88. doi: 10.2217/FMB-2021-0285
163. Ashkenazi-Hoffnung L, Shmueli E, Ehrlich S, Ziv A, Bar-On O, Birk E, et al. Long COVID in children: observations from a designated pediatric clinic. Pediatr Infect Dis J. (2021) 40:E509–11. doi: 10.1097/INF.0000000000003285
164. Esposito S, Principi N, Azzari C, Cardinale F, Di Mauro G, Galli L, et al. Italian intersociety consensus on management of long covid in children. Ital J Pediatr. (2022) 48. doi: 10.1186/S13052-022-01233-6
165. Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatr. (2021) 110:2208–11. doi: 10.1111/APA.15870
166. Stephenson T, Pinto Pereira SM, Roz Shafran R, de Stavola BL, Rojas N, McOwat K, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health. (2022) 6:230–9. doi: 10.1016/S2352-4642(22)00022-0
167. Adab P, Haroon S, O’Hara ME, Jordan RE. Comorbidities and covid-19. BMJ. (2022) 377:o1431. doi: 10.1136/BMJ.O1431
168. Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. (2021) 12:698169/BIBTEX. doi: 10.3389/fmicb.2021.698169
169. Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. (2022) 13:1–11. doi: 10.1038/s41467-022-30836-0
170. Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. (2021) 27:626–31. doi: 10.1038/s41591-021-01292-y
171. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J Infect Dis. (2022) 226:1593–607. doi: 10.1093/INFDIS/JIAC136
172. Khunti K, Davies MJ, Kosiborod MN, Nauck MA. Long COVID - metabolic risk factors and novel therapeutic management. Nat Rev Endocrinol. (2021) 17:379–80. doi: 10.1038/S41574-021-00495-0
173. Frontera JA, Yang D, Lewis A, Patel P, Medicherla C, Arena V, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. (2021) 426. doi: 10.1016/J.JNS.2021.117486
174. Cabrera Martimbianco AL, Pacheco RL, Bagattini Â.M, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int J Clin Pract. (2021) 75(10):e14357. doi: 10.1111/IJCP.14357
175. Rivera-Izquierdo M, Láinez-Ramos-Bossini AJ, de Alba IGF, Ortiz-González-Serna R, Serrano-Ortiz A, Fernández-Martínez NF, et al. Long COVID 12 months after discharge: persistent symptoms in patients hospitalized due to COVID-19 and patients hospitalized due to other causes-a multicentre cohort study. BMC Med. (2022) 20:92. doi: 10.1186/S12916-022-02292-6
176. Florencio LL, Fernández-de-las-Peñas C. Long COVID: systemic inflammation and obesity as therapeutic targets. Lancet Respir Med. (2022) 10:726–7. doi: 10.1016/S2213-2600(22)00159-X
177. O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of Long Covid among hospitalized and non-hospitalized populations: A systematic review and meta-analysis. E Clin Med. (2022) 55. doi: 10.1016/J.ECLINM.2022.101762
178. Estiri H, Strasser ZH, Brat GA, Semenov YR, et al. Evolving phenotypes of non-hospitalized patients that indicate long covid. medRxiv. (2021). doi: 10.1101/2021.04.25.21255923
179. Guedes AR, Oliveira MS, Tavares BM, Luna-Muschi A, dos Santos Lazari C, Montal AC, et al. Reinfection rate in a cohort of healthcare workers over 2 years of the COVID-19 pandemic. Sci Rep. (2023) 13:712. doi: 10.1038/s41598-022-25908-6
180. Boufidou F, Medić S, Lampropoulou V, Siafakas N, Tsakris A, Anastassopoulou C. SARS-coV-2 reinfections and long COVID in the post-omicron phase of the pandemic. Int J Mol Sci. (2023) 24:12962. doi: 10.3390/ijms241612962
181. Bosworth ML, Shenhuy B, Walker S, Nafilyan V, Alwan NA, O’Hara Me, et al. Risk of new-onset Long Covid following reinfection with SARS-CoV-2: community-based cohort study. medRxiv. (2023). doi: 10.1101/2023.04.13.23288522
182. Cegolon L, Mauro M, Sansone D, Tassinari A, Gobba FM, Modenese A, et al. A multi-center study investigating long COVID-19 in healthcare workers from north-eastern Italy: prevalence, risk factors and the impact of pre-existing humoral immunity—ORCHESTRA project. Vaccines. (2023) 11:1769. doi: 10.3390/vaccines11121769
