Abstract
Coelomycete is a general term used for asexual fungi which produce conidia in fruiting bodies: pycnidial, acervular, cupulate, pycnothyria or stromatic conidiomata. The group contains numerous plant pathogenic, saprobic and endophytic species associated with a wide range of hosts. Traditionally, morphological characters and host associations have been used as criteria to identify and classify coelomycetes, and this has resulted in a poor understanding of their generic and species boundaries. DNA based taxonomic studies have provided a better outlook of the phylogenetic and evolutionary trends in coelomycetes. However, the present outcomes represent only a preliminary step towards the understanding of coelomycetes. Many genera have not been revisited since they were first described. The present study revises the classification of the hyaline-spored coelomycetes and provides a modern taxonomic framework based on both morphology and phylogeny. In total, 248 genera were investigated, of which less than 100 are known to have sequence data. Multi-locus sequence data analyses of 28S nrDNA, 18S nrDNA, ITS, RNA polymerase II second largest subunit (rpb2), and part of the translation elongation factor 1-alpha gene (tef1) and β-tubulin (tub2) gene regions were analysed. As a result, three new genera and 23 new species are introduced. In addition, three new links between sexual and asexual genera are provided. There are 138 genera that lack sequence data, and these are treated as Ascomycota, genera incertae sedis. Line drawings and descriptions are provided based on the examination of types and fresh collections and on the literature.
Similar content being viewed by others
Table of contents
Acrocalymma Alcorn & J.A.G. Irwin, Trans. Br. Mycol. Soc. 88(2): 163 (1987)
Acrocalymma aquatica Huang Zhang & K.D. Hyde, Cryptog. Mycol. 33(3): 337 (2012)
Acrocalymma medicaginis Alcorn & J.A.G. Irwin, Trans. Br. Mycol. Soc. 88(2): 163 (1987)
Ajrekarella Kamat & Kalani, Mycopath. Mycol. appl. 24: 300 (1964)
Ajrekarella polychaetriae Kamat & Kalani, Mycopath. Mycol. appl. 24: 300 (1964)
Allantophomopsiella Crous, in CCrous, Quaedvlieg, Hansen, Hawksworth & Groenewald, IMA Fungus 5(1): 180 (2014)
Allantophomopsiella pseudotsugae (M. Wilson) Crous, in Crous, Quaedvlieg, Hansen, Hawksworth & Groenewald, IMA Fungus 5(1): 180 (2014)
Allantophomopsis Petr., Annls. mycol. 23(1/2): 104 (1925)
Allantophomopsis fusiformis (Nag Raj) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 114 (1993)
Alloneottiosporina Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 121 (1993)
Alloneottiosporina thailandica W.J. Li & K.D. Hyde, sp. nov.
Amerosporiopsis Petr., Bot. Arch. 43: 84 (1941)
Amphiporthe Petr., Sydowia 24(1–6): 257 (1971)
Anaphysmene Bubák, Ann Mycol. 4(2): 124 (1906)
Anthracoderma Speg., Boln Acad. nac. Cienc. Córdoba 11(2): 286 (1887) [1888]
Aoria Cif., Atti Ist. bot. Univ. Lab. crittog. Pavia, Ser. 5 19: 89 (1962)
Aphanofalx B. Sutton, Trans. Br. Mycol. Soc. 86(1): 21 (1986)
Apiocarpella Syd. & P. Syd., Ann Mycol. 17(1): 43 (1919)
Apiognomonia Höhn., Ber. dt. bot. Ges. 35(8): 635 (1917)
Apiognomonia errabunda (Roberge ex Desm.) Höhn., Ann Mycol. 16(1/2): 51 (1918)
Apiognomonia pseudohystrix W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Aposphaeria Sacc., Michelia 2(6): 4 (1880)
Aposphaeria pulviscula (Sacc.) Sacc., Michelia 2(6): 4 (1880)
Aposphaeria corallinolutea Gruyter, Aveskamp & Verkley, in Gruyter et al., Stud. Mycol. 75: 28 (2012) [2013]
Aquasubmersa K.D. Hyde & Huang Zhang, Cryptog. Mycol. 33(3): 340 (2012)
Aquasubmersa mircensis K.D. Hyde & Huang Zhang, Cryptog. Mycol. 33(3): 340 (2012)
Aschersonia Mont., Annls Sci. Nat., Bot., sér. 3 10: 121 (1848)
Aschersonia calendulina Hywel-Jones & Mongkols., Mycol. Res. 113(6–7): 687 (2009)
Ascocalyx Naumov, Bolêz. Rast. 14: 138 (1926)
Ascochyta Lib., Pl. crypt. Arduenna, fasc. 1(Praef.): 8 (1830)
Ascochyta herbicola (Wehm.) Qian Chen & L. Cai, Stud. Mycol. 82: 187 (2015)
Ascochyta medicaginicola Qian Chen & L. Cai, Stud. Mycol. 82: 187 (2015)
Ascochyta neopisi W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Ascochytopsis Henn., Bot. Jb. 38: 117 (1905)
Ascodichaena Butin, Trans. Br. Mycol. Soc. 69(2): 249 (1977)
Asteroconium Syd. & P. Syd., Ann Mycol. 1(1): 36 (1903)
Asteromella Pass. & Thüm., in Thümen, Mycoth. Univ., cent. 17: no. 1689 (1880)
Asteromidium Speg., Anal. Soc. cient. argent. 26(1): 66 (1888)
Aurantiosacculus Dyko & B. Sutton, in Dyko, Sutton & Roquebert, Mycologia 71(5): 922 (1979)
Blennoria Moug. & Fr., in Fries, Syst. orb. veg. 1: 366 (1825)
Boeremia Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 36 (2010)
Boeremia exigua (Desm.) Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 37 (2010)
Boeremia exiguavar.heteromorpha (Schulzer & Sacc.) Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 38 (2010)
Boeremia galiicola Jayasiri, Camporesi & K.D. Hyde, in Jayasiri, Hyde, Jones, Jeewon, Ariyawansa, Bhat, Camporesi & Kang, Mycosphere 8(8): 1089 (2017)
Botryocrea Petr., Sydowia 3(1–6): 140 (1949)
Botryosphaeria Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 211 (1863)
Botryosphaeria dothidea (Moug.) Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 212 (1863)
Botryosphaeria kuwatsukai (Hara) G.Y. Sun & E. Tanaka, in Xu, Wang, Ju, Zhang, Biggs,Tanaka & Sun, Fungal Diversity 71: 225 (2014)
Brunneodinemasporium Crous & R.F. Castañeda, in Crous, Verkley, Christensen, Castañeda-Ruíz & Groenewald, Persoonia 28: 128 (2012)
Brunneodinemasporium jonesii Y.Z. Lu, Jian K. Liu & K.D. Hyde, in Lu, Liu, Hyde, Bhat, Xiao, Tian, Wen, Boonmee & Kang, Mycosphere 7(9): 1326 (2016)
Brycekendrickia Nag Raj, Can. J. Bot. 51(7): 1337 (1973)
Brycekendrickia indica Nag Raj, Can. J. Bot. 51(7): 1337 (1973)
Calocline Syd., Ann Mycol. 37(4/5): 417 (1939)
Capnodium Mont., Annls Sci. Nat., Bot., sér. 3, 11: 233 (1849)
Capnodium aciculiforme (Cif., Bat. & Nascim.)W.J. Li & K.D. Hyde, comb. nov.
Capnodium gardeniarum (Bat. & Cif.) W.J. Li & K.D. Hyde, comb nov.
Capnodium paracoartatum Q. Tian, W.J. Li & K.D. Hyde, sp. nov.
Catenophora Luttr., Mycologia 32(4): 535 (1940)
Ceuthodiplospora Died., Ann Mycol. 10(2): 149 (1912)
Chaetasbolisia Speg., Physis, Rev. Soc. Arg. Cienc. Nat. 4(17): 293 (1918)
Chaetoconis Clem., Gen. fung. : 125 (1909)
Chaetoconis vaccinii Melnik & Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 191 (1993)
Chaetomella Fuckel, Jb. nassau. Ver. Naturk. 23-24: 401 (1870) [1869-70]
Chaetophiophoma Speg.,Anal. Mus. nac. B. Aires, Ser. 3 13: 388 (1911)
Chaetophiophoma sorbi W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Chaetophoma Cooke, Grevillea 7(no. 41): 25 (1878)
Chaetoseptoria Tehon, Mycologia 29(4): 444 (1937)
Chaetospermum Sacc., Syll. fung. 10: 706 (1892)
Chaetospermum artocarpi (Nag Raj) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 194 (1993)
Chaetospermum camelliae Agnihothr., Mycopath. Mycol. appl. 16: 115 (1962)
Chaetosphaeronema Moesz, Bot. Közl. 14: 152 (1915)
Chaetosphaeronema hispidulum (Corda) Moesz, Bot. Közl. 14: 152 (1915)
Chaetosticta Petr. & Syd., Ann Mycol. 23(3/6): 270 (1925)
Cheilaria Lib., Pl. crypt. Arduenna, fasc. (Liège) 1(Praef.): 8 (1830)
Choanatiara DiCosmo, in Nag Raj & DiCosmo, Can. J. Bot. 62(4): 709 (1984)
Choanatiara lunata DiCosmo & Nag Raj, in Nag Raj & DiCosmo, Can. J. Bot. 62(4): 712 (1984)
Chondropodiella Höhn., Hedwigia 59(5): 281 (1917)
Ciliochora Höhn., Ber. dt. bot. Ges. 37: 159 (1919)
Ciliosporella Petr., Ann Mycol. 25(3/4): 217 (1927)
Ciliosporella selenospora Petr., Ann Mycol. 25(3/4): 217 (1927)
Ciliosporella italica W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Clohesyomyces K.D. Hyde, Aust. Syst. Bot. 6(2): 170 (1993)
Clohesyomyces aquaticus K.D. Hyde, Aust. Syst. Bot. 6(2): 170 (1993)
Colletotrichum Corda, in Sturm, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 3(12): 41 (1831)
Colletotrichum sansevieriae Miho Nakam. & Ohzono, in Nakamura, Ohzono, Iwai & Arai, J. Gen. Pl. Path. 72(4): 253 (2006)
Collonaemella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 82 (1915)
Colpoma Wallr., Fl. crypt. Germ. (Norimbergae) 2: 422 (1833)
Comatospora Piroz. & Shoemaker, Can. J. Bot. 49: 539 (1971)
Comatospora suttonii Piroz. & Shoemaker, Can. J. Bot. 49: 539 (1971)
Conicomyces R.C. Sinclair, Eicker & Morgan-Jones, Mycologia 75(6): 1100 (1983)
Conicomyces contortus Illman & G.P. White, Can. J. Bot. 63(3): 419 (1985)
Conidiocarpus Woron., Key to fungi (fungi imperfecti) 2: 743 (1917)
Conidiocarpus siamensis (Chomnunti & K.D. Hyde) T. Bose, in Bose, Reynolds & Burbee, Mycologia 106(4): 753 (2014)
Corniculariella P. Karst., Hedwigia 23(4): 57 (1884)
Corniculariella rhamni W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Cornucopiella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 118 (1915)
Cornutispora Piroz., Mycologia 65(4): 763 (1973)
Cornutispora limaciformis Piroz., Mycologia 65(4): 763 (1973)
Crandallia Ellis & Sacc., Bull. Torrey bot. Club 24(10): 466 (1897)
Crinitospora B. Sutton & Alcorn, Trans. Br. Mycol. Soc. 84(3): 437 (1985)
Crinitospora pulchra B. Sutton & Alcorn, Trans. Br. Mycol. Soc. 84(3): 439 (1985)
Crucellisporiopsis Nag Raj, Can. J. Bot. 60(12): 2601 (1983) [1982]
Crucellisporiopsis gelatinosa Nag Raj, Can. J. Bot. 60(12): 2605 (1983) [1982]
Crucellisporiopsis prolongata Brub., Rawla & R. Sharma, Mycotaxon 21: 449 (1984)
Crucellisporium M.L. Farr, in Farr & Horner, Nova Hedwigia 15: 264 (1968)
Crucellisporium africanum Nag Raj, Can. J. Bot. 56(6): 713 (1978)
Cryptomycella Höhn., Mitt. bot. Inst. tech. Hochsch. Wien 2(3): 48 (1925)
Cyclodomus Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 1527 (1909)
Cylindrogloeum Petr., Ann Mycol. 39(4/6): 276 (1941)
Cylindroxyphium Bat. & Cif., Quad. Lab. crittogam., Pavia 31: 77 (1963)
Cystotricha Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 5: 457 (1850)
Cytogloeum Petr., Ann Mycol. 23(1/2): 77 (1925)
Cytonaema Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 131 (1914)
Cytoplacosphaeria Petr., Ann Mycol. 17(2/6): 79 (1920) [1919]
Cytospora Ehrenb., Sylv. mycol. berol. (Berlin): 28 (1818)
Cytospora parasitica C. Norphanphoun, Bulgakov & K.D. Hyde, in Ariyawansa et al., Fungal Diversity[146] (2015)
Cytospora prunicola Norph., Camporesi, T.C. Wen & K.D. Hyde, in Hyde et al., Mycosphere 9(2): 378 (2018)
Cytospora globosa W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Cytospora phialidica W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Cytospora tanaitica C. Norphanphoun, Bulgakov & K.D. Hyde, in Ariyawansa et al., Fungal Diversity [146] (2015)
Cytospora sp.
Cytosporella sycina (Sacc.) Sacc., Syll. fung. 3: 251 (1884)
Cytostagonospora Bubák, Ann Mycol. 14(3/4): 150 (1916)
Darkera H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3052 (1975)
Darkera abietis H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3052 (1975)
Darkera durmitorensis (Karadžić) Crous, in Crous et al., Phytotaxa 202(2): 83 (2015)
Darkera parca H.S. Whitney, J. Reid & Piroz., Canadian Journal of Botany 53: 3053 (1975)
Darkera sp.
Darkera picea H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3053 (1975)
Darkera pseudotsugae (H.S. Whitney, J. Reid & Piroz.) Crous, in Crous et al., Phytotaxa 202(2): 85 (2015)
Dasysticta Speg., Anal. Mus. nac. Hist. nat. B. Aires 23: 108 (1912)
Davisiella Petr., Ann Mycol. 22(1/2): 134 (1924)
Dearnessia Bubák, Hedwigia 58: 25 (1916)
Dendrodomus Bubák, Bot. Közl. 14(3–4): (63) (1915)
Dermea Fr., Syst. orb. veg. 1: 114 (1825)
Dermea cerasi (Pers.) Fr., Syst. orb. veg. 1: 115 (1825)
Diachora Jul. Müll., Jb. wiss. Bot. 25: 623 (1893)
Dialaceniopsis Bat., Anais Soc. Biol. Pernambuco 16(1): 141 (1959)
Diaporthe Nitschke, Pyrenomyc. Germ. 2: 240 (1870)
Diaporthe foeniculina (Sacc.) Udayanga & Castl., in Udayanga, Castlebury, Rossman & Hyde, Persoonia 32: 95 (2014)
Diaporthe arezzoensis W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Didymella Sacc., Michelia 2(no. 6): 57 (1880)
Didymella macrostoma (Mont.) Qian Chen & L. Cai, in Chen, Jiang, Zhang, Cai & Crous, Stud. Mycol. 82: 177 (2015)
Didymochaeta Sacc. & Ellis, Bull. Torrey bot. Club 25: 510 (1898)
Diedickea Syd. & P. Syd., Leafl. of Philipp. Bot. 6: 1931 (1913)
Dilophospora Desm., Annls Sci. Nat., Bot., sér. 2 14: 6 (1840)
Dimastigosporium Faurel & Schotter, Revue Mycol., Paris 30: 156 (1965)
Dinemasporium Lév., Annls Sci. Nat., Bot., sér. 3 5: 274 (1846)
Dinemasporium cruciferum Ellis, Bull. Torrey bot. Club 9: 20 (1882)
Dinemasporium morbidum Crous, in Crous, Verkley, Christensen, Castañeda-Ruíz & Groenewald, Persoonia 28: 131 (2012)
Dinemasporium nelloi W.J. Li, Camporesi & K.D. Hyde, in Liu et al., Fungal Diversity: [18] (2015)
Dinemasporium pingue (Nag Raj) W.J. Li & K.D. Hyde, comb. nov.
Dinemasporium setulosa (B. Sutton) W. J. Li & K. D. Hyde, comb. nov.
Dinemasporium strigosum (Pers.) Sacc., Michelia 2(7): 281 (1881)
Dinemasporium pseudodecipiens A. Hashim. & Kaz. Tanaka, in Hashimoto, Sato, Matsuda, Hirayama, Hatakeyama, Harada, Shirouzu & Tanaka, Mycoscience 56: 95 (2014)
Dinemasporium pseudostrigosum Crous, in Crous, Verkley, Christensen, Castañeda-Ruíz & Groenewald, Persoonia 28: 134 (2012)
Diplocarpon F.A. Wolf, Bot. Gaz. 54: 231 (1912)
Diplocarpon mespili (Sorauer) B. Sutton, The Coelomycetes (Kew): 150 (1980)
Diplosporonema Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 126(4–5): 335 (1917)
Diplozythiella Died., in Sydow, Sydow & Butler, Ann Mycol. 14(3/4): 215 (1916)
Discogloeum Petr., Ann Mycol. 21(3/4): 285 (1923)
Discosporium Höhn., Z. Gärungsphysiol. 5: 196 (1915)
Dothiorina Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 120: 464 (1911)
Drepanopeziza (Kleb.) Jaap, Verh. bot. Ver. Prov. Brandenb. 56: 79 (1914)
Dwayalomella Brisson, Piroz. & Pauzé, Can. J. Bot. 53(23): 2867 (1975)
Dwayalomella vaccinii Brisson, Piroz. & Pauzé, Can. J. Bot. 53(23): 2867 (1975)
Ebollia Minter & Caine, Trans. Br. Mycol. Soc. 74(2): 436 (1980)
Eleutheromyces Fuckel, Jb. nassau. Ver. Naturk. 23-24: 183 (1870) [1869-70]
Eleutheromyces mycophilus (Höhn.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 345 (1993)
Ellula Nag Raj, Can. J. Bot. 58(18): 2013 (1980)
Elongaticonidia W.J. Li, E. Camporesi & K.D. Hyde, gen. nov.
Elongaticonidia rosae W.J. Li, E Camporesi & K.D. Hyde, sp. nov.
Eriospora Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 5: 455 (1850)
Eriosporella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 109 (1916)
Eriosporella calami (Niessl) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 109 (1916)
Erythrogloeum Petr., Sydowia 7(5–6): 378 (1953)
Eutiarosporella Crous, Phytotaxa 202(2): 85 (2015)
Eutiarosporella tritici (B. Sutton & Marasas) Crous, Phytotaxa 202(2): 85 (2015)
Fibulocoela Nag Raj, Can. J. Bot. 56(13): 1491 (1978)
Fibulocoela indica Nag Raj, Can. J. Bot. 56(13): 1491 (1978)
Fuckelia Bonord., Abh. naturforsch. Ges. Halle 8: 135 (1864)
Furcaspora Bonar, Mycologia 57(3): 391 (1965)
Gampsonema Nag Raj, Can. J. Bot. 53(16): 1621 (1975)
Geastrumia Bat., in Batista, Farr & Bezerra, Saccardoa 1: 71 (1960)
Giulia Tassi, Bulletin Labor. Orto Bot. de R. Univ. Siena 6: 92 (1904)
Giulia tenuis (Sacc.) Tassi ex Sacc. & D. Sacc., Syll. fung. 18: 435 (1906)
Gloeosporidina Petr., Ann Mycol. 19(3–4): 214 (1921)
Godronia Moug. & Lév., in Mougeot, Consid. Vég. Vosges: 355 (1846)
Godronia uberiformis J.W. Groves, Can. J. Bot. 43: 1245 (1965)
Groveolopsis Boedijn, Sydowia 5(3–6): 351 (1951)
Groveolopsis pandani (Höhn.) Boedijn, Sydowia 5(3–6): 225 (1951)
Gyrostroma Naumov, Bull. Soc. mycol. Fr. 30(3): 386 (1914)
Hapalosphaeria Syd., in Diedicke & Sydow, Ann Mycol. 6(4): 305 (1908)
Helhonia B. Sutton, The Coelomycetes: 600 (1980)
Hemidothis Syd. & P. Syd., Ann Mycol. 14(1/2): 95 (1916)
Hercospora Fr., Syst. orb. veg. 1: 119 (1825)
Heterosphaeria Grev., Scott. crypt. fl. 1: pl. 103 (1824)
Heterosphaeria linariae (Rabenh.) Rehm, Rabenh. Krypt.-Fl., Edn 2 1.3(lief. 30): 203 (1888) [1896]
Heterosphaeria patella (Tode) Grev., Scott. crypt. fl. 1: pl. 103 (1824)
Heterosphaeria hendersonioides (Fautrey & Lambotte) W.J. Li & K.D. Hyde, comb. nov.
Heterosphaeria umbilicata (Pers.) W.J. Li & K.D. Hyde, comb. nov.
Hypocline Syd., Ann Mycol. 37(3): 245 (1939)
Hypohelion P.R. Johnst., Mycotaxon 39: 221 (1990)
Hysterodiscula Petr., Bot. Arch. 43: 210 (1942) [1941]
Idiocercus B. Sutton, Can. J. Bot. 45(8): 1255 (1967)
Jahniella Petr., Ann Mycol. 18(4/6): 123 (1921) [1920]
Kabatia Bubák, in Kabát & Bubák, Öst. bot. Z. 54: 28 (1904)
Keissleriella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 128(7–8): 582 (1919)
Keissleriella quadriseptata Kaz. Tanaka & K. Hiray., in Tanaka et al., Stud. Mycol. 82: 96 (2015)
Kellermania Ellis & Everh., J. Mycol. 1(12): 153 (1885)
Kendrickomyces B. Sutton, V.G. Rao & Mhaskar, Trans. Br. Mycol. Soc. 67(2): 243 (1976)
Koorchaloma Subram., J. Indian bot. Soc. 32: 124 (1953)
Koorchaloma bambusae Nag Raj, Mycotaxon 19: 175 (1984)
Koorchaloma jamaicensis Nag Raj, Mycotaxon 19: 179 (1984)
Koorchaloma krabiense (Tibpromma & K.D. Hyde) W.J. Li & K.D. Hyde, comb. nov.
Koorchaloma occidentale Nag Raj, Mycotaxon 19: 191 (1984)
Koorchaloma okamurae I. Hino & Katum., Icones Fungorum Bamb. Jap.: 264 (1961)
Leptodermella Höhn., Z. Gärungsphysiol. 5: 212 (1915)
Leptosphaeria Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 234 (1863)
Leptosphaeria sydowii (Boerema, Kesteren & Loer.) Gruyter, Aveskamp & Verkley, in Gruyter et al., Stud. Mycol. 75: 20 (2012) [2013]
Leptothyrina Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 123 (1915)
Leptotrochila P. Karst., Bidr. Känn. Finl. Nat. Folk 19: 245 (1871)
Leptotrochila medicaginis (Fuckel) Schüepp, Phytopath. Z. 36: 253 (1959)
Leptoxyphium Speg., Physis, Rev. Soc. Arg. Cienc. Nat. 4(no. 17): 294 (1918)
Leptoxyphium fumago (Woron.) R.C. Srivast., Arch. Protistenk. 125(1–4): 333 (1982)
Libartania Nag Raj, Can. J. Bot. 57(13): 1390 (1979)
Ligniella Naumov, Mater. Mikol. Fitopat. Ross. 5(1): 5 (1926)
Marasasiomyces Crous, Phytotaxa 202(2): 86 (2015)
Marasasiomyces karoo (B. Sutton & Marasas) Crous, Phytotaxa 202(2): 86 (2015)
Mastigosporella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1, 123: 135 (1914)
Melanops Nitschke ex Fuckel, Jb. nassau. Ver. Naturk. 23-24: 225 (1870) [1869-70]
Melanops fagicola W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Melanops tulasnei Fuckel, Jb. nassau. Ver. Naturk. 23-24: 225 (1870) [1869-70]
Metazythia Petr., Sydowia 4(1–6): 373 (1950)
Micraspis Darker, Can. J. Bot. 41(10): 1390 (1963)
Microdiscula Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1, 124: 142 (1915)
Microperella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 879 (1909)
Mirimyces Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 477 (1993)
Mirimyces pulcher Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 478 (1993)
Monochaetiella E. Castell., Nuovo G. bot. ital. 49: 487 (1943) [1942]
Monochaetiellopsis B. Sutton & DiCosmo, Can. J. Bot. 55(19): 2536 (1977)
Monodia Breton & Faurel, Revue Mycol., Paris 35(1): 23 (1970)
Monodia minor Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 526 (1993)
Monostichella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 95 (1916)
Mucoharknessia Crous, R.M. Sánchez & Bianchin., Phytotaxa 202(2): 86 (2015)
Mucoharknessia anthoxanthi Dissanayake, Camporesi & K.D. Hyde, in Li et al., Fungal Diversity 78: [19] (2016)
Mycotribulus Nag Raj & W.B. Kendr., Can. J. Bot. 48(12): 2219 (1970)
Mycotribulus mirabilis Nag Raj & W.B. Kendr., Can. J. Bot. 48(12): 2219 (1970)
Myriellina Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 100 (1915)
Myxothyrium Bubák & Kabát, Svensk bot. Tidskr. 9: 379 (1915)
Nanoschema B. Sutton, The Coelomycetes: 589 (1980)
Nectria (Fr.) Fr., Summa veg. Scand., Sectio Post. (Stockholm): 387 (1849)
Nectria dematiosa (Schwein.) Berk., N. Amer. Fung.: no. 154 (1873)
Neoascochyta Qian Chen & L. Cai, Stud. Mycol. 82: 198 (2015)
Neoascochyta dactylidis W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Neoascochyta desmazieri (Cavara) Qian Chen & L. Cai, Stud. Mycol. 82: 198 (2015)
Neoascochyta tardicrescens Valenz.-Lopez, Cano, Crous, Guarro & Stchigel, in Valenzuela-Lopez et al. Stud. Mycol. 90: 44 (2017)
Neochaetospora B. Sutton & Sankaran, Mycol. Res. 95(6): 768 (1991)
Neocucurbitaria Wanas., E.B.G. Jones & K.D. Hyde, et al., Mycosphere 8(3): 408 (2017)
Neocucurbitaria sp.
Neodermea W.J. Li, D.J. Bhat & K.D. Hyde, gen. nov.
Neodermea acerina (Peck) Rehm) W.J. Li & K.D. Hyde, comb. nov.
Neodermea rossica W.J. Li, D.J. Bhat & K.D. Hyde, sp. nov.
Neodidymelliopsis Qian Chen & L. Cai, Stud. Mycol. 82: 207 (2015)
Neodidymelliopsis negundinis Manawasinghe, Camporesi & K.D. Hyde, in Hyde et al., Mycosphere 9(2): 295 (2018)
Neodidymelliopsis ranunculi W.J. Li & K.D. Hyde, in Hyde et al., Fungal Diversity: [41] (2016)
Neofusicoccum Crous, Slippers & A.J.L. Phillips, Stud. Mycol. 55: 247 (2006)
Neofusicoccum algeriense Berr.-Tebb. & A.J.L. Phillips, in Berraf-Tebbal, Guerreiro & Phillips, Fungal Diversity 53: 423 (2014)
Neofusicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips, in Crous et al., Stud. Mycol. 55: 248 (2006)
Neofusicoccum sinoeucalypti G.Q. Li & S.F. Chen, in Li et al., Persoonia 40: 88 (2017)
Neogloeosporidina W.J. Li, Camporesi & K.D. Hyde, gen. nov.
Neogloeosporidina pruni W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Neoplaconema B. Sutton, Kew Bull. 31(3): 463 (1977)
Neopyrenochaeta Valenz.-Lopez, Crous, Stchigel, Guarro & Cano, in Valenzuela-Lopez et al., Stud. Mycol. 90: 54 (2017)
Neopyrenochaeta annellidica W.J. Li, Z.H. Zhang & K.D. Hyde, sp. nov.
Neopyrenochaeta chiangraiensis Z.L. Luo, W.J. Li & K.D. Hyde, sp. nov.
Neopyrenochaeta maesuayensis Z.L. Luo, W.J. Li & K.D. Hyde, sp. nov.
Neottiospora Desm., Annls Sci. Nat., Bot., sér. 2 19: 346 (1843)
Neottiospora caricina (Desm.) Höhn., in Weese, Mitt. bot. Inst. tech. Hochsch. Wien 1(3): 78 (1924)
Neozythia Petr., Sydowia 11(1–6): 351 (1958) [1957]
Oncosporella P. Karst., Meddn Soc. Fauna Flora fenn. 14: 105 (1887)
Pestalozziella Sacc. & Ellis ex Sacc., Michelia 2(no. 8): 575 (1882)
Pestalozziella subsessilis Sacc. & Ellis, Michelia 2(no. 8): 575 (1882)
Pestalozziella artocarpi Nag Raj & W.B. Kendr., Can. J. Bot. 50(3): 609 (1972)
Pestalozziella parva Nag Raj, Trans. Br. Mycol. Soc. 52(2): 205 (1969)
Pezicula Tul. & C. Tul., Select. fung. carpol. (Paris) 3: 182 (1865)
Pezicula italica W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Phacidiella P. Karst., Hedwigia 23(6): 85 (1884)
Phacidium Fr., Observ. mycol. (Havniae) 1: 167 (1815)
Phacidium anomala (Nag Raj) W.J. Li & K.D. Hyde, comb. nov.
Phacidium italicum W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Phacidium foliicola (Lib.) W.J. Li & K.D. Hyde, comb. nov.
Phacidium subcorticalis (Fuckel) W.J. Li & K.D. Hyde, comb. nov.
Phellostroma Syd. & P. Syd., Philipp. J. Sci., C, Bot. 9(2): 185 (1914)
Phialophorophoma Linder, Farlowia 1(3): 402 (1944) [1943–1944]
Phloeosporella Höhn., Öst. bot. Z. 66: 106 (1916)
Phlyctaeniella Petr., Ann Mycol. 20(5/6): 323 (1922)
Phlyctema Desm., Annls Sci. Nat., Bot., sér. 38: 16 (1847)
Phlyctema coronillae W.J Li, Camporesi & K.D. Hyde, sp. nov.
Phyllosticta Pers., Traité champ. (Paris): 55, 147 (1818)
Phyllosticta pervincae Bissett & Darbysh., Fungi Canadenses, Ottawa: no. 282 (1984)
Phyllosticta plumbaginicola V.G. Rao, Publicações Inst. Micol. Recife 383: 6 (1963)
Phyllosticta sphaeropsoidea Ellis & Everh., Bull. Torrey bot. Club 10(9): [97] (1883)
Phyllosticta sp.
Pilidium Kunze, in Kunze & Schmidt, Mykologische Hefte (Leipzig) 2: 92 (1823)
Placonema (Sacc. & D. Sacc.) Petr., Ann Mycol. 19(1/2): 60 (1921)
Placothyrium Bubák, Ber. dt. bot. Ges. 34: 302 (1916)
Plectronidiopsis Nag Raj, Can. J. Bot. 57(13): 1397 (1979)
Pleurophoma Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 117 (1914)
Pleurophomopsis Petr., Ann Mycol. 22(1/2): 156 (1924)
Pleurophomopsis sp.
Pleurothyrium Bubák, Ber. dt. bot. Ges. 34: 322 (1916)
Polyphialoseptoria Quaedvl., R.W. Barreto, Verkley & Crous, Stud. Mycol. 75: 355 (2013)
Polyphialoseptoria sp.
Polystigma DC., in de Candolle & Lamarck, Fl. franç., Edn 3 (Paris) 6: 164 (1815)
Pragmopora A. Massal., Framm. Lichenogr.: 12 (1855)
Pragmopora pithya (Fr.) J.W. Groves, Can. J. Bot. 45: 176 (1967)
Proboscispora Punith., Nova Hedwigia 39(1–2): 63 (1984)
Pseudobasidiospora Dyko & B. Sutton, Nova Hedwigia 29(1–2): 168 (1978) [1977]
Pseudobasidiospora caroliniana Dyko & B. Sutton, Nova Hedwigia 29(1–2): 169 (1978) [1977]
Pseudocoleophoma Kaz. Tanaka & K. Hiray., in Tanaka et al., Stud. Mycol. 82: 89 (2015)
Pseudocoleophoma flavescens (Gruyter, Noordel. & Boerema) W.J. Li & K.D. Hyde, comb. nov.
Pseudocoleophoma rusci W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Pseudolachnea Ranoj., Ann Mycol. 8(3): 393 (1910)
Pseudolachnea hispidula (Schrad.) B. Sutton, Mycol. Pap. 141: 167 (1977)
Pseudolachnella Teng, Sinensia, Shanghai 7: 775 (1936)
Pseudolachnella brevifusiformis A. Hashim. & Kaz. Tanaka, in Li et al., Fungal Diversity 78: [74] (2016)
Pseudolachnella coronata (I. Hino & Katum.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 725 (1993)
Pseudolachnella longiciliata (I. Hino & Katum.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 725 (1993)
Pseudolachnella ryukyuensis (I. Hino & Katum.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 729 (1993)
Pseudolachnella setulosa (I. Hino & Katum.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 732 (1993)
Pseudoneottiospora Faurel & Schotter, Revue Mycol., Paris 29(4): 278 (1965) [1964]
Pseudoneottiospora coprophila (Spegazzini) Breton & Faurel apud Nag Raj
Pseudorobillarda M. Morelet, Bull. Soc. Sci. nat. Arch. Toulon et du Var 175: 5 (1968)
Pseudorobillarda bambusae Nag Raj, Morgan-Jones & W.B. Kendr., Can. J. Bot. 50(4): 864 (1972)
Pseudorobillarda eucalypti N. Tangthirasunun & K.D. Hyde, in Tangthirasunun, et al., Phytotaxa 176(1): 255 (2014)
Pseudorobillarda indica Nag Raj, Morgan-Jones & W.B. Kendr., Can. J. Bot. 50(4): 865 (1972)
Pseudorobillarda magna Bianchin., Mycol. Res. 101(10): 1233 (1997)
Pseudorobillarda parasiamensis N. Tangthirasunun, W.J. Li & K.D. Hyde, sp. nov.
Pseudorobillarda phragmitis (Cunnell) M. Morelet, Bull. Soc. Sci. nat. Arch. Toulon et du Var 175: 6 (1968)
Pseudorobillarda sojae Uecker & Kulik, Mycologia 78(3): 450 (1986)
Pseudoseptoria Speg., Anal. Mus. nac. B. Aires, Ser. 3 13: 388 (1911)
Pseudostegia Bubák, J. Mycol. 12(2): 56 (1906)
Pseudothyrium Höhn., in Weese, Mitt. bot. Inst. tech. Hochsch. Wien 4(3): 109 (1927)
Pseudozythia Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 111: 1019 (1902)
Pullospora Faurel & Schotter, Revue Mycol., Paris 29(4): 280 (1965) [1964]
Pullospora macrospora Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 748 (1993)
Pullospora tetrachaeta Faurel & Schotter, Revue Mycol., Paris 29(4): 280 (1965) [1964]
Pycnopeziza W.L. White & Whetzel, Mycologia 30(2): 187 (1938)
Pycnopeziza americanum (Nag Raj) W.J. Li & K.D. Hyde, comb. nov.
Pycnovellomyces R.F. Castañeda, Fungi Cubenses II (La Habana): 16 (1987)
Pycnovellomyces foliicola R.F. Castañeda, Fungi Cubenses II: 16 (1987)
Quaternaria Tul. & C. Tul., Select. fung. carpol. 2: 104 (1863)
Quaternaria quaternata (Pers.) J. Schröt., in Cohn, Krypt.-Fl. Schlesien 3.2(4): 451 (1897) [1908]
Rhabdocline Syd., in Sydow & Petrak, Ann Mycol. 20(3/4): 194 (1922)
Rhabdocline weirii A.K. Parker & J. Reid, Can. J. Bot. 47: 1540 (1969)
Rhabdogloeopsis Petr., Ann Mycol. 23(1/2): 52 (1925)
Rhodesia Grove, British Stem- and Leaf-Fungi (Coelomycetes) 2: 205 (1937)
Rhodesiopsis B. Sutton & R. Campb., Nova Hedwigia 30: 289 (1979) [1978]
Rhodesiopsis gelatinosa B. Sutton & R. Campb., Nova Hedwigia 30: 290 (1979) [1978]
Rhodosticta Woron., Izv. Imp. St.-Peterburgsk. Bot. Sada 11: 13 (1911)
Rhytisma Fr., K. svenska Vetensk-Akad. Handl., ser. 3 40: 104 (181 9)
Rileya A. Funk, Can. J. Bot. 57(1): 7 (1979)
Sakireeta Subram. & K. Ramakr., J. Indian bot. Soc. 36: 83 (1957)
Sakireeta madreeya Subram. & K. Ramakr., J. Indian bot. Soc. 36: 84 (1957)
Sarcophoma Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 75 (1916)
Satchmopsis B. Sutton & Hodges, in Sutton, Nova Hedwigia 26(1): 1 (1975)
Scaphidium Clem., Bot. Surv. Nebraska 5: 5 (1901)
Scopaphoma Dearn. & House, N.Y. St. Mus. Bull. 266: 83 (1925)
Scopaphoma corioli Dearn. & House, N.Y. St. Mus. Bull. 266: 83 (1925)
Scorias Fr., Syst. mycol. 3(2): 269, 290 (1832)
Septopatella Petr., Ann Mycol. 23(1/2): 128 (1925)
Septoria Sacc., Syll. fung. (Abellini) 3: 474 (1884)
Sirococcus Preuss, Linnaea 26: 716 (1855) [1853]
Sirococcus conigenus (Pers.) P.F. Cannon & Minter, Taxon 32(4): 577 (1983)
Sirophoma Höhn., Hedwigia 59(5): 257 (1917)
Siroplacodium Petr., in Rechinger et al., Annln naturh. Mus. Wien 50: 509 (1940)
Sirozythiella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 1532 (1909)
Sphaerellopsis Cooke, Grevillea 12(no. 61): 23 (1883)
Sphaerellopsis anomala Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 896 (1993).
Sphaerellopsis filum (Biv.) B. Sutton, Mycol. Pap. 141: 196 (1977)
Sphaeriothyrium Bubák, Ber. dt. bot. Ges. 34: 299 (1916)
Sphaerographium Sacc., Syll. fung. 3: 596 (1884)
Stagonospora (Sacc.) Sacc., Syll. fung. (Abellini) 3: 445 (1884)
Stagonospora cylindrica (B. Sutton & Alcorn) W.J. Li & K.D. Hyde, comb. nov.
Stagonospora forlicesenensis Phukhams., Camporesi & K.D. Hyde, in Hyde et al., Fungal Diversity: [77] (2016)
Stamnaria Fuckel, Jb. nassau. Ver. Naturk. 23-24: 309 (1870) [1869-70]
Staurophoma Höhn., Denkschr. Kaiserl. Akad. Wiss., Math.-Naturwiss. Kl. 83: 34 (1907)
Stictosepta Petr., Sydowia 17: 230 (1964) [1963]
Stilbophoma Petr., Bot. Arch. 43: 93 (1942) [1941]
Strasseria Bres. & Sacc., in Strasser, Verh. zool.-bot. Ges. Wien 52: 436 (1902)
Strasseriopsis B. Sutton & Tak. Kobay., Mycologia 61(6): 1068 (1970) [1969]
Strigula Fr., Syst. mycol. (Lundae) 2(2): 535 (1823)
Strigula cylindrospora (Syd. & P. Syd.) W.J. Li & K.D. Hyde, comb. nov.
Suttoniella S. Ahmad, Biologia, Lahore 6: 257 (1961) [1960]
Tetranacrium H.J. Huds. & B. Sutton, Trans. Br. Mycol. Soc. 47(2): 202 (1964)
Thoracella Oudem., Ned. kruidk. Archf, 3 sér. 2: 267 (1901)
Thrinacospora Petr., Sydowia 2(1–6): 49 (1948)
Thyronectria Sacc., Grevillea 4(no. 29): 21 (1875)
Thyrsidina Höhn., Ann Mycol. 3(4): 337 (1905)
Tiarosporella Höhn., in Weese, Ber. dt. bot. Ges. 37: 159 (1919)
Tiarosporella graminis (Piroz. & Shoemaker) Nag Raj, Can. J. Bot. 51(12): 2469 (1974) [1973]
Tiarosporella paludosa (Sacc. & Fiori) Höhn., in Weese, Ber. dt. bot. Ges. 37: 159 (1919)
Towyspora Wanasinghe, E.B.G. Jones & K.D. Hyde, in Li et al., Fungal Diversity 78: [32] (2016)
Towyspora aestuari Wanasinghe, E.B.G. Jones & K.D. Hyde, in Li et al., Fungal Diversity 78: [35] (2016)
Tracylla (Sacc.) Tassi, Boll. R. Orto Bot. Siena, 6: 62. (1904); non Tracyella Zambet-takis (nom. illegit.) in Rev. Mycol. 35: 165. (1970)
Trematophoma Petr., Ann Mycol. 22(1/2): 152 (1924)
Tribolospora D.A. Reid, Aust. J. Bot. 14: 31 (1966)
Tryblidiopsis P. Karst. [as ‘Triblidiopsis’], Bidr. Känn. Finl. Nat. Folk 19: 262 (1871)
Tympanis Tode, Fung. mecklenb. sel. (Lüneburg) 1: 24 (1790)
Tympanis spermatiospora (Nyl.) Nyl., Not. Sällsk. Fauna et Fl. Fenn. Förh., Ny Ser. 10: 70 (1868) [1869]
Vestigium Piroz. & Shoemaker, Can. J. Bot. 50(6): 1163 (1972)
Xenidiocercus Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia : 975 (1993)
Yalomyces Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 988 (1993)
Yalomyces pulcher Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 988 (1993)
Yoshinagaia Henn., Hedwigia 43(2): 143 (1904)
Yoshinagaia quercus Henn., Hedwigia 43(2): 143 (1904)
Zelandiocoela Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 990 (1993)
Zelandiocoela ambigua (Nag Raj & W.B. Kendr.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 991 (1993)
Zelosatchmopsis Nag Raj, in Saikawa, Castañeda Ruiz, Kendrick & Nag Raj, Can. J. Bot. 69(3): 633 (1991)
Zelosatchmopsis sacciformis (R.F. Castañeda) Nag Raj & R.F. Castañeda, in Saikawa, Castañeda Ruiz, Kendrick & Nag Raj, Can. J. Bot. 69(3): 633 (1991)
Zunura Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 1006 (1993)
Introduction
Classification of coelomycetes
The term “Coelomycetes” was introduced by Grove (1919), to recognize three genera, Phloeospora, Phyllosticta and Phomopsis (Sutton 1980; Wijayawardene et al. 2016). Subsequently, Grove (1935, 1937) expanded this initial concept and used coelomycetes for all genera that produced conidia within a cavity or cushion-like fungal matrix. Such fruiting bodies (today conidiomata) were termed pycnidia (flask-like structures), acervuli (cushion-like structures) and sporodochium (pulvinate stroma). Grove provided a major morphological account of all British coelomycetes known at that time.
Morphological characters (conidiomata, conidiophores, conidiogenous cells, conidia, behavior in artificial culture) and host association have been the main features used to identify and classify asexual fungi (Morgan-Jones et al. 1972a, b, c, d, e, f; Sutton 1973a, 1977a, 1980; Nag Raj and DiCosmo 1978, 1980; Nag Raj 1981b, 1993; Abbas et al. 1997, 1998). Sutton (1980) published a major revision of coelomycetes, which he divided into six suborders (Blastopycnidiineae, Blastostromatineae Phialopycnidiinea, Phialostromatineae, Thallopycnidiineae, Thallostromatineae), mainly based on conidiogenesis. Nag Raj (1993) provided a major account of coelomycetes that had appendage-bearing conidia. This publication gave diagnostic descriptions and illustrations for 142 genera and over 200 species. The number of coelomycete species grew dramatically, so that by the twenty-first century Kirk et al. (2008) documented approximately 1000 genera (plus 500 synonyms) comprising 7000 species. Although, generations of vast morphological and anatomical data over the years had built up a strong base for taxonomic studies of coelomycetes (Sutton 1980; Nag Raj 1981b, 1993), these lacked satisfactory taxonomic resolution and often resulted in considerable ambiguity and confusion in the intergeneric or interspecific classification of coelomycetes (Crous et al. 2006a; De Gruyter et al. 2009; Hyde et al. 2013).
The suborders described by Sutton (1980) were later shown, from an evolutionary perspective, to be based on an artificial system (De Gruyter et al. 2009, 2010). For instance, genera described in Phialopycnidiinea could be assigned to different orders, such as Botryosphaeriales, Diaporthales, Dothideales, Helotiales and Pleosporales (de Gruyter et al. 2009, 2013).
Coelomycetes is no longer a valid taxonomic name, and the term was actually coined for convenience (Taylor 1995; Kendrick 2000; Wijayawardene et al. 2012a). Members of coelomycetes are distributed in Dothideomycetes, Leotiomycetes, and Sordariomycetes (Wijayawardene et al. 2016), and even Basidiomycetes (Nag Raj 1981b, 1993; Rungjindamai et al. 2008; Tangthirasunun et al. 2014b; He et al. 2019).
Many pleomorphic fungi (e.g., Ascochyta, Broomella, Coniothyrium, Dermea, Didymella, Heterosphaeria, Phoma) can potentially propagate through different modes of reproduction at different geographical locations and at different phases (Nag Raj 1979a, b; Weresub and Pirozynski 1979; Carmichael 1981; Sivanesan 1984; Seifert and Samuels 2000; Li et al. 2015b), failing to reflect in phylogenetic relationships and determine the affiliation between asexual and sexual states.
DNA sequence-based phylogenetic studies have dramatically influenced both the taxonomy and systematics of the coelomycetes since the 1990s (Mills et al. 1992; Sreenivasaprasad et al. 1992; Sherriff et al. 1994; Adams et al. 2002; Shenoy et al. 2007; Cai et al. 2009; De Gruyter et al. 2009, 2013; Cannon et al. 2012; Liu et al. 2012). Molecular data allows linkage of asexual taxa with their sexual morphs (Shenoy et al. 2006, 2010). Considerable re-evaluations are needed to understand the family placements, generic concepts and species limits in coelomycetes (Aveskamp et al. 2008; De Gruyter et al. 2009, 2010; Maharachchikumbura et al. 2011, 2012, 2014; Crous et al. 2012b; Wijayawardene et al. 2012a, b, 2016; Senanayake et al. 2017, 2018). Through a study of the combined LSU and ITS sequence data, Crous et al. (2015b) showed that Tiarosporella is a poly- and paraphyletic genus. Species of this genus recognized by Nag Raj (1993) could be classified in the Botryosphaeriaceae (Dothideomycetes) and Phacidiaceae (Leotiomycetes). Similarly, camarosporium-like, phoma-like, pyrenochaeta-like, and septoria-like taxa were shown to be polyphyletic within Pleosporales (De Gruyter et al. 2010; Quaedvlieg et al. 2013; Crous et al. 2014a, b; Wanasinghe et al. 2014, 2017; Wijayawardene et al. 2014a, c, 2016a, b; Ertz et al. 2015; Phookamsak et al. 2014; Hyde et al. 2016; Crous and Groenewald 2017; Valenzuela-Lopez et al. 2018). More importantly, phylogenetic advancement has now been used to integrate presumably asexual morphs into sexual morphs at the class or higher level (Rungjindamai et al. 2008; Tangthirasunun et al. 2014b; Crous et al. 2015a, b, 2016b; Wijayawardene et al. 2016), order (Tangthirasunun et al. 2014a), family (Liu et al. 2019), genus (Liu et al. 2012; Phillips et al. 2013) and species e.g. Colletotrichum acutatum-Glomerella acutata, Discostroma massarinum- Seimatosporium salicinum (Guerber and Corrcell 2001; Tanaka et al. 2011; Li et al. 2015b).
A major change effecting the nomenclature of coelomycetous fungi is the new ruling in Article 59.1 of International Code of Nomenclature for Algae, Fungi, and Plants (ICN; Melbourne Code) (Hawksworth 2012). These changes, known as “one fungus, one name”, have resulted in the implementation of a single name for one fungus, where formerly separate names were allowed for both asexual and sexual states (Hawksworth 2012; Wingfield et al. 2012). Based on this ruling, several papers have been published with recommendations for use or protection of competing generic names in major groups of Dothideomycetes (Wijayawardene et al. 2014b; Rossman et al. 2015b), Leotiomycetes (Braun 2013; Johnston et al. 2014), Pezizomycetes (Healy et al. 2016) and Sordariomycetes (Stadler et al. 201 3; Rossman et al. 2013; Quandt et al. 2014; Rossman et al. 2015a; Réblová et al. 2016). In an attempt to improve the classification of coelomycetes, Wijayawardene et al. (2016) illustrated, described, and provided taxonomic notes for 235 dematiaceous coelomycetous genera. Of these genera, 152 have been placed in a natural classification system based on molecular data. While 83 genera were treated as Ascomycota, genera incertae sedis, because of a lack of sequence data. Although this study made a major contribution to the taxonomy and classification of coelomycetes, the placement of many genera, especially the hyaline-spored coelomycetes, is still undetermined.
With such vast amounts of literature in disparate publications and huge changes in the taxonomy, phylogeny and nomenclature of coelomycetes, there is a need to compile all data into a single document. The dematacious coelomycetes were monographed by Wijayawardene et al. (2016) and the present paper reviews the genera and species of hyaline-spored taxa of coelomycetes. With the trend towards providing data in readily accessable and updated websites (e.g. phytopathogenic genera—Jayawardena et al. 2019, Dothideomycetes—Pem et al. 2019; Index Fungorum 2020), we will publish a database, www.coelomycetes.org, in the future.
In this study, fresh material was collected from China, Italy, Russia and Thailand. Where possible, specimens of the type species for each genus were examined to determine their morphological characteristics. The primary objectives were to: (1) provide a phylogenetic overview of genera and/or species of hyaline-spored coelomycetes; (2) delimit modern genera or species circumscriptions; (3) resolve nomenclatural issues by identifying redundant names and synonyms; (4) establish taxonomic links between sexual and asexual genera; and (5) provide updated outlines, descriptions, illustrations, and taxonomic notes for these genera and species. Although we have included most coelomycete genera in this monograph, we have not included the coelomycetous asexual morphs of several sexually defined genera (e.g Astrosphaeriella, Phookamsak et al. (2015) and Roussoella (Liu et al. 2014).
Diagnostic characters of coelomycetes
A phylogenetic approach has placed many polyphyletic genera e.g., Camarosporium, Coniothyrium, Phoma, Pyrenochaeta and species complex genera e.g., Colletotrichum, Truncatella into a natural classification (Chen et al. 2015, 2017; Wanasinghe et al. 2017; Valenzuela-Lopez et al. 2018; Liu et al. 2019). However, this approach sometimes adds no further discriminating power to delineate a group of fungi where phylogenetic resolution is weak, there is inadequate taxon sampling, or molecular data is missing (Jeewon and Hyde 2016). Liu et al. (2019) stated that 30 genera were delimited in Sporocadaceae based on multi loci phylogenetic analyses, which is generally congruent with the morphology-based classification system proposed by Nag Raj (1993). Thus, morphological characters of conidiomata, conidiophores, conidiogenous cells, paraphyses, and conidia are also important in the identification of coelomycetous asexual morphs (Sutton 1973b, 1980; Nag Raj 1981b, 1993; Wijayawardene et al. 2016).
Conidiomata
Kendrick (1979) proposed the term conidioma (pl. conidiomata) for asexual fungi that produce conidia in specialized structures. These structures are diverse and vary in form from acervular to cupulate, pycnidial, sporodochial, synnematous and pycnothyrial (Sutton 1980; Nag Raj 1981b, 1993). The typical concepts and characters are outline below:
Acervular conidiomata (Fig. 1a–e) can be subcuticular, intraepidermal, or subepidermal in the host tissues. The hymenium develops beneath overlapping host tissue. Conidiophores or conidiogenous cells are restricted to the basal stroma. At maturity, the overlapping tissues are ruptured by the developing mass of conidia (e.g. Colletotrichum, Rhabdocline, Pycnopeziza) (Sutton 1980; Nag Raj 1981b, 1993).
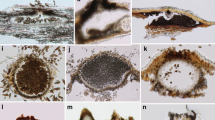
Vertical sections through conidiomata. a–c, e, g Acervular conidiomata, aEriosporella calami, bRhabdocline weirii, c asexual morph of Pycnopeziza americanum (= Acarosporium americanum), eDiplocarpon mespili, gColletotrichum sp. d Cupulate conidiomata in Zelosatchmopsis sacciformis. f, h–v Different shapes of pycnidial conidiomata, fCorniculariella rhamni, h asexual morph of Leptosphaeria sydowii, iChaetosphaeronema hispidulum, jChaetospermum camelliae, kAllantophomopsiella pseudotsugae, lChaetoconis polygoni, mNeofusicoccum sinoeucalypti, nBrycekendrickia indica, oChaetoconis vaccinia, pClohesyomyces aquaticus, qGiulia tenuis, rPseudorobillarda bambusae, sCytospora tanatica, tDarkera abietis, uSphaerellopsis anomala,vPseudocoleophoma rusci. Scale bars a, b, e–i, l–r, t, v = 100 µm, c, s = 500 µm, d, u = 50 µm, j–k = 200 µm
Cupulate conidiomata (Fig. 2d, h–i, n–p) are regarded as a transitional form between synnematal, sporodochial, and pycnidial conidiomata. They are usually superficial, with variable excipular development and adorned with sterile hyphae or setae (e.g. Dinemasporium, Dwayalomella, Koorchaloma, Pseudolachnella, Pseudolachnella) (Okada and Tubaki 1987; Sutton 1980; Nag Raj 1993).
Vertical sections through conidiomata or side view of stalked pycnidia or synnematous conidiomata. a–g, l–m, t–u Pycnidial conidiomata, aMelanops tulasnei, bPhacidium italicum, cMelanops fagicola, dYoshinagaia quercus (asexual morph), e, fCapnodium paracoartatum, gCytospora phialidica, lHeterosphaeria patella (asexual morph), mNeottiospora caricina. t–uConidiocarpus siamensis, h–i, n–p Cupulate conidiomata, hDwayalomella vaccinii,iPseudolachnella setulosa, nPseudolachnella longiciliata, oPseudolachnea hispidula, pPseudolachnella brevifusiformis. j–k Sprodochial conidiomata, jCrucellisporiopsis gelatinosa, kNectria dematiosa. q–s Synnematous conidiomata, qConicomyces contortus, rLeptoxyphium sp., sCrucellisporiopsis prolongata. Scale bars a, c = 200 µm, b–d, k = 500 µm, e, f, r, = 20 μm, g, h, i = 200 µm, j, l–n, p–q, t–u = 100 µm, o = 50 µm
Different types of conidiomatal wall. a, bTextura angularis and textura prismatica in Yoshinagaia quercus. cTextura prismatica in Zelosatchmopsis sacciformis. d, eTextura oblita in Cytospora prunicola. f, gTextura intricata in Cytospora sp. hTextura angularis in Neoascochyta desmazieri. iTextura intricata in Colletotrichum sansevieriae. jTextura globosa in Neoscytalidium orchidacearum. kTextura globosa to textura angularis in Botryosphaeria torilicola. ltextura oblita to textura epidermoidea in Aschersonia calendulina. mTextura porrecta at the periphery in Pseudolachnea hispidula. nTextura intricata to textura oblita in Chaetospermum camelliae. oTextura epidermoidea in Ciliosporella selenospora. Scale bars a–d, f–g, j, l, n–o = 50 µm, e, h, i, k = 20 µm, m = 10 µm
Pycnidial conidiomata (Figs. 1f, h–v, 2a–g, l–m, t–u) can be simple or compound, stromatic, superficial immersed or erumpent, unilocular or multilocular. The conidia form more or less completely enclosed by fungal tissue. Conidiophores or conidiogenous cells line the entire cavity. There is a fairly well defined ostiole through which the conidia are released and well develop wall tissue (e.g. Clohesyomyces, Chaetoconis, Giulia, Heterosphaeria, Melanops, Neottiospora, Yoshinagaia) (Sutton 1980; Nag Raj 1981b, 1993).
Sporodochial conidiomata (Fig. 2j–k) with superficial pulvinate stroma supporting conidiophores or conidiogenous cells on its upper surface and not covered by the substrate, with or without a basal stroma (e.g. Crucellisporiopsis, Nectria) (Sutton 1980; Nag Raj 1993).
Synnematous conidiomata (Fig. 2q–s) consisting of a compacted group of erect and fused hyphae, the apices and occasionally intercalary cells of which function as conidiophores and conidiogenous cells (e.g. Conicomyces, Crucellisporiopsis, Leptoxyphium) (Sutton 1980; Nag Raj 1993).
Pycnothyrial conidiomata are generally flattened, elongated, shield-shaped, hemispherical, superficial, comprised of radiating cells, sometimes with only an upper wall, sometimes also with a basal wall (e.g. Diedickea, Tracylla) (Sutton 1980; Nag Raj 1993) (Fig. 3).
Conidiomatal wall
The wall tissue of coelomycetes have usually been categorized simply as pseudoparenchymatous or plectenchymatous, but they can be actually recognized in detail types (textura angularis, textura epidermoidea, textura intricata, textura globosa, textura prismatica, textura porrecta and textura oblita) (Sutton 1980; Nag Raj 1993).
Conidiophores, conidiogenous cells
In coelomycetes, the conidiophores are mainly hyaline, occasionally pigmented (Annellolacinia, Arthrinium, Xepiculopsis), branched or unbranched, arising from inner layer cells of cavity. In some genera (e.g. Cenangiomyces, Ellula, Fibulocoela and Pycnovellomyces), conidiophores possess clamp connections. In many cases, the conidiophores are reduced to conidiogenous cells (e.g. Ascochyta, Mirimyces, Neodidymelliopsis, Phoma) that are indistinguishable from cells of the wall (Sutton 1980; Nag Raj 1981b, 1993). Three major types of conidiogenous cells are recognized in coelomycetes. They are holoblastic, enteroblastic and thallic (Sutton 1980; Nag Raj 1993; Kendrick 2000). The different types of conidiophores and conidiogenous cells are illustrated in Fig. 4.
Different types of conidiogenous cells and conidiophores. a–b, m Phialidic conidiogenous cells in Colletotrichum sansevieriae and Mirimyces pulcher, respectively. c, f, d–e Annellidic conidiogenous cells in Botryosphaeria artemisiae and Comatospora suttonii, respectively. g, j Sympodial conidiogenous cells in Heterosphaeria umbilicata. h, i Conidiophores with terminal or acropleurogenous conidiogenous cells in Nectria dematiosa and Allantophomopsiella pseudotsugae, respectively. k, l Thallic conidiogenous cells in asexual morph of Pycnopeziza americanum (= Acarosporium americanum). n Conidiophores with clamp connections (arrows) in Pycnovellomyces foliicola. o, p Holoblastic conidiogenous cells in Botryosphaeria dothidea and Melanops fagicola, respectively. Scale bars a–k, n–p = 10 µm, l = 20 µm, m = 5 µm
Conidia
Conidia of coelomycetes display the greatest variation between genera and species. These can be hyaline or pigmented, amerosporous, didymosporous, phragmosporous, dictyosporous (Thyrsidina carneominiata, Yalomyces pulcher), scolecosporous, and even more complex and composite staurosporous forms in (Tribolospora sycopsidis and Geastrumia polystigmatis) and others (Eriosporella calami, Vestigium felicis, Tetranacrium gramineum) (Nag Raj 1981a, b, 1993; Abbas et al. 1997, 1998). The different types of conidia encountered in coelomycetes are illustrated in Figs. 5, 6.
Different types of conidia. a Conidia with single appendage in Rhabdocline weirii. b Conidia with branched appendage in Pestalozziella subsessilis. cPullospora macrospora. dChaetospermum camelliae. eBrycekendrickia indica. fDinemasporium pingue. gPseudobasidiospora caroliniana. h Conidia with mucoid appendage in Neottiospora caricina. i, j Conidia with mucoid appendage in Mucoharknessia anthoxanthi. k, l, m Conidia with mucoid appendage in Tiarosporella paludosa. n, oEutiarosporella tritici. p, qDarkera pseudotsugae and D. picea, respectively. r Muriform conidia with mucoid appendages in Yalomyces pulcher. sAlloneottiosporina thailandica. tClohesyomyces aquaticus. u Conidia with mucoid appendage in Stagonospora forlicesenensis (arrow). v, wColletotrichum sp. xAllantophomopsiella pseudotsugae. yChaetosphaeronema hispidulum. Scale bars a, b, d, f, i–p, t, u = 10 µm, c, e, g, h, q, s, v, w, x, y = 5 µm, r = 20 µm
Different types of conidia. aZelosatchmopsis sacciformis. bDwayalomella vaccinii. dNeoascochyta dactylidis. ePhyllosticta sp. fPhacidium distylii. c, r, sPseudolachnella brevifusiformis, P. longiciliata, P. coronata. g Elongated-shaped conidia in (Elongaticonidia rosae) hCrinitospora pulchra. i, jCornutispora limaciformis. k Y-shaped or digitate, triradiate conidia in Sphaerellopsis anomala. lPseudolachnella setulosa. mChoanatiara lunata. nCorniculariella rhamni. oDermea cerasi. pCryptosporella alni-cordata. q Cruciform conidia in Diplocarpon mespili. tConicomyces contortus. u Tetra-radiate Eriosporella calami. vHeterosphaeria patella. wChaetoconis vaccinia. x Groveolopsis pandani. yAposphaeria corallinolutea. z Conidia of Cytospora globosa. 1Chaetoconis vaccinia. 2Dinemasporium setulosa. Scale bars a–c, f, g, t, v = 5 µm, d, e, h–s, w–z = 10 µm, u = 20 µm
Biology of Coelomycetes
Coelomycetes are geographically widespread and found mainly in tropical and temperate regions, less in antarctic and arctic regions (Sutton 1980). They can be saprobes that recycle carbon and other nutrient elements, or pathogens of terrestrial or aquatic plants, or endophytes (Sutton 1980; Nag Raj 1981b, 1993; Wang et al. 2005; Crouch et al. 2009; Seephueak et al. 2011; Rajagopal et al. 2012; Zhang et al. 2012a; Norphanphoun et al. 2018). Some survive in the soil; others are parasitic on insects or vertebrates, including humans (Nag Raj 1981b, 1993; Roberts and Humber 1981; Chaverri et al. 2008; Ferrer et al. 2009; Udayanga et al. 2011; Li et al. 2016; Tibpromma et al. 2017; Valenzuela-Lopez et al. 2018). Some are fungicolous (Hawksworth 1981a, b; Nag Raj 1993; Sun et al. 2019) or lichenicolous (Vobis and Hawksworth 1981; Diederich et al. 2012; Flakus and Farkas 2013; Ertz et al. 2015; Wijayawardene et al. 2016), while others are mycorrhizal (Oliveira et al. 2014). Several endophytic species may be latent pathogens, existing as symptomless endophytes, but becoming pathogens once the host defense system weakens (Photita et al. 2004); while other endophytes may become saprobes following death of host tissues where they perform as recyclers of dead plant material (Promputtha et al. 2010; Udayanga et al. 2011; Maharachchikumbura et al. 2012; Wijayawardene et al. 2012b; Gomes et al. 2013).
Many species of coelomycetes are plant pathogens of a broad range of hosts, and responsible for numerous diseases, including leaf and fruit spots, root lesions, blight, anthracnose, die-back and canker diseases (Sutton 1980; Aveskamp et al. 2008; Liu et al. 2012; Phillips et al. 2013; Wikee et al. 2013; Wijayawardene et al. 2016; Norphanphoun et al. 2017; Jami et al. 2018a, b; Li et al. 2018; Yang et al. 2018). Diseases caused by coelomycetes may directly influence agricultural industry and result in serious economic loss. It has been estimated that in the Mediterranean region, Australia and Pakistan, blight disease of chickpea caused by Ascochyta rabiei has resulted in up to 100% crop loss (Hamza et al. 2000; Ghazanfar et al. 2010; Wijayawardene et al. 2012b). Some coelomycetes are widely used in disciplines such as biological control (e.g., Ampelomyces quisqualis, Colletotrichum lagenarium, C. gloeosporioides f. sp. aeschynomene, Sphaerellopsis filum), bioremediation and pharmaceutical fields (e.g., anti-cancer drug producing fungi, Bartalinia robillardoides, Pestalotiopsis malicola, Phoma clematidina) (Pei et al. 2003; Kiss et al. 2004; Płachecka 2005, Gangadevi and Muthumary 2008; Bi et al. 2011; Boyette et al. 2011; Wijayawardene et al. 2012a, b). However, the taxonomy of coelomycetes is unsettled and remains problematic, with many of the fungi included yet to find a place in modern fungal classification. Therefore, a modified definition of coelomycetous fungi is not only helpful in taxonomy, but will also be important in plant biosecurity, identification of plant pathogens, and in industry applications.
Hyaline-spored coelomycetes such as Botryosphaeria, Colletotrichum, Cytospora, Diaporthe, Phoma occur in numerous habitats with a worldwide distribution (Nakamura et al. 2006; Liu et al. 2012; Hyde et al. 2014; Dissanayake et al. 2016; Chen et al. 2015, 2017; Norphanphoun et al. 2017, 2018, Ma et al. 2018; Phillips et al. 2013, 2019). Taxa are found as pathogens on economic trees (Prunus, citrus, Huang et al. 2013; Dayarathne et al. 2017; teak, Doilom et al. 2017; walnut Fan et al. 2015a), horticultural plants (Limber 1955; Sutton 1980; Cunnington et al. 2007; Quaedvlieg et al. 2013), crops (Raza et al. 2019) and ornamental trees (Sutton 1980). There are found on monocotyledons, such as bamboo (Dai et al. 2017), palms (Wikee et al. 2012), grasses (Photita et al. 2005; Phookamsak et al. 2014, 2017; Raza et al. 2019) and banana (Photita et al. 2001). They are also saprobes in leaf litter (Sutton 1980; Nag Raj 1993), on substrates in streams and lakes (Cai et al. 2003; Zhang et al. 2012a, b), in marine habitats (Dayarathne et al. 2017), and in soil (Aveskamp et al. 2010; Chen et al. 2015).
Materials and methods
Specimens and isolates
Fresh specimens were collected from various hosts, and other substrata in China, Italy, Russia and Thailand. Type or representative specimens were obtained on loan from various herbaria (http://sweetgum.nybg.org/science/ih/): Cornell University (CUP), U.S.A.; Agriculture and Agri-Food Canada (DAOM), Canada; Harvard University (FH), U.S.A.; Martin-Luther-Universität (HAL), Germany; Hirosaki University (HHUF), Japan; CABI Bioscience UK Centre (IMI), U.K., England; Royal Botanic Gardens (K), U.K., England; Mae Fah Luang University (MFLU), Thailand; Plant Protection Research Institute (PREM), South Africa. Type species were examined and re-described as in Nag Raj (1993), Hyde et al. (2013) and Li et al. (2014). Dried herbarium specimens were rehydrated in water or water containing a few drops of lactic acid, or in a 5% aqueous solution of KOH. Conidomata were sectioned using a razor blade. Thin sections were mounted in water or lactic acid for microscopic study and photomicrography. Conidiophores were stained with cotton blue for checking phialidic conidiogenous cells when necessary. Conidia were stained using India ink to check mucoid sheaths or appendages. Micro-morphological characters (conidiomata, conidiophores, conidiogenous cells, conidia) were examined using a Nikon ECLIPSE 80i and 90i compound microscope and photographed with a Canon 550 D and 600 D digital camera fitted to the microscope. Measurements of morphological structures were made with the Tarosoft (R) v.0.9.7 Image Frame Work program. Photographic plates were edited and combined using Adobe Photoshop CS6 Extended version 13.0.1 software (Adobe Systems, USA). Permanent slides were prepared by adding lactoglycerol and sealing with clear nail polish (Nag Raj 1993; Phookamsak et al. 2014).
Pure cultures from fresh material were made using the single spore method of Chomnunti et al. (2014). Germinated spores were observed with a stereomicroscope and transferred to malt extract agar (MEA) or potato dextrose agar (PDA) for examination of culture characteristics, sporulation and extraction of DNA. Colony colour was determined according to the colour charts of Rayner (1970). The asexual morphs were established in culture using the method of Phookamsak et al. (2015). Specimens are deposited in the herbarium of Mae Fah Luang University (MFLU), Chiang Rai, Thailand, and Kunming Institute of Botany, Chinese Academy of Sciences (KUN). Culture isolates are deposited at Mae Fah Luang University Culture Collection (MFLUCC). Duplicate cultures are deposited in the International Collection of Microorganisms from Plants, Landcare Research, New Zealand (ICMP), Mycothèque de l’Université catholique de Louvain (MUCL), and Kunming Institute of Botany Culture Collection (KUMCC). Faces of Fungi and Index Fungorum numbers were obtained as outlined in Jayasiri et al. (2015) and Index Fungorum (2019).
DNA extraction, PCR amplification and sequencing
DNA extraction was performed from fresh fungal mycelia or conidiomata of dried specimens. Isolates were grown on MEA or PDA at room temperature (25–30 °C), until the colony completely covered the agar surface. The mycelium (at least 50 mg) was scraped off and collected in a 1.5 ml micro-centrifuge tube. Mycelium was ground to a fine powder in liquid nitrogen and genomic DNA was extracted using the Biospin Fungus Genomic DNA Extraction Kit (BioFlux®) following the manufacturer’s protocol (Hangzhou, P.R. China). For dried specimens, for which it was difficult to obtain a culture or where the conidia germinated but then stopped growing on artificial medium, DNA was extracted directly from the conidiomata following the method of Li et al. (2015a, b, c). The materials were surface disinfected with 75% alcohol for 1 min and subsequently rinsed with sterile water for 1 min. Target conidiomata (5–10) were removed to a 1.5 ml micro-centrifuge tube using sterile fine forceps. The genomic DNA was extracted from conidiomata and infusion of conidiomata using the E.Z.N.A. TM Forensic DNA Extraction Kit (OMEGA Bio-Tek, D3591-01, Norcross, GA, U.S.A.) (Li et al. 2015a, c). The DNA products were kept at 4 °C for use in regular work and duplicated at − 20 °C for long-term storage.
DNA amplification was performed by polymerase chain reaction (PCR) using the respective gene primers: the internal transcribed spacers (ITS, ITS1-5.8S nrDNA-ITS2), large subunit nrDNA (LSU, 28S), small subunit nrDNA (SSU, 18S), RNA polymerase II second largest subunit (rpb2), and translation elongation factor 1-alpha gene (tef1) and β-tubulin (tub2). The primer pairs ITS4/ITS5 were used to amplify nuclear ITS regions (White et al. 1990), LR0R/LR5 for LSU (Vilgalys and Hester 1990; Rehner and Samuels 1994), NS1/NS4 for SSU (White et al. 1990), rpb2-5f/rpb2-7cr for rpb2 (Liu et al. 1999; Sung et al. 2007a, b), tef1-728F/tef1-986R (Carbone and Kohn 1999), tef1-983F/tef1-2218R (Rehner and Buckley 2005) and Bt2a/Bt2b (Glass and Donaldson 1995). The amplifications were performed in a 25 μl reaction volume as follows: 1 μg DNA template, 1 μl of each forward and reverse primer, 12.5 μl of 2 × Taq PCR SuperMix (mixture of 0.1 U Taq Polymerase/μl, 500 μm Dntp each, 20 mM Tris–Hcl pH 8.3, 100 Mm KCl, 3 mM MgCl2 and optimized buffer) (TIANGEN BIOTECH Co., Chaoyang District, Beijing, PR China) and 9.5 μl sterilized distilled water (Li et al. 2015c). The PCR thermal cycle program of ITS, nuLSU, nuSSU, tef1 and tub2 genes amplification conditions were provided as: initially 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 30–50 s, annealing at 55 °C for 1 min, elongation at 72 °C for 90 s, and final extension at 72 °C for 5–10 mins (Liu et al. 2012). The PCR thermal cycle program for the partial RNA polymerase second largest subunit (rpb2) was as follows: initially 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 1 min, annealing at 52 °C for 2 min, elongation at 72 °C for 90 s, and final extension at 72 °C for 10 min (Phookamsak et al. 2014). The PCR products were checked on 1% agarose electrophoresis gels stained with anthocyanidin. The PCR products were send to Shang Hai Biological Engineering Technology Co. (Shang Hai, P.R. China) for sequencing.
Sequence alignment and phylogenetic analysis
Forward and reverse of each sequence generated from different primer pairs were edited and assembled using DNASTAR Lasergene SeqMan Pro v. 7.1.0 (44.1). A BLAST search based on LSU sequence data was carried out to reveal the closest matches in GenBank. The sequences generated in this study were supplemented with additional sequences obtained from GenBank based on blast searches and published literature (Supplementary Table 1). The individual sequences (LSU, SSU, ITS, rpb2, tef1, tub2) were aligned singly in MAFFT v. 7 at the web server (http://mafft.cbrc.jp/alignment/server) (Katoh et al. 2013, 2017; Kuraku et al. 2013), and improved manually where necessary in BioEdit v. 7.0.9.0 (Hall 1999) or AliView v. 1.19-betalk (Larsson 2014). The concatenated sequence alignments were obtained from SequenceMatrix v 1.8 (Vaidya et al. 2011). The concatenated alignment was converted to NEXUS file format (.nex) and PHYLIP file format (.phy) for maximum parsimony (MP), Bayesian analyses (BA) and maximum likelihood (RAxML) analysis respectively, using AliView v. 1.19-betalk (Larsson 2014). The NEXUS file was prepared for MrModeltest v. 2.3 after replacing “?” to “-” in notepad and running a single line in Phylogenetic Analysis Using Parsimony (PAUP) v. 4.0b10 (Swofford 2002). The optimal nucleotide substitution model for Bayesian analysis were selected independently for each locus using MrMTgui v. 1.0 with MrModeltest v. 2.3 (Nylander et al. 2008), under the Akaike Information Criterion (AIC) implemented in PAUP v. 4.0b10.
Phylogenetic analyses of both individual and concatenated aligned data were based on Bayesian analyses (BA), maximum likelihood (ML) and maximum parsimony (MP). Maximum likelihood and BA were implemented on the CIPRES Science Gateway v. 3.3 platform (Miller et al. 2010, 2012) using RAxML-HPC BlackBox v. 8.2.10 (Stamatakis 2014) and MrBayes on XSEDE v. 3.2.6 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003), respectively. A GTR+I+G substitution model with 1 000 bootstrap iterations was set for ML analyses. Posterior probabilities (PP) (Rannala and Yang 1996; Zhaxybayeva and Gogarten 2002) were determined by Markov Chain Monte Carlo sampling (BMCMC) in MrBayes v. 3.0b4 (Huelsenbeck and Ronquist 2001). Six simultaneous Markov chains were run for 100 000 000 generations and trees were sampled every 1000th generation. The burn-in fraction was set to 0.2, and the run was automatically stopped when the average standard deviation of split frequencies fell below 0.01.
Maximum parsimony analyses were performed using the heuristic search option with 1000 random taxa addition and tree bisection and reconnection (TBR) as the branch-swapping algorithm. All characters were unordered and of equal weight and gaps were treated as missing data. MaxTrees were unlimited, branches of zero length were collapsed and all multiple equally parsimonious trees were saved. The robustness of the most parsimonious trees was evaluated from 1000 bootstrap replications (Hillis and Bull 1993). Tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI), and log likelihood (− ln L) were calculated for trees generated under different optimality criteria. The Kishino-Hasegawa tests (Kishino and Hasegawa 1989) were performed to determine whether trees were significantly different.
The resulting trees were plotted using FigTree v. 1.4.2 (Rambaut 2014) and edited in Adobe Illustrator® CS6 Tryout v. 16.0.0.
Results
Phylogenetic analyses
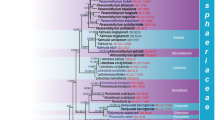
The large number of taxa included in this study lack sequence data for various gene regions, such as SSU, ITS, tub2, tef1 and rpb2. We therefore used LSU sequence data to construct the phylogenetic tree for the classes Agaricomycetes (Basidiomycota), Coniocybomycetes, Dothideomycetes, Eurotiomycetes, Geoglossomycetes, Lecanoromycetes, Leotiomycetes, Lichinomycetes, Orbiliomycetes, Pezizomycetes and Sordariomycetes (Ascomycota) (Fig. 7).
Phylogenetic tree generated from a maximum likelihood analysis based on LSU sequences data of Agaricomycetes (Basidiomycota), Coniocybomycetes, Dothideomycetes, Eurotiomycetes, Geoglossomycetes, Lecanoromycetes, Leotiomycetes, Lichinomycetes, Orbiliomycetes, Pezizomycetes and Sordariomycetes (Ascomycota). Related sequences were obtained from GenBank. Six hundred and twenty-nine strains are included in the analyses, which comprise 978 characters including gaps. Phytophthora moyootj VHS27218 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the maximum parsimony and the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 2919.850785 is presented. The matrix had 245 distinct alignment patterns, with 14.17% of undetermined characters or gaps. Estimated base frequencies were: as follows: A = 0.209309, C = 0.308850, G = 0.253017, T = 0.228825; substitution rates AC = 0.826247, AG = 2.476259, AT = 1.076270, CG = 0.884896, CT = 4.252047, GT = 1.000000; gamma distribution shape parameter α = 0.168455. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 10 changes. Ex-type or ex-epitype strains are in bold. New isolates are in blue
Family, generic and species phylogenies
To better infer delimitation of hyaline-spored coelomycetes at the family or generic level, DNA sequence data of multi-loci (e.g., SSU, ITS, tub2, tef1, and rpb2) were concatenated to construct a phylogenetic tree. The phylogenetic analyses performed in this study see the Supplementary Table 2.
Outline for hyaline-spored coelomycetes
Phylum ASCOMYCOTA Caval-Sm.
Subphylum PEZIZOMYCOTINA O.E. Erikss. & Winka
Class Dothideomycetes sensu O.E. Erikss. & Winka
Subclass Dothideomycetidae P.M. Kirk et al. ex C.l. Schoch et al.
Capnodiales Woron.
Capnodiaceae Höhn. ex Theiss.
Capnodium Mont. 1849
Conidiocarpus Woron. 1917
Leptoxyphium Speg. 1918
Scorias Fr. 1832
Mycosphaerellaceae Lindau
Caryophylloseptoria Verkley, Quaedvl. & Crous 2013
Colletogloeum Petr. 1953
Cytostagonospora Bubák 1916
Dothistroma Hulbary 1941
Geastrumia Bat. 1960
Neoseptoria Quaedvl., Verkley & Crous 2013
Phloeospora Wallr. 1833
Polyphialoseptoria Quaedvl., R.W. Barreto, Verkley & Crous 2013
Ruptoseptoria Quaedvl., Verkley & Crous 2013
Septoria Sacc. 1884
Sphaerulina Sacc. 1878
Stromatoseptoria Quaedvl., Verkley & Crous 2013
Dothideales Lindau
Dothideaceae Chevall.
Cylindroseptoria Quaedvl., Verkley & Crous 2013
Dothidea Fr. 1818
Dothiora Fr. 1849
Saccotheciaceae Bonord.
Pseudoseptoria Speg. 1911
Dothideales, genera incertae sedis
Asteromellopsis H.E. Hess & E. Müll. 1951
Mytilinidiales E. Boehm, C.L. Schoch & Spatafora
Mytilinidiaceae Kirschst. 1924
Pseudocamaropycnis Crous 2016
Pleosporales Luttr. ex M.E. Barr
Acrocalymmaceae Crous & Trakun.
Acrocalymma Alcorn & J.A.G. Irwin 1987
Groveolopsis Boedijn 1951
Aquasubmersaceae A. Hashim. & Kaz. Tanaka
Aquasubmersa K.D. Hyde & Huang Zhang 2012
Cucurbitariaceae Luerss.
Allocucurbitaria Valenz.-Lopez, Stchigel, Guarro & Cano 2017
Neocucurbitaria Wanas., E.B.G. Jones & K.D. Hyde 2017
Paracucurbitaria Valenz.-Lopez, Stchigel, Guarro & Cano 2017
Dictyosporiaceae Boonmee & K.D. Hyde
Pseudocoleophoma Kaz. Tanaka & K. Hiray. 2015
Didymellaceae Gruyter, Aveskamp & Verkley
Allophoma Q. Chen & L. Cai 2015
Ascochyta Lib. 1830
Boeremia Aveskamp, Gruyter & Verkley 201 0
Briansuttonomyces Crous 2016
Calophoma Q. Chen & L. Cai 2015
Cumuliphoma Valenz.-Lopez, Stchigel, Crous, Guarro & Cano 2017
Didymella Sacc. 1880
Ectophoma Valenz.-Lopez, Cano, Crous, Guarro & Stchigel 2017
Epicoccum Link 1816
Heterophoma Q. Chen & L. Cai 2015
Juxtiphoma Valenzuela-Lopez, Cano, Crous, Guarro & Stchigel 2017
Macroventuria Aa 1971
Neoascochyta Q. Chen & L. Cai 2015
Neodidymelliopsis Qian Chen & L. Cai 2015
Nothophoma Qian Chen & L. Cai 2015
Paraboeremia Q. Chen & L. Cai 2015
Phoma Sacc. 1880
Phomatodes Q. Chen & L. Cai 2015
Remotididymella Valenz.-Lopez, Crous, Cano, Guarro & Stchigel 2017
Similiphoma Valenz.-Lopez, Crous, Cano, Guarro & Stchigel 2017
Stagonosporopsis Died. 1912
Vacuiphoma Valenz.-Lopez, Cano, Crous, Guarro & Stchigel 2017
Xenodidymella Q. Chen & L. Cai 2015
Lentitheciaceae Y. Zhang ter, C.L. Schoch, J. Fourn., Crous & K.D. Hyde
Keissleriella Höhn 1919
Pleurophoma Höhn. 1914
Setoseptoria Quaedvl., Verkley & Crous 2013
Towyspora Wanasinghe, E.B.G. Jones & K.D. Hyde 2016
Leptosphaeriaceae Sacc
Acicuseptoria Quaedvl., Verkley & Crous 2013
Leptosphaeria Ces. & De Not. 1863
Querciphoma Crous 2017
Sphaerellopsis Cooke 1883
Lindgomycetaceae K. Hiray., Kaz. Tanaka & Shearer
Clohesyomyces K.D. Hyde 1993
Lophiotremataceae K. Hiray. & Kaz. Tanaka
Atrocalyx A. Hashim. & Kaz. Tanaka 2017
Pseudocryptoclypeus A. Hashim. & Kaz. Tanaka 2017
Massarinaceae Munk
Massarina Sacc. 1883
Stagonospora (Sacc.) Sacc. 1884
Melanommataceae G. Winter
Aposphaeria Sacc. 1880
Neocamarosporiaceae Wanas., Wijayaw., Crous & K.D. Hyde
Dimorphosporicola Crous 2016
Neohendersoniaceae A. Giraldo & Crous
Medicopsis Gruyter, Verkley & Crous 2012
Neopyrenochaetaceae Valenz.-Lopez, Crous, Cano, Guarro & Stchigel
Neopyrenochaeta Valenz.-Lopez, Crous, Stchigel, Guarro & Cano 2017
Parapyrenochaetaceae Valenz.-Lopez, Crous, Stchigel, Guarro & Cano
Parapyrenochaeta Valenz.-Lopez, Crous, Stchigel, Guarro & Cano 2017
Phaeosphaeriaceae M.E. Barr
Alloneottiosporina Nag Raj 1993
Chaetosphaeronema Moesz 1915
Loratospora Kohlm. & Volkm.-Kohlm. 1993
Neosetophoma Gruyter, Aveskamp & Verkley 2010
Neosphaerellopsis Crous & Trakun. 2014
Neostagonospora Quaedvl., Verkley & Crous 2013
Paraphoma Morgan-Jones & J.F. White 1983
Parastagonospora Quaedvl., Verkley & Crous 2013
Phaeosphaeria I. Miyake 1909
Setophoma Gruyter, Aveskamp & Verkley 2010
Vrystaatia Quaedvl., W.J. Swart, Verkley & Crous 2013
Xenoseptoria Quaedvl., H.D. Shin, Verkley & Crous 2013
Pseudopyrenochaetaceae Valenz.-Lopez, Crous, Stchigel, Guarro & Cano
Pseudopyrenochaeta Valenz.-Lopez, Crous, Stchigel, Guarro & Cano 2017
Pyrenochaetopsidaceae Valenz.-Lopez, Crous, Cano, Guarro & Stchigel
Neopyrenochaetopsis Valenz-Lopez, Cano, Guarro & Stchigel 2017
Pyrenochaetopsis Gruyter, Aveskamp & Verkley 2010
Xenopyrenochaetopsis Valenz.-Lopez, Crous, Stchigel, Guarro & Cano 2017
Saccharataceae Slippers, Boissin & Crous
Septorioides Quaedvl., Verkley & Crous 2013
Trematosphaeriaceae K.D. Hyde, Y. Zhang ter, Suetrong & E.B.G. Jones, 2013
Trematosphaeria Fuckel 1870
Pleosporales, genera incetae sedis
Chaetophoma Cooke 1878
Chaetasbolisia Speg. 1918
Foliophoma Crous 2017
Pseudochaetosphaeronema Punith.1979
Phialophorophoma Linder 1944
Strigulales Lücking, M.P. Nelsen & K.D. Hyde
Strigulaceae A.B. Frank 1877
Strigula Fr. 1823
Dothideomycetes, orders incertae sedis
Botryosphaeriales C.L. Schoch, Crous & Shoemaker
Botryosphaeriaceae Theiss. & Syd.
Botryobambusa Phook., Jian K. Liu & K.D. Hyde 2012
Botryosphaeria Ces. & De Not. 1863
Cophinforma Doilom, J.K. Liu & K.D. Hyde 2012
Endomelanconiopsis E.I. Rojas & Samuels 2008
Eutiarosporella Crous 2015
Macrophomina Petr. 1923
Marasasiomyces Crous 2015
Mucoharknessia Crous, R.M. Sánchez & Bianchin. 2015
Neofusicoccum Crous, Slippers & A.J.L. Phillips 2006
Neoscytalidium Crous & Slippers 2006
Sakireeta Subram. & K. Ramakr. 1957
Tiarosporella Höhn. 1919
Melanopsaceae Phillips, Slippers, Boissin & Crous
Melanops Nitschke ex Fuckel 1870
Phyllostictaceae Fr.
Phyllosticta Pers. 1818
Pseudofusicoccum Mohali, Slippers & M.J. Wingf. 2006
Planistromellaceae M.E. Barr
Kellermania Ellis & Everh. 1885
Pseudorobillardaceae Crous
Pseudorobillarda M. Morelet 1968
Dothideomycetes, genera incertae sedis
Asteromella Pass. & Thüm. 1880
Chaetosticta Petr. & Syd. 1925
Kabatia Bubák 1904
Lignosphaeria Boonmee, Thambug. & K.D. Hyde 2015
Neottiosporina Subram. 1961
Class Eurotiomycetes O.E. Erikss. & Winka
Subclass Chaetothyriomycetidae Doweld
Chaetothyriales M.E. Barr
Lyrommataceae Lücking
Lyromma Bat. & H. Maia 1965
Phaeomoniellales K.H. Chen, A.E. Arnold, Gueidan & Lutzoni
Celotheliaceae Lücking, Aptroot & Sipman
Aequabiliella Crous 2015
Celerioriella Crous 2015
Minutiella Crous 2015
Neophaeomoniella Roon.-Lath. & Crous 2015
Paraphaeomoniella Crous 2015
Pseudophaeomoniella Nigro, Antelmi & Crous 2015
Phaeomoniellaceae P.M. Kirk
Phaeomoniella Crous & W. Gams 2000
Xenocylindrosporium Crous & Verkley 2009
Pyrenulales, genus incertae sedis
Xenus Kohlm. & Volkm.-Kohlm. 1992
Class Lecanoromycetes O.E. Erikss. & Winka
Subclass Ostropomycetidae V. Reeb, Lutzoni & Cl. Roux
Ostropales, genera incertae sedis
Elongaticonidia W.J. Li, E. Camporesi & K.D. Hyde
Mulderomyces Crous, Jacq. Edwards & P.W.J. Taylor 2016
Phacidiella P. Karst. 1884
Class Leotiomycetes O.E. Erikss. & Winka
Subclass Leotiomycetidae P.M. Kirk, P. Cannon, Minter & Stalpers
Chaetomellales Crous & Denman
Chaetomellaceae Baral, P.R. Johnst. & Rossman
Chaetomella Fuckel 1870
Pilidium Kunze 1823
Sphaerographium Sacc. 1884
Helotiales Nannf.
Drepanopezizaceae Baral
Diplocarpon F.A. Wolf 1912
Drepanopeziza Kleb. Jaap 1914
Leptotrochila P. Karst. 1871
Godroniaceae Baral
Ascocalyx Naumov 1926
Godronia Moug. & Lév. 1846
Gremmeniella M. Morelet 1969
Heterosphaeriaceae Rehm
Heterosphaeria Grev. 1824
Lachnaceae Raitv.
Crucellisporiopsis Nag Raj 1983
Crucellisporium M.L. Farr 1968
Ploettnerulaceae Kirschst.
Mastigosporium Riess 1852
Sclerotiniaceae Whetzel
Pycnopeziza W.L. White & Whetzel 1938
Helotiales, genera incertae sedis
Cylindrosporium Grev. 1822
Libartania Nag Raj 1979
Vestigium Piroz. & Shoemaker 1972
Leotiales Korf & Lizoň
Cochlearomycetaceae Crous
Satchmopsis B. Sutton & Hodges 1975
Tympanidaceae Baral & Quijada
Pragmopora A. Massal. 1855
Tympanis Tode 1790
Medeolariales Korf
Ascodichaenaceae D. Hawksw. & Sherwood
Ascodichaena Butin 1977
Dermateaceae Fr.
Chaetophiophoma Speg. 1911
Coleophoma Höhn. 1907
Corniculariella P. Karst. 1884
Dermea Fr. 1825
Neodermea W.J. Li, D.J. Bhat & K.D. Hyde 2020
Neofabraea H.S. Jacks. 1913
Neogloeosporidina W.J. Li, Camporesi & K.D. Hyde 2020
Parafabraea Chen, Verkley & Crous 2016
Pezicula Tul. & C. Tul. 1865
Phlyctema Desm. 1847
Pseudofabraea Chen, Verkley & Crous 2016
Micraspidales Quijada & Tanney
Micraspidaceae Quijada & Tanney
Micraspis Darker 1963
Phacidiales Bessey
Helicogoniaceae Baral
Eleutheromyces Fuckel 1870
Phacidiaceae Fr.
Allantophomopsiella Crous 2014
Allantophomopsis Petr. 1925
Darkera H.S. Whitney, J. Reid & Piroz. 1975
Phacidium Fr. 1815
Potebniamyces Smerlis 1962
Rhytismatales M.E. Barr ex Minter
Calloriaceae Baral & G. Marson
Stamnaria Fuckel 1870
Rhytismataceae Chevall.
Colpoma Wallr. 1833
Hypoderma De Not. 1847
Hypohelion P.R. Johnst. 1990
Rhytisma Fr. 1819
Tryblidiopsis P. Karst. 1871
Leotiomycetes, family incertae sedis
Hemiphacidiaceae Korf
Rhabdocline Syd. 1922
Leotiomycetes, genera incertae sedis
Furcaspora Bonar 1965
Monochaetiellopsis B. Sutton & DiCosmo 1977
Class Sordariomycetes O.E. Erikss. & Winka
Subclass Diaporthomycetidae Senan., Maharachch. & K.D. Hyde
Diaporthales Nannf.
Cryphonectriaceae Gryzenh. & M.J. Wingf.
Amphilogia Gryzenh., H.F. Glen & M.J. Wingf. 2005
Aurantiosacculus Dyko & B. Sutton 1979
Aurapex Gryzenh. & M.J. Wingf. 2006
Celoporthe Nakab., Gryzenh., Jol. Roux & M.J. Wingf. 2006
Chrysoporthe Gryzenh. & M.J. Wingf. 2004
Cryphonectria (Sacc.) Sacc. & D. Sacc. 1905
Cryptometrion Gryzenh. & M.J. Wingf. 2010
Diversimorbus S.F. Chen & Jol. Roux 2013
Endothia Fr. 1849
Endothiella Sacc. 1906
Latruncellus M. Verm., Gryzenh. & Jol. Roux 2011
Mastigosporella Höhn. 1914
Microthia Gryzenh. & M.J. Wingf. 2006
Rostraureum Gryzenh. & M.J. Wingf. 2005
Ursicollum Gryzenh. & M.J. Wingf. 2006
Cytosporaceae Fr.
Cytospora Ehrenb. 1818
Waydora B. Sutton 1976
Diaporthaceae Höhn. ex Wehm.
Apioporthella Petr. 1929
Chaetoconis Clem. 1909
Chiangraiomyces Senan. & K.D. Hyde 2017
Diaporthe Nitschke 1870
Hyaliappendispora Senan., Camporesi & K.D. Hyde 2017
Erythrogloeaceae Senan. et al.
Erythrogloeum Petr. 1953
Gnomoniaceae G. Winter
Amphiporthe Petr. 1971
Apiognomonia Höhn. 1917
Asteroma DC. 1815
Cryptosporella Sacc. 1877
Gloeosporidina Petr. 1921
Gnomoniopsis Berl. 1893
Sirococcus Preuss 1855
Lamproconiaceae Norph., T.C. Wen & K.D. Hyde
Hercospora Fr. 1825
Melanconiellaceae Senan., Maharachch. & K.D. Hyde
Melanconiella Sacc. 1882
Prosopidicolaceae Senan. & K.D. Hyde
Prosopidicola Crous & C.L. Lennox 2004
Stilbosporaceae Link
Crinitospora B. Sutton & Alcorn 1985
Sydowiellaceae Lar. N. Vassiljeva
Breviappendix Senan. & K.D. Hyde 2017
Hapalocystis Auersw. ex Fuckel 1863
Tenuiappendicula Senan., Camporesi & K.D. Hyde 2017
Diaporthales, genera incertae sedis
Hypodermina Höhn. 1916
Gyrostroma Naumov 1914
Subclass Hypocreomycetidae O.E. Erikss. & Winka
Glomerellales Chadef. ex Réblová, W. Gams & Seifert
Glomerellaceae Locq. ex Seifert & W. Gams
Colletotrichum Corda 1831
Hypocreales Lindau
Clavicipitaceae O.E. Erikss.
Aschersonia Mont. 1848
Moelleriella Bres. 1897Polynema Lév. 1846
Samuelsia P. Chaverri & K.T. Hodge 2008
Flammocladiaceae Crous, L. Lombard & R.K.
Flammocladiella Crous, L. Lombard & R.K. Schumach 2015
Nectriaceae Tul. & C. Tul.
Nectria (Fr.) Fr. 1849
Thyronectria Sacc. 1875
Stachybotryaceae L. Lombard & Crous
Koorchaloma Subram. 1953
Subclass Sordariomycetidae O.E. Erikss. & Winka
Chaetosphaeriales Huhndorf, A.N. Mill. & F.A. Fernández
Chaetosphaeriaceae Locq.
Brunneodinemasporium Crous & R.F. Castañeda 2012
Conicomyces R.C. Sinclair, Eicker & Morgan-Jones 1983
Dendrophoma Sacc. 1880
Dinemasporium Lév. 1846
Infundibulomyces Plaingam, Somrith. & E.B.G. Jones 2003
Neopseudolachnella A. Hashim. & Kaz. Tanaka 2015
Pseudodinemasporium A. Hashim. & Kaz. Tanaka 2015
Pseudolachnea Ranoj. 1910
Pseudolachnella Teng 1936
Phyllachorales M.E. Barr
Phaeochoraceae K.D. Hyde, P.F. Cannon & M.E. Barr
Cyclodomus Höhn. 1909
Phyllachoraceae Theiss. & P. Syd.
Diachora Jul. Müll. 1893
Rhodosticta Woron. 1911
Subclass Xylariomycetidae O.E. Erikss & Winka
Amphisphaeriales D. Hawksw. & O.E. Erikss.
Phlogicylindriaceae Senan. & K.D. Hyde
Phlogicylindrium Crous, Summerb. & Summerell 2006
Xylariales Nannf.
Amphisphaeriaceae G. Winter
Amphisphaeria Ces. & De Not. 1863
Diatrypaceae Nitschke
Eutypa Tul. & C. Tul. 1863
Quaternaria Tul. & C. Tul. 1863
Microdochiaceae Hern.-Restr., Crous & J.Z. Groenew.
Ciliosporella Petr. 1927
Polystigmataceae Höhn. ex Nannf.
Polystigma DC.1815
Sordariomycetes, orders incertae sedis
Tracyllalales Crous
Tracyllaceae Crous
Tracylla (Sacc.) Tassi 1904
ASCOMYCOTA, genera incertae sedis
Abrothallus De Not. 1845
Agyriellopsis Höhn. 1903
Ajrekarella Kamat & Kalani 1964
Amerosporiopsis Petr. 1941
Anaphysmene Bubák 1906
Anthracoderma Speg. 1887
Aoria Cif. 1962
Aphanofalx B. Sutton 1986
Apiocarpella Syd. & P. Syd. 1919
Aristastoma Tehon 1933
Ascochytopsis Henn. 1905
Asteroconium Syd. & P. Syd. 1903
Asteromidium Speg. 1888
Blennoria Moug. & Fr. 1825
Botryocrea Petr. 1949
Brycekendrickia Nag Raj 1973
Calocline Syd. 1939
Camaropycnis E.K. Cash 1945
Catenophora Luttr. 1940
Ceuthodiplospora Died. 1912
Chaetoseptoria Tehon 1937
Cheilaria Lib. 1830
Choanatiara DiCosmo 1984
Chondropodiella Höhn. 1917
Ciliochora Höhn. 1919
Clypeopycnis Petr. 1925
Collonaemella Höhn. 1915
Comatospora Piroz. & Shoemaker 1971
Cornucopiella Höhn. 1915
Cornutispora Piroz. 1973
Crandallia Ellis & Sacc. 1897
Cryptomycella Höhn. 1925
Cryptosporium Kunze 1817
Cylindrogloeum Petr. 1941
Cylindroxyphium Bat. & Cif. 1963
Cystotricha Berk. & Broome 1850
Cytogloeum Petr. 1925
Cytonaema Höhn. 1914
Cytoplacosphaeria Petr. 1920
Cytosporella Sacc. 1884
Dasysticta Speg. 1912
Davisiella Petr. 1924
Dearnessia Bubák 1916
Dendrodomus Bubák 1915
Dialaceniopsis Bat.1959
Didymochaeta Sacc. & Ellis 1898
Diedickea Syd. & P. Syd. 1913
Dilophospora Desm. 1840
Dimastigosporium Faurel & Schotter 1965
Diplosporonema Höhn. 1917
Diplozythiella Died. 1916
Discogloeum Petr. 1923
Discosporina Höhn. 1927
Discosporium Höhn., Z. 1915
Dothiorina Höhn. 1911
Dwayalomella Brisson, Piroz. & Pauzé 1975
Ebollia Minter & Caine, 1980
Eriospora Berk. & Broome 1850
Eriosporella Höhn. 1916
Fuckelia Bonord. 1864
Gampsonema Nag Raj 1975
Hapalosphaeria Syd. 1908
Helhonia B. Sutton 1980
Hemidothis Syd. & P. Syd. 1916
Hypocline Syd. 1939
Hysterodiscula Petr. 1942
Idiocercus B. Sutton 1967
Jahniella Petr. 1921
Kendrickomyces B. Sutton, V.G. Rao & Mhaskar 1976
Kirstenboschia Quaedvl., Verkley s& Crous 2013
Leptochlamys Died. 1921
Leptodermella Höhn. 1915
Leptothyrina Höhn. 1915
Ligniella Naumov 1926
Metazythia Petr. 1950
Microdiscula Höhn. 1915
Microperella Höhn. 1909
Mirimyces Nag Raj 1993
Monochaetiella E. Castell. 1943
Monodia Breton & Faurel 1970
Monostichella Höhn. 1916
Myriellina Höhn. 1915
Myxothyrium Bubák & Kabát 1915
Nanoschema B. Sutton 1980
Neochaetospora B. Sutton & Sankaran 1991
Neoplaconema B. Sutton 1977
Neottiospora Desm. 1843
Neozythia Petr. 1958
Oncosporella P. Karst. 1887
Pestalozziella Sacc. & Ellis ex Sacc. 1882
Phacostromella Petr. 1955,
Phellostroma Syd. & P. Syd., 1914
Phloeosporella Höhn. 1916
Phlyctaeniella Petr. 1922
Placonema Sacc. & D. Sacc. Petr. 1921
Placothyrium Bubák 1916
Plectronidiopsis Nag Raj 1979
Pleurophomopsis Petr. 1924
Pleurothyrium Bubák 1916
Proboscispora Punith.1984
Pseudobasidiospora Dyko & B. Sutton 1978
Pseudoneottiospora Faurel & Schotter 1965
Pseudostegia Bubák 1906
Pseudothyrium Höhn. 1927
Pseudozythia Höhn.1902
Pullospora Faurel & Schotter 1965
Pycnovellomyces R.F. Castañeda, 1987
Rhabdogloeopsis Petr.1925
Rhodesia Grove 1937
Rhodesiopsis B. Sutton & R. Campb. 1979
Rileya A. Funk 1979
Sarcophoma Höhn. 1916
Scaphidium Clem. 1901
Scopaphoma Dearn. & House 1925
Septocyta Petr. 1927
Septogloeum Sacc. 1880
Septopatella Petr. 1925
Sirophoma Höhn. 1917
Siroplacodium Petr. 1940
Sirozythiella Höhn. 1909
Sphaeriothyrium Bubák 1916
Staurophoma Höhn. 1907
Stictosepta Petr. 1964
Stilbophoma Petr. 1942
Strasseria Bres. & Sacc. 1902
Strasseriopsis B. Sutton & Tak. Kobay. 1970
Suttoniella S. Ahmad 1961
Tetranacrium H.J. Huds. & B. Sutton 1964
Thoracella Oudem. 1901
Thrinacospora Petr. 1948
Thyrsidina Höhn. 1905
Trematophoma Petr. 1924
Tribolospora D.A. Reid 1966
Xenidiocercus Nag Raj 1993
Yalomyces Nag Raj 1993
Yoshinagaia Henn. 1904
Zelandiocoela Nag Raj 1993
Zelosatchmopsis Nag Raj 1991
Zunura Nag Raj 1993
Phylum BASIDIOMYCOTA R.T. Moore
Subphylum AGARICOMYCOTINA Doweld
Class Agaricomycetes Doweld
Subclass Agaricomycetidae Parmasto
Agaricales underw.
Physalacriaceae Corner
Mycotribulus Nag Raj & W.B. Kendr., 1970
Subclass incertae sedis
Order Corticiales K.H. Larss. 2007
Corticiaceae Herter
Giulia Tassi 1904
Sebacinales M. Weiss, Selosse, Rexer, A. Urb. & Oberw.
Sebacinales, genus incertae sedis
Chaetospermum Sacc. 1892
Agaricomycetes, genenra incertae sedis
Cenangiomyces Dyko & B. Sutton 1979
Ellula Nag Raj 1980
Fibulocoela Nag Raj 1978
Pycnovellomyces R.F. Castañeda 1987
Taxonomy
Acrocalymma Alcorn & J.A.G. Irwin, Trans. Br. Mycol. Soc. 88(2): 163 (1987)
Facesoffungi number: FoF 07097
Dothideomycetes, Pleosporomycetidae, Pleosporales, Acrocalymmaceae
Saprobic on terrestrial host plant and freshwater water habit. Sexual morph:Ascomata initially immersed, later becoming erumpent, globose, unilocular, covered with light pale grey hyphae, opening by a centrally located ostiole, with a long break. Ostiole cylindrical, filled with hyaline periphyses. Peridium composed of thick-walled, brown cells of textura angularis. Hymenium composed of numerous, hyaline, filliform, septate, anastomosed pseudoparaphyses and asci. Asci bitunicate, 8-spored, cylindrical, short pedicellate. Ascospores biseriate, initially hyaline, ultimately becoming brown or pale reddish brown, narrowly fusiform, straight or slightly curved, 1–3-septate, constricted at septa, thick-walled, guttulate, enclosed by 1–2 µm wide, hyaline sheath (adapted from Shoemaker et al. 1991). Asexual morph:Conidiomata dark brown to black, solitary to gregarious or confluent, immersed to semi-immersed, stromatic, pycnidial, globose to subglobose, pyriform, unilocular or multi-locular, ostiolate. Ostiole circular, cylindrical, straight or curved, centrally or laterally located. Conidiomatal wall composed of brown to paler, thick-walled cells of textura angularis to textura globosa or textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to subcylindrical or lageniform, smooth-walled. Conidia hyaline, cylindrical to fusiform, aseptate or 1–3-septate, bearing helmet-shaped, mucoid appendage at each end.
Type species: Acrocalymma medicaginis Alcorn & J.A.G. Irwin, Trans. Br. Mycol. Soc. 88(2): 163 (1987)
Notes: The genus Acrocalymma was introduced by Alcorn and Irwin (1987) for a single species, A. medicaginis, which was associated with a root and crown rot disease of Medicago sativa (Fabaceae). Acrocalymma is characterized by globose conidiomata with a long pycnidial beak, and hyaline, or becoming pale brown with age, cylindrical to fusiform, aseptate, or 1–3-septate conidia, bearing an appendage at one or both ends. Zhang et al. (2012a) described an additional species A. aquatica Huang Zhang & K.D. Hyde from Thailand on submerged wood in a freshwater stream. A third species, A. cycadis Crous & R.G. Shivas, was introduced by Crous et al. (2014b), and it is distinct from A. aquatica by its smaller conidia. Trakunyingcharoen et al. (2014) revised the genus Sphaerellopsis, and allied lichenicolous and other genera, and transferred an isolate previously incorrectly identified as S. filum (CBS 317.76) to Acrocalymma, as A. fici Crous & Trakun. They also synonymized the genus Rhizopycnis D.F. Farr under Acrocalymma based on morphology and DNA phylogeny, and proposed a new combination namely, A. vagum for Rhizopycnis vagum (type species of Rhizopycnis). The sexual morph of Acrocalymma medicaginis has been linked to Massarina walkeri by Shoemaker et al. (1991) in culture. However, Trakunyingcharoen et al. (2014) showed that the two isolates which produced sexual morphs in culture are not conspecific with Acrocalymma medicaginis, and made a new combination A. walkeri (Shoemaker, C.E. Babc. & J.A.G. Irwin) Crous & Trakun. (Shoemaker et al. 1991; Trakunyingcharoen et al. 2014). A new family Acrocalymmaceae was introduced in Dothideomycetes to accommodate Acrocalymma.
We re-examined the type material of Acrocalymma medicaginis and provide a description and illustrations. We also illustrate A. aquatica.
Distribution: Australia, India, Spain, Thailand, USA (Alcorn and Irwin 1987; Nag Raj 1993; Trakunyingcharoen et al. 2014, this study).
Acrocalymma aquatica Huang Zhang & K.D. Hyde, Cryptogr. Mycol. 33(3): 337 (2012)
Facesoffungi number: FoF 07098, Fig. 8
Acrocalymma aquatic(MFLU 11-1113, holotype) a Herbarium specimen. b Appearance of black conidiomata on the host. c, d Vertical sections of conidiomata. e–f Section of peridium. g–j Conidiogenous cells and developing conidia. k–o Conidia (note k, m from herbarium specimen MFLU 111137). Scale bars d–c = 100 µm, e–f = 10 µm, g–o = 5µm
Saprobic on submerged wood in freshwater stream. Sexual morph: undetermined. Asexual morph:Conidiomata 120–270 µm diam., 110–250 µm high, black, solitary to gregarious, immersed to partly erumpent, pycnidial, globose to subglobose or irregular in shape, unilocular, glabrous, thick-walled, papillate. Ostiole single, centrally located. Conidiomatal wall 12–27 µm wide, composed of thick-walled cells of textura angularis, with brown to dark brown cells in outer several layers, gradually merging with hyaline cells lining the cavity. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–10 × 2–5 µm, hyaline, enteroblastic, phialidic, cylindrical to lageniform, determinate, formed from inner layers of conidiomata. Conidia 13–17 × 2–5.5 µm (\( \bar{x} \) = 15 × 4 µm; n = 30), hyaline, cylindrical to fusiform, with rounded apex and more or less narrow truncate base, straight, unicellular when young, becoming 1–2-euseptate at maturity, not constricted at the septa, thick-walled, smooth-walled, bearing a flabellate, mucoid appendage at apex.
Material examined: Thailand, Chiang Mai, on submerged wood, 16 November 2010, Huang Zhang d67 (MFLU 11–1113, holotype).
Acrocalymma medicaginis Alcorn & J.A.G. Irwin, Trans. Br. Mycol. Soc. 88(2): 163 (1987)
Facesoffungi number: FoF 07099, Fig. 9
(IMI 165613, ex-isotype) a, b Herbarium specimen. c, d Appearance of dark brown to black conidiomata on the host. e, f, g Vertical sections of conidiomata. h, i, j Section of peridium. k Ostiole note k is examined in lactic acid. l, m, n, o Conidiogenous cells and developing conidia. p, q, r, s Conidia with mucoid appendages (arrows). Scale bars c = 1000 µm, d = 200 µm, e–f = 200 µm, g = 100 µm, h–j = 25 µm, k = 100 µm, l–s = 5 µm
Caulicolous, corticolous on Medicago saliva (Fabaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, solitary to gregarious or confluent, immersed to semi-immersed, stromatic, pycnidial, globose to subglobose, pyriform or irregular in shape, multilocular or unilocular, with locule 125–390 µm diam., 225–475 µm high, often convoluted and irregularly divided, glabrous, thick-walled, with rounded or cylindrical, sinuate neck, ostiolate. Ostiole 95–280 × 65–120 µm, circular, cylindrical, straight or curved, centrally or laterally located. Conidiomatal wall 23–60 µm wide, composed two cell types; (a) outer layers of textura globosa to textura angularis, with dark brown to brown, thick-walled cells; (b) inner and middle layers of textura angularis to textura prismatica with pale brown to hyaline, thick-walled cells. Conidiophores reduced to conidiogenous cells arising all around the cavity of conidiomata. Conidiogenous cells 4–9 × 3–6 µm, hyaline, enteroblastic, phialidic, determinate, ampulliform to subcylindrical or lageniform, with a moderate periclinal thickening in collarette zone. Conidia 13–19 × 4–6 µm (\( \bar{x} \) = 17 × 5 µm; n = 30), hyaline, cylindrical to fusiform, with a rounded or obtuse apex and a narrow truncate base, straight, aseptate or 1–2-septate, not constricted at septa, thick and smooth-walled, bearing helmet-shaped, mucoid appendage at both ends.
Material examined: Australia, Queensland, Hermitage, on PDA, 21 March 1972–17 April 1972, J.A.G. Irwin (IMI 165613, ex-isotype).
Ajrekarella Kamat & Kalani, Mycopath. Mycol. Appl. 24: 300 (1964)
Facesoffungi number: FoF 07101
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, solitary to gregarious, immersed to semi-immersed, occasionally appearing superficial, oval to subglobose, unilocular, often with convoluted locules, thick-walled, glabrous or setose, ostiolate or nonostiolate, dehiscing by an irregular break in the apical wall. Ostiole conical to triangular, single, centrally located. Conidiomatal setae when present, pale brown to dark brown, lining the area of dehiscence, unbranched, septate at base, thick-walled. Conidiomata wall composed of thick-walled, hyaline to brown cells of textura angularis to textura intricata. Conidiophores reduced to conidiogenous cells, or septate and irregularly branched, hyaline, arising at the base and extending up the sides or all around the cavity of the conidiomata. Conidiogenous cells hyaline, enteroblastic, annellidic, ampulliform to subcylindrical, smooth. Conidia hyaline, fusiform to broadly naviculate, or ellipsoid, straight or slightly curved, aseptate, guttulate, bearing tubular, filiform, flexuous appendages at both ends, apical appendage mostly 1, rarely 2, unbranched, arising from the top of the conidia, basal appendages 2–5, eccentric, unbranched or rarely forked.
Type species: Ajrekarella polychaetriae Kamat & Kalani, Mycopath. Mycol. appl. 24: 300 (1964)
Notes: The genus was introduced by Kamat and Kalani (1964) with A. polychaetriae as type species, described from dead twigs of Jasminum calophyllum (Oleaceae) in India. Sutton (1967a) re-described the genus and provided a detailed description and illustration. Nag Raj (1993) added the second species A. stauronemoides collected from Stipa caudata (Poaceae) in Argentina and distinguished by a lack of conidiophores. Dharkar et al. (2009) described a third species A. asetosa from Cyperus nutans (Cyperaceae), which is distinct from other species in lacking conidiomatal setae, and bearing larger conidia. The genus has no molecular data and therefore, it is placed in Ascomycota, genera incertae sedis.
Distribution: Argentina, lndia (Nag Raj 1993, this study).
Key to species of Ajrekarella
1. Conidiomatal setae absent, conidia up to 30 µm…2
1. Conidiomatal setae present, conidia less than 30 µm…A. asetosa
2. Conidiophores reduced to conidiogenous cells, apical appendages 1–2, basal appendages 2–5, without lateral appcndages…A. stauronemoides
2. Conidiophores branched, integrated, apical and basal appendage 1, lateral appcndages 1–3…A. polychaetriae
Ajrekarella polychaetriae Kamat & Kalani, Mycopath. Mycol. Appl. 24: 300 (1964)
Facesoffungi number: FoF 07102, Fig. 10
Ajrekarella polychaetriae (IMI 101970, holotype) a, b Herbarium specimen. c, d Appearance of brown to dark brown conidiomata on the host. e, h–i Sections of peridium. f Ostiole. g, j Vertical section of conidiomata. k–l Conidiophores, conidiogenous cells and developing conidia. m–r Conidia. Scale bars c = 200 µm, d = 500 µm, e = 20 µm, f, h, i = 100 µm, g, j = 200 µm, k–l = 20 µm, m–r = 5 µm
Saprobic on dead stems of Jasminum calophyllum (Oleaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 550–760 µm diam., 220–380 µm high, brown to dark brown, pycnidial, mostly solitary, occasionally gregarious, immersed to semi-immersed, oval to subglobose, unilocular, thick-walled, glabrous, ostiolate. Ostiole 165–390 × 130–320 µm, conical to triangular, single, centrally located. Conidiomatal setae pale brown to dark brown, lining the area of dehiscence, unbranched, septate at base, straight or curved, smooth and thick-walled, tapered to acute apices. Conidiomata wall 10–50 µm wide, composed of thick-walled, hyaline to brown cells of textura angularis. Conidiophores hyaline, septate, irregularly branched, arising from the inner layer cells of conidiomata. Conidiogenous cells 9–20 × 2–4 µm, hyaline, enteroblastic, annellidic, cylindrical to subcylindrical, with up to 4 annellations. Conidia 12–17 × 4–6 µm (\( \bar{x} \) = 14 × 4.8 µm; n = 30), hyaline, fusiform to broadly naviculate, narrowed and truncated at the base, acute at the apex, aseptate, guttulate, bearing tubular, filiform, flexuous appendages (13–24 µm long) at both ends, apical appendage mostly 1, occasionally 2, unbranched, arising from the top of the conidia, basal appendages 2–5, unbranched or rarely forked.
Material examined: India, Poona, on the stems of Jasminum calophyllum (Oleaceae), I.K. Kalani, February 1961 (IMI 101970, holotype).
Notes: The conidiomatal setae presented in the original description (Sutton 1967b, 1980), but not observed in the present study.
Allantophomopsiella Crous, in Crous, Quaedvlieg, Hansen, Hawksworth & Groenewald, IMA Fungus 5(1): 180 (2014)
Facesoffungi number: FoF 07103
Leotiomycetes, Leotiomycetidae, Phacidiales, Phacidiaceae
Saprobic on branches of Pinus sylvestris. Sexual morph: see DiCosmo et al. (1983). Asexual morph:Mycelium immersed, composed of septate, branched, hyphae. Conidiomata dark brown to black, stromatic, pycnidial, solitary to gregarious, or confluent, initially immersed, ultimately becoming erumpent, pulvinate to subconical, irregularly plurilocular, thick-walled, glabrous, papillate, ostiolate. Ostiole single. short-cylindrical, centrally located. Conidiomatal wall composed of thick-walled, dark brown to pale brown cells of textura epidermoidea to textura intricata. Conidiophores formed from the innermost layers of conidiomata, hyaline, subcylindrical, branched or unbranched, septate, often constricted at septa, smooth-walled, invested by mucus. Conidiogenous cells hyaline, enteroblastic, annellidic, lageniform to subcylindrical, discrete or most often integrated, indeterminate, smooth-walled, with even subtending cells developing into new conidiogenous cells. Conidia hyaline, ellipsoid, obovoid or occasionally fusiform, obtuse or acute at apex, narrow and truncate at base, unicellular, thick and smooth-walled, guttulate, bearing a flame-like or irregular, mucoid apical appendage or sometimes, with a short or less conspicuous, mucoid, basal appendage.
Type species: Allantophomopsiella pseudotsugae (M. Wilson) Crous, in Crous, Quaedvlieg, Hansen, Hawksworth & Groenewald, IMA Fungus 5(1): 180 (2014)
Notes: The generic name Allantophomopsiella was proposed by Crous et al. (2014d) to accommodate a segment from Allantophomopsis, with A. pseudotsugae as the type. Allantophomopsiella is similar to Allantophomopsis with its pycnidial, convoluted, irregularly plurilocular conidiomata, annellidic conidiogenous cells and hyaline conidia bearing mucoid appendages of type C at one or both ends (Nag Raj 1993). However, Allantophomopsiella pseudotsugae is distinct from its annellidic conidiogenous cells developing not only from the terminal but also from subtending cells of the conidiophore and in conidial shape (inequilaterally fusiform or navicular). The length of the neck of ampulliform conidiogenous cells vary in view of a number of annellations. The genus is monotypic.
Distribution: Canada, Norway, UK (Hern et al. 2019, this study).
Allantophomopsiella pseudotsugae (M. Wilson) Crous, in Crous, Quaedvlieg, Hansen, Hawksworth & Groenewald, IMA Fungus 5(1): 180 (2014)
≡ Phomopsis pseudotsugae M. Wilson, Trans. R. Scottish Arboricult. Soc. 34(2): 147 (1920)
= Allantophomopsis pseudotsugae (M. Wilson) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 116 (1993)
= Potebniamyces coniferarum (G.G. Hahn) Smerlis, Can. J. Bot. 40: 352 (1962)
Facesoffungi number: FoF 07104, Fig. 11
Allantophomopsiella pseudotsugae (DAOM 129883) a, b Herbarium package and specimen. c–e Appearance of dark brown to black conidiomata on the host. f Ostiole. g Vertical section of conidiomatal wall in ostiolar region. h–j Vertical sections of conidiomata. k–n Conidiophores, conidiogenous cells and developing conidia. o–t Conidia. Scale bars: c–d = 500 µm, e = 200 µm, f–g = 50 µm, h–j = 200 µm, k–m = 10 µm, n–t = 5 µm
Saprobic on branches of Pinus sylvestris. Sexual morph: see DiCosmo et al. (1983). Asexual morph:Mycelium immersed, composed of septate, branched, hyphae. Conidiomata 260–570 µm diam., 300–500 µm high, dark brown to black, stromatic, pycnidial, solitary to gregarious, or confluent, initially immersed, ultimately becoming erumpent, pulvinate to subconical, irregularly plurilocular, thick-walled, glabrous, papillate, ostiolate. Ostiole 60–90 × 60–70 µm, single. short-cylindrical, centrally located. Conidiomatal wall 20–40 µm wide, composed of thick-walled, dark brown to brown cells of textura epidermoidea to textura intricata in the basal and lateral part, passing into thick-walled, pale brown cells of textura epidermoidea in locular wall, with dark brown cells of textura intricata at ostiolar region. Conidiophores formed from the innermost layers of conidiomata, hyaline, subcylindrical, branched or unbranched, septate, often constricted at septa, smooth-walled, invested in mucus. Conidiogenous cells 5–9 × 2–4 μm, hyaline, enteroblastic, annellidic, lageniform to subcylindrical, discrete or most often integrated, indetermined, smooth-walled, with new subtending cells becoming conidiogenous cells. Conidia 5–8 × 2–3 µm (\( \bar{x} \) = 6 × 2.5, n = 30) μm, hyaline, ellipsoid, obovoid or occasionally fusiform, obtuse or acute at apex, narrow and truncate at base, unicellular, thick and smooth-walled, guttulate, bearing a flame-like or irregular, mucoid apical appendage and sometimes, a shorter and less conspicuous, mucoid, basal appendage.
Material examined: Canada, Quebec, Lac-à-la-Tortue, on branches of Pinus sylvestris (Pinaceae), 10 November 1967, E. Smerlis (DAOM 129883).
Allantophomopsis Petr., Annls. mycol. 23(1/2): 104 (1925)
Facesoffungi number: FoF 07105
Leotiomycetes, Leotiomycetidae, Phacidiales, Phacidiaceae
Saprobic on dead leaves and stems of host plant. Sexual morph:Apothecia brown to black, solitary to gregarious, or confluent, subepidermal, immersed to semi-immersed, or erumpent, ellipsoid to conical, unilocular, glabrous, ostiole absent, dehiscing by an irregular split in the upper host tissue. Peridium composed of thick-walled, brown to dark brown cells of textura angularis. Hamathecium comprising paraphyses and asci. Paraphyses hyaline, numerous, slender, branched, anastomosing, septate, often constricted at septa. Asci unitunicate, 8-spored, clavate, rounded at apex, with a distinct apical ring, short-pedicellate, with an amyloid (I +) apical apparatus. Ascospores hyaline, uniseriate, biseriate, or irregularly arranged, ellipsoid to fusiform, unicellular, smooth-walled, guttulate. Asexual morph:Conidiomata brown to dark brown or black, stromatic, pycnidial, solitary to gregarious, subepidermal, immersed to semi-immersed or erumpent, globose to subglobose, irregularly plurilocular, glabrous, papillate, ostiolate. Ostiole single, cylindrical to circular, centrally located. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis to textura epidermoidea, textura globulosa or textura prismatica. Conidiophores formed from the innermost layers of conidiomata wall, hyaline, subcylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, with percurrent proliferation at apex, annellidic, cylindrical to ampulliform, discrete or integrated, smooth-walled. Conidia hyaline, fusiform to occasionally navicular, or irregular, unicellular, smooth-walled, guttulate, bearing a funnel-shaped or irregular, mucoid apical appendage, sometimes with a small, inconspicuous basal appendage (Nag Raj 1993; Crous et al. 2015a).
Type species: Allantophomopsis cytisporea (Fr.) Petr., Ann Mycol. 23(1/2): 104 (1925)
Notes: Allantophomopsis has been revised by several authors. Clements and Shear (1931) considered Allantophomopsis a synonym of Phoma; Sutton (1977a) regarded Allantophomopsis as synonym of Ceuthospora; Carris (1990) demonstrated the synonymy of Apostrasseria with Allantophomopsis. Nag Raj (1993) gave detailed accounts of the genus and its synonyms, and recognized seven species in the genus, viz. A. abietina Nag Raj, A. cytisporea (Fr.) Petr., A. fusiformis (Nag Raj) Nag Raj, A. lycopodina (Höhn.) Carris, A. pseudotsugae (M. Wilson) Nag Raj, A. pusilla (Lib.) Nag Raj and A. robusta (Nag Raj) Nag Raj. Crous et al. (2015a) epitypified the type species, A. cytisporea collected from Latvia on infected berries of Oxycoccus macrocarpus based on morphology and ITS and LSU sequence data. They placed Allantophomopsis in Phacidiaceae (Leotiomycetes) and resurrected A. lunata as a separate species, which had been synonymized under A. cytisporea (Nag Raj 1993). However, Allantophomopsis pseudotsugae had already been designated as the type for the new genus Allantophomopsiella (Crous et al. 2014d). Allantophomopsis fusiformis, which shares a similar form of conidiomata and conidia with the type species A. cytisporea, was listed as a synonym of Phacidium taxicola (Index fungorum 2019). This connection was probably reported because of their association on the same host (Nag Raj 1983; DiCosmo et al. 1983), and no other reason or evidence, however, was provided to support this linkage. Furthermore, the sexual morph of Phacidium sensu stricto is Ceuthospora Grev., which is morphologically distinct from Allantophomopsiella (Crous et al. 2014d). We re-examined the type materials of A. fusiformis and its presumed sexual morph Phacidium taxicola Dearn. & House. and provide a detailed description and photo plates.
Distribution: Austria, Canada, Germany, Latvia, Sweden, UK, USA (Nag raj 1993; Crous et al. 2015a, this study).
Allantophomopsis fusiformis (Nag Raj) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 114 (1993)
≡ Apostrasseria fusiformis Nag Raj, Can. J. Bot. 61: 17 (1983)
= Phacidium taxicola Dearn. & House, Mycologia 18(5): 239 (1926)
Facesoffungi number: FoF 07106, Figs. 12, 13.
Allantophomopsis fusiformis (sexual morph, JD 4882, type) a–c Herbarium package and specimen. d–e Appearance of black apothecia on the host. f, j Section of peridium. g Enlarged view of apical ascus. h–i Vertical sections of apothecia. k–n Asci. o Paraphyses. p–t Ascospores. Scale bars d = 500 µm, e = 200 µm, f, j = 20 µm, g, k–t = 5 µm, h–i = 100 µm
Allantophomopsis fusiformis (asexual morph, JD 4882, type) a–b Herbarium package and specimen. c–d Appearance of dark brown to black conidiomata on the host. e–f Vertical sections of conidiomata. g Ostiole. h–j Conidiophores, conidiogenous cells and developing conidia. k–l Vertical sections of conidiomatal wall. m–u Conidia. Scale bars: e–f = 100 µm, g, k–l = 20 µm, h–j = 10 µm, m–u = 5 µm
Saprobic on leaves and stems of Taxus canadensis. Sexual morph:Apothecia 290–380 µm diam., 130–300 µm high, black at base, brown at upper part in outline, solitary to gregarious, or confluent, subepidermal, initially immersed to semi-immersed, ultimately becoming erumpent, ellipsoid to conical, unilocular, glabrous, ostiole absent, dehiscing by an irregular split in the middle part of overlapping host tissue. Peridium 10–45 µm wide, composed of thick-walled, brown to dark brown cells of textura angularis. Hamathecium comprising paraphyses and asci. Paraphyses 30–55 × 1–3 µm, hyaline, numerous, slender, with an obtuse apex, branched and slightly swollen at base, anastomosing, septate, often constrict at septa. Asci 34–49 × 4–7 µm (\( \bar{x} \) = 41 × 5.6 µm; n = 30), unitunicate, 8-spored, clavate, bluntly rounded at apex, with a distinct apical ring, short-pedicellate, with an amyloid (J+) apical apparatus. Ascospores 7–10 × 2–3.5 µm (\( \bar{x} \) = 8.5 × 3 µm; n = 30), hyaline uniseriate or biseriate, or irregularly arranged, ellipsoid to fusiform, acute at both ends, unicellular, smooth-walled, guttulate. Asexual morph:Conidiomata 170–250 µm diam., 140–200 µm high, brown to dark brown, stromatic, pycnidial, solitary to gregarious, developing in linear series on both sides of the midrib, appearing as slightly elevated, minute, brown to black, rounded specks in surface view, subperidermal, deeply immersed, globose to subglobose, unilocular or irregularly plurilocular, glabrous, papillate, ostiolate. Ostiole 50–70 × 40–55 µm, single. cylindrical to circular, centrally located. Conidiomatal wall 10–30 µm wide, composed of thick-walled, pale brown to hyaline cells of textura angularis to textura prismatica in the basal and lateral part, passing into thick-walled, hyaline cells of textura prismatica in the locular wall, becoming olivaceous-brown cells of textura angularis at ostiolar region. Conidiophores formed from the innermost layers of conidiomata, hyaline, subcylindrical, branched at base, septate, often constricted at septa, smooth-walled. Conidiogenous cells 4–10 × 2–4 μm, hyaline, enteroblastic, annellidic, with 1–3 annellations, cylindrical to ampulliform, discrete or integrated, smooth-walled. Conidia 6–9.5 × 2–3.5 µm (\( \bar{x} \) = 8 × 3 μm, n = 30), hyaline, fusiform to occasionally naviculate, with an acute apex and a narrow truncate base, unicellular, smooth-walled, guttulate, with a funnel-shaped or irregular, mucoid, apical appendage, sometimes with a short, less conspicuous, mucoid, basal appendage.
Material examined: USA, New York, Hamilton Co., Blue Mountain Lake, on leaves and stems of Taxus canadensis (Taxaceae), 7 September 1920, H.D. House (JD 4882, type).
