The following is a guest post from Fulvio Capra who studied Glowing Plants for a project. He is studying for a Bachelor’s degree in Agronomic Sciences and Technologies at UNIVERSITÀ DEGLI STUDI DI TORINO.
Curriculum: Realization and management of green spaces
Year: 2015
SUMMARY
1 - Introduction
1.1 Bioluminescence
1.2 History
of luminescent plants
1.3 Functional
plants
2 – Case study
2.1 Introduction
2.2 Results
2.3
Discussion
3 - Applications
3.1
Glowing Plant
3.2
Bioglow
4 - Potentialities - perspectives
4.1
Curiosities
4.2
Trees like street lamps
4.3 Vertical
green applications
4.4 Possible
controversies
5 - Conclusion
6 - Bibliography
1 - INTRODUCTION
 A
relevant feature of the ornamental plants market is the product innovation. The
client is always looking for new shapes and colors of the flowers or leaves. That’s
why there are several floricultural producers that use hybridization or genetic
engineering to obtain new varieties to commercialize. An example is the
transformation of floral species with a gene _Green Fluorescent Protein _(GFP)
which belongs to a jellyfish. In 2001 a group of scholars tried to prove that
it is possible to use GFP as fluorescent dying for flower petals. One of the
species they studied belonged to the Osteospermum genus, and was named
Fluorescent Daisy. (Pic. 1).
A
relevant feature of the ornamental plants market is the product innovation. The
client is always looking for new shapes and colors of the flowers or leaves. That’s
why there are several floricultural producers that use hybridization or genetic
engineering to obtain new varieties to commercialize. An example is the
transformation of floral species with a gene _Green Fluorescent Protein _(GFP)
which belongs to a jellyfish. In 2001 a group of scholars tried to prove that
it is possible to use GFP as fluorescent dying for flower petals. One of the
species they studied belonged to the Osteospermum genus, and was named
Fluorescent Daisy. (Pic. 1).
The possibility of
producing and commercializing light emitting plants is innovating and could
turn out to be a revolution in the field of ornamental plants.
Recently, a few startups, beginning
from results obtained in experiments conducted in 2010, managed to obtain
autoluminescent plants, with a glow-in-the-dark type of light.
1.1 Bioluminescence
Bioluminescence is defined
as the ability of living organisms to emit light through the chemiluminescence
phenomenon.
The light emission is due
to transfer of electrons from a substrate, in presence of an enzyme called Luciferase.
The electrons are transferred to a lower energetic level, with an output of
energy in the form of light radiation.
It’s a natural phenomenon
spread through different taxonomic groups of living beings, both terrestrial
and marine. Among the animals can be found insects, shellfishes and molluscs
(Pic. 2).




Marine bioluminescent
bacteria can be found both in free form and in symbiosis with fishes or
molluscs. There are three genus: Vibrio (Pic. 3), Photobacterium,
and Xenorhabdus.
There are, moreover, about
70 existing species of bioluminescent fungi, like the Panellus stipticus
(Pic. 4).


A few years back the
bioluminescence phenomenon found application in the healthcare field, in
particular in the fight against cancer. The Bioluminescent Activated
Destruction technique, developed eleven years ago and still in experimental
phase, consists in the transformation of tumorigenic cells so that they express
both the photosensibilizing and the luciferase gene from the firefly
(Pic. 5).
This way the cells,
emitting bioluminescence, commit some sort of suicide because the
photosensibilizing reacts to the luminescence producing toxins.


1.2 History of luminescent plants
When a plant is able to
emit bioluminescent autonomously is defined autoluminescent.
The first experiment
regarding luminescent plants was conducted in 1986 by a group of US scholars
and researchers. They used as a reporter gene the luciferase, which is
essential for the bioluminescence reaction in many living organisms – e.g. the
firefly, Photinus pyralis – and obtained a plant that, due to the
addition of luciferin (substrate of the reaction) and ATP (adenosin
triphosphate), produced a dim light emission.
The first part of the
experiment had the aim of testing the activity of luciferase gene –
responsible for the synthesis of _luciferase _enzyme – in vegetal cells. A
construct of complementar DNA (cDNA) was introduced in protoplasts – wall-free
cells – of Daucus carota through electroporation.


