- 1Department of Neurology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Nursing Department, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Endocrinology Department, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: The prevalence and associated factors of dysphagia in Parkinson's disease (PD) are different in studies conducted in different countries. The purpose of our systematic review and meta-analysis was to evaluate the prevalence of dysphagia in PD and to clarify its associated factors.
Methods: Two researchers systematically searched PubMed, Embase, Web of Science, Cochrane Library, CNKI, Wanfang Database, SinoMed and VIP databases and manually searched references in the retrieved articles to identify potential research subjects. The last search was conducted on June 28, 2022. Finally, a total of 58 studies including 60 observations with 20,530 PD patients were included in our meta-analysis.
Results: The meta-analysis estimated that the pooled prevalence rate of dysphagia in PD was 36.9% (95% CI: 30.7–43.6%) and instrumental examination showed a higher prevalence (57.3%, 95% CI: 44.3–69.1%). Oceania showed the highest prevalence of dysphagia in PD (56.3%) compared to Africa (39.5%), Asia (38.6%), Europe (36.1%) and America (28.9%). Dysphagia in PD was associated with older age, lower body mass index, longer disease duration, higher Hoehn and Yahr stage and levodopa equivalent daily dose, PIGD subtype, severe motor symptoms, drooling and higher levels of depression, and lower quality of life.
Conclusions: In conclusion, our meta-analysis showed that dysphagia occurs in more than one-third of PD patients and was associated with several demographic characteristics and PD-related characteristics, motor symptoms, non-motor symptoms, as well as decreased quality of life. It deserves early screening, diagnosis, and treatment in clinical practice to prevent serious complications from dysphagia.
Introduction
Parkinson's disease (PD) is a neurodegenerative disease characterized by progressive degeneration of dopaminergic neurons in the substantia nigra of the midbrain and multiple system involvement (1). Dysphagia is a common non-motor symptom in patients with Parkinson's disease, which can lead to a range of serious problems such as malnutrition, aspiration pneumonia, prolonged hospital stay, and even increased mortality (2–4). Studies in different countries have reported large differences in the prevalence of dysphagia in PD, and prevalence of 32.0% in Israe (5) and 87.1% in China (6) have been reported. In previous studies, dysphagia in PD patients has been assessed by different methods such as standardized questionnaires (7), water swallowing test (8), interview (9), clinical assessment (10), and instrumental examination (11) etc., with a wide range of results. The review conducted by Kalf et al. reported the prevalence of dysphagia (12), but it was conducted 10 years ago, and many relevant studies have emerged in recent years. Representative estimates of the actual prevalence of dysphagia in PD are still scarce. Therefore, the present study is a necessary step in identifying and reporting on the prevalence of dysphagia in PD in the existing literature. In addition, previous studies have shown that its high prevalence is related to many factors, such as age, male sex, levodopa equivalent daily dose (LEDD), Hoehn and Yahr stage (H-Y stage) and cognitive impairment, etc. (6, 13, 14). However, the results of the association between dysphagia in PD and associated factors have been inconsistent across studies, and there have been few quantitative reviews to clarify the strength of these association. Understanding these related factors can enable clinicians and nurses to detect dysphagia in PD patients as soon as possible, and timely prevent and treat them, thereby preventing the occurrence of related complications. Therefore, a meta-analysis is needed to clarify the associated factors for dysphagia in PD. Given these gaps in the current research on dysphagia in PD, we conducted two systematic reviews with the following objectives: (1) to provide a comprehensive estimate of the prevalence of dysphagia in PD and (2) to calculate a summary effect estimate of the associated factors for dysphagia in PD by summarizing all available data. The results of this study can provide reference for clinical practice related to dysphagia in PD patients.
Methods
Study design and registration
Our meta-analysis adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) (15) statements and were registered with PROSPERO (registration number CRD42022344132).
Search strategy
We searched PubMed, Embase, Web of Science, Cochrane Library, CNKI, Wanfang Database, SinoMed and VIP databases. Our search strategy was as follows: (Parkinson* OR PD OR Paralysis Agitans) AND (dysphagia OR deglutition disorder* OR swallowing dysfunction OR swallowing disorder OR impaired swallowing OR acataposis OR swallow problem*). The last search was conducted on June 28, 2022. In addition, we manually searched the references in the retrieved articles to identify potential research subjects. The detailed search strategy is illustrated in Supplementary Table 1.
Selection criteria
A study was included if (1) patients were diagnosed with PD according to the United Kingdom Brain Bank Criteria (16), the Movement Disorders Society diagnostic criteria (17), or clinically diagnosed by neurologist; (2) dysphagia was assessed according to validated diagnostic or screening modality; (3) study reported the prevalence or associated factors of PD-related dysphagia or provided data that could estimate the prevalence or associated factors of it; (4) search design: cohort, case-control studies or cross-sectional; and (5) published in English or Chinese. Studies were excluded if they (1) were case reports, conference abstracts and reviews; (2) published in different studies with duplicate participants. If multiple studies reported results from the same sample including varying numbers of patients, we would consider the study with the larger sample size.
Data extraction and quality assessment
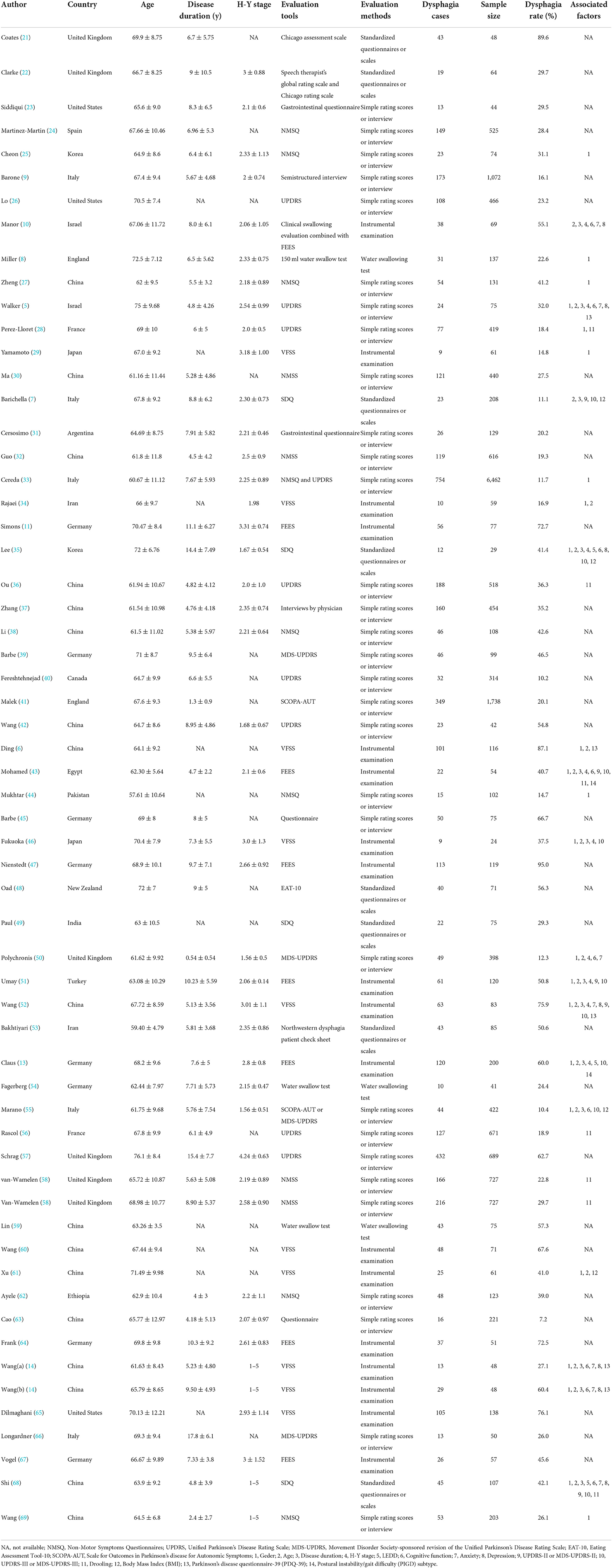
Two authors (SY Gong and Y Gao) performed the literature screening alone according to the inclusion and exclusion criteria, respectively: (1) duplicate studies were removed using EndNote X9 software; (2) the titles and abstracts were screened to exclude obviously irrelevant literature; (3) the full text was read, and the reasons for exclusion were indicated; (4) the references were screened for inclusion. Then, two authors (SY Gong and Y Gao) independently extracted the data from each study in accordance with the predesigned data extraction. The quality of the included studies was assessed using the Newcastle Ottawa Scale (NOS) for cohort or case-control studies and the Agency for Healthcare Research and Quality (AHRQ) for cross-sectional studies. Based on the NOS score, the included eligible studies with 0–3, 4–6, and 7–9 points indicated low, moderate and high quality, respectively. Based on the AHRQ score, the included eligible studies with 0–3, 4–7, and 8–11 points indicated low, moderate and high quality, respectively. Disagreements were resolved through discussion and consultation with a third author (CL Liao). The extracted data included the characteristics of the following categories: author, publication year, country, age, disease duration, H-Y stage, evaluation tools, evaluation methods, dysphagia cases, sample size, dysphagia rate and associated factors.
