Abstract
Alternaria is a plant pathogen and human allergen. Alternaria alternata is one of the most abundant fungal spores in the air. The purpose of this study was to examine whether Alternaria spp. spore concentrations can be used to predict the abundance and spatio-temporal pattern of A. alternata spores in the air. This was investigated by testing the hypothesis that A. alternata dominates airborne Alternaria spp. spores and varies spatio-temporally. Secondarily, we aimed at investigating the relationship between airborne Alternaria spp. spores and the DNA profile of A. alternata spores between two proximate (~ 7 km apart) sites. These were examined by sampling Alternaria spp. spores using Burkard 7-day and cyclone samplers for the period 2016–2018 at Worcester and Lakeside campuses of the University of Worcester, UK. Daily Alternaria spp. spores from the Burkard traps were identified using optical microscopy whilst A. alternata from the cyclone samples was detected and quantified using quantitative polymerase chain reaction (qPCR). The results showed that either A. alternata or other Alternaria species spores dominate the airborne Alternaria spore concentrations, generally depending on weather conditions. Furthermore, although Alternaria spp. spore concentrations were similar for the two proximate sites, A. alternata spore concentrations significantly varied for those sites and it is highly likely that the airborne samples contained large amounts of small fragments of A. alternata. Overall, the study shows that there is a higher abundance of airborne Alternaria allergen than reported by aerobiological networks and the majority is likely to be from spore and hyphal fragments.
Similar content being viewed by others
Introduction
Alternaria is a fungus, which is a plant and animal pathogen and human aeroallergen (Nowicki et al. 2012; Meena et al. 2017; Grinn-Gofroń et al. 2019). Human exposure to Alternaria spores causes allergy and severe asthma hospital admissions in sensitised individuals, more frequently in children (Neukirch et al. 1999; Mitakakis et al. 2001). Traditionally, optical microscopy is the main instrument for monitoring aeroallergens such as Alternaria (West et al. 2017). However, the use of morphological features alone to identify Alternaria spores at species level is unreliable due to their overlapping characteristics (Ammour et al. 2020; West et al. 2017).
Alternaria alternata is among the most prevalent fungal species causing sensitisation to allergy (Mari et al. 2003). It is one of the abundant, allergenic and pathogenic Alternaria species among fungal types whose spores are frequently observed in the air (Gabriel et al. 2015, 2016). However, no study has examined the abundance of A. alternata within the Alternaria genus. A. alternata allergen (Alt a 1) is the most prevalent among patients sensitised to fungal allergy (López Couso et al. 2021). A. alternata has a high spatio-temporal variability due to changing weather, diverse host ranges and abundance of inoculum, making its occurrence, until now, generally unpredictable (Ding et al. 2021). It is unknown whether A. alternata spore concentrations differ between nearby rural and urban areas especially during periods of high spore concentrations. However, the metabarcoding approach used to analyse airborne microorganisms revealed systematic differences between the rural and urban atmosphere and high fungal spore biodiversity (Hanson et al. 2022). Spores of different Fusarium species were concurrently dispersed when meteorological variables simultaneously influenced their development and dispersion (Audenaert et al. 2009; Xu et al. 2005). Simultaneous growth and eventual atmospheric release of different Alternaria species within an area is very likely due to some species being host specific, whilst others may be opportunistic and associated with many hosts (Al-Lami et al. 2019). However, such a simultaneous relationship between A. alternata and Alternaria spp. spores has not been tested before especially for nearby rural and urban areas. Simultaneous dispersal of the spores of different Alternaria species increases exposure and severity of the symptoms of allergy and asthma in sensitised individuals (Damialis and Gioulekas 2006; Madsen et al. 2016). Meanwhile, the asynchronous spore dispersal pattern among the species, as observed between A. alternata and A. solani, prolongs disease virulence and severity in crops as well as the season of allergy (Al-Lami et al. 2020; Ding et al. 2021). Generally, Alternaria spp. spore concentrations have been shown to have a high spatio-temporal variation, due to the effect of the meteorological variables and anthropogenic activities, e.g. crop combine-harvesting that govern their sporulation and dispersal (Escuredo et al. 2011; Recio et al. 2012; Skjøth et al. 2012). However, it is unknown how the meteorological variables and combine-harvesting of crops impact A. alternata spore concentrations, especially at nearby rural and urban sites that are < 10 km apart.
Therefore, the purpose of the study was to understand whether the overall Alternaria spp. spore concentrations (obtained with optical microscopy) can be used to predict the distribution and spatio-temporal pattern of A. alternata spores in an area. This was achieved by examining the relationship between the DNA profile of A. alternata spores and overall Alternaria spp. spore concentrations between two proximate (~ 7 km apart) sites. We tested the hypothesis that airborne Alternaria spore concentrations are dominated by A. alternata and have varying spatio-temporal patterns. This was examined by detecting and quantifying Alternaria spp. spore concentrations using optical microscopy and A. alternata spore DNA profile using qPCR, at two separate nearby locations in one region. The impact of the meteorological variables and crop combine-harvesting on the spatio-temporal variations of the spores was examined.
Materials and methods
Spore sampling instruments and locations
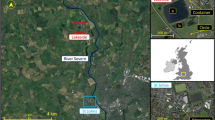
Alternaria spp. spores were sampled using pairs of Hirst-type Burkard 7-day volumetric spore trap (Hirst 1952) and an automatic multi-vial cyclone sampler (Burkard Manufacturing, UK) at the University of Worcester, Worcester, UK. The spores were sampled (Fig. S1) at a rural (Lakeside campus) and an urban site (Worcester campus) continuously for approximately 3 months (Jul–Sep) for three separate years (2016–2018; Table S1) during the main Alternaria spore season in the areas (Apangu et al. 2020). Worcester is an urban area surrounded by agricultural areas comprising permanent orchards for fruit and cider production (Sadyś et al. 2014), crops under rotation (Apangu et al. 2020; Sadyś et al. 2015), grasslands and pasture within the public parks (Sadyś et al. 2016) and small woodlands (Skjøth et al. 2015). The Lakeside site is a rural area whose immediate vicinity had no source of Alternaria spores (e.g. buildings in the south, non-vegetated areas such as hard standing, roadways and a man-made lake in the west). However, areas outside the vicinity comprised potential sources such as pine trees, grassland, mixed arable crop fields, permanent pastures, animal paddocks and patches of trees. Lakeside samplers were approximately 7 km away from Worcester.
Sampling heights were set following the recommendations on the height of pollen/spore sampling (Galán et al. 2014). Samplers at Worcester (52.1970 N, − 2.2421 E) were placed 10 m above ground level (AGL) on the rooftop of the Edward Elgar building (e.g. Sadyś et al. 2014) to capture spores at both local and regional scales. Meanwhile, samplers at Lakeside (52.2544 N, − 2.2537 E) were placed on top of a container (hereafter Lakeside Container) 4 m AGL. Sampling Alternaria spores using the Burkard trap was conducted similarly to the previous studies (Adams-Groom et al. 2020; Apangu et al. 2020). Meanwhile, the particles collected by the cyclone sampler were deposited by centrifugal force through spiralling action into a 1.5 mL microcentrifuge tube, similar to Pashley et al. (2012). The cyclones automatically replace the microcentrifuge tube with a new one at 9:00 am to ensure that daily samples from the cyclones are temporally synchronised across the sampling network (Brennan et al. 2019). The samples of the 7-day Burkard trap at Worcester were unloaded weekly at 9:00 am whilst those at Lakeside Container were emptied at 14:00 and spore data was later converted to match that at Worcester. The Burkard trap samples were stored at 4 °C until they were mounted on microscope slides. The microscope slides were prepared and Alternaria spores were identified and counted according to a standard procedure used for over 50 years in England and other European countries (Adams-Groom et al. 2020; Apangu et al. 2020; BAF 1995; Kasprzyk 2008; Makra et al. 2010; Skjøth et al. 2016). Samples collected with the cyclone sampler were stored at – 20 °C for about a month and then transferred to – 80 °C for long-term storage in order to preserve the spores until the DNA extraction. This procedure limits DNA degradation over time, a common problem with environmental samples (Lear et al. 2018). Here, storage at – 80 °C for a year (Pashley et al. 2012) or several years (Hanson et al. 2022) has successfully been applied to air samples collected with cyclones.
