- 1School of Ecology and Nature Conservation, Beijing Forestry University, Beijing, China
- 2Department of Biology, National Museum of Natural Science, Taichung, Taiwan
- 3Biodiversity Research Center, Academia Sinica, Taipei, Taiwan
- 4Center for Forest Mycology Research, Northern Research Station, U.S. Forest Service, Madison, WI, United States
- 5Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, China
The phylogenetic analyses of the family Irpicaceae were carried out based on a complete global sampling. The dataset that included concatenated ITS1-5.8S-ITS2 and nrLSU sequences of 67 taxa of Irpicaceae from around the world was subjected to the maximum likelihood analyses and Bayesian inference. In the phylogenetic tree, species from 14 genera were distributed in nine clades, among which five genera—Irpex, Phanerochaetella, Byssomerulius, Cytidiella, and Meruliopsis, received high support values. The genus Efibula was shown to be paraphyletic and four subclades could be recognized, while Phanerochaete allantospora, Leptoporus mollis, and several species from Ceriporia and Candelabrochaete formed a large clade with relatively strong support. Based on the molecular and morphological evidence, seven new corticioid species—Candelabrochaete guangdongensis, Efibula grandinosa, E. hainanensis, E. shenghuae, E. taiwanensis, Irpex alboflavescens, and Phanerochaetella sinensis, were revealed from the materials mostly from East Asia. The monotypic genus Flavodontia, newly described from southwestern China, is regarded as a later synonym of Irpex, and the new combination I. rosea is proposed. In addition, Phanerochaetella queletii is proposed for a taxon first described from Italy and newly recorded from China; Phanerochaete jose-ferreirae from Portugal is determined to be a later synonym. Descriptions and illustrations of the new species and the newly combined taxa are presented, and morphological comparisons for the known species of Efibula and Phanerochaetella are provided.
Introduction
The phlebioid clade of Polyporales includes lots of wood-decaying fungi, which were distributed in the three well-supported families: Phanerochaetaceae, Irpicaceae, and Meruliaceae (Floudas and Hibbett, 2015; Miettinen et al., 2016; Justo et al., 2017; Chen et al., 2021). Irpicaceae is a relatively new and small family with 13 corticioid and polyporoid genera accepted at present (Spirin, 2003): Byssomerulius Parmasto, Ceriporia Donk, Crystallicutis El-Gharabawy, Leal-Dutra and G.W. Griff, Cytidiella Pouzar, Efibula Sheng H. Wu, Gloeoporus Mont., Irpex Fr., Leptoporus Quél., Meruliopsis Bondartsev, Phanerochaetella C.C. Chen and Sheng H. Wu, Raduliporus Spirin and Zmitr., Resiniporus Zmitr., and Trametopsis Tomšovský. Species in the family usually have resupinate to effused-reflexed basidiomata, a monomitic hyphal system with simple-septate generative hyphae, colorless, thin-walled, and smooth basidiospores without reactions in cotton blue and Melzer's reagents, and lack cystidia. Ecologically, members of the Irpicaceae are widely distributed from temperate to tropic areas mostly on woody angiosperms and associated with a white rot decay, except for Leptoporus species which are associated with brown-rot decay (Chen et al., 2021).
Irpex was created in 1,825 by Fries and is the generic type of Irpicaceae which included 13 genera. Over time, species with hydnoid to irpicioid and even poroid hymenophores were placed in Irpex. By narrowing the morphological criteria for the genus, species were transferred to other genera and Irpex evolved into a morphologically well-defined genus with supporting phylogenetic evidence. Ryvarden (2020) evaluated the taxonomic status of 180 names described in Irpex and restricted the genus to two species, I. lacteus (Fr.) Fr. and I. hydnoides Y.W. Lim and H.S. Jung. Recently, Chen et al. (2021) showed by phylogenetic analyses of multiple genes that Emmia Zmitr., Spirin and Malysheva, Flavodon Ryvarden and Hydnopolyporus D.A. Reid and Irpex formed a strongly supported clade and placed the first three genera in synonymy under Irpex. They also showed that the genus Efibula was paraphyletic in the phylogenetic tree but lacked sufficient morphological evidence to support the recognition of separate genera (Chen et al., 2021). The polyphyletic genus Ceriporia was distributed in the three families of the phlebioid clade with the type species nested within Irpicaceae. Although lineages in Phanerochaetaceae and Meruliaceae have been resolved, the relationship between Ceriporia s.s. and species of Candelabrochaete s.l. requires further studies (Miettinen et al., 2016; Chen et al., 2020, 2021).
A large number of specimens belonging to Irpicaceae were collected from East Asia by the corresponding author in recent years. In order to further the knowledge of species diversity and taxonomy of Irpicaceae, we carried out complete morphological and molecular phylogenetic studies on our specimens with emphasis on corticioid taxa, and our results are presented below.
Materials and Methods
Specimen Collection
Field trips for specimen collection in many kinds of Nature Reserves and Forest Parks in China and other countries were carried out by the authors. In situ photographs of the fungi were taken with a Canon camera EOS 70D (Canon Corporation, Japan). Fresh specimens were dried with a portable drier (manufactured in Finland). Dried specimens were labeled and then stored in a refrigerator at minus 40 °C for two weeks to kill the insects and their eggs before they were ready for morphological and molecular studies.
Morphological Studies
Voucher specimens are deposited at the herbaria of Beijing Forestry University, Beijing, China (BJFC), Centre for Forest Mycology Research, U.S. Forest Service, Madison, Wisconsin, USA (CFMR), and National Museum of Natural Science, Taichung, Taiwan, China (TNM). Herbarium code designations follow Index Herbarium1 Thin, freehand sections were made from dried basidiomata and mounted in 2% (w/v) aqueous potassium hydroxide (KOH) and 1% (w/v) aqueous phloxine. Amyloidity and dextrinoidity of basidiospores were checked in Melzer's reagent (IKI). Cyanophily of hyphal and basidiospore walls was observed in 1% (weight/volume) cotton blue in 60% (w/v) lactic acid (CB). Microscopic examinations were carried out with a Nikon Eclipse 80i microscope (Nikon Corporation, Japan) at magnifications up to 1,000 ×. Drawings were made with the aid of a drawing tube. The following abbreviations are used: IKI– = neither amyloid nor dextrinoid, CB– = acyanophilous, L = mean spore length, W = mean spore width, Q = L/W ratio, n (a/b) = number of spores (a) measured from number of specimens (b). Color codes and names follow Kornerup and Wanscher (1978).
DNA Extraction and Sequencing
A CTAB plant genomic DNA extraction Kit DN14 (Aidlab Biotechnologies Co., Ltd, Beijing, China) was used to extract total genomic DNA from dried specimens and then amplified by the polymerase chain reaction (PCR), according to the manufacturer's instructions. The ITS1-5.8S-ITS2 region was amplified with the primer pair ITS5/ITS4 (White et al., 1990) using the following protocol: initial denaturation at 95 °C for 4 min, followed by 34 cycles at 94 °C for 40 s, 58 °C for 45 s and 72 °C for 1 min, and final extension at 72 °C for 10 min. The nrLSU D1-D2 region was amplified with the primer pair LR0R/LR72 employing the following procedure: initial denaturation at 94 °C for 1 min, followed by 34 cycles at 94 °C for 30 s, 50 °C for 1 min and 72 °C for 1.5 min, and final extension at 72 °C for 10 min. DNA sequencing was performed at Beijing Genomics Institute, and the sequences were deposited in GenBank3 (Table 1). BioEdit v.7.0.5.3 (Hall, 1999) and Geneious Basic v.11.1.15 (Kearse et al., 2012) were used to review the chromatograms and for contig assembly.
Phylogenetic Analyses
The molecular phylogeny was inferred from a concatenated dataset of ITS-nrLSU sequences of species in the Irpicaceae. Gloeoporus dichrous (Fr.) Bres. and G. pannocinctus (Romell) J. Erikss. were selected as the outgroup (Floudas and Hibbett, 2015; Chen et al., 2021). The ITS and nrLSU sequences were aligned separately using MAFFT v.74 (Katoh et al., 2017) with the G-INS-I iterative refinement algorithm and optimized manually in BioEdit v.7.0.5.3. The separate alignments were then concatenated using Mesquite v.3.5.1 (Maddison and Maddison, 2018). The datasets were deposited in TreeBase5 (submission ID: 29610).
Maximum likelihood (ML) analyses and Bayesian inference (BI) were carried out by using RAxML v.8.2.10 (Stamatakis, 2014) and MrBayes 3.2.6 (Ronquist et al., 2012), respectively. In ML analysis, statistical support values were obtained using rapid bootstrapping with 1000 replicates, with default settings used for other parameters. For BI, the best-fit substitution model was estimated with jModeltest v.2.17 (Darriba et al., 2012). Four Markov chains were run for 8,000,000 generations until the split deviation frequency value was lower than 0.01. Trees were sampled every 100th generation. The first quarter of the trees, which represented the burn-in phase of the analyses, were discarded, and the remaining trees were used to calculate posterior probabilities (BPP) in the majority rule consensus tree.
Results
Phylogenetic Analyses
The concatenated ITS-nrLSU dataset contained 120 ITS and 101 nrLSU sequences from 126 samples representing 69 taxa of Irpicaceae (Table 1). The concatenated dataset had an aligned length of 2220 characters. jModelTest suggested that GTR+I+G was the best-fit model of nucleotide evolution for the concatenated ITS-nrLSU. The average standard deviation of split frequencies of BI was 0.007321 at the end of the run. ML analyses resulted in almost identical tree topology compared to the BI analysis. Only the BI tree is provided in Figure 1 with the likelihood bootstrap values (≥50%, before the slash) and Bayesian posterior probabilities (≥0.95, behind the slash) labeled along the branches.
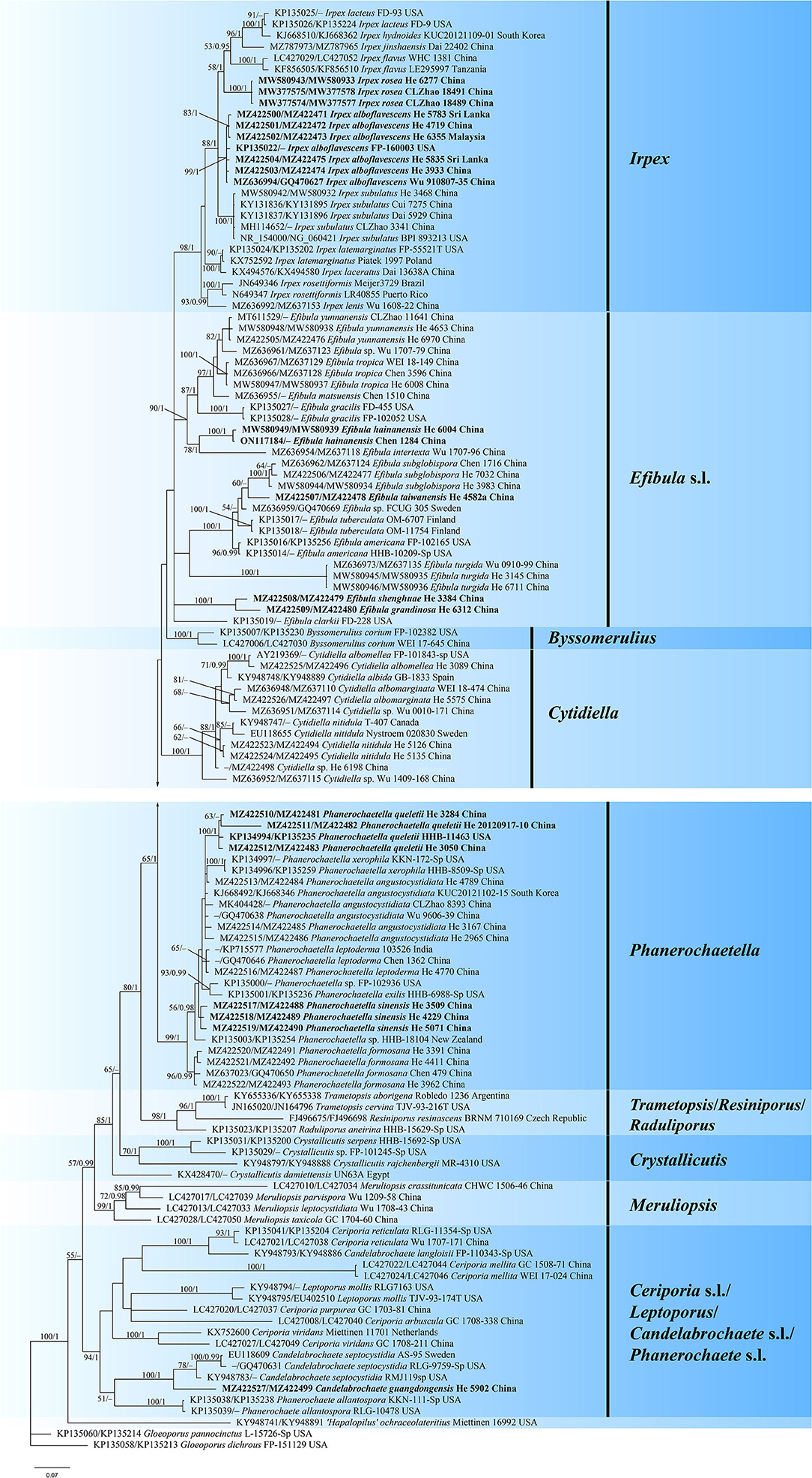
Figure 1. Phylogram of Irpicaceae inferred from Bayesian inference using the concatenated ITS-nrLSU sequences dataset. Branches are labeled with likelihood bootstrap values (≥50%, before the slash) and Bayesian posterior probabilities (≥0.95, after the slash). New species and new combinations are set in bold.