Statistical analysis
We used the software R software package version 4.1.1 to analyze all statistical data. In our study, in the meta-analyses focusing on the prevalence of dysphagia in PD, the effect sizes were defined as the prevalence of dysphagia in PD, If more than one method of assessing dysphagia was used in the same study, the results of the instrumental examination were used. In the meta-analyses of factors associated with dysphagia in PD, when more than 2 studies reported the same associated factor, the effect sizes were defined as the odds ratio (OR), the weighted mean difference (WMD) or standardized mean difference (SMD) and 95% CI. To account for the variability and heterogeneity of the prevalence among the included studies, we analyzed the data using a logit transformation random effects model (18). Due to a wide range of characteristics of the studies included, all analyses were performed using the random-effects model. Statistical heterogeneity between the studies was assessed using the I2 statistic, with I2 values of 25, 50, and 75% indicating low, moderate, and high heterogeneity, respectively (19). Moreover, in the meta-analyses focusing on the prevalence of dysphagia in PD, subgroup analyses were conducted on the continent and evaluation method. To estimate the stability of the overall results and distinguish the potential impact of individual studies, sensitivity analyses were performed by removing each study individually. In addition, the funnel plots and Egger's test were used to assess possible publication bias (20). A P < 0.05 was used for the comparison of the result variables, and the difference was statistically significant.
Results
Search results
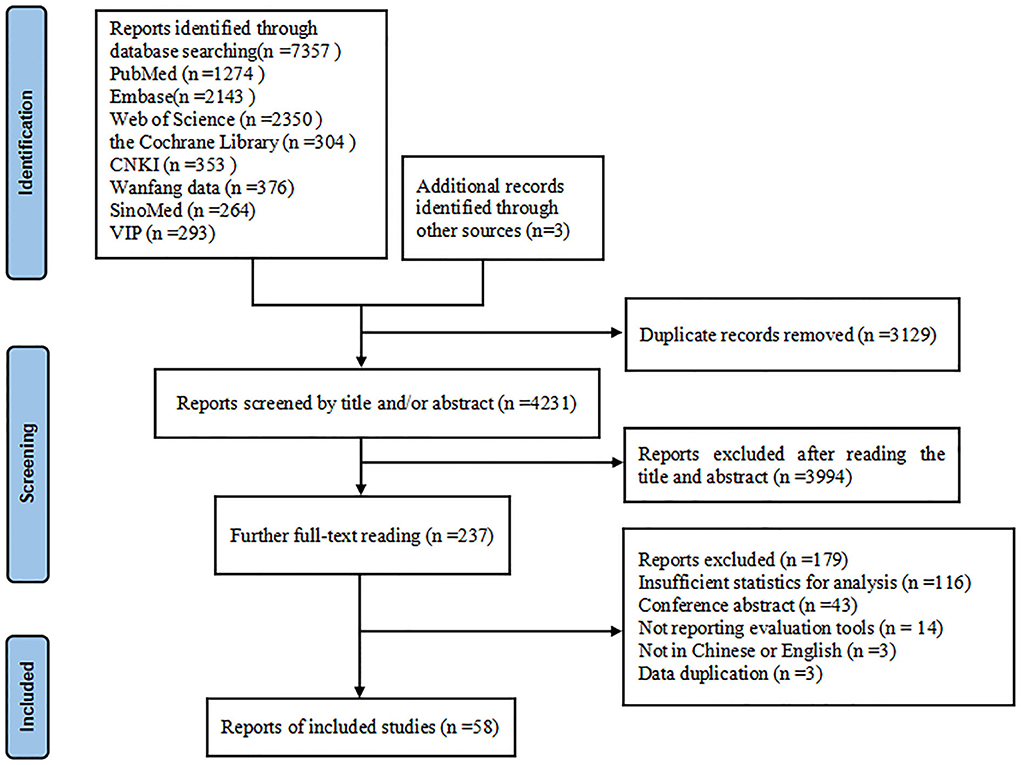
Our research initially retrieved 7,360 articles, of which 3,129 articles were removed due to duplication, and 4,231 articles remained. Among them, 3,994 articles were excluded because their abstracts and titles failed to meet the selection criteria; thus, 237 remained. After we read the full text of the articles, 179 articles were excluded for the following reasons: insufficient statistics for analysis, conference abstract, not reporting evaluation tools, not in Chinese or English, data duplication. Finally, 58 studies (5–11, 13, 14, 21–69) remained and were included in this research. The specific inclusion screening process is shown in Figure 1.
Study characteristics
A total of 58 studies (5–11, 13, 14, 21–69) including 60 observations with 20,530 PD patients were included in the present study. Thirty-seven were cross-sectional studies, 20 were cohort studies, and 1 case-control study. These studies were published between 1997 and 2022, and their sample sizes ranged from 24 to 6,462. Twenty-seven studies were carried out in Asia, 23 in Europe, 5 in America, 2 in Africa and 1 in Oceania. All the included studies reported the prevalence of dysphagia in PD, 27 of which reported on associated factors. In total, 17 studies used instrumental examination as evaluation method, 8 studies used standardized questionnaires or scales, 3 studies used water swallowing test, and 30 studies used simple rating scores or interview, and dysphagia rate ranged from 7.2 to 95.0%. The quality assessment scores of the included studies ranged from 5 to 8 points (median 6 points) based on NOS, indicating moderate quality; and 2–8 points (median 6 points) based on AHRQ, also indicating moderate quality. The detailed NOS or AHRQ scores of the included studies are shown in Supplementary Tables 2, 3. The detailed characteristics of the included reports are described in Table 1.
