Abstract
In recent decades the vitality and physical stability of European ash trees in Germany have been reduced by European ash dieback, especially when associated with stem collar necroses and rots. This study was carried out to investigate the composition of the fungal communities associated with stem collar necroses. Filamentous fungi were isolated from 58 ash trees out of nine forest stands in northern, eastern, and central Germany. Obtained isolates were identified to a genus or species level by means of morphological and molecular analyses. In total 162 morphotypes including endophytic, saprotrophic, and pathogenic fungi were isolated. For 33 species found no prior reports from Fraxinus excelsior were recognised, including Cryptostroma corticale and Diplodia sapinea. None of the identified species were found at all studied sites, though Diplodia fraxini was the most common fungus with regard to frequency within all isolates, occurring at seven sample sites. This species is followed by Hymenoscyphus fraxineus, Armillaria spp., Neonectria punicea, Diaporthe cf. eres, Fusarium cf. lateritium, and Paracucurbitaria sp. in order of frequency within all isolates. The aforementioned species are characterised and analysed in respect to their occurrence in stem collar necroses and at sample sites. The influence of site conditions on the fungal composition was described for five intensively sampled sites with a minimum of five studied trees (Schwansee, Rhüden, Berggießhübel, Satrup, and Schlangen). The sampling site of Schlangen was further subdivided into four subplots with different positions in the terrain. In the remaining four extensive sample sites, either one or two trees, respectively, were sampled and analysed (Oranienbaumer Heide, Woltershausen, Wolfenbüttel, and Neuhege). Over all sample sites, fungal communities of symptomatic stem tissue are similar concerning the most frequent fungi, but vary greatly according to singularly isolated fungi.
Similar content being viewed by others
Introduction
Since the early 1990s, the European ash (Fraxinus excelsior L.) is threatened by European ash dieback, caused by Hymenoscyphus fraxineus (T. Kowalski) Baral, Queloz, & Hosoya (Helotiaceae, Ascomycota). First disease reports came from Poland and Lithuania (Przybyl 2002; Lygis et al. 2005). In Germany, the severe disease was observed since 2002 and the causal agent was first proven in the year 2006 (Heydeck et al. 2005; Schumacher et al. 2007). Meanwhile, this invasive fungal pathogen has become widespread in Europe. Affected European ash (hereafter referred to as ash) trees of all ages show a broad range of symptoms, such as leaf necrosis, wilting, shoot blight, inner bark discolorations, sunken cankers, epicormic shoots, as well as stem collar, and root necrosis (Gross et al. 2014; Langer 2017). Reduction of tree stability and increase of mortality of ash is often connected to stem collar necrosis, which progresses towards xylem and heartwood. Stem collar necrosis can occur on trees with or without crown symptoms of ash dieback, but is often observed on diseased trees (Schumacher et al. 2009; Husson et al. 2012; Enderle et al. 2017; Langer 2017; Meyn et al. 2019). Stem collar necroses are defined as basal lesions with necrotic tissue on the outside and the inside of the stem mainly caused by fungi (Langer 2017). The actual shape of stem collar necroses depends on different factors, such as individual progress or associated fungi. Advanced necroses are often associated with wood rot caused by fungi colonising the stem following the initial infection by H. fraxineus (Langer 2017).
Even though many fungi are reported from F. excelsior (981 different species according to the USDA website, Farr and Rossman 2022, retrieved on 17.06.2022) fungi associated with necrotic stem tissue of ash, specifically in Germany, have rarely been described (Enderle et al. 2017; Langer 2017; Meyn et al. 2019). Most frequently isolated from the necroses at the collar base of diseased ash trees were Armillaria spp., Diaporthe eres Nitschke, Diplodia spp., Fusarium avenaceum (Fr.) Sacc., F. lateritium Nees, Fusarium solani (Mart.) Sacc. (syn. Neocosmospora solani (Mart.) L. Lombard & Crous), H. fraxineus, and Neonectria punicea (J. C. Schmidt) Castl. & Rossman (Lygis et al. 2005; Langer 2017; Meyn et al. 2019; Linaldeddu et al. 2020). A strong evidence for H. fraxineus being the causal agent of ash dieback was given by Chandelier et al. (2016), who proved occurrence of H. fraxineus in the majority of symptomatic tissue of ash stem collar. Langer (2017) confirmed frequent isolation from stem collar necroses and the assignment as primary agent. But not every type of stem collar necrosis must be primarily caused by H. fraxineus. Langer (2017) showed that basal necroses can be caused by Phytophthora under special site conditions, as found in floodplain forests or by Armillaria spp. on weakened ash trees. The path of infection by H. fraxineus still remains unknown and little is understood about the influence of environmental factors on stem collar necroses. Site characteristics, such as moisture content, are assumed to affect disease severity. Kenigsvalde et al. (2010) and Marçais et al. (2016) determined that disease severity correlates positively with soil humidity conditions. It has been suggested that stem collar necroses development and extent is also related to moist conditions or humid topographical positions (Marçais et al. 2016). Therefore the design of this study covers a wide range of water supply types at sampling sites. The composition of the forest stands combined with their nutrient and water availability could be a factor in assessing differences in fungal diversity per stand. Most trees moderately and severely damaged due to ash dieback were observed at forest sites with a high soil organic matter content and a neutral to slightly alkaline soil pH (Turczański et al. 2019). Hence, it can be suspected that fungal composition depends on soil and water availability just as well (Linaldeddu et al. 2011; Salamon et al. 2020).
This study is conducted as part of the demonstration project FraxForFuture and the sub-network FraxPath (Langer et al. 2022). The aims of this research are to fill knowledge gaps concerning the α-diversity of cultivable Dikarya Hibbett, T. Y. James & Vilgalys associated with stem collar necroses of trees affected by ash dieback and the composition of their fungal communities. Therefore, fungi associated with necrotic stem bases of ash were isolated and identified from 58 ash trees in order to determine the continuity and the frequency of H. fraxineus and secondary fungi. The role of the most frequent fungi in the process of stem collar necroses formation is discussed.
Materials and methods
Sampling sites
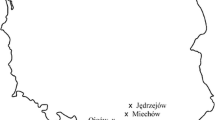
In total, six federal states of Germany (Lower Saxony, North Rhine-Westphalia, Saxony, Saxony-Anhalt, Schleswig-Holstein, and Thuringia) were investigated. Nine mixed broad-leaved forest stands with a substantial share of F. excelsior affected by ash dieback were selected in order to cover different sites with a wide range of soil water supply types (Table 1 and Fig. 1). The sample sites are located in northern, eastern, and central Germany with sub-oceanic to sub-continental temperate zones. All sites are eutrophic and cover the most common substrates of ash stands in Germany. Basic soil and geological data were acquired by using geological maps with a high resolution (scale 1:25000) of the respective federal geology departments and the forest inventory and forest site mapping data sets of the federal forestry authorities. Additional data from soil core sampling, soil profiles, and soil analyses were available for the sampling sites of Rhüden, Berggießhübel, Schwansee, and Schlangen because these sites are part of other studies associated with soil inventories: The site of Rhüden is part of the national forest soil inventory. Schwansee and Berggießhübel correspond to the intensive monitoring plots “TH_1 Schwansee” and “SN_2 Bienhof” of the research cluster FraxForFuture (Langer et al. 2022). The largest forest stand continuously including ash trees investigated in this study (Schlangen) has a pronounced relief and was divided into four subplots to investigate different positions in the terrain. In this case, intensive soil exploration was conducted to differentiate between the four subplots, including pedological assessments and soil sampling from soil profiles.
Sampling sites in Germany divided in intensive (red) and extensive (yellow) sampling sites with a detailed view of the special study site Schlangen and its feature of splitting in four subplots with different terrain positions (resources: QGIS 3.24 © GeoBasis-DE/BKG (2022), DGM1 © GeoBasis NRW (2021))
The shallow sites (Rhüden, Schlangen 2) in exposed terrain positions on limestone with high coarse soil fractions and low water storage capacities represent the driest end of the ecological niche of ash. Several other sites have moderate (Schlangen 3, Schlangen 4, Wolterhausen) or high water storage capacities (Wolfenbüttel, Schlangen 1) because of medium to deep loamy loess covers or colluvial deposits. The remaining sites are primarily characterised by either stagnic soil conditions (Satrup, Berggießhübel), slope water influence (Berggießhübel), or groundwater influence (Oranienbaumer Heide, Schwansee, Neuhege). Soil substrate, water retention capacity, terrain relief, climate, and presence/absence of groundwater or stagnic soil properties (Table 1) were combined to create a ranking of site water supplies of the sampling sites (Online Resource 1 and Online Resource 2).
Between one and nineteen trees were excavated and sampled per site. Hence, the sampling sites were divided into intensive and extensive sampling sites. The intensive sampling sites had a minimum of five and a maximum of 19 sample trees. Intensive sampling was conducted in Rhüden (10 sampled trees), Satrup (5), Berggießhübel (11), Schwansee (8), and Schlangen (19). Each intensive sampling site or subplot was 0.2–0.5 ha in size. The extensive sampling sites with only one or two examined ash trees were taken into account only for fungal occurrence: Woltershausen (2 sampled trees), Wolfenbüttel (1), Neuhege (1), and Oranienbaumer Heide (1). These individual trees were included to increase the sample set and the distribution of investigated stem collar necroses and their associated fungi.
Sampled trees
In total 58 ash trees were sampled, including six trees initially selected as control trees (two in Berggießhübel, one in Satrup, and three in Schlangen; Online Resource 3). The diameter at breast height of the sampled ash trees ranged from approximately 7–25 cm. The age of the sample trees ranged from 15 up to 80 years. The majority was approximately 40 years old. Classification of stem base and crown condition of the studied trees was carried out according to the guidelines of Peters et al. (2021a, b). Additionally, the neighbouring tree species occurring in the studied stands were noted (Table 1).
Ash trees were felled in the years 2020–2021 and cut at least 15 cm above the visible necrotic area. Subsequently, trunk bases and the uppermost parts of the main roots were dug out with picks and shovels. Depending on soil structure (rock content) final roots were cut by chainsaw with a .325-in. Rapid Duro 3 (RD3), 1.6-mm chainsaw chain (STIHL AG & Co. KG, Dieburg, Germany). The stem collars were transported to laboratory in clean and marked plastic bags.
