Abstract
Lichens, which are symbioses of a fungus and one or two photoautotrophs, frequently tolerate extreme environmental conditions. This makes them valuable model systems in astrobiological research to fathom the limits and limitations of eukaryotic symbioses. Various studies demonstrated the high resistance of selected extremotolerant lichens towards extreme, non-terrestrial abiotic factors including space exposure, hypervelocity impact simulations as well as space and Martian parameter simulations. This study focusses on the diverse set of secondary lichen compounds (SLCs) that act as photo- and UVR-protective substances. Five lichen species used in present-day astrobiological research were compared: Buellia frigida, Circinaria gyrosa, Rhizocarpon geographicum, Xanthoria elegans, and Pleopsidium chlorophanum. Detailed investigation of secondary substances including photosynthetic pigments was performed for whole lichen thalli but also for axenically cultivated mycobionts and photobionts by methods of UV/VIS-spectrophotometry and two types of high performance liquid chromatography (HPLC). Additionally, a set of chemical tests is presented to confirm the formation of melanic compounds in lichen and mycobiont samples. All investigated lichens reveal various sets of SLCs, except C. gyrosa where only melanin was putatively identified. Such studies will help to assess the contribution of SLCs on lichen extremotolerance, to understand the adaptation of lichens to prevalent abiotic stressors of the respective habitat, and to form a basis for interpreting recent and future astrobiological experiments. As most of the identified SLCs demonstrated a high capacity in absorbing UVR, they may also explain the high resistance of lichens towards non-terrestrial UVR.
Similar content being viewed by others
Introduction
Lichens are symbiotic associations constituted of a heterotrophic fungus (mycobiont) and a photoautotrophic partner (photobiont). The latter is a eukaryotic green alga or a prokaryotic cyanobacterium but tripartite systems may also occur (Henssen and Jahns 1974). The lichen thallus represents a distinct, species-specific structure that confers emergent morphological and physiological features. Lichens tolerate extreme environmental conditions (Kappen 1973, 1988) as found in highly insolated alpine, polar, and arid habitats (Lange 1992). Due to their extremotolerant, symbiotic, and eukaryotic nature some lichens were established as model organisms in astrobiological research (Sancho et al. 2009; de Vera and Ott 2010; Meeßen et al. 2013b). In this context, the capacity of lichens to endure extreme conditions associated to space exposure experiments or planetary (especially Martian) environment simulations is of special interest (de Vera et al. 2010, 2013 in print). Such factors include cold (Kappen 1993; Dyer and Crittenden 2008), high levels of (ultraviolet) radiation (Lange 1992; Nybakken et al. 2004), infrequent water supply, and extreme drought as found in the dry valley cold deserts in Antarctica (Marchant and Head 2007; Harańczyk et al. 2008; Sun et al. 2010) or in the Andean Atacama (McKay et al. 2003).
To get a deeper insight into lichen extremotolerance, it is essential to assess their limits and limitations towards hostile environmental conditions. That includes testing on single or combined abiotic factors under extreme terrestrial conditions, in space or Mars simulations, but also in space exposure experiments. In this field of astrobiology, remarkable progress was made in various experimental approaches including simulation tests on vacuum, Martian atmosphere, and UVR (often in combination, de Vera et al. 2003, 2004a, b, 2008, 2010; de Vera and Ott 2010; de Vera 2012; de la Torre et al. 2004, 2007; Sánchez et al. 2012), on hypervelocity impacts (Stöffler et al. 2007; Horneck et al. 2008), but also in space experiments as LICHENS II on BIOPAN 5/FOTON M2, LITHOPANSPERMIA and STONE on BIOPAN6/FOTON M3, and LIFE on EXPOSE-E/EuTEF (Sancho et al. 2007; de la Torre et al. 2007, 2010; Raggio et al. 2011; Onofri et al. 2012; Scalzi et al. 2012). Currently, the BIOMEX-experiment is in progress to expose two lichen species to LEO conditions and to simulated Mars conditions for 15–18 months on EXPOSE-R2/Zvezda (ESA call ILSRA-AO 2009). The results of these experiments consistently stress the potential of lichens to resist space or Martian conditions (see references above).
Lichen resistance to extreme environmental stressors is based on the peculiar lichen lifestyle and on manifold adaptations towards harsh conditions experienced in the natural habitat (Kappen 1988; Lange et al. 1999b, 2001). Among others, three adaptive traits are discussed to constitute lichen resistance. First, poikilohydry allows lichens to tolerate drought by passing into an ametabolic state called anhydrobiosis (Kranner et al. 2005). Anhydrobiosis also protects lichens against stressors that come along with desiccation, including high or low temperatures and high UVR-/PAR-levels (Nybakken et al. 2004) while rehydration by liquid water or high air humidity (>80 % to 97 %, Sigfridsson and Oquist 1980; Lange et al. 1986) results in metabolic re-activation. In nature, water availability is often correlated to more moderate climatic conditions, allowing lichens to benefit from favourable conditions and omit unfavourable ones. In the context of astrobiology, the importance of poikilohydry is stressed by recent studies on lichen UVC-resistance in anhydrobiosis and when physiologically active (Sánchez et al. 2013).
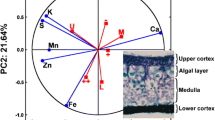
Second is the diverse set of morphological and anatomical properties that shape a lichen thallus. Lichen morphology is clearly linked to the high plasticity of thalline structures adapting lichens to the habitat’s dominant (micro)climatic and orographic factors. The morpho-anatomical diversity is displayed by a broad range of growth types as well as by functional structures (e.g. heteromeric thallus stratification and cortices). The adaptive significance of these structures is a complex issue in lichenology (Jahns 1988) and its relevance for astrobiology was recently considered elsewhere (Meeßen et al. 2013b).
The present study focuses on the third aspect: a high number of secondary lichen compounds (SLCs) of which many are found exclusively in lichens. While some authors estimated about 200 (Rundel 1978) to 600 SLCs (Brodo et al. 2001), Huneck and Yoshimura (1996) compiled more than 1,000 substances reported from lichens. About 90 % of them may be regarded as SLCs and their derivatives, usually constituting 1–6 % and rarely up to 20–42 % of thallus dry weight (Rundel 1978). Three major biosynthetic pathways of SLCs are described (Mosbach 1964, 1967; Henssen and Jahns 1974; Rundel 1978). The most important one by number of compounds is the acetate-polymalonate pathway (also polyketide pathway). It produces aliphatic and mono- to polycyclic aromatic compounds by polymerisation of CoA-bound acetyl-groups with one to several CoA-bound malonyl-groups, partial reduction, and subsequent cyclisation. Common compound classes of this pathway are depsides (e.g. gyrophoric acid), depsidones (e.g. norstictic acid), depsones, anthraquinones (e.g. parietin), xanthones, chromones, and usnic acids. The second one is the shikimate pathway which leads to two compound classes in lichens: terphenylquinones and tetronic acids whose subgroup of pulvinic acid derivatives is of high abundance (e.g. rhizocarpic acid). To synthesise pulvinic acid, phosphoenolpyruvate and erythrose-4-phosphate are transformed via several intermediates as shikimic and polyporic acid. The third one is the mevalonic acid pathway. It starts with the formation of mevalonic acid by condensating three CoA-bound acetyl-groups. Subsequent reduction, decarboxylation, and phosphorylation lead to isopentenyl pyrophosphate as the precursor of steroids and di- or tri-terpenic compounds (e.g. carotenoids). Apart from these major pathways, numerous other compound classes were also produced (Boustie and Grube 2005).
Physiological and ecological functions of SLCs are mostly unknown and thought to be diverse. Considering their high diversity, it is reasonable to assume that single compounds classes are involved in more than one function (Rundel 1978). Besides other hypotheses, four ecological roles are well supported by experimental data: 1) bio-weathering of rock by chelating metal ions, 2) allelopathic effects in terms of antibacterial and antifungal defense, and 3) antiherbivorous effects. In the context of astrobiology, the following role seems to be most relevant: 4) the photoprotective effect of SLCs (Rundel 1978; Fahselt 1994) by absorbing excess doses of PAR and UVR causing photoinhibition/oxidative stress as well as biological damage (Solhaug and Gauslaa 1996; Huneck 1999; Solhaug and Gauslaa 2004; McEvoy et al. 2006). So, photoprotection of the non-photophilic photobionts (as the common genus Trebouxia) and the mycobiont itself is considered to be a main function of pigmented cortices in lichens (Ertl 1951; Jahns 1988). UVR-protection by SLCs is most crucial when the lichen thalli are desiccated and repair mechanisms are not active (Lange et al. 1999a). Thus, SLCs are discussed as a key factor to resist space conditions (de Vera et al. 2003, 2004a, b; de Vera and Ott 2010; Raggio et al. 2011), especially concerning extreme desiccation under vacuum. Indeed, the majority of SLCs show maximal absorption in the UVR range (Hale 1956; BeGora and Fahselt 2001; Solhaug et al. 2003), providing the same advantage that flavonoids confer to higher plants (Jordan 1996). Cortical SLCs are reported to absorb up to 50 % of light influx (Ertl 1951) and show positively correlated concentrations along light gradients (Rundel 1978). They act as filters to provide optimal light levels in the algal layer. Parietin for example, is obligately found in the genera of Xanthoria, Fulgensia, Caloplaca, and Teloschistes, all colonising exposed habitats, while shade-adapted lichens as Lobaria pulmonaria lack any cortical pigments (Gauslaa and Solhaug 2001).
By now, astrobiological studies were performed with eight lichen species whose mycobionts have been ascomycetes (Lecanoromycetes) and whose photobionts have been classified as green algae (Trebouxiophyceae). The species used preferentially are Buellia frigida, Circinaria gyrosa, Rhizocarpon geographicum, Xanthoria elegans, and recently Pleopsidium chlorophanum (de Vera et al. 2012a, 2013 in print). Apart from that, Fulgensia bracteata, Xanthoria parietina, and Peltigera aphthosa were used for particular UVR- and vacuum-simulation studies (de Vera et al. 2004a, b; de Vera and Ott 2010). To continue previous research, the present study will give a comparative approach on potentially photoprotective SLCs in the five space-relevant lichens with special respect to their UV-protective properties. Such approach might help to assess the SLC’s role in extremotolerance and give a valuable basis for interpreting the data of recent and future experiments.