Prevalence of dysphagia in Parkinson's disease
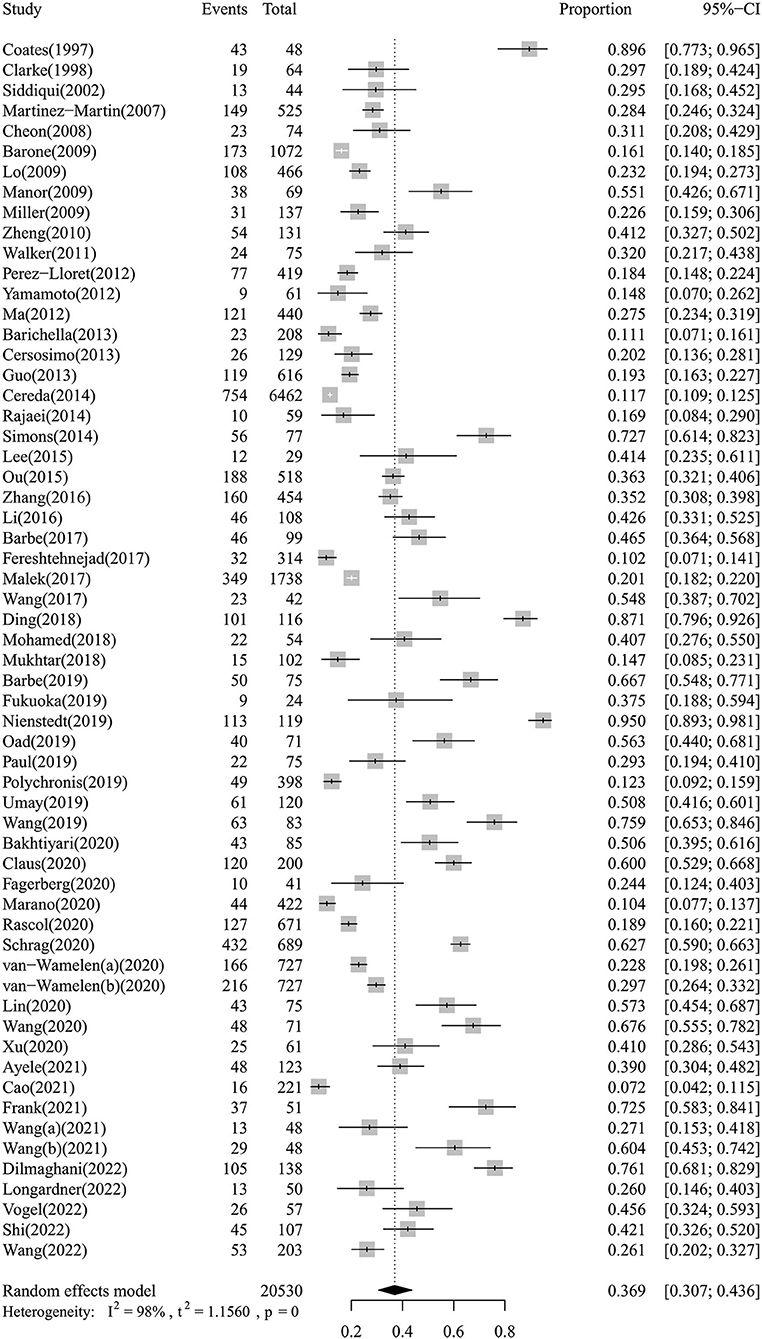
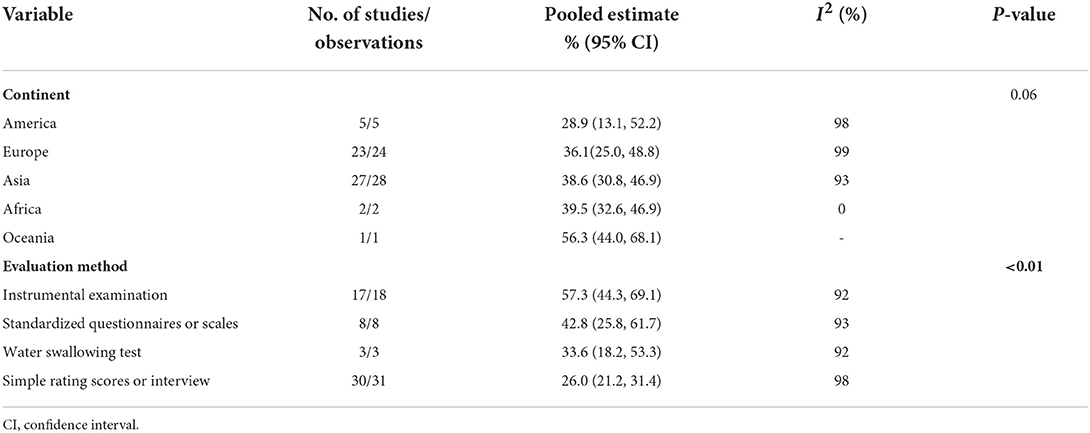
The meta-analysis of the overall prevalence of dysphagia in PD is shown in the forest plot in Figure 2. The random effects meta-analysis estimated that the pooled prevalence rate of dysphagia in PD was 36.9% (95% CI: 30.7–43.6%). There was high heterogeneity among the studies pooled for the meta-analysis (I2 = 98%). The I2 statistic describes the percentage of variation across the studies, and this is due to heterogeneity rather than a chance. The subgroup analyses were then performed by the continent and evaluation method. The subgroup analyses found that the evaluation method to be significant moderators, while the continent was not. Regarding the evaluation method (Supplementary Figure 1), the instrumental examination showed a higher prevalence of dysphagia in PD (57.3%, 95% CI: 44.3–69.1%) compared to the standardized questionnaires or scales (42.8%, 95% CI: 25.8–61.7%), the water swallowing test (33.6%, 95% CI: 18.2–53.3%), and the simple rating scores or interview (26.0%, 95% CI: 21.0–31.4%). Regarding the continent (Supplementary Figure 2), the Oceania showed a higher prevalence of dysphagia in PD (56.3%, 95% CI: 44.0–68.1%) compared to the Africa (39.5%, 95% CI: 32.6–46.9%), the Asia (38.6%, 95% CI: 30.8–46.9%), the Europe (36.1%, 95% CI: 25.0–48.8%), and the America (28.9%, 95% CI: 13.1–52.2%). The subgroup analyses of the prevalence of dysphagia in PD based on random-effect analysis are shown in Table 2.
The results of the sensitivity analysis indicated that our findings were robust and did not depend on a single study. Our pooled estimated prevalence of dysphagia in PD varied between 35.6% (95% CI: 29.9–41.8%) and 37.7% (95% CI: 31.5–44.4%) after removing each study individually. A forest plot for a sensitivity analysis of the prevalence of dysphagia in PD is shown in Figure 3. Regarding publication bias, visual inspection of the funnel plot indicated asymmetry (Supplementary Figure 3), and Egger's test also showed publication bias (P < 0.01).
Factors associated with dysphagia in Parkinson's disease
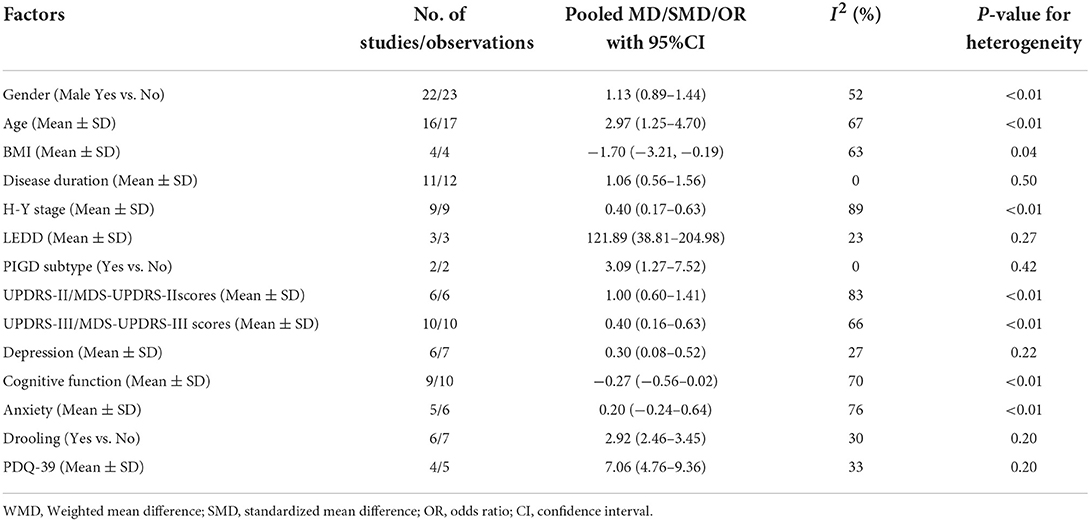
In total, 27 studies (5–8, 10, 13, 14, 25, 27–29, 33–36, 43, 44, 46, 50–52, 55, 56, 58, 61, 68, 69) reported information about the associated factors in PD. Fourteen associated factors had data that could be used in the quantitative meta-analysis. Associated factors for included studies were summarized into the following main categories: demographic characteristics, such as gender, age, and BMI; PD-related characteristics, such as disease duration, H-Y stage, LEDD, and PIGD subtype; motor symptoms, such as UPDRS-II/MDS-UPDRS-II scores, and UPDRS-III/MDS-UPDRS-III scores; non-motor symptoms, such as cognitive function, anxiety, depression, and drooling; as well as quality of life. The meta-analyses of associated factors of dysphagia in Parkinson's disease based on random-effect analysis are shown in Table 3.
Demographic characteristics
Gender, Age and BMI. Twenty-two studies (5, 6, 8, 13, 14, 25, 27–29, 33–35, 43, 44, 46, 50–52, 55, 61, 68, 69) reported information about gender. But, our analysis did not find a significant association between PD with dysphagia and male gender (pooled OR: 1.13, 95% CI: 0.89–1.44) (Supplementary Figure 4A). Sixteen studies (5, 7, 10, 13, 14, 34, 35, 43, 46, 50–52, 55, 61, 68) reported the age of PD patients with dysphagia and those without dysphagia, and the meta-analysis showed that PD patients with dysphagia were older than those without dysphagia (pooled MD: 2.97, 95% CI: 1.25–4.70) (Supplementary Figure 4B). Four studies (7, 35, 55, 61) reported information about BMI. Our meta-analysis revealed that PD patients with dysphagia were lower BMI than those without dysphagia (pooled MD: −1.70, 95% CI: −3.21,−0.19) (Supplementary Figure 4C).
