Abstract
The genus Amorphophallus encompasses some 230 species and is one of the largest genera of the Araceae family. Most species release scents, smelling of carrion, faeces, dung and similar nauseating odours for pollinator attraction and are therefore considered to have evolved a deceptive pollination syndrome. Some of the most iconic members of the genus, such as the A. titanum and A. gigas, are considered to be carrion mimics. Copro-necrophagous insects, beetles and flies in particular, are attracted by these scents and are therefore assumed to act as pollinators. However, many reports and observations on Amorphophallus pollinators are anecdotal in nature or do not distinguish between legitimate pollinators and non-pollinating visitors. Moreover, some published observations are not readily accessible as they are many decades old. Therefore, the available data and information about insect visitors and/or pollinators in the genus Amorphophallus is compiled, reviewed and discussed.
Similar content being viewed by others
Amorphophallus
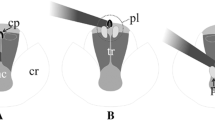
The genus Amorphophallus Blume ex Decne. (Araceae) has a palaeotropical distribution with the majority of species originating in Africa, Continental Asia and Southeast Asia (Claudel et al. 2017). It currently encompasses some 230 validly published species (WCVP 2021; Bustamante et al. 2020; Tamayo et al. 2021). The Amorphophallus inflorescence consists of a spadix surrounded by a spathe (Mayo et al. 1997) (Fig. 1a). The spathe is usually funnel-shaped but may occasionally be differentiated into a limb and a kettle, forming a chamber or a trap (Bröderbauer et al. 2012) (Fig. 1b). The spadix is subdivided into three zones (Fig. 1b). The lowermost zone that bears the female (pistillate) flowers (Fig. 1c), the adjacent zone that bears the male (staminate) flowers and a terminal zone, consisting of the appendix (Fig. 1b) that essentially serves the purpose of scent production and emission (Hetterscheid and Ittenbach 1996; Kite and Hetterscheid 2017).
Amorphophallus johnsonii. a Inflorescence consisting of a spadix surrounded by a constricted spathe, separated into a limb and a base forming a floral chamber. b Same inflorescence cut open, showing the female flowers at the base, followed by the male flower zone and the appendix above. Note the broadened appendix base, which in combination with the constriction and the slippery floral chamber make it difficult for insects to leave once they have entered. c Close-up of the female flower zone and the male flower zone. Note the hair-like papillae covering the base inside the floral chamber. d Section from (c). Extrusion of pollen strands on the second day of anthesis. Scale bars: A = 10 cm. B = 5 cm. C = 1 cm. D = 0.5 cm. Photographs: Cyrille Claudel
Amorphophallus inflorescences are protogynous and anthesis usually lasts for 2 days. On the first day of anthesis the stigmas of the pistillate flowers are receptive. On the second day of anthesis, pollen is released by the staminate flowers (Fig. 1d). Once the pollen is released, the female flowers are no longer receptive and self-pollination is prevented (Mayo et al. 1997; Hesse 2006). Usually, stigma receptivity is announced by the emission of characteristic scent compounds which serve to attract pollinators. In some species, such as A. konjac K. Koch, A. paeoniifolius (Dennst.) Nicolson and A. titanum Becc. ex Arcang, the scent volatilisation is enhanced through heat generation by the appendix (Skubatz et al. 1990; Barthlott et al. 2009; Korotkova and Barthlott 2009; Lamprecht and Seymour 2010).
The most famous species of the genus Amorphophallus are the two giants of the genus, A. titanum and A. gigas Teijsm and Binnend. These species develop large leaves and inflorescences, the latter exceeding three metres height (Hetterscheid 1994; Hetterscheid and Ittenbach 1996; McPherson and Hetterscheid 2011). The inflorescences carry spathes that are inwardly purplish and are accompanied by foul smells of decomposing organic material, such as carrion, and are therefore referred to as “carrion” or “corpse flowers” (Barthlott and Lobin 1998; Barthlott et al. 2009; Chen et al. 2015; Jürgens and Shuttleworth 2016; Raman et al. 2017).
The scent compounds of nearly a hundred Amorphophallus species have been analysed (Kite and Hetterscheid 1997, 2017; Kite et al. 1998; Kakishima et al. 2011; Lamprecht and Seymour 2010; Shirasu et al. 2010; Chen et al. 2015; Raman et al. 2017) and most species release scent types that include “carrion, faeces, urine, dung, fishy, sewerage, nauseating gaseous, rancid cheese, fermenting fruit and mushrooms” (Kite and Hetterscheid 2017). These odour types are effective cues for insects that search for such substrates for feeding, mating or breeding, indicating the deceptive nature of the majority of Amorphophallus species (Kite et al. 1998; Jürgens et al. 2006, 2013; Vereecken and McNeil 2010; Urru et al. 2011; Johnson and Schiestl 2016; Kite and Hetterscheid 2017). The deceived targets are usually Diptera or Coleoptera (Wiens 1978; Faegri and Van der Pijl 1979; Johnson and Schiestl 2016), defining most Amorphophallus species as oviposition-site mimics.
