Abstract
The family Pleosporaceae includes numerous saprobic, opportunistic human, and plant pathogenic taxa. The classification of genera and species Pleosporaceae has been a major challenge due to the lack of a clear understanding of the importance of the morphological characters used to distinguish taxa as well as the lack of reference strains. Recent treatments concluded that Pleospora and some other genera in Pleosporaceae are likely polyphyletic. In order to establish the evolutionary relationships and to resolve the polyphyletic nature of Pleospora and allied genera, we sequenced the 18S nrDNA, 28S nrDNA, ITS, GAPDH, RPB2 and TEF1-alpha gene regions of Pleosporaceae species and phylogenetically analysed this data. Multigene phylogenies strongly support the monophyletic nature of Pleosporaceae among the other families in Pleosporales, and the acceptance of the genera Alternaria, Bipolaris, Clathrospora, Comoclathris, Curvularia, Dactuliophora, Decorospora, Diademosa, Exserohilum, Extrawettsteinina, Gibbago, Neocamarosporium, Paradendryphiella, Platysporoides, Pleospora, Porocercospora, Pseudoyuconia and Pyrenophora. Austropleospora, Dendryphion, Edenia and Macrospora are excluded from the family based on morphology coupled with molecular data. Two novel species, Alternaria murispora in this paper and Comoclathris sedi are introduced. The sexual morph of Alternaria alternata is re-described and illustrated using modern concepts from fresh collections. The paraphyletic nature of Pleospora is resolved based on the available morpho-molecular data, but further sampling with fresh collections, reference or ex-type strains and molecular data are needed to obtain a natural classification of genera and the family.
Similar content being viewed by others
Introduction
Pleosporaceae is the largest family in the Pleosporales and is representative of the order (Zhang et al. 2012; Hyde et al. 2013; Wijayawardene et al. 2014). The classification in the Pleosporaceae has primarily been based on the Pleospora type of centrum development (Dong et al. 1998) and asci that are interspersed with pseudoparaphyses in the ascomata. These pseudoparaphyses originate above the hymenial layer and grow downward among the asci to fuse at the base of the locule (Wehmeyer 1975). Ascomata are perithecial, initially immersed and become erumpent and are usually black and sometimes hairy or setose. Asci are bitunicate and fissitunicate, cylindrical, with an ocular chamber and the hamathecium comprises cellular pseudoparaphyses are. Ascospores are usually brown and phragmosporous or dictyosporous (Dong et al. 1998; Kirk et al. 2001). Species are pathogenic or saprobic on wood and dead herbaceous stems or leaves, as well as pathogenic in humans (Sivanesan 1984; Carter and Boudreaux 2004).
Historic overview of Pleosporaceae
The family Pleosporaceae was introduced by Nitschke (1869) based on the immersed ascomata and presence of pseudoparaphyses, and was classified in Sphaeriales. The family was transferred to Pseudosphaeriaceae and later raised to ordinal rank as the Pseudosphaeriales (Theissen and Sydow 1918). Pleosporaceae, Venturiaceae and Lophiostomataceae were assigned under Pleosporales by Luttrell (1955), while treating Pseudosphaeriales as a synonym of Pleosporales. Luttrell (1973) included eight families in Pleosporales including Pleosporaceae. Preliminary genera added to this family were based on ascospore characteristics, including shape, colour, septation, pigmentation and presence or absence of mucilaginous sheaths (Luttrell 1955, 1973; Wehmeyer 1961, 1975; Eriksson 1981; Sivanesan 1984; Barr 1987b; Abler 2003). Many of these characters were also found in other families, such as Leptosphaeriaceae, Melanommataceae, Phaeosphaeriaceae and Sporormiaceae. This led for confusion in the intergeneric and familial classification (Luttrell 1955, 1973; Wehmeyer 1961, 1975; von Arx and Müller 1975; Sivanesan 1984; Barr 1987a, b; Eriksson and Hawksworth 1986, 1991). Barr (1987b) redefined the Pleosporaceae to include Clathrospora (= Comoclathris), Kirschsteiniothelia, Lewia and Pleospora and grouped Cochliobolus, Pyrenophora and Setosphaeria into the family Pyrenophoraceae. Berbee (1996) disagreed, suggesting that all those genera belong to the Pleosporaceae. Kodsueb et al. (2006) showed the intergeneric relationships and phylogenetic perspectives of the family Pleosporaceae based on sequence analyses of partial 28S rDNA and accepted 14 genera in Pleosporaceae. Based on multi-gene phylogenetic analysis, Zhang et al. (2009) concluded that some species from Lewia, Cochliobolus, Pleospora, Pyrenophora and Setosphaeria resided in the Pleosporaceae. Lumbsch and Huhndorf (2010) accepted 13 genera in Pleosporaceae but Zhang et al. (2012) included only 10 genera by excluding Monascostroma, Kriegeriella, and Zeuctomorpha (see notes below). Hyde et al. (2013) included 23 genera in this family based on morphology coupled with molecular data. Multi-gene phylogenetic studies has shown that the familial placement of Pleosporaceae with respect to other families in order Pleosporales is valid (Lumbsch and Huhndorf 2010; Zhang et al. 2012; Hyde et al. 2013). Major circumscription changes of the genera in Pleosporaceae from 1961 to 2013 are given in Table 1.
Asexual morphs of Pleosporaceae
The asexual morphs of Pleosporaceae can be coelomycetous or hyphomycetous (Zhang et al. 2012; Hyde et al. 2013) and some sexual genera in Pleosporaceae have been linked with asexual morphs (Zhang et al. 2012; Hyde et al. 2013, 2014). The type species of the family Pleospora is linked to Stemphylium, which causes leaf disease (Sivanesan 1984). Bipolaris was shown to be the asexual morph of “Cochliobolus”, and the cause of plant disease or infection in human beings (Khan et al. 2000; Manamgoda et al. 2014). The nomenclatural conflict in this complex was resolved by giving priority to the more commonly used established generic name Bipolaris (Manamgoda et al. 2012a, b; 2014). At the same time, Manamgoda et al. (2012a, b) showed that Curvularia grouped with “Pseudocochliobolus”. The type species of Pleoseptum (P. yuccaesedum A.W. Ramaley & M.E. Barr) has been linked with Camarosporium yuccaesedum Fairm (Ramaley and Barr 1995) and Pyrenophora has the asexual morph Drechslera (Farr et al. 1989). Ariyawansa et al. (2014c) proposed to conserve Pyrenophora over Drechslera by giving priority to the oldest name. Based on the combined gene analysis of GAPDH, RPB2 and TEF1, Woudenberg et al. (2013) synonymised Allewia, Brachycladium, Chalastospora, Chmelia, Crivellia, Embellisia, Lewia, Nimbya, Sinomyces, Teretispora, Ulocladium, Undifilum and Ybotromyces under Alternaria. In same study, Woudenberg et al. (2013) treated the 24 internal clades in the Alternaria complex as sections, which is a continuation of a recent proposal for the taxonomic treatment of lineages in Alternaria. Furthermore, Alternariaster, a genus formerly seen as part of Alternaria was transferred to Leptosphaeriaceae based on molecular data. Recently, new asexual genera were introduced by different researches based on both morphology and phylogeny. i.e. Porocercospora was introduced as a new genus in Pleosporaceae by Amaradasa et al. (2014) to accommodate the buffalo grass false-smut pathogen, while Johnalcornia was introduced to accommodate Bipolaris aberrans, which clusters sister to the newly described Porocercospora (Tan et al. 2014). Paradendryphiella was introduced by Woudenberg et al. (2013) to accommodate two marine Dendryphiella species (D. arenariae Nicot, D. salina (G.K. Sutherl.) Pugh & Nicot) that did not group with the type species D. vinosa (Berk. & M.A. Curtis) Reisinger (Jones et al. 2008; Woudenberg et al. 2013).
Taxa in Alternaria, Bipolaris, Stemphylium and phoma like species are more common asexual morphs in Pleosporaceae and can be saprobic or parasitic on various hosts. Phoma betae A.B... Frank is a notorious pathogen of sugar beet, which causes zonate leaf spot. Alternaria porri (Ellis) Cif., Stemphylium solani G.F. Weber, S. botryosum Wallr and S. vesicarium (Wallr.) E.G. Simmons can cause leaf blight of garlic (Zhang et al. 2009). Phoma incompta Sacc & Martelli is a pathogen on olive, and S. botryosum, the asexual morph of Pleospora herbarum (Pers.) Rabenh, causes leaf disease of olive trees (Malathrakis 1979). Some Curvularia species have been reported as human pathogens, causing respiratory tract, cutaneous, and corneal infections (Carter and Boudreaux 2004).
We have been studying the families of Dothideomycetes based on morphology and molecular phylogeny, in order to provide a natural classification of this large class. Hyde et al. (2013) provide an account of all 105 families of Dothideomycetes, while Wijayawardene et al. (2014) provided an outline of Dothideomycetes and suggestions on the on the names to use where asexual and sexual genera were linked. The largest order in Dothideomycetes is Pleosporales and we also have provided a natural classification of this large order (Ariyawansa et al. 2013a, b, c; Ariyawansa et al. 2014a, b, c, d, e, f; Zhang et al. 2012). Zhang et al. (2012) provided an account for 105 genera in Pleosporales, however, at that time little verified molecular data were available. The aim of the present study was to delineate the phylogenetic lineages within Pleosporaceae, and to build a robust taxonomy to act as a backbone tree for the family. Phylogenetic analysis were conducted using sequence data from parts of the 18S nrDNA (SSU), 28S nrDNA (LSU), the internal transcribed spacer regions 1 and 2 and intervening 5.8S nrDNA (ITS) and RNA polymerase second largest subunit (RPB2) gene regions of extype, reference and putative strains of all available allied genera in Pleosporaceae. We also focused on the phylogenetic lineages within Alternaria and allied genera, and to create a stable taxonomy. Phylogenetic inferences were conducted on sequence data of parts of the SSU, LSU, ITS, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), RPB2 and translation elongation factor 1-alpha (TEF1) gene regions of extype and reference strains of Alternaria species and all available allied genera.
Material and methods
Specimen examination
Fresh material of species of Pleosporaceae were collected in Thailand, Germany and Italy during 2011–2014. Specimens were taken to the laboratory in Ziplock plastic bags. The samples were processed and examined following the method described in Ariyawansa et al. (2013a, b). Fresh and herbarium material were examined under a Motic SMZ 168 dissecting microscope to locate and isolate ascomata fruiting bodies. Hand sections of the fruiting structures were mounted in water for microscopic studies and photomicrography. The taxa were examined using a Nikon ECLIPSE 80i compound microscope and photographed with a Canon 450D digital camera fitted to the microscope. Measurements were made with the Tarosoft (R) Image Frame Work program and images used for figures processed with Adobe Photoshop CS3 Extended version 10.0 software (Adobe Systems, USA). Isolations were made from single ascospores, following a modified method of Ariyawansa et al. (2013b) and Chomnunti et al. (2014). Contents of the sectioned fruiting body were transferred to a drop of sterile water on a flame-sterilized slide. Drops of the spore suspension were pipetted and spread on a Petri-dish containing 2 % water agar (WA) and incubated at 25 °C. Germinated ascospores were transferred singly to MEA media (Alves et al. 2006).
Herbarium specimens were obtained on loan from the Swedish Museum of Natural History (S) and the New York Botanical Garden (NY). Voucher specimens are deposited in the herbarium of Mae Fah Luang University (MFLU), Chiang Rai, Thailand, Kunming Institute of Botany (KIB) and New Zealand Fungal Herbarium-Landcare Research (PDD), New Zealand. Living cultures are deposited at the Mae Fah Luang University Culture Collection (MFLUCC), International collection of microorganisms from plants (ICMP) and Queensland Plant Pathology Herbarium (BRIP), the latter under Material Transfer Agreement No. 4/2010 (MTA). Each genus is listed along with a description of the type species, except in cases where there is only a single species in the genus.
DNA extraction, PCR amplification and sequencing
Single ascospore fungal isolates were grown on MEA or PDA for 28 days at 25 °C in the dark. Genomic DNA was extracted from the growing mycelium using the Biospin Fungus Genomic DNA Extraction Kit (BioFlux®) following the manufacturer’s protocol (Hangzhou, P.R. China). Otherwise DNA extracted directly from ascomata using a DNA extraction kit (E.Z.N.A.® Forensic DNA kit, D3591- 01,Omega Bio-Tek) following Telle and Thines (2008).
The amplification procedure was performed in a 50 μl reaction volume containing 5–10 ng DNA, 0.8 units Taq polymerase, 1X PCR buffer, 0.2 mM d’NTP, 0.3 μM of each primer with 1.5 mM MgCl2 (Cai et al. 2009). The PCR reactions for amplification of the recently ratified universal fungal barcode ITS1-5.8S-ITS2 of the nuclear ribosomal DNA operon (Schoch et al. 2009), were performed under standard conditions (White et al. 1990; Stielow et al. 2010). PCR conditions for amplifying the partial SSU and LSU r-DNA followed the protocol of Phillips et al. (2008). Amplification of GAPDH, RPB2 and TEF1 followed the protocol of Woudenberg et al. (2013). The PCR products were observed on 1 % agarose electrophoresis gels stained with ethidium bromide. Purification and sequencing of PCR products were carried at Shanghai Sangon Biological Engineering Technology and Services Co., (China).
DNA sequence data was obtained from the internal transcribe spacer (ITS), small and large subunits of the nuclear ribosomal RNA genes (SSU, LSU) and the protein coding gene, GAPDH (TUB). Primer sets used for these genes were as follows: ITS: ITS5/ITS4 SSU: NS1/NS4; LSU: LR0R/LR5; RPB2–5F2/fRPB2–7cR (Liu et al. 1999; Sung et al. 2007). The GAPDH region with gpd1 and gpd2 (Berbee et al. 1999) and the TEF1 gene with the primers EF1-728F and EF1-986R (Carbone and Kohn 1999). Primer sequences are available at the WASABI database at the AFTOL website (aftol.org). Sequences are deposited at NCBI GenBank under the accession numbers provided in Supplementary Table 1. Alignments are deposited in TreeBASE.
Sequence alignment and phylogenetic analysis
Multiple sequence alignments were generated with MAFFT v. 6.864b (http://mafft.cbrc.jp/alignment/server/index.html). The alignments were checked visually and improved manually where necessary. Two different datasets were used to estimate two phylogenies; a Pleosporineae family tree and an Alternaria phylogeny. The first tree focuses on phylogenetic placement of Pleosporaceae in sub order Pleosporineae, the second one was generated to show the placement of newly reported Alternaria species and their sexual state. All introns and exons were aligned separately. Regions containing many leading or trailing gaps were removed from the ITS, SSU, LSU, GAPDH, TEF1 and RPB2 alignments prior to tree building. The alignments were checked visually and improved manually where necessary. All sequences obtained from GenBank and used by Ariyawansa et al. (2014a), Boonmee et al. (2014), Hyde et al. (2013), Phookamsak et al. (2014) Schoch et al. (2009), Suetrong et al. (2009), Verkley et al. (2014), and Zhang et al. (2012) are listed in supplementary Table 1.
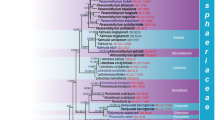
Maximum likelihood analyses including 1000 bootstrap replicates were run using RAxML v. 7.2.6 (Stamatakis 2006; Stamatakis et al. 2008). The online tool Findmodel (http:// www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html) was used to determine the best nucleotide substitution model for each partition. The best scoring tree was selected with a final likelihood value of −19492.551787. The resulting replicates were plotted on to the best scoring tree obtained previously. Maximum Likelihood bootstrap values (ML) equal or greater than 50 % are given below or above each node in black (Fig. 1).
RAxML tree based on a combined dataset of ITS, SSU, LSU and RPB2 of 98 strains representing the Pleosporineae. Bootstrap support values for maximum likelihood greater than 50 % (black) and bayesian posterior probabilities greater than 0.90 (green) below and above the nodes. Halojulella avicenniae is the out group taxon. The original isolate numbers are noted after the species names. Ex-type culture numbers are in bold. Newly generated strains in this study are indicated in red. The type species of each genus is indicated in blue
The model of evolution was performed by using MrModeltest 2.2 (Nylander 2004). Posterior probabilities (PP) (Rannala and Yang 1996; Zhaxybayeva and Gogarten 2002) were determined by Markov Chain Monte Carlo sampling (MCMC) in MrBayes v. 3.0b4 (Huelsenbeck and Ronquist 2001). Six simultaneous Markov chains were run for 5,000,000 generations and trees were sampled every 100th generation and 50,000 trees were obtained. The first 10,000 trees, representing the burn-in phase of the analyses, were discarded while the remaining trees were used for calculating posterior probabilities in the majority rule consensus tree (critical value for the topological convergence diagnostic set to 0.01) (Crous et al. 2006). Bayesian Posterior Probabilities (BYPP) equal or greater than 0.90 is given below or above each node (Fig. 1).
In order to determine the species limits in Pleospora herbarum species complex, Alternaria complex and the newly introduced Comoclathris sedi, we applied the criteria of Genealogical Concordance Phylogenetic Species Recognition (GCPSR) (Taylor et al. 2000; Dettman et al. 2003). Dettman et al. (2003) emphasised that species should be recognised if they satisfy one of two criteria: genealogical concordance or genealogical non-discordance. Clades were genealogically concordant if they were present in at least some of the gene trees and genealogically non-discordant if they were strongly supported (MP ≥ 70 %; ML ≥ 70 %) in a single gene and not contradicted at or above this level of support in any other single gene tree. This criterion prohibited poorly supported non-monophyly at one locus from undermining well-supported monophyly at another locus. Phylogenetic trees and data files were viewed in MEGA v. 5 (Tamura et al. 2011), TreeView v. 1.6.6 (Page 1996) and FigTree v. 1.4 (Rambaut and Drummond 2008).
Results and discussion
Phylogeny
Phylogeny of Pleosporineae
The final Pleosporineae alignment included 98 strains, representing seven families, and consisted of 3170 characters (SSU 941, LSU 863, ITS 493, RPB2 858). In the SSU alignment a large insertion at position 446 in the isolates Chaetosphaeronema hispidulum (Corda) Moesz (CBS 216.75), Phoma fallens Sacc (Pleospora fallens) (CBS 161.78) and Ophiosphaerella herpotricha (Fr.) J. Walker (CBS 620.86) was excluded from the phylogenetic analyses. Four different alignments corresponding to each individual gene, and a combined alignment of the four genes were analysed. Comparison of the alignment properties and nucleotide substitution models are provided in Table 2. Results of the partition-homogeneity test (P = 0.107) indicate that the ITS, LSU, SSU and RPB2 gene trees reflect the same underlying phylogeny. Therefore, these datasets were combined and analyzed by using several tree-building programs, the resulting trees compared and the best tree is presented in Fig. 1. New sequences are deposited in GenBank (Table 1).
The combined ITS, LSU, SSU and RPB2 gene dataset from seven families in the Pleosporales sub order Pleosporaneae is shown in Fig 1. All trees (ML and BYPP) were similar in topology and not significantly different (data not shown). A best scoring RAxML tree is shown in Fig. 1, with the value of −19492.551787. Phylogenetic trees obtained from Maximum Likelihood and Bayesian analysis yielded trees with similar overall topology at subclass and family relationships in agreement with previous work based on Maximum Likelihood analysis (Schoch et al. 2009; Suetrong et al. 2009; Zhang et al. 2012; Ariyawansa et al. 2014a; Boonmee et al. 2014; Hyde et al. 2013, Phookamsak et al. 2014, Verkley et al. 2014; Wijayawardene et al. 2014). The support values for the different phylogenetic methods vary, with the Bayesian posterior probabilities being higher than the RAxML bootstrap support values.
In the multi-locus phylogeny inferred from the combined dataset shown in Fig. 1, several well-supported sub-clades can be identified in the family Pleosporaceae, which are interpreted as appropriate for the delimitation of genera, i.e. Alternaria, Bipolaris, Comoclathris, Curvularia, Decorospora, Dendryphion, Exserohilum, Gibbago, Johnalcornia, Neocamarosporium, Porocercospora, Pleospora and Pyrenophora.
The Pleospora sensu stricto clade comprises of eleven strains along with the type strain of Pleospora herbarum/ Stemphylium herbarum (CBS 191.86) including our strains (MFLUCC 14–0261, MFLUCC 13–0344 and MFLUCC 13–0266). In order to determine the species limits of Pleospora herbarum, a combine gene tree (Fig. 1.) was used. Nodes that were supported (≥70 %) in the combined gene phylogeny were recognised as taxa within Pleospora herbarum sensu stricto. Thus the putative strains of P. tomatonis E.G. Simmons (CBS 109844), P. sedicola E.G. Simmons (CBS 109843), which clustered within Pleospora herbarum sensu stricto are treated as strains of Pleospora herbarum in this study. Further work may yet prove this is a species complex.
A recently introduced genus, Paradendryphiella forms a basal clade to the Pleospora sensu stricto clade including the type strain of Paradendryphiella salina (CBS 302.84) and Dendryphiella salina (CBS 142.60). Another poorly-supported clade forming the major part of the ingroup of the tree comprises the putative strains of Gibbago trianthemae E.G. Simmons (NFCCI 1886 and GT-VM). The Bipolaris clade comprises four strains with B. oryzae (MFLUCC 10–0714), B. cynodontis (Marignoni) Shoemaker (ICMP 6128), B. melinidis Alcorn (BRIP 12898) and B. maydis (Y. Nisik. & C. Miyake) Shoemaker (CBS 134.39, previously known as Cochliobolus heterostrophus). Two monophyletic genera Porocercospora seminalis (Ellis & Everh.) Amaradasa et al., (CPC 21349 and CPC 21332) and Johnalcornia aberrans (Alcorn) Y.P. Tan & R.G. Shivas (BRIP 16281) form well-supported clades sister to the Curvularia, Exserohilum and Bipolaris clades. Curvularia forms a relatively well-supported clade within the family Pleosporaceae. This clade contains three strains with C. lunata (Wakker) Boedijn (CBS 730.96), the type species of Curvularia, and the highly supported clades of the following species: C. ravenelii (M.A. Curtis ex Berk.) Manamgoda et al. (BRIP 13165) and C. heteropogonis Alcorn (CBS 284.91). Exserohilum forms a well-supported clade sister to Curvularia containing the putative strains of Setosphaeria monoceras Alcorn (CBS 154.26), Exserohilum sp. (C950801) and Exserohilum sp. (NK93).
The recently redefined genus Alternaria forms a well-supported clade sister to Pyrenophora containing 18 strains with A. alternata (Fr.) Keissl (CBS 916.96) the type species of Alternaria, along with the three sexual strains of A. alternata (MFLUCC 14–1184, MFLUCC 14–1185 and ICMP). Apart from A. alternata strains, the clade consist of A. arborescens E.G. Simmons (CBS 102605), A. solani (Ellis & G. Martin) L.R. Jones & Grout (CBS 116651), A. macrospora Zimm., (CBS 117228), A. nobilis (Vize) E.G. Simmons (CBS 116490), Lewia infectoria (Fuckel) M.E. Barr & E.G. Simmons (CBS 210.86), Lewia ethzedia E.G. Simmons (CBS 197.86), Chalastospora gossypii (Jacz.) U. Braun & Crous (CPC 15567), Chalastospora obclavata Crous & U. Braun (CBS 124120), Embellisia abundans E.G. Simmons (CBS 534.83), A. anigozanthi R.D. Raabe (CBS 121920), Lewia eureka E.G. Simmons (DAOM 195275) and Crivellia papaveracea (De Not.) Shoemaker & Inderb (CBS 116607). Furthermore the type strain of Xenobotryosphaeria, X. calamagrostidis Quaedvl et al., (CBS 303.71) also clustered within the Alternaria clade.
