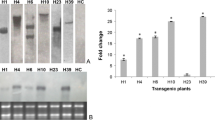
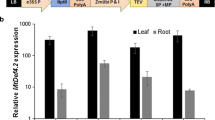
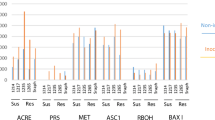
Abstract
A model pathosystem involving Mycosphaerella pinodes, causal agent of Mycosphaerella blight on pea and barrel medic(k) Medicago truncatula has been developed. Nineteen M. truncatula ecotypes were evaluated for disease susceptibility to M. pinodes strain OMP-1, using detached leaves inoculated with a pycnospore suspension. Inoculation of ecotype R108-1 with the pycnospores allowed direct penetration into the host’s epidermal cells, eventually causing leaf spots. Since pycnidia formed abundantly in infected tissues by 3 days post inoculation, M. pinodes is considered to have completed its infection cycle as on a natural host plant. Expression of phenylalanine ammonia-lyase (PAL)-, chalcone synthase (CHS)- and isoflavone reductase (IFR) mRNAs as well as subsequent accumulation of a major phytoalexin, medicarpin, was induced in ecotype R108-1 with an elicitor preparation from M. pinodes, whereas this phytoalexin response was markedly attenuated by supprescins from the same fungus. A transcriptional study of a salicylic acid (SA)-regulated gene encoding pathogenesis-related protein 10-1 (PR10-1) indicated that the suppressor likely interferes with the signal transduction process leading to inducible defenses in M. truncatula. Indeed, the suppressor rendered the host tissues (ecotype R108-1) susceptible to unrelated nonpathogenic fungi, probably through targeting ATPase activity of the host cells. Accordingly, the resistant and susceptible response of pea can be recapitulated in M. truncatula. This model pathosystem thus will allow us to verify the molecular basis underlying the fungal suppressor-mediated plant susceptibility.








Similar content being viewed by others
References
Amano M, Toyoda K, Ichinose Y, Yamada T, Shiraishi T (1995) H+-translocating activity in proteoliposomes reconstituted with pea plasma membrane ATPase and its inhibition by fungal suppressor from Mycosphaerella pinodes. Ann Phytopathol Soc Jpn 61:369–375
Amano M, Toyoda K, Ichinose Y, Yamada T, Shiraishi T (1997) Association between ion fluxes and defense responses in pea and cowpea tissues. Plant Cell Physiol 38:698–706
Barker DG, Bianchi S, Blondon F, Dattée Y, Duc G, Essad S, Flament P, Gallusci P, Génier G, Guy P, Muel X, Tourneur J, Dénarié J, Huguet T (1990) Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium–legume symbiosis. Plant Mol Biol Rep 8:40–49
Bonnin I, Huguet T, Gherardi M, Prosperi JM, Olivieri I (1996) High level of polymorphism and spatial structure in a selfing plant species, Medicago truncatula (Leguminosae), shown using RAPD markers. Am J Bot 83:843–855
Chabaud M, de Carvalho-Niebel F, Barker DG (2003) Efficient transformation of Medicago truncatula cv. Jemalong using the hypervirulent Agrobacterium tumefaciens strain AGL1. Plant Cell Rep 22:46–51
Clulow SA, Lewis BG, Parker ML, Matthews P (1991) Infection of pea epicotyls by Mycosphaerella pinodes. Mycol Res 95:817–820
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411:826–833
El Hadrami A, El Hadrami I, Daayf F (2009) Suppression of induced plant defense responses by fungal and oomycete pathogens. In: Bouarab K, Brisson N, Daayf F (eds) Molecular plant–microbe interactions. CAB International, Wallingford, pp 231–268
Elmore JM, Coaker G (2011) The role of the plasma membrane H+-ATPase in plant–microbe interactions. Mol Plant 4:416–427
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Gutiérrez-Nájera N, Muñoz-Clares RA, Palacios-Bahena S, Ramírez J, Sánchez-Nieto S, Plasencia J, Gavilanes-Ruíz M (2005) Fumonisin B1, a sphingoid toxin, is a potent inhibitor of the plasma membrane H+-ATPase. Planta 221:589–596
Harrison MJ (2000) Molecular genetics of model legumes. Trend Plant Sci 5:414–415
Heath MC (2000) Nonhost resistance and nonspecific plant defenses. Curr Opin Plant Biol 3:315–319
Hoffmann B, Trinh TH, Leung J, Kondorosi A, Kondorosi E (1997) A new Medicago truncatula line with superior in vitro regeneration, transformation, and symbiotic properties isolated through cell culture selection. Mol Plant Microbe Interact 10:307–315