However, there are exceptions, as some Amorphophallus species are sweetly scented. Two clades, containing 13 of the 92 investigated species, release sweet odour types based on aromatic hydrocarbons, such as 1-phenylethanol derivatives or 4-methoxyphenethyl alcohol (Kite and Hetterscheid 1997, 2017). These odour types appear quite different from carrion, dung or other scent types that indicate decomposition of organic matter. However, it must be considered that methoxylated aromatics, 4-methoxyphenethyl alcohol in particular, are strong attractants to various beetle taxa (Dötterl et al. 2012; Tóth et al. 2017; Lohonyai et al. 2018). That said, 4-methoxyphenethyl alcohol does not appear to be related to decomposition processes. However, although pleasantly scented to the human nose, at least one 1-phenylethanol derivative, acetophenone, is a sweet odour that is released during cadaveric decomposition (Buis 2016). Therefore, these contrasting odour types may be very similar from a functional perspective, and a necrophagous insect might be similarly attracted to nauseating odours as to “the sweet stench of decay” (Ollerton and Raguso 2006).
However, the knowledge about pollinators in Amorphophallus is limited, particularly if the large size of the genus and its geographical spread are considered. Some reports consist in casual observations and rely on one single inflorescence. Furthermore, the distinction between insect visitors and pollinators is rarely specified, which makes it challenging to evaluate the plant-pollinator interaction in the genus Amorphophallus. Therefore, the available information is compiled and evaluated to bring together the observations about pollinators in Amorphophallus that are scattered through the literature, often extending over many decades.
Pollinators
Beetles are the main known pollinator group reported for Amorphophallus (Moretto et al. 2019). Commonly, but not exclusively, three Scarabaeoidea families act as Amorphophallus pollinators, according to Moretto et al. (2019). These are the Dynastidae, more precisely the genus Peltonotus in India and South East Asia, the Hybosoridae, i.e., the genus Phaeochrous in Southeast Asia and Africa; and the copro-necrophagous Scarabaeidae in Southeast Asia and India (Moretto et al. 2019). Some Amorphophallus species, such as A. hohenackeri (Schott) Engl. and Gehrm., A. johnsonii N.E. Br., A. konkanensis Hett., Yadav and Patil, A. julaihii Ipor, Tawan and P.C. Boyce, A. sylvaticus (Roxb.) Kunth and A. variabilis Bl. are considered specialists, pollinated by a single beetle species (van der Pijl 1937; Sivadasan and Sabu 1989; Beath 1996; Punekar and Kumaran 2010; Chai and Wong 2019).
However, most Amorphophallus species that have been investigated seem to attract a multitude of insects (van der Pijl 1937; Bogner 1976; Hetterscheid 1994; Beath 1996; Giordano 1999; Jung 2006; Punekar and Kumaran 2010; Gibernau 2011; Chaturvedi 2017; Moretto et al. 2019; Chai and Wong 2019); or, as in the case of A. paeoniifolius, the reported observations are contradictory (Singh and Gadgil 1995; Grimm 2009; Sites 2017). Also, considering the large size of the genus Amorphophallus (> 230 species; WCVP, 2021; Bustamante et al. 2020; Tamayo et al. 2021) very few actual field studies were conducted. As a consequence, actual pollination has rarely been observed and there are even fewer reports that include observations on fruit set, which could validate if the observed insects are truly the pollinators (Singh and Gadgil 1995; Beath 1996; Jung, 2006; Chai and Wong 2019).
In order to evaluate and discuss the reported insect and non-insects visitors and pollinators, all the known pollinators, putative pollinators and visitors of Amorphophallus are listed in Table 1. The distinction between pollinator, putative pollinator and visitor is based on several considerations, first and foremost the observations and statements provided in the references. However, not all references make a distinction between a visitor and a pollinator, and some reports are contradictory. For example, Trigona bees are either not categorised at all, or categorised either as visitors or as pollinators, depending on the report. Similarly, some Diptera have been observed crawling on the stigma but were not reported as pollinators. However, they might contribute to pollination and are classified as putative pollinators in such cases. As for the visitors, they are usually classified in the various reports as such, on the grounds that they never visit the female flower zone, or if they are rare and the visiting organism, such as Arachnida, does not match the pollinating type. However, such visitors may also play a role in pollination as predators.
As previously mentioned, the most common pollinators in Amorphophallus belong to the three beetle families Dynastidae, Hybosoridae and Scarabaeidae (Moretto et al. 2019). However, smaller beetle taxa, i.e., Nitidulidae and Staphylinidae, also visit Amorphophallus inflorescences and act as pollinators (van der Pijl 1937; Punekar and Kumaran 2010; Chen et al. 2015; Chai and Wong 2019). Furthermore, fly pollination has also been mentioned. Amorphophallus angolensis subsp. maculatus (N. E. Br.) Ittenb., A. prainii, A. konjac, A. titanum and A. gomboczianus were reported to be pollinated or at least visited by flies (Gombocz 1936; Bogner 1976; Soepadmo 1973; Chen et al. 2015). Whilst Gombocz (1936) and Soepadmo (1973) only casually mentioned flies as pollinators, Bogner (1976) reported them as pollinators with certainty, together with the beetle Phaeochrous camerunensis. Chen et al. (2015) investigated the olfactory and visual attractors in A. konjac and provided detailed experiments and observations. A total of 12 fly genera belonging to the three families Calliphoridae, Sarcophagidae and Muscidae were recorded to be attracted to A. konjac inflorescences, and the main visitors were species of the fly genera Lucilia and Chrysomya (Chen et al. 2015). However, Chen et al. (2015) did not investigate the fruit set. Moreover, Chen et al. (2015) also mentioned Dermaptera as well as several beetle families as natural pollinators of A. konjac, namely Histeridae, Staphylinidae and Nitidulidae. For the time being therefore, the effectiveness of the recorded fly genera (Chen et al. 2015) acting as pollinators for A. konjac remains unsubstantiated for the time being.
