Abstract
This is a continuity of a series of taxonomic and phylogenetic papers on the fungi where materials were collected from many countries, examined and described. In addition to extensive morphological descriptions and appropriate asexual and sexual connections, DNA sequence data are also analysed from concatenated datasets to infer phylogenetic relationships and substantiate systematic positions of taxa within appropriate ranks. Wherever new species or combinations are proposed, we apply an integrative approach using morphological and molecular data as well as ecological features wherever applicable. Notes on 112 fungal taxa are compiled in this paper including Biatriosporaceae and Roussoellaceae, Didysimulans gen. nov., 81 new species, 18 new host records and new country records, five reference specimens, two new combinations, and three sexual and asexual morph reports. The new species are Amanita cornelii, A. emodotrygon, Angustimassarina alni, A. arezzoensis, A. italica, A. lonicerae, A. premilcurensis, Ascochyta italica, A. rosae, Austroboletus appendiculatus, Barriopsis thailandica, Berkleasmium ariense, Calophoma petasitis, Camarosporium laburnicola, C. moricola, C. grisea, C. ossea, C. paraincrustata, Colletotrichum sambucicola, Coprinopsis cerkezii, Cytospora gelida, Dacrymyces chiangraiensis, Didysimulans italica, D. mezzanensis, Entodesmium italica, Entoloma magnum, Evlachovaea indica, Exophiala italica, Favolus gracilisporus, Femsjonia monospora, Fomitopsis flabellata, F. roseoalba, Gongronella brasiliensis, Helvella crispoides, Hermatomyces chiangmaiensis, H. chromolaenae, Hysterium centramurum, Inflatispora caryotae, Inocybe brunneosquamulosa, I. luteobrunnea, I. rubrobrunnea, Keissleriella cirsii, Lepiota cylindrocystidia, L. flavocarpa, L. maerimensis, Lophiotrema guttulata, Marasmius luculentus, Morenoina calamicola, Moelleriella thanathonensis, Mucor stercorarius, Myrmecridium fluviae, Myrothecium septentrionale, Neosetophoma garethjonesii, Nigrograna cangshanensis, Nodulosphaeria guttulatum, N. multiseptata, N. sambuci, Panus subfasciatus, Paraleptosphaeria padi, Paraphaeosphaeria viciae, Parathyridaria robiniae, Penicillium punicae, Phaeosphaeria calamicola, Phaeosphaeriopsis yuccae, Pleurophoma italica, Polyporus brevibasidiosus, P. koreanus, P. orientivarius, P. parvovarius, P. subdictyopus, P. ulleungus, Pseudoasteromassaria spadicea, Rosellinia mearnsii, Rubroboletus demonensis, Russula yanheensis, Sigarispora muriformis, Sillia italica, Stagonosporopsis ailanthicola, Strobilomyces longistipitatus, Subplenodomus galicola and Wolfiporia pseudococos. The new combinations are Melanomma populina and Rubroboletus eastwoodiae. The reference specimens are Cookeina tricholoma, Gnomoniopsis sanguisorbae, Helvella costifera, Polythrincium trifolii and Russula virescens. The new host records and country records are Ascochyta medicaginicola, Boletellus emodensis, Cyptotrama asprata, Cytospora ceratosperma, Favolaschia auriscalpium, F. manipularis, Hysterobrevium mori, Lentinus sajor-caju, L. squarrosulus, L. velutinus, Leucocoprinus cretaceus, Lophiotrema vagabundum, Nothophoma quercina, Platystomum rosae, Pseudodidymosphaeria phlei, Tremella fuciformis, Truncatella spartii and Vaginatispora appendiculata and three sexual and asexual morphs are Aposphaeria corallinolutea, Dothiora buxi and Hypocrella calendulina.
Similar content being viewed by others
Table of contents
Ascomycota
Dothideomycetes
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss.
Asterinaceae Hansf.
Asterinales genera incertae sedis
491. Morenoina calamicola Konta & K.D. Hyde, in Fungal Diversity 83: 10 (2017), new species
Botryosphaeriales C.L. Schoch, Crous & Shoemaker
Botryosphaeriaceae Theiss. & Syd
492. Barriopsis thailandica Dissanayake, Senan., & K.D. Hyde, in Fungal Diversity 83: 13 (2017), new species
Capnodiales Woron
Mycosphaerellaceae Lindau
493. Polythrincium trifolii Kunze, in Kunze & Schmidt, Mykologische Hefte (Leipzig) 1: 14 (1817), reference specimen
Dothideales Lindau
Dothioraceae Theiss. & Syd.
494. Dothiora buxi Jayasiri, Camporesi & K.D. Hyde, in Fungal Diversity 81: 30 (2016), asexual morph report
Hysteriales Lindau
Hysteriaceae Chevall.
495. Hysterium centramurum Senan., in Fungal Diversity 83: 22 (2017), new species
496. Hysterobrevium mori (Schwein.) E.W.A. Boehm & C.L. Schoch, Stud. Mycol 64: 62 (2009), new host record
Pleosporales Luttr. ex M.E. Barr
Amorosiaceae Thambugala, K. D. Hyde
497. Angustimassarina alni Jayasiri & K.D. Hyde, in Fungal Diversity 83: 26 (2017), new species
498. Angustimassarina arezzoensis Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: XX (2017), new species
499. Angustimassarina premilcurensis Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 29 (2017), new species
500. Angustimassarina italica Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 33 (2017), new species
501. Angustimassarina lonicerae Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 28 (2017), new species
502. Biatriosporaceae K.D. Hyde
Lophiostomataceae Sacc.
503. Berkleasmium ariense Rajeshkumar & Marathe, in Fungal Diversity 83: 35 (2017), new species
504. Platystomum rosae Wanas., Thambug., Camporesi & K.D. Hyde, Fungal Diversity 74: 234 (2015), new host record
505. Sigarispora muriformis Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 37 (2017), new species
506. Vaginatispora appendiculata Wanas., E.B.G. Jones & K.D. Hyde, Studies in Fungi 1 (1): 60 (2016), new host record
Lophiotremataceae K. Hiray. & Kaz. Tanaka.
507. Lophiotrema guttulata Boonmee, Tibpromma & K.D. Hyde, in Fungal Diversity 83: 40 (2017), new species
508. Lophiotrema vagabundum (Sacc.) Sacc., Michelia 1 (4): 338 (1878), new host record
509. Hermatomyces chiangmaiensis J.F. Li, Bhat & K.D. Hyde, in Fungal Diversity 83: 45 (2017), new species
510. Hermatomyces chromolaenae J.F. Li, Mapook & K.D. Hyde, in Fungal Diversity 83: 47 (2017), new species
Melanommataceae G. Winter.
511. Melanomma populina (Died.) Phukhamsakda & K.D. Hyde, in Fungal Diversity 83: 49 (2017), new combination
512. Aposphaeria corallinolutea Gruyter, Aveskamp & Verkley, Stud. Mycol 75: 28 (2012), sexual morph report
Nigrogranaceae Jaklitsch & Voglmayr
513. Nigrograna cangshanensis Z.L. Luo, H.Y. Su & K.D. Hyde, in Fungal Diversity 83: 52 (2017), new species
514. Roussoellaceae J.K. Liu, Phook., D.Q. Dai & K.D. Hyde Q. Tian & K.D. Hyde
Thyridariaceae Q. Tian & K.D. Hyde
515. Parathyridaria robiniae Mapook, Camporesi & K.D. Hyde, in Fungal Diversity 83: 52 (2017), new species
Sub order Massarineae
Didymosphaeriaceae Munk.
516. Paraphaeosphaeria viciae de Silva, Camporesi & K.D. Hyde, in Fungal Diversity 83: 57 (2017), new species
Latoruaceae Crous
517. Pseudoasteromassaria spadicea W. Dong, H. Zhang & K.D. Hyde, in Fungal Diversity 83: 59 (2017), new species
Lentitheciaceae Y. Zhang ter, C.L. Schoch, J. Fourn., Crous & K.D. Hyde.
518. Keissleriella cirsii R.H. Perera, Bulgakov, Wanasinghe & K.D. Hyde, in Fungal Diversity 83: 62 (2017), new species
519. Pleurophoma italica Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 62 (2017), new species
Massarinaceae Munk
520. Pseudodidymosphaeria phlei Phukhams., Camporesi & K.D. Hyde, Fungal Diversity 78: 57 (2016), new host record
Massarineae genera incertae sedis
521. Inflatispora caryotae Wanasinghe & K.D. Hyde, in Fungal Diversity 83: 68 (2017), new species
Suborder Pleosporineae
Didymellaceae Gruyter, Aveskamp & Verkley
522. Ascochyta italica Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 70 (2017), new species
523. Ascochyta medicaginicola Q. Chen & L. Cai, Stud. Mycol. 82: 187 (2015), new host record
524. Ascochyta rosae Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 74 (2017), new species
525. Calophoma petasitis Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 74 (2017), new species
526. Didysimulans Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 76 (2017), new genus
527. Didysimulans italica Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 78 (2017), new species
528. Didysimulans mezzanensis Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 78 (2017), new species
529. Nothophoma quercina (Syd. & P. Syd.) Q. Chen & L. Cai, Stud. Mycol. 82: 213 (2015), new host record
530. Stagonosporopsis ailanthicola Manawasinghe, Camporesi & K.D. Hyde, in Fungal Diversity 83: 81 (2017), new species
Leptosphaeriaceae M.E. Barr
531. Paraleptosphaeria padi Phukhamsakda, Bulgakov, & K.D. Hyde, in Fungal Diversity 83: 82 (2017), new species
532. Subplenodomus galicola Phukhamsakda, Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 86 (2017), new species
Phaeosphaeriaceae M.E. Barr.
533. Entodesmium italica Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 88 (2017), new species
534. Neosetophoma garethjonesii Tibpromma, EBG Jones & K.D. Hyde, in Fungal Diversity 83: 88 (2017), new species
535. Nodulosphaeria guttulatum Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 92 (2017), new species
536. Nodulosphaeria multiseptata Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 92 (2017), new species
537. Nodulosphaeria sambuci Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 93 (2017), new species
538. Phaeosphaeria calamicola Konta & K. D. Hyde, in Fungal Diversity 83: 96 (2017), new species
539. Phaeosphaeriopsis yuccae Dayarathne, Bulgakov, E.B.G. Jones & K.D. Hyde, in Fungal Diversity 83: 96 (2017), new species
Pleosporineae genera incertae sedis
540. Camarosporium laburnicola R.H. Perera, Bulgakov, & K.D. Hyde, in Fungal Diversity 83: 99 (2017), new species
541. Camarosporium moricola Chethana, Bulgakov & K. D. Hyde, in Fungal Diversity 83: 101 (2017), new species
Eurotiomycetes
Eurotiales G.W. Martin ex Benny & Kimbr.
Trichocomaceae E. Fisch.
542. Penicillium punicae Hyang B. Lee, P.M. Kirk & T.T.T. Nguyen, in Fungal Diversity 83: 104 (2017), new species
543. Exophiala italica Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 83: 106 (2017), new species
Mucoromycotina Benny
Mucorales Fr.
Cunninghamellaceae Naumov ex R.K. Benj.
544. Gongronella brasiliensis C.A. de Souza, D.X. Lima & A.L. Santiago, in Fungal Diversity 83: 110 (2017), new species
Rhizopodaceae K. Schum.
545. Mucor stercorarius Hyang B. Lee, P.M. Kirk, K. Voigt & T.T.T. Nguyen, in Fungal Diversity 83: 111 (2017), new species
Sordariomycetes
Diaporthales Nannf.
Gnomoniaceae G. Winter
546. Gnomoniopsis sanguisorbae (Rehm) D.M. Walker, Mycologia 102 (6): 1494 (2010), reference specimen
Sydowiellaceae Lar.N. Vassiljeva
547. Sillia italica de Silva, Camporesi & K.D. Hyde, in Fungal Diversity 83: 117 (2017), new species
Valsaceae Tul. & C. Tul
548. Cytospora gelida Norphanphoun, Bulgakov, T.C. Wen & K.D. Hyde, in Fungal Diversity 83: 120 (2017), new species
549. Cytospora ceratosperma (Tode) G.C. Adams & Rossman, in Rossman, Adams, Cannon, Castlebury & Crous, IMA Fungus 6: 147 (2015), new record
Hypocreales (Lindau) Earle ex Rogerson
Clavicipitaceae Earle.
550. Hypocrella calendulina Hywel-Jones & Mongkolsamrit, Mycol. Res. 113 (6–7): 687 (2009), asexual morph reported
551. Moelleriella thanathonensis Y.P. Xiao, T.C. Wen, & K.D. Hyde, in Fungal Diversity 83: 125 (2017), new species
Stachybotryaceae L. Lombard & Crous
552. Myrothecium septentrionale J.F. Li, Phookamsak & K.D. Hyde, in Fungal Diversity 83: 129 (2017), new species
Glomerellales Chadef. ex Réblová et al.
Glomerellaceae Locq. ex Seifert & W. Gams
553. Colletotrichum sambucicola Jayawardena, Camporesi & K.D. Hyde, in Fungal Diversity 83: 131 (2017), new species
Myrmecridiales Crous
554. Myrmecridium fluviae Hyang B. Lee & T.T.T. Nguyen, in Fungal Diversity 83: 136 (2017), new species
Xylariales Nannf.
Bartaliniaceae Wijayawardene et al.
555. Truncatella spartii Senan., Camporesi & K.D. Hyde, Fungal Diversity 73: 91 (2015), new host record
Xylariaceae Tul. & C. Tul.
556. Rosellinia mearnsii Tennakoon, Phookamsak & K.D. Hyde, in Fungal Diversity 83: 139 (2017), new species
Ascomycota , families incertae sedis
Evlachovaea B.A. Borisov & Tarasov
557. Evlachovaea indica P.N. Singh, A. Baghela, S.K. Singh & S. Amir, in Fungal Diversity 83: 139 (2017), new species
Pezizomycetes
Pezizales J. Schröt.
Helvellaceae Fr.
558. Helvella costifera Nannf., Schriften der Naturforschende Gesellschaft zu Leipzig 1: 114 (1953), reference specimen
559. Helvella crispoides Q. Zhao & K.D. Hyde, in Fungal Diversity 83: 147 (2017), new species
Sarcoscyphaceae Le Gal ex Eckblad
560. Cookeina tricholoma (Mont.) Kuntze, Revis. gen. pl. (Leipzig) 2: 849 (1891), reference specimen
Basidiomycota
Agaricomycetes Doweld
Agaricales Underw.
Agaricaceae Chevall.
561. Amanita cornelii Mehmood, K. Das, Iqbal Hosen, Tulloss, & R.P. Bhatt, in Fungal Diversity 83: 152 (2017), new species
562. Amanita emodotrygon Mehmood, Tulloss, K. Das, Iqbal Hosen & R.P. Bhatt, in Fungal Diversity 83: 157 (2017), new species
563. Lepiota cylindrocystidia Sysouphanthong K.D Hyde & Vellinga, in Fungal Diversity 83: 160 (2017), new species
564. Lepiota flavocarpa Sysouphanthong, K.D. Hyde & Vellinga, in Fungal Diversity 83: 162 (2017), new species
565. Lepiota maerimensis Sysouphanthong K.D Hyde, & Vellinga, in Fungal Diversity 83: 163 (2017), new species
566. Leucocoprinus cretaceus (Bull.) Locq., Bull. mens. Soc. linn. Soc. Bot. Lyon 14: 93 (1945), new record
Entolomataceae Kotl. & Pouzar
567. Entoloma magnum K.N.A. Raj & Manim, in Fungal Diversity 83: 168 (2017), new species
Marasmiaceae Roze ex Kühner.
568. Inocybe brunneosquamulosa K.P.D. Latha & Manim, in Fungal Diversity 83: 172 (2017), new species
569. Inocybe luteobrunnea K.P.D. Latha & Manim, in Fungal Diversity 83: 176 (2017), new species
570. Inocybe rubrobrunnea K.P.D. Latha & Manim, in Fungal Diversity 83: 178 (2017), new species
571. Marasmius luculentus A.K. Dutta, K. Acharya & Antonín, in Fungal Diversity 83: 181 (2017), new species
Mycenaceae Overeem
572. Favolaschia auriscalpium (Mont.) Henn., Botanische Jahrbücher für Systematik Pflanzengeschichte und Pflanzengeographie 22: 93 (1895), new record
573. Favolaschia manipularis (Berk.) Teng, Zhong Guo De Zhen Jun [Fungi of China]: 760 (1963), new record
Physalacriaceae Corner
574. Cyptotrama asprata (Berk.) Redhead & Ginns, Canadian Journal of Botany 58 (6): 731 (1980), new record
Boletales E.-J. Gilbert
Boletaceae Chevall.
575. Austroboletus appendiculatus Semwal, D. Chakr., K. Das, Indoliya, D. Chakrabarty, S. Adhikari & Karunarathna, in Fungal Diversity 83: 189 (2017), new species
576. Boletellus emodensis (Berk.) Singer, Annales Mycologici 40: 19 (1942), new record
577. Rubroboletus demonensis Vasquez, Simonini, Svetasheva, Mikšík & Vizzini, in Fungal Diversity 83: 197 (2017), new species
578. Rubroboletus eastwoodiae (Murrill) Vasquez, Simonini, Svetasheva, Mikšík & Vizzini, in Fungal Diversity 83: 206 (2017), new combination
579. Strobilomyces longistipitatus D. Chakr., K. Das & S. Adhikari, in Fungal Diversity 83: 207 (2017), new species
Cantharellales Gäum.
Clavulinaceae Donk
580. Clavulina grisea Meiras-Ottoni & Gibertoni, in Fungal Diversity 83: 211 (2017), new species
581. Clavulina ossea Meiras-Ottoni & Gibertoni, in Fungal Diversity 83: 211 (2017), new species
582. Clavulina paraincrustata Meiras-Ottoni & Gibertoni, in Fungal Diversity 83: 212 (2017), new species
Polyporales Gäum.
Fomitopsidaceae Jülich
583. Fomitopsis flabellata Soares & Gibertoni, in Fungal Diversity 83: 215 (2017), new species
584. Fomitopsis roseoalba Soares, Ryvarden & Gibertoni, in Fungal Diversity 83: 215 (2017), new species
Polyporaceae Fr. ex Corda
585. Favolus gracilisporus H. Lee, N.K. Kim & Y.W. Lim, in Fungal Diversity 83: 219 (2017), new species
586. Lentinus sajor - caju (Fr.) Fr., Epicrisis Systematis Mycologici: 393 (1838), new record
587. Lentinus squarrosulus Mont., Annales des Sciences Naturelles Botanique 18: 21 (1842), new record
588. Lentinus velutinus Fr., Linnaea 5: 510 (1830), new record
589. Panus subfasciatus Thongbai, Karunarathna & K.D. Hyde, in Fungal Diversity 83: 228 (2017), new species
590. Polyporus brevibasidiosus H. Lee, N.K. Kim & Y.W. Lim, in Fungal Diversity 83: 230 (2017), new species
591. Polyporus koreanus H. Lee, N.K. Kim & Y.W. Lim, in Fungal Diversity 83: 230 (2017), new species
592. Polyporus orientivarius H. Lee, N.K. Kim & Y.W. Lim, in Fungal Diversity 83: 232 (2017), new species
593. Polyporus parvovarius H. Lee, N.K. Kim & Y.W. Lim, in Fungal Diversity 83: 234 (2017), new species
594. Polyporus subdictyopus H. Lee, N.K. Kim & Y.W. Lim, in Fungal Diversity 83: 235 (2017), new species
595. Polyporus ulleungus H. Lee, N.K. Kim & Y.W. Lim, in Fungal Diversity 83: 236 (2017), new species
596. Wolfiporia pseudococos F. Wu, J. Song & Y.C. Dai, in Fungal Diversity 83: 237 (2017), new species
Psathyrellaceae Locq.
597. Coprinopsis cerkezii Tkalčec, Mešić, I. Kušan & Matočec, in Fungal Diversity 83: 239 (2017), new species
Russulales Pers.
Russulaceae Lotsy
598. Russula yanheensis T.C. Wen, K. Hapuarachchi & K.D. Hyde, in Fungal Diversity 83: 244 (2017), new species
599. Russula virescens (Schaeff.) Fr., Anteckningar öfver de i Sverige växande ätliga svampar: 50 (1836), reference specimen
Dacrymycetales Henn.
Dacrymycetaceae J. Schröt.
600. Dacrymyces chiangraiensis Ekanayaka, S. C. Karunarathna, Q. Zhao & K. D. Hyde, in Fungal Diversity 83: 248 (2017), new species
601. Femsjonia monospora Ekanayaka, S. C. Karunarathna, Q. Zhao & K. D. Hyde, in Fungal Diversity 83: 251 (2017), new species
Tremellales Fr.
Tremellaceae Fr.
602. Tremella fuciformis Berk., Hooker’s Journal of Botany and Kew Garden Miscellany 8: 277 (1856), new record
Materials and methods
Sampling, isolation and identification
Specimens in this study were collected from Brazil, China, Croatia, Germany, India, Italy, Korea, Russia, Sri Lanka, Thailand and the UK. Soil samples to isolate Gongronella brasiliensis were collected from Taquaritinga do Norte, state of Pernambuco, Brazil, following methods outlined in Benny (2008). Gongronella brasiliensis was cultured in triplicate, in MEA and PDA, and incubated at 10, 15, 20, 25 and 30 °C, over 7 days. Colony characteristics were recorded, while alternative morphs were induced in culture using sterilized pieces of plant material (Phookamsak et al. 2015). Plant material was examined with a Carl Zeiss GmbH (AxioCam ERC 5 S) stereo microscope. Fruiting bodies of ascomycetes were rehydrated in water, lactic acid or 5% KOH. Cut sections were observed with a compound microscope; micro-morphological characteristics (e.g. ascomata sections, peridium structures, asci and ascospores in ascomycetes and basidia, cystidia, basidiospores in basidiomycetes) were examined, described and photographed. Measurements (e.g. from basidiospores and ascospores) were taken from at least 20 representatives and the mean and the standard deviation for length and width, together with the range of spore quotient (Q, the length/width ratio) and its mean value (Qm) are given. Herbarium specimens and ex-type living cultures were deposited in various collections and are listed under each taxon description. Facesoffungi numbers (FoF) and Index Fungorum (IF) numbers were obtained as explained in Jayasiri et al. (2015) and Index Fungorum (2017). New species were established as per recommendations outlined by Jeewon and Hyde (2016).
DNA extraction, PCR amplification and sequencing
For most ascomycetous fungal samples, total genomic DNA was extracted from fresh fungal mycelium grown on appropriate media at 16 °C or room temperature (as outlined by Jeewon et al. 2002), while for basidiomycetes, dry fruiting bodies were used. DNA was extracted following the instructions of the Biospin Fungus Genomic DNA Extraction Kit (BioFlux®, Hangzhou, P.R. China) or other fungal DNA extraction kits according to the manufacturer’s instructions. When fungi failed to grow in culture, DNA was extracted directly from ascomycete fruiting bodies using aseptic techniques. For Gongronella brasiliensis, fungal biomass was obtained in MEA cultures in test tubes kept at 28 °C for up to six days and genomic DNA extraction of G. brasiliensis was carried out with previously macerated material according to Góes-Neto et al. (2005).
DNA amplification for all samples was performed by Polymerase Chain Reaction (PCR) using universally standard primers. The primers used and PCR conditions are shown in the Table 1. For Gongronella brasiliensis specimens, thermal profiling and amplification reactions of ITS regions were conducted as described by Oliveira et al. (2014) and the final amplicons were purified with the PureLink PCR Purification Kit (Invitrogen), sequenced directly or cloned with a Clone JETTM PCR Cloning Kit (Fermentas; Carlsbad, USA), following the manufacturer’s instructions. The quality of the PCR products was checked by electrophoresis on 1% agarose gels. Purification and sequencing of PCR products (with same primers used in PCR) was done by commercial sequencing providers depending on the regions where the studies were carried out.
Sequence alignment and phylogenetic analyses
A careful verification of sequence data generated in this study was carried out with appropriate reference sequences following BLAST searches in the nucleotide database of GenBank (http://blast.ncbi.nlm.nih.gov/). It was ensured that no erroneous sequences were used in the further analyses and then data were submitted to GenBank. Following sequence verification and Blast search, DNA sequences from appropriate taxonomic ranks were downloaded based on recent publications to construct datasets for phylogenetic analyses. BioEdit sequence alignment editor (Hall 1999), CLUSTALX (Larkin et al. 2007), Mega 6.0.5 (Tamura et al. 2013) and MAFFT: multiple sequence alignment software version 7.215 (Katoh et al. 2002) were used for sequence alignment. Under most circumstances, concatenated DNA datasets were analyzed to generate phylogenetic trees, but in cases of limited availability of DNA sequences from respective gene regions, phylogenies were inferred from single or a combination of two gene datasets and either a consensus of these gene phylogenies or one of the most parsimonious phylogenies was used to infer phylogenetic relationships across taxa sampled. Selection of outgroup(s) for rooting purposes was based on knowledge of potential common ancestors to the in-group as well as taxon sampling from previously published studies. Phylogenetic analyses were performed by neighbor joining (NJ), maximum parsimony (MP), maximum likelihood (RAxML) and Bayesian inference (BI) analyses. The maximum parsimony analyses were performed using PAUP v. 4.0b10 (Swofford 2003), bootstrap analysis with 1000 replicates. All multiple, equally parsimonious trees were saved and descriptive tree statistics for parsimony consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI) were calculated. Other details are as described by Jeewon et al. (2003b, 2004, 2013), Hu et al. (2007) and Promputtha et al. (2007). The robustness of the best parsimonious tree was estimated by a bootstrap (BT) value with 1000 replicates, each with 100 replicates of random stepwise addition of taxa (Liu et al. 2011, 2012). Maximum likelihood (ML) analysis was performed using RAxMLGUI v. 1.3 (Silvestro and Michalak 2010) with 1000 rapid bootstrap replicates. The substitution model comprised a generalized time reversible (GTR) for nucleotides with a discrete gamma distribution (Silvestro and Michalak 2012). Bayesian analyses were performed by MrBayes v. 3.0b4 (Ronquist and Huelsenbeck 2003) with the best-fit model of sequence evolution estimated with MrModeltest 2.2 as described in Nylander et al. (2008). Markov Chain Monte Carlo sampling (BMCMC) was used to determine the posterior probabilities (PP) (Rannala and Yang 1996; Zhaxybayeva and Gogarten 2002) in MrBayes v. 3.0b4 (Ronquist and Huelsenbeck 2003). Six simultaneous Markov chains were run at least 1000,000 generations or depending on individal settings for the fungal group and trees were sampled every 100th/1,000th generation (Cai et al. 2006). Tracer v. 1.6 (Rambaut and Drummond 2003) was used to check the effective sampling sizes (ESS) and burn-in value. The run was stopped automatically as soon as the average standard deviation of split frequencies fell below 0.01 (Maharachchikumbura et al. 2015). Phylograms were visualized in Treeview (Page 2001) or FigTree 1.4.2 (Rambaut 2014) with bootstrap values above or below the nodes and reorganized in Adobe Illustrator CS v. 6., Microsoft Powerpoint (v.2007), portable document format (PDF) and Adobe Photoshop version CS3 (Adobe Systems, USA). All the sequences generated in this study were deposited in GenBank and accession numbers have been provided where appropriate.
Dothideomycetes
Dothideomycetes is the largest class of Ascomycota. In this study, we follow the classifications in the recent studies of Hyde et al. (2013) and Wijayawardene et al. (2014a).
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss.
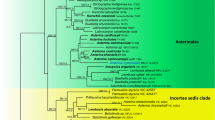
Hyde et al. (2013) accepted Asterinaceae, Aulographaceae, and Parmulariaceae in Asterinales and a revision of genera in Asterinales was provided by Hongsanan et al. (2014). In recent publications members of Asterinales clustered in two clades (Ertz et al. 2016; Hyde et al. 2016). Hyde et al. (2016) treated the Asterinales clade (not clade containing Parmulariaceae) as Asterinales sensu stricto, because most of the Asterinales strains, from both Asterina and Lembosia clustered in this clade and also because the large clade that contains Asterinales as circumscribed by Ertz et al. (2016) does not have any phylogenetic support. Hyde et al. (2016) also synonymized the younger Asterotexiales under Asterinales. In this paper, we provide an updated tree for Asterinales (Fig. 1).
Phylogram generated from maximum likelihood and Bayesian analyses based on LSU sequence data from selected species of Asterinales. The RAxML bootstrap value greater than 50% and the posterior probabilities value ≥0.95 are shown above the nodes. Lichenized taxa are in green and lichenicolous fungi in red. The new isolate is in blue bold and other ex-type strains are in black bold. The tree is rooted with Saccharomyces cerevisiae
Asterinaceae Hansf.
The family Asterinaceae has characteristic superficial, web-like, black colonies on the upper and lower surfaces of leaves (Hyde et al. 2013). Hofmann et al. (2010) placed Asterinaceae in Dothideomycetes based on multi-gene sequences, and this was substantiated by Hongsanan et al. (2014).
Morenoina Theiss
= Aulographella Höhn.
Morenoina was introduced by Theissen (1913) with Morenoina antarctica (Speg.) Theiss. as the type species. Morenoina resembles Aulographum, differing only in the morphology of the scutellum, which comprises inordinately arranged cells and a hypostroma of subcuticular hyphae beneath the thyriothecium (Ellis 1980).
Morenoina calamicola Konta & K. D. Hyde, sp. nov.
Index Fungorum number: IF552661; Facesoffungi number: FoF02757, Fig. 2
Morenoina calamicola (MFLU 15-0013, holotype). a Appearance of thyriothecia on host substrate. b Close up of thyriothecium. c, e Cell walls of thyriothecium with radial arrangement. d Section of thyriothecium. f–i Asci. j–k Ascospores. l Germinated ascospore. m Culture on MEA media. Scale bars a = 500 μm, b = 100 μm, c, d = 20 μm, e–i = 10 μm, j, k = 5 μm, l = 20 μm
Phylogram generated from maximum parsimony analysis of all available type/authentic sequences of Barriopsis based on combined ITS and TEF1 sequence data. Parsimony bootstrap support values for MP ≥ 75% and Bayesian posterior probabilities ≥0.9 are indicated on the nodes. Isolate numbers of all ex-types and reference strains are in bold. Novel species are indicated in blue. The tree is rooted with Sphaeropsis visci
Holotype: MFLU 15-0013
Saprobic on Calamus sp. Sexual morph Thyriothecia 100–325 μm, occurring on host surface, solitary, aggregated, or gregarious, easily removed from the host surface, superficial, ellipsoid, oblong, curved, flat, with longitudinal, slit-like opening, linear fissure, which are branched at the margin, from the center to the outer rim, lacking free hyphae and appressoria at the margin, in transverse Sect. 24–33 µm high × 86–121 µm diam. (\( \bar{x} \) = 27 × 103 μm, n = 5). Upper wall comprising linear cells, with irregular, filiform hyphae, radiating from the center to the outer rim. Asci 13–20 × 10–17 μm (\( \bar{x} \) = 17 × 14 μm, n = 10), 8-spored, bitunicate, globose to subglobose or clavate, or saccate to globose, apedicellate, with a distinct, thickened apical region. Ascospores 9–11 × 3–4 μm (\( \bar{x} \) = 10 × 3.9 μm, n = 10), irregularly arranged, oblong or fusiform, wider at apex, with slightly acute ends, 1–septate, with two large guttules in each cell, hyaline, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on MEA within 24 h and germ tube produced from each cell. Colonies on MEA reaching 3–4 cm diam., after 5 days, grey to olivaceous, dense, with fairly fluffy surface, hyphae, septate, branched and smooth-walled.
Material examined: THAILAND, Chiang Mai Province, on dead stem of Calamus sp. (Arecaceae), 11 August 2014, Sirinapa Konta, P04a (MFLU 15-0013, holotype; ex-type living culture, MFLUCC 14-1162).
GenBank Numbers LSU: KY511424; ITS: KY511430; SSU: KY511427.
Notes: Hongsanan et al. (2014) transferred Morenoina to Aulographaceae based on morphology and phylogeny. In the current phylogenetic analyses, Morenoina calamicola clustered with Melaspilea lekae (Melaspileaceae) (94% in ML, 1.00 in BYPP). The morphology of Morenoina calamicola is distinct and Melaspilea lekae is lichenicolous (Kalb et al. 2012). Morenoina calamicola is similar to Aulographaceae (Asterinales) in lacking appressoria and flattened, elongate thyriothecia, opening by a slit. In the phylogenetic analyses (Fig. 1) Morenoina calamicola is well-separated from Aulographaceae.
Morenoina calamicola is most similar to M. palmicola J. Fröhl., K.D. Hyde & Joanne E. Taylor in characteristics of asci, ascospores and host genus (Calamus australis), but differs in the size and shape of ascospores.
Botryosphaeriales C.L. Schoch et al.
Botryosphaeriales is a diverse order with a worldwide distribution, comprising species that vary from endophytes to pathogens (Slippers and Wingfield 2007; Phillips et al. 2013; Chethana et al. 2016; Daranagama et al. 2016; Dissanayake et al. 2016; Giambra et al. 2016; Konta et al. 2016a, b; Linaldeddu et al. 2016a, b, c; Manawasinghe et al. 2016; Tennakoon et al. 2016; Zhang et al. 2017). Since it was introduced by Schoch et al. (2006) this order has undergone significant taxonomic changes with the addition of several new families. Currently, nine families are recognized, namely, Aplosporellaceae, Botryosphaeriaceae, Endomelanconiopsisaceae, Melanopsaceae, Phyllostictaceae, Planistromellaceae, Pseudofusicoccumaceae, Saccharataceae and Septorioideaceae (Schoch et al. 2006; Minnis et al. 2012; Wikee et al. 2013; Slippers et al. 2013; Wyka and Broders 2016; Dissanayake et al. 2016; Yang et al. 2017), although several authors considered this to be too many (Chethana et al. 2016; Daranagama et al. 2016; Dissanayake et al. 2016; Giambra et al. 2016; Konta et al. 2016a, b; Linaldeddu et al. 2016a, b, c; Manawasinghe et al. 2016; Tennakoon et al. 2016; Liu et al. 2016; Zhang et al. 2017).
Botryosphaeriaceae Theiss. & Syd.
The family Botryosphaeriaceae was described in the 1820s, originally for species of Sphaeria (Fr.) (Crous et al. 2006; Schoch et al. 2006). von Arx and Müller (1954). Barr (1987) included only nine genera, which are mostly different from those of von Arx and Müller (1954). Hawksworth et al. (1995) listed five genera. Lumbsch and Huhndorf (2010) included eleven genera, while Hyde et al. (2011) and Wijayawardene et al. (2012) listed 16 genera. Liu et al. (2012) included 17 genera in the family based on molecular data and examination of generic types. Phillips et al. (2013) provided comprehensive descriptions, notes and phylogenies for the 17 genera and 110 species known from cultures. Twenty-three genera and 187 species of Botryosphaeriaceae were listed by Dissanayake et al. (2016).
Barriopsis A.J.L. Phillips, A. Alves & Crous
Barriopsis was introduced when Phillips et al. (2008) transferred Physalospora fusca to Barriopsis fusca. Stevens (1926) originally placed this species in Physalospora, but was obviously hesitant to do so on account of its brown ascospores. Petrak and Deighton (1952) then transferred it to Phaeobotryosphaeria as P. fusca. Although von Arx and Müller (1954) considered Phaeobotryosphaeria a synonym of Botryosphaeria, Phillips et al. (2008) showed that it is morphologically and phylogenetically distinct from other genera in Botryosphaeriaceae. However, the fungus considered by Stevens (1926) and Petrak and Deighton (1952) does not have apiculi on its ascospores, and thus does not fall within the concept of Phaeobotryosphaeria, which has small, hyaline apiculi on the ascospores. For this reason Phillips et al. (2008) introduced the genus Barriopsis. Dissanayake et al. (2016) list four species.
Barriopsis thailandica Dissanayake, Senan. & K.D. Hyde, sp. nov.
Index Fungorum number: IF551363; Facesoffungi number: 00913, Figs. 4, 5
Etymology: Name refers to the country Thailand, where the fungus was collected. Holotype: MFLU 15-1414
Saprobic on decaying bark of Tectona grandis L.f. Sexual morph Ascostromata 170–220 μm high × 150–170 μm diam. (\( \bar{x} \) = 194 × 160 μm, n = 10), scattered, initially immersed, becoming erumpent through bark when mature, solitary or gregarious, uniloculate, globose to subglobose, or flask-shaped when cut horizontally, coriaceous, ostiolate, papillate. Papilla 70–80 × 60–65 μm (\( \bar{x} \) = 77 × 64 μm, n = 10), short, periphysate. Periphyses 15–20 μm (\( \bar{x} \) = 20 μm, n = 10), brown, curved towards the outside, long, flat, leaf-like. Peridium 20–45 μm wide (\( \bar{x} \) = 30 μm, n = 10) comprising outer, 5–10 layers of brown, thick-walled, large cells of textura angularis to textura globulosa and inner, 4–7 layers of hyaline, thin-walled, small cells of textura angularis to textura globulosa. Hamathecium comprising 2–4 μm wide (\( \bar{x} \) = 3.5 μm, n = 10), hypha-like, numerous, septate, pseudoparaphyses, slightly constricted at septa. Asci 50–110 × 19–21 μm (\( \bar{x} \) = 70 × 21 μm, n = 20), (2) 4 (8)-spored, bitunicate, fissitunicate, cylindric-clavate or clavate, with long or short pedicel, apically wider than base, apex rounded with an ocular chamber. Ascospores 15–20 × 9–11 μm (\( \bar{x} \) = 18 × 10 μm, n = 30), mostly biseriate, rarely overlapping uniseriate, ellipsoidal or rhomboidal, inequilateral, wider in the center, initially hyaline, becoming yellow, pale brown to reddish-brown when mature, aseptate, ends blunt when mature, thick-walled, smooth-walled or verruculose. Asexual morph Conidiomata stromatic, uniloculate, dark brown to black. Hamathecium 20–35 μm long (\( \bar{x} \) = 28 μm, n = 20), hyaline, cylindrical, mostly aseptate, sometimes branched, ends slightly swollen and rounded, arising amongst the conidiogenous cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–7 × 4–6 μm (\( \bar{x} \) = 6.5 × 5 μm, n = 20), hyaline, smooth, cylindrical, slightly swollen at the base, enteroblastic, proliferating percurrently to form one or two closely spaced annellations. Conidia 15–19 × 7.5–8.5 μm (\( \bar{x} \) = 17 × 7.8 μm, n = 20), ellipsoidal at apex, rounded at base, widest at the center, thick-walled, initially hyaline, aseptate, becoming 1-septate, dark brown, striated after released from the conidiomata.
Culture characteristics: Colonies on MEA reaching 3–4 cm diam., after 5 days in the dark at 18 °C, flattened, velvety, lobate, fimbriate, initially white at the edge, becoming olive green in the center, after 7 days becoming greenish-ash, woolly with erect mycelia.
Material examined: THAILAND, Uttaradit Province, on decaying bark of Tectona grandis (Lamiaceae), 12 October 2014, I.C. Senanayake (MFLU 15-1414, holotype), ex-type living culture MFLUCC 14-1190, KUMCC 16-0185.
GenBank Numbers ITS:KY115675; TEF1:KY115676.
Notes: Barriopsis is one of the less well-known genera in the family Botryosphaeriaceae. To date only four species; Barriopsis archontophoenicis, B. fusca, B. iraniana and B. tectonae have been reported for this genus (Phillips et al. 2008; Abdollahzadeh et al. 2009; Doilom et al. 2014, Konta et al. 2016a, b). Barriopsis tectonae has both sexual and asexual morphs (Doilom et al. 2014; Konta et al. 2016a, b). We describe both a sexual and asexual morph for Barriopsis thailandica (Figs. 4, 5). The asexual morph formed in culture grown on MEA (Fig. 5). In the phylogenetic analysis of combined ITS and TEF1 sequence data, this new taxon is related to B. iraniana (Fig. 3), but phylogenetically distinct with very good support (100% maximum parsimony and 1.0 in Bayesian analysis) to justify its establishment as a new species. The small conidial dimensions of B. thailandica (17 × 8 μm, L/W ratio = 2.1) clearly distinguish it from B. iraniana (27 × 16 μm, L/W ratio = 1.6). The asexual morph has not been reported for B. iraniana, whereas that of B. thailandica is reported herein.
Capnodiales Woron.
The order Capnodiales introduced by Woronichin (1925) was accepted in Dothideomycetes based on phylogenetic studies (Schoch et al. 2006, 2009; Crous et al. 2009; Chomnunti et al. 2014). The Capnodiales are mainly defined by their shared ecological niches (Hughes 1976; Crous et al. 2009; Chomnunti et al. 2011; Hyde et al. 2013).
Mycosphaerellaceae Lindau
Mycosphaerellaceae is a large family in the order Capnodiales (class Dothideomycetes). Species in Mycosphaerellaceae have cosmopolitan distributions, occurring on various hosts worldwide (Crous et al. 2009, 2013a, b; Schoch et al. 2009; Farr and Rossman 2012; Hyde et al. 2013; Quaedvlieg et al. 2013). Some genera of Mycosphaerellaceae e.g. Cercospora, Pallidocercospora, Pseudocercospora and Septoria contain important and well known pathogens, causing serious diseases of economic crops (Crous et al. 2007, 2009, 2013a, b; Cheewangkoon et al. 2008; Schoch et al. 2009; Hyde et al. 2013; Quaedvlieg et al. 2013). Several sexual genera in Mycosphaerellaceae are morphologically similar, but can be distinguished by their asexual morphs (Crous et al. 2007, 2009, 2013a, b). Many phylogenetic studies of the asexual genera in Mycosphaerellaceae have been carried out (Crous and Braun 2003; Crous et al. 2007, 2009, 2013a, b; Quaedvlieg et al. 2013). However, phylogenetic affinities of sexual genera such as Achorodothis, Polythrincium (=Cymadothea), Euryachora, Gillotia, Melanodothis, Polyascosporella and Wernerella are largely unresolved partially due to limited availability of collections and DNA sequence data (Hyde et al. 2013). The phylogeny of Polythrincium trifolii is shown in Fig. 6.
The best scoring of the RAxML tree based on analysis of combined dataset of ITS and LSU sequence data. Bootstrap support values for maximum parsimony (right) and maximum likelihood (left) greater than 50% are given above the nodes. Bayesian posterior probabilities (BYPP) greater than 0.95 are shown as bold branches. The tree is rooted to Dissoconium aciculare. The new strain is indicated by bold blue. Ex-type strains are shown in bold
Polythrincium Kunze
Polythrincium was introduced in Kunze and Schmidt (1817) and is typified by P. trifolii Kunze. Polythrincium trifolii is an obligate pathogen causing sooty blotch of clover in temperate regions (Simon et al. 2005a, b, 2009; Rossman et al. 2015). The sexual morph of Polythrincium has been reported as Cymadothea trifolii (Wolf 1935) and it is characterized by small, black, pustulate ascostromata on the lower surface of clover leaflets, bitunicate, clavate asci, lack of pseudoparaphyses and hyaline to subhyaline, oblong to clavate and 1-septate ascospores (Wolf 1935; Simon et al. 2009).
Polythrincium is relatively poorly studied with only seven epithets in Index Fungorum (2017), but rDNA based phylogenies indicate that P. trifolii belongs to the family Mycosphaerellaceae (Simon et al. 2009).
Polythrincium trifolii Kunze, in Kunze & Schmidt, Mykologische Hefte (Leipzig) 1: 14 (1817) Synonym: Cymadothea trifolii (Pers.) F.A. Wolf, Mycologia 27(1): 71 (1935)
Facesoffungi number: FoF 2765, Fig. 7
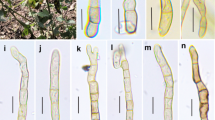
Polythrincium trifolii (HKAS 96227, reference specimen). a–c Appearance of fungal colonies on lower surface of white clover leaves. d, e Section through sporodochia arising from leaf tissue. f, g Conidiophores. h–j Conidiogenous cells with developing conidia. k–q Conidia. Scale bars d–f = 50 µm, g = 20 µm, h–j = 10 µm, k–q = 5 µm
Reference specimen: HKAS 96227
Obligate biotroph causing black blotch or sooty blotch on leaves of Trifolium repens L. Colonies on host substrate brown to dark brown, scattered to gregarious, punctiform, effuse to dense, eventually covering most of the leaf surface. Mycelium immersed, brown to dark brown, smooth-walled. Sexual morph reported as Cymadothea trifolii (Wolf 1935). Asexual morph hyphomycetous forming on lower surface of leaves. Sporodochia 40–120 µm high, 35–80 µm diam., with cushion-like pseudoparenchymatous cells at the base, composed of compact, fasciculate, foot-like cells at the base, with tightly aggregated, parallel, cylindrical to subcylindrical conidiophores. Conidiophores (25–)30–60(–62) × (4.5–)5–7 µm (\( \bar{x} \) = 45.1 × 6.2 µm, n = 50), macronematous, mononematous, caespitose, erect to flexuous, unbranched, aseptate, torsive, brown to dark brown, smooth-walled, largely ampulliform at the base, undulated at the upper part. Conidiogenous cells polyblastic, sympodial, integrated, terminal, cylindrical, undulate, with large, cicatrized scars. Conidia (21–)24–27(–29) × (13–)15–17(–19) µm (\( \bar{x} \) = 25.3 × 16.6 µm, n = 50), acropleurogenous, solitary, simple, cuneiform to pyriform, brown, 1-septate, rough-walled, verruculose, upper cell larger than lower cell.
Material examined: CHINA, Yunnan Province, Kunming Institute of Botany, on lower surface of leaves of white clover Trifolium repens L., (Fabaceae), 19 September 2016, R. Phookamsak, KIB001 (HKAS 96227, reference specimen designed here).
GenBank Numbers HKAS96227A LSU:KY554951; ITS:KY554953. HKAS96227B LSU:KY554952.
Notes: Polythrincium trifolii differs from P. shiraianum Henn. and P. trifolii var. platense Speg. in having larger conidia (P. shiraianum = 15–30 × 7–8 µm and P. trifolii var. platense = 16–18 × 12–14 µm) (Hennings 1905; Spegazzini 1910). Polythrincium lathyrinum Syd. has conidia of a similar size (P. trifolii: (21–)24–27(–29) × (13–)15–17(–19) µm; P. lathyrinum: 22–32 × 15–18 µm), but occurs on Lathyrus maritimus Willd. (Farr and Rossman 2012) (Sydow 1928). while P. trifolii has been reported from Trifolium, Astragalus sinicus L. (from China) and Stemphylium sarcinaeforme (Cavara) Wiltshire. Phylogenetic analyses based on combined LSU and ITS sequence data show that P. trifolii belongs to Mycosphaerellaceae and concurs with Simon et al. (2009). This is the first report on T. repens from China.
Dothideales Lindau
Thambugala et al. (2014b) revised the order Dothideales and synonymized Dothioraceae under Dothideaceae, and accepted only two families in the order.
Dothioraceae Theiss. & Syd.
Dothideaceae was established by Chevallier (1826) as “Dothideae”, and later Fuckel (1870) introduced Dothidea as the type genus and D. gibberulosa (Fr.) Fr. as the type species. The characteristic features of Dothideaceae are immersed to erumpent or superficial, 1 or multi-loculate ascostromata; 8-or polysporous, bitunicate asci and hyaline or brown, transversely septate, sometimes muriform (Dothiora Fr.) ascospores (Thambugala et al. 2014b). Dothideaceae was revised by Thambugala et al. (2014b) who included ten sexual genera and five asexual genera. The phylogeny of Dothiora buxi along with other members of the Dothideaceae is shown in Fig. 8.
RAxML maximum likelihood phylogenetic tree based on analysis of LSU and ITS sequence data from species of Dothideaceae. Maximum likelihood bootstrap values greater than 50% are shown above the nodes. The new isolates are in blue and other ex-type strains are in bold. The tree is rooted with Elsinoë phaseoli
Dothiora Fr.
Dothiora was introduced by Fries (1849) with D. pyrenophora (Fr.) Fr. as the type species. Thambugala et al. (2014b) based a study of Dothiora on both morphology and phylogeny.
Dothiora buxi Jayasiri, Camporesi & K.D. Hyde, in Fungal Diversity 81: 30 (2016)
Facesoffungi number: FoF00078, Fig. 9
Dothiora buxi (MFLU 15-2356, asexual morph). a Conidiomata on the upper leaf surface. b Conidiomata on the lower leaf surface. c–e Close up of conidiomata. f Section of conidiomata. g Cells of peridium. h–o Conidiogenous cells. p–u Conidia. v Germinated conidium. Scale bars a, b = 5 mm, c = 1000 μm, d = 500 μm, e = 200 μm, f = 100 μm, g = 20 μm, h–u = 5 μm, v = 10 μm
Saprobic and/or parasitic on dying leaves and twigs (hemibiotrophic) of Buxus sempervirens L. Sexual morph in Hyde et al. 2016. Asexual morph Conidiomata 182–263 µm high × 383–447 µm diam. (\( \bar{x} \) = 232 × 422 μm, n = 5), pycnidial, globose to subglobose, scattered, solitary, visible as brown to black, pustulate on the lower leaf surface, semi-immersed to erumpent, exposed by breaking leaf epidermis. Peridium 21–43 μm wide, comprising 1–3 layers of thickened, pale brown, cells arranged in textura prismatica. Conidiogenous cells 11–17 × 6–11 µm diam. (\( \bar{x} \) = 14 × 9 μm, n = 10), phialidic, subglobose, hyaline. Conidia 10–16 × 6–19 µm diam. (\( \bar{x} \) = 13 × 7 μm, n = 10), ellipsoid to obovoid, rounded at top, narrow at base, guttulate, smooth, 1-celled, hyaline.
Culture characteristics: Colonies on MEA, reaching 2–2.5 cm diam. after 10 days at 16 °C, flat, margin with entire edge, white, producing conidiomata and numerous conidia.
Material examined: RUSSIA, Rostov Region, Shakhty City, 20th anniversary of Red Army microdistrict, street plantation, street shrubs, dying leaves and twigs (hemibiotrophic) of Buxus sempervirens, 1 May 2015, T. Bulgakov, T421 (MFLU 15-2356, asexual morph); living culture MFLUCC 15-0823.
GenBank Numbers LSU:KY511425; SSU:KY511428 (Fig. 10).
Phylogram generated from maximum parsimony analysis based on LSU, SSU and TEF1 sequence data from taxa of Hysteriaceae. Maximum likelihood bootstrap support values greater than 70% are shown above the nodes. The tree is rooted with Delitschia winteri. Some branches were shortened to fit the page—these are indicated by two diagonal lines with the number of times a branch was shortened indicated next to the lines. The new isolate is in blue
Notes: Fries (1849) introduced the genus Dothiora, with D. pyrenophora (Fr.) Fr. as the type species. In the current study, the phylogenetic analyses indicate that our strain is closely related to Dothiora buxi Jayasiri, Camporesi & K.D. Hyde (MLFU 15-3404) with 92% bootstrap support. The morphology could not be compared as the latter was reported in its sexual morph with no information on its asexual morph (Hyde et al. 2016). However, we found the asexual morph on the same host, Buxus sempervirens. In culture, conidiomata were produced from germinated ascospores after one week’s incubation. Conidiomata, conidiogenous cells, and conidia found in culture are similar to those of D. buxi found on the host. Thus, we introduce the new record of asexual morph of D. buxi based on the morphological and phylogenetic evidence.
Hysteriales Lindau
The order Hysteriales, consisting of a single family, was introduced by Lindau (1896). DNA sequence data showed that Hysteriales should be accommodated in Dothideomycetes (Boehm et al. 2009a, b; Shearer et al. 2009; Suetrong et al. 2009). This order is characterized by carbonaceous navicular ascoma with a longitudinal dehiscent slit and ascospores that are typically dark and vary in septation (Boehm et al. 2009a).
Hysteriaceae Chevall.
Chevallier (1826) introduced the family Hysteriaceae as ‘Hysterineae’. This family has been treated with different genera by authors (Zogg 1962; von Arx and Muller 1975; Kirk et al. 2001; Lumbsch and Huhndorf 2010). Recent multi-gene phylogenetic studies placed Hysteriaceae in Hysteriales, Pleosporomycetidae (Boehm et al. 2009a, b; Hyde et al. 2013; Wijayawardene et al. 2014a; Thambugala et al. 2016).
Hysterium Tode
Hysterium was introduced by Persoon (1797) with H. pulicare Pers. as type species. The genus is characterized by pigmented, versicolorous or concolorous, asymmetric phragmoascospores, three- or more transversely-septate, borne in hysterothecia (Boehm et al. 2009a).
Hysterium centramurum Senan. sp. nov.
Index Fungorum number: IF552708; Facesoffungi number: FoF00164, Figs. 11, 12
Hysterium centramurum (MFLU 14-0096, holotype). a–c Ascomata on substrate. d Cross section of ascoma. e Granulated ends of pseudoparaphyses near to slit. f Pseudoparaphyses. g–i Asci. j Ascospores releasing from the broken asci. k–l Immature ascospores. m–o Ascospores at maturity. p Cup-shaped endoplasts. q–s Fully mature ascospores. t Sheath. Scale bars a, b. 500 µm, c = 250 µm, d = 100 µm, e = 50 µm, f = 10 µm, g–j = 100 µm, k–t = 50 µm
Etymology: The species epithet from the combination of two Latin words “centrum” and “murum”, meaning centre and septum or wall, in reference to the ascospore having a median septum.
Holotype: MFLU 14-0096
Saprobic on decaying wood. Sexual morph Ascomata 0.9–1.8 mm long, 400–450 µm wide, 370–380 µm high (\( \bar{x} \) = 1250 × 400 × 375 µm, n = 20), a hysterothecium, scattered, superficial, base immersed in substrate, elongate and depressed conchate, globose, surface black, shiny, longitudinally striate, apex compressed, opening by longitudinal slit. Periphyses aseptate, hyaline with swollen ends. Peridium 30–45 µm wide (\( \bar{x} \) = 34 µm, n = 10), carbonaceous, brittle, of heavily pigmented, small, prosenchymatous cells. Hamathecium comprising 0.75–0.85 µm wide (\( \bar{x} \) = 0.8 µm), aseptate, branched, trabeculate pseudoparaphyses, borne in a gel matrix which is pinkish and granular above the asci. Asci 170–195 × 30–55 µm (\( \bar{x} \) = 179 × 36 µm, n = 20), 8-spored, bitunicate, oblong to clavate, with a short pedicel, apically thickened, with a distinct ocular chamber. Ascospores 110–130 × 20–25 µm (\( \bar{x} \) = 125 × 23 µm, n = 20), crowded to biseriate, fusiform when young, oblong at maturity, with a single median septum, hyaline to light yellow, wall greatly thickened towards the apex, cytoplasm clearly subdivided into numerous compartments in Melzer’s and Cotton blue reagent, muriform when mature, endoplasts appearing cup-shaped when young, smooth-walled. Sheath present, but not very obvious. Asexual morph coelomycetous. Conidiomata 100–110 µm (\( \bar{x} \) = 107 µm), superficial, solitary or aggregated, black to brown, appearing as a mycelium mass, prosenchymatous, without a prominent wall. Conidiophores 8–10 µm long × 1–2 µm wide (\( \bar{x} \) = 9 × 1 µm), cylindrical, attached to the mycelium, hyaline. Conidiogenous cells 2–2.5 × 1–1.5 µm (\( \bar{x} \) = 2.4 × 1.2 µm), holoblastic, terminal or intercalary on conidiophores, cylindrical, short, smooth, hyaline. Conidia 2–2.5 µm diam. (\( \bar{x} \) = 2.3 µm, n = 20) oval to globose, hyaline, brown when mature.
Culture characteristics: Colonies on MEA, greenish-white, circular, with smooth margin, slow growing, attaining 2 cm diam. within 60 days at 20 °C, tightly arranged, short, aerial mycelium. Orange-brownish exudates released into the media. The mycelial mats produce erumpent, globose, light brownish, viscous droplets when incubated at 20 °C for 3 weeks and later becoming lighter in colour.
Material examined: THAILAND, Chiang Mai Province, Doi Suthep-Pui, Sangasabhasri Lane to Huai Kok Ma Village (N1848.62′, E9854.60′, elev. 1145 m), on decaying wood, 17 December 2012, I.C. Senanayake CHUNI 70 (MFLU 14-0096, holotype, HKAS 82633, isotype), ex-type living culture MFLUCC 12-0808.
GenBank Numbers ITS:KM272258; LSU:KM272256; SSU:KM272257; TEF1:KM277819.
Notes: Hysterium centramurum is morphologically similar to Ostreichnion curtisii (Duby) M.E. Barr and O. thailandicum Tennakoon, Phookamsak & KD Hyde. However, phylogenetically, our strain is not closely related to the type species of Ostreichnion, O. sassafras. Combined gene sequences analysis of LSU, SSU and TEF1 (Fig. 10) places H. centramurum in the genus Hysterium. Morphologically H. centramurum differs from other Hysterium species in having hyaline to light yellow ascospores, surrounded by a sheath, with a median septum. Additionally, the coelomycetous asexual morph for this species was obtained in culture. The major secondary metabolic exudate produced in cultures of H. centramurum was identified by mass spectroscopy and 1D and 2D NMR spectroscopy data analysis as physcion (1,8-dihydroxy-6-methoxy-3-methyl-9,10-dioxoanthracene-2-carboxylic acid), which is useful in the textile industry as a dye and in the pharmaceutical industry as an antibiotic and anti-cancer agent (Velmurugan et al. 2010).
Hysterobrevium mori (Schwein.) E.W.A. Boehm & C.L. Schoch, Stud. Mycol 64: 62 (2009)
Facesoffungi number: FoF 2766, Fig. 13
Saprobic dead branch of Cornus sanguinea L. Sexual morph Ascomata 200–220 µm high × 240–290 µm diam. (\( \bar{x} \) = 209 × 262 μm, n = 5), superficial on wood, elongate, narrowly elliptical to fusiform, straight or irregularly curved, solitary or scattered on the host surface, dull black, carbonaceous, with a conspicuous longitudinal cleft and often with inconspicuous parallel striations, the cleft gradually widening to expose the hymenium, not easier to remove. Peridium 45–65 μm wide, thick, composed of very dark, near opaque, thick-walled brown to black cells, basal region similar, with a layer of hyaline thin-walled cells forming the cavity floor, dull cells arranged in a textura angularis to textura globulosa. Hamathecium of 1.2–2.2 µm wide, long cylindrical, hyaline, smooth, septate, branched, cellular, pseudoparaphyses. Asci 50–70 × 10–20 μm (\( \bar{x} \) = 61 × 16 μm, n = 15), 8-spored, bitunicate, cylindrical to clavate, with short pedicel, tapering to a rounded or irregular base, thick-walled. Ascospores 15–21 × 5–10 μm (\( \bar{x} \) = 19 × 7 μm, n = 20), mostly ellipsoidal, muriform, usually with 3–4 transverse septa and 1–2 longitudinal septa, with slightly paler ends, conical and narrowly rounded at the ends, hyaline, with mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA at 16 °C reaching 4 cm in two weeks, circular with undulate, yellow-grey mycelium, velvety and flat on the media.
Material examined: ITALY, Forlì-Cesena Province, Castrocaro Terme, dead branch of Cornus sanguinea (Cornaceae), 6 January 2012, Erio Camporesi, IT86 (MFLU 15-1476, HKAS 94526, new host record); living culture, MFLUCC 14-0520).
GenBank Numbers LSU:KY496718; ITS:KY496739; SSU:KY501109; RPB2:KY514403.
Notes: Phylogenetically this species resides in a distinct subclade in Hysterobrevium and is H. mori (Fig. 10). Our new isolate from Italy matches H. mori in having ellipsoidal, hyaline, muriform ascospores, with 3–4 transverse septa and 1–2 longitudinal septa that pass through one to two cells, with a mucilaginous sheath. Thus, we confirm (based on both morphological characteristics and phylogenetic data) that our taxon belongs to H. mori even though it was found on a different host (Cornus sanguinea). Previously, H. mori has been reported from many hosts, such as Acer, Amelanchier, Aspidosperma, Castanea, Cercocarpus, Cotinus, Crataegus, Fraxinus, Gleditsia, Juniperus, Melia, Morus, Olea, Ostrya, Pinus, Pistacia, Prunus, Pyrus, Quercus, Rhus, Rubus, Salix, Ulmus, various Fabaceae, Vitis and Ziziphus (Zogg 1962).
Pleosporales Luttr. ex M.E. Barr
For an account of Pleosporales Luttr. ex M.E. Barr see Zhang et al. (2012b) and Hyde et al. (2013).
Amorosiaceae Thambugala & K.D. Hyde
Amorosia was introduced by Mantle et al. (2006) with A. littoralis Mantle & D. Hawksw. Previous studies, based on phylogenetic analysis, have referred it to the Sporormiaceae (Mantle et al. 2006), but Thambugala et al. (2015a) introduced Amorosiaceae as a new family for this species and described three new species of Angustimassarina in this family. We introduce six new species of Angustimassarina based on morphology and phylogeny (Fig. 14).
Phylogram generated from maximum parsimony analysis of combined ITS, SSU, LSU and TEF1 sequence data for Lophiostomataceae and Amorosiaceae. Parsimony bootstrap support values for MP ≥ 70% and Bayesian posterior probabilities ≥0.8 are indicated at the nodes. Isolate numbers of all ex-types and reference strains are in bold. Novel species/new host records are indicated in blue. The tree is rooted with Melanomma pulvis-pyrius
Angustimassarina Thambug., Kaz. Tanaka & K.D. Hyde
Thambugala et al. (2015a) introduced Angustimassarina to accommodate three new ascomycetous species placed in the family Amorosiaceae. The members of the genus are considered as fungicolous or they may be parasitic on other fungi and appear to grow within other ascomata or on other ascomycetes. A synopsis of Angustimassarina species is provided (Table 2).
Angustimassarina alni Jayasiri & K.D. Hyde, sp. nov.
Index Fungorum number: IF552551; Facesoffungi number: FoF 2689, Fig. 15
Etymology: The specific epithet alni is based on the host genus.
Holotype: MFLU 16-2981
Saprobic on Alnus glutinosa (L.) Gaertn. Sexual morph Ascomata 160–250 µm high × 130–200 µm diam. (\( \bar{x} \) = 208 × 188 μm, n = 5), scattered to gregarious, immersed to semi-immersed, carbonaceous, dark brown to black, globose to subglobose. Ostiolar canal inner layer lined with black to dark brown cells, outer layer with dark brown to black pseudoparenchymatous cells, with a pore like opening or opening through cracks in the host surface. Peridium 28–44 µm wide, equally thick, comprising dark brown to black cells of textura angularis. Hamathecium comprising 1–1.5 µm septate, unbranched, cellular pseudoparaphyses, embedded in gelatinous matrix, between and above the asci. Asci 71–89 × 8–10 μm (\( \bar{x} \) = 82 × 9 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindric-clavate, with furcate or knob-like pedicel, rounded at the apex with a minute ocular chamber. Ascospores 19–22 × 3–4 μm diam. (\( \bar{x} \) = 21 × 3.5 μm, n = 30), uni to biseriate, partially overlapping, hyaline, fusiform to cylindrical or ellipsoidal-fusiform, straight to curved, widest at the centre and tapering towards the ends, 3-septate, constricted at the primary septum, smooth-walled, filled with two different sized guttules per cell and surrounded by a mucilaginous sheath. Asexual morph Undetermined.
Material examined: GERMANY, loamy sand, acid, fresh, ditch with alder, 40 m a.s.l., mesotroph, twig of Alnus glutinosa (Betulaceae), 1 March 2014, Rene K. Schumacher 044, (MFLU 16-2981, holotype); ex-type living culture, MFLUCC 15-0184, KUMCC 16-0071.
GenBank Numbers LSU:KY548097; SSU:KY548098; ITS:KY548099.
Culture characteristics: Ascospores germinating on WA within 24 h. Colonies growing on MEA, reaching 5 mm diam. in 1 week at 18 °C. Mycelium superficial, slightly effuse, ashy white, inner brown, sparsely hairy, with undulate edge, lower surface black.
Notes: Angustimassarina alni fits with generic concept of Angustimassarina (Thambugala et al. 2015a). Angustimassarina alni forms a sister clade with Exosporium stylobatum (CBS 16030; asexual morph) and A. premilcurensis (MFLUCC 15-0074. We could not obtain an asexual morph in culture. Angustimassarina alni is similar to A. premilcurensis in having immersed to semi-immersed ascomata, cylindrical to clavate asci and hyaline, fusiform to cylindrical ascospores with a mucilaginous sheath, but differs in having a carbonaceous peridium and different sized ascospores with guttules.
Angustimassarina arezzoensis Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552690; Facesoffungi number: FoF 2769, Fig. 16
Etymology: refers to the Arezzo (Italy) where the fungus was collected.
Holotype: MFLU 14-0681
Saprobic on dead stem of Salvia sp. Sexual morph Ascomata 169–234 µm high × 166–245 µm diam. (\( \bar{x} \) = 208 × 188 μm, n = 4), immersed to erumpent, visible as raised, dark spots on the host surface, uniloculate, subglobose, solitary or in small groups, scattered on the host surface, without papilla, short ostiole in the center, thick-walled, carbonaceous, dark brown or usually black. Peridium 22–41 μm wide, inner cells hyaline to pale brown, outer layer cells brown to dark brown, composed of flattened cells of textura angularis. Hamathecium 1.6–2.9 µm wide, comprising numerous, septate, cylindrical, frequently anastomosing, cellular, pseudoparaphyses. Asci 67–95 × 10–15 μm (\( \bar{x} \) = 82 × 13 μm, n = 15), 8-spored, bitunicate, fissitunicate, broadly cylindrical to cylindric-clavate, with short bulbous, furcate pedicel, rounded at the apex, with a poorly develop ocular chamber. Ascospores 19–21 × 5–6 μm (\( \bar{x} \) = 20 × 6 μm, n = 20), overlapping 1–2-seriate, hyaline, fusiform, usually 3-septate, narrowly fusoid with rounded ends, constricted at the septum, deeply constricted at the central septum, enlarged at the second cell, guttulate, smooth-walled, surrounded by mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA at 16 °C reaching 1.5 cm in 1 week, irregular, whiteish-grey, with undulate edge, lower surface black.
Material examined: ITALY, near Poppi Province, Arezzo, dead stem of Salvia sp. (Lamiaceae), 4 June 2013, Erio Camporesi, IT1321 (MFLU 14-0681, holotype); ex-type living culture, MFLUCC 13-0578; (HKAS 94552 bis, paratype).
GenBank Numbers LSU:KY496722; ITS:KY496743; SSU:KY501113; TEF1:KY514392.
Notes: Angustimassarina arezzoensis clustered with A. populi in phylogeny (Fig. 14), but they are morphologically different (Table 2).
Angustimassarina lonicerae Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552691; Facesoffungi number: FoF 2770, Fig. 17
Angustimassarina lonicerae (MFLU 15-1485, holotype). a, b Appearance of ascomata with ostiole on host substrate. c Section of ascoma. d Section of peridium. e Pseudoparaphyses. f, g Asci. h–k Ascospores. l Germinated ascospore. Scale bars a = 100 μm, b, c = 50 μm, d = 10 μm, e = 2 μm, f, g = 20 μm, h–l = 5 μm
Etymology: named for its occurrence on the host plant (Lonicera sp.).
Holotype: MFLU 15-1485
Saprobic on dead branch of Lonicera sp. Sexual morph Ascomata 193–203 µm high × 170–220 µm diam. (\( \bar{x} \) = 198 × 201 μm, n = 5), semi-immersed to erumpent through host tissue, solitary, globose to subglobose, visible on host surface as raised ostiolar dots, black. Ostioles central, long, conspicuous at the surface, shiny, black. Peridium 10–18 μm wide, thick at the sides, broad at the apex and thinner at the base, comprising brown to dark brown cells of textura angularis, fusing at the outside with the host tissues. Hamathecium comprising 0.7–1.1 µm wide, dense, cylindrical, septate, branched, pseudoparaphyses. Asci 55–81 × 9–13 μm (\( \bar{x} \) = 71 × 12 μm, n = 15), 8-spored, bitunicate, cylindrical, short pedicellate, apex rounded, with a minute ocular chamber. Ascospores 19–25 × 4–7 μm (\( \bar{x} \) = 22 × 6 μm, n = 15), overlapping 1–2-seriate, fusiform, 1–3-septate, guttulate, enlarged at the second cell, constricted at the center, conical at the ends, smooth-walled, hyaline, surrounded by a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 6 cm diam., after 7 days in the dark at 16 °C, irregular, grey, with undulate edge, lower surface black.
Material examined: ITALY, Forlì-Cesena Province, Fiumicello di Premilcuore, on dead branch of Lonicera sp. (Caprifoliaceae), 19 November 2013, Erio Camporesi, IT1524 (MFLU 15-1485, holotype); ex-type living culture, MFLUCC 15-0087; (HKAS 94563 bis, paratype).
GenBank Numbers LSU:KY496724; ITS:KY496759.
Notes: Angustimassarina lonicerae is similar to A. italica however the peridium in A. lonicerae is 10–18 μm wide, with 1-septate ascospores swollen above centre septum, versus 23–40 μm wide in A. italica, and 1–3-septate ascospores enlarged at the second cell. The phylogeny (Fig. 14) also supports this new species as distinct as they cluster in different subclades within Amorosiaceae with good statistical support (86% in ML/1.00 in BYPP)
Angustimassarina premilcurensis Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552692; Facesoffungi number: FoF 2771, Fig. 18
Angustimassarina premilcurensis (MFLU 14-0703, holotype). a, b Appearance of ascomata with erumpent ostiole. c Section of ascoma. d Section of peridium. e Pseudoparaphyses. f–h Ascus with pedicel. i Ocular chamber. j–m Ascospores. n Germinated ascospore. Scale bars a = 500 μm, b = 200 μm, d = 20 μm, e = 2 μm, f–i = 10 μm, j–n = 5 μm
Etymology: refers to the Fiumicello di Premilcuore (Italy) where the fungus was collected.
Holotype: MFLU 14-0703
Saprobic on dead branch of Carpinus betulus L. Sexual morph Ascomata 231–238 µm high × 290–311 µm diam. (\( \bar{x} \) = 235 × 326 μm, n = 5), immersed, solitary or scattered on the host surface, globose to subglobose, black, without papilla, ostiole in the center. Peridium 20–30 μm wide, comprising 2–4 layers of irregular, brown-walled cells arranged in a textura angularis. Hamathecium comprising 0.5–0.9 µm, cylindrical, filamentous, septate, pseudoparaphyses. Asci 64–93 × 11–15 μm (\( \bar{x} \) = 77 × 13 μm, n = 10), 8-spored, bitunicate, cylindrical to cylindric-clavate, short pedicellate or sessile, rounded at the apex, with ocular chamber. Ascospores 19–23 µm × 4–7 μm (\( \bar{x} \) = 21 × 6 μm, n = 15), overlapping bi-seriate, fusiform, 1-septate, constricted at the centre, straight or slightly curved, swollen near the septum, surrounded by mucilaginous sheath, hyaline, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 4 cm diam., after 7 days in the dark at 16 °C, irregular, white–grey, flat on the surface, lower surface black.
Material examined: ITALY, Province of Forlì-Cesena [FC], Fiumicello di Premilcuore, on dead branch of Carpinus betulus L. (Betulaceae), 10 February 2014, Erio Camporesi, IT1716 (MFLU 14-0703, holotype); ex-type living culture, MFLUCC 15-0074; (HKAS 94575 bis, paratype).
GenBank Numbers LSU:KY496725; ITS:KY496745; RPB2:KY514404.
Notes: Angustimassarina premilcurensis differs from other species in Angustimassarina in possessing 1-septate ascospores, constricted and swollen near the septum. In the phylogenetic analyses, A. premilcurensis clustered with Exosporium stylobatum. Exosporium is a hyphomycete (Crous et al. 2011) with more than 120 epithets (Index Fungorum 2017), but DNA sequence data are not available for its type species (Thambugala et al. 2015a). Any conclusive association between A. premilcurensis and Exosporium stylobatum is premature until further DNA sequence data becomes available.
Angustimassarina italica Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552707; Facesoffungi number: FoF 2785, Fig. 19
Angustimassarina italica (MFLU 15-1494, holotype). a Appearance of ascomata on host substrate. b Section of ascoma. c Section of peridium. d Pseudoparaphyses. e, f Asci. g, h Ascospores. i Ascospore in Indian ink to show sheath. j Germinating ascospore. Scale bars b = 40 μm, c = 20 μm, d = 2 μm, e, f = 20 μm, g–j = 5 μm
Phylogenetic tree generated from neighbor joining analysis of LSU sequence data from Zhang et al. (2009a) with Tubeufiaceae (Tubeufia javanica and Tubeufia paludosa) as out group taxa. Bootstrap support values for maximum likelihood greater than 50% are given above the nodes. New sequences are in blue
Sigarispora muriformis (MFLUCC 13-0744, holotype). a Appearance of papilla of erumpent ascomata on host substrate. b Section of ascoma. c Section of peridium. d Pseudoparaphyses. e–g Asci. h–k Ascospores. l Germinated ascospore. Scale bars a, b = 200 μm, c, d = 5 μm, e–h = 10 μm, f, g = 20 μm, h–l = 10 μm
Vaginatispora appendiculata (MFLU 16-2887). a Appearance of ascomata on host substrate. b Section of ascoma. c Close up of ostiole d Section of peridium. e Pseudoparaphyses. f–h Asci. i–m Ascospores (note the ascospore stained in Indian ink to show the mucilaginous sheath in m). n Germinated ascospore. Scale bars a = 500 μm, b = 100 μm, c = 50 μm, d = 20 μm, e = 10 μm, f–h = 20 μm, i–n = 10 μm
Phylogenetic construction using RAxML-based analysis of a combined LSU and SSU, dataset. Bootstrap support values for maximum likelihood (ML, black) equal to or greater than 50% and Bayesian posterior probabilities (BYPP, red) equal to or greater than 0.95 are shown above the nodes. The tree is rooted to Lophiostoma macrostomum. The type strains are in black bold and the newly generated sequences are indicated in blue bold
Lophiotrema guttulata (MFLUCC 10-0929, ex-type living culture on WA). a, b Germinating ascospores. c Single ascospore colonies on MEA. d, e Colonies on MEA from surface and reverse at 4 weeks. f, g Squash mount of immature and mature hyphae. h Growth of asexual morph on plant tissues. i–k Squash mount of vegetative hyphae. Scale bars a, b, f, g, i–k = 10 µm, c, e, h = 10 mm
Phylogenetic tree generated from maximum parsimony analysis based on combined ITS and LSU sequence data of the family Melanommataceae. Bootstrap support values for maximum likelihood and maximum parsimony greater than 50% and Bayesian posterior probabilities greater than 0.80 are indicated above or below the nodes as ML/MP/BYPP. Ex-type strains and reference strains are in bold. The new isolates are in red. The tree is rooted with Pleomassaria siparia (CBS 279.74)
Aposphaeria corallinolutea (MFLU 15-2752, sexual morph). a Host substrate. b, c Appearance of ascomata on host. d Vertical section of ascoma. e Ostioles. f Section through peridium. g Pseudoparaphyses. h–i Developmental stages of asci. j–n Developmental stages of ascospores. o Germinated ascospore. p Culture characters. q Conidiomata produced in culture. r–v Conidiogenesis developing. w Conidia. Scale bars b = 1000 µm, c, q = 500 µm, d = 200 µm, e, g–i = 50 µm, f = 100 µm, j–m, r–w = 5 µm, n, o = 10 µm
RAxML tree based on a combined dataset of LSU, SSU, ITS, RPB2 and TEF1 partial sequences. Bootstrap support values for maximum likelihood (ML) higher than 70% and Bayesian posterior probabilities (BYPP) greater than 0.95 are given above the branches. The ex-type and reference strains are in bold; the new isolates are in blue. The tree is rooted to Melanomma pulvis-pyrius (Melanommataceae)
Nigrograna cangshanensis (HKAS 83978, holotype). a Wood. b, c Appearance of ascomata on wood. d Section of ascoma. e Section of the peridium. f Paraphyses. g–k Asci. l–p Ascospores. q–s Germinating ascospore. t, u Culture grow on MEA. Scale bars d = 100 µm, e = 50 µm, f = 20 µm, g–k = 10 µm, l–p = 5 µm, q–s = 10 µm
Phylogram generated from RAxML based on combined LSU, ITS, TEF1 and RPB2 sequenced data. Maximum likelihood (ML) bootstrap support values greater than 60% and Bayesian posterior probabilities (BYPP) greater than 0.95 are shown at the nodes. The ex-type strains are in bold and the new isolate is in blue. The tree is rooted with Occultibambusa bambusae
Parathyridaria robiniae (MFLU 17-0015, holotype). a, b Appearance of superficial ascomata on substrate. c Section through of ascomata. d Ostiole e Peridium. f Pseudoparaphyses. g, h Asci at immature and mature. i–k Ascospores. l Ascospore surrounded by hyaline gelatinous sheath in Indian ink. Scale bars a, b = 200 µm, c = 100 µm, d, g–h = 50 µm, e = 20 µm, i–l = 10 µm, f = 5 µm
RAxML tree based on a combined dataset of LSU, ITS, SSU and TEF1 partial sequences from Didymosphaeriaceae. Bootstrap support values for maximum likelihood higher than 60% and Bayesian posterior probabilities higher than 0.90 are defined as above the nodes respectively. The tree is rooted to Pleospora herbarum and Pleospora tarda. The new species is in blue
Phylogenetic tree generated from maximum likelihood analysis based on combined LSU, SSU, TEF1 and RPB2 sequence data of the suborder Massarineae. Bootstrap support values for maximum likelihood greater than 70% are indicated above or below the nodes as ML. Ex-type strains and reference strains are in black bold. The new species are in blue. The tree is rooted with Pleospora herbarum and Alternaria alternata
Pseudoasteromassaria spadicea (MFLU 15-2683, holotype). a, b Appearance of conidioma on host substrate. c, d Section of conidioma. e Section of neck. f Section of peridium. g–i Conidiogenous cells and conidia. j Germinated conidium producing light brown pigment. k–p Conidia. q Colony on PDA (from top). r Colony on PDA (from reverse). Scale bars b = 200 μm, c–d = 100 μm, e = 20 μm, f, j = 10 μm, g–i = 5 μm, k–p = 5 μm
RAxML tree based on analysis of a combined LSU, SSU, TEF1 and ITS sequence dataset. Bootstrap support values for maximum likelihood, maximum parsimony higher than 60% and Bayesian posterior probabilities greater than 0.95 are given above each branch. The ex-type and reference strains are in bold; the new species are in blue. The tree is rooted to Massarina eburnea and Massarina cisti in Massarinaceae
Keissleriella cirsii (MFLU 15-2900, holotype). a Type material. b Appearance of ascomata immersed in host substrate. c Section through ascoma. d Section through ostiole with internal setae. e Close up of the peridium. f. Pseudoparaphyses. g, h Asci. i Ascus in Melzer’s reagent. j, k Ascospores. Scale bars b = 500 μm, c = 100 μm, d = 50 e, f = 10 μm, g–k = 20 μm
Inflatispora caryotae (MFLU 16-2886, holotype). a Appearance of ascomata on host substrate. b, c Section of ascoma. c Close up of ostiole. d Section of peridium. e Pseudoparaphyses. f–h Asci. i–k Ascospores. l Germinated ascospore. Scale bars a = 500 μm, b, c = 100 μm, d = 20 μm, e = 10 μm, f–l = 20 μm
Phylogram generated from maximum likelihood analysis of a LSU, ITS, β-tubulin and RPB2 combined dataset for Didymellaceae. Leptosphaeria doliolum is used as the outgroup taxon. Bootstrap support values for maximum likelihood greater than 60% and Bayesian posterior probabilities greater than 0.80 are given at the nodes. The ex-type strains are in bold. Novel species/new host records sequences are in blue
Ascochyta italica (MFLU 14-0724, holotype). a Appearance of ascomata on host substrate. b Section of ascoma. c Section of peridium. d Pseudoparaphyses. e, f Ascus. g–i Ascospores. j Germinating ascospore. Note: stained with cotton blue reagent in f and i. Scale bars b, c = 20 μm, d = 2 μm, e–f = 10 μm, g–j = 5 μm
Phylogenetic tree generated from maximum likelihood analysis based on combined LSU, SSU, ITS, RPB2, and β-tubulin sequence data from Leptosphaeriaceae. Bootstrap support values for maximum likelihood greater than 50% and Bayesian posterior probabilities greater than 0.80 are indicated above or below the nodes as ML/BYPP are indicated above or below the nodes. Ex-type strains and reference strains are in black bold. The new species are in blue. The tree is rooted with Leptosphaeria doliolum (CBS 505.75)
Etymology: refers to the country (Italy) where the fungus was collected.
