Abstract
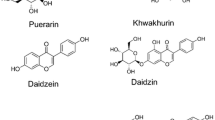
Isoflavonoids, a class of flavonoid polyphenolic compounds, are found primarily in leguminous plants. The chemical structure of isoflavonoid is a rearranged 2-phenyl of the flavonoids to a 3-phenyl by substituting the C-ring. Consuming dietary isoflavonoids provides many health benefits, including protection against age-related diseases, such as post-menopausal problems, osteoporosis, cardiovascular diseases, and hormone-dependent cancer. Since the market for isoflavonoids is growing, there is a need for alternative strategies for producing isoflavonoids from sustainable sources. In vitro suspension cell culture is environmentally friendly, and its conditions can be easily manipulated to enhance the yield of isoflavonoids. This review provides a detailed survey of the strategies used to augment the production of isoflavonoids with a particular emphasis on media optimization, elicitation, application of electric field, and metabolic engineering.
Similar content being viewed by others
Abbreviations
- DW:
-
Dry weight
- FW:
-
Fresh weight
- MS:
-
Murashige and Skoog
- SCC:
-
Suspension cell culture
- 2,4,-D:
-
2,4-Dichlorophenoxyacetic acid
- BA:
-
6-Benzyladenine
- NAA:
-
1-Naphthaleneacetic acid
- TDZ:
-
Thidiazuron
- MJ:
-
Methyl jasmonate
References
Ahmadi-Sakha S, Sharifi M, Niknam V (2016) Bioproduction of phenylethanoid glycosides by plant cell culture of Scrophularia striata Boiss.: from shake-flasks to bioreactor. Plant Cell Tissue Organ Cult 124(2):275–281
Akashi T, Aoki T, Si A (1999) Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice. Plant Physiol 121(3):821–828
Akiyama T, Ishida J, Nakagawa S, Ogawara H, Si W, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262(12):5592–5595
Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, De Aloysio D (1998) The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 91(1):6–11
Algar E, Gutierrez-Mañero FJ, Bonilla A, Lucas JA, Radzki W, Ramos-Solano B (2012) Pseudomonas fluorescens N21.4 metabolites enhance secondary metabolism isoflavones in soybean (Glycine max) calli cultures. J Agric Food Chem 60(44):11080–11087
Ames TT, Worden RM (1997) Continuous production of daidzein and genistein from soybean in a magnetofluidized bed bioreactor. Biotechnol Prog 13(3):336–339
Arias-Castro C, Seragg A, Rodriguez-Mendiola M (1993) The effect of cultural conditions on the accumulation of formononetin by suspension cultures of Glycyrrhiza glabra. Plant Cell Tissue Organ Cult 34(1):63–70
Arya SS, Rookes JE, Cahill DM, Lenka SK (2020) Next-generation metabolic engineering approaches towards development of plant cell suspension cultures as specialized metabolite producing biofactories. Biotechnol Adv 45:107635
Aviezer D, Almon-Brill E, Shaaltiel Y, Galili G, Chertkoff R, Hashmueli S, Galun E, Zimran A (2009) 8. Novel enzyme replacement therapy for Gaucher disease: ongoing phase III clinical trial with recombinant human glucocerebrosidase expressed in plant cells. Mol Genet Metab 2(96):S13–S14
Babich O, Sukhikh S, Pungin A, Ivanova S, Asyakina L, Prosekov A (2020) Modern trends in the in vitro production and use of callus, suspension cells and root cultures of medicinal plants. Molecules 25(24):5805
Basile DV, Akhtari N, Durand Y, Nair M (1993) Toward the production of artemisinin through tissue culture: determining nutrient-hormone combinations suitable for cell suspension cultures. In Vitro Cell Dev Biol Plant 29(3):143–147