Drosophila flies, usually not as pollinators but as visitors, have been documented in A. bulbifer (Roxb.) Bl., A. commutatus (Schott) Engl. (Punekar and Kumaran 2010), A. henryi N.E. Br. (Jung 2006), A. napalensis (Wall.) Bogner and Mayo (Chaturvedi 2017), and A. titanum (Giordano 1999). Likewise, flies from the Calliphoridae and the Muscidae have been observed as visitors in most of these species (Giordano 1999; Jung 2006; Punekar and Kumaran 2010). However, their exact contribution to pollination remains unclear in most cases even though they have occasionally been observed to crawl on the female flowers (Giordano 1999; Punekar and Kumaran 2010).
Moreover, ants (Formicidae) and cockroaches (Blaberidae/Panesthiinae) were observed as visitors in several Indian Amorphophallus species (Punekar and Kumaran 2010). Ants and cockroaches (Blattoidae and Blattodea) were also found as visitors in A. henryi and A. titanum (Jung 2006; Giordano 1999) whereas ants without cockroaches were observed to crawl at the spathe base in A. koratensis (pers. comm. Sutthinut Soonthornkalump).
In A. napalensis, even honey bees (Apis indica) were recorded as flower visitors (Chaturvedi 2017). Also, earwigs (Dermaptera) were reported to be pollinators in A. konjac (Chen et al. 2015), and stingless bees (Trigona spp.) have been reported on several occasions as visitors or putative pollinators in several Amorphophallus species (Hetterscheid 1994; Singh and Gadgil 1995; Giordano 1999; Punekar and Kumaran 2010; Chaturvedi 2017). However, only one study explicitly reported that stingless bees (Trigona sp.) act as pollinators (Punekar and Kumaran 2010). Nevertheless, 14 years earlier, it was questioned if Trigona bees are likely to act reliably as pollinators in Amorphophallus (Hetterscheid and Ittenbach 1996). However, they have been repeatedly observed crawling on both male and female flowers of A. titanum and A. koratensis and carrying pollen (Hetterscheid 1994; Giordano 1999; pers. comm. Sutthinut Soonthornkalump). Moreover, considering the varied trophic preferences of stingless bees (Eltz 2001), it seems at least possible that they are attracted to Amorphophallus species. Recently, two fungi species of Cladosporium have been identified that form a fungal layer at the base of A. titanum inflorescences (Ruprecht et al. 2021). Referring to Sayyad and Mulani (2016), Ruprecht et al. (2021) propose that Cladosporium species grow as endophytes in A. titanum, forming a fungal layer at the spathe base during inflorescence development. If these findings are confirmed in situ, future investigations will have to consider and investigate the impact of fungal layers on pollinator attraction, considering that Trigona collina stingless bees have been observed to harvest mold spores (Rhizopus sp.) (Eltz 2001).
As a side note, most Trigona bees reported as putative pollinators of Amorphophallus have not been identified at the species level (Hetterscheid 1994; Punekar and Kumaran 2010; Chaturvedi 2017). However, the genus Trigona has been extensively revised in the meantime and various Asian species have been transferred to other genera (Michener 2007). For this reason, stingless bees in general are referred to the following pages, unless the species or the genus has been specified.
Recently, stingless bees have been observed visiting the inflorescence of a cultivated plant of A. koratensis Gagn. in large numbers (pers. comm. Sutthinut Soonthornkalump, Prince of Songkla University, Thailand). The bees repeatedly visited the inflorescence and collected pollen, occasionally falling down into the pistillate flower zone. They were identified as Tetragonula species by Kanuengnit Wayo, an entomologist from Prince of Songkla University. Besides the large number of Tetragonula bees, S. Soonthornkalump also observed small numbers of Formicidae at the base of the floral chamber. Interestingly, the bees were still attracted to the inflorescence after it ceased to smell, at least to the human nose. This behaviour has already been observed on behalf of A. titanum (Hetterscheid 1994), making it unclear what exactly attracts the bees. However, pollen has been shown to release fragrances that are attractive to bees but are not perceptible by humans (Dobson and Bergström 2000; Flamini et al. 2002). This could signify that some Amorphophallus species putatively attract two different pollinator guilds, copro-necrophagous insects and stingless bees. However, in the case of A. koratensis, the question if Tetragonula spp. is a pollinating taxon awaits confirmation as there was only one inflorescence, and because the inflorescence is protogynous, the pollination was unsuccessful.
It has been reported in several cases that the pollinating beetles are trapped in the floral chamber until pollen shedding (Sivadasan and Sabu 1989; Beath 1996; Moretto et al. 2019). Although most Amorphophallus species do not form complex traps (Bröderbauer et al. 2012), some still capture visitors or pollinators by means of slippery spathes and/or a floral chamber with a strong constriction, making it difficult for most trapped insects to leave the inflorescence (Sivadasan and Sabu 1989; Beath 1996; Chai and Wong 2019; Moretto et al. 2019). Similarly, or additionally, in some species, such as in A. johnsonii (Beath 1996) (Fig. 1) and A. titanum (van der Pijl 1937), the base of the appendix is broadened and forms an overhanging wall, functioning as an effective obstacle to insects that try to leave. Still, it has also been observed on several occasions that visitors and pollinators were “disinclined” to leave for no apparent reason (Chai and Wong 2019), and according to Beath (1996), it must be assumed that the pollinators are kept by the smell.
