Abstract
The production of a new allohexaploid Brassica crop (2n = AABBCC) is increasingly attracting international interest: a new allohexaploid crop could benefit from several major advantages over the existing Brassica diploid and allotetraploid species, combining genetic diversity and traits from all six crop species with additional allelic heterosis from the extra genome. Although early attempts to produce allohexaploids showed mixed results, recent technological and conceptual advances have provided promising leads to follow. However, there are still major challenges which exist before this new crop type can be realized: (1) incorporation of sufficient genetic diversity to form a basis for breeding and improvement of this potential crop species; (2) restoration of regular meiosis, as most allohexaploids are genetically unstable after formation; and (3) improvement of agronomic traits to the level of “elite” breeding material in the diploid and allotetraploid crop species. In this review, we outline these major prospects and challenges and propose possible plans to produce a stable, diverse and agronomically viable allohexaploid Brassica crop.



Similar content being viewed by others
References
Abdalla MMF, Hermsen JGT (1972) Unilateral incompatibility: hypotheses, debate and its implications for plant breeding. Euphytica 21:32–47
Abel S, Möllers C, Becker H (2005) Development of synthetic Brassica napus lines for the analysis of “fixed heterosis” in allopolyploid plants. Euphytica 146:157–163
Ahmar S, Gill RA, Jung K-H, Faheem A, Qasim MU, Mubeen M, Zhou W (2020) Conventional and molecular techniques from simple breeding to speed breeding in crop plants: recent advances and future outlook. Int J Mol Sci 21:2590
Baker RL, Yarkhunova Y, Vidal K, Ewers BE, Weinig C (2017) Polyploidy and the relationship between leaf structure and function: implications for correlated evolution of anatomy, morphology, and physiology in Brassica. BMC Plant Biol 17:3
Balao F, Herrera J, Talavera S (2011) Phenotypic consequences of polyploidy and genome size at the microevolutionary scale: a multivariate morphological approach. New Phytol 192:256–265
Barker MS, Arrigo N, Baniaga AE, Li Z, Levin DA (2016) On the relative abundance of autopolyploids and allopolyploids. New Phytol 210:391–398
Bernacchi D, Tanksley SD (1997) An interspecific backcross of Lycopersicon esculentum×L. hirsutum: linkage analysis and a QTL study of sexual compatibility factors and floral traits. Genetics 147:861–877
Bhullar R, Nagarajan R, Bennypaul H, Sidhu GK, Sidhu G, Rustgi S, von Wettstein D, Gill KS (2014) Silencing of a metaphase I-specific gene results in a phenotype similar to that of the Pairing homeologous 1 (Ph1) gene mutations. Proc Natl Acad Sci USA 111:14187–14192
Bing DJ, Downey RK, Rakow GFW (1996) Hybridizations among Brassica napus, B. rapa and B. juncea and their two weedy relatives B. nigra and Sinapis arvensis under open pollination conditions in the field. Plant Breed 115:470–473
Bretagnolle F, Thompson JD (1995) Tansley Review No. 78 Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolypoid plants. New Phytol 129:1–22
Chang S, Yang T, Du T, Huang Y, Chen J, Yan J, He J, Guan R (2011) Mitochondrial genome sequencing helps show the evolutionary mechanism of mitochondrial genome formation in Brassica. BMC Genom 12:497