The topology of the tree is similar to those in previous studies (Justo et al., 2017; Chen et al., 2021). For the in-groups, species from 14 genera were distributed in nine clades: Irpex, Efibula, Phanerochaetella, Byssomerulius, Cytidiella, Raduliporus/Resiniporus/Trametopsis, Crystallicutis, Meruliopsis, Ceriporia s.l./Leptoporus/Candelabrochaete s.l./ Phanerochaete s.l. While Irpex, Phanerochaetella, Byssomerulius, Cytidiella, and Meruliopsis received high support values, and the genus Efibula was shown to be polyphyletic. Raduliporus, Resiniporus, and Trametopsis formed a strongly supported clade. The monophyly of Crystallicutis was not well-supported in our tree. Phanerochaete allantospora, Leptoporus mollis, and several species from Ceriporia and Candelabrochaete formed a large clade with relatively strong support. Seven new species—Candelabrochaete guangdongensis, Efibula grandinosa, E. hainanensis, E. shenghuae, E. taiwanensis, Irpex alboflavescens, and P. sinensis, formed distinct lineages in the tree. For Irpex rosea, our sample (He 6277) and the type materials of Flavodontia rosea (Zhao 18489 and Zhao 18491) formed a distinct lineage in the Irpex clade, while for Phanerochaetella queletii, samples from China and USA grouped together with strong support values.
Taxonomy
Candelabrochaete guangdongensis Y. Li and S.H. He, sp. nov.
MycoBank: MB843531
Type—China, Guangdong Province, Shixing County, Chebaling Nature Reserve, on fallen angiosperm trunk, June 14, 2019, He 5902 (BJFC 030777, holotype).
Etymology—Refers to the type locality in Guangdong Province, southern China.
Fruiting body—Basidiomata annual, resupinate, widely effused, closely adnate, inseparable from substrate, ceraceous, first as small patches, later confluent up to 9-cm long, 3-cm wide, up to 250-μm thick in section. Hymenophore smooth, orange [6B(7–8)] to reddish orange [7B(7–8)], turning reddish black in KOH, rarely cracked; margin thinning out, determinate, adnate, concolorous with or darker than hymenophore surface. Context cream.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct; hyphae colorless, slightly to distinctly thick-walled, smooth, usually branched at a right angle, frequently septate, loosely interwoven, 3–6 μm in diam. Subhymenium thickening, composed of collapsed hymenia; hyphae colorless, thin- to slightly thick-walled, vertically arranged, frequently branched and septate, 2–4 μm in diam. Septocystidia abundant, cylindrical, usually sinuous, frequently septate, colorless, slightly thick-walled, smooth, or sometimes slightly encrusted with yellowish granules at the apex, arising from the subiculum, mostly embedded, projecting up to 40 μm beyond hymenium, 145–190 × 6–10 μm. Basidia clavate to subcylindrical, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, 16–24 × 3.5–5 μm. Basidiospores short-cylindrical to allantoid, with an apiculus, colorless, thin-walled, smooth, IKI–, CB–, 4–5 × 1.5–2 μm, L = 4.5 μm, W = 1.7 μm, Q = 2.6 (n = 30/1).
Notes—Candelabrochaete guangdongensis (Figure 2) is characterized by the ceraceous basidiomata with a smooth hymenophore that turns black in KOH, large septocystidia and short-cylindrical to allantoid basidiospores. In the phylogenetic tree (Figure 1), C. guangdongensis is closely related to C. septocystidia (Burt) Burds., and morphologically both species have smooth hymenophore, large septocystidia, and allantoid basidiospores. However, C. septocystidia can be distinguished from C. guangdongensis by having a hymenophore unchanged in KOH, slightly longer basidiospores (4.5–6.5 μm), distinctly encrusted septocystidia, and a distribution in North America and Europe (Burdsall, 1984). Candelabrochaete macaronesica M. Dueñas, Tellería and Melo is similar to C. guangdongensis by sharing smooth hymenophore and large septocystidia but differs in having slightly larger ellipsoid basidiospores and a distribution in Portugal (5–6.5 × 2–3 μm, Dueñas et al., 2008). Candelabrochaete neocaledonica Duhem and Buyck has similar septocystidia and basidiospores with C. guangdongensis but differs in having a distinctly hydnoid hymenophore (Duhem and Buyck, 2011).
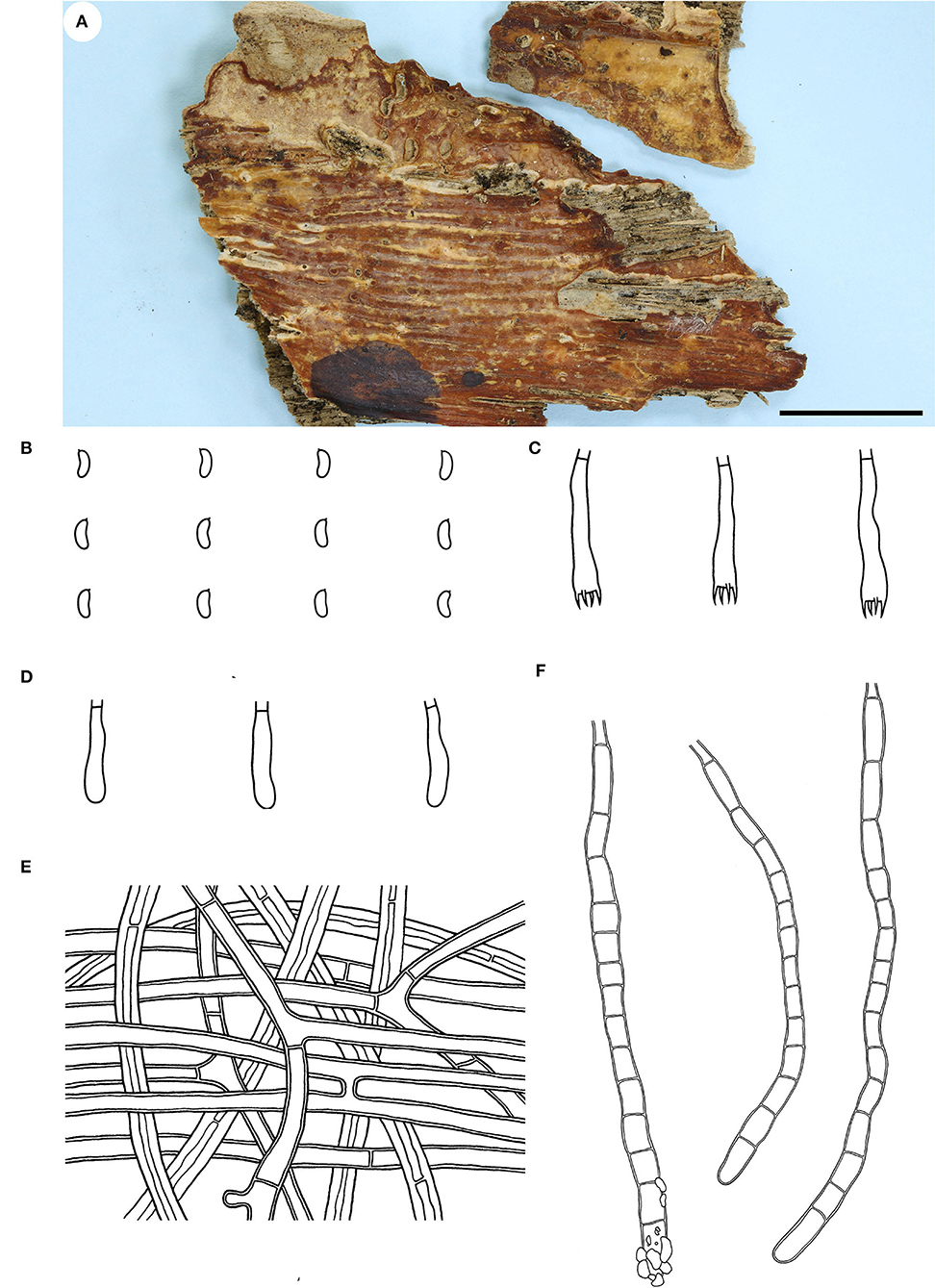
Figure 2. Candelabrochaete guangdongensis (from the holotype He 5902; scale bars: a = 1 cm; b–d, f = 10 μm; e = 20 μm). (A) Basidiomata. (B) Basidiospores. (C) Basidia. (D) Basidioles. (E) Septocystidia. (F) Hyphae from subiculum.
Efibula grandinosa Y. Li and S.H. He, sp. nov.
MycoBank: MB843532
Type—China, Yunnan Province, Shizong County, Junzishan Forest Park, on dead angiosperm branch, November 18, 2019, He 6312 (BJFC 033256, holotype).
Etymology—Refers to the grandinioid hymenophore due to the projecting hyphal pegs.
Fruiting body—Basidiomata annual, resupinate, widely effused, closely adnate, inseparable from substrate, membranaceous, first as small patches, later confluent up to 10-cm long, 3-cm wide, up to 250-μm thick in section. Hymenophore grandinioid with projecting hyphal pegs, pale orange (6A3) to grayish orange [6B(4–5)], slightly darkening in KOH, not cracking upon drying; margin thinning out, adnate, indistinct, paler than hymenophore surface, white. Context orange white.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct; hyphae colorless, thin- to slightly thick-walled, smooth, moderately branched, frequently septate, loosely interwoven, 2.5–3.8 μm in diam. Subhymenium thin; hyphae colorless, thin-walled, smooth, moderately branched and septate, interwoven. Cystidia absent. Hyphal pegs scattered, largely projecting beyond hymenium, composed of many vertically arranged hyphae; hyphae colorless, thin-walled, smooth or occasionally encrusted with crystals, unbranched, infrequently septate. Basidia clavate, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, 36–43 × 5–7 μm. Basidiospores ellipsoid, with an apiculus, colorless, thin-walled, smooth, IKI–, CB–, 6–6.8 (−7) × (3.5–) 3.7–4 (−4.1) μm, L = 6.4 μm, W = 3.9 μm, Q = 1.7 (n = 30/1).
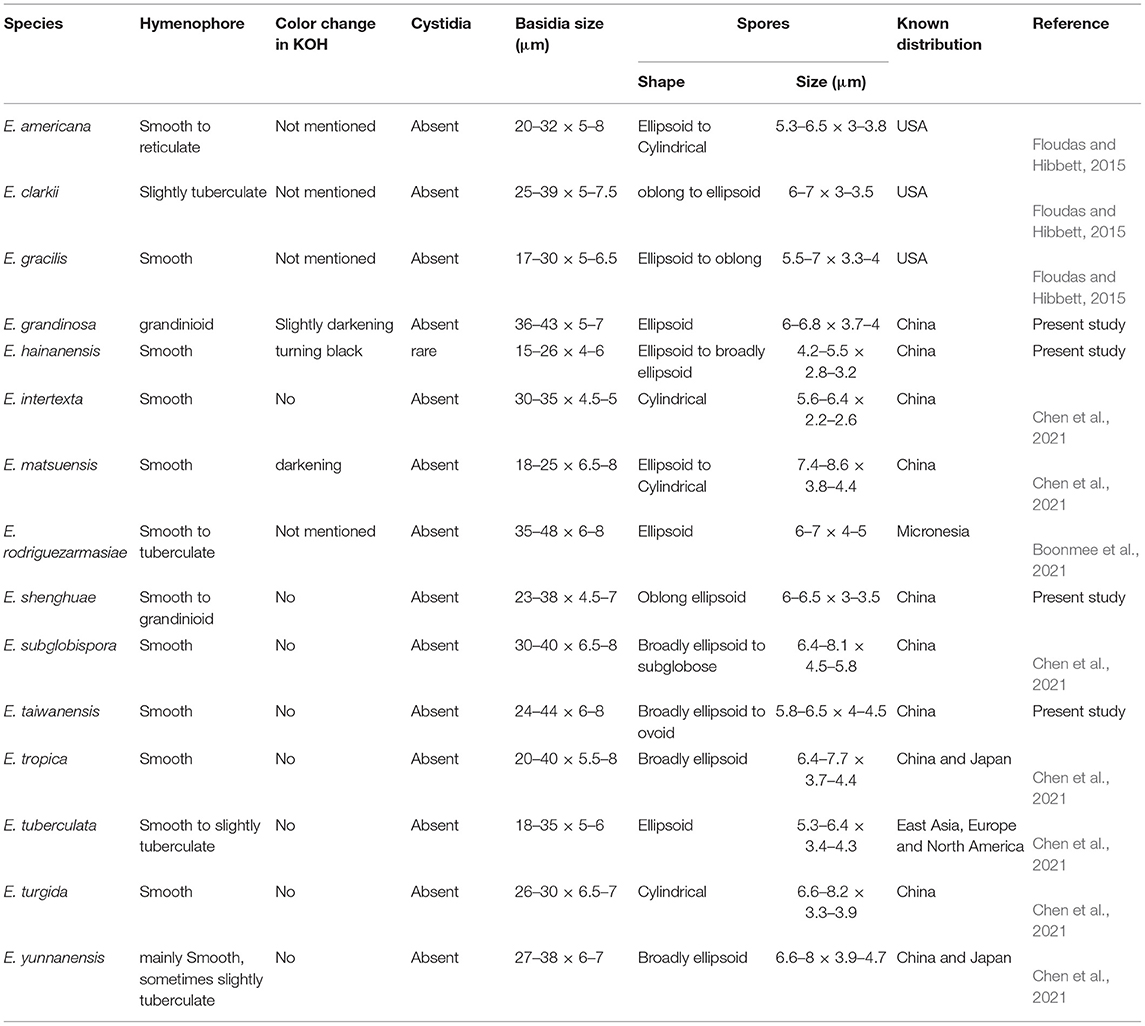
Notes—Efibula grandinosa Figure 3 is characterized by having a grandinioid hymenophore and hyphal pegs. In the phylogenetic tree (Figure 1), E. grandinosa and E. shenghuae formed a strongly supported lineage sister to E. clarkii Floudas and Hibbett. Morphologically, although all the three species have grandinioid or tuberculate hymenophore, E. shenghuae and E. clarkii can be easily distinguished from E. grandinosa by lacking hyphal pegs (Floudas and Hibbett, 2015).