183. Kenny G, McCann K, O’Brien C, O’Broin C, Tinago W, Yousif O, et al. Impact of vaccination and variants of concern on long COVID clinical phenotypes. BMC Inf Dis. (2023) 23:804. doi: 10.1186/s12879-023-08783-y
184. Canas LS, Molteni E, Deng J, Sudre CH, Murray B, Kerfoot E, et al. Profiling post-COVID-19 condition across different variants of SARS-CoV-2: a prospective longitudinal study in unvaccinated wild-type, unvaccinated alpha-variant, and vaccinated delta-variant populations. Lancet. (2023) 5:441–34. doi: 10.1016/S2589-7500(23)00056-0
185. Diexer S, Klee B, Gottschick C, Xu C, Broda A, Purschke O, et al. Association between virus variants, vaccination, previous infections, and post-COVID-19 risk. I J Inf Diseas. (2023) 136:14–21. doi: 10.1016/j.ijid.2023.08.019
186. Perlis RH, Santillana M, Ognyanova K, Safarpour A, Trujillo KL, Simonson MD, et al. Prevalence and correlates of long COVID symptoms among US adults. JAMA. (2022) 5(10):e2238804. doi: 10.1001/jamanetworkopen.2022.38804
187. Fernández-de-Las-Peñas C, Notarte KI, Peligro PJ, Velasco JV, Ocampo MJ, Henry BM, et al. Long-COVID symptoms in individuals infected with different SARS-coV-2 variants of concern: A systematic review of the literature. Viruses. (2022) 14:2629. doi: 10.3390/v14122629
188. Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. (2022) 328:676–8. doi: 10.1001/JAMA.2022.11691
189. Ledford H. How common is long COVID? Why studies give different answers. Nature. (2022) 606:852–3. doi: 10.1038/D41586-022-01702-2
190. Strain WD, Sherwood O, Banerjee A, van der Togt V, Hishmeh L, Rossman J. The impact of COVID vaccination on symptoms of long COVID: an international survey of people with lived experience of long COVID. Vaccines (Basel). (2022) 10(5):652. doi: 10.3390/VACCINES10050652
191. Gao P, Liu J, Liu M. Effect of COVID-19 vaccines on reducing the risk of long COVID in the real world: A systematic review and meta-analysis. Int J Environ Res Public Health. (2022) 19:12422. doi: 10.3390/IJERPH191912422
192. Liu Q, Qin C, Liu M, Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. (2021) 10:132. doi: 10.1186/S40249-021-00915-3
193. Notarte KI, Catahay JA, Velasco JV, Pastrana A, Ver AT, Pangilinan FC, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. E Clin Med. (2022) 53. doi: 10.1016/J.ECLINM.2022.101624
194. Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, et al. Trajectory of long covid symptoms after covid-19 vaccination: community-based cohort study. BMJ. (2022) 377:2022. doi: 10.1136/BMJ-2021-069676
195. Greenhalgh T, Sivan M, Delaney B, Evans R, Milne R. Long covid—an update for primary care. BMJ. (2022) 378:e072117. doi: 10.1136/BMJ-2022-072117
196. Wise J. Covid-19: Long covid risk is lower with omicron than delta, researchers find. BMJ. (2022) 377:o1500. doi: 10.1136/BMJ.O1500
197. Søraas A, Kalleberg KA, Dahl JA, Søraas CL, Myklebust TA, Axelsen E, et al. Persisting symptoms three to eight months after non-hospitalized COVID-19, a prospective cohort study. PloS One. (2021) 16(8):e0256142. doi: 10.1371/JOURNAL.PONE.0256142
198. Arnold D, Milne A, Samms E, Stadon L, Maskell N, Hamilton F. Are vaccines safe in patients with Long COVID? A prospective observational study. medRxiv. (2021). doi: 10.1101/2021.03.11.21253225
199. Yelin D, Margalit I, Yahav D, Runold M, Bruchfeld J. Long COVID-19-it’s not over until? Clin Microbiol Infect. (2021) 27:506–8. doi: 10.1016/J.CMI.2020.12.001
200. Peluso MJ, Deeks SG. Early clues regarding the pathogenesis of long-COVID. Trends Immunol. (2022) 43:268–70. doi: 10.1016/J.IT.2022.02.008