Chatterjee D, Banga S, Gupta M, Bharti S, Salisbury PA, Banga SS (2016) Resynthesis of Brassica napus through hybridization between B. juncea and B. carinata. Theor Appl Genet 129:977–990
Chen J-P, Ge X-H, Yao X-C, Feng Y-H, Li Z-Y (2011a) Synthesis and characterization of interspecific trigenomic hybrids and allohexaploids between three cultivated Brassica allotetraploids and wild species Brassica fruticulosa. Afr J Biotechnol 10:12171–12176
Chen S, Nelson MN, Chèvre A-M, Jenczewski E, Li Z, Mason AS, Meng J, Plummer JA, Pradhan A, Siddique KHM, Snowdon RJ, Yan G, Zhou W, Cowling WA (2011b) Trigenomic bridges for Brassica improvement. Crit Rev Plant Sci 30:524–547
Cheng F, Sun R, Hou X, Zheng H, Zhang F, Zhang Y, Liu B, Liang J, Zhuang M, Liu Y et al (2016) Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat Genet 48:1218–1224
Chiang MS, Chiang BY, Grant WF (1977) Transfer of resistance to race 2 of Plasmodiophora brassicae from Brassica napus to cabbage (B. oleracea var. capitata) I. Interspecific hybridization between B. napus and B. oleracea var. capitata. Euphytica 26:319–336
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846
De Storme N, Geelen D (2013) Sexual polyploidization in plants-cytological mechanisms and molecular regulation. New Phytol 198:670–684
De Storme N, Mason AS (2014) Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr Plant Biol 1:10–33
Dhaliwal I, Mason AS, Banga S, Bharti S, Kaur B, Gurung AM, Salisbury PA, Batley J, Banga SS (2017) Cytogenetic and molecular characterization of B-genome introgression lines of Brassica napus L. G3 (Bethesda) 7:77–86
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87:81–89
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult 104:359–373
Ding M, Chen ZJ (2018) Epigenetic perspectives on the evolution and domestication of polyploid plant and crops. Curr Opin Plant Biol 42:37–48
Ding Y, Mei J, Li Q, Liu Y, Wan H, Wang L, Becker HC, Qian W (2013) Improvement of Sclerotinia sclerotiorum resistance in Brassica napus by using B. oleracea. Genet Resour Crop Evol 60:1615–1619
Dubcovsky J, Dvorak J (2007) Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316:1862–1866
FitzJohn RG, Armstrong TT, Newstrom-Lloyd LE, Wilton AD, Cochrane M (2007) Hybridisation within Brassica and allied genera: evaluation of potential for transgene escape. Euphytica 158:209–230
Gaebelein R, Mason AS (2018) Allohexaploids in the genus Brassica. Crit Rev Plant Sci 37:422–437
Gaebelein R, Alnajar D, Koopmann B, Mason AS (2019a) Hybrids between Brassica napus and B. nigra show frequent pairing between the B and A/C genomes and resistance to blackleg. Chromosome Res 27:221–236
Gaebelein R, Schiessl SV, Samans B, Batley J, Mason AS (2019b) Inherited allelic variants and novel karyotype changes influence fertility and genome stability in Brassica allohexaploids. New Phytol 223:965–978
Geng XX, Chen S, Astarini IA, Yan GJ, Tian E, Meng J, Li ZY, Ge XH, Nelson MN, Mason AS, Pradhan A, Zhou WJ, Cowling WA (2013) Doubled haploids of novel trigenomic Brassica derived from various interspecific crosses. Plant Cell Tissue Organ Cult 113:501–511
Gonzalo A, Lucas M-O, Charpentier C, Sandmann G, Lloyd A, Jenczewski E (2019) Reducing MSH4 copy number prevents meiotic crossovers between non-homologous chromosomes in Brassica napus. Nat Commun 10:2354
Griffiths S, Sharp R, Foote TN, Bertin I, Wanous M, Reader S, Colas I, Moore G (2006) Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature 439:749–752
