Summary
Afrothismia is a genus of non-photosynthetic mycoheterotrophs from the forests of continental tropical Africa. Multiple phylogenetic inferences using molecular data recover the genus as sister to a clade comprising mycoheterotrophic Thismiaceae and the photosynthetic family Taccaceae, contrary to earlier placements of Afrothismia and Thismiaceae within Burmanniaceae. Morphological support for separating Afrothismia from the rest of Thismiaceae has depended on the zygomorphic flowers of Afrothismia (although some species of Thismia are also zygomorphic), and their clusters of root tubers, each with a terminal rootlet. The number of described species of Afrothismia has recently increased from four to 16, with seven more species as yet undescribed; these discoveries have added morphological characters that support its distinction from Thismiaceae. Most notably, the ovary in Afrothismia has a single stalked placenta, and circumscissile fruits from which seeds are exserted by placental elevation (vs in Thismiaceae, three placentas, a deliquescing fruit lid, and seeds not exserted). Afrothismia stamens are inserted in the lower part of the floral tube, where they are attached to the stigma, and individual flowers are subtended by a single large dorsal bract. In contrast, in Thismiaceae, stamens are inserted at the mouth of the tube, free of and distant from the stigma, and each flower is subtended by a loose whorl of (2 –) 3 (– 4) bracts. Here we formally characterise Afrothismiaceae and review what is known of its development, seed germination, interactions with mycorrhizal Glomeromycota, biogeography, phylogeny and pollination biology. All but one (Afrothismia insignis; Vulnerable) of the 13 species assessed on the IUCN Red List of Threatened Species are either Endangered or Critically Endangered; one species (A. pachyantha) is considered extinct.
Similar content being viewed by others
Introduction
The non-photosynthetic and fully mycoheterotrophic genus Afrothismia Schltr. (Schlechter 1906) is currently placed in the achlorophyllous tribe Thismieae (Burmanniaceae), together with Thismia Griff. (Griffith 1845), Haplothismia Airy Shaw (1952), Oxygyne Schltr. (Schlechter 1906), and the more recently described Tiputinia P.E.Berry & C.L.Woodw. (Woodward et al. 2007). Based on early molecular phylogenetic analyses that included plastid rbcL, atpB and nuclear 18S rDNA genes (Caddick et al. 2000a, b), APG II (2003) and subsequent APG classifications (e.g. APG III 2009; APG IV 2016) combined Burmanniaceae sensu stricto with former Thismiaceae into a single family, Burmanniaceae sensu lato. However, the molecular-based analyses used for these classifications included contaminant plastid sequences (Lam et al. 2016), a common issue when using polymerase chain reaction (PCR) amplification to recover plastid DNA sequence data from mycoheterotrophic taxa.
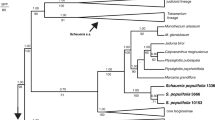
By contrast, morphological analysis (Caddick et al. 2002) indicated the paraphyly of Burmanniaceae s.l. with respect to other Dioscoreales, based partly on the absence of septal nectaries in Thismiaceae (Caddick et al. 2000b, 2002). More recent molecular phylogenetic data based on nuclear and mitochondrial sequences, and whole-plastid genomes (Merckx et al. 2009, 2010, 2014; Lam et al. 2016, 2018; Shepeleva et al. 2020; Lin et al. 2022) endorse earlier treatments (e.g., Jonker 1938; Maas-van der Kamer 1998; APG I 1998) that support the separation of Thismiaceae from Burmanniaceae in different subclades of Dioscoreales, with Thismiaceae instead associated with Taccaceae (e.g., Merckx et al. 2009, 2014). Moreover, studies of Thismiaceae based on mitochondrial and nuclear gene sets (Merckx et al. 2009, 2014, 2017) have shown that the family is itself paraphyletic, with Afrothismia recovered as the sister group of a clade comprising photosynthetic Taccaceae plus the rest of Thismiaceae. Strong support for this arrangement was recently recovered in phylogenomic analyses based on mitochondrial genomes (Lin et al. 2022). A general consensus has therefore emerged that Afrothismia is a phylogenetic lineage that is distinct from Burmanniaceae, Taccaceae and Thismiaceae (Fig. 1).
Higher-order relationships within Dioscoreales, following Lin et al. (2022). Taxa in red are fully mycoheterotrophic (Afrothismia, Thismiaceae s.s.), or include both mycoheterotophic and photosynthetic taxa (Burmanniaceae).
To date, Afrothismia has been distinguished from Thismia by two morphological characters: zygomorphic flowers (but some species of Thismia are zygomorphic), and clusters of root tubers, each with a terminal rootlet (Maas-van der Kamer 1998). However, additional morphological data generated in the last two decades potentially support separate family status for Afrothismia, including new characters associated with the description of numerous new species of Afrothismia (Cheek 2004a; 2007; 2009; Cheek et al. 2019; Cheek & Jannerup 2006; Dauby et al. 2008; Franke 2004; Franke et al. 2004; Maas-van de Kamer 2003; Sainge & Franke 2005; Sainge et al. 2005, 2013) and studies on ontogeny, floral morphology and mycorrhizal organization (Imhof & Sainge 2008; Imhof et al. 2020; Shepeleva et al. 2020). Here, we review these recent studies and present evidence in support of family status for the genus. We formally describe and diagnose Afrothismiaceae as a new, fully mycoheterotrophic and monogeneric family in the order Dioscoreales. An alternative approach of lumping Afrothismia (and other families with varying number of genera) under a very broadly defined Dioscoreaceae would obscure the high level of morphological diversity evident among these families (see, for example, Table 1). Given the unambiguous and substantial differences between the existing, well-recognised related families, including Thismiaceae (itself misplaced under Burmanniaceae in APG IV 2016; see Lin et al. 2022), this justifies recognising Afrothismia at the family level.
The related family Taccaceae Dumort., which is sometimes placed in or near Dioscoreaceae R.Br., is a pantropical family of 10 – 20 species, all placed in Tacca J.R.Forst. & G.Forst. Superficially, these large, pantropical, photosynthetic, terrestrial herbs seem unconnected with the non-photosynthetic and leafless Thismiaceae and Afrothismiaceae. However, both molecular and morphological studies indicate a close relationship between these taxa. As Rübsamen (1986) stated (translated from the German), “…Remarkable parallels exist in the structure of the anthers between Taccaceae and Burmanniaceae: the six stamens (of Tacca) look confusingly similar to those of Afrothismia winkleri (Engl.) Schltr. or Haplothismia; the plate- or umbrella-shaped stigma (or the cap-like appearance of the style) is also reminiscent of the more bowl-shaped stigma of Afrothismia.”
Taxonomy and nomenclature
Nomenclature follows the Code (Turland et al. 2018). Authorship of names follows IPNI (continuously updated). The format of the description follows Cheek et al. (2019). Morphological terms follow Beentje & Cheek (2003). Herbarium codes follow Index Herbariorum (Thiers, continuously updated). All specimens cited have been seen by one of the authors. The conservation assessments cited follow the categories and criteria of the IUCN (International Union for Conservation of Nature) Red List of Threatened Species (2012).
Diagnostic characters for Afrothismia are presented in Table 1 and Figs 2, 3 and 4, including a comparison with Thismiaceae (Haplothismia, Oxygyne, Thismia, Tiputinia) and Taccaceae (Tacca, Fig. 5).
Afrothismia zambesiaca. A habit, flowering stem; B dehisced fruit (showing the placenta with its seeds, exserted by the “placentophore”) on sympodial inflorescence; C seeds, showing an appendage, a possible elaiosome at each end; D dissected flower; E flower in section, reconstructed from D. All from Exell, Mendonça & Wild 1066 (holotype, K). DRAWN BY ANDREW BROWN
Photomicrographs of sections of flowers. A – C Afrothismia hydra Sainge & T.Franke, serial transverse sections of zygomorphic flower bud; A ovary with a single placenta on a long stalk (placentophore); B top of ovary showing base of hollow style and stamens on one side; C hollow style. D, E Thismia aseroe Becc. (K: Dransfield 7340A, Malaysia, Nov. 1993), serial transverse sections of flower bud; D ovary with three equal placentas and numerous ovules (ov) at early developmental stages; E stamen tube with external developing anthers. F – J Thismia sp. (K: Chew, Corner & Stainton 1909, Malaysia, Aug. 1961); F longitudinal section of young flower bud showing apical placentas in ovary and stamen connectives extending downwards towards stigma (not connected with stigma at this stage); G – J serial transverse sections of young flower bud; G three equal placentas in ovary; H top of ovary and style base; J hollow style. K – M Haplothismia exannulata Airy Shaw (K: Abraham & Jacob, India, Oct. 1951), serial transverse sections of flower; K three equal placentas in ovary; L top of ovary with mucilage accumulation around style base; M style with mass of germinating pollen tubes (p) inserted between carpel margins. Labels: ov = ovule, p = pollen-tube mass, st = stamen. Scales = 100 μm.