Microscopic Alternaria spp. spore identification
Alternaria spp. spores from the Burkard trap were identified using an optical microscope (Sadyś et al. 2014) and counted using the 12 transverse method at × 400 magnification according to fungal spore monitoring recommendations in Europe (Galán et al. 2021). This approach is being used for fungal spore monitoring in Worcester, UK (Apangu et al. 2020), Denmark (Skjøth et al. 2012) and Hungary (Paldy et al. 2014). The daily (24 h) mean Alternaria spp. spore concentrations were expressed as spores m−3 of air by multiplying the microscopic spore counts with previously calculated correction factors (Lacey and Allit 1995). To match the 7-day A. alternata spore data (spores, hyphal and mycelial fragments) from the cyclone samplers, weekly mean Alternaria spp. spore concentrations were calculated by summing Alternaria spp. spore concentrations every 7 days and computing their averages.
Cultivation and quantification of A. alternata conidia as reference for qPCR
A. alternata conidia were cultivated and later used in qPCR bioassays to identify and quantify airborne A. alternata spores sampled at Lakeside Container and Worcester. To achieve this, conidia of A. alternata (Culti-Loops™ TX 8025) procured from Fisher Scientific, UK, were grown under sterile conditions on potato dextrose agar for 23 days at 23 °C, similar to Smith et al. (2012). The Culti-Loops™ A. alternata isolate was originally derived from the American Type Culture Collection (ATCC®). To harvest spores, sterile water (10 mL) was added to the culture Petri dish and spore suspensions were obtained by gently scraping the surface of the culture using a sterile L-shaped spreader. Five millilitres of the spore suspension was drawn into a clean and sterile 50 mL microcentrifuge tube. The spore suspension was recovered after 5 min of centrifugation (CL31 Multispeed centrifuge, Thermo Scientific) at 2500 rpm, corresponding to 600 × g. The supernatant was discarded and the pellet was transferred into a clean 2 mL microcentrifuge tube and resuspended in 1 mL sterile water. Ten microliters of the spores from the pellet suspension were counted using an improved Neubauer haemocytometer (Nexcelom Bioscience 2018), similar to Ojaghian et al. (2018), where the Neubauer haemocytometer is one of several types of counting chambers designed for counting cells in a liquid. In this case, the counting chamber has a depth of 0.1 mm. The concentration of A. alternata spores per mL was calculated using the formula below (Nexcelom Bioscience 2018). If the spore concentration is low as in our case, then it is recommended to count all the 9 large squares in the haemocytometer which we did here, whilst the alternative is to count only the central square. The pellet suspension with a known spore concentration was then used for generating standard curves (‘real-time qPCR’ section) for qPCR and used for computing mean spore concentrations in the air samples.
In our case, the spore count in each of the nine grids was 201, 112, 209, 164, 185, 151, 174, 160 and 209, respectively. This corresponds to a mean spore concentration in the pellet suspension of 1.74*106 spore mL−1.
DNA extraction from air samples and culture material
DNA was isolated from the daily air samples collected with multi-vial cyclone samplers and the A. alternata culture material using a commercial protocol (Fast DNA spin kit for soil; MP Biomedicals), similar to previous studies (Chen et al. 2020; Degois et al. 2019; Ettenauer et al. 2012; Fröhlich-Nowoisky et al. 2012). Cell lysis solution (CLS)-Y buffer, which aids release of impacted DNA in the spores through enzymic cell membrane breakdown, was added to the daily air samples (100 μL) and culture material (200 μL). The daily air samples and culture material were then vortexed using the Vortex-Genie 2 vortex mixer (Mo Bio Laboratories, Inc) at a maximum speed (ten) and for 5 min. The daily air samples were pooled every seven consecutive days of spore sampling to form a 7-day pooled air sample of 700 μL, similar to Brennan et al. (2019). The 7-day pooled air samples and culture material were diluted to 1 mL by topping up with 300 and 800 μL of CLS-Y buffer, respectively and samples transferred into a 2 mL lysing matrix A tube (pre-filled with irregularly shaped garnet particles and a single 1/4 inch ceramic sphere). The 7-day pooled air samples and culture material were then homogenised using a FastPrep® instrument (MP Biomedicals) for 40 s at 6 m/s. The rest of the steps in the Fast DNA spin kit protocol were followed for the DNA extraction from the 7-day pooled air samples and culture material. DNA was eluted by resuspending the binding matrix above the SPIN filter in 100 μL of DES (DNase/Pyrogen-free water). The concentration of DNA in the samples was quantified using a Nanodrop 2000c spectrophotometer instrument (Fisher Scientific, UK), similar to previous studies (Degois et al. 2019; Ettenauer et al. 2012; Hanson et al. 2022; Shokere et al. 2009). The DNA was stored at – 20 °C for subsequent analyses.
Real-time qPCR
To detect and quantify the amount of DNA from A. alternata spores in the air samples, qPCR assays were conducted according to experimental conditions stipulated in Black et al. (2013). For each qPCR assay, a 25 μL reaction volume was prepared to contain 2 μL template DNA from air samples, 12.5 μL qPCRbio SyGreen Blue mix (PCR Biosystems, UK), 1.2 μM of each forward and reverse primer and 4.5 μL purified water (Sigma, UK; Black et al. 2013). A. alternata was detected using the primers; Sense 5′ CGA ATC TTT GAA CGC ACA TTG 3′, Antisense 5′ CGC TCC GAA ACC AGT AGG 3′ (Black et al. 2013). To check for the primer specificity to A. alternata, melt curve temperature was analysed and the primer sequences were BLAST searched in the NCBI Genbank database, similar to Patel et al. (2019). DNase-free double-distilled H2O (Clent Life Science, UK) replaced DNA in the master mix as a negative control. The qPCR amplification was conducted in a Roche LightCycler 480 and analysed using Gene Scanning software machine v.1.5 (Roche Molecular Sytems, UK) with each sample and standard prepared from culture extract run in triplicate. DNA in the samples was initially denatured at 95 °C for 4 min. Sample products were detected and amplified at 95 °C for 40 cycles and 10 s, followed by 57 °C for 10 s and 72 °C for 10 s. Melting curve parameters were set at 95 °C for 1 min, followed by 40 °C for 1 min, 60 °C for 1 s and 95 °C with a continuous analysis mode. The products were cooled at 40 °C for 30 s. Fluorescence levels were recorded at the end of each amplification cycle as stipulated in the minimum information for publication of quantitative real-time experiments guideline (Bustin et al. 2009). The qPCR method only works by having a standard curve based on a set (here 7) of known concentrations of A. alternata spores in form of standard dilutions, taken from samples free from both mycelia, spore fragments and other spores. To produce a standard curve, genomic DNA was extracted from a suspension of the A. alternata spores, grown and quantified as described in ‘Cultivation and quantification of A. alternata conidia as reference for qPCR’ section. The standard curve was generated by the Roche LightCycler 480 by plotting the ‘Crossing point’ (Cp) value for each dilution similar to Grinn-Gofroń et al. (2016) and Black et al. (2013). Mean spore concentrations mL−1 were generated using the second derivative maximum method in the LightCycler 480 software by measuring the Cp at which the fluorescent signal generated by amplification reaches the maximum second derivative. In second derivative maximum analysis, the number of spores mL−1 is calculated by polynomial regression of Cp against cycle number.
Mean air spore concentrations were determined against the standard curve generated from the cultured A. alternata spores. In both instances, concentrations were measured as the number of spores mL−1 due to suspension of spores in solution prior to DNA extraction. After quantification, the number of spores m−3 air was calculated from the number of spores mL−1. The cyclone samples 16.5 L air min−1 and operates for 24 h, therefore sampling 23.76 m3 air day−1, or 166.32 m3 air week−1. For simplicity, we will onwards term the DNA spore extraction as A. alternata spore equivalents; calculated as spores m−3 = spores mL−1/166.32 m3 as it should be noted that the DNA extraction and amplification of the air samples will detect both airborne spores and spore/hyphal fragments, whilst the reference samples that were quantified using a haemocytometer did not contain any spore/hyphal fragments. Since 2 μL of the eluted 100 μL (1/50th) DNA of the air samples was used in the qPCR reactions, the weekly air sample (166.32 m3) was divided by 50 = 3.326. Therefore, the weekly mean spore concentration was obtained by dividing the mean spore concentration from the LightCycler by 3.326.
Meteorological data
Two Campbell Scientific meteorological stations were established at Worcester and Lakeside to provide half-hourly meteorological data for the period 2017–2018. Worcester meteorological station was co-located with the Burkard 7-day and cyclone samplers whilst Lakeside Container meteorological station was located 310 m away from the samplers. There was a gap in meteorological data from January 2016 to July 2017 before the acquisition of the Campbell Scientific meteorological instruments. The gap in data was filled with hourly meteorological data obtained from the nearest (20 km away) UK Met station (Pershore Weather Station; MET Office, UK). This was after verifying that relevant weather data from Pershore had a high correlation with Lakeside and Worcester weather data, similar to Skjøth et al. (2016).