PD-related characteristics
Disease duration, H-Y stage, LEDD and PIGD subtype. Of note, our analysis showed that longer disease duration (5, 7, 10, 13, 14, 35, 43, 46, 51, 52, 68), higher H-Y stage (5, 10, 13, 35, 43, 46, 50–52) and higher LEDD (13, 35, 68) were all associated with dysphagia in PD patients. The pooled MDs were 1.06 (95% CI: 0.56–1.56), 0.40 (95% CI, 0.17–0.63) and 121.89 (95% CI: 38.81–204.98), respectively (Supplementary Figures 5A–C). Moreover, two studies (13, 43) reported information about PIGD subtype. Our analysis found a significant association between PD with dysphagia and PIGD subtype (pooled OR: 3.09, 95% CI: 1.27–7.52) (Supplementary Figure 5D).
Motor symptoms
UPDRS-II/MDS-UPDRS-II and UPDRS-III/MDS-UPDRS-III Scores. Our study found that PD patients with dysphagia had higher UPDRS-II/MDS-UPDRS-II scores (7, 43, 50–52, 68) and UPDRS-III/MDS-UPDRS-III scores (7, 13, 35, 43, 46, 50–52, 55, 68) than those without dysphagia. The pooled SMDs were 1.00 (95% CI: 0.60–1.41) (Supplementary Figure 6A) and 0.40 (95% CI, 0.16–0.63) (Supplementary Figure 6B), respectively.
Non-motor symptoms
Depression, Cognitive function, Anxiety and Drooling. Our meta-analysis found that PD patients with dysphagia had slightly higher scores on depression scales (5, 10, 14, 35, 52, 68) than those without dysphagia (pooled SMD: 0.30, 95%CI: 0.08–0.52) (Supplementary Figure 7A). But, our analysis did not find a significant association between PD with dysphagia and cognitive function scores (5, 6, 10, 14, 35, 43, 50, 55, 68) and anxiety scores (10, 14, 52, 68). The pooled SMDs were −0.27 (95% CI: −0.56–0.02) (Supplementary Figure 7B) and 0.20 (95% CI, −0.24–0.64) (Supplementary Figure 7C), respectively. Of note, our meta-analysis of six studies (28, 36, 43, 56, 58, 68) showed that drooling was significantly associated with dysphagia in PD (pooled OR: 2.92, 95% CI: 2.46–3.45) (Supplementary Figure 7D).
Quality of life
Four studies (5, 6, 14, 52) reported PDQ-39 scores in patients with dysphagia and those without dysphagia, and the meta-analysis showed that PD patients with dysphagia were significantly higher (worse) PDQ-39 scores than those without dysphagia (pooled MD: 7.06, 95% CI: 4.76–9.36) (Supplementary Figure 8).
Discussion
Prevalence of dysphagia in Parkinson's disease
The mechanism of dysphagia in PD is unclear. Studies have shown that in the progression of PD, a variety of pathological changes occur in the neuromuscular associated with swallowing function, resulting in impaired central and peripheral swallowing regulation mechanisms, and eventually swallowing disorders (70, 71). PD can impair all phases of swallowing (72). Dysphagia in the oral phase of PD patients is characterized by difficulties in the initiation of swallow, oral residues, piecemeal swallow and premature falling of the food; the pharyngeal phase is characterized by regurgitation of food into the nasal cavity or upper pharynx, pharyngeal residue and penetration/aspiration, and the esophageal phase is characterized by reduced esophageal peristalsis (71). The severity of dysphagia was assessed by FEES, with mild dysphagia presenting only with premature spillage and/or residues, and severe dysphagia presenting with frequent penetration/aspiration events (72). Although dysphagia become apparent in the later stages of PD, they may have been present in the early stages but often go undetected (73).
Our study established that dysphagia in PD is common. We found that the global prevalence of dysphagia in PD was 36.9%, the Oceania was 56.3%, the Africa was 39.5%, the Asia was 38.6%, the Europe was 36.1%, and the America was 28.9%. The high prevalence of dysphagia in PD reminds us that it is very important to screen out high-risk groups of dysphagia early to promote early clinical diagnosis and treatment, thereby preventing serious complications caused by dysphagia. It should be noted that there are few relevant studies from the Oceania and Africa. Therefore, more studies in these regions are warranted to further clarify the prevalence of dysphagia in PD and its associated factors.
In the subgroup analysis of evaluation method, the prevalence of dysphagia in PD patients assessed by simple rating scores or interview, water swallowing test, standardized questionnaires or scales, and instrumental examination increased sequentially (26.0: 33.6: 42.8: 57.3%). As expected, studies using more rigorous assessments reported a higher prevalence of dysphagia in PD patients. Instrumental examinations (VFSS and FEES) provide accurate assessment of swallowing function and are considered the “gold standard” for the diagnosis of dysphagia. VFSS can provide detailed assessment and analysis of the different phases of swallowing (oral and pharyngeal and esophageal phase), as well as the anatomical structures of the tongue, soft palate, pharynx and larynx and the delivery process of the food mass, which is crucial in the diagnosis of silent aspiration (74, 75). FEES can detect residue, penetration and aspiration by observing the function of the structures of the nose, pharynx and larynx during food swallowing and the location and amount of pigmented food mass remaining during feeding (76–78). Our research revealed that only about half of dysphagia were detected by simple rating scores or interview (26.0%), compared with instrumental examination (57.3%). These simple rating scores or interviews rely on the PD patient's self-perception of swallowing and are not reliable modality for identifying dysphagia, consistent with the results of previous studies (47, 79). Therefore, the use of simple rating scores or interview to screen PD patients for dysphagia is not recommended in future studies. Our study showed that the prevalence of dysphagia detected using the water swallowing test was much lower than that of instrumental examination (33.6 vs. 57.3%). The diagnostic accuracy of water swallowing test relies on the retention of cough reflex and pharyngeal-laryngeal sensitivity (80). However, PD patients often have a weak cough reflex and silent aspiration, and the water swallowing test may underestimate the prevalence of dysphagia. The combination of water swallowing test with clinical tests to assess voluntary and/or reflex cough function may be considered in clinical applications to improve accuracy (81). In addition, our study found that the prevalence of dysphagia in PD patients assessed using standardized questionnaires or scales were second only to instrumental examination (42.8 vs. 57.3%). Standardized questionnaires or scales that combine multiple aspects of clinical signs and symptoms, social life, or psychological implications associated with dysphagia, may be considered valid tools for screening for dysphagia (80). Although instrumental examination is indeed an objective and crucial modality for the diagnosis of dysphagia, its clinical application is limited due to its relatively expensive and the limitations of instrumentation and technology (47, 82). If clinical conditions allow, instrumental examination is recommended as the first choice to assess swallowing function in PD patients; if clinical conditions are limited, especially in the epidemiological investigation or screening of dysphagia in PD patients, it may be simpler and more convenient to use standardized questionnaires or scales. If the screening result is positive, further clinical evaluation or instrumental examination should be carried out by specialists to confirm the presence and assess the severity of dysphagia in order to avoid the occurrence of serious complications.
Factors associated with dysphagia in Parkinson's disease
Our study compared PD patients with and without dysphagia showed that PD patients with dysphagia were older and associated with lower BMI. With age, decreased muscle tone in the elderly can lead to decreased chewing and swallowing function, and more prone to dysphagia (83). Several previous studies have also shown that age is significantly associated with dysphagia (84, 85). BMI not only partially reflects the nutritional status of patients, but also correlates with dysphagia. A study by Sakamoto et al. showed that a smaller BMI was significantly associated with dysphagia (86). The review conducted by Simons also showed that low BMI is a highly correlated clinical predictor of severe dysphagia in PD patients (87). This may be related to reduced energy intake due to dysphagia, which in turn leads to weight loss (88). In the present meta-analysis, we observed no significant association between male gender and the prevalence of dysphagia in PD patients based on the random effects model, with moderate heterogeneity (I2 = 52%). However, previous review has shown that gender differences in pathophysiological characteristics of PD patients are manifested by males having greater impairment in the etiology of the disease, possibly due to increased physiologic striatal dopamine levels due to estrogenic activity, which decreases incidence and age of onset in females (89). A study by Dumican et al. showed significantly higher (worse) overall pharyngeal phase dysphagia severity Videofluoroscopic Dysphagia Scale (VDS) scores in men with PD compared to women (90). The inconsistency of our study with the results of previous studies may be related to the inclusion of different swallowing phases in this study, which showed moderate heterogeneity. The association between gender and the different phases of dysphagia in PD patients needs to be further explored in the future.
