Sunflower Production Field Guide - Your "Home Page"
Sunflower Production Field Guide - Your "Home Page"
Sunflower Production Field Guide - Your "Home Page"
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
A-1331 (EB-25 Revised)<br />
Sunfl ower<br />
<strong>Production</strong><br />
SEPTEMBER 2007
Foreword<br />
The fi rst edition of “Sunfl ower <strong>Production</strong> and Marketing<br />
Extension Bulletin 25” was published in 1975.<br />
This publication provided general information for<br />
growers, seedsmen, processors, marketing agencies<br />
and Extension personnel. Revised editions followed in<br />
1978, 1985 and 1994. Interest and knowledge about<br />
sunfl ower production and marketing in the U.S. has<br />
increased greatly in the past 30 years. Marketing and<br />
processing channels have stabilized and have become<br />
fairly familiar to growers since 1985, but pest problems<br />
have shifted and new research information has<br />
become available to assist in production decisions.<br />
This publication is a revision of the “Sunfl ower <strong>Production</strong><br />
and Marketing Bulletin” published in 1994.<br />
The purpose is to update information and provide a<br />
production and pest management guide for sunfl ower<br />
growers. This revised publication is directed primarily<br />
to the commercial production of sunfl ower, not to marketing<br />
and processing. It will attempt to give specifi c<br />
guidelines and recommendations on production practices,<br />
pest identifi cation and pest management, based<br />
on current information.<br />
This publication also is directed primarily toward sunfl<br />
ower production in the northern Great Plains of the<br />
U.S. However, much of the information is relevant to<br />
other production areas. All pesticides recommended<br />
have a U.S. Environmental Protection Agency label<br />
unless otherwise specifi ed. This publication contains<br />
certain recommendations for pesticides that<br />
are labeled ONLY for North Dakota. The users of<br />
any pesticide designated for a state label must have<br />
a copy of the state label in their possession at the<br />
time of application. State labels can be obtained<br />
from agricultural chemical dealers or distributors.<br />
USE PESTICIDES ONLY AS LABELED.<br />
Acknowledgements<br />
The editor is indebted to the contributors for writing<br />
sections of this publication. The editor also appreciates<br />
the efforts made by previous contributors, as<br />
these previous sections often were the starting point<br />
for current sections.<br />
i
ii<br />
Contributors<br />
Roger Ashley, Area Extension Agronomist, Dickinson<br />
Research Extension Center, Dickinson, ND 58601<br />
Duane R. Berglund, Professor Emeritus and Former<br />
Extension Agronomist, NDSU Extension Service,<br />
North Dakota State University, Fargo, ND 58105<br />
Carl Bradley, Former Extension Plant Pathologist,<br />
NDSU Extension Service, North Dakota State<br />
University, Fargo, ND 58105<br />
Gary Brewer, Former Department Chair and Professor,<br />
Department of Entomology, North Dakota State<br />
University, Fargo, ND 58105<br />
Lawrence Charlet, Research Entomologist,<br />
ARS-USDA, North Dakota State University,<br />
Fargo, ND 58105<br />
Greg Endres, Area Extension Agronomist, NDSU<br />
Research Extension Center, Carrington, ND 58421<br />
George Flaskerud, Extension Agricultural Economist,<br />
NDSU Extension Service, North Dakota State<br />
University, Fargo, ND 58105<br />
Dave Franzen, Extension Soils Specialist, NDSU<br />
Extension Service, North Dakota State University,<br />
Fargo, ND 58105<br />
Thomas Gulya, Research Plant Pathologist,<br />
ARS-USDA, North Dakota State University,<br />
Fargo, ND 58105<br />
James Hanzel, Sunfl ower Breeder, Proseed Inc.,<br />
Harvey, ND 58341<br />
Kenneth Hellevang, Extension Agricultural Engineer,<br />
NDSU Extension Service, North Dakota State<br />
University, Fargo, ND 58105<br />
Vernon L. Hofman, Professor Emeritus and Former<br />
Extension Agricultural Engineer, NDSU Extension<br />
Service, North Dakota State University, Fargo, ND<br />
58105<br />
Larry Kleingartner, Executive Director, National<br />
Sunfl ower Association, Bismarck, ND 58503<br />
Jan Knodel, Extension Entomologist, NDSU<br />
Extension Service, North Dakota State University,<br />
Fargo, ND 58105<br />
Greg Lardy, Extension Beef Cattle Specialist,<br />
Department of Animal and Range Sciences,<br />
North Dakota State University, Fargo, ND 58105<br />
George Linz, Wildlife Biologist, USDA-APHIS,<br />
Bismarck, ND 58501<br />
Sam Markell, Extension Plant Pathologist, NDSU<br />
Extension Service, North Dakota State University,<br />
Fargo, ND 58105<br />
Jerry Miller, Retired Sunfl ower Breeder, USDA-ARS,<br />
North Dakota State University, Fargo, ND 58105<br />
John Sandbakken, Marketing Specialist, National<br />
Sunfl ower Association, Bismarck, ND 58503<br />
Tom Scherer, Extension Irrigation Engineer, NDSU<br />
Extension Service, North Dakota State University,<br />
Fargo, ND 58105<br />
Don Tanaka, Soil Scientist, ARS-USDA, Northern<br />
Great Plains Research Laboratory, Mandan, ND<br />
58554<br />
Richard Zollinger, Extension Weeds Specialist,<br />
NDSU Extension Service, North Dakota State<br />
University, Fargo, ND 58105<br />
Former Editors: David W. Cobia, David E. Zimmer,<br />
Marcia McMullen and Duane R. Berglund<br />
Former Contributors: Ron R. Allen, William S. Ball,<br />
James Bauder, Al Black, David W. Cobia, William<br />
Danke, Alan Dexter, Carl Fanning, Gerhardt N. Fick,<br />
Basil Furgala, Phil Glogoza, James Helm, Harvey<br />
J. Hirning, Edna T. Holm, David H. Kinard, Arthur<br />
Lamey, Darnell Lundstrom, Dean McBride, Hugh<br />
McDonald, John Nalewaja, Berlin Nelson, David M.<br />
Noetzel, William K. Pfeifer, Lyle Prunty, Charlie E.<br />
Rogers, LeRoy W. Schaffner, Albert Schneiter, Robert<br />
and Jay Schuler, John T. Schulz, Tommy E. Thompson,<br />
Sebastian Vogel, Howard D. Wilkins, David E.<br />
Zimmer and Joseph C. Zubriski
Sunfl ower<br />
<strong>Production</strong><br />
Edited and Compiled by<br />
Duane R. Berglund<br />
Professor Emeritus and<br />
Former Extension Agronomist<br />
North Dakota State University<br />
Extension Service<br />
Extension Publication A-1331<br />
(EB-25 revised)<br />
September 2007<br />
North Dakota Agricultural<br />
Experiment Station<br />
and<br />
North Dakota State University<br />
Extension Service<br />
North Dakota State University<br />
Fargo, North Dakota 58105<br />
Contents<br />
page<br />
Foreword . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . i<br />
Acknowledgements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . i<br />
Contributors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ii<br />
I. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1<br />
Historical perspective . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1<br />
Taxonomy. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2<br />
Growth stages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2<br />
Growing degree days . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3<br />
II. <strong>Production</strong> . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6<br />
World production . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6<br />
U.S. production . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6<br />
Acreage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6<br />
Seed yields/acre . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7<br />
Pounds of seed production . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7<br />
Processing plants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7<br />
Prices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8<br />
Sunfl ower marketing strategy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9<br />
III. Hybrid Selection and <strong>Production</strong> Practices . . . . . . . . . . . . . . 10<br />
Hybrid selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10<br />
Oilseed (NuSun), traditional and confectionary . . . . . . . . . . . . 11<br />
Criterial for hybrid selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11<br />
Semidwarf sunfl ower . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12<br />
Sunfl ower branching . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13<br />
<strong>Production</strong> practices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13<br />
Seed quality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13<br />
Soils . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13<br />
Soil fertility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13<br />
Fertilizer recommendations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14<br />
Fertilizer application . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15<br />
Water requirements for sunfl ower . . . . . . . . . . . . . . . . . . . . . . . . . . 15<br />
Soil water management for dryland sunfl ower . . . . . . . . . . . . . 16<br />
Irrigation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16<br />
Tillage, seedbed preparation and planting . . . . . . . . . . . . . . . . . 18<br />
Crop rotation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23<br />
Pollination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24<br />
IV. Pest Management. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26<br />
Insects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27<br />
Diseases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54<br />
Weeds . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 80<br />
Birds . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 85<br />
Other . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88<br />
V. Hail Injury . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91<br />
VI. Herbicide Drift and Chemical Residue . . . . . . . . . . . . . . . . . . . . 95<br />
VII. Harvesting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 101<br />
Maturity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 101<br />
Harvesting attachments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 101<br />
Combine adjustments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 103<br />
VIII. Drying and Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105<br />
IX. Feeding Value of Sunfl ower Products<br />
in Beef Cattle Diets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 109<br />
X. U.S. Grades and Standards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 112<br />
XI. Other Information Sources. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 113<br />
Glossary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 115<br />
Appendix 1. Diseases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 117<br />
iii
Introduction<br />
(Duane R. Berglund)<br />
Two primary types of sunfl ower are grown: (1) oilseed<br />
for vegetable oil production and (2) nonoilseed for<br />
human food and bird-food markets (Figure 1). The oilseed<br />
hybrids may be of three fatty acid types: linoleic,<br />
mid-oleic (NuSun) or high oleic. They are usually<br />
black-seeded and have a thin hull that adheres to the<br />
kernel. Seed of the oilseed varieties contains from 38<br />
percent to 50 percent oil and about 20 percent protein.<br />
Some black-seeded oil types go into the hulling market<br />
for birdseed. Nonoilseed sunfl ower also has been<br />
referred to as confectionery sunfl ower, and is usually<br />
white striped and/or comes in large-seeded varieties.<br />
1<br />
2<br />
Nonoilseed sunfl ower generally has a relatively thick<br />
hull that remains loosely attached to the kernel, permitting<br />
more complete dehulling. Seed of the nonoilseed<br />
hybrids generally is larger than that of the oilseed<br />
types and has a lower oil percentage and test weight.<br />
Sunfl ower is a major source of vegetable oil in<br />
the world. Worldwide production of sunfl ower has<br />
increased since the last revision of this publication<br />
and peaked during the 1998-1999 period. The former<br />
Soviet Union remains the highest producer, followed<br />
by Argentina and then the U.S., which is third in<br />
production worldwide. Domestic use and exportation<br />
of nonoilseed sunfl ower also have increased. The majority<br />
of U.S. production of sunfl ower oil is exported,<br />
although domestic use is increasing.<br />
The following chapters provide a historical perspective<br />
and background of the sunfl ower as a viable<br />
economic crop and provide the current information<br />
on worldwide and U.S. production, U.S. production<br />
practices, current pest identifi cation and pest management<br />
practices, hail injury, herbicide use and damage,<br />
harvesting, drying, storing, and U.S. grades and<br />
standards for market.<br />
Historical Perspective<br />
Sunfl ower, native to North America, is the state<br />
fl ower of Kansas and grows wild in many areas of<br />
the U.S. Sunfl ower has a long and varied history as<br />
an economic plant, but the time and place of its fi rst<br />
cultivation is uncertain. Sunfl ower was used by North<br />
■ Figure 1. The two classes of sunfl ower based on seed<br />
characteristics: (1) oilseed hybrids grown as a source<br />
for oil and meal, and (2) nonoilseed hybrids-grown for<br />
human and bird food. Wholeseed and kernel types for<br />
both are shown. (Gerhardt Fick)<br />
Introduction<br />
1
2<br />
American Indians before colonization of the New<br />
World. Spanish explorers collected sunfl ower in North<br />
America and by 1580, it was a common garden fl ower<br />
in Spain (Figure 2). Early English and French explorers,<br />
fi nding sunfl ower in common use by the American<br />
Indians, introduced it to their respective lands. It<br />
spread along the trade routes to Italy, Egypt, Afghanistan,<br />
India, China and Russia. Sunfl ower developed as<br />
a premier oilseed crop in Russia and has found wide<br />
acceptance throughout Europe. Oilseed sunfl ower has<br />
been an economically important crop in the U.S. since<br />
1966. Before 1966, sunfl ower acreage in the U.S. was<br />
devoted primarily to nonoilseed varieties.<br />
The center of sunfl ower origin has been identifi ed<br />
as limited to the western Plains of North America,<br />
but whether the domesticated type originated in the<br />
Southwest or in the Mississippi or Missouri River<br />
valleys has not been determined. The wild form of the<br />
cultivated sunfl ower is well-known, which is not true<br />
with most of our cultivated crop species today.<br />
■ Figure 2. A 1586 drawing of sunfl ower.<br />
(Mattiolus from Heiser)<br />
The American Indians used sunfl ower as a foodstuff<br />
before the cultivation of corn. Sunfl ower also was used<br />
as a medicinal crop, source of dye, oil for ceremonial<br />
body painting and pottery, and as a hunting calendar.<br />
When sunfl ower was tall and in bloom, the bison fed<br />
on it, and according to stories told, the fat and the<br />
meat were good.<br />
Cultivation of sunfl ower was undertaken by New<br />
World settlers as a supplementary food. Later, sunfl<br />
ower was grown primarily as a garden ornament. It<br />
also was grown as an ensilage crop in the late 1800s<br />
and early 1900s.<br />
Expanded world production of sunfl ower resulted<br />
primarily from development of high-oil varieties by<br />
plant scientists and more recently by the development<br />
of hybrids. Sunfl ower is widely grown in the world<br />
where the climates are favorable and a high quality oil<br />
is desired.<br />
Taxonomy<br />
The cultivated sunfl ower (Helianthus annuus L.) is<br />
one of the 67 species in the genus Helianthus. All<br />
are native to the Americas and most are found in the<br />
U.S. It is a member of the Compositae family and<br />
has a typical composite fl ower (Figure 3). Jerusalem<br />
artichoke (H. tuberosus L.), another species, is grown<br />
on a limited basis for food and livestock feed in the<br />
U.S. A few species are grown as ornamentals and the<br />
rest are weeds, usually found in pastures or disturbed<br />
areas.<br />
The basic chromosome number for the Helianthus<br />
genus is 17. Diploid, tetraploid and hexaploid species<br />
are known. The majority of the species are perennial,<br />
with only about a dozen annual species. Plant breeders<br />
have made interspecifi c crosses within the genus and<br />
have transferred such useful characteristics as higher<br />
oil percentage, cytoplasmic male sterility for use in<br />
production of hybrids, and disease and insect resistance<br />
to commercial sunfl ower.<br />
Growth Stages<br />
The division of growth into vegetative and reproductive<br />
stages as developed by Schneiter and Miller is<br />
shown in Figure 4. This scheme is important as it<br />
gives producers, scientists and the industry a common<br />
basis to discuss plant development.
ay fl ower<br />
petal<br />
disk fl orets<br />
receptacle<br />
Table 1. Growing Degree Days: Sunfl ower Growth and Development<br />
Sunfl ower Plant<br />
Ave. days and GDD** units<br />
accum. from planting<br />
GDD<br />
Stage Description units Days<br />
VE Emergence 167 10<br />
V4 4 True Leaves 349 20<br />
V8 8 True Leaves 545 28<br />
V12 12 True Leaves 690 34<br />
V16 16 True Leaves 772 38<br />
V20 20 True Leaves 871 44<br />
R1 Miniature Terminal Bud 919 46<br />
R2 Bud
4<br />
Vegetative Stages<br />
True leaf — 4 cm<br />
V-12<br />
Reproductive Stages<br />
R-5.1<br />
R-7<br />
V-E<br />
V-2<br />
V-4<br />
R-1 R-2<br />
R-3 R-3 Top View R-4 Top View<br />
R-5.5 R-5.9 R-6<br />
R-8 R-9<br />
■ Figure 4. Stages of sunfl ower development.<br />
(A. A. Schneiter and J.F. Miller.)<br />
Less<br />
than<br />
2cm<br />
R-2 R-3<br />
More<br />
than<br />
2cm
Description of sunfl ower growth stage<br />
The total time required for development of a sunfl ower plant and the time between the various<br />
stages of development depends on the genetic background of the plant and growing season environment.<br />
When determining the growth stage of a sunfl ower fi eld, the average development of a<br />
large number of plants should be considered. This staging method also can be used for individual<br />
plants. The same system can be used for classifying either a single head or branched sunfl ower.<br />
In the case of branched sunfl ower, make determinations using only the main branch or head. In<br />
stages R-7 through R-9, use healthy, disease-free heads to determine plant development if possible<br />
because some diseases can cause head discoloration. Also, in a number of recently released and<br />
grown hybrids, the stay-green characteristic is present, which means the yellowing or browning of<br />
the bracts may not be a good indicator of plant maturity.<br />
Stage Description<br />
V (number) These are determined by counting the number of true leaves at least<br />
Vegetative Stages 4 cm in length beginning as V-1, V-2, V-3, V-4, etc. If senescence of<br />
(e.g., V-1, V-2, V-3, etc.) of the lower leaves has occurred, count leaf scars (excluding those<br />
where the cotyledons were attached) to determine the proper stage.<br />
R-1 Reproductive Stages The terminal bud forms a miniature fl oral head rather than a cluster of<br />
leaves. When viewed from directly above, the immature bracts have a<br />
many-pointed starlike appearance.<br />
R-2 The immature bud elongates 0.5 to 2.0 cm above the nearest leaf<br />
attached to the stem. Disregard leaves attached directly to the back of<br />
the bud.<br />
R-3 The immature bud elongates more than 2 cm above the nearest leaf.<br />
R-4 The infl orescence begins to open. When viewed from directly above,<br />
immature ray fl owers are visible.<br />
R-5 (decimal) This stage is the beginning of fl owering. The stage can be divided into<br />
(e.g., R-5.1, R-5.2, R-5.3, etc.) substages dependent upon the percent of the head area (disk fl owers)<br />
that has completed or is in fl owering. Ex. R-5.3 (30%), R-5.8 (80%),<br />
etc.<br />
R-6 Flowering is complete and the ray fl owers are wilting.<br />
R-7 The back of the head has started to turn a pale yellow.<br />
R-8 The back of the head is yellow but the bracts remain green.<br />
R-9 The bracts become yellow and brown. This stage is regarded as<br />
physiological maturity.<br />
From Schneiter, A.A., and J.F. Miller. 1981. Description of Sunfl ower Growth Stages. Crop Sci. 21:901-903.<br />
Growth Stages<br />
5
6<br />
<strong>Production</strong><br />
World <strong>Production</strong><br />
(John Sandbakken and Larry Kleingartner)<br />
The sunfl ower is native to North America but commercialization<br />
of the plant took place in Russia. Sunfl ower<br />
oil is the preferred oil in most of Europe, Mexico and<br />
several South American countries. Major producing<br />
countries or areas are the former Soviet Union,<br />
Argentina, Eastern Europe, U.S., China, France and<br />
Spain (Table 2). These seven countries/areas of the<br />
world produce about 80 percent of the world’s oilseed<br />
and nonoilseed sunfl ower. Historically, the former Soviet<br />
Union has been the No. 1 producer of sunfl ower,<br />
producing about 35 percent of the world’s production<br />
annually. During much of the 1970s, the U.S. was<br />
the world’s second largest producer, but in the 1980s,<br />
Argentina became fi rmly entrenched in second place.<br />
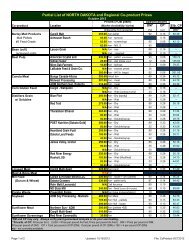
Table 2. World <strong>Production</strong> of All Sunfl ower<br />
U.S. <strong>Production</strong><br />
Acreage<br />
The fi rst sustained commercial production of oilseed<br />
sunfl ower in the U.S. occurred in 1966, when about<br />
6,000 acres were grown. Total combined acreage of<br />
oilseed and nonoilseed sunfl ower increased gradually<br />
in the late 1960s and expanded rapidly in the 1970s,<br />
reaching a peak in 1979 at 5.5 million acres. The U.S.<br />
share of world production has declined in recent years<br />
as production in Argentina and other countries has<br />
increased. During the peak period of U.S. production,<br />
the U.S. produced about 15 percent of the world’s<br />
sunfl ower production. In 2005, the U.S. market share<br />
was only 6 percent.<br />
The rapid acreage increase in the late 1970s was<br />
stimulated by a variety of factors. Favorable yields in<br />
1977 and 1978 brought about by improved hybrids<br />
and favorable weather conditions were key factors,<br />
along with excellent prices when compared with competitive<br />
crops.<br />
1996- 1997- 1998- 1999- 2000- 2001- 2002- 2003- 2004- 2005-<br />
2006-<br />
2007<br />
1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 Forecast<br />
Argentina 5.45 5.68 7.13 5.80 2.94 3.73 3.34 2.98 3.75 3.82 4.20<br />
Eastern Europe 2.92 2.18 2.59 2.75 1.67 1.86 2.02 2.67 2.27 2.11 2.17<br />
European Union 3.87 4.08 3.44 3.10 3.27 3.03 3.72 4.07 4.07 3.72 4.06<br />
China, Peoples<br />
Republic of<br />
1.42 1.17 1.46 1.77 1.95 1.75 1.95 1.82 1.70 1.83 1.85<br />
former USSR 5.37 5.41 5.74 6.89 7.27 4.94 7.19 9.35 8.00 11.32 11.20<br />
United States 1.60 1.67 2.39 1.97 1.61 1.55 1.11 1.21 .93 1.82 .92<br />
India 1.30 1.16 1.17 .87 .81 .73 1.06 1.16 1.45 1.50 1.43<br />
Turkey .67 .67 .85 .82 .63 .53 .83 .56 .64 .80 .90<br />
Other 1.99 1.87 2.83 2.98 3.01 3.55 2.74 3.07 3.58 3.25 3.77<br />
World Sunfl ower 24.63 23.89 27.60 26.95 23.16 21.80 23.95 26.88 26.39 30.16 30.49<br />
<strong>Production</strong><br />
(million metric tons)
Changes in the 1990 government farm program, which<br />
allowed planting fl exibility while providing price support,<br />
led to an increase in sunfl ower acreage in 1991<br />
relative to 1990. The government program established<br />
a marketing loan and a loan defi ciency payment for<br />
sunfl ower and other oilseed crops.<br />
The bulk of U.S. sunfl ower production occurs in North<br />
Dakota, South Dakota, Minnesota, Kansas, Colorado,<br />
Nebraska and Texas. Small acreages are grown<br />
in several other states (Table 3). The majority of the<br />
acreage harvested is for oil production versus nonoil<br />
uses. In 2005, the USDA reported that 2,032,000 acres<br />
of oil sunfl ower and 578,000 of nonoil sunfl ower were<br />
harvested (Table 4).<br />
Seed Yield/Acre<br />
Annual average sunfl ower yields from 1996 to 2005<br />
ranged from 1,140 to 1,564 pounds per acre for<br />
oilseed and from 997 to 1,455 pounds per acre for<br />
Table 3. Total Planted Sunfl ower Acreage by States 1994-2006<br />
nonoilseed sunfl ower. Average yields per acre during<br />
the 1996-2005 period were 1,349 pounds for oilseeds<br />
and 1,220 pounds for nonoilseed sunfl ower (Figure 5).<br />
Pounds of <strong>Production</strong><br />
U.S. production of oilseed sunfl ower ranged from<br />
1,763 million pounds (799,700 metric tons) in 2004 to<br />
4,486 million pounds (2,035,000 metric tons) in 1998<br />
(Table 5). Nonoilseed production ranged from 286<br />
million pounds (130,000 metric tons) in 2004 to 844<br />
million pounds (383,000 metric tons) in 1999.<br />
Processing Plants<br />
Four oil extraction plants in North Dakota, Minnesota<br />
and Kansas process oilseed sunfl ower. These<br />
four plants have a combined crushing capacity of<br />
1,900,000 metric tons per year, according to industry<br />
estimates. Several smaller plants are located throughout<br />
the main sunfl ower production region.<br />
1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006<br />
North Dakota 1,590 1,450 1,180 1,470 1,990 1,700 1,330 1,070 1,370 1,210 880 1,140 900<br />
South Dakota 940 960 700 825 940 920 720 715 640 505 435 550 535<br />
Kansas 260 300 265 200 180 280 250 335 193 215 171 300 152<br />
Minnesota 500 440 150 105 130 130 95 60 70 90 60 135 90<br />
Colorado 100 115 110 85 160 270 220 195 130 130 135 215 100<br />
Texas 34 44 31 88 47 75 60 108 35 59 41 145 54<br />
Nebraska 75 90 47 55 70 101 90 82 60 66 56 99 53<br />
Other States 68 79 53 60 51 77 75 68 60 91 95 125 100<br />
Total U.S.<br />
Thousand Acres<br />
3,567 3,478 2,536 2,888 3,568 3,553 2,840 2,633 2,580 2,344 1,873 2,709 1,984<br />
Table 4. Harvested USA Sunfl ower Acreage 1994-2006<br />
1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006<br />
Oilseed 2,943 2,829 1,934 2,212 2,897 2,695 2,116 2,060 1,815 1,874 1,424 2,032 1,587<br />
Nonoilseed 487 539 545 580 595 746 531 495 365 323 287 578 277<br />
Total<br />
Thousand Acres<br />
3,430 3,368 2,479 2,792 3,492 3,441 2,647 2,555 2,180 2,197 1,711 2,610 1,864<br />
<strong>Production</strong><br />
7
8<br />
Table 5. U.S. Sunfl ower <strong>Production</strong> 1994-2006<br />
■ Figure 5. Average U.S. Sunfl ower Yield 1996-2005<br />
in Pounds Per Acre.<br />
Prices<br />
1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006<br />
Oilseed<br />
---------------------------------------------------- Million Pounds ----------------------------------------------------<br />
4,223 3,398 2,844 2,986 4,486 3,498 2,910 2,804 2,070 2,260 1,763 3,178 1818<br />
Nonoilseed 612 611 716 691 787 844 635 615 420 406 286 841 295<br />
Total 4,835 4,009 3,560 3,677 5,273 4,342 3,545 3,419 2,490 2,666 2,049 4,018 2113<br />
Historically, sunfl ower depended heavily on the<br />
export market for either seed or oil. With the advent<br />
of NuSun and high oleic sunfl ower, the market has<br />
switched almost exclusively to a U.S. and Canadian<br />
market. Both of these oils are very stable and do not<br />
require hydrogenation as do competitive oils, such<br />
as traditional soybean and canola oils, when used in<br />
a frying application. Sunfl ower prices now are more<br />
determined by their relationship to corn oil prices.<br />
Large domestic users tend to buy in advance, thus<br />
prices are not directly affected by the Chicago soybean<br />
oil contract and are not as likely to be as volatile.<br />
More opportunities are available to presell a portion<br />
of the crop well before planting begins. This ensures<br />
a domestic user of a supply and allows a producer to<br />
“lock in” a price for a portion of his production. Storage<br />
of sunfl ower is necessary. The domestic market<br />
needs a 12-month supply of oil and crushers will need<br />
a steady supply of seed. Crushers likely will have to<br />
provide producers with storage premiums for delivery<br />
in the out-of-harvest months. Oilseed sunfl ower producers<br />
have the advantage of multiple market options:<br />
the hulling market, the crush market or the bird food<br />
market. Supply and demand drive prices in all three<br />
markets. These markets are very specifi c and unique,<br />
with different values associated with them. Farmers<br />
should have samples of their crop graded to determine<br />
quality and talk directly to buyers to fi nd out what<br />
they want in terms of seed specifi cations.<br />
Nonoilseed (confection) sunfl ower production is<br />
geared to the “in-shell” markets. Today’s confection<br />
hybrids produce a signifi cant level of large seeds.<br />
Growers often are paid on a percentage of large seed.<br />
Quality standards for confection sunfl ower are high<br />
and allow little tolerance for off-color and insect<br />
damage. Most confection sunfl ower is produced on<br />
a contract basis. The seasonal average price during<br />
the 1994/95 to 2002/03 period ranged from $5.89 to<br />
$12.30 per hundredweight for oilseed sunfl ower and<br />
from $11.90 to $15.20 per hundredweight for nonoilseed<br />
sunfl ower. During that period, the nonoilseed<br />
price exceeded the oilseed price by $3.85 per hundredweight<br />
on average.
Sunfl ower Marketing Strategy<br />
(George Flaskerud)<br />
Sunfl ower marketing strategies usually use the cash<br />
forward contract for locking in a price prior to harvest.<br />
Use of this contract may be appropriate on a portion<br />
of the sunfl ower crop, but so may the use of other marketing<br />
tools, such as hedging with futures or use of<br />
options (puts or calls). The best marketing alternative<br />
depends in part on the basis, which is the relationship<br />
between a cash and futures price.<br />
Since a sunfl ower futures market does not exist, relationships<br />
between the sunfl ower cash price and other<br />
closely related futures markets need to be considered.<br />
Using the futures market of a different commodity for<br />
hedging is cross-hedging, while the cash and futures<br />
price relationship is the cross-basis. Two futures<br />
contracts are examined: soybean oil futures, which<br />
are traded on the Chicago Board of Trade, and canola<br />
futures, which are traded on the Winnipeg Commodity<br />
Exchange.<br />
Historic prices were analyzed during 1997 through<br />
April 2004 to identify patterns and relationships<br />
useful for developing marketing strategies. Prices<br />
were standardized in U.S. dollars per hundredweight<br />
(US$/cwt).<br />
Correlations indicate that changes in NuSun prices<br />
(40 percent oil) at Enderlin, N. D., are the most closely<br />
correlated with canola futures (correlation = .91). Soybean<br />
oil futures were a distant, second-best correlation<br />
(.75). These correlations suggest that canola futures<br />
should provide the most risk reduction for cross-hedging<br />
cash sunfl ower prices. However, with the current<br />
situation for sunfl owers, soybean oil and canola need<br />
to be evaluated to determine which futures contract<br />
likely is to be the most profi table.<br />
Price quotations for canola futures are in Canadian<br />
dollars (C$) per metric ton. The price quotation for<br />
November canola was C$352 on August 25, 2003,<br />
and the exchange rate was 1.41 C$/US$. In U.S.<br />
dollars per hundredweight, this quotation would be<br />
US$11.32 (C$352 divided by 1.41 divided by 22.046<br />
= US$11.32).<br />
Seasonal patterns for Enderlin NuSun prices (Figure<br />
6) revealed a broad range of price behavior during individual<br />
marketing years (October-September). Highs<br />
occurred during August in 2000-01 and 2001-02,<br />
April in 1999-00, October in 1998-99 and November<br />
in 2002-03. The distribution of prices reveals that the<br />
pattern, on average, is to decline to lows in October<br />
and then increase to a peak in June before declining<br />
into the next marketing year.<br />
The range in the monthly average, excluding the low<br />
and the high, was only $.53 per cwt. The within-year<br />
variations were considerably greater. The average<br />
within-year range was $3.07. During 2000-01 and<br />
2001-02, when prices trended up, the average withinyear<br />
range was $3.98. During the other years, the<br />
average within-year range was $2.47.<br />
The tendency for the Enderlin NuSun cross-basis<br />
relative to canola futures (Figure 7) was to decline to a<br />
low in September and to remain nearly as low during<br />
October and November, and then to increase to a high<br />
in February-April before generally declining into the<br />
end of the marketing year. During three of the fi ve<br />
years, the cross-basis was near its low in October. The<br />
range of the cross-basis was the narrowest during September<br />
and the widest during May. During October,<br />
the average cross-basis per cwt ranged from -$1.64 to<br />
-$.16 and averaged -$.99.<br />
Relative to soybean oil futures, the Enderlin average<br />
cross-basis showed a pattern of marketing year lows<br />
per cwt in November (-$9.15), April (-$9.25) and September<br />
(-$9.16) and highs in February (-$8.08) and<br />
July (-$8.30). During October, the average cross-basis<br />
ranged from -$14.47 to -$6.59 and averaged -$8.95.<br />
■ Figure 6. Seasonal Behavior of NuSun Prices at<br />
Enderlin, ND.<br />
<strong>Production</strong><br />
9
10<br />
■ Figure 7. NuSun Cross-Basis at Enderlin, ND<br />
Relative to Nearby WCE Canola Futures.<br />
Variability, as measured by the standard deviation, was<br />
greater for the cross-basis relative to soybean oil futures<br />
than for the cross-basis relative to canola futures.<br />
This suggests lower basis risk when cross-hedging<br />
with canola futures than with soybean oil futures.<br />
From this information, marketing strategies can be<br />
developed. The seasonal pattern for Enderlin NuSun<br />
prices suggests that preharvest sales should be considered<br />
when prices are above the fi ve-year average<br />
price. Prices declined into harvest during two of three<br />
years that prices were above the average early in the<br />
marketing year.<br />
Use of the cash forward contract or cross-hedge would<br />
be appropriate on that portion of the sunfl ower crop<br />
that can be safely produced, i.e., on 20 percent to 40<br />
percent of the crop. The cash forward contract would<br />
be preferred if it refl ects an average or better cross-basis<br />
relative to sunfl ower oil futures or canola futures.<br />
A greater portion of the crop could be sold on a cash<br />
forward contract if it includes an act-of-God clause.<br />
In addition to the cash forward contract or crosshedge,<br />
a call option could be purchased to preserve<br />
upside potential. In the case of the cross-hedge, the<br />
call option would be purchased in the same futures<br />
contract. In the case of the cash forward contract, the<br />
call option could be purchased in either the soybean<br />
oil futures or canola futures. The put option is an alternative<br />
to using a cash forward contract or cross-hedge<br />
in combination with a call option.<br />
For sunfl owers that are not cash forward contracted,<br />
storage is an alternative. On average, storage was profitable<br />
during the 1998-99 to 2002-03 marketing years.<br />
However, the most profi table period of storage varied<br />
considerably. The most profi table sell or store strategy<br />
was to sell the 1998 crop at harvest, store the 1999<br />
crop until January, store the 2000 and 2001 crops until<br />
August and store the 2002 crop for one month. Sell or<br />
store decisions are diffi cult and require frequent evaluation<br />
of fundamentals, cash prices, futures prices,<br />
basis and storage costs.<br />
Additional marketing alternatives are available but beyond<br />
the scope of this article. Further information can<br />
be found in NDSU Extension publication EC-1270,<br />
“Managing Sunfl ower Price Risk.”
Hybrid Selection and<br />
<strong>Production</strong> Practices<br />
Hybrid Selection<br />
(Jerry Miller)<br />
Selection of sunfl ower hybrids to plant is one of the<br />
most important decisions a producer must make<br />
each season (Figure 8). First, three classes of hybrids<br />
- NuSun oilseed, traditional oilseed and confection<br />
hybrids are available,. Second, variables such as yield,<br />
quality factors, maturity, dry down, standability, and<br />
pest and disease tolerance, should be considered.<br />
NuSun Sunfl ower<br />
NuSun oilseed sunfl ower hybrids will produce an oil<br />
quality with more than 55 percent oleic fatty acid.<br />
This oil is in wide demand by the frying food industry<br />
and potentially could be a bottled oil. Some hybrid<br />
seed companies are providing a grower guarantee that<br />
their hybrids will make the minimum oleic grade.<br />
Some processors of NuSun sunfl ower also are providing<br />
contracts for producing seed of this quality. A<br />
premium may be paid to producers for planting NuSun<br />
hybrids.<br />
Traditional Sunfl ower<br />
Traditional oilseed sunfl ower hybrids have a high<br />
linoleic and lower oleic fatty acid quality in contrast<br />
with the NuSun hybrids. Traditional hybrids have been<br />
grown for their multipurpose marketability, with large<br />
export demand and hulling for the kernel market being<br />
most important.<br />
Confection Sunfl ower<br />
Confection sunfl ower hybrids are used primarily for<br />
in-shell and hulled kernel markets. They are characterized<br />
by having large seed, with a distinctive color<br />
striping on the hull. New hybrids with very long, large<br />
seed are in demand for the export market. Producers<br />
must be careful to set their combine concave widths<br />
properly to avoid hull damage on these hybrids. Producers<br />
generally plant confection hybrids at a lower<br />
plant population and increase insect scouting and<br />
control to maintain high kernel quality. Contracts are<br />
available to producers interested in planting confection<br />
hybrids.<br />
Criteria for Hybrid Selection<br />
Growers should use several criteria in hybrid selection.<br />
First, they should take an inventory of available hybrids<br />
being marketed in their area. Seed yield potential<br />
is an important trait to consider when looking at an<br />
available hybrid list. Yield trial results from university<br />
experiment stations, National Sunfl ower Associationsponsored<br />
trials and commercial companies should<br />
identify a dozen or so consistently high yielding<br />
hybrids for a particular area. Results from strip tests or<br />
demonstration plots on or near growers’ farms should<br />
■ Figure 8. A hybrid seed production fi eld of<br />
sunfl ower. Female and male parents are planted in<br />
alternate strips across the fi eld. (Marcia P. McMullen)<br />
Hybrid Selection<br />
11
12<br />
be evaluated. Yield results from previous years on an<br />
individual’s farm and information from neighbors also<br />
are valuable. The best producing hybrids in a region<br />
may produce approximately 2,300 pounds per acre<br />
with good soil fertility and favorable soil moisture, or<br />
more than 3,000 pounds per acre in the most favorable<br />
growing conditions.<br />
Oil percentage should be another trait to consider in<br />
oilseed hybrid selection. Several environmental factors<br />
infl uence oil percentage, but the hybrid’s genetic<br />
potential for oil percentage also is important. Current<br />
hybrids have oil percentages ranging from 38 percent<br />
to more than 50 percent. Domestic oil processors have<br />
been paying a premium based on market price for<br />
more than 40 percent oil (at 10 percent moisture) and<br />
discounts for oil less than 40 percent. Current recommendations<br />
are to select a high-oil hybrid instead of a<br />
low-oil hybrid with the same yield potential, but don’t<br />
sacrifi ce yield in favor of oil content.<br />
Maturity and dry down are important characteristics to<br />
consider when deciding what hybrid to plant. Maturity<br />
is especially important if planting is delayed, being<br />
mindful of the average killing frost in your area. Yield,<br />
oil content and test weight often are reduced when a<br />
hybrid is damaged by frost before it is fully mature.<br />
An earlier hybrid likely will be drier at harvest than a<br />
later hybrid, thus reducing drying costs. Also, consider<br />
planting hybrids with different maturity dates as a production<br />
hedge to spread risk and workload at harvest.<br />
The most economical and effective means to control<br />
sunfl ower diseases and other pests is planting resistant<br />
or tolerant hybrids and considering a minimum<br />
of three to four years’ rotation between successive<br />
sunfl ower crops. Hybrids are available with resistance<br />
to rust, Verticillium wilt and certain races of downy<br />
mildew. New hybrids may be available with tolerance<br />
to Sclerotinia head and stalk disease. Growers should<br />
check with their local seed dealer or sunfl ower seed<br />
company representative to obtain this information.<br />
Stalk quality, another trait to consider, provides resistance<br />
to lodging, various diseases and other pests. Hybrids<br />
with good stalk quality are easier to harvest and<br />
yield losses generally are reduced, withstanding damages<br />
from pests and high winds. Uniform stalk height<br />
at maturity is another important trait to consider.<br />
Hybrid selection may include selecting a hybrid with<br />
resistance to certain herbicides not previously available.<br />
This nontransgenic resistance either was derived<br />
from the wild species of sunfl ower or from mutagenesis.<br />
Sunfl ower hybrids can be sprayed with herbicides<br />
that control various broadleaf and grassy weeds either<br />
by one chemical or by a tank-mix of two chemicals.<br />
This technology will allow broad-spectrum weed control<br />
in minimum-till or no-till sunfl ower production,<br />
as well as with traditional production. Growers should<br />
check with their local seed dealer or sunfl ower seed<br />
company representative to obtain information regarding<br />
availability of these hybrids.<br />
The last item to consider is to purchase hybrid seed<br />
from a reputable seed company and dealer with a good<br />
technical service record. This is particularly important<br />
if producers have any questions regarding production<br />
practices. Companies and seed dealers provide different<br />
services, policies and purchase incentives, including<br />
credit, delivery service and returns.<br />
Semidwarf Sunfl ower<br />
(Duane Berglund)<br />
Semidwarf sunfl ower is 25 percent to 35 percent shorter<br />
than normal height hybrid sunfl ower. Research results<br />
show seed yield and oil content of semidwarf and<br />
normal height sunfl ower are similar in some years but<br />
not always. In drought stress years, seed yield of semidwarf<br />
sunfl ower was signifi cantly less than normalheight<br />
hybrids. Most semidwarf sunfl ower have early<br />
maturity ratings, thus the potential for high yields is<br />
limited, compared with conventional-height sunfl ower.<br />
The semidwarf plant types appear to be less susceptible<br />
to lodging, which could be very important during<br />
years of optimum plant growth or where sunfl ower<br />
is grown under irrigation, in high-plant populations.<br />
Generally, the semidwarf sunfl ower can be planted<br />
in narrowly spaced rows or solid seeded. University<br />
research indicates root penetration and water use<br />
to a depth of 6 feet is similar for normal height and<br />
semidwarf sunfl ower. Beyond 6 feet, root penetration<br />
of the semidwarf may not be as great as that of taller<br />
plant types. Some sunfl ower breeders have observed<br />
that short-stature plants have demonstrated limitations<br />
in head size and ability of the plant to fi ll the center of<br />
the head. Also, slower seedling emergence has been<br />
reported for semidwarfs.