Griffiths AG, Moraga R, Tausen M, Gupta V, Bilton TP, Campbell MA, Ashby R, Nagy I, Khan A, Larking A, Anderson C, Franzmayr B, Hancock K, Scott A, Ellison NW, Cox MP, Asp T, Mailund T, Schierup MH, Andersen SU (2019) Breaking free: the genomics of allopolyploidy-facilitated niche expansion in white clover. Plant Cell 31:1466–1487
Gu HH, Hagberg P, Zhou WJ (2004) Cold pretreatment enhances microspore embryogenesis in oilseed rape (Brassica napus L.). Plant Growth Regul 42:137–143
Gupta PK, Priyadarshan PM (1982) Triticale: present status and future prospects. Adv Genet 21:255–345
Gupta M, Atri C, Agarwal N, Banga SS (2016) Development and molecular-genetic characterization of a stable Brassica allohexaploid. Theor Appl Genet 129:2085–2100
Hansen LB, Siegismund HR, Jørgensen RB (2001) Introgression between oilseed rape (Brassica napus L.) and its weedy relative B. rapa L. in a natural population. Genet Resour Crop Evol 48:621–627
Heinberg R, Lerch D (2010) The post carbon reader: managing the 21st century’s sustainability crisis. Post Carbon Institute, Santa Rosa
Hickey LT, Hafeez AN, Robinson H, Jackson SA, Leal-Bertioli SCM, Tester M, Gao C, Godwin ID, Hayes BJ, Wulff BBH (2019) Breeding crops to feed 10 billion. Nat Biotechnol 37:744–754
Higgins EE, Howell EC, Armstrong SJ, Parkin IAP (2021) A major quantitative trait locus on chromosome A9, BnaPh1, controls homoeologous recombination in Brassica napus. New Phytol 229:3281–3293
Hu D, Zhang W, Zhang Y, Chang S, Chen L, Chen Y, Shi Y, Shen J, Meng J, Zou J (2019) Reconstituting the genome of a young allopolyploid crop, Brassica napus, with its related species. Plant Biotechnol J 17:1106–1118
Hu D, Zhao Y, Shen J, He X, Zhang Y, Jiang Y, Snowdon R, Meng J, Reif JC, Zou J (2021) Genome-wide prediction for hybrids between parents with distinguished difference on exotic introgressions in Brassica napus. Crop J. https://doi.org/10.1016/j.cj.2020.11.002
Jenczewski E, Eber F, Grimaud A, Huet S, Lucas MO, Monod H, Chèvre AM (2003) PrBn, a major gene controlling homeologous pairing in oilseed rape (Brassica napus) haploids. Genetics 164:645–653
Jiang Y, Tian E, Li R, Chen L, Meng J (2007) Genetic diversity of Brassica carinata with emphasis on the interspecific crossability with B. rapa. Plant Breed 126:487–491
Jiang J, Rong H, Ran L, Fan H, Kong Y, Wang Y (2018) Creating favourable morphological and yield variations for rapeseed by interspecific crosses between Brassica rapa and Brassica oleracea. Plant Breed 137:621–628
Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, dePamphilis CW (2011) Ancestral polyploidy in seed plants and angiosperms. Nature 473:97–100
Katche E, Quezada-Martinez D, Katche EI, Vasquez-Teuber P, Mason AS (2019) Interspecific hybridization for Brassica crop improvement. Crop Breed Genet Genom 1:e190007
Katche E, Gaebelein R, Idris Z, Vasquez-Teuber P, Lo Y, Nugent D, Batley J, Mason AS (2021) Stable, fertile lines produced by hybridization between allotetraploids Brassica juncea (AABB) and Brassica carinata (BBCC) have merged the A and C genomes. New Phytol 230:1242–1257
Kemp BP, Doughty J (2003) Just how complex is the Brassica S-receptor complex? J Exp Bot 54:157–168
Kim S, Rayburn AL, Boe A, Lee DK (2012) Neopolyploidy in Spartina pectinata Link: 1. Morphological analysis of tetraploid and hexaploid plants in a mixed natural population. Plant Syst Evol 298:1073–1083