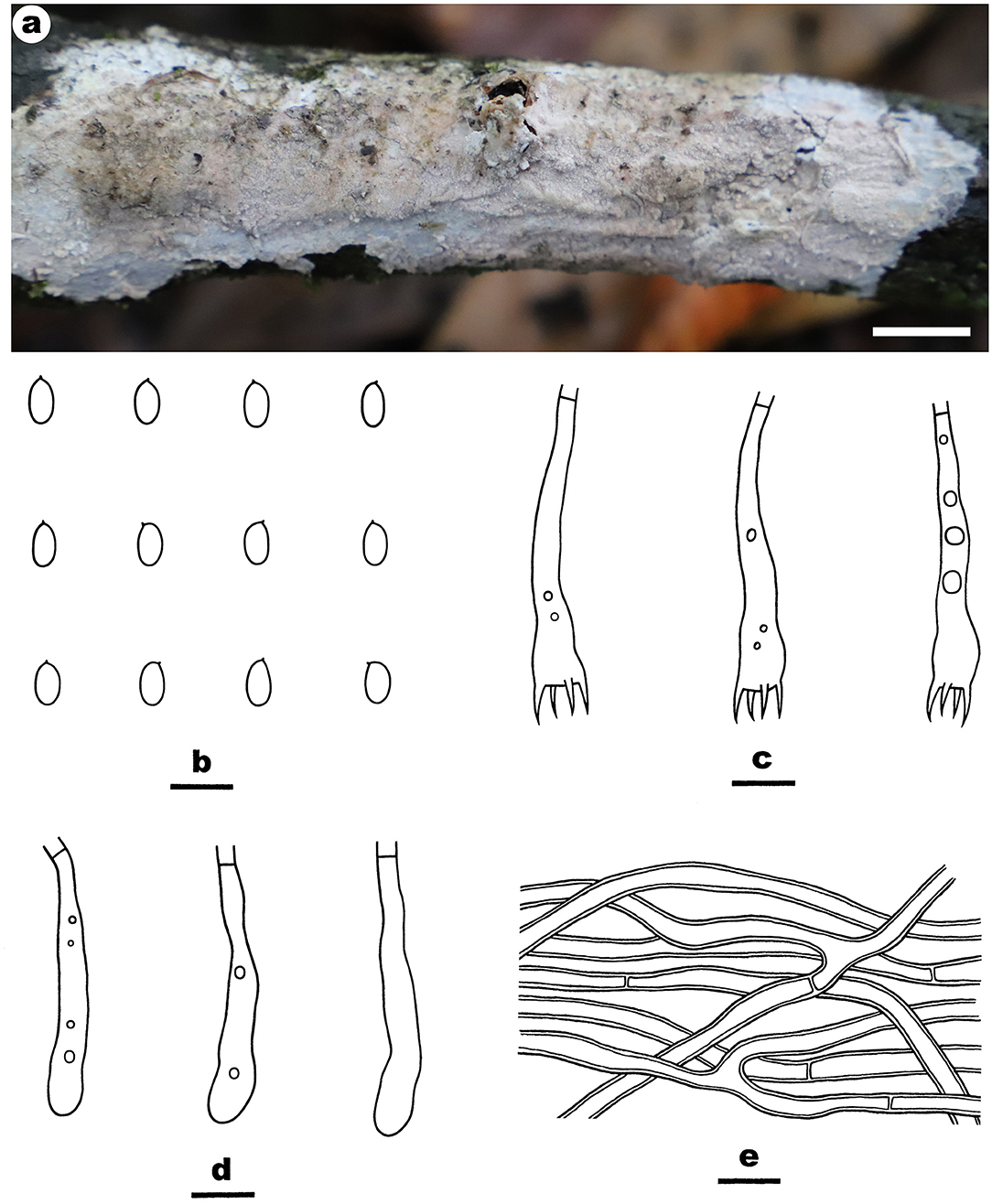
Figure 3. Efibula grandinosa (from the holotype He 6312; scale bars: a = 1 cm; b–e = 10 μm). (A) Basidiomata. (B) Basidiospores. (C) Basidia. (D) Basidioles. (E) Hyphae from subiculum.
Efibula hainanensis Y. Li and S.H. He, sp. nov.
MycoBank: MB843533
Type—China, Hainan Province, Changjiang County, Bawangling Nature Reserve, on dead liana, July 4, 2019, He 6004 (BJFC 030880, holotype).
Etymology—Refers to the type locality in Hainan Province, southern China.
Fruiting body—Basidiomata annual, resupinate, widely effused, closely adnate, inseparable from substrate, membranaceous, first as small patches, later confluent up to 8-cm long, 2.5-cm wide, up to 200-μm thick in section. Hymenophore smooth, pale orange (5A3) to brownish orange [5C(4–5)], turning black in KOH, not cracking upon drying; margin thinning out, adnate, indistinct, paler than hymenophore surface, white to pale yellow. Context pale yellow.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct; hyphae colorless, thin- to thick-walled, usually encrusted with small crystals, straight, rarely branched, infrequently septate, loosely interwoven, 1.8–3.5 μm in diam. Subhymenium thin; hyphae colorless, thin-walled, encrusted with fine crystals, moderately septate. Cystidia rare, subfusiform to subcylindrical, colorless, thin-walled, smooth, with a basal simple septum, embedded or slightly projecting beyond the hymenium, 30–50 × 5–9 μm. Basidia clavate, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, 15–26 × 4–6 μm. Basidiospores ellipsoid to broadly ellipsoid, with an apiculus, colorless, thin-walled, smooth, IKI–, CB–, (4–) 4.2–5.5 (−5.8) × (2.5–) 2.8–3.2 (−3.5) μm, L = 4.7 μm, W = 3.0 μm, Q = 1.6 (n = 30/1).
Additional specimen examined—China, Taiwan, Taitung, Orchid Island, Tienchih, on branch of angiosperm, May 16, 2003; Chen 1284 (TNM F0015135).
Notes—Efibula hainanensis Figure 4 is characterized by having a smooth hymenophore, thin-walled cystidia, and relatively small ellipsoid basidispores. Until now, E. hainanensis is the only species in the genus with cystidia. In the phylogenetic tree (Figure 1), E. hainanensis and E. intertexta (Sheng H. Wu) C.C. Chen and Sheng H. Wu formed a relatively strongly supported lineage. Morphologically, E. intertexta differs from E. hainanensis by having distinctly thickening hymenial layer and cylindrical basidiospores (5.6–6.4 × 2.2–2.6 μm) and lacking cystidia (Chen et al., 2021). Efibula tuberculata (P. Karst.) Zmitr. and Spirin is similar to E. hainanensis by sharing relatively small basidiospores but differs in having a smooth to tuberculate, cracked hymenophore, occasional clamps, and slightly larger basidiospores (Chen et al., 2021).
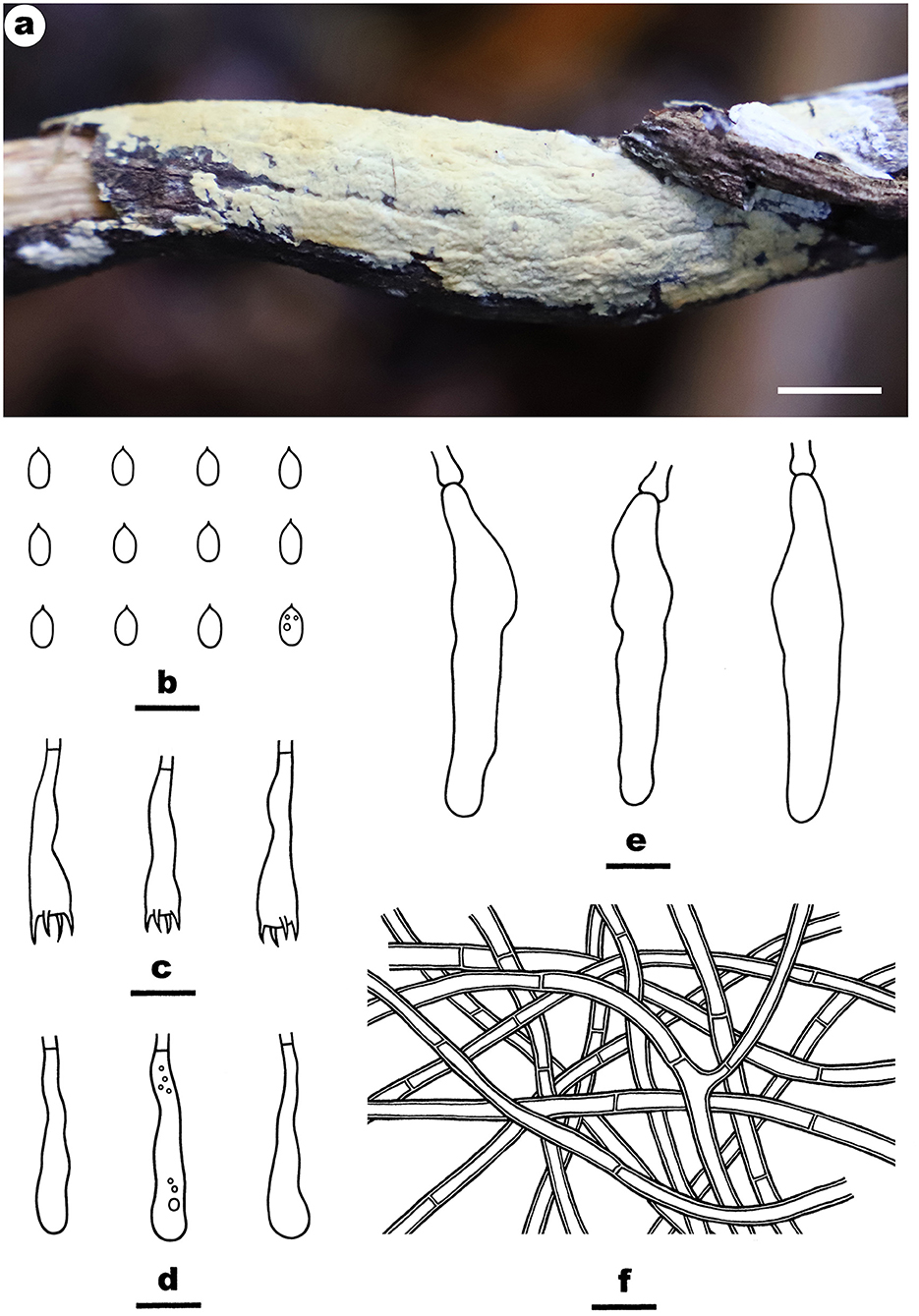
Figure 4. Efibula hainanensis (from the holotype He 6004; scale bars: a = 1 cm; b–f = 10 μm). (A) Basidiomata. (B) Basidiospores. (C) Basidia. (D) Basidioles. (E) Cystidia. (F) Hyphae from subiculum.
Efibula shenghuae Y. Li and S.H. He, sp. nov.
MycoBank: MB843534
Type—China, Yunnan Province, Baoshan County, Gaoligongshan Nature Reserve, on dead branch of Quercus, November 30, 2015, He 3384 (BJFC 021779, holotype; isotype in CFMR).
Etymology—Named to honor Dr. Sheng-Hua Wu (TNM, Taiwan) who established and contributed much to the genus of Efibula.
