- 1Institute of Microbiology, School of Ecology and Nature Conservation, Beijing Forestry University, Beijing, China
- 2College of Biological Science and Engineering, North Minzu University, Yinchuan, China
- 3Faculty of Agronomy and Life Sciences, Zhaotong University, Zheaotong, China
- 4Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Xining, China
- 5China Fire and Rescue Institute, Beijing, China
Two new species of Antrodia, A. aridula and A. variispora, are described from western China. Phylogeny based on a six-gene dataset (ITS + nLSU + nSSU + mtSSU + TEF1 + RPB2) demonstrates that samples of the two species form two independent lineages within the clade of Antrodia s.s. and are different in morphology from the existing species of Antrodia. Antrodia aridula is characterized by its annual and resupinate basidiocarps with angular to irregular pores of 2–3 mm each and oblong ellipsoid to cylindrical basidiospores measuring 9–12 × 4.2–5.3 μm, growing on gymnosperm wood in a dry environment. Antrodia variispora is characterized by its annual and resupinate basidiocarps with sinuous or dentate pores with a size of 1–1.5 mm each and oblong ellipsoid, fusiform, pyriform, or cylindrical basidiospores measuring 11.5–16 × 4.5–5.5 μm, growing on the wood of Picea. The differences between the new species and morphologically similar species are discussed in this article.
Introduction
Antrodia P. Karst. is one of the major groups of fungi causing brown rot, mostly on gymnosperm wood in the Northern Hemisphere (Gilbertson and Ryvarden, 1986; Núñez and Ryvarden, 2001; Ryvarden and Melo, 2017; Wu et al., 2022), and traditionally, the members of the genus are characterized by annual to perennial, leathery, mostly light colored, resupinate to effused-reflexed or distinctly pileate basidiocarps; a dimitic hyphal structure or a few species with a monomitic hyphal system; generative hyphae with clamp connections; skeletal hyphae negative in Melzer’s reagent; some species with amyloid skeletal hyphae; and hyaline, thin-walled basidiospores which are negative in Melzer’s reagent and cotton blue (Spirin et al., 2013; Liu et al., 2022). Recent studies demonstrated that this genus is polyphyletic including 12 small and monophyletic genera (Ortiz-Santana et al., 2013; Spirin et al., 2013; Runnel et al., 2019; Liu et al., 2022), and Antrodia s. str. Has been delimited as the species grouped around Antrodia serpens (Fr.) P. Karst. within the antrodia clade of Polyporales (Hibbett and Donoghue, 2001; Spirin et al., 2013).
During an investigation on polypores in western China, five resupinate, cream to buff specimens were collected from Qinghai Province and Inner Mongolia Autonomous Region, western China. Macromorphology and the ecology of brown rot on gymnosperm wood showed that they belong to Antrodia s.l., and further morphological examination and phylogenetic analysis indicated that they represent two undescribed species of Antrodia s. str. Thus, we describe them as two new species in the present article.
Materials and methods
Morphological studies
All studied specimens are deposited in the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). Morphological descriptions are based on field notes and microscopic examinations of voucher specimens. Special color terms are based on Anonymous (1969) and Petersen (1996). Microscopic structures refer to Spirin et al. (2013), Chen and Cui (2015), and Liu et al. (2022).