Isolation of fungi
In preparation for fungal isolation, tree stems were cleaned with a coarse brush under tap water. After air-drying overnight, the samples were processed in the laboratory. Each sample was photographed for documentation. Bark was removed from symptomatic stem areas at the transition zones of living and dead woody tissue. Depending on the thickness of the bark either a sterilised knife or a scalpel was used for removal of the bark. The exposed tissue was sprayed with 70% ethanol all over the necrosis. For chipping of wood tissue samples a chisel and hammer were used. All tools were sterilised by flame shortly before each use. The top tissue layer of the sample was discarded and only the sterile layer directly underneath was incubated. The number of wood chips taken from each tree varied depending on the size of the necrosis. Three of the 5–10-mm-long wood chips were placed in a 90 mm petri dish containing malt yeast peptone (MYP) agar, modified according to Langer (1994) containing 0.7% malt extract (Merck, Darmstadt, Germany), 0.05% yeast extract (Fluka, Seelze, Germany), 0.1% peptone (Merck) and 1.5% agar (Fluka). Once the surface of necroses was processed, stem collars were cut longitudinally with a band saw and carefully sanded for better visualisation of the discolorations. The longitudinal sections were treated according to the isolation method for the surface of necrosis described above. Wood chips were taken at the edge of the necroses and at the transition areas of different discolorations. The process was repeated with the cross section of the stem (Fig. 2).
Fungi associated with the stem collar necrosis of ash tree number 53 (Online resource 3) from the sampling site Schlangen 2. Isolation loci are numbered. a Sampled stem base from the outside, b longitudinal-section of stem base with visible wood discoloration, c basal cross-section of stem base, and d cross-section of the stem base above ground level every 10–15 cm
The petri dishes containing wood chips were incubated at room temperature under ambient daylight for four weeks. The cultures were checked for isolates once a week. Emerging mycelia of filamentous fungi were sub-cultured into pure cultures. The pure cultures were grouped into morphotypes (MT) based on similarity of colony morphology. At least one representative culture for each MT was stored in MYP slants at 4 °C at the fungal culture collection of the Northwest German Forest Research Institute (NW-FVA). Beside the MT assignment, contaminated or overgrown fungi were summarised under “Fungus sp.”.
The frequency of each isolated fungal MT within all isolates (fMT) was specified as the percentage of this particular MT in all isolates. To measure the ratio of morphotype isolates to isolation attempts, the frequency of isolated fungal MT in relation to the total amount of wood chips (fWC) is used. Continuity of isolated MT is defined as the number of sampled trees where the MT was detected in relation to the total number of sampled trees. Analyses of the fungal diversity found in this study were conducted using RStudio (v. 4.1.2, R Core Team 2021). The packages used were tidyverse (Wickham et al. 2019), ggplot 2 (Wickham 2016), and ggVennDiagram (Gao 2021).
The dependency of fWC of the most commonly isolated fungal MT on site water supply ranks of the intensive sampling sites was tested by using Dirichlet regression (Maier 2014) and the R add-on package DirichletReg (Maier 2021). A non-parametric test is necessary because site water supply is an ordinal variable. The ranks are based on a combination of soil water retention, soil stagnic properties, relief, topography, and climate characteristics of the study sites and correspond to the site descriptions in Table 1 and Online Resource 1.
Molecular analysis
For molecular analysis, at least one representative strain from each MT was chosen. Mycelium was placed in 1.5-ml Eppendorf tubes with three glass beads (3 mm) and 150 μl of TE buffer (10 ml 1 mmol Tris HCl (pH 0.8), 2 ml 0.5 mmol EDTA; Carl Roth, Karlsruhe, Germany), and crushed in a Mixer Mill MM 200 (Retsch, Haan, Germany) with 25 vibrations per second for 90 s. Subsequently, genomic DNA was extracted following the protocol of Izumitsu et al. (2012).
The 5.8S nuclear ribosomal gene with the two flanking internal transcribed spacers ITS-1 and ITS-2 (ITS region) was amplified for all strains using the primer pair ITS-1F (Gardes and Bruns 1993) and ITS-4 (White et al. 1990). Additionally, for a selection of strains belonging to Armillaria, a partial sequence of the translation elongation factor 1α (EF-1α) was amplified using the primer pair EF595F + EF1160R (Kauserud and Schumacher 2001). The PCR mixture consisted of 1 μl of DNA and 19 μl mastermix which contained 2.5 μl 10× PCR reaction buffer (with 20 mM MgCl2, Carl Roth, Karlsruhe, Germany), 1 μl of each primer (10 mmol), 2.5 μl MgCl2 (25 mmol), 0.1 μl Roti®-Pol Taq HY Taq polymerase (Carl Roth, Karlsruhe, Germany) and 2.5 μl of 2 mmol dNTPs (Biozym Scientific GmbH, Hessisch Oldendorf, Germany). Each reaction was topped up to a volume of 20 μl by adding sterile water. A StepOnePlus™ PCR System (Applied Biosystems, Waltham, Massachusetts, US) was used to carry out the DNA amplifications. The PCR conditions for the amplification of the ITS and EF-1α regions were set according to Bien et al. (2020) and Guo et al. (2016), respectively. A 1% agarose gel was used to visualise the PCR products. The products were sent to Eurofins Scientific Laboratory (Ebersberg, Germany) for sequencing. Initially, PCR samples of the ITS region were sequenced using the forward reaction (primer ITS-1F). In case of imprecise results, additionally, reverse reactions (primer ITS-4) were sequenced. PCR products of the EF-1α sequence region were sequenced by the respective forward and reverse reactions. All resulting sequences were visually checked and edited as follows using BioEdit Sequence Alignment Editor (v. 7.2.5; Hall 1999). Consensus sequences were generated, for all strains with forward and reverse sequences available. Defective sequence beginnings and ends were trimmed and erroneous nucleotide allocations corrected. Sequences were submitted to GenBank (Table 2).
Identification of fungi
Only cultivatable Dikarya fungi were investigated, however, yeasts were not taken into account. Only isolates which are clearly growing from the wood chips were determined. Obvious contamination with filamentous fungi was not considered. The genus Trichoderma was not included in the analyses, because it is difficult to assess whether these very fast-growing fungi were contaminations or real outgrowth from the wood.
MT were assigned to fungal taxa based on morphological observation and molecular analysis of representative strains following the method of Guo et al. (2000). For fungal taxon determination blastn searches based on ITS sequences were conducted on the GenBank database (http://www.ncbi.nlm.nih.gov/genbank, Altschul et al. 1997) excluding uncultured/environmental sample sequences from the search. Results were critically interpreted with emphasis on well-curated culture collections, such as the Westerdijk Fungal Biodiversity Collection (CBS). In general, blastn results on a species level below a threshold of 98% identity were not trusted to be accurate enough for final determination. In case no definite affiliation to a specific taxonomic level was possible, for example because of more than one hit with a threshold of over 98% identity, the identification was marked by cf. (confer) to indicate uncertainties. The results were re-checked against literature and previously identified cultures from the institute’s collection for confirmation. In addition to blastn searches, extended analyses for taxon determination on a species level were conducted for isolates belonging to Diplodia and Armillaria due to the considerable number of isolates from these genera. Phylogenetic analyses were conducted based on an ITS sequence-dataset and an ITS-EF-1α concatenated sequence-dataset for Diplodia and Armillaria isolates, respectively, including appropriate reference sequences retrieved from GenBank. Both analyses were performed using RAxML v. 8.2.11 (Stamatakis 2006, 2014) as implemented in Geneious R11 (Kearse et al. 2012) using the GTRGAMMA model with the rapid bootstrapping and search for best scoring ML tree algorithm including 1000 bootstrap replicates (Online Resource 4 and 5).
As a rule, current names were applied according to the nomenclatorial database MycoBank (Robert et al. 2005). Two exceptions from this generally applied rule have been made in the case of the MT designated here as Fusarium solani s. l. and Armillaria gallica. In the case of F. solani the authors are aware that there is a currently unsettled discussion about correct delimitation of this species, or rather species complex. Here we follow the “classic” nomenclature substantiated by Geiser et al. (2021) obverse that promoted by Lombard et al. (2015) and Sandoval-Denis et al. (2019) who place said species complex in the genus Neocosmospora (N. solani (Mart.) L. Lombard & Crous). The currently applied name for A. gallica, according to MycoBank is A. lutea Gillet. However, Marxmüller (1992) stated that A. lutea is a nomen ambiguum and the later introduced name A. gallica Marxm. & Romagn. is to be used (Burdsall and Volk 1993).
Results
Sampled trees
The crown health status regarding ash dieback of the sampled trees ranged from vital and nearly without dieback symptoms to almost dead. All sampled trees (Online Resource 3), except for the six planed control trees, had obvious stem collar necroses through discoloured, sunken, and in some cases ruptured bark. In cases of ruptured bark, subjacent stem collars were neither completely rotting nor dead. During processing of the sample trees in the laboratory two out of the six control trees (trees 32 and 42, Online Resource 3) showed necrotic woody tissue inside the stem base. Consequently, these two trees were transferred to and analysed as sample trees (symptomatic sample trees n = 54, asymptomatic control trees n = 4).
Isolated fungi
In total, 4401 wood chips of stem collar tissue originating from 58 trees were incubated. A total of 1511 isolates (from which 226 were not identifiable due to contaminations; marked as Fungus sp.) from 1413 wood chips (32%) were observed. 958 chips (22%) showed no outgrowth at all after four weeks of incubation, while 960 (22%) chips had been overgrown by fast-growing fungi from adjacent wood chips before outgrowth could be recognised. The remaining 1070 (24%) wood chips were colonised or contaminated by yeasts, mould, or fungi which do not belong to Dikarya. The resulting pure culture isolates were assigned to 162 MT (excluding Trichoderma spp.) and all but ten could at least be identified to a genus level. Eighty-nine isolates could be identified to a species level (Table 2).
The majority of the isolated filamentous fungi from all samples were Ascomycota (132 MT including Trichoderma spp., 77.8%), 36 MT (22.2%) belonged to the phylum of Basidiomycota. Within the Ascomycota, the most frequently observed orders (Fig. 3) were Hypocreales (23.0%) followed by Pleosporales (22.2%), and Helotiales (19.8%). The Basidiomycota fungi were mainly represented by Russulales (36.1%), Agaricales (25.0%), and Polyporales (22.2%).
Despite the diversity of 162 detected fungal MT, only a few fungi occurred with high fMT. Sixty-seven MT (41%) were isolated more than once and from these only 13 fungi (8%) were obtained ten or more times. The remaining 95 fungi (59%) were only isolated once. Between one and 27 different fungi were found per stem collar. On average, nine MT were recorded on each tree. None of the identified fungi were found at all sites (including extensive sampling sites) and merely 46 MT (28%) were found at more than one site. In total, 116 fungi (72%) were found solely at one site (the four subplots of Schlangen are considered one site).