201. Tran VT, Perrodeau E, Saldanha J, Pane I, Ravaud P. Efficacy of COVID-19 vaccination on the symptoms of patients with long COVID: a target trial emulation using data from the ComPaRe e-cohort in France. (2022). doi: 10.21203/RS.3.RS-1350429/V1
202. Ekström S, Andersson N, Lövquist A, Lauber A, Georgelis A, Kull I, et al. COVID-19 among young adults in Sweden: self-reported long-term symptoms and associated factors. Scand J Public Health. (2022) 50:85. doi: 10.1177/14034948211025425
203. Wright J, Astill SL, Sivan M. The relationship between physical activity and long COVID: A cross-sectional study. Int J Environ Res Public Health. (2022) 19(9):5093. doi: 10.3390/IJERPH19095093
204. Yu Z, Ekström S, Bellander T, Ljungman P, Pershagen G, Eneroth K, et al. Ambient air pollution exposure linked to long COVID among young adults: a nested survey in a population-based cohort in Sweden. Lancet Regional Health - Europe. (2023) 28. doi: 10.1016/j.lanepe.2023.100608
205. Brussow H, Timmis K. COVID-19: long covid and its societal consequences. Environ Microbiol. (2021) 23:4077–91. doi: 10.1111/1462-2920.15634
206. Jassat W, Reyes LF, Munblit D, Caoili J, Bozza F, Hashmi M, et al. Long COVID in low-income and middle-income countries: the hidden public health crisis. Lancet. (2023) 402:1115–7. doi: 10.1016/S0140-6736(23)01685-9
207. Nyasulu PS, Tamuzi JL, Erasmus RT. Burden, causation, and particularities of Long COVID in African populations: A rapid systematic review. IJID Reg. (2023) 8:137–44. doi: 10.1016/j.ijregi.2023.08.004
208. Malliarou M, Gagamanou A, Bouletis A, Tzenetidis V, Papathanasiou I, Theodoropoulou M, et al. COVID-19 pandemic and health and social inequalities worldwide: impact and response measures in Greece. Adv Exp Med Biol. (2023) 1425:393–9. doi: 10.1007/978-3-031-31986-0_38
209. Available online at: https://iris.who.int/bitstream/handle/10665/341050/WHO-EURO-2021-2410-42165-58100-eng.pdf?sequence=1 (Accessed 4th of January, 2024).
210. Baraniuk C. Covid-19: How Europe is approaching long covid. BMJ. (2022) 376:o158. doi: 10.1136/BMJ.O158
211. Verger A, Kas A, Dudouet P, Goehringer F, Salmon-Ceron D, Guedj E. Visual interpretation of brain hypometabolism related to neurological long COVID: a French multicentric experience. Eur J Nucl Med Mol Imaging. (2022) 49:3197–202. doi: 10.1007/S00259-022-05753-5
212. Lorenz-Dant K. Germany and the COVID-19 long-term care situation. LTCcovid, International Long Term Care Policy Network, CPEC-LSE (2020). Available at: https://ltccovid.org/wp-content/uploads/2020/05/Germany_LTC_COVID-19-26-May-2020.pdf.
213. Umakanthan S, Monice M, Mehboob S, Jones CL, Lawrence S. Post-acute (long) COVID-19 quality of life: validation of the German version of (PAC19QoL) instrument. Front Public Health. (2023) 11:1163360. doi: 10.3389/FPUBH.2023.1163360
214. Bahmer T, Borzikowsky C, Lieb W, Horn A, Krist L, Fricke J, et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-center, population-based cohort study. EClinicalMedicine. (2022) 51. doi: 10.1016/J.ECLINM.2022.101549
215. Landi F, Gremese E, Bernabei R, Fantoni M, Gasbarrini A, Settanni CR, et al. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. (2020) 32:1613–20. doi: 10.1007/S40520-020-01616-X
216. Fissi S, Gori E, Romolini A. Social media government communication and stakeholder engagement in the era of Covid-19: evidence from Italy. Int J Public Sector Manage. (2022) 35:276–93. doi: 10.1108/IJPSM-06-2021-0145/FULL/PDF
217. Ruiu ML. Mismanagement of Covid-19: lessons learned from Italy. J Risk Res. (2020) 23:1007–20. doi: 10.1080/13669877.2020.1758755