Ishiga Y, Funato A, Tachiki T, Toyoda K, Shiraishi T, Yamada T, Ichinose Y (2002) Expression of the 12-oxophytodienoic acid 10,11-reductase gene in the compatible interaction between pea and fungal pathogen. Plant Cell Physiol 43:1210–1220
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kato T, Shiraishi T, Toyoda K, Saitoh K, Satoh Y, Tahara M, Yamada T, Oku H (1993) Inhibition of ATPase activity in pea plasma membranes by fungal suppressors from Mycosphaerella pinodes and their peptide moieties. Plant Cell Physiol 34:439–445
Kawahara T, Toyoda K, Kiba A, Miura A, Ohgawara T, Yamamoto M, Inagaki Y, Ichinose Y, Shiraishi T (2003) Cloning and characterization of pea apyrases: involvement of PsAPY1 in response to signal molecules from the pea pathogen Mycosphaerella pinodes. J Gen Plant Pathol 69:33–38
Kawahara T, Namba H, Toyoda K, Kasai T, Sugimoto M, Inagaki Y, Ichinose Y, Shiraishi T (2006) Induction of defense responses in pea tissues by inorganic phosphate. J Gen Plant Pathol 72:129–136
Kiba A, Toyoda K, Yamada T, Ichinose Y, Shiraishi T (1995) Specific inhibition of cell wall-bound ATPases by fungal suppressor from Mycosphaerella pinodes. Plant Cell Physiol 36:809–817
Kiba A, Toyoda K, Yamada T, Ichinose Y, Shiraishi T (1996) Species-specific suppression of superoxide-anion generation on surfaces of pea leaves by the suppressor from Mycosphaerella pinodes. Ann Phytopathol Soc Jpn 62:508–512
Kiba A, Miyake C, Toyoda K, Ichinose Y, Yamada T, Shiraishi T (1997) Superoxide generation in extracts from isolated plant cell walls is regulated by fungal signal molecules. Phytopathology 87:846–852
Kiba A, Ohgawara T, Toyoda K, Inoue-Ozaki M, Takeda T, Rao US, Kato T, Ichinose Y, Shiraishi T (2006a) A binding protein for fungal signal molecules in the cell wall of Pisum sativum. J Gen Plant Pathol 72:228–237
Kiba A, Toyoda K, Yoshioka K, Tsujimura K, Takahashi H, Ichinose Y, Takeda T, Kato T, Shiraishi T (2006b) A pea NTPase, PsAPY1, recognizes signal molecules from microorganisms. J Gen Plant Pathol 72:238–246
Masuda Y, Shiraishi T, Ouchi S, Oku H (1983) A rapid and accurate analysis of isoflavonoid phytoalexins by high performance liquid chromatography. Ann Phytopathol Soc Jpn 49:558–560
Matsui H, Nakamura G, Ishiga Y, Toshima H, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y (2004) Structure and expression of 12-oxophytodienoate reductase (subgroup I) genes in pea, and characterization of the oxidoreductase activities of their recombinant products. Mol Gen Genomics 271:1–10
Oku H, Shiraishi T, Ouchi S, Ishiura M, Matsueda R (1980) A new determinant of pathogenicity in plant disease. Naturwissenshaften 67:310–311
Rogers C, Wen J, Chen R, Oldroyd G (2009) Deletion-based reverse genetics in Medicago truncatula. Plant Physiol 151:1077–1086
Shiraishi T, Oku H, Yamashita M, Ouchi S (1978) Elicitor and suppressor of pisatin induction in spore germination fluid of pea pathogen, Mycosphaerella pinodes. Ann Phytopathol Soc Jpn 44:659–665
Shiraishi T, Araki M, Yoshioka H, Kobayashi I, Yamada T, Ichinose Y, Kunoh H, Oku H (1991) Inhibition of ATPase activity in pea plasma membranes in situ by a suppressor from a pea pathogen, Mycosphaerella pinodes. Plant Cell Physiol 32:1067–1075
Shiraishi T, Saitoh K, Kim HM, Kato T, Tahara M, Oku H, Yamada T, Ichinose Y (1992) Two suppressors, supprescins A and B, secreted by a pea pathogen, Mycosphaerella pinodes. Plant Cell Physiol 33:663–667
Shiraishi T, Yamada T, Ichinose Y, Kiba A, Toyoda K (1997) The role of suppressors in determining host–parasite specificities in plant cells. Int Rev Cytol 172:55–93
Shiraishi T, Toyoda K, Kiba A, Kawahara T, Takahashi H, Inagaki Y, Ichinose Y (2005) Defense signaling and the plant cell wall—a new signaling pathway dependent upon inorganic phosphate. In: Tsuyumu S, Leach JE, Shiraishi T, Wolpert T (eds) Genomic and genetic analysis of plant parasitism and defense. APS Press, St. Paul, pp 114–125
Simon-Plas F, Gomes E, Milat ML, Pugin A, Blein JP (1996) Cercospora beticola toxins (X. Inhibition of plasma membrane H+-ATPase by beticolin-1). Plant Physiol 111:773–779
Suzuki T, Maeda A, Hirose M, Ichinose Y, Toyoda K, Shiraishi T (2006) Ultrastructural features of Mycosphaerella pinodes infection and differences in host susceptibility responses among Medicago truncatula ecotypes. J Electron Microsc Technol Med Biol 20:167–168
