Abstract
From 2018 to 2020, Germany experienced periods of exceptional weather conditions. Extremely high summer temperatures and precipitation deficits induced stress and mortality in forest trees. Acer pseudoplatanus (sycamore) was one of the affected tree species. Symptoms of sooty bark disease (SBD) and severe damage of entire stands, both caused by the fungal species Cryptostroma corticale, were reported more frequently. To explore the non-symptomatic distribution of C. corticale, wood cores from visibly healthy sycamore stems were sampled and all outgrowing fungi were identified and recorded. In total, 50 trees, aged 30–65 years, were sampled at five different forest stands, from which 91 endophytic filamentous morphotypes could be isolated. The fungal endophytic community in the woody tissue of the sycamore trees varied greatly at the different sites and between the trees. The number of isolated morphotypes at the different sites ranged from 13 to 44 and no morphotype was found at all sites. At 1.20-m stem height, 3.3 fungi could be isolated from woody tissue per tree on average. The most abundant species isolated from visibly healthy sycamore in regard to both occurrence at the studied sites and continuity was C. corticale. It was recorded at four of the studied forest stands, from 26% of all studied sycamore trees, and had a frequency of 7.85% relative to the 293 isolated filamentous strains that were isolated. The second most abundant species was Xylaria longipes followed by Lopadostoma turgidum. In this study clear evidence for the endophytic lifestyle of C. corticale is presented which thus appears to be spread further than expected based on visible SBD symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sycamore (Acer pseudoplatanus L., Sapindaceae) is a deciduous tree that can be found throughout large parts of Europe (EUFORGEN 2022). In Germany, besides A. pseudoplatanus, two other native Acer species occur, namely Acer platanoides L. and Acer campestre L. Pure maple stands, if found in Germany, are the result of active management, since no natural pure Acer stands exist in this region. According to the phytosociological assignment and syntaxonomical treatment following Runge (1994), maple occurs in subalpine sycamore-beech forests (Aceri-Fagion) or in lime-maple mixed forests (Tilio-Acerion). In German forests, maple is often found in mixed stands, on calcareous soil with good nutrient and good water supply. Acer pseudoplatanus can be found in various forest communities, mainly paired with beech and highly valuable hardwoods (Schmidt 2009). The timber is very durable and thus often used for furniture and floors. Additionally, the occasionally wavy grained wood is used for the manufacture of musical instruments, making sycamore a valuable timber species (EUFORGEN 2022). Sycamore is a very valuable urban tree species as well.
Even though many fungi are reported from A. pseudoplatanus (244 different fungal genera according to the USDA website, Farr and Rossman (2022)), only a few studies focus specifically on endophytes of sycamore or fungi associated with the living woody tissue of sycamore (Kowalski and Kehr 1992; Kelnarová et al. 2017). The available data on fungi associated with A. pseudoplatanus mainly refers to dead wood (Butin and Kowalski 1986; Chlebicki 1988; Unterseher et al. 2005; Brglez et al. 2020a) or to leaves (Schlegel et al. 2018).
According to Petrini (1991), Saikkonen et al. (1998), Arnold and Lutzoni (2007), and Sieber (2007), we consider those fungi as endophytes that spend a significant amount of their life cycle within the host plant tissue without causing any symptoms there. A change of environmental conditions can cause a change in the lifestyle of the fungus from endophytic to pathogenic (Sieber 2007) or to saprotrophic when the host tissue dies (Sun et al. 2011). Some fungi can be endophytic in one tree species and pathogenic in another tree species (Petrini 1991; Saikkonen et al. 2010; Sanz-Ros et al. 2015). In other cases, endophytes can change to a pathogenic lifestyle under the right circumstances, such as stress in the host tree and high summer temperatures (Ragazzi et al. 2003; Hyde and Soytong 2008). Many wood-decay fungi appear to have a transient endophytic lifestyle, which may be in preparation for their saprotrophic life stage (Boddy and Rayner 1983; Parfitt et al. 2010).
As a result of the very warm and dry summers and mild winters in the years 2018–2020, many forest trees started showing signs of stress and mortality. In Germany, these years were characterised by mean daily temperatures during the meteorological summer months (June, July, and August) averaging from 19.3 °C in 2018 (2.2 °C above average of the reference period from 1981 to 2010), to 19.2 °C in 2019 (+2.1 °C above average) and 18.2 °C in 2020 (+1.1 °C above average (DWD 2018, 2019, 2020)). The precipitation sum for the three summer months was 130 l/m2 in 2018, 175 l/m2 in 2019, and 230 l/m2 in 2020, while the required average precipitation for the reference period is 239 l/m2 (DWD 2018, 2019, 2020). As a consequence, German forests developed a soil water deficit resulting in signs of stress in the trees (NW-FVA 2020, 2021). Following these weather extremes, an extraordinary outbreak of sooty bark disease (SBD), caused by the invasive fungus Cryptostroma corticale (Ellis & Everh.) P.H. Greg. & S. Waller (Ellis and Everhart, 1889; Gregory and Waller 1951), was observed more frequently in several regions in Germany (Bork 2018; Rohde et al. 2019; Wenzel et al. 2019; Delb et al. 2019). Symptoms included wilting and dieback in the crown in earlier stages, as well as the production of masses of black conidia under the outer layer of the bark in later stages of the disease (Enderle et al. 2020; Schlößer and Langer 2021).
Cryptostroma corticale is presumed to be opportunistic with endophytic, pathogenic, and saprophytic life stages that react to stress, and it is known to have an optimal growing temperature of 25 °C (Dickenson 1980; Enderle et al. 2020). Currently, there is no published evidence for an endophytic/latent lifestyle of the pathogen, despite occasional isolations of C. corticale from symptomless tissue (e.g. Kelnarová et al. (2017); Tropf (2020)). However, in these cases, the symptomless tissue samples originate from trees already showing SBD symptoms (wood discolouration, defoliation, etc.) in other parts of the tree. As the distance of investigated symptomless samples to symptomatic tissue in no study is communicated, the fungal growth could have simply extended beyond the recognizable necrotic tissue; and therefore, a true endophytic/latent phase expressed by this species is questionable. It is assumed that C. corticale primarily infects the tree through fresh wounds (Townrow 1953; Dickenson 1980) and causes a soft rot in infested tissues of the tree, like many other closely related fungi from the Xylariales (Worrall et al. 1997; Schwarze 2018). The original description of C. corticale by Ellis and Everhart (1889) originated from Canada. The first record in Germany reported by the plant protection office in Berlin dates back to 1964 (Plate and Schneider 1965). In this case, spores of C. corticale were detected on infested firewood originating from trees of the Berlin Tiergarten park area and stored in a basement. Triggered by extremely warm weather and precipitation deficits in the year 2003, several incidences of SBD occurred in this and the following years throughout Germany and Europe (Cech 2004; Engesser et al. 2004; Metzler 2006; Robeck et al. 2008; Langer et al. 2013; Bencheva 2014; Koukol et al. 2014). The recently observed cases of disease from 2018 to 2021 constitute the biggest outbreaks of SBD in Germany yet. Since the data are recorded by each forest protection office in Germany for their respective regions, there is a gap in compiled data about the actual distribution of SBD in German forests. A preliminary distribution map regarding German forests was published by Schlößer and Langer (2021); the updated version is presented in this paper.
The main goals of this research were to undertake a better assessment of (1) fungi associated with woody tissues of Acer pseudoplatanus paired with an investigation into the spread of C. corticale in its endophytic stage, and (2) the current status and distribution of C. corticale as well as the potential risks of SBD in Germany. Therefore, the distribution of SBD in German forests was mapped based on compiled data from forest protection offices in Germany and fungi associated with living sycamore woody stem tissue from forest stands with different health status in respect to SBD were studied. In order to explore fungi associated with living woody tissue of sycamore, as well as the non-symptomatic distribution of C. corticale in visibly healthy trees, a study was conducted examining stem wood cores, following an adjusted version of the method used by Kelnarová et al. (2017). The results of this study are relevant regarding the risk assessment of potential disease outbreaks especially in light of the ongoing climate change.
Materials and methods
Mapping of the sooty bark disease
In order to map the distribution of SBD cases in German forests, three different approaches were perused: (1) infestation data from the federal forest protection institutions of Germany were compiled (deadline 31.05.2021); (2) from May 2020 to September 2020, 123 forest stands with A. pseudoplatanus (aged 20–100 years, with sycamore as a dominant tree species) in Hesse were evaluated with regard to visible symptoms of sooty bark disease; (3) data on cases of SBD in Schleswig-Holstein, Lower Saxony, Hesse, and Saxony-Anhalt were retrieved from the ‘Waldschutzmeldeportal’ Forest Protection Reporting Portal (WSMP) of the Northwest German Forest Research Institute (NW-FVA); and (4) cases of SBD directly reported by forest owners or foresters and checked by the authors between May 2020 and March 2022. Forest owners and foresters can register georeferenced forest damage in the online WSMP. QGIS (v. 3.22.3, www.qgis.org) was used to create a combined map based on a preliminary distribution map (Schlößer and Langer 2021).
