Abstract
Purpose
Hyperaccumulators are plants with the ability to tolerate and accumulate high concentrations of potentially phytotoxic metals. The Australian legume Crotalaria novae-hollandiae accumulates remarkably high concentrations of zinc (Zn), cadmium (Cd) and copper (Cu) in its shoots when growing on metalliferous (Zn-Cd ‘calamine’) soils. This study aimed to investigate zinc-cadmium tolerance in C. novae-hollandiae and to compare it with the closely related, but non-metalliferous, C. cunninghamii.
Methods
Crotalaria cunninghamii and C. novae-hollandiae were exposed to Zn (3–1000 μM) and Cd (0–60 μM) treatments in hydroponics culture. At the end of the experiment, harvested plants were segmented into roots, old and young stems, old and young leaves for elemental analysis with Inductively coupled plasma atomic emission spectroscopy (ICP-AES). Laboratory-based micro-X-ray fluorescence (μXRF) analysis was used to elucidate elemental distribution in a shoot and in leaflets.
Results
Crotalaria cunninghamii accumulated up to 1210 μg Zn g−1 and 47.6 μg Cd g−1 in its leaves, with a 75% reduction in biomass in the Zn treatment. Crotalaria novae-hollandiae accumulated up to 16,600 μg Zn g−1 and 1250 μg Cd g−1, with a 70% increase in biomass when exposed to Zn. The species both exhibited chlorosis and stunted growth in the Cd treatments, while only C. cunninghamii exhibited toxicity symptoms in Zn treatment.
Conclusions
Crotalaria novae-hollandiae has limited tolerance for Cd and based on the accumulation and distribution of foliar Zn and Cd it is suspected that C. novae-hollandiae has different uptake and tolerance mechanisms when compared to other widely studied Zn-Cd hyperaccumulators (such as Noccaea caerulescens and Arabidopsis halleri).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some trace elements, such as zinc (Zn), are micronutrients that are essential for plant growth, whereas others such as cadmium (Cd) are not essential and can be phytotoxic, even at low concentrations (Foy et al. 1978). As a micronutrient, Zn plays a key role as a cofactor in numerous enzymes, most notably a structural role in proteins when interacting with nucleic acids (Clemens 2019). Highly elevated Zn and Cd concentrations in soils can be harmful to plants, causing physiological symptoms such as foliar chlorosis, and ultimately death (Foy et al. 1978). Most plants exhibit Zn toxicity at foliar concentrations of >500 μg Zn g−1 (Chaney 1993). Non-essential transition metals, such as Cd, cause significant cellular damage once their limited binding capacity is exhausted, as they are not guided to sites used by the homeostatic system (Chen et al. 2017). Metallophytes can tolerate higher concentrations of potentially phytotoxic metals than ‘normal’ plants (Baker 1981; Baker 1987). Due to their immobility plants adapt their physiological function in response to environmental factors over generations (Antonovics et al. 1971; Baker et al. 2010; Ernst 2006). Plants that have evolved specialised tolerance mechanisms over millennia in natural metalliferous soils, such as gossans and ultramafic soils, are so-called ‘obligate or true metallophytes’. Whereas ‘pseudo-metallophytes’ are metal tolerant strains of common species that have evolved limited resistance to metals after exposure to (man-made) metal enriched soils (Whiting et al. 2004). The majority of metallophytes are ‘facultative’ that thrive on both metalliferous soils and non-metalliferous soils, whilst ‘obligate metallophytes’ are restricted to metalliferous soils (Baker 1981; Baker and Brooks 1989; Pollard et al. 2014).
A subset of metallophytes are hyperaccumulators which can accumulate metal or metalloid concentrations at orders of magnitude greater levels than non-accumulators (van der Ent et al. 2013). The hyperaccumulation threshold depends on the metal/metalloid, in the case of Zn the hyperaccumulation threshold is 3000 μg Zn g−1 whilst for Cd it is 100 μg Cd g−1 (Krämer 2010; van der Ent et al. 2013). Hyperaccumulation is a rare occurrence which occurs in approximately 0.2% of angiosperms (Cappa and Pilon-Smits 2014). Of the ~700 globally known hyperaccumulators ~3% (~20 species) are Zn hyperaccumulators and ~1% (~7 species) are Cd hyperaccumulators (Reeves et al. 2017). A more recent study identified 10 potential Zn hyperaccumulators in the families Celastraceae, Cunoniaceae, Salicaceae, Rubiaceae and Proteaceae from Papua New Guinea, with Mitragyna speciosa (Rubiaceae) accumulating the highest Zn concentrations (22,990 μg Zn g−1) (Do et al. 2020). However, this was discovered via herbarium X-ray Fluorescence scanning, and still needs to be confirmed by field sampling. About half of the Zn hyperaccumulating species are in the Brassicaceae family (Balafrej et al. 2020; Reeves et al. 2017), including the two most intensively studied model herbaceous species Arabidopsis halleri and Noccaea caerulescens (Assunção et al. 2003; Lochlainn et al. 2011; Mishra et al. 2017; Nouet et al. 2015; Szopiński et al. 2020; Zhao et al. 2000). Arabidopsis halleri and N. caerulescens can accumulate extremely high concentrations of Zn and Cd (in A. halleri up to 53,900 μg Zn g−1 and up to 3640 μg Cd g−1; Stein et al. 2017) and/or Ni (in N. caerulescens up to 16,200 μg Ni g−1; Reeves et al. 2001) when growing on Zn-Pb-Cd-enriched calamine soils or on Ni-Co-Mn-enriched ultramafic soils. Sedum plumbizincicola (Crassulaceae) is another Zn-Cd hyperaccumulator, which can accumulate 7010 μg Cd g−1 and 18,400 μg Zn g−1 in its aerial tissues (Cao et al. 2014). Noccaea caerulescens, as well as A. halleri have the ability to hyperaccumulate Zn when growing on soils with only ‘background Zn concentrations’ (e.g., <300 mg Zn kg−1) (Reeves et al. 2001). The only other known Zn hyperaccumulator which hyperaccumulates Zn on soils with background Zn concentrations is Dichapetalum gelanioides subsp. sumatranum which is capable of attaining up to 10,730 μg Zn g−1 in its leaves on a soil with just 20 mg Zn kg−1 (Nkrumah et al. 2018).