Moreover, recent research indicates that some beetles respond differently to scent compounds, depending on their life-stage. Trumbo and Steiger (2020) investigated the attractiveness of five single scent compounds, as well as mixtures of these five compounds on burying beetles from the genus Nicrophorus. They showed that freshly emerged beetles respond to the scent signatures of well-rotted carcasses whereas beetles in search of a suitable breeding site respond to the scent signatures of fresh carcasses which may serve as food for their own brood. Moreover, flying beetles in search of a breeding place were actually deterred by some compounds, such as dimethyl trisulphide (Trumbo and Steiger 2020). Four out of five of these scent compounds, namely dimethyl monosulphide, dimethyl disulphide, dimethyl trisulphide and s-methyl thioacetate are emitted by Amorphophallus species (Kite and Hetterscheid 1997, 2017). Nearly half of the Amorphophallus species studied by Kite and Hetterscheid (1997, 2017) emit oligosulphides as major scent compounds, often accompanied by s-methyl-thioesters. This underlines the necessity for future research as the specific ratio of the scent compounds might have very different effects on putative pollinators.
In some species, such as A. johnsonii, A. paeoniifolius and A. titanum, the floral chamber was used by insects as a mating place (Beath 1996; Giordano 1999; Grimm 2009; Chai and Wong 2019). Moreover, it has been observed that both the appendix and the pollen has been consumed or harvested by pollinators in A. napalensis (Chaturvedi 2017) and A. commutatus (Punekar and Kumaran 2010). Likewise, fruit bodies are offered in A. hohenackeri (Sivadasan and Sabu 1989; Punekar and Kumaran 2010) and A. konkanensis (Punekar and Kumaran 2010), as is food tissue in A. variabilis (van der Pijl 1937) and stigmatic fluid in A. bulbifer (Punekar and Kumaran 2010), indicating that plant-pollinator interactions in the genus Amorphophallus are diverse and can be based on several, not necessarily mutually exclusive strategies, such as deceit, trapping, provision of a reward or possibly even mutualism. Obviously, some insects are in search of food, whereas others use the floral chamber as a mating place, or both; a behavioural trait known from other plant-pollinator interactions, such as Glaphyridae beetles feeding and mating on large bowl-shaped flowers from Anemone coronaria L. and Papaver umbonatum Boiss. (Keasar et al. 2010). Besides visiting the inflorescence in search of females, some visitors are simply using the inflorescence as a shelter (Wasserman and Itagaki 2003; Fishman and Hadany 2013). However, the purpose of other visitors or pollinators in Amorphophallus remains obscure (van der Pijl 1937; Chai and Wong 2019).
Moreover, the picture is very heterogenous. For example, one of the species, A. commutatus comprises four subspecies and the spectrum of insect visitors or pollinators differs markedly between the four subspecies (Table 1). Judging by the reported visitors/pollinators, it would seem that A. commutatus var. anmodensis is exclusively pollinated by a single beetle species whereas the other three subspecies are visited by a broad spectrum of different taxa (Table 1). It seems surprising that one subspecies is apparently a specialist when it comes to pollinator attraction whereas the other three subspecies are generalists. That said, it is unclear if more than one inflorescence of A. commutatus var. anmodensis has been investigated and therefore more observations are required to validate these observations.
Similarly, there are three species that have been sampled by different investigators. Firstly, A. paeoniifolius that has been sampled five times (Table 1). Although Hybosoridae and Scarabaeidae prevail in most of these reports, other Coleoptera and Diptera have also been observed to visit the inflorescences. However, A. paeoniifolius is a crop plant that is widely distributed in the tropics and its natural distribution is not known (Hetterscheid 2012). It is therefore debatable if the reported insects can be regarded as the natural pollinators, especially as one of the reports explicitly state that some of the wild occurring A. paeoniifolius plants, and all of the cultivated plants, failed to develop fruits (Singh and Gadgil 1995).
Another example is A. titanum in which insect visitors/pollinators in situ have been observed on three occasions, with markedly different results. Hetterscheid (1994) observed only stingless bees during the second day of anthesis but no insects at all on the first day of anthesis. In contrast, van der Pijl report that Diamesus osculans beetles (Silphidae) in particular, as well as Creophilus villipennis beetles (Staphylinidae) have been observed to visit several inflorescences of A. titanum. Lastly, Giordano (1999) observed several A. titanum specimens and reported a multitude of different taxa, including Coleoptera, Diptera and Hymenoptera and also ants, cockroaches and spiders (Table 1).
Lastly, A. variabilis, which has been investigated by Backer (1913) and van der Pijl (1937). At least in this species both authors report a beetle from the Nitidulidae family as the main pollinator. Nonetheless, Backer also reports a second visitor, namely the beetle Philanthus crassicornis (Staphilinidae) that was not observed by van der Pijl (1937).
