Abstract
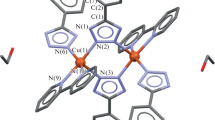
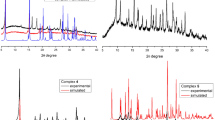
Complex [Cu(phen)2Cl]Cl∙2PABA∙4H2O has been isolated, where phen ligand is 1,10-phenanthroline and PABA is p-aminobenzoic acid. The complex crystallizes in the centrosymetric (monoclinic) space group P2(1)/n. The unit cell dimensions are a = 10.479(4) Å, b = 22.396(7) Å, and c = 16.212(6) Å. The basic unit structure of complex is distorted trigonal bipyramidal. Two hydrogen-bonded PABA molecules are enclosed through π−π interactions in filled aryl box FAB motif made by juxtaposition of four phen from two opposing cations. While π−π interaction between phen causes the formation of offset face-to-face overlap OFF primary motif. The result is formation of extended (OFF-FAB)n chains. Cyclic voltammetry showed one reversible oxidation reduction process followed by one irreversible oxidation process. Thermal analysis indicated successive loss of crystalline water and crystalline PABA molecules, chloride ions, and phen ligands. DFT study indicates that PABA interacts with phen with binding energy 68.87 kJ mol−1 reflecting strong π-stacking. The charge density in HOMO is localized on the metal halogen bond, whereas in LUMO, it is spread over two phen. The vibrational spectrum of complex was calculated at M05-2X/6-31G(d) and compared with the experimental vibrational spectrum data. The inhibitory concentrations IC50 against carcinoma cells A549 (lung adenocarcinoma) and MDA-MB-231 (breast adenocarcinoma) are 3.4 and 4.5 µM, respectively. IC50 values of new complex are comparable to those of cisplatin and smaller than the values for [Cu(phen)2Cl]Cl∙6.5H2O.
Graphic abstract












Similar content being viewed by others
References
Gandra RM, Mc Carron P, Fernandes MF, Ramos LS, Mello TP, Aor AC, Branquinha MH, McCann M, Devereux M, Santos ALS (2017) Front Microbiol 8:1257
Coyle B, Kinsella P, McCann M, Devereux M, O’Connor R, Clynes M, Kavanagh K (2004) Toxicol In Vitro 18:63
Coyle B, Kavanagh K, McCann M, Devereux M, Geraghty M (2003) Biometals 16:321
Viganor L, Howe O, McCarron P, McCann M, Devereux M (2017) Curr Top Med Chem 17:1280
Turel I, Golobič A, Kljun J, Samastur P, Batista U, Sepčić K (2015) Acta Chim Slov 62:337
Raman N, Raja J (2007) Indian J Chem Sect A 46:1611
Chandraleka S, Ramya K, Chandramohan G, Dhanasekaran D, Priyadharshini A, Panneerselvam A (2014) J Saudi Chem Soc 18:953
Byrnes R, Antholine W, Petering D (1992) Free Radical Bio Med 12:457
Devereux M, O’Shea D, O’Connor M, Grehan H, Connor G, McCann M, Rosair G, Lyng F, Kellett A, Walsh M, Thati EB (2007) Polyhedron 26:4073
De Vizcaya-Ruiz A, Rivero-Mueller A, Ruiz-Ramirez L, Kass G, Kelland L, Or R, Dobrota M (2000) Toxicol In Vitro 14:1
Allardyce CS, Dyson PJ (2016) Dalton Trans 45:3201
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C (2014) Chem Rev 114:815
Hussain A, AlAjmi MF, Rehman MT, Amir S, Husain FM, Alsalme A, Siddiqui MA, AlKhedhairy AA, Khan RA (2019) Sci Rep 9:5237
Wehbe M, Lo C, Leung AWY, Dragowska WH, Ryan GM, Bally MB (2017) Invest New Drugs 35:682
de Souza ÍP (2019) Drug Des Int Prop Int J 3:333
Ndagi U, Mhlongo N, Soliman ME (2017) Drug Des Devel Ther 11:599
Hammud HH, Kortz U, Bhattacharya S, Demirdjian S, Hariri E, Isber S, Choi ES, Mirtamizdoust B, Mroueh M, Daher CF (2020) Inorg Chim Acta 506:119533
Lu X, Zhang B, Zhang W, Cheng Y, Sun X (2016) Preparation, crystal structure, and antitumor activity of copper complexes of amino acid and o-phenanthroline or derivatives. Patent CN 105440059, Mar 30, 2016; (2016) Chem Abstr 164: 501169
Saha R, Sengupta S, Dey SK, Steele IM, Bhattacharyya A, Biswas S, Kumar S (2014) RSC Adv 4:49070
