Abstract
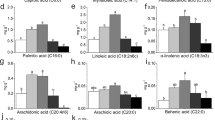
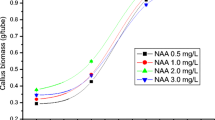
The callus growth kinetics allows identifying the appropriate moment for callus pealing and monitoring the accumulation of primary and secondary metabolites. The physic nut (Jatropha curcas L.) is a plant species used for biofuel production due to its high oil content; however, this plant presents a great amount of bioactive compounds which can be useful for industry. The aim of this research was to establish a calli growth curve and to evaluate the fatty acid profile of crude oil extracted from callus. The callus growth kinetics presented a sigmoid standard curve with six distinct phases: lag, exponential, linear, deceleration, stationary, and decline. Total soluble sugars were higher at the inoculation day. Reducing sugars were higher at the inoculation day and at the 80th day. The highest percentage of ethereal extract (oil content) was obtained at the 120th day of culture, reaching 18 % of crude oil from the callus. The calli produced medium-chain and long-chain fatty acids (from 10 to 18 carbon atoms). The palmitic acid was the fatty acid with the highest proportion in oil (55.4 %). The lipid profile obtained in callus oil was different from the seed oil profile.


Similar content being viewed by others
References
Costa, J. L., Lima, R. P., Silva, A. L. L., Scheidt, G. N., & Erasmo Lemus, E. A. (2011). Initial growth of plants of physic nut under shading in the Gurupi county, Tocantins State, Brazil. Journal of Biotechnology and Biodiversity, 2, 43–47.
Horbach, M. A., Malavasi, U. C., Malavasi, M. M., Ajala, M. C., Lima, P. R., & Schulz, D. G. (2014). Propagation methods for physic nut (Jatropha curcas). Advances in Forestry Science, 1(1), 53–57.
Goel, G., Makkar, H. P. S., Francis, G., & Becker, K. (2007). Phorbol esters: structure, occurrence and biological activity. International Journal of Toxicology, 26, 279–288.
Zhang, X. (2012). The molluscidal activities of different extracts from Jatropha curcas L. against Pomacea canaliculata. Journal of Anhui Agricultural University, 40, 3349–3350.
Devappa, R. K., Rajesh, S. K., Kumar, V., Makkar, H. P., & Becker, K. (2012). Activities of Jatropha curcas phorbol esters in various bioassays. Ecotoxicology and Environmental Safety, 78, 57–62.
Li, J., Yan, F., Wu, F. H., Yue, B. S., & Chen, F. (2004). Insecticidal activity of extracts from Jatropha curcas seed against Lipaphis erysimi. Acta Physica Sinica, 31(3), 289–293.
Kupchan, S. M., Sigel, C. W., Matz, M. J., Gilmore, C. J., & Bryan, R. F. (1976). Structure and stereochemistry of jatrophone, a novel macrocyclic diterpenoid tumor inhibitor. Journal of the American Chemical Society, 98, 2295–2300.
Van den Berg, A. J., Horsten, S. F., Kettenes van den Bosch, J. J., Kroes, B. H., Beukelman, C. J., Loeflang, B. R., & Labadie, R. P. (1995). Curcacycline A: a novel cyclic octapeptide isolated from the latex of Jatropha curcas Linn. FEBS Letters, 358, 215–218.
Lin, J., Yan, F., Tang, L., & Chen, F. (2003). Antitumor effects of curcin from seeds of Jatropha curcas. Acta Pharmacologica Sinica, 24, 241–246.
Reddy MP, Pamidimarri DS (2010) Biology and biotechnological advances in Jatropha curcas - a biodiesel plant. In: (Ramawat K. G. ed.) Desert plants. Springer Berlin Heidelberg. pp. 57-71.
Wei, Q., Liao, Y., Zhou, L. J., Zhou, J. X., Wang, S. H., & Chen, F. (2004). Antifungal activity of curcin from seeds of Jatropha curcas. Chinese Journal of Oil Crop Sciences, 26, 71–75.
Heller, J. (1996). Physic nut. International Plant Genetic Resources Institute, Rome: Jatropha curcas L. Promoting the conservation and use of underutilized and neglected crops.
Scheidt, G. N., Silva, A. L. L., Oliveira, Y., Costa, J. L., Biasi, L. A., & Soccol, C. R. (2011). In vitro growth of Melaleuca alternifolia Cheel in bioreactor of immersion by bubbles. Pakistan Journal of Botany, 43(6), 2937–2939.
Rong, F., & Wang, S. H. (2005). Identification of curcin by western blot in calli generated from explants of Jatropha curcas L. Journal of Sichuan University (Natural Science Edition), 42(1), 211–214.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiology, 15, 473–497.