DNA extraction, amplification, and sequencing
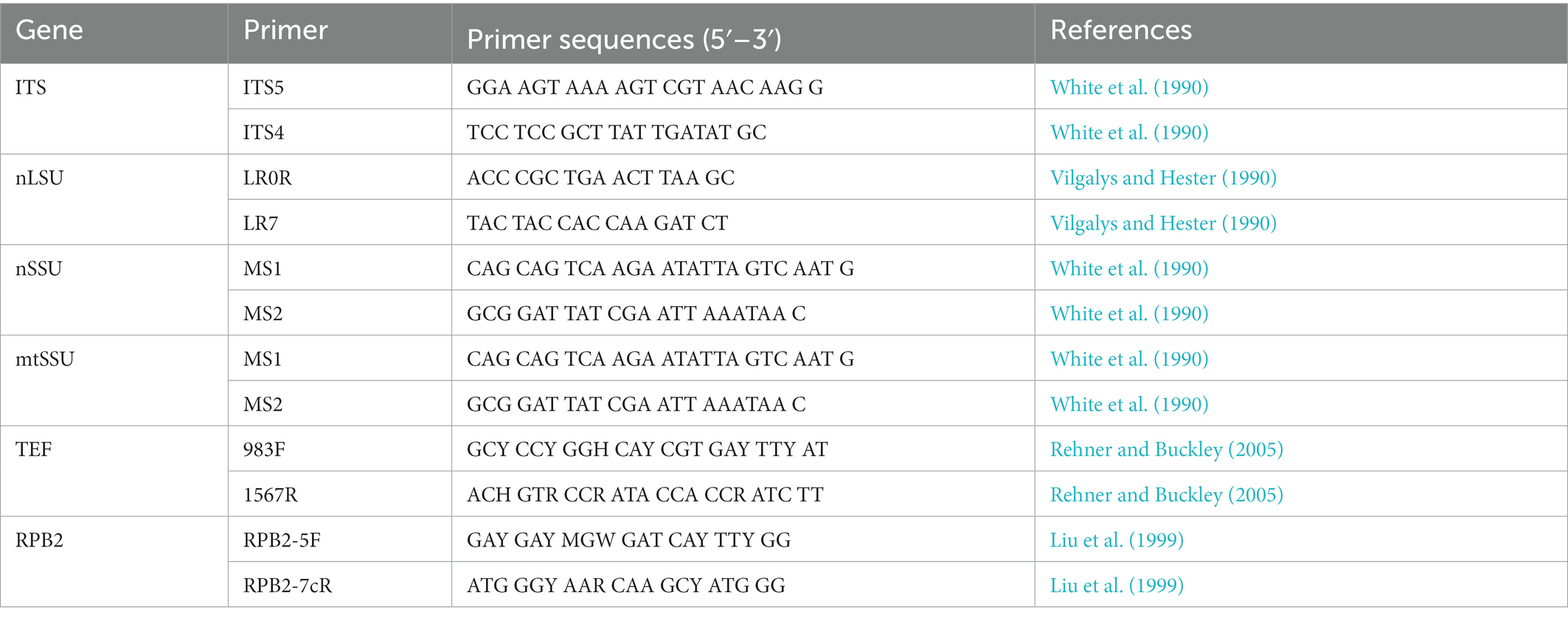
Acetyl trimethylammonium bromide rapid plant genome extraction kit (Aidlab Biotechnologies Co., Ltd., Beijing, China) was used to obtain DNA templates from dried specimens and perform the polymerase chain reaction (PCR) according to the manufacturer’s instructions with some modifications (Chen and Dai, 2021; Liu et al., 2022). The primers of ITS, including nLSU, nSSU, mtSSU, TEF1, and RPB2, for amplifying the DNA regions are mentioned in Table 1. The PCR procedure for ITS, mtSSU, TEF1, and RPB2 follows Liu et al. (2022). All newly generated sequences have been submitted to GenBank and are listed in Table 2.
Phylogenetic analysis
A total of 98 samples of Antrodia and related taxa were used for phylogenetic analysis in this study (Table 2). Oligoporus rennyi (Berk. & Broome) Donk and Postia lactea (Fr.) P. Karst. were selected as outgroups for phylogenetic analysis following Liu et al. (2022), based on the combined datasets of the internal transcribed spacer (ITS) region, the large subunit nuclear ribosomal RNA gene (nLSU), the small subunit nuclear ribosomal RNA gene (nSSU), the small subunit mitochondrial rRNA gene sequences (mtSSU), the translation elongation factor 1-α gene (TEF1), and the second subunit of RNA polymerase II (RPB2). Sequences were aligned with MAFFT v. 7 online1 adjusting the direction of nucleotide sequences according to the first sequence (accurate enough for most cases), selecting the G-INS-i iterative refinement method (Katoh et al., 2017). The aligned sequences were deposited at TreeBase (submission ID 29874).2
The analyses of maximum parsimony (MP), maximum likelihood (ML), and Bayesian inference (BI) refer to Liu et al. (2022).
Results
Phylogeny
The concatenated dataset included 98 ITS sequences, 90 nLSU sequences, 67 nSSU sequences, 64 mtSSU sequences, 70 TEF1 sequences, and 66 RPB2 sequences representing 54 taxa. There are 4,608 characters in the dataset, including 3,044 were constant, 144 were variable and parsimony-uninformative, and 1,420 were parsimony-informative. MP analysis yields a tree (TL = 8,189, CI = 0.323, RI = 0.700, RC = 0.226, HI = 0.677). “GTR + I + G” was the best model for the BI analysis, lset nst = 6, rates = invgamma; prset statefreqpr = dirichlet (1,1,1,1). The average standard deviation of split frequencies in the BI analysis was 0.008957. Branches that received bootstrap support for MP (MP-BS), ML (ML-BS), and BI (BPP) greater than or equal to 50% (MP-BS and ML-BS) and 0.95 (BPP) are considered as significantly supported, respectively.