Bhatia S, Sharma K, Dahiya R, Bera T (2015) Modern applications of plant biotechnology in pharmaceutical sciences. Academic Press, New York
Boonsnongcheep P, Korsangruang S, Soonthornchareonnon N, Chintapakorn Y, Saralamp P, Prathanturarug S (2010) Growth and isoflavonoid accumulation of Pueraria candollei var candollei and P candollei var mirifica cell suspension cultures. Plant Cell Tissue Organ Cult (PCTOC) 101(2):119–126
Bouque V, Bourgaud F, Nguyen C, Guckert A (1998) Production of daidzein by callus cultures of Psoralea species and comparison with plants. Plant Cell Tissue Organ Cult 53(1):35–40
Bulgakov V, Tchernoded G, Mischenko N, Khodakovskaya M, Glazunov V, Radchenko S, Zvereva E, Fedoreyev S, Zhuravlev YN (2002) Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotechnol 97(3):213–221
Camacho-Fernández C, Hervás D, Rivas-Sendra A, Marín M, Seguí-Simarro JM (2018) Comparison of six different methods to calculate cell densities. Plant Methods 14(1):1–15
Carrão-Panizzi MC, Berhow M, Mandarino JMG, Oliveira MCN (2009) Environmental and genetic variation of isoflavone content of soybean seeds grown in Brazil. Pesq Agrop Brasileira 44:1444–1451
Carusi D (2000) Phytoestrogens as hormone replacement therapy: an evidence-based approach. Primary Care Update OB/GYNS 7(6):253–259
Chandran H, Meena M, Barupal T, Sharma K (2020) Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol Rep 26:e00450
Chen L, Teng H, Jia Z, Battino M, Miron A, Yu Z, Cao H, Xiao J (2018a) Intracellular signaling pathways of inflammation modulated by dietary flavonoids: The most recent evidence. Crit Rev Food Sci Nutr 58(17):2908–2924
Chen L, Teng H, Xie Z, Cao H, Cheang WS, Skalicka-Woniak K, Georgiev MI, Xiao J (2018b) Modifications of dietary flavonoids towards improved bioactivity: An update on structure–activity relationship. Crit Rev Food Sci Nutr 58(4):513–527
Chen L, Lin X, Teng H (2020) Emulsions loaded with dihydromyricetin enhance its transport through Caco-2 monolayer and improve anti-diabetic effect in insulin resistant HepG2 cell. J Funct Foods 64:103672
Cherdshewasart W, Sriwatcharakul S (2007) Major isoflavonoid contents of the 1-year-cultivated phytoestrogen-rich herb, Pueraria mirifica. Biosci Biotechnol Biochem 71(10):2527–2533
Cherdshewasart W, Subtang S, Dahlan W (2007) Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J Pharm Biomed Anal 43(2):428–434
Cierna M, Kákoniová D, Liskova D (1991) A medium for rapid plant callus growth. Biologia 46(3):271–272
Daems F, Decruyenaere V, Agneessens R, Lognay G, Romnee JM, Froidmont É (2016) Changes in the isoflavone concentration in red clover (Trifolium pratense L.) during ensiling and storage in laboratory-scale silos. Anim Feed Sci Technol 217:36–44
Daniel S, Tiemann K, Wittkampf U, Bless W, Hinderer W, Barz W (1990) Elicitor-induced metabolic changes in cell cultures of chickpea (Cicer arietinum L.) cultivars resistant and susceptible to Ascochyta rabiei. Planta 182(2):270–278
de Lima PF, Colombo CA, Chiorato AF, Yamaguchi LF, Kato MJ, Carbonell SRAM (2014) Occurrence of isoflavonoids in Brazilian common bean germplasm (Phaseolus vulgaris L.). J Agric Food Chem 62(40):9699–9704
Devi MA, Giridhar P (2014) Isoflavone augmentation in soybean cell cultures is optimized using response surface methodology. J Agric Food Chem 62(14):3143–3149
Devi MA, Kumar G, Giridhar P (2020) Effect of biotic and abiotic elicitors on isoflavone biosynthesis during seed development and in suspension cultures of soybean (Glycine max L.). 3 Biotech 10(3):1–14