Although morphologically similar to Diplodia mutila (Fr.) Mont., in the phylogenetic analysis based on ITS sequences, the majority of Diplodia isolates from this study are placed clearly within a clade of strains of Diplodia fraxini (Fr.) Fr., including its ex-neotype (Online Resource 4 and Online Resource 6). One strain from this study (NW-FVA 5979) is placed within a clade formed by strains of Diplodia sapinea (Fr.) Fuckel including its ex-epitype strain and the ex-type strain of the synonymised Diplodia intermedia. The ITS sequences of this clade differ by one nucleotide, however, the ITS sequence of NW-FVA 5979 is identical to that of the ex-epitype strain of D. sapinea. Preliminary morphological observation of strains of Armillaria indicated affiliation to A. gallica. However, A. gallica cannot be clearly distinguished morphologically from Armillaria cepistipes Velen. (Tsopelas 1999). Of the 35 isolates included in the phylogenetic analysis of Armillaria, 33 isolates are placed within a clade containing reference strains of A. gallica. The two remaining isolates are placed within a clade of A. cepistipes (Online Resource 5 and Online Resource 7). Since only a selection of Armillaria isolates was included in the phylogenetic analysis due to limited lab resources, a clear distinction could not be made for all isolates of this genus. Hence, isolates of Armillaria are combined and referred to as Armillaria spp. in the final assessment of this study.
The most frequent MT isolated were D. fraxini (21.8%), H. fraxineus (13.9%), Armillaria spp. (12.3%), and N. punicea (9.6%), which account for nearly half of the isolated fungi. Perithecia of N. punicea were frequently observed on the ash bark above the necrotic lesions. All other isolated fungi were less frequent with <4% proportion. The most abundant MT in respect to continuity beside the aforementioned D. fraxini (71% continuity), H. fraxineus (53%), Armillaria spp. (50%), and N. punicea (33%), were Diaporthe cf. eres (43% continuity / 3.8% fMT), Fusarium cf. lateritium (34% / 3.5%), and Paracucurbitaria sp. (34% / 2.1%). These MT were also most abundant in regard to occurrence at the nine sample sites (H. fraxineus occurs at eight sites, Diaporthe cf. eres and D. fraxini at seven sites, Fusarium cf. lateritium, N. punicea, and Paracucurbitaria sp. at six sites). When taking into account only the intensive sampling sites with at least five stem collars studied, there is an overlap of five occurring fungi: Diaporthe cf. eres, D. fraxini, H. fraxineus, N. punicea, and Paracucurbitaria sp.
The ash dieback pathogen H. fraxineus was isolated from 31 of the 54 stem collar necroses (57%). It was isolated at all studied sites except at Wolfenbüttel, where only a single tree with stem collar necrosis was sampled. The fungus was detected in both, young and advanced necroses, but not in the four control samples without symptomatic tissue. Hymenoscyphus fraxineus was isolated less frequently at the investigated sites with better water supply (soil water supply and climate combined; Online Resource 2).
147 MT (91%) were only isolated from necrotic stem tissue. Almost one-third of all MT according to their identification are able to decay wood (Table 2). A significant proportion of the isolated species were found here for the first time associated with F. excelsior, for example: three isolates from necrotic stem collar tissue were assigned to the MT identified as Cryptostroma corticale (Ellis & Everh.) P.H. Greg. & S. Waller and one isolate from stem collar tissue was identified as D. sapinea. The isolation of Paracucurbitaria sp. from the examined samples is the first proof of this genus from stem collar necroses of F. excelsior. Furthermore, one isolate from necrotic stem collar tissue was preliminarily assigned to the genus Vexillomyces S. Bien, C. Kraus & Damm based on ITS sequence comparison. Further morphologic as well as multi-locus phylogenetic investigations based on additional DNA regions (ribosomal large subunit, EF-1α, and a 200-bp intron of the glyceraldehyde-3-phosphate dehydrogenase) revealed this isolate to represent a novel yet undescribed species of Vexillomyces (Tan et al. 2022). The MT, which only occurred once in asymptomatic control samples were assigned to Akanthomyces sp., Cephalotrichiella penicillata Crous, and Sclerostagonospora cycadis Crous & G. Okada. The following fungi were isolated from stem collar necroses as well as from symptomless controls: Alternaria infectoria E.G. Simmons, Alternaria sp., Diaporthe cf. eres, Exophiala sp., Hypoxylon fragiforme E.G. Simmons, Jackrogersella sp., Paracucurbitaria sp., Peniophora sp. 1 and sp. 2, Phaeosphaeria sp., Pseudeurotiaceae sp., and Sistotrema oblongisporum M.P. Christ. & Hauerslev.
Fungal communities in stem collar necroses
The number of MT isolated from stem collar necroses at the different sites ranged from one (Wolfenbüttel, one sample tree) to ten (Woltershausen, two sample trees) at the extensive sites and from 29 (Schwansee, eight sample trees) to 87 (Schlangen, 19 sample trees) at the intensive sites. Hence, the fungal communities at the sites studied differed in their species composition and diversity (Fig. 4). In Schlangen 52 MT (32% of all 162 isolated MT of this study) out of 87 MT were found exclusively at this site. Nevertheless, the most common MT in Schlangen are identical with the most frequent MT over all sampling sites (Armillaria spp., D. fraxini, H. fraxineus, and N. punicea). Diplodia fraxini was isolated from almost 80% and H. fraxineus from almost 70% of the trees studied at this site. 54 MT were found in the samples from the site of Berggießhübel and 30 (19%) of those occurred exclusively at this site. Likewise, the most common MT at Berggießhübel were D. fraxini, H. fraxineus, and Armillaria spp. However, N. punicea was isolated only once there. At the Rhüden study site, 32 MT were found, 13 (8%) of which were found exclusively at this site. The most frequent MT were the same as the most abundant MT in respect to continuity from all samples - except N. punicea, which was isolated only once in Rhüden. 31 MT were found in the Satrup samples, 12 of which (8%) exclusively at this site. The most common MT in Satrup were identical with the four most common MT in Schlangen. At Schwansee, 29 fungal MT were isolated in total, 12 of which (8%) were found exclusively at this site. At this location, the most common MT besides D. fraxini and N. punicea were Coprinellus species and F. solani s. l.
Overlap between the fungi isolated from the main sites (with at least five samples) with the indication of how many of the isolated fungi were found at each site and between the different sites (absolute number as well as percantage), empty white areas indicating no overlap between the respective sites. Five fungi at the overlap of all sites are Diaporthe cf. eres, Diplodia fraxini, Hymenoscyphus fraxineus, Neonectria punicea, and Paracucurbitaria sp. (RStudio 4.1.2)
Discussion
Fungi associated with ash stem collars
In total, 162 fungi were isolated from ash stem collars and differentiated within this study. About half of these fungi were isolated from stem collar necroses in comparable studies as well (Lygis et al. 2005; Langer 2017; Meyn et al. 2019; Kranjec Orlović et al. 2020). Though 87 taxa (54%) isolated here were not reported by the aforementioned studies. It has to be taken into account that different sample sizes and sample site numbers lead to differing numbers of species. Considering the mentioned studies, Meyn et al. (2019) had the smallest sample size with four trees at one sample site and reported the smallest diversity with 16 fungal species. Langer (2017) isolated more fungal species (35) from 32 sample trees at seven sample sites. The correlation between sampling size and reported fungal diversity is also confirmed by Kranjec Orlović et al. (2020) with 68 fungal species isolated from 90 sample trees examined at three sample sites. The non-negligible impact of the number of sample sites on the detectable fungal species diversity is shown in this study with 162 MT isolated from a smaller sample size (58 trees), but a higher number of sample sites (9) compared to the study of Kranjec Orlović et al. (2020). Other factors, such as number of the incubated wood chips or the isolation method may also have influence on the number of species isolated. But overall, there seems to be a positive correlation between sample size or number of sampled sites and species richness. Langer (2017) observed that, advanced stem collar necroses result in a higher number of isolated species. This could be confirmed in the present study when taking into account only the stem collar necroses with isolation of H. fraxineus.
Similar to studies focusing on endophytes of tree woody tissues (Bußkamp et al. 2020; Langer et al. 2021) in this study the majority of fungi isolated belong to Ascomycota (77.8%). A reason for the lower frequencies of Basidiomycota compared to Ascomycota might be that fungi belonging to the former often need longer incubation periods in order to grow out from incubated woody tissues (Oses et al. 2008) but since the incubated increment segments were kept for four weeks on nutrient media, it has to be assumed that enough time was given for fungi to grow out. However, the proportion of Basidiomycota (22.2%) in this study is higher than in the aforementioned studies. The reason for this discrepancy might be the focus on different woody tissue types in the mentioned studies and hence detection of differing fungal communities with divergent ecological functions. Basidiomycota isolated from woody tissues are often related to wood rot, because lignin is primarily decomposed by this fungal group and therefore they are more likely to be found in diseased or necrotic rather than asymptomatic woody tissue (Eriksson et al. 1990; Bugg et al. 2011). Hence, the occurrence of white and brown rot fungi in stem collar necroses is not unusual. Typical white rot fungi like Armillaria spp., Coprinellus spp., Bjerkandera adusta (Willd.) P. Karst., Peniophora spp., Trametes versicolor (L.) Lloyd, and few brown rot fungi like Fomitopsis spp. have been isolated from stem collar necroses in this study. The majority of soft rot fungi isolated here pertain to Ascomycota and the following representatives of this group were found: Biscogniauxia nummularia (Bull.) Kuntze, Hypoxylon spp., Jackrogersella sp., Nemania serpens (Pers.) Gray, and Xylaria spp. (all Xylariales). Besides the occurrence of white and brown rot fungi, the frequent association of xylarialean wood decay fungi with stem collar necroses make it plausible that affected ash trees have a massive loss of stability and tend to topple over even without wind as a supporting factor.
Approximately one-third of the isolated MT detected in this study were listed for F. excelsior in the USDA fungal database (Farr and Rossman 2022). Only one of the most frequent MT of this study, N. punicea, is not listed there, but other species from the genus Neonectria are mentioned. However, N. punicea was described as one of the most frequent MT associated with stem collar necroses in the context of ash dieback (Langer 2017; Meyn et al. 2019). The other most abundant MT isolated in our study Armillaria spp., D. fraxini, H. fraxineus, Diaporthe cf. eres, Fusarium cf. lateritium, and Paracucurbitaria sp. were already described to be associated with ash (Chandelier et al. 2016; Haňáčková et al. 2017; Langer 2017; Meyn et al. 2019; Linaldeddu et al. 2020; Kowalski and Bilański 2021; Barta et al. 2022). Langer (2017) investigated stem collar necroses of 32 ash trees and determined the aforementioned species as well—except for Paracucurbitaria sp. Meyn et al. (2019) isolated D. fraxini (labelled as Botryosphaeria stevensii), H. fraxineus, N. punicea, and Diaporthe cf. eres as well. The most commonly isolated species from stem collar necroses in the present study, except Armillaria sp. and Paracucurbitaria sp., were also isolated in high frequency by Linaldeddu et al. (2020), although they focussed on symptomatic branches of damaged ash trees in Italy. The absence of Armillaria sp. in branches was anticipated, because it is a soil-borne root and stem rot fungus, colonising its host through rhizomorph growth (Morrison 2004).
