Cucumber, Squash, Melon & Other Cucurbit Diseases
HGIC 2206
Printer Friendly Version
Many diseases of cucurbits can be prevented or minimized in the home vegetable garden by using the following simple cultural controls:
- Plant certified disease-free seeds.
- Select varieties recommended for South Carolina, especially those with some degree of disease resistance (Table 1).
- Keep the garden and surrounding area free of weeds that harbor insects, which can spread viruses and bacterial wilt.
- Remove plant debris from the garden after harvest, since many diseases survive on plant debris from year to year.
More information about growing cucurbit plants is available in the fact sheets: HGIC 1304, Cantaloupe and Honeydew Melon; HGIC 1309, Cucumber; HGIC 1321, Summer Squash; and HGIC 1325, Watermelon. See also Fact Sheet CE-6 Cucurbit Diseases, an Aid to identification.
Bacterial Wilt
The main symptom of this disease is severe wilting of the vines, followed by rapid death of the plant. The disease is caused by the bacterium Erwinia tracheiphila, and at first may only affect a few vines on a plant. However, as the disease progresses, more leaves wilt, and eventually the entire vine is affected. Bacterial wilt is most severe on cucumber and cantaloupe and less severe on squash, pumpkin and watermelon.
Prevention and Treatment: There is no chemical control for bacterial wilt once plants become infected. The bacteria are carried from plant to plant by striped or spotted cucumber beetles. The beetles spread the wilt bacterium by feeding on infected vines and then feeding on healthy plants.
Bacterial wilt can be reduced in your garden if the beetles are kept under control at the first sign of activity. Insecticides that control striped and spotted cucumber beetles in the home vegetable garden include carbaryl and esfenvalerate (see HGIC 2207, Cucumber, Squash, Melon and Other Cucurbit Insects). Bees pollinate many of these vegetables, so spray all insecticides in the late afternoon. Apply all chemicals according to directions on the label.
Powdery Mildew
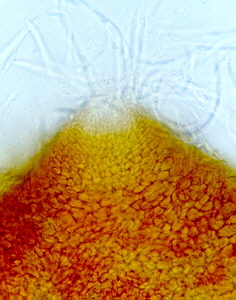
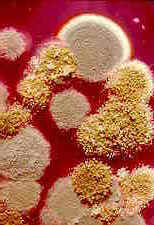
Powdery mildew causes a white powdery growth on the upper surfaces of leaves and on the stems of infected plants. Infected areas are often stunted and distorted and may drop prematurely from the plant. Fruits are usually not directly affected, but their size and growth may be stunted. Powdery mildew is caused by the fungi Erysiphe cichoracearum and Sphaerotheca fuliginea. Infection can occur when temperatures are between 50 and 90 °F, during dry weather with high relative humidity. The disease can be a particular problem on late-planted squash.

Powdery mildew on watermelon leaves.
David B. Langston, University of Georgia, www.insectimages.org
Larger Image (280Kb)
Prevention and Treatment: Powdery mildew-resistant varieties (Table 1) are available for most cucurbits, thus with proper planning, chemical control should not be necessary. Preventative fungicide treatments are available (Table 2) if disease becomes severe enough to warrant chemical control.
Downy Mildew
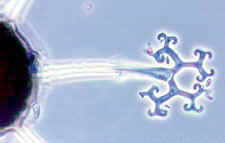
Downy mildew is one of the most important leaf diseases of cucurbits. Typically, symptoms begin as small yellow areas on the upper leaf surface. As lesions expand, they may become brown with irregular margins. Affected areas may grow together, and the entire leaf may wither and die. Infected plants also develop a gray mold on the lower leaf surface. The fruit is not affected, but in the case of cantaloupes, it will be less sweet. This disease is caused by the fungus Pseudoperonospora cubensis and is favored by moist conditions.

Larger Image (509 Kb)

Larger Image (990 Kb)
Downy Mildew on top (left) and bottom (right) of cantaloupe leaf.
Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org
Symptoms on watermelon are different than symptoms on other curcurbits. Leaf spots on watermelon are dark brown and irregular in shape, ranging from oval to angular to retangular. Slight yellowing may be seen around the edges of the spots or in small patches in other parts of the leaf. Leaves infected with downy mildew curl inward as the leaf dies. As on other crops, spores usually are found on the bottom of the leaf, although spores may be formed on top of the leaf in severe infections or foggy weather.
Prevention and Treatment: Use varieties that are resistant to this disease (Table 1). Fungicides are available for the home vegetable garden if disease becomes severe enough to warrant chemical control (Table 2).
Gummy Stem Blight
Gummy stem blight is a stem and leaf disease of cucumber, cantaloupe, pumpkin and watermelon caused by the fungus Didymella bryoniae. This fungus also causes a fruit rot called black rot.
Symptoms include leaves with brown or tan spots of various sizes that may eventually cover the entire leaf. The stems may split to form open wounds called cankers. A brown, gummy substance may be evident on the surface of these open wounds. Infected vines usually wilt after the middle of the season. Infected stems die one after another, and seedlings and entire individual vines may be killed. Affected fruit have irregular circular spots, and a wet rot occurs where the fungus penetrates the rind.