Holotype: MFLU 15-1494
Saprobic on dead branch of Ilex aquifolium L. Sexual morph Ascomata 127–159 µm high × 97–131 µm diam. (\( \bar{x} \) = 143 × 114 μm, n = 5), semi-immersed to erumpent through host tissue, solitary, uniloculate, globose to subglobose, black, without hairs, with long ostiole in the center. Peridium 23–40 μm wide, comprising several layers of hyaline to pale brown cells of textura angularis. Hamathecium 1.1–1.9 µm wide, comprising numerous, aseptate, unbranched, pseudoparaphyses. Asci 78–103 × 10–12 μm (\( \bar{x} \) = 89 × 11 μm, n = 15), 8-spored, bitunicate, cylindrical with bulbous pedicel, rounded at the apex, with a well-developed ocular chamber. Ascospores 15–22 × 3–6 μm (\( \bar{x} \) = 19 × 4 μm, n = 15), overlapping 1-seriate, hyaline, 1-septate, constricted at centre, swollen above centre septum, conical at the ends, guttulate, surrounding by a large mucilaginous sheath, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 5–6 cm diam., after 7 days in the dark at 16 °C, irregular, white–grey to black, flat on the surface, lower surface black.
Material examined: ITALY, Campigna—Santa Sofia, on dead branch of Ilex aquifolium (Aquifoliaceae), 10 October 2014, Erio Camporesi, IT2159 (MFLU 15-1494, holotype); ex-type living culture MFLUCC 15-0082; (HKAS 94605 bis, paratype).
GenBank Numbers LSU:KY496736; ITS:KY496756; SSU:KY501124; TEF1:KY514400.
Notes: Angustimassarina italica is phylogenetically closely related to A. acerina and A. quercicola (Fig. 14). However, it differs in having hyaline, 1-septate ascospores while A. quercicola has brown, 1(–3)-septate ascospores, which are finely verruculose at maturity and A. acerina has brown, 1(–3)-septate ascospores (Thambugala et al. 2015a).
Biatrosporaceae K.D. Hyde
Jaklitsch and Voglmayr (2016) dismissed the family Biatriosporaceae, whose type clustered amongst Nigrograna species, and transferred several species from Biatriospora to Nigrograna. They introduced the family Nigrogranaceae and accepted a single genus Nigrograna based on morphology and phylogenetic analyses and provided DNA sequence data. Jaklitsch and Voglmayr (2016) doubted the validity of the strain of B. marina in GenBank. However, based on the type species, the family Biatriosporaceae is morphologically distinct and the family should be maintained. Nevertheless, we accept that it seems odd that the strain of Biatriospora marina in GenBank clusters with Nigrograna species and thus B. marina needs recollecting for sequence analysis to establish if Nigrograna is related, which morphologically seems unlikely. A phylogenetic tree for Nigrogranaceae is presented in this paper (Fig. 59), where the continued use of Biatriosporaceae, which is unlike Nigrogranaceae based on its morphological distinctiveness, is recommended.
Paraleptosphaeria padi (MFLU 15-2752, holotype). a Host substrate. b, c Close up of ascomata on host. d Vertical section of ascoma. e Vertical section of ascoma stained with cotton blue to show the hyaline inner layer. f Ostioles filled with periphyses. g Section through peridium. h Cellular pseudoparaphyses (stained with cotton blue showing septate). i–k Developmental stages of asci. l–p Developmental stages of ascospores. q Ascospore stained with cotton blue to show septa. Scale bars c = 500 µm, d, e = 200 µm, f = 100 µm, g–k = 50 µm, l–q = 10 µm
Lophiostomataceae Sacc.
The family Lophiostomataceae is an interesting and diverse family in the order Pleosporales, Dothideomycetes. Most members of this family are saprobic and occur mainly on twigs, stems or bark of various woody plants and herbaceous plants in both terrestrial and aquatic environments (Hirayama and Tanaka 2011; Hyde et al. 2013; Ariyawansa et al. 2015c; Thambugala et al. 2015a). Thambugala et al. (2015a) revised the family and accepted 16 genera, including eleven newly introduced genera. In this study, we place two new species and two new host records in Lophiostomataceae (Figs. 14, 20).
Berkleasmium Zobel.
Zobel (1854) established the genus Berkleasmium by re-combining the species Sporidesmium concinnum Berk., and renamed the taxon in honour of Corda, as Berkleasmium cordeanum. Hughes (1958) later corrected this type species name as Berkleasmium concinnum. Moore (1958) and Ellis (1971) accepted this genus for sporodochial taxa that produce dark dictyosporous conidia on short simple unbranched conidiophores or directly on the hyphae. Fourty species are currently listed as legitimate under Berkleasmium in MycoBank (2017). According to Seifertk et al. (2011) the genus Berkleasmium comprises 32 valid species recorded from Africa, Asia, Australasia and North America. Most of these species are recorded from dead substrates, such as dead wood, litter and bark of various plants (Moore 1958; Rao and Rao 1963; Ellis 1971, 1976; Morris 1972; Chouhan and Panwar 1980; Matsushima 1981, 1983; Holubová-Jechová 1987; Yip 1988; Bussaban et al. 2001; Pinnoi et al. 2007; Qu et al. 2014; Hüseyin et al. 2014). The new species B. ariense from India is similar to the type species B. concinnum, however, the conidia are longer. Three species of Berkleasmium were previously recorded from India; B. abuense (current name: Monodictys abuensis), B. osmaniae and B. papillatum (current name: Acrodictys papillata). Phylogenetic affinities of the new taxon with other Berkleasmium species, based on the LSU and ITS gene sequences, is shown in Fig. 20.
Berkleasmium ariense Rajeshkumar & Marathe, sp. nov.
MycoBank number: MB 819049; Facesoffungi number: FoF 2779; Fig. 21
Etymology: ariense, referring to the Agharkar Research Institute, a reputed school of mycology in India.
Holotype: AMH 9828
Saprobic on natural substrates. Sexual morph Undetermined. Asexual morph Conidiomata sporodochial, raised, superficial, scattered, dark brown to blackish, velvety, with immersed mycelia. Stroma rudimentary. Setae and hyphopodia absent. Conidiophores micronematous, short, closely packed together into sporodochia, flexuous, unbranched, 2–5 µm. Conidiogenous cells monoblastic, integrated, terminal, determined. Conidia 119–238 × 41–60 µm, solitary, dry or sticky, acrogenous, simple, clavate, ellipsoidal, oblong or irregular, muriform, pale to dark brown or blackish-brown, hilum not protruding; conidial base 11–15 µm. Young conidia hyaline or subhyaline, 56 × 17 µm.
Culture characteristics: Colonies on MEA slow to medium growing, 30 to 45 mm diam. after 20 days, dark brownish-black, mycelia immersed, centrally fluffy, margin irregular, reverse dark brown to blackish. No sporulation observed.
Material examined: INDIA, Maharashtra, Tamhini Village, on unidentified stem litter, 9 June 2015 (AMH 9828, holotype); ex-type living culture NFCCI 4026; additional culture NFCCI 4027.
GenBank Numbers ITS:KY039163, KY039164; LSU:KY039165, KY039166.
Notes: Our new isolate is morphologically similar and phylogenetically related to Platystomum rosae (strain MFLUCC 15-0633) (Fig. 14), but associated with a different host. Platystomum rosae (holotype) was recorded on Rosa canina L (Rosaceae) and our collection was found on Cornus sp. (Cornaceae).
Platystomum Trevis.
Platystomum was introduced by Trevisan (1877) and is typified by Platystomum compressum. Platystomum compressum was reported as a synonym of Lophiostoma compressum and Thambugala et al. (2015a) accepted Platystomum in Lophiostomataceae as a distinct genus, and accepted five species (Platystomum actinidiae, P. compressum, P. crataegi, P. rosae and P. salicicola), based on new collections and combinations (Fig. 14).
Platystomum rosae Wanas., Thambug., Camporesi & K.D. Hyde, Fungal Diversity 74: 234 (2015)
Facesoffungi number: FoF 2780, Fig. 22
Saprobic on species of Cornus sp., in terrestrial environments. Sexual morph Ascomata 300–500 μm high × 250–450 μm diam. (\( \bar{x} \) = 424.7 × 305.3 μm, n = 5), scattered to gregarious, immersed, coriaceous, dark brown to black, subglobose to conical, ostiolate. Ostioles 150–200 µm long, 40–60 µm diam. (\( \bar{x} \) = 175.4 × 49.1 µm, n = 10), papillate, black, smooth, with short, setae. Peridium 20–25 µm wide at the base, 25–35 µm wide at the sides, broad at the apex, two–layered, outer layer composed of small, blackish to dark brown, thick-walled cells of textura prismatica, inner layer composed of smaller, compressed, hyaline cells. Hamathecium of 1.5–3 μm wide, septate, cellular pseudoparaphyses, embedded in gelatinous matrix between and above the asci. Asci 120–170 × 15–20 μm (\( \bar{x} \) = 144.9 × 17.1 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, pedicel, narrowly rounded at the apex, with an indistinct ocular chamber. Ascospores 19–23 × 7–9 μm (\( \bar{x} \) = 21.2 × 8.4 μm, n = 40), overlapping 1–2-seriate, muriform, mostly ellipsoidal, with 3–4 transverse septa with 1 vertical septum in each row, slightly curved, deeply constricted at the centre septum, slightly constricted at the remaining septa, initially hyaline, becoming brown at maturity, rounded at the ends, without a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: on PDA, reaching 25–30 mm diam. after 21 days at 16 °C, surface dirty green, spreading with moderate aerial mycelium, and even, smooth margins; reverse dark green.
Material examined: ITALY, Province of Forlì-Cesena, near Fiumicello di Premilcuore, on Cornus sp. (Cornaceae), 11 January 2015, Erio Camporesi, IT 2333 (MFLU 15-2569, new host record).
GenBank Numbers LSU:KY264746; ITS:KY264742; SSU:KY264750.
Notes: Our new isolate is morphologically similar and phylogenetically related to Platystomum rosae (strain MFLUCC 15-0633), but associated with a different host. Platystomum rosae (holotype) was recorded on Rosa canina L (Rosaceae), while our collection was found on Cornus sp. (Cornaceae). The phylogenetic placement of this species is shown in Fig. 14.
Sigarispora Thambug. & K.D. Hyde.
Thambugala et al. (2015a) introduced the genus in Lophiostomataceae and it is typified by S. ravennica (Tibpromma et al.) Thambugala & K.D. Hyde. Sigarispora is characterized by immersed to semi-immersed ascomata, a small crest-like ostiole, and brown, cigar-shaped or ellipsoidal-fusiform, multi-septate or muriform ascospores. Seven species are accepted in the genus.
Sigarispora muriformis Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552696; Facesoffungi number: FoF 2781, Fig. 23
Etymology: refers to the muriform ascospores.
Holotype: MFLU 14-0676
Saprobic on dead stem of Helichrysum italicum (Roth) G. Don fil. Sexual morph Ascomata 425–660 µm high (including ostiole) × 335–560 µm diam. (\( \bar{x} \) = 506 × 460 μm, n = 10), semi-immersed on host tissue, solitary, scattered, globose to subglobose, conspicuous at the surface, papillate, carbonaceous. Ostioles central, lacking periphyses in ostiole canal, dark, dull. Peridium 25–47 μm wide, coriaceous to carbonaceous, comprising an outer stratum of thick-walled and black cells arranged in a textura angularis and fusing with host cells, and inner layer of hyaline cells of textura angularis. Hamathecium of 1.9–3.2 µm wide, cylindrical, septate, branched, guttulate, cellular pseudoparaphyses. Asci 84–130 × 12–18 μm (\( \bar{x} \) = 104 × 15 μm, n = 10), 8-spored, bitunicate, cylindric-clavate, slightly curved, long pedicellate, with apex rounded, with a minute ocular chamber. Ascospores 18–28 × 7–12 μm (\( \bar{x} \) = 24 × 9 μm, n = 20), overlapping 2–3-seriate, muriform, ellipsoidal, hyaline then become reddish–brown with age, with 5–8 transverse septa and 2–3 longitudinal septa, guttulate, constricted at the centre septum, conical at the ends, smooth-walled, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 8 cm diam., after 7 days in the dark at 16 °C, surface dark-brown, spreading with moderate aerial mycelium, and even, margins smooth; reverse dark.
Material examined: ITALY, Forlì-Cesena Province, near Premilcuore, on dead stem of Helichrysum italicum (Asteraceae), 24 May 2013, Erio Camporesi, IT1302 (MFLU 14-0676, holotype); ex-type living culture, MFLUCC 13-0744); Ibid. (HKAS 94549 bis, paratype).
GenBank Numbers LSU:KY496719; ITS:KY496740; SSU:KY501110.
Notes: The new species clearly differs from other Sigarispora species in having semi-immersed ascomata, cylindric-clavate asci and ellipsoidal, muriform ascospores, with 5–8 transverse septa and 2–3 longitudinal septa. This species separates from other Sigarispora species in the phylogenetic analyses (Fig. 14). Sigarispora coronillae and S. muriformis have muriform ascospores, but S. coronillae differs from S. muriformis in having brown to dark brown ascospores with 4–6 transverse septa and 2–4 longitudinal septa (Thambugala et al. 2015a).
Vaginatispora K.D. Hyde
Vaginatispora was introduced by Hyde (1995) in Massarinaceae Munk to accommodate Vaginatispora aquatica K.D. Hyde. Vaginatispora was long considered to be a synonym of Massarina (Hyde et al. 1992; Read et al. 1997) but recently Thambugala et al. (2015a) confirmed it as a separate genus in Lophiostomataceae based on both morphological characteristics and phylogeny. Vaginatispora is characterized by ‘depressed globose ascomata, immersed beneath a blackened neck, with a slot-like ostiole, numerous and filamentous pseudoparaphyses, cylindrical to clavate asci and narrowly ellipsoidal, hyaline, 2-celled ascospore with a mucilaginous collar around its equator and a spreading papilionaceous sheath’ (Hyde 1995; Zhang et al. 2014). Currently the genus comprises Vaginatispora appendiculata, V. aquatica, V, armatispora and V. fuckelii.
Vaginatispora appendiculata Wanas., E.B.G. Jones & K.D. Hyde, Studies in Fungi 1 (1): 60 (2016)
Facesoffungi number: FoF 2782, Fig. 24
Saprobic on Calamus rotang L. in terrestrial habitats. Sexual morph Ascomata 250–300 μm high, 360–400 μm diam. (\( \bar{x} \) = 266 × 389 μm, n = 5), scattered to gregarious, immersed, coriaceous to carbonaceous, black, subglobose, ostiolate. Necks 70–90 µm long, 30–50 µm diam., long slot-like, black, filled with dark brown cells. Peridium 9–12 µm wide at the base, 30–40 µm wide at the sides, comprising 3–4 layers, fused at the outside with the host tissue, composed of thick-walled reddish-brown to dark angular or relatively compressed pseudoparenchymatous cells. Hamathecium of 1–1.5 µm wide, filiform, septate, anastomosing, branched, pseudoparaphyses. Asci 115–130 × 14–18 µm (\( \bar{x} \) = 123.6 × 15.19 µm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-obclavate, short pedicellate, apically rounded. Ascospores 40–45 × 6–8 µm (\( \bar{x} \) = 42.7 × 7.2 µm, n = 30), 2–3-seriate, fusiform, narrowing towards the acute ends, 1-septate, slightly wide above and below the septa, constricted at the septum, hyaline, smooth-walled, guttulate, surrounded by a distinct mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: on MEA reaching 2 cm diam. after 2 weeks at 25 °C, dirty white at the beginning and blackish-brown at maturity, convex on the surface, undulate, margins smooth; reverse blackish-green.
Material examined: THAILAND, Chiang Rai Province, Chiang Sean, on dead Calamus rotang stem (Arecaceae), 21 September 2013, Dhanushka Wanasinghe, DHA17 (MFLU 16-2887, new host record); living culture, MFLUCC 13-0835.
GenBank Numbers LSU:KY264745; SSU:KY264749 (Fig. 25).
Notes: As morphological characters examined largely overlap with those of Vaginatispora appendiculata, we report our collection as a new host record of V. appendiculata from Calamus rotang in Thailand. Combined multi-gene based phylogenies also show that both strains (MFLUCC 13-0835 and MFLUCC 11-0083) of V. appendiculata cluster together, but with a low bootstrap support (Fig. 14).
Lophiotremataceae K. Hiray. & Kaz. Tanaka.
Hirayama and Tanaka (2011) introduced the family Lophiotremataceae with its type genus Lophiotrema Sacc. Phylogenetic analyses based on LSU and SSU sequence data supported this new familial placement and its distinctiveness from Lophiostomataceae. Lophiotremataceae comprises Aquasubmersa, Hermatomyces and Lophiotrema (Hyde et al. 2016; Doilom et al. 2017). The genus Hermatomyces was introduced by Spegazzini (1911), with H. tucumanensis as the type species and includes 13 species (Index Fungorum 2017). Morphologically, the genus is characterized by lenticular to cylindrical, muriform conidia, often with subhyaline to pale brown peripheral cells, and dark brown central cells. Conidia are cylindrical and comprise 1–4 columns with 2–11 cells and are irregularly pigmented (Castañeda and Heredia 2000; Leão-Ferreira et al. 2013; Doilom et al. 2017).
Lophiotrema Sacc.
Lophiotrema was proposed by Saccardo (1878) to accommodate species having hyaline ascospores and typified by Lophiotrema nucula, which was excluded from Lophiostoma based on ascospore characteristics. This genus includes many lignicolous bitunicate fungi characterized by immersed-erumpent through woody substrates, dark-pigmented ascomata with ostiolate papilla and fusiform, 1-septate and hyaline ascospores (Barr 1992; Tang et al. 2003; Hirayama and Tanaka 2011). Currently, 161 epithets are listed in Index Fungorum, but only 97 species were accepted in Lophiotrema based on current morphological data and phylogenetic evidence (Hyde et al. 2016; Doilom et al. 2017).
Lophiotrema guttulata Boonmee, Tibpromma & K.D. Hyde, sp.nov.
Index Fungorum number: IF552688; Facesoffungi number: FoF02735; Figs. 26, 27
Etymology: ‘guttulata’ referring to the guttules in this species.
Holotype: MFLU 10-0971
Saprobic on dried terrestrial branch. Sexual morph Ascomata 248.5–310 µm high × 271–340 µm diam. (\( \bar{x} \) = 279 × 305.5 µm, n = 5), uniloculate, immersed, scattered, subglobose, dark brown, ostiolate. Ostioles 98–137 × 29–53 µm diam. (\( \bar{x} \) = 124 × 37 µm, n = 5), plugged by periphyses, apically with a crest-like papilla, dark, carbonaceous, fragile, with irregular opening. Peridium 23–38.5 µm wide, outer layer narrow, composed of dark cells of textura angularis, inner layer of light pigmented to hyaline cells of textura subglobosa. Hamathecium comprising 1–2 µm wide, branched, septate, cylindrical pseudoparaphyses. Asci (78-)90–125 × 8–11.5 µm (\( \bar{x} \) = 96.5 × 9 µm, n = 20), 8-spored, bitunicate, fissitunicate, cylindric-clavate, sessile to short pedicellate, apically rounded with an ocular chamber. Ascospores 23–29 × 4–6 µm (\( \bar{x} \) = 26 × 5 µm, n = 10), uniseriate, ellipsoidal to broadly fusiform, 1-septate, constricted at the septum, with 1–2 guttules, with distinct large guttules in each cell, with terminal appendages, hyaline, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Ascospore germinating on MEA within 12 h. Colonies on MEA reaching 10 mm diam. in 7 days at 28 °C, flat to slightly effuse, entire edge, dark brown. Aerial mycelium radiating outwards, partially superficial, light brown to dark brown, with thick cells, nonsporulating on culture.
Material examined: THAILAND, Chiang Mai Province, Jomthong, Ban Luang, N18°31′E98°29′, on dried branch, 6 October 2010, R. Phookamsak, ITN-02: MFLU 10-0971, holotype; ex-type living culture: MFLUCC 10-0929, BCC 52151.
GenBank Numbers LSU:KY498006; SSU:KY498007.
Notes: Lophiotrema guttulata differs from other species of Lophiotrema in having a protruding ascomatal neck, carbonaceous papilla and lacking a mucilaginous sheath in the ascospores. Phylogenetic analyses of the combined LSU and SSU dataset reveal that L. guttulata is nested independently between L. lignicola and L. neohysterioides and therefore provides evidence to support our new species (Fig. 25).
Lophiotrema vagabundum (Sacc.) Sacc., Michelia 1 (4): 338 (1878)
Facesoffungi number: FoF 2783, Fig. 28
Saprobic on dead stem of Sambucus ebulus L. Sexual morph Ascomata 489–614 µm high × 622–801 µm diam. (\( \bar{x} \) = 551 × 762 μm, n = 5), semi-immersed to erumpent through host tissue, under to clypeus, solitary, uniloculate, globose, conspicuous at the surface, black, subcarbonaceous, without hairs, ostiolate, smooth-walled. Peridium 25–41 μm wide, comprising 5–6 layers of hyaline to reddish-brown cells of textura angularis. Hamathecium 1.1–1.9 µm wide, comprising numerous, aseptate, unbranched, anastomosing pseudoparaphyses. Asci 96–129 × 8–10 μm (\( \bar{x} \) = 118 × 9 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical, furcate, rounded at the ends, short pedicellate, lacking an ocular chamber. Ascospores 22–30 × 4–7 μm (\( \bar{x} \) = 25 × 5 μm, n = 15), overlapping 1–2-seriate, hyaline, 3-septate, constricted at the second septum, swollen at second cell, conical at the ends, guttulate, surrounding by large mucilaginous sheath, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 4–5 cm diam., after 7 days in the dark at 16 °C, flat to slightly effuse, entire edge, light brown to dark brown, not sporulating in culture.
Material examined: ITALY, near San Benedetto in Alpe, on dead stem of Sambucus ebulus (Adoxaceae), 24 October 2014, Erio Camporesi, IT2196 (MFLU 15-1497, new host record); living culture MFLUCC 15-0081, (HKAS 94608 bis).
GenBank Numbers LSU:KY496737; ITS:KY496757; SSU:KY501125; TEF1:KY514401.
Notes: Lophiotrema vagabundum was introduced by Saccardo (1878), based on a sexual morph and was recorded from Vitis coignetiae, Epilobium angustifolium and woody plants. Based on rDNA phylogenetic analyses, the collection of L. vagabundum in this study (MFLUCC 15-0081) clustered with high statistical support to other L. vagabundum strains analyzed (Fig. 25). Morphological characters are also identical to other L. vagabundum strains and therefore we report this collection as a new host record on Sambucus ebulus (Adoxaceae), from Italy.
Hermatomyces Speg.
The genus Hermatomyces was introduced by Spegazzini (1911), with H. tucumanensis as the type species. Currently the genus contains six species (Index Fungorum 2017). Morphologically, the genus is characterized by having lenticular to cylindrical, muriform conidia, often with subhyaline to pale brown peripheral cells, and dark brown central cells. Conidia are cylindrical and comprise 1–4 columns with 2–11 cells and are irregularly pigmented (Castañeda and Heredia 2000; Leão-Ferreira et al. 2013; Doilom et al. 2017). Based on the phylogenetic study by Doilom et al. (2017), Hermatomyces is accommodated in Lophiotremataceae.
Hermatomyces chiangmaiensis J.F. Li, Bhat & K.D. Hyde, sp. nov.
Index fungorum number: IF552306; Facesoffungi number: FoF02480, Fig. 29
Etymology: refers to the location where the fungus was collected, Chiang Mai.
Holotype: MFLU 16-1930
Saprobic on dead hanging leaves of Pandanus sp. Sexual morph Undetermined. Asexual morph Colonies discrete on host, black, powdery. Mycelium immersed to superficial in the substrate, composed of septate, branched, smooth, light brown-coloured hyphae. Conidiophores 3.8–5.3 µm long × 4.5–5 µm diam. (\( \bar{x} \) = 4.8 × 4.7 µm, n = 10), micronematous, mononematous, flexuous, hyaline, smooth, moderately thin-walled, comprising 1–2 subcylindrical to subglobose cells, arising from prostrate, thick-walled hyphae. Conidiogenous cells 3.8–4.5 µm long × 4.8–6.7 µm diam. (\( \bar{x} \) = 4.4 × 5.8 µm, n = 10), monotretic, integrated, terminal, ampulliform to doliiform, sub-hyaline to hyaline, smooth, thin-walled, dark-coloured at conidiogenous loci. Conidia dimorphic, thick-walled, smooth: lenticular conidia (10–)13.5–18 µm long × (7.3–)8.6–10.4 µm diam. (\( \bar{x} \) = 13.5 × 9.6 µm, n = 20), multi-septate, with central cells dark brown to black, with peripheral cells subhyaline to pale brown, slightly constricted at the septa, smooth, in side view composed of one column of 4–6 cells, hyaline to light brown at the lower and upper cells, often carrying remnant of conidiogenous cell at base; cylindrical conidia: 10–16.4 μm high, × 16–19.5 μm wide in broadest part of lower cells, (\( \bar{x} \) = 13.2 × 17.5 μm, n = 20), with 3–4 columns of 2–4 cells arising from a common basal cell, each column, with rectangular to globose cells, constricted at septa, pale to subhyaline, granulate, smooth, terminating with a brown upper cell.
Cultural characteristics: Conidia germinating on PDA within 10 h and germ tubes produced from the apex. Colonies growing on PDA, reaching 5 cm in 14 days at 30 °C, mycelium partly superficial, partly immersed, slightly effuse, cottony, with regular edge, olive-green to grayish-green; sexual or asexual spores not formed within 60 days.
Material examined: THAILAND, Chiang Mai Province, Mae Taeng, Musroom Research Centre, on a dead hanging leaf of Pandanus sp., 25 March 2016, Junfu Li, H-MRC40B (MFLU 16-2930, holotype). ex-type living culture MFLUCC 16-2817, KUMCC (paratype in HKAS).
GenBank Number LSU:KY559394.
Notes: Hermatomyces chiangmaiensis is morphologically similar to H. krabiensis (Tibpromma et al. 2016b) in conidial shape, but the former has smaller and ampulliform to cylindrical conidia.
Hermatomyces chromolaenae J.F. Li, Mapook & K.D. Hyde, sp. nov.
Index Fungorum number: IF552309; Facesoffungi number: FoF02481, Fig. 30
Etymology: refers to the Sai Khu waterfall where the fungus was collected.
Holotype: MFLU 16-1931
Saprobic on dead stem of Chromolaena odorata Linn. Sexual morph Undetermined. Asexual morph Colonies on natural substrate dry, blackish-brown, velvety, circular, doughnut-shaped, dull, consisting of a sterile mycelial outer zone and a round, glistening, abundantly sporulating centre, with conidia readily liberated when agitated. Mycelium 1.3–2.1 μm wide, superficial, composed of a network of branched, septate, brown, thick-walled, smooth hyphae. Conidiophores 3–4.3 μm long × 1.8–1.9 μm wide (\( \bar{x} \) = 3.6 × 1.8 µm, n = 10), micronematous, flexuous, pale brown, smooth, unbranched, arising from prostrate hyphae at the centre of circular colony. Conidiogenous cells holoblastic, monoblastic, integrated, terminal, cylindrical, hyaline to subhyaline. Conidia 9.2–10.4 µm high × 10.2–11.5 µm wide (\( \bar{x} \) = 9.7 × 10.7 µm, n = 20) monomorphic, thick-walled, smooth, disk-shaped, with central cells dark brown to black, with peripheral cells pale brown. Two halves of the disk-shaped conidia symmetrically adpressed, forming a deep constriction at lower and upper end in lateral view, each half with 6–7 cells, hyaline to light brown at lower and upper cells, dark brown in middle cells.
Cultural characteristics: Conidia germinating on PDA within 10 h and germ tubes produced from the apex. Colonies growing on PDA, reaching 5 cm in 14 days at 30 °C, mycelium partly superficial, partly immersed, slightly effuse, cottony, with regular edge, olivaceous-green to grayish-green; sexual or asexual spores not formed within 60 days.
Material examined: THAILAND, Chiang Rai Province, Doi Pui, on a dead (not land) stem of Chromolaena odorata (Asteraceae), 5 October 2016, Junfu Li, DPK4 (MFLU 16-2931, holotype). ex-type living culture MFLUCC 16-2818, KUMCC (paratype in HKAS).
GenBank Number LSU:KY559393.
Notes: Morphologically, Hermatomyces chromolaenae is similar to H. saikhuensis in having dark coloured conidia and conidiophores arising from prostrate hyphae at the centre of a circular colony. However, H. chromolaenae is unique in having darker peripheral cells and smaller conidia and the phylogeny also segregates these two species. A close phylogenetic association, albeit with moderate support, between H. chromolaenae and H. pandanicola is also noted. Hermatomyces pandanicola, however, has dark brown to black central cells in the conidia, with subhyaline to pale brown peripheral cells, and in lateral view obovoid, guttulate, hyaline to light brown at lower and upper ends and dark brown at the central cells.
Melanommataceae G. Winter.
The family Melanommataceae, typified by Melanomma, was established by Winter (1885) to accommodate Bertia, Bombardia, Crotonocarpia, Rosellinia, Melanomma and Melanopsamma. Melanommataceae is characterized by globose or depressed perithecial ascomata, bitunicate and fissitunicate asci, pigmented and phragmosporous ascospore (Tian et al. 2015). Tian et al. (2015) revised this family by combining morphology and multi-locus phylogenetic analyses using LSU, SSU, TEF1 and RPB2 datasets and 20 genera were accepted in the family.
Melanomma Nitschke ex Fuckel
Melanomma was validly published by Fuckel (1870) and is typified by Melanomma pulvis-pyrius (Pers.) Fuckel. Melanomma is characterized by the carbonaceous ascomata, and hyaline or brown, 2–3-septate ascospores (Tian et al. 2015). Asexual morphs of Melanomma were reported as coelomycetous and hyphomycetous (Hyde et al. 2011). In this study, we transfer Aposphaeria populina Died. into Melanomma.
Melanomma populina (Died.) Phukhamsakda & K.D. Hyde, comb. nov.
Index Fungorum number: IF552757; Facesoffungi number: FoF 2887
Basionym: Aposphaeria populina Died., Krypt.-Fl. Brandenburg (Leipzig) 9: 206 (1912)
Notes: Aposphaeria is considered as an asexual genus in the family Melanommataceae. Tian et al. (2015) mentioned Aposphaeria and phoma-like asexual morphs have been reported for Melanomma species (Sivanesan 1984). Additionally, this study reports the sexual state for Aposphaeria; A. corallinolutea based on multi-gene phylogeny and the identity of the asexual morph produced from culture. Diedicke (1912) introduced A. populina as a phoma-like species reported from Populus canadensis. De Gruyter et al. (2013) designed an epitype from the same host, and generated sequences for A. populina. Based on phylogenies, A. populina is related to Melanomma pulvis-pyrius as they clustered together with high statistical support (Tian et al. 2015; Hyde et al. 2016). Given that the sexual morph morphology in A. corallinolutea and M. pulvis-pyrius are different (Mugambi and Huhndorf 2009), and phylogeny refer them to distinct clades, we synonymize Aposphaeria populina under Melanomma populina.
Aposphaeria Sacc.
The genus Aposphaeria was introduced by Saccardo (1880) and has a phoma-like structure. Aposphaeria is characterized by pycnidial, superficial conidioma, hyaline, short conidiophores, enteroblastic, phialidic, discrete, conidiogenous cells, and hyaline, aseptate, and ellipsoidal conidia (Sutton 1980). Tian et al. (2015) reconsidered the family Melanommataceae and as a result of lack of sequence data for A. pulviscula (Sacc.) Sacc (the type species), Aposphaeria was retained in Melanommataceae. Based on the phylogenetic analysis, A. populina is closely related to Melanomma pulvis-pyrius, the type genus of Melanommataceae (82%ML/82%MP/0.90BYPP, Fig. 31) and is a synonym of Melanomma. It is however, important that the type of Aposphaeria is recollected to establish if the genus sensu stricto, belongs in Melanommataceae. In this study, we describe the sexual morph of Aposphaeria corallinolutea, bearing in mind that Aposphaeria sensu stricto may not belong in Melanommataceae.
Aposphaeria corallinolutea Gruyter, Aveskamp & Verkley, Stud. Mycol 75: 28 (2012)
Facesoffungi number: FoF 2787, Fig. 32
Saprobic on the dead twigs of Prunus padus L. Sexual morph Ascomata 194–418 μm high × 183–397 μm wide diam. (\( \bar{x} \) = 306 × 290 μm, n = 5), scattered or clustered in small to large groups, initially immersed, becoming erumpent to superficial, globose, black to dark brown, subcarbonaceous, ostiole central. Ostioles 149 μm high × 119 μm diam., papillate, dark brown, smooth, periphyses filled ostioles cannel. Peridium 35–50 μm wide, up to 72 μm, thick, composed of 9–13 layers of textura angularis, outer region haeavily pigment cells, black to dark-brown, inner layer composed of hyaline gelatinous cells, thin, merging with pseudoparaphyses. Hamathecium comprising numerous, long, 1.3–2.2 μm (\( \bar{x} \) = 1.8 μm, n = 40) wide, broad, transversely septate, branched, trabeculate pseudoparaphyses. Asci 66–154 × 6–14 μm (\( \bar{x} \) = 129 × 12 μm, n = 20), (4)–8-spored, bitunicate, cylindrical, with club-shaped pedicel, apically rounded, with an ocular chamber up to 2–4 μm wide. Ascospores 16–23 × 7–11 μm (\( \bar{x} \) = 19 × 9 μm, n = 50), overlapping uniseriate, hyaline, broad-fusiform, conical at the apex, 1-transversely septate, strongly constricted at the septa, wall rough, not uniform, indentations present when mature. Asexual morph Coelomycetous formed in cultures. Conidiomata 300 μm high, 200 μm diam., pycnidia, dark brown, solitary, aggregated, or clustered in groups, globose, papillate. Pycnidial wall comprising of cells of textura angularis, ostiole absent. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–10–(17) × 1.8–3 μm (\( \bar{x} \) = 9 × 2.5 μm, n = 20), holoblastic, phialidic, hyaline, smooth-walled, discrete. Conidia 3–7 × 1.6–3 μm, 1-celled, hyaline, ellipsoid to oblong, slightly curved, smooth-walled.
Material examined: RUSSIA, Arkhangelsk Region, Arkhangelsk City, Northern Maimaksa urban micro-district, wood waste landfill, on dead branches of Prunus padus (Rosaceae), 11 May 2015, G.V. Okatov, T-583 (MFLU 15-2752, sexual morph); living culture MFLUCC 17-0001.
GenBank Numbers LSU:KY554197; SSU:KY554200; ITS:KY554202; TEF1:KY554205; RPB2:KY554207.
Notes: Aposphaeria corallinolutea was introduced by De Gruyter et al. (2013) based on Pleurophoma sp. cultures in CBS culture collection. Aposphaeria corallinolutea (MFLUCC 16-1059, sexual morph) has characters similar to Melanommataceae such as nearly superficial and carbonaceous ascomata, papillate ostioles, thick-walled peridium, trabeculate pseudoparaphyses, cylindrical uniseriate asci, and 1-septate ascospores. Our collection also produced an asexual morph in culture, which is very similar to the characteristics of A. corallinolutea (CBS 131286, CBS 131286). On the other hand, A. corallinolutea strains are sister taxa to Melanomma but lack support. In the phylogenetic tree, our strain Aposphaeria corallinolutea (MFLUCC 16-1059) clustered together with A. corallinolutea (CBS 131286, CBS 131286) (Fig. 31) and we describe the sexual morph of A. corallinolutea with the support of asexual morph production from culture.
Nigrogranaceae Jaklitsch & Voglmayr
See notes under Biatriosporaceae.
Nigrograna De Gruyter et al.
Nigrograna was described by De Gruyter et al. (2013) as a monotypic genus with Nigrograna mackinnonii as type species. The sexual morph of Nigrograna is characterized by depressed globose to globose, immersed or superficial, black ascomata, ostioles periphysate, Ascospores asymmetric, fusoid to narrowly ellipsoid with the second cell slightly wider than others (Jaklitsch and Voglmayr 2016). The asexual morph of Nigrograna is characterized by pycnidia similar to ascomata and usually co-occurring with them, filiform conidiophores, simple to sparsely branched. Conidia forming on pegs and phialides, oblong, 1-celled, smooth (Jaklitsch and Voglmayr 2016). Five species are accepted in Nigrograna (Jaklitsch and Voglmayr 2016) (Fig. 33).
Nigrograna cangshanensis Z.L. Luo, H.Y. Su & K.D. Hyde, sp. nov.
Index Fungorum number: IF 552681; Facesoffungi number: FoF 2888, Fig. 34
Etymology: With reference to the collection site of this fungus.
Holotype: HKAS 83978
Saprobic on decaying wood. Sexual morph Ascomata 120–135 × 135–155 µm, perithecioid, gregarious, immersed, subglobose or ellipsoidal, black, with an ostiole, neck long. Ostiole mostly central, brittle. Peridium 3-layered, composed of angular cells, outer layer, dark brown, thick-walled cells, middle layer and inner layer, pale brown, with thick-walled cells. Hamathecium composed of 1.5–2.5 µm (\( \bar{x} \) = 2 μm, n = 25) wide, numerous, tapering distally, pseudoparaphyses, longer than asci, embedded in a gelatinous matrix. Asci 50–70 µm long (\( \bar{x} \) = 60 µm, n = 25), 6.5–8.5 µm wide (\( \bar{x} \) = 7.5 µm, n = 25), 8-spored, bitunicate, fissitunicate, clavate to cylindric-clavate, short pedicellate, apically rounded, sometimes with an ocular chamber. Ascospores 11–14 µm long (\( \bar{x} \) = 12.5 µm, n = 35), 3.5–4.5 µm wide (\( \bar{x} \) = 4 µm, n = 35), biseriate, olive-gray to yellowish-brown, 3-septate, constricted at the septa. Ascospores germinate from germ tubes from all four cells. Asexual morph Undetermined.
Material examined: CHINA, Yunnan Province, saprobic on decaying wood submerged in stream in Cangshan Mountain, March 2014, Z.L. Luo, LQXM 25 (HKAS83978, holotype), ex-type living culture, MFLUCC 15-0253.
GenBank Numbers LSU:KY511064; SSU:KY511065; ITS:KY511063; TEF1:KY511066.
Notes: All species of Nigrograna are morphologically very similar and thus, Jaklitsch and Voglmayr (2016) considered them to be cryptic species. Morphologically, N. cangshanensis is very similar to other species in the genus. However, N. cangshanensis differs from N. fuscidula, N. norvegica and N. obliqua in having a thicker peridium. Phylogenetic analysis also showed that N. cangshanensis is distinct from other Nigrograna species (Fig. 33).
Roussoellaceae J.K. Liu, Phook., D.Q. Dai & K.D. Hyde
The family Thyridariaceae was introduced by Hyde et al. (2013) to accommodate the genus Thyridaria based on morphology and phylogenetic analyses with Thyridaria incrustans as the type species. Jaklitsch and Voglmayr (2016) introduced a new genus, Parathyridaria, with two species in the family, and provided a phylogenetic analysis of the family based on the combined ITS, LSU, SSU, RPB2 and EF-1α sequence data. In this study, a new species, Parathyridaria robiniae, is introduced with evidence from molecular data analysis and morphological comparisons. Jaklitsch and Voglmayr (2016) treated Roussoellaceae as a synonym of Thyridariaceae. We obtained a similar topology in our phylogenetic analysis, however, the two families have distinct morphological characters. The families are also well resolved in the analyses. This is in agreement with the results of Liu et al. (2014) where they provide evidence that Roussoellaceae is a good family in Pleosporales with high diversity (Crous et al. 2014; Ariyawansa et al. 2015b; Dai et al. 2017). We follow Liu et al. (2014) and treat Roussoellaceae as a well-resolved family in Pleosporales. The phylogenetic tree, based on combined LSU, ITS, EF-1α and RPB2 sequence data is presented in Fig. 35.
Thyridariaceae Q. Tian & K.D. Hyde
See notes for Roussoellaceae above.
Parathyridaria robiniae Mapook, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552776; Facesoffungi number: FoF 2794, Fig. 36
Etymology: Name reflects the host genus Robinia.
Holotype: MFLU 17-0015
Saprobic on a dead branch of Robinia pseudoacacia L. Sexual morph Ascomata 450–470 µm high × 255–270 µm diam. (\( \bar{x} \) = 455 × 260 µm, n = 5), semi-immersed to immersed, scattered, coriaceous, in groups, ovoid to obpyriform, reddish-brown to brown, ostiole central. Peridium 10–25 µm wide, comprising light brown to hyaline cells of textura angularis. Hamathecium comprising 0.5–1.5 µm wide, cylindrical to filiform, septate, branched, pseudoparaphyses. Asci 105–115 × 10–15 µm (\( \bar{x} \) = 110 × 11.5 µm, n = 10), 8-spored, bitunicate, cylindric-clavate, pedicellate, apically rounded with a small ocular chamber. Ascospores 15–20 × 4–7 µm (\( \bar{x} \) = 18.5 × 5 µm, n = 15), overlapping 1–2-seriate, hyaline, broadly fusiform to inequilateral, 1-septate, constricted at the septa, widest at the centre and tapering towards the narrow ends, smooth-walled, with mucilaginous sheath. Asexual morph Undetermined.
Material examined: ITALY, Forlì-Cesena Province, Collina di Pondo—Santa Sofia, on dead branch of Robinia pseudacacia (Fabaceae), 22 September 2014, E. Camporesi (MFLU 17-0015, holotype), ex-type living culture MFLUCC 14-1119 (isotype in HKAS, under the code of HKAS).
GenBank Numbers LSU:KY511141; ITS:KY511142; TEF1:KY549682.
Notes: Phylogenetic analysis indicates that our collection belongs to the family Thyridariaceae and P. robiniae groups together with members of Parathyridaria a genus that was introduced by Jaklitsch and Voglmayr (2016). The phylogeny also indicates that P. robiniae lies in an independent terminal taxon nested between P. percutanea and P. ramulicola and therefore can be considered as a new species (Fig. 35). Parathyridaria robiniae is also distinct in having 1-septate ascospores surrounded by a hyaline gelatinous sheath.
Massarineae
Massarineae is a suborder of Pleosporales, originally established by Barr (1979) to accommodate Massarinaceae and Arthopyreniaceae (Hawksworth et al. 1983, 1995; Barr 1987; Eriksson and Winka 1998; Kirk et al. 2008; Lumbsch and Huhndorf 2010). The taxonomic framework, phylogenetic relationships, biology, and species diversity of Massarineae are poorly understood (Tanaka et al. 2015; Phukhamsakda et al. 2016).
Didymosphaeriaceae Munk.
The family Didymosphaeriaceae was introduced by Munk (1953) and typified by Didymosphaeria, with D. epidermidis (Fr.) Fuckel as the type species. Ariyawansa et al. (2014a) synonymized Montagnulaceae under Didymosphaeriaceae based on priority of the oldest name. Currently, 25 genera are accommodated in Didymosphaeriaceae (Ariyawansa et al. 2015b; Crous et al. 2015a; Thambugala et al. 2014a; Wijayawardene et al. 2014a; Tanaka et al. 2015; Wanasinghe et al. 2016). We introduce introduce a new species of Paraphaeosphaeria with an updated phylogenetic tree for the family.
Paraphaeosphaeria O.E. Erikss.
Paraphaeosphaeria was introduced by Erikss (1967) and species of this genus are similar to Phaeosphaeria Miyake 1909. Sutton (1980) mentioned that all known species of Paraphaeosphaeria occur on monocotyledons and taxa differ in ascomatal structure, ascospores septation, size, host and in asexual characteristics. Taxonomic studies have also reported that this genus is polyphyletic (Câmara et al. 2002; Checa et al. 2002)
Paraphaeosphaeria viciae de Silva, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552714; Facesoffungi number: FoF 2776, Fig. 38
Etymology: Name reflects the host genus Vicia.
Holotype: MFLU 15-1231
Saprobic on Vicia sp. Sexual morph Undetermined. Asexual morph Conidiomata 113–163 μm diam. semi-immersed, solitary to gregarious, scattered, globose to subglobose, uniloculate, pale brown, ostiolate. Ostioles circular, without papilla. Conidiomata wall 8–13 μm wide, composed of thin-walled, hyaline to light brown cells of textura angularis. Conidiophores indistinct. Conidiogenous cells 3–5 × 2–4 μm (\( \bar{x} \) = 4.1 × 3 μm), enteroblastic, phialidic, cylindrical to subcylindrical, ampulliform, discrete, hyaline, smooth. Conidia 4–5 × 1–2 μm diam. (\( \bar{x} \) = 4.8 × 2.1 μm), subglobose to ellipsoid or obovoid, hyaline to pale, smooth and thick-walled, guttulate. Sexual morph Undetermined.
Culture characteristics: Colonies on PDA 35 mm diam. after 21 days at 25 °C, irregular, flat, smooth surface with undulate edge, white colour colony and reverse pale brown.
Material examined: ITALY, Forlì-Cesena Province, Monte Mirabello-Predappio, on dead branch of Vicia sp. (Fabaceae), 18 May 2015, Erio Camporesi, IT2482 (MFLU 15-1231 holotype), ex-type living culture KUMCC 16-0184.
GenBank Numbers LSU:KY397948; SSU:KY397947; ITS:KY379969.
Notes: Paraphaeosphaeria viciae shares some similarities with P. spartii in having subglobose to ellipsoid or obovoid, aseptate conidia with guttules. Paraphaeosphaeria viciae however, has 113–163 μm diam., semi-immersed, globose to subglobose conidiomata as compared to 200–300 μm diam. in P. spartii (Liu et al. 2015; Wijayawardene et al. 2016). DNA sequence analyses confirm the position of our new taxon in Didymosphaeriaceae (Fig. 37) and indicate the genus is polyphyletic.
Latoruaceae Crous
The family Latoruaceae was introduced by Crous et al. (2015b) and typified by Latorua caligans (Bat. & H.P. Upadhyay) Crous. Four genera Latorua, Matsushimamyces, Polyschema, Pseudoasteromassaria are accommodated in this family. Both sexual morph and asexual morph of the genus Pseudoasteromassaria have been reported and are characterized by fusiform, 1–3-septate, brown ascospores and cylindrical, 6–7-septate, hyaline conidia (Ariyawansa et al. 2015c) (Fig. 39).
Pseudoasteromassaria M. Matsum. & Kaz. Tanaka
Pseudoasteromassaria was introduced by M. Matsum. & Kaz. Tanaka (2015) with Ps. fagi M. Matsum. & Kaz. Tanaka as the type species. The morphological characteristics of this genus are similar to those of Asteromassaria (Pleomassariaceae) (Ariyawansa et al. 2015c).
Pseudoasteromassaria spadicea W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum number: IF 552730; Facesoffungi number: FoF 2825, Fig. 40
Etymology: spadicea in reference to the colour of the germinated conidia.
Holotype: MFLU 15-2683
Saprobic on submerged wood in freshwater habitats. Asexual morph Conidiomata 290–320 μm high × 460–480 μm diam., pycnidial, immersed, solitary or scattered on the host surface, ellipsoidal, unilocular, ostiolate in the center, circular, dark-brown, with a short papilla. Peridium 23–28 μm wide, composing several layers of irregular cells arranged in a textura angularis, brown to dark brown. Conidiophores reduced. Conidiogenous cells phialidic, up to 8 μm long, determinate, discrete, cylindrical or pyramidal, hyaline, smooth, forming from the inner wall cells. Conidia 11–15 × 7–10 μm (\( \bar{x} \) = 13 × 9 μm, n = 20), globose to subglobose or ellipsoidal or obovoid, without septum, swollen with one large guttules in the cell, apex and base of cell rugose or smooth, hyaline, becoming light brown when germinated in culture, thick-walled. Sexual morph Undetermined.
Culture characteristics: on PDA reaching 3 cm diam. after 30 days at 25 °C, with circular colony, edge undulate, surface rough with raised elevation, brown to dark brown.
Material examined: THAILAND, Prachuap Khiri Khan Province, on submerged wood, 30 July 2015, Wei Dong, 34B (MFLU 15-2683, holotype); ex-type living culture MFLUCC 15-0973).
GenBank Numbers LSU:KY522724; SSU:KY522725; ITS:KY522726.
Notes: Phylogenetic analyses of combined genes indicate that Pseudoasteromassaria spadicea clustered with other species of Pseudoasteromassaria and in particular it shares a close affinity to P. fagi. Pseudoasteromassaria spadicea differs from the asexual strain of P. fagi mainly by its globose or obovoid, aseptate conidia and conidial size (11–15 × 7–10 μm vs. (52–)61–71 × 10–12.5 μm) (Ariyawansa et al. 2015c).
Lentitheciaceae Y. Zhang ter, C.L. Schoch, J. Fourn., Crous & K.D. Hyde
The family Lentitheciaceae was introduced by Zhang et al. (2009a) with L. fluviatile (Aptroot & Van Ryck.) K.D. Hyde (2009) as the type species. This family now includes six genera (Wanasinghe et al. 2014).
Keissleriella Höhn.
Keissleriella was introduced by Höhnel (1919) to accommodate Keissleriella aesculi Sacc. (≡Pyrenochaeta aesculi Höhn.) as the type. The genus is characterized by ascomata with ostiolar necks filled with black setae, and 1 to multi-septate, hyaline ascospores (Barr 1990; Liu et al. 2015). Munk (1957) placed the genus in Lophiostomataceae, while von Arx and Muller (1975) placed it in Pleosporaceae. Later Barr (1990) transferred it to Melanommataceae. Lumbsch and Huhndorf (2007) maintained Keissleriella in Massarinaceae, while Zhang et al. (2009b) placed the genus in Lentitheciaceae, and this has been followed by subsequent authors (Hyde et al. 2013; Tanaka et al. 2015; Wijayawardene et al. 2014a; Zhang et al. 2012b) (Fig. 41).
Keissleriella cirsii R.H. Perera, Bulgakov, Wanasinghe & K.D. Hyde, sp. nov.
Index Fungorum number: IF552705; Facesoffungi number: FoF 2767, Fig. 42
Etymology: Named after the host genus.
Holotype: MFLU 15-2900
Saprobic on Cirsium sp. Sexual morph Ascomata 245–370 µm high, 180–368 µm diam. (\( \bar{x} \) = 285 × 255 µm, n = 10), semi-immersed, appearing as black raised spots on the host, solitary, globose to subglobose, wall black, uniloculate, black, ostiolate. Ostioles with dark brown to black setae, Peridium 30–67 µm wide, composed of 4–6 layers of pale brown to brown cells of textura angularis. Hamathecium comprising numerous, 1.5–2.2 µm (n = 25) wide, filamentous, branched, septate, pseudoparaphyses. Asci 110–147 × 6–8.5 µm (\( \bar{x} \) = 130 × 8 µm, n = 30), 8-spored, bitunicate, clavate, with a short furcate pedicel, rounded at the apex. Ascospores 23.5–29 × 5–7 µm (\( \bar{x} \) = 27 × 6 µm, n = 40), uniseriate to biseriate, fusiform, 3–5-septate, constricted at the septa, hyaline, inequilateral, with a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 50–55 mm diam. in 21 days, white, spreading, with moderate aerial mycelium, slightly irregular, with smooth margins.
Material examined: RUSSIA, Voronezh Oblast, Lysogorka, on stems of Cirsium vulgare (Savi) Ten., 25 July 2015, T. Bulgakov, T 755 (MFLU 15-2900, holotype); ex-type living cultures MFLUCC 16-0454, MFLUCC 16-0455.
GenBank Numbers LSU:KY497780; SSU:KY497782; ITS:KY497783; TEF1:KY497786; RPB2:KY497787.
Notes: Keissleriella cirsii is typical of Keissleriella in its ostiolar neck covered by short dark setae (Munk 1953; Tanaka et al. 2015)., K. cirsii is a distinct species in the genus, supported by molecular and morphological characteristics. Keissleriella cirsii clustered as sister to K. taminensis with 100% bootstrap support. Keissleriella cirsii differs from K. taminensis in having larger ascomata and ascospores with fewer longitudinal septa (3–4 vs. 4–5) (Tanaka et al. 2015).
Pleurophoma Höhn.
The genus Pleurophoma, based on P. pleurospora (De Gruyter et al. 2009), lacks any known sexual morph (De Gruyter et al. 2010). We introduce a new species of Pleurophoma italica, and describe both morphs.
Pleurophoma italica Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552689; Facesoffungi number: FoF 2768, Figs. 43, 44
Etymology: refers to the country where the holotype was collected.
Holotype: MFLU 15-1254
Saprobic on cone of Pinus nigra J.F. Arnold lying on the ground. Sexual morph Ascomata 154–167 µm high × 141–155 µm diam. (\( \bar{x} \) = 157 × 148 μm, n = 5), semi-immersed or erumpent, solitary, or in small groups, uniloculate, globose to subglobose, conspicuous at the surface, black, without hairs, ostiolate. Peridium 6–12 μm wide, comprising 4–5 layers of reddish-brown cells of textura angularis. Hamathecium 2.2–3.4 µm wide, comprising dense, aseptate, unbranched pseudoparaphyses. Asci 51–106 × 7–11 μm (\( \bar{x} \) = 72 × 9 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindrical, with short, club-shaped pedicel. Ascospores 13–16 × 3–5 μm (\( \bar{x} \) = 14 × 4 μm, n = 20), overlapping 1–2-seriate, hyaline, fusiform, 1-septate in the center, deeply constricted at the septum, swollen at the first cell, conical at the ends, with guttulate, lacking a mucilaginous sheath. Asexual morph Coelomycetous, produced on sterilized bamboo pieces and pine needles on water agar. Conidiomata 68–105 μm high × 77–98 μm diam., pycnidial, superficial on substrate, solitary, black, dull, uniloculate, globose to subglobose, conspicuous at the surface. Conidiophores reduced to conidiogenous cells arising from the innermost wall-layer cells of the conidiomata. Conidiogenous cells 5.3–8.6 × 3.2–5.1 μm (\( \bar{x} \) = 6.6 × 3.9 μm, n = 20), holoblastic, phialidic, globose to oblong, hyaline, and formed from the inner layer of the pycnidium wall. Conidia 0.9–3 × 0.7–1.8 μm (\( \bar{x} \) = 1.9 × 1.2 μm, n = 40), globose to oval, aseptate, hyaline, smooth-walled, spermatia-like.
Culture characteristics: Colonies on MEA at 16 °C reaching 2.5 cm in 1 week, circular with wavy margin, white–grey, smooth surface and raised.
Material examined: ITALY, near Ortignano-Raggiolo, on cone of Pinus nigra (Pinaceae) on ground, 29 July 2014, Erio Camporesi, IT2024 (MFLU 15-1254, holotype); ex-type living culture MFLUCC 15-0061 (HKAS 94600 bis, paratype).
GenBank Numbers LSU:KY496734; ITS:KY496754; SSU:KY501122; TEF1:KY514398.
Notes: Pleurophoma italica is introduced as a new species with both sexual and asexual morphs. The phylogeny indicates a close relationship of P. italica to P. pleurospora (type species), but constitutes a separate branch (Fig. 41). Pleurophoma italica shares similar asexual morphology with P. pleurospora. However, our taxon also clustered with Keissleriella sparticola, K. cladophila and K. genistae, but the morphological characteristics of sexual morphs are different. In the future, if Keissleriella sparticola, K. cladophila and K. genistae asexual morphs are discovered, and with more phylogenetic evidence, these three species could be synonymized under Pleurophoma. Hence, based on our phylogenetic tree (Fig. 41) we agree with Crous et al. (2015a) that the link between Pleurophoma and Keissleriella needs to be resolved.
Massarinaceae Munk
Details of this family can be seen in Hyde et al. (2013), Tanaka et al. (2015) and Hyde et al. (2016).
Pseudodidymosphaeria Thambug. & K.D. Hyde
The genus Pseudodidymosphaeria Thambugala & K.D. Hyde was introduced by Thambugala et al. (2015b) and accommodated in the family Massarinaceae. Two species are accepted in the genus, viz. P. spartii (Fabre) Thambugala et al. and P. phlei Phukhamsakda et al. (Li et al. 2016).
Pseudodidymosphaeria phlei Phukhams., Camporesi & K.D. Hyde, Fungal Diversity 78: 57 (2016)
Facesoffungi number: FoF 2786, Fig. 45
Saprobic on species of Arundo donax L., in terrestrial environments. Sexual morph Ascomata 300–500 μm high × 250–450 μm diam. (\( \bar{x} \) = 184.6 × 146.2 μm, n = 5), solitary, immersed, coriaceous, dark brown to black, globose to subglobose. Peridium 7–12 µm wide at the base, 12–20 µm wide at the sides, two-layered, and outer layer composed of dark brown, thin-walled cells of textura angularis, inner layer composed of smaller, compressed, hyaline cells. Hamathecium of 1.5–2.5 μm wide, septate, hyaline, cellular pseudoparaphyses, embedded in gelatinous matrix between and above the asci. Asci 100–130 × 12–20 μm (\( \bar{x} \) = 112.9 × 15.1 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, with a short pedicel, narrowly rounded at the apex, with an indistinct ocular chamber. Ascospores 19–22 × 9–11 μm (\( \bar{x} \) = 20.2 × 10.2 μm, n = 40), uniseriate to obliquely uniseriate, slightly overlapping, hyaline when immature, becoming golden brown when mature, ellipsoid, with broadly obtuse ends, 1-septate, upper cell slightly broader than the lower one, constricted at the septum, verrucose, surrounded by a thick mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: on PDA growing slowly, reaching 25–30 mm diam. after 21 days at 16 °C, surface greenish-brown, spreading with moderate aerial mycelium, and uneven, smooth margins; reverse buff.
Material examined: ITALY, Forlì-Cesena, Ravaldino in Monte, on dead Arundo donax stem (Poaceae), 11 November 2014, Erio Camporesi, IT 2233 (MFLU 15-1360, new host record);
GenBank Numbers LSU:KY264748; ITS:KY264744; SSU:KY264752.
Notes: Li et al. (2016) introduced Pseudodidymosphaeria phlei from Phleum pretense L. (Poaceae) collected in Forlì-Cesena Province of Italy as the second species of Pseudodidymosphaeria. Pseudodidymosphaeria phlei is similar to type species, P. spartii, in having globose to subglobose ascomata, cylindric-clavate asci and 1-septate ascospores. Our new isolate is also morphologically similar to P. phlei (strain MFLUCC 14-1061) and phylogenetically groups with 100% ML support. However, it is associated with a different host. Pseudodidymosphaeria phlei (holotype) was recorded on Phleum pretense and our collection was found on Arundo donax (Poaceae).
Massarineae genera incertae sedis
Inflatispora Y. Zhang ter, J. Fourn. & K.D. Hyde
Zhang et al. (2011) introduced Inflatispora as a lignicolous genus of Pleosporales from France to accommodate I. pseudostromatica Y. Zhang ter, J. Fourn. & K.D. Hyde. The genus is characterized by ‘widely porate ascomata, bitunicate asci, 3-septate, hyaline, cylindrical ascospores, with a swollen upper central cell and surrounded by a thin sheath, and narrow cellular pseudoparaphyses that anastomose between and above the asci (Zhang et al. 2011). Inflatispora pseudostromatica resembles species of Nodulosphaeria in having ascospores with an enlarged supramedian cell, but it can be distinguished from Nodulosphaeria by the presence a sheath surrounding the whole ascospore, while Nodulosphaeria has terminal ascospore appendages. The genus Inflatispora seems more typical of Massarineae, but the familial placement is still uncertain. Thus, we maintain the genus in Massarineae genera incertae sedis. It is essential to collect more taxa to establish their placement in Massarineae (Fig. 39).
Inflatispora caryotae Wanasinghe & K.D. Hyde, sp. nov.
Index Fungorum number: IF552767; Facesoffungi number: FoF 2795, Fig. 46
Etymology: refers to the host genus (Caryota).
Holotype: MFLU 16-2886
Saprobic on Caryota sp. in terrestrial habitats. Sexual morph Ascomata 350–400 μm high, 200–300 μm diam. (\( \bar{x} \) = 403 × 260 μm, n = 5), scattered, immersed, black, subglobose, papillate. Peridium 15–25 µm wide at the base, 10–15 µm wide at the sides, comprising 2–3 layers, fused with the host tissue at the outside, composed of thick-walled, black-brown, angular or relatively compressed, pseudoparenchymatous cells. Hamathecium of 1.5–3 µm wide, filiform, septate, anastomosing and branched, pseudoparaphyses. Asci 110–140 × 15–30 µm (\( \bar{x} \) = 122.6 × 23.1 µm, n = 20), 8-spored, bitunicate, fissitunicate, clavate to cylindric-clavate, pedicellate, apically rounded, with an ocular chamber. Ascospores 40–50 × 5–8 µm (\( \bar{x} \) = 46.5 × 6.6 µm, n = 30), 2–3-seriate, hyaline, fusiform, narrow towards and with acute ends, 1-septate, constricted at the septum, guttulate, smooth-walled, surrounded by a distinct mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: on MEA reaching 2 cm diam. after 2 weeks at 25 °C, dirty white at the beginning, with pale brown centre, dirty white towards outer margin at maturity, convex on the surface, undulate, margins smooth; reverse coffee brown.
Material examined: THAILAND, Narathiwat Province, on dead Caryota rachis, August 2013, Saithong Kaewchai, DHA07 (MFLU 16-2886, holotype); ex-type living culture, MFLUCC 13-0825).
GenBank Numbers LSU:KY264747; ITS:KY264743; SSU:KY264751.
Notes: We introduce the second species in Inflatispora, I. caryotae from Thailand based on morphological characteristics coupled with molecular data (Fig. 39). This species morphologically resembles I. pseudostromatica in having fusiform, hyaline ascospores with acute ends and a distinct mucilaginous sheath surrounding the ascospores. However, I. pseudostromatica has semi-immersed ascomata and 3-septate ascospores, while I. caryotae has immersed ascomata and 1-septate ascospores.
Suborder Pleosporineae
Didymellaceae Gruyter, Aveskamp & Verkley
The family Didymellaceae was introduced for the “Didymella clade” to accommodate the type species Didymella exigua and includes phoma and phoma-like genera (De Gruyter et al. 2009). Seventeen genera are presently included in Didymellaceae based on morphology and phylogeny, and these are Allophoma, Ascochyta, Boeremia, Calophoma, Dactuliochaeta, Didymella, Epicoccum, Heterophoma, Leptosphaerulina, Macroventuria, Neodidymelliopsis, Neoascochyta, Nothophoma, Paraboeremia, Phoma, Phomatodes and Xenodidymella (Hyde et al. 2013; Wijayawardene et al. 2014a; Chen et al. 2015). We provide an updated tree for Didymellaceae.
Ascochyta Lib
Ascochyta was introduced by Lib. in (1830) with A. pisi as the type species. A recent study by Aveskamp et al. (2010), reported that Ascochyta is polyphyletic. We introduce two new species and one new host record of Ascochyta and provide a new phylogenetic tree for Didymellaceae (Fig. 47).
Ascochyta italica Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552693; Facesoffungi number: FoF 2772, Fig. 48
Etymology: Named after the country where the fungus occurs.
Holotype: MFLU 14-0724
Saprobic on dead stem of Onobrychis viciifolia Scop. Sexual morph Ascomata 127–161 µm high × 167–196 µm diam. (\( \bar{x} \) = 142 × 187 μm, n = 5), immersed, solitary, or in small groups, uniloculate, globose, conspicuous at the surface, dull black, carbonaceous, without hairs, with short ostiolate. Peridium 16–37 μm wide, comprising 5–6 layers of hyaline to yellow–brown cells of textura angularis. Hamathecium 1.3–2.7 µm wide, comprising few dense, septate, unbranched, pseudoparaphyses. Asci 42–66 × 9–16 μm (\( \bar{x} \) = 59 × 14 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindric-clavate, with short club-shaped pedicel, thick-walled, rounded at the apex. Ascospores 14–20 × 7–10 μm (\( \bar{x} \) = 16 × 8 μm, n = 20), overlapping 1–2-seriate, 2-celled, hyaline, oval to ellipsoid, 1-septate in the centre, deeply constricted at the septum, enlarge at the first cell, rounded to conical at the ends, with small guttules, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 6 cm diam., after 7 days in the dark at 16 °C, circular with entire edge, smooth, convex, white to white–gray with age.
Material examined: ITALY, Premilcuore Province, near Fiumicello, on dead stem of Onobrychis viciifolia (Faboideae), 24 June 2014, Erio Camporesi, IT1954 (MFLU 14-0724, holotype); ex-type living culture MFLUCC 15-0077.
GenBank Numbers LSU:KY496732; ITS:KY496752; SSU:KY501120; RPB2:KY514410; TEF1:KY514397.
Notes: The sexual morph of Ascochyta species have been referred to Didymella and Mycosphaerella (Corlett 1981; Peever et al. 2007). Our phylogeny indicates Ascochyta italica is close to A. viciae (asexual morph) which was collected from the Netherlands on Vicia sepium. There are five base pair differences in RPB2 nucleotide sequences between A. viciae and A. italica.
Ascochyta medicaginicola Q. Chen & L. Cai, Stud. Mycol. 82: 187 (2015)
Facesoffungi number: FoF 2773, Fig. 49
Saprobic on dead branch of Scabiosa sp. Sexual morph Ascomata 143–162 µm high × 144–233 µm diam. (\( \bar{x} \) = 152 × 196 μm, n = 5), semi-immersed to erumpent, visible as raised, dark spots on the host surface, subglobose, solitary or small groups, scattered on the host surface, without papilla, with short ostiole in the centre, thin-walled, carbonaceous, dark brown or usually black. Peridium 17–28 μm wide, thin-walled, inner cells hyaline to pale brown, outer layer cells brown to dark brown, composed of flattened cells of textura angularis. Hamathecium 2.5–3.8 µm wide, comprising numerous, septate, cylindrical, cellular, pseudoparaphyses. Asci 46–67 × 12–15 μm (\( \bar{x} \) = 55 × 14 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate, rounded at the apex with acute ends, lacking an ocular chamber. Ascospores 17–22 × 7–9 μm (\( \bar{x} \) = 19 × 8 μm, n = 15), overlapping 1–2-seriate in the ascus, fusiform, 2-celled, narrowly fusoid with rounded ends, the cells above central septum often broader than the lower ones, constricted at the septum, deeply constricted at the septum, enlarged at the first cell, with small guttules in each cell, hyaline, lacking a mucilaginous sheath, smooth-walled. Asexual morph Undetermined.
Material examined: ITALY, Province of Arezzo, near Moggiona, on dead branch of Scabiosa sp. (Caprifoliaceae), 1 June 2013, Erio Camporesi, IT1314 (MFLU 15-1481, new host record); Ibid. (HKAS 94550 bis).
GenBank Numbers LSU:KY496720; ITS:KY496741; SSU:KY501111.
Notes: Ascochyta medicaginicola isolates sampled for LSU, ITS, β-tubulin and RPB2 based phylogenies clustered in the same subclade with A. medicaginicola var. medicaginicola (Fig. 47). We report our collections as new host records for A. medicaginicola. This is the first report of A. medicaginicola on Scabiosa sp. (Caprifoliaceae) from Italy and first report of a sexual morph for A. medicaginicola.
Ascochyta rosae Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552694; Facesoffungi number: FoF 2774, Figs. 50, 51
Etymology: Named after the host family (Rosaceae).
Holotype: MFLU 14-0723
Saprobic on dead branch of Rubus ulmifolia Sexual morph Ascomata 398–451 µm high × 364–398 µm diam. (\( \bar{x} \) = 446 × 380 μm, n = 5), immersed, solitary, or in small groups, uniloculate, globose, conspicuous at the surface, dull black, without hairs, long ostiolate. Peridium 16–29 μm wide, comprising 4–5 layers of reddish-brown cells of textura angularis. Hamathecium comprising 0.8–2.3 µm wide, dense, septate, unbranched, pseudoparaphyses. Asci 48–74 × 7–9 μm (\( \bar{x} \) = 58 × 8 μm, n = 25), 8-spored, bitunicate, fissitunicate, cylindrical, with short, club-shaped pedicel. Ascospores 13–17 × 3–5 μm (\( \bar{x} \) = 16 × 4.5 μm, n = 20), overlapping 1–2-seriate, fusiform, hyaline, 2-celled, 1-septate in the centre, deeply constricted at the septum, enlarged at the second cell, conical at the ends, with large guttules and with appendages at the ends. Asexual morph Produced on sterilized bamboo pieces with pine needles on water agar. Coelomycetous. Conidiomata 68–105 × 77–98 μm, pycnidial, yellow orange to black, globose, covered by dense vegetative hyphae, uniloculate, solitary to scattered. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5.3–8.6 × 3.2–5.1 μm (\( \bar{x} \) = 6.6 × 3.9 μm, n = 20), holoblastic, annelidic, globose to oblong, hyaline, and formed from the inner layer of pycnidium wall. Conidia 3.2–4.4 × 1.2–1.8 μm (\( \bar{x} \) = 3.7 × 1.5 μm, n = 40), cylindrical, rounded at both ends, sometimes slightly curved, aseptate, hyaline, guttulate.
Culture characteristics: Colonies on MEA reaching 3–4 cm diam., after 7 days in the dark at 16 °C, circular with entire edge, smooth, convex, flossy and velvety, white to yellow-white with age. Asexual morph on WA at room temperature (26 °C), convex, flossy and velvety, yellowish.
Material examined: ITALY, Galeata Province, near Strada San Zeno, on dead branch of Rubus ulmifolia (Rosaceae), 19 June 2014, Erio Camporesi, IT1946 (MFLU 14-0723, holotype); ex-type living culture MFLUCC 15-0063. (HKAS 94596bis, paratype).
GenBank Numbers LSU:KY496731; ITS:KY496751; SSU:KY501119; RPB2:KY514409.
Notes: Ascochyta rosae is introduced with both sexual and asexual morphs. The phylogeny indicates that A. rosae clusters with A. herbicola with high statistical support (100% in ML/1.00 in BYPP) (Fig. 47). Ascochyta rosae and A. herbicola differ in the sizes of conidiomata and conidia morphology. Conidia of A. rosae are smaller than A. herbicola (3.2–4.4 × 1.2–1.8 vs. 7–10.5 × 2–2.5) and the conidia are cylindrical to subcylindrical, without guttules (De Gruyter et al. 1998) while, A. rosae has cylindrical, sometimes slightly curved conidia with guttules. RPB2 nucleotide sequence comparison reveals six base pair differences with Ascochyta herbicola.
Calophoma Q. Chen & L. Cai
Calophoma was introduced by Chen & Cai (2015) with C. clematidina (Thüm.) Q. Chen & L. Cai as the type species. The asexual morph of this genus is characterized by subglobose, subcylindrical, ellipsoidal, somewhat obclavate-fusiform conidia with 0–1-septa and chlamydospores are produced in one species. The sexual morph of this genus has not been reported (Chen et al. 2015).
Calophoma petasitis Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552695; Facesoffungi number: FoF 2775, Fig. 52
Etymology: Named after the host genus.
Holotype: MFLU 15-1489
Saprobic on dead stem of Petasites sp. Sexual morph Ascomata 573–661 µm high × 437–508 µm diam. (\( \bar{x} \) = 624 × 494 μm, n = 5), superficial, flattened at the base, solitary, black subglobose, solitary or aggregated, ostiolate. Peridium 20–50 μm wide, comprising 1–2 layers, the outer layer heavily encrusted with pigments and often longitudinally striate on the surface pigmentation and the inner layer comprising thick-walled, hyaline cells of textura angularis. Hamathecium comprising few, 0.6–1.5 µm wide, septate, guttulate pseudoparaphyses. Asci 60–92 × 9–12 μm (\( \bar{x} \) = 81 × 10 μm, n = 10), 8-spored, bitunicate, cylindrical, slightly curved, with short, furcate pedicel. Ascospores 14–16 × 4–6 μm (\( \bar{x} \) = 15 × 6 μm, n = 20), overlapping 1–2-seriate, 2-celled, hyaline, 1-septate, guttulate, enlarged at the first cell, constricted at the septum, conical at the ends, smooth-walled, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 7 cm diam. after 7 days at 16 °C, edge entire, brown to black mycelium, raised, producing fruiting bodies on media.
Material examined: ITALY, Province of Forlì-Cesena, near Campigna—Santa Sofia, dead stem of Petasites sp. (Asteraceae), 9 June 2014, Erio Camporesi, IT1920 (MFLU 15-1489, holotype); ex-type living culture, MFLUCC 15-0076); Ibid. (HKAS 94594 bis, paratype).
GenBank Numbers LSU:KY496729; ITS:KY496749; SSU:KY501117; RPB2:KY514407.
Notes: Calophoma petasitis is introduced as a new species with the first report of a sexual morph for this genus. The sexual morph of C. petasitis is characterized by subglobose ascomata and smooth-walled, guttulate, hyaline ascospores lacking a mucilaginous sheath. Multigene phylogenetic analyses support the position of the new taxon in Calophoma and its establishment as a new species. Calophoma petasitisi clearly segregates from other known species (Fig. 47). We attempted to induce the asexual morph of this species on WA media and found that they produce ascomata on media.
Didysimulans Tibpromma, Camporesi & K.D. Hyde, gen. nov.
Index Fungorum number: IF552770; Facesoffungi number: FoF 2884
Etymology: refers to the didymella-like characteristics.
Type species: Didysimulans mezzanensis Tibpromma, Camporesi & K.D. Hyde
Saprobic on dead stems. Sexual morph immersed under the host tissue, solitary, black, globose to irregular, conspicuous at the surface, with or lacking ostioles. Peridium composed of brown cells arranged in a textura angularis. Hamathecium comprising dense, septate, guttulate pseudoparaphyses. Asci 8-spored, bitunicate, cylindrical, slightly curved, short pedicellate, apically rounded. Ascospores 2-celled, hyaline, 1-septate, guttulate, enlarged at the first cell, constricted at the septum, conical at the ends, lacking a mucilaginous sheath. Asexual morph Chlamydospores occasionally produced, hyaline to yellow–brown mycelium, in spiral chains.
Notes: Didysimulans is introduced as a new genus based on morphology and phylogenetic support (LSU, ITS, RPB2 and β-tubulin DNA sequence data). The ascomata of Didysimulans are similar to those of Didymellaceae in having a peridium of textura angularis and cylindrical asci with 2-celled, hyaline, 1-septate ascospores. Didysimulans produces chlamydospores with hyaline to yellow–brown mycelium, with spiral chains. Leptosphaerulina (muriform ascospores), Platychora (ascospores septate near the lower end), Monascostroma (ascospores narrowly inequilateral), Macroventuria (ascomata with hairs or setae) and Didymella (ovoid to ellipsoidal ascospores) (Hyde et al. 2013), are all easily distinguishable from Didysimulans.
Didysimulans italica Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552771; Facesoffungi number: FoF 2885, Fig. 53
Etymology: refers to the country where the holotype was collected.
Holotype: MFLU 14-0722
Saprobic on dead stem of Epilobium sp. Sexual morph Ascomata 149–167 µm high × 165–188 µm diam. (\( \bar{x} \) = 159 × 177 μm, n = 5), immersed, solitary or in small groups, uniloculate, globose, conspicuous at the surface, dull, black, smooth, without hairs, ostiolate. Peridium 27–58 μm wide, thick, comprising 5–6 layers of hyaline to dark brown cells of textura angularis. Hamathecium comprising 2.3–3.3 µm wide, dense, numerous, septate, unbranched, pseudoparaphyses. Asci 32–49 × 9–15 μm (\( \bar{x} \) = 42 × 12 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindric, short pedicellate, thick-walled. Ascospores 9–14 × 3–5 μm (\( \bar{x} \) = 11 × 4 μm, n = 20), overlapping 1–2-seriate, 2-celled, hyaline, oval or obclavate, 1-septate, slightly constricted at the septum, enlarged at first cell, conical at the ends, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 4 cm diam., after 7 days in the dark at 16 °C, rhizoid colony with undulate, smooth, raised on surface, white.
Material examined: ITALY, Galeata Province, Passo delle Forche, on dead stem of Epilobium sp. (Onagraceae), 16 June 2014, Erio Camporesi, IT1936 (MFLU 14-0722, holotype); ex-type living culture MFLUCC 15-0059 (HKAS 94595 bis, paratype).
GenBank Numbers LSU:KY496730; ITS:KY496750; SSU:KY501118; RPB2:KY514408.
Notes: Didysimulans italica is introduced as a new species with distinct ascospores which are conical at the ends. Although D. italica is closely related to D. mezzanensis (type species), they can be distinguished by morphology. Ascomata and ascospore characters are different (Figs. 53, 54).
Didysimulans mezzanensis Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552772; Facesoffungi number: FoF 2886, Figs. 54, 55
Etymology: Named after the province which the fungus was found.
Holotype: MFLU 15-1490
Saprobic on dead stem of Thalictrum sp. Sexual morph Ascomata 83–95 µm high × 110–139 µm diam. (\( \bar{x} \) = 89 × 124 μm, n = 5), immersed in host tissues, solitary, black, irregular, conspicuous at the surface, ostiolate. Peridium 6–29 μm wide, composed of two strata, an outer stratum of thin-walled and brown cells arranged in a textura angularis and inner layer of hyaline cells of textura angularis. Hamathecium comprising 0.8–1.2 µm wide, dense, septate, guttulate pseudoparaphyses. Asci 45–60 × 10–13 μm (\( \bar{x} \) = 53 × 12 μm, n = 15), 8-spored, bitunicate, cylindrical, slightly curved, short pedicellate, apically rounded. Ascospores 13–16 × 4–8 μm (\( \bar{x} \) = 15 × 7 μm, n = 20), overlapping 1–2-seriate, 2-celled, hyaline, 1-septate, guttulate, enlarged at the first cell, constricted at the septum, conical at the ends, smooth-walled, lacking a mucilaginous sheath. Asexual morph Colonies superficial to immersed; mycelium composed of septate, branched, thick-walled, smooth, guttulate, yellow green, coiled, smooth, hyphae, producing thick-walled, small chlamydospores; sometimes, hyphae packed into knot-like structures.
Culture characteristics: Ascospore germinating on MEA within 12 h. Colonies on MEA reaching 5 mm diam. in 7 days at 16 °C, rhizoidal with lobate edge, rough, flat on surface, hyaline at beginning and becoming yellow green, with septate, branched, guttulate, hyphae packed in circular knots; not sporulating in culture.
Material examined: ITALY, near Mezzana Province, Trento, dead stem of Thalictrum sp. (Ranunculaceae), 16 July 2014, Erio Camporesi, IT1998A (MFLU 15-1490, holotype); ex-type living culture, MFLUCC 15-0067); Ibid. (HKAS 94598 bis, paratype).
GenBank Numbers LSU:KY496733; ITS:KY496753; SSU:KY501121; RPB2:KY514411.
Notes: Didysimulans mezzanensis is introduced as the type species of Didysimulans. ITS nucleotide sequence comparison reveals eight base pair differences between D. mezzanensis and D. italica.
Nothophoma Q. Chen & L. Cai
The genus Nothophoma was introduced with the type species N. infossa (Ellis & Everh.) Q. Chen & L. Cai. (syn. Phoma infossa) to accommodate five synonymized Phoma species, including Nothophoma anigozanthi (Tassi) Q. Chen & L. Cai (syn. Phoma anigozanthi), Nothophoma arachidis-hypogaeae (V.G. Rao) Q. Chen & L. Cai (syn. Phoma arachidis-hypogaeae), Nothophoma quercina (Syd.) Q. Chen & L. Cai (syn. Phoma fungicola), and Nothophoma gossypiicola (Gruyter) Q. Chen & L. Cai (syn. Phoma gossypiicola) (Chen et al. 2015). This genus is ubiquitous, species-rich and includes many important plant pathogens, some of which are of quarantine concern (Aveskamp et al. 2008, 2010; Chen et al. 2015).