Alloneottiosporina Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 121 (1993)
Facesoffungi number: FoF 07107
Dothideomycetes, Pleosporomycetidae, Pleosporales, Phaeosphaeriaceae
Parasitic on living leaves of undetermined species of bamboo, grass, and Paspalum distichum. Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown or black, pycnidial, separate, gregarious or confluent, immersed to semi-immersed, globose to subglobose, unilocular, glabrous, thick-walled, papillate, ostiolate. Ostiole circular to oval, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown to pale brown or hyaline cells of textura prismatica or textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells of two kinds: (a) those producing macroconidia lining the base and side walls of conidiomata, hyaline, annellidic, ampulliform to lageniform, smooth-walled; (b) those producing microconidia restricted to the roof of the conidiomata near the ostiolar channel, hyaline, ampulliform, conical, lageniform or irregular, discrete, without annellations, smooth-walled. Macroconidia hyaline, ellipsoid, clavate, subcylindrical or fusiform, euseptate, constricted at the septa, smooth-walled, bearing mucoid appendages at both ends, apical appendgaes initially funnel-shaped or widely flared, eventually splitting to become tentaculiform; basal appendage less conspicuous than the apical appendages. Microconidia hyaline, ellipsoid or subglobose to obovate, acute or rounded at the apex, narrow and truncate at the base, smooth-walled.
Type species: Alloneottiosporina carolinensis Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 122 (1993)
Notes: Alloneottiosporina is characterized by hyaline, ellipsoid, clavate, subcylindrical or fusiform, euseptate conidia bearing tentaculiform or widely flared, mucoid appendages at both ends. Nag Raj (1993) discussed the differences between Alloneottiosporina and other morphologically similar genera viz. Neottiosporina Subram., Stagonospora (Sacc.) Sacc., Tiarospora Sacc. & Marchal and Tiarosporella Höhn. Two species were accepted in the genus, namely, A. carolinensis and A. infundibularis, but neither has been studied using molecular data. In the present study, a new species, Alloneottiosporina thailandica strain MFLUCC 15–0576 collected from Thailand is described. In a phylogenetic study, it formed a separate branch upper to Setophoma Gruyter, Aveskamp & Verkley in Phaeosphaeriaceae (Fig. 14). Comparison of A. thailandica with A. carolinensis and other coelomycetes genera indicates that it fits well within the generic concept of Alloneottiosporina. The phylogenetic analysis indicates that Alloneottiosporina may be related to Phaeosphaeriaceae, but in the absence of ex-type cultures, the phylogenetic position of this genus cannot be confirmed.
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS, SSU and tef1 sequences data of Phaeosphaeriaceae. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from (Phookamsak et al. 2017; Zhang et al. 2019) and GenBank. One hundred and six strains are included in the analyses, which comprise 3164 characters including gaps (LSU: 1-834, ITS: 838-1317, SSU: 1326-2269, tef1: 2273-3164). Paraleptosphaeria dryadis CBS 643.86 and Leptosphaeria doliolum CBS 505.75 were used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to the maximum parsimony and the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 23616.706146 is presented. The matrix had 961 distinct alignment patterns, with 20.70% of undetermined characters or gaps. Estimated base frequencies were: A = 0.243007, C = 0.236284, G = 0.267159, T = 0.253551; substitution rates AC = 1.136380, AG = 2.921747, AT = 2.414255, CG = 0.638090, CT = 6.696516, GT = 1.000000; gamma distribution shape parameter α = 0.152855. The maximum parsimonious dataset consisted of constant 2406, 553 parsimony-informative and 205 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 217 steps (CI = 0.295, RI = 0.602, RC = 0.177, HI = 0.705) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.05 changes. The new isolates are shown in blue
Distribution: Australia, USA, Thailand (Nag Raj 1993, this study).
Key to species of Alloneottiosporina
1. Conidia 2–3, unequally septate… 2
1. Conidia 1–septate… A. thailandica
2. Central cell longer than end cells… A. carolinensis
2. Central cell shorter than end cells… A. infundibularis
Alloneottiosporina thailandica W.J. Li & K.D. Hyde, sp. nov.
Index Fungorum number: IF557135, Facesoffungi number: FoF 07108, Fig. 15
Alloneottiosporina thailandica (MFLU 19-2896, holotype) a Herbarium specimen. b, c Black conidiomata on the host. d, e Vertical section of conidiomata. f Vertical section of peridium. k–l, g–j Conidiogenous cells and developing conidia. q–r Germinating spore. m–p, s–v Conidia. w. Culture on PDA. Scale bars: b–c = 200 µm, d–e = 100 µm, f = 20 µm, g–j, m–v, q–r = 5 µm, k–l = 10 µm, w–x = 2 cm
Etymology: Referring to Thailand, where the holotype was collected.
Parasitic on living leaves of grass, forming conspicuous, small, rounded, black conidiomata. Sexual morph: undetermined, Asexual morph:Conidiomata 100–140 µm diam., 80–140 µm high, pycnidial, separate, gregarious to confluent, globose to subglobose, semi-immersed, unilocular, glabrous, ostiolate, thick-walled. Conidiomatal wall 10–20 µm wide, composed dark brown to brown, thick-walled cells of textura angularis in the above half, becoming hyaline, thin-walled cells in the basal part. Ostiole single, inconspicuous, circular, centrally located. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising all around the wall of conidiomata, 3–6.5 × 3–7 µm, hyaline, enteroblastic, ampulliform or short cylindrical, determinate, smooth-walled. Conidia 10–17 × 3.5–4.5 µm (\( \bar{x} \) = 15 × 4 µm; n = 30), hyaline, ellipsoid to ovoid, with a broad and rounded apex, and a narrow and truncate base, 1-septate, slightly constricted at the septum, smooth and thick-walled, guttulate, bearing mucoid appendage at both ends; apical appendage tentaculiform, undulate or widely flared, arising from the partial mucoid sheath which initially enclosed the upper part of young conidium; basal appendage small, inconspicuous, slightly flared .
Culture characters: Colonies on PDA, reaching 5 mm diam. after 5 weeks at 20–25 °C, circular to irregular, with sparse, white aerial mycelium, surface olivaceous-black, reverse similar in colour.
Material examined: Thailand, Chiang Rai, on living leaves of grass, 19 March 2015, Jing-Zu Sun, WJL0030 (MFLU 19-2896, holotype), ex-type living culture MFLUCC 15–0576.
Notes: Alloneottiosporina thailandica shares a similar conidia form with the generic type, A. carolinensis, but can be distinguished by the number of septa. Alloneottiosporina thailandica has a single septum, while A. carolinensis has 2–3 septa. Moreover, A. thailandica has smaller conidia than A. carolinensis [20–30(–32) × 6.5–8.5 µm (\( \bar{x} \) = 25 × 7.5 µm)] and A. infundibularis [15–34 × (8–)9–10 µm (\( \bar{x} \) = 24.5 × 9.5 µm)].
Amerosporiopsis Petr., Bot. Arch. 43: 84 (1941)
Facesoffungi number: FoF 07109, Fig. 16
Amerosporiopsis gaubae (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Saprobic on dead leaves of Sesleria sp. (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata greenish black, pycnidial, solitary, immersed, globose, unilocular, glabrous, ostiolate. Ostiole single, circular, depressed, centrally located. Conidiomatal wall composed of dark brown, thick-walled cells of textura angularis in the upper part, becoming pale brown, thinner-walled at the base. Conidiophores arising from the innermost layers of conidiomata, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, determinate, smooth-walled. Conidia hyaline, unicellular, fusiform to limoniform, with acute apex and protuberant or slightly truncate base, smooth-walled, guttulate (Sutton 1980).
Type species: Amerosporiopsis gaubae Petr., Bot. Arch. 43: 84 (1941)
Notes: This genus has remained monotypic, and no molecular data is available. Amerosporiopsis shares similar morphology of conidia with Disculoides Crous et al. (2012a). The latter has olivaceous conidia and annellidic conidiogenous cells, which differ from the hyaline conidia and phialidic conidiogenous cells in Amerosporiopsis. To clarify the taxonomy of Amerosporiopsis, the type species will have to be recollected and epitypified.
Distribution: Iran (Sutton 1980).
Amphiporthe Petr., Sydowia 24(1–6): 257 (1971)
= Amphicytostroma Petr., Ann Mycol. 19(1/2): 63 (1921)
Facesoffungi number: FoF 4410, Fig. 17
Amphiporthe hranicensis (asexual morph, redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and developing conidia
Sordariomycetes, Sordariomycetidae, Diaporthales, Gnomoniaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: see Petrak (1970) and Senanayake et al. (2018). Asexual morph:Conidiomata stromatic, produced in association with the sexual morph, the locules of which are basal with long ostiolar channels; the stroma subperidermal, periclinal, completely surrounding the ostiolar channels but not extending as deeply as the locules, multilocular but not convoluted, glabrous. Ostiole absent, dehiscence by breakdown of the periderm near the apex of the ascocarp ostioles. Conidiomatal wall composed of pale brown, dense tissue of textura intricata at the base, becoming very diffuse, with little structural organization at the overlapping tissue where mainly consisting of sterile flexuous hyphae formed from very pale brown loose pseudoparenchyma. Conidiophores formed from the basal region of the locular wall, hyaline, filiform, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled, periclinal wall thickened towards the apex. Conidia hyaline, cylindrical, straight or slightly curved, unicellular, smooth-walled, eguttulate (Sutton 1980).
Type species: Amphiporthe hranicensis (Petr.) Petr., Sydowia 24(1–6): 257 (1971)
= Amphicytostroma tiliae (Sacc.) Petr., Ann Mycol. 19(1/2): 63 (1921)
Notes: Amphicytostroma tiliae (Sacc.) Petr. (type of Amphicytostroma) was considered to be the asexual morph of Amphiporthe hranicensis (Petrak 1921, Sutton 1980). Rossman et al. (2015a) reduced Amphicytostroma to a synonym of Amphiporthe, since the latter is a widely used name. Amphiporthe tiliae (Sacc.) Rossman & Castl. was introduced for A. hranicensis (= Diaporthe hranicensis Petr.) and Amphicytostroma tiliae (= Cytospora tiliae Sacc.) (Rossman et al. 2015a). This concept was followed by Wijayawardene et al. (2017b). To avoid confusion with the generic name Amphiporthe, we recommend using the old name A. hranicensis rather than A. tiliae (the new combination taxa). Two species are included in Amphiporthe, i. e., A. hranicensis and, A. raveneliana (Thüm. & Rehm) M.E. Barr. Sogonov et al. (2008) and Senanayake et al. (2017, 2018) showed that A. hranicensis belongs in Gnomoniaceae, but fresh collections and molecular data for asexual morph of A. hranicensis are needed to confirm this connection.
Distribution: Czech Republic, Germany, UK (Sutton 1980; Senanayake et al. 2018).
Anaphysmene Bubák, Ann Mycol. 4(2): 124 (1906)
Facesoffungi number: FoF 07110, Fig. 18
Anaphysmene heraclei (redrawn from Sutton 1972a) a Conidia. b Vertical section of coniodiomata. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, caulicolous or foliicolous, sporodochial or acervular, solitary to gregarious or often confluent, immersed or superficial, thick-walled. Conidiomatal wall composed of textura angularis with dark brown, thick-walled cells. Conidiophores arising from basal layers of stroma, pale brown at the base, becoming much paler towards the apex, cylindrical to subulate, parallel, unbranched, septate, thick- and smooth-walled. Conidiogenous cells pale brown, annellidic, cylindrical, integrated, terminal, smooth-walled, with 1–8 percurrent proliferation. Conidia hyaline, falcate to fusiform or cylindrical, with obtuse apex and truncate base, straight or gently and irregularly curved, 1-septate, smooth-walled, guttulate.
Type species: Anaphysmene heraclei (Lib.) Bubák, Ann Mycol. 4(2): 124 (1906)
Notes: Anaphysmene shares similar conidia with Ascochytopsis and Pseudoseptoria and the differences among these genera see notes of Ascochytopsis. Sutton (1972a) re-described the genus and listed the synonyms. Sutton and Hodges (1990) described a second species A. cupressi B. Sutton & Hodges from needles of Cupressus lusitanica, which is distinguished by its sporodochial conidiomata and 10–14-septate conidia. No molecular data is available for Anaphysmene species.
Distribution: France, Germany (Sutton 1980).
Anthracoderma Speg., Boln Acad. nac. Cienc. Córdoba 11(2): 286 (1887) [1888]
Facesoffungi number: FoF 07111, Fig. 19
Ascomycota, genera incertae sedis
Fungicolous.Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, pycnidial, carbonaceous, solitary to gregarious or confluent, superficial, subspherical in section view, often shortly stipitate, unilocular or multilocular, glabrous. Ostiole absent, opening by irregular splits in the apical wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura epidermoidea in the basal part, progressively becoming paler to hyaline cells of textura angularis in the conidial hymenium. Conidiophores arising from the inner layer cells of locular wall, hyaline to subhyaline, irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, occasionally sympodial, lageniform or irregular, terminal or intercalary and integrated, indeterminate, smooth-walled. Conidia hyaline, fusiform, straight or irregularly curved, aseptate, smooth-walled, eguttulate (Morgan-Jones 1977; Sutton 1980).
Type species: Anthracoderma hookeri Speg., Boln Acad. nac. Cienc. Córdoba 11(2): 286 (1887) [1888]
Notes: Anthracoderma hookeri was collected from a fructification of Cyttaria hookeri (Cyttariaceae) (Sutton 1980). Petrak and Sydow (1935) studied the holotype of Anthracoderma and regarded it as a valid genus. Morgan-Jones (1977) and Sutton (1980) re-described the genus. Besides the type species, another two species are accepted in the genus, A. duvidosum Viégas, and A. selenospermum Speg. The genus has no molecular data and, therefore, fresh collections are needed to epitypify the genus and to place it in its natural classification.
Distribution: Argentina, Brazil, Chile (Sutton 1980, https://www.gbif.org/).
Aoria Cif., Atti Ist. bot. Univ. Lab. crittog. Pavia, Ser. 5 19: 89 (1962)
Facesoffungi number: FoF 07112, Fig. 20
Aoria amphistroma (redrawn from Sutton 1980) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidiomata
Ascomycota, genera incertae sedis
Parasite on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata black, stromatic, solitary, occasionally confluent, subepidermal in origin, immersed, multilocular, glabrous. Ostiole absent, dehiscing by irregular ruptures in the apical wall and overlying host tissue. Conidiomatal wall composed of three cell types: (a) basal wall of textura angularis with thick-walled, dark brown to subhyaline cells; (b) inter-locular tissue of textura porrecta with thick-walled, black to dark brown cells; (c) upper wall of textura angularis with thinner-walled, brown to hyaline cells, which is packed with brown fungal tissue of textura angularis to textura epidermoidea. Conidiophores arising from innermost layer of basal stroma, hyaline, cylindrical to subcylindrical, tapered to the apex, unbranched or branched immediately below the transverse septa, smooth-walled. Conidiogenous cells hyaline, holoblastic, sympodial, lageniform, integrated, indeterminate, loci indistinct, smooth-walled. Conidia hyaline, ellipsoid with broaden and obtuse apex, and narrow, acute base, unicellular, smooth-walled, guttulate (Sutton 1980; Morgan-Jones 1972).
Type species: Aoria amphistroma Cif., Atti Ist. bot. Univ. Lab. crittog. Pavia, Ser. 5 19: 89 (1962)
Notes: Aoria amphistroma is associated with tar spot on leaves of Lyonia sp. (Ericaceae) in Dominica (Sutton 1980). Aoria shares a similar form of conidiomata with the asexual morph of Rhytisma (= Melasmia), but the latter was separated from the former by its enteroblastic, phialidic, conidiogenous cells and cylindrical conidia (Sutton 1980). The genus is monotypic and no molecular data. New collections are needed and subjected to DNA analyses to place Aoria in a natural classification.
Distribution: Dominican Republic (Sutton 1980).
Aphanofalx B. Sutton, Trans. Br. Mycol. Soc. 86(1): 21 (1986)
Facesoffungi number: FoF 07113, Fig. 21
Aphanofalx mali (redrawn from Nag Raj 1993) a Conidia. b Vertical sections of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, scattered to gregarious, immersed to semi-immersed, globose to subglobose or oval, unilocular, glabrous, ostiolate. Ostiole single, circular or oval, centrally located. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis, becoming darker at the ostiolar region. Conidiophores formed from the innermost layer wall cells of the conidiomata, hyaline, short-cylindrical to doliiform, branched, and septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, enteroblastic, annellidic, cylindrical or lageniform, integrated or discrete, indeterminate, smooth-walled, with numerous inconspicuous to conspicuous percurrent proliferations toward apex. Conidia hyaline, fusiform, lunate or irregular, curved, unicellular, smooth-walled, bearing a broad tubular, plectronoid to podiform, unbranched, excentric basal appendage (Nag Raj 1993).
Type species: Aphanofalx mali B. Sutton, Trans. Br. Mycol. Soc. 86(1): 22 (1986)
Notes: Two species, Aphanofalx irregularis B. Sutton & Abbas on twigs of Salvadora oleoides (Salvadoraceae) and A. mali B. Sutton on lenticels of Malus pumila (Rosaceae) were accepted in Aphanofalx (Nag Raj 1993). Aphanofalx shares a similar form of conidiomata, conidiogenous cells and conidia with Pseudoseptoria, but is distinguished by its conidia bearing a basal appendage. There is no molecular data available for this genus.
Distribution: Pakistan, Zambia (Nag Raj 1993).
Apiocarpella Syd. & P. Syd., Ann Mycol. 17(1): 43 (1919)
Facesoffungi number: FoF 07114, Fig. 22
Apiocarpella macrospora (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Sexual morph: undetermined. Asexual morph:Conidiomata brown, pycnidial, solitary, immersed, unilocular, glabrous, ostiolate. Ostiole circular, centrally loacted. Conidiomatal wall composed of thin-walled cells of textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, doliiform to ampulliform, determinate, smooth-walled. Conidia hyaline, clavate, broaden and obtuse at the apex, narrowed and slightly truncate at the base, 1-septate, asymmetrical, smooth-walled, guttulate (Sutton 1980).
Type species: Apiocarpella macrospora (Speg.) Syd. & P. Syd., Ann Mycol. 17(1): 43 (1919)
Notes: Apiocarpella was regarded as a subgeneric taxon of Ascochyta (Mel’nik 1971). However, Ascochyta is polyphyletic, and the species in this genus usually possesses enteroblastic phialidic conidiogenesis, which differs the holoblastic conidiogenesis in Apiocarpella (Sutton 1980). Mel’nik (1971) accepted the generic name for 8 species and extended the range to include species on Gramineae, Leguminosae and Ranunculaceae. Sutton (1980) provided a detailed description and illustration for Apiocarpella. No molecular data is available for this genus. Fresh collections of type species are needed to placed Apiocarpella in a natural group.
Distribution: Argentina, USA (Sutton 1980).
Apiognomonia Höhn., Ber. dt. bot. Ges. 35(8): 635 (1917)
= Discula Sacc., Syll. fung. (Abellini) 3: 674 (1884)
Facesoffungi number: FoF 07115
Sordariomycetes, Diaporthomycetidae, Diaporthales, Gnomoniaceae
Saprobic or parasitic on the host plant. Sexual morph: see Sogonov et al. (2007). Asexual morph:Conidiomata yellowish brown to black, acervular, solitary to gregarious, immersed to erumpent, irregularly round or oval in outline, subglobose in section view, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of the apical wall. Conidiomatal wall composed of thick-walled, yellowish brown cells at the base. Conidiophores restricted to the basal wall, hyaline, cylindrical, septate and branched, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic or annellidic, without or rarely with a few annellidic scars, lageniform to cylindrical, integrated, determinate, smooth-walled, tapered toward the apex. Conidia hyaline, broadly ellipsoid to oval, straight or slightly curved, aseptate, smooth-walled (Sogonov et al. 2007).
Type species: Apiognomonia veneta (Sacc. & Speg.) Höhn., Hedwigia 62: 47 (1920)
Notes: The asexual morph of Apiognomonia has been assigned to Discula Sacc. on twigs, and Gloeosporium Desm. & Mont. on leaves (Sogonov et al. 2007). Sogonov et al. (2007) revised Apiognomonia and confirmed its asexual morph in Discula. They resolved confusion concerning the correct name for the type species and related species of both Apiognomonia and Discula. Apiognomonia veneta and its asexual morph, D. nervisequa (Fuckel) M. Morelet (the earliest available epithet for D. platani), are the correct names for the type species of Apiognomonia and Discula, respectively (Sogonov et al. 2007). Discula umbrinella (Berk. & Broome) M. Morelet, was erroneously applied as the type species of Discula by Sutton (1980) and the erroneously applied asexual morph of A. errabunda (Sogonov et al. 2007). Because Apiognomonia is a well-defined genus (Sogonov et al. 2007, 2008), it was recommended to be used for the holomorph (Rossman et al. 2015a). In this study, three fresh collections from Italy on dead branches of Fagus sp. are introduced as a new species A. pseudohystrix (strain MFLUCC 16-1311 and MFLUCC 16-1140) and an asexual morph of A. errabunda (strain MFLUCC 16-1318). Besides, A. lasiopetali described by Crous et al. (2016a) and A. rigniacensis (Monod 1983) clustered away from the epitype of A. veneta (CBS 119036) (Fig. 23) and are thus excluded from Apiognomonia. More collections are needed to evaluate the generic concept of Apiognomonia.
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tef1 sequences data of Apiognomonia and related genera. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from (Sogonov et al. 2008) and GenBank. Forty-six strains are included in the analyses, which comprises 2846 characters including gaps (ITS: 1–546, LSU: 547–1331, rpb2: 1332–2399, tef1: 2400–2846). Melanconis stilbostoma CBS 109778 was used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 17068.866349 is presented. The matrix had 915 distinct alignment patterns, with 24.78% of undetermined characters or gaps. Estimated base frequencies were: as follows: A = 0.252526, C = 0.250094, G = 0.266606, T = 0.230774; substitution rates AC = 1.207685, AG = 3.220952, AT = 1.086464, CG = 1.001971, CT = 6.577202, GT = 1.000000; gamma distribution shape parameter α = 0.178267. Maximum likelihood bootstrap support values (MLBS) higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.03 changes. Ex-type or ex-epitype strains are in bold. The new isolates are highlighted
Distibution: Belgium, Canada, Czech Republic, France, Germany, Italy, New Zealand, Netherlands, Poland, Russia, Switzerland, UK, USA (Sogonov et al. 2007; this study).
Apiognomonia errabunda (Roberge ex Desm.) Höhn., Ann Mycol. 16(1/2): 51 (1918)
Facesoffungi number: FoF 07116, Fig. 24
Apiognomonia errabunda (MFLU 16-1148) a Herbarium specimen. b–c Appearance of orange to yellowish conidiomata on the host. d–e Vertical section of conidiomata. f, g, h Section of peridium. i–m Conidiophores, conidiogenous cells and developing conidia. n Germinating conidium. o–p Conidia. q–r Culture on MEA. Scale bars b–d = 500 µm, e = 100 µm, f = 20 µm, g–h = 50 µm, i–m = 10 µm, n = 20 µm, o–p = 5 mm, q–r = 20 mm
Saprobic or parasitic on the host plant. Sexual morph: see Sogonov et al. (2007). Asexual morph:Conidiomata 900–1000 µm diam., 450–500 µm high, black, stromatic, pycnidial, solitary, deeply immersed when young, becoming erumpent at maturity, dehiscing by an irregular break in the apical wall, multilocular, convoluted, with locules 200–450 µm diam., 200–400 µm high, ovoid to subglobose, thick-walled, glabrous, smooth-walled. Conidiomatal wall 85–180 µm wide, composed of relatively thick-walled, hyaline cells of textura angularis to textura intricata in the upper part, and thin-walled, hyaline cells of textura angularis in the basal and lateral part. Conidiophores arising from the inner layer cells of the conidiomata, hyaline, cylindrical, branched at the base, septate, smooth-walled. Conidiogenous cells 10–20 × 2–5 µm hyaline, enteroblastic, annellidic, ampulliform to subcylindrical, integrated or discrete, indeterminate, thick- and smooth-walled, tapered towards apex, with several indistinct percurrent proliferations (Fig. 24i, j, k, l). Conidia 10–15 × 3–5 µm (\( \bar{x} \) = 12 × 4 µm; n = 50), hyaline, fusiform to oval or cylindrical, rounded at both ends, straight or slightly curved, unicellular, smooth, guttulate.
Culture characters: colonies on MEA, reaching to 90 mm diam. after 14 d at 25 °C, forming concentric circles, white, flattened, with dense, filamentous, aerial, fluffy hyphae on the surface; margin entire, circular; reverse pale brown; colonies on PDA, reaching 30–40 mm diam. after 14 d at 25 °C, without forming concentric circles, whitish, plane to slightly convex, velutinous to felty, dense in the middle zone, becoming sparse in the marginal zone; margin entire to undulate; reverse whitish to pale brown with age.
Material examined: Italy, Province of Forlì-Cesena, Passo la Calla, on dead branch of Fagus sylvatica (Fagaceae), 5 April 2016, Erio Camporesi, IT2571 (MFLU 16-1148), living culture MFLUCC 16-1318 = ICMP 21528 = KUMCC 16-0073, (KUN, HKAS 93598).
Notes: Conidia of strain (MFLUCC 16-1318) share a similar form (fusiform to oval or cylindrical) and dimension (av. 12.7 × 3.8, Sogonov et al. 2007) to those of the asexual morph of Apiognomonia errabunda. Phylogenetic tree reconstruction based on combined genes of LSU, ITS, rpb2 and tef1 sequence dataset of Apiognomonia and related genera show that MFLUCC 16-1318 clustered with epitype strain (CBS 775.79) of A. errabunda (Fig. 23). Therefore, it is identified as the asexual morph of A. errabunda, and a detailed description and plate are provided.
Apiognomonia pseudohystrix W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557136, Facesoffungi number: FoF 07117, Fig. 25
Apiognomonia pseudohystrix (MFLU 16-1333, holotype) a Herbarium specimen. b, c Appearance of brown conidiomata on the host. d–e Vertical section of conidiomata. f–h Section of peridium. i–m Conidiogenous cells and developing conidia (arrows show two annellides). n Conidia. o–p Culture on PDA. Scale bars b–c = 200 µm, d–e = 500 µm, f, h = 20 µm, g = 50 µm, i–l = 10 µm, m = 5 µm, n = 20 µm, o–p = 20 mm
Etymology: Phylogeny closely related to A. hystrix, but morphology distinct.
Saprobic on dead branch of Ostrya carpinifolia. Sexual morph: undetermined. Asexual morph:Conidiomata 800–950 µm diam., 300–350 µm high, pale brown to brown, stromatic, acervular, solitary, deeply immersed when young, becoming erumpent at maturity, subglobose, multilocular, thick-walled, glabrous. Ostiole absent, dehiscing by irregular breakdown of the overlapping host tissue. Conidiomatal wall 20–150 µm wide, composed of relatively thick-walled, pale brown to hyaline cells of textura intricata in the middle wall (Fig. 25g), gradually merging with thick-walled, pale brown cells of textura angularis in the basal part and inner part of locular wall. Conidiophores arising from the inner layer cells of the conidiomata, reduced to conidiogenous cells or present, hyaline, short cylindrical, branched at the base, smooth-walled. Conidiogenous cells 7–15 × 2–4 µm, hyaline, enteroblastic, annellidic, cylindrical to lageniform, mostly discrete, occasionally integrated, indeterminate, smooth-walled, with 1–2 distinct percurrently proliferations towards apex (Fig. 25k, m). Conidia 14–22 × 3–5 µm (\( \bar{x} \) = 17 × 4 µm; n = 50), hyaline, fusiform, obtuse at the apex, slightly truncate at the base, 1-septate, slightly constricted at the septum, smooth-walled, guttulate.
Culture characters: colonies on PDA, reaching 30–50 mm diam. after 14 d at 25 °C, whitish to pale brown in the middle region, whitish in the margin region, becoming olivaceous brown with age, plane, velutinous to felty, with entire margin; reverse whitish to olivaceous brown with age.
Material examined: Italy, Province of Forlì-Cesena, Santa Sofia, Camposonaldo, on dead branch of Ostrya carpinifolia (Betulaceae), 8 May 2016, Erio Camporesi, IT2962 (MFLU 16-1333, holotype), living culture MFLUCC 16-1140 = ICMP 21546 = KUMCC 16-0096, (KUN, HKAS 101650, isotype); ibid., Predappio, Rocca Delle Caminate, on dead aerial branches of Torilis arvensis (Apiaceae), 2 March 2016, IT2862 (MFLU 16-0936), living culture MFLUCC 16-1311 = ICMP 21535.
Notes: Phylogenetically, Apiognomonia pseudohystrix is related to the sexual morph of A. hystrix (CBS 911.79), but it formed a separated branch (Fig. 23). The asexual morph of A. hystrix is undetermined. Apiognomonia pseudohystrix is distinguished from other taxa of Apiognomonia by its 1-sepate, fusiform conidia.
Aposphaeria Sacc., Michelia 2(6): 4 (1880)
Facesoffungi number: FoF 00756
Dothideomycetes, Pleosporomycetidae, Pleosporales, Melanommataceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: see Tibpromma et al. (2017). Asexual morph:Conidiomata black, stromatic, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed to superficial, globose to subglobose, unilocular or multilocular, glabrous, ostiolate. Ostiole circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores arising from the inner cavity of the conidiomata, hyaline, cylindrical, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled, with terminal or lateral apertures. Conidia hyaline, cylindrical to ellipsoidal, unicellular, smooth, guttulate (Sutton 1980).
Type species:Aposphaeria pulviscula (Sacc.) Sacc., Michelia 2(6): 4 (1880)
Notes: Sutton (1980) and Tian et al. (2015) redescribed and illustrated the generic type of Aposphaeria. More than 200 taxa are included in Aposphaeria, but most of them have not been re-studied (Sutton 1980). De Gruyter et al. (2013) showed that Aposphaeria was phylogenetically related to Melanommataceae, and Tian et al. (2015) accepted Aposphaeria as a separate genus in this family. Hashimoto et al. (2017) re-circumscribed Melanommataceae and accepted Melanomma Nitschke ex Fuckel sensu stricto in the family; Aposphaeria and other genera accepted by Tian et al. (2015) however, were placed in Pleosporales incertae sedis. However, Wanasinghe et al. (2018) adopted a broad concept as did Tian et al. (2015) and included Aposphaeria in Melanommataceae. A fresh collection (MFLU 16-2412) from Russia shares similar mophology and phylogeny with A. corallinolutea (Fig. 26), and is thus an additional collection. We accept Aposphaeria as a member of Melanommataceae, at least until an eptype of A. pulviscula is designated. We provide a detailed description and plate for A. corallinolutea.
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, tef1, rpb2 and SSU sequences data of Aposphaeria and related genera. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from Wanasinghe et al. (2018), Tian et al. (2015) and GenBank. Forty-one strains are included in the analyses, which comprise 3396 characters including gaps (LSU: 1–810, rpb2: 811–1463, tef1: 1464–2416, SSU: 2417–3396). Hysterium angustatum CBS 236.34 and CBS 123334 were used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 14254.356813 is presented. The matrix had 782 distinct alignment patterns, with 28.01% of undetermined characters or gaps. Estimated base frequencies were: as follows A = 0.250571, C = 0.240061, G = 0.275397, T = 0.233970; substitution rates AC = 1.127507, AG = 4.180424, AT = 0.956643, CG = 1.037671, CT = 9.714674, GT = 1.000000; gamma distribution shape parameter α = 0.136490. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.04 changes. Ex-type or ex-epitype strains are in bold. The new isolates are highlighted
Distribution: Australia, France, Germany, India, Italy, Morocco, Netherlands, Norway, Pakistan, Portugal, Russia, Slovenia, Spain, South Africa, Sweden, Thailand, UK, USA (De Gruyter et al. 2013; Li et al. 2016, this study, https://www.gbif.org/).
Aposphaeria pulviscula (Sacc.) Sacc., Michelia 2(6): 4 (1880)
FacesofFungi number: FoF 00757, Fig. 27
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata (30–)54–100 μm diam., (56–)84–133 μm high, brown, pycnidial, solitary to gregarious, semi-immersed to superficial, globose to subglobose, unilocular, glabrous, ostiolate. Ostiole circular, centrally located. Conidiomatal wall 9–13 μm wide, composed of thick-walled, dark brown to brown cells of textura angularis. Conidiophores 1–4 μm wide, arising from the inner cavity of the conidiomata, hyaline, cylindrical, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled, with terminal or lateral apertures. Conidia 2.3–2.8 × 0.8–1.4 μm (\( \bar{x} \) = 2.5 × 1.2 μm, n = 10), hyaline, cylindrical to ellipsoidal, unicellular, smooth, guttulate (Tian et al. 2015).
Material examined: Italy, Padova, on bare wood of Salix udensis (Salicaceae), January 1878, Saccardo (IMI 202557, isotype).
Aposphaeria corallinolutea Gruyter, Aveskamp & Verkley, in Gruyter et al., Stud. Mycol. 75: 28 (2012) [2013]
FacesofFungi number: FoF 01647, Fig. 28
Saprobic on dead twigs of Prunus padus (Rosaceae) or wood of Fraxinus excelsior (Oleaceae) or undetermined host. Sexual morph: see Tibpromma et al. (2017). Asexual morph:Conidiomata 200–400 µm diam., 180–340 µm high, black, stromatic, pycnidial, solitary to gregarious or confluent, subepidermal, initially immersed to semi-immersed, ultimately become erumpent, round and depressed in surface view, globose to subglobose in section view, unilocular or multilocular, glabrous, ostiolate. Ostiole circular, short papillate, centrally located. Conidiomatal wall 20–40 µm wide, composed of thick-walled, dark brown to brown cells of textura angularis in the periphery, gradually merging with thick-walled, hyaline cells towards conidial hymenium. Conidiophores formed from the inner cavity of the conidiomata, hyaline, cylindrical, branched at the base, septate, smooth-walled. Conidiogenous cells 6–15 × 1–2.5 µm, hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled. Conidia 3–5 × 1–2 µm (\( \bar{x} \) = 3.7 × 1.4 µm; n = 30), hyaline, cylindrical to ellipsoidal, unicellular, smooth, with guttule at each end.
Material examined: Russia, on dead stem of undetermined host, 27 May 2016, T.S. Bulgakov, DL 5 (MFLU 16-2412).
Aquasubmersa K.D. Hyde & Huang Zhang, Cryptog. Mycol. 33(3): 340 (2012)
Facesoffungi number: FoF 07118
Dothideomycetes, Pleosporomycetidae, Pleosporales, Aquasubmersaceae
Saprobic on submerged wood in freshwater habit. Sexual morph:Ascomata dark brown to black, solitary to gregarious, immersed to semi-immersed, subglobose, unilocular, glabrous, short papillate, ostiolate. Peridium composed of thick-walled, dark brown to pale brown cells of textura angularis. Hamathecium comprising asci and pseudoparaphyses. Pseudoparaphyses hyaline, subcylindrical, branched, septate. Asci 8-spored, bitunicate, cylindrical, pedicellate, rounded at apex, with an ocular chamber. Ascospores biseriate, hyaline, broadly fusiform with rounded ends, straight or slightly curved, with a median septum, constricted at septum, enclosed by a gelatinous sheath (adapted from Ariyawansa et al. 2015). Asexual morph:Conidiomata dark brown to black, pycnidial, subperidermal, solitary, semi-immersed, subglobose to ellipsoidal, unilocular, glabrous, thick-walled, ostiolate. Conidiomatal wall composed of dark brown to pale brown, thick-walled cells of textura angularis to textura prismatica. Conidiophores arising from inner layer cells of conidiomata, when present, subcylindrical to doliiform, unbranched, septate. Conidiogenous cells hyaline, holoblastic, subcylindrical to lageniform, determinate, discrete or indiscrete, thick and smooth-walled. Conidia hyaline, ellipsoidal, rounded at both ends, or sometimes with papillate bases, smooth, thick-walled, guttulate, with or without gelatinous sheath.
Type species: Aquasubmersa mircensis K.D. Hyde & Huang Zhang, Cryptog. Mycol. 33(3): 340 (2012)
Notes: Ariyawansa et al. (2015) added a second species A. japonica A. Hashim. & Kaz. Tanaka, which has both the sexual and asexual morph. They also showed that Aquasubmersa is close to Lophiotrema (Lophiotremataceae) based on analysis of SSU, LSU and ITS sequence data, but it is distinguished by conidiomata form. Aquasubmersa was assigned to a new family Aquasubmersaceae (Pleosporales) based on SSU, ITS, LSU, tef1, rpb2 sequence data (Hashimoto et al. 2017).
Distribution: Japan, Thailand (Zhang et al. 2012a, b; Ariyawansa et al. 2015).
Aquasubmersa mircensis K.D. Hyde & Huang Zhang, Cryptog. Mycol. 33(3): 340 (2012)
Facesoffungi number: FoF 07119, Fig. 29
Saprobic on submerged wood in a freshwater stream. Sexual morph: undetermined. Asexual morph:Conidiomata 150–250 µm diam., 100–200 µm high, dark brown to black, pycnidial, subperidermal, solitary, semi-immersed, subglobose to ellipsoidal, thick-walled, unilocular, glabrous, ostiolate. Conidiomatal wall up to 20 µm thick, consisting of textura angularis with cells brown to hyaline, thick-walled. Conidiophores arising from inner layer cells of conidiomata, absent or occasionally present, subcylindrical to doliiform, unbranched, septate. Conidiogenous cells 10–15 × 4–7 µm, hyaline, holoblastic, subcylindrical to lageniform, determinate, discrete or integrated, thick-walled, smooth. Conidia 16–21 × 8–11 µm (\( \bar{x} \) = 18 × 9 µm; n = 20), hyaline, ellipsoidal, rounded at both ends, or sometimes with papillate base, smooth, thick-walled, guttulate.
Material examined: Thailand, Chiang Mai, Mushroom Research Centre, on submerged wood, 29 November 2010, Huang Zhang, m3 (MFLU 11–1001, holotype).
Aschersonia Mont., Annls Sci. Nat., Bot., sér. 3 10: 121 (1848)
= Underwoodina Kuntze, Revis. gen. pl. (Leipzig) 3(3): 538 (1891)
Facesoffungi number: FoF 07396
Sordariomycetes, Hypocreomycetidae, Hypocreales, Clavicipitaceae
Saprobic or parasitic on scale insects and whiteflies. Sexual morph: see Chaverri et al. (2008). Asexual morph:Conidiomata reddish brown, formed from the body of an insect, stromatic, pycnidial, rounded or irregular in shape, multilocular, with immersed, globose, subglobose or obpyriform locules arranged peripherally in the same plane or irregularly in the inner stroma, glabrous, with or without ostiole. Conidiomatal wall composed of thick-walled, brown to hyaline cells. Paraphyses arising from the inner wall layer of locules, hyaline, filiform, tapered towards apex. Conidiophores reduced to conidiogeneous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, determinate, smooth-walled. Conidia hyaline, fusiform, straight or slightly curved, thick- and smooth-walled, guttulate, often in brightly colored mass, extruded in copious slime.
Type species: Aschersonia tahitensis Mont., Annls Sci. Nat., Bot., sér. 3 10: 122 (1848)
Notes: Aschersonia, Moelleriella and Samuelsia are entomopathogenic fungi (Chaverri et al. 2008). They are similar in having stromatic, pycnidial, multilocular conidiomata, hyaline, filiform paraphyses and enteroblastic, phialidic conidiogenous cells. Aschersonia and Moelleriella have fusiform conidia, while Samuelsia has allantoid conidia (Chaverri et al. 2008; Tibpromma et al. 2017). Aschersonia was separated from Moelleriella by its sexual morph. Ascospores of Moelleriella usually disarticulate at maturity inside the ascus, whereas the ascospores of Aschersonia do not disarticulate inside the ascus (Chaverri et al. 2008).
Aschersonia was initially recognized as plant parasites (Montagne 1848; Parkin 1906). However, it was reported as entomopathogenic by Webber (1897), and confirmed by Petch (1925). Accounts of the taxonomic history and phylogeny of Aschersonia were provided by Obornik et al. (1999), Sung et al. (2007a, b) and Hyde et al. (2020). Synonym of Aschersonia was provided by Kuntze (1891) as Underwoodina. Hypocrella was considered as the sexual morph of Aschersonia and the preferred name (Rossman et al. 2016a, b). However, Hyde et al. (2020) preferred Aschersonia over Hypocrella as Aschersonia was the earlier name.
Distribution: Argentina, Australia, Brazil, Britain, Canada, Columbia, China, Cuba, Dominican, Ecuador, Guyana, India, Indonesia, Japan, Mauritius, Malaysia, Mexico, Netherlands, New Zealand, Papua New Guinea, Philippines, Poland, Puerto Rico, Senegal, Sir Lank, Sudan, Thailand, USA, Venezuela, Vietnam (Montagne 1848; Hooker 1855; Berkeley and Curtis 1869; Patouillard 1891; Saccardo 1892; 1893, Patouillard and Hariot 1900; Hennings 1902, 1904; Koorders 1907; Spegazzini 1911; Saccardo 1913; Sydow and Sydow 1917, 1914; Petch 1921; Kobayashi 1973; Obornik et al. 1999; Mongkolsamrit et al. 2009; Qiu et al. 2011; Tibpromma et al. 2017).
Aschersonia calendulina Hywel-Jones & Mongkols., Mycol. Res. 113(6–7): 687 (2009)
Facesoffungi number: FoF 2805, Fig. 30
Aschersonia calendulina (MFLU 16-2918, asexual morph) a Herbarium specimens. b, c Appearance of reddish brown conidiomata. d–f Vertical sections of conidiomata. g, h Vertical sections of peridium. i Paraphyses. j, k Conidiogenous cells and developing conidia. l Conidia. Scale bars: b–d = 500 µm, e = 200 µm, f = 100 µm, g–i = 50 µm, j–k = 5 µm, l = 10 µm
Parasitic on unidentified insect. Sexual morph: undetermined. Asexual morph:Conidiomata 1400–2200 × 450–550 µm, reddish brown, with a slimy yellowish mass on the surface of conidiomata, hypophyllous, stromatic, pycnidial, solitary, rounded in outline, roughened, flattened, multilocular, with immersed, globose or subglobose to obpyriform locules, 250–550 µm diam., 500–650 µm high, thick-walled, glabrous, ostiolate. Ostiole well-developed, subcylindrical, single to each locule, centrally located, Conidiomatal wall 100–200 µm wide, composed of thick-walled, brown cells of textura oblita to textura epidermoidea in the outer layers, becoming hyaline cells towards conidial hymenium. Paraphyses 160–300 × 1–2 µm, hyaline, arising from the inner wall layer of locules, hyaline, filiform, tapered towards apex. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 8–15 × 1–2 µm, hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, determinate, smooth. Conidia 11–13 × 2–3 µm (\( \bar{x} \) = 12 × 2.5 µm, n = 50), hyaline, fusiform, unicellular, usually straight, thick- and smooth walled, guttulate.
Material examined: Thailand, Chiang Rai, on unidentified insect on living leaves of bamboo, Jing-Zu Sun, 10 February 2015, MRC15021007 (MFLU 16-2918).
Ascocalyx Naumov, Bolêz. Rast. 14: 138 (1926)
= Bothrodiscus Shear, Bull. Torrey bot. Club 34(6): 312 (1907)
Facesoffungi number: FoF 07120, Fig. 31
Phylogenetic tree generated from a maximum parsimony analysis based on a concatenated alignment of ITS, LSU, rpb2 and tub2 sequences data representing Ascochyta. The newly generated nucleotide sequences were compared against the GenBank database by blast search. Related sequences were obtained from Chen et al. (2015), Tibpromma et al. (2017) and GenBank. Thirty-three strains are included in the analyses, which comprise 2273 characters including gaps (ITS: 1–489, LSU: 490–1344, rpb2: 1345–1940, tub2: 1941–2273). Phoma herbarum CBS 134.96 and P. herbarum CBS 274.37 are used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 5657.513625 is presented. The matrix had 270 distinct alignment patterns, with 16.63% of undetermined characters or gaps. Estimated base frequencies were: A = 0.242420, C = 0.238519, G = 0.274514, T = 0.244547; substitution rates AC = 0.891880, AG = 4.215502, AT = 1.884133, CG = 0.651574, CT = 11.712755, GT = 1.000000; gamma distribution shape parameter α = 0.020000. The maximum parsimonious dataset consisted of constant 1979, 216 parsimony-informative and 78 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 439 steps (CI = 0.749, RI = 0.864, RC = 0.647, HI = 0.251) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes, respectively. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 20.0 changes. Ex-type and reference strains are in bold. New isolates are shown in bold and highlighted with colour
Leotiomycetes, Leotiomycetidae, Helotiales, Godroniaceae
Saprobic or parasitic on the host plant. Sexual morph: see Groves (1936). Asexual morph:Conidiomata dark brownish to black or olivaceous black and fleshy-leathery when moist, sometimes arising on the same stroma as the apothecia, stromatic, pycnidial, solitary to gregarious, superficial, initially globose to subglobose, then opening circularly and becoming cupulate, broadly stipitate, multilocular, glabrous, with numerous locules at one level concealed by incurved margin. Ostiole absent, dehiscence by irregular splits of each locule wall. Conidiomatal wall composed of thick-walled, pale brown cells of textura porrecta to textura epidermoidea at the base, gradually merging with thick-walled, brownish cells of predominantly textura angularis above, becoming darker at the periphery. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from inner layer cells of locular wall, hyaline, holoblastic, sympodial, cylindrical, indeterminate, smooth-walled, with 1–3 flattened, unthickened scars towards the apices. Conidia hyaline, lunate to falcate or cylindrical, with obtuse apex and truncate base, septate, smooth-walled (adapted from Groves 1936, 1968; Nag Raj 1977a, b; Sutton 1980).
Type species: Ascocalyx abietis Naumov, Morbi Plant. Script. Sect. Phytopath. Hort. Bot. Prince. USSR 14: 138 (1925) [1926]
= Bothrodiscus pinicola Shear, Bull. Torrey bot. Club 34(6): 313 (1907)
Notes: In the original description, the host of Bothrodiscus pinicola was said to be Pinus virginiana, but it was later proved to be Abies balsamea (Pinaceae) (Groves 1936). A detailed list of synonyms of the type species was provided by Groves (Groves 1968). The sexual morph of B. pinicola was linked to Ascocalyx abietis Naumov (type of Ascocalyx) on the basis of culture studies (Groves 1936). Groves (1968) provided more evidence on this connection, with two additional taxa of Ascocalyx associated with Bothrodiscus asexual morph. Johnston et al. (2014) and Wijayawardene et al. (2017b) reduced Bothrodiscus to a synonym of Ascocalyx, as the later sexual morph name was widely used. In the present study, we re-describe and re-illustrate the asexual morph of Ascocalyx. It is necessary to incorporate molecular studies to evaluate sexual and asexual morph connections of this genus.
Distribution: Canada, Finland, Japan, Pakistan, Spain, Sweden, Switzerland, Thailand, USA (Groves 1936; Sutton 1980, https://www.gbif.org/).
Ascochyta Lib., Pl. crypt. Arduenna, fasc. 1(Praef.): 8 (1830)
= Didymochaeta Sacc. & Ellis, Bull. Torrey bot. Club 25: 510 (1898)
Facesoffungi number: FoF 07121
Dothideomycetes, Pleosporomycetidae, Pleosporales, Didymellaceae
Saprobic or parasitic on the host plant. Sexual morph: see Chilvers et al. (2009). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, globose or subglobose, unilocular, glabrous, papillate, ostiolate. Ostiole single, circular, papillate or non-papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown to paler cells of textura angularis. Conidiophores arising all around the cavity of the conidiomata, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, annellidic or phialidic, subcylindrical to lageniform or ampuliform to dolliform, determinate, smooth-walled. Conidia hyaline, oblong to cylindrical, straight or slightly curved, 0–2-septate, slightly constricted at the septa, smooth-walled, guttulate (Chen et al. 2015).
Type species: Ascochyta pisi Lib., Pl. crypt. Arduenna, fasc. 1(nos 1–100): no. 59 (1830)
Notes: Most species of Ascochyta have been shown to highly host specific on Campanulaceae, Chenopodiaceae, Leguminosae, Poaceae, Solanaceae and Umbelliferae (Chen et al. 2015, 2017). Ascochyta has pycnidial conidiomata producing hyaline or sometimes pale brown, ovoid, oblong, subcylindrical or ellipsoidal, mostly two-celled conidia (Boerema and Bollen 1975; Boerema et al. 2004). This genus is often confused with Phoma, as both genera are similar in morphology, physiology, pathogenicity and nucleotide sequences (Boerema and Bollen 1975; Mendes-Pereira et al. 1999; Davidson et al. 2009; De Gruyter et al. 2009, 2010; Aveskamp et al. 2010). Chen et al. (2015) clarified the generic circumscriptions of Ascochyta and Phoma, and emended the generic concept for both genera based on multigene sequence data of ITS, LSU, rpb2 and tub2 and morphology characters. Since then, three additional species were described, A. boeremae L.W. Hou et al., A. italica Tibpromma et al. and A. rosae Tibpromma et al. (Chen et al. 2017; Tibpromma et al. 2017). However, A. rosae Tibpromma et al. with both sexual and asexual morph is identical to A. herbicola in morphology and phylogeny, and is synonymized under the latter in the present study. We describe a new taxon A. neopisi on Colutea arborescens (Fabaceae) from Italy.
Distribution: worldwide.
Ascochyta herbicola (Wehm.) Qian Chen & L. Cai, Stud. Mycol. 82: 187 (2015)
= Ascochyta rosae Tibpromma, Camporesi & K.D. Hyde, in Tibpromma et al., Fungal Diversity 83: 74 (2017)
Facesoffungi number: FoF 07122, Fig. 33
Saprobic on the host plant in terrestrial habit. Sexual morph: see Tibpromma et al. (2017). Asexual morph:Conidiomata 190–240 µm diam., 110–230 µm high, brown, pycnidial, scattered, rarely gregarious, semi-immersed to superficial, globose to subglobose, unilocular, thick-walled, glabrous. Ostiole absent, dehiscing by an irregular rupture in the apical wall. Conidiomatal wall 20–50 µm wide, composed of relatively thick-walled, dark brown to brown cells of textura angularis in the basal part from exterior to conidial hymenium, becoming thinner in the upper part. Conidiophores mostly reduced to conidiogenous cells, occasionally present, short, cylindrical, septate and branched at base, formed from inner layers of peridium. Conidiogenous cells 6–13 × 2–4 µm, hyaline, enteroblastic, annellidic, lageniform, attenuated towards apex, discrete or integrated, indeterminate, smooth-walled. Conidia 4–7 × 1.5–2.6 µm (\( \bar{x} \) = 5 × 2 µm; n = 50), hyaline, cylindrical to subcylindrical, rounded at both ends, straight or slightly curved, unicellular, thick- and smooth-walled.
Material examined: Russia, 20th anniversary of Red Army microdistrict, on dead stems of Potentilla sp. (Rosaceae), 21 May 2015, T.S. Bulgakov, T422 (MFLU 15-2126).
Notes: A new specimen (MFLU 15-2126) collected on Potentilla sp. in Russia nested between A. rosae (MFLUCC 15-0063) and A. herbicola (CBS 629.97) (Fig. 32). The sequence (LSU, ITS and rpb2) of the new collection are compared with A. rosae and A. herbicola, and the results showed only three loci (including two gaps) difference between the new collection and A. rosae in rpb2 except at the beginning and end, and also three loci difference between new collection and A. herbicola. In addition, our collection has similar conidia with A. herbicola (5–7(–8.5) × 2–3 µm, av. 6.2 × 2.3 µm (Degruyter et al. 1993). Thus, we treat these three collection as conspecific, and synonymize A. rosae under A. herbicola.
Ascochyta medicaginicola Qian Chen & L. Cai, Stud. Mycol. 82: 187 (2015)
= Phoma medicaginis Malbr. & Roum., in Roumeguère, Fungi Selecti Galliaei Exs. 37: no. 3675 (1886)
= Ascochyta medicaginicola var. macrospora Qian Chen & L. Cai, Stud. Mycol. 82: 187 (2015)
= Ascochyta medicaginicola var. medicaginicola Qian Chen & L. Cai, Stud. Mycol. 82: 187 (2015)
Facesoffungi number: FoF 00423; Fig. 34
Ascochyta medicaginicola (MFLU 14–0812) a Herbarium specimen. b, c Appearance of black conidiomata on the host surface. d Vertical section of conidioma. e, g Section of peridium. f Ostiole. h–k Conidiogenous cells and developing conidia. l–o Conidia. Scale bars: b = 500 µm, c = 100 µm, d = 50 µm, e–g = 25 µm, h–o = 5 µm
Saprobic, endophytic or pathogenic on the host plant in terrestrial habitat (Ellwood et al. 2006; Aveskamp et al. 2008, 2010; Rivera-Orduña et al. 2011). Sexual morph: see Tibpromma et al. (2017). Asexual morph:Conidiomata 150–250 µm diam., 100–150 µm high, dark brown, pycnidial, solitary to gregarious, immersed to semi-immersed, globose, unilocular, thick-walled, ostiolate. Ostiole circular, papillate, centrally located. Conidiomatal wall 15–30 µm wide, composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–7 × 5–8 µm, hyaline, enteroblastic, phialidic, doliiform, determinate, smooth-walled. Conidia 10–20 × 3.5–5.5 µm (\( \bar{x} \) = 15.5 × 5, n = 20), ellipsoidal to ovoid, hyaline, straight or slightly curved, 0–2-septate, constricted at septa, obtuse at both ends, smooth-walled, guttulate.
Material examined: Italy, Province of Ravenna, Zattaglia, on dead twig of Scabiosa sp. (Caprifoliaceae), 30 December 2012, E. Camporesi, IT988 (MFLU 14-0812), living culture, MFLUCC 13-0485, (KUN, HKAS 83971).
Notes: Phoma medicaginis was synonymized under A. medicaginicola var. macrospora by Chen et al. (2015), but this variety together with A. medicaginicola var. medicaginicola are listed as synonyms of Ascochyta medicaginicola in Index Fungorum (2018). We provide a detailed description and photo plate for A. medicaginicola.
Ascochyta neopisi W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557137, Facesoffungi number: FoF 07123, Fig. 35
Ascochyta neopisi (MFLU 16-0291, holotype) a Herbarium package and specimen. b–c Appearance of brown conidiomata on the host. d Vertical section of conidiomata. e–f Section of peridium. g–j Conidiogenous cells and developing conidia. k Germinated conidium. l–m Conidia. n, o Culture on PDA. Scale bars b = 100 µm, c = 200 µm, d = 50 µm, e = 20 µm, f = 10 µm, g–j, l–m = 5 µm, k = 20 mm,n–o = 10 mm
Etymology: Referring to its morphology similar to A. pisi, but phylogeny is distinct
Saprobic on dead stems of Colutea arborescens (Fabaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 80–120 µm diam., 80–100 µm high, brown to black, pycnidial, solitary to gregarious or confluent, immersed, globose, unilocular, thick-walled, glabrous, with a dark brown papillate ostiole. Ostiole short, single, circular to oval, centrally located. Conidiomatal wall 10–20 µm wide, composed of thick-walled, brown cells, gradually merging with hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–13 µm × 5–8.5 µm, hyaline, enteroblastic, phialidic, determinate, doliiform to ampulliform, smooth-walled, arising from the inner cavity of the conidiomata. Conidia 10–15 × 4–6.5 µm (\( \bar{x} \) = 12 × 5 µm; n = 30), hyaline, ellipsoidal to ovoid, obtuse at both ends, straight or slightly curved, 1-septate, constricted at the septum, smooth, guttulate.
Culture characters: Colonies on PDA, reaching 15–20 mm diam. after 7 days at 25–30 °C, with white, flattened, dense, filamentous, aerial, fluffy hyphae on the surface, margin crenate, circular; reverse brown to yellowish in the middle zone, paler toward margins.
Material examined: Italy, Ravenna, Zattaglia, on dead stem of Colutea arborescens (Fabaceae), 30 January 2016, Erio Camporesi, IT2780 (MFLU 16-0291, holotype), living culture MFLUCC 16-1303, (KUM, HKAS 97465, isotype).
Notes: Ascochyta neopisi is similar to A. pisi in having pycnidial conidiomata and 1-septate conidia, but is distinguished by its conidiogenous cells. Ascochyta neopisi has phialidic conidiogenous cells, while A. pisi has annellidic conidiogenous cells. Phylogenetic analyses based on LSU, ITS, rpb2 and tub2 sequence data showed that A. neopisi formed a separate branch basal to A. fabae, A. pisi, A. syringae and A. viciae (Fig. 32).
Ascochytopsis Henn., Bot. Jb. 38: 117 (1905)
Facesoffungi number: FoF 07124, Fig. 36
Ascochytopsis vignae (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat, such as Dolichos kilimandscharicus and Phaseolus lunatus (Fabaceae), or isolated from soil. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, initially closed, then becoming erumpent, cupulate, solitary, superficial, sessile or short-stipitate, globose, unilocular, glabrous. Ostiole absent, dehiscence by irregular split in the apical wall. Conidiomatal wall composed of an out layer of textura globosa with thick-walled, dark brown to pale brown cells, and inner layers of textura angularis with thick-walled, subhyaline cells. Conidiophores arising from the innermost layer of conidiomata, reduced to conidiogenous cells or when present, hyaline, irregular or dolliform, branched at base, septate, smooth-walled. Conidiogenous cells hyaline, phialidic, subcylindrical, integrated, determinate, smooth-walled. Conidia hyaline, fusiform to falcate, apex obtuse, base acute, straight or curved, aseptate, smooth-walled (Sutton 1980).
Type species: Ascochytopsis vignae Henn., Bot. Jb. 38: 117 (1905)
Notes: Anaphysmene, Ascochytopsis and Pseudoseptoria have hyaline, fusiform to falcate conidia. Anaphysmene has acervular conidiomata with 0–1-septate conidia, which differs from the pycnidial conidiomata with unicellular conidia in Ascochytopsis and Pseudoseptoria (Sutton 1980). Ascochytopsis was separated from Pseudoseptoria by its phialidic conidiogenous cells. Five species are included in Ascochytopsis (Wijayawardene et al. 2017). There is no molecular data available for this genus.
Distribution: Japan, Zimbabwe (Sutton 1980).
Ascodichaena Butin, Trans. Br. Mycol. Soc. 69(2): 249 (1977)
= Polymorphum Chevall., J. Phys. Chim. Hist. nat. Arts 94: 32 (1822)
Facesoffungi number: FoF 05926, Fig. 37
Ascodichaena rugosa (asexual morph, redrawn from Morgan-Jones 1974) a Vertical section of conidioma. b Conidiophores, conidiogenous cells, developing conidia and conidia
Leotiomycetes, Leotiomycetidae, Medeolariales, Ascodichaenaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: see Butin (1977) and Hawksworth (1983). Asexual morph:Conidiomata dark brown to black, pycnidial, gregarious, initially immersed, ultimately become erumpent, hysteriform to irregularly disciform, unilocular, glabrous, ostiolate. Ostiole slit-like, opening by a simple, divided or stellate fissure. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura prismatica at the basal part, becoming thick-walled, dark brown to hyaline cells of textura angularis at lateral upper part. Conidiophores arising from the innermost layers of conidiomata, hyaline, cylindrical, branched near the base, 1–5 septate, becoming swollen towards apex, smooth-walled. Conidiogenous cells hyaline, holoblastic, cylindrical, determinate, smooth-walled. Conidia hyaline, globose to ovoid, unicellular, smooth-walled, guttulate, becoming delimited from the conidiogenous cells either by the formation of a thin septum or breaking off with the conidiogenous cell still attached and appearing pedicellate (Hawksworth and Punithalingam 1973; Butin 1977).
Type species: Ascodichaena rugosa Butin, Trans. Br. Mycol. Soc. 69(2): 249 (1977)
= Polymorphum fagineum (Pers.) Chevall., J. Phys. Chim. Hist. nat. Arts 94: 33 (1822)
= Polymorphum quercinum (Pers.) Chevall., J. Phys. Chim. Hist. nat. Arts 94: 33 (1822)
= Polymorphum rugosum (L.) D. Hawksw. & Punith., Trans. Br. Mycol. Soc. 60(3): 503 (1973)
Notes: Polymorphum is characterized by hysteriform to irregularly disciform conidiomata and globose to ovoid, aseptate conidia. Polymorphum was introduced to accommodate two species, P. fagineum and P. quercinum, but neither was selected as type species. Hawksworth and Punithalingam (1973) discussed the synonym of Polymorphum, and lectotypified P. rugosum as a generic type. Hawksworth (1983) revised the nomenclature of Polymorphum and Ascodichaena, and connected the former to the latter sexual morph. He also applied P. quercinum as a generic type of Polymorphum. However, Polymorphum quercinum is a synonym of P. rugosum (Johnston et al. 2014). Based on “one fungus, one name” principle, Johnston et al. (2014) synonymized Polymorphum under Ascodichaena. No molecular data is available for Ascodichaena.
Distribution: Canada, France, Germany, Mexico, Pakistan, UK (Sutton 1980).
Asteroconium Syd. & P. Syd., Ann Mycol. 1(1): 36 (1903)
Facesoffungi number: FoF 07125, Fig. 38
Ascomycota, genera incertae sedis
Parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata acervular, subepidermal, solitary or confluent. Ostiole absent. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, monoblastic or sympodial, cylindrical, integrated, indeterminate, flexuous, smooth-walled. Conidia hyaline, rounded or tapered, with short projections, truncated or uneven at the base, tapered and obtuse at the apex, very thick-walled with much reduced lumina, smooth-walled (Sutton 1980).
Type species: Asteroconium saccardoi Syd. & P. Syd., Ann Mycol. 1(1): 36 (1903)
Notes: Asteroconium is similar to Tribolospora, and the differences between these two genera see notes of Tribolospora. Sutton (1980) recognized two taxa, Asteroconium nothopegiae T.S. Ramakr. et al. on Nothopegia dalzellii (Anacardiaceae) in India and A. saccardoi on leaves of Litsea glaucescens (Lauraceae) in Mexico. Chen et al. (1991) reported that A. saccardoi was associated with white vein disease on Cinnamomum glanduliferum (Lauraceae) in China. The sexual morph of Asteroconium is undetermined, and no molecular data is available.
Distribution: China, India, Japan, Mexico (Sutton 1980; Chen et al. 1991, https://www.gbif.org/).
Asteromella Pass. & Thüm., in Thümen, Mycoth. Univ., cent. 17: no. 1689 (1880)
Facesoffungi number: FoF 06214, Fig. 39
Asteromella ovata (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and developing conidia
Dothideomycetes, genera incertae sedis
Saprobic or parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary to gregarious, immersed, globose, unilocular. Ostiole circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, brown to paler cells of textura angularis. Conidiophores arising from the innermost layer of conidiomata, hyaline, cylindrical, branched at base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, lageniform to subcylindrical, integrated or discrete, indeterminate, smooth-walled, with periclinal thickenings at collarette zone. Conidia hyaline, cylindrical to oval, unicellular, smooth-walled (Sutton 1980; Vanev and van der Aa 1998).
Type species: Asteromella ovata Thüm., Mycoth. Univ., cent. 17: no. 1689 (1880)
Notes: Asteromella species are generally regarded as the microconidial or spermogonial states of Mycosphaerella species (Vanev and Van Der Aa 1998; Crous 2009), but this connection has not been confirmed by molecular data. Ruszkiewicz-Michalska (2016) monographed Asteromella in Poland and detailed the history and generic concept of the genus. Asteromella is currently placed in Dothideomycetes genera incertae sedis (Wijayawardene et al. 2017b). More than 200 taxa of Asteromella are listed in Index Fungorum (2019) and MycoBank (2019), but many of them are illegitimate or invalid. Further studies with a larger number of collections and molecular data are needed to clarify the generic concept of Asteromella.
Distribution: Argentina, Austria, Australia, Belgium, Brazil, China (Taiwan), Costa Rica, Croatia, Czech Republic, Dominican Republic, Ecuador, Finland, France, Gabon, Germany, Greece, Hungary, India, Iran, Italy, Latvia, Malta, Netherlands, Philippines, Poland, Portugal, Russia, Serbia, Spain, Sweden, Switzerland, Tanzania, Turkey, UK, Ukraine, USA, Venezuela (Sutton 1980; Vanev and Van der Aa 1998; Ruszkiewicz-Michalska 2016).
Asteromidium Speg., Anal. Soc. cient. argent. 26(1): 66 (1888)
Facesoffungi number: FoF 07126, Fig. 40
Asteromidium imperspicuum (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on living leaves of Sapindaceae. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, acervular, solitary to gregarious, pulvinate to doliiform, immersed, globose, unilocular, glabrous. Ostiole absent. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, cylindrical, discrete, indeterminate, smooth-walled, with 1–2 sympodial proliferations, scars unthickened, flat. Conidia hyaline, fusiform to cylindrical with obtuse apex and truncate base, 3-septate, smooth-walled (Sutton 1980; Quaedvlieg et al. 2013).
Type species: Asteromidium imperspicuum Speg., Anal. Soc. cient. argent. 26(1): 66 (1888)
Notes: Asteromidium shares similar morphology of conidiogenous cells with Diplosporonema, Phloeosporella, Stictosepta and Septocyta, and the differences among these genera see notes of Phloeosporella. Sutton (1980) provided a detail description and illustration for Asteromidium. There is no molecular data available.
Distribution: Brazil, Paraguay, Sweden (Sutton 1980, https://www.gbif.org/).
Aurantiosacculus Dyko & B. Sutton, in Dyko, Sutton & Roquebert, Mycologia 71(5): 922 (1979)
Facesoffungi number: FoF 04125, Fig. 41
Aurantiosacculus eucalypti (redrawn from Nag Raj and DiCosmo 1980) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia
Sordariomycetes, Sordariomycetidae, Diaporthales, Cryphonectriaceae
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: see Jiang et al. (2019). Asexual morph:Conidiomata bright orange to black, stromatic, pycnidial, solitary to gregarious, intraepidermal, immersed to erumpent, globose and flattened in outline, broadly conical to crateriform in section view, unilocular, glabrous, gelatinous. Ostiole absent, dehiscence by breakdown of the apical wall. Conidiomatal wall composed of thin-walled, hyaline to pale brown cells of textura angularis in the basal wall, becoming thick-walled, gelatinized cells of textura oblita in the upper wall. Conidiophores arising from the inner wall layer of conidiomata, hyaline, cylindrical to subcylindrical, branched, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, enteroblastic, lageniform to subcylindrical, integrated, indeterminate, smooth-walled, with percurrent proliferation several times or with minute periclinal thickening and collarette at the apex. Conidia hyaline, scolecosporous, sigmoid to falcate, tapered at the apex, slightly swollen and bearing a thick truncate scar at the base, unicellular, smooth-walled (Dyko et al. 1979; Nag Raj and DiCosmo 1980)
Type species: Aurantiosacculus eucalypti (Cooke & Massee) Dyko & B. Sutton, in Dyko, Sutton & Roquebert, Mycologia 71(5): 922 (1979)
Notes: Aurantiosacculus was proposed by Dyko et al. (1979) to accommodate a species separated from Protostegia as A. eucalypti, a fungus collected from leaves of Eucalyptus incrassata (Myrtaceae) in Australia. The genus is characterized by bright orange conidiomata and scolecosporous conidia with swollen bases. The conidiogenous cells of Aurantiosacculus eucalypti were described as phialides with several percurrently proliferating towards apex by Dyko et al. (1979) and Nag Raj and DiCosmo (1980). Three additional taxa, A. acutatus Crous & Summerell on E. viminalis and A. eucalyptorum Crous & C. Mohammed on E. globulus, and A. castaneae C.M. Tian & N. Jiang on Castanea mollissima are included (Crous et al. 2012a; Jiang et al. 2019). Aurantiosacculus was placed in Cryphonectriaceae based on the sequence of LSU and ITS (Crous et al. 2012a). More collections of Aurantiosacculus are needed, and an epitype of the generic type is also needed to confirm its placement.
Distribution: Australia, China, Tasmania (Crous et al. 2012a; Jiang et al. 2019).
Blennoria Moug. & Fr., in Fries, Syst. orb. veg. 1: 366 (1825)
Facesoffungi number: FoF 07127, Fig. 42
Blennoria buxi (redrawn from Morgan-Jones et al. 1972b) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic or parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, amphigenous, scattered to gregarious or confluent, semi-immersed, oval, circular to irregular, multilocular, divided into several locules with vertical columns of tissue, glabrous. Ostiole papillate. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis to textura porrecta. Conidiophores arising from the inner cells of the locular walls, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled, with a minute apical channel. Conidia hyaline, cylindrical, with obtuse apex and abruptly tapered base, unicellular, smooth-walled (Morgan-Jones et al. 1972b; Sutton 1972b, 1980)
Type species: Blennoria buxi Fr., Syst. orb. veg. 1: 366 (1825)
Notes: Blennoria was regarded as a synonym of Ceuthospora Grev. (Petrak 1929; Von Arx 1970). Sutton (1972b) and Morgan-Jones et al. (1972b) demonstrated that the genera could be distinguished by the form of conidiomata and conidia. Blennoria has eustromatic conidiomata and cylindrical conidia without apical appendage, whereas Ceuthospora has pseudostromatic conidiomata and cylindrical conidia bearing a minute, fan-shaped, mucilaginous apical appendage. Kirk et al. (2013) accepted Blennoria as a legitimate name, and we follow this concept. Fresh collections are needed and subjected to DNA sequence analyses to determine its placement.
Distribution: France, UK, Switzerland (Sutton 1972b, 1980).
Boeremia Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 36 (2010)
Facesoffungi number: FoF 07128
Dothideomycetes, Pleosporomycetidae, Pleosporales, Didymellaceae
Saprobic or parasitic on the host plant in terrestrial habitat or from soil (Boerema et al. 2004; Shamoun and Zhao 2005; Koike et al. 2006; Aveskamp et al. 2009). Sexual morph: see Aveskamp et al. (2010) and Jayasiri et al. (2017). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious, subperidermal, immersed or semi-immersed, variable in shape and size, mostly globose to subglobose, unilocular, glabrous, ostiolate. Ostiole single, non-papillate or papillate, lined internally with hyaline cells when mature, centrally or laterally located. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis or sometimes textura globosa. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, determinate, doliiform to ampulliform, formed from the inner layer cells of the conidiomata. Conidia hyaline, variable in shape, mostly aseptate, sometimes 1–2-septate, straight or slightly curved, thick and smooth-walled, often guttulate (Aveskamp et al. 2010).
Type species: Boeremia exigua (Desm.) Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 37 (2010)
Notes: Since Boeremia was introduced, 23 species and varieties have been accepted in the genus (Aveskamp et al. 2010; Chen et al. 2015, 2017; Jayasiri et al. 2017). Phylogenetic analyses based on LSU, ITS, rpb2 and tub2 sequence data indicated that a new collection (MFLU 15-1341) clustered with B. galiicola (MFLUCC 15-2279) (Fig. 43). Morphologically, the new collection shares a similar form of conidiomata and conidia with the asexual morph of B. galiicola, but differs in conidial septation and dimensions. However, it should be noted that in some coelomyceyes, conidial septation and dimensions on natural substrate may differ from those found in culture. Based on phylogeny, morphology, and host, this new collection is regarded as conspecific with B. galiicola, and an improved photo plate of the asexual morph is provided. Two strains (CBS 109183, CBS 119730) were named as B. exigua var. coffeae (Chen et al. 2017), but they formed two distinct branches (Fig. 43). Since the B. exigua var. coffeae was originally introduced based on strain CBS 109183 collected from Coffea arabica in Brazil, strain CBS 119730 collected from Camernoon is treated as Boeremia sp. Varieties B. exigua var. exigua (CBS 431.74), B. exigua var. forsythia (CBS 101213, CBS 101197), B. exigua var. gilvescens (CBS 101150) and B. exigua var. viburni (CBS 100354) nested with two strains of B. exigua (CBS 118.38, CBS 119.38) (Fig. 43). Based on phylogeny, these varieties are considered as conspecific species with the generic type B. exigua. Similarly, variety B. exigua var. populi (CBS 100167) is reduced to a synonym of B. exigua var. heteromorpha on the basis of molecular data (Fig. 43)
Phylogenetic tree generated from a maximum parsimony analysis based on a concatenated alignment of LSU, ITS, tub2 and rpb2 sequences data of Boeremia. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Forty-one strains are included in the analyses, which comprise 2379 characters including gaps (LSU: 1–961, ITS: 962–1450, tub2: 1451–1783, rpb2: 1784–2379). Neodidymelliopsis cannabis CBS 234.37 and N. polemonii CBS 109181 are used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 5796.175520 is presented. The matrix had 242 distinct alignment patterns, with 8.74% of undetermined characters or gaps. Estimated base frequencies were: A = 0.239226, C = 0.238771, G = 0.271655, T = 0.250348; substitution rates AC = 1.659257, AG = 4.372503, AT = 2.219281, CG = 0.796064, CT = 14.559800, GT = 1.000000; gamma distribution shape parameter α = 0.020000. The maximum parsimonious dataset consisted of constant 2079, 190 parsimony-informative and 110 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 433 steps (CI = 0.783, RI = 0.793, RC = 0.621, HI = 0.217) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 20 changes. Ex-type strains are in bold. New isolates are shown in bold and blue
Distribution: Worldwide.
Boeremia exigua (Desm.) Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 37 (2010)
Facesoffungi number: FoF 07129
= Boeremia exigua var. forsythiae (Sacc.) Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 37 (2010)
= Boeremia exigua var. viburni (Roum. ex Sacc.) Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 39 (2010)
= Boeremia exigua var. gilvescens Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 37 (2010)
Notes: Description see Aveskamp et al. (2010).
Boeremia exiguavar.heteromorpha (Schulzer & Sacc.) Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 38 (2010)
Facesoffungi number: FoF 07130
= Boeremia exigua var. populi (Gruyter & P. Scheer) Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 39 (2010)
Notes: Description and illustration see Chen et al. (2015).
Boeremia galiicola Jayasiri, Camporesi & K.D. Hyde, in Jayasiri, Hyde, Jones, Jeewon, Ariyawansa, Bhat, Camporesi & Kang, Mycosphere 8(8): 1089 (2017)
Facesoffungi number: FoF 02501, Fig. 44
Saprobic on dead stems of Galium sp. Sexual morph: see Jayasiri et al. (2017). Asexual morph:Conidiomata 200–300 µm diam., 200–250 µm high, black, pycnidial, solitary to gregarious, or confluent, subepidermal or peridermal in origin, immersed to partly erumpent, globose to subglobose, unilocular, glabrous, thick-walled, papillate. Ostiole short, single, centrally located. Conidiomatal wall 30–40 µm wide, composed of thick-walled, dark brown to hyaline cells of textura globosa to textura angularis, becoming thickened at the basal and upper part. Conidiophores occasionally present, hyaline, doliiform to subcylindrical, arising from inner layers of the pycnidial wall. Conidiogenous cells 5–8 × 4.5–7 µm, hyaline, enteroblastic, phialidic, ampulliform, determinate, smooth. Conidia 8–13 × 3–4.5 µm (\( \bar{x} \) = 10 × 3.5 µm; n = 30), hyaline, cylindrical obtuse, 0–1-septate, occasionally slightly constricted at the septum, straight or slightly curved, thick- and smooth-walled, guttulate.
Material examined: Italy. Province of Forlì-Cesena, Bagno di Romagna, near Lago Pontini, on dead stems of Galium sp. (Rubiaceae), 28 April 2015, Erio Camporesi, IT2464 (MFLU 15-1341, holotype); (KUN, HKAS 93620)
Notes: The conidia of this collection (MFLU 15-1341) did not germinate, and the sequence was obtained directly from fruiting bodies.
Botryocrea Petr., Sydowia 3(1–6): 140 (1949)
Facesoffungi number: FoF 07134, Fig. 45
Botryocrea sclerotioides (redrawn from Sutton 1980) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidioma. d Detail of conidiomatal wall and conidiophores
Ascomycota, genera incertae sedis
Saprobic on stems of Astragalus sp. (Fabaceae). Sexual morph: undetermined. Asexual morph:Conidiomata reddish brown, solitary to gregarious, pycnidial, superficial, globose to irregular, unilocular, glabrous. Ostiole absent, dehiscence by irregular split in the apical wall. Conidiomatal wall composed of thick-walled, hyaline cells of textura angularis to textura prismatica. Conidiophores arising from the innermost layers of conidiomata, hyaline, filiform, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, lageniform to cylindrical, integrated or discrete, indeterminate, smooth-walled, with periclinal thickening at collarette zone. Conidia hyaline, fusiform, tapered at the apex, curved, 3-septate, smooth-walled (Sutton 1980).
Type species: Botryocrea sclerotioides (Höhn.) Petr., Sydowia 3(1–6): 141 (1949)
Notes: Botryocrea is a monotypic genus and a shares similar form of conidia with Fusarium (Sutton 1980). Index Fungorum (2019) lists Botryocrea as a synonym of Fusarium, but the fungus is coelomycetous, whereas Fusarium is a hyphomycete. Kirk et al. (2013) did not treat Botryocrea as a valid name. We conclude that Botryocrea is a well-established genus and should be treated as a legitimate name. Fresh collections are needed and subjected to DNA sequence analyses to resolve its placement in a natural classification.
Distribution: Turkey (Sutton 1980).
Botryosphaeria Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 211 (1863)
Facesoffungi number: FoF 00141
Dothideomycetes, Incertae sedis, Botryosphaeriales, Botryosphaeriaceae
Saprobic or parasitic on the host plant. Sexual morph:Ascomata black, eustromatic, solitary to gregarious, sometimes forming botryose clusters, semi-immersed to partly erumpent, globose to subglobose, unilocular or multilocular, glabrous, thick-walled, papillate, ostiolate. Peridium composed of thick-walled, black to dark brown cells arranged in a textura angularis or textura globosa with cells becoming hyaline from outer layers to inner layers. Pseudoparaphyses hyaline, septate, constricted at septa, thin-walled, deliquescing from the basal parts when asci mature. Asci 8-spored, bitunicate, clavate or cylindric-clavate, pedicellate, with a prominent apical chamber, developing on a broad basal hymenial layer. Ascospores hyaline or sometimes becoming pale brown with age, irregularly biseriate in the ascus, ovoid or fusoid or fusoid-ellipsoid, usually widest in the middle, straight or inequilateral, thin- and smooth-walled, 0–2-septate, guttulate (description of sexual morph adapted from Liu et al. 2012; Phillips et al. 2013). Asexual morph:Conidiomata black, stromatic, pycnidial, solitary to gregarious or confluent, immersed in origin, becoming erumpent, unilocular or multi-locular, globose or ovoid to subglobose, thick-walled, glabrous, smooth, papillate, ostiolate. Ostiole circular to subcylindrical, centrally located. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis to textura prismatica or textura globosa. Paraphyses hyaline, filiform, septate, unbranched. Conidiophores sometimes reduced to conidiogenous cells, when present, hyaline, subcylindrical, septate, straight or slightly curved, formed from the most inner wall of the conidiomata. Conidiogenous cells hyaline, holoblastic or enteroblastic, phialidic or annellidic, ampulliform to subcylindrical or lageniform, determinate or indeterminate, discrete or integrated, smooth-walled, with moderate periclinal thickenings at above or percurrently proliferating several times. Conidia hyaline or sometimes becoming olivaceous or darker with age, variable in shape, elliptical to fusiform or clavate, straight or slightly curved, unicellular or 1–4-septate, thick-walled, smooth, bearing irregular mucoid sheath at both ends or apex only. Synasexual morphs present or absent. Spermatophores hyaline, cylindrical to subcylindrical, septate, occasionally branched, smooth-walled. Spermatogenous cells hyaline, cylindrical, discrete or integrated, smooth-walled, holoblastic or proliferating via phialides with periclinal thickenings. Spermatia hyaline, allantoid to rod-shaped, aseptate (the description of synasexual morph from Phillips et al. 2013).
Type species: Botryosphaeria dothidea (Moug.) Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 212 (1863)
Notes: Botryosphaeria was introduced by Cesati & De Notaris in 1863 to accommodate nine species, but the type species was not designated. Botryosphaeria berengeriana De Not., B. dothidea (Moug.) Ces. & De Not. and B. quercuum (Schwein.) Sacc. have been suggested as type species by different authors (von Höhnel 1909; Theissen and Sydow 1915; von Arx and Müller 1954; Barr 1972). However, B. berengeriana and B. quercuum were not one of the original species. Thus, B. dothidea was designated as type species (Barr 1972) and this proposal was followed by later authors (Barr 1972; Crous et al. 2006a; Chen et al. 2011; Liu et al. 2012; Phillips et al. 2013; Slippers et al. 2014). Slippers et al. (2004) epitypified B. dothidea on the basis of both morphology and phylogenetic study and confirmed the placement of the genus.
Botryosphaeria is characterized by sexual morph having hyaline, aseptate ascospores and bitunicate asci. The asexual morphs have been assigned to diplodia-like and fusicoccum-like conidial states (Denman et al. 2000; Crous et al. 2006a; Liu et al. 2012). However, Phillips et al. (2013), restricted its asexual morph in Fusicoccum only and synonymized Fusicoccum under Botryosphaeria on the basis of multi-locus of LSU, ITS, tef-α and tub2 sequences data. In addition, they accepted seven species in Botryosphaeria. Dissanayake et al. (2016) included ten species in the genus. Four additional species have since been recognized, B. pseudoramosa G.Q. Li & S.F. Chen, B. qingyuanensis G.Q. Li & S.F. Chen, B. rosaceae Y.P. Zhou & Ying Zhang, and B. wangensis G.Q. Li & S.F. Chen (Zhou et al. 2017; Li et al. 2018).
In this study, six fresh collections from Italy were studied. The phylogeny derived from the ITS and tef-α dataset shows that these strains grouped with B. agaves, B. auasmontanum F.J.J. Van der Walt et al., B. dothidea,B. mimutispermatia Ariyawansa et al., B. sinensis, and B. wangensis, and presented as polytomy (data not shown here). In an attempt to resolve the polytomy, additional data from the tub2 are included in the phylogenetic analyses (Fig. 46). Compared to the trees generated from ITS and tef-α, the later phylogeny provided better resolution for each species clade in general, but the polytomy is still not completely resolved. Comparative studies of LSU, ITS, tef-α and tub2 sequence data show that these six collections were conspecific species with B. dothidea. The sequence similarities between B. dothidea (CBS 115476) and our collection are shown in the Table 1. Similarly, Botryosphaeria minutispermatia is synonymized under B. dothidea. The sequence similarities between B. dothidea (CBS CBS 115476) and B. minutispermatia (GZCC 16-0013) are 99% (518/521) in ITS (included 3 gaps), 98% (274/280) in tef-α (included 1 gap). Morphologically, Botryosphaeria minutispermatia is similar to B. dothidea in both sexual and asexual morph, and the only difference is the dimension of ascomata, asci and conidia (Ariyawansa et al. 2016). However, it should be noted that the dimension of ascomata, asci, and conidia could be changed with different geography and host. In addition, Botryosphaeria rosaceae is reduced to a synonym of B. kuwatsukai based on both morphology and phylogeny.
Phylogenetic tree generated from a maximum parsimony analysis based on a concatenated alignment of ITS, tef1 and tub2 sequences data of Botryosphaeria. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from (Liu et al. 2012; Phillips et al. 2013; Li et al. 2018) and GenBank. Thirty-five strains are included in the analyses, which comprise 1233 characters including gaps (ITS: 1–522, tef1: 523–813, tub2: 814–1233). Neofusicoccum parvum ATCC 58191 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the maximum parsimony and the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 2919.850785 is presented. The matrix had 245 distinct alignment patterns, with 14.17% of undetermined characters or gaps. Estimated base frequencies were: A = 0.209309, C = 0.308850, G = 0.253017, T = 0.228825; substitution rates AC = 0.826247, AG = 2.476259, AT = 1.076270, CG = 0.884896, CT = 4.252047, GT = 1.000000; gamma distribution shape parameter α = 0.168455. The maximum parsimonious dataset consisted of constant 1055, 80 parsimony-informative and 98 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 217 steps (CI = 0.894, RI = 0.897, RC = 0.802, HI = 0.106) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 10 changes. Ex-type or ex-epitype strains are in bold. New isolates are in blue
Botryosphaeria dothidea (Moug.) Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 212 (1863)
Facesoffungi number: FoF 03512, Figs. 47, 48
Botryosphaeria dothidea (a–e from MFLU 16-0504, f–h from MFLU 16-0936, i–k from MFLU 19-2870/IT217, l–m MFLU 19-2877/IT740) a–c, f, i, l Appearance of dark brown to black conidiomata on the host. d, g, j, m Vertical sections of conidiomata. e, h, k, n Section of peridium. Scale bars c = 500 µm, d = 200 µm, e, j = 50 µm, f, i = 200 µm, g, m = 100 µm, h, k, n = 20 µm
Botryosphaeria dothidea(a, b, d, l from MFLU 19-2873/IT428; c, g, m, r, s from MFLU 19-2877/IT740; e, f, n, o from MFLU 16-0936/IT2862s; h, t, u from MFLU 19-2870/IT217; i–k MFLU 16-0504 IT2795, p, q from MFLU 19-2876/IT734). a–e, g–h Conidiophores, conidiogenous cells and developing conidia. f Paraphyses. i–u Conidia (1–k stained by India ink, arrows show conidial sheath). Scale bars a, b, d, h, i–s = 5 µm, c, e, f, t, u = 10 µm, g = 20 µm
Saprobic on dead stems of host plant in terrestrial habitat. Sexual morph: see Phillips et al. (2013). Asexual morph:Conidiomata 100–2000 µm diam., 70–1000 µm high, black, stromatic, pycnidial, forming a botryose aggregate of up to 50, solitary to gregarious or confluent, immersed in origin, becoming erumpent, multi-locular, globose or ovoid to subglobose, thick-walled, glabrous, smooth, papillate, ostiolate. Ostiole circular to subcylindrical, centrally or laterally located. Conidiomatal wall 30–150 µm wide, composed of thick-walled, dark brown to hyaline cells of textura angularis to textura prismatica or textura globosa. Conidiophores formed from the inner cavity of the conidiomata, reduced to conidiogenous cells, or present, hyaline, cylindrical to subcylindrical. Conidiogenous cells 5–30 × 2–6 µm, hyaline, annelidic, phialidic or holoblastic, ampulliform to subcylindrical, integrated or determinate, smooth. Conidia 13–33 × 4–10 µm, hyaline, fusiform, with a rounded apex and a slightly narrow truncate base, straight or slightly curved, 0–1-septate, thick-walled, smooth, bearing irregular mucoid sheath at each or both ends or without sheath. Synasexual morph not known.
Culture characters: colonies on PDA, reaching 25–30 mm diam. after 14 days at 25 °C, white at first, then olivaceous or dark brown, eventually black, circular, flattened, with aerial hyphae dense, filamentous, cottony, margins undulate to entire. reverse white at first, becoming black in the middle, and forming numerous conidiomata.
Material examined: Italy, province of Forlì-Cesena, Castrocaro Terme, Sorgara, on dead aerial branches of Ailanthus altissima (Simaroubaceae), 26 January 2016, Erio Camporesi, IT2795 (MFLU 16-0504), living culture MFLUCC 16-1304 = KUMCC 16-0077, (KUN, HKAS 97481); ibid., Predappio Alta, on dead aerial stems of Artemisia sp. (Asteraceae), 22 September 2012, Erio Camporesi, IT740 (MFLU 19-2877), living culture MFLUCC 13-0063 = ICMP 20781, (KUN, HKAS 93660); ibid., 7 April 2012, Erio Camporesi, IT217 (MFLU 19-2870), living culture MFLUCC 13-0199 = ICMP 20771 = KUMCC 15-0637, (KUN, HKAS 101687); ibid., Predappio, Rocca delle Caminate, on dead aerial stems of Torilis arvensis (Apiaceae), 2 March 2016, Erio Camporesi, IT2862S (MFLU 16-0936), living culture MFLUCC 16-1312 = ICMP 21536 = KUMCC 16-0084, (KUN, HKAS 101648); ibid., near Castrocaro, on dead aerial stems of Urtica dioica (Urticaceae), 19 September 2012, Erio Camporesi, IT734 (MFLU 19-2876), living culture MFLUCC 13-0206 = ICMP 20780 = KUMCC 15-0613, (KUN, HKAS93659); ibid., Province of Pesaro-Urbino, Monte Nerone, on dead aerial branches of Cornus sanguinea (Cornaceae), 11 June 2012, Erio Camporesi, IT428 (MFLU 19-2873), living culture MFLUCC 13-0202 = ICMP 20773 = KUMCC 15-0605, (KUN, HKAS 93653).
Notes: Botryosphaeria dothidea was associated with a wide range of host plant; the details are provided by Phillips et al. (2013), and will not be present here. The asexual morph of Botryosphaeria dothidea is variable in form and dimension of conidiomata, conidiogenous cells and conidia (Fig. 48 and Table 2).
Botryosphaeria kuwatsukai (Hara) G.Y. Sun & E. Tanaka, in Xu, Wang, Ju, Zhang, Biggs,Tanaka & Sun, Fungal Diversity 71: 225 (2014)
= Botryosphaeria rosaceae Y.P. Zhou & Ying Zhang, in Zhou, Zhang, Dou & Zhang, Mycosphere 8(1): 167 (2017)
Facesoffungi number: FoF 07135
Description and illustration: see Xu et al. (2014), Zhou et al. (2017)
Notes: Botryosphaeria kuwatsukai (LSP 5, CBS 135219) and B. rosaceae (CGMCC3.18008, CGMCC3.18007) show close phylogenetic affinity (Li et al. 2018, and this study). The ITS and tef1 sequences of these two taxa are 100 % similar excluding the beginning and end where there are no base pairs to compare. Morphologically, B. kuwatsukai shows similar form of conidia and paraphyses to B. rosaceae (note: the conidiomata of B. kuwatsukai are not mentioned in detail in Xu et al. (2015) and cannot be compared). Based on above facts, B. kuwatsukai is regarded as conspecific with B. rosaceae. Because B. kuwatsukai has a priority, B. rosaceae is reduced as synonym of B. kuwatsukai.
Brunneodinemasporium Crous & R.F. Castañeda, in Crous, Verkley, Christensen, Castañeda-Ruíz & Groenewald, Persoonia 28: 128 (2012)
Facesoffungi number: FoF 07394, Fig. 49
Brunneodinemasporium brasiliense (redrawn from Crous et al. 2012b) a Conidiomatal seta on the surface of conidioma. b Conidiogenous cells and developing conidia. c Conidia. d Enlarged view of conidiomatal setae
Sordariomycetes, Sordariomycetidae, Chaetosphaeriales, Chaetosphaeriaceae
Saprobic on the host plant in terestrial habitat or on decaying wood in freshwater habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, scattered or aggregated, superficial, unilocular, setose. Conidomatal setae brown to black, abundant, subulate to cylindrical, simple, septate, unbranched, smooth-walled, arising randomly throughout basal stroma. Basal stroma composed of thick-walled cells of textura angularis. Conidiophores lining the basal stroma in a dense layer, hyaline to pure brown, cylindrical, branched, thin- and smooth-walled. Conidiogenous cells hyaline, phialidic, subcylindrical to lageniform, determinate, smooth-walled, periclinal thickening towards apex. Conidia hyaline to pale brown, fusiform, with obtuse apex and truncate base, gently curved or straight, unicellular, bearing single, unbranched, flexuous, tubular appendage at each end.
Types species: Brunneodinemasporium brasiliense Crous & R.F. Castañeda, in Crous, Verkley, Christensen, Castañeda-Ruíz & Groenewald, Persoonia 28: 129 (2012)
Notes: Two taxa are currently recognizedin Brunneodinemasporium, B. brasiliense on decaying leaf in Brazil and B. jonesii on decaying wood in a freshwater stream in China (Crous et al. 2012b; Lu et al. 2016). Brunneodinemasporium jonesii has hyaline to pure brown conidia with mucilaginous balls connecting the conidia in short false chains, whereas B. brasiliense has hyaline to subhyaline conidia with bipolar appendages (Lu et al. 2016).
Distribution: China, Brazi (Crous et al. 2012b; Lu et al. 2016).
Brunneodinemasporium jonesii Y.Z. Lu, Jian K. Liu & K.D. Hyde, in Lu, Liu, Hyde, Bhat, Xiao, Tian, Wen, Boonmee & Kang, Mycosphere 7(9): 1326 (2016)
Facesoffungi number: FoF02638, Fig. 50
Brunneodinemasporium jonesii (GZAAS 16–0062, holotype). a, b Appearance of dark brown to black conidiomata on host surface. c Slide view of conidiomata. d, h, i, j Conidiomatal setae. e, f–g Basal stroma of conidioma bearing conidiophores and conidiogenous cells (stained by cotton blue). k–m Conidia (note conidia are connected by mucilaginous balls). Scale bars: c = 500 µm, d = 100 µm, e–g = 10 µm, h = 50 µm, i = 20 µm, j–m = 5 µm
Saprobic on decaying wood in freshwater stream (Lu et al. 2016). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, with a white to buff slimy conidial mass in center, solitary to gregarious, superficial, cupulate, setose. Conidiomatal wall composed of thick-walled, brown cells of textura angularis at base, become thick-walled, darker cells of textura porrecta towards periphery. Conidiomatal setae composed of two types: (a) dark brown to black, conspicuous, subulate, unbranched, tapered towards the apex, thick-and smooth-walled (Fig. 50) (b) pale brown to brown at base, become hyaline and obtuse at apex, inconspicuous, subcylindrical or irregular, straight or curved, unbranched, septa, smooth-walled (Fig. 50). Conidiophores formed from the innermost layers of basal stroma, hyaline, cylindrical, unbranched or branched, septate, smooth-walled. Conidiogenous cells 7–13 × 1–2.5 µm, hyaline, phialidic, lageniform to subcylindrical, integrated, determinate, smooth-walled, with moderate periclinal thickenings. Conidia 6–9.5 × 1.5–2 µm (\( \bar{x} \) = 8 × 1.7 µm, n = 50), hyaline, fusiform, obtuse at apex, subtruncate at base, straight or curved, unicellular, smooth-walled, aguttulate or guttulate, with mucilaginous balls released at the conidial ends; connecting the conidia in short false chains. Chains of conidia arising from conidiogenous cells aggregated into a conspicuous slimy, dome-shaped mass.
Material examined: China, Guangxi Province, Fang Cheng Gang, on decaying wood in a freshwater stream, 15 May 2016, Yong-Zhong Lu, JHC17-1 (GZAAS 16–0062, holotype).
Brycekendrickia Nag Raj, Can. J. Bot. 51(7): 1337 (1973)
Facesoffungi number: FoF 07136
Ascomycota, genera incertae sedis
Saprobic on fallen and decaying leaves of Bambusa sp. (Poaceae) and Sporobolus sp. (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata pale brown to brown, stromatic, pycnidial, mostly epiphyllous, solitary to gregarious or often confluent, initially immersed, then becoming partly erumpent, globose to subglobose, unilocular or multilocular, glabrous, ostiolate. Ostiole single, funneled to flabellate, centrally located. Conidiomatal wall composed of pale brown to brown, thick-walled cells of textura angularis in the outer layers, and hyaline, thin-walled cells of textura prismatica in the inner layers. Conidiophores arising all around the cavity of the conidiomata, hyaline, branched, septate, invested in a thin, non-persistent mucus. Conidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical to obclavate, discrete or integrated, smooth. Conidia hyaline, navicular, with an obtuse apex and a narrow truncate base, aseptate, slightly curved, eguttulate, smooth-walled, bearing hyaline, branched, filiform, flexuous appendage at both ends.
Type species: Brycekendrickia indica Nag Raj, Can. J. Bot. 51(7): 1337 (1973)
Notes: Brycekendrickia indica was collected on fallen and decaying leaves of Bambusa sp. in India (Nag Raj 1973a). The genus remains monotypic and no molecular data is available.
Distribution: India.
Brycekendrickia indica Nag Raj, Can. J. Bot. 51(7): 1337 (1973)
Facesoffungi number: FoF 07137, Fig. 51
Brycekendrickia indica (DAOM 139472(c), ex-type) a–b Herbarium specimen. c, d Appearance of pale brown to brown conidiomata on sterilized sugarcane leaves. e, f, g Vertical sections of conidiomata. h Ostiole. i–k Section of peridium. l–m Conidiophores, conidiogenous cells and developing conidia. n–q Conidia in 10% KOH and stained by India ink. Scale bars c = 500 µm, d = 200 µm, e–g = 100 µm, h = 50 µm, i, k–m = 10 µm, j = 20 µm, n–q = 5 µm
Saprobic on fallen and decaying leaves of Bambusa sp. (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 250–300 µm diam., 350–450 µm high, pale brown to brown, stromatic, pycnidial, mostly epiphyllous, solitary to gregarious or often confluent, initially immersed, then becoming partly erumpent, globose to subglobose, unilocular or multilocular, glabrous, ostiolate. Ostiole 100–200 × 100–200 µm, single, funneled to flabellate, centrally located. Conidiomatal wall 10–40 µm thick, composed of pale brown to brown, thick-walled cells of textura angularis in the outer layers, and hyaline, thin-walled cells of textura prismatica in the inner layers. Conidiophores arising all around the cavity of the conidiomata, hyaline, branched, septate, invested in a thin, non-persistent mucus. Conidiogenous cells 6–10 × 1–3 µm, hyaline, enteroblastic, phialidic, subcylindrical to obclavate, discrete or integrated, smooth. Conidia 10–14 × 1.5–3 µm (\( \bar{x} \) = 12 × 2 µm; n = 30), hyaline, navicular with an obtuse apex and a narrow truncate base, aseptate, slightly curved, eguttulate, smooth-walled, bearing hyaline, branched, filiform, flexuous appendage at both ends (6 × 16 µm long).
Material examined: India, Bangalore, Lalbagh Gardens, on fallen and decaying leaves of Bambusa sp. (Poaceae), T.R. Nag Raj, 30 October 1970 (DAOM 139472(a), holotype); ibid., culture on PDA, 30 October 1970–30 November 1970 (DAOM 139472(b); ibid., culture on sterilized sugarcane leaf, 5 December 1970–21 December 1970 (DAOM 139472(c), type); ibid. on leaves of Bambusa sp., 3 November 1970, T.R. Nag Raj (DAOM 215548).
Calocline Syd., Ann Mycol. 37(4/5): 417 (1939)
Facesoffungi number: FoF 07138, Fig. 52
Calocline chusqueae (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Conidia
Ascomycota, genera incertae sedis
Parasitic on Chusquea serrulata (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, loosely aggregated into a linear row between veins, pycnidial, immersed to superficial, ampulliform, unilocular, glabrous. Ostiole circular, centrally located. Conidiomatal wall composed of thick-walled, very pale brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform to lageniform, determinate, smooth-walled, with periclinal thickening at collarette zone. Conidia hyaline, oval to cylindrical, unicelullar, smooth-walled (Sutton 1980; Morgan-Jones 1977).
Type species: Calocline chusqueae Syd., Ann Mycol. 37(4/5): 417 (1939)
Notes: Calocline is a monotypic genus. Calocline chusqueae has been reported as a pathogen on leaves of Chusquea serrulata (Poaceae), causing lenticular lesions (Sutton 1980). This genus shares a similar form of conidiomata, conidiogenous cells and conidia with Phoma. Calocline and Phoma are probably congeneric. However, Phoma is a polyphyletic genus; no molecular data is available for Calocline. Therefore fresh collections of type species are needed to clarify the taxonomy of Calocline.
Distribution: Ecuador (Sutton 1980).
Capnodium Mont., Annls Sci. Nat., Bot., sér. 3, 11: 233 (1849)
= Conidioxyphium Bat. & Cif., Quad. Lab. crittogam., Pavia 31: 72 (1963)
Facesoffungi number: FoF 06944
Dothideomycetes, Dothideomycetidae, Capnodiales, Capnodiaceae
Saprobic or parasitic on the host plant. Sexual morph: see Chomnunti et al. (2011). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious, superficial, flask-shaped or elongated, unilocular, glabrous, often with a basal sterile stalk. Ostiole circular, apical, surrounded by hyaline hyphae. Stalk wall composed of thick-walled, dark brown, parallel columns, longitudinally elongated cells of textura porrecta. Pycnidial wall composed of brown to pale brown, cuboid to longitudinally elongated pseudoparenchyma. Conidiophores arising from inner layer of the base and sides of the pycnidial wall, hyaline, cylindrical to subcylindrical, unbranched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to dolliform, integrated, determinate, smooth-walled. Conidia hyaline, ellipsoid to cylindrical, unicellular (Sutton 1980; Chomnunti et al. 2011).
Type species: Capnodium citri Berk. & Desm., in Berkeley, Journal of the Royal Horticultural Society 4: 11 (1849)
Notes: Chomnunti et al. (2011) synonymized Conidioxyphium with Conidiocarpus Woron. Phylogenetic tree reconstruction based on LSU, SSU and ITS sequence data showed that Conidioxyphium gardeniarum (type species of Conidioxyphium) is closely related to Capnodium (Fig. 53). This result is in accordance with Liu et al. (2015a, b). Morphologically, Conidioxyphium gardeniarum has elongated pycnidia adjacent to basal stalk; unlike those species in Conidiocarpus which have globose pycnidia adjacent to upper half part (Sutton 1980; Crous et al. 2009). Based on both morphology and phylogeny, Conidioxyphium is regarded as a synonym of Capnodium, thus a new combination Capnodium gardeniarum is introduced for this taxon. Microxiphium aciculiforme (CBS 892.73) also clustered with Capnodium taxa (Chomnunti et al. 2011; Liu et al. 2015a, b; Hongsanan et al. 2015, this study), thus a new combination Capnodium aciculiforme is introduced for this taxon. Besides, a new species Capnodium paracoartatum is introduced.
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, SSU and ITS sequences data of Capnodiaceae. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from (Hongsanan et al. 2015) and GenBank. Thirty-four strains are included in the analyses, which comprise 2353 characters including gaps (LSU: 1–855, SSU: 856–1908, ITS: 1909–2353). Dissoconium aciculare CBS 342.82 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 5878.599486 is presented. The matrix had 381 distinct alignment patterns, with 23.61% of undetermined characters or gaps. Estimated base frequencies were: A = 0.257003, C = 0.221996, G = 0.280254, T = 0.240748; substitution rates AC = 1.135762, AG = 1.502113, AT = 0.986617, CG = 0.850935, CT = 4.489268, GT = 1.000000; gamma distribution shape parameter α = 0.020000. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes (MPBS/MLBS/BPP). Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.02 changes. Ex-type or ex-epitype strains are in bold. New isolates are in blue
Distribution: France, Germany, Thailand, USA, (Sutton 1980; Chomnunti et al. 2011; Hongsanan et al. 2015, this study).
Capnodium aciculiforme (Cif., Bat. & Nascim.)W.J. Li & K.D. Hyde, comb. nov.
≡Microxiphium aciculiforme Cif., Bat. & Nascim., Publicações Inst. Micol. Recife 47: 3 (1956)
Index Fungorum number: IF557163, Facesoffungi number: FoF 07139
Capnodium gardeniarum (Bat. & Cif.) W.J. Li & K.D. Hyde, comb. nov.
= Conidioxyphium gardeniarum Bat. & Cif. Quad. Lab. crittogam., Pavia 31: 73 (1963)
Index Fungorum number: IF557164, Facesoffungi number: FoF 07140, Fig. 54
Capnodium gardeniarum (redrawn from Sutton 1980) a Conidia b Branched conidiomata. c Conidiophores, conidiogenous cells and developing conidia. d Side view of a conidioma
On leaves of Gardenia jasminoides (Rubiaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 600 × 50 μm, dark brown to black, pycnidial, solitary to gregarious, superficial, ovoid to flask-shaped or elongated, unilocular, glabrous, produced on and merging into a basal sterile stalk, expanding slightly in the locular region, finally with a long tapered rostrate apex, sometimes branched. Ostiole apical, centrally located, surrounded by hyaline hyphae. Stalk wall composed of thick-walled, dark brown, parallel columns, longitudinally elongated cells of textura porrecta. Pycnidial wall composed of cuboid brown pseudoparenchyma at the locular region, becoming pale brown, thin-walled, parallel columns, longitudinally elongated pseudoparenchyma at the rostrate apex region. Conidiophores up to 4 μm wide, arising from inner layer of the base and sides of the pycnidial wall, hyaline, cylindrical to subcylindrical, unbranched, septate, smooth-walled, consisting of several cuboid conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to dolliform, integrated, determinate, smooth-walled, with apical or lateral apertures immediately below septa, occasionally polyphialidic Conidia 4.5–8 × 2–2.5 μm, hyaline, ellipsoid to cylindrical, unicellular, smooth-walled, guttulate (Sutton 1980).
Capnodium paracoartatum Q. Tian, W.J. Li & K.D. Hyde, sp. nov.
Facesoffungi number: FoF 07141, Fig. 55
Capnodium paracoartatum (MFLU 19-2888, holotype) a, b Sooty mould on the surface of host plant. c, d Appearance of dark brown conidiomata on the host surface. e, h–j Slide view of stalked pycnidia. f Sterile mycelium. g Rostrate apex. l–m Ostiole surround by hyaline hyphae. n Conidia. o Germinating conidium. p, q Culture on MEA. Scale bars c–d = 100 μm, e = 50 μm, f–k = 20 μm, l–m, o = 10 μm, n = 5 μm
Etymology: Referring to its morphology similar to C. coartatum, but phylogeny is distinct.
Dothideomycetes, Dothideomycetidae, Capnodiales, Capnodiaceae
Saprobic on sugary exudates from insects growing on the surface of living leaves. Thallus composed of abundant mycelium, growing over the surface of the plant, brown to pale brown, sub-cylindrical, irregularly branched, septate, constricted at the septa, produced numerous pycnidia on the surface of hyphal cells (2–7 μm wide).
Sexual morph: see Chomnunti et al. (2011). Asexual morph:Conidiomata 200–300 × 40–50 μm, brown to dark brown, pycnidial, solitary to gregarious, superficial, flask-shaped to elongated, with a long tapered rostrate apex, lacking basal sterile stalk, unilocular, glabrous. Ostiole apex 40–80 × 10–17 μm, circular, apical, centrally located, surrounded by hyaline hyphae. Stalk and pycnidial wall composed of thick-walled, dark brown cells of textura porrecta to textura angularis at the base and lower half, becoming pale brown, thin-walled, parallel columns, longitudinally elongated pseudoparenchyma toward rostrate apex region. Conidiophores not observed. Conidiogenous cells not observed. Conidia 4–9 × 2–4 μm (\( \bar{x} \) = 6 × 3 μm, n = 20), hyaline, ellipsoid to cylindrical, rounded at both ends, or occasionally tapered at the base, unicellular, smooth-walled, eguttulate.
Material examined: Thailand, Chiang Rai province, Mae Fah Luang University campus, on living leaves of Ficus sp. (Moraceae), 15 March 2014, Qing Tian, MFU07 (MFLU 19-2888, holotype), ex-type living culture MFLUCC 14-0282.
Notes: Capnodium paracoartatum is similar to C. coartatum in possessing flask-shaped to elongated pycnidia with a long tapered rostrate apex lacking a basal sterile stalk. Capnodium paracoartatum is distinguished from C. coartatum by its conidial size. However, it should be noted that conidial size alone is an inefficient character to delineate Capnodium species, because C. paracoartatum and C. coffeicola share similar conidial size (av. 6 μm long). Capnodium paracoartatum differs from C. coffeicola and C. gardeniarum in lacking a basal stalk. Phylogenetically, C. paracoartatum is distinct from other Capnodium species.
Catenophora Luttr., Mycologia 32(4): 535 (1940)
Facesoffungi number: FoF 07142, Fig. 56
Catenophora pruni (redrawn from Sutton 1980) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, acervular, scattered to gregarious or confluent, initially immersed, then become erumpent, depressed globose to pulvinate, glabrous. Ostiole absent. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores arising from the innermost layer of conidiomata, sub-hyaline to hyaline, cylindrical, branched at base, arranged in compact, linear columns, septate, smooth-walled. Conidiogenous cells sub-hyaline to hyaline, holoblastic, cylindrical to doliiform, catenate, integrated, determinate, smooth-walled, with acropleurogenous conidia. Conidia hyaline to sub-hyaline, elliptical with obtuse apex and slightly truncate base, unicelullar, smooth-walled (Luttrell 1940; Nag Raj 1977a; Sutton 1980; Nag Raj and Kendrick 1988).
Type species: Catenophora pruni Luttr., Mycologia 32(4): 536 (1940)
Notes: The asexual morph of Tympanis, Catenophora, Dendrodomus, Pyrenochaeta, Pleurophoma, and Sirophoma form conidiomata with filiform, acropleurogenously branched conidiophores (Sutton 1980; Damm et al. 2010). Catenophora has acervular conidiomata and holoblastic conidiogenous cells, whereas other genera have pycnidial conidiomata and enteroblastic, phialidic conidiogenous cells. Catenophora species are associated with Prunus serrulata (Rosaceae), Yucca sp. and Yucca baccata (Asparagaceae). No molecular data is available for this genus.
Distribution: USA (Nag Raj 1977a, b; Sutton 1980).
Ceuthodiplospora Died., Ann Mycol. 10(2): 149 (1912)
Facesoffungi number: FoF 07143, Fig. 57
Ceuthodiplospora robiniae (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Habit on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, pycnidial, scattered, immersed, globose in section view, unilocular or multilocular, glabrous. Ostiole single, circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown to pale brown cells of textura angularis in the base and lateral part, gradually merging with pale brown to hyaline cells in the conidial hymenium, becoming darker in the ostiolar region. Conidiophores formed from the inner wall layer of the conidiomata, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform, determinate, smooth-walled. Conidia hyaline, cylindrical to ellipsoid, with obtuse apex and slightly truncate base, 0–1-septate, slightly constricted at the septum, smooth-walled, eguttulate (Sutton 1980).
Type species: Ceuthodiplospora robiniae (Bubák) Died., Ann Mycol. 10(2): 149 (1912)
Notes: Ceuthodiplospora was described from twigs of Robinia pseudoacacia (Fabaceae), from the Czech Republic for a single species C. robiniae (Diedicke 1912). The genus has been regarded as a synonym of Cytodiplospora Oudem. (Clements and Shear 1931) and Discella Berk. & Broome (Von Arx 1963), but it was accepted as a separate genus by Sutton (1980). The sexual morph of C. robiniae has been assigned to Pleomassaria robiniae Bubák (1980). However, the type species of Pleomassaria, P. siparia (Berk. & Broome) Sacc. has an asexual morph, Prosthemium botulinum (type of Prosthemium) (Hantula et al. 1998; Tonolo 1956; Tanaka et al. 2005, 2010). Prosthemium species have stellate, brown, multi-septate conidia that are readily distinguished from the cylindrical to ellipsoid, hyaline, 0–1-septate conidia in Ceuthodiplospora. Therefore, Ceuthodiplospora is accepted as a distinct genus (Wijayawardene et al. 2017a, b) and is not a synonym of Pleomassaria (Index Fungorum 2019). The genus has remained monotypic and has no molecular data.
Distribution: Czech Republic (Diedicke 1912; Sutton 1980).
Chaetasbolisia Speg., Physis, Rev. Soc. Arg. Cienc. Nat. 4(17): 293 (1918)
Facesoffungi number: FoF 07144, Fig. 58
Chaetasbolisia erysiphoides (redrawn from Sutton 1980) a Surface view of a conidioma. b Conidia. c Conidiogenous cells and developing conidia. d Conidiomatal seta
Dothideomycetes, Pleosporomycetidae, Pleosporales, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, scattered, superficial, globose, unilocular, setose, ostiolate. Ostiole circular, centrally located. Conidiomatal setae brown, subulate, with acute apex, unbranched, septate, straight or curved, thick-walled, irregularly verrucose. Conidiomatal wall composed of thin-walled, brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the innermost wall cells of the conidiomata, hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled, with minute channel and collarette. Conidia hyaline, oval, with rounded ends, unicellular, smooth-walled, guttulate (Sutton 1980)
Type species: Chaetasbolisia erysiphoides (Griffon & Maubl.) Griffon & Maubl., Physis, Rev. Soc. Arg. Cienc. Nat. 4(17): 293 (1918)
Notes: Chaetasbolisia shares similar characters with Chaetosticta in having pycnidial, setose conidiomata, and phialidic conidiogenous cells, but is distinguished by its conidia form. Seven taxa have been accepted in Chaetasbolisia, but apart from the type species, C. erysiphoides on leaves of Photinia loriformis (Rosaceae), the other taxa have never been restudied (Batista and Ciferri 1963; Sutton 1980; Wijayawardene et al. 2017b). De Gruyter et al. (2009) and Aveskamp et al. (2010) recognized Chaetasbolisia was a member of Didymellaceae based on molecular data of a reference strain (CBS 148.94). Chen et al. (2015) revised Didymellaceae based on multi-gene sequence of LSU, ITS, rpb2 and tub2, and excluded Chaetasbolisia. Chaetasbolisia should be restudied based on epitype and new collections.
Distribution: China (Sutton 1980).
Chaetoconis Clem., Gen. fung. : 125 (1909)
Facesoffungi number: FoF 04172
Sordariomycetes, Diaporthomycetidae, Diaporthales, Diaporthaceae
Saprobic on the host plant in terrestrial habitat, such as on dead stems of Polygonum alpinum, P. polymorphum (Polygonaceae), Rumex acetosa, R. crispus (Polygonaceae) and Vaccinium uliginosum (Ericaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, stromatic, pycnidial, solitary to gregarious, subperidermal, immersed to erumpent, globose to depressed globose or conical in sectional view, unilocular to multilocular, thick-walled, glabrous, ostiolate. Ostiole single or more, cylindrical to subcylindrical, sometimes papillate. Conidiomata wall composed of thick-walled, dark brown cells of textura angularis in the outer layer, gradually merging with pale brown to hyaline cells of textura prismatica towards hymenium. Conidiophores formed from the innermost cells of conidiomata, hyaline, septate, branched, smooth-walled, invested by mucus. Conidiogenous cells enteroblastic, phialidic, hyaline, discrete or integrated, subcylindrical to cylindrical or irregular, smooth-walled. Conidia hyaline, obclavate, ellipsoidal or irregular, septate, thick-walled, guttulate, apical cell attenuated into a tubular, flexuous, unbranched or occasionally forked appendage.
Type species: Chaetoconis polygoni (Ellis & Everh.) Clem., Gen. fung.: 176 (1909).
Notes: Sutton (1968) re-described the genus and accepted Chaetoconis and C. polygoni as validly published, while Pirozynski and Morgan-Jones (1968) gave a detailed list of synonyms of the genus. Nag Raj (1993) accepted two species, C. polygoni and C. vaccinii Melnik & Nag Raj. De Gruyter et al. (2009) and Senanayake et al. (2017) showed that Chaetoconis is related to Diaporthaceae in Diaporthales based on sequence analysis. The sexual morph of Chaetoconis has been connected to Ceriospora polygonacearum (Petr.) Piroz. & Morgan-Jones (Pirozynski and Morgan-Jones 1968), but this connection has not been confirmed by molecular data. We provide detailed descriptions and photo plates for two Chaetoconis species. In the future, more collections need to be brought into culture and subjected to DNA analysis to confirm its generic placement and to link its sexual morph.
Distribution: Germany, Russia, Sweden, UK, USA (Nag Raj 1993, this study).
Key to species of Chaetoconis
1. Conidiomata globose, conidia 2-septate, with unbranched appendages…C. polygoni
1. Conidiomata broadly conical to depressed globose, conidia 4-septate, with forked appendages…C. vaccinii
Chaetoconis polygoni (Ellis & Everh.) Clem., Gen. fung.: 176 (1909)
Facesoffungi number: FoF 03475, Fig. 59
Chaetoconis polygoni (MFLU 17–0965) a Herbarium specimen. b, c, d Appearance of black conidiomata on the host. e, f Vertical sections of conidiomata. g Section of peridium. h Ostiole. i–l Conidiophores, conidiogenous cells and developing conidia. m–s Conidia. Scale bars b = 1000 µm, c–d = 200 µm, e–f = 100 µm, g = 50 µm, h = 20 µm, i = 5 µm, j–s = 10 µm
Saprobic on stem of Rumex acetosa (Polygonaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 200–250 µm diam., 100–150 µm high, dark brown to black, pycnidial, solitary to gregarious or confluent, deeply immersed at beginning, then becoming partly erumpent, globose, unilocular or multilocular, thick-walled, glabrous, ostiolate. Ostiole 50–70 × 50–60 µm, single, cylindrical to subcylindrical, centrally located. Conidiomata wall 20–45 µm wide, composed of thick-walled, dark brown cells of textura angularis in the outer layer, thin-walled, pale brown cells of textura prismatica in the inner layer. Conidiophores 4–10 × 3–9 µm, formed from the innermost cells of conidiomata, hyaline, broadly cylindrical or irregular, septate, branched, smooth-walled, invested by mucus. Conidiogenous cells 8–17 × 2–8 µm, enteroblastic, phialidic, hyaline, subcylindrical to cylindrical or irregular, straight or curved, discrete or mostly integrated, with marked periclinal thickenings in the collarette zone. Conidia 33–48 × 2.5–5 µm (\( \bar{x} \) = 40 × 4 µm; n = 30), hyaline, obclavate, with obtuse or slightly truncate base, 2-septate, guttulate, central cell generally shorter than end cells, apical cell attenuated into a tubular, flexuous, unbranched appendage 18–37 × 1–2 µm.
Material examined: Germany, on the edge of a mixed forest, 39 m asl, sandy, acid, fresh, mesotroph, on stem of Rumex acetosa (Polygonaceae), 9 May 2013, R.K. Schumacher, CHUNI 73 (MFLU 17-0965).
Chaetoconis vaccinii Melnik & Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 191 (1993)
Facesoffungi number: FoF 07145, Fig. 60
Chaetoconis vaccinii (DAOM 215176, holotype) a, b Herbarium package and specimen. c, d Appearance of black conidiomata on the host. e, f Vertical sections of conidiomata. g, i Section of peridium. h Surface view of conidiomatal wall. j–l Conidiogenous cells and developing conidia. m–r Conidia. Scale bars c = 500 µm, d = 200 µm, e–f = 100 µm, g, h = 50 µm, i = 20 µm, j–l = 5 µm, m–r = 10 µm
Saprobic on Vaccinium uliginosum (Ericaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 200–250 µm diam., 100–200 µm high, black, pycnidial, solitary to gregarious, initially immersed, then become sunken and erumpent, broadly conical to depressed globose in sectional view, unilocular, thick-walled, glabrous, ostiolate. Ostiole 80–100 × 30–60 µm, single, long, cylindrical to subcylindrical, eccentric. Conidiomatal wall 10–40 µm wide, composed of a basal textura angularis with thick-walled, pale brown cells, gradually merging with hyaline cells, and a peripheral textura prismatica with thick-walled, dark brown to black cells. Conidiophores formed from the innermost cells of conidiomata, hyaline, septate, branched, smooth-walled, invested in mucus. Conidiogenous cells 10–20 × 2–4 µm, enteroblastic, hyaline, subcylindrical to cylindrical or irregular, discrete or mostly integrated, with minute periclinal thickenings in the collarette zone, with 1–3 percurrent proliferations (Fig. 60k). Conidia 35–76 × 6–9 µm (\( \bar{x} \) = 53 × 7 µm; n = 30), hyaline, obclavate, ellipsoidal or irregular, with narrow and obtuse base, 1–4-septate, mostly 3-septate, thick-walled, guttulate, apical cell attenuated into a tubular, flexuous, unbranched or occasionally forked appendage 20–49 × 2–3 µm (\( \bar{x} \) = 35.5 × 2 µm).
Material examined: Russia, Kamchatka Region, Kronokense Reservation, on Vaccinium uliginosum (Ericaceae), 1 August 1981, N. Vassiljeva (DAOM 215176, holotype).
Chaetomella Fuckel, Jb. nassau. Ver. Naturk. 23-24: 401 (1870) [1869-70] Facesoffungi number: FoF 07146, Fig. 61
Chaetomella oblonga (redrawn from Rossman et al. 2004) a Vertical section of pycnidium. b Vertical section of sporodochium c Conidiophores, conidiogenous cells. d Conidiomatal setae. e Conidia
Leotiomycetes, Leotiomycetidae, Chaetomellales, Chaetomellaceae
Saprobic or parasitic on the host plant in terrestrial habitat or isolated from soil. Sexual morph: undetermined. Asexual morph:Conidiomata two types: pycnidial or sporodochial. Pycnidia initially pale brown, ultimately dark brown to black, superficial, separate or gregarious, oblong, elongate or reniform, rarely globose to irregularly shaped, sessile or short stipitate, unilocular, setose. Pycnidial setae brown, unbranched, straight, curved, or coiled, septate, thick- and smooth-walled, with clavate to acuminate apex. Ostiole absent, dehiscing by an elongate, longitudinal slit in the upper part. Pycnidial wall composed of hyaline, 1–2 layers of thick-walled cells in the outer region, merging with dark brown, 2–4 layers layers of thick-walled cells in the middle region, become hyaline, 3–4 layers layers of thick-walled cells of textura prismatica to textura angularis in the inner region. Conidiophores arising from all around the cavity of pycnidium, often more numerous toward base, hyaline, filiform, cylindrical, branched,. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated, indeterminate, smooth-walled, forming acropleurogenously singly or in pairs below septa and at apices of conidiophores. Conidia hyaline, ellipsoid to fusiform or falcate, broadly rounded to slightly pointed at both ends, straight or slightly curved, unicellular, smooth-walled. Sporodochia pale brown to dark brown, discoid or crown-like at maturity, stalked, opening irregularly at apices with wavy margin, setose. Conidiophores, conidiogenous cells and conidia in sporodochial state similar to those in pycnidia (Rossman et al. 2004)
Type species: Chaetomella oblonga Fuckel, Jb. nassau. Ver. Naturk. 23–24: 402 (1870) [1869-70]
Notes: Chaetomella is characterized by pycnidial or sporodochial conidiomata with brown, unbranched setae, long, acropleurogenous conidiophores, and hyaline ellipsoid to fusiform or falcate, unicellular conidia (Sutton 1980; Rossman et al. 2004). Rossman et al. (2004) revised Chaetomella and listed the synonyms of the genus. They assigned Chaetomella to Leotiomycetes based on SSU and LSU sequence data and accepted four species and one variety e.g., C. acutiseta B. Sutton & A.K. Sarbhoy, C. circinoseta Stolk, C. raphigera Swift, C. oblonga Fuckel, and C. acutiseta var. nigra. Crous et al. (2014b, 2019) added C. zambiensis Crous on undetermined host (Fabaceae). and C. pseudocircinoseta Crous & Carnegie on on leaves of Eucalyptus microcorys (Myrtaceae). The sexual morph of this genus is undetermined.
Distribution: Australia, Canada, USA, India, Zambia (Rossman et al. 2004; Crous et al. 2014b, 2019)
Chaetophiophoma Speg., Anal. Mus. nac. B. Aires, Ser. 3 13: 388 (1911)
Facesoffungi number: FoF 07147
Leotiomycetes, Leotiomycetidae, Medeolariales, Dermateaceae
Saprobic on plant host in terrestrial habitat, forming numerous, conspicuous rounded, black conidiomata. Sexual morph: undetermined. Asexual morph:Conidiomata black, scattered to gregarious, pycnidial, immersed to semi-immersed, globose to subglobose, unilocular, glabrous, thick-walled, smooth. Ostiole absent, dehiscence by breakdown of peridium at upper region. Conidiomata wall composed of thick-walled, hyaline to pale brown cells of textura angularis. Conidiophores arising from the inner layers of pycnidium, hyaline, repeatedly branched at base and above, often fasciculate, septate, with acropleurogenously developing conidia. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, indeterminate, integrated or discrete, thin-walled, smooth, with collarette and narrow channel. Conidia hyaline, fusiform to falcate, aseptate, thick-walled, smooth, guttulate.
Type species: Chaetophiophoma trematis Speg., Anal. Mus. nac. B. Aires, Ser. 3 13: 388 (1911)
Notes: Chaetophiophoma is characterized by pycnidial, globose, pale brown, unilocular conidiomata, branched, septate conidiophores, and hyaline, falcate, unicellular, guttulate conidia. A new collection from Sorbus aucuparia shares similar characters with C. trematis in form of conidiomata, conidiophores, conidiogenous cells and conidia, but differs in dimension of conidiomata and conidia. However, the new collection has larger conidiomata and smaller conidia than C. trematis (conidiomata: 50–75 µm diam., conidia: 16–24 × 2 µm). Unfortunately, molecular data for C. trematis is not available in GenBank, but C. sorbi is introduced as new species based on distinct morphology.
Distribution: Italy, Argentina (Sutton 1980, this study).
Chaetophiophoma sorbi W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557141, Facesoffungi number: FoF 07148, Fig. 62
Chaetophiophoma sorbi(MFLU 15-2273, holotype) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d Vertical section of conidioma. e, f Section of peridium. g–j Conidiophores, conidiogenous cells and developing conidia k–o Conidia. Scale bars d = 100 µm, e = 50 µm, f = 20 µm. g–j = 10 µm. k–o = 5 µm
Etymology: Named after the host genus, Sorbus
Saprobic on dead stems of Sorbus aucuparia (Rosaceae), forming numerous, conspicuous rounded, black, conidiomata. Sexual morph: undetermined. Asexual morph:Conidiomata 200–250 µm diam., 250–300 µm high, black, scattered to gregarious or confluent, pycnidial, immersed to semi-immersed, subglobose, unilocular, glabrous, thick-walled, smooth, papillate. Conidiomata wall 20–1000 µm thick, composed of thick-walled, hyaline to pale brown cells of textura angularis. Conidiophores arising from the inner layers of pycnidium, hyaline, repeatedly branched at the base and above, often fasciculate, septate in association with branches, with acropleurogenous conidia. Conidiogenous cells 8–30 × 1.5–3 µm, hyaline, enteroblastic, phialidic, cylindrical, indeterminate, integrated or discrete, thin-walled, smooth, with collarette and narrow channel. Conidia 13–17 × 2–4 µm (\( \bar{x} \) = 15 × 3 µm; n = 30), hyaline, fusiform to falcate, aseptate, thick-walled, smooth, guttulate.
Material examined: Italy, Forlì-Cesena, near Monte Falco, on dead aerial branches of Sorbus aucuparia (Rosaceae), 8 July 2015, Camporesi Erio, IT2554 (MFLU 15-2273, holotype), (KUN, HKAS 93633, isotype).
Chaetophoma Cooke, Grevillea 7(no. 41): 25 (1878)
Facesoffungi number: FoF 07149, Fig. 63
Chaetophoma quercifolia (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Surface view of conidiomata
Dothideomycetes, Pleosporomycetidae, Pleosporales, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, scattered to gregarious, superficial, globose, unilocular, glabrous. Ostiole absent. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to doliiform, determinate, smooth-walled, with a minute apical channel and collarette. Conidia hyaline, cylindrical, unicelullar, smooth-walled (Sutton 1980).
Type species: Chaetophoma quercifolia Cooke, Grevillea 7(41): 25 (1878)
Notes: Chaetophoma has similar morphology of conidiomata and conidia with Aposphaeria and Asteromella. The conidiophores of Chaetophoma are lacking, while they are present in Aposphaeria and Asteromella (Sutton 1980). De Gruyter et al. (2009) showed that Chaetophoma might be related to Pleosporales based on LSU and SSU sequence data. Similarly, Zhang et al. (2012b) reported that Byssothecium (Massarinaceae, Pleosporales) has a chaetophoma-like asexual morph. The sequence of the type species of C. quercifolia is not available. Therefore Chaetophoma could not be placed in a certain family in Pleosporales. More than 70 taxa have been listed in Chaetophoma (Index Fungorum 2019), but many of them need to be re-studied with fresh collections.
Distribution: Brazil, USA (Sutton 1980; Gruyter et al. 2009).
Chaetoseptoria Tehon, Mycologia 29(4): 444 (1937)
Facesoffungi number: FoF 07150, Fig. 64
Chaetoseptoria vignae (redrawn from Morgan-Jones et al. 1972e) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown, pycnidial, amphigenous, scattered to gregarious, immersed to semi-immersed, globose, unilocular, setose. Ostiole well defined, circular, centrally located. Conidiomatal setae brown, cylindrical to subulate, with obtuse apex, unbranched, sparsely septate, smooth-walled, restricted in ostiolar region. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, ampuliform, determinate, smooth-walled, with a minute apical channel and collarette. Conidia hyaline, acicular, straight or slightly curved, 6–7-septate, smooth-walled, guttulate (Fig. 65).
Type species: Chaetoseptoria vignae Tehon, Mycologia 29(4): 444 (1937)
Notes: Chaetoseptoria shares similar morphology of conidia with Septoria and septoria-like fungi (Neoseptoria, Phlyctaeniella, Polyphialoseptoria, Ruptoseptoria, Sphaerulina), but can be distinguished from these genera by its setose conidiomata (Sutton 1980; Quaedvlieg et al. 2013). Three species are accepted in Chaetoseptoria, C. indica P.K. Khanna, C. vignae Tehon and C. wellmanii J.A. Stev., but none of them has been studied with molecular data. Chaetoseptoria wellmanii and C. vignae have been reported as a pathogen on Fabaceae (Phaseolus vulgaris, Vigna sinensis), causing round to irregular, brown or yellowish leaf spots (Tehon 1937; Yerkes 1956). Chaetoseptoria indica has been reported on decaying shoots of Bothriochloa pertusa (Poaceae) (Khanna 1966).