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7(7):1085
Eibl R, Meier P, Stutz I, Schildberger D, Hühn T, Eibl D (2018) Plant cell culture technology in the cosmetics and food industries: current state and future trends. Appl Microbiol Biotechnol 102(20):8661–8675
Engelmann NJ, Reppert A, Yousef G, Rogers RB, Lila MA (2009) In vitro production of radiolabeled red clover (Trifolium pratense) isoflavones. Plant Cell Tissue Organ Cult 98(2):147–156
Esperanca MN, Mendes CE, Rodriguez GY, Cerri MO, Bettega R, Badino AC (2020) Sparger design as key parameter to define shear conditions in pneumatic bioreactors. Biochem Eng J 157:107529
Espinosa-Leal CA, Puente-Garza CA, García-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248(1):1–18
Fang H, Tong W, Shi LM, Blair R, Perkins R, Branham W, Hass BS, Xie Q, Dial SL, Moland CL (2001) Structure—activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. Chem Res Toxicol 14(3):280–294
Federici E, Touché A, Choquart S, Avanti O, Fay L, Offord E, Courtois D (2003) High isoflavone content and estrogenic activity of 25 year-old Glycine max tissue cultures. Phytochemistry 64(3):717–724
Fedoreyev S, Pokushalova T, Veselova M, Glebko L, Kulesh N, Muzarok T, Seletskaya L, Bulgakov V, Zhuravlev YN (2000) Isoflavonoid production by callus cultures of Maackia amurensis. Fitoterapia 71(4):365–372
Fedoreyev SA, Bulgakov VP, Grishchenko OV, Veselova MV, Krivoschekova OE, Kulesh NI, Denisenko VA, Tchernoded GK, Zhuravlev YN (2008) Isoflavonoid composition of a callus culture of the relict tree Maackia amurensis Rupr. et Maxim. J Agric Food Chem 56(16):7023–7031
Franks P (2004) Nutraceuticals from grains. Elsevier, New York, pp 312–318
Fujita Y (1988) Shikonin: production by plant (Lithospermum erythrorhizon) cell cultures Medicinal and Aromatic Plants I. Springer, New York, pp 225–236
Georgiev V, Slavov A, Vasileva I, Pavlov A (2018) Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng Life Sci 18(11):779–798
Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH (2003) Inactivation of NF-κ B by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 22(30):4702–4709
Goyal S, Ramawat K (2008a) Ethrel treatment enhanced isoflavonoids accumulation in cell suspension cultures of Pueraria tuberosa, a woody legume. Acta Physiol Plant 30(6):849–853
Goyal S, Ramawat K (2008b) Increased isoflavonoids accumulation in cell suspension cultures of Pueraria tuberosa by elicitors. Indian J Biotechnol 7:378–382
Grishchenko O, Kiselev K, Tchernoded G, Fedoreyev S, Veselova M, Bulgakov V, Zhuravlev Y (2013) The influence of the rolC gene on isoflavonoid production in callus cultures of Maackia amurensis. Plant Cell Tissue Organ Cult 113(3):429–435
Gueven A, Knorr D (2011) Isoflavonoid production by soy plant callus suspension culture. J Food Eng 103(3):237–243
Gupta OP, Nigam D, Dahuja A, Kumar S, Vinutha T, Sachdev A, Praveen S (2017) Regulation of isoflavone biosynthesis by miRNAs in two contrasting soybean genotypes at different seed developmental stages. Front Plant Sci 8:567
Gutierrez-Gonzalez JJ, Wu X, Zhang J, Lee JD, Ellersieck M, Shannon JG, Yu O, Nguyen HT, Sleper DA (2009) Genetic control of soybean seed isoflavone content: importance of statistical model and epistasis in complex traits. Theor Appl Genet 119(6):1069–1083