Our meta-analysis showed that PD patients with dysphagia had significantly longer disease duration, higher H-Y stage, and higher LEDD than patients without dysphagia. These PD-related characteristics factors all predict further progression of PD or more severe symptoms. The lack of dopamine in the striatum may impair the intramedullary swallowing network in PD patients. As the disease progresses in PD patients, dopaminergic decline continues, which may lead to a decline in the function of the swallowing system (2, 13). Dysphagia occurs in nearly all patients with advanced PD, and is closely related to the severity of PD (12, 91). Of note, our study revealed that PD patients with PIGD subtype were more vulnerable to dysphagia compared to other PD subtypes. Previous study has also indicated that dysphagia may be more generalized in the PIGD subtype (8), and these findings may be the result of poor coordination of the oropharyngeal muscles with rigidity and prolonged oral transit time (92).
In the present meta-analysis, we observed that PD patients with dysphagia had significantly more severe motor symptoms than without dysphagia. The UPDRS-II/MDS-UPDRS-II score includes the screening items for dysphagia, and this part of the score is closely related to the occurrence of dysphagia. In addition, the results of this study also suggested that PD patients with dysphagia had higher UPDRS-III/MDS-UPDRS-III scores, consistent with previous studies (87). Therefore, PD patients should be closely monitored for the occurrence of dysphagia when aggravation of motor symptoms is found clinically.
Our meta-analysis revealed that PD patients with dysphagia were significantly more depressed than patients without dysphagia. The study conducted by Han et al. suggested that depression levels became more severe as the frequency of dysphagia increased, and depression is a predictor of dysphagia, which may also be a precursor to depression (93). This may be related to the possibility of various oral and pharyngeal symptoms in PD patients with dysphagia, resulting in psychological fear and stigma. In the present meta-analysis cognitive function was not related to dysphagia in PD patients based on the random effects model, with high heterogeneity (I2 = 70%). However, previous studies have shown that dysphagia is associated with the classification of cognitive impairment (93). The study conducted by Yatabe et al. also showed that cognitive decline may be an independent predictor of dysphagia (94). The results of the present study were inconsistent with those of the afore-mentioned studies, which may be attributed to the inconsistency of the included study population (the afore-mentioned study included an elderly population), and the results of the cognitive function assessment in our study with the Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) scale scores, respectively, without exploring the classification of cognitive impairment.
Our analysis did not find a significant association between PD with dysphagia and anxiety based on the random effects model, with high heterogeneity (I2 = 76%). Previous studies have suggested that anxiety in PD should be assessed with tailored screening tools (95). But, the anxiety scores included in our study were all general scales. Tailored screening tools may be needed to further explore the correlation between dysphagia and anxiety in patients with PD. Our study revealed that PD patients with drooling were more vulnerable to dysphagia, compared with PD patients without drooling. Dysphagia in PD patients is associated with oropharyngeal muscle dyskinesia and muscle stiffness caused by damaged basal ganglia (96). The dyskinesia of the throat muscles may be the common pathogenesis of salivation and dysphagia (28). In addition, study has found that the cause of salivation in PD patients may be oral retention caused by related dysphagia, suggesting that salivation is at least a manifestation of subclinical dysphagia (97).
Last, our meta-analysis observed that PD patients with dysphagia had significantly lower quality of life than PD patients without dysphagia. The progression of dysphagia may lead to longer meal times and dietary restrictions, negatively impacting the quality of life of PD patients (80). Several studies have reported that dysphagia can have a negative impact on psychological and social integrations (98, 99), which may indirectly reduce the quality of life of PD patients. In addition, the exacerbation of motor and non-motor symptoms due to difficulty in taking oral antiparkinsonian therapy is another problem that often affects PD patients' quality of life (100). Previous studies have also found that the severity of dysphagia significantly affects the quality of life of PD patients (101, 102). Therefore, in clinical work, early diagnosis and treatment of dysphagia is important to improve the quality of life of PD patients.
Strengths and limitations
To our knowledge, this was the first and most comprehensive meta-analysis to include both the prevalence and associated factors of dysphagia in PD. However, the current meta-analysis had several limitations. First, in a meta-analysis of the prevalence of dysphagia in PD, we observed high heterogeneity and publication bias. The heterogeneity across the studies and publication bias may result from the differences in the study settings, samples and diagnostic methods. Second, in the meta-analysis of the associated factors of dysphagia in PD, we included the associated factors that were not used and adjusted for them in the multivariate analysis, and we did not perform a subgroup analysis, which may lead to some potential bias. Third, our meta-analysis included only those studies published in English or Chinese and ignored studies in other languages. Fourth, we did not search the gray literature, and there may be potential studies that were not included. Fifth, limited by the inconsistent definition of dysphagia severity in the included studies, our study only discussed clinical factors related to PD patients with or without dysphagia, and clinical factors related to the severity and specific phase of dysphagia need to be further explored in the future.
Conclusions
In conclusion, our meta-analysis showed that dysphagia occurs in more than one-third of PD patients and was associated with several demographic characteristics, PD-related characteristics, motor symptoms, non-motor symptoms as well as lower quality of life. It deserves early screening, diagnosis, and treatment in clinical practice to prevent serious complications from dysphagia in PD and improve the quality of life.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
SG and CL contributed to the design. SG and YG helped in statistical analysis and participated in most of the study steps. JLiu, JLi, and XT prepared the manuscript. RT and QR assisted in designing the study and helped in the interpretation of the study. All authors have read and approved the content of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1000527/full#supplementary-material
Supplementary Figure 1. The forest plots for subgroup analysis on the evaluation method of the prevalence of dysphagia in PD based on random-effect analysis.
Supplementary Figure 2. The forest plots for subgroup analysis on the continent of the prevalence of dysphagia in PD based on random-effect analysis.
Supplementary Figure 3. The funnel plot for included studies on prevalence of dysphagia in PD.
Supplementary Figure 4. The forest plot for gender (A), age (B), BMI (C) based on random-effect analysis.
Supplementary Figure 5. The forest plot for disease duration (A), H-Y stage (B), LEDD (C), PIGD subtype (D) based on random-effect analysis.
Supplementary Figure 6. The forest plot for UPDRS-II/MDS-UPDRS-II scores (A), UPDRS-III/MDS-UPDRS-III scores (B) based on random-effect analysis.
Supplementary Figure 7. The forest plot for depression (A), cognitive function (B), anxiety (C), drooling (D) based on random-effect analysis.
Supplementary Figure 8. The forest plot for PDQ-39 scores based on random-effect analysis.
Supplementary Table 1. The detailed search strategy.
Supplementary Table 2. Quality assessment based on NOS.
Supplementary Table 3. Quality assessment based on AHRQ.