Afrothismia baerae. A habit, flowering stem; B cluster of root tubers, each with terminal root; C flower with bract (rear view); D flower, frontal view; E longitudinal section of flower (non-median); F flower-subtending bract; G detail of stamen and connective; H side view of the free part of the stamen showing the expanded filament-connective structure above the anther cells. All from Baer s.n. (K). DRAWN BY MARGARET TEBBS
Tacca leontopetaloides. A habit; B part of leaf; C tuber, scape and cataphyll; D inflorescence at fruiting stage; E flower, side view; F opened flower, ovary and style with 1 inner and 1 outer tepal (× 3); G 3 tepals with anthers in hoods; H ovary and style; J capsule, side view; K seed. A, C, E from Pawek 4171, B, D from Goyder & Paton 3511, F – H from Bingham 10224, J, K from Pawek 2310. DRAWN BY JULIET WILLIAMSON. Reproduced from Wilkin (2009) with permission
Taxonomic Treatment
Afrothismiaceae Cheek & Soto Gomez fam. nov. Type genus: Afrothismia Schltr. (Schlechter 1906).
http://www.ipni.org/urn:lsid:ipni.org:names:77328212-1
A single genus, Afrothismia, restricted to continental tropical Africa.
Description as for the genus (see below).
Afrothismia Schltr. (Schlechter 1906; Jonker 1938; Cowley 1988; Maas-van de Kamer 1998; Cheek 2009). Type of genus: Afrothismia winkleri (Engl.) Schltr. Designated by Jonker (1938: 223).
Perennial non-photosynthetic mycoheterotrophic herbs entirely lacking green tissue, with only the flower or fruit emerging above the leaf-litter. Stem (rhizome) colourless, opaque, succulent, concealed in substrate, spreading more or less horizontally, becoming vertical when flowering, sparsely branched or unbranched. Scale leaves sparse, alternate, ovate-triangular, minute, axillary buds globose, minute. Bulbil clusters subglobose in outline, bulbils 15 - 40, each globose, with an apical rootlet (Fig. 4B).
Inflorescence 1 – few-flowered, terminal or sympodial where more than one flower present (Fig. 2B). Flowers strongly or weakly zygomorphic, lateral flowers subtended by a large dorsal colourless ovate bract (Fig. 2A, 3A, 3B, 4D). Floral tube usually colour-patterned, translucent, white, red and/or purple, erect to horizontal, straight, S-shaped or angled, globose or subcylindrical, constricted or not, separated internally usually by an annulus, into two parts, upper and lower, outer surface smooth, ribbed or papillate; mouth of tube projecting beyond insertion of the lobes, ribbed or not, partly covered with an operculum (corona) or not; aperture orbicular, hemi-orbicular or elliptic; post-anthesis deliquescing. Perianth lobes six, yellow, white, red or purple, patent, forward-directed or curved, equal or unequal, triangular or filiform, entire or lacerate.
Stamens six, inserted on distal part of lower floral tube below the annulus; staminal filaments dorsiventrally slightly flattened or clavate, the apex and basal part of the connective swollen and sometimes geniculate (Fig. 4H), arching inward and downward to stigma, glabrous, papillate or hairy; anther thecae two, elliptic, introrse, separated by and often embedded in the connective; distal connective appendage papillate (Fig. 4G), firmly adnate to stigmatic surface (Fig. 4E); pollen monoporate, surface reticulate.
Ovary inferior, campanulate, unilocular, placentation basal, placenta globose and sometimes 3-lobed, massive, attached at base by cylindrical stalk; ovules numerous, anatropous, often on long funicles (Fig. 3A). Style cylindrical, short, hollow (Fig. 3B, C); stigma obconical, thick, usually slightly 6-lobed, surface densely papillate or hairy (Fig. 4E).
Fruit campanulate, dehiscence circumscissile; the lid of the fruit (ovary roof) detaching completely, exposing the seed-covered placenta which is projected more or less completely above or far above the fruit wall by the elongation of the placental base or placentophore. Seeds numerous, narrowly obovoid or ellipsoid, reticulate, lacking appendages or with a swollen structure (possible elaiosome) at each end. (Fig. 2).
recognition. Afrothismiaceae fam. nov. differing from Thismiaceae J.Agardh in that the ovary has a single stalked, globose placenta (vs three placentas), fruits circumscissile (pyxidium), seeds exserted from fruit by placental elevation (vs seeds included), rootstocks with clusters of globose root tubers, each with a terminal root (vs a single tuber or roots fleshy), stamens inserted in mid or lower part of floral tube, below the annulus (vs inserted near mouth); anther appendages connected to stigma at anthesis (vs stamens distant and free from stigma).
distribution & biogeography. Continental tropical Africa, from Nigeria east to Kenya, south to Malawi. Of the 16 accepted species (Table 2), 12 occur in Cameroon, one of these extending west to Nigeria, another East to Gabon. Gabon has a single endemic species, Uganda another (currently treated at varietal rank), Kenya and Malawi both have one, and Tanzania two. In addition, several undescribed species are known to have been collected in Cameroon (Sainge et al. 2017) and Gabon (MC, pers. obs), and one in Tanzania (Afrothismia “arachnites”; Rübsamen 1986). In Cameroon, the species are concentrated in the Cross-Sanaga Interval (Cheek et al. 2001), where nine of the 12 Cameroon species occur, seven of which are endemic. This region contains the highest vascular plant species and generic diversity per degree square in tropical Africa, with endemic genera such as Medusandra Brenan (Peridiscaceae, Barthlott et al. 1996; Dagallier et al. 2020; Soltis et al. 2007; Breteler et al. 2015). The species of Afrothismia in this region show the full range of floral and root morphology documented in the genus. The genus is unrecorded from the Congo basin and there is a c. 2200 km disjunction between the species of Lower Guinea (West-Central Africa) and the westernmost East African record in Uganda.
The most species-diverse area for Afrothismia is Mt Kupe in Southwest Region, Cameroon, within the Cross-Sanaga Interval. Here five species have been recorded: A. fungiformis Sainge & Kenfack (Sainge et al. 2013), A. hydra Sainge & T.Franke (Onana & Cheek 2011), A. kupensis Cheek & S.A.Williams (Cheek et al. 2019), A. saingei T.Franke (2004) and A. winkleri (Cheek et al. 2004). Two species, A. kupensis and A. saingei, are endemic to Mt Kupe. The Mt Kupe region has been the source of numerous other new species (e.g. Stoffelen et al. 1997; Cheek & Csiba 2002; Cheek 2003) and even new genera, including Kupeantha Cheek (Rubiaceae, Cheek et al. 2018b) and another non-photosynthetic mycoheterotroph, Kupea Cheek & S.A.Williams (Triuridaceae, Cheek et al. 2003), subsequently found to extend to East Africa (Cheek 2004b).
At Mt Kupe, Afrothismia species co-exist with other achlorophyllous mycoheterotrophic plant species in the families Gentianaceae, Burmanniaceae and Triuridaceae, including one site of c. 20 m2 with six mycoheterotrophic species, including Afrothismia kupensis (Cheek & Williams 1999; Cheek 2006), equalling the record in the other most species-diverse site recorded in Africa, at Moliwe, Mt Cameroon (Cheek & Ndam 1996, analysing collection records from Schlechter), which is now converted into agricultural plantations.
The first records of several of the mycoheterotrophs at Mt Kupe were made during the course of intensive botanical surveys conducted over several seasons, including mycoheterotroph specialists, to support conservation management. The surveys usually resulted in a botanical conservation checklist (in the case of Mt Kupe, Cheek et al. 2004). However, similar surveys at several other locations in Cameroon with lowland or submontane forest that might be expected to host Afrothismia failed to uncover any plants from this genus, even during the supposedly appropriate late wet season (Cheek et al. 2000; 2010, 2011; Harvey et al. 2004, 2010). These records suggest that Afrothismia species are not ubiquitous in any apparently suitable habitat, but are genuinely localised and rare, even in Cameroon, where the majority of the known species are recorded.
habitat. Lowland and submontane evergreen forest; c. 200 – 1150 m altitude.
conservation status. Of the 17 accepted Afrothismia taxa (16 species and one variety), only 14 have been assessed for their IUCN Red List extinction risk status (Table 2). One species has been assessed as Vulnerable, two as Endangered and 11 of the 14 are assessed as Critically Endangered, the highest category of threatened status before extinction. In fact, A. pachyantha Schltr. has subsequently been declared extinct (Cheek et al. 2019), based on multiple attempts to relocate the species at its sole locality by teams of mycoheterotroph specialists since 1991. Its habitat has been cleared for plantation and small-holder agriculture, including rubber (Hevea brasiliensis (Willd. ex A.Juss.) Müll.Arg.).