Another difficulty is that the numbers of sampled specimens per site is not always referenced (Bogner 1976; Punekar and Kumaran 2010) and it remains unclear on how many inflorescences these observations are based. In some species, several specimens per population (Beath 1996; Giordano 1999) or several populations per species have been investigated (Bogner 1976; Jung 2006; Punekar and Kumaran 2010). However, other observations rely on observations gathered on behalf of one single inflorescence per Amorphophallus species (Soepadmo 1973; Hetterscheid 1994; Giordano 1999; Grimm 2009; Punekar and Kumaran 2010; Sites 2017; Moretto et al. 2019).
Pollinating predators
One motive, which so far has been widely neglected, is that visitors and pollinators do not approach either the substrate (dung, carrion, etc.) or the mimic (the inflorescence) in themselves, but arrive there to prey on the feeding or mating insects, or insects larvae (Moretto et al. 2019). Apparently, some Amorphophallus species, such as A. titanum (Giordano 1999; Moretto et al. 2019), A. henryi (Jung 2006) and A. commutatus (Punekar and Kumaran 2010), attract different insect groups as well as other arthropods. The attracted and deceived insects might feed on plant resources such as pollen, etc., or use the floral chamber as a mating place or as a shelter. However, some of the attracted insects or arthropods, exemplified by Arachnida, Blattaria, and predatory beetles (Giordano 1999; Jung 2006; Punekar and Kumaran 2010), do not arrive for the plant resources, etc., but rather to prey on the visiting insects.
For example, Creophilus beetles (Staphylinidae) are reported as exclusive pollinators in A. julaihii. However, Creophilus species are well investigated, as they provide useful forensic information; and Creophilus species are generally predators feeding on copro-necrophagous adult insects and on their larvae (Frątczak-Łagiewska et al. 2020). Similar predatory visitors have also been observed in A. titanum (Giordano 1999; Moretto et al. 2019). Fittingly, insect larvae, more specifically maggots, have been reported in inflorescences of A. variabilis and A. commutatus var. commutatus (van der Pijl 1937; Punekar and Kumaran 2010).
Although these records constitute only a few observations, it must be noted that predators such as Arachnida, Blattodea and Formicidae are reported in all of the more detailed observations and investigations (Giordano 1999; Jung 2006; Punekar and Kumaran 2010). This could signify a complex interplay between the inflorescence and its visitors, and it begs the question, which group contributes the most to actual pollination? The insects that are deceived and not rewarded, those feeding on plant resources, or those predating the first two insect groups? And if predators are attracted first in a significant numbers, would other visitors still alight on the inflorescence? A most fascinating scenario would consist of a multitude of attracted insects that constitute the actual reward for a predatory beetle, with prey and predator both potentially acting as pollinators. If such a relationship could be confirmed it would certainly add another dimension to the complexity of deceit flowers. Remarkably, this scenario was proposed as early as 1889 but has not received much attention ever since (Delpino 1889). Engler (1920, pp. 18 and 19 and references herein) gives a brief summary on a scientific dispute between Arcangeli and Delpino. Delpino had observed that Dracunculus vulgaris Schott is exclusively pollinated by flies, whereas Arcangeli reported beetles as the main pollinators (Engler 1920). In 1889, Delpino emphasised the idea that flies are the main pollinators of Dracunculus vulgaris and that the beetles only follow the flies to prey on them. Subsequent investigations revealed that both Diptera as well as Coleoptera can act as pollinators in Dracunculus vulgaris (Engler 1920). However, the impact of predators on pollinators and pollination in deceit flowers still remains to be investigated, at least in Amorphophallus.
Summary and outlook
The aim of this review was to compile, review and discuss the state of the art of insect visitors and pollinators in the genus Amorphophallus. In summary, insect visitors or pollinators are reported for a total of 22 Amorphophallus species, which is less than 10% of the species diversity of the genus (ca. 230 spp.). Moreover, approximately a third of the reported observations were made on behalf of a single Amorphophallus inflorescence in the wild (Table 1). and the actual success of pollination, the fruit set, has been reported or documented in only four cases (Singh and Gadgil 1995; Beath 1996; Jung 2006; Chai and Wong 2019).
A most interesting observation is that stingless bees have been repeatedly observed in different Amorphophallus species, in India, Thailand and Sumatra. This may indicate that their role has to be considered more closely in future studies, particularly in conjunction with the observations made regarding fungal layers at the base of the spathe (Ruprecht et al. 2021).
It becomes evident through the presented data that the knowledge about pollinators in the genus Amorphophallus remains limited. The motives of the visiting insects are often not obvious, i.e., if they are in search of a mating or a breeding place, possibly also attracted by the plant’s food resources or if they are predators in search of prey? Or simply in search of a shelter? However, if no clear motives are discernible, this may as well signify the unspecific attraction of all copro-necrophagous insects or the attraction of unspecialised Coleoptera and Diptera alike. For example, Moretto et al. (2019) identified members of the beetle genus Sphaeridium as pollinators of Amorphophallus. These beetles are ubiquitous in tropical Africa and they are attracted by decomposing organic material of all kinds, such as excrement, carrion, mushrooms, fruits, vegetables.
The only tentative conclusion that can be drawn from the compiled data is that beetles are most likely the main pollinator group in Amorphophallus, and that although various Diptera are attracted by many Amorphophallus species, they seem to contribute less to actual pollination. However, more detailed observations based on larger samplings are required to draw more specific conclusions.