Kumari P, Bisht DS, Bhat SR (2018) Stable, fertile somatic hybrids between Sinapis alba and Brassica juncea show resistance to Alternaria brassicae and heat stress. Plant Cell Tissue Organ Cult 133:77–86
Leitch AR, Leitch IJ (2008) Genomic plasticity and the diversity of polyploid plants. Science 320:481–483
Li M, Qian W, Meng J, Li Z (2004) Construction of novel Brassica napus accessions through chromosomal substitution and elimination using interploid species hybridization. Chromosome Res 12:417–426
Li Q, Chen Y, Yue F, Qian W, Song H (2018) Microspore culture reveals high fitness of B. napus-like gametes in an interspecific hybrid between Brassica napus and B. oleracea. PLoS ONE 13:e0193548
Liu Z, Adamczyk K, Manzanares-Dauleux M, Eber F, Lucas M-O, Delourme R, Chèvre AM, Jenczewski E (2006) Mapping PrBn and other quantitative trait loci responsible for the control of homeologous chromosome pairing in oilseed rape (Brassica napus L.) haploids. Genetics 174:1583–1596
Liu S, Fan C, Li J, Cai G, Yang Q, Wu J, Yi X, Zhang C, Zhou Y (2016) A genome-wide association study reveals novel elite allelic variations in seed oil content of Brassica napus. Theor Appl Genet 129:1203–1215
Lloyd A, Bomblies K (2016) Meiosis in autopolyploid and allopolyploid Arabidopsis. Curr Opin Plant Biol 30:116–122
Lloyd AH, Ranoux M, Vautrin S, Glover N, Fourment J, Charif D, Choulet F, Lassalle G, Marande W, Tran J, Granier F, Pingault L, Remay A, Marquis C, Belcram H, Chalhoub B, Feuillet C, Bergès H, Sourdille P, Jenczewski E (2014) Meiotic gene evolution: can you teach a new dog new tricks? Mol Biol Evol 31:1724–1727
Longin CFH, Reif JC (2014) Redesigning the exploitation of wheat genetic resources. Trends Plant Sci 19:631–636
Majdi M, Karimzadeh G, Malboobi MA, Omidbaigi R, Mirzaghaderi G (2010) Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): morphological, physiological, cytological and phytochemical changes. HortScience 45:16–21
Marchant DB, Soltis DE, Soltis PS (2016) Patterns of abiotic niche shifts in allopolyploids relative to their progenitors. New Phytol 212:708–718
Mason AS, Yan G, Cowling WA, Nelson MN (2012) A new method for producing allohexaploid Brassica through unreduced gametes. Euphytica 186:277–287
Mason AS, Batley J, Bayer PE, Hayward A, Cowling WA, Nelson MN (2014) High-resolution molecular karyotyping uncovers pairing between ancestrally-related Brassica chromosomes. New Phytol 202:964–974
McCarthy EW, Landis JB, Kurti A, Lawhorn AJ, Chase MW, Knapp S, Le Comber SC, Leitch AR, Litt A (2019) Early consequences of allopolyploidy alter floral evolution in Nicotiana (Solanaceae). BMC Plant Biol 19:162
Meng J, Shi S, Gan L, Li Z, Qu X (1998) The production of yellow-seeded Brassica napus (AACC) through crossing interspecific hybrids of B. campesrtis (AA) and B. carinata (BBCC) with B. napus. Euphytica 103:329–333
Morinaga T (1934) Interspecific hybridisation in Brassica VI. The cytology of F1 hybrids of B. juncea and B. nigra. Cytologia 6:62–67
Mwathi MW, Gupta M, Atri C, Banga SS, Batley J, Mason AS (2017) Segregation for fertility and meiotic stability in novel Brassica allohexaploids. Theor Appl Genet 130:767–776
Mwathi MW, Schiessl SV, Batley J, Mason AS (2019) “Doubled-haploid” allohexaploid Brassica lines lose fertility and viability and accumulate genetic variation due to genomic instability. Chromosoma 128:521–532