The legume (Fabaceae) genus Crotalaria has ~600 species occurring mainly in (sub)tropical regions of Africa and Australia with 36 species recorded from the latter (Holland 2002). Some African species of Crotalaria are metallophytes that tolerate high soil cobalt (Co) and Cu concentrations (>5 μg Co g−1 and/or > 50 μg Cu g−1) in the African ‘Copperbelt’ on metalliferous soils (Brooks et al. 1977; Brooks 1979). Crotalaria cobalticola is a Cu-Co obligate hyperaccumulator found in the DR Congo (Brooks et al. 1977; Brooks 1979). In Australia, C. novae-hollandiae was originally discovered to be a Zn hyperaccumulator accumulating up to 8975 μg Zn g−1 from the Dugald River Zn-Pb-Cd gossan in central Queensland (Farago et al. 1977). Recent investigations on the Dugald River site have revealed that C. novae-hollandiae is a polymetallic Zn-Cu-Cd hyperaccumulator that can accumulate up to 16,200 μg Zn g−1, 545 μg Cu g−1 and 170 μg Cd g−1 (Tang et al. 2021). As a species, C. novae-hollandiae occurs across the Northern areas of Australia, and an assessment of over 200 C. novae-hollandiae specimens at the Queensland Herbarium (BRI) using portable X-ray fluorescence (pXRF) for foliar metal concentrations was conducted to identify other instances of metal hyperaccumulation. All samples, bar two, had <20 μg Zn g−1 and the two samples were above the Zn hyperaccumulation threshold (>3000 μg g−1), both of which were subsequently identified to be growing on Zn-Pb gossans 250 km apart (Tang et al. 2021). One of these sites is the Dugald River gossan which is a polymetallic metalliferous outcrop with extremely high soil concentrations of Zn, Pb, Cd and Cu from which a range of metallophytes and hyperaccumulators is known (Farago et al. 1977; Tang et al. 2021). Polymetallic hyperaccumulation of metals is rare, and most hyperaccumulators only accumulate one metal, while some hyperaccumulate two or more metals simultaneouslym such as Zn and Cd (Jhee et al. 2006; McGrath et al. 2001; Yang et al. 2004). It is possible that metal tolerance evolved as an ancestral trait and that many (Australian) species of Crotalaria share the same genetic trait for metal tolerance, but this remains to be tested experimentally.
Crotalaria novae-hollandiae can provide unique insights into polymetallic uptake and hyperaccumulation. As a 2 m tall ligneous perennial legume species that grows across subtropical savannahs on metalliferous and non-metalliferous soils, it is vastly different to the majority of Zn-Cd hyperaccumulators which are small herbaceous plants from temperate climate regions (Holland 2002). This study aimed to investigate the Zn-Cd uptake response of C. novae-hollandiae and a closely related Australian Crotalaria species with similar morphology and similar habitat distribution, C. cunninghamii, to gain insights into: (i) the distribution of Zn-Cd within plant tissue; (ii) the physiological impacts of Zn-Cd; and (iii) the inter-elemental relationships within plant tissue under different treatments.
Materials and methods
Plant material sources and propagation
Crotalaria novae-hollandiae subsp. novae-hollandiae (New Holland Rattlepod) is a native 2 m shrub widespread across the northern half of Australia, growing in sandy soils in warm regions (Fig. 1). Crotalaria cunninghamii (Regal Birdflower) is native and widespread across inland northern Australia. The species is a perennial shrub which can grow up to 3 m in height. Crotalaria novae-hollandiae seeds were collected at the Dugald River gossan (Zn-Pb-Cu), western Queensland, Australia in the second half of June 2018. Upon collection, the seeds were dried at 40 °C for two days and stored in airtight ZIP-lock bags in a desiccator cabinet until used. Seeds of C. novae-hollandiae were grown to mature plants in the glasshouse. Cuttings were then produced by cutting 10–15 cm stem lengths, reducing leaves, and striking in Clonex Red Root Hormone (8 g/L Indole Butyric Acid, Yates, DuluxGroup Pty) and rooted in moistened 50% fine perlite and 50% vermiculite over 30 days. The rooted cuttings were then introduced into hydroponics culture. Crotalaria cunninghamii seeds were purchased from the Nindethana Seed Company (King River WA, Australia). Crotalaria cunninghamii was germinated in a mix of perlite, and vermiculite, and seedlings watered with demineralized water once per day two weeks prior to being transplanted into the hydroponic system. The Crotalaria species were exposed to treatment once plants grew to an average height of 17.5 cm (15–20 cm).