During the experiment
different constructs were tested (Pic. 6). The researchers observed that the
construct (pDO432), containing the whole luciferase gene, plus the promoter and
the nos terminator, was the most efficient for the transformation. If they used
the (pDO435) construct, containing an inverted _luciferase _gene, its
activity was reduced to zero. With the (pDO446) construct, in which the
terminal part (nos 3’) had been removed, the luciferase activity
was reduced to 66%. Finally they tried to remove a small segment belonging to
the luciferase gene, and the luminescence decreased to 8%. Thanks to
these results the researchers acknowledged that the expression of luciferase
was linked to all its components: the 35S promoter, the luciferase gene
and the nos 3’.
The pDO432 construct was
inserted in a plasmid, which was introduced into protoplasts with
electroporation. After 24 hours, the scholars analyzed the results with a
luminometer, and observed a dim light emission when they added ATP and luciferine
as a reaction substrate. At that point they tried to change the quantity of
reagents in the reaction: excesses of substrate lead to a higher number of
extracts containing luciferase activity and thus an augmentation of
light radiation. In absence of luciferine they couldn’t detect any
luminescence. Without ATP and with abundant luciferine they observed a
dim light emission.
Further on the researchers
introduced the same pDO432 plasmid in young Nicotiana tabacum plants,
using the Agrobacterium tumefaciens type transformation, in order to
obtain a stable genetic transformation. The A. tumefaciens containing
the recombinant plasmid was inoculated within leaves disks to get transgenic
tobacco plants. The results were analyzed through the insertion of fragments of
transformed leaves in a reaction tube containing substrate and reading the
quantity of light radiation using a luminometer.
The transgenic plants
obtained after the transformation were grown in a controlled environment.
Afterwards was tested the distribution of luciferase into different
organs. Its activity was detected in leaves, roots and stems, but in different
quantities. Roots and stems showed a greater light intensity than leaves.
Moreover younger organs emitted more light than older ones. The researchers
also discovered that if they added dimethyl sulfoxide (DMSO) and sodium citrate
to the luciferine into the substrate, the light emission improved. Last
they tried to “water” roots of young plants, in sterile conditions, with a
solution of luciferine. The goal was to observe if the same pattern as
before was detected in the _luciferase _activity of different organs. The
outcome was positive: the light emission was more abundant in roots, stems and
young leaves.
The experiments conducted
in 1986 were relevant not only for autoluminescent plants, but also for the
research on DNA and gene expression. The luciferase was employed in many
studies, as a marker gene to observe the expression and the tissue regulation
in different phases of the plant growth.
1.3 Functional plants
The engineered plants –
often grouped within the genetic modified organisms (GMO) – are, for some
aspects, controversial, and the debate on their use is currently on-going. One
of the reasons why Europeans are reluctant to use them is because of a security
principle applied to transgenic food laws. That’s why nowadays many biotech
companies prefer to concentrate their efforts in every different field but
alimentation. This latter is, in facts, only one of the many subjects to which
we can apply biotech processes. Other examples are phytoremediation, the
production of renewable energy and biofuels, using plants to produce vaccines,
therapeutic molecules, industrial enzymes and others (Pic. 7).