Material and Methods
The astrobiologically relevant lichen species investigated in the present study are Buellia frigida Darb. (1910), Circinaria gyrosa Sohrabi (2012), Xanthoria elegans (Link) Th. Fr. (1860), Rhizocarpon geographicum (L.) DC (1805), and Pleopsidium chlorophanum (Wahlenb.) Zopf (1855). A detailed overview on the five lichens and their morphological-anatomical characteristics was offered before (Meeßen et al. 2013b) while basic information of these species is given in Table 1. The photobiont of B. frigida, C. gyrosa, X. elegans and R. geographicum is the photobiont Trebouxia spec., while P. chlorophanum was found to habit two types of photobionts (Meeßen et al. 2013b), one of them recently identified as Trebouxia jamesii, clade S (Sadowsky et al. 2012). The lichen samples were air-dried after collection and stored dark at −25 °C until further investigation; the samples of C. gyrosa were air-dried and kept under dark and dry conditions until testing.
Cultivation of the Symbionts of B. Frigida, X. elegans, and R. geographicum
Three lichen mycobionts were taken in axenic culture to test the production of melanin and carotenoids in their aposymbiotic state. Thus, the mycobionts of B. frigida and X. elegans were isolated from spores, pre-cultured on malt yeast extract agar (MY) at 10 °C and a day/night cycle of 14/10 h at 15–25 μmol m−2 s−1 PPFD for about 12 weeks. Mycelial colonies were transferred to liquid MY-medium and cultured at 12 °C and a day/night cycle of 12/12 h at 15–25 μmol m−2 s−1 PPFD for six additional weeks to increase biomass or to observe changes in the medium (B. frigida, see below). The spore-isolated mycobiont of R. geographicum was completely cultured on solid MY as described above. The photobionts of B. frigida and X. elegans were isolated by previously described methods (Meeßen et al. 2013a; Meeßen and Ott 2013) and cultured in liquid Trebouxia organic medium (TOM) at 12 °C with a day/night cycle of 12/12 h at 15–25 μmol m−2 s−1 PPFD.
Tests on Melanin
Due to the difficulties in directly testing melanin in lichens, a set of chemical tests was used to verify the black colouration of B. frigida thalli, of the pro-/hypothallus as well as the apothecia of R. geographicum, and of both isolated mycobionts as melanin. Melanin is known to be insoluble in water and organic solvents (except e. g. DMSO), soluble in alkali (0.1–1.0 N NaOH or NH4OH) when cold (phaeomelanin but no DOPA- or DHN-melanin) or heated (heterogeneous melanin), precipitated in alkaline FeCl3, and bleached in strong oxidising agents (Bell and Wheeler 1986; Sava et al. 2001). Regarding these characteristics, the solubility of the pigmentation in thallus fragments was compared to pure melanin (≥ 97 %, synthetic, Sigma-Aldrich) in water, ethanol, methanol, acetone, toluol, hexane, chloroform, ethyl acetate, DMSO, 0.2 N NaOH, and 0.2 N NH4OH, precipitation in saturated alkaline FeCl3 (in 0.2 N NaOH), and bleaching in H2O2, as well as in NaOCl. To test the ochre to brownish colour of C. gyrosa on melanic compounds, its thalli were also investigated.
UV/VIS-analysis of Lichen Extracts
Besides the chemical tests on B. frigida, R. geographicum and C. gyrosa, 50-100 mg of dry lichen and mycobiont material were freed from substrate, mixed with 1.0 ml acetone, ethanol, methanol, 0.1 N NaOH, or DMSO (the latter for testing on melanin), respectively and incubated for 12–18 h under inversion. After removing thalli fragments by centrifugation (5 min at 14,000 rpm, Centrifuge 5415C, Eppendorf) the supernatant was analysed in a BioSpectrometer basic (Eppendorf) in a range from λ = 750 to 210, 220, 240, or 250 nm, depending on the absorption properties of the respective solvent. Measured spectra were compared to standards (as melanin, emodin, parietin, atranorin, usnic, and stictic acid) and to UV/VIS absorption data sets from the literature (Huneck and Yoshimura 1996) to identify the SLCs of space-relevant lichens and assess their spectrophotometric properties.
HPLC-analysis of Lichen and Mycobiont Extracts
50–100 mg of dry lichen/mycobiont material were freed from substrate, mixed with 1.0 ml acetone, and powdered for 5 min in a bead mill (MM2, Retsch). After removing thallus fragments by centrifugation (5 min at 14,000 rpm, Centrifuge 5415C, Eppendorf) the supernatant was speed-vac dried for 1 h, redissolved in 1.0 ml methanol (HPLC grade), checked on its concentration on a silica gel 60 F254 TLC sheet (Merck) under 254 and 366 nm (CAMAG UV-cabinet 3, VWR), and diluted appropriately with HPLC-grade methanol. All samples were stored at −25 °C before UV/VIS- and HPLC-analysis. The HPLC analysis of lichen extracts (injection of 20 μl per sample) was performed with a HPLC-system (Dionex P580) coupled to a photodiode array detector (Dionex UVD340S, routine detection at λ = 235, 254, 280, 340 nm). The separation column was prefilled with Eurospher-10 C18 (Knauer) and the following gradient elution program of 60 min at 1.0 ml/min flow rate was used (MeOH, NanoPur© H2O (pH 2 with orthophosphoric acid)); MeOH: 0 min, 10 %; 5 min, 10 %; 35 min, 100 %; 45 min, 100 %). Chromatograms were recorded for all four channels and a complete spectrum (200–595 nm) was taken for every signal with a minimum peak area of ≥ 1.0 [signal]*min at 280 nm. The Chromeleon© V6.30 software (Dionex) was used for examination and subsequent analysis. The collected spectra were matched with an internal UV spectrum library and compared to UV/VIS absorption data sets from literature (Huneck and Yoshimura 1996) to identify the sample’s SLCs.
HPLC-analysis of Photobiont Extracts
In a second approach, the aposymbiotic photobiont cultures of B. frigida and X. elegans were tested on their production of photosynthetic pigments/carotenoids by an alternative HPLC technique which is specialised on photosynthetically relevant pigments including chlorophylls, β-carotene, lutein, neo- and violaxanthin. 100–200 mg of fresh algal material was mixed with 1.0 ml acetone and grinded by a micro-pestle by addition of sterilised quartz sand. The cellular debris was removed by centrifugation and the supernatant was directly transferred to a HPLC-system (Schambeck/Hitachi/Merck) with injection volumes of 20 μl per sample for subsequent pigment determination. The gradient was constituted in a solvent system of acetonitrile : methanol : tris acetate buffer (87:10:3) and methanol : n-hexan (4:1), the retention times were compared to standards and the concentration was determined by peak integration. Alongside tests of axenic mycobiont cultures of B. frigida, R. geographicum, X. elegans, and also of Fulgensia bracteata gave no result and are not presented below.
Results
Chemical Tests on Melanin
In correspondence with the black colouration of the lichen thallus of Buellia frigida as well as of the pro-/hypothallus and the apothecia of Rhizocarpon geographicum, the chemical tests clearly indicate that both species form melanic substances. The test results accord with those of synthetic melanin (Table 2). The mycobionts of both lichen species show similar results and thus demonstrate that also the isolated mycobiont is capable to produce melanin. The cultivation of B. frigida in liquid MY produced a very strong black colouration of the medium, becoming visible after 1 week and blackening the medium within 5 weeks (Figs. 1 and 2). Additionally, the brownish to black colonies of the mycobiont of R. geographicum produced dark areola when cultivated on solid MY-agar, while the black colour of the these fungal colonies is often found to cease during long-term cultivation in the aposymbiotic state (Eva Posthoff, pers. comm.). Melanin-forming fungi and bacteria secrete tyrosinase into the medium that causes intensive dark colouration by forming extracellular melanin if the medium contains hydrolysed proteins such as peptone (Bell and Wheeler 1986). Assuming this mechanism, the observed phenomena gave additional support for the identification of melanin in the mycobionts of B. frigida and R. geographicum.
Despite the ochre to brownish colour of the cortex of Circinaria gyrosa, it was recently demonstrated that this lichen species contains no detectable amounts of organic solvent soluble SLCs by standard thin layer chromatographic detection procedures (Raggio et al. 2011). Interestingly, the chemical tests with C. gyrosa show positive results on melanin. The solubility corresponds to those of the melanin control and the other investigated lichen thalli/mycobionts, especially with respect to a brownish colouration in the solvents DMSO, NaOH, and NH3. Nonetheless the precipitation test with alkaline FeCl3 failed, making a clear identification difficult by these methods. In correspondence with the colour of C. gyrosa observed in the present study, the brownish to ochre thallus colouration of the lichen Lobaria pulmonaria was used as an indicator of melanic compound production (Solhaug et al. 2003)
HPLC-analysis of Lichen Extracts
Buellia frigida
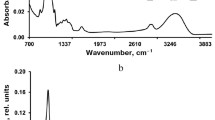
Methanol and acetone extracts of B. frigida showed a widely concurrent chromatographic pattern of mostly minor signals. Three of these signals were of special interest. At first, a strong signal at 26.96 min RT which was detected at all four wavelengths and had an UV/VIS-peak pattern of 210.9(st), 239.2(st), ca. 270(sh), and 310.5(w) nm (Fig. 3a). This signal showed very high similarity to UV/VIS-spectral properties of stictic and norstictic acid (213(4.60), S 237(4.47), S 270(4.10), 313(3.77) nm and 212(4.48), 239(4.40), S 270(4.02), 317(3.70) nm, according to Huneck and Yoshimura 1996). As the chemically closely related stictic acid standard revealed a retention time of 24.63 min in an additional HPLC-run, the present signal was identified as norstictic acid. This result matches with UV/VIS-spectrometry results of crude thallus extract that reveals again a peak at 310 nm (see below) but also with data from literature reporting that norstictic acid is a typical SLC in B. frigida (Øvstedal and Smith 2001). Despite this typical SLC, the MeOH-extracts of B. frigida revealed additional signals after 37.52 and 39.42 min RT with UV/VIS-spectral properties of 227.5(m), ~395(m), 418.1(st), and 444.1(st) nm as well as 266.9(w), ca. 415(m), 441.7(st), and 468.4(st) (Fig. 3b-c) which were clearly identified as carotenoids by their trident peak structure between 395–445 and 415–470 nm, respectively. The identification of carotenoids in lichen thalli raised the question whether these carotenoids are produced by and located in the fungal part of the thallus or if they were a product of the photobiont. Subsequent investigations on the isolated symbionts (see below) clarified the distribution and identity of the carotenoid compounds.