Distribution: Australia, Colombia, Mexico, New Zealand, Nigeria (Yerkes Jr. 1956; https://www.gbif.org/).
Chaetospermum Sacc., Syll. fung. 10: 706 (1892)
Facesoffungi number: FoF 07151
Agaricomycetes, Incertae sedis, Sebacinales, genera incertae sedis
Saprobic on the host plant or submerged wood in freshwater stream. Sexual morph: see Wells and Bandoni (2001) and Kirschner and Oberwinkler (2009). Asexual morph: Conidiomata off white or pearl white when moist, yellowish-brown and waxy when dry, stromatic, pycnidial, solitary to gregarious or confluent, superficial, initially closed, ultimately opening by an irregular fissure in the apical wall, globose to subglobose or irregular in shape, unilocular, with the locule occasionally irregularly divided or convoluted, glabrous. Conidiomata wall heavily gelatinized, composed of hyaline, thick-walled cells of textura intricata to textura oblita. Conidiophores hyaline, loosely aggregated, branched, septate at base, formed from innermost cells of the conidiomata. Conidiogenous cells hyaline, subcylindrical or irregular, discrete, bearing an apical cluster of up to 3 conidia. Conidia hyaline, cylindrical, or broadly ellipsoidal, rounded at both ends, straight or slightly curved, aseptate, smooth, bearing polar or subpolar, filiform, or narrow and attenuated, flexuous, unbranched appendages.
Type species: Chaetospermum chaetosporum (Pat.) A.L. Sm. & Ramsb., Trans. Br. Mycol. Soc. 4(2): 328 (1914) [1913]
Notes: Patouillard (1888) described a fungus from decaying grass as Tubercularia chaetospora Pat. Later, this fungus was designated, as C. tubercularioides Sacc., to be the type species for a new genus Chaetospermum Sacc., because of its distinct morphology (Saccardo 1892). This name was legitimized by changing it to C. chaetosporum by Smith and Ramsbottom (1914). A detailed developmental study of the type species was published by Fonseka (1960), and several generic synonyms were noted by Sutton (1977a). Subsequently, Sutton (1980) accepted three species in the genus namely, C. carneum Tassi, C. chaetosporum and C. gelatinosum Petch, based on distinct conidial dimensions. Nag Rag (1993) revised the genus considering C. gelatinosum as a synonym of Mastigonema gelatinosum (Berk. & Broome) Nag Raj, and C. carneum a nomen dubium. Ciliospora gelatinosa Zimm. and C. elasticae var. artocarpi Koord. previously regarded as a synonym of C. carneum were made as new combinations of C. artocarpi (Nag Raj) Nag Raj and C. camelliae Agnihothr., respectively. Pestalozziella gossypina G.F. Atk. was reduced to a synonym of C. gossypinum (G.F. Atk.) Nag Raj, and a key to four species was provided (Nag Raj 1993).
Chaetospermum has been less well-studied with molecular data. Rungjindamai et al. (2008) showed that Chaetospermum species were related to Sebacinales (Agaricomycetes, Basidiomycota) based on LSU and SSU sequence data, but the type species of Chaetospermum was not included in their study. Crous et al. (2014a) designated a neotype for C. chaetosporum and revealed that it was a member of Sebacinales. In addition, a connection between C. chaetosporum and its sexual morph Efibulobasidium albescens (Sacc. & Malbr.) K. Wells (type species of Efibulobasidium) was made based on ITS sequence data. This agrees with Wells and Bandoni (2001), who recorded a Chaetospermum morph in culture of Efibulobasidium albescens. Kirschner and Oberwinkler (2009) observed both sexual and asexual morphs in the one specimen. According to the International Code of Nomenclature for Algae, Fungi, and Plants (ICN; Melbourne Code), Crous et al. (2014a) recommended the use of the older generic name Chaetospermum over Efibulobasidium.
To date, including C. setosum Rajeshk. et al. (Rajeshkumar et al. 2010) and C. malipoense X.M. Tan & S.X. Guo (Tan et al. 2014), six species are accepted in the genus. We re-examined the type of C. artocarpi and C. camelliae and provide a detailed description; we did not examine the type species, which is not in good condition.
Distribution: China, France, India, Pakistan, Thailand, Switzerland (Rungjindamai et al. 2008; Crous et al. 2014a; Tan et al. 2014).
Chaetospermum artocarpi (Nag Raj) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 194 (1993)
≡ Chaetospermum elasticae var. artocarpi Nag Raj, Proc. Indian Acad. Sci., Sect. B 59: 50 (1964)
Facesoffungi number: FoF 07185
Saprobic on dead rotting leaves of Artocarpus integrifolia (Moraceae). Sexual morph: undetermined. Asexual morph:Conidiomata 300–600 µm diam., 150–300 µm high, salmon pink or fresh coloured to yellowish brown and waxy when dry, off white or pearl white and gelatinous when moist, pycnidial, solitary to gregarious or confluent, subepidermal in origin, globose to subglobose, unilocular, initially closed, ultimately opening by an iregular split in the apical wall, glabrous, thick-walled (Nag Raj 1993). Conidiomatal wall composed of thick-walled, hyaline to dark brown cells of textura intricata to textura oblita. Conidiophores arising from the innermost wall of conidiomata, hyaline, loosely aggregated, branched, septate at the base, invested in gel. Conidiogenous cells hyaline, subcylindrical to narrowly conic or irregular, discrete, bearing an apical cluster of up to 4 conidia resulting from holoblastic- sympodial proliferations. Conidia 16–22 × 4–7 µm (\( \bar{x} \) = 18 × 6 µm; n = 20), hyaline, ellipsoidal to oblong ellipsoidal, or cylindrical, rounded at both ends, straight or slightly curved, smooth, bearing appendages, appendages 8–18 µm long, polar, mostly 3 at each end, occasionally 2, tubular, attenuated, branched, flexuous.
Material examined: India, Kamataka state, Balehonnur, Coffee Research Station, on dead rotting leaves of Artocarpus integrifolia (Moraceae), T.R. Nag Raj, 27 September 1962 (IMI 99088, type).
Chaetospermum camelliae Agnihothr., Mycopath. Mycol. appl. 16: 115 (1962)
Facesoffungi number: FoF 07152, Figs. 66, 67
Chaetospermum camelliae(MFLU13–0287, neotype) a Herbarium specimen. b–e Appearance of yellowish brown conidiomata on the host. f–h Vertical section of conidiomata. i–k Section of peridium. l–n Conidiogenous cells and developing conidia, o–q Conidia. Scale bars b = 1000 µm, c–h = 200 µm, i–j = 100 µm, k = 50 µm, l–q = 10 µm
Chaetospermum camelliae (IMI 76076) a–b Herbarium specimen. c–d Appearance of yellowish brown conidiomata on the host. e Vertical section of conidiomata. f–j Section of peridium. k Conidiogenous cells and developing conidia. l–o Conidia. Scale bars c = 500 µm, d, e = 200 µm, f–i = 50 µm, j–k = 20 µm, l–o = 10 µm
Saprobic on dead leaves. Sexual morph: undetermined. Asexual morph:Conidiomata 350–600 µm diam., 250–350 µm high, yellowish-brown and waxy when dry, pearl white and gelatinous when moist, astromatic, pycnidial, solitary or gregarious, superficial, globose to subglobose, cupulate or bow-shaped when dry, hemispherical in sectional view, initially closed but dehiscing from the sunken wall at upper part, unilocular, glabrous. Conidiomatal wall 20–70 µm wide, intermixed with host cells at base, composed of thick-walled, hyaline cells of textura intricata to textura oblita in gel. Conidiophores formed from the inner wall layer of the conidiomata, hyaline, short, densely aggregated, septate, branched, smooth. Conidiogenous cells 5–20 × 2–5 µm wide, hyaline, holoblastic, discrete, sympodially arranged, cylindrical to subcylindrical, straight or slightly curved. Conidia 20.6–29 µm × 4.3–8.5 (\( \bar{x} \) = 24 × 5.8 µm; n = 30), hyaline, unicellular, cylindrical to subcylindrical, rounded at both ends, straight or slightly curved, guttulate, smooth, bearing 6.5–20 µm long, tubular, unbranched, flexuous, subpolar, 2–4 appendages at each end.
Material examined: Thailand, Chiang Rai, Doi Mae Selong, on unidentified dead leaves, 13 July 2012, N. Tangthirasunun, NTCL097–3 (MFLU13–0287, epitype, but no conidiomata); ibid., NTCL097–2 (MFLU13–0287, neotype); Venezuela, on wood chips, R.W.G. Dennis, 30 June 1958 (IMI 76076).
Chaetosphaeronema Moesz, Bot. Közl. 14: 152 (1915)
Facesoffungi number: FoF 00241
Dothideomycetes, Pleosporomycetidae, Pleosporales, Phaeosphaeriaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph:Ascomata dark brown to black, solitary to gregarious or confluent, visible as spots on the host surface, immersed to semi-immersed, globose to subglobose, unilocular, glabrous, thick-walled, papillate, ostiolate. Ostiole single, cylindrical, centrally located. Peridium composed of thick-walled, dark brown to pale brown or hyaline cells of textura angularis. Hamathecium composed of asci and pseudoparaphyses. Pseudoparaphyses hyaline, broad cellular, septate, constricted at septa, branched, smooth-walled. Asci 8-spored, bitunicate, with indistinct ocular chamber, cylindrical to cylindric-clavate, with rounded apex and short pedicel. Ascospores greenish-yellow, overlapping or lying parallel or spiral, without sheath or appendages, septate, inflated at 10th cell, the inflation more pronounced near the 9th septum, apical part bent or curved (description from Ariyawansa et al. 2014). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious, immersed to semi-immersed, subepidermal in origin, rostrate, globose to subglobose, unilocular or multi-locular, thick-walled, setose or glabrous, ostiolate. Ostiole single, cylindrical to subcylindrical or conical, straight or curved, with long neck lined with periphyses, covering by dark brown to brown setae. Conidiomtal setae dark brown, restricted to the beak, sparse or numerous, septate, unbranched, straight or curved, blunt at base, attenuated towards the upper part. Periphyses hyaline, hyphae-like, subcylindrical, with obtuse apex, unbranched, septate, smooth-walled, formed from inner wall of ostiole. Conidiomatal wall composed of thick-walled, dark brown to brown or hyaline cells of textura angularis to textura prismatica. Conidiophores arising from the innermost layer of conidiomata, absent or present, when present, hyaline, cylindrical, branched irregularly and only sparingly, septate. Conidiogenous cells hyaline, enteroblastic, phailidic or annelidic, cylindrical to subcylindrical, ampuliform or lageniform, determinate, integrated or discrete, smooth-walled, channel and collarette minute, periclinal wall thickened or percurrent proliferation. Conidia hyaline, cylindrical, with obtuse apex, with blunt and truncate base, 1-septate, smooth-walled, often guttulate.
Type species: Chaetosphaeronema hispidulum (Corda) Moesz, Bot. Közl. 14: 152 (1915)
Notes: Chaetosphaeronema is characterized by dark brown, thick-walled, setose conidiomata with long, papillate ostioles, and hyaline, cylindrical, 1-septate conidia (Sutton 1980). Petrak (1944) synonymized C. herbarum (Hollós) Moesz under the type species, and accepted three species in the genus, namely C. collivagum (Petr.) Petr, C. galii (Petr.) Petr. and C. hispidulum. Sutton (1980) accepted only type species in the genus and provided a detailed description and illustration. De Gruyter et al. (2009) included sequence data (LSU and SSU) of type species in phylogeny analyses, and showed that Chaetosphaeronema was related to Phaeosphaeriaceae. However, strain CBS 826.88 isolated from soil showed characteristics of Phoma betae A.B. Frank and Ascochyta caulina (P. Karst.) Aa & Kesteren (pilose pycnidia, hyaline, ellipsoidal and aseptate conidia), which is distinct from the type species where the setose feature is restricted to the ostiolar region, and conidia are 1-septate (Boerema et al. 2004; De Gruyter et al. 2009). Therefore, it was concluded that strain CBS 826.88 was a misidentification. De Gruyter et al. (2010) added species C. oonsii (≡ Phoma coonsii) based on hair-like setae on conidiomata, together with molecular data of LSU and SSU. However, our phylogenetic analyses of LSU, SSU and ITS sequence data show that C. oonsii has closely affinity with Dematiopleospora alliariae Thambug et al. (Fig. 68). Chaetosphaeronema oonsii differs from Chaetosphaeronema by its aseptate conidia. Based on both morphology and phylogeny, C. oonsii is excluded from Chaetosphaeronema, and further study on sexual and asexual morphs of these two taxa is needed.
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, SSU and ITS sequences data representing Chaetosphaeronema and other genera in Phaeosphaeriaceae. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Nineteen strains are included in the analyses, which comprise 2721 characters including gaps (LSU: 1–854, SSU: 855–2144, ITS: 2145–2721). Sulcispora pleurospora MFLUCC 14-0995 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RaxML tree with a final optimization likelihood value of − 6355.103133 is presented. The matrix had 334 distinct alignment patterns, with 23.66% of undetermined characters or gaps. Estimated base frequencies were: A = 0.251913, C = 0.214042, G = 0.272247, T = 0.261798; substitution rates AC = 1.654340, AG = 3.138157, AT = 1.609133, CG = 0.985821, CT = 5.651532, GT = 1.000000; gamma distribution shape parameter α = 0.020000. The maximum parsimonious dataset consisted of constant 2421, 226 parsimony-informative and 74 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 466 steps (CI = 0.796, RI = 0.833, RC = 0.663, HI = 0.204) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.01 changes. Ex-type or reference strains are in bold. New isolates are in blue
Chaetosphaeronema is associated with sexual morph Ophiobolus (Petrak 1944; Zhang et al. 2009, 2012b; Ariyawansa et al. 2014; Phookamsak et al. 2014), but this sexual and asexual morph connection has not been confirmed, because of limited sequence data. Phookamsak et al. (2017) revised Ophiobolus and ophiobolus-like taxa and showed that O. cirsii is closely related to Chaetosphaeronema species and distinct from Ophiobolus sensu stricto. Our phylogenetic results are in accordance with Phookamsak et al. (2017) (Fig. 68). The LSU sequence of Ophiobolus cirsii (MFLUCC 13-0218) is 100% (873/873) similar to C. hispidulum (CBS 216.75), and ITS sequence is distinguished by two gaps (568/570). The SSU sequence of O. cirsii is problematic after 1105bp length and is not included in the analyses. To provide a natural classification for O. cirsii, we synonymize it under Chaetosphaeronema hispidulum.
Two new collections, C. achilleae (MFLUCC 16-0476) and C. hispidulum (CBS 216.75) with glabrous pycnidia cluster with Ophiobolus cirsii (MFLUCC 13-0218), (Fig. 68) with strong bootstrap value (99/100/0.99). Combined with morphological characters, these two collections are recognized as conspecific with C. hispidulum. Chaetosphaeronema achilleae is also reduced to a synonym of the type species.
Distribution: Australia, Germany, India, Italy, Japan, Russia (Gruyter et al. 2010; Quaedvlieg et al. 2013, this study).
Chaetosphaeronema hispidulum (Corda) Moesz, Bot. Közl. 14: 152 (1915)
= Chaetosphaeronema achilleae S.K. Huang & K.D. Hyde, in Hyde et al., Fungal Divers. 80: 1–270 (2016)
= Ophiobolus cirsii (P. Karst.) Sacc., Syll. fung. 2: 341 (1883)
Facesoffungi number: FoF 07153, Figs. 69, 70, 71
Chaetosphaeronema hispidulum (MFLU 15–1922) a, b Herbarium package and specimen. c, d Appearance of dark brown to black coniodiomata on the host, note d black ostiole in surface view. e, f, g Vertical sections of conidiomata. h Conidiomatal setae. i Ostiole. j Vertical section of peridium. k Ostiole with periphyses. l–r Conidiogenous cells and developing conidia (arrows show 2 annellides). s–x Conidia. Scale bars c–d = 200 µm, e–g = 100 µm, h–i, k = 20 µm, j = 50 µm, l–x = 5 µm
Chaetosphaeronema hispidulum (MFLU 16–2275) a Herbarium specimen. b–d Appearance of dark brown coniodiomata on the host. e–g Vertical section of conidiomata. h, k Vertical section of peridium. i–j Ostiole with periphyses. l–p Conidiogenous cells and developing conidia (arrows show 2 annellides). q–s Conidia. t Germinating conidium. u–v Culture on PDA. Scale bars b = 500 µm, c–d = 200 µm. e–g = 100 µm, h, k, s–t = 20 µm, i–j = 50 µm, l–r = 5 µm, u–v = 20 mm
Chaetosphaeronema hispidulum (MFLU 16-1965) a Herbarium specimen. b, c Appearance of dark brown to black coniodiomata on the host. d–f Vertical sections of conidiomata. g Ostiole. h Vertical section of peridium. i–k Conidiogenous cells and developing conidia. l Germinating conidium. m–o Conidia. p–q Culture on PDA. Scale bars b = 200 µm, c–f = 100 µm, g = 50 µm, h = 20 µm, i–k = 5 µm, l–o = 10 µm, p–q = 25 mm
Saprobic on dead stem of Achillea nobilis, Onobrychis sp., Medicago sp. Sexual morph:Ascomata dark brown to black, solitary to gregarious or confluent, visible as spots on the host surface, immersed to semi-immersed, globose to subglobose, unilocular, glabrous, thick-walled, papillate, ostiolate. Ostiole single, cylindrical, centrally located. Peridium composed of thick-walled, dark brown to pale brown or hyaline cells of textura angularis. Hamathecium consisting of asci and pseudoparaphyses. Pseudoparaphyses hyaline, broad cellular, septate, constricted at septa, branched, smooth-walled. Asci 8-spored, bitunicate, with indistinct ocular chamber, cylindrical to cylindric-clavate, with rounded apex and short pedicel. Ascospores greenish yellow, overlapping or lying parallel or spiral, without sheath or appendages, septate, inflated at 10th cell, the inflation more pronounced near the 9th septum, apical part bent or curved (Ariyawansa et al. 2014). Asexual morph:Conidiomata 180–260 µm diam., 150–390 µm high, dark brown to black, pycnidial, solitary to gregarious, deeply immersed with only the dark brown, rostrate ostiole visible in surface view, globose to subglobose, unilocular, setose, thick-walled, ostiolate. Ostiole 140–200 × 70–110 µm, single, cylindrical to obconical, straight or curved, covered by dark brown to brown setae. Conidiomatal setae 70–130 × 4–6 µm, restricted to the beak, dark brown and blunt at base, becoming paler and attenuated towards top, septate, unbranched, smooth-walled. Conidiomatal wall 15–70 µm wide, composed of relatively thick-walled dark brown to pale brown cells of textura angularis in the upper part and ostiolar region, with thick-walled, pale brown to hyaline cells of textura angularis gradually merging with textura prismatica in the basal and lateral part. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–10 × 2–4 µm, hyaline, enteroblastic, annelidic, cylindrical to lageniform, determinate, smooth-walled, with periclinal thickenings at collarette zone, or percurrently proliferating 1–2 times. Conidia 7–12 × 1.6–2.2 µm (\( \bar{x} \) = 10 × 1.9 µm; n = 50), hyaline, cylindrical, rounded at apex, blunt and truncate at base, straight or slightly curved, 1-septate, without constrictions at the septum, often guttulate, smooth-walled.
Culture characters: Strain MFLUCC 17–2503, colonies on PDA, reaching 30–40 mm diam. after 30 d at 20–25 °C, producing globules, pale brown in the middle zone, with dense, flattened, filamentous, cottony, aerial hyphae, brown in the margin area, margin circular, with sparse, filiform aerial hyphae, reverse dark brown; strain MFLUCC 17–1317, colonies on PDA, reaching 50–60 mm diam. after 21 days at 20–25 °C, with pale brown to brown, dense, flattened, filamentous, fluffy, aerial hyphae in the middle zone, white, sparse, circular, entire; aerial hyphae in the margin region, reverse pale brown to brown.
Material examined: Russia, Rostov Region, Oktyabrsky District, southern outskirts of Persianovky settlement, Khoruli gully (rus. balka Khoruli), on dead stems of Achillea nobilis (Asteraceae), 28 April 2015, T.S. Bulgakov, T218 (MFLU 15–1922); ibid., Italy, Province of Forlì-Cesena, Premilcuore, Passo della Braccina, on dead aerial stems of Onobrychis viciifolia (Fabaceae), 31 July 2016, Erio Camporesi, IT3050 (MFLU 16–2275), living culture MFLUCC 17-2503, (KUN, HKAS 101659); ibid., Province of Arezzo, near Poppi, on dead aerial stems of Medicago sp. (Fabaceae), 13 June 2016, Erio Camporesi, IT2998 (MFLU 16-1965), living culture MFLUCC 17–1317 = ICMP 21551 = KUMCC 16-0103, (KUN, HKAS 97468).
Notes: Chaetosphaeronema achilleae was introduced as a new taxon based on its smaller, glabrous conidiomata with textura angularis cells and lack of conidiomatal setae (Hyde et al. 2016). After re-examining the type specimen, we found that it has setose conidiomata with textura angularis to textura prismatica cells. The conidiogenous cells are percurrently proliferating 1–2 times. The LSU and ITS sequences of C. achilleae differ from C. hispidulum in only 2 base pairs. Thus, C. achilleae is considered as conspecific with C. hispidulum. Two new collections were distinguished from C. hispidulum by smaller and glabrous conidiomata, but molecular data proved they are the same species. Thus, setose pycnidia and dimension of conidiomata are not stable characters to delineate the species in Chaetosphaeronema. A revised description and photos are provided for C. hispidulum, and also the measurements of each collection is shown in Table 3.
Chaetosticta Petr. & Syd., Ann Mycol. 23(3/6): 270 (1925)
Facesoffungi number: FoF 07154, Fig. 72
Dothideomycetes, genera incertae sedis
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, pycnidial, scattered, superficial, globose to slightly depressed, membranous, unilocular, setose, ostiolate. Ostiole circular, papillate, centrally located. Conidiomatal setae pale brown, subulate, with obtuse apex, unbranched, septate, smooth-walled. Conidiomatal wall composed of thin-walled, brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the innermost wall cells of the conidioma, hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled, periclinal thickenings at the collarette zone. Conidia hyaline, cylindrical, with rounded ends, aseptate to several septate, smooth-walled, guttulate (Sutton 1980).
Type species: Chaetosticta perforata (Ellis & Everh.) Petr. & Syd., Ann Mycol. 23(3/6): 270 (1925)
Notes: Crane (1971) revised Chaetosticta and designated a lectotype on undersurface of leaves of Cirsium discolor (Asteraceae) for its generic type, C. perforata. The genus was re-described and re-illustrated by Sutton (1980) and Morgan-Jones et al. (1972e). Four taxa are listed in Index Fungorum (2019), but, apart from type species, other taxa have not been re-examined. The sexual morph of Chaetosticta has been assigned to Nematostoma Syd. & P. Syd. and Lasiostemma Theiss., Syd. & P. Syd. (Pseudoperisporiaceae) (Barr 1968; Crane 1971; Kirk et al. 2008; Hyde et al. 2013), but these connections have never been successfully demonstrated by culture studies or other evidence. Thus, we place Chaetosticta in Ascomycota, genera incertae sedis until molecular data is available.
Distribution: Australia, Chian, Brazil, India, Italy, Japan, Malaysia, New Zealand, Romania, Spain, UK, USA, (Sutton 1980, https://www.gbif.org/).
Cheilaria Lib., Pl. crypt. Arduenna, fasc. (Liège) 1(Praef.): 8 (1830)
Facesoffungi number: FoF 07155, Fig. 73
Cheilaria agrostidis (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, acervular, scattered to gregarious, semi-immersed, unilocular, glabrous. Ostiole absent. Conidiomatal wall composed of thick-walled, dark brown to pale brown cells of textura angularis to textura epidermoidea. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, lageniform to cylindrical, determinate, smooth-walled, with a minute apical channel. Conidia hyaline, fusiform, straight or slightly curved, 0–3-septate, smooth-walled, eguttulate (Sutton 1980).
Type species: Cheilaria agrostidis Lib., Pl. crypt. Arduenna, fasc. (Liège) 1: no. 63 (1830)
Notes: Cheilaria resembles Myriellina, and the differences between these two genera see notes of Myriellina. Wijayawardene et al. (2017b) accepted only Cheilaria agrostidis in the genus. This species is associated with leaf lesions on Calamagrostis inexpansa, C. varia, Cymbopogon giganteus, Milium effusum (Poaceae) (Sutton 1980). No molecular data is available for this genus. Fresh collections are needed to place it in a natural group.
Distribution: Germany, Sudan, Switzerland, UK, USA (Sutton 1980).
Choanatiara DiCosmo, in Nag Raj & DiCosmo, Can. J. Bot. 62(4): 709 (1984)
Facesoffungi number: FoF 07156
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious or confluent, initially immersed, ultimately becoming partly erumpent, globose to subglobose, unilocular, glabrous, ostiolate. Ostiole single, papillate, circular or oval, excentric. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis to textura globulos or textura prismatica. Conidiophores arising all around the cavity of the conidiomata, reduced to conidiogenous cells or present, when present, hyaline, cylindrical, unbranched or branched, invested in mucus. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to lageniform, discrete or integrated, smooth-walled, with or without percurrent proliferation. Conidia hyaline, fusiform to naviculate, with an acute apex, and a narrow and truncate base, unicellular, smooth-walled, bearing a funnel-shaped, apical or subapical, mucoid appendage.
Type species: Choanatiara lunata DiCosmo & Nag Raj, in Nag Raj & DiCosmo, Can. J. Bot. 62(4): 712 (1984)
Notes: Choanatiara was collected from fallen leaves of Pinus resinosa (Pinaceae) in Canada. Choanatiara is characterized by unilocular conidiomata with sinuate, lateral neck, more than one distinct tissue type, and fusiform to occasionally navicular, unicellular conidia bearing a funnel-shaped, apical or subapical, mucoid appendage. Two species are accepted in the genus, C. gracilis Nag Raj and C. lunata. No sequence data is available for these two species. We re-examined the type specimens of the twoChoanatiara species and provide detailed descriptions and photo plates.
Distribution: Canada, India.
Key to the species of Choanatiara
1. Conidiomta with lateral ostiole, average size of conidia 15 × 1.8 µm…C. gracilis
1. Conidiomta with central ostiole, average size of conidia 25 × 3 µm…C. lunata
Choanatiara gracilis Nag Raj, in Nag Raj & DiCosmo, Can. J. Bot. 62(4): 712 (1984)
Facesoffungi number: FoF 07157, Fig. 74
Choanatiara gracilis (DAOM 186356, holotype) a, b Herbarium package and specimen. c Appearance of dark brown to black conidiomata on the host. d Vertical section of conidioma. e, f Section of peridium. g–i Conidiogenous cells and developing conidia. j–n Conidia examined using lactophenol cotton blue and India ink.(arrows show appendages). Scale bars d = 100 µm, e–f = 20 µm, g–i = 10 µm, j–n = 5 µm
Saprobic on fruit capsules of Eucalyptus sp. (Myrtaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 200–250 µm diam., 100–200 µm high, dark brown to black, pycnidial, solitary to gregarious, deeply immersed at beginning, then becoming partly erumpent at maturity, subglobose to broadly ellipsoidal, unilocular, thick-walled, glabrous, with a subcylindrical or narrow conical, lateral neck, ostiolate. Ostiole single, circular or oval, excentric. Conidiomatal wall 20–60 µm wide, composed of two distinct tissue layers, an outer layer of textura angularis to textura globulos with thick-walled, dark brown to brown cells, and an inner layer of textura prismatica with thick-walled, large hyaline cells but reduced in size towards the conidial hymenium. Conidiophores arising all around the cavity of the conidiomata, reduced to conidiogenous cells or present, when present, hyaline, broadly cylindrical, unbranched or sparsely branched, invested in mucus. Conidiogenous cells 5–7 × 2–2.5 µm, hyaline, enteroblastic, phialidic, ampulliform to lageniform, discrete or integrated, smooth-walled, proliferating once or twice. Conidia 12–17 × 2–3 µm (\( \bar{x} \) = 15 × 1.8 µm; n = 30), hyaline, fusiform to occasionally naviculate, acute at apex, narrow and truncate at base, aseptate, smooth-walled, bearing a funnel-shaped, apical or subapical, mucoid appendage.
Material examined: India, Bangalore, Lalbagh Gardens, on fruit capsules of Eucalyptus sp. (Myrtaceae), 19 September 1980, T.R. Nag Raj (DAOM 186356, holotype).
Choanatiara lunata DiCosmo & Nag Raj, in Nag Raj & DiCosmo, Can. J. Bot. 62(4): 712 (1984)
Facesoffungi number: FoF 07158, Fig. 75
Choanatiara lunata (DAOM 173904, holotype; DAOM 215182) a–d Herbarium package and specimens (d from DAOM 215182). e, f Appearance of dark brown to black conidiomata on the host. g, h Vertical sections of conidiomata (h from DAOM 215182. i, j Section of peridium. k–p Conidiophores, conidiogenous cells and developing conidia (arrows show conidiogenous cells). q–u Conidia (r–u examined by using lactophenol cotton blue and India ink, arrows show apical appendages). Scale bars e = 500 µm, f = 200 µm, g–h = 100 µm, i–j = 20 µm, k–u = 10 µm
Saprobic on fallen leaves of Pinus resinosa (Pinaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 200–300 µm diam., 200–300 µm high, dark brown to black, pycnidial, mostly solitary, occasionally gregarious, appearing as irregular humps in surface view, deeply immersed at beginning, then becoming partly erumpent at maturity, globose to subglobose, unilocular, thick-walled, glabrous, ostiolate. Ostiole papillate, single, circular or oval, papillate, centrally located or with a lateral sinuate neck. Conidiomatal wall 10–50 µm wide, composed of two distinct tissue layers, an outer layer of textura globulosa thick-walled cells, dark brown becoming lighter toward the interior, and an outer layer of textura angularis to textura intricata thick-walled cells, hyaline. Conidiophores arising from the innermost layers of the conidiomata, hyaline, cylindrical or irregular, branched, septate, invested in mucus. Conidiogenous cells 6–9 × 2–4 µm, hyaline, enteroblastic, phialidic, cylindrical to ampulliform or lageniform, discrete or integrated, smooth-walled. Conidia 23–27 × 2–3 µm (\( \bar{x} \) = 25 × 3 µm; n = 30), hyaline, lunate to fusiform, obtuse at apex, narrow and truncate at base, aseptate, smooth-walled, bearing a funnel-shaped, apical, mucoid appendage.
Material examined: Canada, Ontario, Lambton County, Pinery Provincial Park, on fallen needles of Pinus resinosa (Pinaceae), 23 September 1979, F. DiCosmo (DAOM 173904, holotype); Ontario, Trout Creek, South of Delhi, on fallen leaves of P. resinosa, F. DiCosmo, 31 July 1980 (DAOM 215182).
Chondropodiella Höhn., Hedwigia 59(5): 281 (1917)
Facesoffungi number: FoF 07159, Fig. 76
Chondropodiella clethrincola (redrawn from Morgan-Jones et al. 1972d) a Conidia. b Vertical section of conidioma. c Conidiophores, and conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat, such as Clethra alnifolia, C. acuminata (Clethraceae). Sexual morph: putative sexual morph see Groves (1965). Asexual morph:Conidiomata dark brown, pycnidial, solitary to gregarious, semi-immersed to erumpent, cylindrical to cylindrical-subulate, sometimes bent, unilocular, locule extending the length of the beak and somewhat enlarged in the basal part, glabrous, beaked. Conidiomatal wall composed of thick-walled, hyaline to dark brown, somewhat interwoven to almost parallel hyphae of textura epidermoidea in the outer layer, becoming thick-walled, yellowish hyphae of textura oblita in the wall of the beak. Conidiophores arising from basal and lateral walls, hyaline, simple, branched, septate, smooth-walled. Conidiogenous cells hyaline, holothallic, cylindrical, determinate, smooth-walled. Conidia hyaline, arthric, formed by disarticulation of the conidial chain, produced in simple unbranched chains with the oldest conidium at the apex, cylindrical to ellipsoid, rounded at both ends or occasionally truncate at the base, unicellular, smooth-walled (Groves 1965; Morgan-Jones 1977).
Type species: Chondropodiella clethrincola (Ellis) Höhn., Hedwigia 59(5): 281 (1917)
Notes: The cylindrical to subulate conidiomata with locule extending the length of the beak and somewhat enlarged in the basal part in Chondropodiella is similar to Corniculariella. However, Corniculariella can be distinguished from Chondropodiella by its enteroblastic, phialidic conidiogenous cells and falcate or fusiform to clavate, 1–10-septate, curved conidia (Morgan-Jones et al. 1972d, this study). Chondropodiella has holothallic, cylindrical, septate conidiogenous cells, and hyaline, arthric, aseptate conidia formed by disarticulation of the unbranched conidial chain, with the oldest conidium at the apex, which can easily be confused with Phellostroma (Morgan-Jones et al. 1972d; Sutton 1980). However, the latter can be separated by its stromatic, pycnidial, cushion-shaped, multilocular conidiomata (Morgan-Jones et al. 1972d).
Chondropodiella is monotypic. Chondropodiella clethrincola was generally considered as the microconidial state of species placed in Godronia (Coolce and Ellis 1877; Groves 1965; Sutton 1977a). However, it is unclear whether Chondropodiella is congeneric with Godronia as no DNA sequence data exists for either generic type. Therefore, Chondropodiella should be treated as a legitimate name rather than reduced to a synonym of Godronia (Kirk et al. 2013). Fresh collections of this species are needed to clear up the placement of this genus and its putative sexual morph.
Distribution: Brazil, Sweden, USA (Groves 1965; https://www.gbif.org/).
Ciliochora Höhn., Ber. dt. bot. Ges. 37: 159 (1919)
Facesoffungi number: FoF 07160, Fig. 77
Ciliochora longiseta (redrawn from Nag Raj and DiCosmo 1978) a Conidia. Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat, such as Bambusa sp. (Poaceae), Fagus sylvatica (Fagaceae), Spatholobus littoralis (Fabaceae), Vitex parviflora (Lamiaceae). Sexual morph: undetermined. Asexual morph:Conidiomata brown, stromatic, pycnidial, solitary to gregarious, immersed, irregularly pulvinate or globose to subglobose, unilocular or multilocular, glabrous, with or without a well-developed clypeus. Ostiole absent, dehiscence by irregular splits of the apical wall. Conidiomatal wall composed of thick-walled, brown to pale brown cells of textura angularis to textura globulosa or textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline to subhyaline, enteroblastic, or holoblastic, subcylindrical, lagcniform, broadly ampulliform or irregular, integrated or discrete, determinate or indeterminate, smooth-walled. Conidia hyaline to subhyaline, fusiform to ellipsoidal, with truncate base, unicellular, smooth-walled, bearing tubular, branched or unbranched, flexuous, attenuated, apical appendages. Microconidia occasionally present, hyaline, acerose, straight or curved, unicellular, smooth-walled (Nag Raj and DiCosmo 1978; Nag Raj 1993).
Type species: Ciliochora longiseta (Racib.) Höhn., in Weese, Mitt. bot. Inst. tech. Hochsch. Wien 1(3): 84 (1924)
Notes: Nag Raj (1993) accepted two leaf-inhabiting species, Ciliochora indica (Subram. & K. Ramakr.) Nag Raj and C. longiseta. A third species C. calabrica B. Sutton & Mugnai was described from F. sylvatica in Italy; it is distinguished by its larger conidia and longer appendages (Sutton et al. 1996). The sexual morph of Ciliochora has been assigned in Physalospora (Nag Raj 1993), but there is no molecular data to prove this connection.
Distribution: India, Java, Philippines (Nag Raj 1993).
Ciliosporella Petr., Ann Mycol. 25(3/4): 217 (1927)
Facesoffungi number: FoF 07161
Sordariomycetes, Xylariomycetidae, Xylariales, Microdochiaceae
Saprobic on the host plant (Fabaceae and Myrtaceae) in terrestrial habitats. Sexual morph: undetermined. Asexual morph:Conidiomata pale brown to brown or dark brown, stromatic, pycnidial, solitary to gregarious or confluent, deeply immersed, globose to depressed globose, unilocular or multilocular, glabrous, papillate, ostiolate. Ostiole single, short, rounded, excentric. Conidiomatal wall of textura angularis or textura angularis to textura epidermoideat, thick-walled, brown, becoming paler toward the conidial hymenium. Paraphyses hyaline, filiform, unbranched, smooth-walled, originating from inner layer cells of conidiomata or from conidiophores. Conidiophores formed from the innermost wall layers of conidiomata, reduced to conidiogenous cells, when present hyaline, branched or unbranched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical to lageniform, discrete or integrated, determinate, smooth-walled. Conidia hyaline, fusiform to naviculate, truncate at base, acute at apex, 0–2-septate, attenuated at the apex into a rostrate tubular, unbranched appendage.
Type species: Ciliosporella selenospora Petr., Ann Mycol. 25(3/4): 217 (1927)
Notes: Ciliosporella was described form Trifolium alpestre in Australia (Petrak 1927b). Nag Raj (1974, 1993) re-described and re-illustrated the genus. Yuan and Mohammed (1997) described a second species C. tuberculiformis Z.Q. Yuan & C. Mohammed on Eucalyptus regnans (Myrtaceae) in Tasmania. Ciliosporella tuberculiformis is distinguished from C. selenospora by its larger conidia, host plant and geographical distribution. We re-examined the isotype of C. selenospora, and found it has hyaline, filiform, paraphyses and 0–1-septate conidia. Thus an emended description for Ciliosporella is provided. In addition, two fresh collections on Colutea arborescens and Galega sp. from Italy share similar morphology of conidiomata, conidiogenous cells and conidia with C. selenospora and C. tuberculiformis, but are distinguished by conidia dimensions and septation, as well as conidiomatal structure. Therefore, a new species Ciliosporella italica is introduced for these two strains. Our phylogeny, based on LSU sequence data show that Ciliosporella is related to Sordariomycetes (Fig. 78). Fresh collections of the generic type are needed to confirm its placement.
Phylogenetic tree generated from a maximum likelihood analysis analysis based on the alignment of LSU sequences data representing Microdochiaceae and other families in Xylariales. The newly generated nucleotide sequences were compared against the GenBank database by Blast search. Related sequences were obtained from GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Ninety-six strains are included in the analyses, which comprise 772 characters including gaps. Chaetosphaeria jonesii MFLUCC 15–1015 and C. innumera MR 1175 are used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 7919.785516 is presented. The matrix had 345 distinct alignment patterns, with 2.16% of undetermined characters or gaps. Estimated base frequencies were: A = 0.249993, C = 0.207779, G = 0.298530, T = 0.243698; substitution rates AC = 0.713492, AG = 3.270922, AT = 1.082226, CG = 0.604374, CT = 6.756273, GT = 1.000000; gamma distribution shape parameter α = 0.486612. The maximum parsimonious dataset consisted of constant 484, 233 parsimony-informative and 55 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 1392 steps (CI = 0.300, RI = 0.706, RC = 0.212, HI = 0.700) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MP BS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.04 changes. Ex-type or ex-epitype strains are in bold. New isolates are in blue
Distribution: Austria, Italy (Nag Raj 1993; this study).
Key to species
1. Paraphyses present, conidia 0–2-septate…2
1. Paraphyses absent, conidia aseptate…C. tuberculiformis
2. Conidiophores cylindrical, branched, septate, average size of conidia 27 × 3.5 µm…C. selenospora
2. Conidiophores reduced to conidiogenous cells, average size of conidia 35 × 3µm…C. italica
Ciliosporella selenospora Petr., Ann Mycol. 25(3/4): 217 (1927)
Facesoffungi number: FoF 07162, Fig. 79
Ciliosporella selenospora (FH 01142397, isotype). a, b Herbarium package and specimen. c, d Appearance of pale brown to brown conidiomata on the host. e, f Vertical section of conidiomata. g, h, k Section of peridium. i, j, l–o Conidiophores, conidiogenous cells, paraphyses and developing conidia. p–t Conidia. Scale bars c = 200 µm, d–f = 100 µm, g, i = 20 µm, h, k = 50 µm, j, l–t = 10 µm
Saprobic on dead stems of Trifolium alpestre (Fabaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 150–300 µm diam., 100–150 µm high, pale brown to brown, stromatic, pycnidial, solitary to gregarious or occasionally confluent, deeply immersed, globose to depressed globose, unilocular or multilocular, glabrous, papillate, ostiolate; the locules 60–100 µm diam., 80–100 µm high. Ostiole 30–40 × 20–40 µm, single, short, rounded, excentric. Conidiomatal wall 20–40 µm wide, composed of three types of cells, (a) basal wall of textura angularis to textura epidermoidea with pale to hyaline, thick-walled cells; (b) middle wall of textura epidermoidea with hyaline, thick-walled cells; (c) upper wall which surrounding by brown to pale brown, thick-walled, cells of textura angularis. Paraphyses hyaline, filiform, unbranched, smooth-walled, originating from inner layer of cells of conidiomata or from conidiophores. Conidiophores 10–20 × 2–4 µm, formed from the innermost wall layers of conidiomata, hyaline, branched, septate, smooth-walled. Conidiogenous cells 6–10 × 2–3 µm, hyaline, enteroblastic, phialidic, subcylindrical to lageniform, discrete or integrated, determinate, smooth-walled, with morderate periclinal thickening in the collarette zone. Conidia 23–31 × 3–5 µm (\( \bar{x} \) = 27 × 3.5 µm; n = 30), hyaline, fusiform to navicular, truncate at base, acute at apex, 1-septate, bearing a rostrate, tubular, unbranched appendage at apex (6–11 × 1–2 µm).
Material examined: Austria, Brunn in Mähren, on stems of Trifolium alpestre L. (Fabaceae), May 1926, J. Hruby (FH 01142397, isotype), F. Petrak, Flora Bohemiae et Moraviae exs. #2245.
Ciliosporella italica W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557142, Facesoffunginumber: FoF 07163, Figs. 80, 81
Ciliosporella italica(MFLU 16-1114, holotype) a Herbarium specimen. b, c Appearance of dark brown conidiomata on the host. d, e, h Vertical section of conidiomata. f, g Section of peridium. i Ostiole. j Conidiogenous cells and paraphyses. k–n Conidiogenous cells and developing conidia. o Germinating conidia. p–s Conidia. t Culture on PDA. Scale bars b = 1000 µm, c = 200 µm, d, h = 100 µm, e = 50 µm, f–g = 10 µm, i–j = 20 µm, k–n = 5 µm, o = 50 µm, p–s = 10 µm, t = 20 mm
Ciliosporella italica(MFLU 16-1114, paratype) a Herbarium specimen. b, c, d Appearance of brown to dark brown conidiomata on the host. e, f Vertical sections of conidiomata. g, h Sections of peridium. i Ostiole. j Germinating conidium. k–m Conidiogenous cells, paraphyses and developing conidia. n–q Conidia. r Culture on PDA. Scale bars b = 1000 µm, c = 200 µm, d = 500 µm, e = 100 µm, f–h = 50 µm, i–j = 20 µm, k, l = 5 µm, m, n–q = 10 µm, r = 20 mm
Etymology: Named after the country Italy, where it was collected.
Saprobic on dead stream of Colutea arborescens and Galega sp. (Fabaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 200–250 µm diam., 50–100 µm high, brown to black, stromatic, pycnidial, solitary to gregarious or confluent, immersed, subperidermal, globose to depressed globose, unilocular or multi-locular, thick-walled, glabrous, papillate, ostiolate, the locules 50–80 µm diam., 60–100 µm high. Ostiole 10–30 µm wide, single to each locule, circular, centrally or laterally located. Conidiomatal wall 8–15 µm wide, composed of thick-walled, pale brown to hyaline cells of textura prismatica to angularis, becoming thicker at upper part. Conidiophores reduced to conidiogenous cells. Paraphyses hyaline, filiform, unbranched, smooth-walled, originating from inner layer of cells of conidioma. Conidiogenous cells 6–10 × 2–5 µm, hyaline, enteroblastic, phialidic, cylindrical to lageniform, attenuate towards apex, discrete, determinate, formed from the inner layer cells of the conidiomata. Conidia 30–42 × 2.6–4.3 µm (\( \bar{x} \) = 35 × 3 µm; n = 100), hyaline, fusiform to navicular, with an acute apex and slightly truncate base, mostly 1-septate, occasionally 2-septate, thick-walled, smooth, attenuated at the apex into a rostrate, tubular, unbranched appendage.
Culture characteristics: colony on the PDA, reaching 15–20 mm diam, after 14 days at 25 °C, orange, with white, circular margin, flattened, felt-like, with filamentous, dense, aerial mycelium on the surface, reverse similar in colour.
Material examined: Italy, Province of of Forlì-Cesena, Predappio, San Martino, on dead aerial branches of Colutea arborescens (Fabaceae), 22 March 2016, Erio Camporesi, IT2898 (MFLU 16-1114, holotype), ex-type living culture, MFLUCC 16-1146, ICMP 21541; ibid., Province of Arezzo, Stia, Montemezzano, on dead aerial stems of Galega officinalis (Fabaceae), 15 July 2016, Erio Camporesi, IT3029 (MFLU 16-2154, paratype), ex-paratype living culture, MFLUCC 16-1305.
Notes: Strain (MFLUCC 16-1146) on C. arborescens shares similar characters with strain (MFLUCC 16-1305) on Galega sp., in having stromatic and pycnidial conidiomata with lateral ostiole, phialidic conidiogenous cells and fusiform to navicular, 0–2-septate conidia. Moreover, these two strains are 100% identical in LSU, SSU, ITS, rpb2 sequence data except at the beginning and end regions. Ciliosporella italica differs from C. selenospora in conidomatal structure and conidiohores. The former has textura angularis to epidermoidea wall cells and lacks conidiophores, while the latter has textura angularis to prismatica wall cells and cylindrical, branched, septate conidiophores. Ciliosporella italica is distinguished from C. tuberculiformis by its paraphyses.
Clohesyomyces K.D. Hyde, Aust. Syst. Bot. 6(2): 170 (1993)
Facesoffungi number: FoF 07164
Dothideomycetes, Pleosporomycetidae, Pleosporales, Lindgomycetaceae
Saprobic on submerged wood in a freshwater stream. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, subperidermal, solitary to gregarious, semi-immersed, globose to subglobose, unilocular, glabrous, ostiolate. Ostiole single, papillate, circular, centrally located. Conidiomatal wall composed of brown to pale brown, thick-walled cells of textura angularis to textura porrecta. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, phialidic, with a collarette, cylindrical to subcylindrical or lageniform, determinate, thick- and smooth-walled. Conidia hyaline, ellipsoidal, rounded at both ends, or occasionally truncate at base, 1-septate, slightly constricted at septum, straight or slightly curved, thick- and smooth-walled, guttulate, surrounded by an irregular mucilaginous sheath.
Type species: Clohesyomyces aquaticus K.D. Hyde, Aust. Syst. Bot. 6(2): 170 (1993)
Notes: Clohesyomyces aquaticus was originally reported from Australia on submerged wood in a freshwater stream (Hyde 1993). This species has been also reported in Yunnan, China and Thailand (Cai et al. 2006; Vijaykrishna et al. 2006). Zhang et al. (2012a, b) showed that C. aquaticus was closely related to Lindgomycetaceae (Pleosporales) based on sequence data of LSU and SSU. Clohesyomyces has remained monotypic.
Distribution: Australia, Denmark, France, Germany, Greece, Thailand, UK, (Hyde 1993; Zhang et al. 2012a, b, https://www.gbif.org/).
Clohesyomyces aquaticus K.D. Hyde, Aust. Syst. Bot. 6(2): 170 (1993)
Facesoffungi number: FoF 07165, Fig. 82
Phylogenetic tree inferred from a maximum likelihood analysis based on a concatenated alignment of ITS, GPDH, act and tub sequence data representing Colletotrichum. Related sequences were obtained from Ma et al. (2018), Damm et al. (2019) and GenBank. One hundred and thirty-five strains are included in the analyses, which comprise 1633 characters including gaps (ITS: 1–561, gpdh: 562–859, act: 860–1134, tub: 1135–1633). Monilochaetes infuscans CBS 869.96 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 29658.549798 is presented. The matrix had 1124 distinct alignment patterns, with 13.26% of undetermined characters or gaps. Estimated base frequencies were: A = 0.220113, C = 0.306427, G = 0.241277, T = 0.232183; substitution rates AC = 1.088881, AG = 2.766716, AT = 1.049106, CG = 0.809360, CT = 3.568059, GT = 1.000000; gamma distribution shape parameter α = 0.411952. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.07 changes. Ex-type or ex-epitype strains are in bold. New isolate is in blue
Saprobic on submerged wood in a freshwater stream. Sexual morph: undetermined. Asexual morph:Conidiomata 150–250 µm diam., 200–250 µm high, dark brown to black, pycnidial, subperidermal, solitary to gregarious, semi-immersed, globose to subglobose, unilocular, thick-walled, glabrous, ostiolate. Ostiole single, papillate, circular, centrally located. Conidiomatal wall 20–40 µm wide, consisting of brown to pale brown, thick-walled cells of textura angularis gradually merging with textura porrecta. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 6–15 × 2–10 µm, hyaline, phialidic, cylindrical to subcylindrical or lageniform, determinate, thick- and smooth-walled, with marked periclinal thickening at collarette zone. Conidia 10–23 × 7–10 µm (\( \bar{x} \) = 17 × 8 µm; n = 30), hyaline, ellipsoidal, rounded at both ends, or occasionally truncate at base, 1-septate, slightly constricted at septum, straight or slightly curved, thick-walled, smooth, guttulate, surrounded by an irregular mucilaginous sheath (Fig. 83).
Material examined: Thailand, Chiang Mai, Doi Inthanon, on submerged wood, 16 November 2010, Huang Zhang, d66 (MFLU 11–1112), d66 (MFLU 11–1114).
Colletotrichum Corda, in Sturm, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 3(12): 41 (1831)
Facesofungi number: FoF 00144
Sordariomycetes, Hypocreomycetidae, Glomerellales, Glomerellaceae
Endophytic, parasitic or saprobic on the host plant. Sexual morph: see Yamamoto (1961), Guerber and Correll (2001), Hou et al. (2016), Ma et al. (2018), Damm et al. (2019). Asexual morph:Conidiomata dark brown to black, acervular, solitary, gregarious or confluent, semi-immersed to erumpent, subglobose, glabrous, unilocullar, black, thick–walled, smooth-walled. Conidiomatal wall composed of thick-walled, dark brown cells of textura globulosa to textura epidermoidea or textura porrecta, textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the basal stroma, hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, determinate, smooth-walled. Conidia hyaline, ellipsoid or falcate, straight or slightly curved, thick- and smooth-walled, guttulate. Appressoria brown, entire or with crenate to irregular margins, simple or repeatedly germinating to produce complex columns of several closely connected appressoria (the description of appressoria from Sutton 1980).
Type species: Colletotrichum lineola Corda, in Sturm, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 3(12): 41 (1831)
Notes: Colletotrichum is a coelomycetous genus with Glomerella sexual state (Hyde et al. 2014). There are 193 accepted species in eleven species complexes and 23 accepted singleton species (Hyde et al. 2014; Ma et al. 2018). Colletotrichum species can occur on plants as fungal endophytes, parasites, pathogens or saprobes and are reported from temperature to tropical areas (Vucinic and Latinovic 1997; Huang et al. 2009, 2013; Ma et al. 2018; Rashmi et al. 2019). Colletotrichum species can cause anthracnose which is a devastating disease in many economically important crops (Cai et al. 2009; Cannon et al. 2012). Colletotrichum was regarded as the eighth most important plant pathogenic genus in the world considering their perceived scientific and economic importance (Dean et al. 2012). They have also been found as fungal endophytes in angiosperms, gymnosperms, ferns and lichens (Li et al. 2007; Cannon et al. 2012; Damm et al. 2012a; Hyde et al. 2014). Many Colletotrichum species adopt biotrophic life strategies in living plant tissues (Photita et al. 2004; Yang et al. 2009). Confused morphological features, ambiguous species boundaries and incorrectly named sequences in NCBI often make it difficult to identify species in Colletotrichum (Cai et al. 2009; Hyde et al. 2014; Liu et al. 2016). Therefore, a multi-gene (ITS, GDPH, TUB2, ApMat, GS, ACT) phylogenic analysis method combined with morphological characteristics was proposed for species resolution in this genus (Cai et al. 2009; Hyde et al. 2014; Hou et al. 2016; Ma et al. 2018; Damm et al. 2019). We include a new record of Colletotrichum sansevieriae from Thailand.
Distribution: worldwide (Cannon et al. 2012; Damm et al. 2012a, b, 2019; Liu et al. 2015a, b, 2016).
Colletotrichum sansevieriae Miho Nakam. & Ohzono, in Nakamura, Ohzono, Iwai & Arai, J. Gen. Pl. Path. 72(4): 253 (2006)
Facesoffungi number: FoF 07397, Fig. 84
Colletotrichum sansevieriae (MFLU 19-2898) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d Vertical section of conidioma. e, f Vertical sections of peridium. g–i Conidiogenous cells and developing conidia. j–p Conidia. q Germinating conidium. Scale bars: b = 1000 µm. c = 500 µm, d = 100 µm, e–f = 20 µm, g–i = 10 µm, j–p = 5 µm, q = 20 µm, r = 20 mm
Parasitic on living leaves of Sansevieria trifasciata, causing conspicuous, rounded to oval, black, leaf-spots, forming numerous minute fruiting bodies in concentric zones. Sexual morph: undetermined. Asexual morph:Conidiomata 300–350 µm diam., 300–350 µm high, stromatic, acervular, amphigenous, solitary, gregarious or confluent, semi-immersed, subglobose, glabrous, unilocullar, black, thick- and smooth-walled. Conidiomatal wall 70–140 µm wide, composed of thick-walled, dark brown cells of textura globulosa to loose textura epidermoidea in the outer part, merging with brown to pale brown, thick-walled cells of textura porrecta to textura angularis in the inner part. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the basal regions of stromata, 15–35 × 3–6 µm, hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, determinate, smooth-walled. Conidia 13–23 × 4–7 µm (\( \bar{x} \) = 18 × 5 µm; n = 30), hyaline, ellipsoidal, with rounded ends, sometimes with a narrow and truncate base, straight or slightly curved, thick- and smooth-walled, guttulate.
Culture characteristics: Colonies on PDA, reaching 6–8 cm diam. after 1 week at 25–30 °C, white to brown with age, circular, flattened, dense, slightly cottony, smooth at margins, reverse brown. Sporulation appearing after 4 weeks. Conidiomata formed from mycelium, brown to dark brown, gregarious, globose or subglobose.
Material examined: Thailand, Chiang Rai, on living leaves of Sansevieria trifasciata (Asparagaceae), 10 April 2015, Wen-Jing Li, WJL0035 (MFLU 19-2898), living culture, MFLUCC 15-0579 = ICMP 21855.
Notes: Colletotrichum sansevieriae is host-specific to Sansevieria, causing leaf anthracnose (Nakamura et al. 2006). This species has been reported from Australia, Japan, Korea and USA (Nakamura et al. 2006; Aldaoud et al. 2011; Palmateer et al. 2012; Park et al. 2013; Liu et al. 2014). Our collection MFLUCC 15-0579 clustered with C. sansevieriae with good support (Fig. 83). The conidia dimensions of our collection (13–23 × 4–7 µm) fall into the range of C. sansevieriae (12.5–(18.4)– 32.5 × 3.8–(6.4)–8.8 µm; Nakamura et al. 2006). Based on morphology, phylogeny, habit and host, our collection is identified as a new record of C. sansevieriae from Thailand.
Collonaemella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 82 (1915)
Facesoffungi number: FoF 07166, Fig. 85
Collonaemella microscopica (redrawn from Nag Raj and DiCosmo 1978) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown, stromatic, pycnidial, scattered, semi-immersed, subglobose to subconical, unilocular, glabrous, ostiolate. Ostiole cylindrical to subcylindrical, single, centrally located. Conidiomatal wall composed of thick-walled, brown to sub-hyaline cells of textura globulosa. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical to ampuliform, determinate, smooth-walled, with a minute apical channel and collarttte. Conidia hyaline, fusiform, with acute ends, 1-septate, smooth-walled (Nag Raj and DiCosmo 1984).
Type species: Collonaemella microscopica (Fr.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 82 (1915)
Notes: Nag Raj and DiCosmo (1984) emended the description of Collonaemella and treated it as a monotypic genus. Collonaemella was listed as a synonym of Corniculariella in Index Fungorum (2019), but a comparison of their morphology indicates that they are distinct in the structure of conidiomata and conidia. Collonaemella has an inconspicious beaked conidiomata with textura globulosa cells, and fusiform, 1-septate, fusiform conidia (Sutton 1980). Corniculariella has beaked conidiomata with textura angularis to intricata or porrecta cells, and 1–10-septate, fusiform to clavate conidia. Therefore, Collonaemella is treated as a legitimate name. No sexual morph has been linked to this genus, and no molecular data is available.
Distribution: France, UK (https://www.gbif.org/).
Colpoma Wallr., Fl. crypt. Germ. (Norimbergae) 2: 422 (1833)
= Conostroma Moesz, Bot. Közl. 19: 44 (1921)
Facesoffungi number: FoF 07167, Fig. 86
Colpoma quercinum (asexual morph, redrawn from Sutton 1980) a Conidia. b Conidiophores, conidiogenous cells and developing conidia
Leotiomycetes, Leotiomycetidae, Rhytismatales, Rhytismataceae
Saprobic or parasite on the host plant in terrestrial habitat (Johnston 1991). Sexual morph: see Twyman (1947) and Hou and Piepenbring (2005). Asexual morph:Conidiomata dark brown, stromatic, acervular, solitary, subepidermal in origin, immersed, unilocular or multilocular, glabrous. Ostiole absent, dehiscing by the central column rupturing the bark, then opening widely. Conidiomatal wall composed of thick-walled, dark brown to subhyaline cells of textura angularis. Conidiophores arising from innermost layer of locular walls, hyaline, cylindrical to subcylindrical, branched, smooth-walled. Conidiogenous cells hyaline, holoblastic, sympodial, subcylindrical, integrated, indeterminate, smooth-walled, with up to 3 conidia formed sympodially in a very restricted area at the apices. Conidia hyaline, cylindrical, obtuse at the apex, acute or obtuse at the base, unicellular, smooth-walled, eguttulae (Sutton 1980).
Type species: Colpoma quercinum (Pers.) Wallr., Fl. crypt. Germ. (Norimbergae) 2: 423 (1833)
= Conostroma didymum (Fautrey & Roum.) Moesz, Bot. Közl. 19: 44 (1920)
Notes: Conostroma didymum (Fautrey & Roum.) Moesz, type of Conostroma, has been reported as the asexual morph of Colpoma quercinum, type of Colpoma (Sutton 1980). According to one fungus = one name initiative, Conostroma (1920) was reduced to a synonym of Colpoma (1833) (Wijayawardene et al. 2017a). More than 30 epithets are listed in Index Fungorum (2019), but many of them have not been studied by molecular data. Colpoma was accepted as a member of Rhytismataceae (Leotiomycetes) (Lantz et al. 2011; Ekanayaka et al. 2019), but this genus needs to be redefined based on many fresh collections.
Distribution: Austria, China, France, Germany, Roumania, UK, USA, Czech Republic, New Zealand (Sutton 1980; Johnston 1991; Hou and Piepenbring 2005).
Comatospora Piroz. & Shoemaker, Can. J. Bot. 49: 539 (1971)
Facesoffungi number: FoF 07168
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, scattered, subcuticular in origin, then becoming erumpent, hemispherical, unilocular, glabrous, ostiolate. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis. Ostiole circular, single, papillate, centrally located. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising all around the cavity of conidiomata, hyaline, annellidic, discrete, cylindrical, clavate or ampulliform, smooth-walled, percurrently developing to produce new crops of conidia at higher levels, with marked periclinal thickenings and flared collarettes. Conidia ellipsoid to ovoid, 1-euseptate, hyaline, smooth-walled, bearing up to 8, filiform, flexuous, unbranched, apical, extracellular appendages, arising by differential gelatinization of a conidium sheath.
Type species: Comatospora suttonii Piroz. & Shoemaker, Can. J. Bot. 49: 539 (1971)
Notes: Comatospora can be confused with Crinitospora, as these two genera have annellidic conidiogenous cells and 1-septate conidia bearing apical appendages. Comatospora differs from Crinitospora by its pycnidial conidiomata and conidia appendage type. The conidial appendages of Comatospora are extracellular, originating by structural changes in parts of the conidium sheath, whereas it is cellular, attenuated or filiform, simple or branched, nucleate or enucleate, originating from the upper part of conidium body in Crinitospora (Nag Raj 1993). Comatospora is monotypic and no sexual morph has been linked to it. The fungus was initially described from twigs and leaf scars of withered branches of Picea mariana in Canada (Pirozynski and Shoemaker 1971). Because of lack of molecular data, the placement of this genus is undetermined.
Distribution: Canada.
Comatospora suttonii Piroz. & Shoemaker, Can. J. Bot. 49: 539 (1971)
Facesoffungi number: FoF 07169, Fig. 87
Comatospora suttonii (DAOM 129701, holotype) a–c Herbarium specimen. d–g Appearance of black conidiomata on the host. h–i Vertical section of conidiomata. j Section of peridium. k–n Conidiogenous cells and developing conidia (arrows show annellations). o–s Conidia. Scale bars d–e = 500 µm, f–g = 200 µm, h–i = 100 µm, j = 20 µm, k–s = 10 µm
Saprobic on twigs and leaf scars of withered branches of Picea mariana (Pinaceae). Sexual morph: undetermined. Asexual morph: Conidiomata 200–300 µm diam., 70–150 µm high, black, pycnidial, solitary to gregarious, superficial, hemispherical or pyriform, unilocular, glabrous, opening by an ill-defined pore or papillate, ostiolate. Ostiole oval or circular, centric or eccentric. Conidiomata wall 30–50 µm wide, composed of thick-walled, progressively dark brown to hyaline cells of textura angularis from outer layers to inner layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 10–20 × 4–7 µm, enteroblastic, annelledic, hyaline, cylindrical, clavate or ampulliform, thin-walled and smooth, percurrently proliferating upward several times and producing new conidia at higher level each time. Conidia 7–17 × 4–7 µm (\( \bar{x} \) = 11 × 5 µm; n = 30), hyaline, ellipsoid to ovoid, rounded and broad at apex, narrowed and slightly truncate at base, 1-septate, smooth-walled, and occasionally slightly constricted at septum, guttulate, bearing up to 8, filiform, flexuous, apical appendages.
Material examined: Canada, British Columbia, Victoria, Vancouver Island, Old Fort, on twigs and leaf scars of withered branches of Picea mariana (Pinaceae), J.S. Monts, 8 June 1967 (DAOM 129701, holotype).
Conicomyces R.C. Sinclair, Eicker & Morgan-Jones, Mycologia 75(6): 1100 (1983)
Facesoffungi number: FoF 07170
Sordariomycetes, Sordariomycetidae, Chaetosphaeriales, Chaetosphaeriaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, synnematous, scattered or gregarious, superficial, cornute to subcylindrical, slightly roughened, differentiated into a long, subcylindrical compacted mycelial stalk with a slightly swollen head bearing a concave conidial hymenium, setose; wall of stipe and hymenium composed of thick-walled, brown to pale brown cells of textura intricata to textura porrecta, textura globulosa or textura epidcrmoidea. Conidiomatal setae brown at base, paler and narrow above, straight or curved, unbranched, septate, thick-walled, arising from stroma or stipe. Conidiophores arising from the inner layer cells of the apical part of the stalk, pale brown to hyaline, branched or unbranched, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical, discrete or integrated, smooth-walled, with periclinal wall thickening above. Conidia hyaline, acerose, claviform, or elongate fusiform, with a narrowed apex and truncate base, multiseptate, straight or slightly curved, thick- and smooth-walled, guttulate, bearing single, unbranched, flexuous apical appendage (Nag Raj 1993).
Type species: Conicomyces transvaalensis R.C. Sinclair, Eicker & Morgan-Jones, Mycologia 75(6): 1100 (1983)
Notes: Conicomyces was described as a member of hyphomyctes by Sinclair et al. (1983), Illman and White (1985) and Seifert (1999, 2011), whereas Nag Raj (1993) and Liu et al. (2015a, b) considered it as a coelomycetous taxon. Four species, C. contortus Illman & G.P. White, C. nassensis Seifert, C. pseudotransvaalensis A. Hashim., G. Sato & Kaz. Tanaka and C. transvaalensis, are accepted in the genus. Liu et al. (2015a, b) showed that Conicomyces is related to Chaetosphaeriaceae (Chaetosphaeriales, Sordariomycetes), on the basis of LSU and ITS sequence data. Fresh collections of the generic type are needed to confirm its placement.
Distribution: Austria, Canada, Japan, South Africa, USA (Nag Raj 1993; Liu et al. 2015a, b; this study).
Conicomyces contortus Illman & G.P. White, Can. J. Bot. 63(3): 419 (1985)
Facesoffungi number: FoF 07171, Fig. 88
Conicomyces contortus (DAOM 177281, type) a–b Herbarium package and specimen. c–e Appearance of brown conidiomata on the host. f–h, l Conidiomata with stalk and head. i Conidiomatal setae. j–k, o–p Conidiophores, conidiogenous cells and developing conidia (arrows show phialidic conidiogenous cells). m–n Structure of conidiomatal wall. q–v Conidia. Scale bars c–e = 200 µm, f–g = 100 µm, h = 50 µm, i–k = 10 µm, l = 100 µm, m–n = 20 µm, o–p = 10 µm, q–v = 5 µm
Corticolous or lignicolous.Sexual morph: undetermined. Asexual morph:Conidiomata up to 700 µm long, stromatic, solitary or gregarious, seemingly superficial and spuriously synnematous, but intra-peridermal in origin and coelomycetous in nature, cornute to subcylindrical, slightly roughened, differentiated into a stalk and a conidiiferous head; stalk up to 500 µm long, 100–160 µm wide, black to dark brown for most of its length, becoming paler at upper part, subcylindrical, straight or variously curved, unbranched or branched, thick-walled; head 200–500 µm diam., 100–400 µm wide, slightly swollen, ellipsoidal or depressed globose, bearing a concave conidial hymenium, often with a pointed tip or sometimes flattened above, sparsely setose. Wall of stipe and hymenium composed of thick-walled, brown to pale brown cells of textura intricata to textura porrecta. Conidiomatal setae brown at base, becoming paler and narrower above, scant, septate, unbranched, thick-walled. Conidiophores up to 60 µm long, pale brown to hyaline, arising in the cavity of the conidiomata, more or less compact, mostly irregularly branched, septate, smooth, invested in mucus. Conidiogenous cells hyaline, enteroblastic, subcylindrical to clavate, discrete or integrated, smooth-walled, with periclinal thickenings above. Conidia 60–80 × 3–5 µm (\( \bar{x} \) = 70 × 4 µm; n = 30), hyaline, acerose to elongate fusiform, with a narrowed and obtuse apex, and a narrowed and truncate base bearing minute marginal frills, 10–19-septate, without constrictions at the septa, indistinct or distinct, straight or slightly curved, thick- and smooth-walled, guttulate, bearing a single, tubular, unbranched, flexuous apical appendage (20–40 µm long).
Material examined: Canada, Ontario, Tarzwell, Boreal forest, on rotten, decorticated branches (probably of Populus tremuloides), 3 October 1980, G.P. White (DAOM 177281, type).
Conidiocarpus Woron., Key to fungi (fungi imperfecti) 2: 743 (1917)
= Phragmocapnias Theiss. & Syd., Ann Mycol. 15(6): 480 (1918) [1917]
Facesoffungi number: FoF 06946
Dothideomycetes, Dothideomycetidae, Capnodiales, Capnodiaceae
Habit forming a soot-like coating on the upper surface of leaves. Thallus composed of brown to pale brown, cylindrical, branched, septate hyphae. Sexual morph: see Chomnunti et al. (2011). Asexual morph:Conidiomata brown to dark brown, pycnidial, solitary to gregarious, superficial, unilocular, glabrous, differentiated into a basal stalk and a conidiiferous locule at upper half. Rostrate apex apical, centrally located, surrounded by hyaline hyphae. Stalk and pycnidial wall composed of thick-walled, dark brown to pale brown cells of textura porrecta to textura angularis. Conidiophores formed on inner layers of pycnidial wall. Conidiogenous cells not observed. Conidia hyaline, ellipsoid to cylindrical, unicellular, smooth-walled, eguttulate.
Type species: Conidiocarpus penzigii Woron., Ann Mycol. 25(3/4): 250 (1927)
Notes: Chomnunti et al. (2011) linked Conidiocarpus to its sexual morph Phragmocapnias Theiss. & Syd. based on LSU and SSU sequence data, and reduced the former as a synonym of the latter. Bose et al. (2014) confirmed this connection and reinstated Conidiocarpus as the generic name, because it is an older name. Hongsanan et al. (2015) followed this concept, although Wijayawardene et al. (2017b) recommended using the sexual morph name Phragmocapnias for the holomorph, but without providing a detailed reason. Hongsanan et al. (2015) also introduced a new combination, C. philippinensis for Phragmocapnias philippinensis Hongsanan & K.D. Hyde. However, phylogenetic analyses based on LSU, SSU and ITS sequence data showed that C. philippinensis clustered with Antennariella placitae (CBS 124785) and Chaetocapnodium siamensis (MFLUCC 13-0778) (Fig. 53), thus this taxon is excluded from Conidiocarpus. We use Conidiocarpus in preference over Phragmocapnias. Phragmocapnias asiaticus Chomnunti & K.D. Hyde (MFLUCC10-0062) is synonymized under C. siamensis (Fig. 53), because the sequences similarities between P. asiaticus (MFLUCC10-0062) and C. siamensis (MFLUCC 10-0065) are 100% (422/422) in ITS, 99% (970/975) in LSU and 100% (959/959) in SSU. Phragmocapnias asiaticus has slightly smaller pycnidia and conidia than C. siamensis, but this may not be informative at the species level in Conidiocarpus. In addition, a fresh collection from Thailand is identified as C. siamensis (Fig. 53).
Distribution: Bangladesh, Brazil, Canada, Germany, India, Iran, Myanmar, Seychelles, Thailand, Uganda, Viet Nam (Chomnunti et al. 2011; Hongsanan et al. 2015; https://www.gbif.org/).
Conidiocarpus siamensis (Chomnunti & K.D. Hyde) T. Bose, in Bose, Reynolds & Burbee, Mycologia 106(4): 753 (2014)
≡ Phragmocapnias siamensis Chomnunti & K.D. Hyde, in Chomnunti et al., Fungal Diversity 51(1): 112 (2011)
= Phragmocapnias asiaticus Chomnunti & K.D. Hyde, in Chomnunti et al., Fungal Diversity 51(1): 111 (2011)
Facesoffungi number: FoF 07172, Fig. 89
Conidiocarpus siamensis (MFLU 19-2891) a, b Sooty moulds on the surface of host plant. c, d Appearance of dark brown conidiomata on the host surface. e Pycnidial wall. f Ostiole surrounded by hyaline hyphae. g Network-like hyphae. h–j Slide view of stalked pycnidia. k Rostrate apex. l Germinating conidia. m Conidia. n, o Culture on MEA. Scale bars c–d, i = 100 μm, e–g, j–l = 20 μm, h = 200 μm, m = 10 μm
Corniculariella rhamni (MFLU 16-1329, holotype) a–e Appearance of yellowish, dark brown to black conidiomata on the host. f–j Vertical sections of conidioma. k Section of peridium. l–m Conidiogenous cells and developing conidia. n. Paraphyses. o–q Conidia, r Germinating conidium. s–t Cultures on PDA . Scale bars a, e–i = 200 µm, j–k = 100 µm, l–m, o–q = 20 µm, n = 10 µm, r = 100 µm, s–t = 20 mm
Cornucopiella mirabilis (redrawn from Nag Raj and DiCosmo 1980) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Cornutispora limaciformis (DAOM 138360, holotype) a, b Herbarium specimen. c, d Appearance of black conidiomata on the host. e–h Vertical sections of conidiomata. i, j Section of peridium. k, l Conidiophores, conidiogenous cells and developing conidia. m–t Conidia. Scale bars c = 1000 µm, d = 500 µm, e–f = 500 µm, g–h = 200 µm, i–l = 20 µm, m–t = 10 µm
Crandallia juncicola (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Conidia
Crinitospora pulchra (IMI 259110, holotype) a Herbarium specimen package. b, d–e Appearance of brown conidiomata on grass laid on top of PDA. c, f Appearance of black conidiomata on PDA. g–h Vertical section of conidiomata. i–j, m–n Conidiophores, conidiogenous cells and developing conidia. k–l, o–p Conidia. Scale bars d = 1000 µm, e = 200 µm, f = 500 µm, g–h = 100 µm, i–p = 10 µm
Crucellisporiopsis gelatinosa (DAOM 215303) a, b Herbarium specimen. c–e Appearance of yellowish to orange yellow conidiomata on the host. f–g Vertical section of conidiomata. h–i Section of peridium. j–l Conidiophores, conidiogenous cells and developing conidia. mSterile hyphae. n–p Conidia. Scale bars c = 500 µm, d–e = 200 µm, f–g = 100 µm, h–i = 50 µm, j–p = 10 µm
Crucellisporiopsis prolongata (DAOM 187814, holotype) a, b Herbarium specimen. c, f–g Appearance of straw-coloured stalked pycnidia on the host. d Stalked pycnidium. e Conidiomatal head. h–i Vertical section of a conidiomatal head. j Stalk of conidioma. k–l Conidiophores, conidiogenous cells and developing conidia. m–n, p–q Conidia. o, r Excipulum of conidima. Scale bars c = 1000 µm, d = 100 µm, e–f = 200 µm, h–j = 100 µm, k–l = 20 µm, m, n, p,q = 10 µm, o = 20 µm, r = 10 µm
Cryptomycella pteridis (redrawn from Nag Raj and DiCosmo 1981a) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and conidia. c Conidia
Cyclodomus umbellulariae (redrawn from Nag Raj and DiCosmo 1978) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Condia
Cylindrogloeum trillii (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Cylindroxyphium virginianum (redrawn from Sutton 1980) a Side view of conidioma. b Condia. c Conidiophores, conidiogenous cells and developing conidia
Cystotricha striola (redrawn from Sutton 1980) a Condia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Cytogloeum tiliae (redrawn from Sutton 1980) a Condia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Cytonaema spinella (redrawn from Sutton 1980) a Condia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Cytoplacosphaeria rimosa (redrawn from Sutton 1980) a Conidia. c Conidiogenous cells and developing conidia. b Vertical section of conidioma
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS and rpb2 sequences data of Cytospora. One hundred and fifty-seven strains are included in the analyses, which comprise 3002 characters including gaps (LSU: 1–702, ITS: 703–1577, rpb2: 1578–3002). Diaporthe eres CBS 145040 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 18311.216780 is presented. The matrix has 801 distinct alignment patterns, with 42.53% of undetermined characters or gaps. Estimated base frequencies were: A = 0.248733, C = 0.255482, G = 0.273492, T = 0.222294; substitution rates AC = 1.819004, AG = 5.371596, AT = 1.903512, CG = 1.537203, CT = 13.426713, GT = 1.000000; gamma distribution shape parameter α = 0.576514. The Bayesian posterior probabilities analysis for final split frequency critical value for the topological convergence diagnostic is 0.009997. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MLBS and a posterior probability lower than 0.95 for BYY. Ex-type strains are in bold. New isolates are in blue
Cytospora parasitica(MFLU 14-0788, holotype) a Herbarium specimen. b Appearance of black conidiomata on the host. c Black conidioma with yellow conidia mass. d Vertical section of conidioma. e, g–i Sections of peridium (note e upper wall, h inner wall, g, i basal and lateral wall). f Ostiole. j–l Conidiophores, conidiogenous cells and developing conidia. m Conidia. Scale bars b–c, f = 200 µm, d = 500 µm, e, g = 100 µm, h = 50 µm, i = 20 µm, j–k, m = 10 µm, l = 5 µm
Cytospora prunicola (MFLU 16-0940) a Herbarium specimen. b–d Appearance of brown conidiomata on the host. e, f Vertical sections of conidiomata. g Ostiole. h–j Sections of peridium. k–m Conidiophores, conidiogenous cells and developing conidia. n Conidia. o Geminating conidium. p–q Culture on PDA. Scale bars b–c, f = 200 µm, d–e = 500 µm, g = 100 µm, h–i = 50 µm, j–k = 20 µm, l–o = 10 µm, p–q = 25 mm
Cytospora prunicola (HKAS 93600, new host record) a Herbarium specimen. b, c Appearance of brown conidiomata on the host. d Vertical section of conidiomata. e Ostiole. f–h Sections of peridium. i–j Conidiophores, conidiogenous cells and developing conidia. k Conidia. Scale bars d, h = 200 µm, e = 100 µm, f = 50 µm, g, i = 10 µm, j–k = 5 µm
Cytospora globosa (MFLU 16-2054, holotype). a Herbarium specimen. b–d Appearance of dark brown to black coniodiomata on the host. e, f Vertical sections of conidiomata. g Ostiole. h–j Sections of peridium. k–n Conidiophores, conidiogenous cells and developing conidia. o Conidia. p–q Germinating conidia. r–s Culture on PDA. Scale bars b = 1000 µm, c–d = 500 µm, e–f = 200 µm, g–j = 50 µm, k–q = 10 r–s = 20 mm
Cytospora phialidica(MFLU 16-2442, holotype). a Herbarium specimen. b, c Appearance of brown coniodiomata on the host. d, e Vertical sections of conidiomata. f–i Sections of peridium. j–l Conidiophores, conidiogenous cells and developing conidia. m–p Conidia. Scale bars b = 1000 µm, c–e = 200 µm, f, h = 50 µm, g = 100 µm, i = 20 µm. j–l = 10 µm, m–p = 5 µm
Cytospora tanatica(MFLU 14-0790, holotype) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d Vertical section of conidioma. e Ostiloe. f–h Vertical sections of peridium. i–k Conidiophores, conidiogenous cells and developing conidia. l Conidia. Scale bars b = 1000 µm, c–d = 500 µm, e = 200 µm, f = 100 µm, g–h = 50 µm, i–l = 10 µm
Cytosporasp. (MFLU 19-2857) a Herbarium specimen. b, c Appearance of brown coniodiomata on the host. d, e Vertical sections of conidiomata. f Ostiole. g, h Sections of peridium. i–k Conidiophores, conidiogenous cells and developing conidia. l Conidia. Scale bars c = 500 µm, d–e = 200 µm, f = 100 µm, g–h = 50 µm, i–l = 10 µm
Cytosporella sycina (redrawn from Sutton 1980) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidioma
Cytostagonospora photiniicola (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Darkera abietis (DAOM 145406 a and b, holotype) a, b Herbarium package and specimen. c, d Appearance of black ascomata on the host. e, h Vertical section of conidiomata. f Hamathecium with asci and paraphyses. g Section of peridium. i Paraphyses. j–n Asci. o–s Ascospores. Scale bars c = 500 µm, d, e, h = 200 µm, f = 50 µm, g, i, j–n = 20 µm, o–s = 10 µm
Darkera abietis (asexual morph, DAOM 145416, type) a, b Herbarium package and specimen. c, d Appearance of pale brown to brown conidiomata on the host. e Ostiole. f Vertical section of conidiomata. g–h, k–n Conidiogenous cells and developing conidia. i–j Section of peridium. o–s Conidia (arrows show mucoid appendages). Scale bars e = 50 µm, f = 100 µm, g, i–j = 20 µm, h, k–s = 10 µm
Darkera parca (DAOM 145413, holotype) a, b Herbarium package and specimen. c–d Appearance of brown to dark brown conidiomata on the host. e, f Vertical section of conidiomata. g, h Section of peridium. i–k Asci. l Paraphyses. m–n Asci apex with ascospores. o–q Ascospores. Scale bars c = 1000 µm, d = 500 µm, e–f = 100 µm, g = 50 µm, h = 20 µm, i–q = 10 µm
Darkera parca (asexual morph, DAOM 145413b, holotype) a–b Herbarium package and specimen. c–d Appearance of brown to dark brown conidiomata on the host. e–f, h Vertical section of conidiomata. g, j Section of peridium. i Ostiole. k–o Conidiophores, conidiogenous cells and developing conidia. p–s Conidia. Scale bars c = 500 µm, d = 200 µm, e–f = 100 µm, g, j = 20 µm, h–i = 50 µm, k–s = 10 µm
Darkerasp. (DAOM 145420) a, b Herbarium package and specimen. c, d Appearance of brown to dark brown conidiomata on the host. e–f Vertical section of conidiomata. g Section of peridium. h Ostiole. i–o Conidiogenous cells and developing conidia (arrows show apical mucoid appendages). p–s Conidia. Scale bars c = 500 µm, d = 200 µm, e–f = 100 µm, g–h = 50 µm, i–s = 10 µm
Darkera picea (HKAS 93593) a Material specimen. b Black coniodiomata on the host. c–e Vertical section of conidioma. f Section of peridium. g Ostiole. h–j Conidiogenous cells and developing conidia. k–o Conidia. Scale bars: a = 1000 µm, b = 200 µm, c = 100 µm, d–e, g = 50 µm, f = 20 µm, h–j = 10 um, k–o = 5 µm
Darkera pseudotsugae (DAOM 49190, holotype) a, b Herbarium package and specimen. c, d Appearance of brown to dark brown conidiomata on the host. e Vertical section of conidiomata. f, h Section of peridium. g Ostiole. i–m Conidiogenous cells and developing conidia. n–q Conidia. Scale bars c = 500 µm, d = 200 µm, e = 100 µm, f, h, k = 20 µm, g = 50 µm, i–j = 10 µm, l, n–q = 10 µm, m = 5 µm
Dasysticta sapindophila (redrawn from Sutton 1980) a, b, c Conidiogenous cells and condia. d Surface view of conidioma with setae
Davisiella elymina (redrawn from Sutton 1980) a Condia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidioma
Dearnessia apocyni (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Condia
Dendrodomus annulatus (redrawin from Sutton 1980) a Condia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Saprobic on sugary exudates from insects growing on the surface of living leaves. Thallus composed of brown to pale brown, sub-cylindrical, irregularly branched, septate, network-like hyphe. Sexual morph: see Chomnunti et al. (2011). Asexual morph:Conidiomata 1300–1500 μm high, 90–100 μm wide, brown to dark brown, pycnidial, solitary to gregarious, superficial, differentiated into a stalk and a conidiiferous locule; stalk 760–110 µm long, 40–70 µm wide, black to dark brown, subcylindrical, straight or variously curved, unbranched or branched (Fig. 89h, i), thick-walled; locule 120–260 μm high, 60–90 μm wide, swollen, globose (Fig. 89e. j), bearing pale brown, cylindrical, long rostrate apex. Rostrate apex apical, centrally located, surrounded by hyaline hyphae. Stalk wall composed of thick-walled, dark brown cells of textura porrecta. Pycnidial wall composed of thick-walled, brown to pale brown cells of textura angularis, becoming thin-walled, pale brown cells of texture prismatica towards apex. Conidiophores arising from the inner layers of pycnidial wall. Conidiogenous cells not observed. Conidia 6–11 × 4–5.5 μm (\( \bar{x} \) = 9 × 5 μm, n = 20), hyaline, ellipsoid to cylindrical, rounded at both ends, unicellular, smooth-walled, eguttulate.
Material examined: Thailand, Chiang Rai, Thasud Tha Sut, on living leaves of Ficus sp. (Moraceae), 3 December 2013, Qing Tian, TS02 (MFLU 19-2891), living culture MFLUCC 14-0179.
Corniculariella P. Karst., Hedwigia 23(4): 57 (1884)
= Cornularia Sacc., Syll. fung. 3: 598 (1884)
= Chondropodium Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 45 (1916)
Facesoffungi number: FOF00380
Leotiomycetes, Leotiomycetidae, Medeolariales, Dermateaceae
Saprobic on plant host. Sexual morph: undetermined. Asexual morph:Conidiomata yellowish or dark brown to black, pycnidial, solitary to gregarious or confluent, deeply immersed in origin, becoming partly erumpent at maturity, subglobose or subcylindrical to subconical, slightly swollen at the base or level of the locule, narrow towards the apex, unilocular, the locule in the upper part of the conidiomata, thick-walled, minutely scabrous, usually lacking an ostiole but dehiscing by an irregular split in the apical region, or presenting an ostiole. Ostiole single, centrally located, with a long neck filled with long, hyaline paraphysate hyphae. Conidiomata wall composed of closely interwoven septate hyphae, compacted towards exterior. Conidiophores hyaline, cylindrical, branched, developed from inner layer of the conidiomata. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, indeterminate, discrete or integrated, smooth, moderately thick-walled. Conidia hyaline, falcate or fusiform to clavate, tapering towards both ends, curved, septate, thick-walled, smooth, guttulate.
Type species: Corniculariella abietis P. Karst., Hedwigia 23(4): 57 (1884)
Notes: Corniculariella was introduced by Karsten 1840 based on C. abietis P. Karst. In the same year, Saccardo changed the name of Corniculariella to Cornularia, and described eight species in the genus. Subsequently, several new species were described (Karsten 1890). DiCosmo (1978) revised the genus and accepted seven species viz. C. abietes, C. harpographoidea Dearn. ex DiCosmo, C. hystricina (Ellis) DiCosmo, C. populi DiCosmo, C. pseudotsugae (W.L. White) DiCosmo, C. spina (Berk. & Ravenel) DiCosmo and C. urceola (Höhn.) DiCosmo. In addition, he provided an emended description and a key to the species. Based on the emended conception, Sutton (1991) added C. queenslandica B. Sutton to the genus. Oliveira et al. (2014), described C. brasiliensis I.B. Cunha, et al. based on LSU and ITS sequence data. However, phylogenetic re-assessment of Leotiomycetes using LSU sequence data show that our new species, C. rhamni, is close to Dermea cerasi (Pers.) Fr., (Medeolariales, Dermateaceae), but is distant from C. brasiliensis (Helotiales, Chaetomellaceae) (Fig. 129). Morphologically, C. brasiliensis is similar to the type species C. abietis only in the conidia shape (falcate), but differs in various other ways and are probably not congeneric. Molecular data for the type and other species is not available and therefore classification of Corniculariella cannot be confirmed in the Dermateaceae. Examination of fresh material from the type locality and pure cultures are essential to confirm taxonomy and phylogeny of Corniculariella.
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS and rpb2 sequences data of Dermateaceae. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from (Chen et al. 2016) and GenBank. Sixty-two strains are included in the analyses, which comprise 2465 characters including gaps. Infundichalara microchona CBS 175.74 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 15611.979066 is presented. The matrix had 938 distinct alignment patterns, with 16.00% of undetermined characters or gaps. Estimated base frequencies were: as follows A = 0.251579, C = 0.230991, G = 0.272796, T = 0.244634; substitution rates AC = 1.650679, AG = 3.597104, AT = 0.907930, CG = 0.800954, CT = 8.324139, GT = 1.000000; gamma distribution shape parameter α = 0.201567. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.06 changes. Ex-type or ex-epitype strains are in bold. New isolates are in blue
Distribution: Australia, Austria, Canada, Finland, Italy, UK, USA, (DiCosmo 1978; Sutton 1980; this study; https://www.gbif.org/).
Corniculariella rhamni W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557144, Facesoffungi number: FoF 07173, Fig. 90
Etymology: Named after the host genus from which it was collected, Rhamnus.
Saprobic on dead stems of Rhamnus alpinus (Rhamnaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 300–550 µm diam., 200–1200 µm high, yellowish (Fig. 90b, c) or dark brown to black, pycnidial, solitary to gregarious or confluent, deeply immersed in origin, becoming partly erumpent at maturity, subglobose or subcylindrical to subconical, slightly swollen at the base or level of the locule, narrow towards the apex, unilocular, locule occasionally extending the length of the beak and somewhat enlarged in the basal part, glabrous, beaked, thick-walled, minutely scabrous, lacking an ostiole but dehiscing by an irregular split in the apical wall (Fig. 90a, e), or presenting ostiole (Fig. 90f, i). Ostiole 200–400 × 55–250 µm, cylindrical to subcylindrical, single, centrally located, with a long neck filled with hyaline hyphae. Paraphyses 200–370 × 2–5 µm, hyaline, filiform, septate, unbranched, developed from the upper region of conidiomata. Conidiomata wall 50–200 µm wide, composed of relatively thick-walled, brown to hyaline cells of textura angularis to textura intricata in the basal part, and a thick-walled, pale brown gradually merging with hyaline cells of textura porrecta in the upper part, and hyaline hyphae forming a textura porrecta to textura angularis in the upper part. Conidiophores 10–30 × 2–5 µm, hyaline, cylindrical, branched, formed from inner layers of the conidiomata. Conidiogenous cells 10–40 × 2–4.5 µm, hyaline, enteroblastic, phialidic, cylindrical to lageniform, indeterminate, discrete or integrate, smooth, thick-walled. Conidia 40–65 × 2.6–5 µm (\( \bar{x} \) = 52 × 4 µm; n = 100), hyaline, fusiform to clavate, tapering toward the base, with a pedicel at base, obtuse at apex, curved, 0–3-septate, mostly1-septate, thick-walled, smooth, guttulate.
Culture characters: Colonies on PDA, yellowish, flattened, with filamentous, sparse, aerial hyphae on the surface, margins lobate, reaching 30 mm diam. after 4 weeks at 25–30 °C, reverse yellowish to brown.
Material examined: Italy, Province of Forlì-Cesena, Monte Fumaiolo, on dead aerial branches of Rhamnus alpinus (Rhamnaceae), 11 May 2016, Erio Camporesi, IT2958 (MFLU 16-1329, holotype), ex-type living culture MFLUCC 16-1446 = ICMP 21545; IT2958F (KUN, HKAS 97498), living culture MFLUCC 16-1445; IT2958b (KUN, HKAS 101649), living culture MFLUCC 17-2501.
Notes: Three samples of Corniculariella rhamni (IT2958, IT2958b and IT2958F) were collected from Italy on dead stems of Rhamnus alpinus, of which IT2958 has black, subcylindrcial to subconical conidiomata without an ostiole (Fig. 90a, e), while IT2958F and IT2958b have yellowish conidiomata with long necked ostioles (Fig. 90b–d). Phylogenetic analyses based on multi-gene of LSU, ITS and rpb2 sequence data show that these three collections are conspecific with high bootstrap value (Fig. 90). The discrepancy shown here may reflect intraspecific variation. Moreover, according to the multi-gene dataset, the fixed differences in nucleotides among these collections are relatively small and can be found only in rpb2 sequence region (4/1079 bp). Morphologically, C. rhamni fits well within the generic concept of Curniculariella, comparing with other genera of coelomycetes (DiCosmo 1978; Sutton 1980). Corniculariella rhamni shares similarity with C. abietis in the form of conidiomata, conidiogenous cells and conidia (DiCosmo 1978), but can easily be distinguished by septation of conidia. Corniculariella rhamni produces 0–3-septate conidia, while C. abietis has 7–8 septate conidia. Corniculariella spina has 0–3-septate conidia (DiCosmo 1978; Illman and White 1985), but, C. rhamni has yellowish to black conidiomata and smaller conidiomata than the black conidiomata, 500–1500 µm high in C. spina. Furthermore, C. rhamni has fusiform to clavate conidia with a pedicel, whereas, C. spina has falcate, crescentic to sigmoid conidia. Based on its distinct morphology, C. rhamni is introduced as a new species in Corniculariella.
Cornucopiella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 118 (1915)
Facesoffungi number: FoF 07174, Fig. 91
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat, such as Alnus incana (Betulaceae), Fagus sp. (Fagaceae). Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, solitary to gregarious, superficial, cornute, cylindrical, sessile, unilocular, locule extending deeply, glabrous. Ostiole absent. Conidiomatal wall composed of thick-walled, dark brown to pale brown cells of textura porrecta at the base and lateral part. Conidiophores arising from inner layers of basal and lateral wall, hyaline, cylindrical, repeatedly branched at the base and above, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated, determinate, smooth-walled, collarettes and apertures minute. Conidia hyaline, ellipsoid, rounded at both ends, unicellular, smooth-walled, guttulate (Sutton 1980).
Type species: Cornucopiella mirabilis Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 118 (1915)
Notes: Cornucopiella shares a similar form of conidiomata with Nanoschema. The differences between these two genera and aligned genera are discussed under Nanoschema. Two taxa are accepted in the genus, Cornucopiella fusispora (P. Karst.) Seifert and C. mirabilis, they can be distinguished by dimensions of conidia (Seifert 1985b). No sexual morph has been linked to this genus, nor is molecular data available.
Distribution: Argentina, Austria, Australia, Canada, Chile, Finland, South Africa, Sweden, UK (Sutton 1980; Seifert 1985b; https://www.gbif.org/).
Cornutispora Piroz., Mycologia 65(4): 763 (1973)
Facesoffungi number: FoF 07175
Ascomycota, genera incertae sedis
Fungicolous on apothecia of discomycetes. Sexual morph: undetermined. Asexual morph: Conidiomata black, stromatic, pycnidial, solitary to gregarious or often confluent, deeply immersed, then becoming partly erumpent through the outer layers of the excipulum, unilocular or multi-locular, ovoid to subglobose, glabrous, ostiolate. Ostiole single, cylindrical to irregular, centrally located. Conidiomatal wall composed of thick-walled, dark brown to black or hyaline cells of textura angularis to textura intricata. Conidiophores arising from the innermost wall layer of conidiomata, hyaline, branched, septate, smooth-walled. Conidiogenous cells hyaline, cylindrical, discrete or integrated, indeterminate. Conidia hyaline, conidium body subcylindrical to naviculate or narrow cuneiform, with a small attachment scar, straight or slightly curved, aseptate, bearing appendages at both ends and middle of the conidial body; apical appendages 1, 2 or 3, mostly divergent, sometimes single, unbranched, basal appendage single, mostly unbranched, occasionally divergent at middle part, eccentric.
Types species: Cornutispora limaciformis Piroz., Mycologia 65(4): 763 (1973)
Notes: The genus was introduced by Pirozynski (1973) based on C. limaciformis Piroz., which parasitized apothecia of Therrya fuckelii (Helotiales) on branches of Pinus resinosa (Pinaceae). Cornutispora is characterized by its fungicolous habitat and aseptate, staurospores or y-shaped conidia. Hawksworth (1976) added species, C. lichenicola from a thallus of Parmelia sulcata, which is distinguished by the much smaller conidia. Subsequently, Gierl and Kalb (1993) described C. ciliata Kalb, from apothecia of Dibaeis cretacea in Tasmania. Punithalingam (2003) re-examined the type materials of C. ciliata, C. lichenicola and C. limaciformis, and concluded that the size and shape of the conidia and the two apical bifurcations or segments can be used as criteria to distinquishish Cornutispora species. Cornutispora intermedia and C. pittii were introduced by Punithalingam (2003). Index Fungorum (2019) lists nine species, while Wijayawardene et al. (2017b) estimated there are five species.
We examined the holotype and isotype of Cornutispora limaciformis, of which the latter is not in good condition, and provide a detailed description and photoplates of the genus. Besides, we found that apical appendages or divergent arms vary, and cannot be used to distinguish species. Since, molecular data is lacking for the genus, the natural placement of Cornutispora is unclear.
Distribution: Australia, Brazil, Canada, Denmark, Estonia, France, Germany, Italy, Poland, Spain, Sweden, UK, USA (Nag Raj 1993; Punithalingam 2003; https://www.gbif.org/).
Cornutispora limaciformis Piroz., Mycologia 65(4): 763 (1973)
Facesoffungi number: FoF 07176, Fig. 92
Fungicolous on apothecia of discomycetes. Sexual morph: undetermined. Asexual morph:Conidiomata black, stromatic, pycnidial, solitary to gregarious or often confluent, deeply immersed, then becoming partly erumpent through the outer layers of the excipulum, mostly multi-locular, occasionally unilocular, glabrous, with locule 100–200 µm diam., 100–200 µm high, ovoid to subglobose, thick-walled, ostiolate. Ostiole single, cylindrical to irregular, centrally located. Conidiomatal wall 15–100 µm wide, composed of thick-walled, dark brown to black cells of textura angularis at upper zone, and relatively thick-walled, brown to hyaline cells of textura intricata gradually merging with textura angularis at lateral and basal zone. Conidiophores arising from the innermost layer cells of conidiomata, hyaline, branched, septate, smooth-walled. Conidiogenous cells 6–15 × 1–3 µm, hyaline, cylindrical, discrete or integrated, indeterminate. Conidium body 10–27 × 3–5 µm (\( \bar{x} \) = 16 × 4 µm; n = 30), hyaline, subcylindrical to naviculate or narrow cuneiform with a small attachment scar, straight or slightly curved, aseptate, bearing appendages at both ends and middle of the conidial body; apical appendages 5–11 × 0.7–2, 1 or 2, mostly divergent, sometimes single, unbranched, basal appendage 4–11 × 0.7–1.6, single, mostly unbranched, occasionally divergent at middle part, eccentric.
Material examined: Canada, Ontario, Lake Simcoe District, Midhurst Nursery, on apothecia of Therrya fuckelii (Rhytismataceae), on branches of Pinus resinosa (Pinaceae), 27 May 1971, R.L. Bowser & P.E. Buchan (DAOM 138360, holotype); (IMI 164414a, isotype).
Crandallia Ellis & Sacc., Bull. Torrey bot. Club 24(10): 466 (1897)
Facesoffungi number: FoF 07177, Fig. 93
Ascomycota, genera incertae sedis
Saprobic or parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, solitary, scutate, immersed, circular to subcircular, multilocular, glabrous, ostiolate. Ostiole circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis to textura intricata. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, cylindrical, determinate, smooth-walled. Conidia hyaline, cylindrical with obtuse ends, unicellular, smooth-walled (Powell 1973; Sutton 1980).
Type species: Crandallia juncicola Ellis & Sacc., Bull. Torrey bot. Club 24(10): 466 (1897)
Notes: Crandallia juncicola (type species of Crandallia) was considered as the asexual morph of Duplicaria acuminata Ellis & Everh. (Powell 1973). Duplicaria empetri (Pers.) Fuckel (type species of Duplicaria Fuckel) was considered as the sexual morph of Melasmia empetri Magnus (Lind 1913; Nannfeldt 1932; Darker 1967; Dennis 1978). Based on the differences in host family and asexual morphs, Rossman et al. (2016b) excluded D. acuminata from Duplicaria, and made two new combinations, C. acuminata and C. antarctica. However, Powell (1973) clearly interpreted that M. empetri is not the asexual morph of D. empetri. Therefore, the two combinations introduced by Rossman et al. (2016b) should be regarded as valid names, and D. acuminata should be reserved in Duplicaria until further studies are made. At present, three species are accepted in Crandallia namely, C. anthurii Bat., C. juncicola and C. proteae Marinc., M.J. Wingf. & Crous, but none have been studied with molecular data. It is necessary to obtain fresh material and make cultural connections in other species
Distribution: South Africa, USA (Sutton 1980; Marincowitz et al. 2008).
Crinitospora B. Sutton & Alcorn, Trans. Br. Mycol. Soc. 84(3): 437 (1985)
Facesoffungi number: FoF 04172
Sordariomycetes, Sordariomycetidae, Diaporthales, Stilbosporaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown to black, stromatic, acervular, solitary to gregarious or confluent, immersed to semi-immersed, subglobose, unilocular, thick-walled. Conidiomatal wall composed of thick-walled, hyaline to brown cells of textura angularis to textura globulosa. Conidiophores usually reduced to conidiogenous cells or if present, hyaline, cylindrical, short, unbranched, septate, and arising from the innermost layer cells of conidiomata. Conidiogenous cells hyaline, annellidic, lageniform to cylindrical, smooth-walled. Conidia hyaline, ellipsoid with a rounded apex and a broadly truncate base, 1-septate, slightly constricted at septum, bearing several appendages, unbranched, filiform, divergent, flexuous, and arising in apical crest.
Type species: Crinitospora pulchra B. Sutton & Alcorn, Trans. Br. Mycol. Soc. 84(3): 439 (1985)
Notes: Crinitospora was introduced by Sutton and Alcorn (1985) to accommodate C. pulchra which was initially described from twigs of Mangifera indica in Australia. The genus is characterized by acervular, subglobose conidiomata, annellidic conidiogenous cells and ellipsoid, 1-septate conidia bearing mostly 5–6 apical appendages. Morphologically, C. pulchra is similar to Comatospora suttonii (type species of Comatospora) in form of conidia and conidiogenous cells, but is distinguished by conidiomata structure (acervular in C. pulchra vs pycnidial in C. suttonii). Crous et al. (2014a) epitypified C. pulchra based on an isolate collected from twigs of M. indica in Thailand and accommodated Crinitospora in Melanconidaceae (Diaporthales). Our phylogenetic analysis based on LSU data shows that Crinitospora clustered with Stilbospora with well support (Fig. 7); this result agreed with Senanayake et al. (2017). Therefore, Crinitospora belongs to Stilbosporaceae. The genus is monotypic.
Distribution: Australia, Thailand (Sutton and Alcorn 1985; Crous et al. 2014a).
Crinitospora pulchra B. Sutton & Alcorn, Trans. Br. Mycol. Soc. 84(3): 439 (1985)
Faceoffungi number: FoF 03475, Figs. 94, 95
Saprobic on dead twigs of Mangifera indica . Sexual morph: undetermined. Asexual morph:Conidiomata 200–300 µm diam., 100–150 µm high, brown to black, stromatic, acervular, solitary (Fig. 94d, e), solitary to gregarious and confluent on PDA medium (Fig. 94c, f), semi-immersed initially, dehiscing by an irregular rupture of the overlying tissue, subglobose, unilocular, glabrous, thick-walled. Conidiomatal wall 35–60 µm wide, composed of thick-walled, hyaline to brown cells of textura angularis gradually merging with textura globulosa. Conidiophores arising from the innermost layer cells of conidiomata, reduced to conidiogenous cells or when present, hyaline, cylindrical, short, unbranched, septate. Conidiogenous cells 20–30 × 4–8 µm, hyaline, annellidic, lageniform to cylindrical, smooth-walled. Conidia 24.5–35 × 9–15 µm (\( \bar{x} \) = 29 × 12.7 µm; n = 30), hyaline, ellipsoid with a rounded apex and a broadly truncate base, 1-euseptate, slightly constricted at septum, bearing appendages, with appendages 18–40 µm long, mostly 5–6, occasionally 10, tubular, unbranched, filiform, divergent, flexuous, arising from an apical crest.
Material examined: Australia, Queensland, Bowen, on twigs of Mangifera indica (Anacardiaceae), I.F. Muirhead, 18 December 1980 (IMI 259110, holotype); (CBS 138014, culture ex-epitype).
Crucellisporiopsis Nag Raj, Can. J. Bot. 60(12): 2601 (1983)
Facesoffungi number: FoF 07178
Leotiomycetes, Leotiomycetidae, Helotiales, Lachnaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata stromatic, variable from acervular to sprodochia or synnematous, solitary to gregarious, superficial, erumpent, gelatinous. Conidiomatal wall well developed, composed of a basal wall of textura angularis with relatively thick-walled, hyaline cells, and an excipulum of textura prismatica to textura intricata with thick-walled, hyaline cells. Conidiophores arising in the cavity of the conidiomata, hyaline, septate, branched, smooth-walled, branches terminating in conidiogenous cells and sterile hyphae. Sterile hyphae hyaline, filamentous, unbranched in the base, blunt, irregularly coiled in the apical part, septate. Conidiogenous cells hyaline, cylindrical to subcylindrical, discrete or integrated, indetermined, smooth-walled. Conidia hyaline, four or five radiate, smooth; main axis cylindrical to subcylindrical, 0–1-septate, narrowed and truncate at base, straight or slightly curved, cells unequal, smooth-walled, guttulate, bearing a tubular, unbranched, attenuated appendage; arms three or four, inserted at different loci at the apex of the main axis and separated from it by septa, divergent, attenuated, euseptate, unequal.
Type species: Crucellisporiopsis gelatinosa Nag Raj, Can. J. Bot. 60(12): 2605 (1983) [1982]
Notes: Nag Raj (1982) accepted two taxa, C. cymbionoides Nag Raj collected from unidentified twigs of a tree in Venezuela, and C. gelatinosa Nag Raj collected from decaying leaves of Weinmannia racemosa in New Zealand. Brubacher et al. (198 4) described third species C. prolongata Brub., Rawla & R. Sharma. The fourth species C. marquesiae Crous was added by Crous et al. (2014c) mainly based on the dimensions of its central axis, and lateral radiating arms.
Crucellisporiopsis can be confused with Crucellisporium M.L. Farr and Eriosporella Höhn., because all possess four or five radiate conidia with a 0–1-septate main axis bearing 2–5 divergent arms at the point of succession (Nag Raj 1993). The significant difference between Crucellisporiopsis and Eriosporella is that Eriosporella lacks sterile hyphae at the conidiomatal margin. Crucellisporiopsis has annellidic conidogenous cells while in Crucellisporium they are holoblastic-sympodial (Nag Raj 1982, 1993; Diederich et al. 2001). Crous et al. (2014c) showed that Crucellisporiopsis was related to Hyaloscyphaceae (Helotiales) based on LSU and ITS sequence, but the type species was not included. Ekanayaka et al. (2019) placed Crucellisporiopsis in Lachnaceae. In our study, the type specimen of C. prolongata and one deposited as C. gelatinosa in DAOM were re-examined. Of interest is that C. prolongata has synnematous conidiomata, while C. gelatinosa has acervular conidiomata. Because of the lack of molecular sequence, C. prolongata was maintained as a separate species in Crucellisporiopsis. Once epitypes of these two species are designated, it will be possible to confirm if they are congeneric.
Distribution: India, New Zealand, Zambia (Nag Raj 1993; Crous et al. 2014c; this study)
Crucellisporiopsis gelatinosa Nag Raj, Can. J. Bot. 60(12): 2605 (1983) [1982]
Facesoffungi number: FoF 07179, Fig. 96
Saprobic on decaying leaves of Weinmannia racemosa (Cunoniaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 157–235 µm diam., 143–217 µm high, stromatic, sporodochial, straw-coloured at beginning, becoming yellowish to orange-yellow at maturity, gelatinous crusts, amphigenous, solitary to gregarious, variable from acervular and irregularly divided to shallow-cupulate, initially intraepidermal, then becoming erumpent through the overlying host tissue, thick-walled. Conidiomatal wall 20–60 µm wide, composed of relatively thick-walled, hyaline cells of textura angularis mixed with textura intricata at base, and merging into a moderately developed excipulum of textura prismatica to textura intricata of thick-walled, hyaline cells in the innermost zone. Conidiophores arising in the cavity of the conidiomata, hyaline, septate, branched, smooth-walled, branches terminating in conidiogenous cells and sterile hyphae. Sterile hyphae hyaline, filamentous, unbranched in the base, blunt, irregularly coiled in the apical part, septate (Nag Raj 1993). Conidiogenous cells hyaline, cylindrical to subcylindrical, discrete or integrated, indeterminate, smooth-walled. Conidia hyaline, mostly tetra-radiate, occasionally quinque-radiate; main axis 12–38 × 1–3 µm (\( \bar{x} \) = 24 × 2 µm; n = 30), cylindrical to subcylindrical, aseptate or 1-septate, narrowed and truncate at base, straight or slightly curved, cells unequal, smooth-walled, guttulate, bearing a tubular, unbranched, attenuated appendage; arms 31–68 × 1–2.5 µm (\( \bar{x} \) = 52 × 1.8 µm; n = 30), mostly three, sometimes four, inserted at different loci at the apex of the main axis and separated from it by septa, attenuated, 2–5-septate.
Material examined: New Zealand, South Island, Haast Pass, Westland, on decaying leaves of Weinmannia racemosa (Cunoniaceae), 31 March 1980, B. Kendrick (DAOM 215303).
Crucellisporiopsis prolongata Brub., Rawla & R. Sharma, Mycotaxon 21: 449 (1984)
Facesoffungi number: FoF 07180, Fig. 97
Saprobic on decomposing leaf of an unidentified angiosperm. Sexual morph: undetermined. Asexual morph:Conidiomata straw-coloured, stromatic, cornuted to spuriously synnematoid, solitary to gregarious, erect or variously curved, slightly roughened, differentiated into a stalk and a conidiiferous head; stalk 1.5–2 mm long, 100–300 µm wide, subcylindric, slightly swollen at the base, becoming narrowed above, head 200–300 µm diam., 150–200 µm high, ellipsoidal to subcylindrical, often with a pointed tip, with the concave conidial intricate; excipulum of hyaline, textura porrecta; hymenium of textura prismatica to textura angularis. Conidiophores hyaline, septate, branched, smooth-walled, with up to 3 terminal conidiogenous cells or sterile hyphae, arising in cavity of conidiomata. Sterile hyphae hyaline, clavate to subcylindrical, with an obtuse apex, straight or curved, septate, smooth-walled. Conidiogenous cells 10–25 × 2–3 µm, hyaline, cylindrical to subcylindrical, discrete or integrated, indeterminate, smooth-walled. Conidia hyaline, mostly tetra-radiate, rarely quinque-radiate, guttulate; main axis 11–30 × 2–3 µm (\( \bar{x} \) = 18 × 2.3 µm; n = 30), clavate to obconic with a narrow truncate base, aseptate or 1-septate, cells unequal, straight or curved, bearing a tubular, unbranched, attenuated appendage; arms 47–74 × 1–3 µm (\( \bar{x} \) = 61 × 1.8 µm; n = 30), three, rarely four, delimited from the main axis by septa, straight or curved, each arm 3–5-septate, without constrictions at the septa, arms unequal.
Material examined: India, Himachal Pradesh, Dharampur, Solan, along banks of a stream in predominantly pine forest, on decomposing leaf of an unidentified angiosperm, August 1978, G.S. Rawla (DAOM 187814, holotype).
Crucellisporium M.L. Farr, in Farr & Horner, Nova Hedwigia 15: 264 (1968)
Facesoffungi number: FoF 07181
Leotiomycetes, Leotiomycetidae, Helotiales, Lachnaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata stromatic, acervular, solitary to gregarious, immersed at first, eventually erumpent, rounded in outline. Conidiomatal wall composed of textura globulosa to textura prismatica with thick-walled, pale brown to hyaline cells at basal part, surrounding by a sheath of sterile hyphae. Sterile hyphae pale brown to hyaline, cylindrical, unbranched, septate, smooth-walled. Conidiophores arising from the upper layers of basal wall, hyaline, short, branched or unbranched, septate, smooth-walled. Conidiogenous cells hyaline, subcylindrical to clavate, discrete or integrated, proliferating sympodially to a limited extent. Conidia hyaline, tetra-radiate, occasionally tri-radiate; main axis subcylindrical to clavate, aseptate or 1-euseptate, smooth-walled, bearing a short, excentric, attenuated appendage; arms two or four, subcylindrical, attenuated, aseptate or 1-septate, arising at the apex of the main axis from different loci and delimited from it by septa.
Type species: Crucellisporium selaginellae M.L. Farr, in Farr & Horner, Nova Hedwigia 15: 264 (1968)
Notes: Crucellisporium selaginellae was collected from leaves of Selaginella rupestris (Selaginellaceae) in the USA (Farr and Horner 1968). Nag Raj (1974) revised the genus and provided a description and illustration for the type species. Sutton (1980) regarded Crucellisporium as a synonymy of Belaina Bat. & Peres. However, Belaina was reduced to synonym of Polynema Lév. based on type studies (Nag Raj and Kendrick 1978). They described an additional species C. africanum Nag Raj from the leaves of Dalbergia lactea (Fabaceae) in Tanzania. Marincowitz et al. (2010) introduced the third species C. umtamvunae Marinc., Gryzenh. & M.J. Wingf. and showed that Crucellisporium belongs to Helotiales (Leotiomycetes). Ekanayaka et al. (2019) listed Crucellisporium as a member of Lachnaceae (Helotiales). The Sequence of C. selaginellae is not available. Hence, new collections from type material location are needed and subjected to DNA analysis to confirm its placement.
Distribution: South Africa, Tanzania, USA (Farr and Horner 1968; Nag Raj 1993).
Crucellisporium africanum Nag Raj, Can. J. Bot. 56(6): 713 (1978)
Facesoffungi number: FoF 07182, Fig. 98
Foliicolous.Sexual morph: undetermined. Asexual morph:Conidiomata 100–150 µm diam., 80–100 µm high, olive-brown, stromatic, acervular, inconspicuous, mostly epiphyllous, solitary to gregarious, immersed at first, becoming erumpent at maturity, rounded in outline. Conidiomatal wall 10–30 µm wide, well developed, partly immersed, composed of textura globulosa with cells thick-walled, red-brown in the lower layers, and gradually merging with small, almost hyaline, thick-walled cells of textura prismatica in the upper layers, and surrounded by a sheath of sterile hyphae. Sterile hyphae pale brown in the basal part, becoming hyaline above, cylindrical, with a blunt or rounded apex, unbranched, 3–5-septate, smooth-walled. Conidiophores arising from the upper layers of basal wall, hyaline, short, branched or unbranched, septate, smooth-walled (Nag Raj 1993). Conidiogenous cells hyaline, subcylindrical to clavate, discrete or integrated, indeterminate, smooth-walled. Conidia hyaline, mostly tetra-radiate, occasionally tri-radiate; main axis 9–26 × 1–3 µm (\( \bar{x} \) = 18 × 2 µm; n = 20), subcylindrical to clavate, with a narrow and truncate base, straight or slightly curved, 1-septate, cells unequal, smooth-walled, bearing a tubular, unbranched, excentric, attenuated appendage; arms 12–34 × 1–2.4 µm (\( \bar{x} \) = 28 × 1.6 µm; n = 30), two or three, subcylindrical, attenuated, 1-septate, not constricted at septa, arising at apex of the main axis from different loci and delimited from it by septa.
Material examined: Tanzania, Tanganyika, Kigoma, Mkenke, on leaves of Dalbergia lactea (Fabaceae), 26 January 1964, K. Pirozynski (IMI 106429(d), type).
Cryptomycella Höhn., Mitt. bot. Inst. tech. Hochsch. Wien 2(3): 48 (1925)
Facesoffungi number: FoF 07183, Fig. 99
Sordariomycetes, genera incertae sedis
Saprobic or parasitic on the host plant in terrestrial habitatat. Sexual morph: see Sutton (1980). Asexual morph:Conidiomata dark brown to black, acervular, solitary to gregarious or confluent, semi-immersed, flattened, circular to irregular, unilocular, glabrous. Ostiole absent, opening by longitudinal fissure in apex. Conidiomatal wall composed of thick-walled, dark brown to brown cells of textura prismatica to textura globosa. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, annellidic, cylindrical to lageniform, integrated or discrete, indeterminate, smooth-walled. Conidia hyaline, oblong to fusiform, with a narrow truncate base, unicellular, smooth-walled, guttulate (Morgan-Jones 1977).
Type species: Cryptomycella pteridis (Kalchbr.) Höhn., Mitt. bot. Inst. tech. Hochsch. Wien 2(3): 48 (1925)
Notes: Conidiogenous cells of C. pteridis were described as phailidic by Sutton (1980), while Nag Raj and DiCosmo (1981) described them as annellidic. Because type material is not available, we cannot verify which is correct. The illustration and description are mainly based on Nag Raj and DiCosmo (1981a, b). Cryptomycella pteridis was linked to sexual morph Cryptomycina pteridis (Rebent.) Höhn., a fungus causing a leaf roll disease on bracken fern, Pteridium aquilinum (Dennstaedtiaceae) (Weymeyer 1966; Sutton 1980; Gabel 1993). Hyde et al. (2020) placed Cryptomycella in Sordariomycetes, genera incertae sedis. Three taxa are included in this genus, C. maxima Höhn., C. plumeriae Bat. & Peres and C. pteridis, but none of them has molecular data.
Distribution: Austria, Brazil, Canada, Estonia, Finland, Germany, Latvia, New Zealand, Roumania, South Africa (Sutton 1980; https://www.gbif.org/).
Cyclodomus Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 1527 (1909)
Facesoffunginumber: FoF 07184, Fig. 100
Sordariomycetes, Sordariomycetidae, Phyllachorales, Phaeochorellaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to brownish black, stromatic, pycnidial, scattered to gregarious, immersed, globose, multilocular, glabrous. Ostiole absent, dehiscence by irregular splits of apical wall. Conidiomatal wall composed of thick-walled, brown, vertically arranged cells of textura angularis in the middle part, becoming darker, thick-walled cells of textura angularis to textura globosa at the base and lateral part. Conidiophores reduced to conidiogenous cells or when present, hyaline, cylindrical, unbranched, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to ampuliform, integrated or discrete, determinate, smooth-walled. Conidia hyaline, subcylindrical, obtuse at the apex, narrowed and slightly truncate at the base, smooth-walled, guttulate (Nag Raj and DiCosmo 1978; Sutton 1980).
Type species: Cyclodomus umbellulariae Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 1528 (1909)
Notes: Sutton (1980) redescribed Cyclodomus and accepted the single species C. umbellulariae on dead leaves of Umbellularia californica (Lauraceae). Hyde et al. (1996) added second species C. aequatoriensis K.D. Hyde and linked it to the sexual morph Maculatifrondes aequatoriensis K.D. Hyde based on host association. Although C. aequatoriensis shares a similar structure of conidiomatal wall in the middle part with the type species, it also differs in many ways. Cyclodomus umbellulariae has multilocular conidiomata producing subcylindrical conidia (15–17 × 2–2.5 µm), whereas, C. aequatoriensis has unilocular conidiomata producing ellipsoidal to ovoid conidia (2–3 × 2–2.5 µm). Based on morphology, C. aequatoriensis is excluded from Cyclodomus. Cyclodomus is considered a member of Phyllachoraceae (Hyde et al. 2020). Fresh collections and DNA sequences of Cyclodomus are needed to confirm its placement.
Distribution: Pakistan, USA (https://www.gbif.org/).
Cylindrogloeum Petr., Ann Mycol. 39(4/6): 276 (1941)
Facesoffungi number: FoF 07398, Fig. 101
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown, acervular, scattered or confluent, immersed to erumpent, depressed-globose, unilocular, glabrous. Ostiole absent, opening by irregular splits of apical host tissue. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis. Conidiophores arising from inner layer of the basal part of condioma, hyaline, irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or determinate, smooth-walled. Conidia hyaline, cylindrical to fusiform with acute apex and slightly truncate base, straight or slightly curved, 0–1-septate, smooth-walled, eguttulate (Sutton 1980).
Type species: Cylindrogloeum arcticum Petr., Ann Mycol. 39(4/6): 276 (1941)
Notes: This description is based on Cylindrogloeum trillii (Ell. & Ev.) Arx. Sutton (1980) gave details of the taxonomy of this genus. No sexual morph has been linked to this genus and no molecular data is available.
Distribution: Norway, Sweden, USA (https://www.gbif.org/).
Cylindroxyphium Bat. & Cif., Quad. Lab. crittogam., Pavia 31: 77 (1963)
Facesoffungi number: FoF 07217, Fig. 102
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown, pycnidial, solitary, superficial, cylindrical, unilocular, setose. Ostiole single, circular, centrally located. Conidiomatal setae brown, subulate, unbranched, septate, smooth-walled, restricted to upper half. Conidiomatal wall composed of thick-walled, brown cells of textura angularis. Conidiophores arising from the inner layer of pycnidial wall, reduced to conidiogenous cells or when present, hyaline, cylindrical to dolliform, unbranched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled, apertures terminal or lateral immediately below transverse septa. Conidia hyaline, cylindrical to ellipsoid, unicellular, smooth-walled, eguttulate (Sutton 1980).
Type species: Cylindroxyphium virginianum Bat. & Cif., Quad. Lab. crittogam., Pavia 31: 77 (1963)
Notes: Cylindroxyphium shares similar conidiomata morphology with Capnodium, but the latter has a rostrate apex and lacks conidiomatal setae. Cylindroxyphium is a monotypic genus. Cylindroxyphium virginianum was collected on leaves of Quercus virginiana (Fagaceae) in USA (Sutton 1980). There is no molecular data available for Cylindroxyphium. To clarify the taxonomy of Cylindroxyphium, the type species will have to be recollected, and epitypified.
Distribution: USA (Sutton 1980).
Cystotricha Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 5: 457 (1850)
Facesoffunginumber: FoF 07218, Fig. 103
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary, semi-immersed, oval to elongated, unilocular, glabrous. Ostiole absent, dehiscence by a longitudinal fissure of apical wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura intricata to textura angularis. Conidiophores arising from the inner walls of the conidiomata, pale brown, branched, septate, rough-walled. Conidiogenous cells pale brown, holoblastic, doliiform or irregular, integrated, determinate, lateral or intercalary, rough-walled, with a minute apical channel and collarette. Conidia hyaline, cylindrical to ellipsoid, straight or slightly curved, 1-septate, constricted at septum, smooth-walled, eguttulate (Petch 1943; Sutton 1980).
Type species: Cystotricha striola Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 5: 457 (1850)
Notes: Cystotricha can be confused with Ceuthodiplospora, as they have hyaline, cylindrical to ellipsoid, septate conidia. Cystotricha differs from Ceuthodiplospora by its, pale brown, branched, septate, rough-walled conidiophores, and a feature absent in the latter. Sutton (1980) gave a full account of the taxonomy of Cystotricha, and accepted only the type species in the genus. The sexual morph of C. striola was considered to be Durella compressa (Pers.) Tul. & C. Tul. (Sutton 1980; Petch 1981), but this has not be confirmed by molecular data. Wijayawardene et al. (2017a) placed it in Ascomycota, genera incertae sedis. Fresh collections are required and DNA sequencing to place Cystotricha in a natural classification.
Distribution: Belgium, Estonia, Sweden, UK, (Sutton 1980, https://www.gbif.org/).
Cytogloeum Petr., Ann Mycol. 23(1/2): 77 (1925)
Facesoffungi number: FoF 07219, Fig. 104
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, acervular, solitary, immersed, slightly pulvinate in the centre, elongated, unilocular, glabrous. Ostiole absent, dehiscence by irregular fissure of the bark. Conidiomatal wall composed of thick-walled, brown cells of textura intricata. Conidiophores arising from the inner layer of basal wall of the conidiomata, hyaline, branched, septate, smooth-walled, with conidia formed at the apices of main and lateral branches. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, determinate, smooth-walled, with a minute apical channel and collarette. Conidia hyaline, cylindrical with an obtuse apex and acute base, straight or slightly curved, unicellular, smooth-walled, eguttulate (Sutton 1980).
Type species: Cytogloeum tiliae Petr., Ann Mycol. 23(1/2): 77 (1925)
Notes: Cytogloeum tiliae was collected from twigs of Tilia sp. (Malvaceae) in Germany. The genus remains monotypic, and no molecular data is available. Fresh collections of type species are needed to fix the generic concept of Cytogloeum.
Distribution: Czechia, Germany, Sweden (Sutton 1980, https://www.gbif.org/).
Cytonaema Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 131 (1914)
Facesoffungi number: FoF 07220, Fig. 105
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat, such as Salix alba, Salix sp. (Salicaceae). Sexual morph: undetermined. Asexual morph:Conidiomata brown, stromatic, pycnidial, solitary, semi-immersed, elongate globose, unilocular, glabrous, ostiolate. Ostiole subcylindrical, centrally located, with a rostrate apex extending above the substrate. Conidiomatal wall composed of thick-walled, dark brown to pale brown cells of elongated textura intricate to textura angularis. Conidiophores arising from the inner layer of the conidiomata, hyaline, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, determinate, smooth-walled, formed as short or long lateral branches from the conidiophores immediately below septa, with a minute apical channel and collarette. Conidia hyaline, cylindrical to allantoid, straight or slightly curved, unicellular, smooth-walled, eguttulate (Sutton 1980).
Type species: Cytonaema spinella (Kalchbr.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 84 (1914)
Notes: Cytonaema shares a similar form of conidia with Cytospora, but differs in unilocular conidiomata with rostrate ostiole. Three taxa are placed in the genus, C. proteae Marinc., M.J. Wingf. & Crous, C. quercinum Potl. and C. spinella (Potlaichuk 1964; Sutton 1980; Marincowitz et al. 2008). No sexual morph has been linked to this genus and no molecular data is available. Cytonaema species have been reported as a producer of human cytomegalovirus protease inhibitors (Guo et al. 2000). Future study with a lager number of collections are needed to study the chemistry of Cytonaema.
Distribution: Argentina, Austria, Germany, Italy, New Zealand, Poland, Slovakia, UAS (Sutton 1980; https://www.gbif.org/).
Cytoplacosphaeria Petr., Ann Mycol. 17(2/6): 79 (1920) [1919]
Facesoffungi number: FoF 07221, Fig. 106
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, pycnidial, solitary or aggregated into linear stromata, immersed to semi-immersed, globose, unilocular or multilocular, glabrous, ostiolate. Ostiole one per locule, circular, papillate. Conidiomatal wall composed of thick-walled, dark brown to black cells of textura angularis in the exterior, becoming thin-walled and paler toward the hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to doliiform, determinate, smooth-walled, with a minute apical channel, periclinal thickenings at the collarette zone. Conidia hyaline, fusiform, apex pointed or rounded, base truncate or rounded, straight or slightly curved, 1–3-septate, smooth-walled, eguttulate (Sutton 1980).
Type species: Cytoplacosphaeria rimosa Petr., Ann Mycol. 17(2/6): 79 (1920) [1919]
Notes: Two taxa are accepted in Cytoplacosphaeria, C. phragmiticola Poon & K.D. Hyde on decaying stems and leaf sheaths of Phragmites australis and C. rimosa on culms of Phragmites communis, P. vulgaris (Poaceae) or salt marsh plant host (Sutton 1980; da Luz Calado and Barata 2012; Wijayawardene et al. 2017a). The sexual morph was tentatively regarded as Scirrhia rimosa (Alb. & Schwein.) Fuckel (Petrak 1924), but there is no further reference to this. No molecular data is available.
Distribution: Belgium, Czechia, Latvia, Sweden, UK (https://www.gbif.org/).
Cytospora Ehrenb., Sylv. mycol. berol. (Berlin): 28 (1818)
Facesoffungi number: FoF 01022
Sordariomycetes, Sordariomycetidae, Diaporthales, Cytosporaceae
Saprobic, parasitic on the host plant in terrestrial habitat or on mangroves in marine habitat (Sutton 1980; Norphanphoun et al. 2018; Shang et al. 2019, this study). Sexual morph: see Adams et al. (2005, 2006), Shang et al. (2019). Asexual morph:Conidiomata, dark brown to black, eustromatic, pycnidial, solitary to gregarious or confluent, globose to subglobose, conical or irregular in shape, multilocular and convoluted, thick-walled, glabrous, ostiolate; the locules radiating and enlarging from the centre, separated by conidiomatal wall, but united in the ostiolar region. Ostiole circular, erumpent, prominent, centrally located, with or without a furfuraceous ring. Conidiomatal wall composed of thick-walled, dark brown to subhyaline cells of textura angularis to textura intricata or textura oblita. Conidophores arising from innermost wall layer of locular wall, hyaline, cylindrical to subcylindrical, branched, septate, smooth-walled. Conidogenous cells hyaline, phialidic subcylindrical to lageniform, determinate, integrated or discrete, thick and smooth-walled. Conidia hyaline, allantoid, unicellular, smooth-walled, eguttulate.
Type species: Cytospora chrysosperma (Pers.) Fr., Syst. mycol. (Lundae) 2(2): 542 (1823)
Notes: Cytospora species are widely distributed around the world and occur as endophytes, saprobes or plant pathogens (Adams et al. 2005, 2006; Norphanphoun et al. 2017, 2018; Lawrence et al. 2018; Shang et al 2019). Some species can cause dieback and canker on economically important orchard crops, such as Juglans, Malus, Prunus, Punica, Vitis and Ziziphus (Wang et al. 2011; Palavouzis et al. 2015; Fan et al. 2015a, 2020; Lawrence et al. 2017, 2018; Venter et al. 2017), and ornamental plants, urban and forest trees (Adams et al. 2005, 2006; Fan et al. 2015b, 2020; Norphanphoun et al. 2017, 2018; Jami et al. 2018a, b; Zhu et al. 2018).
Cytospora is characterized by stromatic, pycnidial, unilocular to multilocular and labyrinthine conidiomata with long necks, cylindrical, branched conidophores, usually phialidic conidogenous cells and hyaline, unicellular, allantoid conidia (Sutton 1980; this study). Ascomata are stromatic, perithecial, multilocular, globose to subglobose, with long or short necks, containing 8-spored, unitunicate, clavate, sessile asci with a J- apical ring and hyaline, allantoid, aseptate ascospores (Adams et al. 2005; Norphanphoun et al. 2018; Shang et al. 2019).