The half-hourly and hourly weather data were independently averaged to provide weekly meteorological data for each meteorological station to match the weekly observations of Alternaria spp. and A. alternata spores. Selected meteorological parameters including mean air temperature (°C), pressure (hPa), relative humidity (%), solar radiation (W/m2), rain (mm), wind speed (m/s), wind direction (°), leaf wetness (minutes) and dew point (°C) were extracted for further analyses.
Crop harvest data
To investigate the effect of crop harvesting on Alternaria spp. and A. alternata spore concentrations, reports of weekly crop harvest data were downloaded from the Agriculture and Horticulture Development Board (AHDB; https://ahdb.org.uk/cereals-oilseeds/gb-harvest-progress) and examined, similar to Apangu et al. (2020). AHDB is a statutory levy board and summarises the weekly harvest progress reports for specific regions of Great Britain that are supplied by independent agronomists weekly. The report covers crops such as winter wheat, winter oilseed rape, winter barley, spring wheat and spring barley. The crop harvest reports from the West Midlands region during the period from July to September of 2016 to 2018 were examined to explain the high spore concentrations during the harvest periods-starting with weeks when > 5% of the crops were harvested. To obtain the crop harvest data within 30 km (Avolio et al. 2008) from Worcester and Lakeside Container samplers, the percentage weekly crop harvest data (from AHDB) were multiplied with the land cover data for each crop above, similar to Apangu et al. (2020). The land cover for crops (in ha), produced by the UK Centre for Ecology and Hydrology (https://www.ceh.ac.uk/), was downloaded from the EDINA digimap website (https://digimap.edina.ac.uk/environment) and analysed using Spatial Analyst tool of ArcGIS 10.6.
Statistical analyses of data
The descriptive summaries included start and end of sampling, weekly mean spore concentrations, peak week, peak spore concentration, total spores per site and days with daily spore concentrations above 100 spores m−3 (hereafter high days). For statistical analyses, the Shapiro –Wilk significance test was applied to test for normality of the spore data, similar to Kulik et al. (2015). Spearman’s rank correlation test was chosen after confirming from the Shapiro–Wilk significance test that the spores were not normally and linearly distributed. Spearman’s rank correlation test was performed between meteorological parameters observed at Worcester and Lakeside Container and weekly mean spore concentrations of Alternaria spp. and A. alternata, similar to Olsen et al. (2019) and Grinn-Gofroń et al. (2018). The same correlation analysis was performed between weekly mean A. alternata/Alternaria spp. spore concentrations and crop harvest data. The Spearman’s correlation test was applied to the logarithmically transformed weekly mean A. alternata and Alternaria spp. spore values to determine any relationship between them, similar to previous studies (Ding et al. 2021; Grinn-Gofroń et al. 2016; Pavón et al. 2012). The Wilcoxon signed-rank test was performed to compare the weekly mean A. alternata and Alternaria spp. spore concentrations and the days with daily mean spore concentration above 100 spores m−3 of air (clinical/high days) observed at Worcester and Lakeside Container, similar to previous studies (Kasprzyk and Worek 2006; Trigo et al. 2000). Multiple linear regression was performed to determine the most significant meteorological/harvesting variables contributing to airborne A. alternata spore equivalents and Alternaria spp. spore concentrations, as multiple linear regression is a vital technique to explain complex relationships in fungal spore concentrations (including Alternaria) in aerobiology (Martinez-Bracero et al. 2022). Alternaria spore data was transformed using a logarithmic function [(No. spores m−3) + 1] to obtain a normal distribution of the spore data prior to the multiple regression analyses, similar to previous studies (Angulo-Romero et al. 1999; Recio et al. 2012). All statistical analyses were performed in R v.4.1.3 (R Core Team 2022).
Results
Comparison of Alternaria spp. with A. alternata spore concentrations
A. alternata had a similar distribution pattern to Alternaria spp. spores and simultaneously peaked at both the rural site (Lakeside Container) and urban site (Worcester) in 2016 (Fig. 1a). Moreover, there was a strong and positive linear relationship between Alternaria spp. spore concentrations and A. alternata spore equivalents at Lakeside Container (r = 0.77, p = 0.003) and Worcester (r = 0.95, p < 0.001) in 2016 (Fig. 2a and b). However, despite the positive linear relationship (Fig. 2c–f) in both sites except in Worcester in 2017, A. alternata varied with Alternaria spp. spores in distribution and spatio-temporal pattern in 2017 (Fig. 1b) and 2018 (Fig. 1c) and they moderately-to-weakly correlated at Lakeside Container [2017 (r = 0.42, p = 0.16), 2018 (r = 0.5, p = 0.14)] and Worcester [2017 (r = − 0.38, p = 0.25), 2018 (r = 0.14, p = 0.69)]. A. alternata spore concentrations peaked in July and August whilst Alternaria spp. spores peaked only in August at both the rural (Lakeside Container) and urban (Worcester) sites throughout the sampling periods.
Spatio-temporal variation of the Log transformed weekly mean A. alternata (qPCR) and Alternaria spp. (optical microscopy) spore concentrations detected at Lakeside Container (rural site) and Worcester (urban site) during the same period of harvesting of the cereals and oilseed rape in 2016 and crop areas of main crops grown in a 30 km radius area centred on each of the Lakeside Container or Worcester sampling sites
Spatio-temporal variation of the Log transformed weekly mean A. alternata (qPCR) and Alternaria spp. (optical microscopy) spore concentrations detected at Lakeside Container (rural site) and Worcester (urban site) during the same period of harvesting of the cereals and oilseed rape in 2017 and crop areas of main crops grown in a 30 km radius area centred on each of the Lakeside Container or Worcester sampling sites
Spatio-temporal variation of the Log transformed weekly mean A. alternata (qPCR) and Alternaria spp. (optical microscopy) spore concentrations detected at Lakeside Container (rural site) and Worcester (urban site) during the same period of harvesting of the cereals and oilseed rape in 2018 and crop areas of main crops grown in a 30 km radius area centred on each of the Lakeside Container or Worcester sampling sites
Total and peak A. alternata spore concentration equivalents, based on collections with the cyclones and qPCR, were considerably higher than those of Alternaria spp. based on the collection with the 7-day Burkard trap and the optical microscopy at both the rural (Lakeside Container) and urban (Worcester) sites, except in the period late July to 16 August 2018 (Fig. 1a–c; Table S1). The peak and total spore concentrations of A. alternata spores were dominated by the rural site in 2017 and 2018 (Table S1). Meanwhile, the peak and total spore concentrations of Alternaria spp. spores were dominated by the urban site in 2016 and 2018.
Wilcoxon signed-rank statistical analyses of the weekly mean A. alternata spore equivalents revealed that the rural site was significantly different from the urban site during the 2016 (p = 0.002), 2017 (p = 0.005) and 2018 (p = 0.014) spore seasons. Moreover, analyses of the high days (clinically important days) also showed that the rural site significantly varied from the urban site in 2017 (p = 0.010). However, there was no significant difference in the weekly mean Alternaria spp. spore concentration between the two sites in 2016 (p = 0.077), 2017 (p = 0.067) and 2018 (p = 0.646). Similarly, the sites showed no statistical difference in the high days in 2016 (p = 0.910) and 2018 (p = 0.932).
Using optical microscopy, Alternaria spp. spores were only identified to genus level whilst qPCR detected the specific A. alternata spores from the air samples within the recommended quantification cycle value of < 40 (Fig. S2a) and with high efficiency (2.084; Fig. S2b). The primer pair, as revealed by the melt curve temperature (Fig. S2c), was sufficiently specific and its sequences matched 100% with that of A. alternata (accession numbers OM422689.1 and OM422688.1).
A. alternata spore equivalents and Alternaria spp. spore concentrations during crop harvesting
Overall, analysis of the weekly crop harvest data showed that A. alternata spore equivalents and Alternaria spp. spore concentrations considerably and significantly increased during the harvesting of the cereals and oilseed rape (Fig. 1a–c; Table S2). The minor peak of A. alternata and Alternaria spp. spores at both the rural site and urban site on 14 July 2016 was observed when < 5% of the crops were harvested. However, the major peak of A. alternata and Alternaria spp. spores (11 August 2016) was observed when winter wheat, winter barley and winter oilseed rape were harvested in considerable amounts at both sites in 2016 (Fig. 1a). Harvesting of winter wheat increased Alternaria spp. spore concentrations at both Lakeside Container and Worcester and A. alternata at Lakeside Container in 2017 (Fig. 1b) and 2018 (Fig. 1c). Meanwhile, harvesting of mainly winter barley and winter oilseed rape increased both Alternaria spp. and A. alternata spore concentrations at both Worcester and Lakeside Container in 2017 and 2018, especially around the peak periods.