The Pyrenophora clade comprises five strains with P. phaeocomes (Rebent.) Fr., (DAOM 222769), the type species of Pyrenophora, P. dictyoides A.R. Paul & Parbery (DAOM 63666 and DAOM 75616), Drechslera dematioidea (Bubák & Wróbl.) Scharif (CBS 108963) and P. chaetomioides Speg., (DAOM 208989). The type species of Comoclathris, C. lanata Clem, was not available for study, but the five verified strains of Comoclathris species form a well-supported clade within the family Pleosporaceae. i. e. C. compressa (Harkn.) Shoemaker & C.E. Babc (CBS 156.53 and CBS 157.53) and the novel species C. sedi (MFLUCC 13–0817, MFLUCC 13–0754, MFLUCC 13–0763 and MFLUCC 13–0607) along with a putative strain of Pleospora ambigua (Berl. & Bres.) Wehm (CBS 366.52) cluster in a well-supported clade within the Pleosporaceae outside Alternaria s. str. A putative strain of Pleospora ambigua (CBS 366.52) also clustered with our new species Comoclathris sedi and is thus treated as an additional strain of C. sedi. Furthermore, Comoclathris incompta (Pleospora incompta (Sacc. & Martelli) Gruyter & Verkley) (CBS 467.76) and Comoclathris typhicola (Pleospora typhicola (Cooke) Sacc. 1875) (CBS 132.69) also forms two distinct clades within the Comoclathris clade.
Another well supported clade (Fig. 1) was formed by Decorospora, D. gaudefroyi (Pat.) Inderb et al., (pp4723) the type species, along with two putative strains of Pleospora sp. ATCC MYA-3203 and ATCC MYA-3202 basal to the Comoclathris clade. The Neocamarosporium clade comprises seven strains with Neocamarosporium goegapense Crous & M.J. Wingf. (CPC 23676T), the type species of Neocamarosporium: N. betae (CBS 109410, IMI 156653, ICMP 10945) and N. calvescens (CBS 344.78, CBS 246.79 and CBS 432.77). The type species of Clathrospora, C. elynae, forms a well-supported clade, located basal to the Pleosporaceae (Fig. 1), outside the Alternaria complex.
The genus Edenia, with E. gomezpompae M.C. González et al., as the type (C1cT, CBS 124106, JLCC34533 and 11G048) clusters outside Pleosporaceae and formed a distinct clade in Phaeosphaeriaceae. During preliminary analysis (data not shown), the type strains of Austropleospora osteospermi R.G. Shivas & L. Morin (BRIP 51628) and Dendryphion europaeum Crous & R.K. Schumach (CPC 23231 and CPC 22943) clustered outside the suborder Pleosporineae, thus are excluded from the final analysis (data not shown).
Pleospora fallens (Sacc.) Gruyter & Verkley (CBS 161.78) treated here as Phoma fallens Sacc., forms a basal clade in family Pleosporaceae. Confusion surrounding this species is discussed in the latter part of this study.
Phylogeny of Alternaria
In order to define the phylogeny and taxonomy of Alternaria and allied genera, 114 strains were included in the Alternaria complex alignment. For the Alternaria tree, the gene boundaries were: 1–533 bp for ITS, 539–1080 bp for GPDH, 1087–1941 bp for LSU, 1947–2811 bp for RPB2 and 2817–3080 bp for TEF. All phylogenies, different phylogenetic methods and gene regions or gene combinations used on this dataset (data not shown, trees and alignments deposited in TreeBASE), show a weak support at the deeper nodes of the tree. The only well-supported node (Bayesian posterior probability of 1.0, RAxML Maximum Likelihood support value of 99) in all phylogenies separates Pyrenophora and the Pleospora/Stemphylium clade from the Alternaria complex (Fig. 1). The overall topology of the concatenated genes analysis of ITS, GPDH, LSU, RPB2 and TEF was in agreement to the phylogenetic trees obtained from Maximum Likelihood and Bayesian analysis yielded trees with previous work based on Maximum Likelihood and Bayesian analysis of Woudenberg et al. (2013) and Lawrence et al. (2013).
In the Alternaria clade, six monotypic lineages and 24 internal clades occur consistently in the individual and combined phylogenies, although positions vary between the different gene regions or combinations used and are similar with Woudenberg et al. (2013) and Lawrence et al. (2013). The support values for the different phylogenetic methods vary, with the Bayesian posterior probabilities being higher than the RAxML bootstrap support values (Fig. 2). The recently introduced genus, Xenobotryosphaeria, X. calamagrostidis (CBS 303.71) clustered within the Alternaria complex in sect. Infectoriae, thus giving rise to confusion (further discussed in the Taxonomy section of Alternaria).
RAxML tree based on a combined dataset of ITS, LSU GAPDH, RPB2 and TEF1 of 114 strains representing the Alternaria-complex. Bootstrap support values for maximum likelihood greater than 50 % and Bayesian posterior probabilities greater than 0.90 (green) are indicated below or above the nodes. Pleospora herbarum is the out group taxon. The original isolate numbers are noted after the species names. Newly generated strains in this study are indicated in red
Seven alignments were analysed corresponding to single gene analyses of ITS, SSU, LSU, GAPDH, TEF1 and RPB2 and combined alignments of the both Alternaria and Pleosporineae. Comparison of the alignment properties and nucleotide substitution models are provided in Tables 2 and 3.
Taxonomy
Pleosporaceae Nitschke, Verh. naturh. Ver. preuss. Rheinl. 26: 74 (1869).
Facesoffungi number : FoF 00500
Pathogenic or saprobic on wood and dead herbaceous stems or leaves or pathogen of humans. Sexual morph: Ascomata perithecial, initially immersed and becoming erumpent to nearly superficial, black, globose, subglobose or ovoid, sometimes hairy or setose, ostiolate. Ostiole papillate or apapillate, sometimes with a pore-like ostiole, ostiolar canal filled with or lacking periphyses. Peridium relatively thin, usually thick at the sides, thinner at the base. Hamathecium of hyaline, septate, cellular pseudoparaphyses interspersed with asci. Asci 8-spored, bitunicate, fissitunicate, cylindrical, with or without a pedicel, with an ocular chamber. Ascospores uniseriate or biseriate, partially overlapping, phragmosporous or muriform, brown or pale brown, with or without mucilaginous sheath. Asexual morph: coelomycetous or hyphomycetous, and the conidiogenous cells can be phialidic, annellidic or sympodial blastic.
Type: Pleospora Rabenh. ex Ces. & De Not., Comm. Soc. crittog. Ital. 1(4): 217 (1863)
Genera accepted in Pleosporaceae
Alternaria Nees, Syst. Pilze (Würzburg): 72 (1816) [1816–17].
= Lewia M.E. Barr & E.G. Simmons, in Simmons, Mycotaxon 25(1): 289 (1986).
= Allewia E.G. Simmons, Mycotaxon 38: 260 (1990).
=Brachycladium Corda, Icon. fung. (Prague) 2: 14 (1838).
=Chalastospora E.G. Simmons, CBS Diversity Ser. (Utrecht) 6: 668 (2007).
=Chmelia Svob.-Pol., Biológia, Bratislava 21: 82 (1966).
=Crivellia Shoemaker & Inderb., in Inderbitzin, Shoemaker, O’Neill, Turgeon & Berbee, Can. J. Bot. 84(8): 1308 (2006).
=Embellisia E.G. Simmons, Mycologia 63(2): 380 (1971).
=Nimbya E.G. Simmons, Sydowia 41: 316 (1989).
=Sinomyces Yong Wang bis & X.G. Zhang, Fungal Biology 115(2): 192 (2009).
=Teretispora E.G. Simmons, CBS Diversity Ser. (Utrecht) 6: 674 (2007).
=Ulocladium Preuss, Linnaea 24: 111 (1851).
=Undifilum B.M. Pryor, Creamer, Shoemaker, McLain-Romero & Hambl., Botany 87(2): 190 (2009).
=Ybotromyces Rulamort, Bull. Soc. bot. Centre-Ouest, Nouv. sér. 17: 192 (1986).
Type species: Alternaria alternata (Fr.) Keissl., Beih. bot. Zbl., Abt. 2 29: 434 (1912).
Bipolaris Shoemaker, Can. J. Bot. 37(5): 882 (1959).
= Cochliobolus Drechsler, Phytopathology 24: 973 (1934).
Type species: Bipolaris maydis (Y. Nisik. & C. Miyake) Shoemaker 1959.
Clathrospora Rabenh., Hedwigia 1(18): 116 (1857).
Type species: Clathrospora elynae Rabenh., Hedwigia 1: 116 (1857).
Comoclathris Clem., Gen. fung. (Minneapolis): 37, 173 (1909).
Type species: Comoclathris lanata Clem. [as ‘Comochlatris’], Gen. fung. (Minneapolis): 1–227 (1909).
Curvularia Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13(1): 123 (1933).
= Pseudocochliobolus Tsuda, Ueyama & Nishih., Mycologia 69(6): 1117 (1978) [1977].
Type species : Curvularia lunata (Wakker) Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13(1): 127 (1933).
Dactuliophora C.L. Leakey, Trans. Br. mycol. Soc. 47(3): 341 (1964).
Type species: Dactuliophora tarrii C.L. Leakey, Trans. Br. mycol. Soc. 47(3): 343 (1964).
Decorospora Inderb. et al., in Inderbitzin et al., Mycol. Progr. 1(4): 657 (2002).
Type species: Type species: Decorospora gaudefroyi (Pat.) Inderb. et al., in Inderbitzin et al., Mycol. Progr. 94(4): 657 (2002).
≡ Pleospora gaudefroyi Pat., Tab. analyt. Fung. (Paris)(7): 40 (no. 602) (1886).
Diademosa Shoemaker & C.E. Babc., Can. J. Bot. 70(8): 1641 (1992).
Type species: Diademosa californiana (M.E. Barr) Shoemaker & C.E. Babc. [as ‘californianum’], Can. J. Bot. 70(8): 1641 (1992).
Basionym: Graphyllium californianum M.E. Barr, Mem. N. Y. bot. Gdn 62: 40 (1990).
Exserohilum K.J. Leonard & Suggs, Mycologia 66(2): 289 (1974).
= Setosphaeria K.J. Leonard & Suggs, Mycologia 66(2): 294 (1974).
= Luttrellia Khokhr. & Gornostaĭ, in Gornostaĭ in Azbukina et al. (Eds), Vodorosli, Griby i Mkhi Dal’nego Vostoka [Algae, Fungi and Mosses of the Soviet Far-East] (Vladivostok): 80 (1978).
Type species: Exserohilum turcicum (Pass.) K.J. Leonard & Suggs, Mycologia 66(2): 291 (1974).
Extrawettsteinina M.E. Barr, Contr. Univ. Mich. Herb. 9(8): 538 (1972).
Type species: Extrawettsteinina minuta M.E. Barr, Contr. Univ. Mich. Herb. 9(8): 538 (1972).
Gibbago E.G. Simmons, Mycotaxon 27: 108 (1986).
Type species: Gibbago trianthemae E.G. Simmons, Mycotaxon 27: 108 (1986).
Neocamarosporium Crous & M.J. Wingf., Persoonia, Mol. Phyl. Evol. Fungi 32: 273 (2014).
Type species: Neocamarosporium goegapense Crous & M.J. Wingf., Persoonia, Mol. Phyl. Evol. Fungi 32: 273 (2014).
Paradendryphiella Woudenberg & Crous, Stud. Mycol. 75(1): 207 (2013).
Type species: Paradendryphiella salina (G.K. Sutherl.) Woudenberg & Crous, Stud. Mycol. 75(1): 207 (2013).
Basionym: Cercospora salina G.K. Sutherl., New Phytol. 15: 43 (1916).
Platysporoides (Wehm.) Shoemaker & C.E. Babc., Can. J. Bot. 70(8): 1648 (1992).
Type species: Platysporoides chartarum (Fuckel) Shoemaker & C.E. Babc., Can. J. Bot. 70(8): 1650 (1992).
Basionym: Pleospora chartarum Fuckel, Jb. nassau. Ver. Naturk. 23–24: 133 (1870) [1869–70].
Pleospora Rabenh. ex Ces. & De Not., Comm. Soc. crittog. Ital. 1(4): 217 (1863).
Type species: Pleospora herbarum (Pers.) Rabenh., Klotzschii Herb. Viv. Mycol.: no. 547 (1854).
Basionym: Sphaeria herbarum Pers., Syn. meth. fung. (Göttingen) 1: 78 (1801).
Porocercospora Amaradasa et al., Mycologia 106(1): 81 (2014).
Type species: Porocercospora seminalis (Ellis & Everh.) Amaradasa et al., Mycologia 106(1): 81 (2013).
Basionym: Cercospora seminalis Ellis & Everh., J. Mycol. 4(1): 4 (1888).
Pseudoyuconia Lar.N. Vassiljeva, Nov. sist. Niz. Rast. 20: 71 (1983).
Type species: Pseudoyuconia thalictri (G. Winter) Lar.N. Vassiljeva [as ‘thalicti’], Nov. sist. Niz. Rast. 20: 71 (1983).
Basionym: Leptosphaeria thalictri G. Winter, Hedwigia 10: 40 (1872)
Pyrenophora Fr., Summa veg. Scand., Section Post. (Stockholm): 397 (1849).
= Drechslera S. Ito, Proc. Imp. Acad. Japan 6: 355 (1930).
= Marielliottia Shoemaker, Can. J. Bot. 76(9): 1559 (1999) [1998].
Type species: Pyrenophora phaeocomes (Rebent.) Fr., Summa veg. Scand., Section Post. (Stockholm): 397 (1849).
Basionym: Sphaeria phaeocomes Rebent., Prodr. fl. neomarch. (Berolini): 338 (1804).
Excluded genera
Austropleospora R.G. Shivas & L. Morin et al., Fungal Diversity 40(1): 70 (2010).
Type species: Austropleospora osteospermi R.G. Shivas & L. Morin, in Morin, Shivas, Piper & Tan, Fungal Diversity 40(1): 70 (2010).
Dendryphion Wallr., Fl. crypt. Germ. (Norimbergae) 2: 300 (1833).
Type species: Dendryphion comosum Wallr., Fl. crypt. Germ. (Norimbergae) 2: 300 (1833).
Edenia M.C. González et al., in González et al., Mycotaxon 101: 254 (2007).
Type species: Edenia gomezpompae M.C. González et al., in González et al., Mycotaxon 101: 254 (2007).
Kriegeriella Höhn., Annls mycol. 16(1/2): 39 (1918).
Type species: Kriegeriella mirabilis Höhn., Annls mycol. 16(1/2): 39 (1918).
Macrospora Fuckel, Jb. nassau. Ver. Naturk. 23–24: 139 (1870) [1869–70].
= Nimbya E.G. Simmons, Sydowia 41: 316 (1989).
Type species: Macrospora scirpicola (DC.) Fuckel, Jb. nassau. Ver. Naturk. 23–24: 139 (1870) [1869–70].
Basionym: Sphaeria scirpicola DC., in Lamarck & de Candolle, Fl. franç., Edn 3 (Paris) 2: 300 (1805).
Monascostroma Höhn., Annls mycol. 16(1/2): 160 (1918).
Type species: Monascostroma innumerosum (Desm.) Höhn. [as ‘innumerosa’], Annls mycol. 16(1/2): 160 (1918).
Zeuctomorpha Sivan et al., Bitunicate Ascomycetes and their Anamorphs (Vaduz): 572 (1984).
Type species: Zeuctomorpha arecae Sivan et al., in Sivanesan, Bitunicate Ascomycetes and their Anamorphs (Vaduz): 572 (1984).
Herein we accepted 18 genera in family Pleosporaceae based on morphology coupled with molecular data and exclude 7 genera, which have previously been classified under the family (sensu Hyde et al. 2013; Wijewardana et al. 2014).
Treatment of genera in Pleosporaceae
Alternaria Nees, Syst. Pilze (Würzburg): 72 (1816) [1816–17]
Facesoffungi number: FoF 00501
Pathogenic or saprobic on wood and dead herbaceous stems or leaves. Sexual morph: Ascomata small, solitary to clustered, erumpent to (nearly) superficial at maturity, globose to ovoid, dark brown, smooth, apically papillate, ostiolate. Ostiole papilla short, blunt. Peridium relatively thin. Hamathecium of cellular pseudoparaphyses. Asci (4–6–) 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, straight or somewhat curved, with a short, furcate pedicel and minute ocular chamber. Ascospores ellipsoid to fusoid, muriform, slightly constricted at septa, yellow-brown, without guttules, smooth-walled. Asexual morph: Stroma rarely formed, setae and hyphopodia absent. Conidiophores macronematous, mononematous, simple or irregularly and loosely branched, pale brown or brown, solitary or in fascicles. Conidiogenous cells integrated, terminal becoming intercalary, polytretic, sympodial, or sometimes monotretic, cicatrized. Conidia catenate or solitary, dry, ovoid, obovoid, cylindrical, narrowly ellipsoid or obclavate, beaked or non-beaked, pale or medium olivaceous-brown to brown, smooth or verrucose, with transverse and with or without oblique or longitudinal septa (Woudenberg et al. 2013).
Type species: Alternaria alternata (Fr.) Keissl., Beih. bot. Zbl., Abt. 2 29: 434 (1912), Figs. 3 and 4
Basionym: Torula alternata Fr., Syst. mycol. (Lundae) 3(2): 500 (1832)
Pathogen or saprobe on wood and dead herbaceous stems or leaves. Sexual morph: Ascomata 170–20 × 190–240 μm (\( \overline{x} \) = 190 × 440 μm, n = 10), small, solitary to clustered, erumpent to (nearly) superficial at maturity, globose to ovoid, dark brown, smooth, apically papillate, ostiolate. Ostiole papilla short, blunt. Peridium 38–50 μm (\( \overline{x} \) = 45 μm, n = 10) wide, thin, comprising two cell types, outer layer composed of small heavily pigmented, thick-walled cells of textura angularis, inner layer composed of lightly pigmented or hyaline, thin-walled cells of textura angularis. Hamathecium of 2–2.5 μm (\( \overline{x} \) = 2.5 μm, n = 10) broad, long, cellular pseudoparaphyses. Asci 170–190 × 23–30 μm (\( \overline{x} \) = 180 × 25 μm, n = 20), (4–6–) 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, straight or somewhat curved, with a short, furcate pedicel and minute ocular chamber. Ascospores 37–43 × 13–14 μm (\( \overline{x} \) = 37 × 13.5 μm, n = 40), ellipsoid to fusoid, muriform, slightly constricted at septa, with 3–7 transverse septa, 1–2 series of longitudinal septa through the two original central segments, end cells without septa, or with 1 longitudinal or oblique septum, or with a Y-shaped pair of septa, brown, without guttules, smooth-walled. Asexual morph: Conidiophores 40–50 × 3–6 μm solitary to clustered, simple or branched, straight or flexuous, sometimes geniculate, pale, olivaceous or golden brown, smooth. Conidia 20–63 × 9–18 μm, mostly branched, obclavate, obpyriform, ovoid or ellipsoidal, often with a short conical or cylindrical beak, pale to mid golden brown, up to 8 transverse and usually several longitudinal or oblique septa, smooth or verruculose (Ellis 1971).
Material examined: ITALY, Monte Cervarola, Sestola, on the dead stem, 26 March 2012, E. Camporesi IT181-1 MFLU 14–0755, ICMP, living culture (MFLUCC 14–1184). ITALY, Monte Cervarola, Sestola, on the stem of Achillea sp. (Asteraceae) 15 July 2013, E. Camporesi IT 181–2 MFLU 14–0756 living culture (MFLUCC 14–1185). ITALY, Monte Cervarola, Sestola, on the stem of Portulaca sp. (Portulacaceae), 5 September 2012, E. Camporesi IT 466 (MFLU 14–0757); (KIB, PDD).
Notes: Alternaria was introduced by Nees (1816) and is a ubiquitous genus that includes saprobic, endophytic and pathogenic species associated with a wide variety of substrates (Woudenberg et al. 2013). Recent studies based on DNA data as well as literature reviews revealed multiple paraphyletic genera within the Alternaria complex, and Alternaria species clades that do not always correlate to species-groups based on morphological characteristics (Woudenberg et al. 2013). Based on the combined gene analysis of GAPDH, RPB2 and TEF1, Woudenberg et al. (2013) concluded that the Alternaria clade contains 24 internal clades and six monotypic lineages, the grouping of which are recognised as Alternaria and thus synonymised Allewia, Brachycladium, Chalastospora, Chmelia, Crivellia, Embellisia, Lewia, Nimbya, Sinomyces, Teretispora, Ulocladium, Undifilum and Ybotromyces under Alternaria senso stricto Furthermore, Woudenberg et al. (2013) treated 24 internal clades in the Alternaria complex as sections. Our phylogeny based on analysis of ITS, GAPDH, LSU, RPB2 and TEF1 sequence data (Fig. 2) produced similar results as Woudenberg et al. (2013). We therefore follow their recent proposal for the taxonomic treatment of lineages in Alternaria.
In the present study we collected the sexual morph of Alternaria alternata from Italy in 2013 and 2014. Cultures did not produce the asexual morph but phylogenetically our collections (MFLUCC 14–1185, MFLUCC 14–1184) are 100 % similar with the type strain of Alternaria alternata (CBS 916.96). Therefore here we have given the description of the sexual morph of Alternaria alternata based on our collections.
We carried out a separate phylogenetic study for the Alternaria section to show the placement of some newly introduce species in this study and some sexual reports of Alternaria (Fig. 2). During our phylogenetic analysis we observed that the type strain of Xenobotryosphaeria calamagrostidis (CBS 303.71) forms a clade within the Alternaria section Infectoriae and clustered with Alternaria infectoria.
The sexual morph of Xenobotryosphaeria calamagrostidis is characterised by globose, smooth, superficial ascomata, a peridium with 3–4 layers of brown textura angularis, bitunicate, clavate, short pedicellate, 6–8-spored, asci and multiseriate, hyaline, granular, broadly ellipsoid, aseptate ascospores. This is a clear morphological deviation from the sexual morph of Alternaria infectoria. Xenobotryosphaeria is typical of genera in the Botryosphaeriales, but is phylogenetically distinct (Crous et al. 2006; Phillips et al. 2008; Liu et al. 2012). It also resembles species of Muyocopron (Muyocopronaceae), but the latter genus differs in that it has circular, flattened ascomata, as well as prominent pseudoparaphyses, which are absent in Xenobotryosphaeria. Therefore the confusion surrounding Xenobotryosphaeria remains unresolved.
During the study we found a novel species of Alternaria, A. murispora from Germany and this is described below.
Alternaria murispora Ariyawansa & K.D. Hyde, sp. nov., Fig. 5
Index Fungorum number : IF 550952, Facesoffungi number : FoF 00502
Etymology: Named after its muriform ascospores.
