Abstract
Blueberries (Vaccinium corymbosum L.) are cultivated worldwide and represent an important asset for the Portuguese economy. Pathogen infection and water deficiency are known to affect crops productivity worldwide, thus limiting plant yield or fruit quality. Diaporthe is a cosmopolitan genus comprising plant pathogens and endophytes, that may switch their behavior to a pathogenic phase when the host is under environmental stress (e.g., drought, precipitation). Given the scarce physiological studies on blueberry plant- pathogen interactions, in this study we aimed to evaluate the effect of Diaporthe amygdali and D. eres on 9-month-old clonal blueberries under two different water scenarios: well-watered (WW) and water deficit (WD). Morphological (lesion length) and physiological parameters (water status, leaf gas exchange, photosynthetic pigments, proline, phenolic compounds, flavonoids, starch, total soluble sugars, and lipid peroxidation) were assessed. Our results suggest that the irrigation regime applied was not sufficient to cause severe stress to plants. Under WW conditions, plants inoculated with D. eres may have used malondialdehyde content (MDA) as a signaling molecule. Although D. amygdali has caused plant mortality, this study shows that under WW conditions, plants manage to deal with pathogen attack, maintaining their physiological performance. This study also demonstrates that the interaction between fungal pathogens and water limitation seems to stimulate plant defense, through the accumulation of proline. Our findings offer crucial insights to understand how blueberry plants cope with infection by species of Diaporthe, and how plants can adapt to climate changes in the Mediterranean area (e.g., water scarcity).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blueberries (Vaccinium corymbosum L.) are a small-fruit crop largely cultivated and commercialized due to their fruits’ health benefits (Silva et al., 2020). In Portugal, blueberry production has increased during the last 20 years, being currently the 7th biggest producer worldwide (FAOSTAT, 2022), with a total planted area of 2,490 ha and a production of 15,418 tons (INE, 2021). In Europe the total blueberry production increased from 47,970 tons in 2010 to 168,472 tons in 2020 (FAOSTAT, 2022), due to efforts to develop new genotypes more adapted to several climates, such as the Mediterranean (Mazzoni et al., 2020).
The reported decrease in rainfall has limited the availability of water, which has proven to be a problem in crops from the Mediterranean region (del Pozo et al., 2019). This situation is especially serious for the root-hair-less blueberry plants, which are rapidly affected by water deprivation, leading to a reduction in their growth and fruit quality (Jiménez-Donaire et al., 2020). Because of this, this topic has attracted the attention of researchers in recent years. Recent studies have evaluated the response of blueberry plants to drought stress aiming to understand its effect on yield and fruit quality (Ortega-Farias et al., 2021), to select drought resistant cultivars (Balboa et al., 2020), and to validate if propagation methods have a role in the plants´ response (Mazurek et al., 2021). Although seldom explored, a few studies have assessed blueberry fitness in response to water deficit. These studies reported a decrease in shoot growth (Mingeau et al., 2001), stomatal closure and reduction in gas exchange (Rho et al., 2012), lowered water potentials (Lobos et al., 2018) and decreased chlorophyll concentration (Yu et al., 2015).
The dissemination of pathogens is another threat causing severe economic losses in agriculture (Baldi & Porta, 2020). Diaporthe is one of the most common genera of endophytic fungi or latent pathogens, found in a wide range of hosts (Gomes et al., 2013; Hilário et al., 2020). It is worth mentioning that this “friendly” behavior may switch to a pathogenic stage in situations in which the host fitness may be compromised (e.g., excessive pruning, lack of rainfall) (Hrycan et al., 2020). Most of the studies on the pathogenicity on blueberry plants are solely based on the evaluation of the symptoms and disease development under optimal growth conditions (Cardinaals et al., 2018; Guarnaccia et al., 2021; Hilário et al., 2020). In a recent study, Hilário et al. (2021b) showed that D. amygdali strain CAA958 was one of the most aggressive species on blueberry plants, while Diaporthe eres strain CBS 160.32 (syn. D. vaccinii), no longer listed as a quarantine organism (EU Regulation 2019/2072), was confirmed as a cause of minor symptoms. Although Roloff et al (2004) demonstrated that photosynthetic rates on blueberry plants decreased in the presence of the fungus Septoria albopunctata, the interaction of the pathosystem V. corymbosum/Diaporthe spp. is yet to be unveiled.
In the field, stresses rarely occur independently. Drought stress can have different outcomes on plant performance such as an increase in the plant susceptibility to pathogens thereby triggering disease outbreaks (Ghanbary et al., 2021), can provide endurance to some plants (Pandey et al., 2017) or even reduce disease susceptibility (Swinfield et al., 2012). The combination of both drought stress and fungal infection results in a complex interaction of shared and/or unique molecular and physiological responses (Sinha et al., 2019) as reported for other abiotic combinations (Correia et al., 2018). The downregulation of the photosynthetic machinery (e.g., stomatal closure, turgor loss) (Hazrati et al., 2016; Morales et al., 2013), the upregulation of stress-responsive genes, and increased accumulation of osmoprotectants (e.g., proline, carbohydrates) (Chen & Jiang, 2010) are some of the molecular responses common to both stresses. These outcomes and the molecular responses mentioned above depend on the order in which drought and fungal infection are imposed, the pathogen, and the timing and severity of drought, which prevent solid conclusions being drawn on the host–pathogen interactions (Hossain et al., 2019; Ramegowda & Senthil-Kumar, 2015).
Considering the importance of blueberry, the expansion of this crop into areas with water limitation (Balboa et al., 2020), the cosmopolitan behaviour of species in the genus Diaporthe (Dissanayake et al., 2020) and the increase in plants susceptibility to Diaporthe infection (Hulke et al., 2019; Kim et al., 2015), it is essential to understand the host physiological responses to these environmental stimuli. This knowledge is important to select additional cultivars displaying resistance to drought and to fungal infection, to guarantee the sustainability of the agriculture sector (Toscano et al., 2019).
Therefore, this study was designed to explore if water limitation after inoculation changes disease progression and host–pathogen interaction using two fungal species with different levels of aggressiveness. We aimed to evaluate the physiological performance of V. corymbosum cultivar ‘Duke’ after infection with D. eres strain CBS 160.32 and D. amygdali strain CAA958 under well-watered (WW) and water deficit conditions (WD). Symptom progression and key morpho-physiological indicators of primary (growth) and secondary metabolism (defense) such as the water status content, leaf gas exchange related parameters, phenolic and flavonoids compounds, proline, starch, total soluble sugars and malondialdehyde content were assessed.
Material and Methods
Fungal isolates
Diaporthe amygdali CAA958 was obtained from V. corymbosum plants sampled in a previous study on the species of Diaporthe occurring in association with this host in Portugal (Hilário et al., 2021b). Strain CBS 160.32 (D. eres, syn. D. vaccinii) was obtained from the Westerdijk Fungal Biodiversity Institute, Netherlands. Diaporthe amygdali strain CAA958 was selected once it was shown to be one the most aggressive species on blueberry plants, as described by Hilário et al. (2021b). Diaporthe eres CBS 160.32, formerly recognized as D. vaccinii (Hilário et al., 2021a), and previously listed as a quarantine organism in Europe was also selected as it proved to be less aggressive. Cultures were maintained in 15% glycerol at − 80 °C at the University of Aveiro (Portugal). When needed these were grown on potato dextrose agar for 7 days at 25 °C (PDA, Merck, Germany).
