- 1Cambridgeshire & Peterborough NHS Foundation Trust, Fulbourn Hospital, Cambridge, United Kingdom
- 2Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom
Objectives: Face-to-face healthcare, including psychiatric provision, must continue despite reduced interpersonal contact during the COVID-19 (SARS-CoV-2 coronavirus) pandemic. Community-based services might use domiciliary visits, consultations in healthcare settings, or remote consultations. Services might also alter direct contact between clinicians. We examined the effects of appointment types and clinician–clinician encounters upon infection rates.
Design: Computer simulation.
Methods: We modelled a COVID-19-like disease in a hypothetical community healthcare team, their patients, and patients' household contacts (family). In one condition, clinicians met patients and briefly met family (e.g., home visit or collateral history). In another, patients attended alone (e.g., clinic visit), segregated from each other. In another, face-to-face contact was eliminated (e.g., videoconferencing). We also varied clinician–clinician contact; baseline and ongoing “external” infection rates; whether overt symptoms reduced transmission risk behaviourally (e.g., via personal protective equipment, PPE); and household clustering.
Results: Service organisation had minimal effects on whole-population infection under our assumptions but materially affected clinician infection. Appointment type and inter-clinician contact had greater effects at low external infection rates and without a behavioural symptom response. Clustering magnified the effect of appointment type. We discuss infection control and other factors affecting appointment choice and team organisation.
Conclusions: Distancing between clinicians can have significant effects on team infection. Loss of clinicians to infection likely has an adverse impact on care, not modelled here. Appointments must account for clinical necessity as well as infection control. Interventions to reduce transmission risk can synergize, arguing for maximal distancing and behavioural measures (e.g., PPE) consistent with safe care.
Introduction
Infection Control Strategies for Community Teams
Many countries reduced interpersonal contact to control infection during the COVID-19 pandemic. The UK implemented interpersonal distancing on 16 March 2020 and “lockdown” on 23 March; its National Health Service (NHS) reduced non-critical work and moved towards videoconferencing and telephone assessments where possible (1). However, in times of “lockdown,” some face-to-face consultations remain necessary, including for urgent mental or physical health needs. For community-based health services—including physical care teams, community mental health teams (CMHTs), mental health crisis teams, and other urgent care services—this raises the important question of how to structure patient contacts and clinical teams to minimise infection.
COVID-19 is a respiratory infection transmitted directly by airborne aerosols/droplets from an infectious person and indirectly via contaminated objects (fomites) (2). Asymptomatic people may be infectious (2, 3). Airborne transmission is reduced by asymptomaticity (4), physical distance, time, and personal protective equipment (PPE) (2), and increased by symptoms such as coughing and aerosol-generating procedures (2). Fomite transmission is reduced by handwashing, cleaning/disinfection, and time, which cause viral inactivation (2, 5).
Should community services bring patients to a central base (e.g., clinic) or visit patients at home? In the early phase of the first wave of COVID-19 in the UK (e.g., 8 April 2020), UK guidance advised PPE only for contact with patients having suspected or confirmed COVID-19, rather than for all patients (6). Primary care and outpatient settings should segregate COVID-19 and other patients in time or space, and allocate staff to one group or the other where possible (2). If we assume that clinic settings have appropriate infection control procedures in place, such as physical separation and cleaning, then a key difference between this, and home visits is exposure of the clinician to the home environment. This additional exposure includes other household members and fomites. Does this pose a risk of increased transmission to the clinician and wider community? Is this influenced by different models of service provision?
Other questions relate to clinician–clinician encounters, and the impact of clinical activity on disease transmission to patients and families. To what extent do clinician–clinician and clinician–patient encounters affect spread? This may be important not only for the infection of clinicians, with implications for healthcare capacity, but because clinicians may be in contact with more people than most in a time of “social distancing” and thus might have the potential to infect a relatively large number of patients—one aspect of concern about “super-spreading” (7). Clinicians may also work with patients particularly vulnerable to COVID-19.
Modelling Approach
Epidemiologists have modelled COVID-19 spread across populations, and social measures to reduce spread given limited critical care capacity (4). However, at the time of writing we were unable to find assessments of the quantitative impact of community consultation strategy or clinician-to-clinician contacts on disease spread [via PubMed to 29 March 2020 or via enquiries on 24 March to the NHS Sustainability and Transformation Partnership for Cambridgeshire; search repeated 23 Oct 2020 via PubMed as “community AND covid AND (clinician OR appointment)”]. We therefore simulated a population of clinicians, patients, and patients' families via agent-based modelling (8), seeding the population with an infectious disease with the approximate characteristics of COVID-19. We examined spread under different conditions of interpersonal contacts, representing alternative ways of organising community health services for necessary appointments. We report these simulations and make suggestions for infection control strategies applicable to such services.
We took a broad approach because there were insufficient high-quality data to infer reliable parameters for many aspects of a complex model. Uncertainties include: the infectivity profile over time; airborne transmission rates by contact type and time; the risks of fomite (surface) transmission in different contexts; many details of the network of patient and household community contacts (including community mixing in rural and urban areas, the use of public vs. private transport, and the mix of patient residence between private homes and care facilities); the proportion of people in patients' households who must continue to work; the consequences of antigen and antibody testing; the likelihood of serious morbidity or death following infection; etc. Therefore, we used a standard infectious disease model and applied simple “service-level” manipulations, including appointment type (varying the degree to which clinicians met patients and their family directly) and whether clinicians met each other. We tested the robustness of these effects by varying other inputs to summarise a wide range of unknown factors, such as the baseline and ongoing infection rates, and a form of inter-patient association or clustering. Furthermore, clinical care has competing objectives: to deliver optimal healthcare, to safeguard patients and staff from infection, to ensure the service is robust to staff shortages, to provide continuity of care, etc. An action that improves infection control may have other adverse effects. We did not model a mixed objective function explicitly. Instead, we focus on infection rates (including clinician infection rates) and discuss other potential effects of the service-level manipulations examined.