UV/VIS-spectra from HPLC-analysis of secondary lichen compounds in B. frigida, C. gyrosa, R. geographicum, X. elegans, and P. chlorophanum. a: norstictic acid from B. frigida (RT 26.96 min), b-c: carotenoids from B. frigida (RT 37.52 min, 39.42 min, refer to HPLC-analysis of photobiont extracts), d: unknown anthraquinone from X. elegans (RT 28.80 min), e: emodin from X. elegans (RT 33.53 min), f: parietin from X. elegans (RT 36.91 min), g: psoromic acid from R. geographicum (RT 30.43 min), h: rhizocarpic acid from R. geographicum (RT 34.29 min), i: gyrophoric acid from R. geographicum (RT 35.10 min). The coloured bar indicates the ranges of UVC (200–280 nm), UVB (280–320 nm), and UVC (320–400 nm) according to Tevini and Häder (1985)
Circinaria gyrosa
Methanol as well as acetone extracts evoked similar chromatographic patterns but with poor signal frequency and intensity. Interestingly, the three detectable signals of C. gyrosa extracts appear after very short retention times of 3.03, 4.41, and 7.83 min RT, all showing UV-absorption in the UVC. Their UV/VIS-spectra revealed unspecific peaks of 266.6(st) nm for the first signal, 205.3(st), 271.6(vw), and ~355(vw) for the second substance, as well as 207.7(st) nm for the third one. Identification of these substances was unfeasible with the methods used, while other SLCs were not detected.
Xanthoria elegans
Besides a multitude of minor and medium-derived signals, MeOH as well as acetone extracts revealed three signals of special interest, which were identified as parietin-type anthraquinones by their trident peak pattern between 240 nm and 290 nm and their broad peak around 430 nm (Fig. 3d-f). These signals were detected after 28.80, 33.53, and 36.91 min RT and comprised analogue spectral properties: in the first weak signal UV/VIS-peaks were detected at 208.6(m), 240.6(st), 261.5(sh), ~284(sh), ~340(w), and ~440(w,br) nm, in the second signal the peaks were at 222.4(st), 250.2(m), 269.3(m), ~289(st), and ~434(m,br) nm, and in the third signal UV/VIS-peaks were detected at 222.2(st), 252.2(m), 266.0(m), ~288(m), and ~432(m,br) nm. While the identification of signal 1 is pending, the medium signal 2 was identified as emodin by comparison with an emodin standard that showed the same retention time (33.53 vs. 33.70 min RT) and according spectral properties. Subsequently, the third peak was identified by its spectral properties as parietin, which is in accord with the observation. Being the most prominent peak in the HPLC-analysis is in accordance to the observation that parietin is reported as the major SLC of X. elegans (Øvstedal and Smith 2001; Brunauer et al. 2007). As with B. frigida, MeOH-extracts of X. elegans also revealed the minor signal of a non-polar compound after 39.41 min RT with a UV/VIS-peak pattern of 266.2(w), ~416(m), 441.9(st), and 467.6(st) which was subsequently identified as the same carotenoids. Therefore, samples of the isolated myco- and photobiont of X. elegans were analysed on carotenoids alongside those of B. frigida (see below).
Besides these findings, extracts revealed a signal after 3.0 min RT which is comparable to the one described for C. gyrosa at 3.03 min RT, two signals after 30.48 and 31.43 min RT with some similarity to rhizocarpic acid (UV/VIS-peaks roughly at ~235(m,br), ~280(st,br) and ~360(m,br) nm), and two peaks after 27.04 and 34.11 min RT which are characterised by a strong and broad UV/VIS-peak at ~380 nm (218.0(st), 280(m,br), 380(st,br), 450(sh,br) nm for the first one and 245.6(st), 320(sh,br), 385(st,br), 480(sh,br) nm for the second one). The identification of all these minor signals was not possible with the methods applied.
Rhizocarpon geographicum
The MeOH- and acetone extracts of R. geographicum revealed similar chromatographic patterns after HPLC-analysis. Besides norstictic acid, which is found after 26.96 min RT as described above (Fig. 3a) and also reported for the genus Rhizocarpon (Culberson 1969), three signals of considerable intensity were presented (Fig. 3g-i): The first signal appeared after 30.43 min RT with UV/VIS-peaks of 205.2(st), 240.6(st), and shoulders at ~270(sh) and ~320(sh) nm and the second one after 34.29 min RT with its UV/VIS-peaks at 238.5, 281.0, and 369.4 nm. The third signal was detected after 35.10 min RT with three peaks in the UV at 213.5(st), 276.3(st), and 307.4(m) nm. The first signal which was the most prominent signal in UV-detector channels of 235, 254, and 280 nm, gave good accordance to psoromic acid (UVMeOH: 211(4.64), 240(4.58), S 271(4.18), and 317(3.59) nm, Huneck and Yoshimura 1996) which was repeatedly reported to be an SLC of this species (Culberson 1969; Nash et al. 2001). The second signal represent rhizocarpic acid and was identified by comparison to available UV/VIS absorption data sets from literature (Huneck and Yoshimura 1996), by data from literature on the SLC-inventory of R. geographicum (Culberson 1969; Nash et al. 2001) and UV/VIS-spectra from P. chlorophanum, which reportedly contains rhizocarpic acid as well (see below). Finally, the third signal showed very good accordance with gyrophoric acid (UVMeOH: 214(4.87), 271(4.44), and 305(4.25) nm, according to Huneck and Yoshimura 1996), a SLC also known from R. geographicum (Nash et al. 2001).
Additionally, three minor signals were detected at retention times of 26.96, 27.70, and 32.19 min. The first one gave a clear signal with UV-peaks at 210.8(st), 239.8(st) and 309.5(w) nm but remained unidentified by comparison with known SLCs from R. geographicum. The signal at 27.70 min RT showed high similarity with psoromic acid at 30.43 min RT and the signal at 32.19 min RT with gyrophoric acid at 35.10 min RT. Why both types of spectra were detected at two different retention times remains ambiguous, but this result might point to the presence of derivatives, precursors, and isomers or to chemically related substances with similar spectral characteristics. Barbatic acid, which is also reported as a SLC in R. geographicum (Culberson 1969; Nash et al. 2001) was not detected.
Pleopsidium chlorophanum
MeOH- and acetone extracts of this lichen species revealed a similar chromatographic pattern of five signals of interest: a close double-peak of 26.22 and 26.87 min RT with two similar UV/VIS-spectra of three peaks (228.2(sh), 274.4(br), 328.9(sh) nm). After 33.38 min RT, a very weak signal appeared (signal 3; with the UV/VIS-spectral properties of three peaks at 231.4(st), 282.1(m) and 368.0(m) nm). At least, a strong double-peak appeared at 34.29 and 34.55 min RT, again with two similar UV/VIS-spectra of three broad peaks (238.8(br), 281.6(br), 366.5(br) nm). Especially the three compounds after 33.38, 34.29, and 34.55 min RT revealed a broad absorption range between 400 nm and 200 nm being more pronounced at their peaks of ~239, ~281, and ~367 nm, thus ranging in the UVC, at the UVC/UVB-boarder and in the UVA, respectively (as defined by Tevini and Häder 1985). Signal 4 was identified as rhizocarpic acid by matching with the HPLC-internal UV spectrum library (98,7 % identity), by comparison with available UV/VIS absorption data sets from literature (Huneck and Yoshimura 1996), and by comparison with samples of R. geographicum (34.30 min RT in both, see above) that also contains rhizocarpic acid.
Rhizocarpic acid is reported by Culberson (1969) as a common SLC of the closely related genera Pleopsidium and Acarospora (P. flavum f. chlorophanum (Wahlenb. ex Ach.) syn. to A. chlorophana (Wahlenb. ex Ach.) Mass., according to Culberson (1969). Indeed, rhizocarpic, acaranoic and acarenoic acid are reported as the lichens SLCs under the synonym A. chlorophana (Culberson 1969), while none of the obtained spectra resembled the latter two acids (Huneck and Yoshimura 1996). The signals 3 and 5 show moderate and high UV/VIS-spectral accordance with the spectrum of rhizocarpic acid, respectively, indicating a common chemical structure. Signal 5 shows some accordance with the spectral data of epanorin (Huneck and Yoshimura 1996), which is structurally closely related to rhizocarpic acid and reported as a SLC from A. erythrophora (Nash et al. 2001) and A. rouxii (Knudsen 2007; Knudsen et al. 2008). By the present results, the identity of both signals remains ambiguous, being either derivatives, precursors, and isomers of rhizocarpic acid or being epanorin.
UV/VIS-analysis of Lichen and Mycobiont Extracts
The UV/VIS-analyses confirmed the HPLC-results by providing evidence for the existence of parietin in Xanthoria parietina as well as rhizocarpic acid in Rhizocarpon geographicum and Pleopsidium chlorophanum. In respect to the HPLC-analysis already described above, this section will focus on the contribution of UV/VIS-spectroscopic analysis to the detection of melanin in Buellia frigida, Rhizocarpon geographicum and Circinaria gyrosa and in the mycobionts of the two former lichen species. Figure 4 represents 5 UV/VIS-spectra that were blanked against its solvent DMSO and ranging between 260 and 700 nm. As the y-axis represents arbitrary units only, the DMSO-extract of the isolated mycobiont of B. frigida (Fig. 4a) resembles those of the melanin standard isolated from shrimp (Fig. 4e, provided by Sigma-Aldrich) with a constantly increasing absorption to lower wavelengths. The general pattern in thallus extracts from R. geographicum, C. gyrosa and B. frigida (Fig. 4b-d) is comparable but exhibits additional peaks at 661, 657, and 665 nm as well as at 409, 411, and 408 nm, respectively. These data correspond to the absorption peaks of chlorophyll a from spinach (664 and 417 nm in MeOH, data not shown) and indicate that chlorophylls are co-isolated in DMSO-extracts. In extracts from B. frigida, an additional peak at about 307 nm occurs, which in accordance with the peak at 309 nm from norstictic acid, as already demonstrated as a SLC of B. frigida by HPLC-analysis (see above). To further verify the presence of melanin in the isolated mycobionts of B. frigida and R. geographicum but also in the lichen C. gyrosa, NaOH-extracts were also investigated on the spectral characteristics of melanin (Fig 5). Both mycobiont extracts (Fig. 5c-d) and the extract of C. gyrosa show good accordance with the melanin standard (Fig. 5c) in resembling a constantly increasing absorption with decreasing wavelength.
