Abstract
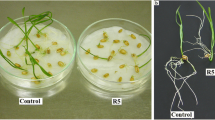
The stress induced by allelochemicals present in stem aqueous extract (SAE) of Nicotiana plumbaginifolia on alterations in growth, ultrastructure on Cassia tora L., and mitotic changes on Allium cepa L. were inspected. Application of SAE at different concentrations (0.5, 1, 2, and 4%) expressively reduced the growth of C. tora in terms of seedling length and dry biomass. Moreover, the ultrastructural variations induced in the epidermis of Cassia leaf (adaxial and abaxial surface) of 15-day-old saplings were analyzed through scanning electron microscopy (SEM). The variations noticed are rupturing and shrinking of cells along epidermis; damaged margins, extensively curled leaf apex along with the appearance of puff-like structures, grooves, and thread-like structures on the leaf surface. The epidermal cells of samples exposed to treatment no longer appear smooth relative to control, besides showing necrosis as well. Upon exposure to different concentrations of extract, A. cepa root tip cells showed aberrations in chromosome arrangement and disparity in the shape of the interphase and prophase nuclei along various phases of mitotic cycle as compared to control. The mitotic index (MI) showed a concentration-dependent decline in onion root tips exposed to SAE. The aberrations appearing frequently were formation of multinucleated cells, sticky metaphase and anaphase with bridges, sticky telophase, disturbed polarity, etc. The results also show the induction of elongated cells, giant cells, and cells with membrane damage by extract treatment. To our knowledge, this is the first gas chromatography-mass spectrometry (GC-MS) analysis of the methanolic extract of N. plumbaginifolia stem. Overall, 62 compounds were reported, covering 99.61% of the entire constituents, which can be considered responsible for the allelopathic suppression of C. tora. The chief component was 4-tert-butylcalix[4]arene with the highest composition of 19.89%, followed by palmitic acid (12.25%), palmitoleic acid (8.23%), precocene 2 (7.53%), isophytyl acetate (4.01%), and betastigmasterol (3.95%).







Similar content being viewed by others
References
Abouziena HF, Haggag WM (2016) Weed control in clean agriculture: a review1. Planta 34(2):377–392
Ajaib M, Fatima S, Kamran SH, Khan KM, Perveen S, Shah S (2016) Comparative antidiabetic evaluation of different parts of Nicotiana Plumbajnifolia in alloxan induced diabetic mice. J Chem Soc Pak 38:1267–1267
Ahmed SM, Abdelgaleil SAM (2005) Antifungal activity of extracts and sesquiterpene lactones from Magnolia grandiflora L. (Magnoliaceae). Int J Agric Biol 7:638–642
Anonymous (1966) Nicotiana L. In: Zaheer SH, Prasad B, Chopra RN, Santapau H, Krishnan MS, Deshaprabhu SB (eds) The wealth of India, raw materials, vol VII. CSIR Publications, New Delhi, India, p 46
Arora K (2013) Mitotic aberrations induced by Cassia ocidentalis L. in Allium cepa L. root tip cells. Indian J Fund Appl Life Sci 3:1–4
Arora N, Pandey-Rai S (2014) GC–MS analysis of the essential oil of Celastrus paniculatus Willd. Seeds and antioxidant, anti-inflammatory study of its various solvent extracts. Ind Crop Prod 61:345–351
Celik TA, Aslanturk OS (2010) Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. J BioMed Biotechnol, Vol 2010, doi:https://doi.org/10.1155/2010/189252,
Bajwa AA (2014) Sustainable weed management in conservation agriculture. Crop Prot 65:105–113
Bergin D (2011) Weed control options for coastal sand dunes: a review. New Zealand Forest research institute LTD, pp. 5–13
Blum U (2011) Plant-plant allelopathic interactions: phenolic acids, cover crops and weed emergence. Springer, London