Plant material
Blueberry plants from cultivar ‘Duke’ (one the most widely cultivated in Portugal), were obtained from micropropagation from the breeding program of Deifil, Biotechnology (Braga, Portugal). After arrival but before transplantation, plants were checked regarding their health status. Well-formed leaves with uniform color, a vigorous growth and a strong root system were considered as criteria for plant quality. Plants with chlorotic and decaying leaves, a low number of leaves and stems with visible necrotic tissues were excluded from the experiment. The selected plants had heights ranging from 50 to 80 cm, and each plant had approximately 10 to 15 leaves. Sixty 9-month-old clonal plants were transplanted to 1L plastic pots filled with 3:2 (w/w) peat:perlite mixture. Potted plants were subjected to a 20-day acclimation period with a temperature of 25/20 °C and a 16/8 h (day/night) photoperiod. During the acclimatation period, plants were watered every 2 days to maintain 75% field capacity (FC) and fertilized two times on the transplantation day and before the inoculation day with a N:P:K nutrient solution (5:8:10; Frutifol L12, Nufarm, Portugal). After the acclimation period, plants were placed inside a climate chamber (Fitoclima 1200, Aralab, Portugal), with a temperature of 25/20 °C, a 16/8 h (day/night) photoperiod, a steady relative humidity (RH) of 60/65% and a photosynthetic photon flux density (PPFD) of approximately 400 µmol m−2 s−1. To minimize the effects of environmental heterogeneity, pots were randomly arranged and moved during the entire experiment.
Plant inoculation procedure
Plant inoculation was initiated by surface disinfection of the stem with 70% ethanol. A shallow wound was made 5 cm above the soil surface using a sterile scalpel, removing the bark, and exposing the cambium. Mycelial plugs (5 mm diameter) from the active margin of one-week-old fungal cultures grown on PDA at 25 °C were placed into the wound with the mycelial surface facing the cambium and sealed with Parafilm® to prevent desiccation. Plugs of sterile PDA were similarly applied into wounds on stems of control plants. Ten replicate plants were randomly assigned to each treatment.
Experimental design
For this experiment, three treatments were considered for each one of the water conditions: well-watered (WW) and water deficit (WD). For each water treatment, ten plants were inoculated with D. amygdali strain CAA958, ten inoculated with D. eres strain CBS 160.32 and ten mock-inoculated. The WW plants were subjected to water supplied every evening until soil water content reached around 75% FC. The WD regime was established by withholding water supply until the soil water content reached around 30% of FC (which lasted 9 days) and thereafter kept at this target. Pots from both water regimes were gravimetrically monitored through daily weighing and watered to maintain the soil water targeted (if necessary). Soil water content corresponding to 100% FC was previously determined by weighing soil-filled pots after full watering (till runoff) and after drying for 3 days at 80 °C.
Each treatment (inoculated and non-inoculated plants) was sampled as soon as 50% mortality was observed in one treatment (Fig. 1). Healthy leaves from five plants (n = 5) displaying typical disease symptoms (dieback and stem necrosis) were selected from each treatment, and immediately frozen in liquid nitrogen and stored at − 80 °C for further analysis.
Disease progression
During the entire experiment, the disease progression was evaluated. The development of external symptoms such as stem lesions, necrosis, foliar chlorosis, and wilting was assessed weekly. At the end of the experiment, which lasted 21 days, the length of the internal lesions was measured by removing the external bark. Pieces of wood from the edges of lesions were immersed in 70% ethanol for 1 min, rinsed in sterile distilled water and blotted dry on sterile filter paper. Disinfected plant tissue was placed on PDA and incubated at room temperature for one week to verify Koch's postulates.
Water status
Relative water content (RWC) was measured according to Smart and Bingham (1974). Three leaf discs of 11 mm diameter were obtained using a cork borer from detached healthy leaves and weighed to obtain fresh weight (FW). Discs were transferred to tubes with distilled water and maintained overnight in the dark at 4 °C. After 24 h, the turgid weight (TW) was registered. Leaf discs were then dried at 50 °C for one week to obtain the dry weight (DW) and shoot water potential (Ψmd, MPa) was measured with a Schölander-type pressure chamber (PMS Instrument Co., Corvallis, OR).
Leaf gas exchange measurements
Stomatal conductance (gs, mmol H2O m−2 s−1), transpiration rate (E, mmol H2O m−2 s−1), net CO2 assimilation rate (A, µmol CO2 m−2 s−1) and intercellular CO2 concentration content (Ci, ppm) were measured in five plants per treatment using an LCpro-SD portable infrared gas analyzer (ADC BioScientific Ltd., UK) equipped with a broad photosynthesis chamber. The following conditions were maintained inside the chamber during all measurements: ambient CO2 concentration and humidity; air flux: 200 μmol/s; block temperature: 26.5 ± 0.7 °C. To determine the saturation light intensity A/PPFD (photosynthetic photon flux density; light response curves of CO2 assimilation), response curves were performed with the following PPFD: 2000, 1500, 1000, 750, 500, 250, 100, 50 and 0 μmol m−2 s−1. After A/PPFD data analysis, punctual measurements at saturation light intensity were performed at 1000 μmol m−2 s−1. Data were recorded when the measured parameters were stable (8–10 min). Measurements were carried out in five biological replicates per treatment and isolate used.
Biochemical parameters
Photosynthetic pigments, total soluble sugars, and starch
Photosynthetic pigments, total soluble sugars and starch were determined following the descriptions of López-Hidalgo et al. (2020). Briefly, 1 mL of cold (4 °C) 80% ethanol was added to 70 mg frozen tissue and ground for 30 s. Samples were centrifuged at 10,000 g for 10 min at 4 °C and the generated pellets (for starch quantification) and supernatants (for photosynthetic pigments and total soluble sugars quantification) were separated and used for further quantifications.
From the supernatant, 300 µl was diluted in 80% cold ethanol (1:1) for photosynthetic pigment determination. Chlorophyll A, chlorophyll b and carotenoid contents were quantified and calculated according to López-Hidalgo et al. (2020). Briefly, 150 µl of the diluted sample was transferred to a 96-well microplate, and absorbances were measured at 470, 649 and 664 nm, in a microplate reader (Synergy HT, BioTek Instruments, Winooski, VT, USA).
Both total soluble sugars and starch were determined using the anthrone method following the procedure described by López-Hidalgo et al. (2020). Samples were transferred to a 96-well microplate and the absorbance was read at 625 nm, in a microplate reader. Total soluble sugar content was calculated against a D-glucose standard curve in 80% ethanol (1–0 mg/ml). Starch content was calculated with a D-glucose in 30% perchloric acid standard curve (1–0 mg/ml).