Hydroponics and metal dosing experimentation
The hydroponic system consisted of four separate rectangular containers (21 cm height × 30 cm width × 40 cm length; 25 L each) filled with a nutrient solution that contained: K (2.5 mM as KNO3), Ca (2.5 mM as Ca(NO3)2·4H2O), P (0.1 mM as K2HPO4), Mg (1 mM as MgSO4·7H2O), Cl (75 μM as KCl), B (10 μM as H3BO3), Mn (4 μM as MnSO4·H2O), Cu (0.5 μM CuSO4·5 H2O), Mo (0.2 μM as Na2MoO4·2 H2O), and Fe (20 μM as C20H20N2O6FeK (FeHBED)) based on Brown et al. (1995). The solution was kept aerated using a 25 cm long air stone at the bottom of each container. The treatment was divided into two phases. The initial phase was to assess the Zn tolerance and uptake of the species based on diethylenetriaminepentaacetic acid (DTPA) extractable Zn soil concentrations found at the Dugald River gossan (min: 1.26 μg Zn g−1; max: 1030 μg Zn g−1; median: 31.6 μg Zn g−1) (Tang et al. 2021). The second phase was to assess Cd tolerance, uptake, and its interaction with Zn at optimum Zn concentrations. The nutrient solution for C. novae-hollandiae was spiked with four levels of Zn treatment using ZnSO4·7H2O at concentrations of 3 μM, 30 μM, 300 μM and 1000 μM. Once the optimum had been identified, a new experiment consisting of four Cd treatments using CdSO4 with optimum Zn concentrations for each species was conducted. The Cd dose rates were: 0 μM, 5 μM, 20 μM and 60 μM. As C. cunninghamii is not of metalliferous origin, a reduced concentration of treatment was used (Phase 1 (Zn): 3 μM, 30 μM, 60 μM, 300 μM; Phase 2 (Cd): 0 μM, 5 μM, 10 μM and 20 μM). To calculate free ionic activity of Zn and Cd a simulation was performed using the software GEOCHEM-EZ 1.0 (available at http://www.PlantMineralNutrition.net). Depending on the treatment, the results show that 67–85% of Zn2+ was present as the free ion, whereas 85% of Cd2+ was present as the free ion and the remainder complexed with HBED or SO4− (Supp Tables 1, 2 and 3). The pH was automatically maintained at the set value of pH 5.8 ± 0.1 with 0.1 M KOH solution using a Bluelab Pro Controller with PeriPod M3 dosing pumps (Bluelab Corporation, Tauranga, New Zealand). The nutrient solutions were changed completely once a week. In each treatment, 12 plants were grown for C. novae-hollandiae, and 8 plants were grown for C. cunninghamii (to reduce overcrowding due to its larger size), in 5 cm round plastic baskets with a foam disk to allow the roots to be immersed in the nutrient solution. Crotalaria novae-hollandiae were grown for 30 days with a 12-12 hr. light-dark photoperiod, under high intensity LED lights Phytomax 400 (Black Dog LED, Niwot, CO, USA), with a photosynthetic photon flux density of 550 μmol m−2 s−1 (measured with an Apogee MQ-500 instrument) at 22/20 °C day/night. Crotalaria cunninghamii had 2-week growth period at pre-treatment levels until plants were of comparable size to C. novae-hollandiae cuttings (approximately 20 cm in height) before being exposed to the treatment followed by a 30-day growth period. At the end of the experiment, the plants were harvested and divided into young and old leaves, young and old stems, pre-treatment stems, and roots. The plant samples were thoroughly rinsed with de-ionised water and dried at 60 °C for 72 hrs in a dehydrating oven.
Laboratory-based μXRF elemental mapping
After 12 weeks of growth in the hydroponics system, elemental maps were obtained on selected leaf samples using μXRF analysis. Leaves of the same age were used as a representative of the plant, the 4th set from the youngest is considered the youngest mature leaf. The University of Queensland microXRF facility contains a modified IXRF ATLAS X system with a 50 W XOS microfocus Mo-target tube producing 17.4 keV X-rays (flux of 2.2 × 108 photons s−1) focussing to 25 μm FWM and two silicon drift detectors (SDD) of 150 mm2 coupled to a XIA Mercury X4 signal processing unit. Samples for analysis were mounted between 4 μm Ultralene thin film stretched over a Perspex frame and sealed airtight to limit evaporation and analysed within 10 minutes after excision. Measurements were conducted atmospheric temperature (~20 °C), using the Mo 25 μm X-ray source at a 50 kV with a per-pixel dwell of 100 ms. The XRF spectra on the UQ microXRF facility were acquired using the instrument control package, Iridium (IXRF systems) from the sum of counts at the position of the principal K-line peak for each element. These were each exported into ImageJ as greyscale 8-bit TIFF files and displayed using ImageJ’s “Fire” lookup table.
Elemental analysis of plant material samples (ICP-AES)
Dried plant material samples (100 mg) were weighed into 6 mL polypropylene tubes, pre-digested for 24 hours using 2 mL 70% HNO3 and then digested at 70 °C for 1 hour followed by an additional 1 hour at 125 °C. The samples were brought to volume (10 mL) using ultra-pure water, filtered, and analysed with inductively coupled plasma atomic emission spectroscopy (ICP-AES, Thermo Scientific iCAP 7400) as described earlier (Tang et al. 2021).
Data processing and statistical analysis
Data obtained from ICP-AES was cleaned and organised in Excel (Microsoft Corporation 2021) and interpreted in R (R Core Team 2020). All values are reported on a dry weight (DW) basis. Results below the Limit of Detection (LOD) were substituted using LOD/√2 in preparation for statistical analysis if +80% of the data were above the LOD (Hornung and Reed 1990). Kruskal-Wallis Chi-squared test was used to identify whether there are any differences in Zn/Cd uptake in plant parts between treatments. A Dunn’s test with Bonferroni correction was used as a post-hoc test to identify where the differences occur. Translocation factor was used to test the efficiency of translocating Zn and Cd from roots to leaf tissues using the calculation: translocation factor metal = old leaf metal / root metal. A Wilcox rank sum test was used to test the differences in translocation factor between species and treatment. A Principal Components Analysis using the prcomp() function in R was conducted to test the inter elemental relationships of metals between treatments.
Results
Zinc and Cd dose effects on overall plant health
Representative images of the Crotalaria species in all treatments are shown in Fig. 2. For C. novae-hollandiae, the root morphology changed substantially in the 1000 μM Zn treatment compared to the control, showing signs of thickened stubbed lateral roots. There were no clear signs of chlorosis in the Zn treatments; however, there were fewer leaves at the highest Zn concentration dose level. When exposed to Cd, the leaves became chlorotic, increasingly with an increase in treatment level. Plants were stunted with curled leaves in the highest Cd treatment level. For C. cunninghamii, there was a major decrease in fine lateral roots in both the Zn and Cd treatments. In the 30 μM Zn treatment, all new foliage was chlorotic. When exposed to Cd, C. cunninghamii exhibited stunted growth and chlorosis in the leaves.