Sampling sites
Wood samples were taken from five forest stands (Table 1) containing sycamore, located in two federal states of Germany. Four of the sites are located in Hesse in the middle of Germany and one, Nehmten, in Schleswig-Holstein, in the very north of Germany. The four Hessian sites were located in the forest departments of Melsungen, Nidda, Fulda, and Beerfelden. Two of the five stands, in Fulda and in Nidda, were visibly affected by SBD at the time of sampling, and several maple trees exhibited black conidia underneath the ruptured bark. In the studied stands in Melsungen, Beerfelden, and Nehmten, no visible signs of SBD were observed. The forest stands without symptoms of SBD were located at different distances from the nearest known infestation point with C. corticale. The studied site in Beerfelden was 200 m away from the next SBD-affected trees, while in Melsungen there was more than 30 km of aerial distance to the closest infested stand. In Schleswig-Holstein, no case of SBD in forests had been reported until June 2022. Forest stands of different health status were chosen in order to check for occurrence of C. corticale in stands without visible signs of SBD.
Isolation of fungi
At each site, ten living and obviously healthy trees, aged 30–65 years and without visible symptoms of SBD, were sampled using increment borers (Haglöf Increment Borer Mora-Coretax, three-edged, 300 mm drilling depth, 5.15 mm diameter). Two 20 cm increments of stem tissue per tree were taken at a 1.20 m stem height above ground following the method of Kelnarová et al. (2017). In Melsungen, the first sampled site, three increments were taken per tree. The bark of the sample trees was sprayed with 70% ethanol at sampling height and wiped with a paper towel. Two borers were used alternately and sterilised by flame shortly before every use. The outer layer of the bark was carefully scraped and the exposed tissue was disinfected with ethanol again. Increments, taken on the vertical axis, were carefully removed from the extractor by disinfected hands and placed into clean and marked plastic tubes. The borer, extractor, and knife were cleaned each time before taking a new sample by spraying with ethanol and wiping away the excess plant material which was followed by flame sterilisation. Samples were cooled during transportation to the laboratory. Sampling was performed from March to September 2021. For each tree, diameter at breast height (1.30 m) as well as the following were recorded: crown vitality, the number of epicormic shoots as an indicator for vitality as well as visible signs of infestation with C. corticale or Stegonsporium pyriforme (Hoffm.) Corda (Online Resource 1).
In the laboratory, each increment was surface sterilised by rinsing the sample with 70% ethanol and left to dry for a few minutes. Increments were cut into 5 mm-long segments and three pieces were placed on each 90 mm petri dish containing malt yeast peptone (MYP) agar, modified according to Langer (1994) containing 0.7% malt extract (Merck, Darmstadt, Germany), 0.05% yeast extract (Fluka, Seelze, Germany), 0.1% peptone (Merck, Darmstadt, Germany), and 1.5% agar (Fluka, Seelze, Germany). From the sampling sites in Fulda, Beerfelden, Nidda, and Nehmten, the increment segments with visible wood discolouration or signs of rot were observed and counted. The petri dishes were incubated at room temperature with ambient daylight for 4 weeks. The cultures were monitored every second day in the first 2 weeks and twice a week in the third and fourth weeks. Emerging mycelia were sub-cultured into pure cultures. The pure cultures were tentatively grouped into morphotypes (MTs) based on morphological observation following the method of Schulthess and Faeth (1998). At least one representative culture for each MT was stored on MYP slants at 4 °C in the fungal culture collection of the NW-FVA.
Frequency of isolated taxa, defined as proportion of isolated strains in relation to the total number of isolated filamentous strains, was calculated. Continuity of isolated taxa, defined as the number of trees from which the fungus was isolated in relation to the total number of trees, was calculated.
Molecular analysis
As a rule, one representative strain per MT was used for genetic analysis and species identification. Mycelium was placed in 1.5 ml Eppendorf tubes with five glass beads (3 mm) and 150 μl of TE buffer (10 ml 1 mmol Tris HCl (pH 0.8), 2 ml 0.5 mmol EDTA; Carl Roth, Karlsruhe, Germany). The mycelium was crushed in a Mixer Mill MM 200 (Retsch, Haan, Germany) with 25 vibrations per second for 90 s. Subsequently, genomic DNA was extracted following the protocol of Izumitsu et al. (2012).
For all strains, the 5.8S nuclear ribosomal gene with the two flanking internal transcribed spacers ITS-1 and ITS-2 (ITS) was amplified using the primer pair ITS-1F (Gardes and Bruns 1993) + ITS-4 (White et al. 1990). For strains belonging to Neonectria, the actin gene (ACT) and portion of the β-tubulin gene (TUB) were additionally amplified using primer pairs Tact1 + Tact2 (Samuels et al. 2006) and T1 (O’Donnell and Cigelnik 1997) + Bt-2b (Glass and Donaldson 1995), respectively. The PCR mixture consisted of 1 μl of DNA and 19 μl mastermix which contained 2.5 μl 10× PCR reaction buffer (with 20 mM MgCl2, Carl Roth, Karlsruhe, Germany), 1 μl of each primer (10 mmol), 2.5 μl MgCl2 (25 mmol), 0.1 μl Roti®-Pol Taq HY Taq polymerase (Carl Roth, Karlsruhe, Germany), and 2.5 μl of 2 mmol dNTPs (Biozym Scientific GmbH, Hessisch Oldendorf, Germany). Each reaction was topped up to a volume of 20 μl by adding sterile water.
A StepOnePlus™ PCR System (Applied Biosystems, Waltham, Massachusetts, USA) was used to carry out the DNA amplifications. The PCR conditions for the amplification of the ITS region were set according to Bien et al. (2020). The amplification conditions for the primer pair Tact1 + Tact2 were as follows: initial denaturation at 94 °C for 10 min; followed by 30 cycles of denaturation at 94 °C for 35 s, annealing at 48 °C for 30 s and extension at 72 °C for 80 s; and a final extension step of 10 min at 72 °C. The amplification conditions for the primer pair T1 + Bt-2b were set according to Cabral et al. (2012b) with the exception of a 60 °C annealing temperature. A 1% agarose gel was used to visualise the PCR products. The products were sent to Eurofins Scientific Laboratory (Ebersberg, Germany) for sequencing. Initially, PCR samples of the ITS region were sequenced using the forward reaction (primer ITS-1F). In case of imprecise results, reverse reactions (primer ITS-4) were sequenced in addition. All other DNA sequence regions were sequenced by the respective forward and reverse reactions. By using BioEdit Sequence Alignment Editor (v. 7.2.5; Hall (1999)), all sequences were visually checked, and defective sequence beginnings and ends trimmed. In case of forward and reverse sequences available, consensus sequences were generated using BioEdit and further processed in the same way. Sequences were submitted to GenBank (Table 2).
Identification of fungi
Morphotypes were assigned to a taxonomic level by molecular analysis of representative strains of each morphotypic group following the method of Guo et al. (2000). The BLAST algorithm (http://www.ncbi.nlm.nih.gov/genbank, Altschul et al. (1997)) was used for fungal taxon determination. The results were re-checked against literature and known cultures for confirmation. BLAST results below a threshold of 98% identity were not trusted to be accurate enough for final determination. Each identification was critically interpreted with emphasis on well-curated culture collections such as the Westerdijk Fungal Biodiversity Collection (CBS). In case no definite affiliation was possible to a specific taxonomic level, the identification was marked by cf. (confer) to indicate uncertainties.
Since BLAST results for different Neonectria and Eutypa strains were inconclusive, additional comprehensive analyses were performed. For strains belonging to Neonectria, final determination of the genus and species was based on two separate comprehensive phylogenetic analyses using the ACT and TUB gene regions, respectively, including reference sequences retrieved from GenBank (data not shown). Similarly, for the final determination of different Eutypa strains, a phylogenetic analysis based on the ITS results was accomplished.
Analysis with R
Analysis of the fungal diversity found in this study was conducted using RStudio V 4.1.2 (R Core Team 2021). The package tidyverse (Wickham et al. 2019) was used, where a distribution chart was created using the function ‘pie’. The packages ggplot 2 (Wickham 2016) and ggVennDiagram (Gao 2021) were used to analyse the overlap between the fungi found at each site. Here the function ‘ggVennDiagram’ was used.