Other locations lost due to agricultural clearance include the type locality of Afrothismia winkleri at Muea in Cameroon (Onana & Cheek 2011). This process represents the main threat faced by Afrothismia species; they are acutely susceptible because most occur in either a single or only two to three locations, and at each location, their population can occupy only 2 – 10 m2. It is expected that additional species will shortly become extinct, if they are not already so. For example, the forest at Mt Kala, type and sole locality for A. amietii Cheek and A. pusilla Sainge & Kenfack, is being cleared for housing development. Afrothismia baerae Cheek has not been seen for 20 years since it was first collected in 2002, despite annual monitoring (Quentin Luke pers. comm. to MC 2022). However, other species not seen for decades, such as A. zambesiaca Cheek, unseen since the type gathering in 1955, cannot be assumed extinct because there have been no targeted efforts to relocate them. In order to support their protection, in 2022, almost all Cameroon Afrothismia species were included within a network of Important Plant Areas (IPAs or TIPAs, Darbyshire et al. 2017; https://www.kew.org/science/our-science/projects/tropical-important-plant-areas-cameroon). However, these species currently lack legal protection. Eight Afrothismia species are included in the Cameroon Red Data Book (Onana & Cheek 2011: 353 – 356).
Non-photosynthetic mycoheterotrophic plants such as Afrothismia have rarely been successfully cultivated, though Merckx et al. (2013) noted that field-sourced plants of A. foertheriana T.Franke, Sainge & Agerer and A. winkleri have been grown in a laboratory. Ideally, success in growing Afrothismia from seed would require both the species of fungal symbiont and suitable autotrophic plant partner(s) already established, although the latter remain unknown. There is no record that this goal has been achieved or even attempted. It is likely that Afrothismia might have orthodox seeds since they are small and dry, but no species are known to be seed-banked, and seed banking is of little value if the seeds cannot be grown to produce viable plants.
The IUCN Red List convention is that species are not considered for inclusion on their website (iucnredlist.org) until they are formally published. This makes it more urgent to publish species so that they can be formally red-listed and afforded a higher level of protection than they would otherwise obtain (Cheek et al. 2020). Therefore, publication of the seven known but still undescribed species of Afrothismia remains a high priority.
etymology. Taken to signify “African Thismia”.
notes. Regarding the typification of Afrothismia, two species were published in the generic protologue (Schlechter 1906). Jonker effectively selected one of these as the lectotype of the genus by designating Afrothismia winkleri as “Type species” (Jonker 1938: 223).
species discovery. Afrothismia winkleri was first published as Thismia winkleri Engl. (Engler 1905) before becoming the foundation of Schlechter’s genus Afrothismia, later joined by A. pachyantha Schltr. (Schlechter 1906). Both species were first collected on the slopes of Mt Cameroon. The third species was collected in the Usambara Mts, now in Tanzania, by Peter (s.n., B), who labelled it as Afrothismia arachnites n. sp., but never formally published it. Until today, this species seems to have been overlooked, except by Rübsamen (1986). Two new taxa were added to the genus more than 80 years later by Cowley (1988), A. winkleri var. budongensis Cowley (Uganda) and A. insignis Cowley (Tanzania). By the end of the 20th century, Afrothismia was known to have only three species, two of which occurred at Mt Cameroon, one endemic (Cheek & Ndam 1996). A large increase in species discovery and publication, arising mainly from Cameroon, began 15 years after Cowley (1988). Afrothismia gesnerioides H.Maas (Maas-van der Kamer 2003) of Cameroon, was soon followed by A. baerae Cheek (2004a) of Kenya, and four species from Southwest Region, Cameroon: A. saingei T.Franke (2004), A. foertheriana T.Franke, Sainge & Agerer (Franke et al. 2004), A. hydra Sainge & T.Franke and A. korupensis Sainge & T.Franke (Sainge et al. 2005; Sainge & Franke 2005). Subsequent species were A. mhoroana Cheek of Tanzania (Cheek & Jannerup 2006), A. amietii Cheek (2007) of Cameroon, A. gabonensis Dauby & Stévart (Dauby et al. 2008) of Gabon, and A. zambesiaca Cheek (2009) of Malawi. The most recently published taxa are three species from Cameroon: A. pusilla Sainge & Kenfack, A. fungiformis Sainge & Kenfack (both Sainge et al. 2013) and A. kupensis Cheek & S.A.Williams (Cheek et al. 2019).
Sainge et al. (2017) mentioned three further still undescribed species (referred to as a, b, d, respectively) that Sainge had collected in Cameroon and a fourth that he had seen from Gabon, which he termed sp. C (Boupouya et al. 674). The first author (MC) has also seen photos of two further undescribed species from the Crystal Mountains of Gabon, Bidault 5044 and Bidault 5478. Additionally, there is Peter s.n. (A. “arachnites”) of Tanzania (Rübsamen 1986). Thus, seven undescribed species remain to be published, which would take the total number of species in the genus Afrothismia and family Afrothismiaceae to 23, exceeding the 20 species currently accepted in the related family Taccaceae (Plants of the World online).
Taking existing point data records for Afrothismia and using Maxent and an ecological niche-modelling approach to map the potential range, Sainge et al. (2017) predicted highly suitable regions where additional species might be found in Sierra Leone, Liberia, Ivory Coast, Nigeria, Cameroon, Equatorial Guinea, Gabon, Republic of Congo and Democratic Republic of Congo. However, regarding the first three countries, which equate to Upper Guinea, no Afrothismia species have been found to date, despite recent discoveries of other achlorophyllous mycoheterophic plant species there (e.g. Cheek & van der Burgt 2010).
Origin and diversification of Afrothismia
Using two different relaxed molecular clock models, the origin of Afrothismia was estimated as 91 ± 11 Mya and 120 ± 11 Mya, with diversification of the genus starting around 50 ± 13 Mya and 78 ± 9 Mya (Merckx 2008; Merckx & Bidartondo 2008; Merckx et al. 2010, 2014, 2017). Afrothismia kupensis diverged 50 Mya (its fungal symbiont 219 Mya), A. korupensis 34 Mya (its symbiont and that of A. foetheriana 122 Mya), while A. hydra and A. winkleri diverged from each other 0.8 Mya (their symbionts 66 Mya).
Floral diversity in Afrothismia
The species of Afrothismia included in the molecular phylogenetic analyses of Merckx & Bidartondo (2008) and Merckx et al. (2009) are representative of the range of variation in floral morphology currently known in Afrothismia. Here, we briefly describe these patterns, referring to other species that share similar morphology, accepting that this similarity might result in part from convergence rather than recent common ancestry, probably associated with attracting pollinators.
To date, no molecular phylogenetic analyses have included all 16 described species of Afrothismia. Merckx & Bidartondo (2008) and Merckx et al. (2009) included five and six species respectively, and Shepeleva et al. (2020) included six species. In these analyses, A. kupensis (as gesnerioides) was sister to the remaining Afrothismia species. Afrothismia gesnerioides and A. kupensis share similar morphology and are probably closely related, both species possessing tepals that are triangular and non-filamentous and a horizontal floral tube that is only slightly sinusoidal in the upper part. All other species possess more-or-less filamentous perianth lobes. In A. korupensis, the floral tube is vertical; the proximal and most of the distal part are aligned on the same axis, and only the mouth and uppermost part of the distal tube is horizontally oriented, with a projecting corona partly occluding the mouth. This pattern, of a vertical floral tube with horizontal mouth, is also seen in A. pusilla, A. fungiformis (both also in Cameroon), A. baerae (Kenya) and A. “arachnites” (Tanzania). Afrothismia foertheriana has a highly reduced distal floral tube that mostly consists of the campanulate proximal part, which is about as wide as long; the lobes and corona bear numerous short projections that are also seen in A. pusilla. Afrothismia amietii of Cameroon also shares this pattern, though the distal part of tube is entirely absent. The remaining species examined, A. hydra and A. winkleri, are notable for the proximal floral tube being ± horizontal, the distal tube being angled vertically, forming an L-shape. This pattern is present, with variations, throughout the range of the genus, occurring also in A. gabonensis Dauby & Stévart (Gabon), A. insignis, A. mhoroana Cheek (both Tanzania) and A. zambesiaca (Malawi). It is also among these species that yellow is included among the flower colours. All other species are coloured in a combination of purple or dark dull red, usually with white (though white is absent from the perianth of A. foertheriana). The flowers of Afrothismia mhoroana are yellow and white in colour, lacking purple or dark red colouring entirely.