UV/VIS-spectra of thallus and mycobiont extracts in DMSO. a: melanin from the mycobiont of B. frigida, b: melanin from R. geographicum, c: melanin from C. gyrosa, d: melanin from B. frigida, e: melanin standard from shrimp (Sigma-Aldrich). The peaks of about 660 and 410 nm in thallus extracts of R. geographicum, C. gyrosa and B. frigida (b-d) indicate co-dissolved chlorophyll while the peak of about 310 nm (d) displays norstictic acid
In contrast, methanolic extracts from isolated mycobionts of B. frigida, F. bracteata, X. elegans from Col du Sanetsch as well as from Zmutt, Zermatt (Fig. 6) revealed different patterns of UV/VIS-absorption. On the one hand, the black mycobiont of B. frigida (Fig. 6a) as well as the pinkish mycobionts of F. bracteata and X. elegans from Col du Sanetsch (Fig. 6b-c) revealed a uniform absorption peak at 265, 263, and 268 nm, respectively. In contrast, the orange coloured mycobiont of X. elegans from Zmutt, Zermatt (Fig. 6d) revealed a peak pattern of 233(st), 263(m), 276(m), 292(m), 380(sh), and 445(br) nm. Except the shoulder at 380 nm, the peaks clearly indicate the presence of parietin in this peculiar mycobiont cultivar corresponding to the findings of HPLC-analysis (see below).
UV/VIS-spectra of mycobiont extracts in MeOH. a: unknown compound from the mycobiont of B. frigida, b: unknown compound from the mycobiont of F. bracteata, c: unknown compound from the mycobiont of X. elegans (Col du Sanetsch), d: emodin from the mycobiont of X. elegans (Zmutt, Zermatt). The unknown compound in all three mycobionts might correspond to those detected in mycobiont extracts of B. frigida by HPLC-analysis (Fig 7a)
To summarize, UV/VIS-spectrometry data supported the identification of melanin in thalli of B. frigida, R. geographicum and C. gyrosa and in the isolated mycobionts of the two former species, as already indicated by the chemical tests described above. Additionally, UV/VIS-spectra stressed site-dependent chemotype differences in the mycobiont of X. elegans.
HPLC-analysis of Other Mycobiont Extract Compounds
Isolated mycobiont cultivars of Xanthoria elegans from Zermatt – a Swiss alpine sampling location where specimen were collected for several space exposure experiments as LIFE on EXPOSE-E/EuTEF (de la Torre et al. 2010; Onofri et al. 2012) – revealed the production of several anthraquinone compounds in the aposymbiotic state. This characteristic was already seen in the respective mycobiont cultures as an intensive reddish to orange colour of the mycelium. However, it was neither observed nor detected in isolated mycobiont cultivars from the Dolomites or from Col du Sanetsch which formed intensely pink colonies. The chromatographs showed four peaks of peculiar interest at retention times (RT) of about 29.38, 32.70, 33.62, and 35.85 min. By comparison with literature data and results from emodin standard as well as whole lichen thallus analysis (see above), the peaks at 33.62 and 35.85 min RT showed best correspondence to emodin and parietin, respectively. The spectral characteristics of the minor peaks at 29.38 and 32.70 min RT point to two yet unspecified anthraquinones. Compared to the HPLC-analysis of the intact lichen X. elegans, the peak identified as emodin (ca. 33.62 min RT) is stronger, while the peak identified as parietin (ca. 35.76 min RT) is less pronounced. In mycobiont extracts of R. geographicum one signal after 21.27 min RT was detected which revealed a peak pattern in UV/VIS-spectrometry of 217.9, 260.3, and 350.8 nm, vaguely resembling those of rhizocarpic or pulvinic acid (according to data from Huneck and Yoshimura 1996).
Apart from that, no spectra of the already described lichen SLCs were detected. However, the spectral properties of one peak in B. frigida mycobiont extracts (peak at 264.4 nm, RT of ~28.4 min RT) point to the aposymbiotic production of a yet unidentified compound absorbing mostly in the UVC range (Fig. 7a). Additionally, the mycobionts of B. frigida, R. geographicum, X. elegans (from Col du Sanetsch, Valais, Switzerland), and F. bracteata uniformly gave a peak at a RT of 42.3 to 42.6 min RT with spectral properties that indicated 22-dehydrocampesterol with a spectral matching factor of 99.3 to 99.6 % (Fig. 7b). Despite the actual identity of this substance remained ambiguous, representatives of the class of triterpenoids were repeatedly reported as compounds of lichens (e.g. in X. parietina, Huneck and Yoshimura 1996).
HPLC-analysis of Photobiont Extracts
The HPLC-analysis and subsequent quantification of the photosynthetic pigments of the two axenic photobiont cultures of Xanthoria elegans and Buellia frigida revealed interesting insights in the photoprotective mechanisms of the lichen photobiont itself. The amount of chlorophyll a (chl a), chlorophyll b (chl b) and β-carotene (β-car) (Table 3) as well as the respective ratios of chl a/chl b and β-car/chl a + b (Table 4) are comparable to the data reported for other lichen photobionts (Bačkor et al. 2010). The amount of the three carotenoids involved in the so-called xanthophyll cycle (violaxanthin - vio, antheraxanthin - ant, and zeaxanthin - zea) as well as the ratio of their total amount (VAZ) to chl a + b is remarkably lower (Table 4), compared to data from other lichen photobionts (Sadowsky and Ott 2012). In photobionts of X .elegans the VAZ/chl a + b-ratio is lower with 36.4 ± 8.70 compared to the photobiont of B. frigida with 55.3 ± 3.20 and the astrobiologically non-relevant Antarctic lichen photobiont of Usnea lambii with 83.6 ± 4.40. Interestingly, such low levels of the xanthophyll cycle pigments are contrasted by high amounts of neoxanthin and lutein (neo, lut, refer to Tables 2 and 3) and by high ratios of neo/chl a + b and lut/chl a + b (Table 4). Photobionts of B. frigida revealed the highest ratio of neo/chl a + b and photobionts of X. elegans the highest ratio of lut/chl a + b, while both ratios are much lower in the comparable data from photobionts of U. lambii. Nonetheless, percentage of the three xanthophyll cycle compounds vio, ant, and zea (Table 5) clearly indicates that the cultivation conditions of both lichen photobionts did not impose light-stress on their photosynthetic apparatus: The high percentage of vio (86.1 + 5.0 in X. elegans and 85.5 + 3.6 in B. frigida), the low percentage of zea (11.8 ± 4.3 and 13.4 ± 3.4, respectively) and the lowest percentage of the intermediate compound ant (2.2 + 0.7 and 1.1 + 0.2) display the relaxed state of the xanthophyll cycle (Table 5, Demmig-Adams and Adams 1996). The de-epoxidation status of the xanthophyll pool (DEPS) was calculated by the equation of Vrábliková et al. (2005) as DEPS = (zea + ant)/(vio + ant + zea). By this approach, the photobionts of X. elegans revealed an DEPS of 0.140 and those of B. frigida showed an DEPS of 0.145. Both values indicate a low activation state of the photoprotective xanthophyll cycle. Compared to the photobiont of U. lambii, the relative amount of ant and thus the DEPS is decreased in both space-relevant photobionts.
Discussion
Melanin in Lichen Thalli and Mycobionts
The results of the chemical tests (Tables 2 and 3) and of UV/VIS-photometry (Figs. 4 and 5) demonstrate that thalli and mycobionts of Buellia frigida, Rhizocarpon geographicum and, putatively, thalli of Circinaria gyrosa produce melanin. Despite being non-essential, melanic pigments are found in animals, plants, fungi, and microorganisms, implying to considerably enhance survival and competitiveness (Bell and Wheeler 1986).
In ascomycetes, melanin is mostly synthesised by tyrosinases in a two-step reaction of tyrosine via 3,4-dihydroxyphenylalanine (DOPA) to dopaquinone and subsequent cyclisation (Beckett et al. 2012), or from 1,8-dihydroxynaphthalene (DHN) via the pentaketide pathway. The respective polyketide synthases are present in most lichens (Muggia and Grube 2010). In lichens, melanin is widely distributed (Beckett et al. 2012), but its type is mostly unknown (Muggia et al. 2009). Tyrosinase activity was found to be high in some taxa but low or absent in others (Beckett et al. 2012). So, different taxa may use different synthetic pathways.
Fungal melanin occurs in two types (Bell and Wheeler 1986). The first type is wall-bound melanin incrusting the outer hyphal cell wall. The blackening of marginal cortical hyphae in B. frigida and of prothalline/apothecial hyphae in R.geographicum (Meeßen et al. 2013b) as well as the dark colour of their hyphae in the aposymbiotic state indicate the presence of cell wall-bound melanin in both lichens. The second state is the formation of heterogenous extracellular melanin by secretion of tyrosinases which synthesise free melanin and turn the medium dark. This behaviour is frequently observed in microbial and fungal cultures (Hollis 1952; Nurudeen and Ahearn 1979; Bell and Wheeler 1986). In the present study the darkening of the medium was observed in liquid cultures of the B. frigida mycobiont and in agar cultures of the R. geographicum mycobiont. So it may be concluded that both fungi form extracellular melanin and secrete enzymes as tyrosinase for its synthesis.
The ecological role of melanin is diverse but predominantly assumed to adapt organisms to “the edge of extreme life” (Wang et al. 2006). Melanogenesis responds to several environmental stresses as toxic metals, hyperosmotic condition, microbe defense, and pH shock, but also to temperature extremes, desiccation, UVR, and ionising radiation (Henson et al. 1999). Thus, melanin-producing organisms are found in extreme habitats as deserts, alpine regions and the upper biosphere. For example, many rock-colonising fungi are highly melanised (Bell and Wheeler 1986), as the extremotolerant Antarctic fungi Cryomyces antarcticus and C. minterii, which serve as model organisms in astrobiological research (Onofri et al. 2012) and will be exposed together with B. frigida and C. gyrosa to LEO and simulated Mars conditions at the ISS in the up-coming BIOMEX-project. In respect to astrobiology, the protective role of melanin to a broad range of environmental stresses and its production as general stress response (Henson et al. 1999) are remarkable, making melanic compounds a class of substances to pay attention to.
Concerning irradiative stress, melanin provides UVR protection for all fungal tissues (Henson et al. 1999) and melanised spores resist UVR, γ-ray, and X-ray treatment better than melanin-deficient ones (Bell and Wheeler 1986), while its synthesis is induced by UVR-dependent DNA-damages (Henson et al. 1999). The underlying mechanism of photoprotection is based on the fact that melanin dissipates light energy at a rate of 99.9 % into heat by internal conversion, which relaxes the excited state of the molecule into vibration states and thus prevents formation of harmful free radicals in the cell (Meredith and Riesz 2004).