It was further checked manually and using RStudio (R Core Team 2021) whether the presence of C. corticale influenced the fungal community using the ‘plot’ function as well as the ‘ddply’ function of the plyr package (Wickham 2011) and a distance matrix using the function ‘vegdist’ from the package vegan (Oksanen et al. 2022) was generated. Additionally, it was analysed whether the third increment taken at Melsungen had a significant influence on the diversity of isolated morphotypes at that site using the ‘plot’ function as well.
Results
Distribution of SBD in German forests
By 31.05.2021, we had received reports of SBD from forests all over Germany with the exception of the federal state of Schleswig-Holstein, where no outbreak has been recorded so far. In total, 403 stands with obvious symptoms of SBD were reported (Fig. 1). Of the 123 evaluated stands in Hesse, 31.71% showed visible symptoms of SBD. At the beginning of 2022, a new collection of SBD reports were registered in the WSMP for Hesse and added to the map.
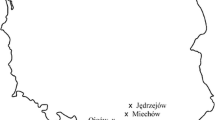
a: Distribution of SBD cases in Germany, reported by 31.05.2021, last updated for Hesse: 31.03.2022, 1 = Melsungen, 2 = Fulda, 3 = Beerfelden, 4 = Nidda, 5 = Nehmten; b: detailed view of the cases in the federal state of Hesse; © GeoBasis-DE / BKG (2021), © HessenForst 2020, last updated: 31.03.2022 (QGIS Desktop 3.22.3);  sampling sites without sooty bark disease,
sampling sites without sooty bark disease,  sampling sites with sooty bark disease,
sampling sites with sooty bark disease,  reported stands with visible sooty bark disease,
reported stands with visible sooty bark disease,  hessian forest department borders,
hessian forest department borders,  borders of the federal states.
borders of the federal states.
Isolated fungi
In total, 4124 segments of stem tissue increments originating from 50 sycamore trees were incubated. From these, 379 mycelial outgrows were observed and 292 of them were transferred to pure cultures (Online Resource 2). Most fungi grew out between weeks 1 and 3 after incubation of the increment segment. When the original plates were discarded, only a few outgrowths had been observed in the preceding week. Some of the 379 outgrows were omitted due to obvious repetitions or contaminations. The resulting pure culture isolates were assigned to 91 morphotypes and all but 14 could be assigned to genus or species level (Table 2, Fig. 2). The majority of the wood tissue increments studied (93.8%, n = 3163 studied increment segments) had no visible discolouration or even signs of rotting. From sampling sites in Fulda, Beerfelden, Nidda, and Nehmten, 28 of the 80 increments showed visible signs of infection, resulting in 195 increment segments with wood discolouration or rot (6.2%, n = 3163). From those segments, yeast grew out in 78 cases and mycelia in 43 cases (nine morphotypes), and no growth was observed in 78 cases. Seven of the isolated morphotypes from discoloured or decayed wood were assigned to genus or species level, namely Biscogniauxia nummularia (Bull.) Kuntze, C. corticale, Diaporthe sp., Furcasterigmium furcatum (C. Moreau & Moreau ex W. Gams) Giraldo López & Crous, Jackrogersella cohaerens (Pers.) L. Wendt, Kuhnert & M. Stadler, Leptosillia muelleri (Duby) Voglmayr & Jaklitsch, and Neonectria sp. The remaining two morphotypes could be assigned to the order of Agaricales (NW-FVA 7192) and the family of Hymenochaetaceae (cf. Inonotus sp. NW-FVA 7019), respectively.
Overview of isolated morphotypes per site visualized by a stacked bar chart, sorted alphabetically by order within the Ascomycota (Capnoidales, yellow; Diaporthales, orange; Helotiales, red; Hypocreales, blue; Pleosporales, green; Xylariales, purple; remaining orders of the Ascomycota, grey, and Basidiomycota, turquoise)
The majority of the isolated filamentous fungi from all samples were Ascomycota (79 taxa, 86.81%), nine taxa (9.89%) belonged to the division of Basidiomycota, and one morphotype was determined to be Mucoromycota (1.1%). The remaining two taxa (2.19%) could not be classified due to unsuccessful DNA extraction, one presumably being a coelomycete fungus. Within the Ascomycota, the most frequently observed orders (Fig. 3) were Pleosporales (26.58%), followed by Xylariales (13.92%) and Hypocreales (12.66%). The Basidiomycota morphotypes were assigned to Agaricales sp. (NW-FVA 7192), Coprinellus micaceus (Bull.) Vilgalys, Hopple & Jacq. Johnson, Coprinellus sp. (NW-FVA 7000), Hymenochaetaceae sp. (NW-FVA 7019), Hypholoma fasciculare (Huds.) P. Kumm., Porostereum spadiceum (Pers.) Hjortstam & Ryvarden, Serpula himantioides (Fr.) P. Karst., Stereum cf. hirsutum (NW-FVA 6270), and Trametes versicolor (L.) Lloyd.
Of the 91 isolated morphotypes detected, 41 (45%) were isolated more than once and only six morphotypes were obtained ten or more times. The remaining 50 morphotypes (55%) were only isolated once. Between 0 and 19 different morphotypes were found in the studied woody tissue per tree. On average, 3.3 morphotypes were recorded on each tree. From four trees, no isolations could be made. None of the isolated morphotypes was found at all sites and merely 15 out of the 91 morphotypes were found at more than one site. In total, 84.62% of the isolated morphotypes were found solely at one site while 63.74% of all isolated morphotypes were isolated from a single tree, respectively. The top ten morphotypes with the highest frequency were Hymenochaetaceae sp. (10.6%), C. corticale (7.85%), Cytospora cf. populina (6.48%), Cytospora cf. rodophila (5.12%), Thyridium vestitum (Fr.) Fuckel (4.78%), Lopadostoma turgidum (Pers.) Traverso (3.41%), Xylaria longipes Nitschke (3.07%), Penicillium sp. (3.07%), and Nectria cinnabarina (Tode) Fr. (3.07%).
The most abundant morphotypes, in regard to both occurrence at the studied sites and continuity, were C. corticale (4 sites, 26% continuity, 7.85% frequency), X. longipes (4, 14%, 3.07%), Lo. turgidum (3, 10%, 3.41%), Cadophora prunicola Damm & S. Bien (3, 6%, 1.02%), Trichoderma sp. (3, 6%, 1.02%), L. muelleri (2, 12%, 2.05%), Diaporthe pustulata Sacc. (2, 6%, 1.02%), Didymella macrostoma (Mont.) Qian Chen & L. Cai (2, 6%, 2.05%), and B. nummularia (2, 6%, 1.71%).
The number of morphotypes isolated at the different sites ranged from 13 to 44. Hence, the fungal communities at the sites studied differed in their species composition and diversity (Fig. 4). In Melsungen, 44 morphotypes (48.35% of all isolated taxa) were found and 36 (39.56%) of those occurred exclusively at this site. The most common isolated morphotypes in Melsungen, sorted by continuity, were C. corticale, Penicillium sp., Cytospora cf. rodophila, Cytospora cf. populina, and T. vestitum. Cryptostroma corticale was isolated from 60% of the trees studied at this site. Twenty-one morphotypes (23.07%) were found in the samples from the stand in Fulda, eleven of which (12.01%) were only found there. The most common species at that site were X. longipes, L. muelleri, Lo. turgidum, D. pustulata, and Neonectria sp. Cryptostroma corticale was not isolated from any tree in Fulda. At the Beerfelden study site, 19 morphotypes (20.9%) were found, 13 of which (14.29%) were found exclusively at that site. The five most frequently found species were Arthrinium rasikravindrae Shiv M. Singh, L.S. Yadav, P.N. Singh, Rah. Sharma & S.K. Singh, Arthrinium cf. marii, L. muelleri, C. corticale, and Lophiostoma carpini Andreasen, Jaklitsch & Voglmayr. Cryptostroma corticale was found in one of the trees studied (10%). Fifteen morphotypes were found in the Nidda samples (16.5%), eight of which (8.8%) were exclusive to that site, namely C. corticale, B. nummularia, Lo. turgidum, Hymenochaetaceae sp., and an unidentified species (NW-FVA 7196). Cryptostroma corticale was isolated from five out of ten trees studied in Nidda (50%). At Nehmten, 13 morphotypes (14.29%), including C. corticale (10%), were isolated in total, eight of which (8.8%) were found exclusively at that site. At that location, only Hypoxylon fragiforme (Pers.) J. Kickx f., was isolated multiple times.