Methods
Experiment 1
Fixed Patient and Disease-Process Parameters
We used a “susceptible–exposed–infectious–recovered” (SEIR) model (9), modified to distinguish the symptomatic and infectious periods (Figure 1A). We modelled individuals stochastically (appropriate for small populations). (a) Susceptible. Every simulated person began in the “susceptible” state. (b) Exposed. If a simulated person was exposed to someone who was infectious at that time, they had a certain chance (described below) of becoming infected. If infected, they entered a latent or incubation period, during which they were not yet infectious to others. (c) Infectiousness and symptomaticity. People became infectious 4.6 days (the latent period) after infection (4). People were randomly assigned to become symptomatic (if infected) with probability p = 0.67, initially based on influenza (10) and close to the 69% (confidence interval 46–92%) estimated for SARS-CoV-2 (11). Otherwise, infection would lead to asymptomatic infectiousness. (d) Duration of infectiousness and symptomaticity, and transition to recovery. People remained infectious for 7 days, an uncertain value based on (12, 13); we used a simple model without temporal variation in infectivity during that time. If symptoms developed, they began 5.1 days after infection (4, 14, 15) and lasted 7 days (12). Recovery precluded re-infection (also uncertain).
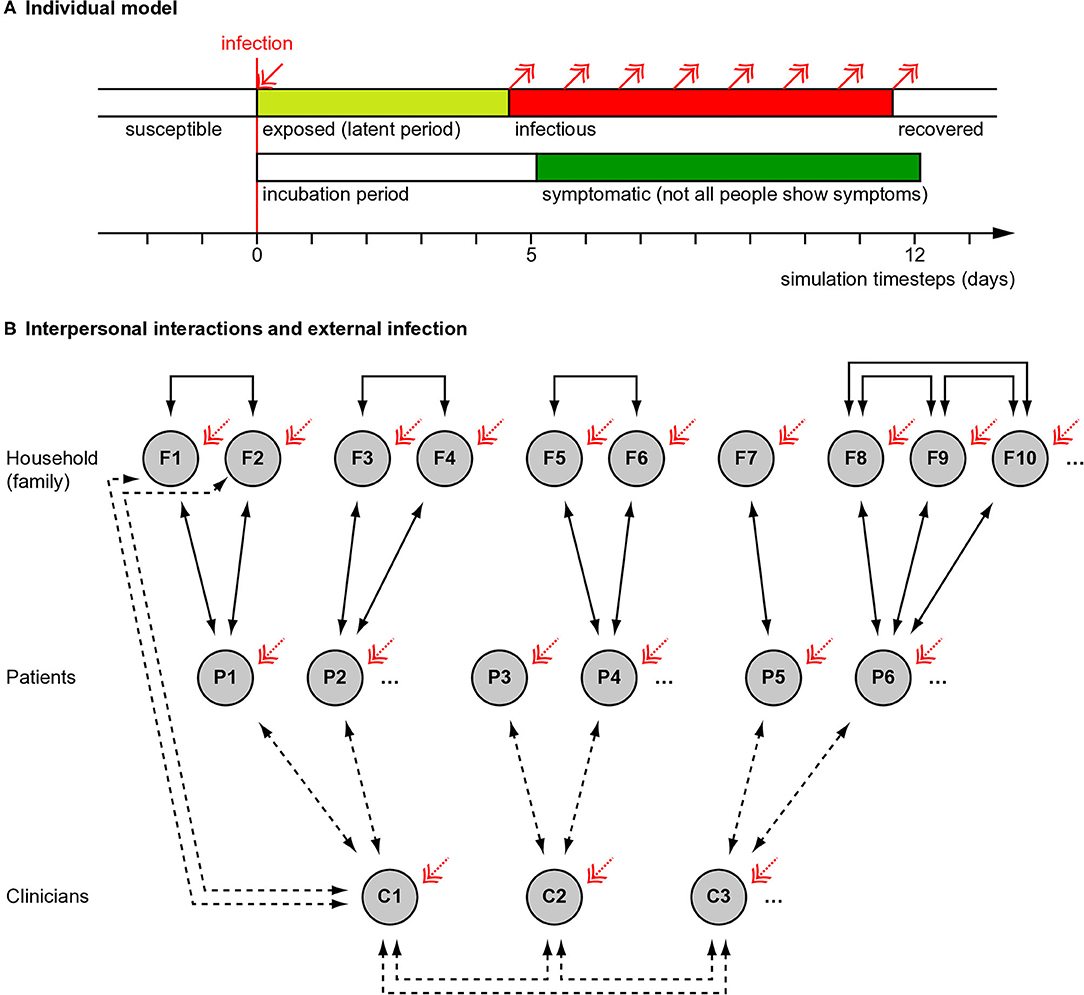
Figure 1. Overview of simulation methods. (A) Individual people followed a “susceptible–exposed–infectious–recovered” model, with full immunity after recovery. Whether an infected person developed symptoms or not was probabilistic (see Methods) and if they developed symptoms, this occurred slightly after the infectious period began. (B) Interaction between people (see Methods). Solid lines show fixed interactions; dashed lines show interactions that were varied. Patients interacted with their household (family) members and family members interacted with each other. Family sizes varied. In different conditions, clinicians did or did not interact directly with each other. Clinicians interacted with their patients, except in the “remote visit” condition. In the “family contact” (e.g., home visit) condition, clinicians also interacted briefly with family (not all such interactions drawn). The initial level of infection and the rate of ongoing “external” infection (red arrows) were varied. Additional manipulations, not shown here, included whether symptoms altered infectiousness in a behavioural, protective way (in contrast to an ever-present biological exacerbating way) and, in Experiment 2, whether patient households were grouped together in larger clusters with mutual interaction.
The transmission risk when a susceptible person was exposed to a symptomatic infectious individual was assumed to be p = 0.2 for 24 h exposure. We reduced this proportionally for shorter periods of exposure: a process with a constant rate of infection (or half-life τ for remaining uninfected), e.g., pinfection(t) = 1–0.5t/τ, is approximately linear in this range. This value is uncertain: the basic reproduction number (R0) estimated for COVID-19 (16–18) implicitly incorporates the duration of infectiousness, the interpersonal contact rate, and the transmission risk per contact (itself depending on contact duration and transmission risk per unit time) (19). An R0-value of 2–3 (16) would be roughly equivalent to p = 0.2 per 24 h exposure if, for example, an infectious person infected 0.35 people/day for 7 days (2.5 people in total), e.g., via contact with susceptible others for 40 person-hours (e.g., 10 people, 4 h each) per day. Asymptomatic people were 50% as infectious biologically as their symptomatic counterparts (4, 10).