Fruiting body—Basidiomata annual, resupinate, widely effused, closely adnate, inseparable from substrate, membranaceous, first as small patches, later confluent up to 8-cm long, 1.5-cm wide, up to 200-μm thick in section. Hymenophore smooth to grandinioid with irregular and scattered granules, orange white (5A2) to pale orange (5A3), unchanged in KOH, not cracking upon drying; margin thinning out, adnate, indistinct, fimbriate, paler than hymenophore, white. Context white.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum indistinct, thin; hyphae colorless, slightly thick-walled, smooth, rarely branched, moderately septate, more or less parallel to substrate, 3–4.5 μm in diam. Subhymenium distinct, thickening, with masses of crystals; hyphae colorless, thin-walled, smooth, densely interwoven, agglutinated, frequently septate, 1.8–3.5 μm in diam. Cystidia absent. Basidia clavate, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, 23–38 × 4.5–7 μm. Basidiospores oblong ellipsoid, with an apiculus, colorless, thin-walled, smooth, IKI–, CB–, 6–6.5 (−6.8) × 3–3.5 (−3.8) μm, L = 6.2 μm, W = 3.2 μm, Q = 1.9 (n = 30/1).
Notes—Efibula shenghuae Figure 5 is characterized by its grandinioid hymenophore, indistinct subiculum, and masses of crystals in subhymenium. In the phylogenetic tree (Figure 1), E. shenghuae and E. grandinosa formed a well-supported lineage sister to E. clarkii. However, E. grandinosa can be easily distinguished from E. shenghuae by having hyphal pegs, while E. clarkii differs from E. shenghuae by having extensively cracked basidiomata and less crystals (Floudas and Hibbett, 2015). Efibula tuberculata is similar to E. shenghuae by sharing smooth to tuberculate hymenophore but differs by having cracked basidiomata and less crystals in section (Chen et al., 2021). Moreover, the two species formed distinct lineages in the tree.
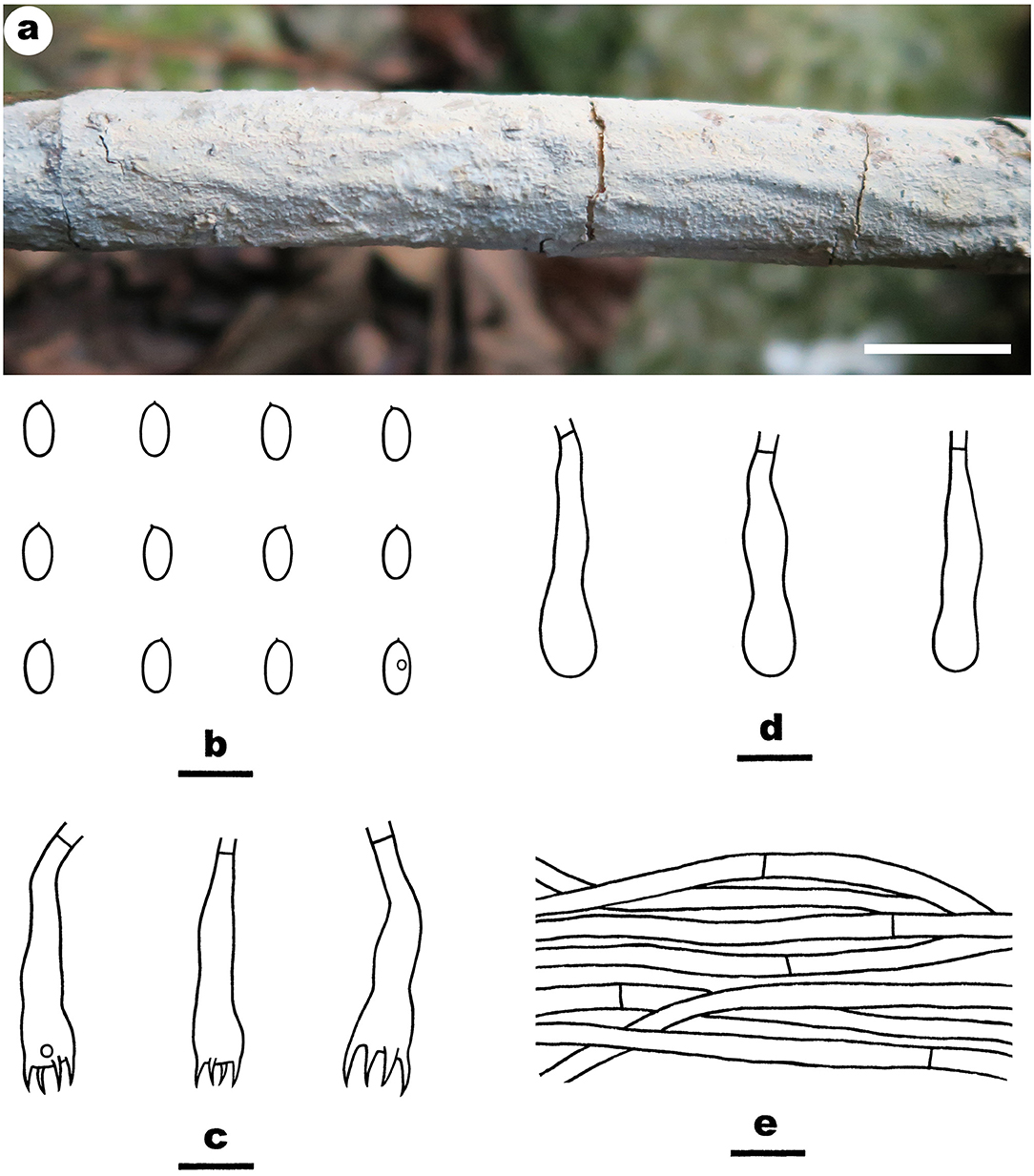
Figure 5. Efibula shenghuae (from the holotype He 3384; scale bars: a = 1 cm; b–e = 10 μm). (A) Basidiomata. (B) Basidiospores. (C) Basidia. (D) Basidioles. (E) Hyphae from subiculum.
Efibula taiwanensis Y. Li and S.H. He, sp. nov.
MycoBank: MB843535
Type—China, Taiwan, Nantou County, Lianhuachi Forest Park, on dead angiosperm branch, December 6, 2016, He 4582a (BJFC 024024, holotype).
Etymology—Refers to the type locality in Taiwan.
Fruiting body—Basidiomata annual, resupinate, widely effused, closely adnate, inseparable from substrate, membranaceous to slightly pellicular, first as small patches, later confluent up to 6-cm long, 1.5-cm wide, up to 150-μm thick in section. Hymenophore smooth, white (5A1) to orange white (5A2), unchanged in KOH, not cracking upon drying; margin thinning out, adnate, indistinct, arachnoid, paler than hymenophore surface, white. Context white.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct, thick; hyphae colorless, thin- to slightly thick-walled, smooth or slightly to heavily encrusted with fine crystals, loosely interwoven, moderately branched and septate, 2.5–4 μm in diam. Subhymenium thin; hyphae colorless, thin-walled, smooth or slightly encrusted with fine crystals, interwoven, 2–3.5 μm in diam. Cystidia absent. Basidia clavate to subcylindrical, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, 24–44 × 6–8 μm. Basidiospores broadly ellipsoid to ovoid, with a distinct apiculus, colorless, thin-walled, smooth, IKI–, CB–, (5.5–) 5.8–6.5 (−7) × 4–4.5 μm, L = 6.2 μm, W = 4.2 μm, Q = 1.5 (n = 30/1).
Notes—Efibula taiwanensis Figure 6 is characterized by having membranaceous to pellicular basidiomata and broadly ellipsoid to ovoid basidiospores. In the phylogenetic tree, E. taiwanensis is sister to E. subglobispora C.C. Chen and Sheng H. Wu and E. americana Floudas and Hibbett. Morphologically, E. subglobispora differs from E. taiwanensis by having larger basidiospores (6.4–8.1 × 4.5–5.8 μm, Chen et al., 2021), while E. americana can be easily distinguished from E. taiwanensis by having smaller ellipsoid to cylindrical basidiospores (5.3–6.5 × 3–3.8) and a distribution in USA (Floudas and Hibbett, 2015).
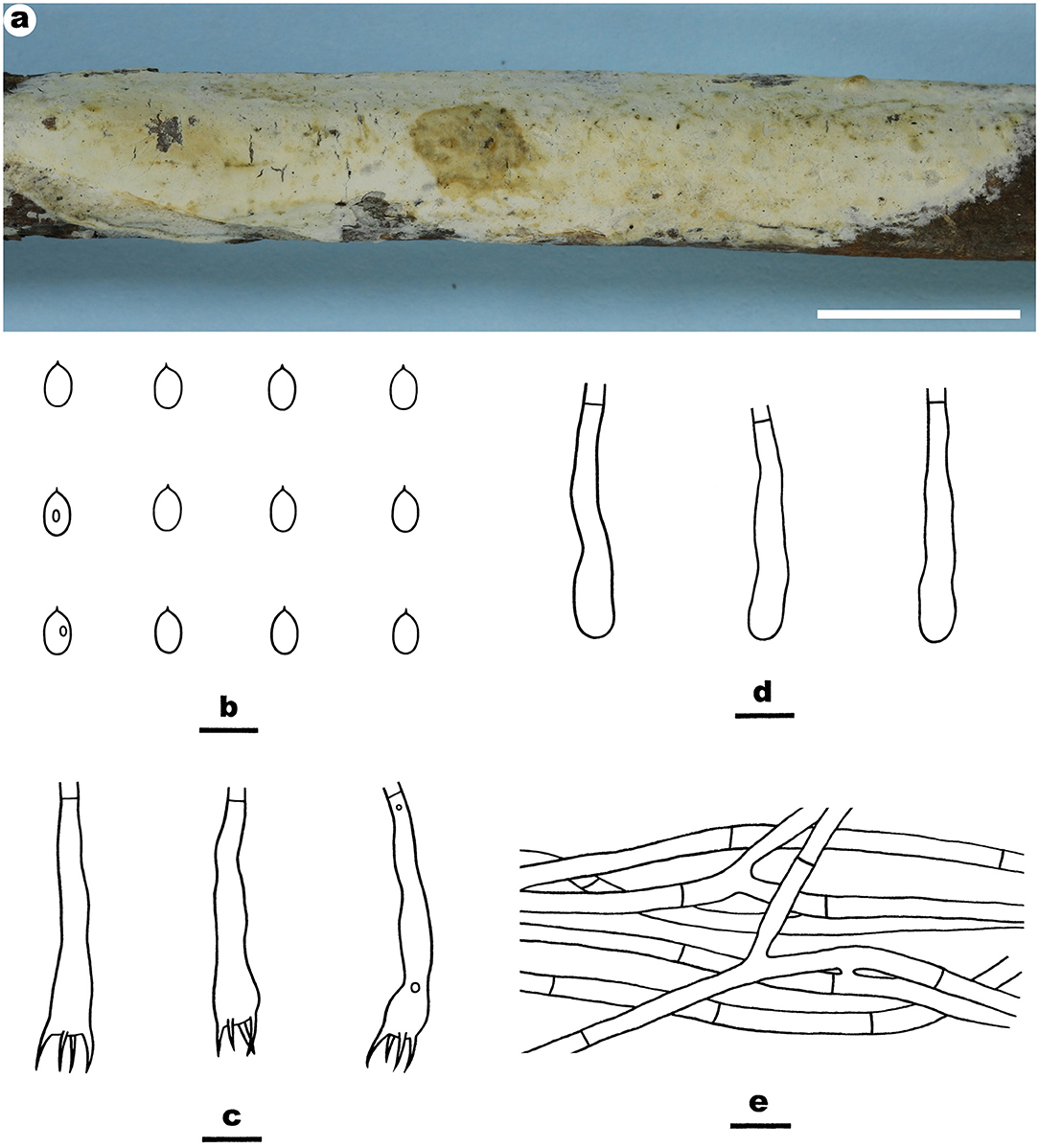
Figure 6. Efibula taiwanensis (from the holotype He 4582a; scale bars: a = 1 cm; b–e = 10 μm). (A) Basidiomata. (B) Basidiospores. (C) Basidia. (D) Basidioles. (E) Hyphae from subiculum.
Irpex alboflavescens Y. Li, Nakasone and S.H. He, sp. nov.
MycoBank: MB843536
Type—China, Hainan Province, Wuzhishan County, Wuzhishan Nature Reserve, on fallen angiosperm trunk, June 10, 2016, He 3933 (BJFC 022435, holotype).
Etymology—Refers to the color of hymenophore, “albo (Lat.)” = white; “flavescens (Lat.)” = yellow.