In conclusion, the plant-pollinator interaction seems to follow a generalist pattern in most of the Amorphophallus species investigated, attracting copro-necrophagous Coleoptera and Diptera alike. Similarly, Gibernau et al. (2010) found an “imperfect discrimination” of quantitative floral traits between fly and beetle-pollinated aroids.
However, attracting a multitude of insects suggests a generalist pollination strategy that is at the same time highly efficient insofar as insects can be attracted anywhere as copro-necrophagous insects are ubiquitous. It might therefore be speculated that relying on this functional group of insects as pollinators, which is available everywhere on earth, might have contributed to the evolutionary success of the genus Amorphophallus, which is the largest palaeotropical aroid genus and the third largest genus of the Araceae altogether (Boyce and Croat (2018 onwards).
References
Backer CA (1913) Het Slangenblad II. Trop Nat 2:177–179
Barthlott W, Lobin W (eds) (1998) Amorphophallus titanium—Tropische und Subtropische Pflanzenwelt. Akademie der Wissenschaft und der Literatur in Mainz. Franz Steiner Verlag, Stuttgart
Barthlott W, Szarzynski J, Vlek P, Lobin W, Korotkova N (2009) A torch in the rain forest: thermogenesis of the Titan arum (Amorphophallus titanum). Plant Biol (stuttg) 11:499–505. https://doi.org/10.1111/j.1438-8677.2008.00147.x
Beath DDN (1996) Pollination of Amorphophallus johnsonii (Araceae) by carrion beetles (Phaeochrous amplus) in a Ghanaian rain forest. J Trop Ecol 12:409–418
Bogner J (1976) Amorphophallus maculatus. Der Palmengarten 3:83–86
Boyce PC, Croat TB (2018) The Überlist of Araceae, Totals for Published and Estimated Number of Species in Aroid Genera. Available from:http://www.aroid.org/genera/20201008Uberlist.pdf. Accessed 9 Sept 2021
Bröderbauer D, Diaz A, Weber A (2012) Reconstructing the origin and elaboration of insect–trapping inflorescences in the Araceae. Am J Bot 99:1666–1679. https://doi.org/10.3732/ajb.1200274
Buis RC (2016) The validation of human decomposition fluid as a cadaver–detection dog training aid. Thesis submitted for the degree of Doctor of Philosophy (Science) Centre for Forensic Science University of Technology, Sydney
Bustamante RAA, Mansibang JA, Hetterscheid WLA, Tamayo MN (2020) Amorphophallus caudatus (Thomsonieae, Araceae), a new species from Camarines Norte, Luzon island, the Philippines. Nordic J Bot. https://doi.org/10.1111/njb.02982
Chai SK, Wong SY (2019) Five pollination guilds of aroids (Araceae) at Mulu National Park (Sarawak, Malaysian Borneo). Webbia 74:353–371
Chaturvedi SK (2017) Pollinators and visitors of Amorphophallus napalensis (Wall.) Bogner and Mayo (Araceae) in Nagaland State, North-East India. Pleione 11:336–340
Chen G, Ma X-K, Jürgens A, Lu J, Liu E-X, Sun W-B, Cai X-H (2015) Mimicking livor mortis: a well-known but unsubstantiated color profile in sapromyiophily. J Chem Ecol 41:808–815. https://doi.org/10.1007/s10886-015-0618-2
Claudel C, Buerki S, Chatrou L, Antonelli A, Alvarez N, Hetterscheid W (2017) Large–scale phylogenetic analysis of Amorphophallus (Araceae) derived from nuclear and plastid sequences reveals new subgeneric delineation. Bot J Linn Soc 184:32–45. https://doi.org/10.1093/botlinnean/box013
Delpino F (1889) Sulla Impollinazione Dell’arum Dracunculus. Malpighia III:385–395
Dobson HEM, Bergström G (2000) The ecology and evolution of pollen odors. Plant Syst Evol 222:63–87. https://doi.org/10.1007/BF00984096
Dötterl S, David A, Boland W, Silberbauer-Gottsberger I, Gottsberger G (2012) Evidence for behavioral attractiveness of methoxylated aromatics in a dynastid scarab beetle-pollinated Araceae. J Chem Ecol 38:1539–1543. https://doi.org/10.1007/s10886-012-0210-y
Eltz T (2001) Ecology of stingless bees (Apidae, Meliponini) in lowland dipterocarp forests in Sabah, Malaysia, and an evaluation of logging impact on populations and communities. Dissertation, Julius-Maximilians-Universität Würzburg
Engler A (1920) Araceae-Aroideae und Araceae-Pistioideae. Das Pflanzenreich 23(73):1–274
Faegri K, van der Pijl L (1979) The principles of pollination ecology, 3rd edn. Pergamon Press, London
Fishman MA, Hadany L (2013) Pollinators’ mating rendezvous and the evolution of floral advertisement. J Theor Biol 316:99–106. https://doi.org/10.1016/j.jtbi.2012.09.006
Flamini G, Cioni PL, Morelli I (2002) Differences in the fragrances of pollen and different floral parts of male and female flowers of Laurus nobilis. J Agric Food Chem 50:4647–4652. https://doi.org/10.1021/jf020269x