Mycorrhizal associations
Merckx & Bidartondo (2008) used combined molecular results from four genes to investigate the phylogeny of several species of Afrothisma and their fungal partners from three locations in Southwest Region Cameroon. The species of Afrothismia included were A. foertheriana, A. hydra, A. korupensis, A. kupensis (as A. gesnerioides) and A. winkleri. All the fungal symbionts were placed in the Glomus sp. A lineage (now identified as Glomeraceae, Krüger et al. 2012) of Glomeromycota, and there was no fungal lineage overlap among the different species of Afrothismia. No other fungi or fungal-like organisms were identified, apart from a stramenopile (non-mycorrhizal, assumed pathogen) in A. foetheriana. Franke et al. (2006), investigated the symbionts of seven taxa of Afrothismia, also from Southwest Region, Cameroon, and also found them to be exclusively the Glomus sp. A. lineage. However, Imhof (2006) reported an unidentified second fungal species in material of A. gesnerioides. A delayed co-speciation pattern between the plant species and the Glomus lineages was revealed (Merckx 2008; Merckx & Bidartondo 2008): the divergence time estimates for the Glomus nodes were older than for their corresponding nodes in Afrothismia.
Glomeromycota, which originated before 400 Mya (Strullu-Derrien et al. 2018), form vesicular arbuscular mycorrhiza with about 90% of all land plants (Cheek et al. 2020). Glomus group A are also symbionts with Taccaceae (Merckx 2008). Many other non-photosynthetic mycoheterotrophic plants also depend on Glomeromycota as symbionts, including Thismiaceae, Burmanniaceae and Triuridaceae. However, non-photosynthetic mycoheterotrophic Ericaceae and some Orchidaceae are mainly ectomycorrhizal and depend on Ascomycota and Basidiomycota as symbionts.
Mycorrhizal structures
A series of studies of the mycorrhizal structures of Afrothismia (Imhof 1999; 2006; Imhof et al. 2013, 2020) also found signs of ongoing evolutionary diversification. The studies involved Afrothismia winkleri (identification to be confirmed, based on Wilks 1179 of Gabon, Imhof 1999), Afrothismia gesnerioides (based on de Winter 91(L), South Region Cameroon, Imhof 2006), Afrothismia kupensis (as A. gesnerioides) and likely Afrothismia winkleri (as Afrothismia saingei) (Imhof et al. 2013), and Afrothismia hydra, A. korupensis, A. gesnerioides and likely Afrothismia winkleri (as Afrothismia saingei) (Imhof et al. 2020).
According to Imhof et al. (2020), the root-shoot combination of Afrothismia winkleri exhibits one of the most complex mycorrhizal colonisation patterns described to date. It shows four different hyphal shapes (straight, looped, inflated coils, degenerating coils) in six separate tissue compartments (filiform root, root epidermis, third root layer, root cortex parenchyma, shoot cortex at root clusters, shoot cortex apart from root clusters). Interconnections between all hyphal shapes demonstrated that they belong to the same fungus. In addition, the long filiform roots of this species were interpreted as being especially efficient in facilitating penetration by fungi. In contrast, the mycorrhizal pattern in A. gesnerioides is comparatively simple, with three hyphal forms in five tissue compartments, and the short blunt roots interpreted as being less efficient. Afrothismia hydra and A. korupensis were found to be intermediate in complexity between the foregoing species.
Imhof et al. (2020) suggested that the differences between four Afrothismia species reflect a transitional change towards increasing functional complexity and strict partitioning of conveyance (straight hyphae) and storage purposes (inflated hyphal coils). They concluded that since investigations on the mycorrhizal structures of Thismia spp. describe a disparate and much less sophisticated colonisation pattern than in Afrothismia, this difference supports taxonomic separation from Thismiaceae.
Ontogeny
Caddick et al. (2000a, b) described comparative floral ontogeny in several taxa of Dioscoreales, including two species of Thismia and four species of Tacca. Nuraliev et al. (2021) provided a detailed description of both flower and inflorescence ontogeny in several species of Thismia, noting variation in placentation in different species, including some in which placentation is parietal at the base and columnar above. Flower buds of Afrothismia hydra, Haplothismia exannulata Airy Shaw and two species of Thismia are illustrated here (Fig. 3). Imhof & Sainge (2008) documented development in Afrothismia hydra from seed to seed-dispersal.
In Afrothismia hydra, seeds germinate with root tissue only, disrupting the seed coat and developing a primary ovoid root tubercle. The hypocotyl, cotyledons and shoot are not visible during germination. A second tubercle arises at the proximal end of the first one and subsequent root tubercles with filiform extensions develop sequentially, resulting in a small root aggregate. The root aggregate enlarges, forming a central axis to which all roots are connected. This axis has a growth pole where new root tubercles arise; it later develops into a stem with scale leaves, finally terminating in a flower. After anthesis, the floral tube disintegrates, leaving a pyxidium which opens by means of a peculiar elongating placenta, which Imhof & Sainge (2008) termed a “placentophore,” also shown in our material of A. hydra (Fig. 3A). The placentophore later elevates the placenta with attached seeds above the flowering level and is interpreted as an adaptation to ombrohydrochory (rain-operated seed dispersal). The placentophore can extend to 4 – 5 times the length of the fruits and seems to be the result of meristem growth rather than cell elongation (Imhof & Sainge 2008).
Pollen structure
To date, the most detailed studies of pollen structure and development in Afrothismia, Thismia and related taxa are those of Rübsamen (1986), Caddick et al. (1998) and Severova et al. (2021). Rübsamen (1986) made an SEM study of the pollen of two species of Afrothismia, A. winkleri (based on Zenker 3613, B) and A. “arachnites” (based on Peter s.n., B), using dried herbarium material. Caddick et al. (1998) described both microsporogenesis and pollen morphology in most genera of Dioscoreales, including Thismia and Tacca. They found that the occurrence of tetragonal tetrads in Thismia indicates successive microsporogenesis, in contrast with simultaneous microsporogenesis in Tacca, although the condition in Afrothismia remains unknown. In Afrothismia, pollen grains are released as single units, as in most Dioscoreales (rarely as tetrads in some Burmanniaceae and Thismia species); they are plano-convex, monoporate (ulcerate), with pollen lengths of the two species 24 μm and 14 – 16 μm, respectively (Rübsamen 1986). The surface sculpture is coarsely reticulate, the muri “caterpillar like,” occasionally with tiny pores, compared with more finely perforate, psilate or reticulate in Thismia and finely reticulate in Tacca (Rübsamen 1986; Caddick et al. 1998; Severova et al. 2021), indicating clear separation between Afrothismia and related taxa. Severova et al. (2021) also noted that Thismia is highly unusual among seed plants in possessing a single aperture in an equatorial position.
Pollination biology
Within Afrothismia, pollination has been recorded only in A. kupensis (Cheek et al. 2019). Observations over a seven-day period of floral visitors to a plot with six flowering plants recorded ten visitors entering the flowers, all representing a species of a mosquito-like insect. Two specimens were caught on departure from the flowers, preserved and found to be carrying pollen consistent with Thismiaceae. The insects were identified as scuttle flies (Phorideae, Diptera), probably the genus Megaselia, which in other plant groups has a mutualism in which pollination is achieved in exchange for the larvae feeding on the decomposing flowers (Sakai 2002; Hall & Brown 1993). It might be expected that other species will have different pollinators because they have pollination structures not seen in A. kupensis, such as brightly coloured yellow flowers (vs dull purple and white) and long filiform antennae-like floral lobes (vs flat, triangular) postulated to disperse pollinator attractant volatiles (Merckx 2008).
Within Thismia, “trap” flowers have been suggested, as pollinators have been observed temporarily restrained inside the hypanthium chamber (Guo et al. 2019; Nuraliev et al. 2021). Self-fertilisation could also occur widely in Dioscoreales, including both Thismia and Afrothismia. The unusual flower structure of Afrothismia indicates this possibility, with anther appendages extending to the stigma surface at anthesis. In contrast, self-fertilisation (autogamy) is strongly indicated by our anatomical sections of Haplothismia exannulata (Fig. 3 K, L) showing a mucilagenous mass of germinating pollen tubes growing directly into the style between the carpel margins. Severova et al. (2021) also commented that the pollen of Thismia is highly unusual for biotically pollinated plants; they followed Yudina et al. (2021) and other authors in suggesting possible autogamy in flowers of Thismia and some other Dioscoreales.
Seed structure and seed dispersal
Rübsamen (1986) described seeds of two species of Afrothismia, A. winkleri (based on Zenker 3613, B) and A. “arachnites” (based on Peter s.n., B), in which the seeds are 0.7 – 0.91 × 0.17 – 0.23 mm, twice the length of the six Thismia species that she described. Measurements of A. hydra (0.7 – 0.8 mm long) also fit this range (Sainge & Franke 2005), but seeds of A. foertheriana and A. zambesiaca are slightly shorter (0.5 – 0.6 mm: Franke et al. 2004; Cheek 2009), and those of A. kupensis much longer (c. 1.5 mm: Cheek et al. 2019). Seed dimensions are not recorded for other Afrothismia species.
