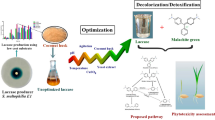
Abstract
Chlorophenols are widely used in industry and are known environmental pollutants. The degradation of chlorophenols is important for environmental remediation. In this study, we evaluated the biodegradation of 2-chlorophenol using crude laccase produced by Myrothecium verrucaria. Atmospheric and room temperature plasma technology was used to increase laccase production. The culture conditions of the M-6 mutant were optimized. Our results showed that corn stover could replace glucose as a carbon source and promote laccase production. The maximum laccase activity of 30.08 U/mL was achieved after optimization, which was a 19.04-fold increase. The biodegradation rate of 2-chlorophenol using crude laccase was 97.13%, a positive correlation was determined between laccase activity and degradation rate. The toxicity of 2-CP was substantially reduced after degradation by laccase solution. Our findings show the feasibility of the use of corn stover in laccase production by M. verrucaria mutant and the subsequent biodegradation of 2-chlorophenol using crude laccase.
Highlights
-
A mutant of M. verrucaria with high laccase production and genetic stability was obtained using ARTP mutagenesis.
-
Culture conditions were optimized, and extracellular laccase activity was increased by corn stover and copper ion supplementation.
-
Crude laccase could degrade 2-chlorophenol by up to 97.13%.








Similar content being viewed by others
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
Zhu MY, Lu J, Dong LC, Hu SH, Peng SC, Zhu CZ (2022) Photochemical transformations of 2, 6-dichlorophenol and 2-chlorophenol with superoxide ions in the atmospheric aqueous phase. J Mol Struct. https://doi.org/10.1016/j.molstruc.2022.132910
Maity D, Kundu P, Adhikari S (2022) Isolation and characterization of 4-chlorophenol degrading bacterial strain from pharmaceutical xenobiotic compounds contaminated soil using enrichment technique. J Indian Chem Soc. https://doi.org/10.1016/j.jics.2021.100336
Zdarta J, Antecka K, Frankowski R, Zgoła-Grze´ skowiak A, Ehrlich H, Jesionowski T (2018) The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.09.213
Gaitan IJ, Medina SC, González JC, Rodríguez A, Espejo AJ, Osma JF, Sarria V, Alméciga-Díaz CJ, Sánchez OF (2011) Evaluation of toxicity and degradation of a chlorophenol mixture by the laccase produced by Trametes pubescens. Bioresource Technol. https://doi.org/10.1016/j.biortech.2010.11.040
Yang Y, Xu J, Guo Y, Wang X, Xiao LP, Zhou J (2022) Biodegradation of lignin into low-molecular-weight oligomers by multicopper laccase-mimicking nanozymes of the Cu/GMP complex at room temperature. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.1c08679
Koyappayil A, Kim HT, Lee MH (2021) ‘Laccase-like’ properties of coral-like silver citrate micro-structures for the degradation and determination of phenolic pollutants and adrenaline. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.125211
Chen ZH, Yao J, Ma B, Liu B, Kim J, Li H, Zhu XZ, Zhao CC, Amde M (2022) A robust biocatalyst based on laccase immobilized superparamagnetic Fe3O4@SiO2–NH2 nanoparticles and its application for degradation of chlorophenols. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.132727
Li H, Wei L, Gu Q, Qiang W, Hu W, Yu X (2013) Acetone, butanol, and ethanol production from cane molasses using Clostridium beijerinckii mutant obtained by combined low-energy ion beam implantation and N-methyl-N-nitro-N-nitrosoguanidine induction. Bioresource Technol. https://doi.org/10.1016/j.biortech.2013.03.084
Zhang X, Zhang XF, Li HP, Wang LY, Zhang C, Xing XH, Bao CY (2014) Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl Microb Biotech. https://doi.org/10.1007/s00253-014-5755-y