218. Nordic solidarity and covid-19. Available online at: https://ams.hi.is/en/publication/83/ (Accessed Aug. 05, 2023).
219. Greve B, Blomquist P, Hvinden B, van Gerven M. Nordic welfare states-still standing or changed by the COVID-19 crisis? Soc Policy Adm. (2021) 55:295–311. doi: 10.1111/SPOL.12675
220. Saunes IS, Vrangbæk K, Byrkjeflot H, Jervelund SS, Birk HO, Tynkkynen LK, et al. Nordic responses to Covid-19: Governance and policy measures in the early phases of the pandemic. Health Policy. (2022) 126:418–26. doi: 10.1016/J.HEALTHPOL.2021.08.011
221. de la Porte C, Jensen MD, Kvist J. Going Nordic—Can the Nordic model tackle grand challenges and be a beacon to follow? Regul Gov. (2023) 17:595–607. doi: 10.1111/REGO.12494
222. Christensen T, Jensen MD, Kluth M, Kristinsson GH, Lynggaard K, Lægreid P, et al. The Nordic governments’ responses to the Covid-19 pandemic: A comparative study of variation in governance arrangements and regulatory instruments. Regul Gov. (2022) 17(3):658–76. doi: 10.1111/REGO.12497
223. Sisó-Almirall A, Brito-Zerón P, Conangla Ferrín L, Kostov B, Moragas Moreno A, Mestres J, et al. Long covid-19: proposed primary care clinical guidelines for diagnosis and disease management. Int J Environ Res Public Health. (2021) 18(8):4350. doi: 10.3390/IJERPH18084350
224. Fernández-de-las-Peñas C, Rodríguez-Jiménez J, Moro-López-Menchero P, Cancela-Cilleruelo I, Pardo-Hernández A, Hernández-Barrera V, et al. Psychometric properties of the Spanish version of the EuroQol-5D-5L in previously hospitalized COVID-19 survivors with long COVID. Sci Rep. (2022) 12. doi: 10.1038/S41598-022-17033-1
225. Pérez-González A, Araújo-Ameijeiras A, Fernández-Villar A, Crespo M, Poveda E, Cohort COVID-19 of the Galicia Sur Health Research Institute. Long COVID in hospitalized and non-hospitalized patients in a large cohort in Northwest Spain, a prospective cohort study. Sci Rep. (2022) 12(1):3369. doi: 10.1038/S41598-022-07414-X
226. Antoniou KM, Vasarmidi E, Russell AM, Andrejak C, Crestani B, Delcroix M, et al. European Respiratory Society statement on long COVID follow-up. Eur Respir J. (2022) 60. doi: 10.1183/13993003.02174-2021
227. Ladds E, Rushforth AA, Wieringa BS, Taylor CS, Rayner DC, Husain EL, et al. Developing services for long COVID: lessons from a study of wounded healers. Clin Med (Lond). (2021) 21:59–65. doi: 10.7861/CLINMED.2020-0962
228. Routen A, O’Mahoney L, Ayoubkhani D, Banerjee A, Brightling C, Calvert M, et al. Understanding and tracking the impact of long COVID in the United Kingdom. Nat Med. (2022) 28:11–5. doi: 10.1038/S41591-021-01591-4
229. Cutler DM. The costs of long COVID. JAMA Health Forum. (2022) 3(5):e221809. doi: 10.1001/JAMAHEALTHFORUM.2022.1809
230. HHS announces the formation of the office of long COVID research and practice and launch of long COVID clinical trials through the RECOVER initiative | HHS.gov. Available online at: https://www.hhs.gov/about/news/2023/07/31/hhs-announces-formation-office-long-covid-research-practice-launch-long-covid-clinical-trials-through-recover-initiative.html (Accessed Aug. 05, 2023).