The current phylogeny placed all samples of Antrodia in a high supported clade (Figure 1). Two new species Antrodia aridula and A. variispora formed two well-supported lineages, respectively (100% MP 100% ML 1.00 BI and 99% MP 100% ML 1.00 BI). The two new species clustered with Antrodia macra (Sommerf.) Niemelä formed a well-supported subclade (96% MP 98% ML 1.00 BI).
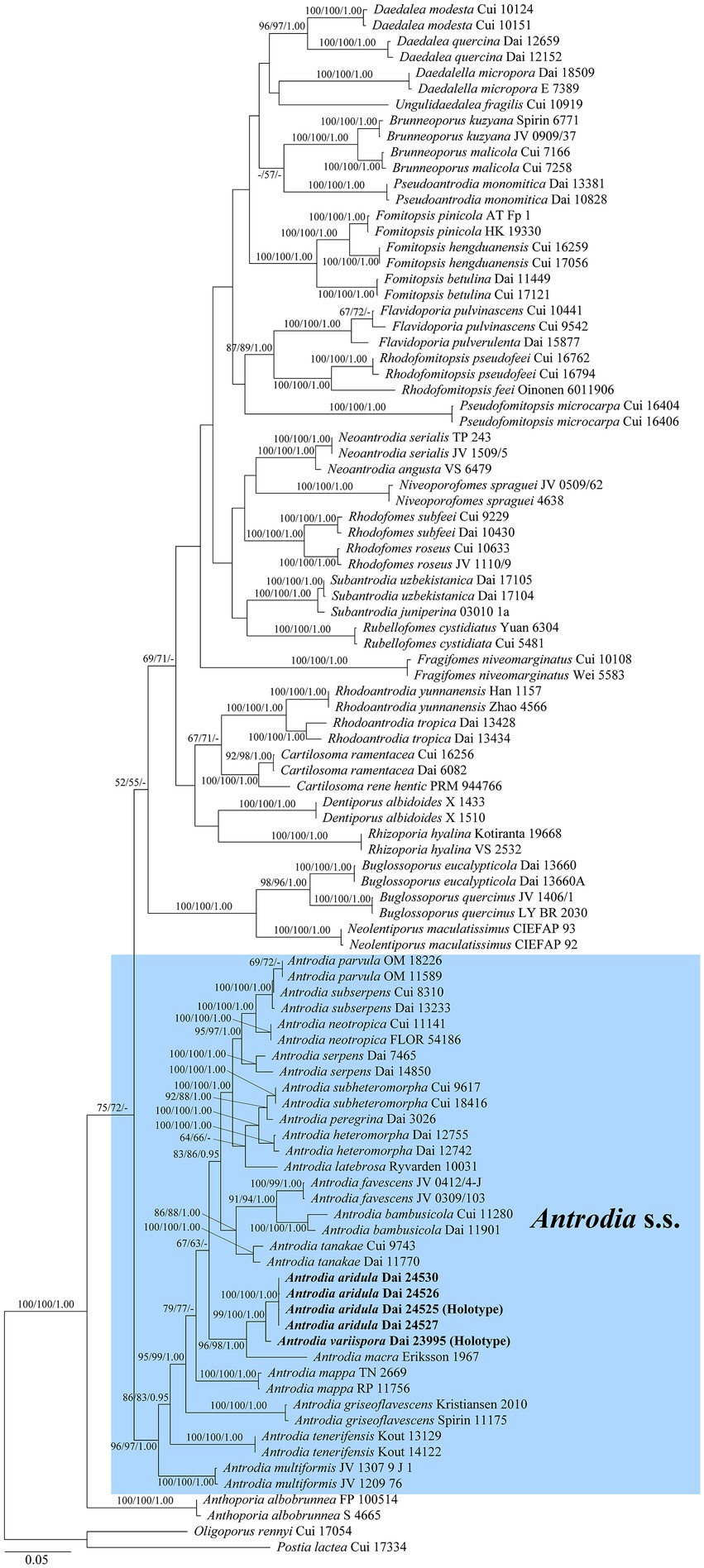
Figure 1. Maximum likelihood illustrating the phylogeny of Antrodia s.s. and related genera based on ITS + nLSU + nSSU + mtSSU + TEF1 + RPB2. Branches are labeled with parsimony bootstrap proportions and maximum likelihood bootstrap higher than 50%, and Bayesian posterior probabilities more than 0.95, respectively.