Borghetti F, Silva LCR, Pinheiro JD, Varella BB, Ferreira AG (2005) Aqueous leaf extract properties of Cerrado species in Central Brazil. In: Harper JDI, An M, Wu H, Kent JH (eds) Proceedings and selected papers: fourth world congress on allelopathy. The George Stutt University, Wagga, pp 388–390
Borghetti F, Lima ECD, Silva LCR (2013) A simple procedure for the purification of active fractions in aqueous extracts of plants with allelopathic properties. Acta Bot Bras 27:50–53
Devi S, Thoppil JE (2016) Cytotoxic studies and phytochemical analysis of Orthosiphon thymiflorus (Roth) sleesen. Int J Pharm Pharm Sci 8(2):249–255
Chauvel B, Guillemin JP, Gasquez J, Gauvrit C (2012) History of chemical weeding from 1944 to 2011 in France: changes and evolution of herbicide molecules. Crop Prot 42:320–326
Duke SO, Dayan FE, Rimando RM, Scharder KK, Aliotta G, Oliva A, Romangini JG (2002) Chemicals from nature for weed management. Weed Sci 50:138–151
Duncan DB (1955) Multiple range and multiple F-tests. Biometrics 11:1–42
Ercoli L, Masoni A, Pampan S, Arduini I (2007) Allelopathic effects of rye, brown mustard and hairyn vetch on redroot pigweed, common lambsquarter and knotweed. Allelopathy J 19:249–256
Farooq M, Jabran K, Cheema ZA, Wahid A, Siddique KHM (2011) The role of allelopathy in agricultural pest management. Pest Manag Sci 67:493–506
Fiskesjo G (1985) The Allium test as a standard in environmental monitoring. Hereditas 102:99–112
Fiskesjo G (1997) Allium test for screening chemicals: evaluation of cytologic parameters. In: Wang W, Gorsuch JW, Hughes JS (eds) Plants for environmental studies. CRC Publishers, Boca Raton, pp 308–333
Gaba S, Gabriel E, Chadœuf J, Bonneu F, Bretagnolle V (2016) Herbicides do not ensure for higher wheat yield, but eliminate rare plant species. Sci Reports 6:30112
Ghareib HRA, Hamed MSA, Ibrahim OH (2010) Antioxidative effects of acetone fraction and vanillic acid from Chenopodium murale on tomato plants. Weed Biol Manag 10:64–72
Grisi PU, Gualtieri SCJG, Pereira VCP, Imatomi SAM (2012) Phytotoxic activity of Sapindus saponaria L. leaf and stem bark on initial growth of Triticum aestivum L. Commun Plant Sci 2:2237–4027
Guglielmini AC, Verdu AM, Satorre EH (2017) Competitive ability of five common weed species in competition with soybean. Int J Pest Manag 2;63(1):30-6
Gulzar S, Siddiqui MB (2014) Evaluation of allelopathic effect of Eclipta alba (L.) Hassk on biochemical activity of Amaranthus spinosus L., Cassia tora L. and Cassia sophera L. Afr. J. Environ Sci Techn 8:1–5
Gulzar A, Siddiqui MB, Bi S (2016) Phenolic acid allelochemicals induced morphological, ultrastructural, and cytological modification on Cassia sophera L. and Allium cepa L. Protoplasma 253(5):1211–1221
Harker KN (2013) Slowing weed evolution with integrated weed management. Can J Plant Sci 93:759–764
Havey MJ (2002) Genome organization in Allium. In: Rabinowitch HD, Currah L (eds) Allium crop science. Recent Advances. CABI Publishing, United Kingdom, pp 59–79
Hussain MI, Gonzalez L, Reigosa MJ (2011) Allelopathic potential of Acacia melanoxylon R. Br. On the germination and root growth of native species. Weed Biol Manag 11:18–28
Ishak MS, Sahid I (2014) Allelopathic effects of the aqueous extract of the leaf and seed of Leucaena leucocephala on three selected weed species. AIP Conference Proceedings 1614(1):659–664
Jabran K, Mahajan G, Sardana V, Chauhan BS (2015) Allelopathy for weed control in agricultural systems. Crop Prot 72:57–65
Javed S, Javaid A, Shoaib A (2014) Herbicidal activity of some medicinal plants extracts against Parthenium hysterophorus L. Pak J Weed Sci Res 20(3):279–291
Khanh TD, Chung MI, Xuan TD, Tawata S (2005) The exploitation of crop allelopathy in sustainable agricultural production. J Agron Crop Sci 191(3):172–184