Nothophoma quercina (Syd. & P. Syd.) Q. Chen & L. Cai, Stud. Mycol. 82: 213 (2015)
Facesoffungi number: FoF 2688, Fig. 56
Basionym: Cicinobolus quercinus Syd., Ann. Mycol. 13: 42 (1915)
≡ Ampelomyces quercinus (Syd.) Rudakov, Mikol. Fitopatol. 13: 109 (1979).
≡ Phoma fungicola Aveskamp et al., Stud. Mycol. 65: 26 (2010)
Saprobic on dead branch of Ulmus × hollandica Mill. Sexual morph Undetermined. Asexual morph Conidiomata produced on surface of culture in PDA, 0.22–0.43 mm (\( \bar{x} \) = 0.28 mm, n = 10) diam., pycnidial, solitary, scattered, globose to irregularly-shaped, black, ostiolate. Pycnidia multi-layered, composed of pale brown, pseudoparenchymatous cells, with thick outer layer and thin inner layer. Conidiogenous cells phialidic, hyaline, simple, doliiform to ampulliform, variable in size. Conidia 2–5.5 × 1–4 µm (\( \bar{x} \) = 3.5 × 2.5 µm, n = 40), variable in size and shape, subglobose to oval or obtuse, initially hyaline, but brown at maturity, aseptate, smooth-walled. Conidial exudates not recorded.
Culture characteristics: Colonies on PDA reaching 80 mm diam. after 7 days at 25 °C, with regular margin, dull white aerial mycelium, surface floccose to downy, greenish-olivaceous to olivaceous near the centre and reverse dark ochreous in the centre and white at the margin.
Material examined: ITALY, Forlì-Cesena Province, San Lorenzo in Noceto—Forlì, on dead branch of Ulmus × hollandica (Ulmaceae), 31 March 2016, Erio Camporesi (MFLU 16-2676, new host record), living culture, MFLUCC 16-1392, KUMCC 16-0128.
GenBank Numbers LSU:KY053897; ITS:KY053896; RPB2: KY053898; β-tubulin: KY053899.
Notes: Nothophoma quercina has been reported as a saprobe from Quercus sp. in Ukraine (Aveskamp et al. 2010; Chen et al. 2015) and as a pathogen causing stem canker and fruit blight on Pistacia vera L. in the USA (Chen et al. 2013). This is the first record of N. quercina on Ulmus species. When comparing our species with the type specimen of N. quercina (CBS 633.92), they are similar in morphology, but differ in their host. The conidiomata are larger, while conidia are smaller compared to the type specimen. Based on our phylogenetic analysis of combined ITS, TEF1, β-tubulin and RPB2 sequence data of Nothophoma species (Fig. 47), our strain (MFLUCC 16-1392) clustered with the ex-type strain of Nothophoma quercina with relatively high bootstrap and Bayesian probabilities (99%MP/1.00BYPP).
Stagonosporopsis Died.
Stagonosporopsis produces dimorphic conidia, and most of these conidia are aseptate, hyaline, ellipsoidal to subglobose, thin-walled and smooth-walled. The other type of conidia are smaller, 1-celled or with up to 3 septa, produced both in vivo and in vitro in the same pycnidium (Chen et al. 2015). Aveskamp et al. (2010) and Chen et al. (2015) have detailed this genus.
Stagonosporopsis ailanthicola Manawasinghe, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552548; Facesoffungi number: FoF 2682, Fig. 57
Etymology: Name reflects the host genus Ailanthus.
Holotype: MFLU 16-2678
Saprobic on dead branch of Ailanthus altissima (Mill.) Swingle. Sexual morph Undetermined. Asexual morph Conidiomata 41–74 μm diam. (\( \bar{x} \) = 55 µm, n = 10), scattered, fusing when mature, globose to subglobose. Pycnidial wall multi-layered, comprising 2–3 layers, heavily pigmented, thick-walled, comprising dark brown celled of textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, solitary, hyaline, smooth-walled. Conidia 3.3–5.9 × 1.7–3.2 μm (\( \bar{x} \) = 4.7 × 2.3 μm, n = 40), aseptate, hyaline, 1-celled, ellipsoid, apex acutely rounded.
Culture characteristics: Colonies on PDA reach 60–65 mm diam., after 7 days at 25 °C. Floccose aerial mycelium covering the whole colony.
Material examined: ITALY, Forlì-Cesena (FC) Province, Via Pietro Nenni—Forlì, on dead branch of Ailanthus altissima (Simaroubaceae), 2 May 2015, Erio Camporesi IT2473, (MFLU 16-2678, holotype); ex-type living culture, MFLUCC 16-1439.
GenBank Numbers ITS:KY100872; TEF1:KY100874; β-tubulin:KY100878; RPB2:KY100876.
Notes: Stagonosporopsis species are characterized by their unique dimorphic conidia, chlamydospores and conidiomata (De Gruyter and Noordeloos 1992; Chen et al. 2015). Molecular data is important to identify and characterize these species. Combined phylogenetic analysis of LSU, ITS, β-tubulin and RPB2 sequence data places S. ailanthicola within the clade of Stagonosporopsis in the Phoma species complex. Stagonosporopsis ailanthicola differs from S. dorenboschii in having chlamydospores and larger conidia. It forms a sister taxon with S. heliopsidis with 100% bootstrap support and 1.00 Bayesian posterior probability (Fig. 47). This is the first host record of Stagonosporopsis species on Ailanthus.
Leptosphaeriaceae M.E. Barr
The family Leptosphaeriaceae was introduced by Barr (1987) in the order Pleosporales and is typified by Leptosphaeria doliolum (Pers.) Ces. & De Not. Taxa commonly occur as saprobes or parasites (Hyde et al. 2013). Members of this family are characterized by superficial or erumpent ascomata, with single papillate ostiole lined with periphyses, a relatively thick peridium wall, bitunicate cylindrical asci and pale brown to brown, transversely septate ascospores (Kodsueb et al. 2006; Ariyawansa et al. 2015a). The asexual morphs of the family Leptosphaeriaceae can be coelomycetous or hyphomycetous (Shenoy et al. 2006; Zhang et al. 2012b; De Gruyter et al. 2013; Ariyawansa et al. 2015a). Currently, seven genera are accepted in the family: Alternariaster, Heterospora, Leptosphaeria, Neophaeosphaeria, Paraleptosphaeria, Plenodomus, and Subplenodomus (Ariyawansa et al. 2015a). We introduce a new species in both Paraleptosphaeria and Subplenodomus. Updated phylogenetic analyses followed taxon sampling as outlined by Ariyawansa et al. (2015a) (Fig. 58).
Paraleptosphaeria Gruyter, Aveskamp & Verkley
Paraleptosphaeria is typified by P. nitschkei (Rehm ex G. Winter) Gruyter, Aveskamp and Verkley. De Gruyter et al. (2013) introduced the genus to accommodate the heterogeneous section of Leptosphaeria and its related species in Leptosphaeriaceae. Paraleptosphaeria is characterized by having superficial or immersed, subglobose ascomata, a thick-walled peridium comprising cells of textura angularis, cylindric-clavate asci and transversly septate ascospores. Ariyawansa et al. (2015a) included six species in this genus.
Paraleptosphaeria padi Phukhamsakda, Bulgakov, Okatov & K.D. Hyde, sp. nov.
Index Fungorum number: IF552709; Facesoffungi number: FoF 2777, Fig. 59
Etymology: The species habitat regarding to the host Prunus padus L.
Holotype: MFLU 15-2756
Saprobic on dead and dying branches of Prunus padus L. Sexual morph Ascomata 288–345 μm high × 330–396 μm diam. (\( \bar{x} \) = 316 × 363 μm, n = 5), solitary, scattered or clustered in small groups, immersed under epidermal layer, superficial on host surface, globose, black to dark brown, coriaceous, ostiole central. Ostioles 119 × 83 μm, papillate, shiny, dark brown, smooth, periphyses filling ostiole channel. Peridium 9–53 μm wide, up to 72 μm at base, outer region heavily pigmented, composed of 6–8 layers of textura angularis, black to dark-brown, 7–17(–20) μm wide cells, inner layer composed of thick hyaline gelatinous cells. Hamathecium comprising numerous, 2–3.1 μm (\( \bar{x} \) = 2 μm, n = 50) wide, long, transversely septate, branched, cellular pseudoparaphyses. Asci 74–127 × 8–14 μm (\( \bar{x} \) = 106 × 11 μm, n = 30), 8-spored, bitunicate, broad-cylindrical, with club-shaped pedicel, apically rounded, ocular chamber clearly visible when immature. Ascospores 16–21 × 6–7 μm (\( \bar{x} \) = 18 × 6 μm, n = 50), overlapping biseriate, pale brown, broad-fusiform, conical at the apex, (2)–3 transversely septate, constricted at the septa, enlarged at the second cell from apex, wall rough, indentations present when mature. Asexual morph Undetermined.
Material examined: RUSSIA, Arkhangelsk Region, Arkhangelsk City, near northern Maimaksa urban microdistrict, wood waste landfill, on dead and dying branches of Prunus padus (Rosaceae), 11 May 2015, G.V. Okatov, T-589 (MFLU 15-2756, holotype).
GenBank Numbers LSU:KY554198; SSU:KY554201; ITS:KY554203; TEF1: KY554206.
Notes: Paraleptosphaeria padi is typical of Paraleptosphaeria based on the thick-walled peridium, immersed under host epidermal layer, scleroplectenchymatous cell layers, broad-cylindrical asci with a bulbous pedicel and transversally septate ascospores (Ariyawansa et al. 2015a). We compared the new species with P. praetermissa that has been reported from Rosaceae. Even though P. padi is similar to P. praetermissa in its peridium, asci, and ascospore characteristics; the ascomata of P. praetermissa are erumpent to immersed, whereas in P. padi they are superficial and smaller in size (300–500 μm high × 350–625 μm wide diam. vs. 288–345 μm high × 330–396 μm wide diam.). Paraleptosphaeria padi also shares similarities with P. dryadis. However, P. dryadis has smaller ascomata (180–275 μm high × 150–250 μm wide and larger ascospores (20–25 μm high × 6–7 μm wide diam. The phylogeny also supports the establishment of P. padi as a new species with high support (Fig. 58).
Subplenodomus Gruyter, Aveskamp & Verkley
Subplenodomus was established by De Gruyter et al. (2013), typified with S. violicola (P. Syd.) Gruyter, Aveskamp & Verkley, to accommodate some Phoma-like species that belong to Leptosphaeriaceae. Based on morphological characterization and multi-gene phylogenetic analysis, Subplenodomus are accepted as asexual morphs of Leptosphaeriaceae (De Gruyter et al. 2013; Hyde et al. 2013; Ariyawansa et al. 2015a). The genus includes S. apiicola, S. drobnjacensis, S. valerianae and S. violicola. Subplenodomus is characterized by thick-walled pycnidia, consisting of pseudoparenchymatous or sometimes scleroplectenchymatous cell types, ostiole, phialidic, ampulliform to doliiform conidiogenous cells and hyaline, aseptate, and ellipsoid conidia (De Gruyter et al. 2013). We reconstruct the phylogeny for Leptosphaeriaceae using a concatenated dataset of RPB2 and β-tubulin. Our new collection of S. galicola (MFLU 15-1368) is close to S. violicola (Fig. 59).
Subplenodomus galicola Phukhamsakda, Tibpromma, Camporesi, & K.D. Hyde, sp. nov.
Index Fungorum number: IF552711; Facesoffungi number: FoF 2778, Fig. 60
Subplenodomus galicola (MFLU 15-1368, holotype) a Host substrate. b Close up of ascomata on host. c Vertical section of ascoma. d Ostioles filled with periphyses. e Section through peridium. f Cellular pseudoparaphyses (stained with cotton blue showing septa). g–i Developmental stages of asci. j–n Developmental stages of ascospores. Scale bars b = 500 µm, c = 200 µm, d, e, g–i = 50 µm, f, j–n = 20 µm
Etymology: The species epithet refers to the host.
Holotype: MFLU 15-1368
Saprobic on dead stem of Galium sp. Sexual morph Ascomata 254–285 μm high × 311–314 μm wide diam. (\( \bar{x} \) = 269 × 313 μm, n = 5), solitary, scattered or sometimes gregarious in small groups, immersed under epidermis layer, superficial on host surface, depressed-globose, ampulliform, base flat, dark brown to brown, coriaceous, ostiole central. Ostioles 70–98 μm high × 98–117 μm diam. (\( \bar{x} \) = 87 × 104 μm, n = 5), papillate, shiny, brown to reddish-brown, smooth, filled with periphyses. Peridium 32–60 μm wide, up to 75 μm at ostiole part, thick, outer region of heavily pigment cells, outer layer of 8–10 layers of black to dark-brown cells of textura angularis, inner layer composed of thick-walled, hyaline, angular cells. Hamathecium comprising numerous, long, 1.5–2.8 μm (\( \bar{x} \) = 2 μm, n = 50) wide, broad, transversely septate, branched, cellular pseudoparaphyses. Asci 66–120 × 12–17 μm (\( \bar{x} \) = 98 × 14 μm, n = 20), 8-spored, bitunicate, broad cylindrical, with club-shaped pedicel, apically rounded, ocular chamber visible when immature. Ascospores 30–40 × 6–9 μm (\( \bar{x} \) = 37 × 7 μm, n = 50), overlapping biseriate, pale brown, broad fusiform, tapering at the ends, with (3)–4 transverse septa, slightly constricted at the septa, enlarged at the second cell from apex, wall rough, indentations present when mature. Asexual morph Undetermined.
Material examined: ITALY, Forlì-Cesena [FC], Tontola di Predappio, on dead stem of Galium sp., 4 April 2014, Erio Camporesi, IT 1797 (MFLU 15-1368, holotype).
GenBank Numbers LSU:KY554199; ITS:KY554204.
Notes: Subplenodomus galicola is similar to other Subplenodomus species in its pseudoparenchymatous peridium cell types (De Gruyter et al. 2013). Subplenodomus galicola also shares similarities with Plenodomus in its cylindric asci and yellowish ascospores with 3–5 transverse septa. However, they are distinct, as the ascomata of S. galicola are, superficial, whereas Plenodomus has immersed to erumpent ascomata (Ariyawansa et al. 2015a). Currently, the species of Subplenodomus are known from herbaceous plants or woody dicotyledonous trees (De Gruyter et al. 2013).
Phaeosphaeriaceae M.E. Barr.
The family Phaeosphaeriaceae, introduced by Barr (1979), was originally characterized by immersed to superficial, globose to subglobose ascomata, short papilla, bitunicate asci and hyaline, yellowish or brown, fusiform to ellipsoidal, filiform, or muriform, septate ascospores (Barr 1979; Shoemaker 1984; Shoemaker and Babcock 1989; Zhang et al. 2012b; Hyde et al. 2013; Phookamsak et al. 2014). Most species in this family are widely distributed on plants as necrotrophic pathogens or as saprobes (Shoemaker and Babcock 1989; Carson 2005; Stukenbrock et al. 2006; Cannon and Kirk 2007). Phaeosphaeriaceae shows diverse morphological characters and comprise more than 35 sexual and asexual genera (Phookamsak et al. 2014; Wijayawardene et al. 2014a; Hyde et al. 2016).
Entodesmium Riess
Entodesmium was introduced by Riess in 1854 with E. rude Riess as the type species. Entodesmium has immersed, globose ascomata, and filiform, multi-septate ascospore, some breaking into part ascospores (Shoemaker 1984; Barr 1992; Zhang et al. 2012b; Phookamsak et al. 2014) (Fig. 61).
RAxML tree based on analysis of a combined dataset of ITS, LSU, SSU and RPB2 partial sequences. Bootstrap support values for maximum likelihood (ML, black) higher than 50% and Bayesian posterior probabilities (BYPP, black) greater than 0.90 are defined as above the nodes. The tree is rooted to Didymella exigua. All type strains are in bold. New species are given in blue
Entodesmium italica Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552697; Facesoffungi number: FoF 2788, Fig. 62
Entodesmium italica (MFLU 14-0716, holotype). a Fruiting bodies on host surface. b Section through ascoma. c Section through papilla. d Section through peridium. e Pseudoparaphyses. f–h Asci. i–k Ascospores. l Germinated ascospore. Scale bars a = 100 μm, b, c = 50 μm, d = 5 μm, e = 5 μm, f–h = 20 μm, i–l = 10 μm
Etymology: refers to the country where the fungus was collected.
Holotype: MFLU 14-0716
Saprobic on decaying plant stems of Lathyrus sp. Sexual morph Ascomata 256–289 µm high, 176–201 µm diam. (\( \bar{x} \) = 266 × 190 µm, n = 5), immersed to semi-immersed, scattered beneath the host periderm or on decorticated wood, visible as small black dots on the host surface, ampulliform, solitary, with central, shiny ostiole. Ostioles central, long, inconspicuous at the surface. Peridium 25–32 µm wide, comprising 3–5 layers, of dark brown cells of textura angularis. Hamathecium comprising numerous, 0.9–3.7 µm wide, filamentous, unbranched, cellular, guttulate pseudoparaphyses. Asci 72–98 × 9–10 µm (\( \bar{x} \) = 89 × 10 µm, n = 10), 8-spored, bitunicate, fissitunicate, cylindric-clavate, with long, club-shaped pedicel. Ascospores 24–30 × 4–5 µm (\( \bar{x} \) = 267 × 4 µm, n = 15), overlapping biseriate, yellow-green, usually 4-septate, constricted or not at the septum, conical at the ends, guttulate, smooth-walled, not separating at the septa, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 3–4 cm diam., after 7 days in the dark at 18 °C, yellowish, irregular, flattened to slightly raised, with rough surface, fairly fluffy, not producing pigment in agar.
Material examined: ITALY, Province of Arezzo, Poppi, on dead stem of Lathyrus sp. (Fabaceae), 14 May 2014, Erio Camporesi, IT1875 (MFLU 14-0716, holotype); living culture, MFLUCC 14-0526; (HKAS 94588 bis, paratype).
GenBank Numbers LSU:KY496727; ITS:KY496747; RPB2:KY514406; TEF1:KY514395.
Notes: Molecular analysis indicates that the new species belongs in the genus Entodesmium in the Phaeosphaeriaceae clade and clusters with E. rude (type) (Fig. 61). Entodesmium rude differs in having longer filiform ascospores that break into part spores (Shoemaker 1984; Phookamsak et al. 2014).
Neosetophoma Gruyter, Aveskamp & Verkley
Neosetophoma is typified by N. samararum (Desm.) and most species are pathogens causing leaf spots of various hosts (Phookamsak et al. 2014). The genus is characterized by globose to irregular conidiomata, with papillate ostioles, and yellowish conidia that are attenuate at one end (De Gruyter et al. 2010) and no sexual morph is known.
Neosetophoma garethjonesii Tibpromma, EBG Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF552698; Facesoffungi number: FoF 2789, Fig. 63
Etymology: Named in honour of Professor E.B. Gareth Jones who turned 80 years old in January 2017 and in recognition of his immense contribution to mycology.
Holotype: MFLU 16-1921
Saprobic on dead stem. Sexual morph Ascomata 403–444 µm high × 332–363 µm diam. (\( \bar{x} \) = 424 × 348 μm, n = 5), globose to subglobose, solitary, dark brown to black, immersed, black, smooth, ostiolate canal filled without sparse periphyses. Peridium 10–30 μm wide, thin, comprising 3–4 layers of brown to reddish-brown cells of textura angularis. Hamathecium of 1.5–2 µm wide, guttulate pseudoparaphyses. Asci 44–75 × 5–9 μm (\( \bar{x} \) = 61 × 7 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical, short pedicellate, thin-walled at the apex, with a minute ocular chamber. Ascospores 16–26 × 3–5 μm (\( \bar{x} \) = 21 × 4 μm, n = 20), overlapping 1–2-seriate, hyaline when young, becoming yellow pale at maturity, 4–5-celled, usually 3-septate, fusiform, narrowly fusoid with rounded ends, constricted at the septum, enlarged at the second cell, guttulate, lacking a mucilaginous sheath, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 3–4 cm diam., after 5 days in the dark at 18 °C, brown to dark-brown irregular, flattened to slightly raised, with rough surface, fairly fluffy.
Material examined: UK, Hampshire, the New Forest, on dead stem of unknown plant, 30 March 2014, EBG Jones, GJ004, (MFLU 16-1921, holotype); (HKAS 94525 bis, paratype), ex-type living culture MFLUCC 14-0528.
GenBank Numbers LSU:KY496738; ITS:KY496758; SSU:KY501126; TEF1:KY514402.
Notes: The phylogenetic analyses place N. garethjonesii in Neosetophoma as a new species with good statistical support (95% in ML/0.99 in BYPP) (Fig. 61). The sexual morph of Neosetophoma had not been previously reported (De Gruyter et al. 2010, Liu et al. 2015). Neosetophoma garethjonesii is introduced as a new species and is the first report of the sexual morph of Neosetophoma.
Nodulosphaeria Rabenh.
The genus Nodulosphaeria was introduced by Rabenhorst (1858) and typified with N. hirta Rabenh. The characteristics of this genus are ascomata lined with brown setae, and three- to multi-septate ascospores, with a swollen cell and some with terminal appendages (Holm 1961; Shoemaker 1984; Mapook et al. 2016). Clements and Shear (1931) considered Nodulosphaeria a synonym of Leptosphaeria, but Holm (1957) showed Nodulosphaeria was different to Leptosphaeria. Recent studies by Phookamsak et al. (2014), Ariyawansa et al. (2015a) and Mapook et al. (2016) included a reference strain of Nodulosphaeria modesta, morphological characteristics and investigation of the familial placement and generic affinities based on combined DNA sequence data.
Nodulosphaeria guttulatum Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552699; Facesoffungi number: FoF 2790, Fig. 64
Nodulosphaeria guttulatum (MFLU 14-0712, holotype). a Appearance of ascomata on host substrate. b Section of ascoma. c Section of peridium. d Hairs. e Ostiole. f Pseudoparaphyses. g–i Ascus. j–l Ascospores. m Germinating ascospore. Scale bars b = 50 μm, c–e = 10 μm, f = 5 μm, g–l = 20 μm, j–m = 10 μm
Etymology: ‘guttulatum’ referring to ascospore containing numerous guttules.
Holotype: MFLU 14-0712
Saprobic on dead stem of Scabiosa sp. Sexual morph Ascomata 197–218 µm high × 163–244 µm diam. (\( \bar{x} \) = 206 × 199 μm, n = 5), immersed, solitary, or in small groups, uniloculate, globose to subglobose, conical at the base, black, hairy. Ostiole papillate, protruding, with numerous, short, brown to dark brown setae. Peridium 15–30 μm wide, comprising 6–7 layers of hyaline to reddish-brown cells of textura angularis. Hamathecium 2.1–3.9 µm wide, comprising dense, numerous, aseptate, distinctly guttulate pseudoparaphyses. Asci 40–89 × 10–15 μm (\( \bar{x} \) = 71 × 13 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindric-clavate, with long, club-shaped pedicel, thick-walled at the apex, with a minute ocular chamber. Ascospores 29–38 × 5–6.5 μm (\( \bar{x} \) = 33 × 6 μm, n = 15), overlapping 1–2-seriate, hyaline when young, becoming golden yellow at maturity, fusiform, 4-septate, slightly constricted at the septum, enlarged at the second cell from apex, conical and narrowly rounded at the ends, with numerous guttulate, with circular sheath at both ends. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 3–4 cm diam., after 7 days in the dark at 16 °C, white–gray to dark, cottony, flat, dense, with sparse aerial mycelium on the surface.
Material examined: ITALY, Predappio Province, Rocca delle Caminate, on dead stem of Scabiosa sp. (Caprifoliaceae), 21 April 2014, Erio Camporesi, IT1826 (MFLU 14-0712, holotype); ex-type living culture MFLUCC 15-0069 (HKAS bis, paratype).
GenBank Numbers LSU:KY496726; ITS:KY496746; SSU:KY501115; RPB2:KY514405; TEF1:KY514394.
Notes: Nodulosphaeria guttulatum differs from N. aconita, N. modesta, N. scabiosae, and N. spectabilis in having ascospores that are slightly constricted at the septa, enlarged at the second cell from the apex, numerous guttules, and with a circular sheath at both ends. Phylogenetic analyses indicate that N. guttulatum is closely related to N. multiseptata with high support (90% in ML/0.98 in BYPP) (Fig. 61), but is morphologically different.
Nodulosphaeria multiseptata Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552700; Facesoffungi number: FoF 2791, Fig. 65
Nodulosphaeria multiseptata (MFLU 14-0720, holotype). a Appearance of ascomata on host substrate. b Section of ascoma. c Section of peridium. d, e Section of ostiole. f Pseudoparaphyses. g, h Asci. i, j Ascospores. k Germinating ascospore. Scale bars a = 200 μm, b = 50 μm, c = 10 μm, d, e = 20 μm, f = 5 μm, g–k = 20 μm
Etymology: refers to the ascospore morphology.
Holotype: MFLU 14-0720
Saprobic on dead stem of Sambucus ebulus L. Sexual morph Ascomata 232–260 µm high × 267–272 µm diam. (\( \bar{x} \) = 246 × 269 μm, n = 5), immersed to semi-immersed, solitary, uniloculate, globose to subglobose, black, smooth-walled. Ostiole 60–74 × 83–102 μm (\( \bar{x} \) = 67 × 96 μm, n = 5), papillate, protruding from the centre of ascomata, with numerous, short, internal brown to dark brown setae. Peridium 19–27 μm wide, comprising of dark brown cells of textura angularis. Hamathecium 3.3–4.5 µm wide, comprising numerous, aseptate, distinctly guttulate, pseudoparaphyses. Asci 74–127 × 17–21 μm (\( \bar{x} \) = 100 × 19 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindric-clavate, with short, club-shaped pedicel, thick-walled at the apex, with a minute ocular chamber. Ascospores 56–70 × 5–6 μm (\( \bar{x} \) = 65 × 6 μm, n = 20), overlapping 1–2-seriate, hyaline when young, becoming golden yellow at maturity, fusiform, 12–14-septate, slightly constricted at the septum, enlarged at fifth cell from apex, conical and narrowly rounded at the ends, guttulate, with mucilaginous appendages at the ends. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 8 cm diam. after 7 days at 16 °C, whitish-gray, cottony, dense, with sparse aerial mycelium on the surface.
Material examined: ITALY, Province of Arezzo, near Ortignano-Raggiolo, on dead stem of Sambucus ebulus (Adoxaceae), 25 May 2014, Erio Camporesi, IT1889 (MFLU 14-0720, holotype); ex-type living culture MFLUCC 15-0078 (HKAS 94591, paratype).
GenBank Numbers LSU:KY496728; ITS:KY496748; SSU:KY501116; TEF1:KY514396.
Notes: In the phylogenetic analysis N. multiseptata clustered with N. guttulatum with high statistical support (90% in ML, 0.98 in BYPP), but the species are different. Nodulosphaeria guttulatum has 4-septate, fusiform ascospores, enlarged at the second cell from the apex, with conical and narrowly rounded ends (Fig. 65).
Nodulosphaeria sambuci Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552701; Facesoffungi number: FoF 2792, Fig. 66
Nodulosphaeria sambuci (HKAS94536, holotype). a Appearance of ascomata on host substrate. b Section of ascoma. c, d Section of ostiole. e Section of peridium. f Pseudoparaphyses. g, h Asci. i–k Ascospores. l Germinating ascospore. Scale bars a = 100 μm, b = 50 μm, c–e = 20 μm, f = 5 μm, g, h = 20 μm, i–l = 10 μm
Etymology: named for its occurrence on the host plant.
Holotype: HKAS94536
Saprobic on dead stem of Sambucus ebulus L. Sexual morph Ascomata 204–220 µm high × 173–215 µm diam. (\( \bar{x} \) = 213 × 198 μm, n = 5), superficial, solitary, or in small groups, uniloculate, globose to subglobose, conspicuous at the surface, black, hairy. Ostioles 78–92 × 107–126 μm (\( \bar{x} \) = 86 × 117 μm, n = 5), papillate, protruding from the centre of the ascomata, with numerous, short, brown to dark brown setae. Peridium 23–44 μm wide, comprising of light-brown to dark brown cells of textura angularis. Hamathecium 1.1–3.4 µm wide, comprising dense, numerous, aseptate, distinctly guttulate pseudoparaphyses. Asci 53–80 × 7–10 μm (\( \bar{x} \) = 68 × 9 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindric-clavate, with small furcate pedicel, apically rounded, with a minute ocular chamber. Ascospores 32–35 × 3–5 μm (\( \bar{x} \) = 34 × 4 μm, n = 15), overlapping 1–2-seriate, hyaline when young, becoming golden yellow at maturity, fusiform, 8-septate, slightly constricted at the septum, enlarged at four cell, conical and narrowly rounded at the ends, guttulate, with small mucilaginous appendages at both ends. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 4 cm diam. after 7 days at 16 °C, gray to dark, cottony, flat, dense, with sparse aerial mycelium on the surface
Material examined: ITALY, Province of Forlì-Cesena, Monte Fumaiolo, on dead stem of Sambucus ebulus, 10 July 2012, Erio Camporesi, IT558 (HKAS 94536, holotype); ex-type living culture MFLUCC 15-0068.
GenBank Numbers LSU:KY496721; ITS:KY496742; SSU:KY501112.
Notes: Nodulosphaeria sambuci shares similarities with species having 8-septate ascospores (Nodulosphaeria derasa, N. dolioloides and N. pellita), but those species lack molecular data. It is phylogenetically closely related to N. senecionis with high statistical support (100% in ML, 1.00 in BYPP). However, N. sambuci has fusiform, golden yellow ascospores, that are enlarged at the fourth cell from the apex, and conical and narrowly rounded at the ends, while N. senecionis has long fusiform to almost cylindrical, yellowish brown ascospores, enlarged at the third cell from the apex and tapering towards the rounded ends (Ariyawansa et al. 2015c).
Phaeosphaeria I. Miyake
Phaeosphaeria was established by Miyake (1909) with P. oryzae as the type species. The epitypification of Phaeosphaeria oryzae I. Miyake and Phaeoseptoria papayae Speg. are confirmed through sexual and asexual morph connections and based on evidence from phylogenetic analysis (Quaedvlieg et al. 2013; Phookamsak et al. 2014).There are 203 epithets for Phaeosphaeria in Index Fungorum (2017) with 19, 363 nucleotide sequences available in GenBank.
Phaeosphaeria calamicola Konta & K. D. Hyde, sp. nov.
Index Fungorum number: IF552662; Facesoffungi number: FoF02758, Fig. 67
Phaeosphaeria calamicola (MFLU 15-0019, holotype). a Appearance of ascomata on host substrate. b Close up of ascomata. c Section of ascoma. d Peridium. e Pseudoparaphyses. f–j Asci. k–o Ascospores. Note sheath in o in India Ink. Scale bars a = 500 μm, b = 100 μm, c = 50 μm, d = 20 μm, e = 10 μm, f–j = 20 μm, k–o = 10 μm
Etymology: Referring to the host substrate (Calamus sp.).
Holotype: MFLU 15-0019
Saprobic on Calamus sp. Sexual morph Ascomata 76–110 µm high × 103–125 µm diam. (\( \bar{x} \) = 95 × 117 μm, n = 5), solitary, scattered, semi-immersed, subglobose, with ostiole at the centre. Peridium 9–14 μm wide, composed of outer layers of light brown, thick-walled, cells of textura angularis, inner layers of hyaline and thin-walled cells. Hamathecium 1–3 μm wide, comprising dense, numerous, septate, pseudoparaphyses. Asci 41–63 × 8–11 μm (\( \bar{x} \) = 54 × 9 μm, n = 10), 8-spored, bitunicate, overlapping 2-seriate cylindric-clavate, short-pedicellate, apically rounded, lacking a distinct ocular chamber. Ascospores 18–22 µm high × 4–5 µm diam. (\( \bar{x} \) = 20 × 5 μm, n = 10), 2–3-seriate, initially hyaline, becoming pale brown at maturity, fusiform, 3-septate, guttulate, rounded at the ends, surrounded by a thick mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Ascospore germinating on MEA within 24 h and germ tube produced from both ends of the ascospore. Colonies on MEA reaching 3–4.5 cm diam. after 4 weeks at 25 °C, white from the centre to margins and slightly fluffy at the margins, dense, septate, branched, smooth-walled.
Material examined: THAILAND, Chiang Rai Province, Doi Chang, on dead stem of Calamus sp. (Arecaceae), 8 November 2014, Sirinapa Konta, DC02a (MFLU 15-0019, holotype); ex-type living culture, MFLUCC 14-1168.
GenBank Numbers LSU:KY511423; ITS:KY511429; SSU:KY511426.
Notes: Phaeosphaeria calamicola clustered with P. chiangraina in the Phaeosphaeria clade with high support (95% ML, 0.97 BYPP). The phylogenetic analyses (Fig. 61) indicate that P. calamicola belongs in Phaeosphaeria within Phaeosphaeriaceae. Phaeosphaeria calamicola differs from P. chiangraina, P. papayae and P. oryzae in its much smaller ascomata, asci and ascospores.
Phaeosphaeriopsis M.P.S. Câmara, M.E. Palm & A.W. Ramaley
Phaeosphaeriopsis, typified by P. glaucopunctata, was introduced to accommodate non-congeneric species of Paraphaeosphaeria by Câmara et al. (2003) and followed by Phookamsak et al. (2014).
Phaeosphaeriopsis yuccae Dayarathne, Bulgakov, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF552710; Facesoffungi number: FoF 2793, Fig. 68
Phaeosphaeriopsis yuccae (MFLU 15-2938, holotype) a, b Appearance of conidiomata on Yucca sp. c Vertical section of conidioma. d Peridium. e–g Developing stages of conidia on conidiogenous cells. h–k Conidia. l Germinating conidium. m, n Cultures on PDA (m upper, n lower). Scale bars b = 500 µm, c = 100 µm, d = 20 µm, e = 10 µm. f, g, l = 5 µm, h–k = 2 µm
Etymology: Named referring to the host genus.
Holotype: MFLU 15-2938
Saprobic on dying leaves of Yucca sp. Sexual morph Undetermined. Asexual morph Conidiomata 97–161.5 μm high × 104–178 μm diam., pycnidial, solitary, semi-immersed to immersed, visible as slightly, raised, small black spots on host surface, globose to subglobose or cup-shaped when sectioned, with centrally placed ostiole. Conidiomata wall 13–34 μm wide, composed of 3–4 layers of brown cells, arranged in textura angularis, outer layers comprising 2–3 layers of brown to dark brown, thick-walled cells, inner layer comprising 2–3 layers of hyaline, thin-walled cells. Conidiophores simple, rarely branched, doliiform to cylindrical or ampulliform, septate, hyaline, mostly reduced to conidiogenous cells. Conidiogenous cells 3–5 × 2–4 μm, holoblastic, phialidic, single, discrete, sometimes integrated, ampulliform or cylindric-clavate, hyaline, arising from basal stratum. Conidia 2–3.5 × 2–3 μm (\( \bar{x} \) = 2.9 × 2.8 μm, n = 30), initially hyaline, becoming brown to dark brown, globose to subglobose, aseptate, smooth-walled.
Culture characters: Colonies on potato dextrose agar (PDA) 24–30.5 mm diam. after 14 days at 25–30 °C, white to cream at the margins, pale yellowish to yellowish-white, white at the centre; reverse white to cream at the margins, brown to orange-brown in the centre and pale yellowish to yellowish at the centre; medium dense, irregular, flattened to slightly raised, with rough surface, undulate edge with entire to slightly radiating margins, floccose to fairly fluffy, no pigments produced in agar.
Material examined: RUSSIA, Rostov Region, Rostov-on-Don City, Botanical Garden of Southern Federal University, ornamental nursery, (47.2371121′E, 39.6443796′N), on Yucca sp. (Agavaceae), 22 July 2015, Timur Bulgakov (MFLU 15-2938, holotype); ex-type living culture, MFLUCC 16-0558.
GenBank Numbers SSU:KY554480; LSU:KY554481; ITS:KY554482.
Notes: Phaeosphaeriopsis yuccae is characterized by having pycnidial conidiomata, doliiform to cylindrical or ampulliform conidiophores and globose to subglobose, dark brown conidia at maturity. Paraphaeosphaeria species differ in their asexual morph characteristics, including conidiomatal structure, type of conidiogenesis, and conidial morphology. Some asexual morphs are Coniothyrium-like, whereas others are more typical of Microsphaeropsis Höhn (Câmara et al. 2003). This new strain is Coniothyrium-like and morphologically most similar to asexual morph of Phaeosphaeriopsis dracaenicola in conidiomata, conidiophores and conidial nature, but they are phylogenetically distant in our phylogram (Fig. 62). According to phylogenetic analyses P. phacidiomorpha has a close affinity to P. yuccae, but the two strains are well-resolved with high statistical support (83% ML, 1.00 BYPP) (Fig. 61). Furthermore, the conidiomata of P. phacidiomorpha is cylindrical, dark brown and 3 mm long and typical of the Microsphaeropsis-like asexual morph of Phaeosphaeriopsis (Farr et al. 2006; Câmara et al. 2003).
Pleosporineae genera incertae sedis
Camarosporium Schulzer
Camarosporium was established by Schulzer (1870), with Camarosporium quaternatum as the type species. The genus Camarosporium has been referred to different families (Liu et al. 2015; Wijayawardene et al. 2014b, c, 2015, 2016; Ariyawansa et al. 2015b). Currently, there are more than 500 names listed in the genus (Index Fungorum 2017). Based on multi-locus phylogeny, Wijayawardene et al. (2014c) placed Camarosporium sensu stricto in Pleosporineae, Pleosporales. This genus has been reported as the asexual morph of many important fungal families and orders including Cucurbitariaceae, Leptosphaeriaceae, Phaeosphaeriaceae and Botryosphaeriales (Kirk et al. 2008; Wijayawardene et al. 2012; Hyde et al. 2013) (Fig. 69).
Maximum likelihood tree based on combined LSU, SSU and ITS sequenced data of Camarosporium sensu stricto. Maximum likelihood and parsimony bootstrap support values greater than 50% are indicated near the nodes, and branches with Bayesian posterior probabilities greater than 0.95 are given in bold. The ex-types are in bold and newly introduced species are in blue. The scale bar indicates 0.01 changes. The tree is rooted with Pyrenochaeta lycopersici (CBS 306.65)
Camarosporium laburnicola R.H. Perera, Bulgakov, & K.D. Hyde, sp. nov.
Index Fungorum number: IF552706; Facesoffungi number: FoF 2784; Fig. 70
Camarosporium laburnicola (MFLU 15-1522, holotype). a, b Appearance of ascomata on host substrate. c Vertical section through ascomata. d Close up of ostiole. e Close up of the peridium. f Pseudoparaphyses. g–i Immature and mature asci. j–n Immature and mature ascospores. o Germinating ascospore. p, q Colonies on MEA. Scale bars b = 2 cm, b = 200 μm, d–i = 50 μm, j–o = 10 μm
Holotype: MFLU 15-1522
Etymology: Named after the host genus on which the fungus occurs.
Saprobic on branches of Laburnum anagyroides Medik. Sexual morph Ascomata 280–370 µm high, 220–320 µm diam. (\( \bar{x} \) = 331.4 × 274.2 µm, n = 5), erumpent through the host epidermis, globose to subglobose or obovoid, solitary or arranged in groups of 2–22, black, Ostioles central, short, periphysate. Periphyses cellular, aseptate, with a blunt apex, hyaline. Peridium 22–69 µm wide, thick, comprising 8–14 layers, outer layer heavily pigmented, thin- walled, comprising dark brown cells of textura globulosa to textura angularis, inner layer composed of hyaline to brown thin-walled cells of textura globulosa to textura angularis. Hamathecium comprising numerous, 1.1–3.1 µm (n = 30) wide, filamentous, branched, aseptate, pseudoparaphyses. Asci 125–150 × 9–11 µm (\( \bar{x} \) = 134 × 9.8 µm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical, thick-walled at the apex, with a minute ocular chamber. Ascospores 15–21 × 6–8 µm (\( \bar{x} \) = 17.4 × 6.6 µm, n = 40), uniseriate to overlapping uniseriate, muriform, ellipsoidal, 6–7 transversely septate, with 5–6 vertical septa constricted at the septa, initially hyaline, becoming yellowish-brown at maturity, conical and narrowly rounded at the ends, without a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 40–45 mm diam. in 28 days, pinkish-grey, spreading with moderate aerial mycelium, slightly irregular, margins smooth, with underneath pinkish-grey.
Material examined: RUSSIA, Rostov Region, Rostov-na-Donu City, Botanical garden of Southern Federal University, Higher Park, dead branches of Laburnum anagyroides Medik. (=Cytisus laburnum L.), 5 March 2014, T. Bulgakov, T-81 (MFLU 15-1522, holotype; ibid. HKAS 89691, isotype); ex-type living culture MFLUCC 14-0565).
GenBank Numbers LSU:KY497779; SSU:KY497781; ITS:KY497784; TEF1:KY497785.
Notes: Camarosporium uniseriatum clusters with two coelomycetous species, C. clematidis and C. spartii with high bootstrap support (Fig. 69). Camarosporium uniseriatum, C. elongatum and C. arezzoensis, are the sexual morphs reported for the genus Camarosporium (Mirza 1968; Hyde et al. 2013; Tibpromma et al. 2016a; Thambugala et al. 2016). Camarosporium laburnicola differs from C. elongatum and C. arezzoensis in having smaller asci and ascospore with a different number of longitudinal and transverse septa. Even though asci and ascospore sizes overlap, C. laburnicola can be distinguished from C. uniseriatum which has 3–5 transverse septa and 1–2(–3) longitudinal septa; (Thambugala et al. 2016). However, there are three coelomycetous Camarosporium species (i.e. C. cytisi, C. laburni, C. laburnicum) reported from the same host (Laburnum spp.) for which molecular data are unavailable (Saccardo 1882, 1892).
Camarosporium moricola Chethana, Bulgakov & K. D. Hyde, sp. nov.
Index Fungorum number: IF552502; Facesoffungi number: FoF 2687, Fig. 71
Camarosporium moricola (MFLU 15-2075, holotype). a Appearance of conidiomata on dead branch of Morus alba. b Immersed conidiomata on the host surface. c Longitudinal section of a conidioma. d Longitudinal section of a conidioma wall. e Longitudinal section of a conidioma wall showing cell organization. f Conidiogenous cells with developing conidia. g Conidia. h–i Different septation patterns on conidia. j–k Upper view (j) and the reverse view (k) of the colony on PDA. Scale bars a = 2 mm, b = 0.5 mm, c = 50 μm, d, e = 20 μm, f, g = 10 μm, h, i = 5 μm
Etymology: The specific epithet moricola is named after the host genus Morus.
Holotype: MFLU 15-2075
Necrotrophic on dead and dying branches of Morus alba L. Sexual morph Undetermined. Asexual morph Conidiomata 150–340 µm (\( \bar{x} \) = 206 µm, n = 10) diam., pycnidial, solitary, scattered, immersed, unilocular, globose, black, with a papillate ostiolate. Pycnidial wall 25–47 µm (−67 µm at apex), multi–layered, with outer layer composed of 3–4 layers of thick, dark brown cells, with inner 4–6 layers of hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–10 × 3–7 µm (\( \bar{x} \) = 7.6 × 5.3 µm, n = 20), enteroblastic, phialidic, doliiform, hyaline, smooth-walled, formed from the inner most layer of the pycnidial wall, with an opening of 2.3 µm diam. Conidia 8–15 × 4–7.5 µm (\( \bar{x} \) = 11.1 × 5.4 µm, n = 30), oblong, ellipsoid, straight or rarely slightly curved, initially hyaline, pale to dark brown at maturity, smooth-walled, rounded at both ends, muriform, with 1–3 transverse septa and 1–2 longitudinal septa.
Culture characteristics: Colonies on PDA, circular, fimbriate, margin rough, with both surfaces greyish-white at the margin and grey olivaceous towards the centre, reaching 3 cm diam. after 7 days at 20 °C.
Material examined: RUSSIA, Rostov Region, Shakhty City, Grushevka steppe slopes near Grushevsky pond, shrubbery, on dead and dying branches of Morus alba L. (Moraceae), 14 May 2015, T. S. Bulgakov (MFLU 15-2075, holotype), ex-type living culture, MFLUCC 16-1396, KUMCC 16-0132; RUSSIA, Rostov Region, Krasnosulinsky District, Donskoye forestry, artificial, forest edge, on dead and dying branches of Morus alba L. (Moraceae), 18 June 2015, T. S. Bulgakov (MFLU 15-2222), ex-type living culture, MFLUCC 16-1397, KUMCC 16-0133; RUSSIA, Rostov region, Krasnosulinsky District, Donskoye forestry, ravine forest edge, on dead and dying branches of Morus alba L. (Moraceae), 18 June 2015, T. S. Bulgakov (MFLU 15-2223, paratype), ex-type living culture, MFLUCC 16-1398, KUMCC 16-0134.
GenBank Numbers MFLUCC 16-1396 LSU:KY053890; ITS:KY053887; SSU:KY053893. MFLUCC 161397 LSU:KY053891; ITS:KY053888; SSU:KY053894. MFLUCC 16-1398 LSU:KY053892; ITS:KY053889; SSU:KY053895.
Notes: Based on phylogenetic analyses and morphological comparison, our collection belongs to Camarosporium sensu stricto, which is a distinct lineage in Pleosporinae, Pleosporales. In our phylogenetic analyses of combined LSU, SSU and ITS sequence data of Camarosporium sensu stricto (Fig. 52), our collection of C. moricola clustered in a well-defined monophyletic clade sister to C. robiniicola (MFLUCC 13-0527) and C. aureum (MFLUCC 14-0620) with relatively high bootstrap and Bayesian probabilities (90% MP/97% ML/1.00 BYPP). Camarosporium moricolais distinct from C. robiniicola as the latter has obclavate conidiophores and larger conidia (18–28 × 7–11 μm) with 4–6 transverse septa and 1 longitudinal septum (Wijayawardene et al. 2014b, c). Camarosporium moricola differs from C. aureum in having smaller conidiomata (400–550 × 350–550 μm), smaller conidia ((19–)–19.5–22.5(–24) × 8–9(–10) μm) with 4–5 transverse septa, with 2–3 longitudinal septa (Liu et al. 2015). Camarosporium moricola also shows morphological similarities to its sister taxa, C. clematidis and C. spartii. Our species is distinct from C. clematidis by having conidiomata (370–425 × 350–380 μm), enteroblastic, annellidic conidiogenous cells and wider conidia 2–3 transverse and 1 longitudinal septa (Wijayawardene et al. 2014c). They differ from C. spartii by not having annelidic conidiogenous cells, larger conidia (16–13 × 6–7 μm), 3–4 transverse septa and 2–3 longitudinal septa (Wijayawardene et al. 2014b).
Eurotiomycetes O.E. Erikss. & Winka.
Eurotiomycetes was introduced by O.E. Erikss. & Winka (1997). Eurotiomycetes is a monophyletic class (Geiser et al. 2006) comprising three major clades, Chaetothyriomycetidae, Eurotiomycetidae, and Mycocaliciomycetidae. Some are human pathogens, many of the black yeasts, and some lichenized families (Kirk et al. 2008).
Eurotiales G.W. Martin ex Benny & Kimbr.
Eurotiales was introduced by G.W. Martin and validated Benny & Kimbr in 1980. The order contains three families (Elaphomycetaceae, Monascaceae and Trichocomaceae) of well known and common fungi, particularly those with phialidic Aspergillus and Penicillium asexual stages (Kirk et al. 2008).
Trichocomaceae E. Fisch.
Trichocomaceae was introduced by Fischer (1897). Species of Trichocomaceae are economically important in industry and medicine (Houbraken and Samson 2011). Phylogenetic relationships are well known for only certain genera of Trichocomaceae (Aspergillus, Penicillium and Paecilomyces) (Peterson 2000a, b; Samson et al. 2004; Peterson 2008; Samson et al. 2009), and the relationships are still poorly studied (Houbraken and Samson 2011).
Section Exilicaulis
Penicillium Link.
Species of Penicillium (Trichocomaceae, Eurotiales) are well known and abundant in soil, air, indoor environments and in contaminated foods (Frisvad and Samson 2004; Samson et al. 2010). Their main function in nature is the decomposition of organic materials and many are known for the postharvest decay of fruits and as mycotoxin producers on various foods (Frisvad and Samson 2004; Houbraken et al. 2016).
The genus Penicillium is divided into four subgenera—Aspergilloides, Penicillium, Biverticillata and Furcatum comprising 25 sections (Visagie et al. 2014). Section Exilicaulis was described by Pitt (1980), and is characterized by monoverticillate conidiophores and typified by P. restrictum. Recently, Houbraken and Samson (2011) revised the classification of the genus Penicillium based on phylogenetic data. They redefined section Exilicaulis to include species with biverticillate conidiophores, such as P. corylophilum, P. melinii, and P. raciborskii. Recently, section Exilicaulis was revised using three genes (BenA, CaM, and RPB2) to comprise eight clades defined by the inclusion of the following species: P. melinii, P. corylophilum, P. restrictum, P. citreonigrum, P. decumbens and P. parvum (Visagie et al. 2016).
As far as can be determined, only four species of Penicillium. P. daejeonium, P. koreense, P. samsonianum and P. jejuense, have been reported as new from Korea: P. daejeonium from grape and schisanda fruit, P. koreense from soil, P. samsonianum from stems and leaves of Viscum album var. coloratum, and P. jejuense from marine environments of Jeju Island.
During an investigation of the fungi associated with pomegranate fruit in Korea, a new species was isolated and is described here based on morphological characteristics and phylogenetic analyses (Fig. 72).
Phylogenetic tree of Penicillium punicae CNUFC-FP2-1 and CNUFC-FP2-2 and related species within the P. corylophilum clade of the sect. Exilicaulis based on maximum likelihood analysis of combined datasets for β-tubulin, CaM and RPB2. Sequence of Penicillium restrictum CBS 367.48 was used as outgroup taxon. Numbers at the nodes indicate the bootstrap values (>50%) from 1000 replications. The bar indicates the number of substitutions per position. New taxa are in blue and ex-type strains in bold
Penicillium punicae Hyang B. Lee, P.M. Kirk & T.T.T. Nguyen, sp. nov.
MycoBank number: MB 818233; Facesoffungi number: FoF 2797, Fig. 73
Penicillium punicae (CNUFC-FP2-1, holotype). a, d Colonies in malt extract agar. b, e Colonies in yeast extract sucrose agar. c, f Colonies in Czapek yeast autolysate agar. (a–c obverse view, d–f: reverse view). (h–m, SEM). g Fruit rot inside of pomegranate stored in fridge. h–l Verticillate conidiophore shapes and phialides on metula. m Conidia. Scale bars h–j, l = 10 µm, k = 5 µm, m = 2 µm
Etymology: punicae. Referring to pomegranate fruit from which the species was first isolated.
Holotype: CNUFC-FP2-1
Colonies grow rapidly on MEA, reaching 35–37 mm diam. at 25 °C in 7 days, green in the center with a white margin; reverse white. Conidiophores mostly biverticillate, with some triverticillate; stipes rough, 2–4.5 μm wide. Metulae 2–4 per stipe. Vesicles subglobose to globose when present. Phialides smooth ampulliform, 4–7 per metula, 6–10.5 × 1.5–3 μm. Conidia slightly roughened, globose, 2–3.5 μm × 2–3.5 μm.
Material examined: REPUBLIC OF KOREA, Jeonnam Province, Gwangju (3510′N 12655′E), from a fruit of Punica granatum (pomegranate) from a grocery store and then stored in the fridge, 5 February 2016 (CNUFC-FP2-1, preserved as glycerol stock at −80 °C in the Chonnam National University Fungal Collection; isotype in Culture Collection of National Institute of Biological Resources (NIBR), Incheon); living culture (ex-type) deposited at Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC:SF:12421).
GenBank Numbers CNUFC-FP2-1 RPB2:KX839675; CaM:KX839671; β-tubulin:KX839673. CNUFC-FP2-2 RPB2:KX839670; CaM:KX839672; β-tubulin:KX839674.
Culture characteristics: The isolate was observed to grow over a wide range of temperatures with varying growth rates on MEA (malt extract agar), CYA (Czapek yeast autolysate agar), and YES (yeast extract sucrose agar). The average growth rates of CNUFC-FP2-1 on MEA, CYA, and YES were 5, 3, and 2.5 mm per 24 h, respectively. Optimal growth was observed around 25 °C, slow growth was observed at below 20 °C, and restricted growth at 37 °C.
Notes: Penicillium punicae is distinct from P. cravenianum and P. pagulum, growing rapidly when cultivated on MEA and CYA; whereas P. cravenianum and P. pagulum have restricted growth on MEA, and CYA, respectively. The number of phialides per metula and metulae are fewer than in P. cravenianum and P. pagulum. In the phylogenetic tree based on multiple genes, the strain formed a separate branch from other species of Penicillium sect. Exilicaulis and is considered to represent a new species.
Exophiala J.W. Carmich.
Exophiala was introduced by Carmichael (1966) to accommodate the type species E. salmonis from cerebral lesions of Salmo clarkii. It is characterized by melanin or melanin-like pigments in the cell walls, annelidic conidiogenesis and an abundance of budding cells (de Hoog and Hermanides-Nijhof 1977; Haase et al. 1999). Exophiala includes mostly black yeast asexual morphs belonging to Chaetothyriales (Untereiner et al. 1995; de Hoog et al. 2011; Zeng et al. 2014), occasionally linked to peculiar coelomycetes synanamorphs, viz. E. placitae Crous & Summerell (Crous et al. 2007). The sexual morph of Exophiala has been reported as Capronia (Herpotrichiellaceae, Chaetothyriales) (Carmichael 1967; Hironaga et al. 1981; de Hoog et al. 2011). They are present in extreme ecological niches causing animal and human diseases (Li et al. 2008, 2009; Sudhadham et al. 2008; de Hoog et al. 2011; Najafzadeh et al. 2013; Wen et al. 2015), in soil, rock surfaces, air, rhizosphere, and plant tissues (Addy et al. 2005; Julou et al. 2005; Bates et al. 2006; Neubert et al. 2006; Bukovská et al. 2010; de Hoog et al. 2011; Seyedmousavi et al. 2011; Ferrari et al. 2011; Isola et al. 2013) (Fig. 74).
RAxML tree based on a combined dataset of LSU, SSU, ITS and TEF1 of Exophiala. Bootstrap support values for maximum parsimony and maximum likelihood higher than 70% and Bayesian posterior probabilities greater than 0.95 are defined above the nodes respectively. The tree is rooted to Cyphellophora laciniata (CBS 190.61). Ex-type and ex-epitype strains are in bold. Newly generated sequences are blue
Exophiala italica Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552702; Facesoffungi number: FoF 2798, Figs. 75, 76
Etymology: refers to the country where the fungus was collected.
Holotype: MFLU 15-2551
Saprobic on dead branch of Cytisus scoparius (L.) Link. Sexual morph Ascomata 391–489 µm high × 388–417 µm diam. (\( \bar{x} \) = 438 × 398 μm, n = 5), semi-immersed to erumpent through host tissue, solitary, uniloculate, oval, conspicuous at the surface, black, without papilla, ostiole with reddish to brown setae in centre. Peridium 14–22 μm wide, thin, comprising 6–7 layers of hyaline to pale brown cell of textura angularis. Hamathecium of 1.6–2.4 µm wide, numerous, septate, branched, pseudoparaphyses. Asci 62–100 × 8–12 μm (\( \bar{x} \) = 77 × 10 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindric, with a club-shaped pedicel, thin-walled, round at the apex. Ascospores 13–22 × 3–7 μm (\( \bar{x} \) = 19 × 5 μm, n = 20), overlapping 1-seriate, fusiform, hyaline, 1-septate in the centre, apical cell longer than basal cell, conical at the ends, guttulate, smooth-walled, surrounded by large mucilaginous sheath. Asexual morph Mycelium consisting of smooth, branched, septate, pale brown, 1.2–3.5 μm wide hyphae, forming hyphal strands. Conidiophores subcylindrical, medium brown, smooth, consisting of a supporting cell and a single conidiogenous cell, or reduced to a conidiogenous cell, straight to curved. Conidiogenous cells 10.6–26.5 × 1.2–2.7 µm (\( \bar{x} \) = 17.74 × 1.78 μm, n = 20), phialidic, pale to medium brown, ellipsoid or subclavate, smooth, with a somewhat flared collarette. Conidia 2.1–5.2 × 1.7–3.4 μm (\( \bar{x} \) = 3.36 × 2.44 μm, n = 40), 1-celled, ellipsoid or oval, smooth-walled, guttulate, hyaline, becoming pale with age, becoming aggregated in chains.
Culture characteristics: Colonies reaching 9 cm diam. after 1 week at 16 °C, colonies erumpent, spreading, with sparse to dense aerial mycelium on water agar media with sterile toothpicks and pine needles, thin to wide, smooth, sporulation sparse.
Material examined: ITALY, near Passo la Calla, on dead branch of Cytisus scoparius (Fabaceae), 25 September 2013, Erio Camporesi, IT1464 (MFLU 15-2551, holotype); ex-type living culture MFLUCC 16-0245, (HKAS 94561bis, paratype).
GenBank Numbers LSU:KY496723; ITS:KY496744; SSU:KY501114; TEF1:KY514393.
Notes: We introduce a new species based on both morphology and phylogeny. Exophiala italica was found in Italy, where the sexual morph is characterized by semi-immersed to erumpent oval ascomata, ostiole, cylindric asci, club-shaped pedicel and 2-celled hyaline ascospores, constricted at the second septum. The species was mainly identified based on phylogenetic analyses with high statistical support (96% in ML/1.00 in BYPP) and clustered with E. hongkongensis. We compared the asexual morph of E. italica and found that E. hongkongensis is different (see Woo et al. 2013). E. italica is the first sexual morph report in Exophiala based on morphological characteristics and molecular data (Fig. 74).
Mucoromycotina Benny
Mucorales Fr.
Mucorales has the largest number of species within the Mucoromycota and is characterized by the formation of coenocytic hyphae with septa either at the base of the reproductive structures or irregularly distributed in old colonies (Santiago et al. 2013). Species of this order are mostly saprotrophic, and are commonly isolated from soil, stored grains, plants, and animal excrement, especially that of herbivores and rodents (Santiago et al. 2013; de Souza et al. 2016). The genus Gongronella, proposed by Ribaldi (1952), belongs to the phylum Mucoromycota, subphylum Mucoromycotina, order Mucorales and family Cunninghamellaceae (Benny et al. 2016; Spatafora et al. 2016). It includes species characterized by relatively slow growing colonies that form poorly developed stolons and rhizoids, as well as erect or circinately branched sporangiophores. The sporangia are small with a deliquescing or breaking wall, and the columellae are reduced in size, with the presence of an apophysis meaning the sporangium never has a pyriform shape. The sporangiospores are 1-celled, hyaline, smooth and variously shaped, while the brown to black zygospores are borne aerially, and have parallel suspensors devoid of appendages (Hesseltine and Ellis 1964).
Cunninghamellaceae Naumov ex R.K. Benj.
Gongronella Ribaldi
Gongronella was established to accommodate a single species: G. urceolifera Ribaldi (currently G. butleri Lendn.), previously allocated within Mortierellaceae. The presence of a distinct globose apophysis and the reduced size of the columellae were the main characteristics in the introduction of the genus (Upadhyay 1969). Peyronel and Dal Vesco (1955) and Pici (1955) transferred Absidia butleri Lendn. to Gongronella, based on the presence of a characteristic apophysis similar to those observed in the type species. Later, Hesseltine and Ellis (1961) proposed the addition of another species, G. lacrispora Hesselt. & J.J. Ellis, and recently, three new species have been described (Adamčík et al. 2015; Ariyawansa et al. 2015c; Li et al. 2016). Currently, the genus includes five species: G. butleri, G. guangdongensis F. Liu, T.T. Liu & L. Cai, G. koreana Hyang B. Lee & T.T.T. Nguyen, G. lacrispora and G. orasabula Hyang B. Lee, K. Voigt, P.M. Kirk & T.T.T. Nguyen. Species of Gongronella have traditionally been distinguished based on their morphology and sporangial stages. In recent years, however, molecular analysis has been used as an additional tool to delimitate species, causing an increase in the number of new taxa (Adamčík et al. 2015; Ariyawansa et al. 2015c; Li et al. 2016).
Gongronella brasiliensis C.A. de Souza, D.X. Lima & A.L. Santiago, sp. nov.
Index Fungorum number: IF552552; Facesoffungi number: FoF 2699, Fig. 78 Etymology: Brasiliensis, referring to country where the species was first isolated.
Gongonella brasiliensis (URM 7487, holotype). a–c Sporangiophores with variously shaped apophysis and sporangia. d Branched sporangiophore with apophysis and columella. e Branched sporangiophore with two septa (arrows), sporangia and a small columella with collarette. f Simple sporangiophore with apophysis and columella. g Simple sporangiophore with apophysis and inconspicuous columella with collarette (arrow). h Giant cells. i Sporangiospores. Scale bars a, b, d = 25 μm, c, e–i = 20 μm
Holotype: URM 7487
Colonies white, cottony, low and exhibiting slow growth (6 cm diam. and 1–2 mm in height) after 7 days on MEA at 25 °C. Reverse cream to buff with irregular margins. Rhizoids frequent, slightly branched, long or short, up to 45 μm length, hyaline. Stolons present, 2–6 μm diam., hyaline, generally coenocytic, although some septa can be observed in old colonies. Odourless. Sporangiophores erect, straight or slightly recumbent, smooth-walled, hyaline, 26.5–320 × 2.5–5 μm; solitary, arising from stolons, or in whorls of two, often with a single branch, predominantly terminating in a sporangium, while those emerging directly from aerial mycelia branch repeatedly. Some branches end in a sterile sporangial, globose, 5–17 μm diam. One or two septa may form below the apophysis. Sporangia first yellowish then becoming light brownish, globose, subglobose, 9.5–30 μm diam., smooth-walled, some leaving collars. Columellae hyaline to light grey, smooth-walled, globose, subglobose, (3–)4–8(–9) μm or conical-cylindrical, 1.5–2.5 × 2–3 μm, some so small that they are almost inconspicuous, up to 1 μm diam., some with an evident collar. Apophysis hyaline, smooth-walled, variable in shape, globose, (3–)4–5(–6) μm diam., vase-shaped, (3–)4 × 12(–14.5) μm and ellipsoidal, 5–10(–12) × 3–7(–8.5) μm. Sporangiospores variable in shape, hyaline, smooth-walled, some containing oil droplets, reniform, 1.5–4 × 1.5–2.5 μm, ellipsoid to fusiform, 2–6.5 × 1.5–3 μm, ellipsoid with a flattened end, some almost falciform, 2.5–7.5 × 1.5–4. Chlamydospores present in the aerial mycelium, globose, subglobose and doliiform. Giant cells globose, subglobose, ovoid, some hypha-like and irregularly swollen, up to 48 μm diam. Zygosporangia not observed.
Media and temperature tests: On MEA at 10 °C lack of growth and sporulation. At 15 °C—colonies with slow growth, reaching 3.6 cm in diam. after 192 h; poor sporulation. At 20 °C—low (1–2 mm in 168 h) colonies with slow growth (4.3 cm in 168 h); good sporulation. Sporangiophores with mainly single branches, rarely multi-branched; Chlamydospores present, globose to subglobose; giant cells absent. At 25 °C—low (1–2 mm in 168 h) colonies, better growth (6 cm in 168 h); excellent sporulation. At 30 °C—good growth 5.6 cm in 168 h; excellent sporulation. The growth of G. brasiliensis on PDA was slightly slower than on MEA at all tested temperatures.
Material examined: BRAZIL, Taquaritinga do Norte, state of Pernambuco (752′28″S, 3559′25″W), in soil samples. Soil, 5.XI.2015, leg. C.A.F de Souza (URM 7487, holotype). Gene bank numbers ITS:KY114930; KY114931.
Notes: According to the phylogenetic (ITS) and morphophysiological analyses, G. brasiliensis exhibits well-supported genetic datasets and morphological characteristics that differentiate it from other species of the genus (Fig. 78). Gongronella brasiliensis produces elliptical apophysis, conical-cylindrical columellae and giant cells not so far reported for this genus. G. brasiliensis may have been previously confused with G. butleri because of the branching pattern of the sporangiophores and for the size and shape of the sporangia. However, only the former produces elliptical apophysis, conical-cylindrical columellae and giant cells (Fig. 77). Additionally, colonies of G. brasiliensis are persistently white, in contrast to the colonies of G. butleri that are at first white, later becoming olive-buff or olive-gray (Upadhyay 1969). In most species of Gongronella, one septum is commonly observed below the sporangia. The presence of two septa delimiting the sporangia was only observed in G. orasabula and G. brasiliensis. Additionally, the sporangiospores of G. brasiliensis are variable in shape and larger than those observed in G. butleri, G. guangdongensis, G. koreana and G. orasabula (Figs. 79, 80).
Phylogenetic tree of Mucor stercorarius CNUFC-UK2-1 and CNUFC-UK2-2 based on maximum likelihood analysis of ITS sequence data. Sequences of Syncephalastrum racemosum were used as the outgroup taxon. Numbers at the nodes indicate the bootstrap values (>50%) from 1000 replications. The bar indicates the number of substitutions per position. The new species is in blue and ex-type strains in bold
Phylogenetic tree of Mucor stercorarius CNUFC-UK2-1 and CNUFC-UK2-2 and related species based on maximum likelihood analysis of SSU and LSU, Actin TEF1 sequence data. Sequences of Umbelopsis nana and U. isabellina were used as outgroup taxa. Numbers at the nodes indicate the bootstrap values (>50%) from 1000 replications. The bar indicates the number of substitutions per position. The new species is in blue and ex-type strains in bold
Rhizopodaceae K. Schum.
Mucor Fresen.
The genus Mucor includes primarily saprotrophic species characterized by the production of fast-growing colonies, simple or branched sporangiophores without basal rhizoids, non-apophysate sporangia, and seldomly produced zygospores which are borne from opposed suspensors, possess a thick pigmented and ornamented zygosporangium (Schipper and Samson 1978). The species within the genus can be easily isolated from soil, dung, water, stored grains and other plant parts (Benny 2008; Li et al. 2016). Mucor spp. are commonly reported in different herbivore faeces (Thilagam et al. 2015; Li et al. 2016; Nguyen et al. 2016). On these substrates, Zygomycetes usually appear first, followed by ascomycete and finally basidiomycete fungi. Among the Zygomycetes found in dung, species of Mucor were dominant (Benny 2008). In Korea, a small number of Mucorales have reported from animal fecal (Li et al. 2016; Nguyen et al. 2016).
Traditional taxonomy of species of Mucor was based on morphological characteristics such as the size and shape of sporangia, as well as the mode of reproduction (sexual or asexual). Recently, molecular data have been used to evaluate mucoralean species (Hoffmann et al. 2013; Walther et al. 2013; Ariyawansa et al. 2015c).
During a study on the Mucorales from animal faecal samples in Korea, a species of Mucor that differed morphologically and phylogenetically from other species was isolated and is thus described as new.
Mucor stercorarius Hyang B. Lee, P.M. Kirk, K. Voigt & T.T.T. Nguyen, sp. nov.
MycoBank number: MB 818383; Facesoffungi number: FoF 2799, Fig. 81
Mucor stercorarius (CNUFC-UK2-1, holotype). a Colony on synthetic mucor agar. b Sporangium with sporangial wall net. c Sporangium without wall net. d Spore bearing sporangium on sporangiophore. e–j Columellae with clear collar present at the apex of the sporangiophores. Scale bars b = 15 μm, c–j = 20 μm
Etymology: stercorarius. Named for rat dung from which the species was first collected.
Holotype: CNUFC-UK2-1
Colonies growing fast on SMA, white, grayish-white in reverse, reaching 42–45 mm diam. at 20 °C after 2 days of incubation. Sporangiophores 4.5–7 μm wide, erect, branched sympodially. Sporangia globose to subglobose, yellow, multi-spored, reaching 30–38.5 × 27.5–37 μm; sporangial wall at maturity fully deliquescent, leaving a small collar on the sporangiophore. Columellae of diverse shape, globose to subglobose or pyriform, 15.5–22 × 17–25 μm. Sporangiospores ellipsoidal, 6.5–10 × 3.5–6 μm. Zygospores 38–83 × 45–86 μm.
Material examined: REPUBLIC OF KOREA, Jeonnam Province, garden of the Chonnam National University located in Gwangju (3510′N 12655′E), from a rat fecal sample, 24 February 2016 (CNUFC-UK2-1 preserved as glycerol stock at −80 °C in the Chonnam National University Fungal Collection (CNUFC); isotype in Culture Collection of National Institute of Biological Resources (NIBR), Incheon; living culture (ex-type) deposited at Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC:SF:12422).
GenBank Numbers CNUFC-UK2-1 LSU:KX839685; SSU:KX839684; ITS:KX839689; TEF1:KX839681; Actin:KX839688. CNUFC-UK2-2 LSU:KX839682; SSU:KX839686; ITS:KX839680; TEF1:KX839687; Actin: KX839683.
Culture characteristics: The isolate grew over a wide range of temperatures with varying growth rates on SMA, PDA (potato dextrose agar), and MEA (malt extract agar) of 21.5, 17.5 and 23 mm per 24 h, respectively. Optimal growth was 20–25 °C, slow growth at 5 °C, and no growth at 37 °C.
Notes: Mucor stercorarius is similar in morphology and closely related to M. genevensis but differs by forming smaller sporangia. Columellae are diverse in shape and smaller than those of M. genevensis; sometimes a, bell-shaped apophysis was present. Moreover, the new species grows and sporulates at 35 °C, whilst M. genevensis does not. In the phylogenetic tree of ITS and multiple genes, the strain forms a branch separate from other species of Mucor, supporting its establishment as a new species.
Sordariomycetes O.E. Erikss. & Winka
Sordariomycetes is the second largest class of Ascomycota (Hyde et al. 2013) including 6 subclasses, 32 orders, 105 families and 1331 genera (Maharachchikumbura et al. 2016). The Sordariomycetes have a cosmopolitan distribution comprising terrestrial and aquatic taxa (Jones et al. 2015; Pratibha et al. 2014). Species are characterized by perithecial ascomata and inoperculate unitunicate asci (Zhang et al. 2006) or non-fissitunicate asci (Kirk et al. 2008). The class includes many important plant pathogens, endophytes, saprobes, epiphytes, coprophilous, fungicolous, and lichenized or lichenicolous taxa (Maharachchikumbura et al. 2016) (Figs. 79, 80).
Diaporthales Nannf.
The Diaporthales was introduced by Nannfeldt (1932), and includes a number of plant pathogenic fungi and many asexually reproducing fungi (Castlebury et al. 2002; Rossman et al. 2007).
Gnomoniaceae G. Winter
Gnomoniaceae was introduced by G. Winter (1886) with Gnomonia Ces. & De Not. 1863 as the type genus. Species in Gnomoniaceae can exist as saprobes as well as cause serious tree diseases (Rossman et al. 2007). They are characterized by ascomata that are generally immersed, solitary, without a stroma, or aggregated with a rudimentary stroma (Sogonov et al. (2008).The genera of Gnomoniaceae were listed by Sogonov et al. (2008) (Fig. 82).
Consensus tree resulting from a maximum likelihood analysis of a combined TEF1 and ITS sequence alignment for taxa of Gnomoniopsis. Species are indicated in coloured blocks. RAxML bootstrap support values (ML above 50) and Bayesian posterior probabilities (BYPP above 90%) are given at the nodes (ML/BYPP). The scale bar represents the expected number of changes per site. The tree is rooted to Gnomonia gnomon AFTOL ID 952. New sequences are in blue bold
Gnomoniopsis Stoneman
Gnomoniopsis Berl. was introduced based on the type species G. chamaemori (Fr.) Berl. (Barr 1978; Monod 1983; Walker et al. 2010). Gnomoniopsis is characterized by having small, black perithecia, solitary or in groups in a minimal stroma, and 1-septate, oval to fusiform ascospores. Sogonov et al. (2008) identified some Sirococcus species as the asexual morph of Gnomoniopsis. Most species of Gnomoniopsis occur on plants of the Fagaceae, Onagraceae and Rosaceae. Gnomoniopsis also contains economically important pathogens of rosaceous crop plants, including blackberry, raspberry, and strawberry (Bolay 1971; Monod 1983; Maas 1998). An updated phylogeny of Gnomoniopsis with new isolates based on a combined gene analysis is presented (Fig. 83).
Gnomoniopsis sanguisorbae (MFLU 15-1255, reference specimen). a, b Appearance of ascomata on host substrate. c Section of ascoma. d Ostiole. e Section of peridium. f Pseudoparaphyses. g–i Ascus. j–m Ascospores. n Germinating ascospore. Note: stained with cotton blue in i, j, l and m. Scale bars c = 100 μm, d, e = 10 μm, f = 2 μm, g–i = 5 μm, j–n = 2 μm
Gnomoniopsis sanguisorbae (Rehm) D.M. Walker, Mycologia 102 (6): 1494 (2010)
Facesoffungi number: FoF 2800, Fig. 83
Reference specimen: MFLU 15-1255
Saprobic on dead stem of Sanguisorba minor Scop. Sexual morph Ascomata 320–432 µm high × 397–466 µm diam. (\( \bar{x} \) = 388 × 442 μm, n = 5), immersed to semi-immersed, solitary, or in small groups, uniloculate, globose, conspicuous at the surface, black, without hairs, long ostiolate. Peridium 15–21 μm wide, comprising 3–4 layers of pale brown cells of textura prismatica. Hamathecium 0.5–1.2 µm wide, numerous, comprising dense, septate, unbranched, paraphyses. Asci 15–19 × 2–5 μm (\( \bar{x} \) = 17 × 4 μm, n = 25), 8-spored, bitunicate, fissitunicate, cylindric to cylindric-clave, short pedicellate, thin-walled, round at the apex. Ascospores 6–10 × 1–3 μm (\( \bar{x} \) = 9 × 2 μm, n = 20), overlapping 1–2-seriate, hyaline, 1-septate, constricted at the septum, first cell longer than second cell, round at the ends, guttulate, smooth-walled, lacking amucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA, reaching 6 cm diam. after 7 days at 16 °C, producing dense mycelium, circular, rough margin white–gray with raised on media surface.
Material examined: ITALY, Premilcuore Province, Castel dell’ Alpe, on dead stem of Sanguisorba minor (Rosaceae), 22 August 2014, Erio Camporesi, IT2058 (MFLU 15-1255, HKAS 94604, reference specimen designated here); living culture MFLUCC 15-0062.
GenBank Numbers LSU:KY496735; ITS:KY496755; SSU:KY501123; TEF1:KY514399.
Notes: Walker et al. (2010) chose a lectotype of G. sanguisorbae but their illustration is not clear. However, our phylogenetic tree shows that our strain groups with other strains of G. sanguisorbae. We also compared the morphology of our strain with G. sanguisorbae and found that it matches. We therefore designate G. sanguisorbae (MFLU 15-1255) as a reference specimen (sensu Ariyawansa et al. 2014b).
Sydowiellaceae Lar.N. Vassiljeva
Sydowiellaceae was established in Diaporthales by Lar.N. Vassiljeva (1987) based on S. fenestrans (Duby) Petr. (Rossman et al. 2007, Senanayake et al. 2017). The family accommodates 15 genera with 43 species, but several taxa do not share any clear morphological features in common (Rossman et al. 2007; Liu et al. 2015; Maharachchikumbura et al. 2015, 2016; Senanayake et al. 2017). Members of Sydowiellaceae are found on herbaceous, dicotyledonous plants and hardwood trees as pathogens and saprobes (Rossman et al. 2007).
Sillia P. Karst.
Sillia was introduced by P. Karsten (1873), with the type S. ferruginea (Pers.) P. Karst. There are six species listed in Index Fungorum (2017) and Senanayake et al. (2017) added a new species, S. karstenii. Sillia is characterized by stromata with numerous ascomata with long cylindrical necks and stromatic tissues, which turn to red in 10% KOH (Iznova and Rukseniene 2012). A revised phylogeny among members of the Diaporthales is presented in Fig. 84
Consensus tree resulting from a maximum likelihood analysis of combined LSU and ITS sequence alignment of genera of Diaporthales. Genera in Sydowiellaceae are indicated in blue and ash blocks. Maximum parsimony bootstrap support (MPB above 50%), RAxML bootstrap support (MLB above 50%), and Bayesian posterior probabilities (PP above 0.9) are given at the nodes (MPB/MLB/PP). The scale bar represents the expected number of changes per site. The tree is rooted to Gnomonia gnomon (CBS 829.79; CBS 199.53). Ex-type strains are in bold and new strains are in blue bold
Sillia italica de Silva, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552716; Facesoffungi number: FoF 2801, Fig. 85
Sillia italica (MFLU 16-0056, holotype). a Appearance of ascomata erumpent through cracks in bark on branch of Corylus sp. b, c Vertical sections of stromata and ascomata. d Peridium. e–h Asci. i Pseudoparaphyses. i–n Ascospores. Scale bars b, c = 80 μm, d = 30 μm, e–h = 80 μm, i = 20 μm, j–n = 80 μm
Etymology: Referring to the country where the fungus was first collected.
Holotype: MFLU 16-0056
Saprobic on dead branch of Corylus sp. (Corylaceae). Sexual morph Stroma erumpent from cracks, up to 1–2.5 mm in diam., black on the surface. Ascomata 480–745 µm high × 136–346 µm diam. (\( \bar{x} \) = 640 × 242 µm, n = 10), semi-immersed in black stromatic tissues gregarious, globose to subglobose, ostiolate, soft and found in a valsoid arrangement. In 10% KOH solution stroma becomes brownish-red. Ostioles 330–570 μm long, cylindrical, forming at the center of the ascomata, raised from the host surface. Peridium 20–34 μm thick, with outer layer of brown and thick-walled cells of textura prismatica; inner layer of hyaline and thin-walled cells of textura angularis. Paraphyses 4–7 µm wide, cylindrical, cellular, septate, mixed with asci. Asci 147–234 × 10–15 μm (\( \bar{x} \) = 190 × 13 µm, n = 25), 8-spored, unitunicate, narrowly clavate, straight or somewhat curved, with a short obtuse pedicel. Ascospores 99–128 × 3.1–4.6 µm (\( \bar{x} \) = 109 × 3 µm, n = 30), fasciculate, hyaline, filiform, elongate, narrow and curved at the ends, guttulate, smooth-walled, with 3 transverse septa. Asexual morph Undetermined.
Material examined: ITALY, Forlì-Cesena Province, Monte Mirabello–Predappio, on dead twig of Corylus sp. (Corylaceae), 11 January 2016, Erio Camporesi, IT2754 (MFLU 16-0056 holotype, BBH isotype).
GenBank Numbers LSU:KY397949; ITS:KY523484.
Notes: Sillia italica strains analyzed herein are sister taxa to S. ferruginea, S. karstenii and this relationship is well-supported (Fig. 84). This close phylogenetic affinity is also supported by morphological similarities. Both S. ferruginea and S. italica become reddish in 10% KOH solution (Iznova and Rukseniene 2012). Sillia italica however, has some distinct characters as compared to S. ferruginea. Stromatic tissues of S. ferruginea comprise black tissues on the surface and inner yellow stromatic tissues mixed with black tissues (Iznova and Rukseniene 2012), while S. italica contains black stromatic tissues. The ascomata arrangement of S. italica is valsoid, whereas in S. ferruginea it is diatrypoid. Sillia italica has clavate asci similar to S. ferruginea, but they are longer (147–234 µm vs. 96–115 µm). Ascospores of S. italica are 99–128 µm long with 3 septa, while in S. ferruginea they are 65–72 µm long with 3–5 septa. Senanayake et al. (2017) introduced Sillia karstenii which morphologically differs from S. italica in having large ascomata with very poorly developed stromata. The phylogenetic analysis of Senanayake et al. (2017) also showed that S. italica is phylogenetically distinct from other Sillia species (Fig. 86).
Phylogram generated from one of 1000 most parsimonious trees based on combined ITS, LSU, RPB2 and Actin sequence data from Cytospora isolates. The tree is rooted to Phomopsis vaccinii (ATCC 18451). Maximum likelihood bootstrap values ≥60% are given at the nodes. The species obtained in this study are in blue and ex-type are in blue bold. Ex-type taxa are in bold
Cytospora Ehrenb.