Crops harvested significantly correlated with A. alternata spore equivalents and Alternaria spp. spore concentrations (Table S2). However, winter oilseed rape and winter barley were the significant and most frequent contributors to airborne A. alternata spore equivalents at any of the two sites; meanwhile, simultaneous harvesting of all the crops during a week (total weekly harvest) and of winter wheat were the significant and greatest contributors to Alternaria spp. spore concentrations.
The multiple linear regression found that none of the crops had a significant effect on A. alternata spore equivalents or Alternaria spp. spore concentration (Table 1). However, the model showed winter barley and total weekly harvest as potential candidates that could have contributed to A. alternata spore equivalents and Alternaria spp. spore concentrations. Overall, the model showed that crop harvesting contributed 13.2% (R2 = 0.132) and 46.7% (R2 = 0.467) of the variation in A. alternata spore equivalents and Alternaria spp. spore concentrations respectively.
Relationship between meteorological parameters and Alternaria spp./A. alternata spore concentrations
Overall, the peak A. alternata and Alternaria spp. spore concentrations were observed when high relative humidity (above 70%) and air temperature (above 15 °C) and low rain (below 20 mm) were recorded at both Lakeside Container and Worcester in the 3 years of observation (Fig. S3a–c). However, there was an anomaly in 2017 where high A. alternata spore concentration was observed at Worcester when a high amount of rain (above 40 mm) was recorded, whilst Alternaria spp. spore concentration remained low at both sites.
Several meteorological variables significantly correlated with Alternaria spp. spore concentrations and A. alternata spore equivalents at either the rural (Lakeside Container) or the urban (Worcester) site. Spearman’s correlation test (Table S3) found that wind direction correlated with A. alternata spore equivalents at only the rural site in 2017. Air temperature significantly correlated with either Alternaria spp. spore concentration or A. alternata spore equivalents at either the rural or urban site during the three years of observation. Dew point temperature significantly corrected with both Alternaria spp. spore concentration and A. alternata spore equivalents at the rural site in 2017 and 2018 whilst it correlated with Alternaria spp. spore concentration at the urban site in 2018. Relative humidity was significantly and inversely correlated with A. alternata spore equivalents at the rural site in 2016 and 2017 and the urban site in 2018. Meanwhile, relative humidity was significantly and inversely correlated with Alternaria spp. spore concentration at the rural site in 2017 and 2018. Solar radiation was significantly correlated with Alternaria spp. spore concentration at the rural site in 2017 and 2018 and at the urban site in 2018, whereas solar radiation significantly correlated with A. alternata spore equivalents at only the rural site in 2017. Leaf wetness significantly correlated with only A. alternata spore equivalents at the rural site in 2017 and 2018. Overall, the air temperature was the weather variable that was most frequently associated with either A. alternata spore equivalents or Alternaria spp. spore concentration at either the rural or urban site.
Multiple linear regression found that wind direction and air temperature significantly contributed to the airborne A. alternata spore equivalents (Table 2(a)); meanwhile, wind direction, wind speed, dew point temperature and relative humidity significantly increased Alternaria spp. spore concentrations (Table 2(b)) at all the sites. Overall, the model showed that weather contributed 23.9% (R2 = 0.239) and 46.3% (R2 = 0.463) of the variation in A. alternata spore equivalents and Alternaria spp. spore concentrations respectively.
Discussion
Relationship between airborne A. alternata and Alternaria spp. spores
We partly accepted the hypothesis that the high Alternaria spore concentrations in 2016 were mainly contributed by A. alternata. The high A. alternata spore equivalents (measured with qPCR) compared to Alternaria spp. spore concentrations (measured with microscopy) is partly attributed to the fact that qPCR estimates spore numbers based on the quantification of the total genomic DNA in air samples, which includes the spores, hyphae and their fragments (Zeng et al. 2006). Fungal fragments can significantly exceed intact spores by concentration (Green et al. 2005). Aerosolization experiments have shown a 300-fold increase in the fungal fragments (Górny et al. 2002). Grewling et al. (2020) reported a low amount of the allergen Alt a1 in spore or hyphal fragments compared to intact Alternaria spores. However, it has been suggested that hyphal fragments from A. alternata contain few or no Alt a 1 allergens (Saha et al. 2022), whilst other allergens may be present. Pashley et al. (2012) found a discrepancy between optical microscopy and the DNA-based approach in the detection of Botrytis spores in air samples and attributed this to DNA from teleomorphs and hyphal fragments, since DNA-based analysis does not distinguish between DNA from spores or hyphae. Consequently, these fungal fragments may rapidly increase concentrations of allergenic and pathogenic fungal particles in the air, especially in agricultural areas that undergo crop combine-harvesting (Ammour et al. 2020; Lee and Liao 2014; Olsen et al. 2019; Tomlin et al. 2020). Importantly, as in our study, Pashley et al. (2012) used a cyclone sampler for the DNA extraction and a Hirst type volumetric trap for the microscopic counting of spores, both from Burkard. According to the manufacturer, the cyclones will be efficient in collecting small particles, whilst the same is not stated for the Hirst type volumetric trap. Therefore, it is likely that the high levels of A. alternata detected by the qPCR assays in all the air samples collected with the cyclone sampler from both the rural and urban sites could have included fragments of A. alternata spores or high amounts of hyphae. Fragments of Alternaria spores or hyphae can contain both DNA and several allergens and the hyphae may grow when they meet a susceptible plant host (Rosemond and Kramer 1971). They can penetrate further into the lungs compared with large spores that are intact and are in some environments suspected to be present in much higher concentrations than intact spores (Green et al. 2006). Future studies should consider the quantification of fungal fragments and hyphae using both qPCR and immunological approaches using samplers that efficiently capture this small particle fraction to identify their contribution to the dispersal of aeroallergens and pathogens.
The dominance of A. alternata spores over other Alternaria species spores is partly attributed to weather and crop harvesting, since wind direction, air temperature and potentially harvesting of winter barley were found to significantly contribute to A. alternata spore equivalents. Previous studies also found similar results regarding the positive effect of air temperature, wind speed, relative humidity and precipitation on airborne Alternaria spore concentrations (Angulo-Romero et al. 1999; Rodríguez-Rajo et al. 2003; Recio et al. 2012; Sidel et al. 2015; Ščevková et al. 2019; Fagodiya et al. 2022). However, none had included crop harvest data in the model analyses. This is the first study that reports on the contribution of crop harvesting to A. alternata spore concentrations.
During crop harvesting, there are harvest intervals when crops for consumption cannot be sprayed with fungicides and growers rely on good weather to proceed with harvesting, as was demonstrated with the significant correlations between A. alternata/Alternaria spp. spore concentrations and weather/crop harvest variables in our study. The 2016 results show that the temporal variations of A. alternata spore concentrations can be used as a proxy to predict other pathogenic and allergenic Alternaria species in an environment. To our knowledge, this is the first study that used the DNA of A. alternata spores to estimate the spore concentration of other Alternaria species in the air. A. alternata is considered a world-wide pathogen in agriculture (e.g. Kgatle et al. 2019). It is also found to be the most prevalent indoor Alternaria species in the USA (Woudenberg et al. 2015) and unlike many other Alternaria species A. alternata is not host specific. Therefore, it may be hypothesized that A. alternata may be a reasonable proxy to predict the development of a large range of pathogenic and allergenic Alternaria species within large parts of the world. A. alternata should therefore be a target for further investigations and in particular forecasting models.
An anomaly of high A. alternata and low Alternaria spp. spore concentrations during or after rain was observed in 2017. The low Alternaria spp. spore concentration is attributed to the scavenging effect of rain on larger bioaerosols such as intact Alternaria spp. spores including A. alternata (O’Connor et al. 2014), whilst spore fragments or small hyphae may escape this scavenging due to their smaller particle size (Berthet et al. 2010; Miki 2023). Raindrops, through spore dislodgement and with the support of wind, could have dispersed the already existing large amounts of A. alternata spores and spore/hyphal fragments on soil and vegetation or those in raindrops as was observed with fungal and bacterial spores (Joung et al. 2017; Zhai et al. 2018). The high A. alternata spore concentration after rain is due to sporulation and faster growth of the spores immediately after deposition on the right host and in a conducive environment, e.g. humidity (Humpherson-Jones and Phelps 1989; Hjelmroos 1993). Blagojević et al. (2020) showed that A. alternata spores grow faster than other common Alternaria species, e.g. A. brassicae, A. brassicicola and A. japonica over a wide range of temperature. Despite the complicating factors, then the most probable explanation of the anomaly seen in 2017 is how the scavenging effect by rain can efficiently remove larger intact spores, whilst a fraction of the smaller spore fragments and hyphae can remain airborne.