Khanh TD, Elzaawely AA, Chung IM, Ahn JK, Tawata S, Xuan TD (2007) Role of allelochemicals for weed management in rice. Allelopathy J 19(1):85–96
Khalila A,, Maslata A , Hafiza A, Mizyedb S, Ashram M (2014) Zeitschrift für Naturforschung C, 64: (3-4) 167–175
Knapp S, Clarkson JJ (2004) (1642) Proposal to conserve the name Nicotiana plumbaginifolia against N. pusilla, N. humilis and N. tenella (Solanaceae). Taxon 53(3):844–846
Kong CH, Li HB, Hu F, Xu XH (2006) Allelochemicals released by rice roots and residues in soil. Plant Soil 288:47–56
Kumar DS, Chakrabarty D, Verma AK, Banerji BK (2011) Gamma ray induced chromosomal aberrations and enzyme related defense mechanism in Allium cepa L. Caryologia 64(4):388–397
Ladhari A, Omezzine F, DellaGreca M, Zarrelli A, Haouala SZR (2013) Phytotoxic activity of Cleome arabica L. and its principal discovered active compounds. South Afr J Bot 88:341–351
Li ZH, Wang Q, Ruan X, Pan CD, Jiang DA (2010) Phenolics and plant allelopathy. Molecules 15(12):8933–8952
Liu Q, Lu D, Jin H, Yan Z, Li X, Yang X, Guo H, Qin B (2014) Allelochemicals in the rhizosphere soil of Euphorbia himalayensis. J Agri Food Chem 62:8555–8561
Miyake Y. (2009) Plant growth inhibitor. Japan patent no 2009274970. Tokyo: Japan patent office
Mohamed FI, El-Ashry ZM (2012) Cytogenetic effect of allelochemicals Brassica nigra L. extracts on Pisum sativum L. World Appl Sci J 20(3):344–353
Muscolo A, Panuccio MR, Sidari M (2001) The effect of phenols on respiratory enzymes in seed germination respiratory enzyme activities during germination of Pinus laricio seeds treated with phenols extracted from different forest soils. Plant Growth Regul 35:31–35
Mushtaq W, Quratul-Ain, Siddiqui MB (2018) Screening of alleopathic activity of the leaves of Nicotiana plumbaginifolia Viv. on some selected crops in Aligarh, Uttar Pradesh, India. Int J Photochem Photobiol 2(1):1–4
Nefic H, Musanovic J, Metovic A, Kurteshi K (2013) Chromosomal and nuclear alterations in root tip cells of Allium cepa L. induced by alprazolam. Med Arch 67(6):388
Nwakanma NMC, Okoli BE (2010) Cytological effects of the root extracts of Boerhaavia diffusa on root tips of Crinum jagus. Eurasia J Biosci 4:105–111
Oerke EC (2006) Crop losses to pests. J Agr Sci 144(1):31–43
Ogata T., Hamachi M., Nishi K. (2008) Organic herbicide for Paddy field. Japan patent no 2008050329. Tokyo: Japan patent office
Omezzine F, Ladhari A, Haouala R (2014) Physiological and biochemical mechanisms of allelochemicals in aqueous extracts of diploid and mixoploid Trigonella foenum-graecum L. South Afr J Bot 93:167–178
Pan L, Li X, Yan Z, Guo H, Qin B (2015) Phytotoxicity of umbelliferone and its analogs: structure-activity relationships and action mechanisms. Plant Physiol Biochem 97:272–277
Pawlowski A, Kaltchuk-Santos E, Zini CA, Caramao CB, Soares GLS (2012) Essential oils of Schinus terebinthifolius and S. molle (Anacardiaceae): mitodepressive and aneugenic inducers in onion and lettuce root meristems. South Afr J Bot 80:96–103
Pina GDO, Borghetti F, Silveira CES e, Pereira LAR (2009) Effects of Eugenia dysenterica leaf extracts on the growth of sesame and radish. Allelopathy J 23:313–322
Proestos C, Sereli D, Komaitis M (2006) Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem 95:44–52
Rawat LS, Maikhuri RK, Negi VS, Bahuguna YM, Pharswan DS, Maletha A (2016) Allelopathic performance of medicinal plants on traditional oilseed and pulse crop of Central Himalaya, India. Natl Acad Sci Lett 39(3):141–144
Reigosa MJ, Souto XC, Gonz’lez L (1999) Effect of phenolic compounds on the germination of six weeds species. J Chem Ecol 28:83–88
Rice EL (1974) Allelopathy. Academic Press, New York, p 353
