Abstract
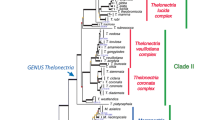
Specimens regarded as Thelonectria discophora (Thelonectria, Nectriaceae, Hypocreales) constitute a conspicuous group of saprobic fungi on decaying plant material, characterized by red perithecia each with a broad mammiform (nipple-like) apex. The asexual state is characterized by a cylindrocarpon-like morphology, with 3–5 septate macroconidia, unicellular microconidia and chlamydospores that are rarely produced in culture. In the past, T. discophora was regarded as one species with a wide geographic distribution. However, a recent study rejected the monophyly and cosmopolitan distribution of this species, and showed the existence of at least 16 cryptic species distributed in three main groups. By combining the results of phylogenetic analyses of six nuclear loci and morphological studies, we revised the taxonomy of the T. discophora species complex, resulting in the description of 12 new species and four new combinations based on historic names. Even though molecular phylogenetic analyses strongly support the segregation of these species, and are in agreement with previous studies, individual diagnostic morphological characters for each species could not be identified. However, discrete morphological traits corresponding to each of the three main groups of species were discovered. Lineages could be differentiated based on the average values of morphological traits as well as the presence/absence of characteristic asexual propagules and colony growth at 30C. Descriptions, illustrations are provided for the recognized species.









Similar content being viewed by others
References
Booth C (1966) The genus Cylindrocarpon. Mycol Pap 104:1–56
Brayford D (1991) Nectria canker. In: Ellis MA, Converse RH, Williams RN, Williamson B (eds) Compendium of raspberry and blackberry diseases and insects. American Phytopathological Society Press, St. Paul, p 20
Brayford D, Samuels GJ (1993) Some didymosporous species of Nectria with non-microconidial Cylindrocarpon anamorphs. Mycologia 85:612–637
Brayford D, Honda BM, Mantiri FR, Samuels GJ (2004) Neonectria and Cylindrocarpon: the Nectria mammoidea group and species lacking microconidia. Mycologia 96:572–597
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 85:612–637
Carriconde F, Gardes M, Jargeat P, Heilmann-Clausen J, Mouhamadou B, Gryta H (2008) Population evidence of cryptic species and geographical structure in the cosmopolitan ectomycorrhizal fungus Tricholoma scalpturatum. Microb Ecol 56:513–524
Castlebury LA, Rossman AY, Sung G-H, Hyten AS, Spatafora JW (2004) Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol Res 108:1–9
Cedeño L, Carrero C, Quintero K, Pino H, Espinoza W (2004) Cylindrocarpon destructans var. destructans and Neonectria discophora var. rubi associated with black foot rot on blackberry (Rubus glaucus Benth.) in Merida, Venezuela. Interciencia 29:455–460
Chaverri P, Vilchez B (2006) Hypocrealean (Hypocreales, Ascomycota) fungal diversity in different stages of succession in a tropical forest in Costa Rica. Biotropica 38:531–543
Chaverri P, Salgado C, Hirooka Y, Rossman AY, Samuels GJ (2011) Delimitation of Nectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Stud Mycol 68:57–68
Dahlberg A, Mueller GM (2011) Applying IUCN red-listing criteria for assessing and reporting on the conservation status of fungal species. Fungal Ecol 4:147–162
Dettman JR, Jacobson DJ, Taylor JW (2003) A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution 57:2703–2720
Dingley JM (1951) The Hypocreales of New Zealand. II. The genus Nectria. Trans R Soc NZ 79:177–202
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:326–631
Finlay BJ (2002) Global dispersal of free-living microbial eukaryote species. Science 296:1061–1063
Giraud T, Refregier G, Le Gac M, De Vienne DM, Hood ME (2008) Speciation in fungi. Fungal Genet Biol 45:791–802
Guu J-R, Ju Y-M, Hsieh H-J (2007) Nectriaceous fungi collected from forest in Taiwan. Bot Stud 48:187–203
Hawksworth DL (2011) A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. IMA Fungus 2:155–162
Hirooka Y, Kobayashi T (2007) Taxonomic studies of nectrioid fungi in Japan. I: the genus Neonectria. Mycoscience 48:53–62
Hirooka Y, Rossman AY, Samuels GJ, Lechat C, Chaverri P (2012) A monograph of Allantonectria, Nectria and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Stud Mycol 71:1–210
Hudson RR, Boos DD, Kaplan NL (1992) A statistical test for detecting geographic subdivision. Mol Biol Evol 9:138–151
Huelsenbeck JP, Rannala B (2004) Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Syst Biol 53:904–913
Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP (2001) Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294:2310–2314
James TY, Porter D, Hamrick JL, Vilgalys R (1999) Evidence for limited intercontinental gene flow in the cosmopolitan mushroom Schizophyllum commune. Evolution 53:1665–1677
Leigh JW, Susko E, Baumgartner M, Roger AJ (2008) Testing congruence in phylogenomic analysis. Syst Biol 57:104–115
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Loytynoja A, Goldman N (2005) An algorithm for progressive multiple alignment of sequences with insertions. Proc Natl Acad Sci U S A 102:10557–10562
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York, 521 pp
Nirenberg HI (1976) Untersuchungen uber die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitt Biol Bundesanst Land- Forstw Berlin-Dahlem 169:1–117
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 7:103–117