Hagimori M, Matsumoto T, Obi Y (1982) Studies on the production of Digitalis cardenolides by plant tissue culture: II. Effect of light and plant growth substances on digitoxin formation by undifferentiated cells and shoot-forming cultures of Digitalis purpurea L. grown in liquid media. Plant Physiol 69(3):653–656
Hellwig S, Drossard J, Twyman RM, Fischer R (2004) Plant cell cultures for the production of recombinant proteins. Nat Biotechnol 22(11):1415–1422
Hibino K, Ushiyama K (1999) Commercial production of ginseng by plant tissue culture technology plant cell and tissue culture for the production of food ingredients. Springer, New York, pp 215–224
Hoeck JA, Fehr WR, Murphy PA, Welke GA (2000) Influence of genotype and environment on isoflavone contents of soybean. Crop Sci 40(1):48–51
Holland T, Sack M, Rademacher T, Schmale K, Altmann F, Stadlmann J, Fischer R, Hellwig S (2010) Optimal nitrogen supply as a key to increased and sustained production of a monoclonal full-size antibody in BY-2 suspension culture. Biotechnol Bioeng 107(2):278–289
Jeong YJ, An CH, Park SC, Pyun JW, Lee J, Kim SW, Kim H-S, Kim H, Jeong JC, Kim CY (2018) Methyl jasmonate increases isoflavone production in soybean cell cultures by activating structural genes involved in isoflavonoid biosynthesis. J Agric Food Chem 66(16):4099–4105
Joyce SM, Cassells AC, Jain SM (2003) Stress and aberrant phenotypes in vitro culture. Plant Cell Tissue Organ Cult 74(2):103–121
Jung W, Yu O, Lau SMC, O’Keefe DP, Odell J, Fader G, McGonigle B (2000) Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat Biotechnol 18(2):208–212
Karwasara VS, Dixit VK (2012) Culture medium optimization for improved puerarin production by cell suspension cultures of Pueraria tuberosa (Roxb. ex Willd.) DC. In Vitro Cell Dev Biol Plant 48(2):189–199
Kašparová M, Siatka T, Klimešová V, Dušek J (2012) New synthetic pyridine derivate as potential elicitor in production of isoflavonoids and flavonoids in Trifolium pratense L. suspension culture. Sci World J 2012:5. https://doi.org/10.1100/2012/746412
Kaufman PB, Duke JA, Brielmann H, Boik J, Hoyt JE (1997) A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: implications for human nutrition and health. J Altern Complement Med 3(1):7–12
Kieran P, MacLoughlin P, Malone D (1997) Plant cell suspension cultures: some engineering considerations. J Biotechnol 59(1–2):39–52
Kirakosyan A, Kaufman P, Nelson RL, Kasperbauer MJ, Duke JA, Seymour E, Chang SC, Warber S, Bolling S (2006) Isoflavone levels in five soybean (Glycine max) genotypes are altered by phytochrome-mediated light treatments. J Agric Food Chem 54(1):54–58
Kirchhoff J, Raven N, Boes A, Roberts JL, Russell S, Treffenfeldt W, Fischer R, Schinkel H, Schiermeyer A, Schillberg S (2012) Monoclonal tobacco cell lines with enhanced recombinant protein yields can be generated from heterogeneous cell suspension cultures by flow sorting. Plant Biotechnol J 10(8):936–944
Kokotkiewicz A, Luczkiewicz M, Kowalski W, Badura A, Piekus N, Bucinski A (2013) Isoflavone production in Cyclopia subternata Vogel (honeybush) suspension cultures grown in shake flasks and stirred-tank bioreactor. Appl Microbiol Biotechnol 97(19):8467–8477
Korsangruang S, Soonthornchareonnon N, Chintapakorn Y et al (2010) Effects of abiotic and biotic elicitors on growth and isoflavonoid accumulation in Pueraria candollei var. candollei and P. candollei var. mirifica cell suspension cultures. Plant Cell Tiss Organ Cult 103:333–342. https://doi.org/10.1007/s11240-010-9785-6