Rübsamen (1986) characterised the seeds of Afrothismia as elongated, with 3 – 5 cells along the longitudinal axis, and having rows of epidermal cells that are twisted either clockwise or counter-clockwise. The epidermal cells are elongate with raised anticlinal walls showing a suture. The outer periclinal wall is smooth to verrucose, usually collapsed, showing the parallel-bar-like thickenings on the inner periclinal wall (Table 82 f – h in Rübsamen 1986).
Burmanniaceae and Thismiaceae are usually described as having “dust seeds,” which are typically small, elongated and dispersed abiotically (Leake 1994). Dust seeds represent a characteristic feature of either fully or partially mycoheterotrophic plants (Eriksson & Kainulainen 2011). In forest floor habitats of Thismia, even breezes are usually absent, so Maas et al. (1986) considered that the seeds could be dispersed in runnels of water. Further studies have indicated a “splash-cup” (ombrohydrochoric) mechanism of seed dispersal in Thismia (Stone 1980; Coelho et al. 2021). However, seeds of Afrothismia are mostly larger than those of Thismia. In both A. zambesiaca (Cheek 2009) and A. “arachnites” (Cheek, pers. obs. 2022) the base and apex of the seed have attached white bodies, possibly elaiosomes (Fig. 2C), though these structures were not mentioned by Rübsamen (1986). The appendages could indicate ant dispersal, rather than water dispersal. In all known Afrothismia species where fruits have been observed, the seeds are elevated above the fruit on a placentophore, and so the splash-cup mechanism suggested for Thismia appears highly unlikely in Afrothismia.
Conclusions
Our study presents clear evidence for recognising Afrothismia at the family level. With the addition of Afrothismiaceae, at least seven families can now be recognised and identified in Dioscoreales (Fig. 1 Lin et al. 2022) using the characters in the identification key above and Table 1). Classifications in APG III and IV (2009, 2016) included Afrothismia and Thismiaceae in Burmanniaceae sensu lato and incorporated Trichopodaceae Hutch. and Taccaceae into Dioscoreaceae. Each of these families has strong molecular phylogenetic support and differ morphologically from each other, so there is no justification for lumping them; to do so would maintain a Burmanniaceae and Dioscoreaceae that would be paraphyletic and/or internally morphologically discordant.
Nonetheless, there remain additional outstanding family-level classification issues in Dioscoreales, particularly on the status of Stenomeris (e.g., Soto Gomez 2020). These questions will be addressed in future studies. Setting aside its seven undescribed species (Table 2), and based on only its 16 accepted species, Afrothismia (Afrothismiaceae) represents the most species-diverse fully mycoheterotrophic genus and family in Africa. In recent years, many facets of the biology of this fascinating genus have been uncovered, yet important aspects remain imperfectly known, such as pollination biology, microsporogenesis, cytology, and seed dispersal. Fully mycoheterotrophic taxa effectively parasitise soil fungi that obtain their nutrients from nearby autotrophic plants, but in the case of Afrothismia, the relevant autotrophic plant species remain unknown.
Perhaps the highest priority for the family must be to protect from extinction the known species, to conserve them in their natural habitats (e.g., by including them in Important Plant Areas, Darbyshire et al. 2017) and to develop species conservation action plans to improve the likelihood of their survival (e.g. Couch et al. 2022), which is crucial since ex situ conservation is currently impossible. Until species are documented, described and known to science, it is difficult to assess them for their IUCN Red List conservation status, and therefore the possibility of conserving them is reduced (Cheek et al. 2020). Documented extinctions of plant species continue. In the Cross-Sanaga Interval of Cameroon, the centre of diversity for Afrothismia (including half the accepted species), the most documented global species extinction is another fully mycoheterotrophic species, Oxygyne triandra Schltr. (Thismiaceae, Cheek & Onana 2011; Onana & Cheek 2011; Cheek et al. 2018a). Examples of species becoming extinct before they are known to science appear to be on the increase. In Cameroon, inside the Cross-Sanaga Interval, examples are Vepris bali Cheek and Monanthotaxis bali Cheek (Cheek et al. 2018c; Cheek et al. 2023). In all cases, anthropogenic habitat clearance for agriculture has been the cause of these extinctions.
For its size, Afrothismia must be among the most highly threatened genera on the planet, because 11 of the 14 assessed species are globally Critically Endangered, the highest level of threat (Table 2). Although only one of these species is considered extinct, several others have not been seen alive in decades. Searching for these long-lost species is urgent. Accepted species numbers for Afrothismia have increased in the last 20 years by 300% (from 4 to 16). It can be projected that more species await discovery as long as unsurveyed suitable habitat survives, and it is vital to find and protect this endemic and significant African family.
References
Airy Shaw, H. K. (1952). A new genus and species of Burmanniaceae from South India. Kew Bull. 7: 277 – 279. https://www.jstor.org/stable/4109280
Angiosperm Phylogeny Group, APG I. (1998). An ordinal classification for the families of flowering plants. Ann. Missouri Bot. Gard. 85: 531 – 553. https://www.jstor.org/stable/2992015
____ (2003). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 141: 399 – 436. https://doi.org/10.1046/j.1095-8339.2003.t01-1-00158.x
____ (2009). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 161: 105 – 121. https://doi.org/10.1111/j.1095-8339.2009.00996.x
____ (2016). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 181: 1 – 20. https://doi.org/10.1111/boj.12385
Barthlott, W., Lauer, W. & Placke, A. (1996). Global distribution of species diversity in vascular plants: towards a world map of phytodiversity. Erdkunde 50: 317 – 327. https://doi.org/10.3112/erdkunde.1996.04.03
Beentje, H. & Cheek, M. (2003). Glossary. In: H. Beentje (ed), Flora of Tropical East Africa. Balkema, Lisse. https://doi.org/10.1201/9781482283808
Breteler, F. J., Bakker, F. T. & Jongkind, C. C. H (2015). A synopsis of Soyauxia (Peridiscaceae, formerly Medusandraceae) with a new species from Liberia. Plant Ecol. Evol. 148: 409 – 419. https://doi.org/10.5091/plecevo.2015.1040
Caddick, L. R., Furness, C. A. Stobart, K. L. & Rudall, P. J. (1998). Microsporogenesis and pollen morphology in Dioscoreales and allied taxa. Grana 37: 321 – 336. https://doi.org/10.1080/00173139809362687
____, Rudall, P. J., Wilkin, P. & Chase, M. W. (2000a). Yams and their allies: systematics of Dioscoreales, pp. 475 – 487. Monocots: Systematics and Evolution 1. CSIRO Publishing, Victoria.
____ (2000b). Floral morphology and development in Dioscoreales. Feddes Repert. 111: 189 – 230. https://doi.org/10.1002/fedr.20001110313
____, ____, ____, Hedderson, T. A. & Chase, M. W. (2002). Phylogenetics of Dioscoreales based on combined analyses of morphological and molecular data. Bot. J. Linn. Soc. 138: 123 – 144. https://doi.org/10.1046/j.1095-8339.2002.138002123.x
Cheek, M. (2003). A new species of Ledermanniella (Podostemaceae) from western Cameroon. Kew Bull. 58: 733 – 737. https://www.jstor.org/stable/4111153
____ (2004a). A new species of Afrothismia (Burmanniaceae) from Kenya. Kew Bull. 58: 951 – 955. https://www.jstor.org/stable/4111208
____ (2004b). Kupeaeae, a new tribe of Triuridaceae from Africa. Kew Bull. 58: 939 – 949. https://www.jstor.org/stable/4111207
____ (2004c). Afrothismia pachyantha. The IUCN Red List of Threatened Species. 2004:e. T39539A10246294. https://www.iucnredlist.org/species/39539/10246294. [Accessed 14 Aug. 2022]
____ (2006). African Saprophytes: new discoveries. In: S. A. Ghazanfar & H. J. Beentje (eds), Taxonomy and ecology of African plants, their conservation and sustainable use. Proceedings of the 17th AETFAT Congress, Addis Ababa, Ethiopia. Royal Botanic Gardens, Kew.
____ (2007). Afrothismia amietii (Burmanniaceae), a new species from Cameroon. Kew Bull. 61: 605 – 607. https://www.jstor.org/stable/20443306
____ (2009). Burmanniaceae. In: J. R. Timberlake (ed.), Flora Zambesiaca 12 (2): 145 – 148. Published by the Royal Botanic Gardens, Kew for the Flora Zambesiaca Managing Committee.