Taxonomy
Antrodia aridula Y.C. Dai, H.M. Zhou, Y.D. Wu & Shun Liu, sp. nov. Figures 2, 3.
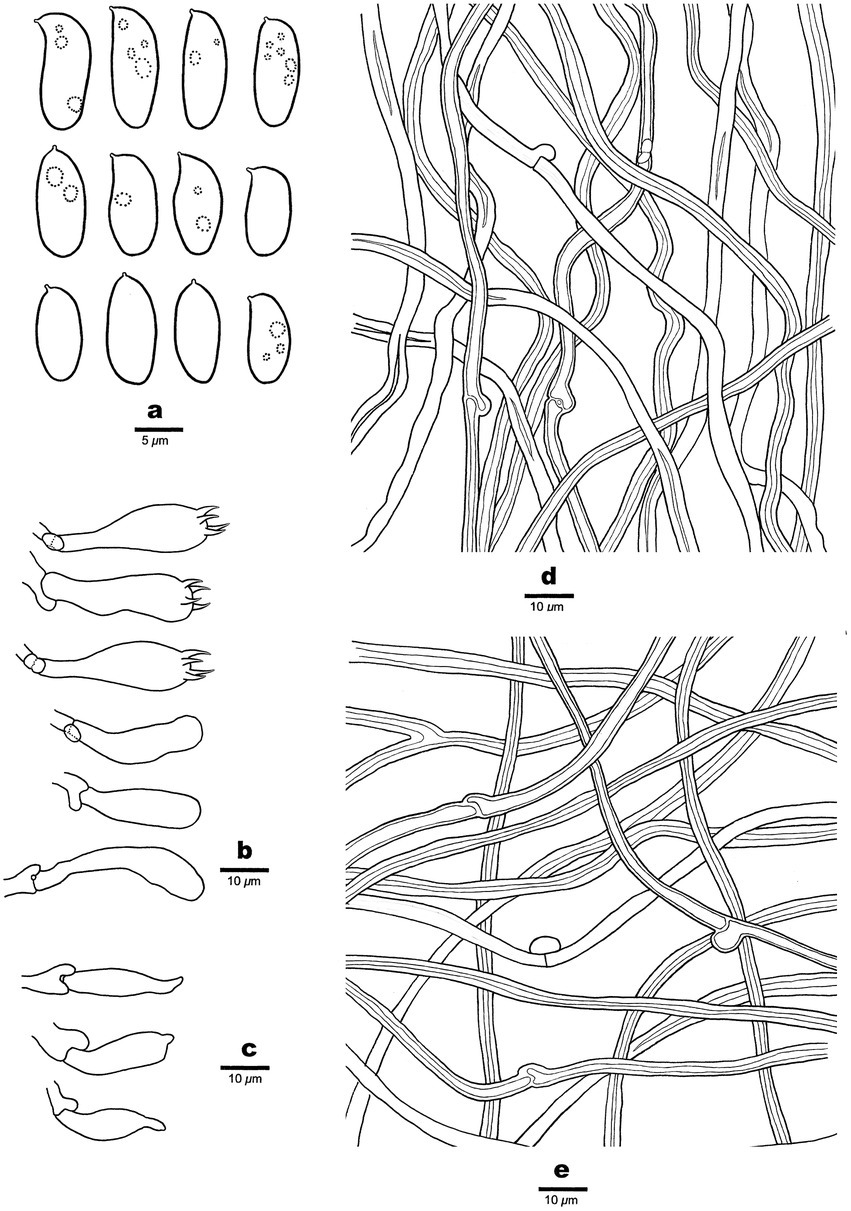
Figure 3. Microscopic structures of Antrodia aridula (holotype, Dai 24525). (A) Basidiospores. (B) Basidia and basidioles. (C) Cystidioles. (D) Hyphae from trama. (E) Hyphae from subiculum.
MycoBank number: 846495.
Holotype: China. Inner Mongolia Autonomous Region, Alxa Left Banner, Helanshan Nature Reserve, elev. 2270 m, N 38.865282, E 105.899814, on fallen trunk of Picea crassifolia, 17 September 2022, Dai 24525 (BJFC).
Etymology: Aridula (Lat.): Referring to the species that prefer to the dry environment in Helan Mts. around the desert area of the Inner Mongolia Autonomous Region.