Biomass production, and Zn and cd concentrations in plant tissues
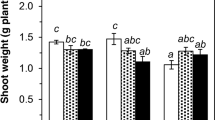
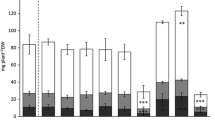
The biomass and metal concentrations in plant tissues are given in Figs. 3, 4 and 5, Supp Tables 4, 5, 6 and 7 and Supp Fig. 3. In the control Zn treatment, C. cunninghamii grew 320% more biomass than its starting weight compared to C. novae-hollandiae which only increased 63%. However, the foliar biomass of C. cunninghamii decreased by 80% and 75% in the Cd and Zn treatments, respectively, compared to the control. The majority of Zn accumulated in the root (~5270 μg g−1), followed by old stem (~2120 μg g−1), old leaves (~1210 μg g−1), young stem (~560 μg g−1), and young leaves (~795 μg g−1). Zinc concentrations in leaves were well below the hyperaccumulation threshold of foliar 3000 μg Zn g−1. For Cd treatment, Cd was efficiently translocated from root to shoot while Zn was accumulated in the roots before being translocated to the shoots in the Zn treatment. Cadmium accumulation was primarily in the roots (~240 μg g−1) followed by the old stem (~200 μg g−1), young stem (~110 μg g−1), old leaves (~47.6 μg g−1), and young leaves (~19.4 μg g−1). Zinc accumulation was significantly lower in the roots (−70%) and old leaves (−80%) but higher in the old stem once Cd was introduced (difference in median to the control for old stem Zn uptake: +60%, +100%, +130%; 5, 10, 20 Cd μM, respectively).
The biomass of C. novae-hollandiae increased with increasing Zn treatment, with an optimal Zn concentration being between 30 and 300 Zn μM. The average foliar biomass was 70% greater than the control between the 30–300 Zn μM treatment range. However, foliar biomass decreased significantly at 1000 Zn μM by 30% compared to the control. Second to roots (~30,600 μg g−1), C. novae-hollandiae accumulated most Zn in the old leaves (~16,600 μg g−1), followed by old stems (~9160 μg g−1), young leaves (~6660 μg g−1), and young stems (~3680 μg g−1). A significant amount of overlap in Zn uptake occurred in the roots between the treatments with root saturation occurring between 30 and 300 Zn μM (Supp Table 8). All other plant parts accumulated more Zn with increasing Zn treatment (3–1000 μM: 40-fold increase in μg Zn g−1). Hyperaccumulation of Zn was achieved at 150 Zn μM (3830 μg g−1). Similar to C. cunninghamii, C. novae-hollandiae decreased in biomass when exposed to the Cd treatment (−60%). However, the decrease in biomass was less than in C. cunninghamii despite C. novae-hollandiae being exposed to 300-fold more Cd (Supp Fig. 8). Cadmium accumulation was approximately 5-fold greater in C. novae-hollandiae than in C. cunninghamii for the same treatment level (20 μM). Root Cd accumulation was uniform between 20 and 60 Cd μM. However, Cd accumulation in aerial tissues increased significantly between 20 and 60 Cd μM when compared to 5–20 Cd μM (Supp Table 8). Unlike C. cunninghamii, C. novae-hollandiae achieved Cd accumulation beyond the hyperaccumulation threshold (>100 μg Cd g−1) in its leaves at 5 Cd μM, with up to 1250 μg Cd g−1 in old leaves in the 60 Cd μM treatment. Cadmium exposure decreased Zn accumulation in the roots (0–60 Cd μM: −75% Zn). However, a test of difference shows that Zn concentrations remained relatively stable between the aerial tissues (Supp Table 8).
Inter-elemental relationships in plant tissues
The results of the principal components analysis (PCA) showing inter-elemental relationships and effects of treatment are given in Fig. 6 and Supp Table 10. Groupings primarily influenced by the treatments are clear: for the Zn treatment of C. novae-hollandiae, the first principal component (PC) explains 50% of the variation which is driven by Zn, PC2 explains an additional 17% of the variation which is driven by phosphorus (P) and potassium (K). For C. cunninghamii in Zn treatment, PC1 is also driven by Zn but only accounts for 35% of the variation, and PC2 explains an additional 33% of the variation which is driven by calcium (Ca)-manganese (Mn)-K. A large proportion of the variance (89%) is explained by Cd for C. novae-hollandiae, PC2 accounts for an additional 5% variation which is driven by two groups, Zn and Mg-Mn. Unlike C. novae-hollandiae, PC1 in C. cunninghamii accounts for 54% for the variation is still driven by Zn but with stronger influences from Ca and Mn and PC2 which accounts for an additional 30% variation is driven by Cd.
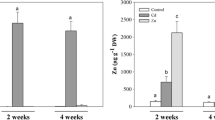
Zinc and Cd translocation from roots to shoots
The results for the translocation factors are given in Fig. 7. In the Zn treatment, C. cunninghamii had decreasing translocation factors with an increase in Zn exposure, whilst C. novae-hollandiae had increasing translocation factors with an increase in Zn exposure. This is reflected by significant differences in the translocation factors between the species at 3 μM Zn (C. cunninghamii translocation factor: 0.713; C. novae-hollandiae translocation factor: 0.157) and the 300 μM Zn treatment (C. cunninghamii translocation factor: 0.258; C. novae-hollandiae translocation factor: 0.808), whilst translocation factors were both relatively low and not significantly different in 30 μM Zn (C. cunninghamii: 0.1; C. novae-hollandiae: 0.151) (Supp Table 9). For C. cunninghamii, the translocation of Cd in Cd treatment remained low (<0.1) and decreased with an increase in Cd treatment (5 μM Cd: 0.0462; 20 μM Cd: 0.00716). There was a significant difference in Cd translocation factors between both species at the respective Cd treatment levels (p < 0.05), with C. novae-hollandiae having relatively higher translocation factors (Supp Table 9). There was no difference in the translocation factors of Cd between 5 and 20 Cd μM for C. novae-hollandiae but there was an increase in translocation factors between 20 and 60 Cd μM (Supp Table 9). There was no difference in the translocation factors for Zn for both species in Cd treatment, with only a marginally significant difference in 5 Cd μM with C. cunninghamii having a higher translocation factor.