Zhang ZC, ManzoorShah A, Mohamed H, Zhang Y, Sadaqat B, Tsiklauri N, Sadunishvili T, Song YD (2022) Improved laccase production in Pleurotus djamor RP by atmospheric and room temperature plasma (ARTP) mutagenesis. Electron J Biotechn. https://doi.org/10.1016/j.ejbt.2022.03.005
Othman AM, Mahmoud M, Abdelraof M, Karim GSAA, Elsayed AM (2021) Enhancement of laccase production from a newly isolated Trichoderma harzianum S7113 using submerged fermentation: optimization of production medium via central composite design and its application for hydroquinone degradation. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2021.09.207
Suman SK, Malhotra M, Khichi SS, Ghosh S, Jain SL (2020) Optimization and kinetic modeling of Trametes maxima IIPLC-32 laccase and application in recalcitrant dye decolorization. New J Chem. https://doi.org/10.1039/D0NJ05179A
Unuofin JO, Okoh AI, Nwodo UU (2019) Maize stover as a feedstock for enhanced laccase production by two gammaproteobacteria: a solution to agroindustrial waste stockpiling. Ind Crop Prod. https://doi.org/10.1016/j.indcrop.2018.12.043
Zeng S, Zhao J, Xia L (2017) Simultaneous production of laccase and degradation of bisphenol A with Trametes versicolor cultivated on agricultural wastes. Bioproc Biosys Eng. https://doi.org/10.1007/s00449-017-1783-1
Kumar VV, Venkataraman S, Kumar PS, George J, Rajendran DS, Shaji A, Lawrence N, Saikia K, Rathankumar AK (2022) Laccase production by Pleurotus ostreatus using cassava waste and its application in remediation of phenolic and polycyclic aromatic hydrocarbon-contaminated lignocellulosic biorefinery wastewater. Environ Pollut. https://doi.org/10.1016/j.envpol.2022.119729
Xu L, Sun K, Wang F, Zhao L, Hu J, Ma H, Ding Z (2020) Laccase production by Trametes versicolor in solid-state fermentation using tea residues as substrate and its application in dye decolorization. J Environ Manag. https://doi.org/10.1016/J.JENVMAN.2020.110904
Kannaiyan R, Mahinpey N, Kostenko V, Martinuzzi RJ (2015) Nutrient media optimization for simultaneous enhancement of the laccase and peroxidases production by coculture of Dichomitus squalens and Ceriporiopsis subvermispora. Biotechnol Appl Bioc. https://doi.org/10.1002/bab.1263
Lu Y, Wang L, Ma K, Li G, Zhang C, Zhao H, Lai Q, Li HP, Xing XH (2011) Characteristics of hydrogen production of an Enterobacter aerogenes mutant generated by a new atmospheric and room temperature plasma (ARTP). Biochem Eng J. https://doi.org/10.1016/j.bej.2011.02.020
Majumder A, Gupta AK, Sillanpää M (2022) Insights into kinetics of photocatalytic degradation of neurotoxic carbamazepine using magnetically separable mesoporous Fe3O4 modified Al-doped ZnO: delineating the degradation pathway, toxicity analysis and application in real hospital wastewater. Colloid Surface A. https://doi.org/10.1016/j.colsurfa.2022.129250
Swain G, Sonwani RK, Singh RS, Jaiswal RP, Tai BN (2021) A comparative study of 4-chlorophenol biodegradation in a packed bed and moving bed bioreactor: performance evaluation and toxicity analysis. Environ Technol Inno. https://doi.org/10.1016/j.eti.2021.101820
Komariah LN, Arita S, Rendana M, Ramayanti C, Suriani NL, Erisna D (2022) Microbial contamination of diesel-biodiesel blends in storage tank; an analysis of colony morphology. Heliyon. https://doi.org/10.1016/j.heliyon.2022.e09264
Zhuo R, He F, Zhang X, Yang Y (2015) Characterization of a yeast recombinant laccase rLAC-EN3-1 and its application in decolorizing synthetic dye with the coexistence of metal ions and organic solvents. Biochem Eng J. https://doi.org/10.1016/j.bej.2014.09.004
Shi H, Peng J, Li J, Wang Z, Gao S (2016) Laccase-catalyzed removal of the antimicrobials chlorophene and dichlorophen from water: reaction kinetics, pathway and toxicity evaluation. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2016.05.064