References
1. Kalia LV, Lang AE. Parkinson's disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
2. Suttrup I, Warnecke T. Dysphagia in Parkinson's disease. Dysphagia. (2016) 31:24–32. doi: 10.1007/s00455-015-9671-9
3. Lin CW, Chang YC, Chen WS, Chang K, Chang HY, Wang TG. Prolonged swallowing time in dysphagic Parkinsonism patients with aspiration pneumonia. Arch Phys Med Rehabil. (2012) 93:2080–4. doi: 10.1016/j.apmr.2012.07.010
4. Di Luca DG, McArthur EW, Willis A, Martino R, Marras C. Clinical and economic outcomes associated with dysphagia in hospitalized patients with Parkinson's disease. J Parkinsons Dis. (2021) 11:1965–71. doi: 10.3233/JPD-212798
5. Walker RW, Dunn JR, Gray WK. Self-reported dysphagia and its correlates within a prevalent population of people with Parkinson's disease. Dysphagia. (2011) 26:92–6. doi: 10.1007/s00455-010-9317-x
6. Ding X, Gao J, Xie C, Xiong B, Wu S, Cen Z, et al. Prevalence and clinical correlation of dysphagia in Parkinson disease: a study on Chinese patients. Eur J Clin Nutr. (2018) 72:82–6. doi: 10.1038/ejcn.2017.100
7. Barichella M, Cereda E, Madio C, Iorio L, Pusani C, Cancello R, et al. Nutritional risk and gastrointestinal dysautonomia symptoms in Parkinson's disease outpatients hospitalised on a scheduled basis. Br J Nutr. (2013) 110:347–53. doi: 10.1017/S0007114512004941
8. Miller N, Allcock L, Hildreth AJ, Jones D, Noble E, Burn DJ. Swallowing problems in Parkinson disease: frequency and clinical correlates. J Neurol Neurosurg Psychiatry. (2009) 80:1047–9. doi: 10.1136/jnnp.2008.157701
9. Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord. (2009) 24:1641–9. doi: 10.1002/mds.22643
10. Manor Y, Balas M, Giladi N, Mootanah R, Cohen JT. Anxiety, depression and swallowing disorders in patients with Parkinson's disease. Parkinsonism Relat Disord. (2009) 15:453–6. doi: 10.1016/j.parkreldis.2008.11.005
11. Simons JA, Fietzek UM, Waldmann A, Warnecke T, Schuster T, Ceballos-Baumann AO. Development and validation of a new screening questionnaire for dysphagia in early stages of Parkinson's disease. Parkinsonism Relat Disord. (2014) 20:992–8. doi: 10.1016/j.parkreldis.2014.06.008
12. Kalf JG, de Swart BJ, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson's disease: a meta-analysis. Parkinsonism Relat Disord. (2012) 18:311–5. doi: 10.1016/j.parkreldis.2011.11.006
13. Claus I, Muhle P, Suttrup J, Labeit B, Suntrup-Krueger S, Dziewas R, et al. Predictors of pharyngeal dysphagia in patients with Parkinson's disease. J Parkinsons Dis. (2020) 10:1727–35. doi: 10.3233/JPD-202081
14. Wang P, Wang B, Chen X, Xiong B, Xie F, Wu S, et al. Six-year follow-up of dysphagia in patients with Parkinson's disease. Dysphagia. (2021) 37:1271–8. doi: 10.1007/s00455-021-10387-0
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
16. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. (1992) 55:181–4. doi: 10.1136/jnnp.55.3.181
17. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
18. Yunitri N, Chu H, Kang XL, Jen HJ, Pien LC, Tsai HT, et al. Global prevalence and associated risk factors of posttraumatic stress disorder during COVID-19 pandemic: a meta-analysis. Int J Nurs Stud. (2022) 126:104136. doi: 10.1016/j.ijnurstu.2021.104136
19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
21. Coates C, Bakheit AM. Dysphagia in Parkinson's disease. Eur Neurol. (1997) 38:49–52. doi: 10.1159/000112902
22. Clarke CE, Gullaksen E, Macdonald S, Lowe F. Referral criteria for speech and language therapy assessment of dysphagia caused by idiopathic Parkinson's disease. Acta Neurol Scand. (1998) 97:27–35. doi: 10.1111/j.1600-0404.1998.tb00605.x
23. Siddiqui MF, Rast S, Lynn MJ, Auchus AP, Pfeiffer RF. Autonomic dysfunction in Parkinson's disease: a comprehensive symptom survey. Parkinsonism Relat Disord. (2002) 8:277–84. doi: 10.1016/S1353-8020(01)00052-9
24. Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson's disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. (2007) 22:1623–9. doi: 10.1002/mds.21586
25. Cheon SM, Ha MS, Park MJ, Kim JW. Nonmotor symptoms of Parkinson's disease: prevalence and awareness of patients and families. Parkinsonism Relat Disord. (2008) 14:286–90. doi: 10.1016/j.parkreldis.2007.09.002
26. Lo RY, Tanner CM, Albers KB, Leimpeter AD, Fross RD, Bernstein AL, et al. Clinical features in early Parkinson disease and survival. Arch Neurol. (2009) 66:1353–8. doi: 10.1001/archneurol.2009.221
27. Zheng J, Sun SG, Qiao X. Prevalence and its impacting factors of non-motor symptoms in Parkinson's disease. Chin J Geriatr. (2010) 29:796–9. doi: 10.3760/cma.j.issn.0254-9026.2010.10.002
28. Perez-Lloret S, Nègre-Pagès L, Ojero-Senard A, Damier P, Destée A, Tison F, et al. Oro-buccal symptoms (dysphagia, dysarthria, and sialorrhea) in patients with Parkinson's disease: preliminary analysis from the French COPARK cohort. Eur J Neurol. (2012) 19:28–37. doi: 10.1111/j.1468-1331.2011.03402.x
29. Yamamoto T, Ikeda K, Usui H, Miyamoto M, Murata M. Validation of the Japanese translation of the swallowing disturbance questionnaire in Parkinson's disease patients. Qual Life Res. (2012) 21:1299–303. doi: 10.1007/s11136-011-0041-2
30. Ma JH, Zhou HQ, Sun F, An J, Chen B, et al. Prevalence of non-motor symptoms in 440 patients with Parkinson's disease and their correlative factors. Chin J Neuromed. (2012) 11:1225–8. doi: 10.3760/cma.j.issn.1671-8925.2012.12.009
31. Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutiérrez C, et al. Gastrointestinal manifestations in Parkinson's disease: prevalence and occurrence before motor symptoms. J Neurol. (2013) 260:1332–8. doi: 10.1007/s00415-012-6801-2
32. Guo X, Song W, Chen K, Chen X, Zheng Z, Cao B, et al. Disease duration-related differences in non-motor symptoms: a study of 616 Chinese Parkinson's disease patients. J Neurol Sci. (2013) 330:32–7. doi: 10.1016/j.jns.2013.03.022
33. Cereda E, Cilia R, Klersy C, Canesi M, Zecchinelli AL, Mariani CB, et al. Swallowing disturbances in Parkinson's disease: a multivariate analysis of contributing factors. Parkinsonism Relat Disord. (2014) 20:1382–7. doi: 10.1016/j.parkreldis.2014.09.031
34. Rajaei A, Azargoon SA, Nilforoush MH, Barzegar Bafrooei E, Ashtari F, Chitsaz A. Validation of the persian translation of the swallowing disturbance questionnaire in Parkinson's disease patients. Parkinsons Dis. (2014) 2014:159476. doi: 10.1155/2014/159476
35. Lee KD, Koo JH, Song SH, Jo KD, Lee MK, Jang W. Central cholinergic dysfunction could be associated with oropharyngeal dysphagia in early Parkinson's disease. J Neural Transm. (2015) 122:1553–61. doi: 10.1007/s00702-015-1427-z
36. Ou R, Guo X, Wei Q, Cao B, Yang J, Song W, et al. Diurnal drooling in Chinese patients with Parkinson's disease. J Neurol Sci. (2015) 353:74–8. doi: 10.1016/j.jns.2015.04.007
37. Zhang S, Ou R, Chen X, Yang J, Zhao B, Yuan X, et al. Correlative factors of cognitive dysfunction in PD patients: a cross-sectional study from Southwest China. Neurol Res. (2016) 38:434–40. doi: 10.1080/01616412.2016.1139320
38. Li LX, Piao YS, Yu SY, Hu Y, Zuo LJ, Yu QJ, et al. Analysis of nutritional status and its influence factors in patients with Parkinson disease complicated with cognitive impairment. Pract Geriatr. (2016) 30:588–591. doi: 10.3969/j.issn.1003-9198.2016.07.019