Functional plants is one
of the subject of studying that are gathered within the synthetic biology. The National
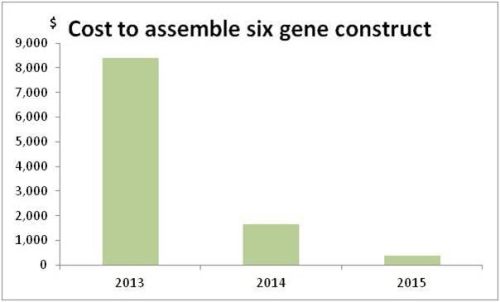
Human Genome Research Institute’s data indicate that the cost for DNA
sequencing is falling rapidly. In 2001 the price for a base pair was equal to
10.000 $, in 2011 was only 0,1 $ (Pic. 8). That means that the price for
reading and writing DNA sequences has fallen a hundred thousand times in ten
years. Synthetic biology benefited of this: lower costs encouraged many startups
aiming at applying this new technology to fields like biofuels, human
healthcare, alimentation and many more. One of the projects that are now
feasible thanks to synthetic biology is the development of autoluminescent
plants.



2 - CASE STUDY
2.1 Introduction
The 1986 experiment was
crucial to help discovering the activity of luciferase in plants, and
also allowed the codification of light emission as a marker. However, it had
two main flaws: the quantity of light was too low to be detected by naked eye
(the researchers used a luminometer), plus to obtain luminescence was needed
the addition of luciferine.
Like has previously been
said, the bioluminescence reaction is typical of many different species of
living beings, like bacteria, in which the reaction is encoded by a lux
operon, including luxA, luxB, luxC, luxD, luxE genes. In this case the
substrate isn’t luciferine but a flavin mononucleotide and a long-chain
aldehyde.
luxA and luxB genes
code the α and β subunits of the bacterial
luciferase, luxC, luxD and luxE code enzymes involved in
the synthesis of the aldehyde substrate employed in the luminescence reaction.
The bioluminescence phenomenon is due to an oxidation reaction of the aldehyde
and the reduced flavin mononucleotide (FMNH-) conducted by molecular
oxygen. The overall reaction is the following: FMNH- + H+
- RCHO + O2 à FMN + RCOOH + H2O + hv. The products of this
reaction are the oxidized flavin mononucleotide (FMN), a long-chain fatty acid,
water and blue-green light. In most of the luminescent bacteria there is a lux
operon consisting of 5 components: luxCDABE. In some marine bacteria,
like Photobacterium leiognathi (Pic. 9) and Vibrio fisheri, an
additional gene can be found, the luxG. Its amino acid sequence is
similar to the Fre. A flavin reductase isolated in Escherichia coli. Studies
conducted in 2007 actually proved that luxG gene of the P. Leiognathi
behaves as a flavin reductase.


In 2010 a group of
scientists performed an experiment with the intent of obtaining a full
autoluminescent plant – and thus get past the limitations emerged during the
previous experiment. They exploited the bioluminescence mechanism of marine
bacteria, the P. leiognathi. They inserted the lux operon in the
chloroplast genome of tobacco plants, Nicotiana tabacum, and managed to
create the first autoluminescent plant, containing the bacterial luciferase
and capable of emitting light visible by naked eye. For the first time was
proved that a higher plant was able to reproduce a complex enzymatic pathway
originated from a distant, unrelated organism (P. leiognathi is a
prokaryote).
2.2 Results
The scholars created two
transplastomic lines of N. tabacum. The first one, called “line A”,
contained the lux operon in the rps12/TrnV locus of the
chloroplast genome. The second, “line B”, had the lux operon inserted in
a more transcriptionally active locus, the TrnI/TrnA. In both cases the
spectinomycin was used as a selection marker. To enable the entry of the lux
genes in the chloroplast, homologous recombination sites were used, whose ends
were recognized by the respective ends of the chloroplast genome. To obtain
transplastomic plants, the transformed chloroplasts were shot in N. Tabacum
cells using microbombardment methods. Afterwards, the researchers made some
analyses to ensure that the plants that survived the spectinomycin selection
were actually transplastomic. Through a junction PCR (Pic. 10) they separated
transformed plants from those spectinomycin-resistant because of mutating
portions of the ribosomal RNA.