Cytospora Ehrenb., Sylv. mycol. berol. (Berlin): 2 (1818)
Cytospora was introduced by Ehrenberg (1818) and is an important pathogenic genus causing canker disease on branches leading to dieback on a wide range of plants. (Adams et al. 2005, 2006; Hyde et al. 2016; Norphanphoun et al. 2017). See Norphanphoun et al. (2017) for a recent account of the genus. An updated tree for the genus is provided in Fig. 87.
Cytospora gelida (MFLU 15-3779, holotype). a Appearance of fruiting bodies in wood. b Fruiting bodies on substrate. c Close up of fruiting body. d Cross section of the conidioma. e Ostiole. f Peridium. g, h, i Conidiophores with conidiogenous cells. j Conidia. k, l Culture characters on MEA. Scale bars a = 2 mm, b = 1 mm, c = 500 µm, d = 200 µm, e = 100 µm, f = 50 µm, g = 20 µm, h, i = 5 µm, j = 10 µm
Cytospora gelida Norphanphoun, Bulgakov, T.C. Wen & K.D. Hyde, sp. nov.
Index Fungorum number: IF552712; Facesoffungi number: FoF 2802, Fig. 88
Cytospora ceratosperma (MFLU 15-3757). a Appearance of fruiting bodies in wood. b Fruiting bodies on substrate. c Close up of fruiting body. d Cross section of the ascomata. e Peridium. f Ostiole. g, h, i Asci. j Ascus strain in Melzer’s reagent. k Paraphyses. l Apical ring. m, n, o, p Ascospores. q, r Culture characters on MEA. Scale bars a = 2 mm, b = 1 mm, c, d = 500 μm, e = 50 μm, f = 100 μm, g–j = 10 μm, k = 50 μm, l–p = 5 μm
Etymology: the Latin epithet “gelida” meaning colonies on media look like ice.
Holotype: MFLU 15-3779, Fig. 87
Pathogen on twigs and branches of Cotinus coggygria Scop. Sexual morph Undetermined. Asexual morph Conidiomata 650–1000 µm diam. × 350–450 µm high. immersed in host tissue, solitary, scattered, erumpent, unilocular, with ostiolate. Ostiole 300–400 µm diam. at the same level as the disc surface. Peridium comprising several layers of cell of textura angularis, with inner most layer thick, brown, outer layer dark brown to black. Conidiophores branched, reduced to conidiogenous cells. Conidiogenous cells branched, blastic, enteroblastic, phialidic, hyaline, smooth. Conidia (5.3–)5.7–8 × 1.4–1.8(–2) µm (\( \bar{x} \) = 6.9 × 1.8 µm, n = 30) 1-celled, allantoid to subcylindrical, hyaline, smooth-walled.
Culture characteristics: Colonies on MEA, reaching 4 cm diam. after 7 days at 25 °C, producing dense mycelium, circular, rough margin white mycelium, without aerial mycelium: reverse of the colony whitish-yellow
Material examined: RUSSIA, Rostov Region, Krasnosulinsky District, Donskoye forestry, lining-out nursery, trees and shrubs, dying twigs and branches of Cotinus coggygria (Anacardiaceae), 27 October 2015, T. Bulgakov; T-1117 (MFLU 15-3779, holotype), ex-type living culture, MFLUCC 16-0634, KUMCC.
Notes: In the phylogenetic analyses (Fig. 87), C. gelida groups with C. cotini (strain MFLUCC 14-1050) and C. ribis (strain 284-2). Cytospora gelida can be distinguished from C. cotini by its multi-loculate (>4) conidiostromata, while C. cotini has 2–3 locules in the conidiostromata. The conidia of C. gelida are larger than C. cotini (6.9 × 1.8 vs. 5.9 × 1.2 µm). Cytospora gelida differs from C. cotini in the polymorphic nucleotides of ITS, RPB2 and Actin sequence data. In ITS it differs with two polymorphisms, and RPB2 in 17 polymorphisms.
Cytospora ceratosperma (Tode) G.C. Adams & Rossman, in Rossman, Adams, Cannon, Castlebury & Crous, IMA Fungus 6: 147 (2015)
Facesoffungi number: FoF 2803, Fig. 88
Pathogen on twigs and branches of Acer platanoides L. Sexual morph: Ascomata 300–450 µm high × 500–550 µm diam. (\( \bar{x} \) = 300 × 500 μm, n = 5), immersed in host tissue, scattered, erumpent through the surface of bark, lenticular, extending to a large circular area, with 3–6-ostioles per group. Locules dark brown, arranged circularly, flask-shaped to sphaerical with one ostiole. Ostiole 450–550 µm diam. numerous, dark brown to black, at the same level as the disc, occasionally area below disc a lighter entostroma. Peridium composing several layers of irregular cells arranged in a textura angularis, with inner most layer thick, dark brown, outer layer dark brown to black. Hamathecium long cylindrical, cellular, anastomosed, paraphyses. Asci (58–)60–65 × 10–11(–12) μm (\( \bar{x} \) = 63 × 11 μm, n = 15), 6–8-spored, unitunicate, clavate to elongate obovoid, with apical ring J-, short pedicel late or sessile. Ascospores (11–)12.2–15 × 3.1–4(–4.2) μm (\( \bar{x} \) = 14.5 × 3.7 μm, n = 20), biseriate, elongate-allantoid, 1-celled hyaline, lacking a mucilaginous sheath, smooth walled. Asexual morph: Undetermined.
Culture characteristics: Colonies on MEA, reaching 5 cm diam. after 7 days at 25 °C, producing dense mycelium, circular, rough margin white, without aerial mycelium.
Material examined: RUSSIA, Rostov Region, Krasnosulinsky District, Donskoye forestry, Kabanya Balka (Boar gully), artificial forest, dying twigs and branches (necrotrophic) on Acer platanoides (Sapindaceae), 27 October 2015, T. Bulgakov; T-1095 (MFLU 15-3757, new record); living culture, MFLUCC 16-0625, KUMCC.
Notes: Cytospora ceratosperma has been reported on hosts worldwide (Ahmad 1969; Pantidou 1973; Simonyan 1981; Truszkowska and Chlebicki 1983; Cannon et al. 1985; Spielman 1985; Ahmad et al. 1997; Dudka et al. 2004; Mulenko et al. 2008; Fotouhifar et al. 2010; Rossman et al. 2015). The characters of the sexual morph in our collection indicate it belongs to Cytospora in having a large circular area, with 3–6-ostioles per group, with asci (\( \bar{x} \) = 63 × 11 μm) and ascospores (\( \bar{x} \) = 14.5 × 3.7 μm). In the phylogenetic analyses of our collection (strain MFLUCC 16-0625), groups with C. rosarum (strain 218), collected from Rosa canina (Fotouhifar et al. 2010) (Fig. 86). The current study represents the first record of maple canker caused by C. ceratosperma in Russia.
Cytospora ceratosperma has not been epitypified and therefore we treat MFLUCC 16-0625 as a reference specimen (Ariyawansa et al. 2014b). Fresh collections of C. ceratosperma from the type location and host are needed for epitypification.
Hypocreales Lindau
Bionectriaceae, Clavicipitaceae, Cordycipitaceae, Flammocladiaceae, Hypocreaceae, Nectriaceae, Niessliaceae, Ophiocordycipitaceae, Stachybotryaceae and Tilachlidiaceae are included in the order Hypocreales (Maharachchikumbura et al. 2015). Hypocreales are highly diverse and found in the tropics and subtropics (Põldmaa 2011). They are characterized by pigment producing, brightly coloured perithecial ascomata, and typically ostiolate perithecial fruiting body (Rogerson 1970; Rehner and Samuels 1995).
Clavicipitaceae Earle
The families Clavicipitaceae, Cordycipitaceae and Ophiocordycipitaceae were recognized by Sung et al. (2007) based on phylogenetic analyses. The family Clavicipitaceae (Hypocreales) is a very heterogeneous group comprising obligate saprotrophes, parasites and symbionts with insects and, fungi or grasses, rushes or sedges (Sung et al. 2007; Schardl et al. 2013; Kepler et al. 2013; Li et al. 2016). Maharachchikumbura et al. (2015) listed 48 genera under this family.
Species of Hypocrella, Moelleriella and Aschersonia belong to the family Clavicipitaceae and are pathogens of scale insects and whiteflies and are common in tropical regions (Chaverri et al. 2008). Most species of Hypocrella produce an Aschersonia asexual morph that is characterized by pycnidia or acervuli on a stroma, often filled with brightly coloured, slimy conidia (White et al. 2003; Chaverri et al. 2005). Moelleriella was described by Bresadola (1896) and it produces filiform, multi-septate ascospores, that disarticulate at the septa within the ascus and an aschersonia-like asexual morph with fusoid conidia (Chaverri et al. 2008) (Fig. 89).
Phylogram of Hypocrella, Samuelsia, Moelleriella and Aschersonia generated from maximum likelihood analysis of RPB1, RPB2 and TEF1 sequence data. Shimizuomyces paradoxus Kobayasi is used as outgroup taxon. Maximum likelihood bootstrap values greater than 50% and Bayesian posterior probabilities over 0.5 are indicated above the nodes. The new species and epitype species are indicated in blue
Hypocrella calendulina Hywel-Jones & Mongkols., Mycol Res 113 (6–7): 687 (2009)
Facesoffungi number: FoF 2805, Fig. 90
Hypocrella calendulina (MFLU 16-2918, asexual morph). a Habitat. b Appearance of brownish conidiomata on the host. c, d Vertical section of conidiomata. e, g, h Base of conidioma with conidiophores, conidiogenous cells and developing conidia. f Paraphyses arising from the hymenium of the conidioma. i–n Immature to mature conidia. o Germinating conidium. p, q Culture on PDA medium notes q reverse. Scale bars b = 1000 µm, c = 200 µm, d = 500 µm, e, g = 20 µm, f = 100 µm, h = 50 µm, i–o = 5 µm, p–q = 1 cm
Parasitic on scale insect nymph (Hemiptera), forming yellow to brownish stromata on the underside of bamboo leaves. Sexual morph See Mongkolsamrit et al. 2009. Asexual morph Coelomycetous. Conidiomata 1.4–2.2 × 0. 45–0.55 mm (\( \bar{x} \) = 1.784 × 0.493 mm, n = 10), reddish-brown, solitary, irregular in shape, surface roughened, flattened pulvinate, multi-loculate, locules 520–638 × 385–455 µm (\( \bar{x} \) = 579 × 420 µm, n = 20), brown, immersed, globose or subglobose to obpyriform, ostiolate. Ostioles subcylindrical, single, well-developed, centrally located. Wall of conidiomata 19–29 µm wide, composed of relatively thick-walled, brown cells of textura oblita in the outer layers and thin-walled, hyaline cells of textura angularis in the inner layers. Paraphyses 163–308 × 1.2–1.6 µm (\( \bar{x} \) = 236 × 1.4 µm, n = 150), hyaline, filiform, tapering at the apices, arising from the hymenium of the conidioma. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 8–14 × 1–1.5 µm (\( \bar{x} \) = 11 × 1.2 µm, n = 90), hyaline, phialidic, cylindrical to subcylindrical, broad at base, discrete, smooth, arising from the inner layers of conidioma. Conidia 11–13 × 2.1–2.9 µm (\( \bar{x} \) = 12 × 2.5 µm, n = 150), hyaline, fusiform, unicellular, straight or slightly curved, guttulate, thick-and smooth-walled, accumulating in a slimy yellowish mass above the conidioma.
Culture characteristics: Cultures were obtained from germinating ascospores and conidia. The ascospores and conidia germinated within 48 h on PDA. Colonies on PDA were irregular and approx. 2 cm diam. after 4 weeks at 20 °C. The colonies derived from germinating ascospores or conidia formed a compact mycelium. The colonies have rough surface, viscous texture, knobly protuberances and curled margin. The conidial mass was pale yellow to yellow appearing as abundant slimy masses scattered over the surface of stromatic colonies.
Material examined: THAILAND, Chiang Rai Province, on insect nymph (Hemiptera) on bamboo leaf, 10 February 2015, Jingzu Sun & Yuanpin Xiao, MRC 15021007 (MFLU 16-2918, asexual morph report) MFLU 16-2919, MFLU 16-2919, MFLU 16-2920, MFLU 16-2921, living culture, MFLUCC 17-0057, MFLUCC 17-0058, MFLUCC 17-0059, MFLUCC 17-0060. GenBank Numbers RPB1:KY646196; RPB2:KY646197; TEF1:KY646198.
Notes: The sexual and asexual morphs of Hypocrella calendulina were introduced by Mongkolsamrit et al. (2009) and is a widespread species in Thailand, which can be found throughout the year (Mongkolsamrit et al. 2009). In this study, for the first time we provide, illustrations of the asexual morph of this species, which includes wall of conidiomata, conidiogenous cells and conidia.
Moelleriella thanathonensis Y.P. Xiao, T.C. Wen & K.D. Hyde, sp nov.
Index Fungorum number: IF552794; Facesoffungi number: FoF 2804, Fig. 91
Moelleriella thanathonensis (MFLU 16-2922, holotype). a Habitat. b Appearance of yellow to yellowish conidiomata. c, d Vertical section of conidiomata. e–g Base of conidioma with conidiogenous cells, conidiophores and developing conidia. h–n Immature to mature conidia. o–r Culture on PDA medium, notes p r reverse. Scale bars b, c = 1000 µm, d = 500 µm, e = 50 µm, f–g = 20 µm, h–n = 5 µm, o–p = 5 cm, q–r = 1 cm
Etymology: The species epithet refers to Headquarter of Thanathon orchard, the location of the holotype collection.
Holotype: MFLU 16-2922
Parasitic on an unidentified insect, forming yellow to yellowish conidiomata on the underside of leaves. Sexual morph Undetermined. Asexual morph Coelomycetous, Conidiomata 1.6–1.7 × 0.7–0.9 mm (\( \bar{x} \) = 1.65 × 0.8 mm, n = 10), yellow to orange, solitary or gregarious, with pycnidium-like depression in the stroma, lacking well-defined walls, irregular in shape, surface pruinose, flattened pulvinate, base slightly constricted, surrounded by a white hyphae, up to 3 mm wide, multi-locular, with locules, 309–443 × 234–342 µm (\( \bar{x} \) = 376 × 288 µm, n = 20), globose to subglobose, thick-walled., surface minutely tomentose, covered with confluent conidial masses that are deep yellow to orange yellow. Wall of conidiomata 25–58 µm wide, thin, composed of thick-walled, brown to hyaline cells. Paraphyses 43–61 × 0.9–1.5 µm (\( \bar{x} \) = 52 × 1.2 µm, n = 90), arising from the hymenium of the conidioma, filiform, tapering at the apices. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 13–18 × 1–1.4 µm (\( \bar{x} \) = 15.6 × 1.2 µm, n = 60), hyaline, phialidic, cylindrical, undetermined, discrete, thick and smooth-walled. Conidia 11–13.5 × 1.6–2.1 µm (\( \bar{x} \) = 13 × 1.9 µm, n = 150), hyaline, fusiform, small, 1-celled, often brightly yellow in mass.
Culture characteristics: Cultures were obtained from germinating conidia. The conidia germinated within 24 h on PDA. The colonies were 2 cm diam. after 4 week at 25 °C. The colonies were circular, umbonate, with entire margin and cream to pale yellow conidial masses formed in two circular areas on the stromatic colonies.
Material examined: THAILAND, Chiang Rai Province, Headquarter of Thanathon orchard, on an unidentified insect, Yuanpin Xiao, 10 February 2015, MRC 15021007, MRC 15021007 (MFLU 16-2922, holotype); (MFLU 16-2923, paratypes), ex-type living cultures, MFLUCC 17-0057, MFLUCC 17-0058, MFLUCC 17-0059, MFLUCC 17-0060).
GenBank Numbers PBP1:KY646195; RPB2:KY646199; TEF1:KY646200.
Notes: The sexual morph of this species was not found in the field although several attempts were made to find it throughout the year. Moelleriella thanathonensis is similar to three widespread species in Thailand, namely Moelleriella raciborskii (Zimm.) P. Chaverri, M. Liu & K.T. Hodge, M. libera (Syd. & P. Syd.) P. Chaverri & M. Liu and Aschersonia placenta Berk. Aschersonia placenta is the asexual morph of Moelleriella raciborskii (syn. Hypocrella raciborskii Zimm.) (Liu et al. 2009). Moelleriella libera is the sexual morph of Aschersonia aleyrodis Webber (Chaverri et al. 2008). Our new species M. thanathonensis is characterized by multi-locular conidiomata, fusiform conidia and phialidic conidiogenous cells. In this paper, we provide a synopsis of morphologically similar asexual morph species to M. thanathonensis in Table 3. Phylogenetic analysis of combined TEF1, RPB1 and RPB2 sequence data (Fig. 89) also provides evidence that M. thanathonensis is a new species.
Stachybotryaceae L. Lombard & Crous
Stachybotryaceae was introduced with its type genus Stachybotrys (Corda 1837) and is typified by S. chartarum (Ehrenb.) S. Hughes (Wang et al. 2015). Presently, 33 genera are accepted in the family Stachybotryaceae (Lombard et al. 2016; Lin et al. 2016) (Fig. 92).
Phylogenetic construction using RAxML analysis of the combined LSU and SSU, dataset. Bootstrap support values for maximum likelihood (black) equal to or greater than 50% and Bayesian posterior probabilities (red) equal to or greater than 0.95 are shown above the nodes. The tree is rooted to Valsonectria pulchella and Scopinella solani. The type strains are in black bold and the new species is in blue bold
Myrothecium Tode
Myrothecium was introduced by Tode (1790) with M. inundatum Tode as the type species. Species of this genus are saprobes in the soil and plant pathogens (Ellis and Ellis 1985; Watanabe 1994; Chen et al. 2016). More than 30 species of this genus have been recorded worldwide (Seifertk et al. 2011; Chen et al. 2016; Lombard et al. 2016).
Myrothecium septentrionale J.F. Li, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF 552310; Facesoffungi number: FoF02482, Fig. 93
Etymology: “septentrionale” means “northern” in Latin, named after the location where the species collected, northern Thailand.
Holotype: MFLU 16-2889
Saprobic on dead hanging leaves of Pandanus sp. Sexual morph Undetermined. Asexual morph Colonies effuse on host, grey. Mycelium superficial on the substrate, composed of aseptate, unbranched, smooth, subhyaline hyphae. Conidiophores 8–11 µm long × 1–1.6 µm diam. (\( \bar{x} \) = 9.8 × 1.2 µm, n = 20), macronematous, mononematous, solitary, erect, scattered, straight to curved, subhyaline to hyaline, smooth, thin-walled, aseptate, unbranched, arising from a stromatic base. Conidiogenous cells 1–1.5 µm long × 1–1.6 µm diam. (\( \bar{x} \) = 1.3 × 1.3 µm, n = 20), phialidic, terminal, globose to ellipsoid, hyaline, smooth and thin-walled. Conidia 8.6–12 µm long × 1.2–2.1 µm diam. (\( \bar{x} \) = 10.5 × 1.6 µm, n = 20) solitary, acrogenous, hyaline, smooth-walled, 0–1-septate, ampulliform to doliiform, chiefly subcylindrical. Conidial secession rhexolytic.
Cultural characteristics: Conidia germinating on PDA within 14 h and germ tubes produced from the apex. Colonies growing on PDA, reaching 5 cm in 14 days at 30 °C, mycelium partly superficial, partly immersed, slightly effuse, powdery, vertical, with regular edge, maroon to yellowish-red; sexual or asexual spores not formed within 60 days.
Material examined: THAILAND, Chiang Mai Province, Mae Taeng, dead hanging leaves of Pandanus sp. (Pandanaceae), 25 March 2016, Junfu Li, H-MRC 41B (MFLU 16-2889, holotype), ex-type living culture MFLUCC 16-2819, KUMCC; (isotype in HKAS).
GenBank Numbers KY559395.
Notes: Myrothecium septentrionale is similar to M. chiangmaiense D.Q. Dai & K.D. Hyde in morphology. However, M. septentrionale and M. chiangmaiense are phylogenetically distinct and the latter is basal to M cinctum and M. gramineum, while M. septentrionale is closely related to M. roridum with high support (Fig. 92). Myrothecium septentrionale differs from M. roridum in having larger ampulliform conidia (8.6–12 × 1.2–2.1 µm vs. 5–6 × 1–1.2 µm), while M. roridum has cylindrical conidia.
Glomerellaceae Locq. ex Seifert & W. Gams
Glomerellaceae is a monotypic family which mainly comprises plant pathogens. The family was invalidly published by Locquin (1984) and was validated in Zhang et al. (2006). This family was accepted as one of the four families of Glomerellales in Maharachchikumbura et al. (2015). This family is characterized by the Colletotrichum asexual morph and the Glomerella sexual morphs, which are now named as Colletotrichum (Hyde et al. 2014; Maharachchikumbura et al. 2015).
Colletotrichum Corda.
This genus was introduced by Corda (1831), for C. lineola Corda (Damm et al. 2009). Colletotrichum species are mainly plant pathogens, but also endophytes and saprobes (Yang et al. 2009; Hyde et al. 2009; Cannon et al. 2012; Jayawardena et al. 2016a, b). Kirk et al. (2001, 2008), Réblová et al. (2011) referred Colletotrichum to the family Glomerellaceae and Maharachchikumbura et al. (2016) further confirmed the familial affinities of this genus (Fig. 94).
Phylogram generated from maximum parsimony analysis based on combined ITS, GADPH, CHS, Actin and TUB2 sequenced data from species of the dematium complex. Maximum parsimony bootstrap support values greater than 75% and Bayesian posterior probabilities greater than 0.80 are shown above the branches. The ex-type strains are in bold and the new species is in blue. The tree is rooted with Colletotrichum nigrum
Colletotrichum sambucicola Jayawardena, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552553; Facesoffungi number: FoF 2683, Fig. 95
Colletotrichum sambucicola (MFLU 16-2675, holotype). a Appearance of conidiomata on host. b Base of setae. c Tip of setae. d Setae with conidiophores. e, f Conidiogenous cells. g, h Conidia. i Upper view of 7 days old culture. j reverse view of 7 days old culture. Scale bars a = 0.2 mm, b = 20 µm, d = 10 µm scale bar of b applies to c, e and scale bar of d applies to f, g, h
Holotype: MFLU 16-2675
Saprobic on dead branch of Sambucus ebulus L. Sexual morph Undetermined. Asexual morph Conidiomata 0.13–0.4 mm (\( \bar{x} \) = 0.27 mm, n = 10) diam., black, acervulus, oval, solitary, gregarious. Setae straight or ± bent, abundant, dark brown, becoming paler towards the apex, opaque, smooth walled, septate, 1–6-septate, 110–145 μm long, base cylindrical, slightly inflated, 6.1–8.5 μm diam., apex acute to rounded, smooth. Conidiophores simple, to 20 μm long, hyaline to pale brown, smooth-walled. Conidiogenous cells 9–16 × 2–4 μm (\( \bar{x} \) = 11.9 × 2.9 μm, n = 20), phialidic, hyaline, smooth-walled, cyllindrical to slighty inflated, opening 1–2 μm wide, collarette or periclinal thickening observed. Conidia 12.2–29.7 × 3–5 μm (\( \bar{x} \) = 17.7 × 3.8 μm, n = 15), L/W ratio 4.7, hyaline, smooth or verruculose, aseptate, curved, both sides gradually tapering towards the round to slightly acute apex and truncate base, guttulate. Appressoria not observed.
Culture characteristics: Colonies on PDA flat with entire margin, aerial mycelium sparse, short, pale olivaceous-grey, colony surface buff, some sectors dark grey-olivaceous to dark-olivaceous-grey, iron-grey acervuli can be observed mainly on the edge of the colony. Reverse olivaceous green, concentric rings can be clearly observed, reaching 40–55 mm in 7 days at 25 °C.
Material examined: ITALY, Province of Forlì-Cesena [FC], Meldola, on dead branch of Sambucus ebulus (Adoxaceae), 30 March 2016, Erio Camporesi IT 2902 (MFLU 16-2675, holotype); ex-type living culture, MFLUCC 16-1388, KUMCC 16-0127.
GenBank Numbers ITS:KY098781, KY595193; GAPDH: KY098780, KY595192; CHS:KY098779, KY595190; Actin: KY098778, KY595190; TUB2: KY098782, KY595194 respectively.
Notes: Colletotrichum dematium species complex is mainly characterized by species with curved conidia (Damm et al. 2009; Jayawardena et al. 2016a, b). Colletotrichum sambucicola falls within the C. dematium species complex clade and forms a separate branch with 100% bootstrap support and 1.00 Bayesian posterior probability and is a sister taxon to C. anthrisci (Fig. 95). Among the species with angular conidia, conidia of C. anthrisci have the highest L/W ratio (7.8) and the apex is strongly pointed. Colletotrichum sambucicola differs from C. anthrisci in having conidiogenous cells with a distinct collarette, as well as by having smaller conidia. A BLASTn search of NCBI GenBank with the ITS sequence of MFLUCC 16-1388 showed 99% similarity to quite a number of Colletotrichum species with angular conidia. The closet match in a BLASTn search in GenBank with the GAPDH sequence of MFLUCC 16-1388 was GenBank GU228237 (98% identity, three bp differences) and CHS GU228335 (98% identity 4–7 bp differences). The newly described taxon differs from its sister taxon C. anthrisci in six bp in ITS, eight bp in GAPDH, four bp in CHS, 39 bp in Actin and four bp in TUB2 (Fig. 96).
Phylogram generated from maximum likelihood analysis based on ITS sequence data from species of Bartaliniaceae. Maximum likelihood bootstrap support values higher than 70% and Bayesian prosterior probabilities greater than 0.96 are indicated above or below the branches. The new isolate is shown in blue bold and ex-type strains in black bold. The tree is rooted to Pseudopestalotiopsis theae
Xylariales Nannf.
Xylariales was introduced by Nannf. 1932 and this order is the largest order of perithecial ascomycetes. Familial relationships in this order have been investigated (e.g. Tang et al. 2009) and currently 22 families are accepted Maharachchikumbura et al. (2016).
Bartaliniaceae Wijayaw. et al.
Based on morphology and DNA sequence data, Senanayake et al. (2015) introduced the family Bartaliniaceae to accomodate the genera Bartalinia, Broomella, Dyrithiopsis, Hyalotiella, Truncatella and Zetiasplozna. Later Wijayawardene et al. (2016) included the genera Doliomyces Steyaert and Morinia Berl. & Bres. Doliomyces is known to be morphologically similar to the other genera of the family but there is no sequence data yet to confirm this placement (Wijayawardene et al. 2016). Morinia Berl. & Bres. has uniquely distinct morphological features but phylogenetically groups within Bartaliniaceae (Wijayawardene et al. 2016). Bartaliniaceae is phylogenetically related to the families Sporocadaceae, Pestalotiopsidaceae and Robillardaceae (Jeewon et al. 2002, 2003a; Senanayake et al. 2015; Maharachchikumbura et al. 2016).
Truncatella Steyaert
The genus Truncatella, typified by T. truncata was established by Steyaert (1949) to accommodate distinct species having 3-septate, verruculose, pigmented conidia (Steyaert 1949; Jeewon et al. 2002; Maharachchikumbura et al. 2012, 2015). The genus was previously placed in Amphisphaeriaceae, Xylariales (Jeewon et al. 2003a; Lumbsch and Huhndorf 2010), and later included in Bartaliniaceae by Senanayake et al. (2015) based on both morphological features and molecular evidence. Several studies have contributed to the phylogenetic understanding of Truncatella, such as its affinity to Bartalinia (Jeewon et al. 2002; Lee et al. 2006; Maharachchikumbura et al. 2012, 2014a, b, 2016, 2017; Senanayake et al. 2015; Maharachchikumbura et al. 2017) and Neotruncatella (Hyde et al. 2016). The sexual morph of Truncatella remains largely unresolved, however Senanayake et al. (2015) illustrated both the sexual and asexual morph for T. spartii.
Truncatella spartii Senan., Camporesi & K.D. Hyde, Fungal Diversity 73: 91 (2015) Facesoffungi number: FoF 00685, Fig. 97
Truncatella spartii (KUMCC 15-0127). a Appearance of conidiomata on cone of Picea sp. b Close-up of conidiomata. c, d Cross section of conidioma. e Conidioma wall. f–i Developing conidia from conidiogenous cells. j–m Conidia. n Germinating spores. Scale bars c, d = 50 µm, e–i, n = 10 µm, j–m = 20 µm
Saprobic on cone of Piceae sp., appearing as black, irregular lesions on the host surface. Sexual morph See Senanayake et al. 2015. Asexual morph Conidiomata (240–)320–370(–433) μm diam., 160–200 μm, (\( \bar{x} \) = 340 × 180 μm, n = 8), pycnidial or acervular, semi-immersed to superficial, becoming erumpent at maturity, globose with a flattened base, solitary to aggregated, black. Conidiomata wall consisting of outer light brown to hyaline cells of textura angularis and inner hyaline cells of textura angularis to textura globulosa. Conidiophores 2.5–4 μm long, hyaline, cylindrical, simple or branched. Conidiogenous cells 8.5–14.2 μm long hyaline, simple, filiform, annelidic, indeterminate, integrated. Conidia 17.5–24.8 × 5.3–7.2 μm (\( \bar{x} \) = 21.5 × 6.5 μm, n = 30), broadly fusiform, 3-septate, slightly constricted at the mid septum, verruculose, median cells brown to yellowish-brown, 10.5–15 μm long, thick-walled, globules present, basal cell hyaline, conical tapering towards the base with an acute apex, apical cell hyaline, bearing 3 appendages. Appendages 10–13.5 μm long, hyaline, simple, filiform, similar in length or shorter than conidia.
Culture characteristics: On PDA, slow growing, attaining 15 mm in 7 days at 18 °C, circular, margin serrate, from above white, reverse greyish-white.
Material examined: ITALY, Province of Forlì-Cesena [FC], near Camposonaldo—Santa Sofia, on dead cone attached to Picea excelsa (Pinaceae), 6 May 2013, Camporesi Erio, IT1226 (HKAS96302, new host record), living culture KUMCC 15-0127.
GenBank Numbers ITS:KY 474383.
Notes: This is the first record of Truncatella sparti on Picea excelsa. This species was introduced by Senanayake et al. (2015) from the host Spartium with both sexual and asexual morphs illustrated. Truncatella spartii is closely related to T. hartigii (Tubeuf) Steyaert, T. helichrysi (Severini) B. Sutton and T. restionacearum S. Lee & Crous, but differs in the apical cell characteristics of its conidia (Lee et al. 2006; Senanayake et al. 2015).
Myrmecridiales Crous
Myrmecridium Arzanlou, W. Gams & Crous.
The genus Myrmecridium was described by Arzanlou et al. (2007) with the type species M. schulzeri. To date, 10 species of Myrmecridium have been described (Peintner et al. 2016). Species belonging to this genus are characterized by the production of obovoid or fusiform conidia, tapering towards a narrowly truncate base, hyaline mycelium with pale to unpigmented, and pimple-like denticles. They are frequently isolated from soils and plant tissues (Crous et al. 2011; Jiea et al. 2013; Peintner et al. 2016). Some species possess potent antimicrobial activity (Zhang et al. 2012a). Molecular data have been used to evaluate species of Myrmecridium (Crous et al. 2015a; Réblová et al. 2016; Peintner et al. 2016). In a previous study a new species, Myrmecridium fluviae, was reported from a freshwater sample from Yeongsan River in Gwangju, Korea (Hyde et al. 2016).
While examining the diversity of fungi from freshwater sample of Yeongsan River in Gwangju, Korea, a new species was isolated and is described here based on morphological characteristics and phylogenetic analyses (Fig. 98).
Phylogenetic tree of Myrmecridium fluviae CNUFC-YR61-1 and CNUFC-YR61-2 and related species based on maximum likelihood analysis of a ITS, b LSU sequences. Sequences of Veronaeopsis simplex were used as outgroup. Numbers at the nodes indicate the maximum likelihood bootstrap values (>50%) from 1000 replications. The bar indicates the number of substitutions per position. New taxa are in blue and ex-type strains in bold
Myrmecridium fluviae Hyang B. Lee & T.T.T. Nguyen, sp. nov.
MycoBank number: MB 818334; Facesoffungi number: FoF 2806, Fig. 99
Myrmecridium fluviae (CNUFC-YR61-1, holotype). a, d Colonies in potato dextrose agar (PDA) b, e Colonies in oatmeal agar (OA) c, f Colonies in malt extract agar (MEA) (a–c obverse view, d–f reverse view), (g–q light microscope, r–v SEM). g–q Conidia and conidiophores (i, j, s, t lateral production of spore on conidiophore and k–n, r apical production of conidia on conidiophore). v Rachis with scattered, pimple-shaped denticles (yellow arrow). Scale bars g = 50 μm, h–q = 20 μm, r–v = 3 μm
Etymology: fluviae. Referring to fresh water from which the species was first isolated.
Holotype: CNUFC-YR61-1
Sexual morph Undetermined. Asexual morph Colonies on OA growing very slowly, grey olivaceous, reaching 15–17 mm diam. at 25 °C after 7 days incubation; reverse olivaceous black with a white margin. Conidiophores arising vertically from aerial hyphae, straight to irregular, branched, septate, varying greatly in length, 3–4.5 μm diam. Conidiogenous cells cylindrical, holoblastic, polyblastic, forming a rachis with scattered pimple-shaped denticles. Conidia produced laterally or apically, obovoid to fusoid, wall smooth to slightly rough, 7.5–12 × 3–4 μm. On PDA growth is faster than on OA and MEA, greenish-olivaceous, reverse olivaceous-buff; on MEA, surface greyish-sepia.
Material examined: REPUBLIC OF KOREA, Jeonnam Province, Yeongsan River located in Gwangju (3510′N 12655′E), from a freshwater sample, 15 February 2016 (CNUFC-YR61-1, preserved as glycerol stock at −80 °C in the Chonnam National University Fungal Collection; isotype in Culture Collection of Nakdonggang National Institute of Biological Resources (NNIBR), Sangju, Gyeongbuk Province); living culture (ex-type) deposited at Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC:SF:12423).
GenBank Numbers CNUFC-YR61-1 LSU:KX839677; ITS:KX839678. CNUFC-YR61-2 LSU:KX839676; ITS:KX839679.
Culture characteristics: The isolate grew over a wide range of temperatures with varying growth rates on PDA, MEA and OA with average growth rates of 2.8, 2.5, and 2.2 mm per 24 h, respectively, for CNUFC-YR61-1. Optimal growth around 25 °C to 27 °C, slow growth at 10 °C, and no growth at 35 °C.
Notes: Myrmecridium fluviae is morphologically distinct from other species, especially from M. phragmitis and M. flexuosum by having larger conidia with smooth to slightly rough-walls, sometimes forming a spiny layer. Our phylogenies provide sufficient evidence to justify the new species, albeit not strong, possibly due to inadequate taxon sampling and sequence data from other genes.
Xylariaceae Tul. & C. Tul.
The family Xylariaceae was introduced by Tulasne and Tulasne (1963) using the term “Xylariei” and the rank of the name was not certain as it did not address the family concept (Stadler et al. 2013). Even though the exact number of currently accepted taxa varies, the recent outline of Xylariaceae (Maharachchikumbura et al. 2015) accepted 87 genera. The segregation of Xylariaceae into the subfamilies Hypoxyloideae and Xylarioideae have been supported by molecular data (Tang et al. 2009; Stadler et al. 2013; Daranagama et al. 2015; Maharachchikumbura et al. 2015). The xylariaceae are mainly saprobes, while several species can also be found as pathogens and endophytes.
Rosellinia De Not.
Rosellinia was introduced by De Notaris (1844) with R. aquila (Fr.) Ces. & De Not. as the type species. According to the world monograph by Petrini (2013) only 142 species are accepted, of which 37 species are described as new species (Fig. 100).
Consensus tree resulting from a maximum likelihood analysis of a combined ITS, RPB2 and LSU sequence alignment for taxa in Rosellinia and other selected isolates of Xylariaceae. Type species are indicated in black bold and the new species is in blue bold. Maximum Parsimony bootstrap support values (MP above 50%), RAxML bootstrap support values (ML above 50) and Bayesian posterior probabilities (BYPP above 0.9) are given at the nodes (MP/ML/BYPP). The scale bar represents the expected number of changes per site. The tree is rooted to Lopadostoma turgidum CBS 133207
Rosellinia mearnsii Tennakoon, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF552779; Facesoffungi number: FoF 2718, Fig. 101
Etymology: Name reflects the host Acacia mearnsii, from which the holotype was collected.
Holotype: MFLU 16-1382
Saprobic on dead stem of Acacia mearnsii De Wild. Sexual morph Stromata globose, with a conical pointed top, 700–750 µm high × 550–580 µm diam. (\( \bar{x} \) = 735 × 565 μm, n = 10), black, shiny, solitary, aggregated into small groups, carbonaceous, flattened base, surrounded by grey to pale brown, woolly to felty subiculum, confined to the stromatal base, ostiolate. Ostioles black, distinctively papillate. Ascomata, globose, uni-loculate, 550–625 µm high × 390–485 µm diam. (\( \bar{x} \) = 576 × 445 μm, n = 20). Peridium black, thick-walled, with several compressed cell layers, carbonaceous. Ascomata 600–650 × 550–580 µm (\( \bar{x} \) = 635 × 565 μm, n = 10), nearly semiglobose, surface convex, dark, smooth, with a central black papilla and ostiole. Peridium black, thick walled, compressed and carbonaceous. Paraphyses at base (3.6–)3.8–4.6(–4.9) μm wide (\( \bar{x} \) = 4.2 μm), distinct septate, slightly longer than asci, filamentous, unconstructed and hyaline. Asci (198.2–)202–224(–232.5) × (9.6–)11.5–14.2(–15.5) μm (\( \bar{x} \) = 215 × 12.5μm, n = 20) 8-spored, bearing part cylindrical, short pedicellate, with barrel-shaped, subapical, J + ring, 15.6–15.9 µm high, 7.9–8.4 µm diam., apically rounded with well-developed ocular chamber. Ascospores (44.7–)46.2–53.4(–56.3) × (7.9–)8.2–8.8(–9.1) μm (\( \bar{x} \) = 49.3 × 8.6 μm, n = 20), overlapping, inequilaterally fusiform, uni-seriate, initially hyaline to slight brown, becoming brown colour, aseptate, straight to curved, smooth walled with prominent guttules. Asexual morph Undetermined.
Material examined: CHINA, Yunnan Province, Xishuangbanna, Nabanhe, dead branch of Acacia mearnsii (Mimosaceae), 20 November 2015, D.S. Tennakoon, DXH 21 (MFLU 16-1382, holotype; HKAS 93712, isotype).
GenBank Numbers LSU:KY514060; ITS:KY514059; RPB2:KY514061.
Notes: Rosellinia mearnsii resembles R. lamprostoma in having asymmetrically ellipsoidal to slightly fusoid, light brown ascospores (Petrini 2013). However, R. mearnsii differs from R. lamprostoma in its globose ascomata and prominent guttules in the ascospores, whereas stromata in R. lamprostoma are conical, pear shaped and ascospores with inconspicuous guttules (Petrini 2013). The size ranges of ascomata also differ (550–625 μm high × 390–485 μm diam., in R. mearnsii and 700–1050 μm high × 625–1250 μm diam., in R. lamprostoma). Rosellinia mearnsii is also reminiscent to R. capetribulensis, which has fusiform, brown ascospores and a barrel-shaped apical ring (Bahl et al. 2005). However, in R. capetribulensis, the asci are clavate as compared to the cylindrical asci in R. mearnsii. Although the ascospores in R. mearnsii and R. capetribulensis different in length, the spores of R. capetribulensis are 103–155 × 15–22.5 μm, whereas those in R. mearnsii are 46.2–53.4 × 8.2–8.8 μm. Furthermore, Rosellinia mearnsii resembles R. chiangmaiensis in having ellipsoidal to slightly fusoid, brown ascospores, but R. chiangmaiensis can easily be distinguished from R. mearnsii in having an inverted hat-shaped apical ring and ascospores with a thin mucilaginous sheath (Li et al. 2016).
Phylogenetic analyses of combined ITS, LSU and RPB2 sequence data show that R. mearnsii clusters with R. lamprostoma with strong support (100% ML, 100% MP, 1.00 BYPP, Fig. 101). Besides the morphological differences, R. mearnsii differs from R. lamprostoma in having six ITS base pair and seven RPB2 base pair differences.
Sordarimycetes genera, incertae sedis
Evlachovaea B.A. Borisov & Tarasov
The genus Evlachovaea was established by Borisov and Tarasov (1999) and since then this genus is a monotypic with E. kintrischica Borisov and Tarasov as the type species. Only one species (Evlachovaea kintrischica B.A. Borisov & Tarasov 1999) has so far been reported from this genus (Index Fungorum 2017) (Figs. 102, 103).
Phylogram generated from maximum likelihood method based on ITS sequences: The tree with the highest log likelihood (-2225.7653) is shown. One-thousand bootstrap replicates were analyzed to obtain nodal support values. The novel species is shown in blue. The tree is rooted with Paecilomyces carneus, P. lilacinus, P. marquandii and Metarhizium anisopliae. Phylogenetic analyses were conducted in MEGA6 (Tamura et al. 2013)
Phylogram generated from maximum likelihood method based on TEF1 sequences. The evolutionary history was inferred by using the maximum likelihood method based on the Kimura 2-parameter model (Kimura 1980). The tree with the highest log likelihood (−3415.2087) is shown. One-thousand bootstrap replicates were analyzed to obtain nodal support values. The novel species is shown in blue. The tree is rooted with Paecilomyces lilacinus, P. marquandii and Metarhizium robertsii
Ascomycota families incertae sedis
Evlachovaea indica P.N. Singh, A. Baghela, S.K. Singh & S. Amir, sp. nov.
MycoBank number: MB 816184; Facesoffungi number: FoF 2807, Figs. 104, 105
Evlachovaea indica (AMH 9760, holotype) on PDA. a Fungal growth appeared near prothorax (arrow). b Colony morphology (front view). c Colony morphology (reverse view). d Basipetal chain of conidia on PDA showing zipper pattern. e enlarged view of cylindrical and elongated conidia. f Conidiophores producing phialides in whorls with intact conidia. g Enlarged view of phialides and intact conidia. h Branched conidiophore bearing conidia in basipetal chains. i Apical region of conidiophore extends to form long whip like structure producing unilateral short phialides (arrow). j Chlamydospores in chain. Scale bars d–j = 10 µm
Etymology: specific epithet ‘indica’ refers to the country of origin.
Holotype: AMH 9760
On live Mantispa sp. in terrestrial habitats. Asexual morph Conidiophores mononematous, unbranched to branched, sometimes apical region extends to form long whip like structure producing solitary and short conidiogenous cells unilaterally or verticillately, smooth-walled, hyaline, 25–80 × 1.5–2.5 µm (\( \bar{x} \) = 47 × 3.29 µm, n = 30). Conidiogenous cells phialides in whorls of 2–8, or more, occasionally solitary on conidiophores or arising from a vegetative hypha, with slightly inflated and narrowed towards base, smooth-walled, hyaline, tapering with long slender neck, 8–40 × 1–2.39 µm (\( \bar{x} \) = 16.24 × 1.49 µm, n = 30). Conidia produced in long basipetal chains, forming a zip-like pattern, oval or subglobose, broadly ellipsoidal to clavate, smooth-walled, hyaline, 1.75–9 × 1–3 µm (\( \bar{x} \) = 6.40 × 2.07 µm, n = 30). Sexual morph Undetermined.
Culture characteristics: on semi-synthetic agar medium-sucrose (HAM–S) reaching 5.5 cm diam. in 14 days at 25 °C. After two weeks of incubation with irregular margin, smooth surface, cottony, puffy, umbonate to pulvinate, white (1A1-2) towards margin, pinkish-white at centre (7A2), rosy vinaceous with age, raised, sometimes reaching about 1 cm high, and touching upper lid of Petri plates. Reverse light yellow to pale orange (4A4–5A3) towards centre, yellowish-white (4A2) towards margin, wrinkled; hyphae septate, unbranched to branched, pigmented, smooth and thin walled 2.5–4.35 µm wide.
Material examined: INDIA, Maharashtra, Pune District, on live Mantispa sp. (Mantispidae) 30 August 2012, P.N. Singh; (AMH 9760 holotype-dried colony on PDA); ex-type living culture, NFCCI 3970, National Fungal culture Collection of India-WDCM 932);
GenBank Numbers ITS:KU983517; TEF1:KU983516.
Notes: Colony characters and growth rates of E. indica vary when grown on HAM medium supplemented with different carbon sources, viz., arabinose, galactose, glucose, glycerol, fructose, lactose, maltose and sucrose (Fig. 106). Arabinose favored maximum radial growth of colony (56–60 mm) on pre-defined incubation conditions of 25 °C, and for 14 days, followed by galactose (55–56 mm), lactose (54–56 mm), sucrose (52–55 mm). Colony characters and growth pattern of E. indica resembles colony characters of E. kintrischica, but the growth rate was faster in E. indica compared to E. kintrischica on most of the carbon sources used in present study. In addition, E. indica and E. kintrischica expressed very prominent wrinkles on reverse sides of the colony on glucose, glycerol, and sucrose. Evlachovaea indica expressed less prominent wrinkles on maltose compared to distinctly prominent in E. kintrischica. Both the species did not produce wrinkles on galactose and lactose. Besides, E. indica produced no wrinkles on fructose, while it has been reported prominent in E. kintrischica.
Evlachovaea indica is morphologically distinct in having smaller and slender conidiophores, and significantly larger conidia from the type species, E. kintrichica, and from Evlachovaea-like isolates group I and II (Humber et al. 2013) in having longer conidia and conidiogenous cells. However, length–width ratio of conidia (L/W 1.8–3.4) and conidiogenous cells (L/W 4.8–32.9) in E. indica is significantly greater as compared to L/W ratio of conidia (L/W 1.3–2.5) and conidiogenous cells (L/W 2–7) of E. kintrichica. Proposed species is also distinct from type species in lacking synnemata and triangular or pomegranate like conidia (Borisov and Tarasov 1999).
The internal transcribed spacer (ITS)-rDNA and translation elongation factor (TEF1) regions have been shown to be taxonomically and phylogenetically informative for various entomopathogenic fungi such as Beauveria, Cordyceps, Isaria, Metarhizium and Paecilomyces (Liu et al. 2002; Luangsa-ard et al. 2005). These two gene sequences were also found to be useful in unambiguously placing the proposed isolate in a unique place in the phylogenetic trees. Though, it shows some level of relatedness with Evlachovaea-like isolates group I, is still distinct from Evlachovaea-like isolates group I, II, and Evlachovaea kintrischica. The phylogenetic analysis clearly establishes Evlachovaea indica as a novel species. Humber et al. (2013), utilized above mentioned genes to place the evlachovaea-like Brazilian isolates to Cordycipitaceae. However, they could not determine the correct species identifications, possibly due to the unavailability of TEF1 and other gene sequences of species of Isaria and other allied taxa. This indicates that a comprehensive analysis of more of genes, in addition to, ITS and TEF1 and their phylogenetic analysis needs to be undertaken to resolve the phylogenetic ambiguity of this group of fungi. Until the phylogenetic ambiguity is resolved, the present isolate is placed as a novel species under the genus Evlachovaea Borsov and Tarasov. To our knowledge, this fungus was isolated for the first time from a four-winged live Mantispa sp. collected from Western Ghats region in India. Moreover, this genus is being reported for the first time from India.
Pezizales J. Schröt.
Helvellaceae Fr.
Helvella L. 1753
Species of Helvella L. are widely distributed in the northern hemisphere (e.g. Hyde et al. 2016; Zhao et al. 2015, 2016a, b, c). Since Linnaeus (1753) proposed the generic name Helvella, numerous studies have been conducted to address the taxonomic issue and infrageneric division in Asia, Europe and North America (e.g. Dissing 1966; Weber 1972; Häffner et al. 1987; Abbott and Currah 1997; Hyde et al. 2016; Zhao et al. 2015, 2016a, b, c).
Helvella costifera Nannf., Schriften der Naturforschenden Gesellschaft zu Leipzig 1: 114 (1953)
Facesoffungi number: FoF03127, Fig. 107
Reference specimen: HKAS 87704
Symbiotic in the coniferous, mixed or deciduous forests. Sexual morph Pileus shallowly cupulate to cupulate, margin expanding at maturity, 0.5–2 cm high, 0.5–4 cm broad, hymenium even, pale grey to greyish-brown when fresh, dark brown to blackish-brown when dried, receptacle surface above concolorous with hymenium, lower part white to cream, pubescent, with branched, sharp or rarely blunt ribs, ribs reaching from the half to the pileus edge. Stipe 0.5–6 cm long, 0.2–2 cm diam., expanding and merging with apothecium, below equal or base rarely slightly enlarged, costate or lacunose, sharp-edged ribs, white to light yellow brown, pubescent. Medullary excipulum 350–550 µm broad, of textura intricata, hyaline, composed of 3–5 µm diam., hyaline, walls thickened, J+. Ectal excipulum 80–110 µm broad, of textura angularis, yellowish-brown, terminal cells 15–20 × 7–12 μm, blue in cotton blue, J−. Stipitipellis 80–100 µm, of textura angularis, yellowish-brown, terminal cells 16–25 × 9–15 μm, blue in cotton blue, J−. Asci pleurorhynchous, 8-spored, subcylindrical to clavate, with apex rounded, 230–300 × 12–16 µm, J− in Melzer’s reagent, blue in cotton blue. Paraphyses filiform, 3–4 µm diam., slightly exceeding the asci, apex enlarged, 6–7 µm diam., brown, J−. Ascospores [60/2/2, in H2O] 15–19 × 10–13(–13.5) μm [Q = 1.20–1.64, Q = 1.39 ± 0.08)], broadly ellipsoid to ellipsoid, smooth-walled under the light microscope, uniguttulate. Asexual morph Undetermined.
Habitat: Scattered or gregarious on soil in coniferous forests.
Distribution: Europe (Dissing 1966; Häffner et al. 1987); North America (Abbott and Currah 1997).
Material examined: CHINA, Yunnan Province, Panlong County, under Pinus spp., alt. 2000 m, 10 July, 2014, Qi Zhao 1643 (HKAS 87704, reference specimen designed here), Qi Zhao 1645 (HKAS 87706).
Notes: Helvella costifera has a greyish hymenium, pubescent receptacle surface with blunt edged ribs and a non-lacunose stipe, pleurorhynchous asci, broadly ellipsoid ascospores 15–19 × 10–13(–13.5) µm, slightly enlarged paraphyses (6–7 µm), and ectal excipulum J+ in Melzer’s reagent. Helvella costifera can be confused with H. acetabulum, which also have cupulate pileus and ribbed stipe (Dissing 1966; Häffner et al. 1987; Abbott and Currah 1997). However, H. acetabulum is distinguished by its brownish cup-shaped pileus and ribbed or lacunose stipe, with ribs reaching to the half or to the receptacle surface edge (Abbott and Currah 1997).
Helvella crispoides Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF 551935; Facesoffungi number: FoF 01338, Fig. 108
Etymology: crispoides is proposed because of the eastern Asian mushroom’s similarity to Helvella crispa.
Holotype: HKAS 90535
Sexual morph Pileus irregularly lobed to saddle-shaped, up to 4 cm high, 1–3 cm broad; margin inflexed to receptacle surface when young, expanded at maturity; hymenium undulate-rugose, cream, becoming brownish-yellow when dried; receptacle surface pubescent, with blunt ribs covering almost 1/3 of the pileus, grey, becoming greyish when dried. Stipe 7–10 cm long, 1–2 cm thick, deeply ribbed, with cross-veins and pockets, finely pubescent, slightly brittle, white, becoming cream when dried; basal mycelium white. Medullary excipulum 280–450 µm broad, of textura intricata, hyaline, composed of 2.5–4 µm hyphae broad, J−. Ectal excipulum 80–150 µm broad, outermost cells catenuliform in long fascicled tufts, hyaline, evenly blue in cotton blue, with 23–55 × 9–18 µm cylindrical to subclavate, slightly thick-walled, J− end cells. Stipitipellis 60–100 µm, hyaline, terminal cells 22–32 × 10–18 μm, clavate, J−. Asci 250–290 × 13–15 µm, pleurorhynchous, 8-spored, subcylindrical to clavate. Paraphyses filiform, 4–5 µm broad, slightly exceeding the asci, apex enlarged, 6–7.5 µm broad, slightly blue in cotton blue, J−. Ascospores [100/4/4, in H2O] 15–17(–17.5) × 9.5–11.5(–12) μm [Q = (1.4) 1.45–1.7(–1.78), Q = 1.56 ± 0.09)], ellipsoid, smooth-walled under the light microscope, uniguttulate. Asexual morph Undetermined.
Habitat: Solitary or gregarious on the ground. In northern Thailand in conifer forest dominated by Pinus kesiya, and in southwestern China in conifer forest dominated by P. yunnanensis Franch.
Distribution: Only known from Eastern Asia.
Material examined: THAILAND, Chiang Mai Province, Chiang Mai County, Mushroom Research Center, in coniferous forest dominated by Pinus kesiyi, alt. 640 m, 31 August 2015, Qi Zhao 2661 (HKAS 90535, holotype). CHINA, Yunnan Province, Yulong County, alt. 1840 m, 21 Aug 2010, Q. Zhao 8292 (HKAS 69968).
Notes: Gross morphological differences between H. crispoides and H. pseudoreflexa Zhao et al. (2015) are subtle, the broad medullary excipulum of H. crispoides is usually 280–450 µm, while in the latter species it is generally 200–280 µm. The main differences between them are probably their ecological preferences, H. crispoides is associated with coniferous forests, while H. pseudoreflexa is mainly distributed in deciduous forests (Zhao et al. 2015). Both H. crispoides and H. papuensis Weber exhibit an irregular saddle-shaped pileus, which is deeply ribbed, with cross-veins and stipe pockets. However, H. papuensis is a tropical species originally described from Papua New Guinea, in the southern hemisphere by Dissing (1979). It is diagnosed by oxide yellow to brownish-yellow hymenium, with a villose to tomentose sterile surface which is concolorous or slightly darker than the hymenium surface (Dissing 1979). Helvella crispoides has a cream hymenium and a grey, pubescent, receptacle surface.
Sarcoscyphaceae Le Gal ex Eckblad
Cookeina Kuntze
Cookeina is a genus of cup fungi in the family Sarcoscyphaceae, members can be found in tropical and subtropical regions of the world. Species in the Cookeina have a deep, cup-shaped to funnel-shaped fruiting bodies, or apothecia, may be found on fallen branches of angiosperms, trunks, and sometimes on fruits (Weinstein et al. 2002) (Fig. 109).
Phylogenetic relationships inferred from maximum parsimony analysis of ITS-rDNA sequences of 22 taxa. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The new Sri Lankan record: Cookeina tricholoma having GenBank Accession Number KY649459 and Herbarium Number MFLU 12-2390 is shown in bold and blue. The tre is rooted with Microstoma floccosum. Evolutionary analysis was conducted in PAUP 4.0b 10
Cookeina tricholoma (Mont.) Kuntze, Revis. gen. pl. (Leipzig) 2: 849 (1891)
Facesoffungi number: FoF 3136, Figs. 110, 111
Apothecia deeply cup-shaped, 4–16 mm diam. and up to 43 mm high, more or less long stalked, funnel-shaped. Hymenial surface smooth, pale yellow-orange or pale pink-orange to orange-reddish. Outer surface covered with long, whitish or very faintly orange brown hairs (1–2 μm diam.), obviously denser at the margin, grouped in bundles, concolorous to the hymenial surface, sometimes rough; margin smooth, whole, often introflexed. Stalk cylindrical, long, slightly expanded at the top, about 3 mm diam., smooth, white-cream. Flesh whitish or very pale whitish-orange, waxy. Ascospores variable in size, 33–36 × 13–16 μm, fusiform, few irregularly citriform, occasionally with slightly mucronate extremities, with 10–20 longitudinal thin striations, straight and more or less parallel, wall up to 2–3 μm thick, usually with a large cylindrical-ellipsoidal oil drop, rarely 2 oil-drops, hyaline, uniseriate in the asci. Asci 300–400 × 15–20 μm, 8-spored, cylindrical, wall 1–2 μm thick, with rapidly tapered base that ends in a thin and usually curved appendage 15–35 μm long, not amyloid, suboperculate. Paraphyses 2–3 μm diam., thread-like, cylindrical, simple or pluri-forked. Subhymenium not noticeable. Medullar excipulum 230–300 μm thick; textura intricata, hyphae of 6–9 μm diam., interwoven, variously anastomosed, septate, hyaline or slightly yellowish. Ectal excipulum 300–350 μm thick; textura globulosa, cells 18–30 μm diam., rounded, rarely angular, globose or slightly ellipsoidal, wall thin or somewhat thickened, grey-brown or pale brown. Presence of catenulate marginal cells, apex irregularly shaped or enlarged. Hairs up to 260 μm long, 40–50 μm large at the apex and 100–150 μm at the base, arising from the medullar excipulum, formed by more or less parallel fenced hyphae 10–15 μm wide, cylindrical or slightly enlarged in some points, with rounded, capitulate tip, irregularly thickened in some points, wall 2–4 μm thick, septate, yellow–brown, less dense near the top and more numerous and shorter near the base.
Habit, habitat and distribution: as groups of more or less close fruit-bodies, usually on dead or decaying wood, May–August. Our collection was collected on a decaying wood at Hanthana Mountains Range, Peradeniya.
Specimens examined: SRI LANKA, Kandy District, Hanthana Mountains Range, 19 June 2012, Samantha C. Karunarathna (MFLU 12-2390, reference specimen designated here).
GenBank Number ITS:KY649459.
Notes: The main distinguishing characteristics of C. tricholoma are pale yellow-orange apothecia and large ascospores. However, colorful C. sulcipes (Berk.) Kunze, are usually orange-red, cherry-red or scarlet, might be confused with C. tricholoma; its ascospores, although with the same kind of ornamentation, are smaller in size (25–30 × 11–14 μm). This is the first report of C. tricholoma with the molecular phylogenetic confirmation from Sri Lanka (Fig. 111). We therefore designate it as a reference specimen (sensu Ariyawansa et al. 2014b).
Basidiomycota
Agaricomycetes Doweld
Agaricales Underw.
Agaricaceae Chevall.
The family Agaricaceae was introduced by the French botanist François Fulgis Chevallier in 1826 (Chevallier 1826). Named after the type genus Agaricus, the family was originally circumscribed by Carl Linnaeus in 1753 in his work on Species Plantarum.
Amanita Pers.
The morphologically diverse and monophyletic genus Amanita Pers. (Persoon 1797; Bas 1969; Zhang et al. 2004; Wolfe et al. 2012b; Tulloss et al. 2016) is dominantly mycorrhizal however, at least one species is saprobic (Wolfe et al. 2012a) and another three dozen or so are non-mycorrhizal with precise trophic modes unclear. The genus is divided into two subgenera: Amanita subg. Amanita (with inamyloid spores) comprising three sections, viz sect. Amanita, sect. Caesareae Singer, sect. Vaginatae sensu Yang (1997) and A. subg. Lepidella (E.-J. Gilbert) Veselý emend. Corner & Bas (with amyloid spores) includes four sections namely, sect. Amidella (E. J. Gilbert) Konrad & Maubl., sect. Lepidella sensu Bas (1969), sect. Phalloideae (Fr.) Quél., and sect. Validae (Fr.) Quél. More than 530 species are described worldwide with valid accepted names and about 50 taxa are reported from India (Yang 2000; Bhatt et al. 2003; Tulloss and Yang 2016). Here, we describe two new species belonging to subg. Amanita sect. Vaginatae. They were collected from the northwestern Indian Himalayas and are presented here with morphological and molecular data. The respective single nrLSU gene tree is presented in Fig. 112.
RAxML tree and putative relationships of Amanita subg. Amanita sect. Vaginatae inferred from analysis of LSU sequence data. Bootstrap support values (>50%) obtained from maximum likelihood analysis are shown above or below the branches at the nodes. The new taxa are highlighted in bold and blue font in the tree. GenBank numbers accession numbers are provided after each species name and followed by country of origin. Amanita caesareae (Scop.) Pers. served as the outgroup taxon. The tree is artificial in that it embodies two somewhat distant clusters of taxa associated with the two new taxa by morphology and/or BLAST searches. This results in a compact single figure and, also, in low support for nodes near the tree’s root
Amanita cornelii Mehmood, K. Das, Iqbal Hosen, Tulloss, & R.P. Bhatt, sp. nov.
MycoBank number: MB 817887; Facesoffungi number: FoF 2946, Figs. 113, 114 Etymology: in honor of Cornelis Bas for his major revision of the study of Amanita.
Holotype: CAL 1337
Pileus 40–60 mm wide, initially convex then plano-convex, umbonate, dark brown (8F4–6) over centre, otherwise having brown to greyish-brown (6D3) rather coarse and irregular radial streaks on pallid ground, smooth, tacky, shiny. Pileus context 3–5 mm thick, thinning evenly toward margin, white, unchanging when exposed or bruised. Margin short-striate up to 10 mm, non-appendiculate. Universal veil on pileus absent. Lamellae free, subdistant (7–8 per 10 mm at margin), 3 mm broad, white (1A1) in mass and in side view. Lamellulae scarce, truncate, of at least 2 lengths, unevenly distributed. Stipe 100–120 × 8–12 mm, slightly tapering upward, with white (2A1) ground, densely covered by light brown to gray (6F3–5) fibrils arranged in “flame” or “zebroid” pattern, with fibrils darkening to nearly black where handled. Stipe context white, hollow, unchanging when bruised or exposed. Partial veil absent. Universal veil at stipe base saccate, 33–39 × 18–24 mm, white (1A1–2A1) thick, membranous, persistent. Spore print white. Odor indistinct. Taste not recorded. Basidiospores [50/2/1] (8–)9–11.5(–13.5) × (7.5–)8–10.5(–13) μm (L = 9–10 μm; L′ = 9.31 μm; W = 8–10 μm; W′ = 8.92 μm; Q = (1–)1.02–1.10(–1.25); Q = 1.05–1.11; Q′ = 1.06), hyaline, colourless, thin walled, inamyloid, globose to subglobose, rarely broadly ellipsoid, with uniguttulate contents, apiculus lateral to sublateral 1.5 × 1 μm. Basidia (39–)42–51(–57) × (11–)12–14(–15) μm, 2- to 4-spored, thin-walled, colourless; sterigmata up to 4 × 1.5 μm. Clamp connections not observed at the base of Basidia. Subhymenium wst-near = 52–75 μm thick, wst-far = 60–88 μm, Basidia arising from small inflated cells (up to 12 × 14 μm wide). Lamellae edge sterile; inflated cell clavate or pyriform, 35–50 × 22–31 μm, colourless, thin walled; clamp connections not observed Hymenophoral trama bilateral, divergent; wcs = 50–80 μm; well rehydrated, filamentous undifferentiated hyphae (3–16) μm wide; vascular hyphae 11–15 × 3–4 μm wide. Pileipellis 130–195 μm thick, in two layers, with gelatinized colorless suprapellis (40–55 μm thick), and ungelatinized subpellis (95–130 μm thick); ungelatinized filamentous undifferentiated hyphae 2–6 μm wide, subradially arranged densely arranged in subpellis, with brown intracellular pigment vascular hyphae 7–10 × 2.8–3.5 μm. Pileus trama filamentous, undifferentiated hyphae 2.5–16 μm wide, constricted at septa, thin-walled, branching, forming loose open matrix; acrophysalides common up to 120 × 23 μm, thin-walled, hyaline, vascular hyphae rare up to 15 × 5 μm. Exterior surface of universal veil (on stipe base) filamentous, undifferentiated hyphae 3–12 μm wide; inflated cells subclavate (up to 84 × 34 μm), narrowly ellipsoid to elongate (e.g. 120 × 18 μm). Interior surface of universal veil (on stipe base) filamentous, undifferentiated hyphae 3–9 μm wide; inflated cells subglobose to ovoid (up to 127 × 119 μm), infrequent. Stipe context longitudinally acrophysalidic; filamentous undifferentiated hyphae (2–9 μm wide); acrophysalides dominating, 151–190 × 31–42 μm; clamp connections not observed.
Habitat and distribution: Under Quercus sp., in temperate mixed forest dominated by Abies and Quercus.
Material examined: INDIA, Uttarakhand, Rudraprayag District, Baniyakund, 2630 m, N3028.914′ E7910.854′, 14 July 2015, T. Mehmood, TM 15–625 (CAL 1337, holotype); 18 July 2015, ibid. (TM 15–693, paratype).
GenBank Number ITS: KX528072.
Notes: The combination of macro- and micromorphological features such as inamyloid spores, saccate volva, absence of a bulb and absence of Basidia clamps place Amanita cornelii in Amanita [subg. Amanita] sect. Vaginatae sensu Yang (Yang 1997). In the field the present species is distinct from other known species of Amanita sect. Vaginatae by its pileus with coarse, irregular, radial, brown streaks. In eastern Asia, Amanita cornelii might be confused with Amanita brunneofuliginea Zhu L. Yang (Yang 1997) (originally described from China); however, the latter has a pileus that is blackish-brown to brownish-black over the centre. The latter also differs by lacking coarse, irregular, brown stripes on the pale background on the pileus, bearing a white to dirty white patches of universal veil on the pileus, its having a saccate volva which is dirty white with plentiful pale-leather to orange spots on its outer surface and by its subglobose to broadly ellipsoid basidiospores “(10–)10.5–13(–14) × (9–)9.5–12(–12.5) μm, (Q = (1.04–)1.06–1.24(–1.26); Q′ = 1.13 ± 0.06)” (Yang 1997). Amanita lignitincta Zhu L. Yang (Yang 1997) (originally described from China), is somewhat similar to the present taxon with a wood brown to leather brown pileus; but it differs by its slightly depressed pileus, a contrastingly darker ring–like zone at the inner end of its marginal striations, by its larger basidiospores “(9.5–)10–13(–13.5) × (8.5–)9–12(–13) µm” (Yang 1997) and by lacking the unusual striping pattern of the present species. Our nrLSU-tree clearly indicates the genetic dissimilarities of Amanita cornelii from six taxa. BLAST search indicates relative closeness (96% identity with 95% query coverage) of A. cornelii to A. friabilis (GenBank Numbers KU248120, KU248119); (96% identity to 96% query coverage) of A. cornelii to A. basiana (GenBank Numbers KP258987); (96% identity with 94% query coverage) of A. cornelii to A. affin. fulva (GenBank Numbers HQ539697); (96% identity with 95% query coverage) of A. cornelii to A. olivaceogrisea (GenBank Numbers KU867877); (95% identity to 95% query coverage) of A. cornelii to A. affin. ceciliae (GenBank Numbers KU139438, KU139439); (97% identity with 95% query coverage) of A. cornelii to A. populiphila (GenBank Numbers KP221314, KP221304).
The European A. basiana Tulloss & M. Traverso (Tulloss and Traverso 2000) and A. friabilis (Karst.) Bas (Bas 1974) are easily segregated from the present species because, in those two taxa, hyphae provide extensive and persistent interconnection between the pileipellis and the universal veil (Bas 1974; Tulloss and Traverso 2000, 2001). Moreover, A. friabilis is separated from A. cornelii by its subglobose to elongate spores “Q = (1.06–)1.13–1.62(–1.89); Q = 1.23–1.43; Q′ = 1.34” (www.amanitaceae.org/?Amanita%20friabilis); and A. basiana also differs from the present taxon due to its broadly ellipsoid to ellipsoid spores Q = (1.08–)1.13–1.44(–2.34); Q = 1.21–1.33(–1.36); Q′ = 1.27 (www.amanitaceae.org/?Amanita%20basiana).
Volval differences also serve to segregate species that could be mistaken for A. ceciliae (European) and A. olivaceogrisea (European) from the present species. It should be noted that many species in eastern Asia, eastern North America and other regions have been mistaken for A. ceciliae in the past. This group have friable volva’s that quickly become gray with age often leaving warts and small patches on the pileus, fragments on the lower stipe, and a white cupulate structure on the stipe’s base (Bas 1984; Tulloss 1994, 2001). Amanita olivaceogrisea also differs from Amanita cornelii by its subglobose to broadly ellipsoid spores showing slightly higher Q values, i.e. Q = (1.03–)1.04–1.14(–1.27); Q = 1.09–1.11; Q′ = 1.10 (www.amanitaceae.org/?Amanita%20olivaceogrisea).
Amanita affin. fulva (North America) possesses an orange-brown pileus that becomes paler at the margin and brown to dark brown in the center, lacks the irregular striping pattern of the present species, and has a white external volva surface that becomes orange-brown to rusty in numerous spots. The matching GenBank Numbers sequence is a nearly perfect match to sequences derived from Newfoundland material presently treated under a provisional name by Tulloss (www.amanitaceae.org/?Amanita%20daimonioctantes).
Amanita populiphila Tulloss & Moses (Tulloss and Moses 1995), described from North America differs from the present taxon by an unstriped, white to pale yellowish pileus that takes on rusty stains with time; cream-colored Lamellae having pale orange or pinkish tint; occurrence of plentiful lamellulae and a weakly structured, white volva that tends to change color as the pileus does. Amanita populiphila has only been recorded with species of Populus [cottonwood and aspen] and has subglobose spores with slightly higher Q values than that of the present taxon; Q = (1–)1.04–1.20(–1.61); Q = (1.06–)1.08–1.15(–1.19); Q′ = 1.11. (www.amanitaceae.org/?Amanita%20populiphila). Macro- and micromorphology coupled with the LSU-based genetic distances, segregate Amanita cornelii from previously known species of Amanita sect. Vaginatae.
Amanita emodotrygon Mehmood, Tulloss, K. Das, Iqbal Hosen & R.P. Bhatt sp. nov.
Index Fungorum number: IF552463; Facesoffungi number: FoF 2947, Figs. 115, 116
Etymology: emodos (Grk., India) + trygon (Grk., dove), to indicate possible genetic grouping with North American A. trygonion Tulloss, Pastorino & Kudzma nom. prov. (www.amanitaceae.org/?Amanita%20trygonion)
Holotype: CAL 1338
Pileus 96–132 mm wide, initially convex then campanulate, initially brownish-grey to greyish-brown (7E2–3) and finally olive (1E4–4E7) smooth, dry, shiny. Pileus context 8–10 mm thick, first thinning slowly then rapidly toward margin, membranous at margin, off-white, unchanging when bruised or exposed. Margin with distinct regular veins or striations and corresponding grooves (up to 36 mm wide few veins forked more toward margin, non-appendiculate, whitish to greyish in between the grooves. Universal veil on pileus absent Lamellae free, crowded (11–13 per 10 mm at margin), 6 mm broad, initially white (1A1) fading pale yellow with age. Lamellulae truncate, of 3–4 lengths, unevenly distributed. Stipe 150–183 × 16–19 mm, equal or slightly tapering upward initially off-white then greenish-grey to greyish-green (1C2–3), with olive brown tinge toward the base. Context off-white, hollow, stuffed in button stage. Partial veil absent. Universal veil at stipe base saccate, 33–41 × 19–29 mm, white to off-white (1A1–2A1) with many olive-brown spots on exterior surface when age, bilimbate, membranous, thick at bottom thin toward margin. Spore print cream. Odor indistinct. Taste not recorded. Basidiospores [60/3/1] (8.5–)9.8–12(–13.5) × (8–)9.5–11(–13) μm (L = 10–11.5 μm; L′ = 10.9 μm; W = 9.5–10.5 μm; W′ = 10.2 μm; Q = (1–)1.05–1.12(–1.17); Q = 1.05–1.11; Q′ = 1.08), globose to subglobose, rarely broadly ellipsoid, inamyloid, hyaline, colourless, thin walled, with uniguttulate contents, apiculus lateral to sublateral to 1.5 × 1.2 μm. Basidia (46–)52–65(–70) × (14–)14.5–16(–17) μm, 2- to 4-spored, thin-walled, colorless; sterigmata 4–6 × 1.5–2 μm. Clamp connections not observed at the base of Basidia. Subhymenium wst-near = 35–65 µm; wst-far = 50–75 μm, Basidia arising mostly from inflated cells to irregular cell (up to 10 × 8 μm wide). Lamellae edge sterile; inflated cells; pyriform or clavate, 43–48 × 13–22 μm, colourless, thin-walled, infrequent; clamp connections not observed. Hymenophoral trama bilateral, divergent; wcs = 70–90 μm filamentous undifferentiated hyphae (4–9) µm wide; inflated cells 19–27 × 38–45 μm; vascular hyphae not observed. Pileipellis 125–190 μm thick, in two layers; slightly gelatinized colorless suprapellis (30–60 μm thick) of radially arranged filamentous undifferentiated hyphae 1–3 μm wide, thin-walled, colourless; nongelatinized subpellis (90–130) μm thick); filamentous undifferentiated hyphae 2–7 μm wide, subradially and compactly arranged with brown intracellular pigment; vascular hyphae not observed. Pileus trama filamentous, hyphae 2–12 μm wide, constricted at septa, slightly thick-walled, hyaline, branching, acrophysalides common up to 135 × 31 μm, slightly thick-walled, vascular hyphae up to 17 × 3.5 μm. Exterior surface of universal veil (on stipe base) filamentous, undifferentiated hyphae 4–12 µm wide, slightly thick walled, septate and frequently branched, colourless, hyaline; globose to subglobose cells up to 102 × 108 μm; ellipsoid cells up to 116 × 39 μm. Interior surface of universal veil (on stipe base) filamentous, undifferentiated hyphae 4–8 µm wide; inflated cells subglobose cells to 45 × 41 μm, clavate cells up to 78 × 23 μm, infrequent. Stipe context longitudinally acrophysalidic; filamentous hyphae 4–9 μm wide, acrophysalidic dominating, (151–190 × 31–42 μm); Clamp connections not observed.
Habitat and distribution: Solitary to scattered, under Pinus roxburghii in temperate coniferous forest of western Himalaya.
Specimen examined: India, Uttararakhand, Rudraprayag, Hariyali Devi Forest, 1651 m, N3015.955′E7903.719′, 27 July 2015, T. Mehmood, TM 15-659 (CAL 1338, holotype).
GenBank Number ITS:KX539266.
Notes: The combination of macro- and micromorphological features such as longitudinally acrophysalidic stipe context; bilateral, divergent lamella trama, inamyloid spores, absence of a stipe bulb and absence of Basidia clamps place Amanita emodotrygon in Amanita [subg. Amanita] sect. Vaginatae Sensu Yang (Yang 1997).
In the field this species is distinct from other known species of Amanita sect. Vaginatae by its greyish-brown to olive campanulate pileus, which is deeply grooved towards the margin, crowded white lamellae that becomes pale yellowish with age and occurrence in a coniferous forest under Pinus roxburghii. Micromorphologically, presence of globose to subglobose spores, branched, septate and thick-walled, filamentous hyphae in the universal veil at the stipe base are also quite striking. Based on the key in Tulloss (1994) and (www.amanitaceae.org/?stirps%20Pachyvolvata), there is a group of North American and European taxa that are morphologically similar to A. emodotrygon in having a large, robust basidiome and universal veil-strips Pachyvolvata of Tulloss. The relevant section of the key is largely based on cap colour and spore size and shape. Two of the taxa are described as having an olivaceous tint to the cap. Two are described as having a ring around the inner ends of marginal striations. Hence, gross morphology of this group is similar to that of A. emodotrygon. One of the species has both the cited pileus characters and is, hence, most similar to the present taxon:
Amanita magnivolvata Aalto (1974) (originally reported from Finland) is segregated from A. emodotrygon by its gray ring on the inner edge of the marginal striations, white Lamellae becoming gray with age and a white exterior surface of the universal veil, developing small, rusty, yellow spots. It occurs in mixed forest including Alnus, Betula, and Picea in Scandinavia and has been reported from Italy (Liguria). Its spores are subglobose to broadly ellipsoid and have comparatively higher Q values than occur in the present taxon-Q = (1.03–)1.06–1.36(–1.52); Q = 1.18. (www.amanitaceae.org/?Amanita%20magnivolvata)
Our nrLSU-tree clearly indicates the genetic dissimilarities of Amanita emodotrygon from three taxa. BLAST search indicates relative closeness (98% identity with 98% query coverage) of Amanita emodotrygon to Amanita trygonion (GenBank Numbers KU186810); (94% identity with 94% query coverage) of Amanita emodotrygon with Amanita penetratrix (GenBank Numbers KU186834, KU186833, KU186832, KU186831); (93% identity to 93% query coverage) of Amanita emodotrygon to Amanita myrmeciae (GenBank Numbers KU852505 and KU186806).
The relatively small number of taxa that have been sampled for nrLSU in section Vaginatae and the great difficulty in dealing with the genus by morphological means alone create an unusual situation in the present case, i.e. all of the sequences for which close BLAST comparisons exist in the case of A. emodotrygon are taxa described provisionally. Hence, we can say, at the time of writing, the present taxon is quite distinct from all published species of sect. Vaginatae for which sequence data exists.
Amanita trygonion Tulloss, Pastorino & Kudzma, nom. prov. is a small gray species of the sandy coastal plain in southeastern Texas, USA, where it occurs in Pinus dominated mixed forest and has subglobose to broadly ellipsoid spores with higher Q values than those of the present taxon-Q = (1.05–)1.10–1.33(–1.40); Q = 1.17–1.20; Q′ = 1.19. (www.amanitaceae.org/?Amanita%20trygonion)
Amanita penetratrix Tulloss & Kudzma, nom. prov. is a large species with a hard-fleshed, prominent umbo in the center of a gray to gray-brown to white pileus. It is known from a few sites in the northeastern USA. It occurs in mixed forest including Pinus strobus, Betula, Fagus, and Quercus and has spores that are dominantly subglobose to broadly ellipsoid with higher Q values than those of the present taxon-Q = (1.04–)1.05–1.25(–1.34); Q = 1.09–1.16; Q′ = 1.12. The primordium of this mushroom develops deep in forest soil. The unusual umbo allows penetration of up to 154 mm of soil dense with roots and is reminiscent of the perforatorium of species of Termitomyces. (www.amanitaceae.org/?Amanita%20penetratrix)
Amanita myrmeciae Tulloss, Kudzma & Albertella, nom. prov. is known only from association with eucalypts in the Blue Mountains of New South Wales, Australia. This species is unique among those discussed here by being a significant food source for ants of the genus Myrmecia. The species is known from one specimen with a light brown to brown pileus 62 mm wide and has subglobose spores with slightly higher Q values than those of the present taxon—Q = 1.06–1.18; Q 1.10. (www.amanitaceae.org/?Amanita%20myrmeciae)
Lepiota (Pers.) Gray
Lepiota (Pers.) Gray (Agaricaceae) is a large group of saprotrophic basidiomycetes in temperate and tropical areas, comprising six sections and an estimated 500 species (Singer 1986; Vellinga 2004; Kirk et al. 2008). A tree to three new species from Thailand is provided below (Fig. 117).
Bayesian tree based on ITS sequence alignment of Lepiota species represent from each section. Support values from Bayesian posterior probabilities values (BYPP) more than 0.95 are given above tree branches, and bootstrap percentage more than 50% from maximum likelihood analyses are indicated below the tree branches. New sequences from Thailand are as bold. Agaricus bisporus and Clarkeinda trachodes are outgroup taxa
Lepiota cylindrocystidia Sysouphanthong & K.D. Hyde, sp. nov.
Index Fungorum number: IF552903; Facesoffungi number: FoF 2948, Figs. 118, 119
Etymology: The name of this species “cylindricystidia” comes from its cylindrical Cheilocystidia.
Holotype: MFLU 12-2035
Pileus 16 mm, broadly convex with inflexed margin, covered with spine-like or pyramidal squamules over the whole surface, slightly crowded at center and more distant towards margin, squamules darker or yellowish-brown, light brown to golden-brown (5D5–8, 5E5–6) on greyish-yellow to greyish-orange (4B4–5, 5B4–5) fibrillose or floccose background; at marginal zone, fibrillose, concolorous with background. Lamellae white, free, ventricose, 1.5–2 mm wide, crowded, with white eroded edge. Stipe 38 × 2.5–3 mm, cylindrical or slightly wider at base and apex, covered with crowded fibrillose squamules from annular zone downward base, with squamules at basal zone, concolorous with those on pileus, with white to yellowish-white to pale yellow (4A2–3) fibrillose at annular zone upward from apex. Annulus an annular zone with fibrillose and fibrillose squamules as referred to on stipe. Context in pileus white, 3 mm wide; white and hollow in stipe. Taste and smell not observed. Spore print white. Basidiospores [25, 1, 1] 4–5 × 3–3.2 μm, avl × avw = 4.62 × 3.1 μm, Q = 1.33–1.56, avQ = 1.49, in side-view ellipsoid ovoid, in frontal view ellipsoid, hyaline, thin-walled, dextrinoid, congophilous, cyanophilous, not metachromatic. Basidia 20–26 × 5–6.5 μm, clavate, 4-spored, hyaline, thin-walled. Lamella edge sterile. Cheilocystidia 22–34 × 7–17 μm, mostly cylindrical, rarely narrowly clavate, hyaline, thin-walled, often branch and septate. Pleurocystidia absent. Pileus covering an epithelium made up of globose elements in lower layer (10–20 μm), with subglobose to oblong elements and often with subclavate elements in upper layer (8–32 × 5–20 μm, thick-walled, parietal and intracellular pigment in upper elements and parietal in lower elements. Stipe covering in squamules an epithelium similar to pileus covering. Clamp-connections present in all tissues.