These values may be biologically inaccurate, and we did not implement realistic intersubject variability in these parameters. More sophisticated models exist (20). However, the absolute rate of infection was not our chief concern (as above); rather, we focused on the effect of service arrangements (described below). Relative differences due to service organisation were assumed to be ordinally independent of the absolute transmission risk or other biological parameters.
Fixed Population Parameters
We simulated encounters between clinicians, patients, and patients' household contacts (Figure 1B). A team of 20 clinicians was simulated, representing a multidisciplinary team (MDT). Each clinician saw five new patients per day, without follow-up appointments. Every clinician–patient interaction lasted 1 h, based on a common UK “new patient” appointment slot in psychiatry. Patients were assumed to come from independent households. Patients could live with others (referred to here as family); the number of family members per patient was drawn from a Poisson distribution with λ = 1.37, from the mean UK household size of 2.37 (21). Everyone in a household was assumed to interact daily and every household contact was assumed to last 8 h/day. Clinicians' households were not simulated, for simplicity (though the likely effect of adding clinician households would be to increase clinician infection rates slightly). People were assumed to follow household social isolation, i.e., with the exception of clinician encounters, there were no explicit household–household contacts. In addition, “external” infection was simulated (see below).
If a clinician became symptomatic, they were assumed to cease work for the duration of their symptoms and not meet others in the model during that time. Their appointments were reassigned to other clinicians (who therefore saw more patients, without limit). Symptomatic patients and family continued to interact with each other, and symptomatic patients were assumed still to require clinical care.
We simulated a population of mean size 14,240 (20 clinicians, 6,000 patients, and on average 8,220 family members) for 60 consecutive days (1–60, with no allowance for weekends). Days were simulated discretely; i.e., contacts were simulated (effectively) simultaneously for a given day, without regard to time of day.
Manipulations
The following aspects were varied, in all possible combinations. Variable ranges were chosen to represent plausible extremes.
• Appointment type (“AT”). In the “patient only” (PO) condition, clinicians interacted only with the patient. This was intended to represent patients coming alone to a clinic, not interacting with others, with physical distancing, and decontamination of clinic environments. In the “family contact” (FC) condition, clinicians also interacted for 0.2 h (12 min) with each family member of the patient. This was intended to represent a home visit, but might also represent family accompanying the patient to a clinic and being present for a collateral history. In the “remote visit” (RV, e.g., videoconferencing) condition, no direct clinician–patient, or clinician–family contact occurred.
• Clinician–clinician meetings (“CM”). In the “clinician meeting” condition, all clinicians met up for 1 h/day (e.g., for a handover or MDT meeting). In the “no clinician meeting” condition, clinicians did not interact with each other in person (e.g., used videoconferencing instead).
• Baseline infection rate (“BL”). On day 0, the day before interpersonal contact simulation began, either 1 or 5% of the population were infected at random, reflecting approximate confidence interval (CI) extremes for the UK, 28 March 2020 (22). Clinicians and others had an equal chance of initial infection.
• External infection (“EX”). Every person had a 0, 0.5, 1, or 2% chance each day of becoming infected from external sources (e.g., in supermarkets, on public transport, etc.). The external infection rate is not constant in an epidemic, being affected by prevalence and the rate of external contacts; the upper figure of 2% was chosen to represent a very high value (e.g., if 10% of “external” people were infectious and each modelled person had 24 person-hours of “external” contact per day at transmission risk p = 0.2 per person-day exposure as above).
• Protective behavioural effect of symptoms upon transmission risk (“SX”). Being symptomatic has biological consequences (modelled above) but also social consequences. For example, overt illness may increase physical distancing or cause clinicians to use PPE, reducing infection risks. We chose values to represent plausible extremes of any such effect. In one condition, being symptomatic had an effect to reduce transmission risk to 10% of what it would otherwise have been (“behavioural symptom effect present”); the relative risk of infection with H7N7 avian influenza A is ~9% when using a respirator (23). Alternatively, the transmission risk was unmodified (“behavioural symptom effect absent”). If present, this effect applied to all interpersonal contacts (which may be unrealistic in that family members are unlikely to use PPE with each other; thus, any such behavioural effects may be smaller than those modelled).
Simulation
We simulated each condition 2,000 times, using Python (https://www.python.org/).
Analysis
We used R (https://www.r-project.org/) to analyse the total number of (a) people and (b) clinicians infected by the end of the virtual experiment. We used a generalised linear model (GLM) with a Poisson distribution and a log link function, then analysis of deviance with type III sums of squares and α = 0.05. However, the statistical power of a simulation is arbitrary (being determined by the effect size and the number of runs); thus, we present CIs and do not report exact p-values, reporting instead “p < α” for 10−3 ≤ p < 0.05; “p ≪ α” for 10−5 ≤ p < 10−3; “p ⋘ α” for p < 10−5. A significant interaction term implies non-additive effects of factors on the linear predictor, but because the predictors were on a log scale with respect to the dependent variable, interactions imply non-multiplicative effects of experimental factors on the number of people infected.
Experiment 2
Not all patients live in small households: some live in much larger shared living facilities, such as care homes. Clustering might be used as a route out of “lockdown.” Does such patient clustering amplify any infection-transmitting effect of clinicians, who are unusually mobile people during times of “lockdown” and may travel between clusters? We examined whether the effects of appointment type and clinician meetings depended on the degree of patient clustering in such “hubs,” which we modelled as large households with multiple patients and their virtual “families” (or, similarly, care staff). We implemented this simply, by aggregating virtual households: thus, n = 1 patients plus their family (household) per cluster as in Experiment 1, or n = 2 households per cluster, etc. For simplicity, all co-residents were assumed to interact with each other, as patients and family would in a single-patient household, and clinicians were assumed to have “family-level” contact with everyone else in the cluster during FC-type visits. The selection of patients requiring a visit on any 1 day was random; those patients were then assigned cyclically to available (not sick) clinicians.