Overlap between the fungi isolated from each site with the indication of how many of the isolated fungi were found at each site and between the different sites (absolute number as well as percentage); green filling, fungi isolated just at the respective site; blue, fungi isolated from two sites; yellow, fungi isolated at three sites; red, fungi isolated from four sites (RStudio 4.1.2)
According to the statistical analyses including the distance matrix, C. corticale appears to have no significant influence on the fungal community observed in this study. However, eleven fungi only grew out from sampled trees where no C. corticale was found (Agaricales sp., Angustimassarina sp., A. rasikravindrae, Cladosporium sp. 1, D. cf. rudis, D. pustulata, L. muelleri, Lo. turgidum, Neocucurbitaria acerina Wanas., Camporesi, E.B.G. Jones & K.D. Hyde, Neodidymelliopsis sp., and Neosetophoma cf. italica). Only two fungi (B. nummularia and Capronia sp.) grew out from trees with C. corticale. The third increment taken per tree at Melsungen, increasing the sampling size in contrast to the other sampling sites, had no significant influence on the result of Melsungen being the most diverse site.
Discussion
Composition of isolated fungi
Similar to other studies on fungi isolated from tree woody tissues (Singh et al. 2017; Bußkamp 2018; Ghobad-Nejhad et al. 2018; Langer et al. 2021), in this study mainly Ascomycota (85.17%) and significantly less Basidiomycota (9.89%) were isolated. This also corresponds to studies focusing on fungi colonising different tree tissues (Petrini and Fisher 1988; Kowalski and Kehr 1992; Peršoh et al. 2010; Martínez-Álvarez et al. 2012; Sanz-Ros et al. 2015). Basidiomycota associated with woody tissues are found less frequently in living trees, a reason for this could be that a number of them is involved in wood decay (Langer et al. 2021).
Sieber (2007) concluded that the endophytic fungal communities in Aceraceae are mainly dominated by species belonging to Diaporthales. Furthermore, Pleosporales and Xylariales can be dominant endophytes in angiosperms. The morphotypes identified in this study mainly belong to Pleosporales, followed by Xylariales and Hypocreales. Isolates belonging to the Diaporthales constitute only the fourth most common group. In comparable studies focusing solely on woody sycamore tissue (e.g. Butin and Kowalski (1986), Kowalski and Kehr (1992), Unterseher et al. (2005), Brglez et al. (2020a)) the most commonly isolated orders include Diaporthales, Helotiales, Hypocreales, and Pleosporales. Diaporthales did not unequivocally dominate in any of these studies. This discrepancy in the fungal species composition could be explained by the fact that Sieber (2007) focused on different forest trees and fungi isolated from leaves and woody tissue except roots. The composition of fungal orders inhabiting leaves and woody tissue, as subsumed by Sieber (2007), might not represent the composition solely in woody tissue of Acer trees in specific. The fungal community of this and the aforementioned studies were not dominated by a few host-specific species as stated by Sieber (2007). In our study, only five species (C. corticale, L. muelleri, Lo. turgidum, Penicillium sp., and X. longipes) were found in 10% or more of the examined trees. Only one of these is host genus-specific, namely the most abundant, invasive species C. corticale. This might be due to the small geographical region that the analysed sites are located in, in comparison to the geographical area covered in the study of Sieber (2007), as well as the diversity of the studied plants or even the differing sampling and isolation methods used.
The total amount of isolated morphotypes from woody sycamore tissue in this study (91) is significantly higher than that in previous investigations, where 10–52 different morphotypes were detected (Butin and Kowalski 1986; Kowalski and Kehr 1992; Unterseher et al. 2005; Brglez et al. 2020a). This difference in diversity can be explained by the larger sampling size and the greater number of sites studied here in contrast to the other studies.
Many of the detected morphotypes in our study were single isolates, which may indicate a sporadic occurrence or sampling bias since only ten trees per site were studied at one specific height. However, it cannot be ruled out that these fungi occur in other stands as well as in higher abundancy and simply were not isolated from the sampled material. Due to the rather small sample size of two or three increments compared to the entire wood body of the tree, the listed fungi are likely just a small fraction of the present fungal community. Additionally, it is to be expected that the composition of fungi might differ within the tree, depending on the host tissue type (Gennaro et al. 2003) and tree age (Halley et al. 1994; Maherali and Klironomos 2007). Furthermore, a possible underestimation of fungal diversity in the studied trees may occur since not all fungi are detectable through standardised culture-based methods or in general (Guo et al. 2001; Allen et al. 2003; Unterseher 2007; Muggia et al. 2017). The composition of the forest stands combined with the nutrient and water availability could also be a factor in assessing the differences in fungal diversity per stand. The stand in Nehmten, stocked only with sycamore, had the lowest fungal diversity of all studied sites and at the same time the lowest nutrient availability.
The composition of fungi isolated in this study differed significantly between the studied forest sites, with very little overlap between the sites. It can be assumed that adding another differing site an entirely new set of fungal wood inhabitants not recorded in this study could be revealed. While some of the isolated fungi (29.1%) were already described as associated with maple (Ellis and Ellis 1985; Butin and Kowalski 1986; Chlebicki 1988; Kowalski and Kehr 1992; Unterseher et al. 2005; Brglez et al. 2020a), most were not recorded in the aforementioned articles (70.9%). Only ten of the detected species (C. corticale, D. pustulata, D. rudis, D. macrostroma, Eutypa maura (Fr.) Fuckel, N. cinnabarina, N. acerina, Petrakia irregularis Aa, T. vestitum, and X. longipes) were listed for A. pseudoplatanus in the USDA fungal database (Farr and Rossman 2022). Three of them were also reported from sycamore in Germany. This further illustrates the current lack of knowledge about endophytes, specifically in woody tissue of A. pseudoplatanus in Germany. According to our data, no difference in the fungal composition per site was observed between trees with C. corticale and trees without. These results should be verified by more comprehensive studies on a larger scale.
Function of the isolated fungi
Since our goal was to isolate fungi that were expressing an endophytic life stage in A. pseudoplatanus wood, including the check for the presence of C. corticale, the far majority of the wood samples we isolated came from trees and tissue that were not exhibiting any SBD symptoms. However, a significant number of fungi have been found to switch between different lifestyles (Promputtha et al. 2010; Álvarez-Loayza et al. 2011; Eaton et al. 2011; O’Connell et al. 2012; Kuo et al. 2015). They live inside their host endophytically, inducing no visible symptoms; however, they become pathogenic, if the host plant is exposed to stress, e.g. drought (Desprez-Loustau et al. 2006; Slippers and Wingfield 2007). Therefore, inferences about the specific function of each species within the temporal-spatial succession of fungal communities linked to healthy wood of A. pseudoplatanus trees in Germany must be taken cautiously.
The ten most abundant fungi, including C. corticale, were isolated from healthy wood tissue and are discussed hereinafter. It must be assumed that all of them exhibit an endophytic life stage, even though three of them were also isolated from discoloured tissue once as well (B. nummularia, C. corticale, and L. muelleri). This observation for the latter three species is supported by their affiliation to the Xylariales, which is an order hosting many wood-decaying fungi with endophytic life stages (Hendry et al. 2002; Bußkamp et al. 2020). Xylaria longipes was the second most abundant species in this study and is a known endophyte in a number of different tree species. Xylaria species often grow and sporulate on lying deadwood and stumps (Scholtysik et al. 2013). The third most abundant morphotype, assigned to Lo. turgidum, belongs to the Xylariales as well, and has to the authors’ knowledge not been reported from Acer before. Cadophora prunicola, also belonging to the ten most abundant morphotypes, is only known from its first description on Prunus (Bien and Damm 2020a), besides our study. This is the first report of this helotialean species from A. pseudoplatanus. For C. prunicola, an endophytic life stage is probable, since several other fungi belonging to the Helotiales are known endophytes (Petrini and Fisher 1988; Langer et al. 2021). Species of Trichoderma can occur as endophytes as described by Evans et al. (2003), while the superordinate order Hypocreales is a group hosting several other endophytic species as well. The isolation of Diaporthe pustulata in this study was of no surprise since it was originally described from A. pseudoplatanus (Gomes et al. 2013). Species belonging to the Diaporthales can occur as endophytes (Suryanarayanan 2011; Bußkamp et al. 2020; Langer et al. 2021). Therefore and because it was isolated from healthy tissue, D. pustulata might also have an endophytic stage in its life cycle. The pleosporalean fungus Didymella macrostroma is presumed to have an endophytic life stage as well, since many other fungi in the Pleosporales have endophytic life stages (Langer et al. 2021). Biscogniauxia nummularia is a known and widespread multi-host endophyte isolated from several different tree species (Petrini-Klieber 1985; Chapela and Boddy 1988; Chapela 1989; Nugent et al. 2005; Bußkamp et al. 2020) belonging to the Xylariales as well, and very common on beech trees (Chapela and Boddy 1988; Chapela 1989). To the authors’ knowledge, this fungus has not been reported from Acer before.