Habitat and distribution: Growing solitary, on humus-rich soil with decaying leaves and wood in deciduous forest; only known from Mae Taeng District, Chiang Mai Province, Thailand.
Material examined: THAILAND, Chiang Mai Province, Mae Taeng District, Forest of Pha Deng Village, N19°07′13.7″, E98°43′52.9″, alt. 905 m, 30 June 2011, P. Sysouphanthong (MFLU 12-2035, holotype).
GenBank Number ITS:JN224828.
Notes: All materials of Lepiota cylindrocystidia are recognized as having a yellow basidiomata, with cylindrical cheilocystidia.The ITS sequence data from the holotype clustered with species in sect. Echinatae and related to, but distinct from species with ovoid basidiospores.
Lepiota flavocarpa Sysouphanthong & K.D. Hyde sp. nov.
MycoBank number: MB 519962; Facesoffungi number: FoF 2949, Figs. 118, 120
Etymology: named because of its yellow basidiomata.
Holotype: MFLU 10-0581
Pileus 20–45 mm, convex at first, then umbonate with low umbo, applanate to plano-concave with straight margin, at center covered by crowded and large pyramidal warts, with or without recurred tips, and distant towards margin, dark yellow to yellowish-brown (4C8, 5D8), on yellow to vivid yellow (3A6–8), fibrillose back ground, when mature, trips of pyramids around margin fragile, pyramids becoming large squamules, and later fibrillose squamules; margin appendiculate with fibrillose or cortinate, concolorous with back ground or yellowish-white to pale yellow (1A2–3). Lamellae free, crowded, ventricose, 4–8 mm wide, concolorous with pileus background, consistent, turning brownish-red to dark red (10C6–8) with KOH. Stipe 40–40 × 4–8 mm, cylindrical or slightly tapering to apex, background fibrillose, concolorous with pileus, completely covered with crowded lanate flocculate or squamules from base upward to annular zone, then absent toward apex, at annular zone with concolorous fibrillose or cortinate as on pileus margin. Annulus an annular zone, cortinate or fibrillose, yellowish-white to pale yellow (1A2–3). Context in pileus concolorous with surface, up to 5 mm wide; in stipe concolorous with surface, hollow. Taste and smell not observed. Spore print white. Basidiospores [50, 2, 2] 4–4.5 × 3–3.2 μm, avl × avw = 4.24 × 3.1 μm, Q = 1.33–1.40, avQ = 1.36, in side-view ellipsoid ovoid, in frontal view ellipsoid, dextrinoid, congophilous, cyanophilous, not metachromatic in Cresyl Blue. Basidia 16–20 × 6–10 μm, narrowly clavate, 4-spored, hyaline to pale yellow-walled. Lamella edge sterile. Cheilocystidia 20–28 × 7–13 μm, clavate, fusiform or utriform, hyaline to pale yellow and thin-walled. Pleurocystidia absent. Pileus covering a sub hymeniderm or epithecium made up of agglutinate chains of mostly subglobose to oblong elements, 35–75 × 20–27 μm, sometimes globose, 18–25 μm, yellow to pale brown parietal and intracellular pigments. Stipe covering a sub hymeniderm made up of subglobose to oblong, 30–60 × 18–25 μm. Clamp connections present in all tissues.
Habitat and distribution: growing on a small group, on humus-rich soil mixed decayed leaves and branches; in deciduous forest dominated by Dipterocarpus and Castanopsis spp.; only found in rainy season (June–August) of Hui Kok Ma, Doi Suthep District, Chiang Mai, Thailand.
Material examined: THAILAND, Chiang Mai Province, Muang District, Hui Kok Ma od Doi Suthep, N18°48′62″, E98°54′60″, alt. 1145 m, 20 June 2010, P. Sysouphanthong, P63 (MFLU 10-0581, holotype); ibid., 18 June 2010, P. Sysouphanthong, P47 (MFLU 10-0565); ibid., 19 June 2010, P. Sysouphanthong, P50 (MFLU 10-0568); ibid., 27 August 2010, P. Sysouphanthong, P110 (MFLU 10-0628).
GenBank Number ITS:KP348285.
Notes: All material of Lepiota flavocarpa is recognized as having a yellow basidiomata, with dark yellow to yellowish-brown pyramidal squamules on the pileus, turning brownish-red to dark red in KOH for whole basidiomata. The characters of ovoid Basidiospores and the subhymenidermal pileus covering made up of globose to oblong elements located this species in sect. Echinatae. The ITS sequence data from the holotype clustered with species in sect. Echinatae and related to, but distinct from species with ovoid basidiospores.
Lepiota maerimensis Sysouphanthong & K.D Hyde, sp. nov.
Index Fungorum number: IF552904; Facesoffungi number: FoF 2950, Fig. 121
Etymology: The name of this species “cylindricystidia” comes from its cylindrical Cheilocystidia
Holotype: MFLU 12-2036
Pileus 8–17 mm, at first parabolic to broadly convex, expanding umbonate with low umbo, inflexed margin; with warts, or pyramidal squamules, larger at centre and smaller towards margin, brown and turning dark brown (6F5–8, 6E4–6), on orange-white (5A2) background; margin cortinate in young samples, squamulose, fibrillose. Lamellae free, crowded, ventricose, 1.5–2 mm wide, white, yellowish-white when mature, with white serrulate edge. Stipe 28–32 × 1.8–2 mm, cylindrical, with bulb, 2.2–2.4 mm wide, completely floccose or with crowded squamules from annular zone toward base, light brown (6D5–6), white to yellowish-white (4A2) at apex, hollow. Annulus an annular zone, floccose or squamulose, light brown (6D5–6). Context white in pileus; white in stipe. Smell and taste not observed. Spore print white. Basidiospores [25] 4–5 × 2.8–3 µm, avl × avw = 4.3 × 3 µm, Q = 1.1–1.6, Qav = 1.4, oblong ovoid in side-view, oblong in frontal view, thick-walled, hyaline, not dextrinoid, congophilous, cyanophilous, not metachrometic. Basidia 10–12.5 × 4–5 µm, clavate, 4-spored. Lamella edge sterile. Cheilocystidia 10–22 × 4–9 µm, clavate, narrowly clavate, rarely utriform, hyaline, thin-walled. Pileus covering an epithelium made up of several chains elements, terminal elements 10–25 × 7–17 µm, globose to ellipsoid, clavate, subclavate, with brown walls, slightly thick-walled; under layer with hyaline hyphae, cylindrical, up to 4 µm wide. Clamp connections present.
Habitat and distribution: growing solitary or in small groups, saprotrophic on rich humus soil, sand or loamy-clayey, nutrient-rich soil.
Material examined: THAILAND, Chiang Mai Province, Mae Rim District, Mae Sa Valley, 12 July 2008, P. Sysouphanthong (MFLU 12-2036, holotype).
GenBank Number ITS:KP348284.
Notes: Lepiota microspila, a species from Sri Lanka is similar to L. maerimensis in its ochraceous tawny to rufous squamules on the pileus and stipe covering. Lepiota microspila differs from L. maerimensis in having a smaller spore size (3.3–4.5 × 1.7–2.3 (3.8 × 2) µm, shorter Basidia (6.5–8 × 3.3.5 µm), and thicker wall of elements in the pileus covering (up to 1 µm). Unfortunately, Cheilocystidia of Lepiota microspila were not recovered in the Sri Lankan material (Pegler 1972). Some species of section Echinatae from India are compared with this species, for example Natarajan and Manjula (1982) described Lepiota subrufa from South India and it differs from L. maerimensis in its pale red background on the pileus and stipe, and reddish-brown pyramidal squamules, pastel red Lamellae and dull red membranous annulus. Lepiota babruka is also similar to L. maerimensis in having brown to dark brown pyramidal squamules on the pileus, but it differs in lacking a squamulose stipe and yellowish-white to pale yellow lamellae (Arun Kumar and Manimohan 2009) (Fig. 121).
Phylogenetic relationships inferred from maximum parsimony analysis of ITS-rDNA sequences of 11 taxa. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The new Sri Lankan record: Leucocoprinus cretaceus having GenBank Accession Number KY649466 and Herbarium Number MFLU 12-1893 (ITS-rDNA) is shown in bold and blue. The topology is rooted with Leucoagaricus gongylophorus. Evolutionary analysis was conducted in PAUP 4.0b 10
Leucocoprinus Pat.
Leucocoprinus belongs to the family Agaricaceae. Its best-known member is the yellow pot-plant mushroom Leucocoprinus birnbaumii, found worldwide. The type species of this genus is L. cepistipes (Sowerby) Pat.
Leucocoprinus cretaceus (Bull.) Locq., Bull. mens. Soc. linn. Soc. Bot. Lyon 14: 93 (1945)
Facesoffungi number: FoF 3137, Figs. 122, 123, 124
Pileus 3–6 cm broad, hemispherical when young, expanding to planoconvex to campanulate, margin decurved, slightly sulcate-striate, when young with many, dense, soft floccules, readily collapsing or wearing off to leave farinose covering; light buff to white at center, white elsewhere. Lamellae distantly free, rather broad, thin; white, edge slightly fimbriate. Stipe 5–8 cm long, 4–6 mm broad at apex, gradually enlarged downward to a broadly clavate to somewhat fusiform 6–14 mm broad base; sometimes coarsely farinose to slightly flocculose-farinose below annulus, subfarinose above; white to ivory yellow tinted. Annulus white, very soft, rather flaring, median to superior. Basidiospores 8–11 × 5–8 μm, Q = 1.32–1.47, metachromatic, dextrinoid, ellipsoid to amygdaliform, thick-walled, with an apical germ pore covered with a hyaline lens. Basidia 16–22 × 9–12 μm, pyriform to clavate with a bulbous base, 4-spored, surrounded by 4 pseudoparaphyses. Sterigmata 1–2 × 0.7–1.5 μm. Pseudoparaphyses 10–13 × 7–10 μm, sphaeropedunculate to broadly clavate to pyriform, very broad point of attachment. Cheilocystidia 27–75 × 7–18 μm, thin-walled, hyaline, subcylindrical to narrowly fusiform to slightly narrowly lageniform, mucronate, rarely obtuse, moderately pedicellate. Pileus covering a sparse layer of terminal cells essentially absent on mature pilei, most prevalent near the center of young pilei, 45–117 × 7–9 μm, cylindrical to narrowly lageniform, with flexuous necks. Stipe covering structure like that of pileus, with terminal cells even more sparse. Stipitipellis a cutis of narrowly cylindrical, 5–8 μm broad elements. Clamp connections absent.
Habit, habitat and distribution: as a group, usually on manure or wood chips, May–August. Our collection was collected on cow manure at Hanthana Mountains Range, Peradeniya.
Specimen examined: SRI LANKA, Kandy District, Hanthana Mountains Range, 6 June 2012, Samantha C. Karunarathna (MFLU 12-1893, new record).
GenBank Number ITS:KY649466.
Notes: This is widely distributed in North America and this is the first report of L. cretaceus with molecular phylogenetic confirmation from Sri Lanka.
Entolomataceae Kotl. & Pouzar
Entolomataceae is a large family of pink-spored terrestrial gilled mushrooms introduced by Kotl. and Pouzar in 1972 and typified by Entoloma. The family is highly variable in terms of sporocarp morphology and micromorphology (Noordeloos 2004). This family contains four genera and 1071 species (Kirk et al. 2008).
Entoloma Fr. ex P. Ku mm.
The genus Entoloma is characterized by pink or pinkish-brown spore print and basidiospores that are angular in all views (Noordeloos 1992; Noordeloos and Gates 2012). There is no agreement regarding the limits of the genus Entoloma as some authors have set a very wide limit to the genus (e.g. Donk 1949; Romagnesi 1978; Noordeloos 1981, 1992, 2004; Co-David et al. 2009; Noordeloos and Gates 2012) and others have applied a very restricted concept of the genus and have introduced several segregate genera such as Alboleptonia, Claudopus, Inocephalus, Leptonia, Nolanea, and Pouzarella (e.g. Largent 1974; Mazzer 1976; Baroni et al. 2011). In the course of a continuing study on the Entolomataceae of Kerala State, India, we came across a species of Entoloma, which was found to be new to science. It is herein formally described as new based on both morphology and molecular phylogeny. Maximum likelihood analysis placed the new species within a clade that represents the subgenus Entoloma with significant (74%) ML bootstrap support (Fig. 125).
ITS-based phylogram generated from maximum likelihood analysis using RAxML-HPC2 v. 8.2.8 (Stamatakis 2014) on XSEDE platform employed in CIPRES Science Gateway web server (Miller et al. 2010) depicting the placement of Entoloma magnum (KY002062) within Entoloma subg. Entoloma. Bootstrap support values ≥50% are indicated at the nodes. The tree is rooted with Rhodocybe luteobrunnea, R. tugrulii, Clitopilus prunulus and C. reticulosporus
Entoloma magnum K.N.A. Raj & Manim., sp. nov.
MycoBank number: MB 818742; Facesoffungi number: FoF02637; Figs. 126, 127
Etymology: The specific epithet refers to the large size of the basidiocarp.
Holotype: CAL 1385
Basidiocarp large, tricholomatoid. Pileus 102 mm diam., somewhat plano-convex with a low central umbo surrounded by a shallow depression; surface dark brown (7F4/OAC636) at the centre and light brown (7D4/OAC659) towards the margin, not hygrophanous, tacky, finely radially wrinkled, innately fibrillose, plicate-striate towards the margin; margin somewhat incurved to almost straight, finely crenate, fissile. Lamellae narrowly adnate to adnate, moderately crowded, subventricose, orange white (6A2/OAC634), up to 13 mm wide, with lamellulae of 3 lengths; edge crenate to somewhat wavy, concolorous with the sides. Stipe 99 × 18 mm, central, terete, slightly tapering towards both the apex and the base, robust, solid; surface yellowish-brown (5D4/OAC743) towards the apex, whitish towards the base, appressed-fibrillose all over; base with scanty basal mycelium. Odour and taste not distinctive.
Basidiospores 7–10 × 7–9 (8.6 ± 0.73 × 7.6 ± 0.66) µm, Q = 1–1.2, Qm = 1.13, predominantly pentagonal or occasionally 4- or 6-angled in profile, isodiametric to sub-isodiametric, hyaline, thick-walled. Basidia 31–57 × 9–11 µm, clavate, hyaline, thin-walled, 4-spored; sterigmata up to 7 µm long. Lamella-edge fertile. Cheilocystidia and pleurocystidia absent. Lamellar trama subregular; hyphae 4–7 µm wide, pale brownish-yellow, thin-walled. Subhymenium inconspicuous. Pileus trama subregular; hyphae 7–13 µm wide, pale yellow, thin-walled. Pileipellis a transition between an ixocutis and an ixotrichoderm; hyphae 5–10 µm wide, with a pale brownish-yellow wall pigment, thin-walled. Stipitipellis a cutis rarely disrupted with flaring out hyphae; hyphae 5–12 µm wide, pale brownish-yellow, thin-walled. Caulocystidia absent. Oleiferous hyphae present in both lamellar and pileus trama. Clamp connections observed on all hyphae.
Habitat: on soil, among litter, solitary.
Material examined: INDIA, Idukki District, Munnar, Eravikulam National Park, 6 July 2011, K.N. Anil Raj AR683 (CAL 1385, holotype).
GenBank Number ITS:KY002062.
Notes: Entoloma magnum is characterized by a large, tricholomatoid basidiocarp; a brown-coloured, umbonate pileus; adnexed to narrowly adnate lamellae; a fleshy-fibrous stipe; isodiametric to sub-isodiametric basidiospores; absence of cheilocystidia; a pileipellis that is a transition between an ixocutis and an ixotrichoderm and the presence of clamp connections on all hyphae. Owing to these characters, E. magnum fits into the section Entoloma of the subgenus Entoloma (Noordeloos 2004; Co-David et al. 2009; Noordeloos and Gates 2012).
Entoloma brihadum Manim., A.V. Joseph & Leelav., a species previously described from Kerala (Manimohan et al. 1995), somewhat resembles E. magnum in having robust, tricholomatoid basidiocarps with a subumbonate pileus, crowded lamellae, a fertile lamella-edge, and absence of caulocystidia. However, E. brihadum has a smaller (50 mm), pinkish-white, glabrous pileus with pellucid striations, free to adnexed lamellae, a pinkish-white, and glabrous stipe, smaller and exclusively quadrate basidiospores (6–8 × 6–7 µm) and a cutis-type pileipellis composed of hyaline hyphae.
Entoloma myochroum Noordel. & E. Ludw., a species from Germany (Noordeloos 2004), is close to E. magnum in having almost similar-sized basidiocarps, a concave and glabrous pileus, adnate lamellae with concolorous edges, a solid stipe with a fibrillose surface, almost similar-sized basidiospores (7–10 × 6.5–8.5 µm) with 4–6 angles, a fertile lamella-edge and clamped hyphae. However, that species has a mouse-grey-coloured pileus, a flexuous or irregularly shaped stipe, a cutis-type pileipellis made up of hyphae with intracellular pigment and lamellar and pileus trama with short inflated elements.
In a BLASTn search using the ITS sequence (628 bp) derived from E. magnum, the closest hit was E. ochreoprunuloides (GenBank Number KC710092; 92% identity). Entoloma ochreoprunuloides Morgado & Noordel., is a species from Germany (Noordeloos 2004; Morgado et al. 2013), resembles E. magnum in having a similar-shaped, brownish pileus with radially wrinkled and innately fibrillose surface, isodiametric basidiospores, a fertile-lamella edge, and clamped hyphae. However, E. ochreoprunuloides differs from E. magnum owing to its smaller-sized and differently-coloured basidiocarps, emarginate lamellae, smaller (5.9–7.1 × 5.7–7.2 µm) basidiospores and hyphae of the pileipellis with intracellular pigment.
In the phylogram (Fig. 126) generated from the ML analysis, E. magnum nested within a clade, which represents the subgenus Entoloma. Within this clade, E. magnum, E. luteobasis, E. ochreoprunuloides, E. madidum and collections of E. bloxamii formed a subclade. Within this subclade, E. magnum was differentiated as an independent lineage separated from other species with significant (74%) bootstrap support.
Inocybaceae Jülich
The type genus of the Inocybaceae, Inocybe, had traditionally been placed within the Cortinariaceae family (Kirk et al. 2008, Singer 1986). Despite this, Jülich placed the genus in its own family, the Inocybaceae (Jülich 1982). Members of this family have a widespread distribution in tropical and temperate areas (Cannon & Kirk 2007).
Inocybe (Fr.) Fr.
The genus Inocybe (Inocybaceae) is a species-rich genus of Agaricales and is well-known for their ectomycorrhizal ecology and toxicity of most species (Matheny 2009). Matheny (2009) proposed seven major clades or lineages consisting of Inocybe and its allies within the family Inocybaceae. Of the seven clades or lineages (Inocybe s. str., Nothocybe, Pseudosperma, Mallocybe, Inosperma, Auritella and Mallocybella), Auritella and Mallocybella were formally recognised as distinct genera, Auritella and Tubariomyces (Matheny and Bougher 2006a, b; Alvarado et al. 2010). Species of Inocybe are characterised by mostly brownish or rarely whitish basidiomata occasionally with a purplish or lilac hue, a fibrillose-rimose or squamulose pileus, brownish lamellae, brown spore-print, a fibrillose-pruinose stipe at times with a distinct marginate-bulbous base, a characteristic odour, smooth, warty, nodulose or spinulose basidiospores and metuloidal cystidia often with crystalloid deposits at their apices. Several species are devoid of metuloidal cystidia and they are characterised by abundant, thin-walled cheilocystidia (Matheny 2005; Larsson et al. 2009). During the course of our studies on this genus in Kerala State, India, we discovered several new species of Inocybe. Three of these species belonging to the Pseudosperma clade (Fig. 128) are described here.
Phylogram generated from maximum likelihood (RAxML) analysis based on RPB2 sequence data matrix for 34 Inocybe species. Sequences of Inocybe species belonging to the Pseudosperma clade used in this study have been selected from a previous analysis of Kropp et al. (2013). Values at nodes indicate bootstrap support. BS values ≥50% are shown. Inocybe luteobrunnea, I. brunneosquamulosa and I. rubrobrunnea are in pink to highlight its phylogenetic position in the tree. The tree is rooted with I. adaequata and I. calamistrata of Inosperma clade
Inocybe brunneosquamulosa K.P.D. Latha & Manim., sp. nov.
MycoBank number: MB 816735; Facesoffungi number: FoF: 2176, Figs. 129, 130
Etymology: referring to the brown squamules on the pileus surface.
Holotype: CAL 1308
Basidiocarps small. Pileus 7–11 mm diam., convex with a small umbo when young, becoming broadly convex still with a small umbo at maturity; surface initially dark brown (6F8/OAC636) on the squamules and brownish-orange (6C4/OAC653) elsewhere, becoming dark brown (6F7/OAC637) on the squamules, greyish-orange (6B3/OAC633) on the fibrils and brownish-grey (6C2/OAC662) elsewhere at maturity, appressed- to recurved-squamulose all over when young, becoming appressed- to recurved squamulose on and around the umbo and appressed-fibrillose and rimose towards the margin; margin incurved, becoming decurved, crenate or wavy. Lamellae sinuate, close, initially orange grey (6B2/OAC634), becoming brownish-orange (6C4/OAC655) or light brown (6D4/OAC686) at maturity, up to 1.5 mm wide, with lamellulae of 1 length; edges fimbriate, whitish. Stipe 17–19 × 1–2 mm, central, terete, equal, cartilaginous, solid; surface brownish-orange (6C3/OAC633) all over, appressed-fibrillose in most parts, slightly-recurved fibrillose and finely pruinose towards the apex; base not enlarged. Odour and taste not distinctive. Basidiospores 8–10 × 5–6.5 (9 ± 0.6 × 5.9 ± 0.4) µm, Q = 1.3–1.9, Qm = 1.5, smooth, ellipsoid to subphaseoliform, slightly thick-walled, yellowish-brown. Basidia 21–30 × 11–13 µm, clavate, thin-walled, hyaline, 4-spored; sterigmata up to 4.5 µm long. Pleurocystidia absent. Lamella-edge heterogeneous. Cheilocystidia 17–39 × 11–18 µm, versiform: clavate, utriform, fusiform, cylindrical with an obtuse apex, occasionally subglobose or rarely pedicellate or septate, hyaline with faint hyaline encrustations, thin- to slightly thick-walled. Lamellar trama subregular; hyphae 6–15 µm wide, thin-walled, hyaline or pale yellow. Subhymenium pseudoparenchymatous. Pileus trama subregular, composed of both narrow and inflated hyphae; hyphae 3–30 µm wide, pale yellow, thin-walled. Pileipellis a cutis frequently disrupted with trichodermal patches, often a perfect trichoderm at the centre; hyphae 8–16 µm wide, thin- to slightly thick-walled, with a brown wall pigment and dense, yellowish-brown or brown spiral encrustations; terminal cells 25–52 × 7–11 µm, clavate or cylindrical with an obtuse apex, thin- to slightly thick-walled. Stipitipellis a cutis often disrupted by loose hyphal projections scattered over the entire surface of the stipe and with bunches of caulocystidia confined to the extreme stipe apex; hyphae 5–11 µm wide, thin- to slightly thick-walled, with a pale yellowish-brown wall pigment and faint hyaline encrustations; terminal cells, 21–49 × 5–7 µm, cylindrical or flexuous-cylindric, slightly thick-walled, with a pale yellowish-brown wall pigment and faint hyaline encrustations. Caulocystidia 22–50 × 11–14 µm, catenulate, clavate, inflated clavate or obovoid, rarely septate, hyaline, occasionally with faint, hyaline encrustations, thin- to slightly thick-walled. Stipe trama hyphae with dense, yellowish-brown oleaginous contents. Clamp connections seen on all hyphae.
Habitat: on the ground, scattered around Vateria indica (Dipterocarpaceae) trees.
Specimen examined: INDIA, Kerala State, Ernakulam District, Kochi, Thevakkal, Ponnakkudam Kavu sacred grove, 25 August 2014, K.P.D Latha DKP264 (CAL 1308, holotype).
GenBank Numbers ITS: KX073582; LSU: KX073586; RPB2: KX073589.
Notes: Small basidiocarps with a dark brown, squamulose and fibrillose-rimose pileus; a fibrillose stipe with a finely pruinose apex and an abruptly ending base; smooth, ellipsoid to subphaseoliform basidiospores; a hymenium devoid of pleurocystidia; versiform cheilocystidia with occasional, faint, hyaline encrustations; a cutis-type pileipellis which is disrupted with trichodermal patches and a cutis-type stipitipellis disrupted by loose, hyphal projections and often with caulocystidia at the extreme stipe apex are the salient features of I. brunneosquamulosa. Inocybe fuscospinulosa, a species originally described from Indonesia (Horak 1980b) and also reported from Sri Lanka (Pegler 1986), seems to be somewhat similar to I. brunneosquamulosa in having a pileus of rather similar colour and surface features, a fimbriate lamella-edge, a fibrillose stipe, a hymenium devoid of pleurocystidia, a trichoderm-type pileipellis and the presence of cheilocystidia. Inocybe fuscospinulosa, however, is distinguished from the I. brunneosquamulosa in having larger basidiocarps with a densely squamulose pileus, crowded, adnexed, tobacco brown lamellae, a reddish-brown tinted stipe with occasional scales, smaller (6.5–8 × 4–5 µm) and ovoid basidiospores, cylindric to subfusoid cheilocystidia devoid of encrustations and a stipitipellis lacking caulocystidia. Inocybe brunneosquamulosa is also somewhat similar to I. umbrinovirens E. Horak, a species so far known only from Papua New Guinea (Horak 1980b), in having a somewhat similar-coloured pileus with almost similar surface features, a fibrillose stipe, smooth basidiospores, a hymenium devoid of pleurocystidia, the presence of cheilocystidia with encrusting pigment and a trichoderm-type pileipellis. However, the characters such as the larger basidiocarps with differently-shaped pileus, chocolate brown, adnexed, crowded lamellae, a hollow stipe with a greenish base, larger (10–12.5 × 7–8.5 µm) and ovoid basidiospores, larger basidia, cheilocystidia that are terminal elements of lamellar trama, the absence of caulocystidia and a strong odour make I. umbrinovirens different from I. brunneosquamulosa.
Inocybe squamata J.E. Lange, a species widespread in Europe and USA (Cripps 1997) and also reported from Kerala (Pradeep and Vrinda 2010; Mohanan 2011) is similar to I. brunneosquamulosa in having a pileus of somewhat similar texture, rather similarly attached lamellae, a fibrillose stipe, subphaseoliform basidiospores, a hymenium devoid of pleurocystidia, the presence of cheilo- and caulocystidia and similar type of pileipellis. However, I. squamata has larger basidiocarps with a differently-coloured pileus, thick, broad, yellow brown lamellae, a longer, white stipe, larger basidiospores (9–11.5 (13) × (5) 5.5–6.5 µm), occasional 2-spored basidia and narrowly clavate cheilo- and caulocystidia lacking encrustations.
Comparison of the ITS (679 bp), LSU (935 bp) and RPB2 (699 bp) sequences derived from I. brunneosquamulosa with the nucleotide sequences available in GenBank Numbers showed that I. brunneosquamulosa has distinct sequences. Inocybe species MCA562 resulted as the closest hit in megablast searches with ITS (GenBank Numbers JQ408785; Identities = 630/682 (92%)), LSU (GenBank Numbers JN975016; Identities = 921/936 (98%)) and RPB2 (GenBank Numbers JQ421077; Identities = 673/699 (96%)) sequences. Inocybe species MCA562 is a collection from Japan, but its morphological features are unavailable for comparison as it remains unpublished.
The phylogenetic placement of I. brunneosquamulosa is shown in the phylogram (Fig. 128) generated from the ML analysis of RPB2 sequence data matrix. In the ML analysis, I. brunneosquamulosa nested in the Pseudosperma clade with maximum support (100% ML) where it paired with Inocybe species MCA562 and had a strong support (96% ML).
Inocybe luteobrunnea K.P.D. Latha & Manim., sp. nov.
MycoBank number: MB 816734; Facesoffungi number: FoF 2177; Figs. 131, 132
Etymology: referring to the yellowish-brown pileus.
Holotype: CAL 1260
Basidiocarps small. Pileus 6–14 mm diam., narrowly conical when very young, becoming conico-convex and finally convex with a small umbo; surface brown (6F6/OAC636) on the squamules and yellowish-brown (5D8/OAC775) elsewhere when young, becoming dark brown (6F7/OAC639) at the centre and on the squamules and brownish-orange (5C4, 5C5/OAC806) elsewhere at maturity, with appressed- to slightly recurved, minute squamules on and around the umbo, appressed-fibrillose towards the margin; margin incurved when young, becoming decurved to somewhat straight with age, crenate or somewhat wavy, finely fissile. Lamellae emarginate, subventricose, rarely furcate, close, greyish-orange (5B3, 5B4/OAC793), up to 2 mm wide, with lamellulae of 3 lengths; edges fimbriate, rather whitish. Stipe 13–22 × 2–2.5 mm, central, equal, fistulose; surface initially orange grey (6B2/OAC634), becoming greyish-orange (5B2/OAC675) at maturity, appressed-fibrillose all over, finely pruinose towards the apex; base somewhat bulbous, not marginate-bulbous. Odour and taste not distinctive.
Basidiospores 7–8 (9) × 5–6 (6.5) (7.9 ± 0.7 × 5.9 ± 0.5) µm, Q = 1.2–1.6, Qm = 1.4, smooth, ovoid to amygdaliform, slightly thick-walled, pale yellowish-brown. Basidia 23–34 × 8–10 µm, clavate, thin-walled, hyaline, 4-spored or rarely 2-spored; sterigmata up to 5 µm long. Mature basidia slightly projecting beyond the hymenial surface. Pleurocystidia absent. Lamella-edge sterile with abundant cheilocystidia. Cheilocystidia 17–52 × 9–15 µm, versiform: clavate, inflated clavate, cylindrical or cylindrical with apical constriction, cylindro-flexuous, cylindrical with irregular constriction, narrowly utriform, utriform with a median constriction, ovoid, fusiform, submoniliform or short-pedicellate, often septate, thin- to slightly thick-walled, hyaline. Lamellar trama subregular, composed of both narrow and inflated hyphae; hyphae 3–25 µm wide, thin- to slightly thick-walled, hyaline, at times with faint, hyaline encrustations especially towards the edge of the hymenium. Subhymenium poorly developed. Pileus trama subregular; hyphae 9–28 µm wide, pale yellow, thin- to slightly thick-walled. Pileipellis a cutis often transitioning to a trichoderm towards the centre; hyphae 4–11 µm wide, tangled, slightly thick-walled, with a pale-yellow wall pigment and brown spiral encrustations. Stipitipellis a cutis frequently disrupted with bunches of caulocystidia towards the apex; hyphae 5–12 µm wide, thin-to slightly thick-walled, with a pale-yellow wall pigment and hyaline encrustations, some hyphae with dense, yellowish-brown, amorphous contents towards the base. Caulocystidia 13–74 × 7–13 µm, versiform: clavate, narrowly clavate, moniliform, submoniliform, cylindrico-flexuous, cylindrical with an obtuse apex, utriform with a median constriction, cylindrical or ovoid with a rostrate apex, often septate, thin- to slightly thick-walled, hyaline. Clamp connections observed on all hyphae.
Habitat: scattered among bryophytes, on a mud wall.
Material examined: INDIA, Kerala State, Idukki District, Munnar, on the way to Mattupetti top hill station, 31 August 2013, K. P. Deepna Latha DKP167 (CAL 1260, holotype); GenBank Numbers ITS: KX073580; LSU: KX073584; RPB2: KX073588; INDIA, Kerala State, Idukki District, Munnar, on the way to Mattupetti top hill station, 9 November 2013, K. P. Deepna Latha DKP251 (CAL 1260, holotype; CAL 1261, paratype).
GenBank Numbers ITS: KX073581; LSU: KX073585.
Notes: Inocybe luteobrunnea is characterised by a yellowish-brown pileus with a fibrillose to minutely squamulose surface; a fibrillose stipe with a finely pruinose apex and a bulbous base; smooth, ovoid to amygdaliform basidiospores; a hymenium lacking pleurocystidia; abundant, versiform cheilocystidia; subregular lamellar trama with faint hyaline encrustations; a pileipellis that is a cutis with a transition to a trichoderm towards the centre and a cutis-type stipitipellis frequently disrupted with bunches of caulocystidia confined to the stipe apex. Inocybe palaeotropica E. Turnbull & Watling, a widespread species reported from Singapore, Malaysia, Sabah, Papua New Guinea, Solomon Islands (Horak 1980b) and also recorded from Kerala by Vrinda et al. (1997, as I. umbrina Massee), shares a few characters with I. luteobrunnea such as a similar-shaped pileus, similar-sized basidiospores (7.5–10.5 × 4.5–6 µm), a sterile lamella-edge and caulocystidia on the stipe apex. Inocybe palaeotropica, however, has larger basidiocarps with dissimilar colour and surface features, adnexed lamellae, a fibrillose stipe devoid of apical pruinosity, ovoid to short ellipsoid basidiospores, only 4-spored basidia, clavate to cylindro-clavate cheilocystidia, hyphae of lamellar trama lacking encrustations, a cutis-type pileipellis and smaller, cylindro-clavate caulocystidia. Inocybe luteobrunnea is somewhat similar to I. fuscospinulosa Corner & E. Horak, an Indonesian species (Horak 1980b) and also reported from Sri Lanka (Pegler 1986), in having a somewhat similar-shaped pileus, a fibrillose stipe, almost similar-sized basidiospores (6.5–8 × 4–5 µm), cheilocystidia, a hymenium devoid of pleurocystidia and an almost similar pileipellis structure. However, I. fuscospinulosa differs from I. luteobrunnea owing to its larger basidiocarps with conspicuous, erect, spiny squamules on the pileus, crowded, adnexed, tobacco-brown lamellae, a red-brown tinged stipe with occasional brown squamules and without a distinct bulbous base, ovoid basidiospores, consistently 4-spored basidia, larger, differently-shaped cheilocystidia and a stipitipellis devoid of caulocystidia.
The distinctive status of (CAL 1260: 675 bp; CAL 1261: 674 bp), LSU (CAL 1260: 838 bp; CAL 1261: 746 bp) and RPB2 (CAL 1260: 651 bp) sequences of I. luteobrunnea was confirmed in the BLASTn searches. An unnamed Australian species, Inocybe species AU95 was the closest hit in a megablast search for ITS (GenBank Numbers KP636851; Identities = 540/588 (92%)), LSU (GenBank Number KP171053; Identities = 831/839 (99%)) and RPB2 (GenBank Number KM555145; Identities = 638/651 (98%)) sequences.
The phylogram generated from the Maximum Likelihood (ML) analysis (Fig. 129) depicts the relative placement of I. luteobrunnea. The ML analysis placed I. luteobrunnea in the Pseudosperma clade with full support (100% ML) based on RPB2 sequence data matrix. Within this clade, I. luteobrunnea clustered with an unnamed Australian species, Inocybe species AU95 (KM555145) with maximum support (100% ML). The macro-morphological and microscopic data of that species are not available for comparison.
Inocybe rubrobrunnea K.P.D. Latha & Manim., sp. nov.
MycoBank number: MB 816736; Facesoffungi number: FoF: 2175; Figs. 133, 134
Etymology: referring to the reddish-brown colour of the pileus.
Holotype: CAL 1307
Basidiocarps small. Pileus 6–12 mm diam., somewhat hemispherical or paraboloid when young, becoming convex or plano-convex with a small umbo; surface reddish-brown (8E5, 8E6/OAC609, OAC610) when young, becoming dark brown (7F7/OAC621) at the centre and on the squamules, orange grey (6B2) elsewhere, appressed- to slightly recurved squamulose and appressed-fibrillose all over when young, becoming appressed- to recurved squamulose on and around the centre, appressed-fibrillose and rimulose towards the margin; margin initially incurved, becoming decurved to somewhat straight with age, crenate. Lamellae sinuate or emarginate, close, initially greyish-orange (6B3/OAC654), becoming brownish-orange (6C4/OAC695), up to 1.5 mm wide, with lamellulae of 3 lengths; edges fimbriate, whitish. Stipe 11–20 × 1–2 mm, central, terete, equal or slightly tapered towards the base, cartilaginous, solid; surface brownish-orange (6C3/OAC633), fading towards the apex, appressed-fibrillose all over, slightly pruinose at the apex; base slightly enlarged. Odour and taste not distinctive.
Basidiospores 7–9 × 5–6 (8.32 ± 0.63 × 5.57 ± 0.43) µm, Q = 1.2–1.7, Qm = 1.4, smooth, ellipsoid to slightly phaseoliform, slightly thick-walled, pale yellowish-brown. Basidia 18–27 × 9–12 µm, clavate, thin-walled, hyaline, 4-spored; sterigmata up to 3 µm long. Pleurocystidia absent. Lamella-edge heterogeneous. Cheilocystidia 17–44 × 6–15 µm, abundant, versiform: narrowly clavate, clavate, cylindrical, oblong, ellipsoid, fusiform, obovoid, cylindrical with a median constriction or with a subcapitate apex, occasionally septate, exuding some amorphous material at the apex, slightly thick-walled (up to 1 µm thick), hyaline or rarely with pale yellowish-brown, amorphous contents, occasionally with faint, hyaline encrustations. Lamellar trama subregular, composed of both narrow and inflated hyphae; hyphae 3–23 µm wide, thin- to slightly thick-walled, hyaline. Subhymenium pseudoparenchymatous. Pileus trama subregular; hyphae 6–27 µm wide, hyaline or pale yellow, thin-walled. Pileipellis a cutis often disrupted with bundles of tangled ascending hyphae towards the centre; hyphae 5–10 µm wide, with a pale brownish-yellow wall pigment and brown spiral encrustations. Stipitipellis a cutis composed of hyaline or pale yellow hyphae (3–10 µm wide), devoid of encrustations, frequently disrupted by bundles of loose, tangled hyphae towards the apex (2–6 µm wide), with a pale-yellow wall pigment and pale yellow spiral encrustations, thin- to slightly thick-walled, occasionally with yellowish-brown amorphous contents. Caulocystidia absent. Clamp connections observed on all hyphae.
Habitat: on soil, solitary or in small groups, near Hopea ponga (Dipterocarpaceae) trees.
Specimen examined: INDIA, Kerala State, Wayanad District, Muthanga, Muthanga Wildlife Sanctuary, 21 August 2013, K. P. Deepna Latha DKP142 (CAL 1307, holotype).
GenBank Numbers ITS:KX073583; LSU:KX073587; RPB2:KX073590.
Notes: Small basidiocarps with an appressed-fibrillose to squamulose pileus; a stipe with a slightly enlarged base and a fibrillose surface; smooth, ellipsoid to slightly phaseoliform basidiospores; a hymenium devoid of pleurocystidia; versiform cheilocystidia exuding some amorphous material at the apex; a cutis-type pileipellis disrupted with bundles of tangled ascending hyphae; and a cutis-type stipitipellis often disrupted by bundles of loose, tangled hyphae towards the apex are the diagnostic features of I. rubrobrunnea.
Following the key of Kobayashi (2002), I. rubrobrunnea keys out close to I. quercina Hongo, a species known from Japan, because of its reddish-brown pileus with almost similar size, shape and surface features, a whitish lamella-edge, a solid stipe with a fibrillose surface, almost similar-shaped basidiospores, the absence of pleurocystidia and the presence of cheilocystidia. However, the characters such as the adnexed lamellae that become red when cut, a longer stipe, larger basidiospores (8.2–10.8 × 4.8–5.5 µm) and basidia, smaller cheilocystidia devoid of encrustations, a cutis-type pileipellis lacking encrustations, the presence of caulocystidia and a strong odour of that species make it different from I. rubrobrunnea. Inocybe fuscospinulosa, a species originally described from Indonesia (Horak 1980b) and also reported from Sri Lanka (Pegler 1986), has a pileus with dark brown squamules, a whitish lamella-edge, basidiospores of similar size (7–9 × 4.2–5.5 µm) and shape and the absence of pleuro- and caulocystidia. However, that species differs in having larger basidiocarps with a campanulate to applanate pileus, a white context discolouring purplish-red on exposure, cheilocystidia devoid of encrustations and a trichoderm-type pileipellis.
Comparison of the ITS (676 bp), LSU (924 bp) and RPB2 (702 bp) sequences of I. rubrobrunnea with the nucleotide sequences of taxa available in GenBank Numbers suggests that it has distinct sequences. In a megablast search of the GenBank Numbers database using ITS sequence of I. rubrobrunnea, the closest hit was Inocybe species MCA562 (GenBank Numbers JQ408785; Identities = 616/668 (92%)) followed by Inocybe species AU43 (GenBank Numbers KJ729878; Identities = 605/659 (92%)). An undescribed Inocybe species, Inocybe species AU44 was the closest hit in BLASTn search with LSU (GenBank Numbers KJ729906; Identities = 896/925 (97%)) sequence. Inocybe species AU43 (GenBank Numbers KJ729935; Identities = 670/702 (95%)) resulted as the closest hit in BLASTn search with RPB2 sequences. But, the details of Inocybe species MCA562, an unnamed Inocybe collection from Japan and Inocybe species AU43 and Inocybe species AU44, another unnamed collection from Australia, are not available for comparison.
The RPB2-based ML phylogeny (Fig. 128) placed Inocybe rubrobrunnea in the Pseudosperma clade with full support (100% ML). Within this clade, I. rubrobrunnea clustered with Inocybe species AU43 with a significant support (95% ML).
Marasmiaceae Roze ex Kühner.
The family Marasmiaceae is characterized by white spores. The members of this family mostly have tough stems and the capability of shrivelling up during a dry period and later recovering. According to Kirk et al. (2008), the family contains 54 genera and 1590 species.
Marasmius Fr.
Marasmius is a genus of mushroom-forming fungi in the family Marasmiaceae. It contains about 500 species (Kirk et al. 2008) of which a few, such as Marasmius oreades, are edible. However, most members of this genus are small, unimpressive, brown mushrooms. Their humble appearance contributes to them not being readily noticeable to the layman, and therefore these mushrooms are seldom collected by mushroom hunters. Quite a few of the species are known to grow in the characteristic fairy ring pattern (Fig. 135).
Phylogram generated from maximum likelihood (RAxML) analysis using a GTR + I + G model of nucleotide evolution based on ITS for 55 Marasmius and two outgroup sequences (Crinipellis brunneipurpurea and C. malesiana). Maximum likelihood bootstrap support values greater than 50% and Bayesian posterior probabilities greater than 0.5 are indicated above or below the nodes. Numbers to the left of/are ML bootstrap percentages, and those to the right are Bayesian posterior probabilities. Sequences used in this study mostly have been sampled from a previous study (Tan et al. 2009; Wannathes et al. 2009). The newly generated sequences are in blue, and the newly described taxa is placed in bold font to highlight its phylogenetic position in the tree. GenBank accession numbers for all of the sequences are indicated in the tree. G—sect. Globulares; L—sect. Leveilleani; MM—sect. Marasmius subsect. Marasmius; MS—sect. Marasmius subsect. Sicciformes; N—sect. Neosessiles; SA—sect. Sicci ser. Atrorubentes; SH—sect. Sicci ser. Haematocephali; SL—sect. Sicci ser. Leonini; and SS—sect. Sicci ser. Spinulosi
Marasmius luculentus A.K. Dutta, K. Acharya & Antonín, sp. nov.
MycoBank number: MB 816959; Facesoffungi number: FoF 2192, Figs. 136, 137
Etymology: referring to the beautiful (luculentus) appearance of the pileus.
Holotype: CUH AM120
Pileus 5–10 mm in diam., conic to hemispherical when very young, becoming convex in age, with a small conic to convex papilla that ranges from reddish-brown (8D6) to violet brown (10E5–6, 11E6–7) when young, but later turning brown (7D7, 7E8) to dark brown (7F7) at maturity, very rarely forming a central dot, often with a depression around the papilla; striate to plicate up to center, margin often crenate; surface dry, glabrous, hygrophanous, brownish-orange (7C4) to light brown (7D5–6) when very young, later reddish-grey (7–8B2) and finally turning white (1A1) to off-white at maturity, greyish-yellow (1–2B4, 4B4–5) on drying; context very thin, white to cream. Lamellae adnexed or adnate to a collarium, distant to subdistant (L = 12–14, l = 0), white (1A1), regular, slightly intervenose, edge concolorous. Stipe 5–9(–12) × 0.1–0.3 mm, central, glabrous, wiry, pliant, cylindric, equal, simple and insititious on the substratum, dark brownish-black to black overall, often accompanied by black rhizomorphs, 30–42 mm long. Odour and taste indistinct. Spore print white.
Basidiospores (9.3–)10–10.3–10.5(–11) × (3.9–)4.3–4.8–5.4(–5.8) µm, Q = 1.7–2.2–2.6, ellipsoid to ellipsoid-fusoid, smooth, hyaline, IKI-, thin-walled. Basidia not observed. Basidioles 24–25 × 5.5–7 µm, fusoid to clavate, hyaline, thin-walled. Lamellae edge sterile. Pleurocystidia absent. Cheilocystidia composed of Siccus-type broom cells; main body 14–18 × 4–6(–7.5) µm, sphaeropedunculate to (broadly) clavate or irregular in outline, hyaline, apically thick-walled; apical setulae 2–3.5(–5.5) µm long, cylindrical or irregular in outline, obtuse, pale yellow to light brown in KOH, thick-walled. Pileipellis a hymeniform layer, composed of Siccus-type broom cells; main body (17–)19–23(–25) × (7–)8–11(–14.5) µm, sphaeropedunculate to clavate, broadly clavate, often branched, pale yellow to light brownish in KOH, thick-walled; apical setulae (1.5–)3–4(–5.5) µm long, cylindrical, obtuse, thick-walled. Pileus trama composed of 4.5–7.5 µm broad, interwoven, cylindrical, hyaline, inamyloid, thin-walled hyphae. Lamellar trama hyphae 4–6.5 µm broad, interwoven, cylindrical, hyaline, inamyloid, thin-walled. Stipitipellis hyphae 5.5–6.5 µm broad, parallel to subparallel, cylindrical, smooth, non-gelatinous, hyaline to pale yellow in KOH, non-dextrinoid to weakly dextrinoid, thick-walled. Stipe trama hyphae 6.8–7.5 µm broad, parallel to subparallel, cylindrical, smooth, non-gelatinous, hyaline, inamyloid, thin- to thick-walled. Caulocystidia absent. Clamp connections present in all tissues.
Material examined: INDIA, West Bengal, North-24-parganas, Barasat, Berunanpukhuria, N22°44′22.7832”, E88°26′25.2384”, 10.809 m alt., on dried leaves of Bambusa bambos (L.) Voss plant, A.K. Dutta & S. Paloi, 25 June 2015, AKD 100/2015 (CUH AM120, holotype). INDIA, West Bengal, North-24-parganas, Barasat, Berunanpukhuria, N 22 44′21.642”, E 88 26′26.6676”, ca 10.747 m alt., on dried leaves of B. bambos plant, A.K. Dutta & S. Paloi, 29 June 2015, AKD 132/2015 (CUH AM125, paratype).
GenBank Numbers CUH AM120 ITS: KX138604; LSU:KX138606. CUH AM125 ITS:KX138605.
Notes: Distinctive features of M. luculentus include a small basidiomes with a convex, plicate, light brown to reddish-grey pileus when young that later turns into white to off-white, with a reddish-brown to brown or dark brown central papilla, collariate and distant to subdistant, often intervenose lamellae (12–14), a small central, insititious stipe arising directly from the substratum, obtuse apical setulae of the pileipellis broom cells and cheilocystidia of the Siccus-type, and moderately-sized basidiospores (10.3 × 4.8 µm; mean Q = 2.2). These combinations of macro- and micro-morphological features easily categorizes the present specimen belonging to sect. Marasmius and subsect. Sicciformes Antonín (Wannathes et al. 2009).
Being a well representative of sect. Marasmius, the new Indian species is morphologically similar to several other species. Marasmius conicopapillatus Henn., described from Cameroon for the first time (Hennings 1895) and later subsequently reported to occur in other African countries (Antonín 2007), Bolivia (Singer 1976), Uganda (Pegler 1977) and Java (Desjardin et al. 2000) differs by its much smaller pileus (1–7 mm diam.) coloured pure white to off-white when young that turns cream-buff at maturity, non-intervenose lamellae, and a considerably larger stipe (12–30 mm long, Desjardin et al. 2000; 22–35 mm long, Pegler 1977) coloured white towards apex. Marasmius pallenticeps Singer, originally described from Argentina, has a much smaller pileus (1–2 mm diam.), a copper brown to reddish-brown coloured stipe that arise directly from the rhizomorphs, and distinctly smaller basidiospores (7–8.5 × 2.7–4.5 µm; Singer 1976). Marasmius chrysochaetes Berk. & M.A. Curtis differs by its much smaller pileus (1.2–2 mm), a longer stipe (22–25 mm long), and differently sized basidiospores (9–10 × 3–3.5 µm, Dennis 1951; 8–9 × 3.5–4.2 µm, Singer 1976). Marasmius stypinoides Petch, described from Hakgala, differs by its much smaller (up to 5 mm diam.) and minutely rugulose pileus, a longitudinally striate and initially white stipe, and a serrate lamellae edge (Petch 1948). Marasmius gracilichorda Corner has smaller basidiomes with a minutely velutinous pileus surface coloured brownish-orange to dark brown overall, yellowish-white lamellae with brownish edges (Tan et al. 2009).
Among other species that grows on bamboo leaves, Marasmius kuthubutheenii Y.S. Tan, Desjardin, Vikineswary & Noorlidah differs by its much smaller (1–3 mm) pileus with a minutely pruinose surface coloured brownish-orange to light brown overall and a very smaller stipe (2–4 mm long; Tan et al. 2009). As revealed in Fig. 136, M. luculentus appears to be phylogenetically close to several other taxa like M. nigrobrunneus (Pat.) Sacc., M. ruforotula Singer, and M. subruforotula Singer. Marasmius nigrobrunneus, originally described from Vietnam and previously reported from India, differs by its smaller pileus (1–4 mm diam.) coloured greyish-black to brown black with slightly paler striae, a long slender stipe (up to 73 mm long), and somewhat differently sized basidiospores (Singer 1976; Manimohan and Leelavathy 1989; Antonín and Buyck 2006; Antonín 2007). Marasmius ruforotula has a greyish-orange to brownish-orange pileus with a black papilla, and yellowish-white lamellae, and presence of numerous black rhizomorphs (Tan et al. 2009). Marasmius subruforotula has a minutely velutinous pileus surface coloured greyish-brown to orange brown often with white striae, discoloured and pale orange coloured lamellae edge, slightly smaller basidiospores with a mean of 9.5 × 4.5 µm (Q mean 2.1), and habitat on dicotyledonous leaves or on wood (Pegler 1977; Antonín 2007; Wannathes et al. 2009).
The new species is macro-morphologically similar to M. capillaris, M. limosus, M. rotalis, and M. rotula, but the latter have cheilocystidia and pileipellis broom cells of the Rotalis-type, and belong in subsect. Marasmius (Petch 1948; Gilliam 1976; Singer 1976; Pegler 1977, 1986; Desjardin 1989; Bas et al. 1995; Corner 1996).
Mycenaceae Overeem
The Mycenaceae is a family of fungi in the order Agaricales which the members are saprobic, have a cosmopolitan distribution, and are found in almost all ecological zones. According to Kirk et al. (2008), the family contains 10 genera and 705 species. This is one of the several families that were separated from the Tricholomataceae as a result of phylogenetic analyses.
Favolaschia (Pat.) Pat.
Favolaschia is a genus of mushrooms in the family Mycenaceae. The genus has a widespread distribution, and contains about 50 species worldwide (Kirk et al. 2008) (Fig. 138).
Phylogenetic relationships inferred from maximum parsimony analysis of ITS-rDNA sequences of 31 taxa. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The new Sri Lankan records: Favolaschia auriscalpium having GenBank Accession Number KY649461 and Herbarium Number MFLU 12-1891; Favolaschia manipularis having GenBank Accession Number KY649462 and Herbarium Number MFLU 12-1172 (ITS-rDNA) are shown in bold and blue. The topology is rooted with Mycena crocata. Evolutionary analysis was conducted in PAUP 4.0b 10
Favolaschia auriscalpium (Mont.) Henn., Botanische Jahrbücher für Systematik Pflanzengeschichte und Pflanzengeographie 22: 93 (1895)
Facesoffungi number: FoF 3138, Figs. 139, 140
Pileus small, orange, becoming yellow and ultimately dirty pallid when dry, orbicular in outline, convex, glabrous. Pores concolorous or yellowish, some irregularly elongated, dried 0.2–0.4 mm wide, frequently hexagonal. Pseudostipe concolorous with the pileus, laterally attached to the pileus, subvelutinous, insititious, up to 1.8 × 0.3 mm. Basidiospores 8–11 × 5–7 µm, ellipsoid, sometimes slightly constricted in the middle or reniform, smooth, amyloid. Basidia 23–26 × 7–8 µm, 4–spored, rarely 2–spored, mostly clavate or constriction in the middle. Gloeocystidia few or none. Hyphae filamentous, hyaline, truly gelatinized, thin walled in parts of the trama, non-gelatinized and often broader and somewhat thick-walled in other parts, predominantly in the pseudostipe, all hyphae inamyloid, and clamp connections present. Gloeocystidia 21–35 × 9–22 µm, mostly clavate or ventricose to subvesiculose, rarely cylindrical, hyaline to yellowish. Dermatocystidia 19–29 × 6–14 µm, hyaline, thin-walled, erect, subvesiculose or broadly ventricose, deep purplish-pink in Cresyl blue mounts, obtuse or rarely somewhat mucronate, crowded at the epicutis, otherwise few.
Habit, habitat and distribution: as a group on dead wood, inflorescences, leaf sheaths or petioles of Palmae, May–September. Our collection was collected on a decaying wood near Hanthana Mountains Range, Peradeniya.
Specimens examined: SRI LANKA, Kandy District, Hanthana Mountains Range, 30 June 2012, Samantha C. Karunarathna (MFLU 12-1891, new record).
GenBank Number ITS:KY649461.
Notes: This species was first described from Gulf Coast of South America and this is the first report of F. auriscalpium with the molecular phylogenetic confirmation from Sri Lanka. Favolaschia auriscalpium is morphologically closely related to F. calocera R. Heim. but F. calocera differs by having 2 spored, clavate, 38–40 × 7–10 μm size basidia whereas F. auriscalpium has 4 spored, clavate 23–26 × 7–8 µm size basidia. Phylogenetic analysis (Fig. 138) also supports to place the species in F. auriscalpium.
Favolaschia manipularis (Berk.) Teng, Zhong Guo De Zhen Jun [Fungi of China]: 760 (1963)
Facesoffungi number: FoF 3139, Figs. 141, 142
Pileus 0.3–2.5 cm in diam., conico-campanulate to convex with conical umbo, translucently reticulate, hygrophanous, white pruinose, whitish, when moist greyish to hyaline, when dried up purely white to yellowish. Hymenophore tubular, adnate or adnate-emarginate with a slightly decurrent tooth. 1–5 mm long, white, arranged as radial rows, 5–7 in a row, with angular-round pores of 0.5–1.5 mm wide. Stipe 20–65 × 0.5–3 mm, hollow, hyaline to white, completely white, pruinose cylindrical, thickened in the base. Odour not distinct. Basidiospores 6–8 × 4–5 μm, Q = 1.2–1.6, smooth, white, ellipsoid to broadly ellipsoid and amyloid. Basidia 16–18 × 6–8 μm, narrowly clavate, clamped, with up to 6 μm long sterigmata. Cheilocystidia 51–75 × 7–13 μm, sterile lamellae edge, lageniform, subcylindrical or subclavate, mostly diverticulate or irregularly branched in the apex, simple lageniform with more or less developed neck. Pleurocystidia not visible. Hyphae of the pileipellis 5–16 μm wide, clamped, with diverticulate terminal cells. Pileocystidia 25–34 × 8–11 μm, abundant, irregularly shaped, lageniform to subclavate, with excrescences. Hyphae of the cortical layer of the stipe 3–8 μm wide, clamped, with diverticulate terminal cells. Caulocystidia 24–39 × 6–8 μm, abundant, irregularly shaped.
Habit, habitat and distribution: as a group, usually on dead or decaying wood, May–August. Our collection was collected on a decaying wood at Hanthana Mountains Range, Peradeniya.
Specimens examined: SRI LANKA, Kandy District, Hanthana Mountains Range, 3 June 2012, Samantha C. Karunarathna (MFLU 12-1172, new record)
GenBank Number ITS:KY649462.
Notes: The mycelium and fruiting bodies of the mushroom are bioluminescent. This is found in Australasia, Malaysia, and the Pacific islands and this is the first report of F. manipularis with the molecular phylogenetic confirmation from Sri Lanka.
Physalacriaceae Corner
Physalacriaceae is a family of mushrooms in the order Agaricales which was originally defined by the English mycologist E.J.H. Corner in 1970 (Corner 1970). Species in this family have a widespread distribution, ranging from the Arctic, to the tropics and from marine sites and fresh waters to semiarid forests.
Cyptotrama Singer
Cyptotrama is a genus of mushrooms in the family Physalacriaceae. The type species of the genus is Cyptotrama macrobasidia Singer. According to Kirk et al. (2008), 15 species have been recorded worldwide (Fig. 143).
Phylogenetic relationships inferred from maximum parsimony analysis of ITS-rDNA sequences of 52 taxa. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The new Thai record: Cyptotrama asprata having GenBank Accession Number KY649460 and Herbarium Number MFLU 12-1892 is shown in bold and blue. The topology is rooted with Marasmius epiphyllus. Evolutionary analysis was conducted in PAUP 4.0b 10
Cyptotrama asprata (Berk.) Redhead & Ginns, Canadian Journal of Botany 58 (6): 731 (1980)
Facesoffungi number: FoF 3140, Figs. 144, 145
Pileus 1–5 cm in diam., convex, hemispherical to applanate, whitish, cream to yellow, densely covered by orange, golden yellow coloured squamules, becoming whitish-yellow when mature. Lamellae adnate to slightly decurrent, height up to 5 mm, distant, white to cream, with lamellulae. Stipe 1–6 cm in length, 0.1–0.6 cm in diam., floccose, cylindrical or enlarged at base, fistulose, concolorous with pileus. Context thin, white. Odour indistinct. Basidiospores (7–11 × 4.5–8.5 µm, Q = 1.2–1.9 µm, Qm = 1.4), non-amyloid, non-dextrinoid, thin-walled, smooth, ellipsoid-limoniform to broadly ellipsoid, colourless and hyaline. Basidia 4-spored, 35–60 × 6–8 µm, clavate, colourless or hyaline; sterigmata 4–6 µm long. Basidioles abundant, 40–60 × 4–8 µm, fusiform with subacute apices, thin walled, colourless. Cheilocystidia and pleurocystidia present and similar in size and form, scattered, 50–90 × 8–17 µm, fusiform with acute to subacute apices, thin-to slightly thick-walled, colourless or hyaline. Pileocystidia absent. Squamules on pileus composed of inflated chained, narrowly clavate, cylindrical to fusiform cells, 18–65 × 3–16 µm, thick-walled, golden yellow to brownish-yellow. Pileipellis under squamules an epithelium, composed of 1–4 layers, broadly ellipsoid, subglobose to obovoid cells, 24–40 × 12–28 µm, thin to somewhat thick walled, nearly colourless. Squamules on stipe composed of interwoven, filamentous to narrowly cylindrical hyphae 2–5 µm wide, thin walled, colourless to yellowish, hyaline, with few terminal cells, 25–40 × 5–10 µm, slightly thick walled. Stipitipellis composed of vertically arranged, colourless to yellowish filamentous hyphae, 3–10 µm in diam. Stipe trama composed of vertically arranged, colourless or hyaline hyphae, 3–16 µm in diam. Clamp connections abundant.
Habit, habitat and distribution: solitary on a dead wood, widely distributed in tropical, subtropical or even temperate regions of many parts of the world, May–September. Our collection was collected on a decaying wood near Doi Suthep Pui national park, Chiang Mai.
Specimens examined: THAILAND, Chiang Mai Province, Doi Suthep Pui national park, 30 July 2012, Samantha C. Karunarathna (MFLU 12-1892, new record).
GenBank Number ITS:KY649460.
Notes: Cyptotrama asprata was first described from Sri Lanka. It is widely distributed in South and Southeast Asia (Sri Lanka and Laos), East Asia (China, Japan and Korea), New Zealand, Hawaii, and Central America (Martinique and France) (Berkeley 1847; Redhead and Ginns 1980; Yang 1990; Moreau et al. 2015; Qin and Yang 2016). Cyptotrama chrysopepla morphologically resembles to C. asprata because of its’ gold yellow and scruffy appearance, but differs by having smaller squamules, hymeniderm pileipellis, thick walled elements in squamules of stipe, the non-acute subcapitate pleurocystidia and larger basidiospores (8.5–13 × 6.5–10 µm). This is the first report of C. asprata with the molecular phylogenetic confirmation from Thailand.
Boletales E.-J. Gilbert
Boletaceae Chevall.
Boletaceae is a family of mushrooms, first described by the French botanist François Fulgis Chevallier in 1826 as a family distinct from Agaricaceae, primarily characterized by developing their spores in small pores on the underside of the mushroom, instead of gills, as are found in agarics. As a whole, the typical members of the family are commonly known as boletes.
Austroboletus
The genus Austroboletus (Corner) Wolfe was established by Wolfe (1979) with the boletoid mushrooms showing the combination of striking morphological features: white to pale cream coloured tube and pore surface which gradually turn pink to pink-rose or sometimes vinaceous on bruising; lacunose to lacerate or reticulate stipe surface; spore print pink to vinaceous or purple brown to rust cinnamon, basidiospores with warted to pitted or reticulate ornamentations; ixotrichodermium or trichodermium nature of pileipellis (Wolfe 1979; Das and Dentinger 2015). According to recent phylogenetic analysis of the members of Boletaceae, this genus is placed under subfamily Austroboletoideae (Wu et al. 2014). About 30 species are reported globally (Kirk et al. 2008) in this genus. Here we describe a new Austroboletus species with the support of morphology and phylogeny (Fig. 146) which was collected from Uttarakhand of India.
Phylogenetic relationships inferred from neighbour-joining analysis of ITS-rDNA sequences of 22 taxa. The optimal tree with the sum of branch length = 0.82684195 is shown. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The novel Indian species: Austroboletus appendiculatus having GenBank Accession Number KX530028 (ITS-rDNA) is shown in bold and blue. The topology is rooted with Boletus edulis. Evolutionary analysis was conducted in MEGA 6.0 (Tamura et al. 2013)
Austroboletus appendiculatus Semwal, D. Chakr., K. Das, Indoliya, D. Chakrabarty, S. Adhikari & Karunarathna, sp. nov.
MycoBank number: MB 817672; Facesoffungi number: FoF 2952, Figs. 147, 148 Etymology: referring to stipes (of basidiomata) with appendages.
Austroboletus appendiculatus (CAL 1304, holotype). a–c Fresh basidiomata in the field and base camp. d Hymenophoral trama. e Terminal cells of hyphae of pileipellis. f Cross section through stipitipellis showing anastomosing ridges. g, h Caulocystidia. i Basidiospores. j, k SEM micrograph of basidiospores. Scale bars a = 100 mm, d = 50 µm, e, g–j = 10 µm, f = 100 µm, k = 5 µm
Holotype: CAL 1304
Diagnosis: is distinct from the closest species A. subvirens by larger and differently coloured pileus, longer and wider stipe, presence of pleurocystidia and caulocystidia and putative association with Shorea sp.
Pileus 75–90 mm. diam.; convex when young, to plano-convex, sometimes uplifted with age; surface areolate-squamose, then disrupted into several patches or segments with the expansion exposing yellowish-orange to greyish-yellow (4B7–4A7, 4B6) subpellis that becoming paler towards margin as pastel yellow to butter yellow, light yellow (3A4–4A5, 4A4); segments of suprapellis light brown to brown, cottony, slightly viscid when wet; margin plane, regular. Pore surface pinkish when young, gradually on bruising or maturity greyish-orange to brownish-orange (5B3–6C3), reddish-grey (12B2), or darker to brown, but paler toward the stipe juncture as light orange grey (6B2), depressed at junction to stipe, unchanging on bruising, pores often up to 2 mm broad at maturity, angular to rectangular, attached. Tubes 12–16 mm long, concolorous to slightly paler then pore surface. Stipe 80–120 × 20–30 mm, central, terete or more or less fusoid towards down, surface distinctly covered with pale orange to light orange (4–5A2–4) or sometimes yolk yellow (4B8) anastomosing broad ridges or appendages forming elevated or flaring to wing-like network (on yellowish-white background) which gradually becoming thin (less elevated) reticulum towards apex, but, never with typical lacerate surface, base with white mycelia elements; ridges cottony, darker with age as golden yellow to brownish-yellow (5B5–6, 5C6). Context white, unchanging, fluffy, partly hollow in stipe. Odour mushroomoid. Taste indistinct. Spore print rust brown to reddish-brown (6E8–8E8). Basidiospores 14.2–14.8–16.5 × 7.3–8–9.1 µm (n = 20, Q = 1.68–1.83–1.97), broadly fusoid to amygdaliform, depressed suprahilar part, surface appears warted by disruption of the outer wall, deeply pitted to form punctuate-warted; under SEM, pits minute at both the fusoid ends, gradually with intricate furrows towards mid region, at mid region with more broader and deeper pits forming warty surface with truncate warts, with disrupted adaxial surface. Basidia 22–32 × 12–16 µm, 4–spored, clavate. Pleurocystidia rare. Tube trama boletoid, gelatinous. Pileipellis trichoderm, composed of erect, septate hyphae; terminal cells cylindrical with rounded apex, width 6–9 µm. Stipitipellis fertile, composed of caulocystidia and caulobasidia. Caulocystidia 40–70 × 11–20 µm, clavate to subclavate, subventricose to appendiculate. Caulobasidia 30–45 × 9–12 µm, 2- to 4-spored, clavate.
Habitat and distribution: Under Shorea robusta Gaertn. in subtropical broadleaf forest.
Material examined: INDIA, Uttarakhand, Dehradun District, Tapovan forest, 650 m, 17 July 2010, K.C. Semwal, KCS 1401 (CAL 1304, holotype; isotype: BSHC).
GenBank Numbers ITS:KX530028.
Notes: Austroboletus appendiculatus is typically featured by combination of characters: brownish areolate-squamose pileus with exposed yellowish subpellis, distinctively reticulate-appendicute and non-lacerate stipe surface, pinkish pore surface that becomes brownish to greyish on bruising or after maturity, partly hollow stipe, almond-shaped deeply pitted basidiospores with distinct suprahilar depression and prominently disrupted adaxial surface and occurrence with putative association of Shorea in subtropical broadleaf forest.
In the field, Austroboletus subvirens (Hongo) Wolfe [≡Porphyrellus subvirens Hongo], A. lacunosus (Kuntze) T.W. May & A.E. Wood [≡A. cookie (Sacc. & Syd.) Wolfe] and A. novae-zelandiae (McNabb) Wolfe [≡Porphyrellus novaezelandiae McNabb] are quite similar (as far as the stipe surface is concerned) to the present taxon. But, A. subvirens (probably the closest species being reported earlier from Japan, Papua New Guinea, China, Americas and Australia and labelled the Australian Material here with GenBank Numbers KP242207, KP242214, KP242212 and KP242210 in Fig. 146) has distinctively smaller (25–60 mm) and differently coloured pileus, shorter and thinner stipe (50–90 × 4–10 mm), and lacks pleuro-, cheilo- and caulocystidia (Wolfe 1979; Jian-Zhe 1985). Moreover, A. subvirens are found to be associated with the trees of Fagaceae in Australasia and Americas and Acacia, Allocasuarina and Eucalyptus in Australia (Wolfe 1979; Horak 1980a, c; Halling et al. 2006) whereas, A. appendiculatus (present species) grows under Shorea robusta (Dipterocarpaceae). Austroboletus novae-zelandiae (reported from New Zealand and New Caledonia and labelled with GenBank Numbers accession number HM060327 in Fig. 146) is separated from the present species by distinctively shorter and slender stipe (70 × 8 mm), bigger (17–19.5 × 8–10.4 µm) basidiospores (Wolfe 1979). Austroboletus lacunosus (reported from Australia and labelled with GenBank Accession Number KC552014 in Fig. 146) can also be separated from the present taxon by smaller pileus (15–25 mm), and stipe (20–50 × 5–6 mm) and smaller (32.5–56 × 10–15.5 µm) caulocystidia (Wolfe 1979).
Only two other species, hitherto known from India: A. olivaceoglutinosus K. Das & Dentinger (labelled with GenBank Numbers KM597478 and KM597479 in Fig. 146) and A. gracilis (Peck) Wolfe can easily be differentiated in the field from the present novel species. A. olivaceoglutinosus which is found in subalpine Himalayan region under Tsuga and Picea has distinguishingly long and slender basidiomata (Pileus 30–55 mm diam., stipe 100–180 × 13–17 mm), highly glutinous olive coloured pileus, lacerate (never with ridges) stipe surface and narrower (12.7–19 × 5.9–7.7 μm) basidiospores showing different ornamentation pattern (Das and Dentinger 2015) whereas, A. gracilis has different pilear surface (subtomentose, velvety or fibrillose), smaller caulocystidia: “14–42(–57) × 4–14 μm” and different pattern (“rugulose punctate”) of spore ornamentation, different host associations (Wolfe 1979; Lakhanpal 1996).
Considering the spore ornamentation, A. fusisporus (reported from Japan and China and labelled with GenBank Numbers AB509830 and JX889719 in Fig. 146) also appears close to A. appendiculatus but the former has altogether different and very small (pileus 5–20 mm, stipe 20–45 × 2–4 mm) basidiomata (Wolfe 1979).
Our ITS phylogeny (Fig. 146) were obtained by the neighbour-joining analysis criterion considering the all available ITS sequences available in GenBank and/or appeared in BLAST search with the sequence of our Indian material. The new sequence forming a separate clade is clustered (80% bootstrap) with the sequences of A. subvirens derived from Australian Material (Fig. 146). These two species in turn is clustered (80% bootstrap) with A. festivus (sequence derived from specimen collected from Guyana).
Unique combination of macro- and micromorphological features coupled with ITS phylogeny clearly support the novelty of Austroboletus appendiculatus.
Boletellus Murrill
Boletellus is a genus of mushrooms in the family Boletaceae (Binder and Hibbett 2006). The genus was first introduced by American mycologist William Alphonso Murrill in 1909 (Murrill 1909). The genus contains about 50 species, has a widespread distribution, especially in subtropical regions (Kirk et al. 2008) (Fig. 149).
Phylogenetic relationships inferred from maximum parsimony analysis of ITS-rDNA sequences of 28 taxa. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The new Sri Lankan record: Boletellus emodensis having GenBank Accession Number KY649458 and Herbarium Number MFLU 12-1214 is shown in bold and blue. The topology is rooted with Boletus sp. Evolutionary analysis was conducted in PAUP 4.0b 10
Boletellus emodensis (Berk.) Singer, Annales Mycologici 40: 19 (1942)
Facesoffungi number: FoF 3141, Figs. 150, 151
Basidiomata (small to medium-sized. Pileus 3–9 cm in diam., convex to applanate; surface smooth and dry, purple to dull red colour, fading to pale brown with age, finely tomentose when young, then peeling or cracking into large squamules; at first margin extended into a fake veil and covering the pores, then radially splitting, appendiculate with fake veil remnants; context 0.5–1.2 cm thick in the pileus center, yellowish, strongly and rapidly turn to blue when bruised or injured. Hymenophore poroid, yellowish, strongly and rapidly turn to blue when bruised or injured; pores 0.1–0.2 cm diam., simple and angular. Stipe central, 6–8 × 0.7–1 cm, cylindrical, firm, solid; surface concolorous with the pileus, dry, fibrous but apex yellowish; context yellow, strongly and rapidly turn to blue when bruised or injured. Annulus not present. Basidia 33–55 × 10–18 μm, clavate, colourless to yellowish-brown in KOH, mostly 4-spored. Basidiospores 18–24 × 8–11 μm, Q = 1.9–2.8, Qm = 2.28, yellowish-brown in KOH, elongate-ellipsoid with longitudinal ridges, 6–9 ridges visible in lateral view; ridges up to 0.9–1.6 μm in height. Tube trama made up of thin-walled, colourless to yellowish hyphae 5–13 μm in width. Cheilocystidia 46–69 × 10–18 μm, thin-walled, subfusiform to fusiform, colourless or yellowish-brown in KOH. Pleurocystidia 68–92 × 10–20 μm, subfusiform to fusiform, colourless to yellowish-brown in KOH. Pileipellis is an intricate trichoderm composed of interwoven, thin-walled, yellowish-brown hyphae; terminal cells 18–36 × 6–12 μm, with round to obtuse apex. Pileal trama composed of thin walled, colourless to yellowish hyphae 4–16 μm diam. Clamps absent.
Habit and habitat: Solitary or gregarious on soil, tree stumps or rotten wood in forests dominated by Fagaceae
Distribution: originally described from India; then reported from southeast Asia, east Asia and Australia, this is the first report from Sri Lanka with morphological characteristics and phylogenetic data.
Specimens examined: SRI LANKA, Kandy District, Hanthana Mountains Range, 10 June 2012, Samantha C. Karunarathna (MFLU 12-1214, new record).
GenBank Number ITS:KY649458.
Notes: Boletellus emodensis is characterized by its purple to dull red pileus, yellowish context in both pileus and stipe that rapidly and strongly turn to blue when injured or bruised, ridged basidiospores with fine striations on the ridges can be observed under the high power of light microscope. This is the first report of B. emodensis with the molecular phylogenetic confirmation (Fig. 149) from Sri Lanka.
Rubroboletus Kuan Zhao & Zhu L. Yang
The genus Rubroboletus (Zhao et al. 2014), typified by R. sinicus (W.F. Chiu) Kuan Zhao et Zhu L. Yang, was established to accommodate Boletaceae with reddish pileal surface, an orange, orange-red to blood red surface of the hymenophore, yellow tubes, pink to red net or spots on the stipe, a bluish colour-change when injured, a non-amyloid context (even if the original diagnosis says “not observed”), smooth spores, olive-brown in deposit, and an interwoven trichodermal pileipellis (Fig. 152).
Phylogeny of the genus Rubroboletus based on a Bayesian and Maximum Likelihood Inference analysis of a ITS dataset. Bayesian posterior probability values (in bold) ≥0.7 and maximum likelihood bootstrap (ML) values ≥50% are shown on the branches. Thickened branches indicate BYPP ≥ 0.95 and ML support ≥70%. Dashed branches indicate BYPP ≥ 0.95 and ML bootstrap support <70%. Nodes that receive BYPP < 0.95 but with ≥70% ML are indicated by small black-filled squares. Newly sequenced collections are in bold. Caloboletus calopus (HM347645) was chosen as the outgroup taxon
Rubroboletus demonensis Vasquez, Simonini, Svetasheva, Mikšík & Vizzini, sp. nov.
= Boletus rhodopurpureus f. polypurpureus s. Ruiz Fernandez & Ruiz Pastor, Guía Micologica Tomo n. 4, Supl. Orden Boletales en España: 62 (2006)
= Boletus rubrosanguineus s. Calzada Dominguez 2007, Guía de los Boletos de España y Portugal: 163 (2007)
= Boletus legaliae s. Rodà, Funghi Aspromontani Comparati Boletales: 96 (right), 97 (top), countercover (2012)
Index Fungorum number: IF552526; Facesoffungi number: FoF 2953, Figs. 153, 154, 155
Rubroboletus demonensis (GS10244, holotype). Basidiomes from the main collections. a 9 October 2013, C.da Piano Torre (Madonie), Castelbuono (PA), with Quercus pubescens s.l. and Q. ilex. b 5 September 2013, Portella dell’Obolo (Nebrodi), Capizzi (ME), with Q. pubescens s.l. c 4 August 2014, near Village Nimea, Komotini, Rhodope Regional Unit, with Q. petraea ssp. medwediewii. d 15 September 2013, Portella dell’Obolo (Nebrodi), Capizzi (ME), with Q. pubescens s.l. e 15 September 2013, Portella dell’Obolo (Nebrodi), Capizzi (ME), with Q. pubescens s.l. f 27 June 2009, Portella Sella Maria (Nebrodi), Cesarò (ME), with Q. pubescens and Q. virgiliana. g 30 June 2008, pizzo Inferno (Nebrodi), Floresta (ME), with Castanea sativa and Pinus nigra ssp. calabrica. h GS10274. i GS10244 (Holotype). j GS10708. k GS10278. l GS10242. Bars 50 mm
Rubroboletus demonensis (GS10244, holotype). Microscopic structures. a Pileipellis in 3% NH3 + Congo Red, with Differential Interference Contrast (DIC) (GS10244). b Spores in 3% NH3 (GS10244). c left Spores in 3% NH3 (GS10274). c right Abnormal spores in 3% NH3 (GS10243). d Basidia in 3% NH3 + Congo Red (GS10245). e Facial cystidia in 3% NH3 + Congo Red, with DIC (GS10245). f Marginal cystidia in 3% NH3 + Congo Red, with DIC (GS10245). Bars 10 μm
Distribution of spore size of R. demonensis (7 collections), compared with R. legaliae (13 collections) and R. rubrosanguineus (20 collections), using “isoprobability ellipses”. It is shown the distribution of the average values of spore size for any of the collections, at the confidence of 68% (corresponding to 1st deviation, left side) and at the confidence of 95% (correspondent to 2 × SD, right side)
Etymology: the specific epithet of the name of the taxon comes from the Latin word “demonensis” and refers to the ancient name “Valdemone”, which was one of the three valleys (“valli”) or real domains (“reali dominii”) in which the region Sicily was subdivided from the Muslim domination to the Bourbon period; the “Valdemone”, with respect to the “Val di Noto” and the “Val di Mazzara”, constitutes the north-eastern part of the Sicily, an area that closely corresponds to the actual habitat of R. demonensis. Furthermore, the epithet “demonensis” well reminds the peculiar features of the species, i.e. the flaming red colour of the cap and the pores, features shared with other close “devilish” species, belonging to the same genus (R. satanas). Moreover, unforeseeable circumstances would have wanted that one of the first growth stations of R. demonensis had been the locality “Pizzo Inferno” (i.e.: Hell Peak) on the Nebrodi chain, in the common of Floresta.