Conidia and ascospores in many Cytospora species are similar in shape and dimension, and specific morphological distinctions are limited (Adams et al. 2005, 2006; Norphanphoun et al. 2017; Fan et al. 2020). This lack of distinct morphological characters caused Cytospora taxonomy to be largely dependent on host association, leading to many of the described species being identifiable only by host. However, this reliance on host association provides a far from natural classification, as several species are not restricted to a single specific host, or a single host may have more than one Cytospora species (Norphanphoun et al. 2017; Lawrence et al. 2018, this study). Adams et al. (2002, 2005, 2006), applied molecular data to identify Cytospora species and overcome these difficulties. Approximately 650 epithets of Cytospora are listed in Index Fungorum (2019), but many are considered as synonyms of previously described taxa or treated as dubious (Kirk et al. 2008). However, more than 150 species are estimated in Cytospora (Kirk et al. 2008; Norphanphoun et al. 2017, 2018; Lawrence et al. 2017, 2018; Fan et al. 2018; Zhu et al. 2018; Fan et al. 2020). The taxonomy and nomenclature of Cytospora has been discussed in Norphanphoun et al. (2017), Lawrence et al. (2018), and will not be presented here. We describe two new taxa, C. globosa and C. phialidica, from Italy based on morphology and phylogeny, and one new host record of C. prunicola. We also provide a detailed description and illustration for an unidentified Cytospora and describe and illustrate two other species (C. parasitica and C. tanatica).
Distribution: worldwide.
Cytospora parasitica C. Norphanphoun, Bulgakov & K.D. Hyde, in Ariyawansa et al., Fungal Diversity[146] (2015)
Facesoffungi number: FoF 01022, Fig. 108
Parasitic on dying branches of Malus cf. domestica, causing canker of trees. Sexual morph: undetermined. Asexual morph:Conidiomata 950–1120 µm diam., 520–630 µm high, black, eustromatic, pycnidial, usually separate, immersed, conical, multilocular and convoluted, with locules 60–260 µm diam., 100–360 µm high, radiating and enlarging from the centre, separated by inner walls, but uniting in the ostiolar region, thick-walled, ostiolate, surrounded by yellow conidial mass at the tip. Ostiole 250–400 × 110–240 µm, single, circular, centrally located, prominent. Conidiomatal wall 10–250 µm thick, composed of thick-walled, pale brown to dark brown cells of textura angularis in the basal and lateral part, becoming thick-walled cells of textura intricata at the upper region. Conidophores arising from the inner wall layer of locular wall, hyaline, cylindrical to subcylindrical, branched repeatedly, smooth-walled. Conidogenous cells 8–15 × 2–3 µm, enteroblastic, phialidic, lageniform to subcylindrical, integrated or discrete, determinate, smooth-walled, with a minute collarette and narrow channel near apex. Conidia 4.8–7 × 1.3–2 µm (\( \bar{x} \) = 5.7 × 1.8 µm; n = 30), hyaline, allantoid, unicellular, thin-walled, smooth-walled, eguttulate.
Material examined: Russia, Rostov Region, Shakhty City, near Grushevsky pond, grove of feral trees in gully, on dead and drying branches of Malus cf. domestica (Rosaceae), 18 May 2014, T. Bulgakov (MFLU 14–0788, holotype).
Cytospora prunicola Norph., Camporesi, T.C. Wen & K.D. Hyde, in Hyde et al., Mycosphere 9(2): 378 (2018)
Facesoffungi number: FoF04097, Figs. 109, 110
Saprobic on dead stems or bark. Sexual morph: see Shang et al. (2019). Asexual morph:Conidiomata 1270–1600 µm diam., 450–520 µm high, brown, eustromatic, pycnidial, usually solitary, at maturity furfuraceous, immersed to erumpent, globose to subglobose in outline, conical in section view, multilocular, convoluted, thick-walled, glabrous, ostiolate. Ostiole 300–350 × 250–460 µm, single, circular, prominent, centrally located. Conidiomatal wall 30–200 µm wide, composed of dark brown, thick-walled cells of textura angularis to textura intricta in the basal and periclinal wall, gradually merging with brown or olivaceous cells of textura oblita in the middle wall, becoming rather thick-walled, dark brown cells of textura angularis at ostiolar region. Conidiophores formed from inner cells of the locular walls, hyaline, cylindrical, usually branched, septate, smooth-walled. Conidiogenous cells 7–14 × 1–3 µm, hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, integrated or discrete, determinate, smooth-walled, with minute collarette near apex. Conidia 3–5 × 1–2 µm (\( \bar{x} \) = 4.6 × 1.4 µm; n = 30), hyaline, allantoid, aseptate, smooth-walled, eguttulate.
Culture characters: Colonies on PDA, reaching 3–4 mm diam. after 14 d at 25–30 °C, white at first, become dark brown with age, felty, flattened, dense, with undulate to lobate margin, reverse dark brown.
Material examined: Italy, Province of Forlì-Cesena, Premilcuore, Valbura, on dead land branches of Ostrya carpinifolia (Betulaceae), 1 March 2016, Erio Camporesi, IT2871 (MFLU 16-0940), living culture MFLUCC 16-1315 = ICMP 21537 = KUMCC 16-0085; ibid. Passo della Braccina, on dead land branches of Fagus sylvatica (Fagaceae), 10 August 2015, Erio Camporesi, IT2580 (KUN, HKAS 93600, new host record).
Notes: Two collections (MFLU 16-0940/IT2871, HKAS 93600/IT2580) clustered with C. prunicola (MFU 17-0995, MFLUCC 18-1200), C. gutnerae, and C. terebinthi (Fig. 107). The sequence of our collection HKAS 93600/IT2580 shares 100% similarity with C. prunicola (MFU 17-0995) in LSU (808/808), ITS (517/517), and rpb2 (778/778) regions. The sequence similarities between our strain (MFLUCC 16-1315) and C. prunicola (MFU 17-0995) are 99% (576/578, 2 gaps) in ITS, 99% (807/810, 22 gaps) in LSU, and 100% (778/778) in rpb2 region.Our collections have similar form of conidiomata, conidiogenous cells and conidia, as well as conidial dimensions with C. prunicola (Table 4). Therefore, our collections are considered conspecific with C. prunicola. Cytospora prunicola was described from Prunus sp. and Ostrya carpinifolia (Hyde et al. 2018; Shang et al. 2019). The collection (HKAS 93600/IT2580) from Fagus sylvatica is regarded as a new host record.
Cytospora globosa W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557145, Facesoffungi number: FoF 07222, Fig. 111
Etymology: Name refers to its globose conidiomata.
Saprobic on dead needles of Abies alba. Sexual morph: undetermined. Asexual morph:Conidiomata 400–550 µm diam., 350–550 µm high, dark brown to black, pycnidial, solitary, immersed to erumpent, globose in section view, unilocular, convoluted, thick-walled, glabrous, ostiolate. Ostiole 150–200 × 100–220 µm, single, cylindrical, prominent, centrally located. Conidiomatal wall 10–60 µm wide, composed of brown to olivaceous, thick-walled cells of textura angularis to textura intricta in the outer and middle wall, becoming thicker, darker towards ostiolar region. Conidiophores arising from inner wall layer of locules, hyaline, cylindrical, verticillately branched at base and above, septate, smooth-walled. Conidiogenous cells 5–17 × 1–2 µm, hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, determinate, formed as short or long lateral branches from the conidiophores immediately below septa, with minute collarette at the apex. Conidia 4–6.5 × 1–2 µm (\( \bar{x} \) = 5 × 1.6 µm; n = 50), hyaline, allantoid, unicellular, smooth-walled, eguttulate.
Culture characters: Colonies on PDA, reaching 5 mm diam. after 2 days at 25–30 °C, white at first, becoming dark brown with age, cottony, flattened, dense, with entire to undulate margin, reverse pale brown.
Material examined: Italy, Province of Forlì-Cesena, Monte Fumaiolo, on dead aerial needles of Abies alba (Pinaceae), 2 July 2016, Erio Camporesi, IT3019 (MFLU 16-2054, holotype), ex-type living culture MFLUCC 16-1153 = KUMCC 16-0105; (KUM, HKAS 97500, isotype)
Notes: Collection MFLUCC 16-1153/IT3019 clustered with Cytospora sp. UCPH-DK-7 and formed a separated branch within Cytospora, with high support (Fig. 107). The morphology of conidiomata, conidiogenous cells and conidia in many Cytospora species are indistinguishable. Therefore, molecular data (LSU, ITS, rpb2) are used to delimit Cytospora species. Cytospora abietis (= Valsa abietis) and C. friesii (= V. friesii) were collected from Abies alba, but they separated from our collection by ITS sequence (other protein gene not available) (Adams et al. 2002, 2006). The sequence similarities between these two strains (CBS 185.42, CBS 194.42) and our collection MFLUCC 16-1153 are only 93% (511/551, 19 gaps) and 96% (528/551, 14 gaps) in ITS gene region. Therefore, our strain together with Cytospora sp. UCPH-DK-7 is introduced as a new species, Cytospora globosa.
Cytospora phialidica W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557146, Facesoffungi number: FoF 07399, Figs. 112, 113
Etymology: Name refers to its phialidic conidiogenous cells.
Saprobic on dead stems of Alnus glutinosa. Sexual morph: undetermined. Asexual morph:Conidiomata 950–1100 µm diam., 400–500 µm high, brown, eustromatic, pycnidial, solitary to gregarious, at maturity furfuraceous, immersed to erumpent, globose to subglobose in section view, multilocular, convoluted, thick-walled, glabrous, ostiolate. Ostiole 200–250 × 70–100 µm, single to each locule, circular, prominent, centrally located. Conidiomatal wall 35–120 µm wide, composed of dark brown, thick-walled cells of textura angularis to textura intricta in outer layer, becoming hyaline towards conidial hymenium. Conidiophores arising from inner wall layer of locules, hyaline, cylindrical, branched at base, septate, smooth-walled. Conidiogenous cells 6–15 × 1–2 µm, hyaline, enteroblastic, polyphialidic, cylindrical to subcylindrical, usually integrated, indeterminate, smooth-walled, with 2 conidia formed sympodially in a very restricted area at the apices. Conidia 3.5–5 × 1–2 µm (\( \bar{x} \) = 4 × 1.5 µm; n = 50), hyaline, allantoid, aseptate, smooth-walled, eguttulate.
Culture characters: Colonies on PDA, reaching 4–5 mm diam. after 14 days at 25–30 °C, white to brown with age, velutinous to felty, flattened, dense, with rounded margin, reverse greyish red (10C4), sporulation after 4 weeks.
Material examined: Italy, Province of Forlì-Cesena, Bagno di Romagna, Lago Pontini, on dead aerial stems of Alnus glutinosa (Betulaceae), 11 August 2016, Erio Camporesi, IT3067 (MFLU 16-2442, holotype), ex-type living culture MFLUCC 17-2498, (KUM, HKAS 101662).
Notes: Phylogenetic analyses based on LSU, ITS and rpb2 shows that collection MFLUCC 17-2498 grouped with Cytospora sp. I126, C. melanodiscus (Jimslanding2, Worrall2b), but it formed a separated branch above Cytospora sp. I126 (Fig. 107). Our collection MFLUCC 17-2498 has polyphialidic conidiogenous cells, while other Cytospora species have monophialidic conidiogenous cells (Sutton 1980; Adams et al. 2006; Norphanphoun et al. 2017, 2018; Fan et al. 2020). The ostiole in many Cytospora species unite as a single channel in the upper region, but our collection has a single ostiole to each locule. Therefore, our collection is introduced as new taxon.
Cytospora tanaitica C. Norphanphoun, Bulgakov & K.D. Hyde, in Ariyawansa et al., Fungal Diversity [146] (2015)
Facesoffungi number: FoF 01021, Fig. 114
Parasitic and saprobic on branches. Sexual morph: undetermined. Asexual morph:Conidiomata 920–1250 µm diam., 450–500 µm high, black, eustromatic, pycnidial, solitary, immersed to erumpent, conical to subglobose, multilocular, thick-walled, ostiolate. Ostiole 200–370 × 70–250 µm, single, cylindrical to subcylindrical, centrally located. Conidiomatal wall 40–270 µm thick, composed of brown to dark brown, thick-walled cells of textura angularis to porrecta at base and lateral region, becoming thicker, dark brown cells of textura intricata to textura angularis at upper region. Conidophores formed from inner layer of locular wall, hyaline, cylindrical to subcylindrical, branched, smooth-walled. Conidogenous cells 6–11 × 1–2 µm, hyaline, enteroblastic, phialidic, subcylindrical, occasionally lageniform, integrated or discrete, determinate, thick and smooth-walled, with collarette and minute channel. Conidia 3.5–5.5 × 1.1–2.2 µm (\( \bar{x} \) = 4.7 × 1.7 µm; n = 30), hyaline, allantoid, unicellular, eguttulate, smooth-walled.
Material examined: Russia, Rostov Region, Shakhty City, Central Park, on dead branches of Betula pubescens, 5 May 2014, T. Bulgakov (MFLU 14-0790, holotype).
Cytospora sp.
Facesoffungi number: FoF 07224, Fig. 115
Saprobic on dead twigs of Platanus orientalis. Sexual morph: undetermined. Asexual morph:Conidiomata 720–850 µm diam., 400–520 µm high, brown, pycnidial, usually solitary, deeply immersed at first, becoming erumpent, furfuraceous at maturity, subglobose in section view, multilocular, convoluted, thick-walled, glabrous, ostiolate. Ostiole 350–400 × 200–300 µm, single, circular, prominent, centrally located. Conidiomatal wall 20–140 µm wide, composed of brown, thick-walled cells of textura angularis to textura intricta. Conidiophores arising from inner wall layer of locules, hyaline, cylindrical, branched at base and above, septate, smooth-walled. Conidiogenous cells 5–12 × 1–2 µm, hyaline, enteroblastic, phialidic, cylindrical to doliiform, integrated or discrete, determinate, with a short terminal or lateral branch formed immediately below a septum, thickening at collarette zone. Conidia 4–8 × 1–2 µm (\( \bar{x} \) = 6 × 1.5 µm; n = 50), hyaline, cylindrical or allantoid, with obtuse ends, straight or curved, unicellular, smooth-walled, eguttulate.
Material examined: Germany, on dead twigs of Platanus orientalis (Platanaceae), 3 March 2013, René K. Schumacher, D5 (MFLU 19-2857).
Notes: The conidiomatal structure of our collection is close to C. ulmi (Norphanphoun et al. 2017). However, our collection has cylindrical to doliiform, acropleurogenous conidiogenous cells, a feature lacking in C. ulmi. As our collection lacks protein gene (e.g., rpb2), and it cannot be separated well from other Cytospora taxa (Fig. 107), we keep it as an unidentified taxon. Fresh collections of this taxon are needed to clarify its placement.
Cytosporella Sacc., Syll. fung. (Abellini) 3: 251 (1884)
Facesoffungi number: FoF 07225, Fig. 116
Ascomycota, genera incertae sedis
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary, immersed to semi-immersed, pulvinate, multilocular, convoluted, glabrous. Ostiole absent, dehiscence by breakdown of the upper wall. Conidiomatal wall composed of thick-walled, dark brown to black cells of textura angularis in the exterior, becoming thick-walled, brown to paler cells of textura intricata towards locular wall. Conidiophores arising from inner layers of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled, with acropleurogenous conidia. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to doliiform, integrated or discrete, indeterminate, smooth-walled, often formed from lateral branches immediately below transverse septa, with a minute apical channel and collarette. Conidia hyaline, ellipsoidal, unicellular, smooth-walled, eguttulate (Sutton 1980).
Type species: Cytosporella sycina (Sacc.) Sacc., Syll. fung. 3: 251 (1884)
Notes: More than 60 Cytosporella epithets are listed in Index Fungorum (2019). The convoluted conidiomata in Cytosporella resembles Cytospora. The latter has allantoid conidia, which differs from the ellipsoidal conidia in Cytosporella. Wijayawardene et al. (2017b) placed Cytosporella in Gnomoniaceae (Diaporthales, Sordariomycetes). There is no molecular data available for type species, C. sycina. To clarify the taxonomy of Cytosporella, the type species will have to be recollected, and epitypified, so that authentic cultures and DNA barcodes will become available to fix the genetic concept.
Distribution: Brazil, Cuba, Cyprus, Czechia, Dominican Republic, France, Germany, Hungary, India, Italy, New Zealand, Pakistan, Portugal, Russian, Spain, South Africa, Sweden, Switzerland, USA (https://www.gbif.org/).
Cytostagonospora Bubák, Ann Mycol. 14(3/4): 150 (1916)
Facesoffungi number: FoF 07226, Fig. 117
Dothideomycetes, Dothideomycetidae, Capnodiales, Mycosphaerellaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, amphigenous, solitary, immersed, globose, unilocular, glabrous, clypeate. Ostiole circular, papillate to shortly rostrate, depressed, central, situated within the clypeus. Conidiomatal wall composed of thick-walled, dark brown cells of textura globulosa in the exterior, becoming hyaline cells of textura angularis toward hymenium. Conidiophores arising from inner layers of conidiomata, reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, ampuliform to lageniform, smooth-walled. Conidia hyaline, filiform, with obtuse apex and truncate base, 0–2-septate, not constricted at septa, smooth-walled, eguttulate (Sutton 1980).
Type species: Cytostagonospora photiniicola Bubák, Ann Mycol. 14(3/4): 150 (1916)
Notes: Cytostagonospora was treated as a synonym of Septoria by von Arx (1983), while it was retained as a separate genus by Sutton (1980). Quaedvlieg et al. (2013) obtained a culture of C. martiniana and clarified the phylogenetic position of Cytostagonospora, in Mycosphaerellaceae (Capnodiales), which is distinct from Septoria. However, no molecular data is available for the type species occurring on Photinia serrulata, Austria. Fresh material is needed to confirm its placement.
Distribution: Canada, Italy, New Zealand, Pakistan (https://www.gbif.org/)
Darkera H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3052 (1975)
Facesoffungi number: FoF 07263
Leotiomycetes, Leotiomycetidae, Phacidiales, Phacidiaceae
Foliicolous, occurring on needles of coniferous hosts, such as Abies balsamea, Picea glauca, P. engelmanni, P. excels, Pseudotsuga menziesii. Sexual morph:Apothecia black, solitary to gregarious, or confluent, subhypodermal, immersed to semi-immersed, then becoming erumpent, ellipsoid to elongate, unilocular, glabrous, ostiole absent, opening by a median longitudinal slit and raising the overlying host tissue on both sides of the central fissure. Peridium composed of textura angularis with thick-walled and brown to pale brown cells. Hamathecium consisting of paraphyses and asci. Paraphyses hyaline, numerous, slender, with occasionally slightly swollen and obtuse apex, septate, anastomosing, branched, without sheath, or enveloped by a thin mucilaginous sheath. Asci bitunicate, fussitunicate, with an ocular chamber, 8-spored, clavate to broadly clavate, sessile or sometimes with short or broadly short-cylindrical pedicel, lacking pore structure, releasing ascospores by apical wall irregularly rupturing. Ascospores hyaline to pale brown, or pale brown to olivaceous brown, uniseriate or biseriate, or irregularly arranged, rounded or broadly ellipsoidal, subreniform or allantoid, sometimes slightly tapering with rounded ends, unicellular, smooth-walled or with bright, crack-like decorations on the surface. Asexual morph:Conidiomata brown to dark brown, pycnidial, amphigenous, epiphyllous or hypophyllous, solitary to gregarious or confluent, subepidermal, deeply immersed, raising the epidermis into brown swellings, then piercing it by a lateral neck, globose to subglobose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, cylindrical to subcylindrical, laterally or centrally located. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis to textura porrecta, textura intricata or textura epidermoidea cells. Conidiophores arising from the innermost layers of conidiomata, reduced to conidiogenous cells, or occasionally present, hyaline, subcylindrical, unbranched, septate, with or without constriction at septa. Conidiogenous cells hyaline, holoblastic or enterolastic, phialidic or annellidic, cylindrical to subcylindrical, ampuliform to lageniform, determinate or indeterminate, discrete or integrated, smooth-walled, with or without proliferation. Conidia hyaline, subcylindrical, or cylindrical to clavate, with a rounded apex, and narrow and truncate base, sometimes bulging in the middle, bearing a widely flared, or funnel-shaped to irregular, undulated, mucoid, apical appendage resulting from the eversion of a mucoid sheath enveloping the upper part of developing conidium.
Type species: Darkera parca H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3053 (1975)
Notes: The genus Dakera was introduced by Whitney et al. (1975) to accommodate D. abietis H.S. Whitney et al. and D. parca. The detailed taxonomy of Dakera has been discussed by Whitney et al. (1975). The asexual morph of Dakera has been assigned to tiarosporella-like species based on host association, but this connection has never been confirmed via culture studies. Crous et al. (2015b) regarded this link as probably correct, because tiarosporella-like morphs have been linked to more than one species (Whitney et al. 1975). A group of fungi share similar morphology with Tiarosporella paludosa (Sacc. & Fiori) Höhn., but cluster to the Phacidiaceae Crous et al. (2015b). Five species are accepted in the genus, D. abietis, D. durmitorensis (Karadžić) Crous, D. parca, D. picea H.S. Whitney et al. and D. pseudotsugae (H.S. Whitney, J. Reid & Piroz.) Crous. In this study, two specimens collected from Italy on dead needles of Picea excelsa Wender. (Pinaceae) correspond very well with D. picea in morphology and phylogeny. These two collections are regarded as conspecific with D. picea. Since no photo of conidiomata structure from nature was given in Crous et al. (2015b), we provide a plate and detailed description for D. picea. In addition, we re-examined the type specimen of both sexual and asexual morphs of D. abietis and D. parca as well as D. pseudotsugae. Of interest is that Dakera species has more than two kinds of conidiomatal wall, viz. textura intricata to textura porrecta, textura epidermoidea or textura angularis, and these kinds of wall cells are often mixed together. Whereas, Tiarosporella species only have textura angularis to textura prismatica wall cells. This apparent difference may be a valuable character to distinguish Dakera from Tiarosporella. Further collections are needed to epitypify and to confirm the placement of Dakera species.
Distribution: Canada, Finland, Norway, Siberia, Switzerland (Crous et al. 2015b, this study).
Darkera abietis H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3052 (1975)
= Tiarosporella abietis H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3055 (1975)
Facesoffungi number: FoF 07264, Figs. 118, 119
Foliicolous, occurring on needles of Abies balsamea. Sexual morph:Apothecia 300–550 µm diam., 100–500 µm high, black, solitary to gregarious, or confluent, subhypodermal, immersed to semi-immersed, then becoming erumpent, ellipsoid to elongate, unilocular, glabrous, ostiole absent, dehiscence of the apothecia by an irregular split in the apical wall. Peridium 20–40 µm wide, composed an outer pseudoparenchyma of compact textura angularis with thick-walled and brown to pale brwon cells in the basal part, passing into brown to dark brown cells of textura angularis in the lateral part. Hamathecium consisting of paraphyses and asci. Paraphyses 40–90 × 2–4 µm, hyaline, numerous, slender, with occasionally slightly swollen base and obtuse apex, becoming free at the upper end only as the apothecium matures, branched at base, anastomosing, septate, often constrict at septa. Asci 40–90 × 10–25 µm (\( \bar{x} \) = 66 × 18.5 µm; n = 30), bitunicate, fussitunicate, with an ocular chamber, 8-spored, clavate to broadly clavate, with blunt and rounded apex, sessile or sometimes with broadly short-cylindrical pedicel, lacking pore structure, ascospores released by apical wall irregularly rupturing in the middle part. Ascospores 19–28 × 7–18 µm (\( \bar{x} \) = 23 × 12 µm; n = 30), hyaline to pale brown, uniseriate or biseriate, or irregularly arranged, rounded or broadly ellipsoid, sometimes slightly tapering with rounded ends, unicellular, smooth-walled. Asexual morph: Coelomycetous. Conidiomata 200–300 µm diam., 140–300 µm high, pale brown to brown, pycnidial, mostly epiphyllous, sometimes amphigenous, solitary to gregarious, subepidermal, deeply immersed, raising the epidermis into brown swellings, then piercing it by a lateral neck, globose to subglobose, unilocular, glabrous. Ostiole single, cylindrical to subcylindrical, laterally located. Conidiomatal wall 20–70 µm wide, composed of thick-walled, pale brown to hyaline cells of textura angularis in the basal part, becoming relatively thick-walled, brown to pale brown cell of compact textura angularis to textura porrecta or textura intricata in the upper and lateral part. Conidiophores arising from the innermost layers of conidiomata, reduced to conidiogenous cells, or occasionally present, hyaline, subcylindrical, unbranched, septate, constricted at septa. Conidiogenous cells 7–20 × 3–4 µm, hyaline, enteroblastic, cylindrical to conical, determined, smooth-walled, without proliferation. Conidia 39–51 × 6–10 µm (\( \bar{x} \) = 46 × 7.5 µm; n = 30), hyaline, subcylindrical to subclavate, with a rounded apex and a narrow, truncate base bearing minute marginal frills, straight or slightly curved, unicellular, smooth-walled, guttulate, bearing an irregular, widely flared, mucoid, apical appendage.
Material examined: Canada, Ontario, Geraldton District, Rod Lack, on needles of Abies balsamea (Pinaceae), 22 July 1961, Jansons, Griffin & Lynn (DAOM 145406 a & b, holotype); Alberta, Slave Lake, Smith, 2 mile N. W. of Lawrence L. campground, on needles of A. balsamea, 22 May 1968, C. Layton (DAOM 145416, type).
Darkera durmitorensis (Karadžić) Crous, in Crous et al., Phytotaxa 202(2): 83 (2015)
≡ Tiarosporella durmitorensis Karadžić, European Journal of Forest Pathology 28: 148 (1998)
Facesoffungi number: FoF 07265
Description and illustration see Karadžić (1998)
Notes: Tiarosporella durmitorensis was introduced as a new species in Tiarosporella by Karadžić (1998). Crous et al. (2015b) transferred it to Darkera on the basis of its morphology (large unilocular conidiomata and long, wide conidia) and ecology (pathogenic fungus on needles of Abies alba).
Darkera parca H.S. Whitney, J. Reid & Piroz., Canadian Journal of Botany 53: 3053 (1975)
= Tiarosporella parca (Berk. & Broome) H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3055 (1975)
Facesoffungi number: FoF 07266, Figs. 120, 121
Foliicolous, occurring on needles of Picea glauca. Sexual morph:Apothecia 200–300 µm diam., 100–150 µm high, brown to dark brown, acervulus, subhypodermal, initially immersed, ultimately becoming erumpent, solitary to gregarious, ellipsoid to elongate, unilocular, glabrous, ostiole absent, dehiscence by a lateral overlying host tissue. Peridium 10–40 µm wide, composed of an outer pseudoparenchyma of compact textura angularis with thick-walled and pale brown to hyaline cells in the basal part, passing into thick-walled, brown to dark brown cells of textura angularis in the lateral and upper part. Hamathecium consisting of paraphyses and asci. Paraphyses 50–90 × 2–4 µm, hyaline, numerous, slender, with occasionally slightly swollen apex, septate, branched, enveloped by a thin mucilaginous sheath. Asci 70–120 × 17–24 µm (\( \bar{x} \) = 96 × 20 µm; n = 20), bitunicate, with an ocular chamber, 8-spored, clavate to broadly clavate, with rounded apex, sessile or sometimes with short pedicel, lacking pore structure, releasing ascospores by apical wall irregularly rupturing. Ascospores 20–34 × 9–13 µm (\( \bar{x} \) = 27 × 11 µm; n = 30), pale brown to olivaceous-brown, uniseriate or uniseriate at base, then becoming biseriate at upper half of asci, or irregularly arranged, subreniform or allantoid, or elliptical to broadly elliptical and slightly tapering with rounded ends, unicellular, smooth-walled at beginning, late with bright, crack-like decorations at ascospore surface. Asexual morph: Coelomycetous. Conidiomata 200–250 µm diam., 60–120 µm high, amphigenous, pycnidial, solitary to gregarious, hypophyllous in origin, raising the epidermis into dark brown swellings, then piercing the epidermis and forming an ostiole, deeply immersed, ellipsoidal, unilocular, glabrous. Ostiole single, cylindrical, laterally located. Conidiomatal wall 10–30 µm wide, composed of thick-walled, hyaline cells of textura porrecta to textura intricata in the basal part, passing into thick-walled, brown to pale brown cell of textura anularis in the upper part and ostiolar region. Conidiophores reduced to conidiogenous cells, or occasionally present, hyaline, subcylindrical, branched, septate, arising from the innermost layers of conidiomata. Conidiogenous cells 7–15 × 3–5 µm, hyaline, enteroblastic, annellidic, cylindrical to lageniform, occasionally conical, discrete or sometimes integrated, smooth-walled, with 1–3 percurrent proliferations (Fig. 121l, m). Conidia 37–54 × 8–14 µm (\( \bar{x} \) = 42 × 11 µm; n = 50), hyaline, subcylindrical, sometimes bulging in the middle part, with rounded apex, and sometimes narrow, truncate base bearing marginal frills, straight or slightly curved, unicellular, smooth-walled, bearing an irregular, mucilaginous appendage which initially envelopes upper half, eventually becoming an everted mucilaginous sheath at apex.
Material examined: Canada, Alberta, Two Lakes Rd., 68 mile S.W. of Wembley, on needles of Picea glauca (Moench) Voss (Pinaceae), 4 July 1965 J. Petty & G.J. Smith (DAOM 145413, holotype), (DAOM 145413b, holotype of asexual morph).
Darkera sp.
Fig. 122
Foliicolous, occurring on needles of Picea engelmanni. Sexual morph: undetermined. Asexual morph:Conidiomata 200–300 µm diam., 150–200 µm high, dark brown to black, amphigenous, pycnidial, solitary to gregarious or confluent, hypophyllous, deeply immersed, later opening a circular pore in the middle part of overlapping host tissue, but conidia released via lateral ostiole, depressed globose in sectional view, unilocular, glabrous. Ostiole single, subcylindrical, laterally located. Conidiomatal wall 10–100 µm wide, composed of thick-walled, pale brown to hyaline cells of textura angularis in the basal and lateral part, passing into relatively thick-walled, pale brown to brown cells of textura intricata to textura epidermoidea in the upper part, becoming dark brown cells of textura angularis in the ostiolar region. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–15 × 3–7 µm, hyaline, enterolastic, phialidic, lageniform to conical, determined, smooth-walled. Conidia 21–35 × 5.7–8.6 µm (\( \bar{x} \) = 27 × 7.3 µm; n = 30), hyaline, subcylindrical, with rounded apex, and narrow, truncate base bearing marginal frills, straight, unicellular, smooth-walled, bearing a funnel-shape or irregular, mucoid, apical appendage.
Material examined: Canada, Alberta, 31 mile N. W. of Rocky Mountain Ranger Station, on needles of Picea engelmanni Engelm. (Pinaceae), 7 July 1966, J. Petty & F. March (DAOM 145420).
Notes: Collection DAOM 145420 was deposited as Darkera parca by Whitney et al. (1975), but it has smaller conidia than DAOM 145413b (holotype, 37–54 × 8.3–13.8 µm (\( \bar{x} \) = 42 × 10.7 µm). In addition, collection DAOM 145420 has phialidic conidiogenous cells whereas they are distinctly annellidic in DAOM 145413b. These two collection are probably not conspecific, but lacking molecular data, this hypothesis cannot be confirmed.
Darkera picea H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3053 (1975)
Facesoffungi number: FoF 07267, Fig. 123
Saprobic on dead needles of Picea excels, dark brown fruiting bodies, in a linear series. Sexual morph: undetermined. Asexual morph:Conidiomata 250–400 µm high, 200–300 µm diam., pycnidial, separate or gregarious, globose, subglobose to ovoid, immersed, subepidermal, unilocular, glabrous, ostiolate, thick-walled. Conidiomatal wall 20–35 µm wide, composed of cells of outer textura intricata and inner textura epidermoidea, thick-walled, dark brown to pale brown. Ostiole 20–40 µm wide, centrally or laterally located, long, subcylindrical, single or sometimes 2-ostiolate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–6.5 µm long × 3–7 µm wide, hyaline, holoblastic, lageniform to subcylindrical, smooth, arising from inner layers of conidiomata. Conidia 20–28 × 3.5–4.5 µm (\( \bar{x} \) = 25.5 × 4 µm; n = 30), subcylindrical, straight or slightly curved, with a rounded apex, and narrow and truncate base often with minute frills, unicellular, hyaline, smooth, thick-walled, bearing an irregular, widely flared, undulated, mucoid, apical appendage resulting from the eversion of a mucoid sheath enveloping the upper part of developing conidium.
Material examined: Italy, Province of Trento, Mezzana, Marilleva 1400, on dead and not land needles of Picea abies (Pinaceae), 20 August 2014, Erio Camporesi, IT2050 (KUN, HKAS 93593), IT2050A (MFLU 19-2868).
Notes: Our collection is morphologically conforms to the type of Darkera picea.
Darkera pseudotsugae (H.S. Whitney, J. Reid & Piroz.) Crous, in Crous et al., Phytotaxa 202(2): 85 (2015)
≡Tiarosporella pseudotsugae H.S. Whitney, J. Reid & Piroz., Can. J. Bot. 53(24): 3057 (1975)
Facesoffungi number: FoF 07268, Fig. 124
Foliicolous, occurring on leaves of Pseudotsuga menziesii. Sexual morph: undetermined. Asexual morph:Conidiomata 200–250 µm diam., 150–200 µm high, brown to dark brown, hypophyllous, pycnidial, solitary to gregarious, subepidermal, deeply immersed, globose to subglobose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, cylindrical to circular, centrally located. Conidiomatal wall 30–50 µm wide, composed of thick-walled, pale brown cells of textura intricata in the basal part, passing into relatively thick-walled, brown to hyaline cell of textura intracata mixed with textura epidermoidea in the upper half part, becoming dark brown textura intracata at ostiolar region. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–15 × 3–6 µm, hyaline, enteroblastic, ampuliform to lageniform or conical, discrete or integrated, smooth-walled. Conidia 42–55 × 5–7.5 µm (\( \bar{x} \) = 50 × 6 µm; n = 30), hyaline, cylindrical to clavate, with bulging and rounded apex, attenuated below to a narrow, truncate base, straight or slightly curved, unicellular, smooth-walled, bearing a funnel-shaped to irregular, mucoid appendage at apex, or occasionally a subcylindrical mucoid appendage at base (Fig. 124q).
Material examined: Canada, Alberta, Edith Cavell turnoff, Jasper, on leaf of Pseudotsuga menziesii (Pinaceae), 26 July 1974, R.J. Bourchier (DAOM 49190, holotype).
Dasysticta Speg., Anal. Mus. nac. Hist. nat. B. Aires 23: 108 (1912)
Facesoffungi number: FoF 07269, Fig. 125
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, pycnidial, solitary, superficial, amongst leaf hairs, globose, unilocular, setose. Conidiomatal setae hyaline, tapered, flexuous, septate, thick-walled. Ostiole absent. Conidiomatal wall composed of thick-walled, pale brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled, formed directly from the cells comprising the pycnidial wall. Conidia hyaline, cylindrical, unicellular, smooth-walled, often with a large central guttule (Sutton 1980).
Type species: Dasysticta sapindophila Speg., Anal. Mus. nac. Hist. nat. B. Aires 23: 108 (1912)
Notes: Dasysticta sapindophila was collected on leaves of Serjania caracasana (Sapindaceae) in Argentina. The genus remains monotypic, and there is no molecular data available. Dasysticta can be confused with Chaetasbolisia, as they have pycnidial, globose, unilocular, setose conidiomata, phialidic, doliiform to ampulliform conidiogenous cells and hyaline, unicellular conidia. Dasysticta was separated from Chaetasbolisia by its hyaline, tapered, flexuous conidiomatal setae up to 650 µm long, and cylindrical conidia (Sutton 1980). Dasysticta has brown, subulate conidiomatal setae up to 60 µm long, and oval conidia.
Distribution: Argentina.
Davisiella Petr., Ann Mycol. 22(1/2): 134 (1924)
Facesoffungi number: FoF 07270, Fig. 126
Ascomycota, genera incertae sedis
Fungicolous, parasitic in the locules of Phyllachora on Poaceae (Greene 1949). Sexual morph: undetermined. Asexual morph:Conidiomata hyaline, either enclosed by perithecia or immersed in clypear tissue of Phyllachora, pycnidial, solitary, rarely aggregated, globose to pyriform, unilocular, glabrous. Ostiole single, circular, centrally located. Conidiomatal wall composed thick-walled, hyaline cells of textura angularis. Conidiophores lining all around the cavity of conidiomata, hyaline, cylindrical to lageniform, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to doliiform, integrated to discrete, determinate, smooth-walled, with terminal or lateral apertures formed immediately below septa. Conidia hyaline, fusiform to cylindrical or irregular in shape, with rounded apex and truncate base, straight or curved, 2–3-septate, smooth-walled, guttulate (Sutton 1980; Morgan-Jones et al. 1986).
Type species: Davisiella elymina (Davis) Petr., Ann Mycol. 22(1/2): 134 (1924)
Notes: Sutton (1980) revised Davisiella and accepted three species in the genus. Davisiella is characterized by its fungicolous habit (Sutton 1980; Sun et al. 2019). Davisiella domingensis was recorded from several Phyllachora species on different grasses in Dominica (Petrak and Ciferri 1932; Cole and Kendrick 1981). Davisiella botryodiplodiae was described from conidiomata of Botryodiplodia theobromae Pat. (Ahmad 1961), while D. elymina was described from Phyllachora on Muhlenbergia racemosa (Poaceae) in the USA (Sutton 1980). No molecular data is available for Davisiella.
Distribution: Dominican Republic, Pakistan, USA.
Dearnessia Bubák, Hedwigia 58: 25 (1916)
Facesoffungi number: FoF 07271, Fig. 127
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, amphigenous, solitary, immersed to erumpent, globose, unilocular, glabrous. Ostiole single, circular, centrally located. Conidiomatal wall composed of thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells formed from the inner cells of the pycnidial wall, hyaline, holoblastic, doliiform to ampulliform or subcylindrical, determinate, smooth-walled. Conidia hyaline, cylindrical or irregular, with rounded apex and truncate base, straight or irregular, 1–4-septate, smooth-walled, guttulate (Sutton 1980, Quaedvlieg et al. 2013).
Type species: Dearnessia apocyni Bubák, Hedwigia 58: 25 (1916)
Notes: Dearnessia remains monotypic and is associated with lesions on Apocynum androsaemifolium (Apocynaceae) in Canada. Quaedvlieg et al. (2013) re-examined the holotype and re-described the genus. No molecular data is available.
Distribution: Canada (Sutton 1980).
Dendrodomus Bubák, Bot. Közl. 14(3–4): (63) (1915)
Facesoffungi number: FoF 07272, Fig. 128
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary to gregarious, superficial, globose to collabent, unilocular, glabrous. Ostiole single, circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the periphery, becoming thin-walled, paler and then hyaline, small cells toward hymenium. Conidiophores formed from the inner wall of conidiomata, hyaline, cylindrical, flexuous, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical or ampulliform, integrated or discrete, determinate, smooth-walled, with terminal or lateral apertures and formed immediately below transverse septa, periclinal thickening at the collarette zone. Conidia hyaline, ellipsoid to cylindrical, with rounded apex and slightly truncate base, straight or curved, unicellular, smooth-walled, guttulate (Sutton 1980).
Type species: Dendrodomus annulatus Bubák, Bot. Közl. 14(3–4): (63) (1915)
Notes: Dendrodomus remains monotypic, and the type species is associated with Scrophularia bosniaca (Scrophulariaceae) (Sutton 1980). No molecular data is available for this genus. Dendrodomus shares similar morphology with Sirophoma. The differences between these two genera are discussed under Sirophoma.
Distribution: Yugoslavia (Sutton 1980).
Dermea Fr., Syst. orb. veg. 1: 114 (1825)
= Gelatinosporium Peck, Ann. Rep. Reg. Univ. St. N.Y. 25: 84 (1873) [1872]
= Foveostroma DiCosmo, Can. J. Bot. 56(14): 1682 (1978)
Facesoffungi number: FoF 07400
Leotiomycetes, Leotiomycetidae, Medeolariales, Dermateaceae
Saprobic on plant host in terrestrial habitat. Sexual morph:Apothecia dark brown to black, erumpent, seperate or caespitose, circular to undulate, sessile or narrowed below to substipitate, hard, leathery in consistency, softer and more fleshy when moist. Hymenium at first concave, becoming plane or convex, roughened, sometimes cracked, occasionally slightly umbilicate, black, olivaceous to greenish when moist. Asci cylindrical-clavate, mostly 8-spored. Ascospore hyaline to yellow-brown, ellipsoid to ellipsoid-fusiform, 1–7-septate. Paraphyses numerous, filiform, exceeding the asci in length and forming an epithecium (Groves 1946). Asexual morph:Conidiomata yellowish to brown, solitary to gregarious, subperidermal in origin, immersed to partly erumpent at maturity, stromatic, subglobose to depressed globose, multilocular, thick-walled, glabrous. Ostiole short, centrally located. Conidiomata wall composed of thick-walled, hyaline cells of textura intricata to textura epidermodea. Conidiophores hyaline, cylindrical to subcylindrical, septate, broader and branched at base, formed from inner cells of conidiomata. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, discrete or integrated, indeterminate, with periclinal thickening in the collarette zone, smooth. Conidia hyaline, elongate-fusiform to subfiliform, tapering towards both ends, curved or straight, aseptate or 1-septate, smooth, guttulate.
Type species: Dermea cerasi (Pers.) Fr., Syst. orb. veg. 1: 115 (1825)
Notes: Dermea was proposed by Fries (1825) to accommodate a group of inoperculate Discomycetes, but he did not designate a type species for this genus. Subsequently, Tulasnes (1865) chose D. cerasi (Pers.) Fr. as the type species, because it is one of the most frequently collected and best known Dermea. According to Article 20 of the International Rules of Nomenclature, Seaver and Velasquez (1933) agreed that D. cerasi can be accepted as the type of genus. Based on D. cerasi, 16 species from North American were recognized in Dermea by Groves (1940, 1946).
Groves (1946) linked Dermea cerasi to its asexual morph Micropera drupacearum Lév. which is now synonymized under Foveostroma drupacearum (Lév.) DiCosmo based on a culture study. Therefore, he thought that the asexual morph of Dermea species belonged to Foveostroma DiCosmo, with the exception of D. acerina (Peck) Rehm, whose asexual morph was considered to be in Naemosphaera P. Karst. (Groves 1946; DiCosmo 1978). Since the morphology of conidia (shape) is remarkably constant, Grove (1946) divided the genus into four groups, viz. Cerasi group, Padi group, Prunastri group and the fourth group consists of the single species D. acerina (Peck) Rehm. However, this classification has not been confirmed by molecular study (Abeln et al. 2000). In addition, characters of sexual morph that are thought to be specific for a certain group appeared to be polyphyletic. For example, D. libocedri J.W. Groves and D. viburni J.W. Groves both belong to Cerasi group, but the asci of D. libocedri tend to approach certain species of the Prunastri group in shape, while D. viburni are more like those of the Padi group (Groves 1946).
In this study, phylogenetic tree reconstructed utilizing multi-gene of LSU, ITS and rpb2 sequence data show that two new collections from Italy cluster with D. cerasi with high bootstrap value Fig. 129. Morphologically, these two strains largely resemble Foveostroma drupacearum, type species of the genus, in the form of conidiomata, conidiogenous cells and conidia, but differ in dimensions of conidiomata. However, structure of conidiomata in Foveostroma species are varied and this cannot be used as a criterion in species or generic identification. Combining both phylogeny and morphology outlined above, Foveostroma is confirmed as the asexual morph of Dermea and this agrees with the concept of Groves (1946).
Gelatinosporium is similar to asexual morph of Dermea (= Foveostroma drupacearum) in having pulvinate, unilocular to multilocular conidiomata without preformed ostioles, opening by breakdown of the apical wall, and phialidic conidiogenous cells and fusiform to lunate, 1-septate conidia. The only differences between these two genera are the conidiomatal structure. Gelatinosporium has pale brown to hyaline cells of textura oblita in the basal part, while Dermea has hyaline cells of textura intricata to textura epidermodea in the basal part (Sutton 1980; this study). However, the conidiomatal wall structure as single characters have insufficient value to separate genera in coelomycetes, therefore Gelatinosporium is reduced to a synonym of Dermea.
Distribution: Austria, Belgium, Canada, Hungary, Italy, Sweden, UK, USA, (Groves 1946; Sutton 1980; this study).
Dermea cerasi (Pers.) Fr., Syst. orb. veg. 1: 115 (1825)
≡ Peziza cerasi Pers., Neues Mag. Bot. 1: 115 (1794)
= Foveostroma drupacearum (Lév.) DiCosmo, Can. J. Bot. 56(14): 1682 (1978)
Facesoffungi number: FoF 07273, Fig. 130
Dermea cerasi (MFLU 16-1286, reference specimen) a Herbarium specimen. b–d Appearance of yellowish to black conidiomata on the host. e–h, m Vertical section of conidiomata. i Section of peridium. j–k Conidiophores, conidiogenous cells and developing conidia. l Germinating conidium. n–q Conidia. r–s Culture on PDA medium. Scale bars b–d = 1000 µm, e, h = 500 µm, f, g, m = 200 µm, i–l, n–q = 20 µm, r–s = 20 mm
Saprobic on the host plant. Sexual morph:Apothecia 1–3 mm diam., up to 1.5 mm high, erumpent, gregarious, separate or sometimes in small clusters, ± sessile or pedicellate, with a plane to undulate margin, at first yellowish brown and scurfy, eventually black and glabrous, leathery to horny in consistency, becoming more fleshy-leathery when moist. Hymenium at first concave, becoming plane or convex, roughened, sometimes cracked, occasionally slightly umbilicate, black, olivaceous to greenish when moist, margin at first thick, raised, furfuraceous, finally glabrous and disappearing, openings of the pycnidial cavities often appearing as yellowish wrinkles around the margin. Paraphyses 1.5–2 µm wide, hyaline, filiform, septate, unbranched, broadened at apex, narrowed at base, adhering to form a yellowish epithecium. Asci (75–)90–120(–150) × 10–13(–15) µm, 8-spored, cylindric-clavate, tapering below into a short stalk. Ascospores (12–)15–20(–25) × (4–)5–7.5 µm, hyaline to yellowish brown, irregularly biseriate, ellipsoidal-fusiform, straight or slightly curved, 0–4-septate (Groves 1946). Asexual morph:Conidiomata 1000–2000 µm diam., 300–650 µm high, yellowish to brown, solitary to gregarious, subperidermal in origin, immersed to partly erumpent at maturity, stromatic, subglobose to depressed globose, multilocular, thick-walled, glabrous. Ostiole short, centrally located. Conidiomata wall 40–189 µm wide, composed of thick-walled, hyaline cells of textura intricata to textura epidermodea. Conidiophores hyaline, cylindrical to subcylindrical, septate, broadened and branched at base, formed from inner cells of conidioma. Conidiogenous cells 11–25 µm long, 2–4 µm wide, hyaline, entoroblastic, phialidic, cylindrical to subcylindrical, discrete, determinate, with periclinal thickening in the collarette zone, smooth. Conidia 43–62 × 3–5 µm (\( \bar{x} \) = 53.5 × 3.6 µm; n = 30), hyaline, elongate-fusiform to subfiliform, tapering towards both ends, curved or almost straight, aseptate or 1-septate, smooth, guttulate.
Culture characteristics: Colony on PDA reaching 30–40 mm diam., after 14 d at 25 °C, white to pale grey, flattened, felt-like, with undulate to lobate margin, with dense, filamentous aerial mycelium on the surface, reverse pale brown.
Material examined: Italy, Province of Forlì-Cesena, Bagno di Romagna, near Riofreddo, on dead land branches of Prunus avium (Rosaceae), 25 April 2016, Erio Camporesi, IT2856B (MFLU 16-1286, reference specimen designated here), living culture MFLUCC 16-1147 = ICMP 21533 = KUMCC 16-0081, (KUN, HKAS 101646); ibid., Province of Forlì-Cesena, Campigna, on dead land branches of P. avium (Rosaceae), 22 Febrary 2016, Erio Camporesi, IT2856 (MFLU 16-0929), living culture MFLUCC 16-1148 = ICMP 21534 = KUMCC 16-0082.
Diachora Jul. Müll., Jb. wiss. Bot. 25: 623 (1893)
Facesoffungi number: FoF 07274, Fig. 131
Diachora onobrychidis (asexual morph, redrawn from Nag Raj 1993) a Conidia. b Vertical section of conidioma. c, d Conidiophores, conidiogenous cells and developing conidia
Sordariomycetes, Sordariomycetidae, Phyllachorales, Phyllachoraceae
Saprobic or parasitic on the host plant. Sexual morph: see Müller (1986). Asexual morph:Conidiomata black, stromatic, acervular, solitary to gregarious or confluent, immersed to erumpent, irregular in shape, unilocular, glabrous. Ostiole absent, dehiscence by irregular longitudinal fissures in the upper wall. Conidiomatal wall composed of thick-walled, brown or dark brown to almost black cells of textura angularis in the basal part, and thin-walled, black cells gradually merging with black discoloured upper epidermal wall and cuticle in the upper part. Conidiophores arising from inner layer cells of basal stroma, hyaline, cylindrical, branched at the base, septate, aggregated into a dense palisade, invested in mucus. Conidiogenous cells hyaline, enteroblastic, polyphialidic, cylindrical to broad obclavate, integrated or discrete, determinate, smooth-walled, periclinal wall thickened towards apex. Conidia hyaline, narrowly obpyriform to fusiform or irregular, truncate at the base, unicellular, smooth-walled, bearing filiform, irregular, unbranched, cellular, apical appendage (Sutton 1967a, 1980; Nag Raj 1993).
Type species: Diachora onobrychidis (DC.) Jul. Müll., Jb. wiss. Bot. 25: 623 (1893)
Notes: Diachorella onobrychidis (DC.) Höhn., generic type of Diachorella was generally considered to be the asexual morph of Diachora onobrychidis, generic type of Diachora (Sutton 1980; Nag Raj 1993; Maharachchikumbura et al. 2016). Réblová et al. (2016) recommended using the name Diachora for the holomorph, because of its priority and being widely known. The asexual morph of Diachora (= Diachorella) shares similar conidia morphology with Monochaetiella and Neoplaconema. Neoplaconema has pycnidial conidiomata, which differs from the acervular conidiomata in Diachora and Monochaetiella (Nag Raj 1993). Diachora was separated from Monochaetiella by its polyphialidic conidiogenous cells. The genus has never been studied with molecular data. It is necessary to obtain fresh collections of both morphs to confirm their relationship.
Distribution: Algeria, Austria, Canada, China, Commonwealth of Independent States (former U.S.S.R.), France, Germany, Hungary, Iraq, Italy, Japan, Malta, Portugal, Romania, Spain, Turkey (Nag Raj 1993; Wijayawardene et al. 2017b).
Dialaceniopsis Bat., Anais Soc. Biol. Pernambuco 16(1): 141 (1959)
Facesoffungi number: FoF 07275, Fig. 132
Dialaceniopsis landolphiae (redrawn from Sutton 1980) a Condia. b Surface view of conidioma with setae. c Conidiogenous cells and developing conidia
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of ITS, tef1, tub2, his3 and cal sequences data of Diaporthe. Related sequences were obtained from Gao et al. (2017), Yang et al. (2018) and GenBank. 245 strains are included in the analyses, which comprise 2725 characters including gaps (ITS: 1-502, tef1: 503-952, tub2: 953-1702, his3: 1703-2201, cal: 2202-2725). Diaporthella corylina CBS 121124 was used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 71030.968640 is presented. The matrix had 1887 distinct alignment patterns, with 27.35% of undetermined characters or gaps. Estimated base frequencies were: A = 0.212900, C = 0.331400, G = 0.233928, T = 0.221772; substitution rates AC = 1.213593, AG = 3.478420, AT = 1.305051, CG = 0.983866, CT = 4.519164, GT = 1.000000; gamma distribution shape parameter α = 0.462047. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.09 changes. Ex-type or ex-epitype strains are in bold. The new isolates are shown in blue
Ascomycota, genera incertae sedis
Hypophyllous on Landolphia ugandensis.Sexual morph: undetermined. Asexual morph:Conidiomata brown, pycnidial, superficial, globose, collapsing to collabent at maturity, unilocular, setose, ostiolate. Ostiole single, circular, centrally located. Conidiomatal setae dark brown, paler near the apices, radiated to reflexed, straight or flexuous, septate, smooth-walled, often adherent to the superficial mycelium. Conidiomatal wall composed of thin-walled, dark brown cells of textura angularis. Conidiophores formed from the innermost layer wall cells of the conidiomata, reduced to conidiogenous cells. Conidiogenous cells pale brown to hyaline, enteroblastic, annellidic, doliiform to ampulliform, indeterminate, smooth-walled, with 1–4 conspicuous percurrent proliferations toward apex. Conidia hyaline, fusiform, with obtuse apex and truncate base, 1-septate, smooth-walled, eguttulate (Sutton1980) (Fig. 133).
Type species: Dialaceniopsis landolphiae Bat., Anais Soc. Biol. Pernambuco 16(1): 141 (1959)
Notes: Dialaceniopsis is a monotypic genus. The sexual morph of Dialaceniopsis landolphiae was referred to Rhytidenglerula landolphiae (Hansf.) Arx (Sutton 1980). However, Sivanesan (1984) assigned the asexual morph of Rhytidenglerula to Capnodiastrum Speg. Wijayawardene et al. (2017b) kept Dialaceniopsis as a legitimate name and placed it in Ascomycota, genera incertae sedis, because of lack of molecular data. Future studies with fresh collections and cultures are needed.
Distribution: Uganda (Sutton 1980).
Diaporthe Nitschke, Pyrenomyc. Germ. 2: 240 (1870)
Facesoffungi number: FoF 02106
Sordariomycetes, Diaporthomycetidae, Diaporthales, Diaporthaceae
Saprobic, parasitic, or endophytic on the host plant. Sexual morph: see Senanayake et al. (2017, 2018). Asexual morph:Conidiomata brown to dark brown, solitary to gregarious or confluent, immersed to erumpent, pycnidial, globose to subglobose, unilocular, multilocular or convoluted, thick-walled, glabrous, ostiolate. Ostiole usually single, centrally or laterally located. Conidiomatal wall composed of thick-walled, brown cells of textura angularis. Conidiophores arising from the inner layer cells of the conidiomata, hyaline, cylindrical to subcylindrical, septate, branched at base or above, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, usually integrated, determinate, smooth-walled, with periclinal thickenings towards apex. Conidia hyaline, of two types, but in some species with intermediates between the two, (a) α-conidia, hyaline, fusiform, straight, guttulate or eguttulate, aseptate, smooth-walled; (b) β-conidia, hyaline, filiform, straight or hamate, aseptate, smooth-walled, eguttulate.
Type species: Diaporthe eres Nitschke, Pyrenomyc. Germ. 2: 245 (1870)
Notes: Species of Diaporthe have broad host ranges and a worldwide distribution (Udayanga et al. 2012b; Gao et al. 2017; Senanayake et al. 2018). They have been reported as innocuous endophytes isolated from asymptomatic plant tissues (Murali et al. 2006; Botella and Diez 2011a, b; Gomes et al. 2013), saprobes that colonize and degrade dead leaves, bark, twigs (Udayanga et al. 2012b; Senanayake et al. 2018), and destructive plant pathogens (Ménard et al. 2014; Guarnaccia et al. 2016; Torres et al. 2016), or even causing health problems in humans and other mammals (Sutton et al. 1999; Udayanga et al. 2011). Many Diaporthe species are associated with dieback, cankers, fruit and root rots, leaf spots, blights, decay and wilt on cultivated crops, trees, and ornamentals, leading to serious diseases and significant yield losses (Uecker 1988; Mostert et al. 2001; Van Rensburg et al. 2006; Diogo et al. 2010; Santos et al. 2010, 2011; Thompson et al. 2011; Udayanga et al. 2011, 2012a, 2014, 2015; Grasso et al. 2012; Gomes et al. 2013; Huang et al. 2015; Guarnaccia et al. 2016; Torres et al. 2016; Gao et al. 2017). Some endophytic species have been reported as producers of novel compounds with medicinal, agricultural and industrial applications (Bandre and Sasek 1977; Dettrakul et al. 2003; Lin et al. 2005; Kumaran and Hur 2009; Jordaan et al. 2006; Ash et al. 2010; Udayanga et al. 2011; Gomes et al. 2013).
The taxonomic history, nomenclature, and phylogeny of Diaporthe were detailed in Udayanga et al. (2012b), Gomes et al. (2013) and Gao et al. (2017). More than 1000 epithets of Diaporthe are listed in Index Fungorum (2020), but only one-fifth of taxa have been well-studied with molecular data (Udayanga et al. 2012b, 2014; Gomes et al. 2013; Gao et al. 2017; Senanayake et al. 2017, 2018; Yang et al. 2018). Recently, several new species are introduced to Diaporthe based on morphology and multigene sequence data (Yang et al. 2018; Zhu et al. 2019). We introduced a new species Diaporthe arezzoensis from Cytisus sp., and report an additional collection of D. foeniculina from Italy.
Distribution: worldwide.
Diaporthe foeniculina (Sacc.) Udayanga & Castl., in Udayanga, Castlebury, Rossman & Hyde, Persoonia 32: 95 (2014)
≡ Phoma foeniculina Sacc., Michelia 2(no. 6): 95 (1880)
= Diaporthe theicola Curzi, Atti Ist. bot. R. Univ. Pavia, 3 Sér. 3: 60 (1927)
= Phomopsis theicola Curzi, Atti Ist. bot. R. Univ. Pavia, 3 Sér. 3: 65 (1927)
= Diaporthe neotheicola A.J.L. Phillips & J.M. Santos, Fungal Diversity 34: 120 (2009)
Faces of funginumber: FoF 02183, Fig. 134
Diaporthe foeniculina (MFLU 16-1132) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d, e Vertical sections of conidiomata. f Ostiole. g, h Section of peridium. i–k Conidiophores, conidiogenous cells and developing conidia. l Germinating conidium. m–p Conidia. q Culture on PDA. Scale bars b = 1000 µm, c = 500 µm, d–e = 200 µm, f–h = 50 µm, i = 5 µm, j–k, m = 10 µm, l = 20 µm, n–p = 5 µm, q = 10 mm
Saprobic or parasitic on the host plant. Sexual morph: see Udayanga et al. (2014). Asexual morph:Conidiomata 400–600 µm diam., 300–650 µm high, black, solitary to gregarious or confluent, immersed, subperidermal in origin, immersed to partly erumpent at maturity, pycnidial, globose to subglobose, unilocular or multilocular, thick-walled, glabrous. Ostiole 25–60 µm wide, 1–2 ostiolar canals, centrally or laterally located. Conidiomatal wall 40–150 µm wide, composed of thick-walled, brown cells of textura angularis at periclinal wall, thickening at basal and upper zone. Conidiophores hyaline, short, cylindrical to subcylindrical, branched at base, arising from the inner layer cells of the conidiomata. Conidiogenous cells 5–15 ×2–4 µm, hyaline, entoroblastic, phialidic, integrated, determinate, cylindrical to subcylindrical, tapered to the apices, with minute collarette, smooth-walled. Conidia 6–10 × 2–4 µm (\( \bar{x} \) = 7.7 × 3.2 µm; n = 30), hyaline, fusiform, ellipsoid, subcylindrical, straight or slightly curved, unicellular, smooth-walled, guttulate. Beta conidia not observed.
Culture characteristics: Colony on PDA, reaching 20–30 mm diam, after 7 days at 25 °C, white, flattened, fluffy, with undulate to crenate margin, with dense, filamentous aerial mycelium on the surface, reverse white.
Material examined: Italy, Province of Forlì-Cesena, Santa Sofia, Campigna, on dead land branches of Prunus avium (Rosaceae), 23 March 2016, Erio Camporesi, IT2856A (MFLU 16-1132), living culture, MFLUCC 16-1144 = ICMP 21532 = KUMCC 16-0080.
Notes: This species has been reported in Argentina, Australia, France, Greece, Italy, New Zealand, Portugal, Spain, South Africa, and USA, with a wide host range, such as Actinidiaceae, Anacardiaceae, Apiaceae, Ebenaceae, Fabaceae, Fagaceae, Grossulariaceae, Hydrangeaceae, Juglandaceae, Nyctaginaceae, Oleaceae, Onagraceae, Rosaceae, Rutaceae, Sapindaceae, Theaceae and Vitaceae (Udayanga et al. 2014). Diaporthe foeniculina is a pathogen on temperate and tropical fruits and can cause a stem-end rot of Citrus limonia (Udayanga et al. 2014).
Our collection is a saprobe collected on Prunus avium.
Diaporthe arezzoensis W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557207, Facesoffungi number: FoF 07277, Figs. 135, 136
Diaporthe arezzoensis(MFLU 19-2880, holotype) a Herbarium specimen. b, c Appearance of brown to dark brown conidiomata on the host. d, e Vertical sections of conidiomata. f Ostiole. g Section of peridium. h–l Conidiogenous cells and developing conidia. m Germinating conidium. n–o Conidia. Scale bars b = 1000 µm, c = 300 µm, d–e = 200 µm, f–g = 50 µm, h–o = 10 µm
Diaporthe arezzoensis(MFLU 19-2883) a Herbarium specimen. b, c Appearance of brown to dark brown conidiomata on the host. d Vertical section of conidioma. e Loculus. f Section of peridium. g Ostiole. h–k Conidiogenous cells and developing conidia. l, p β-conidia. m–o α-conidia. Scale bars b = 500 µm, c–d = 200 µm, e = 100 µm, f–g = 50 µm, h–p = 10 µm
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tub2 sequences data representing Didymella. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from Chen et al. (2015, 2017) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Eighty five strains are included in the analyses, which comprise 2259 characters including gaps (LSU: 1–836, ITS: 837–1330, tub2: 1331–1663, rpb2: 1664–2259). Allophoma labilis CBS 124.93 is used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 12867.361882 is presented. The matrix had 507 distinct alignment patterns, with 4.64% of undetermined characters or gaps. Estimated base frequencies were: as follows: A = 0.235641, C = 0.245246, G = 0.276046, T = 0.243067; substitution rates AC = 1.018337, AG = 5.020873, AT = 1.570404, CG = 0.725578, CT = 10.612461, GT = 1.000000; gamma distribution shape parameter α = 0.107496. The maximum parsimonious dataset consisted of constant 1801, 382 parsimony-informative and 76 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 1968 steps (CI = 0.350, RI = 0.730, RC = 0.255, HI = 0.650) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.02 changes. Ex-type or reference strains are in bold. New isolates are shown in blue
Etymology: Refers to the name of the province in Italy where the fungus was collected
Saprobic on dead stems of Cytisus sp. Sexual morph: undetermined. Asexual morph:Conidiomata 350–400 µm diam., 300–450 µm high, brown to dark brown, pycnidial on the upper surface of stem, solitary, initially closed, later opening to become disciform to cupulate, immersed to erumpent, globose to subglobose, unilocular, motilocular or convoluted, ostiolate. Ostiole circular, centrally or laterally located. Conidiomatal wall 20–240 µm wide, composed of thick-walled, dark brown cells of textura intricata in the outer wall layer, becoming pale brown cells of textura angularis towards inner layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 16–30 × 2–6 µm, hyaline, enteroblastic, phialidic, subcylindrical, tapered to the apices, determinate, unbranched, smooth-walled, with periclinal thickenings at collarette zone. Conidia hyaline, of two types, (a) α-conidia 13–20 × 5–9 µm (\( \bar{x} \) = 16 × 7 µm; n = 50), fusiform to oblong, straight, aseptate, thick- and smooth-walled eguttulate; (b) β-conidia, 22–30 × 1–3 µm (\( \bar{x} \) = 26 × 2 µm; n = 50), filiform, acute at both ends, straight or more often hamate, aseptate, eguttulate.
Material examined: Italy, Province of Arezzo, near Croce di Pratomagno, on dead land branches of Cytisus sp. (Faboideae), 1 October 2012, Erio Camporesi, IT752 (MFLU 19-2880, holotype), ex-type living culture MFLUCC 15-0127 = ICMP 21837 = KUMCC 15-0581, (KUN, HKAS93664, isotype), ibid., Province of Arezzo, Bagno di Cetica, 7 October 2012, Erio Camporesi, IT792 (MFLU 19-2883), (KUN, HKAS 93606).
Notes: The description of our collections fits well within the generic concept of asexual morphs of Diaporthe. These collections have much larger, wider and thick-walled α-conidia than other taxa. In the phylogeny, our strains formed a separated branch (Fig. 133).
Didymella Sacc., Michelia 2(no. 6): 57 (1880)
Facesoffungi number: FoF 07278
Dothideomycetes, Pleosporomycetidae, Pleosporales, Didymellaceae
Saprobic or parasitic on terrestrial host plants or from air (Chen et al. 2017). Sexual morph: see Chen et al. (2015). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, globose to subglobose, or irregular in shape, unilocular, glabrous, ostiolate or poroid, sometimes with elongated necks. Ostiole single or multi-ostiolate, centrally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores formed in the inner cavity of conidiomata, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled. Conidia hyaline or occasionally pale brown, variable in shape, i.e. cylindrical to subcylindrical, or ellipsoidal to subglobose, or fusiform, oblong, ovoid, sometimes allantoid, 0–1-septate, smooth-walled, guttulate (Chen et al. 2015; Jayasiri et al. 2017) (Fig. 137).
Type species: Didymella exigua (Niessl) Sacc., Syll. fung. 1: 553 (1882)
Notes: Chen et al. (2015) delineated Didymella to accommodate the species (Peyronellaea and several phoma-like species), which have close phylogenetic affinity with D. exigua. Fifty-four species are accepted in Didymella (Chen et al. 2015, 2017; Crous et al. 2018a). Members of this genus are cosmopolitan, having been reported in Africa (Egypt, Ethiopia, South Africa), Asia (China, India), Europe (Belgium, France, Germany, Italy, Romania, Russia, Spain, Switzerland, The Netherlands, UK), North America (USA, Canada), Oceania (Australia, New Zealand) and South America (Peru) (Aveskamp et al. 2010; Chen et al. 2015, 2017; Jayasiri et al. 2017; Crous et al. 2018a). We add an additional collection of D. macrostoma on Vicia sp. in Italy, and provide a detailed description and photo plate.
Distribution: worldwide.
Didymella macrostoma (Mont.) Qian Chen & L. Cai, in Chen, Jiang, Zhang, Cai & Crous, Stud. Mycol. 82: 177 (2015)
Facesoffungi number: FoF 07279, Fig. 138
Didymella macrostoma(MFLU 15-1344) a Herbarium package and specimens. b, c Appearance of black coniodiomata on the host. d Surface view of conidioma. e, f Vertical section of conidiomata. g Section of peridium. h–j Conidiogenous cells and developing conidia k–s Conidia. Scale bars d–f = 100 µm, g = 10 µm, h–s = 5 µm
Saprophytic on dead stems of Vicia sp., forming conspicuous rounded, black conidiomata. Sexual morph: see Jayasiri et al. (2017). Asexual morph:Conidiomata 200–230 µm diam., 100–200 µm high, black, pycnidial, solitary to gregarious or occasionally confluent, immersed to semi-immersed, globose to subglobose, unilocular, glabrous, ostiolate. Ostiole circular, papillate, laterally or centrically located. Conidiomatal wall 10–20 µm wide, composed of thick-walled, dark brown to hyaline cells of texura angularis. Conidiophores formed from the innermost layer of wall cells, reduced to conidiogenous cells. Conidiogenous cells 2–6 × 3–6 µm, hyaline, enteroblastic, phialidic, ampuliform, smooth-walled, with a periclinal wall thickening at the tip. Conidia 4–9 × 3–4 µm (\( \bar{x} \) = 6 × 3.3 µm; n = 50), hyaline, fusiform to oblong, or oval, or irregular in shape, straight or slightly curved, 0–1-septate, smooth-walled.
Material examined: Italy, Province of Arezzo, Quota, Casuccia di Micheli, on dead aerial stems of Vicia sp. (Fabaceae), 1 June 2015, Erio Camporesi, IT2514 (MFLU 15-1344), (KUN, HKAS93628).
Notes: The sequence of this collection (MFLU 15-1344) was obtained via direct sequence from fruiting bodies.
Didymochaeta Sacc. & Ellis, Bull. Torrey bot. Club 25: 510 (1898)
Facesoffungi number: FoF 07280, Fig. 139
Didymochaeta americana (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary to gregarious, semi-immersed to superficial, globose, unilocular, glabrous. Ostiole single, circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura globulosa, often pruinose in the periphery, becoming thinner-walled, paler and then hyaline, small cells of textura angularis towards hymenium. Conidiophores formed from the inner wall of conidiomata, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform, determinate, smooth-walled, with minute collarette but prominent channel and thickened periclinal walls. Conidia hyaline, cylindrical, obtuse at both ends, straight, 1–2-septate, constricted at the septa, smooth-walled, guttulate (Sutton 1980).
Type species: Didymochaeta americana Ellis & Sacc., Bull. Torrey bot. Club 25: 510 (1898)
Notes: Sutton (1980) gave a full account of Didymochaeta, and accepted four species in the genus, of which D. fraserae (Ellis & Everh.) B. Sutton, was assigned as the generic type, and D. americana was listed as a synonym of the type species. According to the priority principle (Hawksworth 2012), D. americana is presently selected as generic type. Didymochaeta shares similar form of conidiomata, conidiogenous cells and conidia with Ascochyta. The only difference between these two genera is the conidiomatal wall structure. Didymochaeta has conidiomatal wall of textura globulosa to textura angularis, and often pruinose in the periphery, whereas Ascochyta has conidiomatal wall of textura angularis. Ascochyta and Didymochaeta might be taxonomically congeneric. However, Ascochyta is a polyphyletic genus as Phoma (Aveskamp et al. 2010); no molecular data is available for Didymochaeta. Therefore, fresh collections of D. americana are needed to place Didymochaeta in a natural group.
Distribution: Argentina, USA.
Diedickea Syd. & P. Syd., Leafl. of Philipp. Bot. 6: 1931 (1913)
Facesoffungi number: FoF 07308, Fig. 140
Diedickea singularis (redrawn from Nag Raj 1977a) a Vertical section of conidioma. b Conidiogenous cells and developing conidia formed from the upper wall. c Enlarged view of conidiomatal suface. d Conidia
Ascomycota, genera incertae sedis
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnothyrial, solitary to gregarious or confluent, superficial, orbicular in outline, hemispherical in section, unilocular, glabrous. Ostiole absent, opening by a stellate split in the apical wall. Conidiomatal wall membranous, only a few cells thick, with cells of outer layer more or less elongated, dark brown, thick-walled; only a thin pellicle covers the floor of the pycnothyrium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform, determinate, smooth-walled, with minute collarette and narrow channel. Conidia hyaline, oblong, with obtuse apex and truncate base, straight, unicellular, smooth-walled, guttulate, discharged in a mucoid mass (adapted from Morgan-Jones 1977).
Type species: Diedickea singularis Syd. & P. Syd., Leafl. Philipp. Bot. 6: 1931 (1913)
Notes: Diedickea is characterized by pycnothyrial conidiomata without an ostiole, but opening by a stellate split in the apical wall, and phialidic, ampulliform conidiogenous cells lining only the cavity of the upper wall. This genus share similar morphology of conidiomata with Abropelta, Peltistromella, Poropeltis, and Tracylla. The differences among these genera are discussed under Tracylla. Morgan-Jones (1977) re-described and illustrated the genus. Four taxa are listed in Index Fungorum (2019). The biology of Diedickea species is poorly known. Bonar (1942) reported D. piceae Bonar was associated with yellowing and discoloration of the leaves and early leaf fall of Picea sitchensis (Pinaceae). The taxonomy of Diedickea needs further investigation based on fresh collections and molecular data.
Distribution: Canada, Dominican Republic, Sierra Leone.
Dilophospora Desm., Annls Sci. Nat., Bot., sér. 2 14: 6 (1840)
Facesoffungi number: FoF 06234, Fig. 141
Dilophospora alopecuri (redrawn from Nag Raj 1993) a Conidia. b Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, aggregated into elongate stromata between host veins, immersed, globose to subglobose, unilocular or multilocular, glabrous. Ostiole single to each locule, circular, papillate. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the exterior, becoming thick-walled, brown to dark brown elements of textura intricata on the exterior of conidiomatal wall and between aggregated conidiomata, merging with paler then hyaline cells toward hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to lageniform, occasionally doliiform, discrete, determinate, smooth-walled, invested in mucus. Conidia hyaline, cylindrical, obtuse at both ends, 0–3-septate, smooth-walled, bearing appendages at each end; appendages tubular, 2–5, mostly dichotomously or irregularly branched, occasionally unbranched, maintaining protoplasmic continuity with the conidium body (Nag Raj 1993).
Type species: Dilophospora alopecuri (Fr.) Fr., Summa veg. Scand., Sectio Post.: 419 (1849)
Notes: Dilophospora was proposed by Desmazières (1840) for graminicolous pycnidial fungi, with D. graminis Desm. as type species, which is now known as Dilophospora alopecuri (Fr.) Fr. (Bessey 1906), based on Sphaeria alopecuri Fries (1828). Walker and Sutton (1974) revised Dilophospora and accepted only D. alopecuri in the genus, and listed D. graminis as a synonym. Nag Raj (1993) provided a detailed description and illustration for the generic type. The hyaline, cylindrical, septate conidia with dichotomously or irregularly branched appendages in Dilophospora resembles Pseudolachnella (especially for, P. coronata, P. brevifusiformis) (Nag Raj 1993). Pseudolachnella has cupulate, setose conidiomata, which differs in Dilophospora that are pycnidial, glabrous.
The sexual morph of D. alopecuri was assigned to Lidophia graminis (Sacc.) J. Walker & B. Sutton, as the latter with pseudothecia was found to occur mixed in the same stroma of D. alopecuri pycnidia. However, this connection has not been confirmed experimentally (Walker and Sutton 1974). Fresh collections of type species are needed to clarify the taxonomy of Dilophospora.
Distribution: Austria, Australia, Belgium, Canada, Commonwealth of Independent States (former U.S.S.R), Czech Republic, France, Germany, India, Iraq, Japan, Latvia, New Zealand, Romania, UK, USA, Yugoslavia (Walker and Sutton Nag Raj 1993; Riley 1994).
Dimastigosporium Faurel & Schotter, Revue Mycol., Paris 30: 156 (1965)
Facesoffungi number: FoF 07309, Fig. 142
Dimastigosporium musimonum (redrawn from Nag Raj 1993) a Vertical section of conidioma. b Conidiomatal setae. c Conidiophores, conidiogenous cells and developing conidia. d Mature conidia
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU and ITS sequences data representing Dinemasporium and allied genera. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from GenBank. Thirty-eight strains are included in the analyses, which comprise 1409 characters including gaps. Thozetella nivea is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 3713.080557 is presented. The matrix had 254 distinct alignment patterns, with 6.36% of undetermined characters or gaps. Estimated base frequencies were: A = 0.219860, C = 0.301533, G = 0.264091, T = 0.214516; substitution rates AC = 1.310261, AG = 1.406775, AT = 2.357796, CG = 1.386339, CT = 4.661447, GT = 1.000000; gamma distribution shape parameter α = 0.267389. The maximum parsimonious dataset consisted of 330 constant, 151 parsimony-informative and 62 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 623 steps (CI = 0.552, RI = 0.781, RC = 0.431, HI = 0.448) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen indicates a value lower than 50% for MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.05 changes. Ex-type and reference strains are in bold. New isolates are in bold and blue
Phylogenetic tree inferred from a maximum likelihood analysis based on ITS sequence data of Dinemasporium and allied genera. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from Hashimoto et al. (2015a) and GenBank. Fifty-three strains are included in the analyses, which comprise 543 characters including gaps. Thozetella nivea EU825201 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 3743.997685 is presented. The matrix had 258 distinct alignment patterns, with 6.36% of undetermined characters or gaps. Estimated base frequencies were: A = 0.219860, C = 0.301533, G = 0.264091, T = 0.214516; substitution rates AC = 1.306238, AG = 1.425648, AT = 2.393712, CG = 1.384533, CT = 4.702082, GT = 1.000000; gamma distribution shape parameter α = 0.275440. The maximum parsimonious dataset consisted of 326 constant, 151 parsimony-informative and 66 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 627 steps (CI = 0.555, RI = 0.781, RC = 0.433, HI = 0.445) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen indicates a value lower than 50% for MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.05 changes. Ex-type and reference strains are in bold. New isolates are in bold and blue
Ascomycota, genera incertae sedis
Coprophilous. Sexual morph: undetermined. Asexual morph: Conidiomata greenish brown to black, stromatic, cupulate, scattered, sessile, superficial, initially closed, eventually opening, setose. Conidiomatal setae brown to dark brown, divergent, septate, arising from the tissue of the periclinal wall. Conidiomatal wall composed of thick-walled, brown, pseudoparenchymatous cells at the base. Conidiophores arising from inner layer cells of conidioma, hyaline, cylindrical branched, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, cylindrical, attenuated towards apex, integrated or discrete, determinate, smooth-walled. Conidia hyaline, pyriform or subcylindrical, unicellular, smooth-walled, bearing appendages at each end or only at base; apical appendage absent or present, single, unbranched, arising as tubular extension of the conidium body; basal appendages, 2–4, unbranched, tubular, divergent, maintaining protoplasmic continuity with the conidium body (Yadav and Bhat 2009; Nag Raj 1993).
Type species: Dimastigosporium musimonum Faurel & Schotter, Revue Mycol., Paris 30: 157 (1965)
Notes: Dimastigosporium was proposed by Faurel and Schotter (1965) for a single species D. musimonum, a fungus collected on dung of Ammotragus lervia (wild sheep) from Central Africa. Nag Raj (1993) provided a revised description and detailed illustration for this genus. The second taxon D. yanense S.K. Yadav & Bhat was described from dung of cattle in India, which is distinguished from type species by its smaller conidia with an apical and three basal appendages (Yadav and Bhat 2009). Only the two species are accepted, but no molecular data is available. Dimastigosporium is compared with several coprophilous fungi. The details see notes of Pseudoneottiospora (Fig. 143).
Distribution: Central Africa, India (Nag Raj 1993; Yadav and Bhat 2009).
Dinemasporium Lév., Annls Sci. Nat., Bot., sér. 3 5: 274 (1846)
Facesoffungi number: FoF 01763
Sordariomycetes, Sordariomycetidae, Chaetosphaeriales, Chaetosphaeriaceae
Saprobic on various herbaceous and woody substrata. Sexual morph:Ascomata subepidermal, on leaf sheaths and stems, immersed to semi-immersed, solitary to gregarious, globose to elongated, unilocular, glabrous, thick-walled composed of brown elongate polygonal cells. Asci 4–8-spored, unitunicate, cylindrical to subcylindrical, pedicellate, rounded above. Ascospores hyaline, uniseriate, rarely sub-biseriate, narrowly elliptical to spindle-shaped or slightly inequilateral, smooth-walled, biguttulate. Paraphyses absent (Webster 1955). Asexual morph:Conidiomata black, stromatic, cupulate, pulvinate in surface view, initially closed, ultimately becoming erumpent and often covered with a mass of conidia that is glutinous and pale pink when moist, but waxy yellowish brown and crustose when dry, solitary to gregarious, superficial to semi-immersed, oval to rounded or irregular in outline, unilocular, setose. Conidomatal setae composed of two types, (a) arising from basal stroma, conspicuous, subcylindrical, unbranched, septate, dark brown to black, blunt at the base, pale brown, tapered towards the apex, rigid, thick- and smooth-walled; (b) arising from excipular elements, pale brown to brown, inconspicuous, subulate, flexuous, unbranched, aseptate, smooth-walled. Conidiomatal wall composed of a thin hypostroma and well-developed excipulum. Conidiophores lining the cavity of the conidiomata, hyaline, cylindrical to subcylindrical, branched, smooth-walled, invested in mucus. Conidiogenous cells hyaline, phialidic, cylindrical to subcylindrical or lageniform, discrete or integrated, indeterminate, smooth-walled. Conidia hyaline, ellipsoid, allantoid to naviculate or fusiform, unicellular, bearing branched or unbranched, single or multiple, bipolar or lateral appendages (Fig. 144).
Types species: Dinemasporium strigosum (Pers.) Sacc., Michelia 2(7): 281 (1881)
= Dinemasporium graminum (Lib.) Lév., Annls Sci. Nat., Bot., sér. 3 5: 274 (1846)
Notes: Léveillé (1846) introduced Dinemasporium with D. graminum (Lib.) Lév. (= D. strigosum (Pers.) Sacc.) as type species. The genus is characterized by superficial, cupulate, setose conidiomata which are initially closed, becoming erumpent, and unicellular conidia with bipolar appendages (Léveillé 1846). Two additional species were added, D. platense Speg. and D. cruciferum Ellis, and the generic concept was expanded to accommodate species with both bipolar and lateral conidial appendages (Spegazzini 1880; Ellis 1882). Conidial appendages are seen as taxonomically informative in separating species and genera (Sydow et al. 1916; Kalani 1964; Nag Raj 1993; Duan et al. 2007). Saccardo (1884a) established the subgenus Stauronema to accommodate taxa with conidia bearing apical, basal and lateral appendages. Stauronema was elevated to generic level by Sydow et al. (1916) on the basis of insertion of conidial appendages. Sutton (1980) accepted this proposal and narrowed circumscription of Dinemasporium to include only species with aseptate conidia. Crous (2012a) reduced Stauronema to a synonym of Dinemasporium based on molecular data (LSU, ITS), and concluded that appendage morphology alone cannot be a defining feature at generic level. Hashimoto et al. (2015a) confirmed this conclusion and added Diarimella Sutton as a synonym because diarimella-like species nested within Dinemasporium. Therefore, the generic concept of Dinemasporium is expanded to accommodate species having conidia with unicellular, unbranched or branched, single or multiple, bipolar and lateral appendages at each end.
Culture studies showed that Dinemasporium strigosum was associated with Phomatospora dinemasporium J. Webster, a species having 4–8-spored, unitunicate, cylindrical, pedicellate, asci with rounded apex and hyaline, uniseriate, narrowly elliptical to spindle-shaped conidia (Webster 1995). However, Rappaz (1992) excluded P. dinemasporium from Phomatospora Sacc. because it lacked ornamented ascospores and an ascus apical ring. He also connected P. berkeleyi Sacc. (generic type of Phomatospora) and P. arenaria Sacc., E. Bommer & M. Rousseau to Sporothrix Hektoen & C.F. Perkins asexual morph (hyphomycetes), which is in conflict with coelomycetous asexual morph of P. dinemasporium (Fallah and Shearer 1998; Senanayake et al. 2016). The sexual morph of Dinemasporium has not been subsequently reported. More collections of phomatospora-like taxa are needed to confirm the connection with Dinemasporium species.
We re-examined the type species of Dinemasporium strigosum, D. cruciferum Ellis (= Stauronema cruciferum) and D. setulosa (= Diarimella setulosa B. Sutton) and provide a detailed description and photo plates. Four collections from Italy are also identified as conspecific with D. pseudostrigosum, D. pseudodecipiens and D. morbidum. Dinemasporium pseudodecipiens is considered to be a complex species, because of its varied conidiomatal setae morphology and conidial dimensions. Additional fresh collections are needed to confirm this.
Distribution: Argentina, Canada, China, Commonwealth of Independent States (Former U.S.S.R.), Cuba, Czech Republic, France, Ghana, India, Italy, Japan, Netherlands, Nigeria, Sierra Leone, USA (Nag Raj 1993; Duan et al. 2007; Crous et al. 2012b, 2014b; Hashimoto et al. 2015a; this study).
Dinemasporium cruciferum Ellis, Bull. Torrey bot. Club 9: 20 (1882)
≡ Stauronema cruciferum (Ellis) Syd., P. Syd. & E.J. Butler, Ann Mycol. 14(3/4): 217 (1916)
Facesoffungi number: FoF 07310, Fig. 145
Dinemasporium cruciferum (FH 01142403, isotype). a, b Herbarium specimen. c Appearance of black conidiomata on the host. d Conidioma with setae. e–f Setae. g Conidiomatal wall. h–k Conidiophores, conidiogenous cells and developing conidia. l–o Conidia. Scale bars: c = 100 µm, d = 50 µm, e–f = 20 µm, g–k = 10 µm, l–o = 5 µm
Saprobic on dead leaves of grasses. Sexual morph: undetermined. Asexual morph:Conidiomata up to 450 µm wide, and 250 µm deep, black, stromatic, cupulate or obconical in surface view, solitary to gregarious, superficial but semi-immersed in origin, setose. Conidiomatal wall composed of two types; (a) basal stroma 30–60 µm thick, of textura angularis, cells thick-walled, often pale brown to brown and encrusted in lower layers, becoming paler, smooth and thin-walled only near the conidial hymenium; (b) excipulum 20–30 µm thick, of textura prismatica, with thick-walled and dark brown to brown cells in the external layers, thin-walled, hyaline in the inner layers. Conidomatal setae 10–130 × 2–7 µm, honey-brown to dark brown, thick and smooth-walled, arising from the basal stroma and excipulum; those arising from basal stroma subulate or cylindrical, acute or blunt at apices, multiseptate, straight or divergent; those arising from excipular elements incurved over the conidial hymenium, with fewer septa. Conidiophores lining the cavity of the conidioma, hyaline, cylindrical to subcylindrical, septate, branched, smooth-walled, invested in mucus. Conidiogenous cells 9–20 × 2–2.5 µm, hyaline, lageniform to subcylindrical, discrete or integrated, indeterminate, thickened at periclinal collarette zone, smooth-walled. Conidia 7–10 × 2–4 µm (\( \bar{x} \) = 9 × 3 µm; n = 50), hyaline, fusiform to naviculate, with a narrow, truncate base, aseptate, bearing unbranched, attenuated, flexuous appendages; apical and basal appendages 6–16 (\( \bar{x} \) = 9) µm long, single; basal appendage excentric; lateral appendages 3–10 (\( \bar{x} \) = 6) µm long, 1–3, inserted about 2–3 µm below the apex,
Material examined: USA, New Jersey, New Field, on dead leaves of various grasses, June 1881 (FH 01142403, isotype, Ellis & Everhart - North American Fungi #755)
Notes: The isotype was in poor condition, thus the description of conidiomata and conidiomatal wall are based on Nag Raj (1993).
Dinemasporium morbidum Crous, in Crous, Verkley, Christensen, Castañeda-Ruíz & Groenewald, Persoonia 28: 131 (2012)
Facesoffungi number: FoF 07311, Fig. 146
Dinemasporium morbidum (MFLU 19-2884) a Herbarium specimen b, c Appearance of black conidiomata on the host. d Squashed conidioma with setae. e Vertical section of conidioma. f Tissue details of basal stroma and excipulum. g–i Conidiophores, conidiogenous cells and developing conidia. j–k Conidia. l Germinated conidium. m–n Cultures on PDA. Scale bars b–c = 100 µm, d = 200 µm, e = 100 µm, f = 20 µm, g–l = 5 µm, m–n = 20 mm
Saprobic on dead stems of Lolium sp. Sexual morph: undetermined. Asexual morph: Conidiomata 200–300 µm diam., 150–300 µm high, dark brown to black, solitary to gregarious, initially closed, becoming wide open at maturity, cupulate, superficial, unilocular, crateriform in section view, setose. Conidiomatal wall 20–60 μm wide, composed of a basal stroma of textura angularis with thick-walled, pale brown to hyaline cells; and a well-developed excipulum of textura porrecta with dark brown to hyaline cells. Conidiomatal setae 250–500 × 7–10 μm, subulate, broad and dark brown at the base, becoming pale brown, and narrowed towards apex, unbranched, smooth-walled. Conidiophores hyaline, cylindrical to subcylindrical, branched at the base, septate, arising from the innermost cells of the basal stroma and the elements of the excipulum. Conidiogenous cells 6–10 × 1–2 µm, hyaline, phialidic, subcylindrical, discrete, rarely integrated, determinate, smooth-walled. Conidia 9–12 × 2–3 µm (\( \bar{x} \) = 11 × 2.5 µm; n = 30), hyaline, naviculate to fusiform or ellipsoid, with obtuse apex and base, unicellular, smooth-walled, guttulate, bearing a single, unbranched, tubular appendage at each end, 4–9 (\( \bar{x} \) = 6.5) µm long.
Material examined: Italy, Province of Perugia, near Niccone, on dead aerialstems of Lolium sp. (Poaceae), 6 November 2012, Erio Camporesi, IT884 (MFLU 19-2884), living culture MFLUCC 13-0401 = KUMCC 15-0622, (KUN, HKAS 101670).
Notes: Strain (MFLUCC 13-0401) shares similar morphology of conidiomata, conidiogenous cells, conidia and conidial dimension with D. morbidum [(10–)12–14(–15) × (2.5–)3 µm]. Phylogenetically, this strain shows close affinity with D. morbidum (Fig. 144). Based on both morphology and phylogeny, this strain is regarded as conspecific with D. morbidum.
Dinemasporium nelloi W.J. Li, Camporesi & K.D. Hyde, in Liu et al., Fungal Diversity: [18] (2015)
Facesoffungi number: FoF 00424, Fig. 147
Dinemasporium nelliana (MFLU 14–0811, holotype) a Specimen. b, c Appearance of black conidiomata on the host surface. d Vertical section of conidioma. e Section of peridium. f Conidiomatal seta. g–i Conidiogenous cells and developing conidia. j–n Conidia. Scale bars: b = 500 µm, c = 100 µm, d = 50 µm, e = 10 µm, f = 20 µm, g–n = 5 µm
Saprobic on dead stems of Dactylis glomerata, forming conspicuous, rounded to irregular, black, conidiomata. Sexual morph: undetermined. Asexual morph: Conidiomata 250–350 µm diam., 100–200 µm high, pycnidial, superficial, erumpent, cupulate when dry, solitary, scattered or gregarious, black, with stroma cells of textura angularis at the base and a lateral excipulum of textura porrecta or textura intricata of pale to dark brown cells. Setae divergent, stiff, brown, smooth-walled, septate, tapering towards an acute apex. Conidiophores cylindrical, septate, smooth, thick-walled, hyaline, arising from basal and periclinal wall cells. Conidiogenous cells 10–15 × 1.5–3.5 µm, phialidic, cylindrical, hyaline, smooth-walled, tapering toward the apex. Conidia 10–20 × 2.5–3.5 µm (\( \bar{x} \) = 15 × 3 µm; n = 20), blastic-phialidic, unicellular, allantoid or lenticular, guttulate, hyaline, smooth-walled, with a single unbranched setula at each end; setula 5–15 × 0.5–1.5 µm.
Material examined: Italy, Province Forlì-Cesena, Castrocaro Terme, Converselle, on dead stems of Dactylis glomerata (Poaceae), 1 December 2012, Erio Camporesi, IT934 (MFLU 14–0811, holotype), ex-type living culture, MFLUCC 13–0482, (KUN, HKAS 83970, isotype).
Dinemasporium pingue (Nag Raj) W.J. Li & K.D. Hyde, comb. nov.
≡ Stauronema pingue Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 908 (1993)
Facesoffungi number: FoF 07312, Fig. 148
Dinemasporium pingue (DAOM 215301, holotype) a–c Herbarium specimen. d–g Appearance of black conidiomata on the host. h, l Setae. i–k Vertical sections of conidiomata. m–o Conidiophores, conidiogenous cells and developing conidia. p Basal wall structure. q–s Conidia. Scale bars: d = 500 µm, e–g = 200 µm, h, l = 20 µm, i–k = 100 µm, m–o = 10 µm, p = 50 µm, q–s = 10 µm
Saprobic on decaying fragments of culms and leaf blades of an undetermined grass and on leaves of Dactylis hispanica (Poaceae). Sexual morph: undetermined. Asexual morph: Conidiomata 300–600 µm diam., 200–350 µm high, black, stromatic, cupulate, pulvinate in surface view, solitary to gregarious, superficial to semi-immersed, with a thin hypostroma, oval to rounded or irregular in outline, unilocular, setose, covered with a mass of conidia that is glutinous and pale pink when moist, but waxy yellowish brown and crustose when dry. Conidiomatal wall 10–30 μm thick, composed thick-walled, brown to pale brown cells of textura globulosa to textura angularis in the lower layers, thin-walled and paler above; excipulum composed of textura prismatica below, externally cells thick-walled, pale brown to hyaline giving rise to setae above. Conidomatal setae 88–212 ×2–8 μm, pale brown to brown, but paler towards the apex, cylindrical to subulate, septate, thick-walled, minutely rough-walled, arising from the basal stroma as well as from the excipular elements. Conidiophores lining the cavity of the conidiomata, hyaline, sometimes pale brown in the basal part, cylindrical to subcylindrical, broader at base, septate, irregularly branched, smooth-walled, invested in mucus. Conidiogenous cells 7–22 × 1–3 μm = (\( \bar{x} \) = 11 × 2 μm), hyaline, lageniform to subcylindrical, discrete or integrated, thickened at periclinal collarette zone, indeterminate, smooth-walled. Conidia 8–12 × 3–5 μm (\( \bar{x} \) = 10 × 4 μm), mostly hyaline, but sometimes pale brown at maturity, ellipsoid, with an acute apex and a narrow, truncate base. bearing unbranched, occasionally forked, narrow and attenuated, flexuous appendages at each end; apical appendage single, arising from the apex; basal appendages mostly 3, rarely 4; one centrally located; others excentric.
Material examined: India, Bangalore, Rajajinagar, on decaying fragments ofculms and leaf blades of an undetermined grass, 10 October 1970, T.R. Nag Raj (N17) (DAOM 215301, holotype).
Dinemasporium setulosa (B. Sutton) W. J. Li & K. D. Hyde, comb. nov.
≡ Diarimella setulosa B. Sutton, The Coelomycetes: 454 (1980)
Index Fungorum number: IF557166, Facesoffungi number: FoF 07313, Fig. 149
Dinemasporium setulosa (DAOM 215192) a–c Herbarium specimen. d Appearance of black conidiomata on the host. e Vertical section of conidioma. f–h Setae. i–k Conidiophores, conidiogenous cells and developing conidia (i in 10% KOH and India ink). l–o Conidia. Scale bars d = 500 µm, e = 100 µm, f–o = 10 µm
Saprobic on decaying sliver of Bambusa sp. (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 300–350 µm diam., 200–300 µm high, black, stromatic, solitary to aggregated or often confluent, semi-immersed to superficial, elongate to oval or irregular in outline, pulvinate to hysteriform in surface view, unilocular, setose. Conidiomatal wall 10–40 µm wide, composed of thick-walled, pale brown cells of textura angularis at base and sides, gradually merging with thick-walled, darker cells of textura porrecta at the periphery. Conidiomatal setae composed of two types, arising from the external textura porrecta: (a) 60–250 × 3–8 μm, dark brown to black, conspicuous, subcylindrical, unbranched, septate, tapered towards the apex, rigid, thick- and smooth-walled; (b) 20–80 × 1–6 μm, pale brown to brown, inconspicuous, subulate, straight or curved, unbranched, without septa, smooth-walled. Conidiophores formed from the innermost layers of conidiomata, hyaline, subcylindrical, straight or curved, branched, smooth-walled. Conidiogenous cells 6–10 × 1–2.5 µm, hyaline, lageniform to subcylindrical with moderate periclinal thickenings, straight or with slightly curved apices, discrete or integrated, smooth-walled. Conidia 4–6.5 × 1–3 µm (\( \bar{x} \) = 5 × 2 µm; n = 50), hyaline, allantoid to naviculate or fusiform, rounded at apex, and narrowed and truncate at base, guttulate, bearing unbranched appendages, filiform, flexuous, tubular at both ends (1–3µm long).
Material examined: India, Karnataka State, Rajajinagar, Bangalore, on decaying sliver of Bambusa sp. (Poaceae) on ground, 13 December 1970, T.R. Nag Raj (DAOM 215192).
Dinemasporium strigosum (Pers.) Sacc., Michelia 2(7): 281 (1881)
Facesoffungi number: FoF 07314, Fig. 150
Dinemasporium strigosum (Asexual morph, DAOM 215548) a Herbarium specimen. b–d Appearance of dark brown to black conidiomata on the host. e Conidioma with setae. f–g Vertical section of conidiomata. h, l Setae and surface view of excipulum. i Section of peridium. j–k Conidiophores, conidiogenous cells and developing conidia. m–p Conidia. Scale bars b–g = 200 µm, h = 50 µm, i, l = 20 µm, j–k = 10 µm, m–p = 5 µm
Saprobic on various grasses or on rabbit dung (Nag Raj 1993). Sexual morph:Ascomata 120–300 µm diam., subepidermal, on leaf sheaths and stems, immersed to semi-immersed, solitary to gregarious, globose to elongated, unilocular, glabrous, thick-walled composed of brown elongate polygonal cells. Asci 68–84 × 4–5 µm, 4–8-spored, unitunicate, cylindrical to subcylindrical, pedicellate, rounded above. Ascospores 9–16 × 3–4 µm, hyaline, uniseriate, rarely sub-biseriate, narrowly elliptical to spindle-shaped or slightly inequilateral, smooth-walled, biguttulate. Paraphyses absent (description from Webster 1955). Asexual morph: Coelomycetous. Conidiomata 300–450 µm diam., 250–300 µm high, dark brown to black, stromatic, solitary to gregarious, cupulate, superficial, unilocular, globose to crateriform in sectional view, setose with a central, white, gloeoid mass of conidia, thick-walled. Conidiomatal wall composed of two types: (a) basal stroma 20–40 μm wide, moderately developed, of textura angularis with pale brown to hyaline cells; (b) excipulum 10–25 μm wide, of textura porrecta, elements septate, moderately thick- and smooth-walled, dilute brown to hyaline cells. Conidiomatal setae 100–600 × 3–20 μm, dark brown below, brown above, subulate, erect or slightly curved, unbranched, smooth-walled, arising from the excipulum and elements of the basal stroma, often interspersed irregularly through the latter, less conspicuous and sparsely septate when arising from the excipular elements. Conidiophores hyaline, cylindrical to subcylindrical, branched, septate, arising from the innermost cells of the basal stroma and elements of the excipulum. Conidiogenous cells 6–15 × 1–2.5 µm, hyaline, subcylindrical to lageniform with marked periclinal thickenings in the collarette zone, straight or slightly curved, discrete or integrated, indeterminate, smooth-walled. Conidia 7–11 × 1.5–3 µm (\( \bar{x} \) = 8 × 2 µm; n = 30), hyaline, naviculate to fusiform or ellipsoid, obtuse or acute at apex, narrow and truncate at base, unicellular, smooth-walled, guttulate, bearing a single, unbranched, tubular appendage at each end, 5–12 (\( \bar{x} \) = 9) µm long.
Material examined: India, Bangalore, Lalbagh Gdns., on leaves of Bambusa sp., 3 November 1970, T.R. Nag Raj (DAOM 215548).
Notes: We re-examined the type specimen of Brycekendrickia indica and found an additional species on Bambusa sp.. A comparison morphology study of this species with previous known Dinemasporium species, showed that it shares similar morphology of conidiomata, conidiogenous cells and conidia with the generic type, D. strigosum [8–10(–11) × 1.5–2 µm (\( \bar{x} \) = 9 × 1.8 µm] (Nag Raj 1993).
Dinemasporium pseudodecipiens A. Hashim. & Kaz. Tanaka, in Hashimoto, Sato, Matsuda, Hirayama, Hatakeyama, Harada, Shirouzu & Tanaka, Mycoscience 56: 95 (2014)
Facesoffungi number: FoF 07315, Figs. 151, 152
Dinemasporium pseudodecipiens (MFLU 15-3635) a Herbarium specimen b, c Appearance of black conidiomata on the host d Vertical section of conidiomata e Excipular elements and seta f Tissue details of basal stroma and excipulum g Superficial cells of conidioma h Conidiomatal seta i–l Discrete conidiogenous cells and developing conidia m-q Conidia. Scale bars d = 100 µm, e–h = 20 µm, i–l = 10 µm, m–q = 5 µm
Dinemasporium pseudodecipiens (MFLU 19-2865) a, b Appearance of brown to dark brown coniodiomata on the host. c Vertical section of conidioma. d Section of peridium. e Setae. f, g Conidiogenous cells and developing conidia. h–k Conidia. Scale bars: c = 200 µm, d = 50 µm, e = 20 µm, f–g = 10 µm, h–k = 5 µm
Saprobic on dead stems or branches of Colutea arborescens and Fraxinus ornus. Sexual morph: undetermined. Asexual morph:Conidiomata 200–400 µm diam., 100–300 µm high, brown to black, superficial, solitary to gregarious, occasionally confluent, pulvinate, oval to rounded in outline, cupulate, with incurved margins, unilocular, setose. Conidiomatal setae composed of two types; (a) 150–400 × 2–14 μm, dark brown to black, conspicuous, subulate, unbranched, septate, tapered towards the apex, rigid, thick- and smooth-walled, arising from basal stroma; (b) 50–100 × 1.5–4 μm, pale brown to brown, inconspicuous, subcylindrical, with obtuse and paler apex, unbranched, smooth-walled, arising from lateral excipulum, occasionally from outer layers of the stroma. Conidiomatal wall composed of basal stroma 20–70 µm thick, of textura intricata with cells brown to pale brown, thick-walled, and well developed excipulum 10–30 µm thick, of textura porrecta with hyaline to pale brown, thick-walled cells. Conidiophores arising in the cavity of the conidiomata in a closely packed palisade, hyaline, subcylindrical, irregularly branched. Conidiogenous cells 10–20 × 2–4 µm, hyaline, discrete, subcylindrical with moderate periclinal thickenings in the collarette zone, thick-walled, smooth. Conidia 5–12 × 2–5 µm (\( \bar{x} \) = 8 × 3 µm; n = 50), hyaline, naviculate to subellipsoidal, narrowed and rounded at both ends, thick-walled, smooth, bearing 2–7 µm long, unbranched, excentric appendage at each end.
Material examined: Italy, Province of Forlì-Cesena, Predappio, San Martino, on dead aerial branches of Colutea arborescens (Fabaceae), 29 October 2015, Erio Camporesi, IT2679 (MFLU 15-3635), (KUN, HKAS 93646); ibid., Trivella di Predappio, on dead aerial branches of Fraxinus ornus (Oleaceae), 26 February 2014, Erio Camporesi, IT1743 (MFLU 19-2865).
Notes: Two fresh collections (MFLU 15-3635/IT2679), MFLU 19-2865/IT1743) show similar morphology of conidomata, conidiogenous cells and conidia with D. pseudodecipiens. However, MFLU 15-3635/IT2679 has two types of conidiomatal setae (dark brown, erect, acuminate setae and pale brown, flexuous setae) which differ from MFLU 19-2865/IT1743 and MAFF 244352 which are black, subulate. Moreover, MFLU 15-3635/IT2679 has slightly larger conidia than MFLU 19-2865 (IT1743) and MAFF 244352 (Table 5). Phylogenetically, these two collections clustered with D. pseudodecipiens (MAFF 244351, MAFF 244352) (Fig. 144). Therefore, these two collections are identified as D. pseudodecipiens, and the differences noted above may reflect intra-species variation. We conclude that D. pseudodecipiens is a complex species, and that conidiomatal setae and conidia dimensions cannot be used to distinguish Dinemasporium species.
Dinemasporium pseudostrigosum Crous, in Crous, Verkley, Christensen, Castañeda-Ruíz & Groenewald, Persoonia 28: 134 (2012)
Facesoffungi number: FoF 07316, Fig. 153
Dinemasporium pseudostrigosum (MFUL 15-0588) a Herbarium specimen. b, c Appearance of brown to dark brown conidiomata on the host. d, f Conidiomatal setae. e Vertical section of conidioma. g–h Conidiogenous cells and developing conidia. i Germinating conidium. j–l Conidia. m–n Culture on PDA. Scale bars: b = 500 µm, c = 200 µm, d = 50 µm, e = 100 µm, f, i = 20 µm, g–h = 10 um, j–l = 5 um, m–n = 20 mm
Saprobic on dead stems of Elymus farctus. Sexual morph: undetermined. Asexual morph:Conidiomata 200–300 µm diam., 200–250 µm high, brown to dark brown, superficial, solitary to gregarious, pulvinate, oval to rounded in outline, cupulate in section view, unilocular, setose. Conidiomatal setae 100–200 × 5–15 µm, subulate, brown and blunt at base, becoming paler and acute towards apex, arising from lateral excipulum and basal stroma. Conidiomatal wall 10–30 µm thick, composed basal stroma of textura angularis with thin-walled, hyaline to pale brown cells, and lateral excipulum of textura porrecta with dark brown to hyaline cells. Conidiophores hyaline, cylindrical, irregularly branched, mostly arising from inner layers of basal stroma, occasionally from lateral excipulum. Conidiogenous cells 10–20 × 1–2.5 µm, hyaline, phialidic, integrated or discrete, determinate, subcylindrical, thick and smooth-walled, with moderate periclinal thickenings in the collarette zone. Conidia 9–15 × 2–3 µm (\( \bar{x} \) = 12 × 2.5 µm; n = 30), hyaline, naviculate to fusiform, rounded at the apex, truncate at the base, unicellular, thick- and smooth-walled, guttulate, bearing 3–9 µm long, unbranched, tubular, apical appendage and excentric basal appendage.
Material examined: Italy, Province of Ravenna, Collinaccia-Castrocaro Terme, on dead aerial stems of Elymus farctus (Poaceae), 28 January 2015, Erio Camporesi, IT2352 (MFLU 15-0588), living culture MFLUCC 15-0130 = KUMCC 15-0616.
Notes: Strain (MFLUCC 15-0130/IT2352) shows similar form of conidiomata, conidiogenous cells and conidia with the type of D. pseudostrigosum, but the type has longer appendages [(11–)13–15(–17) µm] (Crous et al. 2012b). Phylogenetically, this strain clusters to D. pseudostrigosum with high bootstrap support (Fig. 144). We conclude that the length of appendage cannot be used as a defining feature at species level in Dinemasporium.
Diplocarpon F.A. Wolf, Bot. Gaz. 54: 231 (1912)
= Entomosporium Lév., Bull. Soc. bot. Fr. 3: 31 (1857) [1856]
Facesoffungi number: FoF 07317
Leotiomycetes, Leotiomycetidae, Helotiales, Drepanopezizaceae
Parasitic or saprobic on the host plant in terrestrial habitat. Sexual morph: see Wolf (1912). Asexual morph:Conidiomata yellowish brown to dark brown, stromatic, acervular, amphigenous, but mostly epiphyllous, solitary to gregarious or confluent, subcuticular in origin, oval to rounded or occasionally sunken in the middle zone or irregular in outline, rugose, dehiscing by an irregular break in the host cuticle. Conidiomatal wall mixed with host plant tissue, of a loose textura angularis, with thick-walled, pale brown to brown cells and encrusted in the lower layers, becoming hyaline toward in the upper layers. Conidiophores hyaline, branched at the base, septate, formed from the innermost layer of wall cells. Macroconidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, determinate, smooth-walled. Macroconidia hyaline, with two large vertically arranged central cells and two to three small lateral cells arising at the upper part of central cells; basal central cell short cylindrical to globose, with truncate or obtuse base, bearing minute marginal frills; apical central cell globose with obtuse apex; lateral cells mostly 2, sometimes 1–3, globose to ellipsoidal, smaller than the apical and basal central cells; apical and each of lateral cells bearing a single, tubular, septate, unbranched, attenuated, flexuous appendages. Microconidial synanamorph occasionally developing in the same or separate conidiomata. Microconidiogenous cells hyaline, annelidic, cylindrical to lageniform, broader at base, discrete or integrated, indeterminate, smooth-walled. Microconidia hyaline, narrowly ellipsoidal, truncated at base, obtuse at apex, unicellular, smooth-walled.
Type species: Diplocarpon rosae F.A. Wolf, Bot. Gaz. 54: 231 (1912)
Notes: The sexual morph of Entomosporium mespili has been reported as Diplocarpon mespili (Suton 1980). Entomosporium mespili type species of Entomosporium is associated with leaf and fruit lesions of many host plants such as Amelanchier oreophila, A. alnifolia, Aronia melanocarpa (Rosaceae), Cydonia oblonga, Crataegus, Eriobotrya japonica etc. (Sutton 1980). Accepting this connection, Johnston et al. (2014) synonymized Entomosporium under Diplocarpon. However, this connection has not been confirmed by molecular sequence data. Ekanayaka et al. (2019) placed Diplocarpon in Drepanopezizaceae. Fresh collections are needed to approve the connection between sexual and asexual morphs.
Distribution: Australia, Austria, Canada, Cyprus, France, Germany, India, Italy, Kenya, Malawi, Romania, USA, Zimbabwe (Sutton 1980; Nag Raj 1993, this study).
Diplocarpon mespili (Sorauer) B. Sutton, The Coelomycetes (Kew): 150 (1980)
= Entomosporium mespili (DC.) Sacc., Michelia 2(no. 6): 115 (1880)
Facesoffungi number: FoF 07318, Fig. 154
Diplocarpon mespili (asexual morph, FH 01142401). a, b Herbarium specimen. c, d Appearance of yellowish brown to brown conidiomata (arrows) on the host. e–g Vertical section of conidiomata. h–l Conidiophores, conidiogenous cells and developing conidia. m–p, r–u Macroconidia. q Microconidia. Conidia. Scale bars c–d = 200 µm, e–g = 100 µm, h–p, r–u = 10 µm, q = 5 µm
Parasitic on the host plant. Sexual morph: see Sutton (1980). Asexual morph:Conidiomata 100–750 µm diam., 30–100 µm high, yellowish brown to dark brown, stromatic, acervular, amphigenous, but mostly epiphyllous, solitary to gregarious or confluent, subcuticular in origin, oval to rounded or occasionally sunken in the middle zone (Fig. 154d) or irregular in outline, rugose, dehiscing by an irregular break in the host cuticle. Conidiomatal wall 10–70 µm wide, mixed with host plant tissue, of a loose textura angularis, with thick-walled, pale brown to brown cells and encrusted in the lower layers, becoming hyaline towards the upper layers. Conidiophores formed from the innermost layer of wall cells, hyaline, branched at the base, septate. Macroconidiogenous cells 7–12 × (3–)4–5 µm, hyaline, enteroblastic, phialidic, cylindrical, determinate, smooth-walled. Macroconidia hyaline, cruciform, mostly 4-celled, occasionally 3–6-celled; basal cell 6–12 × 4–7 (\( \bar{x} \) = 8.6 × 5.5 µm; n = 30), short cylindrical to globose, with truncate or obtuse base, bearing minute marginal frill; apical cell 7–15(–18) × (6.5–)7–9 (\( \bar{x} \) = 11 × 8; n = 30), globose with obtuse apex; lateral cells 3.5–7.5 × 3–5(–5.5) (\( \bar{x} \) = 5.3 × 4 µm; n = 30), mostly 2, sometimes 1–3, globose to ellipsoidal, smaller than the apical and basal cells, formed from the upper part of the basal cell; apical and each lateral cell bearing a single, tubular, unbranched, attenuated, flexuous appendage, 2–4 (\( \bar{x} \) = 53) µm long. Microconidial synanamorph occasionally developing in the same or separate conidiomata. Microconidiogenous cells 6–10 × 2–3 hyaline, annelidic, cylindrical to lageniform, broader at base, discrete or integrated, indetermined, smooth-walled. Microconidia (3–)4–6 × 1–1.5(–2) (\( \bar{x} \) = 3.5 × 1.3 µm; n = 30), hyaline, narrowly ellipsoidal, truncated at base, obtuse at apex, unicellular, smooth-walled.
Material examined: Canada, Ontario, Byron nr. London, on leaf of Amelanchier canadensis (Rosaceae), 16 August 1910, J. Dearness (FH 01142401, Kabát & Bubák - Fungi Imperfecti Ex s. #826).
Diplosporonema Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 126(4–5): 335 (1917)
Facesoffungi number: FoF 07319, Fig. 155
Diplosporonema delastrei (redrawn from Sutton 1980) a Conidiophores, conidiogenous cells and developing conidia. b Conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown, acervular, solitary, immersed to erumpent, globose to subglobose, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of apical wall. Conidiomatal wall composed of thin-walled, pale brown to hyaline cells of textura angularis. Conidiophores arising from inner cells of basal stroma, hyaline, cylindrical to subcylindrical, branched at the base and above, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, sympodial, subcylindrical, integrated or discrete, indeterminate, smooth-walled, with 1–2 conidia formed sympodially from broad, flat, unthickened, scarcely protuberant scars. Conidia hyaline, clavate, with an obtuse apex and a truncate base, 1–2-septate, smooth-walled (adapted from Sutton 1980).
Type species: Diplosporonema delastrei (Lacroix) Höhn. ex Petr., Sydowia 1(1–3): 74 (1947)
Notes: Diplosporonema delastrei is associated with host plants, such as Agrostemma githago, Silene spp., Melandrium sp. and Caryophyllaceae (Sutton 1980). The genus is monotypic, and there is no molecular data available.
Distribution: Austria, Canada, Czech Republic, Latvia, USA (Sutton 1980).
Diplozythiella Died., in Sydow, Sydow & Butler, Ann Mycol. 14(3/4): 215 (1916)
Facesoffungi number: FoF 07320, Fig. 156
Diplozythiella bambusina (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata very pale brown, pycnidial, solitary to gregarious, immersed, globose to irregular, unilocular, glabrous. Ostiole indistinct. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis. Conidiophores lining all around the cavity of conidiomata, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, discrete, determinate, with minute apical collarette and prominent channel, with periclinal thickening around the channel. Conidia hyaline, fusiform, narrowed and obtuse at the apex, truncate at the base, 1-septate, smooth-walled (Sutton 1980).
Type species: Diplozythiella bambusina Died., in Sydow, Sydow & Butler, Ann Mycol. 14(3/4): 215 (1916)
Notes: The genus remains monotypic, and there is no molecular data available. Diplozythiella bambusina is a pathogen and associated with lesions on leaves of Bambusa (Poaceae) (Sutton 1980). Diplozythiella shares similar form of conidia with Microperella. The differences between these two genera were shown under Microperella.
Distribution: India (Sutton 1980).
Discogloeum Petr., Ann Mycol. 21(3/4): 285 (1923)
Facesoffungi number: FoF 07321, Fig. 157
Discogloeum veronicarum (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Saprobic or parasitic on the host plant (Morgan-Jones 1971). Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, acervular, solitary to gregarious or confluent, immersed to erumpent, circular, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of apical host tissue. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis. Conidiophores formed from the inner cells of basal stroma, hyaline, cylindrical, branched and septate only at the base, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, cylindrical to obovoid, obtuse at the apex, truncated at the base, unicellular or faintly 1–3-septate, smooth-walled (Morgan-Jones 1977; Sutton 1980).
Type species: Discogloeum veronicarum (Ces.) Petr., Ann Mycol. 21(3/4): 285 (1923)
Notes: Discogloeum was revised by Arx (1957a, 1970) and Sutton (1980), and four species were accepted namely, D. comari (Allesch.) Arx, D. concentricum (Heald & F.A. Wolf) Morgan-Jones, D. veronicae (Lib.) Petr. and D. veronicarum (Ces.) Petr. Discogloeum veronicarum is associated with leaf lesions on Veronica tournefortii (Plantaginaceae), while the ecology of other species is unclear (Sutton 1980). No sexual morph has been linked to Discogloeum species, and no molecular data is available. To clarify the taxonomy of Discogloeum, many species will have to be recollected, and epitypified.
Distribution: Germany (Sutton 1980).
Discosporium Höhn., Z. Gärungsphysiol. 5: 196 (1915)
= Chondroplea Kleb., Phytopath. Z. 6: 291 (1933)
Facesoffungi number: FoF 07322, Fig. 158
Discosporium hyalinum (redrawn from Nag Raj and DiCosmo 1978) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and conidia
Ascomycota, genera incertae sedis
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph: Conidiomata dark brown to black, stromatic, scattered to gregarious, immersed, discoid, orbicular to irregular in outline, unilocular or plurilocular, convoluted, glabrous. Ostiole absent, dehiscence by irregular breakdown of upper tissues. Conidiomatal wall composed of thick-walled cells of textura angularis to textura intricata. Conidiophores arising from inner layer cells of locular wall, pale brown to brown, cylindrical to subcylindrical, simple or branched, septate, thick- and rough-walled for most of their length. Conidiogenous cells hyaline or occasionally subhyaline at the base, enteroblastic, annellidic, subcylindrical, integrated or discrete, indeterminate, smooth-walled, with several percurrent proliferations. Conidia hyaline, subglobose to obovoid or napiform, with a slightly truncate base, unicellular, smooth-walled, eguttulate (Nag Raj and DiCosmo 1978; Sutton 1980).
Type species: Discosporium hyalinum (Ellis) Höhn., Z. Gärungsphysiol. 5: 196 (1915)
Notes: Klebahn (1933) introduced Chondroplea Kleb., with C. populea (Sacc. & Briard) Kleb. as type species. Petrak (1957b) noted that C. populea is taxonomically identical to the generic type of Discosporium, and he reduced Chondroplea as a synonym of Discosporium, following priority. Sutton (1980) revised Discosporium and accepted three taxa namely, D. eugeniae (Allesch.) B. Sutton, D. hyalinum (= D. populeum (Sacc.) B. Sutton) and D. tremuloides (Ellis & Everh.) B. Sutton. Three species were added, D. acaciae (Sutton & Davison 1983), D. eucalypti (Sutton & Davison 1983) and D. minutum (Marincowitz et al. 2008). The sexual morph of Discosporium has been assigned to Cenangium Fr. in Leotiomycetes (Hanlin 1997), Cryptodiaporthe Petr. (now Plagiostoma Fuckel) (Sutton 1980; Mejía et al. 2011) and Diplacella Syd. in Sordariomycetes (Maharachchikumbura et al. 2015, 2016). However, none of these connections have been successfully demonstrated in culture. There is no molecular data available for the genus and therefore we retain Discosporium as the legitimate name. Future studies with fresh collections and cultures of Discosporium species are needed.
Distribution: Australia, Austria, Canada, Germany, Hungary, Kenya, Romania, South Africa, UK, USA, (Sutton 1980; Sutton and Davison 1983; Marincowitz et al. 2008).
Dothiorina Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 120: 464 (1911)
Facesoffungi number: FoF 07323, Fig. 159
Dothiorina tulasnei (redrawn from 1977a) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidiomata
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata greenish to brownish and fleshy when moist, becoming black and carbonaceous when dried, stromatic, pycnidial, sessile, scattered to gregarious or confluent, immersed to superficial or erumpent through bark, subspherical to moriform, plurilocular, with numerous irregularly oval or pyriform pycnidial locules at different levels, glabrous, ostiolate. Ostiole single to each locule, circular, centrally located. Conidiomatal wall composed of thin-walled, gelatinous anastomosing, dark brown to black cells of textura oblita in the exterior, becoming somewhat parallel, greenish to light brown cells in the inner locular wall. Conidiophores arising from the innermost layer cells of the locular wall, hyaline to subhyaline, subcylindrical to irregular, repeatedly branched, septate, with granular ornamentation towards the base. Conidiogenous cells hyaline, enteroblastic, phialidic, with long, cylindrical necks and cylindrical to slightly ampulliform venter, integrated, determinate, tapered at the apices, smooth-walled. Conidia hyaline, in chains, cylindrical to allantoid, produced by ring wall building within phialides, forming basipetal chains of up to five conidia into the necks, unicellular, smooth-walled, guttulate (Sutton 1980; Nag Raj 1977a, b; Sánchez and Bianchinotti 2007).
Type species: Dothiorina tulasnei (Sacc.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 120: 464 (1911)
Notes: von Höhnel (1911) introduced Dothiorina to accommodate a single species D. tulasnei, a fungus collected on fallen branches of Nothofagus pumilio (Nothofagaceae) from Argentina. Dothiorina is characterized by glabrous, plurilocular conidiomata with numerous irregularly pycnidial locules at different vertical levels and unusual conidiogenesis (ontogeny enteroblastic by ring wall building within phialides, forming basipetal chains of up to five conidia into the cylindrical necks) (Sánchez and Bianchinotti 2007). Two additional species have been added to the genus namely, D. discoidea (von Höhnel 1925) and D. subcarnea (Riedl 1977). Sánchez and Bianchinotti (2007) revised the genus and excluded D. discoidea, because of its distinct morphology of conidiomata and conidiogenous cells (setose, cupuliform conidiomata, phialides with periclinal thickening and solitary conidia). Dothiorina subcarnea was regarded as a doubtful taxon as the type material was lost. Thus, only the generic type is accepted in Dothiorina. The sexual morph of Dothiorina tulasnei was generally considered as Chlorociboria aeruginascens (Nyl.) Kanouse ex C.S. Ramamurthi et al. (Berthet 1964; Dixon 1975; Hanlin 1997; Nag Raj 1977a, b; Sutton 1980; Gamundí et al. 2004). According to this connection, Johnston et al. (2014) preserved Chlorociboria as generic name over the older name Dothiorina, as Chlorociboria is a well-known name. However, the connection between Chlorociboria and Dothiorina was only based on the proximity of apothecia and conidiomata on natural substratum and it has never been successfully demonstrated in culture (Sánchez and Bianchinotti 2007). Thus, we keep Dothiorina as a legitimate name, as does Kirk et al. (2013), until molecular data becomes available.
Distribution: Argentina, Austria, USA (Sanchez et al. 2007).
Drepanopeziza (Kleb.) Jaap, Verh. bot. Ver. Prov. Brandenb. 56: 79 (1914)
= Gloeosporidiella Petr., Hedwigia 62: 318 (1921)
Facesoffungi number: FoF 07351, Fig. 160
Drepanopeziza ribis (redrawn from Morgan-Jones et al. 1972c) a Macroconidia. b microconidia. c Vertical section of conidioma. d Conidiophores, conidiogenous cells and developing conidia
Leotiomycetes, Leotiomycetidae, Medeolariales, Dermateaceae
Saprobic or pathogenic on the host plant in terrestrial habitat. Sexual morph: see Rimpau (1961), Spiers and Hopcroft (1998). Asexual morph:Conidiomata brown to black, acervular, solitary, gregarious or confluent, semi-immersed to superficial, unilocular. Ostiole absent, dehiscing by irregular rupture of overlapping host tissue. Conidiomatal wall composed of thick-walled, brown to dark brown cells of textura angularis in basal layers. Conidiophores formed from the upper layer cells of the basal wall, hyaline, cylindrical or irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, monophialidic, ampulliform to subcylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, fusiform or falcate, unicellular, smooth-walled (Morgan-Jones et al. 1972c; Sutton 1980).
Type species: Drepanopeziza ribis (Rehm ex Kleb.) Höhn., Ann Mycol. 15(5): 332 (1917)
= Gloeosporidiella ribis (Lib.) Petr., Hedwigia 62: 318 (1921)
Notes: Morgan-Jones et al. (1972c) and Sutton (1980) re-described and illustrated the genus. It is characterized by acervular, conidiomata, phialidic conidiogenous cells and hyaline, fusiform or falcate, aseptate conidia. The sexual morph of Gloeosporidiella was correlated with Drepanopeziza by von Arx (1957a, b), and this connection was confirmed by Rimpau (1961). According to “one fungi = one name” principle, Rossman et al. (2016a) conserved the generic name Gloeosporidiella for the holomorph, as it has priority and is well-defined. Species of Drepanopeziza (= Gloeosporidiella) are associated with leaf lesions on Ribes (Grossulariaceae) (1957a, b; Sutton 1980).
Distribution: Italy, France, Germany, Netherlands, Portugal, Sweden, UK, USA, (Sutton 1980; Spiers and Hopcroft 1998).
Dwayalomella Brisson, Piroz. & Pauzé, Can. J. Bot. 53(23): 2867 (1975)
Facesoffungi number: FoF 07352
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, acervular or sporodochial, rounded to oval, containing numerous minute conidiomata in concentric zonations, mostly gregarious, sometimes solitary, multilocular, setose. Conidiomatal wall composed of thick-walled, pale brown cells of textura intricata to textura angularis at base, becoming thick-walled, brown cells of textura angularis to textura porrecta toward upper zone. Conidiomatal setae dark brown below, becoming brown above, marginal, subulate, erect, unbranched, 1-septate only at the base, smooth-walled, Conidiophores hyaline, cylindrical to subcylindrical, branched, septate, arising from the upper layer of cells of the basal stroma. Conidiogenous cells phialidic, hyaline, cylindrical to subcylindrical, straight or slightly curved, discrete or integrated, indeterminate, smooth-walled. Conidia hyaline, cylindrical, rounded at both ends, but with a protuberant base, 1-septate in the median part, sometimes unicellular, smooth-walled and occasionally slightly constricted at the septum, guttulate, bearing appendages at both ends; apical appendages 1–3, tubular. divergent, filiform, flexuous, arising from the apical or subapical zone of conidia, basal appendage, tubular, unbranched, filiform, flexuous.
Type species: Dwayalomella vaccinii Brisson, Piroz. & Pauzé, Can. J. Bot. 53(23): 2867 (1975)
Notes: Dwayalomella has hyaline, cylindrical conidia bearing two or three subpolar appendages at each end, and superficially resembles those of Infundibulomyces cupulatus Plaingam et al. (type species of Infundibulomyces) (Clark and Lott 1989; Nag Raj 1993; Plaingam et al. 2003). However, they can be distinguished by form of conidiogenous cells and conidiomata. Dwayalomella species possess enteroblastic, phialidic conidiogenous cells and acervular conidiomata which appear to be sporodochial in culture, with conidiomatal setae, while Infundibulomyces has holoblastic conidiogenous cells and cupulate conidiomata lacking setae. Two species are accepted in Dwayalomella, viz. Dwayalomella setulosa Carris and D. vaccinia, but no molecular sequence is available.
Distribution: Canada.
Dwayalomella vaccinii Brisson, Piroz. & Pauzé, Can. J. Bot. 53(23): 2867 (1975)
Facesoffungi number: FoF 07353, Fig. 161
Dwayalomella vaccinii (DAOM 147569, holotype) a–c Herbarium specimen. d–f Appearance of dark brown to black conidiomata on culture and stems of Vaccinium angustifolium. g–h Vertical section of conidiomata. i–k, n–o Conidiophores, conidiogenous cells and developing conidia. l Section of peridium. m, p Setae. q–u Conidia (u is in 10% KOH and India ink). Scale bars d–h = 200 µm, i–k, n–o = 10 µm, l–m, p = 20 µm, q–u = 5 µm
Isolated in culture from stems of Vaccinium angustifolium (Ericaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 200–300 µm diam., 100–250 µm high, dark brown to black, seemingly sporodochial, reportedly acervular, rounded to oval, containing numerous minute conidiomata in concentric zonations, mostly gregarious, sometimes solitary, multilocular, setose. Conidiomatal wall 40–100 μm wide, composed of thick-walled, pale brown cells of textura intricata to textura angularis at base, becoming thick-walled, brown cells of textura angularis to textura porrecta toward upper zone. Conidiomatal setae 80–150 × 2–5 μm wide, dark brown below, becoming brown above, marginal, subulate, erect, unbranched, 1-septate only at the base, smooth-walled. Conidiophores hyaline, cylindrical to subcylindrical, branched, septate, arising from the upper layer of cells of the basal stroma. Conidiogenous cells 10–20 × 1.5–3 µm, hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, straight or slightly curved, discrete or integrated, indeterminate, smooth-walled. Conidia 7–12 × 1–3 µm (\( \bar{x} \) = 9 × 2 µm; n = 30), hyaline, cylindrical, rounded at both ends, but with a protuberant base, 1-septate in the median part, sometimes unicellular, smooth-walled and occasionally slightly constricted at the septum, guttulate, bearing appendages at both ends; apical appendages 6–11 (\( \bar{x} \) = 9) µm long, 1–3, tubular. divergent, filiform, flexuous, arising from the apical or subapical zone of conidia; basal appendages 4–7 (\( \bar{x} \) = 5.6) µm long, 2–3, tubular, unbranched, filiform, flexuous.
Material examined: Canada, Quebec, Chicoutimi Co., St-Leon-de-Labrecque (48°41′, 71°31′), isolated in culture from stems of Vaccinium angustifolium (Ericaceae), 21 May 1971, J. D. Brisson & R. Brousseau (DAOM 147569, holotype).
Ebollia Minter & Caine, Trans. Br. Mycol. Soc. 74(2): 436 (1980)
Facesoffungi number: FoF 07354
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata orange-yellow or yellowish white, stromatic, pycnidial, initially immersed, ultimately erumpent, ellipsoid to elongate in outline, cylindrical to broadly conical in section view, unilocular to plurilocular, glabrous, thick-walled, lacking an ostiole but dehiscing by an irregular break in the apical wall, and then opening wide and appearing acervuloid. Conidiomatal wall composed of thick-walled, hyaline cells of textura intricata to textura angularis. Conidiophores arising in the cavity of the conidioma, hyaline, branched and broader at base, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, enteroblastic, cylindrical to subcylindrical, discrete or integrated, indeterminate, with or without percurrent proliferation. Conidia hyaline, filiform to elongated obclavate or vermiform, attenuated towards the apex, truncated at base, variously curved, multiseptate, slightly constricted at the septa, smooth-walled, guttulate, apical cell attenuated into a tubular appendage (Nag Raj and DiCosmo 1982; Nag Raj 1993).