Within the genus Armillaria, A. gallica was the most frequently isolated species in the present study. Additionally to our isolations, mycelial fans and rhizomorphs of Armillaria spp. were observed at all sample sites of this study and the majority of further studied ash stands diseased by ash dieback. Armillaria species are common soil colonisers in Europe and therefore are probably existing in most forest sites even before ash dieback occurred (Morrison 2004; Lygis et al. 2005; Bakys et al. 2009b). They are considered secondary pathogens and wood-decaying fungi infecting stressed trees, which explains their occurrence in advanced stem necroses and root rot (Chandelier et al. 2016). On the one hand, Armillaria spp. can colonise stem collars after the necrosis has already formed by H. fraxineus. On the other hand, they are also able to independently attack a weakened ash tree without a stem collar necroses due to H. fraxineus (Langer 2017). As in our study, the occurrence of A. gallica and A. cepistipes associated with stem collar necroses of trees affected by ash dieback has been shown by Chandelier et al. (2016) in Belgium. Enderle et al. (2017) also detected A. gallica in stem collar rots in south-western Germany. These results are in contrast to investigations by Lygis et al. (2005), who determined A. cepistipes as most frequent in Lithuania. Nevertheless, regardless of which of the two species caused infection, Armillaria spp. most likely accelerate the decline of ash dieback-affected ash trees (Chandelier et al. 2016) and reduce stem stability.
The most frequently isolated species in our study D. fraxini has been recognised as the dominant species in comparable studies as well. Linaldeddu et al. (2020) determined that many reports of D. fraxini on ash have earlier been assigned to D. mutila s. l. Phylogenetic analyses showed, that most of the Diplodia strains isolated in this study, although morphologically similar to D. mutila, certainly match with D. fraxini. It is an aggressive pathogen known to cause bark lesions and wood discoloration or to enlarge necroses, which are primarily caused by H. fraxineus (Alves et al. 2014; Linaldeddu et al. 2020, 2022). Kowalski et al. (2017) classified it as the second most pathogenic fungus after H. fraxineus, though it was not mentioned as a frequent coloniser of F. excelsior before ash dieback disease occurred (Kowalski et al. 2016). These facts might indicate that infections with H. fraxineus facilitate the colonisation of affected ash trees by D. fraxini. Another possible explanation for the more frequent occurrence of D. fraxini could be global warming because this species benefits from warm temperatures of around 25 °C (Alves et al. 2014). In our opinion, D. fraxini plays an important role in ash dieback disease and contributes undoubtedly to a greater damage extent, in particular at stem collar necroses. Besides the latter very frequent Diplodia species, to the knowledge of the authors, this is the first report of D. sapinea on ash. In contrast to the study by Linaldeddu et al. (2020), the species D. subglobosa could not be isolated in our analysis, maybe because they investigated branches and not stem collar necroses.
Neonectria punicea has a large host spectrum, including F. excelsior (Hirooka et al. 2013). However, this fungus has rarely been documented from this particular host species before (Langer 2017; Meyn et al. 2019; Karadžić et al. 2020). N. punicea was found to be associated with stem collar necroses and cankers of European ash in Germany (Langer 2017; Meyn et al. 2019) and it is able to cause necroses in juvenile ash trees (Karadžić et al. 2020). Its perithecia were observed frequently on the bark above the necrotic ash tissue (ibid. and Karadžić et al. 2020). Neonectria punicea is mainly known to be a secondary pathogen, but can also express an endophytic lifestyle (Langer 2017). Species of the genus Neonectria invade through natural entrances, like lenticels or artificial wounds, for infection (Flack and Swinburne 1977; Salgado-Salazar and Crouch 2019).
The isolation of strains assigned to Diaporthe cf. eres were made from diseased and also from healthy woody ash tissue in this study. This is in agreement with insights that Diaporthe eres can live as a plant pathogen, endophyte, or saprotroph and has a wide host range as well as a widespread distribution (Udayanga et al. 2014; Linaldeddu et al. 2020). This species often produces its tiny fruit bodies on dead woody tissues (Kowalski et al. 2016). In a study by Kowalski et al. (2017), D. eres showed the least virulence and caused significantly milder disease symptoms on F. excelsior plants than the other tested fungal species. Diaporthe eres could be considered a weak pathogen in comparison to ash dieback on F. excelsior. In case of tree weakening by H. fraxineus the early endophytic presence of D. eres favours a fast pathogenic attack (Kowalski et al. 2016).
In this study Fusarium cf. lateritium Nees has been isolated frequently from symptomatic tissue and once from healthy wood tissue. The species is already known from F. excelsior in association with bacterial ash canker (Riggenbach 1956) but its virulence seems to be low in comparison with other fungal species (Bakys et al. 2009b). Kowalski et al. (2017) showed, that F. lateritium causes none or only small necroses on F. excelsior. In general, Fusarium spp. have a wide host range and are reported as the most common endophytes in ash bark and wood (Kowalski and Kehr 1992; Sieber 2007; Bakys et al. 2009b; Kowalski et al. 2016). The facts described above, indicate that F. lateritium is able to colonise the bark and woody tissue of ash independently of H. fraxineus. In association with ash dieback though it is more likely that the species contributes to the stem collar necroses as secondary pathogen. Thereby, it is non-essential, whether acceleration of ash dieback is established by shifting from endophytic to pathogenic lifestyle or colonising the tree as a secondary pathogen after tree weakening.
As far as it is known, the isolation of Paracucurbitaria sp. from the examined samples is the first proof of this genus in stem collar necroses. It was not isolated by Langer (2017) and Meyn et al. (2019) from rootstock. However, Kowalski and Bilański (2021) detected Paracucurbitaria sp. in previous year’s ash leaf petioles in Poland, Barta et al. (2022) isolated it from ash twigs in Slovakia, and Haňáčková et al. (2017) reported Paracucurbitaria corni (Bat. & A. F. Vital) Valenz.-Lopez, Stchigel, Guarro & Cano as an endophyte of ash leaves and seeds. Therefore the occurrence of species from the genus Paracucurbitaria in plant material of F. excelsior is not striking, but its high frequency in stem collar necroses was unanticipated. It can be assumed that the high frequency of Paracucurbitaria sp. is no coincidence, because its detection in stem collar necroses of ash is increasing in ongoing research at the NW-FVA since sampling for this study.
Besides D. sapinea, there are a few species, which, to the knowledge of the authors, have not been previously reported from F. excelsior (Table 2). One of them is C. corticale, known as the causal agent of the sooty bark disease on maples. Its main host is Acer pseudoplatanus L., but it has been proven that C. corticale can colonise other maple species as well as Aesculus hippocastanum L. (Enderle et al. 2020). This species was found at sampling sites with sycamore. In addition to the first reports of ash as a host, one strain belonging to the genus Vexillomyces was isolated and recognised as undescribed species. The genus Vexillomyces was described in 2020 for two species (V. palatinus, V. verruculosus) isolated from spore traps attached to vine shoots. No host organism is known for these species. Later several species of Claussenomyces and Tympanis were transferred to the genus (Baral and Quijada 2020). The respective species are known from dead or living angiosperm and gymnosperm wood, however, only for V. atrovirens (syn. Claussenomyces atrovirens) an affiliation to the host genus Fraxinus could be recognised (Dennis 1986).
Role of Hymenoscyphus fraxineus in stem collar necroses
The ash dieback pathogen H. fraxineus could not be isolated from all of the 54 symptomatic stem collars. Only in about half of the trees, the fungus could be determined. It has been already reported by several authors, that H. fraxineus could not be frequently isolated from symptomatic tissue of ash (Przybyl 2002; Bakys et al. 2009a; Enderle et al. 2017). A possible explanation for this could be its slow growth, unfavourable sampling conditions for the pathogen or too advanced necroses with antagonistic activity of other colonisers (Kowalski and Holdenrieder 2009; Hauptman et al. 2013; Gross et al. 2014; Langer 2017). Often, H. fraxineus could be solely isolated from recently discoloured woody tissues of the stem collar necroses (Fig. 2) and is probably supressed in the older parts of the necroses already colonised by secondary fungi. The aforementioned reasons might have contributed to the moderate isolation success of the ash dieback pathogen in this study. Perhaps, fungal community analysis by means of culture-independent methods, such as high throughput sequencing or qPCR could detect H. fraxineus more frequently than by culture based isolation, since these methods have the potential to detect inactively present fungi or even DNA residues if the initial fungus has been suppressed by secondary invaders (Lindahl et al. 2013). Our results on the fMT and continuity of the association and localisation of H. fraxineus in basal stem necroses support the assumption, that this pathogen is very often the main or primary causal agent triggering stem collar necroses. Either way, H. fraxineus is confirmed as the main pathogenic agent of the ash dieback epidemic (Kowalski 2006; Bakys et al. 2009a; Kowalski and Holdenrieder 2009; Gross et al. 2014). The only lack of evidence for H. fraxineus at the study site Wolfenbüttel could be explained by the meagre sample size. It can be assumed that H. fraxineus may have been isolated if a larger wood chip number or sample tree size was examined. According to information from a co-researcher in FraxPath, H. fraxineus was present in branches of the sample trees at the study site Wolfenbüttel (Maia Ridley, personal communication).
Fungal communities in stem collar necroses
The observation of significant differences in the occurrence of fungal taxa between the investigated forest sites is consistent with the results of Bilański and Kowalski (2022). In the study of Meyn et al. (2019) only two species were found in all sample trees and many of the identified fungi were single isolates. Similarly, Kranjec Orlović et al. (2020) revealed just few predominating taxa representing half of all fungal isolates from stem bases of Fraxinus angustifolia Vahl. In addition, species represented only by a single isolate make up one-third of all isolates in the study by the latter authors. The fact, that the composition of fungi isolated in this study differs with only a little overlap between the sampling sites, leads to the assumption that adding further sampling sites would reveal new sets of fungi not recorded in this study.
Relation of the most common fungi to the site characteristics
Independent of age class and site conditions, European ash trees can be vulnerable to an infection by H. fraxineus (Pautasso et al. 2013). However, the extent of ash dieback in the crown and stem collar necroses and tree mortality, most likely depend on many different factors. Susceptibility of ash trees to the pathogen, the range of subsequent colonising fungal species (Langer et al. 2022), tree vitality, or the environmental context of the forest site and stand (Havrdová et al. 2017) are some examples. Ash tree vitality is encouraged at fertile and (moderately) wet soils, conditions which are preferred by ash (Walentowski et al. 2017). It has to be taken into account that for this study only a selection of forest sites from a rather narrow area out of the wide range of European ash was investigated. An optimal soil and water supply with a sufficient percentage of ash trees was fundamental. Furthermore, the selection of sample trees was subjected to different restrictions. For example the condition of a diameter at breast height less than 25 cm because of logistics and processing abilities in the lab. Besides that, trees with very advanced necroses like completely necrotic or rotten stem base or dead trees were not suitable for investigation.