Mwathi MW, Gupta M, Quezada-Martinez D, Pradhan A, Batley J, Mason AS (2020) Fertile allohexaploid Brassica hybrids obtained from crosses between B. oleracea and B. juncea via ovule rescue and colchicine treatment of cuttings. Plant Cell Tissue Organ Cult 140:301–313
Niemann J, Kotlarski S, Wojciechowski A (2014) The evaluation of self-incompatibility and crossability in choosen Brassica species based on the observation of pollen tubes growth and seed set. Acta Sci Pol Agric 13:51–59
Nishiyama I, Sarashima M, Matsuzawa Y (1991) Critical discussion on abortive interspecific crosses in Brassica. Plant Breed 107:288–302
O’Mara JG (1953) The cytogenetics of Triticale. Bot Rev 10:587–605
Oettler G (2005) The fortune of a botanical curiosity - Triticale: past, present and future. J Agric Sci 143:329–346
Osman K, Higgins JD, Sanchez-Moran E, Armstrong SJ, Franklin FCH (2011) Pathways to meiotic recombination in Arabidopsis thaliana. New Phytol 190:523–544
Paritosh K, Gupta V, Yadava SK, Singh P, Pradhan AK, Pental D (2014) RNA-seq based SNPs for mapping in Brassica juncea (AABB): synteny analysis between the two constituent genomes A (from B. rapa) and B (from B. nigra) shows highly divergent gene block arrangement and unique block fragmentation patterns. BMC Genom 15:396
Paritosh K, Pradhan AK, Pental D (2020a) A highly contiguous genome assembly of Brassica nigra (BB) and revised nomenclature for the pseudochromosomes. BMC Genom 21:887
Paritosh K, Yadava SK, Singh P, Bhayana L, Mukhopadhyay A, Gupta V, Bisht NC, Zhang J, Kudrna D, Copetti D, Wing RA, Lachagari VBR, Pradhan AK, Penta D (2020b) A chromosome-scale assembly of allotetraploid Brassica juncea (AABB) elucidates comparative architecture of the A and B genomes. Plant Biotechnol J 19:602–614
Parkin IAP, Koh C, Tang H, Robinson SJ, Kagale S, Clarke WE, Town CD, John N, Krishnakumar V, Bidwell SL, Denoeud F, Belcram H et al (2014) Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol 15:R77
Pelé A, Rousseau-Gueutin M, Chèvre A-M (2018) Speciation success of polyploid plants closely relates to the regulation of meiotic recombination. Front Plant Sci 9:907
Pires JC, Conant GC (2016) Robust yet fragile: expression noise, protein misfolding, and gene dosage in the evolution of genomes. Annu Rev Genet 50:113–131
Pradhan A, Plummer JA, Nelson MN, Cowling WA, Yan G (2010) Trigenomic hybrids from interspecific crosses between Brassica napus and B. nigra. Crop Pasture Sci 61:464–474
Prakash S, Takahata Y, Kirti PB, Chopra VL (1999) Cytogenetics. In: Gómez-Campo C (ed) Biology of Brassica coenospecies. Elsevier Science Publishers BV, Amsterdam, pp 59–106
Qin H, Zhang W, Wang M, Xiong S, Hu D, Sun X, Hu L, Meng J, Zou J (2019) Characterizing glucosinolates of four Brassica species and interspecific transferring of specific glucosinolates. J Plant Genet Resour 21:94–104 (in Chinese)
Rahman MH (2001) Production of yellow-seeded Brassica napus through interspecific crosses. Plant Breed 120:463–472
Rahman MH (2002) Fatty acid composition of resynthesized Brassica napus and trigenomic Brassica void of genes for erucic acid in their A genomes. Plant Breed 121:357–359
Raman R, Qiu Y, Coombes N, Song J, Kilian A, Raman H (2017) Molecular diversity analysis and genetic mapping of pod shatter resistance loci in Brassica carinata L. Front Plant Sci 8:1765
Ramsey J, Schemske DW (2002) Neopolyploidy in flowering plants. Annu Rev Ecol Syst 33:589–639