Type species: Ebollia valdiviensis (Speg.) Minter & Caine, Trans. Br. Mycol. Soc. 74(2): 436 (1980)
Notes: Ebollia was introduced by Minter and Caine (1980) for a species, Trichocrea valdiviensis Speg. segregated from Trichocrea Marchal. The genus is characterized by orange-yellow, embedded to erumpent pycnidia, monoblastic, integrated, cylindrical conidiogenous cells and hyaline, elongated obclavate, multiseptate conidia (Minter and Caine 1980). Nag Raj (1993) added a second species, E. vermispora Nag Raj to the genus, which differs from the type species by its apical appendage. Ebollia has never been studied with molecular sequence, thus new collections of these two species are needed to confirm its placement in a natural classification.
Distribution: India, USA.
Key to species of Ebollia
1. Conidia up to 70 µm long, apical cells attenuated into a tubular appendage…E. vermispora
1. Conidia less than 70 µm long, lacking apical appendage…E. valdiviensis
Ebollia vermispora Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 342 (1993)
Facesoffungi number: FoF 07355, Fig. 162
Ebollia vermispora (DAOM 215251, holotype) a, b Herbarium package and specimen. c, d Appearance of yellowish white conidiomata on the host. e–g Vertical section of conidiomata. h Conidiomatal wall. i–l Conidiophores, conidiogenous cells and developing conidia. m–p Conidia. Scale bars e–g = 100 µm, h–j = 10 µm, k–p = 5 µm
Saprobic on dead bamboo twigs kept in moist chamber for 7 days. Sexual morph: undetermined. Asexual morph:Conidiomata 100–350 diam., 100–250 µm wide, appearing as yellowish white blisters, stromatic, pycnidial, solitary to gregarious or confluent, immersed in origin, becoming partly erumpent late, ellipsoid to elongate in outline, cylindrical to broadly conical or irregular in section view, unilocular or multilocular, locule often irregularly divided and convoluted, glabrous, thick-walled, lacking an ostiole but dehiscing by an irregular break in the apical wall. Conidiomatal wall 10–30 µm wide, composed of thick-walled, hyaline cells of textura intricata in outer layers, gradually merging with thin-walled, hyaline cells of textura angularis in innermost layers. Conidiophores arising in the cavity of the conidiomata, hyaline, branched and broader at base, septate, smooth-walled, invested in mucus. Conidiogenous cells 4–10 × 1–2 µm, hyaline, enteroblastic, subcylindrical, straight or slightly curved, sometimes narrowed at middle part, discrete or integrated, indeterminate, percurrently proliferating several times. Conidia 67–112 × 2–3 µm (\( \bar{x} \) = 91 × 2.3 µm; n = 30), hyaline, vermiform, with an obtuse base, variously curved, attenuated towards the acuminate apex, 7–12-septate, slightly constricted at the septa, smooth-walled, guttulate, with apical cell attenuated into a tubular appendage; conidia extruded in a gloeoid mass or in long cirrhi.
Material examined: India, Tamilnadu, Coonoor, Nilgiri Hills, on dead bamboo twigs kept in moist chamber for 7 days, 28 September 1980, T.R. Nag Raj (DAOM 215251, holotype).
Eleutheromyces Fuckel, Jb. nassau. Ver. Naturk. 23-24: 183 (1870) [1869-70]
Facesoffungi number: FoF 07356
Leotiomycetes, Leotiomycetidae, Phacidiales, Helicogoniaceae
Fungicolous on agarics. Sexual morph: undetermined. Asexual morph:Conidiomata yellowish brown, salmon or black, pycnidial, solitary to gregarious, deeply immersed or immersed to semi-immersed, oval or globose to subglobose, unilocular, glabrous, gelatinous, thick- and smooth-walled, papillate, ostiolate. Ostiole circular, single, centrally located. Conidiomatal wall composed of textura angularis with thick-walled cells, dark brown to paler towards the conidial hymenium. Conidiophores arising all around the cavity of the conidiomata, hyaline, often branched and broader at base, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical to lageniform, integrated with the conidiogenous loci immediately below the septa, smooth-walled. Conidia hyaline, ellipsoidal or lenticular or cylindrical, unicellular, bearing tubular, attenuated appendages at each end, appendages delimited from the conidium body by septa; basal appendage developing before the conidium body.
Type species: Eleutheromyces subulatus (Tode) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 183 (1870) [1869-70]
Notes: Fuckel (1870) proposed Eleutheromyces based on E. subulatus, a fungus reported from North America and Europe on decayed fleshy fungi (Sutton 1980; Sigler 1990; Tsuneda et al. 1997). Seeler (1943) described conidiogenesis in E. subulatus as phialidic. He also redescribed the genus Eleutheromycella (type species E. mycophila Höhn.) and differentiated it from Eleutheromyces by its darker, shorter pycnidia and longer, more slender conidia. This generic concept was endorsed by Sutton (1980). However, Nag Raj (1993) did not agree and regarded these two as congeneric, since both share similar form of conidiomata, conidiogenous cells and conidia. The difference noted by Seeler (1943) is therefore used only at the species level. Sigler (1990) reported a Hyphozyma synasexual morph for E. subulatus. Subsequently, Tsuneda et al. (1997) found that this morph causes black spot disease on Lentinula edodes (Berk.) Pegler. Crous et al. (2014a) epitypified E. subulatus with the strain CBS 113.86 (incertae sedis, Helotiales), and two cultures listed in CBS collection as E. subulatus (CBS 113.86 and CBS 458.88) were found to be phylogenetically and morphologically distinct. Crous et al. (2015d) added an additional species, E. pseudosubulatus Crous & A. Giraldo found on Lactarius scrobiculatus (Scop.) Fr., based on its narrower conidia with longer appendages. We re-examined the type species of E. mycophila and found that it shares similar morphology of conidiomata, conidiogenous cells and conidia with E. pseudosubulatus, except the condiomata are dark brown to black in E. mycophila and salmon in E. pseudosubulatus. Moreover, conidia of E. pseudosubulatus (5–6 × 1.5–2 µm) fall into the range of E. mycophila (4–13 × 1–3.5 µm). Therefore, E. pseudosubulatus is reduced to synonymy under E. mycophila, and the colour differences noted here may reflect intraspecific variation. Additional collections need to be studied to verify this hypothesis. Thus, two species are accepted in the genus, viz. E. mycophila and E. subulatus.
Distribution: Austria, Canada, Germany, France, Latvia, Sweden, UK (Nag Raj 1993; Crous et al. 2014a, 2015d, this study).
Eleutheromyces mycophilus (Höhn.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 345 (1993)
= Eleutheromycella mycophila Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 117: 1023 (1908)
= Eleutheromyces pseudosubulatus Crous & A. Giraldo, in Crous, et al., Sydowia 67: 102 (2015)
Facesoffungi number: FoF 07357, Fig. 163
Eleutheromyces mycophilus (FH 00290437, holotype). a, b Herbarium specimen. c–e Appearance of dark brown conidiomata on the host. f Ostiole. g–h Vertical section of conidiomata. i Section of peridium. j–m Conidiophores, conidiogenous cells and developing conidia. n–q Conidia. Scale bars c–e, g–h = 100 µm, f = 50 µm, i = 20 µm, j, n–o, q = 10 µm, k–m, p = 5 µm
Fungicolous on rotting basidiomata of Trametes versicolor and Lactarius scrobiculatus (Russulaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 250–500 µm diam., 200–400 µm high, dark brown to black, pycnidial, solitary to gregarious, deeply immersed or immersed to semi-immersed, oval or globose to subglobose, unilocular, glabrous, gelatinous, thick- and smooth-walled, papillate, ostiolate. Ostiole circular, single, centrally located. Conidiomatal wall 19–66 µm wide, composed of textura angularis with cells thick-walled, dark brown to paler towards the conidial hymenium. Conidiophores arising all around the cavity of the conidiomata, hyaline, often branched and broader at base, variously curved, septate, smooth-walled. Conidiogenous cells 6–10 × 2–4 µm, hyaline, enteroblastic, phialidic, subcylindrical to lageniform, with apical periclinal thickening at the collarette zone, integrated, indeterminate, smooth-walled. Conidia body 4–13 × 1–3.5 µm (\( \bar{x} \) = 6.5 × 2 µm; n = 50), hyaline, ellipsoidal or lenticular or cylindrical, unicellular, bearing tubular, attenuated appendages at each end, apical appendage 7–18 (\( \bar{x} \) = 13) µm long, basal appendage 2–5 (\( \bar{x} \) = 3) µm long.
Material examined: Austria, Wiener-Wald, Gcorgenberg, Purkersdorf, on rotting bsidiomata of Trametes versicolor (L.) Lloyd, 2 August 1907, v. Hohnel (FH 00290437, holotype).
Ellula Nag Raj, Can. J. Bot. 58(18): 2013 (1980)
Faces of fungi number: FoF 07358, Fig. 164
Ellula guaduae (redrawn from Nag Raj 1993) a Vertical section of conidioma. b Conidia with appendages. c Conidiophores with clamp connections at basal septa and conidia
Basidiomycota, genera incertae sedis
Saprobic on dead culms of Guadua sp. (Poaceae). Sexual morph: undetermined. Asexual morph: Conidiomata pearl white when moist but dirty yellow when dry, stromatic, pycnidial, linearly disposed, subepidermal in origin, erumpent, gelatinous, pulvinate or cornuted, globose to subglobose in section view, unilocular or multilocular, glabrous, ostiolate. Ostiole centrally located, with an initially narrow, but ultimately wider pore, lined by densely packed, columnar bundles of hyphae with clamp connections. Conidiomatal wall composed of thick-walled, hyaline, septate hyphae of textura intricata in the exterior, gradually merging with hyaline hyphae of textura epidermoidea toward hymenium. Conidiophores arising from inner layer cells of conidiomata, hyaline, cylindrical or irregular, unbranched or rarely branched, septate, smooth-walled, with clamp connections. Conidiogenous cells hyaline, holoblastic, cylindrical, integrated or discrete, determinate, smooth-walled, arising from near clamp connections along the length of the conidiophores. Conidia hyaline, 2-septate, with a larger ellipsoidal to subcylindrical central cell, and a shorter, terete cell on either end of the central cell, with an attenuated, unbranched, apical appendage, smooth-walled, guttulate (Nag Raj 1980, 1993).
Type species: Ellula guaduae (Viégas) Nag Raj, Can. J. Bot. 58(18): 2014 (1980)
Notes: Ellula is a monotypic genus and there is no molecular data available. The genus is characterized by pycnidia with a central pore, cylindrical conidiophores bearing clamp connections, and ellipsoidal to subcylindrical conidia with an unbranched, attenuated, flexuous appendage. Ellula is similar to Cenangiomyces, Fibulocoela and Pycnovellomyces in having cylindrical conidiophores bearing clamp connections. The differences among these genera are discussed under Fibulocoela. Fresh collections are needed to clarify the taxonomy of Ellula.
Distribution: Brazil (Nag Raj 1980, 1993).
Elongaticonidia W.J. Li, E. Camporesi & K.D. Hyde, gen. nov.
Lecanoromycetes, Ostropomycetidae, Ostropales, genera incertae sedis
Index Fungorum number: IF557147, Facesoffungi number: FoF 07359
Etymology: Referring to the conidial shape is elongated.
Saprobic on dead spines of Rosa canina. Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, solitary to gregarious, subepidermal, immersed, globose to subglobose, ostiolate. Ostiole short, centrally located. Conidiomatal wall composed of thick-walled, dark brown to brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells forming from inner layers of conidiomata, pale brown to hyaline, forming from inner wall layers of conidiomata, pale brown to hyaline, holothallic, ampulliform to cylindrical, discrete, determinate, smooth-walled, consisting of basal thicker-walled cells which become flared at the inception of conidiogenesis. Conidia hyaline, arthric, formed by disarticulation of the conidial chain, produced in simple unbranched chains with the youngest conidium at the base, elongated, 1-septate, deeply constricted at septum, bearing unbranced, cellular, flexuous, appendages.
Type species: Elongaticonidia rosae W.J. Li, E Camporesi & K.D. Hyde, sp. nov.
Notes: Phylogenetic study based on LSU sequence data shows Elongaticonidia close to Mulderomyces (Fig. 7), but it formed a seprate branch in Ostropales (Lecanoromycetes). Morpholgically, Elongaticonidia is distinct from Mulderomyces in form of conidia and conidiogenous cells. Elongaticonidia has holothallic conidiogenous cells and elongated 1-septate, deeply constricted at septum conidia bearing 4–8, unbranded, flexuous appendages. Mulderomyces has proliferating sympodial, subcylindrical conidiogenous cells and cylindrical, 2–6-septate, prominently constricted at septa, with mature conidia breaking into phragmospores (Crous et al. 2016b).
Distribution: Italy.
Elongaticonidia rosae W.J. Li, E Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557148, Facesoffungi number: FoF 07360, Fig. 165
Elongaticonidia rosae (MFLU 19-2869, holotype) a Herbarium specimen. b–d Appearance of black conidiomata on the host. e Vertical section of conidiomata. f–g Sections of peridium. h–j Conidiogenous cells and developing conidia. k–p Conidia. q Germinating conidium. Scale bars b–d = 200 µm, e = 100 µm, f–g, i–j, o, q = 10 µm, h, k–n, p = 5 µm
Etymology: Referring to the host genus from which it was collected, Rosa
Saprobic on dead spines of Rosa canina. Sexual morph: undetermined. Asexual morph:Conidiomata 100–300 µm diam., 100–250 µm high, black, pycnidial, solitary to gregarious, subepidermal, immersed, globose to subglobose, ostiolate. Ostiole short, centrally located. Conidiomatal wall 20–40 µm wide, composed of thick-walled, dark brown to brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells forming from inner wall layers of conidiomata, pale brown to hyaline, holothallic, ampulliform to cylindrical, discrete, determinate, smooth-walled, consisting of basal thicker-walled cells which become flared at the inception of conidiogenesis. Conidia 12.9–17 × 2.2–5.5 µm (\( \bar{x} \) = 14.7 × 4 µm; n = 30), hyaline, arthric, formed by disarticulation of the conidial chain, produced in simple unbranched chains with the youngest conidium at the base, elongated, 1-septate, deeply constricted at septum, rounded at both ends, bearing 4–8, mostly 7, unbranced, cellular, flexuous, appendages distributed uniformly all over.
Material examined: Italy, Province of Forlì-Cesena, Santa Sofia, Campigna, on dead aerial spines of Rosa canina (Rosaceae), 2 October 2014, Erio Camporesi, IT2146 (MFLU 19-2869, holotype).
Notes: Germinating conidia stop to growing, thus the sequence of Elongaticonidia rosae was obtained via direct sequence from fruiting bodies.
Eriospora Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 5: 455 (1850)
Facesoffungi number: FoF 07361, Fig. 166
Eriospora leucostoma (redrawn from Sutton 1980) a Conidia. b, c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, solitary, subepidermal, immersed, globose, unilocular, occasionally convoluted, glabrous, ostiolate. Ostiole single, circular, papillate, centrally located. Conidiomatal wall composed of hyaline cells of loose textura intricata in the base, appearing thicker-walled, pale to dark brown cells in the lateral and upper part. Conidiophores repeatedly branched to form an apparently reticulate layer lining the whole pycnidial wall, hyaline, irregular, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, polyblastic, sympodial, subcylindrical or irregular, integrated, indeterminate, smooth-walled, with several small conidiogenous loci towards the apices. Conidia hyaline, filiform, straight or curved, up to 8-septate, smooth-walled, euguttulate (Sutton 1980).
Type species: Eriospora leucostoma Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 5: 455 (1850)
Notes: Eriospora was re-described by Petch (1943) and Sutton (1980). More than 20 epithets are listed in Index Fungorum (2019), but most of them have not been restudied. Wijayawardene et al. (2017b) accepted only the type species in the genus. Further studies with fresh collections and molecular data are needed to redefine this genus.
Distribution: UK (Sutton 1980).
Eriosporella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 109 (1916)
Facesoffungi number: FoF 07362
Ascomycota, genera incertae sedis
Saprobic on leaves of Calamus sp. (Arecaceae). Sexual morph: see (Nag Raj 1993). Asexual morph:Conidiomata straw-coloured, acervular, amphigenous but predominantly epiphyllous, solitary to gregarious, rounded to oval or square to irregular in outline, unilocular, thick-walled, glabrous. Conidiomatal wall composed of thin-walled, hyaline cells of textura angularis mixed with host cells. Conidiophores arising all around the cavity of the conidiomata, hyaline, septate, branched or unbranched, smooth-walled, invested in a thin layer of mucus. Conidiogenous cells hyaline, narrowly conical, ampulliform or lageniform, discrete or integrated, smooth-walled (Nag Raj 1993). Conidia hyaline, tetraradiate, main axis cylindrical to obconical, with a truncate base bearing minute marginal frills, aseptate or 1-septate, smooth-walled; 3-armed, with arms inserted at the apex of the main axis and separated from it by septa, divergent, broader at base and attenuated towards the apex, flexuous, unequal, septate, guttulate, smooth-walled; central arm 5–7-septate other arms 2–4-septate.
Type species: Eriosporella calami (Niessl) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 109 (1916)
Notes: Eriosporella is characterized by acervular, immersed conidiomata and hyaline, tetraradiate conidia composed of a basal main axis bearing single appendage and three apical, flexuous, unequal, septate arms. Although Sutton (1980) and Van der Aa and Van Oorschot (1985) regarded the conidiogenous cells as phialides, Nag Raj and DiCosmo 1981a) and Nag Raj (1993) described them as annellides with up to three annellations. We re-examined the type specimen but it is in poor condition and we could not confirm if it is phiallidic or annellidic.
Dai et al. (2014) described a second species E. bambusicola D.Q. Dai, et al. on the basis of its tetraradiate conidia. However, they did not pay much attention to other characters of the conidiomata and conidiogenous cells. This species is, in fact, a hyphomycetes with sporodochial fruiting bodies distinct from the acervular, immersed conidiomata in Eriosporella. In addition, E. bambusicola has sympodial, monoblastic, rarely polyblastic conidiogenous cells. Eriosporella bambusicola matches Tricornispora bambusae Bonar (type species of Tricornispora) (Van der AA and Van Oorschot 1985). The conidia of E. bambusicola are 4.5–8.5 × 2.5–5 µm (main axis) and 42.5–60 × 3–5 µm (arms), which is similar to T. bambusae 9–12 × 4–4.5 (main axis) and (15–) 40–75 × 3–4.5 µm (arms). Therefore, Eriosporella bambusicola is regarded as a synonym of Tricornispora bambusae. Eriosporella therefore remains monotypic.
Distribution: Austria, India, Japan (Nag raj 1993, this study).
Eriosporella calami (Niessl) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 109 (1916)
Facesoffungi number: FoF 07363, Fig. 167
Saprobic on leaves of Calamus sp. (Arecaceae). Sexual morph: see (Nag Raj 1993). Asexual morph:Conidiomata 550–700 µm diam., 70–200 µm high, straw-coloured, acervular, amphigenous but predominantly epiphyllous, solitary to gregarious, rounded to oval or square to irregular in outline, unilocular, thick-walled, glabrous. Conidiomatal wall 10–30 μm wide, composed of thin-walled, hyaline cells of textura angularis mixed with host cells. Conidiophores arising all around the cavity of the conidiomata, hyaline, septate, branched or unbranched, smooth-walled, invested in a thin layer of mucus. Conidiogenous cells hyaline, narrowly conical, ampulliform or lageniform, discrete or integrated, smooth-walled. Conidia hyaline, tetraradiate; main axis 6–10 × 1.5–3 µm (\( \bar{x} \) = 8.5 × 2 µm; n = 30), cylindrical to obconical, with a truncate base bearing minute marginal frills, aseptate or 1-septate, smooth-walled, most often drawn out into a narrow, tubular appendage up to 3 μm long; arms three, 32–79 × 1–2.4 µm (\( \bar{x} \) = 55 × 1.8 µm; n = 30), inserted at the apex of the main axis and separated from it by septa, divergent, broader at base and attenuated towards the apex, flexuous, unequal, septate, guttulate, smooth-walled; central arm 5–7-septate and the other arms 2–4-septate.
Material examined: India, Calcutta, on leaves of Calamus sp. (Arecaceae), S. Kurz (DAOM 745784, lectoisotype).
Erythrogloeum Petr., Sydowia 7(5–6): 378 (1953)
Facesoffungi number: FoF 07364, Fig. 168
Erythrogloeum hymenaeae (redrawn from Sutton 1980) a Conidiogenous cells and developing conidia. b Conidia
Sordariomycetes, Sordariomycetidae, Diaporthales, Erythrogloeaceae
Parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata brown, stromatic, acervular, solitary, subepidermal, immersed to erumpent, subglobose, unilocular, glabrous. Ostiole absent, rupturing by means of irregular splits. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis in the base, appearing dark brown to black at the side. Conidiophores formed from the inner cells of conidiomata, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, lageniform to cylindrical, discrete, determinate, smooth-walled, with periclinal thickenings at channel wall. Conidia hyaline, ellipsoid to ovoid, rounded at the apex, narrow and truncated at the base, unicellular, smooth-walled, guttulate or aguttulate (Sutton 1980; Crous et al. 2012a).
Type species: Erythrogloeum hymenaeae Gonz. Frag. & Cif. ex Petr., Sydowia 7(5–6): 379 (1953)
Notes: Two species were accepted in Erythrogloeum, namely E. hymenaeae (generic type) and E. pini-acicola H.C. Evans (Sutton 1980; Evans 1984), of which E. hymenaeae is a well-known pathogen of Hymenaea spp. in South America (Petrak 1953; Ferreira et al. 1992). The phylogenetic position of Erythrogloeum is rather obscure although Crous et al. (2012a) designated an epitype for E. hymenaeae, and placed Erythrogloeum in Diaporthales based on ITS and LSU sequence data. The sexual morph of Erythrogloeum is undetermined.
Distribution: Brazil, Costa Rica, Dominican Republic, Nicaragua (Sutton 1980; Crous et al. 2012a).
Eutiarosporella Crous, Phytotaxa 202(2): 85 (2015)
Facesoffungi number: FoF 07365
Dothideomycetes, Incertae sedis, Botryosphaeriales, Botryosphaeriaceae
Saprobic or parasitic on the host plant in terrestrial habitat, such as Celtis africana (Cannabaceae), Dactylis glomerata (Poaceae), Triticum aestivum (Poaceae), and Vachellia karroo (Fabaceae) (Dissanayake et al. 2016). Sexual morph: see Li et al. (2016). Asexual morph:Conidiomata pale brown to brown, stromatic, pycnidial, mostly epiphyllous, solitary to gregarious or often confluent, initially immersed, then becoming partly erumpent, globose to subglobose, unilocular or multilocular, glabrous, ostiolate. Ostiole single, funneled to flabellate, centrally located. Conidiomatal wall composed of pale brown to brown, thick-walled cells of textura angularis in the outer layers, and hyaline, thin-walled cells of textura prismatica in the inner layers. Conidiophores arising all around the cavity of the conidiomata, hyaline, branched, septate, invested in thin, non-persistent mucus. Conidiogenous cells enteroblastic, hyaline, subcylindrical to obclavate, discrete or integrated, smooth. Conidia hyaline, navicular with an obtuse apex and a narrow truncate base, aseptate, slightly curved, aguttulate, smooth-walled, bearing hyaline, branched, filiform, flexuous appendage at both ends.
Type species: Eutiarosporella tritici (B. Sutton & Marasas) Crous, Phytotaxa 202(2): 85 (2015)
Notes: Sutton and Marasas (1976) described Tiarosporella tritici based on its large conidia and pulvinate conidiomata. Crous et al. (2015b) showed that T. africana, T. tritici, and T. ubis-rosarum clustered apart from Tiarosporela sensu stricto and formed a well-supported clade in Botryosphaeriaceae. In accordance with its distinct phylogeny, they introduced a new genus Eutiasporella to accommodate these three species. Li et al. (2016) added E. dactylidis with sexual morph in Eutiarosporella. We re-examined the holotype of E. tritici and found that the conidia also bear a basal appendage which is evanesced at maturity. We provide additional morphological details of conidia and conidiomatal structure, which were not available previously (Sutton 1980; Nag Raj 1993; Crous et al. 2015b). Seven species, i. e. T. africana, E. dactylidis, E. darliae, T. tritici, E. tritici-australis, T. ubis-rosarum, and E. pseudodarliae, are accepted in Eutiarosporella (Crous et al. 2015b; Thynne et al. 2015; Dissanayake et al. 2016).
Distribution: Australia, Italy, South Africa (Crous et al. 2015b; Thynne et al. 2015; Li et al. 2016).
Eutiarosporella tritici (B. Sutton & Marasas) Crous, Phytotaxa 202(2): 85 (2015)
≡ Tiarosporella tritici B. Sutton & Marasas, Trans. Br. Mycol. Soc. 67(1): 74 (1976)
Facesoffungi number: FoF 07366, Fig. 169
Eutiarosporella tritici (PREM 44966, holotype) a, b Herbarium specimen. c, d Appearance of brown conidiomata on the host. e, f Vertical section of conidiomata (e in 10% KOH, f in lactic acid). g upper locule. h Section of peridium. i Mycelium on the surface of conidioma. j–n Conidiogenous cells and developing conidia. o–t Conidia. Scale bars c = 1000 µm, d = 500 µm, e–f = 200 µm, g = 100 µm, h–i = 50 µm, j–t = 10 µm
Sexual morph: undetermined. Asexual morph:Conidiomata 200–900 µm diam., 170–850 µm high, brown to dark brown or black, pycnidial, single or up to 5 aggregated in large, pulvinate, botryose, superficial or partly immersed at basal stroma, subglobose, cylindrical to subcylindrical, or irregular in shape, slightly swollen at the base or level of the locule, narrow towards the apex, unilocular, covered by hyphae, with cylindrical, straight or slightly curved neck, ostiolate. Ostiole 130–350 × 70–140 µm, single, cylindrical to subcylindrical, centrally located. Hyphae hyaline to pale brown, septate, cylindrical, simple, straight or flexuous, smooth or verruculose, arising from upper layers of conidiomata. Conidiomatal wall 50–80 µm wide, composed of dark brown to pale brown, thick-walled cells of textura angularis in the outer layers, and hyaline, thick-walled cells of textura prismatica in the inner layers. Conidiophores formed lining the innermost layers of conidiomata, reduced to conidiogenous cells or distinct, when present, hyaline, dolliform, unbranched, septate, smooth-walled. Conidiogenous cells 10–20 × 3–5 µm, hyaline, enteroblastic, phialidic, subcylindrical, occasionally broad at base, straight or slightly curved, thick- and smooth-walled. Conidia 24–35 × 6.5–15 µm (\( \bar{x} \) = 30 × 11 µm; n = 30) (excluding the sheath), hyaline, fusiform, broad at middle part, unicellular, mostly straight, thick-walled, surrounded by a 1–3 µm wide sheath except both ends, bearing hyaline, flame-like, or circular sector-like mucoid appendage only at apex or both ends (10–35 × 8–22 µm).
Material examined: South Africa, Orange Free State, Heilbron, on Triticum aestivum (Poaceae), 18 January 1973, W.F.O. Marasas (PREM 44966, holotype).
Fibulocoela Nag Raj, Can. J. Bot. 56(13): 1491 (1978)
Facesoffungi number: FoF 07367
Basidiomycota, Agaricomycetes, genenra incertae sedis
Foliicolous. Sexual morph: undetermined. Asexual morph:Conidiomata stromatic, pycnidial, solitary to gregarious or confluent, subepidermal, deeply immersed in origin, later yellowish brown conidial mass visible in surface view, globose to subglobose in section view, multilocular, gelatinous, glabrous, thick-walled, ostiolate. Ostiole single, oval to cylindrical, centrally located. Conidiomatal wall composed of an outer, hyaline, loose, clamp-bearing hyphae, and an inner densely packed textura intricata to textura epidermoidea. Conidiophores arising from the innermost wall layers of conidiomata, hyaline, branched or unbranched, septate with a clamp connection at each septum, smooth-walled, invested in a thin, non-persistent layer of mucus. Conidiogenous cells hyaline, cylindrical to subcylindrical, terminal, but also lateral when arising from the clamp connections along the length of the conidiophores, smooth-walled. Conidia hyaline, cylindrical to subcylindrical, with rounded apex and slightly truncate base, straight or slightly curved, aseptate, smooth-walled, bearing a single, unbranched, attenuated, tubular, flexuous apical appendage; conidia extruded from conidiomata in long cirrhi.
Type species: Fibulocoela indica Nag Raj, Can. J. Bot. 56(13): 1491 (1978)
Notes: The genus Fibulocoela was proposed by Nag Raj (1978) with F. indica as type species. The genus is characterized by pycnidial conidiomata, branched conidiophores with a clamp connection at each septum and cylindrical to subcylindrical conidia bearing a single apical appendage. Clamp connections in the coelomycetous asexual morph indicate its basidiomycetous affinity. Based on this structure, Nag Raj (1993) designated four coelomycetous genera Cenangiomyces Dyko & B. Sutton, Ellula Nag Raj, Fibulocoela and Pycnovellomyces R.F. Castañeda as Basidiomycota, No teleomorph has been linked to these genera and no sequence data are available. Fibulocoela can be easily distinguished from the other basidiomycetous genera by its conidiomata and conidia. Cenangiomyces has pulvinate to cornute conidiomata with a shallow-cupulate head bearing the conidial hymenium, and cylindrical conidia with tubular appendage at each end (Dyko and Sutton 1980). Ellula has subcylindrical or ellipsoid conidia with pairs of closely spaced eusepta and apical and basal appendages (Nag Raj 1980). Pycnovellomyces has unique conidiomatal features (pycnidial, sessile, more or less globose, with irregularly branched fascicles of intertwined asperate hyphae) (Nag Raj et al. 1989). Fibulocoela is monotypic. To fix the genera concept of Fibulocoela, fresh collections of F. indica will have to be epitypified.
Distribution: Cuba.
Fibulocoela indica Nag Raj, Can. J. Bot. 56(13): 1491 (1978)
Facesoffungi number: FoF 07368, Fig. 170
Fibulocoela indica (DAOM 215274) a, b Herbarium package and specimen. c Appearance of yellowish white conidial mass on the host. d, e Vertical section of conidiomata. f Conidiomatal wall. g–j Conidiophores, conidiogenous cells and developing conidia (arrows indicate clamp connections). k–n Conidia. Scale bars c = 200 µm, d–e = 100 µm, f = 10 µm, g = 50 µm, h–n = 5 µm
Foliicolous. Sexual morph: undetermined. Asexual morph:Conidiomata 120–300 diam., 100–240 µm high, stromatic, pycnidial, solitary to gregarious or confluent, subepidermal, deeply immersed in origin, later yellowish white conidial mass visible in surface view, globose to subglobose in section view, multilocular, gelatinous, glabrous, thick-walled, ostiolate. Ostiole single, oval to cylindrical, centrally located. Conidiomatal wall 10–15 µm wide, composed of an outer, hyaline, loose network of clamp-bearing hyphae, and an inner densely packed textura intricata to textura epidermoidea. Conidiophores arising from the innermost wall layers of conidiomata, hyaline, cylindrical to subcylindrical, sometimes broader at top, forming oval to rounded structure (Fig. 170i, j), branched or unbranched, septate with a clamp connection at each septum, smooth-walled, invested in a thin, non-persistent layer of mucus. Conidiogenous cells holoblastic, sympodial, hyaline, cylindrical to subcylindrical, terminal, later becoming lateral when arising from the clamp connections along the length of the conidiophores, smooth-walled. Conidia 13–19 × 1–3 µm (\( \bar{x} \) = 16 × 2 µm; n = 30), hyaline, cylindrical to subcylindrical, with rounded apex and slightly truncate base, straight or slightly curved, aseptate, smooth-walled, bearing a single, unbranched, attenuated, tubular, flexuous apical appendage (9–17 µm long); conidia extruded from conidiomata in long cirrhi.
Material examined: Cuba, Cienaga de Zapata, on decaying leaves of Bucida palustris Borhidi & O.Muñiz (Combretaceae), 29 April 1988, R. F. Castafieda C 88/166 (ex INIFAT #1038) (DAOM 215274).
Fuckelia Bonord., Abh. naturforsch. Ges. Halle 8: 135 (1864)
Facesoffungi number: FoF 07401, Fig. 171
Fuckelia ribis (redrawn from Sutton 1980) a Conidia. b Conidiophores and conidiogenous cells. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Habit on the host plant, Ribes sp., R. rubrum (Grossulariaceae). Sexual morph: putative sexual morph Godronia ribis (Groves 1965). Asexual morph:Conidiomata grey to grey brown, sometimes almost concolorous, eustromatic, pycnidial, with or without a basal, central foot, separate or caespitose, peridermal to subperidermal, superficial, pulvinate or verruciform to discoid, multilocular, convoluted, glabrous, locules developing in the upper part, ovoid to irregular, unequal in size. Ostiole absent, rupturing surface by means of irregular splits of upper walls of locules. Conidiomatal wall composed of thick-walled, hyaline to yellowish or brownish, interwoven hyphae of textura epidermoidea at the basal part, becoming thick-walled, dark brown cells of textura angularis towards upper part in the exterior, gradually merging with thin-walled, pale brown cells of textura angularis or intricata in the interlocular wall. Conidiophores formed from the inner cells of locular wall, hyaline, cylindrical or irregular, branched irregularly above and below, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, lageniform to cylindrical, integrated, less often discrete, determinate, smooth-walled, with apical or lateral apertures on small branches immediately below septa, periclinal thickenings at channel wall. Conidia hyaline, ellipsoid, rounded at the apex, narrow and truncate at the base, 1-septate, smooth-walled, guttulate (adapted from Groves 1965; Sutton 1980).
Type species: Fuckelia ribis (Fr.) Bonord., Abh. naturforsch. Ges. Halle 8: 135 (1864)
Notes: The type species Fuckelia ribis has been generally considered as asexual morph of Godronia ribis (Fr.) Seaver (Cash 1934; Seaver 1945; Groves 1965). Accordingly, Johnston et al. (2014) recommended the use of Godronia over Fuckelia for the holomorph. Chondropodiella and Topospora were also treated as taxonomically congruent with Godronia by Johnston et al. (2014). However, there is considerable difference in conidiomata, conidiogenous cells and the conidia among these three genera. Chondropodiella has cylindrical-subulate, unilocular, beaked conidiomata, holothallic conidiogenous cells and arthric, cylindrical to ellipsoid, aseptate conidia. Topospora has globose to oval or obpyriform, unilocular conidiomata, phialidic conidiogenous cells and cylindrical to fusiform, 3-septate conidia. Fuckelia has multilocular, convoluted conidomata with or without basal central foot, phialidic conidiogenous cells and ellipsoid, 1-septate conidia. The connection between F. ribis and Godronia ribis relies on constant association of both morphs but has not been confirmed by culture studies or other evidence. Therefore, Fuckelia should be treated as a legitimate name as suggested by Kirk (2013), until molecular evidence is available.
Distribution: Austria, UK (Sutton 1980).
Furcaspora Bonar, Mycologia 57(3): 391 (1965)
Facesoffungi number: FoF 07402, Fig. 172
Furcaspora pinicola (redrawn from Nag Raj 1974) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidioma
Leotiomycetes, Leotiomycetidae, order incertae sedis
Saprobic or parasitic on the host plant in terrestrial habitat, such as Abies concolor (Pinaceae), Eucalyptus globulus (Myrtaceae), Pinus monophylla (Pinaceae). Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, stromatic, pycnidial, immersed to innate erumpent, globose to subglobose, unilocular, glabrous. Ostiole absent, rupturing surface by means of irregular splits of upper walls, and ultimately becoming wide open. Conidiomatal wall composed of thick-walled, pale brown cells of textura angularis in the exterior, gradually merging with paler coloured cells towards hymenium. Conidiophores formed from the inner layer cells of conidiomatal wall, hyaline, cylindrical or irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, sympodial, subcylindrical, integrated or discrete, determinate, smooth-walled, producing 1–3 conidia sympodially or synchronously from different loci at the apices. Conidia hyaline, triradiate, aseptate, smooth-walled; main axis cylindrical, with fusiform arms; each arm with a terminal extracellular filiform, tubular appendage.
Type species: Furcaspora pinicola Bonar, Mycologia 57(3): 391 (1965)
Notes: Furcaspora shares hyaline, triradiate conidia with Suttoniella, but it can be distinguished from the latter by the form of conidiomata and conidiogenous cells. The differences between these two genera are discussed under Suttoniella. Nag Raj 1993) revised Furcaspora and accepted a single species in the genus. Crous et al. (2007) included molecular data for Furcaspora and introduced a new species, F. eucalypti Crous & Verkley. Furcaspora was placed in Leotiomycetes, order incertae sedis. Fresh collection of F. pinicola are needed to place Furcaspora in a natural group.
Distribution: Australia, USA (Nag Raj 1993; Crous et al. 2007).
Gampsonema Nag Raj, Can. J. Bot. 53(16): 1621 (1975)
Facesoffungi number: FoF 07403, Fig. 173
Gampsonema exile (redrawn from Nag Raj 1993) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and developing conidia. d Conidiomatal wall with tissue details and conidiophores
Ascomycota, genera incertae sedis
Saprobic on dead bark of Eucalyptus eximia and dead leaves of E. grandis, E. paniculata, E. robusta, E. saligna and Eucalyptus sp. (Myrtaceae) (Nag Raj 1993). Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, scattered, immersed to innate erumpent, globose, unilocular, glabrous, ostiolate. Ostiole single, circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the exterior, becoming thin-walled, brown to pale brown cells of textura prismatica in the inner part. Conidiophores formed from the inner wall layer of conidiomata, hyaline, cylindrical or irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, sympodial, ampulliform or cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, naviculate or narrow-fusiform, rounded at the apex and blunt at the base, slightly curved, 2-septate, smooth-walled; with 2 apical appendages; appendages filiform, unbranched, reflexed, more or less lateral, delimited from conidia by septa (adapted from Nag Raj 1993).
Type species: Gampsonema exile (Tassi) Nag Raj, Can. J. Bot. 53(16): 1623 (1975)
Notes: Gampsonema is monotypic and no molecular data is available. This genus can be confused with Hyalotiella, as they have pycnidial conidiomata, holoblastic, sympodial conidiogenous cells and septate conidia with apical appendages. Gampsonema has hyaline, naviculate or narrow-fusiform, 2-septate, conidia bearing 2 unbranched, apical appendages, whereas Hyalotiella has pale brown except for the hyaline apical cell, 3–4-septate, unequal cells conidia bearing branches 2–4, mostly 3, divergent, apical appendages (Nag Raj 1993; Li et al. 2015b). This genus need to be revised based on fresh collections and molecular data.
Distribution: Australia, Brazil, India, USA (Sutton 1980; Nag Raj 1993).
Geastrumia Bat., in Batista, Farr & Bezerra, Saccardoa 1: 71 (1960)
Facesoffungi number: FoF 07406, Fig. 174
Dothideomycetes, Dothideomycetidae, Capnodiales, Mycosphaerellaceae
Saprobic or parasitic on the host plant. Mycelium superficial, composed of brown to pale brown, branched, septate hyphae (Pirozynski 1971; Sutton 1980). Sexual morph: undetermined. Asexual morph: Conidiomata dark brown to black, pycnidial, discrete or irregularly scattered, superficial, sessile, at first closed and globose, later opening widely and become cupulate, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of the apical wall. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis in the basal part, becoming one cell thick, dark brown cells of textura globulosa at the lateral and upper part. Conidiophores formed from the repent hyphae at the base and at the point of their transformation into the covering wall, hyaline, branched and septate at the base, tapered towards the apex, smooth-walled. Conidiogenous cells hyaline, holoblastic, narrowly ampulliform to cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, staurosporous, composite, consisting of 6–14 arms, fusiform, straight or slightly curved, up to 15-eptate invested in mucus, and remaining attached to a common branched, long-pedicellate basal cell even after liberation (Nag Raj and DiCosmo 1978).
Type species: Geastrumia polystigmatis Bat. & M.L. Farr, in Batista, Farr & Bezerra, Saccardoa 1: 71 (1960)
Notes: Geastrumia polystigmatis was collected from living leaves of Andira jamaicensis (Fabaceae) in the Dominican Republic (Pirozynski 1971). The genus is characterized by pycnidial, superficial conidiomata, holoblastic conidiogenous cells and staurosporous conidia with 4–16 arms, fusiform arms. Santos (2011) described two unnamed taxa Geastrumia sp. 1 and Geastrumia sp. 2 from leaves of Salacia crassifolia (Celastraceae) in Brazil. Geastrumia sp. 2 differs from G. polystigmatis and Geastrumia sp. 1 by its larger conidiomata and brown conidia. Geastrumia sp. 1 has shorter conidial arms than the other taxa. Ismail et al. (2016) included molecular data for G. polystigmatis and showed that is related to Mycosphaerellaceae. Fresh collections of the unnamed taxa are needed to confirm generic placement.
Distribution: Brazil, Dominican Republic, Thailand (Pirozynski 1971; Santos 2011).
Giulia Tassi, Bulletin Labor. Orto Bot. de R. Univ. Siena 6: 92 (1904)
Facesoffungi number: FoF 07404
Agaricomycetes, Incertae sedis, Corticiales, Corticiaceae
Saprobic on dead stems of Lepidium graminifolium (Brassicaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious, subepidermal in origin, deeply immersed, ellipsoid, with a globose to subglobose venter and a lateral, elongated, curved neck, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, subcylindrical, laterally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis to textura primatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, phialidic, ampulliform to conical, smooth-walled. Conidia hyaline, cylindrical, rounded at apex, blunt and truncate at base, straight or slightly curved, aseptate, smooth-walled, bearing 3–7 flexuous, unbranched, tubular, apical appendages.
Type species: Giulia tenuis (Sacc.) Tassi ex Sacc. & D. Sacc., Syll. fung. 18: 435 (1906)
Notes: Tassi (1904) proposed a new genus Nematospora Tassi for a segregated species from Leptostroma Sacc. (as N. tenuis). Nematospora Tassi is a homonym of Nematospora Peglion (Peglion 1901). Thus, a new name Giulia Tassi (1904) was proposed but, Tassi did not explicitly transfer N. tenuis into this genus, an omission that was corrected by Saccardo and Saccardo (1906). Pirozynski and Shoemaker (1971) re-described the type species of Giulia and transferred the genus from Leptostromataceae to Phomaceae. Rungjindamai et al. (2008) included a sequence of G. tenuis, which was isolated from the sheath of Bambusa arundinacea Retz. (Poaceae) from Thailand, in a molecular analysis and showed it clustered with the Corticiaceae (Corticiales, Basidiomycota). The genus is monotypic.
Distribution: Italy, Thailand (Rungjindamai et al. 2008, this study).
Giulia tenuis (Sacc.) Tassi ex Sacc. & D. Sacc., Syll. fung. 18: 435 (1906)
Facesoffungi number: FoF 07405, Fig. 175
Giulia tenuis (DAOM 130457, ex type slides, h redrawn from Morgan-Jones 1974) a–c Herbarium package and slides. d–e Vertical section of conidiomata. f Conidiomatal wall. g–h Conidiophores, conidiogenous cells and developing conidia. i–m Conidia. Scale bars d–e = 100 µm, f = 10 µm, h–m = 10 µm
Saprobic on dead stems of Lepidium graminifolium. Sexual morph: undetermined. Asexual morph:Conidiomata 150–250 diam., 150–200 µm high, dark brown to black, pycnidial, solitary to gregarious, subepidermal in origin, deeply immersed, ellipsoid, with a globose to subglobose venter and a lateral, elongated, curved neck, unilocular, glabrous, thick-walled, ostiolate. Ostiole 100–140 × 30–70 µm, single, subcylindrical, laterally located. Conidiomatal wall 20–40 µm wide, composed of thick-walled, dark brown to pale brown cells of textura angularis in the outer layers, and thick-walled, hyaline cells of textura primatica in the inner layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, phialidic, ampulliform to conical, smooth-walled. Conidia 11–24 × 4–8 µm (\( \bar{x} \) = 18 × 5 µm; n = 30), hyaline, cylindrical, rounded at apex, blunt and truncate at base, straight or slightly curved, aseptate, smooth-walled, bearing 3–7 flexuous, unbranched, tubular, apical appendage (10–20 µm long).
Material examined: Italy, Padua, on dead stems of Lepidium graminifolium (Brassicaceae), September 1879, P.A. Saccardo (DAOM 130457, ex-type slides).
Gloeosporidina Petr., Ann Mycol. 19(3–4): 214 (1921)
Facesoffungi number: FoF 04286, Fig. 176
Gloeosporidina moravica (redrawn from Morgan-Jones et al. 1972e) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and developing conidia
Sordariomycetes, Diaporthomycetidae, Diaporthales, Gnomoniaceae
Saprobic or parasitic on host plant in terrestrial habitats, such as Cercocarpus betuloides (Rosaceae), Cryptomeria japonica (Cupressaceae), Lasiopetalum macrophyllum (Malvaceae), Sargassum natans (Sargassaceae), Quercus robur (Fagaceae). Sexual morph: see Senanayake et al. (2018). Asexual morph:Conidiomata brown to black, acervular, solitary to gregarious, subepidermal to epidermal, immersed, subglobose in section view, unilocular, glabrous. Ostiole absent, dehiscence irregular rupture of the upper overlapping host tissue. Conidiomatal wall composed of hyaline to subhyaline cells of textura angularis in the basal part. Conidiophores arising from the innermost layer cells of the basal stroma, hyaline to pale brown, cylindrical to subcylindrical, sparsely branched, septate, smooth-walled. Conidiogenous cells hyaline to pale brown, enteroblastic, monophialidic, cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, subglobose to elliptical or pyriform, obtuse at the apex, narrow and truncate at the base, unicellular, smooth-walled, guttulate (Sutton 1980).
Type species: Gloeosporidina moravica Petr., Ann Mycol. 19(3–4): 214 (1921)
Notes: Gloeosporidina was re-described and re-illustrated by Sutton and Pollack (1973), Sutton (1980), Morgan-Jones et al. (1972e) and Senanayake et al. (2018). Gloeosporidina shares a similar morphology of conidiomata and conidia with the asexual morph of Elsinoe Racib. (= Sphaceloma), but can be distinguished by conidiogenous cells. Gloeosporidina has monophialidic, cylindrical, determinate conidiogenous cells, whereas Elsinoe has polyphialidic, ampulliform to doliiform, indeterminate conidiogenous cells with 1–3 loci. Sutton (1980) accepted four taxa namely G. canthiicola B. Sutton, G. cecidii (Kohlm.) B. Sutton, G. cercocarpi (Ellis & Everh.) B. Sutton & Pollack and G. moravica. Subsequently, three species were added, G. lasiopetali B. Sutton (1991), G. cryptomeriae Kubono (1993), and G. platani Butin & Kehr (1998). Gloeosporidina platani was shown to be part of the life cycle of Apiognomonia veneta by Butin and Kher (1998), and was reduced to a synonym of it. Six taxa are listed in Index Fungorum (2019). Gloeosporidina has been described as a spermatial state of Diaporthaceae by von Arx (1970), and connected to Stromatinia (Boud.) Boud. by Kubono and Hosoya (1994). However, these connections have never been successfully demonstrated. Senanayake et al. (2018) placed Gloeosporidina in Gnomoniaceae and provided a description and illustration of the sexual morph of G. moravica. There is no molecular data available for Gloeosporidina. Fresh collections and cultures are needed to place Gloeosporidina in a natural group.
Distribution: Australia, Czech Republic, Japan, Zambia (Sutton 1980; Kubono 1994; Senanayake et al. 2018).
Godronia Moug. & Lév., in Mougeot, Consid. Vég. Vosges: 355 (1846)
= Topospora Fr., Fl. Scan.: 347 (1836)
Facesoffungi number: FoF 07407
Leotiomycetes, Leotiomycetidae, Helotiales, Godroniaceae
Saprobic or parasitic on the host plant in terrestrial habitats (Cash 1934; Strømeng and Stensvand 2011). Sexual morph: see Groves (1965). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious or confluent, at first immersed, eventually erumpent and appearing superficial, globose to oval or obpyriform, unilocular, glabrous, ostiolate. Ostiole single, circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura oblita to textura angularis. Conidiophores arising from the inner layers of the conidiomata wall, hyaline, cylindrical, branched at the base and above, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical to lageniform or irregular, integrated or discrete, determinate, smooth-walled. Conidia hyaline, cylindrical or fusiform, with obtuse apex and narrow and truncate base, septate, smooth-walled, eguttulate (adapted from Sutton 1980; Nag Raj and DiCosmo 1981a, b).
Type species: Godronia muehlenbeckii Moug. & Lév., in Mougeot, Consid. Vég. Vosges: 355 (1846)
Notes: Groves (1965) linked Topospora uberiformis (Kunze) Fr. (type of Topospora) to the sexual morph of Godronia uberiformis on Ribes sp. (Grossulariaceae). Johnston et al. (2014) regarded these two genera as taxonomically congruent, and recommended to use sexual morph name Godronia Moug. & Lév. for the holomorph. Konrad et al. (2007) showed that T. myrtilli grouped with G. cassandrae based on SSU sequence data, thus providing more evidence of connection between Topospora and Godronia. However, the type species of Topospora has not been linked to Godronia. To clarify the taxonomy of Godronia, the type species of both sexual and asexual morph will have to be recollected, and epitypified.
Distribution: Austria, Canada, Finland, Germany, Latvia, Netherlands, Russia, Sweden, Switzerland, UK, USA (Groves 1965; Sutton 1980; Konrad et al. 2007).
Godronia uberiformis J.W. Groves, Can. J. Bot. 43: 1245 (1965)
= Topospora uberiformis (Kunze) Fr., Summa veg. Scand., Sectio Post. 69: 415 (1849) [1848].
Facesoffungi number: FoF 07408, Fig. 177
Godronia uberiformis (asexual morph, redrawn from Nag Raj and DiCosmo 1981a) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Saprobic or parasitic on the host plant. Sexual morph: see Groves (1965). Asexual morph:Conidiomata up to 800 µm wide, 1350 µm high, dark brown to black, pycnidial, solitary to gregarious, at first immersed, eventually erumpent and appearing superficial, globose to oval or obpyriform, unilocular, glabrous, ostiolate. Ostiole single, circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown to black cells of textura oblita in the exterior, becoming thin-walled, subhyaline to hyaline cells of textura angularis towards hymenium. Conidiophores up to 35 µm long, 2.5–3 µm wide, arising from the inner layers of basal wall and part way up the sides of the conidiomata, hyaline, cylindrical, branched at the base and above, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical to lageniform, integrated or discrete, determinate, smooth-walled, with prominent collarettes. Conidia 17–22.5 × 3–3.5 µm, hyaline, cylindrical to fusiform, with obtuse apex and narrow and truncate base, 3-septate, smooth-walled, eguttulate (Sutton 1980, Nag Raj and DiCosmo 1981a, b).
Groveolopsis Boedijn, Sydowia 5(3–6): 351 (1951)
Dothideomycetes, Pleosporomycetidae, Pleosporales, Acrocalymmaceae
Facesoffungi number: FoF 07412
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, solitary to gregarious or confluent, immersed, globose or oval to irregular, unilocular, thick-walled, glabrous, ostiolate. Ostiole short, circular to oval, centrally located. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura globulosa to textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to subcylindrical, smooth, arising from the inner wall layers of conidiomata. Conidia hyaline, obclavate, narrow and truncate at base, attenuated towards the apex, 0–1-septate, smooth, guttulate, bearing a cup-like or irregular, mucoid, apical appendage.
Type species: Groveolopsis pandani (Höhn.) Boedijn, Sydowia 5(3–6): 225 (1951)
Notes: Wijayawardene et al. (2017a) estimated that there are six species in Groveolopsis, but they did not provide any details on species names. Groveolopsis remains monotypic (Morgan-Jones 1977; Sutton 1980; Nag Raj 1993, Index Fungorum 2019, MycoBank 2019). A collection made during the present study (MFLU13-0309) shares similar morphology of conidiomata and conidial dimensions with Groveolopsis pandani, and therefore, it is regarded as conspecific with the type species.
Based on a blast search of NCBIs GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Acrocalymma vagum voucher INBio:483A (GenBank KU204582.1; Identities = 371/405(92%), 12/405(2%), A. fici BR68 (GenBank MN637807.1; Identities = 372/406(92%), 15/406(3%), Acrocalymma sp. LS83 (GenBank MK715136.1; Identities = 370/404(92%), 11/404(2%)), and A. medicaginis G838 (GenBank MK247909.1; Identities = 370/404(92%), 11/404(2%)). As other sequences (e.g., LSU, SSU) are not available for G. pandani. Groveolopsis is temporarily placed in Acrocalymmaceae (Pleosporales) based on ITS sequence data. Groveolopsis is similar to Acrocalymma in having pycnidial conidiomata produced hyaline conidia with flared mucoid apical and basal appendages (Trakunyingcharoen et al. 2014). However, Groveolopsis has obclavate, 0–1-septate conidia, which differ from Acrocalymma in that conidia are cylindrical to fusoid, 0–3-septate. To fix the genetic concept of Groveolopsis, fresh collections of G. pandani from its original location will have to be epitypified.
Distribution: Indonesia, Thailand (Sutton 1980; this study).
Groveolopsis pandani (Höhn.) Boedijn, Sydowia 5(3–6): 225 (1951)
Facesoffungi number: FoF 07413, Fig. 178
Groveolopsis pandani(MFLU 13-0309) a, b Herbarium specimens. c, d Appearance of black conidiomata on the host. e, f Vertical sections of conidiomata. g, h Sections of peridium. i–o Conidiogenous cells and developing conidia. p–v Conidia (arrows indicate basal appendages). Scale bars c = 1000 µm, d–f = 100 µm, g = 20 µm, i–j = 5 µm, k–o, p–v = 10 µm
Parasitic on living leaves of Pandanus sp. (Pandanaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 130–160 µm diam., 100–140 µm high, black, pycnidioid, solitary to gregarious or confluent, immersed, globose or oval to irregular, unilocular, thick-walled, glabrous, with a dark brown papillate ostiole. Ostiole short, single, circular to oval, centrally located. Conidiomatal wall 8–30 µm wide, composed of thick-walled, brown cells of textura globulosa at the outer layers, and thick-walled, hyaline cells of textura prismatica at inner layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 6–15 × 3–8 µm, hyaline, enteroblastic, phialidic, ampulliform to subcylindrical, smooth, arising from the inner wall layers of conidiomata. Conidia 36–55.4 × 5–9 µm (\( \bar{x} \) = 44 × 6.7 µm; n = 30), hyaline, obclavate, narrow and truncate at the base, attenuated towards the apex, usually asepatte, occasionally 1-septate, smooth-walled, guttulate, bearing a cup-like or irregular, mucoid, apical appendage; basal appendages present or absent.
Material examined: Thailand, Chiang Mai, on living leaves of Pandanus sp. (Pandanaceae), 4 February 2012, N. Tangthirasunun, NTP008–1 (MFLU13-0309).
Gyrostroma Naumov, Bull. Soc. mycol. Fr. 30(3): 386 (1914)
Facesoffungi number: FoF 07409
Sordariomycetes, Sordariomycetidae, Diaporthales, genera incertae sedis
Saprobic on bark of Abies sibirica (Pinaceae). Sexual morph: undetermined. Asexual morph:Conidiomata off white, extensive, stromatic, pycnidial, scattered to gregarious or confluent, initially immersed, ultimately become erumpent, pulvinate, globose to subglobose, unilocular or multilocular, glabrous. Ostiole absent, dehiscence by irregular breakdown of the locular walls. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores formed from the inner layer of locular wall, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, determinate, smooth-walled. Conidia hyaline, oblong to ellipsoidal, unicellular, smooth-walled, eguttulate (adapted from Hirooka et al. 2012).
Type species: Gyrostroma sinuosum Naumov, Bull. Soc. mycol. Fr. 30(3): 386 (1914)
Notes: Seeler (1940b) revised Thyronectria and linked it to the asexual morph Gyrostroma, based on culture studies and both apothecia and conidiomata found compactly associated. Two new species, Gyrostroma austroamericanum Seeler and G. missouriense Seeler were introduced as asexual morph of T. austroamericana (Speg.) Seeler and T. missouriensis (Ellis & Everh.) Seaver, respectively (Seeler 1940b). Hirooka et al. (2012) re-examined the type specimen of G. sinuosum and suggested that it may be a member of Diaporthales rather than hypocrealean. Gyrostroma austroamericanum and G. missouriense were transferred to Pleonectria (synonym of Thyronectria) by Hirooka et al. (2012). Wijayawardene et al. (2017) synonymized Kaskaskia (represented by K. gleditsiae Born & J.L. Crane) under Gyrostroma. However, Bedker and Wingfield (1983) synonymized Kaskaskia gleditsiae under Gyrostroma austroamericanum on the basis of morphology and culture study. Thus, Kaskaskia is a synonym of Thyronectria, rather than a synonym of Gyrostroma. It is necessary to obtain fresh collections and obtain DNA sequences to confirmed placement of Gyrostroma and allied genera.
Illustration of Gyrostroma sinuosum see Hirooka et al. (2012).
Distribution: Europe (Hirooka et al. 2012).
Hapalosphaeria Syd., in Diedicke & Sydow, Ann Mycol. 6(4): 305 (1908)
Facesoffungi number: FoF 07410, Fig. 179
Hapalosphaeria deformans (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Parasitic on anthers of Rubus fruticosus, R. idaeus and Rubus spp. (Rosaceae) (Sutton 1980). Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, pycnidial, scattered or gregarious, immersed to erumpent, globose, unilocular, glabrous. Ostiole absent, dehiscence by a relatively wide apical opening. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis. Conidiophores formed from the inner wall layer of conidiomata, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform or dolliform, determinate, smooth-walled. Conidia hyaline, globose, unicellular, smooth-walled, eguttulate.
Type species: Hapalosphaeria deformans (Syd. & P. Syd.) Syd., in Diedecke & Sydow, Ann Mycol. 6(4): 305 (1908)
Notes: Hapalosphaeria is monotypic and no molecular data is available. This genus shares similar form of conidiomata, conidiogenous cells and conidia with phoma-like fungi. However, phoma-like fungi are polyphyletic. Species within this group have been assigned to various families such as Cucurbitariaceae, Didymellaceae, Pleosporineae, Pseudopyrenochaetaceae and Pyrenochaetopsidaceae (De Gruyter et al. 2010; Chen et al. 2015, 2017; Valenzuela-Lopez et al. 2018). Thus, it is difficult to place Hapalosphaeria in a natural group based on morphological characters. Fresh collections of H. deformans are needed to epitypify the genus.
Distribution: Germany, UK (Sutton 1980; Wijayawardene et al. 2017).
Helhonia B. Sutton, The Coelomycetes: 600 (1980)
Facesoffungi number: FoF 07411, Fig. 180
Helhonia rhamnigena (redrawn from Sutton 1980) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Saprobic on stems of Sambucus nigra (Adoxaceae). Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, pycnidial, scattered or gregarious, subepidermal to epidermal, immersed to erumpent, subglobose, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of apical wall. Conidiomatal wall composed of thin-walled, hyaline cells of textura angularis in the basal part, becoming thick-walled, pale brown cells at the upper part. Conidiophores arising from the inner wall layer of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to ampulliform, integrated, less often discrete, determinate, smooth-walled, with minute collarette and channel. Conidia hyaline, cylindrical to fusiform, tapered at one or both ends, 1-septate, smooth-walled, guttulate (adapted from Sutton 1980).
Type species: Helhonia rhamnigena (Fautrey) B. Sutton, The Coelomycetes: 600 (1980)
Notes: Helhonia is monotypic. This genus can easily be confused with Cylindrogloeum, Davisiella, Diplozythiella and Placonemina, because all have septate, fusiform conidia. However, characters of conidiomata, conidiophores and conidiogenous cells and habitat can differentiate these genera. Helhonia has pycnidial conidiomata, while Cylindrogloeum has acervular conidiomata. Davisiella has two types of conidiomatal wall (hyaline, rather thick-walled cells of textura angularis in the outer layers, becoming smaller and compact cells towards inner layers), and 2–3-septate conidia. The conidiomatal wall of Helhonia consists of thin-walled, hyaline cells of textura angularis in the basal part, becoming thick-walled, pale brown cells at the upper part, and the conidia are 1-septate. Davisiella species are fungicolous, while Helhonia rhamnigena is saprobic. In Diplozythiella and Placonemina, the conidiophores are reduced to conidiogenous cells, while in Helhonia, they are hyaline, branched, septate. It is difficult to clarify relationships among these genera due to a lack of molecular data. Fresh collections of Cylindrogloeum, Davisiella, Diplozythiella and Placonemina are needed to place them in a natural classification.
Distribution: France (Sutton 1980).
Hemidothis Syd. & P. Syd., Ann Mycol. 14(1/2): 95 (1916)
Facesoffungi number: FoF 07415, Fig. 181
Hemidothis miconiae (redrawn from Morgan-Jones et al. 1972d) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic or parasitic on Miconia sp. (Melastomataceae) or Lauraceae in the tropics (Hanlin 1997). Sexual morph: undetermined. Asexual morph:Conidiomata black, stromatic, pycnidial, gregarious, superficial, discoid, orbicular to irregular, multilocular, glabrous. Ostiole absent, dehiscence by breakdown of upper wall. Conidiomatal wall composed of thick-walled, pale brown to brown cells of textura angularis. Conidiophores arising from the inner layers of locular wall, hyaline, cylindrical to irregular, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to ampulliform, integrated, determinate, smooth-walled. Conidia hyaline, long-fusiform, curved to sigmoid, 3-septate, smooth-walled, guttulate (adapted from Morgan-Jones et al. 1972d).
Type species: Hemidothis miconiae Syd. & P. Syd., Ann Mycol. 14(1/2): 95 (1916)
Notes: Hyaline, long-fusiform, curved to sigmoid conidia and phialidic conidiogenous cells of Hemidothis resembles asexual morph of Dermea (= Foveostroma). Dermea has stromatic, pulvinate, furfuraceous or pruinoseconidiomata produced 1-sepate conidia, whereas Hemidothis has stromatic, pycnidial conidiomata produced 3-sepate conidia (Nag Raj and DiCosmo 1980; this study).
Hemidothis has been poorly studied, and the sexual morph is unclear. Petrak (1927a, 1929), Sydow (1930) and Burton and Gwendolyn (1943) showed the connection between Hemidothis and Bagnisiopsis Theiss. & Syd. However, Hanlin (1997) listed Hemidothis as the asexual morph of Coccodiella Hara represented by C. melastomatum (Lév.) I. Hino & Katum., which causes lesions on leaves of Miconia sp. Six epithets are listed in Index Fungorum (2019), but none of them been revised with molecular data. Therefore, fresh material and DNA sequence analyses are needed to clarify the taxonomy of Hemidothis.
Distribution: Brazil, Dominican Republic, India, Venezuela.
Hercospora Fr., Syst. orb. veg. 1: 119 (1825)
= Rabenhorstia Fr., Summa veg. Scand., Sectio Post. (Stockholm): 410 (1849)
Facesoffungi number: FoF 02250
Sordariomycetes, Diaporthomycetidae, Diaporthales, Lamproconiaceae
Saprobic on the host plant. Sexual morph: see Norphanphoun et al. (2016) and Senanayake et al. (2018). Asexual morph:Conidiomata dark blackish brown, stromatic, solitary, subepidermal to epidermal, immersed, applanate but markedly erumpent in the centre, unilocular or multilocular, convoluted, glabrous. Ostiole absent, dehiscence irregular. Conidiomatal wall composed of thick-walled, pale brown cells of textura angularis in the basal part, becoming dark brown cells of textura intricata in the lateral wall, consisting entirely of textura oblita and projecting as a cap over the dehiscent area in the upper part. Conidiophores arising from the innermost cell layer of the basal and lateral wall, hyaline, cylindrical to subcylindrical, branched extensively at the base, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, holoblastic, cylindrical, integrated or discrete, determinate, smooth-walled, with terminal or lateral conidia formed from a branch immediately below a transverse septum. Conidia hyaline, ellipsoid, occasionally with truncate base, unicellular, contents granular, often with a central guttule, thick- and smooth-walled, produced in mucilage but without any mucilaginous envelope or appendage at maturity (Sutton 1980).
Type species: Hercospora tiliae (Pers.) Tul. & C. Tul., Select. fung. carpol. 2: 154 (1863)
= Rabenhorstia tiliae (Pers.) Fr., Summa veg. Scand., Sectio Post. (Stockholm): 410 (1849)
Notes: Rabenhorstia tiliae (Pers.) Fr. (type of Rabenhorstia), was considered as the asexual morph of Hercospora tiliae (type of Hercospora), since both fruiting bodies are frequently found together (Sutton 1980). Norphanphoun et al. (2016) and Senanayake et al. (2018) re-examined Hercospora and provided detailed descriptions of both sexual and asexual morphs. Hercospora was placed in Lamproconiaceae (Diaporthales) based on sequence data of LSU region. However, molecular data for its asexual morph is not available. Fresh collections and cultures of both morphs are necessary to confirm this connection.
Illustration of Hercospora tiliae see Senanayake et al. (2018).
Distribution: Austria, Canada, Norway, UK, USA, Sweden (Sutton 1980; Senanayake et al. 2018).
Heterosphaeria Grev., Scott. crypt. fl. 1: pl. 103 (1824)
= Heteropatella Fuckel, Jb. nassau. Ver. Naturk. 27-28: 54 (1874) [1873-74]
Facesoffungi number: FoF 07419
Leotiomycetes, Leotiomycetidae, Helotiales, Heterosphaeriaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph:Apothecia dark brown to black, solitary, sessile, seated on a more or less distinct subiculum of brown, septate hyphae, which penetrate the host tissue, globose to subglobose. Excipulum 2-layered, outer layers crust-like, composed of thin-walled, dark brown, isodiametrical cells of textura globosa to textura angularis, inner layers composed of relatively thick-walled, hyaline, agglutinated, orientated to somewhat interwoven and anastomosing, short, cartilaginous celled-hyphae, gradually merging with thin-walled, hyphae of textura porrecta towards the margin; inside of the apothecial margin with few, free hyphal tips or bordered with regularly arranged, bristle-like hyphae. Paraphyses hyaline, filiform, unbranched, 1–3-septate, apex enlarged club-like, exceeding the asci. Asci cylindrical to clavate, with trapezoidal apex and an apical pore blued by iodine. Ascospores hyaline, irregularly biseriate above, uniseriate at the base, ellipsoidal, often slightly inequilateral, aseptate, or sometimes 1-septate, usually with a large guttule at both ends (Leuchtmann 1987). Asexual morph:Conidiomata black, stromatic, pycnidial, cupulate, sometimes with laciniate margins after dehiscence, solitary to gregarious or confluent, semi-immersed to superficial, unilocular, glabrous, ostiolate or ostiole absent, papillate, thick-walled. Ostiole cylindrical to obconic, single, centrally or excentricity located, sometimes with an operculum. Conidiomatal wall composed of an outer textura angularis to textura globosa, with thick-walled, dark brown to black cells, and an inner textura prismatica, merging with innermost, hyaline, thin layers of hyaline cells of textura angularis near the conidial hymenium. Conidiophores hyaline, subcylindrical, branched, septate, lining the base and most of the way up the side walls of the conidiomata. Conidiogenous cells hyaline, cylindrical, discrete, smooth-walled. Conidiogenesis: ontogeny holoblastic by apical wall-building in the first conidium and replacement wall-building in subsequent conidia; maturation by moderate diffuse wall-building synchronous with conidium ontogeny; delimitation by a transverse septum; secession schizolytic; proliferation of conidiogenous cells holoblastic-sympodial; collarettes, periclinal thickenings, annellations, and regeneration of conidiogenous cells absent (Nag Raj 1993). Conidia hyaline, fusiform, 1–4-septate, slightly constricted at septa, guttulate, smooth, bearing appendages, basal cell obconic with a truncate base, median cells mostly 2–3, sometimes 4, cylindrical to doliiform, apical cell narrow conical and attenuated into an unbranched, cellular appendage, basal appendage attenuated, unbranched, excentric.
Type species: Heterosphaeria patella (Tode) Grev., Scott. crypt. fl. 2: 103 (1823)
Notes: Morphological criteria alone has resulted in a poor understanding in Heterospheria. For example, Rehm (1896) placed Heterospheria in the family Heterosphaeriaceae, Boudier (1907) in Patellariaceae and von Höhnel 1918a, b) in Dermateaceae as it is closely related to Pyrenopeziza Fuckel. Leuchtmann (1987) revised Heterosphaeria and accepted eight species in the genus.
The asexual morph of Heterosphaeria was discovered by Tulasne in 1865 on the same substrate, with asci in apothecia and separate conidiomata (Buddin and Wakefield 1926; Leuchtmann 1987). Grove (1937) linked Heterosphaeria linariae to the asexual morph Heteropatella lacera (type species of Heterosphaeria). However, Leuchtmann (1987) linked H. patella to Heteropatella lacera based on culture studies. Ekanayaka et al. (2019) and Kuntida et al. (2019) showed that Heteropatella lacera was the correct asexual morph of H. linariae based on molecular data, and this connection was confirmed in the present study (Fig. 182).
Phylogenetic tree generated from a maximum likelihood (RAxML) analysis based on a concatenated alignment of LSU and ITS sequence data of Heterosphaeria. Fifteen samples are included in the analyses, which comprise 1319 characters including gaps (LSU: 1–839, ITS: 840–1319). Mycochaetophora gentianae (MAFF 239231) is the outgroup taxon. The tree topology from the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 2537.429196 is presented. The matrix had 245 distinct alignment patterns, with 14.17% of undetermined characters or gaps. Estimated base frequencies were: A = 0.244624, C = 0.220369, G = 0.287242, T = 0.247764; substitution rates AC = 0.875045, AG = 1.047921, AT = 0.702980, CG = 0.162225, CT = 3.36711, GT = 1.000000; gamma distribution shape parameter α = 0.020000. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 10 changes. Ex-type or ex-epitype strains are in bold. New isolates are in blue
Fresh collections from Italy were studied. The phylogeny derived from LSU and ITS sequence data shows that these collections cluster with H. linariae (MFLU 15-2764, CBS 636.86, CBS 163.27), H. patella (GM 2014-08-04-1) and Heterosphaeria sp. (KP 2019a), but are unresolved. Because of the lack of suitable genes (e.g., rpb2) for most strains in this clade, it is difficult to resolve the relationship. Comparison of LSU, ITS and rpb2 sequence data and morphological studies show that MFLU 19-2866/IT1847 and MFLU 16-1154/IT2922 are the asexual morph of H. linariae (see notes under H. linariae). Heterosphaeria sp. (KP 2019a) is considered as conspecific with H. linariae, and the sequence similarites between these two strains are 99% (687/689) in LSU (without gaps) and 99% (741/748) in ITS (including 1 gap). We also describe an asexual morph for H. patella (see notes below), and provide sequence data for H. umbilicata.
Distribution: Austria, Czechoslovakia, Germany, Italy, Latvia, Romania, Russia, Sweden, UK, (Nag Raj 1993; Ekanayaka et al. 2019, this study).
Heterosphaeria linariae (Rabenh.) Rehm, Rabenh. Krypt.-Fl., Edn 2 1.3(lief. 30): 203 (1888) [1896]
= Heterosphaeria ovispora Leuchtm., Mycotaxon 28(2): 272 (1987)
= Heteropatella lacera Fuckel, Jb. nassau. Ver. Naturk. 27-28: 54 (1874) [1873-74]
Facesoffungi number: FoF 05874, Figs. 183, 184
Heterosphaeria linariae (asexual morph, FH 01142398, isotype) a, b Herbarium specimen. c, d Appearance of black conidiomata on the host surface. e–f, h Vertical sections of conidiomata. g Section of peridium. i–l Conidiophores, conidiogenous cells and developing conidia. m–p Conidia. Scale bars c–f = 200 µm, g–h = 50 µm, i = 20 µm, j–p = 10 µm
Heterosphaeria linariae (asexual morph, MFLU 16-1154, reference specimen). a Herbarium specimen. b, c Appearance of black conidiomata on the host. d, g Section of peridium. e Vertical section of conidiomata. f Ostiole. h–k Conidiophores, conidiogenous cells and developing conidia. l–p Conidia. Scale bars a, e = 100 µm, b = 500 µm, c = 200 µm, d, f = 50 µm, g = 20 µm, h = 5 µm, i–p = 10 µm
Saprobic on dead stems of Euphorbia sp. and Linaria vulgaris. Sexual morph: see Leuchtmann (1987) and Ekanayaka et al. (2019). Asexual morph:Conidiomata 150–420 µm diam., 300–400 µm high, pycnidial, globose to subglobose or irregular in outline, semi-immersed to superficial, separate to gregarious or confluent, unilocular, glabrous, thick-walled, ostiolate, rugose. Ostiole 70–210 µm long, 80–150 µm wide, cylindrical or funnelled, single, centrally or excentricity located. Conidiomatal wall 10–70 µm wide, composed of dark brown, thick-walled cells of textura angularis to textura globosa. Conidiophores up to 70 µm long, hyaline, branched, septate, formed from the innermost layer of wall cells. Conidiogenous cells 8–25 × 2–3 µm, hyaline, enteroblastic, phialidic, cylindrical, smooth, with a slightly wider apex, proliferating, sympodially 2–4 times. Conidia 44–68 × 2–4 µm (\( \bar{x} \) = 53 × 3 µm; n = 50), hyaline, fusiform, mostly 4-septate, occasionally 3-septate, slightly constricted at septa, guttulate, smooth-walled, bearing appendages, basal cell 2.4–10 µm (\( \bar{x} \) = 7 µm), obconic with a truncate base, median cells with a total length 9–25.6 µm (\( \bar{x} \) = 15), mostly 3, occasionally 2 or 4, cylindrical to doliiform, apical cell narrow conical and attenuated into an unbranched, cellular appendage 25–44 × 1–2 µm (\( \bar{x} \) = 36 × 1.5 µm; n = 30), basal appendage 5–25 × 1–2 µm (\( \bar{x} \) = 12 × 1.5 µm; n = 30), filiform, unbranched, attenuated, excentric.
Material examined: Austria, Nassau, Dombachsgraben, on dead stem of Linaria vulgaris Hill (Plantaginaceae) (FH 01142398, Fuckel -Fungi Rhenani Edn. I, #2565, isotype); Italy, Province of Forlì-Cesena, Bagno di Romagna, Alfero, on dead aerial stems of Silene sp. (Caryophyllaceae), 6 April 2016, Nello Camporesi, IT2922 (MFLU 16-1154, reference specimen designated here), (KUN, HKAS 97463); ibid., Province of Arezzo, Pieve Santo Stefano, Montalone, on dead aerial stems of Euphorbia sp. (Euphorbiaceae), 2 May 2014, Erio Camporesi, IT1847 (MFLU 19-2866).
Notes: The sequences of our collection were obtained by direct sequencing. The LSU sequence of (MFLU 19-2866/IT1847) and (MFLU 16-1154/IT2922) is 99% or 100% similar to H. linariae (CBS 163.27, MFLU 15-2764, CBS 636.86, KP 2019a). There are four base pairs difference between the ITS (including 2 gaps) and rpb2 sequence of (MFLU 16-1154/IT2922) and H. linariae (CBS 163.27). The sequence similarities among these strians are shown in Table 6. The description of (MFLU 16-1154/IT IT2922) and (MFLU 19-2866/IT1847) is in accordance with the asexual morph of H. linariae (Figs. 183 and 184). The dimensions of these two collections are in the range of the asexual morph of H. linariae [(43–64 × (2.5–)3–4 (\( \bar{x} \) = 53.5 × 3.5 µm)] (Nag Raj 1993), and 42–78.5 × 2.6–4 (\( \bar{x} \) = 55.6 × 3.2 µm) (this study).
Heterosphaeria patella (Tode) Grev., Scott. crypt. fl. 1: pl. 103 (1824)
Facesoffungi number: FoF 07095, Fig. 185
Heterosphaeria patella (asexual morph, MFLU 15-2272) a Herbarium specimen. b, c Appearance of black coniodiomata on the host. d, e Vertical section of conidiomata. f Section of peridium. g–i Conidiophores, conidiogenous cells and developing conidia. j–m Conidia. Scale bars d–e = 100 µm, f = 20 µm, g–j = 10 µm, k–m = 5 µm
Saprobic on dead stems of Malva sp. (Malvaceae). Sexual morph: see Leuchtmann (1987). Asexual morph:Conidiomata 100–200 µm diam., 100–150 µm high, black, discoid in outline, solitary to gregarious or occasionally confluent, pycnidial, globose to subglobose in section view, semi-immersed to superficial, unilocular, lacking obvious setae, with brown to dark brown hyphae developing from conidiomatal base on substrate. Ostiole absent. Conidiomatal wall 20–30 µm wide, composed of thick-walled, dark brown cells of textura globulosa at outer part, gradually merging with thin-walled, pale brown to hyaline cells of textura angularis lining the cavity. Conidiophores lining the base and sides of the conidiomatal cavity, hyaline, septate and branched, smooth, invested in mucus. Conidiogenous cells 10–20 × 2–5 µm, hyaline, holoblastic, sympodial, cylindrical to subcylindrical, terminal to integrated, indeterminate, smooth, with 2 to several, small, indistinct protuberant conidiogenous loci towards the apices. Conidia 18–26 × 2.5–4 µm (\( \bar{x} \) = 21 × 3 µm; n = 30), hyaline, fusiform, 1–2-septate, slightly constricted at the septa, guttulate, smooth, thick-walled, without appendage, basal cell long obconic with a truncate base, apical cell shortly rostrate.
Material examined: Italy, Province of Forlì-Cesena, Bagno di Romagna, near Lago Pontini, on dead aerial stems of Malva sp. (Malvaceae), 1 July 2015, Erio Camporesi, IT2551 (MFLU 15-2272), (KUN, HKAS 93632).
Notes: Our collection is considered as the asexual morph of Heterosphaeria patella with sequence similarities between MFLU 15-2272/IT2551, and H. patella (MF196187) of 100% (734/734) in LSU and 99% (479/482) in ITS. The asexual morph of H. patella differs from asexual morph of H. linariae in the form of conidiomata and conidia. Heterosphaeria patella has brown to dark brown hyphae developing from the conidiomatal base, while this character is absent in H. linariae. In addition, Heterosphaeria linariae has 3–4-septate conidia bearing appendages at each end, whereas H. patella has 1–2-septate conidia without appendages. The differences between H. linariae and H. patella may correlate with protein gene sequence (e.g., rpb2). We found that there are 55 base pairs differences in rpb2 sequence between MFLU 15-2272/IT2551 and MFLU 19-2866/IT1847. Hence it is necessary to include higher resolution protein coding genes to better understand the taxonomy of species in Heterosphaeria.
Heterosphaeria hendersonioides (Fautrey & Lambotte) W.J. Li & K.D. Hyde, comb. nov.
≡ Heteropatella hendersonioides Fautrey & Lambotte, Revue mycol., Toulouse 18(no. 72): 143 (1896)
Index Fungorum number: IF 557323, Facesoffungi number: FoF 07420, Fig. 186
Heterosphaeria hendersonioides (FH 01142402, isotype). a, b Herbarium specimen. c, d Appearance of black conidiomata on the host. e, f Vertical section of conidiomata. g, h Section of peridium. i Ostiole. j–l Conidiophores, conidiogenous cells and developing conidia. m–q Conidia. Scale bars c–f = 200 µm, g–h = 50 µm, i–j = 20 µm, k–q = 10 µm
Saprobic on dry stems of Bupleurum falcatum (Apiaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 250–530 µm diam., 100–420 µm high, dark brown to black, pycnidial but appearing sunken in the middle zone after discharge of the operculum, solitary or gregarious, semi-immersed to superficial, globose to subglobose, unilocular, glabrous, thick-walled, ostiolate. Ostiole short, rounded, single, centrally located. Conidiomatal wall 60–200 µm wide, composed of two types, (a) basal and lateral wall of textura angularis to textura globose with dark brown, thick-walled cells in outer layers, becoming textura intricata gradually merging textura epidermoidea with pale brown to hyaline, relatively thick-walled cells in inner layers; (b) upper wall of textura angularis with thick-walled, brown cells in outer layers, becoming hyaline towards inner layers. Conidiophores up to 70–100 µm long, formed from the base and side wall of conidiomata, hyaline, branched at base, septate, invested in mucus. Conidiogenous cells 10–16 × 2–5 µm, hyaline, enteroblastic, cylindrical, smooth-walled, indeterminate, discrete or proliferating sympodially 1–2 times. Conidia 25–43 × 3–5.6 µm (\( \bar{x} \) = 35 × 4.5 µm; n = 50), hyaline, fusiform, curved, 1–4-septate, slightly constricted at septa, guttulate, smooth-walled, bearing appendages, basal cell 2–4 µm long (\( \bar{x} \) = 3.5 µm), obconic with a truncate base, 3 median cells with a total length 14–18.5 (x = 16.7 µm), cylindrical to doliiform, apical cell narrow conical and attenuated into an unbranched, cellular appendage 12–21 µm long, basal appendage 4–23 µm long, 1–5, filiform, unbranched or branched, attenuated, excentric, inserted at different loci.
Material examined: France, Côted’Or, Montagne de Bard, on dry stems of Bupleurum falcatum (Apiaceae), June 1896, F. Fautrey (FH 01142402, isotype), C. Roumeguère - F. Gallici. Exs. #7227.
Notes: Heterosphaeria hendersonioides fits well within the generic concept of Heterosphaeria except for the basal appendages of the conidia. However, this is a reasonable character to distinguish it from other Heterosphaeria species. Fresh collections of H. hendersonioides are necessary to better understand its placement.
Heterosphaeria umbilicata (Pers.) W.J. Li & K.D. Hyde, comb. nov.
≡ Peziza umbilicata Pers., Mycol. eur.1: 323 (1822)
= Heteropatella umbilicata (Pers.) Grove, J. Bot., Lond. 56: 319 (1918)
Index Fungorum number: IF557168, Facesoffungi number: FoF 07421, Fig. 187
Heterosphaeria umbilicata (MFLU 19-2874, reference specimen, a, c, d, i, k, p, w–z from MFLU 19-2874/IT507, e, j,o, t–v from MFLU 19-2875/IT656, b, f–h, 1–n, q–s from MFLU 19-2878/IT742) a, b Herbarium specimen. c–e Appearance of dark brown to black conidiomata on the host. f Ostiole. g Section of peridium. h–k Vertical sections of conidiomata. l–p Conidiophores, conidiogenous cells and developing conidia. q–z Conidia. Scale bars b = 500 µm, c–d = 100 µm, e = 200 µm, h–i, k = 100 µm, f, g = 20 µm, j = 50 µm, l–z = 10 µm
Saprobic on dead stems of herbaceous plant forming conspicuous, rounded to oval, erumpent, black, fruiting bodies. Sexual morph: undetermined. Asexual morph:Conidiomata 100–500 µm diam., 70–400 µm high, pycnidial, globose to subglobose, semi-immersed to superficial, separate to gregarious or confluent, unilocular, glabrous, thick-walled, ostiolate. Ostiole 60–130 µm long, 50–120 µm wide, cylindrical, single, centrally or excentricity located. Conidiomata 20–90 µm wide, composed of outer several layers of textura globosa to textura angularis, with thick-walled, dark brown cells, and inner layers of textura angularis, with thick-walled, hyaline cells. Conidiophores hyaline, branched, septate, formed from the innermost layer of wall cells. Conidiogenous cells 9–30 × 1–4 µm, hyaline, enteroblastic, cylindrical to subcylindrical, denticulate, integrated to occasionally discrete, smooth-walled, with a slightly wider apex, proliferating sympodially 2–3 times. Conidia 22–46 × 2–4 µm (\( \bar{x} \) = 34 × 3 µm; n = 50), hyaline, long-fusiform to naviculate, 1–3-septate, slightly constricted at septa, guttulate, smooth-walled, bearing appendages, basal cell 4–12 µm long (\( \bar{x} \) = 7 µm), obconic with a truncate or rounded base, median cells mostly 2, occasionally 1, together 13–20 µm long (\( \bar{x} \) = 14 µm), cylindrical to doliiform, apical cell narrow conical and attenuated into an unbranched, cellular appendage, with a total length 8–22 µm (\( \bar{x} \) = 14 µm), basal appendage often lacking, but when present, tubular, unbranched, attenuated, 1–3.8 µm long (\( \bar{x} \) = 2.6 µm).
Material examined: Italy, Province of Trento, Vermiglio, near Passo del Tonale, on dead aerial stems of Plantago sp. (Plantaginaceae), 28 June 2012, Erio Camporesi, IT507 (MFLU 19-2874, reference specimen); ibid., Province of Trento, Mezzana, Marilleva, on dead aerial stems of Heracleum sphondylium (Apiaceae), 9 August 2012, Erio Camporesi, IT656 (MFLU 19-2875), (KUN, HKAS93657); ibid., Province of Forlì-Cesena, Predappio Alta, on dead aerial stems of Torilis arvensis (Apiaceae), 22 September 2012, Erio Camporesi, IT742 (MFLU 19-2878), (KUN, HKAS93661).
Notes: Three specimens (MFLU 19-2874/IT507, MFLU 19-2875/IT656 and MFLU 19-2878/IT742) were collected from Italy on dead stems of Plantago sp., Heracleum sphondylium and Torilis arvensis, respectively. These collections have similar form of conidiomata, conidiogenous cells and conidia as well as conidial dimensions, MFLU 19-2874/IT507 (22–39 × 2–3 µm (\( \bar{x} \) = 32 × 2.4 µm; n = 50), MFLU 19-2875/IT656 (22–41 × 2.6–4 µm (\( \bar{x} \) = 34 × 3 µm; n = 50), MFLU 19-2878/IT742 (23–46 × 2.5–4 µm (\( \bar{x} \) = 35 × 3.4 µm; n = 50). The descriptions of these collections match the concept of H. umbilicata, and the only distinguishing character is their conidial dimensions. However, it should be noted that this character may not be very reliable, for example, Nag Raj (1993) gave conidia dimensions for H. umbilicata of 33–49(–59) × 2–3 (\( \bar{x} \) = 41.8 × 2.6 µm), while Sutton (1980) gave 17–22 × 2–2.5 µm. Based on morphological information, these collections are regarded as conspecific with H. umbilicata.
These specimens were difficult to culture; the sequence of collection MFLU 19-2874/IT507 was obtained by direct sequencing, but we were unable to obtain sequence data for IT656 and MFLU 19-2878/IT742. Hetersphaeria umbilicata was originally collected from Europe and is associated with various herbaceous plants, e.g., Angelica sylvestris (Apiaceae), Campanula uniflora (Campanulaceae) and Oxyria digyna (Polygonaceae). Because the type specimen lacks sequence data, we designate our collection as a reference specimen.
Hypocline Syd., Ann Mycol. 37(3): 245 (1939)
Facesoffungi number: FoF 07414, Fig. 188
Hypocline penniseti (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on Pennisetum sp. (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, stromatic, pycnidial, scattered, immersed or semi-immersed, elongated to subglobose, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of apical wall. Conidiomatal wall composed of thin-walled, hyaline cells of textura angularis in the base part, becoming thick-walled, with brown cells at the upper part. Conidiophores arising from inner wall layer of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, polyblastic, sympodial, integrated, determinate, smooth-walled, with 1–3 apical slightly protruding, conidiogenous loci without scars. Conidia hyaline, cylindrical, with obtuse apex and truncate base, unicellular, smooth-walled, eguttulate.
Type species: Hypocline penniseti Syd., Ann Mycol. 37(3): 246 (1939)
Notes: Hypocline is monotypic, and is associated with lesions on Pennisetum sp. in Uganda (Sutton 1980). This genus shares similar morphology of conidiogenous cells with Rhabdogloeopsis. The differences between these genera are detailed under notes of Rhabdogloeopsis. There is no molecular data available for this genus. Fresh collections of H. penniseti are needed to place this genus in a natural group.
Distribution: Uganda (Sutton 1980).
Hypohelion P.R. Johnst., Mycotaxon 39: 221 (1990)
= Leptostroma Fr., Observ. mycol. (Havniae) 1: 196 (1815)
Faces of fungi number: FoF 07416, Fig. 189
Hypohelion scirpinum (asexual morph, redrawn from Sutton 1980) a Conidia. b Conidiogenous cells. c Vertical section of conidioma
Leotiomycetes, Leotiomycetidae, Rhytismatales, Rhytismataceae
Saprobic on the host plant in terrestrial habitats, such as Arundinaria gigantea (Poaceae), Scirpus lacustris (Cyperaceae), S. validus, Stephanandra incisa (Rosaceae) (Sutton 1980; Wang et al. 2014a, b) Sexual morph: see Grove (1937). Asexual morph:Conidiomata black, subepidermal, pycnidial, immersed, circular, occasionally elongated, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of overlying wall. Conidiomatal wall composed of 1-cell thick, dark brown cells of textura angularis or prismatica in the upper part, becoming thick-walled, dark brown to hyaline cells of textura angularis in the basal part. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from inner layer cells of basal stroma, hyaline, holoblastic, sympodial to synchronous, filiform, integrated or discrete, indeterminate, smooth-walled, with several, unthickened conidiogenous loci towards the apices. Conidia hyaline, ellipsoid, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Hypohelion scirpinum (DC.) P.R. Johnst., Mycotaxon 39: 221 (1990)
= Leptostroma scirpinum Fr., Syst. mycol. (Lundae) 2(2): 598 (1823)
Notes: Leptostroma scirpinum (type of Leptostroma) was generally considered as the asexual morph of Hypohelion scirpinum by several authors (Grove 1937; Sutton 1980; Minter 1997). Johnston et al. (2014) regarded these two genera as taxonomically congeneric and recommended to use Hypohelion for the holomorph, because the latter name is well-established. The asexual morph of Hypohelion (= Leptostroma) is similar to Polystigma (= Polystigmina) in having holoblastic, sympodial to synchronous, indeterminate conidiogenous cells. However, Polystigma has filiform, hamate, tapered towards the apices, guttulate conidia, whereas Hypohelion has ellipsoid, eguttulate conidia (Sutton 1980; Dayarathne et al. 2017). There is no molecular data available for asexual morph of H. scirpinum (= L. scirpinum). To clarify the taxonomy of Hypohelion, many species will have to be recollected, and epitypified, so that authentic cultures and molecular data will become available to fix the genetic concept.
Distribution: Canada, China, Germany, Hungary, Latvia, Sweden, USA (Sutton 1980; Lantz et al. 2011, Wang et al. 2014a, b).
Hysterodiscula Petr., Bot. Arch. 43: 210 (1942) [1941]
Facesoffunginumber: FoF 07417, Fig. 190
Hysterodiscula empetri (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiophores. c Conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitats, such as Empetrum nigrum (Ericaceae), E. hermaphroditicum (Sutton 1980). Sexual morph: undetermined. Asexual morph:Conidiomata black, stromatic, pycnidial, solitary to gregarious or confluent, immersed, elongated or irregular in shape, irregularly multilocular, glabrous. Ostiole absent, dehiscence by longitudinal fissures in the upper wall. Conidiomatal wall composed of thin-walled, pale brown to hyaline cells of textura angularis in the basal and lateral part, becoming thicker, dark brown cells in the upper part. Conidiophores arising all around the locular cavity, hyaline, cylindrical, branched at the base, septate, with acrogenous conidia. Conidiogenous cells hyaline, holoblastic, cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, cylindrical to clavate, obtuse at apex, narrow and truncate at base, unicellular, smooth-walled.
Type species: Hysterodiscula empetri Petr., Bot. Arch. 43: 211 (1942) [1941]
Notes: Petrak (1942) found that Melasmia empetri Magnus differs from typical members of the genus in form of conidiomata, conidiophores and conidia. He proposed a new genus Hysterodiscula to accommodate this species and another species H. kalmiae. Sutton (1980) re-described and illustrated H. empetri and provided a detailed description for the genus. The sexual morph of H. empetri has been assigned to Duplicaria empetri (Pers.) Fuckel (type species of Duplicaria Fuckel) by several authors (Lind 1913; Nannfeldt 1932; Darker 1967; Dennis 1968). However, Powell (1973) rejected the connection of M. empetri with D. empetri. Wijayawardene et al. (2017b) accepted only H. kalmiae Petr. in the genus. We accept two species in the genus, which has not been studied with molecular data.
Distribution: Finland, France, Germany, Latvia, Sweden (Sutton 1980).
Idiocercus B. Sutton, Can. J. Bot. 45(8): 1255 (1967)
Facesoffungi number: FoF 07418, Fig. 191
Idiocercus pirozynskii (redrawn from Nag Raj 1993) a Vertical section of conidioma. b Partial enlarged sectional view of the wall tissue near the ostiolar region. c Microconidiogenous cells and microconidia. d Macroconidiogenous cells with developing conidia. e Macroconidia
Ascomycota, genera incertae sedis
Saprobic or parasitic on leaves of Harungana madagascariensis (Hypericaceae) and Eucalyptus gracilis (Myrtaceae) (Sutton 1967a; Swart 1988; Crous et al. 1990). Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, solitary, immersed, globose to subglobose, unilocular, glabrous, ostiolate. Ostiole single, circular, papillate. Conidiomatal wall composed of thin-walled, brown cells of textura prismatica in the basal and lateral part, becoming thick-walled, dark brown cells of textura globulosa at ostiolar region. Conidiophores often reduced to conidiogenous cells. Conidiogenous cells arising from inner wall layer of conidiomata, hyaline, enteroblastic, annellidic, indeterminate, smooth-walled, and of two kinds: (a) those producing macroconidia lining the base and up the side walls of the conidioma, subcylindrical to irregular; (b) those producing microconidia restricted to the inner layer of the conidiomatal wall close to the ostiolar region, subcylindrical to broadly ampulliform. Macroconidia hyaline, clavate to ellipsoid, unicellular, smooth-walled, with or without a tubular, conical, short apical appendage. Microconidia hyaline, unicellular, smooth-walled.
Type species: Idiocercus pirozynskii B. Sutton, Can. J. Bot. 45(8): 1256 (1967)
Notes: Sutton (1967a, b) introduced Idiocercus to accommodate I. pirozynskii (generic type) and I. macarangae (T.S. Ramakr.) B. The third species I. australis (Cooke) H.J. Swart was described from Eucalyptus gracilis (Myrtaceae) by Swart (1988). Nag Raj (1993) re-examined the type specimen and emended the description and illustration of Idiocercus. Idiocercus australis was excluded from the genus because of its conidial morphology. Idiocercus macarangae was designated as the type for the new genus Xenidiocercus Nag Raj. Wu and Sutton (1995) and Wijayawardene et al. (2017b) accepted I. australis as a separate species in Idiocercus. We check the description and illustration of I. australis and then excluded this species from Idiocercus as Nag Raj (1993). Idiocercus pirozynskii has annellidic conidiogenous cells with widely-spaced, flared annellations, which differs those conidiogenous cells in I. australis no flared annellations. In addition. Idiocercus pirozynskii has hyaline, clavate to ellipsoid, unicellular conidia with or without tubular, conical apical appendage. However Idiocercus australis has cylindrical to long clavate or slightly elliptical conidia lacking an apical appendage (Crous et al. 1990). There is no sexual morph linked to Idiocercus, and no molecular data is available.
Distribution: Sierra Leone, Tanzania (Nag Raj 1993).
Jahniella Petr., Ann Mycol. 18(4/6): 123 (1921) [1920]
Facesoffungi number: FoF 07395, Fig. 192
Jahniella bohemica (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary, subepidermal to epidermal, immersed, globose in sectional view, unilocular, glabrous, ostiolate. Ostioles single, circular, papillate, centrally located, with a distinct channel. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the exterior, becoming thicker, pale brown to hyaline cells towards conidial hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the inner wall layer of conidiomata, hyaline, holoblastic, ampulliform, discrete, indeterminate, smooth-walled. Conidia hyaline, cylindrical to fusiform, tapered towards apex, truncate at the base, straight or slightly curved, 3–4-septate, smooth-walled, eguttulate.
Type species: Jahniella bohemica Petr., Ann Mycol. 18(4/6): 123 (1921) [1920]
Notes: Jahniella was re-described by Sutton (1980) and Quaedvlieg et al. (2013). Three taxa (e.g., J. bohemica Petr., J. campanulae-cervicariae (Vestergr.) Petr., J. hordei Petr.) are accepted in the genus, but none of them have been studied with molecular data. Jahniella is similar to Septoria in having hyaline, multi-septate conidia, but it can be separated from the latter by conidiogenous cells. Septoria has proliferate sympodially and percurrently conidiogenous cells, a feature absent in Jahniella (Sutton 1980; Quaedvlieg et al. 2013). This taxon needs recollection and study. This taxon needs recollection and study.
Distribution: Czech Republic, France, Germany, Russia, Ukraine (Quaedvlieg et al. 2013; Wijayawardene et al. 2017).
Kabatia Bubák, in Kabát & Bubák, Öst. bot. Z. 54: 28 (1904)
Facesoffungi number: FoF 07422, Fig. 193
Kabatia spp. (redrawn from Sutton 1980, a, b, e K. periclymeni, c, f K. lonicerae var. involucratae, d K. latemarensis) a Surface view of conidioma. b Vertical section of conidioma. c Conidiogenous cells and developing conidia. d–f Conidia
Dothideomycetes, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, solitary, superficial, rounded in outline, subglobose in section view, unilocular. Ostiole absent, dehiscence by radial splitting between the hyphae of the upper wall from the centre to the periphery. Conidiomatal wall composed of thin-walled, hyaline, large cells of textura angularis in the basal part, thick-walled, dark brown cells converging towards the centre and remaining distinctly hyphal in the upper part. Conidiophores arising from the inner cell layer of the basal stroma, reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, doliiform to cylindrical, determinate, smooth-walled. Conidia hyaline, clavate, fusiform, cylindrical or falcate, truncate at the base, tapered towards apex, 0–2-septate near the apices, smooth-walled, rarely guttulate.
Type species: Kabatia latemarensis Bubák, in Kabát & Bubák, Öst. bot. Z. 54: 28 (1904)
Notes: Kabatia was revised by Conners (1959) and Sutton (1980), and ten species were accepted in the genus. These taxa are associated with leaf lesions of plants in Caprifoliaceae (e.g., Lonicera caerulea, L. canadensis, L. conjugialis, L. involucrata, L. oblongifolia, L. utahensis). Kabatia is characterized by unilocular conidiomata with radiating hyphal structure in the upper wall, and fusiform to falcate conidia with 0–2 septa near the apices (Sutton 1980). The sexual morph has been assigned to Guignardia Viala & Ravazby Reusser (1964) and Discosphaerina Höhn. by Sivanesan (1984). However, these connections have not been satisfactory demonstrated. Molecular studies are necessary to link its correct sexual morph.
Distribution: Armenia, Canada, China, Czechoslovakia, Germany, Italy, Japan, Poland, Russia, Spain, Scotland, Switzerland, Turkey, USA (Sutton 1980).
Keissleriella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 128(7–8): 582 (1919)
Facesoffungi number: FoF 07424
Dothideomycetes, Pleosporomycetidae, Pleosporales, Lentitheciaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: see Barr (1990), Tanaka et al. (2015), Wanasinghe et al. (2018). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, globose to subglobose, unilocular, glabrous, ostiolate. Ostiole single, circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores arising from the inner wall layer of the conidiomata, hyaline, cylindrical, septate, branched and septate. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, or ampuliform, integrated or discrete, determinate, smooth-walled. Conidia hyaline or brown, cylindrical or oblong, 0–1-septate, guttulate, smooth-walled. Spermatia absent or present, hyaline, cylindrical or bone-shaped, smooth-walled.
Type species: Keissleriella aesculi (Höhn.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 128(7–8): 582 (1919)
Notes: Over 40 taxa are accepted in the genus (Tanaka et al. 2015; Tibpromma et al. 2017; Wanasinghe et al. 2018). Zalasky (1974) linked K. emergens to Diplodia tumefaciens (Shear) Zalasky. Sivanesan (1984) connected K. alpina and K. cladophila to Dendrophoma, and K. gallica to Ascochyta. However, Ascochyta, Diplodia and Dendrophoma are confirmed in Didymellaceae (Aveskamp et al. 2010; Chen et al. 2015, 2017; Valenzuela-Lopez et al. 2018), Botryosphaeriaceae (Liu et al. 2012; Phillips et al. 2013; Slippers et al. 2013) and Chaetosphaeriaceae (Crous et al. 2012b), respectively. Tanaka et al. (2015) introduced three new taxa in Keissleriella, and described the asexual morph of K. quadriseptata as having hyaline to brown, aseptate to 1-septate, cylindrical conidia from culture. Crous et al. (2015a) and Tibpromma et al. (2017) noted that Pleurophoma and Keissleriella might be related based on their phylogenies, but that this needed further investigation. We found that a fresh collection on Ammophila arenaria shares similar morphology with the generic type of Pleurophoma (P. pleurospora), but can be distinguished by phylogeny (Fig. 194). Based on phylogeny, this strain is regarded as the microconidial or spermogonial state of K. quadriseptata. Sufficient samples of pleurophoma-like asexual morph are needed to clarify the relationship between Keissleriella and Pleurophoma.
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, SSU, ITS, tef1 (2218R/983F) sequences data of Keissleriella and related genera in Lentitheciaceae. Fifty-four strains are included in the analyses, which comprise 3210 characters including gaps (LSU: 1–815, SSU: 816-1763, ITS: 1764–2296, tef1: 2297–3210). Massarina eburnea (CBS 473.64, H 3953) is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 13200.401362 is presented. The matrix had 800 distinct alignment patterns, with 25.51% of undetermined characters or gaps. Estimated base frequencies were: A = 0.237554, C = 0.251673, G = 0.273334, T = 0.237438; substitution rates AC = 1.079965, AG = 1.791604, AT = 1.565961, CG = 1.634128, CT = 8.355853, GT = 1.000000 gamma distribution shape parameter α = 0.163425. Maximum maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.02 changes. Ex-type strains are in bold. New isolates are shown in bold and blue
Distribution: Canada, Italy, Japan, Russia, Netherlands, UK (Zalasky 1974; Crous et al. 2014b; Liu et al. 2015a, b; Tanaka et al. 2015; Tibpromma et al. 2017; Wanasinghe et al. 2018, this study).
Keissleriella quadriseptata Kaz. Tanaka & K. Hiray., in Tanaka et al., Stud. Mycol. 82: 96 (2015)
Facesoffungi number: FoF 07425, Fig. 195
Keissleriella quadriseptata (MFLU 19-2871) a, b Appearance of dark brown to black conidiomata on the host. c Vertical section of peridium. d Vertical section of conidioma. e–i Conidiophores, conidiogenous cells and developing conidia. j Germinating conidium. k Conidia. l, m Culture on PDA. Scale bars: a–b = 200 µm, c = 50 µm, d = 100 µm, e = 20 µm, f–i, k = 10 µm, j = 20 µm, l–m = 10 mm
Saprobic on dead stem of Ammophila arenaria.Sexual morph: see Tanaka et al. (2015), Asexual morph:Conidiomata 150–300 μm diam., 200–400 μm high, dark brown to black, pycnidial, solitary, semi-immersed, globose in section view, unilocular, ostiolate. Ostiole 60–90 μm long, 50–75 μm wide, papillate, centrally located. Conidiomatal wall 10–25 μm wide, composed of dark brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, lageniform. Conidia 25–32 × 6–8.5 μm (\( \bar{x} \) = 28.4 × 7.2 μm, n = 30), hyaline to brown with age, cylindrical, with rounded apex, slightly truncate at base, straight, 0–1-septate, smooth-walled (re-described from Tanaka et al. (2015). Spermatogonia 100–140 µm diam., 150–200 µm high, dark brown to black, pycnidial, solitary to gregarious, globose to subglobose, immersed to semi-immersed, unilocular, glabrous, ostiolate. Ostiole single, papillate, circular, centrally located. Wall 20–30 µm wide, composed of thick-walled, dark brown cells of textura angularis in the exterior, becoming hyaline cells towards conidial hymenium. Spermatophores arising all around the cavity of the conidiomata, hyaline, cylindrical, branched at the base and above, septate, smooth-walled. Spermatogenous cells 5–7 × 3–5 µm, hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, determinate, smooth-walled, with periclinal wall thickenings at the collarette zone, with terminal apertures or lateral apertures formed from subtending cells of conidiophores immediately below the transverse septa. Spermatia 3–4 × 2–3 µm (\( \bar{x} \) = 3.5 × 2.5 µm; n = 30), hyaline, cylindrical to oblong, aseptate, guttulate, smooth-walled.
Material examined: Italy, Province of Ravenna, Lido di Dante, on dead aerial stems of Ammophila arenaria (Poaceae), 27 January 2015, Erio Camporesi, IT2355 (MFLU 19-2871), living culture MFLUCC 15-0131 = ICMP 21845 = KUMCC 15-0585, (KUN, HKAS 101689).
Notes: Phylogenetic tree reconstruction based on LSU, SSU, ITS, tef1 sequence dataset shows that strain MFLUCC 15-0131/IT2355 is close to K. quadriseptata. The sequence similarities between MFLUCC 15-0131/IT2355 and K. quadriseptata (KT 2292) are 99% (847/850) in LSU, 99% (492/496) in ITS, 99% (1017/1028) in SSU, and 98% (903/924) in tef1. Our strain has hyaline, cylindrical to oblong, aseptate, guttulate spermatia which is similar to these of spermatial morph in K. gloeospora and K. taminensis (Tanaka et al. (2015).
Kellermania Ellis & Everh., J. Mycol. 1(12): 153 (1885)
Facesoffungi number: FoF 06690, Fig. 196
Kellermania yuccigena (redrawn from Morgan-Jones et al. 1972c) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Conidia
Dothideomycetes, Incertae sedis, Botryosphaeriales, Planistromellaceae
Saprobic on plants in terrestrial habitats, such as Asparagaceae, subfamilies Agavoideae and Nolinoideae (Nag Raj 1993; Minnis et al. 2012; Crous et al. 2014a). Sexual morph: see Monkai et al. (2013). Asexual morph:Conidiomata dark brown, pycnidial, solitary to aggregated, immersed, globose in section view, unilocular or multilocular, glabrous, ostiolate. Ostiole single, circular or oval, non-papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown to brown cells of textura prismatica in the exterior, gradually merging with thin-walled, hyaline cells of textura angularis in the conidial hymenium, becoming thicker, pale brown to hyaline cells towards conidial hymenium, with columnar, thin-walled, hyaline cells surrounding the ostiole. Conidiophores reduced to conidiogenous cells, invested in mucus. Conidiogenous cells hyaline, holoblastic, discrete, determinate, often of two kinds, those producing macroconidia, arising from the basal and lateral wall, cylindrical to subcylindrical, smooth-walled; those producing microconidia restricted in the inner wall around the ostiolar region, ampulliform to broadly ampulliform, smooth-walled. Macroconidia hyaline, cylindrical to narrowly clavate, truncate at the base, unicellular or euseptate, bearing single, multiple, stout and unbranched, attenuated, flexuous apical appendages or lacking appendages. Microconidia hyaline, cylindrical to ellipsoidal or irregular, rounded and blunt at the apex, truncate at the base, unicellular, smooth-walled.
Type species: Kellermania yuccigena Ellis & Everh., J. Mycol. 1(12): 154 (1885)
Notes: Kellermania was revised by Sutton (1968, 1980), Morgan-Jones et al. (1972a) and Nag Raj (1993). Minnis et al. (2012) included molecular data and placed Kellermania in Planistromellaceae (Botryosphaeriales). They stated that conidial appendages as single characters have insufficient value to separate genera in coelomycetes, and then reduced Alpakesa Subram. & K. Ramakr. and Piptarthron Mont. ex Höhn. to synonymy with Kellermania. Crous et al. (2014a) designated an epitype on leaves of Yucca rostrata (Asparagaceae) from USA and confirmed the placement of Kellermania as in Minnis et al. (2012). Wijayawardene et al. (2017b) estimated there to be 20 species in Kellermania. The sexual morph of Kellermania has been assigned to Planistroma A.W. Ramaley and Planistromella A.W. Ramaley (1991, 1992, 1993, 1995, 1998), but they both were reduced to synonymy with Kellermania (Minnis et al. 2012). Monkai et al. (2013) considered Planistroma as a separated genus based on LSU and ITS sequence data.
Minnis et al. (2012) adopted a broad generic circumscription for Kellermania, including single, multiple, or no conidial appendages taxa. This broad generic concept can easily lead to confusion between Kellermania and other genera, such as Brencklea, Crinitospora and Parastagonospora. Brencklea is similar to K. yuccigena in septate conidia bearing an attenuated, cellular, apical appendage, but it can be distinguished from the later by its hyaline to pale brown, fusiform to naviculate conidia. Crinitospora has hyaline, 1-euseptate conidia bearing multiple, apical appendages (Type A, see Nag Raj 1993), which are similar to K. uniseptata (Minnis et al. 2012). However, Crinitospora can be distinguished from K. uniseptata by its annellidic conidiogenous cells and acervular conidiomata. Parastagonospora share similar morphology of conidia (cylindrical, euseptate, lacking conidial appendages) with K. yuccifoliorum, K. confusa and K. macrospora. However, they differ in phylogeny (Parastagonospora belongs to Planistromellaceae, and Parastagonospora belongs to Phaeosphaeriaceae). The conidia of Kellermania are variable in shape, septate, appendages. In the future, a large number of collections of Kellermania species are needed to clarify the generic boundary.
Distribution: Algeria, France, Mexico, USA (Nag Raj 1993; Minnis et al. 2012; Monkai et al. 2013; Crous et al. 2013, 2014a).
Kendrickomyces B. Sutton, V.G. Rao & Mhaskar, Trans. Br. Mycol. Soc. 67(2): 243 (1976)
Facesoffungi number: FoF 07423, Fig. 197
Kendrickomyces indicus (redrawn from Sutton 1980) a Conidia. b Conidiophores and conidiogenous cells. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Saprobic on dead branches of Terminalia belerica (Combretaceae) and parasitic on fruits of Ziziphus jujuba (Rhamnaceae) (Sutton 1980; Mandal and Dasgupta 1984). Sexual morph: undetermined. Asexual morph:Conidiomata stromatic, pycnidial, at maturity furfuraceous, solitary to gregarious or confluent, immersed, globose to collabent, multilocular, glabrous, ostiolate. Locules initiated in the upper layer, aggregated into 1–2 separate groups within the stroma, vertically elongated, regularly arranged. Ostiole single to each locule at first, then merging into a single communal ostiole, papillate, circular. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the outer peripheral wall, gradually merging with thick-walled cells of textura oblita in the middle layer, becoming progressively looser towards upper region of the conidiomata. Conidiophores arising from the inner layer of locular wall, hyaline, cylindrical or irregular, irregularly branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, integrated, determinate, smooth-walled, with periclinal thickening, with minute channel and collarette. Conidia hyaline, allantoid, unicellular, smooth-walled, guttulate.
Type species: Kendrickomyces indicus B. Sutton, V.G. Rao & Mhaskar, Trans. Br. Mycol. Soc. 67(2): 244 (1976)
Notes: Kendrickomyces is characterized by complex multilocular conidiomata, furfuraceous ostioles, phialidic conidiogenous cells and falcate conidia (Sutton et al. 1976). The furfuraceous ostioles in Kendrickomyces can easily be confused with asexual morphs of Phacidium (especially for P. italica), Cytospora and Waydora. However, these genera can be separated by differences in conidiomata and conidia. The asexual morph of Phacidium has fusiform to ellipsoid or cylindrical conidia bearing funnel-shaped mucoid appendages. While the conidia of Cytospora, Kendrickomyces, and Waydora are allantoid, and lack conidial appendages (Nag Raj 1993, this study). Kendrickomyces can be distinguished from Cytospora and Waydora by its complex multilocular conidiomata. Kendrickomyces has definite locular chambers surrounding by ecto- or endostroma, and distinct locular channels uniting into a main ostiole. Whereas the conidiomata of Cytospora and Waydora are convoluted and lacunose (Sutton et al. 1976; Norphanphoun et al. 2017). Cytospora differs from Waydora in multiple wall layers and locular organization in the stromata (Sutton et al. 1976; Senanayake et al. 2018). Two species are accepted in the genus, K. brevisporus N.C. Mandal & M.K. Dasgupta and K. indicus, but neither has molecular data.
Distribution: India (Sutton 1980).
Koorchaloma Subram., J. Indian bot. Soc. 32: 124 (1953)
= Pseudoornatispora Tibpromma & K.D. Hyde, in Tibpromma et al., Fungal Diversity, [115] (2018)
Facesoffungi number: FoF 06611
Sordariomycetes, Hypocreomycetidae, Hypocreales, Stachybotryaceae
Saprobic on plants in terrestrial or marine habit. Sexual morph:Perithecia dark brown for most part, becoming paler in the papilla neck and ostiolar region, collapsing when dry, discrete, globose to subglobose or pyriform, unilocular, setose, ostiolate. Ostiole circular or oval, filled with hyaline periphysis. Perithecial setae arising all around the perithecial wall in the middle part, cylindrical, septate, dark brown and thick-walled in the basal part, becoming paler and thin-walled towards apex, with swollen and bulbous terminal cells. Peridium composed of thick-walled, brown to dark brown cells of textura angularis in the exterior, gradually merging with paler or hyaline cells of textura prismatica. Asci hyaline, 8-spored, unitunicate, clavate, apical apparatus non-amyloid. Ascospores hyaline, fusiform or slightly sigmoid, occasionally lunate, 1-sepate, smooth or verrucose, guttulate (Nag Raj 1984; Tibpromma et al. 2018). Asexual morph:Conidiomata brightly coloured, stromatic, spuriously sporodochioid or shallow-cupulate with varying degrees of excipular development, superficial, gelatinous or not, setose. Conidiomatal wall consisting of two parts: (a) basal stroma with thick-walled, brown cells of textura angularis; (b) excipulium with thick-walled, pale brown to hyaline cells of textura intricata to textura oblita or textura intricata. Conidiomatal setae dark brown to black at the base, hyaline at the apex, apical cell often inflated and showing percurrent growth, subulate to subcylindrical, straight or slightly curved, marginal and irregularly interspersed, septate. Conidiophores reduced to conidiogenous cells or present, hyaline, cylindrical or lageniform, branched, septate, formed from the basal stroma. Conidiogenous cells short, holoblastic, subcylindrical, hyaline, smooth, arising from inner layers of conidiomata. Conidia fusiform, with an apiculate apex and a narrow truncate base, aseptate, hyaline, thick-walled, smooth, guttulate, bearing a flared, mucoid appendage at both ends (Nag Raj 1993).
Type species: Koorchaloma madreeya Subram., J. Indian bot. Soc. 32: 124 (1953)
Notes: Koorchaloma species are commonly found as saprobes, and have been reported in Argentina, China, Jamaica, Japan, India, Lithuania, USA and Thailand (Nag Raj 1993; Allegrucci et al. 2011, this study). This genus was originally regarded as hyphomycetous (Subramanian and Ramakrishnan 1953, 1956). However, Nag Raj (1984, 1993) revised the genus and demonstrated that the conidiomata of the generic type are indeed cupulate conidiomata with moderate development of an excipulum. Therefore, they placed it in coelomycetes and recognized five taxa. Six additional taxa, K. dimorpha Matsush., K. europaea Treigienė, K. galateae Kohlm. & Volkm.-Kohlm., K. novojournalis Yanna, K.D. Hyde & Goh, K. scutiae Allegr., Eliades & Aramb., and K. spartinicola V.V. Sarma et al. were added (Yanna et al. 1998a; Kohlmeyer and Volkmann-Kohlmeyer 2001; Matsushima 2003; Treigienė 2006; Allegrucci et al. 2011).
Based on culture studies, Koorchaloma was connected to a Kananascus Nag Raj sexual morph by Nag Raj (1984). Kananascus is characterized by globose to subglobose, unilocular, setose perithecia, unitunicate, 8-spored asci with J- apical rings and hyaline, fusiform, 1-septate, verrucose or smooth-walled ascospores. Tibpromma et al. (2018) described a new genus Pseudoornatispora on Pandanus sp. (Pandanaceae) in Thailand, with P. krabiense as the type species. However, the morphology of the sexual morph of P. krabiense is similar to the type species of Kananascus, K. verrucisporus Nag Raj. The only difference between these two species is that Pseudoornatispora krabiense has filiform, cellular, branched or unbranched paraphyses and fusiform ascospores with a mucilaginous sheath, while these characters are absent in K. verrucisporus. In addition, the asexual morph of P. krabiense also shares a similar form of conidiomata, conidiogenous cells and conidia with Koorchaloma species (especially with K. okamurae). We note that the conidia shown by Tibpromma et al. (2018; Fig. 87e–h) do not have mucoid appendages, but they can be seen in Fig. 87b, c. Phylogenetic analyses of LSU, ITS and rpb2 sequence data show that P. krabiense (MFLUCC16-0317, MFLUCC 16-0318) clustered with K. spartinicola (SAP130) and K. bambusae (MFLU 19-2899) with high bootstrap support (Fig. 198). However, Kananascus sp. (SAP141) formed a distinct branch in Stachybotryaceae (Fig. 198). Due to a lack of sequence data for Kananascus verrucisporus, it is difficult to know if Kananascus sp. (SAP141) is related. Considering the morphology and phylogeny, Pseudoornatispora is considered to be congeneric with Koorchaloma and Koorchaloma (1953) is protected over Pseudoornatispora (2018).
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of ITS, LSU and rpb2 sequences data representing Koorchaloma and other genera in Stachybotriaceae. Seventy-seven strains are included in the analyses, which comprise 2112 characters including gaps (LSU: 1–827, rpb2: 828–1548, ITS: 1549–2112). Single gene analyses were carried out and compared with each species, topology of the tree, and clade stability. Calonectria ilicicola CBS 190.50, Fusarium sambucinum CBS 146.95 and Nectria cinnabarina AR4477 are used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 23154.202969 is presented. The matrix had 882 distinct alignment patterns, with 13.84% of undetermined characters or gaps. Estimated base frequencies were: A = 0.248526, C = 0.253298, G = 0.284160, T = 0.214016; substitution rates AC = 1.616659, AG = 4.226940, AT = 1.302843, CG = 1.028656, CT = 8.666029, GT = 1.000000; gamma distribution shape parameter α = 0.592271. The maximum parsimonious dataset consisted of constant 1310, 676 parsimony-informative and 126 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 4601 steps (CI = 0.290, RI = 0.683, RC = 0.198, HI = 0.710) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.05 changes. Ex-type strains are in bold. New isolates are shown in red
Buchan et al. (2002) placed Koorchaloma in Trichosphaeriaceae (Trichosphaeriales, Sordariomycetes) based on ITS sequence of K. spartinicola. However, our phylogeny shows that Koorchaloma is related to Stachybotryaceae. It is necessary to collect and designate an epitype of K. madreeya from its original host and area to confirm its placement.
Distribution: India, Jamaica, Japan, Thailand, (Nag Raj 1993; Tibpromma et al. 2018, this study).
Koorchaloma bambusae Nag Raj, Mycotaxon 19: 175 (1984)
Facesoffungi number: FoF 07433, Figs. 199, 200
Koorchaloma bambusae (DAOM 187208, holotype) a, b Herbarium specimen. c–e Appearance of dirty yellow to brownish yellow conidiomata on the host. f Conidioma mounted in 10% KOH. g–h Setae. i Sterile hyphae at excipulum. j–l Conidiophores, conidiogenous cells and developing conidia. m–s Conidia. Scale bars: c–e = 200 µm, f = 100 µm, g–h = 20 µm, i–l = 10 µm, m–s = 5 µm
Koorchaloma bambusae (MFLU 19-2899) a Specimen. b, c Appearance of brownish coniodiomata on the host. d Conidioma mounted in water. e Conidiomatal setae. f Conidiomatal wall. g–i Conidiogenous cells and developing conidia. j–p Conidia. Scale bars: b = 1000 µm, c–d = 200 µm, e = 50 µm, f = 20 µm, g–j = 10 um, k–p = 5 µm
Saprobic on dead leaves and culms of Bambusa sp. (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata up to 150 μm long and up to 100 μm wide, off-white when moist, dirty yellow to brownish yellow when dry, stromatic, shallow-cupulate, amphigenous when foliicolous, solitary to gregarious and often confluent, superficial, appearing as pulvinate, gelatinous, flat crusts, rounded to oval in outline, occasionally elongate, setose. Conidiomatal wall composed of two types: (a) basal wall of textura angularis with thick-walled, dark brown cells; (b) reduced excipulum of textura oblita with pale brown cells in the outer layers gradually merging with a loose textura intricata with hyaline cells in the inner layers. Conidiomatal setae 65–180 μm long, 3–13 μm wide, dark brown, becoming progressively paler towards the apex, cylindrical, slightly inflated at base and apex, straight or variously curved, up to 3-septate, unbranched, thick-walled, interspersed through the basal stroma. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 8–14 × 2.5–5 µm (\( \bar{x} \) = 10 × 3 μm), hyaline, cylindrical to ampulliform or lageniform, with minute periclinal thickenings, discrete, smooth-walled. Conidia 10–16 × 4–6 µm (\( \bar{x} \) = 13 × 5 µm, n = 150), mostly hyaline, fusiform, with an apiculate apex and a narrow truncate base, aseptate, thick- and smooth-walled, guttulate, bearing flared apical and basal mucoid appendages.
Material examined: Jamaica, Maggotty, on dead leaves and culms of Bambusa sp. (Poaceae), 30 May 1978, T.R. Nag Raj (DAOM 187208, holotype); Thailand, Chiang Rai, on dead leaves of bamboo, 19 April 2014, Sun-Jun Zu, WJL0037 (MFLU 19-2899), (KUN, HKAS 93583).
Notes: The new collection (MFLU 19-2899/WJL037) from Thailand is similar to the type of Koorchaloma bambusae in having yellow to brownish yellow, shallow-cupulate, setose conidiomata with a basal stroma composing cells of textura angularis to textura intricta, phialidic, cylindrical to ampulliform conidiogenous cells and fusiform conidia bearing mucoid appendages. The new collection shares similar conidial dimensions to the conidia of the holotype of K. bambusae (wjl037: 10–16 × 4–6 µm (\( \bar{x} \) = 14 × 5 µm, DAOM 187208: 11–14 × 4–5.5 µm (\( \bar{x} \) = 12 × 5 µm). According to morphology, habit and host, our collection is identified as a new record of K. bambusae from Thailand.
Koorchaloma jamaicensis Nag Raj, Mycotaxon 19: 179 (1984)
Facesoffungi number: FoF 07434, Fig. 201
Koorchaloma jamaicensis (DAOM 187210, type) a, b Herbarium specimen. c–e Appearance of dark brown to black conidiomata on the host. f Surface of conidiomata. g Setae. h Conidioma mounted in 10% KOH. i–l Conidiophores, conidiogenous cells and developing conidia. m–p Conidia. Scale bars: c–d = 500 µm, e = 200 µm, f = 50 µm, g = 20 µm, h = 100 µm, i–l = 10 µm, m–p = 5 µm
Saprobic on blades of grass. Sexual morph: see Nag Raj (1984). Asexual morph:Conidiomata 100–300 µm diam., 30–50 µm deep (conidiomatal dimension from Nag Raj 1993, excluding the conidial mass), dark brown to black, stromatic, shallow-cupulate, amphigenous, solitary to gregarious and occasionally confluent, superficial, appearing as pulvinate, gelatinous, flat crusts, rounded to cylindrical or irregular in outline, setose. Conidiomatal wall composed of two types: (a) basal wall of textura angularis with thick-walled, dark brown cells; (b) excipulum reduced, of textura oblita with pale brown cells in the outer layers gradually merging with a loose textura intricata with hyaline cells in the inner layers. Conidiomatal setae 100–200 μm long, 3–12 μm wide, marginal and irregularly interspersed, dark brown to black in the basal part, becoming paler above, with a somewhat bulbous, almost colourless, thin-walled, occasionally percurrent growth of terminal cell, subulate to subcylindrical, straight or variously curved, unbranched, septate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 8–12 × 2–3 μm, hyaline, ampulliform to lageniform, with minute periclinal thickenings in the collarette zone, discrete, smooth-walled. Conidia 15–20 × 3–4 (\( \bar{x} \) = 17 × 3.4) μm, hyaline, naviculate, occasionally fusiform, with an obtuse apex and a narrow truncate base, aseptate, thick- and smooth-walled, guttulate, bearing initially funnel-shaped appendages, then splitting into several radiating strands at each end.
Material examined: Jamaica, Montego Bay, Seawind Beach Resort area, on blades of undetermined grass, 27 May 1978, T.R. Nag Raj (DAOM 187210, type).
Koorchaloma krabiense (Tibpromma & K.D. Hyde) W.J. Li & K.D. Hyde, comb. nov.
= Pseudoornatispora krabiense Tibpromma & K.D. Hyde, in Tibpromma et al., Fungal Diversity: [115] (2018)
Index Fungorum number: IF557169, Facesoffungi number: FoF 07435
Description and illustration: See Tibpromma et al. (2018)
Notes: The differences between sexual morph and other Koorchaloma species has been discussed above (see notes under Koorchaloma genera). The conidia and conidiomata of K. krabiense (= P. krabiense) are similar to K. okamurae, but can be distinguished by the conidiophores. Koorchaloma okamurae has hyaline, cylindrical, branched conidiogenous cells, while this character is absent in K. krabiense.
Koorchaloma occidentale Nag Raj, Mycotaxon 19: 191 (1984)
Facesoffungi number: FoF 07436, Fig. 202
Koorchaloma occidentale (DAOM 187212, type) a, b Herbarium specimen. c, d Appearance of yellow to brownish yellow conidiomata on the host. e, f Conidiomata mounted in 10% KOH. g Excipular elements. h, i Conidiophores and conidiogenous cells. j–n Conidia. Scale bars: c–d = 200 µm, e–f = 100 µm, g = 20 µm, h–n = 10 µm
Saprobic on blades of Gramineae. Sexual morph: undetermined. Asexual morph:Conidiomata 80–180 µm diam., 40–70 µm deep (conidiomatal dimension from Nag Raj 1993, excluding the conidial mass), with yellow to brownish yellow crusts, stromatic, shallow-cupulate, mostly solitary, sometimes gregarious or confluent, superficial, appearing as pulvinate, gelatinous, rounded to oval in outline, setose. Conidiomatal wall composed of two types: (a) basal wall of textura angularis with hyaline cells; (b) excipulum moderately developed, of textura oblita in the outer layers merging with textura intricata in the inner layers. Conidiomatal setae 80–160 μm long, 3–10 μm wide, interspersed throughout the conidioma, brown in the basal part, becoming pale yellow to almost colourless at the apex, subulate with a slightly bulbous base and an acute apex, straight or variously curved, unbranched, septate, with the septa indistinct in the upper part, often showing percurrent growth. Conidiophores hyaline, cylindrical to subcylindrical, branched, septate, invested in mucus. Conidiogenous cells 12–17 × 2–3 μm, hyaline, ampulliform to lageniform or obclavate, with minute collarettes, discrete or sometimes integrated, smooth-walled. Conidia 8–14 × 2–4 (\( \bar{x} \) = 11 × 3) μm, hyaline, fusiform, with an acute apex and a narrow truncate and often apiculate base, aseptate, smooth-walled, guttulate, bearing a mucoid appendage at each end.
Material examined: Jamaica, Montego Bay, Seawind Beach Resort area, on blades of Gramineae, 27 May 1978, T.R. Nag Raj (DAOM 187212, type).
Koorchaloma okamurae I. Hino & Katum., Icones Fungorum Bamb. Jap.: 264 (1961)
Facesoffungi number: FoF 07437, Fig. 203
Koorchaloma okamurae (DAOM 187213, isotype) a, b Herbarium specimen. c–e Appearance of yellowish to orange-yellow conidiomata on the host. f Conidioma mounted in 10% KOH. g–j Conidiophores, conidiogenous cells and developing conidia. k–l Setae. m–r Conidia (p–r staining in lactophenol cotton blue and India ink). Scale bars: c–d = 500 µm, e = 200 µm, f = 100 µm, g–r = 10 µm
Saprobic on dead culms of Sinobambusa tootsik (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 150–300 µm diam., or up to 900 µm diam, when confluent, 35–45 µm deep (conidiomatal dimension from Nag Raj 1993, excluding the conidial mass) yellowish to orange-yellow, stromatic, shallow-cupulate, solitary to gregarious or occasionally confluent, superficial, appearing as pulvinate, gelatinous, rounded to oval or heart-shaped in outline, setose. Conidiomatal wall composed of two types: (a) basal wall of textura angularis with hyaline cells; (b) excipulum well developed, of textura intricata with septate, branched, hyaline, hyphoid elements. Conidiomatal setae up to 200 µm long, 9–10 µm wide at the base, 5–7 µm wide above (conidiomatal setae dimension from Nag Raj 1993), marginal, brown to dark brown at the base, becoming paler and slightly attenuated towards the apex, cylindrical to subcylindrical, straight or slightly curved, unbranched, septate. Conidiophores hyaline, cylindrical to subcylindrical, branched, septate, formed from the innermost cells of the basal stroma. Conidiogenous cells 14–30 × 2–4 μm, hyaline, subcylindrical, with marked periclinal thickenings and flared collarettes, discrete, smooth-walled. Conidia 10–14 × 3–5.5 (\( \bar{x} \) = 12 × 4.5) μm, hyaline, fusiform, obtuse at the apex, narrowed and slightly truncate at the base, aseptate, thick and smooth-walled, guttulate, bearing a funnel-shaped, mucoid appendage at each end.