Occurrence of SBD and spread of C. corticale
The observed widespread occurrence of SBD in Germany coincides with information from neighbouring central European countries (Oliveira Longa et al. 2016; Kelnarová et al. 2017; Cech 2019; Queloz et al. 2020). As shown by the SBD reports documented in this study, there are no cases of the disease in the forests of the most northern federal state of Germany, Schleswig-Holstein. This might be due to a more maritime and thus more humid climate. When comparing the site conditions of the forest stand where the fungal wood inhabitants were isolated, it can also be observed that the site in Nehmten, Schleswig-Holstein, has a different bedrock and higher water storing capacity than the other sites (Table 1). Additionally, the site in Nehmten is influenced by groundwater due to being located in a lake region. This indicates that even in drought periods, the plant water availability is more stable, resulting in less plant stress. The climate data for the summers 2018 and 2019 show that temperatures in Hesse were in general higher, as well as in relation to the average reference period with less precipitation in comparison to those in Schleswig-Holstein (DWD 2018, 2019), indicating potential higher drought stress and thus more favourable circumstances for the outbreak of SBD in Hesse, especially with reference to the respective site conditions (Table 1).
The results of Kelnarová et al. (2017) indicate an endophytic life stage of C. corticale as it could be detected in part from healthy woody tissue and in a quarter of all studied urban sycamore trees in Prague. However, the study only states that wood discolouration was observed in 59% of all sampled trees and it was not clarified whether C. corticale was isolated from discoloured or healthy tissue, only a positive correlation between wood discolouration and the presence of C. corticale was mentioned. This assumption is confirmed by our observations of the latent non-symptomatic stage of C. corticale in forest trees in Germany. This is the first verification of an endophytic life stage for C. corticale. As shown in this study, 26% of all observed, apparently healthy sycamores harbour C. corticale non-symptomatically, regardless of the occurrence of external symptoms in the studied forest stands or area. This leads to the assumption that latent infection with C. corticale is widespread in Germany. The risk of mortality due to C. corticale for apparently healthy sycamore trees rises with the increase of years with drought and extraordinary high temperatures, since SBD is triggered by these circumstances (Dickenson 1980; Ogris et al. 2021). Even though only vital trees with no signs of distress or injury were chosen for our sampling, 35% of the increments exhibited wood discolouration and/or wood rot. In Kelnarová et al. (2017), discolouration was observed in 59% of all sampled trees and was correlated strongly with the presence of C. corticale. In contrast, in this study, C. corticale was isolated only once from discoloured woody tissue. Besides C. corticale, other wood decay fungi such as B. nummularia, Hymenochaetaceae sp., and J. cohaerens were associated with the discoloured tissues. However, the amount of discoloured tissue observed in this study was rather low, due to our focus on vital trees.
C. corticale was isolated from two of the three stands without obvious symptoms of SBD (Beerfelden, Melsungen, and Nehmten). Especially the proof of C. corticale in Nehmten was surprising since symptoms of SBD have not yet been reported from forests in the entire federal state of Schleswig-Holstein. The detection of C. corticale in Beerfelden was unexpected, due to the high wood quality and tree vitality of the sampled stand. In contrast, the isolation of C. corticale in Nidda was expected, due to obvious symptoms of SBD at the site. The fungus was not detected at the stand of Fulda despite obvious symptoms. Similar to the results of Kelnarová et al. (2017), who used both a culture and a non-culture–based isolation method, the number of trees C. corticale was isolated from in our study was higher than expected from the occurrence of SBD with the exception of Fulda. The continuity of C. corticale in the presented study is 26%, similar to the results of Kelnarová et al. (2017) where the continuity, based on both isolation methods combined, was 25%.
Conclusion
The results in this paper illustrate once more that C. corticale can be isolated from healthy woody tissue of sycamore, thus confirming a latent life stage of this pathogenic fungus. It can be assumed that the current occurrence of the endophytic stage of C. corticale associated with A. pseudoplatanus is more widely spread than expected. This increases the risk of further outbreak of SBD in forests in the light of climate change. Therefore, the spread of C. corticale should be further investigated in Germany as well as in Europe in order to estimate the potential risk for Acer trees in this region. The presented data functions as a basis in this endeavour. An increase of negative effects due to climate change, such as rising temperature and insufficient precipitation, needs to be expected for the future, and constitutes major risks for the forest sector. The detected isolated morphotypes could be a basis for future studies concerning potential biological control agents against C. corticale.
Data availability
The DNA sequences generated in this study were deposited in GenBank (https://www.ncbi.nlm.nih.gov; Table 2). All sampling data is provided in the online resources (ESM 1 and ESM 2).
References
Allen TR, Millar T, Berch SM, Berbee ML (2003) Culturing and direct DNA extraction find different fungi from the same ericoid mycorrhizal roots. New Phytol 160:255–272. https://doi.org/10.1046/j.1469-8137.2003.00885.x
Altschul SF, Madden TL, Schäffer AA et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein databases search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Álvarez-Loayza P, White JF, Torres MS et al (2011) Light converts endosymbiotic fungus to pathogen, influencing seedling survival and niche-space filling of a common tropical tree, Iriartea deltoidea. PLoS One 6:e16386. https://doi.org/10.1371/journal.pone.0016386
Anderson IC, Parkin PI (2007) Detection of active soil fungi by RT-PCR amplification of precursor rRNA molecules. J Microbiol Methods 68:248–253. https://doi.org/10.1016/j.mimet.2006.08.005
Andreasen M, Skrede I, Jaklitsch WM et al (2021) Multi-locus phylogenetic analysis of lophiostomatoid fungi motivates a broad concept of Lophiostoma and reveals nine new species. Persoonia - Mol Phylogeny Evol Fungi. https://doi.org/10.3767/persoonia.2021.46.09
Arnold AE, Lutzoni F (2007) Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88:541–549
Aveskamp MM, de Gruyter J, Woudenberg JHC et al (2010) Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60. https://doi.org/10.3114/sim.2010.65.01
Bencheva S (2014) First report of Cryptostroma corticale (Ellis & Everh.) P.H. Greg. & S. Waller on Acer platanoides L. in Bulgaria. Silva Balcania 15:101–104
Bensch K, Groenewald JZ, Meijer M et al (2018) Cladosporium species in indoor environments. Stud Mycol 89:177–301. https://doi.org/10.1016/j.simyco.2018.03.002
Bien S, Damm U (2020a) Arboricolonus simplex gen. et sp. nov. and novelties in Cadophora, Minutiella and Proliferodiscus from Prunus wood in Germany. MycoKeys 63:119–161. https://doi.org/10.3897/mycokeys.63.46836
Bien S, Damm U (2020b) Prunus trees in Germany - a hideout of unknown fungi? Mycol Prog 19:667–690. https://doi.org/10.1007/s11557-020-01586-4
Bien S, Kraus C, Damm U (2020) Novel collophorina-like genera and species from Prunus trees and vineyards in Germany. Persoonia - Mol Phylogeny Evol Fungi 45:46–67. https://doi.org/10.3767/persoonia.2020.45.02
Bills GF, Platas G, Peláez F, Masurekar P (1999) Reclassification of a pneumocandin-producing anamorph, Glarea lozoyensis gen. et sp. nov., previously identified as Zalerion arboricola. Mycol Res 103:179–192. https://doi.org/10.1017/S095375629800687X