Micro-X-ray fluorescence spectrometry elemental mapping
Laboratory-based micro-X-ray fluorescence (μXRF) analysis results for leaves are shown in Figs. 8, 9 and 10. In Fig. 8, it can be seen that C. novae-hollandiae had no obvious signs of toxicity in the colour photo (such as yellowing/chlorosis). The elemental maps show that Zn was more enriched in the old leaves compared to young leaves. Zinc was evenly distributed in the leaf of C. novae-hollandiae in the 30 μM Zn treatment. However, in the 150 μM Zn treatment (Fig. 9), Zn accumulated in clusters within the lamina. Zinc was more evenly distributed at 300 μM Zn, however, at a much higher intensity compared to 30 μM Zn and high concentrations in the margin. In the 1000 μM Zn treatment, Zn decreased in the mid-rib followed by the veins, with the highest Zn in the lamina and margin. Under 30–150 μM Zn, Ca was low in the midrib and veins, but high in the lamina. However, at 300–1000 μM Zn, C. novae-hollandiae had much lower concentrations of Ca in the lamina. There was no major change in K distribution in the leaf of C. novae-hollandiae under any of the Zn treatments. It can be seen in the colour photo (Fig. 9), that C. cunninghamii was chlorotic in all of Zn treatments (30–300 μM Zn). In the lower Zn treatments (3–30 μM Zn), Zn concentrations were low throughout the leaf. Zinc was slightly enriched in the midrib, veins, and the lamina at 300 μM Zn. There was significant variability in Ca and K distribution and concentration between the treatments with no clear visible trends.
There were no differences in Zn distribution in the leaf of C. cunninghamii in the respective Cd treatments (Fig. 10). Zinc was enriched in the veins, but relatively depleted in the lamina. Leaf Ca and K were enriched in the base and areas adjacent to the veins when exposed to Cd treatments. For C. novae-hollandiae, Zn accumulation in the lamina decreased but increased in the midrib and veins when exposed to Cd treatments. In the highest Cd treatment (60 μM Cd), Ca was highly concentrated in the lamina but low in the mid-rib and veins whereas K was very low in the lamina and higher in the mid rib. In the lower Cd treatments (0–20 μM Cd), Ca and K had similar enrichment patterns.
Discussion
In hydroponics, Zn accumulates primarily in the old leaves of C. novae-hollandiae. This may be an adaptation to reduce Zn toxicity, by reducing the total concentration of Zn within the plant. The non-metallophyte C. cunninghamii had a different response to Zn; despite Zn accumulation being restricted to the roots with foliar concentrations below the level of toxicity, C. cunninghamii still showed signs of toxicity (e.g., chlorosis and stunted growth). Cadmium did not stimulate biomass growth in C. novae-hollandiae. However, C. novae-hollandiae accumulated very high foliar Cd concentrations. Like C. cunninghamii, C. novae-hollandiae decreased in biomass when exposed to Cd treatment, though at a much lower rate, which may be indicative of some level of tolerance. For C. cunninghamii, Cd accumulation was largely restricted to the stems with lower foliar concentrations.
Crotalaria novae-hollandiae accumulated foliar Zn (up to 16,600 μg Zn g−1) similar to what was found in nature at the Dugald River gossan (~16,200 μg Zn g−1) (Tang et al. 2021). However, Cd shoot concentrations well exceeded in-situ concentrations (maximum foliar concentration at Dugald River gossan: 170 μg Cd g−1 and maximum foliar concentration in hydroponics: 1250 μg Cd g−1) due to Cd exposure being significantly greater in the experimental treatment than at the Dugald River (soil DTPA-extractable Cd at Dugald River: 6.59 μg Cd g−1; hydroponics dose rate: 60 μM Cd) (Tang et al. 2021). Crotalaria novae-hollandiae had a much higher tolerance to Zn (30–1000 μM Zn), whereas C. cunninghamii exhibited signs of toxicity with chlorotic leaves in the 30 μM Zn treatment. Crotalaria cunninghamii is generally a faster growing species than C. novae-hollandiae; however, Zn treatment reduced growth, whilst it strongly stimulated growth in C. novae-hollandiae. Stimulatory effects on plant biomass caused by (toxic) metals have been observed in other metallophytes, but the physiological causes are still uncertain (Tang et al. 2009). One hypothesis postulated for the stimulatory response of metallophytes/hyperaccumulators to metals is an extreme form of hormesis (Roosens et al. 2003; Tang et al. 2009; Salinitro et al. 2021).
Laboratory μXRF elemental maps of C. novae-hollandiae shoot and leaflets show that old leaves are major Zn sinks, which suggests limited phloem redistribution. One physiological advantage of allocating Zn to the old leaves is the potential for it to remove excess Zn with natural leaf shedding (Ernst et al. 1992). Crotalaria cunninghamii is less tolerant to Zn whereas C. novae-hollandiae shows no visual signs of Zn toxicity, which suggests an inherent ability to inhibit Zn accumulation in the chloroplast (Bayçu et al. 2017; Sitko et al. 2017; Szopiński et al. 2020). Crotalaria novae-hollandiae shows a clearer trend of Zn being distributed into the lamina whilst C. cunninghamii restricts the majority of Zn within its veins. Vacuolar compartmentalisation and organic acid complexation are two key mechanisms of Zn tolerance in plants (Chardonnens et al. 1999; Clemens 2001; Ernst et al. 1992; Hall 2002; Verkleij et al. 1998). The Metal Tolerance Protein 1 (MTP1) is highly expressed in Zn hyperaccumulators (N. goesingensis, N. caerulescens, Sedum alfredii and A. halleri) as a vacuolar transporter associated with Zn tolerance (Gustin et al. 2009; Krämer 2005; Milner and Kochian 2008; Zhang et al. 2011). However, C. novae-hollandiae had previously been identified to accumulate Zn in the phloem and cell walls and not in the vacuoles (Farago et al. 1977). In addition, the majority of Zn is in a water-soluble form and present in non-specific sites (70% of total foliar Zn), while the insoluble component is accumulated in carbohydrates associated with the cell wall at non-metal specific sites and can be exchanged with Ca ions (Farago et al. 1977).
