Abstract
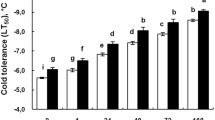
The exploitation of symbiotic interactions between fungi and plants, coupled with the application of osmoprotectants such as trehalose (Tre), presents a promising strategy for mitigating environmental stress. To determine the mechanism of Serendipita indica and Tre-mediated cold stress tolerance, a comparative experiment was designed to study the impact of S. indica, Tre and their combination on tomato plants grown under cold stress. The results showed that cold stress significantly decreased biomass, relative water content, photosynthetic pigments and elements concomitantly with increasing antioxidant activities, malondialdehyde (MDA), electrolyte leakage, hydrogen peroxide and proline content. Meanwhile, S. indica and Tre treatments promoted biomass and enhanced carbohydrate, protein, proline, potassium, phosphorous, antioxidant enzymes and photosynthetic pigments content under cold stress. Furthermore, single or dual application of endophyte and Tre mitigated physiological disorders induced by cold stress and increased the integrity of cell membranes by decreasing hydrogen peroxide, MDA, and electrolyte leakage (EL). Our findings suggest that S. indica and Tre combination could significantly promote cold stress tolerance compared with single treatment. This study is novel in showing the cold adaptation of tomato plants by combination use of S. indica and Tre, which can be a promising strategy for improving cold tolerance. The underlying molecular mechanisms of sugar–fungus interaction must be further investigated.





Similar content being viewed by others
Data Availability
The data presented in this study are available upon request from the corresponding author.
Code Availability
Not applicable.
References
Liu T et al (2020) H2O2 and NO are involved in trehalose-regulated oxidative stress tolerance in cold-stressed tomato plants. Environ Exp Bot 171:103961. https://doi.org/10.1016/j.envexpbot.2019.103961
Dreyer A, Dietz K-J (2018) Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 7(11):169
Wang M, Zhang S, Ding F (2020) Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants 9(3):218. https://doi.org/10.3390/antiox9030218
Fernandez O et al (2010) Trehalose and plant stress responses: friend or foe? Trends Plant Sci 15(7):409–417
Kosar F et al (2019) Trehalose: a key organic osmolyte effectively involved in plant abiotic stress tolerance. J Plant Growth Regul 38(2):606–618. https://doi.org/10.1007/s00344-018-9876-x
Ding F, Wang R (2018) Amelioration of postharvest chilling stress by trehalose in pepper. Sci Hortic 232:52–56. https://doi.org/10.1016/j.scienta.2017.12.053
Paul MJ et al (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59:417–441. https://doi.org/10.1146/annurev.arplant.59.032607.092945
Zhu X, Song F, Liu F (2017) Arbuscular mycorrhizal fungi and tolerance of temperature stress in plants. In: Wu Q-S (ed) Arbuscular mycorrhizas and stress tolerance of plants. Springer, Singapore, pp 163–194
Jafari M et al (2018) Inoculation and co-inoculation of alfalfa seedlings with root growth promoting microorganisms (Piriformospora indica, Glomus intraradices and Sinorhizobium meliloti) affect molecular structures, nutrient profiles and availability of hay for ruminants. Anim Nutr 4(1):90–99. https://doi.org/10.1016/j.aninu.2017.08.008
Jangir P et al (2021) Role of Serendipita indica in enhancing drought tolerance in crops. Physiol Mol Plant Pathol 116:101691. https://doi.org/10.1016/j.pmpp.2021.101691
Azizi M, Fard EM, Ghabooli M (2021) Piriformospora indica affect drought tolerance by regulation of genes expression and some morphophysiological parameters in tomato (Solanum lycopersicum L.). Sci Hortic 287:110260. https://doi.org/10.1016/j.scienta.2021.110260
Abdelaziz ME et al (2019) Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci Hortic 256:108532. https://doi.org/10.1016/j.scienta.2019.05.059
Karimi R, Amini H, Ghabooli M (2022) Root endophytic fungus Piriformospora indica and zinc attenuate cold stress in grapevine by influencing leaf phytochemicals and minerals content. Sci Hortic 293:110665. https://doi.org/10.1016/j.scienta.2021.110665
Ghabooli M, Kaboosi E (2022) Alleviation of the adverse effects of drought stress using a desert adapted endophytic fungus and glucose in tomato. Rhizosphere 21:100481. https://doi.org/10.1016/j.rhisph.2022.100481
Barrs H, Weatherley P (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15(3):413–428
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1. https://doi.org/10.1104/pp.24.1.1
Bao A-K et al (2009) Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci 176(2):232–240. https://doi.org/10.1016/j.plantsci.2008.10.009
Ershadi A, Karimi R, Mahdei KN (2016) Freezing tolerance and its relationship with soluble carbohydrates, proline and water content in 12 grapevine cultivars. Acta Physiol Plant 38(1):2
Velikova V, Loreto F (2005) On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ 28(3):318–327
Dubois M, Gilles KA, Hamilton JK, Smith RPA, F, (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207. https://doi.org/10.1007/BF00018060
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59(2):309–314. https://doi.org/10.1104/pp.59.2.309
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Elsevier, pp 764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