Frątczak-Łagiewska K, Grzywacz A, Matuszewski S (2020) Development and validation of forensically useful growth models for Central European population of Creophilus maxillosus L. (Coleoptera: Staphylinidae). Int J Legal Med 134:1531–1545. https://doi.org/10.1007/s00414-020-02275-3
Gibernau M (2011) Pollinators and visitors of aroid inflorescences: an addendum. Aroideana 34:70–83
Gibernau M, Chartier M, Barabé D (2010) Recent advances towards an evolutionary comprehension of Araceae pollination. In: Seberg O, Peterson G, Barfod AS, Davis JI (eds) Diversity, phylogeny and evolution in the monocotyledons. Aarhus University Press, Denmark
Giordano C (1999) Observations on Amorphophallus titanum (Becc.) Becc.ex Arcangeli in the Forest of Sumatra. Aroideana 22:10–19
Gombocz E (1936) Ein neuer Amorphophallus. Ann Hist-Nat Musei Hung 30:1–3
Grimm R (2009) Peltonotus nasutus Arrow, 1910 und Phaeochrous-Arten als Bestäuber von Amorphophallus paeoniifolius (Araceae) in Thailand (Coleoptera: Scarabaeidae). Entomol Z Insektenbörse 119(4):167–168
Hesse M (2006) Reasons and consequences of the lack of a sporopollenin ektexine in Aroideae (Araceae). Flora 201:421–428. https://doi.org/10.1016/j.flora.2005.10.002
Hetterscheid WLA (1994) Sumatran Amorphophallus adventures: 20 August–1 September 1993. Aroideana 17:61–77
Hetterscheid WLA (2012) Amorphophallus. In: Boyce PC, Sookchaloem D, Hetterscheid WLA, Gusman G, Jacobsen N, Idei T, Nguyen VD (eds) Araceae. Flora of Thailand 11. The Forest Herbarium, Bangkok, pp 218–232
Hetterscheid WLA, Ittenbach S (1996) Everything you always wanted to know about Amorphophallus, but were afraid to stick your nose into. Aroideana 19:7–131
Johnson SD, Schiestl FP (2016) Floral mimicry. Oxford University Press, New York
Jürgens A, Shuttleworth A (2016) Carrion and dung mimicry in plants. In: Benbow ME, Tomberlin JK, Tarone AM (eds) Carrion ecology, evolution, and their applications. CRC Press, Boca Raton, pp 361–386
Jürgens A, Dötterl S, Meve U (2006) The chemical nature of fetid floral odours in stapeliads (Apocynaceae–Asclepiadoideae–Ceropegieae). New Phytol 172:452–468. https://doi.org/10.1111/j.1469-8137.2006.01845.x
Jürgens A, Wee S-L, Shuttleworth A, Johnson SD (2013) Chemical mimicry of insect oviposition sites: a global analysis of convergence in angiosperms. Ecol Lett 16:1157–1167. https://doi.org/10.1111/ele.12152
Jung M-J (2006) The Pollination Biology of Amorphophallus henryi N. E. Br. Master thesis, National Cheng–Kung University, Taiwan
Kakishima S, Terajima Y, Murata J, Tsukaya H (2011) Infrared thermography and odour composition of the Amorphophallus gigas (Araceae) inflorescence: the cooling effect of the odorous liquid. Plant Biol (stuttg) 13:502–507. https://doi.org/10.1111/j.1438-8677.2010.00399.x
Keasar T, Harari AR, Sabatinelli G, Keith D, Dafni A, Shavit O, Zylbertal A, Shmida A (2010) Red anemone guild flowers as focal places for mating and feeding by Levant glaphyrid beetles. Biol J Linn Soc 99:808–817. https://doi.org/10.1111/j.1095-8312.2010.01384.x
Kite GC, Hetterscheid WLA (1997) Inflorescence odours of Amorphophallus and Pseudodracontium (Araceae). Phytochemistry 46:71–75. https://doi.org/10.1016/S0031-9422(97)00221-5
Kite GC, Hetterscheid WLA (2017) Phylogenetic trends in the evolution of inflorescence odours in Amorphophallus. Phytochemistry 142:126–142. https://doi.org/10.1016/j.phytochem.2017.06.006
Kite GC, Hetterscheid WLA, Lewis M, Boyce PC, Ollerton J, Cocklin E, Diaz A, Simmonds MSJ (1998) Inflorescence odours and pollinators of Arum and Amorphophallus (Araceae). In: Owens S, Rudall P (eds) Reproductive biology in systematics, conservation and economic botany. Kew Publishing, Kew, pp 295–315
Korotkova N, Barthlott W (2009) On the thermogenesis of the Titan arum (Amorphophallus titanum). Plant Signal Behav 4:499–505. https://doi.org/10.4161/psb.4.11.9872
Lamprecht I, Seymour RS (2010) Thermologic investigations of three species of Amorphophallus. J Therm Anal Calorim 102:127–136. https://doi.org/10.1007/s10973-010-0891-9
Lohonyai Z, Vuts J, Fail J, Tóth M, Imrei Z (2018) Field response of two cetoniin chafers (Coleoptera, scarabaeidae) to floral compounds in ternary and binary combinations. Acta Phytopathol Entomol Hung 53:259–269
Mayo SJ, Bogner J, Boyce PC (1997) The genera of Araceae. Royal Botanic Gardens, Kew
McPherson S, Hetterscheid WLA (2011) Amorphophallus in the wild and in cultivation. Plantsman 10:90–97
Michener CD (2007) The bees of the world, 2nd edn. John Hopkins University Press, Baltimore