Sunfl ower Branching<br />
(Duane Berglund)<br />
Sunfl ower branching is an undesirable trait in commercial<br />
sunfl ower production. It can be caused by the<br />
genetics of a hybrid, environmental infl uences and<br />
herbicide injury.<br />
Branching of various degrees can occur in sunfl ower,<br />
ranging from a single stem with a large single infl orescence<br />
in cultivated types to multiple branching<br />
from axils of most leaves on the main stem in the wild<br />
species. Branch length varies from a few centimeters<br />
to a distance longer than the main stem. Branching<br />
may be concentrated at the base or top of the stem or<br />
spread throughout the entire plant. Generally, heads<br />
on branches are smaller than heads on the main stem.<br />
Occasionally, some fi rst-order branches have a terminal<br />
head almost as large as the main head. In most<br />
wild species, the head on the main stem blooms fi rst,<br />
but generally is no larger than those on the branches.<br />
Studies on the genetics of top branching have shown<br />
that it is dominant over nonbranching and is controlled<br />
by a single gene. Sunfl ower literature reports that top<br />
branching in cultivated sunfl ower is controlled by a<br />
single dominant gene, but branching in wild species is<br />
controlled by duplicate dominant genes.<br />
Source: Sunfl ower Technology and <strong>Production</strong>,<br />
ASA monograph number 35.<br />
■ Figure 9. Soil Tests are the most reliable means<br />
for growers to determine fertilizer needs to obtain<br />
projected yield goals. (Dave Franzen)<br />
<strong>Production</strong> Practices<br />
Seed Quality<br />
(Duane Berglund)<br />
High quality, uniform seed with high germination,<br />
known hybrid varietal purity and freedom from weed<br />
seeds and disease should be selected to reduce production<br />
risks. The standard germination test provides an<br />
indication of performance under ideal conditions but<br />
is limited in its ability to estimate what will happen<br />
under stress. Accelerated aging is another method<br />
used to evaluate seed vigor. Any old or carry-over<br />
seed should have both types of tests conducted. Seed<br />
is sold on a bag weight basis or by seed count. Seed<br />
size designations are fairly uniform across companies.<br />
Most seed is treated with a fungicide and insecticide<br />
to protect the germinating seedling. Seed should be<br />
uniformly sized to allow precision in the planting<br />
operation.<br />
Soils<br />
(David Franzen)<br />
Sunfl ower is adapted to a variety of soil conditions,<br />
but grows best on well-drained, high water-holding<br />
capacity soils with a nearly neutral pH (pH 6.5-7.5).<br />
<strong>Production</strong> performance on high-stress soils, such as<br />
those affected by drought potential, salinity or wetness,<br />
is not exceptional but compares favorably with<br />
other commercial crops commonly grown.<br />
Soil Fertility<br />
(David Franzen)<br />
Sunfl ower, like other green plants, requires at least 16<br />
elements for growth. Some of these, such as oxygen,<br />
hydrogen and carbon, are obtained from water and the<br />
air. The other nutrients are obtained from the soil. Nitrogen,<br />
phosphorus and sulfur are frequently defi cient<br />
in soils in any climatic zone. Potassium, calcium and<br />
magnesium are frequently defi cient in high-rainfall<br />
areas. Defi ciencies of iron, manganese, zinc, copper,<br />
molybdenum, boron and chlorine are uncommon but<br />
can appear in many climatic zones.<br />
A sunfl ower yield of 2,000 pounds per acre requires<br />
approximately the same amount of nitrogen, phosphorus<br />
and potassium as 40 bushels per acre of wheat.<br />
Hybrid Selection and <strong>Production</strong> Practices<br />
13
14<br />
The nutrient content of the soil, as determined by a<br />
soil test, is the only practical way to predict probability<br />
of a response to applied nutrients (Figure 9). A soil<br />
test will evaluate the available nutrients in the soil and<br />
classify the soil as very low (VL), low (L), medium<br />
(M), high (H) or very high (VH) in certain nutrients.<br />
A fi eld classifi ed as very low in a nutrient will give a<br />
yield response to applied fertilizer 80 percent to 100<br />
percent of the time. A yield response is not always<br />
obtained because soil moisture or some other environmental<br />
factor may become limiting. A fi eld classifi ed<br />
as low will respond to applied fertilizer 40 percent<br />
to 60 percent of the time, a medium testing fi eld will<br />
respond to added fertilizer 10 percent to 20 percent<br />
of the time and a high-testing fi eld will respond to<br />
applied fertilizer only occasionally. <strong>Field</strong>s testing very<br />
high will not respond because the reserve of nutrients<br />
in the soil is adequate for optimum plant growth and<br />
performance.<br />
Fertilizer Recommendations<br />
(David Franzen)<br />
Soil tests have been developed to estimate sunfl ower’s<br />
potential response to fertilizer amendments. The most<br />
important factors in the fertilizer recommendations<br />
are the yield goal and the level of plant-available soil<br />
nutrients. In most climatic zones, predicting yield<br />
is impossible. Past yield records are a reasonable<br />
estimate of potential yield for the coming year. A yield<br />
goal for sunfl ower should be more optimistic than the<br />
average yield, and should approach the past maximum<br />
yield obtained by the grower on the same or a similar<br />
soil type. Nutrients not used by a crop in a dry growing<br />
season usually are not lost and can be used by the<br />
following crop.<br />
From an economic standpoint, having a yield goal that<br />
is somewhat high is much more benefi cial for a grower<br />
than having a goal that is too low. A low yield goal in<br />
a good growing season easily can mean lost income of<br />
$30 to $40 per acre. In contrast, a high yield goal in a<br />
dry growing season will result in a loss of only $1to<br />
$2 in additional interest on the cost of unused nutrients<br />
since most of the nutrients will be available to the<br />
subsequent crop.<br />
The amounts of nitrogen, phosphorus and potassium<br />
recommended for various sunfl ower yield goals and<br />
soil test levels are shown in Table 6. For yield goals<br />
not shown in the table, use the formulas at the base<br />
of the table. The data in this table are based on the<br />
amount of nitrate-nitrogen (NO 3 -N) in pounds per<br />
acre found in the top 2 feet of soil, the parts per million<br />
(ppm) of phosphorus (P) extracted from the top<br />
6 inches of soil by the 0.5N sodium bicarbonate, and<br />
the ppm of potassium (K) extracted by neutral normal<br />
ammonium acetate in the top 6 inches of soil<br />
Other nutrients are not usually defi cient for sunfl ower.<br />
On sandy slopes and hilltops, sulfur may be a problem;<br />
however, sulfur would not be expected to be defi -<br />
cient in higher organic matter, depressional soils. The<br />
sulfur soil test is a poor indicator of the probability of<br />
response to sulfur fertilizers. Sunfl ower has not been<br />
shown to be responsive to the application of other<br />
nutrients, including micronutrients in the state.<br />
Table 6. Nitrogen (N), phosphate (P 2 O 5 ) and<br />
potash (K 2 O) recommendations for sunfl ower in<br />
North Dakota.<br />
Yield<br />
Soil N<br />
plus<br />
fertilizer<br />
Soil Test Phosphorus, ppm<br />
VL L M H VH<br />
Goal N<br />
lb/acre-<br />
Bray-1 0-5 6-10 11-15 16-20 21+<br />
lb/acre 2 ft. Olsen 0-3 4-7 8-11 12-15 16+<br />
- - - - - - lb P2O5 /acre - - - - - -<br />
1,000 50 20 15 9 4 0<br />
1,500 75 31 22 14 5 0<br />
2,000 100 41 30 18 7 0<br />
2,500 125 51 37 23 9 0<br />
Nitrogen recommendation = 0.05 YG - STN - PCC<br />
(Bray-1) Phosphate recommendation = (0.0225-0.0011 STP)YG<br />
(Olsen) Phosphate recommendation = (0.0225-0.0014 STP)YG<br />
Yield<br />
Soil Test Potassium, ppm<br />
Goal 0-40 41-80 81-120 121-160 161+<br />
lb/acre VL L M H VH<br />
1,000 36 25 14 3 0<br />
1,500 53 37 21 5 0<br />
2,000 71 50 28 6 0<br />
2,500 89 62 35 8 0<br />
(K, ammonium acetate extractant) Potash recommendation =<br />
(0.04100-0.00027 STK)YG<br />
YG = Yield Goal<br />
PCC = Previous Crop Credit<br />
STN is the amount of NO 3 -N in the top 2 feet of soil<br />
STP, STK = Soil Test P or K, respectively<br />
VL,L,M, H,VH = very low, low, medium, high and very high,<br />
respectively
Fertilizer Application<br />
(Dave Franzen)<br />
Germinating sunfl ower seed is similar to corn in its<br />
reaction to seed-placed fertilizer. Application of more<br />
than 10 pounds per acre of nitrogen (N) plus potash<br />
(K 2 O) in a 30-inch row will result in reduced stands<br />
or injured seedlings. Dry soil conditions can increase<br />
the severity of injury. In row widths narrower than<br />
30 inches, rates of N plus K 2 O can be proportionally<br />
higher. For improved fertilizer rate fl exibility, starter<br />
fertilizer should be placed in bands at least 2 to 3<br />
inches from the seed row.<br />
Producers have several good reasons to apply nitrogen<br />
in the fall, such as availability of labor, soil conditions,<br />
etc. However, the general principle with respect<br />
to nitrogen application is: The longer the time period<br />
between application and plant use, the greater the<br />
possibility for N loss. In other words, use judgment<br />
in making a decision on time of N application. In the<br />
case of sandy soils, fall application of N is not recommended.<br />
In many instances, a side-dress application of<br />
N when the sunfl ower plants are about 12 inches high<br />
may be preferable.<br />
Phosphate and potash may be fall or spring applied<br />
before a tillage operation. These nutrients are not<br />
readily leached from soil because they form only<br />
slightly soluble compounds or attach to the soil. The<br />
phosphate and potash recommendations in Table 6.<br />
are broadcast amounts. The recommendations for soil<br />
that tests very low and low in P and K can be reduced<br />
by one-third the amount in the table when applied in a<br />
band at seeding. In minimum or no-till systems, phosphate<br />
and potash may be applied in a deeper band to<br />
reduce the buildup of nutrients at the soil surface that<br />
occurs with these systems. However, most comparisons<br />
among deep, shallow and surface applications<br />
have shown little difference in crop response.<br />
Water Requirements for Sunfl ower<br />
(Duane Berglund)<br />
Sunfl ower has deep roots and extracts water from<br />
depths not reached by most other crops; thus it is<br />
perceived to be a drought-tolerant crop. Sunfl ower has<br />
an effective root depth around 4 feet, but can remove<br />
water from below this depth. Research on side-by-side<br />
plots has shown that sunfl ower is capable of extracting<br />
more water than corn from an equal root zone volume.<br />
With its deep root system, it also can use nitrogen and<br />
other nutrients that leach below shallow-root crops;<br />
thus it is a good crop to have in a rotation.<br />
Seasonal water use by sunfl ower averages about 19<br />
inches under irrigated conditions. Under dryland<br />
conditions, sunfl ower will use whatever stored soil<br />
moisture and rain that it receives during the growing<br />
season. When access to water is not limited, small<br />
grains use 2 to 3 inches less total water than sunfl ower<br />
during the growing season, whereas soybean water use<br />
is slightly greater. Corn uses 1 to 4 inches, and sugar<br />
beets use 2 to 6 inches more than sunfl ower, respectively,<br />
during the growing season.<br />
These total water use values are typical for nondrought<br />
conditions in southeastern North Dakota. Small grains<br />
use the least total water since they have the fewest<br />
number of days from emergence to maturity. Sunfl ower<br />
and soybean have an intermediate number of days<br />
of active growth and corresponding relative water use.<br />
Corn ranks above sunfl ower in growth days and water<br />
use, while sugar beets rank highest in both categories.<br />
However, water use effi ciency does vary among these<br />
crops. Comparative water use effi ciency measured as<br />
grain (pounds per acre or lb/A) per inch of water used<br />
on three dryland sites and two years in eastern North<br />
Dakota was 119, 222, 307, 41, 218, 138, and 127 for<br />
sunfl ower, barley, grain corn, fl ax, pinto bean, soybean<br />
and wheat, respectively. These results indicated that<br />
corn had the highest water use effi ciency, sunfl ower<br />
and wheat were intermediate and fl ax the lowest.<br />
(Source: M. Ennen. 1979. Sunfl ower water use in<br />
eastern North Dakota, M.S. thesis, North Dakota State<br />
University).<br />
Fertility has little infl uence on total water use, but<br />
as fertility increases, water use effi ciency increases<br />
because yield increases. Yield performance has been<br />
shown to be a good indicator of water use effi ciency<br />
of sunfl ower hybrids; higher yielding hybrids exhibit<br />
the highest water use effi ciency.<br />
<strong>Production</strong> Practices<br />
15
16<br />
Soil Water Management<br />
for Dryland Sunfl ower<br />
(Duane Berglund)<br />
Management practices that promote infi ltration of<br />
water in the soil and limit evaporation from the soil<br />
generally will be benefi cial for sunfl ower production<br />
in terms of available soil moisture. Leaving stubble<br />
during the winter to catch snow and minimum tillage<br />
are examples. Good weed control also conserves<br />
moisture for the crop. The use of post-applied and preemergence<br />
herbicides with no soil incorporation also<br />
conserves moisture when growing sunfl ower.<br />
Sunfl ower has the ability to exploit a large rooting<br />
volume for soil water. <strong>Field</strong>s for sunfl ower production<br />
should be selected from those with the greater waterholding<br />
capacity and soils without layers that may<br />
restrict roots. Water-holding capacity depends mainly<br />
on soil texture and soil depth. The loam, silt loam,<br />
clay loam and silty clay loam textures have the highest<br />
water-holding capacities. Water-holding capacity of<br />
the soils in any fi eld can be obtained from county soil<br />
survey information available from local Natural Resources<br />
Conservation Service (NRCS) USDA offi ces.<br />
Sampling or probing for available soil moisture before<br />
planting also can help select fi elds for sunfl ower<br />
production. With other factors being equal, fi elds with<br />
the most stored soil moisture will have potential for<br />
higher yields. Where surface runoff can be reduced<br />
or snow entrapment increased by tillage or residue<br />
management, increases in stored soil moisture should<br />
occur and be benefi cial to a deep-rooted crop such as<br />
sunfl ower.<br />
Irrigation Management<br />
(Tom Scherer)<br />
Irrigation of sunfl ower by commercial growers is not<br />
common, but sunfl ower will respond to irrigation.<br />
Data collected by the USDA Farm Service Agency<br />
(FSA) for irrigated crops in North Dakota shows that<br />
an annual average of about 1,500 acres of sunfl ower<br />
are irrigated each year. Data for irrigated and dryland<br />
oil-type variety trials between 1975 and 1994 from the<br />
Carrington Research Extension Center show an aver-<br />
age yield differential of about 500 pounds per acre.<br />
However, some years the irrigated trials yielded more<br />
than 1,500 pounds per acre more than the dryland<br />
plots, and some years the dryland plots actually had<br />
greater yield than the irrigated plots.<br />
Irrigated sunfl ower seasonal water use averages about<br />
19 inches. With good water management, average<br />
water use will increase from about 0.03 inch per day<br />
soon after emergence to more than 0.27 inch per day<br />
from head emergence to full seed head development.<br />
However, during July and August, water use on a hot,<br />
windy day can exceed 0.32 inch.<br />
Research by Stegman and Lemert of NDSU has<br />
demonstrated the yield potential of sunfl ower grown<br />
under optimum moisture conditions and the effect of<br />
water stress at different growth stages. Sunfl ower yield<br />
is most sensitive to moisture stress during the fl owering<br />
period (R-2 to R-5.9 reproductive stages) and least<br />
sensitive during the vegetative period (emergence to<br />
early bud). A 20 percent reduction of irrigation water<br />
application from plant emergence to the R-2 stage<br />
resulted in only a 5 percent reduction in yield, but a<br />
20 percent reduction in irrigation water application<br />
during the R-2 to R-5.9 period resulted in a 50 percent<br />
yield reduction.<br />
If soil water content is near fi eld capacity at planting,<br />
research indicates that the fi rst irrigation could be<br />
delayed until the root zone soil moisture is about 70<br />
percent depleted. However, if pumping capacity is low<br />
(less than 800 gallons per minute, or gpm, for a 128acre<br />
center pivot), a lesser depletion is advisable due<br />
to inadequate “catch-up capacity.” Irrigations during<br />
the critical bud to ray-petal appearance (R-2 to R-5.0)<br />
period should be scheduled to maintain a low soil<br />
moisture stress condition (35 percent to 40 percent<br />
depletion). Irrigation should be avoided from R-5.1<br />
to R-5.9 because of the susceptibility of the sunfl ower<br />
plant to head rot from Sclerotinia (white mold). Irrigate<br />
just before fl owering in the bud stages R-3 to<br />
R-4. Soil moisture depletion again can approach 70<br />
percent during late seed fi ll and beyond with little or<br />
no depression in yield.<br />
Yield increases due to irrigation depend on several<br />
factors. Soil water-holding capacity and precipitation<br />
are two of the most important. Research indicates that<br />
the seed yield versus crop water use (ET) exhibits a<br />
linear relationship with a slope averaging 190 pounds<br />
per acre-inch. This means every additional inch of
water applied by irrigation will increase seed yield<br />
by about 190 pounds per acre. Remember that the<br />
research was performed on the loam and sandy-loam<br />
soils of Carrington and Oakes, N.D. A yield increase<br />
of 50 percent or more with irrigation may be expected<br />
almost every year on coarse-textured soils. However, a<br />
seed yield increase from irrigation may not always occur<br />
on soils with higher water-holding capacities and<br />
with adequate precipitation. Adequate soil fertility is<br />
very important in achieving the higher yield potential<br />
under irrigation.<br />
Management of applied irrigation water requires the<br />
combination of periodic soil moisture measurement<br />
with a method of irrigation scheduling. Soil moisture<br />
can be measured or estimated in a variety of ways. The<br />
simplest is the traditional “feel” method that is an art<br />
developed through time with extensive use and experience.<br />
For most irrigation water management applications,<br />
either the resistance block type of soil moisture<br />
measurement or tensiometers should be used. These<br />
are relatively inexpensive and require little labor to<br />
use effectively.<br />
The soil water balance method of irrigation scheduling,<br />
otherwise known as the checkbook method, is<br />
popular and well-documented. With this method,<br />
a continuous account is kept of the water stored in<br />
the soil. Soil water losses due to crop use and soil<br />
surface evaporation are estimated each day based on<br />
the maximum temperature and the days since crop<br />
emergence. Precipitation and irrigation are measured<br />
and added to the soil water account each day. Errors in<br />
estimating water use will accumulate through time, so<br />
periodically measuring the moisture in the soil profi le<br />
is necessary. Detailed instructions for using the checkbook<br />
method are published (North Dakota Extension<br />
publication AE-792). A computer program using this<br />
method also is available from the NDSU Agricultural<br />
and Biosystems Engineering Department.<br />
Another form of irrigation scheduling is to use estimated<br />
daily water use values for sunfl ower (Table 7).<br />
This method, sometimes called the “water use replacement<br />
method,” is based on obtaining daily estimates<br />
of sunfl ower water use and accurately measuring the<br />
amount of rain received on the fi eld. Irrigations are<br />
scheduled to replace the amount of soil moisture used<br />
by the sunfl ower minus the amount of rain received<br />
since the last irrigation. Estimates of daily sunfl ower<br />
water use can be obtained several ways. AE-792 has<br />
a table for sunfl ower water use throughout the season<br />
based on weeks’ past emergence and maximum daily<br />
air temperature. More accurate sunfl ower water use<br />
values, based on measured weather variables from<br />
the North Dakota Agricultural Weather Network<br />
(NDAWN), are available at the NDAWN Web site:<br />
http://ndawn.ndsu.nodak.edu/. Click on “Applications”<br />
on the left side of the home screen. Sunfl ower crop<br />
water use estimates can be obtained for the current<br />
growing season (between emergence and harvest)<br />
or for past growing seasons if you want to do some<br />
comparisons.<br />
Table 7. Average daily water use for sunfl ower in inches per day based on maximum daily air<br />
temperature and weeks past emergence. For example, during the eighth week after emergence,<br />
if the daily air temperature were 85 degrees on a particular day, sunfl ower water use for that<br />
day would be 0.25 inch.<br />
Maximum<br />
Daily Air<br />
Temperature<br />
Week After Emergence<br />
°F 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15<br />
50 to 59 0.01 0.03 0.05 0.06 0.08 0.08 0.08 0.08 0.08 0.08 0.08 0.07 0.06 0.04 0.03<br />
60 to 69 0.02 0.05 0.08 0.10 0.12 0.14 0.14 0.14 0.13 0.13 0.13 0.12 0.10 0.07 0.04<br />
70 to 79 0.03 0.07 0.11 0.15 0.17 0.19 0.19 0.19 0.19 0.18 0.17 0.16 0.13 0.10 0.06<br />
80 to 89 0.03 0.09 0.14 0.19 0.22 0.25 0.25 0.25 0.24 0.23 0.22 0.21 0.17 0.13 0.07<br />
Above 90 0.04 0.11 0.17 0.23 0.27 0.30 0.30 0.30 0.29 0.29 0.27 0.26 0.21 0.15 0.09<br />
Bud Ray Flower 100% Ray<br />
Anther Petal<br />
Drop<br />
<strong>Production</strong> Practices<br />
17
18<br />
Tillage, Seedbed Preparation<br />
and Planting<br />
(Roger Ashley and Don Tanaka)<br />
Sunfl ower, like other crops, requires proper seedbed<br />
conditions for optimum plant establishment. Errors<br />
made at planting time may be compounded throughout<br />
the growing season. Seedbed preparation, soil tilth,<br />
planting date, planting depth, row width, seed distribution<br />
and plant population should be nearly correct as<br />
conditions permit.<br />
Tillage and Seedbed Preparation<br />
Tillage traditionally has been used to control weeds<br />
and incorporate herbicides in preparation for planting.<br />
When tillage is used in low rainfall areas, producers<br />
must take care to control weeds while leaving as much<br />
of the previous crop’s residue intact as possible. Tillage<br />
never should occur when soils are too wet. Soils<br />
that are tilled when too wet and then dry will crust,<br />
turn lumpy and generally provide for poor seedbed<br />
conditions for germination and establishment.<br />
Maintaining a moist seedbed is important if producers<br />
expect to have uniform germination and emergence<br />
across the fi eld (Figure 10). Poor germination<br />
and emergence will infl uence the need for and the<br />
effectiveness of future management practices. Excessive<br />
tillage should be avoided where tillage is used<br />
to prepare the seedbed or to incorporate preplant<br />
herbicides. Excessive tillage will break down soil<br />
structure, cause compaction and crusting problems,<br />
reduce aeration, restrict water movement and provide<br />
■ Figure 10. No-till one pass seeding systems<br />
preserve soil moisture and ground cover when water<br />
is limited. (Roger Ashley)<br />
conditions favorable for infection by downy mildew or<br />
other soil-borne diseases. Breakdown of soil structure<br />
also causes reduced nutrient and water uptake and<br />
reduces yield.<br />
Tillage and planting equipment is available to provide<br />
systems with varying levels of surface residue for<br />
sunfl ower production. <strong>Production</strong> systems can range<br />
from conventional-till, where the quantity of surface<br />
residue covers less than 30 percent of the soil surface,<br />
to no-till, where the quantity of surface residue covers<br />
more than 60 percent of the soil surface.<br />
Conventional-till <strong>Production</strong> Systems<br />
Conventional-till production systems usually involve<br />
two or more tillage operations for weed control, incorporation<br />
of pre-emergence herbicides and incorporation<br />
of the previous crops residues. Pre-emergence<br />
herbicides may be incorporated with a tandem disk,<br />
chisel or sweep plows, disk harrow, long-tine harrow,<br />
rolling harrow or air seeders with sweeps in different<br />
sequences or combinations. Tillage sequences are<br />
determined by herbicide label requirements by the<br />
quantity of crop residue present at the beginning of<br />
the tillage operation, and by the seedbed requirements<br />
needed to match planting equipment capabilities.<br />
Conventional tillage systems, with or without preemergence<br />
or post-emergence herbicide, may include<br />
the option of row cultivation once or twice during the<br />
early growing season before the sunfl ower reaches a<br />
height too tall for cultivation. A rotary hoe or harrow<br />
can be used just before sunfl ower emergence and/or at<br />
the V-4 to V-6 development stage. Harrowing or rotary<br />
hoeing between emergence and the V-4 stage can result<br />
in injury or death of the sunfl ower plant. Depending<br />
on planting depth and stage of crop development,<br />
stand losses are generally less than 5 percent if the<br />
sunfl ower crop has at least two fully expanded leaves.<br />
Proper adjustment of the harrow or rotary hoe will<br />
maximize damage to the weeds and minimize injury<br />
to the sunfl ower crop.<br />
Minimum-till <strong>Production</strong> Systems<br />
Minimum-till production systems use subsurface<br />
implements with wide sweeps, such as an undercutter<br />
(Figure 11), or harrow systems for application<br />
and incorporation of herbicides. These production<br />
systems leave between 30 percent and 60 percent of<br />
the soil surface covered by crop residue after planting.<br />
Minimum-tillage sunfl ower production systems rely
on a good long-term crop rotation to help control diffi<br />
cult weeds. These systems rely on two incorporations<br />
of granular herbicides, such as Trifl uralin 10G and<br />
Sonalan 10G. Herbicides can be applied in late fall<br />
(late October) or early spring (mid to late April) and<br />
incorporated with either an undercutter (at a soil depth<br />
of 2 inches) or a harrow system. A second incorporation<br />
just before or at sunfl ower planting is performed<br />
with an undercutter, harrow or air seeder with sweeps<br />
(Figure 12). The time between the application/fi rst<br />
incorporation and the second incorporation should<br />
be at least three weeks to increase the opportunity for<br />
precipitation to occur and rewet the treated soil layer.<br />
Rewetting the treated soil layer dissolves and activates<br />
the herbicide granules before the second incorporation.<br />
■ Figure 11. Undercutter sweep machine used to<br />
prepare seedbed, control small weeds and conserves<br />
soil moisture. (Al Black)<br />
■ Figure 12. Air-seeder with sweeps used for<br />
minimum till sunfl ower planting. (Roger Ashley)<br />
Air Drill Use<br />
Solid-seeded sunfl ower has become popular with<br />
producers in some regions. Air drills commonly are<br />
used to plant solid-seeded stands. Advantages listed by<br />
producers include 1) improved utilization of equipment<br />
already owned and 2) ease of changing between<br />
crops.<br />
Suggested adjustments to the air drill when planting<br />
sunfl ower include: 1) Use the proper metering roller;<br />
2) Slow the metering roller speed; 3) Calibrate the<br />
drill. Run through the calibration cycle10 times and<br />
then three additional times to check for consistently<br />
metered weights; 4) Recalibrate the drill every time<br />
variety or seed lot changes; 5) Reduce airfl ow. Provide<br />
the minimum amount of air to move seed and fertilizer<br />
to the opener so the seed is not damaged; 6) Don’t<br />
place all of your seed in the bin after the drill is calibrated.<br />
Place a couple of bags in the bin and run until<br />
the low seed light appears. Then place another bag in<br />
the hopper and run until the low seed light appears.<br />
Calculate the number of acres seeded. If you appear<br />
to be planting the correct seeding rate, place one more<br />
bag of seed in the hopper and run until the low seed<br />
light appears. If this seeding rate is correct, fi ll the<br />
seed bin and plant the rest of the fi eld.<br />
No-till <strong>Production</strong> Systems<br />
No-till is a production system without primary or<br />
secondary tillage prior to, during or after crop establishment.<br />
No-till systems rely heavily on diverse crop<br />
rotations and pre- and postemergence herbicides, and<br />
minimize soil disturbance to control weeds. Crop rotations<br />
should include cool-season grass and broadleaf<br />
crops, as well as warm-season grass and broadleaf<br />
crops. These production systems maintain at least 60<br />
percent surface covered by crop residue after planting.<br />
Crop residues protect soils from erosion, control<br />
weeds, suppress evaporation and improve soil water<br />
infi ltration. Pre- and postemergence herbicide choices<br />
provide producers options that were not available just<br />
a few years ago. Residual herbicides can be applied<br />
in a timely manner to get adequate precipitation for<br />
activation. Burn-down applications of glyphosate or<br />
paraquat are needed before or shortly after planting<br />
but before emergence of the crop to control emerged<br />
weeds and volunteer crop. Spartan (sulfentrazone), a<br />
residual broadleaf weed herbicide, may be tank-mixed<br />
with glyphosate to control broadleaf weeds for six to<br />
eight weeks. At the present time, postemergence herbi-<br />
<strong>Production</strong> Practices<br />
19
20<br />
cides such as Poast (sethoxydim) and Clethodim provide<br />
excellent grassy weed control and Assert (imazamethabenz)<br />
will control wild mustard. Clearfi eld<br />
sunfl ower varieties were selected for tolerance of the<br />
herbicide Beyond (imazamox) and will allow postemergence<br />
control of both broadleaf and grassy weeds.<br />
However, ALS (acetolactate synthase)-resistant kochia<br />
is not controlled with this herbicide. For current information<br />
on registered sunfl ower herbicides, contact<br />
your county agent or weed control specialist.<br />
Planting sunfl ower in a no-till production system may<br />
require the addition of residue managers to move<br />
a minimum of crop residue from the seed row so<br />
double-disc openers can place seed properly for seed<br />
to soil contact. Poor placement will delay or prevent<br />
germination, emergence and establishment of the<br />
crop. This, in turn, may lead to weed, insect and harvest<br />
management problems. Single-disc openers and<br />
narrow-point hoe openers have been used successfully<br />
to seed sunfl ower. Minimizing soil disturbance is important.<br />
Soil disturbance can bury weed seed, placing<br />
them in a favorable position to survive, germinate and<br />
establish. Weed seed left on the soil surface is exposed<br />
to weather, insects, birds and fungi and rapidly will<br />
lose viability. Low-disturbance no-till production systems<br />
provide the best opportunity for adequate seed<br />
zone soil water at planting.<br />
No-till and One-pass Seeding<br />
When low-disturbance openers, such as the single-disc<br />
style, are used, seed should be placed at least 2 inches<br />
deep. In undisturbed silt loam soils, the dry/wet inter-<br />
face usually will be found about 1 inch below the soil<br />
surface. In coarse-textured soils, this interface will be<br />
deeper. Planting at or just below this dry/wet interface<br />
will result in poor and/or uneven germination, resulting<br />
in either germinating seed running out of water<br />
and dying before the plant has a chance to establish<br />
or seed lying in dry soil until adequate rainfall is<br />
received.<br />
One-pass seeding operations utilizing high-disturbance<br />
openers (sweeps, hoes and narrow points) can<br />
produce uneven stands under dry conditions. Seeding<br />
depth is more variable with these types of openers,<br />
compared with the single-disc style, and moisture<br />
conditions will be more variable. Long, dry periods<br />
at planting do occur in western North Dakota. Soils<br />
will dry below acceptable levels for germination to<br />
the depth of the disturbed soil in the seedbed. Uneven<br />
germination, emergence, plant stands and plants at<br />
different stages of maturity will occur unless adequate<br />
moisture is received shortly after planting to rewet the<br />
seedbed.<br />
Anhydrous ammonia (Figure 13) sometimes is applied<br />
in one-pass seeding operations with openers specifi -<br />
cally designed for ammonia application at the time of<br />
seeding. If moisture is suffi cient and application rates<br />
do not exceed 50 pounds, little damage to germinating<br />
seed will occur. However, if the seedbed is abnormally<br />
dry and/or the soil does not seal properly between the<br />
anhydrous ammonia band and the seed, damage to<br />
germinating seed will occur.<br />
More recently, no-till row planters with row cleaners<br />
ahead of double-disc openers are equipped with either<br />
■ Figure 13. This one-pass seeding operation is seeding directly into wheat stubble.<br />
Anhydrous ammonia and seed are banded through special openers. Glyphosate and<br />
sulfentrazone were applied three days prior to seeding. (Roger Ashley)
a liquid or dry fertilizer attachment. These attachments<br />
band nitrogen fertilizers separately from the<br />
seed band, eliminating injury to the germinating seed.<br />
Planting Dates<br />
Sunfl ower may be planted during a wide range of<br />
dates. In the northern Great Plains, planting may<br />
extend from May 1 until late June. Early maturing<br />
hybrids should be selected for late planting or replanting<br />
in northern areas. Planting may be as early as two<br />
weeks before the last killing frost and as late as 100<br />
days before the fi rst killing frost in the fall.<br />
Growing conditions during the season will affect yield,<br />
oil content and fatty acid composition. High temperatures<br />
during seed formation have been identifi ed as<br />
the main environmental factor affecting the ratio of<br />
linoleic and oleic acid content. Therefore, the optimum<br />
planting date will be dependent upon the variety<br />
and location, as well as weather conditions during<br />
the growing season. Variety genetics also affect oleic<br />
content, so select adapted varieties for your area. High<br />
yields may be obtained from very early planting dates,<br />
but yields may be reduced by increased pest problems.<br />
If sunfl ower is seeded early in narrow rows and weeds<br />
are controlled early with preplant and post-plant herbicide<br />
products, early canopy closure should control<br />
late-germinating weeds, eliminating the need for herbicides<br />
or cultivation later in the season. Also, early<br />
planting provides producers the opportunity to harvest<br />
high quality seed earlier with less cost required for<br />
postharvest handling. Late-June plantings often result<br />
in lower yields and oil content. In addition, when harvest<br />
is delayed by weather, mechanical drying of seed<br />
■ Figure 14. Solid seeded sunfl ower stand<br />
established using an air drill. (Roger Ashley)<br />
is required, thus adding to production expenses. The<br />
fatty acid profi le also is affected by planting date. In a<br />
three-year planting date study in southwestern North<br />
Dakota, oleic fatty acid content was greatest when<br />
the planting date occurred around May 23 and lowest<br />
when the planting date was later than June 10.<br />
Soil temperature at the 4-inch depth should be at a<br />
minimum of 45 F for planting. A temperature near 50<br />
F is required for germination. Periods of soil temperature<br />
below 50 F delay germination and extend the<br />
period of susceptibility to seedling diseases, such as<br />
downy mildew, and to herbicide injury.<br />
Row Spacing and Plant Population<br />
Sunfl ower will perform well in a wide range of plant<br />
populations and plant spacing. When adequate weed<br />
control exists, no yield differences have been detected<br />
between sunfl ower seeded in rows and solid seeded.<br />
<strong>Field</strong>s with a row spacing of less than 20 inches are<br />
considered to be solid seeded (Figure 14). In 2003, the<br />
National Sunfl ower Association fi eld survey found 70<br />
percent of the sunfl ower fi elds surveyed in southwestern<br />
North Dakota to have been solid seeded. Equidistant<br />
spacing of seeds should produce a uniform sunfl<br />
ower stand, which makes maximum use of resources,<br />
such as water, nutrients and sunlight. Seed spacing to<br />
achieve the desired plant population is listed in Table<br />
8. Table 8 assumes seed germination is 90 percent<br />
and a 10 percent stand reduction will occur between<br />
emergence and harvest. The seed spacing must be<br />
adjusted with lower or higher germination rates, and<br />
thus spacing between seed.<br />
Desired seed spacing may be calculated using the following<br />
formula:<br />
SS = (6,272,640/RS)/(PP/(GR x SR)<br />
Where:<br />
SS = in row seed spacing in inches<br />
RS = between row spacing in inches<br />
PP = desired plant population at harvest<br />
GR = germination rate as a decimal. For example,<br />
if germination is 95 percent, then germination<br />
rate = .95.<br />
SR = stand reduction as a decimal. This reduction<br />
is a result of other factors between germination<br />
and fi nal harvest population. For example,<br />
if a 10 percent reduction is expected,<br />
then 100 percent - 10 percent = 90 percent,<br />
or .9.<br />
<strong>Production</strong> Practices<br />
21
22<br />
Sunfl ower plants will compensate for differences in<br />
plant population by adjusting seed and head size. As<br />
plant population decreases, seed and head size will<br />
increase. Oilseed hybrids generally are planted at<br />
higher populations than confection varieties, as the<br />
size of harvested seed is less important. Plant populations<br />
for oilseed sunfl ower should be between 15,000<br />
and 25,000 plants per acre, with adjustments made<br />
for soil type, rainfall potential and yield goal. Lower<br />
populations are recommended for soils with lower<br />
water-holding capacity and if normal rainfall is inconsistent<br />
or inadequate. Confection sunfl ower should<br />
be planted at populations between 14,000 and 20,000<br />
plants per acre. Preharvest dry down is more rapid in<br />
higher plant populations because of the smaller head<br />
size. However, higher plant populations may result in<br />
increased lodging and stalk breakage. Producers who<br />
solid seeded sunfl ower use seeding rates of 18,000 to<br />
23,000 plants per acre.<br />
Proper planting equipment adjustment and operation<br />
is one of the most important management tasks in<br />
sunfl ower production. Plateless and cyclo air planters<br />
have been used effectively to get good seed distribution.<br />
Double-seed drops should be avoided and planter<br />
adjustments should be made. Conventional plate<br />
planters will provide good seed distribution by using<br />
correct planter plates, properly sized seed and proper<br />
seed knockers. Commercial seed companies have plate<br />
recommendations for all seed sizes. Grain drills and<br />
air seeders may be used for seeding, although uniform<br />
depth of planting and seed spacing may be a problem<br />
unless proper adjustments and modifi cations are made.<br />
Postharvest Tillage<br />
After harvest, tillage of sunfl ower stalks is not<br />
recommended because snow-trapping potential is<br />
diminished, thereby reducing soil water conservation<br />
potential during the winter for the following crop.<br />
Also, because of the nature of sunfl ower residues, a<br />
late harvest followed by late fall tillage leaves the soil<br />
extremely susceptible to wind and water erosion.<br />
Table 8. Seed spacing required for various populations, assuming<br />
90 percent germination and 10 percent stand loss.<br />
Plants<br />
Row Spacing<br />
per acre 7.5 12 18 22 28 30 36<br />
- - - - - - - inches between seeds in the row - - - - - - -<br />
12,000 56.5 35.3 23.5 19.2 15.1 14.1 11.8<br />
14,000 48.4 30.2 20.2 16.5 13.0 12.1 10.1<br />
16,000 42.3 26.5 17.6 14.4 11.3 10.6 8.8<br />
17,000 39.8 24.9 16.6 13.6 10.7 10.0 8.3<br />
18,000 37.6 23.5 15.7 12.8 10.1 9.4 7.8<br />
19,000 35.7 22.3 14.9 12.2 9.6 8.9 7.4<br />
20,000 33.9 21.2 14.1 11.5 9.1 8.5 7.1<br />
21,000 32.3 20.2 13.4 11.0 8.6 8.1 6.7<br />
22,000 30.8 19.2 12.8 10.5 8.2 7.7 6.4<br />
23,000 29.5 18.4 12.3 10.0 7.9 7.4 6.1<br />
24,000 28.2 17.6 11.8 9.6 7.6 7.1 5.9<br />
25,000<br />
Feet per<br />
27.1 16.9 11.3 9.2 7.3 6.8 5.6<br />
1/1,000 acre 69.7 43.6 29 23.8 18.7 17.4 14.5<br />
Highlighted seed spacings provide nearly equal-distant spacing between plants for a given row<br />
spacing and plant population.
Crop Rotation<br />
(Greg Endres)<br />
Having a proper rotation sequence with all crops,<br />
including sunfl ower, is important. Research shows that<br />
sunfl ower seed yield is greater following most other<br />
crops than sunfl ower (Tables 9 and 10).<br />
Growers who do not rotate sunfl ower fi elds likely will<br />
be confronted with one or more of the following yieldreducing<br />
problems:<br />
1. Disease and disease-infested fi elds<br />
2. Increased insect risk<br />
3. Increasing populations of certain types of weeds<br />
4. Increased populations of volunteer sunfl ower<br />
5. Soil moisture depletion.<br />
6. Phytotoxicity or allelopathy of the sunfl ower<br />
residue to the sunfl ower crop<br />
Therefore, producers have many valid reasons for<br />
rotating sunfl ower fi elds.<br />
Table 9. Seed yield of sunfl ower based on<br />
previous crop, Crookston, Minn., 1972-78.<br />
Previous<br />
Seed yield/acre (pounds)<br />
4-yr.<br />
crop 1973 1975 1977 1978 ave.<br />
Sunfl ower 852 1,338 1,852 1,781 1,456<br />
Potato 908 1,279 2,348 1,605 1,535<br />
Sugar beet 770 1,683 2,358 2,168 1,745<br />
Pinto bean 946 1,410 2,282 1,674 1,578<br />
Wheat 1,284 1,549 2,339 1,655 1,706<br />
LSD .05 240 121 292 132<br />
Table 10. Seed yield of various crops following<br />
sunfl ower, Mandan, N.D., 1999-2000.<br />
Previous crop Seed yield<br />
Sunfl ower 870 lbs/A<br />
Canola 1,200 lbs/A<br />
Flax 22.5 bu/A<br />
Soybean 35.2 bu/A<br />
<strong>Field</strong> pea 41.7 bu/A<br />
HRS wheat 49.2 bu/A<br />
Barley 70.0 bu/A<br />
Risks of sunfl ower disease will be greatly magnifi ed<br />
by short sequencing of sunfl ower in a crop rotation.<br />
Sclerotinia or white mold (wilt, stem rot and head rot)<br />
is the primary disease concern with a poor sunfl ower<br />
rotation. Other disease concerns with improper rotations<br />
include Verticillium wilt, Phoma and premature<br />
ripening. Rotations of at least three- or four-year<br />
spacings between sunfl ower or other Sclerotiniasusceptible<br />
crops (e.g., canola, dry bean, soybean)<br />
are recommended to help reduce disease risk. The<br />
sunfl ower disease section in this publication contains<br />
specifi cs on the characteristics and methods of control<br />
for each disease.<br />
Crop rotation may help reduce but will not prevent<br />
insect problems in sunfl ower. Proper rotations help<br />
reduce populations of insects that overwinter in the<br />
soil or sunfl ower plant residue. Crop rotation will not<br />
reduce damage from insects that migrate into an area<br />
from other geographic regions or from fi elds planted<br />
to sunfl ower the previous year that are in proximity<br />
to current-season fi elds. Rotations recommended<br />
for reducing sunfl ower disease risks also will reduce<br />
insect risks.<br />
Rotation of other crops with sunfl ower can reduce<br />
the buildup of many weed species. Also, proper crop<br />
rotation increases weed management options, including<br />
cultural, mechanical and chemical weed control.<br />
Consult records of previous fi eld management to<br />
determine if long-residual herbicides that would<br />
adversely affect sunfl ower production were used.<br />
Volunteer sunfl ower also can become a serious weed<br />
problem in repeat sunfl ower and other crops. For additional<br />
details. refer to the weed management section<br />
of this publication, herbicide labels and NDSU Extension<br />
Service publication W-253, “North Dakota Weed<br />
Control <strong>Guide</strong>.”<br />
Different patterns of soil moisture utilization are important<br />
considerations when planning sunfl ower rotations.<br />
Sunfl ower is a deep-rooted and full-season crop.<br />
Sunfl ower has intermediate water use requirements,<br />
compared with other crops (see water requirements<br />
section in this publication). Sunfl ower is relatively<br />
tolerant to effects of short water-stress periods, espe-<br />
<strong>Production</strong> Practices<br />
23
24<br />
cially if the moisture stress occurs before the crop is in<br />
the reproductive stages. In limited-moisture growing<br />
seasons, sunfl ower following a shallower rooted and<br />
short-season crop (e.g., small grain, canola, fl ax) will<br />
allow the sunfl ower to extract residual water from a<br />
greater depth in the soil profi le.<br />
Continuous cropping of crops such as corn, wheat and<br />
soybeans results in yields that are depressed below<br />
the level obtained when a crop is rotated with other<br />
crops. The same effect is observed with sunfl ower,<br />
even when variable factors such as fertility, disease,<br />
insects and moisture are well-managed. This response<br />
with continuous cropping is known as allelopathy.<br />
Toxic materials in the sunfl ower residue, development<br />
of antibodies and the increase of soil-borne disease<br />
organisms all have been suggested as causes for<br />
allelopathy in sunfl ower. This effect has been demonstrated<br />
repeatedly and emphasizes a need to rotate<br />
sunfl ower.<br />
Suggested sunfl ower rotations for North Dakota<br />
will vary somewhat by geographic region, primarily<br />
because of different precipitation zones. For practical<br />
purposes, the state can be divided into east and west<br />
regions. Suggested rotations for these regions are<br />
listed in Table 11. Rotations may be varied by substituting<br />
other crops in the rotation, but the time spacing<br />
of the sunfl ower crop should be observed strictly or<br />
increased. See NDSU Extension Service publication<br />
EB-48, “Crop Rotations for Increased Productivity,”<br />
for additional details on crop rotation.<br />
Table 11. Sunfl ower rotation examples for North Dakota.<br />
Year West East<br />
Pollination<br />
(Gary Brewer)<br />
1 Sunfl ower Sunfl ower Sunfl ower Sunfl ower<br />
2 Small grain Pulse crop* Soybean* Small grain<br />
3 Pulse crop* Small grain Small grain Soybean *<br />
4 Flax or small grain Corn<br />
*medium susceptibility to Sclerotinia.<br />
Native sunfl ower and the early varieties of sunfl ower<br />
were self-incompatible and required insect pollination<br />
for economic seed set and yields. However, because<br />
numbers of pollinators were often too low to ensure<br />
adequate seed set, current hybrids have been selected<br />
for and possess high levels of self-compatibility.<br />
Although self-compatible sunfl ower hybrids usually<br />
outproduce self-incompatible cultivars, modern<br />
hybrids benefi t from insect pollination.<br />
The agronomic value of insect pollinating activities to<br />
current hybrids varies among hybrids, fi elds and years.<br />
Controlled studies indicate that in most sunfl ower<br />
hybrids, seed set, seed oil percentage, seed yields and<br />
oil yields increase when pollinators (primarily bees)<br />
are present. Literature reports indicate that yield could<br />
increase as much as 48.8 percent and oil percentage<br />
could increase 6.4 percent in bee-exposed hybrids.<br />
However, despite the increases in yield and oil concentration<br />
that occur, the benefi t of insect pollination<br />
of sunfl ower often is overlooked (Figure 15).<br />
Seed companies and growers who produce F1 hybrid<br />
seed for planting must use bees to transfer pollen from<br />
the male parent to the female parent. Although native<br />
wild bees are often better pollinators of sunfl ower<br />
than honey bees, the honey bee is the only managed<br />
pollinator of sunfl ower available. However, if pollen<br />
sources other than sunfl ower are nearby, the honey bee
■ Figure 15. Bees and hives are occasionally placed<br />
in sunfl ower fi elds to boost the native population to<br />
assure a better seed set. (Reid Bevis)<br />
will forage sunfl ower primarily for nectar and will not<br />
transfer sunfl ower pollen effi ciently.<br />
Honey bee colonies are placed in seed production<br />
fi elds at a rate of one hive per one to two acres. A bee<br />
density of more than 20 bees per 100 heads in bloom<br />
is needed to transfer suffi cient pollen from the male<br />
line to the female sterile line. Placement of honey bee<br />
colonies will depend upon proximity and acreage of<br />
competing nectar and pollen sources. With no competition,<br />
all honey bee colonies are placed at one end<br />
of the target fi eld. With competing nectar and pollen<br />
sources, placement of honey bee colonies at 800-foot<br />
intervals may be necessary.<br />
Sunfl ower genotypes vary in their attractiveness to the<br />
honey bee. Honey bees prefer short corolla length,<br />
unpigmented stigmas, many stomata on the nectary<br />
and high sucrose content of the nectar. Glandular<br />
trichomes on the anthers and ultraviolet refl ecting and<br />
adsorbing pigments also may be important in honey<br />
bee preference. Although some crops, such as oilseed<br />
rape, have been selected for honey bee preference, this<br />
has not been done with sunfl ower.<br />
Maximum seed yields often require the use of<br />
insecticides to protect the crop from insect competitors.<br />
Unfortunately, many of the major insect pests of<br />
sunfl ower attack the crop when it is fl owering. Thus,<br />
insecticides used to control the pest also harm pollinating<br />
bees. If pollinator activity is decreased, yield<br />
and oil percentage may decline. The hazards to honey<br />
bees can be minimized with adequate communication<br />
and cooperation among beekeepers, growers and pesticide<br />
applicators. Beekeepers must inform applicators<br />
of the location of apiaries and be prepared to move or<br />
protect colonies. When insecticide spraying is justifi<br />
ed, applicators must make every attempt to notify<br />
beekeepers in advance.<br />
<strong>Production</strong> Practices<br />
25
26<br />
Pest Management<br />
(Janet Knodel and Larry Charlet)<br />
Integrated Pest Management<br />
Sunfl ower can be a high-risk crop because of potential<br />
losses from diseases, insects, birds and weeds. These<br />
potential risks require that growers follow integrated<br />
pest management (IPM) practices. IPM is a sustainable<br />
approach to managing pests by combining<br />
biological, cultural, physical and chemical tools in a<br />
way that minimizes economic, health and environmental<br />
risks to maintain pest populations below levels<br />
that cause unacceptable losses to crop quality or yield.<br />
The concept of pest management is based on the fact<br />
that many factors interact to infl uence the abundance<br />
of a pest. Control methods vary in effectiveness, but<br />
integration of these various population-regulating<br />
factors can minimize the number of pests in sunfl ower<br />
and reduce the cost of managing pest populations<br />
without unnecessary crop losses. IPM also recommends<br />
the judicious use of chemical pesticides when<br />
needed and suggests ways to maximize effectiveness<br />
and minimize impact on nontarget organisms and the<br />
environment.<br />
Economic Injury Level<br />
and Economic Threshold Levels<br />
One major component of a pest management program<br />
is determining when tactics should be implemented<br />
to prevent economic loss. Economic loss results when<br />
pest numbers increase to a point where they cause<br />
crop losses that are greater than or equal to the cost of<br />
controlling the pest. An economic injury level (EIL) is<br />
defi ned as the level of pests that will cause economic<br />
damage. An EIL recognizes that treatment is justifi ed<br />
for some pest population levels while others are not of<br />
economic importance.<br />
An economic threshold level (ETL) is the level or<br />
number of pests at which tactics must be applied to<br />
prevent an increasing pest population from causing<br />
economic losses. Usually the ETL is lower than the<br />
EIL. The ETL has been defi ned most extensively for<br />
insect pests; fewer ETLs have been established for<br />
other types of pests. The ETL varies signifi cantly<br />
among different pests and also can vary during different<br />
developmental stages of the crop. Crop price, yield<br />
potential, crop density, cost of control and environmental<br />
conditions infl uence the ETL and EIL. Generally,<br />
the ETL increases as cost of control increases and<br />
decreases as the crop value increases.<br />
Monitoring Pest Population Levels<br />
In general, fi elds should be evaluated regularly to determine<br />
pest population levels. A weekly fi eld check is<br />
usually suffi cient, but fi eld checks should be increased<br />
to two or three times a week if the number of pests is<br />
increasing rapidly or if the number is approaching an<br />
economic threshold level. Pests should be identifi ed<br />
accurately because economic threshold levels and con-
trol measures vary for different organisms. In addition,<br />
when insects pests are monitored, many insects are<br />
benefi cial and may help reduce numbers of injurious<br />
insects; recognizing which are pests and those that are<br />
benefi cial is important.<br />
Tools of Pest Management<br />
Tools of pest management include many tactics, of<br />
which pesticides are only one. These tactics can be<br />
combined to create conditions that are the least conducive<br />
for pest survival. Chemical or biological pesticides<br />
are used when pests exceed economic threshold<br />
levels; sometimes they are necessary when control is<br />
needed quickly to prevent economic losses.<br />
Some of the tools or components of pest management<br />
that can be used to reduce pest populations are:<br />
Biological Controls<br />
Benefi cial insects<br />
Benefi cial pathogens<br />
Host Resistance<br />
Cultural Controls<br />
Planting and harvest dates<br />
Crop rotation<br />
Tillage practices<br />
Mechanical/Physical Controls<br />
Temperature<br />
Weather events<br />
Trapping<br />
Chemical Controls<br />
Pesticides<br />
Attractants<br />
Repellents<br />
Pheromones<br />
Summary<br />
Growers should examine their operations and<br />
minimize pest damage by adopting integrated pest<br />
management practices based on the use of economic<br />
threshold levels, when available, plus carefully<br />
monitoring and combining various control methods.<br />
Signifi cant progress with sunfl ower pest management<br />
has been made and undoubtedly will continue to be<br />
made in the future to aid successful sunfl ower production.<br />
The following sections provide current information<br />
on management of insects, diseases, weeds, birds<br />
and other sunfl ower pests. A growing season calendar<br />
shows the major sunfl ower pest problems and time of<br />
occurrence in the northern Great Plains production<br />
area (Figure 16).<br />
■ Figure 16. A growing season calendar indicating<br />
time of occurrence of major sunfl ower pests.<br />
(J. Knodel)<br />
Pest Management<br />
27
28<br />
Quick Reference <strong>Guide</strong> to Major Sunfl ower Insects<br />
The information presented on this page is designed to be a quick reference for growers, crop consultants, fi eld scouts and<br />
others. Since this information is very brief, the user should refer to the following pages for more detailed data on life cycles,<br />
damage, descriptions, etc.<br />
Insects Description Occurrence, Injury and Economic Threshold (ET)<br />
Cutworms Dirty gray to gray brown. ET – 1 per square foot or 25 percent to 30 percent stand<br />
(several species) Grublike larva, 0.25 to 1.5 inches in length. reduction. Appear in early spring when plants are in the<br />
seedling stage, chewing them at or slightly above ground.<br />
Palestriped Adult: 1/8 inch long and shiny black, with two white ET – 20 percent of the seedling stand is injured and at risk<br />
Flea Beetle stripes on the back. The hind legs are enlarged and to loss due to palestriped fl ea beetle feeding. Scout for<br />
modifi ed for jumping. fl ea beetles by visually estimating population on seedlings<br />
or using yellow sticky cards placed close to the ground.<br />
Sunfl ower Beetle Adult: reddish-brown head, cream back with three ET – 1 to 2/seedling (adults), or 10 to 15/seedling<br />
dark stripes on each wing cover. Body 0.25 to 0.5 (larvae). Adults appear in early June, larvae shortly<br />
inch long. Larva: yellowish green, humpbacked in thereafter. Both adults and larvae chew large holes<br />
appearance, 0.35 inch in length. in leaves.<br />
Sunfl ower Adult: wingspread 0.63 to 0.75 inch, gray brown ET – none. First generation adults appear in late May to<br />
Bud Moth with two dark transverse bands on forewings. mid-June. Second generation adults appear in midsummer.<br />
Larva: Cream-colored body (0.33 to 0.4 inch) with Larvae from fi rst generation damage terminals and stalks,<br />
a brown head. whereas second generation larvae feed in receptacle area.<br />
Longhorned Adult: pale gray and 5/8 inch (6 to 11 mm) in No scouting method or ET has been developed. Adults are<br />
Beetle (Dectes) length, with long gray and black banded antennae. present from late June through August. Larvae tunnel and<br />
Larvae: yellowish with fl eshy protuberances on the feed in the petioles and stem pith and girdle the base of<br />
fi rst seven abdominal segments (1/3 to 1/2 inch) plants. Stalks often break at the point of larval girdling.<br />
Sunfl ower Stem Adult: small (0.19 inch long) weevil with a ET – 1 adult/3 plants in late June to early July. Adults<br />
Weevil gray-brown background and white dots on the back. appear in mid to late June, with larvae in stalks from<br />
early July to late summer.<br />
Thistle Caterpillar Adult: wingspread of 2 inches, upper wing surface brown ET – 25 percent defoliation, provided that most of the<br />
(Painted Lady with red and orange mottling and white and black spots. larvae still are less than 1.25 inches in length. Adults<br />
Butterfl y) Larva: brown to black, spiny, with a pale yellow stripe appear in early to mid-June, with larvae appearing<br />
on each side, 1.25 to 1.5 inches in length. shortly thereafter. Larvae chew holes in leaves.<br />
Sunfl ower Midge Adult: small (0.07 inch), tan, gnatlike insect. ET – none. Adult emergence begins in early July. Larvae<br />
Larva: cream or yellowish, 0.09 inch long, tapered at feed around head margin and at the base of the seeds,<br />
front and rear. causing shrinkage and distortion of heads.<br />
Sunfl ower Seed Adults: the red sunfl ower seed weevil is about 0.12 ET – generally 8 to 14 adult red sunfl ower weevils per head<br />
Weevils inch long and rusty in color. The gray sunfl ower seed (oil) and one per head (confectionery). Adults appear in<br />
weevil is about 0.14 inch long and gray in color. late June to early July. Treat for red sunfl ower seed weevil<br />
Larvae: both species are cream-colored, legless and at R5.1 to R5.4. Larvae feed in seeds from mid to late<br />
C-shaped. summer.<br />
Sunfl ower Moth Adult: body is 0.38 inch long, with 0.75 inch ET – 1 to 2 adults/5 plants at onset of bloom. Adults are<br />
wingspread. Color is buff to gray. Larva: brown head migratory and usually appear in early to mid-July. Larvae<br />
capsule with alternate dark and light lines running<br />
longitudinally, 0.75 inch in length.<br />
tunnel in seeds from late July to late August.<br />
Banded Sunfl ower Adult: small 0.25-inch straw-colored moth with brown ET – See banded sunfl ower moth section for egg or adult<br />
Moth triangular area on forewing. Larva: in early growth sampling methods for determining ET. Sampling should be<br />
stage, off-white, changing to red and then green color conducted in the late bud stage (R-3), usually during midat<br />
maturity, 0.44 inch in length. July. Adults appear about mid-July to mid-August. Larvae<br />
present in heads from mid-July to mid-September.<br />
Lygus Bug Adult: small (0.2 inch in length), cryptically colored ET - for CONFECTION SUNFLOWERS ONLY –<br />
insects with a distinctive yellow triangle or “V” on 1 Lygus bug per 9 heads. Two insecticide sprays are<br />
the wings and vary in color from pale green to dark recommended: one application at the onset of pollen shed<br />
brown. Nymph (immature stages): usually green and or 10 percent bloom, followed by a second treatment<br />
similar in appearance to the adults, but lack wings. 7 days later.<br />
Sunfl ower Adult: metallic, black, 0.25-inch long body with a long ET – none. Adults appear in mid to late July and create<br />
Headclipping “snout.” Larvae: 0.25 inch in length. feeding punctures around stalk just below the heads.<br />
Weevil Heads drop off.<br />
NOTE: The insects discussed above are listed in the order that they likely are to occur throughout the growing season; however, the various insects<br />
may or may not appear, depending upon overwintering survival and environmental conditions as the season progresses. The table is intended<br />
simply as a guide to when fi elds should be checked for possible presence of the various insects known to infest sunfl owers.