Picture 10B shows different
fragments of the genome with the respective expected size, expressed in
kilobase (kb). The primers used were designed so that they had one end in the
chloroplast genome and the other into the exogen DNA. As further proof of the
transformation, luxC e luxB were amplified. The results are
shown on the gel (Pic. 10B), prooving that the amplifications on the wild-type
of each fragment wasn’t detected.
In order to verify the
transformation the researchers performed DNA blot analysis on the Wild-type
and on the two lines, line A and line B (Pic. 11). The DNA was cut with
endonucleases Smal, and the hybridation was done with two different
probes, one for the line A, one for the line B.


They also performed a
similar DNA blot analysis using probes specifically to ascertain whether the
insertion of the aadA (selection marker) and the lux operon into the
plastidial genome had been successful. (Pic. 12).


The luminosity was
quantified by placing shoots of transformed plants and of wild types in a
scintillator. This latter is a machine that counts the number of photons and
displays a result measured in number of counted photons per minute. The count
lasted twenty minutes, in which the luminescence emitted by the two lines of
transformed N. Tabacum performed a decreasing trend, probably due to the
exhaustion of oxygen within the vial. The oxygen constitutes a reagent in the
luminescence reaction. In the line B the lux operon was inserted in a
locus more transcriptionally active, thus the number of counted photons was 25
times higher than line A. the starting data was 82 million of photons per
minute, after twenty minutes it was down to 60 million (Pic. 13). The
researchers grew the plants until they obtained adult plants, and observed that
the luminescence emitted was enough to be appreciated in dark conditions by
naked eye.


2.3 Discussion
The studies conducted in
2010 achieved an important result: for the first time was obtained an
autoluminescent plant whose light was visible by naked eye in the dark. The
potential developments of this experiment are numerous and in great part still
unexplored. The scholars understood that the mechanism that permitted the
tobacco plants to acquire bioluminescence, typical of a marine bacterium, is
shared among all vegetal species. Consequently, the same process can be applied
to other plants. Considering that the light emission can be changed by using
different promoters and that the color can be modified, as well as the parts of
the plant in which the luminescence is expressed, these studies are expected to
generate many great applications in the floriculture and nursery world.
Being a transformation
that concerns only the chloroplast DNA, not the nuclear DNA, we use the name of
chloroplastic transformation, from which stems the name of transplastomic
plants. The plastids are the class of organelle to which belong the
chloroplasts, and they are motherly inherited in most of the angiosperm plants.
From an environmental point of view this is a safe approach: the transformed
plant won’t contain transgenic DNA in the pollen, so it won’t be able to convey
it to other plants.
3 - APPLICATIONS
3.1 Glowing Plant
Glowing Plant is an organization funded
in California in 2013 by Anthony Evans and Kyle Taylor (Pic. 14). Their
starting point was the experiment of 2010.


In the same year was also
developed a project hosted by the University of Cambridge and called iGEM.
The researchers inserted genes responsible for the luminescence in the firefly
into an Escherichia coli bacteria. Afterwards they inserted the lux
operon taken from a marine bacteria called Vibrio fischeri into a
different E. coli.


The results showed that
the light emission was abundant, sufficient to permit the reading of a text
using only the bacteria as a light source. Most importantly, they managed to
obtain different luminescence colors (Pic. 15).
Evans and Taylor had the
bright idea of joining the experiment conducted on N. Tabaci –
previously described – with the iGEM project, with the intent of
creating an autoluminescent plant and improve as much as possible the light
quantity.
In April 2013 they
launched an online fundraising, using the kickstarter website, to
finance the production of autoluminescent plants, and they gathered almost half
a million dollars. Five months later the two of them founded an organization, G_lowing
plant_. The goal was to benefit of the positive entourage that was rising
around them and their product, and in the meantime keep the research up in order
to further improve the luminescence and develop new products. They obtained
120.000 $ financed by Y Conbinator, an organization of people who decide
to back up startups in which they see a potential. Today Glowing Plant
has an online store selling seeds and seedlings of autoluminescent plants (Arabidopsis
thaliana, Pic. 16 and 17) and maker kits for those who would like to try
and replicate the experiment by themselves and obtain their own autoluminescent
plants.




