Abstract
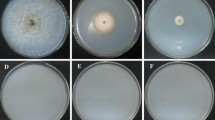
Mal secco disease, caused by the pathogenic fungus Phoma tracheiphila, is a devastating disease of susceptible citrus species, especially lemon. To study the molecular interactions between the pathogen and its host, a method for identifying the genes involved in pathogenicity is needed. This work describes the transformation of P. tracheiphila phialoconidia by Agrobacterium tumefaciens, and the generation of mutated P. tracheiphila isolates exhibiting reduced virulence on rough lemon seedlings. A rapid, replicable, and reliable method for screening P. tracheiphila mutants to assess their virulence by using rough lemon seedlings was developed. Among 2263 transformants obtained, three were non-virulent and 43 displayed reduced virulence. In addition, one of the transformants, which exhibited virulence similar to that of the wild type, was used for in planta visualization of the fungus progression through the plant xylem. To our knowledge, this is the first report of A. tumefaciens-mediated transformation of P. tracheiphila, and subsequent screening of the transformants to identify non-virulent mutants.






Similar content being viewed by others
References
Balmas, V., Scherm, B., Ghignone, S., Salem, A. O. M., Cacciola, S. O., & Migheli, Q. (2005). Characterisation of Phoma tracheiphila by RAPD-PCR, microsatellite-primed PCR and ITS rDNA sequencing and development of specific primers for in planta PCR detection. European Journal of Plant Pathology, 111, 235–247.
Bugg, T. D. (2003). Dioxygenase enzymes: catalytic mechanisms and chemical models. Tetrahedron, 59, 7075–7101.
Chet, I., Havkin, D., & Katan, J. (1978). The role of catechol in inhibition of Fusarium wilt. Phytopathologische Zeitschrift, 91, 60–66.
Chorin, M., Pinkas, J., & Bental, A. (1966). [Wilting disease in citrus.] The National and University Agricultural Press, 5, 348–350 (in Hebrew).
Chorin, R., & Chorin, M. (1956). Mal secco of citrus in Israel and neighbouring countries. Bulletin of the Research Council of Israel, 5D, 176–182.
Cogoni, C., & Macino, G. (1999). Gene silencing in Neurospora crassa requires a protein homologous to RNA-dependent RNA polymerase. Nature, 399, 166–169.
Demontis, M. A., Cacciola, S. O. M., Orrù, V. B., Chessa, V., Maserti, B. E., Mascia, L., et al. (2008). Development of real-time PCR systems based on SYBR® Green I and TaqMan® technologies for specific quantitative detection of Phoma tracheiphila in infected Citrus. European Journal of Plant Pathology, 120, 339–351.
EPPO, & CABI. (1990). Data sheet on quarantine pests. Deuterophoma tracheiphila (Phoma tracheiphila) (pp. 1–4). Wallingford, UK: CAB International.
Ezra, D., Kroitor, T., & Sadowsky, A. (2007). Molecular characterization of Phoma tracheiphila, causal agent of mal secco disease of citrus, in Israel. European Journal of Plant Pathology, 118, 183–191.
Ezra, D., Skovorodnikova, J., Kroitor-Keren, T., Denisov, Y., & Liarzi, O. (2010). Development of methods for detection and Agrobacterium-mediated transformation of the sterile, endophytic fungus Muscodor albus. Biocontrol Science and Technology, 20, 83–97.
Fogliano, V., Marchese, A., Scaloni, A., Ritieni, A., Visconti, A., Randazzo, G., et al. (1998). Characterization of a 60 kDa phytotoxic glycoprotein produced by Phoma tracheiphila and its relation to malseccin. Physiological and Molecular Plant Pathology, 53, 149–161.
Fulci, V., & Macino, G. (2007). Quelling: post-transcriptional gene silencing guided by small RNAs in Neurospora crassa. Current Opinions in Microbiology, 10, 199–203.
Graniti, A., & Perrota, G. (1988). Phoma tracheiphila (Petri) Kanchaveli & Gikashvili (pp. 396–398). In I. M. Smith, J. Dunez, R. A. Lelliott, D. H. Phillips, & S. A. Archer (Eds.), European handbook of plant diseases. Oxford, UK: Blackwell Scientific Publications.
Inoue, L., Ohara, T., Namikv, F., & Tsuge, T. (2001). Isolation of pathogenicity mutants of Fusarium oxysporum f. sp. melonis by insertional mutagenesis. Journal of General Plant Pathology, 67, 191–199.
Kritzman, G., & Chet, I. (1980). The role of phenols in the pathogenicity of Botrytis allii. Phytoparasitica, 8, 27–37.
Kumar, D., & Gupta, R. K. (2006). Biocontrol of wood rotting fungi. Indian Journal of Biotechnology, 5, 20–25.
Licciardello, G., Grasso, F. M., Bella, P., Cirvilleri, G., Grimaldi, V., & Catara, V. (2006). Identification and detection of Phoma tracheiphila, causal agent of citrus mal secco disease, by real-time polymerase chain reaction. Plant Disease, 90, 1523–1530.
Migheli, Q., Balmas, V., Olga Cacciola, S., Pane, A., Ezra, D., & di San Lio, G. M. (2009). Mal secco disease caused by Phoma tracheiphila: A potential threat to lemon industry worldwide. Plant Disease, 93, 852–867.
Nicasio, T., Del Bosco, F. S., Nigro, F., & Ippolito, A. (2000). Response of cybrids and a somatic hybrid of lemon to Phoma tracheiphila infections. HortScience, 35, 125–127.
Palm, M. E. (1987). Pests not known to occur in the United States or of limited distribution, no. 91: Phoma tracheiphila. In US Department of Agriculture APHIS-PPQ (p. 14). Assessment Support Staff PPQ, APHIS, USDA, Beltsville, MD, USA.
Retig, N., & Chet, I. (1974). Catechol-induced resistance of phenols to Fusarium wilt. Physiological Plant Pathology, 4, 469–475.
Reverberi, M., Betti, C., Fabbri, A. A., Zjalic, S., Spadoni, S., Mattei, B., et al. (2008). A role for oxidative stress in the Citrus limon / Phoma tracheiphila interaction. Plant Pathology, 57, 92–102.
Rollo, F., Roberto, S., & Torchia, P. (1990). Highly sensitive and fast detection of Phoma tracheiphila by polymerase chain reaction. Applied Microbiology and Biotechnology, 32, 572–576.
Solel, Z. (1976). Epidemiology of Mal secco disease of lemons. Phytopathology, 85, 90–92.
Solel, Z., Pinkas, J., & Loebenstein, G. (1972). Evaluation of systemic fungicides and mineral oil adjuvants for the control of mal secco disease of lemon plants. Phytopathology, 62, 1007–1013.
Spiegel, P., & Solel, Z. (1973). [Examination and investigation of lemon species for tolerance to Mal secco.] Bet Dagan, Israel: The Agricultural Research Organization Press, 5, 349–356 (in Hebrew).
Timmer, L. W., Garnsey, S. M., & Graham, J. H. (1988), Compendium of citrus diseases, 2nd ed. St. Paul, MN, USA: APS Press.
Acknowledgments
This work was partially financed by the Chief Scientist of the Israel Ministry of Agriculture and Rural Development (MOARD), research project number 132-1243-06. Contribution No. 523/13 from the Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kroitor-Keren, T., Liarzi, O., Gat, T. et al. Identification and characterization of Phoma tracheiphila mutants impaired in pathogenicity following Agrobacterium-mediated mutagenesis. Phytoparasitica 41, 491–502 (2013). https://doi.org/10.1007/s12600-013-0328-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-013-0328-7