We varied the number of patients per household (“NPH,” 1–10, as a discrete predictor), AT, and CM. We held all other parameters constant at values suggested by Experiment 1 to give high power to detect such effects (no behavioural symptom effect; BL 1%; EX 0%).
Deterministic SEIR Model
To examine the basis of the synergy between interventions observed in Experiments 1–2, we ran deterministic plain SEIR models using the EpiDynamics R package. This form of model represents SEIR state changes via differential equations governed by rate parameters. Solutions to a system of differential equations are deterministic and represent the expected consequences, in the limit, of a large number of stochastic events. The SEIR model assumes that (a) subjects move from the susceptible (S) state to the exposed-but-not-yet-infectious (E) state in a manner governed by the number of infectious (I) people present and a transmission rate parameter β (representing a combination of the degree of interpersonal contact and the biological infectivity); (b) subjects move from the E (exposed/incubation period) state to the infectious (I) state according to a further rate parameter, σ; and (c) that people move from the I state to the recovered (R) state according to a recovery rate parameter, γ. Recovered people are assumed to be immune and therefore not susceptible to further infection. SEIR models may also add new people to the model (“birth”) or remove them via death (not used in the present experiment). This simple deterministic model does not consider the timing, symptom, or interpersonal contact structure used in our agent-based model. We varied the proportion exposed at time t = 0 (1 or 5%, cf. Experiment 1) and the transmission rate β. Constants were: tfinal = 1,000 (for asymptote); birth/death rate μ = 0; exposed-to-infectious rate σ = 1/5; recovery rate γ = 1/7. We examined the cumulative proportion infected (1–susceptible).
Patient and Public Involvement
Several of the authors have self-isolated as patients during the pandemic. Patients were not otherwise involved.
Results
Whole-Population Infection Rates
In Experiment 1, whole-population infection was dominated by baseline and external infection rates (with infection spreading primarily via intra-household contacts), plus the behavioural response to symptoms (all p ⋘ α), with only very small contributions from the appointment type and clinician–clinician meetings (Figure 2A). That is, neither appointment type nor clinician meetings had any appreciable effect on the total number of people infected. Appointment type and clinician meetings had effects (e.g., AT × CM × BL × EX × SX interaction, p < α), but these effects were very small (the overall difference in the proportion infected was 0.01 percentage points between FC and RV conditions; Figure 2A). The beneficial effects of symptom-induced protective behaviour were proportionally greater in conditions with lower external infection rates (BL × EX × SX, p ⋘ α; Figure 2A).
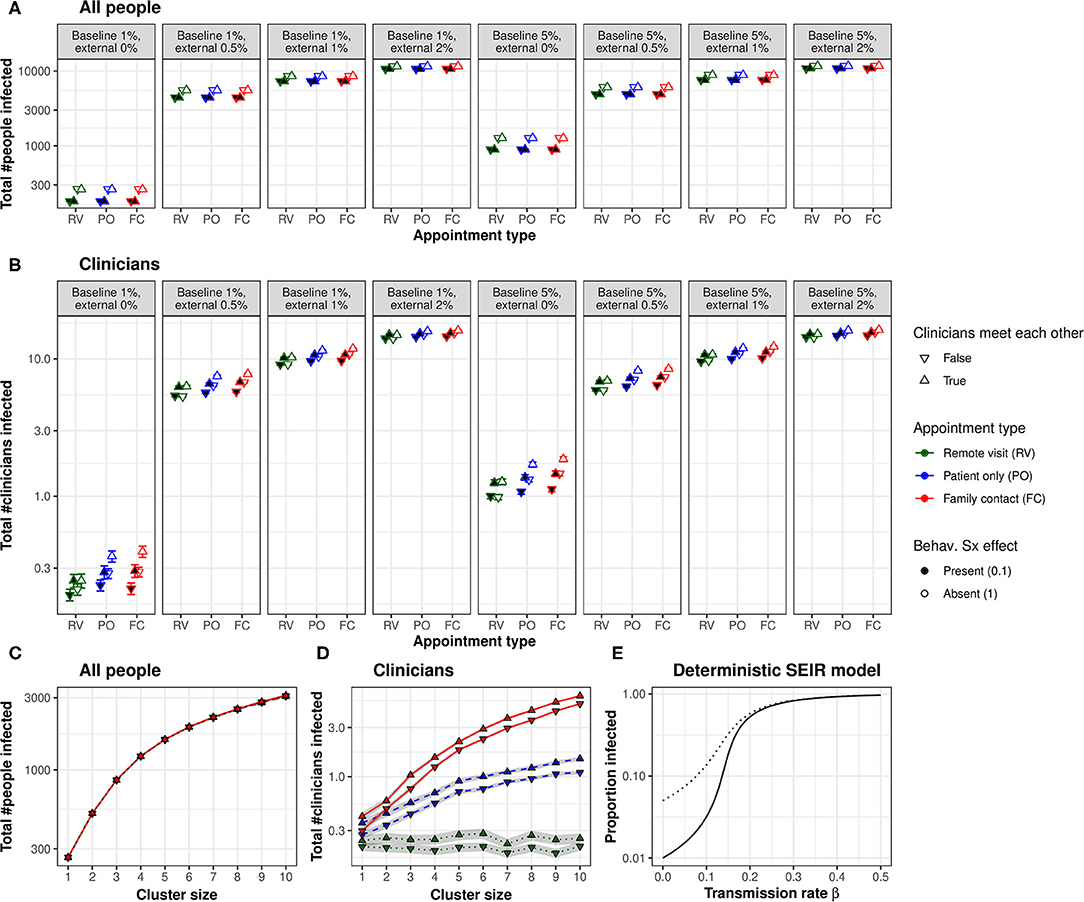
Figure 2. (A) Appointment type and clinician–clinician meetings had no substantial effect on whole-population infection. A protective behavioural response to symptoms had a proportionally greater effect at lower external infection rates. (B) Appointment type and clinician meetings had more substantial effects on the infection of clinicians. Moving to lower-contact appointment types and reducing clinician meetings showed synergy with a protective behavioural response to symptoms. (C) Clustering patient households together increased whole-population transmission. (D) Clustering increased clinician infection and substantially magnified the effect of appointment type. The beneficial effect of eliminating face-to-face clinician–clinician meetings was proportionally greater in low-clustering conditions, but this effect was very small. “Cluster size” refers to the number of patients (and their virtual families, or care home staff) per interacting “household”. (E) Changes in transmission rate have non-linear effects on the total proportion of the population infected, shown in a deterministic standard SEIR model (solid line, 1% initially exposed; dotted line, 5% initially exposed). This effect underlies the synergy between measures that can be taken to reduce the transmission of infection: small changes in transmission risk sometimes cause dramatic changes in total infection. [RV, remote visits; PO, patient only; FC, family contact. Logarithmic scale. Error bars/ribbons are 95% CIs. “Behav. Sx effect” refers to the behavioural effect upon transmission when an infected person shows symptoms; either this reduces the transmission risk to 10% of its former value, e.g., via enhanced physical distancing or PPE, or has no effect].