The model results suggest that, apart from its wide host range, the weather and crop harvesting, there are other significant and unknown underlying variables that were contributing to the dominance and abundance of A. alternata spores or spore/hyphal fragments over other Alternaria spp. spores in 2016. Previous studies also showed that the type of fungal species, weather conditions and mechanical disturbance of the spore source, could determine the simultaneous release and dominance of one type of species over others (Grewling et al. 2020; Madsen et al. 2016). Apart from the dominance and abundance of A. alternata spore equivalents, this study also demonstrated that the spores of A. alternata and other Alternaria species can be simultaneously released from their sources and have a similar distribution pattern when the weather, i.e. air temperature and relative humidity, is suitable for them all and crop harvesting takes place. This suggests the natural co-existence of A. alternata with other Alternaria species when they have similar growth and environmental conditions that facilitate their simultaneous dispersal. This complements and extends findings in Hanson et al.’s (2022) study, also undertaken in this rural – urban study region, that found several Alternaria species to be present at the same time. Audenaert et al. (2009) and Xu et al. (2005) also found positive co-existence patterns among Fusarium species, i.e. F. poae, F. avenaceum and F. culmorum. The simultaneous release of A. alternata and other Alternaria species also demonstrates the extent of fungal diversity in the air and its implication on human health and agriculture/forestry. Sensitised people can be simultaneously exposed to different fungal species in the air (Madsen et al. 2016). Hong et al. (2005) showed that 37 different Alternaria species including A. alternata, A. brassicicola, A. tenuisima and A. infectoria contain the major Alternaria allergen Alt a 1. Significantly high fungal diversity associated with an increase in symptoms of respiratory infections was observed in schoolchildren in fungal-infested and moisture-damaged schools (Meklin et al. 2005). Furthermore, different Alternaria species including A. alternata, A. solani and A. infectoria were isolated from closely located potato fields, signifying the potential of simultaneous infection by different Alternaria species for total yield loss in crops (Leiminger et al. 2014; Belosokhov et al. 2017). Knowledge of the co-existence of pathogenic and allergenic fungal communities is important for effective disease management, forecasting and allergy prevention.
Spatio-temporal variation between A. alternata and Alternaria spp. spore concentrations
We partly rejected the hypothesis that the high Alternaria spore concentrations in 2017 and 2018 were mainly contributed to by A. alternata. This suggests that other Alternaria species were the most dominant and in higher abundance than A. alternata during the spore seasons of 2017 and 2018. Weather played a significant role in the dominance and high abundance of other Alternaria species. Variation in the spatio-temporal pattern and peak weeks of Alternaria spp. compared to A. alternata spores is in line with Pashley et al. (2012), who attributed the differences to weather variables such as precipitation, relative humidity and wind speed. The spatio-temporal variations and non-significant relationships suggest that, besides A. alternata, there were other Alternaria species in the urban (Worcester) and rural (Lakeside Container) sites with differing spatio-temporal patterns that were being detected by microscopy. Studies of spatio-temporal patterns in Alternaria are rare. Ding et al. (2021) studied the spatio-temporal pattern of A. alternata and A. solani in potato fields in Wisconsin, USA, and found that A. alternata spores were always dispersed in late June whilst A. solani started later and both varied spatially, owing to differences in host ranges of the two species and variation in abundance of inoculum at the different potato fields. Although the urban (Worcester) and the rural (Lakeside Container) sites generally had ecosystems with similar hosts (grasslands and farmland; Apangu et al. 2020; Rowney et al. 2021), differences in the ecosystem conditions between the two sites could have caused the spatio-temporal variations of Alternaria spp. This was demonstrated by Reis and Boiteux (2010) who found that, although A. brassicae and A. brassicicola are both major pathogens of the Brassicaceae, A. brassicicola was more prevalent in Brassica oleracea complex whilst A. brassicae infects mostly the B. rapa complex, thus attacking the crops at different times of the growing season. Similarly, Schiro et al. (2018) found that Alternaria spp. spore distribution and abundance were strongly influenced by local ecological conditions within a topographically heterogeneous field of wheat.
The spatio-temporal variations in this study in 2017 and 2018 also demonstrate a lack of co-existence between A. alternata and other Alternaria species on the same hosts. A lack of co-existence and co-dispersal between fungal species may occur due to competition for space, antibiosis, differences in growth conditions and dissimilarities in response to environmental requirements (Nicolaisen et al. 2014). Antagonistic relationships due to antibiosis have been found within Alternaria spp. and between Alternaria spp. and other fungal species, e.g. Fusarium culmorum, Botrytis cinerea and Cladosporium herbarum (Kilic et al. 2010; Lević et al. 2012; Liggitt et al. 1997; Nicolaisen et al. 2014). A consequence of the non-significant relationship and antagonistic spatio-temporal pattern between A. alternata and Alternaria spp. spore concentrations is that allergy/asthma patients and farmers/foresters will observe many extended days of clinical and pathological significance. In general, there will be spatio-temporal differences in sporulation and release of spores from different Alternaria species into the atmosphere because different species of Alternaria are located on different substrates and will be at different stages of growth and in competition with other microbes especially during crop senescence and will respond differently to weather cues.
Difference between A. alternata and Alternaria spp. spore concentration in nearby rural and urban sites
The statistically similar Alternaria spp. spore concentrations at both sites was expected, since the two sites are closely located and generally have similar vegetation cover and crop production near the spore traps. Antón et al. (2021) found similar results for two samplers that were located 1.4 km apart. However, statistical analyses revealed that the rural site had significantly (p < 0.05) different A. alternata spore concentrations from the urban site in all the 3 years of spore observation. Moreover, the two sites also significantly varied from each other during the high days in 2017. Similarly, previous studies that examined Alternaria spp. spore concentrations between nearby (< 10 km) rural and urban sites also found statistically significant difference in spore concentrations and diversity between such sites (Hanson et al. 2022; Kasprzyk and Worek 2006; Lin et al. 2018; Oliveira et al. 2009). The variation between such nearby sites could be attributed to the difference in the density of crops near the traps, their spore emission potential (Apangu et al. 2020) and the effect of mechanical farming operations and local weather on Alternaria sporulation and spore release (Schiro et al. 2018; Skjøth et al. 2012, 2016). In examining the A. alternata pattern for two nearby (~ 7 km apart) sites, this study extends the previous studies of closely located sites that focused on only Alternaria spp. spores in general and suggests that care should be taken when comparing Alternaria spp. spores from several sites for application in clinical or pathological studies as the species composition and abundance can vary between nearby sites.
Comparison of optical microscopy with qPCR method in detecting Alternaria spp. and A. alternata spores
High concentrations of A. alternata spore equivalents from the qPCR, compared to the Alternaria spp. concentrations using microscopy, may partly be attributed to the fact that optical microscopy cannot always discriminate the different Alternaria species (Luo et al. 2007), whereas the DNA analysis can. Moreover, Parker et al. (2014) and Pizolotto et al. (2022) found very high concentrations of Sclerotinia sclerotiorum and Erysiphe betae, respectively, using qPCR in contrast to microscopy. Differences may also be attributed to variation in the sampling efficiencies of Burkard cyclones (16.5 L/min) and spore traps (10 L/min), which vary with particle size. The Burkard multi-vial cyclone sampler has a high sampling efficiency (93.82–100%), especially for small-sized (≤ 1 µm) bioaerosols (De Linares et al. 2022; Humbal et al. 2018; West et al. 2008) compared with the Burkard 7-day sampler that is most efficient for collecting particles with an aerodynamic diameter of > 5.2 µm (Ščevková and Kovac 2019). Almaguer et al. (2020) analysed fungal fragments alongside mature spores and found that hyphal fragments of Cladosporium and Leptosphaeria were abundant in the air and had no diurnal pattern. Their abundance and lack of diurnal pattern suggest that they are constantly abundant (day and night) in the air and hence can be captured by the cyclones. This puts further emphasis on the need to quantify fungal fragments in the air.