Fruiting body—Basidiomata annual, resupinate, widely effused, adnate, separable from substrate, coriaceous, first as small patches, becoming confluent up to 15-cm long, 5-cm wide, up to 350-μm thick in section. Hymenophore smooth or slightly tuberculate when fresh, pale orange (5A3) to grayish orange [5B(4–5)], slightly darkening in KOH, rarely cracked; margin thinning out, adnate or sometimes elevated and curved inside exposing substrate upon drying, indistinct, fimbriate, paler than hymenophore, white to orange white. Context white.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct; hyphae colorless, slightly thick-walled, usually encrusted with fine crystals, loosely interwoven, infrequently branched, moderately septate, 2–5 μm in diam. Subhymenium distinct, thickening; composed of lamprocystidia and hyphae; hyphae colorless, slightly thick-walled, smooth, interwoven, slightly agglutinated, moderately branched and septate, 2–4 μm in diam. Lamprocystidia numerous, metuloid, colorless, thick-walled, heavily encrusted, embedded or slightly projecting beyond the hymenium, 35–70 × 8–16 μm (crystals included). Basidia subcylindrical, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, 13–26 × 3.2–6.5 μm. Basidiospores ellipsoid to broadly ellipsoid, with a distinct apiculus, colorless, thin-walled, smooth, IKI–, CB–, (3.8–) 4.2–5.8 (−6) × (2.5–) 2.8–3.5 (−3.8) μm, L = 4.8 μm, W = 3.1 μm, Q = 1.4–1.6 (n = 120/4).
Additional specimens examined—China, Guangxi Autonomous region, Longzhou County, Nonggang Nature Reserve, on dead angiosperm branch, 22 July 2012, He 20120722–4 (BJFC 014505, CFMR); on fallen wood of rattan, June 4, 2015, Dai 15296 (BJFC 019407); Huanjiang County, Mulun Nature Reserve, on dead angiosperm branch, July 10, 2017, He 4719 (BJFC 024238) and He 4724 (BJFC 024243); Guizhou Province, Libo County, Maolan Nature Reserve, on dead angiosperm branch, June 15, 2016, He 3782 (BJFC 022281) and He 3791 (BJFC 022290); Hunan Province, Shimen County, Hupingshan Nature Reserve, on fallen angiosperm trunk, July 6, 2015, He 2278 (BJFC 020733, CFMR); Jiangxi Province, Lianping County, Jiulianshan Nature Reserve, on dead angiosperm branch, August 13, 2016, He 4311 (BJFC 023753). Malaysia, Sembilan, Semenyih, Broga Hill, on dead angiosperm branch, 3 December 2019, He 6355 (BJFC 033299). Sri Lanka, Central Province, Kandy, Udawattakele Royal Forest Park, on dead angiosperm branch, March 2, 2019, He 5767 (BJFC 030634); Avissawella, Salgala Forest, on fallen angiosperm branch, March 3, 2019, He 5783 (BJFC 030650); Western Province, Ingiriya, Dombagaskanda Forest Reserve, on dead angiosperm branch, February 27, 2019, He 5732 (BJFC 030599); Mitirigala Nissarana vanaya Forest Monastery, on dead angiosperm branch, March 4, 2019, He 5835 (BJFC 030702). Thailand, Chiang Rai, Campus of Mae Fah Luang University, on dead angiosperm branch, July 21, 2016, He 4037 (BJFC 023476).
Notes—Irpex alboflavescens Figures 7, 8 is characterized by having smooth hymenophores, metuloid cystidia, and ellipsoid to broadly ellipsoid basidiospores. In the phylogenetic tree (Figure 1), samples of I. alboflavescens from China (including Taiwan), Malaysia, Sri Lanka, Thailand, and USA formed a well-supported distinct lineage sister to I. lacteus, the type of the genus. According to the phylogenetic analysis results, Chen et al. (2021) treated Emmia, Flavodon, and Hydnopolyporus as synonyms of Irpex, which now includes species with smooth, poroid, labyrinthine, irpicoid, hydnoid to irregular hymenophore configuration. Irpex alboflavescens is similar to I. lenis C.C. Chen and Sheng H. Wu that also has a smooth hymenophore, but the latter species differs in lacking cystida and having larger basidiospores (6.2–7.2 × 4.6–5.2 μm, Chen et al., 2021). Moreover, I. lenis is phylogenetically distant from I. alboflavescens (Figure 1).
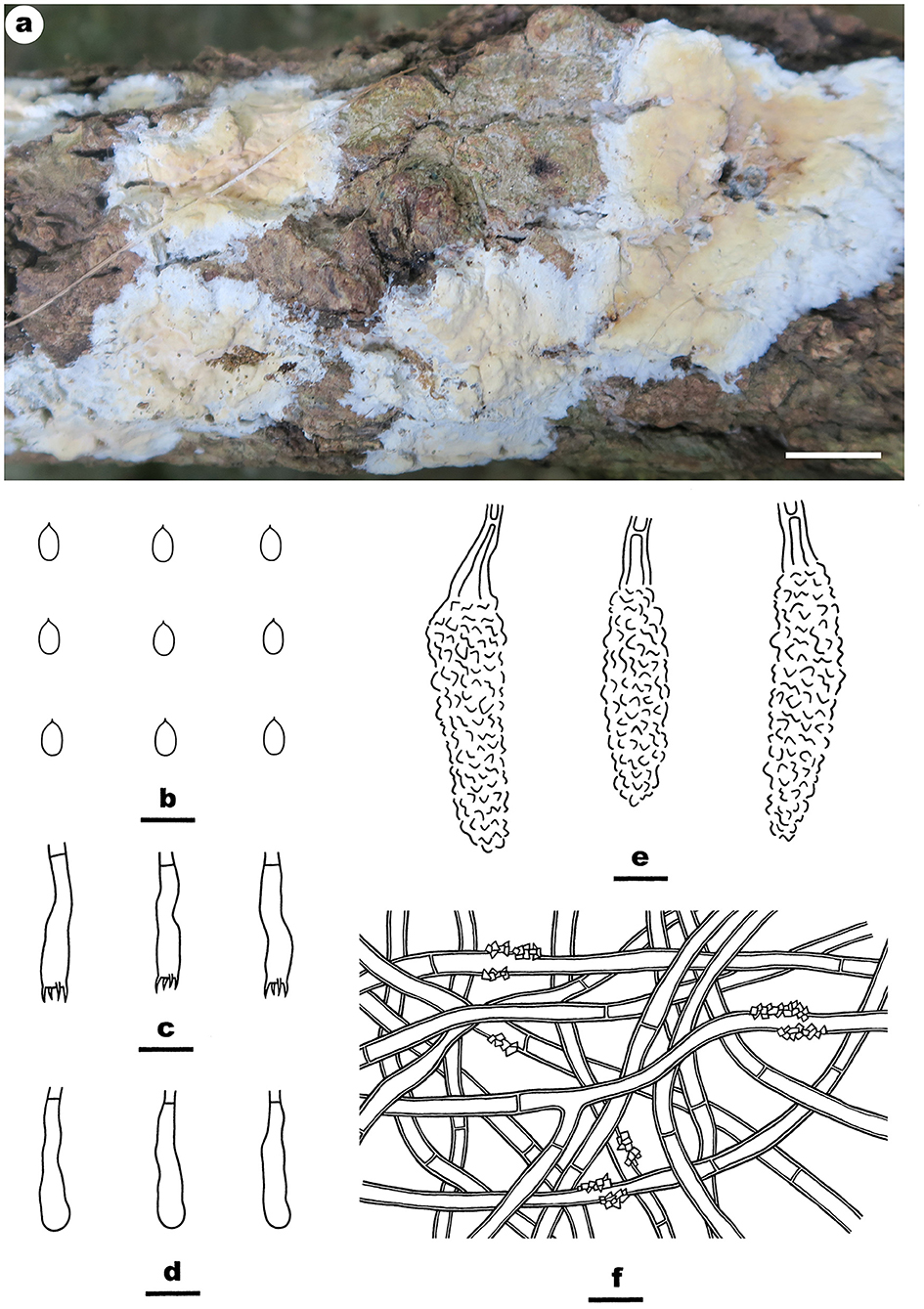
Figure 7. Irpex alboflavescens (from the holotype He 3933; scale bars: a = 1 cm; b–f = 10 μm). (A) Basidiomata. (B) Basidiospores. (C) Basidia. (D) Basidioles. (E) Lamprocystidia. (F) Hyphae from subiculum.

Figure 8. Basidiomata of Irpex alboflavescens (scale bars: A–E = 1 cm). (A) He 2278; (B) He 4719; (C) He 5783; (D) He 6355; (E) He 5732.
Irpex rosea (C.L. Zhao) Y. Li and S.H. He, comb. nov.
MycoBank: MB844001
= Flavodontia rosea C.L. Zhao, Mycotaxon 136: 762, 2022 [2021]. [MB# 838323]
Fruiting body—Basidiomata annual, resupinate to effused–reflexed with slightly elevated margin, adnate, easily separated from substrate, coriaceous, first as small patches, later confluent up to 10-cm long, 3-cm wide, up to 700-μm thick in section. Hymenophore smooth, odontioid or irpicoid, grayish orange [6B(4–5)] to grayish red [7B(4–5)], turning light brown in KOH, rarely cracked; margin thinning out, adnate or slightly elevated and curved inside, indistinct, concolorous with or paler than hymenophore surface. Context cream.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Basal layer distinct, brown; hyphae yellowish-brown, thick-walled, smooth, agglutinated, parallel to substrate, rarely branched, infrequently septate, up to 7 μm in diam. Subiculum distinct, thick; hyphae colorless, thick-walled, smooth, rarely branched, infrequently septate, slightly agglutinated, parallel to substrate, 3–7 μm in diam. Subhymenium distinct, thick; hyphae colorless, thin-walled, smooth, moderately branched and septate, slightly agglutinated, more or less vertically arranged, 2–4.5 μm in diam. Cystidia absent. Basidia clavate to subcylindrical, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, 19–28 × 4–6 μm Basidiospores broadly ellipsoid to ovoid, with a distinct apiculus, colorless, thin-walled, smooth, IKI–, CB–, (3.5–) 4–5 (−5.5) × (2.8–) 3–4 μm, L = 4.7 μm, W = 3.4 μm, Q = 1.4 (n = 30/1).
Notes—Wang and Zhao (2022) built a new monotypic genus Flavodontia Figures 9, 10 C.L. Zhao for the species F. rosea collected from Yunnan Province, southwestern China, mainly based on molecular evidence; however, our phylogenetic analyses using an expanded dataset of Irpicaceae demonstrated that the species was nested within the Irpex clade, which has been shown to include taxa from Emmia, Flavodon, and Hydnopolyporus (Chen et al., 2021). Morphologically, I. rosea has effused-reflexed coriaceous basidiomata with smooth, odontioid, or irpicoid hymenophores, simple-septate generative hyphae, broadly ellipsoid basidiospores and lacks cystidia, which fits well with the characters of Irpex. Thus, we propose the new combination and treat Flavodontia as a later synonym of Irpex.
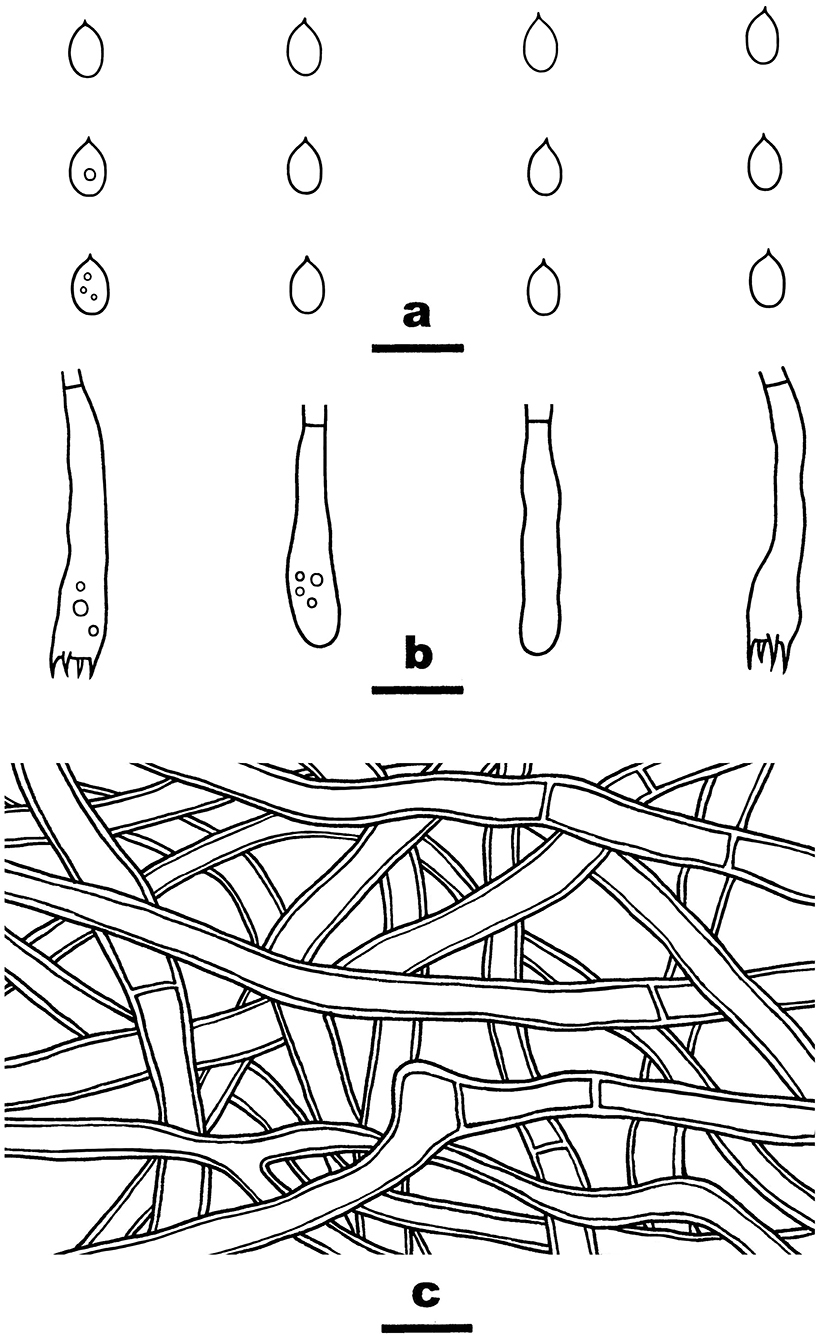
Figure 10. Irpex rosea (from He 6277; scale bars: a–c = 10 μm). (A) Basidiospores. (B) Basidia and basidioles. (C) Hyphae from subiculum.