Our preliminary results indicate that H. fraxineus was isolated less frequent at sites with higher water availability (Online Resource 2). This is in accordance with the guess that the fungal composition of stem collar necroses depends on soil and water availability of the forest stand (Linaldeddu et al. 2011; Salamon et al. 2020). As mentioned before, this assumption refers only to the selection of the investigated forest sites. One explanation could be, that secondary fungi have more favourable conditions at sites with higher water availability and thus are able to overgrow H. fraxineus faster than at drier sites.
For the other most common fungi Armillaria spp., Diaporthe cf. eres, D. fraxini, Fusarium cf. lateritium, N. punicea, and Paracucurbitaria sp., Dirichlet regression indicated no correlation between fWC and water supply rank of the site (Online Resource 2). Assuming that H. fraxineus as the sole pathogen influences the extent of damage caused by stem collar necrosis, this this would be in contrast to the suggestion of several authors that stands with wet soil conditions show a higher probability that the individual trees affected by H. fraxineus exhibit greater damage (Gross et al. 2014; Erfmeier et al. 2019). At Schwansee, the wettest sampling site, H. fraxineus was only isolated twice. However, the stem collar necroses were most advanced at these sampling trees, where a lower isolation rate of H. fraxineus was generally expected, as mentioned previously.
It was noticeable, that D. fraxini and N. punicea had a significantly different fWC at the various sampling sites (Online Resource 2), but there was no indication for a correlation with the site characteristics water supply, soil and bedrock, climate, or mixture of trees. However, it was observed that ash trees with a low fWC of D. fraxini had a thinner bark. Compared with D. fraxini and N. punicea, the MT Diaporthe cf. eres, Fusarium cf. lateritium, and Paracucurbitaria sp. had a consistent fWC over all sites. But Fusarium cf. lateritium was not isolated at Satrup and at the valley bottom in Schlangen. Due to the lower amount of isolations in this study, the authors assume there is also a lower probability of occurrence in stem collar necroses.
Armillaria species were not present at all studied sites and could not be isolated from the trees in Schwansee. This result is contradictory to those of Enderle et al. (2017), who found older necroses to be more often colonised by Armillaria spp. The progress of the necroses formation was clearly visible by their partially ruptured wood surface and presence of fruiting bodies on the necrotic stem areas of wood decay fungi, such as Coprinellus sp. and Xylaria polymorpha (Pers.) Grev. (Liers et al. 2011). Furthermore, the absence of Armillaria spp. isolates in Schwansee, the most moist of all sampling sites which is influenced by its ground water, do not correspond to preference of Armillaria species for continuously moist soil conditions (Whiting and Rizzo 1999). A possible explanation for the lack of this species in Schwansee, could be the specific forest site background as a former lake. The area was earlier used as fishpond until the eighteenth century. Thus, the soil was subjected to special formation conditions (Welk 2017) and perhaps it was not possible for Armillaria spp. to colonise the soil like in other forest sites.
Many of the other MT detected in this study were isolated just once, which may indicate no direct correlation with the investigated forest sites, thus site characteristics like soil and water supply relatedness cannot be assumed. However, it cannot be ruled out that the one-time isolated fungi occur in other forest sites, than the investigated ones, too. As well as a higher abundancy is theoretical possible. Additionally, it is to be expected that the composition of fungi might differ according to tree age, tree species composition, forest management type, season, and the like (Scholtysik et al. 2013; Tomao et al. 2020). For example, a more diverse tree species composition at a forest site could contribute to the occurrence of a wider spectrum of fungi colonising a tree (Cavard et al. 2011; Kowalski et al. 2016; Krah et al. 2018; Tomao et al. 2020). This is confirmed by the isolation of sycamore-typical fungi like C. corticale und Cy. rubronotata from F. excelsior in stands with maple trees. It is furthermore supported by the fact that the most mixed intensive sampling site of Berggießhübel has one of the highest fungal diversity in relation to its sample tree amount. In addition to its diverse tree species composition, Berggießhübel is the most eastern sampling site. Satrup is the most northern sampling site and shows high fungal diversity despite its smallest sample tree amount. This observation suggests that widely varying sites in Germany lead to differing fungal communities. Furthermore, a possible underestimation of fungal diversity in the studied trees may occur since not all fungi are detectable through standardised culture based methods or in general (Guo et al. 2001; Allen et al. 2003; Unterseher 2011; Muggia et al. 2017).
Conclusion and outlook
This study provided new insights into the fungal diversity and communities of endophytes, primary and secondary pathogens, wood-decaying fungi, and saprotrophic fungi associated with stem collar necroses of European ash trees. A rich fungal composition inhabiting symptomatic stem tissue has been revealed with four frequent species occurring at most of the studied forest sites, but with little overlap between the sites. The fungal species richness detected in this study (162) is considerably higher compared to previous investigations in which 16–75 different species were detected (Lygis et al. 2005; Enderle et al. 2017; Langer 2017; Meyn et al. 2019). This difference in diversity can be explained by the larger sampling size (not only tree number, but also amount of wood chips taken) and the partially greater number of sites studied. Single trees with only about 20 studied chips of stem collar tissue each (Oranienbaumer Heide, Wolfenbüttel) had the fewest amount of isolated MT. Further studies on stem collar necroses can increase the knowledge of fungal biodiversity on F. excelsior, as clearly demonstrated by the newly described species, Vexillomyces fraxinicola (Tan et al. 2022), which was collected in this study.
The ash dieback pathogen was isolated from only about half of the trees sampled. Different reasons like its slow growth can cause a low isolation rate of the primary pathogen. Nevertheless, it is possible that stem collar necroses are commonly initiated by this fungus. The occurrence of several pathogenic fungi from necrotic stem tissue of ash beside H. fraxineus is striking, because of their high fMT. It was shown that the different fungal communities of the sample trees are largely dominated by three MT (D. fraxini, Armillaria spp. and N. punicea) next to H. fraxineus representing almost 50% of all isolates. They are considered to play a major role in the progression of stem collar necroses and rot and therefore also contribute to a loss of tree stability. The remaining fungi which were isolated from the stem collars necroses turned out to be very diverse with much lower fMT, in the majority of cases were represented with only one isolate. Overall, the synergistic interaction of different pathogens in the context of ash dieback, for example H. fraxineus and D. fraxini or N. punicea, can lead to a larger damage in contrast to infection by only one pathogen (Marçais et al. 2010). In this context, N. punicea poses a serious threat to planted ash forests and natural regenerations of F. excelsior, especially if another host tree species, such as European beech (Fagus sylvatica L.) is in mixture. European beech is potentially an inoculum reservoir of N. punicea for future infections of ash stem collars (Karadžić et al. 2020). Therefore, in the future, the susceptibility of ash to form stem collar necroses and to be diseased by D. fraxini and N. punicea should be considered in breeding programmes to develop more resistant ash trees in relation to ash dieback.
However, stem collar necrosis types caused by other fungi than H. fraxineus or Phytophthora spp. (Langer 2017), should not be disregarded. The results of this study show, that at least one fungal pathogen can be found in the necrosis without evidence of H. fraxineus. For example, one of the control samples, which turned out to have necrotic tissue inside the wooden body, was colonised by Armillaria sp. In this case, it is likely that the fungus attacked the weakened tree independently of a pre-colonisation of the stem collar by H. fraxineus.
Since in this study no correlation between the site factors and fungal occurrence could be calculated because most of the isolated fungi were only detected once, further studies should be carried out at additional comparable forest sites. Inventories of stem collar necroses at a higher number of locations may reveal dependence of MT to forest side conditions and their individual role in the fungal communities in detail. Future studies need to be conducted in order to estimate potentially high-risk characteristics of forest sites for pronounced and fast-advancing stem collar necroses and rot. Additionally, the investigation of genotypes of H. fraxineus associated with single-stem collar necroses could help to better understand the path of infection with H. fraxineus and the secondary colonisation by other fungi.
Data availability
The DNA sequences generated in this study were deposited in GenBank (https://www.ncbi.nlm.nih.gov; Table 2). All sampling data is provided in the online resources (ESM 3). The fungal strains are stored in the strain collection of the NW-FVA. Text and images are permanently stored on an internal drive of the NW-FVA.