Antony Evans, CEO of Glowing
plant, participated in two TEDx conference talks, one in Brussels and one
in Kuala Lumpur, demonstrating his startup’s work (Pic. 18). In these occasions
he explained how it was possible to obtain their product, and exhorted the
listeners to pay attention to the synthetic biology and the results that could
be achieved. He talked about the possibility that, one day, streets could be
lighted by glowing trees. He underlined the need to start a debate on topics
that could change drastically men’s life, like the fact that in a few years
from today we’ll be able to synthesize a whole human genome with just a few thousand
dollars, given the needed ethical considerations.


3.2 Bioglow
Dr. Alexander Krichevsky
is one of the researchers that participated in the 2010 experiment on
autoluminescent tobacco. Consequently to the achievements he applied the same
procedure to Nicotiana alata and other plants within the same family
with the aim of producing autoluminescent plants. He partnered with the
entrepreneur Tal Eidelberg and together they founded the startup Bioglow,
which in 2013 commercialized the very first autoluminescent plant, called Starlight
Avatar (Pic. 19).


The features of this plant
are comparable to the tobacco plant yielded by the experiments. The ideal
temperature for its growth is around 25°Celsius, it’s designed for indoor
environments and its average lifespan is two to three months. The team of Bioglow
researchers is presently working for a new generation of autoluminescent plants.
They have two main goals:
improving the light emission and developing new cultivars with different
species and different luminescence color. The aim is yielding new plants
capable of lighting up gardens, parking lots, streets, in order to reduce the
electricity and coal energy consumption (Pic. 20).


They also believe that in
the future it will be possible to obtain species having luminescence of
different colors between the different organs of the same plant, or capable of activating
the luminescence in response of an outer stimulation, such as pollution or a
hand-touch.
4 - POTENTIALITIES - PERSPECTIVES
4.1 Curiosities
Many could wonder about
the look of autoluminescent plants if they were tall as trees, and about their appearance
if they were actually employed in a landscape project.
In Avatar, science
fiction movie directed in 2009 by James Cameron, part of the scenography is
composed by plants light-emitting, yielding some bright scenic effects (Pic.
21).


Is it possible that the
1986 experiment on luciferase and its employment on plants could have
influenced the director’s choices in merit of scenography? Or that the film Avatar,
aired in 2009, could have awoken the interest of those scientists that one year
later experimented the bioluminescence of Photobacterium leiognathi in tobacco plants?
4.2 Trees like street lamps
The Studio Roosegaarde
is a social design lab based in Netherlands and China, and has developed two
pictures to show their idea of luminescent trees (Pic. 22 e 23).


For now the idea of using
trees as street lamps is unfeasible, the light output is still too small. However,
there are good reasons to believe that light emission will improve swiftly and
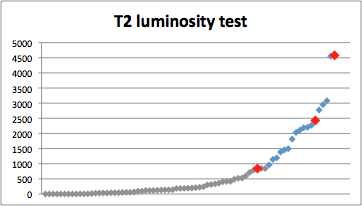
soon someone may be able to obtain true autoluminescent trees. For instance, Glowing
Plant has more than quadruplicated the light emitted in only three months (Pic.
24 e 25). This improvement was achieved after the shipment of the first
transplastomic Arabidopsis thaliana, therefore it will be applied to following generations of
luminescent plants.




That of synthetic biology
– and thus autoluminescent plants – is a very interesting market. It strikes
that many of the projects in this field are not tied to multinational
corporations, but based on public research or crowdfunding started by small
biotech companies recently born. This was possible thanks to the already cited
drop of the costs for DNA sequencing and for the obtainment of engineered
plants (Pic. 26) – which resulted in costs for genetic engineering that are now
much more accessible by new companies.


The autoluminescent plants
that have been obtained so far are designed for a domestic environment, as
ornament or as dim light to be used at night.
4.3 Vertical green applications
In the last years the
attention towards a green environment has substantially increased. The number
of areas dedicated to parks and gardens has grown, especially in cities
economically wealthy. In recent times new ways of using the vegetation have
been tested, for example the vertical green. The French botanist Patrick Blanc
is considered the father of this method. We can see an application at the Bosco
Verticale, a project designed for the 2015 Universal Exposition in Milan (Pic.
27).