Malondialdehyde (MDA) content
Lipid peroxidation, estimated by the amount of malondialdehyde (MDA), was quantified using the method described by Heath and Packer (1968), with minor modifications. Briefly, MDA was extracted from 75 mg of frozen leaves, homogenized in 5 mL of TCA (trichloroacetic acid) 0.1% (v/v) and centrifuged at 12,000 g for 15 min at 4 °C. Afterwards, 1 ml of supernatant was mixed with 4 ml of 0.5% of thiobarbituric acid (TBA) in 20% TCA and incubated at 95 °C for 30 min. The absorbance was read at 532 and 600 nm. MDA content was determined applying the formula described by Heath and Packer (1968).
Proline quantification
Proline content was determined as described by Bates et al. (1973) with slight modifications. Plant tissue (70 mg) from five biological replicates per treatment was homogenized with 1.5 ml of sulphosalicylic acid (3%, w/v). Following centrifugation (10 min, 10,000 g, 4 °C), 1 ml of supernatant was collected, and 1 ml of ninhydrin acid and 1 ml of glacial acetic acid were added. After incubation at 100 °C and cooling, 2 ml of toluene were added to the solution and absorbance was read at 520 nm. Free proline content was calculated against a D-proline standard curve (0–0.5 mg/ml).
Total phenolic content and flavonoids quantification
Extraction was conducted as described by Dinis et al. (2012) with slight modifications. Briefly, 40 mg of frozen leaves were homogenized in 1.5 mL of 70% methanol and kept in an orbital shaker at 700 rpm for 1 h at 25 °C. Samples were centrifuged at 10,000 g at 4 °C for 15 min and the supernatant was collected. This process was repeated at least 4 times, until we obtained a final volume of 6 ml. Samples were then stored at − 80 °C for further quantification.
Total phenolic content was estimated by Folin–Ciocalteu’s method adapted from Singleton et al. (1999). A total of 20 µL of supernatant, 90 µL of distilled water and 10 µL of Folin–Ciocalteau reagent solution were added to a 96-well microplate and left in the dark at room temperature for 6 min. Then, 80 µL of 7% sodium carbonate solution were added to each well and incubated in the dark at room temperature for 2 h. The absorbance was measured at a wavelength of 750 nm. Gallic acid was used as a standard to build a calibration curve (0–1 mg/mL).
Total flavonoid content was measured following the aluminum chloride colorimetric assay adapted from Chang et al. (2002). A total of 60µL of supernatant 28 µL of 5% sodium nitrite solution were added to a 96-well microplate and left in the dark at room temperature for 6 min. Then, 28 µL of 10% aluminum chloride solution was added to each well and incubated again for 6 min in the dark. After that, 120 µL of 4% sodium hydroxide solution was added to each well and gently shook. The absorbance was measured at 370 nm. Catechin was used as a standard to calculate the calibration curve (0–0.5 mg/mL).
Statistical analysis
Prior to analysis, data were checked for normality with the Shapiro–Wilk test. As data met ANOVA assumptions, a two‐way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests was employed to identify significant differences between plants inoculated with D. eres CBS 160.32 and D. amygdali CAA958 under WW and WD conditions (p ≤ 0.05) (Table 1). Considering that plant response was dependent on the inoculation (Table 2), data were analyzed, and one‐way ANOVA followed by a Tukey test as a post-hoc multiple comparisons (p ≤ 0.05) was performed to evaluate the fungal effect in each water treatment (Table 2). All analyses were performed using SigmaPlot for Windows (Systat Software for Windows v. 14.5 Systat Software Inc., Chicago, IL, USA).
RESULTS
Disease progression
Twenty-one days after inoculation, mortality rates (50%) occurred only for plants inoculated with D. amygdali under WD conditions (Fig. 2). Besides the development of necrosis in the inoculation site, plants showed wilting of leaves and discoloration of the outer epidermis. The remaining plants that survived, like in the other treatments, did not show any of these symptoms, besides the development of lesions around the inoculation point at the end of the experiment. Diaporthe eres did not cause the death of any plant.
Necrosis length
Overall, D. amygdali produced larger lesion lengths in both WW and WD conditions (Fig. 3) alongside wilting of leaves and necrosis of the internal vascular tissues. Moreover, D. eres (1.0 ± 0.3 cm) and D. amygdali (1.4 ± 0.3 cm) exhibited also significantly larger necrosis under WD conditions, compared to the WW treatment (0.8 ± 0.3 cm, p = 0.001; 1.2 ± 0.6 cm, p = 0.043, respectively) (Fig. 3).
Internal lesion lengths caused by Diaporthe eres and D. amygdali under well-watered (WW) and water deficit (WD) conditions. Data are presented as mean ± SD. Capital and lowercase letters indicate differences between inoculated plants within each water treatment (p ≤ 0.05). The asterisks indicate differences between WW and WD for a given treatment (*p ≤ 0.05. ***p ≤ 0.001)
Water potential
No significant differences were observed within the WD treatment (Fig. 4). Non-inoculated plants under WD treatment displayed a decrease in water potential (Ψmd = –0.2 MPa), compared to those non-inoculated under WW conditions (Ψmd = –0.3 MPa). Moreover, the inoculation with D. eres influenced that water potential, since an increase was observed under WW conditions (Ψmd = –0.4 MPa) (Fig. 4, Table 1).
Shoot water potential of blueberry plants (Ψmd) under well-watered (WW) and water deficit (WD) conditions and inoculated with Diaporthe eres and D. amygdali. Data are presented as mean ± SD. Capital and lowercase letters indicate differences between inoculated plants within each water treatment (p ≤ 0.05). The asterisk indicates differences between WW and WD for a given treatment (*p ≤ 0.05)
Relative water content (RWC)
No significant differences of RWC were observed within the WW treatment (Fig. 5). Under WD conditions, inoculation with both fungal species induced a significant decrease in RWC values, compared to the non-inoculated plants.
Relative water content (RWC) of blueberry plants under well-watered (WW) and water deficit (WD) conditions and inoculated with Diaporthe eres and D. amygdali. Data are presented as mean ± SD. Capital and lowercase letters indicate differences between inoculated plants within each water treatment (p ≤ 0.05)
Leaf gas exchange measurements
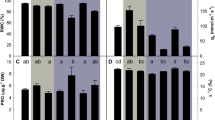
A negative impact of water treatment and inoculation on leaf gas exchange parameters (Table 1) was evident. Under WW conditions, CO2 assimilation rate (A) (Fig. 6A), stomatal conductance (gs) (Fig. 6C) and transpiration rate (E) (Fig. 6D), were affected after inoculation with both D. eres and D. amygdali. An increase in intercellular CO2 concentration (Ci) was observed upon inoculation with D. eres (Fig. 6B). Under WD conditions, plants showed a similar response profile. Inoculated plants presented higher Ci values in the WD treatment, compared to the non-inoculated plants, whereas A, gs and E rates were negatively affected by both fungal species. Non-inoculated plants showed a decrease in A under WD conditions (3.1 ± 1.2 µmol CO2 m−2 s−1), compared to the WW conditions (5.3 ± 1.6 µmol CO2 m−2 s−1). Additionally, plants inoculated with D. amygdali and exposed to WD showed a significantly lower rate of the foliar CO2 assimilation (1.4 ± 0.4 µmol CO2 m−2 s−1) when compared to the respective treatment under WW conditions (2.6 ± 1.0 µmol CO2 m−2 s−1, p = 0.034).