____ (2017). Afrothismia korupensis. The IUCN Red List of Threatened Species 2017: e.T110096021A110096027. https://doi.org/10.2305/IUCN.UK.2017-3.RLTS.T110096021A110096027.en. [Accessed 14 Aug. 2022]
____ (2018a). Afrothismia amietii. The IUCN Red List of Threatened Species 2018: e.T110075845A110075847. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T110075845A110075847.en. [Accessed 14 Aug. 2022]
____ (2018b). Afrothismia foertheriana. The IUCN Red List of Threatened Species 2018: e.T110096961A110096966. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T110096961A110096966.en. [Accessed 14 Aug. 2022]
____ (2018c). Afrothismia gesnerioides. The IUCN Red List of Threatened Species 2018: e.T110096839A110096841. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T110096839A110096841.en. [Accessed 14 Aug. 2022]
____ & Csiba, L. (2002). A new epiphytic species of Impatiens (Balsaminaceae) from western Cameroon. Kew Bull. 57: 669 – 674. https://www.jstor.org/stable/4110997
____ & Jannerup, P. (2006). A new species of Afrothismia (Burmanniaceae) from Tanzania. Kew Bull. 60: 593 – 596. https://www.jstor.org/stable/25070246
____ & Lovell, R. (2020). Afrothismia hydra. The IUCN Red List of Threatened Species 2020: e.T110096897A110096899. https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T110096897A110096899.en. [Accessed 14 Aug. 2022]
____ & Ndam, N. (1996). Saprophytic Flowering Plants of Mount Cameroon. Pp. 612 – 617. In: L. J. G. van der Maesen, X. M. van der Burgt & J. M. van Medenbach de Rooy (eds), The biodiversity of African plants (Proceedings XIV AETFAT Congress). Kluwer, Dordrecht. https://doi.org/10.1007/978-94-009-0285-5_74
____ & Onana, J. M. (2011). Extinct species in Cameroon: Oxygyne triandra. In: Red Data Species in Cameroon, a Guide for Secondary School Teachers. Royal Botanic Gardens, Kew.
____ & Rokni, S. (2017). Afrothismia saingei. The IUCN Red List of Threatened Species 2017: e.T110096780A110096785. https://doi.org/10.2305/IUCN.UK.2017-3.RLTS.T110096780A110096785.en. [Accessed 14 Aug. 2022]
____ & Rotton, H. (2022). Afrothismia kupensis. The IUCN Red List of Threatened Species 2022: e.T200535052A200535056. https://doi.org/10.2305/IUCN.UK.2022-1.RLTS.T200535052A200535056.en. [Accessed 14 Aug. 2022]
____ & Williams, S. (1999). A review of African saprophytic flowering plants. Pp. 39 – 49. In: J. Timberlake & S. Kativu (eds), African Plants. Biodiversity, Taxonomy & Uses. Proceedings of the 15th AETFAT Congress at Harare, Zimbabwe. Royal Botanic Gardens, Kew.
____ & van der Burgt, X. (2010). Gymnosiphon samoritoureanus (Burmanniaceae) a new species from Guinea, with new records of other achlorophyllous heteromycotrophs. Kew Bull. 65: 83 – 88. https://doi.org/10.1007/s12225-010-9180-9
____, Alvarez-Agiurre, M. G., Grall, A., Sonké, B., Howes, M. J. R. & Larridon, I. (2018b). Kupeantha (Coffeeae, Rubiaceae), a new genus from Cameroon and Equatorial Guinea. PLoS One 13: 20199324. https://doi.org/10.1371/journal.pone.0199324
____, Darbyshire, I. & Onana, J. M. (2023). Discovery and conservation of Monanthotaxis bali (Annonaceae) a new Critically Endangered (possibly extinct) montane forest treelet from Bali Ngemba, North West Region, Cameroon. Kew Bull. 78: 259 – 270. https://doi.org/10.1007/s12225-023-10117-9
____, Etuge, M. & Williams, S. (2019). Afrothismia kupensis sp. nov. (Thismiaceae), Critically Endangered, with observations on its pollination and notes on the endemics of Mt Kupe, Cameroon. Blumea 64: 158 – 164. https://doi.org/10.3767/blumea.2019.64.02.06
____, Gosline, G. & Onana, J. M. (2018c). Vepris bali (Rutaceae), a new critically endangered (possibly extinct) cloud forest tree species from Bali Ngemba, Cameroon. Willdenowia 48: 285 – 292. https://doi.org/10.3372/wi.48.48207
____, Harvey, Y. & Onana, J. M. (2010). The plants of Dom, Bamenda Highlands, Cameroon, A Conservation Checklist. Royal Botanic Gardens, Kew.
____, ____ & ____ (2011). The Plants of Mefou Proposed National Park, Yaoundé, Cameroon, A Conservation Checklist. Royal Botanic Gardens, Kew.
____, Mackinder, B., Gosline, G., Onana, J. M. & Achoundong, G. (2001). The phytogeography and flora of western Cameroon and the Cross River-Sanaga River interval. Syst. Geogr. Pl. 71: 1097 – 1100. https://www.jstor.org/stable/3668742
____, Nic Lughadha, E., Kirk, P., Lindon, H., Carretero, J., Looney, B., Douglas, B., Haelewaters, D., Gaya, E., Llewellyn, T., Ainsworth, M., Gafforov, Y., Hyde, K., Crous, P., Hughes, M., Walker, B. E., Forzza, R. C., Wong, K. M. & Niskanen, T. (2020). New scientific discoveries: plants and fungi. Plants, People Planet 2: 371 – 388. https://doi.org/10.1002/ppp3.10148
____, Onana, J. M. & Pollard, B. J. (2000). The Plants of Mount Oku and the Ijim Ridge, Cameroon, a Conservation Checklist. Royal Botanic Gardens, Kew.
____, Pollard, B. J., Darbyshire, I, Onana, J. M. & Wild, C. (2004). The Plants of Kupe, Mwanenguba and the Bakossi Mts, Cameroon. A Conservation Checklist. Royal Botanic Gardens, Kew.
____, Tsukaya, H., Rudall, P. J. & Suetsugu, K. (2018a). Taxonomic monograph of Oxygyne (Thismiaceae), rare achlorophyllous mycoheterotrophs with strongly disjunct distribution. PeerJ 6: e4828. DOI: https://doi.org/10.7717/peerj.4828
____, Williams, S. & Etuge, M. (2003). Kupea martinetugei, a new genus and species of Triuridaceae from western Cameroon. Kew Bull. 58: 225 – 228. https://www.jstor.org/stable/4119366
Coelho, C. P., Sousa, I. P., Silva, G. E., Rocha, D. I., Azevedo, M. O. & Guilherme, F. A. G. (2021). Ombrohydrochory in Thismia panamensis (Standley) Jonk: a mycoheterotrophic species in Brazilian Cerrado forests. Plant Biology 23: 630 – 635. https://doi.org/10.1111/plb.13250
Couch, C., Molmou, D., Magassouba, S., Doumbouya, S., Diawara, M., Diallo, M. Y., Keita, S. M., Koné, F., Diallo, M. C., Kourouma, S., Diallo, M. B., Keita, M. S., Oularé, A., Darbyshire, I., Gosline, G., Nic Lughadha, E., van der Burgt, X., Larridon, I. & Cheek, M. (2022). Piloting development of species conservation action plans in Guinea. Oryx 57 (4). 497 – 506. https://doi.org/10.1017/S0030605322000138
Cowley, E. J. (1988). Burmanniaceae. In: R. M. Polhill (ed.), Flora of Tropical East Africa. Balkema, Rotterdam.
Dagallier, L. P., Janssens, S. B., Dauby, G., Blach‐Overgaard, A., Mackinder, B. A., Droissart, V., Svenning, J. C., Sosef, M. S., Stévart, T., Harris, D. J. & Sonké, B. (2020). Cradles and museums of generic plant diversity across tropical Africa. New Phytol. 225: 2196 – 2213. https://doi.org/10.1111/nph.16293
Darbyshire, I., Anderson, S., Asatryan, A., Byfield, A., Cheek, M., Clubbe, C., Ghrabi, Z., Harris, T., Heatubun, C. D., Kalema, J., Magassouba, S., McCarthy, B., Milliken, W., Montmollin, B. de, Nic Lughadha, E., Onana, J. M., Saıdou, D., Sarbu, A., Shrestha, K. & Radford, E. A. (2017). Important Plant Areas: revised selection criteria for a global approach to plant conservation. Biodivers. Conserv. 26: 1767 – 1800. https://doi.org/10.1007/s10531-017-1336-6.
Dauby, G., Parmentier, I. & Stévart, T. (2008). Afrothismia gabonensis sp. nov. (Burmanniaceae) from Gabon. Nord. J. Bot. 25: 268 – 271. https://doi.org/10.1111/j.0107-055X.2008.00119.x
Drenth, E. (1972). A revision of the family Taccaceae. Blumea 20: 367 – 406.