References
Alborés S (2018) Biodiversity and antimicrobial activity of Antarctic fungi from the Fildes Peninsula, King George Island. Sydowia Int J Mycol 185–192. https://doi.org/10.12905/0380.sydowia70-2018-0185
Alimadadi N (2019) Molecular identification of some wild medicinal macrofungi from Northern Iran. Stud Fungi 4:26–36. https://doi.org/10.5943/sif/4/1/4
Allen TR, Millar T, Berch SM, Berbee ML (2003) Culturing and direct DNA extraction find different fungi from the same ericoid mycorrhizal roots. New Phytol 160:255–272. https://doi.org/10.1046/j.1469-8137.2003.00885.x
Altschul SF, Madden TL, Schäffer AA et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein databases search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Alves A, Correia A, Phillips A (2006) Multi-gene genealogies and morphological data support Diplodia cupressi sp. nov., previously recognized as D. pinea f. sp. cupressi, as a distinct species. Fungal Divers 23:1–15
Alves A, Linaldeddu BT, Deidda A et al (2014) The complex of Diplodia species associated with Fraxinus and some other woody hosts in Italy and Portugal. Fungal Divers 67:143–156. https://doi.org/10.1007/s13225-014-0282-9
Bakys R, Vasaitis R, Barklund P et al (2009a) Investigations concerning the role of Chalara fraxinea in declining Fraxinus excelsior. Plant Pathol 58:284–292. https://doi.org/10.1111/j.1365-3059.2008.01977.x
Bakys R, Vasaitis R, Barklund P et al (2009b) Occurrence and pathogenicity of fungi in necrotic and non-symptomatic shoots of declining common ash (Fraxinus excelsior) in Sweden. Eur J For Res 128:51–60
Baral HO, Quijada L (2020) Nomenclatural novelties. Index Fungorum 454:1–3
Barta M, Pastirčáková K, Ostrovský R et al (2022) Culturable endophytic fungi in Fraxinus excelsior and their interactions with Hymenoscyphus fraxineus. Forests 13:1098. https://doi.org/10.3390/f13071098
Bien S, Damm U (2020a) Arboricolonus simplex gen. et sp. nov. and novelties in Cadophora, Minutiella and Proliferodiscus from Prunus wood in Germany. MycoKeys 63:119–161. https://doi.org/10.3897/mycokeys.63.46836
Bien S, Damm U (2020b) Prunus trees in Germany - a hideout of unknown fungi? Mycol Prog 19:667–690. https://doi.org/10.1007/s11557-020-01586-4
Bien S, Kraus C, Damm U (2020) Novel collophorina-like genera and species from Prunus trees and vineyards in Germany. Persoonia 45:46–67. https://doi.org/10.3767/persoonia.2020.45.02
Bilański P, Kowalski T (2022) Fungal endophytes in Fraxinus excelsior petioles and their in vitro antagonistic potential against the ash dieback pathogen Hymenoscyphus fraxineus. Microbiol Res 257:126961. https://doi.org/10.1016/j.micres.2022.126961
Bills GF, Platas G, Overy DP et al (2009) Discovery of the parnafungins, antifungal metabolites that inhibit mRNA polyadenylation, from the Fusarium larvarum complex and other Hypocrealean fungi. Mycologia 101:449–472. https://doi.org/10.3852/08-163
Blumenstein K, Bußkamp J, Langer GJ et al (2021) The Diplodia tip blight pathogen Sphaeropsis sapinea is the most common fungus in Scots pines’ mycobiome, irrespective of health status - a case study from Germany. J Fungi 7:607. https://doi.org/10.3390/jof7080607
Bugg TDH, Ahmad M, Hardiman EM, Rahmanpour R (2011) Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep 28:1883–1896. https://doi.org/10.1039/C1NP00042J
Burdsall HH, Volk TJ (1993) The state of taxonomy of the genus Armillaria. McIlvainea 11:4–12
Bußkamp J, Langer GJ, Langer EJ (2020) Sphaeropsis sapinea and fungal endophyte diversity in twigs of Scots pine (Pinus sylvestris) in Germany. Mycol Prog 19:985–999. https://doi.org/10.1007/s11557-020-01617-0
Cabral A, Groenewald JZ, Rego C et al (2012) Cylindrocarpon root rot: multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycol Prog 11:655–688. https://doi.org/10.1007/s11557-011-0777-7
Cavard X, Macdonald SE, Bergeron Y, Chen HYH (2011) Importance of mixedwoods for biodiversity conservation: evidence for understory plants, songbirds, soil fauna, and ectomycorrhizae in northern forests. Environ Rev 19:142–161. https://doi.org/10.1139/a11-004
Chandelier A, Gerarts F, Martin GS et al (2016) Temporal evolution of collar lesions associated with ash dieback and the occurrence of Armillaria in Belgian forests. For Pathol 46:289–297. https://doi.org/10.1111/efp.12258
Chen C, Verkley GJM, Sun G et al (2016) Redefining common endophytes and plant pathogens in Neofabraea, Pezicula, and related genera. Fungal Biol 120:1291–1322. https://doi.org/10.1016/j.funbio.2015.09.013
Crous PW, Schumacher RK, Wingfield MJ (2015) Fungal systematics and evolution: FUSE 1. Sydowia Int J Mycol 81–118. https://doi.org/10.12905/0380.sydowia67-2015-0081
Crous PW, Shivas RG, Quaedvlieg W et al (2014) Fungal Planet description sheets: 214-280. Persoonia 32:184–306. https://doi.org/10.3767/003158514X682395
Crous PW, Wingfield MJ, Guarro J et al (2013) Fungal Planet description sheets: 154–213. Persoonia 31:188–296. https://doi.org/10.3767/003158513X675925
Denman S, Barrett G, Kirk SA et al (2017) Identification of Armillaria species on declined oak in Britain: implications for oak health. Forestry 90:148–161. https://doi.org/10.1093/forestry/cpw054
Dennis RWG (1986) Fungi of the Hebrides. Royal Botanic Gardens, Kew
Douanla-Meli C, Langer E (2012) Diversity and molecular phylogeny of fungal endophytes associated with Diospyros crassiflora. Mycology 3:175–187. https://doi.org/10.1080/21501203.2012.705348
Dufresne PJ, Moonjely SS, Ozaki K et al (2017) High frequency of pathogenic Aspergillus species among nonsporulating moulds from respiratory tract samples. Med Mycol 55:233–236. https://doi.org/10.1093/mmy/myw064
Enderle R, Riebesehl J, Becker P, Kehr R (2020) Rußrindenkrankheit an Ahorn - Biologie, Pathologie und Entsorgung von Schadholz. In: Dujesiefken D (ed) Jahrbuch der Baumpflege 2020, 24th edn. Haymarket Media, Braunschweig, pp 85–100
Enderle R, Sander F, Metzler B (2017) Temporal development of collar necroses and butt rot in association with ash dieback. IForest - Biogeosci For 10:529–536. https://doi.org/10.3832/ifor2407-010
Erfmeier A, Haldan KL, Beckmann L-M et al (2019) Ash dieback and its impact in near-natural forest remnants – a plant community-based inventory. Front Plant Sci 10:658. https://doi.org/10.3389/fpls.2019.00658
Eriksson K-E, Blanchette RA, Ander P (1990) Biodegradation of lignin. In: Timell TE (ed) Microbial and enzymatic degradation of wood and wood components, 1st edn. Springer, Heidelberg, pp 225–333
Farr DF, Rossman AY (2022) Fungal databases - fungus-host distributions. In: US Natl. Fungus Collect. Fungal Database. https://nt.ars-grin.gov/fungaldatabases/fungushost/fungushost.cfm. Accessed 4 May 2022
Flack NJ, Swinburne TR (1977) Host range of Nectria galligena Bres. and the pathogenicity of some Northern Ireland isolates. Trans Br Mycol Soc 68:185–192. https://doi.org/10.1016/S0007-1536(77)80007-7
Fukasawa Y (2018) Fungal succession and decomposition of Pinus densiflora snags. Ecol Res 33:435–444. https://doi.org/10.1007/s11284-017-1557-x
Gao C-H (2021) ggVennDiagram: a “ggplot2” implement of Venn diagram. In: GgVennDiagram Ggplot2 Implement Venn Diagr. https://CRAN.R-project.org/package=ggVennDiagram
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Geiser DM, Al-Hatmi AMS, Aoki T, et al (2021) Phylogenomic analysis of a 55.1-kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani species complex. Phytopathology® 111:1064–1079. https://doi.org/10.1094/PHYTO-08-20-0330-LE
Gomes RR, Glienke C, Videira SIR et al (2013) Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31:1–41. https://doi.org/10.3767/003158513X666844
Griffin MA, Spakowicz DJ, Gianoulis TA, Strobel SA (2010) Volatile organic compound production by organisms in the genus Ascocoryne and a re-evaluation of myco-diesel production by NRRL 50072. Microbiol 156:3814–3829. https://doi.org/10.1099/mic.0.041327-0
Gross A, Holdenrieder O, Pautasso M et al (2014) Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol Plant Pathol 15:5–21. https://doi.org/10.1111/mpp.12073
Guo LD, Hyde KD, Liew ECY (2000) Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol 147:617–630
Guo LD, Hyde KD, Liew ECY (2001) Detection and taxonomic placement of endophytic fungi within frond tissues of Livistona chinensis based on rDNA sequences. Mol Phylogenet Evol 20:1–13. https://doi.org/10.1006/mpev.2001.0942
Guo T, Wang HC, Xue WQ et al (2016) Phylogenetic analyses of Armillaria reveal at least 15 phylogenetic lineages in China, seven of which are associated with cultivated Gastrodia elata. PLoS One 11:e0154794. https://doi.org/10.1371/journal.pone.0154794
Haelewaters D, Dirks AC, Kappler LA et al (2018) A preliminary checklist of fungi at the Boston Harbor Islands. Northeast Nat 25:45–76. https://doi.org/10.1656/045.025.s904
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98. https://doi.org/10.14601/Phytopathol_Mediterr-14998u1.29
Haňáčková Z, Havrdová L, Černý K et al (2017) Fungal endophytes in ash shoots - diversity and inhibition of Hymenoscyphus fraxineus. Balt For 23:89–106
Hauptman T, Piškur B, de Groot M et al (2013) Temperature effect on Chalara fraxinea: heat treatment of saplings as a possible disease control method. For Pathol 43:360–370. https://doi.org/10.1111/efp.12038
Havrdová L, Zahradník D, Romportl D et al (2017) Environmental and silvicultural characteristics influencing the extent of ash dieback in forest stands. Balt For 23:168–182
Heydeck P, Bemmann M, Kontzog H-G (2005) Triebsterben an Gemeiner Esche (Fraxinus excelsior) im nordostdeutschen Tiefland. Forst und Holz 60:505–506
Hirooka Y, Rossman AY, Zhuang W-Y et al (2013) Species delimitation for Neonectria coccinea group including the causal agents of beech bark disease in Asia, Europe, and North America. Mycosystema 32:485–517
Hosseini B, Voegele RT, Link TI (2021) Establishment of a quadruplex real-time PCR assay to distinguish the fungal pathogens Diaporthe longicolla, D. caulivora, D. eres, and D. novem on soybean. PLoS One 16:e0257225. https://doi.org/10.1371/journal.pone.0257225
Husson C, Caël O, Grandjean JP et al (2012) Occurrence of Hymenoscyphus pseudoalbidus on infected ash logs. Plant Pathol 61:889–895. https://doi.org/10.1111/j.1365-3059.2011.02578.x