Insects<br />
(Janet Knodel and Larry Charlet)<br />
Sunfl ower plays host to a number of insect pests. In<br />
the major sunfl ower producing areas of the Dakotas,<br />
Minnesota and Manitoba, approximately 16 species of<br />
sunfl ower insects can cause plant injury and economic<br />
loss, depending on the severity of infestation. However,<br />
during any one growing season, only a few species<br />
will be numerous enough to warrant control measures.<br />
The sunfl ower insects of major importance in the<br />
northern Great Plains have been sunfl ower midge,<br />
Contarinia schulzi Gagne'; sunfl ower beetle, Zygogramma<br />
exclamationis (Fabricius); sunfl ower stem<br />
weevil, Cylindrocopturus adspersus (LeConte); red<br />
sunfl ower seed weevil, Smicronyx fulvus LeConte; and<br />
the banded sunfl ower moth, Cochylis hospes Walsingham.<br />
Recently, Lygus bugs have been an economic<br />
problem for the confection and hulling sunfl ower seed<br />
market. Populations of the long-horned sunfl ower stem<br />
girdler, Dectes texanus LeConte, have been increasing<br />
in North and South Dakota.<br />
Infestation of sunfl ower insects must be monitored<br />
regularly, usually weekly, to determine the species<br />
present and if populations are at economic threshold<br />
levels. Furthermore, proper timing of insecticidal<br />
treatment is essential to maximize control.<br />
The following sections provide information on the<br />
identifi cation, life cycle, damage, scouting methods,<br />
economic threshold levels and management of some<br />
of the most common insect pests of sunfl ower in the<br />
northern Great Plains. A preliminary quick reference<br />
guide to sunfl ower insects is available.<br />
Sunfl ower pests are not distributed evenly throughout<br />
a fi eld, and fi elds should be checked in several locations.<br />
Some insect pests, such as banded sunfl ower<br />
moth, are concentrated in areas of a fi eld or are more<br />
abundant near the edge of a fi eld than in the middle.<br />
Determining the extent of a pest population on the<br />
basis of what is found in only one or two small areas<br />
of a fi eld is impossible. At least fi ve sites per 40-acre<br />
fi eld should be monitored to collect good information<br />
on the nature and extent of a pest infestation.<br />
Sampling sites should be at least 75 feet in from the<br />
fi eld margin to determine whether an entire fi eld or a<br />
portion of the fi eld requires treatment. When infestations<br />
occur primarily along fi eld margins, delineating<br />
those and treating as little of the fi eld as needed to<br />
provide economic control may be possible. In most<br />
cases, 20 plants per sampling site should be examined<br />
in the Z or X pattern as shown (Figure 17).<br />
Crop consultants who are trained in pest management<br />
scouting may be hired. Consultants should be able to<br />
identify pest and benefi cial insects and provide information<br />
about pest management.<br />
■ Figure 17. The X and Z scouting patterns.<br />
Insects<br />
29
30<br />
■ Wireworms<br />
Species: various<br />
Description: Wireworm larvae (Figure 18) are hard,<br />
smooth, slender, wirelike worms varying from 1.5 to 2<br />
inches (38 to 50 mm) in length when mature. They are<br />
a yellowish white to a coppery color with three pairs<br />
of small, thin legs behind the head. The last body segment<br />
is forked or notched.<br />
Adult wireworms (Figure 19) are bullet-shaped, hardshelled<br />
beetles that are brown to black and about ½<br />
inch (13 mm) long. The common name “click beetle”<br />
is derived from the clicking sound that the insect<br />
makes when attempting to right itself after landing on<br />
its back.<br />
■ Figure 18. Wireworm larvae. (Mark Boetel)<br />
■ Figure 19. Click beetle or adult wireworm.<br />
(Roger Key, http://www.insectimages.org)<br />
Life Cycles: Wireworms usually take three to four<br />
years to develop from the egg to an adult beetle. Most<br />
of this time is spent as a larva. Generations overlap, so<br />
larvae of all ages may be in the soil at the same time.<br />
Wireworm larvae and adults overwinter at least 9 to<br />
24 inches (23 to 61 cm) deep in the soil. When soil<br />
temperatures reach 50 to 55 F (10 to 13 C) during the<br />
spring, larvae and adults move nearer the soil surface.<br />
Adult females emerge from the soil, attract males<br />
to mate, then burrow back into the soil to lay eggs.<br />
Females can re-emerge and move to other sites, where<br />
they burrow in and lay more eggs. This behavior<br />
results in spotty infestations throughout a fi eld. Some<br />
wireworms prefer loose, light and well-drained soils;<br />
others prefer low spots in fi elds where higher moisture<br />
and heavier clay soils are present.<br />
Larvae move up and down in the soil profi le in<br />
response to temperature and moisture. After soil<br />
temperatures warm to 50 F (10 C), larvae feed within<br />
6 inches (15 cm) of the soil surface. When soil temperatures<br />
become too hot (>80 F, 27 C) or dry, larvae<br />
will move deeper into the soil to seek more favorable<br />
conditions. Wireworms infl ict most of their damage in<br />
the early spring, when they are near the soil surface.<br />
During the summer months, the larvae move deeper<br />
into the soil. Later as soils cool, larvae may resume<br />
feeding nearer the surface, but the amount of injury<br />
varies with the crop.<br />
Wireworms pupate and the adult stage is spent within<br />
cells in the soil during the summer or fall of their fi nal<br />
year. The adults remain in the soil until the following<br />
spring.<br />
Damage: Wireworm infestations are more likely to<br />
develop where grasses, including grain crops, are<br />
growing. Wireworms damage crops by feeding on the<br />
germinating seed or the young seedling. Damaged<br />
plants soon wilt and die, resulting in thin stands. In a<br />
heavy infestation, bare spots may appear in the fi eld<br />
and reseeding is necessary.<br />
Scouting Method: Decisions to use insecticides for<br />
wireworm management must be made prior to planting.<br />
No rescue treatments are available for controlling<br />
wireworms after planting. Producers have no easy
way to determine the severity of infestation without<br />
sampling the soil. Infestations vary from year to year.<br />
Considerable variation may occur both within and<br />
between fi elds.<br />
Sometimes the past history of a fi eld is a good indicator,<br />
especially if wireworms have been a problem in<br />
previous seasons. Also, crop rotation may impact<br />
population levels.<br />
Two sampling procedures are available. One procedure<br />
relies on the use of a corn-wheat seed mixture used as<br />
bait, placed in the soil, which attracts the wireworms<br />
to the site (Figure 20). The other involves digging and<br />
sifting a soil sample for the presence of wireworms.<br />
Economic Threshold: If the average density is greater<br />
than one wireworm per bait station, the risk of crop<br />
injury is high and a soil insecticide should be used<br />
at planting to protect the sunfl ower. If no wireworms<br />
are found in the traps, risk of injury is low; however,<br />
wireworms still may be present but were not detected<br />
by the traps. When digging soil samples, 12 or more<br />
wireworms in 50 3-inch by 3-inch (8 cm by 8 cm)<br />
samples, is likely to result in damage to sunfl ower.<br />
Management: Seeds should be treated with an approved<br />
insecticide for protection of germinating seeds<br />
and seedlings.<br />
■ Figure 20. Wireworm bait station. (Extension<br />
Entomology)<br />
■ Seedcorn Maggot<br />
Species: Delia platura (Meigan)<br />
Description: Seedcorn maggots are larvae of small<br />
fl ies resembling housefl ies. The adult is a light gray<br />
fl y about 0.2 inch (5 mm) long. Larvae are white,<br />
cylindrical, tapered anteriorly and also about 0.2 inch<br />
(5 mm) long (Figure 21). Larvae can be found inside<br />
damaged seeds or in the soil nearby.<br />
Life Cycle: After soil temperatures reach 50 F (10 C)<br />
in the spring, the fl ies emerge, mate and then deposit<br />
eggs in soil, especially where high organic matter<br />
exists. Eggs hatch in a few days and the maggots burrow<br />
into seeds. Infected seeds often do not emerge,<br />
resulting in stand loss. Even when infested seeds do<br />
germinate, plants may be weakened. No effective preplant<br />
monitoring techniques are available for seedcorn<br />
maggots. <strong>Field</strong>s with extensive decaying organic matter,<br />
such as those that are heavily manured or where<br />
a cover crop has been turned under, are particularly<br />
attractive to egg-laying fl ies.<br />
Damage: The fi rst sign of seedcorn maggot damage is<br />
areas in the fi eld where seedlings have not emerged.<br />
Seedcorn maggots hollow out seeds or eat portions of<br />
seedlings. Damage is most common in early plantings<br />
while the soil is cool, and if organic matter is high,<br />
such as when a green plant material is plowed into a<br />
fi eld before planting. Females are strongly attracted to<br />
decaying organic matter for laying eggs.<br />
Scouting Method: Scouting to determine the risk of<br />
seedcorn maggot infestation has not been developed.<br />
■ Figure 21. Seed corn maggot larvae. (Ric Bessin,<br />
University of Kentucky)<br />
Insects<br />
31
32<br />
■ Cutworms<br />
Species:<br />
Darksided cutworm Euxoa messoria (Harris)<br />
Redbacked cutworm Euxoa ochrogaster (Guenee)<br />
Dingy cutworm Feltia jaculifera (Walker)<br />
Description: Darksided cutworm — Forewings<br />
of the adult darksided cutworm are usually light,<br />
powdery and grayish brown with indistinct markings<br />
(Figure 22). The larvae are pale brown dorsally and<br />
white on the ventral areas (Figure 23 ). Sides have numerous<br />
indistinct stripes. At maturity, they are about<br />
1.25 to 1.5 inches (32 to 38 mm) long and 0.19 inch (5<br />
mm) wide.<br />
Redbacked cutworm — The forewings of the adult<br />
redbacked cutworm are reddish brown with characteristic<br />
bean-shaped markings (Figure 24). The larvae are<br />
dull gray to brown with soft, fl eshy bodies and may be<br />
1 to 1.25 inches (25 to 32 mm) long when fully grown<br />
(Figure 25). Larvae can be distinguished by two dull<br />
reddish stripes along the back.<br />
Dingy cutworm — Forewings are dark brown with<br />
bean-shaped markings as in the redbacked cutworm<br />
adults (Figure 26). Hind wings in the male are whitish<br />
with a broad, dark border on the outer margin; in the<br />
female they are uniform dark gray. The larvae have<br />
a dull, dingy, brown body mottled with cream color.<br />
The dorsal area is pale with traces of oblique shading<br />
(Figure 27).<br />
Life Cycles: The female darksided and redbacked cutworm<br />
moths deposit eggs in the soil in late July and<br />
early August. The eggs remain dormant until the onset<br />
of warm weather the following spring. The larvae of<br />
both species emerge from late May to early June. They<br />
continue to feed and grow until about the end of June,<br />
when fully grown larvae pupate in earthen cells near<br />
the soil surface. The pupal period lasts about three<br />
weeks. Both species have one generation per year.<br />
The adult dingy cutworms emerge in August and are<br />
active until mid-October, with peak activity in September.<br />
Eggs are deposited in plants in the Compositae<br />
family in the fall. The larvae develop to the second<br />
or third instar in the fall and overwinter in the soil.<br />
Pupation occurs in the spring to early summer. One<br />
generation of this species is produced per year.<br />
■ Figure 22. Adult - Darksided cutworm<br />
Euxoa messoria. (Extension Entomology)<br />
■ Figure 23. Larva - Darksided cutworm<br />
Euxoa messoria. (Extension Entomology)<br />
■ Figure 24. Adult - Redbacked cutworm<br />
Euxoa ochrogaster. (Extension Entomology)<br />
■ Figure 25. Larva - Redbacked cutworm<br />
Euxoa ochrogaster. (Extension Entomology)
Damage: Cutworm damage normally consists of<br />
crop plants being cut off from 1 inch (25 mm) below<br />
the soil surface to as much as 1 to 2 inches (25 to 50<br />
mm) above the soil surface. Young leaves also may be<br />
severely chewed as a result of cutworms (notably the<br />
darksided species) climbing up to feed on the plant<br />
foliage.<br />
Most cutworm feeding occurs at night. During the<br />
daytime, the cutworms usually will be just under the<br />
soil surface near the base of recently damaged plants.<br />
Wilted or dead plants frequently indicate the presence<br />
of cutworms. Cut-off plants may dry and blow<br />
away, leaving bare patches in the fi eld as evidence of<br />
cutworm infestations.<br />
Scouting Method: Sampling should begin as soon as<br />
sunfl ower plants emerge, and fi elds should be checked<br />
at least twice per week until approximately mid-June.<br />
The Z pattern should be used in scouting fi elds for<br />
cutworms, with sampling points one and two near the<br />
margin as indicated in Figure 17.<br />
Stand reduction is determined by examining 100<br />
plants per fi ve sampling sites for a total of 500 plants.<br />
■ Figure 26. Adult - Dingy cutworm Feltia jaculifera.<br />
(Extension Entomology)<br />
■ Figure 27. Larva - Dingy cutworm Feltia jaculifera.<br />
(Extension Entomology))<br />
A trowel or similar tool should be used to dig around<br />
damaged plants to determine if cutworms are present,<br />
since missing plants in a row do not necessarily<br />
indicate cutworm damage (damage may be caused by<br />
a defective planter, rodents or birds).<br />
The Z pattern should be used again to determine<br />
cutworm infestation level by examining fi ve 1-squarefoot<br />
(30 by 30 cm) soil samples per site (in the row)<br />
for a total of 25 samples.<br />
Economic Threshold: One larva per square foot (30<br />
by 30 cm) or 25 percent to 30 percent stand reduction.<br />
Management: Several different insecticides are registered<br />
for cutworm control in sunfl ower. Postemergent<br />
treatment with an insecticide provides quick control of<br />
surface feeding cutworms.<br />
■ Palestriped Flea Beetle<br />
Species: Systena blanda (Melsheimer)<br />
Description: The adult is about 1/8 inch (3.2 mm)<br />
long and shiny black, with two white stripes on the<br />
back. The hind legs are enlarged and modifi ed for<br />
jumping (Figure 28).<br />
Life Cycle: The life cycle of palestriped fl ea beetles<br />
on sunfl ower fi elds is poorly understood. However, the<br />
adult fl ea beetles seem to overwinter in the fi eld under<br />
soil clods, fi eld debris and crop residues. They become<br />
active again in the spring, perhaps feeding fi rst on<br />
alfalfa and weeds before moving to and feeding on<br />
sunfl ower seedlings in June. They have been observed<br />
feeding on sunfl ower through July. Palestriped fl ea<br />
beetles have a wide host range, which includes various<br />
weeds, potato, tomato, carrot, peanut, corn, oat,<br />
cotton, pea, beans, strawberry, watermelon, grape and<br />
■ Figure 28. Adult - Palestriped fl ea beetle.<br />
(Michael Catangui, SDSU)<br />
Insects<br />
33
34<br />
pumpkin. Palestriped fl ea beetles are considered an<br />
important pest of commercially grown vegetables in<br />
some areas of the U.S. Recently, palestriped fl ea beetles<br />
have been observed delaying regrowth of alfalfa<br />
and also were observed feeding on soybean seedlings<br />
in eastern South Dakota.<br />
Damage: Palestriped fl ea beetles chew on the cotyledons,<br />
leaves and hypocotyls of sunfl ower seedlings,<br />
causing them to wilt and die. Injured leaves become<br />
riddled with holes, giving them a “lacey” appearance<br />
(Figure 29). The sunfl ower plant is most sensitive to<br />
palestriped fl ea beetle injury from seedling emergence<br />
(V-E) through the four-leaf stage (V-4). Signifi cant<br />
stand losses may result from heavy feeding injury by<br />
the palestriped fl ea beetles.<br />
■ Figure 29. Damaged sunfl ower leaves by<br />
palestriped fl ea beetle. (Michael Catangui, SDSU)<br />
Scouting Method: Surveys may be accomplished by<br />
using yellow sticky cards placed close to the ground<br />
(Figure 30). Sampling seedlings for beetles also can<br />
aid in estimating populations and feeding injury<br />
levels. Palestriped fl ea beetles move very fast and are<br />
hard to count directly on the seedlings or catch with<br />
an insect net.<br />
Economic Threshold: Control is recommended when<br />
20 percent of the seedling stand is injured and at risk<br />
to loss due to palestriped fl ea beetle feeding. This economic<br />
threshold is a guideline based on published hail<br />
injury data that predicts potential yield loss relative to<br />
seedling stand loss.<br />
Management: Palestriped fl ea beetles are hard to<br />
control with chemical insecticides; research has shown<br />
that treatments may provide up to 75 percent control<br />
of adults.<br />
■ Figure 30. Yellow sticky trap for monitoring<br />
palestriped fl ea beetles. (Michael Catangui, SDSU)
■ Sunfl ower Beetle<br />
Species: Zygogramma exclamationis (Fabricius)<br />
Description: The sunfl ower beetle is associated<br />
exclusively with sunfl ower. Adults (Figure 31) closely<br />
resemble adult Colorado potato beetles and may be<br />
confused with potato beetles. However, sunfl ower<br />
beetles are smaller and do not feed on potatoes, and<br />
Colorado potato beetles do not feed on sunfl ower. The<br />
head of the adult is reddish brown and the thorax (area<br />
between head and abdomen) is pale cream-colored<br />
with a reddish-brown patch at the base. Each front<br />
wing cover is cream-colored and has three dark stripes<br />
that extend its length. A shorter lateral stripe ends at<br />
the middle of the wing in a small dot that resembles<br />
■ Figure 31. Adult - Sunfl ower beetle Zygogramma<br />
exclamationis. (Extension Entomology)<br />
■ Figure 32. Larva - Sunfl ower beetle Zygogramma<br />
exclamationis. (Larry Charlet)<br />
an exclamation point. The beetle is ¼ to ½ inch (6 to<br />
12 mm) long and 3/32 to 3/16 inch (2 to 4 mm) wide.<br />
Eggs are about 1/16 inch (1.5 to 2 mm) long, cigarshaped<br />
and cream yellow. Sunfl ower beetle larvae<br />
are yellowish green with a brown head capsule and<br />
humpbacked in appearance. Newly hatched larvae are<br />
about 1/16 inch (1.5 to 1.75 mm long), and will reach<br />
a length of about an inch (8 to 10 mm) when fully<br />
developed (Figure 32).<br />
Life Cycle: The sunfl ower beetle has one generation<br />
per year in North Dakota. The adults overwinter in the<br />
soil, emerging in late May or early June. Shortly after<br />
emergence, the beetles begin to feed, mate and lay<br />
eggs singly on stems and undersides of leaves. Adults<br />
live for about 8½ weeks and lay eggs for a six- to seven-week<br />
period. Each female lays approximately 850<br />
eggs, with a range of 200 to 2,000 eggs. Eggs hatch<br />
into larvae in about one week (Figure 33). The larvae<br />
have four instars, which feed and are present in fi elds<br />
for about six weeks. When mature, the larvae enter the<br />
soil to pupate in earthen cells. The pupal stage lasts<br />
from 10 days to two weeks. Adults of the new generation<br />
emerge and feed for a short period on the bracts<br />
of the sunfl ower head or on the uppermost leaves of<br />
the plant before re-entering the soil to overwinter.<br />
Damage: Adult sunfl ower beetles damage plants soon<br />
after they emerge from overwintering. Damage to<br />
cotyledons is generally slight, but the fi rst true leaves<br />
may be severely damaged or completely consumed.<br />
<strong>Field</strong>s may be severely defoliated if beetles are numerous.<br />
Adults feed predominately on leaf margins while<br />
■ Figure 33. Eggs - Sunfl ower beetle. (Larry Charlet)<br />
Insects<br />
35
36<br />
larvae feed on the entire leaf surface. When larvae<br />
are numerous, damaged leaves take on a lacy appearance.<br />
Most larval feeding occurs at night, and adults<br />
will feed during the day. During the daytime, larvae<br />
typically rest in the terminal growth area, where they<br />
are easily found in leaf axils and fl ower buds. If larval<br />
feeding is severe, defoliation can reduce yield due to<br />
poor seed set or fi ll.<br />
The late summer generation of emerging sunfl ower<br />
beetle adults and late-maturing larvae rarely causes<br />
economic damage to the sunfl ower crop. However, in<br />
some cases, they have been abundant enough to cause<br />
feeding injury on late-planted sunfl ower.<br />
Scouting Method: Sampling sites should be at least<br />
75 to 100 feet (23 to 31 m) from the fi eld’s margins<br />
when determining if an entire fi eld should be treated.<br />
Adults and/or larvae should be counted on 20 plants at<br />
each of fi ve sampling sites along an X pattern for a total<br />
of 100 plants. The average number of adults and/or<br />
larvae per plant then should be determined.<br />
The average percent defoliation of plants is determined<br />
when damage is evident in the fi eld by examining<br />
20 plants per fi ve sampling sites for a total of 100<br />
plants (Figure 34).<br />
Economic Threshold: As sunfl ower plants develop,<br />
they can tolerate more feeding damage. In the seedling<br />
stage, one to two adults per seedling is the recommended<br />
economic threshold. For larvae, the treatment<br />
threshold is when populations reach 10 to 15 larvae<br />
per plant, or when approximately 25 percent defoliation<br />
occurs on the upper eight to 12 leaves (active<br />
growing part). Management normally is advised if<br />
defoliation reaches the 25 percent to 30 percent level<br />
at the late vegetative and early bud stages and it appears<br />
(based on larval size of less than ¼ inch or 6<br />
mm) that more defoliation will occur on the actively<br />
growing part of sunfl ower plant. However, if defoliation<br />
is 25 percent and the majority of larvae are about<br />
1/3 inch (8 mm) long, they have reached maturity and<br />
soon will stop feeding. Then, management probably is<br />
not warranted.<br />
Management: Insecticide seed treatments and foliar<br />
insecticides are effective in reducing spring populations<br />
of the adult sunfl ower beetle. Application of a<br />
foliar insecticide is recommended only when beetle<br />
populations have reached an economic threshold<br />
level in a fi eld. Insecticides are effective in prevent-<br />
ing economic loss when applied to actively feeding<br />
adults and/or larvae. Adult and larval populations of<br />
sunfl ower beetle decrease as planting date is delayed.<br />
Defoliation also is lower at the later planting dates.<br />
As a result, delayed planting is effective in preventing<br />
yield reductions caused by sunfl ower beetle feeding,<br />
but may make fi elds more attractive to later season<br />
insects, such as red sunfl ower seed weevil. Spring<br />
or fall cultivation does not reduce the overwintering<br />
populations of sunfl ower beetle adults or infl uence the<br />
pattern of emergence from the soil during the spring<br />
and summer. Sunfl ower hybrids with resistance to the<br />
sunfl ower beetle are not available. Natural enemies include<br />
parasites of the eggs, larvae and adults. General<br />
predators, such as ladybird beetles, carabid beetles,<br />
lacewings, stink bugs, nabids and anthocorids, destroy<br />
both eggs and larvae of the sunfl ower beetle.<br />
■ Figure 34. Percent defoliation of sunfl ower leaves.<br />
(Extension Entomology)
■ Sunfl ower Bud Moth<br />
Species: Suleima helianthana (Riley)<br />
Description: Sunfl ower bud moths have a wingspread<br />
of about 0.63 inch (16 to 18 mm). Each gray-brown<br />
forewing has two dark transverse bands (Figure 35).<br />
One band extends across the middle of the wing and<br />
the second band is near the wing tip. The larva has<br />
a dark head capsule with a smooth, cream-colored<br />
body and is 0.31 to 0.43 inch (8 to 11 mm) at maturity<br />
(Figure 36).<br />
Life Cycle: Two generations of sunfl ower bud moth<br />
are produced per year in North Dakota. Adults emerge<br />
from overwintering pupae during the last week of May<br />
to mid-June.<br />
A few days after adult emergence, eggs are deposited<br />
on the terminals of immature sunfl ower or on the receptacle<br />
of mature sunfl ower. Eggs also are deposited<br />
in leaf axils. The hatched larvae begin tunneling into<br />
the sunfl ower plant. The initial infestation in mid-June<br />
is characterized by an entrance hole surrounded by<br />
black frass, or insect excrement.<br />
Mature larvae pupate within the sunfl ower plant. Pupae<br />
move to the opening of the entrance holes formed<br />
in the stem or head tissue so that adults can emerge<br />
easily.<br />
The second-generation adults appear in July and August.<br />
Infestation by the second-generation larvae is not<br />
economically important.<br />
Damage: In early planted sunfl ower, 65 percent to 85<br />
percent of the infestations occur in the stalks. In lateplanted<br />
sunfl ower, most infestations occur in the pith<br />
areas of the head.<br />
Up to 4,000 larvae per acre have been reported in<br />
North Dakota and 24,000 larvae per acre have been<br />
reported in Texas. Despite these high populations,<br />
economic loss due to this insect has been minimal.<br />
The only time yield loss is noticeable is when larvae<br />
burrow into unopened buds, preventing proper head<br />
development. The larvae normally do not feed on<br />
developing seeds but confi ne feeding activities to<br />
the fl eshy part of the head. Yield loss has not been<br />
economically signifi cant, although injury by the larva<br />
produces malformations in both the head and stalk.<br />
■ Figure 35. Adult - Sunfl ower bud moth Suleima<br />
heliathana. (Extension Entomology)<br />
■ Figure 36. Larva - Sunfl ower bud moth Suleima<br />
heliathana. (Extension Entomology)<br />
Scouting Method: A fi eld monitoring scheme for<br />
this insect has not been established since it is not of<br />
economic signifi cance.<br />
Economic Threshold: None established.<br />
Management: Insecticide use has not been warranted<br />
for control of sunfl ower bud moth.<br />
Insects<br />
37
38<br />
■ Long-horned Sunfl ower Stem<br />
Girdler or Longhorned Beetle<br />
Species: Dectes texanus LeConte<br />
Description: The adult is pale gray and 5/8 inch (6 to<br />
11 mm) in length, with long gray and black banded<br />
antennae. (Figure 37) Eggs are about 0.1 inch (1.9<br />
mm) long and elongate, and turn dark yellow prior to<br />
hatch. Mature larvae are yellowish and 1/3 to 1/2 inch<br />
(7 to 13 mm) in length. Larvae bear fl eshy protuberances<br />
on the fi rst seven abdominal segments. (Figure<br />
38).<br />
Life Cycle: Adults appear in mid-June to early July in<br />
the southern Plains. Emergence continues through August,<br />
with 50 percent emerged by mid-July in Texas.<br />
Eggs are laid four to eight days after mating and eggs<br />
are deposited singly in leaf petioles. Approximately<br />
■ Figure 37. Adult - Longhorned beetle Dectes<br />
texanus. (Extension Entomology)<br />
■ Figure 38. Larva - Longhorned beetle Dectes<br />
texanus. (Extension Entomology)<br />
50 eggs are laid per female, with about one-third viable.<br />
Eggs hatch in six to 10 days. Larvae tunnel and<br />
feed in the petioles and stem pith and fi nally move to<br />
the base of the plant to overwinter. Larvae develop<br />
through six instars. In late summer, the mature larvae<br />
girdle the inside of the lower stalk or root crown, move<br />
below the girdle and pack frass into the tunnels. Stalks<br />
often break at the point of girdling, leaving the larva<br />
protected in its frass-packed tunnel during the winter.<br />
The larvae are cannibalistic and stalks usually harbor<br />
only a single larva, even though several may have<br />
hatched in a stalk. This insect has one generation per<br />
year. Host plants include sunfl ower, soybean, ragweed<br />
and cocklebur.<br />
Damage: Plant damage due to adult feeding appears<br />
to be insignifi cant since the scars do not penetrate the<br />
cortex nor encircle the stalk. Larval feeding is apparent<br />
when stalks lodge at the point of the girdle, about<br />
2.5 to 3.5 inches (7 to 9 cm) above the soil surface.<br />
Scouting Method: None has been developed.<br />
Economic Threshold: None established.<br />
Management: In the southern Plains, later planting<br />
dates and fall or winter tillage have reduced sunfl ower<br />
infestations by this pest. Perennial sunfl ower species<br />
are resistant to stalk infestation, indicating the possibility<br />
of breeding cultivars resistant to the longhorned<br />
sunfl ower stem girdler. Chemical treatments on<br />
soybean and sunfl ower are ineffective against larvae<br />
and were determined to be impractical against adults<br />
because of the extended emergence period. When<br />
larvae are present in the stalks, plants do not always<br />
lodge. Utilizing lower plant populations that encourage<br />
thicker stalks may help reduce damage from<br />
lodging. If fi elds are suspected to be infested, prompt<br />
harvesting will limit losses from lodging.
■ Sunfl ower Maggots<br />
Species:<br />
Sunfl ower receptacle maggot, Gymnocarena diffusa<br />
(Snow)<br />
Sunfl ower maggot, Strauzia longipennis (Wiedemann)<br />
Sunfl ower seed maggot, Neotephritis fi nalis (Loew)<br />
Description: The adult forms of all three sunfl ower<br />
maggots (fl ies) have wings with a distinct brown or<br />
yellowish-brown pattern. The name “picture-wing fl y”<br />
has been given to fl ies of this type. While all three fl y<br />
species are similar in appearance, they do have distinguishing<br />
differences.<br />
Gymnocarena diffusa - This species is the largest of<br />
the three, with a body about 0.4 inch (10 mm) long<br />
and a wing span of approximately 0.75 inch (19 mm)<br />
(Figure 39). The eyes of this species are bright green<br />
and the wings have a yellowish-brown and somewhat<br />
mottled appearance. G. diffusa larvae attain a length of<br />
nearly 0.31 inch (8 mm) at maturity. The larvae taper<br />
from the front to rear and are yellowish white (Figure<br />
40).<br />
Strauzia longipennis - Adults of this species have a<br />
wing spread of about 0.5 inch (13 mm) and a body<br />
0.25 inch (6 mm) long (Figure 41). The wings bear<br />
broad, dark bands that form a fairly distinct F-shaped<br />
mark near the tips. The larvae of S. longipennis are<br />
creamy white, headless and legless, as are the other<br />
two species (Figure 42). They taper slightly at both<br />
ends and attain a length of about 0.28 inch (7 mm) at<br />
maturity.<br />
Neotephritis fi nalis - This sunfl ower maggot is the<br />
smallest of the three species, with the adult having<br />
a body length of about 0.25 inch (6 mm) and a wing<br />
span of approximately 0.28 inch (7 mm) (Figure<br />
43). The wings have a brown lacelike appearance. N.<br />
fi nalis larvae attain a length of 0.19 inch (4.5 mm) at<br />
maturity. The small, brown pupa of N. fi nalis is found<br />
in the face of the sunfl ower bud, usually surrounded by<br />
a small number of damaged fl orets (Figure 44).<br />
Life Cycles: Adults of G. diffusa emerge in late June<br />
to early July after sunfl ower buds reach 2 to 4 inches<br />
(5 to 10 cm) in diameter. Eggs are laid on the bracts<br />
■ Figure 39. Adult - Sunfl ower receptacle maggot<br />
Gymnocarena diffusa. (Extension Entomology)<br />
■ Figure<br />
40. Larva<br />
- Sunfl ower<br />
receptacle<br />
maggot<br />
Gymnocarena<br />
diffusa.<br />
(Extension<br />
Entomology)<br />
■ Figure 41. Adult - Sunfl ower maggot Strauzia<br />
longipennis. (Extension Entomology)<br />
■ Figure 42. Larva - Sunfl ower maggot Strauzia<br />
longipennis. (Extension Entomology)<br />
Insects<br />
39
40<br />
of the developing sunfl ower heads. Egg laying occurs<br />
from mid-July through August. The hatched larvae<br />
tunnel into the spongy tissue of the receptacle. Damage<br />
to the head is negligible. After 30 days, the mature<br />
larvae cut a small emergence hole on the underside of<br />
the receptacle and drop into the soil to pupate. Overwintering<br />
pupae are found about 7.5 inches (19 cm)<br />
deep in the soil by August or early September. Some<br />
larvae will pupate in the sunfl ower head. Only one<br />
generation per year occurs in North Dakota.<br />
Strauzia longipennis has one generation per year. This<br />
insect overwinters as a larva in plant debris in the<br />
soil. Pupation and adult emergence is completed in<br />
early June. Females lay eggs in stem tissue of young<br />
sunfl ower, and larvae feed in the pith tissue for much<br />
of the growing season.<br />
■ Figure 43. Adult - Sunfl ower seed<br />
maggot Neotephritis fi nalis. (Extension<br />
Entomology)<br />
■ Figure 44. Pupae - Sunfl ower seed maggot<br />
Neotephritis fi nalis. (Extension Entomology)<br />
Unlike the other two species of sunfl ower maggots,<br />
two complete generations per year of N. fi nalis occur<br />
in North Dakota. Adults of N. fi nalis emerge during<br />
the fi rst week of July. Egg deposition occurs on the corolla<br />
of incompletely opened sunfl ower infl orescences.<br />
The total larval period is 14 days. The fi rst generation<br />
of N. fi nalis pupates in the head; the second generation<br />
overwinters in the soil as pupae.<br />
Damage: Damage by sunfl ower maggots has been<br />
negligible.<br />
The maggots of Gymnocarena diffusa feed on the<br />
spongy receptacle tissue of the sunfl ower head and<br />
feeding may cause partially deformed heads. Larvae<br />
do not feed on developing seeds.<br />
The magnitude of damage to sunfl ower seeds by N.<br />
fi nalis larvae depends largely on the stage of larval<br />
and seed development. Seed sterility occurs when<br />
newly hatched larvae tunnel into the corolla of young<br />
blooms. Observations indicate that a single larva feeding<br />
on young fl owers will tunnel through 12 ovaries.<br />
Mature larvae feeding on older sunfl ower heads will<br />
destroy only one to three seeds.<br />
While infestation levels of S. longipennis occasionally<br />
have reached nearly 100 percent, damage from larval<br />
feeding is usually light. Part of a commercial sunfl<br />
ower fi eld next to a grassed waterway or other water<br />
source sometimes supports a higher than usual infestation.<br />
Under these conditions, high larval numbers of<br />
eight to 10 per stalk may be found and stalk breakage<br />
can occur. Stalk breakage of up to 30 percent of the<br />
plants has been recorded.<br />
Scouting Method: A scouting method has not been<br />
developed for sunfl ower maggots because of the negligible<br />
injury caused by these insects.<br />
Economic Threshold: None established.<br />
Management: Insecticide use has not been warranted<br />
for control of sunfl ower maggots.
■ Sunfl ower Stem Weevil<br />
Species: Cylindrocopturus adspersus (LeConte)<br />
Description: Adult sunfl ower stem weevils are about<br />
3/16 inch (4 to 5 mm) long and grayish brown, with<br />
varying-shaped white spots on the wing covers and<br />
thorax (Figure 45). The snout, eyes and antennae are<br />
black. The snout is narrow and protrudes down and<br />
backward from the head. Eggs are deposited inside the<br />
epidermis of sunfl ower stems and are very small (0.51<br />
mm long by 0.33 mm wide), oval and yellow, making<br />
them diffi cult to see. The larvae are ¼ inch (5 to 6<br />
mm) long at maturity, legless and creamy white with<br />
a small, brown head capsule (Figure 46). They are<br />
normally in a curled or C-shaped position within the<br />
sunfl ower stalk. Pupae are similar to the adult in size<br />
and creamy white<br />
Life Cycle: Only one generation occurs per year. Larvae<br />
overwinter in sunfl ower stalks and crown roots and<br />
pupate in the spring, and adults emerge in mid to late<br />
June, feeding on the epidermal tissue of the sunfl ower<br />
foliage and stem. This feeding does not affect plant<br />
vigor. Mating occurs soon after emergence of adults.<br />
Just prior to egg laying, females descend to the lower<br />
portion of the plant to deposit eggs individually in the<br />
stem tissue. Approximately 50 percent of oviposition<br />
occurs by mid-July. Upon hatching in early July, the<br />
fi rst instar (larval growth stage) larvae feed on subepidermal<br />
and vascular tissue. Feeding is concentrated in<br />
the pith tissue as the larvae develop to third and fourth<br />
instar stages. By the last week in August, the larvae<br />
descend while feeding to just above the soil surface.<br />
A chamber is constructed in the stem, and the weevil<br />
overwinters there as a fi fth instar larva. Pupation of<br />
the overwintering larva occurs the following year in<br />
early June.<br />
Damage: Adult sunfl ower stem weevil feeding causes<br />
minor damage to the stem and leaf tissue of the plant.<br />
More importantly, adult weevils have been implicated<br />
in the epidemiology of the sunfl ower pathogen<br />
Phoma black stem (Phoma macdonaldii Boerma) and<br />
charcoal stem rot (Macrophomina phaseolina (Tassi)<br />
Goid).<br />
Larval injury can cause the stem to weaken from tunneling,<br />
pith destruction and especially by construction<br />
of overwintering chambers at the stalk base. At larval<br />
infestations of 20 to 25 or more per stalk, the plants<br />
run a risk of stalk breakage and loss of the entire capitual<br />
(head). Risk of breakage is greatest when plants<br />
are under drought stress and/or during periods of high<br />
winds. The breakage typically occurs at or slightly<br />
above the soil line, in contrast to breakage attributed<br />
to a stalk disease, which normally occurs farther up on<br />
the stalks.<br />
Scouting Methods: <strong>Field</strong> monitoring for sunfl ower<br />
stem weevils to estimate population size is important.<br />
However, adults are diffi cult to see on the plants<br />
due to their small size, cryptic color and “play dead”<br />
behavior. They are inactive on the plant or fall to the<br />
ground when disturbed and remain motionless. Adults<br />
can be found on both surfaces of the leaves, the lower<br />
portions of the stem, in leaf axils, within the dried<br />
■ Figure 45. Adult - Sunfl ower stem weevil<br />
Cylindrocopturus adspersus. (Extension Entomology)<br />
■ Figure 46. Larva - Sunfl ower stem weevil<br />
Cylindrocopturus adspersus. (Extension Entomology)<br />
Insects<br />
41
42<br />
cotyledons or in soil cracks at the base of the sunfl<br />
ower plant. Yellow sticky traps were unsuccessful in<br />
relating captured adult numbers to larval infestations.<br />
Sampling for the larval stage is diffi cult since they<br />
develop totally within the sunfl ower plant. The only<br />
method for detecting the presence of larvae is to split<br />
the sunfl ower stem, a time-consuming process.<br />
<strong>Field</strong> scouting for adults should begin when plants<br />
are in the eight- to 10-leaf stage, developmental stage<br />
V-8 to V-10, or late June to early July, and continue<br />
until mid-July. Select sampling sites 70 to 100 feet in<br />
from the fi eld margin. Count the number of adults on<br />
fi ve plants at fi ve randomly selected sampling sites<br />
throughout the fi eld for a total of 25 plants. Calculate<br />
the average number of weevils per plant. Use an X<br />
pattern (or W pattern) to space sample sites throughout<br />
the entire fi eld. When scouting for stem weevils,<br />
approach plants carefully and slowly to avoid disturbing<br />
the adults.<br />
Economic Threshold: Average fi eld counts of one<br />
adult sunfl ower stem weevil per three plants can result<br />
in damaging larval densities of more than 40 larvae<br />
per stalk at the end of the season.<br />
Management: Insecticidal treatment, if needed based<br />
on fi eld counts, should be initiated in late June or<br />
early July before signifi cant egg laying has occurred.<br />
Cultural control tactics, including delayed planting,<br />
altered plant population and tillage, are useful for<br />
managing the sunfl ower stem weevil. Delayed planting<br />
of sunfl owers until late May or early June has been<br />
effective in reducing densities of larvae in the stem.<br />
Reducing plant population results in an increased<br />
stalk diameter and, as a result, decreases damage from<br />
lodging. Combinations of disking to break up stalks<br />
and moldboard plowing to bury them at a depth of 6<br />
inches (15 cm) can increase larval/pupal mortality and<br />
severely impact the emergence of adult stem weevils.<br />
Otherwise, larvae/pupae are physically protected in<br />
the woody stalks. Survival is affected only by performing<br />
both operations. Greenhouse and fi eld experiments<br />
have shown resistance to feeding, oviposition<br />
and larval development in many native species of<br />
sunfl ower. <strong>Field</strong> research for resistant sunfl ower germplasm<br />
is under way. Natural enemies of the sunfl ower<br />
stem weevil include parasitic wasps that attack both<br />
the egg and larval stages.<br />
■ Black Sunfl ower Stem Weevil<br />
Species: Apion occidentale (Fall)<br />
Description: Adults are black and only 0.1 inch (2.5<br />
mm) long from the tip of the snout to the tip of the<br />
abdomen (Figure 47). The snout is very narrow and<br />
protrudes forward from the head, which is small in relation<br />
to the rather large, almost globose body. Larvae<br />
of A. occidentale are very similar in appearance to C.<br />
adspersus, except they are only 0.1 to 0.12 inch (2.5 to<br />
3 mm) long at maturity and yellowish (Figure 48).<br />
Life Cycle: Apion occidentale overwinters as an adult<br />
in soil, plant residue, sod and weed clusters and begins<br />
to emerge and feed on volunteer sunfl ower as soon<br />
as the plants reach the early seedling stage. Females<br />
deposit eggs under the epidermis of the stem or leaf<br />
petioles. Larvae emerging from these eggs tunnel in<br />
the pith area of the stem, pupate and emerge as adults<br />
in early August. Little or no adult activity is observed<br />
■ Figure 47. Adult - Black sunfl ower stem weevil<br />
Apion occidentale. (Extension Entomology)<br />
■ Figure 48. Larva - Black sunfl ower stem weevil<br />
Apion occidentale. (Extension Entomology)
for about two weeks in late July and early August.<br />
Black sunfl ower stem weevil adults emerging in August<br />
also feed on the leaves and stems of the plant, but<br />
as the plant matures and the leaves begin to die, the<br />
adults move under the bracts of the sunfl ower head,<br />
where they can be observed feeding until the plants<br />
are harvested.<br />
Damage: Adult feeding generally is considered as insignifi<br />
cant mechanical injury. Like the sunfl ower stem<br />
weevil, the black sunfl ower stem weevil is suspected<br />
of vectoring Phoma black stem disease in sunfl ower<br />
fi elds. In situations of extremely high populations<br />
feeding on seedling sunfl owers, stand loss has occurred.<br />
However, in most cases, populations are too<br />
low to cause economic damage and stalk tunneling<br />
only results in minor injury to the plant.<br />
Scouting Method: A scouting method has not been<br />
developed for the black sunfl ower stem weevil.<br />
Economic Threshold: None established.<br />
Management: Recommendations for insecticidal control<br />
of this insect have not been developed.<br />
■ Figure 49. Adult - Sunfl ower root weevil Baris<br />
strenua. (Extension Entomology)<br />
■ Sunfl ower Root Weevil<br />
Species: Baris strenua (LeConte)<br />
Description: Adults are rather robust-looking weevils,<br />
with a somewhat oval-shaped body (Figure 49). They<br />
are 0.25 inch (6 mm) long and have a short, almost<br />
blunt, downward projecting snout. Their coloration is<br />
dull black in contrast to the shiny, black appearance<br />
of A. occidentale. Baris strenua larvae are similar in<br />
appearance to C. adspersus larvae but much larger and<br />
are not located in the sunfl ower stalk (Figure 50).<br />
Life Cycle: Adult root weevils emerge during the latter<br />
part of June. They feed on sunfl ower foliage in early<br />
morning and late afternoon. About two weeks after<br />
emergence, the adults begin to congregate around the<br />
root zone near the soil surface. Continued feeding and<br />
copulation occur during this period. Feeding activity<br />
during this period produces callus tissue, under<br />
which the bright yellow eggs are deposited two or<br />
three at a time. Hatching of the larvae normally occurs<br />
during the second week in July. Baris strenua larvae<br />
are not very mobile. Most of the feeding (consisting<br />
of circular tunnels) and development to fourth instar<br />
takes place in the same area where hatching occurs. At<br />
about the time that the fourth larval stage is reached<br />
in late August to early September, the plant becomes<br />
signifi cantly dehydrated and encapsulation of the<br />
larvae within a “soil cocoon” begins. This “larval<br />
cocoon” overwinters among the remaining roots in the<br />
soil. Overwintering larvae have been recovered from a<br />
depth of 15 inches (38 cm) in North Dakota.<br />
Damage: The sunfl ower root weevil adult, like the<br />
other two stem weevils, causes negligible mechanical<br />
injury to the foliage of the sunfl ower plant. The destruction<br />
of root tissue by the larvae of the sunfl ower<br />
root weevil causes the plants to wilt and lodge if the<br />
infestation is severe. The damage to fi elds attacked by<br />
the weevil tends to be localized.<br />
Scouting Method: A scouting method has not been<br />
developed because damage caused by this pest has<br />
been minor.<br />
Economic Threshold: None established.<br />
Management: Insecticide use has not been warranted<br />
for the control of the sunfl ower root weevil.<br />
■ Figure 50. Larva - Sunfl ower root weevil Baris<br />
strenua. (Extension Entomology)<br />
Insects<br />
43
44<br />
■ Thistle Caterpillar (Painted Lady)<br />
Species: Vanessa cardui (Linnaeus)<br />
Description: The body of the adult is about 1 inch<br />
(25 mm) long with a wingspread of about 2 inches<br />
(50 mm) (Figure 51). The upper wing surfaces are<br />
brown with red and orange mottling and white and<br />
black spots. The undersides of the wings are marble<br />
gray, buff and white. Each hind wing possesses a row<br />
of four distinct and obscure eyespots. Eggs are small,<br />
spherical and white. The larvae are brown to black and<br />
spiny, with a pale yellow stripe on each side (Figure<br />
52). When mature, the larvae are 1.25 to 1.5 inches<br />
(32 to 38 mm) long. The chrysalis, or pupa, is molten<br />
gold and about 1 inch (25 mm) long.<br />
■ Figure 51. Adult - Painted lady butterfl y Vanessa<br />
cardui. (Extension Entomology)<br />
■ Figure 52. Larva - Painted lady butterfl y Vanessa<br />
cardui. (Extension Entomology)<br />
Life Cycle: The painted lady butterfl y is indigenous<br />
to the southern U.S. and migrates annually to the<br />
northern U.S. and Canada. The painted lady breeds in<br />
the north-central states and Canada, migrates south for<br />
the winter and returns to the northern areas in early<br />
June. Eggs are laid on Canada thistle, wild and cultivated<br />
sunfl ower, and many other host plants. Hatching<br />
occurs in about one week. Larvae feed on sunfl ower<br />
until they reach maturity in late June or early July.<br />
Chrysalids are formed and hang from the leaves of the<br />
plant. Butterfl ies will emerge in about 10 days from<br />
the chrysalid and a second generation begins.<br />
Damage: The caterpillars (larvae) feed on the leaves<br />
and, when numerous, may defoliate infested plants.<br />
The larvae produce a loose silk webbing that covers<br />
them during their feeding activity. Black fecal pellets<br />
produced by the larvae often are found in proximity to<br />
the webbing.<br />
The effect of defoliation by the larvae on the yield of<br />
sunfl ower is similar to that described for defoliation by<br />
sunfl ower beetle larvae.<br />
Scouting Method: Sampling sites should be at least<br />
75 to 100 feet (23 to 31 m) from the fi eld margins<br />
when collecting data to determine whether an entire<br />
fi eld should be treated. Infestations frequently will be<br />
concentrated in areas of a fi eld where Canada thistle<br />
plants are abundant. Plants should be examined carefully<br />
for the presence of eggs and/or larvae.<br />
The fi eld should be monitored by using the X pattern,<br />
counting 20 plants per sampling site for a total of 100<br />
plants to determine percent defoliation (Figure 35).<br />
Economic Threshold: The threshold is 25 percent defoliation,<br />
provided that most of the larvae are still less<br />
than 1.25 inch (32 mm) long. If the majority of the<br />
larvae are 1.25 to 1.5 inches (32 to 38 mm) long, most<br />
of the feeding damage already will have occurred and<br />
treatment is not advised.<br />
Management: Insecticide use generally has not been<br />
warranted for control of larvae of the painted lady.<br />
However, instances of high localized infestations have<br />
occurred within certain fi elds where spot treating may<br />
be necessary. Disease outbreaks, indicated by dying<br />
larvae present on leaves, often occur when large populations<br />
are present.