Material examined: Japan, Kyoto Pref., Kyoto-si, Arashiyama, on dead culms of Sinobambusa tootsik (Makino) Makino (Poaceae), 27 August 1960, H. Okamura & H. Muroi (DAOM 187213, isotype).
Leptodermella Höhn., Z. Gärungsphysiol. 5: 212 (1915)
Facesoffungi number: FoF 07426, Fig. 204
Leptodermella incarnata (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of ACT, LSU and ITS sequences data representing Leptosphaeria and allied genera. Thirty-two strains are included in the analyses, which comprise 1618 characters including gaps. Alternariaster helianthi CBS 327.69 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 4834.043321 is presented. The matrix had 298 distinct alignment patterns, with 12.75% of undetermined characters or gaps. Estimated base frequencies were: A = 0.234301, C = 0.235164, G = 0.276778, T = 0.253757; substitution rates AC = 1.699909, AG = 1.997824, AT = 1.681020, CG = 0.712676, CT = 4.465480, GT = 1.000000; gamma distribution shape parameter α = 0.074292. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.02 changes. Ex-type and reference strains are in bold. New isolates are in bold and blue
Leptosphaeria sydowii(MFLU 15-1240) a Herbarium specimen. b–e Appearance of black conidiomata on the host. f–h Vertical sections of conidiomata. i, j Section of peridium. k–q Conidiogenous cells and developing conidia. r Conidia. Scale bars c = 500 µm, d–e = 200 µm, f–h = 100 µm, i–j = 20 µm, k–q = 5 µm, r = 10 µm
Leptothyrina rubi (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Leptotrochila medicaginis (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and developing conidia
Libartania laserpitii (redrawn from Nag Raj and DiCosmo 1980) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Conidia
Ligniella atrata (redrawn from Nag Raj and DiCosmo 1981a) a Conidia. b Vertical section of conidioma. c Conidiophores and conidia
Mastigosporella hyalina (redrawn from Nag Raj 1993) a Macroconidia and microconidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS, tef1 and tub2 sequences data of Melanopsaceae and related taxa. Nineteen strains are included in the analyses, which comprise 2155 characters including gaps (LSU: 1–857, ITS: 858–1412, tef1: 1413–1720, tub2: 1721–2155). Saccharata proteae CBS 115206 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the maximum parsimony and the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 7148.390120 is presented. The matrix had 554 distinct alignment patterns, with 11.45% of undetermined characters or gaps. Estimated base frequencies were: A = 0.217404, C = 0.277507, G = 0.285037, T = 0.220052; substitution rates AC = 1.336840, AG = 2.207893, AT = 1.652168, CG = 1.519891, CT = 5.976852, GT = 1.000000; gamma distribution shape parameter α = 0.213950. The maximum parsimonious dataset consisted of constant 1591, 414 parsimony-informative and 150 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 874 steps (CI = 0.840, RI = 0.921, RC = 0.774, HI = 0.160) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.03 changes. Ex-type or ex-epitype strains are in bold. New isolates are in blue
Melanops fagicola (MFLU 19-2860, holotype, MFLU 19-2862, paratype) a, d Herbarium packages and specimens. b, c, e–g Appearance of brown to dark brown conidiomata on the host. h, k, n Vertical sections of peridium. i, j, m Vertical sections of conidiomata. l Ostiole. o–q Conidiogenous cells and developing conidia. r Paraphyses. s Germinated conidium. t–w Conidia (arrows show mucus sheath). x, y Culture on PDA. Scale bars c = 500 µm, e–g, i, m = 200 µm, h, k, n = 50 µm, j = 100 µm, l, o, r–s = 20 µm, p–q, t–w = 10 µm, x–y = 50 mm
Melanops tulasnei(MFLU 19-2889) a Herbarium specimen. b–f Appearance of dark brown to black conidiomata on the host. g–i Vertical sections of conidiomata. j–k Vertical section of peridium. l, r Paraphyses. m, o, p Conidiogenous cells and developing conidia. n Immature asci. q, s–v Conidia (arrows show mucus sheath). Scale bars b = 1000 µm, c–f = 500 µm, g–h = 200 µm, i = 100 µm, j–k = 50 µm, l, n, p, q, s–v = 20 µm, m, o, r = 10 µm
Metazythia caespitosa (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidiomata
Micraspis acicola (asexual morph, redrawn from Nag Raj and DiCosmo 1980) a Vertical section of conidioma. b Conidiomatal wall. c Conidiophores, conidiogenous cells and developing conidia. d Conidia
Microdiscula rubicola (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Microperella quercus (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Mirimyces pulcher (DAOM 215253, holotype) a, b Herbarium package and specimen. c Appearance of dark brown conidiomata on the host. d–f Vertical section of conidiomata. g, i, j Conidiomatal wall. h, k–q Conidiogenous cells and developing conidia. r–x Conidia. Scale bars c = 200 µm, d–f = 100 µm, g, j = 20 µm, h, k–q = 5 µm, i = 10 µm, r–x = 5 µm
Monochaetiella hyparrheniae (redrawn from Morgan-Jones 1977) a Conidia. b Partial view of vertical section of conidioma. c Conidiogenous cells and developing conidia
Monochaetiellopsis themedae (redrawn from Nag Raj 1993) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Monodia minor (DAOM 215298, type) a–c Herbarium package and specimen (c dried cultures). d Side view of a conidioma. e, f Appearance of pale brown to brown conidiomata on YPSS agar. g–i Vertical section of conidiomata. j Conidiomatal wall. k Enlarged view of neck surface. l–p Conidiophores and conidiogenous cells. q–t Conidia. Scale bars d = 50 µm, e = 200 µm, f, g = 100 µm, h–i = 50 µm, j, m = 10 µm, k = 20 µm, l, n–t = 5 µm
Monostichella robergei (redrawn from Morgan-Jones et al. 1972a) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Phylogenetic tree generated from a maximum parsimony analysis based on a concatenated alignment of LSU, ITS, tef1 and tub2 sequences data of Mucoharknessia and allied genera in Botryospaheriaceae. Nineteen strains are included in the analyses, which comprise 2030 characters including gaps (LSU: 1–798, ITS: 799–1296, tef1: 1297–1590, tub2: 1591–2030). Pseudofusicoccum stromaticum CBS 117448 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the maximum parsimony and the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 7197.586893 is presented. The matrix had 522 distinct alignment patterns, with 17.13% of undetermined characters or gaps. Estimated base frequencies were: A = 0.221011, C = 0.276126, G = 0.285433, T = 0.217430; substitution rates AC = 0.988201, AG = 1.394582, AT = 0.789575, CG = 1.615438, CT = 3.517628, GT = 1.000000; gamma distribution shape parameter α = 0.173546. The maximum parsimonious dataset consisted of constant 1523, 377 parsimony-informative and 130 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 912 steps (CI = 0.770, RI = 0.812, RC = 0.625, HI = 0.230) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 40 changes. Ex-type or ex-epitype strains are in bold. New isolates are shown in blue
Mucoharknessia anthoxanthi (MFLU 15–3477, holotype) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d, e Vertical section of conidiomata. f Vertical section of peridium. g–k Conidiogenous cells and developing conidia. l–p Conidia (l–o are mounted in India ink). Scale bars b–c = 200 µm, d–e = 50 µm, f = 20 µm, g–k = 5 µm, l–p = 10 µm
Mucoharknessia anthoxanthi (MFLU 17-2782) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d, e Vertical sections of conidiomata. f Section of peridium. g–j Conidiogenous cells and developing conidia. k–n Conidia. Scale bars: a = 1000 µm. b = 500 µm. c–e = 200 µm. f = 20 µm. g–n = 10 µm
Mycothibulus mirabilis (IMI 128041, holotype, DAOM 124817, isotype) a–c Herbarium specimen (b from DAOM 124817). d, e Appearance of dark brown to black conidiomata on the host. f Yellow conidial mass at the tip of conidiomata. g, o Section of peridium. h Ostiole. i–k Vertical sections of conidiomata (k from DAOM 124817). l–n Paraphyses, conidiophores, conidiogenous cells and developing conidia (l from DAOM 124817, arrows show paraphyses). p–u Conidia. Scale bars e = 100 µm, f = 200 µm, g = 10 µm, h = 50 µm, i–k = 100 µm, l–m, o = 20 µm, n, p–u = 5 µm
Myriellina cydoniae (redrawn from Sutton 1980) a Conidiophores, conidiogenous cells and developing conidia. b Conidia. c Vertical section of conidioma
Myxothyrium leptideum (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Conidia
Nanoschema elaeocarpi (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Phylogenetic tree generated from a maximum parsimony analysis based on a concatenated alignment of act, ITS, LSU, rpb1, tef1 and tub2 sequences data of Nectria and Thyronectria. Related sequences were obtained from GenBank, Hirooka et al. (2012), and Zeng et al. (2018). Forty-nine strains are included in the analyses, which comprise 3880 characters including gaps (act: 1–584, tef1: 585–1417, ITS: 1418–1926, LSU: 1927–2725, rpb1: 2726–3349, tub2: 3350-3880). Hydropisphaera fungicola CBS 122304 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 26949.773295 is presented. The matrix had 1430 distinct alignment patterns, with 17.43% of undetermined characters or gaps. Estimated base frequencies were: A = 0.226408, C = 0.275328, G = 0.265729, T = 0.232535; substitution rates AC = 0.727729, AG = 2.16045, AT = 1.276972, CG = 0.791319, CT = 4.544990, GT = 1.000000; gamma distribution shape parameter α = 0.210600. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MP BS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.05 changes. Ex-type strains are in bold. New isolates are shown in bold and highlighted with colour
Nectria dematiosa (asexual morph, MFLU 16-1131) a Herbarium specimen. b, c Appearance of orange to red conidiomata on the host. d, e Vertical sections of conidiomata. f–h Section of peridium. i–p Conidiophores, conidiogenous cells and developing conidia. q Germinating conidia. r–t Conidia. Scale bars d–e = 500 µm, f = 25 µm, g–i, k = 50 µm, j, l, m, q = 20 µm, n–p, s–t = 5 µm, r = 10 µm
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tub2 sequences data of Neoascochyta. Related sequences were obtained from Chen et al. (2015), Valenzuela-Lopez et al. (2018) and GenBank. Thirty-tree strains are included in the analyses, which comprise 2384 characters including gaps (LSU: 1–962, ITS: 963–1453, tub2: 1454–1788, rpb2: 1789–2384). Neodidymelliopsis cannabis CBS 234.37 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 6875.550512 is presented. The matrix had 367 distinct alignment patterns, with 14.52% of undetermined characters or gaps. Estimated base frequencies were: A = 0.239145, C = 0.239844, G = 0.278891, T = 0.242119; substitution rates AC = 2.076679, AG = 6.350673, AT = 2.034061, CG = 0.968881, CT = 11.538616, GT = 1.000000; gamma distribution shape parameter α = 0.020000. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes, respectively. Hyphen (“–”) indicates a value lower than 50% for MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.02 changes. Ex-type and reference strains are in bold. New isolates are shown in bold and blue
Neoascochyta dactylidis (MFLU 19-2859, holotype) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d Vertical section of conidioma. e Vertical sections of peridium. f Ostiole. g–j Conidiogenous cells and developing conidia. k Germinating conidium. l–o Conidia. Scale bars: b = 500 µm, c = 100 µm, d = 50 µm, e = 20 µm, f = 50 µm, g = 5 µm, h–o = 10 µm
Neoascochyta desmazieri (MFLU 19-2881) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d Vertical section of conidioma; e. Vertical section of peridium. f–g, i–j Conidiogenous cells and developing conidia. h Germinated conidium. k–m Conidia. n Culture on PDA. Scale bars d = 50 µm, e, h = 20 µm, f–g, i–j = 5 µm, k–m = 10 µm, n = 25 mm
Neoascochyta tardicrescens (MFLU 19-2885) a Herbarium specimen. b, c Appearance of dark brown conidiomata on the host. d Vertical section of conidioma. e Vertical section of peridium. f–g, i–j Conidiogenous cells and developing conidia. h Ostiole. k Germinating conidium. l–n Conidia. o–r Culture on PDA. Scale bars b = 200 µm, c = 100 µm, d, h, k = 20 µm, e = 10 µm, f–g = 5 µm, i–j = 2 µm, l–n = 5 µm, o–r = 25 mm
Neochaetospora quezelii (redrawn from Nag Raj 1993) a Vertical section of conidioma. b Enlarged surface view of ostiolar setae and parietal cells. c Conidiogenous cells and developing conidia. d Conidia
Neocucurbitariasp. (DAOM 215329) a Herbarium specimen. b, c Appearance of dark brown to black conidiomata on dung. d, e Vertical sections of conidiomata. f, h Sections of peridium. g Setae. i, j, l Conidiogenous cells and developing conidia. k Ostiole. m, n Conidia. Scale bars: d–e = 200 µm, f–g, i, l = 20 µm, h = 50 µm, j–k = 10 µm, m–n = 5 µm
Neodermea rossica (MFLU 15-2190, holotype). a, b Herbarium specimen. c, d Appearance of black conidiomata on the host. e, f Vertical sections of conidiomata. g–i Section of peridium. j–l Conidiophores, conidiogenous cells and developing conidia. m–q Conidia. Scale bars e–f = 200 µm, g–i = 20 µm, j–l = 5 µm, m–q = 10 µm
Phylogenetic tree generated from a maximum parsimony analysis based on a concatenated alignment of LSU, ITS, tub2 and rpb2 sequences data representing Neodidymelliopsis. Twenty strains are included in the analyses, which comprise 2284 characters including gaps (LSU: 1–850, ITS: 851–1341, tub2: 1342–1622, rpb2: 1623–2284). Neoascochyta exitialis CBS 118.40 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 5257.245362 is presented. The matrix had 236 distinct alignment patterns, with 18.75% of undetermined characters or gaps. Estimated base frequencies were: A = 0.237842, C = 0.242381, G = 0.275497, T = 0.244280; substitution rates AC = 1.760157, AG = 5.616104, AT = 1.877732, CG = 1.140385, CT = 13.721726, GT = 1.000000; gamma distribution shape parameter α = 0.059875. The maximum parsimonious dataset consisted of constant 1971, 130 parsimony-informative and 183 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 405 steps (CI = 0.862, RI = 0.855, RC = 0.737, HI = 0.138) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 20 changes. Ex-type strains are in bold. New isolates are shown in bold and blue
Neodidymelliopsis negundinis (MFLU 16-1123) a Herbarium specimen. b–d Appearance of brown conidiomata on the host. e–h Vertical sections of conidiomata. i, j Section of peridium. k–n Conidiogenous cells and developing conidia. o Germinating conidium. p–r Conidia. s Cultures on PDA. Scale bars b–c = 500 µm, d–e = 200 µm, f–h = 100 µm, i–j, o = 20 µm, k–n, p–r = 5 µm, s = 20 mm
Phylogenetic tree generated from a maximum parsimony analysis based on a concatenated alignment of tub2, ITS, tef1 and rpb2 sequences data representing Neofusicoccum. Eighty-one strains are included in the analyses, which comprise 1722 characters including gaps (tub2: 1–402, ITS: 403–911, tef1: 912–1157, rpb2: 1158–1722). Botryosphaeria dothidea CBS 100564 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 7424.635437 is presented. The matrix had 507 distinct alignment patterns, with 17.63% of undetermined characters or gaps. Estimated base frequencies were: A = 0.212116, C = 0.296588, G = 0.271129, T = 0.220167; substitution rates AC = 0.911239, AG = 4.043983, AT = 0.610024, CG = 0.981397, CT = 7.171228, GT = 1.000000; gamma distribution shape parameter α = 0.253755. The maximum parsimonious dataset consisted of constant 1226, 298 parsimony-informative and 198 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 902 steps (CI = 0.655, RI = 0.850, RC = 0.557, HI = 0.345) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 20 changes. Ex-type strains are in bold. New isolates are in blue
Neofusicoccum parvum (a–c, j, m, p, y–z from MFLU 19-2858, d–f, k–l, q–s, v–x from MFLU 16-0999, g–i, n–o, t–u, 1–2 from MFLU 19-2861). a, d Herbarium specimens. b–c, e–i Appearance of brown to dark brown conidiomata on the host j–n Vertical sections of conidiomata. o–q Vertical section of peridium. r–u Conidiogenous cells and developing conidia. v–z Conidia. Scale bars b, e, h = 500 µm, c, f, i–k = 200 µm, g = 1000 µm, l, n = 100 µm, m = 50 µm, o–q = 20 mm, r–u = 10 mm, v–z = 5µm
Neofusicoccum sinoeucalypti (MFLU 19-2886) a Herbarium specimen. b–c Appearance of dark brown to black conidiomata on the host. d–e Vertical sections of conidiomata. f–h Sections of peridium. i–j Conidiogenous cells and developing conidia. k–l Conidia. m Culture on PDA. Scale bars d–e = 100 µm, f–g = 50 µm, h = 20 µm, i–j = 10 µm, k–l = 5 µm, m = 20 mm
Neofusicoccum sinoeucalypti (MFLU 19-2887) a Herbarium specimen. b Appearance of black conidiomata on the host. c, g Vertical sections of conidiomata. d Ostiole. e, f Sections of peridium. h–l Conidiogenous cells and developing conidia. m Germinating conidium. n–o Conidia. p Culture on PDA. Scale bars c = 100 µm, d, f = 20 µm, e, g = 50 µm, h–l, n–o = 5 µm, m = 10 µm, p = 20 mm
Neogloeosporidina pruni (MFLU 16-2153, holotype) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d, e Vertical sections of conidiomata. f–i Conidiophores, conidiogenous cells and developing conidia. j–n Conidia. Scale bars b, d = 100 µm, c = 200 µm, e = 50 µm, f, j–n = 5 µm, g–i = 10 µm
Neoplaconema napelli (redrawn from Nag Raj 1993) a Vertical section of conidioma. b Conidia. c Conidiogenous cells and developing conidia
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tub2 sequences data representing Neopyrenochaetaceae and related families Pseudopyrenochaetaceae and Pyrenochaetopsidaceae. Related sequences were obtained from Valenzuela-Lopez et al. (2018) and GenBank. Seventeen strains are included in the analyses, which comprise 2630 characters including gaps. Cucurbitaria berberidis CBS 130007 is used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 10079.517896 is presented. The matrix had 685 distinct alignment patterns, with 13.61% of undetermined characters or gaps. Estimated base frequencies were: A = 0.241850, C = 0.243521, G = 0.270693, T = 0.243935; substitution rates AC = 1.357249, AG = 3.565695, AT = 1.463719, CG = 1.023510, CT = 8.198681, GT = 1.000000; gamma distribution shape parameter α = 0.186797. The maximum parsimonious dataset consisted of constant 1895, 569 parsimony-informative and 166 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 1363 steps (CI = 0.731, RI = 0.826, RC = 0.604, HI = 0.269) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“-”) indicates a value lower than 50% for MP BS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.04 changes. Ex-type strains are in bold. New isolates are in bold and blue
Neopyrenochaeta annellidica(MFLU 11-1105, holotype) a, b Appearance of dark brown to black conidiomata on the host (the conidiomata with white conidial mass is in moist condition). c, f, g Sections of peridium. d, e Vertical sections of conidiomata. h, i Conidiogenous cells and developing conidia. j–l Conidia. m Germinating conidium. n–o Culture on PDA . Scale bars c, g = 20 µm, d–e = 100 µm, f = 50 µm, h–l = 10 µm, m = 20 µm, n–o = 25 mm
Neopyrenochaeta chiangraiensis(MFLU 15-0086, holotype) a, b, d Appearance of dark brown to black conidiomata on the host. c Seta. e Surface view of conidioma with setae. f Section of peridium. h, i Vertical sections of conidiomata. g Germinating conidium. j, k Conidiogenous cells and developing conidia. l–r Conidia. Scale bars b, d–e = 100 µm, c, f–g, l = 20 µm, h–i = 50 µm, j–k, m–r = 5 µm
Neopyrenochaeta maesuayensis(MFLU 15-0078, holotype) a, b Appearance of black conidiomata on the host. c Setae. d Section of peridium. e Vertical section of dried conidioma. f Conidiogenous cells and developing conidia. g, i–m Conidia. h Germinating conidium. Scale bars c, e = 50 µm, d, g = 20 µm, f = 5 µm, h = 50 µm, i–m = 5 µm
Neottiospora caricina (DAOM 59000) a, b Herbarium package and specimen. c–e Appearance of olivaceous green to black conidiomata on the host. f, g Vertical sections of conidiomata (g from DAOM 81836). h–j Conidiomatal wall (j from DAOM 81836). k–n Conidiophores, conidiogenous cells and developing conidia. o–u Conidia. Scale bars c = 500 µm, d = 200 µm, e–g = 100 µm, h, j = 20 µm, i = 50 µm, k–n = 10 µm, o–u = 5 µm
Neottiospora caricina (DAOM 110354) a–d Herbarium package and specimen. e–g Appearance of black conidiomata on the host (e on sterilized grass in agar). h, k Conidiomatal wall (k on sterilized grass in agar). i–j Vertical sections of conidiomata. l–q Conidiophores, conidiogenous cells and developing conidia. r–v Conidia. Scale bars e = 200 µm, i–j = 100 µm, k = 50 µm, h, l–m = 10 µm, n–v = 5 µm
Neozythia nectrioidea (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiophores and developing conidia
Oncosporella punctiformis (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Pestalozziella subsessilis (FH 01142400, type). a, b Herbarium package and specimen. c, d Appearance of yellowish brown to brown conidiomata (arrows) on the host. e, f Vertical sections of conidiomata (e in 10% KOH, f in lactic acid). g Vertical section of peridium. h–l Conidiophores, conidiogenous cells and developing conidia (arrows show conidiogenous loci). m–o Conidia. Scale bars c = 500 µm, d–f = 100 µm; g–h = 20 µm. i–o = 10 µm
Pestalozziella artocarpi (DAOM 134442, holotype) a, b Herbarium package and specimen. c–d Appearance of brown conidiomata on the host. e, f Vertical sections of conidiomata. g, h Conidiomatal wall. i–k Conidiophores, conidiogenous cells and developing conidia. l–o Conidia. Scale bars c = 500 µm, d–f = 100 µm, g–h = 20 µm, i–j = 20 µm, k = 5 µm, l–o = 10 µm
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS and rpb2 sequences data of Pezicula. 55 strains are included in the analyses, which comprise 2468 characters including gaps (LSU: 1–573, ITS: 574–1415, rpb2: 1416–2468). Parafabraea caliginosa CBS 124806 and Parafabraea eucalypti CBS 124810 are used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 11646.896746 is presented. The matrix had 717 distinct alignment patterns, with 16.09% of undetermined characters or gaps. Estimated base frequencies were: A = 0.250189, C = 0.231401, G = 0.274025, T = 0.244386; substitution rates AC = 1.878061, AG = 3.979139, AT = 0.989967, CG = 0.932632, CT = 10.871716, GT = 1.000000; gamma distribution shape parameter α = 0.141239. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MLBS and a posterior probability lower than 0.95 for BYY. Ex-type strains are in bold. New isolates are in blue
Ascomycota, genera incertae sedis
Saprobic on dead stems of Matricaria suaveolens (Asteraceae).Sexual morph: undetermined. Asexual morph:Conidiomata pale orange, acervular, solitary, epidermal to subepidermal, immersed to erumpent, circular, unilocular, glabrous. Ostiole absent, dehiscence by irregular breakdown of upper host tissue. Conidiomatal wall composed of thin-walled, hyaline cells of textura angularis in the basal part. Conidiophores arising from the inner layer of the basal stroma, reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, cylindrical, determinate, smooth-walled. Conidia hyaline, ellipsoid to oval-ellipsoid, with rounded apex and truncate base, unicellular, thick- and smooth-walled, wall of two layers, the outer projecting as a basal frill, guttulate (Sutton 1980).
Type species: Leptodermella incarnata (Bres.) Höhn., Z. Gärungsphysiol. 5: 212 (1915)
Notes: Only Leptodermella incarnata is accepted in Leptodermella by Wijayawardene et al. (2017b). Leptodermella incarnata shares similar char Johnston et al. (2004) acters of conidia (hyaline, aseptate, ellipsoid to ovoid conidia with two layers wall) with asexual morph of Hercospora tiliae (Lamproconiacea, Diaporthales, Sordariomycetes) and Pezicula italica (Dermateaceae, Helotiales, Leotiomycetes). However, they can be distinguished by conidiomata, conidiophores and conidiogenous cells. The conidiophores of Hercospora tiliae and Pezicula italica are reduced to conidiogenous cells, whereas they are hyaline, septate, and branched at the base in H. tilia (Sutton 1980; Senanayake et al. 2018). Leptodermella incarnata has acervular conidiomata and holoblastic, cylindrical conidiogenous cells. Hercospora tiliae has pycnidial, ostiolate conidiomata with superficial cap of sterile tissues surrounding ostiole, and holoblastic, cylindrical conidiogenous cells. Pezicula italica has pycnidial conidiomata with a large ostiole, and enteroblastic, phialidic conidiogenous cells (Fig. 264). No sexual morph has been linked to Leptodermella and no molecular data is available. Fresh collections of Leptodermella incarnate are needed to place this genus in a natural classification.
Distribution: Latvia (Sutton 1980).
Leptosphaeria Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 234 (1863)
Facesoffungi number: FoF 04369
Dothideomycetes, Pleosporomycetidae, Pleosporales, Leptosphaeriaceae
Saprobic or parasitic on stems and leaves of herbaceous or woody plants in terrestrial habitats. Sexual morph: see Sivanesan (1984), Hyde et al. (2013), Ariyawansa et al. (2015). Asexual morph:Conidiomata black, pycnidial, solitary to gregarious or confluent, superficial, globose to depressed globose, with a flattened base and cylindrical neck, unilocular, glabrous, ostiolate. Ostiole subcylindrical to irregular, centrally located. Conidiomatal wall composed of thick-walled, dark brown to brown then hyaline cells of textura globosa to textura. Conidiophores formed from the inner cavity of the conidiomata, reduced to conidiogenous cells or if present, hyaline, cylindrical, septate, branched, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to subcylindrical, determinate, smooth-walled. Conidia hyaline, cylindrical to subcylindrical or ellipsoidal, unicellular, smooth, guttulate.
Type species: Leptosphaeria doliolum (Pers.) Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 234 (1863)
Notes: Sivanesan (1984) provided a full account of the taxonomy, biology, pathology and asexual morph of Leptosphaeria. Ariyawansa et al. (2015) evaluated the genus with molecular data and circumscribed Leptosphaeria sensu stricto to accommodate twelve species. The asexual morph of Leptosphaeria has been assigned to several genera, namely Coniothyrium Corda (Punithalingam 1980; Sivanesan 1984), Diplodina Westend. (Holm 1957; Sivanesan 1984), Phaeoseptoria Speg., (Hughes 1949; Webster l955, Webster and Hudson 1957), Phoma Sacc. (Hudson 1960; Lucas and Webster 1967), Scolecosporiella Petr. (Müller 1953; Lucas and Webster 1967; Sutton 1980), Septoria Sacc. (Sutton and Waterston 1966; Sivanesan 1984), and Stagonospora (Sacc.) Sacc. (Müller 1950; Webster and Hudson 1957; Lucas and Webster 1967; Hsieh 1979). However, except for the phoma-like asexual morph, other genera have not been successfully linked with Leptosphaeria sensu stricto (de Gruyter et al. 2013; Ariyawansa et al. 2015). In this study, a fungus collected on Petasites sp. is morphologically and phylogenetically 100 % similar to L. sydowii, thus reprsenting an additional collection of L. sydowii. In addition, the sexual moph L. ebuli (MFLUCC 14 0828), which was described from Sambucus ebulus (Adoxaceae) by Liu et al. (2015a, b), clustered with four strains of L. sydowii with 100MLBS/1BPP (Fig. 205). Therefore, this strain is regarded as the sexual morph of L. sydowii and L. ebuli is reduced to synonymy with L. sydowii, as the latter has priority.
Distribution: England, Italy (Ariyawansa et al. 2015; Dayarathne et al. 2015, this study).
Leptosphaeria sydowii (Boerema, Kesteren & Loer.) Gruyter, Aveskamp & Verkley, in Gruyter et al., Stud. Mycol. 75: 20 (2012) [2013]
= Leptosphaeria ebuli Jayasiri, Camporesi & K.D. Hyde, Fungal Diversity 72: 110 (2015)
Facesoffungi number: FoF 07428, Fig. 206
Saprobic on dead stem of Petasites sp. (Asteraceae). Sexual morph: undetermined. Asexual morph:Conidiomata 250–400 µm diam., 200–450 µm high, black, pycnidial, solitary to gregarious or confluent, superficial, globose to depressed globose, with flattened base, unilocular, thick-walled, glabrous, smooth, with a distinct rostrate, long neck. Ostiole 100–200 µm long and 30–80 µm wide, subcylindrical to irregular, with a tube-shaped pore, straight or curved, centrally located. Conidiomatal wall 50–100 µm wide, composed of an outer textura globosa to textura angularis with dark brown to brown cells, and an inner textura angularis with hyaline cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–8 × 2–6 µm, hyaline, enteroblastic, phialidic, ampulliform to subcylindrical, determinate, with moderate periclinal thickening in the collarette zone, smooth, formed from the inner cavity of the conidiomata. Conidia 4.3–6 × 1.6–3 µm (\( \bar{x} \) = 5 × 2 µm; n = 30), hyaline, cylindrical to subcylindrical, rounded at both ends, unicellular, smooth-walled, guttulate.
Material examined: Italy, Province of Forlì-Cesena, near Monte Falco, on dead aerial stems of Petasites sp. (Asteraceae), 8 April 2015, Erio Camporesi, IT2484 (MFLU 15-1240), (KUN, HKAS 93623).
Notes: Leptosphaeria sydowii was introduced based on Phoma sydowii Boerema, Kesteren & Loer. (de Gruyter et al. 2013). This species is commonly found in Europe and was associated with perennial species of Senecio (Asteraceae) and other Asteraceae (Boerema et al. 2004). Leptosphaeria sydowii is characterised by pycnidial, globose to depressed globose conidiomata with flattened base and pronounced cylindrical neck, and hyaline, cylindrical to ellipsoidal conidia. Our collection (MFLU 15-1240) fits well with the description provided by Boerema et al. (2004) and thus represents an additional collection of L. sydowii. The sexual morph of L. sydowii has been linked to L. senecionis (Fuckel) G. Winter, because these two taxa are often associated (Holm 1957; Boerema et al. 2004). In this study, Leptosphaeria sydowii was connected to its sexual morph based on phylogenic analyses (see morphology in Liu et al. 2015a, b).
Leptothyrina Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 123 (1915)
Facesoffungi number: FoF 07427, Fig. 207
Ascomycota, genera incertae sedis
Saprobic or parasitic on the host plant in terrestrial habitat, for example, Rubus sp. (Rosaceae). Sexual morph: undetermined. Asexual morph:Conidiomata black, amphigenous, subepidermal, immersed, circular, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of the apical wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the upper part, becoming pale brown, larger cells in the basal part. Conidiophores arising from the innermost layer of cells of the basal stroma, hyaline, short-cylindrical, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, polyblastic, sympodial to synchronous, cylindrical to lageniform, integrated or discrete, indeterminate, smooth-walled, with 1–2 small, unthickened conidiogenous loci at the apices. Conidia hyaline, cylindrical to fusiform, tapered and curved towards the base, obtuse at the apex, unicellular, smooth-walled, eguttulate.
Type species: Leptothyrina rubi (Duby) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 123 (1915)
Notes: Leptothyrina is characterized by immersed, acervular-like conidiomata, sympodial, flask-shaped conidiogenous cells and short-cylindrical conidia. The asexual morphs of Coccomyces De Not. and Hypoderma De Not. share a similar form of conidiomata, conidiogenous cells and conidia (Johnston 1986, 1990). Due to a lack of molecular data, Leptothyrina is placed in Ascomycota, genera incertae sedis following (Wijayawardene et al. 2017).
Distribution: Switzerland (Sutton 1980).
Leptotrochila P. Karst., Bidr. Känn. Finl. Nat. Folk 19: 245 (1871)
= Sporonema Desm., Annls Sci. Nat., Bot., sér. 3 8: 172, 182 (1847)
Facesoffungi number: FoF 07429
Leotiomycetes, Leotiomycetidae, Helotiales, Drepanopezizaceae
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: see Karsten (1871). Asexual morph:Conidiomata brown, pycnidial, solitary to gregarious or confluent, immersed, subglobose, unilocular, convoluted, glabrous. Ostiole absent, dehiscence by breakdown of apical wall in the middle part. Conidiomatal wall composed of thick-walled, pale brown to hyaline, compact cells of textura angularis in the basal and lateral part, becoming dark brown, larger cells towards the apex. Conidiophores arising from inner layers of the basal and lateral wall of conidiomata, hyaline, cylindrical or irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, indeterminate, smooth-walled, with periclinal thickening at collarette zone. Conidia hyaline, fusiform to cylindrical or irregular, unicellular, smooth-walled, eguttulate.
Type species: Leptotrochila radians (Roberge ex Desm.) P. Karst., Bidr. Känn. Finl. Nat. Folk 19: 245 (1871)
Notes: More than 50 taxa have been described in Sporonema (http://www.indexfungorum.org/names/Names.asp). Limber (1955) studied and compared 18 species of Sporonema while Sutton (1980) gave a detailed generic description, but only the type species was treated in his study. The type species of Sporonema, S. phacidioides, was connected to Leptotrochila medicaginis (Fuckel) Schüepp (Sutton 1980) and therefore, Johnston et al. (2014) synonymized Sporonema under Leptotrochila. Leptotrochila shares a similar form of conidia with phoma-like fungi, but it can be separated from the latter by its convoluted conidiomata. There is no molecular data available for asexual morph of Leptotrochila. Fresh collections are needed to better understand this genus and confirm the connection between Leptotrochila and Sporonema.
Distribution: Austria, Australia, Canada, Dominica, Czechoslovakia, Cyprus, India, Iraq, Israel, Italy, Kenya, Latvia, Malawi, New Zealand, Tanzania, UK, USA, Zambia (Sutton 1980).
Leptotrochila medicaginis (Fuckel) Schüepp, Phytopath. Z. 36: 253 (1959)
= Sporonema phacidioides Desm., Annls Sci. Nat., Bot., sér. 3 8: 172, 182 (1847)
Facesoffungi number: FoF 07430, Fig. 208
Saprobic or parasitic on the host plant in terrestrial habitat, such as Medicago sativa, M. lupulina (Fabaceae). Sexual morph: see Sutton (1980) and Semeniuk (1982). Asexual morph:Conidiomata up to 150 µm diam., 100 µm deep, brown, pycnidial, solitary to gregarious or confluent, immersed, subglobose, unilocular, convoluted, glabrous. Ostiole absent, dehiscence by breakdown of apical wall in the middle part. Conidiomatal wall composed of thick-walled, pale brown to hyaline, compact cells of textura angularis in the basal and lateral part, becoming dark brown, with larger cells towards the apex. Conidiophores arising from inner layers of the basal and lateral wall of conidiomata, hyaline, cylindrical or irregular, branched, septate, smooth-walled. Conidiogenous cells 7–15 × 2–3 µm hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, indeterminate, smooth-walled, with periclinal thickening at collarette zone. Conidia 4–5.5 × 2–2.5 µm, hyaline, fusiform to cylindrical or irregular, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980).
Leptoxyphium Speg., Physis, Rev. Soc. Arg. Cienc. Nat. 4(no. 17): 294 (1918)
Facesoffungi number: FoF 06949
Dothideomycetes, Dothideomycetidae, Capnodiales, Capnodiaceae
Saprobic on sugary exudates from insects growing on the surface of living leaves. Thallus composed of grey brown to brown, cylindrical, branched, septate, network-like mycelium. Sexual morph: undetermined. Asexual morph:Conidiomata grey-brown, synnematous, gregarious, superficial, arising from aggregated hyphae, base bulbous, comprising parallel hyphae, straight to slightly flexuous, sometimes with helical twisting, differentiated into a basal stalk and a funnel-shaped, cupulate head. Stalk and pycnidial wall composed of thick-walled, dark brown to brown cells of textura porrecta. Conidiophores formed on inner layers of pycnidial wall. Conidiogenous cells not observed. Conidia hyaline, ellipsoid, unicellular, smooth-walled, eguttulate (Hughes 1976; Chomnunti et al. 2011).
Type species: Leptoxyphium graminum (Pat.) Speg., Physis, Rev. Soc. Arg. Cienc. Nat. 4(no. 17): 294 (1918)
Notes: Twenty taxa are accepted in Leptoxyphium, but few have been studied with molecular data (Cheewangkoon et al. 2009; Crous et al. 2011b; Chomnunti et al. 2011; Yang et al. 2014). Chomnunti et al. (2011) regarded Leptoxyphium as a separate genus in Capnodiaceae based on LSU and SSU sequence data. The phylogeny derived from a LSU, SSU and ITS dataset shows that our strain (MFLUCC 14-0189/UiTM05) clustered with L. cacuminum (MFLUCC10-0049, MFLUCC10-0086, MFLUCC 10-0059), L. glochidion (IFRDCC 2651) and L. fumago (CBS 123.26) (Fig. 53). Comparison of LSU, SSU and ITS sequence data of these strains show they are conspecific. The sequence similarities among these strains are shown in Table 7. They share a similar form of pycnidia and conidia, and the only difference among these strains are pycnidial and conidial dimensions (Table 8). However, this difference is considered to be intraspecies variation. The name Leptoxyphium fumago has priority, thus L. cacuminum and L. glochidion are reduced to synonymy with L. fumago.
Distribution: Australia, China, Madagascar, Thailand (Cheewangkoon et al. 2009; Crous et al. 2011a; Chomnunti et al. 2011; Yang et al. 2014)
Leptoxyphium fumago (Woron.) R.C. Srivast., Arch. Protistenk. 125(1–4): 333 (1982)
= Leptoxyphium cacuminum Chomnunti & K.D. Hyde, in Chomnunti et al., Fungal Diversity 51(1): 114 (2011)
= Leptoxyphium glochidion H. Yang & K.D. Hyde, in Yang, Ariyawansa, Wu & Hyde, Phytotaxa 178(1): 177 (2014)
Facesoffungi number: FoF 07432, Fig. 209
Saprobic on sugary exudates from insects growing on the surface of living leaves. Thallus composed of brown to pale brown, sub-cylindrical, irregularly branched, septate, network-like hyphae. Sexual morph: undetermined. Asexual morph:Conidiomata 120–170 × 20–40 μm, grey-brown, synnematous, gregarious, superficial, arising from aggregated hyphae, differentiated into a basal stalk and a head; stalk 70–130 µm long, 10–40 µm wide, dark brown, base bulbous, comprising parallel hyphae, straight to slightly flexuous, sometimes with helical twisting, branched; head 30–50 μm high, 25–35 μm wide, funnel-shaped, cupulate (Fig. 209e. j), bearing a conidial hymenium. Conidiomatal wall composed of thick-walled, dark brown to pale brown cells of textura porrecta. Conidiophores arising from the inner layers of pycnidial wall. Conidiogenous cells not observed. Conidia 4–9 × 2.5–4 μm (\( \bar{x} \) = 3 × 4 μm, n = 30), hyaline, ellipsoid, rounded at both ends, sometimes narrow at the middle part, straight or slightly curved, unicellular, smooth-walled, guttulate.
Material examined: Malaysia, Selangor, Shah Alam, Universiti Teknologi MARA campus, on living leaves of Citrus sp. (Rutaceae), 31 January 2014, Qing Tian, UiTM05 (MFLU 19-2892), living culture MFLUCC 14-0189.
Libartania Nag Raj, Can. J. Bot. 57(13): 1390 (1979)
Facesoffungi number: FoF 07431, Fig. 210
Leotiomycetes, Leotiomycetidae, Helotiales, genera incertae sedis
Saprobic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata brown to black, pycnidial, solitary to gregarious, immersed to semi-immersed or erumpent, globose to subglobose in section view, unilocular, glabrous. Ostiole absent, dehiscence by longitudinal dehiscence of apical wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the exterior, becoming thin-walled, pale brown to hyaline cells towards hymenium. Conidiophores arising all around the cavity of conidiomata, reduced to conidiogenous cells. Conidiogenous cells subhyaline to hyaline, holoblastic, monoblastic or asynchronously polyblastic, ampulliform to subcylindrical or irregular, integrated or discrete, determinate, smooth-walled, invested in mucus. Conidia hyaline, subcylindrical to fusiform, with a narrow truncate base, septate, smooth-walled, bearing tubular, branched or unbranched, attenuated, apical appendages which are separated from the conidium body by a septum; basal appendage absent or present, hyaline, short, eccentric (Nag Raj 1979a, 1993).
Type species: Libartania laserpitii (Bres.) Nag Raj, Can. J. Bot. 57(13): 1390 (1979)
Notes: Libartania was introduced by Nag Raj (1979a) to accommodate two taxa L. laserpitii (Bres.) Nag Raj and L. themedae (Hansf.) Nag Raj. The genus is characterized by pycnidial conidiomata, monoblastic or asynchronously polyblastic conidiogenous cells and subcylindrical to fusiform, septate conidia, bearing branched or unbranched apical appendages, rarely with a basal appendage (Nag Raj 1979a, 1993). With the third species L. phragmiticola Nag Raj included, the generic concept was broadened to accommodate species with a persistent basal appendage (Nag Raj 1993). Lee and Crous (2003) added an additional species L. ischyrolepis S.J. Lee & Crous, which is distinguished from other species by its conidiomatal wall structure. Wijayawardene et al. (2017b) estimated only two taxa in Libartania, but neither listed species name nor gave any reasons. We accept four species listed above until the molecular data of these taxa are available. The sexual morph of Libartania was assigned in Phragmiticola Sherwood (Nag Raj 1993), but this connection needs verification.
Distribution: Australia, Canada, Italy, Latvia and South Africa (Nag Raj 1993; Lee and Crous et al. 2003).
Ligniella Naumov, Mater. Mikol. Fitopat. Ross. 5(1): 5 (1926)
Facesoffungi number: FoF 07438, Fig. 211
Ascomycota, genera incertae sedis
Saprobic on wood of Betula sp. (Betulaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, stromatic, pycnidial, scattered, initially immersed, then erumpent and appearing superficial, irregular, pulvinate, rugose, multilocular, glabrous. Ostiole single to each locule, circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura globulosa in the exterior, gradually merging with pale brown cells of textura globulosa to textura epidermoidea towards the inner layer at the basal stroma, becoming hyaline cells of textura angularis in the locular wall. Conidiophores formed from the inner layer cells of locular wall, hyaline, cylindrical or irregular, simple or branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to cylindrical, discrete, determinate, smooth-walled. Conidia hyaline, cylindrical or bacilliform, unicellular, smooth-walled.
Type species: Ligniella atrata Naumov, Mater. Mycol. Phytopath. Leningrad 5(1): 5 (1926)
Notes: Ligniella has been poorly studied. Nag Raj and DiCosmo (1981a) re-described and illustrated Ligniella, and stated that L. atrata might be identical to Amphicytostroma tiliae (= Amphiporthe hranicensis). Index Fungorum (2019) listed Ligniella as a synonym of Discula (asexual morph of Apiognomonia). Comparative morphology study of Amphicytostroma, asexual morph of Apiognomonia and Ligniella has shown that Ligniella shares similar characters with Amphicytostroma and asexual morph of Apiognomonia in phialidic conidiogenous cells but differs in many ways in conidiomata and conidial structures. Ligniella has irregular, pulvinate, rugose, multilocular, conidiomata with a rather thick basal stroma, which differs from the subglobose, unilocular or multilocular, and convoluted in asexual morph of Apiognomonia, and multilocular but not convoluted in Amphicytostroma (Sutton 1980; Sogonov et al. 2007, this study). The conidia of Ligniella are cylindrical or bacilliform, and aseptate which resemble the cylindrical and aseptate conidia in Amphicytostroma, but differ from the broadly ellipsoid to oval or fusiform and 0–1-aseptate in asexual morph of Apiognomonia (Sogonov et al. 2007; this study). Maharachchikumbura et al. (2015) placed Ligniella in Sordariomycetes, genera incertae sedis. We accept Ligniella as a legitimate name and place it in Ascomycota, genera incertae sedis until molecular data is available.
Distribution: Russia.
Marasasiomyces Crous, Phytotaxa 202(2): 86 (2015)
Facesoffungi number: FoF 07440
Dothideomycetes, Incertae sedis, Botryosphaeriales, Botryosphaeriaceae
Saprobic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, semi-immersed, subglobose to pyriform, unilocular, setose, thick-walled, ostiolate. Ostiole subcylindrical, with an elongated neck, single, centrally located. Setae brown to dark brown, septate, branched, smooth or verruculose-walled. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, determinate, cylindrical, arising from the inner cavity of conidiomata. Conidia hyaline, subcylindrcial to fusiform, unicellular, smooth, bearing a cone-like, mucoid apical appendage.
Type species: Marasasiomyces karoo (B. Sutton & Marasas) Crous, Phytotaxa 202(2): 86 (2015)
Notes: The monotypic genus Marasasiomyces was introduced by Crous et al. (2015b) based on M. karoo (= Tiarosporella graminis var. karoo B. Sutton & Marasas). The genus is similar to Eutiasporella in its long-necked, hairy conidiomata, and holoblastic conidiogenous cells. The significant difference between these two genera is that Eutiasporella forms pulvinate, botryose conidiomata in clusters, while it is not the case in Marasasiomyces.
Distribution: South Africa.
Marasasiomyces karoo (B. Sutton & Marasas) Crous, Phytotaxa 202(2): 86 (2015)
≡ Tiarosporella graminis var. karoo B. Sutton & Marasas, Trans. Br. Mycol. Soc. 67(1): 73 (1976)
Facesofungi number: FoF 07441, Fig. 212
Saprobic on dead stems of Eriocephalus sp. (Asteraceae). Sexual morph: undetermined. Asexual morph:Conidiomata 150–700 µm diam., 440–550 µm high, dark brown to black, semi-immersed, subglobose to pyriform, unilocular, setose, thick-walled, ostiolate. Ostiole 210–330 × 80–140 µm, single, subcylindrical, with an elongated neck rounded at the apex, centrally located. Setae 3–4 µm wide, brown to dark brown, septate, branched, smooth to verruculose. Conidiomata wall 30–60 µm wide, composed of thick-walled, brown to dark brown cells of textura angularis in outer part, thick-walled, pale brown to hyaline cells of loose textura angularis in inner part. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 10–20 × 2–3 µm, hyaline, holoblastic, determinate, cylindrical, arising from the inner cavity of conidiomata (Crous et al. 2015b). Conidia 19–23.5 × 4.7–8 µm (\( \bar{x} \) = 21 × 6 µm; n = 10), hyaline, subcylindrcial to fusiform, rounded at the apex, slightly truncated at the base, unicellular, straight or slightly curved, smooth, bearing a cone-like, mucoid apical appendage.
Material examined: South Africa. Cape Province: Colesberg, on dead stems of Eriocephalus sp. (Asteraceae), W.F.O. Marasas, February 1971 (IMI 186782, isotype).
Mastigosporella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1, 123: 135 (1914)
Facesoffungi number: FoF 04157, Fig. 213
Sordariomycetes, Sordariomycetidae, Diaporthales, Cryphonectriaceae
Foliicolous on plants, such as Anisophyllea sp. (Anisophylleaceae), Nyssa biflora and N. sylvatica (Nyssaceae), Quercus coccinea (Fagaceae). Sexual morph: see Senanayake et al. (2018). Asexual morph:Conidiomata yellowish brown to dark brown, pycnidial, scattered to gregarious, immersed to erumpent, oval to irregular in section view, unilocular, glabrous, ostiolate. Ostiole single, papillate, centrally located. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura globulosa to textura angularis in the base and lateral part, becoming darker towards ostiolar region. Conidiophores arising from the innermost layer wall cells of conidiomata, mostly reduced to conidiogenous cells or present, hyaline, sparsely septate and branched, invested in mucus. Conidiogenous cells hyaline, enteroblastic, annellidic, lageniform to subcylindrical, discrete or occasionally integrated, indeterminate, smooth-walled. Macroconidia hyaline or pale brown, narrowly ellipsoid to fusiform, with a narrow truncate base, unicellular, smooth-walled, guttulate, bearing an unbranched, attenuated apical appendage arising as a tubular extension of the conidium body. Microconidia hyaline, cylindrical to clavate with a rounded or blunt apex and a narrow truncate base, unicellular, smooth-walled (Nag Raj 1993; Crous et al. 2018a).
Type species: Mastigosporella hyalina (Ellis & Everh.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1, 123: 135 (1914)
Notes: Nag Raj and DiCosmo (1981a) and Nag Raj (1993) re-described Mastigosporella and accepted two species, M. hyaline on leaves of Quercus coccinea (Fagaceae) from the USA and M. nyssae Nag Raj & DiCosmo on leaves and petioles of Nyssa sylvatica (Nyssaceae) from Georgia. They also listed the presumed sexual morph as Wuestneiopsis J. Reid & Dowsett (Reid and Dowsett 1990; Nag Raj 1993). Crous et al. (2013) added a third species, M. anisophylleae Crous, on Anisophyllea sp. (Anisophylleaceae) from Zambia, which has larger conidia than other Mastigosporella species. Crous et al. (2013) also showed Mastigosporella was related to Cryphonectriaceae instead of Wuestneiopsis (Melanconidaceae), based on LSU and ITS sequence data. Rossman et al. (2015a) added an additional species M. georgiana (J.H. Mill. & G.E. Thomps.) Rossman & Crous based on Gnomoniella georgiana J.H. Mill. & G.E. Thomps. Crous et al. (2018a) add the fourth species, M. pigmentata V.P. Abreu & O.L. Pereira which has pale brown conidia and placed Mastigosporella in Harknessiaceae. Senanayake et al. (2018) described the sexual morph of Mastigosporella and placed this genus in Cryphonectriaceae. Mastigosporella and Harknessia were considered to be closely related (Nag Raj and DiCosmo 1981b; Senanayake et al. 2018). We checked the description and illustration of these two genera and found that they are totally different in conidial form. Mastigosporella has hyaline to pale brown, narrowly ellipsoid to fusiform, aseptate conidia with attenuated apical appendages. On the other hand, Harknessia has brown, globose to subglobose, aseptate conidia or are variable in shape, with or without longitudinal bands, bearing a cellular, cylindrical to subcylindrical, basal appendage, occasionally with an apical appendage (Nag Raj 1993).
Distribution: Brazil, USA and Zambia (Nag Raj 1993; Crous et al. 2013, 2018a).
Melanops Nitschke ex Fuckel, Jb. nassau. Ver. Naturk. 23-24: 225 (1870) [1869–70]
Facesoffungi number: FoF 07442
Dothideomycetes, Incertae sedis, Botryosphaeriales, Melanopsaceae
Saprobic on dead wood or stems of Fagus sylvatica and Quercus robur. Sexual morph: Ascomata black, stomatic, initially immersed, ultimately erumpent, solitary to gregarious, rounded in surface view, multilocular, glabrous, ostiolate. Ostioles circular, centrally located on each locule. Peridium composed of textura angularis with thick-walled, brown cells. Pseudoparaphyses hyaline, hyphae-like, septate, constricted at septa. Asci 8-spored, bitunicate, fissitunicate, clavate, pedicellate, apically rounded with an ocular chamber. Ascospores hyaline, irregularly biseriate in the ascus, ellipsoid to rhomboid, obtuse at both ends, widest in the middle, unicellular, smooth- and thin-walled, with a mucilaginous sheath (description from Phillips and Alves 2009; Liu et al. 2012). Asexual morph:Conidiomata dark brown to black, eustromatic, scattered to gregarious, or confluent, subperidermal in origin, immersed to partly erumpent, multi-locular, thick-walled, smooth, glabrous, ostilate. Ostiole circular, papillate, central on each locule. Conidiomatal wall composed of outer layer of thick-walled, dark brown, occluded cells of textura angularis to textura globose; and inner layer of pale brown to hyaline, thick-walled cells of textura prismatica. Conidiophores arising from innermost wall of conidiomata, reduced to conidiogenous cells or present, when present, hyaline, cylindrical to subcylindrical, blunt and branched at base, smooth-walled. Conidiogenous cells hyaline, enteroblastic or holoblastic, cylindrical or subcylindrical, determinate or indeterminate, discrete or integrated, smooth-walled. Conidia hyaline, cylindrical, aseptate, obtuse or acute at apex, narrowed and truncate at base, surrounded by a persistent mucus sheath, guttulate.
Type species: Melanops tulasnei Fuckel, Jb. nassau. Ver. Naturk. 23–24: 225 (1870) [1869-70]
Notes: Melanops is characterized by large ascomata and conidiomata that occur within the same stroma and ellipsoid to rhomboid ascospore and very large fusiform conidia enclosed in a mucus sheath (Phillips and Pennycook 2004; Phillips and Alves 2009; Liu et al. 2012). Classification based on morphology has resulted in a great deal of confusion in Melanops. Melanops tulasnei was originally described as Dothidea melanops Tul. & C. Tul. by Tulasne (1856). Winter (1887) made a new combination Botryosphaeria melanops (Tul. & C. Tul.) G. Winter to accommodate D. melanops. Von Arx and Müller (1954) synonymized B. melanops under B. quercum. Phillips and Pennycook (2004) suggested that the correct name for this species is B. melanops and designated a specimen in PAD as neotype. Phillips and Alves (2009) epitypified the type species M. tulasnei and considered Melanops to be a genus in Botryosphaeriaceae. This concept was followed by Liu et al. (2012) and Hyde et al. (2013). However, Slippers et al. (2013) introduced a new family Melanopsaceae to accommodate monotypic genus Melanops based on DNA sequence data of six loci (SSU, LSU, ITS, tef1, tub2, mtSSU) analyses. Phillips et al. (2019) agreed with Slippers et al. (2013) and accepted Melanopsaceae as a separate group in Botryosphaeriales based on ITS and LSU sequence data, and evolutionary divergence times. Jiang et al. (2018) described two taxa, M. castaneicola on Castanea mollissima, and M. chinensis on Quercus sp.
Two samples collected on Fagus sylvatica from Italy and one collected on Quercus robur from Russia, clustered with M. tulasnei and an undescribed Melanops sp. with high bootstrap support (100/100/1) (Fig. 214). Combined with morphological characters, the two collections from Italy are recognized as conspecific taxa and introduced as a new species M. fagicola, while the one from Russia is identified as M. tulasnei.
Distribution: China, Germany, Italy, Russia (Phillips and Alves 2009; Jiang et al. 2018, this study).
Melanops fagicola W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557149, Facesoffungi number: FoF 07443, Fig. 215
Etymology: Referring to the host from which it was collected, Fagus.
Saprobic on dead stems of Fagus sylvatica. Sexual morph: undetermined. Asexual morph:Conidiomata 845–1270 µm diam., 370–470 µm high, brown, eustromatic, scattered to gregarious, rarely confluent, subperidermal in origin, immersed to partly erumpent, globose to subglobose, multi-locular, thick-walled, smooth, glabrous, ostiolate, with up to 15 locules, 130–220 µm diam., 280–370 µm high, divided by pale brown to hyaline wall. Ostiole 80–120 × 40–80 µm, circular, papillate. Conidiomatal wall 50–180 µm wide, composed of outer several layers of thick-walled, dark brown cells of textura globosa to angularis, with inner layers pale brown to hyaline, thick-walled cells of textura prismatica. Paraphyses 40–70 × 2–4 µm, hyaline, inconspicuous, filiform, swollen and branched at the base, attenuating above, obtuse at the apex, septate, smooth-walled. Conidiophores reduced to conidiogenous cells, arising from innermost wall layer of locules. Conidiogenous cells 13–20 × 3–6 μm, hyaline, holoblastic, cylindrical, determinate, smooth-walled. Conidia 37–52.5 × 9.5–15.5 µm (\( \bar{x} \) = 46 × 12.3 µm; n = 30), hyaline, cylindrical, unicellular, with rounded and obtuse apex, and slightly narrowed and truncate base, sometimes swollen in the middle part, surrounded by a persistent mucus sheath, with small granular contents or large guttules.
Culture characteristics: colony on PDA, reaching 15–20 mm diam. after four weeks at 20–25 °C, white to grey at first, then becoming grey, eventually black, with filamentous, dense, flattened, felt-like, aerial mycelium on the surface, reverse similar in colour, without sporulation.
Material examined: Italy, Province of Forlì-Cesena, Premilcuore, near Castel dell’Alpe, on dead aerial branches of Fagus sylvatica (Fagaceae), 20 January 2013, Erio Camporesi, IT1134bis (MFLU 19-2860, holotype), ex-type living culture MFLUCC 13-0504 = ICMP 21847 = KUMCC 15-0656, (KUN, HKAS 95003); ibid., 30 March 2013, Erio Camporesi, IT1159 (MFLU 19-2862, paratype), (KUN, HKAS 93591, paratype).
Notes: Melanops fagicola is similar to M. tulasnei with very large conidia surrounded by a mucoid sheath, but differs in conidiogenous cells and paraphyses. Melanops fagicola has holoblastic conidiogenous cells and inconspicuous paraphyses, while M. tulasnei has enteroblastic, annellidic conidiogenous cells and dense paraphyses (Phillips and Alves 2009). Phylogenetically, these two collections formed a separate branch between M. tulasnei and an undescribed Melanops species (Fig. 214).
Melanops tulasnei Fuckel, Jb. nassau. Ver. Naturk. 23-24: 225 (1870) [1869-70]
Facesoffungi number: FoF 07444, Fig. 216
Saprobic on dead twigs of Quercus robur. Sexual morph: see Phillips and Alves (2009) and Phillips et al. (2013). Asexual morph:Conidiomata 1000–1500 µm diam., 500–750 µm high, dark brown to black, stromatic, mostly solitary, occasionally gregarious, subepidermal, semi-immersed to partly erumpent, rounded in surface view, multi-locular, thick-walled, glabrous, ostiolate, with locules 100–400 µm diam., 200–550 µm high, globose to subglobose, divided by pale brown to hyaline wall. Ostiole 80–150 × 50–100 µm, multi-ostiolar, circular, papillate. Conidiomatal wall 20–105 µm wide, composed an outer layer of textura globosa to textura angularis with relatively thick-walled, dark brown to black cells, and an inner layer of textura prismatica with thick-walled, pale brown to hyaline cells. Paraphyses 100–150 × 1–2 µm, hyaline, conspicuous, filiform, septate, branched irregularly, arising between conidiophores. Conidiophores reduced to conidiogenous cells, lining the base and side walls of conidiomata. Conidiogenous cells 20–40 × 1.5–3.5 µm, hyaline, holoblastic, cylindrical to subcylindrical, determinate, smooth-walled. Conidia 53–85 × 8–11 µm (\( \bar{x} \) = 61 × 9 µm; n = 30), hyaline or occasionally pale brown, elongate fusiform, attenuating towards each end, obtuse at the apex and truncate at the base, unicellular, enclosed in mucus sheath (Fig. 216v).
Culture characteristics: Colonies reaching 15–20 mm diam. after four weeks at 20–25 °C, white at first, then becoming grey or olivaceous, eventually black, circular to irregular, raised, with aerial hyphae, dense, filamentous, cottony; margins undulate to lobate, reverse white at first, then becoming black; sporulation after 12 months.
Material examined: Russia, on dead twigs of Quercus robur (Fagaceae), 8 May 2014, T.S. Bulgakov, T48 (MFLU 19-2889, living culture MFLUCC 16-1194, (KUN, HKAS 101667).
Notes: Strain (MFLUCC 16-1194) clustered with two strains of M. tulasnei (CBS 116806, CBS 116805) (Fig. 214). Immature asci were found in the same stroma with the asexual morph (Fig. 216n). The asexual morph, except paraphyses and conidial dimensions which are much larger than the epitype strain M. tulasnei (37.2–)45–46.8(–53) × (7.2–)9.1–9.7 (–12.1), is in other aspects almost similar. Phillips and Alves (2009) noted that the conidial dimensions of M. tulasnei is variable and the difference can be regarded as intra-species variation. In addition, the range of conidial dimension of M. fagicola and M. tulasnei overlap, but they are phylogenetically distinct. Therefore, the new collection is recognized as conspecific with M. tulasnei, and the difference might be caused by different geography. Here, we note that conidial dimensions alone are not informative at the species level in Melanops.
Metazythia Petr., Sydowia 4(1–6): 373 (1950)
Facesoffungi number: FoF 07439, Fig. 217
Ascomycota, genera incertae sedis
Hyperparasitic on fungal stroma on Chusquea serrulata (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary to gregarious, immersed to erumpent, globose to subglobose, unilocular or sometimes multilocular, glabrous. Ostiole single to each pycnidium, circular, centrally located. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis. Conidiophores arising from the inner layers of conidiomal wall, hyaline, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled, with markedly periclinal thickened, minute collarette and aperture. Conidia hyaline, cylindrical to fusiform, occasionally irregular, unicellular, smooth-walled, biguttulate (Sutton 1980).
Type species: Metazythia caespitosa Petr., Sydowia 4(1–6): 373 (1950)
Notes: Metazythia is monotypic. The conidiomata, conidiophores, conidiogenous cells and conidia of Metazythia resemble phoma-like taxa, such as Allophoma, Ascochyta, Boeremia, Didymella, Phoma. and the genus may be congenic with some of them. However, no molecular data is available and fresh collections of M. caespitosa are needed.
Distribution: Ecuador (Sutton 1980).
Micraspis Darker, Can. J. Bot. 41(10): 1390 (1963)
Facesoffungi number: FoF 07446, Fig. 218
Leotiomycetes, Leotiomycetidae, Micraspidales, Micraspidaceae
Parasitc on the host plant. Sexual morph: see Darker (1963), Quijada et al. (2020). Asexual morph:Conidiomata black, pycnidial, amphigenous, scattered to gregarious, immersed, elliptical to irregular in outline, flattened, unilocular, glabrous. Ostiole absent, dehiscence by a longitudinal fissure of the apical wall. Conidiomatal wall composed of thick-walled, dark brown to black cells of textura angularis. Conidiophores arising from the inner wall layer of conidiomata, hyaline, cylindrical, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, fusiform to cylindrical or falcate, with obtuse apex and truncate base, straight or slightly curved, 1–3-septate, smooth-walled, guttulate (Darker 1963; Nag Raj and DiCosmo 1980).
Type species: Micraspis acicola Darker, Can. J. Bot. 41(10): 1390 (1963)
Notes: Micraspis is represented by M. acicula, described on Picea mariana (Pinaceae) from northern Ontario (Darker 1963). Another fungus Periperidium Darker, typified by P. acicola Darker, was described on the same host and it was regarded as a possible asexual morph (Darker 1963). Johnston et al. (2014) regarded Micraspis and Periperidium as congeneric, and recommended to use the name of Micraspis for the holomorph. Micraspis was originally placed in Phacidiaceae (Phacidiales) (Darker 1963). Later it was transferred to Tympaneae (DiCosmo et al. (1983) and Tympanidaceae (Phacidiales) Baral 2016). Quijada et al. (2020) revised Micraspis based on multigene phylogenetic analysis and morphological evidence, and introduced a new family Micraspidaceae (Micraspidales) for this genus. They also confirmed the connection between M. acicola and its purported asexual morph (= P. acicola) on symptomatic needles.
Micraspis acicola has hyaline, fusiform to cylindrical or falcate conidia and phialidic conidiogenous cells, which can be confused with Corniculariella and the asexual morph of Cryptosporella and Dermea. These genera are mainly separated by the conidiomatal structure and conidial septation. The conidiomata of Micraspis acicula are immersed, flattened, unilocular, while they are subcylindrical to conical or rostrate, and unilocular in Corniculariella, furfuraceous, discoid to subglobose, and unilocular in Cryptosporella, and furfuraceous or pruinose, pulvinate, and multilocular in Dermea (Nag Raj 1993, this study). In addition, the conidia of Micraspis are 1–3-septate, while they are 1–10-septate in Corniculariella, aseptate in Cryptosporella, and 0–1-septate in Dermea.
Distribution: Canada, Denmark, Ireland, Russia, UK USA (Nag Raj and DiCosmo 1980; Wijayawardene et al. 2017; Quijada et al. 2020).
Microdiscula Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1, 124: 142 (1915)
Facesoffungi number: FoF 07445, Fig. 219
Ascomycota, genera incertae sedis
Saprobic on dead stems of Rubus fruticosus (Rosaceae), and unidentified plant. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, stromatic, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, globose to subglobose, multilocular, convoluted, glabrous, ostiolate. Ostiole single, circular, papillate, centrally or laterally located. Conidiomatal wall composed of thick-walled, brown to pale brown cells of textura angularis to textura intricata. Conidiophores arising from the innermost layer wall of conidiomata, hyaline, branched septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, cylindrical to fusiform or allantoid, unicellular, smooth-walled, eguttulate (Sutton 1980).
Type species: Microdiscula rubicola (Bres.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1, 124: 142 (1915)
Notes: Microdiscula is similar to Cytospora in having pycnidial, multilocular, convoluted, ostiolate conidiomata and phialidic, cylindrical, integrated or discrete conidiogenous cells. The main difference separating these two genera is conidial form (cylindrical to fusiform in Microdiscula, allantoid in Cytospora). Sutton (1980) re-described and illustrated the generic type M. rubicola. Another taxon M. phragmitis (Westend.) Höhn. has remained unexamined. Fresh collections are needed to better understand Microdiscula species.
Distribution: Germany (Sutton 1980).
Microperella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 879 (1909)
Facesoffungi number: FoF 07447, Fig. 220
Ascomycota, genera incertae sedis
Habitat on leaves of Quercus glauca (Fagaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, pycnidial, solitary, superficial, irregularly pulvinate to hemispherical, often irregularly lobed and elevated at the periphery and depressed towards the centre, multilocular, with subglobose or irregular locules, glabrous. Ostiole absent, dehiscence by irregular splits of upper locular wall. Conidiomatal wall composed of thick-walled, pale brown cells, in vertical parallel arrangement, of textura prismatica to textura angularis beneath the locules, becoming relatively thick-walled, dark brown cells in the periphery. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the inner layer of locular wall, hyaline, holoblastic, ampulliform to lageniform, determinate, smooth-walled. Conidia hyaline, fusiform, attenuated obtuse towards apex, truncate at the base, 1-septate, slightly constricted at the septum, smooth-walled, eguttulate (Sutton 1980).
Type species: Microperella quercus Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 879 (1909)
Notes: The genus is monotypic. Microperella is similar to Diplozythiella in having hyaline, fusiform, 1-septate conidia. Microperella possesses irregularly pulvinate to hemispherical, multilocular conidiomata often irregularly lobed and elevated at the periphery, and holoblastic conidiogenous cells. On the other hand, Diplozythiella has globose to irregular, unilocular conidiomata, and enteroblastic, phialidic conidiogenous cells (Sutton 1980). There is no molecular data available for the genus. Fresh collections and cultures are needed to place it in a natural group.
Distribution: Japan (Sutton 1980).
Mirimyces Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 477 (1993)
Facesoffungi number: FoF 07448
Ascomycota, genera incertae sedis
Saprobic on decaying leaves of Mimusops sp. (Sapotaceae). Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, mostly gregarious, subepidermal in origin, deeply immersed, obpyriform to globose or irregular, unilocular or plurilocular, glabrous, thick-walled, ostiolate. Ostiole single, cylindrical to subcylindrical, centrally located. Conidiomatal wall composed of textura angularis to textura prismatica with thick-walled, dark brown to hyaline cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, ampulliform, doliiform, lageniform, or subcylindrical, with periclinal thickenings at collarette zone. Conidia hyaline, ovoid to ellipsoid or fusiform, occasionally wall bulging in the middle or lower half, unicellular, thin- and smooth-walled, guttulate, bearing deeply infundibuliform, cylindrical or irregular, mucoid apical and basal appendages.
Type species: Mirimyces pulcher Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 478 (1993)
Notes: Mirimyces is a monotypic genus. It can easily be confused with the asexual morph of Phacidium. They share similar form of conidiomata and conidia, but can be distinguished by conidiogenous cells and conidial appendages. Mirimyces has ampulliform, doliiform, lageniform conidiogenous cells, with flared collarettes and conspicuous periclinal thickenings. The asexual morph of Phacidium has cylindrical, subcylindrical conidiogenous cells without flared, thickened collarettes. In addition, conidia of Mirimyces bear apical and basal appendages (arising from structure changes in appendages primordia delimited on the developing conidium), but conidia of the asexual morph of Phacidium have apical appendages (originating by eversion of partial and full mucoid sheath). No sexual morph has been linked to Mirimyces and no molecular data is available in GenBank.
Distribution: Cuba.
Mirimyces pulcher Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 478 (1993)
Facesoffungi number: FoF 07449, Fig. 221
Saprobic on decaying leaves of Mimusops sp. (Sapotaceae) Sexual morph: undetermined. Asexual morph:Conidiomata 90–250 diam., 80–230 µm high, brown to dark brown, pycnidial, mostly gregarious, subepidermal in origin, deeply immersed, obpyriform to globose or irregular, unilocular or plurilocular, glabrous, thick-walled, ostiolate. Ostiole single, cylindrical to subcylindrical, centrally located. Conidiomatal wall 10–40 µm wide, composed of an outer layer of textura angularis with thick-walled, dark brown to hyaline cells, and an inner layer of textura prismatica with thin-walled, hyaline cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–7 × 2–4 µm, arising from the innermost wall layers of conidiomata, hyaline, ampulliform, doliiform, lageniform, or subcylindrical, with flared collarettes and conspicuous periclinal thickenings (Fig. 221k, l, m, n), thick- and smooth-walled. Conidia 10–19 × 2–3.5 µm (\( \bar{x} \) = 16 × 3 µm; n = 30), hyaline, ovoid to ellipsoid or fusiform, occasionally wall bulging in the middle or lower half, unicellular, thin- and smooth-walled, guttulate, bearing a deeply infundibuliform, cylindrical or irregular, mucoid apical and basal appendage.
Material examined: Cuba, Havana, Santiago de Cuba, INIFAT campus, on decaying leaves of Mimusops sp. (Sapotaceae), 7 March 1989, T.R. Nag Raj, DAOM 215253, holotype).
Monochaetiella E. Castell., Nuovo G. bot. ital. 49: 487 (1943) [1942]
Facesoffungi number: FoF 07450, Fig. 222
Ascomycota, genera incertae sedis
Parasitic on leaves of Hyparrhenia cymbaria, H. cymbaria, H. filipendula, H. rufa, H. variabilis, Hyparrhenia spp. (Poaceae) (Nag Raj 1993). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, acervular, solitary or confluent, epidermal to subepidermal, immersed, linear, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of apical wall. Conidiomatal wall composed of thin-walled, pale brown to brown cells of textura prismatica in the base wall, merging with thick-walled, brown to dark brown cells of textura angularis to textura globulosa in the lateral wall. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the inner wall layer of the basal stroma, pale brown, with verruculose walls in the basal part, becoming thin-walled, paler and smooth above, enteroblastic, phialidic, lageniform to subcylindrical or cylindrical, discrete, determinate. Conidia hyaline, fusiform to navicular, truncate at the base, unicellular, smooth-walled, guttulate, bearing a cellular, tubular, apical appendage (Sutton and Dicosm 1977; Nag Raj 1993).
Type species: Monochaetiella hyparrheniae E. Castell., Nuovo G. bot. ital. 49: 487 (1943) [1942]
Notes: Kandaswamy and Sundaram (1957) and Punithalingam (1969) described two taxa from graminicolous hosts in India, namely, M. themedae M. Kandasw. & Sundaram and M. cymbopogonis Punith. & Sarwar. Sutton and Dicosm (1977) revised Monochaetiella and showed that M. themedae and M. cymbopogonis were distinct from the type species in conidiogenous cells and conidia. They proposed a new genus, Monochaetiellopsis B. Sutton & DiCosmo to accommodate these two species, and designated M. themedae as the generic type. This concept was followed by later authors (Sutton 1980; Nag Raj 1993). Monochaetiella is monotypic. The type species Monochaetiella hyparrheniae was associated with leaf lesions on Hyparrhenia rufa in Ethiopia.
Monochaetiella has hyaline, fusiform to navicular conidia bearing an apical appendage, which can be confused with Ciliosporella, Monochaetiellopsis and Tracylla (T. aristata). However, Ciliosporella has pycnidial, multilocular conidiomata with paraphyses, which differs from the acervular conidiomata in Monochaetiella and Monochaetiellopsis. Tracylla possesses superficial, pycnothyrial conidiomata with a shield connected to the immersed mycelium by a central supporting column of cells, which is different from other three genera (Nag Raj 1993). Monochaetiellopsis can be distinguished from Monochaetiella by its sympodial conidiogenous cells and 1–2-septate conidia with septa close to the base and apex. No molecular data is available for Monochaetiella, and fresh collections are needed to place it in a natural classification.
Distribution: Ethiopia, Sudan, Uganda, Zambia (Nag Raj 1993).
Monochaetiellopsis B. Sutton & DiCosmo, Can. J. Bot. 55(19): 2536 (1977)
Facesoffungi number: FoF 07451, Fig. 223
Leotiomycetes, Leotiomycetidae, Helotiales, genera incertae sedis
Parasitic on leaves of Cymbopogon citratus, C. nardus, Heteropogon contortus and Themeda tremula (Poaceae) (Sutton and DiCosmo 1977). Sexual morph:Hypnotheca graminis (Tommerup 1970). Asexual morph:Conidiomata pale brown, acervular, solitary or confluent, epidermal to subepidermal, immersed, linear, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of overlapping tissue. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis in the basal part. Conidiophores arising from the inner wall layer of the basal stroma, hyaline, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, polyblastic, sympodial or synchronous, cylindrical to lageniform, integrated or discrete, indeterminate, smooth-walled, with minute conidiogenous loci towards the apices. Conidia hyaline, fusiform, straight or slightly curved, 1–2-septate, with a very small, short basal cell, a long median cell and a long cellular, flexuous, unbranched, apical appendage (Sutton and Dicosm 1977; Sutton 1980; Nag Raj 1993).
Type species: Monochaetiellopsis themedae (M. Kandasw. & Sundaram) B. Sutton & DiCosmo, Can. J. Bot. 55(19): 2541 (1977)
Notes: Monochaetiellopsis differs from Monochaetiella by having pale brown conidiomata, polyblastic synchronous or sympodial conidiogenous cells and septate conidia. Three species are accepted in the genus, all are associated with leaf lesions on graminicolous hosts (Sutton and DiCosmo 1977). The sexual morph of Monochaetiellopsis was connected to Hypnotheca graminis Tommerup (1970), based on a morphological link between the two morphs and cross-inoculation experiments. Because of a lack of molecular data, Monochaetiellopsis is placed in Helotiales, incertae sedis based on morphology of the sexual morph (Johnston et al. 2014; Wijayawardene et al. 2017).
Distribution: Australia, India and Tanzania (Nag Raj 1993).
Monodia Breton & Faurel, Revue Mycol., Paris 35(1): 23 (1970)
Facesoffungi number: FoF 07453
Ascomycota, genera incertae sedis
Coprophilous. Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, scattered to gregarious, immersed to semi-immersed, with globose to subglobose venter, unilocular, glabrous, thick-walled, ostiolate. Ostiole dark brown to almost black, circular or oval, with a subcylindrical neck, bearing irregular shaped, curved setae. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores arising from the innermost wall layers of conidiomata, hyaline, cylindrical to subcylindrical, branched, septate, invested in mucus. Conidiogenous cells hyaline, subcylindrical with the conidiogenous loci immediately beneath the septa, integrated, indeterminate, terminal or lateral, smooth-walled. Conidia hyaline, ellipsoid to fusiform or occasionally cylindrical, unicellular, guttulate, bearing 2–3, tubular, unbranched, flexuous appendages at each end.
Type species: Monodia elegans Breton & Faurel, Revue Mycol., Paris 35(1): 24 (1970)
Notes: Monodia elegans was collected from dung of hares (Breton and Faurel 1970). The second species was described from dung of Trichosurus vulpecula in Australia (Nag Raj 1993). Monodia is characterized by coprophilous habitat and pycnidial, globose conidiomata with cylindrical necks, occasionally bearing setae, and subcylindrical conidiogenous cells with the conidiogenous loci immediately beneath the septa, and subcylindrical, unicellular conidia with two appendages at each end. The generic type cannot be located; thus we re-examined the type species of M. minor and provide a detailed description and photo plate. No molecular data is available for this genus.
Monodia can easily be confused with Pullospora, because they are both coprophilous and share similar morphology of conidiomata and conidia. The significant characters that differentiate these two genera are associated with conidiomatal setae, conidiogenous cells and the number of conidial appendages. Monodia has globose to subglobose conidiomata bearing setae around the conidiomatal wall or at the ostiolar region, and phialidic, subcylindrical conidiogenous cells, and ellipsoid to fusiform conidia with 2 appendages at each end, or occasionally 1 or 3 at the base. Pullospora has globose to subglobose or irregular, glabrous conidiomata, annellidic, subcylindrical to lageniform, conidiogenous cells, and cylindrical to subcylindrical conidia with a primary centric basal appendage and 2–4 unbranched, secondary appendages at each end.
Distribution: Australia, Chad, Namibia (Nag Raj 1993).
Monodia minor Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 526 (1993)
Facesoffungi number: FoF 07454, Fig. 224
Coprophilous. Sexual morph: undetermined. Asexual morph:Conidiomata 50–160 diam., 50–200 µm high, pale brown to brown with black tip on YPSS agar, pycnidial, scattered to gregarious, immersed to semi-immersed, with globose to subglobose venter, unilocular, glabrous, thick-walled, ostiolate. Ostiole 25–60 × 25–50 µm, circular or oval, with a subcylindrical or irregular neck, dark brown to almost black neck covered by variously curved setae. Conidiomatal wall 10–20 µm thick, composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores arising from the innermost wall layers of conidiomata, hyaline, cylindrical to subcylindrical, branched and broader at the base or above, septate, often constricted at septum, smooth-walled, invested in mucus. Conidiogenous cells hyaline, subcylindrical, integrated, indeterminate, terminal or lateral, smooth-walled. Conidia 8–10 × 2.5–4 µm (\( \bar{x} \) = 9 × 3 µm; n = 30), hyaline, ellipsoid to fusiform or occasionally cylindrical, with obtuse apex, and slightly truncate base, unicellular, guttulate, bearing filiform, tubular, unbranched, flexuous appendages at each end; apical appendages 10–30 µm long, mostly 2, arising from lateral part of upper half, basal appendages 5–25 µm long, 1–3, one centrally located, other two arising from lateral part.
Material examined: Australia, New South Wales, dried cultures on YPSS agar (28 January 1982–19 February 1982) isolated from dung of Trichosurus vulpecula (brush tail possum), August 1981, A. Bell (DAOM 215298, type).
Monostichella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 95 (1916)
= Cryptocline Petr., Ann Mycol. 22(3/6): 402 (1924)
Facesoffungi number: FoF 07452, Fig. 225
Ascomycota, genera incertae sedis
Saprobic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata acervular, scattered to gregarious or confluent, subepidermal to epidermal, immersed, usually circular, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of overlying host tissue. Conidiomatal wall composed of thin-walled, pale brown to hyaline cells of textura angularis in the basal part. Conidiophores usually reduced to conidiogenous cells. Conidiogenous cells arising from the inner layer cells of the basal stroma, hyaline, enteroblastic, phialidic, lageniform to cylindrical, discrete, determinate, smooth-walled. Conidia hyaline, ovoid, ellipsoidal or somewhat pyriform, with truncate base and obtuse apex, unicellular, smooth-walled (Sutton 1980; Morgan-Jones et al. 1972a).
Type species: Monostichella robergei (Desm.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 95 (1916)
Notes: Monostichella was re-described and re-illustrated by Sutton (1980) and Morgan-Jones et al. (1972a). Conidia ranging in shape from ellipsoid to pyriform in Monostichella invite comparison with Cryptocline, Erythrogloeum, and Gloeosporidina (Sutton 1980). However, Erythrogloeum possesses eustromatic, pycnidial conidiomata, which distinguish it from the acervular conidiomata in Cryptocline, Gloeosporidina and Monostichella. Gloeosporidina has hyaline to pale brown, cylindrical to subcylindrical, branched conidiophores, which are usually reduced to conidiogenous cells in Cryptocline and Monostichella. Cryptocline has been distinguished from Monostichella by its flared conidiogenous cells, but this minor difference cannot be used to separate these two genera. Therefore, we synonymise Cryptocline with Monostichella, because the latter name has priority. Erythrogloeum and Gloeosporidina were placed in Diaporthales based on molecular data (Crous et al. 2012a; Senanayake et al. 2018), and we speculate that Monostichella might be related to Diaporthales. Wijayawardene et al. (2017b) estimated 15 taxa in the genus. Further studied with fresh collections and cultures are needed.
Distribution: Austria, Canada, Czechoslovakia, Hungary, India, Iraq, Italy, Germany, Romania, USA (Sutton 1980).
Mucoharknessia Crous, R.M. Sánchez & Bianchin., Phytotaxa 202(2): 86 (2015)
Facesoffungi number: FOF 01651,
Dothideomycetes, Incertae sedis, Botryosphaeriales, Botryosphaeriaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, scattered to gregarious or confluent, semi-immersed, globose to subglobose, unilocular or multilocular, glabrous, ostiolate. Ostiole short, circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis to textura globosa. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the innermost layers of conidiomata, hyaline, lageniform to subcylindrical, determinate, thick- and smooth-walled, with or without percurrently proliferation at apex, with or without flared collarette. Conidia brown or hyaline, ellipsoidal to oval, clavate or subcylindrical, thick- and smooth-walled, or smooth to finely verruculose, lacking striations, unicellular, straight or slightly curved, bearing two kinds type of apical appendage: (a) type B, arising from mucilaginous material around developing conidia gradually receding towards conidium apex, hyaline, mucilaginous, irregular, smooth, extracellular (Crous et al. 2015b); (b) type C, originating by eversion of partial or full mucoid sheath, widely flared, cup-like, cone-shaped or irregular, extracellular; basal appendage absent or present, tubular, thin-walled, smooth, hyaline, often collapsing.
Type species: Mucoharknessia cortaderiae Crous, R.M. Sánchez & Bianchin., Phytotaxa 202(2): 86 (2015).
Notes: Mucoharknessia was introduced by Crous et al. (2015b) based on M. cortaderiae. The type species is characterized by its conidia, which are brown, oval to ellipsoidal, thick-walled, smooth to finely verruculose, lacking striations, with extracellular apical appendage (type B, see Nag Raj 1993). Li et al. (2016) described a new species M. anthoxanthi Dissan., Camporesi & K.D. Hyde based on phylogeny, but its morphology is similar to tiaropsorella-like species. A phylogenetic tree reconstruction based on LSU, ITS, tef1 and tub2 sequences shows that two fresh collections on Dactylis glomerata from Italy have a close affinity with M. cortaderiae and M. anthoxanthi (Fig. 226). The fresh collections are very similar to M. anthoxanthi, except for conidiomatal structure. The fresh collections have dark brown, rounded, multilocular, papillate conidiomata in a linear series on the host surface, in contrast those of M. anthoxanthi, which were described as scattered to gregarious, unilocular, initially closed, then becoming partly erumpent (Fig. 227b, c). However, sequences of the new collections are 100% (559/559) similar to M. anthoxanthi in LSU gene region, and differ in four base pairs (including three gaps) in ITS. Based on both phylogeny and morphology, the new collection is regarded as conspecific with M. anthoxanthi. The difference of conidiomatal morphology is thought to reflect the intra-species variation. Two species are accepted in the genus, and a key to the species is provided.
Distribution: Argentina, Italy (Crous et al. 2015b; Li et al. 2016, this study).
Key to species
1. Conidia hyaline, subcylindrical to ellipsoidal, smooth-walled, with mucilaginous, apical appendage of type C…M. anthoxanthi
1. Conidia brown, oval to ellipsoidal, smooth to finely verruculose, with apical appendage of type B…M. cortaderiae
Mucoharknessia anthoxanthi Dissanayake, Camporesi & K.D. Hyde, in Li et al., Fungal Diversity 78: [19] (2016)
Facesoffungi number: FoF 07456, Figs. 227, 228
Saprobic on dead stems of Anthoxanthum odoratum and Dactylis glomerata. Sexual morph: undetermined. Asexual morph:Conidiomata up to 600 µm diam., 170–250 µm high, brown to dark brown, forming in a linear series on the host surface, or solitary, closed when young, partly erumpent when mature, stromatic, pycnidial, globose or subglobose, superficial or semi-immersed, unilocular or multi-locular, glabrous, ostiolate. Ostiole single to each locule, short, circular, papillate, centrally located. Conidiomatal wall 20–35 µm wide, composed of thick-walled, dark brown to pale brown cells of textura angularis in the outer layers, becoming hyaline cells of textura prismatica in the inner layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 6–20 × 2–6 µm, arising from innermost layers of conidiomata, hyaline, enteroblastic, phialidic, subcylindrical, determinate, smooth-walled. Conidia 18–31 × 6–9.5 µm (\( \bar{x} \) = 24 × 7.6 µm; n = 50), hyaline, ellipsoidal to clavate, rounded at apex, narrowly truncate at the base, unicellular, thick- and smooth-walled, bearing a cone-shaped, or widely flared to cup-like or irregular, undulate, mucoid, apical appendage, arising as segments of a partial mucoid sheath which initially enclosed the upper part of young conidium, basal usually appendages absent.
Material examined: Italy, Province of Forlì-Cesena, Passo delle Forche - Galeata, on dead aerial stems of Anthoxanthum odoratum (Poaceae), 24 November 2012, Erio Camporesi IT981 (MFLU 15–3477, holotype); ibid., on dead aerial stems of Dactylis glomerata (Poaceae), 21 April 2014, Erio Camporesi, IT1828a (MFLU 17-2782), IT1828b (KUN, HKAS 93589).
Notes: We re-examined the type specimen and provide an improved photo plate (Figs. 227, 228). For the fresh collection, despite several attempts we could not isolate it into culture. Therefore, DNA was extracted directly from the conidiomata of dried specimens, and its morphology and phylogeny results correspond to the Mucoharknessia species.
Mycotribulus Nag Raj & W.B. Kendr., Can. J. Bot. 48(12): 2219 (1970)
Facesoffungi number: FoF 07459
Agaricomycetes, Agaricomycetidae, Agaricales, Physalacriaceae
Saprobic on dead leaves of plant host. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious, immersed to semi-immersed, conical to subglobose, unilocular, thick-walled, glabrous, ostiolate. Ostiole cylindrical, single, centrally located. Conidiomata wall composed of thick-walled, hyaline to brown cells of textura angularis. Conidiophores hyaline, unbranched or sparsely branched, intermingled with paraphyses formed from the inner layers of the conidiomata. Paraphyses hyaline, filamentous, branched or unbranched, septate, narrowed at the base, broad and lobed or irregular at the apices. Conidiogenous cells hyaline, holoblastic, subcylindrical, to obclavate, discrete, smooth. Conidia hyaline, fusiform to navicular, pointed at apex, narrowed and somewhat truncated at base, aseptate, smooth, guttulate, bearing filiform, tubular, flexuous appendages at both ends, apical appendage single, polar, unbranched, straight or slightly curved, basal appendages mostly 2–3, invested laterally slightly above the truncate base, unbranched, divergent, straight or often curved.
Type species: Mycotribulus mirabilis Nag Raj & W.B. Kendr., Can. J. Bot. 48(12): 2219 (1970)
Notes: Few coelomycetous genera viz. Cenangiomyces, Chaetospermum, Ellula, Fibulocoela, Giulia, Mycotribulus and Pycnovellomyces have been reported as asexual morphs of Basidiomycota (Dyko and Sutton 1979b; Nag Raj 1979b, 1980, 1993; Rungjindamai et al. 2008). Only Chaetospermum, Giulia and Mycotribulus are confirmed by molecular sequences (Rungjindamai et al. 2008; Crous et al. 2014a; Tangthirasunun et al. 2014b). Mycotribulus was introduced by Nag Raj and Kendrick (1970) to accommodate M. mirabilis collected from rotting leaves of Eucalyptus sp. This species is probably cosmopolitan (China, India, Venezuela and South Africa and Thailand) and associated with several host plants namely, Apodytes abbottii Potg. & A. E.van Wyk, Eucalyptus camaldulensis Dehnh., Mangifera indica L. and Syzygium cordatum Hochst. ex Krauss (Nag Raj 1993; Crous 1993; Marincowitz et al. 2010). Rungjindamai et al. 2008) located Mycotribulus in Physalacriaceae (Agaricales) on the basis of SSU and LSU sequence data. Crous et al. (2014a) epitypified this genus and confirmed the result of Rungjindamai et al. (2008), and introduced the second species, M. indonesiae Crous.
Mycotribulus can be confused with Ajrekarella, Dinemasporium and Polynema because of the conidia being hyaline, fusiform to navicular or ellipsoid with appendages at each end. However, Mycotribulus and Ajrekarella have pycnidial conidiomata, which are distinct from the cupulate conidiomata in Dinemasporium and Polynema. Mycotribulus differs from Ajrekarella by its paraphyses.
Distribution: China, India and Venezuela (Crous et al. 2014a, this study).
Mycotribulus mirabilis Nag Raj & W.B. Kendr., Can. J. Bot. 48(12): 2219 (1970)
Facesoffungi number: FoF 07460, Fig. 229
Saprobic on dead rotting leaves of Eucalyptus sp. Sexual morph: undetermined. Asexual morph:Conidiomata 200–300 µm diam., 150–200 µm high, dark brown to black, pycnidial, amphigenous, solitary to gregarious, initially immersed, becoming partly erumpent at maturity, conical to subglobose, unilocular, thick-walled, glabrous, ostiolate, surrounded by yellow conidial mass at the tip. Ostiole 60–90 µm long × 50–70 µm wide, cylindrical, single, centrally located. Conidiomata wall 10–25 µm wide, composed of an outer textura angularis with thick-walled, brown cells, and inner layer of hyaline cells. Conidiophores hyaline, unbranched or sparsely branched and septate at the base, intermingled with paraphyses lining the cavity of the conidiomata. Paraphyses 15–60 × 1.5–2.5 µm, hyaline, filamentous, unbranched or branched, septate, gradually widening at the deeply lobed apex to a width of 1.5–3.5 µm, with the apical lobes irregularly curved. Conidiogenous cells 9–17 × 2–2.5 µm, hyaline, holoblastic, cylindrical to subcylindrical, smooth. Conidia 12.5–17.6 × 2.6–5.6 µm (\( \bar{x} \) = 15 × 4 µm; n = 50), hyaline, fusiform to naviculate, pointed at apex, narrowed and somewhat truncated at base, aseptate, smooth, guttulate, bearing filiform, tubular, flexuous appendages at both ends, apical appendage 8–16 µm long, single, polar, unbranched, straight or slightly curved, basal appendages 7–14 µm long, mostly 2–3, invested laterally slightly above the truncate base, unbranched, divergent, straight or often curved.
Material examined: India, Karnataka State, Balehonnur, Coffee Research Station, on rotting leaves of Eucalyptus sp. (Myrtaceae), T.R. Nag Raj, 12 November 1963 (IMI 128041, holotype), (DAOM 124817, isotype).
Myriellina Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 100 (1915)
Facesoffungi number: FoF 07455, Fig. 230
Ascomycota, genera incertae sedis
Habit on leaves of Cydonia vulgaris (Rosaceae), Imperata cylindrica (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata brown, acervular, solitary, subepidermal, immersed to erumpent, linear in outline, subglobose in section view, unilocular, glabrous. Ostiole indistinct, dehiscence by irregular rupture of the apical wall. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis extending into the hypodermis. Conidiophores arising from the inner cell layers of basal stroma, hyaline, cylindrical, simple or branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform or irregular, integrated or discrete, determinate, smooth-walled. Conidia hyaline, cylindrical to fusiform, tapered towards apex, truncate at the base, 1–5-septate (mostly 3-septate), smooth-walled.
Type species: Myriellina cydoniae (Desm.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 124: 100 (1915)
Notes: Sutton (1980) re-described Myriellina and provided a detailed description for the genus. Sankaran and Sutton (1991) described the second species M. imperatae B. Sutton & Sankaran from Imperata cylindrica (Poaceae) in Australia. Myriellina imperatae differs from M. cydoniae by its larger conidia; (15.5–)22–40.5 × 1.5–2 µm in M. imperatae, and 9–17 × 2.5–3 µm in M. cydoniae (Sutton 1980; Sankaran and Sutton 1991). Both Myriellina species are associated with leaf lesions (Sutton 1980; Sankaran and Sutton 1991).
Myriellina is similar to Cheilaria in having acervular conidiomata and fusiform, septate conidia. The differences between those two genera are basal stroma and conidial septation. Cheilaria has basal stroma composed of rather thick-walled, dark brown to pale brown cells of textura angularis to textura epidermoidea, and 0–3-septate (usually 2-septate) conidia. Myriellina has basal stroma composed of thin-walled, pale brown cells of textura angularis, and 1–5-septate (usually 3-septate) conidia. However, conidial septation is not a sufficient character for generic delineation (Minnis et al. 2012). Thus, Cheilaria and Myriellina might be taxonomically congeneric. The differences of basal stroma between these two genera can be used to delineate taxa at the species level. There is no molecular data available for Cheilaria or Myriellina. Therefore, fresh collections are needed to verify our hypothesis.
Distribution: Australia, Italy (Sutton 1980; Sankaran and Sutton 1991).
Myxothyrium Bubák & Kabát, Svensk bot. Tidskr. 9: 379 (1915)
Facesoffungi number: FoF 07457, Fig. 231
Ascomycota, genera incertae sedis
Saprobic on dead leaves of Vaccinium vitis-idaea (Ericaceae).Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, stromatic, pycnidial, hypophyllous, solitary to gregarious or confluent, epidermal to subepidermal, immersed, subglobose, unilocular or multi-locular, glabrous. Ostiole absent, dehiscence by irregular split of apical wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the inner wall layer of conidiomata, hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled. Conidia hyaline, cylindrical to fusiform, with obtuse ends, straight, unicellular, smooth-walled, with several large guttules (adapted from Sutton 1980).
Type species: Myxothyrium leptideum (Fr.) Bubák & Kabát, Svensk bot. Tidskr. 9: 379 (1915)
Notes: Sutton (1980) re-described Myxothyrium and accepted a single species. The second species M. camelliarum was added by Zhao and Zhao (2012), but this has not been re-examined. Wijayawardene et al. (2017b) accepted only the type species in the genus. Myxothyrium can be confused with Phoma in form of conidiomata, conidiogenous cells and conidia, and it may be taxonomically congeneric with Phoma. Sequence data is not available for Myxothyrium and fresh collections are needed to verify the relationship between Myxothyrium and Phoma.
Distribution: Sweden (Sutton 1980).
Nanoschema B. Sutton, The Coelomycetes: 589 (1980)
Facesoffungi number: FoF 07458, Fig. 232
Ascomycota, genera incertae sedis
Parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, tubular or cupulate, attached to the substrate by a small hypostroma, solitary, superficial, unilocular. Ostiole absent. Conidiomatal wall composed of thin-walled, brown to hyaline cells of textura angularis in the basal region, pale brown cells of textura porrecta in the lateral region, becoming shortly fimbriate in the apex. Conidiophores arising from the inner wall layer of basal stroma, hyaline, cylindrical, irregularly branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated, determinate, smooth-walled. Conidia hyaline, ellipsoid, rounded at the apex, narrowed and truncate at the base, straight or slightly curved, 1-septate, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Nanoschema elaeocarpi B. Sutton, The Coelomycetes: 589 (1980)
Notes: Nanoschema is similar to Cornucopiella, Dinemasporium, Hoehneliella, Infundibulomyces, Oncosporella, Pseudolachnea, Satchmopsis and Stevensonula with tubular or cupulate conidiomata (Sutton 1980; Cole and Kendrick et al. 1981; Sutton and Pascoe 1987; Nag Raj 1993, this study). However, Infundibulomyces, Oncosporella, and Satchmopsis can be distinguished from other genera by their glabrous conidiomatal wall. Nanoschema has ellipsoid, 1-septate conidia, which can be distinguished from ellipsoid, unicellular conidia in Cornucopiella, hyaline, ellipsoid, allantoid to naviculate or fusiform conidia bearing appendages in Dinemasporium and Pseudolachnea, hyaline to brown, 1-septate conidia with branched or unbranched appendages in Hoehneliella, and dark brown, broadly fusiform to mostly reniform conidia with appendages at each end in Stevensonula. Nanoschema is a monotypic genus. Nanoschema elaeocarpi is associated with leaf lesions on Elaeocarpus sphaericus (Elaeocarpaceae) in Solomon Islands. There is no molecular data available.
Distribution: UK (Sutton 1980).
Nectria (Fr.) Fr., Summa veg. Scand., Sectio Post. (Stockholm): 387 (1849)
Facesoffungi number: FoF 02122
Sordariomycetes, Hypocreomycetidae, Hypocreales, Nectriaceae
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: see Hirooka et al. (2011, 2012). Asexual morph:Conidiomata orange to yellowish, or red-brown to black, or orange to red, stromatic, variable from pycnidial to synnematous or sporodochial, sessile to long stipitate, intra-epidermal in origin, erumpent, solitary or gregarious, emerging from ascomatal cluster or independently, basal stroma well developed. Wall of basal stroma composed relatively thick-walled, hyaline to pale brown cells of textura angularis to textura prismatica or textura epidermodea to textura intricata; Wall of stalk composed thick-walled, brown cells of textura porrecta, covered by brown to pale brown hyphae. Conidiophores arising in the cavity of the conidiomata, hyaline, branched, septate, smooth-walled, branches terminating in conidiogenous cells and sterile hyphae, invested in mucus. Sterile hyphae present or absent, hyaline, filiform, unbranched or dichotomously branched, straight or curved, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to subcylindrical or lageniform, discrete or integrated, determinate, smooth-walled, with or without acropleurogenous branches. Conidia hyaline, ellipsoidal to subglobose, obovate, or cylindrical, or sometimes allantoid, straight or slightly curved, unicellular, smooth-walled (Hirooka et al. 2012).
Type species: Nectria cinnabarina (Tode) Fr., Summa veg. Scand., Sectio Post. (Stockholm): 388 (1849)
Notes: Nectria can be confused with Albonectria, Allantonectria, Geejayessia, and Thyronectria, because it has lightly to brightly coloured, ostiolate, perithecial, unilocular or multilocular ascomata, and unitunicate asci (Lombard et al. 2015). However, Nectria has coelomycetous asexual morphs, which can be separated from those genera with hyphomycetous asexual morphs, such as Albonectria and Geejayessia which are associated with fusarium-like asexual morphs, and Allantonectria with trichoderma-like asexual morph (Schroers et al. 2011; Hirooka et al. 2011, 2012; Lombard et al. 2015). Thyronectria differs from Nectria by its ellipsoidal, fusiform to long-fusiform, cylindrical, muriform ascomata budding to produce hyaline bacillar ascoconidia (Hirooka et al. 2012; Jaklitsch and Voglmayr 2014).
Clements and Shear (1931) designated N. cinnabarina as a legitimate lectotype for Nectria. More than 1000 species have been described in the genus, and most of them have been allocated in Bionectriaceae and Nectriaceae (Rehner and Samuels 1995; Rossman et al. 1999, 2013; Schroers 2001; Hirooka et al. 2010, 2012; Lombard et al. 2015). The concept of Nectria was restricted to a narrow sense by Rossman (1989); Rossman et al. (1999) and Hirooka et al. (2012). Thus, 31 taxa are recognized in Nectria sensu stricto, of which 29 were described and illustrated by Hirooka et al. (2012), and another two (N. triseptata and N. tibetensis) by Zeng and Zhuang (2015) and Zeng et al. (2018). The asexual morph of Nectria has been assigned to Gyrostroma Naumov with immersed pycnidia, Tubercularia Tode with synnematal and sporodochial conidiomata, and Zythiostroma Höhn. with superficial pycnidia (Seifert 1985a; Rossman 1989; Rossman et al. 1999). Hirooka et al. (2012) revised Nectria and restricted the asexual morph of Nectria sensu stricto to tubercularia-like fungi, except for N. magnispora (pycnidial conidiomata), based on both morphological characteristics and molecular sequence data. Considering Nectria is a well-studied genus and has priority, the asexual morph Tubercularia was recognized as synonym of Nectria (Rossman et al. 2013; Lombard et al. 2015). In the present study, four asexual morph isolates collected from Italy share similar form of conidiomata, conidiogenous cells and conidia with N. dematiosa. Phylogenetically, these strains also clustered with N. dematiosa (A.R. 2699, CBS 126570, MAFF 241430) with 99% (MLBS) (Fig. 233). Based on both phenotypic and genotypic characters, these four strains are considered conspecific with N. dematiosa, and a detailed description and plate are provided.
Distribution: Argentina, Austria, Brazil, Bolivia, Cameroon, Canada, Chile, China, Croatia, Colombia, Costa Rica, Cuba, Czech Republic, Denmark, Dominica, Ecuador, El Salvador, Finland, France, French Guiana, Gabon, Germany, Ghana, Greece, Guadeloupe, Guatemala, Guyana, India, Indonesia, Ireland, Italy, Jamaica, Japan, Malaysia, Martinique, Mexico, Montenegro, New Zealand, Netherlands, Panama, Papua New Guinea, Paraguay, Peru, Philippines, Poland, Puerto Rico, Spain, Sri Lanka, Thailand, Tanzania, Uganda, UK, Ukraine, USA, Venezuela (Hirooka et al. 2011, 2012).
Nectria dematiosa (Schwein.) Berk., N. Amer. Fung.: no. 154 (1873)
Faces of fungi number: FoF 07461, Figs. 234, 235
Saprobic on the host plant in terrestrial habit. Sexual morph: see Hirooka et al. (2011, 2012). Asexual morph:Conidiomata 120–300 µm diam., 100–250 µm high, sporodochial, sessile, orange to yellowish or red, mostly solitary, occasionally gregarious or confluent, semi-immersed to erumpent, pulvinate, subepidermal, cup-shaped, subglobose, thick-walled. Conidiomatal wall 20–40 µm wide, middle wall composed of thick-walled, hyaline cells of textura angularis to tight textura prismatica towards upper part; basal wall composed of thick-walled, pale brown cells of textura angularis; lateral wall adjacent to host tissue composed of thick-walled, hyaline to light brown cells of textura epidermodea to textura intricata. Conidiophores up to 150 µm long, 2–3 µm wide, formed from the upper cells of conidiomata, hyaline, cylindrical to subcylindrical, densely branched and swollen at base, septate, straight or flexuous, flared towards the apices, smooth-walled, with acropleurogenous conidiogenous cells. Conidiogenous cells 3–10 × 2–5 µm, hyaline, enteroblastic, phialidic, short cylindrical or subcylindrical, determinate, integrated or discrete, arising from apical or on short lateral branches which form immediately below septa. Conidia 13–17 × 2–5.5 µm (\( \bar{x} \) = 15 × 4 µm; n = 30), hyaline, cylindrical to subcylindrical or fusiform, with a rounded apex and a narrow truncate base, straight or slightly curved, unicellular, thick- and smooth-walled, accumulating in a slimy orange mass above the conidioma.
Culture characteristiers: Colony on PDA, reaching 50–60 mm diam. after 14 days at 25–30 °C, white to whitish saffron with age, flattened, dense, radial, slightly cottony with aerial mycelium on the surface, with rounded margins, sporulating at 4 weeks, reverse white.
Material examined: Iatly, Province of Forlì-Cesena, Santa Sofia, near Corniolo, on dead aerial branches of Populus tremula (Salicaceae), 23 February 2016, Camporesi Erio, IT2848 (MFLU 16-0921), living culture MFLUCC 16-1141 = KUMCC 16-0078, (KUN, HKAS 97474); ibid., Santa Sofia, Campigna, on dead aerial branches of Acer pseudoplatanus (Sapindaceae), 2 April 2016, IT2917 (MFLU 16-1131), living culture MFLUCC 16-1143 = ICMP 21543 = KUMCC 16-0092, (KUN, HKAS 97460); ibid., Monte Fumaiolo, on dead aerial branches of Rhamnus alpinus (Rhamnaceae), 3 July 2016, IT3017 (MFLU 16-2053), living culture MFLUCC 16-1309 = ICMP 21552 = KUMCC 16-0104; ibid., Province of Arezzo, near Casuccia di Micheli, on dead aerial branches of Crataegus sp. (Rosaceae), 30 May 2016, Erio Camporesi, IT2986 (MFLU 16-1951), living culture MFLUCC 16-1307 = ICMP 21550 = KUMCC 16-0101, (KUN, HKAS 97501).
Notes: Nectria dematiosa was epitypified by Hirooka et al. (2011) based on the collection on Morus sp. (Moraceae) from the USA with a detailed description and illustration. This species has wide distribution (Canada, China, Finland, Japan, New Zealand, Poland, USA) and is associated with a broad range of host plants (Acer macrophyllum, A. pseudoplatanus, Acer sp., Morus sp., Prunus tenella, Ribes sp., Rosa sp., Sambucus nigra ssp. canadensis, and Weigela coraeensis). In this study, four additional strains collected on A. pseudoplatanus, Crataegus sp., P. tremula, and R. alpinus from Italy are added.
Neoascochyta Qian Chen & L. Cai, Stud. Mycol. 82: 198 (2015)
Facesoffungi number: FoF 07462
Dothideomycetes, Pleosporomycetidae, Pleosporales, Didymellaceae
Saprobic on terrestrial plants or from soil or human bodies (Valenzuela-Lopez et al. 2018). Sexual morph: see Chen et al. (2015). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, globose to subglobose or ovoid, unilocular, glabrous, ostiolate. Ostiole single, centrally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis to textura globosa. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform to ampulliform, or short obpyriform, determinate, smooth-walled, formed in the inner cavity of conidiomata. Conidia hyaline, variable in shape, cylindrical to subcylindrical, or obclavate-ovoid to ellipsoidal, or fusiform, 0–1-septate, smooth-walled, guttulate.
Type species: Neoascochyta exitialis (Morini) Qian Chen & L. Cai, Stud. Mycol. 82: 200 (2015)
Notes: The species of Neoascochyta are cosmopolitan, and have been reported from Argentina, Austria, Belgium, China, Italy, Germany, Netherlands, New Zealand, Norway, South Africa, Sweden, Switzerland, USA (Chen et al. 2015, 2017; Valenzuela-Lopez et al. 2018). Twelve species are listed in Index Fungorum (2019), of which Neoascochyta adenii Crous & Cheew. clustered away from Neoascochyta species in our phylogenetic tree (data not shown here). Morphologically, N. adenii has setose conidiomata which are absent in other taxa of Neoascochyta, thus N. adenii is excluded from Neoascochyta. In addition, a new species Neoascochyta dactylidis and additional collections of N. desmazieri and N. tardicrescens are described from natural substrates.
Distribution: worldwide.
Neoascochyta dactylidis W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557150, Facesoffungi number: FoF 07463, Figs. 237, 238
Etymology: Name refers to Dactylis, the host genus from which this fungus was collected.
Saprobic on dead stems of Dactylis glomerata. Sexual morph: undetermined. Asexual morph:Conidiomata 100–125 µm high, 100–130 µm diam., dark brown to black, pycnidial, solitary, gregarious or confluent, deeply immersed with black spots on the host surface, globose, unilocular, glabrous, ostiolate. Conidiomatal wall 10–20 µm wide, composed of brown to dark brown, thick-walled cells of textura globosa in the exterior, becoming thin-walled, pale brown to hyaline cells of textura angularis in the conidial hymenium. Ostiole single, circular, papillate, centrally located. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–9 × 3.5–10 µm, hyaline, enteroblactic, phialidic, short, originating from the innermost cell layer of conidiomata. Conidia 20–29 × 5–8 µm (\( \bar{x} \) = 25 × 7 µm; n = 50), hyaline, cylindrical to fusiform, with narrow and rounded ends or sometime broad and truncate at base, 1-septate, straight or slightly curved, thick- and smooth-walled, with two large guttules in each cell.
Culture characters: Colonies on PDA, reaching 12–15 mm diam. after 7 days at 25–30 °C, white to pale brown in the middle zone, paler toward margins, with orange mycelium mass in the middle zone after 4 weeks, umbonate, dense, reverse brown, with white margins.
Material examined: Italy, Province of Forlì-Cesena, Predappio, near Monte Colombo, on dead aerial stems of Dactylis glomerata (Poaceae), 2 February 2013, Erio Camporesi, IT1038 (MFLU 19-2859, holotype), ex-type living culture MFLUCC 13-0495, (KUN, HKAS 101674, isotype).
Notes: Neoascochyta dactylidis shows a closely affinity with N. europaea, N. exitialis and N. graminicola, but constitutes a separate branch (Fig. 236). Morphologically, it fits well with the concept of Neoascochyta, but can be distinguished by its larger conidia with four large guttules. Based on both phylogeny and morphology, N. dactylidis is described as new taxon in Neoascochyta.
Neoascochyta desmazieri (Cavara) Qian Chen & L. Cai, Stud. Mycol. 82: 198 (2015)
Facesoffungi number: FoF 07464, Fig. 239
Habit on dead stems of Fabaceae and Poaceae (Chen et al. 2015) or on human (Valenzuela-Lopez et al. 2018). Sexual morph: undetermined. Asexual morph:Conidiomata 100–150 µm diam., 80–100 µm high, black, pycnidial, mostly solitary, superficial or semi-immersed, globose or subglobose, unilocular, thick-walled, ostiolate, papillate. Conidiomatal wall 15–30 µm wide, composed of textura angularis with thick-walled, dark brown, larger cells becoming paler and compact towards conidial hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–6 × 3–6 µm, arising all around the basal region of the conidiomata, hyaline, enterblastic, phialidic, dolliform or ampulliform to broadly conical, determinate, smooth-walled, formed from the innermost layer of cells of the pycnidial wall. Conidia 12–14 × 2–4 µm (\( \bar{x} \) = 13 × 3; n = 50), hyaline, cylindrical, rounded at both ends, straight, 1-septate, smooth-walled, guttulate.
Culture characters: Colonies on PDA, reaching 40–50 mm diam. after 7 days at 25–30 °C, white to pale grey in the middle zone, becoming darker with age, velutinous to felty, flattened, dense, subsuperficial, reverse black in the middle region, paler toward margins. Margins even, entire, neat.
Material examined: Italy, Province of Arezzo, Stia, Montemezzano, on dead aerial stems of Vicia sp. (Fabaceae), 3 October 2012, Erio Camporesi, IT769 (MFLU 19-2881), living culture MFLUCC 13-0395 = ICMP 20787, (KUN, HKAS 93667).
Notes: Neoascochyta desmazieri has also been isolated from the head, respiratory tract, and toe nail of humans (Valenzuela-Lopez et al. 2018).
Neoascochyta tardicrescens Valenz.-Lopez, Cano, Crous, Guarro & Stchigel, in Valenzuela-Lopez et al. Stud. Mycol. 90: 44 (2017)
Facesoffungi number: FoF 07465, Fig. 240
Saprobic on dead leaves of Dactylis glomerata. Sexual morph: undetermined. Asexual morph:Conidiomata 75–140 µm diam., 70–120 µm high, pycnidial, scattered or gregarious, immersed, with dark brown to black spots on the host surface, globose to subglobose, unilocular, thick-walled, ostiolate. Ostiole circular, papillate, centrally located. Conidiomatal wall 10–20 µm wide, composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–7 × 3–6 µm, hyaline, enteroloblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled, formed from the inner wall cells of the pycnidium. Conidia 9–17.5 × 3–5 µm (\( \bar{x} \) = 13 × 4; n = 50), hyaline, fusiform, straight, obtuse at both ends, smooth-walled, guttulate.
Culture characters: Colonies on PDA, reaching 20–30 mm diam. after 7 days at 25–30 °C, white to darker in the middle zone, becoming grey to black with age, velutinous to felty, flattened, dense, subsuperficial, reverse black, with fimbriae margins; sporulating after 3 months.
Material examined: Italy, province of Forlì-Cesena, Civitelle di Romagna, Bonalda, on dead aerial leaves of Dactylis glomerata (Poaceae), 2 December 2012, Erio Camporesi, IT945 (MFLU 19-2885), living culture MFLUCC 13-0483 = ICMP 20793; (KUN, HKAS 101699).
Notes: Neoascochyta tardicrescens has also been isolated from human feet (Valenzuela-Lopez et al. 2018).
Neochaetospora B. Sutton & Sankaran, Mycol. Res. 95(6): 768 (1991)
≡ Chaetospora Faurel & Schotter, Revue Mycol., Paris 30(3): 149 (1965) [1964]
Facesoffungi number: FoF 07474, Fig. 241
Ascomycota, genera incertae sedis
Coprophilous on rodent dung (e.g., Massoutiera harterti). Sexual morph: undetermined. Asexual morph: Conidiomata black, pycnidial, innate-erumpent, globose, unilocular, setose, ostiolate. Ostiole single, circular, centrally located. Conidiomatal setae dark brown below, tapered and paler towards apex, subulate to irregular, rigid, straight or slightly curved, unbranched, smooth-walled, mostly restricted at ostiolar region, occasionally on the pycnidial wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from inner cell layer of conidiomata, hyaline, enteroblastic, phialidic, lageniform to cylindrical, discrete, determinate, smooth-walled, with flared collarettes. Conidia hyaline, cylindrical to fusiform, with truncate base, 2-septate, smooth-walled, bearing 3–5, unbranched, septate, flexuous, apical appendages arising from different loci of bulbous apical cell (Nag Raj 1993).
Type species: Neochaetospora quezelii (Faurel & Schotter) B. Sutton & Sankaran, Mycol. Res. 95(6): 768 (1991)
≡ Chaetospora quezelii Faurel & Schotter, Revue Mycol., Paris 29(3): 149 (1965) [1964]
Notes: Neochaetospora is a monotypic genus. The genus is similar to Pseudoneottiospora in having cylindrical or subcylindrical to fusiform conidia bearing unbranched, flexuous appendages. However, Neochaetospora possesses globose, setose conidiomata and 2-septate conidia with bulbous apical cell bearing 3–5-septate, apical appendages. Pseudoneottiospora has globose, glabrous conidiomata and 1–3-septate conidia with cylindrical or dolliform apical cell bearing 3–4-aseptate apical appendages (Nag Raj 1993). Neochaetospora has not been studied with molecular data. Future studies with fresh collections are needed to place it in a natural group.
Distribution: Sahara (Africa) (Nag Raj 1993).
Neocucurbitaria Wanas., E.B.G. Jones & K.D. Hyde, et al., Mycosphere 8(3): 408 (2017)
Facesoffungi number: FoF 02902
Dothideomycetes, Pleosporomycetidae, Pleosporales, Cucurbitariaceae
Saprobic on the host plant or animal dung or isolated from human body. Sexual morph: see Wanasinghe et al. (2017). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, globose to subglobose, unilocular, papillate, setose, ostiolate. Ostiole circular, single, centrally located. Conidiomatal setae brown, septate, branched, smooth-walled. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores usually reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical to lageniform, arising from the inner wall layer of the conidiomata. Conidia hyaline, cylindrical or ellipsoidal, unicellular, guttulate, smooth-walled.
Type species: Neocucurbitaria unguis-hominis (Punith. & M.P. English) Wanas., E.B.G. Jones & K.D. Hyde et al., Mycosphere 8(3): 412 (2017)
= Pyrenochaeta unguis-hominis Punith. & M.P. English, Trans. Br. Mycol. Soc. 64(3): 539 (1975)
Notes: Neocucurbitaria can be confused with Pyrenochaeta as they both have setose pycnidia producing hyaline, unicellular conidia. The conidiophores of Neocucurbitaria are usually unbranched, or reduced to conidiogenous cells, whereas those of Pyrenochaeta are branched, filiform, septate, and acropleurogenous (Sutton 1980; Wanasinghe et al. 2017; De Gruyter et al. 2010). Neocucurbitaria was placed in Cucurbitariaceae (Wanasinghe et al. 2017). However, the placement of Pyrenochaeta remains ambiguous after several phylogenetic studies and needs further revision (Aveskampet al. 2010; De Gruyter et al. 2010; Valenzuela-Lopez et al. 2018).
Twenty-two species are accepted in Neocucurbitaria (Index Fungorum 2019). They are saprobes or human pathogens, for example, N. unguis-hominis can cause infection of human skin and nails (de Gruyter et al. 2010; Toh et al. 2016). We examined a fungus on rodent dung, which shows a similar morphology of conidiomata, conidiogenous cells, and conidia with Neocucurbitaria. However, it lacks molecular data and thus we keep it as Neocucurbitaria sp. The taxonomy of this species needs further investigations based on fresh collections and phylogenetic inferences.
Distribution: Australia, Israel, Canada, Germany, Greece, Italy, Netherlands, Montenegro, Spain, Ukraine, USA (Wanasinghe et al. 2017; Valenzuela-Lopez et al. 2018; Crous et al. 2019).
Neocucurbitaria sp.
Fig. 242
Saprobic on rodent dung. Sexual morph: undetermined. Asexual morph: Coelomycetous. Conidiomata 120–250 µm diam., 120–250 µm high, dark brown to black, pycnidial, solitary to gregarious, immersed to semi-immersed, globose to subglobose or oval, unilocular, thick-walled, papillate, setose, ostiolate. Ostiole circular, single, centrally located. Conidiomatal setae 2–4 µm wide, septate, branched. Conidiomatal wall 14–30 µm wide, composed of thick-walled, brown to pale brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells comprised of two types: (a) 6–25 × 1.5–3.5 µm, hyaline, subcylindrical to lageniform, with marked periclinal thickenings at collarette zone, arising from the inner-most cells of the conidiomata at base and lateral part; (b) 3.5–7 × 2–3 µm, cylindrical, pale brown at base, becoming hyaline above, formed at upper zone close to ostiole. Conidia 3.5–7 × 2–3 µm (\( \bar{x} \) = 5 × 2 µm; n = 30), hyaline, cylindrical to oval or circular, unicellular, obtuse at each end, guttulate, smooth-walled.
Material examined: USA, California, San Bernardino Co., 1 mile North of Mt. Baldy, on rodent dung, 11 April 1972, D. Malloch (DAOM 215329).
Neodermea W.J. Li, D.J. Bhat & K.D. Hyde, gen. nov.
Index Fungorum number: IF557151, Facesoffungi number: FoF 07471
Etymology: Named after its morphology similar to Dermea.
Leotiomycetes, Leotiomycetidae, Medeolariales, Dermateaceae
Saprobic on plant host. Sexual morph:Apothecia black or drak brown, solitary or gregarious, erumpent, circular or undulate, sessile, narrowed below, leathery to horny in consistency, becoming more fleshy-leathery when moist. Hymenium at first concave, becoming plane or slightly convex, the margin at first thick, raised, later almost disappearing. Paraphyses hyaline, filiform, branched, septate, apex slightly swollen and glued together forming a yellowish epithecium. Tissue of hypothecium compact, pseudoparenchymatous, brownish. Asci 8-spored, cylindrical to clavate, short, stalked. Ascospores hyaline when young, becoming yellowish at maturity, uniseriate to biseriate, oblong-ellipsoid to ellipsoid-fusiform, 1–3-septate, straight or slightly curved (adapted from Grove 1946). Asexual morph:Conidiomata dark brown to black, stomatic, pycnidial, solitary or gregarious, immersed to semi-immersed, erumpent, globose to subglobose, unilocular or multi-locular, thick-walled, ostiolate. Ostiole cylindrical, single, with wide channel, centrally located. Conidiomata wall composed of hyaline to brown, thick-walled cells of textura angularis. Conidiophores arising from the innermost wall layer of conidiomata, hyaline, cylindrical, septate. Conidiogenous cells enteroblastic, phialdic, cylindrical, usually integrated, determinate, smooth. Conidia hyaline, oblong-ellipsoid, rounded at both ends, or with a narrow and truncate base, straight or slightly curved, unicellular, guttulate, smooth-walled. Microconidia absent or present, hyaline, filiform, aseptate, straight or curved.
Type species: Neodermea rossica W.J. Li, D.J. Bhat & K.D. Hyde, sp. nov.
Notes: Dermea acerina (Peck) Rehm was introduced by Peck (1878) as Tympanis acerina Peck based on morphology of apothecia. Subsequently, Saccardo (1889) transferred D. acerina to Scleroderris (Fr.) Bonord., although it differs from Scleroderris species in oblong-ellipsoid to ellipsoid-fusiform ascospores (Grove 1946). Rehm (1912) transferred it toDermea based on characters of apothecia, asci and ascospores. However, D. acerina has oblong-ellipsoid conidia, which is similar in form to conidia of Pezicula species, rather than D. cerasi (elongate-fusiform to subfiliform conidia), and this has resulted in a confusion of the generic concept. Groves (1938, 1941) established the connection between D. cerasi and Naemosphaera acerina (Peck) Höhn., based on culture studies. A phylogenetic tree for Dermateaceae based on multi-locus sequence data shows that D. acerina CBS 161.38 is distinct from D. cerasi and forms a separate clade with two new collections (Fig. 129). Neodermea is introduced as a new genus to accommodate N. acerina and N. rossica. Neodermea rossica is designated as the type species.
Distribution: Canada, Italy, USA (Grove 1946; Verkley et al. 2003, this study)
Neodermea acerina (Peck) Rehm) W.J. Li & K.D. Hyde, comb. nov.
≡ Tympanis acerina Peck, Ann. Rep. N.Y. St. Mus. nat. Hist. 31: 48 (1878)
= Dermea acerina (Peck) Rehm, Ber. bayer. bot. Ges. 13: 197 (1912)
Index Fungorum number: IF557170, Facesoffungi number: FoF 07472
Description and illustrations see Grove (1946).
Neodermea rossica W.J. Li, D.J. Bhat & K.D. Hyde, sp. nov.
Index Fungorum number: IF557152, Facesoffungi number: FoF 07473, Fig. 243
Etymology: Named after country Russia from which it was collected.
Saprophytic on dead stem of Acer tataricum (Sapindaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 370–450 µm diam., 200–350 µm high, dark brown to black, stomatic, pycnidial, solitary or gregarious, deeply immersed in origin, becoming partly erumpent at maturity, globose to subglobose, unilocular or multi-locular, thick-walled, ostiolate. Ostiole cylindrical, single, with wide channel, centrally located. Conidiomata wall 30–90 µm wide, composed of hyaline to brown, rather thick-walled cells of textura angularis to textura prismatica at basal region, and brown to dark brown cells of textura angularis at upper and lateral region. Conidiophores hyaline, cylindrical, septate, arising from the inner layers of conidiomata. Conidiogenous cells 3–15 × 2–4 µm, enteroblastic, phialidic, cylindrical, discrete or integrated, indeterminate, smooth. Conidia 14–25 × 4–8 µm (\( \bar{x} \) = 17 × 6.5 µm; n = 30), hyaline, oblong-ellipsoid, rounded at both ends, or narrowed and truncate at base, straight or slightly curved, unicellular, guttulate, smooth. Microconidia absent.
Material examined: Russia, Rostov region, Donskoye forestry, on dead stems of Acer tataricum (Sapindaceae), 18 June 2015, T.S. Bulgakov, T486B (MFLU 15-2190, holotype), ex-type living culture MFLUCC 17-2506 = KUMCC 15-0635, T486 (MFLU 19-2890).
Notes: Neodermea rossica is similar to N. acerina in its oblong-ellipsoid, straight or slightly curved, unicelluar conidia. However, those two taxa are separated by conidiomatal structure and microconidia. Neodermea acerina has slender, beaked conidiomata up to 1.5 mm in length and 300–650 µm wide, which is longer and wider than the globose to subglobose conidiomata with cylindrical ostiole in N. rossica (Grove 1946). Neodermea acerina also has microconidia, which are absent in N. rossica.
Neodidymelliopsis Qian Chen & L. Cai, Stud. Mycol. 82: 207 (2015)
Facesoffungi number: FoF 07518, Fig. 244
Dothideomycetes, Pleosporomycetidae, Pleosporales, Didymellaceae
Saprobic on terrestrial plants or from soil in desert (Chen et al. 2015, 2017) Sexual morph: see Chen et al. (2015). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed or erumpent, globose to subglobose, unilocular or multilocular, glabrous, ostiolate. Ostiole single, papillate or with elongate necks, centrally or laterally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells formed in the inner cavity of conidiomata, hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled. Conidia hyaline to pale brown or pale yellowish, variable in shape, ellipsoidal to ovoid, or cylindrical, allantoid, 0–1-septate, smooth-walled, guttulate. Chlamydospores present or absent, brown, globose to oval, intercalary or terminal, single or in chains, sometimes dictyochlamydospores (Chen et al. 2015).
Type species: Neodidymelliopsis cannabis (G. Winter) Qian Chen & L. Cai, Stud. Mycol. 82: 207 (2015)
Notes: Neodidymelliopsis species have been reported from Canada, Israel, Italy, Iran, Russia, the Netherlands, and UK, and are associated with many host plants viz. Berberidaceae, Combretaceae, Juglandaceae, Moraceae, Myrtaceae, Polemoniaceae, Ranunculaceae, Rutaceae, Sapindaceae and Urticaceae (Chen et al. 2015, 2017; Hyde et al. 2016, 2017, 2018; Ahmadpour et al. 2017). Ten taxa are listed in Index Fungorum (2019). Phylogenetic analyses based on LSU, ITS, tub2 and rpb2 sequence data shows that N. farokhinejadii (SCUA-4-And-Con, SCUA 6-EZ-Jug, SCUA-8-Ahv-Cit), N. longicolla (CBS 382.96, UTHSC: DI16-322) and N. ranunculi (MFLUCC 13-0490) clustered together with high support (Fig. 244). Morphologically, N. longicolla and N. ranunculi share similar form of conidiomata and conidia (pycnidial conidiomata, hyaline to brown, ellipsoidal to cylindrical, 1-septate conidia). The differences between these two taxa are conidial dimension and ostiole. Neodidymelliopsis longicolla has cylindrical, much longer ostiole and larger conidia than N. ranunculi (12–15(–16.5) × 4–7 μm vs. 7.5–10 × 3–5 μm). It should be noted that in some coelomycetes, the conidiomata that develop in culture differ from those occurring in nature, and the conidia dimensions have also proven not to be very reliable (Cole and Kendrick 1981; Verkley et al. 2014). Therefore, N. farokhinejadii, N. longicolla and N. ranunculi are considered conspecific and the first two names are reduced to synonyms of N. ranunculi. We also introduce an additional strain of N. negundinis from Sophora japonica in Italy and provide an improved photo plate for this species.
Distribution: Canada, Israel, Italy, Netherlands, UK, USA, (Chen et al. 2015, 2017; Hyde et al. 2016; Valenzuela-Lopez et al. 2018).
Neodidymelliopsis negundinis Manawasinghe, Camporesi & K.D. Hyde, in Hyde et al., Mycosphere 9(2): 295 (2018)
Facesoffungi number: FoF 03891, Fig. 245
Saprobic on dead stems of Sophora japonica. Sexual morph: undetermined. Asexual morph:Conidiomata 310–350 µm diam., 130–190 µm high, black, pycnidial, solitary to gregarious or confluent, immersed in origin, becoming erumpent, unilocular or multi-locular, with locules 140–250 µm diam., 110–190 µm, globose to subglobose, thick-walled, glabrous, papillate. Ostiole short, single, circular to cylindrical, centrally located. Conidiomatal wall 15–40 µm wide, composed of thick-walled, brown, gradually merging with hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–9 × 3–6 µm, arising from the inner cavity of conidiomata, hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled. Conidia 4.8–7.5 × 3–4 µm (\( \bar{x} \) = 6 × 3.7 µm; n = 30), hyaline, ellipsoidal to ovoid, obtuse at both ends, straight or slightly curved, aseptate, smooth-walled, guttulate.
Culture characters: colonies on PDA, reaching 40 mm diam. after 4 weeks at 25–30 °C, with brown to olivaceous, flattened, dense, filamentous, aerial, fluffy hyphae on the surface; margins white, circular, entire; reverse olivaceous.
Material examined: Italy, Province of Forlì-Cesena, Forlì, Via Pietro Nenni, on dead aerial branches of Sophora japonica (Fabaceae), 3 April 2016, Erio Camporesi, IT2907 (MFLU 16-1123), living culture MFLUCC 16-1316 = KUMCC 16-0091.
Notes: Neodidymelliopsis negundinis was original described from dead and dying twigs and branches of Acer negundo (Sapindaceae) in Russia (Hyde et al. 2018). Thus our collection is a new host record from Italy.
Neodidymelliopsis ranunculi W.J. Li & K.D. Hyde, in Hyde et al., Fungal Diversity: [41] (2016)
= Neodidymelliopsis longicolla L.W. Hou, Crous & L. Cai, in Chen et al., Stud. Mycol. 87: 153 (2017)
= Neodidymelliopsis farokhinejadii Ahmadp. & Mehr.-Koushk., Sydowia 69: 175 (2017)
Notes: Description and illustration see Hyde et al. (Hyde et al. 2016).