Penn O, Privman E, Ashkenazy H, Landan G, Graur D, Pupko T (2010) GUIDANCE: a web server for assessing alignment confidence scores. Nucleic Acids Res 38(Web Server issue):W23–W28
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Pringle A, Baker DM, Platt JL, Wares JP, Latgé JP, Taylor JW (2005) Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evolution 59:1886–1899
Queloz V, Sieber TN, Holdenrieder O, McDonald BA, Grunig CR (2011) No biogeographical pattern for a root-associated fungal species complex. Glob Ecol Biogeogr 20:160–169
Rambaut A (2005) FigTree v1.3.1. http://tree.bio.ed.ac.uk/software/figtree/
Rambaut A, Drummond AJ (2007) Tracer v. 1.5. http://beast.bio.ed.ac.uk/Tracer
Rayner RW (1970) A mycological colour chart. Surrey, United Kingdom, Commonwealth Mycological Institute Kew, 34 pp
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Roper M, Pepper RE, Brenner MP, Pringle A (2008) Explosively launched spores of ascomycetes fungi have drag-minimizing shapes. Proc Natl Acad Sci U S A 105:20583–20588
Roper M, Seminara A, Bandi MM, Cobb A, Dillard HR, Pringle A (2010) Dispersal of fungal spores on a cooperatively generated wind. Proc Natl Acad Sci U S A 107:17474–17479
Rossman AY, Seifert KA, Samuels GJ, Minus AW, Schroers HJ, Lombard L, Crous PW, Poldma K, Cannon PF, Summerbell RC, Geiser DM, Zhuang W, Hirooka Y, Herrera C, Salgado-Salazar C, Chaverri P (2013) Genera in Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales) proposed for acceptance or rejection. IMA Fungus 4:41–51
Rydholm C, Szakacs G, Lutzoni F (2006) Low genetic variation and no detectable population structure in Aspergillus fumigatus compared to closely related Neosartorya species. Eukaryot Cell 5:650–657
Salgado-Salazar C, Rossman AY, Samuels GJ, Capdet M, Chaverri P (2012) Multigene phylogenetic analyses of the Thelonectria coronata and T. veuillotiana species complexes. Mycologia 104:1325–1350
Salgado-Salazar C, Rossman AY, Chaverri P (2013) Not as ubiquitous as we thought: taxonomic crypsis, hidden diversity and cryptic speciation in the cosmopolitan fungus Thelonectria discophora (Nectriaceae, Hypocreales, Ascomycota). PLoS ONE 8(10):e76737. doi:10.1371/journal.pone.0076737
Samuels GJ, Brayford D (1994) Species of Nectria (sensu lato) with red perithecia and striate ascospores. Sydowia 46:75–161
Samuels GJ, Doi Y, Rogerson CT (1990) Hypocreales. In: Samuels GJ (ed) Contributions toward a mycobiota of Indonesia: Hypocreales, synnematous Hyphomycetes, Aphyllophorales, Phragmobasidiomycetes, and Myxomycetes. New York Botanical Garden, New York, pp 6–108
Samuels GJ, Dodd S, Lu B-S, Petrini O, Schroers H-J, Druzhinina I-S (2006) The Trichoderma koningii aggregate species. Stud Mycol 56:67–133
Seifert KA, Rossman AY (2011) How to describe a new fungal species. IMA Fungus 1:109–116
Silvestro D, Michalak I (2012) raxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12:335–337
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Taylor JW, Turner E, Townsend JP, Dettman JR, Jacobson D (2006) Eukaryotic microbes, species recognition and the geographic limits of species: examples from the kingdom Fungi. Philos Trans R Soc Lond B Biol Sci 361:1947–1963
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: A guide to methods and applications. Academic, New York, pp 315–322
Yu J-H, Keller N (2005) Regulation of secondary metabolism in filamentous fungi. Annu Rev Phytopathol 43:437–458
Zeng Z-Q, Zhuang W-Y (2013) Four new taxa of Ilyonectria and Thelonectria (Nectriaceae) revealed by morphology and combined ITS and B-tubulin sequence data. Phytotaxa 85:15–25
Acknowledgments
This study was funded by a grant from United States National Science Foundation (PEET program) DEB-0925696: “Monographic Studies in the Nectriaceae, Hypocreales: Nectria, Cosmospora, and Neonectria” to University of Maryland (P. Chaverri, G.J. Samuels & A.Y. Rossman). Special thanks to Christian Feuillet for helping with Latin names. We are indebted to the Genetic Resources Collection at CABI UK for providing various cultures, Dr. Carlos Mendez (University of Costa Rica) for providing transportation during collecting trips in Costa Rica, Dr. Andrea Romero for her invaluable help during fieldwork in Argentina, Dr. Teresa Iturriaga for collaborating and organizing the collecting trip in Venezuela, Dr. Margaret Dick for providing specimens from New Zealand, Andres de Errasti for providing the specimen and culture from Chile, and Dr. Guu for providing specimens and cultures from Taiwan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(DOCX 165 kb)
Online Resource 2
Majority rule Bayesian phylogram showing relationships among isolates of T. discophora-like and T. beijingensis and T. yunnanica species based on the concatenated analysis of ITS and tub loci. Thick branches indicate Bayesian posterior probabilities >0.95 and ML bootstrap >70 %. No thick branches indicate branch was not recovered/supported. (JPEG 63 kb)
Online Resource 3
Average colony growth of T. discophora species complex under different temperatures. Bar indicates 95 % confidence interval. (JPEG 874 kb)
Rights and permissions
About this article
Cite this article
Salgado-Salazar, C., Rossman, A.Y., Samuels, G.J. et al. Phylogeny and taxonomic revision of Thelonectria discophora (Ascomycota, Hypocreales, Nectriaceae) species complex. Fungal Diversity 70, 1–29 (2015). https://doi.org/10.1007/s13225-014-0280-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-014-0280-y