Eastern Arc Mountains & Coastal Forests CEPF Plant Assessment Project. (2009a). Afrothismia baerae. The IUCN Red List of Threatened Species 2009: e.T158066A5185926. https://doi.org/10.2305/IUCN.UK.2009-2.RLTS.T158066A5185926.en. [Accessed 14 Aug. 2022]
Eastern Arc Mountains & Coastal Forests CEPF Plant Assessment Project. (2009b). Afrothismia insignis. The IUCN Red List of Threatened Species 2009: e.T158049A5184943. https://doi.org/10.2305/IUCN.UK.2009-2.RLTS.T158049A5184943.en. [Accessed 14 Aug. 2022]
Engler, A. (1905). Thismia winkleri Engler eine neue Africanische Burmanniaceae. Bot. Jahrb. Syst. 38: 89 – 91. https://www.biodiversitylibrary.org/item/136899#page/101/mode/1up
Eriksson, O. & Kainulainen, K. (2011). The evolutionary ecology of dust seeds. Perspect. Pl. Ecol. Evol. Syst. 13: 73 – 87. https://doi.org/10.1016/j.ppees.2011.02.002
Franke, T. (2004). Afrothismia saingei (Burmanniaceae, Thismieae), a new myco-heterotrophic plant from Cameroon. Syst. Geogr. Pl. 74: 27 – 33. https://www.jstor.org/stable/3668554
____, Beenken, L., Döring, M., Kocyan, A. & Agerer, R. (2006). Arbuscular mycorrhizal fungi of the Glomus-group A lineage (Glomerales; Glomeromycota) detected in myco-heterotrophic plants from tropical Africa. Mycol. Progr. 5: 24 – 31. https://doi.org/10.1007/s11557-006-0500-2
____, Sainge, M. & Agerer, R. (2004). A new species of Afrothismia (Burmanniaceae, tribe Thismieae) from the western foothills of Mt Cameroon. Blumea 49: 451 – 456. https://doi.org/10.3767/000651904X484397
Griffith, W. (1845). On the root-parasites referred by authors to Rhizantheae, and their allies. Proc. Linn. Soc. London 1: 216 – 222. https://www.biodiversitylibrary.org/item/34955#page/238/mode/1up
Guo, X., Zhao, Z., Mar, S. S., Zhang, D. & Saunders, R. M. K. (2019). A symbiotic balancing act: arbuscular mycorrhizal specificity and specialist fungus gnat pollination in the mycoheterotrophic genus Thismia (Thismiaceae). Ann. Bot. 124: 331 – 342. https://doi.org/10.1093/aob/mcz087
Hall, D. W. & Brown, B. V. (1993). Pollination of Aristolochia littoralis (Aristolochiales: Aristolochiaceae) by Males of Megaselia spp. (Diptera: Phoridae). Ann. Entomol. Soc. Amer. 86: 609 – 613. https://doi.org/10.1093/aesa/86.5.609
Harvey, E. K. & Cheek, M. (2021). Afrothismia pusilla. The IUCN Red List of Threatened Species 2021: e.T183090383A183090536. https://doi.org/10.2305/IUCN.UK.2021-3.RLTS.T183090383A183090536.en. [Accessed 14 Aug. 2022]
Harvey, Y. B., Pollard, B. J., Darbyshire, I., Onana, J. M. & Cheek, M. (2004). The Plants of Bali Ngemba Forest Reserve. Cameroon: A Conservation Checklist. Royal Botanic Gardens, Kew.
____, Tchiengue, B. & Cheek, M. (2010). The Plants of the Lebialem Highlands, a Conservation Checklist. Royal Botanic Gardens, Kew.
Imhof, S. (1999). Anatomy and mycotrophy of the achlorophyllous Afrothismia winkleri. New Phytol. 144: 533 – 540. https://doi.org/10.1046/j.1469-8137.1999.00532.x
____ (2006). Two distinct fungi colonize roots and rhizomes of the myco-heterotrophic Afrothismia gesnerioides (Burmanniaceae). Canad. J. Bot. 84: 852 – 861. https://www.ingentaconnect.com/content/cndscipub/cjb/2006/00000084/00000005/art00014
____ & Sainge, M. N. (2008). Ontogeny of the mycoheterotrophic species Afrothismia hydra (Burmanniaceae). Bot. J. Linn. Soc. 157: 31 – 36. https://doi.org/10.1111/j.1095-8339.2008.00787.x
____, Massicotte, H. B., Melville, L. H. & Peterson, R. L. (2013). Subterranean morphology and mycorrhizal structures, pp. 157 – 213. In: V. S. F. T. Merckx (ed.), Mycoheterotrophy: The Biology of Plants Living on Fungi. Springer, New York.
____, Feller, B. & Heser, A. (2020). Morpho-anatomical differences among mycoheterotrophic Afrothismia spp. (Thismiaceae) indicate an evolutionary progression towards improved mycorrhizal benefit. Mycorrhiza 30: 397 – 405. https://doi.org/10.1007/s00572-020-00951-1
IPNI (continuously updated). The International Plant Names Index. http://ipni.org/ [Accessed 14 Aug. 2022]
IUCN (2012). IUCN Red List Categories and Criteria: Version 3.1. 2nd edition. IUCN, Gland and Cambridge. Available from: http://www.iucnredlist.org/ [Accessed 14 Aug. 2022]
IUCN SSC East African Plants Red List Authority. (2013). Afrothismia winkleri. The IUCN Red List of Threatened Species 2013: e.T39540A45338733. [Accessed 14 Aug. 2022]
Jonker, F. P. (1938). Monograph of the Burmanniaceae. Meded. Bot. Mus. Herb. Rijks Univ. Utrecht 51: 1 – 279. https://repository.naturalis.nl/pub/534971
Krüger, M., Krüger, C., Walker, C., Stockinger, H. & Schüssler, A. (2012). Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol. 193: 970 – 984. https://doi.org/10.1111/j.1469-8137.2011.03962.x
Kubitzki, K. (1998). Taccaceae, pp. 425 – 428. In: K. Kubitzki (ed), The families of Vascular Plants. 3. Flowering Plants. Monocotyledons. Lilianae (excluding orchids). Springer, Berlin.
Lam, V. K., Merckx, V. S. & Graham, S. W. (2016). A few‐gene plastid phylogenetic framework for mycoheterotrophic monocots. Amer. J. Bot. 103: 692 – 708. https://doi.org/10.3732/ajb.1500412
____, Darby, H., Merckx, V. S. F. T, Lim, G., Yukawa, T., Neubig, K. M., Abbott, J. R., Beatty, G. E., Provan, J., Gomez, M. S. & Graham, S. W. (2018). Phylogenomic inference in extremis: a case study with mycoheterotroph plastomes. Amer. J. Bot. 105: 480 – 494. https://doi.org/10.1002/ajb2.1070
Leake, J. R. (1994). The biology of myco-heterotrophic (“saprophytic”) plants. New Phytol. 127: 171 – 216. https://doi.org/10.1111/j.1469-8137.1994.tb04272.x
Lin, Q., Braukmann, T. W., Soto Gomez, M., Mayer, J. L. S., Pinheiro, F., Merckx, V. S., Stefanović, S., & Graham, S. W. (2022). Mitochondrial genomic data are effective at placing mycoheterotrophic lineages in plant phylogeny. New Phytol. 236: 1908 – 1921. https://doi.org/10.1111/nph.18335
Maas-van de Kamer, H. (1998). Burmanniaceae. In: K. Kubitzki (ed.), The Families and Genera of Vascular Plants III. Monocots, Lilianae (except Orchidaceae). Springer, Berlin.
____ (2003). Afrothismia gesnerioides, another new species of Afrothismia (Burmanniaceae) from tropical Africa. Blumea 48: 475 – 478. https://doi.org/10.3767/000651903X489429
Maas, P. J., Maas-van de Kamer, H., Van Benthem, J., Snelders, H. C. M. & Rübsamen, T. (1986). Burmanniaceae [Monograph 42]. Flora Neotropica 1 – 189.
Merckx, V. S. F. T. (2008). Mycoheterotrophy in Dioscoreales. PhD Thesis. Katholieke Universiteit Leuven.
____ & Bidartondo, M. I. (2008). Breakdown and delayed cospeciation in the arbuscular mycorrhizal mutualism. Proc. Roy. Soc. London, Ser. B, Biol. Sci. 275: 1029 – 1035. https://doi.org/10.1098/rspb.2007.1622
____, Smets, E. F. & Specht, C. D. (2013). Biogeography and Conservation, pp. 103 – 154. In: V. S. F. T. Merckx (ed.), Mycoheterotrophy: The Biology of Plants Living on Fungi. Springer, New York.