Effects of Appointment Type on Clinician Infection
In contrast, appointment type had more substantial effects on clinician infection rates (Figure 2B), which depended upon external infection rates and the behavioural symptom effect (AT, p ⋘ α; AT × EX × SX, p ⋘ α). As expected from the number of contacts involved, infection rates were consistently ranked “family contact” (e.g., home visit)>“patient only” (e.g., clinic)>“remote” for all conditions. However, these effects were lessened at high external infection rates. We calculated the “new infection” rate as the final number of clinicians infected minus the number infected on day 0. We examined new infections in the PO condition as a proportion of the FC condition (PO new infections ÷ FC new infections) and similarly for RV vs. FC. With 0% external infection, appointment type had a substantial effect (PO 82% of FC; RV 22% of FC); with 2% external infection, this was much less (PO 99% of FC, RV 95%). The effects of appointment type were larger without a behavioural symptom effect (Figure 2B).
Effects of Clinician–Clinician Meetings on Clinician Infection
There was a strong effect of clinician meetings, more pronounced at low levels of external infection (Figure 2B; CM, p ⋘ α; CM × EX, p ⋘ α). We calculated the effect's magnitude as “new infections without clinician meetings ÷ new infections with clinician meetings” (“new infections” defined as before). The effect of eliminating clinician meetings was much larger with external infection at 0% (RV 0%, PO 36%, and FC 38%) than at 2% (RV 94%, PO 95%, and FC 95%), and numerically larger with a behavioural symptom effect [e.g., for 0% EX and PO appointments, eliminating clinician meetings cut new infections to 25% (of that with meetings) when there was a behavioural symptom effect, but only to 47% without that behavioural effect].
Effects of Symptom-Related Behaviour on Clinician Infection
Symptom-triggered behaviour had substantial effects on clinician infection rates (Figures 2A,B; SX, p ⋘ α; AT × EX × SX, p ⋘ α), as it did for whole-population rates. The beneficial effects of symptom-triggered behaviour were proportionally greater with lower external infection rates, for higher-risk appointment types, and without clinician meetings.
Impact of Patient Clustering
Figures 2C,D shows Experiment 2's results. Predictably, greater clustering increased infection rates (whole-population and clinicians, NPH, p ⋘ α). The effects of appointment type and clinician meetings on whole-population infection (AT × CM × NPH, p ⋘ α) were very small. The effects of appointment type on clinician infection were substantially magnified by greater clustering (AT × NPH, p ⋘ α).
Synergy Between Service Manipulations
Many of the effects of the modelled variables upon clinician infections were synergistic. Preventing physical clinician meetings had a greater proportional effect when external infection rates were lower, and with a behavioural protective response to symptoms (as above). Moving to lower-contact appointment types had a greater proportional effect when external infection rates were lower. Not all manipulations were synergistic (e.g., appointment types had a greater proportional effect without a behavioural response to symptoms, but both manipulations were nonetheless helpful). In a simple deterministic SEIR model, linear changes in transmission rate had non-linear effects on the cumulative infection rate (Figure 2E), which is consistent with the synergies observed in the full model.
Discussion
Summary
We modelled a hypothetical community clinical team, under different baseline and external infection rates. The fictional team organised its patient assessments in controlled environments in which only clinician–patient contacts occurred (e.g., managed clinics), visits in which some clinician–family contacts occurred (e.g., home visits or family present for a collateral history), or remote assessments in which the clinician and patient did not meet physically (e.g., videoconferencing). Clinicians met daily in person, or refrained from doing so. Under our assumptions, these service arrangements had only a very small impact on infection rates across the population studied (clinicians, patients, and family together; Figures 2A,C), but some had a substantial effect on clinician infection rates (Figures 2B,D). Clinicians may sometimes be a scarce resource, so higher clinician infection rates may have wider adverse effects on population health through lack of clinician availability. Behavioural measures to reduce transmission in response to overt symptoms also had substantial effects on infection rates, despite a period of “silent” infectiousness and some infectious people never exhibiting symptoms.
In our model, the infection risk to clinicians of appointment type directly reflected the degree of contacts with patients and family. This was expected, but we have quantified the relative importance of structural service manipulations. Eliminating daily face-to-face clinician–clinician meetings also had a noticeable effect on clinician infection rates, which was most pronounced, proportionally, with the lowest-risk patient encounter types. Combined risk reduction methods interacted with each other, sometimes having more than a multiplicative effect, and were disproportionately more effective than each alone. All these effects lessened with increasing rates of infection from outside the modelled population. The impact of clinical service organisation on whole-population infection was small likely because the maximum possible number of contact hours between clinicians and patients in our model was realistically small relative to those for patient–family interactions. Patient clustering increased whole-population infection and magnified the effect of appointment type on clinician infection, even for “patient-only” appointments. In our model, it was possible for multiple clinicians to visit a cluster; restricting which clinicians visit which clusters may be another important factor to consider. Clustering or “hub” effects may also occur in other ways not modelled here—such as patients being in receipt of multiple services from one or several provider organisations—and would similarly serve to increase transmission further. Changes in “lockdown” practices may affect external infection rates or clustering, requiring services to adapt to changing public health policy. Multiple small improvements in infection control can have a total effect greater than the sum of its parts (Figure 2E).