Kubeš J, Tůmová L, Martin J, Vildová A, Hendrychová H, Sojková K (2014) The production of isoflavonoids in Genista tinctoria L. cell suspension culture after abiotic stressors treatment. Nat Prod Res 28(24):2253–2263
Kumar G, Saad KR, Arya M, Puthusseri B, Mahadevappa P, Shetty NP, Giridhar P (2021) The Synergistic role of serotonin and melatonin during temperature stress in promoting cell division, ethylene and isoflavones biosynthesis in Glycine max. Curr Plant Biol 26:100206
Lagari VS, Levis S (2014) Phytoestrogens for menopausal bone loss and climacteric symptoms. J Steroid Biochem Mol Biol 139:294–301
Lee EK, Jin YW, Park JH, Yoo YM, Hong SM, Amir R, Yan Z, Kwon E, Elfick A, Tomlinson S (2010) Cultured cambial meristematic cells as a source of plant natural products. Nat Biotechnol 28(11):1213–1217
Li L, Zhang C (2006) Production of puerarin and isoflavones in cell suspension cultures of Pueraria Iobata (Willd.): effects of medium supplementation with casein hydrolysate and coconut milk. J Environ Biol 27(1):21
Li J, Xu F, Ji D, Tian C, Sun Y, Mutanda I, Ren Y, Wang Y (2021) Diversion of metabolic flux towards 5-deoxy (iso) flavonoid production via enzyme self-assembly in Escherichia coli. Metab Eng Commun 13:e00185
Liu H, Li L (2002) Cell cultures of Pueraria lobata (Willd.): growth production of isoflavones puerarin. S Afr J Bot 68(4):542–544
Liu CJ, Huhman D, Sumner LW, Dixon RA (2003) Regiospecific hydroxylation of isoflavones by cytochrome p450 81E enzymes from Medicago truncatula. Plant J 36(4):471–484
Liu Q, Liu Y, Li G, Savolainen O, Chen Y, Nielsen J (2021) De novo biosynthesis of bioactive isoflavonoids by engineered yeast cell factories. Nat Commun 12(1):6085. https://doi.org/10.1038/s41467-021-26361-1
Łuczkiewicz M, Głód D (2003) Callus cultures of Genista plants—in vitro material producing high amounts of isoflavones of phytoestrogenic activity. Plant Sci 165(5):1101–1108
Magy B, Tollet J, Laterre R, Boutry M, Navarre C (2014) Accumulation of secreted antibodies in plant cell cultures varies according to the isotype, host species and culture conditions. Plant Biotechnol J 12(4):457–467
Motolinía-Alcántara EA, Castillo-Araiza CO, Rodríguez-Monroy M, Román-Guerrero A, Cruz-Sosa F (2021) Engineering considerations to produce bioactive compounds from plant cell suspension culture in bioreactors. Plants 10(12):2762
Noh D, Choi JG, Huh E, Oh MS (2018) Tectorigenin, a flavonoid-based compound of leopard lily rhizome, attenuates UV-B-induced apoptosis and collagen degradation by inhibiting oxidative stress in human keratinocytes. Nutrients 10(12):1998
Okura A, Arakawa H, Oka H, Yoshinari T, Monden Y (1988) Effect of genistein on topoisomerase activity and on the growth of [Val 12] Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun 157(1):183–189
Ong SKL, Shanmugam MK, Fan L, Fraser SE, Arfuso F, Ahn KS, Sethi G, Bishayee A (2019) Focus on formononetin: anticancer potential and molecular targets. Cancers 11(5):611
Picherit C, Coxam V, Bennetau-Pelissero C, Kati-Coulibaly S, Davicco MJ, Lebecque P, Barlet JP (2000) Daidzein is more efficient than genistein in preventing ovariectomy-induced bone loss in rats. J Nutr 130(7):1675–1681
Qiao F, Jiang X, Cong H, Sun H, Li L, Nick P (2018) Cell shape can be uncoupled from formononetin induction in a novel cell line from Callerya speciosa. Plant Cell Rep 37(4):665–676