Foliar net CO2 assimilation rate (A) (A), intercellular CO2 concentration (Ci) (B), stomatal conductance (gs) (C) and transpiration rate (E) (D) of blueberry plants under well-watered (WW) and water deficit (WD) conditions and inoculated with Diaporthe eres and D. amygdali. Data are presented as mean ± SD. Capital and lowercase letters indicate differences between inoculated plants within each water treatment (p ≤ 0.05). The asterisk indicates differences between WW and WD for a given treatment (*p ≤ 0.05)
Photosynthetic pigments content
Under WW conditions, no significant differences were found in chlorophyll b (Fig. 7B) and carotenoid (Fig. 7C) content in inoculated plants, regardless of the fungal species. However, Diaporthe inoculation produced an effect on chlorophyll a (Table 1). This pigment was significantly smaller in plants inoculated with D. eres (3.4 ± 0.5 nmol gFW−1) and D. amygdali (4.9 ± 1.9 nmol gFW−1), than in non-inoculated plants (7.8 ± 0.8 nmol gFW−1) (Fig. 7A). In plants under WD conditions, no significant differences in chlorophyll a were observed (Fig. 7A). Additionally, the interaction between the two factors (inoculation and water regime) had a negative impact on chlorophyll b and carotenoid content. Plants inoculated with D. eres under WW conditions had significantly higher contents of chlorophyll b (4.6 ± 0.4 nmol gFW−1) and carotenoids (2.4 ± 0.1 nmol gFW−1), compared to the respective treatment under WD conditions (chlorophyll b: 3.1 ± 0.6 nmol gFW−1, p = 0.011; carotenoids: 1.8 ± 0.5 nmol gFW−1, p = 0.048, Fig. 7B, Table 2).
Chlorophyll a, b, and carotenoids content of blueberry plants under well-watered (WW) and water deficit (WD) conditions and inoculated with Diaporthe eres and D. amygdali. Data are presented as mean ± SD. Capital and lowercase letters indicate differences between inoculated plants within each water treatment (p ≤ 0.05). The asterisk indicates differences between WW and WD for a given treatment (*p ≤ 0.05)
Malondialdehyde (MDA) content
Inoculation with D. eres under WW conditions influenced the MDA content (Fig. 8, Table 2). Significant differences were found in D. eres inoculated plants under WW conditions, exhibiting higher MDA content (60.1 ± 7.3 nmol gFW−1) when compared to the WD scenario (22.2 ± 6.4 nmol gFW−1, p = 0.001) (Fig. 8, Table 2). Moreover, a significant interaction between the factors was found (Table 1). Under WW conditions, plants inoculated with D. eres showed significantly higher MDA content when compared to those inoculated with D. amygdali (23.5 ± 12.2 nmol gFW−1) and to the non-inoculated plants (26.4 ± 10.6 nmol gFW−1). No significant differences were observed in WD treatments.
MDA content of blueberry plants under well-watered (WW) and water deficit (WD) conditions and inoculated with Diaporthe eres and D. amygdali. Data are presented as mean ± SD. Capital and lowercase letters indicate differences between inoculated plants within each water treatment (p ≤ 0.05). The asterisks indicate differences between WW and WD for a given treatment (***p ≤ 0.001)
Phenolic compounds and flavonoids content
Under WW conditions, the phenolic content of plants inoculated with D. eres (38.6 mg gFW−1) and D. amygdali (35.1 mg gFW−1) was significantly smaller, compared to the non-inoculated plants (43.2 mg gFW−1) (Fig. 9B). No significant differences were observed in the flavonoid content within each treatment (Fig. 9A). Additionally, the availability of water also had an effect on the antioxidant content of blueberries (Table 1). In non-inoculated plants and under WW conditions, the content of phenolic compounds (43.2 ± 7.4 mg gFW−1) and flavonoids (27.4 ± 6.8 mg gFW−1) were significantly higher compared to the non-inoculated plants under WD conditions (34.3 ± 5.7 mg gFW−1, p = 0.017; 20.9 ± 4.9 mg gFW−1, p = 0.031) respectively (Fig. 9B, Table 2).
Phenolic compounds (A) and flavonoids (B) content of blueberry plants under well-watered (WW) and water deficit (WD) conditions and inoculated with Diaporthe eres and D. amygdali. Data are presented as mean ± SD. Capital and lowercase letters indicate differences between inoculated plants within each water treatment (p ≤ 0.05). The asterisk indicates differences between WW and WD for a given treatment (*p ≤ 0.05)
Proline
The water treatment did not affect the proline content, whereas an interaction was evident between inoculation and the leaf gas exchange parameters (Table 1). Regarding the WW treatment, no significant differences were observed for proline content (Fig. 10) in inoculated and non-inoculated plants. Proline content was significantly higher in WD treated plants inoculated with D. eres (105.3 ± 4.0 mg g−1, p ≤ 0.001) and D. amygdali (104.0 ± 10.7 mg g−1, p ≤ 0.001) (Table 2). However, WD non-inoculated plants maintained the same profile as WW plants.
Proline content of blueberry plants under well-watered (WW) and water deficit (WD) conditions and inoculated with Diaporthe eres and D. amygdali. Data are presented as mean ± SD. Capital and lowercase letters indicate differences between inoculated plants within each water treatment (p ≤ 0.05). The asterisks indicate differences between WW and WD for a given treatment (***p ≤ 0.001)
Total soluble sugars and starch content
The inoculation with D. amygdali showed a negative effect on the total soluble sugar content (Table 2). Plants inoculated with this fungus under WW conditions showed lower content of soluble sugars (0.6 ± 0.1 mg gFW−1), compared to the non-inoculated plants (0.9 ± 0.09 mg gFW−1). Moreover, a significant interaction was observed between the inoculation and water regime factors. Plants under WD conditions and inoculated with D. amygdali exhibited higher total sugar content (1.2 ± 0.1 mg gFW−1), compared to those exposed to WW (0.6 ± 0.1 mg gFW−1, p = 0.003), while plants inoculated with D. eres showed lower sugars content (0.6 ± 0.03 mg gFW−1) compared to the respective WW condition (1.0 ± 0.2 mg gFW−1, p = 0.005).
Regarding the starch content, no significant differences were observed in starch content within plants under WW conditions (Fig. 11B). Nevertheless, the starch content in plants exposed to the WD treatment was significantly affected after inoculation with D. amygdali only. Additionally, a significant interaction between water and inoculation was found (Table 2). Inoculated plants with D. amygdali exhibited a significant increase in starch content (1.3 ± 0.5 mg gFW−1), compared to the respective WW treatment (0.7 ± 0.4 mg gFW−1, p = 0.047 (Fig. 11).