Holotype: MFLU 14–0758
Saprobic on dead stem. Sexual morph: Ascomata 110–160 × 100–200 μm (\( \overline{x} \) = 130 × 150 μm, n = 10), small, scattered, erumpent to (nearly) superficial at maturity, globose or spheroid, dark brown, smooth, apically papillate, ostiolate. Ostiole papilla short and blunt. Peridium 7–15 μm (\( \overline{x} \) = 12 μm, n = 10), thin, comprising two cell types, outer layer composed of small heavily pigmented, thick-walled cells of textura angularis, inner layer composed of lightly pigmented or hyaline, thin-walled cells of textura angularis. Hamathecium of 1–2.5 μm (\( \overline{x} \) = 1.8 μm, n = 20), cellular, septate, pseudoparaphyses branching and anastomosing between and above asci. Asci 75–106 × 11–16 μm (\( \overline{x} \) = 92 × 14 μm, n = 20), (4–6–) 8-spored, bitunicate, fissitunicate, cylindrical to subcylindrical, straight or somewhat curved, with a short, furcate pedicel and minute ocular chamber. Ascospores 16–18 × 5–7 μm (\( \overline{x} \) = 17 × 6 μm, n = 40), overlapping seriate, ellipsoid to fusoid, muriform, initially 3-transseptate become 4–5 at maturity, constricted at the central septum, pale brown when immature, dark brown at maturity, without guttules, smooth-walled, with a 1–2.5 μm wide sheath. Asexual morph: undetermined.
Material examined: GERMANY, Frankfurt, on dead stem, 28 November 2013, A. D Ariyawansa (MFLU 14–0758, holotype).
Notes: Single spore isolation was not successful and therefore DNA was extracted directly from the fruiting body of the fungus. Therefore no living culture is available.
Notes: The novel species Alternaria murispora is introduced here based on both morphology and phylogeny. Alternaria murispora fits well with the general concept of Alternaria in having superficial, globose to ovoid ascomata with short, blunt ostiole, cellular pseudoparaphyses, cylindrical asci with a short, furcate pedicel and ellipsoid to fusoid, brown ascospores which are slightly constricted at the septa. Morphologically A. murispora is closely related to A. conjuncta E.G. Simmons (= Lewia scrophulariae (Desm.) M.E. Barr & E.G. Simmons), but differs in having comparatively smaller asci (75–106 μm versus 120–140 μm), smaller ascospores (16–18 μm versus 23–25 μm) and in the number of septa at maturity (3–5 μm versus 6–7 μm). This is also supported phylogenetically. i.e. A. conjuncta forms a sister clade to A. californica E.G. Simmons & S.T. Koike, while A. murispora forms a sister clade with A. triticina Prasada & Prabhu, in section Infectoriae (Fig. 2).
Bipolaris Shoemaker, Can. J. Bot. 37(5): 882 (1959)
= Cochliobolus Drechsler, Phytopathology 24: 973 (1934)
Facesoffungi number : FoF 00503
Pathogenic or saprobic on wood and dead herbaceous stems or leaves. Sexual morph Ascomata brown or black, immersed, erumpent, partially embedded or superficial, free or on flat stroma, mostly globose to ellipsoidal, sometimes flask-shaped or flattened on hard substrata, smooth or covered with vegetative filaments. Ostiole arising centrally, papillate or with a neck. Peridium comprising pseudoparenchymatous cells of equal thickness or slightly thickened at apex. Hamathecium dense septate, filiform, branched pseudoparaphyses. Asci 2–8 spored, bitunicate, fissitunicate, clavate, cylindrical-clavate or broadly fusoid, straight or slightly curved, thin-walled, often becoming more or less distended prior to dehiscence, short pedicellate, rounded at apex. Ascospores fasciculate, filiform or flageliform, hyaline or sometimes pale yellow or pale brown at maturity, septate, helically coiled within ascus, degree of ascospore coiling moderate to very strongly coiled, sometimes with free ends, often with a thin mucilaginous sheath (modified from Manamgoda et al. 2012). Asexual morph Conidiophores pale to dark brown, single, branched, sometimes arranged in small groups, straight to flexuous or geniculate with smooth or verrucose conidiogenous node. Conidia mostly curved, canoe-shaped, fusoid or obclavate, rarely straight, 3–14 pseudoseptate (usually more than 6), hyaline, pale or dark brown, reddish brown or pale to deep olivaceous, germinating by production of one or two germination tubes by polar cells. Hilum often slightly protruding or truncate sometimes inconspicuous. Septum ontogeny first septum median to sub median, second septum delimits basal cell and third delimits distal cell.
Type species: Bipolaris maydis (Y. Nisik. & C. Miyake) Shoemaker, Canad. J. Bot. 33: 882 (1959), Fig. 6.
a-h: Bipolaris luttrellii (holotype of dried culture of Cochliobolus luttrellii , j-n), Bipolaris maydis (neotype of Bipolaris maydis, a–h) a. Ascomata on host substrate. b. Section of the ascomata. c. Close up of the peridium d-e. Subcylindrical asci with a short pedicle note: helical arrangement of the ascospores j. Conidiophore. k-n. Conidia. Scale bars: b = 100 μm, c = 10 μm, d–g = 60 μm, h–j = 30 μm
Basionym: Helminthosporium maydis Y. Nisik. & C. Miyake, Journal of Plant Protection, Tokyo13: 20 (1926).
≡ Drechslera maydis (Y. Nisik. & C. Miyake) Subram.& B.L. Jain, Curr. Sci. 35: 354 (1966)
= Helminthosporium maydis Brond., Ill. Iconogr. Microscop.Cryptog. France 15. 1856–1857 (as ‘Helmisporium’), nom. rej.prop. (Rossman, Manamgoda & Hyde, 2013b)
= Ophiobolus heterostrophus Drechsler in J. Agric. Res. 31: 701. 1925, nom. rej.prop (Rossman et al., 2013b)
= Cochliobolus heterostrophus (Drechsler) Drechsler, Phytopathology 24: 973 (1934).
Facesoffungi number : FoF 00504
Pathogenic or saprobic on wood and dead herbaceous stems or leaves. Sexual morph of Bipolaris luttrellii on Sach’s agar medium: Ascomata 260–370 μm (\( \overline{x} \) = 316 μm, n = 10) diam., superficial or slightly immersed, black, sub globose to ellipsoidal. Ostiole, subconical to campanulate, ostiolar beak and upper part of ascomata covered by densely arranged setae. Pseudoparaphyses filiform, hyaline, septate. Asci 140–205 × 18–26 μm (\( \overline{x} \) = 178 × 22 μm, n = 20), 1–8-spored, bitunicate, fissitunicate, hyaline, subcylindrical, short pedicellate. Ascospores 180–285 × 6–8 μm (\( \overline{x} \) = 235 × 7 μm, n = 20), filiform, hyaline, tapered slightly towards apex and base, tightly coiled inside ascus, sometimes slightly coiled to straight at upper most part, (7–)8(−12)-distoseptate. Asexual morph of Bipolaris maydis on PDA: Conidiophores 105–470 × 5–7 μm (\( \overline{x} \) = 286 μm, n= 20), usually arising singly or in small groups, simple or rarely branched, septate, straight or flexuous, geniculate at upper part,olivaceous brown. Conidiogenous nodes dark brown, distinct. Occasionally secondary sporulation observed. Conidia 66–102 × 14–18 μm (\( \overline{x} \) = 94 × 16 μm, n = 40) μm, pale to mid dark brown, smooth, slightly curved, fusiform, distoseptate. Hilum distinct, 3–5 μm wide, germination tubes arising from both ends of conidia.
Material examined: AUSTRALIA, on Dactyloctenium aegyptium, 3 June1985, J.L. Alcorn, (BRIP 14791, holotype of dried culture of Cochliobolus luttrellii) and USA, North Carolina, isolated from Zea mays L?, Olin Yoder C5, resulting from six crosses, culture sporulating on Zea mays (BPI 892696, neotype of Bipolaris maydis).
Notes: Bipolaris was introduced by Shoemaker (1959) and it is considered an important plant pathogen associated with over 60 host genera (Sivanesan 1987; Manamgoda et al. 2011; Agrios 2005; Hyde et al. 2014). Cochliobolus Drechsler (1934) is the sexual stage of Bipolaris. There was no clear morphological boundary between the asexual genera Bipolaris and Curvularia, and some species show intermediate morphology which thus caused confusion for many plant pathologists and mycologists for their correct identification (Sivanesan 1987; Manamgoda et al. 2011; Hyde et al. 2014). Based on combined gene analysis of rDNA ITS (internal transcribed spacer), LSU, GPDH and EF1-α, Manamgoda et al. (2012) resolved the taxonomic confusion in Bipolaris and Curvularia complex. Multilocus phylogeny showed that Bipolaris and Curvularia form two well supported clades in previous studies (Manamgoda et al. 2011, 2012) as well as in the present study. Further, the nomenclatural conflict in this complex was resolved giving priority to the more commonly used established generic names Bipolaris and Curvularia thus Cochliobolus was synonymised under Bipolaris. Recently Manamgoda et al. (2014) revised the genus Bipolaris based on DNA sequence data derived from living cultures of fresh isolates, available ex-type cultures from worldwide collections and observation of type and additional specimens. They accepted 47 species in the genus Bipolaris and clarify the taxonomy, host associations, geographic distributions and species’ synonymies while epi- or neotypes were designated for Bipolaris cynodontis (Marignoni) Shoemaker, B. oryzae (Breda de Haan) Shoemaker, B. victoriae (F. Meehan & H.C. Murphy) Shoemaker, B. yamadae (Y. Nisik.) Shoemaker and B. zeicola (G.L. Stout) Shoemaker.
In our phylogeny, Bipolaris forms a robust clade sister to Curvularia and Porocercospora. Therefore we accept Bipolaris as a well established genus in Pleosporaceae based on both morphology and phylogeny.
Clathrospora Rabenh., Hedwigia 1(18): 116 (1857)
Facesoffungi number : FoF 00505
Saprobic on wood and stems. Sexual morph: Ascomata semi-immersed, scattered on putrid host stems and foliage, brown to blackish brown, subglobose or nearly globose, with a central sunken ostiole open via a circular lid, asci and pseudoparaphyses forming at the base of the peridium. Peridium composed of 3–5 layers of brown, relatively thick-walled cells of textura angularis, inner cells flattened, thin-walled and lighter. Hamathecium composed of dense, hyaline, filiform, pseudoparaphyses which are longer than the asci. Asci 8-spored, bitunicate, fissitunicate, thick-walled, cylindrical to clavate, with a short pedicle and shallow ocular chamber. Ascospores biseriate, fusiform, muriform, 7-transseptate, two or many rows of longitudinal septa, applanate, constricted only at the central septum, dark brown to brown, surrounded by a thin, hyaline mucilaginous sheath. Asexual morph: Alternaria like (Zhang et al. 2012).
Type species: Clathrospora elynae Rabenh., Hedwigia 1: 116 (1857), Fig. 7.
Clathrospora elynae (isotype) a. Herbarium material. b. Close up of ascomata. c. Section of the ascoma d. Close up of the peridium. e. Hyaline, filiform, pseudoparaphyses. f–h. Cylindrical to clavate asci with a short pedicle and ocular chamber. i-k. Dark brown to brown muriform ascospores surrounded by a thin, hyaline mucilaginous sheath. Scale bars: b = 100 μm, c = 10 μm, d–g = 60 μm, h–j = 30 μm
≡ Clathrospora elymae Rabenh. (1857).
≡ Pleospora elynae (Rabenh.) Ces. & De Not., Commentario della Società Crittogamologica Italiana 1 (4): 218 (1863).
Facesoffungi number : FoF 00506
Saprobic on wood and stems. Sexual morph: Ascomata 220–140 × 145–175 μm (\( \overline{x} \) = 170 × 150 μm, n = 10), semi-immersed, scattered on the putrid host stems and foliage, subglobose or nearly globose, brown to blackish brown, with a central sunken ostiole open via a circular lid. Peridium 20–55 μm (\( \overline{x} \) = 38, n = 20), composed of 3–5 layers of brown, relatively thick-walled cells of textura angularis, inner cells flattened, thin-walled and lighter. Hamathecium composed of dense, 2–3 μm diam (\( \overline{x} \) = 2, n = 20), hyaline, filiform, pseudoparaphyses, longer than the asci. Asci 160–230 × 24–48 μm (\( \overline{x} \) = 190 × 35 μm, n = 20), 8-spored, bitunicate, fissitunicate, thick-walled, cylindrical to clavate, with a short pedicle and minute ocular chamber. Ascospores 40–65 × 18–27 μm (\( \overline{x} \) = 53 × 23 μm, n = 40), biseriate, fusiform, muriform, 7-transseptate, two or many rows of longitudinal septa, constricted only at the central septum, applanate, dark brown to brown, surrounded by a thin, hyaline mucilaginous sheath. Asexual morph: Alternaria-like (Zhang et al. 2012).
Material examined: SWITZERLAND, on the stem of Carex curvula, September 1898, Winter (BPI 627748, isotype).
Notes: Shoemaker and Babcock (1992) assigned Clathrospora to Diademaceae and included an additional nine species and provided a key to the genus based on the number of septa and length of ascospores. Clathrospora was characterized by ascomata circular lid-like opening and applanate, muriform ascospores. Currently, 50 Clathrospora species are listed in the genus in Index Fungorum (2015). Molecular studies based on combine gene analysis showed that two putative strains of Clathrospora, C. elynae (CBS 196.54) and C. diplospora (IMI 68086) clustered in Pleosporaceae (Ariyawansa et al. 2014a). We obtained similar results in the phylogenetic tree produced from combined ITS, nrLSU, nrSSU and RPB2 sequence analysis (Fig. 1). Clathrospora elynae, the type of Clathrospora, formed a separate clade with low bootstrap support (58 %) within Pleosporaceae. Based on the phylogenetic result together with the morphological characters (slightly papillate ostiole with applanate, muriform ascospores and Alternaria- like asexual morph) we refer Clathrospora to Pleosporaceae.
Comoclathris Clem., Gen. fung. (Minneapolis): 37, 173 (1909)
= Platyspora Wehm., World Monograph of the Genus Pleospora and its Segregates: 254 (1961).
Facesoffungi number : FoF 00507
Saprobic on dead wood or stems. Sexual morph: Ascomata semi-immersed to superficial, scattered or aggregated, subglobose or nearly globose, brown to blackish brown coriaceous, ascomata opening via a large circular aperture or lid. Peridium comprising 3–4 layers of brown, relatively thick-walled cells of textura angularis. Hamathecium composed of dense, hyaline, filiform, septate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate with an ocular chamber. Ascospores uniseriate or partially overlapping, fusiform, muriform, applanate, brown to reddish-brown, surrounded by a thick, hyaline, mucilaginous sheath. Asexual morph: Alternaria-like (Zhang et al. 2012).
Type species: Comoclathris lanata Clem. [as ‘Comochlatris’], Gen. fung. (Minneapolis): 1–227 (1909), Fig. 8.
Comoclathris lanata (holotype). a. Herbarium material showing habit of fungus on host stem. b. Erumpent ascomata. c. Section through ascoma. Note the arrangement of asci and external setae. d. Section showing peridial cells of ascoma. e. Hyaline, filform, pseudoparaphyses. f. Asci with short knob-like pedicels and shallow ocular chamber. g–i. Muriform, applanate ascospores with a thick sheath. Scale Bars: b =200 μm, c = 40 μm, d–e = 10 μm, f =20 μm, g–i = 10 μm
Facesoffungi number : FoF 00508
Saprobic on dead stem. Sexual morph: Ascomata 175–245 × 142–197 μm (\( \overline{x} \) = 205 × 159 μm, n = 20), scattered or aggregated on the host stem, subglobose or nearly globose, superficial, coriaceous, covered with a pale membrane, brown to blackish brown, with a central ostiole. Peridium 10–22 μm wide, comprising 3–4 layers of brown, relatively thick-walled cells of textura angularis, inner cells flattened, thin-walled and lighter. Hamathecium composed of dense 2–4 μm wide, septate, hyaline, filiform, pseudoparaphyses longer than the asci. Asci 108–149 × 20–30 μm (\( \overline{x} \) = 24 × 125 μm, n = 20), 8-spored, bitunicate, fissitunicate, thick-walled, cylindrical to cylindro-clavate, with a short knob-like pedicel, and indistinct shallow ocular chamber. Ascospores 20–32 × 8–13 μm (\( \overline{x} \) = 12 × 28 μm, n = 20 ), 1–2 overlapping seriate, fusiform, muriform, with 4–5-transverse septa and 1–2-longitudinal septa, not constricted at the septa, applanate, brown to reddish-brown, surrounded by a distinct, hyaline, mucilaginous 3–8 μm wide sheath. Asexual morph: undetermined.
Material examined: USA, Colorado, on stem of Leptotaenia multifida Nutt (Umbelliferae), 8 July 1907, F.E. & E.S. Clements (COLO 62872, holotype).
Notes: Comoclathris, typified by Comoclathris lanata, was introduced by Clements (1909). The genus is characterized by ascomata with circular lid-like openings and applanate reddish-brown to dark reddish-brown, muriform ascospores, with single longitudinal septa (Ariyawansa et al. 2014a). Zhang et al. (2012) tentatively placed Comoclathris in the Pleosporaceae based on Alternaria-like asexual morphs and this was followed by Woudenberg et al. (2013). Comoclathris shares common characters with Pleospora herbarum, the type of Pleospora, in having cylindrical to cylindro-clavate asci with an ocular chamber and muriform, brown or pale brown ascospores, with or without sheath. Comoclathris and Pleospora differ in the opening of ascomata (opening via a large circular aperture or lid versus opening by a central pore and applanate ascospores). Comoclathris and Pleoseptum share similar characters in having globose, black, ascomata and cylindrical to cylindro-clavate asci with muriform, yellowish to dark brown ascospores. Comoclathris differs from Pleoseptum in having superficial ascomata with circular lid-like openings composed of comparatively thin peridium and applanate and fusiform ascospores surrounded by a distinct hyaline, mucilaginous thick sheath (Ariyawansa et al. 2014a). In Pleoseptum ascomata are immersed, usually with a papillate apex, with a relatively broad peridium and ovoid to fusoid ascospores (Ariyawansa et al. 2014a). Comoclathris was considered to differ from Clathrospora as in the latter genus species have two or more rows of longitudinal septa as compared with a single row in Comoclathris (Ariyawansa et al. 2014a). Shoemaker and Babcock (1992) provided a key to 21 species of Comoclathris. Presently 32 epithets are listed for Comoclathris in Index Fungorum (2015).
Molecular data for Comoclathris lanata, the type species of Comoclathris, is not available. Two putative strains of Comoclathris compressa (CBS 157.53 and CBS 156.53) however, cluster together in a well-supported clade within the family Pleosporaceae (Ariyawansa et al. 2014a). Based on the phylogenetic result, coupled with the morphological characters (Alternaria-like asexual morph), we agree with Ariyawansa et al. (2014a), Zhang et al. (2012) and Woudenberg et al. (2013) in placing Comoclathris in Pleosporaceae, but re-collection of the type species and epitypification or a reference specimen (Ariyawansa et al. 2014a) with molecular data is essential to establish the correct placement of the genus.
During this study we have proposed several new combinations in order to resolve the paraphyletic nature of Pleospora and to make a stable taxonomy for the family Pleosporaceae. Pleospora incompta (Sacc. & Martelli) Gruyter & Verkley) (CBS 467.76) was introduced by de Gruyter et al. (2013) in order to accommodate Phoma incompta Sacc. & Martelli. In the same study Pleospora typhicola Cook was proposed by de Gruyter et al. (2013) to accommodate Phoma typhina (Sacc. & Malbr.) van der Aa & Vanev in the family Pleosporaceae but during our study Pleospora typhicola (Cooke) Sacc. 1875) (CBS 132.69) and Pleospora incompta (CBS 467.76) form separate clades within the genus Comoclathris (Fig. 1). Therefore we provide two new combinations namely Comoclathris incompta and C. typhicola to accommodate Pleospora incompta and P. typhicola in Comoclathris.
Comoclathris incompta (Sacc. & Martelli) Ariyawansa & K. D. Hyde, comb. nov.,
Fungorum number: IF 550953
Basionym: Phoma incompta Sacc. & Martelli, Syll. Fung. 10: 146.1892.
≡ Pleospora incompta (Sacc & Martelli) Gruyter & Verkley, in Gruyter et al., Stud. Mycol. 75: 25 (2012).
Comoclathris typhicola (Cooke) Ariyawansa & K. D. Hyde, comb. nov.,
Fungorum number: IF 550954
Basionym: Sphaeria typhicola Cooke, Grevillea 5: 121. 1877.
≡ Pleospora typhicola (Cooke) Sacc., Reliq. Libert 2: no. 152 (1875)
≡ Clathrospora typhicola (Cooke) Höhn., Ann. Mycol. 16: 88. 1918.
≡ Pyrenophora typhicola (Cooke) E. Müll., Sydowia 5: 256. 1951.
≡ Macrospora typhicola (Cooke) Shoemaker & C.E. Babc., Canad. J. Bot.
70: 1644. 1992.
= Phyllosticta typhina Sacc. & Malbr., Sacc., Michelia 2: 88. 1880.
≡ Phoma typhina (Sacc. & Malbr.) van der Aa & Vanev, A revision of the
species described in Phyllosticta: 468. 2002.
= Phoma typharum Sacc., Syll. Fung. 3: 163. 1884.
We also collected a novel species of Comoclathris, C. sedi from Italy and this is described below.
Type species: Comoclathris sedi Wanasinghe, Ariyawansa, E. Camporesi & K.D. Hyde, sp. nov., Fig. 9.
Comoclathris sedi (holotype). a. Habit of the fungus on host stem. b. Superficial ascomata. c. Thick section through ascoma. d. Dark brown setae. e. Hyaline pseudoparaphyses. f-h. Asci with shallow ocular chamber. i-k. Muriform ascospores with thick sheath. Scale Bars: b = 200 μm, c = 40 μm, f–h = 10 μm, i–k = 20 μm
Index Fungorum number : IF 550955, Facesoffungi number : FoF 00509
Etymology: The specific epithet sedi is based on the host genus from which the fungus was isolated.
Holotype: MFLU 14 0758
Saprobic on dead stem. Sexual morph: Ascomata 200–250 × 290–350 μm (\( \overline{x} \) = 230 × 320 μm, n = 10), scattered or aggregated on the host stem, subglobose or nearly globose, superficial, coriaceous, brown to blackish brown with a blunt ostiole. Peridium 20–38 μm (\( \overline{x} \) = 25 μm, n = 10) wide, comprising 3–4 layers of brown, relatively thick-walled cells of textura angularis, inner cells flattened, thin-walled and lighter. Hamathecium composed of dense 1.5–2.5 μm (\( \overline{x} \) = 2 μm, n = 10) wide, septate, hyaline, filiform, pseudoparaphyses longer than the asci. Asci 80–110 × 16–18 μm (\( \overline{x} \) = 98 × 18 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short knob-like pedicel, and indistinct shallow ocular chamber. Ascospores 19–20 × 8–10 μm (\( \overline{x} \) = 20 × 19 μm, n = 40 ), 1–2 overlapping seriate, fusiform, muriform, with 4–5-transverse septa and 1–2-longitudinal septa, not constricted at the septa, brown to reddish brown, surrounded by a distinct, hyaline, mucilaginous 5–9 μm wide sheath. Asexual morph: undetermined.