____, ____ & Kellogg, E. A. (ed.) (2014). Thismia americana, the 101st anniversary of a botanical mystery. Int. J. Pl. Sci. 175: 165 – 175. https://doi.org/10.1086/674315
____, Bakker, F. T., Huysmans, S. & Smets, E. (2009). Bias and conflict in phylogenetic inference of myco‐heterotrophic plants: a case study in Thismiaceae. Cladistics 25: 64 – 77. https://doi.org/10.1111/j.1096-0031.2008.00241.x
____, Huysmans, S. & Smets, E. (2010). Cretaceous origins of myco-heterotrophic lineages in Dioscoreales, pp. 39 – 53. In: Diversity, phylogeny, and evolution in the Monocotyledons. Aarhus University Press.
____, Gomes, S. I., Wapstra, M., Hunt, C., Steenbeeke, G., Mennes, C. B., Walsh, N., Smissen, R., Hsieh, T-H., Smets, E. F. & Bidartondo, M. I. (2017). The biogeographical history of the interaction between mycoheterotrophic Thismia (Thismiaceae) plants and mycorrhizal Rhizophagus (Glomeraceae) fungi. J. Biogeogr. 44: 1869 – 1879. https://doi.org/10.1111/jbi.12994
Mwangoka, M., Ndangalasi, H., Kindeketa, W., Kabuye, C., Minani, V., Kelbessa, E., Gereau, R. E., Malombe, I., Luke, W. R. Q. & Kalema, J. (2021). Afrothismia mhoroana. The IUCN Red List of Threatened Species 2021: e.T179900A1593676. https://doi.org/10.2305/IUCN.UK.2021-3.RLTS.T179900A1593676.en. [Accessed 14 Aug. 2022]
Nuraliev, M. S., Yudina, S. V., Shepeleva, E. A., Truong, B. V., Do, T. X., Beer, A. S. & Remizowa, M. V. (2021). Floral structure in Thismia (Thismiaceae: Dioscoreales): new insights from anatomy, vasculature and development. Bot. J. Linn. Soc. 195: 501 – 531. https://doi.org/10.1093/botlinnean/boaa066
Onana, J. M. & Cheek, M. (2011). Red Data Book of the Flowering Plants of Cameroon, IUCN Global Assessments. Royal Botanic Gardens, Kew.
Plants of the World Online (POWO): https://powo.science.kew.org/ [Accessed 14 Aug. 2022]
Rübsamen, T. (1986). Morphologische, embryologische und systematische Untersuchungen an Burmanniaceae und Corsiaceae. Diss. Bot. 92. J. Cramer, Berlin.
Sainge, M. N. & Franke, T. (2005). A new species of Afrothismia (Burmanniaceae) from Cameroon. Nord. J. Bot. 23: 299 – 303. https://doi.org/10.1111/j.1756-1051.2003.tb00398.x
____, Franke, T. & Agerer, R. (2005). A new species of Afrothismia (Burmanniaceae, tribe Thismieae) from Korup National Park, Cameroon. Willdenowia 35: 287 – 291. https://doi.org/10.3372/wi.35.35209
____, Kenfack, D. & Chuyong, G. B. (2013). Two new species of Afrothismia (Thismiaceae) from southern Cameroon. Kew Bull. 68: 591 – 597. https://doi.org/10.1007/s12225-013-9478-5
____, Chuyong, G. B. & Peterson, A. T. (2017). Endemism and geographic distribution of African Thismiaceae. Plant Ecol. Evol. 150: 304 – 312. https://doi.org/10.5091/plecevo.2017.1196
Sakai, S. (2002). Aristolochia spp. (Aristolochiaceae) pollinated by flies breeding on decomposing flowers in Panama. Amer. J. Bot. 89: 527 – 534. https://doi.org/10.3732/ajb.89.3.527
Schlechter, R. (1906). Burmanniaceae africanae. Bot. Jahrb. Syst. 38:140 – 141. https://www.biodiversitylibrary.org/item/700#page/153/mode/1up
Severova, E. E., Polevova, S. V., Yudina, S. V., Truong, B. V., Do, T. X., Chantanaorrapint, S., Suetsugu, K., Guo, X., Schelkunov, M. I. & Nuraliev, M. S. (2021). Palynological study of Asian Thismia (Thismiaceae: Dioscoreales) reveals an unusual pollen type. Pl. Syst. Evol 307, 54: 1 – 19. https://doi.org/10.1007/s00606-021-01778-9
Shepeleva, E. A., Schelkunov, M. I., Hroneš, M., Sochor, M., Dančák, M., Merckx, V. S. F. T., Kikuchi, I. A. B. S., Chantanaorrapint, S., Suetsugu, K., Tsukaya, H., Mar, S. S., Luu, H. T., Li, H. Q., Logacheva, M. D. & Nuraliev, M. S. (2020). Phylogenetics of the mycoheterotrophic genus Thismia (Thismiaceae: Dioscoreales) with a focus on the Old World taxa: delineation of novel natural groups and insights into the evolution of morphological traits. Bot. J. Linn. Soc. 193: 287 – 315. https://doi.org/10.1093/botlinnean/boaa017
Soltis, D. E., Clayton, J. W., Davis, C. C., Wurdack, K. J., Gitzendanner, M. A., Cheek, M., Savolainen, V., Amorim, A. M. & Soltis, P. S. (2007). Monophyly and relationships of the enigmatic family Peridiscaceae. Taxon 56: 65 – 73. https://www.jstor.org/stable/25065736
Soto Gomez, M. (2020). Phylogenomic studies of the monocot sister orders Pandanales and Dioscoreales. PhD thesis, The University of British Columbia, Vancouver, BC.
Stoffelen, P., Cheek, M., Bridson, D. & Robbrecht, E. (1997). A new species of Coffea (Rubiaceae) and notes on Mt Kupe, Cameroon. Kew Bull. 52: 989 – 994. https://www.jstor.org/stable/4117826
Stone, B. C. (1980). Rediscovery of Thismia clavigera (Becc.) F.V.Mueller (Burmanniaceae). Blumea 26: 419 – 425.
Strullu-Derrien, C., Selosse, M. A., Kenrick, P. & Martin, F. M. (2018). The origin and evolution of mycorrhizal symbioses: from palaeomycology to phylogenomics. New Phytol. 220: 1012 – 1030. https://doi.org/10.1111/nph.15076
Thiers, B. (continuously updated). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Available from: http://sweetgum.nybg.org/ih/ [Accessed Feb. 2022]
Turland, N. J., Wiersema, J. H., Barrie, F. R., Greuter, W., Hawksworth, D. L., Herendeen, P. S., Knapp, S., Kusber, W. H., Li, D. Z., Marhold, K., May, T. W., McNeill, J., Monro, A. M., Prado, J., Price, M. J. & Smith, G. F. (eds) (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress. Shenzhen, China, July 2017. Regnum Veg. 159. Koeltz Botanical Books, Glashütten. https://doi.org/10.12705/Code.2018
Watson, L. & Dallwitz, M. J. (1992 onwards). The families of flowering plants: descriptions, illustrations, identification, and information retrieval. Version: 5th August 2022. delta-intkey.com.
Whittaker, A. & Cheek, M. (2021). Afrothismia fungiformis. The IUCN Red List of Threatened Species 2021: e.T192483831A192597361. https://doi.org/10.2305/IUCN.UK.2021-2.RLTS.T192483831A192597361.en [Accessed 14 Aug. 2022]
Wilkin, P. (2009). Dioscoreaceae, pp 109 – 140. In: J. W. Timberlake & E. S. Martins, Flora Zambesiaca 12. Published by the Royal Botanic Gardens, Kew for the Flora Zambesiaca Managing Committee.
Woodward, C. L., Berry, P. E., van de Kamer, H. M. & Swing, K. (2007). Tiputinia foetida, a new mycoheterotrophic genus of Thismiaceae from Amazonian Ecuador, and a likely case of deceit pollination. Taxon 56: 157 – 162. https://www.jstor.org/stable/25065746
Yudina, S. V., Vislobokov, N. A. & Nuraliev, M. S. (2021). Evidence of a mixed pollination strategy in Vietnamese species of Thismia (Thismiaceae: Dioscoreales). Wulfenia 28: 109 –128. https://msu-botany.ru/gallery/159%20Yudina%20et%20al.,%202021%20-%20Thismia%20pollination.pdf
Acknowledgements
The first author (MC) thanks R. Vogt (B) for access to material of Afrothismia ‘arachnites’, Janis Shillito for typing and Anne Marshall for retrieval of literature. We are grateful to three anonymous reviewers for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheek, M., Soto Gomez, M., Graham, S.W. et al. Afrothismiaceae (Dioscoreales), a new fully mycoheterotrophic family endemic to tropical Africa. Kew Bull 79, 55–73 (2024). https://doi.org/10.1007/s12225-023-10124-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-023-10124-w