Jiang S, Ren DJ, Wang ZB, Zhang SQ, Zhang XQ, Chen WS (2022) Improved stability and promoted activity of laccase by one-pot encapsulation with Cu (PABA) nanoarchitectonics and its application for removal of Azo dyes. Ecotox Environ Safe. https://doi.org/10.1016/j.ecoenv.2022.113366
Songulashvili G, Elisashvili V, Wasser S, Nevo E, Hadar Y (2006) Laccase and manganese peroxidase activities of Phellinus robustus and Ganoderma adspersum grown on food industry wastes in submerged fermentation. Biotechnol Lett. https://doi.org/10.1007/s10529-006-9109-4
Hao J, Song F, Huang F, Yang C, Zhang Z, Zheng Y, Tian X (2007) Production of laccase by a newly isolated deuteromycete fungus Pestalotiopsis sp. and its decolorization of azo dye. J Ind Microbiol Biotechnol. https://doi.org/10.1007/s10295-006-0191-3
Bonugli-Santos RC, Durrant LR, Sette LD (2010) Laccase activity and putative laccase genes in marine-derived basidiomycetes. Fungal Biol. https://doi.org/10.1016/j.funbio.2010.08.003
Rashid J, Barakat MA, Mohamed RM, Ibrahim IA (2014) Enhancement of photocatalytic activity of zinc/cobalt spinel oxides by doping with ZrO2 for visible light photocatalytic degradation of 2-chlorophenol in wastewater. J Photoch Photobio A. https://doi.org/10.1016/j.jphotochem.2014.03.017
Divya LM, Prasanth GK, Sadasivan C (2013) Isolation of a salt tolerant laccase secreting strain of Trichoderma sp. NFCCI-2745 and optimization of culture conditions and assessing its effectiveness in treating saline phenolic effluents. J Environ Sci. https://doi.org/10.1016/S1001-0742(12)60321-0
Castaño JD, Cruz C, Torres E (2015) Optimization of the production, purification and characterization of a laccase from the native fungus Xylaria sp. Biocatal Agr Biotech. https://doi.org/10.1016/j.bcab.2015.09.012
Triarsi SW, Jun O, Hiromi T (2010) Characterization of alkaliphilic laccase activity in the culture supernatant of Myrothecium verrucaria 24G–4 in comparison with bilirubin oxidase. Fems Microb Lett. https://doi.org/10.1016/S0378-1097(03)00892-9
Zhao D, Zhang X, Cui D (2012) Characterisation of a novel white laccase from the deuteromycete fungus Myrothecium verrucaria NF-05 and its decolourisation of dyes. PLoS ONE. https://doi.org/10.1371/journal.pone.0038817
Podieiablonskaia EV, Kolomytseva MP, Myasoedova NM (2017) Myrothecium verrucaria F-3851, a producer of laccases transforming phenolic compounds at neutral and alkaline conditions. Microbiology. https://doi.org/10.1134/S0026261717030146
Sun J, Guo N, Niu LL, Wang Q, Zang Y, Zu Y, Fu Y (2017) Production of laccase by a new Myrothecium verrucaria MD-R-16 isolated from Pigeon Pea [Cajanus cajan (L.) Millsp.] and its application on dye decolorization. Molecules. https://doi.org/10.3390/molecules22040673
Agrawal K, Bhardwaj N, Kumar B (2018) Process optimization, purification and characterization of alkaline stable white laccase from Myrothecium verrucaria ITCC-8447 and its application in delignification of agroresidues. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2018.12.108
Ren F, Chen L, Tong Q (2017) Highly improved acarbose production of actinomyces through the combination of ARTP and penicillin susceptible mutant screening. World J Microb Biotech. https://doi.org/10.1007/s11274-016-2156-7
Liu X, Lv J, Xu J (2017) Erythritol production by Yarrowia lipolytica mutant strain M53 generated through atmospheric and room temperature plasma mutagenesis. Food Sci Biotech. https://doi.org/10.1007/s10068-017-0116-0
Zou Z, Zhao Y, Zhang T, Xu J, He A (2018) Efficient isolation and characterization of a cellulase hyperproducing mutant strain of Trichoderma reesei. J Microb Biotech. https://doi.org/10.4014/jmb.1805.05009
Zheng S, Jiang B, Zhang T, Chen J (2020) Combined mutagenesis and metabolic regulation to enhance D-arabitol production from Candida parapsilosis. J Ind Microb Biotech. https://doi.org/10.1007/s10295-020-02278-4