Tadege M, Wen J, He J, Tu H, Kwak Y, Eschstruth A, Cayrel A, Endre G, Zhao PX, Chabaud M, Ratet P, Mysore KS (2008) Large-scale insertional mutagenesis using Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J 54:335–347
Takahashi H, Toyoda K, Hirakawa Y, Morishita K, Kato T, Inagaki Y, Ichinose Y, Shiraishi T (2006) Localization and responsiveness of a cowpea apyrase VsNTPase1 to phytopathogenic microorganisms. J Gen Plant Pathol 72:143–151
Toyoda K, Shiraishi T, Yoshioka H, Yamada T, Ichinose Y, Oku H (1992) Regulation of polyphosphoinositide metabolism in pea plasma membranes by elicitor and suppressor from a pea pathogen, Mycosphaerella pinodes. Plant Cell Physiol 33:445–452
Toyoda K, Kawahara T, Ichinose Y, Yamada T, Shiraishi T (2000) Potentiation of phytoalexin accumulation in elicitor-treated epicotyls of pea (Pisum sativum) by a diacylglycerol kinase inhibitor. J Phytopath 148:633–636
Toyoda K, Kawamoto Y, Kawanishi Y, Niwa M, Takahashi H, Suzuki T, Kasai T, Takahara H, Amano M, Inagaki Y, Ichinose Y, Shiraishi T (2011) Suppression of defense—the role of fungal suppressors in conditioning plant susceptibility. In: Wolpert T, Shiraishi T, Collmer A, Akimitsu K, Glazebrook J (eds) Genome-enabled analysis of plant–pathogen interactions. APS Press, St. Paul, pp 139–147
Toyoda K, Yasunaga E, Niwa M, Ohwatari Y, Nakashima A, Inagaki Y, Ichinose Y, Shiraishi T (2012) H2O2 production by copper amine oxidase, a component of the ecto-apyrase (ATPase)-containing protein complex(es) in the pea cell wall, is regulated by an elicitor and a suppressor from Mycosphaerella pinodes. J Gen Plant Pathol 78:311–315
Uppalapati SR, Toyoda K, Ishiga Y, Ichinose Y, Shiraishi T (2004) Differential regulation of MBP kinases by a glycoprotein elicitor and a polypeptide suppressor from Mycosphaerella pinodes in pea. Physiol Mol Plant Pathol 64:17–25
Yamada T, Hashimoto H, Shiraishi T, Oku H (1989) Suppression of pisatin, phenylalanine ammonia-lyase mRNA, and chalcone synthase mRNA accumulation by a putative pathogenicity factor from the fungus Mycosphaerella pinodes. Mol Plant-Microbe Interact 2:256–261
Yoshioka H, Shiraishi T, Yamada T, Ichinose Y, Oku H (1990) Suppression of pisatin production and ATPase activity in pea plasma membranes by orthovanadate, verapamil and a suppressor from Mycosphaerella pinodes. Plant Cell Physiol 31:1139–1146
Yoshioka H, Shiraishi T, Kawamata S, Nasu K, Yamada T, Ichinose Y, Oku H (1992a) Orthovanadate suppresses accumulation of phenylalanine ammonia-lyase mRNA and chalcone synthase mRNA in pea epicotyls induced by elicitor from Mycosphaerella pinodes. Plant Cell Physiol 33:201–204
Yoshioka H, Shiraishi T, Nasu K, Yamada T, Ichinose Y, Oku H (1992b) Suppression of the activation of chitinase and β-1,3-glucanase in pea epicotyls by orthovanadate and a suppressor elicitor from Mycosphaerella pinodes. Ann Phytopathol Soc Jpn 58:405–410
Zhu H, Choi HK, Cook DR, Shoemaker RC (2005) Bridging model and crop legumes through comparative genomics. Plant Physiol 137:1189–1196
Zipfel C (2009) Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol 12:414–420
Acknowledgments
We gratefully acknowledge the late Prof. Dr. A. Kondorosi (Institut des Sciences Vegetales, Centre National de la Rescherche Scientifique, Gif-sur-Yvette, CEDEX France), Prof. Dr. M. J. Harrison (Boyce Thompson Institute for Plant Research, Cornell University, Tower Road Ithaca, NY, USA), Dr. J.M. Prosperi (Institut National de la Rescherche Agronomique, Centre de Montpellier, France) and Dr. R. Jonson (National Germplasm Resources Laboratory, USDA, ARS, Beltsville, MD, USA) for giving us M. truncatula seeds of the core collection accessions. This research was supported in part by the Grants-in-Aid for Scientific Research (No. 22580051) from the Japan Society for Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toyoda, K., Ikeda, S., Morikawa, Ji. et al. The Medicago truncatula–Mycosphaerella pinodes interaction: a new pathosystem for dissecting fungal-suppressor-mediated disease susceptibility in plants. J Gen Plant Pathol 79, 1–11 (2013). https://doi.org/10.1007/s10327-012-0405-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-012-0405-7