Ferreira LC et al (2010) Nitric oxide reduces oxidative stress generated by lactofen in soybean plants. Pestic Biochem Physiol 97(1):47–54. https://doi.org/10.1016/j.pestbp.2009.12.003
Abdel-Shafy HI, Hegemann W, Teiner A (1994) Accumulation of metals by vascular plants. Environ Manag Health 5(2):21–24. https://doi.org/10.1108/09566169410057137
Ahmadvand G, Hajinia S (2018) Effect of endophytic fungus Piriformospora indica on yield and some physiological traits of millet (Panicum miliaceum) under water stress. Crop Pasture Sci 69(6):594–605. https://doi.org/10.1071/CP17364
Su Z-z et al (2017) Piriformospora indica promotes growth, seed yield and quality of Brassica napus L. Microbiol Res 199:29–39
Kovi MR, Ergon Å, Rognli OA (2016) Freezing tolerance revisited—effects of variable temperatures on gene regulation in temperate grasses and legumes. Curr Opin Plant Biol 33:140–146. https://doi.org/10.1016/j.pbi.2016.07.006
Liu T et al (2021) Trehalose triggers hydrogen peroxide and nitric oxide to participate in melon seedlings oxidative stress tolerance under cold stress. Environ Exp Bot 184:104379. https://doi.org/10.1016/j.envexpbot.2021.104379
Paul MJ, Watson A, Griffiths CA (2020) Trehalose 6-phosphate signalling and impact on crop yield. Biochem Soc Trans 48(5):2127–2137. https://doi.org/10.1042/bst20200286
Prasad R et al (2013) Root endophyte Piriformospora indica DSM 11827 alters plant morphology, enhances biomass and antioxidant activity of medicinal plant Bacopa monniera. J Basic Microbiol 53(12):1016–1024. https://doi.org/10.1002/jobm.201200367
Saksena HB et al (2020) The versatile role of glucose signalling in regulating growth, development and stress responses in plants. J Plant Biochem Biotechnol. https://doi.org/10.1007/s13562-020-00614-4
Akram NA et al (2016) Trehalose pretreatment induces drought tolerance in radish (Raphanus sativus L.) plants: some key physio-biochemical traits. Acta Physiol Plant 38(1):1–10. https://doi.org/10.1007/s11738-015-2018-1
Ghabooli M et al (2020) Effect of Piriformospora indica inoculation on some morphophysiological parameters in licorice (Glycyrrhiza glabra L.) under drought stress. Iran J Plant Physiol 10(4):3379–3389. https://doi.org/10.30495/ijpp.2020.677198
Ali Q, Ashraf M (2011) Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. J Agron Crop Sci 197(4):258–271. https://doi.org/10.1111/j.1439-037X.2010.00463.x
Abdallah MMS, Abdelgawad ZA, El-Bassiouny HMS (2016) Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. S Afr J Bot 103:275–282. https://doi.org/10.1016/j.sajb.2015.09.019
Khalid M et al (2018) An endosymbiont Piriformospora indica reduces adverse effects of salinity by regulating cation transporter genes, phytohormones, and antioxidants in Brassica campestris ssp. chinensis. Environ Exp Bot 153:89–99. https://doi.org/10.1016/j.envexpbot.2018.05.007
Nanda R, Agrawal V (2018) Piriformospora indica, an excellent system for heavy metal sequestration and amelioration of oxidative stress and DNA damage in Cassia angustifolia Vahl under copper stress. Ecotoxicol Environ Saf 156:409–419. https://doi.org/10.1016/j.ecoenv.2018.03.016
Sadak MS (2019) Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull Natl Res Centre 43(1):1–10. https://doi.org/10.1186/s42269-019-0098-6
Mitra D et al (2021) Amelioration of thermal stress in crops by plant growth-promoting rhizobacteria. Physiol Mol Plant Pathol 115:101679. https://doi.org/10.1016/j.pmpp.2021.101679
Pradhan S et al (2019) Low temperature stress induced physiological and biochemical alterations in papaya genotypes. S Afr J Bot 123:133–141. https://doi.org/10.1016/j.sajb.2019.02.004
Kaplan F et al (2007) Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J 50(6):967–981. https://doi.org/10.1111/j.1365-313X.2007.03100.x
Mesa T et al (2022) Differential physiological response to heat and cold stress of tomato plants and its implication on fruit quality. J Plant Physiol 268:153581. https://doi.org/10.1016/j.jplph.2021.153581
Tsai H-J et al (2020) Piriformospora indica symbiosis improves water stress tolerance of rice through regulating stomata behavior and ROS scavenging systems. Plant Signal Behav 15(2):1722447. https://doi.org/10.1080/15592324.2020.1722447
Luo Y et al (2010) Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol Plant 54(3):495–501. https://doi.org/10.1007/s10535-010-0087-y
Sadak MS, El-Bassiouny HMS, Dawood MG (2019) Role of trehalose on antioxidant defense system and some osmolytes of quinoa plants under water deficit. Bull Natl Res Centre 43(1):5. https://doi.org/10.1186/s42269-018-0039-9
Ngwene B et al (2016) Phosphate utilization by the fungal root endophyte Piriformospora indica. Plant Soil 405(1):231–241
Yang L et al (2014) Exogenous trehalose largely alleviates ionic unbalance, ROS burst, and PCD occurrence induced by high salinity in Arabidopsis seedlings. Front Plant Sci. https://doi.org/10.3389/fpls.2014.00570
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
MG and RK conceived and designed the main content. EK conducted the experiments. MG and EK wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaboosi, E., Ghabooli, M. & Karimi, R. Combined Effect of Trehalose and Serendipita indica Inoculation Might Participate in Solanum lycopersicum Induced Cold Tolerance. Curr Microbiol 80, 224 (2023). https://doi.org/10.1007/s00284-023-03335-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03335-8