Conclusion
In summary, the study found high spore concentration of A. alternata spore equivalents (using qPCR) compared to Alternaria spp. (from optical microscopy), and this was likely due to spores and spore/hyphal fragments. The spore numbers should be used with caution because in most cases a fraction of the spore count obtained with the optical microscope originates from other Alternaria species. Besides A. alternata, many other Alternaria species remain unidentified in spore counts by microscopy. Furthermore, spore material may be present as fragments with the potential to penetrate further into the lungs. These fragments are not counted and are also less likely to be captured by the impaction technique used to deliver material on microscope slides. The spatio-temporal invariability in 2016 and variations in 2017 and 2018 in the distribution of A. alternata from other Alternaria species were due mainly to weather variations, especially mean air temperature and relative humidity and harvesting of cereals and oilseed rape. Future studies should examine such relationships between particular species of Alternaria and other pathogenic and allergenic fungal species, at a higher temporal resolution (less than a week) and with more emphasis on spore fragments. The rural site significantly varied from the urban site in A. alternata spore concentration even though they were closely located (approximately 7 km apart) and this was attributed to differences in local weather and harvesting activities.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Adams-Groom B, Skjøth CA, Selby K et al (2020) Regional calendars and seasonal statistics for the United Kingdom’s main pollen allergens. Allergy Eur J Allergy Clin Immunol 75:1492–1494. https://onlinelibrary.wiley.com/doi/full/10.1111/all.14168
Al-Lami HFD, You MP, Barbetti MJ (2019) Incidence, pathogenicity and diversity of Alternaria spp. associated with Alternaria leaf spot of canola (Brassica napus) in Australia. Plant Pathol 68:492–503. https://doi.org/10.1111/ppa.12955
Al-Lami HFD, You MP, Mohammed AE, Barbetti MJ (2020) Virulence variability across the Alternaria spp. population determines incidence and severity of Alternaria leaf spot on rapeseed. Plant Pathol 69:506–517. https://doi.org/10.1111/ppa.13135
Almaguer M, Díaz L, Fernández-González M, Valdéz E (2020) Allergenic fungal spores and hyphal fragments in the aerosol of Havana, Cuba. Aerobiologia 36:441–448. https://doi.org/10.1007/s10453-020-09643-x
Ammour MS, Bove F, Toffolatti SL, Rossi V (2020) A real-time PCR assay for the quantification of Plasmopara viticola Oospores in grapevine leaves. Front Microbiol 11:1–9. https://doi.org/10.3389/fpls.2020.01202
Angulo-Romero J, Mediavilla-Molina A, Domínguez-Vilches E (1999) Conidia of Alternaria in the atmosphere of the city of Cordoba, Spain in relation to meteorological parameters. Int J Biometeorol 43:45–49
Antón SF, Reyes ES, de la Cruz DR et al (2021) Comparison of Alternaria spore levels between two areas within the same city (Salamanca, Middle West Spain). Aerobiologia (bologna) 37:809–824. https://doi.org/10.1007/s10453-021-09725-4
Apangu GP, Frisk CA, Adams-Groom B et al (2020) Air mass trajectories and land cover map reveal cereals and oilseed rape as major local sources of Alternaria spores in the Midlands, UK. Atmos Pollut Res 11:1668–1679. https://doi.org/10.1016/j.apr.2020.06.026
Audenaert K, van Broeck R, van Bekaert B et al (2009) Fusarium head blight (FHB) in Flanders: population diversity, inter-species associations and DON contamination in commercial winter wheat varieties. Eur J Plant Pathol 125:445–458. https://doi.org/10.1007/s10658-009-9494-3
Avolio E, Pasqualoni L, Federico S et al (2008) Correlation between large-scale atmospheric fields and the olive pollen season in Central Italy. Int J Biometeorol 52:787–796. https://doi.org/10.1007/s00484-008-0172-5
BAF (1995) Airborne pollens and spores: a guide to trapping and counting. BAF, The British Aerobiology Federation, Aylesford
Belosokhov AF, Chudinova EM, Kokaeva LY et al (2017) Alternaria spp. and Colletotrichum coccodes in potato leaves with early blight symptoms. Sixt Euroblight Work Aarhus, Denmark 181–190
Berthet S, Leriche M, Pinty JP et al (2010) Scavenging of aerosol particles by rain in a cloud resolving model. Atmos Res 96:325–336. https://doi.org/10.1016/j.atmosres.2009.09.015
Black J, Dean T, Byfield G et al (2013) Determining fungi rRNA copy number by PCR. J Biomol Tech 24:32–38. https://doi.org/10.7171/jbt.13-2401-004
Blagojević JD, Vukojević JB, Ivanović ZS (2020) Occurrence and characterization of Alternaria species associated with leaf spot disease in rapeseed in Serbia. Plant Pathol 69:883–900. https://doi.org/10.1111/ppa.13168
Brennan GL, Potter C, de Vere N et al (2019) Temperate airborne grass pollen defined by spatio-temporal shifts in community composition. Nat Ecol Evol 3:750–754. https://doi.org/10.1038/s41559-019-0849-7
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
Chen L, Fang K, Dong XF et al (2020) Characterization of the fungal community in the canopy air of the invasive plant Ageratina adenophora and its potential to cause plant diseases. PLoS ONE 15:1–16. https://doi.org/10.1371/journal.pone.0230822
Damialis A, Gioulekas D (2006) Airborne allergenic fungal spores and meteorological factors in Greece: forecasting possibilities. Grana 45:122–129. https://doi.org/10.1080/00173130600601005
De Linares C, Navarro D, Puigdemunt R, Belmonte J (2022) Airborne Alt a 1 dynamic and its relationship with the airborne dynamics of Alternaria conidia and Pleosporales spores. J Fungi 8:1–12. https://doi.org/10.3390/jof8020125
Degois J, Simon X, Bontemps C et al (2019) Characterization of experimental complex fungal bioaerosols: impact of analytical method on fungal composition measurements. Aerosol Sci Technol 53:146–159. https://doi.org/10.1080/02786826.2018.1557320
Ding S, Meinholz K, Gevens AJ (2021) Spatiotemporal distribution of potato-associated Alternaria species in Wisconsin. Plant Dis 105:149–155. https://doi.org/10.1094/PDIS-11-19-2290-RE
Escuredo O, Seijo MC, Fernández-González M, Iglesias I (2011) Effects of meteorological factors on the levels of Alternaria spores on a potato crop. Int J Biometeorol 55:243–252. https://doi.org/10.1007/s00484-010-0330-4
Ettenauer JD, Piñar G, Lopandic K et al (2012) Microbes on building materials—evaluation of DNA extraction protocols as common basis for molecular analysis. Sci Total Environ 439:44–53. https://doi.org/10.1016/j.scitotenv.2012.09.005
Fagodiya RK, Trivedi A, Fagodia BL (2022) Impact of weather parameters on Alternaria leaf spot of soybean. Sci Rep 12:1–10. https://doi.org/10.1038/s41598-022-10108-z
Fröhlich-Nowoisky J, Burrows SM, Xie Z et al (2012) Biogeography in the air: fungal diversity over land and oceans. Biogeosciences 9:1125–1136. https://doi.org/10.5194/bg-9-1125-2012
Gabriel MF, Postigo I, Gutiérrez-Rodríguez A et al (2015) Development of a PCR-based tool for detecting immunologically relevant Alt a 1 and Alt a 1 homologue coding sequences. Med Mycol 53:636–642. https://doi.org/10.1093/mmy/myv022
Gabriel MF, Postigo I, Tomaz CT, Martínez J (2016) Alternaria alternata allergens: markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ Int 89–90:71–80. https://doi.org/10.1016/j.envint.2016.01.003
Galán C, Smith M, Thibaudon M et al (2014) Pollen monitoring: minimum requirements and reproducibility of analysis. Aerobiologia 30:385–395. https://doi.org/10.1007/s10453-014-9335-5
Galán C, Smith M, Damialis A et al (2021) Airborne fungal spore monitoring: between analyst proficiency testing. Aerobiologia (Bologna) 2021:1–11. https://doi.org/10.1007/s10453-021-09698-4
Górny RL, Reponen T, Willeke K et al (2002) Fungal fragments as indoor air biocontaminants. Appl Environ Microbiol 68:3522–3531. https://doi.org/10.1128/AEM.68.7.3522-3531.2002
Green BJ, Sercombe JK, Tovey ER (2005) Fungal fragments and undocumented conidia function as new aeroallergen sources. J Allergy Clin Immunol 115:1043–1048. https://doi.org/10.1016/j.jaci.2005.02.009
Green BJ, Tovey ER, Sercombe JK et al (2006) Airborne fungal fragments and allergenicity. Med Mycol 44:245–255. https://doi.org/10.1080/13693780600776308