Material examined: ITALY, Stavel, Ortignano-Raggiolo, on stem of Sedum sp. (Crassulaceae), 6 June 2012, E. Camporesi IT 416, (MFLU 14 0759, holotype) – extype living culture (MFLUCC 13 0817, BRIP); ITALY, Monte Cervarola, Sestola, on dead branch of Clematis vitalba (Ranunculaceae), 21 July 2012, E. Camporesi IT 589, (KIB, paratype) – extype living culture (ICMP); ITALY, Almazzago, on dead stem of Rosa sp. (Rosaceae), 8 August 2013, N. Camporesi IT 1408 (MFLU14-0760) – living culture (MFLUCC 13 0763).
Notes: The novel taxon, Comoclathris sedi was initially isolated on dead stem of Sedum sp. But later the same species was isolated from Clematis vitalba, Rosa sp. and Digitalis sp. and they are morphologically and phylogenetically identical. Comoclathris sedi shows similarities with Comoclathris, in having globose, black, ascomata with setae and cylindrical to cylindro-clavate asci having knob-like pedicel with muriform, yellowish to dark brown applanate ascospores. Our new species differs from other species of Comoclathris in having superficial, 200–250 × 290–350 μm ascomata () with distinct ostioles, , a peridium comprising a single layer of brown, relatively thick-walled cells of textura angularis, and fusiform to ellipsoidal, muriform, brown to reddish brown ascospores with 2–5-transverse septa and 1–4-longitudinal septa. The phylogenetic analysis of combined ITS, LSU, SSU nrDNA and RPB2 sequence data provides strong evidence that Comoclathris sedi belongs in Pleosporaceae and forms a robust clade within the genus Comoclathris sister to Comoclathris compressa (CBS 157.53 and CBS 156.53) with relatively high bootstrap support (Fig. 1); thus a new species is proposed.
Curvularia Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13(1): 123 (1933)
= Pseudocochliobolus Tsuda, Ueyama & Nishih., Mycologia 69(6): 1117 (1978) [1977]
= Curvusporium Corbetta [as ‘Curvosporium’], Riso 12(3): 28, 30 (1963)
= Malustela Bat. & J.A. Lima, Publções Inst. Micol. Recife 263: 5 (1960)
Facesoffungi number : FoF 00510
Pathogenic or saprobic on wood and dead herbaceous stems or leaves or humans. Sexual morph: Ascomata superficial, globose to ellipsoidal, dark brown to black, free or frequently developing from columnar stromata or flat stromata, with a well defined ostiolar neck. Peridium coriaceous, carbonaceous, pseudoparenchymatous. Hamathecium comprising filiform, septate and sometimes branched pseudoparaphyses,. Asci 1–8-spored, bitunicate, fissitunicate, cylindrical to cylindrical clavate, pedicel short and minute ocular chamber. Ascospores fasciculate, usually parallel, loosely coiled or highly coiled at the extremities of the ascus, filamentous, filiform to flageliform and somewhat tapered at the extremities, 3–20 septate, hyaline or somewhat pigmented at maturity. Asexual morph: Conidiophores branched or unbranched, straight to flexuous, septate, smooth to verruculose, often geniculate sometimes nodulose. Conidiogenous cells polytretic, integrated, sometime when mature becoming intercalary, sympodial, cylindrical with smooth to verrucose conidiogenous nodes. Conidia straight oblong, ellipsoidal, clavate, fusiform, subcylindrical or lunate, rounded at the ends or sometimes tapering slightly towards the base, pale brown, medium reddish brown to dark brown, 3–10 distoseptate (usually 3–5), conidial wall smooth to verrucose. Hilum protuberant in some species. Stromata formed in some species (obtained from Manamgoda et al. 2012).
Type species : Curvularia lunata (Wakker) Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13(1): 127 (1933), Fig. 10.
≡ Acrothecium lunatum Wakker, De ziekten van het suikerriet op Java, die niet door dieren veroorzaakt worden: 196 (1898).
= Helminthosporium curvulum Sacc., Atti della Accademia Scientifica Veneto-Trentino-Istriana 10: 89 (1916).
= Helmisporium curvulum Sacc. (1916).
Facesoffungi number : FoF 00511
Pathogenic or saprobic on wood and dead herbaceous stems or leaves. Sexual morph not observed in culture. Asexual morph: Conidiophores 39–430 × 4–9 μm, branched or unbranched, straight to flexuous, septate, smooth to verruculose, often geniculate sometimes nodulose. Conidiogenous cells 4–20 × 3–13 μm, polytretic, integrated, sometime when mature becoming intercalary, sympodial, cylindrical, cicatrized or sometimes swollen. Conidiogenous nodes smooth to verrucose. Conidia 21–31 × 9–13 μm, straight oblong, ellipsoidal, clavate, fusiform, sub-cylindrical or lunate, rounded at the ends or sometimes tapering slightly towards the base, pale brown, medium reddish brown to dark brown, 3–10 distoseptate (usually 3–5), conidial wall smooth to verrucose.
Material examined: THAILAND, Chiang Rai, on stem of Zea mays, 8 July 2012, D.S Manamgoda (MFLU 10–0555) – extype living culture (MFLUCC 12–0181).
Notes: Curvularia was introduced by Boedijin (1933) and it is an important pathogen in humans and plants (Sivanesan 1987; Agrios 2005, Manamgoda et al. 2012, 2014). Bipolaris and Curvularia share many morphological similarities (Sivanesan 1987). There are some Bipolaris species with short, straight conidia showing intermediate conidial characters between these two genera (Manamgoda et al. 2012). Confusion surrounding Bipolaris and Curvularia was resolved by Manamgoda et al. (2012) based on combined genes of ITS, GPDH, LSU, EF1-α sequence data thus Manamgoda et al. (2012) revealed that the traditionally circumscribed Bipolaris and Curvularia cannot be combined into a single monophyletic genus (Goh et al. 1998; Shimizu et al. 1998; Berbee et al. 1999). A similar phylogeny was found in the present study, where Bipolaris and Curvularia form two well-supported clades in the family Pleosporaceae, thus we accept Curvularia as a separate genus in Pleosporaceae.
Dactuliophora C.L. Leakey, Trans. Br. mycol. Soc. 47(3): 341 (1964).
Facesoffungi number : FoF 00512
Parasitic on leaves. Mycelium generally immersed, hyaline and diffuse in the leaf tissues, aggregated irregularly in the epidermal or deeper leaf tissues into plectenchymic masses from which sclerotiophores and sclerotia develop, ‘sclerotiophore’ remains after the disjunction of the sclerotium as a superficial or occasionally more or less immersed structure, being the external continuation of the immersed aggregation. Hyphae at the circumference are dematiaceous and relatively large while those in the centre of the ring so formed are generally paler and relatively smaller. Sclerotia maybe glaborus, hispidulous, hispid or sparsely setose, spherical to subspherical, ellipsoidal, pyriform or rostrate, wholly or partly composed of dematiaceous cells on the outside, hyaline and undifferentiated, separates from sclerotiophore by the fracture of many thin-walled cells joining the base of the mature sclerotium to the centre of the sclerotiophore. Note: No other structures have been observed other than sclerotia (Leakey 1964).
Type species: Dactuliophora tarrii C.L. Leakey, Trans. Br. mycol. Soc. 47(3): 343 (1964), Fig. 11.
Facesoffungi number : FoF 00513
Parasitic on leaves. Leaf spots broadly zonate on upper leaf surface, rosette-like altering white and brown bands, lower surface densely covered with sclerotiophores and sclerotia, zonation less distinct. Mycelium immersed, hyaline, generally diffused throughout the tissues in the leaf spot and aggregated in plectenchymic masses beneath the sclerotiophores. Sclerotiophores consist of a fully erumpent ring of dematiaceous hyphae continuous with a subepidermal plectenchymic mycelial mass. Sclerotia 35–135 μm diam., scattered, specially on the paler parts of the leaf spot, generally hypophyllous but occasionally amphigenous, spherical to subspherical, bearing 0–10 (usually 3–7) scattered setae or sometimes glabrous, dematiaceous external cells (8–12 μm diam.), internal cells hyaline, undifferentiated. Sclerotial setae 3–4 μm diam., when present more or less cylindrical but sometimes slightly sinuate, slightly attenuated distally to a blunt tip, dematiaceous or hyaline, 0–13 septate (Leakey 1964).
Notes: Dactuliophora was introduced by Leakey (1964) to accommodate four species of Dactuliophora , namely D. elongata C.L. Leakey, D. glycines C.L. Leakey, D. harrisiae C.L. Leakey and D. tarrii C.L. Leakey and typified with D. tarrii (Leakey 1964). The genus is characterised on the basis of the production of a cup-like ‘sclerotiophores’, which are parasitic on several economically important crops (Leakey 1964). Wijayawardene et al. (2014) placed Dactuliophora in Pleosporaceae thus we follow this classification. Currently five Dactuliophora species are listed in Index Fungorum (2015) including D. anuae S.M. Singh., and no molecular data is available for any of these species. Therefore fresh collections of the type species of the genus are needed so that molecular data can be obtained to verify the natural taxonomic affinities of this genus.
Decorospora Inderb et al., in Inderbitzin et al., Mycol. Progr. 1(4): 657 (2002).
Facesoffungi number : FoF 00514
Saprobic on dead wood and maritime plants in marine habitats. Sexual morph: Ascomata subglobose to ellipsoidal, immersed, ostiolate, epapillate or short papillate, carbonaceous, black. Peridium composed of thick-walled cells with large lumina, forming a textura angularis in longitudinal section. Hamathecium composed of septate, branch pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, clavate, short pedicellate, thick-walled, with a clear ocular chamber. Ascospores biseriate, ellipsoidal, muriform, brown, covered by a gelatinous sheath that is slightly constricted around the centre and drawn out at each apex into 2 or rarely 3 subconical extensions. Asexual morph: undetermined.
Decorospora gaudefroyi (Pat.) Inderb. et al., in Inderbitzin et al., Mycol. Progr. 1(4): 657 (2002), Fig. 12
Decorospora gaudefroyi (holotype). a. Ascomata on the host surface. b. Peridium cells from surface view. c. Section of ascoma. d. Peridium. e–f. Branched pseudoparaphyses. g. Young asci amongst pseudoparaphyses. h. Tip of the ascus. Note the ocular chamber. i. Pedicellate ascus base. j–l. Ascospores. k, l Ascospores stained in Indian ink. Note the sheath. Scale bars: a = 300 μm, b = 10 μm, c = 50 μm, d − e = 10 μm, f = 5 μm, g = 30 μm, h − l = 10 μm
Basionym: Pleospora gaudefroyi Pat., Tabl. analyt. Fung. France (Paris) 10: 40 (no. 602) (1886)
= Pleospora salsolae Fuckel var. schoberiae (Sacc.) Sacc., Syll. fung. (Abellini) 2: 248 (1883)
≡ Pleospora schoberiae (Sacc.) Berl., Icon. fung. (Abellini) 2: 23 (1895)
= Pleospora lignicola J. Webster & M. T. Lucas, Trans. Br. mycol. Soc. 44(3): 431 (1961)
= Pleospora salicorniae Jaap, Verh. Bot. Ver. Prov. Brandenburg 49, 16. 1907 (non Pleospora salicorniae P. A. Dang. 1888)
= Pleospora herbarum var. salicorniae Jaap, Annls mycol. 14(1/2): 17 (1916).
Facesoffungi number : FoF 00515
Saprobic on dead wood in marine habitats. Sexual morph: Ascomata perithecioid, small, 200–250 μm in diam., globose to subglobose, immersed to erumpent, becoming superficial on the substrate at maturity, carbonaceous, black, solitary or several clustered, short ostiolate, papillate. Ostiole central, short, brown to black. Peridium thin, composed of thick-walled cells, forming a textura angularis in both surface view and longitudinal section, up to 12 μm. Hamathecium composed of 2 μm wide, hypha-like, cellular, septate, branched pseudoparaphyses anastomosing above the asci. Asci 80–125 × 15–25 μm (\( \overline{x} \) = 104 × 18 μm, n = 10), 8-spored, bitunicate, fissitunicate, clavate, short pedicellate, thick-walled with large lumina, apically rounded with an ocular chamber. Ascospores 20–25 × 7–10 μm (\( \overline{x} \) = 21.2 × 9 μm, n = 10), biseriate, ellipsoidal, muriform, 6–7 transverse septa and 2–3 longitudinal septa in each segment, slightly constricted at the centre transverse septum, brown, covered by a gelatinous thick sheath with 2 or rarely 3 extensions. Asexual morph: undetermined.
Material examined: France, Marais de la Pointe de Touquet, Etaples, on Suaeda maritima (L.) Dumort, 15 Aug. 1879, O. Hariot (PC 0084490, holotype)
Notes: Decorospora gaudefroyi (Pat.) Inderbn et al., is a marine ascomycete firstly described as Pleospora gaudefroyi Pat. from northern France in 1886 and described by Inderbn et al. (2002). Yusoff et al. (1994) described the ascospores of P. gaudefroyi at the ultrastructural level without the unfolded sheath extensions. Based on analysis of partial SSU and ITS ribosomal DNA gene data transferred P. gaudefroyi to a new genus Decorospora, as it did not group in Pleospora. Morphological characters of D. gaudefroyi include black, erumpent ascomata becoming superficial on the substrate at maturity, septate and branched pseudoparaphyses, fissitunicate, clavate asci with thick wall, as well as yellow-brown ascospores with seven transverse and 1–3 longitudinal septa with a gelatinous sheath with a tripartite outer boundary. The characteristic sheath makes these species easy to distinguish from any other marine ascomycetes. D. gaudefroyi was considered to be a synonym of Pleospora herbarum (Fr.) Rabh. which is the type species of Pleospora (Yusoff et al. 1994). However, the presence of the ascospore sheath with its apical extensions and its marine habitat distinguish it from other marine Pleospora species (Inderbn et al. (2002). Decorospora is placed in Pleosporaceae, which is well-supported by partial SSU and ITS ribosomal DNA sequences.
During our phylogenetic analysis, the type strain of Decorospora gaudefroyi (pp4723) forms a robust clade basel to Comoclathris. Furthermore the putative strains of Pleospora sp. ATCC MYA-3203 and Pleospora sp. ATCC MYA-3202 are included. Thus we renamed them as Decorospora sp. in this study.
Diademosa Shoemaker & C.E. Babc., Can. J. Bot. 70(8): 1641 (1992).
Facesoffungi number : FoF 00516
Saprobic on stems and wood. Sexual morph: Ascomata immersed, initially erumpent becoming superficial, scattered, depressed-globose, some flattened at the base, opening a disc-like lid of brown prismatic cells with setae. Peridium composed of brown pseudoparenchyma cells of textura angularis. Hamathecium comprising numerous, septate, hyaline, cellular pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, clavate with short narrow pedicel and minute ocular chamber. Ascospores biseriate, partially overlapping, fusiform, straight, frequently circular in section but narrowing to one end, with transverse and vertical septa, pale brown to dark brown, smooth walled. Asexual morph: undetermined.
Type species: Diademosa californiana (M.E. Barr) Shoemaker & C.E. Babc. [as ‘californianum’], Can. J. Bot. 70(8): 1641 (1992), Fig. 13
Diademosa californiana (holotype) a, b. Ascomata on host substrate.c. Section of ascoma. d. Section of peridium. e. Septate, hyaline, cellular pseudoparaphyses f. Light to dark brown setae. g, h. Ascus with minute pedicel bearing 8 irregularly arranged ascospores. i-k. Ascospores. l. Ascospore stained in Indian ink and showing narrow sheath. Scale bars: d–e = 200 μm, f–h = 10 μm, g–h = 50 μm, i–l = 10 μm
Basionym: Graphyllium californianum M.E. Barr, Mem. N. Y. bot. Gdn 62: 40 (1990).
Facesoffungi number : FoF 00517
Saprobic on stem and wood. Sexual morph: Ascomata 200–365 × 240–425 μm (\( \overline{x} \) = 315 × 275 μm, n = 10), immersed, initially erumpent becoming superficial, scattered, depressed-globose, some flattened at the base, opening a disc-like lid of brown prismatic cells with setae. Peridium 24–59 μm (\( \overline{x} \) = 32 μm, n = 20), composed brown pseudoparenchyma cells of textura angularis. Hamathecium of dense, 2–3 μm diam. (\( \overline{x} \) = 2 μm, n = 20), numerous, septate, hyaline, cellular pseudoparaphyses. Asci 140–175 × 24–28 μm (\( \overline{x} \) = 150 × 26 μm, n = 20), 8-spored, bitunicate, fissitunicate, clavate with short narrow pedicel and minute ocular chamber. Ascospores 50–65 × 26–32 μm (\( \overline{x} \) = 57 × 29 μm, n = 40), biseriate or discontinuously arranged, partially overlapping, fusiform, straight, cylindrical, frequently circular in end view with transverse and vertical septa, muriform, constricted at first septum, pale brown to dark brown, smooth walled with narrow sheath. Asexual morph: undetermined.
Material examined: USA, Bump-Cold Boiling Lake Trail, Lassen Volcanic National Park, Shasta, California, on branch of Wyethia Nutt (Asteraceae), 12 July 1966, W.B. Cooke & D.L. Hawksworth. (NY, holotype).
Notes: Diademosa was established by Shoemaker and Babcock (1992) and typified by D. californiana, based on the ascomatal opening via a circular lid and ascospores being frequently circular in end view. Diademosa californiana was initially introduced as Graphyllium californianum by Barr (1990) and referred to Hysteriaceae based on the pore or slit like opening. Re-examination of the type specimens by Shoemaker and Babcock (1992) concluded that Diademosa opened by a flat lid similar with Diadema and assigned it in to Diademaceae. The lid is hard to observe in sections unless they are mounted directly in lactic acid because excessive swelling occurs in water (Shoemaker and Babcock 1992). Diademosa differs from Comoclathris in having cylindrical ascospores which are frequently circular in section, but narrow of one end, as compared with the flattened ascospores of Comoclathris. Ariyawansa et al. (2014a) placed Diademosa in Pleosporaceae based on its similarities with other genera in this family, but confirmation of the phylogenetic status of this genus depends on recollecting the fungus and epitypification with molecular sequences.
Exserohilum K.J. Leonard & Suggs, Mycologia 66(2): 289 (1974)
= Setosphaeria K.J. Leonard & Suggs, Mycologia 66(2): 294 (1974).
= Luttrellia Khokhr. & Gornostaĭ, in Gornostaĭ in Azbukina et al. (Eds), Vodorosli, Griby i Mkhi Dal’nego Vostoka [Algae, Fungi and Mosses of the Soviet Far-East] (Vladivostok): 80 (1978)
Facesoffungi number : FoF 00518
Saprobic or pathogenic on leaves or wood. Sexual morph: Ascomata superficial, globose or ellipsoid with or without an ostiole. Ostiole surrounded by simple, short, rigid, brown hairs, similar hairs scattered over surface of upper part of ascomata. Peridium coriaceous-carbonaceous, pseudoparenchymous. Pseudoparaphyses filamentous. Asci 1–8-spored, bitunicate, cylindrical to cylindrical-clavate. Ascospores hyaline, fusoid, trans-septate, surrounded by a mucilaginous sheath. Asexual morph: Conidiophores cylindrical, simple, olivaceous brown, upwardly geniculate. Conidia porogenous, acrogenous and pseudopleurogenous, subclylindrical to fusoid or broad obclavate-rostrate, pseudoseptate, olivaceous to brown, hilum protruding from basal cell, germinating by bipolar germ tubes.
Exserohilum turcicum (Pass.) K.J. Leonard & Suggs, Mycologia 66(2): 291 (1974), Fig. 14
≡ Helminthosporium turcicum Pass., Boln Comiz. Agr. Parmense: 3 (1876).
≡ Bipolaris turcica (Pass.) Shoemaker, Canadian Journal of Botany 37 (5): 884 (1959).
≡ Drechslera turcica (Pass.) Subram. & B.L. Jain, Current Science 35 (14): 355 (1966).
≡ Luttrellia turcica (Pass.) Khokhr., Vodorosli, Griby i Mkhi Dal’nego Vostoka: 81 (1978).
= Helminthosporium inconspicuum Cooke & Ellis, Grevillea 6 (39): 88 (1878).
= Trichometasphaeria turcica Luttr., Phytopathology 48(5): 282 (1958).
Facesoffungi number : FoF 00519
Pathogenic on living leaves of Zea mays. Sexual morph: undetermined. Asexual morph: Conidiophores (111-)147–164(−215) × 7.3–8.5(−11) μm (\( \overline{x} \) = 158 × 8.2 μm, n = 20) erect singly or in groups of 2–5, long, straight or flexuous, cylindrical, unbranched, or branched below, 2–5-septate, grayish brown, inner wall layers of conidiogenous cell are continuous with the conidial wall, pale to medium brown. Conidia (41-)112–127 × (17-)22–23(−29) μm (\( \overline{x} \) = 99 × 23 μm, n = 20), ellipsoid-fusiform, widest at the middle and tapering toward ends, long, straight or slightly curved, 2–8-distoseptate, some euseptate, grayish brown, thick-walled, smooth, scars thickened.
Material examined: ZAMBIA, Katete, on living leaves of Zea mays (Poaceae), 9 February 1957, A. Angus, NY (Ex herbarium: IMI 69726).
Notes: The genus Exserohilum was proposed by Leonard and Suggs (1974) to accommodate species which were previously classified in Bipolaris, in which the conidial hilum was distinctly protuberant. In the same study, Leonard and Suggs (1974) introduced a new genus Setosphaeria to place the sexual morphs of Exserohilum, which is segregated from Keissleriella on the basis of lack of a clypeus, lysigenous development of the ostiole, occurrence of setae on the perithecial wall, the absence of periphyses in the ostiole and the hyphomycetous conidial states. As a genus can now only have one name, Wijayawardene et al. (2014) proposed to conserved Exserohilum over Setosphaeria on the basis having of more epithets (Index Fungorum 2015), and is more widely use in the literature.
During our phylogenetic study putative strains of Exserohilum monoceras (= Setosphaeria monoceras CBS 154.26), Exserohilum sp. (C950801) and Exserohilum sp. (NK93) formed a well-supported clade sister to Curvularia. Therefore based on phylogeny and morphology we accept Exserohilum as a separate genus in the family Pleosporaceae.
Extrawettsteinina M.E. Barr, Contr. Univ. Mich. Herb. 9(8): 538 (1972).
Facesoffungi number : FoF 00520
Saprobic on dead leaves and wood. Sexual morph: Ascomata scattered, erumpent to superficial, globose or conical, dark brown to black, carbonaceous. Papilla black, with a pore-like ostioles, ostiolar canal filled with periphyses. Peridium a single layer composed of compressed, thick-walled cells, fusing with the host at the outside. Hamathecium of dense broad, cellular pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, apex rounded with and a minute ocular chamber with short pedicel. Ascospores uniseriate, slightly overlapping, ellipsoid, obovate-clavate, 3-septate, slightly to not constricted at the septum, wall smooth. Asexual morph: undetermined.