Hyperaccumulators are characterised by their ability to accumulate very high concentrations of metal(loid)s into their aerial tissues (Roosens et al. 2003; van der Ent et al. 2013). Therefore, hyperaccumulators have translocation factors >1, which is indicative of efficient root-to-shoot translocation of metals (Baker 1981; Gupta et al. 2016). As expected, the translocation factors for the non-metallophyte, C. cunninghamii, were low when exposed to Zn or Cd treatment. However, for the hyperaccumulator C. novae-hollandiae, Zn-Cd translocation factors increased with increasing Zn-Cd treatment levels. Nevertheless, the translocation factors for C. novae-hollandiae did not exceed 1, suggesting relatively limited Zn-Cd translocation. However, C. novae-hollandiae did surpass the Zn hyperaccumulation threshold by 5.5-fold and as such confirmed its status as a Zn hyperaccumulator under experimental conditions (Tang et al. 2021). The increase in Zn translocation factors for C. novae-hollandiae in Cd treatments indicates that Cd facilitates Zn translocation. However, the underlying mechanisms are not presently known. The role of ZIP (Zrt-Irt-like Protein) transporters in the uptake of Zn in hyperaccumulating metallophytes has been well studied and may be expressed in C. novae-hollandiae (Corso and de la Torre 2020; Merlot et al. 2021; Singh et al. 2019; Sytar et al. 2020). However, unlike other well-known Zn hyperaccumulators (A. halleri and N. caerulescens), C. novae-hollandiae has a much lower translocation factor and does not accumulate Zn or Cd when grown on soils or substrates with low Zn or Cd status (Bert et al. 2003; Peer et al. 2006; Zhao et al. 2000).
Crotalaria novae-hollandiae and C. cunninghamii are both sensitive to Cd. However, C. novae-hollandiae accumulated Cd well beyond the hyperaccumulation threshold although its tolerance threshold may be lower than expected. Unlike Zn, Cd does not have a specific transporter and uptake is via the binding of non-specific transporters of essential elements with great attention being given to ZIP transporters (Huang et al. 2020). Iron transporters (IRT) have also received attention for their possible involvement in Cd uptake in the N. caerulescens ‘Ganges’ population of Southern France. IRT1, which is primarily expressed in the root epidermis, has been found to increase root Fe uptake in the Ganges population when exposed to Cd (Halimaa et al. 2019; Vert et al. 2002). The mechanisms of Cd uptake in C. novae-hollandiae may differ as no significant change in root Fe-uptake occurred with exposure to Cd. Zinc and Cd share chemical similarities, though Cd is more chemically similar to Fe which can result in preferential uptake via the same pathways and transporters without altering Fe uptake or the Fe transport system if it is not saturated (Nouet et al. 2015). As Zn is hyperaccumulated by C. novae-hollandiae, Cd may also be inadvertently hyperaccumulated and no specific mechanism of Cd detoxification has evolved. However, C. novae-hollandiae is still able to accumulate up to 170 μg Cd g−1 in its old leaves growing naturally in soils with up to 6.59 mg Cd kg−1 bioavailable (DTPA-extractable Cd) with no visible signs of toxicity (Tang et al. 2021). It may, therefore, be classified as a (rather weak) Cd hyperaccumulator; and its tolerance range is clearly not as great as other well-known Zn-Cd hyperaccumulators (with foliar concentrations in N. caerulescens of up to 2890 μg Cd g−1 and in A. halleri with up to 3600 μg Cd g−1) (Bert et al. 2003; Reeves et al. 2017). In C. novae-hollandiae leaves, Cd appears to restricts Zn to the mid-rib and veins from entering the lamina, which is most clear in the highest Cd treatment (60 μM in solution) and K concentrations are reduced throughout the leaf (Fig. 10). This contrasts with A. halleri where Zn is restricted from translocation to the veins (Morina and Küpper 2020). Cadmium increased Ca in C. novae-hollandiae and has also been shown to alleviate toxicity effects of Zn-Cd in N. caerulescens (Bayçu et al. 2017). However, Ca in C. novae-hollandiae decreased under Zn treatment which may indicate that the tolerance mechanisms for Zn and Cd differ; but as Cd is able to replace Zn in the roots, the Zn-Cd transport pathways may be the same (Bayçu et al. 2017). As the patterns of uptake and mechanisms of tolerance appear to differ between C. novae-hollandiae and other well studied Zn-Cd hyperaccumulators, further research is required to provide insights at the cellular and subcellular levels using synchrotron-based techniques, and molecular approaches to identify putative transporters.
Conclusions
Crotalaria novae-hollandiae is one of the few polymetallic (Zn-Cd-Cu) hyperaccumulators known, and the only known Zn hyperaccumulating ligneous legume species growing in tropical savannahs (although there is a Zn hyperaccumulating legume, Anthyllis vulneraria, from southern France; Escarré et al. 2010). The species has wide distribution across Northern Australia and its tolerance to dry climates makes it a strong contender for use in mine rehabilitation and/or phytoextraction. Crotalaria novae-hollandiae can accumulate high foliar concentrations of Zn (up to ~16,600 μg g−1) and Cd (up to 1250 μg g−1) in its leaves when growing on metalliferous soils or mine wastes. There is, hence, potential for producing “green” zinc oxide derived from its harvested biomass in a phytoextraction operation. This could be used in a variety of applications including sunscreens, cosmetics, and supplements, and potentially generate an estimated revenue of USD$6000 per ha per year (at $195 per kg for high-purity zinc oxide), excluding production and processing costs. Obviously, the final product should be extremely low in Cd for any such health product applications.
The Zn treatment was found to have a positive impact on growth when compared to the control until toxicity at 1000 μM Zn. The physiological reasons for this response are unknown, as such concentrations are far beyond the essential Zn requirement. Cadmium induces foliar chlorosis even at low dose rates (5 μM Cd in solution) and its tolerance and accumulation in nature (maximum of 170 μg Cd g−1 in leaves) are not as high as other Zn-Cd hyperaccumulators growing in situ (S. plumbizincicola 7010 μg Cd g−1; N. caerulescens 2890 μg Cd g−1; A. halleri 3600 μg Cd g−1). However, C. novae-hollandiae can still be classified as a Cd hyperaccumulator as it exceeds the hyperaccumulation threshold of 100 μg Cd g−1 when growing in the natural habitat. The research on C. novae-hollandiae is still in the early stages and further investigations employing state of the art techniques, including synchrotron μXRF analysis and molecular techniques, are expected to provide more detailed insights into the fundamental mechanisms underlying Zn-Cd hyperaccumulation in C. novae-hollandiae.