■ Sunfl ower Midge<br />
Species: Contarinia schulzi Gagne'<br />
Description: The tan body of the adult sunfl ower<br />
midge is about 0.07 inch (1.69 mm) long, with a<br />
wingspan of about 0.19 inch (4 mm) (Figure 53). The<br />
wings are transparent with no markings except the<br />
veins. The larvae attain a length of nearly 0.09 inch<br />
(2.42 mm) at maturity and they are cream to yellowish<br />
orange when fully grown (Figure 54). They are<br />
tapered at the front and rear, with no legs or apparent<br />
head capsule.<br />
Life Cycle: The sunfl ower midge overwinters in the<br />
soil as a cocooned larva and pupates during June and<br />
July in North Dakota and Minnesota. Typically, the<br />
initial peak of fi rst-generation adult emergence occurs<br />
in early to mid-July. A second peak occurs about seven<br />
to 10 days later. They prefer to lay eggs on sunfl ower<br />
buds with a diameter greater than 1 inch (25 mm).<br />
Larvae initially feed on margins of the head between<br />
the bracts surrounding the heads. Larvae migrate to<br />
the base of the developing seeds and to the center<br />
of the head as it develops. Presence of the larvae<br />
frequently is determined by necrotic areas at the base<br />
of or between the bracts. As midge larvae mature,<br />
they move to the surface of the head and drop to the<br />
ground. A partial, second generation occurs in August.<br />
Second-generation adults oviposit among the seeds.<br />
Damage: Damage to sunfl ower is a result of fi rstgeneration<br />
larval feeding in developing heads. When<br />
populations are low, damage is restricted to the base<br />
of the bracts of the head and causes slight localized<br />
necrosis but little if any economic loss. When many<br />
larvae are present, feeding prevents ray petal formation<br />
and distorts the growth of the developing sunfl<br />
ower head. If the abnormal growth is severe, the back<br />
of the head overgrows the front and little or no seed<br />
production occurs (Figure 55). If an infestation occurs<br />
in the early bud stage, the bud may be killed.<br />
Often midge damage is restricted to fi eld margins<br />
or small portions of fi elds and economic losses are<br />
minimal. However, when populations are very heavy,<br />
damage will extend throughout the fi eld and substantial<br />
economic losses occur. The extent of damage from<br />
second generation larvae is unknown.<br />
Scouting Method: None established.<br />
Economic Threshold: None established.<br />
Management: Because effective chemical and other<br />
controls are not available, sunfl ower midge management<br />
relies on cultural practices done prior to planting.<br />
If a midge infestation is anticipated, new fi elds<br />
should be established away from fi elds damaged the<br />
previous season. To minimize the risk of all plantings<br />
being at their most susceptible stage at midge emergence,<br />
several planting dates should be used. If available,<br />
growers should consider using a tolerant hybrid.<br />
■ Figure<br />
53. Adult<br />
- Sunfl ower<br />
midge<br />
Contarinia<br />
schulzi.<br />
(Extension<br />
Entomology)<br />
■ Figure 54. Larva - Sunfl ower midge Contarinia<br />
schulzi. (Extension Entomology)<br />
■ Figure 55. Severe damage to receptacle and seed<br />
development occurs when midge infection is high.<br />
(Extension Entomology)<br />
Insects<br />
45
46<br />
■ Red Sunfl ower Seed Weevil<br />
Species: Smicronyx fulvus LeConte<br />
Description: Red sunfl ower seed weevil adults are 0.1<br />
to 0.12 inch (2.5 to 3.06 mm) long and reddish brown<br />
(Figure 56). The larvae are small, 0.10 inch (2.54 mm)<br />
long, cream-colored, legless and C-shaped (Figure 57).<br />
Life Cycle: Red sunfl ower seed weevil emergence<br />
occurs in late June and early July. The newly emerged<br />
adults feed on sunfl ower buds or fl oral tissues. Once<br />
pollen is available, the adults include it in their diet.<br />
Females need to feed on sunfl ower pollen for several<br />
days prior to egg deposition. Eggs are deposited<br />
within young developing seeds. Normally a single<br />
egg is placed in each seed, although 8 percent to 12<br />
percent of the seeds may contain several eggs.<br />
■ Figure 56. Adult - Red sunfl ower seed weevil<br />
Smicronyx fulvus. (Extension Entomology)<br />
■ Figure 57. Larva - Red sunfl ower seed weevil<br />
Smicronyx fulvus. (Extension Entomology)<br />
The small, white eggs hatch in approximately one<br />
week. The larvae consume a portion of the kernel, and<br />
this feeding causes economic damage. After completion<br />
of larval development, the majority of the larvae<br />
drop to the ground. Larval drop occurs from mid-<br />
August through September. The larvae overwinter in<br />
the soil at a depth of about 6 inches (15 cm). Larvae<br />
pupate in late June of the following year and the pupal<br />
period lasts about one week. A single generation per<br />
year is produced in North Dakota.<br />
Damage: While the kernel of some seeds may be<br />
totally eaten, most seeds are only partially consumed.<br />
The separation of undamaged from weevil-damaged<br />
seed is diffi cult.<br />
Most larvae drop from the head to the soil after completing<br />
their development, but a small percentage may<br />
remain in the seed and are present at harvest. Growers<br />
who encounter a seed weevil infestation may want to<br />
delay harvest to allow most of the weevil larvae to exit<br />
the seeds to avoid having larvae in the harvest bin.<br />
Larvae that are still in the seed at bin fi lling time are<br />
done feeding and can cause heating and moisture<br />
problems. Larvae harvested with the seed cannot be<br />
controlled until they have completed development and<br />
have emerged from the infested seeds. Once emerged,<br />
they are susceptible to fumigation. But fumigation<br />
normally is not recommended. However, the most<br />
advantageous time to initiate control of seed weevil is<br />
in the fi eld when the adult weevils are active, but prior<br />
to egg deposition.<br />
Economic Thresholds: The economic threshold varies<br />
with differences in plant population, the cost of insecticide<br />
application and the market price of sunfl ower.<br />
The procedure for calculating the economic threshold<br />
is discussed in the NDSU Extension sunfl ower seed<br />
weevil publication (E-817). Currently, an infestation<br />
level of four to seven seed weevil adults per head in<br />
oil sunfl ower or one seed weevil per head in confectionery<br />
sunfl ower is the average economic threshold.<br />
The optimal period for insecticide treatment is when<br />
at least three out of 10 plants in the fi eld are at early<br />
bloom (R-5.1 to R-5.4, Figure 4) and the economic<br />
threshold has been reached. If spray application is<br />
delayed past when more than four out of 10 plants are<br />
at stage R-5.4, many eggs already will be laid in the<br />
developing seeds and those eggs and larvae cannot be<br />
controlled. If fi elds are sprayed too early, reinfesta-
tion may occur in areas with a high weevil population.<br />
After spraying, fi elds should be rechecked periodically<br />
to determine if reinfestation is reaching the economic<br />
threshold. Continue rechecking until most of the heads<br />
in the fi eld have reached the R-5.7 stage. At that stage,<br />
most eggs already will have been laid and most seeds<br />
will be too mature to be suitable for further red seed<br />
weevil egg oviposition.<br />
Scouting Method: Begin by taking samples from 12<br />
plants, three plants from each of the four fi eld sides.<br />
Sampling sites should be at least 75 feet (21 m) in<br />
from fi eld borders, which often have an inordinately<br />
high number of weevils. The total number of weevils<br />
counted should be compared to the sequential sampling<br />
table in the most recent NDSU Extension sunfl<br />
ower seed weevil publication (E-817). According to<br />
the table, take one of three possible actions: Stop sampling,<br />
no action is needed; stop sampling and treat;<br />
or, take more samples because a decision cannot be<br />
reached. When populations are low or high, sequential<br />
sampling allows a quick decision with few samples.<br />
If populations are near the economic threshold, more<br />
precision is needed to making an accurate determination<br />
and more samples are required.<br />
NOTE: To more precisely check individual sunfl ower<br />
heads for red sunfl ower seed weevils, the face of the<br />
heads should be sprayed with a commercial formulation<br />
of mosquito repellent containing diethyl toluamide<br />
(DEET). This will cause the weevils to move<br />
out from between the fl orets where they can be more<br />
accurately counted. Consult the most recent NDSU<br />
Extension sunfl ower seed weevil publication (E-817)<br />
for a table to convert the visual counts to the absolute<br />
number of weevils (both counted and uncounted).<br />
Management: Several federally registered insecticides<br />
are available for control of sunfl ower seed weevils in<br />
the U.S. Early planting of sunfl ower reduces achene<br />
damage caused by the red sunfl ower seed weevil without<br />
causing a measurable reduction in oil content and<br />
achene weight.<br />
Surrounding a sunfl ower fi eld with a ring of early<br />
blooming sunfl ower effectively can trap immigrating<br />
red sunfl ower seed weevils into a small portion<br />
of the fi eld, where they can be controlled effi ciently.<br />
The trap cropping method given in publication E-817<br />
is as effective and more cost effi cient than standard<br />
insecticide treatment for control of red sunfl ower seed<br />
weevils.<br />
■ Gray Sunfl ower Seed Weevil<br />
Species: Smicronyx sordidus LeConte<br />
Description: Adults of the gray sunfl ower seed weevil<br />
are slightly larger (0.14 inch long) than S. fulvus and<br />
gray (Figure 58). The larvae are small, 0.12 inch<br />
long (3.1 mm), cream-colored, legless and C-shaped<br />
(Figure 59).<br />
Life Cycle: Gray sunfl ower seed weevil emergence occurs<br />
in late June and early July and reaches 50 percent<br />
emergence about 10 days before the red sunfl ower<br />
seed weevil. The newly emerged adults feed on fl oral<br />
buds. Oviposition occurs on fl owers in the bud stage<br />
and before red sunfl ower seed weevil oviposition<br />
begins. Female gray sunfl ower seed weevils do not lay<br />
as many eggs as do females of the red sunfl ower seed<br />
weevil.<br />
■ Figure 58. Adult - Gray sunfl ower seed weevil<br />
Smicronyx sordidus. (Extension Entomology)<br />
■ Figure 59. Larva - Gray sunfl ower seed weevil<br />
Smicronyx sordidus. (Extension Entomology)<br />
Insects<br />
47
48<br />
The larvae feed in a single achene, and infested<br />
achenes are enlarged and protrude above surrounding<br />
uninfested achenes. The majority of the larvae drops<br />
to the ground from mid-August through September<br />
and overwinters in the soil. Larvae pupate in late June<br />
and a single generation per year is produced in North<br />
Dakota.<br />
Damage: Seeds infested by the gray seed weevil lack<br />
a kernel and, due to their light weight, the seeds may<br />
be lost during the harvesting process. Because of their<br />
low population levels and low fecundity, the gray sunfl<br />
ower seed weevil usually does not cause economic<br />
damage, especially in oil sunfl ower fi elds. In confection<br />
fi elds, however, populations of the gray sunfl ower<br />
seed weevil may be suffi ciently high to warrant treatment<br />
at the late bud stage (R-3 to R-4).<br />
As with the red sunfl ower seed weevil, larvae normally<br />
drop from the head to the soil after completing their<br />
development. Larvae that do not emerge will present<br />
the grower with the same problem as unemerged red<br />
sunfl ower seed weevil larvae.<br />
Scouting Method: Normally, gray sunfl ower seed<br />
weevil populations are too low to cause economic<br />
damage. However, if an area has had a history of high<br />
populations, fi elds, especially confection fi elds, should<br />
be sampled beginning at bud stage R-2 (Figure 4).<br />
Sampling should be done as for the red sunfl ower seed<br />
weevil and continue until plants are blooming.<br />
Economic Thresholds: None established.<br />
Management: Several insecticides are federally registered<br />
for control of sunfl ower seed weevils in the U.S.<br />
If fi elds are to be treated with insecticides, they should<br />
be sprayed while the plants are still in early bud stage.<br />
By late bud stage, most oviposition already will have<br />
occurred.<br />
■ Sunfl ower Moth<br />
Species: Homoeosoma electellum (Hulst)<br />
Description: The adult is a shiny gray to grayish tan<br />
moth about 0.38 inch (9 mm) long, with a wingspan<br />
of about 0.75 inch (19 mm) (Figure 60). The hind<br />
wings are devoid of markings; however, the forewings<br />
have a small, dark, discal dot near the center of each<br />
wing and two or three small, dark dots near the leading<br />
margin of each wing. When at rest, the wings are<br />
held tightly to the body, giving the moth a somewhat<br />
cigar-shaped appearance. The larva has alternate dark<br />
and light-colored longitudinal stripes on a light brown<br />
body (Figure 61). The larva is about 0.75 inch (19<br />
mm) long at maturity.<br />
■ Figure 60. Adult - Sunfl ower moth Homoeosoma<br />
electellum. (Extension Entomology)<br />
■ Figure 61. Larva - Sunfl ower moth Homoeosoma<br />
electellum. (Extension Entomology)
Life Cycle: Sunfl ower moth migrations from the<br />
south-central U.S. normally appear in North Dakota<br />
in early to mid-July. The moths are highly attracted<br />
to sunfl ower that is beginning to bloom. Individual<br />
female moths will deposit up to 30 eggs per day on<br />
the surface of open sunfl ower heads. The eggs hatch<br />
within 48 to 72 hours and the newly emerged larvae<br />
feed on pollen and fl orets. The larvae begin tunneling<br />
into seeds upon reaching the third instar (larval<br />
growth stage). This tunneling continues throughout<br />
the remainder of larval development. Larval development<br />
from hatching to full maturity takes about 15 to<br />
19 days.<br />
Damage: The young larvae of the sunfl ower moth feed<br />
primarily on fl orets and pollen. Older larvae tunnel<br />
through immature seeds and other parts of the head. A<br />
single larva may feed on three to 12 seeds and forms<br />
tunnels in both the seeds and head tissue. Larvae spin<br />
silken threads, which bind with dying fl orets and frass<br />
to give the head a trashy appearance. Severe larval infestations<br />
can cause 30 percent to 60 percent loss, and<br />
in some cases, the entire head can be destroyed. Sunfl<br />
ower infested with sunfl ower moth has an increased<br />
incidence or risk of Rhizopus head rot.<br />
Scouting Method: Sampling sites should be at least<br />
75 to 100 feet (23 to 31 m) from fi eld margins. The X<br />
pattern should be used in monitoring a fi eld, counting<br />
moths on 20 heads per sampling site for a total of 100<br />
heads. Scouting is most accurate in the early morning<br />
or late evening, when moths are active. Sex pheromone<br />
lures are available commercially for monitoring<br />
with traps to indicate their arrival and local populations.<br />
Insecticide applications should be considered<br />
when pheromone trap catches average four moths per<br />
trap per day from the R-3 through R-5 growth stages.<br />
Economic Threshold: The economic threshold for<br />
sunfl ower moth is one to two adults per fi ve plants at<br />
the onset of bloom or within seven days of the adult<br />
moth’s fi rst appearance. If using pheromone traps,<br />
consider the threshold mentioned in the Scouting<br />
Method section.<br />
Management: A number of federally labeled insecticides<br />
are registered for control of the sunfl ower moth.<br />
■ Banded Sunfl ower Moth<br />
Species: Cochylis hospes Walsingham<br />
Description: The adult has a dark band across the buff<br />
or yellowish-tan forewings (Figure 62 ). The wingspan<br />
is about 0.5 inch (13 mm). Early instar larvae are<br />
off-white; late instar larvae are pinkish to red with a<br />
brown head capsule (Figure 63). Larvae will be about<br />
0.44 inch (11 mm) at maturity.<br />
Life Cycle: The life cycle of the banded sunfl ower<br />
moth is similar to that of the sunfl ower moth, except<br />
that the adults emerge from local overwintering sites<br />
rather than migrating into North Dakota. Banded<br />
■ Figure 62. Adult and eggs - Banded sunfl ower moth<br />
Cochylis hospes. (Extension Entomology)<br />
■ Figure 63. Larva - Banded sunfl ower moth Cochylis<br />
hospes. (Extension Entomology)<br />
Insects<br />
49
50<br />
sunfl ower moths begin to emerge from the soil about<br />
mid-July and are present in the fi eld until mid-August.<br />
Adults tend to congregate in fi eld margins on weeds or<br />
adjacent crops during the day and then move into the<br />
crop in the evening. Within a week after emergence,<br />
they begin to lay eggs on the outside of the bracts of<br />
the sunfl ower head. Eggs may be found through early<br />
August and hatch in fi ve to eight days. Larvae develop<br />
through fi ve instars and are present in sunfl ower heads<br />
from mid-July to mid-September. After feeding to<br />
maturity, larvae drop to the ground and spin cocoons<br />
in the soil to overwinter. Pupation takes place in late<br />
June or early July the following year. The pupal period<br />
lasts about 12 days.<br />
Damage: The newly hatched larvae move from the<br />
bracts to the fl orets of the sunfl ower head, where they<br />
enter open fl orets to feed. When the eggs hatch, young<br />
larvae feed on bract tissue before moving into the<br />
head. A sunfl ower head is susceptible to infestation<br />
only during the fl owering period. The larvae feed in<br />
the fl orets until the third instar. During later stages<br />
of larval development, the insect tunnels through the<br />
base of the fl oret into the seed. The larvae may consume<br />
part or all of the contents of the developing seed.<br />
The larvae usually enter near the top of the seed and<br />
leave by way of the same opening after the contents<br />
are eaten. Each larva may destroy several (fi ve to<br />
seven) seeds. Small areas of silken webbing on mature<br />
sunfl ower heads indicate the presence of banded sunfl<br />
ower moth larvae within the head.<br />
Adult Scouting Method and Economic Threshold:<br />
Sampling sites should be at least 75 to 100 feet from<br />
the fi eld margins. In monitoring a fi eld, use the X<br />
pattern (Figure 17), counting moths on 20 plants per<br />
sampling site to obtain the total number of moths per<br />
100 plants. Sampling should be conducted in the late<br />
bud stage (R3), usually during mid-July. If treatment<br />
is warranted, it should be applied at the R5.1 sunfl ower<br />
plant growth stage (when 10% of head area has disk<br />
fl owers that are fl owering or completed fl owering.)<br />
During the day (late morning to early afternoon) the<br />
moths remain quiet, resting on upper or lower surfaces<br />
of the leaves of sunfl ower plants. When disturbed,<br />
they fl utter from plant to plant. When sampling for<br />
moths during the day, the decision to treat or not is<br />
based on comparing the mean number of adult moths<br />
in the fi eld to the EIL for moths. The EIL number is<br />
the number of moths per head that will, if not man-<br />
aged, result in seed damage with a value equal to the<br />
cost of treatment. Use the following formula based on<br />
treatment costs, plant population and market price to<br />
determine the adult EIL for day sampling.<br />
EIL<br />
(moths per 100 plants) =<br />
[[<br />
(Treatment Cost ($)/Market Price) x 582.9 – 0.7<br />
Plant Population<br />
The constants in the formula simplify the calculation<br />
and include the amount of loss attributable to each<br />
banded sunfl ower moth larva produced per plant.<br />
A sample calculation of the EIL based on moth sampling<br />
for the following conditions is given below.<br />
Insecticide treatment cost = $8/acre<br />
Market price = $0.09/lb.<br />
Plant population = 20,000/acre<br />
[[[ [<br />
EIL = ($8/$0.09) x 582.9 – 0.7<br />
20,000<br />
= 19 moths per 100 plants<br />
For this set of variables, an infestation of about 1.9<br />
moths per 100 plants will result in suffi cient larvae<br />
to destroy seeds in the sunfl ower head equal to the $8<br />
treatment cost per acre in a fi eld of 20,000 plants per<br />
acre with a market value of 9 cents per pound. If the<br />
adult population has reached or exceeded this level,<br />
then the growner should consider the use of a chemical<br />
insecticide to prevent larval seed damage.<br />
Egg Scouting Method and Economic Threshold:<br />
Banded sunfl ower moth eggs can be counted accurately<br />
using a low power magnifi er. A head-mounted 3.5X<br />
magnifi er is recommended. Egg counts should be<br />
made when most of the sunfl ower plants are at stage<br />
R-3. However, buds should be selected randomly to<br />
avoid bias. Sampling for banded sunfl ower moth egg<br />
populations in commercial fi elds should be conducted<br />
as follows:<br />
1. Divide each side of the fi eld to be surveyed into<br />
1,312-foot (400 m) sections.<br />
2. Sample the center of each 1,312-foot (400 m)<br />
section at 20 feet (6 m) into the fi eld from the<br />
fi eld margin.<br />
3. Randomly select fi ve buds at each sample site.<br />
4. Randomly select six bracts from the outer whorl<br />
on each bud and count the banded moth eggs.<br />
Average the egg counts from the fi ve buds.<br />
Compare the average egg count to the EIL.
Economic Injury Level (EIL) is the number of eggs<br />
per six bracts.<br />
TC<br />
EIL =<br />
V x PP x 0.00078<br />
V = Market value per lb<br />
PP = Plant population per acre<br />
TC = Treatment cost<br />
Example: TC = $8, V = $0.10, and PP = 16,000<br />
The EIL is 6.4 eggs per six bracts.<br />
Economic Distance (ED) is the distance into a fi eld<br />
from a sample site on the fi eld edge where an economically<br />
damaging population is expected to extend.<br />
ED gives you the capability to diagram the extent of<br />
the EIL within a fi eld. Economic Distance:<br />
ED = e<br />
[[<br />
EIL – 1.29<br />
(E)<br />
– .194<br />
E = Average six bract egg count at 20 feet (6 m)<br />
*EIL based on eggs per six bracts<br />
Economic Distance Example:<br />
<strong>Field</strong> Size: 800 m by 800 m with average egg<br />
counts of 15 per six bracts per sample site.<br />
The EIL is 6.4 eggs per six bracts.<br />
The ED is 280 feet.<br />
In this case, only 37 percent of the fi eld would<br />
need treatment, resulting in a savings of 63 percent.<br />
Management: Deep fall plowing of sunfl ower stubble<br />
in Manitoba has reduced moth emergence the following<br />
season by about 80 percent; however, this is not<br />
practical for areas practicing conservation tillage. Research<br />
in North Dakota has demonstrated that delaying<br />
planting of sunfl ower until late May or early June<br />
helps reduce infestation levels of the banded sunfl ower<br />
moth. Parasitic wasps attack both the eggs and larvae<br />
of the moth. Predators also consume eggs and larvae.<br />
Since banded sunfl ower moths have a tendency to<br />
congregate around fi eld margins, perimeter spraying<br />
has been used with some success. This will minimize<br />
insecticide treatment costs and impact on pollinators.<br />
■ Lygus bugs<br />
Species: Tarnished plant bug, Lygus lineolaris (Palisot<br />
de Beauvois) and other Lygus species<br />
Description: The most common species occurring in<br />
sunfl ower fi elds is the tarnished plant bug. It attacks at<br />
least 385 different plant species and occurs in 39 U.S.<br />
states and fi ve Canadian provinces. Adults (Figure 64)<br />
are small, cryptically colored insects with a distinctive<br />
yellow triangle or “V” on the wings and 0.2 inch (4 to<br />
5 mm) in length. They vary in color from pale green to<br />
dark brown. The immature stages, or nymphs (Figure<br />
65), are similar in appearance to the adults, but lack<br />
wings and are usually green in color. They often are<br />
confused with aphids, but lygus move much more<br />
rapidly.<br />
■ Figure 64. Adult - Lygus bug (Tarnished plant bug)<br />
Lygus lineolaris. (Scott Bauer, http://www.ars.usda.gov/<br />
is/graphics/photos/insectsimages.new.htm)<br />
■ Figure 65. Nymphs - Lygus bug Lygus lineolaris.<br />
(Scott Bauer, http://www.ars.usda.gov/is/graphics/photos/<br />
insectsimages.new.htm)<br />
Insects<br />
51
52<br />
Life Cycle: Adults overwinter in plant debris along<br />
fi eld margins and shelterbelts. Populations probably<br />
move to sunfl ower from alfalfa, canola or other<br />
crops when those plants either have senesced or been<br />
harvested. Sticky trap catches in North Dakota showed<br />
that lygus bugs were present throughout the reproductive<br />
growth stages of sunfl ower. These insects produce<br />
at least two generations per year in the northern<br />
Plains. The biology of other Lygus species is similar.<br />
Damage: Oilseed sunfl ower are not believed to be<br />
at risk to damage from Lygus feeding at this time.<br />
The presence of scarring on confection or nonoilseed<br />
sunfl ower seeds, known as kernel brown spot (Figure<br />
66), is caused by lygus bugs feeding on the developing<br />
seed. The quality issue is signifi cant because processors<br />
discount the fi nished product with only 0.5 percent<br />
damage. The incidence of damage in 2006 ranged<br />
between 1 percent and 5 percent in some production<br />
areas of the northern Plains. Lygus feed preferentially<br />
on either the developing reproductive organs or on the<br />
apical meristematic and leaf primordial tissue, causing<br />
a necrosis around the feeding site due to the injection<br />
■ Figure 66. Kernel brown spot caused by Lygus bug.<br />
(Larry Charlet)<br />
of enzymes. This tissue destruction causes the brown<br />
spot on the sunfl ower kernel, resulting in a bitter taste<br />
to the seeds. Greenhouse and fi eld studies showed that<br />
33 to 38 seeds were damaged per adult lygus bug, and<br />
that all reproductive growth stages (R-4 to R-5) were<br />
vulnerable to attack. Damage was reduced if heads<br />
were infested after fl owering was completed (R-6 to<br />
R-7).<br />
Scouting Method: A scouting method has not been<br />
developed for lygus bug in sunfl ower.<br />
Economic Threshold: Approximately 36 seeds are<br />
damaged by each adult. Therefore, 0.5 percent damage<br />
on heads with 800 seeds would occur with feeding on<br />
only four seeds per head. Thus, populations of adult<br />
lygus at levels of one per nine heads could result in<br />
economic loss to the producer through the reduction<br />
of seed quality.<br />
Management: Lygus can be treated at the same time<br />
confection sunfl ower is treated for other insects, such<br />
as the seed weevil and banded sunfl ower moth. Two<br />
treatments are recommended to suffi ciently protect<br />
confection sunfl ower heads from insect feeding: one<br />
application at the onset of pollen shed, or approximately<br />
10 percent bloom, followed by a second treatment<br />
seven days later. This program should control<br />
insects adequately on confection sunfl ower throughout<br />
fl owering, minimizing the potential feeding damage.
■ Sunfl ower Headclipping Weevil<br />
Species: Haplorhynchites aeneus (Boheman)<br />
Description: The sunfl ower headclipping weevil adult<br />
is shiny black (Figure 67). The weevil is about 0.31<br />
inch (8 mm) long from the tip of the snout to the rear<br />
of the abdomen. The area behind the head and thorax<br />
is large and “squared” in relation to the narrow and<br />
prolonged head and snout.<br />
Headclipping weevil larvae are cream-colored, somewhat<br />
C-shaped and grublike and 0.16 to 0.24 inch (4<br />
to 6 mm) long (Figure 68).<br />
Life Cycle: Adults emerge in mid-July and are active<br />
for a two- to three-week period. The females feed on<br />
pollen and nectar of fl owering heads. In preparation<br />
for egg laying, the female makes one nearly complete<br />
row of feeding punctures around the circumference of<br />
the stalk just below the head and then lays an egg in<br />
the head. The girdled head subsequently falls to the<br />
ground, where larval development and overwintering<br />
occur.<br />
Damage: Head clipping by H. aeneus is the most<br />
apparent type of damage caused by this weevil and<br />
frequently occurs along fi eld margins. The percent<br />
of “clipped heads” in a fi eld is normally very low (1<br />
percent to 3 percent). However, losses up to 25 percent<br />
have been reported in individual fi elds (Figure 69).<br />
Scouting Method: The weevils’ presence is determined<br />
using the X scouting pattern. If the adults are<br />
encountered only periodically throughout the sampling<br />
sites, controls should not be necessary.<br />
Economic Threshold: None established.<br />
Management: Insecticide use has not been warranted<br />
for control of the sunfl ower headclipping weevil.<br />
■ Figure 67. Adult - Sunfl ower headclipping weevil<br />
Haplorhynchites aeneus. (Extension Entomology)<br />
■ Figure 68. Larva- Sunfl ower headclipping weevil<br />
Haplorhynchites aeneus. (Extension Entomology)<br />
■ Figure 69. Sunfl ower headclipping weevil damage.<br />
(Extension Entomology)<br />
Insects<br />
53
54<br />
Diseases of Sunfl ower<br />
(Carl Bradley, Sam Markell and Tom Gulya)<br />
Sunfl ower (Helianthus annuus) is unique in that it<br />
is one of the few crop plants that are native to North<br />
America. The genus Helianthus comprises more than<br />
60 annual and perennial species, with one to several<br />
species found in every state. Since wild sunfl ower<br />
is a native plant, a native population of diseases and<br />
insects that can attack cultivated sunfl ower also is<br />
present in most areas of the U.S. Sunfl ower is also<br />
unusual in that it is both a fi eld crop, with two distinct<br />
subtypes (oil and confection), and is grown as a<br />
garden fl ower and for the cut-fl ower industry. At least<br />
30 diseases, caused by various fungi, bacteria and<br />
viruses, have been identifi ed on wild or cultivated<br />
sunfl ower, but fortunately, only a few are of economic<br />
signifi cance as far as causing yield losses. When considering<br />
sunfl owers as an ornamental plant, even small<br />
spots on the foliage are enough to reduce marketability,<br />
and thus proper disease identifi cation is necessary<br />
to decide upon appropriate disease management. See<br />
Appendix 1 for a listing of all known sunfl ower diseases<br />
that occur in the world.<br />
The most important diseases in the northern Great<br />
Plains are Sclerotinia wilt, Sclerotinia head rot and<br />
downy mildew. Rust, especially on confection sunfl<br />
owers, and Phomopsis stem canker are important in<br />
some years, but are of less overall concern. Phoma<br />
black stem is almost universally prevalent, but is not<br />
thought to cause yield losses. Leaf diseases caused by<br />
Alternaria, Septoria and powdery mildew, head rots<br />
caused by Botrytis and Erwinia, and Verticillium leaf<br />
mottle are diseases that either are observed infrequently<br />
in the northern Great Plains or have not occurred<br />
with suffi cient intensity to be considered serious.<br />
The most important sunfl ower disease in both the<br />
central Great Plains (Kansas, Nebraska, Colorado,<br />
Texas) and California is Rhizopus head rot. Sclerotinia<br />
head rot and wilt, downy mildew and Phoma black<br />
stem are of sporadic importance. In the central Great<br />
Plains, rust can be severe on sunfl ower grown under<br />
center pivot irrigation, and Phomopsis stem canker has<br />
been of concern in years of plentiful rainfall. Under<br />
drought conditions, charcoal rot is also of concern in<br />
the central Great Plains. In California, rust, followed<br />
by various stalk rots, are the most prevalent diseases<br />
after Rhizopus head rot. For complete details on the<br />
incidence and severity of sunfl ower diseases, visit the<br />
National Sunfl ower Association Web site (www.sunfl<br />
owernsa.com), where the proceedings of the annual<br />
“Sunfl ower Research Workshop” are posted.<br />
The production of ornamental sunfl owers in the U.S.<br />
is scattered across at least 41 states, and includes both<br />
fi eld and greenhouse production. Greenhouse-grown<br />
sunfl ower is prone to Pythium and Phytophthora root<br />
rot and Botrytis blight, as well as the range of sunfl<br />
ower-specifi c pathogens. <strong>Field</strong>-grown ornamental<br />
sunfl ower, especially in the Midwest, is prone to the<br />
same pathogens that attack oilseed sunfl ower. Sclerotinia<br />
wilt, rust, powdery mildew, Rhizopus head rot,<br />
southern blight and root knot nematodes are cited as<br />
the major diseases. Since cosmetic appearance is so<br />
important on ornamental crops, even diseases considered<br />
as minor on an oilseed sunfl ower crop are of<br />
consequence to ornamental sunfl ower. Thus, obscure<br />
diseases, such as leaf smut, cocklebur rust and petal<br />
blight, have been cited as causing losses to ornamental<br />
sunfl ower in the U.S. and abroad.<br />
Effective control measures for most sunfl ower diseases<br />
are:<br />
• Planting resistant hybrids<br />
• A minimum rotation of four years between successive<br />
sunfl ower crops<br />
• Seed treatment for control of downy mildew and<br />
damping-off<br />
• Tillage to bury crop residue that may harbor<br />
pathogens<br />
• Foliar fungicides for rust and other foliar diseases
Many sunfl ower diseases are controlled by single<br />
dominant genes for resistance (e.g., downy mildew,<br />
Verticillium leaf mottle). Most sunfl ower hybrids in<br />
the U.S. have resistance to Verticillium leaf mottle,<br />
several races of downy mildew and several races<br />
of rust. Unfortunately, the rust and downy mildew<br />
pathogens continue to evolve and new races are found<br />
periodically. This requires commercial seed companies<br />
to add new genes to their hybrids so their hybrids can<br />
remain resistant to the ever-changing pathogens. Some<br />
other disease organisms, such as Sclerotinia, require<br />
multiple genes for resistance, which makes development<br />
of resistant hybrids much more diffi cult. Great<br />
strides have been made in making sunfl ower hybrids<br />
more tolerant of both Sclerotinia wilt (root rot) and<br />
Sclerotinia head rot. No current hybrids can be considered<br />
immune to either type of Sclerotinia infection,<br />
but then again, breeders have so far been unable to<br />
develop immune varieties for other Sclerotinia-susceptible<br />
crops. Disease reaction varies widely among<br />
hybrids, and growers are encouraged to consult seed<br />
companies and Extension or public research personnel<br />
for recommended disease-resistant varieties. Producers<br />
also should remember that disease response is<br />
highly infl uenced by environment. Disease evaluations<br />
made in only one location may not accurately predict a<br />
hybrid’s performance in all areas.<br />
Crop rotation helps reduce populations of many important<br />
sunfl ower pathogens in the soil. Most sunfl ower<br />
diseases are caused by pathogens specifi c to sunfl<br />
ower. Sclerotinia, however, attacks many crops, and<br />
susceptible crops (such as mustard, canola, crambe,<br />
soybean and dry edible bean) should be interspersed<br />
in rotation with corn and cereals, which are nonhosts<br />
of Sclerotinia. Rotation time away from sunfl ower is<br />
infl uenced by the occurrence and severity of diseases<br />
noted in the current year. Regular monitoring of fi elds<br />
and maintaining accurate records are also important<br />
in determining rotation practices. Rotation will have<br />
a minimal effect on foliar diseases since the pathogen<br />
spores may be carried by wind from distant fi elds.<br />
Currently, only two fungicides are employed for foliar<br />
disease control, with several others in the registration<br />
process. Consult state Extension publications for more<br />
details.<br />
The arrangement of the sunfl ower diseases in the<br />
following section is based on time of appearance<br />
during the growing season and the plant part affected.<br />
Early season diseases (e.g., downy mildew and apical<br />
chlorosis) are covered fi rst, followed by foliar diseases<br />
(including virus diseases), stalk and root infecting<br />
diseases (including nematodes) and fi nally, head rots<br />
and other diseases of mature plants.<br />
For additional information and photos of sunfl ower<br />
diseases, visit the National Sunfl ower Association<br />
Web site (www.sunfl owernsa.com) and the Web<br />
site of the USDA Sunfl ower Research Unit in<br />
Fargo, N.D. (www.ars.usda.gov/main/site_main.<br />
htm?modecode=54420520), as well as the heavily referenced<br />
chapter on sunfl ower diseases in “Sunfl ower<br />
Technology and <strong>Production</strong>” edited by A.A. Schneiter<br />
and published by the Agronomy Society of America<br />
as Monograph No. 35. For current information on<br />
the disease resistance of commercial hybrids, please<br />
consult NDSU publication A652, “Hybrid Sunfl ower<br />
Performance Testing,” available in hard copy or on<br />
the Web at www.ext.nodak.edu/extpubs/plantsci/rowcrops/a652.pdf.<br />
Diseases<br />
55
56<br />
I. Early Season Diseases<br />
■ Downy Mildew<br />
Description: Downy mildew has been observed on<br />
cultivated and wild sunfl ower throughout the U.S.<br />
and was quite common before the advent of resistant<br />
hybrids and the use of fungicides as seed treatments. It<br />
is most serious in areas with fl at topography or heavy,<br />
clay soils that foster waterlogged conditions conducive<br />
for disease development.<br />
Typical systemic symptoms in seedlings include<br />
dwarfi ng and yellowing (chlorosis) of the leaves<br />
(Figure 70) and the appearance of white, cottony<br />
masses (fungal mycelium and spores) on the lower and<br />
sometimes upper leaf surface during periods of high<br />
humidity or dew (Figure 71). Most infected seedlings<br />
are killed, but those that survive will produce stunted<br />
■ Figure 70. Dwarfi ng and discoloration of sunfl ower<br />
resulting from infection by downy mildew.<br />
(D.E. Zimmer)<br />
■ Figure 71. Lower surface leaves on downy mildew<br />
infected plants frequently exhibit a white cottony<br />
growth of fungus. (D.E. Zimmer)<br />
plants with erect, horizontal heads with little, if any,<br />
seed (Figure 72). When seedlings are infected several<br />
weeks after emergence, or a fungicide seed treatment<br />
inhibits rather than prevents infection, the plants usually<br />
start showing symptoms at the four-, six- or eightleaf<br />
stage. This situation is referred to as “delayed<br />
systemic infection.” These plants are characterized by<br />
some degree of stunting, with typical downy mildew<br />
leaf symptoms starting at some level in the plant (with<br />
lower leaves appearing normal). If susceptible plants<br />
are exposed to the mildew pathogen after the seedling<br />
stage, they also may develop a thickened, clublike<br />
root and become stunted, but may not show foliar<br />
symptoms. All infected plants serve to perpetuate the<br />
pathogen in the soil and are more prone to drought<br />
stress and lodging.<br />
Sunfl ower plants also display localized foliar lesions<br />
due to airborne downy mildew spores. The infected<br />
spots are generally small, angular lesions (delimited<br />
by veinlets) with white sporulation on the underside of<br />
the lesion. These local lesions may coalesce into larger<br />
lesions, but they rarely result in a systemic infection,<br />
and thus have minimal impact upon yield.<br />
Dwarfi ng and distortion of leaves also are symptoms<br />
typical of herbicide drift damage, especially from<br />
2,4-D and related phenoxy compounds, and may be<br />
confused with downy mildew symptoms (Figure 73).<br />
Herbicide damage, however, never will exhibit the<br />
white appearance (fungal mycelium and spores) on<br />
the underside of the leaves nor the chlorosis typical of<br />
downy mildew.<br />
■ Figure 72. Plants infected with downy mildew<br />
seldom produce heads; when they do, the heads<br />
do not nod but remain erect and produce little or no<br />
seed. (T. Gulya)
Disease Cycle: Downy mildew is caused by the obligate<br />
fungus Plasmopara halstedii, which is soil-borne,<br />
wind-borne and seed-borne. Sunfl ower plants are susceptible<br />
to systemic infection before the seedling roots<br />
exceed 2 inches in length. This short period may range<br />
up to a maximum of two to three weeks, depending on<br />
soil temperature and moisture. Cool, water-saturated<br />
soil during this period greatly favors infection. The<br />
fungus may persist in the soil for fi ve to 10 years as<br />
long-lived oospores. While this downy mildew fungus<br />
does not infect any crops other than sunfl ower and<br />
Jerusalem artichoke, weeds in the Compositae family,<br />
such as marsh elder, are susceptible, and thus may<br />
serve as reservoirs for the fungus.<br />
Sunfl ower planted on land with no previous sunfl ower<br />
history occasionally has shown downy mildew infection.<br />
There are three principle ways downy mildew<br />
may occur in fi elds with no previous history of sunfl<br />
ower. Windblown and soil-borne spores account for<br />
the majority of such infections. Spores of the fungus<br />
occurring on volunteer sunfl ower or wild annual<br />
sunfl ower, even a few miles distant, may be blown to<br />
newly planted fi elds and result in substantial infection<br />
under favorable conditions of cool, water-logged soils.<br />
Spores also may adhere to soil particles and move to<br />
neighboring fi elds during dust storms. Water running<br />
through an infested fi eld also may carry mildew<br />
spores into a previously disease-free fi eld.<br />
Modern seed production practices, coupled with stringent<br />
inspection of certifi ed seed, virtually eliminate<br />
the possibility of introducing downy mildew into a<br />
“clean” fi eld via infected seed. Seed from infected<br />
■ Figure 73. Damage to sunfl ower from 2,4-D or<br />
growth regulator type herbicides may be mistaken<br />
for downy mildew symptom. (D.E. Zimmer)<br />
plants is generally either nonviable or so light in<br />
weight that it is separated during seed processing.<br />
A slight possibility remains that viable seed may be<br />
produced on infected plants. These seeds may produce<br />
healthy plants, systemically infected seedlings<br />
or plants with latent infection in which the fungus is<br />
localized in the roots and does not produce symptoms<br />
on the leaves. These latent infections, however, will<br />
help perpetuate the disease in the fi eld.<br />
Damage: Severely infected plants may die before or<br />
shortly after emergence or in the seedling stage. The<br />
few plants reaching maturity seldom produce viable<br />
seed. Heads on these plants typically face straight up,<br />
rendering them extremely vulnerable to bird feeding.<br />
Yield losses from downy mildew can be substantial,<br />
depending on the percentage of infected plants and<br />
their distribution within the fi eld. If infected plants<br />
are scattered randomly throughout a fi eld, yield losses<br />
probably will not be observed unless infection exceeds<br />
15 percent. Sunfl ower have excellent compensating<br />
ability when healthy plants are adjacent to infected<br />
plants. When the disease is in a localized area, such<br />
as a low spot in a fi eld, and all plants are infected, the<br />
resultant yield loss is much greater.<br />
Management: The continued discovery of new downy<br />
mildew races and the occurrence of mildew strains<br />
resistant to Apron XL (mefenoxam) and Allegiance<br />
(metalaxyl) seed treatment have altered management<br />
strategies. When these seed treatments were effective,<br />
seed companies had no need to develop resistant<br />
varieties. Now most seed companies are developing<br />
hybrids that use multirace immunity, which should be<br />
effective despite the development of new races. Not<br />
all hybrids in a company’s lineup will have downy<br />
mildew resistance, however. At least two dozen races<br />
of the fungus have been identifi ed in the U.S., but annually,<br />
usually two to three races make up the majority<br />
of races. Fortunately, lines released by the USDA are<br />
available that confer resistance to all known races.<br />
With the appearance of downy mildew strains that<br />
are insensitive to metalaxyl and mefenoxam fungicide<br />
seed treatments (Apron XL, Allegiance), efforts<br />
are under way to fi nd replacement seed treatments.<br />
The fungicide azoxystrobin (Dynasty) recently has<br />
been labeled for use on sunfl ower as a seed treatment<br />
Diseases<br />
57
58<br />
for downy mildew suppression. As other effective<br />
chemicals are registered for use as sunfl ower seed<br />
treatments, the most effective management strategy<br />
would be to use two fungicides at the same time. A<br />
two-fungicide treatment will improve disease control<br />
and probably be less costly, in addition to delaying the<br />
development of fungicide resistance in downy mildew.<br />
Seed-applied fungicides will protect against root infection<br />
by all races, and thus will augment protection<br />
offered by resistant hybrids.<br />
Seed applications, however, will not protect against<br />
foliar infection. Since the fungicides are water-soluble,<br />
they can be washed off shallow-planted seed with<br />
excessive rainfall, resulting in poor disease control.<br />
Selection of downy mildew-resistant hybrids is the<br />
most effective way of controlling downy mildew.<br />
Seed companies are actively incorporating genes that<br />
control all known races, and these hybrids will be<br />
immune, at least until a new race emerges. Additional<br />
management practices that minimize downy mildew<br />
problems include extended crop rotations, eradication<br />
of volunteer sunfl ower, avoiding poorly drained fi elds<br />
or those with excessive low areas and delaying planting<br />
until warm soil temperatures foster rapid seedling<br />
growth.<br />
■ Apical Chlorosis<br />
Description: Apical chlorosis is the one of two bacterial<br />
diseases of sunfl ower that is noticed with any<br />
regularity in the U.S. The causal organism is Pseudomonas<br />
syringae pv. tagetis. Apical chlorosis is striking<br />
and seldom goes unnoticed. The major symptom of<br />
the disease is the extreme bleaching or chlorosis of<br />
the upper leaves (Figure 74). Apical chlorosis may be<br />
distinguished from iron chlorosis or nitrogen defi -<br />
ciency by the complete lack of green pigment and the<br />
uniformity of the chlorosis. With mineral defi ciencies,<br />
the veins characteristically remain green. In addition,<br />
the white leaves affected by apical chlorosis never<br />
will “regreen,” while those due to mineral defi ciencies<br />
will.<br />
Disease Cycle: Apical chlorosis occurs only during<br />
the vegetative growth stage when leaves are actively<br />
expanding. It is most severe in young seedlings during<br />
cold weather and in water-logged soils.<br />
Damage: Plants affected by apical chlorosis usually<br />
will produce new green leaves in several weeks<br />
with little discernible effect other than striking white<br />
leaves in the middle of the plant. Thus, yield reductions<br />
due to apical chlorosis are minimal. However,<br />
if long periods of cold spring temperatures coincide<br />
with water-logged soils, seedlings affected by apical<br />
chlorosis may die. Yield losses still will be minimal as<br />
healthy neighboring plants should compensate for the<br />
dead seedlings.<br />
Management: No hybrids are completely immune to<br />
apical chlorosis. The only control recommendation<br />
at present is to follow a four-year rotation to avoid<br />
increasing the population of the bacteria in the soil.<br />
Roguing (identifying and removing) infected plants<br />
in seed production fi elds will eliminate the disease<br />
and minimize the possibility of infected seed. This<br />
same bacterium will produce apical chlorosis on other<br />
Compositae weeds, such as thistles, ragweed, other<br />
Helianthus species (including cultivated Jerusalem<br />
artichoke), marigold and zinnia. Thus, controlling volunteer<br />
sunfl owers and Compositae weeds, as potential<br />
disease reservoirs, will help minimize soil populations<br />
of this bacterium.<br />
■ Figure 74. Apical chlorosis is characterized by an<br />
extreme bleaching or chlorosis of the upper leaves.<br />
(William K. Pfeifer)
II. Foliar Diseases<br />
■ Rust<br />
On most crops, rust refers to a single fungal species.<br />
On sunfl ower, the main fungus causing rust is Puccinia<br />
helianthi, which is worldwide in distribution and<br />
causes economic losses. In North America, four other<br />
Puccinia species are found on wild and cultivated sunfl<br />
ower: P. canaliculata (nutsedge rust), P. enceliae, P.<br />
massalis and P. xanthii (cocklebur rust), plus one rust<br />
from another genus, Coleosporium helianthi. The following<br />
discussion will deal mainly with P. helianthi,<br />
with minimal descriptions of the other rust fungi.<br />
Description: Rust occurs in all sunfl ower production<br />
areas of the U.S. and Canada and also is widespread<br />
on wild sunfl ower. Most oilseed hybrids have<br />
had good resistance to the prevailing rust races, but<br />
changes in the rust population in the last decade<br />
have resulted in greater rust severity and occasionally<br />
in substantial losses in seed yield or seed quality.<br />
Confection hybrids are generally more susceptible<br />
to rust (and other diseases) than oilseed hybrids. At<br />
least 25 different rust races have been identifi ed in<br />
the U.S., which makes breeding for rust resistance a<br />
challenge. Rust, incited by the fungus Puccinia helianthi,<br />
is characterized by cinnamon-colored spots or<br />
uredial pustules, which primarily occur on the leaves<br />
(Figure 75) but also on the stems, petioles, bracts and<br />
the back of the head under severe infestations. The<br />
initial appearance of rust is determined by adequate<br />
rainfall and warm temperatures, so the disease usually<br />
occurs in late summer in the northern Great Plains.<br />
The uredial pustules turn black with the advent of cool<br />
temperatures as the brown urediospores are replaced<br />
by black overwintering teliospores.<br />
Disease Cycle: This rust completes its entire life cycle<br />
on sunfl ower and does not require an alternate host, as<br />
do some cereal rusts. Puccinia helianthi overwinters<br />
on plant debris as teliospores (thick walled, resting<br />
spores). These spores germinate in the spring to<br />
produce basidiospores that infect volunteer seedlings<br />
or wild sunfl owers. This initial infection results in the<br />
formation of pycnia (generally on the underside of<br />
leaves), which, in turn, leads to aecial pustules, generally<br />
on the upper surfaces of leaves or cotyledons. The<br />
aecia are small (1/8 inch), orange cup-shaped pustules<br />
that may occur singly or in small clusters (Figure 76).<br />
The aeciospores are spread by wind to other sunfl ower<br />
plants, where they initiate the cinnamon-brown uredial<br />
pustules. The uredial stage is the repeating portion of<br />
the rust life cycle. Rust multiplies rapidly under favorable<br />
conditions of warm temperatures and either rain<br />
or dew. Thus, even in dry years, if night temperatures<br />
are low enough to promote dew formation on leaves,<br />
this minimal amount of leaf wetness will be suffi cient<br />
to initiate rust infection. Excessive rates of nitrogen<br />
fertilization and abnormally high seeding rates result<br />
in excessive foliage, which increases humidity within<br />
the canopy and favors rust development.<br />
Damage: Rust not only reduces yield, but also reduces<br />
oil, seed size, test weight and kernel-to-hull ratios.<br />
Late-planted fi elds of susceptible hybrids are generally<br />
more severely damaged by rust than earlier-planted<br />
fi elds. Irrigated fi elds also are apt to have more severe<br />
infection as the constantly wet leaves provide an ideal<br />
environment for the rust fungus to multiply.<br />
■ Figure 75. Rust occurs most commonly on leaves<br />
and after fl owering. The cinnamon-red pustules<br />
produce summer spores; the black pustules occur late<br />
fall and produce overwintering spores. (D.E. Zimmer)<br />
■ Figure 76. Aecial cups of Puccinia helianthi.<br />
(T. Gulya)<br />
Diseases<br />
59
60<br />
Management: The most effective way to avoid loss<br />
from rust is by planting rust-resistant hybrids. With<br />
sunfl owers grown under center pivot irrigation, night<br />
irrigation fosters more rust infection since the spores<br />
germinate best in the dark. Headline (pyraclostrobin)<br />
and Quadris (azoxystrobin) are registered for control<br />
of rust on sunfl ower, and additional fungicides may<br />
become registered. Refer to the most current edition<br />
of the “North Dakota <strong>Field</strong> Crop Fungicide <strong>Guide</strong>”<br />
(PP-622) available from the NDSU Extension Service<br />
to view fungicide products registered on sunfl ower.<br />
The injury threshold (i.e., disease severity above<br />
which fungicide spraying is warranted to minimize<br />
yield loss) developed for using tebuconazole is when<br />
the rust severity on the upper four leaves is 3 percent<br />
or greater. High rust severity on lower leaves has<br />
less impact upon seed yields, as the upper leaves are<br />
the ones supplying most of the photosynthate for the<br />
developing seeds.<br />
Sunfl ower rust, like cereal rusts, occurs as many different<br />
“physiological races,” which constantly change.<br />
Thus, hybrids selected for rust resistance eventually<br />
become susceptible as rust races change. Seed companies<br />
continually are testing their hybrids in different<br />
locations to determine rust resistance against the<br />
various races from region to region. Rust evaluations<br />
made under conditions of natural infection or with<br />
artifi cial inoculation of specifi c races also are done<br />
by university research centers, and this information<br />
frequently is found in the NDSU publication “Hybrid<br />
Sunfl ower Performance Testing” (A652). A few other<br />
management practices, besides rust-resistant hybrids,<br />
that can minimize rust are available. Destruction of<br />
volunteer plants and wild annual sunfl ower occurring<br />
in the vicinity of commercial fi elds as early in the<br />
spring as possible will reduce sources of inoculum.<br />
High rates of nitrogen fertilizer and high plant populations<br />
both foster dense canopy development that in<br />
turn create ideal conditions for rust infection, and thus<br />
should be avoided if rust is of concern.<br />
Other Rusts: As mentioned previously, four other<br />
Puccinia species and one Coleosporium species of<br />
rust can infect sunfl ower in parts of the U.S. None<br />
of them have yet to be reported to be of economic<br />
signifi cance on cultivated sunfl ower, but they might<br />
be considered potential nuisance foliar pathogens<br />
on ornamental sunfl owers. As sunfl ower production<br />
expands into new areas, especially in the south-central<br />
and southwestern U.S., the possibility of other Puccinia<br />
species attacking cultivated sunfl ower increases.<br />
Puccinia xanthii, commonly known as cocklebur<br />
rust, is easy to distinguish from P. helianthi based on<br />
pustule size. This rust is microcyclic, with only telia<br />
and basidiospores, and does not exhibit the fi ve spore<br />
stages of a full-cycle (macrocyclic) rust such as P.<br />
helianthi. On sunfl ower, the telial pustules are few in<br />
number, range from 1/4 to 3/8 inch in diameter, are<br />
distinctly puckered (convex) and bear a layer of dark<br />
brown spores only on the underside of leaves. As the<br />
teliospores germinate in place, the spore layer changes<br />
from brown to gray. As little as two to three hours of<br />
dew at temperatures of 68 to 77 F is suffi cient for P.<br />
xanthii infection. Sunfl ower is minimally susceptible<br />
to this rust. The main host for this rust is cocklebur<br />
(Xanthium species), with some authors also listing<br />
ragweed (Ambrosia). This rust has been seen only<br />
once on sunfl ower, and only in North Dakota.<br />
Puccinia enceliae and P. massalis are two rusts<br />
that are recorded on wild Helianthus species in the<br />
southwestern U.S. and have been shown to infect<br />
cultivated sunfl ower in greenhouse tests. No reports<br />
of them being identifi ed on or causing yield losses to<br />
cultivated sunfl ower are known. Puccinia massalis is<br />
reported only on Texas blueweed (Helianthus ciliaris)<br />
in the Rio Grande Valley of Texas and New Mexico.<br />
Uredial pustules are indistinguishable from those of P.<br />
helianthi. Positive identifi cation of P. massalis requires<br />
microscopic examination of teliospores for the placement<br />
of germ pores. Puccinia enceliae occurs on wild<br />
Helianthus and dessert shrubs in the genera Viguiera<br />
(goldeneye and resin bush), Encelia (brittlebush) and<br />
Tithonia (Mexican sunfl ower) in the desert regions of<br />
western U.S. and northern Mexico. Uredial and telial<br />
pustules of P. enceliae on sunfl ower are similar in<br />
appearance to those of P. helianthi. Positive identifi cation<br />
of P. enceliae requires microscopic examination<br />
of teliospores. Neither of these two Puccinia species<br />
has been observed on sunfl ower in the northern Great<br />
Plains.<br />
Puccinia canaliculata is a full-cycle, heteroecious rust<br />
that has its aecial stage on sunfl ower and cocklebur<br />
(Xanthium strumarium), and its uredial and telial stage
on sedges of the genus Cyperus. This rust had been<br />
considered as a possible mycoherbicide for control of<br />
the noxious weed yellow nutsedge. On sunfl ower, the<br />
aecial pustules look very similar to the telial pustules<br />
of P. xanthii in shape, with the main difference that the<br />
P. canaliculata aecia on the underside of the leaf are<br />
orange, as compared with the dark brown of the telia<br />
of P. xanthii. This rust has been observed in Kansas in<br />
a sunfl ower fi eld infested with nutsedge. Since the rust<br />
requires both hosts to complete its life cycle, elimination<br />
of the nutsedge effectively will limit this rust<br />
infection of sunfl ower.<br />
Coleosporium species are commonly referred to as<br />
pine needle rusts and are heteroecious rusts (requiring<br />
two different hosts to complete their life cycle).<br />
Coleosporium helianthi has its uredial and telial stages<br />
on sunfl ower, and the remainder of its life cycle on<br />
two- and three-needle pines, such as Jack pine, Virginia<br />
pine and loblolly pine. The rust is of minimal economic<br />
importance in pine plantations and never has<br />
been observed on cultivated sunfl ower. On Jerusalem<br />
artichoke (and other perennial Helianthus species), C.<br />
helianthi infections can be quite severe and potentially<br />
cause yield losses. Preliminary greenhouse tests have<br />
shown that cultivated sunfl ower is susceptible, with<br />
little differences between commercial hybrids. The<br />
most effective way to prevent C. helianthi infection<br />
on sunfl ower is to avoid planting near shelter belts or<br />
areas with two-needle pines.<br />
■ Albugo or “White” Rust<br />
Description: White rust is one of the rarest sunfl<br />
ower diseases in North America, but is considered a<br />
potentially serious disease in countries such as South<br />
Africa and Argentina. Despite the word “rust” in the<br />
common name, this disease is caused by a pathogen<br />
more closely related to downy mildew. White rust has<br />
been recorded on sunfl ower in North America only<br />
in western Kansas, eastern Colorado and adjacent<br />
Nebraska during the late 1990s and seldom is seen<br />
in this area. In North Dakota, it has been recorded on<br />
ragweed (Ambrosia spp.), various sage and sagebrush<br />
(Artemisia spp.) and goatsbeard (Tragopogon). White<br />
rust has not been recorded on either wild sunfl ower or<br />
cultivated sunfl ower in the northern Great Plains or in<br />
Canada on sunfl ower.<br />
Foliar lesions, consisting of raised, chlorotic pustules<br />
up to 3/8 inch in diameter, are the most commonly<br />
seen symptom on sunfl ower in the U.S. (Figure 77). A<br />
dull, white layer of spores forms in these pustules on<br />
the underside of the leaf, which could be mistaken for<br />
local lesions caused by downy mildew. If the Albugo<br />
pustules are numerous enough, they will merge as they<br />
enlarge, and entire areas of the leaf will turn necrotic<br />
as secondary fungi colonize the pustules.<br />
One striking feature often seen with Albugo is that a<br />
single horizontal “layer” of leaves in the crop canopy<br />
usually is affected, with a sharp demarcation of<br />
healthy leaves above and below the affected leaves.<br />
This delimited layer of infected leaves suggests that<br />
environmental conditions and Albugo spores were<br />
■ Figure 77. Albugo lesions on upper leaf surface (left) and lower leaf surface (right). (T. Gulya)<br />
Diseases<br />
61
62<br />
present for a very short time, thus only one layer of<br />
leaves shows symptoms. Albugo also can cause lesions<br />
on stems, petioles, bracts and the back of heads. The<br />
lesions on stems (Figure 78), petioles and the back of<br />
the heads are much different from those on leaves, and<br />
appear to be dark, bruiselike lesions. This appearance<br />
is due to the presence of black oopsores just beneath<br />
the epidermis. No white sporangia ever are seen in<br />
the stem or petiole lesions. Stem lesions often are<br />
colonized by other fungi and may lead to stalk rot and<br />
lodging by the secondary fungi. Petiole lesions may<br />
girdle the petiole and cause the affected leaf to wilt,<br />
thus causing considerable damage and yield loss.<br />
Disease Cycle: The causal agent of white rust is the<br />
obligate fungus Albugo tragopogonis, which recently<br />
has been reclassifi ed as Pustula tragopogonis. In<br />
addition to sunfl ower, this fungus infects cocklebur<br />
(Xanthium), groundsel (Senecio), marsh elder (Iva),<br />
ragweed (Ambrosia) and several other weedy Composites.<br />
The fungus appears to have host-specifi c races,<br />
however, so white rust occurring on one host genus<br />
may not infect other genera or cause only minimal<br />
infection. Albugo overwinters as oospores in infected<br />
plant debris. Rain splashes the oospores onto nearby<br />
seedlings, where they germinate to form motile zoospores<br />
that enter through stomates. The pustules that<br />
develop contain masses of dry, white asexual sporangia.<br />
The pustules rupture to release sporangia that are<br />
windblown to other plants to continue leaf infection.<br />
Sporangia also can initiate infection on stems, petioles<br />
and bracts. Optimal conditions for Albugo infection<br />
■ Figure 78. Albugo lesions on stems. (T. Gulya)<br />
are cool nights (50 to 60 F) with rain or dew, but lesion<br />
development is favored by warm days (70 to 80<br />
F).<br />
Damage: Foliar infection by Albugo seldom causes<br />
yield loss, although the symptoms are quite noticeable.<br />
Lesions on stems and petioles, which fortunately<br />
are seen seldom in the U.S., are much more serious.<br />
Petiole lesions lead to defoliation, with signifi cant<br />
yield losses, and stem lesions may become colonized<br />
by other fungi and result in lodging. Albugo infections<br />
on the head may result in seed infection, which would<br />
be of concern in seed production fi elds.<br />
Management: <strong>Field</strong> trials have shown that sunfl ower<br />
plants can tolerate a high proportion of foliage infection<br />
by Albugo before yield losses are observed. Stem<br />
and petiole lesions caused by Albugo are more serious,<br />
as stem lesions can result in lodging and petiole<br />
lesions will result in defoliation. In countries where<br />
Albugo is serious, commercial seed companies have<br />
made the effort to develop resistant hybrids. In the<br />
U.S., no information is available regarding the reaction<br />
of hybrids to white rust. Evaluations of USDA breeding<br />
material have shown that resistance probably is<br />
controlled by several genes, each governing infection<br />
of leaves, stem, petioles and heads. Since Albugo is an<br />
Oomycete, like downy mildew, the same fungicides<br />
that control downy mildew have been effective against<br />
white rust. Thus, seed treatment with metalaxyl or<br />
mefenoxam will offer protection against both systemic<br />
infection and foliar infection for a limited time. Rotation<br />
is of limited use with Albugo, as the spores can be<br />
windblown from adjacent fi elds.