Holotype: MCVE 29081 (GS10244)
Pileus 60–150(–240) mm broad, initially hemispherical, then convex, pulvinate, in the end almost applanate in mature basidiomes. Margin ± regular in young, soon uneven, ±undulate-lobate, sometimes distinctly lobate in mature specimens. Colour at the very beginning whitish, pale grey, pale ochre (4/A1–B2), soon, starting from the margin, dirty pale pink-lilac (5–12/A1–B2), then strongly variable from light grey (5–7/B1–C2), to pink-lilac (7–12/B2–C3), to purple-red (10–12/B3–C6, 11–12/C6–D8) depending on environmental conditions; with wet weather or in shady woods, the pileus shows particularly bright colours, tending to purple-blood red, quite uniformly distributed on the pileus surface (11–12/C8–D8). In some occurrences, red colour seems not to develop completely both in pores and stipe, and in meantime also the pileus colour stabilizes on a pink-lilac uniform colour (8–11/A3–B4), that becomes blood red when bruised. Conversely, in dry weather or in areas exposed to the sun, pileus colour is much more variable, with flesh pink colours sometimes fading to brownish-grey or to paler tone tending to cream-whitish (7–9/B2–D3). However, in the most of the basidiomes observed, large intensely reddish areas or wide blood red spots persist, even more intense following to scrubbing, touching or other manipulation. Surface typically tomentose and dry at the beginning, sometimes smooth and viscous in wet weather; the cuticle is not detachable from the underlying flesh. Often, in mature large basidiomes, the pileus appears pleasantly bright shining red, darker when bruised. Looking closely at the pileus surface, also with the aid of a lens, minute, in relief, concolorous scales can be noticed. This feature is shared with the close species R. rubrosanguineus, R. legaliae, R. pulcherrimus and R. sinicus. Stipe 80–120(–150) × 40–80(–90) mm, bulky, robust and wide, cylindrical, often gradually enlarged or clavate towards the base, sometimes also obese, not rooting. Surface in rare case yellow orange at the very beginning (4–6/A4–A6), with purplish base (9–10/A4–B6), then, passing through intermediate orange-red tones (7–8/A4–B6), in the end bright red, blood red, purple red, often darker at the base (9–10B7–D8, 9–11D6–D8), often with an evident deep yellow, or orange-yellow band (5–15 mm wide) in the upper part. In some xanthoid aspects stipe does not develop red tones in the upper half, remaining yellow or orange yellow, but the stipe base is anyway red, purple-red, blood red. Bitten areas often show the deep yellow flesh colour, giving a chromatic effect reminiscent of R. rhodoxanthus. A sharp net, with regular meshes, is well distributed over at least ¾ of the height, darker than the colour of the ground; the meshes are usually larger and the ribs usually thicker than those of B. rubrosanguineus or B. legaliae, giving to the net a general rougher appearance with respect to the ones of the close species. The extreme base of the stipe is covered with a whitish grainy, furfuraceous over layer, particularly evident in basidiomes collected on moist grounds. Tubes 5–12(–20) mm, having average length, free at the stipe, from deep yellow to olive green, discolouring blue when cut. Pores small, round, usually from the beginning purple-red, dark red (10–11/C7–D8, 11–12/D7–F8), dull blue when touched, in aged basidiomes orange-yellow towards the margin of the pileus. In some collections, young specimens have yellow pores, that with age develop only an orange, red–orange colour, reminiscent that one of typical R. legaliae. Flesh firm, yellow, deep yellow, lemon yellow (3/A4–A8), particularly intensive in both stipe and pileus wounds; when exposed, quickly turning sky-blue, blue, deep blue (21–23/A4–B6), due to discolouring not particularly intensive, sometimes weak, then fading to a pale grey-cream colour. In some cases, in the exposed pileus or mostly in the lower part of the stipe some beetroot shades may appear. Sub-hymenophoral layer concolorous. Taste sweetish, slightly acidic. Smell weak, fungal and pleasant. Spore print tobacco-brown. Amyloid reaction in the flesh at the stipe base, according to Imler’s procedure (Imler 1950): the tissues at the stipe base are not amyloid in all the collections if examined microscopically. As in R. legaliae and R. rubrosanguineus, sometimes the amyloid reaction could be macroscopically “positive” (Cheype 1983) due to the absorption of the Melzer’s regent in the tissues. Basidiospores smooth, fusiform ellipsoid, with a more or less pronounced suprahilar depression, (12.5–)12.6–13.6(–13.9) × (4.6–)4.7–5.1(–5.1) µm (363/7/7), on average 13.1 ± 0.5 × 4.9 ± 0.2 µm, Q = (2.53–)2.57–2.79(–2.89), Qm = 2.68 ± 0.11, from light yellow to yellow–brownish in KOH. They may have different shape in different collections, despite the molecular investigation has confirmed them as belonging to the same species. In some collections, they are ellipsoid (Fig. 156b), more elongate with respect to those of R. legaliae, smaller both in width and in length with respect to those of R. rubrosanguineus but with almost the same Q ratio; in other collections, they are more fusiform, with the apex opposed to apiculum more tapered and acuminate (Fig. 156c). In some collections, lots of irregular spores, with various, irregular protuberances, may also occur (Fig. 156c(right)). Basidia mostly 4-spored, hyaline, (23.8–)31.3–47.7(–62.6) × (4.2–)7.7–12.7(–13) µm (34/2/2). Facial cystidia fusiform, versiform, sometimes lageniform, hyaline, (15.5–)29.5–44.7(–50.7) × (4.2–)5.3–7.7(–9.5) (64/2/2). Marginal cystidia similar to facial cystidia but smaller, mostly versiform than fusiform or lageniform, hyaline (15.8–)20–29 (37.2) × (3.5–)4.3–6.3(–7.9) (46/2/2). Pileipellis an entangled trichodermium, tending to a cutis, often gelatinized in mature individuals, consisting of thin, cylindrical elements, collapsing gradually during development of the basidiomes. Terminal elements (33.3–)32.9–42.9(–44.3) × (5–)5.2–5.9(–6.9) µm (138/5/5), Q = (6.6–)6.4–8.1, Qm = 7.3, with rounded or tapered tip, sometimes clavate, rarely capitulate or pear-shaped, with pale yellow vacuolar pigment and incrusted pigmented here and there, mostly in the deeper and closer to hypoderma hyphae. Hymenophoral trama boletoid in the sense of Singer (1965, 1967). Clamp connections absent.
Phylogram generated from neighbour-joining analysis based on ITS-rDNA sequences: Phylogenetic relationships inferred from neighbour-joining analysis of ITS sequences of 25 taxa. The optimal tree with the sum of branch length = 0.79320854 is shown. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The novel species having GenBank Numbers KX364694 (ITS-rDNA) is shown in bold and blue font. Boletus edulis (European material with GenBank Numbers AY278764) was considered as the out group. Evolutionary analysis was conducted in MEGA6 (Tamura et al. 2013)
Habitat: Rubroboletus demonensis is typical in acid and silicic soils, thermophilic, in summer. It grows in groups of small basidiomes in warm deciduous forests, in mountains or in some cases in mixed deciduous and coniferous woods (Pinus nigra and Taxus baccata), never with pure conifers. It prefers mesic forests of deciduous oaks (Quercus pubescens sensu lato, Q. cerris, Q. congesta and Q. virgiliana), rarely with holm (Q. ilex). It is common in chestnut woods (Castanea sativa), pure or mixed, and at higher elevations with beech (Fagus sylvatica). It is usually collected in June after the late spring rains and, not liking the summer drought, it disappears during the hottest period of July and August, then appears again in September. During the warmer autumns, it can be observed until the first half of October.
Distribution: R. demonensis was found in a rather delimited areal of the South Italy [(Sila Plateau (“Altopiano della Sila”) in Calabria] and Sicily, where it inhabits the mesomediterranean and supramediterranean zone of the mountain strip, characterized by a humid Mediterranean climate, with large rainfalls and Mediterranean humid forest well represented by deciduous forests of oak (Q. pubescens s.l.) and chestnut (C. sativa), some rare patches of evergreen forest with Q. ilex, with the presence of Pinus nigra ssp. calabrica, in an altitudinal range between 1200 m and 1600 m a.s.l.. Sicily seems to be the southern limit of distribution of the species. A unique collection from Greece (04.08.2014, near Nimea Village, Komotini, Rhodope Regional Unit, coord. 4112′42.2″N, 2531′16.5″E, alt. 650 m a.s.l., leg. D. Tragkos) with Q. petraea ssp. medwediewii, proved only by photographic material, represents the most eastern collection. The findings in Spain under Q. faginea and Q. ilex, until now attributed to Imperator rhodopurpureus var. polypurpureus (Ruiz Fernandez & Ruiz Pastor 2006) or R. rubrosanguineus (Calzada Dominguez 2007), seem likely referable to R. demonensis, and, if confirmed, might represent the western (Zamora, Salamanca) and northern (Navarra, Palencia, Huelva) limits of the distribution of the species. The main collection sites in Sicily are the north-eastern mountain range (Appennino Siciliano), the Nebrodi chain and the Madonie chains, but it has also been reported on the Etna volcano massif, where it grows even at higher altitudes. The most of the collections from Sicily comes from the commons of Cesarò, Floresta, Maniace and Troina in the Nebrodi Mountains Park, in the province of Messina.
Edibility: like other closely related species in this genus, it is probably poisonous if consumed raw.
Material examined: (GS refers to the personal herbarium of G. Simonini): Rubroboletus demonensis. ITALY—CALABRIA. 20 June 1999, Gambarie (RC), with C. sativa, coord. 3810′N, 1550′E, alt. 1300 m a.s.l., leg. C. Lavorato, GS2129. 9 June 2013, S. Domenica di Terreti (RC), with C. sativa, coord. 3810′21″N, 1547′48″E, alt. 900 m a.s.l., leg. P. Rodà, GS10708. SICILY. 26 September 2014, Portella Sella Maria (Nebrodi), Cesarò (ME), with F. sylvatica and Q. pubescens, coord. 3754′1″N, 1439′17″E, alt.1400 m a.s.l., leg. G. Vasquez, GS10400; 19 June 2015, Lago Biviere di Cesarò (Nebrodi), Cesarò (ME), with F. sylvatica, coord. 3757′6″N, 1442′50″E, alt. 1290 m a.s.l., leg. M.G. Pulvirenti, GS10242; 22 June 2015, Portella Sella Maria (Nebrodi), Cesarò (ME), with F. sylvatica and Q. pubescens, coord. 3753′57″N, 1439′15″E, alt. 1470 m a.s.l., leg. D. Milazzo, GS10243; 28 June 2015, Portella Sella Maria (Nebrodi), Cesarò (ME), with F. sylvatica and Q. pubescens, coord. 3754′1″N, 1439′17″E, alt. 1420 m a.s.l., leg. G. Vasquez, MCVE 29081, (GS10244, holotype); 20 August 2015, Piano della Cicogna, Cesarò (ME), with Q. cerris, coord. 3752′55″N, 1439′32″E, alt. 1350 m a.s.l., leg. M.G. Pulvirenti, GS10274; 20 September 2015, Piano della Cicogna, Cesarò (ME), with Q. cerris, coord. 3752′59″N, 1439′38″E, alt. 1320 m a.s.l., leg. M.G. Pulvirenti, GS10278.
Additional material examined (MM and JLC refer to the personal herbaria of M. Mikšík and J.L. Cheype): Rubroboletus legaliae. CZECH REPUBLIC—17 July 2005, Lednice, chateau park, with Q. robur, 4848′44″N, 1648′25″E, alt. 230 m a.s.l., leg. M. Mikšík, MM 17705B2; 26 July 2008, Bechov-Svobodín, Mladá Boleslav, with Q. petraea and Carpinus betulus, 5026′7″N, 154′52″E, alt. 250 m a.s.l., leg. M. Mikšík, MM 26708B1; 6 August 2012, Dlouhopolsko, Nymburk, “Kněžičky” reserve, with Q. petraea, 509′40″N, 1520′24″E, alt. 240 a.s.l., leg. M. Mikšík, MM 6812B2; 9 August 2012, Dlouhopolsko, Nymburk, “Kněžičky” reserve, with Q. petraea, 509′46″N, 1519′47″E, alt. 250 a.s.l., leg. D. Kvasnička, MM 9812B3; 5 September 2015, Prodašice, Mladá Boleslav City, with Q. petraea and C. betulus, 5021′41″N, 157′44″E, alt. 250 m a.s.l., leg. J. Schneider, MM 5915B4. SLOVAK REPUBLIC—8 July 2008, Hontianske Nemce, Krupina, “Štiavnické vrchy″Natural reserve, with Q. pubescens, 4818′7″N, 1858′58″E, alt. 337 a.s.l., leg. M. Mikšík, MM 8708B1. HUNGARY—27 July 1997, Pilisszentkereszt, Budapest, with Tilia sp., Q. cerris, Q. petraea, coord. 4742′N, 1855′E, alt. 450 m a.s.l., leg. A. Kiss, GS1881. ITALY—EMILIA-ROMAGNA. 16 September 1983, Pulpiano, Viano (RE), with Q. cerris, coord. 4430′32″N, 1033′31″E, alt. 514 m a.s.l., leg. unknown, GS12. SARDINIA. 31 July 1990, Case Floris, Desulo (NU), with Q. pubescens and C. sativa, coord. 4000′N, 912′E, alt. 1066 a.s.l., leg. G. Simonini, GS735; 9 November 1994, Cala Gonone, Dorgali (NU), with Q. ilex, coord. 4016′22″N, 936′47″E, alt. 187 m a.s.l., leg. A. Montecchi, GS1310. SICILY. 21 October 2013, Portella dei Bufali (Nebrodi), Cesarò (ME), with Q. cerris and Q. pubescens, coord. 3752′17″N, 1441′17″E, alt. 1180 m a.s.l., leg. G. Simonini, GS10112; 3 October 2014, Portella dei Bufali (Nebrodi), Cesarò (ME), with Q. pubescens and Q. cerris, coord. 3752′17″N, 1441′17″E, alt. 1180 m a.s.l., leg. G. Vasquez, GS10304; 28 June 2015, Portella Sella Maria (Nebrodi), Cesarò (ME), with F. sylvatica and Q. pubescens, coord. 3754′1″N, 1439′17″E, alt. 1420 m a.s.l., leg. G. Vasquez, GS10245. RUSSIA—23 July 1983, vic. of township Soglasie, Penza Oblast, with Q. robur on the carbonaceous soil, leg. A.I. Ivanov, Le 200041; 8 August 2008, Voronka River Reservoir, vic. of Podgorodnie Datchi Village, Leninsky District, Tula Oblast, with Q. robur, coord. 54 6′5″N, 3732′53″E, alt. 150 m a.s.l., leg. T. Svetasheva, GS10317; 14 July 2011 (also 16 August 2011, 27 July 2012, 5 August 2012), protected area “Nizovie Plushchan river”, “Galichia Gora” Reserve, Krasninsky District, Lipetsk Oblast, with Q. robur, leg. T. Svetasheva, L. Sarycheva; 1 September 2012, vic. of village Podgorodnie Datchi, Leninsky District, Tula Oblast, with Q. robur, coord. 54 6′5″N, 3732′53″E, leg. T. Svetasheva, GS10311; 21 September 2012, vic. of khutor Leshchevsky, Leninsky District, Volga-Akthuba Floodplain, Volgograd Oblast, with Q. robur, coord. 4831′37.44″N, 4451′42.90″E, alt. 30 m a.s.l., leg. T. Svetasheva, GS10308; 14 October 2013, loc. Shutov Ugol, vic. of khutor Leshchevsky, Leninsky District, Volga–Akthuba Floodplain, Volgograd Oblast, with Q. robur, coord. 4831′50.29″N, 4455′25.94″E, alt. 20 m a.s.l., leg. T. Svetasheva, GS10304. Rubroboletus rubrosanguineus. AUSTRIA—10 August 1998, Thurntaler, Sillian, with Picea abies, coord. 4645′54″N, 1224′39″E, alt. 1800 m a.s.l., leg. G. Simonini, GS1930. CZECH REPUBLIC—17 August 2014, Francova Lhota, Beskydy Mountains protected area, with P. abies and Abies alba, alt. 650 m a.s.l., leg. J. Polcak, GS10285. FRANCE—date unknown, loc. unknown, with P. abies, leg. J.L. Cheype, HOLOTYPE JLC 83209. ITALY—EMILIA-ROMAGNA 20 July 1987, Le Borelle, Villa Minozzo (RE), with F. sylvatica, coord. 4418′N, 1024′E, alt. 1300 m a.s.l., leg. L. Cocchi, GS405; 31 July 1987, Le Borelle, Villa Minozzo (RE), with F. sylvatica, coord. 4418′N, 1024′E, alt. 1300 m a.s.l., leg. L. Cocchi, GS410; 1 September 1991, Costa delle Veline, Villa Minozzo (RE), with F. sylvatica, coord. 4416′N, 1025′E, alt. 1700 m a.s.l., leg. A. Montecchi, GS828; 8 August 1995, M. Prampa, Villa Minozzo (RE), with F. sylvatica, coord. 4420′N, 1026′E, alt. 1300 m a.s.l., leg. L. Cocchi, GS1387. TRENTINO–ALTO ADIGE 13 August 1997, Pochi, Salorno (BZ), with P. abies and F. sylvatica, coord. 4614′N, 1115′E, alt. 1400 m a.s.l., leg. K. Kob, GS1818; 15 August 1998, Fai della Paganella, Andalo (TN), with F. sylvatica and P. abies, coord. 4611′N, 1104′E, alt. 1100 m a.s.l., leg. M. Comuzzi, GS1960; 28 August 1999, Vipiteno (BZ), with P. abies, coord. 4654′N, 1125′E, alt. 1150 m a.s.l., leg. unknown, GS2131; 25 August 1999, Waldheim, Sarnonico (TN), with A. alba, coord. 4625′N, 1110′E, alt. 1230 m a.s.l., leg. G. Simonini, GS2282. FRIULI VENEZIA–GIULIA, 27 June 1998, Bosco Taggers, Lauco (UD), with P. abies, coord. 4625′N 1257′E, alt. 950 m a.s.l., leg. G. Zecchin, GS1899; 27 June 1998, Bosco Taggers, Lauco (UD), with P. abies, coord. 4625′N 1257′E, alt. 950 m a.s.l., leg. G. Zecchin, GS1900. VENETO, 10 July 1998, Val di Cadore (BL), with P. abies, coord. 4624′N, 12 19′E, alt. 950 m a.s.l., leg. P. Gaggio, GS1911; 11 July 1998, Val di Cadore (BL), with P. abies, coord. 4624′N, 12 19′E, alt. 950 m a.s.l., leg. P. Gaggio, GS1912; 11 July 1998, Val di Cadore (BL), with P. abies, coord. 4624′N, 1218′E, alt. 920 m a.s.l., leg. P. Gaggio, GS1913; 12 July 1998, Val di Cadore (BL), with P. abies, coord. 4624′N, 1219′E, alt. 930 m a.s.l., leg. P. Gaggio, GS1917; 12 July 1998, Val di Cadore (BL), with P. abies, coord. 4625′N, 1218′E, alt. 750 m a.s.l., leg. P. Gaggio, GS1918; 21 August 2012, Padola (BL), with A. alba and F. sylvatica, coord. 4635′N, 1228′E, alt. 1550 m a.s.l., leg. A. Testoni, GS10097. RUSSIA—19 August 2009, Arkhys, Teberda Reserve, valley of Kyzgich river, Karachay–Cherkessia Republic, Caucasus, with Abies nordmanniana. and F. sylvatica, coord. 4340′N, 4153′E, alt. 1050 m a.s.l., leg. T. Svetasheva, A. Kovalenko, GS10315 and GS10316.
GenBank Number ITS:KY677920.
Notes: During mycological surveys and mapping of Northeast of Sicily, in the territory between mountain ranges of Nebrodi, Peloritani and Madonie and the massif of Mount Etna, several collections of a Boletaceae apparently close to Rubroboletus rubrosanguineus (Cheype) Kuan Zhao & Zhu L. Yang of Northern Italy were observed. They grew exclusively with broadleaved trees and in the first instance they were included in the large colour variability of R. legaliae (Pilát & Dermek) Della Maggiora & Trassin. In the meantime, other sporadic finds of the same bolete occurred in the region Calabria, in the areas of Gambarie and Sila Piccola (Vasquez 2014). However, since the first findings, it was observed that these collections differ from these two neighbouring taxa in chorological-ecological, anatomical and macroscopic characters. The sharp delineation of the new species has become clear in additional collections (Vasquez 2012, 2013), to which some microscopic and molecular differences from the other close, but different species have been later added. Based on the BLASTn results (sequences were selected based on the greatest similarity) and the recent phylogenetic study focused on Rubroboletus (Zhao et al. 2014), sequences were retrieved from GenBank number and UNITE (http://unite.ut.ee/) databases for the comparative phylogenetic analysis with the newly generated sequences. Caloboletus calopus (HM347645) was chosen as an outgroup species. Phylogenetic analyses were performed using the Bayesian Inference (BI) (MrBayes v. 3.2.2, Ronquist et al. 2012) and maximum likelihood (ML) (RAxML v. 7.0.4, Stamatakis 2006; RAxML version 8, Stamatakis 2014) approaches. The ITS data set comprised 52 taxa (24 from GenBank number and 14 from UNITE). BI and ML trees were congruent and only the Bayesian tree with both BYPP (Bayesian Posterior Probabilities) and MLB (Maximum Likelihood Bootstrap) values is shown in Fig. 152. In the analysis, the six sequences of R. demonensis form a highly-supported clade (BYPP = 1, MLB = 100); it resulted as a distinct species, sister (with low support, BYPP = 89, MLB = 54) to R. eastwoodiae. Distinctive features of R. demonensis are the overall purple blood red colour (general appearance close to R. rubrosanguineus with brighter colours), combined with frequent orange-yellow collar in the upper part of the stipe, growth with broadleaved trees (Quercus, Castanea, Fagus) and spores narrower than in the closer species, with a higher Q ratio (13.1 ± 0.5 × 4.9 ± 0.2 µm, Qm = 2.68 ± 0.11). As in the most of the boletes, red colours can be in some situations attenuate: this originates xanthoid aspect, whose appearance is quite close to R. legaliae. R. legaliae has a paler, whitish, greyish-cream pileus tending to pink or reddish [R. legaliae f. spinarii (Hlaváček) Mikšík], more velutinous, and shares in many occurrences in Sicily the same environment of R. demonensis. It has also shorter but stouter in average spores (12.5 ± 0.4 × 5.5 ± 0.1 µm, Q = 2.29 ± 0.05) (533/13/13) and generally paler yellowish-orange pores, even if some bright red pores form do exist. It grows with broadleaved trees, mainly with oaks, in neutral-acidocline or basicline soils (Lannoy and Estades 2001; Estades and Lannoy 2004; Muñoz 2005; Andersson 2013; Halama 2016). It is nevertheless much more widely distributed in Europe and its areal extends North to Denmark, East to Penza region (Russia), South to Sicily (Italy) and West to Baixo Alentejo (Portugal), including: Austria, Bulgaria, Corse, Croatia, Czech Republic, Denmark, France, Germany, Great Britain, Hungary, Ireland, Italy, Montenegro, Norway, Portugal, Romania, Russia, Sardinia, Serbia, Sicily, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey (Courtecuisse and Duhem 2011; Halama 2016). Its occurrence in Russia, until now has been overlooked (Courtecuisse and Duhem 2011; Halama 2016), but this species is known in Russia as rather rare but typical for the middle and southern oakeries, associated with Quercus robur in regions of Penza, Tula (Svetasheva 2010), Volgograd, Stavropol, Lipetsk (Sarycheva and Svetasheva 2015). R. rubrosanguineus is a rare bolete growing in submontane and mountainous regions, on alkaline soil, in spruce and firs (Abies, Picea) woods, also mixed with beech (Fagus), rarely in pure Fagus woods and if so in the areas formerly populated by Abies; it has pileus colour more purplish than R. demonensis, without so intensive and bright red tones (Walty 1969; Cheype 1983; Lannoy and Estades 2001; Estades and Lannoy 2004; Muñoz 2005). It is widespread along the Alpine arch but its distribution western limit is constituted by Spain (Palazon 2006) and reaches the most northern distribution in the West Carpathians (Beskydy Mountains, Czech Republic), even if a doubtful record could set this limit to Belgium (Noordeloos 2010); at East, it extends to Russian Caucasus (Kiyashko 2012; Svetasheva and Kovalenko 2013). The southern limit seems to be constituted by the Italian Appennini chain, in Emilia-Romagna region, where it grows with Fagus in the areas earlier populated by Abies alba. So, its distribution includes Austria, Belgium, Bosnia and Hercegovina, Bulgaria, Croatia, Czech Republic, France, Germany, Greece, Italy, Monte Negro, Poland, western part of Russia, Slovakia, Slovenia, Spain, Switzerland (Courtecuisse and Duhem 2011). R. rubrosanguineus presents spores as slender as those of R. demonensis, but longer and broader (14 ± 0.8 × 5.4 ± 0.3 µm, Qm = 2.58 ± 0.12, (618/20/20).
From both R. rubrosanguineus and R. legaliae, R. demonensis can be distinguished by the net (reticulum) with larger meshes and thicker ribs. R. rubrosanguineus has also significantly broader pileipellis terminal elements (47.7 ± 4.9 × 7.5 ± 0.5 µm, Qm = 6.73 ± 0.49 (42/4/4)) with respect to the other two boletes (37.9 ± 5 × 5.5 ± 0.4 µm, Qm = 7.24 ± 0.89 (138/5/5) and 32.8 ± 0.1 × 5.7 ± 0., 5 µm Qm = 6.15 ± 0.51 (49/2/2) respectively for R. demonensis and R. legaliae). R. pulcherrimus (Thiers & Halling) D. Arora, N. Siegel & J.L. Frank is a North-American species apparently very similar, growing in in the North Western mixed forests of the Coastal Chain (Arora et al. 1999; Desjardin et al. 2015); it has much larger spores (14.3 ± 1.1 × 5.8 ± 0.1 µm, Q = 2.48 ± 0.15 (106/4/4) (Arora et al. 1999).
The phylogenetically closest related Boletus eastwoodiae (Murrill) Sacc. & Trotter differs in the pale greyish pileus, becoming olive-buff-grey and developing pink tones especially along the margin. The stipe is massive, abruptly bulbous with a ventricose base, with pinkish-grey colour and a concolorous or vinaceous reticulation (Bessette et al. 2000; Desjardin et al. 2015). Due to the molecular evidence offered in our work, Suillellus eastwoodiae Murrill is here combined in the genus Rubroboletus.
Rubroboletus eastwoodiae (Murrill) Vasquez, Simonini, Svetasheva, Mikšík & Vizzini, comb. nov.
Index Fungorum number: IF552780; Facesoffungi number: FoF 2813, Fig. 155
Basionym: Suillellus eastwoodiae Murrill, N. Amer. Fl. (New York) 9 (3): 152 (1910)
It is worth noting that the combination R. eastwoodiae (Murrill) Arora, C.F. Schwarz & J.L. Frank (Frank 2015) is invalid (wrong citation of the basionym). Curiously, R. demonensis shares both the growth area and edaphic-environmental conditions of another Mediterranean species very common in Sicily and Calabria, Boletus flavosanguineus Lavorato & Simonini: frequently these boletes are observed growing together (pers. obs.).
The analysis of the spore size distribution with the method of the “isoprobability ellipses” (Simonini 1992, 1998; Consiglio and Simonini 2005) (Fig. 155) is based on average values of 20 collections of R. rubrosanguineus, 13 collections of R. legaliae, and 7 collections of R. demonensis. This comparison allows to analyse better the differences of the spores both in size and shape. It could be observed that spores of R. demonensis and R. legaliae are clearly separated each other even at confidence level of 95%, having R. demonensis clearly shorter and narrower spores. R. rubrosanguineus has some overlapping with the other species only at confidence level of 95%, while at 68% there is almost no overlapping: R. demonensis has narrower and stouter spores with respect to R. rubrosanguineus, and the latter has slender and longer spores with respect to R. legaliae. The difference in width of the spores of R. rubrosanguineus and R. legaliae is statistically not significant.
Strobilomyces Berk.,
The genus Strobilomyces Berk. is a member of the family Boletaceae and characterized by greyish or blackish basidiomata; presence of fibrillose squamules or scales on pilear surface; stipe dry, woolly to shaggy, with or without annulus; ornamented basidiospores (Bessette et al. 2010; Das et al. 2014). This genus includes nearly 40 taxa (Gelardi et al. 2012; Wu et al. 2014; Antonín et al. 2015) around the globe. During a macrofungal survey to East District of Sikkim (a small Himalayan state in India), an interesting member this genus was collected and here it is described as a novel taxon (S. longistipitatus) with its morphology and phylogeny (Fig. 157).
Phylogram generated from neighbour-joining analysis based on LSU-rDNA sequences: Phylogenetic relationships inferred from neighbour-joining analysis of partial nuclear large subunit ribosomal RNA gene sequences of 15 taxa. The optimal tree with the sum of branch length = 0.27898332 is shown. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The novel species having GenBank Numbers KX364695 (LSU-rDNA) is shown in bold and blue font. Boletus edulis (European material with GenBank Numbers AF050643) was considered as the out group. Evolutionary analysis was conducted in MEGA6 (Tamura et al. 2013)
Strobilomyces longistipitatus D. Chakr., K. Das & S. Adhikari, sp. nov.
MycoBank number: MB 817357; Facesoffungi number: FoF 2954, Figs. 158, 159 Etymology: referring to the very long stipe (of basidiomata)
Strobilomyces longistipitatus (CAL 1336, holotype). a, c Fresh basidiomata in the field and basecamp. b Pore surface. d Pileipellis. e Terminal and subterminal cells of hyphal elements of pileipellis. f Transverse section through tube showing hymenophoral trama and hymenium. g Pleurocystidia. h SEM image of Basidiospore. Scale bars d = 500 µm; e = 50 µm; f = 100 µm; g = 10 µm; h = 5 µm
Phylogenetic tree of the Clavulina obtained by analyses from rDNA sequences. Taxa with two accession numbers were analysed by concatenated ITS and partial LSU rDNA; both regions ITS and partial LSU rDNA was used to analyse the other individuals. Support values (from top) are maximum likelihood, maximum parsimony and Bayesian analyses. Sequences obtained in this study are in boldface. Only support values of at least 50% are shown. The tree was rooted with Hydnum albomagnum, H. repandum and H. umbilicatum
Holotype: CAL 1336
Diagnosis: Distinct from the European species Strobilomyces strobilaceous by its exceptionally long stipe, significantly short tubes and frequent occurrence of tongue-like appendages on the wall of the ridges of basidiospores. Pileus 37–50 mm. diam.; convex when young, becoming pyramidal to convex with maturity; surface densely squamulose to floccose in numerous conical clusters of somewhat repent pattern, greyish-brown to violet brown (11E3–11F4) to blackish, reddish with KOH; margin wavy with sterile flap of tissue, brownish-black. Pore surface covered with thick cottony partial veil when young, white to dingy white; depressed near stipe, yellowish-white (3A2), turning rusty or darker after bruising; pore 2 mm, simple, angular. Tube 6 mm long, sinuate, chalky white or yellowish-white (3A2), turning reddish-orange after bruising. Stipe 110–200 × 17–28 mm, central, dark grey to (11F4) or black; surface cotton like or floccose with white basal mycelium. Context solid in pileus and stipe; context in pileus and stipe chalky white but immediately turning pastel red (8A4) and then greyish-red (8C5) or darker on exposure, turning orange (6A7) with KOH, greyish-green (25E5) with FeSO4, greyish-green (25D4) with guiacol. Spore print blackish-brown. Odour like Boletus edulis. Basidiospores 10–11.1–12.7 × 8–9.1–9.9 µm (n = 20, Q = 1.16–1.22–1.33), broadly ellipsoid to ellipsoid, ornamentation composed of high ridges forming complete reticulum; under SEM with broad and confluent ridges (of irregularly wavy walls) forming complete reticulations and with frequent tongue like erect appendages on the wall of ridges, blackish-brown. Basidia 40–58 × 14–18 µm, 2 to 4 spored, clavate to subclavate. Pleurocystidia 34–75 × 8–17 µm, pigmented, emergent 15–30 µm, common, subfusiform to fusiform, ventricose to ventricose with somewhat moniliform or capitate head; content dense, slightly fibrillose. Tube edge fertile, composed of basidia, cystidia. Cheilocystidia 39–65 × 11–13 µm, subclavate to subventricose, some are appendiculate, mostly hyaline. Tube trama divergent, tramal hyphae 5–13.5 µm wide, gelatinous, encrusted, septate, branched. Pileipellis 1000–1200 µm thick, trichododerm to palisadoderm, composed of erect elements, sometimes septate; terminal cells up to 20 µm wide, slightly thick walled (up to 0.8 µm), mostly with rounded to fusoid apices, brown pigmented, sometimes faintly encrusted; finely warted when observed under SEM. Stipitipellis trichoderm to palisadoderm, hyphae brown pigmented, wall up to 1 µm thick, often minutely encrusted; fertile, with basidia and cystidia. Caulobasidia 4-spored, similar to tube basidia. Caulocystidia 23–65 × 14–20 µm, clavate to subclavate, or fusoid to ventricose. Clamp connections not found.
Habitat and distribution: Under Abies densa in subalpine mixed (broadleaf and coniferous) forest.
Material examined: INDIA, Sikkim, East District, Memainchu, 3692 m, N2721′25.0″ E8836′24.9″, 4 July 2015, D. Chakraborty, DC 15-010 (CAL 1336, holotype).
GenBank Numbers ITS:KX364694.
Notes: Characteristic features of S. longistipitatus are presence of short pileus with almost blackish dense squamules on surface in numerous conical clusters arranged repently (never erect), exceptionally long stipe (3–4 times or more of pileus diameter) without any striation or reticulation on the surface, distinguishingly short white sinuate tubes which becomes reddish-orange on bruising, basidiospores with complete reticulum with the confluent ridges and frequent occurrence of tongue-like appendages on the ridge-walls and occurrence in association with the coniferous trees.
Our ITS based phylogeny (Fig. 156) clearly supports the existence of two distinct and strongly supported clades (Clade A and Clade B) in Strobilomyces which are also reported repeatedly by earlier workers (Gelardi et al. 2012; Antonín et al. 2015) however, another clade represented by the only species S. annulatus Corner from Malaysia (sequence of ITS region is not yet available in GenBank) is also known when phylogeny is conducted based on partial sequences of the largest subunit of RNA polymerase II (RPB2) as shown by Gelardi et al. (2012) and Antonín et al. (2015). Here, Clade A includes S. confusus Singer, S. seminudus Hongo, S. verruculosus Hirot. Sato where basidiospores are never reticulated as also stated by Gelardi et al. (2012) and Antonín et al. (2015). Distinguishingly, Clade B represents S. strobilaceous (Scop.: Fr) Berk., S. appendiculatus sp. nov., S. echinocephalus Gelardi & Vizzini., S. pteroreticulosporus Antonín & Vizzini, S. mirandus Corner where basidiospores are typically reticulate (Gelardi et al. 2012, Antonín et al. 2015). Basidiospore-ornamentation pattern is known to be the reliable parameter in the delimitation of major groups of Strobilomyces. Recently, on the basis of morphology and ITS data Petersen et al. (2012) showed the existence of only a single species of Strobilomyces in Europe, as S. strobilaceus. Therefore, we considered (Fig. 156) only the sequences derived from European collections as S. strobilaceus sensu stricto in our phylogeny. Our LSU based phylogeny which was conducted considering all the Strobilomyces LSU sequences appeared on BLAST (or available in GenBank) search with our specimen (GenBank Numbers KF112459, KF112460, KF112463, KF030345, DQ534626) and some other species of Boletaceae also clearly supports the position of the present specimen amongst Strobilomyces and confirms the novelty (Fig. 157). Here three sequences labelled as Strobilomyces sp. (Wu et al. 2014) are placed in the weakly supported sister clade of present species (94–95% identity with S. longistipitatus for 91% query coverage using BLAST).
Morphologically, this Indian specimen is quite similar to S. strobilaceous (an European species represented here by GenBank Numbers JQ319001, JQ318998, JQ318984, JQ318975, JQ318977 and JQ318988 in Fig. 156) and S. echinocephalus (reported from China) but, the former (probably the closest relatives of present species) differs from S. longistipitatus by its considerably shorter stipe [60–140 mm and 80–150 mm as mentioned by Breitenbach and Kränzlin (1991) and Knudsen and Vesterholt (2012) respectively] and longer tubes (10–15 mm) whereas, the latter is distinct from the present Indian specimen by its much shorter stipe (74–105 mm), presence of striation on stipe apex and longer tube (9 mm). Phylogenetically, both the species are well distinct as shown in Fig. 156. Three more species: S. alpinus, S. pteroreticulosporus, S. mirandus are also partly close to S. longistipitatus. Strobilomyces alpinus can be separated in field by the presence of longitudinal striations on stipe and unchanging exposed context and larger spores (12.5–15 µm) (Gelardi et al. 2012). Strobilomyces pteroreticulosporus is distant by its pileus surface which is covered with pyramidal fibrillose dirty whitish scales, longer (30 mm) tubes and shorter (100–140 mm) stipe (Antonín et al. 2015) whereas, S. mirandus has yellow or golden orange to brownish-orange pileus, longer (up to 10 mm) tubes and distinctly smaller (40–70 mm) stipe (Sato et al. 2005).
Strobilomyces polypyramis Hook. f., another species recently reported from the state Sikkim can easily be separated by presence of erect, small conical to pyramidal, pointed or rarely spinoid black scales, longer tube (up to 13 mm), shorter stipe (80 mm), absence of complete reticulum in basidiospores (Horak 1980a, c; Das et al. 2014). Strobilomyces dryophilus Cibula & N.S. Weber (96% identity with S. longistipitatus for 95% query coverage using BLAST) and S. floccopus were also appeared close (though well separated) in the LSU phylogeny along with our Indian material (Fig. 157). But, S. dryophilus is morphologically distinct from S. longistipitatus by its whitish ground coloured pileus with coarse, woolly appressed or erect, greyish-pink or pinkish-brown scales, 10–17 mm long tubes, and much shorter (40–80 mm) stipe (Bessette et al. 2010). Whereas, S. floccopus (sequence derived from European material) is considered as the synonym (Petersen et al. 2012; Gelardi et al. 2012) of S. strobilaceous (morphological comparison given in the earlier paragraph).
According to unique combination of morphological data and phylogenetic analyses of ITS and LSU sequences, our specimen from India should be treated as an independent species, proposed as S. longistipitatus which is placed in Clade B (Fig. 156) along with other species featured with reticulate basidiospores.
Cantharellales Gäum.
Clavulinaceae Donk
Clavulinaceae was described in 1970 by Donk to accommodate the genus Clavulina, based on bisterigmate, secondarily septate, and stichic basidia. Currently, it includes four genera and belongs to Cantharellales (Kirk et al. 2008). It is characterized by having simple, branched or resupinate basidiomata, monomitic hyphal system, clamped or not hyphae, and cystidia that is present in a few species. The basidia are clavate or subcylindric, with sterigmata generally incurved and the basidiospores are usually globose, subglobose to ellipsoid, smooth, guttulate and inamyloid. They are reported as saprobes, ectomycorrhizal and lichenized (Corner 1950; Donk 1964; Moncalvo et al. 2006; Bernicchia and Gorjón 2010).
Clavulina J. Schröt.
Clavulina J. Schröt. accommodates species usually with branched basidiomata, occasionally simple, infundibuliform, effused-coralloid or resupinate, typically with two smooth, hyaline, guttulate basidiospores per basidia, and cornute sterigmata. Additionally, a septum is sometimes formed at the base of the basidia after the release of basidiospores (Corner 1950, 1970; Petersen 1988; Thacker and Henkel 2004; Henkel et al. 2005; Uehling et al. 2012a). Clavulina is a genus of ectomycorrhizal fungi with about 75 species widely distributed mainly in tropical regions (Uehling et al. 2012b). Studies about the phylogeny of the genus show its monophyly within Cantharellales (Thacker and Henkel 2004; Moncalvo et al. 2006; Olariaga et al. 2009; Uehling et al. 2012a, b).
Clavulina grisea Meiras-Ottoni & Gibertoni, sp. nov.
MycoBank number: MB 818101; Facesoffungi number: FoF 2955, Fig. 161
Etymology: grisea (Greek) = greyish, referring to the colour of the basidiomata.
Holotype: URM 89967
Basidiomata weakly branched or simple, solitary or in small groups, 2.5–8 cm, branches polychotomous, tips unique or bifurcated to cristae, consistency fleshy when fresh, brittle to crumbling when dry, grey to brownish-grey (5C1, 5C2, 5D1) when fresh, stipe well to not delimited 1–2.3 cm, white, beige to light brown (6A1, 6E3, 6E4) when fresh, young specimens white turning ochraceus (5A1, 5C3, 5C4) towards the apex of the branches, hymenium amphigenous developing just below the branching point and extending to the apex of the branches. Basidiospores globose to subglobose, 5.5–8 (L = 6.63) × 4–6.5 (W = 5.67) μm, Q = 1.18, smooth, hyaline in water, pale yellowish in KOH 3%, with one large oleiferous guttule, thin-walled, with short apiculus (0.7–1 μm), IKI-. Hyphal system monomitic, tramal hyphae smooth, parallel, hyaline, 2–10 μm, slightly inflated, clamp connections abundant, subhymenium tightly interwoven. Cystidia hymenial hyaline, cylindrical with homogeneous to granular cytoplasmic contents, 85–98 × 5, 5–10 μm, no clamp connection at the base. Basidia elongate-clavate, approximately 31 × 5.5–6 μm, hyaline, guttulate, with 2 cornute sterigmata, 4–6 μm, clamped at the base, but agglutinated and difficult to observe.
Material examined: BRAZIL, Acre: Sena Madureira, Floresta Nacional de São Francisco, January 2015, A. Meiras-Ottoni & S.O. Almeida (URM 89966); BRAZIL, Acre: Sena Madureira, Reserva Extrativista Cazumbá-Iracema, January 2015, A. Meiras-Ottoni & S.O. Almeida (URM 89967, holotype; isotype in O); BRAZIL, Acre: Sena Madureira, Reserva Extrativista Cazumbá-Iracema, January 2015, A. Meiras-Ottoni & S.O. Almeida (URM 89968, paratype).
GenBank Numbers URM 89967 ITS:KX811199, URM 89968 ITS:KX811184.
Notes: This species is characterized by the slender, flexible, fleshy and greyish basidiomata when fresh, brittle when dry, and by the hymenial, hyaline cystidia with homogeneous to granular contents. Clavulina grisea composed of several solitary basidiomata growing on soil covered by rotten leaves and branches.
Other species of Clavulina with greyish basidiomata and cystidia have been reported by Corner et al. (1956), Corner (1970) and Petersen (1983). Clavulina hispidulosa Corner, however, has subhyaline to pale yellow or pale brownish, subglobose to obovoid, larger basidiospores (8–12 × 7–10.5 μm), while C. griseopurpurascens Corner has hyaline, usually ellipsoid basidiospores (7.5–8.7 × 6–7 μm).
The specimens of C. grisea are grouped in a well-supported clade (100% ML/100% MP/1.00BYPP) sister of C. cristata (80% ML) (Fig. 160). Clavulina cristata (Holmsk.) J. Schröt. is characterized by white, often becoming yellowish, ochraceous or fuliginous basidiomata, branches with acute tips usually becoming cristate, and larger, slightly thick-walled basidiospores (7–11 × 6.5–10 μm).
Another greyish species in the phylogenetic analyses (Fig. 160) are C. cinerea (Bull.) J. Schröt., C. craterelloides Thacker & T.W. Henkel and C. griseohumicola T.W. Henkel, Meszaros & Aime, all without cystidia. Additionally, C. cinerea has yellow or ochraceous, subglobose or broadly ellipsoid, basidiospores (6.5–11 × 6–10 μm); C. craterelloides has infundibuliform basidiomata, and hyaline, subglobose to broadly ellipsoid, basidiospores (6.5) 7.5–8 × (5.5) 6–7 (7.5) μm; and C. griseohumicola has dark bluish-grey, simple basidiomata, and subglobose basidiospores (8–9.5 × 7–9 μm) (Fig. 161).
Clavulina ossea Meiras-Ottoni & Gibertoni, sp. nov.
MycoBank number: MB 818103; Facesoffungi number: FoF 2956; Figs. 162
Etymology: ossea (Latin) = bone, referring to appearance basidiomata after drying.
Holotype: URM 89970
Basidiomata branched, solitary or in small groups, with fleshy consistency, –10 cm, polychotomous branches, the tips of branches are unique or rarely cristate, becoming dark, stem visible, sterile, when fresh dark brown (8F6), branches brownish-lilac grey (10C2, 16B2), hymenium amphigenous starting just below the branch point and extending to the apex of the branches. Basidiospores globose to subglobose, 6–9 (L = 7.66) × 5–7.5 (W = 6.56) μm, Q = 1.14, smooth, hyaline, with one large oleiferous guttule, thin-walled, with short apiculus (0.2–1 μm), IKI-. Hyphal system monomitic, tramal hyphae smooth sometimes verrucous, parallel, hyaline, 3–10 μm, some hyphae are wide but not inflated, clamp connections abundant, subhymenium tightly interwoven. Cystidia absent. Basidia cylindrical, 21–44 × 5–6 μm, hyaline, multi-guttulate, with 2 cornute sterigmata, 4–5 μm, postpartal septa observed on most basidia.
Material examined: BRAZIL, Paraíba: Areia, Parque Estadual Mata do Pau-Ferro, June 2015, C.O. Mendonça (URM 89970, holotype; isotype in O).
GenBank Number ITS:KX811197.
Notes: This species is characterized by the robust, fleshy, and pale lilac basidiomata when fresh, resembling bones when dry, and hyphae occasionally incrusted. The new species is composed of several solitary or in pairs basidiomata, scattered on soil covered by rotten leaves and branches. Of the species of Clavulina with similar colour (vinaceous, lilac or purple tints), C. incrustata can be distinguished by the basidiomata with very rare clamp connections and ferruginous brown, incrusted hyphae (Wartchow 2012); C. amethystina (Bull.) Donk by the ovoid-ellipsoid or subglobose, slightly larger basidiospores (7–11(–12) × 6–8 μm), while C. amethystinoides (Peck) Corner by the subglobose to broadly ellipsoid (7–10 × 6–8 μm). In all phylogenetic analyses, C. ossea comes an isolated position within the genus (Fig. 160).
Clavulina paraincrustata Meiras-Ottoni & Gibertoni, sp. nov.
MycoBank number: MB 818102; Facesoffungi number: FoF 2957, Figs. 163
Etymology: paraincrustata (Greek + Latin) = similar to Clavulina incrustata, referring to the extracellular incrustations on the hyphae of the context.
Holotype: URM 89969
Basidiomata branched in caespitose clusters, more rarely solitary or in pairs, somewhat fused basally, initially monopodial but branching early, with fleshy consistency, −8 cm, branches occasionally hollow, tips of branches cristate, stipe visible, sterile, when fresh brownish-orange (6C7, 6C8), branches violet brown (11F8), hymenium unilateral, appearing initially above first branching point. Basidiospores globose to subglobose, 6–8 (L = 7.49) × 5–7 (W = 6.32) μm, Q = 1.20, smooth, hyaline, with one large oleiferous guttule, thin-walled, with short apiculus(–1 μm), IKI-. Hyphal system monomitic, tramal hyphae frequently incrusted, parallel, very compact, brownish, 2–10 μm, not inflated, without clamp connections. Cystidia absent. Basidia elongate-clavate, 31–48 × 4, 5–7 μm, hyaline or with granular cytoplasmic contents, with 2 cornute sterigmata, rarely 3, 4–5 μm, postpartal septa observed on most basidia.
Specimen examined: BRAZIL, Paraíba: Rio Tinto e Mamanguape, Reserva Biológica Guaribas, May 2015, A. Meiras-Ottoni (URM 89969, holotype; isotype in O).
Additional specimen examined: Brazil, Pernambuco: Igarassu, Refúgio Ecológico Charles Darwin, May 2010, F. Wartchow (URM 82947).
GenBank Numbers ITS:KX811201; LSU:KX811196.
Notes: This species is characterized by the robust and violet brown basidiomata when fresh, brittle when dry, and incrusted hyphae. It is composed of several solitary or in pairs basidiomata, scattered on soil covered by rotten leaves and branches.
Wartchow (2012) described C. incrustata Wartchow, the first species of Clavulina with incrusted hyphae, based in material collected in Brazil. Despite being microscopically similar, it differs from C. paraincrustata by the less robust, paler basidiomata, and the amphigenous hymenium. DNA of the type was tentatively extracted, without success.
Clavulina paraincrustata was grouped (94% ML/94% MP/1.00BYPP) with C. craterelloides and C. rosiramea Uehling, T.W. Henkel & Aime (Fig. 160), from which can be distinguished by the lack of incrusted hyphae. Additionally, C. craterelloides has infundibuliform basidiomata, subglobose to broadly ellipsoid basidiospores [(6.5) 7.5–8 × (5.5) 6–7 (7.5) μm], while C. rosiramea has simple or slightly branched basidiomata, with orange tints, subglobose to slightly triangular [(6.5) 7–9 (10) × (6) 7–8 (9) μm].
Polyporales Gäum.
Fomitopsidaceae Jülich
Fomitopsidaceae is a family of Polyporales and currently comprises 24 genera and 197 species (Kirk et al. 2008), with Fomitopsispinicola (Sw.) P. Karst as the type species. The species are characterized by the basidiomata annual or perennial, resupinate, applanate, effuse or pileate, woody, leathery or corky in texture. The hymenium is poroid, and the hymenial surface is usually pale or brownish. The hyphal system is di- or trimitic and clamp connections are usually present (Cannon and Kirk 2007). Most genera of Fomitopsidaceae such as Antrodia P. Karst. Fomitopsis P. Karst., Daedalea Pers., Laetiporus Murrill are of ecological, evolutionary and economic importance because this group has many forest pathogens and cause brown rot which also play an important role in carbon sequestration (Fukami et al. 2010; Ortiz-Santana et al. 2013; Zhou and Wei 2011).
Fomitopsis P. Karst.
Fomitopsis includes species with basidiomata sessile to effused-reflexed, rarely annual to perennial, varying from white to purple, and di- to trimitichyphal system with clamped generative hyphae. The basidiospores are smooth and hyaline, thin-walled, ranging from subglobose to cylindrical (Gilbertson and Ryvarden 1986; Ryvarden and Gilbertson 1993; Dai 2012). It is a cosmopolitan genus, causing brown rot in the substrates, and several species were used as biological models in genetic analyses, as well as ecological, biotechnological and taxonomic studies (Högberg et al. 1999; Chlebicki et al. 2003; Aime et al. 2007; Watanabe et al. 2010) (Fig. 164).
Maximum likelihood phylogenetic tree of the concatenated ITS1, 5.8S, ITS2 and partial 28S rDNA sequences. Bootstrap supporting values (1000 replicates) and posterior probabilities (PP) from Bayesian analysis to each node are shown from left to right. Only bootstrap values above 50% and PP above 0.75 are provided. The new species described in this study are highlighted in blue. The tree was rooted with Grifola sordulenta and Perenniporia ochroleuca
Fomitopsis flabellata Soares & Gibertoni, sp. nov.
MycoBank number: MB 817408; Facesoffungi number: FoF 2958, Figs. 165
Etymology: flabella (Latin) = The name refers to the fan-shaped basidiomata.
Holotype: URM 89405
Basidiomata annual, biannual, pileate, dimidiate, solitary or in small groups or clusters. Pileus 0.2–2 cm wide, up to 2 cm long, 0.3 mm thick at base, upper surface purple (9.1E purple) when fresh and dried, azonate to zonate, glabrous to slightly velutinate. Margin obtuse, entire. Pore surface pinkish-brown to lilac-violaceous (9.4D pinkish-brown, 11.5E lilac violaceous), pores round to angular (3–4 mm), dissepiment thin and entire. Context purple brownish (9.1F brownish), 0.1 mm thick.
Basidiospores cylindrical to sub-cylindrical, 4–5 (6.5) × 2–2.5 μm, smooth, thin-walled, IKI–. Basidia clavate with four sterigmata, 8–10 (12) × 4–5 μm. Hyphal system trimitic, generative hyphae clamped, thin-walled, 2–3 μm in diam.; skeletal hyphae dominant, thick-walled, hyaline, 3–5 μm in diam.; binding hyphae thick-walled, hyaline, 1.5–2.5 μm in diam. Cystidia absent.
Specimens examined: BRAZIL, AMAPÁ: Porto Grande, Floresta Nacional do Amapá, October 2014, A. Soares (URM 89405, holotype; Isotype in O). BRAZIL, Amapá: Porto Grande, Floresta Nacional do Amapá, September 2013, A.M. Soares (URM 84210).
GenBank Numbers ITS:KX423688; LSU:KX423686.
Notes: Fomitopsis flabellate can be recognized by the small, usually fan-shaped, coriaceous, brown-vinaceous basidiomata. Fomitopsis lilacinogilva (Berk.) J.E. Wright & J.R. Deschamps and F. cupreorosea (Berk.) Carranza & Gilbn. have a similar colour, but differ by the larger and thicker basidiomata (2–20 × 2–9 × 0.5 × 10 cm; 5–16.5 × 2.6–10.3 × 0.3–2.5 cm, respectively). Besides, the pores in F. lilacinogilva are slightly labyrinthiform (1–2 mm) and sub-daedaleoid, becoming sinuous-daedaleoid in F. cupreorosea (1–3 mm), while in F. flabellata they are round to angular. Fomitopsis flabellata is placed as a basal taxon of the Rhodofomitopsis sensu stricto, but with low statistical support (Fig. 164). For the time being, we prefer to keep this species in Fomitopsis until new sequences are added to the Antrodia clade phylogeny.
Fomitopsis roseoalba Soares, Ryvarden & Gibertoni, sp. nov.
MycoBank number: MB 812876; Facesoffungi number: FoF 2959, Fig. 166
Etymology: The name refers to the colour of basidiomata when fresh, with pore surface white, and upper surface, when present, pink to lilac.
Holotype: URM 86923
Basidiomata annual, pileate, resupinate to effused-reflexed. When resupinate, up to 32 cm long, 11 cm wide, 0.3 cm thick at centre. Pileus when present, 0.2–7 cm wide, 5 cm long, 0.3 cm thick at base, upper surface white to pink (3.1A white, 7.4B pink) when fresh, becoming cream to greyish when dried (3.3A pale yellow, 1.1C pastel grey), slightly velutinate. Context white to cream (3.1A white, 3.3A pale yellow), 0.4 cm thick. Margin narrow in resupinate specimens, obtuse in pileate specimens. Pore surface white to cream (3.1A white, 3.3A pale yellow) when fresh and ochraceous when dried (4.4A light yellow), pores circular to angular (4–6 mm), dissepiment thin and entire.
Basidiospores ellipsoid to sub-cylindrical, 3–4 × 1.8–2 μm, smooth, thin-walled, IKI. Hyphal system trimitic, generative hyphae clamped, thin-walled, 2.5–3 μm in diam.; skeletal hyphae dominant, thick-walled, hyaline, 3.75–5 μm in diam.; binding hyphae thick-walled, hyaline, 1.5–2 μm in diam. (Fig. 166a–f). Cystidia absent. Basidia clavate with four sterigmata, 10–12 × 5–7 μm.
Material examined: BRAZIL, Amapá: Porto Grande, Floresta Nacional do Amapá, on dead hardwood, 00 58. 25.8 N and 051 41 73.6 W, February 2014, A. Soares, AS 1496 (URM 86923, holotype, Isotype O); Porto Grande, Floresta Nacional do Amapá, on dead hardwood, September 2013, A. Soares (URM 86913), A. Soares (URM 86926); February 2014, A. Soares (URM 86918), A. Soares, AS 1431 (URM 86920) A. Soares, AS 1457 (URM 86921), A. Soares, AS 1566 (URM 86922), A. Soares (URM 86923), A. Soares (URM 86925); Brazil, Pará: Melgaço, Floresta Nacional de Caxiuanã, July 2006, T.B. Gibertoni (URM 79982, 79984, 79985, 79986, 79987, 79988, 79989, 79990), March 2007, T.B. Gibertoni (URM 79983, 79991, 79993, 79994, 79995, 79996), August 2007, T.B. Gibertoni (URM 79997, 79998, 79999, 80000, 80001, 80002, 80003, 80004), February 2008, T.B. Gibertoni (URM 79992, 80005, 80006, 80007, 80008), August 2013, A. Soares (URM 86912) A. Soares (URM 86914) A. Soares (URM 86915), A. Soares (URM 86916).
GenBank Numbers ITS:KT189139; LSU:KT189141.
Notes: This species is easily recognized by the white to pink, pileate to effuse-reflexed basidiomata. Of the neotropical species, F. nivosa (Berk.) Gilbertson and Ryvarden has similar whitish colour and morphology (pileate, and sometimes effused-reflexed), but is separated by having cylindrical basidiospores (6–9 × 2–3 μm) and smaller pores (6–8 mm). Despite herbaria revision and several collections in other parts of the Brazilian Amazonia, F. roseoalba was only found in the Eastern Brazilian Amazonia, what indicates that this species may be restricted to this region. According to our molecular analyses, F. roseoalba grouped into a well-supported clade (81/0.99) with one unidentified, endophytic Fomitopsis isolated from Hevea brasiliensis sapwood collected in Brazil (Fomitopsis sp. NHB31) (Martin et al. 2015). This isolate may represent an endophytic stage of F. roseoalba. The F. roseoalba clade grouped in a major clade (98/1.00) with F. subtropica B.K. Cui, Hai J. Li and M.L. Han and two unidentified, endophytic Fomitopsis isolated from Elaeis guineensis from Thailand (Fomitopsis sp. 9 V 3.1 and Fomitopsis sp. 7R 8.1) (Rungjindamai et al. 2008), all included in the Fomitopsis sensu stricto (75/1.00).When in a resupinate form, F. roseoalba is whitish as F. subtropica, and both have similar basidiospores (cylindrical to oblong-ellipsoid 3–4.7 × 1.7–2.3 μm in F. subtropica). Fomitopsis subtropica, however, has smaller pores (6–9 mm) and the pileus, when present, is white to cream (Li et al. 2013b). Li et al. (2013b) suggested that the two specimens of Fomitopsis sp. from Thailand (7R 8.1, 9 V 3.1) could be F. subtropica, because of the high supporting values uniting them in their study (100/1.00). Although the phylogenetic relationship of these three Fomitopsis species was strongly supported, our analyses indicates that F. subtropica, F. roseoalba and Fomitopsis sp. (7R 8.1, 9 V 3.1) are clustered in an independent way (Fig. 164).
Polyporaceae Fr. ex Corda
Favolus (Fr.) Sotome & T. Hatt.
The genus Favolus, typified by F. brasiliensis (Fr.) Fr, is a wood decaying fungus within the Polyporaceae, Polyporales. Previously, Núñez and Ryvarden (1995) treated Favolus as one of the six infrageneric groups of the genus Polyporus, and recently Sotome et al. (2013) proposed as a new genus based on phylogenetic analyses of LSU and ITS regions and morphological characters such as a radially striate pileus, and lack of distinct cutis of agglutinated hyphae. This genus is well supported by multi-gene phylogenies (Binder et al. 2013; Seelan et al. 2015) (Fig. 167).
Favolus gracilisporus H. Lee, N.K. Kim & Y.W. Lim, sp. nov.
Index Fungorum number: IF552508; Facesoffungi number: FoF 2626, Fig. 168
Etymology: referring to a slenderer spore relative to other Favolus species.
Holotype: SFC20130704–40
Basidiocarps annual, lateral stipitate; pileus circular to flabellate, up to 40 mm in diam., up to 3.5 mm thick; pileal surface ivory to pale buff, with age becoming brown, fibrillose with flattened, radially striate, azonate, slightly glabrous, margin concolorous, acute, lacerate. Hymonophore cream to pale brown when fresh, pores diamond-shaped, radially elongated, 2–3 mm long, 1–2 mm wide near stipe to 2–3 per mm at margin; dissepiments thin and lacerate with age. Context in pileus pale tan to ivory, corky, brittle when dry, up to 0.5 mm thick. Tube continuous with the context, up to 2–3 mm thick. Stipe lateral, buff, glabrous, up to 7 mm long and 6 mm thick. Hyphal system dimitic; generative hyphae hyaline in KOH, thin-walled, 3–4 μm in diam., septa with clamps, skeletal-binding hyphae thick-walled, aseptate, much branched, with tapering apices, 3–6 μm diam. Cystidia absent. Basidia clavate, 4–sterigmate (up to 4.5 μm), 27–35 × 5.7–8 μm, with a basal clamp. Basidiospores cylindric, hyaline, smooth, IKI-, 7.9–9.7 × 2.5–3.3 μm. L = 9 μm, W = 2.9 μm, Q = 3.11 (n = 20/1).
Habitat: solitary to gregarious on dead wood of hardwood.
Material examined: KOREA, Gyeongsangbuk-do, Yecheon-gun, Mt. Hagga, on rotten angiosperm stump, 4 July 2013, Y.W. Lim (SFC20130704–40, holotype).
GenBank Number ITS:KY038472.
Notes: Favolus gracilisporus shares similar characteristics with Favolus brasiliensis (Fr.) Fr., Favolusroseus Lloyd, and Neofavolusalveolaris (DC.) Sotome & T. Hatt. such as lateral stipitate basidiocarps with radially elongated and hexagonal pores; however, F. gracilisporus has distinctly larger basidia (27–35 × 5.7–8 μm) compared to F. roseus (18–23.8 × 4.5–7 μm, Sotome et al. 2013) and N. alveolaris (17.5–26 × 4–7 μm, Sotome et al. 2013). Microscopic features of F. brasiliensis are somewhat similar to F. gracilisporus, but the latter species has a larger basidiospore Q value (3.11) than F. brasiliensis (Q = 2.75, Sotome et al. 2013). Phylogenetic analysis based on the ITS region indicated that F. gracilisporus separated distinctly from the other three species (Fig. 167).
Lentinus Fr.
Lentinus (Fr.) Quel is a cosmopolitan genus with an estimated 63 species (Kirk et al. 2008) and 629 records under the name of Lentinus in the Index Fungorum (2017) and, species are able to survive over a wide temperature range, are abundant in boreal, temperate and tropical regions (Pegler 1983; Corner 1981; Karunarathna et al. 2011) (Fig. 169).
Phylogenetic relationships inferred from maximum parsimony analysis of ITS-rDNA sequences of 36 taxa. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The new Sri Lankan records: Lentinus sajor-caju, having GenBank Number KY649463 and herbarium number MFLU 12-1478; Lentinus squarrosulus, having GenBank Number KY649464 and Herbarium Number MFLU 12-1228; Lentinus velutinus, having GenBank Number KY649465 and Herbarium Number MFLU 12-1123 are shown in bold and blue. The topology is rooted with Panus lecomtei. Evolutionary analysis was conducted in PAUP 4.0b 10
Lentinus sajor - caju (Fr.) Fr., Epicrisis Systematis Mycologici: 393 (1838)
Facesoffungi number: FoF 3142, Figs. 170, 171
Pileus 3–9 cm diam., coriaceous drying hard and rigid, soft, convex with a deeply umbilicate centre then cyathiform to infundiliform, or eccentric and flabelliform, surface very variable in colour, cream colour, pale ochraceous, more or less fuliginous, or umbrinous, dry glabrous and smooth or sometimes with small, appressed, darker squamules especially towards the centre, often finely radially striate, rimose in old specimens; margin initially incurved to involute soon straight, very thin, smooth, undulating and lobed. Lamellae deeply decurrent, not furcate, whitish, concolorous with the pileus, or becoming darker towards the edge, often darkening on drying, narrow to sub-linear, 0.5–3 mm broad, densely crowded, with lamellulae of 4–6 lengths; edge entire or finely denticulate. Stipe central, eccentric or lateral, short, 0.5–3 × 0.5–1.5 cm, cylindric with an abrupt base, solid; surface concolorous with the pileus, at times blackening especially towards the base. Annulus present, attached towards stipe apex, firm, white to fulvous, either well formed, slight or commonly weathered away. Context up to 8 mm thick at the centre but very thin over the hymenophore, tough and pliant drying hard and horny, white, consisting of a very tightly woven, dimitic hyphal system with skeleton-ligative hyphae. Generative hyphae 2–5 μm diam. not inflated, very thin-walled, frequently branching with clamp connections. Skeleto-ligative hyphae dominant, 2–8 μm diam., hyaline or pale yellowish, with a thick wall of 3 μm and often a very narrow lumen, comprising a skeletal element, 100–400 μm long, bearing two to several tapering ligative branches, up to 400 μm long, which are themselves dichotomously divided, sometimes the branches are very short, numerous and nodulose to produce a coralloid appearance. Basidiospores 5–9 × 1.5–2.5 μm, Q = 3.23, narrowly cylindric, often curved, hyaline, thin-walled, with few contents. Basidia 15–20 × 3.5–5 μm, very narrow, clavate, cylindric, bearing 4 sterigmata. Lamella-edge a broad sterile zone, with scattered or clustered Cheilocystidia and numerous, emergent skeleto-ligative branches. Cheilocystidia 20–25 × 4–6 μm, clavate, often sinuous or nodulose, hyaline, thin-walled. Hyphal pegs abundant, 50–100 × 20–40 μm, truncate cylindric, consisting of fascicles of up to 50 narrow hyaline hyphae, extending up to 80 μm beyond the basidia. Hymenophoral trama irregular, hyaline, broad, of radiate construction, very compactly interwoven with few interhyphal spaces, and ligative branches frequently penetrating the hymenium. Subhymenial layer often indefinite or indistinct, becoming pseudoparanchymatous when well developed. Pileipellis an epicutis, 15–20 μm thick, of agglutinated, radiating generative hyphae, often with a brown encrustation and sometimes a brown membrane pigment. Smell mushroomy and an excellent edible species.
Habit, habitat and distribution: as a cluster, usually on dead or decaying wood, May–August. Our collection was collected on a decaying wood near Peradeniya Royal Botanic Gardens.
Specimens examined: SRI LANKA, Kandy District, near Peradeniya Royal Botanic Gardens, 23 June 2012, Samantha C. Karunarathna (MFLU 12-1478).
GenBank Number ITS:KY649463.
Notes: Lentinus sajor-caju is one of the most common macrofungi of the palaeotropical forests, with the distribution extending from equatorial and southern Africa, although less frequent in West Africa to throughout south-east Asia and down to the north-east corner of Australia. This is the first report of L. sajor-caju with the molecular phylogenetic confirmation from Sri Lanka Pegler (1983) has previously reported it (Fig. 169).
Lentinus squarrosulus Mont., Annales des Sciences Naturelles Botanique 18: 21 (1842)
Facesoffungi number: FoF 3143, Figs. 172, 173
Basidiocarps moderate size. Pileus 2–8 cm diam., thin and pliant, convex soon depressed; surface milk white, fuscous brown cream coloured or pinkish-buff, radially innate striate, with concentric zones of small squamules, margin downcurved. Lamellae deeply decurrent, occasionally slightly interveined towards the stipe attachment, white to pale buff, thin, 2–3 mm broad, moderately crowded, 4–5 tiers of lamellulae, edge entire or finely denticulate on large specimens. Stipe 1.5–5 cm × 2–6 mm, central or excentric, rarely lateral, tapering below, firm, solid, cylindric; surface concolorous with pileus. Context 2–3 mm thick, fleshy coriaceous, white, consisting of a dimitic hyphal system with generative and skeletal hyphae. Generative hyphae 2–6 µm diam., not inflated or scarcely inflated, hyaline, thin walled, frequently branched, with prominent clamp connexions. Skeletal hyphae 2–10 µm diam., with a hyaline, thickened wall, but always with a continuous lumen, and normally branching. Basidiospores 5.5–7.5 × 1.8–2.7 µm [n = 40, (6.2 × 2.3 µm), Q = 2.69], straight, cylindrical, hyaline, thin walled with few contents. Basidia 15–21 × 4–6 µm, narrowly clavate, bearing 4 sterigmata. Hyphal pegs abundant.
Habit, habitat and distribution: as a cluster, usually on dead or decaying wood, May–August. Our collection was collected on a decaying wood near Peradeniya Royal Botanic Gardens.
Specimens examined: SRI LANKA, Kandy District, near Peradeniya Royal Botanic Gardens, 13 June 2012, Samantha C. Karunarathna (MFLU 12-1228).
GenBank Number ITS:KY649464.
Notes: This is the first report of L. squarrosulus with the molecular phylogenetic confirmation from Sri Lanka after Pegler (1983) has previously reported it.
Lentinus velutinus Fr., Linnaea 5: 510 (1830)
Facesoffungi number: FoF 3144, Figs. 174, 175 Pileus 2–10 cm diam., thin, coriaceous, deeply umbilicate to broadly infundibuliform or cyathiform; surface uniformly pale greyish-cinnamon to rufous or tawny brown, fuscous at the centre, at times almost chestnut brown, dry, uniformly velutinate to short hispid or subsquamulose-furfuraceous, not zonate or obscurely so, neither strongly striate nor sulcate except in very old specimens; margin thin, at first strongly involute, reflexed at maturity, densely ciliate, at times fissile and fenestrated. Lamellae arcuate, short decurrent, not anastomosing, pale buff to cinereous brown, fulvescent, often tinted dark violaceous brown, narrow, 1–1.5 mm wide, moderately to densely crowded, with lamellulae of 3–4 lengths; edge entire. Stipe central, eccentric or lateral, slender, 2–25 cm × 2–10 mm, cylindric, expanding slightly at both apex and base, solid; surface concolorous with the pileus or more often darker, uniformly and persistently velutinate with the indumentums extending into the bases of lamellae, generally arising from a pseudosclerotium. Pseudosclerotium 2–10 × 1–4 cm, rarely larger, irregularly fusoid, comprising sclerified wood impregnated with hyphae, surface pale greyish-brown, smooth. Context thin, up to 1 mm thick, white, drying rigid, consisting of a dimitic hyphal system with generative hyphae.
Generative hyphae 2–4 μm diam., narrow, not inflated, hyaline, thin-walled, rather sparingly branched, with clamp connections. Skeletal hyphae, 2–4 μm diam., cylindric, hyaline, with a thickened wall of 1–2 μm and a narrow, continuous lumen, unbranched, arising in either an intercalary or terminal position, and then with an obtusely rounded apex. Basidiospore print pale buff to cream colour. Basidiospores 5–7 × 3–4 μm, Q = 1.87, short, oblong-cylindric, hyaline, thin-walled, with few contents. Basidia 18–22 × 4–5 μm, narrowly cylindric, bearing 4 sterigmata. Lamella-edge sterile, with numerous basidioles and scattered sclerocystidia. Basidioles 16–30 × 4–8 μm, sinuous clavate or fusoid, constricted, often nodulose, hyaline, thin-walled. Sclerocystidia present on lamella-edge and more especially on sides of lamellae, fairly numerous to very abundant, 20–64 × 3–12 μm, initially clavate and thin-walled with refractive gloeo-contents, soon developing a thickened wall of 2–2.5 μm, hyaline or brownish, not or scarcely projecting beyond the basidia. Hymenophoral trama irregular, of radiate construction, hyaline, similar in structure to the context. Subhymenial layer little developed, 5–12 μm wide, interwoven. Pileipellis a trichodermal palisade producing erect, loose fascicles, 30–1000 μm long, of brown, thick walled, generative hyphae, 3–6 μm diam., with clamp connections, and an obtusely rounded apex, arising from a gelatinized hypodermium of radially repent generative hyphae. Stipitipellis similar to the pileipellis. No special smell; edible when it is young.
Habit, habitat and distribution: solitary, usually on dead or decaying wood, May–August. Our specimen was collected on a decaying wood near Peradeniya Royal Botanic Gardens.
Specimens examined: SRI LANKA, Kandy District, near Peradeniya Royal Botanic Gardens, 11 June 2012, Samantha C. Karunarathna (MFLU 12-1123).
GenBank Number ITS:KY649465.
Notes: The wide distribution of Lentinus velutinus complex has resulted in a range of variations and in some instances constant morphological differences limited to restricted geographical areas. This is the first report of L. velutinus with the molecular phylogenetic confirmation from Sri Lanka after Pegler (1983) has previously reported it.
Panus Fr.
The generic delineation and phylogenetic relationships between Lentinus and Panus and their accepted allies Neolentinus, Heliocybe, and Pleurotus has been controversial (Corner 1981; Pegler 1983; Redhead and Ginns 1985; Singer 1975, 1986). Corner (1981) treated Lentinus and Panus as separate genera on the basis of their hyphal system and some species were placed in Pleurotus by Singer (1975), whereas, Pegler (1983) treated Panus as a subgenus of Lentinus and included several species of Singer’s Pleurotus in Panus (Fig. 176).
Phylogenetic tree generated with RAxML (GRT-GAMMA) based on ITS sequence data aligned with MAFFT. Bootstrap values higher than 70% are shown above or beneath the branches at nodes. The tree is rooted with Polyporus conifericola KU189783, Polyporus tubiformis AB587634 and Polyporus melanopus AF516569. Panus subfasciatus LT614958 (Ex-type) is highlighted in blue. GenBank accession numbers are provided after each species name
Panus subfasciatus Thongbai, Karun., C. Richter & K.D. Hyde
Index Fungorum number: IF 552827; Facesoffungi number: FoF 02915, Fig. 177
Etymology: subfasciatus refers to the similarity to P. fasciatus
Holotype: MFLU 16-2129
Basidiomata (Fig. 177a) small-sized, often short and stocky, 1.2–2.5 × 0.5–1.0 cm in diameter, cylindric, brownish violet to purple brown (11D5–6) when young, greyish ruby to ruby (12D7–8) when old, leathery, solid, dry; surface concolorous with the pileus, velutinate, fully covered with short and soft hairs, brownish grey (11C2); context white to pale purplish, consisting a dimitic hyphal system with skeletal hyphae. Pileus 2.5–5.5 cm in diameter, thin, slightly umbilicate centre, coriaceous, infundibuliform; surface brownish grey to violet brown (10E3–4), often purplish fuscous especially towards the margin, velutinate with numerous hispid squamules forming erect hairs or hispid-strigose; margin at first involute, then hispid. Lamellae 0.3 mm broad, with 3–5 tiers of lamellulae, deeply decurrent, purplish black, moderately distant to moderately crowded.
Basidiospores (Fig. 177b–e) (5.03–)5.28–6.44(–6.56) × (3.04–)3.33–4.16(–4.44) μm (n = 40) Lm = 5.73 μm, Wm = 3.61 μm, Q = (1.34–)1.43–1.92(–1.95), Qm = 1.60 μm, ellipsoid to elongate, hyaline, thin walled, with few contents. Basidia (Fig. 177f–i) 17–21 × 7–10 μm, clavate to elongate clavate, 4-spored or occasionally 2-spored, with sterigmata up to 6 μm long; clamps present at base of basidia. Lamella edge sterile with abundant cheilocystidia. Cheilocystidia (Fig. 177l) 24–28 × 15–25 μm, clavate, hyaline, thin-walled. Sclerocystidia abundant on the sides of the lamellae, 32–40(–55) × 6–9(–12) μm, initially clavate, thin- walled. Pileipellis an epicutis of impact, interwoven, slightly thick-walled; generative hyphae 2.4–5 μm wide, branching, with clamp connections. Generative hyphae (Fig. 177j) 2.5–3 μm, inflated, undifferentiated hyphae, hyaline, thin-walled, branching, with clamp connections. Skeletal hyphae (Fig. 177k) 2.5–3.5 μm, cylindrical, hyaline, slightly thick-walled.
Habitat: On dead and rotting wood in forest of Fagaceae.
Material examined: THAILAND, Chiang Rai Province, along the road number 1149, Mae Fah Luang Sub-District, Mae Fah Luang District, 17 June 2014, collector Benjarong Thongbai (MFLU 16-2129, holotype).
GenBank Number ITS:LT614958.
Notes: Panus subfasciatus is characterized by its brownish violet to purple brown young basidomata. Later they turn greyish ruby, leathery, solid, dry and are fully covered with short brownish grey hairs. In some aspects it bears a superficial resemblance to Panus fasciatus (Berk.) Singer and Lentinus concentricus Karunarathna, K.D. Hyde & Zhu L. Yang. Panus subfasciatus is easily distinguished from P. fasciatus with its ochraceous basidiomata and abundance of sclerocystidia, which are clavate or fusoid, 22–32 × 4–9 μm (Corner 1981; Pegler 1983). Panus subfasciatus differs from L. concentricus by the latter having yellowish brown-clay, velvety, concentrically zonate of basidiomata and absence of sclerocystidia (Karunarathna et al. 2011). Panus subfasciatus also bears a superficial resemblance to Panus conchatus (Bull.) Fr. which also has purplish brown basidiomata but is lacking cilia. The phylogenetic trees obtained from RAxML analyses are shown in Fig. 176. Panus subfasciatus was statistically supported in the Polyporaceae. In the major clade, P. subfasciatus clustered in a subclade that predominantly contained Panus species and Lentinus strigosus, while a second subclade exclusively contained Lentinus species. Panus subfasciatus appeared most similar to P. conchatus (unpublished according to the entry in GenBank) and a yet undescribed Panus species (BS = 82%). The phylogenetic tree suggests that the nrITS region may be useful for preliminary phylogenetic analyses, also because this is the most frequent DNA locus from which references are available. However, an expanded taxon sampling, best supported by a multi gene genealogy, should be carried out to redefine the boundaries between Lentinus and Panus. The new fungus was recently referred to as Lentinus cf. fasciatus in a report of a new secondary metabolite named lentinulactam (Helaly et al. 2016). Nevertheless, after the outcome of the phylogenetic study we preferred to place it in the genus Panus because it is appears closer to the type species of that genus, P. conchatus.
Polyporus P. Micheli ex Adans.
Polyporus is a well-known genus of wood rotting fungi, which produce stipitate basidiocarps with dimitic hyphal system that causes white rot (Ryvarden 1991; Ryvarden and Gilvertson 1994; Núñez and Ryvarden 1995; Sotome et al. 2007). Núñez and Ryvarden (1995) divided this genus into six infrageneric groups based on macro-morphological characters and recent phylogenetic analysis showed that Polyporus sensu lato is separated into at least five well-defined clades as follows: Dendropolyporus, Favolus, Neofavolus, Polyporellus, and Polyporus (Sotome et al. 2008; Krüger et al. 2006; Sotome et al. 2013; Binder et al. 2013; Seelan et al. 2015). Morphological characteristics and phylogenetic analysis of six new species from Korea are described below. The phylogenetic tree is presented in Fig. 178.
Polyporus brevibasidiosus H. Lee, N.K. Kim & Y.W. Lim, sp. nov.
Index Fungorum number: IF552513; Facesoffungi number: FoF 2631, Fig. 179
Etymology: referring to the characteristic short basidia.
Holotype: SFC20130808-24
Basidiocarps annual, central stipitate; Pileus circular, plane then funnel-shaped, up to 30 mm diam., 1.5 mm thick; pileal surface light buff in the center, brown near margin, fibrillose with flattened, radially striate, glabrous, margin reddish-black, undulate, enrolled. Hymonophore white, cream to brown; pores fine, rounded to angular, 8–9 per mm. Context in pileus pale buff, corky, up to 1 mm thick. Tube continuous with the context, up to 0.5 mm thick. Stipe central, brown to dark brown, smooth to longitudinally wrinkled, apex distinct from the pore, up to 40 mm long and 2 mm thick. Hyphal system dimitic; generative hyphae hyaline in KOH, thin-walled, 2.5–3 μm in diam., septa with clamps, skeletal-binding hyphae thick-walled, aseptate, much branched, with tapering apices, 3–6 μm diam. Cystidia absent. Basidia clavate, 4–sterigmate (up to 2.3 μm), 15–16.6 × 6.3–7.4 μm, with a basal clamp. Basidiospores cylindric, hyaline, smooth, IKI–, 6.3–7.4 × 2.8–3.3 μm. L = 6.77 μm, W = 2.86 μm, Q = 2.37 (n = 20/1).
Habitat: solitary to gregarious on dead wood of hardwood.
Material examined: KOREA, Gyeongsangbuk-do, Sangju-si, Mt. Noem, on fallen hardwood branch, 8 August 2013, H. Lee, W. J. Kim & S-Y. Oh (SFC20130808-24, holotype). KOREA, Gangwon-do, Inje-gun, Baekdam temple, on fallen hardwood branch, 9 July 1999, Y. W. Lim (SFC19990709-26, paratype).
GenBank Number ITS:KY038474.
Notes: This species shares similar characteristics with many Polyporus species including eccentric stipe, thin pileus, and small pores. Although the smaller pileus (less than 3 cm), pores (8–9 mm) and basidia (15–16.6 × 6.3–7.4 μm) of P. brevibasidiosus are distinguishable characters, morphology alone is not sufficient for species level identification. ITS sequence analysis is useful to confirm its identity and phylogenetic position.
Polyporus koreanus H. Lee, N.K. Kim & Y.W. Lim, sp. nov.
Index Fungorum number: IF552509; Facesoffungi number: FoF00556; Fig. 180
Etymology: referring to the first reported species of this genus found in Korea.