Total soluble sugars (A) and starch (B) content of blueberry plants under well-watered (WW) and water deficit (WD) conditions and inoculated with Diaporthe eres and D. amygdali. Data are presented as mean ± SD. Capital and lowercase letters indicate differences between inoculated plants within each water treatment (p ≤ 0.05). The asterisks indicate differences between WW and WD for a given treatment (**p ≤ 0.01. ***p ≤ 0.001)
Discussion
To our knowledge, this is the first study to explore the pathosystem V. corymbosum/Diaporthe spp., under two water regimes. Our results indicate that although D. eres and D. amygdali were able to colonize host tissue, infected blueberry plants responded differently to the two pathogens. This study also highlights the role of water availability in infection of blueberry plants by Diaporthe that seems to occur in a pathogen type-dependent manner.
Water deficit conditions impacted plant physiological performance. Some studies have shown that under 45–50% of FC, blueberry plants suffer moderate water stress, which negatively affects their physiological and biochemical characteristics (Balboa et al., 2020; Chen et al., 2017). For instance, Chen et al. (2017) showed that the chlorophyll content of blueberry decreased, while the MDA content increased under drought stress conditions. Additionally, Balboa et al. (2020) demonstrated that proline content increased in all cultivars tested under WD conditions. Rho et al. (2012) proved that cultivar ‘Bluecrop’ was highly sensitive to WD, given the observed rapid stomatal closure and reduced gas exchange. Lobos et al. (2018) also demonstrated that under severe WD conditions, cultivar ‘Brigitta’ displays water potentials ranging from –0.9 MPa to –1.2 MPa. However, our results indicate that although we have applied a stricter water regime (30% FC), this was not sufficient for plants of cultivar ‘Duke’ to reflect severe changes at physiological and biochemical levels, given for instance the observed high values of water potential (ranging from –0.2 MPa to –0.4 MPa).
One of the main effects of the water regime applied was a decrease in phenolic compounds and flavonoids. Although the flavonoid and phenolic compound content can accumulate in plants under abiotic stresses, such as drought, (Laoué et al., 2022; Naikoo et al., 2019), environmental stress can also cause a decrease in the content of phenols (Król et al., 2014). Therefore, it is suggested that the antioxidant system component was not activated, and thus was not involved in plant defense as a strategy to cope with water limitation. Moreover, the leaf gas exchange was negatively affected by the water regime applied through the decrease in gs, E, A rates which might be considered as a water saving strategy (Bertolino et al., 2019; Morato de Moraes et al., 2020). Such results might explain the ability of cultivar ‘Duke’ to adapt to water-stress conditions (Molnar et al., 2022).
Under WW conditions, blueberry plant responses depend on the pathogen inoculated. Based on the lesion lengths observed in this study, D. amygdali CAA958 was the most aggressive, while D. eres CBS 160.32 caused minor symptoms as previously demonstrated by Hilário et al., (2021a, 2021b), suggesting that strain CBS 160.32 does not seem to pose a threat to blueberry plants. One of the plant responses to pathogen infection is the imbalance of photosynthesis (Rojas et al., 2014). The results showed that plants inoculated with both D. eres and D. amygdali under WW conditions exhibited a decrease in A, gs and E, and an increase in Ci. The increase of Ci may be caused by a reduction in the activity of photosynthetic machinery components that limited CO2 assimilation probably due to oxidative stress (Munné-Bosch & Peñuelas, 2004). This may suggest that D. eres and D. amygdali were able to negatively affect the photosynthetic apparatus of blueberries as reported for other pathosystems (Amaral et al., 2019). Moreover, Roloff et al. (2004) showed that infection of blueberry plants by Septora albopunctata had a significant negative effect on photosynthesis, with net assimilation rate decreasing as disease severity increased. Furthermore, the observed decrease in chlorophyll a after D. eres and D. amygdali infection may also help explaining the decrease in photosynthetic performance. Chlorophylls are the main pigments in the plant photosynthetic apparatus, responsible for energy absorption and the transfer of electron chains (ETR) (Gu et al., 2017). The decrease in chlorophyll a content may lead to a lower capacity to absorb energy, thus causing a decrease in the net CO2 assimilation rate, as observed in our study. The photosynthetic capacity of maize leaves is inhibited by infection with Colletotrichum musae and Fusarium moniliforme and is accompanied by a decrease in chlorophyll content (Costa Pinto et al., 2000). Another aspect to consider is that the production of phytotoxins by fungi (e.g., alternariol), may have an impact on the photosynthetic apparatus (Demuner et al., 2013). Therefore, as suggested by Linaldeddu et al. (2009), the decrease in the net photosynthetic rate and the total chlorophyll, regardless of the lesion size observed, could be explained by the production of diffusible toxins.
Stress usually leads to oxidative stress induced by increased reactive oxygen species (ROS) production, which is indicated by the accumulation of MDA (Xu & Zhou, 2006). However, it is important to note that lipid peroxidation can also result from increased lipoxygenase activity, caused by a pathogen invasion (Morales & Munné-Bosch, 2019). In fact, in this study we observed an increase in MDA content in plants under WW conditions and inoculated with D. eres. Besides the minor symptoms and the null mortality observed, it is suggested that MDA might have acted as a signaling molecule rather than a response to lipid peroxidation (Morales & Munné-Bosch, 2019) in plants inoculated with D. eres.
It is recognized that sugars play a key role in plant defense, supplying energy for cellular defense against pathogens (Nabavi et al., 2020). Nevertheless, either a decrease or an increase may happen in the level of sugars in infected tissues (Berger et al., 2007). Our results show that the total soluble sugar content decreased in those plants under WW conditions and infected with D. amygdali. Considering that this pathogen was shown to be the most aggressive, it is plausible that the enhanced sugar metabolism from the plant to cope with fungal infection may have caused changes in the composition of carbohydrates, thus causing a reduction of their content (Morkunas & Ratajczak, 2014).
Water deficit conditions may change pathogen behavior impacting plant responses. Previous studies have shown that low water availability results in physiological and metabolic changes in several agricultural crops, weakening the host defense and increasing disease susceptibility (Ghanbary et al., 2021). Such changes can cause oxidative damage to membrane lipids and decreases in stomatal conductance, photosynthetic rate, and chlorophyll concentration (Ghanbary et al., 2017; Yang & Luo, 2021). Although investigations into the effects of stress scenarios on disease development caused by Diaporthe are limited, stress of the host plant (e.g., transplant shock, excessive pruning, freeze injury) may affect the transition from the endophytic to pathogenic phase (Hrycan et al., 2020). Hulke et al. (2019) found that increases in average precipitation led to enhanced disease incidence of Phomopsis stem blight of sunflowers caused by D. helianthi. Moreover, Kim et al. (2015) found that severe frost and frequent wind events increased the susceptibility of Citrus trees to D. citri infection. In the present study, under WD conditions, D. amygdali CAA958 not only caused larger lesions than D. eres, but also caused 50% plant mortality. Thus, it is suggested that water availability may be considered a key factor that enhances the pathogenicity of D. amygdali. Moreover, the mortality observed might also have been caused by the presence of phytotoxins such as the Fusicoccin, identified in D. amygdali and recently detected on the genome of D. amygdali strain CAA958 (Hilário et al., 2022). Although this toxin induces opening of stomata and uncontrolled transpiration (Marra et al., 2021), we observed stomatal closure through the decrease of gs and E in plants inoculated with D. amygdali. However, this hypothesis cannot be overlooked, and further studies should be considered to evaluate the effect that Fusicoccin may pose to blueberry plants. Despite the differences in aggressiveness, inoculated plants with both pathogens under WD conditions showed a decrease in A, gs and E, and an increase in Ci when compared to WD non-inoculated plants, revealing stomatal limitations and impacts at metabolic level. For instance, plants inoculated with D. amygdali CAA958 reflected an increase in proline, starch, and total soluble sugars content, whereas plants inoculated with D. eres CBS 160.32 showed a decrease in total soluble sugar content.