In lichens, melanic compounds screen excessive irradiation from the whole thallus when produced in the upper cortex (McEvoy et al. 2007) and the quantities of melanin often result in completely black thalli (Gauslaa and Solhaug 2001), as both was observed in B. frigida. Cortical melanin synthesis in the lichens Lobaria pulmonaria and Cetraria islandica was found to be induced by UVB rather than by UVA or PAR (Solhaug et al. 2003; Nybakken et al. 2004). The screening capacity of melanic compounds was assessed in both lichens by direct measurement of cortical transmittance in a wavelength range from 230–1.000 nm and the results indicated that fungal cortical melanin strongly absorbs UVB, UVA, and also PAR (Nybakken et al. 2004). As seen from the presented UV/VIS-spectrographs (Figs. 4 and 5), melanic compounds from B. frigida, R. geographicum and C. gyrosa continuously absorb high energy photons at higher rates than low energy photons and basically resemble what was found by cortical transmittance measurements by Nybakken et al. (2004). The screening effect of melanin is the more efficient the more hazardous the insolated wavelength is, implying a higher resistance against excessive light where these pigments are abundant (Gauslaa and Solhaug 2001). This conclusion is matched by recent investigation on B. frigida (Meeßen et al. 2013b) that demonstrated a correlation between the melanic pigmentation of the upper cortex and the abundance of algal cells: The photobiont-rich, marginal areolae were shielded by a pronounced black cortex, while the photobiont-deficient inner areolae mostly lacked melanin incrustation. Thus, melanin protects the underlying photobiont of B. frigida against excessive solar radiation as already concluded for other lichens (Gauslaa and Solhaug 2001; Vrábliková et al. 2005). For the putatively melanised cortex of C. gyrosa, a corresponding effect can be assumed that supports the photoprotective effect of the wide, dense, and intensely gelatinised subcortex (Meeßen et al. 2013b). Additionally, the intense pigmentation of apothecia (B. frigida, R. geographicum), spores (B. frigida) and prothalli (R. geographicum) points to a protection of generative as well as growing tissues. While melanin synthesis is an active physiological process requiring hydration (McEvoy et al. 2007), the UVB transmittance is low in dry cortices (Solhaug et al. 2003), indicating efficient UVR-shielding during anhydrobiosis.
Anthraquinones in Lichen Thalli and Mycobionts
Anthraquinones, as parietin and emodin, are widely distributed in plants, fungi, and lichens (Sargent et al. 1970; Locatelli et al. 2009; Brunauer et al. 2007; Huneck and Yoshimura 1996) and were demonstrated to possess a wide range of biological activities including antifungal and antimicrobial activity (summarized in Locatelli et al. 2009).
In the space-relevant lichen species Xanthoria elegans (Brunauer et al. 2007), X. parietina (Solhaug and Gauslaa 1996) and Fulgensia bracteata, the dominant SLC is parietin. As other SLCs like atranorin, usnic acid and melanin, parietin is preferentially located in the upper cortical layer (Nybakken et al. 2004; Meeßen et al. 2013b) and its synthesis is clearly induced by UVB but not by UVA and PAR (Solhaug et al. 2003; Nybakken et al. 2004) The induction of parietin synthesis by, and its strong absorbance of UVB suggest a UVR-protective effect (Solhaug and Gauslaa 1996; McEvoy et al. 2007), especially shielding the underlying photobiont against excessive UVR (Gauslaa and Solhaug 2001). Additionally, the spectral absorption properties of parietin (Fig. 3f) indicate a protective effect against blue light PAR. So, it also protects the photosynthetic apparatus of the photobiont against excess blue light (Solhaug et al. 2003) that may lead to photo-oxidative stress, photodamage and subsequent reduction of carbon fixation. In vitro, the UVB-absorbance of parietin is as high as its blue light absorbance and it reduces photosynthetic O2 evolution in vivo, reflecting its substantial PAR-screening capabilities (Solhaug and Gauslaa 1996). The peculiar features of parietin—location, UVB- and PAR-protection, environmentally responded synthesis—are crucial aspects to explain the resistance and survival capacity of X. elegans in space experiments (de Vera et al. 2003, 2004a, b, 2008; Onofri et al. 2012).
Besides parietin and emodin, several of their derivatives were detected in X. elegans (Brunauer et al. 2007). Their study revealed diverging quantities and qualities in anthraquinone synthesis in different developmental stages and cultivation conditions of X. elegans as lichen thalli, relichenisation stages, and mycobiont cultures. Axenic mycobiont cultures turned from light pink into dark orange after about 3 months (Brunauer et al. 2007). This effect was observed in the present study in mycobiont cultures from Zermatt but in contrast, the cultures from Col du Sanetsch and the Dolomites did not change colour, irrespective of the duration of cultivation. Several lichen species reveal different chemical phenotypes (Henssen and Jahns 1974) and these differences were thought to be due to different genetic backgrounds. Our results suggest a site-dependent fixation in the different mycobiont cultivars from Zermatt, the Dolomites and Col du Sanetsch. According to Brunauer et al. (2007), high ecological amplitudes might be facilitated by a flexible secondary metabolism, especially in respect of colonising extreme habitats. The differences between the mycobiont cultivars of X. elegans found in the present study stress this interpretation. In the present HPLC-analyses of lichen extracts more parietin was detected than emodin. In contrast, emodin was more abundant than parietin in those mycobiont cultures from Zermatt that changed their colour from pink to orange. Emodin and the minor peaks at 29.38 and 32.70 min RT point to three anthraquinone precursors of parietin-synthesis which are accumulated in the aposymbiotic mycobiont but not processed to parietin. In the lichen itself, emodin content is reduced and more parietin is formed, becoming the most abundant SLC of X. elegans (Øvstedal and Smith 2001).
Other Secondary Lichen Compounds
Most secondary lichen compounds strongly absorb UVR and confer protection against harmful UVR-quantities (Solhaug et al. 2003). The cortically deposited SLCs as melanin in B. frigida and parietin in X. elegans protect especially the photobiont cells in the layer below the cortex (Gauslaa and Solhaug 2001). For R. geographicum and P. chlorophanum the likewise located rhizocarpic acid confers a comparable protection for the photobiont. Interestingly, the alga-rich areolae/lobes of R. geographicum and P. chlorophanum contain rhizocarpic acid while the regenerative and growing parts of R. geographicum that consist of fungal tissue only (prothallus and apothecia) are melanised (Meeßen et al. 2013b). This observation may stress the protective effect of rhizocarpic acid for this peculiar photobiont while marginal areolae in B. frigida are intensely pigmented with melanin, indicating different levels of light-adaption in both photobionts. In accordance with reports from literature (Øvstedal and Smith 2001) and besides the cortically deposited melanin, norstictic acid was identified as an additional SLC of B. frigida. In general, the co-occurrence of several SLCs in lichens—melanin and norsticitc acid in B. frigida, several anthraquinones in X. elegans, melanin, rhizocarpic, psoromic and gyrophoric acid in R. geographicum—may lead to an additive effect that increases UVR-shielding by broadening the range and intensity of absorbance and impose a crucial advantage when facing harsh terrestrial or, in the case of recent studies, space conditions. The co-occurrence of several SLCs of the same chemical class was already shown for the stictic-norstictic acid chemosyndrome in Lobaria pulmonaria, where a total of seven compounds was detected and discussed to confer UVB-tolerance (McEvoy et al. 2007). Besides the dominant and mostly cortically located SLCs as melanin, parietin and rhizocarpic acid, the vast majority of them is colourless but with strong UVB-absorbance bands and located in the medulla or deposited in the algal layer (Fahselt and Alstrup 1997). This organisation may constitute additional UVR-protection beyond the protective effect of cortical SLCs alone (McEvoy et al. 2007) and may influence the quality of light reaching the photobiont. The location of norstictic acid in B. frigida, emodin in X. elegans, as well as psoromic and gyrophoric acid in R. geographicum should be interpreted in this context.
Carotenoids in the Photobionts of Xanthoria elegans and Buellia frigida
The results of the HPLC-based quantification of photosynthetic relevant carotenoids give a first indication that the photobionts of some lichens are adapted to extreme insolation conditions independent from or additional to the photoprotective strategies of the complete thallus. Despite similar light absorption properties, every carotenoid plays a distinguishable role to support and protect the thylakoid-bound photosystems of chloroplasts (Dall’Osto et al. 2006). The accessory pigment β-carotene (β-car) is part of the light harvesting complex (LHC) and feeds the central chl b with absorbed light energy. Additionally, β-car protects the photosystem from photo-oxidative destruction by excessive insolation. It efficiently quenches reactive oxygen species (ROS) and singlet oxygen, but also relaxes excited triplet chlorophyll by dissipating its energy into heat (Telfer et al. 1994; Trebst 2003). A process that prevents excess light stress is the enzymatic xanthophyll cycle which is initiated by light-driven acidification of the thylakoid lumen and subsequent activation of the violaxanthin de-epoxidase. The enzyme converts thylakoid-located violaxanthin (vio) via antheraxanthin (ant) to zeaxanthin (zea) (Demmig-Adams and Adams 1996) which quenches excess excitation of the reaction centers and acts as an antioxidant (Havaux and Niyogi 1999; Havaux et al. 2007).
In X. elegans and B. frigida photobionts, the level of β-car did not differ significantly from those reported in other lichens (Sadowsky and Ott 2012) and the constituents of the xanthophyll cycle were present in even lower ratios to chl a + b (Table 5). Thus, the influence of β-car and the xanthophyll cycle does not explain the peculiar resistance of X. elegans photobionts towards space and simulation experiments (de la Torre et al. 2004, 2010; Sancho et al. 2007, 2009; Raggio et al. 2011; Onofri et al. 2012; de Vera et al. 2003, 2004a, b, 2008).
In contrast, the high levels of neoxanthin (neo) in B. frigida and lutein (lut) in X. elegans likely confer photoprotection. Lut is the most abundant xanthophyll in higher plants, followed by neo and vio (Dall’Osto et al 2006, 2007). Both, lut and neo act as antioxidants within the photosystem II supercomplex by scavenging ROS generated from the incomplete quenching of triplet chlorophyll (Dall’Osto et al. 2006; Croce et al. 1999). While carotenoids are essential to protect chloroplasts from photoxidative stress (Niyogi 1999), neo is not essential for plant survival under mild environmental conditions but the resistance of neo-deficient mutants to photo-oxidative stress is decreased (Dall’Osto et al 2007). Thus, the elevated ratios of neo and lut to chl a + b might indicate that both xanthophyll levels are benefical under extreme environmental conditions and thus may partially explain the resistance of the lichens X. elegans and B. frigida towards real and simulated space parameters. Recent studies on thalli of R. geographicum and C. gyrosa (Sánchez et al. 2013, in print) confirm higher contents of lut (about 40 and 33 μg/g TDW, respectively) compared to β-car (about 10 and 5 μg/g TDW, respectively) by a factor of 4–6, as also found in X. elegans and B. frigida (refer to Tables 2 and 3). X. elegans and B. frigida were shown to contain high levels of parietin and melanin respectively and thus absorb substantial amounts of blue light (see above, Figs. 3f and 4d). Nonetheless, both lichen photobionts revealed high levels of carotenoids, indicating that an additional photoprotection in the alga is necessary.