Boddy L, Rayner ADM (1983) Origins of decay in living deciduous trees: the role of moisture content and a re-appraisal of the expanded concept of tree decay. New Phytol 94:623–641. https://doi.org/10.1111/j.1469-8137.1983.tb04871.x
Bork K (2018) Rußrindenkrankheit an Ahorn – Erstfund in Bayern. AFZ – Der Wald 20:40–41
Brglez A, Piškur B, Ogris N (2020a) Eutypella parasitica and other frequently isolated fungi in wood of dead branches of young sycamore maple (Acer pseudoplatanus) in Slovenia. Forests 11:467. https://doi.org/10.3390/f11040467
Brglez A, Piškur B, Ogris N (2020b) In vitro interactions between Eutypella parasitica and some frequently isolated fungi from the wood of the dead branches of young sycamore maple (Acer pseudoplatanus). Forests 11:1072. https://doi.org/10.3390/f11101072
Bueno E, Martin KR, Raguso RA et al (2020) Response of wild spotted wing Drosophila (Drosophila suzukii) to microbial volatiles. J Chem Ecol 46:688–698. https://doi.org/10.1007/s10886-019-01139-4
Bußkamp J (2018) Schadenserhebung, Kartierung und Charakterisierung des „Diplodia-Triebsterbens“ der Kiefer, insbesondere des endophytischen Vorkommens in den klimasensiblen Räumen und Identifikation von den in Kiefer (Pinus sylvestris) vorkommenden Endophyten. Dissertation, Universität Kassel
Bußkamp J, Langer GJ, Langer EJ (2020) Sphaeropsis sapinea and fungal endophyte diversity in twigs of Scots pine (Pinus sylvestris) in Germany. Mycol Prog 19:985–999. https://doi.org/10.1007/s11557-020-01617-0
Butin H, Kowalski T (1986) Die natürliche Astreinigung und ihre biologischen Voraussetzungen. Eur J For Pathol 16:129–138. https://doi.org/10.1111/j.1439-0329.1986.tb01053.x
Cabral A, Groenewald JZ, Rego C et al (2012a) Cylindrocarpon root rot: multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycol Prog 11:655–688. https://doi.org/10.1007/s11557-011-0777-7
Cabral A, Rego C, Nascimento T et al (2012b) Multi-gene analysis and morphology reveal novel Ilyonectria species associated with black foot disease of grapevines. Fungal Biol 116:62–80. https://doi.org/10.1016/j.funbio.2011.09.010
Cech TL (2004) Bermerkenserte Pilzkrankheiten in 2004. Forstsch Aktuell 32:31–34
Cech TL (2019) Rußrindenkrankheit bedroht Ahornbestände in Laubwäldern im Osten Niederösterreichs. Forstsch Aktuell 65:24
Chapela IH (1989) Fungi in healthy stems and branches of American beech and aspen: a comparative study. New Phytol 113:65–75. https://doi.org/10.1111/j.1469-8137.1989.tb02396.x
Chapela IH, Boddy L (1988) Fungal colonization of attached beech branches. I. Early stages of development of fungal communities. New Phytol:39–45. https://doi.org/10.1111/j.1469-8137.1988.tb00235.x
Chlebicki A (1988) Some ascomycetous fungi or their anamorphs occurring on trees in Poland I. Acta Mycol 24:77–92
Crous P, Wingfield MJ, Schumacher RK et al (2020) New and interesting fungi. 3. Fungal Syst Evol 6:157–231. https://doi.org/10.3114/fuse.2020.06.09
Crous PW, Schumacher RK, Akulov A et al (2019) New and interesting fungi. 2. Fungal Syst Evol 3:57–134. https://doi.org/10.3114/fuse.2019.03.06
Crous PW, Schumacher RK, Wingfield MJ et al (2015) Fungal systematics and evolution: FUSE 1. Sydowia 67:81–118. https://doi.org/10.12905/0380.sydowia67-2015-0081
Delb H, Grüner J, Reinhold J, et al (2019) Waldschutzsituation 2018/2019 in Rheinland-Pfalz und Saarland. 2019 22–25
Desprez-Loustau M-L, Marçais B, Nageleisen L-M et al (2006) Interactive effects of drought and pathogens in forest trees. Ann For Sci 63:597–612. https://doi.org/10.1051/forest:2006040
Dickenson SJ (1980) Biology of Cryptostroma corticale and the sooty bark disease of sycamore. Dissertation, University of Bath
DWD (2018) Deutschlandwetter im Sommer 2018. DWD (Deutscher Wetterdienst), Offenbach
DWD (2019) Deutschlandwetter im Sommer 2019. DWD (Deutscher Wetterdienst), Offenbach
DWD (2020) Deutschlandwetter im Sommer 2020. DWD (Deutscher Wetterdienst), Offenbach
Eaton CJ, Cox MP, Scott B (2011) What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci 180:190–195. https://doi.org/10.1016/j.plantsci.2010.10.002
Ellis JB, Everhart BM (1889) New species of hyphomycetes fungi. J Mycol 5:68–72
Ellis MB, Ellis PJ (1985) Microfungi on land plants: an identification handbook. Croom Helm Ltd., London
Enderle R, Riebesehl J, Becker P, Kehr R (2020) Rußrindenkrankheit an Ahorn - Biologie, Pathologie und Entsorgung von Schadholz. In: Jahrbuch der Baumpflege 2020, 24th edn. Haymarket Media, Braunschweig, pp 85–100
Engesser R, Forster B, Meier E, Odermatt O (2004) Forstschutzsituation 2003 in der Schweiz. AFZ – Der Wald 59:385–387
EUFORGEN (2022) Acer pseudoplatanus - sycamore. In: Euforgen. http://www.euforgen.org/species/acer-pseudoplatanus/. Accessed 25 Mar 2022
Evans HC, Holmes KA, Thomas SE (2003) Endophytes and mycoparasites associated with an indigenous forest tree, Theobroma gileri, in Ecuador and a preliminary assessment of their potential as biocontrol agents of cocoa diseases. Mycol Prog 2:149–160. https://doi.org/10.1007/s11557-006-0053-4
Farr DF, Rossman AY (2022) Fungal databases - fungus-host distributions. In: US Natl. Fungus Collect. Fungal database. https://nt.ars-grin.gov/fungaldatabases/fungushost/fungushost.cfm. Accessed 4 May 2022
Frisvad JC, Houbraken J, Popma S, Samson RA (2013) Two new Penicillium species Penicillium buchwaldii and Penicillium spathulatum , producing the anticancer compound asperphenamate. FEMS Microbiol Lett 339:77–92. https://doi.org/10.1111/1574-6968.12054
Gao C-H (2021) ggVennDiagram: A “ggplot2” implement of Venn Diagram. In: GgVennDiagram Ggplot2 implement Venn Diagr. https://CRAN.R-project.org/package=ggVennDiagram
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gennaro M, Gonthier P, Nicolotti G (2003) Fungal endophytic communities in healthy and declining Quercus robur L. and Q. cerris L. trees in northern Italy. J Phytopathol 151:529–534. https://doi.org/10.1046/j.1439-0434.2003.00763.x
Ghobad-Nejhad M, Meyn R, Langer E (2018) Endophytic fungi isolated from healthy and declining Persian oak (Quercus brantii) in western Iran. Nova Hedwigia 107:273–290. https://doi.org/10.1127/nova_hedwigia/2018/0470
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Glynou K, Thines M, Maciá-Vicente JG (2018) Host species identity in annual Brassicaceae has a limited effect on the assembly of root-endophytic fungal communities. Plant Ecol Divers 11:569–580. https://doi.org/10.1080/17550874.2018.1504332
Gomes RR, Glienke C, Videira SIR et al (2013) Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia Mol Phylogeny Evol Fungi 31:1
Gregory PH, Waller S (1951) Cryptostroma corticale and sooty bark disease of sycamore (Acer pseudoplatanus). Trans Br Mycol Soc 34:579–597. https://doi.org/10.1016/S0007-1536(51)80043-3
Guo LD, Hyde KD, Liew ECY (2000) Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol 147:617–630
Guo LD, Hyde KD, Liew ECY (2001) Detection and taxonomic placement of endophytic fungi within frond tissues of Livistona chinensis based on rDNA sequences. Mol Phylogenet Evol 20:1–13. https://doi.org/10.1006/mpev.2001.0942
Haenzi M, Cochard B, Chablais R et al (2021) Neofusicoccum parvum, a new agent of sequoia canker and dieback identified in Geneva, Switzerland. Forests 12:434. https://doi.org/10.3390/f12040434
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98. https://doi.org/10.14601/Phytopathol_Mediterr-14998u1.29
Halley JM, Comins HN, Lawton JH, Hassell MP (1994) Competition, succession and pattern in fungal communities: towards a cellular automaton model. Oikos 70:435–442. https://doi.org/10.2307/3545783
Hendry SJ, Boddy L, Lonsdale D (2002) Abiotic variables effect differential expression of latent infections in beech (Fagus sylvatica). New Phytol 155:449–460. https://doi.org/10.1046/j.1469-8137.2002.00473.x
Hosoya T, Hosaka K, Nam KO (2018) A check list of non-lichenised fungi occurring on Fagus crenata, a tree endemic to Japan. Mycology 9:29–34. https://doi.org/10.1080/21501203.2017.1363092