Under abiotic or biotic stresses, plants remobilize starch to provide energy and carbon, when photosynthesis may be limited (Thalmann & Santelia, 2017). According to our results, under WD conditions, plants inoculated with D. amygdali CAA958 seem to invest their photo-assimilated energy (total soluble sugars and starch) to maintain cell turgor and to protect them against oxidative stress and membrane injury (Chen & Jiang, 2010). Proline accumulation has been reported as a key metabolite, functioning as a compatible osmolyte and a signaling molecule that contributes to cellular osmotic adjustment, ROS detoxification and protection of membrane integrity (Hayat et al., 2012; Kavi Kishor & Sreenivasulu, 2014). Our results showed high levels of proline in plants inoculated under WD conditions. Such a result is corroborated by Balboa et al. (2020), who also showed the same trend in different blueberry cultivars, under WD stress. This suggests that proline may act more as a signaling molecule rather than an osmoprotectant after pathogen infection and under water limitation (Hayat et al., 2012). Although MDA content was lower in plants inoculated with D. amygdali CAA958 and D. eres CBS 160.32 under WD conditions, this does not imply that an increase in ROS production did not occur: 1) the presence of scavenging enzymes in fungal pathogens are determinant in detoxification of ROS to ensure a successful infection, as already reported in the fungus Magnaporthe oryzae (Apel & Hirt, 2004; Huang et al., 2011, 2019); 2) proline accumulation may act as an antioxidant to scavenge intracellular ROS (Hossain et al., 2014).
Conclusions
The study of the pathogens and plants as an interacting system, coupled with experimental systems with stresses combinations, is essential to unveil how plants cope with a pathogen attack or global change scenarios. The present study is the first to explore the pathosystem V. corymbosum/Diaporthe spp., under WW and WD conditions. Our study evidenced that D. eres CBS 160.32, previously regarded as a quarantine organism, was the least aggressive species and therefore may not be a major threat to blueberry plants. Nevertheless, our results highlighted that D. eres CBS 160.32 was able to alter practically all parameters analyzed, on both WD and WW treatments, which may eventually impact the health of the plants. Therefore, this suggests that lesion size is not related to physiological alterations, and thus cannot be considered as the main measure of pathogen aggressiveness. Our findings also support that D. eres CBS 160.32 and D. amygdali CAA958 may act as latent pathogens, given the minor symptoms and null mortality rates under WW conditions. Nevertheless, as plants inoculated with D. amygdali CAA958 showed 50% mortality under WD conditions, it is suggested that water availability may be a key factor for the pathogenicity of this species.
Furthermore, future studies including RNA sequencing (RNA-Seq) technology should be conducted to unveil blueberry defense mechanisms upon fungal infection and water availability. This approach coupled with more physiological studies, may support the selection of resistant traits to design innovative plant protection strategies under global change scenarios that may hamper agriculture sustainability.
Data availability
The datasets generated and analyzed in this study are available from the corresponding author on reasonable request.
References
Amaral, J., Correia, B., António, C., Rodrigues, A. M., Gómez-Cadenas, A., Valledor, L., Hancock, R. D., Alves, A., & Pinto, G. (2019). Pinus susceptibility to pitch canker triggers specific physiological responses in symptomatic plants: An integrated approach. Frontiers in Plant Science, 10, 509.
Apel, K., & Hirt, H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55, 373–399.
Balboa, K., Ballesteros, G. I., & Molina-Montenegro, M. A. (2020). Integration of physiological and molecular traits would help to improve the insights of drought resistance in highbush blueberry cultivars. Plants, 9, 1457.
Baldi, P., & La Porta, N. (2020). Molecular approaches for low-cost point-of-care pathogen detection in agriculture and forestry. Frontiers in Plant Science, 11, 570862.
Bates, L. S., Waldren, R.P., & Teare, 1973I. D. Rapid determination of free proline for water-stress studies. Plant Soil, 39, 205e207.
Berger, S., Sinha, A. K., & Roitsch, T. (2007). Plant physiology meets phytopathology: Plant primary metabolism and plant–pathogen interaction. Journal of Experimental Botany, 58, 4019–4026.
Bertolino, L. T., Caine, R. S., & Gray, J. E. (2019). Impact of stomatal density and morphology on water-use efficiency in a changing world. Frontiers in Plant Science, 10, 225.
Cardinaals, J., Wenneker, M., Voogd, J. G. B., & van Leeuwen, G. C. M. (2018). Pathogenicity of Diaporthe spp. on two blueberry cultivars (Vaccinium corymbosum). EPPO Bulletin, 48, 128–134.
Chang, C.-C., Yang, M.-H., Wen, H.-M., & Chern, J.-C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10, 178–182.
Chen, H., & Jiang, J.-G. (2010). Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environmental Reviews, 18, 309–319.
Chen, X., Qiu, L., Guo, H., Wang, Y., Yuan, H., Yan, D., & Zheng, B. (2017). Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Brazilian Journal of Botany, 40, 841–851.
Correia, B., Hancock, R. D., Amaral, J., Gomez-Cadenas, A., Valledor, L., & Pinto, G. (2018). Combined drought and heat activates protective responses in Eucalyptus globulus that are not activated when subjected to drought or heat stress alone. Frontiers in Plant Science, 9, 819.
Costa Pinto, L. S. R., Azevedo, J. L., Pereira, J. O., Carneiro Vieira, M. L., & Labate, C. A. (2000). Symptomless infection of banana and maize by endophytic fungi impairs photosynthetic efficiency. New Phytologist, 147, 609–615.
del Pozo, A., Brunel-Saldias, N., Engler, A., Ortega-Farias, S., Acevedo-Opazo, C., Lobos, G. A., Jara-Rojas, R., & Molina-Montenegro, M. A. (2019). Climate change impacts and adaptation strategies of agriculture in Mediterranean-climate regions (MCRs). Sustainability, 11, 2769.
Dinis, L. T., Oliveira, M. M., Almeida, J., Costa, R., Gomes-Laranjo, J., & Peixoto, F. (2012). Antioxidant activities of chestnut nut of Castanea sativa Mill. (cultivar ‘Judia’) as function of origin ecosystem. Food Chemistry, 132, 1–8.