Samans B, Chalhoub B, Snowdon RJ (2017) Surviving a genome collision: genomic signatures of allopolyploidization in the recent crop species Brassica napus. Plant Genome-US. https://doi.org/10.3835/plantgenome2017.02.0013
Scheffler JA, Dale PJ (1994) Opportunities for gene transfer from transgenic oilseed rape (Brassica napus) to related species. Transgenic Res 3:263–278
Schelfhout CJ, Snowdon R, Cowling WA, Wroth JM (2006) Tracing B-genome chromatin in Brassica napus × B. juncea interspecific progeny. Genome 49:1490–1497
Schiessl S-V, Katche E, Ihien E, Chawla HS, Mason AS (2018) The role of genomic structural variation in the genetic improvement of polyploid crops. Crop J 7:127–140
Schranz ME, Osborn TC (2000) Novel flowering time variation in the resynthesized polyploid Brassica napus. J Hered 91:242–246
Sharma BB, Kalia P, Singh D, Sharma TR (2017) Introgression of black rot resistance from Brassica carinata to cauliflower (Brassica oleracea botrytis group) through embryo rescue. Front Plant Sci 8:1255
Soltis PS, Soltis DE (2000) The role of genetic and genomic attributes in the success of polyploids. Proc Natl Acad Sci USA 97:7051–7057
Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng C, Sankoff D, dePamphilis CW, Wall PK, Soltis PS (2009) Polyploidy and angiosperm diversification. Am J Bot 96:336–348
Song Q, Chen ZJ (2015) Epigenetic and developmental regulation in plant polyploids. Curr Opin Plant Biol 24:101–109
Song K, Lu P, Tang K, Osborn TC (1995) Rapid genome change in synthetic polyploids of Brassica and its implications for polyploid evolution. Proc Natl Acad Sci USA 92:7719–7723
Song J-M, Liu D-X, Xie W-Z, Yang Z, Guo L, Liu K, Yang Q-Y, Chen L-L (2021) BnPIR: Brassica napus pan-genome information resource for 1689 accessions. Plant Biotechnol J 19:412–414
Stebbins GL (1950) Variation and evolution in plants. Oxford University Press, London
Szadkowski E, Eber F, Huteau V, Lodé M, Coriton O, Jenczewski E, Chèvre AM (2011) Polyploid formation pathways have an impact on genetic rearrangements in resynthesized Brassica napus. New Phytol 191:884–894
Tank DC, Eastman JM, Pennell MW, Soltis PS, Soltis DE, Hinchliff CE, Brown JW, Sessa EB, Harmon LJ (2015) Nested radiations and the pulse of angiosperm diversification: increased diversification rates often follow whole genome duplications. New Phytol 207:454–467
Tanksley SD, McCouch SR (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Teng C, Niu Y, Du D, Yu Q, Zhao Z (2018) Production and genetic analyses of novel Brassica rapa L. introgressions from interspecific crosses with Brassica juncea L. landraces native to the Qinghai-Tibet Plateau. Euphytica 214:23
Tester M, Langridge P (2010) Breeding technologies to increase crop production in a changing world. Science 327:818–822
Tian E, Jiang Y, Chen L, Zou J, Liu F, Meng J (2010) Synthesis of a Brassica trigenomic allohexaploid (B. carinata × B. rapa) de novo and its stability in subsequent generations. Theor Appl Genet 121:1431–1440
U N, (1935) Genome-analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn J Bot 7:389–452
Udagawa H, Ishimaru Y, Li F, Sato Y, Kitashiba H, Nishio T (2010) Genetic analysis of interspecific incompatibility in Brassica rapa. Theor Appl Genet 121:689–696
Wall JR, York TL (1960) Gametic diversity as aid to interspecific hybridization in Phaseolus and in Cucurbita. Proc Am Soc Hort Sci 75:419–428