Specimen examined—China, Yunnan Province, Xichou County, Xiaoqiaogou Forest Park, on dead angiosperm stump, November 16, 2019, He 6277 (BJFC 033221).
Phanerochaetella sinensis Y. Li and S.H. He, sp. nov.
MycoBank: MB843539
Type—China, Jiangxi Province, Yifeng County, Guanshan Nature Reserve, on dead angiosperm branch, August 9, 2016, He 4229 (BJFC 023671, holotype).
Etymology—Refers to the type locality in China.
Fruiting body—Basidiomata annual, resupinate, widely effused, closely adnate, inseparable from substrate, membranaceous to coriaceous, first as small patches, later confluent up to 10-cm long, 2-cm wide, up to 300-μm thick in section. Hymenophore smooth, orange white (5A2) to brownish orange [5C(3–4)], unchanged in KOH, slightly cracked to densely and deeply cracked; margin thinning out or abrupt, adnate or slightly elevated with age, paler than or concolorous with hymenophore. Context white.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum indistinct, thin; hyphae colorless, thick-walled, smooth, rarely branched, infrequently septate, more or less agglutinated, parallel to substrate, 3–5 μm in diam. Subhymenium thickening; hyphae colorless, thin- to thick-walled, smooth, moderately branched and septate, agglutinated, densely interwoven, 2.5–4.5 μm in diam. Lamprocystidia arising from subhymenium, narrowly clavate, colorless, thick-walled, heavily encrusted, mostly embedded, 30–70 × 5–10 μm. Basidia clavate to subcylindrical, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, 20–35 × 4–6 μm. Basidiospores cylindrical, with an apiculus, colorless, thin-walled, smooth, IKI–, CB–, (5.8–) 6–7.2 (−7.8) × 2–3 (−3.2) μm, L = 6.6 μm, W = 2.5 μm, Q = 2.3–2.9 (n = 120/4).
Additional specimens examined—China, Gansu Province, Tianshui County, Maijishan Forest Park, On dead liana, August 8, 2015, He 2484 (BJFC 020937, CFMR); Hubei Province, Wufeng County, Chaibuxi Forest Park, on dead angiosperm branch, August 15, 2017, He 5071 (BJFC 024589) and He 5073 (BJFC 024591); Yunnan Province, Luquan County, Zhuanlong Town, on dead angiosperm branch, December 4, 2015, He 3509 (BJFC 021907, CFMR).
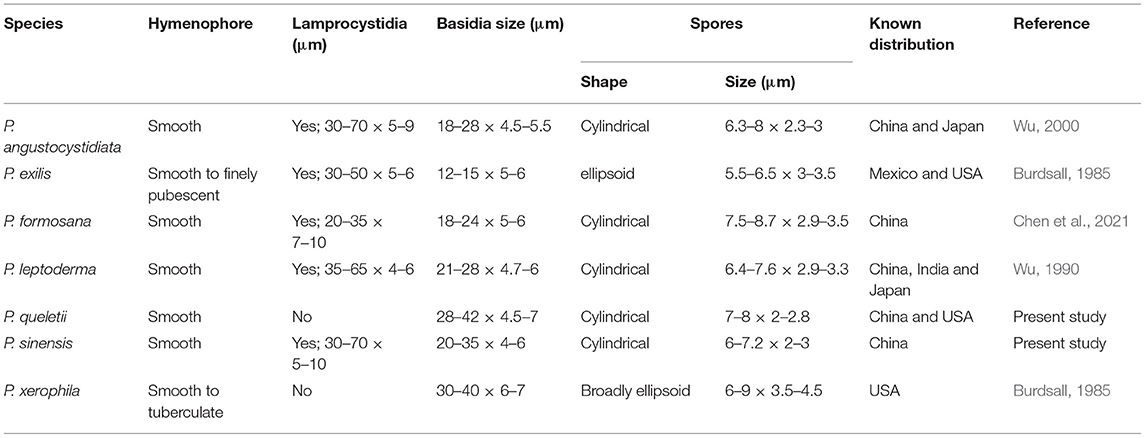
Notes—Phanerochaetella sinensis Figure 11 is characterized having a cracked hymenophore, lamprocystidia, and cylindrical basidiospores. In the phylogenetic tree (Figure 1), three samples of P. sinensis formed a distinct lineage sister to P. exilis (Burt) C.C. Chen and Sheng H. Wu, which differs in having smaller lamprocystidia (30–50 × 5–6 μm) and ellipsoid basidiospores (5.5–6.5 × 3–3.5 μm, Burdsall, 1985). Phanerochaetella angustocystidiata and P. sinensis are almost inseparable in micromorphology, but differing in color (ivory or light cream in the former) and thickness (up to 200-μm thick in the former). Meanwhile, they formed distinct lineages in the tree (Wu, 2000).
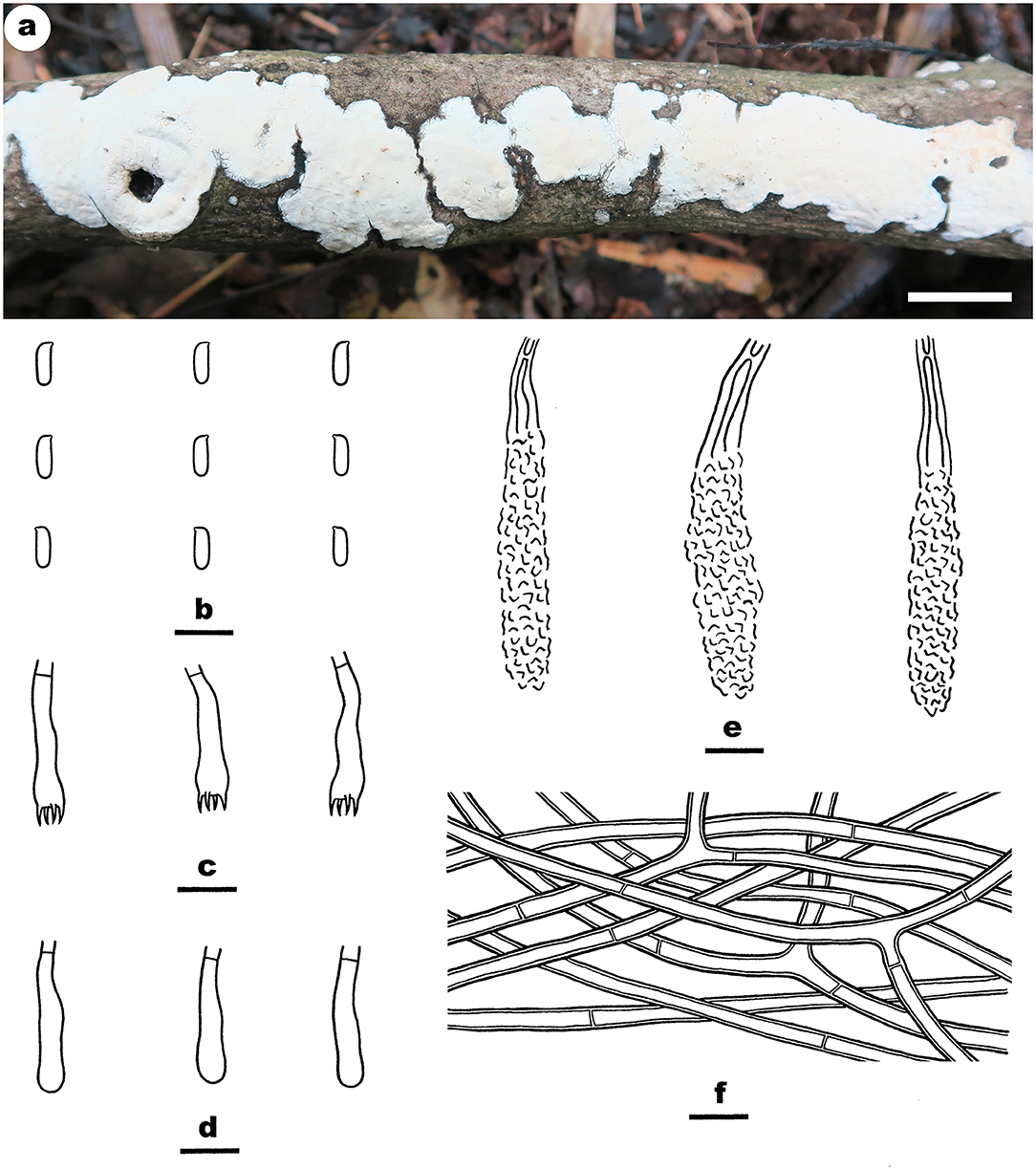
Figure 11. Phanerochaetella sinensis (from the holotype He 4229; scale bars: a = 1 cm; b–f = 10 μm). (A) Basidiomata. (B) Basidiospores. (C) Basidia. (D) Basidioles. (E) Lamprocystidia. (F) Hyphae from subiculum.
Phanerochaetella queletii (Bres.) Y. Li, Nakasone and S.H. He, comb. nov.
MycoBank: MB844002
= Corticium queletii Bres., Nuovo Giornale Boanico Italiano 8: 10, 1901. [MB# 183260]
= Phanerochaete queletii (Bres.) Nakasone, Cryptogamie Mycologie 29(3): 237, 2008. [MB# 512577]
= Phanerochaete jose-ferreirae (D.A. Reid) D.A. Reid, Acta Botanica Croatica 34: 135, 1975. [MB# 319714]
=Corticium jose-ferreirae D.A. Ried, Revista Biolgia 5: 140, 1965. [MB# 311853]
Fruiting body—Basidiomata annual, resupinate, widely effused, adnate, separable from substrate, membranaceous to coriaceous, first as small patches, later confluent up to 8-cm long, 4-cm wide, up to 350-μm thick in section. Hymenophore smooth, pale orange (5A3) to grayish orange [5B(4–5)], unchanged in KOH, not cracking or slightly cracked with age; margin thinning out, adnate or loose and slightly elevated with age, fimbriate and paler than hymenophore surface when juvenile, becoming indistinct and concolorous when mature. Context white.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct, up to 250-μm thick; hyphae colorless, thin- to slightly thick-walled, slightly encrusted with fine crystals, infrequently branched, moderately septate, loosely interwoven, 2.5–5 μm in diam. Subhymenium indistinct, thin; hyphae colorless, thin-walled, smooth, infrequently branched, moderately septate, loosely interwoven, 2–4.5 μm in diam. Cystidia absent. Basidia clavate to subcylindrical, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, 28–42 × 4.5–7 μm. Basidiospores cylindrical, with an apiculus, colorless, thin-walled, smooth, IKI–, CB–, (6.8–) 7–8 (−8.2) × 2–2.8 (−3) μm, L = 7.6 μm, W = 2.4 μm, Q = 3.2–3.3 (n = 90/3).
Type specimens examined—Italy, Vallombrosa, ad ramos corticates Abietis pectinate, Nov 1899, Martielli (BPI 0282568, holotype of C. queletii). Portugal, Serra da Arrabida, on (bark of) fallen branch, 10 May 1964, D.A. Reid (K(M) 146494, holotype of C. jose-ferrieriae).
Additional specimens examined—China, Beijing, Mentougou District, Lingshan Scenic Spot, on dead angiosperm branch, April 10, 2022, He 7467 (BJFC 038602); Inner Mongolia Autonomous Region, Genhe County, Greater Khingan Nature Reserve, on dead Salix branch, October 17, 2015, He 3050 (BJFC 021440, CFMR); Yakeshi County, Tulihe Forest Park, on dead Salix branch, October 18, 2015, He 3065 (BJFC 021455, CFMR); Sichuan Province, Xiaojin County, Jiajin Mountains, on dead angiosperm branch, September 17, 2012, He 20120917-10 (BJFC 014601); Yunnan Province, Baoshan County, Gaoligongshan Nature Reserve, On dead branch of Alniphyllum, November 28, 2015, He 3284 (BJFC 021679, CFMR).