As isolated xanthophylls predominantly absorb light between 400 and 550 nm (Fig. 3b-c, Ruban et al. 1993), their protective effect is likely due to their function as efficient quenchers of blue light. As reviewed for Antarctic cyanolichens, high levels of carotenoids may play a role in quenching excess light and protect the photosystem under extreme environmental conditions (Wynn-Williams and Edwards 2000). Besides these effects, the spectral properties of β-car were also discussed in the context of lichen UVR resistance (Wynn-Williams and Edwards 2000). Despite these findings, the recent study gives no hint on a UVR-protective role of carotenoids beyond its role in the photosynthetic apparatus itself. While some axenically cultured mycobionts were demonstrated to produce carotenoids of unknown identity (Henriksson and Pearson 1968; Renner and Gerstner 1994), HPLC-analysis of axenic mycobiont cultures of X. elegans, B. frigida, R. geographicum but also of Fulgensia bracteata did not give any hint on the production of carotenoids by the mycobiont itself. The detection of xanthophylls in thallus extracts of X. elegans and B. frigida can be explained by their elevated levels in the incorporated photobiont. Thus, a carotenoid-conferred shielding effect for the mycobiont, and subsequently the whole lichen thallus, is not supported by the present data.
Consequences for Astrobiological Research
The effect and importance of SLCs is stressed by the results of two previous experiments: After simulation experiments with monochromatic UVC254 nm on lichen thalli (Wieners 2005; de Vera and Ott 2010) revealed the positive effect of UVR-protective compounds when comparing the post-exposition vitality of the lichens Buellia frigida (melanin and norstictic acid), Xanthoria elegans (parietin) and Peltigera aphthosa (traces of tenuiorin and gyrophoric acid only, Brodo et al. 2001). While the myco- as well as the photobionts of the first two species showed no significant loss of vitality after irradiation with the maximum dose of 2.018 J*m−2, the symbionts of P. aphthosa suffered a loss of almost 95 % vitality after 504 J*m−2. Moreover, differences in SLCs composition and distribution (see above) may also help to explain the better performance of PSII activity of X. elegans after 1.5 years in LEO-space, compared to Rhizocarpon geographicum under the same experimental conditions (Onofri et al. 2012).
In the context of astrobiology, this study highlights the diversity of SLCs in space-relevant lichens. Thus, several aspects have to be taken into account when designing, performing, comparing, and interpreting experiments on lichens. To summarize, 1) the set of SLCs is very different in the species used to date, 2) the concentration and even the presence of SLCs in lichen samples may vary in respect to the site of sample collection, and 3) the methods to detect the different SLCs have to be adapted appropriately. Moreover, the mechanisms and capacities of photo-/UVR-protection by these SLCs may be different with respect to three aspects: 4) to their UVR-absorbing range and capacity (as indicated by comparing the diverse UV/VIS-spectra, refer to Fig. 3), 5) to their variable intrathalline location (e. g. in B. frigida: melanin in cortical areas above the photobiont; in R. geographicum: melanin in regenerative structures and the basal prothallus but not above its algal layer), and 6) to their distribution among the symbionts. While cortical pigments protect both symbionts below the cortex, the distribution of carotenoids within the thalli of B. frigida and X. elegans was shown to be restricted to the photobiont. Thus, carotenoids impose additional protection against high light and UVR stress in the photosynthetic apparatus of the photobiont but do not confer direct protection to the mycobiont. The manifold combinations of SLCs found in lichens 7) may have an additive protective effect. A single SLC, even the most dominant one, may not be sufficient to explain high survival capacity alone. Additionally, 8) the functional roles of SLCs may go beyond mere UVR-protection and have to be considered carefully: parietin is also shielding excess PAR (Solhaug and Gauslaa 2004; Solhaug et al. 2003) while melanin confers resistance against other stresses (Bell and Wheeler 1986; Henson et al. 1999). High UVR-absorbtion properties do not deny other functions as bioweathering, antibacterial, and antiherbivorous effects (as reviewed in Rundel 1978) which may feedback on the lichens resistance to extreme environmental conditions and thus should be assessed separately. Finally, 9) the protective role of SLCs is linked to the thallus morphology and anatomy of the respective lichen investigated, as demonstrated by C. gyrosa whose large and dense subcortex (refer to Meeßen et al. 2013b) comes along with a stunning lack of SLCs. Thus, generalisations with respect to the extent and quality of the protective role have to be avoided. The identity, concentration, spatial and temporal distribution of SLCs, as well as interactions and additive effects between SLCs and morphological-anatomical structures should be considered separately for every lichen species.
Moreover, the role of SLCs as biomarkers in astrobiology has to be discussed. Biomarkers are substances or their degradation products that indicate the presence of life in recent or past times and are exclusively formed by biological activity (Brack et al. 1998; Westall et al. 2000). SLCs comprise a rich set of biogenic substances found in extremotolerant lichens and thus are suitable candidates for further research on their stability under extreme environmental factors (e.g. in Antarctica, Edwards et al. 2005), on geological timescales, and on their degradation properties. Previous studies evaluated the chance to detect secondary or other lichen-associated compounds by in situ applicable instrumentation such as Raman-spectrometry for Mars lander missions as ExoMars (Edwards et al. 2005; Böttger et al. 2011; Böttger et al. 2013 in print). Parietin was detected in Xanthoria mawsonii, X. elegans and Caloplaca spp., and rhizocarpic acid was detected in Rhizocarpon sp. as well as in Acarospora sp. (Edwards et al. 2005; Holder et al. 2000), while β-carotene was found to be a key biomarker in most lichen species investigated (X. mawsonii, X. elegans, Caloplaca spp., Rhizocarpon sp. and Acarospora sp., Edwards et al. 2005; C. gyrosa, Böttger et al. 2013 in print) but also in cyanobacteria as Chroococcidiopsis sp. (Wynn-Williams and Edwards 2000) and Nostoc commune (Böttger et al. 2011), which is frequently found as a lichen photobiont. Additional lichen-associated Raman biomarkers were found to be calcium oxalates (Holder et al. 2000; Edwards et al. 2005; Böttger et al 2013 in print) and chitin (Böttger et al 2013 in print). Therefore, substances as chlorophyll a, β-carotene, chitin, cellulose, parietin, and melanin which can be—among many organisms—found in lichens will be subdued to space and Martian analogue conditions during the BIOMEX experiment onboard the International Space Station (ISS, ESA-ILSRA 2009-0834, de Vera et al. 2012b) and subsequently analysed on their post-flight detectability and degradation.
Abbreviations
- CoA:
-
Coenzyme A
- DEPS:
-
De-epoxidation status of the xanthophyll pool
- DMSO:
-
Dimethyl sulfoxide
- EtOH:
-
Ethanol
- HPLC:
-
High performance liquid chromatography
- LEO:
-
Low Earth orbit
- LHC:
-
Light harvesting complex
- MeOH:
-
Methanol
- MY:
-
Malt yeast extract
- PAR:
-
Photosynthetically active radiation (400–700 nm)
- PS II:
-
Photosystem II
- ROS:
-
Reactive oxygen species
- SEM:
-
Scanning electron microscopy
- SLC:
-
Secondary lichen compound
- TDW:
-
Thallus dry weight
- TLC:
-
Thin layer chromatography
- UV/VIS:
-
Ultra violet/visible light
- UVR:
-
Ultra-violet radiation (100–400 nm)
- VAZ:
-
Xanthophyll cycle of viola-, anthera-, and zeaxanthin
References
Bačkor M, Klemová K, Bačkorová M, Ivanova V (2010) Comparison of the phytotoxic effects of usnic acid on cultures of free-living alga Scenedesmus quadricauda and aposymbiotically grown lichen photobiont Trebouxia erici. J Chem Ecol 36(4):405–411
Beckett RP, Minibayeva FV, Liers C (2012) Occurrence of high tyrosinase activity in the non-Peltigerales lichen Dermatocarpon miniatum (L.) W.Mann. Lichenologist 44(6):827–832
BeGora MD, Fahselt D (2001) Usnic acid and atranorin concentrations in lichens in relation to bands of UV irradiance. Bryologist 104(1):134–140
Bell AA, Wheeler MH (1986) Biosynthesis and functions of fungal melanins. Ann Rev Phytopath 24:411–451
Böttger U, de Vera JP, Fritz J, Weber I, Hübers HW, Schulze-Makuch D (2011) Optimizing the detection of carotene in cyanobacteria in a martian regolith analogue with a Raman spectrometer for the ExoMars mission. Planet Space Sci 60(1):356–362
Böttger U, Meeßen J, de la Torre R, Frias JM, Rull F, Sánchez FJ, Hüber HW, de Vera JP (2013) Raman spectroscopic analysis of Circinaria gyrosa as a candidate for BIOMEX. Int J Astrobiol (in print)
Boustie J, Grube M (2005) Lichens - a promising source of bioactive secondary metabolites. Plant Genet Resour 3(2):273–287
Brack A, Clancy P, Fitton B, Hoffmann B, Horneck G, Kurat G, Maxwell J, Ori G, Pillinger C, Raulin F, Thomas N, Westall F (1998) Search for life on Mars. Biol Sci Space 12(2):119–123
Brodo IM, Sharnoff SD, Sharnoff S (2001) Lichens of North America. Yale University Press, New Haven, London, pp 503–522
Brunauer G, Hager A, Grube M, Türk R, Stocker-Wörgötter (2007) Alterations in secondary metabolism of aposymbiotically grown mycobionts of Xanthoria elegans and cultured resynthesis stages. Plant Physiol Biochem 45:146–151