Basidiocarps: Annual, resupinate, tightly attached on wood, leathery when fresh, hard corky when dry, up to 5 cm long, 2 cm wide, and 0.5-mm thick at the center. Pore surface cream when fresh, becoming cream to buff when dry; sterile margin thinning out, white, up to 1 mm wide; pores angular to irregular, 2–3 per mm; dissepiments thin, lacerate. Subiculum cream, hard corky, paler contrast with tubes, up to 0.1-mm thick. Tubes concolorous with pores, hard corky, up to 0.4-mm long.
Hyphal structure: Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae IKI–, CB–; tissue unchanged in KOH.
Subiculum: Generative hyphae hyaline, thin-to slightly thick-walled, rarely branched, 2–3 μm in diam; skeletal hyphae dominant, hyaline, thick-walled with a narrow lumen to subsolid, rarely branched, 2–5 μm in diam.
Tubes: Generative hyphae frequent, hyaline, thin-to slightly thick-walled, rarely branched, 2–3 μm in diam; skeletal hyphae dominant, hyaline, thick-walled with a narrow lumen to subsolid, rarely branched, interwoven, 3–4 μm in diam. Cystidia absent; cystidioles present, fusoid, thin-walled, 21–26 × 5–7 μm. Basidia clavate, with a basal clamp connection and four sterigmata, 23–30 × 9–10 μm; basidioles in shape similar to basidia, but smaller.
Spores: Basidiospores oblong ellipsoid to cylindrical, gently arcuate and tapering toward the apiculus, hyaline, thin-walled, smooth, sometimes within a few small guttules, IKI–, CB–, (8.5–)9–12(−12.8) × (4–)4.2–5.3(−5.6) μm, L = 10.31 μm, W = 4.7 μm, Q = 2.14–2.22 (n = 90/3).
Additional specimens examined (paratypes): China. Inner Mongolia Autonomous Region, Alxa Left Banner, Helanshan Nature Reserve, elev. 2270 m, N 38.865282, E 105.899814, on fallen trunk of Picea crassifolia, 17 September 2022, Dai 24526 (BJFC), Dai 24527 (BJFC); Helanshan Forest Park, elev. 2070 m, N 38.971847, E 105.905048, on fallen branch of Pinus tubaeliformis, 18 September 2022, Dai 24530 (BJFC).
Notes: Antrodia aridula is characterized by its annual and resupinate basidiocarps with angular to irregular pores of 2–3 per mm, a dimitic hyphal structure, the presence of fusoid cystidioles, basidiospores oblong ellipsoid to cylindrical, gently arcuate and tapering toward the apiculus, 9–12 × 4.2–5.3 μm, and growing on gymnosperm wood in a dry environment of West China. Antrodia aridula and Cartilosoma ramentaceum (Berk. & Broome) Teixeira (≡ Antrodia ramentacea (Berk. & Broome)) Donk have similar pores and growing on the wood of Pinus, but the latter differs from the former by gelatinous basidiocarps, smaller basidiospores (6.2–8.1 × 2.1–2.7 μm vs. 9–12 × 4.2–5.3 μm) and wider geographical distribution (Niemelä, 2005). Cartilosoma is different from Antrodia by completely resupinate and soft basidiocarps when fresh, and gelatinous hymenophore (Liu et al., 2022). Antrodia aridula is similar to A. macra (Sommerf.) Niemelä in macromorphology, and both species are closely related (Figure 1), but the latter species has smaller basidiospores (7.4–11 × 3–4.3 μm vs. 9–12 × 4.2–5.3 μm, Niemelä, 2005), and growing on Salix and Populus only (Ryvarden and Melo, 2017). Antrodia aridula is also closely related to Antrodia variispora (Figure 1), but the latter species has bigger and variable basidiospores (9–12 × 4.2–5.3 μm vs. 11.5–16 × 4.5–5.5 μm).
Ecologically, Antrodia aridula grows on fresh fallen gymnosperm trunks and branches in a dry environment, indicating a pioneer decayer in the coniferous forests of western China.
Antrodia variispora Y.C. Dai, H.M. Zhou, Y.D. Wu & Shun Liu, sp. nov. Figures 4, 5.