What result could be
obtained if, hypothetically, autoluminescent plants were to be used in a
project such as this one? Would it be esthetically appreciable? Considering the
great number of plants, would the light emission be enough to grant at least
the lighting of the outer portion?
If we could find answers
to these questions the upsides would be numerous, starting with energy savings.
For example, the lights placed on top of skyscrapers to signal their position
could be, one day, replaced by plants.
4.4 Possible controversies
People’s opinion about
autoluminescent plants is definitely influenced by the general debate on GMOs. In
the beginning the discussion had a scientific connotation, but lately has
acquired ethical and political implications. Some examples are the thriving of
an anti-GMO publicity (Pic. 28), or the debate on the role of the main
multinational companies commercializing plants, like Monsanto. Regarding
autoluminescent plants, great part of those who contributed to the debate
expressed negativity due to a general skepticism concerning all genetic
transformations. The protest has also risen on internet. While in the spring of
2013 Anthony Evans has risen almost half a million dollars with the Kickstarter
fundraising site, GMO antagonists launched a Kickstopper campaign (Pic.
- and asked Kickstarter not to back up Glowing Plant’s project.




Their goal was to hamper
all synthetic biology experiments, and Antony Evan’s project was symbolic,
because it was the first time that a Syn Bio experiment collected such a great
amount of money. At any rate the attempt to hinder Glowing Plant and
prevent the money raising was unsuccessful, and the Kickstopper campaign
failed.
In a previous chapter was
discussed the chloroplastic transformation and the inheritance of plastids, to
explain why transformed plants can’t convey the hexogen genes to others. This
is true for most of the angiosperms, however there can be some environmental
risks if someone was to apply this transformation method to plants having paternal
inheritance of plastids. For this reason the maker kit (Pic. 30) commercialized
by Glowing Plant has received many critics, motivated by the fact that
everyone can buy this do-it-yourself kit and pretend to be a genetic
hybridizer, even without any base knowledge of genetic or molecular biology.
The risk of transgenes transmission concerns, among the others, genus like Oenothera,
Hypericum, Medicado – plants with biparental inheritance – and other
species like Actinidia deliciosa – with paternal inheritance (Pic. 31).