231. Hopkins Tanne J. Covid-19: US agency launches raft of clinical trials of treatments for long covid. BMJ. (2023) 382:1797. doi: 10.1136/bmj.p1797
232. Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. (2022) 43:1157–72. doi: 10.1093/EURHEARTJ/EHAC031
233. Ahamed J, Laurence J. Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches. J Clin Invest. (2022) 132(15):e161167. doi: 10.1172/JCI161167
234. Stefanou MI, Palaiodimou L, Bakola E, Smyrnis N, Papadopoulou M, Paraskevas GP, et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis. (2022) 13:20406223221076890. doi: 10.1177/20406223221076890
235. DeMars J, Brown DA, Angelidis I, Jones F, McGuire F, O’Brien KK, et al. What is safe long COVID rehabilitation? J Occup Rehabil. (2023) 33:227–30. doi: 10.1007/S10926-022-10075-2
236. Barbara C, Clavario P, De Marzo V, Lotti R, Guglielmi G, Porcile A, et al. Effects of exercise rehabilitation in patients with long coronavirus disease 2019. Eur J Prev Cardiol. (2022) 29:E258–60. doi: 10.1093/EURJPC/ZWAC019
237. Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. (2022) 101:593–601. doi: 10.1159/000522118
238. Goel N, Goyal N, Nagaraja R, Kumar R. Systemic corticosteroids for management of ‘long-COVID’: an evaluation after 3 months of treatment. Monaldi Arch Chest Dis. (2021) 92. doi: 10.4081/MONALDI.2021.1981
239. Koc HC, Xiao J, Liu W, Li Y, Chen G. Long COVID and its management. Int J Biol Sci. (2022) 18:4768–80. doi: 10.7150/IJBS.75056
240. Thye AYK, Law JW-F, Tan LT-H, Pusparajah P, Ser H-L, Thurairajasingam S, et al. Psychological symptoms in COVID-19 patients: insights into pathophysiology and risk factors of long COVID-19. Biol (Basel). (2022) 11(1):61. doi: 10.3390/BIOLOGY11010061
241. Tosato M, Calvani R, Picca A, Ciciarello F, Galluzzo V, Coelho-Júnior HJ, et al. Effects of l-arginine plus vitamin C supplementation on physical performance, endothelial function, and persistent fatigue in adults with long COVID: A single-blind randomized controlled trial. Nutrients. (2022) 14(23):4984. doi: 10.3390/NU14234984
242. Vollbracht C, Kraft K. Oxidative stress and hyper-inflammation as major drivers of severe COVID-19 and long COVID: implications for the benefit of high-dose intravenous vitamin C. Front Pharmacol. (2022) 13:899198/BIBTEX. doi: 10.3389/fphar.2022.899198
243. Piazza M, Di Cicco M, Pecoraro L, Ghezzi M, Peroni D, Comberiati P. Long COVID-19 in children: from the pathogenesis to the biologically plausible roots of the syndrome. Biomolecules. (2022) 12(4):556. doi: 10.3390/BIOM12040556
244. Plassmeyer M, Alpan O, Corley MJ, Premeaux TA, Lillard K, Coatney P, et al. Caspases and therapeutic potential of caspase inhibitors in moderate-severe SARS-CoV-2 infection and long COVID. Allergy. (2022) 77:118–29. doi: 10.1111/ALL.14907
245. Hollifield M, Cocozza K, Calloway T, Lai J, Caicedo B, Carrick K, et al. Improvement in long-COVID symptoms using acupuncture: A case study. Med Acupunct. (2022) 34:172–6. doi: 10.1089/ACU.2021.0088
246. Satoh T, Trudler D, Oh CK, Lipton SA. Potential therapeutic use of the rosemary diterpene carnosic acid for alzheimer’s disease, parkinson’s disease, and long-COVID through NRF2 activation to counteract the NLRP3 inflammasome. Antioxidants (Basel). (2022) 11(1):124. doi: 10.3390/ANTIOX11010124
247. Vaes AW, Goërtz YMJ, Van Herck M, Machado FVC, Meys R, Delbressine JM, et al. Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. (2021) 7:00141–2021. doi: 10.1183/23120541.00141-2021
248. Bateman L, Bested AC, Bonilla HF, Chheda BV, Chu L, Curtin JM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. (2021) 96:2861–78. doi: 10.1016/j.mayocp.2021.07.004
249. Hohberger B, Harrer T, Mardin C, Kruse F, Hoffmanns J, Rogge L, et al. Case report: neutralization of autoantibodies targeting G-protein-coupled receptors improves capillary impairment and fatigue symptoms after COVID-19 infection. Front Med (Lausanne). (2021) 8:754667. doi: 10.3389/fmed.2021.754667
250. Xie Y, Choi T, Al-Aly Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19. medRxiv. (2022). doi: 10.1101/2022.11.03.22281783
251. Zubchenko S, Kril I, Nadizhko O, Matsyura O, Chopyak V. Herpesvirus infections and post-COVID-19 manifestations: a pilot observational study. Rheumatol Int. (2022) 42:1523–30. doi: 10.1007/s00296-022-05146-9
252. Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo SO, Watson LR, et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc Diabetol. (2022) 21:148. doi: 10.1186/s12933-022-01579-5
253. A new treatment for Long Covid (2021). The ME Association. Available online at: https://meassociation.org.uk/2021/10/a-new-treatment-for-long-covid/ (Accessed Aug. 21, 2023).