Extrawettsteinina minuta M.E. Barr, Contr. Univ. Mich. Herb. 9(8): 538 (1972), Fig. 15
Extrawettsteinina minuta (NY, 4278). a, b. Herbarium material. c. Fruiting bodies on the host. d, e. Sections of ascomata. f. Cellular pseudoparaphyses. g. Ascus with short pedicel. h-k. Ellipsoid, obovate-clavate, 3-septate ascospores. Scale bars: d = 80 μm, e = 50 μm, f = 10 μm, g = 30 μm, h–k = 5 μm
≡ Kriegeriella minuta (M.E. Barr) Arx & E. Müll., Stud. Mycol. 9: 88 (1975)
Facesoffungi number : FoF 00521
Saprobic on dead leaves of Juniperus communis. Sexual morph: Ascomata 100–150 × 210–350 μm (\( \overline{x} \) = 120 × 280 μm, n = 10), scattered, erumpent to superficial, globose or conical, dark brown to black, carbonaceous. Papilla black, with a pore-like ostioles, ostiolar canal filled with periphyses. Peridium 10–25 μm (\( \overline{x} \) = 21 μm, n = 10), a single layer composed of compressed, thick-walled cells, fusing with the host at the outside. Hamathecium of dense 1–1.5 μm (\( \overline{x} \) = 1.25 μm, n = 20), broad, cellular pseudoparaphyses. Asci 75–90 × 6–8 μm (\( \overline{x} \) = 80 × 7 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical, apex rounded with and a minute ocular chamber with short pedicel. Ascospores 10–12 × 4–5 μm (\( \overline{x} \) = 11.5 × 4 μm, n = 10), uniseriate, slightly overlapping, ellipsoid, obovate-clavate, 3-septate, slightly to not constricted at the septum, wall smooth. Asexual morph: undetermined.
Material examined: USA, Vermont, Stow Pinnacle trail, on dead leaves of Juniperus communis L (Cupressaceae), 11 July 1964, H.E and M.E Bigelow (NY, 4278).
Notes: During our study we observed the holotype of Extrawettsteinina minuta (NY, 01103003) collected by H. E. Bigelow (19 August 1957) from Juniperus communis but we could not find any ascomata in the type material. Therefore we examined another collection of E. minuta (NY, 4278), which was collected from same host and by the same collector as present in Fig. 15.
Extrawettsteinina was introduced by Barr (1972) and typified by E. minuta. The genus was introduced to accommodate three species, i.e. E. minuta, E. pinastri M.E. Barr and E. mediterranea (E. Müll.) M.E. Barr, which are saprobic on the dead leaves of gymnosperms and angiosperms, in North America and Europe (Barr 1972). Subsequently, a fourth species was introduced, viz. E. andromedae (Auersw.) M.E. Barr (based on Wettsteinina andromedae) by Barr (1987a). Extrawettsteinina is characterized by superficial, conical ascomata, containing a few saccate bitunicate asci and ellipsoidal, obovate-clavate, septate, smooth and hyaline ascospores which turn dull brown at maturity (Barr 1972). The diagnostic character of Extrawettsteinina is its conic ascomata, which are superficial on the substrate, and radiating arrangement of wall cells, which makes it distinguishable from comparable genera such as Stomatogene and Wettsteinina. Morphologically, Extrawettsteinina is comparable with Kriegeriella. In particularly, E. pinastri could not be distinguished from K. transiens or K. mirabilis. Thus, K. transiens, including Extrawettsteinina pinastri, was treated as synonyms of K. mirabilis, and was included in the section of Kriegeriella (von Arx and Müller 1975; Barr 1975). The other section of Kriegeriella, Extrawettsteinina, includes two previous Kriegeriella species, i.e. K. minuta and K. mediterranea. Barr (1987b) introduced a family, Kriegeriellaceae (Dothideales) to accommodate Kriegeriella and Extrawettsteinina. This proposal has rarely been followed.
In this study we tentatively refer Extrawettsteinina in Pleosporaceae following Zhang et al. (2012) and Lumbsch and Huhndorf (2010). Currently no molecular data is available for this genus therefore generic type needs to be collected, isolated and sequenced to show the natural classification of this genus.
Gibbago E.G. Simmons, Mycotaxon 27: 108 (1986)
Facesoffungi number : FoF 00522
Saprobic or pathogenic on leaves and wood. Sexual morph: undetermined. Asexual morph: Conidiophores solitary or 2–4 loosely fasciculate, erect, rarely distantly branched, simple with a single apical conidiogenous locus, then often proliferating by means of a secondary conidiophore that arises immediately below the apical cell of the existing conidiophore, septate, slightly pigmented. Conidia initially solitary, ellipsoid, beakless, pigmented, becoming transversely and longitudinal septate, with apical cells swelling slightly and producing secondary conidia similar to the initial ones (Simmons 1986).
Type species: Gibbago trianthemae E.G. Simmons, Mycotaxon 27: 108 (1986), Fig. 16
Facesoffungi number : FoF 00523
Notes: Gibbago was introduced by Simmons (1986) and typified by G. trianthemae. The genus is characterised by erect, simple with a single apical conidiogenous locus, septate, slightly pigmented, having solitary conidiophores with ellipsoid, beakless conidia and producing secondary conidia. Gibbago was assigned to Pleosporaceae by Simmons (1986) based on its similarities with Alternaria, Embellisia, Ulocladium and Stemphylium and this was followed by Wijayawardene et al. (2014). During our phylogenetic analysis, the putative strains of Gibbago trianthemae GT-VM and NFCCI 1886 forms a distinct clade basel to Paradendryphiella and Pleospora clades with low boostrap support. Therefore based on morphology coupled with molecular data, we tentatively refer Gibbago in Pleosporaceae, but this needs to be confirmed with further sequences of the monotypic genus and type species G. trianthemae.
Johnalcornia Y.P. Tan & R.G. Shivas, in Tan et al., Australas. Pl. Path.: 10.1007/s13313-014-0315-6 (2014).
Facesoffungi number : FoF 00524
Type species: Johnalcornia aberrans (Alcorn) Y.P. Tan & R.G. Shivas, in Tan et al., Australas. Pl. Path.: 10.1007/s13313-014-0315-6 (2014), Fig. 17
≡ Bipolaris aberrans Alcorn, Mycotaxon 39: 364 (1990)
≡ Cochliobolus aberrans Alcorn, Mycotaxon 39: 362 (1990)
Facesoffungi number : FoF 00525
Parasitic on leaves. Sexual morph: Ascomata globose with a short neck. Asci cylindrical to fusoid, straight or curved. Ascospores hyaline, filiform, tightly coiled. Asexual morph: Conidiophores solitary or in fascicles, simple or branched, straight to flexuous, apically geniculate, cylindrical, smooth. Conidiogenous cells integrated, cylindrical, proliferating sympodially, smooth to verruculose, with thickened and conspicuous scars. Conidia solitary, straight to curved, smooth, hilum inconspicuous, distoseptate, germinating from both polar cells, first conidial septum median or submedian, second conidial septum delimiting the apical cell, third septum basal (Description obtained from Tan et al. 2014).
Notes: Johnalcornia was introduced by Tan et al. (2014) to accommodate Bipolaris aberrans and is monotypic. Combined gene analysis of EF-1α, GAPDH, ITS and LSU has shown that J. aberrans forms a sister clade to the newly described genus Porocercospora. Johnalcornia differs from Porocercospora in having distinctive conidia-like chlamydospores as well as comparatively thick-walled, geniculate conidiophores, with conidiogenous cells that have conspicuous scars. Johnalcornia further differs from related genera by forming the second conidial septum in the apical cell. In our phylogenetic analysis we also obtain the similar results where the type strain of J. aberrans forms a distinct clade sister to Porocercospora thus we accept Johnalcornia as a separate genus in the family Pleosporaceae.
Marielliottia Shoemaker, Can. J. Bot. 76(9): 1559 (1999) [1998].
Facesoffungi number : FoF 00526
Pathogenic on cereals, grasses and seeds or saprobic on wood and leaves. Sexual morph: undetermined. Asexual morph: Conidiophores solitary to clustered, erect, septate, straight, brown, closely geniculate from sympodial growth or distantly nodose, apex truncate or inflated, forming conidia through a terminal pore, with flat, non-protruding scars, smooth or sometimes slightly roughened. Conidiogenous cells tretic, integrated, terminal, sympodially proliferating, cylindrical or swollen, cicatrized or not cicatrized. One species additionally produces massive, subulate macroconidiophores radiating from a pseudoparenchymatic base. Conidia porogenous, first septum formed near base, transversely 3-septate, exceptionally 2-septate when immature in one species and sometimes up to 5-septate in one species, brown, straight, ovoid or obovoid; scar pore-like, basal, dark brown, not protruding, set off by a hyaline zone, atrium type (Alcorn 1983) or flat in a slightly papillate base. Germ tubes usually two from the basal cell, rarely one from the apex, but never from the central cells (Description obtained from Shoemaker 1998).
Type species: Marielliottia biseptata (Sacc. & Roum.) Shoemaker, Can. J. Bot. 76(9): 1560 (1999) [1998], Fig. 18
≡ Helminthosporium biseptatum Sacc. & Roum., Revue Mycol. (Toulouse): 56 (1881).
≡ Brachysporium biseptatum (Sacc. & Roum.) Sacc., Sylloge Fungorum 4: 428 (1886).
≡ Drechslera biseptata (Sacc. & Roum.) M.J. Richardson & E.M. Fraser, Transactions of the British Mycological Society 51 (1): 148 (1968).
≡ Pyrenophora biseptata (Sacc. & Roum.) Crous, CBS Biodiversity Series 13: 257 (2013)
= Helminthosporium biforme E.W. Mason & S. Hughes, Transactions of the British Mycological Society 30: 114 (1948).
= Drechslera biforme (E.W. Mason & S. Hughes) Subram. & B.L. Jain (1966).
Facesoffungi number : FoF 00527
Saprobic on stems. Sexual morph: undetermined. Asexual morph: Conidiophores two types. Macronematous conidiophores from stroma, single to many in a cluster, erect to divergent, long, broad at base, gradually narrowed towards apex, closely septate at intervals of 10–18 mm, dark chocolate brown; sporogenous area gnarled, frequently bent, with numerous dark brown scars, apex truncate, bearing an “ear” of conidia, 100–800 × 10–15 μm (at base), 9–11 μm wide near middle, 6–8 μm wide near apex. Conidiogenous cells tretic, integrated, terminal, sympodially proliferating, cylindrical, cicatrized, stromata shallow ellipsoidal to depressed globose, 100–250 × 100–150 μm. Micronematous conidiophores arising from hyaline to pale yellow hyphae (in culture), slender at base, sparingly septate, short, erect, sporogenous area gnarled, densely scarred, dark chocolate brown, usually simple, rarely branched, 15–105 × 5– 8 μm. Conidiogenous cells tretic, integrated, terminal, sympodially proliferating, cylindrical, cicatrized. Conidia obovoid, 3-septate, uppermost septum inconspicuous or lacking in some, rarely constricted at septa, thick walled, light to dark olive brown except lighter basal cell, narrowed to base, bearing a broad black nonprotruding scar 3–4.5 μm wide, (21–)32–45(−49) × (9–)11–13(−17) μm. Germination by one or two (three) oblique tubes near scar, rarely one hypha from apex and very rarely through the scar (Shoemaker 1998).
Notes: Marielliottia was introduced by Shoemaker (1998) to accommodate three species formerly treated in Drechslera namely D. biseptata (Sacc. & Roum.) M.J. Richardson & E.M. Fraser, D. dematioidea (Bubák & Wróbl.) Scharif and D. triseptata (Drechsler) Subram. & B.L. Jain. The genus is typified with Marielliottia biseptata. The species differ from Drechslera in having mostly three-septate, obovoid to ovoid conidia that germinate primarily from the basal cell, occasionally from the apical cell, and not from the central cells (Shoemaker 1998). These fungi are occasional parasites of cereals and grasses, are sometimes seed borne, and may infect non-grass plants (Shoemaker 1998).
Currently no sequence data is available for Marielliottia species in GenBank. Therefore, fresh collections of the type species of the genus are needed so that molecular data can be obtained to verify the natural taxonomic affinities of this genus. Based on the morphological similarities with Drechslera, we tentatively classified Marielliottia in Pleosporaceae.
Neocamarosporium Crous & M.J. Wingf., Persoonia 32: 273 (2014).
Facesoffungi number : FoF 00528
Pathogenic or saprobic on dying leaves and stems. Sexual morph: undetermined. Asexual morph: Conidiomata brown to black, immersed, becoming erumpent, globose with papillate apex and central ostiole; wall of 3–6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner layer of conidioma, separate, hyaline, smooth, ampulliform; proliferating several times percurrently near apex, or at the same level, giving rise to prominent periclinal thickening. Conidia solitary, initially hyaline, aseptate, thick-walled, developing a central septum and then becoming muriformly septate, shape variable from globose to obovoid to ellipsoid, golden brown, finely roughened, thick-walled (Description obtained from Crous et al. 2014).
Type species: Neocamarosporium goegapense Crous & M.J. Wingf., Persoonia, Mol. Phyl. Evol. Fungi 32: 273 (2014), Fig. 19
Neocamarosporium goegapense (Redrawn from the holotype of Neocamarosporium goegapense in Crous et al. 2014). Scale bars: 10 μm
Facesoffungi number : FoF 0529
Pathogenic on dying leaves of Mesembryanthemum sp. (Aizoaceae). Sexual morph: undetermined. Asexual morph: Conidiomata 300 μm diam, brown to black, immersed, becoming erumpent, globose with papillate apex and central ostiole. Conidiomata wall consists of 3–6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 7–9 × 5–6 μm, lining the inner layer of conidioma, separate, hyaline, smooth, ampulliform, proliferating several times percurrently near apex, or at the same level, giving rise to prominent periclinal thickening. Conidia (15–) 20–22(−24) × 15–17(−19) μm, solitary, initially hyaline, aseptate, thick-walled, developing a central septum and then becoming muriformly septate, shape variable from globose to obovoid to ellipsoid, golden brown, finely roughened, thick-walled. (Description obtained from Crous et al. 2014)
Notes: Neocamarosporium was introduced by Crous et al. (2014) and typified with Neocamarosporium goegapense. Neocamarosporium is morphologically similar to Camarosporellum, though the latter appears to have holoblastic conidiogenesis. Based on a megablast search of NCBIs GenBank nucleotide database of LSU and ITS sequences, Crous et al. (2014) referred Neocamarosporium to Pleosporaceae. Crous et al. (2014) also suggested that phylogenetically, Neocamarosporium is allied to a clade containing taxa accommodated in Phoma, Chaetosphaeronema and Pleospora, but morphologically quite distinct. In our phylogenetic analysis we observed that the type strain of Neocamarosporium goegapense (CBS 138008) forms a robust clade sister to the Clathrospora and Decorospora clades. Furthermore, putative strains of Phoma betae (CBS 109410), Pleospora betae (IMI 156653) and Pleospora bjoerlingii (ICMP10945 reside within the Neocamarosporium clade. de Gruyter et al. (2013) reported Phoma betae (CBS 109410) as the asexual state of Pleospora betae (IMI 156653), thus they synonymised Phoma betae under Pleospora betae. Furthermore based on the morphological and DNA data they synonymised Pleospora bjoerlingii under Pleospora betae. Therefore in this study we introduce Neocamarosporium betae to accommodate these species in genus Neocamarosporium.
Neocamarosporium betae (Berl.) Ariyawansa & K. D. Hyde, comb. nov.,
Index Fungorum number: IF 550956
Basionym: Pyrenophora echinella var. betae Berl., Nuovo Giorn.Bot. Ital. 20: 208. 1888.
≡ Pleospora betae (Berl.) Nevod., Grib. ross. Exs., No. 247. 1915.
= Pleospora betae Björl., Bot. Not. 1944: 218. 1944. (later homonym), nom.
illeg.
≡ Pleospora bjoerlingii Byford, Trans. Brit. Mycol. Soc. 46: 614. 1963.
= Phoma betae A.B... Frank, Z. Rúbenzucker-Ind. 42: 904, tab. 20. 1892.
= Phyllosticta betae Oudem., Ned. Kruidk. Arch. Ser. 2, 2: 181. 1877.
= Gloeosporium betae Dearn. & E.T. Barthol., Mycologia 9: 356. 1917.
During our study the putative strains of Pleospora chenopodii (CBS 344.78), P. calvescens (CBS 246.79) and P. halimiones (CBS 432.77) also forms a separate clade basel to the type species of Neocamarosporium, N. goegapense. de Gruyter et al. (2013) treated these species as separate species but our phylogeny clearly show that these three species can be treated a one species thus we propose Neocamarosporium calvescens to accommodate Pleospora chenopodii, P. calvescens and P. halimiones.
Neocamarosporium calvescens (Fr. ex Desm.) Ariyawansa & K.D. Hyde, comb. nov.,
Index Fungorum number: IF 550957
Basionym: Sphaeria calvescens Fr. ex Desm., Ann. Sci. Nat., Bot., 2e Sér., 19: 353. 1843.
≡ Pleospora calvescens (Fr. ex Desm.) Tul. & C. Tul., Selecta Fung. Carpol. 2: 266. 1863.
≡ Pyrenophora calvescens (Fr. ex Desm.) Sacc., Syll. Fung. 2: 279. 1883.
≡ Chaetoplea calvescens (Fr. ex Desm.) Clem., Gen. Fung., 2nd Ed.: 275. 1931.
≡ Leptosphaeria calvescens (Fr. ex Desm.) Crivelli, Ueber Heterog. Ascomycet. Pleospora: 177. 1983.
= Phloeospora chenopodii Ellis & Kellerm., J. Mycol. 4: 26. 1888, [as “Phleospora”].
≡ Pleospora chenopodii (Ellis & Kellerm.) Gruyter & Redhead, Index Fung. 205: 1. 2014.
= Pleospora halimiones Gruyter & Verkley, Stud. Mycol. 75: 25. 2012.
Paradendryphiella Woudenberg & Crous, Stud. Mycol. 75(1): 207 (2013)
Facesoffungi number : FoF 00530
Saprobic or parasitic in terrestrial and marine habitats. Sexual morph: undetermined. Asexual morph: Conidiophores simple or branched, septate or not, straight or flexuous, often nodose with conspicuous, subhyaline, brown pigmentation at the apical region; at times reduced to conidiogenous cells. Conidiogenous cells terminal or lateral, with denticles aggregated at apex, with prominent conidial scars, thickened but not darkened; sometimes proliferating with a new head or a short, inconspicuous sympodial rachis. Conidia produced holoblastically, on narrow denticle, smooth, cylindrical to obclavate, straight or slightly flexuous, 1–7 transverse septa, pale to medium brown, often with dark septa (often constricted), and a darkened zone of pigmentation at the apex, and at the hilum, which is thickened, and somewhat protruding, with a minute marginal frill (Description obtained from Woudenberg et al. 2013).
Type species: Paradendryphiella salina (G.K. Sutherl.) Woudenberg & Crous, Stud. Mycol. 75(1): 207 (2013), Fig. 20
≡ Cercospora salina G.K. Sutherl., New Phytol. 15: 43 (1916).
≡ Dendryphiella salina (G.K. Sutherl.) Pugh & Nicot, Transactions of the British Mycological Society 47 (2): 266 (1964).
≡ Scolecobasidium salinum (G.K. Sutherl.) M.B. Ellis, More dematiaceous Hyphomycetes: 192 (1976).
Facesoffungi number : FoF 0531
Saprobic or parasitic in marine habitats. Sexual morph: undetermined. Asexual morph: Conidiophores 30–40 × 2–4 μm, simple or branched, septate or not, straight or flexuous, often nodose with conspicuous, brown pigmentation at the apical region; at times reduced to conidiogenous cells. Conidiogenous cells terminal or lateral, with denticles aggregated at apex, with prominent conidial scars, thickened but not darkened; sometimes proliferating with a new head or a short, inconspicuous sympodial rachis. Conidia 16–65 × 5–9 μm, produced holoblastically, on narrow denticle, smooth-walled, cylindrical to obclavate, straight or slightly flexuous, 1–7 transverse septa, pale to medium brown, often with dark septa (often constricted), and a darkened zone of pigmentation at the apex, and at the hilum, which is thickened, and somewhat protruding, with a minute marginal frill (Description of Scolecobasidium salinum obtained from Ellis 1976).
Notes: Paradendryphiella was introduced by Woudenberg et al. (2013) to accommodate two species Dendryphiella arenariae and D. salina (= Cercospora salina). Paradendryphiella is characterised by simple or branched conidiophores with a new head or a short, inconspicuous sympodial rachis conidiogenous cells and holoblastically produced cylindrical to obclavate, straight or slightly flexuous, 1–7 transverse septa, pale to medium brown conidia. The phylogenetic trees show that Paradendryphiella nested within the family Pleosporaceae and clustered sister to Pleospora senso stricto clade in Jones et al. (2008), Woudenberg et al. (2013) and as well as in this study. Therefore we accept Paradendryphiella as separate genus in the family Pleosporaceae.
Platysporoides (Wehm.) Shoemaker & C.E. Babc., Can. J. Bot. 70(8): 1648 (1992).
Facesoffungi number : FoF 00532
Saprobic in terrestrial habitats. Sexual morph: Ascomata small, scattered, immersed, semi-immersed to nearly superficial, globose, subglobose, black, smooth; apex with a protruding papilla and pore-like ostiole, without periphyses. Peridium thin, composed of a few layers of textura angularis. Hamathecium of numerous, cellular pseudoparaphyses, anastomosing above the asci, septate. Asci bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel and minute ocular chamber. Ascospores broadly ellipsoid, reddish brown, muriform. Asexual morph: undetermined
Type species: Platysporoides chartarum (Fuckel) Shoemaker & C.E. Babc., Can. J. Bot. 70(8): 1650 (1992), Fig. 21
Platysporoides chartarum (holotype). a, b. Specimen and herbarium material. c. Ascomata on substrate. d. Section through ascoma. e. Upper walled cells of ascoma. f. Peridium. g. Ascus when immature in Melzer’s reagent. h. Ascus when immature in Cotton Blue reagent. i. Ascus at maturity. j. Hamathecium in Cotton Blue reagent. k. Ocular chamber in Melzer’s reagent. l. Ascospores at maturity. m, n. Ascospore when immature with Sheath. o. Ascospore when immature in Cotton Blue reagent. p. Ascospore at maturity with sheath in India Ink. Scale bars: d = 40 μm, e–f = 10 μm, g–i = 100 μm, j = 10 μm, k–p = 5 μm
Basionym: Pleospora chartarum Fuckel, Jb. nassau. Ver. Naturk. 23–24: 133 (1870) [1869–70]
≡ Clathrospora chartarum (Fuckel) O.E. Erikss., Arkiv før Botanik 6 (4–5): 352 (1967)
Facesoffungi number : FoF 00533
Saprobic in terrestrial habitats. Sexual morph: Ascomata 150–230 μm × 180–260 μm, scattered, immersed, semi-immersed to rarely superficial, globose, subglobose, black, smooth; apex with a protruding papilla, ostiolate. Ostiole comprises apex with a protruding papilla and without periphyses. Peridium 8–22 μm wide, composed of 2–4 layers of brown cells of textura angularis, cells 5–9 μm diam., cell wall 1–2.5 μm thick. Hamathecium of 2–3 μm broad, long cellular pseudoparaphyses, anastomosing above the asci, septate. Asci 110–140 × 12.5–16.5 μm (\( \overline{x} \) = 121:5 × 14:7 mm, n = 10), (6-)8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel, 8–17 μm long,and minute ocular chamber. Ascospores 20–26 × 8–11 μm (\( \overline{x} \) = 23:7 × 9 μm, n = 10), obliquely uniseriate and partially overlapping, flattened, broadly ellipsoid in front view, reddish brown, muriform, 3 transverse septa, one longitudinal septum in each central cell, 1 oblique septum in each end cell, constricted at all septa, granulate, with a 2–3 μm wide mucilaginous sheath. Asexual morph: undetermined.