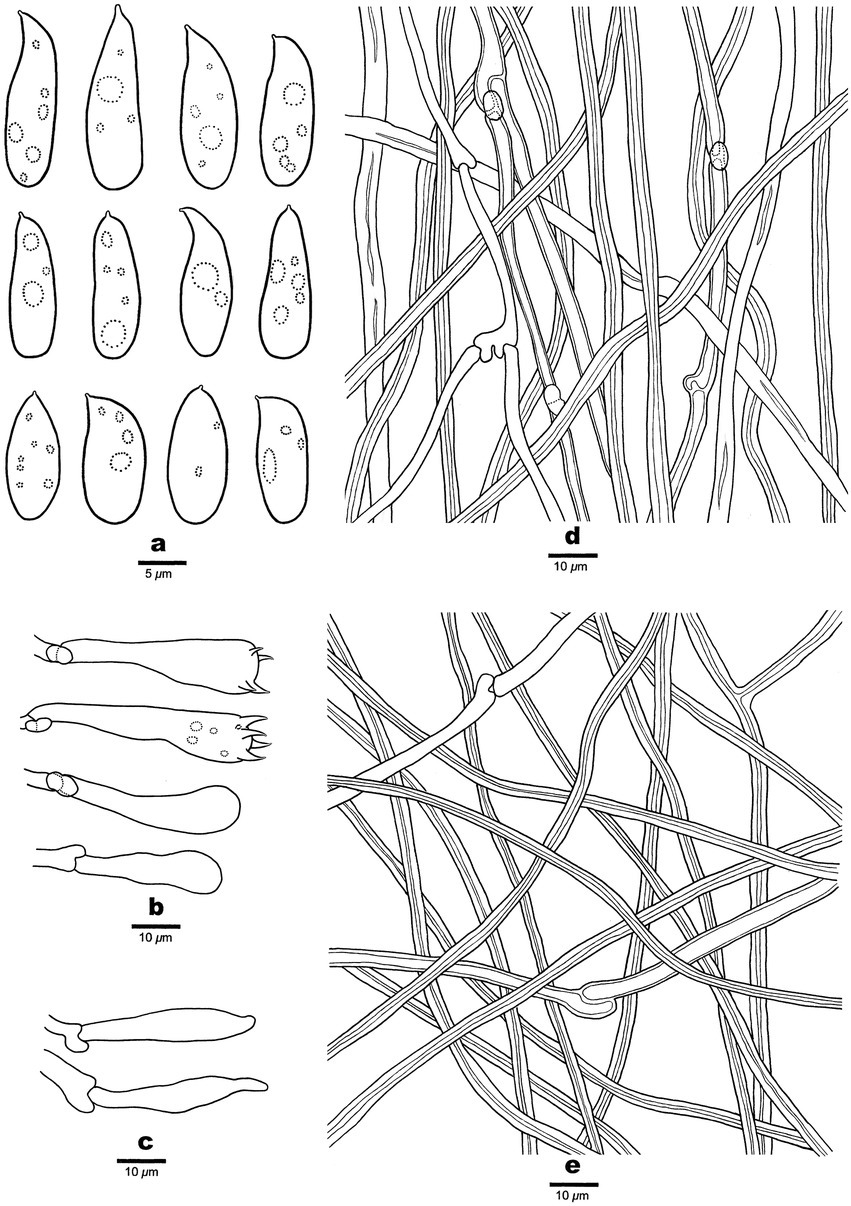
Figure 5. Microscopic structures of Antrodia variispora (holotype, Dai 23995). (A) Basidiospores. (B) Basidia and basidioles. (C) Cystidioles. (D) Hyphae from trama. (E) Hyphae from subiculum.
MycoBank number: 846496.
Holotype: China. Qinghai Province, Nangqian County, Baizha Forest Park, elev. 4090 m, N 31.855040, E 96.467402, on stump of Picea likiangensis var. balfouriana, 7 August 2022, Dai 23995 (BJFC).
Etymology: Variispora (Lat.): Referring to the species having variable basidiospores.
Basidiocarp: Annual, resupinate, tightly attached to wood, leathery when fresh, hard corky to rigid when dry, up to 16 cm long, 6 cm wide, and 1.3-mm thick at the center. Pore surface cream when fresh, becoming cinnamon-buff when dry; sterile margin very narrow to almost lacking; pores sinuous or dentate, (0.5–)1–1.5(−2) per mm; dissepiments thin, lacerate. Subiculum cream, hard corky, paler contrast with tubes, up to 0.3-mm thick. Tubes concolorous with pores, rigid, up to 1-mm long.
Hyphal structure: Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae IKI–, CB–; tissue unchanged in KOH.
Subiculum: Generative hyphae infrequent, hyaline, thin-to slightly thick-walled, rarely branched, 2–4 μm in diam; skeletal hyphae dominant, hyaline, thick-walled with a narrow lumen to subsolid, rarely branched, interwoven, 2.5–4 μm in diam.