Implications
These results emphasise and quantify an obvious point that minimising contact with additional people, such as household contacts of a patient, contributes to infection control. Family contacts might occur during a home visit, but also if family accompany patients to a clinic. Videoconferencing or other remote assessment obviously provides the best infection control of the methods modelled here. However, there are trade-offs between infection control and clinical care for different appointment types, which must be judged by individual teams and clinicians. In particular, the choice between home visits and clinic assessments is complex and goes beyond the “family contact” aspects modelled here (Table 1). Other infection control differences include exposure to others during transport (likely favouring home visits by clinicians), and the risk of fomite transmission in either environment (hard to quantify, but potentially less predictable and greater for home visits). Relevant clinical differences go beyond infection control (Table 1), and appointment types should be tailored to the clinical situation; for example, face-to-face contact is more likely to be required for patients with higher-risk mental states, if there are particular safeguarding concerns, and for first contacts (vs. follow-up appointments) (Table 1).
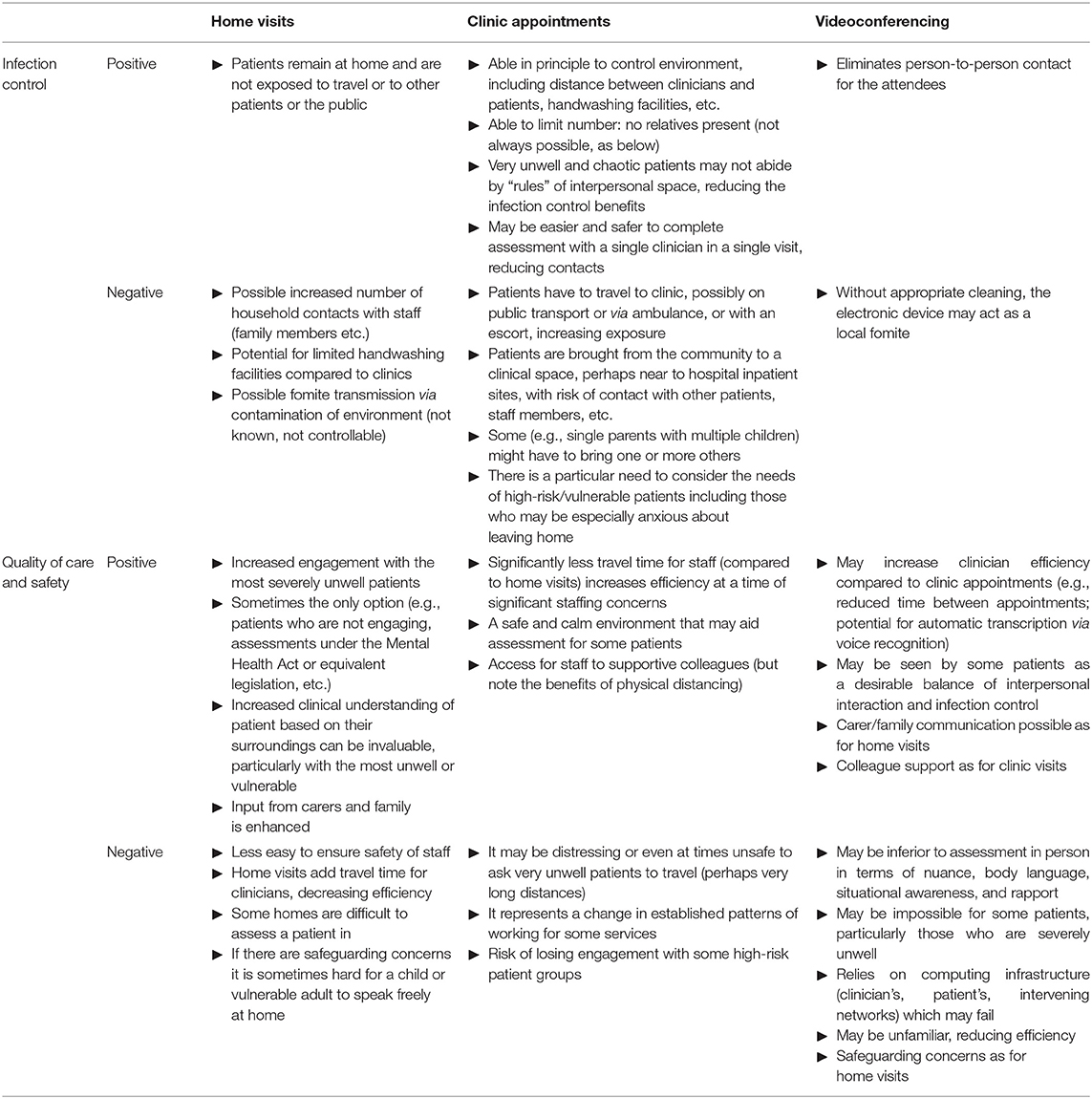
Table 1. Infection control and clinical factors to consider for home visits, clinic appointments, and videoconferencing.
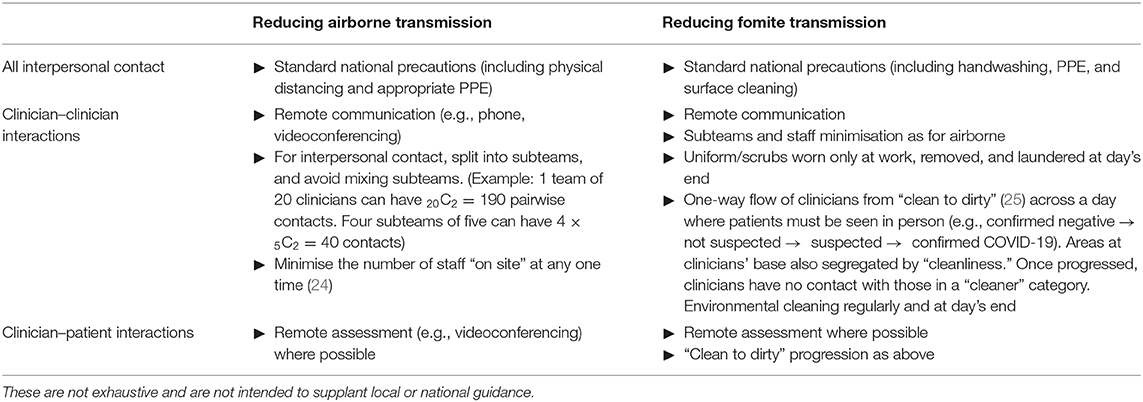
A striking result was the degree to which clinician–clinician meetings affected clinician infection rates, in some cases synergistically with other infection control measures. UK guidelines in wave 1 of COVID-19 (2) included patient segregation, and segregation of primary care staff for COVID-19 and other patients, but did not recommend the segregation of all clinical staff, or all staff, from each other as far as is possible. Reducing contact between staff may be practical. Dividing a clinical team into subteams may provide partial benefits (Table 2). Fomite transmission is also a risk to clinical teams, though this risk may have been overestimated (26): precautions available to clinical teams against fomite transmission overlap with but have some distinct elements from those for airborne transmission (Table 2).