Gao X, Liu E, Yin Y, Yang L, Ho C (2020) Enhancing activities of salt-tolerant proteases secreted by Aspergillus oryzae using atmospheric and room-temperature plasma mutagenesis. J Agr Food Chem. https://doi.org/10.1021/acs.jafc.9b08116
Cai M, Wu Y, Qi H, He J, Qiao M (2021) Improving the level of the tyrosine biosynthesis pathway in Saccharomyces cerevisiae through HTZ1 knockout and atmospheric and room temperature plasma (ARTP) mutagenesis. ACS Synth Biol. https://doi.org/10.1021/acssynbio.0c00448
Zong H, Zhan Y, Li X, Peng J, Feng FQ, Li D (2012) A new mutation breeding method for Streptomyces albulus by an atmospheric and room temperature plasma. Afr J Microb Res. https://doi.org/10.5897/AJMR11.1251
Martin-Sampedro R, Eugenio ME, Macaya-Sanz D, Martin JA, Fillat U, Martinez MJ, Ibarra D (2016) Screening of eucalyptus wood endophytes for laccase activity. Proc Biochem. https://doi.org/10.1016/j.procbio.2016.02.006
Cambria MT, Ragusa S, Calabrese V, Cambria A (2011) Enhanced laccase production in white-rot fungus Rigidoporus lignosus by the addition of selected phenolic and aromatic compounds. Appl Biochem Biotech. https://doi.org/10.1007/s12010-010-9049-2
Debnath R, Saha T (2020) An insight into the production strategies and applications of the ligninolytic enzyme laccase from bacteria and fungi. Biocatal Agr Biotech. https://doi.org/10.1016/j.bcab.2020.101645
Adekunle AE, Guo C, Liu CZ (2017) Lignin-enhanced laccase production from Trametes versicolor. Waste Biomass Valor. https://doi.org/10.1007/s12649-016-9680-4
Feng X, Chen H, Xun D, Yao S (2013) Enhancement of laccase activity by marine-derived deuteromycete Pestalotiopsis sp. J63 with agricultural residues and inducers. Chin J Chem Eng. https://doi.org/10.1016/S1004-9541(13)60567-4
Li S, Tang B, Liu Y, Chen A, Tang W, Wei S (2016) High-level production and characterization of laccase from a newly isolated fungus Trametes sp. LS-10C. Biocat Agr Biotech. https://doi.org/10.1016/j.bcab.2016.10.008
Zhuo R, Yuan P, Yang Y, Zhang S, Ma F, Zhang X (2017) Induction of laccase by metal ions and aromatic compounds in Pleurotus ostreatus HAUCC 162 and decolorization of different synthetic dyes by the extracellular laccase. Biochem Eng J. https://doi.org/10.1016/j.bej.2016.09.016
Xu R, Zhou Q, Li F, Zhang B (2013) Laccase immobilization on chitosan/poly (vinyl alcohol) composite nanofibrous membranes for 2,4-dichlorophenol removal. Chem Eng J. https://doi.org/10.1016/j.cej.2013.02.074
Gonzalez LF, Sarria V, Sanchez OF (2010) Degradation of chlorophenols by sequential biological-advanced oxidative process using Trametes pubescens and TiO2/UV. Bioresource Technol. https://doi.org/10.1016/j.biortech.2009.12.130
Agrawal K, Shankar J, Kumar R (2020) Insight into multicopper oxidase laccase from Myrothecium verrucaria ITCC-8447: a case study using in silico and experimental analysis. J Environ Sci Heal B. https://doi.org/10.1080/03601234.2020.1812334
Funding
This work was supported by Jilin Scientific and Technological Development Program, Grant/Award number: 20210101036JC and National College Students Innovation and Entrepreneurship Project in China.
Author information
Authors and Affiliations
Contributions
ZCG and MJL wrote the manuscript; XQW and YSW conducted experiments; ZQW and MYJ contributed new reagents or analytical tools; XYC, XXY and SST analyzed data; GC and YJS conceived and designed the experiments. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Ethical statement
None required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gou, ZC., Lu, MJ., Cui, XY. et al. Enhanced laccase production by mutagenized Myrothecium verrucaria using corn stover as a carbon source and its potential in the degradation of 2-chlorophen. Bioprocess Biosyst Eng 45, 1581–1593 (2022). https://doi.org/10.1007/s00449-022-02767-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02767-z