Tubes: Generative hyphae frequent, hyaline, thin-to slightly thick-walled, frequently branched, 2.5–4 μm in diam; skeletal hyphae dominant, hyaline, thick-walled with a narrow lumen to subsolid, occasionally branched, interwoven, 3–5 μm in diam. Cystidia absent; cystidioles present, fusoid, thin-walled, 22–41 × 4.5–5 μm. Basidia clavate to pyriform, with a basal clamp connection and four sterigmata, 24–35 × 7–10 μm; basidioles in shape similar to basidia, but smaller.
Spores: Basidiospores variable, oblong ellipsoid, fusiform, pyriform or cylindrical, gently arcuate and tapering toward the apiculus, hyaline, thin-walled, smooth, usually within a few small guttules, IKI–, CB–, (11–)11.5–16(−18.5) × 4.5–5.5(−5.8) μm, L = 13.47 μm, W = 5.12 μm, Q = 2.63 (n = 30/1).
Notes: Antrodia variispora is characterized annual and resupinate basidiocarps with sinuous or dentate pores of 1–1.5 per mm, a dimitic hyphal structure, the presence of fusoid cystidioles, basidiospores variable, oblong ellipsoid, fusiform, pyriform or cylindrical, 11.5–16 × 4.5–5.5 μm, and growing on the wood of Picea in West China. Antrodia variispora is similar to Adustoporia sinuosa (Fr.) Audet (≡ Antrodia sinuosa (Fr.) P. Karst.) in macromorphology, but the latter species has distinctly smaller basidia (11–15 × 4–5 μm vs. 24–35 × 7–10 μm, Ryvarden and Gilbertson, 1993) and smaller basidiospores (4.9–6 × 1.4–1.8 μm vs. 11.5–16 × 4.5–5.5 μm, Niemelä, 2005). Adustoporia differs from Antrodia s. str. by pale brown pore surface when fresh, smaller basidia (15–22 × 4–5 μm), smaller basidiospores (4–6 × 1–2 μm, Audet, 2017b). Antrodia variispora and Dentiporus albidoides (A. David & Dequatre) Audet (≡ Antrodia albidoides A. David & Dequatre) have similar pore and basidiospore dimensions (Ryvarden and Melo, 2017), but the latter species has skeletocystidia, uniformed cylindrical basidiospores, and growing on angiosperm wood in Mediterranean area (Ryvarden and Melo, 2017). In addition, phylogenetically both species are distantly related (Figure 1). The genus Dentiporus differs from Antrodia s. str by often resupinate basidiocarps usually with rose pink tinted pore surface and round to irpicoid pores (Audet, 2017a). Antrodia variispora is closely related to A. macra (Figure 1), but A. macra has regularly cylindrical to oblong ellipsoid basidiospores which are smaller than those in Antrodia variispora (7.4–11 × 3–4.3 μm vs. 11.5–16 × 4.5–5.5 μm, Niemelä, 2005).
Ecologically, Antrodia variispora grows on a large stump of Picea likiangensis var. balfouriana in a virgin forest, and we tried to find more samples in a similar environment of the same forest, and unfortunately, we did not find the second sample. Thus, Antrodia variispora seems to be a rare species in old growth forests.
Discussion
Adding the two new species from China, the definition of Antrodia s. str. is modified as follows: Basidiocarps annual, resupinate to effused-reflexed, soft corky to leathery when fresh, become corky to hard corky or rigid when dry; pileal surface glabrous or matted, white, cream to brownish gray; pore surface white to cream when fresh, buff to pale brown upon drying; pores round, angular, sinuous or dentate; subiculum or context cream, corky; hyphal system dimitic with clamped generative hyphae; skeletal hyphae IKI−, CB−; cystidia absent; fusoid cystidioles present or absent; basidiospores long (6.5–16 μm), oblong ellipsoid, cylindrical, fusiform or pyriform, hyaline, thin-walled, smooth, IKI−, CB−; causing a brown rot.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article.
Author contributions
Y-CD coordinated the project and designed the experimental plan. H-MZ, Y-CD, and SL analyzed the data. Y-CD, X-JD, and H-GL collected the samples from the field. H-MZ and Y-CD wrote the original draft. H-MZ, Y-CD, Y-DW, and SL reviewed and edited the manuscript. Y-CD acquired funding. All authors contributed to the article and approved the submitted version.