Notes—Phanerochaetella queletii Figures 12, 13 is characterized by basidiomata with smooth to tuberculate hymenophores that often are rimose to reveal the white context and margins that are typically distinct and abrupt that often are slightly detached and incurved, cylindrical basidiospores, and lacking cystidia. Its preferred habitat is small, corticate branches of woody angiosperms, rarely on gymnosperms. This widely distributed species was first described from Italy but is known throughout Europe and is frequently collected in the upper Midwest in the USA. This is the first record this species in China. There is variability in the hymenophore ranging from smooth to distinctly tuberculate, sparsely to highly rimose, and nearly white and cream to brownish orange. Similarly, the margin may be narrowly adnate, white, and fimbriate to abrupt, slightly detached and incurved. Variability in basidiospore length and width was also observed. The description above is based on the Chinese specimens only that appear to have slightly narrower basidiospores than reported earlier. Despite the basidiospore size difference between the type specimens of C. queletii and C. jose-ferrieriae, their overwhelming similarities in basidiomata texture and color, hymenophore configuration, and other microscopic features indicate that they are conspecific. For additional descriptions and illustrations, see Eriksson et al. (1978), Nakasone (2008), and Bernicchia and Gorjón (2010).
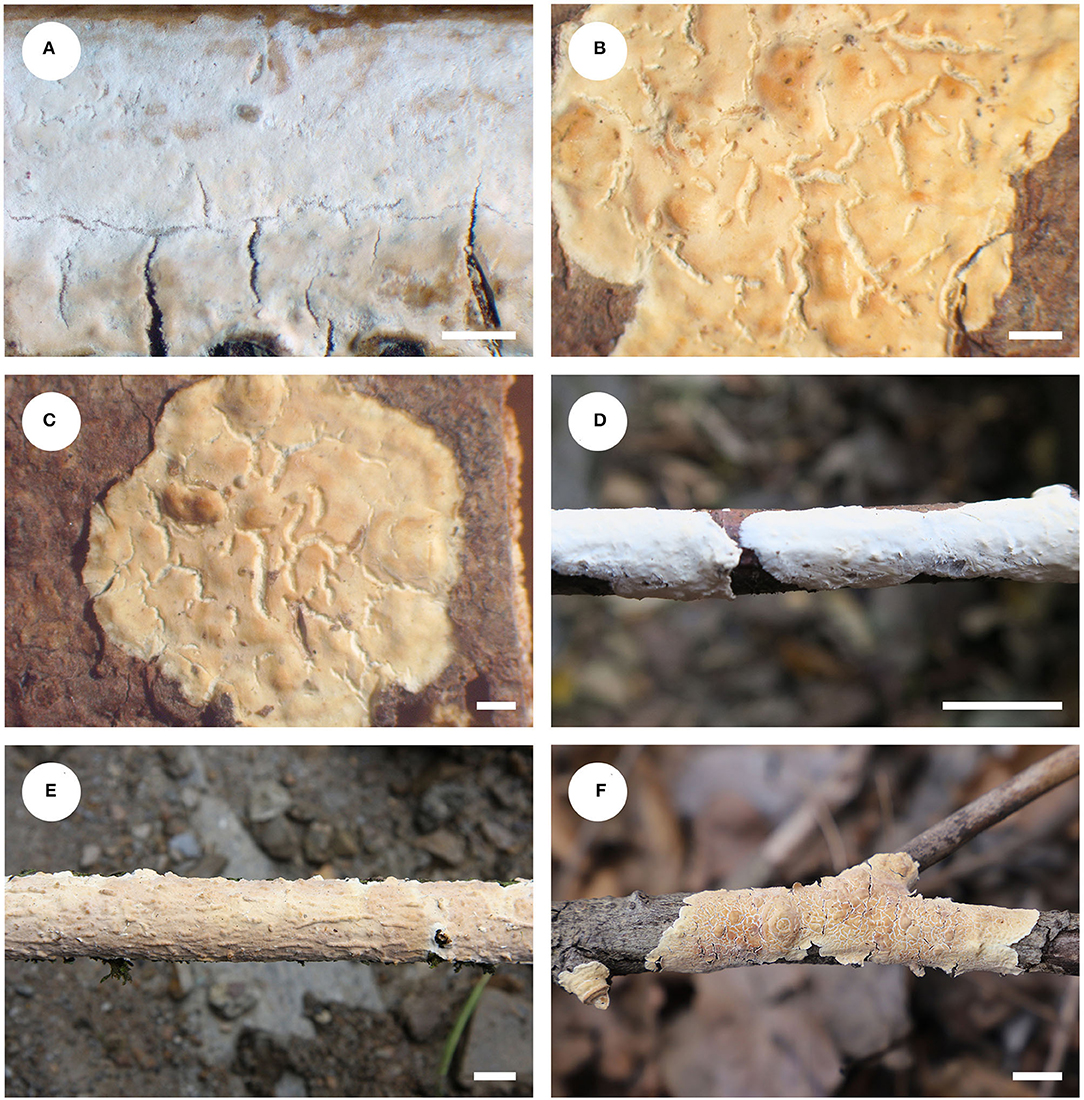
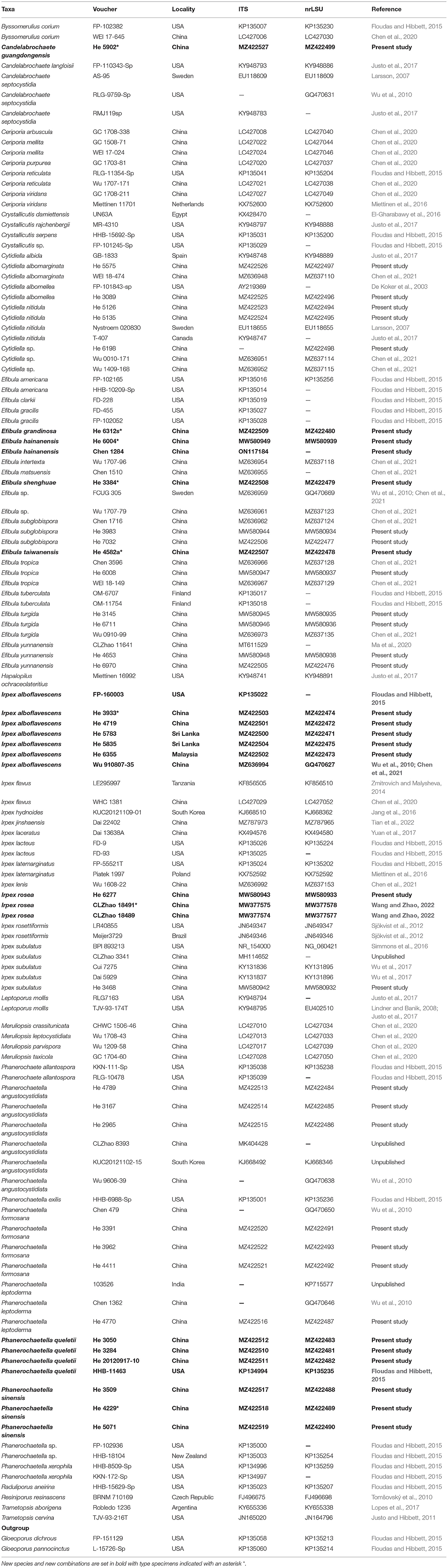
Figure 12. Basidiomata of Phanerochaetella queletii (scale bars: A–F = 1 cm). (A) K(M) 146494 (holotype of Corticium jose-ferrieriae); (B) BPI 282568 (holotype of C. queletii); (C) F11364 (isotype of C. queletii); (D) He 3284; (E) He 20120917-10; (F) He 7467.
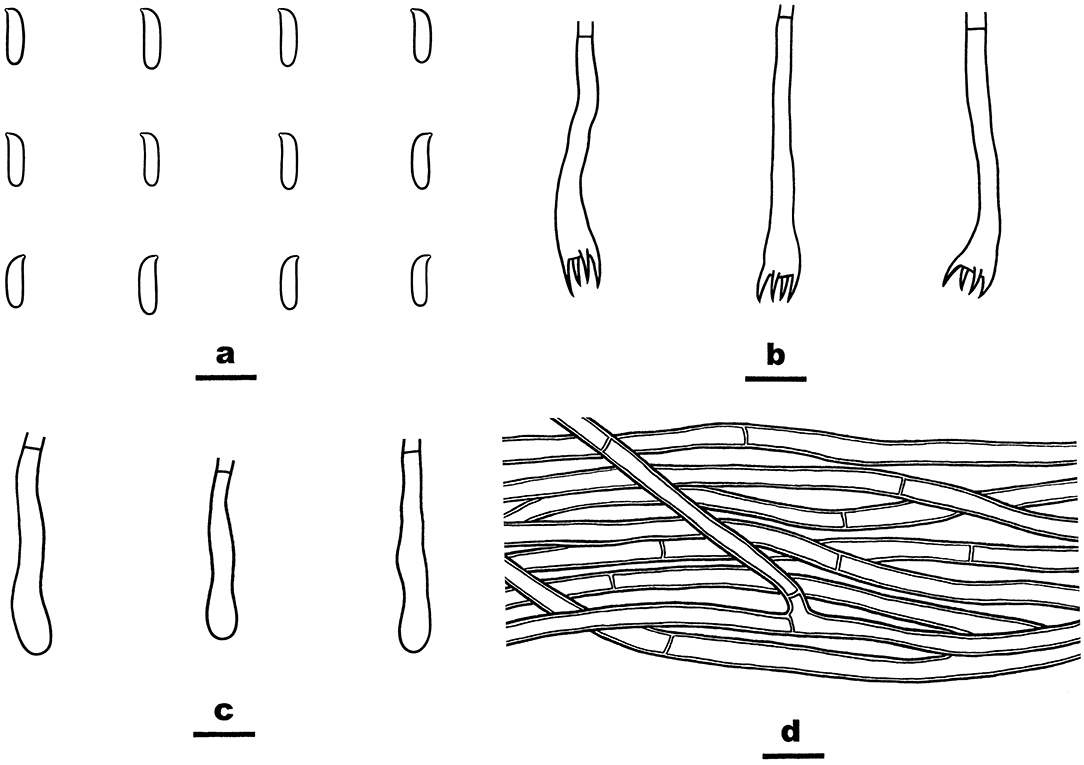
Figure 13. Phanerochaetella queletii (from He 3284; scale bars: a–d = 10 μm). (A) Basidiospores. (B) Basidia. (C) Basidioles. (D) Hyphae from subiculum.
In the phylogenetic tree (Figure 1), three samples of P. queletii from China and one sample from USA (HHB-11463) formed a strongly support lineage, which is sister to P. xerophila (Burds.) C.C. Chen and Sheng H. Wu from the Sonoran Desert on southwestern USA. and P. angustocystidiata (Sheng H. Wu) C.C. Chen and Sheng H. Wu from East Asia. Morphologically, both P. xerophila and P. queletii lack cystidia, but the former species has broadly ellipsoid basidiospores (6–9 × 3.5–4.5 μm, Burdsall, 1985). Phanerochaetella angustocystidiata has cylindrical basidiospores that are slightly smaller than in P. queletii and develops lamprocystidia (Wu, 2000).
Discussion
The species diversity, taxonomy, and phylogeny of the phlebioid clade in Polyporales were intensively studied recently by many authors, and a large number of new taxa from East Asia were described (Floudas and Hibbett, 2015; Miettinen et al., 2016; Justo et al., 2017; Ma and Zhao, 2019; Chen et al., 2020, 2021; Xu et al., 2020; Wang and Zhao, 2021; Zhao et al., 2021; Tian et al., 2022). This study furthers our knowledge of this group with the addition of seven new corticioid species in the Irpicaceae. There is no doubt that more new taxa will be revealed as more surveys are carried out in areas of Asia with the aid of molecular evidence. Our phylogenetic analysis results supported to place the newly described monotypic genus, Flavodontia, in synonymy under Irpex. We also demonstrated that one species, namely, P. queletti, has a wide distribution throughout the north temperate region from China to Europe and North America.
The results of our phylogenetic analyses of Irpicaceae are consistent with that presented by Chen et al. (2021). In both studies, clades of the five genera—Irpex, Phanerochaetella, Byssomerulius, Cytidiella, and Meruliopsis, received strong support values, whereas Efibula was shown to be paraphyletic with species distributed in four subclades. According to the phylogenetic results, Irpex now includes species with smooth, poroid, labyrinthine, irpicoid, hydnoid to irregular hymenophore configurations. However, there are still many old names in Irpex that need to be studied by using modern taxonomic methods and systems. For Efibula, there are no distinct morphological characters to divide it into small genera at present (Table 2). The newly erected genus, Phanerochaetella, contains several species with diverse micromorphology: lamprocystidia present or absent and basidiospores from broadly ellipsoid to cylindrical (Table 3). Three species of Candelabrochaete formed two distinct lineages in the Ceriporia/Candelabrochaete s.l./ Leptoporus/Phanerochaete allantospora clade, but their generic position remains unresolved since the type species, C. africana Boidin, was not nested within the phlebioid clade (Justo et al., 2017; Chen et al., 2021).