Wang X et al (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1039
Wang A, Kang L, Li P, Li Z (2016) Review on new germplasm development in Brassica napus through hybridization in China (in Chineae). Chin J Oil Crop Sci 38:691–698
Washburn JD, Birchler JA (2014) Polyploids as a “model system” for the study of heterosis. Plant Reprod 27:1–5
Wei Z, Wang M, Chang S, Wu C, Liu P, Meng J, Zou J (2016) Introgressing subgenome components from Brassica rapa and B.carinata to B.juncea for broadening its genetic base and exploring intersubgenomic heterosis. Front Plant Sci 17:1677
Wei N, Cronn R, Liston A, Ashman T-L (2019) Functional trait divergence and trait plasticity confer polyploid advantage in heterogeneous environments. New Phytol 221:2286–2297
Xiao J, Grandillo S, Ahn SN, McCouch SR, Tanksley SD, Li J, Yuan L (1996) Genes from wild rice improve yield. Nature 384:223–224
Xiong Z, Gaeta RT, Pires JC (2011) Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus. Proc Natl Acad Sci USA 108:7908–7913
Xu L, Najeeb U, Tang GX, Gu HH, Zhang GQ, He H, Zhou WJ (2007) Haploid and doubled haploid technology. Adv Bot Res 45:181–216
Yang S, Vanderbeld B, Wan J, Huang Y (2010) Narrowing down the targets: towards successful genetic engineering of drought-tolerant crops. Mol Plant 3:469–490
Yang J, Liu D, Wang X, Ji C, Cheng F, Liu B, Hu Z, Chen S, Pental D, Ju Y, Yao P, Li X, Xie K, Zhang J, Wang J, Liu F, Ma W, Shopan J, Zheng H, Mackenzie SA, Zhang M (2016a) The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat Genet 48:1225–1232
Yang S, Chen S, Geng XX, Yan G, Li ZY, Meng JL, Cowling WA, Zhou WJ (2016b) The first genetic map of a synthesised allohexaploid Brassica with A, B and C genomes based on simple sequence repeat markers. Theor Appl Genet 129:689–701
Yang S, Chen S, Zhang K, Li L, Yin Y, Gill RA, Yan G, Meng J, Cowling WA, Zhou W (2018) A high-density genetic map of an allohexaploid Brassica doubled haploid population reveals quantitative trait loci for pollen viability and fertility. Front Plant Sci 9:1161
Yang S, Gill RA, Zaman QU, Ulhassan Z, Zhou W (2020a) Insights on SNP types, detection methods and their utilization in Brassica species: Recent progress and future perspectives. J Biotechnol 324:11–20
Yang Y, Yan G, Li Z, Yuan J, Wei X, Wei F, Tian B, Xie Z, Shi G, Zhang X, Cao G (2020b) Cytological atlas at meiosis reveals insights into pollen fertility in synthetic Brassica allotriploids between allotetraploid B. carinata and diploid B. rapa. Plant Physiol Biochem 148:237–245
Yao X, Ge X, Li Z (2012) Different fertility and meiotic regularity in allohexaploids derived from trigenomic hybrids between three cultivated Brassica allotetraploids and B. maurorum. Plant Cell Rep 31:781–788
Zhang GQ, Zhou WJ, Gu HH, Song WJ, Momoh EJJ (2003) Plant regeneration from the hybridization of Brassica juncea and B. napus through embryo culture. J Agron Crop Sci 189:347–350
Zhang GQ, Tang GX, Song WJ, Zhou WJ (2004) Resynthesizing Brassica napus from interspecific hybridization between Brassica rapa and B. oleracea through ovary culture. Euphytica 140:181–187
Zhang X, Liu T, Li X, Duan M, Wang J, Qiu Y, Wang H, Song J, Shen D (2016) Interspecific hybridization, polyploidization, and backcross of Brassica oleracea var. alboglabra with B. rapa var. purpurea morphologically recapitulate the evolution of Brassica vegetables. Sci Rep 6:18618