Hyde KD, Soytong K (2008) The fungal endophyte dilemma. Fungal Divers 33:163–173
Itagaki H, Hosoya T (2021) Lifecycle of Pyrenopeziza protrusa (Helotiales, Dermateaceae sensu lato) in Magnolia obovata revealed by field observation and molecular quantification. Mycoscience 62:373–381. https://doi.org/10.47371/mycosci.2021.08.001
Izumitsu K, Hatoh K, Sumita T et al (2012) Rapid and simple preparation of mushroom DNA directly from colonies and fruiting bodies for PCR. Mycoscience 53:396–401
Jaklitsch WM, Fournier J, Rogers JD, Voglmayr H (2014) Phylogenetic and taxonomic revision of Lopadostoma. Persoonia - Mol Phylogeny Evol Fungi 32:52–82. https://doi.org/10.3767/003158514X679272
Jaklitsch WM, Voglmayr H (2016) Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Stud Mycol 85:35–64. https://doi.org/10.1016/j.simyco.2016.09.002
Jankowiak R (2005) Fungi associated with Ips typographus on Picea abies in southern Poland and their succession into the phloem and sapwood of beetle-infested trees and logs. For Pathol 35:37–55. https://doi.org/10.1111/j.1439-0329.2004.00395.x
Jankowiak R, Bilański P, Paluch J, Kołodziej Z (2016) Fungi associated with dieback of Abies alba seedlings in naturally regenerating forest ecosystems. Fungal Ecol 24:61–69. https://doi.org/10.1016/j.funeco.2016.08.013
Kelnarová I, Černý K, Zahradník D, Koukol O (2017) Widespread latent infection of Cryptostroma corticale in asymptomatic Acer pseudoplatanus as a risk for urban plantations. For Pathol 47:e12344. https://doi.org/10.1111/efp.12344
Koukol O, Kelnarová I, Černý K (2014) Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. For Pathol 45:21–27. https://doi.org/10.1111/efp.12129
Kowalski T, Bilański P (2021) Fungi detected in the previous year’s leaf petioles of Fraxinus excelsior and their antagonistic potential against Hymenoscyphus fraxineus. Forests 12:1412. https://doi.org/10.3390/f12101412
Kowalski T, Kehr RD (1992) Endophytic fungal colonization of branch bases in several forest tree species. Sydowia 44:137–168
Kruys Å, Castlebury LA (2012) Molecular phylogeny of Sydowiellaceae—resolving the position of Cainiella. Mycologia 104:419–426. https://doi.org/10.3852/11-163
Kuhnert E, Fournier J, Peršoh D et al (2014) New Hypoxylon species from Martinique and new evidence on the molecular phylogeny of Hypoxylon based on ITS rDNA and β-tubulin data. Fungal Divers 64:181–203. https://doi.org/10.1007/s13225-013-0264-3
Kuo H-C, Hui S, Choi J et al (2015) Secret lifestyles of Neurospora crassa. Sci Rep 4:5135. https://doi.org/10.1038/srep05135
Langer GJ (1994) Die Gattung Botryobasidium DONK (Corticiaceae, Basidiomycetes). J. Cramer, Berlin, Stuttgart
Langer GJ (2017) Collar rots in forests of Northwest Germany affected by ash dieback. Balt For 23:4–19
Langer GJ, Bressem U, Habermann M (2013) Vermehrt Pilzkrankheiten an Bergahorn in Nordwestdeutschland. AFZ - Der Wald 6:22–26
Langer GJ, Bußkamp J, Blumenstein K, Terhonen E (2021) Fungi inhabiting woody tree tissues – stems, branches, and twigs. In: Forest microbiome. Elsevier, London, San Diego, Cambridge, Oxford, pp 175–205
Li WJ, McKenzie EHC, Liu JK et al (2020) Taxonomy and phylogeny of hyaline-spored coelomycetes. Fungal Divers 100:279–801. https://doi.org/10.1007/s13225-020-00440-y
Lygis V, Vasiliauskas R, Larsson K-H, Stenlid J (2005) Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand J For Res 20:337–346
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748. https://doi.org/10.1126/science.1143082
Martínez-Álvarez P, Rodríguez-Ceinós S, Martín-García J, Diez JJ (2012) Monitoring endophyte populations in pine plantations and native oak forests in Northern Spain. For Syst 21:373–382. https://doi.org/10.5424/fs/2012213-02254
Metzler B (2006) Cryptostroma corticale an Bergahorn nach dem Trockenjahr 2003. Mitteilungen Biol Bundesanst Für Land- Forstwirtsch 400:161–162
Muggia L, Kopun T, Grube M (2017) Effects of growth media on the diversity of culturable fungi from lichens. Molecules 22:824. https://doi.org/10.3390/molecules22050824
Nugent LK, Sihanonth P, Thienhirun S, Whalley AJS (2005) Biscogniauxia: a genus of latent invaders. Mycologist 19:40–43. https://doi.org/10.1017/S0269915X05001060
NW-FVA (2020) Waldschutzinfo Nr. 1 / 2020 - Witterung 2019. Nordwestdeutsche Forstliche Versuchsanstalt, Göttingen
NW-FVA (2021) Waldschutzinfo Nr. 1 / 2021 Witterung 2020. Nordweestdeutsche Forstliche Versuchsanstalt, Göttingen
O’Connell RJ, Thon MR, Hacquard S et al (2012) Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat Genet 44:1060–1065. https://doi.org/10.1038/ng.2372
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 7:103–116. https://doi.org/10.1006/mpev.1996.0376
Ogris N, Brglez A, Piškur B (2021) Drought stress can induce the pathogenicity of Cryptostroma corticale, the causal agent of Sooty Bark Disease of sycamore maple. Forests 12:377. https://doi.org/10.3390/f12030377
Oksanen J, Simpson GL, Guillaume Blanchet F, et al (2022) vegan: community ecology package. In: Vegan community ecol. package. https://CRAN.R-project.org/package=vegan. Accessed 20 Sep 2022
Oliveira Longa CM, Vai N, Maresi G (2016) Cryptostroma corticale in the northern Apennines (Italy). Phytopathol Mediterr 55:136–138. https://doi.org/10.14601/Phytopathol_Mediterr-17164
Parfitt D, Hunt J, Dockrell D et al (2010) Do all trees carry the seeds of their own destruction? PCR reveals numerous wood decay fungi latently present in sapwood of a wide range of angiosperm trees. Fungal Ecol 3:338–346. https://doi.org/10.1016/j.funeco.2010.02.001
Peršoh D, Melcher M, Flessa F, Rambold G (2010) First fungal community analyses of endophytic ascomycetes associated with Viscum album ssp. austriacum and its host Pinus sylvestris. Fungal Biol 114:585–596. https://doi.org/10.1016/j.funbio.2010.04.009
Petrini O (1991) Fungal endophytes of tree leaves. In: Andrews HJ, Hirano SS (eds) Microbial ecology of leaves. Springer-Verlag, New York and Berlin, pp 179–197
Petrini O, Fisher PJ (1988) A comparative study of fungal endophytes in xylem and whole stem of Pinus sylvestris and Fagus sylvatica. Trans Br Mycol Soc 91:233–238. https://doi.org/10.1016/S0007-1536(88)80210-9
Petrini-Klieber LE (1985) Untersuchungen über die Gattung Hypoxylon (Ascomycetes) und verwandte Pilze. Dissertation, ETH Zürich
Plate HP, Schneider R (1965) Ein Fall von asthmaartiger Allergie, verursacht durch den Pilz Cryptostroma corticale. Nachrichtenblatt Dtsch Pflanzenschutzdienstes 17:100–101
Promputtha I, Hyde KD, McKenzie EHC et al (2010) Can leaf degrading enzymes provide evidence that endophytic fungi becoming saprobes? Fungal Divers 41:89–99. https://doi.org/10.1007/s13225-010-0024-6
Quaedvlieg W, Verkley GJM, Shin HD et al (2013) Sizing up Septoria. Stud Mycol 75:307–390
Queloz V, Forster B, Beenken L, et al (2020) Waldschutzüberblick 2019. Eidg. Forschungsanstalt für Wald, Schnee und Landschaft WSL, CH-8903 Birmensdorf
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Ragazzi A, Moricca S, Capretti P et al (2003) Differences in composition of endophytic mycobiota in twigs and leaves of healthy and declining Quercus species in Italy. For Pathol 33:31–38. https://doi.org/10.1046/j.1439-0329.2003.3062003.x
Robeck P, Heinrich R, Schumacher J et al (2008) Status der Rußrindenkrankheit des Ahorns in Deutschland. In: Jahrbuch der Baumpflege. Haymarket Media GmbH, Braunschweig, pp 238–244
Rohde M, Langer G, Hurling R, Plašil P (2019) Waldschutzsituation 2018 in Nordwestdeutschland. AFZ – Der Wald 74:38–41
Runge F (1994) Die Pflanzengesellschaften Mitteleuropas, 12/13 edn. Aschendorff Verlag, Münster (Westfalen)
Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Annu Rev Ecol Syst 29:319–343
Saikkonen K, Saari S, Helander M (2010) Defensive mutualism between plants and endophytic fungi? Fungal Divers 41:101–113. https://doi.org/10.1007/s13225-010-0023-7
Samuels GJ, Dodd SL, Lu BS et al (2006) The Trichoderma koningii aggregate species. Stud Mycol 56:67–133. https://doi.org/10.3114/sim.2006.56.03