In case someone wanted to
use a genetic transformation technique and be certain that no gene will pass to
other plants, he could use the Cytoplasmic Male Sterility (CSM). Producing CSM
in plants is, evidently, a process that not everyone can operate, consequently
not all those who buy a Maker Kit.
The critics directed to
autoluminescent plants had the effect of making the debate on engineered
organisms laws start anew. In Europe exists a “precaution principle”, therefore
every GMO, before its commercialization, has to be approved and properly labeled.
In the USA, on the contrary, the laws are based on a “substantial equivalence”
principle, consequently every GMO product is treated equally to its equivalent
non-GMO, as long as it doesn’t cause health issues of some kind. Many US
citizens criticize this and they’d prefer that in the States existed laws
comparable to the European ones. Many people complain about the inexistence of
regulation of products such as autoluminescent plants or _Maker Kit_s.
Another criticism aimed at glowing
plants concerns environmental consequences. Some are afraid that bringing
luminescence in plants could alter the balance of the ecosystems, for instance
the habits of night insects and birds, or even the offset of the two phases of
the photosynthesis, the light one and the dark one.
5 - CONCLUSIONS
Synthetic biology could be
a turning point in fields like ornamental green and functional plants. Glowing
plants are an example of how mankind can do its best to find functionality and
esthetical beauty in the surrounding world. It’s something that men have always
done, since ancient times.
Autoluminescent plants are
considered safe for man and the environment. Their commercialization was
possible because the Animal Plant Health and Inspection Service (APHIS) decided
against regulating the glowing plants. This decision has been publically shown
by Glowing Plant, whose product, Arabidopsis thaliana, has been
approved, since it doesn’t contain any organism considered noxious, unknown or
unclassified. In facts, regulated products are restricted because they are
considered as plant weed or as pathology vectors.
The rules concerning the
maker kit are the same, therefore it can be commercialized. The problem may
arise in case someone misused it, willingly or not. In that case it could cause
a serious damage for the environment and for biodiversity
It’s crucial that biotech
research is carried out, and possibly financed by public authorities, free from
interest of profit. The importance of public research is linked to the possibility
of ameliorating the world that surrounds us, particularly concerning ornamental
and functional aspects. In order to do so, we need rules that ought to be
transformed in laws, and controls, all to ensure that the discoveries are safe
and ethically acceptable.
At the same time it is key
that scientific knowledge and research results are spread and shared with all
humanity. Maybe biotech runs way faster than what public opinion is ready to
accept: often innovations are faced with hostility by people, and frequently
the reason is lack of information.
There’s also an attempt to
democratize synthetic biology: there is an ongoing digitization of genetic
engineering. The goal is to make these tools available to all, so that
developing an application in biology will become as easy as creating an
application for computers and other electronic devices.
It’s a time of great
changes, mankind has the opportunity to model it and bend it to his wishes. We
only have to decide if we want to be a part of the innovation or if we prefer
that others decide for us.
6 - BIBLIOGRAFY
-
Mercuri A,
Sacchetti A, De Benedetti L, Schiva T, Alberti S, 2002, Green Fluorescent
Flowers, Plant Science 162
-
Bioluminescence:
http://en.wikipedia.org/wiki/Bioluminescence
-
Bioluminescent
Activated destruction:
http://www.ucl.ac.uk/news/news-articles/news-releases-archive/fireflycancer
-
Ow D.
W. , Wood K. V. , DeLuca M, De Wet J. R. , Helinski D. R., Howell S. H., 1986, Transient
and Stable Expression of the Firefly Luciferase Gene in Plant Cells and
Trangenic Plants, Science, 234: 856-859
-
Krichevsky
A, Meyers B, Vainstein A, Maliga P, Citovsky V, 2010, Autoluminescent plants,
PLoS ONE, 5 (11): e15461
-
Nijvipakul
S, Wongratana J, Suadee C, Entsch B, Ballou D, Chaiyen P, 2008, LuxG Is a
Functioning Flavin Reductase for Bacterial Luminescence, Journal of
Bacteriology VOL. 190: 1531–1538
-
Verma
D, Daniell H, 2007, Chloroplast Vector System for Biotechnology Applications,
Plant Physiology, 145: 1129–1143
-
Glowing Plant: http://www.glowingplant.com/
-
E.
Glowli
Experiment: http://2010.igem.org/Team:Cambridge
-
Bioglow:








 A
relevant feature of the ornamental plants market is the product innovation. The
client is always looking for new shapes and colors of the flowers or leaves. That’s
why there are several floricultural producers that use hybridization or genetic
engineering to obtain new varieties to commercialize. An example is the
transformation of floral species with a gene _Green Fluorescent Protein _(GFP)
which belongs to a jellyfish. In 2001 a group of scholars tried to prove that
it is possible to use GFP as fluorescent dying for flower petals. One of the
species they studied belonged to the Osteospermum genus, and was named
Fluorescent Daisy. (Pic. 1).
A
relevant feature of the ornamental plants market is the product innovation. The
client is always looking for new shapes and colors of the flowers or leaves. That’s
why there are several floricultural producers that use hybridization or genetic
engineering to obtain new varieties to commercialize. An example is the
transformation of floral species with a gene _Green Fluorescent Protein _(GFP)
which belongs to a jellyfish. In 2001 a group of scholars tried to prove that
it is possible to use GFP as fluorescent dying for flower petals. One of the
species they studied belonged to the Osteospermum genus, and was named
Fluorescent Daisy. (Pic. 1).