Funding
The research was supported by the National Natural Science Foundation of China (Project Nos. 32161143013, 31870007). We are grateful to Zhan-Bo Liu was a companion on the field trips.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Anonymous (1969). Flora of British Fungi. Colour Identification Chart. London: Her Majesty’s Stationery Office.
Audet, S. (2017a). New genera and new combinations in Antrodia s. l. or Polyporus s.l., or new families in the Polyporales. Mushrooms Nomencl. 3:1.
Audet, S. (2017b). New genera and new combinations in Antrodia s.l. or Polyporus s.l., or new families in the Polyporales. Mushrooms Nomencl. 11:1.
Chen, Y. Y., and Cui, B. K. (2015). Phylogenetic analysis and taxonomy of the Antrodia heteromorpha complex in China. Mycoscience 57, 1–10. doi: 10.1016/j.myc.2015.07.003
Chen, J. J., and Dai, Y. C. (2021). Two new species of Physisporinus (Polyporales, Basidiomycota) from Yunnan, Southwest China. Mycol. Prog. 20, 1–10. doi: 10.1007/s11557-020-01647-8
Gilbertson, R. L., and Ryvarden, L. (1986). North American polypores 1. Abortiporus-Lindtneria. Oslo: Fungiflora, 1–433.
Hibbett, D. S., and Donoghue, M. J. (2001). Analysis of character correlations among wood decay mechanisms, mating systems, and substrate ranges in Homobasidiomycetes. Syst. Biol. 50, 215–242. doi: 10.1080/10635150151125879
Katoh, K., Rozewicki, J., and Yamada, K. D. (2017). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166. doi: 10.1093/bib/bbx108
Liu, S., Chen, Y. Y., Sun, Y. F., He, X. L., Song, C. G., Si, J., et al. (2022). Systematic classification and phylogenetic relationships of the brown-rot fungi within the Polyporales. Fungal Divers. doi: 10.1007/s13225-022-00511-2
Liu, Y. J., Whelen, S., and Hall, B. D. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 16, 1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092
Núñez, M., and Ryvarden, L. (2001). East Asian polypores 2. Polyporaceae s. lato. Synop. Fungorum 14, 170–522.
Ortiz-Santana, B., Lindner, D. L., Miettinen, O., Justo, A., and Hibbett, D. S. (2013). A phylogenetic overview of the Antrodia clade (Basidiomycota, Polyporales). Mycologia 105, 1391–1411. doi: 10.3852/13-051
Petersen, J. H. (1996). The Danish mycological Society’s colour-chart. Greve: Foreningen til Svampekundskabens Fremme.
Rehner, S. A., and Buckley, E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97, 84–98. doi: 10.3852/mycologia.97.1.84
Runnel, K., Spirin, V., Miettinen, O., Vlasák, J., Dai, Y. C., Ryvarden, L., et al. (2019). Morphological plasticity in brown-rot fungi: Antrodia is redefined to encompass both poroid and corticioid species. Mycologia 111, 871–883. doi: 10.1080/00275514.2019.1640532
Spirin, V., Vlasák, J., Niemelä, T., and Miettinen, O. (2013). What is Antrodia sensu stricto? Mycologia 105, 1555–1576. doi: 10.3852/13-039
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics” in PCR Protocols: A Guide to Methods and Applications. eds. M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (New York, NY: Academic Press), 315–322.
Keywords: fomitopsidaceae, multigene, taxonomy, wood-inhabiting fungi, phylogeny
Citation: Zhou H-M, Liu S, Deng X-J, Liu H-G, Xing R, Dai Y-C and Wu Y-D (2023) Two new species of Antrodia (Polyporales, Basidiomycota) in western China. Front. Microbiol. 14:1102575. doi: 10.3389/fmicb.2023.1102575
Edited by:
Ji-Chuan Kang, Guizhou University, ChinaReviewed by:
Victor Manuel Bandala, Instituto de Ecología (INECOL), MexicoQiang Li, Chengdu University, China
Copyright © 2023 Zhou, Liu, Deng, Liu, Xing, Dai and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Cheng Dai, ✉ yuchengdai@bjfu.edu.cn; Ying-Da Wu, ✉ wydbjfu@163.com
†These authors have contributed equally to this work and share first authorship
 Hong-Min Zhou
Hong-Min Zhou Shun Liu
Shun Liu Xiao-Juan Deng2
Xiao-Juan Deng2 Hong-Gao Liu
Hong-Gao Liu Rui Xing
Rui Xing