Moretto P, Cosson B, Krell F-T, Aristophanous M (2019) Pollination of Amorphophallus barthlottii and A. abyssinicus subsp. akeassii (Araceae) by dung beetles (Insecta: Coleoptera: Scarabaeoidea). Cathar La Rev 18:19–36
Ollerton J, Raguso RA (2006) The sweet stench of decay. New Phytol 172:382–385. https://doi.org/10.1111/j.1469-8137.2006.01903.x
Punekar SA, Kumaran KPN (2010) Pollen morphology and pollination ecology of Amorphophallus species from North Western Ghats and Konkan region of India. Flora 205:326–336. https://doi.org/10.1016/j.flora.2009.12.024
Raman V, Tabanca N, Demirci B, Khan IA (2017) Studies on the floral anatomy and scent chemistry of titan arum (Amorphophallus titanum, Araceae). Turk J Biol 41:63–74. https://doi.org/10.3906/bot-1604-34
Ruprecht UW, Socher SA, Dötterl S (2021) Unexpected occurrence of Cladosporium spp. on the inner side of the spathe of the titan arum Amorphophallus titanum. Acta Mycol. https://doi.org/10.5586/am.563
Sayyad S, Mulani RM (2016) Endophytic fungal diversity in corms of Amorphophallus sylvaticus (Roxb.) Kunth. Int J Eng Res 4:32–34
Shirasu M, Fujioka K, Kakishima S, Nagai S, Tomizawa Y, Tsukaya H, Murata J, Manome Y, Touhara K (2010) Chemical identity of a rotting animal–like odor emitted from the inflorescence of the titan arum (Amorphophallus titanum). Biosci Biotechnol Biochem 74:2550–2554. https://doi.org/10.1271/bbb.100692
Singh SN, Gadgil M (1995) Ecology of Amorphophallus species in Uttara Kannada District of the Karnataka State, India: implications for conservation. Aroideana 18:5–20
Sites RW (2017) The Aroid Scarab Peltonotus nasutus (Coleoptera: Scarabaeidae) in Thailand and its association with Amorphophallus paeoniifolius (Araceae). Nat Hist Bull Siam Soc 62:107–109
Sivadasan M, Sabu T (1989) Beetle pollination—cantharophily—in Amorphophallus hohenackeri (Araceae). Aroideana 12:32–37
Skubatz H, Nelson TA, Dong AM, Meeuse BJD, Bendich AJ (1990) Infrared thermography of Arum lily inflorescences. Planta 182:432–436
Soepadmo E (1973) A short note on Amorphophallus cf. prainii Hook.f. Malay Nat J 25:133–134
Tamayo MN, Magtoto LM, Sumalinog MS, Reyes TD, Austria CM (2021) Amorphophallus calcicolus (Thomsonieae, Araceae), a new species from the Bohol island, Central Visayas, Philippines. Phytotaxa 489:229–235
Tóth M, Szarukán I, Nagy A, Furlan L, Benvegnù I, Rak Cizej M, Ábri T, Kéki T, Körösi S, Pogonyi A, Toshova T, Velchev D, Atanasova D, Kurtulus A, Kaydan BM, Signori A (2017) European corn borer (Ostrinia nubilalis Hbn., Lepidoptera: Crambidae): comparing the performance of a new bisexual lure with that of synthetic sex pheromone in five countries. Pest Manag Sci 73:2504–2508
Trumbo ST, Steiger S (2020) Finding a fresh carcass: bacterially derived volatiles and burying beetle search success. Chemoecology 30:287–296. https://doi.org/10.1101/2020.01.25.919696
Urru I, Stensmyr MC, Hansson BS (2011) Pollination by brood–site deception. Phytochemistry 72:1655–1666. https://doi.org/10.1016/j.phytochem.2011.02.014
van der Pijl L (1937) Biological and physiological observations on the inflorescence of Amorphophallus. Recl Trav Bot Néerl 34:57–67
Vereecken NJ, McNeil JN (2010) Cheaters and liars: chemical mimicry at its finest. Can J Zool 88:725–752. https://doi.org/10.1139/Z10-040
Wasserman SL, Itagaki H (2003) The olfactory responses of the antenna and maxillary palp of the fleshfly, Neobellieria bullata (Diptera: Sarcophagidae), and their sensitivity to blockage of nitric oxide synthase. J Insect Physiol 49:271–280. https://doi.org/10.1016/S0022-1910(02)00288-3
WCVP (2021) World Checklist of Vascular Plants, version 2.0. Facilitated by the Royal Botanic Gardens, Kew. http://wcvp.science.kew.org/. Accessed 14 Feb 2021.
Wiens D (1978) Mimicry in plants. Evol Biol 11:365–403
Acknowledgements
Cyrille Claudel expresses his gratitude to Sutthinut Soonthornkalump for kindly sharing his observations. Moreover, to Simcha Lev-Yadun for his friendship and many constructive comments. To Deni Bown for making corrections taste sweet. And to Mr. John Tan from Singapore for his support. Lastly, to the two anonymous reviewers for their highly valued suggestions.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Dagmar Voigt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Claudel, C. The many elusive pollinators in the genus Amorphophallus. Arthropod-Plant Interactions 15, 833–844 (2021). https://doi.org/10.1007/s11829-021-09865-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-021-09865-x