■ Alternaria Leaf and Stem Spot<br />
Description: Alternaria leaf spot is a ubiquitous<br />
disease on senescing leaves and generally of little<br />
concern, but under warm, humid conditions it can be a<br />
serious defoliating disease. The Midwest has two main<br />
species of Alternaria: A. helianthi and A. zinniae, of<br />
which A. helianthi is the more prevalent and more<br />
serious. In addition, several other Alternaria species<br />
have been reported on sunfl ower, including A. alternata,<br />
A. helianthicola, A. helianthinfi ciens and A. protenta.<br />
Alternaria helianthi and A. zinniae both produce<br />
dark brown spots on leaves. These spots are irregular<br />
in size and shape with a very dark border and a gray<br />
center (Figure 79). The spots on young plants may<br />
have a yellow halo. Leaf lesions may coalesce, causing<br />
leaves to wither. Stem lesions begin as dark fl ecks<br />
that enlarge to form long, narrow lesions (Figure<br />
80). These stem lesions often coalesce to form large<br />
blackened areas, resulting in stem breakage. Stem<br />
lesions are distributed randomly on the stem and are<br />
not associated with the point of attachment of the leaf<br />
petiole. Brown, sunken lesions also may form on the<br />
back of the head, especially following any mechanical<br />
damage such as that caused by hail or birds. The<br />
leaf and stem lesions caused by the various Alternaria<br />
species are similar and thus not diagnostic. Therefore,<br />
microscopic examination is required to distinguish<br />
which Alternaria species is present.<br />
Disease Cycle: All Alternaria fungi overwinter on<br />
diseased stalks. They can be seed-borne at low levels,<br />
■ Figure 79. Leaf lesions caused by Alternaria<br />
hellanthi. (B.D. Nelson)<br />
although seed is a relatively unimportant source of<br />
the inoculum under most conditions. Seedling blights<br />
caused by Alternaria may develop when sunfl ower<br />
plants emerge in rainy weather on Alternaria-infested<br />
soil. However, plants at the fl owering to maturing<br />
stage are more susceptible than plants in the vegetative<br />
or budding stage. Saffl ower and cocklebur also can be<br />
alternate hosts of A. helianthi.<br />
Disease development in A. helianthi is favored by<br />
77 to 82 F temperatures and at least 12 hours of wet<br />
foliage. Extended wet periods of three to four days can<br />
cause serious losses as the spots enlarge.<br />
Damage: The primary damage that all Alternaria<br />
species cause is the leaf blights that lead to defoliation<br />
which increases the potential for yield loss. In<br />
the northern Great Plains, the climate is usually not<br />
conducive for Alternaria epidemics, and Alternaria<br />
generally affects only the lower, senescing leaves.<br />
However, in warmer climates with plentiful rainfall,<br />
the potential for defoliation by Alternaria species<br />
and subsequent yield loss is much greater. In addition<br />
to the direct yield loss caused by foliar Alternaria<br />
infection, this fungus also has been noted to cause<br />
blemishes on the achenes. While this damage may be<br />
only superfi cial, if the achenes are on confection sunfl<br />
owers destined for human consumption, the impact<br />
of “achene blemish” can be signifi cant. This achene<br />
■ Figure 80. Stem lesions caused by Alternaria<br />
hellanthi. (T. Gulya)<br />
Diseases<br />
63
64<br />
blemish, currently noted only on sunfl ower grown<br />
in Israel, looks very similar to “kernel black spot,”<br />
which is caused by feeding by the tarnished plant bug<br />
(Lygus), with no involvement from Alternaria.<br />
Management: Alternaria leaf blights are considered a<br />
major disease in subtropical sunfl ower growing areas,<br />
where yield losses may range from 15 percent to 90<br />
percent, but are much less serious in temperate areas<br />
of the U.S. However, severe epidemics have been<br />
observed on sunfl ower in the eastern and southeastern<br />
portions of the U.S. Management practices to minimize<br />
Alternaria problems include crop rotation and<br />
burying of infested crop refuse to hasten decomposition.<br />
Consult university Extension publications, such<br />
as NDSU’s PP-622, “<strong>Field</strong> Crop Fungicide <strong>Guide</strong>,” for<br />
current recommendations of foliar fungicides registered<br />
for use on sunfl ower for Alternaria control. Seed<br />
treatments with metalaxyl or mefenoxam offer no<br />
control of Alternaria seedling blights.<br />
■ Septoria Leaf Spot<br />
Description: Septoria leaf spot develops fi rst on the<br />
lower leaves and spreads to the upper leaves. The spots<br />
begin as water-soaked areas (greasy green in appearance).<br />
The spots become angular, with tan centers<br />
and brown margins (Figure 81). A narrow yellow halo<br />
often surrounds young spots. Mature leaf spots may<br />
contain tiny black specks, the fungal fruiting bodies<br />
(pycnidia), which are visible with a 5X to 10X hand<br />
lens. The presence of pycnidia is the best means of<br />
distinguishing leaf spots caused by Septoria from<br />
those caused by Alternaria.<br />
Disease Cycle: Septoria leaf spot can be caused by<br />
two Septoria species, S. helianthi. and S. helianthina,<br />
of which the former is the major species in the U.S.<br />
Septoria can be seed-borne and also can survive on<br />
infected sunfl ower crop refuse. Septoria leaf spot<br />
may appear anytime during the growing season and is<br />
favored by moderately high temperatures and abundant<br />
rainfall. As such, the disease potentially is more<br />
severe in southern growing areas, compared with the<br />
northern Great Plains.<br />
Damage: In the temperate climate of the Midwest,<br />
Septoria leaf spot usually causes little damage. Severe<br />
Septoria infection may cause some defoliation, but if<br />
this affects only the lower leaves on mature plants, the<br />
impact upon yield will be minimal.<br />
Management: Crop rotation, incorporation of sunfl<br />
ower residue and clean seed are the best means of<br />
managing Septoria leaf spots. Although resistance to<br />
Septoria has been identifi ed in breeding material, the<br />
infrequent occurrence of Septoria has not warranted<br />
the development of resistant hybrids.<br />
■ Figure 81.Septoria leaf spot. Note small black<br />
pycnidia in lesions. (T. Gulya)
■ Powdery Mildew<br />
Description: Powdery mildew, caused by the fungus<br />
Erysiphe cichoracearum, can be found in most fi elds<br />
after full bloom. The symptoms are distinctive and<br />
easy to recognize: a dull white to gray coating of the<br />
leaves, starting as individual circular spots and eventually<br />
merging to cover the entire leaf (Figure 82). This<br />
coating is the scant mycelial growth of the fungus<br />
on the leaf surface. Severely infected areas senesce<br />
prematurely and dry up. Normally the lower leaves are<br />
more heavily infected than the upper leaves. In other<br />
countries, two other powdery mildew fungi have been<br />
documented on sunfl ower: Sphaerothecia fuliginea<br />
and Leveillula taurica. These both exist in the U.S.,<br />
but to date have not been documented on sunfl ower.<br />
Disease Cycle: Powdery mildew seldom is seen until<br />
late in the growing season, as senescing leaves are<br />
most susceptible to infection. While leaves are the<br />
most common plant part affected, powdery mildew<br />
also will form on bracts and the backs of heads. The<br />
powdery coating seen is a combination of scant mycelia<br />
and spores of the asexual stage, which is referred to<br />
as Oidium. As the season progresses, the fungus forms<br />
small (pinhead-sized) black cleistothecia, the sexual<br />
fruiting bodies.<br />
Damage: Powdery mildew generally occurs late<br />
enough in the season that control measures are not<br />
needed. Sunfl ower cultivars differ widely in reaction<br />
to powdery mildew. On ornamental sunfl ower, especially<br />
those grown in the greenhouse, powdery mildew<br />
is common. Although the main impact of the disease<br />
is cosmetic, this alone can cause economic losses.<br />
Management: Powdery mildew is seldom a problem<br />
on cultivated sunfl ower in the Midwest, but may be<br />
of concern in more humid areas and on the southern<br />
Plains states. On ornamental sunfl owers, powdery<br />
mildew can be minimized by adequate air movement,<br />
allowing the leaves to dry, and by the use of registered<br />
fungicide sprays specifi c for powdery mildew.<br />
■ Figure 82. Powdery mildew with fungus producing<br />
white, powdery spores on leaf surface. (Reu V. Hanson)<br />
■ Diseases Caused by Viruses and<br />
Phytoplasmas<br />
Description: Several viruses have been reported<br />
on sunfl ower from other countries and the warmer<br />
regions of the U.S. (Florida), but no reports of viruses<br />
occurring on sunfl ower in the northern Great Plains<br />
have been confi rmed. Wild sunfl ower is a host of<br />
Tobacco ring spot virus in the Rio Grande Valley, and<br />
Cucumber mosaic virus has been reported on sunfl ower<br />
in Maryland. Viruses reported on sunfl ower outside<br />
the U.S. include Tobacco streak virus, Tomato big bud<br />
virus, Sunfl ower rugose mosaic virus, and Tomato<br />
spotted wilt virus. Confi rmation/identifi cation of a<br />
virus as the causal agent is based on observation of the<br />
viral particles using an electron microscope, detailed<br />
chemical analysis of the viral components or serological<br />
identifi cation.<br />
The only well-documented virus found on sunfl ower<br />
in the U.S. is Sunfl ower mosaic virus (SuMV), currently<br />
found only in southern Texas on both cultivated<br />
sunfl ower and wild Helianthus species. Symptoms of<br />
SuMV are a mottled pattern of light green and normal<br />
green areas on the leaf, referred to as mosaic (Figure<br />
83). Affected plants may die in the seedling stage or<br />
live to maturity, with all leaves affected.<br />
Aster yellows is a disease at fi rst believed to be caused<br />
by a virus but which has since been identifi ed as a<br />
phytoplasma. Phytoplasmas are living cells, in contrast<br />
to viruses, and are intermediate in size between<br />
viruses and bacteria. Symptoms on sunfl ower include<br />
yellowing of leaves and/or the head, which often occurs<br />
in sectors. A characteristic symptom is a wedgeshaped<br />
portion of the head that remains green and<br />
bears small leaves rather than fl oral parts (Figure 84),<br />
a condition termed “phyllody.”<br />
Diseases<br />
65
66<br />
■ Figure 83. Sunfl ower mosaic virus. (T. Gulya)<br />
Disease Cycle: SuMV is spread primarily by aphids<br />
but is also seed-borne to a small extent. Seedlings less<br />
than a month old are the most susceptible, and mosaic<br />
symptoms appear within a week after aphid transmission.<br />
Affected leaves will retain the mosaic pattern for<br />
the life of the plant, but no stunting due to the virus is<br />
discernible.<br />
The aster yellows phytoplasma is transmitted only by<br />
the aster leaf hopper (Macrosteles quadrilineatus) and<br />
occurs on a wide variety of plants. Symptoms generally<br />
appear at fl owering, and affected heads will show<br />
the symptoms for the remainder of the summer.<br />
Damage: SuMV, which currently is found only in<br />
Texas, can substantially reduce yield in individual<br />
plants. No fi elds have been observed with high incidence.<br />
This disease is also of quarantine signifi cance,<br />
and many countries will not accept seed from fi elds<br />
with any level of SuMV. Aster yellows, which occurs<br />
throughout the Midwest, is sporadic in occurrence<br />
and is generally more of a novelty than of economic<br />
consequence.<br />
■ Figure 84. Aster yellows. Note wedged<br />
shaped portion of head which remains<br />
green. (Donald Henne)<br />
Management: As both SuMV and aster yellows are<br />
spread by insects, the easiest means of minimizing<br />
both diseases is to control their insect vectors. Varietal<br />
differences to aster yellows have been noted. In contrast,<br />
no resistance to SuMV is available in commercial<br />
hybrids, although resistance has been found in<br />
wild Helianthus species from Texas.<br />
■ Other Miscellaneous<br />
Foliar Diseases<br />
The diseases mentioned above are the most likely leaf<br />
diseases to be encountered in the main sunfl ower-producing<br />
areas of the Midwest. As sunfl ower production<br />
expands into other areas, a possibility exists of other<br />
fungi causing leaf spots. Some of the fungi recorded<br />
to cause leaf spots on wild sunfl ower in other areas of<br />
the U.S. include Ascochyta compositarum, Cercospora<br />
helianthi, C. pachypus, Colletotrichum helianthi, Entyloma<br />
compositarum (leaf smut), Epicoccum neglectum,<br />
Itersonilla perplexans, Myrothecium roridum,<br />
Phialophora asteris (Phialophora yellows), Phyllosticta<br />
wisconsinensis and Sordaria fi micola. Check out<br />
the “Sunfl ower Diseases” chapter by Gulya et al. in<br />
“Sunfl ower Technology and <strong>Production</strong>,” published by<br />
the Agronomy Society of America, for more details on<br />
these minor foliar pathogens.
III. Stalk- and Root-infecting<br />
Diseases<br />
■ Sclerotinia Wilt<br />
Description: Sclerotina wilt usually is observed fi rst<br />
as plants start to fl ower. The fi rst symptoms include a<br />
sudden wilting (Figure 85) with no other leaf symptoms,<br />
and a characteristic stalk lesion at the soil line.<br />
The length of time from the fi rst sign of wilt to plant<br />
death may be as little as four to seven days. The stalk<br />
lesions that form at the soil line are tan to light-brown<br />
and eventually may girdle the stem (Figure 86). Under<br />
very wet soil conditions stalks and roots may be<br />
covered with white mycelia and hard black structures<br />
called sclerotia (Figure 87). Sclerotia are irregularshaped<br />
structures which range in size and shape from<br />
spherical and 1/8 inch in diameter to cylindrical or<br />
Y-shaped and up to 1 inch in length. Sometime a series<br />
of dark “growth” rings produced by the daily extension<br />
of the fungus can be observed.<br />
Disease Cycle: Sclerotinia sclerotiorum overwinters as<br />
sclerotia in the soil or in plant debris. When sunfl ower<br />
roots grow near sclerotia, the sclerotia are stimulated<br />
to germinate, and the resulting mycelium infects the<br />
lateral roots. The fungus grows along the root system<br />
to the tap root and up into the stem, and the plant wilts<br />
and dies. Contact between roots of adjacent plants<br />
within rows allows the fungus to spread from plant to<br />
plant. The fungus does not move between conventionally<br />
spaced rows. Sunfl ower is the only crop that S.<br />
sclerotiorum consistently infects through the roots.<br />
Other susceptible crops are infected mainly by spores<br />
on above-ground parts of the plant.<br />
Sclerotia are formed in the decayed stem pith and on<br />
the roots as the plant dies. These sclerotia then are<br />
returned to the soil during tillage operations and serve<br />
as sources of inoculum for the next susceptible crop.<br />
Sclerotia can survive in the soil for fi ve or more years,<br />
with a portion of them dying each year if they fail to<br />
infect a host. The higher the inoculum density (i.e., the<br />
number of sclerotia in the soil), the longer the period a<br />
■ Figure<br />
85. Sudden<br />
wilting is a<br />
characteristic<br />
symptom of<br />
Sclerotinia<br />
wilt.<br />
(B.D. Nelson)<br />
■ Figure 86. Basal canker formed from Sclerotinia<br />
wilt infection. (B.D. Nelson)<br />
■ Figure 87.<br />
Dense white<br />
mold may form<br />
on the surface<br />
of the basal<br />
canker. Hard<br />
black bodies<br />
called sclerotia<br />
also form on the<br />
outside and the<br />
inside of stems.<br />
(D.E. Zimmer)<br />
Diseases<br />
67
68<br />
fi eld will remain infested. Soil moisture and temperature<br />
during the growing season are not critical factors<br />
affecting wilt incidence. Plant population, in the range<br />
from 15,000 to 30,000 plants per acre on 30- or 36inch<br />
rows, is not a factor affecting disease incidence,<br />
although solid seeding should be avoided. Lodging of<br />
wilted plants, however, increases at high plant populations<br />
due to smaller stem diameter.<br />
Damage: Historically, wilt is the most prevalent of<br />
the Sclerotinia diseases, with the disease found in one<br />
out of two fi elds in the Dakotas, and about 3 percent<br />
of the crop affected. Wilt occurs whenever sunfl ower<br />
is planted on Sclerotinia-infested soil and can cause<br />
severe yield loss. Infected plants die rapidly, and if<br />
this occurs before the seed is fully mature, the result is<br />
a loss of seed yield accompanied by lower test weight<br />
and lower oil content. Since plant death occurs late in<br />
the season, healthy adjacent plants have little opportunity<br />
to compensate for the loss of the Sclerotinia-infected<br />
plant. On the average, infected plants yield less<br />
than 50 percent of healthy plants. Equally important,<br />
however, is that Sclerotinia wilt leads to increased<br />
numbers of sclerotia in the soil, thus contaminating<br />
the fi eld for all susceptible crops that would be in the<br />
rotation.<br />
Management: Sclerotinia wilt is a diffi cult disease<br />
to manage for several reasons. First, the fungus has a<br />
very broad host range and basically is able to infect all<br />
broadleaf crops to some extent. Rotation to minimize<br />
Sclerotinia is most effective with cereal grains and<br />
corn. Second, since the sclerotia can persist in the soil<br />
for long periods of time, long rotations away from<br />
broadleaf crops are necessary to minimize sclerotial<br />
populations in the soil. This may not be feasible<br />
because repeated cropping of cereal grains will lead to<br />
a buildup of cereal diseases, especially Fusarium head<br />
blight. Sunfl ower breeders have strived to increase the<br />
level of resistance to Sclerotinia wilt and tremendous<br />
improvements have been made, but no immune hybrids<br />
are on the market yet. Based on several years of<br />
tests with artifi cial inoculations, commercial hybrids<br />
with extremely good levels of resistance are available.<br />
Information on hybrid ratings for Sclerotinia stalk<br />
rot resistance is found in NDSU publication A-652,<br />
“Hybrid Sunfl ower Performance Testing.”<br />
Lastly, chemical control is not an option since the<br />
sclerotia are low in number and scattered throughout<br />
the soil. The most effective chemicals would be soil<br />
fumigants used for nematocide control, and even if<br />
the chemicals were registered on sunfl ower, the cost<br />
would be prohibitive. Two management strategies hold<br />
promise to minimize, but not eliminate Sclerotinia.<br />
One is the use of biocontrol. Various fungi, termed<br />
mycoparasites because they feed upon other fungi,<br />
have been shown to attack Sclerotinia. One such<br />
fungus is Coniothryium minitans, which is found in<br />
the registered product called “Contans.” If this product<br />
is applied to the soil (and preferably to the crop before<br />
disking), the mycoparasite Coniothryium will colonize<br />
the sclerotia and kill the sclerotia in several months<br />
rather than years. This will allow shortened rotations<br />
to be used, but replanting a Sclerotinia-infested fi eld to<br />
a susceptible crop (e.g., dry bean, canola, sunfl ower)<br />
the following year still is inadvisable.<br />
Another management option is tillage, and this is at<br />
the center of two schools of thought. One opinion is<br />
that deep tillage (inversion of the soil profi le via moldboard<br />
plowing) will put the sclerotia deep into the<br />
soil in an anaerobic environment where they are more<br />
prone to bacterial degradation and are out of the plant<br />
root zone. If deep tillage is used, producers should<br />
practice reduced tillage in the following years to prevent<br />
bringing the buried sclerotia back to the surface.<br />
The second school of thought is to let the sclerotia<br />
remain on the soil surface, where they are subject both<br />
to weathering and attack by other fungi. No conclusive<br />
evidence is available to show that either no-till or deep<br />
tillage produces signifi cantly less Sclerotinia wilt with<br />
sunfl ower, although research on soybeans strongly<br />
favors no-till to reduce sclerotia numbers.
■ Sclerotinia Middle Stalk Rot<br />
and Head Rot<br />
Description: Middle stalk rot is the disease least often<br />
caused by Sclerotinia, and is fi rst observed in the middle<br />
to upper portion of the stalk at or before fl owering.<br />
Midstalk rot begins with infection of the leaf, and the<br />
fungus progresses internally through the petiole until<br />
it reaches the stem (Figure 88). Symptoms of Sclerotinia<br />
leaf infection are not unique enough to identify<br />
the fungus, but once the stem lesion forms, the<br />
symptoms are identical with the lesion formed by root<br />
infection. The characteristic pith decay and formation<br />
of sclerotia both within the stem and sometimes on the<br />
exterior are highly diagnostic. The stalk usually lodges<br />
at the lesion site and the leaves above the canker die.<br />
With time, the fungus completely disintegrates the<br />
stalk, and the affected area will have a shredded appearance,<br />
as only the vascular elements of the stem<br />
remain.<br />
The fi rst symptoms of head rot usually are the appearance<br />
of water-soaked spots or bleached areas<br />
on the back of the heads. The fungus can decay the<br />
entire head, with the seed layer falling away completely,<br />
leaving only a bleached, shredded skeleton<br />
interspersed with large sclerotia (Figure 89). These<br />
bleached, skeletonized heads, which resemble straw<br />
brooms, are very obvious in the fi eld, even from a<br />
distance. During harvest, infected heads often shatter<br />
and any remaining seeds are lost. The large sclerotia<br />
in the heads may be 0.5 inch (12 mm) or greater in<br />
diameter and many are harvested along with the seed<br />
(Figure 90).<br />
Disease Cycle: If soil is very wet for seven to 14 days,<br />
sclerotia in the upper several inches of soil can germinate<br />
to form small mushrooms called apothecia. These<br />
apothecia produce ascospores for a week or more. The<br />
ascospores can originate within the sunfl ower fi eld or<br />
can be blown in from adjacent fi elds. Thus, sunfl ower<br />
fi elds with no history of Sclerotinia can became affected<br />
by head and middle stalk rot. Apothecia are<br />
not usually observed until after the crop canopy has<br />
completely covered the rows. Apothecia are more<br />
likely to form in crops with dense canopies, such as<br />
small grains, and the resultant spores can be blown a<br />
distance to nearby sunfl ower fi elds (Figure 91). Ascospores<br />
require both free water (dew or rain) and a food<br />
base such as dead or senescing plant tissue to germinate<br />
and infect. The fungus cannot penetrate unbroken<br />
■ Figure 88.<br />
Middle stalk<br />
rot occurs<br />
via ascospore<br />
infection.<br />
(B.D. Nelson)<br />
■ Figure 89. Head rot showing skeleton head fi lled<br />
with sclerotia. (B.D. Nelson)<br />
■ Figure 90. Sunfl ower seed contaminated with<br />
sclerotia.<br />
Diseases<br />
69
70<br />
■ Figure 91. Apothecia in fi eld of soybean plants.<br />
(B.D. Nelson)<br />
tissue. Midstalk infection may result from either leaf<br />
infection or infection at the leaf axil. Head infection<br />
actually starts as ascospores colonize the dead fl orets<br />
and pollen on the face of the head. Thus, when lesions<br />
are seen on the back of the head, several weeks have<br />
elapsed since infection took place.<br />
Damage: Middle stalk rot is the least often seen phase<br />
of Sclerotinia diseases. Head rot incidence fl uctuates<br />
dramatically, dependent entirely upon weather conditions.<br />
In dry years, head rot is entirely absent, while in<br />
years and locations where rainfall is frequent during<br />
and after fl owering, head rot may be present in nearly<br />
all fi elds to some degree. Currently the incidence of<br />
head rot and wilt in the Dakotas is about equal, with<br />
approximately 3 percent of the crop affected by each<br />
disease. Yield loss from head rot on an individual<br />
plant may range from minimal to total loss since the<br />
affected head may disintegrate and drop all of the seed<br />
on the ground prior to harvest. Intact but diseased<br />
heads will have light and fewer seeds, with lower oil<br />
concentration, and also will shatter during harvest.<br />
The sclerotia that form in the diseased stalks and<br />
heads are returned to the soil at harvest, thus contaminating<br />
the fi eld for subsequent broadleaf crops.<br />
Management: The same comments made about Sclerotinia<br />
wilt also apply to head rot management, with<br />
some exceptions. Since ascospores that cause head<br />
rot can be blown into a fi eld, rotation will have less<br />
consistent impact upon head rot, even though it may<br />
reduce the levels of sclerotia in the fi eld. Anything<br />
that can minimize the crop canopy will help modify<br />
the environment necessary for ascospore infection.<br />
Thus, lower plant populations will facilitate more air<br />
movement and hasten leaf drying. Moderate levels<br />
of nitrogen fertilization also will minimize excessive<br />
foliage, but this needs to be counterbalanced with<br />
adequate fertilization to optimize yields.<br />
One of the most important tools for managing all<br />
Sclerotinia diseases is monitoring the incidence of<br />
Sclerotinia diseases on any preceding crop. If Sclerotinia<br />
is observed on a crop, the grower then knows<br />
that planting any susceptible crop in that fi eld the<br />
next year is imprudent. The number of years necessary<br />
to rotate away from susceptible broadleaf crops<br />
(while the population of sclerotia declines) depends<br />
upon the initial Sclerotinia incidence. As a general<br />
rule of thumb, most researchers suggest that four<br />
years away from broadleaf crops should reduce the<br />
sclerotial population to below a threshold level. Since<br />
sunfl ower is the only crop prone to root infection, and<br />
root infection can happen even in dry years, sunfl ower<br />
obviously would be the worst crop choice to plant in<br />
a known infested fi eld, with dry bean and canola following<br />
closely behind. As stated earlier, the best crops<br />
to break the Sclerotinia cycle are monocots (small<br />
grains, corn, sorghum).<br />
Sunfl ower hybrids do exhibit different degrees of susceptibility<br />
to head rot, but no totally resistant hybrids<br />
are available. An extensive testing program is under<br />
way to evaluate commercial hybrids for head rot resistance.<br />
Several university research centers have established<br />
mist-irrigated plots, which when coupled with<br />
artifi cial inoculations with ascospores, have produced<br />
high levels of infection to accurately assess hybrid<br />
disease response. After the most resistant hybrids have<br />
been tested in multiple locations, this information will<br />
be published in the NDSU publication “Hybrid Sunfl<br />
ower Performance Testing” (A652), also available<br />
online at www.ag.ndsu.edu/pubs/plantsci/rowcrops/<br />
a652.pdf. No chemical is registered for control of head<br />
rot in the U.S. Even if a chemical were registered, it<br />
would have to be applied as a preventative because<br />
when symptoms become visible, the infection already<br />
took place two to three weeks earlier and the fungus<br />
has become well-established in the head.<br />
For more information on sclerotinia, consult NDSU<br />
Extension Service publication “Sclerotinia Disease<br />
of Sunfl ower” (PP-840), also viewable on the Internet<br />
at www.ag.ndsu.edu/pubs/plantsci/rowcrops/pp840w.<br />
htm.
■ Stem Rots Caused by Sclerotinia<br />
Minor and Sclerotium Rolfsii<br />
Description: Stem rots caused by Sclerotinia minor<br />
or Sclerotium rolfsii are very similar in appearance<br />
and will be covered together. Sclerotinia minor on<br />
sunfl ower is seen in California and the southern Great<br />
Plains, but has not been reported in the northern<br />
Great Plains. Stem rot caused by Sclerotium rolfsii,<br />
also called Southern blight, primarily is observed on<br />
sunfl ower in warm climates such as California, Florida<br />
and irrigated fi elds in the southern Great Plains. Sclerotium<br />
rolfsii has not been observed on sunfl ower in<br />
the northern Great Plains, but has been noted in nursery<br />
stock in Minnesota, suggesting it could become<br />
established in the northern latitudes. Both fungi have<br />
a very broad host range encompassing many broadleaf<br />
crops.<br />
The symptoms of both Sclerotinia minor and Sclerotium<br />
rolfsii are outwardly very similar to the root<br />
infection and wilt caused by Sclerotinia sclerotiorum.<br />
Affected plants have a water-soaked lesion on the stem<br />
at the soil line that turns light brown. Under humid<br />
conditions, white mycelium also may be found on the<br />
lesion. In some instances, the lesion also may have a<br />
series of dark rings due to the diurnal growth pattern<br />
of the fungus. Plants infected with either fungus wilt<br />
and die suddenly, without any distinctive foliar symptoms,<br />
within a week of the onset of wilting. The major<br />
fi eld sign to distinguish S. sclerotiorum from S. minor<br />
is the size and shape of the sclerotia. In S. minor, the<br />
sclerotia are always round and generally less than 1/12<br />
inch (2mm) in diameter (Figure 92), and thus much<br />
smaller than sclerotia of S. sclerotiorum. Sclerotia of<br />
Sclerotium rolfsii are also round and the same size (<<br />
1/12 inch) as those of S. minor, but Sclerotium rolfsii<br />
sclerotia are tan to light brown. Sclerotia of these<br />
two fungi can be found within pith tissue and on the<br />
surface of the tap root.<br />
Disease Cycle: Both fungi overwinter in infected plant<br />
debris or can persist for several years as free sclerotia<br />
in the soil. The sclerotia germinate in response to root<br />
exudates to form mycelia that infect roots of adjacent<br />
sunfl ower plants. As the fungus moves up the root system<br />
and reaches the tap root and the stem, it produces<br />
toxins and oxalic acid that cause the plant to wilt.<br />
Both fungi are capable of infecting adjacent sunfl ower<br />
plants by root-to-root spread. Neither fungus produces<br />
any spores to initiate leaf or head infection, in contrast<br />
to S. sclerotiorum.<br />
Damage: Both Sclerotinia minor and Sclerotium rolfsii<br />
are as potentially damaging as S. sclerotiorum, with<br />
the caveat that the fi rst two species only cause a root<br />
rot and subsequent wilt. Plants infected near anthesis<br />
will suffer substantial seed losses, and if the affected<br />
plants lodge, further yield losses occur. Additionally,<br />
the sclerotia produced by these two fungi will contaminate<br />
the fi eld and make subsequent crops of other<br />
host plants prone to infection.<br />
Management: Crop rotation, elimination of infected<br />
residue and weed control will help reduce disease<br />
caused by either fungus. Since both fungi are highly<br />
aerobic, deep plowing (>12 inches), especially if<br />
complete inversion of the soil profi le is possible, will<br />
remove most of the sclerotia from the root zone and<br />
place the sclerotia where they are most vulnerable to<br />
attack by soil bacteria. Differences in hybrid susceptibility<br />
have not been investigated in the U.S. because<br />
neither disease is present in the major sunfl ower<br />
production areas.<br />
■ Figure 92. Sclerotia of Sclerotinia<br />
sclerotiorum (left) and S. minor (right)<br />
from sunfl ower stalks. Ruler at top in<br />
centimeters. (T. Gulya)<br />
Diseases<br />
71
72<br />
■ Charcoal Rot<br />
Description: Charcoal rot is caused by Macrophomina<br />
phaseolina, a fungus that attacks about 400<br />
plant species, including sunfl ower, dry bean, soybean,<br />
corn and sorghum. Charcoal rot is found throughout<br />
the Great Plains, but the disease is most common and<br />
severe in southern areas such as Texas, Kansas and<br />
Nebraska. Charcoal rot has been found on sunfl ower<br />
in western North Dakota and north-central South<br />
Dakota recently, and also on corn and soybean in both<br />
states. Charcoal rot generally appears after fl owering<br />
but seedling blights have been reported. Symptoms on<br />
stalks appear as silver-gray lesions near the soil line<br />
(Figure 93), which eventually decay the stem and tap<br />
root, leaving a shredded appearance. Stems are hollow<br />
and rotted, and lodge easily. Plants show poor seed fi ll<br />
and undersized heads. Seed yield, test weight and oil<br />
concentration are reduced. Numerous tiny black fungus<br />
bodies, called microsclerotia, form on the outside<br />
of the stalk and in the pith. To the unaided eye, the microsclerotia<br />
look like pepper grains; with a 5X to 10X<br />
lens they are clearly distinguishable as black, spherical<br />
sclerotia. Another unique characteristic of charcoal rot<br />
is the compressing of pith tissue into horizontal layers,<br />
like a stack of separated coins (Figure 94). This is a<br />
diagnostic characteristic of the disease.<br />
Disease Cycle: The primary source of inoculum is<br />
sclerotia in the soil, but Macrophomina also can be<br />
seed-borne. Upon stimulation by nearby root exudates,<br />
the sclerotia germinate to form mycelium that<br />
colonizes the roots. Macrophomina may colonize<br />
roots early in the season, but disease symptoms do<br />
not manifest themselves until anthesis. Once the root<br />
system is colonized, the fungus enters the stem and<br />
colonizes the vascular system, resulting in wilt and<br />
partial degradation of the pith. Disease development<br />
is favored by soil temperatures above 85 F. Moisture<br />
stress during the post-fl owering period greatly favors<br />
disease development.<br />
Damage: Post-fl owering stresses due to high plant<br />
population or drought coupled with heavy applications<br />
of nitrogen fertilizer, hail or insect damage promote<br />
disease development and accentuate the impact of<br />
charcoal rot. Yield losses can be signifi cant if disease<br />
incidence is high, as infected plants die before seed set<br />
is complete.<br />
Management: Crop rotation, balanced fertilizer programs<br />
and practices to reduce moisture stress all help<br />
minimize the impact of charcoal rot. Certain hybrids<br />
offer some resistance, possibly through drought tolerance.<br />
Since the fungus also attacks corn, sorghum and<br />
soybeans, not growing sunfl ower and these crops in<br />
successive years on the same ground would be advisable<br />
if charcoal rot has been observed.<br />
■ Figure 93. Silver grey discoloration of lower stem<br />
caused by charcoal rot compared with healthy green<br />
stalk (left). (T. Gulya)<br />
■ Figure 94. Charcoal rot affected stalk split apart to<br />
reveal characteristic compression of pith into layers.<br />
(T. Gulya)
Texas Root Rot<br />
Description: Texas root rot, or cotton root rot, is a<br />
soil-borne fungal disease found only in Texas, New<br />
Mexico, Arizona, southeastern California and northern<br />
Mexico. The causal agent, Phymatotrichopsis<br />
omnivora (synonym: Phymatotrichum omnivorum) has<br />
a very broad host range of more than 1,800 species of<br />
broadleaf herbaceous crops and weeds. On sunfl ower,<br />
as with most crops, the initial symptom is wilting followed<br />
quickly by death of affected plants. The disease<br />
in Texas develops in late spring and usually occurs in<br />
circular spots in the fi eld, which enlarge after rain or<br />
irrigation. No diagnostic symptoms appear on leaves<br />
or stems, but white mycelial strands on roots, visible<br />
with a 5X to 10X hand lens, are characteristic of this<br />
fungus. Following rain or irrigation, the fungus may<br />
produce white mycelial mats up to 12 inches in diameter<br />
and up to ¾ inch thick on the soil surface.<br />
Disease Cycle: The Texas root rot fungus survives for<br />
many years in the soil as sclerotia. These germinate to<br />
produce mycelial strands that can grow some distance<br />
in the soil until root contact is made. This ability also<br />
allows the fungus to spread from plant to plant in a<br />
row via overlapping root systems. The disease is most<br />
severe in moist, warm (80 F or higher at a 1-foot<br />
depth) soils that are high pH and high clay content.<br />
Sclerotia, at fi rst white and turning tan to dark brown,<br />
are round and generally greater than 1/12 inch in diameter.<br />
Sclerotia may form in the soil away from plant<br />
roots. On roots, the sclerotia may form at irregular<br />
intervals, giving the appearance of a string of beads.<br />
Damage: Like other root-infecting, stalk-rotting fungi,<br />
Phymatotrichum can cause considerable yield losses<br />
if plants are infected near bloom. Even if seed yield is<br />
not reduced, the fungal infection will lower oil content<br />
and result in lower test weight. In fi eld trials in western<br />
Texas, disease incidences up to 60 percent were<br />
observed, which cut yields by at least half.<br />
Management: The fungus persists in the soil for many<br />
years as sclerotia, which are the propagules that infect<br />
subsequent crops. Resistance has not been observed<br />
in sunfl ower. The best disease management is rotation<br />
with nonhosts, such as corn, sorghum, small grains or<br />
grass. Planting after cotton or alfalfa, especially where<br />
Texas root rot was observed, would be the worst-case<br />
scenario.<br />
■ Phoma Black Stem<br />
Description: Phoma black stem, caused by the soilborne<br />
fungus Phoma macdonaldii, is characterized<br />
by large, jet black lesions on the stem, sometimes<br />
reaching 2 inches in length. In addition, the fungus<br />
produces lesions on the leaves, on the back of the head<br />
and at the base of the stalk. The typical stem lesions<br />
originate with leaf infections that progress down the<br />
petiole to the stalk. Under favorable conditions, the<br />
leaf wilts, the petiole turns uniformly black and the<br />
stem lesions expand to form a large, shiny, black patch<br />
with defi nite borders (Figure 95). Small circular fruiting<br />
bodies of the fungus are produced on the surface<br />
of the stem, but these are inconspicuous to the naked<br />
eye and require a hand lens to observe.<br />
Disease Cycle: Phoma infection occurs throughout the<br />
growing season, although it usually is not noticed until<br />
the stem lesions become obvious later in the summer.<br />
The fungus overwinters in infected debris and conidia<br />
are spread by splashing rain. Insects such as Apion<br />
and Cylindrocopturus stem weevils also can carry<br />
Phoma spores both internally and externally. Adult<br />
weevils feeding on the leaves cause leaf lesions, while<br />
contaminated larvae spread the fungus as they tunnel<br />
throughout the stem. Disease transmission through<br />
infected seed is of minor importance.<br />
■ Figure 95. Large black lesions associated with<br />
the point of attachment of the leaf to the stem is a<br />
characteristic symptom of Phoma black stem.<br />
(D.E. Zimmer)<br />
Diseases<br />
73
74<br />
Damage: Phoma black stem is the most widespread<br />
stalk disease noted on sunfl ower in the northern Great<br />
Plains, but yield losses attributable solely to Phoma<br />
generally are considered minimal. Infected plants may<br />
produce smaller heads with reduced seed yield and oil.<br />
Phoma stem lesions are generally superfi cial and do<br />
not result in pith damage or lodging. However, if stem<br />
weevil larva tunneling spreads Phoma spores in the<br />
pith, extensive pith degeneration can occur.<br />
Management: No control measures are totally effective.<br />
A four-year rotation to other crops will minimize<br />
the concentration of Phoma within the soil. Control<br />
of stem weevils can help reduce transmission of the<br />
fungus. However, such control is not recommended<br />
solely for management of Phoma. No hybrids have<br />
been identifi ed as being immune to the disease, but<br />
some hybrids are more tolerant than others.<br />
■ Phomopsis Stem Canker<br />
Description: Phomopsis stem canker, caused by Phomopsis<br />
helianthi (sexual stage = Diaporthe helianthi)<br />
is a serious disease that fi rst was observed in Europe<br />
in the late 1970s and in the U.S. in 1984. The distinguishing<br />
feature of the disease is the large tan to light<br />
brown lesion or canker that typically surrounds the<br />
leaf petiole (Figure 96). Compared with Phoma black<br />
stem, the Phomopsis lesion is much larger, reaching 6<br />
inches in some cases, is brown rather than black and<br />
typically has a sunken border. Phomopsis also causes<br />
more extensive pith degradation than Phoma, so the<br />
■ Figure 96. Phomopsis is characterized by the<br />
large light brown lesion or canker which typically<br />
surrounds the leaf petiole. (T. Gulya)<br />
stalk may be crushed with moderate thumb pressure.<br />
Phomopsis-infected plants also are more prone to<br />
lodging than Phoma-infected plants.<br />
Disease cycle: The fungus overwinters predominantly<br />
as perithecia of Diaporthe in infected plant debris.<br />
The ascospores released from the perithecia are<br />
rain splashed or windblown onto leaves. The infection<br />
starts on the margins of lower leaves. A brown<br />
necrotic area develops and may be bordered by a<br />
chlorotic margin. The infection spreads down through<br />
the veins to the petiole and fi nally to the stem. These<br />
symptoms look similar to those of Verticillium leaf<br />
mottle, but with Verticillium, the necrotic areas are<br />
between the veins. Stem lesions usually do not appear<br />
until fl owering. Girdling stem lesions result in wilting<br />
and make the plant more prone to lodging. The disease<br />
may be diffi cult to identify when both Phoma and<br />
Phomopsis are present, in which case the stem lesion<br />
may be intermediate in color between the black lesion<br />
associated with Phoma and the brown typically associated<br />
with Phomopsis. In these cases, microscopic<br />
identifi cation of the fungus is necessary.<br />
Damage: Phomopsis stem canker has been found in<br />
both the central and northern areas of the Great Plains.<br />
Since the fungus is specifi c to sunfl ower, it likely<br />
would not be found in areas without a history of sunfl<br />
ower production. The disease is most severe under<br />
conditions of prolonged high temperatures and high<br />
rainfall. Yield losses result from smaller heads and<br />
lighter seed, and from lodging due to weakened stems,<br />
which can be quite extensive.<br />
Management: Since the fungus overwinters in infected<br />
sunfl ower debris on the soil surface, thorough<br />
disking in the fall to bury plant residue and crop<br />
rotation can reduce disease incidence and severity.<br />
Leaving crop residue on the soil surface would foster<br />
the best development of Phomopsis. Most U.S. sunfl<br />
ower companies are trying to incorporate some levels<br />
of Phomopsis resistance into their hybrids, using<br />
parental lines developed in Europe, where the disease<br />
is particularly severe. No U.S. commercial hybrids are<br />
immune to Phomopsis stem canker, nor are any fungicides<br />
registered in the U.S. for control of Phomopsis.<br />
Please consult NDSU publication A-652 for information<br />
on ratings of commercial hybrids to Phomopsis.