Irinyi L, Serena C, Garcia-Hermoso D et al (2015) International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database - the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med Mycol 53:313–337. https://doi.org/10.1093/mmy/myv008
Izumitsu K, Hatoh K, Sumita T et al (2012) Rapid and simple preparation of mushroom DNA directly from colonies and fruiting bodies for PCR. Mycosci 53:396–401
Jaklitsch WM, Checa J, Blanco MN et al (2018) A preliminary account of the Cucurbitariaceae. Stud Mycol 90:71–118. https://doi.org/10.1016/j.simyco.2017.11.002
Jaklitsch WM, Voglmayr H (2016) Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Stud Mycol 85:35–64. https://doi.org/10.1016/j.simyco.2016.09.002
Jang Y, Jang S, Min M et al (2015) Comparison of the diversity of basidiomycetes from dead wood of the Manchurian fir (Abies holophylla) as evaluated by fruiting body collection, mycelial isolation, and 454 sequencing. Microb Ecol 70:634–645. https://doi.org/10.1007/s00248-015-0616-5
Jankowiak R, Ciach M, Bilański P, Linnakoski R (2019a) Diversity of wood-inhabiting fungi in woodpecker nest cavities in southern Poland. Acta Mycol 54:1126. https://doi.org/10.5586/am.1126
Jankowiak R, Strzałka B, Bilański P et al (2019b) Ophiostomatoid fungi associated with hardwood-infesting bark and ambrosia beetles in Poland: taxonomic diversity and vector specificity. Fungal Ecol 39:152–167. https://doi.org/10.1016/j.funeco.2019.02.001
Karadžić D, Stanivuković Z, Milanović S et al (2020) Development of Neonectria punicea pathogenic symptoms in juvenile Fraxinus excelsior trees. Front Plant Sci 11:592260. https://doi.org/10.3389/fpls.2020.592260
Kauserud H, Schumacher T (2001) Outcrossing or inbreeding: DNA markers provide evidence for type of reproductive mode in Phellinus nigrolimitatus (Basidiomycota). Mycol Res 105:676–683. https://doi.org/10.1017/S0953756201004191
Kearse M, Moir R, Wilson A et al (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kenigsvalde K, Arhipova N, Laiviņš M, Gaitnieks T (2010) Fungus Chalara fraxinea as a causal agent for ash decline in Latvia. For Sci 21:110–120
Kotiranta H, Larsson K-H (2013) Sistotrema luteoviride sp. nov. (Cantharellales, Basidiomycota) from Finland. Acta Mycol 48:219–225. https://doi.org/10.5586/am.2013.023
Kowalski T (2006) Chalara fraxinea sp. nov. associated with dieback of ash in Poland. For Pathol 36:264–270. https://doi.org/10.1111/j.1439-0329.2006.00453.x
Kowalski T, Bilański P (2021) Fungi detected in the previous year’s leaf petioles of Fraxinus excelsior and their antagonistic potential against Hymenoscyphus fraxineus. Forests 12:1412. https://doi.org/10.3390/f12101412
Kowalski T, Bilański P, Kraj W (2017) Pathogenicity of fungi associated with ash dieback towards Fraxinus excelsior. Plant Pathol 66:1228–1238. https://doi.org/10.1111/ppa.12667
Kowalski T, Holdenrieder O (2009) Pathogenicity of Chalara fraxinea For Pathol 39:1–7. https://doi.org/10.1111/j.1439-0329.2008.00565.x
Kowalski T, Kehr R (1992) Endophytic fungal colonization of branch bases in several forest tree species. Sydowia 44:137–168
Kowalski T, Kraj W, Bednarz B (2016) Fungi on stems and twigs in initial and advanced stages of dieback of European ash (Fraxinus excelsior) in Poland. Eur J For Res 135:565–579. https://doi.org/10.1007/s10342-016-0955-x
Krah F-S, Seibold S, Brandl R et al (2018) Independent effects of host and environment on the diversity of wood-inhabiting fungi. J Ecol 106:1428–1442. https://doi.org/10.1111/1365-2745.12939
Kranjec Orlović J, Moro M, Diminić D (2020) Role of root and stem base fungi in Fraxinus angustifolia (Vahl) dieback in Croatian floodplain forests. Forests 11:1–15. https://doi.org/10.3390/f11060607
Lakshman D, Vieira P, Pandey R et al (2017) Symptom development in response to combined infection of in vitro-grown Lilium longiflorum with Pratylenchus penetrans and soilborne fungi collected from diseased roots of field-grown lilies. Plant Dis 101:882–889. https://doi.org/10.1094/PDIS-09-16-1336-RE
Langer GJ (1994) Die Gattung Botryobasidium DONK (Corticiaceae, Basidiomycetes). J. Cramer, Berlin, Stuttgart
Langer GJ (2017) Collar rots in forests of Northwest Germany affected by ash dieback. Balt For 23:4–19
Langer GJ, Bußkamp J (2021) Fungi associated with woody tissues of European beech and their impact on tree health. Front Microbiol 12:702476. https://doi.org/10.3389/fmicb.2021.702467
Langer GJ, Bußkamp J, Terhonen E, Blumenstein K (2021) Fungi inhabiting woody tree tissues - stems, branches, and twigs. In: Asiegbu FO, Kovalchuk A (eds) Forest Microbiome Forest Microbiology. Forest Microbiology, Tree Microbiome: Phyllosphere, Endosphere, vol 1, pp 175–205
Langer GJ, Fuchs S, Osewold J, et al (2022) FraxForFuture - research on European ash dieback in Germany. J Plant Dis Prot https://doi.org/10.1007/s41348-022-00670-z
Liers C, Arnstadt T, Ullrich R, Hofrichter M (2011) Patterns of lignin degradation and oxidative enzyme secretion by different wood- and litter-colonizing basidiomycetes and ascomycetes grown on beech-wood. FEMS Microbiol Ecol 78:91–102. https://doi.org/10.1111/j.1574-6941.2011.01144.x
Liew ECY, Aptroot A, Hyde KD (2002) An evaluation of the monophyly of Massarina based on ribosomal DNA sequences. Mycologia 94:803–813. https://doi.org/10.1080/15572536.2003.11833174
Linaldeddu BT, Bottecchia F, Bregant C et al (2020) Diplodia fraxini and Diplodia subglobosa: the main species associated with cankers and dieback of Fraxinus excelsior in north-eastern Italy. Forests 11:883. https://doi.org/10.3390/f11080883
Linaldeddu BT, Bregant C, Montecchio L et al (2022) First report of Diplodia fraxini and Diplodia subglobosa causing canker and dieback of Fraxinus excelsior in Slovenia. Plant Dis 106:26–29. https://doi.org/10.1094/PDIS-06-21-1204-SC
Linaldeddu BT, Sirca C, Spano D, Franceschini A (2011) Variation of endophytic cork oak-associated fungal communities in relation to plant health and water stress. For Pathol 41:193–201. https://doi.org/10.1111/j.1439-0329.2010.00652.x
Lindahl BD, Nilsson RH, Tedersoo L et al (2013) Fungal community analysis by high-throughput sequencing of amplified markers – a user’s guide. New Phytol 199:288–299. https://doi.org/10.1111/nph.12243
Lombard L, van der Merwe NA, Groenewald JZ, Crous PW (2015) Generic concepts in Nectriaceae. Stud Mycol 80:189–245. https://doi.org/10.1016/j.simyco.2014.12.002
Lutz T, Langer G, Heinze C (2022) Complete genome sequence of a new quadrivirus infecting a member of the genus Thelonectria. Arch Virol 167:691–694. https://doi.org/10.1007/s00705-021-05353-y
Lygis V, Vasiliauskas R, Larsson K-H, Stenlid J (2005) Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand J For Res 20:337–346. https://doi.org/10.1080/02827580510036238
Maier M (2014) DirichletReg: dirichlet regression for compositional data in R
Maier MJ (2021) DirichletReg: dirichlet regression. R package version 0.7-1. https://github.com/maiermarco/DirichletReg
Malloch D, Sigler L, Hambleton S et al (2016) Fungi associated with hibernating bats in New Brunswick caves: the genus Leuconeurospora. Botany 94:1171–1181. https://doi.org/10.1139/cjb-2016-0086
Marçais B, Caël O, Delatour C (2010) Interaction between root rot basidiomycetes and Phytophthora species on pedunculate oak. Plant Pathol 60:296–303. https://doi.org/10.1111/j.1365-3059.2010.02378.x
Marçais B, Husson C, Godart L, Caël O (2016) Influence of site and stand factors on Hymenoscyphus fraxineus-induced basal lesion. Plant Pathol 65:1452–1461. https://doi.org/10.1111/ppa.12542
Marxmüller H (1992) Some notes on the taxonomy and nomenclature of five European Armillaria species. Mycotaxon 44:267–274
Meyn R, Langer GJ, Gross A, Langer EJ (2019) Fungal colonization patterns in necrotic rootstocks and stem bases of dieback-affected Fraxinus excelsior L. For Pathol 49:e12520. https://doi.org/10.1111/efp.12520
Min YJ, Park MS, Fong JJ et al (2014) Diversity and saline resistance of endophytic fungi associated with Pinus thunbergii in coastal shelterbelts of Korea. J Microbiol Biotechnol 24:324–333. https://doi.org/10.4014/jmb.1310.10041
Moiseenko KV, Glazunova OA, Shakhova NV et al (2019) Fungal adaptation to the advanced stages of wood decomposition: insights from the Steccherinum ochraceum. Microorganisms 7:527. https://doi.org/10.3390/microorganisms7110527
Moreno G, Blanco M-N, Checa J et al (2011) Taxonomic and phylogenetic revision of three rare irpicoid species within the Meruliaceae. Mycol Prog 10:481–491. https://doi.org/10.1007/s11557-010-0717-y
Morrison DJ (2004) Rhizomorph growth habit, saprophytic ability and virulence of 15 Armillaria species. For Pathol 34:15–26. https://doi.org/10.1046/j.1439-0329.2003.00345.x
Muggia L, Kopun T, Grube M (2017) Effects of growth media on the diversity of culturable fungi from lichens. Molecules 22:824. https://doi.org/10.3390/molecules22050824
Oses R, Valenzuela S, Freer J et al (2008) Fungal endophytes in xylem of healthy Chilean trees and their possible role in early wood decay. Fungal Divers 33:77–86
Palmer JM, Jusino MA, Banik MT, Lindner DL (2018) Non-biological synthetic spike-in controls and the AMPtk software pipeline improve mycobiome data. PeerJ 6:e4925. https://doi.org/10.7717/peerj.4925
Panno L, Bruno M, Voyron S et al (2013) Diversity, ecological role and potential biotechnological applications of marine fungi associated to the seagrass Posidonia oceanica. New Biotechnol 30:685–694. https://doi.org/10.1016/j.nbt.2013.01.010
Pautasso M, Aas G, Queloz V, Holdenrieder O (2013) European ash (Fraxinus excelsior) dieback – a conservation biology challenge. Biol Conserv 158:37–49. https://doi.org/10.1016/j.biocon.2012.08.026
Pažoutová S, Šrůtka P, Holuša J et al (2010) The phylogenetic position of Obolarina dryophila (Xylariales). Mycol Prog 9:501–507. https://doi.org/10.1007/s11557-010-0658-5
Peters S, Langer G, Kätzel R (2021a) Eschentriebsterben - Kriterien zur Schadensbonitur an Eschen, 1st edn. Fachagentur für nachwachsende Rohstoffe e.V. (FNR), Gülzow-Prüzen
Peters S, Langer G, Kätzel R (2021b) Bonitur geschädigter Eschen im Kontext des Eschentriebsterbens. AFZ-DerWald 76:28–31
Phookamsak R, Hyde KD, Jeewon R et al (2019) Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers 95:1–273. https://doi.org/10.1007/s13225-019-00421-w