Material examined: GERMANY, Rhineland-Palatinate, on dead leaves, 1894, Herbier Barby-Boissier (HUH 00290381, holotype).
Notes: Platysporoides was introduced as a subgenus of Pleospora by Wehmeyer (1961) and was typified by Pleospora chartarum. Shoemaker and Babcock (1992) raised Platysporoides to generic rank and placed it in the Pleosporaceae based on its “applanodictyospore” and “terete pored beak of the ascomata”. Currently, eleven species are included in this genus (Shoemaker and Babcock 1992). Another comparable pleosporalean family is Diademaceae, which is distinguished from Platysporoides by its ascoma opening as “an intraepidermal discoid lid” (Shoemaker and Babcock 1992). Aigialus grandis is another pleosporalean fungus with flattened and muriform ascospores as well as papilla and ostioles, which belongs to Aigialaceae, a phylogenetically well supported marine family (Suetrong et al. 2009). Thus, it is highly likely that flattened and muriform ascospores have evolved on separate occasions and not taxonomically informative.
Currently no molecular data is available for this genus and therefore it is essential to epitypify the generic type with fresh collections. Therefore molecular data can be used to resolve the correct natural classification of Platysporoides. We tentatively place Platysporoides in Pleosporaceae based on its similarities with the other genera of this family, such as, in having globose to subglobose ascomata with pore-like ostiole, a peridium composed of a few cell layers of textura angularis, cellular pseudoparaphyses, fissitunicate, cylindrical to cylindro-clavate asci with broadly ellipsoid, reddish brown, muriform ascospores.
Pleospora Rabenh. ex Ces. & De Not., Comm. Soc. crittog. Ital. 1(4): 217 (1863)
= Stemphylium Wallr., Fl. crypt. Germ. (Norimbergae) 2: 300 (1833)
= Cleistotheca Zukal, Österreichische Botanische Zeitschrift 53 (5): 163 (1893)
= Cleistothecopsis Stevens & True, Illinois Agric. Exper. Stat. Bull. Nr. 220: 507 (1919).
Facesoffungi number : FoF 00534
Habitat terrestrial or aquatic, saprobic or parasitic on stems and leaves. Sexual morph: Ascomata small to medium-sized, immersed, erumpent to superficial, base not easy to remove from the substrate, broadly to narrowly oblong and flattened, ostiolate. Ostiole papillate, black, smooth, ostiolar canal filled with hyaline cells. Peridium thin, usually with two layers, thick at the sides and thinner at the base, outer layer composed of heavily pigmented, thick-walled cells of textura angularis, inner layer composed of hyaline thin-walled cells of textura angularis, coriaceous. Hamathecium of cellular, hyaline, septate, broad, dense pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical to clavate, with furcate pedicel and minute ocular chamber. Ascospores uniseriate or partially overlapping, mostly ellipsoidal, muriform, brown or pale brown, with or without sheath. Asexual morph: Stemphylium. Mycelium immersed or nearly superficial, brown. Stroma sometimes present. Conidiophores macronematous, mononematous, scattered or caespitose, unbranched or rarely loosely branched, straight or flexuous, usually nodose with a number of vesicular swellings, pale to mid brown or olivaceous brown, smooth or in part verruculose. Conidiogenous cells monoblastic, integrated, terminal, percurrent, at first clavate or subsphearical with the wall at the apex thin. Conidia solitary, dry, acrogenous, oblong, rounded at the ends, ellipsoidal, obclavate or subsphearical, pale to mid dark or olivaceous brown, smooth, verrucose or echinulate, muriform, often constricted at one or more of the septa, cicatrized at the base. Colonies effuse, grey, brown, olivaceous brown or black, velvety or cottony (Ellis 1971).
Type species: Pleospora herbarum (Pers.) Rabenh., Klotzschii Herb. Viv. Mycol. 2: no. 547 (1854), Fig. 22
Pleospora herbarum (MFLU12-2216). a. Ascomata on host substrate. b. Close up of ascoma .c . Section of ascoma. d. Close up of the peridium. e. Cellular, hyaline, septate, broad pseudoparaphyses. f–h. Asci with short, broad pedicel bearing 8 ascospores i–l. Mature and immature ascospores with mucilaginous sheath. Stemphylium sp. m–p. Conidiophores with pale to mid dark or olivaceous brown, smooth conidia. Scale bars: c = 100 μm, d = 50 μm, e = 20 μm, f–g = 60 μm, i–k = 10 μm, l–o = 10 μm
≡ Sphaeria herbarum Pers., Syn. meth. fung. (Göttingen) 1: 78 (1801)
Facesoffungi number : FoF 00535
Habitat terrestrial, marine, saprobic or parasitic on stems and leaves. Sexual morph: Ascomata 110–240 × 250–460 μm small to medium-sized, immersed, erumpent to superficial, base not easy to remove from the substrate, broadly to narrowly oblong and flattened, ostiolate. Ostiole papillate, black, smooth, ostiolar canal filled with hyaline cells. Peridium 30–50 μm (\( \overline{x} \) = 42 μm, n = 10) thin, usually with two layers, thick at the sides and thinner at the base, outer layer of heavily pigmented thick-walled cells of textura angularis, inner layer composed of hyaline thin-walled cells of textura angularis, coriaceous. Hamathecium of 2–3 μm (\( \overline{x} \) = 2.5 μm, n = 10) cellular, hyaline, septate, broad, dense pseudoparaphyses. Asci 100–225 × 25–30 μm (\( \overline{x} \) = 142 × 28 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to clavate, with furcate pedicel and minute ocular chamber. Ascospores 25–40 × 12–15 μm (\( \overline{x} \) = 33 × 14 μm, n = 40), uniseriate or partially overlapping, mostly ellipsoidal, muriform, brown or pale brown, with or without sheath. Asexual morph: Stemphylium. Conidiophores 4–6 μm (\( \overline{x} \) = 5 μm, n = 10) macronematous, mononematous, scattered or caespitose, unbranched or rarely loosely branched, straight or flexuous, usually nodose with a number of vesicular swellings, pale to mid brown or olivaceous brown, smooth or in part verruculose. Conidiogenous cells monoblastic, integrated, terminal, percurrent, at first clavate or subsphearical with the wall at the apex thin. Conidia 16–22 × 11–18 μm (\( \overline{x} \) = 21 × 16 μm, n = 20) solitary, dry, acrogenous, oblong, rounded at the ends, ellipsoidal, obclavate or subspherical, pale to mid dark or olivaceous-brown, smooth, verrucose or echinulate, muriform, often constricted at one or more of the septa, cicatrized at the base. Colonies effuse, grey, brown, olivaceous-brown or black, velvety or cottony.
Material examined: ITALY, Forlì-Cesena, Montevescovo, on dead stem of Brassica nigra (Brassicaceae), 01 January 2012, E. Camporesi IT 82 (MFLU 12–2216) – living culture (MFLUCC 14–0261); ITALY, Tessello - Cesena, on dead stem of Cirsium sp (Asteraceae), 16 December 2013, E. Camporesi IT 956 (MFLU 14–0762) – living culture (MFLUCC 13–0344); ITALY, Massera - Predappio, on dead fruits of Lunaria rediviva (Brassicaceae), 16 December 2013, E. Camporesi IT 958 (MFLU 14–0763) – living culture (MFLUCC 13–0266, ICMP)
Notes: Pleospora was originally described by Rabenhorst (1857) and is typified by Pleospora herbarum (Pers.) Rabenh., (Kirk et al. 2008). Initially the genus was included in Sphaeriales and later Pseudosphaeriales and Pleosporales, respectively (Wehmeyer 1961). All species belonging to Pleospora have muriform ascospores (Wehmeyer 1961, 1975). Pseudoparaphyses arrangement (downward growing) within the ascomata of “Pleospora-type” development (Luttrell 1951) is considered to be the main feature for the genus. Various authors had included and excluded different species in Pleospora at various times. Barr (1981) placed Curreya in Pleospora however, von Arx and van der Aa (1983) treated it as separate genus, because of its Coniothyrium asexual morph. Petrak (1952) transferred Graphyllium to Pleospora, and noted that the elongate ascomata and closely grouped rows of small ascomata are not sufficient to recognize the genus. Barr (1987b, 1990b) supported this proposal. Due to the heterogenous nature of Pleospora several subgenera have been included. i.e. Teichosporoides contains species of Pleospora with immersed ascomata, and Pleosphaeria with superficial and setose ascomata (Wehmeyer 1961). Pleospora species have a wide host range, especially on monocotyledons as well as dicotyledonous plants (Wehmeyer 1975).
There are numerous species of Dothideomycetes that have muriform ascospores and have at one time or another been placed in Pleospora (Wehmeyer 1961). Pleospora is however, rather distinctive and should be confined to species with characters that are similar to Pleospora herbarum. The ascomata in P. herbarum are immersed and usually become erumpent, the peridium is unusual in having three strata, a thin inner layer of thin-walled, hyaline to light brown flattened cells, a relatively wide central layer of thin-walled, hyaline to light brown angular cells, and an outer very thin black amorphous cells, which gives the blackened colour to the ascomata. The asci are broadly clavate and have a distinctive, squarish, wide, ocular chamber and ascospores are muriform with at least three longitudinal septa per transverse row, and surrounded by a mucilaginous sheath. The asexual morph is Stemphyllium (Zhang et al. 2012; Hyde et al. 2013). For this reason several Pleospora-like species are now transferred to other genera (e.g. Curreya).
Based on our phylogenetic assessment we proposed to confine Pleospora to one well-supported clade based on the type strains of P. herbarum (CBS 191.86) along with the other alternative strains of P. herbarum used in this study (P. herbarum MFLUCC 13–0344, P. herbarum MFLUCC 13–0266 and P. herbarum (MFLUCC 14–0261), sister to Paradendryphiella clade applying GCPSR (Taylor et al. 2000; Dettman et al. 2003). By implementing this, we treat some putative strains, such as P. tomatonis (CBS109844), and Pleospora sedicola (CBS109843) as alternative strains of P. herbarum. Furthermore we treated the putative strains of Pleospora papaveracea (CBS432.50), Pleospora herbarum (DAOM195299), Pleospora tarda (CBS 714.68) and Pleospora halophila (CBS410.73) as separate species which clustered outside the P. herbarum sensu stricto.
Wijayawardene et al. (2014) proposed to conserve Stemphyllium over Pleospora because Stemphyllium has priority as it is an older name, is better established in the literature than Pleospora (more than double the number of hits on Google Scholar), and is a well-known genus to the plant pathology community, and is well circumscribed on a molecular basis (Lawrence et al. 2012). In this study the first author prefers to keep Pleospora because the entire family Pleosporaceae, as well as the order Pleosporales, is based on the Pleospora-type of centrum development.
Porocercospora Amaradasa et al., Mycologia 106(1): 81 (2014)
Facesoffungi number : FoF 00536
Type species: Porocercospora seminalis (Ellis & Everh.) Amaradasa et al., Mycologia 106(1): 81 (2013), Fig. 23
≡ Cercospora seminalis Ellis & Everh., J. Mycol. 4(1): 4 (1888)
≡ Sporidesmium seminale (Ellis & Everh.) U. Braun, Cryptogamie Mycologie 20 (3): 175 (1999).
Facesoffungi number : FoF 588.
Parasitic on grasses. Sexual morph: undetermined. Asexual morph: Conidiophores 450–500 × 5–8 μm, intermingled among hyphae, subcylindrical, medium brown, smooth to finely verruculose, branched above, thin-walled, septate. Conidiogenous cells 20–30 × 5–7 μm, subcylindrical, medium brown, smooth to finely verruculose, apex rounded, monotretic, with a central pore, indistinct, not darkened or thickened. Conidia 60–90 × 7–8 μm solitary, medium brown, thick-walled, finely verruculose, obclavate to cylindro-obclavate, with short conidia obovoid to subcylindrical, transversely multi-distoseptate; apex subobtuse, base obconically truncate, with hila having a distinct central brown pore, thickened and darkened (Description obtained from Amaradasa et al. 2014).
Notes: Porocercospora was introduced by Amaradasa et al. (2014) to accommodate Cercospora seminalis which causes false smut in buffalograss (Buchloe¨ dactyloides). The genus is characterised by densely aggregated and repeatedly branched conidiophores arising from a brown stroma, monotretic conidiogenous cells with inconspicuous loci, and scolecosporous conidia with distosepta, and thickened, darkened hila. DNA sequence data in the present study as well as in the previous studies (Amaradasa et al. 2014) indicated that Porocercospora seminalis is phylogenetically close to but distinct from the genera Bipolaris and Curvularia. In our phylogeny the type strain of Porocercospora seminalis (CPC 21332) forms a distinct clade sister to Johnalcornia. Therefore we accept the placement of Porocercospora as separate genus in Pleosporaceae.
Pseudoyuconia Lar.N. Vassiljeva, Nov. sist. Niz. Rast. 20: 71 (1983).
= Barrella Ahn & Shearer, Canadian Journal of Botany 73 (4): 578 (1995).
Facesoffungi number : FoF 00537
Saprobic on dead wood. Sexual morph: Ascomata semi-immersed to superficial, globose to subglobose. Ostiole central when mature, ostiolar canal filled with periphyses. Peridium comprising of thick-walled cell, forming a textura angularis. Hamathecium comprising hyaline, cellular, septate pseudoparaphyses. Asci 8-spored, bitunicate, broadly cylindrical or clavate to obovoid, with a minute pedicel, apically rounded end, with an ocular chamber. Ascospores broadly ellipsoidal, pale brown to light brown, 2-septate. Asexual morph: undetermined
Type species: Pseudoyuconia thalictri (G. Winter) Lar.N. Vassiljeva [as ‘thalicti’], Nov. sist. Niz. Rast. 20: 71 (1983), Fig. 24
≡ Leptosphaeria thalictri G. Winter, Hedwigia 10: 40 (1872)
≡ Metasphaeria thalictri (G. Winter) Sacc., Sylloge Fungorum 2: 156 (1883)
≡ Scleropleella thalictri (G. Winter) Höhn., Annales Mycologici 18 (1–3): 76 (1920)
≡ Buergenerula thalictri (G. Winter) E. Müll., Sydowia 4 (1–6): 307 (1950)
≡ Pseudoyuconia thalicti (G. Winter) Lar.N. Vassiljeva (1983)
≡ Barrella thalictri (G. Winter) Ahn & Shearer, Can. J. Bot. 73(4): 578 (1995)
Facesoffungi number : FoF 00538
Saprobic on dead wood. Sexual morph: Ascomata 137–186 × 221–137 μm semi-immersed to superficial, globose to subglobose, black. Ostiole central present when mature, ostiolar canal filled with periphyses. Peridium 19–25 μm wide, 3–4 layered, comprising of thick-walled cell, forming a textura angularis. Hamathecium 2–3 μm wide, comprising hyaline, cellular, septate pseudoparaphyses anastomosing above the asci. Asci 80–119 × 17–23 μm (\( \overline{x} \) = 97 × 20 μm, n = 20), 8-spored, bitunicate, broadly cylindrical or clavate to obovoid, with a minute pedicel, apically rounded end, with an ocular chamber. Ascospores 21–25 × 9–12 μm (\( \overline{x} \) = 23 × 11 μm, n = 20), broadly ellipsoidal, pale brown to light brown, 2-septate, slightly constricted at each septum. Asexual morph: undetermined.
Material examined: RUSSIA, Magadan region, Magadan vicinity, Snow Valley, 8 July 1974, on Thalictrum contortum L. (Ranunculaceae), L. Vasilyeva (VLA P/187).
Notes: Pseudoyuconia, a monotypic genus, was introduced by Vassiljeva (1983) to accommodate Leptosphaeria thalictri G. Winter, and was typified by P. thalictri. Currently, Pseudoyuconia is included in Pleosporaceae (Lumbsch and Huhndorf 2010). No molecular data is available for this genus. Therefore, fresh collections of the type species of the genus are needed so that molecular data can be obtained to verify the natural taxonomic affinities of this genus. Based on the similarities with the other genera of the family Pleosporaceae, such as, in having globose to subglobose ascomata with central ostiole filled with periphyses, peridium composed cells of textura angularis we tentatively place Pseudoyuconia in Pleosporaceae.
In this study we could not locate the type material therefore our understanding is based on the collection of Vasilyeva (1983), which was observed by Vasilyeva (1983) when she was introduced this genus.
Pyrenophora Fr., Summa veg. Scand., Section Post. (Stockholm): 397 (1849).
= Drechslera S. Ito, Proc. Imp. Acad. Japan 6: 355 (1930).
Facesoffungi number : FoF 00009
Saprobic or pathogenic on leaves and stems. Sexual morph: Ascomata immersed, becoming erumpent to near superficial, solitary or scattered, globose to subglobose, broadly or narrowly conical, smooth-walled, ostiolate. Ostiole papillate, covered with brown to reddish-brown setae, which are darkened at the base. Peridium comprising 2–4 layers of brown, thick-walled cells of textura angularis. Pseudoparaphyses not observed. Asci 8-spored, bitunicate, fissitunicate, clavate to sub-cylindrical, with a short, broad pedicel, with a distinct ocular chamber surrounded by a large apical ring. Ascospores 2–3-seriate, muriform, constricted at the septum, smooth-walled, surrounded by a mucilaginous sheath. Asexual morph: hyphomycetous Conidiophores macronematous, mononematous, sometimes caespitose, straight or flexuous, often geniculate, unbranched or in a few species loosely branched, brown, smooth in most species. Conidiogenous cells polytretic, integrated, terminal, frequently becoming intercalary, sympodial, cylindrical, cicatrized. Conidia solitary, in certain species also sometimes catenate or forming secondary conidiophores which bear conidia, acropleurogenous, simple, straight or curved, clavate, cylindrical, rounded at the ends, ellipsoidal, fusiform or obclavate, straw-coloured or pale to dark brown or olivaceous brown, sometimes with cells unequally coloured, the end cells then being paler than intermediate ones, mostly smooth, rarely verruculose, pseudoseptate (description of asexual morph obtained from Ellis 1971).
Type species: Pyrenophora phaeocomes (Rebent.) Fr., Summa veg. Scand., Section Post. (Stockholm): 397 (1849), Fig. 25
Pyrenophora phaeocomes (neotype) a. Fungus on host substrate. b. Ascomata on substrate. c. Section through ascoma. d. Brown setae. e. Peridium. f, g. Asci with a distinct ocular chamber surrounded by a large, distinct, apical ring. h–j. Immature and mature ascospores. Scale bars: c = 100 μm, d–e = 50 μm, f–g = 40 μm, h–j = 10 μm
Basionym: Sphaeria phaeocomes Rebent., Prodr. fl. neomarch. (Berolini): 338 (1804)
≡ Ceuthospora phaeocomes (Rebent.) Fr., J. Taihoku Soc. Agric.: 119 (1825)
≡ Pleospora phaeocomes (Rebent.) Fr., Summa veg. Scand., Section Post. (Stockholm): 398 (1849).
Facesoffungi number : FoF 00010
Saprobic on dead leaves. Sexual morph: Ascomata 380–450 × 370–430 μm (x = 395 × 380 μm, n = 10), solitary or scattered, initially immersed, becoming erumpent to near superficial, globose to subglobose, broadly or narrowly conical, coriaceous, smooth-walled, ostiolate. Ostiole usually broadly papillate, central ostiolar canal filled with periphyses and covered with setae. Setae brown to reddish brown, darkened at the base, septate and tapered towards the apex. Peridium 40–70 μm (x = 45 μm, n = 20) wide, comprising two types of cells, outer cells of 1–2 layers of heavily pigmented cells of textura angularis, inner layer composed of small, light brown to hyaline cells of textura angularis. Pseudoparaphyses not observed. Asci 300–400 × 130–160 μm (x = 345 × 140 μm, n = 20), 8-spored, bitunicate, fissitunicate, clavate to subcylindrical, with a short, broad pedicel, thickened and rounded at apex with a distinct ocular chamber surrounded by a large, distinct, apical ring. Ascospores 78–96 × 27–34 μm (x = 88 × 30 μm, n = 40), biseriate to overlapping triseriate, ellipsoidal with broadly rounded ends, hyaline to light brown when immature, becoming brown to chestnut brown when mature, muriform with 5–6 transverse septa, and single longitudinal septa in one or all cells, constricted at the septa, smooth-walled, relatively thick-walled, with a 5–9 μm thick mucilaginous sheath. Asexual morph of Drechslera teres (Fig. 26); Conidiophores 150–200 × 7–11 μm, solitary or in groups of 2–3, straight or flexuous, sometimes geniculate, often swollen at the base, pale to mid brown or olivaceous brown. Conidia 70–160 × 16–23 μm, straight, cylindrical, rounded at the ends, subhyaline to straw-coloured, smooth, 1–10 (4–6), pseudoseptate, frequently with constriction.
Notes: The asexual morph of Pyrenophora teres, Drechslera teres is described and illustrated here in Fig. 25 because in original description of P. phaeocomes only mentored that it has Drechslera- like asexual morph without giving a species name (Sivanasen 1984).
Material examined: SWEDEN, on leaves of Anthoxanthum (Poaceae), 7 August 1951, J. Ax. Nannfeldt (UPS 170980, neotype).
Notes: Based on both morphology and molecular data, Pyrenophora has been assigned to Pleosporaceae (Zhang et al. 2012). The genus is characterized by immersed, erumpent to nearly superficial ascomata, asci usually with a large apical ring with a clear ocular chamber, lack of pseudoparaphyses and muriform, terete ascospores (Sivanesan 1984). Currently 199 species of Pyrenophora are listed in Index Fungorum (2015). Over 3000 hits are found in GenBank including the putative strain of Pyrenophora phaeocomes (DAOM 222769), which is the type species for this genus. In the present study the genus Pyrenophora clusters in the suborder Pleosporineae of the family Pleosporaceae with a relatively high bootstrap support (Fig. 1, 60 %). Further phylogenetic analysis shows that sexual Pyrenophora morphs cluster with asexual Drechslera morphs, i.e. Pyrenophora dictyoides (DAOM 75616) clusters with Drechslera dictyoides (DAOM 63666). The putative strain of Pyrenophora phaeocomes (DAOM 222769), which is the type species of the genus, clusters with other Pyrenophora species.
As a genus can now only have one name, Ariyawansa et al. (2014c) proposed to synonymise Drechslera under Pyrenophora by giving the priority for the oldest name. This was followed by Wijayawardene et al. (2014).