Neofusicoccum Crous, Slippers & A.J.L. Phillips, Stud. Mycol. 55: 247 (2006)
Facesoffungi number: FoF 00153
Dothideomycetes, Incertae sedis, Botryosphaeriales, Botryosphaeriaceae
Saprobic or parasitic on the host plant. Sexual morph:Ascomata forming single or botryose aggregates up to 10, dark brown to black, stomatic, solitary or gregarious or confluent, at first immersed to semi-immersed, then becoming erumpent through the bark, globose with a short, conical papilla, unilocular or multilocular, thick-walled, ostiolate. Peridium composed of thick-walled, dark brown to hyaline cells of textura angularis. Pseudoparaphyses absent or present, when present, hyaline, filiform, septate, rarely branched. Asci 8-spored, bitunicate fissitunicate, clavate to cylindro-clavate, short pedicellate, apically rounded with well developed ocular chamber. Ascospores hyaline, uni-seriate to 2–3-seriate, fusoid to ovoid, smooth, aseptate, occasionally becoming 1–2-septate, thick and rough-walled (adapted from Liu et al. 2012; Phillips et al. 2013). Asexual morph:Conidiomata black, pycnidioid, solitary to gregarious or confluent, stromatic, immersed to erumpent, globose to subglobose or obpyriform, unilocular to multi-locular, glabrous, thick- and smooth-walled, papillate, ostiolate. Ostiole cylindrical or circular, straight or curved, centric or excentric. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis or textura angularis to textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform to subcylindrical, indeterminate, smooth-walled. Conidia usually hyaline, fusiform to ovoid or elongate, aseptate, guttulate, smooth-walled. Synasexual morph Conidia brown, subglobose to obpyriform, with obtuse apex and truncate base, 1–3 transverse septa, 1–2 longitudinal septa, and 1–2 oblique septa (description of synasexual adapted from Phillips et al. 2013).
Type species: Neofusicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips, Stud. Mycol. 55: 248. 2006.
Notes: Neofusicoccum species are emerging as a common and cosmopolitan species on a wide variety of hosts, for instance, N. australe occurs on 41 hosts, N. luteum (31), and N. parvum (55) (Liu et al. 2012; Phillips et al. 2013; Mehl et al. 2017). Some species are aggressive plant pathogens e.g., N. batangarum on seeds of Schinus terebinthifolius (Anacardiaceae) and N. kwambonambiense on Syzygium cordatum (Myrtaceae), N. parvum on grapevines and Eucalyptus (Myrtaceae) (Phillips 1998; Shetty et al. 2011; Pavlic et al. 2009; Li et al. 2018).
Neofusicoccum was introduced by Crous et al. (2006a) to accommodate a group of asexual fungi being morphologically similar to Botryosphaeria, but phylogenetically distinct. In their study, 13 species were accepted in the genus, with N. parvum designated as the type. The sexual morph of N. parvum was described by Liu et al. (2012) from Linum usitatissimum (Linaceae), with globose to subglobose ascomata, 8-spored, butunicate asci and hyaline, fusiform to clavate ascospores. Species of Neofusicoccum share similar characters in hyaline to pale brown conidia, enteroblastic, subcylindrical conidiogenous cells, and forming synasexual morph (pale brown, transverse septate or muriform conidia) in culture (Phillips et al. 2013). However, most of these characters are overlapping, and can be very difficult to distinguish from one to another, and the use of molecular methods with multi-gene sequence data is required to separate species. Phillips et al. (2013) expanded Neofusicoccum to accommodate 22 species based on both morphology and phylogeny studies, and a key to the species was provided. Yang et al. (2017) added four species to the genus i.e., N. buxi Crous, N. pistaciae (Zachos, Tzav.-Klon. & Roubos) Crous, N. pistaciarum Tao Yang & Crous, and N. stellenboschiana Tao Yang & Crous. They recommended that in addition to ITS-tef1 sequence data, data from tub2-rpb2 are essential for species separation and polytomy resolution in Neofusicoccum. Five fresh collections from Italy and Thailand are identified as N. parvum and N. sinoeucalypti on the basis of combined ITS, tef1, tub2 and rpb2 sequence data (Fig. 246). Neofusicoccum italicum and N. pandanicola are reduced to synonymy with N. algeriense and N. parvum. Forty-three species are recognized in Neofusicoccum (Phillips et al. 2013; Marques et al. 2013; Berraf-Tebbal et al. 2014; Dissanayake et al. 2016; Marin-Felix et al. 2017; Yang et al. 2017; Zhang et al. 2017; Jami et al. 2018a, b; Li et al. 2018).
Distribution: This genus has been reported across six continents and more than 29 countries (Sakalidis et al. 2013, Pavlic-Zupanc et al. 2015).
Neofusicoccum algeriense Berr.-Tebb. & A.J.L. Phillips, in Berraf-Tebbal, Guerreiro & Phillips, Fungal Diversity 53: 423 (2014)
= Neofusicoccum italicum Dissan. & K.D. Hyde, in Marin-Felix et al., Stud. Mycol. 86: 170 (2017)
Description and illustration see Berraf-Tebbal et al. (2014)
Notes: Neofusicoccum italicum was distinguished from N. algeriense by its slightly smaller conidia (Marin-Felix et al. 2017). However, with more taxa or isolates added, conidia dimensions have become a poor character in species delineation, and this is also verified with N. parvum and N. sinoeucalypti. The tef1 sequence of N. italicum is 100 % (220/220) similar to N. algeriense. Similarly, there are two gap differences in ITS region (508/510) between N. algeriense and N. italicum. Other sequences of N. italicum are unavailable for comparison. Based on ITS and tef sequence data, N. italicum is reduced to a synonym of N. algeriense.
Neofusicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips, in Crous et al., Stud. Mycol. 55: 248 (2006)
= Neofusicoccum pandanicola Tibpromma & K.D. Hyde, in Tibpromma et al., Fungal Diversity: [60] (2018)
Facesoffungi number: FoF 02411, Figs. 247, 248
Saprobic or parasitic on the host plant in terretrial habitat, such as Platanus hybrid (Platanaceae), Pinus nigra (Pinaceae), and Ulmus × hollandica (Ulmaceae) (Phillips et al. 2008, this study). Sexual morph: undetermined. Asexual morph:Conidiomata 200–800 µm diam., 170–250 µm high, brown to dark brown, stromatic, pycnidial, solitary to gregarious or confluent, at first deeply immersed, then becoming erumpent, unilocular or multi-locular, with locules 90–170 µm diam., 150–200 µm wide?, globose to subglobose, thick-walled, glabrous, smooth, papillate, ostiolate. Ostiole conical or circular, centrically located. Conidiomatal wall 30–90 µm wide, composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 7–20 × 3–10 µm, hyaline, enteroblastic, phialidic, ampulliform to subcylindrical, indeterminate, discrete, smooth, and arising from the inner cavity of the conidiomata. Conidia 12–31 × 5–9.8 µm (\( \bar{x} \) = 18 × 7 µm; n = 30), hyaline, fusiform or ovoid or elongate, with a rounded or occasionally rhomboidal apex and a slightly narrow truncate base, straight or slightly curved, unicellular, guttulate, thick- and smooth-walled.
Culture characters: Colonies on PDA, reaching 30–40 mm diam. after 7 days at 25 °C, initially white tuning olivaceous-grey after 20 d and then becoming black with age, with moderately dense, filamentous, aerial, fluffy hyphae on the surface, margin entire, reverse similar in colour. Colonies on MEA, reaching 40–50 mm diam. after 7 days at 25 °C, forming concentric circles, initially white tuning buff in the middle zone and then becoming black, moderately dense, appressed mycelial mat with irregular very dense, filamentous, cottony aerial aggregations, margin curled, reverse similar in colour.
Material examined: Italy, Province of Forlì-Cesena, Forlì, Viale dell’Appennino, on dead aerial branches of Platanus hybrida (Platanaceae), 9 March 2016, Erio Camporesi, IT2877 (MFLU 16-0999), living culture MFLUCC 16-1447 = ICMP 21539 = KUMCC 16-0087; ibid., on dead aerial stems of Torilis arvensis (Apiaceae), 21 March 2013, Erio Camporesi, IT1137 (MFLU 19-2861), living culture MFLUCC 13-0505, (KUN, HKAS 101681); ibid., San Lorenzo in Noceto, on dead aerial branches of Ulmus × hollandica (Ulmaceae), 16 January 2013, Erio Camporesi, IT1012 (MFLU 19-2858), living culture MFLUCC 13-0488 = ICMP 20797, (KUN, HKAS 101673).
Notes: Neofusicoccum parvum is a well-known pathogen and capable of causing dieback, blossom blight, fruit stem-end rot on grapevines, pine, Eucalyptus and mango (Slippers et al. 2005; Golzar and Burgess 2011; Iturritxa et al. 2011; Úrbez-Torres et al. 2013; Carlucci et al. 2013; Marques et al. 2013; Phillips et al. 2013; Li et al. 2018). Neofusicoccum parvum has been found in 90 hosts across six continents and 29 countries (Sakalidis et al. 2013). This taxon is a species complex, with more than 30 strains recognized. These isolates shown a wide range of variation among conidiomatal structure and conidial dimensions e.g., conidiomata (unilocular or multi-locular), conidia (hyaline or pale brown, aseptate or 1–2-septate, ellipsoidal, fusiform, ovoid or elongate) (Pavlic et al. 2009; Abdollahzadeh et al. 2013; Phillips et al. 2013).
Three fresh collections (MFLUCC 13-0488/IT1012, MFLUCC 13-0505/IT1137 and MFLUCC 16-1447/IT2877) from Italy show a close phylogenetic affinity with N. parvum (CBS 110301, CMW9081) and N. pandanicola (KUMCC 17 0184) (Fig. 246). Strains MFLUCC 13-0488/IT1012 and MFLUCC 16-1447/IT2877 have larger, mutilocular conidiomata, while MFLUCC 13-0505/IT1137 has smaller, unilocular conidiomata (Fig. 247). The conidial dimensions of these isolates are also different, e.g., MFLUCC 13-0488/IT1012 (15–26.7 × 6–9.8 µm (\( \bar{x} \) = 20 × 7.5 µm; n = 30), MFLUCC 16-1447 /IT2877 (15–23 × 4–8 µm (\( \bar{x} \) = 19 × 7 µm; n = 30), MFLUCC 13-0505/IT1137 (12.3–30.7 × 5–8.2 µm (\( \bar{x} \) = 18 × 6.7 µm; n = 30). Because the morphology of these three collections is variable, thus conidiomatal structure and conidial size cannot be applied as criteria for the species delineation of Neofusicoccum. We therefore regard these three collections as conspecific with N. parvum on the basis of phylogeny. Neofusicoccum pandanicola described from fallen dead and decaying leaves of Pandanus sp. (Tibpromma et al. 2018), clustered with ex-type strain of N. parvum (CMW9081). In addition, the ITS sequence of N. pandanicola (GenBank: MH275072) shows 100% (494/494) similar to other isolates of N. parvum (GenBank: MN537040, MN475209, MN475208). The tef1 sequence of N. pandanicola (MH412778) cannot be aligned with other species of Neofusicoccum, and it is not included in phylogenetic analyses. Other sequences of N. pandanicola are unavailable for comparison. Based on ITS sequence data, N. pandanicola is reduced to a synonym of N. parvum.
Neofusicoccum sinoeucalypti G.Q. Li & S.F. Chen, in Li et al., Persoonia 40: 88 (2017)
Facesoffungi number: FoF 07477, Figs. 249, 250
Pathogenic on living leaves of Dieffenbachia sp. or saprobic on dead, leathery stem of herbaceous plant. Sexual morph: undetermined. Asexual morph:Conidiomata 90–170 µm diam., 85–180 µm high, dark brown to black, stromatic, pycnidial, gregarious, subepidermal, deeply immersed to semi-immersed, with a blackened tip above (Fig. 250), obpyriform, unilocular or multilocular, glabrous, ostiolate. Ostiole 10–15 × 7–9 µm, cylindrical or oval, with a long channel, straight or curved, centrally located. Conidiomatal wall 15–40 µm wide, outer layers composed of thick-walled, brown cells of textura angularis to textura prismatica (Fig. 249) or textura angularis (Fig. 250) in the basal and lateral part, inner wall with thin-walled, hyaline cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 6–17 × 1–5 µm, hyaline, phialidic, unbranched, vase-shaped to cylindrical, with periclinal wall thickening at channel and collarette zone, smooth-walled. Conidia 11.3–21 × 4–6.6 µm (\( \bar{x} \) = 16 × 5.3 µm; n = 30), hyaline, elongate to fusiform, with rounded apex and blunt base, or narrow and obtuse apex and base, smooth-walled. Dichomera-like synasexual morph not observed.
Culture characters: Colonies on PDA, reaching 40–50 mm diam. after 7 d at 25 °C, white to grey in the first 14 d, and then becoming black with age, with sparse to moderately dense, appressed mycelial mat in center with sparse tufts of aerial mycelium around the edges, margin rounded, reverse similar in colour.
Material examined: Thailand, Chiang Rai, on living leaves of Dieffenbachia sp. (Dieffenbachieae), 1 July 2012, Wu Shi-Ping, J06 (MFLU 19-2886), living culture MFLUCC 13-0356 = KUMCC 15-0577; ibid., on dead leathery stemes of herbaceous plant, 5 July 2012, Narumon Tangthirasunun, J11 (MFLU 19-2887), living culture MFLUCC 17-2505 = KUMCC 15-0597
Notes: Isolate MFLUCC 13-0356 is parasitic on living leaves of Dieffenbachia sp. causing conspicuous, rounded to oval, brown, leaf spots of 1–3 cm diam., containing numerous minute conidiomata in concentric zones (Fig. 249). Isolate MFLUCC 17-2505 is saprobic on dead, leathery stems of herbaceous plant, forming conspicuous, black conidiomata on the upper surface of host (Fig. 250). Morphologically, isolate MFLUCC 13-0356 differs from MFLUCC 17-2505 in conidiomata structure and form of conidiogenous cells; MFLUCC 13-0356 has unilocular conidiomata with textura angularis to textura prismatica cells and holoblastic conidiogenous cells, while MFLUCC 17-2505 has multilocular conidiomata with textura angularis cells and phialidic conidiogenous cells. However, these two isolates share similar conidia dimensions of 11.3–21 × 4.3–6.6 µm (\( \bar{x} \) = 15.8 × 5.3 µm; n = 30) in isolate MFLUCC 13-0356 and 12.3–19.3 × 4–6.6 µm (\( \bar{x} \) = 16 × 5.3 µm; n = 30) in MFLUCC 17-2505. Phylogenetic analyses of ITS, tef1, tub2 and rpb2 sequence data show that these two isolates are closely related and clustered with N. sinoeucalypti (Fig. 246). Based on phylogeny, isolates MFLUCC 13-0356 and MFLUCC 17-2505 are recognized as N. sinoeucalypti. The differences noted on morphology and hosts might reflect intraspecific variation, and suggest that N. sinoeucalypti is a plurivorous species. Our collections are new records in Thailand.
Neogloeosporidina W.J. Li, Camporesi & K.D. Hyde, gen. nov.
Index Fungorum number: IF557154, Facesoffungi number: FoF 07519
Etymology: Named after its morphology similar with Gloeosporidina.
Leotiomycetes, Leotiomycetidae, Medeolariales, Dermateaceae
Saprobic on plant host in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown, eustromatic, pycnidial, scattered, rarely gregarious, subepidermal or subperidermal in origin, immersed to partly erumpent, globose to subglobose, unilocular, thick-walled, smooth, glabrous. Ostiole absent, dehiscing by an irregular rupture in the apical region, then becoming wide open. Conidiomatal wall composed of brown, thick-walled cells of compressed textura angularis. Conidiophores mostly reduced to conidiogenous cells, occasionally present, short, cylindrical, septate and branched at base, formed from inner layers of conidiomata. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, usually integrated, determinate, smooth-walled. Conidia hyaline, ellipsoid to ventricose, often with a blunt apiculus, unicellular, thick- and smooth -walled.
Type species: Neogloeosporidina pruni W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Notes: Neogloeosporidina pruni was collected from Italy. The sequence was obtained directly from conidiomata. Multi-locus phylogeny reveals N. pruni clusters in a well-supported branch basal to Dermea and Corniculariella (Fig. 129). Neogloeosporidina pruni shares similar characters with Gloeosporidina in having elliptical conidia, and enteroblastic, phialidic, integrated conidiogenous cells, but it can be distinguished by its eustromatic, pycnidial conidiomata. Combining both morphology and phylogeny, Neogloeosporidina is introduced as a new genus.
Distribution: Italy.
Neogloeosporidina pruni W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557153, Facesoffungi number: FoF 07476, Fig. 251
Etymology: Named after the host from which it was collected, Prunus.
Saprobic on dead stems of Prunus avium (Rosaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 100–400 µm diam., 90–230 µm high, brown, eustromatic, pycnidial, scattered, rarely gregarious, subepidermal or subperidermal in origin, immersed to partly erumpent, globose to subglobose, unilocular, thick-walled, smooth, glabrous. Ostiole absent, dehiscing by an irregular rupture in the apical wall, then becoming wide open. Conidiomata wall 20–30 µm wide, composed of brown, thick-walled cells of compressed textura angularis, Conidiophores mostly reduced to conidiogenous cells, occasionally present, short, cylindrical, septate and branched at base, formed from inner layers of conidiomata. Conidiogenous cells 10–35 × 2.1–3.9 µm, hyaline, enteroblastic, cylindrical, discrete or integrated, indeterminate, smooth-walled. Conidia 11–18 × 4.5–9 µm (\( \bar{x} \) = 14 × 6.9 µm; n = 30), hyaline, ellipsoid to ventricose, often with a blunt apiculus, unicellular, thick-walled, smooth.
Material examined: Italy, Province of Forlì-Cesena, Santa Sofia, Campigna, on dead land branches of Prunus avium (Rosaceae), 26 July 2014, Erio Camporesi, IT2016 (MFLU 16-2153, holotype), (KUN, HKAS 101641, isotype).
Neoplaconema B. Sutton, Kew Bull. 31(3): 463 (1977)
Faceoffungi number: FoF 07475, Fig. 252
Ascomycota, genera incertae sedis
Habit on branches of Aconitum napellum, A. toxicum (Ranunculaceae), and on cankered branch of Eucalyptus nitens (Myrtaceae) (Nag Raj 1993; Yuan and Mohammed 1997). Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, solitary to gregarious, epidermal to subepidermal, immersed to erumpent, subglobose, unilocular, sometimes convoluted, glabrous, ostiolate. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown to pale brown cells of textura angularis. Conidiophores arising from the inner wall layer of conidiomata, hyaline, cylindrical to dolliform, branched at the base, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, enteroblastic, phialidic, lageniform to cylindrical, integrated or discrete, determinate, smooth-walled, with minute, periclinal wall thickened channel and collarette. Conidia hyaline, fusiform to subcylindrical or ellipsoidal, rounded at the apex, slightly truncate at the base, straight or slightly curved, unicellular, smooth-walled, guttulate, bearing a flexuous, curved, unbranched or occasionally forked, cellular appendage separated at the base by a septum (adapted from Nag Raj 1993).
Type species: Neoplaconema napelli (Maire & Sacc.) B. Sutton, Kew Bull. 31(3): 463 (1977)
Notes: Neoplaconema was proposed to accommodate a species segregated from Placonema (Sacc. & D. Sacc.) Petr., as N. napelli. This genus has unicellular conidia with an apical appendage similar to Fibulocoela and Rhabdogloeum. However, Rhabdogloeum has acervular conidiomata distinguished from the pycnidial conidiomata in Fibulocoela and Neoplaconema. Fibulocoela has conidiophores with a clamp connection at each septum, a feature not found in Neoplaconema (Nag Raj 1993, this study). Two species are accepted in Neoplaconema, N. cymbiforme Z.Q. Yuan & C and N. napelli, but neither have molecular data. Fresh collections are needed to place Neoplaconema in a natural group.
Distribution: Australia, Germany, Romania (Sutton 1977b; Yuan and Mohammed 1997).
Neopyrenochaeta Valenz.-Lopez, Crous, Stchigel, Guarro & Cano, in Valenzuela-Lopez et al., Stud. Mycol. 90: 54 (2017)
Facesoffungi number: FoF 07389
Dothideomycetes, Pleosporomycetidae, Pleosporales, Neopyrenochaetaceae
Saprobic on the host plant or on submerged stems in freshwater habit, on screen of a mobile phone or from air, or soil (Boerema et al. 2004; Marincowitz et al. 2008; Crous et al. 2015c) Sexual morph: undetermined. Asexual morphConidiomata brown, pycnidial, solitary, semi-immersed to superficial, globose to subglobose, unilocular, setose, ostiolate. Ostiole circular, papillate or non-papillate, centrally or laterally located. Setae conspicuous or inconspicuous, pale brown to black at the base, paler towards apex, unbranched, septate. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis to textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells formed in the inner cavity of conidiomata, hyaline, enteroblastic, annellidic or phialidic, doliiform to ampulliform or lageniform, determinate or indeterminate, smooth-walled. Conidia hyaline, ovoid to ellipsoidal or subcylindrical, guttulate.
Type species: Neopyrenochaeta acicola (Moug. & Lév.) Valenz.-Lopez, Crous, Stchigel, Guarro & Cano, in Valenzuela-Lopez et al., Stud. Mycol. 90: 54 (2017)
Notes: Valenzuela-Lopez et al. (2018) proposed Neopyrenochaeta to accommodate four species namely, N. acicola (Moug. & Lév.) Valenz.-Lopez et al., N. fragariae Valenz.-Lopez et al., N. inflorescentiae (Crous, Marinc. & M.J. Wingf.) Valenz.-Lopez et al. and N. telephoni (Roh. Sharma, Kurli, Sonawane, Shouche & Rahi) Valenz.-Lopez et al. The genus has similar morphology of conidiomata and conidia with Pyrenochaeta De Not., but differs in phylogeny. Three fresh collections from freshwater in Thailand showed close phylogenetic affinity with Neopyrenochaeta species. These three collections are introduced as new species viz. N. annellidica, N. chiangraiensis and N. maesuayensis (Fig. 253).
Neopyrenochaeta annellidica W.J. Li, Z.H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum number: IF557155, Facesoffungi number: FoF 07481 Fig. 254
Etymology: Referring to its annellidic conidiogenous cells.
Saprobic on submerged decaying wood in freshwater habitat. Sexual morph: undetermined. Asexual morph:Conidiomata 180–250 µm diam., 180–240 µm high, dark brown when moist, with white conidial mass surrounding ostiole, becoming black when dry, pycnidial, mostly solitary, occasionally gregarious, semi-immersed to superficial, globose to subglobose, unilocular, setose, ostiolate. Ostiole circular, papillate, centrally or laterally located. Setae up to 60 µm long, inconspicuous, pale brown to black, unbranched, septate, tapered toward apex, originating from the surface of the conidiomatal wall. Conidiomatal wall 10–40 µm wide, composed of thick-walled, brown to dark brown cells of textura angularis in the outer layers, becoming thinner, hyaline cells of textura prismatica towards conidial hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 7–20 × 5–9 µm, formed in the inner cavity of conidiomata, hyaline, enteroblastic, annellidic, doliiform to ampulliform, indeterminate, smooth-walled, with percurrent proliferation up to 2 times. Conidia 9–15 × 6–8 µm (\( \bar{x} \) = 11 × 6.5 µm; n = 50), hyaline, oval or oblong, rounded at the apex, sometimes narrowed at the base, unicellular, thick- and smooth-walled, multi-guttulate.
Culture characters: colonies on PDA, white to pale grey in the middle region, white in the edges, velutinous to felty, flattened, dense, with undulate margin, reverse dark brown in the middle region, white in the edges.
Material examined: Thailand, Chiang Mai, on submerged decaying wood in freshwater water, 29 November 2010, Huang Zhang, D53 (MFLU 11-1105, holotype), ex-type living culture MFLUCC 11-0087.
Notes: Phylogenetic analyses based on LSU, ITS, rpb2, tub2 sequence data shows that Neopyrenochaeta annellidica and N. maesuayensis clustered with N. telephoni, but formed two separate branches (Fig. 253). Neopyrenochaeta annellidica and N. maesuayensis differ from N. telephoni (= Pyrenochaeta telephoni Roh. Sharma et al.) in conidiomatal setae. The former have one setae type distributed on all sides, while the latter has two types: one type stiff, long, mainly concentrated around the ostiole, the other short, soft, spread all over the surface (Crous et al. 2015c). Neopyrenochaeta annellidica has annellidic conidiogenous cells which are distinct from the phialidic type in N. maesuayensis (without acropleurogenous conidiogenous cells) and N. telephoni (having terminal and apical apertures). Therefore, N. annellidica and N. maesuayensis are introduced as new species.
Neopyrenochaeta chiangraiensis Z.L. Luo, W.J. Li & K.D. Hyde, sp. nov.
Index Fungorum number: IF557156, Faceoffungi number: FoF 07482, Fig. 255
Etymology: Named after the city from where this fungus was collected, Chiangrai.
Saprobic on submerged decaying wood in freshwater habitat. Sexual morph: undetermined. Asexual morph:Conidiomata 120–130 µm diam., 80–130 µm high, dark brown to black, pycnidial, solitary, superficial, globose to subglobose, unilocular, setose, ostiolate. Ostiole circular, papillate, centrally located. Setae 65–120 × 2–4 µm, conspicuous, dark brown at the base, becoming paler and tapered toward apex, subulate, straight or slightly curved, unbranched, septate, originating from the surface of the conidiomatal wall. Conidiomatal wall 15–30 µm wide, composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–7 × 2–3 µm, formed in the inner cavity of conidiomata, hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, smooth-walled, with periclinal thickenings towards apex at the collarette zone. Conidia 4–6 × 2–3 µm (\( \bar{x} \) = 4.5 × 2.4 µm; n = 50), hyaline, oval or oblong, rounded at the both ends, unicellular, thick- and smooth-walled, with one large or many smaller guttules.
Material examined: Thailand, Chiang Rai, on submerged decaying wood in freshwater pool, 1 October 2013, Asanka Ranjana Bandara, ZL-17 (MFLU 15-0086, holotype), ex-type living culture MFLUCC 13-0881, (KUN, HKAS 86461, isotypes).
Notes: Neopyrenochaeta chiangraiensis shows a closely affinity with the generic type N. acicola, but formed a separate branch. Neopyrenochaeta chiangraiensis shares similar characters with N. acicula in having globose to subglobose conidiomata with setae and subglobose to ellipsoidal conidia with several small or large guttules (conidia: 3.5–5(–6) × 1.5–3 µm), but is distinct in dimensions of setae. Neopyrenochaeta chiangraiensis has shorter setae than N. acicula (up to 200 µm).
Neopyrenochaeta maesuayensis Z.L. Luo, W.J. Li & K.D. Hyde, sp. nov.
Index Fungorum number: IF557157, Facesoffungi number: FoF 07483 Fig. 256
Etymology: Referring to the county from where it was collected, Mae Suay.
Saprobic on submerged decaying wood in freshwater habitat. Sexual morph: undetermined. Asexual morph:Conidiomata 50–120 µm diam., 50–125 µm high, dark brown to black, pycnidial, solitary, superficial, with cylindrical neck in surface view, globose, unilocular, setose, thick-walled, ostiolate. Ostiole circular to cylindrical, centrally located. Conidiomatal wall 10–30 µm wide, composed of brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 2–3 × 1–1.5 µm, formed in the inner cavity of conidiomata, hyaline, enteroblastic, phialidic, ampulliform to cylindrical, determinate, smooth-walled. Conidia 3–4 × 2–3 µm (\( \bar{x} \) = 3.7 × 2.5 µm; n = 50), hyaline, oblong to short cylindrical, blunt and rounded at both ends or slightly narrow at the base, straight, unicellular, thick- and smooth-walled.
Material examined: Thailand, Mae Suay, on submerged decaying wood in freshwater stream, 22 November 2013, K.D. Hyde, ZL-20 (MFLU 15-0078, holotype), ex-type living culture MFLUCC 14-0043, (KUN, HKAS 86453, isotypes).
Neottiospora Desm., Annls Sci. Nat., Bot., sér. 2 19: 346 (1843)
Facesoffungi number: FoF 07520
Ascomycota, genera incertae sedis
Saprobic on plants, such as Carex acutiformis, C. appropinquata, C. elata, C. pendula, C. riparia (Cyperaceae), Iris pseudacorus (Iridaceae), Panicum maximum (Poaceae) and Typha latifolia (Typhaceae) (Sutton 1980; Nag Raj 1993, this study). Sexual morph: undetermined. Asexual morph:Conidiomata olivaceous green to black, pycnidial, scattered to gregarious or confluent, deeply immersed, ellipsoidal to globose, unilocular, glabrous, thick-walled, ostiole absent, but with a circular operculum, dehiscence by forming two lines between the operculum and upper conidiomatal wall. Conidiomatal wall composed of thick-walled, olivaceous green to hyaline cells of textura angularis to textura prismatica. Conidiophores arising all around cavity of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, clavate to subcylindrical, discrete or integrated, indeterminate, with periclinal thickenings at collarette zone, smooth-walled. Conidia hyaline, fusiform to ellipsoidal, straight or slightly curved, aseptate, smooth-walled, guttulate, bearing an appendage at base, initially funnel-shaped but ultimately splitting into tentacular strands.
Type species: Neottiospora caricina (Desm.) Höhn., in Weese, Mitt. bot. Inst. tech. Hochsch. Wien 1(3): 78 (1924)
Notes: The generic name Neottiospora was introduced by Desmazièrcs (1843) with N. caricum Desm. as the type species, collected from Carex sp. (Cyperaceae) in France. Berkeley and Broome (1850) synonymized Sphaeria caricina Desm. with N. caricum. von Höhnel (1924) re-examined both type materials and concluded that the species are conspecific and named it as Neottiospora caricina (Desm.) Höhn. Höhnel’s finding was confirmed by Cunnell (1957) and this name was adopted in later studies (Cunnell 1957; Pirozynski and Shoemaker 1971; Nag Raj 1973b; 1993; Sutton 1980).
The genus is characterized by deeply immersed, ellipsoidal to globose conidiomata lacking ostioles, but with a circular operculum, and dehiscence by forming two lines between the operculum and upper conidiomatal wall. The conidia are fusiform to ellipsoidal, unicellular, with a basal appendage which is initially funnel-shaped, but ultimately splits into tentacular strands. The origin and position of the conidia appendages has been the subject of controversy (von Höhnel 1924; Grove 1935; Arnaud 1952; Subramanian and Ramakrishnan 1953, 1957). Cunnell (1957) gave a detailed account of structure and ontogeny of this genus and showed that the appendages are located at the base. This was confirmed by Shoemaker (1965) and verified by Pirozynski and Shoemaker (1971) as well as in the current study. Neottiospora can be confused with Tiarosporella because both genera have fusiform to ellipsoidal, unicellular conidia bearing appendages. However, Tiarosporella has its appendages located at the conidial apex and the conidiomata lack a circular operculum.
Over 15 taxa have been described in Neottiospora, but some have been transferred to other genera. von Höhnel (1919, 1924) referred N. paludosa, N. schizochlamys and N. arenaria Syd. & P. Syd. to Tiarosporella. Subramanian and Ramakrishnan (1954) made N. yuccaefolia Hall the type species of a new genus Alyalcesn Subram. & K. Ramakr., and transferred N. coprophila Speg. to Robillarda Sacc. Pirozynski and Shoemaker (1971) showed that N. geranii (J. Schröt.) H.C. Greene and N. umbellifernrurn H.C. Greene are probably nearer to Pestalozziella Sacc. & Ellis ex Sacc. Nag Raj (1993) accepted only N. caricina in the genus. Neottiospora has not been studied by molecular data, and its placement in a natural classification is undetermined. We provide a detailed description and illustration for this genus.
Distribution: Austria, Canada, Germany, France, Uganda, UK (Sutton 1980; Nag Raj 1993, this study).
Neottiospora caricina (Desm.) Höhn., in Weese, Mitt. bot. Inst. tech. Hochsch. Wien 1(3): 78 (1924)
Facesoffungi number: FoF 07521, Figs. 257, 258
Foliicolous. Sexual morph: undetermined. Asexual morph:Conidiomata 110–310 diam., 120–270 µm high, olivaceous green to black, pycnidial, scattered to gregarious or confluent, subepidermal in origin, deeply immersed, ellipsoidal to globose, unilocular, glabrous, thick-walled, ostiole absent, but with a circular operculum, dehiscence by forming two lines between the operculum and upper conidiomatal wall (Fig. 257g, h). Conidiomatal wall 10–25 µm wide, composed of thick-walled, olivaceous green to hyaline cells of textura angularis to textura prismatica (Figs. 257j and 258h) at lateral and basal part, becoming relatively thick-walled, pale olivaceous green to hyaline cells of compact textura angularis at upper part. Conidiophores arising all around cavity of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells 10–20 × 2–3 µm, hyaline, enteroblastic, phialidic, clavate to subcylindrical, discrete or integrated, indeterminate, with periclinal thickenings at collarette zone, smooth-walled. Conidia 9–15 × 3–5 µm (\( \bar{x} \) = 13 × 4 µm; n = 30), hyaline, fusiform to ellipsoidal, with an acute apex and a slightly truncate base, straight or slightly curved, aseptate, smooth-walled, guttulate, bearing an appendage at base, initially funnel-shaped but ultimately splitting into tentacular strands.
Material examined: on leaves of Carex pendula Huds. and C. riparia (R. Br.) Poir. (Cyperaceae), J.B. Mougeot, C. Nestler & W.P. Schimper - Stirpes Cryptogamae Vogeso-Rhenanae, Fasc. August 1850, (DAOM 59000); Austria, Wienerwald, Georgenberg nr. Purkersdorf, on leaves of Carex pendula, C. Keissler, (DAOM 81836); Canada, Ontario, Lanark County, Christie Lake, on leaves of Carex sp., 25 September 1965, R.A. Shoemaker, (DAOM 110354); cultivated on sterilized grass in agar at room temperature in light (21 October 1965–7 December 1965) (DAOM 110354).
Neozythia Petr., Sydowia 11(1–6): 351 (1958) [1957]
Facesoffungi number: FoF 07522, Fig. 259
Ascomycota, genera incertae sedis
Saprobic on dead stems of Astragalus sp. (Fabaceae). Sexual morph: undetermined. Asexual morph:Conidiomata very pale brown, pycnidial, solitary to gregarious, subepidermal, immersed, globose to subglobose, unilocular, glabrous. Ostiole absent, dehiscence by break down of the apical wall. Conidiomatal wall composed of thin-walled, hyaline cells of textura angularis. Conidiophores arising from the inner wall layer of conidiomata, hyaline, irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, meristematic, thallic, integrated, fragmenting, determinate, smooth-walled. Conidia hyaline, cylindrical, ellipsoid or irregular in shape, 0–1-septae, continuous or constricted at the septa, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Neozythia nectrioidea (Petr.) Petr., Sydowia 11(1–6): 351 (1958) [1957]
Notes: Sutton (1980) re-described Neozythia and accepted one species, N. handelii (Bubák) Petr. (1959). He reduced N. nectrioidea to a synonym of N. handelii. Wijayawardene et al. (2017b) selected the earlier name N. nectrioidea (Petrak 1957a) as the generic type. Neozythia is similar to the asexual morph of Pycnopeziza (= Acarosporium Bubák & Vleugel ex Bubák), Barnettella D. Rao & P.Rag. Rao, Phacidiella P. Karst., Phragmotrichum Kunze, Sirozythiella Höhn., Staninwardia B. Sutton and Trullula Ces. in having thallic conidiogenous cells (Sutton 1980; Nag Raj 1993, Wijayawardene et al. 2016). However, these genera are separated by conidia morphology. Neozythia has hyaline, cylindrical, ellipsoid or irregular in shape, 0–1-septae conidia. The asexual morph of Pycnopeziza has hyaline, cylindrical or allantoid, 1–3-septate conidia bearing filiform, flexuous appendages. Barnettella has dark brown, muriform conidia connected by several disjunctor cells. Phacidiella has hyaline, long doliiform or cylindrical, straight, unicellular conidia. Phragmotrichum has brown, concolourous or versicolourous, cymbiform, fusiform or ellipsoidal or muriform conidia. Sirozythiella has hyaline, long doliiform, straight or slightly curved, 0-2-euseptate conidia. Staninwardia has pale brown, doliiform to clavate, 1-septate, verruculose conidia with a mucilaginous sheath. Trullula has pale brown, regularly oblong, aseptate, smooth-walled conidia (Sutton 1980). No molecular data is available for Neozythia. Fresh collections are needed to place it in a natural group.
Distribution: Kurdistan (Sutton 1980).
Oncosporella P. Karst., Meddn Soc. Fauna Flora fenn. 14: 105 (1887)
Faceoffungi number: FoF 07479, Fig. 260
Ascomycota, genera incertae sedis
Saprobic on dead wood of Populus sp. (Salicaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, solitary, superficial, cupulate. Ostiole absent. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis in the basal region, becoming thick-walled, dark brown cells of textura porrecta in the lateral region. Conidiophores arising from the inner wall layer of basal stroma, pale brown to hyaline, cylindrical, branched and septate at the base, smooth-walled. Conidiogenous cells hyaline, holoblastic, sympodial, cylindrical to irregular, integrated, indeterminate, smooth-walled, with 1–3 flat, unthickened, slightly protuberant scars towards the apices. Conidia hyaline, cylindrical to subcylindrical or lunate, rounded at the apex, truncate at the base, straight or slightly curved, up to 7-septate, smooth-walled, guttulate (Sutton 1980).
Type species: Oncosporella punctiformis P. Karst., Meddn Soc. Fauna Flora fenn. 14: 105 (1887)
Notes: Oncosporella is a monotypic genus. This genus has hyaline, cylindrical to subcylindrical, multi-septate conidia, which is similar to Parastagonospora (e.g., P. caricis) and asexual morph of Ascocalyx (= Bothrodiscus). However, Parastagonospora has enteroblastic, phialidic, determinate conidiogenous cells distinguished from the holoblastic, sympodial, integrated, indeterminate conidiogenous cells in asexual morph of Ascocalyx and Oncosporella. The asexual morph of Ascocalyx can be distinguished from Oncosporella by its stromatic, pycnidial conidiomata with broadly stipitate and numerous locules at one level concealed by incurved margin. Oncosporella has not been linked to its sexual morph, or studied by molecular methods. Fresh collections are needed to place it in a natural group.
Distribution: Finland (Sutton 1980)
Pestalozziella Sacc. & Ellis ex Sacc., Michelia 2(no. 8): 575 (1882)
Facesoffungi number: FoF 07486
Ascomycota, genera incertae sedis
Parasitic on leaves of leaves of Geranium carolinianum (Geraniaceae). Sexual morph: undetermined. Asexual morph:Conidiomata yellowish brown to brown, stromatic, pycnidial, epiphyllous, scattered to gregarious, deeply immersed, globose to subglobose, unilocular or multilocular, glabrous, papillate, ostiolate. Conidiomatal wall composed of textura angularis with thick-walled, hyaline to pale brown cells. Conidiophores arising from the innermost layers of basal wall, hyaline, unbranched, doliiform to short cylindrical or irregular. Conidiogenous cells hyaline, holoblastic, sympodial, lageniform to irregular, determinate, smooth-walled. Conidia hyaline, ovoid to cylindrical, aseptate, straight or slightly curved, smooth-walled, bearing an apical appendage that is excentric, branching irregularly or dichotomously, 2–6, filiform, flexuous, and divergent.
Type species: Pestalozziella subsessilis Sacc. & Ellis, Michelia 2(no. 8): 575 (1882)
Notes: Pestalozziella subsessilis Sacc. & Ellis. was associated with leaf spots on Geranium carolinianum. In addition to type species, nine taxa have been described in or transferred to this genus. Nag Raj and Kendrick (1972) revised the genus and accepted four species, P. andersonii Ellis & Galloway, P. artocarpi Nag Raj & W.B. Kendr., Ellis & Galloway, P. parva Nag Raj, and P. subsessilis Sacc. & Ellis. Pestalozziella ambigua Höhn. and P. gossypina G.F. Atk. were transferred to Chaetospermum Sacc. while P. circularis Cooke & Massee was made the type species of a new genus Coma Nag Raj & W.B. Kendr. Pestalozziella geranii-pusilli was reduced to synonymy with P. subsessilis. In spite of the revisions by Nag Raj and Kendrick (1972), the description of Pestalozziella is still ambiguous. Species in Pestalozziella have pycnidial or acervular conidiomata, and sympodial, enteroblastic (phialidic) or holoblastic conidiogenous cells. In addition, conidial appendages are dichotomous or branching from a single point, apical or subapical.
We re-examined the type material of P. artocarpi and of the generic type species, P. subsessilis, and found that P. artocarpi does not fit well within the genus. Pestalozziella subsessilis has yellowish brown to brown, stromatic, pycnidial, unilocular or multilucular conidiomata, and ovoid to cylindrical, unicellular conidia with eccentric, dichotomous, apical appendages. Pestalozziella artocarpi has pearl white to brown, pycnidial, unilocular conidiomata, and narrow cylindrical conidia bearing 2–4, attenuated, divergent, apical appendages. Therefore P. artocarpi should be excluded from Pestalozziella. Similarly, P. andersonii and P. parva are excluded from Pestalozziella on the basis of their distinct morphology. P. andersonii has acervular conidiomata and holoblastic conidiogenous cells without apical scars (conidiogenous loci), while Pestalozziella parva has clavate, cylindrical, subcylindrical, ovoid or fusiform conidia bearing 2–4, divergent, attenuated, apical appendages. However, the description of these three species (P. andersonii, P. artocarpi and P. parva) and lack of molecular sequence data does not enable us to place them in any appropriate genera. Therefore, it is essential to re-collect the type species, as well as other species, and to subject them to DNA analysis to confirm their placement.
Distribution: USA.
Pestalozziella subsessilis Sacc. & Ellis, Michelia 2(no. 8): 575 (1882)
Index Fungorum number: IF 186684 Facesoffungi number: FoF 07487, Fig. 261
Foliicolous on leaves of Geranium carolinianum (Geraniaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 120–290 µm diam., 70–120 µm high, yellowish brown to brown, stromatic, pycnidial, epiphyllous, scattered to gregarious, deeply immersed, globose to subglobose, unilocular to multilocular, glabrous, papillate, ostiolate. Conidiomatal wall 7–26 µm wide, composed of textura angularis with cells in the outer layers moderately thick-walled and pale brown, and in the inner layers relatively thin-walled, hyaline. Conidiophores hyaline, unbranched, doliiform to short cylindrical or irregular, and arising from the innermost layers of basal wall. Conidiogenous cells 5–13 × 2–4 µm, hyaline, holoblastic, sympodial, ampulliform to lageniform or irregular, indeterminate, smooth-walled, with 1 to many, unthickened conidiogenous loci. Conidia 16–23 × 5–8 µm (\( \bar{x} \) = 19 × 7 µm; n = 30), hyaline, ovoid to cylindrical with a rounded apex and a narrowly truncate or obtuse base, aseptate, straight or slightly curved, smooth-walled, bearing an apical appendage, apical appendages 17–23 × 0.6–1.4 µm (\( \bar{x} \) = 19 × 1 µm), hyaline, excentric, branching irregularly or dichotomously, 2–6, filiform, flexuous, divergent.
Material examined: USA, New Jersey, Newfield, on leaves of Geranium carolinianum (Geraniaceae), 23 May 1882 (FH 01142400, type).
Excluded species
Pestalozziella andersonii Ellis & Galloway, J. Mycol. 5(2): 65 (1889)
Description and illustration see Nag Raj (1993)
Pestalozziella artocarpi Nag Raj & W.B. Kendr., Can. J. Bot. 50(3): 609 (1972)
Facesoffungi number: FoF 07488, Fig. 262
Saprobic on dead, rotting leaves of Artocarpus integrifolia (Moraceae). Sexual morph: undetermined. Asexual morph:Conidiomata 200–400 diam., 150–250 µm high, off-white, pearl white when moist but brown when dry, amphigenous, stromatic, pycnidial, solitary to gregarious or confluent, subepidermal, initially immersed, ultimately erumpent, subglobose to irregular, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, cylindrical to circular, centrally located. Conidiomatal wall 9–20 µm wide, composed of thick-walled, pale brown cells of textura intricata on the exterior, gradually merging with hyaline cells of textura prismatica in outer layers. Conidiophores formed from the inmost wall layers of conidiomata, hyaline, cylindrical to subcylindrical, irregularly branched, broader at base, septate, smooth-walled, invested in mucus. Conidiogenous cells 28–40 × 1.5–3 µm, hyaline, enteroblastic, cylindrical, discrete or integrated, indeterminte. Conidia 19–27 × 3–5 µm (\( \bar{x} \) = 21 × 4 µm; n = 30), hyaline, narrow cylindrical, with a rounded apex and a narrow truncate base, straight or slightly curved, unicellular, smooth-walled, bearing apical appendages; appendages 21–37 × 1–3 µm including the length prior to branching, branches 2–4, attenuated, divergent, tubular, flexuous.
Material examined: India, Bangalore, Hebbal, on dead, rotting leaves of Artocarpus integrifolia (Moraceae), 24 July 1967, T.R. Nag Raj (DAOM 134442, holotype).
Notes: The conidiogenous cells of Pestalozziella artocarpi were illustrated as holoblastic, sympodial, cylindrical to subcylindrical, with many, unthickened conidiogenous loci (Sutton 1980). However, the holoblastic, sympodial conidiogenous cells were not described or shown in the illustration of P. artocarpi (Nag Raj 1993), or in this study (Fig. 262j).
Pestalozziella parva Nag Raj, Trans. Br. Mycol. Soc. 52(2): 205 (1969)
Description and illustration see Nag Raj (1993)
Pezicula Tul. & C. Tul., Select. fung. carpol. (Paris) 3: 182 (1865)
Facesoffungi number: FoF 07484
Leotiomycetes, Leotiomycetidae, Medeolariales, Dermateaceae
Endophytic, saprobic or pathogenic on plant host in terrestrial habitat. Sexual morph: See Chen et al. (2016). Asexual morph:Conidiomata dark brown to black, acervular to stromatic, solitary, gregarious or confluent, immersed or erumpent, cylindrical to subglobose or conical, unilocular or multilocular, dehiscence irregular, but some species forming a massive erumpent stroma and dehisence by a large ostiole (P. pruinosa and P. italica), thick-walled. Conidiomata wall composed of thick-walled, hyaline to pale brown cells of textura angularis. Conidiophores reduced to conidiogenous cells or present. Conidiogenous cells enteroblastic, phialidic discrete or integrated, indeterminate, proliferating percurrently, cylindrical to subcylindrical, hyaline. Macroconidia oblong to cylindrical, hyaline, straight, apex obtuse, base abruptly tapered to a distinct truncate scar, aseptate, smooth, thick-walled, guttulate or eguttulate. Microconidia present or absent, hyaline, cylindrical, rounded at the apex, truncate at the base, unicellular, smooth, thin-walled, contents granular (Sutton 1980; Verkley 1999).
Type species:Pezicula carpinea (Pers.) Tul. ex Fuckel, Jb. nassau. Ver. Naturk. 23-24: 279 (1870) [1869-70]
Notes: Since Pezicula was introduced, many species have been described under this genus (Verkley 1999; Abeln et al. 2000; Verkley et al. 2003; Johnston et al. 2004; Yuan and Verkley 2014). The asexual morph of Pezicula is associated with Cryptosporiopsis Bubák & Kabát., which was introduced in 1912 based on C. scutellata, the sexual morph of which is Pezicula ocellata (Yuan and Verkley 2014; Chen et al. 2016). Chen et al. (2016) revised the genus based on both morphology and phylogeny, designated an epitype for P. carpinea and delineated the boundary of the genus. Cryptosporiopsis was synonymized under this genus. There are 27 species accommodated in Pezicula, including a new species which is introduced in this paper.
Distribution: Austria, Belgium, Canada, China, Denmark, France, Germany, Italy, Norway, Poland, Portugal, Russia, UK, USA, Switzerland, The Netherlands (Grove et al. 1946; Abeln et al. 2000; Chen et al. 2016; this study).
Pezicula italica W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557158, Facesoffungi number: FoF 07485, Fig. 264
Etymology: Named after the country from which it was collected.
Saprobic on dead stem of Corylus sp., forming conspicuous, rounded to irregular, black conidiomata. Sexual morph: undetermined. Asexual morph: Conidiomata 100–200 µm diam., 200–450 µm high, pycnidial, solitary, gregarious or confluent, semi-immersed, cylindrical to subglobose, erumpent when mature, dark brown to black, subglobose to subcylindrical, unilocular, thick-walled. Ostiole cylindrical, single. Conidiomatal wall 20–40 µm wide, composed of thick-walled, hyaline to pale brown cells of textura angularis at the base, thick-walled, dark brown cells of textura angularis at periclinal layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–6 × 3–4 µm, hyaline, enteroblastic, phialidic, indeterminate, cylindrical to subcylindrical. Conidia 20–28 × 3.5–4.5 µm (\( \bar{x} \) = 25.5 × 4 µm; n = 30), hyaline, oblong to cylindrical, straight, aseptate, smooth, thick-walled.
Material examined: Italy, Province of Arezzo, Passo della Consuma, on dead aerial branches of Corylus sp. (Betulaceae), 11 August 2014, Erio Camporesi, IT2047 (MFLU 16-1284, holotype), (KUN, HKAS 101686, isotype); IT2047S (MFLU 19-2867, paratype).
Notes: Phylogenetic analysis base on multi-gene of LSU, ITS and rpb2 sequence data shows that two strains of Pezicula italica are distinct from any other Pezicula species and formed a branch above P. corylina J.W. Groves (Fig. 263). Pezicula italica shares similar characters with P. corylina and P. pruinosa Farl. in structure of conidiomata and conidia. However, P. italica has smaller conidiomata and narrower conidia than P. corylina (conidiomata 0.2–0.5 mm diam., 0.3–0.5 mm high; conidia 19–23.5 × 7.5–8.5 µm) and P. pruinosa (conidiomata up to 500 µm diam. at the base, tapering to 250 µm diam., 5–2 mm high; conidia: 24–26 × 9–10 µm).
Phacidiella P. Karst., Hedwigia 23(6): 85 (1884)
Faceoffungi number: FoF 07480, Fig. 265
Phacidiella salicina (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. b Conidiophores, conidiogenous cells and developing conidia
Phylogenetic tree inferred from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS and rpb2 sequence data representing Phacidiaceae. Forty-two strains are included in the analyses, which comprise 1717 characters including gaps (LSU: 1–820, ITS: 824–1360, rpb2: 1364–1717). Phlyctema vincetoxici CBS1 23726 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 5050.835095 is presented. The matrix had 291 distinct alignment patterns, with 7.35% of undetermined characters or gaps. Estimated base frequencies were: A = 0.248949, C = 0.228937, G = 0.272671, T = 0.249442; substitution rates AC = 0.869096, AG = 2.172385, AT = 1.173382, CG = 0.427568, CT = 12.344727, GT = 1.000000; gamma distribution shape parameter α = 0.114625. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.03 changes. Ex-type and reference strains are in bold. New isolates are shown in bold and blue
Lecanoromycetes, Ostropomycetidae, Ostropales, genera incertae sedis
Saprobic on the host plants, such as Artemisia vulgaris (Asteraceae), Asperula asperne (Rubiaceae), Eucalyptus sp. (Myrtaceae), Podocarpus latifolius (Podocarpaceae), Salix caprea, S. viminalis (Salicaceae) and Vitis vinifera (Vitaceae) (Sutton 1980; Purohit and Chawla 1989; Crous et al. 2007, 2014d). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, eustromatic, pycnidial, usually solitary, initially immersed, ultimately becoming erumpent, globose to cupulate, ostiole absent, dehiscence by rupture of the upper wall. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis in the basal part becoming thick-walled, darker cells of textura porrecta in the lateral wall. Conidiophores arising from innermost wall layer of the conidiomata, hyaline, cylindrical, septate, smooth-walled, usually branched at the base. Conidiogenous cells hyaline, holothallic, cylindrical, integrated, indeterminate, smooth-walled. Conidia hyaline, arthric, doliiform, truncated at both ends except for apical conidia which are subacute at the apices, unicellular, straight, smooth-walled, guttulate, formed by disarticulation of the conidiogenous cells from the conidiophore, produced in long, branched chains with the oldest conidia at the apex (adapted from Sutton 1980).
Type species: Phacidiella salicina P. Karst., Hedwigia 23(6): 85 (1884)
Notes: Sutton (1980) revised the generic concept of Phacidiella and accepted three species, P. asperulina (Bubák) B. Sutton P. salicina and P. tomispora (Berl. & Bres.) B. Sutton. Purohit and Chawla (1989) and Crous et al. (2007, 2014d) added three species, P. eucalypti Crous, P. podocarpi Crous & A.R. Wood and P. viticola Purohit & G.C. Chawla. However, Wijayawardene et al. (2017b) suggested that only P. salicina be accepted in the genus, but without giving any reasons. Here we accepted the six species mentioned above in Phacidiella (Sutton 1980; Purohit and Chawla 1989; Crous et al. 2007, 2014d).
The sexual morph of Phacidiella has been linked to Phacidiopycnis Potebnia, (= Potebniamyces) (Smerlis 1962; Johnston et al. 2014) and Pyrenopeziza (Sutton 1980), but these connections have not been proven by culture and molecular data. Crous et al. (2007, 2014d) showed that Phacidiella is related to Ostropales (Lecanoromycetes) based on ITS and LSU sequence data. However, the sequence data of type species P. salicina is not available, thus fresh collections are needed to confirm its placement.
Distribution: Austria, Finland, India, Kurdistan, Latvia, South Africa, Sweden (Sutton 1980; Purohit and Chawla 1989; Crous et al. 2007, 2014d).
Phacidium Fr., Observ. mycol. (Havniae) 1: 167 (1815)
Facesoffungi number: FoF 07500
Leotiomycetes, Leotiomycetidae, Phacidiales, Phacidiaceae
Foliicolous or caulicolous. Sexual morph:Ascomata amphigenous, solitary to gregarious, rounded, initially immersed, later rupturing host tissues by irregular stellate splits. Peridium composed of dark brown pseudoparenchymatous cells of textura globosa in outer layers, and pale brown to hyaline cells in inner layers. Hymenium composed of asci and paraphyses. Paraphyses exceeding asci, hyaline, branched, septate, tubular, filiform, invested in mucilage, and frequently anastomosing. Asci clavate, 4–8-spored, with an amyloid (I+) dehiscence ring (in Melzer’s reagent). Ascospores hyaline, uni- to biseriate, ellipsoid to ellipsoid-fusoid, aseptate, smooth-walled, lacking mucoid appendages (adapted from DiCosmo et al. 1984). Asexual morph:Conidiomata pale brown, dark brown or black, variable, stromatic, pycnidial, solitary, gregarious or confluent, immersed to semi-immersed, or erumpent, multi-locular to unilocular, globose to subglobose, thick-walled, ostiolate. Ostiole circular or cylindrical to subcylindrical, uni-ostiolate or multi-ostiolate, with relatively long neck or short neck. Conidiomatal wall composed of thick-walled, dark brown cells of textura globulosa to textura angularis in outer layers, and inner layers of thick-walled, hyaline cells of textura angularis, textura epidermoidea, textura oblita or textura prismatica. Conidiophores arising from the inner cells of the locular walls, hyaline, branched or unbranched, septate. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, subcylindrical, lageniform, ampulliform or subclavate, discrete or integrated, indeterminate, smooth-walled. Conidia hyaline, fusiform to ellipsoid, or cylindrical, with broad and obtuse base, narrowed apex, aseptate, smooth-walled, bearing fan-shaped, funnel-shaped or irregular, mucoid appendage.
Type species: Phacidium lacerum Fr., Observ. mycol. (Havniae) 2: 312 (1818)
Notes: The genus Ceuthospora was published by Fries (1825) to accommodate Sclerotium inclusum J.C. Schmidt & Kunze ex Fr. and Sphaeria phaeocomes Rebent. Greville (1828) listed three species in Ceuthospora, C. phacidioides Grev., C. lauri Grev. and C. phaeocomes (Rebent.) Fr., of which C. phaeocomes was later selected as the lectotype for Ceuthospora by Fries (1832). Fries (1849) proposed the generic name Pyrenophora, and referred both Sclerotium inclusum and Sphaeria phaeocomes to the genus. The generic name Ceuthspora thus has not been correctly typified. Sutton (1972b) discussed the nomenclatural complexities of Ceuthospora and proposed the conservation of Ceuthospora Grev. 1827 with C. lauri as the lectotype species, over Ceuthospora Fr. 1825 with C. phaeocomes as the lectotype species. According to this concept, more than 100 taxa were included in Ceuthopsora, but no revision has yet been attempted (Sutton 1980). Nag Raj (1993) revised the genus based on some taxa and provided a key to 11 species.
The sexual morph of Ceuthospora has traditionally been linked to Phacidium because of the association of the two phases on the host, or the occurrence of reproductive structure of both morphs within the same stroma (DiCosmo et al. 1983, 1984; Nag Raj 1993). However, these presumptive connections have not been confirmed by molecular data or cultural studies. In light of the previous studies (Hahn 1957; Nag Raj 1993), Crous et al. (2014d) designated a neotype for Phacidium lacerum, the type species of Phacidium and synonymized Ceuthospora under the genus. Crous et al. (2014d) also included eight species in Phacidium with molecular data. In this study, multi-gene analyses of ITS, LSU and rpb2 sequence data were implemented to classify Phacidium species. One new species is introduced in this genus, P. italicum based on combination of morphology and phylogeny, and four new combinations i.e. P. anomala, P. foliicola, P. innumera and P. subcorticalis are included.
Distribution: Argentina, Austria, Belgium, Canada, Chile, China, Czechoslovakia, Denmark, Finland, Germany, Italy, Portugal, Spain, Sweden, Switzerland, UK, USA, Tasmania, the Netherlands (Nag Raj 1993; Crous et al. 2014d, this study).
Phacidium anomala (Nag Raj) W.J. Li & K.D. Hyde, comb. nov.
≡ Ceuthospora anomala Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia (Ontario): 163 (1993)
Index Fungorum number: IF557172, Facesoffungi number: FoF 07501, Figs. 267, 268
Phacidium anomala (MFLU 19-2903, reference specimen) a Herbarium specimen. b, c Appearance of black coniodiomata on the host. d, e Sections of conidiomata. f, i Sections of peridium. g–h, j–k Conidiophores, conidiogenous cells and developing conidia. l–o Conidia. Scale bars: d = 100 µm, e = 200 µm, f–h = 20 µm, i = 10 µm, j–o = 5 um
Saprobic on dead leaves of Osmanthus sp. (Oleaceae) and Dactylis (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 250–300 µm diam., 170–250 µm high, dark brown to black, stromatic, pycnidial, epiphyllous, amphigenous on leaves, solitary, gregarious or confluent, subepidermal, immersed to semi-immersed, globose to subglobose, multilocular, with 3–10 locules; individual locules subglobose, 250–300 µm diam., 170–250 µm high, each with an independent ostiole. Ostioles 25–35 µm wide, centrally located, circular, rounded. Conidiomata wall 30–45 µm wide, composed of two types of pseudoparenchymatous cells, forming several inner layers of hyaline, thick-walled cells of textura epidermoidea, and several outer layers of brown, thick-walled cells of textura angularis. Conidiophores 20–30 × 3–4 µm, arising from inner wall layer of the conidiomata, hyaline, subcylindrical, septate, sympodially branched at the base and above, thick-walled. Conidiogenous cells 5–7 × 3–5 µm, hyaline, cylindrical, integrated, determinate, smooth-walled. Conidia 20–30 × 3.5–4.5 µm (\( \bar{x} \) = 25 × 4 µm; n = 30), hyaline, cylindrical to subcylindrical, with obtuse apex, and narrow, truncate base, unicellular, thick- and smooth-walled, bearing a cone-shaped or irregular, undulated, mucoid apical appendage resulting from the eversion of a mucoid sheath enveloping the upper part of developing conidium.
Culture characters: Colonies on PDA, reaching 8–9 cm after 14 d at 25–30 °C, white to flavescent with age, ultimately becoming brown to dark brown, circular, flattened, dense, slightly cottony with aerial mycelium on the surface, rounded and smooth at margins, reverse pale brown. Mycelium composed of hyaline to pale brown, cylindrical, branched, septate hyphae. Sporulation appearing after 4 weeks. Conidiomata formed from mycelium, black, pycnidial, solitary or gregarious, globose or irregular, multilocular, thick-walled. Conidiophores and conidiogenous cells not observed. Conidia 12–14 × 2–4.6 µm (\( \bar{x} \) = 12 × 3 µm; n = 30), hyaline, cylindrical, unicellular, smooth-walled, guttulate, without mucoid apical appendage.
Material examined: China, Province of Yunnan, Kunming, on dead leaves of Osmanthus sp. (Oleaceae), 20 June 2015, Wen-Jing Li, WJL0080 (MFLU 19-2903, reference specimen designated here), living culture MFLUCC 16-1151 = ICMP 21556 = KUMCC 15-0650; ibid., on dead leaves of Dactylis sp. (Poaceae), 20 June 2015, Wen-Jing Li, living culture MFLUCC 16-1155 = ICMP 21555, WJL0080C1 (MFLU 19-2904).
Notes: Our collections are similar to P. anomala in having immersed to semi-immersed, multilocular conidiomata with independent ostioles, sympodially branched conidiophores and cylindrical to subcylindrical, unicellular conidia with a cone-shaped, mucoid apical appendage. The differences between our strains and P. anomala are conidiomatal and conidial dimensions. Our strains have smaller conidiomata and larger conidia than P. anomala (conidiomata up to 1000 µm, conidia 9–15.5 × 2(–3) µm (\( \bar{x} \) = 12 × 2 µm). However, the conidial dimensions of our collections from culture are similar to P. anomala. Therefore, we suggest that conidial dimensions only are not a sufficient character to separate taxa in Phacidium. Phylogenetic analysis based on multi-locus sequence of LSU, ITS and rpb2 shows that P. anomala formed a separated branch in Phacidium (Fig. 266). Phacidium anomala has been reported from Argentina, Austria, Canada, Denmark, Germany, Italy, UK, and our collection from China is regarded as a new record.
Phacidium italicum W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557159, Facesoffungi number: FoF 07502, Figs. 269, 270
Phacidium italicum (MFLU 16-2155, holotype) a Herbarium specimen. b–g Appearance of black conidiomata on the host. h, i Vertical sections of conidioma. j locules. k, n Sections of peridium. l, m Ostiole. o, p Conidiophores, conidiogenous cells and developing conidia. q–t Conidia. Scale bars: b = 1000 µm, c–d = 200 µm, e–i = 500 µm, j = 100 µm, k–o = 50 µm, p = 20 µm, q–t = 10 µm
Phacidium italicum (MFLUCC 16-1314) a Germinating conidium. b, c Culture on PDA. d, e Appearance of brown conidiomata on mycelium. f Vertical section of conidioma. g Conidiomatal wall. h Conidiogenous cells and developing conidia. Scale bars: a = 20 µm, b–c = 20 mm, d = 1000 µm, e = 500 µm, f = 200 µm, g–h = 10 µm
Etymology: Named after country from where it was collected.
Saprobic on dead stems of Rubus sp. (Rosaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 1500–3570 µm long, 730–1120 µm wide, black, pseudostromatic, solitary or gregarious, oval to irregular in outline, semi-immersed to superficial, ostiolate, thick-walled, glabrous, multi-locular, with less than 10 locules embedded in the pseudostroma, individual locules 80–430 µm diam., 120–460 µm wide, with irregular or globose to subglobose, each with an ostiole. Ostioles 140–240 µm long, 20–70 µm wide, centrally located, circular, rounded, with relatively long necks, opening into a mutual area of dehiscence marked by white furfuraceous border. Conidiomatal wall 20–120 µm wide, composed of outer layers of thick-walled, dark brown to black cells of textura angularis, inner layers of thick-walled, pale brown to hyaline cells of textura epidermoidea to textura porrecta. Conidiophores arising from the innermost layer of the locular walls, hyaline, branched, septate, smooth-walled. Conidiogenous cells 8–20 × 2–3 µm, hyaline, enteroblastic, phailidic, cylindrical to subcylindrical with marked periclinal thickenings in the collarette zone, smooth-walled. Conidia 10–16 × 2–3.6 µm (\( \bar{x} \) = 13.5 × 3 µm; n = 30), hyaline, cylindrical to subcylindrical, with a broad and occasionally protuberant base, and a rounded apex, unicellular, smooth, thick-walled, bearing a cone-shaped or irregular, subapical, mucoid appendage.
Culture characters: Colonies on PDA, reaching 3–4 cm after 7 d at 25–30 °C, white to brown with age, circular, flattened, dense, felty, with aerial mycelium on the surface, rounded and smooth at margins, reverse brown. Mycelium composed of hyaline to pale brown, cylindrical, branched, septate hyphae. Sporulation appearing after 4 weeks. Conidiomata formed from mycelium, brown, pycnidial, solitary or gregarious, globose or irregular, unilocular to multilocular, thick-walled. Conidiophores hyaline.
Material examined: Italy, Province of Arezzo, on dead aerial branches of Rubus sp. (Rosaceae), 13 July 2016, Erio Camporesi, IT3034 (MFLU 16-2155, holotype), ex-type living culture MFLUCC 16-1314 = ICMP 21553 = KUMCC 16-0108, (KUN, HKAS 101655, isotype).
Notes: The dimensions of Phacidium italicum (10–16 × 2–3.6 µm) fall into the variation of asexual morph of P. lauri (7–17 × 2–3 µm, Nag Raj 1993), but the latter has up to 10 individual locules, while P. italicum has less than 10 locules, mostly 5–6-locules. Phylogenetic analyses of multi-locus show that P. italicum is closer to P. distylii than to P. lauri. Therefore, P. italicum is introduced as a new species.
Phacidium innumerum (Massee) Crous, in Crous, et al., Stud. Mycol. 94: 223 (2019)
≡ Ceuthospora innumera Massee, Bull. Misc. Inf., Kew: 182 (1899)
Index Fungorum number: IF832040, Facesoffungi number: FoF 07503, Fig. 271
Phacidium innumera (K(M) 190756, holotype) a, b Herbarium package and specimen. c Vertical section of conidiomata. d, e Appearance of brown to black conidiomata on the host. f Horizontal view of the locules. g Section of peridium. h, i Conidiophores, conidiogenous cells and developing conidia. j–o Conidia. Scale bars c–d = 100 µm, e = 500 µm, f = 200 µm, g–i = 20 µm, j–o = 10 µm
Saprobic on dead leaves of Eucalyptus sp. (Myrtaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 340–430 µm diam., 200–300 µm high, brown to black, stromatic, pycnidial, amphigenous but predominantly hypophyllous, solitary to gregarious, occasionally confluent, deeply immersed with only the red-brown neck of the collection chamber extending above the ruptured host tissue, globose to subglobose or ellipsoid, multilocular, thick-walled, glabrous, ostiolate. Ostiole 140–220 × 88–150 µm, oval or circular, centric or acentric, opening into the base of the locule, not associated with furfuraceous cells in the area of dehiscence. Conidiomata wall 15–30 µm wide, composed of an outer textura angularis with thick-walled, brown to dark brown cells near the ostiole, and an inner textura prismatica gradually merging with textura angularis with thick-walled, hyaline to brown cells at basal and lateral part. Conidiophores reduced to conidiogenous cells or sparsely septate and branched at the base, hyaline, arising from the innermost wall layer of conidiomata. Conidiogenous cells 6–20 × 1–4 µm, hyaline, enteroblastic, ampulliform to subcylindrical with minute periclinal thickenings. Conidia 15–22 × 2.7–4 µm (\( \bar{x} \) = 19 × 3 µm; n = 30), hyaline, subcylindrical, broad blunt or rounded at the base, attenuated towards at the apex, aseptate, smooth-walled, bearing a funnel-shaped, mucoid apical appendage.
Material examined: Tasmania, on dead leaves of Eucalyptus sp. (Myrtaceae), Rodway (K(M) 190756, holotype).
Phacidium foliicola (Lib.) W.J. Li & K.D. Hyde, comb. nov.
≡ Cytospora foliicola Lib., Pl. crypt. Arduenna, fasc. (Liège) 1(nos 1-100): no. 64 (1830)
= Ceuthospora foliicola (Lib.) Cooke, Grevillea 8(no. 46): 60 (1879)
Index Fungorum number: IF557173, Facesoffungi number: FoF 07504, Fig. 272
Phacidium foliicola (FH). a, b Herbarium package and specimen. c, d Appearance of brown to dark brown conidiomata on the host. e Vertical section of conidiomata. f–g, i Section of peridium. h Ostiole. j–m Conidiophores and conidiogenous cells. Scale bars c–d = 200 µm, e = 100 µm, f, h = 50 µm, g, i = 20 µm, j–m = 20 µm
Saprobic on plants, such as Berberis microphylla (Berberidaceae), B. vulgaris, Calocedrus decurrens (Cupressaceae), Cornus mas (Cornaceae), Genista sagittalis (Fabaceae), Hedera helix (Araliaceae), Photinia serrulata (Rosaceae), Vinca minor (Apocynaceae) (Nag Raj 1993, this study). Sexual morph: undetermined. Asexual morph:Conidiomata 300–360 µm diam., 140–180 µm high, black, solitary to gregarious or confluent, pycnidial, initially deeply immersed in the host mesophyll, then becoming partly erumpent and raising the host epidermis into subconical swellings, multilocular, with locules 50–130 µm diam., 100–170 µm high, globose to subglobose, glabrous, thick-walled, ostiolate. Ostioles circular, centrally located. Conidiomatal wall 15–40 µm wide, composed of pale brown, thick-walled cells of textura globulosa in the outer layer, become hyaline cells of textura angularis in the inner layer. Conidiophores arising around the cavity of the locules, hyaline, cylindrical to subcylindical, straight or curved, septate, branched, smooth-walled. Conidiogenous cells 26–28 × 1–3 µm, hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, usually integrated, smooth-walled, with periclinal thickenings. Conidia 10–14 × 2–2.5 (\( \bar{x} \) = 12 × 2.5 µm), hyaline, subcylindrical, rounded at both ends, with a minute dehiscence scar, straight or slightly curved, smooth, bearing a funnel-shaped or irregular, mucoid, apical appendage.
Material examined: Germany, Königstein, on leaves of Vinca minor (Apocynaceae), October 1885, W. Krieger, (FH).
Notes: The description of the conidia is adapted from Nag Raj (1993).
Phacidium subcorticalis (Fuckel) W.J. Li & K.D. Hyde, comb. nov.
≡ Ceuthospora subcorticalis Fuckel, Hedwigia 3: 160 (1864)
Index Fungorum number: IF557174, Facesoffungi number: FoF 07505, Figs. 273, 274
Phacidium subcorticalis (MFLU 19-2900, reference specimen) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d, e Vertical sections of conidiomata. f Ostiole. g, h Section of peridium. i–k Conidiogenous cells and developing conidia. l–p Conidia. Scale bars: d = 200 µm, e = 100 µm, f–g = 20 µm, h–p = 10 µm
Saprobic on dead leaves of Distylium racemosum (Hamamelidaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 250–300 µm diam., 170–250 µm high, black, stromatic, pycnidial, amphigenous on leaves, scattered to gregarious, subepidermal, initially immersed, then becoming partly erumpent, pulvinate to conical, irregularly plurilocular; individual locules irregular or globose to subglobose, 250–300 µm diam., 170–250 µm high, each with an ostiole. Ostioles 25–35 µm wide, centrally located, circular, rounded. Conidiomatal wall 20–35 µm wide, composed of outer several layers of thick-walled, brown cells of textura angularis, inner several layers, of thick-walled, hyaline cells of textura epidemoidea. Conidiophores 20–28 × 3.5–4.5 µm, arising from the inner wall layer of the conidiomata, hyaline, subcylindrical, septate, branched at the base. Conidiogenous cells 5–7 × 3–5 µm, hyaline, enteroblastic, phialidic, usually integrated, indeterminate, cylindrical, smooth. Conidia 20–28 × 3.5–4.5 µm (\( \bar{x} \) = 25.5 × 4 µm; n = 30), hyaline, cylindrical to subcylindrical, rounded at apex, narrowly truncate at the base, unicellular, thick- and smooth-walled, bearing a cone-shaped or irregular, subapical, mucoid appendage.
Culture characters: Colonies on PDA, reaching 7–8 cm after 7 d at 25–30 °C, white to brown with age, circular, flattened, dense, slightly cottony to felty, rounded and smooth at margins, reverse brown to dark brown. Mycelium composed of pale brown, cylindrical, branched, septate hyphae. Sporulation appearing after 4 weeks. Conidiomata formed from mycelium, brown to black, pycnidial, solitary or gregarious, globose or subglobose, multilocular, thick-walled. Conidia not observed.
Material examined: China, Yunnan, Kunming, on dead leaves of Distylium racemosum (Hamamelidaceae), 19 June 2014, Wen-Jing Li, WJL0040 (MFLU 19-2900, reference specimen designated here), living culture MFLUCC 15-0581 = KUMCC 15-0648; ibid., on dead leaves of D. racemosum (Hamamelidaceae), 25 June 2015, Wen-Jing Li, living culture MFLUCC 16-1149 = ICMP 21554 = KUMCC 15-0609, WJL0041 (MFLU 19-2901), WJL0041A (MFLU 19-2902).
Notes: A comparative study of the species described as asexual morphs of Phacidium (= Ceuthospora) show that our collections are similar to P. subcorticalis in form and dimensions of conidiomata and conidiogenous cells, but differs in dimensions of conidia. Our collections have much larger conidia than P. subcorticalis (10.5–14 × 2–2.5 (\( \bar{x} \) = 12 × 2.5). However, the conidia dimensions could reflect hosts or geographic distribution. In addition, under P. anomala we have mentioned that conidial dimension alone cannot be used to distinguish species of Phacidium. Phacidium subcorticalis has been found on fallen leaves and inner surface of bark of Betula alba (Betulaceae) from Austria, Belgium, Finland (Nag Raj 1993). Therefore, our collection is regarded as a new record from China.
Phellostroma Syd. & P. Syd., Philipp. J. Sci., C, Bot. 9(2): 185 (1914)
Faceoffungi number: FoF 07489, Fig. 275
Phellostroma hypoxyloides (redrawn from Morgan-Jones 1974) a Vertical section of conidiomata. b, c Enlarged view of conidiomatal wall. d Conidia. e Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on dead fructification of Areca sp. (Arecaceae) (Sutton and Kobayashi 1969). Sexual morph: undetermined. Asexual morph:Conidiomata black, stromatic, pycnidial, solitary, superficial, cushion-shaped, multilocular, glabrous, ostiolate. Ostiole single to each locule, cylindrical, with long neck of varying length, centrally located. Conidiomatal wall composed of relatively thick-walled, brown to pale brown cells of textura angularis in most parts of the conidiomata, becoming dark brown cells toward periphery, merging with thick-walled, paler cells of textura prismatica in the inner locular wall. Conidiophores arising from inner layer of the locular wall, hyaline, cylindrical to subcylindrical, septate, smooth-walled. Conidiogenous cells hyaline, holothallic, cylindrical, determinate, smooth-walled. Conidia hyaline, arthric, elliptical, somewhat truncated at both ends, unicellular, straight, smooth-walled, guttulate, formed by disarticulation of the conidiogenous cells from the conidiophore, produced in long, branched chains with the youngest conidia at the base (adapted from Morgan-Jones 1974).
Type species: Phellostroma hypoxyloides Syd. & P. Syd., Philipp. J. Sci., C, Bot. 9(2): 185 (1914)
Notes: Phellostroma was described from dead fructification of Areca sp., with P. hypoxyloides as type species (Sydow and Sydow 1914). Phellostroma resembles the asexual morph of Pycnopeziza, Phacidiella, Sirozythiella, Trullula and Vouauxiella Petr. & Syd., in having arthric conidia formed by disarticulation of the conidial chain, with the oldest conidium at the apex (Morgan-Jones 1974; Sutton 1980). However, the asexual morph of Pycnopeziza has cylindrical conidia bearing appendages, which is absent in Chondropodiella, Phacidiella, Phellostroma, Sirozythiella, Trullula and Vouauxiella. Vouauxiella andTrullula have brown or dark brown conidia, which distinguish them from the hyaline conidia in Chondropodiella, Phacidiella, Phellostroma, and Sirozythiella (Sutton 1980; Wijayawardene et al. 2016). Phellostroma differs from Chondropodiella, Phacidiella and Sirozythiella by its cushion-shaped, multilocular, glabrous, ostiolate conidiomata. Sirozythiella was separated from Chondropodiella and Phacidiella by its septate conidia, and textura angularis to textura intricata conidiomatal wall (Morgan-Jones 1974; Sutton 1980). Chondropodiella differs from Phacidiella by its cylindrical to cylindrical-subulate, beaked conidiomata with unbranched conidial chains (Morgan-Jones 1974; Sutton 1980).
von Höhnel (1918b) studied the type collection of P. hypoxyloides and considered the fungus to be an ascomycete. Sutton and Kobayashi (1969) re-examined the type species and could not find any structures of asci and ascospores, and considered it might be a coelomocyte. Morgan-Jones (1974) provided a detailed description and illustration of P. hypoxyloides. The second species, P. tsugae Tak. Kobay. was described from Tsuga sieboldii by Kobayashi (1964). However, this taxon was proved to not be congeneric with the type species by Sutton and Kobayashi (1969), because of its distinct morphology of conidiogenous cells (holoblastic) and conidia (ovoid to broadly fusiform with appendages at each end). Phellostroma tsugae was therefore designated as the type species for a new genus Strasseriopsis. Phellostroma is monotypic and no molecular data is available. Fresh collections are needed to place Phellostroma in a natural group.
Distribution: Philippines (Sutton and Kobayash 1969).
Phialophorophoma Linder, Farlowia 1(3): 402 (1944) [1943–1944]
Faceoffungi number: FoF 07490
Dothideomycetes, Pleosporomycetidae, Pleosporales, genera incertae sedis
Saprobic on the host plant or in marine habitat. Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, scattered to gregarious, immersed to semi-immersed, obpyriform, unilocular, glabrous, ostiolate. Ostiole single, cylindrical to circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown to black cells of textura globulosa in the exterior, becoming hyaline to subhyaline towards the conidial hymenium. Conidiophores usually reduced to conidiogenous cells. Conidiogenous cells arising from the inner wall layers of conidiomata, hyaline, enteroblastic, annellidic, lageniform to subcylindrical, integrated, indeterminate, smooth-walled, proliferating percurrently 1–2 times. Conidia hyaline, ellipsoid to obovoid, unicellular, smooth-walled, guttulate (adapted from Nag Raj and DiCosmo 1984).
Type species: Phialophorophoma litoralis Linder, Farlowia 1(3): 403 (1944) [1943–1944]
Notes: Barghoorn and Linder (1944) proposed Phialophorophoma to accommodate the single species P. litoralis collected from wood pilings in USA. The conidiogenous cells of Phialophorophoma were described and illustrated as enteroblastic, phialidic, cylindrical to lageniform, integrated, with terminal or lateral apertures immediately below transverse septa (Morgan-Jones 1977; Sutton 1980). Conidiophores are hyaline, septate, branched at the base (Sutton 1980). Nag Raj and DiCosmo (1984) re-examined the holotype of P. litoralis and found that its conidiogenous cells are annellidic, proliferating percurrently 1–2 times, and its conidiophores are usually absent. Aveskamp et al. 2010) showed Phialophorophoma is related to Cucurbitariaceae based on LSU and SSU sequence data, while Wijayawardene et al. (2017b) placed it in Pleosporales, genera incertae sedis. Fresh collections of type species are needed to place Phialophorophoma in a natural group.
Distribution: Yugoslavia, UK, USA (Sutton 1980; Nag Raj and DiCosmo 1984).
Illustration was provided by Nag Raj and DiCosmo (1984).
Phloeosporella Höhn., Öst. bot. Z. 66: 106 (1916)
Faceoffungi number: FoF 07491, Fig. 276
Phloeosporella ceanothi (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, acervular, solitary to gregarious, unilocular, glabrous. Ostiole absent. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, sympodial, lageniform to cylindrical, indeterminate, smooth-walled. Conidia hyaline, cylindrical to subcylindrical, tapered gradually towards obtuse apex, truncated at the base, 1–5-septate, straight or slightly curved, smooth-walled, guttulate (adapted from Sutton 1980).
Type species: Phloeosporella ceanothi Höhn., Öst. bot. Z. 66: 106 (1916)
Notes: Johnston et al. (2014) recommended using the generic name Blumeriella Arx over Phloeosporella because P. padi (Lib.) Arx was referred to as macroconidial morph of B. jaapii. In addition, B. jaapii is in common use for the widespread shot-hole disease on Prunus. However, there is no molecular data to prove Blumeriella and Phloeosporella are congeneric. Phloeosporella is a polyphyletic genus and a culture of its type species is not available (Quaedvlieg et al. 2013). Therefore, we accept Phloeosporella as the legitimate name.
Phloeosporella resembles Asteromidium, Diplosporonema, Stictosepta and Septocyta in having holoblastic, sympodial conidiogenous cells with 1–2, unthickened apical scars. Stictosepta and Septocyta have stromatic, pycnidial, convoluted conidiomata, which differ from the acervular conidiomata in Asteromidium Phloeosporella and Diplosporonema (Sutton 1980). Phloeosporella was separated from Asteromidium and Diplosporonema by its cylindrical to subcylindrical, 1–5-septate conidia.
Fourteen taxa are accepted in Phloeosporella (Index Fungorum 2019) and most of them are associated with leaf lesions on Fabaceae, Lamiaceae, Rhamnaceae, and Rosaceae (Sutton 1980).
Distribution: India, USA (Sutton 1980)
Phlyctaeniella Petr., Ann Mycol. 20(5/6): 323 (1922)
Faceoffungi number: FoF 07492, Fig. 277
Phlyctaeniella humuli (redrawn from Sutton 1980) a Conidiophores, conidiogenous cells and conidia. b Conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat, such as Humulus lupulus (Cannabaceae), Eucalyptus obliqua, Eucalyptus sp. (Myrtaceae) (Sutton 1980; Tiwari et al. 2012). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary to gregarious or confluent, immersed to erumpent, globose, unilocular, glabrous. Ostiole indistinct, dehiscence by rupture of the apical wall. Conidiomatal wall composed of thin-walled, hyaline cells of textura angularis in the basal and lateral part, becoming pale brown, larger cells above. Conidiophores arising from the inner wall layer of basal stroma, hyaline, cylindrical to irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, ampulliform to cylindrical or irregular, integrated or discrete, determinate, smooth-walled, markedly tapered at the apices, with apical or lateral apertures, collarette minute. Conidia hyaline, filiform to fusiform, with narrowed and rounded apex, and truncate base, straight or slightly curved, multiseptate, smooth-walled, guttulate (adapted from Sutton 1980).
Type species: Phlyctaeniella polonica Petr., Ann Mycol. 20(5/6): 323 (1922)
Notes: Phlyctaeniella is similar to Septoria in having pycnidial conidiomata and filiform to fusiform conidia, but it can be separated from the latter by its branched, septate conidiophores, whereas conidiophores are usually absent in Septoria (Tiwari et al. 2012; Quaedvlieg et al. 2013). Sutton (1980) re-described the genus based on P. humuli Petr.; the generic type has not been re-examined. Tiwari et al. (2012) introduced a new species from Eucalyptus sp. in India, and provided a key to five species of Phlyctaeniella. There is no molecular data available for Phlyctaeniella. Thus, fresh material and sequencing is needed to place Phlyctaeniella in a natural group.
Distribution: Austria, India, USA (Sutton 1980, Tiwari et al. 2012).
Phlyctema Desm., Annls Sci. Nat., Bot., sér. 38: 16 (1847)
Facesoffungi number: FoF 07493
Leotiomycetes, Leotiomycetidae, Medeolariales, Dermateaceae
Saprobic, parasitic or endophytic on plant host in terrestrial habitat, such as Aconitium napellus (Ranunculaceae), Coronilla sp. (Fabaceae), Erigeron sp. (Asteroideae), Malus pumila, M. sylvestris (Rosaceae) (Sutton 1980; Chen et al. 2016, this study). Sexual morph:Apothecia circular or irregular and merged, sessile, slightly convex, developing from acervuloid stromata. Asci 8-spored, cylindrical-clavate, inoperculate, apical apparatus turning blue in iodine. Ascospores hyaline, elongated ellipsoid, rounded or somewhat pointed at both ends, straight or slightly curved, aseptate when young, septate at maturity. Paraphyses hyaline, numerous, filiform, branched, septate, slightly swollen at apical cell (Verkley 1999). Asexual morph:Conidiomata yellowish brown to brown, pycnidial, solitary or gregarious, immersed to semi-immersed, globose to subglobose, unilocular, thick-walled, glabrous. Ostiole absent, dehiscence by rupture of the overlying tissues. Conidiomata wall composed of thick-walled, dark brown cells of textura angularis in outer layers, becoming thin-walled, hyaline cells in inner layers. Conidiophores arising from the inner wall layers of conidiomata, hyaline, cylindrical to subcylindrical, broaden at the base, branched irregularly. Macroconidiogenous cells hyaline, enteroblastic, phialidic, ampuliform to subcylindrical, usually intergrated, determinate, with a wide channel and minute collaratte and periclinal wall thickening. Macroconidia hyaline, subcylindrical to fusiform, usually curved, aseptate, guttulate, smooth-walled. Microconidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical to ampuliform, smooth-walled. Microconidia hyaline, filiform with rounded ends, unicellular, straight or curved.
Type species: Phlyctema vagabunda Desm., Annls Sci. Nat., Bot., sér. 3 8: 16 (1847)
Notes: The filiform, curved microconidia of Phlyctema can be confused with Diaporthe and Libertella. However, Diaporthe was seprated from Phlyctema by its fusiform to oval or ellipsoid macroconidia. Libertella lacks macroconidia, but differs from Phlyctema by its holoblastic, sympodial, integrated conidiogenous cells with 2 to several, slightly protuberant conidiogenous loci (Sutton 1980; this study).
More than 80 species have been recognized in Phlyctema although most have not been re-examined (Index Fungorum 2019). The sexual morph of P. vagabunda was assigned to Neofabraea alba (E.J. Guthrie) Verkley, making Phlyctema a synonym of Neofabraea (Verkley 1999; Johnston et al. 2014). Chen et al. (2016) treated Phlyctema and Neofabraea as separate genera based on morphological characters and multi-gene phylogenetic analyses. We utilized multi-gene analyses to delineate genera in Dermateaceae and also showed Phlyctema to be distinct from Neofabraea. A new collection from dead stems of Coronilla sp. clusters with Phlyctema sensu stricto with high bootstrap value (Fig. 129), and it is introduced as a new species in Phlyctema (see notes under P. coronillae).
Distribution: Australia, Canada, Chile, Czechia, Eire, France, Germany, New Zealand, South Africa, Tasmania, UK, USA (Sutton 1980; Chen et al. 2016).
Phlyctema coronillae W.J Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557160, Facesoffungi number: FoF 07494, Fig. 278
Phlyctema coronillae (MFLU 15-1243, holotype) a Herbarium specimen. b, c Appearance of brown to dark brown coniodiomata on the host. d Vertical section of conidioma. e Section of peridium. f–h Conidiophores, conidiogenous cells and developing conidia. i–m Conidia. Scale bars d = 100 µm, e = 50 µm, f–m = 5 µm
Etymology: Named after the host genus Coronilla, from which it was collected.
Saprobic on dead stems of Hippocrepis emerus (Fabaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 230–260 µm diam., 80–110 µm high, brown to dark brown, pycnidial, solitary or gregarious, subglobose, thick-walled, unilocular, glabrous. Ostiole absent, dehiscence by longitudinal fissure. Conidiomatal wall 10–20 µm wide, composed of pale brown to hyaline, thick-walled cells of textura prismatica. Conidiophores formed from the inner wall layers of conidiomata, hyaline, cylindrical, repeatedly branched at the base and above, usually fasciculate, septate in association with branches, tapered towards the apices, smooth-walled. Conidiogenous cells 5–16 × 2–3 µm, hyaline, holoblastic, subcylindrical, discrete or integrated, indeterminate, thick-walled, smooth. Conidia 12–29 × 2–3 µm (\( \bar{x} \) = 24 × 2 µm, n = 30), hyaline, falcate to fusiform, obtuse at each end, aseptate, smooth, thin-walled, occasionally guttulate.
Material examined: Italy, Province of Forlì-Cesena, Santa Sofia, Corniolo, on dead aerial branches of Coronilla emerus (Fabaceae), 18 May 2015, Erio Camporesi, IT2495 (MFLU 15-1243, holotype).
Notes: Phlyctema coronillae shares similar form of conidiomata, conidiogenous cells and conidia with P. vagabunda, but it can be differentiated by conidial dimensions. P. coronillae has shorter and narrower conidia than P. vagabunda (20–34 × 2.6–4 µm). Phylogenetic analyses also support them as separate species (Fig. 129).
Phyllosticta Pers., Traité champ. (Paris): 55, 147 (1818)
Dothideomycetes, Incertae sedis, Botryosphaeriales, Phyllostictaceae
Saprobic, parasitic or endophytic on wide range of hosts from trees to ornamentals or crops (Crous et al. 2006a; Wikee et al. 2013; Hern et al. 2019, this study). Sexual morph: see Wikee et al. (2013). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary or gregarious, subepidermal, immersed to semi-immersed, globose or subglobose, unilocular or multilocular, glabrous, ostiolate. Ostiole single, circular, centrical. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura globulosa to textura prismatica Conidiophores usually reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, annellidic, lageniform to subcylindrical, indeterminate, smooth-walled, percurrently proliferating. Conidia hyaline, subglobose, ovoid or obovoid, ellipsoidal or pomiform, subcylindrical, unicellular, smooth-walled, guttulate, surrounded by a mucoid envelope and bearing single, unbranched, attenuated, mucoid apical appendage. Spermatogenous cells hyaline, enteroblastic, phialidic, subcylindrical to ampulliform or lageniform, determinate, smooth-walled. Spermatia hyaline, cylindrical or dumbbell-shaped with rounded or blunt ends, aseptate (description of spermatogenous cells and spermatia adapted from Nag Raj (1993) and Wikee et al. (2013).
Type species: Phyllosticta convallariae Pers., Traité champ. (Paris): 148 (1818)
= Phyllosticta cruenta (Fr.) J. Kickx f., Mém. Acad. R. Sci. Lett. Arts. Brux. 23: 22 (1849)
Notes: Phyllosticta is a morphologically unique and ecologically important genus. The genus is characterized by unilocular, ostiolate ascomata with ellipsoid, or ellipsoid-fusoid to limoniform ascospores surrounded by a mucilaginous sheath, and ellipsoid-fusoid to ovoid, occasionally subcylindrical, aseptate conidia encased in a mucoid sheath and often with an apical appendage, as well as cylindrical or dumbbell-shaped spermatia (Nag Raj 1993; Liu et al. 2012; Wikee et al. 2013; Hern et al. 2019). Many Phyllosticta species are associated with leaf spots and various fruit diseases (Wicht et al. 2012; Pu et al. 2008; Wong et al. 2012; Wu et al. 2014), of which P. citricarpa, the cause of citrus black spot is regarded as a quarantine pest in Europe and the USA (Baayen et al. 2002; Glienke et al. 2011; Wikee et al. 2013; Guarnaccia et al. 2017).
More than 3000 epithets are listed in Index Fungorum (2019), but many of them have been transferred to another genus (Van der Aa and Vanev 2002; Crous et al. 2006a; Liu et al. 2012; Wikee et al. 2013). Wikee et al. (2013) revised Phyllosticta and transferred it to the resurrected family Phyllostictaceae from Botryosphaeriaceae. About 71 taxa have been studied by molecular means (Hern et al. 2019). We re-examined the herbarium specimens of P. plumbaginicola and P. sphaeropsoidea and provide detailed descriptions. We also introduce a new record of P. pervincae from Italy based on morphological characters. A new collection from Thailand, responsible for Musa sp. freckle disease is described. However, it lacks suitable gene sequences (e.g., ITS, act, gapdh, tef1), and thus we keep it as Phyllosticta sp., until molecular data is available.
Distribution: worldwide (Nag Raj 1993; Wikee et al. 2013; Liu et al. 2012; Hern et al. 2019).
Phyllosticta pervincae Bissett & Darbysh., Fungi Canadenses, Ottawa: no. 282 (1984)
Facesoffungi number: FoF 07507, Fig. 279
Phyllosticta pervincae (MFLU 19-2879) a Herbarium specimen. b, c Appearance of dark brown to black conidiomata on the host. d Vertical section of conidioma. e Ostiole. f, g Sections of peridium. h–j, m Conidiogenous cells and developing conidia. k–l, n–p Conidia. Scale bars b–c = 250 µm, d = 50 µm, e–g = 20 µm, h–j, m = 5 µm, k–l, n–p = 10 µm
Saprobic or parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata 100–150 diam., 100–120 µm high, dark brown to black, pycnidial, solitary to gregarious, immersed to semi-immersed, subglobose, unilocular, glabrous, ostiolate. Ostiole circular, centrical. Conidiomatal wall 15–40 µm wide, composed of thick-walled, dark brown cells of textura angularis in the outer layers, becoming thin-walled, hyaline cells towards conidial hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–6.5 × 2–4 µm, hyaline, enteroblastic, annellidic, flask-shaped or cylindrical, smooth-walled, percurrently proliferating, formed from the inner wall layers of conidiomata. Conidia 10–13 × 6–10 µm (\( \bar{x} \) = 11.5 × 7 µm; n = 30), hyaline, elliptical to ovoid, rounded at each end, aseptate, smooth-walled, with a mucoid sheath up to 2–4 µm thick, and an mucoid, apical appendage (7–15 µm long). Spermatia not observed.
Material examined: Italy, Province of Forlì-Cesena, Modigliana, Montebello, on dead aerial branches of Cornus sanguinea (Cornaceae), 23 September 2012, Erio Camporesi, IT748 (MFLU 19-2879).
Notes: Phyllosticta pervincae was originally reported from branches of Vinca difformis (Apocynaceae) in Portugal. This species is also associated with brown, diffuse leaf spots on Vinca minor in the USA (Nag raj 1993). Our collection shares similar form of conidiomata, conidiogenous cells and conidia with P. pervincae. In addition, it has similar conidial dimensions with P. pervincae [(6.5–)9–15 × (4.5–)6–7.5(–9), \( \bar{x} \) = 12 × 6.7 µm] and is, therefore, considered conspecific with P. pervincae.
Phyllosticta plumbaginicola V.G. Rao, Publicações Inst. Micol. Recife 383: 6 (1963)
Facesoffungi number: FoF 07508, Fig. 280
Phyllosticta plumbaginicola (DAOM 215565) a, b Herbarium package and specimen. c–e Appearance of black conidiomata on the host. f Vertical section of conidioma. g Section of peridium. h–n Conidiogenous cells and developing conidia (arrows show annellides). o–s Conidia. Scale bars c = 1000 µm, d = 500 µm, e = 100 µm, f = 50 µm, g = 20 µm, h–s = 5 µm
Parasitic on the leaves of Plumbago zeylanica (Plumbaginaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 50–100 diam., 55–100 µm high, dark brown to black, pycnidial, solitary to gregarious, subepidermal, deeply immersed, globose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, circular, centrical. Conidiomatal wall 10–25 µm wide, composed of thick-walled, brown cells of textura globulosa on the exterior, gradually merging with hyaline cells of textura prismatica in inner layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 6–12 × 2.5–5 µm, hyaline, enteroblastic, lageniform to subcylindrical, straight or slightly curved, thin- and smooth-walled, percurrently proliferating 1–2 times (Fig. 280h–j), formed from the innermost wall layer of conidiomata. Conidia 7.5–11 × 5–7 µm (\( \bar{x} \) = 9 × 6 µm; n = 30), hyaline, oval to ellipsoid, pomiform or subcylindrical to subglobose, with a truncate base bearing minute marginal frills, aseptate, smooth-walled, guttulate, enclosed in a mucoid envelope 1–4 µm wide, bearing appendage at apex; appendage 9.5–16 µm long. single, unbranched, attenuated, mucoid. Spermatia not observed.
Material examined: India, Bangalore, Nat. Tuberculosis Inst., on leaving leaves of Plumbago zeylanica (Plumbaginaceae), 24 November 1968, K.M. Ponnappa (DAOM 215565, part of original collection of Phyllostictina plumbaginis).
Notes: Phyllosticta plumbaginicola has been reported in India, and is responsible for oval to irregular, straw-coloured leaf spots with tan coloured margins (Nag Raj 1993).
Phyllosticta sphaeropsoidea Ellis & Everh., Bull. Torrey bot. Club 10(9): [97] (1883)
Facesoffungi number: FoF 07509, Fig. 281
Phyllosticta sphaeropsoidea (DAOM 215312) a, b Herbarium package and specimen. c–d Appearance of pale brown to brown conidiomata on the host. e–f Vertical sections of conidiomata. g–h Sections of peridium. i Ostiole. j–m Conidiogenous cells and developing conidia. n–s Conidia. Scale bars c = 200 µm, d = 100 µm, e–f = 100 µm, g–h = 10 µm, i = 20 µm, j–s = 5 µm
Parasitic on leaves of Aesculus hippocastanum (Sapindaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 80–100 diam., 90–130 µm high, pale brown to brown, pycnidial, amphigenous, predominantly epiphyllous, solitary to gregarious or confluent, subepidermal, initially immersed, ultimately becoming erumpent, globose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, circular, centrical. Conidiomatal wall 6–30 µm wide, composed of thin-walled, hyaline cells of textura prismatica in the basal and lateral part, becoming thick-walled, dark brown to black cells of textura angularis in the ostiolar region. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 6–17 × 4–7 µm, hyaline, enteroblastic, short cylindrical to subcylindrical, smooth-walled, with periclinal thickenings at upper part (Fig. 281j). Conidia 8.6–16.6 × 9–14 µm (\( \bar{x} \) = 13 × 10 µm; n = 30), hyaline, ellipsoidal to obovoid or globose, bulging in the upper half, rounded and occasionally invaginated at apex, truncate at base and bearing minute marginal frills, enclosed in a distinct mucoid envelope 0.7–2 µm wide, with a single, unbranched, attenuated, mucoid, apical appendage (5.5–8 µm long). Spermatogenous cells hyaline, ampulliform to lageniform or subcylindrical, with periclinal thickenings, smooth-walled. Spermatia hyaline, bacillar with a narrowed apical part and a swollen, truncate basal part bearing minute marginal frills, unicellular, smooth-walled (description of spermatogenous cells and spermatia adapted from Nag Raj 1993).
Material examined: Canada, Ontario, Waterloo, University of Waterloo campus, on leaves of Aesculus hippocastanum (Sapindaceae), 27 July 1988, T.R. Nag Raj (DAOM 215312).
Notes: Phyllosticta sphaeropsoidea has been recoded from Austria, Canada, and USA. This species is associated with leaf blotch disease on A. glabra and A. hippocastanum. The infection is often severe enough to cause premature defoliation (Nag Raj 1993).
Phyllosticta sp.
Fig. 282
Phyllostictasp. (MFUL 19-2897) a Herbarium specimen. b, c Appearance of black conidiomata on the host. d, e Vertical sections of conidiomata. f Vertical section of peridium. g–i Conidiogenous cells and developing conidia. j Germinating conidium. k–o Conidia. Scale bars: b = 500 µm, c = 200 µm, d–e = 100 µm, f, j = 20 µm, g–i, k–o = 10 µm
Parasitic on living leaves of Musa sp., forming conspicuous, small, rounded, black conidiomata. Sexual morph: undetermined, Asexual morph:Conidiomata 70–140 µm diam., 80–120 µm high, brown to black, pycnidial, amphigenous, usually gregarious, immersed to semi-immersed, globose to depressed globose, unilocular, glabrous, ostiolate. Ostiole circular, excentrical. Conidiomatal wall 10–20 µm wide, composed of thick-walled, dark brown cells of textura angularis in the outer layer, become hyaline cells in the inner layer. Ostiole centrally located, circular, papillate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–5 × 3–7 µm, hyaline, enteroblastic, annellidic, lageniform or subcylindrical, indeterminate, smooth-walled, proliferating once or twice. Conidia 7–12 × 5–6.5 µm (\( \bar{x} \) = 10 × 6 µm; n = 30), hyaline, ellipsoid, subcylindrical to subglobose with a truncate base, aseptate, thick- and smooth-walled, enclosed in a 1.5–2 µm wide mucoid envelope, with a 10–17 µm long, unbranched, whip-like, apical appendage arising from the apex. Spermatia not observed.
Material examined: Thailand, Chiang Rai, on living leaves of Musa sp., 7 April 2015, Wen-Jing Li, WJL0032 (MFLU 19-2897), (KUN, HKAS 93581).
Notes: The germinating conidia stop growing on PDA. Therefore, the sequence of our collection was obtained directly from conidiomata. Our collection shares a similar habit with P. musarum which is associated with banana freckle disease (Wong et al. 2012). This collection also share similar morphology of conidiomata and conidia with P. musarum, but it was distinguished from the latter [conidia: (12–)13–16(–20)×(7–)9–10(–11)] by its smaller conidia (Wong et al. 2012). The sequences similar between our collection and P. musarum is 98% in LSU (886/904). However, as it lacks suitable genes (e.g., ITS, act, gapdh, tef1), we keep it as Phyllosticta sp.
Pilidium Kunze, in Kunze & Schmidt, Mykologische Hefte (Leipzig) 2: 92 (1823)
= Discohainesia Nannf., Nova Acta R. Soc. Scient. upsal., Ser. 4 8(no. 2): 88 (1932)
= Hainesia Ellis & Sacc., in Saccardo, Syll. fung. (Abellini) 3: 698 (1884)
= Sclerotiopsis Speg., Anal. Soc. cient. argent. 13(1): 14 (1882)
Facesoffungi number: FoF 07495, Fig. 283
Pilidium acerinum (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Leotiomycetes, Leotiomycetidae, Chaetomellales, Chaetomellaceae
Parasitic on the host plant or isolated from soil. Sexual morph: see Rossman et al. (2004). Asexual morph:Conidiomata pycnidial or sporodochial; pycnidia pale brown when young, dark brown to black at maturity, solitary to gregarious, subepidermal to epidermal, immersed to superficial, globose, subglobose, obpyriform or oblong, sessile, unilocular, glabrous. Ostiole indistinct, opening by a stellate slit or irregular rupture. Pycnidial wall composed of thick-walled, pale brown to dark brown cell of textura angularis in the exterior, gradually merging with hyaline cells in the conidial hymenium. Conidiophores formed from the inner wall layer of conidiomata, hyaline, cylindrical, branched and septate at the base, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, lageniform to cylindrical, usually integrated, determinate, smooth-walled, with a minute apical channel and collarette. Conidia hyaline, fusiform to falcate or cymbiform, with obtuse base and acute apex, unicellular, smooth-walled, eguttulate. Sporodochia pale luteous, solitary, superficial, globose to cupulate, discoid, with irregularly wavy margin, slimy, stalk pale brown near base, becoming dark brown at apex. Conidiophores, conidiogenous cells and conidia similar to those in pycnidia (adapted from Sutton 1980; Rossman et al. 2004).
Type species: Pilidium acerinum (Alb. & Schwein.) Kunze, in Kunze & Schmidt, Mykologische Hefte (Leipzig) 2: 92 (1823)
Notes: Pilidium is similar to Chaetomella in having two types of conidiomata (pycnidium or sporodochium), acropleurogenous conidiogenous cells, and hyaline, usually fusiform to falcate, unicellular conidia. However, Chaetomella was separated from Pilidium by its setose conidiomata and molecular data.
The nomenclatural and taxonomic history of Pilidium was provided by Shear and Dodge (1921). Sutton (1980) revised Pilidium and accepted two species, P. acerinum and P. concavum (Desm.) Höhn. Pilidium acerinum has been reported primarily on leaves of deciduous trees, e.g., Eucalyptus grandis (Crous 1991; Farr et al. 2004). This species was studied by Palm (1991) who determined that light-colored, fleshy, discoid, stalked sporodochia of Hainesia lythri is a synanamorph of the black, enclosed pycnidia of P. concavum (Rossman et al. 2004). The synonymy of Pilidium was provided by Sutton (1980), Rossman et al. (2004), Johnston et al. (2014) and Wijayawardene et al. (2017b) as Discohainesia, Hainesia and Sclerotiopsis.
Rossman et al. (2004) designated an epitype for P. acerinum and placed Pilidium in Chaetomellaceae (Leotiomycetes) based on molecular data. They also assigned the sexual morph of Pilidium to Discohainesia. Subsequently, several new species were included in the genus, P. pseudoconcavum Crous (2013), P. lythri (Desm.) Rossman (2014), P. eucalyptorum Crous & M.J. Wingf. (2015), P. septatum A. Giraldo & Crous (2017). Six taxa are accepted in the genus. Pilidium species are commonly plant pathogens causing leaf spots, root lesions and tan-brown rot of fruits or isolated from soil (Sutton and Gibson 1977; Rossman et al. 2004; Karimi et al. 2016).
Distribution: Austria, Brazil, Canada, China, Eire, France, Germany, Iran, Italy, Japan, Lithuania, Mozambique, New Zealand, Netherlands, Philippines, Portugal, Spain, South Africa, UK, USA, Thailand (Sutton 1980; Rossman et al. 2004; Crous et al. 2013, 2015a, b, c, d, e, 2017; Karimi et al. 2016; Marin-Felix et al. 2017).
Placonema (Sacc. & D. Sacc.) Petr., Ann Mycol. 19(1/2): 60 (1921)
Facesoffungi number: FoF 07496, Fig. 284
Placonema bambusacearum (redrawn from Morgan-Jones et al. 1972d) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Saprobic on the host plants, such as Arthrostylidium longiflorum, A. racemiflorum, Bambusa sp., Merostachys speciosa (Poaceae) (Nag Raj 1993). Sexual morph: undetermined. Asexual morph:Conidiomata brown to black, stromatic, pycnidial, solitary to gregarious, immersed, elongate to oval in outline, globose to subglobose in section view, unilocular or multilocular, glabrous. Ostiole indistinct, dehiscence by irregular rupture of overlapping tissue. Conidiomatal wall composed of thick-walled, dark brown to pale brown then hyaline cells of textura prismatica to textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, annellidic, cylindrical to lageniform or irregular, integrated or discrete, indeterminate, smooth-walled, proliferating percurrently, arising from the inner wall layer of conidiomata. Macroconidia hyaline, sometimes pale brown to hyaline, fusiform, ovoid, ellipsoid or naviculate, unicellular, smooth-walled, bearing one filiform, unbranched, apical appendages and an eccentric basal appendage. Microconidia when present, hyaline, acerose, unicellular, smooth-walled (adapted from Morgan-Jones et al. 1972d; Nag Raj 1993).
Type species: Placonema bambusacearum (Sacc., Syd. & P. Syd.) Petr., Ann Mycol. 19(1/2): 60 (1921)
Notes: The hyaline, sometimes pale brown to hyaline, fusiform, ovoid, ellipsoid or naviculate, unicellular conidia bearing one filiform, unbranched, apical appendage and an eccentric basal appendage invites a comparison with Annellolacinia. However, Annellolacinia possesses acervular conidiomata with brown to dark brown, subulate, often nodulose, unbranched setae, which differs from the stromatic, pycnidial, glabrous conidiomata in Placonema (Nag Raj 1993). Moreover, Placonema has hyaline, acerose, unicellular microconidia, which are usually absent in Annellolacinia.
Placonema was elevated to generic rank from Placosphaeria (De Not.) Sacc. by Petrak (1921) to accommodate P. bambusacearum. The second species P. napelli was described on Aconitum napellus (Ranunculaceae) from Germany. Sutton (1977b) revised Placonema and transferred P. napelli to a newly introduced genus Neoplaconema because of its distinct conidia and conidiogenous cells morphology. Neoplaconema napelli was separated from Placonema by its phialidic conidiogenous cells with distinct collarettes, and fusiform, subcylindrical to ellipsoidal conidia with an, unbranched or branched appendage. Sutton (1977b) also reduced Shanoria Subram. & K. Ramakr. and Stigmateopsis Bat. to synonyms of Placonema. Nag Raj (1993) re-visited Placonema and provided detailed description and illustration for three species, P. austroamericanum Nag Raj, P. bambusacearum and P. bambusae (Bat.) Nag Raj. The sexual morph of Placonema has been assigned to Phyllachora (Farr 1968), but this connection is based on host association, and has not been confirmed by culture studies or other evidence. Therefore, fresh material of these taxa are needed to resolve the placement of Placonema species and its sexual morph.
Distribution: Brazil, El Salvador, Panama, Paraguay, Venezuela (Nag Raj 1993).
Placothyrium Bubák, Ber. dt. bot. Ges. 34: 302 (1916)
Facesoffungi number: FoF 07497, Fig. 285
Placothyrium athyrinum (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Ascomycota, genera incertae sedis
Saprobic on dead stems of Athyrium filix-femina (Athyriaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, stromatic, pycnidial, solitary to gregarious or confluent, epidermal to subepidermal, immersed, globose to subglobose in section view, unilocular or occasionally multilocular, glabrous. Ostiole indistinct, dehiscence by irregular rupture of the apical wall. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores arising from the inner wall layer of conidiomata, hyaline, cylindrical to irregular, branched at the base and above, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, holoblastic, cylindrical to lageniform or irregular, integrated, determinate, smooth-walled, terminal or lateral immediately below transverse septa. Conidia hyaline, filiform, tapered towards each end, 0–2-septate, smooth-walled (Sutton 1980).
Type species: Placothyrium athyrinum Bubák, Ber. dt. bot. Ges. 35(3): 302 (1917)
Notes: Placothyrium shows a similar conidial morphology with the asexual morph of Sphaerulina (such as S. oxyacanthae, S. rhabdoclinis), Pleurothyrium and Septoria as it has hyaline, filiform, septate conidia (Sutton 1980, Quaedvlieg et al. 2013). Placothyrium and Pleurothyrium have conidiophores, which are absent in the asexual morph of Sphaerulina and Septoria. Pleurothyrium differs from Placothyrium by its conidiophores without a gelatinous sheath covering and up to 8-septate, much longer conidia (Sutton 1980). Placothyrium is monotypic and no molecular data is available for this genus. Fresh collections are needed to place it in a natural group.
Distribution: Germany (Sutton 1980).
Plectronidiopsis Nag Raj, Can. J. Bot. 57(13): 1397 (1979)
Facesoffungi number: FoF 07499, Fig. 286
Plectronidiopsis chilensis (redrawn from Nag Raj 1979a) a Conidia. b Vertical section of a conidioma. c Enlarged view of a conidiomatal seta. d Conidiophores and developing conidia
Ascomycota, genera incertae sedis
Saprobic on culms of Hierochloe utriculata (Poaceae) (Nag Raj 1979a). Sexual morph: undetermined. Asexual morph:Conidiomata black, acervular, solitary to gregarious, semi-immersed, linear or oval in outline, patelliform to scutelliform in section view, unilocular, setose. Ostiole absent. Conidiomatal setae pale brown to brown at the base, paler to hyaline towards apex, cylindrical, straight or curved, aseptate, marginal. Conidiomatal wall composed of thick-walled, pale brown cells of textura angularis in the basal layer, becoming darker at the originating of conidiomatal setae. Conidiophores arising from inner wall layer of basal stroma, hyaline, cylindrical to dolliform, septate, unbranched, smooth-walled. Conidiogenous cells hyaline, enteroblastic, annellidic, lageniform to subcylindrical, usually integrated, indeterminate, smooth-walled. Conidia hyaline, cylindrical, with attenuated apex and truncate base, 1-septate, smooth-walled, bearing apical, basal and lateral flexuous appendages, arising as tubular extensions of the conidium body. Apical appendage single; basal appendages 2–3, inserted slightly above the truncate base; lateral appendages 1–3, inserted mostly on the apical cell, occasionally with another on the basal cell just below the median septum (adapted from Nag Raj 1979a, 1993).
Type species: Plectronidiopsis chilensis (Speg.) Nag Raj, Can. J. Bot. 57(13): 1397 (1979)
Notes: Nag Raj (1979a) proposed Plectronidiopsis to accommodate a segregated species from Dilophospora, as P. chilensis. Dilophospora differs from Plectronidiopsis by its globose, glabrous conidiomata and 3-septate conidia with circumpolar and branched dichotomously or irregularly appendages. Plectronidiopsis can be confused with Dwayalomella in having acervular, setose conidiomata and hyaline, cylindrical, 1-septate conidia with appendages. However, the conidia of Plectronidiopsis are separated from those of Dwayalomella in the number and disposition of the filiform appendages. The annellidic conidiogenous cells in Plectronidiopsis also separate it from the phialidic ones in Dwayalomella. The genus is monotypic and no molecular data is available. It is necessary to collect fresh sample of Plectronidiopsis chilensis to place Plectronidiopsis in a natural group and delineate its boundaries.
Distribution: Chile (Nag Raj 1979a).
Pleurophoma Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 117 (1914)
Facesoffungi number: FoF 07498, Fig. 287
Pleurophoma pleurospora (redrawn from Sutton 1980) a Conidia. b Vertical section of coniodiomata. c Conidiophores, conidiogenous cells and developing conidia
Dothideomycetes, Pleosporomycetidae, Pleosporales, Lentitheciaceae
Saprobic on the host plant in terrestrial habitat, such as Acacia glaucoptera (Fabaceae), Pinus nigra (Pinaceae), Salix fragilis (Salicaceae) (Sutton 1980; Crous et al. 2016a; Tibpromma et al. 2017). Sexual morph: see Crous et al. (2015d) and Tibpromma et al. (2017). Asexual morph:Conidiomata black, pycnidial, solitary, superficial, globose to subglobose, unilocular, glabrous, ostiolate. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, brown to pale brown then hyaline cells of textura angularis. Conidiophores arising from the inner wall layer of the conidiomata, hyaline, long, filiform, septate, branched. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, determinate, smooth-walled, with apical apertures or lateral apertures which arise immediately below transverse septa, collarette and channel minute. Conidia hyaline, cylindrical to ellipsoid, unicellular, smooth-walled.
Type species: Pleurophoma pleurospora (Sacc.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 117 (1914)
Notes: Sutton (1980) provided a detailed description and illustration for Pleurophoma, and accepted eight species in the genus. Pleurophoma has been disputed with Phoma taxa because of overlapping morphology of conidiophores and conidia (Sutton 1980; Boerema et al. 2004; de Gruyter et al. 2010). De Gruyter et al. (2013) fixed the generic concept by designating a lectotype for P. pleurospora and placed this genus in Lentitheciaceae. Subsequently, Crous et al. (2015d, 2016a) and Tibpromma et al. (2017) included three additional taxa, P. ossicola Crous, Krawczynski & H.-G. Wagner, P. acaciae Crous, and P. italica Tibpromma, Camporesi & K.D. Hyde.
Pleurophoma can be confused with the microconidial or spermogonial states of Keissleriella as they share similar morphology of conidiomata, conidiophores, conidiogenous cells and conidia (this study). Phylogenetically, Keissleriella species clustered with Pleurophoma species, but without support (Fig. 194). We suspect that Keissleriella and Pleurophoma may be related, but this hypothesis needs adequate collection and culture of fresh specimens to clarify.
Distribution: Australia, Germany, Italy (Crous et al. 2015d, 2016a; this study).
Pleurophomopsis Petr., Ann Mycol. 22(1/2): 156 (1924)
Facesoffungi number: FoF 07510
Ascomycota, genera incertae sedis
Saprobic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, scattered, superficial, globose in section view, unilocular, glabrous. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores formed from the inner wall layer of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, spherical, unicellular, smooth-walled, often with a single guttule (adapted from Sutton 1980).
Type species: Pleurophomopsis salicicola Petr., Ann Mycol. 22(1/2): 156 (1924)
Notes: Sutton (1980) revised Pleurophomopsis based on P. salicina Petr. He noted that if P. salicina is not congeneric with the type P. salicicola, then this generic account needs to be modified. Seven taxa, P. eucalypti Petr., P. lignicola Petr., P. nypae K.D. Hyde & B. Sutton, P. salicicola Petr., P. salicina Petr., P. strictae S. Ahmad, and P. valentina Urries were included in Pleurophomopsis, but most of them have not been examined (Sutton 1980; Hyde and Sutton 1992). The sexual morph of Pleurophomopsis was reported in Lophiostoma Ces. & De Not. (Leuchtmann 1985; Zhang et al. 2009), but this connection has never been successfully demonstrated. Wijayawardene et al. (2017b) placed Pleurophomopsis in Ascomycota, incertae sedis, since there is a lack of molecular data. Fresh collections of generic type and other taxa are needed to redefine the generic concept.
We examined a species, which shares a similar form of conidiomata and conidia with P. salicina, but distinguishable by conidial dimensions. Our collection has slightly larger conidia than P. salicina (2–3 µm long) (Sutton 1980). Because of lack of molecular data, this species is regarded as Pleurophomopsis sp., until an appropriate neo- and epitypification is fixed.
Distribution: China, Czechoslovakia, Italy, Pakistan, Spain (Sutton 1980; Hyde and Sutton 1992, this study).
Pleurophomopsis sp.
Fig. 288
Saprobic on dead aerial spines of Rosa canina. Sexual morph: undetermined. Asexual morph:Conidiomata 200–250 µm diam., 100–150 µm high, dark brown to black, pycnidial, scattered to gregarious, initially immersed, later becoming erumpent and exposed, globose, unilocular, glabrous. Ostiole absent, dehiscing by an irregular rupture of the apical wall. Conidiomatal wall 30–50 µm wide, composed of thick-walled, brown to dark brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 10–15 × 2–4 µm, hyaline, enteroblastic, phialidic, lageniform to cylindrical, occasionally integrated, determinate, with marked periclinal thickenings at apex, smooth-walled. Conidia 4–6 × 2–3 µm (\( \bar{x} \) = 5 × 2.5 µm; n = 20), hyaline, ellipsoidal to rounded, aseptate, thick- and smooth-walled.
Material examined: Italy, Province of Forlì-Cesena, Pieve di Rivoschio, on dead aerial spines of Rosa canina (Rosaceae), 28 January 2015, Erio Camporesi, IT2360 (MFLU 19-2872).
Pleurothyrium Bubák, Ber. dt. bot. Ges. 34: 322 (1916)
Facesoffungi number: FoF 07506, Fig. 289
Pleurothyrium longissimum (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on dead stems of Athyrium filix-femina.Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, stromatic, pycnidial, solitary, subepidermal, immersed, applanate, multilocular, glabrous. Ostiole indistinct, dehiscence by irregular rupture of the apical wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis concentrated in the epidermis in the upper part, becoming hyaline in the lower part, gradually merging pale brown, irregular pseudo-parenchyma between locules. Conidiophores arising from the inner basal wall layer of the locules, hyaline, cylindrical or irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, cylindrical, integrated, determinate, smooth-walled, terminal or lateral immediately below transverse septa. Conidia hyaline, filiform, attenuated towards each end, truncate at the base, up to 8-septate, smooth-walled (adapted from Sutton 1980).
Type species: Pleurothyrium longissimum (Lib.) Bubák, Ber. dt. bot. Ges. 34: 322 (1916)
Notes: Pleurothyrium resembles Megaloseptoria and Stictosepta in having hyaline, multiseptate conidia. However, Megaloseptoria differs in that the conidiomata are superficial, aggregated in a black stroma, and conidia are large, 30–40-septate (Quaedvlieg et al. 2013). In Stictosepta the conidiogenous cells are holoblastic, sympodial or synchronous, with two small, unthickened, apical conidiogenous loci, whereas in Pleurothyrium the conidiogenous cells are holoblastic, cylindrical, terminal or lateral immediately below transverse septa (Sutton 1980). Pleurothyrium is monotypic and no molecular data is available. Fresh collections are needed to place it in a natural group.
Distribution: Austria (Sutton 1980).
Polyphialoseptoria Quaedvl., R.W. Barreto, Verkley & Crous, Stud. Mycol. 75: 355 (2013)
Facesoffungi number: FoF 07511
Dothideomycetes, Dothideomycetidae, Capnodiales, Mycosphaerellaceae
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown, pycnidial, solitary to gregarious, immersed to erumpent, globose, unilocular, glabrous, ostiolate. Ostiole circular, centrally located. Conidiomatal wall composed of thick-walled, brown to pale brown cells of textura angularis. Conidiophores arising from the inner wall layer of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, phialidic or sympodial, subcylindrical to ampulliform, determinate or indeterminate, discrete or integrated. Conidia hyaline, scolecosporous or cylindrical to subcylindrical, with subobtuse apex, irregularly curved, multi-septate, smooth-walled, granular to guttulate (adapted from Quaedvlieg et al. 2013).
Notes: Polyphialoseptoria was introduced to accommodate two septoria-like taxa, P. tabebuiae-serratifoliae Quaedvlieg, Alfenas & Crous collected on Tabebuia serratifolia (Bignoniaceae) and P. terminaliae collected on Terminalia catappa (Combretaceae) (Quaedvlieg et al. 2013). The third species, P. natalensis Crous, was described from an unidentified plant in South Africa (Crous et al. 2018b). These species are associated with leaf spots. We examined a herbarium specimen, which shows a similar form of conidiomata and conidia with Polyphialoseptoria species, but unlike the previously described species it has phialidic conidiogenous cells.
Based on a megablast search of NCBIs GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Polyphialoseptoria natalensi (GenBank NR_161137; Identities = 463/478(97%), 4 gaps (0 %)), P. terminaliae (GenBank NR_156559; Identities = 451/463(97%), 3 gaps (0 %)), Septoria sp. (GenBank DQ897651; Identities = 457/473(97%), 3 gaps (0 %)), and P. tabebuiae-serratifoliae (GenBank NR_156558; Identities = 437/452(97%), 2 gaps (0 %)). However, as our strain lacks LSU sequence and other gene regions (e.g., rpb2, tef1 and tub2), we cannot confirm its placement. Thus this species was kept as Polyphialoseptoria sp.
Distribution: Brazil, South Africa, Thailand (Quaedvlieg et al. 2013; Crous et al. 2018b; this study).
Polyphialoseptoria sp.
Fig. 290
Polyphialoseptoriasp. (MFLU 13-0246) a Herbarium specimen. b–c Appearance of dark brown to black conidiomata on the host. d–e Vertical sections of conidiomata. f Vertical section of peridium. g Ostiole. h–j Conidiophores, conidiogenous cells and developing conidia. k–p Conidia. Scale bars: b = 500 µm, c = 200 µm, d–e = 100 µm, f = 20 µm, g = 50 µm, h–p = 5 µm
Habit on leaves of unidentified plant host. Sexual morph: undetermined. Asexual morph:Conidiomata 160–200 µm diam., 150–250 µm high, dark brown to black, pycnidial, usually solitary, immersed to erumpent, globose to subglobose, unilocular, glabrous, thin-walled, ostiolate. Ostiole circular, centrally located. Conidiomatal wall 10–25 µm wide, composed of thin-walled, brown to pale brown cells of textura angularis. Conidiophores arising from the inner wall layer of conidiomata, hyaline, short cylindrical, septate, smooth-walled. Conidiogenous cells 6–14 × 1–3 µm, hyaline, enteroblastic, phialidic, cylindrical to ampulliform, determinate, discrete, smooth-walled. Conidia 14.5–30 × 1.6–3 µm (\( \bar{x} \) = 21 × 2 µm; n = 30), hyaline, subcylindrical, with obtuse apex, and subtruncate base, curved, 1–3-septate, smooth-walled.
Material examined: Thailand, Chiang Mai, on leaves of unidentified plant host, 4 February 2012, N. Tangthirasunun, NTCL038-2F (MFLU 13-0246).
Polystigma DC., in de Candolle & Lamarck, Fl. franç., Edn 3 (Paris) 6: 164 (1815)
= Polystigmina Sacc., Syll. fung. (Abellini) 3: 622 (1884)
Facesoffungi number: FoF03519, Fig. 291
Polystigma rubrum (asexual morph: redrawn from Sutton 1980) a Conidiophores, conidiogenous cells and developing conidia. b Conidia
Sordariomycetes, Sordariomycetidae, Xylariales, Polystigmataceae
Parasitic on the host plant. Sexual morph: see Cannon (1996). Asexual morph:Conidiomata yellow-orange, pseudostromatic, pycnidial, scattered to gregarious, immersed, globose in section view, unilocular to multilocular or convoluted, glabrous. Ostiole single to each locule, circular, depressed, centrally located. Conidiomatal wall composed of thin-walled, hyaline cells of textura angularis. Conidiophores formed from the inner wall layer of the conidiomata, hyaline, cylindrical to subcylindrical, straight or slightly curved, branched, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, sympodial to synchronous, cylindrical or irregular, integrated or discrete, indeterminate, smooth-walled, with several irregular nodulose conidiogenous loci towards apices. Conidia hyaline, filiform, hamate, tapered towards the apices, unicellular, smooth-walled, guttulate (adapted from Sutton 1980).
Type species: Polystigma rubrum (Pers.) DC., in de Candolle & Lamarck, Fl. franç., Edn 3 (Paris) 5/6: 164 (1815)
= Polystigmina rubra (Pers.) Sacc., Syll. fung. (Abellini) 3: 622 (1884)
Notes: Polystigmina rubra is the type species of Polystigmina. Species of Polystigmina has been linked to sexual morph in Polystigma (Sutton 1980; Cannon 1996; Maharachchikumbura et al. 2015; Réblová et al. 2016; Dayarathne et al. 2017). The correlation between these two genera has been confirmed by inoculation tests, field observations and DNA sequence (Suzuki et al. 2008; Habibi and Banihashemi 2016). Polystigmina is reduced to a synonym of Polystigma, as the later has priority and is of economic importance (Réblová et al. 2016; Wijayawardene et al. 2017b). The asexual morph of Polystigma has hyaline, hamate conidia, which can be confused with asexual morph of Tryblidiopsis pinastri (= Tryblidiopycnis pinastri). However, the latter is distinguished by its black conidiomata and sympodial conidiogenous cells that are percurrent, rather than proliferate sympodially.
The genus Polystigma is polyphyletic, with species clustering in Phyllachorales, Trichosphaeriales and Xylariales (Habibi et al. 2015; Mardones et al. 2017). Dayarathne et al. (2017) designated a reference specimen for P. rubrum, and placed Polystigma in Polystigmataceae (Xylariales) with descriptions, illustrations and molecular data. Polystigma species are serious leaf pathogens, causing almond red leaf blotch on almonds, Prunus cerasifera, and P. ssiori (Rosaceae) (Suzuki et al. 2008; Habibi and Banihashemi 2016; Dayarathne et al. 2017).
Distribution: Worldwide (Farr and Rossman 2016)
Pragmopora A. Massal., Framm. Lichenogr.: 12 (1855)
= Pragmopycnis B. Sutton & A. Funk, Can. J. Bot. 53(6): 522 (1975)
Facesoffungi number: FoF 07512, Fig. 292
Pragmopora pithya (asexual morph, redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Leotiomycetes, Leotiomycetidae, Leotiales, Tympanidaceae
Saprobic on the host plant. Sexual morph: see Massalongo (1855). Asexual morph:Conidiomata black, stromatic, pycnidial, solitary, superficial, short stipitate, subglobose, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of the apical wall. Conidiomatal wall composed of thick-walled, dark brown to black cells of textura angularis. Conidiophores arising from inner layers of basal stroma, hyaline, irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, polyphialidic, cylindrical to lageniform, integrated, indeterminate, smooth-walled. Conidia hyaline, fusiform to allantoid, unicellular, smooth-walled, guttulate (adapted from Sutton 1980).
Type species: Pragmopora amphibola A. Massal., Framm. Lichenogr.: 13 (1855)
Notes: Pragmopycnis isa monotypic genus and its single species, P. pithya was linked to the sexual morph Pragmopora pithya (Fr.) Groves (Sutton and Funk 1975). Johnston et al. (2014) synonymized Pragmopycnis under Pragmopora, and recommended to use Pragmopora for the holomorph, because it has priority and is most frequently cited.
The stipitate conidiomata in the asexual morph of Pragmopora led to a comparison with Tympanis (= Sirodothis). Pragmopora has solitary pycnidia, ramifying conidiophores, and polyphialidic conidiogenous cells with at least 15 conidia, whereas Tympanis has aggregated stromata, multiseptate, sparingly branched conidiophores and monophialidic conidiogenous cells (Sutton and Funk 1975; Sutton 1980). Moreover, the conidia of Pragmopora tend to be allantoid, which is similar to Cytospora, rather than cylindrical as in Tympanis. Molecular sequences of the asexual morph of Pragmopora are not available. It is necessary to obtain fresh material to confirm the connection between Pragmopora and Pragmopycnis.