Dissanayake, A. J., Chen, Y. Y., & Liu, J. K. J. (2020). Unravelling Diaporthe species associated with woody hosts from Karst Formations (Guizhou) in China. Journal of Fungi, 6, 251.
Demuner, A. J., Barbosa, L. C. A., Miranda, A. C. M., Geraldo, G. C., da Silva, C. M., Giberti, S., Bertazzini, M., & Forlani, G. (2013). The fungal phytotoxin alternariol 9-methyl ether and some of its synthetic analogues inhibit the photosynthetic electron transport chain. Journal of Natural Products, 76, 2234–2245.
FAOSTAT. (2022). Food and Agriculture Organization of the United Nations. Retrieved 13 March 2022 from http://www.fao.org/faostat/en/#home
Ghanbary, E., Fathizadeh, O., Pazhouhan, I., Zarafshar, M., Tabari, M., Jafarnia, S., Parad, G. A., & Bader, M. K. F. (2021). Drought and pathogen effects on survival, leaf physiology, oxidative damage, and defense in two middle eastern oak species. Forests, 12, 247.
Ghanbary, E., Kouchaksaraei, M. T., Mirabolfathy, M., Modarres Sanavi, S. A. M., & Rahaei, M. (2017). Growth and physiological responses of Quercus brantii seedlings inoculated with Biscogniauxia mediterranea and Obolarina persica under drought stress. Forest Pathology, 47, e12353.
Gomes, R. R., Glienke, C., Videira, S. I. R., Lombard, L., Groenewald, J. Z., & Crous, P. W. (2013). Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia, 31, 1–41.
Gu, J., Zhou, Z., Li, Z., Chen, Y., Wang, Z., Zhang, H., & Yang, J. (2017). Photosynthetic properties and potentials for improvement of photosynthesis in pale green leaf rice under high light conditions. Frontiers in Plant Science, 8, 1082.
Guarnaccia, V., Brondino, L., Garibaldi, A., & Gullino, M. L. (2021). Leaf anthracnose and defoliation of blueberry caused by Colletotrichum helleniense in Northern Italy. Phytopathologia Mediterranea, 60, 479–491.
Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J., & Ahmad, A. (2012). Role of proline under changing environments. Plant Signaling & Behavior, 7, 1456–1466.
Hazrati, S. Z., Tahmasebi-Sarvestani, S. A., Modarres-Sanavy, M., Mokhtassi-Bidgoli, A., & Nicola, S. (2016). Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiology and Biochemestry, 106, 141–148.
Heath, R. L., & Packer, L. 1968 Photoperoxidation in isolated chloroplasts. Archives of Biochemistry and Biophysics, 1968. 125, 189e198.
Hilário, S., Amaral, A. I., Gonçalves, M. F. M., Lopes, A., Santos, L., & Alves, A. (2020). Diaporthe species associated with twig blight and dieback of Vaccinium corymbosum in Portugal, with description of four new species. Mycologia, 112, 293–308.
Hilário, S., Gonçalves, M. F. M., Fidalgo, C., Tacão, M., & Alves, A. 2022 Genome analyses of two blueberry pathogens: Diaporthe amygdali CAA958 and Diaporthe eres CBS 160.32. Journal of Fungi, 8, 804.
Hilário, S., Gonçalves, M. F. M., & Alves, A. (2021a). Using genealogical concordance and coalescent-based species delimitation to assess species boundaries in the Diaporthe eres complex. Journal of Fungi, 7, 507.
Hilário, S., Santos, L., & Alves, A. (2021b). Diversity and Pathogenicity of Diaporthe Species Revealed from a Survey of Blueberry Orchards in Portugal. Agriculture, 11, 1271.
Hossain, M. A., Hoque, M. A., Burritt, D. J., & Fujita, M. (2014). Proline protects plants against abiotic oxidative stress: Biochemical and molecular mechanisms. In P. Ahmad (Ed.), Oxidative damage to plants (pp. 477–522). Academic Press, Elsevier Inc.
Hossain, M., Veneklaas, E. J., Hardy, G. E. S. J., & Poot, P. (2019). Tree host–pathogen interactions as influenced by drought timing: Linking physiological performance, biochemical defence and disease severity. Tree Physiology, 39, 6–18.
Huang, K., Czymmek, K. J., Caplan, J. L., Sweigard, J. A., & Donofrio, N. M. (2011). Suppression of plant-generated reactive oxygen species is required for successful infection by the rice blast fungus. Virulence, 2, 559–562.
Huang, H., Ullah, F., Zhou, D. X., Yi, M., & Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Frontiers in Plant Science, 10, 800.
Hulke, B. S., Markell, S. G., Kane, N. C., & Mathew, F. M. (2019). Phomopsis stem canker of sunflower in North America: Correlation with climate and solutions through breeding and management. Oilseeds & Fats, Crops and Lipids, 26, 13.
Hrycan, J., Hart, M., Bowen, P., Forge, T., & Urbez-Torres, J. R. (2020). Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease development and symptom expression. Phytopathologia Mediterranea, 59, 395–424.
INE. (2021). Portal do Instituto Nacional de Estatística: Estatísticas agrícolas 2020. Retrieved 13 March 2022 from https://www.ine.pt/xurl/pub/437147278
Jiménez-Donaire, M. D. P., Giráldez, J. V., & Vanwalleghem, T. (2020). Impact of climate change on agricultural droughts in Spain. Water, 12, 3214.
Kavi Kishor P.,B., & Sreenivasulu N 2014 Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant, Cell & Environment 37, 300-311.
Kim, K. H., Kim, G. H., Son, K. I., & Koh, Y. J. (2015). Outbreaks of yuzu dieback in Goheung Area: Possible causes deduced from weather extremes. The Plant Pathology Journal, 31, 290.
Król, A., Amarowicz, R., & Weidner, S. (2014). Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiologiae Plantarum, 36, 1491–1499.
Laoué, J., Fernandez, C., & Ormeño, E. (2022). Plant flavonoids in mediterranean species: A focus on flavonols as protective metabolites under climate stress. Plants, 11, 172.
Linaldeddu, B. T., Sirca, C., Spano, D., & Franceschini, A. (2009). Physiological responses of cork oak and holm oak to infection by fungal pathogens involved in oak decline. Forest Pathology, 39, 232–238.
Lobos, T. E., Retamales, J. B., Ortega-Farías, S., Hanson, E. J., López-Olivari, R., & Mora, M. L. (2018). Regulated deficit irrigation effects on physiological parameters, yield, fruit quality and antioxidants of Vaccinium corymbosum plants cv. Brigitta. Irrigation Science, 36, 49–60.
López-Hidalgo, C., Meijón, M., Lamelas, L., & Valledor, L. (2021). The rainbow protocol: A sequential method for quantifying pigments, sugars, free amino acids, phenolics, flavonoids and MDA from a small amount of sample. Plant, Cell & Environment, 6, 1977–1986.