Excluded genera
Austropleospora R.G. Shivas & L. Morin, in Morin et al., Fungal Diversity 40(1): 70 (2010).
Facesoffungi number : FoF 00539
Type species: Austropleospora osteospermi R.G. Shivas & L. Morin, Morin et al., Fungal Diversity 40(1): 70 (2010), Fig. 27
Austropleospora osteospermi (holotype) a. Herbarium material. b, c. Ascomata on host surface. d. Section through ascoma. e. Section through ostiole. f. Peridium. g. Pseudoparaphyses h. Immature ascus. i. Mature bitunicate asci. j. Apex of ascus. k. Ascospores. k. Pycnidia on host. l. Section of pycnidium. m. Pycnidial wall. o. Conidiogenous cells and developing conidia. n. Brown 3-septate conidia. Scale bars: d = 50 μm, e–f = 25 μm, g = 10 μm, h–i = 25 μm, k = 15 μm, l, m = 20 μm, n = 5 μm
Facesoffungi number : FoF 00540
Parasitic on stem and leaves of Chrysanthemoides monilifera (Asteraceae). Sexual morph: Ascomata 75–110 × 130–200 μm (x = 100 × 146 μm, n = 10), subglobose, sometimes slightly flattened, solitary or in groups, scattered, immersed immediately below the stem epidermis, ostiole 60–90 μm long, with a protruding neck. Peridium 8–18 μm (x = 12 μm, n = 10) wide, composed of dark brown to black cells of textura angularis. Hamathecium of 2–3 μm wide, dense, filamentous, anastomosing, aseptate, hyaline pseudoparaphyses. Asci 75–120 × 13–18 μm (x = 92 × 16 μm, n = 20), bitunicate, fissitunicate, 6–8-spored, cylindrical to clavate, rounded at the apex and minute ocular chamber with a short, broad, pedicel. Ascospores 16.5 − 21× 6–8.4 μm (x = 18 × 7.7 μm, n = 25), biseriate to overlapping uniseriate, ellipsoidal, yellowish brown, muriform, mostly with 3-transverse septa, 0–2 longitudinal septa, slightly constricted at median septum, not or very slightly constricted at other septa, apex rounded to slightly tapered, base tapered to rounded, smooth. Asexual morph: Conidiomata 75–110 × 100–130 μm (x = 88 × 115 μm, n = 10), pycnidial, globose, superficial on stem, immersed in the host tissue and becoming erumpent at maturity, globose, dark brown in the erumpent part, with a single ostiole. Conidiomata wall 9–16 μm wide, brown to reddish-brown, thin-walled, comprising several layers with cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 10–12 × 2.5 − 3.5 μm (x = 11 × 2 μm, n = 15), inconspicuously annellidic, discrete, cylindrical. Conidia 14–18 × 4.8 − 6.5 μm (x = 15.8 × 5.7 μm, n = 25), cylindrical to narrowly ellipsoidal, initially hyaline and aseptate, becoming yellowish-brown at maturity, mostly transversely 1–3-septate, ends rounded.
Material examined: AUSTRALIA, NSW Bellinger Head, Kalang river mouth, on stem and leaves of Chrysanthemoides monilifera ssp. rotundata (L.) Norlindh (Asteraceae). 5 March 2008, L. Morin (BRIP 52234, holotype).
Notes: Austropleospora was introduced by Morin et al. 2010 to accommodate A. osteospermi on Chrysanthemoides monilifera ssp. rotundata (Asteraceae) and it is monotypic. In the same study Shivas and Morin (in Morin et al. 2010) observed Hendersonia osteospermi Wakef. on the same host and identified it as the asexual morph of A. osteospermi both in culture and by DNA sequence analysis. Based on ITS sequence analysis, Morin et al. (2010) placed Austropleospora under Pleosporales without assigning it to any family. Austropleospora is classified in Dothideomycetes genera incertae sedis in Index Fungorum (2015). After re-examineing the holotype of A. osteospermi, Thambugala et al. (2014b) tentatively referred Austropleospora in Pleosporaceae based on morphological similarities. During our preliminary phylogenetic analysis (data not shown), we observed that the type strain of A. osteospermi (BRIP 51628) forms a distinct clade in family Didymosphaeriaceae. Therefore we exclude Austropleospora from Pleosporaceae and refer it to Didymosphaeriaceae.
Dendryphion Wallr., Fl. crypt. Germ. (Norimbergae) 2: 300 (1833)
Dendryphiella Bubák & Ranoj., in Ranojevic, Annls mycol. 12(4): 417 (1914)
Dwayamala Subram., J. Indian bot. Soc. 35: 475 (1956)
Entomyclium Wallr., Fl. crypt. Germ. (Norimbergae) 2: 189 (1833)
Facesoffungi number : FoF 00541
Type species: Dendryphion comosum Wallr., Fl. crypt. Germ. (Norimbergae) 2: 300 (1833)
= Dendryphion curtum Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 7: 176 (1851)
= Dendryphion curtum Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 7: 176 (1851) var. curtum
= Dendryphion curtum var. ramosius Sacc., Syll. fung. (Abellini) 4: 490 (1886)
Facesoffungi number : FoF 00542
Notes: Dendryphion was introduced by Wallroth (1833) and is typified by Dendryphion comosum. The genus Dendryphion is characterised by having apically branched conidiophores with polytretic dark scars, and chains of brown, septate (didymo or cheiro) conidia (Crous et al. 2014). The genus Brachycladium had been synonymised with Dendryphion by Ellis (1971), was resurrected for the asexual morph based on polyphyly within Dendryphion and morphological distinction from type species, D. comosum (Crous et al. 2014). The type species of Brachycladium, B. penicillatum, resides in Alternaria sect. Crivellia in this study as well as in Woudenberg et al. (2013). Brachycladium is therefore a synonymy of Alternaria instead of Dendryphion. Although there are more than 80 names recorded in Index Fungorum (2015), Seifert et al. (2011) confined them to six species. Inderbitzin et al. (2006) showed that Dendryphion was polyphyletic thus the natural classification of the genus has become confused. Currently, no sequence data is available in GenBank for the generic type of Dendryphion. Recently, Crous et al. (2014) introduced a new species of Dendryphion, D. europaeum with molecular and morphological data, and placed it species in the suborder Pleosporineae, family Pleosporaceae. Considering the morphological key provided by Siboe et al. (1999), Crous et al. (2015) confirmed that D. europaeum appears to be distinct from the currently recognised Dendryphion species based on its conidial morphology. During our preliminary analysis we observed that D. europaeum forms a robust clade outside the suborder Pleosporineae. Therefore we tentatively exclude Dendryphion from Pleosporaceae and place it in Pleosporales, genera incertae sedis. However, the placement of Dendryphion in Pleosporales can only be confirmed by phylogenetic analysis, including sequencing the generic type of Dendryphion (D. europaeum).
Edenia M.C. González et al., in González et al., Mycotaxon 101: 254 (2007)
Type species: Edenia gomezpompae M.C. González et al., in González et al., Mycotaxon 101: 254 (2007)
Facesoffungi number : FoF 00543
Notes: Edenia was introduced by González et al. (2007) and is typified by Edenia gomezpompae. This endophytic fungus was isolated from living leaves of Callicarpa acuminta Kunth (González et al. 2007). González et al. (2007) placed Edenia in Pleosporaceae based on the ITS sequence data. In our phylogeny we observed that the type strain of Edenia gomezpompae (C1c) along with putative strains JLCC 34533, CBS 124106 and 11G048 form a robust clade in family Phaeosphaeriaceae outside Pleosporaceae. Therefore we exclude Edenia from Pleosporaceae and placed it in the Phaeosphaeriaceae.
Kriegeriella Höhn., Annls mycol. 16(1/2): 39 (1918).
Type species: Kriegeriella mirabilis Höhn., Annls mycol. 16(1/2): 39 (1918).
Facesoffungi number : FoF 00544
Notes: Kriegeriella was introduced by von Höhnel (1918b) in order to accommodate K. mirabilis and K. transiens Höhn. The genus Kriegeriella was typified with K. mirabilis and referred to Microthyriaceae by Höhnel 1918b. It is characterised by superficial, subglobose, black thyrothecia-like ascomata, cellular pseudoparaphyses, bitunicate, obpyriform asci bearing hyaline, multi-septate ascospores which turn brown when mature. Subsequently, Kriegeriella was assigned to the subfamily of Aulographiodeae (Microthyriaceae) (Batista et al. 1959), Asterinaceae (Hemisphaeriales) (Luttrell 1973) and Pseudosphaeriaceae (Dothideales) (Barr 1975). Barr (1987b) introduced a family, i.e. Kriegeriellaceae (Dothideales) to accommodate Kriegeriella and Extrawettsteinina. This scheme is rarely followed, and Kriegeriella is generally classified under Pleosporaceae (Pleosporales) (Eriksson 2006; Lumbsch and Huhndorf 2007, 2010).
Zhang et al. (2012) excluded Kriegeriella from the family Pleosporaceae and concluded that it is not a typical genus of the family. Furthermore, Zhang et al. (2012) suggested that Kriegeriella might belong to Microthyriaceae, as it would be unusual in Pleosporaceae in having thyrothecium-like ascomata and 5–6-septate ascospores. Therefore we exclude Kriegeriella from Pleosporaceae, following the opinion of Zhang et al. (2012).
Macrospora Fuckel, Jb. nassau. Ver. Naturk. 23–24: 139 (1870) [1869–70].
= Nimbya E.G. Simmons, Sydowia 41: 316 (1989)
Facesoffungi number : FoF 00545
Saprobic on stems or wood. Sexual morph: Ascomata immersed to nearly superficial, subglobose. Ostiole central, present when mature, ostiolar canal filled with periphyses. Peridium comprising thick-walled cells, forming a textura angularis. Hamathecium comprising hyaline, unbranched, septate, pseudoparaphyses. Asci 8-spored, bitunicate, broadly cylindrical or clavate to obovoid, with a knob-like pedicel at the base, apically rounded, with an ocular chamber. Ascospores broadly ellipsoidal, brown to dark brown, multi septate. Asexual morph: undetermined.
Type species: Macrospora scirpicola (DC.) Fuckel, Jb. nassau. Ver. Naturk. 23–24: 139 (1870) [1869–70], Fig. 28
Macrospora scirpicola (holotype) a. Herbarium material. b. Appearance of ascomata on host. c. Neck with periphyses. d. Vertical section through ascoma. e. Section of peridium. f. Pseudoparaphyses. g, h. Immature asci. i–k. Mature asci. l. Immature ascospore. m–p. Mature ascospores. Scale bars: b = 500 μm, g–k = 50 μm, d–e = 30 μm, c = 20 μm, f = 20 μm, l–p = 20 μm
Basiyonym: Sphaeria scirpicola DC., in Lamarck & de Candolle, Fl. franç., Edn 3 (Paris) 2: 300(1805)
≡ Clathrospora scirpicola (DC.) Höhn., Annls mycol. 18(1/3): 77 (1920)
≡ Macrospora scirpicola (DC.) Fuckel, Jb. nassau. Ver. Naturk. 23–24: 139 (1870)
≡ Pleospora scirpicola (DC.) P. Karst., Bidr. Känn. Finl. Nat. Folk 23: 72 (1873)
= Pleospora scirpicola var. scirpicola (DC.) P. Karst., Bidr. Känn. Finl. Nat. Folk 23: 72 (1873)
≡ Pyrenophora scirpicola (DC.) E. Müll., Sydowia 5(3–6): 256 (1951)
= Sphaeria culmorum Wallr., Fl. crypt. Germ. (Norimbergae) 2: 770 (1833)
Facesoffungi number : FoF 00546
Saprobic on dead stems. Sexual morph: Ascomata 83–101 × 72–79 μm, immersed in host tissues, subglobose, brown to dark brown, ostiolate. Ostiole central when mature, ostiolar canal 31–36 μm wide at the base, with periphyses. Peridium 9–25 μm wide, comprising 2–3 layers of thick-walled cells, forming a textura angularis, brown to dark brown at outside and hyaline at the inner layer. Hamathecium of 2–4 μm wide, thick-walled, cellular, hyaline, unbranched, septate, pseudoparaphyses. Asci (26–) 64–88 × 9–13 μm, 8-spored, bitunicate, broadly cylindrical or clavate to obovoid, with a knob-like pedicel at the base, apically rounded, with an ocular chamber. Ascospores 16–28 × 8–12 μm., 2–4 overlapping seriate, broadly ellipsoidal, ends rounded, straight or curved, brown to dark brown, 3-septate, constricted at the central cell, dark brown at the septum, second cell from above widest. Asexual morph: undetermined.
Material examined: FRANCE, collector unspecified, Exsiccati title: Plantes Cryptogames De France, Exsiccati Number: 1778 (NY 0098598, holotype).
Notes: Macrospora had been assigned to Diademaceae based on its applanate and muriform ascospores with 1-row of longitudinal septa, with a sheath, 2–3 μm wide and constricted at the first septum and ascospores that are paler and larger than those of Comoclathris (Shoemaker and Babcock 1992). Macrospora was however, considered as a synonym of Pyrenophora by Eriksson and Hawksworth (1991) which was assigned to Pleosporaceae, and this proposal has been widely followed (Eriksson 2006; Lumbsch and Huhndorf 2010). Ariyawansa et al. (2014a) suggested that Macrospora scirpicola is neither typical of Diademaceae nor Pleosporaceae. Macrospora is compatible with Thyridaria in having immersed, subglobose ascomata with 2–3 layers, of thick-walled cells of textura angularis and cylindrical asci bearing phragmospores, but differs in having cellular pseudoparaphyses and asci with a with a knob-like or furcate pedicel.
Currently no molecular data is available for any Macrospora species in GenBank. Therefore based on its similarities with Thyridaria, we tentatively place Macrospora in Thyridariaceae, but fresh collections of the type species of the genus are needed so that molecular data can be obtained to verify its natural taxonomic affinities.
Monascostroma Höhn., Annls mycol. 16(1/2): 160 (1918).
Facesoffungi number : FoF 00547
Type species: Monascostroma innumerosum (Desm.) Höhn. [as ‘innumerosa’], Annls mycol. 16(1/2): 160 (1918).
Basionym: Hendersonia innumerosa Desm., Annls Sci. Nat., Bot., sér. 3 16: 10 [repr.] (1851)
≡ Stagonospora innumerosa (Desm.) Sacc., Sylloge Fungorum 3: 451 (1884)
Facesoffungi number : FoF 00548
Notes: Monascostroma was introduced by Desmazières (1851) to accommodate M. innumerosum (= Hendersonia innumerosa). Eriksson and Hawksworth (1998) and Kodsueb et al. (2006) included Monascostroma in Pleosporaceae, while later researchers classified Monascostroma in Didymellaceae (Zhang et al. 2009, 2012; Schoch et al. 2009; Lumbsch and Huhndorf 2010).
The putative strain of Monascostroma innumerosum (CBS 345.50) forms a robust clade in family Didymellaceae (Zhang et al. 2009, 2012; Schoch et al. 2009; Hyde et al. 2013). Thus we also accept the genus Monascostroma in the Didymellaceae following other published work (Zhang et al. 2009, 2012; Schoch et al. 2009; Hyde et al. 2013).
Zeuctomorpha Sivan et al., in Sivanesan, Bitunicate Ascomycetes and their Anamorphs (Vaduz): 572 (1984).
Facesoffungi number : FoF 00549
Type species: Zeuctomorpha arecae Sivan et al., in Sivanesan, Bitunicate Ascomycetes and their Anamorphs (Vaduz): 572 (1984).
Facesoffungi number : FoF 00550
Notes: Zeuctomorpha, a monotypic genus typified by Z. arecae, was formally treated as a member of the Pleosporaceae by Sivanesan (1984), based on its superficial setose ascomata, clavate, asci, ellipsoid and 1-septate ascospores, and presence of pseudoparaphyses. Eriksson and Hawksworth (1998) classified Zeuctomorpha in Pleosporaceae and this was followed by Kirk et al. (2001), Kodsueb et al. (2006) and Lumbsch and Huhndorf (2010).
Zhang et al. (2012) suggested that Zeuctomorpha was unusual amongst the Pleosporaceae with its hairy superficial ascomata, few pseudoparaphyses, broadly clavate to obclavate asci and 1-septate pigmented ascospores. Furthermore, Zhang et al. (2012) concluded that all of these morphological characters are most comparable with species of Acantharia, which might be closely related to Venturiaceae, but this needs to be confirmed by molecular data. Therefore, based on the morphology we also recommend to exclude Zeuctomorpha from Pleosporaceae without assigning it to any family.
Concluding remarks
Molecular data coupled with morphology has provided the basis for the modern classification of the kingdom fungi, but have some restrictions in application (Hibbett et al. 2007; Ariyawansa et al. 2014a; Boonmee et al. 2014; Hyde et al. 2014; Nilsson et al. 2014; Schoch et al. 2014; Thambugala et al. 2014a). The most important and disconcerted problem is that the phylogeny inferred from any gene may not divulge the evolution history of the organism (Uilenberg et al. 2004; Ariyawansa et al. 2014a). Therefore polyphasic taxonomy, including genotypical and phenotypical characteristics are recommended in all future studies (Uilenberg et al. 2004; Ariyawansa et al. 2014a, Udayanga et al. 2014a). The genome also needs to be evaluated (Uilenberg et al. 2004; Ariyawansa et al. 2014a). Another main problem is most early studies is that they focused on techniques and used strains of fungi that in most cases were not carefully referenced thus creating confusion as explained in Ariyawansa et al. (2014a). Most strains in GenBank are named without attached voucher material and it impractical to confirm their characters to ensure correct identification.
There have only been a few molecular analysis of Pleosporaceae as compared to morphological studies (Kodsueb et al. 2006). Genera with bitunicate asci and brown muriform ascospores viz Murispora, Pleospora , Pyrenophora and Tremateia were generally classified under Pleosporaceae. Molecular studies have shown that these particular morphological characters have evolved in different families such as Aigialaceae, Amniculicolaceae, Didymellaceae, Lophiostomataceae, Montagnulaceae and Sporormiaceae (Zhang et al. 2012a, Hyde et al. 2013). Recently published papers such as Zhang et al. (2012a), Woudenberg et al. (2013) and Hyde et al. (2013), did not discuss details of the family Pleosporaceae and related genera. Woudenberg et al. (2013) was mainly focused on the genus Alternaria, while Zhang et al., (2012a) gave a detail account of sexual morphs only. Hyde et al. (2013) provided a key to the genera in Pleosporaceae in work on Dothideomycetes.
Fourteen clades of Pleosporaceae have been identified by our analysis based on ITS, LSU, SSU and RPB2 gene data, which are treated as suitable for the delimitation of genera (Figure 5.1). Although relationships among these clades may be weakly supported and some may vary in detail, some tentative conclusions can be drawn. Several current taxonomic hypotheses are supported by our molecular data, and this makes it possible to propose some taxonomic hypotheses about the relationships among the genera in Pleosporaceae. By combining multi-locus DNA sequencing with detailed morphological analyses, we were able to define and formally propose two new species, four combinations and several sexual morphs among the taxa in the family Pleosporaceae. In our study we accept 16 sexual or asexual genera and exclude three genera from the family Pleosporaceae based on morphology coupled with molecular data.
In previous treatments Pleospora species were considered as paraphyletic within the family Pleosporaceae (Kodsueb et al. 2006, Inderbitzin et al. 2009). Several studies have confirmed this opinion and have led to the removal of Pleospora species to other genera (Crivelli 1983, Inderbitzin et al. 2002, 2006; Leuchtmann 1984, Zhang et al. 2009b). In the present study we were able to resolve the paraphyletic nature of the genus by following Woudenberg et al. (2013) and Wijayawardene et al. (2014), where they have treated the genus based on the generic type Pleospora herbarum (CBS 191. 86T). Therefore in order to create a stable phylogenetic taxonomy, several putative strains of Pleospora which clustered with the type strain of Pleospora herbarum (CBS 191.86) were classified under P. herbarum sensu stricto (P. tomatonis CBS109844 and P. sedicola CBS109843). The putative strains of P. papaveracea CBS432.50, P. tarda CBS 714.68 and P. halophila CBS410.73 were treated under the genus Pleospora as separate species but this needs to be clarified with morphology because currently these strains are not attached to any herbarium material.
Some putative strains of Pleospora species other than species discussed in the above paragraph were assigned to different genera in Pleosporaceae based on their molecular data. Pleospora fallens (Sacc.) Gruyter & Verkley was introduced by de Gruyter et al. (2013) in order to accommodate Phoma fallens Sacc., because during their study the strain of Phoma fallens (CBS 284.70) clustered within the family Pleosporaceae. Woudenberg et al. (2013) and Ariyawansa et al. (2014a) showed that this strain forms a separate clade in the family Pleosporaceae basel to the other genera of the family. Importantly Pleospora fallens clustered outside the Pleospora sensu stricto. Therefore here we propose to keep the name Phoma fallens instead of Pleospora fallens. By taking this approach we were able to resolve the paraphyletic nature of Pleospora within the family Pleosporaceae
In recent years, DNA based studies revealed multiple non-monophyletic genera within the Alternaria complex, and Alternaria species clades that do not always correlate to species-groups based on morphological characteristics (Pryor and Bigelow 2003; Inderbitzin et al. 2006; Pryor et al. 2009; Runa et al. 2009; Wang et al. 2011; Lawrence et al. 2012; Woudenberg et al. 2013). A separate phylogeny was prepared to show the placement of the recently revised sections in Alternaria (Figure 5.2). The well-supported node for the Alternaria clade obtained in the present study, and the low bootstrap support at the deeper nodes within the Alternaria complex is similar to previous phylogenetic studies. (Pryor and Bigelow 2003; Inderbitzin et al. 2006; Pryor et al. 2009; Runa et al. 2009; Wang et al. 2011; Lawrence et al. 2012; Woudenberg et al. 2013). We also observed the same result as Woudenberg et al. (2013) where the Alternaria clade contains 24 internal clades and six monotypic lineages, the assemblage of which is recognised as Alternaria. Furthermore we introduced one new species (A. sedi) and several collections of the sexual morph of A. alternata from Italy are also introduced in the study.
The explanation of Pleosporaceae in the current work is sustained by a well-supported phylogenetic nodes in multiple analyses coupled with morphology. We pursue the precedence introduced by Lawrence et al. (2013) and Woudenberg et al. (2013) to assign the taxonomic status of sections of Alternaria for the different clades found. In here also we show the significance of having accurate DNA sequence data attached with both correct taxonomic names and clearly annotated specimen data. For end-users, this also results in a more stable and understandable taxonomy and nomenclature. Furthermore we gave a well resolved back-bone tree for the family Pleosporaceae, representing all the sexual and asexual genera, which currently have DNA sequences.