39. Barbe AG, Bock N, Derman SH, Felsch M, Timmermann L, Noack MJ. Self-assessment of oral health, dental health care and oral health-related quality of life among Parkinson's disease patients. Gerodontology. (2017) 34:135–43. doi: 10.1111/ger.12237
40. Fereshtehnejad SM, Skogar Ö, Lökk J. Evolution of Orofacial Symptoms and Disease Progression in Idiopathic Parkinson's Disease: longitudinal Data from the Jönköping Parkinson Registry. Parkinsons Dis. (2017) 2017:7802819. doi: 10.1155/2017/7802819
41. Malek N, Lawton MA, Grosset KA, Bajaj N, Barker RA, Burn DJ, et al. Autonomic dysfunction in early Parkinson's disease: results from the United Kingdom tracking Parkinson's study. Mov Disord Clin Pract. (2016) 4:509–16. doi: 10.1002/mdc3.12454
42. Wang CM, Shieh WY, Weng YH, Hsu YH, Wu YR. Non-invasive assessment determine the swallowing and respiration dysfunction in early Parkinson's disease. Parkinsonism Relat Disord. (2017) 42:22–7. doi: 10.1016/j.parkreldis.2017.05.024
43. Mohamed AB, Mohamed GF, Elnady HM, Sayed MA, Imam AM, Hassan MM, et al. Evaluation of dysphagia in different phenotypes of early and idiopathic Parkinsonism. Egypt J Neurol Psychiatr Neurosurg. (2018) 54:28. doi: 10.1186/s41983-018-0031-1
44. Mukhtar S, Imran R, Zaheer M, Tariq H. Frequency of non-motor symptoms in Parkinson's disease presenting to tertiary care centre in Pakistan: an observational, cross-sectional study. BMJ Open. (2018) 8:e019172. doi: 10.1136/bmjopen-2017-019172
45. Barbe AG, Ludwar L, Scharfenberg I, Hellmich M, Dano R, Barbe MT, et al. Circadian rhythms and influencing factors of xerostomia among Parkinson's disease patients. Oral Dis. (2019) 25:282–9. doi: 10.1111/odi.12942
46. Fukuoka T, Ono T, Hori K, Wada Y, Uchiyama Y, Kasama S, et al. Tongue pressure measurement and videofluoroscopic study of swallowing in patients with Parkinson's disease. Dysphagia. (2019) 34:80–8. doi: 10.1007/s00455-018-9916-5
47. Nienstedt JC, Bihler M, Niessen A, Plaetke R, Pötter-Nerger M, Gerloff C, et al. Predictive clinical factors for penetration and aspiration in Parkinson's disease. Neurogastroenterol Motil. (2019) 31:e13524. doi: 10.1111/nmo.13524
48. Oad MA, Miles A, Lee A, Lambie A. Medicine administration in people with Parkinson's disease in New Zealand: an interprofessional, stakeholder-driven online survey. Dysphagia. (2019) 34:119–28. doi: 10.1007/s00455-018-9922-7
49. Paul BS, Singh T, Paul G, Jain D, Singh G, Kaushal S, et al. Prevalence of malnutrition in Parkinson's disease and correlation with gastrointestinal symptoms. Ann Indian Acad Neurol. (2019) 22:447–52. doi: 10.4103/aian.AIAN_349_18
50. Polychronis S, Dervenoulas G, Yousaf T, Niccolini F, Pagano G, Politis M. Dysphagia is associated with presynaptic dopaminergic dysfunction and greater non-motor symptom burden in early drug-naïve Parkinson's patients. PLoS ONE. (2019) 14:e0214352. doi: 10.1371/journal.pone.0214352
51. Umay E, Ozturk E, Gurcay E, Delibas O, Celikel F. Swallowing in Parkinson's disease: how is it affected? Clin Neurol Neurosurg. (2019) 177:37–41. doi: 10.1016/j.clineuro.2018.12.015
52. Wang W, Ma YM, Sun L, Wang Y, Chen WG, et al. Clinical analysis of dysphagia in patients with Parkinson's disease. Chin J Rehabil. (2019) 34:646–8. doi: 10.3870/zgkf.2019.12.008
53. Bakhtiyari J, Mehri A, Maroufizadeh S, Amanian, H. Drooling in Parkinson's disease: a multifactorial symptom. Arch Neurosci. (2020) 7:e99300. doi: 10.5812/ans.99300
54. Fagerberg P, Klingelhoefer L, Bottai M, Langlet B, Kyritsis K, Rotter E, et al. Lower energy intake among advanced vs. early Parkinson's disease patients and healthy controls in a clinical lunch setting: a cross-sectional study. Nutrients. (2020) 12:2109. doi: 10.3390/nu12072109
55. Marano M, Gupta D, Motolese F, Rossi M, Luccarelli V, Altamura C, et al. Excessive daytime sleepiness is associated to the development of swallowing impairment in a cohort of early stage drug naïve Parkinson's disease patients. J Neurol Sci. (2020) 410:116626. doi: 10.1016/j.jns.2019.116626
56. Rascol O, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, et al. Excessive buccal saliva in patients with Parkinson's disease of the French COPARK cohort. J Neural Transm. (2020) 127:1607–17. doi: 10.1007/s00702-020-02249-0
57. Schrag A, Hommel ALAJ, Lorenzl S, Meissner WG, Odin P, Coelho M, et al. The late stage of Parkinson's -results of a large multinational study on motor and non-motor complications. Parkinsonism Relat Disord. (2020) 75:91–6. doi: 10.1016/j.parkreldis.2020.05.016
58. van Wamelen DJ, Leta V, Johnson J, Ocampo CL, Podlewska AM, Rukavina K, et al. Drooling in Parkinson's disease: prevalence and progression from the non-motor international longitudinal study. Dysphagia. (2020) 35:955–61. doi: 10.1007/s00455-020-10102-5
59. Lin JB, Cai CS, Guo QY. Nutritional status and related factors in patients with Parkinson's disease. Pract Clin Med. (2020) 21:8–10. doi: 10.13764/j.cnki.lcsy.2020.01.003
60. Wang Y, Wang SY, Sun L, Shen LH, Chen WG. Application of depression drinking water test and EAT-10 in dysphagia in Parkinson's disease. Med J Commun. (2020) 34:289–91. doi: 10.19767/j.cnki.32-1412.2020.03.023
61. Xu LE, Zahng S, Jiang BZ, Hou BN, Yang M, Guo SY, et al. Caiteng's rating method in screening swallowing function of Parkinson's disease and its correlation with long-term nutritional status of patients. Zhejiang Med. (2020) 42:1598–602+1608. doi: 10.12056/j.issn.1006-2785.2020.42.15.2019-3255
62. Ayele BA, Zewde YZ, Tafesse A, Sultan A, Friedman JH, Bower JH. Non-motor symptoms and associated factors in Parkinson's disease patients in Addis Ababa, Ethiopia: a multicenter cross-sectional study. Ethiop J Health Sci. (2021) 31:837–46. doi: 10.4314/ejhs.v31i4.19
63. Cao Y, Li G, Xue J, Zhang G, Gao S, Huang Y, et al. Depression and related factors in patients with Parkinson's disease at high altitude. Neuropsychiatr Dis Treat. (2021) 17:1353–62. doi: 10.2147/NDT.S300596
64. Frank U, Radtke J, Nienstedt JC, Pötter-Nerger M, Schönwald B, Buhmann C, et al. Dysphagia Screening in Parkinson's Disease. A diagnostic accuracy cross-sectional study investigating the applicability of the Gugging Swallowing Screen (GUSS). Neurogastroenterol Motil. (2021) 33:e14034. doi: 10.1111/nmo.14034
65. Dilmaghani S, Atieh J, Khanna L, Hosfield EA, Camilleri M, Katzka DA. Severity of dysphagia is associated with hospitalizations and mortality in patients with Parkinson's disease. Neurogastroenterol Motil. (2022) 34:e14280. doi: 10.1111/nmo.14280
66. Longardner K, Merola A, Litvan I, De Stefano AM, Maule S, Vallelonga F, et al. Differential impact of individual autonomic domains on clinical outcomes in Parkinson's disease. J Neurol. (2022) 269:5510–20. doi: 10.1007/s00415-022-11221-9
67. Vogel A, Claus I, Ahring S, Gruber D, Haghikia A, Frank U, et al. Endoscopic characteristics of dysphagia in multiple system atrophy compared to Parkinson's disease. Mov Disord. (2022) 37:535–44. doi: 10.1002/mds.28854
68. Shi XX, Zheng JH, Ma JJ, Wang ZD, Sun WH, Li MJ, et al. Risk factors of dysphagia in Parkinson's disease. Chin Gen Pract. (2022) 25:669–74. doi: 10.12114/j.issn.1007-9572.2021.02.066
69. Wang YJ, Liu WG, Yu CY, Yan L, Zhang L, Zhang WB, et al. The characteristics and gender differences of non-motor symptoms in early diagnosed Parkinson's disease. J Apoplexy Nervous Dis. (2022) 39:28–32. doi: 10.19845/j.cnki.zfysjjbzz.2022.0007