■ Verticillium Leaf Mottle<br />
Description: Verticillium wilt, or more accurately,<br />
leaf mottle, is caused by the soil-borne fungus Verticillium<br />
dahliae. The fungus has a wide host range<br />
and causes wilt of several other cultivated plants and<br />
weeds. Potato is the other important crop host of<br />
Verticillium in the northern Great Plains. Verticillium<br />
leaf mottle typically causes necrosis between the main<br />
leaf veins with yellow margins. The contrast between<br />
the necrotic tissue surrounded by chlorosis and the<br />
healthy green leaf tissue is striking and quite diagnostic.<br />
Symptoms begin on the lower leaves and progress<br />
slowly upward (Figure 97) and may encompass all<br />
leaves. Affected leaves rapidly become completely dry,<br />
but do not wilt to the same degree as with Sclerotinia<br />
wilt. Thus, the term leaf mottle may be more appropriate<br />
than Verticillium wilt. Symptoms usually are not<br />
observed until fl owering, but under severe conditions,<br />
they may occur as early as the six-leaf stage. The<br />
vascular system of infected plants may be discolored<br />
brown, visible as a brown ring in a cross-section of the<br />
stem. The pith of severely diseased plants is blackened<br />
with a layer of tiny black fruiting bodies (microsclerotia).<br />
These microsclerotia are much smaller than<br />
microsclerotia of charcoal rot and are not visible with<br />
a hand lens. Under a microscope, Verticillium microsclerotia<br />
are irregular to club-shaped (< 0.1 mm long),<br />
while charcoal rot microsclerotia are more uniformly<br />
spherical and larger (0.1 to 1.0 mm in diameter). Another<br />
fungus, Phialophora asteris, causes quite similar<br />
symptoms on sunfl ower. This fungus does not form<br />
microsclerotia, which is one way to distinguish it from<br />
Verticillium.<br />
Life Cycle: Verticillium overwinters as mycelium or<br />
microsclerotia in infected plant debris. The microsclerotia<br />
germinate in response to root contact and colonize<br />
the root system. As the fungus reaches the tap<br />
root and lower stem, toxins produced by the fungus<br />
are translocated to the leaves to produce the chlorotic<br />
and necrotic areas between the veins. Verticillium<br />
remains within the stem tissue and cannot be isolated<br />
from symptomatic leaves. The fungus is isolated most<br />
easily from stems and petioles of infected plants. No<br />
involvement of conidia occurs in disease development,<br />
although the fungus does produce conidia in culture.<br />
Damage: Sunfl owers infected with Verticillium usually<br />
die before seeds are completely mature, and thus yield<br />
losses result from smaller head size, lighter test weigh<br />
and reduced oil concentration. The stems of Verticillium-infected<br />
plants are weakened as the pith shrinks,<br />
and are more prone to lodging.<br />
Management: Resistance to Verticillium dahliae is<br />
controlled by a single dominant gene (V-1), and most<br />
U.S. oilseed hybrids contain this resistance. However,<br />
a new strain of Verticillium that is able to overcome<br />
the V-1 gene recently was identifi ed both in the U.S.<br />
and Canada. Thus, hybrids that previously were considered<br />
resistant have shown symptoms of Verticillium<br />
wilt due to infection by this new strain. Confection<br />
hybrids as a group are more susceptible to Verticillium<br />
than are oilseed hybrids. Verticillium leaf mottle is a<br />
serious disease on lighter soils with a history of sunfl<br />
ower cropping, and is seen less frequently on heavy,<br />
clay soils. This disease will cause some yield loss each<br />
time a susceptible crop is planted, as the fungus can<br />
persists for fi ve to 10 years as microsclerotia.<br />
■ Figure 97. Plants infected with Verticillium<br />
wilt show interveinal necrosis with yellow<br />
margins. (T. Gulya)<br />
Diseases<br />
75
76<br />
■ Bacterial Stalk Rot<br />
Description: Bacterial stalk rot occurs sporadically in<br />
the Great Plains and generally is not considered a major<br />
sunfl ower disease. The pathogen is Erwinia carotovora,<br />
a bacterium that causes soft rot on potato and<br />
other vegetables. Typical disease symptoms are stem<br />
discoloration (dark brown to black), often centered<br />
on a petiole axil, and a wet, slimy, soft rot of internal<br />
stem tissue. A pungent odor, reminiscent of rotting<br />
potatoes, is also characteristic. Due to pressure from<br />
the gas produced by the bacteria, the stem may be split<br />
open. Symptoms may extend down the stem into the<br />
roots. The bacterium also can infect the head, causing<br />
a wet, slimy rot of the receptacle. Infected stems are<br />
prone to lodging, and infected heads quickly fall apart.<br />
After the plant dies, the affected stalk or head dries up<br />
and may leave little indication of prior mushy rot.<br />
Disease Cycle: The main source of the bacteria is<br />
infested plant residue on the soil surface. The bacteria<br />
enter the plant through wounds caused by insects, hail<br />
and windblown sand; they cannot penetrate unbroken<br />
epidermis like fungal pathogens. Extended wet<br />
and warm periods favor disease development. Most<br />
diseased plants are observed later in the season. Young<br />
plants are more resistant to stalk decay than plants<br />
nearing senescence. Varietal differences in susceptibility<br />
to bacterial stalk rot are reported.<br />
Damage: Bacterial stalk rot is seen infrequently. Plants<br />
affected by this disease will be killed and produce<br />
little or no seed, but disease incidence within a fi eld is<br />
generally low.<br />
Management: While differences in resistance have<br />
been observed, little information is available on the<br />
resistance of current hybrids. Control of stem feeding<br />
insects will minimize the potential of insect transmission.<br />
■ Nematode Diseases<br />
Description: Nematodes are microscopic, nonsegmented<br />
roundworms that can cause serious damage to<br />
the roots of many crops. While many different types of<br />
nematodes have been found in sunfl ower fi elds, their<br />
economic impact on the plant is undocumented or<br />
highly variable.<br />
Genera of nematodes that have been reported on sunfl<br />
ower in the northern Great Plains include Heliocotylenchus,<br />
Tylenchrohynchus, Paratylenchus, Hoplolaimus<br />
and Xiphinema; the fi rst two genera are the most<br />
widely distributed in North Dakota, while Paratylenchus<br />
is the dominant nematode in South Dakota.<br />
All these nematodes are ectoparasites, meaning that<br />
they feed either on the root surface or burrow partially<br />
into the roots. Root-knot nematodes (Meloidogyne<br />
spp.), damaging pests of many crops, have been<br />
reported on sunfl ower in Florida and in warm areas of<br />
other countries but have not been recorded on sunfl<br />
ower in the upper Great Plains states. Sunfl ower is<br />
not a host for the soybean cyst nematode (Heterodera<br />
glycines), making sunfl ower suitable for rotation with<br />
soybean where the cyst nematode is a problem.<br />
Disease Cycle: Nematodes overwinter in the soil as<br />
eggs and colonize plant root systems throughout the<br />
growing season. Symptoms caused by nematodes are<br />
not distinctive and mimic those due to drought and<br />
nutrient defi ciencies. In severe infestations, the foliage<br />
wilts and turns yellow, and stunting may occur. The<br />
pattern of affected plants in the fi eld may have little or<br />
no relationship with topography. Examination of roots<br />
is necessary to prove nematode damage. Identifi cation<br />
of nematodes to genus requires their extraction from<br />
soil and roots, followed by microscopic examination.<br />
Damage: High populations of nematodes have caused<br />
yield reductions in greenhouse studies.<br />
Management: Applications of nematocides in fi eld<br />
trials have produced variable yield responses. No<br />
nematocides are registered for use on sunfl ower, and<br />
the potential cost return makes their use questionable.<br />
Tolerance to nematode damage appears related to the<br />
extensive root system of sunfl ower.
IV. Head Rots and Diseases<br />
of Mature Plants<br />
■ Head Rots, Other Than Sclerotinia<br />
Description: Several head rots (other than Sclerotinia)<br />
occur on sunfl ower in the U.S., and these are caused<br />
by several fungi, including Rhizopus, Botrytis and the<br />
bacterium Erwinia, covered previously.<br />
Rhizopus head rot, caused by Rhizopus arrhizus and<br />
R. stolonifera, was considered a sporadic disease in<br />
the Great Plains, but recent surveys have shown it to<br />
be the most widespread disease in the central Great<br />
Plains (Figure 98). Initial symptoms are brown, watery<br />
spots on the receptacle. Rhizopus species rot the soft<br />
tissues of the head, turning it brown and mushy. A<br />
threadlike, whitish fungal mycelium develops on and<br />
within the receptacle. Tiny black pinhead-sized fruiting<br />
structures (sporangia) form within the head tissue,<br />
giving the appearance of pepper grains.<br />
Botrytis head rot is caused by the widespread fungus<br />
Botrytis cinerea, and is distinguished from Rhizopus<br />
by the gray “fuzz” on the heads (caused by mycelium<br />
and spores). Heads affected by Botrytis eventually will<br />
disintegrate and may contain small sclerotia, similar in<br />
size to those caused by Sclerotinia.<br />
Bacterial head rot caused by Erwinia carotovora is<br />
rare in the Great Plains. This disease is characterized<br />
by a slimy, wet, brownish rot of the head with no fungus<br />
growth or spores in the tissues. Often such heads<br />
have a putrid odor.<br />
Disease Cycle: Rhizopus enters the head through<br />
wounds caused by hail, birds and insects and has been<br />
associated with head moth and midge damage. The<br />
susceptibility of heads increases from the bud stage up<br />
to the full bloom and ripening stages. Disease development<br />
is most rapid in warm, humid weather. Once<br />
the head is fully colonized and all tissue killed, the<br />
head dries up and becomes hard and “mummifi ed.”<br />
Botrytis infects sunfl ower heads during cool, wet<br />
weather and requires organic debris, such as fl ower<br />
parts or senescing tissue, to initiate growth. Late attacks<br />
start from the senescent petals and head bracts<br />
and may be serious during a wet fall and late harvesting.<br />
Head rot symptoms start as brown spots on the<br />
back of the head, which is identical to the initial symptoms<br />
of all head-rotting fungi. These spots become<br />
covered with gray powdery Botrytis conidia, giving<br />
the head a “fuzzy” appearance. These spores generally<br />
form on the surface tissues and not inside the tissues,<br />
as with Rhizopus. In wet weather, the infection spreads<br />
throughout the tissues, and the head becomes a rotten,<br />
spongy mass.<br />
Bacterial head rot, as with bacterial stalk rot, is caused<br />
when windblown or rain-splashed bacteria fall on<br />
wounds in the head caused by hail, insects or birds.<br />
No fruiting structures are associated with bacterial<br />
diseases, but the putrid odor associated with bacterial<br />
rotting is distinctive enough for identifi cation.<br />
Damage: Disease incidence for all three described<br />
head rots is generally low throughout the northern<br />
Great Plains, while Rhizopus head rot is common in<br />
the High Plains. Individual heads affected by any of<br />
the head rots will have lighter test weight seed, lower<br />
oil content and reduced seed yield. In severe cases,<br />
the affected heads may be entirely lost. Seeds from<br />
heads infected by any of these fungi or bacteria will<br />
have higher free fatty acid content, resulting in a bitter<br />
taste. Thus, head rots of confection sunfl ower may<br />
cause losses due to lowered quality factors, even in<br />
cases where actual seed yield losses are minimal.<br />
Management: Insect control may help minimize<br />
Rhizopus head rot, but will not offer as much disease<br />
reduction as fungicide sprays. In the U.S., no fungicides<br />
are registered for control of any head rot, except<br />
Sclerotinia. No practices are recommended to control<br />
bacterial head rot, other than to minimize head-feeding<br />
insects that might transmit the bacterium.<br />
■ Figure 98. Rhizopus head rot is characterized by<br />
a dark brown, peppery appearance of tissues in the<br />
receptacle. (T. Gulya)<br />
Diseases<br />
77
78<br />
Weeds<br />
(Richard Zollinger)<br />
Weeds compete with sunfl ower, causing poor growth<br />
and yield losses. Yield loss from weed competition<br />
depends on weed species, time of infestation,<br />
weed density and climatic conditions. All weeds are<br />
competitors. However, in the northern region of the<br />
U.S., wild mustard, wild oats and kochia, which grow<br />
rapidly early in the season, appear more competitive<br />
than foxtail on a per-plant basis.<br />
A comprehensive weed management program consisting<br />
of cultural and/or chemical controls is needed to<br />
maximize yields. Sunfl ower is a good competitor with<br />
weeds. However, this competitive advantage occurs<br />
only after plants are well-established. The fi rst four<br />
weeks after emergence are most critical in determining<br />
weed competition damage, so early weed control<br />
is essential. Weeds competing longer than four weeks<br />
cause important yield loss even if they are removed.<br />
All chemical recommendations for weed control have<br />
a U.S. federal label unless otherwise specifi ed. All<br />
recommended herbicides have federal registration at<br />
the time of printing, and rates listed are label rates at<br />
time of printing. Consult the current issue of NDSU<br />
Extension publication W-253, “North Dakota Weed<br />
Control <strong>Guide</strong>,” or appropriate Extension publications<br />
from other states for current labeled products, rates<br />
and method of application.<br />
WILD MUSTARD (Sinapis arvensis) is a major weed<br />
that infests sunfl ower. Wild mustard is not controlled<br />
by most of the herbicides commonly used in sunfl ower.<br />
Wild mustard emerges early and appears to be most<br />
competitive with sunfl ower when the early season is<br />
cool. The cool condition favors wild mustard, but not<br />
sunfl ower growth. Late seeding with seedbed tillage<br />
to control early emerged wild mustard can reduce<br />
infestations. However, wild mustard may continue to<br />
emerge with timely rains and remain a problem even<br />
with late seeding. Assert (imazamethabenz) is the only<br />
herbicide registered for use in conventional sunfl ower<br />
to control wild mustard. Wild mustard can be controlled<br />
easily in Clearfi eld sunfl ower with Beyond<br />
(imazamox) and in Express-resistant sunfl ower with<br />
Express (tribenuron).Wild mustard is controlled effectively<br />
by herbicides used in other crops in the rotation.<br />
Wild mustard seed can remain viable in the soil for<br />
many years, so plants allowed to produce seed can<br />
cause an infestation for many subsequent years.<br />
WILD OAT (Avena fatua) is another cool-season weed<br />
that is abundant in North Dakota and causes important<br />
yield losses, especially in early seeded sunfl ower. Wild<br />
oat germinates early in the spring, and germination<br />
and emergence generally stop when soil becomes<br />
warm. Delayed seeding reduces wild oat infestations.<br />
Wild oat can infest late-seeded sunfl ower when cool<br />
and moist conditions occur at or after seeding. Wild<br />
oat is controlled to various degrees by several registered<br />
herbicides (Table 12).<br />
GREEN FOXTAIL (Setaria viridis) and YELLOW<br />
FOXTAIL (Setaria lutescens) are the most abundant<br />
grassy weeds in North Dakota. Both green and yellow<br />
foxtail occur throughout the state. Green foxtail has<br />
been more abundant, but yellow foxtail is the dominant<br />
species in many areas because herbicides giving<br />
less control of yellow foxtail have been used in crops.<br />
The two species have similar appearance, but yellow<br />
foxtail has a fl at stem with long hairs at the base of<br />
the leaves, a more brushlike spike and a larger seed.<br />
Green foxtail has a round stem with no hair on the<br />
leaves. Foxtail is a warm-season plant, and germination<br />
and emergence do not occur until the soil reaches<br />
60 degrees Fahrenheit. Many sunfl ower herbicides<br />
give excellent control of foxtail species (Table 12).<br />
KOCHIA (Kochia scoparia) is considered the worst<br />
weed problem of sunfl ower in North Dakota. Kochia<br />
is a highly competitive weed that emerges during cool<br />
periods early in the spring or later with warm temperatures<br />
and adequate moisture. Most kochia has become<br />
resistant to ALS (acetolactate synthase) herbicides and<br />
no registered herbicides in sunfl ower give adequate<br />
control. Beyond herbicide in Clearfi eld sunfl ower is<br />
an ALS herbicide and will not control ALS-resistant<br />
kochia. Soil-applied Spartan (sulfentrazone) controls<br />
ALS-resistant and susceptible kochia when activated<br />
by suffi cient moisture after application. Kochia seeds<br />
do not have a long residual life in the soil. Good<br />
control of kochia in the crop prior to sunfl ower emergence<br />
or control before seeding will reduce the kochia<br />
infestation.
RUSSIAN THISTLE (Salsola iberia) is most common<br />
in the drier western areas of North Dakota. Russian<br />
thistle germinates throughout the season. Germination<br />
is rapid, so light rains anytime will promote a new<br />
fl ush of Russian thistle growth. Competition data on<br />
losses from Russian thistle in sunfl ower are not available.<br />
The plants are normally small and competition<br />
usually is not expected. However, Russian thistle is<br />
drought tolerant and losses may be severe, even from<br />
a small number of plants under conditions of limited<br />
moisture.<br />
Table 12. Relative effectiveness of herbicides for various weeds.<br />
Herbicide<br />
Green/yellow foxtail<br />
Wild oat<br />
Wild buckwheat<br />
Comn. cockle bur<br />
Preplant herbicides<br />
Glyphosate E E F-G E F-E P-E G-E E F-G E G-E G-E G-E<br />
Paraquat G G F F-G G-E E G E G-E E E - P<br />
Soil-applied herbicides<br />
Eptam (EPTC) E G-E F P F F N P F G P N N<br />
Prowl<br />
(pendimethalin) G-E P P N F G N N N G F-G N N<br />
Sonalan<br />
(ethalfl uralin) E P-F P-F N F E N N P G-E G-E N N<br />
Dual Magnum<br />
(s-metolachlor) G-E P P N P P-F N P P F-G P N N<br />
Spartan<br />
(sulfentrazone) P N P-F N E E P-G P-F E E G-E G-E N<br />
Trifl uralin E P P N F G N N N F-G F-G N N<br />
POST-applied herbicides<br />
Assert<br />
(imazamethabenz) P G-E F-G P N P N E N P P-F N N<br />
Beyond* (imazamox) E E P G-E E1 F G-E E E E G-E P N<br />
Poast (sethoxydim) E G-E1 N N N N N N N N N N N<br />
Select (clethodim) E E N N N N N N N N N N N<br />
* = Clearfi eld sunfl ower.<br />
1 = Herbicides will not control resistant biotypes.<br />
E = Excellent, G = Good, F = Fair, P = Poor and N = None.<br />
The ratings in the table indicate relative effectiveness, with effectiveness of each herbicide varying with environment and<br />
method of application.<br />
Kochia<br />
OTHER WEEDS important in sunfl ower are wild<br />
buckwheat (Polygonum convolvulus), redroot pigweed<br />
(Amaranthus retrofl exus), common lambsquarters<br />
(Chenopodium album), fi eld bindweed (Convolvulus<br />
arvensis), Canada thistle (Cirsium arvense), cocklebur<br />
(Xanthium strumarium), marshelder (Iva xanthifolia),<br />
biennial wormwood (Artemisia biennis), nightshades<br />
(Solanum spp.) and wild sunfl ower. Some of these<br />
weeds are controlled partially by soil-applied trifl ualin<br />
or Sonalan (ethalfl uralin), but these products cannot<br />
be used in no-till sunfl ower production because of<br />
Comn. lambsquarters<br />
Marshelder<br />
Wild mustard<br />
Nightshade spp.<br />
R. root pigweed<br />
Russian thistle<br />
Bien. wormwood<br />
Canada thistle<br />
Weeds<br />
79
80<br />
their soil incorporation requirement. Pre-emergence<br />
Spartan controls most small-seeded broadleaf weeds<br />
and suppresses wild buckwheat, marshelder and<br />
foxtail. However, no herbicides are available for selective<br />
control of wild buckwheat, Canada thistle, fi eld<br />
bindweed, cocklebur, marshelder or wild sunfl ower.<br />
Beyond in Clearfi eld sunfl ower controls most annual<br />
grass and broadleaf weeds except ALS-resistant<br />
weeds, including kochia, but has no activity on perennial<br />
broadleaf weeds. Weeds for which no herbicides<br />
are available need to be controlled in previous crops in<br />
rotation, or through tillage or the use of herbicides in<br />
or between other crops in the rotation.<br />
The sunfl ower yield loss from individual weeds varies<br />
with the weed species, environment and time of weed<br />
emergence relative to the crop. Sunfl ower yield losses<br />
from several weeds at various infestations are presented<br />
in Figure 99. The values are averages from several<br />
years and losses from an individual weed would vary<br />
with conditions. A weed that emerges before the sunfl<br />
ower would be more competitive than one emerging<br />
after sunfl ower establishment, and an environment that<br />
favored the growth of the weed would cause a greater<br />
loss than if the environment favored the sunfl ower.<br />
■ Weed Management<br />
(Richard Zollinger)<br />
Cultural<br />
Cultural weed control requires an integrated system of<br />
tillage operations. Weeds must be controlled in other<br />
crops in the rotation to reduce the potential infestation<br />
level in sunfl ower. Preplant, pre-emergence and<br />
postemergence tillage practices all must be followed<br />
for effective weed control using only tillage. Poor<br />
timing or missing any tillage operation can reduce<br />
the effectiveness of the cultural weed control program<br />
drastically.<br />
Preplant tillage can control one or more weed fl ushes.<br />
Sunfl ower should be planted immediately after the last<br />
tillage operation so the crop can germinate rapidly and<br />
compete more favorably. Weeds frequently emerge before<br />
sunfl ower, especially during cool weather. These<br />
weeds can be controlled by pre-emergence harrowing.<br />
Postemergence mechanical weed control consists<br />
of harrowing and cultivating. Small weeds can be<br />
controlled by harrowing after the sunfl ower is in the<br />
four- to six-leaf stage (V-4 to V-6) and can resist burial<br />
and breaking (Figure 100). Postemergence harrowing<br />
should be done across rows and preferably on a warm,<br />
clear day to assure suffi cient weed kill with the least<br />
damage to the sunfl ower. Sunfl ower seedlings, which<br />
are strongly rooted, can be harrowed three to fi ve<br />
times during the four- to six-leaf stage (V-4 toV-6).<br />
The harrow should be kept free of trash. Spring tooth<br />
harrows are recommended; solid spike-tooth harrows<br />
should not be used, as excessive damage may result.<br />
■ Figure 99. Percent reduction in sunfl ower seed yield from several weeds. (J.D. Nalewaja)
■ Figure 100. This fi eld was harrowed before<br />
sunfl ower emergence and was being harrowed at<br />
about the four leaf stage to control seedling weeds.<br />
(GTA-Farmers Union)<br />
The direction of travel during harrowing is determined<br />
by considering the stand, weed growth and herbicide<br />
treatment. Harrowing diagonally to the rows will give<br />
better in-the-row weed control than with the row harrowing.<br />
However, sunfl ower damage will occur from<br />
the tractor wheels with diagonal harrowing. Harrowing<br />
may be necessary if a soil-applied herbicide was<br />
not activated by rainfall, if a fi eld previously treated<br />
with a herbicide has weeds resistant to the herbicide<br />
or if adverse climatic conditions reduce herbicide effectiveness.<br />
If the herbicide is band-applied, harrowing<br />
should be parallel to the rows to prevent dilution<br />
with untreated soil. A rotary hoe also is effective for<br />
postemergence weed control, but weeds must be just<br />
emerging for good control. Setting the harrow or<br />
“weighting” the rotary hoe to do the most damage<br />
to weeds and the least damage to sunfl ower can be<br />
accomplished on a “try-and-adjust” system. Postemergence<br />
harrowing will kill some sunfl ower (5 percent<br />
to 8 percent loss can be expected), so if this system<br />
of weed control is planned, the sunfl ower should be<br />
seeded at higher rates than normal.<br />
After postemergence harrowing, weed control for<br />
the remainder of the season depends on the row-crop<br />
cultivator. During the fi rst cultivation, producers must<br />
take care not to cover the sunfl ower. One to three or<br />
more cultivations may be necessary, depending on<br />
the weed situation in the fi eld. Lateral sunfl ower roots<br />
are shallow and can be damaged easily by cultivating<br />
too deeply and too closely to the plants. Cultivation<br />
should be no closer to the row center than the leaf<br />
spread of the plants. During later cultivations, soil may<br />
be thrown into the row to bury weed seedlings and<br />
provide the sunfl ower extra support.<br />
Registration of Spartan and Clearfi eld sunfl ower will<br />
allow and encourage no-till sunfl ower production. Notill<br />
farming increases dependence on chemicals and<br />
increases selection pressure for resistant weeds.<br />
Chemical<br />
The most effective weed management is accomplished<br />
by an integrated system that uses both cultural and<br />
chemical control. Preplant cultural practices to reduce<br />
weed seed populations, pre-emergence tillage and<br />
postemergence cultivation may be needed to supplement<br />
the herbicides under adverse climatic conditions<br />
and to control late-emerging weeds or weeds that are<br />
not controlled by herbicides. Herbicides vary in their<br />
effectiveness against various weeds (Table 12).<br />
Preplant and Pre-emergence<br />
GLYPHOSATE at 1 to 2 pt/A of 3 lb/gal concentrate<br />
(0.38 to 0.75 lb ai/A) is registered for the control<br />
of emerged weed seedlings before, during or after<br />
planting but before the crop emerges. Glyphosate is<br />
a nonselective, systemic, nonresidual herbicide, so<br />
treatment must be made before sunfl ower emergence<br />
and after weeds emerge. Several formulations are<br />
available, so follow label directions for rates, weed<br />
sizes, application volume and addition of nonionic<br />
surfactant.<br />
PARAQUAT at 2.5 to 4 pt/A (0.63 to 1 lb ai/A) is<br />
registered for the control of emerged weed seedlings<br />
before, during or after planting but before the crop<br />
emerges. Paraquat is a nonselective contact herbicide,<br />
so treatment must be before sunfl ower emergence.<br />
However, application must be after weed emergence,<br />
as paraquat has no soil residual to control late-emerging<br />
weeds. A nonionic surfactant at 0.25 percent v/v<br />
should be added to the spray solution to increase spray<br />
droplet contact with the leaf surface and retention by<br />
the leaf. Spray should be applied at 20 gallons per<br />
acre by ground equipment or 5 gallons per acre by air.<br />
Paraquat is a restricted-use herbicide.<br />
Weeds<br />
81
82<br />
EPTAM (EPTC) at 2.5 to 3.5 pt/A (2 to 3 lb ai/A)<br />
applied before planting or at 4.5 to 5.25 pt/A or 20 to<br />
22.55 lb 10G per acre applied after Oct. 15 controls<br />
some annual grass and broadleaf weed species. Eptam<br />
is degraded within three weeks after application. The<br />
3 pound- per-acre rate spring applied occasionally<br />
has caused sunfl ower injury on coarse-textured, low<br />
organic matter soils. The risk of sunfl ower injury can<br />
be reduced by using lower rates on these soils. Immediate<br />
and thorough incorporation is essential, as the<br />
herbicide is volatile. A 15-minute delay in incorporation<br />
during warm weather with moist soil may result<br />
in signifi cant vapor loss and poor weed control. Proper<br />
incorporation can be accomplished by tandem disking<br />
twice in cross directions (4 to 6 inches deep) or by<br />
any other method that thoroughly mixes the chemical<br />
within the top 3 inches of soil. Eptam generally<br />
gives good short-term weed control but is weak on<br />
wild mustard, Russian thistle, common cocklebur,<br />
smartweed and all perennial broadleaf weeds. Wild oat<br />
control is good.<br />
PROWL or PROWL H20 (pendimethalin) at 2.4 to<br />
3.6 pt/A EC, 2.1 to 3 pt/A ACS preplant incorporated<br />
or pre-emergence in no-till sunfl ower is registered for<br />
control of most grass and certain broadleaf weeds in<br />
sunfl ower. Prowl can be applied in the fall at 2.4 to<br />
4.25 pt/A and incorporated when soil temperature is<br />
less than 45 degrees. Prowl is registered only as an incorporated<br />
treatment for conventionally tilled sunfl ower<br />
because of greater consistency of weed control and<br />
greater crop safety. Prowl plus Spartan controls many<br />
grass and broadleaf weeds in no-till sunfl ower. No-till<br />
sunfl ower is treated with higher rates of Prowl than<br />
conventionally tilled sunfl ower. The higher rates help<br />
overcome the reduced control from pre-emergence vs.<br />
PPI treatment and from Prowl being absorbed on the<br />
crop residue. Prowl is registered at 2.4 pt/A in no-till<br />
sunfl ower for coarse-textured soils with less than 3<br />
percent organic matter and 3.6 pt/A for all other soils,<br />
including coarse-textured soils with greater than 3<br />
percent organic matter.<br />
Prowl may be applied up to 30 days before seeding<br />
no-till sunfl ower. Spray volume greater than 20 gallons<br />
per acre should be used to aid penetration to the<br />
soil in fi elds with high amounts of crop residue. Prowl<br />
does not control emerged weeds, so either glyphosate<br />
or paraquat would be needed to control emerged<br />
weeds in no-till sunfl ower prior to planting. Prowl has<br />
state registration for no-till sunfl ower in North Dakota,<br />
South Dakota and Minnesota.<br />
SONALAN (ethalfl uralin) at 1.5 to 3 pt/A (0.55 to<br />
1.15 lb ai/A) preplant incorporated in the spring is<br />
registered for the control of annual grass and some<br />
small-seeded broadleaf weeds in sunfl ower. The<br />
granular formulation (Sonalan 10G) can be applied<br />
in the spring or fall between Oct. 10 and Dec. 31 at<br />
5.5 to 11.5 lb 10G/A. Sonalan does not control wild<br />
mustard. The fi rst incorporation into the soil may be<br />
delayed no longer than two days after application.<br />
The second incorporation should be delayed three<br />
to fi ve days after the fi rst. Sonalan has a shorter soil<br />
residual than trifl uralin and is slightly more effective<br />
in controlling wild oats, Russian thistle and a few<br />
other broadleaf weeds. Sonalan is registered for tank<br />
mixtures with Eptam.<br />
Recent labeling allows use of Sonalan in reducedtillage<br />
systems for suppression of foxtail species.<br />
Sonalan 10G may be applied at 7.5 to 12.5 pounds per<br />
acre in the fall to small-grain stubble and incorporated<br />
once in the fall and once in the spring with a V-blade<br />
prior to planting sunfl ower. Sonalan 10G may be<br />
applied in the spring and incorporated twice using a Vblade.<br />
For spring applications, a delay of at least three<br />
weeks between incorporations should be observed<br />
unless a minimum of 0.5 inch of precipitation occurs<br />
after the fi rst incorporation. The delay then may be<br />
shortened to 10 days. The incorporations should be<br />
made at approximately 5 mph using a V-blade implement<br />
with 12- to 32-inch-wide sweeps. Both incorporations<br />
should be no deeper than 2 to 2.5 inches.<br />
DUAL MAGNUM (s-metolachlor) at 1 to 2 pt/A (0.95<br />
to 1.9) preplant incorporated or preplant will control<br />
green foxtail and several other weeds, such as pigweed<br />
and lambsquarters. It will not control wild mustard or<br />
wild oats. Incorporation improves weed control and<br />
consistency of control. It requires soil moisture for<br />
activation and better weed management. Use higher<br />
rates for clay soils high in organic matter.<br />
SPARTAN (sulfentrazone) at 3 to 8 fl oz F (1.5 to 4<br />
oz ai/A) applied pre-emergence controls most annual<br />
small-seeded broadleaf weeds, such as kochia, pigweed<br />
species, lambsquarters, nightshade, smartweed,<br />
Russian thistle and biennial wormwood, and may<br />
suppress buckwheat, mustard, ragweed and Russian<br />
thistle. Spartan may provide some grass but no peren-
nial weed control. Adjust rate based on organic matter<br />
and use higher rates if applied up to 30 days prior to<br />
planting. Sunfl ower has good tolerance to Spartan<br />
on medium to fi ne-textured soils with organic matter<br />
above 3 percent. Crop injury may occur on soils<br />
with low organic matter and soil pH greater than 8.0,<br />
especially on calcareous outcropping. Do not use on<br />
coarse-textured soils with less than 1 percent organic<br />
matter. Closely furrow at planting to avoid injury. Poor<br />
growing conditions at and following sunfl ower emergence,<br />
cold temperatures, soil compaction or a rate too<br />
high based on soil type and organic matter may result<br />
in sunfl ower injury. Consistent weed control greatly<br />
depends on at least 0.75 inch rainfall shortly after application<br />
and before weeds emerge. Spartan is a PPO<br />
inhibitor mode-of-action herbicide in which no weed<br />
resistance has been documented.<br />
TRIFLURALIN at 1 to 2 pt/A or 5 to 10 lb 10G/A<br />
(0.5 to 1 lb ai/A) is a preplant incorporated herbicide<br />
for grass and certain broadleaf weed control in<br />
sunfl ower. Incorporation should be by tandem disk or<br />
fi eld cultivator twice in cross directions (4 to 6 inches<br />
deep) at about 6 mph. Thorough incorporation is essential<br />
for optimum, consistent weed control. Trifl uralin<br />
is less volatile than EPTC (Eptam). Immediate<br />
soil incorporation is preferred, but with cold, dry soil<br />
and low wind, incorporation may be delayed up to 24<br />
hours. The lower rate should be used on soils of coarse<br />
texture and low organic matter. Trifl uralin gives seasonlong<br />
control of some annual grass and broadleaf<br />
weeds. Wild mustard is not controlled, and wild oat<br />
control is poor.<br />
Postemergence<br />
ASSERT (imazamethabenz) at 0.6 to 0.8 pt/A (0.19<br />
to 0.25 lb ai/A) controls wild mustard in sunfl ower.<br />
Assert should be applied before sunfl ower exceeds 15<br />
inches in height. Wild mustard should be in the rosette<br />
stage but prior to bloom. Sunfl ower injury may occur<br />
from Assert if applied during high temperatures and<br />
humidity.<br />
POAST (sethoxydim) at 0.5 to 1.5 pt/A (0.1 to 0.3 lb<br />
ai/A) applied postemergence in sunfl ower controls annual<br />
grasses and suppresses quackgrass. Oil adjuvant<br />
should be included at 1 qt/A. Poast at 0.5 pt/A controls<br />
wild proso millet; at 1 pt/A controls volunteer corn,<br />
green and yellow foxtail, and barnyardgrass; and at<br />
1.5 pt/A controls wild oats and volunteer cereals.<br />
Quackgrass that is 6 to 8 inches tall can be suppressed<br />
with Poast at 1.5 pt/A. Quackgrass regrowth should<br />
be treated at 1 pt/A. Cultivation between 14 to 21 days<br />
after application will improve quackgrass control. The<br />
addition of 2 to 4 quarts per acre of liquid nitrogen<br />
solution or 2.5 pounds per acre of ammonium sulfate<br />
in addition to the oil adjuvant may increase control of<br />
volunteer corn, cereal grains and quackgrass. Sunfl<br />
ower should not be harvested before 70 days after<br />
application.<br />
CLETHODIM (several trade names) at 6 to 8 fl oz<br />
or SELECT MAX (clethodim) at 9 to 32 fl oz/A (1<br />
to 3.9 oz ai/A) applied postemergence in sunfl ower<br />
controls annual grasses, volunteer cereals and perennial<br />
grasses, including quackgrass. See label rates to<br />
control individual type of grasses. Oil adjuvant should<br />
be included at 1 qt/A. oz/A. Cultivation between 14<br />
and 21 days after application will improve quackgrass<br />
control. The addition of 2 to 4 qt/A of liquid nitrogen<br />
solution or 2.5 lb/A of ammonium sulfate in addition<br />
to the oil adjuvant may increase grass control. Sunfl<br />
owers should not be harvested before 70 days after<br />
application.<br />
■ Herbicide-resistant Sunfl ower<br />
Clearfi eld sunfl ower<br />
BEYOND (imazamox) at 4 fl oz/A (0.5 oz ai/A) applied<br />
postemergence to Clearfi eld sunfl ower varieties<br />
from the two- to eight-leaf stage controls most annual<br />
grass and broadleaf weeds. Apply with NIS at 0.25<br />
percent v/v alone or with UAN liquid fertilizer at 1 to<br />
2 qt/A. Beyond will not control wild buckwheat, biennial<br />
wormwood, large common lambsquarters, Canada<br />
thistle or ALS-resistant weeds, including kochia.<br />
Clearfi eld sunfl ower can be planted on land previously<br />
treated with Assert or Pursuit to reduce or eliminate<br />
injury from long residual sulfonylurea herbicides.<br />
Clearfi eld sunfl ower may facilitate no-till sunfl ower<br />
production.<br />
EXPRESS (tribenuron) at 0.25 to 0.5 oz/A (0.188<br />
to 0.38 oz ai/A) applied postemergence to Express<br />
Sun sunfl ower varieties will control annual broadleaf<br />
weeds, including wild mustard. It will not control<br />
ALS-resistant weeds, including kochia or grass weeds.<br />
Control or suppression of Canada thistle can be expected<br />
at the higher rate. Apply early postemergence<br />
to Express-resistant sunfl ower in the one-leaf stage<br />
Weeds<br />
83
84<br />
but prior to bud formation. Broadleaf weeds should<br />
be 3 inches or less in height. Apply with MSO-type<br />
oil adjuvants at 1 percent v/v. NIS or petroleum oil<br />
adjuvants are not prohibited.<br />
Sequential applications are allowed but observe a 14day<br />
interval between applications and do not exceed<br />
1 oz/A during any growing season. Allow a 70-day<br />
preharvest interval. Express Sun application may help<br />
facilitate no-till sunfl ower production.<br />
■ Preharvest Application<br />
GRAMOXONE INTEON (paraquat) at 1.5 to 2 pt/A<br />
(0.375 to 0.5 lb ai/A) can be used as a harvest aid in<br />
oilseed sunfl ower. Application should be made when<br />
the backside of the sunfl ower heads is yellow, bracts<br />
are turning brown and seed moisture is less than 35<br />
percent. Paraquat can be used on both confectionary<br />
and oilseed hybrid cultivars. Apply with a nonionic<br />
surfactant at 1 to 2 pints per 100 gallon of water. A<br />
seven-day interval must elapse between application<br />
and harvest. Paraquat is a restricted-use herbicide.<br />
DREXEL DEFOL (sodium chlorate) at 1 to 2 gal/A (6<br />
to 12 lb ai/A) can be used as a desiccant with both oilseed<br />
and confectionary sunfl ower. Application should<br />
be made when the backside of the sunfl ower heads is<br />
yellow, bracts are turning brown and seed moisture is<br />
less than 35 percent. Apply at 20 to 30 gallons per acre<br />
by ground and 5 to 10 gallons per acre by air.<br />
Roundup Preharvest Application in Sunfl ower<br />
Monsanto has issued a Supplemental label allowing<br />
certain applications of glyphosate (Roundup) for control<br />
of annual and perennial weeds in sunfl ower. Apply<br />
no more than a total of 22 fl oz of the 4.5 lb. acid<br />
equivalent/gal formulation at preharvest. See label for<br />
rates suggested.<br />
For preharvest use in sunfl ower, apply for weed<br />
control, NOT crop desiccation when sunfl ower plants<br />
are physiologically mature. Apply when the backsides<br />
of sunfl ower heads are yellow and bracts are turning<br />
brown and seed moisture is less than 35%. Generally<br />
the dry chaffy material from the disk fl owers on the<br />
head can be easily rubbed off by hand and expose the<br />
seeds at this stage of maturity. Allow a minimum of 7<br />
day preharvest interval (PHI) for sunfl ower following<br />
application.<br />
For post-harvest weed control, the products may be<br />
applied after harvest of sunfl ower. Higher rates may be<br />
required for control of large weeds, which were growing<br />
in the crops at the time of harvest. Tank mixtures<br />
with 2,4-D or dicamba may be used after harvest.<br />
Always follow the pesticide label when applying any<br />
product to sunfl ower.<br />
■ Control of Volunteer Sunfl ower<br />
in Crops<br />
Crops following sunfl ower often are infested with<br />
volunteer sunfl ower plants. In small grains, 2,4-D<br />
and MCPA at rates of at least 1 pt/A are needed for<br />
consistent control. Bromoxynil plus MCPA at 0.5 pt/A<br />
+ 0.5 pt/A has given excellent, consistent volunteer<br />
sunfl ower control. Dicamba plus MCPA at 4 fl oz/A +<br />
0.5 pt/A has given good control. Several sulfonylurea<br />
herbicides plus 2,4-D or MCPA, and Curtail or Curtail<br />
M also control sunfl ower. Sunfl ower can emerge from<br />
deep in the soil, and these late-emerging plants may<br />
escape an early herbicide application. However, delaying<br />
treatment until all sunfl ower emerge may result in<br />
poor control and some yield loss from competition.<br />
Some judgment is needed to determine the proper<br />
time for application, and two applications may be<br />
needed in some situations.<br />
In corn, preplant Hornet (fl umetsulam plus clopyralid),<br />
postemergence bromoxynil, Basagran (bentazon),<br />
dicamba, Distinct (dicamba + difl ufenzopyr),<br />
Hornet, Callisto (mesotrione), Permit (halosulfuron),<br />
NorthStar (dicamba + primisulfuron) and Option<br />
(foramsulfuron) control volunteer sunfl ower. Volunteer<br />
sunfl ower also can be controlled with glyphosate in<br />
Roundup Ready corn.<br />
In soybean, preplant Pursuit Plus (imazethapyr +<br />
pendimethalin), Gangster (fl umioxazin + cloransulam),<br />
postemergence bentazon, Result (bentazon<br />
+ sethoxydim), FirstRate (cloransulam), Pursuit<br />
(imazethapyr), Raptor (imazamox) and glyphosate<br />
in Roundup Ready soybean will control volunteer<br />
sunfl ower.<br />
Refer to the herbicide label or the most current<br />
edition of the “North Dakota Weed Control <strong>Guide</strong>,”<br />
NDSU Extension publication W-253, for rates,<br />
adjuvants and application guidelines.