Przybyl K (2002) Fungi associated with necrotic apical parts of Fraxinus excelsior shoots. For Pathol 32:387–394. https://doi.org/10.1046/j.1439-0329.2002.00301.x
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Version 4.1.2. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Riggenbach A (1956) Untersuchung über den Eschenkrebs. Doctoral Thesis, ETH Zürich. https://doi.org/10.3929/ethz-a-000294364
Rivedal HM, Stone AG, Severns PM, Johnson KB (2020) Characterization of the fungal community associated with root, crown, and vascular symptoms in an undiagnosed yield decline of winter squash. Phytobiomes J 4:178–192. https://doi.org/10.1094/PBIOMES-11-18-0056-R
Robert V, Stegehuis G, Stalpers J (2005) The MycoBank engine and related databases. In: MycoBank Database. https://www.mycobank.org/. Accessed 15 Sep 2022
Rongfeng X, Bo L, YuJing Z et al (2018) Spatial distribution characteristics of fungal population in microbial fermentation bed for pig rearing. Chin J Eco-Agric 26:493–504. https://doi.org/10.13930/j.cnki.cjea.170904
Salamon S, Mikołajczak K, Błaszczyk L et al (2020) Changes in root-associated fungal communities in Triticum aestivum ssp. spelta L. and Triticum aestivum ssp. vulgare L. under drought stress and in various soil processing. Plos One 15:e0240037. https://doi.org/10.1371/journal.pone.0240037
Salgado-Salazar C, Crouch JA (2019) Genome resources for the stem and bark canker pathogens Corinectria fuckeliana, Neonectria hederae and N. punicea. Plant Dis 103:389–391. https://doi.org/10.1094/PDIS-05-18-0904-A
Sandoval-Denis M, Lombard L, Crous PW (2019) Back to the roots: a reappraisal of Neocosmospora. Persoonia 43:90–185. https://doi.org/10.3767/persoonia.2019.43.04
Schoch CL, Robbertse B, Robert V et al (2014) Finding needles in haystacks: linking scientific names, reference specimens and molecular data for Fungi. Database 2014:bau061. https://doi.org/10.1093/database/bau061
Scholtysik A, Unterseher M, Otto P, Wirth C (2013) Spatio-temporal dynamics of endophyte diversity in the canopy of European ash (Fraxinus excelsior). Mycol Prog 12:291–304. https://doi.org/10.1007/s11557-012-0835-9
Schumacher J, Kehr R, Leonhard S (2009) Mycological and histological investigations of Fraxinus excelsior nursery saplings naturally infected by Chalara fraxinea. For Pathol 40:419–429. https://doi.org/10.1111/j.1439-0329.2009.00615.x
Schumacher J, Wulf A, Leonhard S (2007) Erster Nachweis von Chalara fraxinea T. KOWALSKI sp. nov. in Deutschland – ein Verursacher neuartiger Schäden an Eschen. Nachrichtenblatt Dtsch Pflanzenschutzdienstes 59:121–123
Sieber TN (2007) Endophytic fungi in forest trees: are they mutualists? Fungal Biol Rev 21:75–89. https://doi.org/10.1016/j.fbr.2007.05.004
Simon UK, Groenewald JZ, Crous PW (2009) Cymadothea trifolii, an obligate biotrophic leaf parasite of Trifolium, belongs to Mycosphaerellaceae as shown by nuclear ribosomal DNA analyses. Persoonia 22:49–55. https://doi.org/10.3767/003158509X425350
Šišić A, Baćanović-Šišić J, Al-Hatmi AMS et al (2018) The “forma specialis” issue in Fusarium: a case study in Fusarium solani f. sp. pisi. Sci Rep 8:1252. https://doi.org/10.1038/s41598-018-19779-z
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Sun L, Zhu J, Li X et al (2014) Diversity of endophytic fungi associated with Ferula sinkiangensis. Acta Microbiol Sin 54:936–942
Tan YP, Bishop-Hurley SL, Shivas RG et al (2022) Fungal Planet description sheets: 1436-1477. Persoonia 49:261–350. https://doi.org/10.3767/persoonia.2022.49.08
Tibpromma S, Hyde KD, Jeewon R et al (2017) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261. https://doi.org/10.1007/s13225-017-0378-0
Tomao A, Antonio Bonet J, Castaño C, de-Miguel S (2020) How does forest management affect fungal diversity and community composition? Current knowledge and future perspectives for the conservation of forest fungi. For Ecol Manag 457:117678. https://doi.org/10.1016/j.foreco.2019.117678
Travadon R, Lecomte P, Diarra B et al (2016) Grapevine pruning systems and cultivars influence the diversity of wood-colonizing fungi. Fungal Ecol 24:82–93. https://doi.org/10.1016/j.funeco.2016.09.003
Tsopelas P (1999) Distribution and ecology of Armillaria species in Greece. Eur J For Pathol 29:103–116. https://doi.org/10.1046/j.1439-0329.1999.00139.x
Turczański K, Rutkowski P, Dyderski M et al (2019) Soil pH and organic matter content affects European ash (Fraxinus excelsior L.) crown defoliation and its impact on understory vegetation. Forests 11:22. https://doi.org/10.3390/f11010022
Udayanga D, Castlebury LA, Rossman AY et al (2014) Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex. Fungal Divers 67:203–229. https://doi.org/10.1007/s13225-014-0297-2
Unterseher M (2011) Fungal Biodiversity Research – from cultivation of foliar tree endophytes to the combination of ecological theory and next generation sequencing. Doctoral Thesis, Ernst Moritz Arndt Universität Greifswald. https://doi.org/10.13140/RG.2.1.2045.3526
Verkley GJM, Dukik K, Renfurm R et al (2014) Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota). Persoonia 32:25–51. https://doi.org/10.3767/003158514X679191
Videira SIR, Groenewald JZ, Braun U et al (2016) All that glitters is not Ramularia. Stud Mycol 83:49–163. https://doi.org/10.1016/j.simyco.2016.06.001
Voglmayr H, Aguirre-Hudson MB, Wagner HG et al (2019) Lichens or endophytes? The enigmatic genus Leptosillia in the Leptosilliaceae fam. nov. (Xylariales), and Furfurella gen. nov. (Delonicicolaceae). Persoonia 42:228–260. https://doi.org/10.3767/persoonia.2019.42.09
Volobuev S, Shakhova N (2022) Towards the discovery of active lignocellulolytic enzyme producers: a screening study of xylotrophic macrofungi from the Central Russian Upland. Iran J Sci Technol Trans Sci 46:91–100. https://doi.org/10.1007/s40995-021-01245-7
Vu D, Groenewald M, de Vries M et al (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol 92:135–154. https://doi.org/10.1016/j.simyco.2018.05.001
Walentowski H, Falk W, Mette T et al (2017) Assessing future suitability of tree species under climate change by multiple methods: a case study in southern Germany. Ann For Res 60:101–126. https://doi.org/10.15287/afr.2016.789
Wanasinghe DN, Phukhamsakda C, Hyde KD et al (2018) Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers 89:1–236. https://doi.org/10.1007/s13225-018-0395-7
Wang W-J, Li Y, Wang X-L et al (2015) Neotypification of Paecilomyces hepiali (Hypocreales). TAXON 64:147–150. https://doi.org/10.12705/641.28
Wang X, Pecoraro L (2021) Analysis of soil fungal and bacterial communities in tianchi volcano crater, northeast China. Life 11:280. https://doi.org/10.3390/life11040280
Welk A (2017) Managementplan (Fachbeitrag Offenland) für das FFH-Gebiet 165 “Schwansee.” Management plan final report, RANA - Büro für Ökologie und Naturschutz on behalf of the Thüringer Landesanstalt für Umwelt und Geologie, Halle (Saale)
Wendt L, Sir EB, Kuhnert E et al (2018) Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol Prog 17:115–154. https://doi.org/10.1007/s11557-017-1311-3
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl 18:315–322
Whiting EC, Rizzo DM (1999) Effect of water potential on radial colony growth of Armillaria mellea and A. gallica isolates in culture. Mycologia 91:627–635. https://doi.org/10.1080/00275514.1999.12061061
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wickham H, Averick M, Bryan J et al (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Yang L, Peng M, Shah SS, Wang Q (2017) Transcriptome sequencing and comparative analysis of Piptoporus betulinus in response to birch sawdust induction. Forests 8:374. https://doi.org/10.3390/f8100374
Yokoya K, Postel S, Fang R, Sarasan V (2017) Endophytic fungal diversity of Fragaria vesca, a crop wild relative of strawberry, along environmental gradients within a small geographical area. PeerJ 5:e2860. https://doi.org/10.7717/peerj.2860
Zare R, Gams W, Culham A (2000) A revision of Verticillium sect. Prostrata I. Phylogenetic studies using ITS sequences. Nova Hedwigia 71:465–480. https://doi.org/10.1127/nova/71/2000/465
Zeng Z-Q, Zhuang W-Y (2016) A new species of Cosmospora and the first record of sexual state of C. lavitskiae. Mycol Prog 15:59. https://doi.org/10.1007/s11557-016-1201-0
Zhang W, Groenewald JZ, Lombard L et al (2021) Evaluating species in Botryosphaeriales. Persoonia 46:63–115. https://doi.org/10.3767/persoonia.2021.46.03
Zhao P, Crous PW, Hou LW et al (2021) Fungi of quarantine concern for China I: Dothideomycetes. Persoonia 47:45–105. https://doi.org/10.3767/persoonia.2021.47.02
Zlatković M, Keča N, Wingfield MJ et al (2016) Botryosphaeriaceae associated with the die-back of ornamental trees in the Western Balkans. Antonie Van Leeuwenhoek 109:543–564. https://doi.org/10.1007/s10482-016-0659-8
Acknowledgements
Many thanks to Peter Gawehn, Annette Ihlemann, Martina Hille, Daniel Gaunitz, and Dr. Holger Sennhenn-Reulen for their technical and statistical support. Additionally, the authors are grateful to the Forestry departments of Grünenplan, Erfurt-Willrode, Liebenburg, Neustadt, Satrup, and Nassesand for granting permission to sample the trees. Special thanks to Maia Ridley for proof-reading the manuscript. We would also like to thank the reviewers for their valuable advice on how to improve the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The project receives funding via the Waldklimafonds (WKF) funded by the German Federal Ministry of Food and Agriculture (BMEL) and the Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV) administrated by the Agency for Renewable Resources (FNR) under grant agreement No. 2219WK22A4.
Author information
Authors and Affiliations
Contributions
The study including sampling, lab work, and analysis was primarily conducted by S. Peters with support from G. Langer, S. Bien and S. Fuchs. The first draft of the manuscript was written by S. Peters and revised by G. Langer, S. Bien, J. Bußkamp, and E. Langer.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Claus Baessler
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peters, S., Fuchs, S., Bien, S. et al. Fungi associated with stem collar necroses of Fraxinus excelsior affected by ash dieback. Mycol Progress 22, 52 (2023). https://doi.org/10.1007/s11557-023-01897-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-023-01897-2