The molecular evidence has brought significant changes and increased our understanding in the taxonomy of Irpicaceae. The morphological circumscriptions of some genera became broader, for example, Irpex now contains species with poroid, labyrinthine, irpicoid, hydnoid to irregular hymenophore, and Efibula is shown to contain species with or without horizontally arranged subicular hyphae. Species with simple-septate hyphae and without cystidia can be found in Efibula, Irpex, and Phanerochaetella. To determine important and useful morphological characters for distinguishing those genera and resolve infra-generic phylogeny, additional taxa from these genera from other regions should be included in the future phylogenetic studies. In addition, comparative morphological analyses of fruitbody features such as subiculum and subhymenium thickness, construction, and texture in addition to basidia, cystidia, and basidiospore shape and size are important areas of consideration in future studies. Information on habitat and distribution may be useful for understanding species delimitation and phylogeny of species within a genus.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, see the Table 1 included in article.
Author Contributions
YL performed the phylogenetic analyses and did most of the measurements, descriptions, and illustrations. S-HH designed the research, collected most of the specimens, and wrote the text. C-CC provided with some specimens and sequences. KN examined materials from USA and revised the text. H-XM helped in field trips. All authors contributed to the article and approved the submitted version.
Funding
Financial support was provided by the National Natural Science Foundation of China (Nos. 31870011 and 31750001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to express their deep appreciation to Prof. Sheng-Hua Wu (National Museum of Natural Science, Taiwan, China) for providing instructions of the research.
Abbreviations
ITS, internal transcribed spacer; nrLSU, nuclear ribosomal large subunit; BJFC, herbarium of Beijing Forestry University, Beijing, China; CFMR, Centre for Forest Mycology Research, U.S. Forest Service, Madison, Wisconsin, USA; TNM, National Museum of Natural Science, Taichung, Taiwan, China; KOH, 2% (w/v) potassium hydroxide; IKI, Melzer's reagent; CB, cotton blue; IKI–, neither amyloid nor dextrinoid; CB–, acyanophilous; L, mean spore length; W, mean spore width; Q, L/W ratio, n (a/b), number of spores (a) measured from number of specimens (b); CTAB, cetyltrimethylammonium bromide; DNA, deoxyribonucleic acid; PCR, polymerase chain reaction; ML, maximum likelihood; BI, Bayesian inference; BPP, Bayesian posterior probability.
Footnotes
1. ^http://sweetgum.nybg.org/science/ih/
2. ^http://www.biology.duke.edu/fungi/mycolab/primers.htm
3. ^https://www.ncbi.nlm.nih.gov/
References
Boonmee, S., Wanasinghe, D. N., Calabon, M. S., Huanraluek, N., Chandrasiri, S. K. U., Jones, G. E. B., et al. (2021). Fungal diversity notes 1387–1511: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 111, 1–335. doi: 10.1007/s13225-021-00489-3
Burdsall, H. H (1984). The genus Candelabrochaete (Corticiaceae) in North America and a note on Peniophora mexicana. Mycotaxon. 19, 389–395.
Burdsall, H. H (1985). A contribution to the taxonomy of the genus Phanerochaete (Corticiaceae, Aphyllophorales). Mycologia Memoirs. 10, 1–165.
Chen, C. C., Chen, C. Y., Lim, Y. W., and Wu, S. H. (2020). Phylogeny and taxonomy of Ceriporia and other related taxa and description of three new species. Mycologia. 112, 64–82. doi: 10.1080/00275514.2019.1664097
Chen, C. C., Chen, C. Y., and Wu, S. H. (2021). Species diversity, taxonomy and multi-gene phylogeny of phlebioid clade (Phanerochaetaceae, Irpicaceae, Meruliaceae) of Polyporales. Fungal Divers. 111, 337–442. doi: 10.1007/s13225-021-00490-w
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Meth. 9, 772. doi: 10.1038/nmeth.2109
De Koker, T. H., Nakasone, K. K., Haarhof, J., Burdsall, H. H., and Janse, B. J. (2003). Phylogenetic relationships of the genus Phanerochaete inferred from the internal transcribed spacer region. Mycological Research 107, 1032–1040. doi: 10.1017/S095375620300827X
Dueñas, M., Telleria, M. T., Melo, I., Rodríguez-Armas, J. L., and Beltrán-Tejera, E. (2008). A new species of Candelabrochaete (Polyporales, Basidiomycota). Mycotaxon. 103, 299–305.
Duhem, B., and Buyck, B. (2011). Candelabrochaete neocaledonica sp. nov. de Nouvelle-Calédonie. Cryptogamie Mycologie. 1, 25–32. doi: 10.7872/crym.v32.iss1.2012.025
El-Gharabawy, H. M., Detheridge, A.ndrew., El-Fallal, A. A., El-Sayed, A. K. A., and Griffith, G. (2016). Analysis of wood decay and ligninolysis in Polyporales from the Nile Delta region of Egypt. Mycosphere. 7, 392–404. doi: 10.5943/mycosphere/7/4/1
Eriksson, J., Hjortstam, K., and Ryvarden, L. (1978). The Corticiaceae of North Europe 5. Mycoaciella – Phanerochaete. Oslo: Fungiflora.
Floudas, D., and Hibbett, D. S. (2015). Revisiting the taxonomy of Phanerochaete (Polyporales, Basidiomycota) using a four gene dataset and extensive ITS sampling. Fungal Biol. 119, 679–719. doi: 10.1016/j.funbio.2015.04.003
Hall, T. A (1999). Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 41, 95–98.
Jang, Y., Jang, S., Lee, J., Lee, H., Lim, Y. W., Kim, C., et al. (2016). Diversity of wood-inhabiting polyporoid and corticioid fungi in Odaesan National Park, Korea. Mycobiology. 44, 217–236. doi: 10.5941/MYCO.2016.44.4.217
Justo, A., and Hibbett, D.S. (2011). Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset. Taxon 60, 1567–1583. doi: 10.1002/tax.606003
Justo, A., Miettinen, O., Floudas, D., Ortiz-Santana, B., Sjökvist, E., Lindner, D., et al. (2017). A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biology 121, 798–824. doi: 10.1016/j.funbio.2017.05.010
Katoh, K., Rozewicki, J., and Yamada, K. D. (2017). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinf. bbx108. doi: 10.1093/bib/bbx108
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinf. 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Kornerup, A., and Wanscher, J. H. (1978). Methuen handbook of colour. 3rd. London: E. Methuen and Co., Ltd.
Larsson, K. H (2007). Re-thinking the classification of corticioid fungi. Mycological Res. 111, 1040–1063. doi: 10.1016/j.mycres.2007.08.001
Lindner, D. L., and Banik, M. T. (2008). Molecular phylogeny of Laetiporus and other brown rot polypore genera in North America. Mycologia. 100, 417–430. doi: 10.3852/07-124R2
Lopes, M. T., Urcelay, C., and Robledo, G. L. (2017). New insights on Trametopsis Tomšovský (Polyporales Gäum) based on phylogenetic evidences and morphological analyses of neotropical species. Phytotaxa. 311, 155–167. doi: 10.11646/phytotaxa.311.2.3
Ma, R. X., Shi, Z. J., and Zhao, C. L. (2020). A new species of Efibula (Polyporales, Basidiomycota) from China. Phytotaxa. 451, 238–244. doi: 10.11646/phytotaxa.451.3.7
Ma, X., and Zhao, C. L. (2019). Crepatura ellipsospora gen. et sp. nov. in Phanerochaetaceae (Polyporales, Basidiomycota) bearing a tuberculate hymenial surface. Mycological Prog. 18, 785–793. doi: 10.1007/s11557-019-01488-0
Maddison, W. P., and Maddison, D. R. (2018). Mesquite: a modular system for evolutionary analysis. Available online at: http://www.mesquiteproject.org
Miettinen, O., Spirin, V., Vlasák, J., Rivoire, B., Stenroos, S., and Hibbett, D. (2016). Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota). MycoKeys. 17, 1–46. doi: 10.3897/mycokeys.17.10153
Nakasone, K. K (2008). Type studies of corticioid hymenomycetes described by bresadola. Cryptogamie Mycologie. 29, 231–257.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Hohna, S., et al. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Simmons, D. R., You, L., Craig, C. B., and Jiri, H. (2016). Flavodon ambrosius sp. nov., a basidiomycetous mycosymbiont of Ambrosiodmus ambrosia beetles. Mycotaxon. 131, 277–285. doi: 10.5248/131.277
Sjökvist, E., Larsson, E., Eberhardt, U., Ryvarden, L., and Larsson, K. H. (2012). Stipitate stereoid basidiocarps have evolved multiple times. Mycologia. 104, 1046–1055. doi: 10.3852/11-174
Stamatakis, A (2014). RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Tian, X. M., Man, X. W., and Liu, Z. B. (2022). Irpex jinshaensis sp. nov. and I. subulatus comb. nov. (Irpicaceae, Polyporales), evidenced by morphological characters and phylogenetic analysis. Phytotaxa. 533, 73–82. doi: 10.11646/phytotaxa.533.1.4
Tomšovský, M., Menkis, A., and Vasaitis, R. (2010). Phylogenetic relationships in European Ceriporiopsis species inferred from nuclear and mitochondrial ribosomal DNA sequences. Fungal Biol. 114, 350–358. doi: 10.1016/j.funbio.2010.02.004
Wang, D. Q., and Zhao, C. L. (2021). Morphological and phylogenetic evidence for recognition of two new species of Phanerochaete from East Asia. J. Fungi. 7, 1063. doi: 10.3390/jof7121063
Wang, H., and Zhao, C. L. (2022). Flavodontia rosea gen. and sp. nov. from southwestern China. Mycotaxon 136, 755–767. doi: 10.5248/136.755
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics”, in PCR Protocols: A Guide to Methods and Applications, Innis, M. A., Gelfand, D. H., Sninsky, J. J., and White, T. J. (eds.). San Diego: Academic Press. p 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wu, F., Chen, J. J., Ji, X. H., Vlasak, J., and Dai, Y. C. (2017). Phylogeny and diversity of the morphologically similar polypore genera Rigidoporus, Physisporinus, Oxyporus, and Leucophellinus. Mycologia 109, 749–765. doi: 10.1080/00275514.2017.1405215
Wu, S. H (1990). The Corticiaceae (Basidiomycetes) subfamilies Phlebioideae, Phanerochaetoideae and Hyphodermoideae in Taiwan. Acta Botanica Fennica. 142, 1–123.
Wu, S. H., Nilsson, H. R., Chen, C. T., Yu, S. Y., and Hallenberg, N. (2010). The white-rotting genus Phanerochaete is polyphyletic and distributed throughout the phleboid clade of the Polyporales (Basidiomycota). Fungal Divers. 42, 107–118. doi: 10.1007/s13225-010-0031-7
Xu, Y. L., Cao, Y. F., Nakasone, K. K., Chen, C. C., and He, S. H. (2020). Taxonomy and phylogeny of Phanerochaete sensu stricto (Polyporales, Basidiomycota) with emphasis on Chinese collections and descriptions of nine new species. Mycosphere. 11, 1527–1552. doi: 10.5943/mycosphere/11/1/12
Yuan, Y., Ji, X. H., Wu, F., and Chen, J. J. (2017). Ceriporia albomellea (Phanerochaetaceae, Basidiomycota), a new species from tropical China based on morphological and molecular evidences. Phytotaxa. 298, 20–28. doi: 10.11646/phytotaxa.298.1.2
Zhao, Y. N., He, S. H., Nakasone, K. K., Wasantha Kumara, K. L., Chen, C. C., Liu, S. L., et al. (2021). Global phylogeny and taxonomy of the wood-decaying fungal genus Phlebiopsis (Polyporales, Basidiomycota). Front. Microbiol. 12, 622460. doi: 10.3389/fmicb.2021.622460
Keywords: corticioid fungi, East Asia, Phanerochaete s.l., phlebioid clade, white rot, wood-decaying fungi
Citation: Li Y, He S-H, Chen C-C, Nakasone KK and Ma H-X (2022) Global Taxonomy and Phylogeny of Irpicaceae (Polyporales, Basidiomycota) With Descriptions of Seven New Species and Proposals of Two New Combinations. Front. Microbiol. 13:911978. doi: 10.3389/fmicb.2022.911978
Received: 03 April 2022; Accepted: 17 May 2022;
Published: 20 June 2022.
Edited by:
George Tsiamis, University of Patras, GreeceReviewed by:
Shaghayegh Nasr, Iranian Biological Resource Center (IBRC), IranLuca Roscini, University of Perugia, Italy
Copyright © 2022 Li, He, Chen, Nakasone and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang-Hui He, heshuanghui@bjfu.edu.cn
 Yue Li
Yue Li Shuang-Hui He
Shuang-Hui He Che-Chih Chen
Che-Chih Chen Karen K. Nakasone
Karen K. Nakasone Hai-Xia Ma
Hai-Xia Ma