Zhang L, Cai X, Wu J, Liu M, Grob S, Cheng F, Liang J, Cai C, Liu Z, Liu B, Wang F, Li S, Liu F, Li X, Cheng L, Yang W, Li M, Grossniklaus U, Zheng H, Wang X (2018) Improved Brassica rapa reference genome by single-molecule sequencing and chromosome conformation capture technologies. Hortic Res 5:50
Zhao J, Wang X, Deng B, Lou P, Wu J, Sun R, Xu Z, Vromans J, Koornneef M, Bonnema G (2005) Genetic relationships within Brassica rapa as inferred from AFLP fingerprints. Theor Appl Genet 110:1301–1314
Zhao Q, Zou J, Meng J, Mei S, Wang J (2013) Tracing the transcriptomic changes in synthetic trigenomic allohexaploids of Brassica using an RNA-Seq approach. PLoS ONE 8:e68883
Zhou J, Tan C, Cui C, Ge X, Li Z (2016) Distinct subgenome stabilities in synthesized Brassica allohexaploids. Theor Appl Genet 129:1257–1271
Zhu JS, Struss D, Röbbelen G (1993) Studies on resistance to Phoma lingam in Brassies napus-Brassica nigra addition lines. Plant Breed 111:192–197
Zou J, Zhu J, Huang S, Tian E, Xiao Y, Fu D, Tu J, Fu T, Meng J (2010) Broadening the avenue of intersubgenomic heterosis in oilseed Brassica. Theor Appl Genet 120:283–290
Zou J, Fu D, Gong H, Qian W, Xia W, Pires JC, Li R, Long Y, Mason AS, Yang T-J, Lim YP, Park BS, Meng J (2011) De novo genetic variation associated with retrotransposon activation, genomic rearrangements and trait variation in a recombinant inbred line population of Brassica napus derived from interspecific hybridization with Brassica rapa. Plant J 68:212–224
Zou J, Hu D, Mason AS, Shen X, Wang X, Wang N, Grandke F, Wang M, Chang S, Snowdon RJ, Meng J (2018) Genetic changes in a novel breeding population of Brassica napus synthesized from hundreds of crosses between B. rapa and B. carinata. Plant Biotechnol J 16:507–519
Acknowledgements
This review paper was based on discussion in meetings held at the International Rapeseed Congress in Berlin in June 2019 and also at Justus Liebig University after the Congress. The co-authors from China and Germany were supported for their work by the National Key Research and Development Program of China (2018YFD0100601), the Sino-German Research Project (GZ 1362; DFG MA6473/3-1), the Science and Technology Department of Zhejiang Province (14th 5-Year New Oil Crops Breeding), the Natural Science Foundation of Zhejiang Province (LQ20C130006), and the Jiangsu Collaborative Innovation Center for Modern Crop Production (JCIC-MCP). The Mason lab is partially funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy–EXC 2070–390732324.
Author information
Authors and Affiliations
Contributions
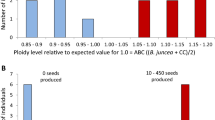
KZ, ASM, JZ, DQM and WZ conceptualized the manuscript; KZ, ASM, MAF, JZ and WZ drafted the manuscript; DQM and ASM prepared Fig. 1, KZ and FI prepared Fig. 2, KZ, DQM and DH prepared Fig. 3, and KZ and SY prepared Table 1. ASM, DQM, JZ and WZ contributed to critical revisions of the manuscript. All authors approved the final version for submission.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared that they have no competing interest.
Additional information
Communicated by Rajeev K. Varshney.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, K., Mason, A.S., Farooq, M.A. et al. Challenges and prospects for a potential allohexaploid Brassica crop. Theor Appl Genet 134, 2711–2726 (2021). https://doi.org/10.1007/s00122-021-03845-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-021-03845-8