Data availability
The data generated during the current study are available from the corresponding author on reasonable request.
References
Antonovics J, Bradshaw AD, Turner RG (1971) Heavy metal tolerance in plants. Adv Ecol Res 7:1–85
Assunção AGL, Schat H, Aarts MGM (2003) Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol 159:351–360
Baker AJM (1981) Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutr 3(1-4):643–654
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1(2):81–126
Baker AJ, Ernst WH, van der Ent A, Malaisse F, Ginocchio R (2010) Metallophytes: the unique biological resource, its ecology and conservational status in Europe, Central Africa and Latin America. Ecol Ind Pollut 18:7–40
Balafrej H, Bogusz D, Triqui ZEA, Guedira A, Bendaou N, Smouni A, Fahr M (2020) Zinc hyperaccumulation in plants: a review. Plants 9(5):562
Bayçu G, Gevrek-Kürüm N, Moustaka J, Csatári I, Rognes SE, Moustakas M (2017) Cadmium-zinc accumulation and photosystem II responses of Noccaea caerulescens to Cd and Zn exposure. Environ Sci Pollut Res Int 24(3):2840–2850
Bert V, Meerts P, Saumitou-Laprade P, Salis P, Gruber W, Verbruggen N (2003) Genetic basis of Cd tolerance and hyperaccumulation in Arabidopsis halleri. Plant Soil 249(1):9–18
Brooks RR (1979) Indicator plants for mineral prospecting—a critique. J Geochem Explor 12:67–78
Brooks RR, McCleave JA, Malaisse F (1977) Copper and cobalt in African species of Crotalaria L. Proc R Soc Lond 197(1127):231–236
Brown SL, Chaney RL, Angle JS, Baker AJM (1995) Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens grown in nutrient solution. Soil Sci Soc Am J 59(1):125–133
Cao D, Zhang H, Wang Y, Zheng L (2014) Accumulation and distribution characteristics of zinc and cadmium in the hyperaccumulator plant Sedum plumbizincicola. Bull Environ Contam Tox 93(2):171–176
Cappa JJ, Pilon-Smits EA (2014) Evolutionary aspects of elemental hyperaccumulation. Planta 239(2):267–275
Chaney RL (1993) Zinc phytotoxicity. In: Robson AD (ed) Zinc in soil and plants. Kluwer Academic Publishers, Dordrecht
Chardonnens AN, Koevoets PLM, van Zanten A, Schat H, Verkleij JAC (1999) Properties of enhanced tonoplast zinc transport in naturally selected zinc-tolerant Silene vulgaris. Plant Physiol 120:779–785
Chen YT, Wang Y, Yeh KC (2017) Role of root exudates in metal acquisition and tolerance. Curr Opin Plant Biol 39:66–72
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Clemens S (2019) Metal ligands in micronutrient acquisition and homeostasis. Plant Cell Environ 42(10):2902–2912
Corso M, de la Torre VSG (2020) Biomolecular approaches to understanding metal tolerance and hyperaccumulation in plants. Metallomics 12(6):840–859
Do C, Abubakari F, Corzo Remigio A, Brown GK, Casey LW, Burtet-Sarramegna V, Gei V, Erskine PD, van der Ent A (2020) A preliminary survey of nickel, manganese and zinc (hyper) accumulation in the flora of Papua New Guinea from herbarium X-ray fluorescence scanning. Chemoecology 30(1):1–13
Ernst WH (2006) Evolution of metal tolerance in higher plants. For Snow Landsc Res 80(3):251–274
Ernst WHO, Verkleij JAC, Schat H (1992) Metal tolerance in plants. Acta Bot Neerl 41:229–248
Escarré J, Lefèbvre C, Raboyeau S et al (2010) Heavy metal concentration survey in soils and plants of the les malines mining district (Southern France): implications for soil restoration. Water Air Soil Pollut 216:485–504
Farago ME, Clark AJ, Pitt MJ (1977) Plants which accumulate metals. Part I. The metal content of three Australian plants growing over mineralised sites. Inorganica Chim Acta 24:53–56
Foy CD, Chaney RL, White MC (1978) The physiology of metal toxicity in plants. Annu Rev Plant Physiol 29:511–566
Gupta N, Ram H, Kumar B (2016) Mechanism of zinc absorption in plants: uptake, transport, translocation and accumulation. Rev Environ Sci Bio Technol 15:89–109
Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE (2009) MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J 57(6):1116–1127
Halimaa P, Blande D, Baltzi E, Aarts MG, Granlund L, Keinänen M, Kärenlampi SO, Kozhevnikova AD, Peräniemi S, Schat H, Seregin IV (2019) Transcriptional effects of cadmium on iron homeostasis differ in calamine accessions of Noccaea caerulescens. Plant J 97(2):306–320
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Holland AE (2002) A review of Crotalaria L. (Fabaceae: Crotalarieae) in Australia. Austrobaileya 6:293–324
Hornung RW, Reed LD (1990) Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51
Huang X, Duan S, Wu Q, Yu M, Shabala S (2020) Reducing cadmium accumulation in plants: structure–function relations and tissue-specific operation of transporters in the spotlight. Plants 9(2):223
Jhee EM, Boyd RS, Eubanks MD (2006) Effectiveness of metal–metal and metal–organic compound combinations against Plutella xylostella: implications for plant elemental defense. J Chem Ecol 32(2):239–259
Krämer U (2005) MTP1 mops up excess zinc in Arabidopsis cells. Trends Plant Sci 10:313–315
Krämer U (2010) Metal hyperaccumulation in plants. Ann Rev Plant Biol 61:517–534
Lochlainn SÓ, Bowen HC, Fray RG, Hammond JP, King GJ, White PJ, Graham NS, Broadley MR (2011) Tandem quadruplication of HMA4 in the zinc (Zn) and cadmium (Cd) hyperaccumulator Noccaea caerulescens. PLoS One 6(3):17814
McGrath SP, Zhao FJ, Lombi E (2001) Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant Soil 232(1):207–214
Merlot S, de la Torre VSG, Hanikenne M (2021) Physiology and molecular biology of trace element hyperaccumulation. In: van der Ent A, Echevarria G, Baker AJM, Morel JL (eds) Agromining: farming for metals. Springer, Cham
Milner MJ, Kochian LV (2008) Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann Bot 102:3–13