254. Wood E, Hall KH, Tate W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 ‘long-haulers. Chronic Dis Transl Med. (2021) 7:14–26. doi: 10.1016/j.cdtm.2020.11.002
255. The C-MORE/PHOSP-COVID Collaborative Group. “Multiorgan MRI findings after hospitalization with COVID-19 in the UK (C-MORE): a prospective, multicentre, observational cohort study. Lancet. (2022) 11:1003–19. doi: 10.1016/S2213-2600(23)00262-X
256. Aiyegbusi OL, Hughes SE, Turner G, Cruz Rivera S, McMullan C, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. (2021) 114:428–42. doi: 10.1177/01410768211032850/ASSET/IMAGES/LARGE/10.1177_01410768211032850-FIG3.JPEG
257. Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med. (2021) 21:e63. doi: 10.7861/CLINMED.2020-0896
258. Nurek M, Rayner C, Freyer A, Taylor S, Järte L, MacDermott N, et al. Recommendations for the recognition, diagnosis, and management of long COVID: a Delphi study. Br J Gen Pract. (2021) 71:E815–25. doi: 10.3399/BJGP.2021.0265
259. Brown DA, O’Brien KK. Conceptualizing Long COVID as an episodic health condition. BMJ Glob Health. (2021) 6:e007004. doi: 10.1136/BMJGH-2021-007004
260. Wolf S, Zechmeister-Koss I, Erdös J. Possible long COVID healthcare pathways: a scoping review. BMC Health Serv Res. (2022) 22:1076. doi: 10.1186/S12913-022-08384-6
261. Luo WR, Wu XM, Wang W, Yu JL, Chen QQ, Zhou Q, et al. Novel coronavirus mutations: Vaccine development and challenges. Microb Pathog. (2022) 173:105828. doi: 10.1016/J.MICPATH.2022.105828
262. SARS-coV-2 variant classifications and definitions. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (Accessed Sep. 13, 2023).
263. Fatemeh M, Zelikovsky A, Mangul S, Chowell G, Skums P, et al. Community structure and temporal dynamics of SARS-CoV-2 epistatic network allows for early detection of emerging variants with altered phenotypes. bioRxyv. (2023). doi: 10.1101/2023.04.02.535277
264. Will BA.2.86 (‘Pirola’), the new coronavirus variant, increase COVID-19 cases? > news, in: Yale medicine. Available online at: https://www.yalemedicine.org/news/new-covid-variant-ba286-pirola (Accessed Sep. 13, 2023).
Keywords: SARS-CoV-2, Inflammatory markers, brain fog, vascular injury, PASC
Citation: Gheorghita R, Soldanescu I, Lobiuc A, Caliman Sturdza OA, Filip R, Constantinescu – Bercu A, Dimian M, Mangul S and Covasa M (2024) The knowns and unknowns of long COVID-19: from mechanisms to therapeutical approaches. Front. Immunol. 15:1344086. doi: 10.3389/fimmu.2024.1344086
Received: 24 November 2023; Accepted: 14 February 2024;
Published: 04 March 2024.
Edited by:
Valentina Mazzotta, National Institute for Infectious Diseases Lazzaro Spallanzani (IRCCS), ItalyReviewed by:
Mohan Tulapurkar, University of Maryland, United StatesLaurissa Ouaguia, Agilent Technologies, United States
Copyright © 2024 Gheorghita, Soldanescu, Lobiuc, Caliman Sturdza, Filip, Constantinescu – Bercu, Dimian, Mangul and Covasa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mihai Covasa, mcovasa@westernu.edu
 Roxana Gheorghita
Roxana Gheorghita Iuliana Soldanescu3
Iuliana Soldanescu3 Andrei Lobiuc
Andrei Lobiuc Olga Adriana Caliman Sturdza
Olga Adriana Caliman Sturdza Roxana Filip
Roxana Filip Adela Constantinescu – Bercu
Adela Constantinescu – Bercu Mihai Covasa
Mihai Covasa