Study Limitations
The biological parameters we took for COVID-19 spread were estimates from the published literature in the first half of 2020 and in some cases are subject to high uncertainty; likewise our estimates of initial and ongoing infection rates. Disease parameters were constant across subjects, other than symptomaticity given infection, which was stochastic. A paucity of contacts outside the household is highly atypical but corresponds to current UK policy, if not necessarily universal practice. The behavioural effect of symptoms upon transmission risk was modelled in the same way for household contacts as for clinician–patient contacts, which is unrealistic in that clinicians are more likely to have access to PPE and rules mandating its use. Clinicians' households were not modelled and would tend to increase clinician infection rates (particularly if clinicians share a household). We also modelled a series of one-off patient assessments; many patients, of course, are seen repeatedly by their clinical teams, or see many different healthcare teams routinely. However, while any of these aspects might limit generalisability, all were constant across conditions.
Similarly, the classes of models used carry limitations. For example, both the stochastic and deterministic SEIR models assume that infection/recovery precludes subsequent reinfection. Reinfection with SARS-CoV-2 is uncommon but possible (27). Similarly, the stochastic model assumed fixed times (e.g., for incubation/latency) and probabilities (e.g., of transmission or of becoming symptomatic), while the deterministic SEIR model assumes constant state transition rates. In the real world, such quantities are not fixed; for example, some individuals may transmit infection at higher rates than others, for example by being highly mobile within a population (“superspreading”) (28). Real-world transmission dynamics may bring further non-linearity. While such considerations may again limit generalisability of our findings, they do not prevent comparison across simulated conditions as in the present study.
More important for the present study are limitations relating to differences between conditions. In the “family contact” (e.g., home visit) condition, we made assumptions about the duration of contact with family members, including that this plausibly encompassed the degree of surface as well as airborne transmission, and we assumed clinicians were not fomite vectors for direct transfer of virus between homes. In the “patient only” (e.g., clinic) condition, we assumed full segregation and cleaning between patients, with no inter-patient virus transfer. In the “no clinicians meeting” condition, we assumed a lack of fomite transmission between clinicians. Any of these may be unrealistic, limiting generalisability. Fomite transmission in either situation would likely increase infection rates and alter the impact of appointment type upon infection. We make our source code open for others to test different assumptions.
Conclusions
Staff segregation, as well as appointment type and additional protective measures when meeting overtly symptomatic people, may have important effects on COVID-19 transmission in community clinical teams. Infection control manipulations can synergize with each other, suggesting that maximal implementation of such measures should be adopted to the degree possible. Appointment types must nevertheless meet the clinical need as well as infection control guidelines.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: Source code is available at https://github.com/RudolfCardinal/covid.
Author Contributions
CW and CD posed the question. RC performed the computational modelling and analysis. RC and CM-S drafted the manuscript. All authors contributed to, edited, and approved the final manuscript.
Funding
RC's, SC's, and SB's research is supported by the UK Medical Research Council (Grant No. MC_PC_17213 to RC). LS is funded by Alzheimer's Research UK (ARUK-SRF2017B-1). SRC is funded by a Wellcome Trust Clinical Fellowship (110049/Z/15/Z). This research was supported in part by the NIHR Cambridge Biomedical Research Centre; the views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Conflict of Interest
RC consults for Campden Instruments Ltd and receives royalties from Cambridge University Press, Cambridge Enterprise, and Routledge. BU is clinical director of the Windsor Unit at Fulbourn Hospital, which delivers clinical trials in dementia/mild cognitive impairment for academic and commercial organisations without personal benefit, and is the clinical lead for dementia for the UK National Institute for Health Research (NIHR) Clinical Research Network in the East of England. SRC consults for Promentis and Ieso Digital Health.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mai Wong, Ruaidhri McCormack, Petra Vertes, Hisham Ziauddeen, and Carol Brayne for helpful discussion. A version of this manuscript has been released as a preprint (29).
References
1. NHS England, NHS Improvement. Next Steps on NHS Response to COVID-19. (2020). Available online at: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/20200317-NHS-COVID-letter-FINAL.pdf (accessed March 18, 2020).
2. Department for Health and Social Care, Public Health Wales Public Health Agency Northern Ireland, Health Protection Scotland Public Health England. COVID-19: Guidance for Infection Prevention and Control in Healthcare Settings. (2020). Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/876577/Infection_prevention_and_control_guidance_for_pandemic_coronavirus.pdf (accessed March 29, 2020).
3. Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. (2020) 323:1406–7. doi: 10.1001/jama.2020.2565
4. Ferguson NM, Laydon D, Nedjati-Gilani G, Imai N, Ainslie K, Baguelin M, et al. Report 9: Impact of Non-Pharmaceutical Interventions (NPIs) to Reduce COVID-19 Mortality and Healthcare Demand. Imperial College London (2020). Available online at: https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf
5. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. (2020) 104:246–51. doi: 10.1016/j.jhin.2020.01.022
6. Public Health England, Academy of Medical Royal Colleges, Public Health Wales, Health Protection Scotland, Public Health Agency Northern Ireland NHS. Recommended PPE for Primary, Outpatient, Community and Social Care by Setting, NHS and Independent Sector. (2020). Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/877599/T2_Recommended_PPE_for_primary_outpatient_and_community_care_by_setting_poster.pdf
7. Yu ITS, Sung JJY. The epidemiology of the outbreak of severe acute respiratory syndrome (SARS) in Hong Kong–what we do know and what we don't. Epidemiol Infect. (2004) 132:781–6. doi: 10.1017/S0950268804002614
8. Bonabeau E. Agent-based modeling: methods and techniques for simulating human systems. Proc Natl Acad Sci USA. (2002) 99 (Suppl. 3):7280–7. doi: 10.1073/pnas.082080899
9. Hethcote HW. The mathematics of infectious diseases. SIAM Rev. (2000) 42:599–653. doi: 10.1137/S0036144500371907
10. Halloran ME, Ferguson NM, Eubank S, Longini IM, Cummings DAT, Lewis B, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci USA. (2008) 105:4639–44. doi: 10.1073/pnas.0706849105
11. Nishiura H, Kobayashi T, Suzuki A, Jung SM, Hayashi K, Kinoshita R, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis. (2020) 94:154–5. doi: 10.1016/j.ijid.2020.03.020
12. NHS. Self-Isolation If You or Someone You Live with has Symptoms [CoronaVirus (COVID-19)]. (2020). Available online at: https://www.nhs.uk/conditions/coronavirus-covid-19/self-isolation-advice/ (accessed March 28, 2020).
13. Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. (2020) 20:656–7. doi: 10.1016/S1473-3099(20)30232-2
14. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
15. Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. (2020) 172:577–82. doi: 10.7326/M20-0504
16. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. (2020) 27:taaa021. doi: 10.1093/jtm/taaa021
17. Zhang S, Diao M, Yu W, Pei L, Lin Z, Chen D. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the diamond princess cruise ship: a data-driven analysis. Int J Infect Dis. (2020) 93:201–4. doi: 10.1016/j.ijid.2020.02.033
18. Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. (2020) 92:214–7. doi: 10.1101/2020.01.23.916395
19. Delamater PL, Street EJ, Leslie TF, Yang YT, Jacobsen KH. Complexity of the basic reproduction number (R0). Emerging Infect Dis. (2019) 25:1–4. doi: 10.3201/eid2501.171901
20. Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dörner L, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. (2020) 368:eabb6936. doi: 10.1126/science.abb6936
21. UK Office for National Statistics. Dataset: Families and Households. (2019). Available online at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/families/datasets/familiesandhouseholdsfamiliesandhouseholds (accessed March 28, 2020).
22. Flaxman S, Mishra S, Gandy A, Unwin HJT, Coupland H, Mellan TA, et al. Report 13: Estimating the Number of Infections and the Impact of Non-Pharmaceutical Interventions on COVID-19 in 11 European Countries. Imperial College London (2020). Available online at: https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-Europe-estimates-and-NPI-impact-30-03-2020.pdf
23. te Beest DE, van Boven M, Bos MEH, Stegeman A, Koopmans MPG. Effectiveness of personal protective equipment and oseltamivir prophylaxis during avian influenza A (H7N7) epidemic, the Netherlands, 2003. Emerging Infect Dis. (2010) 16:1562–8. doi: 10.3201/eid1610.091412
24. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management. Anesth Analg. (2020) 131:37–42. doi: 10.1213/ANE.0000000000004829
25. Health and Safety Executive. Managing Infection Risks When Handling the Deceased: Guidance for the Mortuary, Post-Mortem Room and Funeral Premises, and During Exhumation (Hsg283). (2018). Available online at: https://www.hse.gov.uk/pUbns/priced/hsg283.pdf (accessed March 30, 2020).
26. Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect Dis. (2020) 20:892–3. doi: 10.1016/S1473-3099(20)30561-2
27. Lumley SF, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. (2021) 384:533–40. doi: 10.1056/NEJMoa2034545
28. Lemieux JE, Siddle KJ, Shaw BM, Loreth C, Schaffner SF, Gladden-Young A, et al. Phylogenetic analysis of SARS-CoV-2 in Boston highlights the impact of superspreading events. Science. (2020) 371:eabe3261. doi: 10.1126/science.abe3261
29. Cardinal RN, Meiser-Stedman CE, Christmas DM, Price AC, Denman C, Underwood BR, et al. Simulating a community mental health service during the COVID-19 pandemic: effects of clinician–clinician encounters, clinician–patient–family encounters, symptom-triggered protective behaviour, and household clustering. medRxiv. (2020). doi: 10.1101/2020.04.27.20081505
Keywords: COVID-19/SARS-CoV-2, infection control, community mental health team, computer simulation, susceptible–exposed–infectious–recovered model, personal protective equipment, clustering
Citation: Cardinal RN, Meiser-Stedman CE, Christmas DM, Price AC, Denman C, Underwood BR, Chen S, Banerjee S, White SR, Su L, Ford TJ, Chamberlain SR and Walsh CM (2021) Simulating a Community Mental Health Service During the COVID-19 Pandemic: Effects of Clinician–Clinician Encounters, Clinician–Patient–Family Encounters, Symptom-Triggered Protective Behaviour, and Household Clustering. Front. Psychiatry 12:620842. doi: 10.3389/fpsyt.2021.620842
Received: 23 October 2020; Accepted: 03 February 2021;
Published: 25 February 2021.
Edited by:
Christos Theleritis, National and Kapodistrian University of Athens, GreeceReviewed by:
Kostas Siarkos, National and Kapodistrian University of Athens, GreeceLucia Sideli, University of Cagliari, Italy
Copyright © 2021 Cardinal, Meiser-Stedman, Christmas, Price, Denman, Underwood, Chen, Banerjee, White, Su, Ford, Chamberlain and Walsh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rudolf N. Cardinal, rnc1001@cam.ac.uk
 Rudolf N. Cardinal
Rudolf N. Cardinal Caroline E. Meiser-Stedman1
Caroline E. Meiser-Stedman1 Shanquan Chen
Shanquan Chen Soumya Banerjee
Soumya Banerjee Li Su
Li Su