Mishra S, Mishra A, Küpper H (2017) Protein biochemistry and expression regulation of cadmium/zinc pumping ATPases in the hyperaccumulator plants Arabidopsis halleri and Noccaea caerulescens. Front Plant Sci 8:835
Morina F, Küpper H (2020) Direct inhibition of photosynthesis by Cd dominates over inhibition caused by micronutrient deficiency in the Cd/Zn hyperaccumulator Arabidopsis halleri. Plant Physiol Biochem 155:252–261
Nkrumah PN, Echevarria G, Erskine PD, van der Ent A (2018) Contrasting nickel and zinc hyperaccumulation in subspecies of Dichapetalum gelonioides from Southeast Asia. Sci Rep 8(1):1–15
Nouet C, Charlier JB, Carnol M, Bosman B, Farnir F, Motte P, Hanikenne M (2015) Functional analysis of the three HMA4 copies of the metal hyperaccumulator Arabidopsis halleri. J Exp Bot 66(19):5783–5795
Peer WA, Mahmoudian M, Freeman JL, Lahner B, Richards EL, Reeves RD, Murphy AS, Salt DE (2006) Assessment of plants from the Brassicaceae family as genetic models for the study of nickel and zinc hyperaccumulation. New Phytol 172(2):248–260
Pollard AJ, Reeves RD, Baker AJ (2014) Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci 217:8–17
Reeves RD, Schwartz C, Morel JL, Edmondson J (2001) Distribution and metal-accumulating behavior of Thlaspi caerulescens and associated metallophytes in France. Int J Phytoremediation 3:145–172
Reeves RD, Baker AJM, Jaffré T, Erskine PD, Echevarria G, van der Ent A (2017) A global database for hyperaccumulator plants of metal and metalloid trace elements. New Phytol 218:407–411
Roosens N, Verbruggen N, Meerts P, Ximénez-Embún P, Smith JAC (2003) Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from western Europe. Plant Cell Environ 26:1657–1672
Salinitro M, Mattarello G, Guardigli G, Odajiu M, Tassoni A (2021) Induction of hormesis in plants by urban trace metal pollution. Sci Rep 11:20329. https://doi.org/10.1038/s41598-021-99657-3
Singh R, Jha AB, Misra AN, Sharma P (2019) Adaption mechanisms in plants under heavy metal stress conditions during phytoremediation. In: Pandey VC, Bauddh K (eds) Phytomanagement of polluted sites. Elsevier, Amsterdam
Sitko K, Rusinowski S, Kalaji HM, Szopiński M, Małkowski E (2017) Photosynthetic efficiency as bioindicator of environmental pressure in A. halleri. Plant Physiol 175:290–302
Stein RJ, Höreth S, de Melo JRF, Syllwasschy L, Lee G, Garbin ML, Clemens S, Krämer U (2017) Relationships between soil and leaf mineral composition are element-specific, environment-dependent and geographically structured in the emerging model Arabidopsis halleri. New Phytol 213:1274–1286
Sytar O, Ghosh S, Malinska H, Zivcak M, Brestic M (2020) Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol Plant 173(1):148–166
Szopiński M, Sitko K, Rusinowski S, Zieleźnik-Rusinowska P, Corso M, Rostański A, Rojek-Jelonek M, Verbruggen N, Małkowski E (2020) Different strategies of Cd tolerance and accumulation in Arabidopsis halleri and Arabidopsis arenosa. Plant Cell Environ 43(12):3002–3019
Tang YT, Qiu RL, Zeng XW, Ying RR, Yu FM, Zhou XY (2009) Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ Exp Bot 66(1):126–134
Tang RH, Erskine PD, Nkrumah PN, Echevarria G, van der Ent A (2021) Soil-plant relationships of metallophytes of the zinc-lead-copper Dugald River gossan. Plant Soil, Queensland. https://doi.org/10.1007/s11104-021-05209-z
van der Ent A, Reeves RD, Baker AJM, Pollard J, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362(1–2):319–334
Verkleij JAC, Koevoets PLM, Blake-Kalff MMA, Chardonnens AN (1998) Evidence for an important role of the tonoplast in the mechanism of naturally selected zinc tolerance in Silene vulgaris. J Plant Physiol 153:188–191
Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14(6):1223–1233
Whiting SN, Reeves RD, Richards D, Johnson MS, Cooke JA, Malaisse F, Paton A, Smith JAC, Angle JS, Chaney RL, Ginocchio R (2004) Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restor Ecol 12(1):106–116
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259(1):181–189
Zhang M, Senoura T, Yang X, Nishizawa NK (2011) Functional analysis of metal tolerance proteins isolated from Zn/Cd hyperaccumulating ecotype and non-hyperaccumulating ecotype of Sedum alfredii Hance. FEBS Lett 585:2604–2609
Zhao FJ, Lombi E, Breedon T, McGrath SP (2000) Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ 23:507–514
Acknowledgements
Roger Tang is the recipient of a UQ Graduate School Scholarship (UQGSS) from The University of Queensland. We thank Lachlan Casey (University of Queensland) for support with the laboratory μXRF analysis. We also thank Aleksei Tatarnikov for his assistance in the laboratory during the study. We acknowledge the support of the AMMRF at the Center for Microscopy and Microanalysis at the University of Queensland.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
RHT and AVDE designed and conducted the experiment. RHT collected the samples and undertook the chemical analysis of the samples. AVDE undertook μXRF analysis of the samples. RHT performed data processing and analysis. All authors contributed to writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest relevant to the content of this article.
Additional information
Responsible Editor: Fangjie Zhao.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 12400 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, R.H., Nkrumah, P.N., Erskine, P.D. et al. Polymetallic (zinc and cadmium) hyperaccumulation in the Australian legume Crotalaria novae-hollandiae compared to Crotalaria cunninghamii. Plant Soil 479, 589–606 (2022). https://doi.org/10.1007/s11104-022-05547-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05547-6