Ralston L, Subramanian S, Matsuno M, Yu O (2005) Partial reconstruction of flavonoid and isoflavonoid biosynthesis in yeast using soybean type I and type II chalcone isomerases. Plant Physiol 137(4):1375–1388
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21(2):182
Rani D, Meelaph T, Kobtrakul K, Vimolmangkang S (2018) Optimizing Pueraria candollei var. mirifica cell suspension culture for prolonged maintenance and decreased variation of isoflavonoid from single cell lines. Plant Cell Tissue Organ Cult 134(3):433–443
Rani D, Meelaph T, Kobtrakul K, De-Eknamkul W, Vimolmangkang S (2020) Yeast extract elicited isoflavonoid accumulation and biosynthetic gene expression in Pueraria candollei var mirifica cell cultures. Plant Cell Tissue Organ Cult 141(3):661–667
Rani D, Buranasudja V, Kobtrakul K, De-Eknamkul W, Vimolmangkang S (2021a) Elicitation of Pueraria candollei var. mirifica suspension cells promises antioxidant potential, implying antiaging activity. Plant Cell Tissue Organ Cult 145(1):29–41
Rani D, Kobtrakul K, Luckanagul JA, Thaweesest W, Rojsitthisak P, De-Eknamkul W, Vimolmangkang S (2021b) Differential gene expression levels, chemical profiles, and biological activities of Pueraria candollei var. mirifica callus cultures at different growth stages. Plant Cell. Tissue Organ Cult 147:61–72
Reis A, Scopel M, Zuanazzi JAS (2018) Trifolium pratense: friable calli, cell culture protocol and isoflavones content in wild plants, in vitro and cell cultures analyzed by UPLC. Rev Bras 28(5):542–550
Reiter RJ, Paredes SD, Korkmaz A, Jou MJ, Tan DX (2008) Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip Toxicol 1(2):137
Saisavoey T, Thongchul N, Sangvanich P, Karnchanatat A (2014) Effect of methyl jasmonate on isoflavonoid accumulation and antioxidant enzymes in Pueraria mirifica cell suspension culture. J Medi Plants Res 8(9):401–407
Sansanelli S, Zanichelli D, Filippini A, Ferri M, Tassoni A (2014) Production of free and glycosylated isoflavones in in vitro soybean (Glycine max L.) hypocotyl cell suspensions and comparison with industrial seed extracts. Plant Cell Tissue Organ Cult 119(2):301–311
Santos RB, Abranches R, Fischer R, Sack M, Holland T (2016) Putting the spotlight back on plant suspension cultures. Front Plant Sci 7:297
Sato M, Hosokawa M, Doi M (2011) Somaclonal variation is induced de novo via the tissue culture process: a study quantifying mutated cells in Saintpaulia. PLoS ONE 6(8):e23541
Schroder J (1999) The chalcone/stibene synthase-type family of condensing enzymes. Comprehensive natural products chemistry. Polyketides Other Second Matab Include Fatty Acids Their Deriv 1:749–771
Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD (2005) S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr 81(5):1072–1079
Shaaltiel Y, Bartfeld D, Hashmueli S, Baum G, Brill-Almon E, Galili G, Dym O, Boldin-Adamsky SA, Silman I, Sussman JL (2007) Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J 5(5):579–590
Sharma V, Ramawat KG (2013) Isoflavonoids. In: Ramawat K, Mérillon JM (eds) Natural products. Springer, Berlin Heidelberg
Sharma V, Goyal S, Ramawat KG (2009) Scale up production of isoflavonoids in cell suspension cultures of Pueraria tuberosa grown in shake flasks and bioreactor. Eng Life Sci 9(3):267–271
Shinde A, Malpathak N, Fulzele D (2008) Production of phytoestrogens by plant cell and tissue cultures: recent scenario and exciting prospects. Pharmacogn Rev 2(3):43
Shinde AN, Malpathak N, Fulzele DP (2009) Optimized production of isoflavones in cell cultures of Psoralea corylifolia L. using elicitation and precursor feeding. Biotechnol Bioprocess Eng 14(5):612–618