References
Abler R (2003) Global change and local places: estimating, understanding, and reducing greenhouse gases
Agrios GN (2005) Plant pathology, 5th edn. California Academic Press, Elsevier San Diego
Alves A, Correia A, Phillips AJL (2006) Multi-gene genealogies and morphological data support Diplodia cupressi sp. nov., previously recognized as D. pinea f. sp. cupressi, as a distinct species. Fungal Divers 23:1–15
Amaradasa BS, Madrid H, Groenewald JZ, Crous PW, Amundsen K (2014) Porocercospora seminalis gen. et comb. nov., the causal organism of buffalograss false smut. Mycologia 106(1):77–85
Ariyawansa HA, Jones EBG, Suetrong S, Alias SA, Kang JC, Hyde KD (2013a) Halojulellaceae a new family of the order Pleosporales. Phytotaxa 130:14–24
Ariyawansa HA, Kang JC, Alias SA, Chukeatirote E, Hyde KD (2013b) Towards a natural classification of Dothideomycetes 1: the genera Dermatodothella, Dothideopsella, Grandigallia, Hysteropeltella and Gloeodiscus (Dothideomycetes incertae sedis). Phytotaxa 147(2):35–47
Ariyawansa HA, Maharachchikumbura SSN, Karunarathne SC, Chukeatirote E, Bahkali AH, Kang JC, Bhat JD, Hyde KD (2013c) Deniquelata barringtoniae from Barringtonia asiatica, associated with leaf spots of Barringtonia asiatica. Phytotaxa 105:11–20
Ariyawansa H, Phookamsak R, Tibpromma S, Kang JC, Hyde KD (2014a) A molecular and morphological reassessment of Diademaceae. Sci World J 2014:1–11
Ariyawansa HA, Camporesi E, Thambugala KM, Mapook A, Kang JC, Alias SA, Chukeatirote E, Thines M, Mckenzie EHC, Hyde KD (2014b) Confusion surrounding Didymosphaeria — phylogenetic and morphological evidence suggest Didymosphaeriaceae is not a distinct family. Phytotaxa 176(1):102–119
Ariyawansa HA, Hawksworth DL, Hyde KD, Jones EBG, Maharachchikumbura SSN, Manamgoda DS, Thambugala KM, Udayanga D, Camporesi E, Daranagama A, Jayawardena R, Liu JK, McKenzie EHC, Phookamsak R, Senanayake IC, Shivas RG, Tian Q, Xu JC (2014c) Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Divers 69(1):57–91
Ariyawansa HA, Kang JC, Alias SA, Chukeatirote E, Hyde KD (2014d) Pyrenophora. Mycosphere 5(2):351–362
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Chukeatirote E, Kang JC, Xu JC, McKenzie EHC, Jones EBG, Hyde KD (2014e) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Divers 68:69–104
Ariyawansa HA, Thambugala KM, Kang JC, Alias SA, Chukeatirote E, Hyde KD (2014f) Towards a natural classification of Dothideomycetes 2: The genera Cucurbidothis, Heterosphaeriopsis, Hyalosphaera, Navicella and Pleiostomellina (Dothideomycetes incertae sedis). Phytotaxa 176(1):7–17
Barr ME (1972) Preliminary studies on the Dothideales in temperate North America. Contrib Univ Mich Herb 9:523–638
Barr ME (1975) A note on Extrawettsteinina. Mycotaxon 2:104–106
Barr ME (1981) The genus Curreya: an example of taxonomic confusion in the Ascomycetes. Mycologia 73:599–609
Barr ME (1987a) New taxa and combinations in the Loculoascomycetes. Mycotaxon 29:501–505
Barr ME (1987b) Prodromus to class loculoascomycetes. Amherst. University of Massachusetts, Massachusetts
Barr ME (1990) Some dictyosporous genera and species of Pleosporales in North America. Mem N Y Bot Gard 62:1–92
Batista AC, Costa CA, Peres GEP, Leal FB (1959) Novos e antigos fungos Microthyriaceae. Anais Soc Biol Pernambuco 16:129–140
Berbee ML (1996) Loculoascomycete origins and evolution of filamentous ascomycete morphology based on 18S rRNA gene sequence data. Mol Biol Evol 13:462–470
Berbee ML, Pirseyedi M, Hubbard S (1999) Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91:964–977
Boedijn KB (1933) Uebr Einige Phragmosporen Dematiazeen. Bull Jard Bot Buitenz 13(3 Sér):123
Boonmee S, Rossman AY, Lui JK, LiWJ DDQ, Bhat JD, Jones EBG, McKenzie EHC, Xu JC, Hyde KD (2014) Tubeufiales, ord. nov. integrating sexual and asexual generic names. Fungal Divers 68(1):239–298
Cai L, Wu WP, Hyde KD (2009) Phylogenetic relationships of Chalara and allied species inferred from ribosomal DNA sequences. Mycol Prog 8(2):133–143
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Carter E, Boudreaux C (2004) Fatal cerebral phaeohyphomycosis due to Curvularia lunata in an immunocompetent patient. J Clin Microbiol 42(11):5419–5423
Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Peršoh D, Dhami MK, Alis AS, Xu J, Liu X, Stadler M, Hyde KD (2014) The sooty moulds. Fungal Divers 66:1–36
Clements FE (1909) The genera of fungi. The HW Wilson Company
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO et al (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
Crous PW, Shivas RG, Quaedvlieg W, van der Bank M, Zhang Y, Summerell BA, Tan YP (2014) Fungal Planet description sheets: 214–280. Persoonia 32:184
de Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2013) Redisposition of phoma-like anamorphs in pleosporales. Stud Mycol 75:1–36
Desmazières JBHJ (1851) Dix-neuvième notice sur les plantes cryptogames récemment découvertes en France. Ann Sci Nat Botanique 16:296–330
Dettman JR, Jacobson DJ, Turner E, Pringle A, Taylor JW (2003) Reproductive isolation and phylogenetic divergence in Neurospora: comparing methods of species recognition in a model eukaryote. Evolution 57:2721–2741
Dong JW, Chen WD, Crane JL (1998) Phylogenetic studies of the Leptosphaeriaceae, Pleosporaceae and some other Loculoascomycetes based on nuclear ribosomal DNA sequences. Mycol Res 102:151–156
Drechsler C (1934) Phytopathological and taxonomical aspects of Ophilobolus, Pyrenophora, Helminthosporium and a new genus Cochliobolus. Phytopathology 24(9):953–981
Ellis MB (1971) Dematiaceous hyphomycetes. CMI, Kew
Eriksson OE (1981) The families of bitunicate ascomycetes. Opera Bot 60:1–220
Eriksson OE (2006) Outline of ascomycota – 2006. Myconet 12:1–82
Eriksson OE, Hawksworth DL (1986) An alphabetical list of the generic names of ascomycetes – 1986. Syst Ascomyc 5:3–111
Eriksson OE, Hawksworth DL (1991) Outline of the ascomycetes – 1990. Syst Ascomyc Reprint of Volumes 1–4 (1982–1985) 9: 39–271
Eriksson OE, Hawksworth DL (1998) Outline of the ascomycetes −1998. Syst Ascomyc 16:83–296
Farr DF, Bills GF, Chamuris GP, Rossman AY (1989) Fungi on plants and plant products in the United States. APS Press, St. Paul
Goh KT, Hyde KD, Lee KLD (1998) Generic distinction in Helminthosporium complex based on restriction analysis of the nuclear ribosomal RNA gene. Fungal Divers 1:85–107
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lucking R, Lumbsch HT, Lutzoni F, Matheny PB, Mclaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Koljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley- Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schussler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N (2007) A higher-level phylogenetic classification of the fungi. Mycol Res 111:509–547
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M (2013) Families of dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AWAM, Dissanayake AJ, Glockling SL, Goonasekara ID, Gorczak M, Hahn M, Jayawardena RS, van Kan JAL, Laurence MH, Lévesque CA, Li X, Liu JK, Maharachchikumbura SSN, Manamgoda DS, Martin FN, McKenzie EHC, McTaggart AR, Mortimer PE, Nair PVR, Pawłowska J, Rintoul TL, Shivas RG, Spies CFJ, Summerell BA, Taylor PWJ, Terhem RB, Udayanga D, Vaghefi N, Walther G, Wilk M, Wrzosek M, Xu JC, Yan JY, Zhou N (2014) One stop shop: backbones trees for important phytopathogenic 5 genera: I (2014). Fungal Divers 67(1):21–125
Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Berbee ML (2002) Decorospora, a new genus for the marine ascomycete Pleospora gaudefroyi. Mycologia 94(4):651–659
Inderbitzin P, Shoemaker RA, O’Neill NR, Turgeon BG, Berbee ML (2006) Systematics and mating systems of two fungal pathogens of opium poppy: the heterothallic Crivellia papaveracea and a homothallic species with a Brachycladium papaveris asexual state. Can J Bot 84:1304–1326
Index Fungorum (2015) http://www.indexfungorum.org
Jones EB, Stanley SJ, Pinruan U (2008) Marine endophyte sources of new chemical natural products: a review. Bot Mar 51(3):163–170
Khan JA, Hussain ST, Hasan S, McEvoy P, Sarwari A (2000) Disseminated bipolaris infection in an immunocompetent host: an atypical presentation. J Pak Med Assoc 50:68–71
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Dictionary of the fungi 9th edn. CABI, Wallingford
Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EHC, Hyde KD, Jeewon R (2006) The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia 98:571–583
Lawrence DP, Park MS, Pryor BM (2012) Nimbya and Embellisia revisited, with nov. comb for Alternaria celosiae and A. perpunctulata. Mycol Prog 11:799–815
Lawrence DP, Gannibal PB, Peever TL, Pryor BM (2013) The sections of Alternaria: formalizing species-groups concepts. Mycologia 105:530–546
Leakey CLA (1964) Dactuliophora, a new genus of mycelia sterilia from tropical Africa. Trans Br Mycol Soc 47(3):341–IN10
Leonard KJ, Suggs EG (1974) Setosphaeria prolata, the ascigerous state of Exserohilum prolatum. Mycologia 281–297
Leuchtmann A (1984) Über Phaeosphaeria Miyake und andere bitunicate Ascomyceten mit mehrfach querseptierten Ascosporen. Sydowia 37:75–194
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol 16:1799–1808
Liu JK, Phookamsak R, Mingkhuan M, Wikee S, Li YM et al (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Lumbsch HT, Huhndorf SM (eds.) (2007) Outline of ascomycota −2007. Myconet 13:1–58
Lumbsch HT, Huhndorf SM (2010) Outline of ascomycota – 2009. Fieldiana Life Earth Sci 1:1–60
Luttrell ES (1951) Taxonomy of the pyrenomycetes. Univ Mo Stud 24:1–120
Luttrell ES (1955) The ascostromatci ascomycetes. Mycologia 47:511–532
Luttrell ES (1973) Loculoascomycetes. In: Ainsworth GC, Sparrow FK, Sussman AS (eds) The fungi, an advanced treatise, a taxonomic review with keys: ascomycetes and fungi imperfecti. Academic, New York
Malathrakis NE (1979) A study of an olive tree disease caused by the fungus Phoma incompta Sacc. et Mart. Dissertation, Agricultural College of Athens
Manamgoda DS, Cai L, Bahkali AH, Chukeatirote E, Hyde KD (2011) Cochliobolus: an overview and current status of species. Fungal Divers 51:3–42
Manamgoda DS, Cai L, McKenzie EHC, Crous PW, Madrid H, Chukeatirote E et al (2012) A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia complex. Fungal Divers 56(1):131–144
Manamgoda DS, Rossman AY, Castlebury LA, Crous PW, Madrid H, Chukeatirote E, Hyde KD (2014) The genus bipolaris. Stud in mycol. doi:10.1016/j.simyco.2014.10.002
Morin L, Shivas RG, Piper MC, Tan YP (2010) Austropleospora osteospermi gen. et sp. nov. and its host specificity and distribution on Chrysanthemoides monilifera ssp. rotundata in Australia. Fungal Divers 40(1):65–74
Nilsson RH, Hyde KD, Pawłowska J, Ryberg M et al (2014) Improving ITS sequence data for identification of plant pathogenic fungi. Fungal Divers 67(1):11–19
Nitschke TRJ (1869) Grundlage eines Systems der Pyrenomyceten Verh Naturhist Vereines Preuss Rheinl 26:70–77
Nylander JAA (2004) MrModeltest 2.0. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Petrak F (1952) Ergebnisse einer Revision der Grundtypen verschiedener Gattungen der Askomyzeten und Fungi Imperfecti. Sydowia 6:336–343
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A et al (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21:29–55
Phookamsak R, Liu JK, McKenzie EH, Manamgoda DS, Ariyawansa H, Thambugala KM et al (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68(1):159–238.
Pryor BM, Bigelow DM (2003) Molecular characterization of Embellisia and Nimbya species and their relationship to Alternaria, Ulocladium and Stemphylium. Mycologia 95:1141–1154
Pryor BM, Creamer R, Shoemaker RA, McLain-Romero J, Hambleton S (2009) Undifilum, a new genus for endophytic Embellisia oxytropis and parasitic Helminthosporium bornmuelleri on legumes. Botany 87:178–194
Ramaley AW, Barr ME (1995) New dictyosporous species from leaves of Agavaceae. Mycotaxon 54:75–90
Rambaut A, Drummond A (2008) FigTree: Tree figure drawing tool, version 1.2. 2. Institute of Evolutionary Biology, University of Edinburgh
Rannala B, Yang Z (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Runa F, Park M, Pryor B (2009) Ulocladium systematics revisited: phylogeny and taxonomic status. Mycol Prog 8:35–47
Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, de Gruyter J, de Hoog GS, Dixon LJ, Grube M, Gueidan C, Harada Y, Hatakeyama S, Hirayama K, Hosoya T, Huhndorf SM, Hyde KD, Jones EBG, Kohlmeyer J, Kruys Å, Li YM, Lücking R, Lumbsch HT, Marvanová L, Mbatchou JS, McVay AH, Miller AN, Mugambi GK, Muggia L, Nelsen MP, Nelson P, Owensby CA, Phillips AJL, Phongpaichit S, Pointing SB, Pujade-Renaud V, Raja HA, Rivas Plata E, Robbertse B, Ruibal C, Sakayaroj J, Sano T, Selbmann L, Shearer CA, Shirouzu T, Slippers B, Suetrong S, Tanaka K, Volkmann-Kohlmeyer B, Wingfield MJ, Wood AR, Woudenberg JHC, Yonezawa H, Zhang Y, Spatafora JW (2009) A class–wide phylogenetic assessment of dothideomycetes. Stud Mycol 64:1–15
Schoch CL, Robbertse B, Robert V, Vu D, Cardinali G, Irinyi L, Meyer W, Nilsson RH, Hughes K, Miller AN, Kirk PM, Abarenkov K, Aime MC, Ariyawansa HA, Bidartondo M, Boekhout T, Buyck B, Cai Q, Chen J, Crespo A, Crous PW, Damm U, De Beer ZW, Dentinger BTM, Divakar PK, Dueñas M, Feau N, Fliegerova K, García MA, Ge Z-W, Griffith GW, Groenewald JZ, Groenewald M, Grube M, Gryzenhout M, Gueidan C, Guo L, Hambleton S, Hamelin R, Hansen K, Hofstetter V, Hong S-B, Houbraken J, Hyde KD, Inderbitzin P, Johnston PR, Karunarathna SC, Kõljalg U, Kovács GM, Kraichak E, Krizsan K, Kurtzman CP, Larsson K-H, Leavitt S, Letcher PM, Liimatainen K, Liu J-K, Lodge DJ, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, Manamgoda D, Martín MP, Minnis AM, Moncalvo J-M, Mulè G, Nakasone KK, Niskanen T, Olariaga I, Papp T, Petkovits T, Pino-Bodas R, Powell MJ, Raja HA, Redecker D, Sarmiento-Ramirez JM, Seifert KA, Shrestha B, Stenroos S, Stielow B, Suh S-O, Tanaka K, Tedersoo L, Telleria MT, Udayanga D, Untereiner WA, Uribeondo JD, Subbarao KV, Vágvölgyi C, Visagie C, Voigt K, Walker DM, Weir BS, Weiß M, Wijayawardene NN, Wingfield MJ, Xu JP, Yang ZL, Zhang N, Zhuang W-Y, Federhen S (2014) Finding needles in haystacks: linking scientific names, reference specimens and molecular data for Fungi. Database 2014: bau061
Seifert KA, Morgan-Jones G, Gams W, Kendrick WB (2011) The genera of hyphomycetes. CBS Biodiversity Series no. 9. Utrecht, CBS-KNAW Fungal Biodiversity Centre
Shimizu K, Tanaka C, Peng YL, Tsuda M (1998) Phylogeny of Bipolaris inferred from nucleotide sequences of Brn1, a reductase gene involved in melanin biosynthesis. J Gen Appl Microbiol 44:251–25
Shoemaker RA (1959) Nomenclature of Drechslera and Bipolaris, grass parasites segregated from Helminthosporium. Can J Bot 37:879–887
Shoemaker RA (1998) Marielliottia, a new genus of cereal and grass parasites segregated from Drechslera. Can J Bot 76(9):1558–1569
Shoemaker RA, Babcock CE (1992) Applanodictyosporous Pleosporales: Clathrospora, Comoclathris, Graphyllium, Macrospora, and Platysporoides. Can J Bot 70:1617–1658
Siboe GM, Kirk PM, Cannon PF (1999) New dematiaceous hyphomycetes from Kenyan rare plants. Mycotaxon 73:283–302
Simmons EG (1986) Gibbago, a new phaeodictyoconidial genus of hyphomycetes. Mycotaxon 27:107–111
Sivanesan A (1984) The bitunicate ascomycetes and their anamorphs. J. Cramer, Vaduz
Sivanesan A (1987) Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycol Pap 158:1–261
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–269
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–777
Stielow B, Bubner B, Hensel G, Munzenberger B, Hoffmann P et al (2010) The neglected hypogeous fungus Hydnotrya bailii Soehner (1959) is a widespread sister taxon of Hydnotrya tulasnei (Berk.) Berk. and Broome (1846). Mycol Prog 9:195–203
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann- Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG (2009) Molecular systematics of the marine dothideomycetes. Stud Mycol 64:155–173
Sung G-H, Sung J-M, Hywel-Jones NL, Spatafora JW (2007) A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Mol Phylogenet Evol 44:1204–1223
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Tan YP, Madrid H, Crous PW, Shivas RG (2014) Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australas Plant Path 1–15
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM et al (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32
Telle S, Thines M (2008) Amplification of cox2 (620 bp) from 2 mg of up to 129 years old herbarium specimens, comparing 19 extraction methods and 15 polymerases. PLoS One 3:e3584
Thambugala KM, Ariyawansa HA, Li YM, Boonmee S, Hongsanan S, Tian Q, Singtripop C, Bhat DJ, Camporesi E, Jayawardena R, Liu ZY, Chukeatirote E, Hyde KD (2014a) Dothideales. Fungal Divers 68(1):105–158
Thambugala KM, Singtripop C, Chunfang Y, Mckenzie EH, Liu ZY, Chukeatirote E, Hyde KD (2014b) Towards a natural classification of Dothideomycetes 7: the genera Allosoma, Austropleospora, Dangeardiella, Griggsia and Karschia (Dothideomycetes incertae sedis). Phytotaxa 181(1):34–46
Theissen F, Sydow H (1918) Vorentw?rfe zu den Pseudosphaeriales. Ann Mycol 16:1–34
Udayanga D, Castlebury LA, Rossman AY, Chukeatirote E, Hyde KD (2014) Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex. Fungal Divers 67(1):203–229
Uilenberg G, Thiaucourt F, Jongejan F (2004) On molecular taxonomy: what is in a name? Exp Appl Acarol 32(4):301–312
Vassiljeva LN (1983) De Buergenerula thalictri (Wint.) E. Müller. Nov Sist Nizsh Rast 20:70–72
Verkley GJM, Dukik K, Renfurm R, Göker M, Stielow JB (2014) Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota). Persoonia 32:25–51
von Arx JA, Müller E (1975) A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Stud Mycol 9:1–159
von Arx JA, van der Aa HA (1983) Notes on Curreya (Ascomycetes, Dothideales). Sydowia 36:1–5
von Höhnel F (1918) Dritte vorläufige Mitteilung mycologischer Ergebnisse (nr. 201–304). Ber Deut Bot Ges 36:309–317
Wang Y, Geng Y, Ma J, Wang Q, Zhang X-G (2011) Sinomyces: a new genus of anamorphic Pleosporaceae. Fungal Biol 115:188–195
Wehmeyer LE (1961) A world monograph of the genus pleospora and its segregates. University of Michigan Press, Michigan
Wehmeyer LE (1975) The pyrenomycetous fungi. Mycologia Memoir No. 6. The New York Botanical Garden. J. Cramer, Germany
White TJ, Bruns TD, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: Guide Methods Appl 18:315–322
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Chomnunti P, Dai DQ, D’souza MJ, Diederich P, Dissanayake A, Doilom M, Hongsanan S, Jones EBG, Groenewald JZ, Jayawardena R, Lawrey JD, Liu JK, Lücking R, Madrid H, Manamgoda DS, Muggia L, Nelsen MP, Phookamsak R, Suetrong S, Tanaka K, Thambugala KM, Wikee S, Zhang Y, Aptroot A, Ariyawansa HA, Bahkali AH, Bhat JD, Gueidan C, De Hoog GS, Knudsen K, McKenzie EHC, Miller AN, Mortimer PE, Wanasinghe DN, Phillips AJL, Raja HA, Slippers B, Shivas RS, Taylor JE, Wang Y, Woudenberg JHC, Piątek M, Cai L, Jaklitsch WM, Hyde KD (2014) Naming and outline of dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers 69(1):1–55
Woudenberg JHC, Groenewald JZ, Binder M, Crous PW (2013) Alternaria redefined. Stud Mycol 75:171–212
Yusoff M, Moss ST, Jones EBG (1994) Ascospore ultrastructure of Pleospora gaudefroyi (Pleosporaceae, Loculoascomycetes, Ascomycotina). Can J Bot 72(1):1–6
Zhang Y, Schoch CL, Fournier J, Crous PW, De Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Hyde KD (2009) Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012) Pleosporales. Fungal Divers 52:1–225
Zhaxybayeva O, Gogarten JP (2002) Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genomics 3(1):4
Acknowledgments
MFLU grant number 56101020032 is thanked for supporting studies on Dothideomycetes. We are grateful to the Mushroom Research Foundation, Chiang Rai, Thailand for supporting studies on Dothideomycetes. Kevin D. Hyde thanks the Chinese Academy of Sciences, project number 2013T2S0030, for the award of Visiting Professorship for Senior International Scientists at Kunming Institute of Botany. Jian-Chu Xu and Peter E Mortimer would like to thank Humidtropics, a CGIAR Research Program that aims to develop new opportunities for improved livelihoods in a sustainable environment, for partially funding this work. H.A Ariyawansa and J.C. Kang are grateful to the agricultural science and technology foundation of Guizhou province (Nos. NY[2013]3042 ), the international collaboration plan of Guizhou province (No. G [2012]7006) and the innovation team construction for science and technology of Guizhou province (No. [2012]4007) from the Science and Technology Department of Guizhou province, China. Hiran Ariyawansa is grateful to A.D Ariyawansa, D.M.K Ariyawansa and Dhanuska Udayanga for their valuable suggestions. E.B. Gareth Jones is supported by the Distinguished Scientist Fellowship Program (DSFP), King Saud University, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 253 kb)
Rights and permissions
About this article
Cite this article
Ariyawansa, H.A., Thambugala, K.M., Manamgoda, D.S. et al. Towards a natural classification and backbone tree for Pleosporaceae . Fungal Diversity 71, 85–139 (2015). https://doi.org/10.1007/s13225-015-0323-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-015-0323-z