Holotype: SFC20150813-58
Basidiocarps annual, central stipitate; Pileus circular, plane and somewhat depressed, funnel-shaped in the center, up to 70 mm in diam., 2 mm thick; pileal surface light grey to pale buff, with age becoming brown and dark brown, fibrillose with flattened, radially striate, slightly glabrous; margin ochreous, sharp and undulate. Hymonophore white, cream, buff to brown; pores fine, rounded to angular, 6–7 per mm. Context in pileus pale buff, corky, up to 1.5 mm thick. Tube slightly darker than context, up to 0.5 mm thick. Stipe central, brown, dark reddish-brown to black, smooth to longitudinally wrinkled, apex distinct from the pore, upper portion of blackish stipe usually covered by pores, up to 50 mm long and 9 mm thick. Hyphal system dimitic; generative hyphae hyaline in KOH, thin-walled, 2.8–3.5 μm in diam., septa with clamps, skeletal-binding hyphae thick-walled, aseptate, much branched, with tapering apices, 3–6 μm diam. Cystidia absent. Basidia clavate, 4–sterigmate (up to 3.4 μm), 21–25.7 × 5.6–8 μm, with a basal clamp. Basidiospores cylindric, hyaline, smooth, IKI–, 5.7–6.9 × 2.1–2.3 μm. L = 6.34 μm, W = 2.24 μm, Q = 2.83 (n = 20/1).
Habitat: solitary on dead wood of hardwood or on the ground.
Material examined: KOREA, Gyeonggi-do, Goyang-si, West Five Royal Tombs, on fallen hardwood branch, 13 August 2015, N.G. Kim, J. E. Eom & M. J. So (SFC20150813-58, holotype); KOREA, Gyeongsangbuk-do, Ulleung-gun, Na-ri basin, on fallen hardwood branch, 15 August 1995, Y.W. Lim (SFC19950815-35, paratype); KOREA, Gyeongsangnam-do, Hamyang-gun, Mt. Jiri, on rotten stump of Fraxinus mandshurica, 25 August 2004, J. S. Lee & K. M. Kim (SFC20040825-187, paratype); KOREA, Gyeonggi-do, Uiwang-si, Mt. Cheonggye, on fallen hardwood branch, 20 August 2015, N. G. Kim & H. J. Cho (SFC20150820-61, paratype).
GenBank Numbers SFC20150813-58 ITS:KY038479. SFC19950815-35 ITS:KY038461. SFC20040825-187 ITS:KY038462. SFC20150820-61 ITS:KY038480.
Notes: This species is often confused with P. melanopus, P. ulleungus and P. varius because of similar basidiocarps and microscopic features; however, the smaller basidiospore of Polyporus koreanus (5.7–6.9 × 2.1–2.3 μm) is distinguishable from P. melanopus (7–9 × 3–3.5 μm), P. ulleungus (6.8–8 × 2.3–3.3 μm) and P. varius (9–12 × 2.5–3 μm) (Gilbertson and Ryvarden 1987).
Polyporus orientivarius H. Lee, N.K. Kim & Y.W. Lim, sp. nov.
Index Fungorum number: IF552514; Facesoffungi number: FoF 2632, Fig. 181
Etymology: referring to P. varius, but with Far East Asian distribution.
Holotype: SFC20130704-20
Basidiocarps annual, central to laterally stipitate; Pileus circular to dimidiate, plane then funnel-shaped, up to 70 mm diam., 2.5 mm thick; pileal surface cream to pale buff, with age becoming brown and dark brown, fibrillose with flattened, radially striate, slightly glabrous, margin ochreous, sharp and undulate. Hymenophore white, cream, buff to brown; pores fine, rounded to angular, 6–7 per mm. Context in pileus pale buff, corky, up to 1 mm thick. Tube continuous with the context, up to 1.5 mm thick. Stipe central, brown to dark brownish-black, longitudinally wrinkled, apex distinct from the pore, up to 20 mm long and 9 mm thick. Hyphal system dimitic; generative hyphae hyaline in KOH, thin-walled, 2.8–3.5 μm in diam., septa with clamps, skeletal-binding hyphae thick-walled, aseptate, much branched, with tapering apices, 3–6 μm diam. Cystidia absent. Basidia clavate, 4-sterigmate, 21–25 × 5.7–8 μm, with a basal clamp. Basidiospores cylindric, hyaline, smooth, IKI-, 5.1–6.8 × 2.3–2.5 μm. L = 5.7 μm, W = 2.4 μm, Q = 2.4 (n = 20/1).
Habitat: solitary to gregarious on dead wood of hardwood.
Material examined: KOREA, Gyeongsangbuk-do, Yecheon-gun, Mt. Hagga, on fallen hardwood branch, 4 July 2013, Y.W. Lim (SFC20130704-20, holotype); ibid. (SFC20130704-06, paratype); KOREA, Gyeongsangbuk-do, Sangju-si, Seongjubong Natural Recreation Forest, on fallen hardwood branch, 9 August 2013, H. Lee, W. J. Kim & S-Y. Oh (SFC20130809-44); KOREA, Jeju-do, Seogwipo-si, Hannam Experimental Forest, on rotten hardwood stump, 7 August 2012, Y. W. Lim, H. Lee, W. J. Kim & J. S. Gyeong (SFC20120807-12); KOREA, Chungcheongnam-do, Boryeong-si, Seongjusan Natural Recreation Forest, on fallen hardwood branch, 21 August 2012, Y. W. Lim, H. Lee, W. J. Kim & Y. J. Min (SFC20120821-36); ibid. (SFC20120821-59); KOREA, Jeollabuk-do, Namwon-si, Mt. Jiri, on fallen hardwood branch, 20 July 2005, H. S. Jung (SFC20050720-60); ibid., 22 July 2004, J. S. Lee & S. H. Hong (SFC20040722-26, paratype).
GenBank Numbers SFC20130704-20 ITS:KY038470. SFC20130704-06 ITS:KY038469. SFC20130809-44 ITS:KY038475. SFC20120807-12 ITS:KY038465). SFC20120821-36 ITS: KY038468. SFC20050720–60 ITS:KY038463.
Notes: This species has variable basidiocarp shapes similar to Polyporus varius. Therefore, many of specimens in Korea had been misidentified as Polyporus varius. Two types of P. varius sequences were reported in a previous study (Sotome et al. 2008). The first included sequences from the USA, Russia, and Japan (Sotome et al. 2011; Dai et al. 2014) and the second was unique to Japan (AB587635, Sotome et al. 2011). Phylogenetic analysis based on ITS sequences showed that P. orientivarius formed a monophyletic group with the second type of P. varius sequence and was distantly related to P. varius (Fig. 3). In addition, P. varius has smaller pores (7–9 mm) and longer basidiospores (9–12 × 2.5–3 μm) (Gilbertson and Ryvarden 1987) than those of P. orientivarius. Therefore, both morphological and molecular evidence support P. orientivarius as a new species that is clearly separate from P. varius.
Polyporus parvovarius H. Lee, N.K. Kim & Y.W. Lim, sp. nov.
Index Fungorum number: IF552511; Facesoffungi number: FoF 2629, Fig. 182
Etymology: referring to the similar but smaller basidiocarps compared to P. varius.
Holotype: SFC20120814-33
Basidiocarps annual, central stipitate; Pileus circular, plane then funnel-shaped, up to 35 mm diam., 1.5 mm thick; pileal surface light buff to brown, fibrillose with flattened, radially striate, glabrous; margin reddish-brown, enrolled, undulate. Hymonophore white, cream to brown; pores fine, rounded to angular, 6–7 per mm. Context in pileus pale buff, corky, up to 1 mm thick. Tube continuous with the context, up to 0.5 mm thick. Stipe central, brown to dark brown, smooth to longitudinally wrinkled, apex distinct from the pore, upper portion of blackish stipe usually covered by decurrent pores, up to 40 mm long and 2 mm thick. Hyphal system dimitic; generative hyphae hyaline in KOH, thin-walled, 2.5–3 μm in diam., septa with clamps, skeletal-binding hyphae thick-walled, aseptate, much branched, with tapering apices, 3–6 μm diam. Cystidia absent. Basidia clavate, 4-sterigmate (up to 3.4 μm), 23.8–26.3 × 7.4–8.5 μm, with a basal clamp. Basidiospores cylindric, hyaline, smooth, IKI-, 7.4–8.6 × 3.5–4.5 μm. L = 7.52 μm, W = 4.25 μm, Q = 1.78 (n = 20/1).
Habitat: solitary to gregarious on dead wood of hardwood.
Material examined: KOREA, Gyeongsangbuk-do, Ulleung-gun, Na-ri basin, on fallen hardwood branch, 14 August 2012, Y. W. Lim, W. J. Kim & S-Y. Oh (SFC20120814-33, holotype); KOREA, Gangwon-do, Inje-gun, on rotten wood below litter layer, 1 July 2014, Y. W. Lim & H. J. Cho (SFC2040701-19, paratype).
GenBank Numbers ITS:KY038466.
Notes: Polyporus parvovarius is similar to P. badius, P. elegans and P. varius in its central stipitate basidiocarps, blackish stipe, and microscopic features. Simple septate generative hyphae separate P. badius from the other species. P. parvovarius has larger basidia (23.8–26.3 × 7.4–8.5 μm) than P. elegans (15–20 × 6–7 μm) and P. varius (18–23 × 5–7 μm). Phylogenetic analysis of the ITS region showed that P. parvovarius is closely related to P. brevibasidiosus (Fig. 178). However, they are distinguishable by pore, basidia, and basidiospore size. P. parvovarius has larger than those of P. brevibasidiosus.
Polyporus subdictyopus H. Lee, N.K. Kim & Y.W. Lim, sp. nov.
Index Fungorum number: IF552512; Facesoffungi number: FoF 2630, Fig. 183
Etymology: referring to similar morphology and close phylogenetic relationship with P. dictyopus.
Holotype: SFC20130719-53
Basidiocarps annual, central to laterally stipitate; Pileus circular to dimidiate, plane then funnel-shaped, up to 60 mm diam., 2.5 mm thick; pileal surface whitish when juvenile to pale buff, brown and dark brown with age, fibrillose with flattened, radially striate, slightly glabrous, margin ochreous, sharp and undulate. Hymonophore white, cream, buff to brown; pores fine, rounded to angular, 8–10 per mm. Context in pileus pale buff, corky, up to 2 mm thick. Tube continuous with the context, up to 0.5 mm thick. Stipe central, brown to dark brownish-black, smooth to longitudinally wrinkled, apex distinct from the pore, pores traces observed at upper portion of blackish stipe, up to 15 mm long and 6 mm thick. Hyphal system dimitic; generative hyphae hyaline in KOH, thin-walled, 1.7–3 μm in diam., septa with clamps, skeletal-binding hyphae thick-walled, aseptate, much branched, with tapering apices, 3–5 μm diam. Cystidia absent. Basidia clavate, 4–sterigmate (up to 7.9 μm), 21–23 × 5.1–6.9 μm, with a basal clamp. Basidiospores cylindric, hyaline, clear one or two oil drops, smooth, IKI–, 6.8–9.1 × 2.2–2.8 μm. L = 7.39 μm, W = 2.34 μm, Q = 3.16 (n = 20/2).
Habitat: solitary to gregarious or frequently concrescent on dead wood of hardwood, occasionally on conifer (SFC20110818–02).
Material examined: KOREA, Gyeongsangbuk-do, Sangju-si, Mt. Noem, on fallen hardwood branch, 19 July 2013, H. Lee, W. J. Kim & Y. J. Min (SFC20130719-53, holotype); KOREA, Gangwon-do, Wonju-si, Mt. Chiak, on dead conifer, 18 August 2011, Y.W. Lim (SFC20110818-02, paratype); KOREA, Gyeongsangbuk-do, Yecheon-gun, Mt. Hagga, on fallen hardwood branch, 4 July 2013, Y.W. Lim (SFC20130704-39); KOREA, Jeollabuk-do, Jinan-gun, Unjangsan Natural Recreation Forest, on fallen hardwood branch, 23 July 2014, J. Y. Park, H. Lee & H. J. Cho (SFC20140723-08); KOREA, Jeju-do, Jeju-si, Bijarim, on fallen hardwood branch, 30 June 2015, Y. W. Lim, N. G. Kim & H. Lee (SFC20150630-48); KOREA, Chungcheongbuk-do, Boeun-gun, Mt. Songni, on dead trunk of oak, 25 July 2015, H. Lee (SFC20150725-05).
GenBank Numbers SFC20130719-53 ITS:KY038473. SFC20110818-02 ITS:KY038464. SFC20130704-39 ITS:KY038471. SFC20140723-08 ITS:KY038476. SFC20150630-48 ITS:KY038477. SFC20150725-05 ITS:KY038478).
Notes: Basidiocarps of this species are most similar to those of P. badius. P. badius is easily distinguished by its lack of clamp connection. In a phylogenetic analysis, P. badius formed a separate clade within the Melanopus clade, but P. subdictyopus is located in a sister clade together with P. dictyopus (Fig. 178). Basidiocarps shape and microscopic features of P. dictyopus are hard to distinguish except its pantropical distribution. One or two oil drops in the basidiospores of P. subdictyopus differentiate it from P. dictyopus.
Polyporus ulleungus H. Lee, N.K. Kim & Y.W. Lim, sp. nov.
Index Fungorum number: IF552510; Facesoffungi number: FoF 2628, Fig. 184
Etymology: very common on Ulleung Island.
Holotype: SFC20120814-41
Basidiocarps annual, central stipitate; Pileus circular, plane then infundibuliform, up to 110 mm wide, 3 mm thick; pileal surface light brown to dark blackish-brown in the center, fibrillose with flattened, radially striate, slightly glabrous, margin ochreous, sharp and undulate. Hymonophore white to brown; pores fine, rounded to angular, 5–6 per mm. Context in pileus pale buff, corky, up to 2 mm thick. Tube white and cream when young, ochreous with age or dry, up to 1 mm thick. Stipe central, brown to dark brownish-black, smooth to longitudinally wrinkled, apex distinct from the pore, upper portion of blackish stipe usually covered by pores, up to 30 mm long and 8 mm thick. Hyphal system dimitic; generative hyphae hyaline in KOH, thin-walled, 1.7–3 μm in diam., septa with clamps, skeletal-binding hyphae thick-walled, aseptate, much branched, with tapering apices, up to 6 μm diam. Cystidia absent. Basidia clavate, 4–sterigmate (up to 3.4 μm), 23.4–25.7 × 5.8–6.9 μm, with a basal clamp. Basidiospores cylindric, hyaline, smooth, IKI-, 6.8–8 × 2.3–3.3 μm. L = 7.06 μm, W = 2.85 μm, Q = 2.50 (n = 20/2).
Habitat: solitary to gregarious on dead wood of hardwood.
Material examined: KOREA, Gyeongsangbuk-do, Ulleung-gun, Na-ri basin, on fallen hardwood branch, 14 August 2012, Y.W. Lim, W. J. Kim & S-Y. Oh (SFC20120814-41, holotype); KOREA, Gangwon-do, Inje-gun, on fallen branch of Betula platyphylla var. japonica, 2 September 2015, Y. W. Lim & H. Lee (SFC20150902-99, paratype).
GenBank Numbers SFC20120814-41 ITS:KY038467. SFC20150902-99 ITS:KY038481.
Notes: Polyporus ulleungus is commonly found in the Na-ri Basin of Ulleung Island and has larger basidiocarps (SFC20120814-41; 110 mm across) than other species collected in inland area (SFC20150902-99; 35 mm across). Polyporus ulleungus is most similar to P. koreanus and P. melanpus. It has larger pore (5–6 mm) than those of P. koreanus (6–7 mm) and P. melanpus (6–8 mm). In a phylogenetic analysis, P. ulleungus formed a distinct clade with strong support (100% ML, Fig. 179) and clearly separated from P. koreanus and P. melanpus (Fig. 178).
Wolfiporia Ryvarden & Gilb.
Wolfiporia is a genus of mushrooms in the family Polyporaceae. The genus was introduced by Ryvarden and Gilbertson (1984) to contain the type species Wolfiporia cocos (currently known as W. extensa) and W. dilatohypha (Fig. 185).
Maximum likelihood phylogenetic tree of the concatenated ITS1, 5.8S, ITS2 and partial 28S rDNA sequences. Bootstrap supporting values (1000 replicates) and posterior probabilities (PP) from Bayesian analysis to each node are shown from left to right. The new species Wolfiporia pseudococos described in this study is highlighted in blue. The tree was rooted with Grifola sordulenta and Laetiporus sulphureus
Wolfiporia pseudococos F. Wu, J. Song & Y.C. Dai, sp. nov.
MycoBank number: MB 818729; Facesoffungi number: FoF 2961, Figs. 186, 187
Etymology: referring to the similarity of Wolfiporia cocos.
Holotype: BJFC01938
Basidiocarp annual, resupinate, soft corky and without odour or taste when fresh, corky to fragile when dry, up to 10 cm long, 4 cm wide, 6 mm thick at centre. Margin thin, usually pores extend to the very edge. Pore surface cream to ash-grey when fresh, becoming pinkish-buff to cinnamon-buff when dry, not glancing; pores round, 1–3 per mm; dissepiments thick, even to slightly lacerate. Subiculum cinnamon-buff, corky, up to 1.5 mm; tubes corky to fragile, buff, up to 4.5 mm long. Hyphal system dimitic in all parts, generative hyphae with simple septa, skeletal hyphae dominant, all hyphae IKI-, CB-, weakly inflated in KOH. Subicular hyphal structure homogeneous, hyphae strongly interwoven; generative hyphae occasionally present, hyaline, thin-walled, occasionally branched, frequently simple septate, 4–6 µm in diam.; skeletal hyphae dominant, hyaline, thick-walled with a distinct wide lumen, usually winding, frequently branched, occasionally simple septate, 5–7 µm in diam. Tramal generative hyphae frequent, hyaline, thin-walled, occasionally branched, frequently simple septate, 3–5 µm in diam.; tramal skeletal hyphae frequent, hyaline, thick-walled with a wide lumen, winding, occasionally branched and simple septate, 4–6 µm in diam. Cystidia absent, cystidioles present; Basidia clavate, with four sterigmata and a basal simple septum, 18–25 × 10–14 µm, basidioles in shape similar to basidia but slightly smaller. Basidiospores ellipsoid to broadly ellipsoid, tapering at apiculus, hyaline, thin-walled, IKI-, CB-, (7.6–)7.9–9.5(–9.7) × (2.9–)3–3.8 µm, L = 8.55 µm, W = 3.17 µm, Q = 2.7 (n = 30/1).
Specimen examined: CHANA, Hainan Province, Ledong County, Jianfengling Nature Reserve, On dead angiosperm tree, 1 June 2015, Dai 15269 (BJFC019380, holotype).
GenBank Numbers ITS:KX354451.
Notes: The phylogenetic analysis of ITS sequence (Fig. 186) shows that the new species, Wolfiporia pseudococos, is closely related to W. cocos (F.A. Wolf) Ryvarden & Gilb, the type species of the genus Wolfiporia. Sequence data from three specimens of W. Cocos also clustered in a distinct lineage with strong support (100% BS, 1 BYPP), and the sequence of the new species formed a terminal lineage. Morphologically, Wolfiporia pseudococos is distinguished from W. cocos by the presence of cystidioles and larger basidiospores (7.9–9.5 × 3–3.8 µm vs. 6.5–8.1 × 2.8–3.1 µm). In addition, Wolfiporia cocos usually grows on conifers in temperate areas, but Wolfiporia pseudococos has a distribution in tropics and growing on angiosperm trees.
Psathyrellaceae Locq.
The family Psathyrellaceae is a family of dark-spored agarics that generally have rather soft, fragile fruiting bodies, and are characterized by black, dark brown, rarely reddish, or even pastel-coloured spore prints.
Coprinopsis P. Karst., Acta Soc. Fauna Flora Fenn. 2 (1): 27 (1881)
During the last 25 years, molecular phylogenetic analyses have made great impact on our understanding of phylogenetic relatonships among coprinoid fungi (Hopple and Vilgalys 1994, 1999; Moncalvo et al. 2002). The analyses have showed that the genus Coprinus Pers. sensu lato is not monophyletic. A minority of Coprinus species (including the type species C. comatus (O.F. Müll.) Pers.) is clustered near the genus Agaricus L., while the great majority of Coprinus species is grouped into three clades closely related to the genus Psathyrella (Fr.) Quél. Based on molecular studies, Redhead et al. (2001) transferred the majority of Coprinus species into three genera (Coprinellus P. Karst., Coprinopsis, and Parasola Redhead, Vilgalys & Hopple) within the newly proposed family Psathyrellaceae Vilgalys, Moncalvo & Redhead. Consequently, the genus Coprinopsis includes five subsections of Coprinus sensu lato, Alachuani Singer, Atramentarii (Fr.) Konrad & Maubl., Lanatuli J.E. Lange, Narcotici Uljé & Noordel., and Nivei Citérin. Further phylogenetic research (Larsson and Örstadius 2008; Padamsee et al. 2008; Örstadius et al. 2015) revealed that the genus Psathyrella is not monophyletic either. Based on these studies, some Psathyrella species belong to the genus Coprinopsis (Fig. 188).
Maximum likelihood phylogenetic tree inferred from the dataset of ITS1–5.8S-ITS2 gene sequences from Coprinopsis cerkezii sp. nov. and related species. The new species is shown in blue. Maximum likelihood bootstrap values greater than 50% are indicated at the nodes. The tree is rooted with Coprinellus xanthothrix and C. domesticus. The bar indicates the number of nucleotide substitutions per site
Coprinopsis cerkezii Tkalčec, Mešić, I. Kušan & Matočec, sp. nov.
MycoBank number: MB 818295; Facesoffungi number: FoF 2962, Figs. 189, 190
Etymology: Described in honour of Mr. Milan Čerkez for his great contribution to the study of coprinoid and coprophilous fungi in Croatia.
Holotype: CNF 1/7253
Pileus 3–11 mm broad when expanded, spherical at first, then ellipsoid, paraboloid, convex, at maturity plano-convex with depressed centre, applanate or plano-concave, strongly plicate-sulcate up to 85% of the radius at maturity, edge strongly crenate, sometimes radially splitting with age, not hygrophanous, non-deliquescent, surface dry, at first entirely covered with upper velar layer composed of fibrils mostly directed downwards (more developed in the lower part of the pileus), tufts and warts in the central zone, upper velar layer tearing soon (mostly remaining in the form of velar patches, tufts and fibrils) and exposing whitish to cream granulose lower velar layer, fibrils white to light orange-brown, tufts and warts pale to medium orange-brown to rusty-orange, darker at centre, often paler towards the margin. Lamellae free, distant, L = 18–32, l = 0–1, up to 1.5 mm broad, sometimes forked, often deliquescent at the edge, white at first, becoming rusty-brown or dark (chocolate) brown at maturity, sometimes whitish to greyish till maturity, with whitish and flocculose edge (when not deliquescent). Stipe 10–50 × 0.5–1.2 mm, often with swollen base (up to 2 mm), slightly tapering towards the apex, central, hollow, dry, densely hairy at first, later floccose to flocculose, white to cream when young, then subhyaline, orange-brown to rusty-orange at the base, velar hairs sometimes forming volva-like basal structure. Context fragile, very thin in the pileus, whitish. Odour and taste not observed.
Basidiospores [300/6/4] (4.8–)5.3–6.8–8.3(–9.1) × (2.7–)2.8–3.2–3.6(–3.8) µm, average = 6.2–7.6 × 3–3.4 µm, Q = (1.48–)1.66–2.12–2.59(–2.90), Qav = 1.98–2.47 (very variable among different basidiocarps), narrowly ellipsoid, elongate ellipsoid, cylindrical or subovoid in frontal view, ellipsoid, amygdaliform, phaseoliform or allantoid in side view, smooth, moderately thick-walled (ca. 0.5 µm), with distinct and central (sometimes slightly eccentric), 1–1.5 µm wide germ-pore, rusty-brown in H2O and NH4OH, medium brown in KOH, non-amyloid and non-dextrinoid. Basidia 8–24 × 4–8 μm, narrowly to broadly clavate, 4-spored, thin-walled, hyaline, surrounded by 3–5 hymenophysalides (pseudoparaphyses). Hymenophysalides 10–21 × 7–18 μm, subglobose, (broadly) ellipsoid, broadly clavate or broadly oblong, thin-walled, hyaline, fully developed only in mature basidiocarps. Lamellar edge sterile with crowded cheilocystidia. Cheilocystidia 10–65 × 10–35 μm, very variable in size and shape, globose, subglobose, ellipsoid, clavate, ovoid, obovoid, conical, obtuse-conical, utriform or broadly lageniform, thin- to moderately thick-walled (up to 0.7 µm), hyaline. Pleurocystidia absent. Pileipellis a cutis, composed of repent, hyaline, thin-walled, 1.5–7 μm wide hyphae. Veil on pileus consists of two layers; lower layer completely covering pileipellis and predominantly composed of globose to broadly ellipsoid, subhyaline to pale yellow–brown, thin- to thick-walled (up to 1.2 µm), often minutely encrusted, 15–55 µm wide cells, intermixed with some hyphoid elements; upper layer tearing during the development, composed of long, (sub) cylindrical, non-diverticulate, hyaline to pale yellow–brown, thin-walled, mostly minutely to coarsely encrusted, 2–18 µm wide cells, ± intermixed with inflated (ellipsoid, fusoid, ovoid, clavate, subglobose or globose) cells, subhyaline to light yellow–brown, thin- to thick-walled (up to 1.2 µm), often minutely to coarsely encrusted, up to 50 µm wide. Stipitipellis a cutis of parallel, repent, thin-walled, hyaline, 2–12 μm wide hyphae. Veil on stipe composed of (sub) cylindrical and inflated (ellipsoid, fusoid, ovoid, clavate, subglobose or globose) cells, hyaline to light yellow-brown, thin-walled, minutely encrusted, 2–35 µm wide. Clamp connections absent.
Ecology: In groups on deer dung heaps; among mosses in forest of Abies alba, Picea abies, and Fagus sylvatica and among grasses and sedges in a thicket of Salix aurita, Betula sp., and Alnus glutinosa.
Known distribution: Croatia, mountainous area of Primorje-Gorski Kotar County, found at two localities 20 km apart.
Material examined: CROATIA, Primorje-Gorski Kotar County, near Skrad, about 1 km SE of Rogi, 4525′13″N, 1452′59″E, 725 m a.s.l., in forest of Abies alba, Picea abies, and Fagus sylvatica, on deer dung, 14 May 2010 in culture (substrate coll. 27 March 2010), leg. M. Čerkez (CNF 1/7253, holotype); ibid., 4525′18″N, 1452′51″E, 695 m a.s.l., 22 April 2010 in culture (substrate coll. 27 March 2010), leg. M. Čerkez (CNF 1/7252); CROATIA, Primorje-Gorski Kotar County, about 4 km W-NW of Fužine, 4519′16″N, 1440′15″E, 745 m a.s.l., among grasses and sedges, thicket of Salix aurita, Betula sp., and Alnus glutinosa, on deer dung, 13 May 2008 in culture (substrate coll. 30 April 2008), leg. M. Čerkez (CNF 1/5209); ibid., 22 May 2008 in culture (substrate coll. 30 April 2008), leg. M. Čerkez (CNF 1/5216).
GenBank Number ITS:KX869912.
Notes: Based on a megablast search of NCBIs GenBank numbers nucleotide database, the closest hits using the ITS1-5.8S-ITS2 sequence for our holotype collection (CNF 1/7253, GenBank Numbers KX869912) are Coprinopsis (Coprinus) cortinata (J.E. Lange) Gminder (GenBank Numbers JF907847; Identities = 619/702 (88%), Gaps = 29/702 (4%)), Coprinopsis (Coprinus) bellula (Uljé) P. Roux & Eyssart. (GenBank Numbers FN430682; Identities = 621/705 (88%), Gaps = 30/705 (4%), and Coprinopsis utrifer (Watling) Redhead, Vilgalys & Moncalvo (GenBank Numbers FN396410; Identities = 609/688 (89%), Gaps = 28/688 (4%)). Other three collections of C. cerkezii (CNF 1/5209, 1/5216, 1/7252) have identical ITS and LSU sequences. The phylogenetic analysis using maximum likelihood method was performed with MEGA6 software. The ITS sequence from the holotype of Coprinopsis cerkezii was combined with sequences of all closely related species and some other taxa of the genus Coprinopsis downloaded from GenBank number database. Analysed sequences belong to the species from subsections Alachuani, Atramentarii, Lanatuli, Narcotici, and Nivei (Uljé 2005) of the genus Coprinus sensu lato, as well as to a few Coprinopsis species transferred from the genus Psathyrella (Örstadius et al. 2015). Sequences of Coprinellus species (C. xanthothrix (Romagn.) Vilgalys, Hopple & Jacq. Johnson and C. domesticus (Bolton) Vilgalys, Hopple & Jacq. Johnson) were used as outgroup. In phylogenetic analysis Coprinopsis cerkezii clustered with other members of subsect. Nivei of the genus Coprinus sensu lato (Fig. 188), in accordance with its morphological characters.
Coprinopsis cerkezii is primarily characterised by small and delicate basidiocarps, a strongly plicate-sulcate pileus with an orange-brown to rusty-orange upper veil, narrow and elongated spores without myxosporium, an abundance of spherical pileal velar elements lacking nipple-shaped warts, an absence of pleurocystidia and clamp-connections, and by living on dung. Comparison of C. cerkezii with similar taxa is based on descriptions in the following literature: Bisby et al. (1929), Orton and Watling (1979), Pegler (1986), Doveri (2004), Uljé (2005). Five species morphologically similar to C. cerkezii, C. bellula, C. candidata (Uljé) Gminder & T. Böhning, C. coniophora (Romagn.) Redhead, Vilgalys & Moncalvo, C. cortinata, and Coprinus furfurellus (Berk. & Broome) Pegler, differ by living on soil or wood, by presence of clamp-connections, and by absence of orange-brown tones in pileus. From above mentioned species, Coprinus furfurellus possess the most similar basidiospores (6–7 × 3.5–4 µm), but the average Q is smaller (1.73). Three other similar species live on dung as Coprinopsis cerkezii. Coprinus parvisporus Buller differs by having white pileus, less elongated spores (6 × 3.5 µm), presence of pleurocystidia, and larger spherical elements of pileal veil (60–80 µm). Coprinopsis pseudcortinata (Cacialli, Caroti & Doveri) Doveri, Granito & Lunghini differs by having white to greyish pileus, broader (3.5–4.5 µm) and less elongated spores (Q = 1.5–2.05), presence of pleurocystidia and smaller number of lamellae (L = 6–12). Coprinopsis utrifer differs by having broader (4–5.5 µm) and less elongated spores (Q = 1.3–1.7) and by presence of clamp-connections and pleurocystidia. ITS Sequences of Coprinopsis candidata, C. pseudcortinata, Coprinus furfurellus, and C. parvisporus are not included in phylogenetic analysis since the sequences of these species are not present in GenBank.
Russulales Pers.
Russulaceae Lotsy
Russulaceae is one of the dominant and morphologically diverse families of mushrooms in the order Russulales, with roughly 1900 known species and a worldwide distribution (Kirk et al. 2008). Apart from its three corticoid genera, i.e. Boidinia, Gloeopeniophorella and Pseudoxenasma (Larsson and Larsson 2003; Miller et al. 2006), this family has four predominantly agaricoid genera: Lactifluus, Lactarius, Multifurca and Russula (Buyck et al. 2008, 2010), some of which may also contain secotioid-hypogeous or pleurotoid species.
Russula Pers.
The genus Russula Pers. has a worldwide distribution and can be easily identified with its colourful fruiting bodies (Kirk et al. 2008). Russula is widely distributed from Western Europe to North America in the northern hemisphere (Romagnesi 1967; Singer 1986; Sarnari 1998; Miller and Buyck 2002; Bau et al. 2008). The genus contains more than 200 species generally accepted in Europe (Sarnari 1998, 2005) and more than 750 species worldwide (Kirk et al. 2008). Keys to Russula being complex since of this huge taxonomic diversity. They play a crucial beneficial role in forest ecosystems as ectomycorrhizal symbionts (Li et al. 2013a). Russula species were used as traditional food and medicine in China for a long history. Twenty-two medicinal species and 82 edible species have been reported in China (Dai et al. 2009; Li et al. 2010). Southwestern China represents one of the world’s biodiversity “hotspots” and has a high diversity of macro-fungi (Yuan and Dai 2008), but only a few russulacean species have been identified from this region. However, some new species and varieties have been reported from Southern and Southwestern China (Wang et al. 2009). In this contribution one new species belong to genus Russula is introduced from Guizhou Province, China (Fig. 191).
Phylogram generated from Maximum parsimony (PAUP) analysis based on 5.8S-ITS rDNA sequence data. Bootstrap support values for maximum likelihood (black) and maximum parsimony (blue) greater than 75% are indicated above the nodes. The tree is rooted with Albatrellus ovinus. The strain numbers are mentioned after the species names. The new species is indicated in red and ex-type strains are indicated in black bold
Russula yanheensis T.C. Wen, K. Hapuarachchi & K.D. Hyde, sp. nov.
Index Fungorum number: IF 552563; Facesoffungi number: FoF: 2707, Fig. 192
Etymology: refers to the type collecting site “Yanhe County”, Guizhou Province, China
Holotype: GACP13100308
Basidiocarps small-sized or medium-sized. Pileus 32–70 mm diam., plano-convex, sometimes slightly depressed above the stipe, surface smooth, sticky, broad, fleshy, firm, hemispherical when young, then expanded with depressed centre, depressed weakly in the center when mature, extreme margin becoming somewhat sulcate with age, glabrous, shining. Pileus peeling 1/2 to the disc; margin even, crack when mature. Lamellae regular, adnate, crowded, pale yellowish (2A2), brittle, edge entire. Stipe 23–42 × 13–25 mm, clavate (bottom slightly stigmas), enlarged towards the base, centrally attached, orange white (6A2) when young, pale coralline (9B7) when old, cylindrical to subcylindrical, stuffed, slightly longitudinally wrinkled long and slender, flushed with pink Context compact, 0.1–1.5 cm thick from stipe top to pileus center, not firm, colour unchanged when bruised. Basidiospores ornamentation amyloid, 5.87–8.26 × 4.88–7.31 µm (\( \bar{x} \) = 6.23 × 7.11 μm, Q = 1.01–1.32, n = 28), subglobose to spherical, ellipsoid, colourless, without oil droplets, moderately large and distant amyloid warts. Basidia, broadly tapered towards the base, 48.7–77 × 10.3–12.05 µm (\( \bar{x} \) = 60 × 11.42 μm, Q = 4.32–5.58, n = 10), 4-spored, clavate, hyaline, and smooth. Hymenophoral trama cellular 18.52–33.20 × 16.02–28.58 μm (\( \bar{x} \) = 26.82 × 22.28 μm, Q = 0.99–1.45, n = 20), subglobose to spherical, ellipsoid, colourless, smooth. Cheilocystidia 73.52–103.79 × 10.29–13.57 μm (\( \bar{x} \) = 94.75 × 11.74 μm, Q = 6.32 × 11, n = 8), clavate, fusiform to subfusiform, papillae at the top, thin-wall, hyaline, smooth. Pilocystidium rare, emergent, 85.5–113 × 4.1–8.5 μm (\( \bar{x} \) = 95.28 × 6.7 μm, Q = 10.62–15.94, n = 7) clavate, thin-wall, hyaline, smooth, sometimes with a frayed small appendage and dense crystal inclusions, projecting above the sub hymenium. Pileipellis an ixotrichoderm, composed of 4–6 µm thick, cylindrical, rarely swollen hyphae with intracellular reddish pigmentation; terminal cells. Often inflating up to 7–10 (12) µm thick and with brownish intracellular pigmentation, some fusiform and with zebroid-encrustations. 19.65–7.87 × 5.92–2.1 μm (\( \bar{x} \) = 16.77 × 3.05 μm, Q = 2–11.2, n = 9). Stipitipellis a cutis with some hyphal ends ascending; hyphae 4–6 µm diam., some hyphae near surface yellowish-white; caulocystidia absent.
Habitat and distribution: On the ground, accompanied in humus rich soil with over heavily rotted litter in forest, mossy temperate mixed coniferous forests, solitary, producing basidiomata from summer to late autumn, only found in “Yanhe County”, Guizhou Province, China.
Material examined: CHINA, Guizhou Province, Coniferous mixed rainforest, 28″N 108 “E, elev. 850 m, 3 October 2013, collector T.C Wen (GACP13100308, holotype). CHINA, Guizhou Province, Coniferous mixed rainforest, 28″N 108 “E, elev. 850 m, 3 October 2013, collector T.C Wen (GACP13100319, paratype).
GenBank Number ITS:KY195927.
Notes: Russula yanheensis is morphologically and phylogenetically close to Russula pulchra Burl. Russula pulchra has 50–100 mm diameter pileus, stipe 30–70 mm long, ellipsoid basidiospores 6.5–9 × 5.5–7.5 µm; and pilocystidia absent (Binion et al. 2008), but R. yanheensis has 32–70 mm diameter pileus, stipe 23–42 mm long, ellipsoid to subglobose to globose basidiospores 5.8–8.3 × 4.8–7.3 µm, i.e. having smaller pileus, stipe and basidiospores, and the different shapes of basidiospores, presence of pilocystidia. Russula yanheensis is also morphologically similar to R. flavisiccans Bills but the latter species differs from our species by its pileus colour, pilocystidia and cheilocystidia shapes and sizes, further, the stipe never flushes with pink (Kuo and Methven 2014). Further, R. yanheensis is phylogenetically closely related to R. sichuanensis G.J. Li & H.A. Wen, but its morphological characteristics are very different from the latter species by its colour, pilocystidia and cheilocystidia shapes and sizes.
Russula virescens (Schaeff.) Fr., Anteckningar öfver de i Sverige växande ätliga svampar: 50 (1836)
Facesoffungi number: FoF 3145, Figs. 193, 194
Pileus 4–16 cm, when young round to convex, when mature broadly convex to flat to uplifted with a shallow depression, dry, velvety, surface soon cracking up into small patches, green to yellowish-green, margin not lined; the skin peeling towards the center. Lamellae attached to the stem or at maturity nearly free, close or crowded, white to cream. Stipe 2–11 cm long, 2–5 cm thick, dry, smooth, white, brittle, discolouring brownish with age but not with bruising or cutting. Context white, brittle, thick, not changing when sliced. Odour and taste not distinctive, taste mild. Spore print white. Basidiospores 6–9 × 5–7 μm, elliptical to subglobose, warts 0.5 μm high, connectors almost absent, scattered, or creating moderately reticulated areas. Pleurocystidia very few. Pileipellis a cutis overlaid with epithelium-like areas or crustose patches composed of elements of chained cells diminishing in width from base to tip, with the terminal cell projecting an extension that is frequently elongated and tapered. Pileocystidia cylindric with capitate apices, positive with sulphovanillin.
Habit, habitat and distribution: gregariously or solitary, usually mycorrhizal with hardwoods, May–August. Our collection was collected associated with Dipterocarpus sp. at Peradeniya Royal Botanic Gardens.
Specimens examined: SRI LANKA, Kandy District, near Peradeniya Royal Botanic Gardens, 2 June 2012, Samantha C. Karunarathna (MFLU 12-1894, new record).
GenBank Number ITS:KY649467.
Notes: This has a widespread distribution in Asia, having been recorded from India, Malaysia, Korea, the Philippines, Nepal, China, Thailand, and Vietnam. It is also found in North Africa and Central America. This is the first report of R. virescens with the molecular phylogenetic confirmation from Sri Lanka.
Dacrymycetales Henn.
Dacrymycetaceae J. Schröt.
The family Dacrymycetaceae includes wood-decaying fungi that cause brown-rot (Oberwinkler 1993; Kirk et al. 2001). This family includes eight genera: Calocera, Cerinomyces, Dacrymyces, Dacryopinax, Ditiola, Femsjonia, Guepiniopsis and Heterotextus (Shirouzu et al. 2009). Basidiomata of Dacrymycetaceae are characterized by gelatinized yellow to orange basidiomata with Y-shaped basidia (Kennedy 1958).
Dacrymyces Nees.
Dacrymyces is a genus in the order Dacrymycetales of Dacrymycetes. The genus contains about 39 widely distributed species (Kirk et al. 2008) (Fig. 195).
The best scoring RAxML maximum likelihood phylogenetic tree based on a combined ITS and SSU sequence dataset. Strain/culture numbers are given following the taxon names. The newly generated sequences are in blue. Bootstrap support values for maximum likelihood >60% are given near the nodes. The tree is rooted with Exidia uvapsassa (AFTOL ID 461)
Dacrymyces chiangraiensis Ekanayaka, S.C. Karunarathna, Q. Zhao & K D. Hyde, sp. nov.
Index Fungorum number: IF552549; Facesoffungi number: FoF 2696, Fig. 196
Morphology of Dacrymyces chiangraiensis (MFLU 16-0572, holotype). a Substrate. b Basidiomata on wood. c Cross section of basidiome. d Close up of the hymenium layer and interscal tissue. e Close up of hymenium layer. f Septate, branched hyphae. g, h Probasidia. i, j Developing basidia. k, l Ellipsoid basidiospores. Scale bars a = 1000 µm, b = 200 µm, = 200 µm, c = 400 µm, d = 100 µm, e = 100 µm, f = 15 µm, g–j = 20 µm, k, l = 5 µm
Etymology: Name reflects the province, where the type species was collected
Holotype: MFLU 16-0572
Saprobic on wood, stems and twigs. Sexual morph Basidiomata 478–482 × 908–915 µm (\( \bar{x} \) = 480.8 × 911.6 µm, n = 10) superficial, mostly solitary, sometimes gregarious, pulvinate, yellow to orange. Sterile parts of basidiocarps covered with simple or branched, tightly packed, cylindrical, septate, hyaline, thin-walled marginal hyphae. Internal hyphae branched, thin walled, gelatinous, septate, hyaline, loosely packed, clamp connections absent. Hymenium composed of dikaryophyses and basidia. Dikaryophyses 2–5.5 µm (\( \bar{x} \) = 4 µm, n = 20) branched, septate, thick-walled, hyaline. Hymenium hyaline, limited to the upper surface of the basidiocarp. Probasidia 32–53 × 7–10 µm (\( \bar{x} \) = 41.4 × 8.5 µm, n = 20) hyaline, guttulate, cylindrical to clavate, thin-walled, becoming bifurcate. Basidiospore 19–23 × 6.5–8 µm (\( \bar{x} \) = 20.9 × 7.3 µm, n = 20), allantoid, somewhat bent, hyaline, with many transverse septa, smooth walled. Asexual morph undetermined.
Material examined: THAILAND, Mae Fah Luang University, Chiang Rai Province, 22 December 2014, A.H. Ekanayaka, (MFLU 16-0572, holotype).
GenBank Number ITS:KY498587.
Notes: Dacrymyces chiangraiensis is phylogenetically close to D.san-augustinii. However, they are separated with strong bootstrap support. Morphologically D. chiangraiensis is similar to D. san-augustinii in having yellow to orange pulvinate basidiomata, but they differ in the size of the basidiomata and colour of probasidia and basidiospores. Dacrymyces san-augustinii has larger basidiomata (1–2 mm high, 1–3 mm diam.) with pale yellow probasidia, and subhyaline, smaller basidiospores (15.5–23.6 × 4.6–6.8 µm) (McNabb 1973). Dacrymyces chiangraiensis differs from D. novae-zelandiae in having yellow to orange non-gelatinized basidiomata with smaller probasidia (Ellis and Ellis 1990; Shirouzu et al. 2009)
Femsjonia Fr.
The genus Femsjonia was established by Fries with the type species Femsjonia luteoalba Fr. However, the systematic position of this genus was not clear and linked with Peziza radiculata as the appearance of fruiting body is similar to discomycetes (McNabb 1965). Currently this genus classified within Dacrymycetaceae (Dacrymycetales, Dacrymycetes, Agaricomycotina). The genus Femsjonia is characterized by discoid, brightly coloured basidiomata with thick-walled internal hyphae and globose to sub-globose basidia (McNabb 1965; Shirouzu et al. 2009).
Femsjonia monospora Ekanayaka, S.C. Karunarathna, Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF552550; Facesoffungi number: FoF 2697, Fig. 197
Etymology: Name reflects the aseptate basidiospores.
Holotype: MFLU 16-0608
Saprobic on wood, stems and twigs. Basidiomata superficial, gregarious, discoid, sessile, bright yellow, orange near the base, sterile surface rough tomentose, whitish at the margins of the hymenial surface. Hymenium yellow. Internal hyphae branched, thin-walled, septate, hyaline, loosely packed. Probasidia 100–150 × 7–10 µm (\( \bar{x} \) = 139.6 × 8.2 µm, n = 20) cylindrical, yellow, thin-walled, becoming bifurcate. Basidiospores 25–30 × 10–15 µm (\( \bar{x} \) = 28.5 × 12.5 µm, n = 20), subglobose, guttulate, aseptate, yellowish, smooth-walled.
Material examined: CHINA, Yunnan province, Kunming, 10 November 2015, S. C. Karunarathna (NB130), (MFLU 16-0608, holotype).
GenBank Number ITS:KY498588.
Notes: Femsjonia monospora distinct from all the other species in the genus by having larger probasidia and aseptate, guttulate basidiospores (McNabb 1965; Liu et al. 1988; Shirouzu et al. 2009). The phylogenetic comparisons among Femsjonia species are not possible due to the lack of sequence data.
Tremellales Fr.
Tremellaceae Fr.
This family was erected by Fries in 1821 based on macromorphology of its members (Fries 1821). This family currently comprises 18 genera and 250 valid species (Kirk et al. 2008).
Tremella Pers.,
Tremella was one of the original genera created by Linnaeus in his Species Plantarum of 1753. All Tremella species are parasites of other fungi and most produce anamorphic yeast states. Basidiocarps, when produced, are gelatinous and are colloquially classed among the “jelly fungi”. Over 100 species of Tremella are currently recognized worldwide (Kirk et al. 2008). Two species, T. fuciformis and T. aurantialba, are commercially cultivated for food (Fig. 198).
Phylogenetic relationships inferred from maximum parsimony analysis of ITS-rDNA sequences of 40 taxa. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The new Sri Lankan record: Tremella fuciformis having GenBank Number KY649461 and Herbarium Number MFLU 12-1895 is shown in bold and blue. The topology is rooted with Filobasidium floriforme. Evolutionary analysis was conducted in PAUP 4.0b 10
Tremella fuciformis Berk., Hooker’s Journal of Botany and Kew Garden Miscellany 8: 277 (1856)
Facesoffungi number: FoF 3146, Figs. 199, 200
Basidiocarps foliose, lobes caespitose, wet, gelatinous but fairly hard, crisped, composed of graceful lobes, lobes generally very thin, flower like, translucent whitish, up to about 8 cm across and 5 cm high, surface smooth and shiny, generally associated with Hypoxylon sp. Spore print white. Basidiospores 7–15 × 5–9 µm, Qm = 1.2–1.6 µm, broadly ellipsoid to ellipsoid, smooth, often germinating by repetition, budding or sometimes germ tubes. Basidia mostly 4–spored but occasionally 2 or 3 spored, becoming longitudinally 4–septate with maturity, probasidia typically clavate becoming subglobose to ellipsoid with maturity, 11–16 × 8–14 µm, with sterigmata up to 50 × 4 µm. Conidia absent. Vesicles absent. Hyphidia absent. Hyphae 2–6 µm diam. in inner part of basidiocarps, close to substrate 12 µm diam. Haustorial hyphae in basal part close to substrate, often branched. Clamp connections present.
Habit, habitat and distribution: Tremella fuciformis, following its host, fruit bodies are typically found on dead, attached or recently fallen branches of broadleaf trees. This fungus commonly prefers tropical and subtropical ecosystems, May–September. This species is mainly in tropical and subtropical ecosystems, but does occur temperate areas in Asia including Thailand; Laos and China, and North America. It is found in South and Central America, the Caribbean, parts of North America, sub-Saharan Africa, southern and eastern Asia, Australia, New Zealand and the Pacific Islands. Our collection was found on a fallen dead tree branch near Hanthana Mountains Range, Peradeniya, Sri Lanka.
Specimens examined: SRI LANKA, Kandy District, Hanthana Mountains Range, 1 June 2012, Samantha C. Karunarathna (MFLU 12-1895, new record).
GenBank Number ITS:KY649468.
Notes: The species is distributed mainly in tropical and subtropical areas, but extends into temperate areas in Asia and North America. It is known throughout South and Central America, the Caribbean, parts of North America, sub-Saharan Africa, southern and eastern Asia, Australia, New Zealand, and the Pacific Islands. This is the first report of T. fuciformis with the molecular phylogenetic confirmation from Sri Lanka.
References
Aalto M (1974) Amanita magnivolvata sp. nova (Agaricales). Karstenia 14:93–96
Abbott SP, Currah RS (1997) The Hevellaceae: systematic revision and occurrence in northern and northwestern North America. Mycotaxon 62:1–125
Abdollahzadeh J, Goltapeh EM, Javadi A, Shams-Bakhsh M, Zare R, Phillips AJL (2009) Barriopsis iraniana and Phaeobotryon cupressi, two new species of the Botryosphaeriaceae from trees in Iran. Persoonia 23:1–8
Adamčík S, Cai L, Chakraborty D, Chen XH, Cotter H, Van T, Dai DQ, Dai YC, Sas K, Deng C, Ghobad-Nejhad M, Hyde KD, Langer E, Latha KPD, Liu F, Liu SL, Liu T, Wei LV, Shu-Xia LV, Machado AR, Pinho DB, Pereira OL, Prasher IB, Rosado AWC, Qin J, Qin WM, Verma RK, Wang Q, Yang ZL, Yu XD, Zhou LW, Buyck B (2015) Fungal biodiversity profiles 1–10. Cryptogam Mycol 36:121–166
Adams GC, Wingfield MJ, Common R, Roux J (2005) Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud Mycol 52:1–144
Adams GC, Roux J, Wingfield MJ (2006) Cytospora species (Ascomycota, Diaporthales, Valsaceae): introduced and native pathogens of trees in South Africa. Australas Plant Pathol 35:521–548
Addy HD, Piercey MM, Currah RS (2005) Microfungal endophytes in roots. Can J Bot 83:1–13
Ahmad S (1969) Fungi of West Pakistan, supplement 1. Society at the Biological Laboratories, Government College, Lahore
Ahmad S, Iqbal SH, Khalid AN (1997) Fungi of Pakistan. Sultan Ahmad Mycological Society of Pakistan, Department of Botany, University of the Punjab, Lahore
Aime L, Ryvarden L, Henkel TW (2007) Studies in Neotropicalpolypores 22. Additional new and rare species from Guyana. Synop Fungorum 23:15–31
Alvarado P, Manjon JL, Matheny PB, Esteve-Raventos F (2010) Tubariomyces, a new genus of Inocybaceae from the Mediterranean region. Mycologia 102:1389–1397
Andersson M (2013) A rare bolete, Boletus legaliae, in the Royal Garden of Drottningholm, Stockholm. Sven Mykol Tidskr 34(3):9–13
Antonín V, Buyck B (2006) Marasmius (Basidiomycota, Marasmiaceae) in Madagascar and the Mascarenes. Fungal Divers 23:17–50
Antonín V (2007) Monograph of Marasmius, Gloiocephala, Palaeocephala and Setulipes in tropical Africa. Fungus Flora Trop Afr 1:1–177
Antonín V, Vizzini A, Ercole E, Leonardi M (2015) Strobilomyces pteroreticulosporus (Boletales), a new species of the S. strobilaceus complex from the Republic of Korea and remarks on the variability of S. confuses. Phytotaxa 219(1):78–86
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Mapook A, Chukeatirote E (2014a) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (=Montagnulaceae). Fungal Divers 68:69–104
Ariyawansa HA, Hawksworth DL, Hyde KD, Jones EG, Maharachchikumbura SS, Manamgoda DS, Thambugala KM, Udayanga D, Camporesi E, Daranagama A, Jayawardena R (2014b) Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Divers 69(1):57–91
Ariyawansa HA, Phukhamsakda C, Thambugala KM, Bulgakov TS, Wanasinghe DN, Perera RH, Mapook A, Camporesi E, Kang JC, Jones EG, Bahkali AH (2015a) Revision and phylogeny of Leptosphaeriaceae. Fungal Divers 74:19–51
Ariyawansa HA, Thambugala KM, Manamgoda DS, Jayawardena R, Camporesi E, Boonmee S, Wanasinghe DN, Phookamsak R, Hongsanan S, Singtripop C, Chukeatirote E (2015b) Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers 71(1):85–139
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen JJ, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBG, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel- Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal-Forno M, Dollhofer V, Fliegerová K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytövuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Thorsten Lumbsch H, Matsumura M, Moncada B, Nuankaew S, Parnmen S, Santiago ALCMA, Sommai S, Song Y, de Souza CAF, de Souza- Motta CM, Su HY, Suetrong S, Wang Y, FongWS YH, Zhou LW, Réblová M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH, Miettinen O, Spirin V, Hernawati (2015c) Fungal diversity notes 111–252 taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:27–274
Arora D, Ferrarese G, Simonini G (1999) Boletus pulcherrimus Boletus rubrosanguineus: lo stesso taxon? Micol Veget Medit 14(2):116–141
Arun Kumar TK, Manimohan P (2009) The genus Lepiota (Agaricales, Basidiomycota) in Kerala State, India. Mycotaxon 107:105–138
Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin HD, Crous PW (2007) Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Stud Mycol 58:57–93
Aveskamp MM, de Gruyter J, Crous PW (2008) Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Divers 31:1–18
Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM, Crou PW (2010) Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Bahl J, Jeewon R, Hyde KD (2005) Phylogeny of Rosellinia capetribulensis sp. nov. and its allies (Xylariaceae). Mycologia 97:1102–1110
Baroni TJ, Hofstetter V, Largent DL, Vilgalys R (2011) Entocybe is proposed as a new genus in the Entolomataceae (Agaricomycetes, Basidiomycota) based on morphological and molecular evidence. N Am Fungi 6:1–19
Barr ME (1978) The Diaporthales in North America with emphasis on Gnomonia and its segregates. Mycol Mem 7:1–232
Barr ME (1979) A classification of Loculoascomycetes. Mycologia 71:935–957
Barr ME (1987) Prodomus to class Loculoascomycetes. Published by the Author, Amherst
Barr ME (1990) Melanommatales (Loculoascomycetes). N Am Flora Ser II 13:1–129
Barr ME (1992) Notes on the Lophiostomataceae (Pleosporales). Mycotaxon 45:191–221
Bas C (1969) Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 5:285–579
Bas C (1974) A rare and widespread Amanita associated with Alnus. Bulletin Mensuel de la Société Linnéenne de Lyon 43:17–23
Bas C (1984) On the correct name of ‘Amanita inaurata Secr.’. Persoonia Mol Phylogeny Evol Fungi 12:192–193
Bas C, Kuyper TW, Noordeloos ME, Vellinga EC (1995) Flora Agaricina Neerlandica—3, Tricholomataceae. A. A. Balkema, Rotterdam
Bates ST, Reddy GSN, Garcia-Pichel F (2006) Exophiala crusticola anam. nov. (affinity Herpotrichiellaceae), a novel black yeast from biological soil crusts in the Western United States. Int J Syst Evol Microbiol 56:2697–2702
Bau T, Li Y, Irina AG, Eugenia MB, Wasiliy AS (2008) Common wild edible mushroom resource of Russia. Edible Fungi China 27:9–13
Benny GL (2008) Methods used by Dr. R. K. Benjamin, and other mycologists, to isolate Zygomycetes. Aliso 26:37–61
Benny GL, Smith ME, Kirk PM, Tretter ED, White MM (2016) Challenges and Future Perspectives in the Systematics of Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina. In: Li D-W (ed) Biology of microfungi, fungal biology. Springer, Basel
Berkeley MJ (1847) Decades of fungi XII–XIV. Lond J Bot 6:312–326
Bernicchia A, Gorjón SP (2010) Corticiaceae s.l. Fungi Europaei, vol 12. Ed Candusso, Italia
Bessette AE, Roody WC, Bessette AR (2000) North American Boletes. Syracuse University Press, Syracuse
Bessette AE, Roody WC, Bessette AR (2010) North American Boletes: a color guide to fleshy pored mushrooms. Syracuse University Press, Syracuse
Bhatt RP, Tulloss RE, Semwal KC, Bhatt VK, Moncalvo JM, Stephenson SL (2003) Amanitaceae from India. A critically annotated checklist. Mycotaxon 88:249–270
Binder M, Hibbett DS (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98:971–981
Binder M, Justo A, Riley R, Salamov A, Lopez-Giraldez F, Sjökvist E, Copeland A, Foster B, Sun H, Larsson E, Larsson KH, Townsend J, Grigoriev IV, Hibbett D (2013) Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 105:1350–1373
Binion DE, Stephenson SL, Roody WC, Burdsall HH Jr., Vasilyeva LN, Miller OK Jr. (2008) Macrofungi associated with oaks of Eastern North America. West Virginia University Press, Morgantown
Bisby GR, Buller AHR, Dearness J (1929) The fungi of Manitoba. Longmans, Green and Co, London
Boehm EW, Schoch CL, Spatafora JW (2009a) On the evolution of the Hysteriaceae and Mytilinidiaceae (Pleosporomycetidae, Dothideomycetes, Ascomycota) using four nuclear genes. Mycol Res 113:461–479
Boehm EWA, Mugambi G, Miller AN, Huhndorf S, Marincowitz S, Schoch CL, Spatafora JW (2009b) A molecular phylogenetic reappraisal of the Hysteriaceae, Mytilinidiaceae and Gloniaceae (Pleosporomycetidae, Dothideomycetes) with keysto world species. Stud Mycol 64:49–83
Bolay A (1971) Contribution a la connaissance de Gnomonia comari Karsten (syn. G. fructicola [Arnaud] Fall). Etude taxonomique, phytopathologique et recherches sur sa croissance in vitro. Ber Schweizerischen Bot Ges 81:398–482
Borisov BA, Tarasov KL (1999) Notes on biodiversity of causal agents of invertebrate mycoses in Adjaria (south-western Georgia). I. Evlachovaea kintrischica gen. et sp. nov. (Hyphomycetes) from Kintrishi Reservation. Mikologiya i Fitopatologiya 33(4):248–256
Breitenbach J, Kränzlin F (1991) Fungi of Switzerland, vol. 3, Boletes and Agarics 1st part. Mykologia, Lucerne, Switzerland
Bresadola J (1896) Fungi Tridentini. 195 plates. 1881–1900. Contains figures of Hydnaceae. Fungi Brasilienses. Hedwigia 35:276–302
Bukovská P, Jelínková M, Hršelová H, Sýkorová Z, Gryndler M (2010) Terminal restriction fragment length measurement errors are affected mainly by fragment length, G+C nucleotide content and secondary structure melting point. J Microbiol Methods 82:223–228
Bussaban B, Lumyong S, Lumyong P, Mckenzie EHC, Hyde KD (2001) A synopsis of the genus Berkleasmium with two new species and new records of Canalisporium caribense from Zingiberaceae in Thailand. Fungal Divers 8:73–85
Buyck B, Hofstetter V, Eberhardt U, Verbeken A, Kauff F (2008) Walking the thin line between Russula and Lactarius: the dilemma of Russula subsect. Ochricompactae. Fungal Divers 28:15–40
Buyck B, Hofstetter V, Verbeken A, Walleyn R (2010) (1919) Proposal to conserve Lactarius nom. cons. (Basidiomycota) with a conserved type. Taxon 59(1):295–296
Cai L, Jeewon R, Hyde KD (2006) Phylogenetic investigations of Sordariaceae based on multiple gene sequences and morphology. Mycol Res 110:137–150
Calzada Dominguez A (2007) Guía de los Boletos de España y Portugal. Náyade Editorial, Medina del Campo, Valladolid
Câmara MPS, Palm ME, van Berkum P, O’Neill NR (2002) Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia 94:630–640
Câmara MP, Ramaley AW, Castlebury LA, Palm ME (2003) Neophaeosphaeria and Phaeosphaeriopsis, segregates of Paraphaeosphaeria. Mycol Res 107:516–522
Cannon PF, Kirk PM (2007) Fungal families world, 7th edn. CAB International, Wallingford
Cannon PF, Hawksworth DL, Sherwood-Pike MA (1985) The British Ascomycotina. An annotated checklist. Commonwealth Mycological Institute, Kew, Surrey
Cannon PF, Damm U, Johnston PR, Weir BS (2012) Colletotrichum current status and future directions. Study Mycol 73:181–213
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Carmichael JW (1966) Cerebral mycetoma of trout due to a Phialophora-like fungus. Sabouraudia 6:120–123
Carmichael JW (1967) Cerebral mycetoma of trout due to a Phialophora-like fungus. Sabouraudia. J Med Vet Mycol 5(2):120–123
Carson ML (2005) Yield loss potential of Phaeosphaeria leaf spot of maize caused by Phaeosphaeria maydis in the United States. Plant Dis 89:986–988
Castañeda RF, Heredia G (2000) Two new dematiaceous hyphomycetes on Cyathea from Mexico. Cryptogam Mycol 21:221–228
Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN (2002) A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94:1017–1031
Chaverri P, Bischoff JF, Evans HC, Hodge KT (2005) Regiocrella a new entomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia 97:1225–1237
Chaverri P, Liu M, Hodge KT (2008) A monograph of the entomopatho- genic genera Hypocrella, Moelleriella and Samuelsia gen. nov. (Ascomycota, Hypocreales, Clavicipitaceae) and their aschersonia-like anamorphs in the Neotropics. Stud Mycol 60:1–66
Checa J, Ramaley AW, Palm-Hernández ME, Câmara MP (2002) Paraphaeosphaeria barrii, a new species on Yucca schidigera from Mexico. Mycol Res 106:375–379
Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, To-Anan C (2008) Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21:77–91
Chen Q, Jiang JR, Zhang GZ, Cai L, Crous PW (2015) Resolving the Phoma enigma. Stud Mycol 82:137–217
Chen Y, Ran SF, Dai DQ, Wang Y, Hyde KD, Wu YM, Jiang YL (2016) Mycosphere Essays 2. Myrothecium. Mycosphere 7:64–80
Chen ZH, Zhang P, Zhang ZG (2013) Investigation and analysis of 102 mushroom poisoning cases in southern China from 1994 to 2012. Fungal Divers 64:123–131
Chethana KWT, Phillips AJL, Zhang W, Chen Z, Hao YY, Hyde KD, Li XH, Yan JY (2016) Mycosphere Essays 5: Is it important to name species of Botryosphaeriaceae? Mycosphere 7:870–882
Chevallier FF (1826) Flore Générale des Environs de Paris [General Flora of the Area Around Paris]. Ferra Jeune, France, pp 1–674
Cheype JL (1983) Validation de Boletus rubrosanguineus (Walty). Doc Mycol 52:53–54
Chlebicki A, Mukhin VA, Ushakowa N (2003) Fomitopsisofficinalis on Siberian Larch in Urals. Mycologist 17:116–120
Chomnunti P, Schoch CL, Aguirre-Hudson B, KoKo TW, Hongsanan S, Jones EBG, Kodsub R, Chukeatirote E, Bahkali AH, Hyde KD (2011) Capnodiaceae. Fungal Divers 51:103–134
Chomnunti P, Hongsanan S, Hudson BA, Tian Q, Peršoh D, Dhami MK, Alias AS, Xu J, Liu X, Stadler M, Hyde KD (2014) The sooty moulds. Fungal Divers 66:1–36
Chouhan JS, Panwar KS (1980) Hyphomycetes of Mount Abu-V. Indian Phytopathol 33:285–291
Clements FE, Shear CL (1931) Genera of fungi, 2nd edn. H.W. Wilson, New York
Co-David D, Langeveld D, Noordeloos ME (2009) Molecular phylogeny and spore evolution of Entolomataceae. Persoonia 23:147–176
Consiglio G, Simonini G (2005) Trattamento statistico delle dimensioni sporali. In: Consiglio G, Antonini D, Antonini M (eds) Il Genere Cortinarius, parte III. Centro Studi Micologici AMB, pp 33–44
Corda ACI (1831) Die Pilze Deutschlands. In: Sturm J (ed) Deutschlands Flora in Abbildungen nach der Natur mit Beschreibungen, vol 12(3). Sturm, Nurnberg, pp 33–64
Corda ACJ (1837) Icones fungorum hucusque cognitorum, vol 1. J.G. Calve, Prague
Corlett M (1981) A taxonomic survey of some species of Didymella and Didymella-like species. Can J Bot 59:2016–2042
Corner EJH (1950) A monograph of Clavaria and allied genera. Oxford University Press, Oxford
Corner EJH (1970) Supplement to a monograph of Clavaria and allied genera. Beihefte zur Nova Hedwigia 33:1–299
Corner EJH (1981) The agaric genera Lentinus, Panus, and Pleurotus with particular reference to Malaysian species. Nova Hedwigia 69:1–169
Corner EJH (1996) The agaric genera Marasmius, Chaetocalathus, Crinipellis, Heimiomyces, Resupinatus, Xerula and Xerulina in Malesia. Beih Nova Hedwigia 111:1–175
Corner EJH, Thind KS, Anand GPS (1956) The Clavariaceae of the Mussoorie hills (India). II. Trans Br Mycol Soc 39(4):475–484
Courtecuisse R, Duhem B (2011) Guide des Champignons de France et d’Europe. Delachaux et Niestlé, Paris
Cripps CL (1997) The genus Inocybe in Montana aspen stands. Mycologia 89:670–688
Crous PW, Braun U (2003) Mycosphaerella and its anamorphs: 1. Names published in Cercospora and Passalora. CBS biodiversity series no Centraalbureau voor Schimmelcultures, Utrecht
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess TI, Barber PA, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
Crous PW, Braun U, Groenewald JZ (2007) Mycosphaerella is polyphyletic. Stud Mycol 58:1–3
Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hoog GS, Groenewald JZ (2009) Phylogenetic lineages in the Capnodiales. Stud Mycol 64:17–47
Crous PW, Summerell BA, Shivas RG, Romberg M, Mel’nik VA, Verkley GJM, Groenewald JZ (2011) Fungal planet description sheets: 92–106. Persoonia 27:130–162
Crous PW, Quaedvlieg W, Sarpkaya K, Can C, Erkılıç A (2013a) Septoria-like pathogens causing leaf and fruit spot of pistachio. IMA Fungus 4:187–199
Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJ, Shin HD, Nakashima C, Groenewald JZ (2013b) Phylogenetic lineages in Pseudocercospora. Stud Mycol 75:37–114
Crous PW, Wingfield MJ, Schumacher RK, Summerell BA, Giraldo A, Gené J, Guarro J, Wanasinghe DN, Hyde KD, Camporesi E, Garethjones EB (2014) Fungal planet description sheets: 281–319. Persoonia 33:212–289
Crous PW, Wingfield MJ, Guarro J, Hernández-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Dueñas M, Dutta AK (2015a) Fungal planet description sheets: 320–370. Persoonia 34:167–266
Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernández-Restrepo M, Jaklitsch WM, Lebrun M-H, Schumacher RK, Stielow JB (2015b) The genera of fungi-fixing the application of the type species of generic names-G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 6:163–198
Dai DQ, Phookamsak R, Wijayawardene NN, Li WJ, Bhat DJ, Xu JC, Taylor JE, Hyde KD, Chukeatirote E (2017) Bambusicolous fungi. Fungal Divers 82:1–105
Dai YC (2012) Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 53:49–80
Dai YC, Yang ZL, Cui BK, Yu CJ, Zhou LW (2009) Species diversity and utilization of medicinal mushrooms and fungi in China (review). Int J Med Mushrooms 11:287–302
Dai YC, Xue HJ, Vlasák J, Rajchenberg M, Wang B, Zhou LW (2014) Phylogeny and global diversity of Polyporus group Melanopus (Polyporales, Basidiomycota). Fungal Diver 64:133–144
Damm U, Woudenberg JHC, Cannon PF, Crous PW (2009) Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers 39:45–87
Daranagama DA, Camporesi E, Tian Q, Liu X, Chamyuang S, Stadler M, Hyde KD (2015) Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Divers 73:203–238
Daranagama DA, Jones EBG, Liu XZ, To-anun C, Stadler M, Hyde KD (2016) Mycosphere Essays 13—do xylariaceous macromycetes make up most of the Xylariomycetidae? Mycosphere 7:582–601
Das K, Dentinger BTM (2015) Austroboletus olivaceoglutinosus, a new mushroom species from Sikkim, India with a distinctive green, glutinous pileus. Kew Bull 70:15
Das K, Hembrom ME, Parihar A, Mishra D, Sharma JR (2014) Strobilomyces polypyramis—rediscovery of a wild mushroom from Sikkim, India. Indian J Plant Sci 3(2):13–18
De Gruyter J, Noordeloos ME (1992) Contributions towards a monograph of Phoma (Coelomycetes)-I. 1. Section Phoma: taxa with very small conidia in vitro. Persoonia 15:71–92
De Gruyter J, Noordeloos ME, Boerema GH (1998) Contributions towards a monograph of Phoma (Coelomycetes)—I. 3. Section Phoma: taxa with conidia longer than 7 μm. Persoonia 16:471–490
De Gruyter J, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009) Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycol Res 113:508–519
De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2010) Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102:1066–1081
De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2013) Redisposition of phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36
de Hoog GS, Hermanides-Nijhof EJ (1977) The black yeasts and allied hyphomycetes. Mycologia 69:1242–1244
de Hoog GS, Vicente VA, Najafzadeh MJ, Harrak MJ, Badali H, Seyedmousavi S (2011) Waterborne Exophiala species causing disease in coldblooded animals. Persoonia 27:46–72
De Notaris G (1844) Cenno sulla tribu dei pirenomiceti sferiacei e descrizione di alcuni nuovi generi. Giornale Botanico Italiano 1:322–335
de Souza CAF, Lima DX, Gurgel LMS, Santiago ALCM (2016) Coprophilous Mucorales (ex Zygomycota) from three areas in the semi-arid of Pernambuco. Brazil. Braz J Microbiol 48(1):1–8
Dennis RWG (1951) Some tropical American Agaricaceae referred by Berkeley and Montagne to Marasmius, Collybia or Heliomyces. Kew Bull 6(3):387–410
Desjardin DE (1989) The Genus Marasmius from the southern Appalachian Mountains. Doctoral dissertations, University of Tennessee, Knoxville
Desjardin DE, Retnowati A, Horak E (2000) Agaricales of Indonesia: 2. A preliminary monograph of Marasmius from Java and Bali. Sydowia 52:92–193
Desjardin DE, Wood MG, Stevens FA (2015) California mushrooms. Timber Press, Portland, Oregon
Diedicke (1912) Kryptogamen-Flora der Mark Brandenburg 9(5):206
Dissanayake AJ, Camporesi E, Hyde KD, Phillips AJL, Fu CY, Yan JY, Li XH (2016) Dothiorella species associated with woody hosts in Italy. Mycosphere 7:51–63
Dissing H (1966) genus Helvella in Europe with special emphasis on the species found in Norden. Dansk Botanisk Arkiv. 25(1):1–172
Dissing H (1979) Helvella papuensis, a new species from Papua New Guinea. Sydowia Annales Mycologici, Beihefte 8:156–161
Doilom M, Shuttleworth L, Roux J, Chukeatirote E, Hyde KD (2014) Barriopsis tectonae sp. nov. a new species of Botryosphaeriaceae from Tectona grandis (teak) in Thailand. Phytotaxa 176:081–091
Doilom M, Dissanayake AJ, Wanasinghe DN, Boonmee S, Liu JK, Bhat DJ, Taylor JE, Bahkali AH, McKenzie EHC, Hyde KD (2017) Microfungi on Tectona grandis (teak) in Northern Thailand. Fungal Divers 82:107–182
Donk MA (1949) New and revised nomina generica conservanda proposed for Basidiomycetes (Fungi). Bull Jard Bot 18:83–168
Donk MA (1964) A conspectus of the families of Aphyllophorales. Persoonia Mol Phylogeny Evol Fungi 3(2):199–324
Doveri F (2004) Fungi fimicoli italici. A.M.B., Vicenza
Dudka IO, Heluta VP, Tykhonenko YY, Andrianova TV, Hayova VP, Prydiuk MP, Dzhagan VV, Isikov VP (2004) Fungi of the Crimean peninsula. Institute of Botany, National Academy of Sciences of Ukraine, Ukraine
Ehrenberg CG (1818) Sylvae mycologicae Berolinenses. Bruschke, Berlin, pp 1–32
Ellis JP (1980) The genus Morenoina in Britain. Trans Br Mycol Soc 74:297–307
Ellis MB (1971) Dematiaceous Hyphomycetes. Commonwealth Mycological lnstitute, Kew, Surrey
Ellis MB (1976) More dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey
Ellis MB, Ellis JP (1990) Fungi without gills (Hymenomycetes and Gasteromycetes): an identification handbook. Springer, Berlin
Ellis NB, Ellis JP (1985) Microfungi on land plants—an identification handbook. Macmillan Publishing, New York
Erikss OE (1967) Arkiv før Botanik 6(4–5):405
Eriksson OE, Winka K (1998) Families and higher taxa of Ascomycota. Myconet 1(2):17–24
Ertz D, Heuchert B, Braun U, Freebury CE, Common RS, Diederich P (2016) Contribution to the phylogeny and taxonomy of the genus Taeniolella, with a focus on lichenicolous taxa. Fungal Biol 120:1416–1447
Estades A, Lannoy G (2004) Les bolets européens. Bull Mycol Bot Dauphiné-Savoie 44(3):3–79
Farr DF, Rossman AY (2012) Fungal databases, systematic mycology and microbiology laboratory, ARS, USDA. https://nt.ars-grin.gov/fungaldatabases/. Retrieved 24 Nov 2016
Farr DF, Aime MC, Rossman AY, Palm ME (2006) Species of Colletotrichum on Agavaceae. Mycol Res 110:1395–1408
Ferrari BC, Zhang C, van Dorst J (2011) Recovering greater fungal diversity from pristine and diesel fuel contaminated sub-Antarctic soil through cultivation using both a high and a low nutrient media approach. Front Microbiol 2:217
Fischer E (1897) Plectacineae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol I. Engelmann, Leipzig
Fotouhifar KB, Hedjaroude GA, Leuchtmann A (2010) ITS rDNA phylogeny of Iranian strains of Cytospora and associated teleomorphs. Mycologia 102:1369–1382
Frank JL (2015) Nomenclatural novelties. Index Fungorum 248:1
Fries EM (1821) Systema mycologicum, vol. 1. Ex Officina Berlingiana, Lund & Greifswald
Fries EM (1849) Summa vegetabilium scandinaviae. Part 2. Bonnier, Leipzig
Frisvad JC, Samson RA (2004) Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud Mycol 49:1–174
Fuckel L (1870) Symbolae mycologicae. Beiträge zur Kenntniss der Rheinischen Pilze. Jb nassau Ver Naturk 23–24:212
Fukami T, Dickie IA, Wilkie JP, Paulus BC, Park D, Roberts A, Buchanan PK, Allen RB (2010) Assembly history dictates ecosystem functioning: evidence from wood decomposer communities. Ecol Lett 13:675–684
Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, Hofstetter V, Fraker E, Schoch CL, Tibell L, Untereiner WA, Aptroot A (2006) Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98:1053–1064
Gelardi M, Vizzini A, Ercole E, Voyron S, Wu G, Liu XZ (2012) Strobilomyces echinocephalus sp. nov. (Boletales) from south-western China, and a key to the genus Strobilomyces worldwide. Mycol Prog 12:575–588
Giambra S, Piazza G, Alves A, Mondello V, Berbegal M, Armengol J, Burruano S (2016) Botryosphaeriaceae species associated with diseased loquat trees in Italy and description of Diplodia rosacearum sp. nov. Mycosphere 7:978–989
Gilbertson RL, Ryvarden L (1986) North American polypores. Abortiporus-Lindtneria. Fungiflora, Oslo
Gilbertson RL, Ryvarden L (1987) North American polypores: Megasporoporia-Wrightoporia. Fungiflora, Oslo
Gilliam MS (1976) The genus Marasmius in the Northeastern United States and adjacent Canada. Mycotaxon 4:1–144
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Góes-Neto A, Loguercio-Leite C, Guerrero RT (2005) DNA extraction from frozen field collected and dehydrated herbarium fungal basidiomata: performance of SDS and CTAB-based methods. Biotemas 18:19–32
Haase G, Sonntag L, Melzer-Krick B, de Hoog GS (1999) Phylogenetic inference by SSU-gene analysis of members of the Herpotrichiellaceae with special reference to human pathogenic species. Stud Mycol 43:80–97
Häffner J, Stangl J, Sedlmeier A, Geh G, Krieglsteiner GJ (1987) Di Gattung Helvella: morphologie und taxonomie. Deutsche Gesellschaft fur Mycologie
Halama M (2016) Rubroboletus le-galiae (Boletales, Basidiomycota), a species new for Poland. Acta Mycol 50(2):1066
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid Symp Ser 41:95–98
Halling RE, Osmundson TW, Neves MA (2006) Austroboletus mutabilis sp. nov. from northern Queensland. Muelleria 24:31–36
Hawksworth DL, Sutton BC, Ainsworth GC (1983) Ainsworth and Bisby’s dictionary of the fungi, 7th edn. Commonwealth Mycological Institute, Kew
Hawksworth DL, Kirk PM, Sutton BC, Pegler DN (1995) Ainsworth & Bisby’s dictionary of the fungi, 8th edn. CAB International, Wallingford
Helaly SE, Richter C, Thongbai B, Hyde KD, Stadler M (2016) Lentinulactam, a hirsutane sesquiterpene with an unprecedented lactam modification. Tetrahedron Lett 57(52):5911–5913
Henkel TW, Meszaros R, Aime MC, Kennedy A (2005) New Clavulina species from the Pakaraima Mountains of Guyana. Mycol Prog 4:343–350
Hennings P (1895) Fungi camerunenses I. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 22:72–111
Hennings P (1905) Fungi japonici VI. Bot Jahrb Syst 37:156–166
Hesseltine CW, Ellis JJ (1961) Notes on Mucorales, especially Absidia. Mycologia 53:406–426
Hesseltine CW, Ellis JJ (1964) The genus Absidia: Gongronella and cylindrical-spored species of Absidia. Mycologia 56:568–601
Hirayama K, Tanaka K (2011) Taxonomic revision of Lophiostoma and Lophiotrema based on reevaluation of morphological characters and molecular analyses. Mycoscience 52:401–412