Grewling Ł, Bogawski P, Szymańska A et al (2020) Particle size distribution of the major Alternaria alternata allergen, Alt a 1, derived from airborne spores and subspore fragments. Fungal Biol 124:219–227. https://doi.org/10.1016/j.funbio.2020.02.005
Grinn-Gofroń A, Sadyś M, Kaczmarek J et al (2016) Back-trajectory modelling and DNA-based species-specific detection methods allow tracking of fungal spore transport in air masses. Sci Total Environ 571:658–669. https://doi.org/10.1016/j.scitotenv.2016.07.034
Grinn-Gofroń A, Bosiacka B, Bednarz A, Wolski T (2018) A comparative study of hourly and daily relationships between selected meteorological parameters and airborne fungal spore composition. Aerobiologia 34:45–54. https://doi.org/10.1007/s10453-017-9493-3
Grinn-Gofroń A, Nowosad J, Bosiacka B et al (2019) Airborne Alternaria and Cladosporium fungal spores in Europe: forecasting possibilities and relationships with meteorological parameters. Sci Total Environ 653:938–946. https://doi.org/10.1016/j.scitotenv.2018.10.419
Hanson MC, Petch GM, Ottosen T-B, Skjøth CA (2022) Climate change impact on fungi in the atmospheric microbiome. Sci Total Environ 830:1–10. https://doi.org/10.1016/j.scitotenv.2022.154491
Hirst JM (1952) An automatic volumetric spore trap. Ann Appl Biol 39:257–265. https://doi.org/10.1111/j.1744-7348.1952.tb00904.x
Hjelmroos M (1993) Relationship between airborne fungal spore presence and weather variables: Cladosporium and Alternaria. Grana 32:40–47. https://doi.org/10.1080/00173139309436418
Hong SG, Cramer RA, Lawrence CB, Pryor BM (2005) Alt a 1 allergen homologs from Alternaria and related taxa: analysis of phylogenetic content and secondary structure. Fungal Genet Biol 42:119–129. https://doi.org/10.1016/j.fgb.2004.10.009
Humbal C, Gautam S, Trivedi U (2018) A review on recent progress in observations, and health effects of bioaerosols. Environ Int 118:189–193. https://doi.org/10.1016/j.envint.2018.05.053
Humpherson-Jones FM, Phelps K (1989) Climatic factors influencing spore production in Alternaria brassicae and Alternaria brassicicola. Ann Appl Biol 114:449–458. https://doi.org/10.1111/j.1744-7348.1989.tb03360.x
Joung YS, Ge Z, Buie CR (2017) Bioaerosol generation by raindrops on soil. Nat Commun 8(14668):1–10. https://doi.org/10.1038/ncomms14668
Kasprzyk I (2008) Non-native Ambrosia pollen in the atmosphere of Rzesz (SE Poland); Evaluation of the effect of weather conditions on daily concentrations and starting dates of the pollen season. Int J Biometeorol 52:341–351. https://doi.org/10.1007/s00484-007-0129-0
Kasprzyk I, Worek M (2006) Airborne fungal spores in urban and rural environments in Poland. Aerobiologia 22:169–176. https://doi.org/10.1007/s10453-006-9029-8
Kgatle MG, Flett B, Truter M et al (2019) Distribution of Alternaria leaf blight of sunflowers caused by Alternaria alternata in South Africa. J Agric Rural Dev Trop Subtrop 120:71–77. https://doi.org/10.17170/kobra-20190613558
Kilic M, Ufuk Altintas D, Yilmaz M et al (2010) The effects of meteorological factors and Alternaria spore concentrations on children sensitised to Alternaria. Allergol Immunopathol (Madr) 38:122–128. https://doi.org/10.1016/j.aller.2009.09.006
Kulik T, Treder K, Załuski D (2015) Quantification of Alternaria, Cladosporium, Fusarium and Penicillium verrucosum in conventional and organic grains by qPCR. J Phytopathol 163:522–528. https://doi.org/10.1111/jph.12348
Lacey J, Allit U (1995) Airborne pollen and spores: a guide to trapping and counting. The British Aerobiology Federation, Harpenden
Lear G, Dickie I, Banks J et al (2018) Methods for the extraction, storage, amplification and sequencing of DNA from environmental samples. N Z J Ecol 42:1A–50A. https://doi.org/10.20417/nzjecol.42.9
Lee SA, Liao CH (2014) Size-selective assessment of agricultural workers’ personal exposure to airborne fungi and fungal fragments. Sci Total Environ 466–467:725–732. https://doi.org/10.1016/j.scitotenv.2013.07.104
Leiminger J, Bäßler E, Knappe C et al (2014) Quantification of disease progression of Alternaria spp. on potato using real-time PCR. Eur J Plant Pathol 141:295–309. https://doi.org/10.1007/s10658-014-0542-2
Lević J, Stanković S, Krnjaja V et al (2012) Relationships of mycobiota on rachides and kernels of wheat. Eur J Plant Pathol 134:249–256. https://doi.org/10.1007/s10658-012-9982-8
Liggitt J, Jenkinson P, Parry DW (1997) The role of saprophytic microflora in the development of Fusarium ear blight of winter wheat caused by Fusarium culmorum. Crop Prot 16:679–685. https://doi.org/10.1016/S0261-2194(97)00039-2
Lin WR, Wang PH, Tien CJ et al (2018) Changes in airborne fungal flora along an urban to rural gradient. J Aerosol Sci 116:116–123. https://doi.org/10.1016/j.jaerosci.2017.11.010
López Couso VP, Tortajada-Girbés M, Gil DR et al (2021) Fungi sensitization in spain: importance of the Alternaria alternata species and its major allergen alt a 1 in the allergenicity. J Fungi 7:1–11. https://doi.org/10.3390/jof7080631
Luo Y, Ma Z, Reyes HC et al (2007) Quantification of airborne spores of Monilinia fructicola in stone fruit orchards of California using real-time PCR. Eur J Plant Pathol 118:145–154. https://doi.org/10.1007/s10658-007-9124-x
Madsen AM, Larsen ST, Koponen IK et al (2016) Generation and characterization of indoor fungal aerosols for inhalation studies. Appl Environ Microbiol 82:2479–2493. https://doi.org/10.1128/AEM.04063-15
Makra L, Sánta T, Matyasovszky I et al (2010) Airborne pollen in three European cities: detection of atmospheric circulation pathways by applying three-dimensional clustering of backward trajectories. J Geophys Res Atmos 115. https://doi.org/10.1029/2010JD014743
Mari A, Schneider P, Wally V et al (2003) Sensitization to fungi: epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin Exp Allergy 33:1429–1438. https://doi.org/10.1046/j.1365-2222.2003.01783.x
Martinez-Bracero M, Markey E, Clancy JH et al (2022) Airborne fungal spore review, new advances and automatisation. Atmosphere 13:1–26
Meena M, Gupta SK, Swapnil P et al (2017) Alternaria toxins: potential virulence factors and genes related to pathogenesis. Front Microbiol 8:1–14. https://doi.org/10.3389/fmicb.2017.01451
Meklin T, Potus T, Pekkanen J et al (2005) Effects of moisture-damage repairs on microbial exposure and symptoms in schoolchildren. Indoor Air, Suppl 15:40–47. https://doi.org/10.1111/j.1600-0668.2005.00357.x
Miki K (2023) Particle motion determines the types of bioaerosol particles in the stratosphere. Int J Astrobiol 1–11. https://doi.org/10.1017/S1473550422000441
Mitakakis TZ, Clift A, McGee PA (2001) The effect of local cropping activities and weather on the airborne concentration of allergenic Alternaria spores in rural Australia. Grana 40:230–239. https://doi.org/10.1080/001731301317223268
Neukirch C, Henry C, Leynaert B et al (1999) Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol 103:709–711
Nexcelom Bioscience (2018) How to count cells using a haemocytometer. https://www.nexcelom.com/applications/cellometer/cell-counting/counting-mammalian-cells-using-a-hemacytometer/. Accessed 24 Mar 2019
Nicolaisen M, Justesen AF, Knorr K et al (2014) Fungal communities in wheat grain show significant co-existence patterns among species. Fungal Ecol 11:145–153. https://doi.org/10.1016/j.funeco.2014.06.002
Nowicki M, Nowakowska M, Niezgoda A, Kozik E (2012) Alternaria black spot of crucifers: Symptoms, importance of disease, and perspectives of resistance breeding. Veg Crop Res Bull 76:5–19. https://doi.org/10.2478/v10032-012-0001-6
O’Connor DJ, Sadyś M, Skjøth CA et al (2014) Atmospheric concentrations of Alternaria, Cladosporium, Ganoderma and Didymella spores monitored in Cork (Ireland) and Worcester (England) during the summer of 2010. Aerobiologia 30:397–411. https://doi.org/10.1007/s10453-014-9337-3
Ojaghian S, Mirzaei A, Ling W (2018) Forecasting potato white mold by assessment of ascospores in Iran fields. J Plant Prot Res 58:168–175. https://doi.org/10.24425/122932