Birds<br />
(George M. Linz and Jim Hanzel)<br />
Sunfl ower, due to the easy accessibility and high<br />
nutritional value of its seed, is particularly vulnerable<br />
to damage by birds (Figure 101). Seeds are exposed<br />
and the large head serves as a perch during feeding.<br />
Sunfl ower seed is a preferred bird food because the<br />
seed contains many proteins and fats essential to their<br />
growth, molt, fat storage and weight maintenance<br />
processes. Although many species of birds feed in maturing<br />
sunfl ower fi elds, the greatest losses are caused<br />
by migrating fl ocks of red-winged blackbirds, yellow-headed<br />
blackbirds and common grackles (Figure<br />
102). Signifi cant losses can occur in fi elds near cattail<br />
marshes.<br />
■ Figure 101. Sunfl ower may be depredated by birds.<br />
Birds perch on sunfl ower heads and pluck the seeds.<br />
(Reu V. Hanson)<br />
■ Migrating and Feeding<br />
Habits of Blackbirds<br />
The adult male blackbird is the fi rst of his species<br />
to arrive in the spring. He establishes a territory and<br />
awaits the arrival of the females. As females arrive,<br />
they disperse to the males’ territories and breeding<br />
takes place. Each female produces a clutch of three<br />
or four eggs. Nests are built in dense vegetation, most<br />
often in cattails, which have an abundant food supply.<br />
Their diet throughout the nesting season includes<br />
insects, weed seeds and waste grains.<br />
Following nesting in July, blackbirds form large fl ocks<br />
and begin feeding in grain fi elds. Blackbirds start<br />
feeding on sunfl ower seed soon after the petals begin<br />
to wilt and cause most of the damage during the following<br />
three weeks. Peak concentrations of blackbirds<br />
occur in mid-September in the northern growing area<br />
(Figure 103). This period coincides with the time that<br />
sunfl ower nears physiological maturity. Most often,<br />
the birds roost in the cattail marshes at night and move<br />
to the fi eld for feeding during the day.<br />
Blackbirds feed on insects and weed seeds in small<br />
grain, corn or sunfl ower fi elds before these crops are<br />
vulnerable to damage. They become used to feeding in<br />
a certain location and include sunfl ower seeds in their<br />
■ Figure 102. The red-winged blackbird is the most<br />
serious bird pest of sunfl ower in the northern Plains.<br />
(Reu V. Hanson)<br />
Birds<br />
85
86<br />
■ Figure 103. Blackbirds cause the most damage in<br />
early to mid-September. (Reu V. Hanson, George Linz)<br />
diets as the crop matures. Efforts made by the producer<br />
to move birds from a fi eld often are unsuccessful<br />
because the birds are in the habit of feeding there.<br />
Management<br />
Blackbirds are protected under the Migratory Bird<br />
Treaty Act. However, Section 21.43, Title 50 CFR,<br />
provides: “A federal permit shall not be required<br />
to control yellow-headed, red-winged, tri-colored<br />
red-winged, and Brewer’s blackbirds, cowbirds, all<br />
grackles, crows and magpies when found committing<br />
or about to commit depredations upon ornamental or<br />
shade trees, agricultural crops… .” Cultural practices<br />
in combination with mechanical and chemical harassment<br />
practices should be used to control blackbirds.<br />
Cultural Control<br />
A combination of cultural practices may be used to reduce<br />
the risk of bird damage to sunfl ower. If possible,<br />
sunfl ower should not be planted near cattail marshes<br />
or woodlots. Unplanted access trails allow easier access<br />
to fi elds while scaring blackbirds from the center<br />
of the fi eld. Planting should be done at the same time<br />
as neighbors because earlier and later ripening fi elds<br />
take more damage.<br />
Weed and insect control should begin early. Insects<br />
and weeds in the crop are often an attractive food<br />
source for blackbirds before the crop reaches a suscep-<br />
tible stage. Once blackbirds have developed patterns<br />
in insect-infested or weedy fi elds, they will begin to<br />
include the maturing cultivated crops in their diet.<br />
The plow-down of harvest stubble should be delayed<br />
until after sunfl ower harvest. Crop stubble serves as<br />
an alternate feeding area for harassed birds and other<br />
wildlife. Sunfl ower should be harvested as early as<br />
possible to avoid prolonged exposure to bird damage.<br />
Desiccation to advance harvest will reduce exposure<br />
to birds.<br />
Cattail Management<br />
Dense cattail marshes serving as roosting sites for<br />
blackbirds can be managed with a registered aquatic<br />
herbicide (e.g., glyphosate) to remove cattails used<br />
by these birds (Figure 104). Generally, cattails must<br />
be treated one year before sunfl ower is planted in the<br />
vicinity of the marsh to allow time for the cattails to<br />
decompose. However, herbicide applications made<br />
in mid-July might reduce blackbird use of the marsh<br />
in the year of application. The herbicide should be<br />
applied from mid-July to late August to at least 70<br />
percent of the marsh with an agriculture spray plane<br />
or helicopter (Figure 105). Use 2 quarts of herbicide<br />
per acre. Managing these marshes reduces blackbird<br />
use and improves the habitat for other more desirable<br />
wildlife, such as waterfowl. North Dakota/South<br />
Dakota Wildlife Services, telephone (701) 250-4405,<br />
is a unit within the U.S. Department of Agriculture’s<br />
Animal and Plant Health Inspection Service. It operates<br />
a cost-share cattail management program in North<br />
Dakota and South Dakota.<br />
Decoy Crops<br />
Blackbirds can be attracted readily to small plots of<br />
oilseed sunfl ower or other desirable crops planted near<br />
traditional wetland and tree roost sites. This strategy<br />
can be effective for the protection of high-valued<br />
confectionery and oilseed varieties. The plots must<br />
produce suffi cient seeds to feed the expected population<br />
of blackbirds. Each bird can eat about 1 pound<br />
of sunfl ower seeds. Thus, if a grower expects 30,000<br />
blackbirds, then a 20-acre plot must produce about<br />
1,500 lb/acre to feed the birds for a season. These<br />
plots also provide essential food and cover for other<br />
migrating and game birds. The North Dakota/South<br />
Dakota Wildlife Service’s National Wildlife Research<br />
Center, at telephone (701) 250-4469, is developing<br />
and refi ning the decoy crop concept. A cost-share<br />
program is available to sunfl ower growers.
Birds are kept out of sunfl ower fi elds most successfully<br />
by starting methods to frighten them as soon as the<br />
birds are seen in the vicinity, regardless of their diet.<br />
Various ways of moving birds mechanically are listed.<br />
Use of .22-caliber Rifl e<br />
This method should be used only where legal and safe.<br />
One rifl eman can protect 100 acres by fi ring from a<br />
high position into the midst of settling birds. Several<br />
more rounds fi red into the lifting fl ock often will send<br />
them on their way. Rifl emen must use extreme care<br />
with the use of rifl es since the bullet may carry a mile<br />
or more. Sometimes good results can be obtained with<br />
this method if used consistently.<br />
■ Figure 104. Cattails used by roosting blackbirds<br />
can be removed by aquatic herbicide (e.g.<br />
glyphosate). (George Linz)<br />
■ Figure 105. An aquatic herbicide such as Rodeo<br />
(glyphosate) should be applied by airplane or<br />
helicopter. (James Hanzel)<br />
Automatic Exploders (Figure 106)<br />
Automatic exploders or bird-scaring cannons automatically<br />
detonate a gas to produce an extremely loud<br />
explosion. These devices range from relatively simple<br />
mechanisms to deluxe models with photoelectric regulators<br />
and programmable fi ring sequences. The device<br />
should be operated before birds begin to arrive from<br />
their roosting area at sunrise and continued as long as<br />
birds are in the fi eld. It should be shut off at night. The<br />
exploder should be placed on a stand above the crop.<br />
It should be adjusted to fi re slowly, about every four to<br />
fi ve minutes. The exploder should be moved every two<br />
or three days, as birds will become accustomed to the<br />
noise if operated in the same location day after day.<br />
One exploder can protect 10 to 20 acres, especially if<br />
used with other mechanical devices and shooting.<br />
Electronic Frightening Devices<br />
Devices that broadcast distress calls of blackbirds are<br />
marginally effective and their application is somewhat<br />
limited because of their high cost and limited broadcast<br />
range. Furthermore, because they make extensive<br />
use of batteries, sophisticated electronic equipment<br />
and loud speakers, they are subject to vandalism and<br />
theft.<br />
Pyrotechnic Devices<br />
These include cracker-shells, fl ares, whistlers (fi red<br />
or pistol launched) and fi recrackers. Most of these<br />
products are effective in startling birds and are used<br />
commonly by many growers. These devices must be<br />
used with care, however, because of the potential for<br />
■ Figure 106. Gas exploder, when properly located<br />
and moved within the fi eld every 2 to 3 days can<br />
reduce bird damage. (Reu V. Hanson)<br />
Birds<br />
87
88<br />
mishaps. Safety glasses and hearing protectors are<br />
strongly recommended since these devices occasionally<br />
detonate prematurely. They also may be a fi re<br />
hazard during dry periods.<br />
Shotgun<br />
This tool is costly and ineffective as a direct control<br />
device. Killing a few birds has little if any direct effect<br />
on the rest of the fl ock. However, shotguns can be<br />
used to reinforce automatic exploders and pyrotechnic<br />
devices.<br />
Airplane Hazing<br />
Harassing feeding blackbirds with airplanes sometimes<br />
can be a marginally effective method of chasing<br />
fl ocks from sunfl ower fi elds. This technique is<br />
especially effective if combined with other mechanical<br />
methods, such as shotguns and pyrotechnic devices.<br />
Check with local authorities for permits needed to<br />
conduct low-level fl ying.<br />
Repellents<br />
Avitrol, a chemical frightening agent, and Bird Shield,<br />
a chemical repellent, are the only chemicals registered<br />
for management of blackbirds in sunfl ower. Avitrol<br />
is a cracked-corn bait in which one out of every 100<br />
particles is treated with the active ingredient 4-aminopyridine.<br />
The bait is applied by airplane or ground<br />
vehicle along access lanes placed in fi elds. When a<br />
blackbird eats one or more treated particles, it fl ies erratically<br />
and emits distress calls. This abnormal behavior<br />
sometimes causes the remaining birds in the fl ock<br />
to leave the fi eld. Bird Shield is a newly registered<br />
product that is formulated with the active ingredient<br />
methyl anthranilate. Research results to date indicate<br />
that the effi cacy of both Avitrol and Bird Shield are<br />
inconsistent.<br />
Best results are obtained by using an integrated pest<br />
management system that includes controlling insects<br />
and weeds that might attract blackbirds prior to sunfl<br />
ower ripening and by using a combination of harassment<br />
devices. Any device used must be operated when<br />
the birds are in the fi eld.<br />
Other Pests and Damage<br />
(Duane R. Berglund)<br />
Several sources of sunfl ower injury exist. Some of<br />
them are confused with damage from insects or diseases.<br />
Rabbits<br />
Rabbits will start foraging soon after seedling emergence,<br />
especially near the edges of fi elds. They will<br />
tend to concentrate on one row and apparently eat<br />
their fi ll, then leave until the next feeding period. Continued<br />
feeding by rabbits has been observed until the<br />
plants are 8 to 10 inches tall. Rabbit feeding on such<br />
large plants may be confused with deer. However, deer<br />
can be detected by their tracks.<br />
Deer<br />
Deer begin foraging on sunfl ower plants when the<br />
plants reach 8 to 10 inches and continue through<br />
harvest. They feed in areas near cover, such as wooded<br />
areas. All leaves of young plants will be consumed<br />
below the growing point. Heads will be foraged until<br />
near maturity and seeds until harvest. Often deer will<br />
knock down the stalk to facilitate foraging.<br />
Gophers and Mice<br />
Gopher and mouse damage usually is seen just after<br />
planting. It generally occurs next to overgrazed pastures,<br />
grassland recently converted to cropland and<br />
fi elds next to abandoned areas. The seed will be dug<br />
up, split open with the kernel consumed and the hull<br />
left on the soil. Several seeds in a row will be eaten.<br />
Seedlings are eaten occasionally when they are 2 to 3<br />
inches tall. If the growing point is consumed, the seedling<br />
gradually dies. Shooting or rodenticide-treated<br />
oats will control gophers and mice.
Lightning<br />
Lightning damage sometimes is mistaken for a<br />
disease. It is distinguished from disease damage by<br />
the sudden death of the plants in the affected area<br />
and the fact that both sunfl ower and weeds (not grass,<br />
however) are killed (Figure 107). Near the edge of the<br />
area, plants are wilted but not dead, and the stalks may<br />
have a brown to blackened pith. The area may be as<br />
large as 50 to 100 feet in diameter. The affected area<br />
usually is circular and does not increase in size after<br />
the fi rst two weeks. Flags may be placed at the edges<br />
of the affected area to observe if the damage gradually<br />
progresses beyond the fl ags. If damage does gradually<br />
extend beyond the fl ags, this could indicate damage<br />
from a source other than lightning.<br />
Flooding<br />
Soils should have good drainage for sunfl ower production,<br />
but the crop doesn’t differ greatly from most<br />
other crops. In fl ooded sunfl ower, research found that<br />
ethylene increased in the stems and roots below the<br />
water. Later, chlorophyll breakdown and leaf epinasty<br />
resulted. Sunfl ower plants fl ooded longer than three<br />
days may not recover. Cool, cloudy days during the<br />
fl ooding period reduce the damage, whereas hot and<br />
sunny days may hasten the death of plants.<br />
Heat Canker<br />
Warm temperatures and sunny days can result in heat<br />
canker injury to young sunfl ower seedlings growing<br />
■ Figure 107. Two spots in sunfl ower fi eld damaged<br />
by lightening. (Terry Gregoire)<br />
in black or dark, moist soils. Hot temperatures at the<br />
soil line cause cell death in the young stem and the<br />
plants will show bands of yellowing and constricting.<br />
In severe cases, the constricted area completely girdles<br />
the stem at the soil line and the plant topples over. The<br />
sunfl ower seedling will not recover since the growing<br />
point is above this site. Plant populations can be<br />
reduced signifi cantly in some cases.<br />
Frost Damage<br />
Sunfl ower seedlings in the cotyledonary stage (VE)<br />
can withstand temperatures down to 26 degrees Fahrenheit<br />
when just emerging from the soil. Sunfl ower<br />
in the V-1, V-2 and V-3 stages become less tolerant to<br />
frost as they grow and develop. The terminal bud can<br />
be frost damaged in seedlings with two, four and six<br />
true leaves. This early frost damage and killing of the<br />
terminal bud can result in excessive branching as the<br />
sunfl ower grows and develops.<br />
Sunfl ower is most susceptible at the bud (R-4) and<br />
pollination stages (R-5.0 to R-5.9) of development.<br />
Temperatures of 30 F or less can cause damage to the<br />
anthers and stigmas of the pollinating disk fl owers.<br />
(See Figure 108 for frost-damaged sunfl ower head).<br />
Sunfl ower has a composite type fl ower. Several rows<br />
of showy yellow ray fl owers encircle the head and<br />
commonly are called the “petals,” although each is an<br />
individual fl ower. The center portion of the head, and<br />
by far the greater part, is composed of inconspicuous<br />
individual fl owers, one for each seed that may develop.<br />
These disk fl owers mature in circles from the outside<br />
■ Figure 108. Frost damage in the center third of<br />
sunfl ower head. (Duane Berglund)<br />
Other Pests and Damage<br />
89
90<br />
of the fl ower head to the center, so that at various<br />
stages, the disk fl owers ready for pollination appear as<br />
a yellow circular band in the brownish or dark center<br />
of the head. These disk fl owers are sensitive to frost.<br />
The result of the frost damage in the fl owering period<br />
is circular bands of undeveloped seed that would vary<br />
with individual fl ower heads from a band around the<br />
outside edge to an area in the center. Unopened buds<br />
are less susceptible to frost than the opened fl ower<br />
heads. Growers can determine the extent of injury by<br />
cutting the surface of the fl ower head.<br />
Once pollination is completed and 10 to 14 days after<br />
petal drying occurs, the sunfl ower plants can withstand<br />
frost temperatures as low as 25 F and have only<br />
minor damage. Twenty-fi ve degrees Fahrenheit at the<br />
bud stage often will damage the stalk below the bud<br />
and seeds will not develop. If hard frosts do occur,<br />
many times only the seed in the center of the head (the<br />
last to pollinate) will be affected.<br />
When sunfl ower heads start to turn yellow on the<br />
backside and the bracts are drying and turning brown,<br />
most risk of frost damage is very minimal.<br />
In nonoilseed sunfl ower, frost damage can cause quality<br />
problems by causing a dark brown to blackened<br />
nutmeat to result during the roasting process. For<br />
the birdseed market, light-weight sunfl ower seed and<br />
brown seeds are the result of frost damage and will be<br />
discounted.<br />
For oilseed sunfl ower, reduced test weight per bushel<br />
and lower oil percent may result from a frosted immature<br />
sunfl ower crop.
Hail Injury<br />
(Duane R. Berglund)<br />
Hail storms can and will cause different types of<br />
sunfl ower plant injury. Plant death, damage to the<br />
terminal bud, physical injury to the stalk and head,<br />
and defoliation are all types of injury that can infl uence<br />
yield. Variables such as hailstone size and degree<br />
of hardness, speed and density, storm duration and<br />
plant environmental status, such as whether the leaves<br />
are fl accid or turgid, infl uence the type and degree of<br />
crop injury. The stage of plant development is also an<br />
important factor. Figures 109 and 110 illustrate two<br />
distinct types of hail damage. As shown in Figure 109,<br />
almost all heads have been destroyed, while plants<br />
shown in Figure 108 have a high level of defoliation<br />
with the heads still attached.<br />
One of the major factors causing differential growth<br />
and yield response is the stage at which the injury<br />
occurred. Data were obtained from a sunfl ower dateof-planting<br />
study at Carrington, N.D. Five sunfl ower<br />
hybrids sown at six planting dates between May 1<br />
and June 20 were damaged by a hail storm on Aug. 6.<br />
Stages of plant development at the time of the storm<br />
were from R-1 to R-7. Data were taken approximately<br />
■ Figure 109. Heads destroyed by hail stones.<br />
(A.A. Schneiter)<br />
one week after the storm. Average percent defoliation<br />
from all planting dates was similar at about 26.4 percent.<br />
An average of 4.7 stalk and head stone bruises<br />
occurred per nondestroyed plant. The percent of plants<br />
destroyed and the percent of the remaining plants with<br />
heads broken off or bent over but attached decreased<br />
with plant maturity (Table 13).<br />
Defoliation: Reduced yield as a result of defoliation<br />
depends on the amount of leaf loss and the stage at<br />
which it occurs. Stages R-1 through R-6 appear to<br />
be the most sensitive to defoliation since much of<br />
the photosynthate produced at this time is directed to<br />
head development. At early and late stages of plant<br />
development, high levels of defoliation may not have a<br />
major impact on seed yield. Approximate yield reductions<br />
due to varying degrees of random defoliation<br />
■ Figure 110. Sunfl owers defoliated by hail.<br />
(A.A. Schneiter)<br />
Hail Injury<br />
91
92<br />
Table 13. Effect of hail injury on sunfl ower at several<br />
stages of plant development.<br />
Injury on nondestroyed plants<br />
Approx. % Heads<br />
development % broken over %<br />
Date stage when Plants % Heads but still Attached<br />
planted injured destroyed broken off attached heads<br />
May 1 R-7 19.2 7.6 14.3 78.0<br />
May 9 R-6 24.1 6.3 17.1 76.6<br />
May 21 R-5 23.9 17.1 17.8 65.2<br />
May 30 R-3 29.6 16.1 17.0 66.9<br />
June 10 R-2 55.7 36.4 23.7 39.9<br />
June 20 R-1 60.7 39.9 22.6 37.6<br />
at several stages of growth are presented in Table 14.<br />
These values are based on investigations conducted at<br />
Fargo and Carrington and are the best estimates available<br />
on the effect of defoliation for average growing<br />
conditions.<br />
Stand Reduction: Plant death as a result of hail injury<br />
is a common occurrence, especially at early stages of<br />
development when plants are small. At early stages<br />
of plant development, before plants begin competing<br />
with each other, yield losses due to stand reduction<br />
caused by hail are not different than those that would<br />
occur due to reduced seeding rates. If the amount of<br />
stand reduction is signifi cant and/or occurs when the<br />
plant has begun to develop and compete with neighboring<br />
plants, the remaining uninjured plants cannot<br />
compensate enough and yields will be reduced. Losses<br />
due to stand reduction increase as the plant matures<br />
since it decreases the time for remaining plants to<br />
compensate.<br />
Approximate yield reductions from variable levels<br />
of random stand reduction at several stages of plant<br />
development are presented in Table 15. These values<br />
are based on studies conducted at Carrington and<br />
Fargo. These values represent direct stand reduction<br />
where the plants have been destroyed and no longer<br />
are competing with uninjured plants for light, water or<br />
nutrients.<br />
Injured Plants: In addition to stand reduction and<br />
defoliation, injuries such as terminal bud removal or<br />
injury and stem breakage or bruising may occur as<br />
a result of hail. An example of a living but severely<br />
injured plant is the gooseneck shown in Figure 111.<br />
Plants that are injured but living sometimes may reduce<br />
total crop yield more than if they had been completely<br />
destroyed since they continue to compete with<br />
uninjured plants for space, light and nutrients but do<br />
not produce an equal yield. Competition from injured<br />
plants may reduce the ability of noninjured plants to<br />
compensate for the hail-damaged plants.<br />
The response of plants to a hail injury, such as<br />
terminal bud removal, varies depending on the stage<br />
■ Figure 111. Goose-neck and stem bruising caused<br />
by hail injury. (A.A. Schneiter)
at which the injury occurs. When plants are injured<br />
in this manner at vegetative (V) stages, they usually<br />
develop branches that produce small seed-bearing<br />
heads. When injury to the terminal bud occurs during<br />
the early reproductive (R) stages, a greater percentage<br />
of the plants may die. When injury occurs near or after<br />
fl owering, the plants usually remain green and continue<br />
to live but do not produce seed. A similar type of<br />
response can be evident when plants have been injured<br />
by the head-clipping weevil; however, the injury from<br />
the head-clipping weevil is a straight cut across the<br />
stalk.<br />
The effect of bruising by hailstones is diffi cult to<br />
determine. If the amount of stalk bruising is such that<br />
the plant does not weaken or break during the remainder<br />
of its development prior to combine harvest,<br />
the effects on yield may be minimal. Stalk injury of<br />
such magnitude or at a specifi c location on the plant<br />
Table 14. Approximate percent yield reduction from the indicated percent total leaf area<br />
destroyed at several stages of sunfl ower plant development.<br />
Stage * 5<br />
Percent Leaf Area Destroyed<br />
10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100<br />
- - - - - - - - - - - - - - - - - - - - Approximate percent yield loss - - - - - - - - - - - - - - - - - - - -<br />
V-E to V-3<br />
(6-26 days)<br />
0 0 0 1 1 1 2 2 2 3 3 3 4 4 5 7 8 10 12 15<br />
V-4 to V-5<br />
(27-29 days)<br />
0 0 0 1 2 2 2 2 3 4 4 4 5 5 7 9 12 14 17 21<br />
V-6 to V-8<br />
(30-34 days)<br />
0 0 0 1 2 2 2 2 3 4 4 5 6 6 8 10 14 16 19 22<br />
V-9 to V-11<br />
(35-39 days)<br />
0 0 1 2 3 3 4 4 4 5 5 5 5 7 9 11 14 17 21 24<br />
V-12 to V-(N)<br />
(40-43 days)<br />
0 1 2 3 4 4 5 5 5 6 7 7 9 12 15 18 22 26 31 35<br />
R-1<br />
(44-51 days)<br />
0 2 3 4 5 6 6 6 7 7 8 9 13 16 20 24 29 34 40 47<br />
R-2<br />
(52-58 days)<br />
0 2 3 4 6 8 9 10 11 12 13 14 16 18 23 30 37 45 55 65<br />
R-3<br />
(59-67 days)<br />
0 2 5 8 10 15 17 19 21 24 28 32 38 44 51 59 68 78 88 99<br />
R-4<br />
(68-75 days)<br />
0 2 4 5 7 10 12 12 15 18 22 27 34 39 45 53 61 72 85 99<br />
R-5<br />
(76-84 days)<br />
0 1 2 3 5 7 8 10 13 16 20 25 32 37 43 49 55 67 78 90<br />
R-6<br />
(85-92 days)<br />
0 0 1 1 3 3 4 8 11 15 19 24 29 35 41 46 53 63 72 80<br />
R-7<br />
(93-102 days)<br />
0 0 1 1 1 3 5 7 8 10 11 13 14 16 17 18 19 20 21 22<br />
R-8<br />
(103-110 days)<br />
0 0 1 1 1 2 2 3 4 5 6 7 7 8 9 9 10 10 10 11<br />
R-9<br />
(111-maturity)<br />
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0<br />
*Number of days after planting for development to a specifi c stage will vary signifi cantly depending on environmental conditions<br />
and the hybrid. Interpolating percent of loss between stages may be necessary.<br />
Hail Injury<br />
93
94<br />
resulting in nonharvestable heads certainly would have<br />
an effect on yield. Physical injury by hailstones on<br />
the back of a sunfl ower head at or near anthesis can<br />
result in Rhizopus head rot, especially if wet or humid<br />
conditions are present. Physical injury can occur as a<br />
result of bird, insect or hailstone damage. Increased<br />
dead plant tissue resulting from a hail storm, especially<br />
on the back of a head, may increase the chance<br />
of white mold infection.<br />
Table 15. Approximate percent yield reduction from the Indicated percent stand reduction at<br />
several stages of sunfl ower plant development.<br />
Stage * 5<br />
Percent Stand Reduction<br />
10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100<br />
- - - - - - - - - - - - - - - - - - - - Approximate percent yield loss - - - - - - - - - - - - - - - - - - - -<br />
V-E to V-3<br />
(6-26 days)<br />
0 1 2 3 4 8 10 11 12 12 13 14 16 18 24 32 43 58 77 100<br />
V-4 to V-5<br />
(27-29 days)<br />
0 1 2 3 4 8 10 11 12 12 13 14 16 18 24 32 43 58 77 100<br />
V-6 to V-8<br />
(30-34 days)<br />
0 1 2 3 4 8 10 11 12 12 13 14 16 18 24 33 43 58 77 100<br />
V-9 to V-11<br />
(35-39 days)<br />
0 1 2 3 4 8 10 11 12 12 13 14 16 19 25 33 44 59 77 100<br />
V-12 to V-(N)<br />
(40-43 days)<br />
0 1 2 3 4 8 10 12 12 13 14 15 17 21 27 35 46 60 78 100<br />
R-1<br />
(44-51 days)<br />
1 2 5 9 12 14 15 16 17 18 19 21 25 29 35 43 53 66 81 100<br />
R-2<br />
(52-58 days)<br />
2 3 7 9 13 17 19 21 23 24 26 28 31 35 40 47 57 68 83 100<br />
R-3<br />
(59-67 days)<br />
4 7 11 13 15 17 21 24 27 29 31 34 37 41 46 53 61 72 84 100<br />
R-4<br />
(68-75 days)<br />
5 10 14 18 20 22 25 27 29 32 35 38 42 47 53 60 68 77 88 100<br />
R-5<br />
(76-84 days)<br />
5 10 14 19 20 24 28 31 35 39 42 45 49 54 60 66 73 81 90 100<br />
R-6<br />
(85-92 days)<br />
5 10 15 19 22 26 31 35 39 44 48 52 56 62 68 73 79 85 93 100<br />
*Number of days after planting for development to a specifi c stage will vary signifi cantly depending on environmental conditions<br />
and hybrid. It may be necessary to interpolate percent of loss between stages.
Herbicide Drift and<br />
Chemical Residue<br />
Herbicide Drift<br />
Herbicide drift is the movement of herbicide from<br />
target areas to areas where herbicide application<br />
was not intended. Herbicide drift generally is caused<br />
by movement of spray droplets or by movement of<br />
herbicide vapors. Herbicide granules or dried particles<br />
of herbicide may move short distances in high winds<br />
but are not considered important sources of herbicide<br />
drift.<br />
Sunfl ower is susceptible to many of the postemergence<br />
herbicides commonly used on crops grown in proximity<br />
to sunfl ower. Herbicides that may damage sunfl<br />
ower include most all ALS (acetolactate synthase)<br />
herbicides (Accent, Ally, Amber, Beacon, Express,<br />
Affi nity, Pursuit and Raptor), atrazine, dicamba, bentazon,<br />
Bronate, Buctril, Curtail, glyphosate, MCPA,<br />
paraquat, Stinger, 2,4-D and Tordon.<br />
Sunfl ower yield may be severely reduced by 2,4-D or<br />
dicamba (Figure 112). The amount of loss varied from<br />
25 percent to 82 percent, depending on the sunfl ower<br />
growth stage when the herbicide was applied in tests.<br />
Sunfl ower yield loss averaged from three rates each of<br />
2,4-D and dicamba to simulate spray drift was greatest<br />
when the herbicides were applied in the bud stage<br />
and least when applied during bloom. Sunfl ower in<br />
the V-2 to V-4 leaf stage was affected less than larger<br />
prebloom sunfl ower.<br />
Only a small portion of an applied herbicide drifts<br />
from the target area. However, some nontarget areas<br />
can receive rather high doses of herbicide since herbicide<br />
drift can accumulate in the nontarget areas. Herbicide<br />
accumulated in downwind areas occasionally<br />
may exceed the rate applied to the target fi eld. A small<br />
portion of the herbicide applied with each sprayer pass<br />
may accumulate in an adjoining fi eld. Also, a taller<br />
crop, such as sunfl ower, may intercept more spray<br />
drift than a shorter crop, such as wheat or barley. The<br />
amount of herbicide that contacts the sunfl ower and<br />
the environment during and following application<br />
infl uences the yield loss caused by herbicide drift. For<br />
example, experiments at North Dakota State University<br />
showed that 2,4-D at 0.5 ounce per acre applied<br />
to 12- to 14-leaf sunfl ower caused a 5 percent loss<br />
in 1973 and 93 percent loss in 1978. Low but equal<br />
levels of drift may cause very different effects on<br />
sunfl ower yield, depending on environment. Sunfl ower<br />
injury from herbicide drift will be greatest with warm<br />
temperatures and good soil moisture.<br />
Sunfl ower may exhibit herbicide injury symptoms<br />
without a yield loss. Experiments at North Dakota<br />
State University indicated that sunfl ower height<br />
reduction, as compared with undamaged sunfl ower,<br />
caused by 2,4-D, MCPA or dicamba was signifi cantly<br />
correlated with sunfl ower yield loss. Drift of 2,4-D,<br />
■ Figure 112. Sunfl ower yield loss from herbicides<br />
applied at various growth stages averaged over 2,4-D<br />
rates of 0.5, 1 and 2 oz./A and dicamba at 0.1, 0.5<br />
and 1.0 oz/A. (A. Dexter)<br />
Herbicide Drift and Chemical Residue<br />
95
96<br />
MCPA or dicamba, which causes a sunfl ower height<br />
reduction, also would be expected to reduce yield.<br />
However, typical injury symptoms can occur without<br />
height reduction and this would not be expected to<br />
reduce yield.<br />
MCPA, 2,4-D, dicamba and Tordon are growth-regulator<br />
herbicides and all produce similar symptoms<br />
on sunfl ower. Sunfl ower exhibits epinasty, which is<br />
an abnormal bending of stems and/or leaf petioles,<br />
shortly after contact with a growth-regulator herbicide.<br />
Figure 113 shows the bending of sunfl ower only<br />
24 hours after 2,4-D application while Figure 114<br />
shows the abnormal twisting of leaf petioles several<br />
days after 2,4-D application. Sunfl ower growth often<br />
is slowed or stopped by growth-regulator herbicides.<br />
■ Figure 113. Bending of sunfl ower only 24 hours<br />
after 2,4-D application. (A. Dexter)<br />
■ Figure 114. Abnormal twisting of leaf petioles<br />
several days after 2,4-D application. (A. Dexter)<br />
Leaves that develop after contact with a growthregulator<br />
herbicide often are malformed. Leaves may<br />
have more parallel vein patterns and abnormal leaf<br />
shapes (Figure 115). A higher degree of injury from<br />
a growth-regulator herbicide may stop plant growth<br />
totally. Some plants die without further growth, some<br />
will remain green and not grow the rest of the season,<br />
and some plants will begin growing later after the<br />
herbicide is partially metabolized. Sunfl ower in Figure<br />
116 did not grow for several days and then produced a<br />
stalk and fl ower.<br />
Affected plants also may branch and produce multiple<br />
heads when growth resumes (Figure 117). Root<br />
■ Figure 115. Growth regular herbicides may cause<br />
leaves of sunfl ower to have more parallel vein<br />
patterns and abnormal leaf shapes. (A. Dexter)<br />
■ Figure 116. Interrupted growth of sunfl ower due to<br />
exposure to growth regulator herbicide. (A. Dexter)
growth may be retarded by growth-regulator herbicides<br />
and abnormal lumps or knots may develop on<br />
sunfl ower roots (Figure 118). Sunfl ower contacted by<br />
growth-regulator herbicides during the bud or fl owering<br />
stage may develop malformed heads or heads with<br />
sterility (Figure 119).<br />
■ Figure 117. Multiple branching and heading of<br />
sunfl ower due to exposure to growth regulator<br />
herbicides. (A. Dexter)<br />
■ Figure 118. Abnormal lumps and knots on<br />
sunfl ower roots developed after exposure to growth<br />
regulator herbicides. (A. Dexter)<br />
Sunfl ower plants contacted with a growth-regulator<br />
herbicide will not develop all of the symptoms shown<br />
in Figures 112 through 117. Considerable variation<br />
in symptomology can occur, depending on herbicide<br />
rate, sunfl ower stage and environment.<br />
Symptoms similar to those shown in Figures 114 to<br />
119 also may be produced by soil residues of herbicide.<br />
MCPA and 2,4-D have very short soil residual,<br />
but damage to sunfl ower could occur if they were<br />
applied just before planting or emergence. Tordon<br />
has a long soil residual and can carry over from the<br />
previous year and cause sunfl ower injury. Dicamba at<br />
rates used in small grains would not be expected to<br />
carry over to the next year. Dicamba used in the fall at<br />
rates necessary to control certain perennial weeds may<br />
persist in the soil and damage sunfl ower the next year.<br />
Accent, Ally, Amber, atrazine, Beacon and Pursuit are<br />
long residual herbicides that may persist for more than<br />
one year at levels that can injure sunfl ower. The length<br />
of persistence can be affected by pH (high pH causes<br />
longer residue of some herbicides), application rate<br />
and soil moisture.<br />
The symptoms from these herbicides are not similar to<br />
symptoms from growth-regulator herbicides. Sunfl ower<br />
affected by Accent, Ally, Amber, Beacon or Pursuit<br />
emerge and become well-established. Chlorosis starts<br />
on the young leaves often with a distinct yellowing.<br />
Top growth and roots may be severely stunted. Plants<br />
may remain small for several weeks or they may die.<br />
■ Figure 119. Sunfl owers contacted by growth<br />
regulator herbicides during bud or fl owering stage<br />
developed malformed heads or heads with sterility.<br />
(A. Dexter)<br />
Herbicide Drift<br />
97
98<br />
Low doses may cause temporary stunting and plants<br />
later may begin to grow normally. Sunfl ower affected<br />
with atrazine emerge and look normal for a short time.<br />
Leaf burn starts on the outer edges of the oldest leaves<br />
and progresses toward the middle of the leaf. Veinal<br />
areas are the last to turn brown in an affected leaf.<br />
The risk of sunfl ower injury from herbicide drift is<br />
infl uenced by several factors.<br />
Spray particle size: Spray drift can be reduced by<br />
increasing droplet size since larger droplets move<br />
laterally less than small droplets. Droplet size can be<br />
increased by reduced spray pressure; increased nozzle<br />
orifi ce size; use of special nozzles, such as “Raindrop,”<br />
“LFR,” “XR” or “LP;” use of additives that<br />
increase spray viscosity; rearward nozzle orientation;<br />
and increased nozzle pressure on aircraft. Research<br />
has shown that increasing nozzle pressure in a rearward-oriented<br />
nozzle in a high-speed air stream will<br />
produce larger droplets and less fi nes. This is due to<br />
less secondary wind shear, and does not happen with<br />
ground sprayers.<br />
Spray pressure with standard fl at-fan nozzles should<br />
not be less than 25 pounds per square inch (psi)<br />
because the spray pattern from the nozzles will not<br />
be uniform at lower pressures. The “XR” and “LP”<br />
nozzles are designed to give a good spray pattern at 15<br />
to 50 psi. Operating at a low spray pressure results in<br />
larger spray droplets. Some postemergence herbicides,<br />
such as bentazon, require small droplets for optimum<br />
performance, so techniques that increase droplet size<br />
may reduce weed control with certain herbicides. Herbicides<br />
that readily translocate, such as 2,4-D, MCPA,<br />
dicamba and Tordon, are affected little by droplet size<br />
within a normal droplet size range, so drift control<br />
techniques would not be expected to reduce weed control<br />
with these herbicides. Glyphosate is translocated<br />
readily, so droplet size has minimum effect on weed<br />
control. However, glyphosate is inactivated partially<br />
by increased water volume and spray volume recommendations<br />
on the label should be followed.<br />
Herbicide volatility: All herbicides can drift in spray<br />
droplets, but some herbicides are suffi ciently volatile<br />
to cause plant injury from drift of vapor or fumes. The<br />
ester formulations of 2,4-D and MCPA may produce<br />
damaging vapors, while the amine formulations are<br />
essentially nonvolatile. Dicamba is a volatile herbicide<br />
and can drift in droplets or vapor.<br />
Herbicide vapors may cause crop injury over greater<br />
distances than spray droplets. However, spray droplets<br />
can move long distances under certain environmental<br />
conditions, so crop injury for a long distance does not<br />
necessarily result from vapor drift. A wind blowing<br />
away from a susceptible crop during herbicide application<br />
will prevent damage from droplet drift, but a later<br />
wind shift toward the susceptible plants could move<br />
damaging vapors into the susceptible crop.<br />
Herbicide volatility increases with increasing temperature.<br />
The so-called high-volatile esters of 2,4-D or<br />
MCPA may produce damaging vapors at temperatures<br />
as low as 40 degrees Fahrenheit, while low-volatile<br />
esters may produce damaging vapors between 70 and<br />
90 F. The soil surface temperature often is several<br />
degrees warmer than air temperature, so a low-volatile<br />
ester could be exposed to temperature high enough to<br />
cause damaging vapors, even when the air temperature<br />
was less than 70 F.<br />
Wind velocity and air stability: Wind, or the<br />
horizontal movement of air, is widely recognized as<br />
an important factor affecting spray drift. However,<br />
vertical movement of air also has a large infl uence on<br />
damage to nontarget plants from spray drift. An air<br />
mass with warmer air next to the ground and decreasing<br />
temperature with increasing elevation will be unstable.<br />
That is, the warm air will rise and the cool air<br />
will sink, providing vertical mixing of air. Stable air,<br />
often called an inversion, occurs when air temperature<br />
increases or changes little as elevation increases. With<br />
these temperature relationships, very little vertical<br />
movement of air occurs since cool air will not rise into<br />
warmer air above. Spray droplets or vapor are carried<br />
aloft and dispersed away from susceptible plants with<br />
unstable air conditions. With stable air (inversion),<br />
small spray droplets may be suspended just above the<br />
ground in the air mass, move long distances laterally<br />
and be deposited on susceptible plants.
Low wind velocity in combination with unstable air<br />
generally will result in very little damaging spray drift.<br />
However, low wind velocity with stable air (inversion)<br />
can result in severe damage over a long distance. Crop<br />
injury has been observed two miles or more from<br />
the site of application with 10 mph or slower winds,<br />
small spay droplets, stable air, highly susceptible crops<br />
and nonvolatile but highly active herbicides. Longdistance<br />
drift can occur with particle drift as well as<br />
vapor drift.<br />
Stable air usually can be identifi ed by observing dust<br />
off a gravel road or smoke from a fi re or smoke bomb.<br />
Smoke or dust moving horizontally and staying close<br />
to the ground would indicate stable air. Fog also would<br />
indicate stable air. Herbicide application should be<br />
avoided during stable air conditions unless spray drift<br />
is not a concern.<br />
Distance between nozzle and target (boom<br />
height): Less distance between the droplet release<br />
point and the target will reduce spray drift. Less<br />
distance means less time to travel from nozzle to<br />
target, so less drift occurs. Small spray droplets have<br />
little inertial energy, so a short distance from nozzle to<br />
target increases the chance that the small droplets can<br />
reach the target. Also, wind velocity often is greater as<br />
height above the ground increases, so reduced nozzle<br />
height will reduce the wind velocity affecting the<br />
spray droplets.<br />
Shielded sprayers: Shielded sprayers utilize some<br />
type of shielding to protect spray droplets from wind.<br />
The effectiveness of the shields varies, depending<br />
on the design of the shield, wind velocity and wind<br />
direction relative to the sprayer. Drift from shielded<br />
sprayers has varied from about 50 percent to more<br />
than 95 percent less than from similar nonshielded<br />
sprayers in experiments with various shield designs<br />
and conditions.<br />
Herbicide drift can reduce sunfl ower yield severely.<br />
The risk of herbicide drift can be reduced greatly<br />
by increasing droplet size, reducing nozzle height,<br />
using nonvolatile herbicides, avoiding spraying during<br />
temperature inversions, using shielded sprayers<br />
and spraying when the wind is blowing away from a<br />
susceptible crop.<br />
Chemical Residue in the<br />
Tank and Sprayer Cleanout<br />
Crop injury from a contaminated sprayer may occur<br />
when a herbicide not registered on sunfl ower was used<br />
previously in the sprayer. The risk of damage is greatest<br />
when the previous herbicide is highly phytotoxic to<br />
sunfl ower in small amounts. Rinsing with water is not<br />
adequate to remove all herbicides. Some herbicides<br />
have remained tightly adsorbed in sprayers through<br />
water rinsing and even through several tank loads of<br />
other herbicides. Then, when a tank load of solution<br />
including an oil adjuvant or nitrogen solution was put<br />
in the sprayer, the herbicide was desorbed, moved into<br />
the spray solution and damaged susceptible crops.<br />
Highly active herbicides that have been diffi cult to<br />
wash from sprayers and have caused crop injury include<br />
ALS herbicides (Accent, Ally, Beacon, Express,<br />
Pursuit and Raptor), and growth-regulator herbicides<br />
(2,4-D and dicamba).<br />
Herbicides that are diffi cult to remove from sprayers<br />
are thought to be attaching to residues remaining<br />
from spray solutions that deposit in a sprayer. The<br />
herbicide must be desorbed from the residue or the<br />
residue removed in a cleaning process so the herbicide<br />
can be removed from the sprayer. Sprayer cleanout<br />
procedures are given on many herbicide labels and the<br />
procedure on the label should be followed for specifi c<br />
herbicides. The following procedure is given as an<br />
illustration of a thorough sprayer cleanup procedure<br />
that would be effective for most herbicides.<br />
Step 1. Drain tank and thoroughly rinse interior<br />
surfaces of tank with clean water. Spray<br />
rinse water through the spray boom. Suffi<br />
cient rinse water should be used for fi ve<br />
minutes or more of spraying through the<br />
boom.<br />
Step 2. Fill the sprayer tank with clean water and<br />
add a cleaning solution (many herbicide<br />
labels provide recommended cleaning solutions).<br />
Fill the boom, hoses and nozzles<br />
and allow the agitator to operate for 15<br />
minutes.<br />
Step 3. Allow the sprayer to sit for eight hours<br />
while full of cleaning solution. The cleaning<br />
solution should stay in the sprayer for<br />
eight hours so that the herbicide can be<br />
fully desorbed from the residues inside the<br />
sprayer.<br />
Chemical Residue<br />
99
100<br />
Step 4. Spray the cleaning solution out through<br />
the booms.<br />
Step 5. Remove nozzles, screens and fi lters and<br />
clean thoroughly. Rinse the sprayer to<br />
remove cleaning solution and spray rinsate<br />
through the booms.<br />
Common types of cleaning solutions are chlorine<br />
bleach, ammonia and commercially formulated tank<br />
cleaners. Chlorine lowers the pH of the solution,<br />
which speeds the degradation of some herbicides.<br />
Ammonia increases the pH of the solution, which<br />
increases the solubility of some herbicides. Commercially<br />
formulated tank cleaners generally raise pH and<br />
act as detergents to assist in removal of herbicides.<br />
Read the herbicide label for recommended tank cleaning<br />
solutions and procedures. WARNING: Never mix<br />
chlorine bleach and ammonia, as a dangerous and<br />
irritating gas will be released.<br />
Sprayers should be cleaned as soon as possible after<br />
use to prevent the deposit of dried spray residues. If a<br />
sprayer will remain empty overnight without cleaning,<br />
fi ll the tank with water to prevent dried spray deposits<br />
from forming. A sprayer kept clean is essential to<br />
prevent damage from herbicide contamination.