Costa, J. L., Silva, A. L. L., Scheidt, G. N., Erasmo, E. A. L., & Soccol, C. R. (2010). In vitro establishment of seeds of physic nut (Jatropha curcas L.) - Euphorbiaceae. Caderno de Pesquisa Série Biologia, 22, 5–11.
Soomro, R., & Memon, R. A. (2007). Establishment of callus and suspension culture in Jatropha curcas. Pakistan Journal of Botany, 39, 2431–2441.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Stein, W., & Moore, S. (1948). A modified ninhydrin reagent for photometric determination of amino acids and related compounds. The Journal of Biological Chemistry, 176, 367–372.
Yemm, E. W., & Willis, A. J. (1954). The estimation of carbohydrates in plant extracts by anthrone. The Biochemical Journal, 57(3), 508–514.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Biochemistry, 31, 426–428.
AOAC. (1990). Official methods of analysis of the Association of Official Analytical Chemists (15th ed., pp. 369–406). Association of Official Analytical Chemists: Washington.
Varian. NIst 98 MS Library Database, ver. 1.7.USA, 1998.
Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectrometry (4th ed.). Carol Stream: Allured Publishing Corporation.
EMBRAPA. (1990). Núcleo Tecnológico para Informática. Campinas: SOC–Software Científico.
Smith, R.H Plant tissue culture: techniques and experiments. San Diego: Academic, 1992. 171p.
Nogueira, R. C., Paiva, R., Lima, E. C., Soares, G. A., Oliveira, L. M., Santos, B. R., Emrich, E. B., & Castro, A. H. F. (2008). Curva de crescimento e análises bioquímicas de calos de murici-pequeno (Byrsonima intermedia A. Juss.). Revista Brasileira de Plantas Medicinais, Botucatu, 10(1), 44–48.
Serra, A. G. P., Paiva, R., & Paiva, P. D. O. (2000). Análises bioquímicas de calos formados de explantes foliares de castanha do Brasil (Bertholletia excelsa H.B.K.). Ciencia e Agrotecnologia, 24(40), 833–840.
Santos-Filho, P. R., Santos, B. R., Barbosa, S., Vieira, L. R., Freitas, N. C., Dias, D. F., & Santos, M. H. (2014). Growth curve, biochemical profile and phytochemical analyses in calli obtained from the procambium segments of Bacupari. Brazilian Archives of Biology and Technology, 57, 326–333.
Carvalho, D. C., Silva, A. L. L., Schuck, M. R., Purcino, M., Tanno, G. N., & Biasi, L. A. (2013). Fox grape cv. Bordô (Vitis labrusca L.) and grapevine cv. Chardonnay (Vitis vinifera L.) cultivated in vitro under different carbohydrates, amino acids and 6-benzylaminopurine levels. Brazilian Archives of Biology and Technology, 56(2), 191–201.
López-Villalobos A, Dodds PF, Hornung R (2011) Lauric acid improves the growth of zygotic coconut (Cocos nucifera L.) embryos in vitro.Plant Cell Tiss Organ Cult. 106, 317-327.
Li, Y. L., Zhang, P., & He, Y. (2006). Perspective of the development and application of Jatropha curcas in the dry-hot valley of Panzhihua. Guangxi Trop Agri., 2, 39–40.
Halder, T., & Gadgil, V. N. (1984). Comparison of fatty acid patterns in plant parts and respective callus cultures of Cucumis melo. Phytochemistry, 23(8), 1790–1791.
Correa, S. M., & Atehortúa, L. (2012). Lipid profile of in vitro oil produced through cell culture of Jatropha curcas. Journal of AOAC International, 95, 1161–1169.
Martínez-Herrera, J., Siddhuraju, P., Francis, G., Dávila-Ortíz, G., & Becker, K. (2006). Chemical composition, toxic/antimetabolic constituents, and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico. Food Chemistry, 96(1), 80–89.
Acknowledgments
The authors thank CNPq (the National Council for Scientific and Technological Development) and CAPES (Coordination for the Improvement of Higher Level -or- Education - Personnel) for the Post-Doctoral, Ph. D., and M. Sc. fellowships. Moreover, the authors thank the Fundação Araucária for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Luz Costa, J., da Silva, A.L.L., Bier, M.C.J. et al. Callus Growth Kinetics of Physic Nut (Jatropha curcas L.) and Content of Fatty Acids from Crude Oil Obtained In Vitro. Appl Biochem Biotechnol 176, 892–902 (2015). https://doi.org/10.1007/s12010-015-1618-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1618-y