Oliveira M, Ribeiro H, Delgado JL, Abreu I (2009) Seasonal and intradiurnal variation of allergenic fungal spores in urban and rural areas of the North of Portugal. Aerobiologia 25:85–98. https://doi.org/10.1007/s10453-009-9112-z
Olsen Y, Gosewinkel UB, Skjøth CA et al (2019) Regional variation in airborne Alternaria spore concentrations in Denmark through 2012–2015 seasons: the influence of meteorology and grain harvesting. Aerobiologia 35:533–551. https://doi.org/10.1007/s10453-019-09587-x
Paldy A, Bobvos J, Fazekas B, Manyoki G, Malnasi T, Magyar D (2014) Characterisation of the pollen season by using climate specific pollen indicators. Eur J Occup Environ Med 20:199–214
Parker ML, McDonald MR, Boland GJ (2014) Evaluation of air sampling and detection methods to quantify airborne ascospores of Sclerotinia sclerotiorum. Plant Dis 98:32–42. https://doi.org/10.1094/PDIS-02-13-0163-RE
Pashley CH, Fairs A, Free RC, Wardlaw AJ (2012) DNA analysis of outdoor air reveals a high degree of fungal diversity, temporal variability, and genera not seen by spore morphology. Fungal Biol 116:214–224. https://doi.org/10.1016/j.funbio.2011.11.004
Patel V, Chevignon G, Manzano-Marın A et al (2019) Cultivation-assisted genome of candidatus Fukatsuia symbiotica; the enigmatic “X-type” symbiont of aphids. Genome Biol Evol 11:3510–3522. https://doi.org/10.1093/gbe/evz252
Pavón M ángel, González I, Martín R, García Lacarra T (2012) ITS-based detection and quantification of Alternaria spp. in raw and processed vegetables by real-time quantitative PCR. Food Microbiol 32:165–171.https://doi.org/10.1016/j.fm.2012.05.006
Pizolotto CA, Harrington M, Brown L et al (2022) A real-time PCR assay for Erysiphe betae and its effectiveness when used with different spore trapping methods. Eur J Plant Pathol 162:329–341. https://doi.org/10.1007/s10658-021-02405-6
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 12 May 2021
Recio M, del Mar TM, Docampo S et al (2012) Analysis of the predicting variables for daily and weekly fluctuations of two airborne fungal spores: Alternaria and Cladosporium. Int J Biometeorol 56:983–991. https://doi.org/10.1007/s00484-011-0509-3
Reis A, Boiteux L (2010) Alternaria species infecting Brassicaceae in the Brazilian Neotropics: geographical distribution, host range and specificity. J Plant Pathol 92:661–668
Rodríguez-Rajo FJ, Frenguelli G, Jato MV (2003) Effect of air temperature on forecasting the start of the Betula pollen season at two contrasting sites in the south of Europe (1995–2001). Int J Biometeorol 47:117–125. https://doi.org/10.1007/s00484-002-0153-z
Rosemond JS, Kramer LC (1971) Identifying hyphal fragments in the atmosphere. Trans Kansas Acad Sci 74:48–51
Rowney FM, Brennan GL, Skjøth CA et al (2021) Environmental DNA reveals links between abundance and composition of airborne grass pollen and respiratory health. Curr Biol 31:1–9. https://doi.org/10.1016/j.cub.2021.02.019
Sadyś M, Skjøth CA, Kennedy R (2014) Back-trajectories show export of airborne fungal spores (Ganoderma sp.) from forests to agricultural and urban areas in England. Atmos Environ 84:88–99. https://doi.org/10.1016/j.atmosenv.2013.11.015
Sadyś M, Skjøth CA, Kennedy R (2015) Determination of Alternaria spp. habitats using 7-day volumetric spore trap, hybrid single particle lagrangian integrated trajectory model and geographic information system. Urban Clim 14:429–440. https://doi.org/10.1016/j.uclim.2014.08.005
Sadyś M, Adams-Groom B, Herbert RJ, Kennedy R (2016) Comparisons of fungal spore distributions using air sampling at Worcester, England (2006–2010). Aerobiologia 32:619–634. https://doi.org/10.1007/s10453-016-9436-4
Saha A, Strader M, Green B et al (2022) Proteomic evaluation of Alternaria alternata spores, hyphae, and commercial allergen extracts. J Allergy Clin Immunol 149:AB217. https://doi.org/10.1016/J.JACI.2021.12.711
Ščevková J, Kovac J (2019) First fungal spore calendar for the atmosphere of Bratislava, Slovakia. Aerobiologia 35:343–356
Ščevková J, Hrabovský M, Kováč J, Rosa S (2019) Intradiurnal variation of predominant airborne fungal spore biopollutants in the Central European urban environment. Environ Sci Pollut Res 26:34603–34612. https://doi.org/10.1007/s11356-019-06616-7
Schiro G, Verch G, Grimm V, Müller MEH (2018) Alternaria and Fusarium fungi: differences in distribution and spore deposition in a topographically heterogeneous wheat field. J Fungi 4. https://doi.org/10.3390/jof4020063
Shokere LA, Holden MJ, Ronald Jenkins G (2009) Comparison of fluorometric and spectrophotometric DNA quantification for real-time quantitative PCR of degraded DNA. Food Control 20:391–401. https://doi.org/10.1016/j.foodcont.2008.07.009
Sidel FFB, Bouziane H, Trigo MdM et al (2015) Airborne fungal spores of Alternaria, meteorological parameters and predicting variables. Int J Biometeorol 59:339–346.https://doi.org/10.1007/s00484-014-0845-1
Skjøth CA, Sommer J, Frederiksen L, Gosewinkel Karlson U (2012) Crop harvest in Denmark and Central Europe contributes to the local load of airborne Alternaria spore concentrations in Copenhagen. Atmos Chem Phys 12:11107–11123. https://doi.org/10.5194/acp-12-11107-2012
Skjøth CA, Baker P, Sadyś M, Adams-Groom B (2015) Pollen from alder (Alnus sp.), birch (Betula sp.) and oak (Quercus sp.) in the UK originate from small woodlands. Urban Clim 14:414–428. https://doi.org/10.1016/j.uclim.2014.09.007
Skjøth CA, Damialis A, Belmonte J et al (2016) Alternaria spores in the air across Europe: abundance, seasonality and relationships with climate, meteorology and local environment. Aerobiologia 32:3–22. https://doi.org/10.1007/s10453-016-9426-6
Smith DJ, Jaffe DA, Birmele MN et al (2012) Free tropospheric transport of microorganisms from Asia to North America. Microb Ecol 64:973–985. https://doi.org/10.1007/s00248-012-0088-9
Tomlin JM, Jankowski KA, Rivera-Adorno FA et al (2020) Chemical imaging of fine mode atmospheric particles collected from a research aircraft over agricultural fields. ACS Earth Sp Chem 4:2171–2184. https://doi.org/10.1021/acsearthspacechem.0c00172
Trigo MD, Toro FJ, Recio M, Cabezudo B (2000) A statistical approach to comparing the results from different aerobiological stations. Grana 39:252–258
West JS, Atkins SD, Emberlin J, Fitt BDL (2008) PCR to predict risk of airborne disease. Trends Microbiol 16:380–387. https://doi.org/10.1016/j.tim.2008.05.004
West JS, Canning GGM, Perryman SA, King K (2017) Novel technologies for the detection of Fusarium head blight disease and airborne inoculum. Trop Plant Pathol 42:203–209
Woudenberg JHC, Seidl MF, Groenewald JZ et al (2015) Alternaria section Alternaria: species, formae speciales or pathotypes? Stud Mycol 82:1–21. https://doi.org/10.1016/j.simyco.2015.07.001
Xu XM, Parry DW, Nicholson P et al (2005) Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. Eur J Plant Pathol 112:143–154. https://doi.org/10.1007/s10658-005-2446-7
Zeng QY, Westermark SO, Rasmuson-Lestander Å, Wang XR (2006) Detection and quantification of Cladosporium in aerosols by real-time PCR. J Environ Monit 8:153–160. https://doi.org/10.1039/b509515h
Zhai Y, Li X, Wang T et al (2018) A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ Internat 113:74–90. https://doi.org/10.1016/j.envint.2018.01.007
Acknowledgements
This study was funded by the European Commission through a Marie Curie Career Integration Grant (Project ID CIG630745 and Acronym SUPREME) awarded to Carsten A. Skjøth and that co-financed the Ph.D. project awarded to Godfrey P. Apangu.
Author information
Authors and Affiliations
Contributions
Godfrey P. Apangu, Mary Hanson and Carsten A. Skjøth contributed to the study conception and design. Material preparation, data collection and analysis were performed by all the authors. The first draft of the manuscript was written by Godfrey P. Apangu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Apangu, G.P., Frisk, C.A., Adams-Groom, B. et al. Using qPCR and microscopy to assess the impact of harvesting and weather conditions on the relationship between Alternaria alternata and Alternaria spp. spores in rural and urban atmospheres. Int J Biometeorol 67, 1077–1093 (2023). https://doi.org/10.1007/s00484-023-02480-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-023-02480-w