Leaf spots caused by gummy stem blight on watermelon leaf.
Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org
Larger Image (259 Kb)
To distinguish gummy stem blight on watermelon from downy mildew, look at the size, shape, and position of leaf spots. Leaf spots of gummy stem blight are larger than individual spots of downy mildew. Some leaf spots of gummy stem blight have a ringed or target look. Gummy stem blight also can be found on the petioles (leaf stems) and the mid vein of leaves as a water-soaked or reddish-brown wet spot.
Prevention and Treatment: There are no varieties that are resistant to this disease. This disease may be seed-borne, so purchase seed from a reputable source. Remove and destroy all plant debris in the garden, since the disease can survive on plant debris from year to year. Rotate crops with nonhost plants, such as corn, for two or more years as an effective way of reducing the incidence of this disease. Avoid wetting the leaves when watering. If disease is severe enough to warrant chemical control, preventative fungicides are available (Table 2).

Symptoms of anthracnose on watermelon.
Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org
Larger Image (343 Kb)
Anthracnose
Anthracnose is caused by the fungus Colletotrichum obiculare, and requires rainy, cool weather for several days for the disease to develop. The first symptoms of anthracnose are spots on the leaves that begin as yellowish or water-soaked areas. Spots enlarge and turn brown to black. The diseased tissue dries and the center of the spots fall out giving a “shot-hole” appearance. Infected fruits have black, circular, sunken cankers of different sizes.
Prevention and Treatment: Remove and destroy old cucurbit vines and residues, since this is where the fungus survives the winter. Rotation of crops in the garden is also important to minimize disease. Purchase seeds from a reputable source, since the disease can be seed-borne. If the disease is severe enough to warrant the use of fungicides, several are available for home garden use (Table 2).
Alternaria Leaf Spot
This disease is caused by the fungus Alternaria cucumerina and causes small, circular, tan spots to appear on the leaves, which later enlarge to 1½ inches or more in diameter. Definite concentric rings and margins appear that give the area a “bull’s eye” appearance. Leaf drop can be severe. Bright sunshine, frequent dews or rain, and temperatures between 60 and 90 °F favor disease development.

Alternaria leaf spot on cantaloupe.
Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org
Larger Image (379 Kb)
Prevention and Treatment: Remove and destroy all infected plant residues at the end of the gardening season, since the fungus survives thewinter on plant residue. The disease is easily spread by tools, wind, splashing water or insects. Rotation of crops and seed treatment will also help. When this disease occurs consistently in the garden, a preventative fungicide program can be followed (Table 2).
Fusarium Wilt
This disease is caused by the fungus, Fusarium oxysporum forma specialis melonis. It attacks the roots of the plant and moves into the stems. Older, established plants that are infected become stunted, wilt and eventually die. Wilt symptoms develop in one or more laterals, usually starting at the vine tips. A white mold may develop on dead vines. Affected seedlings will damp-off (rot at the soil line), wilt and die. On runners near the crown of the plant, brown streaks may be evident. Roots will have a honey brown discoloration inside.
Prevention and Treatment: This fungus can survive in the soil for many years. Planting resistant varieties (Table 1) is critical in preventing this disease. Careful water management is also important in minimizing root stress. There are no chemical treatments available for control.
Viruses
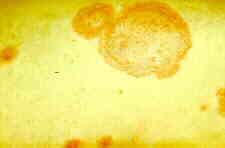
There are several common viruses that can affect cucurbits, including cucumber mosaic virus (CMV) and watermelon mosaic virus (WMV). Infected plants may be stunted or have leaves that are mottled, crinkled, or a light green color. Fruits may be irregular in shape, mottled or warty. Various insects transmit these viruses.

Cucumber mosaic virus (CMV) on squash.
Division of Plant Industry Archive,
Florida Department of Agriculture and Consumer Services,
www.insectimages.orgLarger Image (111 Kb)
Prevention and Treatment: There are no chemicals available to kill viruses. Chemical control of the insects that spread the viruses may minimize the disease. This control method is difficult, because infection occurs immediately after an insect feeds, and insects migrate freely between plants. A good control strategy is to maintain healthy and vigorous plants, plant recommended varieties and monitor your garden for any unusual symptoms as they occur. Keep the area clear of weeds that can harbor insects. Choosing separate areas for early and late plantings may help to reduce virus severity in the late plantings.
Blossom-End Rot
Blossom-end rot appears as a dark-colored dry rot on the end of the fruit where the flower was. The problem is caused by a lack of calcium in the developing fruit. It is an indication that calcium is lacking in the soil or that the plant does not have the ability to take up enough calcium. When growth is rapid, not enough calcium may be delivered to the blossom end of the developing fruit.

Blossom end rot on watermelon.
Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org
Larger Image (361 Kb)
Prevention and Treatment: Help prevent blossom-end rot by having your soil tested before planting through your local county Extension office, and lime according to recommendations. Always maintain an adequate supply of moisture, especially during fruit growth. Mulch plants to prevent rapid drying of the soil and water plants during extended dry periods. Use gypsum (1-2 pounds per 100 square feet) as a supplement to liming on calcium deficient soil. Lime and/or gypsum should be applied before planting. Do not overfertilize plants with excessive nitrogen or potassium. Excess amounts of these nutrients reduce the uptake of calcium in the plant. When plants are dark green, extra fertilizer should not be applied.
Treatment of affected plants includes applying a calcium solution to prevent additional fruits from being damaged. Spray or drench the foliage with a calcium nitrate or calcium chloride (three tablespoons per gallon of water) solution. Premixed solutions are also available. Follow the instructions on the label. Removing fruit with symptoms is recommended.