Croce R, Remelli R, Varotto C, Breton J, Bassi R (1999) The neoxanthin binding site of the major light harvesting complex (LHCII) from higher plants. FEBS Letters 456:1–6
Culberson CF (1969) Chemical and botanical guide to lichen products. University of North Carolina Press, Chapel Hill
Dall’Osto L, Cazzaniga S, North H, Marion-Poll A, Bassi R (2007) The Arabidopsis aba4-1 mutant reveals a specific function for neoxanthin in protection against photo-oxidative stress. The Plant Cell 19(3):1048–1064
Dall’Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R (2006) Lutein is needed for efficient chlorophyll triplet quenching in the major LHC II antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol 6:32–37
Demmig-Adams B, Adams WW (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
de la Torre NR, Sancho LG, Pintado A, Rettberg P, Rabbow E, Panitz C, Deutschmann U, Reina M, Horneck G (2007) BIOPAN experiment LICHENS on the Foton M2 mission: pre-flight verification tests of the Rhizocarpon geographicum-granite ecosystem. Adv Space Res 40(11):1665–1671
de la Torre R, Horneck G, Sancho LG, Pintado A, Scherer K, Facius R, Deutschmann U, Reina M, Baglioni P, Demets R (2004) Studies of lichens from high mountain regions in outer space: the BIOPAN experiment. Proceedings of the third European Workshop on Astrobiology. ESA SP-545, ESA Publications Division, ESTEC, Noordwijk, pp 193–194
de la Torre R, Sancho LG, Horneck G, de los Ríos A, Wierzchos J, Olsson-Francis K, Cockell C, Rettberg P, Berger T, de Vera JP, Ott S, Frías JM, Gonzalez PM, Lucas MM, Reina M, Pintado A, Demets R (2010) Survival of lichens and bacteria exposed to outer space conditions – Results of the Lithopanspermia experiments. Icarus 208(2):735–748
de Vera JP (2012) Lichens as survivors in space and on Mars. Fungal Ecol 5:472–479
de Vera JP, Horneck G, Rettberg P, Ott S (2003) The potential of the lichen symbiosis to cope with the extreme conditions of outer space I. Influence of UV radiation and space vacuum on the vitality of lichen symbiosis and germination capacity. Int J Astrobiol 1:285–293
de Vera JP, Horneck G, Rettberg P, Ott S (2004a) The potential of the lichen symbiosis to cope with the extreme conditions of outer space II: germination capacity of lichen ascospores in response to simulated space conditions. Adv Space Res 33:1236–1243
de Vera JP, Horneck G, Rettberg P, Ott S (2004b) In the context of panspermia: May lichens serve as shuttles for their bionts in space? Proceedings of the third European Workshop on Astrobiology. ESA SP-545, ESA Publications Division, ESTEC, Noordwijk, pp 197-198
de Vera JP, Möhlmann D, Butina F, Lorek A, Wernecke R, Ott S (2010) Survival potential and photosynthetic activity of lichens under Mars-like conditions: a laboratory study. Astrobiology 10(2):215–227
de Vera JP, Ott S (2010) Resistance of symbiotic eukaryotes. Survival to simulated space conditions and asteroid impact cataclysms. In: Seckbach J & Grube M (eds) Symbioses and stress: Joint ventures in biology. Cellular Origin, Life in Extreme Habitats and Astrobiology 17: 595-611
de Vera JP, Rettberg P, Ott S (2008) Life at the limits: capacities of isolated and cultured lichen symbionts to resist extreme environmental stresses. Orig Life Evol Biosph 38:457–468
de Vera JP, Schulze-Makuch D, Khan A, Lorek A, Koncz A, Möhlmann D, Spohn T (2012a) The adaptation potential of extremophiles to Martian surface conditions and its implication for the habitability of Mars. EGU General Assembly, p 2113
de Vera JP, Schulze-Makuch D, Khan A, Lorek A, Koncz A, Möhlmann D, Spohn T (2013) Adaptation of an Antarctic lichen to Martian niche conditions can occur within 34 days. Planetary and Space Science (in print)
de Vera JP, Boettger U, de la Torre R, Sánchez FJ, Grunow D, Schmitz N, Lange C, Hübers HW, Billi D, Baqué M, Rettberg P, Rabbow E, Reitz G, Berger T, Möller R, Bohmeier M, Horneck G, Westall F, Jänchen J, Fritz J, Meyer C, Onofri S, Selbmann L, Zucconi L, Kozyrovska N, Leya T, Foing B, Demets R, Cockell CS, Bryce C, Wagner D, Serrano P, Edwards HGM, Joshi J, Huwe B, Ehrenfreund P, Elsaesser A, Ott S, Meessen J, Feyh N, Szewzyk U, Jaumann R, Spohn T (2012b) Supporting Marsexploration: BIOMEX in Low Earth Orbit and further astrobiological studies on the Moon using Raman and PanCam technology. Planet Space Sci 74(1):103–110
Dyer P, Crittenden P (2008) Antarctic lichens: life in the freezer. Microbiology Today, May 2008, pp 74-77
Edwards HGM, Moody CD, Jorge Villar SE, Wynn-Williams DD (2005) Raman spectroscopic detection of key biomarkers of cyanobacteria and lichen symbioses in extreme Antarctic habitats: evaluation for Mars lander missions. Icarus 174:560–571
Ertl L (1951) Über die Lichtverhältnisse in Laubflechten. Planta 39:245–270
Fahselt D (1994) Secondary biochemistry of lichens. Symbiosis 16:117–165
Fahselt D, Alstrup V (1997) Visualization of extracellular deposits in recent and subfossil Umbilicaria hyperborea. Lichenologist 29(6):547–557
Gauslaa Y, Solhaug KA (2001) Fungal melanins as a sun screen for symbiotic green algae in the lichen Lobaria pulmonaria. Oecologia 126:462–471
Hale ME (1956) Ultraviolet absorption spectra of lichen depsides and depsidones. Science 123(3199):671
Harańczyk H, Pytel M, Pater Ł, Olech A (2008) Deep dehydration resistance of antarctic lichens (genera Umbilicaria and Ramalina) by proton NMR and sorbtion isotherm. Antactic Science. Cambridge University Press, Vol. 20 (6): 527-535
Havaux M, Dall’Osto L, Bassi R (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol 145(4):1506–1520
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects from photo-oxidative damage by more than one mechanism. Proc Nat Acad Sci 96:8762–8767
Henriksson E, Pearson LC (1968) Carotenoids extracted from the mycobionts of Collema tenax, Baeomyces roseus, and some other lichens. Svensk Botanisk Tidskrift 62:441
Henson JH, Butler MJ, Day AW (1999) The dark side of the mycelium: melanins of phytopathogenic fungi. Ann Rev Phytopathol 37:447–471
Henssen A, Jahns HM (1974) Lichenes. Eine Einführung in die Flechtenkunde. Georg Thieme Verlag, Stuttgart, pp 11-71
Holder JM, Wynn-Williams DD, Rull Perez F, Edwards HGM (2000) Raman spectroscopy of pigments and oxalates in situ within epilithic lichens. New Phytol 145:271–280
Hollis JP (1952) Studies on Streptomyces scabies. I. Variability in a melanin-indicator medium. Phytopathology 42:273–276
Horneck G, Stöffler D, Ott S, Hornemann U, Cockell CS, Moeller R, Meyer C, de Vera JP, Fritz J, Schade S, Artemieva NA (2008) Microbial rock inhabitants survive hypervelocity impacts on Mars-like host planets: first phase of lithopanspermia experimentally tested. Astrobiology 8(1):17–44
Huneck S, Yoshimura I (1996) Identification of lichen substances. Springer, Berlin, pp 1–9
Huneck S (1999) The significance of lichens and their metabolites. Naturwissenschaften 86(12):559–570
Jahns HM (1988) The lichen thallus. In: Galun M (ed) CRC handbook of lichenology, vol I, CRC Press. Boca Ranton, Florida, pp 95–143
Jordan BR (1996) The effects of Ultraviolet-B radiation on plants: A molecular perspective. In: Callow JA (ed), Advances in botanical research. Academic Press, Vol. 22 pp. 97-162
Kappen L (1973) Environmental response and effects. Response to extreme environments. In: Ahmadjian V, Hale ME (eds) The Lichens. Academic, New York, pp 346–348
Kappen L (1988) Ecophysiological relationships in different climatic regions. In: Galun M (ed) CRC handbook of lichenology, vol II. CRC Press, Boca Ranton, pp 37–99
Kappen L (1993) Plant activity under snow and ice, with particular reference to lichens. Arctic 46(4):297–302
Knudsen K (2007) Acarospora. In: Nash et al. (eds) Lichen Flora of the Greater Sonoran Region, Vol. 3. pp 1-38
Knudsen K, Elix JA, Reeb V (2008) A preliminary study of the genus Acarospora in South America. Opuscula Philolichenum 5:1–22
Kranner I, Cram WJ, Zorn M, Wornik S, Yoshimura I, Stabentheiner E, Pfeifhofer HW (2005) Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. Proc Nat Acad Sci 102(8):3141–3146
Lange OL (1992) Pflanzenleben unter Stress. Würzburg. Echter Würzburg Fränkische Gesellschaftsdruckerei und Verlag, pp 213-217
Lange OL, Green TGA, Heber U (2001) Hydration-dependent photosynthetic production of lichens: what do laboratory studies tell us about field performance. J Exp Bot, Spec Issue 52(363):2033–2042
Lange OL, Green TGA, Reichenberger H (1999a) The response of lichen photosynthesis to external CO2 concentration and its interaction with thallus water-status. J Plant Physiol 154:157–166
Lange OL, Kilian E, Ziegler H (1986) Water vapor uptake and photosynthesis of lichens: performance differences in species with green and blue-green algae as phycobionts. Oecologia 71:104–110
Lange OL, Leisner JMR, Bilger W (1999b) Chlorophyll fluorescence characteristicsof the cyanobacterial lichen Peltigera rufescens under field conditions. II. Dial and annual distribution of metabolic activity and possible mechanisms to avoid photoinhibition. Flora 194:413–430
Locatelli M, Tammaro F, Menghini L, Carlucci G, Epifano F, Genovese S (2009) Anthraquinone profile and chemical fingerprint of Rhamnus saxatilis L. from Italy. Phytochem Lett 2:223–226
Marchant DR, Head JW III (2007) Antarctic dry valleys: microclimate zonation, variable geomorphic processes, and implications for assessing climate change on Mars. Icarus 192:187–222
McEvoy M, Nybakken L, Solhaug KA, Gauslaa Y (2006) UV triggers the synthesis of the widely distributed secondary lichen compound usnic acid. Mycol Progress 5:221–229