70. Minagi Y, Ono T, Hori K, Fujiwara S, Tokuda Y, Murakami K, et al. Relationships between dysphagia and tongue pressure during swallowing in Parkinson's disease patients. J Oral Rehabil. (2018) 45:459–66. doi: 10.1111/joor.12626
71. Kwon M, Lee JH. Oro-pharyngeal dysphagia in Parkinson's disease and related movement disorders. J Mov Disord. (2019) 12:152–60. doi: 10.14802/jmd.19048
72. Warnecke T, Oelenberg S, Teismann I, Hamacher C, Lohmann H, Ringelstein EB, et al. Endoscopic characteristics and levodopa responsiveness of swallowing function in progressive supranuclear palsy. Mov Disord. (2010) 25:1239–45. doi: 10.1002/mds.23060
73. Pflug C, Bihler M, Emich K, Niessen A, Nienstedt JC, Flügel T, et al. Critical dysphagia is common in Parkinson disease and occurs even in early stages: a prospective cohort study. Dysphagia. (2018) 33:41–50. doi: 10.1007/s00455-017-9831-1
74. Gates J, Hartnell GG, Gramigna GD. Videofluoroscopy and swallowing studies for neurologic disease: a primer. Radiographics. (2006) 26:e22. doi: 10.1148/rg.e22
75. Allen J, Blair D, Miles A. Assessment of videofluoroscopic swallow study findings before and after cricopharyngeal myotomy. Head Neck. (2017) 39:1869–75. doi: 10.1002/hed.24846
76. Langmore SE. History of fiberoptic endoscopic evaluation of swallowing for evaluation and management of pharyngeal dysphagia: changes over the years. Dysphagia. (2017) 32:27–38. doi: 10.1007/s00455-016-9775-x
77. Leder SB, Murray JT. Fiberoptic endoscopic evaluation of swallowing. Phys Med Rehabil Clin N Am. (2008) 19:787–801, viii–ix. doi: 10.1016/j.pmr.2008.05.003
78. Hiss SG, Postma GN. Fiberoptic endoscopic evaluation of swallowing. Laryngoscope. (2003) 113:1386–93. doi: 10.1097/00005537-200308000-00023
79. Buhmann C, Flügel T, Bihler M, Gerloff C, Niessen A, Hidding U, et al. Is the Munich dysphagia Test-Parkinson's disease (MDT-PD) a valid screening tool for patients at risk for aspiration? Parkinsonism Relat Disord. (2019) 61:138–43. doi: 10.1016/j.parkreldis.2018.10.031
80. Cosentino G, Avenali M, Schindler A, Pizzorni N, Montomoli C, Abbruzzese G, et al. A multinational consensus on dysphagia in Parkinson's disease: screening, diagnosis and prognostic value. J Neurol. (2022) 269:1335–52. doi: 10.1007/s00415-021-10739-8
81. Mari F, Matei M, Ceravolo MG, Pisani A, Montesi A, Provinciali L. Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. (1997) 63:456–60. doi: 10.1136/jnnp.63.4.456
82. Cardoso AR, Guimarães I, Santos H, Carvalho J, Abreu D, Gonçalves N, et al. Cross-cultural adaptation and validation of the Swallowing Disturbance Questionnaire and the Sialorrhea Clinical Scale in Portuguese patients with Parkinson's disease. Logoped Phoniatr Vocol. (2021) 46:163–70. doi: 10.1080/14015439.2020.1792979
83. Mello RP, Xavier MO, Tomasi E, Gonzalez MC, Demarco FF, Bielemann RM. Dysphagia perception among community-dwelling older adults from a municipality in Southern Brazil. Dysphagia. (2021) 37:879–88. doi: 10.1007/s00455-021-10347-8
84. Yang C, Pan Y. Risk factors of dysphagia in patients with ischemic stroke: a meta-analysis and systematic review. PLoS ONE. (2022) 17:e0270096. doi: 10.1371/journal.pone.0270096
85. Iruthayarajah J, McIntyre A, Mirkowski M, Welch-West P, Loh E, Teasell R. Risk factors for dysphagia after a spinal cord injury: a systematic review and meta-analysis. Spinal Cord. (2018) 56:1116–23. doi: 10.1038/s41393-018-0170-3
86. Sakamoto Y, Oyama G, Umeda M, Funahara M, Soutome S, Nakamura W, et al. Effect of decreased tongue pressure on dysphagia and survival rate in elderly people requiring long-term care. J Dent Sci. (2022) 17:856–62. doi: 10.1016/j.jds.2021.09.031
87. Simons JA. Swallowing dysfunctions in Parkinson's disease. Int Rev Neurobiol. (2017) 134:1207–38. doi: 10.1016/bs.irn.2017.05.026
88. Kashihara K. Weight loss in Parkinson's disease. J Neurol. (2006) 253(Suppl. 7):VII38–41. doi: 10.1007/s00415-006-7009-0
89. Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2007) 78:819–24. doi: 10.1136/jnnp.2006.103788
90. Dumican M, Watts C, Drulia T, Zhang Y. Dysphagia presentation, airway invasion, and gender differences in a clinically based sample of people with Parkinson's disease. Dysphagia. (2022). doi: 10.1007/s00455-022-10472-y. [Epub ahead of print].
91. Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson's disease. Age Ageing. (2006) 35:614–8. doi: 10.1093/ageing/afl105
92. Park H, Lee JY, Shin CM, Kim JM, Kim TJ, Kim JW. Characterization of gastrointestinal disorders in patients with parkinsonian syndromes. Parkinsonism Relat Disord. (2015) 21:455–60. doi: 10.1016/j.parkreldis.2015.02.005
93. Yang EJ, Kim KW, Lim JY, Paik NJ. Relationship between dysphagia and mild cognitive impairment in a community-based elderly cohort: the Korean longitudinal study on health and aging. J Am Geriatr Soc. (2014) 62:40–6. doi: 10.1111/jgs.12606
94. Yatabe N, Takeuchi K, Izumi M, Furuta M, Takeshita T, Shibata Y, et al. Decreased cognitive function is associated with dysphagia risk in nursing home older residents. Gerodontology. (2018) 35:376–81. doi: 10.1111/ger.12366
95. Leentjens AF, Dujardin K, Pontone GM, Starkstein SE, Weintraub D, Martinez-Martin P. The Parkinson Anxiety Scale (PAS): development and validation of a new anxiety scale. Mov Disord. (2014) 29:1035–43. doi: 10.1002/mds.25919
96. Volonté MA, Porta M, Comi G. Clinical assessment of dysphagia in early phases of Parkinson's disease. Neurol Sci. (2002) 23(Suppl. 2):S121–2. doi: 10.1007/s100720200099
97. Nicaretta DH, Rosso AL, Mattos JP, Maliska C, Costa MM. Dysphagia and sialorrhea: the relationship to Parkinson's disease. Arq Gastroenterol. (2013) 50:42–9. doi: 10.1590/S0004-28032013000100009
98. Han M, Ohnishi H, Nonaka M, Yamauchi R, Hozuki T, Hayashi T, et al. Relationship between dysphagia and depressive states in patients with Parkinson's disease. Parkinsonism Relat Disord. (2011) 17:437–9. doi: 10.1016/j.parkreldis.2011.03.006
99. Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, et al. The relationship between quality of life and swallowing in Parkinson's disease. Mov Disord. (2009) 24:1352–8. doi: 10.1002/mds.22617
100. Lorefält B, Granérus AK, Unosson M. Avoidance of solid food in weight losing older patients with Parkinson's disease. J Clin Nurs. (2006) 15:1404–12. doi: 10.1111/j.1365-2702.2005.01454.x
101. Cilia R, Cereda E, Klersy C, Canesi M, Zecchinelli AL, Mariani CB, et al. Parkinson's disease beyond 20 years. J Neurol Neurosurg Psychiatry. (2015) 86:849–55. doi: 10.1136/jnnp-2014-308786
Keywords: Parkinson's disease, dysphagia, prevalence, associated factors, meta-analysis
Citation: Gong S, Gao Y, Liu J, Li J, Tang X, Ran Q, Tang R and Liao C (2022) The prevalence and associated factors of dysphagia in Parkinson's disease: A systematic review and meta-analysis. Front. Neurol. 13:1000527. doi: 10.3389/fneur.2022.1000527
Received: 22 July 2022; Accepted: 21 September 2022;
Published: 06 October 2022.
Edited by:
Emilia Michou, University of Patras, GreeceReviewed by:
Paul Muhle, University Hospital Münster, GermanyIrene Battel, Trinity College Dublin, Ireland
Copyright © 2022 Gong, Gao, Liu, Li, Tang, Ran, Tang and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunlian Liao, 300259@hospital.cqmu.edu.cn
†ORCID: Chunlian Liao orcid.org/0000-0001-6375-6436
 Siyuan Gong1
Siyuan Gong1 Chunlian Liao
Chunlian Liao