Bond AD (2007) CrystEngComm 9:833
Zaworotko M, Hammud H, Kravtsov V (2007) Chem Crystallogr 27:219
Abraham RJ, Eivazi F, Pearson H, Smith KMJ (1976) Chem Soc Chem Commun 698–699
Abraham RJ, Eivazi F, Pearson H, Smith KM (1976) J Chem Soc Chem Commun 699–701
Hunter CA, Sanders JKM (1990) J Am Chem Soc 112:5525
Shao Y, Yin G-Z, Ren X, Zhang X, Wang J, Guo K, Li X, Wesdemiotis C, Zhang W-B, Yang S, Zhu M, Sun B (2017) RSC Adv 7:6530
Shao C, Grune M, Stolte M, Wurthner F (2012) Chem Eur J 18:13665
Hammud HH, El Hamaoui B, Noubani NH, Feng X, Wu Z-S, Müllen K, Ayub K (2018) Nanosci Nanotechnol-Asia 8:263
Onawumi OO, Adekunle FA, Ibrahim AO, Rajasekharan MV, Odunola OA (2010) Synth React Inorg Met-Org Chem 40:78
Liping L, Shidong Q, Pin Y, Miaoli Z (2004) Acta Cryst E60:m574
Sundholm D, Sundberg M, Uggla R (1998) J Phys Chem A 102:137
Suezawa H, Yoshida T, Umezawa Y, Tsuboyama S, Nishio M (2002) Eur J Inorg Chem 12:3148–3155
Bogdanovic G, Spasojevic-de Brie A, Zaric S (2002) Eur J Inorg Chem 7:1599–1602
Janiak C (2000) J Chem Soc Dalton Trans 21:3885–3896
Kabbani A, Zaworotko M, Abourahma H, Walsh R, Hammud H (2004) J Chem Crystallogr 34:749
Horn C, Berben L, Chow H, Scudder M, Dance I (2002) Cryst Eng Comm 4:7
Russell V, Scudder M, Dance I (2001) J Chem Soc. Dalton Trans 6:789–799
Horn C, Scudder M, Dance I (2000) Cryst Eng Comm 2:196
Shaabani B, Mirtamizdoust B, Viterbo D, Croce G, Hammud H, Hojati-lalemi P, Khandar A (2011) Z Anorg Allg Chem 637:713
Zaworotko M, Hammud H, Kabbani A, McManus G, Ghannoum A, Masoud M (2009) J Chem Crystallogr 39:853
Mirtamizdoust B, Trávnícˇek Z, Hanifehpour Y, Talemi P, Hammud H, Joo SW (2017) Ultrason Sonochem 34:255
Chai J-D, Head-Gordon M (2008) Phys Chem Chem Phys 10:6615
Becke AD (1993) J Chem Phys 98:5648
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Perdew JP, Burke K, Ernzerhof M (1997) Phys Rev Lett 78:1396
Zhao Y, Schultz NE, Truhlar DG (2006) J Chem Theory Comput 2:364
Lakshmipraba J, Arunachalam S, Gandi DA, Thirunalasundari T (2011) Eur J Med Chem 46:3013
Low ML, Cheang CW, Ng PY, Ooi IH, Maah MJ, Chye SM, Tan KW, Ng SW, Ng CH (2017) J Coord Chem 70:223
Mejia C, Ruiz-Azuara L (2008) Pathol Oncol Res 14:467
Kumar V, Mudgal MM, Rani N, Jha A, Jaggi M, Singh AT, Sanna VK, Singh P, Sharma PK, Irchhaiya R, Burman AC (2009) J Enzym Inhib Med Chem 24:763
Al-Omair MA (2019) Arab J Chem 12:1061
Mroueh M, Daher C, Hariri E, Demirdjian S, Isber S, Choi ES, Mirtamizdoust B, Hammud HH (2015) Chem Biol Interact 231:53
Hammud HH, Nemer G, Sawma W, Touma J, Barnabe P, Bou-Mouglabey Y, Ghannoum A, El-Hajjar J, Usta J (2008) Chem Biol Interact 173:84
Iglesias S, Alvarez N, Torre MH, Kremer E, Ellena J, Ribeiro RR, Barroso RP, Costa-Filho AJ, Kramer MG, Facchin G (2014) J Inorg Biochem 139:117
Alvarez N, Noble C, Torre MH, Kremer E, Ellena J, de Araujo MP, Costa-Filho AJ, Mendes LF, Kramer MG, Facchin G (2017) Inorg Chim Acta 466:559
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 09, Revision C.01. Gaussian Inc, Wallingford
Acknowledgements
The authors acknowledge the Deanship of Scientific Research at King Faisal University, Kingdom of Saudi Arabia for the financial support under Nasher Track (Grant No. 186060).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hammud, H.H., McManus, G.J., Zaworotko, M.J. et al. The co-crystal of copper(II) phenanthroline chloride complex hydrate with p-aminobenzoic acid: structure, cytotoxicity, thermal analysis, and DFT calculation. Monatsh Chem 152, 323–336 (2021). https://doi.org/10.1007/s00706-021-02742-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02742-6