Sanz-Ros AV, Müller MM, San Martín R, Diez JJ (2015) Fungal endophytic communities on twigs of fast and slow growing Scots pine (Pinus sylvestris L.) in northern Spain. Fungal Biol 119:870–883. https://doi.org/10.1016/j.funbio.2015.06.008
Schlegel M, Queloz V, Sieber TN (2018) The endophytic mycobiome of European ash and sycamore maple leaves – geographic patterns, host specificity and influence of ash dieback. Front Microbiol 9:2345. https://doi.org/10.3389/fmicb.2018.02345
Schlößer R, Langer G (2021) Verbreitung der Rußrindenkrankheit des Ahorns in Deutschland. AFZ – Der Wald 24:28–32
Schmidt PA (2009) Der Bergahorn – eine typische Mischwald-Baumart süd-mitteleuropäischer Bergwälder. In: Bayerische Landesanstalt für Wald und Forstwirtschaft (LWF) (ed) Beiträge zum Bergahorn. Freising, pp 13–18
Scholtysik A, Unterseher M, Otto P, Wirth C (2013) Spatio-temporal dynamics of endophyte diversity in the canopy of European ash (Fraxinus excelsior). Mycol Prog 12:291–304. https://doi.org/10.1007/s11557-012-0835-9
Schulthess FM, Faeth SH (1998) Distribution, abundances, and associations of the endophytic fungal community of Arizona fescue (Festuca arizonica). Mycologia 90:569–578. https://doi.org/10.1080/00275514.1998.12026945
Schwarze FWMR (2018) Diagnose und Prognose der Fäuledynamik in Stadtbäumen. MycoSolutions AG, ST. Gallen
Senanayake I, Al-Sadi AM, Bhat JD et al (2016) Phomatosporales ord. nov. and Phomatosporaceae fam. nov., to accommodate Lanspora, Phomatospora and Tenuimurus, gen. nov. Mycosphere 7:628–641. https://doi.org/10.5943/mycosphere/7/5/8
Sieber TN (2007) Endophytic fungi in forest trees: are they mutualists? Fungal Biol Rev 21:75–89. https://doi.org/10.1016/j.fbr.2007.05.004
Singh DK, Sharma VK, Kumar J et al (2017) Diversity of endophytic mycobiota of tropical tree Tectona grandis Linn.f.: spatiotemporal and tissue type effects. Sci Rep 7:3745. https://doi.org/10.1038/s41598-017-03933-0
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21:90–106. https://doi.org/10.1016/j.fbr.2007.06.002
Spies CFJ, Mostert L, Carlucci A et al (2020) Dieback and decline pathogens of olive trees in South Africa. Persoonia - Mol Phylogeny Evol Fungi 45:196–220. https://doi.org/10.3767/persoonia.2020.45.08
Sun X, Guo LD, Hyde KD (2011) Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Divers 47:85–95. https://doi.org/10.1007/s13225-010-0086-5
Suryanarayanan TS (2011) Diversity of fungal endophytes in tropical trees. In: Pirttilä AM, Frank AC (eds) Endophytes of forest trees. Springer Netherlands, Dordrecht, pp 67–80
Townrow JA (1953) The Biology of Cryptostroma corticale and the sooty bark disease of sycamore. Report on forest research, London, pp 118–120
Travadon R, Lecomte P, Diarra P, et al (2015) Influence of pruning systems on trunk pathogens and other fungi colonizing grapevine wood. Am Phytopathol Soc Abstr 68-O
Tropf J (2020) Mykologische und histologische Untersuchungen im Zusammenhang mit der Rußrindenkrankheit, verursacht durch Cryptostroma corticale. Master’s Thesis, Albert-Ludwigs-Universität Freiburg
Trouillas FP, Gubler WD (2004) Identification and characterization of Eutypa leptoplaca, a new pathogen of grapevine in Northern California. Mycol Res 108:1195–1204. https://doi.org/10.1017/S0953756204000863
Untereiner WA, Naveau FA (1999) Molecular systematics of the Herpotrichiellaceae with an assessment of the phylogenetic positions of Exophiala dermatitidis and Phialophora americana. Mycologia 91:67–83. https://doi.org/10.1080/00275514.1999.12060994
Unterseher M (2007) Fungal biodiversity research – from cultivation of foliar tree endophytes to the combination of ecological theory and next generation sequencing. Ernst Moritz Arndt Universität Greifswald, Dissertation
Unterseher M, Otto P, Morawetz W (2005) Species richness and substrate specificity of lignicolous fungi in the canopy of a temperate, mixed deciduous forest. Mycol Prog 4:117–132. https://doi.org/10.1007/s11557-006-0115-7
Utermann C, Echelmeyer VA, Oppong-Danquah E et al (2020) Diversity, bioactivity profiling and untargeted metabolomics of the cultivable gut microbiota of Ciona intestinalis. Mar Drugs 19:6. https://doi.org/10.3390/md19010006
Větrovský T, Kolařík M, Žifčáková L et al (2016) The rpb2 gene represents a viable alternative molecular marker for the analysis of environmental fungal communities. Mol Ecol Resour 16:388–401. https://doi.org/10.1111/1755-0998.12456
Videira SIR, Groenewald JZ, Braun U et al (2016) All that glitters is not Ramularia. Stud Mycol 83:49–163. https://doi.org/10.1016/j.simyco.2016.06.001
Voglmayr H, Aguirre-Hudson MB, Wagner HG et al (2019) Lichens or endophytes? The enigmatic genus Leptosillia in the Leptosilliaceae fam. nov. (Xylariales), and Furfurella gen. nov. (Delonicicolaceae). Persoonia - Mol Phylogeny Evol Fungi 42:228–260. https://doi.org/10.3767/persoonia.2019.42.09
Vu GM, de Vries M et al (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol 92:135–154. https://doi.org/10.1016/j.simyco.2018.05.001
Wanasinghe D, Phookamsak R, Jeewon R et al (2017) A family level rDNA based phylogeny of Cucurbitariaceae and Fenestellaceae with descriptions of new Fenestella species and Neocucurbitaria gen. nov. Mycosphere 8:397–414. https://doi.org/10.5943/mycosphere/8/4/2
Wenzel A, Thiel J, Stürtz M (2019) Waldschutzsituation 2018/19 in Thüringen. AFZ - Der Wald 74:26–29
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl 18:315–322
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wickham H (2011) The split-apply-combine strategy for data analysis. J Stat Softw 40:1–29
Wickham H, Averick M, Bryan J et al (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Worrall JJ, Anagnost SE, Zabel RA (1997) Comparison of wood decay among diverse lignicolous fungi. Mycologia 89:199–219
Wysoczański W, Węgrzyn E, Lembicz M, Jaroszewicz B (2021) Fungal microbiota in seeds, seedlings and mature plants of raspberry (Rubus ideaus L.). Eur J Plant Pathol 161:815–820. https://doi.org/10.1007/s10658-021-02364-y
Zhang W, Groenewald JZ, Lombard L et al (2021) Evaluating species in Botryosphaeriales. Persoonia - Mol Phylogeny Evol Fungi 46:63–115. https://doi.org/10.3767/persoonia.2021.46.03
Zhao P, Luo J, Zhuang W et al (2011) DNA barcoding of the fungal genus Neonectria and the discovery of two new species. Sci China Life Sci 54:664–674. https://doi.org/10.1007/s11427-011-4184-8
Zhou LW, Vlasak JJ, Vlasak J (2014) Inonotus andersonii and I. krawtzewii: another case of molecular sequencing-based diagnosis of morphologically similar species. Chiang Mai J Sci 41:789–797
Acknowledgements
The authors thank Peter Gawehn, Annette Ihlemann, Martina Hille, and Ulrike Frieling for their technical support. We also thank all employees of HessenForst involved in this study for their cooperation. Special thanks go to Maia Ridley for proofreading the manuscript. Thanks also go to the reviewers and the editors for their valuable recommendations to improve the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Project in which this study was conducted is funded by the Hessian Ministry of the Environment, Climate Protection, Agriculture and Consumer Protection and located at the Northwest German Forest Research Institute (NW-FVA).
Author information
Authors and Affiliations
Contributions
The study including sampling, lab work, and analysis was primarily conducted by R. Schlößer with support from G. Langer and S. Bien. The first draft of the manuscript was written by R. Schlößer and revised by S. Bien, G. Langer, and E. Langer.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Claus Baessler
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlößer, R., Bien, S., Langer, G.J. et al. Fungi associated with woody tissues of Acer pseudoplatanus in forest stands with different health status concerning sooty bark disease (Cryptostroma corticale). Mycol Progress 22, 13 (2023). https://doi.org/10.1007/s11557-022-01861-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-022-01861-6