Maturity<br />
Harvesting<br />
(Vern Hofman)<br />
Sunfl ower in the northern Great Plains production area<br />
usually is ready for harvest in late September or October,<br />
with a growing season of approximately 120 days.<br />
The growing season may vary in length, depending on<br />
summer temperatures, relative moisture distribution<br />
and fertility levels. The sunfl ower plant is physiologically<br />
mature when the back of the head has turned<br />
from green to yellow and the bracts are turning brown<br />
(Stage R-9), about 30 to 45 days after bloom, and seed<br />
moisture is about 35 percent.<br />
Desiccants can be applied to the crop after physiological<br />
maturity to speed the dry-down process. The<br />
chemical compounds act much like a frost to kill the<br />
green tissue on the plant and accelerate its drying.<br />
After applications of a desiccant, dry down of the seed<br />
is not as rapid as the dry down of the plant. Growers<br />
often are tempted to apply desiccants too early when<br />
potential loss factors are present. Application of a desiccant<br />
before the plant reaches physiological maturity<br />
will reduce yield and lower oil percentage. Drying is<br />
facilitated in most years by a killing frost, but if frost<br />
occurs too early, yield and oil percentages are reduced.<br />
Seed shattering loss during harvest and loss from birds<br />
may be reduced by harvesting sunfl ower at moisture<br />
contents as high as 25 percent. Sunfl ower seed from<br />
the combine then is dried in a grain dryer to 9.5 percent,<br />
which is considered a safe storage level.<br />
Harvesting Attachments<br />
Combines suitable for threshing small grains can<br />
be adapted to harvest sunfl ower. A variety of header<br />
attachments are available, with many operating on a<br />
head stripper principle.<br />
The attachments are designed to gather only the sunfl<br />
ower heads and eliminate as much stalk as possible.<br />
Major components of this attachment are catch pans,<br />
a defl ector and a small reel. Long catch pans extend<br />
ahead of the cutter bar to catch the seed as it shatters.<br />
The defl ector mounted above the catch pans pushes<br />
the stalk forward until only the heads remain above<br />
the cutter bar. As the heads move below the defl ector,<br />
the stems contact the cutter bar and are cut just below<br />
the head. A small reel, mounted directly behind the<br />
defl ector, pushes the heads into the combine feeder.<br />
Catch pans are available in various widths. These<br />
range from narrow 9-inch pans spaced on 12-inch<br />
centers (Figure 120) to 37-inch pans spaced on 40inch<br />
centers (Figure 121). The narrow 9-inch pans can<br />
operate on any row spacing, while the wider and more<br />
effi cient 30- to 40-inch spaced pans are limited to a<br />
fi xed-row spacing.<br />
The defl ector consists of a curved piece of sheet metal<br />
the full width of the combine head. It is attached to the<br />
reel support arms above the catch pans. The reel for<br />
the unit is mounted directly behind the defl ector and<br />
usually consists of three or four arms. The reel is usually<br />
16 to 20 inches in diameter and mounted 4 to 5<br />
inches above the catch pans, so when the heads come<br />
in contact with the reel, they are pushed back into the<br />
feeder. The shield and reel can handle tall plants while<br />
taking only a minimum length of stalk with the head,<br />
allowing harvest when the seed is dry but stalk moisture<br />
may remain above 50 percent. Cleaner threshing<br />
also is accomplished when only the head enters the<br />
machine.<br />
Harvesting<br />
101
102<br />
Optional forward rotating stalk-walker shafts, introduced<br />
from Argentina and used mainly in the southern<br />
Plains, can be mounted under the cutter bar to reduce<br />
plugging of stalk slots between pans. The stalk-walker<br />
pulls sunfl ower stalks and weeds down so that only the<br />
sunfl ower head is fed into the combine. Stalk-walkers<br />
are reported to be especially useful in fi elds with tall<br />
weeds.<br />
A rotating drum with metal projections that replaces<br />
the defl ector bar and reel often is used (Figure 122).<br />
The projections are triangular-shaped pieces of strap<br />
iron welded to its surface. As the drum rotates, the<br />
projections pass through the slots between the catch<br />
pans to remove any stalks that may cause clogging.<br />
The smooth drum acts as a defl ector bar to strip stalks<br />
■ Figure 120. Catch pans in narrow 9 inch pans<br />
spaced on 12 inch centers. (V. Hofman)<br />
■ Figure 121. Catch pans in wide 37 inch pans<br />
spaced on 36 inch centers. (V. Hofman)<br />
until one of the projections catches a head and pushes<br />
it into the cutter bar and into the combine header.<br />
Row-crop units mounted on combine headers have<br />
been used successfully to harvest sunfl ower seed<br />
(Figure 123). One unit uses gathering belts, one on<br />
each side of the row, to draw the stalk into the cutting<br />
unit and the header. A large quantity of stalk passes<br />
through the machine with this unit and may increase<br />
the foreign matter in the seed, but this unit works well<br />
picking up lodged sunfl ower and getting the heads into<br />
the machine.<br />
Another type of header uses a short section of screw<br />
conveyor to pull the stalks into the cutter bar and the<br />
combine header. This unit also works well for picking<br />
up lodged sunfl ower.<br />
■ Figure 122. Rotating drum with metal projections.<br />
(V. Hofman)<br />
■ Figure 123. All crop header used to harvest<br />
sunfl ower. (V. Hofman)
Combine Adjustments<br />
Forward Speed: A combine’s forward speed usually<br />
should average between 3 and 5 miles per hour.<br />
Optimum forward speed usually will vary depending<br />
upon moisture content of the sunfl ower seed and<br />
yield of the crop. Forward speed should be decreased<br />
as moisture content of the seed decreases to reduce<br />
the shatter loss as the heads feed into the combine.<br />
Faster forward speeds are possible if the moisture of<br />
the seed is between 12 percent and 15 percent. The<br />
higher speeds should not overload the cylinder and the<br />
separating area of the combine except in an extremely<br />
heavy crop. Seed having 12 percent to 15 percent<br />
moisture will thresh from the head very easily as it<br />
passes through the cylinder.<br />
Cylinder Speed: After the sunfl ower heads are<br />
separated from the plant, they should be threshed at<br />
a cylinder speed operating as slow as possible. The<br />
normal cylinder speed should be about 300 revolutions<br />
per minute (rpm), depending upon the condition<br />
of the crop and the combine being used. This cylinder<br />
speed is for a combine with a 22-inch-diameter<br />
cylinder to give a cylinder bar travel speed of 1,725<br />
feet per minute. Combines with smaller cylinders<br />
will require a faster speed and combines with a larger<br />
cylinder diameter will require a slower speed. Rotary<br />
combines, as well as conventional machines, should<br />
have similar cylinder travel speeds. A rotary combine<br />
with a 30-inch cylinder will need to be operated at<br />
220 rpm to have a cylinder bar speed of 1,725 feet per<br />
minute. A combine with a 17-inch cylinder will need<br />
to operate at 390 rpm to have a cylinder bar speed of<br />
1,725 feet per minute.<br />
If a combine cylinder operates at speeds of 400 to 500<br />
rpm, giving a cylinder bar speed of more than 2,500<br />
feet per minute, very little seed should be cracked or<br />
broken if the moisture content of the seed is above 11<br />
percent. Cylinder bar speeds of more than 3,000 feet<br />
per minute should not be used because they will cause<br />
excessive broken seed and increased dockage. Excess<br />
dockage and broken seed may overload the sieves and<br />
the return elevator.<br />
Concave Adjustment: Sunfl ower threshes relatively<br />
easily. When crop moisture is at 10 percent or less,<br />
conventional machines should be set wide open to<br />
give a cylinder-to-concave spacing of about 1 inch<br />
at the front of the cylinder and about 0.75 inch at<br />
the rear. A smaller concave clearance should be used<br />
only if some seed is left in the heads. If the moisture<br />
percentage of the crop is between 10 percent and 12<br />
percent, rather than increase the cylinder speed, the<br />
cylinder-to-concave clearance should be decreased<br />
to improve threshing. If seed moisture exceeds 15<br />
percent to 20 percent, a higher cylinder speed and a<br />
closer concave setting may be necessary, even though<br />
foreign material in the seed increases. Seed breakage<br />
and dehulling may be a problem with close concave<br />
settings. Make initial adjustments as recommended<br />
in the operator’s manual. Final adjustments should be<br />
made based on crop conditions.<br />
Rotary combines should be set to have a rotor-to-concave<br />
spacing of about 0.75 to 1 inch. Making initial<br />
settings as recommended in the operator’s manual usually<br />
is best. Final adjustments should be made based<br />
on crop conditions.<br />
Fan Adjustment: Oilseed and nonoilseed sunfl ower<br />
weigh about 28 to 32 pounds per bushel and 22 to 26<br />
pounds per bushel, respectively. The seed is relatively<br />
light compared with other crops, so excessive<br />
wind may blow seed over the chaffer and sieve. Seed<br />
forced over the sieve and into the tailings auger will<br />
be returned to the cylinder and may be dehulled. Only<br />
enough wind to keep the trash fl oating across the sieve<br />
should be used. The chaffer and sieve should be adjusted<br />
to minimize the amount of material that passes<br />
through the tailings elevator.<br />
When the combine is adjusted correctly to thresh<br />
sunfl ower seed, the threshed heads will come through<br />
only slightly broken and with only unfi lled seed<br />
remaining in the head. Cylinder concaves and cleaning<br />
sieves usually can be set to obtain less than 5 percent<br />
dockage. Improper settings will crush the seed but<br />
leave the hull intact. Proper setting is critical, especially<br />
for nonoilseed sunfl ower that is used for the human<br />
food market. The upper sieve should be open enough<br />
to allow an average seed to pass through on end, or<br />
be set at a ½- to 5/8- inch opening. The lower sieve<br />
should be adjusted to provide a slightly smaller opening,<br />
or about 3/8 inch wide. The fi nal adjustments will<br />
depend on the amount of material returning through<br />
the tailings elevator and an estimation of the amount<br />
of dockage in the grain tank. Some operators are able<br />
to adjust and operate their machine to allow only 2<br />
percent to 3 percent dockage in the seed.<br />
Harvesting<br />
103
104<br />
<strong>Field</strong> Loss<br />
The harvested yield of sunfl ower can be increased by<br />
making necessary adjustments following a determination<br />
of fi eld loss. Three main sources of loss are: (a)<br />
loss in the standing crop ahead of the combine, (b)<br />
header loss as the crop enters the machine and (c)<br />
threshing and separating loss. The loss found in any<br />
of these three areas will give the combine operator a<br />
good estimate of sources of seed loss and the adjustments<br />
necessary to minimize seed loss.<br />
Loss occurring in any of these areas may be estimated<br />
by counting the seed on the soil surface in a squarefoot<br />
area. Ten seeds per square foot equal approximately<br />
1 hundredweight (cwt) per acre loss if seed<br />
loss is uniform throughout the entire fi eld.<br />
The loss in the standing crop is estimated by counting<br />
the seed in a 1-square-foot area ahead of the machine<br />
at several different places in the fi eld. Header loss can<br />
be calculated by counting seed in a 1-square-foot area<br />
behind the head under the combine and subtracting the<br />
standing crop loss. The loss in combine separation can<br />
be found by counting the seed in a 1-square-foot area<br />
directly behind the rear of the combine and subtracting<br />
the shatter loss and the header loss found under the<br />
machine. The count made directly behind the combine<br />
will be concentrated, so an adjustment must be made<br />
to equalize the loss over the entire width of cut. The<br />
result should be divided by the ratio:<br />
Width of Header Cut (feet)<br />
Width of Rear of Combine (feet)<br />
The answer is the adjusted separator loss for the width<br />
of cut. This result must be divided by 10 to obtain the<br />
combine separator loss in cwt per acre. The total loss<br />
in cwt per acre is determined by adding the seed loss<br />
in the standing crop, header loss and separator loss<br />
and dividing this answer by 10. The percentage loss<br />
can be found by dividing the total cwt per acre by the<br />
yield in cwt per acre.<br />
Harvest without some seed loss is almost impossible.<br />
Usually a permissible loss is about 3 percent. Loss as<br />
high as 15 percent to 20 percent has occurred with a<br />
well-adjusted combine if the ground speed is too fast,<br />
resulting in machine overload.
Drying and Storage<br />
(Kenneth Hellevang)<br />
Harvesting sunfl ower at higher moisture contents<br />
normally results in higher yields and less fi eld loss.<br />
Early harvest also reduces exposure to late-season wet<br />
and cold weather. Frequently, mechanical drying is<br />
required so harvesting can be completed.<br />
Natural-air, low-temperature and high-temperature<br />
bin, batch (Figure 124) and continuous-fl ow dryers<br />
can be used to dry sunfl ower.<br />
Natural-air and low-temperature bin drying is energy<br />
effi cient if designed properly and permits rapid harvest<br />
since bins can be fi lled at the harvest rate. Drying<br />
will take three to six weeks, depending on the initial<br />
moisture content, airfl ow rate and outdoor temperature.<br />
Required airfl ow rates and drying time for drying<br />
oil sunfl ower at various moisture contents using air at<br />
47 degrees Fahrenheit and 65 percent relative humid-<br />
■ Figure 124. A high temperature column dryer used<br />
for drying sunfl ower. (K. Hellevang)<br />
ity (average North Dakota conditions for October) are<br />
shown in Table 16. Drying times will be twice as long<br />
at 27 degrees due to the reduced moisture-holding<br />
capacity at colder temperatures. Heating the air more<br />
than about 5 degrees normally causes overdrying.<br />
Table 16. Recommended airfl ow rates and drying<br />
times for natural-air drying oilseed sunfl ower in<br />
October. (47 F and 65 percent relative humidity).<br />
Fan Time<br />
Moisture Airfl ow<br />
Content (cfm/bu) hours days<br />
17% 1.00 648 27<br />
15% 1.00 480 20<br />
0.75 720 30<br />
0.50 960 40<br />
13% 1.00 336 14<br />
0.75 504 21<br />
0.50 672 28<br />
Add enough heat when needed to dry the sunfl ower to<br />
the safe storage moisture content. Generally, enough<br />
heat to warm the air about 5 degrees is the maximum<br />
amount required. As a rule of thumb, about 2 kilowatts<br />
(kW) of heater will be required per fan motor<br />
horsepower. The equation for calculating the heat<br />
requirement in Btu is: Btu/hr = cfm x 1.1 x temperature<br />
increase. Convert Btu to kW by dividing by 3,413<br />
Btu/kW. Refer to NDSU Extension Service publication<br />
EB-35, “Natural Air and Low Temperature Crop<br />
Drying,” and publication AE-701, “Grain Drying,” for<br />
more information on drying sunfl ower.<br />
A perforated fl oor is recommended. Since air does the<br />
drying, making sure air reaches all the sunfl ower is<br />
imperative. The uniform airfl ow distribution required<br />
for drying is more diffi cult to achieve with ducts than<br />
Drying and Storage<br />
105
106<br />
with perforated fl oors. However, drying can be done<br />
successfully if ducts are spaced no more than onehalf<br />
the grain depth apart and the distance from the<br />
duct to bin wall does not exceed one-fourth the grain<br />
depth. Provide 1 square foot of duct or fl oor perforated<br />
surface area for each 25 cubic feet per minute (cfm) of<br />
airfl ow. One square foot of bin exhaust opening should<br />
be provided for each 1,000 cfm of airfl ow.<br />
Drying temperatures up to 220 F do not appear to<br />
have an adverse effect on oil percentage or fatty<br />
acid composition. High drying temperatures for the<br />
nonoil varieties may cause the kernels to be steamed,<br />
wrinkled or even scorched.<br />
Column batch and bin batch dryers should be operated<br />
at 180 and 120 F, respectively. Continuous-fl ow and<br />
recirculating batch dryers may be operated at temperatures<br />
up to about 200 F. Temperatures in excess of 110<br />
F should not be used to dry sunfl ower seed for seeding<br />
purposes.<br />
Fire hazards exist in dryers used for sunfl ower. Very<br />
fi ne hairs or fi bers from the seed are rubbed loose<br />
during handling and commonly are found fl oating in<br />
the air around the dryer. These hairs or fi bers or other<br />
plant materials may be ignited when drawn through<br />
the drying fan and open burner. A fi re hazard is present<br />
unless these ignited particles burn themselves out<br />
before contacting the sunfl ower seed.<br />
The fi re hazard is decreased if the fans of a portable<br />
dryer are turned into the wind to draw clean air that<br />
does not contain fi ne hair or fi bers and by pointing<br />
stationary dryers into the prevailing wind. A moveable<br />
air intake duct may be placed on the burner intake<br />
to draw clean air away from the dryer. However, the<br />
duct must be large enough to not restrict the airfl ow<br />
because drying speed will be reduced if the airfl ow is<br />
reduced.<br />
Clean the dryer, air ducts and area around the dryer<br />
at least daily. Frequently remove the collection of<br />
sunfl ower lint on the dryer column and in the plenum<br />
chamber, as this material becomes extremely dry and<br />
can be ignited during dryer operation. A major concern<br />
is that some sunfl ower seeds will hang up in the<br />
dryer or be stopped by an accumulation of fi nes and<br />
become overdried. Make sure the dryer is completely<br />
cleaned out after each batch, and check a continuousfl<br />
ow dryer regularly (at least hourly) to see that the<br />
sunfl ower seed is moving.<br />
High-speed dryers are like a forge when a fi re gets<br />
going. However, fi res can be controlled if they are<br />
noticed immediately, which makes constant monitoring<br />
necessary. Many fi res can be extinguished by just<br />
shutting off the fan to cut off the oxygen. A little water<br />
applied directly to the fi re at the early stages may<br />
extinguish it if shutting off the fan fails to do so. A fi re<br />
extinguisher for oil-type fi res should be used for oil<br />
sunfl ower fi res. Many dryers are designed so that sunfl<br />
ower can be unloaded rapidly in case of a fi re, before<br />
the dryer is damaged. In some dryers, just the part of<br />
the dryer affected by the fi re needs to be unloaded.<br />
Measuring Moisture Content<br />
Measuring the moisture content of sunfl ower immediately<br />
after removal from the dryer results in only an<br />
estimation. As moisture is removed from the sunfl ower<br />
seed, the hull dries fi rst and the kernels dry last. Moisture<br />
testers used by local grain elevators and farm<br />
operators generally result in a reading that is lower<br />
than the actual moisture percentage when moisture is<br />
measured while the moisture variation exists. The initial<br />
moisture content of the sunfl ower and the temperature<br />
of the drying air infl uence the amount of error.<br />
A number of operators have reported that sunfl ower<br />
removed from the dryer at 9 percent to 10 percent<br />
moisture (according to the moisture tester) would be<br />
up to 12 percent moisture later. The moisture rebound<br />
can be estimated by placing a sample from the dryer<br />
in a covered jar and then rechecking the moisture after<br />
12 hours.<br />
<strong>Guide</strong>lines for drying sunfl ower are:<br />
• The area around the dryer and the plenum chamber<br />
should be cleaned thoroughly.<br />
• The fan must be fed clean air without seed hairs.<br />
• A continuous fl ow in all sections of recirculating<br />
batch and continuous-fl ow dryers should be maintained.<br />
Uneven fl ow will cause overdried spots<br />
and increase fi re hazard.<br />
• Drying equipment must not be left unattended<br />
day or night.<br />
• The dried sunfl ower should be cooled to air temperature<br />
before storing.
Storage<br />
Farm structures that are structurally adequate to store<br />
other grains are adequate for storing sunfl ower due to<br />
sunfl ower’s light test weight, Figure 125.<br />
Seed should be cleaned for storage. Fines tend to concentrate<br />
in the center of the bin if a distributor is not<br />
used. Since this material tends to be wetter, this area<br />
is more prone to storage problems. Also, airfl ow will<br />
be restricted by the fi nes, limiting cooling by aeration<br />
in the center of the bin. Large pieces of head, stalk<br />
and corolla tubes, which frequently adhere to the seed,<br />
should be removed because they are higher in moisture<br />
than the seed.<br />
Oil sunfl ower should not be stored above 10 percent<br />
moisture during the winter and 8 percent during the<br />
summer. Nonoilseed sunfl ower should not be stored<br />
■ Figure 125. Structures adequate to store other<br />
grains are adequate for sunfl ower. (K. Hellevang)<br />
■ Figure 126. Aeration is critical for proper storage. (K. Hellevang)<br />
above 11 percent moisture during the winter and 10<br />
percent during the summer. Sunfl ower can be stored<br />
for short periods in the fall at 12 percent with adequate<br />
airfl ow to keep the seeds cool. Resistance of<br />
oilseed sunfl ower to fungal infection during storage at<br />
10 percent moisture is equal to wheat resistance at 15<br />
percent stored moisture.<br />
Aeration to control seed temperature is essential.<br />
Aeration fans normally are sized to provide 0.05 to 0.2<br />
cfm/bu. (0.15 to 0.6 cfm per cwt) of sunfl ower (Figure<br />
126). Sunfl ower should be rotated between bins during<br />
the storage period when aeration is not available.<br />
Cooling sunfl ower reduces the potential for sunfl ower<br />
deterioration from insects and mold. Sunfl ower should<br />
be cooled to 40 degrees or below before or soon after<br />
it is put in the bin and to about 25 degrees for winter<br />
storage. Insects become dormant and will not cause<br />
damage or multiply if seed temperature is below about<br />
40 F.<br />
Moisture and heat accumulate in the peak due to<br />
moisture migration, which results in crusting, spoilage<br />
and increased possibility of insect infestations (Figure<br />
127). This can be prevented by cooling the sunfl ower<br />
using aeration.<br />
Bins should be checked initially every two weeks<br />
for moisture condensation on the roof, crusting and<br />
changes in temperatures within the pile. Any of these<br />
conditions could indicate the presence of mold or insects.<br />
If the sunfl ower has started to heat, it should be<br />
cooled immediately. The sunfl ower should be checked<br />
at least monthly after the seeds have been cooled to<br />
about 25 F for winter storage and a history of temperature<br />
and moisture content has been developed.<br />
Drying and Storage<br />
107
108<br />
■ Figure 127. Moisture migration leads to increased<br />
moisture in top center of stored sunfl ower.<br />
(K. Hellevang)<br />
Seed lots containing a high percentage of hulled seed<br />
or immature seed, such as seed resulting from an<br />
early frost, tend to deteriorate in storage, affecting oil<br />
quality.<br />
Refer to NDSU Extension Service publication<br />
AE-791, “Crop Storage Management,” for more<br />
information on aeration and storage management.
Feeding Value of Sunfl ower<br />
Products in Beef Cattle Diets<br />
(Greg Lardy)<br />
Sunfl ower Meal<br />
Nutrients in sunfl ower meal can vary depending on<br />
several factors. The amount and composition of meal<br />
is affected by oil content of the seed, extent of hull<br />
removal and effi ciency of oil extraction. The proportion<br />
of hull removed before processing differs among<br />
crushing plants. In some cases, a portion of the hulls<br />
may be added back to the meal after crushing. The<br />
amount of hull or fi ber in the meal is the major source<br />
of variation in nutrients (Table 17).<br />
Pre-press solvent extraction of whole seeds with no<br />
dehulling produces meal with a crude protein content<br />
of 25 percent to 28 percent, partial dehulling yields<br />
34 percent to 38 percent crude protein content and<br />
completely dehulled sunfl ower meal commonly yielding<br />
40-plus percent crude protein. Sunfl ower meal<br />
is marketed and shipped as meal or pellets. Protein<br />
required by rumen microbes can be provided in the<br />
form of rumen-degradable protein from sunfl ower<br />
meal. Heat treatment or toasting of meal from the<br />
solvent extraction process may increase the propor-<br />
Table 17. Nutrient content of solvent-extracted<br />
sunfl ower meal based on amount of hulls<br />
retained.<br />
No Hulls Partially<br />
Removed Dehulled Dehulled<br />
Dry Matter, % 90.0 90.0 90.0<br />
Percent, Dry Matter Basis<br />
Crude Protein 28.0 34.0 41.0<br />
Fat 1.5 0.8 0.5<br />
Crude Fiber 24.0 21.0 14.0<br />
Ash 6.2 5.9 5.9<br />
Calcium 0.36 0.35 0.34<br />
Phosphorus 0.97 0.95 1.30<br />
Potassium 1.07 1.07 1.07<br />
Magnesium 0.80 0.79 0.79<br />
Hesley (ed), National Sunfl ower Association, 1994.<br />
tion of undegradable protein. Sunfl ower meal is more<br />
ruminally degradable (74 percent of crude protein)<br />
than either soybean meal (66 percent) or canola meal<br />
(68 percent; Table 18).<br />
Sunfl ower meal has a lower energy value than either<br />
canola or soybean meal (Table 18). Energy varies<br />
substantially with fi ber level and residual oil content.<br />
Higher levels of hulls included in the fi nal meal product<br />
lower the energy content and reduce bulk density.<br />
The mechanical process of oil extraction leaves more<br />
residual oil in the meal, often 5 percent to 6 percent<br />
or more, depending on the effi ciency of the extraction<br />
process. Elevated oil content in mechanically extracted<br />
meals provides greater energy density. Pre-press<br />
solvent extraction reduces residual oil to 1.5 percent<br />
or less.<br />
Sunfl ower Meal in Beef Cattle Diets<br />
Sunfl ower meal can be used as the sole source of<br />
supplemental protein in beef rations. In trials comparing<br />
sunfl ower meal with other protein sources, equal<br />
animal performance commonly is observed based on<br />
isonitrogenous diets from different sources.<br />
Cows consuming low-quality forages, such as winter<br />
range, crop aftermath or other low-quality forages, can<br />
utilize supplemental degradable protein to increase<br />
total intake, forage digestibility and performance.<br />
Protein can be supplemented with a number of feeds,<br />
coproducts or oilseed meals. Least costly sources are<br />
critical to profi tability, and sunfl ower meal often is<br />
very competitively priced per unit protein. Sunfl ower<br />
meal has been used widely in beef cow supplementation<br />
programs but few research trials document<br />
comparative animal performance.<br />
Feeding Value of Sunfl ower Products in Beef Cattle Diets<br />
109
110<br />
Sunfl ower Silage<br />
Table 18. Protein and energy fractions for sunfl ower meal, soybean meal<br />
and canola meal.<br />
Sunfl ower Soybean Canola<br />
Item Meal Meal<br />
Dry Matter Basis, %<br />
Meal<br />
Crude Protein 26.0 49.9<br />
Crude Protein, %<br />
40.9<br />
Rumen Degradable 74.0 66.0 68.0<br />
Rumen Undegradable 26.0 34.0 22.0<br />
Dry Matter Basis, %<br />
Crude fi ber 12.7 7.0 13.3<br />
Neutral Detergent Fiber 40.0 14.9 27.2<br />
Acid Detergent Fiber 30.0 10.0 17.0<br />
Net Energy, Maintenance, Mcal/lb 0.67 0.93 0.73<br />
Net Energy, Gain, Mcal/lb 0.40 0.64 0.45<br />
Total Digestible Nutrients 65 84 69<br />
Adapted from NRC, 1996.<br />
Sunfl ower silage can make a suitable feed for beef<br />
cows; however, high moisture levels can be a challenge<br />
since sunfl owers typically don’t dry down well.<br />
Consequently, dry feed must be added to the silage<br />
pile to reduce the moisture level to a point where seepage<br />
is not a major problem.<br />
Table 19 gives the estimated nutrient content of sunfl<br />
ower silage produced from either low-oil or high-oil<br />
varieties of sunfl ower. Depending on what other feeds<br />
are mixed in the silage pile, nutrient contents may<br />
change.<br />
Blending corn and sunfl ower silages together can help<br />
alleviate the moisture problem. Producers also may<br />
consider waiting seven to 10 days following a killing<br />
frost to facilitate dry down. Blending dry forage into<br />
the silage pile also can reduce moisture content. To<br />
minimize seepage problems, the moisture level should<br />
be 65 percent or less.<br />
Whole Sunfl ower Seeds<br />
When economical, whole sunfl ower seeds can be used<br />
as a source of energy and protein in beef cattle diets<br />
(Table 19). Fat levels can be quite high in whole seeds;<br />
consequently, amounts fed should be restricted based<br />
on fat content of the seed. Typically, no more than 4<br />
percent supplemental fat should be added to cow diets<br />
to reduce the potential for any detrimental effects on<br />
fi ber digestion. This will result in inclusion levels of<br />
approximately 10 percent of the diet.<br />
Sunfl ower Residue<br />
Sunfl ower residue is useful for aftermath grazing by<br />
beef cows. Nutritional value of the head is greater<br />
than the stalk. Supplementation may be required if<br />
the volume of residue is limited and nutrient quality<br />
decreases rapidly after head material is consumed.<br />
Sunfl ower Screenings<br />
Sunfl ower screenings from both confection and oil<br />
seed plants are often available at competitive prices.<br />
Nutrient content varies widely with the amount of<br />
meats, which are high in fat and protein, and hull,<br />
which is low in nutrient content and digestibility.<br />
Screenings are best used in modest growing or maintenance<br />
diets when animal performance is not critical.<br />
The presence of sclerotia bodies does not appear to be<br />
a problem for palatability, nutrient content or animal<br />
performance.<br />
Sunfl ower Hulls<br />
Sunfl ower hulls are low in protein and energy and<br />
should be used only as a bedding source.
Summary<br />
That sunfl ower meal is a useful protein source for<br />
growing and fi nishing cattle is apparent from the<br />
limited research. Similarly, beef cows can be provided<br />
supplemental protein effectively with sunfl ower meal.<br />
Sunfl ower meal may be especially useful in diets<br />
where degradable protein is required, such as lower-<br />
Table 19. Nutrient content of sunfl ower products.<br />
quality forage or high corn fi nishing rations. The increased<br />
bulk of this relatively high-fi ber meal may affect<br />
logistics, but ruminants are positioned to be more<br />
tolerant of high fi ber levels than other species. Other<br />
sunfl ower products can be used effectively in ruminant<br />
diets, given appropriate performance expectations.<br />
NE m , NE g ,<br />
DM, % TDN, % Mcal/lb Mcal/lb CP, % ADF, % Ca, % P, %<br />
Sunfl ower Hulls* 90.0 40.0 0.41 0.00 5.0 63.0 0.00 0.114<br />
Sunfl ower Screenings* 87.0 64.0 0.66 0.39 11.1 29.0 0.72 0.42<br />
Sunfl ower Seed, Confectionary* 94.9 83.0 0.93 0.63 17.9 39.0 0.18 0.56<br />
Sunfl ower Seeds, Oil Type* 94.9 121.0 1.42 1.03 17.9 39.0 0.18 0.56<br />
Sunfl ower Silage, Low-oil Variety** 30.0 61.0 0.61 0.69 11.1 42.0 0.8 0.3<br />
Sunfl ower Silage, High-oil Variety** 30.0 66.0 0.35 0.42 12.5 39.0 1.50 0.3<br />
*Adapted from Lardy and Anderson, 2003.<br />
**Adapted from Park et al., 1997.<br />
Feeding Value of Sunfl ower Products in Beef Cattle Diets<br />
111
112<br />
U.S. Grades and Standards<br />
for Sunfl ower<br />
(Duane R. Berglund)<br />
Defi nition of Sunfl ower Seed<br />
Grain that, before the removal of foreign material,<br />
consists of 50 percent or more of cultivated sunfl ower<br />
seed (Helianthus annuus L.) and not more than 10<br />
percent of other grains for which standards have been<br />
established under the U.S. Grain Standards Act.<br />
Defi nition of Other Terms<br />
Cultivated sunfl ower seed - Sunfl ower seed grown<br />
for oil content. The term seed in this and other<br />
defi nitions related to sunfl ower seed refers to both the<br />
kernel and hull, which is a fruit or achene.<br />
Damaged sunfl ower seed - Seed and pieces of<br />
sunfl ower seed that are badly ground-damaged,<br />
badly weather-damaged, diseased, frost-damaged,<br />
heat-damaged, mold-damaged, sprout-damaged or<br />
otherwise materially damaged.<br />
Dehulled seed - Sunfl ower seed that has the hull<br />
completely removed from the sunfl ower kernel.<br />
Foreign material - All matter other than whole<br />
sunfl ower seeds containing kernels that can be<br />
removed from the original sample by use of an<br />
approved device and by handpicking a portion of the<br />
sample according to procedures prescribed in the<br />
USDA’s Federal Grain Inspection Service instructions.<br />
Heat-damaged sunfl ower seed - Seed and pieces<br />
of sunfl ower seed that are materially discolored and<br />
damaged by heat.<br />
Hull (husk) - The ovary wall of the sunfl ower seeds.<br />
Kernel - The interior contents of the sunfl ower seed<br />
that are surrounded by the hull.<br />
Basis of Determination<br />
Each determination of heat-damaged kernels, damaged<br />
kernels, test weight per bushel and dehulled<br />
seed is made on the basis of the grain when free from<br />
foreign material. Other determinations not specifi cally<br />
provided for in the general provisions are made on the<br />
basis of the grain as a whole, except the determination<br />
of odor is made on either the basis of the grain as a<br />
whole or the grain when free from foreign material.<br />
Table 20 lists the U.S. grade requirements for sunfl<br />
ower according to the Federal Grain and Inspection<br />
Service. These requirements became effective Sept. 1,<br />
1984. The table lists the minimum limit for test weight<br />
and the maximum for damaged and dehulled seed.<br />
U.S. grades for both oilseed and nonoilseed classes of<br />
sunfl ower are determined with the requirements listed<br />
below.<br />
Table 20. Grade and grade requirements<br />
for sunfl ower.<br />
Minimum<br />
Maximum limits of<br />
damaged sunfl ower seed<br />
test weight Heat Dehulled<br />
Grade per bushel damage Total seed<br />
(pounds) - - - - - (percent) - - - - -<br />
U.S. No. 1 25.0 0.5 5.0 5.0<br />
U.S. No. 2 25.0 1.0 10.0 5.0<br />
U.S. Sample grade – U.S. sample grade shall be sunfl ower<br />
seed that:<br />
(1) Does not meet the requirements for the grades U.S.<br />
Nos. 1 or 2; or<br />
(2) In a 600-gram sample, contains eight or more stones<br />
that have aggregate weight in excess of 0.20 percent of<br />
the sample weight, two or more pieces of glass, three<br />
or more crotalaria seeds (Crotalaria spp.), two or more<br />
castor beans (Ricinus Communis), four or more particles<br />
of an unknown substance(s), or 10 or more rodent<br />
pellets, bird droppings or an equivalent quantity of<br />
other animal fi lth; or<br />
(3) Has a musty, sour or commercially objectionable<br />
foreign odor; or<br />
(4) Is heating or otherwise of distinctly low quality.<br />
Source: Federal Grain and Inspection Service-USDA
Other Information Sources<br />
Major Organizations and<br />
Information Sources<br />
■ Public Research and<br />
Nonprofi t Associations<br />
Supporting the efforts of private companies in the<br />
development of the sunfl ower industry are public-supported<br />
research institutions and nonprofi t associations.<br />
The following is a list and brief description of the<br />
functions and purposes of such groups:<br />
State Agricultural Experiment Stations<br />
and Cooperative Extension Service<br />
The Agricultural Experiment Stations, which conduct<br />
research, and Cooperative Extension Services, which<br />
deliver adult education programs in the major sunfl<br />
ower production areas, are at:<br />
Colorado State University, Fort Collins, CO 80523<br />
Kansas State University, Manhattan, KS 66506<br />
North Dakota State University, Fargo, ND 58105<br />
South Dakota State University, Brookings, SD<br />
57006<br />
Texas A & M University, College Station, TX 77843<br />
(branch stations at Lubbock and Bushland)<br />
University of California, Davis, CA 95616<br />
University of Minnesota, St. Paul, MN 55108, and<br />
Crookston, MN 56716<br />
Extension Publications<br />
Many Extension publications giving specifi c information<br />
on sunfl ower production, insect pests, diseases,<br />
herbicide use, marketing and food use are available.<br />
These are readily available through your county or<br />
state Extension offi ces.<br />
Federal Research<br />
The Agricultural Research Service (ARS) of the<br />
USDA, working with state agricultural experiment stations<br />
and cooperative Extension personnel in the major<br />
sunfl ower-producing areas, have developed inbred<br />
lines for hybrids, conducted research and provided<br />
information on production, utilization and marketing<br />
aspects. The main USDA-ARS sunfl ower unit is at the<br />
Northern Crops Science Laboratory on the campus<br />
of North Dakota State University, Fargo, ND 58105,<br />
www.fargo.ars.usda.gov.<br />
Agriculture and Agri-Food Canada<br />
Unit 100-101 Route 100<br />
Morden, Manitoba, R6M 1Y5 CANADA<br />
www.agr.gc.ca/<br />
The National Sunfl ower Association<br />
4023 State St. N.<br />
Bismarck, ND 58502-0690<br />
Tel: (888) 718-7033 or (701) 328-5100<br />
http://sunfl owernsa.com/<br />
The National Sunfl ower Association was organized in<br />
1975 to promote the sunfl ower industry and to solve<br />
common problems. This association is intended to<br />
represent the interest of growers, processors and seed<br />
companies, including country elevators, exporters<br />
and other merchandisers. It publishes The Sunfl ower<br />
magazine and sponsors The Sunfl ower Research Forum,<br />
a meeting devoted to scientifi c reports and topics<br />
of general interest.<br />
National Sunfl ower Association of Canada<br />
Box 1269<br />
Carman, MB R0G 0J0<br />
Phone: (204) 745-6776<br />
info@canadasunfl ower.com<br />
www.canadasunfl ower.com/<br />
U.S. Grades and Standards / Other Information Sources<br />
113
114<br />
The International Sunfl ower Association<br />
12 Avenue George V<br />
75008 Paris, France<br />
The objective of the International Sunfl ower Association<br />
is to improve international cooperation and to<br />
exchange information in the promotion of research<br />
of agronomics, processing techniques and nutrition<br />
associated with the production, marketing, processing<br />
and use of sunfl ower. This objective is accomplished<br />
by sponsoring and publishing the proceedings of international<br />
conferences and by publishing the Sunfl ower<br />
Newsletter, a quarterly periodical.<br />
■ Sources of Information<br />
of General Interest<br />
Governmental Statistical Reporting Services<br />
Sunfl ower production and farm price statistics are<br />
released by the following state and federal offi ces<br />
of Economics, Statistics and Cooperative Extension<br />
Service, USDA:<br />
North Dakota Agricultural Statistics Service<br />
P.O. Box 3166<br />
Fargo, ND 58108-3166<br />
South Dakota Agricultural Statistics Service<br />
Box 31 Drawer V<br />
Sioux Falls, SD 57101<br />
Minnesota Agricultural Statistics Service<br />
Seventh and Roberts Street<br />
Metro Square, Suite 270<br />
St. Paul, MN 55101<br />
Texas Agricultural Statistics Service<br />
Box 70<br />
Austin, TX 78767<br />
National Agricultural Statistics Service<br />
U.S. Department of Agriculture<br />
Washington, D.C. 20250<br />
Sunfl ower Technology and <strong>Production</strong><br />
ASA Monograph 35. 1997, 834 pages. Albert A.<br />
Schneiter, editor. This monograph text is the extensive<br />
revision of the original Agronomy Monograph 19<br />
Sunfl ower Science and Technology. The monograph<br />
has many contributing authors addressing and reporting<br />
all the scientifi c work on sunfl ower that is in the<br />
literature. It has become the “bible of knowledge and<br />
information” for all who wish to read and study about<br />
the sunfl ower plant, its origin, development, production<br />
and utilization.<br />
The Sunfl ower<br />
(by C.B. Heiser Jr. from Univ. of Oklahoma Press,<br />
Norman, OK 73069, for $10.95)<br />
This 200-page book, authored by a botanist and written<br />
in layman’s language, covers historical development,<br />
the distribution and interrelationships of<br />
sunfl ower species. It describes sunfl ower as an international<br />
commercial crop, as ornamentals and as weeds.<br />
The Sunfl ower<br />
(4023 State St. N., Bismarck, ND 58501-0690)<br />
The Sunfl ower magazine is published nine times<br />
a year by the National Sunfl ower Association of<br />
America. It is intended for growers, merchandisers,<br />
processors, hybrid seed companies, researchers and<br />
other legitimate interests in the sunfl ower industry.<br />
Sunfl ower Directory<br />
(4023 State St. N., Bismarck, ND 58501-0690, $10)<br />
The directory includes more than 150 companies, in<br />
addition to several trade organizations, government<br />
agencies and universities, which have a unique function<br />
in the sunfl ower industry. More than 400 individuals<br />
having expertise in sunfl ower also are listed.<br />
High Plains Sunfl ower <strong>Production</strong> Handbook<br />
2005, 44 pages. (Kansas State University- Agric. Experiment<br />
Station and Cooperative Extension Service).<br />
Joint publication of Kansas State University, Colorado<br />
State University, University of Nebraska, University<br />
of Wyoming and USDA-ARS Central Plains Research<br />
Center, Akron, Colo. Also supported by: Kansas<br />
Sunfl ower Commission, National Sunfl ower Association<br />
– High Plains Committee and Colorado Sunfl ower<br />
Administrative Committee.
Glossary<br />
Achene — The sunfl ower fruit consisting of hull<br />
and “seed;” a small, dry, one-seeded fruit that<br />
does not open at maturity.<br />
Apothecium (pl. apothecia) — Cup- or saucershaped<br />
fruiting structure of some fungi.<br />
Ascospore — Fungal spore borne in a structure<br />
within the apothecium.<br />
Annuala — Plant in which the entire life cycle is<br />
completed in a single growing season.<br />
Bract — Modifi ed, reduced leaf structure beneath<br />
ray fl owers on sunfl ower head.<br />
Canker — Sharply defi ned dead area of tissue on<br />
stem.<br />
Corolla — Collective term for petals of the<br />
sunfl ower.<br />
Cytoplasmic Male Sterility — Male<br />
sterility inherited through hereditary units in<br />
the cytoplasm, rather than through nuclear<br />
inheritance.<br />
Defoliate — To remove leaves of a plant.<br />
Dehull — Removal of outer seed coat (hull) from<br />
the “seed.”<br />
Depredation — A plundering or despoiling;<br />
robbery.<br />
Desiccant — A dry-down or defoliating chemical.<br />
Disk Flower — Tubular fl owers that compose the<br />
central part of the sunfl ower head; produce the<br />
seeds.<br />
Fungicide — A chemical or physical agent that<br />
kills fungi.<br />
Fungus (pl. fungi) — A group of organisms that<br />
lack chlorophyll and that obtain food through<br />
absorption, frequently from plants.<br />
Herbicide — A chemical or physical agent that<br />
kills plants.<br />
High Oleic — Oilseed sunfl ower that contains a<br />
trait for high oleic fatty acid content in its oil.<br />
A premium oil used in the snack food industry.<br />
Host — The organism affected by a parasite or<br />
disease.<br />
Hybrid — The offspring of two unlike parents.<br />
Insecticide — A chemical or physical agent that<br />
kills insects.<br />
Instar — Any stage of insect development; larval<br />
growth stage.<br />
Involucral Bract — An individual bract within a<br />
distinct whorl of bracts that subtend the fl owering<br />
part of a plant.<br />
Kernel — Term used for true seed in processing,<br />
preferred to “nutmeat.” The sunfl ower seed is<br />
neither “nut” nor “meat.”<br />
Larva (pl. larvae) — The preadult form of an insect.<br />
Nonoilseed — Preferred term, equivalent to nonoil<br />
sunfl ower or confectionery sunfl ower.<br />
NuSun — Term that describes the new mid-oleic<br />
sunfl ower oil. It is lower in saturated fat (less<br />
that 10 percent) than linoleic sunfl ower oil and<br />
has higher oleic levels (55 percent to 75 percent)<br />
with the remainder being linoleic (15 percent to<br />
35 percent)<br />
Oilseed — Preferred term, equivalent to oil<br />
sunfl ower.<br />
Open Pollinated — Naturally pollinated by selfi ng<br />
or crossing between two related strains.<br />
Perennial — A plant that continues its growth from<br />
year to year, not dying after once fl owering.<br />
Glossary<br />
115
116<br />
Petiole — The stalk of the leaf.<br />
pH — Expression of acidity or alkalinity of soil or<br />
water.<br />
Physiological Maturity — Stage at which a seed<br />
has reached its maximum dry weight.<br />
Pollinator — Insect that carries pollen from plant<br />
to plant.<br />
Pupa (pl. pupae) — The stage between larva and<br />
adult in some insects.<br />
Ray Flower — Flattened, ray shaped fl owers on<br />
margins of sunfl ower head. Commonly referred to<br />
as the petals. These are sterile and do not produce<br />
achenes.<br />
Receptacle — Fleshy, thickened part of sunfl ower<br />
head just above the stem that bears the fl ower<br />
parts.<br />
Sclerotium (pl. sclerotia) — The hard, resting<br />
bodies of certain fungi.<br />
“Seed” — True seed in sunfl ower is the kernel;<br />
however, “seed” commonly is used to describe<br />
the kernel plus hull, which is equivalent to the<br />
achene.<br />
Self Compatability — <strong>Production</strong> of fruits and<br />
normal seeds following self pollination.<br />
Sporea — Reproductive structure of fungi.<br />
Sunfl ower — The preferred term, equivalent to<br />
sunfl owers.<br />
Sun Oil — The preferred term, equivalent to<br />
sunfl ower oil, sunfl ower seed oil.<br />
Variety — A subdivision of a species; a distinct<br />
group of organisms.<br />
Volunteer Plant — Plant arising from seed<br />
dispersed from a previous crop.
Appendix 1<br />
Diseases of Sunfl ower<br />
(Oilseed, Confection and Ornamental)<br />
(Helianthus annuus L.)<br />
■ BACTERIAL DISEASES<br />
Apical chlorosis Pseudomonas syringae pv. tagetis (Hellmers) Young et al.<br />
Bacterial leaf spots Pseudomonas syringae pv. aptata (Brown and Jamieson)Young et al.<br />
P. cichorii (Swingle) Stapp<br />
P. syringae pv. helianthi (Kawamura) Young et al.<br />
P. syringae pv. mellea (Johnson) Young et al.<br />
Bacterial wilt Pseudomonas solanacearum (Smith) Smith<br />
Crown gall Agrobacterium tumefaciens (Smith and Townsend) Conn<br />
Bacterial stalk rot Erwinia carotovora subsp. carotovora (Jones) Bergey et al.<br />
E. carotovora subsp. atroseptica (van Hall) Dye<br />
■ FUNGAL DISEASES<br />
Alternaria leaf blight, stem spot and head rot<br />
Alternaria alternata (Fr.:Fr.) Keissl. = A. tenuis Nees<br />
A. helianthi (Hansf.) Tub. and Nish. = Helminthosporium helianthi Hansf.<br />
A. helianthicola Rao and Rajagopalan<br />
A. helianthinfi ciens Simmons et al.<br />
A. protenta Simmons<br />
A. zinniae M.B. Ellis<br />
Charcoal rot Macrophomina phaseolina (Tassi) Goidanich<br />
= Sclerotium bataticola Taubenhaus<br />
= Rhizoctonia bataticola (Taubenhaus) E.J. Butler<br />
Downy mildew Plasmopara halstedii (Farl.) Berl. and De Toni in Sacc.<br />
Fusarium stalk rot Fusarium equiseti (Corda) Sacc. = (teleomorph: Gibberella intricans Wollenweb.)<br />
F. solani (H. Mart.) Sacc.<br />
= (teleomorph: Nectria haematococca Berk. and Broome)<br />
Microdochium tabacinum (Van Beyma) Arx<br />
= Fusarium tabacinum (Van Beyma) Gams<br />
= (teleomorph: Monographella cucumerina (Lindfors) Arx)<br />
Fusarium wilt Fusarium moniliforme J. Sheld.<br />
= (teleomorph: Gibberella fujikuroi (Sawada) Ito in Ito and K. Kimura)<br />
F. oxysporum Schlechtend.:Fr.<br />
Gray mold Botrytis cinerea Pers.:Fr.<br />
= (teleomorph: Botryotinia fuckeliania (de Bary) Whetzel)<br />
Appendix 1<br />
117
118<br />
Leaf smut Entyloma compsitarum Farl.<br />
= (anamorph: Cercosperella columbrina Ell. and Ever.<br />
Myrothecium leaf spot Myrothecium roridum Tode:Fr.<br />
M. verrucaria (Albertini and Schwein.) Ditmar:Fr.<br />
Petal blight Itersonilia perplexans Derx.<br />
Phialophora yellows Phialophora asteris (Dowson) Burge and Isaac<br />
Phoma black stem Phoma macdonaldii Boerema<br />
= (teleomorph: Leptosphaeria lindquistii Frezzi)<br />
= P. oleracea Sacc. var. helianthi-tuberosi Sacc.<br />
Phomopsis stem canker Phomopsis helianthi M. Muntanola-Cvetkovic et al.<br />
= (teleomorph: Diaporthe helianthi M. Munt.Cvet. et al.)<br />
Phytophthora stem rot Phytophthora spp. P. drechsleri Tucker<br />
Powdery mildew Erysiphe cichoracearum DC. = (anamorph: Oidium asteris-punicei Peck)<br />
E. cichoracearum DC. var. latispora U. Braun<br />
(anamorph: Oidium latisporum U. Braun)<br />
Leveillula compositarum Golovin f. helianthi<br />
L. taurica (Lév.) G. Arnaud<br />
(anamorph: Oidiopsis sicula Scalia)<br />
Sphaerotheca fuliginea (Schlechtend.:Fr.) Pollacci<br />
Pythium seedling blight Pythium spp.<br />
P. aphanidermatum (Edson) Fitzp.<br />
P. debaryanum Auct. non R. Hesse<br />
P. irregulare Buissman<br />
Rhizoctonia seedling blight<br />
Rhizoctonia solani Kühn<br />
(teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk)<br />
Rhizopus head rot Rhizopus arrhizus A. Fischer = R. nodosus Namyslowski<br />
R. microsporus Tiegh.<br />
R. stolonifer (Ehrenb.:Fr.) Vuill = R. nigricans Ehrenb.<br />
Rusts<br />
(common sunfl ower rust) Puccinia helianthi Schwein.<br />
(cocklebur rust) P. xanthii Schwein.<br />
(nutsedge rust) P. canaliculata (Schw.) Lagerh.<br />
(unnamed rusts) P. massalis Arthur, P. enceliae Diet. and Holw.<br />
Pine needle rust Coleosporium helianthi (Schwein.) Arth.<br />
Sclerotinia basal stalk rot and wilt, mid-stalk rot, head rot<br />
Sclerotinia sclerotiorum (Lib.) de Bary<br />
Sclerotinia basal stalk rot and wilt<br />
Sclerotinia minor Jagger<br />
Southern blight Sclerotium rolfsii Sacc.<br />
(teleomorph: Athelia rolfsii (Curzi) Tu and Kimbrough)<br />
Septoria leaf spot Septoria helianthi Ellis and Kellerm.<br />
S. helianthina (Petrov and Arsenijevic)<br />
Texas or cotton root rot Phymatotrichopsis omnivora (Duggar) Hennebert<br />
= Phymatotrichum omnivorum Duggar<br />
Verticillium leaf mottle Verticillium dahliae Kleb.<br />
White rust Albugo tragopogonis (Pers.) S.F. Gray<br />
= Pustula tragopogonis (Pers.) Thines
Misc. foliar pathogens Ascochyta compositarum J.J. Davis<br />
Cercospora helianthi Ell. and Ever.<br />
C. pachypus Ell and Kellerman<br />
Colletotrichum helianthi J.J. Davis<br />
Epicoccum neglectum Desm.<br />
Phyllossticta wisconsinensis H.C Green<br />
Sordaria fi micola (Rob. ex Desm.) Ces. and Not.<br />
■ NEMATODES, PARASITIC<br />
Dagger, American Xiphinema americanum Cobb<br />
Pin Paratylenchus projectus Jenkins<br />
Lesion Pratylenchus spp.<br />
P. hexincisus Taylor and Jenkins<br />
Reniform Rotylenchulus spp.<br />
Rotylenchulus reniformis Linford and Oliviera<br />
Root knot Meloidogyne arenaria (Neal) Chitwood<br />
M. incognita (Kofoid and White) Chitwood<br />
M. javanica (Treub) Chitwood<br />
Spiral Helicotylenchus sp.<br />
Stunt Tylenchorhynchus nudus Allen<br />
Quinisulcius acutus (Allen) Siddiqi<br />
■ VIRUS and PHYTOPLASMA DISEASES.<br />
Aster yellows<br />
Sunfl ower mosaic potyvirus (SuMV)<br />
Sunfl ower chlorotic mottle virus (SuCMoV)<br />
Cucumber mosaic virus (CMV)<br />
Tobacco mosaic virus (TMV)<br />
Tobacco ringspot nepovirus (TRSV)<br />
Tobacco streak lilarvirus (TSV)<br />
Tomato spotted wilt tospovirus (TSWV)<br />
Appendix 1<br />
119
120
121
Featuring<br />
Hybrid Seed<br />
Herbicide Drift<br />
Storage<br />
Harvesting<br />
Insects<br />
Bird Damage<br />
Diseases<br />
Grading<br />
<strong>Production</strong><br />
Hail Injury<br />
Weed Control<br />
The NDSU Extension Service does not endorse commercial products or companies even though reference may be made to tradenames,<br />
trademarks or service names.<br />
This publication may be copied for noncommercial, educational purposes in its entirety with no changes. Requests to<br />
use any portion of the document (including text, graphics or photos) should be sent to permission@ndsuext.nodak.edu. Include exactly<br />
what is requested for use and how it will be used.<br />
For more information on this and other topics, see: www.ag.ndsu.edu<br />
County commissions, North Dakota State University and U.S. Department of Agriculture cooperating. Duane Hauck, director, Fargo, N.D. Distributed in furtherance of<br />
the acts of Congress of May 8 and June 30, 1914. We offer our programs and facilities to all people regardless of race, color, national origin, religion, gender, disability,<br />
age, veteran’s status or sexual orientation; and are an equal opportunity institution. This publication will be made available in alternative formats for people with<br />
disabilities upon request, (701) 231-7881. 4M-9-07