McEvoy M, Gauslaa Y, Solhaug KA (2007) Changes in the pools of depsidones and melanins, and their function during growth and acclimation under contrasting natural light in the lichen Lobaria pulmonaria. New Phytologist 175:271–282
McKay CP, Friedmann EI, Gomez-Silva B, Caceres-Villanueva L, Andersen DT, Landheim R (2003) Temperature and moisture conditions for life in the extreme arid region of the Atacama Desert: Four years of observations including the El Nino of 1997-1998. Astrobiology 3(2):393–406
Meeßen J, Eppenstein S, Ott S (2013a) Recognition mechanisms during the pre-contact state of lichens: II. Influence of algal exudates and ribitol on the response of the mycobiont of Fulgensia bracteata. Symbiosis 59(3):131–143
Meeßen J, Ott S (2013) Recognition mechanisms during the pre-contact state of lichens: I. Mycobiont-photobiont interactions of the mycobiont of Fulgensia bracteata. Symbiosis 59(3):121–130
Meeßen J, Sánchez FJ, Brandt A, Balzer EM, de la Torre R, Sancho LG, de Vera JP, Ott S (2013b) Extremotolerance and resistance of lichens: comparative studies on five species used in astrobiological research. I. Morphological and anatomical characteristics. Orig Life Evol Biosph 43(3):283–303
Meredith P, Riesz J (2004) Radiative relaxation quantum yields for synthetic eumelanin. Photochem Photobiol 79(2):211–216
Mosbach K (1964) On the biosynthesis of lichen substances. Part 1. The depside gyrophoric acid. Acta Chem Scand 18:329–334
Mosbach K (1967) On the biosynthesis of lichen substances. Part 4. The formation of pulvic acid derivatives by isolated lichen fungi. Acta Chem Scand 21:2331–2334
Muggia L, Grube M (2010) Type II polyketide synthases in lichen mycobionts. Fungal Biology 114:379–385
Muggia L, Schmitt I, Grube M (2009) Lichens as a treasure chest of natural products. SIM News 59:85–97
Nash TH, Ryan BD, Gries C, Bugartz F (eds.) (2001) Lichen Flora of the Greater Sonoran Desert Region. Vol 3. Tempe, Arizona, pp 85-97
Nurudeen TA, Ahearn DG (1979) Regulation of melanin production by Cryptococcus meoformans. J Clin Microbiol 10:724–729
Nybakken L, Solhaug KA, Bilger W, Gauslaa Y (2004) The lichens Xanthoria elegans and Cetraria islandica maintain a high protection against UV-B radiation in Arctic habitats. Oecologia 140:211–216
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359
Onofri S, de la Torre R, de Vera JP, Ott S, Zucconi L, Selbmann L, Scalzi G, Vankateswaran KJ, Rabbow E, Sánchez Iñigo FJ, Horneck G (2012) Survival of rock-colonizing organisms after 1.5 years in outer space. Astrobiology 12(5):508–516
Øvstedal DO, Lewis Smith RI (2001) Lichens of Antarctica and South Georgia. A guide to their identification and ecology. Cambridge University Press, pp 66-365
Raggio J, Pintado A, Ascaso C, de la Torre R, de los Ríos A, Wierzchos J, Horneck G, Sancho LG (2011) Whole Lichen Thalli survive exposure to space conditions: results of Lithopanspermia experiment with Aspicilia fruticulosa. Astrobiology 11(4):281–292
Renner B, Gerstner E (1994) Stoffwechselunterschiede zwischen dem lichenisierten und dem isolierten Mycobionten von Baeomyces rufus (Huds.) Rebent. Zeitschrift für Pflanzenphysiologie 107(1):47–57
Ruban AV, Horton P, Young AJ (1993) Aggregation of higher plant xanthophylls: differences in absorption spectra and in the dependency on solvent polarity. J Photochem Photobio B 21(2–3):229–234
Rundel PW (1978) The ecological role of secondary lichen substances. Biochem Syst Ecol 6(3):157–170
Sadowsky A, Ott S (2012) Photosynthetic symbionts in Antarctic terrestrial ecosystems: the physiological response of lichen photobionts to drought and cold. Symbiosis 58:81–90
Sadowsky A, Hussner A, Ott S (2012) Submersion tolerance in a habitat of Stereocaulon paschale (Stereocaulaceae) and Cladonia stellaris (Cladoniaceae) from the high-mountain region Rondane, Norway. Nova Hedwigia 94(3–4):1–12
Sánchez FJ, Mateo-Martí E, Raggio J, Meeßen J, Martínez-Frías J, Sancho LG, Ott S, de la Torre R (2012) The resistance of the lichen Circinaria gyrosa (nom. provis.) towards simulated Mars conditions – a model test for the survival capacity of an eukaryotic extremophile. Planet Space Sci 72(1):102–110
Sánchez FJ, Meeßen J, Ruiz MC, Sancho LG, Ott S, Vílchez C, Horneck G, Sadowsky A, de la Torre R (2013) UV-C tolerance of symbiotic Trebouxia sp. in the space-tested lichens species Rhizocarpon geographicum and Circinaria gyrosa: role of hydration state and cortex/screening substances. Int J Astrobiol. doi:10.1017/S147355041300027X
Sancho LG, de la Torre R, Horneck G, Ascaso C, de los Ríos A, Pintado A, Wierzchos J, Schuster M (2007) Lichens survive in space: results from 2005 LICHENS experiment. Astrobiology 7(3):443–454
Sancho LG, de la Torre R, Pintado A (2009) Lichens, new and promising material from experiments in astrobiology. Fungal Biol Rev 22:103–109
Sargent MV, Smith DN, Elix JA (1970) The minor anthraquinones of Xanthoria parietina (L.) Beltram, the chlorination of parietin, and the synthesis of fragilin and 7-chloroemodin. J Chem Soc C 2:307–311
Sava VM, Yang SM, Hong MY, Yang PC, Huang GS (2001) Isolation and characterization of melanic pigments derived from tea and tea polyphenols. Food Chem 73:177–184
Scalzi G, Selbmann L, Zucconi L, Rabbow E, Horneck G, Albertano P, Onofri S (2012) LIFE Experiment: isolation of cryptoendolithic organisms from Antarctic colonized sandstone exposed to space and simulated Mars conditions on the International Space Station. Orig Life Evol Biosph 42:253–262
Sigfridsson B, Oquist G (1980) Preferential distribution of excitation energy into photosystem I of desiccated samples of the lichen Cladonia impexa and the isolated lichen-alga Trebouxia pyriformis. Physiol Plant 49(4):329–335
Sohrabi M (2012) Taxonomy and phylogeny of the manna lichens and allied species (Megasporaceae). PhD thesis, Publications in Botany from the University of Helsinki. http://urn.fi/URN:ISBN:978-952-10-7400-4
Solhaug KA, Gauslaa Y (1996) Parietin, a photoprotective secondary product of the lichen Xanthoria parietina. Oecologia 108:412–418
Solhaug KA, Gauslaa Y (2004) Photosynthates stimulate the UV-B induced fungal anthraquinone synthesis in the foliase lichen Xanthoria parietina. Plant Cell Environ 27:167–178
Solhaug KA, Gauslaa Y, Nybakken L, Bilger W (2003) UV-induction of sun-screening pigments in lichens. New Phytol 158:91–100
Stöffler D, Horneck G, Ott S, Hornemann U, Cockell CS, Moeller R, Meyer C, de Vera JP, Fritz J, Artemieva NA (2007) Experimental evidence for the potential impact ejection of viable microorganisms from Mars and Mars-like planets. Icarus 189:585–588
Sun HJ, Nienow JA, McKay CP (2010) The antarctic cryptoendolithic microbial ecosystem. In: Doran PT, Lyons WB, McKnight DM (eds) Life in antarctic deserts and other cold dry environments - astrobiological analogs. Cambridge University Press, pp 110-138
Telfer A, Dhami S, Bishop SM, Phillips D, Barber J (1994) β-carotene quenches singlet oxygen formed by isolated photosystem II reaction centers. Biochemistry 33(48):14469–14474
Tevini DP, Häder M (1985) Allgemeine photobiologie. Georg Thieme Verlag, Stuttgart, pp 25–26
Trebst A (2003) Function of β-carotene and tocopherol in photosystem II. Zeitschrift für Naturforschung C 58(9/10):609–620
Vrábliková H, Barták M, Wonisch A (2005) Changes in glutathione and xanthophyll cycle pigments in the high light-stressed lichens Umbilicaria antarctica and Lasallia pustulata. J Photo Biol 79:35–41
Wang H, Pan Y, Tang X, Huang Z (2006) Isolation and characterization of melanin from Osmanthus fragrans seeds. LWT 39:496–502
Westall F, Steele A, Toporski J, Walsh M, Allen C, Guidry S, McKay D, Gibson E, Chafetz H (2000) Polymeric substances and biofilms as biomarkers in terrestrial materials: Implications for extraterrestrial samples. J Geophys Res 105(NoE10):24511–24527
Wieners PC (2005) Das evolutionäre Potential der Flechtensymbiose gegenüber Extrembedingungen. Diplomarbeit, Heinrich-Heine-Universität, Düsseldorf, pp 34–41
Wynn-Williams DD, Edwards HGM (2000) Proximal analysis of regolith habitats and protective biomolecules in situ by Raman Laser Spectroscopy: overview of terrestrial Antarctic habitats and Mars analogues. Icarus 144:486–503
Acknowledgements
The authors would like to express their gratitude to the German Federal Ministry of Economics and Technology (BMWi) and the German Aerospace Center (DLR) for funding the work of Joachim Meeßen (50BW1153), to the Spanish Instituto Nacional de Técnica Aeroespacial (INTA) for granting a PhD scholarship to Francisco Javier Sánchez, and to ESA and the DLR for supporting the space experiment BIOMEX (ESA-ILSRA 2009-0834, P-I Dr. J.-P. de Vera). Samples of B. frigida and P. chlorophanum were collected by S. Ott during the GANOVEX 10 expedition which was funded by the German Research Foundation (DFG, OT 96/15-1) in the framework of the Antarctic Priority Program 1158. We would also like to thank Dr. Abdessamad Debbab and Andreas Marmann from the Institute of Pharmaceutical Biology and Biotechnology (HHU Düsseldorf) and Eva Posthoff for their friendly support as well as the anonymous reviewers for their comments and suggestions. Some results of this study were presented on the 12th European Workshop on Astrobiology (P6.16, EANA 2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meeßen, J., Sánchez, F.J., Sadowsky, A. et al. Extremotolerance and Resistance of Lichens: Comparative Studies on Five Species Used in Astrobiological Research II. Secondary Lichen Compounds. Orig Life Evol Biosph 43, 501–526 (2013). https://doi.org/10.1007/s11084-013-9348-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-013-9348-z