Si H, Liu D (2008) Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J Nutr 138(2):297–304
Sibao C, Dajian Y, Shilin C, Hongxi X, Chan AS (2007) Seasonal variations in the isoflavonoids of Radix Puerariae. Phytochem Anal Int J Plant Chem Biochem Tech 18(3):245–250
Simmler C, Pauli GF, Chen SN (2013) Phytochemistry and biological properties of glabridin. Fitoterapia 90:160–184
Simons R, Gruppen H, Bovee TF, Verbruggen MA, Vincken J-P (2012) Prenylated isoflavonoids from plants as selective estrogen receptor modulators (phytoSERMs). Food Funct 3(8):810–827
Sivanandhan G, Selvaraj N, Ganapathi A, Manickavasagam M (2014) Enhanced biosynthesis of withanolides by elicitation and precursor feeding in cell suspension culture of Withania somnifera (L.) Dunal in shake-flask culture and bioreactor. PLoS ONE 9(8):e104005
Skalicky M, Kubes J, Hejnak V, Tumova L, Martinkova J, Martin J, Hnilickova H (2018) Isoflavones production and possible mechanism of their exudation in Genista tinctoria L. suspension culture after treatment with vanadium compounds. Molecules 23(7):1619
Smetanska I (2008) Production of secondary metabolites using plant cell cultures. Food biotechnology. Springer, Berlin, Heidelberg, pp 187–228
Smith ML, Mason HS, Shuler ML (2002) Hepatitis B surface antigen (HBsAg) expression in plant cell culture: kinetics of antigen accumulation in batch culture and its intracellular form. Biotechnol Bioeng 80(7):812–822
Tekoah Y, Shulman A, Kizhner T, Ruderfer I, Fux L, Nataf Y, Bartfeld D, Ariel T, Gingis-Velitski S, Hanania U (2015) Large-scale production of pharmaceutical proteins in plant cell culture—the protalix experience. Plant Biotechnol J 13(8):1199–1208
ten Hoopen HJ, van Gulik WM, Schlatmann JE, Moreno PR, Vinke J, Heijnen J, Verpoorte R (1994) Ajmalicine production by cell cultures of Catharanthus roseus: from shake flask to bioreactor. Plant Cell Tissue Organ Cult 38(2):85–91
Titova M, Kochkin D, Fomenkov A, Ivanov I, Kotenkova E, Kocharyan G, Dzhivishev E, Mekhtieva N, Popova E, Nosov A (2021) Obtaining and characterization of suspension cell culture of Alhagi persarum Boiss. et Buhse: a producer of Isoflavonoids. Russ J Plant Physiol 68(4):652–660
Tůmová L, Tůma J, Hendrychova H (2014) Effect of ultrasound on the isoflavonoid production in Genista tinctoria L. suspension cultures. Pharmacogn Mag 10(Suppl 2):S425
Udomsuk L, Juengwattanatrakul T, Jarukamjorn K, Putalun W (2012) Increased miroestrol, deoxymiroestrol and isoflavonoid accumulation in callus and cell suspension cultures of Pueraria candollei var. mirifica. Acta Physiol Plant 34(3):1093–1100
Udomsin O, Yusakul G, Kraithong W, Udomsuk L, Kitisripanya T, Juengwatanatrakul T, Putalun W (2019) Enhanced accumulation of high-value deoxymiroestrol and isoflavonoids using hairy root as a sustainable source of Pueraria candollei var. mirifica. Plant Cell Tissue Organ Cult 136(1):141–151
Udomsin O, Yusakul G, Kitisripanya T, Juengwatanatrakul T, Putalun W (2020) The deoxymiroestrol and isoflavonoid production and their elicitation of cell suspension cultures of Pueraria candollei var. mirifica: from shake flask to bioreactor. Appl Biochem Biotechnol 190(1):57–72
Veitch NC (2007) Isoflavonoids of the Leguminosae. Nat Prod Rep 24(2):417–464