Hironaga M, Watanabe S, Nishimura K, Miyaji M (1981) Annellated conidiogenous cells in Exophiala dermatitidis, agent of phaeohyphomycosis. Mycologia 73:1181–1183
Hofmann TA, Kirschner R, Piepenbring M (2010) Phylogenetic relationships and new records of Asterinaceae (Dothideomycetes) from Panama. Fungal Divers 43(1):39–53
Hoffmann K, Pawlowska J, Walther G, Wrzosek M, de Hoog GS, Benny GL, Kirk PM, Voigt K (2013) The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies. Persoonia 30:57–76
Högberg N, Holdenrieder O, Stenlid J (1999) Population structure of the wood decay fungus Fomitopsispinicola. Heredity 83:354–360
Höhnel FXR (1919) Fragmente zur Mykologie (XXIII. Mitteilung, Nr. 1154 bis 1188). Sitzungsber Akad Wiss Wien Math Naturwiss Kl Abt 1 128:535–625
Holm L (1957) Nomenclatural notes on Pyrenomycetes. Taxon 24:475–488
Holm L (1961) Taxonomical notes on Ascomycetes. IV. Notes of Nodulosphaeria Rbh. Sven Bot Tidskr 55:63–80
Holubová-Jechová V (1987) Studies on Hyphomycetes from Cuba V. Six new species of Dematiaceous Hyphomycetes from Havana Province. Ceska Mykologie 41:29–36
Hongsanan S, Li YM, Liu JK, Hofmann T, Piepenbring M, Bhat JD, Boonmee S, Doilom M, Singtripop C, Tian Q, Mapook A, Zeng XY, Bahkali AH, Xu JC, Mortimer PE, Wu XH, Yang JB, Hyde KD (2014) Revision of genera in Asterinales. Fungal Divers 68:1–68
Hopple JS, Vilgalys R (1994) Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 86:96–107
Hopple JS, Vilgalys R (1999) Phylogenetic relationships in the mushroom genus Coprinus and dark-spored allies based on sequence data from the nuclear gene coding for the large ibosomal subunit RNA: divergent domains, outgroups, and monophyly. Mol Phylogenet Evol 13:1–19
Horak E (1980a) Indian Boletales and Agaricales, revisions and new taxa. Sydowia 33:80–110
Horak E (1980b) Inocybe (Agaricales) in Indomalaya and Australasia. Persoonia Mol Phylogeny Evol Fungi 11:1–37
Horak E (1980c) Supplementary remarks to Austroboletus (Corner) Wolfe (Boletaceae). Sydowia 33:71–87
Houbraken J, Samson RA (2011) Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol 70:1–51
Houbraken J, Wang L, Lee HB, Frisvad JC (2016) New sections in Penicillium containing novel species producing patulin, pyripyropens or other bioactive compounds. Persoonia 36:299–314
Hu H, Jeewon R, Zhou D, Zhou T, Hyde KD (2007) Phylogenetic diversity of endophytic Pestalotiopsis species in Pinus armandii and Ribes spp.: evidence from rDNA and β-tubulin gene phylogenies. Fungal Divers 24:1–22
Hughes SJ (1958) Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Can J Bot 36:727–836
Hughes SJ (1976) Sooty moulds. Mycologia 68:693–820
Humber RA, Rocha LF, Inglis PW, Kipnis A, Luz C (2013) Morphology and molecular taxonomy of Evlachovaea like fungi and the status of this unusual conidial genus. Fungal Biol 117:1–12
Hüseyin E, Selçuk F, Churakov BP (2014) A new species of Berkleasmium from Ulyanovsk, Russia. Mycosphere 5:462–466
Hyde KD (1995) Tropical Australasian fungi. IX. Vaginatispora aquatica gen. et sp. nov. Nova Hedwigia 61:233–241
Hyde KD, Vrijmoed LLP, Chinnaraj S, Jones EBG (1992) Massarina armatispora sp. nov., a new intertidal ascomycete from mangroves. Bot Mar 35:325–328
Hyde KD, Cai L, McKenzie EHC, Yang YL, Zhang JZ, Prihastuti H (2009) Colletotrichum: a catalogue of confusion. Fungal Divers 39:1–17
Hyde KD, McKenzie EHC, KoKo TW (2011) Towards incorporating anamorphic fungi in a natural classification—checklist and notes for 2010. Mycosphere 2:1–88
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monka J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Begoña AH, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukea E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JK, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, Mckenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zang M (2013) Families of dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AWAM, Dissanayake AJ, Glockling SL, Goonasekara ID, Gorczak M, Hahn M, Jayawardena RS, van Kan JAL, Laurence MH, Lévesque CA, Li XH, Liu JK, Maharachchikumbura SSN, Manamgoda DS, Martin FN, McKenzie EHC, McTaggart AR, Mortimer PE, Nair PVR, Pawłowska J, Rintoul TL, Shivas RG, Spies CFJ, Summerell BA, Taylor PWJ, Terhem RB, Udayanga D, Vaghefi N, Walther G, Wilk M, Wrzosek M, Xu JC, Yan JY, Zhou N (2014) One stop shop: backbones trees for important phytopathogenic genera: I. Fungal Divers 67:21–125
Hyde KD, Hongsanan S, Jeewon R, Bhat DJ, McKenzie EHC, Jones EBG, Phookamsak R, Ariyawansa HA, Boonmee S, Zhao Q, Abdel-Aziz FA, Abdel-Wahab MA, Banmai S, Chomnunti P, Cui BK, Daranagama DA, Das K, Dayarathne MC, de Silva NI, Dissanayake AJ, Doilom M, Ekanayaka AH, Gibertoni TB, Góes-Neto A, Huang SK, Jayasiri SC, Jayawardena RS, Konta S, Lee HB, Li WJ, Lin CG, Liu JK, Lu YZ, Luo ZL, Manawasinghe IS, Manimohan P, Mapook A, Niskanen T, Norphanphoun C, Papizadeh M, Perera RH, Phukhamsakda C, Richter C, de Santiago ALCMA, Drechsler-Santos ER, Senanayake IC, Tanaka K, Tennakoon TMDS, Thambugala KM, Tian Q, Tibpromma S, Thongbai B, Vizzini A, Wanasinghe DN, Wijayawardene NN, Wu HX, Yang J, Zeng XY, Zhang H, Zhang JF, Bulgakov TS, Camporesi E, Bahkali AH, Amoozegar AM, Araujo-Neta LS, Ammirati JF, Baghela A, Bhatt RP, Bojantchev S, Buyck B, da Silva GA, de Lima CLF, de Oliveira RJV, de Souza CAF, Dai YC, Dima B, Duong TT, Ercole E, Mafalda-Freire F, Ghosh A, Hashimoto A, Kamolhan S, Kang JC, Karunarathna SC, Kirk PM, Kytövuori I, Lantieri A, Liimatainen K, Liu ZY, Liu XZ, Lücking R, Medardi G, Mortimer PE, Nguyen TTT, Promputtha I, Raj KNA, Reck MA, Lumyong S, Shahzadeh-Fazeli SA, Stadler M, Soudi MR, Su HY, Takahashi T, Tangthirasunun N, Uniyal P, Wang Y, Wen TC, Xu JC, Zhang ZK, Zhao YC, Zhou JZ, Zhu L (2016) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 80:1–270
Imler L (1950) Recherches sur les bolets. Bull. Soc. Mycol. Fr. 66(4):177–203
Index Fungorum (2017) http://www.indexfungorum.org/Names/Names.asp
Isola D, Selbmann L, de Hoog GS, Fenice M, Onofri S, Prenafeta- Bold FX, Zucconi L (2013) Isolation and screening of black fungi as degraders of volatile aromatic hydrocarbons. Mycopathologia 175:369–379
Iznova T, Rukseniene J (2012) Ascomycetes species new to Lithuania. Botanica Lithuanica 18:35–39
Jaklitsch WM, Voglmayr H (2016) Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Stud Mycol 85:35–64
Jayasiri SC, Hyde KD, Abd-Elsalam KA, Abdel-Wahab MA, Ariyawansa HA, Bhat J, Buyck B, Dai YC, Ertz D, Hidayat I, Jeewon R, Jones EBG, Karunarathna SC, Kirk P, Lei C, Liu JK, Maharachchikumbura SSN, McKenzie E, Ghobad-Nejhad M, Nilsson H, Pang KL, Phookamsak R, Rollins AW, Romero AI, Stephenson S, Suetrong S, Tsui CKM, Vizzini A, Wen TC, De Silva NI, Promputtha I, Kang JC (2015) The Facesoffungi database: fungal names linked with morphology, molecular and human attributes. Fungal Divers 74:3–18
Jayawardena RS, Hyde KD, Damm U, Cai L, Liu M, Li XH, Zhang W, Zhao WS, Yan JY (2016a) Notes on currently accepted species of Colletotrichum. Mycosphere 7:1192–1260
Jayawardena RS, Hyde KD, Jeewon R, Li XH, Liu M, Yan JY (2016b) Mycosphere essay 6: why is it important to correctly name Colletotrichum species? Mycosphere 7:1076–1092
Jeewon R, Hyde KD (2016) Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7:1669–1677
Jeewon R, Liew ECY, Hyde KD (2002) Phylogenetic relationships of Pestalotiopsis and allied genera inferred from ribosomal DNA sequences and morphological characters. Mol Phylogenet Evol 25:378–392
Jeewon R, Liew ECY, Hyde KD (2003a) Molecular systematics of the Amphisphaeriaceae based on cladistic analyses of partial LSU rDNA gene sequences. Mycol Res 107(12):1392–1402
Jeewon R, Liew EC, Simpson JA, Hodgkiss IJ, Hyde KD (2003b) Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Mol Phylogenet Evol 27(3):372–383
Jeewon R, Liew ECY, Hyde KD (2004) Phylogenetic evaluation of species nomenclature of Pestalotiopsis in relation to host association. Fungal Divers 17:39–55
Jeewon R, Ittoo J, Mahadeb D, Jaufeerally-Fakim Y, Wang HK, Liu AR (2013) DNA based identification and phylogenetic characterisation of endophytic and saprobic Fungi from Antidesma madagascariense, a medicinal plant in mauritius. J Mycol. doi:10.1155/2013/781914
Jian-Zhe Y (1985) Notes on the genus Austroboletus in China. Agarica 6(12):80–89
Jiea CY, Zhoua QX, Zhao WS, Jiang YL, Hyde KD, Mckenzie EHC, Wang Y (2013) A new Myrmecridium species from Guizhou, China. Mycotaxon 124:1–8
Jones EBG, Suetrong S, Sakayaroj J, Bahkali AH, Abdel-Wahab MA, Boekhout T, Pang KL (2015) Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers 73:1–72
Julou T, Burghardt B, Gebauer G, Bervellier D, Damesin C, Selosse MA (2005) Mixotrophy in orchids: insights froma comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytol 166:639–653
Kalb K, Buaruang K, Mongkolsuk P, Boonpragob K (2012) New or otherwise interesting lichens. VI, including a lichenicolous fungus. Phytotaxa 42:35–47
Karsten PA (1873) Mycologia fennica. Pars secunda. Pyrenomycetes. Bidrag till Kännedom av Finlands Natur och Folk 23:1–252
Karunarathna SC, Yang ZL, Zhao RL, Vellinga EC, Bahkali AH, Chukeatirote E, Hyde KD (2011) Three new species of Lentinus from northern Thailand. Mycol Prog 10:389–398
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066
Kennedy LL (1958) The genera of the Dacrymycetaceae. Mycologia 50:874–895
Kepler R, Ban S, Nakagiri A, Bischoff J, Hywel-Jones N, Owensby CA, Spatafora JW (2013) The phylogenetic placement of hypocrealean insect pathogens in the genus Polycephalomyces: an application of One Fungus One Name. Fungal Biol 117:611–622
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Ainsworth and Bisby’s dictionary of the fungi, 8th edn. CABI Publishing, London
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Ainsworth & Bisby’s dictionary of the fungi, 10th edn. CABI, Wallingford
Kiyashko AA (2012) Boletus rubrosanguineus (Walty) ex Cheype, 1983//Red Data Book of Republic of Adygheya. Rare and threatened representatives of the regional fauna and flora. Maykop (in Russian)
Knudsen H, Vesterholt J (2012) Funga nordica. Nordsvamp, Copenhagen
Kobayashi T (2002) The Taxonomic studies of the genus Inocybe. Beih Nova Hedwigia 124:1–246
Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EH, Hyde KD, Jeewon R (2006) The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia 98:571–583
Konta S, Phillips AJL, Bahkali AH, Jones EBG, Eungwanichayapant DP, Hyde KD, Boonmee S (2016a) Botryosphaeriaceae from palms in Thailand—Barriopsis archontophoenicis sp. nov, from Archontophoenix alexandrae. Mycosphere 7:921–932
Konta S, Hongsanan S, Phillips AJ, Jones EBG, Boonmee S, Hyde KD (2016b) Botryosphaeriaceae from palms in Thailand II-two new species of Neodeightonia, N. rattanica and N. rattanicola from Calamus (rattan palm). Mycosphere 7:950–961
Kropp BR, Matheny PB, Hutchison LJ (2013) Inocybe section Rimosae in Utah: phylogenetic affinities and new species. Mycologia 105:728–747
Krüger D, Petersen RH, Hughes KW (2006) Molecular phylogenies and mating study data in Polyporus with special emphasis on group “Melanopus” (Basidiomycota). Mycol Prog 5:185–206
Kunze G, Schmidt JC (1817) Mykologische Hefte 1:1–109
Kuo M, Methven AS (2014) Mushrooms of the Midwest. University of Illinois Press, Urbana
Lakhanpal TN (1996) Mushrooms of India: Boletaceae, vol I. APH Publishing Corporation, New Delhi
Lannoy G, Estades A (2001) Les Bolets. Flore Mycologique d’Europe 6. CRDP, Amiens
Largent DL (1974) Rhodophylloid fungi of the Pacific coast (United States) IV: infrageneric concepts in Entoloma, Nolanea, and Leptonia. Mycologia 66:987–1021
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Larsson E, Larsson KH (2003) Phylogenetic relationships of russuloid basidiomycetes with emphasis on aphyllophoralean taxa. Mycologia 95:1037–1065
Larsson E, Örstadius L (2008) Fourteen coprophilous species of Psathyrella identified in the Nordic countries using morphology and nuclear rDNA sequence data. Mycol Res 112:1165–1185
Larsson E, Ryberg M, Moreau PA, Mathiesen AD, Jacobsson S (2009) Taxonomy and evolutionary relationships within species of section Rimosae (Inocybe) based on ITS, LSU and mtSSU sequence data. Persoonia 23:86–98
Leão-Ferreira SM, Gusmão LFP, Castañeda Ruiz RF (2013) Conidial fungi from the semi-arid Caatinga biome of Brazil. Three new species and new records. Nova Hedwigia 96:479–494
Lee S, Crous PW, Wingfield MJ (2006) Pestalotioid fungi from Pestiona in the Cape Floral Kingdom. Stud Mycol 55:175–187
Li DM, de Hoog GS, Lindhardt Saunte DM, Gerrits van den Ende AHG, Chen XR (2008) Coniosporium epidermidis sp. nov., a new species from human skin. Stud Mycol 61:131–136
Li DM, Li RY, Hoog GD, Wang YX, Wang DL (2009) Exophiala asiatica, a new species from a fatal case in China. Med Mycol 47(1):101–109
Li GJ, Li SF, Wen HA (2010) The Russula species resource and its economic values of China. Acta Edulis Fungi 17(sppl):155–160
Li GJ, Zhao D, Li SF, Yang HJ, Liu XZ (2013a) Russula changbaiensis sp. nov. from northeast China. Mycotaxon 124(1):269–278
Li HJ, Han ML, Cui BK (2013b) Two new Fomitopsis species from southern China based on morphological and molecular characters. Mycol Prog 12:709–718
Li GJ, Hyde KD, Zhao RN, Hongsanan S, Abdel-Aziz FA, Abdel-Wahab MA, Alvarado P, Alves-Silva G, Ammirati JF, Ariyawansa HA, Baghela A, Bahkali AH, Beug M, Bhat DJ, Bojantchev D, Boonpratuang T, Bulgakov TS, Camporesi E, Boro MC, Ceska O, Chakraborty D, Chen JJ, Chethana KWT, Chomnunti P, Consiglio G, Cui BK, Dai DQ, Dai YC, Daranagama DA, Das K, Dayarathne MC, Crop ED, De Oliveira RJV, de Souza CAF, de Souza JI, Dentinger BTM, Dissanayake AJ, Doilom M, Drechsler-Santos ER, Ghobad-Nejhad M, Gilmore SP, Góes-Neto A, Gorczak M, Haitjema GH, Hapuarachchi KK, Hashimoto A, He MQ, Henske JK, Hirayama K, Iribarren MJ, Jayasiri SC, Jayawardena RS, Jeon SJ, Jerónimo GH, Jesus AL, Jones EBG, Kang JC, Karunarathna SC, Kirk PM, Konta S, Kuhnert E, Langer E, Lee HS, Lee HB, Li WJ, Li XH, Liimatainen K, Lima DX, Lin CG, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Lücking R, Lumbsch HT, Lumyong S, Leaño EM, Marano AV, Matsumura M, McKenzie EHC, Mongkolsamrit S, Mortimer PE, Nguyen TTT, Niskanen T, Norphanphoun C, O’Malley MA, Parnmen S, Pawłowska J, Perera RH, Phookamsak R, Phukhamsakda C, Pires-Zottarelli CLA, Raspé O, Reck MA, Rocha SCO, de Santiago ALCMA, Senanayake IC, Setti L, Shang QJ, Singh SK, Sir EB, Solomon KV, Song J, Srikitikulchai P, Stadler M, Suetrong S, Takahashi H, Takahashi T, Tanaka K, Tang LP, Thambugala KM, Thanakitpipattana D, Theodorou MK, Thongbai B, Thummarukcharoen T, Tian Q, Tibpromma S, Verbeken A, Vizzini A, Vlasák J, Voigt K, Wanasinghe DN, Wang Y, Weerakoon G, Wen HA, Wen TC, Wijayawardene NN, Wongkanoun S, Wrzosek M, Xiao YP, Xu JC, Yan JY, Yang J, Yang SD, Hu Y, Zhang JF, Zhao J, Zhou LW, Peršoh D, Phillips AJL, Maharachchikumbura SSN (2016) Fungal divers notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 78:1–237
Lib Pl (1830) crypt. Arduenna (Liège), fasc. 1(Praef.):8
Lin CG, McKenzie EHC, Bhat DJ, Ran SF, Chen Y, Hyde KD, Li DW, Wang Y (2016) Stachybotrys-like taxa from karst areas and a checklist of stachybotrys-like species from Thailand. Mycosphere 7:1273–1291
Linaldeddu BT, Deidda A, Scanu B, Franceschini A, Alves A, Abdollahzadeh J, Phillips AJL (2016a) Phylogeny, morphology and pathogenicity of Botryosphaeriaceae, Diatrypaceae and Gnomoniaceae associated with branch diseases of hazelnut in Sardinia (Italy). J Plant Pathol 146:259–279
Linaldeddu BT, Alves A, Phillips AJL (2016b) Sardiniella urbana gen. et sp. nov., a new member of the Botryosphaeriaceae isolated from declining Celtis australis trees in Sardinian streetscapes. Mycosphere 7:893–905
Linaldeddu BT, Maddau L, Franceschini A, Alves A, Phillips AJL (2016c) Botryosphaeriaceae species associated with lentisk dieback in Italy and description of Diplodia insularis sp. nov. Mycosphere 7:962–977
Lindau G (1896) Hysteriineae. In: Engler A, Prantl K (eds) Naturliche Pflanzenfamilien. I. Teil, I, vol 1. Abteilung, pp 265–278
Linnaeus CV (1753) Species plantarum, 2 vols. Laurentii Salvii, Holmiae
Liu B, Fan L, Tao K (1988) Five new species of Dacrymycetaceae from China. Acta Mycol Sin 7:1–6
Liu JK, Phookamsak R, Jones EBG, Zhang Y, Ko-Ko TW, Hu HL, Boonmee S, Doilom M, Chukeatirote E, Bahkali AH, Wang Y, Hyde KD (2011) Astrosphaeriella is polyphyletic, with species in Fissuroma gen. nov., and Neoastrosphaeriella gen. nov. Fungal Divers 51:135–154
Liu JK, Phookamsak R, Doilom M, Wiki S, Mei LY, Ariyawansa HA, Boonmee S, Chomnunti P, Dai DQ, Bhat DJ, Romero AI, Xhuang WY, Monkai J, Jones EBG, Chukeatirote E, KoKo TW, Zhoa YC, Wang Y, Hyde KD (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Liu JK, Phookamsak R, Dai DQ, Tanaka K, Jones EG, Xu JC, Chukeatirote E, Hyde KD (2014) Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoella gen. nov., Roussoella and Roussoellopsis. Phytotaxa 181:1–33
Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SSN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, Dsouza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Persˇoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe DN, Wisitrassameewong K, Zeng XY, Abdel- Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Li XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2015) Fungal Divers notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Liu M, Chaverri P, Hodge KT (2006) A taxonomic revision of the insect biocontrol fungus Aschersonia aleyrodis, its allies with white stromata and their Hypocrella sexual states. Mycol Res 110:537–554
Liu NG, Ariyawansa HA, Hyde KD, Maharachchikumbura SSN, Zhao RL, Phillips AJL, Jayawardena RS, Thambugala KM, Dissanayake AJ, Wijayawardene NN, Liu JK, Liu ZY, Jeewon R, Jones EBG, Jumpathong J (2016) Perspectives into the value of genera, families and orders in classification. Mycosphere 7:1649–1668
Liu Y, Steenkamp ET, Brinkmann H, Forget L, Philippe H, Lang BF (2009) Phylogenomic analyses predict sister group relationship of nucleariids and fungi and paraphyly of zygomycetes with significant support. BMC Evol Biol 9:272
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808
Liu ZY, Liang ZQ, Liu AY, Yao YJ, Hyde KD, Yu ZN (2002) Molecular evidence for teleomorph anamorph connections in Cordyceps based on ITS-5.8S rDNA sequences. Mycol Res 106:1100–1108
Locquin M (1984) Mycologie Générale et Structurale. Masson, Paris
Lombard L, Houbraken J, Decock C, Samson RA, Meijer M, Réblová M, Groenewald JZ, Crous PW (2016) Generic hyper-diversity in Stachybotriaceae. Persoonia 36:156–246
Luangsa-ard JJ, Hywel-Jones NL, Manoch L, Samson RA (2005) On the relationships of Paecilomyces sect. Isarioidea species. Mycol Res 109:581–589
Lumbsch HT, Huhndorf SM (2007) Outline of Ascomycota. Myconet 13:1–99
Lumbsch HT, Huhndorf SM (2010) Myconet volume 14. Part one. Outline of Ascomycota 2009. Part Two. Notes on Ascomycete systematics. Nos. 4751–5113. Fieldiana Life Earth Sci 1:1–64
Maas JL (1998) Compendium of strawberry diseases, 2nd edn. APS Press, St Paul, pp 1–98
Maharachchikumbura SSN, Guo LD, Cai L, Chukeatirote E, Wu WP, Sun X, Crous PW, Bhat DJ, McKenzie EHC, Bahkali AH, Hyde KD (2012) A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers 56:95–129
Maharachchikumbura SSN, Guo L-D, Chukeatirote E, Hyde KD (2014a) Improving the backbone tree for the genus Pestalotiopsis; addition of P. steyaertii and P. magna sp. nov. Mycol Prog 3:617–624
Maharachchikumbura SSN, Hyde KD, Groenewald JZ, Xu J, Crous PW (2014b) Pestalotiopsis revisited. Stud Mycol 79:121–186
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang S-K, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu J-K, Liu Z-Y, Norphanphoun C, Shenoy BD, Xiao Y, Bahkali AH, Kang J, Somrothipol S, Suetrong S, Wen T, Xu J (2015) Towards a natural classification and backbone tree for Sodariomycetes. Fungal Divers 72:199–301
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat DJ, Dayarathne MC, Huang SK, Norphanphoun C, Senanayake IC, Perera RH, Shang QJ, Xiao YP, D’souza MJ, Hongsanan S, Jayawardena RS, Daranagama DA, Konta S, Goonasekara ID, Zhuang WY, Jeewon R, Phillips AJL, Abdel-Wahab MA, Al-Sadi AM, Bahkali AH, Boonmee S, Boonyuen N, Cheewangkoon R, Dissanayake AJ, Kang JC, Li QR, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Pang KL, Phookamsak R, Promputtha I, Suetrong S, Stadler M, Wen TC, Wijayawardene NN (2016) Families of Sordariomycetes. Fungal Divers 79:1–317
Maharachchikumbura SS, Larignon P, Al-Sadi AM, Zuo-Yi LIU (2017) Characterization of Neopestalotiopsis, Pestalotiopsis and Truncatella species associated with grapevine trunk diseases in France. Phytopathol Mediterr 55(3):380–390
Manawasinghe IS, Phillips AJL, Hyde KD, Chethana KWT, Zhang W, Zhao WS, Yan JY, Li XH (2016) Mycosphere Essays 14: assessing the aggressiveness of plant pathogenic Botryosphaeriaceae. Mycosphere 7(7):883–892
Manimohan P, Leelavathy KM (1989) Marasmius species new to India. Sydowia 41:185–199
Manimohan P, Joseph AV, Leelavathy KM (1995) The genus Entoloma (Agaricales) in Kerala State, India. Mycol Res 99:1083–1097
Mantle PG, Hawksworth DL, Pazoutova S, Collinson LM, Rassing BR (2006) Amorosia littoralis gen. sp. nov., a new genus and species name for the scorpinone and caffeine-producing hyphomycete from the littoral zone in The Bahamas. Mycol Res 110(12):1371–1378
Mapook A, Boonmee S, Ariyawansa HA, Tibpromma S, Campesori E, Jones EG, Bahkali AH, Hyde KD (2016) Taxonomic and phylogenetic placement of Nodulosphaeria. Mycol Prog 15(4):1–15
Martin R, Gazis R, Skaltsas D, Chaverri P, Hibbett D (2015) Unexpected diversity of basidiomycetous endophytes in sapwood and leaves of Hevea. Mycologia 107(2):284–297
Matheny PB (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol Phylogenet Evol 35:1–20
Matheny PB (2009) A phylogenetic classification of the Inocybaceae. McIlvainea 18:11–21
Matheny PB, Bougher NL (2006a) The new genus Auritella from Africa and Australia (Inocybaceae, Agaricales): molecular systematics, taxonomy and historical biogeography. Mycol Prog 5:2–17
Matheny PB, Bougher NL (2006b) Validation of Auritella. Mycotaxon 98:231–233
Matsushima T (1981) Matsushima Mycological Memoirs 2. Matsushima, Kobe, Japan
Matsushima T (1983) Matsushima Mycological Memoirs 3. Matsushima, Kobe
Mazzer SJ (1976) A monographic study of the genus Pouzarella, a new genus in the Rhodophyllaceae, Agaricales, Basidiomycetes. Bibliotheca Mycologica 46:1–191
McNabb RFR (1965) Taxonomic studies on the Dacrymycetaceae. VI. Femsjonia Fries”. NZ J Bot 3(3):223–228
McNabb RFR (1973) Taxonomic studies in the Dacrymycetaceae. VIII. Dacrymyces Nees ex Fries. NZ J Bot 11(3):461–524
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop (GCE). Institute of Electrical and Electronics Engineers, New Orleans, LA, 14 Nov, pp 1–8
Miller SL, Buyck B (2002) Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol Res 106:259–276
Miller SL, Larsson E, Larsson KH, Verbeken A, Nuytinck J (2006) Perspectives in the new Russulales. Mycologia 98:960–970
Minnis AM, Kennedy AH, Grenier DB, Palm ME, Rossman AY (2012) Phylogeny and taxonomic revision of the Planistromellaceae including its coelomycetous anamorphs: contributions towards a monograph of the genus Kellermania. Persoonia 29:11–28
Mirza F (1968) Taxonomic investigations on the ascomycetous genus Cucurbitaria. Nova Hedwigia 16:161–213
Miyake I (1909) Studies on the parasitic fungi of rice in Japan. Bot Mag Tokyo 23:85–97
Mohanan C (2011) Macrofungi of Kerala. KFRI handbook no. 27, Kerala Forest Research Institute, Peechi
Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime CM, Hofsetter V, Verduin SJ, Larsson E, Baroni TJ, Thorn GR, Jacobsson S, Clémencon H, Miller OK (2002) One hundred and seventeen clades of euagarics. Mol Phylogenet Evol 23:357–400
Moncalvo JM, Nilsson RH, Koster B, Dunham SM, Bernauer T, Matheny PB, McLenon T, Margaritescu S, Weiß M, Garnica S, Danell E, Langer G, Langer E, Larsson E, Larsson KH, Vilgalys R (2006) The cantharelloid clade: dealing with incongruent gene trees and phylogenetic reconstruction methods. Mycologia 98:937–948
Mongkolsamrit S, Luangsa-Ard JJ, Spatafora JW, Sung GH, Hywel-Jones NL (2009) A combined ITS rDNA and β-tubulin phylogeny of Thai species of Hypocrella with non-fragmenting ascospores. Mycol Res 113(6):684–699
Monod M (1983) Monographie taxonomique des Gnomoniaceae (Ascomycétes de l’ordre des Diaporthales I). Beihefte Sydowia 9:1–315
Moreau PA, Vila J, Aime MC, Antonín V, Horak E, Pérez-Butrόn JL, Richard F, Urban A, Welti S, Vizzini A (2015) Cibaomyces and Cyptotrama, two new genera for Europe, and an emendation of Rhizomarasmius (Basidiomycota, Physalacriaceae). Mycol Prog 14(2):1–16
Moore RT (1958) Deuteromycetes I: the complex. Mycologia 50:681–692
Morgado LN, Noordeloos ME, Lamoureux Y, Geml J (2013) Multi-gene phylogenetic analyses reveal species limits, phylogeographic patterns, and evolutionary histories of key morphological traits in Entoloma (Agaricales, Basidiomycota). Persoonia Mol Phylogeny Evol Fungi 31:159–178
Morris EF (1972) Costa Rican Hyphomycetes. Mycologia 64:887–896
Mugambi GK, Huhndorf SM (2009) Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae re-circumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota). Stud Mycol 64:103–121
Mulenko W, Majewski T, Ruszkiewicz-Michalska M (2008) A preliminary checklist of micromycetes in Poland. W. Szafer Institute of Botany, Polish Academy of Sciences, Krakow
Munk A (1953) The system of the Pyrenomycetes. A contribution to a natural classification of the group Sphaeriales sensu Lindau. Dansk Botanisk Arkiv 15:1–163
Munk A (1957) Danish Pyrenomycetes. Dan Bot Ark 17:1–491
Muñoz JA (2005) Boletus s.l. (excl. Xerocomus). Fungi Europaei 2. Edizioni Candusso, Alassio
Murrill WA (1909) The Boletaceae of North America: I. Mycologia 1(1):4–18
MycoBank (2017) http://www.mycobank.org/quicksearch.aspx
Najafzadeh MJ, Dolatabadi S, Keisari MS, Naseri A, Feng P, De Hoog GS (2013) Detection and identification of opportunistic Exophiala species using the rolling circle amplification of ribosomal internal transcribed spacers. J Microbiol Methods 94:338–342
Nannf (1932) Nova Acta Regiae Societatis Scientiarum Upsaliensis 8(2):66
Natarajan K, Manjula B (1982) South Indian Agaricales: XI. Mycologia 74:130–137
Neubert K, Mendgen K, Brinkmann H, Wirsel SGR (2006) Only a few fungal species dominate highly diverse mycofloras associated with the common reed. Appl Environ Microbiol 72:1118–1128
Nguyen TTT, Lee SH, Bae S, Jeong SJ, Mun HY, Lee HB (2016) Characterization of two new records of zygomycete species belonging to undiscovered taxa in Korea. Mycobiology 44:29–37
Noordeloos ME (1981) Entoloma subgenera Entoloma and Allocybe in the Netherlands and adjacent regions with a reconnaissance of its remaining taxa in Europe. Persoonia 11:153–256
Noordeloos ME (1992) Entoloma s.l. Fungi Europaei, vol. 5. Giovanna Biella, Italy
Noordeloos ME (2004) Entoloma s.l. Fungi Europaei, vol 5a. Edizione Candusso, Alassio
Noordeloos ME (2010) Hoe raak ik thuis in de boleten—8. De roodporieboleten (sectie Luridi). Coolia 53(2):53–71
Noordeloos ME, Gates GM (2012) The Entolomataceae of Tasmania. Fungal diversity research series, vol 22. Springer, London
Norphanphoun C, Doilom M, Daranagama DA, Phookamsak R, Wen TC, Bulgakov TS, Hyde KD (2017) Revisiting the genus Cytospora and allied species. Mycosphere 8:51–97
Núñez M, Ryvarden L (1995) Polyporus (Basidiomycotina) and related genera. Synop Fungorum 10:1–85
Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24:581–583
Oberwinkler F (1993) Diversity and phylogenetic importance of tropical heterobasidiomycetes. Br Mycol Soc Symp 19:121
O’Donnell K (1993) Fusarium and its near relatives. Wallingford, UK
Olariaga I, Begoña MJ, García-Etxerbarria K, Salcedo I (2009) Species delimitation in the European species of Clavulina (Cantharellales, Basidiomycota) inferred from phylogenetic analyses of ITS region and morphological data. Mycol Res 113:1261–1270
Oliveira RJV, Lima TEF, Cunha IB, Coimbra VRM, Silva GA, Bezerra JL, Cavalcanti MAQ (2014) Corniculariella brasiliensis, a new species of coelomycetes in the rhizosphere of Caesalpinia echinata (Fabaceae, Caesalpinioideae) in Brazil. Phytotaxa 178(3):197–204
Örstadius L, Ryberg M, Larsson E (2015) Molecular phylogenetics and taxonomy in Psathyrellaceae (Agaricales) with focus on psathyrelloid species: introduction of three new genera and 18 new species. Mycol Prog 14:25
Ortiz-Santana B, Lindner DL, Miettinen O, Justo A, Hibbett DS (2013) A phylogenetic overview of the Antrodia clade (Basidiomycota, Polyporales). Mycologia 105:1391–1411
Orton PD, Watling R (1979) Coprinaceae 1: Coprinus. British Fungus Flora. Agarics and Boleti 2. Royal Botanic Garden, Edinburgh
Padamsee M, Matheny PB, Dentinger BT, McLaughlin DJ (2008) The mushroom family Psathyrellaceae: evidence for large-scale polyphyly of the genus Psathyrella. Mol Phylogenet Evol 46:415–429
Page RDM (2001) Tree View: tree drawing software for Apple Macintosh and Windows. http://taxonomy.zoology.gla.ac.uk/rod/treeview.html
Palazon FL (2006) Setas para todos. Pirineo, Huesca
Pantidou ME (1973) Fungus-host index for Greece. Benaki Phytopathol. Inst., Kiphissia
Peever TL, Barve MP, Stone LJ, Kaiser WJ (2007) Evolutionary relationships among Ascochyta species infecting wild and cultivated hosts in the legume tribes Cicereae and Vicieae. Mycologia 99:59–77
Pegler DN (1972) A revision of the genus Lepiota from Ceylon. Kew Bull 27:155–202
Pegler DN (1977) A preliminary agaric flora of East Africa. Kew Bull Addit Ser 6:1–615
Pegler DN (1983) The genus Lentinus: a world monograph. Kew Bull Addit Ser 10:1–281
Pegler DN (1986) Agaric flora of Sri Lanka. Kew Bull Addit Ser 12:1–519
Peintner U, Knapp M, Fleischer V, Walch G, Dresch P (2016) Myrmecridium hiemale sp nov from snow-covered alpine soil is the first eurypsychrophile in this genus of anamorphic fungi. Int J Syst Evol Microbiol 66(7):2592–2598
Persoon CH (1797) Tentamen dispositionis methodicae fungorum in classes, ordines genera et familias. Lipsiae
Petch T (1948) A revision of the Ceylon Marasmii. Trans Br Mycol Soc 31:19–44
Petersen RH (1983) Notes on clavarioid fungi XVIII. A preliminary outline of Clavulina in southeastern Australia. Nova Hedwigia 37:19–35
Petersen RH (1988) The clavarioid fungi of New Zealand. Bull NZ Dep Ind Res 236:1–170
Petersen RH, Hughes KW, Adamčík S, Tkalčec Z, Mešić A (2012) Typification of three European species epithets attributable to Strobilomyces (Boletales). Czech Mycol 64(2):141–163
Peterson SW (2000a) Phylogenetic analysis of Penicillium species based on ITS and LSU-rDNA nucleotide sequences. In: Samson RA, Pitt JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Plenum Press, New York, pp 163–178
Peterson SW (2000b) Phylogenetic relationships in Aspergillus based on rDNA sequence analysis. In: Samson RA, Pitt JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Plenum Press, New York, pp 323–355
Peterson SW (2008) Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100:205–226
Petrak F, Deighton FC (1952) Beiträge zur Pilzeflora von Sierra Leone. Sydowia 6:309–322
Petrini LE (2013) Rosellinia—a world monograph. Bibl Mycol 205:1–410
Peyronel B, Dal Vesco G (1955) Ricerche sulla microflora di un terreno agrario presso Torino. Allionia 2:357–417
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia Mol Phylogeny Evol Fungi 21:29–55
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167
Phookamsak R, Liu JK, McKenzie EHC, Manamgoda DS, Ariyawansa HA, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68:159–238
Phookamsak R, Norphanphoun C, Tanaka K, Dai DQ, Luo ZL, Liu JK, Su HY, Bhat DJ, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2015) Towards a natural classidication of Astrosphaeriella-like species; introducing Astrosphaeriellaceae and Pseudoastrosphaeriellaceae fam. nov and Astrosphaeriellaceaeopsis, gen. nov. Fungal Divers 74:143–197
Phukhamsakda C, Hongsanan S, Ryberg M, Ariyawansa HA, Chomnunti P, Bahkali AH, Hyde KD (2016) The evolution of Massarineae with Longipedicellataceae fam. nov. Mycosphere 7:1713–1731
Pici G (1955) Qualche osservazione sopra due Mucoraceae. Atti Inst Bot Univ Pavia 13:38–44
Pinnoi A, Jeewon R, Sakayaroj J, Hyde KD, Jones EG (2007) Berkleasmium crunisia sp. nov. and its phylogenetic affinities to the Pleosporales based on 18S and 28S rDNA sequence analyses. Mycologia 99:378–384
Pitt JI (1980) The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London
Põldmaa K (2011) Tropical species of Cladobotryum and Hypomyces producing red pigments. Stud Mycol 31(68):1–34
Pradeep CK, Vrinda KB (2010) Ectomycorrhizal fungal diversity in three different forest types and their association with endemic, indigenous and exotic species in the Western Ghat forests of Thiruvananthapuram district, Kerala. J Mycopathol Res 48:279–289
Pratibha J, Prabhugaonkar A, Hyde KD, Bhat DJ (2014) Phylogenetic placement of Bahusandhika, Cancellidium and Pseudoepicoccum (asexual Ascomycota). Phytotaxa 176:68–80
Promputtha I, Lumyong S, Dhanasekaran V, McKenzie EHC, Hyde KD, Jeewon R (2007) A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microb Ecol 53(4):579–590
Qin J, Yang ZL (2016) Cyptotrama (Physalacriaceae, Agaricales) from Asia. Fungal Biol 120:513–529
Qu C, Yin G, Zhao G, Cui B, Liu X (2014) Three new species of Berkleasmium (Hyphomycetes) from China. Nova Hedwigia 98:151–161
Quaedvlieg W, Verkley GJM, Shin H-D, Barretto RW, Alfenas AC, Swart WJ, Groenewald JZ, Crous PW (2013) Sizing up Septoria. Stud Mycol 75:307–390
Rabenhorst (1858) Herb. Myc. Ed., 2:725
Rambaut A (2014) FigTree v1.4: tree figure drawing tool. http://treebio.ed.ac.uk/software/figtree/
Rambaut A, Drummond AJ (2003) Tracer: MCMC trace analysis tool. University of Oxford, Oxford
Rannala B, Yang Z (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Rao PR, Rao D (1963) Berkleasmium zobel from India. Mycopathologia 22:311–314
Read SJ, Jones EBG, Moss ST (1997) Ultrastructural observation of asci, ascospores and appendages of Massarina armatispora (Ascomycota). Mycoscience 38:141–146
Réblová M, Gams W, Seifert KA (2011) Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Stud Mycol 68:163–191
Réblová M, Fournier J, Štěpánek V (2016) Two new lineages of aquatic ascomycetes: Atractospora gen. nov. and Rubellisphaeria gen. et sp. nov., and a sexual morph of Myrmecridium montsegurinum sp. nov. Mycol Prog 15(3):1–18
Redhead SA, Ginns J (1980) Cyptotrama asprata (Agaricales) from North America and notes on the five other species of Cyptotrama sect. Xerulina. Can J Bot 58(6):731–740
Redhead SA, Ginns JH (1985) A reappraisal of agaric genera associated with brown rots of wood. Trans Mycol Soc Jpn 26:349–381
Redhead SA, Vilgalys R, Moncalvo JM, Johnson J, Hopple JS Jr (2001) Coprinus Pers. and the disposition of Coprinus species sensu lato. Taxon 50:203–241
Rehner SA (2001) Primers for elongation factor 1-alpha (EF1-alpha). http://www.aftol.org/pdfs/EF1primer.pdf
Rehner SA, Samuels GJ (1995) Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Can J Bot 31(73):816–823
Ribaldi MS (1952) Sopra un interessante Zigomicete terricolo: Gongronella urceolifera n. gen. et n. sp. Riv Biol 49:157–166
Rogerson CT (1970) The hypocrealean fungi (ascomycetes, Hypocreales). Mycologia 1:865–910
Romagnesi H (1967) Les Russules d’Europe et d’Afrique du Nord. Bordas, Paris
Romagnesi H (1978) les fondements de la taxonomie des rhodophylles et leur classification. Nova Hedwigia 59:1–80
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Rossman AY, Farr DF, Castlebury LA (2007) A review of the phylogeny and biology of the Diaporthales. Mycoscience 48:135–144
Rossman AY, Crous PW, Hyde KD, Hawksworth DL, Aptroot A, Bezerra JL, Bhat JD, Boehm E, Braun U, Boonmee S, Camporesi E, Chomnunti P, Dai DQ, D’souza MJ, Dissanayake A, Jones EBG, Groenewald JZ, Hernández-Restrepo M, Hongsanan S, Jaklitsch WM, Jayawardena R, Li WJ, Kirk PM, Lawrey JD, Mapook A, McKenzie EHC, Monkai J, Phillips AJL, Phookamsak R, Raja HA, Seifert KA, Senanayake IC, Slippers B, Suetrong S, Taylor JE, Thambugala KM, Tian Q, Tibpromma S, Wanasinghe DN, Wijayawardene NN, Wikee S, Woudenberg JHC, Wu HX, Yan J, Yang T, Zhang Y (2015) Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus 6:507–523
Ruiz Fernandez JM, Ruiz Pastor E (2006) Guia Micologica Tomo n. 4, Supl. Orden Boletales en España, Imagen. Bilbao: 62
Rungjindamai N, Pinruan U, Choeyklin R, Hattori T, Jones EBG (2008) Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis, in Thailand. Fungal Divers 33:139–161
Ryvarden L (1991) Genera of polypores, nomenclature and taxonomy. Synop Fungorum 5:1–363
Ryvarden L, Gilbertson RE (1984) Mycotaxon 19:141
Ryvarden L, Gilbertson RL (1993) European polypores 1. Abortiporus-Lindtneria. Synop Fungorum 6:1–387
Ryvarden L, Gilvertson RL (1994) European polypores, part 2. Fungiflora, Oslo
Saccardo PA (1878) Fungi Veneti novi vel critici vel mycologiae Venetae addendi. Ser. IX. Michelia 1(4):407
Saccardo PA (1880) Conspectus generum fungorum italiae inferiorum nempe ad Sphaeropsideas, Melanconieas et Hyphomyceteas pertinentium, systemate sporologico dispositu. Michelia 2:1–38
Saccardo PA (1882) Fungi Gallici lecti a Cl. viris P. Brunaud, C.C. Gillet, Abb. Letendre, A. Malbranche, J. Therry & Dom. Libert. Series IV. Michelia 2(8):583–648
Saccardo PA (1892) Supplementum Universale, Pars II. Discomyceteae-Hyphomyceteae. Sylloge Fungorum 10:1–964
Saccardo PA (1899) Sylloge fungorum 14:1–1316
Samson RA, Seifert KA, Kuijpers AFA, Houbraken JAMP, Frisvad JC (2004) Phylogenetic analysis of Penicillium subgenus Penicillium using partial β-tubulin sequences. Stud Mycol 49:175–200
Samson RA, Houbraken J, Varga J, Frisvad JC (2009) Polyphasic taxonomy of the heat resistant ascomycete genus Byssochlamys and its Paecilomyces anamorphs. Persoonia 22:14–27
Samson RA, Houbraken J, Thrane U (2010) Food and indoor fungi. CBS KNAW Biodiversity Center, Utrecht
Santiago ALCMA, Santos PJP, Maia LC (2013) Mucorales from the semiarid of Pernambuco, Brazil. Braz J Microbiol 44:299–305
Sarnari M (1998) Monografia illustrate de genere Russula in Europa. Tomo Primo. AMB, Centro Studi Micologici, Trento
Sarnari M (2005) Monografia illustrate de genere Russula in Europa. Tomo Secondo. AMB, Centro Studi Micologici, Trento
Sarycheva LA, Svetasheva TY (2015) Boletus legaliae Pilát//Red Data Book of Lipetsk region. Plants, fungi, lichenes, 2nd edn. Lipetsk:536–537 (in Russian)
Sato H, Hattori T, Kurogi S, Yumoto T (2005) Strobilomyces mirandus Corner, a new record from Japan. Mycoscience 46:102–105
Schardl CL, Young CA, Hesse U, Amyotte SG, Andreeva K, Calie PJ, Fleetwood DJ, Haws DC, Moore N, Oeser B, Panaccione DG (2013) Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet 9(2):e1003323
Schipper MAA, Samson RA (1978) One certain species of Mucor with a key to all accepted species. Stud Mycol 17:1–52
Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98:1041–1052
Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, de Gruyter J, de Hoog GS, Dixon LJ, Grube M, Gueidan C, Harada Y, Hatakeyama S, Hirayama K, Hosoya T, Huhndorf SM, Hyde KD, Jones EBG, Kohlmeyer J, Kruys A, Li YM, Lücking R, Lumbsch HT, Marvanová L, Mbatchou JS, McVay AH, Miller AN, Mugambi GK, Muggia L, Nelsen MP, Nelson P, Owensby CA, Phillips AJL, Phongpaichit S, Pointing SB, Pujade-Renaud V, Raja HA, Rivas Plata E, Robbertse B, Ruibal C, Sakayaroj J, Sano T, Selbmann L, Shearer CA, Shirouzu T, Slippers B, Suetrong S, Tanaka K, Volkmann-Kohlmeyer B, Wingfield MJ, Wood AR, Woudenberg JHC, Yonezawa H, Zhang Y, Spatafora JW (2009) A class-wide phylogenetic assessment of Dothideomycetes. Stud Mycol 64:1–15
Schulzer S (1870) Mykologische Beiträge. Verhandlungen der Zoologisch-Botanischen Gesellschaft Wien 20:635–658
Seelan JSS, Justo A, Nagy LG, Grand EA, Redhead SA, Hibbett D (2015) Phylogenetic relationships and morphological evolution in Lentinus, Polyporellus and Neofavolus, emphasizing southeastern Asian taxa. Mycologia 107:460–474
Seifertk K, Morgan-Jones G, Gams W, Kendrick B (2011) The genera of Hyphomycetes. CBS-KNAW Fungal Biodiversity Centre, Utrecht
Senanayake IC, Maharachchikumbura SSN, Hyde KD, Bhat JD, Jones EBG, McKenzie EHC, Dai DQ, Daranagama DA, Dayarathne MC, Goonasekara ID, Konta S, Li WJ, Shang QJ, Stadler M, Wijayawardene NN, Xiao YP, Norphanphoun C, Li QR, Liu XZ, Bahkali AH, Kang JC, Wang Y, Wen TC, Wendt L, Xu JC, Camporesi E (2015) Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers 73:73–144
Senanayake IC, Maharachchikumbura SSN, Jeewon R, Promputtha I, Al-Sadi AM, Camporesi E, Hyde KD (2017) Morphophylogenetic study of Sydowiellaceae reveals several new genera. Mycosphere 8(1):172–217
Seyedmousavi S, Badali H, Chlebicki A, Zhao J, Prenafeta-Boldú FX, de Hoog GS (2011) Exophiala sideris, a novel black yeast isolated from environments polluted with toxic alkyl benzenes and arsenic. Fungal Biol 115:1030–1037
Shearer CA, Raja HA, Miller AN, Nelson P, Tanaka K, Hirayama K, Marvanova L, Hyde KD, Zhang Y (2009) The molecular phylogeny of freshwater Dothideomycetes. Stud Mycol 64:145–153
Shenoy BD, Jeewon R, Wu WP, Bhat DJ, Hyde KD (2006) Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycol Res 110:916–928
Shoemaker RA (1984) Canadian and some extralimital Nodulosphaeria and Entodesmium species. Can J Bot 62:2730–2753
Shoemaker RA, Babcock CE (1989) Phaeosphaeria. Can J Bot 67:1500–1599
Shirouzu T, Hirose D, Tokumasu S (2009) Taxonomic study of the Japanese Dacrymycetes. Persoonia 23:16
Silvestro D, Michalak I (2010) raxmlGUI: a graphical front-end for RAxML. http://sourceforge.net/projects/raxmlgui/
Silvestro D, Michalak I (2012) RaxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12:335–337
Simon UK, Bauer R, Rioux D, Simard M, Oberwinkler F (2005a) The intercellular biotrophic leaf pathogen Cymadothea trifolii locally degrades pectins, but not cellulose or xyloglucan in cell walls of Trifolium repens. New Phytol 165:243–260
Simon UK, Bauer R, Rioux D, Simard M, Oberwinkler F (2005b) The vegetative life-cycle of the clover pathogen Cymadothea trifolii as revealed by transmission electron microscopy. Mycol Res 109:764–778
Simon UK, Groenewald JZ, Crous PW (2009) Cymadothea trifolii, an obligate biotrophic leaf parasite of Trifolium, belongs to Mycosphaerellaceae as shown by nuclear ribosomal DNA analyses. Persoonia 22:49–55
Simonini G (1992) Il valore statistico delle dimensioni sporali nei boleti. Atti riguardanti il V Seminario su Russulales e Boletales. AMB Reggio Emilia:16–28
Simonini G (1998) Una metodologia per la descrizione e la rappresentazione delle dimensioni sporali (Applicata al caso delle Boletaceae). Pagine di Micologia 9:1–24
Simonyan SA (1981) Mycoflora of botanical gardens and arboreta in Armenia. Hayka:232
Singer R (1965) Die Röhrlinge, Teil I. J. Klinkhartdt. Bad Heilbrunn:131
Singer R (1967) Die Röhrlinge, Teil II, J. Klinkhartdt. Bad Heilbrunn:151
Singer R (1975) The Agaricales in modern taxonomy, 3rd edn. J. Cramer, Vaduz
Singer R (1976) Marasmieae (Basidiomycetes-Tricholomataceae). Flora Neotropica Monogr 17:1–347
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Sven Koeltz Scientific Books, Königstein
Sivanesan A (1984) The bitunicate ascomycetes and their anamorphs. J Cramer, Vaduz
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21(2):90–106
Slippers B, Boissin E, Phillips A, Groenewald J, Lombard L, Wingfield M, Postma A, Burgess T, Crous P (2013) Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Stud Mycol 76:31–49
Sogonov MV, Castlebury LA, Rossman AY, Mejía LC, White JF Jr (2008) Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Stud Mycol 62:1–77
Sotome K, Hattori T, Kakishima M (2007) Polyporus phyllostachydis sp. nov. with notes on other rhizophilic species of Polyporus (Basidiomycota, Polyporaceae). Mycoscience 48:42–46
Sotome K, Hattori T, Ota Y, To-anun C, Salleh B, Kakishima M (2008) Phylogenetic relationships of Polyporus and morphologically allied genera. Mycologia 100:603–615
Sotome K, Hattori T, Ota Y (2011) Taxonomic study on a threatened polypore, Polyporus pseudobetulinus, and a morphologically similar species, P. subvarius. Mycoscience 52:319–326
Sotome K, Akagi Y, Lee SS, Ishikawa NK, Hattori T (2013) Taxonomic study of Favolus and Neofavolus gen. nov. segregated from Polyporus (Basidiomycota, Polyporales). Fungal Divers 58:245–266
Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY (2016) A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108(5):1028–1046
Spegazzini C (1910) Mycetes Argentinenses (series V). Anales Mus Nac Hist Nat Buenos Aires 20(13):329–467
Spegazzini C (1911) Hermatomyces Speg. Anales Mus Nac Hist Nat Buenos Aires 20(13):445
Spielman LJ (1985) A monograph of Valsa on hardwoods in North America. Can J Bot 63(8):1355–1378
Stadler M, Kuhnert E, Peršoh D, Fournier J (2013) The Xylariaceae as model example for a unified nomenclature following the “One Fungus-One Name” (1F1N) concept. Mycology 4:5–21
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9):1312–1313
Stevens NE (1926) Two species of Physalospora on citrus and other hosts. Mycologia 18:206–217
Steyaert RL (1949) Contributions á lé tude monographique de Pestalotia de Not. et Monochaetia Sacc. (Truncatella gen. nov. et Pestalotiopsis gen. nov.). Bulletin du Jardin botanique de l’É tat a Bruxelles 19:285–354
Stiller JW, Hall BD (1997) The origin of red algae: Implications for plastid evolution. Proc Natl Acad Sci USA 94(9):4520–4525
Stukenbrock EH, Banke S, McDonald BA (2006) Global migration patterns in the fungal wheat pathogen Phaeosphaeria nodorum. Mol Ecol 15:2895–2904
Sudhadham M, Prakitsin S, Sivichai S, Chaiyarat R, Dorrestein GM, Menken SBJ, De Hoog G (2008) The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud Mycol 61:145–155
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG (2009) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173
Sung G, Hywel-Jones NL, Sung J-M, Luangsa-Ard JJ, Shrestha B, Spatafora JW (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57:5–59
Sutton BC (1980) The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew
Svetasheva TY (2010) Boletus legaliae Pilát//Red Data Book of Tula region: plants and fungi./Tula Region Administration, Department of the Tula Region on ecology and natural resources—Tula, Grif et K:327 (in Russian)
Svetasheva TY, Kovalenko AE (2013) Boletus rubrosanguineus Cheype//Red Data Book of Karachay-Cherkessia Republic—Cherkessk:201 (in Russian)
Swofford DL (2003) PAUP: phylogenetic analysis using parsimony, version 4.0 b10. Sinauer Associates, Sunderland, MA. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3348772
Sydow H (1928) Fungi Chilenses a cl. E. Werdermann lecti. Pars prima. Ann Mycol 26(1–2):100–126
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tan YS, Desjardin DE, Perry BA, Vikineswary S, Noorlidah A (2009) Marasmius sensu stricto in Peninsular Malaysia. Fungal Divers 37:9–100
Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y, Shirouzu T (2015) Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud Mycol 82:75–136
Tang AMC, Hyde KD, Corlett RT (2003) Diversity of fungi on wild fruits in Hong Kong. Fungal Divers 14:165–185
Tang AMC, Jeewon R, Hyde KD (2009) A re-evaluation of the evolutionary relationships within the Xylariaceae based on ribosomal and protein-coding gene sequences. Fungal Divers 34:127–155
Templeton MD, Rikkerink EHA, Solon SL, Crowhurst RN (1992) Cloning and molecular characterisation of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 122:225–230
Tennakoon DS, Hyde KD, Phookamsak R, Wanasinghe DN, Camporesi E, Promputtha I (2016) Taxonomy and Phylogeny of Juncaceicola gen. nov. (Phaeosphaeriaceae, Pleosporinae, Pleosporales). Cryptogam Mycol 37:135–156
Thacker JR, Henkel TW (2004) New species of Clavulina from Guyana. Mycologia 96:650–657
Thambugala KM, Daranagama DA, Camporesi E, Singtripop C, Liu ZY, Hyde KD (2014a) Multi-locus phylogeny reveals the sexual state of Tiarosporella in Botryosphaeriaceae. Cryptogam Mycol 35:359–367
Thambugala KM, Ariyawansa HA, Li YM, Boonmee S, Hongsanan S, Tian Q, Singtripop C, Bhat DJ, Camporesi E, Jayawardena R, Liu ZY, Xu JC, Chukeatirote E, Hyde KD (2014b) Dothideales. Fungal Divers 68:105–158
Thambugala KM, Hyde KD, Tanaka K, Tian Q, Wanasinghe DN, Ariyawansa HA, Jayasiri SC, Boonmee S, Camporesi E, Hashimoto A, Hirayama K, Schumacher RK, Promputtha I, Liu ZY (2015a) Towards a natural classification and backbone tree for Lophiostomataceae, Floricolaceae, and Amorosiaceae fam. nov. Fungal Divers 74:199–266
Thambugala KM, Chunfang Y, Camporesi E, Bahkali AH, Liu ZY, Hyde KD (2015b) Pseudodidymosphaeria gen. nov. in Massarinaceae. Phytotaxa 231:271–282
Thambugala KM, Bulgakov TS, Eungwanichayapant PD, Liu ZY, Hyde KD (2016) Camarosporium uniseriatum nom. nov., from Celtis occidentalis in European Russia. Stud Fungi 1:90–98
Theissen F (1913) Lembosia-Studien. Ann Mycol 11(5):424–467
Thilagam L, Nayak BK, Nanda A (2015) Studies on the diversity of coprophilous microfungi from hybrid cow dung samples. Int J PharmTech Res 8:135–138
Tian Q, Liu JK, Hyde KD, Wanasinghe DN, Boonmee S, Jayasiri SC, Luo ZL, Taylor JE, Phillips AJL, Bhat DJ, Li WJ, Ariyawansa H, Thambugala KM, Jones EBG, Chomnunti P, Bahkali AH, Xu JC, Camporesi E (2015) Phylogenetic relationships and morphological reappraisal of Melanommataceae (Pleosporales). Fungal Divers 74:267–324
Tibpromma S, Wijayawardene NN, Manamgoda DS, Boonmee S, Wanasinghe DN, Camporesi E, Yang JB, Hyde KD (2016a) Camarosporium arezzoensis on Cytisus sp., an addition to sexual state of Camarosporium sensu stricto. Saudi. J Biol Sci 23(1):1–8
Tibpromma S, Bhat J, Doilom M, Lumyong S, Nontachaiyapoom S, Yang JB, Hyde KD (2016b) Three new Hermatomyces species (Lophiotremataceae) on Pandanus odorifer from Southern Thailand. Phytotaxa 275(2):127–139
Tode HJ (1790) Fungi Mecklenburgenses Selecti. Fasc. 1. Nova Fungorum Genera Complectens. i–viii, 1–50, plates:1–7
Trevisan V (1877) Note sur la tribu des Platystomees de la famille des Hypoxylacées. Bull Soc R Bot Belg 16:14–20
Truszkowska W, Chlebicki A (1983) Pyrenomycetes pogorza cieszynskiego. I. Acta Mycol 19:3–19
Tulasne R, Tulasne C (1963) Selecta Fungorum Carpologia 2:1–316
Tulloss RE (1994) Type studies in Amanita section Vaginatae I: some taxa described in this century (studied 1–23) with notes on description of basidiospores and refractive hyphae in Amanita. Mycotaxon 52:305–396
Tulloss RE (2001) Amanita olivaceogrisea a little-known species found in Britain. Field Mycol 2(3):99–100
Tulloss RE, Moses E (1995) Amanita populiphila a new species from the central United States. Mycotaxon 53:455–466
Tulloss RE, Traverso M (2000) Illustrazioni di una nuova specie di Amanita dedicata al dr. Cornelis Bas di Leiden. Bolletino del Gruppo Micologico G. Bresadola 43(2):151–153
Tulloss RE, Traverso M (2001) Amanita basiana a new species from pure Pinus forest and resembling the Alnus-associated species Amanita friabilis. Mycotaxon 77:47–55
Tulloss RE, Yang ZL (eds) (2016) Studies in the Amanitaceae. www.amanitaceae.org
Tulloss RE, Kuyper TW, Vellinga EC, Yang ZL, Halling RE, Geml J, Sánchez-Ramírez S, Gonçalves SC, Hess J, Pringle A (2016) The genus Amanita should not be split. Amanitaceae 1(3):1–16
Uehling JK, Henkel TW, Aime MC, Smith ME (2012a) New species of Clavulina with effused or resupinate basidiomata from the Guiana Shield. Mycologia 104:547–556
Uehling JK, Henkel TW, Aime MC, Vilgalys R, Smith ME (2012b) New species and distribution records for Clavulina (Cantharellales, Basidiomycota) from the Guiana Shield, with a key to the lowland neotropical taxa. Fungal Biol 116:1263–1274
Uljé CB (2005) 1. Coprinus Pers. In: Noordeloos ME, Kuyper TW, Vellinga EC (eds) Flora Agaricina Neerlandica 6. Taylor & Francis, Boca Raton, pp 22–109
Untereiner WA, Strauss NA, Malloch D (1995) A molecular-morphotaxonomic approach to the systematics of the Herpotrichiellaceae and allied black yeasts. Mycol Res 99:897–913
Upadhyay HP (1969) Soil fungi from north-east and north Brazil-VII. The genus Gongronella. Nova Hedwigia 17:65–73
Vasquez G (2012) Indagini micologiche sulle Boletales del territorio siciliano. Annales Confederationis Europaeae Mycologiae Mediterraneensis 2009. Alaimo, Palermo, pp 69–81
Vasquez G (2013) Indagini micologiche sulle Boletales Epigee del territorio siciliano—mappatura e censimento delle specie. Tesi di Dottorato di Ricerca (PhD thesis), XXVI Ciclo. Università degli Studi di Catania
Vasquez G (2014) Indagini Micologiche sulle Boletales Epigee del Terittorio Siciliano-mappatura e censimento delle specie [doctoral thesis]. Univ Studi Catania, Catania
Vellinga EC (2004) Genera in the family Agaricaceae-evidence from nrITS and nrLSU sequences. Mycol Res 108:354–377
Velmurugan P, Kamala-Kannan S, Balachandar V, Lakshmanaperumalsamy P, Chae JC, Oh BT (2010) Natural pigment extraction from five filamentous fungi for industrial applications and dyeing of leather. Carbohydr Polym 79(2):262–268
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Visagie CM, Houbraken J, Frisvad JC, Hong SB, Klaassen CHW, Perrone G, Seifert KA, Varga J, Yaguchi T, Samson RA (2014) Identification and nomenclature of the genus Penicillium. Stud Mycol 78:343–371
Visagie CM, Seifert KA, Houbraken J, Samson RA, Jacobs K (2016) A phylogenetic revision of Penicillium sect. Exilicaulis, including nine new species from fynbos in South Africa. IMA Fungus 7:75–117
von Arx JA, Müller E (1954) Die Gattungen der amerosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz 11(1):1–434
von Arx JA, Muller E (1975) A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Stud Mycol 9:1–159
Vrinda KB, Pradeep CK, Abraham TK (1997) Some Inocybes new to India. J Econ Taxon Bot 21(1):41–45
Walker DM, Castlebury LA, Rossman AY, Sogonov MV, White JF (2010) Systematics of genus Gnomoniopsis (Gnomoniaceae, Diaporthales) based on a three gene phylogeny, host associations and morphology. Mycologia 102(6):1479–1496
Walther G, Pawłowska J, Alastruey-IzquierdoA WM, Rodriguez- Tudela JL, Dolatabadi S, Chakrabarti A, de Hoog GS (2013) DNA barcoding in Mucorales: an inventory of biodiversity. Persoonia 30:11–47
Walty H (1969) Tavole Svizzere di funghi, vol. III, 3rd edn. tav. 39
Wanasinghe DN, Jones EBG, Camporesi E, Boonmee S, Ariyawansa HA, Wijayawardene NN, Hyde KD (2014) An exciting novel member of Lentitheciaceae in Italy from Clematis vitalba. Cryptogam Mycol 35:323–337
Wanasinghe DN, Jones EBG, Camporesi E, Dissanayake AJ, Kamolhan S, Mortimer PE, Xu J, Abd-Elsalam KA, Hyde KD (2016) Taxonomy and phylogeny of Laburnicola gen. nov. and Paramassariosphaeria gen. nov. (Didymosphaeriaceae, Massarineae, Pleosporales). Fungal Biol 120(11):1354–1373
Wang L, Zhuang WY (2004) Designing primer sets for amplification of partial calmodulin genes from penicillia. Mycosystema 23(4):466–473
Wang XH, Yang ZL, Li YC, Knudsen H, Liu PG (2009) Russula griseocarnosa sp. nov. (Russulaceae, Russulales), a commercially important edible mushroom in tropical China: mycorrhiza, phylogenetic position, and taxonomy. Nova Hedwigia 88:269–282
Wang Y, Hyde KD, McKenzie EHC, Jiang YL, Li DW, Zhao DG (2015) Overview of Stachybotrys (Memnoniella) and current species status. Fungal Divers 71:17–83
Wannathes N, Desjardin DE, Hyde KD, Perry BA, Lumyong S (2009) A monograph of Marasmius (Basidiomycota) from Northern Thailand based on morphological and molecular (ITS sequences) data. Fungal Divers 37:209–306
Wartchow F (2012) Clavulina incrustata, a new species from Pernambuco, Brazil. Cryptogam Mycol 33(1):105–113
Watanabe T (1994) Two new species of homothallic Mucor in Japan. Mycologia 86:691–695
Watanabe T, Shitan N, Suzuki S, Umezawa T, Shimada M, Yazaki K, Hattori T (2010) Oxalate efflux transporter from the brown rot fungus Fomitopsis palustris. Appl Environ Microbiol 76:7683–7690
Weber NS (1972) The genus Helvella in Michigan. Mich Bot 11:147–201
Weinstein RN, Pfister DH, Iturriaga T (2002) A phylogenetic study of the genus Cookeina. Mycologia 94(4):673–682
Wen YM, Rajendran RK, Lin YF, Kirschner R, Hu S (2015) Onychomycosis associated with Exophiala oligosperma in Taiwan. Mycopathologia 181(1):83–88
White JF Jr, Bacon CW, Hywel-Jones NL, Spatafora JW (2003) Clavicipitalean fungi: evolutionary biology, chemistry, biocontrol and cultural impacts. CRC Press, Boca Raton
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wijayawardene NN, Mckenzie EHC, Hyde KD (2012) Towards incorporating anamorphic fungi in a natural classification-checklist and notes for 2011. Mycosphere 3(2):157–228
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Dai DQ, Dsouza MJ, Diederich P, Dissanayake A, Doilom M, Hongsanan S, Jones EBG, Groenewald JZ, Jayawardena R, Lawrey JD, Liu JK, Lücking R, Madrid H, Manamgoda DS, Muggia L, Nelsen MP, Phookamsak R, Suetrong S, Tanaka K, Thambugala KM, Wanasinghe DN, Wikee S, Zhang Y, Aptroot A, Ariyawansa HA, Bahkali AH, Bhat DJ, Gueidan C, Chomnunti P, De Hoog GS, Knudsen K, Li WJ, McKenzie EHC, Miller AN, Phillips AJL, Piątek M, Raja HA, Shivas RS, Slippers B, Taylor JE, Tian Q, Wang Y, Woudenberg JHC, Cai L, Jaklitsch WM, Hyde KD (2014a) Naming and outline of Dothideomycetes-2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55
Wijayawardene NN, Hyde KD, Bhat DJ, Camporesi E, Schumacher RK, Chethana KWT, Wikee S, Bahkali AH, Wang Y (2014b) Camarosporium-like species are polyphyletic in Pleosporales; introducing Paracamarosporium and Pseudocamarosporium gen. nov. in Montagnulaceae. Cryptogam Mycol 35:177–198
Wijayawardene NN, Bhat DJ, Hyde KD, Camporesi E, Chethana KWT, Tangthirasunun N, Wang Y (2014c) Camarosporium sensu stricto in Pleosporineae, Pleosporales with two new species. Phytotaxa 183(1):16–26
Wijayawardene NN, Hyde KD, Bhat DJ, Goonasekars ID, Nadeeshan D, Camporesi E, Schumacher RK, Wang Y (2015) Additions to brown spored coelomycetous taxa in Massarinae, Pleosporales: introducing Phragmocamarosporium gen. nov. and Suttonomyces gen. nov. Cryptogam Mycol 36:213–224
Wijayawardene NN, Hyde KD, Wanasinghe DN, Papizadeh M, Goonasekara ID, Camporesi E, Bhat DJ, McKenzie EHC, Phillips AJL, Diederich P, Tanaka K, Li WJ, Tangthirasunun N, Phookamsak R, Dai DQ, Dissanayake AJ, Weerakoon G, Maharachchikumbura SSN, Hashimoto A, Matsumura M, Bahkali AH, Wang Y (2016) Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers 77:1–316
Wikee S, Lombard L, Nakashima C, Motohashi K, Chukeatirote E, Cheewangkoon R, McKenzie EHC, Hyde KD, Crous PW (2013) A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Stud Mycol 76:1–29
Winter G (1885) Pilze—Ascomyceten. In: GL Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz 1:65–528
Winter G (1886) Rabenhorst’s Kryptogamen-Flora, Pilze—Ascomyceten 1(2):570
Wolf FA (1935) Morphology of Polythrincium, causing sooty blotch of clover. Mycologia 27(1):58–73
Wolfe CB (1979) Austroboletus and Tylopilus subgenus Porphyrellus with emphasis on North American taxa. Bibl Mycol 69:1–132
Wolfe BE, Kuo M, Pringle A (2012a) Amanita thiersii is a saprotrophic fungus expanding itsrange in the United States. Mycologia 104:22–23
Wolfe BE, Tulloss RE, Pringle A (2012b) The irreversible loss of a decomposition pathway marks the single origin of an ectomycorrhizal symbiosis. PLoS ONE 7(7):539–597
Woo PC, Ngan AH, Tsang CC, Ling IW, Chan JF, Leung SY, Yuen KY, Lau SK (2013) Clinical spectrum of Exophiala infections and a novel Exophiala species, Exophiala hongkongensis. J Clin Microbiol 51(1):260–267
Woronichin NN (1925) Über die Capnodiales. Ann Mycol 23:174–178
Woudenberg JHC, Aveskamp MM, De Gruyter J, Spiers AG, Crous PW (2009) Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 22(1):56–62
Wu G, Feng B, Xu J, Zhu XT, Li YC, Zeng NK, Hosen IMD, Yang ZL (2014) Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers 69:93–115
Wyka SA, Broders KD (2016) The new family Septorioideaceae, within the Botryosphaeriales and Septorioides strobi as a new species associated with needle defoliation of Pinus strobus in the United States. Fungal Biol 120(8):1030–1040
Yang T, Groenewald JZ, Cheewangkoon R, Jami F, Abdollahzadeh J, Lombard L, Crous PW (2017) Families, genera and species of Botryosphaeriales. Fungal Biol. doi:10.1016/j.funbio.2016.11.001
Yang YL, Liu ZY, Cai L, Hyde KD, Yu ZN, McKenzie EHC (2009) Colletotrichum anthracnose of Amaryllidaceae. Fungal Divers 39:123–146
Yang ZL (1997) Die Amanita-Arten von Südwestchina. Bibl Mycol 170:1–240
Yang ZL (1990) Several noteworthy higher fungi from southern Yunnan, China. Mycotaxon 38:407–416
Yang ZL (2000) Species diversity of the genus Amanita (Basidiomycetes) in China. Acta Bot Yunnan 22:135–142
Yip HY (1988) Berkleasmium correae sp. novo on leaf hairs of Correa lawrenciana. Australas Plant Pathol 17:31–33
Yuan HS, Dai YC (2008) Polypores from northern and central Yunnan Province, Southwestern China. Sydowia 60:147–159
Zeng JS, Feng PY, Gerrits van denEnde AHG, Xi LY, Harrak MJ, de Hoog GS (2014) Multilocus analysis of the Exophiala jeanselmei clade containing black yeasts involved in opportunistic disease in humans. Fungal Divers 65:3–16
Zhang LF, Yang JB, Yang ZL (2004) Molecular phylogeny of eastern Asian species of Amanita (Agaricales, Basidiomycota): taxonomic and biogeography implications. Fungal Divers 17:219–238
Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung GH (2006) An overview of the systematics of the Sordariomycetes based on four-gene phylogeny. Mycologia 98:1076–1087
Zhang Y, Schoch CL, Fournier J, Crous PW, De Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD (2009a) Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang Y, Wang HK, Fournier J, Crous PW, Jeewon R, Pointing SB, Hyde KD (2009b) Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Divers 38:25–51
Zhang Y, Fournier J, Bahkar AH, Hyde KD (2011) Inflatispora, a novel lignicolous genus of Pleosporales from France. Sydowia 62:287–295
Zhang XY, Bao J, Wang GH, He F, Xu XY, Qi SH (2012a) Diversity and antimicrobial activity of culturable fungi isolated from six species of the South China Sea gorgonians. Microb Ecol 64:617–627
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012b) Pleosporales. Fungal Divers 53(1):1–221
Zhang H, Hyde KD, Zhao Y, McKenzie EH, Zhou D (2014) Freshwater ascomycetes: Lophiostoma vaginatispora comb. nov. (Dothideomycetes, Pleosporales, Lophiostomaceae) based on morphological and molecular data. Phytotaxa 176:184–191
Zhang M, He W, Wu JR, Zhang Y (2017) Two new species of Spencermartinsia (Botryosphaeriaceae, Botryosphaeriales) from China. Mycosphere 7(7):942–949
Zhao K, Wu G, Jang L (2014) A new genus, Rubroboletus, to accommodate B. sinicus and its allies. Phytotaxa 188(2):61–77
Zhao Q, Tolgor B, Zhao YC, Yang ZL, Hyde KD (2015) Species diversity within the Helvella crispa group (Ascomycota: Helvellaceae) in China. Phytotaxa 239:130–142
Zhao Q, Sulayman M, Zhu XT, Zhao YC, Yang ZL, Hyde KD (2016a) Species clarification of the culinary Bachu mushroom in Western China. Mycologia 108(4):828–836
Zhao Q, Zhang XL, Li SH, Chai HM, Bahkali AH, Hyde KD (2016b) New species and records of saddle fungi (Helvella, Helvellaceae) from Jiuzhaigou Natural Reserve, China. Mycoscience 57(6):422–430
Zhao Q, Brooks S, Zhao YC, Yang ZL, Hyde KD (2016c) Morphology and phylogenetic position of Wynnella subalpina sp. nov. (Helvellaceae) from western China. Phytotaxa 270(1):41–48
Zhaxybayeva O, Gogarten JP (2002) Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genom 3(1):4
Zhou LW, Wei YL (2011) Changbai wood-rooting fungi 16. A new species of Fomitopsis (Fomitopsidaceae). Mycol Prog 11:435–441
Zobel (1854) Icones fungorum hucusque cognitorum 6:4
Zogg H (1962) Die Hysteriaceae s. str. und Lophiaceae. Beiträge zur Kryptogamenflora der Schweiz 11(3):1–190
Acknowledgements
Saowaluck Tibpromma would like to thank the Molecular Biology Experimental Center at Kunming Institute of Botany for facilities for molecular work, the Mushroom Research Foundation (MRF), Chiang Rai, Thailand for the financial support of her study and Shaun Pennycook is thanked for nomenclatural advice. K.D. Hyde would like to thank the Thailand Research Fund Grant No. RSA5980068 entitled “Biodiversity, phylogeny and role of fungal endophytes on above parts of Rhizophora apiculata and Nypa fruticans”, the Chinese Academy of Sciences, Project Number 2013T2S0030, for the award of Visiting Professorship for Senior International Scientists at Kunming Institute of Botany and National Research Council of Thailand (Mae Fah Luang University) for a grants “Biodiversity, phylogeny and role of fungal endophytes of Pandanaceae” (Grant No.: 592010200112), “Diseases of mangrove trees and maintenance of good forestry practice” (Grant No.: 60201000201) for supporting this study. S.C. Karunarathna, P.E. Mortimer and J.C. Xu would like to thank the World Agroforestry Centre, East and Central Asia Office; Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Science; the Chinese; Ministry of Science and Technology, under the 12th 5-year National Key Technology Support Program (NKTSP)2013 BAB07B06 integration and comprehensive demonstration of key technologies on Green Phosphate-mountaion Construction and the CGIAR Research Program 6: Forest, Trees and Agroforestry for partial funding. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group NO (RG-1436-025). Financial support by the German Academic Exchange Service (DAAD) and the Thai Royal Golden Ph.D. Jubilee-Industry program (RGJ) for a joint TRF-DAAD PPP (2012–2014) academic exchange grant to K.D. Hyde and M. Stadler, and the RGJ for a personal grant to B. Thongbai (No. Ph.D/0138/2553 in 4.S.MF/53/A.3) is gratefully acknowledged. Chayanard Phukhamsakda (PHD/0020/2557) acknowledges the Royal Golden Jubilee Ph.D. Program under the Thailand Research Fund. Mingkwan Doilom acknowledges the Royal Golden Jubilee Ph.D. Program (PHD./0072/2553 in 4.S.M.F./53/A.2) under the Thailand Research Fund. Ausana Mapook is grateful to Research and Researchers for Industries (RRI) PHD57I0012. Rungtiwa Phookamsak expresses sincere appreciation to The CAS President’s International Fellowship for Postdoctoral Researchers (Project No. 2017PB0072). Qi Zhao thanks the National Natural Science Foundation of China (No. 31360015) and the CAS/SAFEA International Partnership Program for Creative Research Teams, and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSCX2-EW-Z-9 and KIB2016002). André Luiz Cabral Monteiro de Azevedo Santiago, Carlos Alberto Fragoso de Souza, Diogo Xavier Lima, Rafael José Vilela de Oliveira and Gladstone Alves da Silva would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordination for the Improvement of Higher Education Personnel) (CAPES) and the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (Foundation for the support of Science and Technology of the state of Pernambuco) (FACEPE) for the postgraduate scholarships awarded to Diogo X. Lima and Carlos A. F. de Souza, respectively. We would also like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development) (CNPq) and FACEPE for financial support through the projects: ‘Mucoromycotina in upland forests in the semi-arid region of Pernambuco’ (CNPq-458391/2014-0), and ‘Diversity of Mucoromycotina in different ecosystems of the Pernambuco Atlantic Rainforest’ (FACEPE—APQ 0842-2.12/14). H.B. Lee was supported by the Graduate Program for the Undiscovered Taxa of Korea, and by the Project on Survey and Discovery of Indigenous Fungal Species of Korea funded by NIBR and Project on Discovery of Fungi from Freshwater and Collection of Fungarium funded by NNIBR of the Ministry of Environment (MOE), and in part by a fund from National Institute of Animal Science under Rural Development Administration, Republic of Korea. Z.L Luo and H.Y Su would like to thank the National Natural Science Foundation of China (Project ID: 31460015) for financial support on Study of the distribution pattern and driving factors of aquatic fungal diversity in the region of Three Parallel Rivers. Saranyaphat Boonmee thanks the National Research Council of Thailand, project number 2560A30702021 and the Thailand Research Fund, project number TRG5880152 for providing financial support. C.G. Lin and Y. Wang thank the grant from the National Natural Science Foundation of China (No. NSFC 31560489) and Fundamental Research on Science and Technology, Ministry of Science and Technology of China (2014FY120100) and Mr. Jingzu Sun thank for the National Natural Science Foundations of China (No. 31600024). Wei Dong thanks the for National Natural Science Foundation of China (Project ID: NSF 31500017 to Huang Zhang). P.N. Singh, A. Baghela, S.K. Singh, and S. Aamir thank the Director, MACS’ Agharkar Research Institute, Pune, India for providing facilities and Rajendra Singh (Department of Zoology, DDU Gorakhpur University, UP, India) for identification of insect-host. Saisamorn Lumyong and Rene K. Schumacher are thanked for valuable suggestions and collecting specimens. K.N.A. Raj acknowledges support from the University Grants Commission (UGC), India, in the form of a Rajiv Gandhi National Fellowship (Grant No. F.14-2(SC)/2009 (SA-III)). K.N.A. Raj also acknowledges the permissions given to him for collecting agaric specimens from the forests of Kerala by the Principal Chief Conservator of Forests, Government of Kerala (WL12-4042/2009 dated 5 August 2009). K.P.D. Latha acknowledges the financial support from the Kerala State Council for Science, Technology and Environment (KSCSTE) in the form of a PhD fellowship (Grant No. 001/FSHP/2011/CSTE). K.P.D. Latha also acknowledges the permission (No. WL10-4937/2012, dated 3-10-2013) given to her by the Principal Chief Conservator of Forests, Government of Kerala, to collect agaric specimens from the forests of Kerala. Zdenko Tkalčec has been partially supported by Croatian Science Foundation under the project HRZZ-IP-11-2013-2202 (ACCTA) and is grateful to Milan Čerkez for his great contribution to the study of coprinoid and coprophilous fungi in Croatia. Vladimir Antonín thank the Moravian Museum by the Ministry of Culture of the Czech Republic as part of its long-term conceptual development programme for research institutions (DKRVO, ref. MK000094862). T.C. Wen, Y.P. Xiao, C. Norphanphoun and K.K. Hapuarachchi are grateful to the National Natural Science Foundation of China (No. 31460012) and the Science and Technology Foundation of Guizhou Province (No. [2016]2863). Y.W. Lim would like to thanks NIBR supporting the Project on Survey and Discovery of Indigenous Fungal Species of Korea. Kanad Das and Dyutiparna Chakraborty are thankful to the Director, Botanical Survey of India (BSI) and Scientist-in-Charge, BSI, Gangtok for providing facilities during this study. Sinchan Adhikari, Joydeep Karmakar and Tapas Kumar Bandyopadhyay would like to acknowledge DST-PURSE and DST-FIST for providing central instrumentation facilities and Alan JL Phillips acknowledges the Biosystems and Integrative Sciences Institute (BioISI, FCT/UID/Multi/04046/2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tibpromma, S., Hyde, K.D., Jeewon, R. et al. Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 83, 1–261 (2017). https://doi.org/10.1007/s13225-017-0378-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-017-0378-0