Marra, A., Camoni, A. L., Visconti, A., Fiorillo, A., & Evident, A. (2021). The surprising story of Fusicoccin: A wilt-inducing phytotoxin, a tool in plant physiology and a 14-3-3-targeted drug. Biomolecules, 11, 1393.
Mazurek, M., Siekierzyńska, A., Jacek, B., & Litwińczuk, W. (2021). Differences in response to drought stress among highbush blueberry plants propagated conventionally and by tissue culture. Plant Biosystems - an International Journal Dealing with All Aspects of Plant Biology, 155, 172–178.
Mazzoni L Balducci F Di Vittori Scalzo J Capocasa F Zhong C. F., Forbes-Hernandez T, Y., Giampieri F M Battino Mezzetti B 2020 Yield and nutritional quality of highbush blueberry genotypes trialled in a Mediterranean hot summer climate Journal of the Science of Food and Agriculture 100, 3675-3686.
Mingeau, M., Perrier, C., & Ameglio, T. (2001). Evidence of drought-sensitive periods from flowering to maturity on highbush blueberry. Scientia Horticulturae, 89, 23–40.
Molnar, S., Clapa, D., & Mitre, V. (2022). Response of the five highbush blueberry cultivars to in vitro induced drought stress by polyethylene glycol. Agronomy, 12, 732.
Morales, C. G., Pino, M. T., & del Pozo, A. (2013). Phenological and physiological responses to drought stress and subsequent rehydration cycles in two raspberry cultivars. Scientia Horticulturae, 162, 234–241.
Morales, M., & Munné-Bosch, S. (2019). Malondialdehyde: Facts and artifacts. Plant Physiology, 180, 1246–1250.
Morato de Moraes, D. H., Mesquita, M., Bueno, A. M., Flores, R. A., Elias de Oliveira, H. F., de Lima, F. S. R, de Melo Prado, R., & Battisti, R. Combined effects of induced water deficit and foliar application of silicon on the gas exchange of tomatoes for processing. Agronomy, 2020 10, 1715.
Morkunas, I., & Ratajczak, L. (2014). The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiologiae Plantarum, 36, 1607–1619.
Munné-Bosch, S., & Peñuelas, J. (2004). Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Science, 166, 1105–1110.
Naikoo, M. I., Dar, M. I., Raghib, F., Jaleel, H., Ahmad, B., Raina, A., Khan, F. A., & Naushin, F. (2019). Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In M. I. R. Khan, P. S. Reddy, A. Ferrante, & N. A. Khan (Eds.), Plant Signaling Molecules (pp. 157–168). Woodhead Publishing.
Nabavi, S., Samec, D., Tomczyk, M., Milella, L., Russo, D., Habtemariam, S., Suntar, I., Rastrelli, L., Daglia, M., Xiao, J., Giampieri, F., Battino, M., Sobarzo-Sanchez, E., Nabavi, S. F., Yousefi, B., Jeandet, P., Xu, S., & Shirooie, S. (2020). Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnology Advances, 39, 107461.
Ortega-Farias, S., Espinoza-Meza, S., López-Olivari, R., Araya-Alman, M., & Carrasco-Benavides, M. (2021). Effects of different irrigation levels on plant water status, yield, fruit quality, and water productivity in a drip-irrigated blueberry orchard under Mediterranean conditions. Agricultural Water Management, 249, 106805.
Pandey, P., Irulappan, V., Bagavathiannan, M. V., & Senthil-Kumar, M. (2017). Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Frontiers in Plant Science, 8, 537.
Ramegowda, V., & Senthil-Kumar, M. (2015). The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. Journal of Plant Physiology, 176, 47–54.
Rho, H., Yu, D. J., Kim, S. J., & Lee, H. J. (2012). Limitation factors for photosynthesis in ‘Bluecrop’ highbush blueberry (Vaccinium corymbosum) leaves in response to moderate water stress. Journal of Plant Biology, 55, 450–457.
Rojas, C. M., Senthil-Kumar, M., Tzin, V., & Mysore, K. (2014). Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Frontiers in Plant Science, 5, 17.
I Roloff H Scherm M W Iersel Van 2004 Photosynthesis of blueberry leaves as affected by Septoria leaf spot and abiotic leaf damage Plant Disease 88, 397-401.
SC Silva E M Veiga M Morais, R M Calhau C & M Pintado Health promoting properties of blueberries: A review Critical Reviews in Food Science and Nutrition 60 2020 181 200.
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Sinha, R., Irulappan, V., Mohan-Raju, B., Suganthi, A., & Senthil-Kumar, M. (2019). Impact of drought stress on simultaneously occurring pathogen infection in field-grown chickpea. Scientific Reports, 9, 1–15.
Smart, R. E., & Bingham, G. E. (1974). Rapid estimates of relative water content. Plant Physiology, 53, 258–260.
Swinfield, T., Lewis, O., Bagchi, R., & Freckleton, R. (2012). Consequences of changing rainfall for fungal pathogen-induced mortality in tropical tree seedlings. Ecology and Evolution, 2, 1408–1413.
Thalmann, M., & Santelia, D. (2017). Starch as a determinant of plant fitness under abiotic stress. New Phytologist, 214, 943–951.
Toscano, S., Ferrante, A., & Romano, D. (2019). Response of Mediterranean ornamental plants to drought stress. Horticulturae, 5, 6.
Xu, Z. Z., & Zhou, G. S. (2006). Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta, 224, 1080–1090.
Yang, H., & Luo, P. (2021). Changes in photosynthesis could provide important insight into the interaction between wheat and fungal pathogens. International Journal of Molecular Sciences, 22, 8865.
Yu, D. J., Rho, H., Kim, S. J., & Lee, H. J. (2015). Photosynthetic characteristics of highbush blueberry (Vaccinium corymbosum cv. Bluecrop) leaves in response to water stress and subsequent re-irrigation. The Journal of Horticultural Science and Biotechnology, 90, 550–556.
Acknowledgements
Thanks are due to the Portuguese Foundation for Science and Technology (FCT/MCTES) for the financial support to CESAM (Centro de Estudos do Ambiente e do Mar) (UIDP/50017/2020 + UIDB/50017/2020 + LA/P/0094/2020), and the PhD grants of Sandra Hilário (SFRH/BD/137394/2018) and Pedro Monteiro (SFRH/BD/143879/2019).
Funding
Open access funding provided by FCT|FCCN (b-on). Conceptualization: A.A., L.S., S.H.; Methodology: A.A., G.P., S.H.; Formal analysis and investigation: P.M., S.H.; Writing-original draft: S.H.; Writing-review & editing: A.A., G.P., L.S., P.M., S.H; Funding acquisition: A.A.; Resources: A.A., G.P; Supervision: A.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All principles of ethical and professional conduct have been followed during this research and elaboration of this manuscript.
Informed consent
All authors have reviewed the manuscript and approved its submission to the European Journal of Plant Pathology.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hilário, S., Pinto, G., Monteiro, P. et al. The impact of two Diaporthe species on Vaccinium corymbosum physiological performance under different water availability scenarios. Eur J Plant Pathol 166, 161–177 (2023). https://doi.org/10.1007/s10658-023-02651-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02651-w