Rueda-Ayala V, Rasmussen J, Gerhards R, Fournaise NE (2011) The influence of post-emergence weed harrowing on selectivity, crop recovery and crop yield in different growth stages of winter wheat. Weed Res 51(5):478–488
Sadia S, Qureshi R, Khalid S, Nayyar BG, Zhang JT (2015) Role of secondary metabolites of wild marigold in suppression of Johnson grass and Sun spurge. Asian Pac J Trop Biomed 5(9):733–737
Salarti MA, Kato-Noguchi H (2010) Allelopathic potential of methanol extract of Bangladesh rice seedlings. Asian J Crop Sci 2(2):70–77
Sarwar N, Jamil FF, Parveen R (2001) Accumulation of phytoalexins and phenylalanine ammonia lyase in chickpea after inoculation with Ascochyta rabiei and their role in defence mechanism. Pak J Bot 33:373–382
Schulz M, Kussmann P, Knop M, Kriegs B, Gresens F, Eichert T, Ulbrich A, Marx F, Fabricius H, Goldbach H, Noga G (2007) Allelopathic monoterpenes interfere with Arabidopsis thaliana cuticular waxes and enhance transpiration. Plant Signal Behav 2:231–239
Sharma S, Devkota A (2015) Allelopathic potential and phytochemical screening of four medicinal plants of Nepal. Sci World 12(12):56–61
Singh HP, Batish DR, Kohli RK (2003) Allelopathic interactions and allelochemicals: new possibilities for sustainable weed management. Crit Rev Plant Sci 22(3–4):239–311
Singh A, Singh D, Singh NB (2009) Allelochemical stress produced by aqueous leachate of Nicotiana plumbaginifolia Viv. Plant Gr Reg 58:163–171
Singh A, Singh D, Singh NB (2015) Allelopathic activity of Nicotiana plumbaginifolia at various phenological stages on sunflower. Allelopath J 1:36(2)
Sodaeizadeh H, Rafieiolhossaini M, Damme PV (2010) Herbicidal activity of a medicinal plant, Peganum harmala L., and decomposition dynamics of its phytotoxins in the soil. Ind Crop Prod 31(2):385–394
Soltys D, Langwald AR, Gniazdowska A, Wiśniewska A, Bogatek (2011) Inhibition of tomato (Solanum lycopersicum L.) root growth by cyanamide is due to altered cell division, phytohormone balance and expansin gene expression. Planta 236:1629–1638
Taek–Keun OH, Shinogi Y, Chikushi J, Yong–Hwan LE, Choi B (2012) Effect of aqueous extract of biochar on germination and seedling growth of lettuce (Lactuca sativa L.). J Fac Agr Kyushu Univ 57(1):55–60
Teerarak M, Laosinwattana C, Charoenying P (2010) Evaluation of allelopathic, decomposition and cytogenetic activities of Jasminum officinale L. f. var. grandiflorum (L.) Kob. on bioassay plants. Bioresour Technol 101:5677–5684
Tuyen PT, Xuan TD, Tu Anh TT, Mai Van T, Ahmad A, Elzaawely AA, Khanh TD (2018) Weed suppressing potential and isolation of potent plant growth inhibitors from Castanea crenata Sieb. et Zucc. Molecules 23(2):345
Vyvyan JR (2002) Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 58:1631–1646
Young SL, Pierce FJ, Nowak P (2014) Introduction: scope of the problem—rising costs and demand for environmental safety for weed control. Automation: the future of weed control in cropping systems. Springer, pp. 1-8
Zimdahl RL (2013) Fundamentals of weed science, 4th edn. Academic Press, San Diego, p 664
Zeng RS (2014) Allelopathy—the solution is indirect. J Chem Ecol 40(6):515–516
Zhang XH, Lang DY, Chen J, Zhao YS, Wu XL, Fu XY (2014) Autotoxicity of aqueous extracts from plant of cultivated Astragalus membranaceus var. mongholicus. Zhong yao cai = J Chinese Med Mat 37(2):187–191
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mushtaq, W., Ain, Q., Siddiqui, M.B. et al. Cytotoxic allelochemicals induce ultrastructural modifications in Cassia tora L. and mitotic changes in Allium cepa L.: a weed versus weed allelopathy approach. Protoplasma 256, 857–871 (2019). https://doi.org/10.1007/s00709-018-01343-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-01343-1