Veremeichik G, Grigorchuk V, Silanteva S, Shkryl Y, Bulgakov D, Brodovskaya E, Bulgakov V (2019) Increase in isoflavonoid content in Glycine max cells transformed by the constitutively active Ca2+ independent form of the AtCPK1 gene. Phytochemistry 157:111–120
Veremeichik G, Grigorchuk V, Butovets E, Lukyanchuk L, Brodovskaya E, Bulgakov D, Bulgakov V (2021) Isoflavonoid biosynthesis in cultivated and wild soybeans grown in the field under adverse climate conditions. Food Chem 342:128292
Vitale DC, Piazza C, Melilli B, Drago F, Salomone S (2013) Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet 38(1):15–25
Wang H, Li Q, Chen H (2012) Genistein affects histone modifications on Dickkopf-related protein 1 (DKK1) gene in SW480 human colon cancer cell line. PLoS ONE 7(7):e40955
Wang S, Wang Y, Pan MH, Ho CT (2017) Anti-obesity molecular mechanism of soy isoflavones: weaving the way to new therapeutic routes. Food Funct 8(11):3831–3846
Wu Z, Song L, Feng S, Liu Y, He G, Yioe Y, Liu SQ, Huang D (2012) Germination dramatically increases isoflavonoid content and diversity in chickpea (Cicer arietinum L.) seeds. J Agric Food Chem 60(35):8606–8615
Xiao Y, Zhang S, Tong H, Shi S (2018) Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother Res 32(3):384–394
Xing T, Wang XJ, Malik K, Miki BL (2001) Ectopic expression of an Arabidopsis calmodulin-like domain protein kinase-enhanced NADPH oxidase activity and oxidative burst in tomato protoplasts. Mol Plant Microbe Interact 14(10):1261–1264
Xu J, Ge X, Dolan MC (2011) Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures. Biotechnol Adv 29(3):278–299
Xu J, Zhang N (2014) On the way to commercializing plant cell culture platform for biopharmaceuticals: present status and prospect. Pharm Bioprocess 2(6):499–518. https://doi.org/10.4155/pbp.14.32
Yancheva S, Kondakova V (2018) Plant tissue culture technology: present and future development. Bioprocessing of plant in vitro systems. Phytochemical reference series. Springer, Cham, pp 39–63
Ye H, Huang LL, Chen SD, Zhong JJ (2004) Pulsed electric field stimulates plant secondary metabolism in suspension cultures of Taxus chinensis. Biotechnol Bioeng 88(6):788–795
Zacharius RM, Kalan EB (1990) Isoflavonoid changes in soybean cell suspensions when challenged with intact bacteria or fungal elicitors. J Plant Physiol 135(6):732–736
Zhang T, We C, Ren Y, Feng C, Wu H (2017) Advances in airlift reactors: modified design and optimization of operation conditions. Rev Chem Eng 33(2):163–182
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23(4):283–333
Zhu Y, Chen Y, Zhang X, Xie G, Qin M (2020) Copper stress-induced changes in biomass accumulation, antioxidant activity and flavonoid contents in Belamcanda chinensis calli. Plant Cell Tissue Organ Cult 142:299–311
Acknowledgements
DR was supported by the Rachadapisek Sompote Fund for Postdoctoral Fellowships from the Graduate School of Chulalongkorn University, Thailand.
Funding
Funding was provided by Rachadapisek Sompote Fund for Postdoctoral Fellowships from the Graduate School of Chulalongkorn University, Thailand.
Author information
Authors and Affiliations
Contributions
DR Conceptualization, investigation, writing original draft and editing. SV Conceptualization, reviewing draft, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rani, D., Vimolmangkang, S. Trends in the biotechnological production of isoflavonoids in plant cell suspension cultures. Phytochem Rev 21, 1843–1862 (2022). https://doi.org/10.1007/s11101-022-09811-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-022-09811-6