Distribution: northern hemisphere (Wijayawardene et al. 2017)
Pragmopora pithya (Fr.) J.W. Groves, Can. J. Bot. 45: 176 (1967)
= Pragmopycnis pithya B. Sutton & A. Funk, Can. J. Bot. 53(6): 522 (1975)
Habit on twigs of Pseudotsuga menziesii (Pinaceae) (Sutton and Funk 1975). Sexual morph: see Sutton and Funk (1975). Asexual morph:Conidiomata 200–500 µm diam., brown, stromatic, pycnidial, solitary, sparse, superficial, short stipitate, subglobose, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of the apical wall. Conidiomatal wall composed of thick-walled, dark brown to black cells of textura angularis in the exterior, becoming thinner, paler towards conidial hymenium. Conidiophores arising mainly from inner layers of basal stroma, hyaline, irregular, branched irregularly and ramifying throughout the locule, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, polyphialidic, cylindrical to lageniform, integrated, indeterminate, smooth-walled, terminal or intercalary, with minute apertures and collarettes. Conidia 3–4 × 1 µm, hyaline, fusiform to allantoid, unicellular, smooth-walled, guttulate (adapted from Sutton and Funk 1975).
Proboscispora Punith., Nova Hedwigia 39(1–2): 63 (1984)
(non Proboscispora S.W. Wong & K.D. Hyde, 1999)
Facesoffungi number: FoF 07531, Fig. 293
Proboscispora manihotis (redrawn from Nag Raj 1993) a Vertical section of conidioma. b Tissue details of the conidiomatal wall. c Microconidiogenous cells and microconidia. d Macroconidia. e Macroconidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on Manihot esculenta (Euphorbiaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, scattered to gregarious, deeply immersed with only the papillate neck visible in surface view, ovoid to subglobose in section view, unilocular, glabrous, ostiolate. Ostiole single, circular or oval, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura intricata in the exterior, thin-walled, hyaline cells of textura angularis in the conidial hymenium, becoming thick-walled, dark brown to black in the ostiolar region. Conidiophores arising from the inner wall layer of conidiomata, hyaline, moniliform, short, sparsely septate and branched, smooth-walled, invested in mucus.Conidiogenous cells hyaline, holoblastic smooth-walled, of two kinds; macroconidiogenous cells doliiform to short-cylindric or moniliform, integrated or discrete, determinate, lining the base and most of the side walls; microconidiogenous cells ampulliform to short-cylindric, lining the ostiolar channel. Macroconidia hyaline, cylindrical to ellipsoidal, with truncate base, 0–1-septate, smooth-walled, guttulate, enclosed in a thin-walled, tubular sheath, which extends at the apex into a conical or attenuated, unbranched appendage. Microconidia hyaline, globose to subglobose, unicellular, smooth-walled (adapted from Nag Raj 1993).
Type species: Proboscispora manihotis Punith., Nova Hedwigia 39(1–2): 64 (1984)
Notes: Proboscispora was introduced for a coelomycetous fungus, P. manihotis on Manihot esculenta (Euphorbiaceae) in Papua New Guinea (Punithalingam 1984). Wong and Hyde (1999) introduced Proboscispora to accommodate an ascomycetous fungus, P. aquatica S.W. Wong & K.D. Hyde collected on wood submerged in streams in Australia and the Philippines. Because Proboscispora Punith. (1984) has priority, Proboscispora S.W. Wong & K.D. Hyde (1999) is therefore illegitimate. Proboscispora aquatica was later transferred to a newly named genus Pseudoproboscispora Punith. (Punithalingam 1999; Campbell et al. 2003).
Proboscispora remains monotypic. There are morphological similarities between Idiocercus and Proboscispora with respect to their pycnidial, subglobose conidiomata with darker wall cells towards ostiolar region, and hyaline macroconidia with apical appendage, as well as hyaline, globose to subglobose, unicellular microconidia (Nag Raj 1993). The differences are in conidia septation, conidiophores and conidiogenous cells. The cylindrical to ellipsoidal, 0–1-septate conidia, moniliform conidiophores and holoblastic conidiogenous cells in Proboscispora are in contrast to the clavate to ellipsoid, unicellular conidia, and enteroblastic, annellidic conidiogenous cells in Idiocercus. The conidiophores of Idiocercus are also reduced to conidiogenous cells. These two genera have not been studied with molecular data. Future collections are needed to place them in a natural group and to link to their sexual morph.
Distribution: Papua New Guinea (Punithalingam 1984).
Pseudobasidiospora Dyko & B. Sutton, Nova Hedwigia 29(1–2): 168 (1978) [1977]
Facesoffungi number: FoF 07532
Ascomycota, genera incertae sedis
Foliicolous. Sexual morph: undetermined. Asexual morph:Conidiomata pycnidial, amphigenous, solitary to gregarious, subepidermal, deeply immersed, with only the rostrate neck emerging well above the leaf surface, flask-shaped or rounded, globose to subglobose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, centrally located, with a conical to subcylindrical neck. Conidiomatal wall composed of textura globulosa to textura angularis, with large, thick-walled and dark brown to hyaline cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, ampulliform to lageniform with long narrow neck, discrete, smooth-walled. Conidia hyaline, naviculate, unicellular, smooth-walled, guttulate, bearing a polar, funnel-shape or irregular mucoid appendage.
Type species: Pseudobasidiospora caroliniana Dyko & B. Sutton, Nova Hedwigia 29(1–2): 169 (1978) [1977]
Notes: The monotypic genus Pseudobasidiospora is characterized by pycnidioid, mostly immersed conidiomata with only the rostrate neck emerging above the substrate. The conidiogenous cells usually have a doliiform base. Conidia are naviculate, bearing a mucoid appendage at each end, and formed eccentrically on the tip of filiform conidiogenous cells (Dyko 1979; Campbell 1979; Nag Raj 1993). No sexual morph has been link to this genus and no molecular data is available.
Distribution: Canada, USA (Nag Raj 1993).
Pseudobasidiospora caroliniana Dyko & B. Sutton, Nova Hedwigia 29(1–2): 169 (1978) [1977]
Facesoffungi number: FoF 07533, Fig. 294
Pseudobasidiospora caroliniana (DAOM 215283) a–c Herbarium package and specimen. d, i, j Conidiomatal wall. e, f Rostrate neck emerging well above the leaf surface. g, h Vertical sections of conidiomata. k–n Conidiogenous cells and developing conidia. o–u Conidia. Scale bars d, i, j = 20 µm, e–f = 200 µm, g–h = 50 µm, k–n = 10 µm, o–u = 5 µm
Phylogenetic tree generated from a maximum parsimony analysis based on a concatenated alignment of LSU and ITS sequences data representing Pseudocoleophoma and other genera in Dictyosporiaceae. Twenty-one strains are included in the analyses, which comprise 1348 characters including gaps (LSU: 1-804, ITS: 808-1348). Single gene analyses were carried out and compared with each species, topology of the tree, and clade stability. Periconia igniaria CBS 379.86 and P. igniaria CBS 845.96 are used as the outgroup taxa. The tree topology of the maximum likelihood analysis is similar to either the maximum parsimony or the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 6403.070805 is presented. The matrix had 428 distinct alignment patterns, with 7.96% of undetermined characters or gaps. Estimated base frequencies were: A = 0.239455, C = 0.235655, G = 0.280944, T = 0.243945; substitution rates AC = 1.619880, AG = 2.365441, AT = 2.177466, CG = 0.447534, CT = 6.044894, GT = 1.000000; gamma distribution shape parameter α = 0.171874. The maximum parsimonious dataset consisted of constant 968, 291 parsimony-informative and 89 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 982 steps (CI = 0.601, RI = 0.675, RC = 0.406, HI = 0.399) in the first tree. Maximum parsimony (MPBS) and maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“-”) indicates a value lower than 50% for MP BS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.03 changes. Ex-type strains are in bold. New isolates are shown in and highlighted with colour
Saprobic on leaf litter of Acer sp. (Aceraceae). Sexual morph: undetermined. Asexual morph:Conidiomata 110–160 diam., 100–170 µm high, pycnidial, amphigenous, solitary to gregarious, subepidermal, deeply immersed, with only the rostrate neck emerging well above the leaf surface, flask-shaped or rounded (Fig. 294e, f), globose to subglobose, unilocular, glabrous, thick-walled, ostiolate. Ostiole 30–70 × 40–50 µm, single, centrally located, with a conical to subcylindrical neck. Conidiomatal wall 15–50 µm wide, composed of an outer textura globulosa to textura angularis, with large, thick-walled and dark brown to pale brown cells, and inner textura angularis, with smaller and compact, thick-walled, hyaline cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, consisting of two parts, base 3–7 × 1–4 µm, ampulliform to lageniform or rounded, bulging, upper half 10–20 × 1–2 µm, cylindrical to subcylindrical, narrowed, smooth-walled, arising from the innermost wall layer of conidiomata. Conidia 10–15 × 3–5 µm (\( \bar{x} \) = 12 × 4 µm; n = 50), hyaline, naviculate with slightly apiculate ends, aseptate, smooth-walled, guttulate, bearing mucoid appendage(s) at each end, or occasionally at one end only; appendages initially funnel-shape, later arising by eversion and subsequent irregular splitting of a mucoid sheath enveloping the developing conidia.
Material examined: Canada, Ontario, Waterloo, Laurel Creek Conservation area, on Acer sp. leaf litter from bottom of dried up pond, September 1979, J. Michaelides (DAOM 215283).
Pseudocoleophoma Kaz. Tanaka & K. Hiray., in Tanaka et al., Stud. Mycol. 82: 89 (2015)
Facesoffungi number: FoF 07534
Dothideomycetes, Pleosporomycetidae, Pleosporales, Dictyosporiaceae
Saprobic on the host plant or on submerged stems in freshwater or isolated from soil (De Gruyter et al. 2013; Tanaka et al. 2015; Hyde et al. 2016). Sexual morph: see Tanaka et al. (2015). Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious or confluent, immersed to erumpent, globose to subglobose or ovoid, unilocular, glabrous, ostiolate. Ostiole single, cylindrical to sybcylindrical or obconic, centrally located. Conidiomatal wall composed of thick-walled, dark brown to brown then hyaline cells of textura angularis to textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform, lageniform to subcylindrical, determinate, smooth-walled, formed in the inner cavity of conidiomata. Conidia hyaline, cylindrical to subcylindrical or fusiform, unicellular, smooth-walled (Fig. 295).
Type species: Pseudocoleophoma calamagrostidis Kaz. Tanaka & K. Hiray., in Tanaka et al., Stud. Mycol. 82: 89 (2015)
Notes: Pseudocoleophoma was proposed by Tanaka et al. (2015) to accommodate P. calamagrostidis Kaz. Tanaka & K. Hiray. on dead leaves of Calamagrostis matsumurae and P. polygonicola Kaz. Tanaka & K. Hiray. on dead stems of a polygonaceous plant. The genus is characterized by immersed to erumpent, globose to depressed globose ascomata, fissitunicate, 8-spored, pedicellate asci and fusiform, 1-septate ascospores with a conspicuous sheath, pycnidial, subglobose conidiomata with a long, distinct ostiole, phialidic, doliiform to lageniform conidiogenous cells, and hyaline, cylindrical conidia (Tanaka et al. 2015). Hyde et al. (2016) added an additional species P. typhicola Kamolhan, Banmai et al. on submerged stems of Typha latifolia (Typhaceae) in freshwater from the UK. In this study, strain (MFLUCC 16-1444) collected from Ruscus sp. in Italy is introduced as a new species P. rusci on the basis of both morphology and phylogeny (see notes below). In addition, Paraconiothyrium flavescens, introduced by de Gruyter et al. (2013) based on Phoma flavescens Gruyter, Noordel. & Boerema clustered with Pseudocoleophoma species with high bootstrap support (99Ml/98Mp/1BB). Thus, a new combination Pseudocoleophoma flavescens is introduced to accommodate this species. Five species are accepted in Pseudocoleophoma.
Distribution: Italy, Japan, Netherlands, UK (de Gruyter et al. 2013; Tanaka et al. 2015; Hyde et al. 2016; this study).
Pseudocoleophoma flavescens (Gruyter, Noordel. & Boerema) W.J. Li & K.D. Hyde, comb. nov.
≡ Phoma flavescens Gruyter, Noordel. & Boerema, Persoonia 15(3): 375 (1993)
= Paraconiothyrium flavescens (Gruyter, Noordel. & Boerema) Verkley & Gruyter, in Gruyter et al., Stud. Mycol. 75: 25 (2012) [2013]
Index Fungorum number: IF557175, Facesoffungi number: FoF 07536
Habit: isolated of a soil sample. Sexual morph: undetermined. Asexual morph:Conidiomata 20–140 µm diam., pycnidioid, solitary or confluent, globose, with a rather indistinct, non-papillate ostiole, glabrous or covered by hyphae. Conidia 4–7 × 2–3.5 µm hyaline, ellipsoidal with 2 very large polar guttules, unicellular, thick-walled, smooth-walled (Boerema et al. 2004).
Pseudocoleophoma rusci W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF557161, Facesoffungi number: FoF 07537, Fig. 296
Pseudocoleophoma rusci (MFLU 16-0292, holotype) a Herbarium package and specimens. b, c Appearance of brown to dark brown conidiomata on the host. d–f Vertical sections of conidiomata. g–i Section of peridium. j–m Conidiogenous cells and developing conidia. n Germinating conidium. o Conidia. p, q Culture on PDA . Scale bars b = 500 µm, c = 200 µm, d–f = 100 µm, g, i, n = 20 µm, h = 50 µm, j–m, o = 5 µm
Etymology: Referring to the host from which it was isolated, Ruscus.
Saprobic on dead leaves of Ruscus aculeatus (Asparagaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 130–200 µm diam., 250–330 µm high, brown, pycnidial, solitary to gregarious, deeply immersed, with only the rostrate neck emerging well above the leaf surface, globose, subglobose or ovoid, unilocular, glabrous, thick-walled, ostiolate. Ostiole 110–180 × 60–80 µm, flask-shaped, sybcylindrical, obconic, centrally located. Conidiomatal wall 10–70 µm wide, composed of relatively thick-walled, brown to dark brown cells of textura angularis in the outer layers, merging with hyaline cells in the inner layers at upper part, comprising thick-walled, hyaline cells of textura prismatica at basal and lateral part. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–9 × 3–7 µm, hyaline, enteroblastic, phialidic, doliiform, ampulliform to subcylindrical, determinate, smooth-walled, and formed from the inner wall layer of conidiomata. Conidia 8–14 × 3–6 µm (\( \bar{x} \) = 10 × 4 µm; n = 30), hyaline, cylindrical to subcylindrical or fusiform, with a rounded apex and a slightly narrow truncate base, straight or slightly curved, unicellular, thick- and smooth -walled.
Culture characters: Colonies on PDA, reaching 5 mm diam. after 14 d at 25–30 °C, pale brown to brown, flattened, dense, with undulate margin, reverse dark brown, with white margins.
Material examined: Italy, Province of Forlì-Cesena, Forlì, Farazzano, on dead aerial cladodes of Ruscus aculeatus (Asparagaceae), 11 January 2016, Erio Camporesi, IT2781 (MFLU 16-0292, holotype), living culture MFLUCC 16-1444 = KUMCC 16-0076, (KUN, HKAS 97472, isotype).
Notes: In the phylogenetic tree reconstruction based on ITS and LSU sequence data, P. rusci clustered with P. calamagrostidis, P. polygonicola, P. typhicola and P. flavescens with high bootstrap support (99ML/98MP/1BB). In addition, P. rusci formed a separate branch below P. calamagrostidis and P. polygonicola (Fig. 295). Morphologically, P. rusci shares similar characters with the generic type species in having pycnidial, globose conidiomata with long, cylindrical ostioles, phialidic conidiogenous cells and hyaline cylindrical conidia, but it is distinguished from the type species and other Pseudocoleophoma taxa by its conidia dimensions. Pseudocoleophoma rusci has slightly longer and wider conidia than P. calamagrostidis (av. 8.6 × 2.2 μm), shorter and wider conidia than P. polygonicola (av. 14.4 × 3.4 μm), and wider conidia than P. typhicola (av. 10 × 3 μm). Thus, P. rusci is introduced as a new species.
Pseudolachnea Ranoj., Ann Mycol. 8(3): 393 (1910)
Facesoffungi number: FoF 07538
Sordariomycetes, Sordariomycetidae, Chaetosphaeriales, Chaetosphaeriaceae
Saprobic on angiosperms and monocots (Nag Raj 1993). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, superficial or subcuticular, scattered or gregarious, at first closed, later opening wide to become shallow-cupulate with incurved margins, flattened, unilocular, setose. Conidiomatal wall consisting of basal stroma and excipulium; basal stroma composed of thick-walled, brown cells of textura angularis in the outer layers, becoming hyaline cells of textura porrecta towards conidial hymenium; excipulum well developed, composed of thick-walled, dark brown to hyaline cells of textura porrecta, giving rise to numerous setae. Conidiomatal setae restricted to the margin of the conidiomata, dark brown, subulate with blunt or acute apices, incurved or divergent, unbranched, smooth-walled. Conidiophores arising from the uppermost cells of the basal stroma and the inner cells of the excipulum, hyaline, cylindrical, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, usually integrated, smooth-walled. Conidia hyaline, fusiform to naviculate, usually curved, 1-euseptate, thick- and smooth-walled, guttulate, bearing an unbranched, short, cellular, filiform appendage at each end.
Type species: Pseudolachnea hispidula (Schrad.) B. Sutton, Mycol. Pap. 141: 167 (1977)
Notes: The similarities and differences between Pseudolachnea and Pseudolachnella were discussed under notes of Pseudolachnella. Wijayawardene et al. (2017b) estimated five species in Pseudolachnea, of which only P. fraxini Crous and P. hispidula were studied by molecular data (Crous et al. 2012b; Hashimoto et al. 2015b). A fresh collection on Clematis vitalba in Italy shows similar morphology and phylogeny, as well as conidial dimensions with P. hispidula, and thus it is conspecific with P. hispidula. Pseudolachnea hispidula has been reported from Abies sp. (Pinaceae), Morus bombycis (Moraceae), Phytolacca sp. (Phytolaccaceae), Robinia pseudoacacia (Fabaceae), Salix sp. (Salicaceae), and Sorghum halepense (Poaceae). Our collection is therefore regarded as a new host record.
Distribution: Canada, Czechoslovakia, Italy, Japan, USA, Sweden (Nag Raj 1993; Crous et al. 2012b; Hashimoto et al. 2015b, this study).
Pseudolachnea hispidula (Schrad.) B. Sutton, Mycol. Pap. 141: 167 (1977)
= Pseudolachnea bubakii Ranoj., Ann Mycol. 8(3): 393 (1910)
Facesoffungi number: FoF 07539, Fig. 297
Pseudolachnea hispidula (MFLU 19-2863) a Herbarium specimen. b, c Appearance of black coniodiomata on the host. d Vertical section of conidioma. e, f Vertical section of peridium. g–i Conidiophores, conidiogenous cells and developing conidia. j–l Conidia. m–n Culture. Scale bars b = 200 µm, c = 500 µm, d = 100 µm, e–f = 20 µm, g–i = 10 µm, j–l = 5 µm, m–n = 10 mm
Saprobic on aerial branches of Clematis vitalba. Sexual morph: undetermined. Asexual morph:Conidiomata 130–150 µm high, 100–140 µm diam., dark brown to black, superficial or subcuticular, scattered or gregarious, initially closed, ultimately opening wide and appearing shallow-cupulate, unilocular, setose. Ostiole absent, dehiscence by rupture of the apical wall. Conidiomatal wall composed of thick-walled, brown cells of textura angularis at the periphery, becoming hyaline cells of textura porrecta towards conidial hymenium, and extending to textura porrecta in the excipulum. Conidiomatal setae up to 70 µm long, restricted to the outer wall, at first incurved over the conidiogenous fertile region, later becoming divergent, dark brown at the base, becoming paler towards apex, subulate with blunt or acute apices, smooth-walled. Conidiophores arising from the uppermost cells of the basal stroma and inner wall layer of the excipulum, hyaline, cylindrical, branched at the base, smooth-walled. Conidiogenous cells 10–25 × 1–2 µm, hyaline, enteroblastic, phialidic, cylindrical, usually integrated, smooth-walled, with periclinal wall-thickening at the collarette zone. Conidia 12–15 × 1.5–2.3 µm (\( \bar{x} \) = 13.5 × 2 µm; n = 20), hyaline, fusiform to naviculate, obtuse at both ends, 1-septate, thick and smooth-walled, guttulate, bearing an unbranched, short, cellular, filiform apical appendage and an excentric basal appendage.
Culture characters: Colonies on PDA, reaching 10 mm diam. after 7 days at 25 °C, circular, white, flattened, felt-like, sparse, aerial, surface, with entire edge; reverse cinnamon in the middle area, white in edge.
Material examined: Italy, Province of Forlì-Cesena, Civitella di Romagna, Voltre, on dead aerial branches of Clematis vitalba (Ranunculaceae), 2 December 2013, Erio Camporesi, IT1553 (MFLU 19-2863), living culture MFLUCC 15-0583.
Pseudolachnella Teng, Sinensia, Shanghai 7: 775 (1936)
Facesoffungi number: FoF 07542
Sordariomycetes, Sordariomycetidae, Chaetosphaeriales, Chaetosphaeriaceae
Saprobic on bamboo hosts, such as Phyllostachys nigra var. henonis, Phyllostachys sp., Pleioblastus hindsii, P. linearis, P. simonii, Sasa kurilensis, Sinobambusa tootsik (Poaceae) (Nag Raj 1993; Hashimoto et al. 2015b). Sexual morph: undetermined. Asexual morph: Conidiomata dark brown to black, stromatic, cupulate, solitary to gregarious, or confluent, initially closed and pulvinate, conical to subcupulate, later opening wide to become shallow-cupulate with incurved margins, unilocular, setose. Conidiomatal wall composed of basal stroma and excipulium; basal stroma of textura angularis to textura intricata or textura epidermoidea with thick-walled, brown cells; excipulum well developed, of textura porrecta with thick-walled and dark brown to hyaline cells, giving rise to numerous setae. Conidiomatal setae restricted to the margin of the conidiomata, at first incurved over the conidial hymenium, then divergent, cylindrical, straight or slightly curved, unbranched, aseptate, becoming progressively paler towards the interior, thick-walled, acute and narrowed at apex. Conidiophores arising from the innermost layers of basal stroma and excipulum, hyaline, cylindrical to subcylindrical, broader at base, branched. Conidiogenous cells hyaline, enteroblastic, phialidic or annelidic, cylindrical to lageniform, indeterminate, discrete or integrated, smooth, thick-walled. Conidia hyaline, fusiform to naviculate or cylindrical, 3–7-septate, straight or curved, guttulate, smooth-walled, bearing flexuous, branched or unbranched, centric or excentric, single or multi-appendages, arising from the top of the apical cell or adjacent to apical and basal cell.
Type species: Pseudolachnella scolecospora (Teng & C.I. Chen) Teng, Sinensia, Shanghai 7: 775 (1936)
Notes: Pseudolachnella was proposed by Teng (1936) for Pseudolachnella scolecospora (Teng & C.I. Chen) Teng, a segregated species from Pseudolachnea Ranoj. The genus is characterized by cupulate, setose conidiomata and multi-septate conidia, and its host specific habitat (occurring on bamboo host only) (Nag Raj 1993; Hashimoto et al. 2015b). Pseudolachnella can be easily confused with Pseudolachnea, since both genera have cupulate, conidiomata with numerous, black setae. Sutton (1977a, 1980) regarded these two as congeneric and synonymized Pseudolachnella under Pseudolachnea. However, Nag Raj separated Pseudolachnella from Pseudolachnea on the basis of the conidial septation (1-sepate in Pseudolachnea vs multi-sepate in Pseudolachnella). This generic concept was followed by Zhao et al. (2004) and Sato et al. (2008). In addition, they described two new species, P. vermospora R.L. Zhao, et al. and P. yakushimensis G. Sato et al. Recently, Hashimoto et al. (2015b) revised Pseudolachnea and Pseudolachnella based on molecular data and morphology, and concluded that differences in conidiomata structure (thickness of basal stroma and excipulum) between these genera are more reliable indicators of evolutionary relationship than conidial septation. In addition, eight species were described from bamboo host in Pseudolachnella. Li et al. (2016) added an additional species P. brevifusiformis A. Hashim. & Kaz. Tanaka from a dead sheath of Pleioblastus linearis (Hack.) Nakai. Pseudolachnella guaviyunis Marinc. et al. (Crous et al. 2014b) was excluded from the genus, based on its distinct phylogeny and morphology (pale brown smooth to verruculous conidia). We re-examined several type species of Pseudolachnella which are available, and found that the conidia of P. brevicoronata (62–90.5 × 2–3 µm (\( \bar{x} \) = 72.5 × 2.3 µm; n = 50) fall into the variation of P. coronata 57–95 × 2–3 µm (\( \bar{x} \) = 81 × 2.4 µm; n = 50). Moreover, they share similar form of conidiomata, conidiogenous cells and conida, as well as dimensions of conidiomatal wall (20–30(–37.5) µm wide in P. brevicoronata vs 19–42 µm in P. coronata) and conidiogenous cells (8.5–15 × 1.5–2 µm in P. brevicoronata vs 7–16 × 1–2 µm in P. coronata). Pseudolachnella brevicoronata is therefore regarded as conspecific with P. coronata. Sixteen species are accepted in the genus, viz. P. asymmetrica A. Hashim et al., P. botulispora A. Hashim. et al., P. brevifusiformis A. Hashim et al., P. campylospora A. Hashim. et al., P. complanata A. Hashim. et al., P. corona ta, P. falcatispora A. Hashim et al., P. fusiformis A. Hashim.et al., P. indica (V.G. Rao & Varghese) Nag Raj, P. longiciliata (I. Hino & Katum.) Nag Raj, P. pachyderma A. Hashim. et al., P. ryukyuensis (I. Hino & Katum.) Nag Raj, P. scolecospora (Teng & C.I. Chen) Teng, P. setulosa (I. Hino & Katum.) Nag Raj, P. vermospora, P. yakushimensis.
Distribution: China, India, Japan (Nag Raj 1993; Hashimoto et al. 2015b, this study).
Pseudolachnella brevifusiformis A. Hashim. & Kaz. Tanaka, in Li et al., Fungal Diversity 78: [74] (2016)
Facesoffungi number: FoF 02029, Fig. 298
Pseudolachnella brevifusiformis(HHUF 30495, holotype, HHUF 30496, paratype) a–c Herbarium package and specimens. d, e Appearance of black conidiomata on the host. f Conidiomatal setae. g Vertical section of conidioma. h, i Section of peridium. j–m Conidiophores, conidiogenous cells and developing conidia, n–s Conidia. Scale bars f–g = 100 µm, h–i = 20 µm, j–s = 5 µm
Saprobic on dead sheath of Pleioblastus linearis. Sexual morph undetermined. Asexual morphConidiomata 230–900 µm diam., 170–350 µm high, stromatic, solitary or gregarious, initially conical, then subcupulate, subglobose to oval, unilocular, dark brown to black, setose. Conidiomatal setae up to 100 µm long, numerous, restricted to the margin of the conidiomata, at first incurved over the conidial hymenium, then divergent, cylindrical, straight or slightly curved, unbranched, aseptate, pale brown to dark brown, thick-walled, acute and narrowed at apex. Conidiomatal wall 10–35 µm wide, composed of thick-walled, brown cells of textura intricata to textura epidermoidea. Conidiophores 7.5–14 × 1.6–14 µm, formed from inner layers of the conidiomata, hyaline, cylindrical to subcylindrical, broader at base, branched, smooth-walled. Conidiogenous cells 7–2 × 2–4 µm, hyaline, enteroblastic, phialidic, cylindrical to lageniform, with periclinal thickening in the collarette zone, indeterminate, usually integrated, thick- and smooth-walled. Conidia 3–15 × 3–4.5 µm (\( \bar{x} \) = 12 × 4 µm; n = 30), hyaline, circular or cylindrical, rounded at apex, truncate and narrow at base, 0–3-septate, straight, guttulate, smooth, bearing appendages at both ends; apical appendage 3–6 µm long, 2–6-unbranched, flexuous, centric or excentric, arising from the top of the apical cell or adjacent to apical cell, basal appendage 3–6 µm long, 2–4-unbranched.
Material examined: Japan, Okinawa, Kunigami, Yona, Mt. Fuenchiji, on dead sheath of Pleioblastus linearis, 19 May 2015, K. Tanaka, A. Hashimoto, M. Matsumura, KT 3536 (HHUF 30495, holotype); KT 3537 (HHUF 30496, paratype).
Pseudolachnella coronata (I. Hino & Katum.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 725 (1993)
≡ Chaetopatella coronata I. Hino & Katum., J. Jap. Bot. 33: 239 (1958)
Facesoffungi number: FoF 07543, Fig. 299
Pseudolachnella coronata (DAOM 215567, type) a, b Herbarium package and specimen. c, d, f Appearance of dark brown to black conidiomata on the host. e, j–m, o Conidiophores, conidiogenous cells and developing conidia. g–h Vertical sections of conidiomata. i Section of peridium. n Setae. p–u Conidia. Scale bars c–d, f = 200 µm, e, i, n = 20 µm, g–h = 50 µm, j–l, o–u = 10 µm, m = 5 µm
Foliicolous, occurring on leaf sheaths. Sexual morph: undetermined. Asexual morph:Conidiomata 140–230 diam., 90–230 µm high, dark brown to black, cupulate, cylindrical or conical in outline, mostly solitary, occasionally gregarious, superficial, subcupulate in section view, unilocular, setose. Conidiomatal setae dark brown for the most part, paler towards the apex, cylindrical with narrow and obtuse apex, septate at upper half or unevenly distributed, often constricted at septum, thick- and smooth-walled, restricted to the periphery of the conidiomata, at first incurved over the conidial hymenium, then divergent. Conidiomatal wall 20–40 µm wide, composed of thick-walled, dark brown to almost black cells of textura intricata to textura angularis in the lower and lateral parts, and gradually merging with thin-walled, pale brown cells of textura angularis towards the conidial hymenium. Conidiophores arising from innermost layers of basal wall, hyaline, cylindrical to subcylindrical, irregularly branched, septate, smooth-walled. Conidiogenous cells 7–16 × 1–2 µm, hyaline, enteroblastic, lageniform to subcylindrical, discrete or integrated, indeterminate, with marked periclinal thickenings in the collarette zone (Fig. 299l). Conidia 57–95 × 1.7–3.4 µm (\( \bar{x} \) = 81 × 2 µm; n = 50), hyaline, subcylindrical, gradually tapering towards apex, narrow and truncate at base, straight or slightly curved, 7–12-septate, with or without slight constrictions at the septa, smooth-walled, bearing appendages at both ends; apical appendages mostly 2 or 3, occasionally 4, dichotomously, trichotomously or irregularly branched, branches 6–12 µm long; basal appendages single, excentric, branched to 3–5 branches (2–6 µm long).
Material examined: Japan, Kyushu, Miyazaki,Takaharu-Tyo, on leaf sheaths of Pleioblastus hindsii (Poaceae), 10 May 1958, H. Kato, (DAOM 215567, part of type of Chaetopatella coronata).
Pseudolachnella longiciliata (I. Hino & Katum.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 725 (1993)
≡ Chaetopatella longiciliata I. Hino & Katum., J. Jap. Bot. 33: 238 (1958)
Facesoffungi number: FoF 07544, Fig. 300
Pseudolachnella longiciliata (DAOM 215568, type) a–c Herbarium package and specimens. d, f–h Appearance of black conidiomata on the host. e Setae. i–j Vertical sections of conidiomata. k–l, n–o Conidiophores, conidiogenous cells and developing conidia. m Section of peridium. p–s Conidia. Scale bars d = 500 µm, f–h = 200 µm, i–j = 100 µm, e = 50 µm, k–l, n–s = 10 µm, m = 20 µm
Caulicolous. Sexual morph: undetermined. Asexual morph:Conidiomata 200–360 diam., 95–230 µm high, dark brown to black, cupulate, oval to elongate or cylindrical or conical in outline, solitary to gregarious, superficial, broadly conical in sectional view, unilocular, thick-walled, setose, ostiolate. Ostiole single, laterally located, with subcylindrical, long neck. Conidiomatal setae up to 150 µm long, 3–5 µm wide, dark brown at base, becoming paler above, subcylindrical, with an acute apex, unbranched, septate, thick- and smooth-walled, arising from the basal part and lateral excipulum. Conidiomatal wall 20–40 µm wide, composed of two types of cells; (a) basal stroma of textura angularis to textura intricata, with thick-walled, dark brown cells in lower layers, gradually merging with thin-walled, pale brown cells of textura intricata near the conidial hymenium; (b) excipulum of textura prismatica, with thick-walled and dark brown to brown cells in the external layers, thin-walled, hyaline in the inner layers. Conidiophores arising from innermost layer of basal wall and lateral excipulum, hyaline, subcylindrical, irregularly branched, septate, thick- and smooth-walled. Conidiogenous cells 7–20 × 2–4 µm, hyaline, enteroblastic, phialidic, lageniform to subcylindrical, usually integrated, indeterminate, with marked periclinal thickenings in the collarette zone (Fig. 300k) and percurrently proliferating 1–2 times (Fig. 300l). Conidia 60–83 × 2–5 µm (\( \bar{x} \) = 73 × 3 µm; n = 30), hyaline, subcylindrical with a tapering apex and a truncate base, straight or undulate, 10–17-septate, slightly constricted at the septa, smooth-walled, guttulate, bearing appendages at both ends or only at one end; apical appendage 10–40 µm long, single, unbranched, filiform, flexuous; basal appendage when present, 4–16 µm long, single, unbranched, filiform, flexuous, eccentric.
Material examined: Japan, Akita, Kitakita-gun, Mt. Nyutozan, on dead stems of Sasa kurilensis (Poaceae), 4 August 1957, H. Muroi (DAOM 215568, part of type of Chaetopatella longiciliata).
Pseudolachnella ryukyuensis (I. Hino & Katum.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 729 (1993)
≡ Chaetopatella ryukyuensis I. Hino & Katum., Bull. Faculty of Agriculture, Yamaguchi University 11: 10 (1960)
Facesoffungi number: FoF 07545, Fig. 301
Pseudolachnella ryukyuensis (DAOM 215569, type) a, b Herbarium package and specimen. c–e Appearance of black conidiomata on the host. f Conidiomatal wall in slide view. g, h Conidiomatal setae. i Conidiogenous cells and developing conidia. j–m Conidia. Scale bars c = 500 µm, d–e = 200 µm, f = 50 µm, g–i = 20 µm, j–m = 10 µm
Caulicolous and foliicolous. Sexual morph: undetermined. Asexual morph:Conidiomata 200–350 diam., 120–200 µm high, dark brown to black, cupulate, circular to oval or elongate, or irregular in outline, solitary to gregarious, superficial, conical in sectional view, unilocular, thick-walled, setose, papillate (Fig. 301d). Conidiomatal setae 100–300 µm long, up to 5 µm wide at the base, narrowing to 2–3.5 µm, dark brown below, becoming paler above, subulate to cylindrical with an acute apex, unbranched, septate, thick- and smooth-walled, arising from the basal part. Conidiomatal wall composed of thick-walled, dark brown to brown cells of textura angularis to textura intricata in the basal part of the outer layers, gradually merging with pale brown to hyaline cells towards the conidial hymenium. Conidiophores arising from innermost layer of basal wall, hyaline, subcylindrical, branched at the base, septate, thick- and smooth-walled. Conidiogenous cells 10–15 × 2.5–3 µm, hyaline, enteroblastic, phialidic, lageniform to subcylindrical, integrated, indeterminate, with marked periclinal thickenings in the collarette zone. Conidia 30–40 × 2.5–3 µm (\( \bar{x} \) = 35.7 × 2.7 µm; n = 30), hyaline, subcylindrical to fusiform, with a blunt or rounded apex, and a narrow and truncate base bearing distinct hilum, straight or slightly curved at the lower half, aseptate or 3–5-septate, slightly constricted at the septa, guttulate, bearing appendages at both ends; apical appendages 4–6 µm long, 1–3, attenuated, flexuous, dichotomously branched; basal appendage 3–4 µm long, single, unbranched, excentric (measurement from Nag Raj (1993), as type specimen is not in good condition).
Material examined: Japan, Okinawa, Okinawa Island, Mt. Yonahadake, on dead culms and leaf-sheaths of Pleioblastus linearis (Poaceae), 26 July 1959, H. Muroi (DAOM 215569, type).
Pseudolachnella setulosa (I. Hino & Katum.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 732 (1993)
≡ Heteropatella setulosa I. Hino & Katum., Bull. Faculty of Agriculture, Yamaguchi University 5: 234 (1954)
Facesoffungi number: FoF 07546, Fig. 302
Pseudolachnella setulosa (DAOM 215570, type) a, b, e Herbarium package and specimen. c, d, f, g Appearance of dark brown to black conidiomata on the host. h Conidiomatal setae. i, l Vertical sections of conidiomata. j–k, m–n Conidiophores, conidiogenous cells and developing conidia (arrow shows periclinal thickenings in the collarette zone). o–s Conidia (arrows show short appendages). Scale bars c–d = 1000 µm, f–g = 200 µm, h = 50 µm, i, l = 200 µm, j–k, m = 10 µm, n–s = 5 µm
Caulicolous, on dead culms. Sexual morph: undetermined. Asexual morph:Conidiomata 500–850 diam., 200–400 µm high, dark brown to black, cupulate, oval to elongate or subcylindrical or rounded in outline, solitary to gregarious or confluent, superficial, subcylindrical to conical with a tip in sectional view (Fig. 302i), unilocular, thick-walled, setose. Conidiomatal setae up to 200 µm long, 4–7 µm wide, dark brown to amber brown in the external part, becoming brown to pale towards conidial hymenium, subulate with acute apex, unbranched, septate, thick-walled with narrow lumen. Conidiomatal wall 10–50 µm wide, composed of basal stroma of textura angularis with thick-walled, brown to almost black cells in the lower layers, lacking excipulum. Conidiophores arising from innermost layer of basal wall, hyaline, subcylindrical, branched, septate, thick- and smooth-walled. Conidiogenous cells 6–10 × 1–3 µm, hyaline, enteroblastic, phialidic, lageniform, usually integrated, indeterminate, with marked periclinal thickenings in the collarette zone (Fig. 302n). Conidia 31–53 × 1.5–3 µm (\( \bar{x} \) = 42 × 2.3 µm; n = 30), hyaline, subcylindrical, with a narrow and obtuse apex, and a truncate base bearing a distinct hilum, mostly 7-septate, occasionally 5–6-septate, with or without constriction at septa, guttulate, smooth-walled, bearing appendages at both ends or only at one end; apical appendage 1–2 µm long, single, filiform, unbranched, basal appendage 1–2 µm long, excentric.
Material examined: Japan, Yamaguti, Simonoseki-si, Tyohu, on dead culms of Pleioblastus simonii (Poaceae), 31 March 1954, K. Katumoto (DAOM 215570, type).
Pseudoneottiospora Faurel & Schotter, Revue Mycol., Paris 29(4): 278 (1965) [1964]
Faces of fungi number: FoF 07540
Ascomycota, genera incertae sedis
Coprophilous on dung of sheep and rabbit (Nag Raj 1993). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, scattered to gregarious, immersed to semi-immersed, globose to subglobose, ovoid, pyriform or irregular in section view, unilocular, glabrous, ostiolate. Ostiole circular or oval, centrally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis to textura globulosa or textura prismatica in basal and lateral part, becoming darker at the ostiolar region. Conidiophores arising from inner wall layer of conidiomata, hyaline, cylindrical to subcylindrical, branched, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, enteroblastic, annellidic, lageniform to cylindrical, integrated or discrete, indeterminate, smooth-walled. Conidia hyaline, cylindrical to fusiform, with narrow truncate base, straight or bent, 1–3-septate, uneven, smooth-walled, guttulate, bearing apical appendages; appendages 2–4, filiform, tubular, flexuous, unbranched, attenuated toward apex (Morgan-Jones 1977; Nag Raj 1993).
Type species: Pseudoneottiospora cunicularia Faurel & Schotter, Revue Mycol., Paris 29(4): 279 (1965) [1964]
Notes: Pseudoneottiospora is a coprophilous genus with conidial appendages, which led to a comparison with Dimastigosporium, Monodia, Pirispora, Pullospora and Quezeua. However, Dimastigosporium was separated from other genera by its stromatic, cupulate, superficial, setose conidiomata. Monodia is distinguished by its pycnidial, setose conidiomata. The type specimens of Pirispora and Quezeua do not exist in any herbaria (Nag Raj 1993). Quezeua has black pycnidial conidiomata without basal tissue but anchored to the substrate by brown mycelium, and hyaline ellipsoid-fusiform, 1-septate conidia with three appendages at each end (Nag Raj 1993). Pirispora differs from these genera by its hyaline, cuneiform, aseptate conidia with a tubular basal appendage, separated from the conidium body by a septum, and three similar, but divergent, appendages maintaining protoplasmic continuity with the conidium body (Nag Raj 1993). Pseudoneottiospora differs from Pullospora by its cylindrical to fusiform, 1–3-septate, uneven conidia with 2–4, tubular, apical appendages.
The type specimen of Pseudoneottiospora cunicularia was destroyed during the Algerian uprising (Morgan-Jones 1977), therefore, the description is based on P. coprophila and P. patouillardii Nag Raj. Pseudoneottiospora coprophila was initially proposed by Breton and Faurel (1967) on the basis of Neottiospora coprophila Speg., but this proposal was not validly published. Nag Raj (1993) considered P. coprophila is valid name and provided a detailed description and illustration. However, the name of Pseudoneottiospora coprophila was not listed in Index Fungorum and MycoBank. Thus we need to include this name to Index Fungorum and MycoBank following Nag Raj’s (1993) comments. No molecular sequence data is available for this genus.
Distribution: Italy, Tunisia (Nag Raj 1993).
Pseudoneottiospora coprophila (Spegazzini) Breton & Faurel apud Nag Raj
Facesofungi number: FoF 07541, Fig. 303
Pseudoneottiospora coprophila (redrawn from Nag Raj 1993) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and conidia
Coprophilous on dung of sheep (Nag Raj 1993). Sexual morph: undetermined. Asexual morph:Conidiomata 110–230 µm wide, 100–220 µm deep, dark brown to black, pycnidial, scattered to gregarious, immersed, ovoid or globose, unilocular, glabrous, ostiolate. Ostiole circular or oval, centrally located. Conidiomatal wall 10–15, composed of thick-walled, dark brown to hyaline cells of textura angularis to textura globulosa in basal and lateral part, becoming darker at the ostiolar region. Conidiophores arising from inner wall layer of conidiomata, hyaline, cylindrical to subcylindrical, branched, septate, smooth-walled,. Conidiogenous cells 7–14 × 1–2 µm, hyaline, enteroblastic, annellidic, subcylindrical to obclavate, usually integrated, indeterminate, smooth-walled. Conidia 13.5–20 × 1.5–2 (\( \bar{x} \) = 17.4 × 1.6) µm, hyaline, obclavate, with a broad apex and narrow, truncate base, straight or curved, 1-septate, uneven, smooth-walled, guttulate, bearing 2–4, filiform, tubular, flexuous, unbranched, apical appendages (adapted from Nag Raj 1993).
Pseudorobillarda M. Morelet, Bull. Soc. Sci. nat. Arch. Toulon et du Var 175: 5 (1968)
Facesoffungi number: FoF 07559
Dothideomycetes, Pleosporomycetidae, Incertae sedis, Pseudorobillardaceae
Caulicolous, foliicolous, humicolous, glumicolous, lichenicolous. Sexual morph: undetermined. Asexual morph:Conidiomata yellowish brown to brown or dark brown, black, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, globose to subglobose, unilocular, glabrous, ostiolate. Ostiole single, circular to subcylindrical, centrally or laterally placed. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis to textura prismatica. Paraphyses hyaline, filiform, septate, branched or unbranched. Conidiophores reduced to conidiogenous cells. invested in mucus. Conidiogenous cells hyaline, enteroblastic, phialidic or annellidic, cylindrical to ampulliform, determinate or indeterminate, thick and smooth-walled, arising all around the cavity of conidioma. Conidia hyaline, subcylindrical to fusiform or ellipsoidal, 0–4-septate, slightly constricted at the septa, guttulate, thick- and smooth-walled, bearing 2–6, flexuous, divergent, extracellular, appendages at one end.
Type species: Pseudorobillarda phragmitis (Cunnell) M. Morelet, Bull. Soc. Sci. nat. Arch. Toulon et du Var 175: 6 (1968)
Notes: The members of Pseudorobillarda are widespread. They have been reported in Argentina, Canada, Cuba, Germany, India, Nigeria, Thailand, UK, Ukraine and USA (Nag Raj 1972, 1993; Sutton 1980; Bianchinotti 1997; Vujanovic and St-Arnaud 2003; Plaingam et al. 2005; Tangthirasunun et al. 2014a). Pseudorobillarda taxa can be saprobic, pathogenic, endophytic as well as lichenicolous and humicolous (Petrini 1986; Nag Raj 1993; Bianchinotti 1997; Boom et al. 1998; Vujanovic and St-Arnaud 2003; Kadowaki et al. 2014). The genus is characterized by pycnidial, globose to subglobose conidia with or without paraphyses, phialidic or annellidic conidiogenous cells and subcylindrical to fusiform, aseptate or septate conidia.
Pseudorobillarda was introduced by Morelet (1968) to accommodate P. muhlenbergiae (R. Sprague) M. Morelet and P. phragmitis, with the latter as the type species. Subsequently, Nag Raj (1972) proposed the same name Pseudorobillarda Nag Raj, Morgan-Jones & W.B. Kendr. for four taxa namely, P. agrostidis (R. Sprague) Nag Raj et al. on Agrostis tenuis, P. bambusae Nag Raj et al. on Bambusa sp., P. indica Nag Raj et al. on unidentified plant and P. phragmitis on Phragmites communis. Nag Raj et al. (1973) recognized that Pseudorobillarda Nag Raj et al. was a homonym of Pseudorobillarda M. Morelet and clarified the nomenclature of the generic name as well as the species in Pseudorobillarda. Nag Raj (1993) made a major revision for Pseudorobillarda and accepted eight taxa. Another major revision of this genus was carried out by Plaingam et al. (2005), they recognized 14 species in Pseudorobillarda and provided a synoptic table for all described taxa. Tangthirasunun et al. (2014a) described an additional species, P. eucalypti Tangthir. & K.D. Hyde from eucalyptus in Thailand and placed Pseudorobillarda in Dothideomycetes incertae sedis on basis of LSU sequence data; this result agreed with Rungjindamai et al. (2012). Crous et al. (2014a) showed that Pseudorobillarda taxa are related to Pleosporales, but our phylogenetic analysis based on LSU sequence showed that this genus distinct to Pleosporales, and we placed Pseudorobillarda in Dothideomycetes incertae sedis.
In the phylogenetic tree reconstruction based on LSU, SSU, tef1 (2218F/986R) and rpb2 sequence data, species of Pseudorobillarda presented two clades (A, B) with high support (Fig. 304). Clade A contains taxa which lack paraphyses and the conidia are aseptate or occasionally 3-septate. Clade B contains taxa that have paraphyses, and the conidia are 1–3 septate. This result indicates that paraphyses might correlate with phylogeny, and it can be used as a character at species level identification. Isolates HKAS 93638/IT2601 and MFLU 19-2895 grouped with P. phragmitis (CBS 398.61, CBS 842.84, IA04, IA10) and P. siamensis (BCC12531), respectively, but these two isolates constitute separate branches (Fig. 304). A comparison HKAS 93638/IT2601 morphology with known species concluded that this isolate is conspecific with P. magna Bianchin. Furthermore, three unnamed taxa (MFLUCC 12-0423, MFLUCC 12-0422, MFLUCC 12-0316) clustered with P. sojae (BCC20495, MFLUCC12-0421). We re-examined herbarium specimen of these three taxa and considered they are conspecific with P. sojae. In addition, strain (MFLUCC 12-0414) which was identified as P. siamensis by Tangthirasunun et al. (2014a) formed a separated branched within clade A. Combined with morphology of P. siamensis (BCC12531), strain MFLUCC 12-0414 is considered a new species, Pseudorobillarda parasiamensis. Moreover, we re-examined the available type specimens of Pseudorobillarda species and accepted 16 taxa, P. agrostidis, P. aquatica, P. asparagi, P. bambusae, P. eucalypti, P. indica, P. jaczewskii, P. magna, P. monica, P. parasiamensis, P. peltigerae, P. phragmitis, P. setariae, P. siamensis, P. sojae, P. texana.
Phylogenetic tree generated from a maximum parsimony analysis based on a concatenated alignment of LSU, SSU, ITS and rpb2 sequences data of Pseudorobillarda (Dothideomycetes, Pseudorobillardaceae) and Hysteriales, Mytilinidiales and Valsariales in Dothideomycetes. Twenty-seven strains are included in the analyses, which comprise 3182 characters including gaps. Single gene analyses were carried out and compared with each species, topology of the tree, and clade stability. Didymella exigua CBS 183.55 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 14268.459665 is presented. The matrix had 1013 distinct alignment patterns, with 36.78% of undetermined characters or gaps. Estimated base frequencies were: A = 0.258512, C = 0.218094, G = 0.266371, T = 0.257023; substitution rates AC = 1.318990, AG = 2.552498, AT = 1.506346, CG = 0.816243, CT = 7.03383, GT = 1.000000; gamma distribution shape parameter α = 0.210032. Maximum likelihood (MLBS) bootstrap support values higher than 50% and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.04 changes. Ex-type strains are in bold. New isolates are in bold and blue
Distribution: Cuba, Germany, India, Italy, Nigeria, Thailand, UK, Ukraine, USA (Nag Raj 1993; Tangthirasunun et al. 2014a; this study).
Pseudorobillarda bambusae Nag Raj, Morgan-Jones & W.B. Kendr., Can. J. Bot. 50(4): 864 (1972)
Facesoffungi number: FoF 07560, Fig. 305
Pseudorobillarda bambusae (DAOM 134454, ex-type) a, b Herbarium package and specimen. c, d Appearance of yellowish brown to brown conidiomata on sterilized sugarcane leaves of Saccharum officinarum. e–g Vertical sections of conidiomata. h, i Sections of peridium. j–k, m–p Conidiogenous cells, developing conidia and paraphyses. l Ostiole. q–u Conidia. Scale bars c–d = 200 µm, e–g = 100 µm, h–i = 50 µm, j–k = 10 µm, l = 20 µm, m–u = 5 µm
Foliicolous. Sexual morph: undetermined. Asexual morph:Conidiomata 100–340 µm diam., 120–250 µm high, yellowish brown to brown, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, with an oval to globose or subglobose venter and a cylindrical to subcylindrical neck in section view, unilocular, glabrous, ostiolate. Ostiole 65–150 × 60–90 µm, single, circular to subcylindrical, centrally or laterally placed. Conidiomatal wall 10–30 µm wide, composed of thick-walled, brown to hyaline cells of textura angularis to textura prismatica. Paraphyses 30–60 × 1–2 µm, hyaline, filiform, septate, unbranched, mixed with conidiophores. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–8 × 2–4 µm, hyaline, enteroblastic, phialidic, short cylindrical or ampulliform, determinate, with marked periclinal thickenings at collarette zone (Fig. 305m–p), arising from the inner wall layer of conidiomata. Conidia 16–20 × 3–5 µm (\( \bar{x} \) = 17 × 3.5 µm; n = 30), hyaline, subcylindrical to fusiform, with a subacute apex, and a blunt base, straight or slightly curved, 1-septate, slightly constricted at the septum, guttulate, thick- and smooth-walled, bearing 2–3, flexuous, divergent, extracellular, apical appendages (14–20 µm long).
Material examined: India, Bangalore, Lalbagh Gardens., cultured on sterilized sugarcane leaves of Saccharum officinarum (Poaceae), (29 December 1970 to 15 January 1971), T.R. Nag Raj (DAOM 134454, ex-type).
Pseudorobillarda eucalypti N. Tangthirasunun & K.D. Hyde, in Tangthirasunun, et al., Phytotaxa 176(1): 255 (2014)
Facesoffungi number: FoF 07561, Fig. 306
Pseudorobillarda eucalypti(MFLU 13–0275, ex-holotype) a Herbarium specimen. b, c Appearance of black conidiomata on toothpicks. d, e Vertical sections of conidiomata. f–g Vertical sections of peridium. h–k Conidiogenous cells and developing conidia. l–o Conidia. Scale bars b = 500 µm, c = 200 µm, d–e = 100 µm, f = 20 µm, g = 50 µm, h–o = 10 µm
Saprobic on dead leaves of eucalyptus. Sexual morph: undetermined. Asexual morph:Conidiomata 255–330 µm diam., 350–500 µm high, black, pycnidial, scattered to gregarious, semi-immersed to superficial, subglobose to obclavate, unilocular, glabrous, thick-walled, ostiolate. Ostiole 160–190 × 60–90 µm, single, subcylindrical, centrally located, with a long ostiolar canal. Conidiomatal wall 30–120 µm thick, composed of thick-walled, brown to hyaline cells of textura angularis in the exterior, gradually merging with textura porrecta or textura prismatica towards conidial hymenium. Paraphyses absent. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 6–17 × 2–5 µm, hyaline, enteroblastic, phialidic, short-cylindrical, ampulliform, lageniform, thick-walled, smooth. Conidia 15–18 × 5–8 µm (\( \bar{x} \) = 17 × 6 µm; n = 30), hyaline, subcylindrical to fusiform, rounded at apex, truncated at base, aseptate, guttulate, thick-walled, smooth, bearing 2–3-unbranched, filiform, attenuated, flexuous, apical appendages extracellular in the upper half of apical cell (10–19 µm long).
Material examined: Thailand. Sakaeo, Pang Sida, on dead leaves of eucalyptus, 17 June 2012, N. Tangthirasunun (MFLU 13–0275, ex- holotype).
Pseudorobillarda indica Nag Raj, Morgan-Jones & W.B. Kendr., Can. J. Bot. 50(4): 865 (1972)
Facesoffungi number: FoF 07562, Fig. 307
Pseudorobillarda indica (DAOM 134448, ex-type) a, b Herbarium package and specimen. c, d Appearance of dark brown to brown conidiomata on PDA. e, f Vertical sections of conidiomata. g, l Paraphyses. h Section of peridium. i–k Conidiophores, conidiogenous cells and developing conidia. m–p Conidia. Scale bars c–d = 200 µm, e–f = 100 µm, g, i–l = 10 µm, h = 20 µm
Foliicolous. Sexual morph: undetermined. Asexual morph:Conidiomata 150–300 µm diam., 200–350 µm high, brown to dark brown, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, oval to rounded, with a short-cylindrical neck in surface view, globose to subglobose, occasionally conical in sectional view, unilocular, glabrous, ostiolate. Ostiole 50–60 × 30–40 µm, single, circular to subcylindrical, centrally or laterally located. Conidiomatal wall 10–30 µm wide, composed of thick-walled, brown to hyaline cells of textura prismatica. Paraphyses 30–60 × 1–2 µm, hyaline, filiform, septate, branched and bulging at base, smooth-walled, mixed with conidiophores. Conidiophores arising from the inner wall layers of conidiomata, hyaline, dolliform to subcylindrical, thick- and smooth-walled, invested in mucus. Conidiogenous cells 6–9 × 3–5 µm, hyaline, enteroblastic, phialidic, cylindrical to ampulliform, swollen at base, indeterminate, discrete or integrated, with marked periclinal thickenings at collarette zone (Fig. 307i–l). Conidia 16–24 × 2.4–4 µm (\( \bar{x} \) = 20 × 3 µm; n = 30), hyaline, subcylindrical to fusiform, subacute at apex, blunt and truncate at base, straight or slightly curved, 1-septate, occasionally 3-septate, constricted at the septa, guttulate, thick- and smooth-walled, bearing at one end 3, occasionally 2–4, flexuous, divergent, extracellular appendages (11–23 µm long).
Material examined: India, Bangalore, Rajajinagar, 19 October 1970 to 16 November 1970, T.R. Nag Raj (DAOM 134448, cultured on PDA from type).
Pseudorobillarda magna Bianchin., Mycol. Res. 101(10): 1233 (1997)
Facesoffungi number: FoF 07563, Fig. 308
Pseudorobillarda magna (HKAS 93638) a Herbarium package and specimen. b, c Appearance of black coniodiomata on the host. d Vertical section of conidioma. e Ostiole. f Section of peridium. g–i Conidiogenous cells, developing conidia and paraphyses. j–m Conidia. Scale bars d = 50 µm, e = 20 µm, f–m = 10 µm
Saprobic on dead branches of Cornus sanguinea. Sexual morph: undetermined. Asexual morph:Conidiomata 160–220 µm diam., 130–200 µm high, dark brown, pycnidial, separate to gregarous, immersed to semi-immersed, globose, unilocular, glabrous, thick-walled, ostiolate. Ostiole 15–50 × 20–60 µm, single, circular, centrally located, filled with hyaline, short, cylindrical periphyses. Conidiomatal wall 20–50 µm wide, composed of brown to hyaline, thick-walled cells of texura angularis. Paraphyses 20–60 × 1–2 µm, hyaline, subcylindrical, broader at the base, septate, branched or unbranched, smooth and thick-walled, hyphae-like, originating from the inner wall layer cells of basal and lateral wall. Conidiophores formed from inner wall layer of most part of conidiomata, except ostiolar region. Conidiogenous cells 3–7 × 2–2.5 µm, hyaline, enteroblastic, annellidic, cylindrical, smooth-walled, with periclinal thickenings on wall towards apex, or occasionally 1–2 times percurrently proliferating. Conidia 22–28 × 3–5 µm (\( \bar{x} \) = 25 × 4.5 µm; n = 50), hyaline, fusiform, narrowed above, blunt at the base, straight or slight curved, mostly 3-septate, occasionally 1-septate, slightly constricted, smooth-walled, guttulate, bearing 5–6 unbranched, filiform, flexuous apical appendages (17–26 µm long).
Culture characters: Colonies on PDA, reaching 10–20 mm diam. after 4 weeks at 20–25 °C, white at the beginning, become light brown in the middle zone, white to pale grey at the margin with age, flattened, with velutinous, flexuous aerial mycelium on the surface, margin undulate, reverse brown.
Material examined: Italy, Province of Forlì-Cesena, near Corniolo, on dead aerial branches of Cornus sanguinea (Cornaceae), 8 September 2015, Erio Camporesi, IT2601 (KUN, HKAS 93638), living culture KUMCC 15-0633.
Notes: Our collection clusters to P. phragmitis (CBS 398.61, CBS 842.84), but with low support (Fig. 304). Morphologically, it is similar to P. magna in having 1–3-septate conidia with up 5 apical appendages. Moreover, it shares similar conidial dimensions with P. magna (23–30 × 5–6.5 µm (\( \bar{x} \) = 27 × 5.4). Pseudorobillarda magna has been recorded from Geoffroea decorticans (Fabaceae) in Argentina, thus our collection is considered as a new record from Italy.
Pseudorobillarda parasiamensis N. Tangthirasunun, W.J. Li & K.D. Hyde, sp. nov.
Index Fungorum number: IF557162, Facesoffungi number: FoF 07564, Fig. 309
Pseudorobillarda parasiamensis(MFLU 13–0275, holotype) a Herbarium specimen. b, c Appearance of black conidiomata with rostrate ostiolar neck protruding above the surface of the leaf. d Vertical section of conidioma. e, f Vertical sections of peridium. g–i Conidiogenous cells and developing conidia (arrow shows annellide). j–s Conidia. Scale bars d = 100 µm, e–f = 20 µm, g–s = 10 µm
Etymology: Referring to its morphology similar to P. siamensis, but phylogeny distinct.
Saprobic on dead leaves. Sexual morph: undetermined. Asexual morph:Conidiomata 140–160 µm diam., 100–130 µm high, black, foliicolous, scattered, with the venter immersed in the host leaf and only part of the top of the long, rostrate ostiolar neck protruding above the surface of the leaf, subglobose to obclavate in section view, unilocular, thick-walled, glabrous, ostiolate. Ostiole single, subcylindrical to obconic, centrally located, with a very long ostiolar canal. Conidiomatal wall 20–30 µm thick, composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–10 × 2–4 µm, hyaline, enteroblastic, phialidic, subcylindrical, sometimes proliferating 2–3 times, thick- and smooth -walled. Conidia 15–23 × 6–8 µm (\( \bar{x} \) = 20 × 7 µm; n = 30), hyaline, fusiform to ellipsoidal, rounded at apex, truncated at base, unicellular, guttulate, thick-walled, smooth, bearing 2–4-unbranched, flexuous, acellular appendages in the upper half of apical part (12–23 µm long).
Material examined: Thailand, Nakhonratchasima, Khao Yai, on unidentified dead leaves, 16 June 2012, N. Tangthirasunun NTCL082-3 (MFLU 13–0273, holotype).
Notes: Strain MFLUCC 12–0414 was identified as Pseudorobillarda siamensis by Tangthirasunun et al. (2014a). This strain is morphologically related to P. siamensis, as both have pycnidial conidiomta with long subcylindrical to obconic ostiolar neck and fusiform to broadly ellipsoidal conidia. However, MFLUCC 12–0414 has slightly shorter conidia and is 0–3-septate whereas P. siamensis conidia are 20–26 × 6–7.5 µm (av. 22.6 × 6.8 µm) and aseptate. Phylogenetically these two strains are distinct from each other (Fig. 304)
Pseudorobillarda phragmitis (Cunnell) M. Morelet, Bull. Soc. Sci. nat. Arch. Toulon et du Var 175: 6 (1968)
Facesoffungi number: FoF 07565, Fig. 310
Pseudorobillarda phragmitis (IMI 70768, holotype) a, b Herbarium package and specimen. c, d Appearance of brown conidiomata on PDA. e, f Vertical sections of conidiomata. g, i Sections of peridium. h Locules. j–m Conidiophores, conidiogenous cells and developing conidia (arrows show annellide). n Paraphyses and conidiophores. o–t Conidia. Scale bars c = 200 µm, d–f = 100 µm, g–h = 50 µm, i = 20 µm, j–m, o–t = 5 µm, n = 10 µm
Saprobic on dead stems of Phragmites communis (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 150–250 µm diam., 110–250 µm high, yellowish brown to brown, pycnidial, solitary to gregarious or confluent, deeply immersed, globose or depressed globose with cylindrical, laterally placed neck in section view, unilocular to multilocular, often convoluted and irregularly divided, glabrous, thick-walled, ostiolate. Ostiole 60–125 × 35–90 µm, cylindrical, straight, laterally located. Conidiomatal wall 20–60 µm wide, composed of outer layers of textura angularis, with brown, thick-walled cells, gradually merging with pale brown to hyaline, thick-walled cells of textura angularis to textura prismatica. Paraphyses 15–30 × 1–2 µm, hyaline, filiform, septate, unbranched. Conidiophores arising from the inner wall layer of conidiomata, hyaline, cylindrical, broader at base, septate, branched, smooth-walled (Fig. 310m). Conidiogenous cells 5–10 × 2–4 µm, hyaline, enteroblastic, annellidic, subcylindrical or lageniform, indeterminate, discrete or integrated, with 2–3percurrent proliferation . Conidia 14.5–20 × 2–4 µm (\( \bar{x} \) = 17 × 3 µm; n = 50), hyaline, fusiform, with a rounded or obtuse apex, straight, 1-septate, not constricted at the septum, guttulate, thick- and smooth-walled, bearing 2–5, flexuous, divergent, extracellular, apical appendages (10–20 µm long).
Material examined: UK, Middlesex, near Staines, on dead stems of Phragmites communis (Poaceae), July 1955, G.J. Cunnell (IMI 70768, holotype).
Notes: Three strains (IA04, IA10, CBS 842.84) were named as P. phragmitis in GenBank, but they formed a separated branch below type strain (CBS 398.61) (Fig. 304). Thus, these strains are not conspecific with P. phragmitis, and a morphology study of them is needed to clarify their identification.
Pseudorobillarda sojae Uecker & Kulik, Mycologia 78(3): 450 (1986)
Facesoffungi number: FoF 07566, Fig. 311
Pseudorobillarda sojae (DAOM 215313) a, b Herbarium package and specimen. c, d Appearance of dark brown to black specks beneath the epidermis. e Side view of conidioma. f, g Vertical sections of conidiomata. h, i Sections of peridium. j–l Conidiogenous cells and developing conidia. m–o Conidia. Scale bars c = 200 µm, d–f = 100 µm, g = 50 µm, h–i = 20 µm, j–o = 5 µm
Caulicolous to foliicolous. Sexual morph: undetermined. Asexual morph:Conidiomata 100–250 µm diam., 100–300 µm high, fuscous, pycnidial, scattered to gregarious, subepidermal, deeply immersed, appearing as dark brown to black specks beneath the epidermis in surface view (Fig. 311c, d), with a globose or depressed globose or irregular in shape venter, and an oval or subcylindrical neck, unilocular, glabrous, thick-walled, ostiolate.Ostiole 50–95 × 40–60 µm, circular to subcylindrical, single, centrally or laterally located. Conidiomatal wall 10–30 µm wide, composed of thick-walled, dark brown to brown cells of textura angularis in the outer layers, and thick-walled, hyaline cells of textura prismatica in the inner layers. Paraphyses absent. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–7 × 3–5 µm, hyaline, enteroblastic, phialidic, short cylindrical to subcylindrical, determinate, with marked periclinal thickenings at collarette zone (Fig. 311j), arising from the inner wall layer of conidiomata. Conidia 13.5–19 × 3–5 µm (\( \bar{x} \) = 16.5 × 4 µm; n = 50), hyaline, subcylindrical to fusiform, with a bluntly rounded apex, and a broadly truncate base, unicellular, guttulate, thick- and smooth-walled, bearing 3–4, flexuous, divergent, extracellular, apical appendages (10–21 µm long).
Material examined: Cuba, Pinar del Rio, on decaying leaves of unidentified angiosperm, 1 June 1988, T.R. Nag Raj (DAOM 215313).
Notes: Pseudorobillarda sojae was described from soybean stems on water agar in the USA (Uecker and Kulik 1986). Abbas et al. (2002) excluded P. sojae from Pseudorobillarda, because it lacks paraphyses and conidia are aseptate, a feature which is present in P. phragmitis. However, Plaingam et al. (2005) and Tangthirasunun et al. (2014a) showed P. sojae was phylogenetically related to the type species and included the species in Pseudorobillarda. Three unnamed Pseudorobillarda species (MFLUCC 12-0423, MFLUCC 12-0422, MFLUCC 12-0316) clustered phylogenetically with P. sojae. and morphologically, P. sojae, and the three unnamed strains share similar form of conidiomata, conidiogenous cells and conidia dimensions with P. sojae. Therefore, these three strains are regarded as conspecific with P. sojae. A synopsis of all P. sojae strains is provided (Table 9).
Pseudoseptoria Speg., Anal. Mus. nac. B. Aires, Ser. 3 13: 388 (1911)
Facesoffungi number: FoF 00134, Fig. 312
Pseudoseptoria donacis (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia
Dothideomycetes, Dothideomycetidae, Dothideales, Saccotheciaceae
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, scattered to gregarious, immersed to erumpent, globose in section view, unilocular, glabrous, ostiolate. Ostiole single, circular, centrally located. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells formed from the innermost layer of wall cells of the conidiomata, hyaline, enteroblastic, annellidic, subcylindrical to ampulliform, indeterminate, smooth-walled, with numerous inconspicuous or conspicuous percurrent proliferations towards apex. Conidia hyaline, falcate, fusiform, tapered towards each end, unicellular, smooth-walled, guttulate (adapted from Sutton 1980).
Type species: Pseudoseptoria donacis (Pass.) B. Sutton, Mycol. Pap. 141: 169 (1977)
Notes: Pseudoseptoria species are plant pathogens on Poaceae (Slopekl and Labun 1992; Carmona et al. 1996; Sutton 1980; Quaedvlieg et al. 2013). The genus is characterized by pycnidial conidiomata, annellidic conidiogenous cells and falcate, fusiform conidia. Sutton (1980) revised the genus and accepted five species, P. bromigena (Sacc.) B. Sutton, P. donacis (Pass.) B. Sutton, P. everhartii (Sacc. & P. Syd.) B. Sutton, P. obtusa (R. Sprague & Aar.G. Johnson) B. Sutton, P. stomaticola (Bäumler) B. Sutton. An additional species P. usneae (Vouaux) D. Hawksw. was later added (Hawksworth 1981a, b). Quaedvlieg et al. (2013) included molecular data and placed Pseudoseptoria in Dothioraceae. They also introduced two new species, P. collariana Quaedvl., Verkley & Crous and P. obscura Quaedvl., Verkley & Crous. Wijayawardene et al. (2018) placed Pseudoseptoria in Saccotheciaceae, and accepted eight species in the genus. Fresh collections of the generic type P. donacis are needed to confirm its placement.
Distribution: Canada, Cyprus,, Ethiopia, Hungary, Iran, Ireland, Libya, Pakistan, UK, USA (Sutton 1980; Quaedvlieg et al. 2013).
Pseudostegia Bubák, J. Mycol. 12(2): 56 (1906)
Facesoffungi number: FoF 07547, Fig. 313
Pseudostegia nubilosa (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Saprobic on dead leaves of Carex sp. (Cyperaceae) (Sutton 1980). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, solitary or confluent, subepidermal, immersed, applanate, disciform, unilocular, glabrous. Ostiole indistinct, dehiscence by circumscissile rupture of the upper wall lifting off a flap of tissue. Conidiomatal wall composed of thick-walled, dark brown cells concentrated in the epidermis in the upper part, becoming thick-walled, brown, relatively large cells of textura globulosa in the lower part, gradually diminishing in size and pigmentation towards the conidiogenous cell region. Conidiophores reduced to conidiogenous cells. Conidiogenous cells pale brown, enteroblastic, phialidic, ampulliform to cylindrical, determinate, smooth-walled, with conspicuous, periclinal wall thickened channel and collarette, arising from the inner wall layer of basal stroma. Conidia hyaline, falcate, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Pseudostegia nubilosa Bubák, J. Mycol. 12(2): 56 (1906)
Notes: Pseudostegia is monotypic. The hyaline, falcate, unicellular conidia in Pseudostegia invite a comparison with Colletotrichum and Pseudoseptoria. Pseudoseptoria has pycnidial conidiomata and annellidic, subcylindrical to ampulliform conidiogenous cells, which differs from the acervular conidiomata and phialidic, ampulliform to cylindrical conidiogenous cells in Colletotrichum and Pseudoseptoria (Sutton 1980). Pseudoseptoria was separated from Colletotrichum by its method of conidiomatal wall dehiscence (Sutton 1980). However, this character alone may not be enough to separate it from Colletotrichum. An epitype of Pseudostegia nubilosa is needed to confirm its placement.
Distribution: USA (Sutton 1980).
Pseudothyrium Höhn., in Weese, Mitt. bot. Inst. tech. Hochsch. Wien 4(3): 109 (1927)
Facesoffungi number: FoF 07548, Fig. 314
Pseudothyrium polygonati (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Conidia
Ascomycota, genera incertae sedis
Saprobic on stems of Polygonatum officinale (Asparagaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, stromatic, pycnidial, solitary, rarely confluent, subepidermal, immersed, subglobose in section view, unilocular, glabrous. Ostiole indistinct, dehiscence by irregular rupture of the apical wall in the middle part. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the upper and basal part, becoming thin-walled, hyaline cells of textura prismatica towards hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampulliform, determinate, smooth-walled, and arising from the inner wall layer of basal stroma. Conidia hyaline, cylindrical, acicular, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Pseudothyrium polygonati (Tassi) Höhn., in Weese, Mitt. bot. Inst. tech. Hochsch. Wien 4(3): 109 (1927)
Notes: Arx (1957a) reduced Pseudothyrium to a synonym of Cylindrosporella Höhn., while Sutton (1980) reinstated Pseudothyrium as a valid genus, accepting a single species in the genus. Pseudothyrium is similar to Erythrogloeum in having stromatic, pycnidial conidiomata and phialidic conidiogenous cells, but it can be distinguished from the latter by cylindrical conidia. There is no molecular data available for this genus. Fresh collections are needed to place it in a natural group.
Distribution: Latvia (Sutton 1980).
Pseudozythia Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 111: 1019 (1902)
Facesoffungi number: FoF 07549, Fig. 315
Pseudozythia pusilla (redrawn from Nag Raj and DiCosmo 1980) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant (Kellerman and Ricker 1905). Sexual morph: undetermined. Asexual morph:Conidiomata pycnidial, solitary, superficial, pulvinate, globose to subglobose, unilocular, glabrous, with a short pseudoparenchymatous stalk. Ostiole absent, dehiscent by irregular splits of the apical wall. Conidiomatal wall composed of thin-walled, subhyaline to hyaline cells of textura angularis in the basal part, gradually merging with closely packed, septate, hyaline hyphae of textura intricata to textura prismatica in the periclinal part. Conidiophores arising from inner layer cells of basal stroma, hyaline, cylindrical to irregular, branched, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, occasionally sympodial, subcylindrical, integrated or discrete, determinate, smooth-walled, producing conidia terminally or immediately below the transverse septa. Conidia hyaline, fusiform, with inconspicuous truncate basal scars, straight or slightly curved, unicellular, smooth-walled, guttulate (adapted from Nag Raj and DiCosmo 1980).
Type species: Pseudozythia pusilla Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 111: 1020 (1902)
Notes: Pseudozythia was re-described and re-illustrated by Nag Raj and DiCosmo (1980). The genus remains monotypic. Pseudozythia pusilla closely resembles the synanamorph of Pilidium concavum in having stalked conidiomata and cylindrical, branched conidiophores and enteroblastic, phialidic, integrated or discrete, apical or acropleurogenous conidiogenous cells (Palm 1991). The only difference between these two species is the conidia of Pseudozythia pusilla are fusiform while those of Pilidium concavum are fusiform to falcate or cymbiform. Pseudozythia might be congeneric with Pilidium, but this needs molecular data of Pseudozythia pusilla to confirm.
Distribution: Europe (Wijayawardene et al. 2017).
Pullospora Faurel & Schotter, Revue Mycol., Paris 29(4): 280 (1965) [1964]
Facesoffungi number: FoF 07554
Ascomycota, genera incertae sedis
Coprophilous. Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, solitary to gregarious, immersed to semi-immersed, ellipsoidal, globose to subglobose or irregular, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, cylindrical, centrally located. Conidiomatal wall composed of thick-walled, dark brown to brown cells of textura angularis. Conidiophores formed from the inner wall layer of conidiomata, hyaline, cylindrical to subcylindrical, straight or slightly curved, branched, septate, invested in mucus. Conidiogenous cells hyaline, enteroblastic, annellidic, subcylindrical to lageniform, integrated or terminal, indeterminate smooth-walled, with percurrent proliferations. Conidia hyaline, cylindrical to subcylindrical, aseptate, thick- and smooth-walled, bearing a primary eccentric basal appendage and 2–4 unbranched, tubular, filiform, subpolar appendages.
Type species: Pullospora tetrachaeta Faurel & Schotter, Revue Mycol., Paris 29(4): 280 (1965) [1964]
Notes: Pullospora species are coprophilous on rodent and jackal dung. Nag Raj (1993) added a second species P. macrospora Nag Raj. The genus is characterized by pycnidial conidiomata, subcylindrical to lageniform, discrete or integrated conidiogenous cells and unicellular, subcylindrical conidia bearing a primary centric basal appendage and 2–4 unbranched, secondary subpolar appendages. No sexual morph has been linked to this genus and no molecular data is available (Nag Raj 1993).
Distribution: France, USA.
Key to species of Pullospora
1. Conidia up to 18 μm long, surrounded by sheath, with centric basal appendage…P. tetrachaeta
1. Conidia less than 18 μm long, usually without sheath and centric basal appendage…P. macrospora
Pullospora macrospora Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 748 (1993)
Facesoffungi number: FoF 07555, Fig. 316
Pullospora macrospora (DAOM 215329, holotype) a–c Herbarium package and specimens. d Appearance of brown to dark brown conidiomata with papillae on the dung. e Surface view of conidiomatal wall. f–g Sections of peridium. h–i Vertical sections of conidiomata. j–n Conidiophores, conidiogenous cells and developing conidia. o–t Conidia. Scale bars: d = 200 µm, e–g = 20 µm, h–i = 100 µm, j–o = 10 µm, p–t = 5 µm
Saprobic on rodent dung. Sexual morph: undetermined. Asexual morph:Conidiomata 130–240 µm diam., 140–260 µm high, brown to dark brown, pycnidial, solitary to gregarious, immersed to semi-immersed, ellipsoidal, venter globose to subglobose or irregular, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, cylindrical, centrally located. Conidiomatal wall 10–45 µm wide, composed of thick-walled, dark brown to brown cells of textura angularis in outer layers, thin-walled, paler in the inner layers, becoming much darker towards neck region. Conidiophores arising from the inner wall layer of conidiomata, hyaline, cylindrical to subcylindrical, straight or slightly curved, branched, septate, invested in mucus. Conidiogenous cells 10–30 × 2–6 μm, hyaline, subcylindrical to lageniform, integrated or terminal, smooth-walled, indeterminate, proliferating several times. Conidia 18–28 × 4–7 μm (\( \bar{x} \) = 22 × 5 μm), hyaline, cylindrical to subcylindrical, broadly rounded above, cuneate at the base, aseptate, smooth-walled, bearing primary and secondary cellular appendages; primary appendage centric, reduced to a short, truncate protuberance; secondary appendages 21–43 μm (\( \bar{x} \) = 35 μm) long, mostly 3, sometimes 2 or 4 near the apex and base, subpolar, unbranched, filamentous, attenuated at the ends, slightly wider in the middle, flexuous.
Material examined: USA, California, San Bernardino Co., 1 mile North of Mt. Baldy, on rodent dung, 11 April 1972, D. Malloch (DAOM 215329, holotype).
Pullospora tetrachaeta Faurel & Schotter, Revue Mycol., Paris 29(4): 280 (1965) [1964]
Facesoffungi number: FoF 07556, Fig. 317
Pullospora tetrachaeta (DAOM 215328, holotype) a, b Herbarium package and specimens. c, d Appearance of dark brown to black conidiomata on the dung. e, f Vertical sections of conidiomata. g Section of peridium. h–l Conidiophores, conidiogenous cells and developing conidia. m–q Conidia. Scale bars: d = 200 µm, e–f = 50 µm, g = 10 µm, h–q = 5 µm
Saprobic on rabbit dung. Sexual morph: undetermined. Asexual morph:Conidiomata 100–200 µm diam., 80–150 µm high, dark brown to black, pycnidial, solitary to gregarious, or confluent, immersed to semi-immersed, or immersed with only the short neck visible in surface view, globose to subglobose, unilocular, glabrous, with cylindrical to somewhat obconic neck, thick-walled, ostiolate. Ostiole single, circular or oval, centrally located. Conidiomatal wall 10–20 µm wide, composed of thick-walled, dark brown to brown cells of textura angularis in outer layers, thin-walled, paler in the inner layers, becoming much darker in the neck region. Conidiophores formed from inner wall layer of conidiomata, hyaline, cylindrical, straight or slightly curved, branched, septate, invested in mucus. Conidiogenous cells 7–15 × 2–3 μm, hyaline, subcylindrical to lageniform, discrete or integrated, indeterminate, smooth-walled. Conidia 9–17 × 3–5 μm (\( \bar{x} \) = 14 × 4 μm), hyaline, cylindrical to elongate-ellipsoid, rounded at both ends, aseptate, smooth-walled, surrounded by sheath, bearing primary and secondary cellular appendages; primary appendage 2–4 μm (\( \bar{x} \) = 3 μm) long, only at the base, single, tubular, unbranched, at the broadest point; secondary appendages 13–28 μm (\( \bar{x} \) = 21 μm) long, 2–4 near the apex, 3–4 near the base, subpolar, unbranched, filiform, attenuated at each end, slightly broad in the middle, flexuous.
Material examined: France, Les-Baux-en-Provence, on rabbit dung, 22 October 1974, W. Gams and D. Malloch (DAOM 215328, holotype).
Pycnopeziza W.L. White & Whetzel, Mycologia 30(2): 187 (1938)
= Acarosporium Bubák & Vleugel ex Bubák, Ber. dt. bot. Ges. 29: 384 (1911)
Facesoffungi number: FoF 07550
Leotiomycetes, Leotiomycetidae, Helotiales, Sclerotiniaceae
Saprobic or parasitic on wide range of host plants, e.g., Acer rubrum, Alnus rugosa Betula pubescens, Hedera helix, Picea pungens, Populus tremuloides, Salix caprea (Sutton 1980; Nag Raj 1993). Sexual morph: see White and Whetzel (1938). Asexual morph: Conidiomata dark brown to black, stromatic, pycnidial, solitary to gregarious, initially subepidermal, becoming erumpent, globose to subglobose or irregularly collapsed, or cupulate, unilocular, glabrous, thick-walled, lacking ostiole, but dehiscing by irregular split of overlapping host tissue. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis to textura globulosa. Conidiophores developing from the upper cells of the basal stroma in a palisade along the base of the conidiomata, hyaline, cylindrical to subcylindrical, septate, dichotomously to subdichotomously branched, smooth-walled. Conidiogenous cells hyaline, holothallic, integrated, determinate, smooth-walled. Conidia arthric, formed by disarticulation of the conidial chain, produced in complex, repeatedly branched chains with the oldest conidium at the base, hyaline, cylindrical or allantoid, initially with truncate ends, eventually becoming obtuse, 1–3-septate, smooth, bearing filiform, flexuous, attenuated, cellular appendages, arising from apical and subapical or sometime from basal region.
Type species: Pycnopeziza sympodialis W.L. White & Whetzel ex B. Sutton, The Coelomycetes: 34 (1980)
= Acarosporium sympodiale Bubák & Vleugel ex Bubák, Ber. dt. bot. Ges. 29: 385 (1911).
Notes: Acarosporium was introduced by Bubák and Vleugel in 1911, with the type species A. sympodiale, occurring on dead leaves of Betula odorata Bechst. von Höhnel (1920) introduced a new species A. austriacum Höhn., which is morphologically similar to the type taxon. Sutton (1980) subsequently synonymized A. austriacum under A. sympodiale and recognized only two species, including A. quisquiliaris W.L. White & Whetzel ex B. Sutton, the conidia of which lack appendages. Nag Raj (1993) recognized three taxa namely, A. americanum Nag Raj, A. quisquiliaris and A. sympodiale and gave a detailed account of conidium formation. Ihlen and Tønsberg (1998) and Johnston et al. (2014) included A. lichenicola.
Acarosporium sympodiale has been connected to the sexual morph Pycnopeziza sympodialis (generic type of Pycnopeziza) (White and Whetzel 1938, Whetzel and White 1940; Sutton 1980). Johnston et al. (2014) synonymized Acarosporium under Pycnopeziza because it has available sequence data and the name was widely used (Holst-Jensen et al. 1997, 2004; Wijayawardene et al. 2017b; Pärtel et al. 2017). However, lacking an available molecular sequence for the asexual morph, the relationship of these two genera remains uncertain. New collections are required to delineate the circumscription of the genus.
Distribution: Belarus, Canada, Germany, Italy, Norway, Sweden, USA (White and Whetzel 1938; Nag Raj 1993; Holst-Jense et al. 1997).
Pycnopeziza americanum (Nag Raj) W.J. Li & K.D. Hyde, comb. nov.
≡ Acarosporium americanum Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 98 (1993)
Index Fungorum number: IF557176, Facesoffungi number: FoF 07551, Fig. 318
Foliicolous, occuring on male inflorescence of Acer rubrum.Sexual morph: undetermined. Asexual morph: Conidiomata 820–1060 µm diam., 200–700 µm high, dark brown to black, stromatic, pycnidial, solitary to gregarious, initially closed, ultimately becoming erumpent, globose to hemispherical, unilocular, glabrous, thick- and smooth-walled, lacking a definite ostiole, but opening by irregular splits in the apical wall, then wide open and appearing cupulate. Conidiomatal wall 40–110 µm wide, composed of thick-walled, dark brown cells of textura angularis to textura globulosa in the outer brittle layer, gradually merging with thin-walled, pale brown to hyaline cells towards conidial hymenium. Conidiophores hyaline, cylindrical to subcylindrical, septate, dichotomously to subdichotomously branched, developing from the upper cells of the basal stroma, packed in a dense palisade. Conidiogenous cells hyaline, holothallic, integrated, determinate, septate, smooth-walled. Conidia 17–22.5 × 3–5 µm (\( \bar{x} \) = 19.5 × 3.7; n = 30), hyaline, cylindrical to allantoid, initially truncate at both ends, then becoming rounded or truncate, mostly 1–3-septate, catenate, smooth-walled, bearing appendages; appendage 20–40 × 1–3 µm, filiform, flexuous, tapering at distal end, slightly broad in the lower half, usually 2, but occasionally 3 or 4 arising from apical and subapical region of the terminal cell.
Material examined: USA, West Virginia, Morgantown, on male inflorescence of Acer rubrum (Aceraceae), 4 June 1937, Whetzel, Orton & Hoffmaster (CUP 25842, holotype).
Pycnovellomyces R.F. Castañeda, Fungi Cubenses II (La Habana): 16 (1987)
Facesoffungi number: FoF 07552
Agaricomycetes, genera incertae sedis
Saprobic on dead leaves of Mimusops commersonii. Sexual morph: undetermined. Asexual morph:Conidiomata pale ochraceous to pale brown, pycnidial, solitary to gregarious, intraepidermal in origin, becoming erumpent later, rounded in outline, sessile, globose to subglobose in section view, unilocular, ornamented with long, dichotomously or irregularly branched and intertwined aseptate hyphae, dehiscing by an irregular split in the apical wall of overlapping tissue. Conidomatal hyphae composed of two types; (a) pale brown subcylindrical or irregular in shape, fascicular, irregularly branched, intertwined, asperate, arising from upper periphery, or occasionally in the middle; (b) hyaline, cylindrical or irregular in shape, irregularly branched, septate, with numerous thorns on the surface arising from upper and lateral periphery, or surface of pale brown hyphae. Conidiomatal wall composed of pale brown to hyaline cells of textura angularis to prismatica or intricata. Conidiophores arising in a basal cushion, made up of fused hyphae with clamp connections, hyaline, subcylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, determinate, with marked periclinal thickenings at collarette zone. Conidia hyaline, cylindrical with obtuse to angular ends, straight, unicellular, guttulate, thick- and smooth-walled, bearing subpolar, filiform, flexuous, unbranched or dichotomously or trichotomously branched appendages at both ends.
Type species: Pycnovellomyces foliicola R.F. Castañeda, Fungi Cubenses II (La Habana): 16 (1987)
Notes: Pycnovellomyces is monotypic. This genus is a rather bizarre coelomycetes, and is characterized by its unique conidiomatal features being golden yellow or brown, unilocular, sessile with pale brown to golden yellow, single or fasciculate, septate, flexuous, tuberculate to echinulate hyphae at the upper periphery, its holoblastic conidiogenous cells, and its hyaline conidia with 1–2 bifurcate, tubular appendages at both ends (Castañeda 1987; Nag Raj 1993, this study). Its cylindrical, unicellular conidia with appendages leads to a comparison with Chaetospermum. However, Chaetospermum has white to yellowish brown or pearl, glabrous conidiomata, holoblastic, sympodial conidiogenous cells, and unbranched conidial appendages (Nag Raj 1993, this study). Nag Raj (1989) re-examined the genus and emended the generic description, assigning it to Basidiomycota on the basis of its conidiophores with clamp connections. These clamp connections have also been reported in Cenangiomyces, Ellula, and Fibulocoela. The differences between Pycnovellomyces and these genera were discussed under Fibulocoela. No molecular data is available for Pycnovellomyces, and fresh collections are needed to link its basidiomycetous affinities.
Distribution: Cuba.
Pycnovellomyces foliicola R.F. Castañeda, Fungi Cubenses II: 16 (1987)
Facesoffungi number: FoF 07553, Fig. 319
Pycnovellomyces foliicola (DAOM 199973, isotype) a, b Herbarium package and specimen. c, e, f Appearance of yellowish brown to brown conidiomata with yellowish brown hyphae on the host. d, j Enlarged sectional views of lateral wall. g Cutout view of conidiomata showing internal details. h Yellowish brown hyphae on the conidiomata surface. i Vertical section of conidioma. j Enlarged sectional views of upper wall with hyaline hyphae. k Hyaline, asperate hyphae from conidiomatal wall surface. l Enlarged sectional views of basal wall. m–p Conidiophores, conidiogenous cells and developing conidia (arrows show conidiophores with clamp connections). q–t Conidia (arrows show appendages). Scale barsc = 1000 µm, d = 20 µm, e–g, i = 500 µm, h = 200 µm, j–m = 20 µm, n–p = 10 µm, q–t = 5 µm
Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS and tub2 sequences data of Diatrypaceae. Seventy-one strains are included in the analyses, which comprise 3002 characters including gaps (ITS: 1–702, LSU: 703–1577, tub2: 1578–3002). Lopadostoma turgidum CBS 133207 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 17348.916958 is presented. The matrix had 1156 distinct alignment patterns, with 65.86% of undetermined characters or gaps. Estimated base frequencies were: A = 0.232389, C = 0.232389, G = 0.251650, T = 0.260002; substitution rates AC = 0.957438, AG = 2.339970, AT = 2.339970, CG = 0.829454, CT = 4.380498, GT = 1.000000; gamma distribution shape parameter α = 0.441031. The Bayesian posterior probabilities analysis for final split frequency critical value for the topological convergence diagnostic is 0.009796. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MLBS and a posterior probability lower than 0.95 for BYY. Ex-type strains are in bold. New isolates are in blue
Saprobic on dead leaves of Mimusops commersonii. Sexual morph: undetermined. Asexual morph:Conidiomata 1000–1500 µm diam., 900–1000 µm high, pale ochraceous or pale brown when dry, gelatinous and orange-yellow when moistened, pycnidial, solitary to gregarious, intraepidermal in origin, becoming erumpent later, rounded in outline, sessile, bearing asperate hyphae on the surface of conidiomata (Fig. 319h), globose in sectional view, slightly flattened or with a slight depression in the centre of the upper wall, unilocular, ostiole absent, but dehiscing by an irregular split in the upper wall. Conidiomatal hyphae composed of two types; (a) pale brown, 300–700 × 10–50 µm, subcylindrical or irregular in shape, bulging at base, tapering towards apex, fascicular, irregularly branched, intertwined, asperate, arising from upper periphery, or occasionally in the middle (Fig. 319h); (b) hyaline, 6–30 × 3–7 µm, cylindrical or irregular in shape, bluntly rounded at apex, irregularly branched, septate, with numerous thorns on the surface (Fig. 319j, k), arising from upper and lateral periphery, or surface of pale brown hyphae. Conidiomatal wall composed of three types; (a) upper wall 30–40 µm wide, thick-walled, pale brown to hyaline cells of textura angularis (Fig. 319j); (b) lateral wall 30–60 µm wide, thick-walled, pale brown to hyaline cells of loose textura prismatica (Fig. 319d); (c) basal wall 50–90 µm wide, relatively thick-walled, pale brown to hyaline cells of textura angularis to textura porrecta in the lower layers, and gradually merging with thick-walled, hyaline textura intricata clamp-bearing hyphae near conidial hymenium (Fig. 319l). Conidiophores arising in a basal cushion, made up of fused hyphae with clamp connections (Fig. 319m), hyaline, subcylindrical, branched, septate, smooth-walled. Conidiogenous cells 10–30 × 2–3 µm, hyaline, holoblastic, cylindrical to lageniform, determinate, with marked periclinal thickenings at collarette zone (Fig. 319p). Conidia 10–17 × 1.5–3 µm (\( \bar{x} \) = 13.5 × 2.3 µm; n = 30), hyaline, cylindrical with obtuse to angular ends, straight, unicellular, guttulate, thick- and smooth-walled, bearing subpolar, tubular appendages at both ends; appendages 8–13 µm long, mostly 2, occasionally 3 at the apical end, 1–2 at the basal end, filiform, flexuous, unbranched or dichotomously or trichotomously branched.
Material examined: Cuba, province of Havana, Santiago de las Vegas, on dead leaves of Mimusops commersonii (Sapotaceae), 26 April 1985, R.F. Castafieda Ruiz, (DAOM 199973, isotype).
Quaternaria Tul. & C. Tul., Select. fung. carpol. 2: 104 (1863)
= Libertella Desm., Annls Sci. Nat., sér. 1 19: 275 (1830)
Facesoffungi number: FoF 07557
Sordariomycetes, Xylariomycetidae, Xylariales, Diatrypaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: see Mehrabi et al. (2016). Asexual morph:Conidiomata brown, acervular, separate or gregarious, subperidermal, immersed, simple or irregular in shape, unilocular, glabrous, thick-walled. Conidiomatal wall composed of pale brown to hyaline, thick-walled cells of texura angularis to intricata at the basal part. Conidiophores hyaline, branched, fasciculate, septate in association with branches, tapered towards the apices, formed from the inner cells of basal stroma. Conidiogenous cells hyaline, sympodial, cylindrical, integrated, indeterminate, smooth, thick-walled. Conidia hyaline, falcate or filiform, curved, aseptate, thin-walled, smooth, eguttulate.
Type species: Quaternaria quaternata (Pers.) J. Schröt., in Cohn, Krypt.-Fl. Schlesien 3.2(4): 451 (1897) [1908]
Notes: Libertella is characterized by acervular conidiomata, holoblastic, sympodial, integrated conidiogenous cells and hyaline, falcate or filiform conidia (Sutton 1980). Saccardo (1880) listed L. faginea Desm. collected on Fagus sylvatica as the generic type, while Clements and Shear (1931) gave L. betulina Desm., collected on Betula sp. as lectotype species. Sutton (1980) revised Libertella and considered L. faginea representative of the genus, following Saccardo’s choice, because of its priority.
Libertella is a polyphyletic genus. The species in this genus have been linked to several sexual morphs genera in Xylariales (Grove 1937; Maharachchikumbura et al. 2015). Libertella faginea was linked to Q. quaternata (= Q. persoonii Tul.), type of Quaternaria (Saccardo 1882). Libertella betulina was linked to Diatrype stigma (Hoffm.) Fr. (Grove 1937; Croxall 1950). However, those connections have not been proven by molecular data. A coelomycetous fungus collected on Fagus sylvatica (Fagaceae) in Italy nested between two strains of Q. quaternata (EL60C, GNF13) with bootstrap value of 100 (MLBS) and 1 (BSPP) in Fig. 320. Our collection is similar to L. faginea in form of conidiomata, conidiogenous cells and conidia, and the only difference is conidial dimensions. However, the conidial dimensions could be different because of different geographical factors, host or measuring methods. For example, conidia dimensions of L. faginea have been given as 16 × 3–4 µm (Saccardo 1882), 17–26 9 × 0.5–0.75 µm (Grove 1937), 14–17 × 1.5 µm (Sutton 1980), and 15–22 × 1–2 µm (this study). Therefore, our collection is considered conspecific to L. faginea. Libertella is also reduced to a synonym of Quaternaria rather than Diatrype as suggested by Maharachchikumbura et al. (2015) based on morphology and phylogeny.
The taxonomy, nomenclature, and phylogeny of Quaternaria have been stated by Rappaz (1987), Gams (1994), Acero et al. (2004), and Vasilyeva (2011), and will not be presented again. Fresh collections are needed to investigate its boundary and relations with related genera.
Distribution: France, Germany, Iran, Italy, Romania, Slovakia, Switzerland, UK, Ukraine, Hungary (Adamčíková et al. 2011, Mehrabi et al. 2016; this study).
Quaternaria quaternata (Pers.) J. Schröt., in Cohn, Krypt.-Fl. Schlesien 3.2(4): 451 (1897) [1908]
= Quaternaria persoonii Tul. & C. Tul., Select. fung. carpol. 2: 105 (1863)
= Libertella faginea Desm., Annls Sci. Nat., sér. 1 19: 276 (1830)
Facesoffungi number: FoF 07558, Fig. 321
Quaternaria quaternata(MFLU 15-2605) a Herbarium package and specimens. b, c Appearance of brown conidiomata on the host. d Vertical section of conidiomata. e Section of peridium. f–k Conidiophores, conidiogenous cells and developing conidia (arrows show conidiogenous loci). l–q Conidia. Scale bars: d = 1000 μm, e = 200 μm, f, l = 20 μm, g–k = 10 μm, m–q = 5 μm
Saprobic on dead stems of Fagus sylvatica (Fagaceae). Sexual morph: see Mehrabi et al. (2016). Asexual morph:Conidiomata 800–2000 µm diam., 400–600 µm high, brown, acervular, subepidermal, separate or gregarious, immersed, subglobose or irregular in shape, unilocular, glabrous, thick-walled. Conidiomatal wall 30–50 µm wide, composed of pale brown to hyaline, thick-walled cells of texura angularis to texura intricata at the basal stroma. Conidiophores hyaline, subcylindrical, fasciculate, branched, septate in association with branches, tapered towards the apices, formed from the inner cells of basal stroma. Conidiogenous cells 14–26 × 1–2 µm, hyaline, holoblastic, sympodial, cylindrical, integrated, indeterminate, thick- and smooth-walled. Conidia 15–22 × 1–2 µm (\( \bar{x} \) = 19 × 1.5 µm; n = 30), hyaline, falcate, rounded at each end, curved, aseptate, thin-walled, smooth, eguttulate.
Material examined: Italy, Province of Forlì-Cesena, Passo la Calla, on dead land branches of Fagus sylvatica (Fagaceae), 3 August 2015, Erio Camporesi, IT2577 (MFLU 15-2605), (KUN, HKAS 93599).
Notes: The asexual morph of Quaternaria quaternata resembles Tryblidiopsis pinastri (= Tryblidiopycnis pinastri Höhn.) in having holoblastic, sympodial conidiogenous cells and falcate conidia, but it is distinguished by conidiomatal structure. Quaternaria quaternata has acervular, unilocular conidiomata, whereas T. pinastri has pycnidial, unilocular to multilocular conidiomata with thick basal stroma.
Rhabdocline Syd., in Sydow & Petrak, Ann Mycol. 20(3/4): 194 (1922)
= Meria Vuill., C. r. hebd. Séanc. Acad. Sci., Paris 122: 546 (1896)
= Rhabdogloeum Syd., in Sydow & Petrak, Ann Mycol. 20(3/4): 215 (1922)
Facesoffungi number: FoF 07572
Leotiomycetes, Leotiomycetidae, Incertae sedis, Hemiphacidiaceae
Saprobic or parasitic on the host plant in terrestrial habitat. Sexual morph: see Parker and Reid (1969). Asexual morph: two types (a) sporodochial asexual morph (e.g. Meria), see Sherwood-Pike et al. (1986) and Gernand et al. (1997); (b) acervular asexual morph (e.g. Rhabdogloeum). Conidiomata pale ochraceous to orange-yellow, gelatinous amphigenous, acervular, solitary to gregarious or confluent, rounded to oval, unilocular, glabrous, thick-walled, dehiscing by a split in the host epidermis. Ostiole absent. Conidiomatal wall composed of hyaline, thin-walled cells of textura angularis at base. Conidiophores arising from the upper cells of the basal stroma, hyaline, branched, septate. Conidiogenous cells hyaline, cylindrical to ampulliform, discrete or sometimes integrated, smooth-walled, proliferating one or two times. Conidia hyaline, cylindrical to ellipsoid, straight or slightly curved, narrowed at middle zone, aseptate, smooth-walled, guttulate, bearing a filiform, curved, apical, mucoid appendage.
Type species: Rhabdocline pseudotsugae Syd., Ann Mycol. 20(3/4): 194 (1922)
Notes: Rhabdogloeum was introduced by Sydow and Petrak (1922) to accommodate a single species R. pseudotsugae, collected on living leaves of Pseudotsuga taxifolia in North America, together with a new genus Rhabdocline described from same host. Therefore, Sydow (1922) suggested Rhabdocline to be the sexual morph of Rhabdogloeum. Two additional species, R. abietinum Dearn. collected on leaves of Abies fraseri and R. hypophyllum D.E. Ellis & L.S. Gill collected on leaves of P. taxifolia were included in Rhabdogloeum (Dearness 1928; Ellis and Gill 1945). However, Rhabdogloeum abietinum was later considered a synonym of Rhabdogloeopsis balsameae, and R. hypophyllum was transferred to Cryptocline Petrak (Nag Raj and Morgan-Jones 1973).
Parker and Reid (1969) reported that Rhabdocline weirii A.K. Parker & J. Reid was associated with Rhabdogloeum pseudotsugae based on culture study. Therefore, Rhabdogloeum was reduced to a synonym of Rhabdocline (Johnston et al. 2014, Wijayawardene et al. 2017). Except for Rhabdogloeum asexual morph, Rhabdocline species have also been reported to have a Meria asexual morph based on ITS sequence data (Sherwood-Pike et al. 1986; Gernand et al. 1997; Stone and Gernandt 2005). Meria is a hyphomycetous taxon which has sporodochial conidiomata, and oblong conidia without appendages, while Rhabdogloeum is a coelomycetous taxon with acervular conidiomata producing cylindrical to ellipsoid conidia with apical appendages (Sherwood-Pike et al. 1986, this study). The asexual morph of R. weirii (= Rhabdogloeum pseudotsugae) has not been studied by molecular data, thus further investigations based on fresh collections and phylogenetic inferences with multi-loci sequence are needed. We re-examined herbarium specimen of asexual Rhabdocline weirii and provide a detailed description and plate for further reference.
Distribution: Canada, USA (Parker and Reid 1969; Sherwood-Pike et al. 1986, this study)
Rhabdocline weirii A.K. Parker & J. Reid, Can. J. Bot. 47: 1540 (1969)
= Rhabdogloeum pseudotsugae Syd., in Sydow & Petrak, Ann Mycol. 20(3/4): 215 (1922)
Facesoffungi number: FoF 07573, Fig. 322
Rhabdocline weirii (asexual morph, DAOM 215573) a, b Herbarium package and specimen. c–f Appearance of orange-yellow conidiomata with yellowish conidial mass on host. g–j Vertical sections of conidiomata. k–o Conidiophores, conidiogenous cells and developing conidia (k, o stained by 10% KOH and India ink). p–t Conidia. Scale bars c = 1000 µm, d–f = 200 µm, g–j = 100 µm, k–t = 10 µm
Parasitic on leaves of Pseudotsuga menziesii.Sexual morph: see Parker and Reid (1969). Asexual morph:Conidiomata 420–550 µm diam., 180–230 µm high, pale ochraceous to orange-yellow, gelatinous amphigenous, pycnidial, solitary to gregarious or confluent, rounded to oval, unilocular, glabrous, thick-walled, dehiscing by a split in the host epidermis which often remains as a flap covering the conidioma, with yellowish conidia mass at lateral zone of conidiomata (Fig. 322d–f). Ostiole absent. Conidiomatal wall 10–20 µm wide, composed of thin-walled, hyaline, cells of textura angularis at base. Conidiophores arising from the upper cells of the basal stroma, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells 5–15 × 2–6 µm, hyaline, enteroblastic, phialidic, cylindrical to ampulliform, discrete or sometimes integrated, smooth-walled, proliferating once or twice. Conidia 11–17 × 3–6 µm (\( \bar{x} \) = 13.5 × 5 µm; n = 50), hyaline, cylindrical to ellipsoid, straight or slightly curved, narrowed at middle zone, obtuse or rounded at the apex, truncate at the base, aseptate, smooth-walled, guttulate, bearing a filiform, curved, apical, mucoid appendage 7–18 µm (\( \bar{x} \) = 12 µm) long.
Material examined: Canada, British Columbia, Langford, on leaves of Pseudotsuga menziesii (Pinaceae), June 1966, A.K. Parker (DAOM 215573).
Rhabdogloeopsis Petr., Ann Mycol. 23(1/2): 52 (1925)
Facesoffungi number: FoF 07574, Fig. 323
Rhabdogloeopsis balsameae (redrawn from Morgan-Jones et al. 1972c) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and conidia
Ascomycota, genera incertae sedis
Habit on Abies balsamea and A. fraseri (Pinaceae). Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, acervular, hypophyllous, solitary to gregarious, subepidermal, immersed, linear or oval, unilocular, glabrous. Ostiole absent. dehiscence by irregular splits of the overlapping host epidermis. Conidiomatal wall composed of thin-walled, pale brown to hyaline cells of textura angularis in the basal part. Conidiophores arising from the upper cells of the basal stroma, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, sympodial, cylindrical, slightly curved, integrated, indeterminate, smooth-walled, with 1–3 sympodial, unthickened proliferations towards the apices. Conidia hyaline, fusiform, sometimes broader at the middle zone, obtuse at the apex, slightly truncate the base, aseptate, smooth-walled, guttulate (adapted from Morgan-Jones et al. 1972c; Sutton 1980).
Type species: Rhabdogloeopsis balsameae (Davis) Petr., Ann Mycol. 23(1/2): 52 (1925)
Notes: Petrak (1925) proposed Rhabdogloeopsis to accommodate a segregated species Gloeosporium balsameae Davis as R. balsameae which was collected form Abies balsamea in Wisconsin. This genus was re-described by Nag Raj and Morgan-Jones (1973). While the illustration is purported to be of Rhabdogloeopsis balsameae it is, in fact, of asexual morph of Rhabdocline weirii (= Rhabdogloeum pseudotsugae), and it was amended by Sutton (1980) and Nag Raj (1993). Rhabdogloeopsis is characterized by holoblastic conidiogenous cells with 1–3 sympodial, unthickened proliferations towards the apices and fusiform, aseptate conidia (Bonar 1962; Sutton 1980). The holoblastic, sympodial, conidiogenous cells in Rhabdogloeopsis closely resemble Hypocline. The later has eustromatic conidiomata with upper and basal wall cells of textura angularis producing cylindrical conidia, which differs from the acervular conidiomata producing fusiform conidia in Rhabdogloeopsis (Sutton 1980; Morgan-Jones et al. 1972c).
Bonar (1962) and Korf (1962) made a connection between asexual morph Rhabdogloeopsis balsameae (≡ Gloeosporium balsameae) and sexual morph Stegopezizella balsameae (= Sarcotrochila balsameae (Davis) Korf), based on both morphs occurring on same host. However, this connection has not been proven by culture study. Thus Rhabdogloeopsis is accepted as a legitimate name following Kirk et al. (2013) and Wijayawardene et al. (2017b), rather than a synonym of Sarcotrochila (Index Fungorum 2019). It is necessary to obtain fresh material of type species and to make cultural connections in other species.
Distribution: USA (Sutton 1980).
Rhodesia Grove, British Stem- and Leaf-Fungi (Coelomycetes) 2: 205 (1937)
Facesoffungi number: FoF 07576, Fig. 324
Rhodesia subtecta (redrawn from Sutton 1980) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Habit on leaves and stems of Ammophila arenaria (Poaceae) (Sutton 1980). Sexual morph: undetermined. Asexual morph:Conidiomata brown, acervular, solitary to gregarious, subepidermal, immersed, elongate to subglobose in section view, unilocular, glabrous. Ostiole absent, dehiscence by irregular rupture of the overlapping tissue. Conidiomatal wall composed of pale brown cells of textura angularis in the basal part. Conidiophores arising from the inner wall layer of basal stroma, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled, with minute channel and evident collarette, periclinal wall thickened. Conidia hyaline, oval, tapered to apex, slightly truncate at the base, unicellular, smooth-walled, guttulate (adapted from Sutton 1980).
Type species: Rhodesia subtecta (Roberge ex Desm.) Grove, British Stem- and Leaf-Fungi (Coelomycetes) 2: 205 (1937)
Notes: Only one species was accepted by Index Fungorum (2019) and Sutton (1980), but Wijayawardene et al. (2017b) estimated two species in the genus. Rhodesia is similar to Asteroma in having acervular conidiomata and phialidic, cylindrical conidiogenous cells. The oval conidia in Rhodesia differs from those in Asteroma that are cylindrical to fusiform and acicular or even more broadly fusiform (Sutton 1980). The genus has never been studied by molecular data. Therefore, a fresh collection of the type species is needed to resolve its taxonomic placement.
Distribution: Germany (Sutton 1980).
Rhodesiopsis B. Sutton & R. Campb., Nova Hedwigia 30: 289 (1979) [1978]
Facesoffungi number: FoF 07578
Ascomycota, genera incertae sedis
Saprobic on leaves of host plant. Sexual morph: undetermined. Asexual morph:Conidiomata orange-yellow, acervular, scattered to gregarious, initially immersed, becoming erumpent, circular to oval or irregular in outline, unilocular, glabrous, gelatinous, dehiscing by an irregular split in the overlying host tissue. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis in the basal part. Conidiophores arising from the inner wall layer of basal stroma, hyaline, cylindrical to subcylindrical, branched, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, enteroblastic, annellidic, clavate to subcylindrical, integrated, indeterminate, smooth-walled, with percurrent proliferation. Conidia hyaline, ellipsoid to fusiform, with an obtuse or acute apex and a narrow and truncate base, unicellular, thick- and smooth-walled, guttulate, bearing a infundibuliform, flame-like or irregular, mucoid appendage at one end or both ends.
Type species: Rhodesiopsis gelatinosa B. Sutton & R. Campb., Nova Hedwigia 30: 290 (1979) [1978]
Notes: Rhodesiopsis is a monotypic genus. The ellipsoid to fusiform conidia with mucoid, apical appendage can be confused with those of Allantophomopsiella, Allantophomopsis and Zelandiocoela. However, Rhodesiopsis can be separated from these genera by its acervular conidiomata and annelidic conidiogenous cells with up to 7 annellations (Nag Raj 1993). There is no molecular data for Rhodesiopsis. Therefore, new collections are needed to epitypify the genus and to place it in a natural classification.
Distribution: UK.
Rhodesiopsis gelatinosa B. Sutton & R. Campb., Nova Hedwigia 30: 290 (1979) [1978]
Facesoffungi number: FoF 07579, Fig. 325
Rhodesiopsis gelatinosa (IMI 202761C, holotype) a, b Herbarium package and specimens. c–e Appearance of orange-yellow conidiomata on the host. f Vertical section of conidioma. g, h Conidiophores, conidiogenous cells and developing conidia. i–n Conidia. Scale bars: c–e = 200 µm, f = 100 µm, g–h = 10 µm, i–n = 5 µm
Saprobic on leaves of Phormium tenax (Asphodelaceae) and Poa oliosa (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 400–700 µm diam., 120–200 µm high, orange-yellow, acervular, scattered to gregarious, initially immersed, becoming erumpent, circular to oval or irregular in outline, unilocular, glabrous, gelatinous, thick-walled, lacking an ostiole, but dehiscing by an irregular split in the overlying host tissue. Conidiomatal wall 1 0–40 µm wide, composed of thick-walled, brown to hyaline cells of textura angularis in the basal part. Conidiophores arising from the inner wall layer of basal stroma, hyaline, cylindrical to subcylindrical or irregular, branched, septate, smooth-walled, invested in mucus. Conidiogenous cells 10–20 × 3–4 μm, hyaline, enteroblastic, annellidic, clavate to subcylindrical, integrated, indeterminate, smooth-walled, with up to seven percurrent proliferations. Conidia 8–12 × 3–5 μm (\( \bar{x} \) = 10 × 4 μm, n = 30), hyaline, ellipsoid to fusiform, with an obtuse or acute apex and a narrow and truncate base, unicellular, thick- and smooth-walled, guttulate, bearing an infundibuliform, flame-like or irregular, mucoid apical appendage and sometimes, a shorter and less conspicuous, mucoid, basal appendage.
Material examined: UK, Cornwall, Lizard, on the leaves of Phormium tenax (Asphodelaceae), B.C. Sutton, 18 April 1976 (IMI 202761C, holotype).
Rhodosticta Woron., Izv. Imp. St.-Peterburgsk. Bot. Sada 11: 13 (1911)
Facesoffungi number: FoF 07575, Fig. 326
Rhodosticta caraganae (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and developing conidia
Sordariomycetes, Sordariomycetidae, Phyllachorales, Phyllachoraceae
Parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata yellowish brown, stromatic, pycnidial, usually epiphyllous, scattered to gregarious, immersed, globose to irregular in shape, multilocular, occasionally convoluted, glabrous. Ostiole single to each locule, circular to irregular. Conidiomatal wall composed of thin-walled, loose, hyaline cells of textura angularis in the base and lateral part, merging with a few septate, unbranched, pale brown, hyphal elements extending towards and lining the ostiole. Conidiophores formed from the inner wall layer of the conidiomata, hyaline, cylindrical to subcylindrical, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, usually integrated, determinate, smooth-walled, apertures terminal or on short lateral branches which form immediately below septa, with flared collarettes and marked periclinal thickenings around the apertures. Conidia hyaline, cuneiform to obovoid, rounded at the apex, blunt and truncate at the base, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980; Nag Raj and DiCosmo 1982).
Type species: Rhodosticta caraganae Woron., Izv. Imp. St.-Peterburgsk. Bot. Sada 11: 13 (1911)
Notes: Rhodosticta was re-described and illustrated by Sutton (1980) and Nag Raj and DiCosmo (1982). The sexual morph of Rhodosticta has been assigned to Apiosphaeria Höhn. (Sydow 1930, 1935; Hanlin 1997) and Polystigma DC. (von Höhnel 1917; Sutton 1980; Cannon and Kirk 2007). Dianese et al. (1994) studied the asexual morph of A. guaranitica (Speg.) Höhn. (type species of Apiosphaeria) and stated that Oswaldina icarahyensis Rangel was its asexual morph, rather than R. caraganae. The connection between Rhodosticta and Polystigma has not been successfully demonstrated. Maharachchikumbura et al. (2015) listed Rhodosticta as a member of Chaetosphaeriaceae (Chaetosphaeriales, Sordariomycetes). However, Wijayawardene et al (2017a, b) placed it in Phyllachoraceae (Phyllachorales, Sordariomycetes) and included two taxa. Rhodosticta caraganae has been reported to be associated with leaf lesions on Caragana frutescens (Fabaceae) (Sutton 1980). There is no molecular data available for Rhodosticta. Fresh collections are needed to place it in a natural group.
Distribution: USA (Sutton 1980).
Rhytisma Fr., K. svenska Vetensk-Akad. Handl., ser. 3 40: 104 (1819)
Facesoffungi number: FoF 07577, Fig. 327
Rhytisma acerinum (asexual morph, redrawn from Sutton 1980) a Detail of conidiomatal wall. b Conidia. c Conidiophores, conidiogenous cells and developing conidia. d Vertical section of a conidioma
Leotiomycetes, Leotiomycetidae, Rhytismatales, Rhytismataceae
Saprobic or parasitic on the host plant. Sexual morph: see Cannon and Minter (1984), Weber and Webster (2002). Asexual morph:Conidiomata black, stromatic, solitary to gregarious or confluent, epidermal, immersed to semi-immersed, circular to irregular in outline, applanate, unilocular or multilocular, glabrous, ostiolate. Ostiole circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the upper part, becoming thicker, pale brown cells in the basal part, in which are scattered dark brown sclerotized cells. Conidiophores formed from the inner wall layer cells of basal stroma, hyaline, cylindrical to irregular, branched and septate at the base and irregularly above, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, determinate, smooth-walled, with periclinal wall thickened towards apex. Conidia hyaline, cylindrical or slightly tapered to the base, straight or slightly curved, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980; Weber and Webster 2002).
Type species: Rhytisma acerinum (Pers.) Fr., K. svenska Vetensk-Akad. Handl., ser. 3 40: 104 (1819)
Notes: The generic type of Melasmia, M. acerina Lév. was generally considered as asexual morph of R. acerinum (Darker 1967; Sutton 1980; Cannon and Minter 1984). Both sexual and asexual morphs were associated with tar spots on leaves of host plants in family Sapindaceae (Sutton 1980; Weber and Webster 2002; Suto 2009; Wang et al. 2009; Masumoto et al. 2014, 2015). Based on “one fungus = one name” principle, Rhytisma was proposed for conservation over Melasmia (Johnston et al. 2014, Wijayawardene et al. 2017). The asexual morph of Rhytisma resembles Aoria in having stromatic, glabrous conidiomata as well as the same habit causing tar spots on leaves, but the latter was distinguished from the former by its holoblastic, sympodial conidiogenous cells and ellipsoid conidia (Sutton 1980). There is no molecular data available for Melasmia species. It is necessary to obtain fresh collections and cultures to confirm connection between Rhytisma and Melasmia.
Distribution: worldwide (Wijayawardene et al. 2017).
Rileya A. Funk, Can. J. Bot. 57(1): 7 (1979)
Facesoffungi number: FoF 07580, Fig. 328
Rileya piceae (redrawn from Nag Raj and DiCosmo 1980) a Vertical section of conidioma. b Enlarged view of conidiomatal wall. c Conidia. d Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, scattered to gregarious, semi-immersed, innate-erumpent, globose, carbonaceous, unilocular, glabrous, ostiolate. Ostiole single, slightly papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown to brown cells of textura globulosa to textura angularis in the exterior, gradually merging with relatively thinner, hyaline cells of textura prismatica in the conidial hymenium. Conidiophores arising from inner layer cells of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, enteroblastic, annellidic, cylindrical, integrated or discrete, indeterminate, smooth-walled. Conidia hyaline, elongate-fusiform, truncate at the base, terminal, scolecosporous, with the distosepta thinner than the periclinal wall, smooth-walled, apical cell attenuated into a long tubular, flexuous appendage (adapted from Nag Raj and DiCosmo 1980; Nag Raj 1993).
Type species: Rileya piceae A. Funk, Can. J. Bot. 57(1): 7 (1979)
Notes: Rileya is monotypic. Rileya piceae was collected from living branches of Picea sitchensis (Pinaceae). The genus is characterized by globose, carbonaceous, pycnidial conidiomata, annellidic, cylindrical conidiogenous cells and scolecosporous, distoseptate conidia with a long tubular, flexuous apical appendage (Funk 1979; Nag Raj 1993). Rileya shares similar distoseptate conidia with Coryneum (e.g. C. megaspermum, C. stromatoideum, C. umbonatum), and Massariothea. However, Massariothea has paraphyses which are absent in Coryneum and Rileya. Coryneum differs from Rileya by its acervular conidiomata and brown, narrowly to broadly fusiform or globose conidia without an appendage (Sutton 1980; Nag Raj 1993). There is no molecular data available for Rileya. Fresh collections are needed to place it in a natural group.
Distribution: Canada (Nag Raj 1993).
Sakireeta Subram. & K. Ramakr., J. Indian bot. Soc. 36: 83 (1957)
Facesoffungi number: FoF 07583
Dothideomycetes, Incertae sedis, Botryosphaeriales, Botryosphaeriaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph: Conidiomata brown to dark brown, pycnidial, subepidermal, usually aggregated, immersed, globose to depressed globose, unilocular to irregular multi-locular, glabrous, ostiolate, thick-walled. Conidiomatal wall composed of thick-walled, brown cell of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, cylindrical to ampulliform. Conidia hyaline, subcylindrical to narrowly ellipsoidal or clavate, rounded at the apex, slightly truncated at the base, unicellular, initially bearing a cone-shaped, mucoid, apical sheath which splits up selectively forming three or four, tentaculiform, undulate appendages.
Type species: Sakireeta madreeya Subram. & K. Ramakr., J. Indian bot. Soc. 36: 84 (1957)
Notes: Nag Raj (1973a, b) re-described the generic type and showed it was similar to Tiarosporella paludosa in having subcylindrical to clavate conidia, with an everted cone-like appendage that later splits into 3–4 tentacular appendages, but it has smaller conidia. On this basis S. madreeya was referred to T. madreeya. The concept was followed by later authors (Sutton and Marasas 1976; Sutton 1980; Nag Raj 1993). Crous et al. (2015b) resurrected the genus Sakireeta based on T. madreeya, which has multilocular conidiomata embedded in a brown stroma. Sakireeta remains monotypic. To provide further taxonomy and phylogeny studies of Sakireeta, recollecting material from type localities and isolating the organism into pure culture is essential.
Distribution: India.
Sakireeta madreeya Subram. & K. Ramakr., J. Indian bot. Soc. 36: 84 (1957)
≡ Tiarosporella madreeya (Subram. & K. Ramakr.) Nag Raj, Can. J. Bot. 51(12): 2470 (1974) [1973]
Facesoffungi number: FoF 07584, Fig. 329
Sakireeta madreeya (MUBL 631 = MII 196129,holotype, f–h redrawn from Nag Raj 1993). a Herbarium specimen. b Additional slide. c Dark brown to black conidioma. d–e Conidia. Scale bars c = 100 µm, d–e, g–h = 10 µm, f = 50 µm
Saprobic on dead culms of Aristida setacea. Sexual morph: undetermined. Asexual morph: Conidiomata brown to dark brown, pycnidial, aggregated, immersed, globose to depressed globose, mostly irregularly multilocular in a stroma, glabrous, ostiolate, thick-walled. Conidiomata wall composed of thick-walled, brown cells of textura angularis in the outer layers, becoming hyaline cells towards conidial hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 8–10 × 2.5–4 µm, hyaline, holoblastic, cylindrical to ampulliform, determinate, smooth-walled, and arising all around the cavity of the conidioma. Conidia 17.5–26 × 4.5–9.7 µm (\( \bar{x} \) = 20 × 6.5 µm; n = 30), hyaline, subcylindrical to narrowly ellipsoidal or clavate, rounded at the apex, slightly truncated at the base, unicellular, initially bearing a cone-shaped, mucoid, apical sheath which splits into three or four, tentaculiform, undulate appendages.
Material examined: India, Madras, Choolai, on dead culms of Aristida setacea (Poaceae), K. Ramakrishnan, 27 November 1951 (MUBL 631 = MII 196129, holotype).
Sarcophoma Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 75 (1916)
Facesoffungi number: FoF 07581, Fig. 330
Sarcophoma endogenospora (redrawn from Sutton 1980) a Conidia. b Conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Saprobic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata yellowish brown to dark brown, stromatic, solitary, immersed, globose to subglobose, unilocular, glabrous. Ostiole absent, dehiscence by irregular split of the apical wall. Conidiomatal wall composed of thick-walled, pale brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, mono- or polyphialidic, ampulliform to doliiform, integrated or discrete, determinate, smooth-walled, periclinal wall thickened towards apex. Conidia hyaline, ellipsoid to obpyriform, with obtuse apex and narrow and truncate base, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Sarcophoma endogenospora Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 125(1–2): 76 (1916)
Notes: Morgan-Jones (1971), Sutton (1980) and Sivanesan (1984) considered S. miribelii (Fr.) Höhn. as the generic type and listed S. endogenospora as a synonym. Wijayawardene et al. (2017b) reinstated S. endogenospora as the generic type as it has priority. Three taxa were accepted in Sarcophoma, S. endogenospora, S. eriogoni (Ellis & Everh.) Arx and S. garganica (Sacc. & D. Sacc.) Arx (Sutton 1980; Wijayawardene et al. (2017b). Sarcophoma endogenospora (= S. miribelii) has been linked to the sexual morph Guignardia miribelii Aa based on culture study (Van der Aa 1975). However, G. miribelii was synonymized under Discosphaerina miribelii (Aa) Sivan. (Hyponectriaceae, Xylariales) by Sivanesan (1984). Due to a lack of culture and molecular data of the type species, the placement of Sarcophoma and its sexual morph are uncertain. Fresh collections are needed to place it in a natural group.
Distribution: Austria, France, Germany, Hungary, Italy, Latvia, Romania, UK (van der Aa 1975; Sutton 1980).
Satchmopsis B. Sutton & Hodges, in Sutton, Nova Hedwigia 26(1): 1 (1975)
Facesoffungi number: FoF 07582, Fig. 331
Satchmopsis brasiliensis (redrawn from 1977a) a Conidia. c Conidiophores, conidiogenous cells and developing conidia. b Enlarged view of lateral wall
Scaphidium boutelouae (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Conidia
Leotiomycetes, Leotiomycetidae, Leotiales, Cochlearomycetaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to brown, stromatic, superficial, subepidermal, sessile, infundibuliform to cupulate or cylindrical, unilocular. Ostiole absent, dehiscence by irregular splits of the apical wall. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis in the basal part, becoming vertically elongated, adpressed, parallel, dark brown hyphae of textura porrecta in the lateral part. Conidiophores arising from the upper cells of the basal stroma, hyaline, cylindrical, branched towards base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to long lageniform, integrated or discrete, determinate, smooth-walled, with minute collarettes. Conidia hyaline, cylindrical or acerose, unicellular, smooth-walled (adapted from Nag Raj 1977a) (Fig. 332).
Type species: Satchmopsis brasiliensis B. Sutton & Hodges, in Sutton, Nova Hedwigia 26(1): 3 (1975)
Notes: The genus was introduced by Sutton (1975) to accommodate a single species S. brasiliensis collected on dead leaves of Eucalyptus sp. in Brazil. The genus is characterized by sessile conidiomata with a one-cell thick periclinal wall composed of vertically elongated, almost parallel pseudoparenchyma, phialidic conidiogenous cells and hyaline, cylindrical, unicellular conidia (Nag Raj 1977a, b; Saikawa et al. 1991). Sutton and Pascoe (1987) included S. australiensis B. Sutton & Pascoe with an extensive geographical distribution in North, Central and South America, Asia, and Australasia. Castañeda (1987) added a third species, S. sacciformis R.F. Castañeda, which is distinguished by its falcate, 1-septate conidia with basal appendage, and a periclinal wall that is pale brown in the lower part, and dark brown in the apical part. However, S sacciformis was later designated as the type species of Zelosatchmopsis by Saikawa et al. (1991), based on its distinct conidial morphology. Another taxon, S. cubensis R.F. Castañeda & W.B. Kendr. was described from a dead leaf of Citharexylum fruticosum (Verbenaceae) in Cuba (Castañeda and Kendrick 1991). Crous et al. (2006b) showed that S. brasiliensis was close to several families (Dermateaceae (ITS), Tympanidaceae, Pseudeurotiaceae, and Vibrisseaceae (LSU) and Phacidiaceae (SSU) in Helotiales, but its definite placement cannot be confirmed. Ekanayaka et al. (2019) revised Leotiomycetes and placed Satchmopsis in Cochlearomycetaceae (Leotiales). The taxonomy of Satchmopsis warrants further investigations based on fresh collections and phylogenetic inferences with multi-loci.
Distribution: USA, Australasia, Brazil, Colombia, Cuba, Indonesia (Sutton 1975; Sutton and Pascoe 1987; Crous et al. 2006b).
Scaphidium Clem., Bot. Surv. Nebraska 5: 5 (1901)
Facesoffungi number: FoF 07585, Fig. 332
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, solitary to gregarious, immersed to semi-immersed, globose to subglobose, unilocular, glabrous. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the exterior, becoming paler towards the hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, annellidic, cylindrical to lageniform, determinate, smooth-walled, proliferating percurrently with up to four annellations. Conidia hyaline, fusiform, with rounded apex and truncate base, 1-septate, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Scaphidium boutelouae Clem., Bot. Surv. Nebraska 5: 5 (1901)
Notes: The conidia of Scaphidium were described as yellow, fusiform-obclavate, curved, and 1-septate by Sprague (1950). Sutton (1980) revised the genus and described the conidia as hyaline, fusiform, with a rounded apex and truncate base, and 1-septate. Scaphidium shares similar form of conidiomta and conidia, as well as habit with Diplozythiella. Both genera can cause lesions on leaves, such as S. boutelouae produces linear brown or grey lesions with brown borders on Bouteloua curtipendula (Poaceae), while Diplozythiella bambusina was associated with Bambusa (Sprague 1950; Sutton 1980). Scaphidium was separated from Diplozythiella by its annellidic conidiogenous cells. Scaphidium is monotypic, and no molecular data is available. Fresh collection of the type is needed to place it in a natural group.
Distribution: USA (Sutton 1980).
Scopaphoma Dearn. & House, N.Y. St. Mus. Bull. 266: 83 (1925)
Facesoffungi number: FoF 07586
Ascomycota, genera incertae sedis
Hyperparasitic on Polyporus sp. (Polyporaceae). Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, papillate ostioles visible in surface view, globose to subglobose, unilocular, glabrous, thick-walled, ostolate. Ostiole central, circular, papillate. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores arising all around the cavity of the conidiomata, hyaline, branched, septate. Conidiogenous cells hyaline, subcylindrical to lageniform, integrated, indeterminate, smooth-walled, Conidia hyaline, ellipsoidal to subcylindrical, straight or slightly curved, 1-septate, apical cell attenuated apically into a flexuous, filiform, tubular, unbranched appendage, basal cell bearing an unbranched, tubular appendage separated from the conidium body by a septum.
Type species: Scopaphoma corioli Dearn. & House, N.Y. St. Mus. Bull. 266: 83 (1925)
Notes: Scopaphoma is a monotypic genus. Scopaphoma corioli is fungicolous on Polyporus sp. and shares similar morphology with Eleutheromyces mycophilus, a fungicolous species on agarics, in having pycnidial conidiomata, and subcylindrical to lageniform, integrated conidiogenous cells. However, they can be differentiated from each other by conidial form. Scopaphoma corioli has ellipsoidal to subcylindrical, 1-septate conidia, while Eleutheromyces mycophilus has ellipsoidal, lenticular or cylindrical, unicellular conidia. No sexual morph has been linked to this genus and no molecular data is available. Fresh collections are needed to place it in a natural group.
Distribution: Canada, USA (Nag Raj 1993; this study).
Scopaphoma corioli Dearn. & House, N.Y. St. Mus. Bull. 266: 83 (1925)
Facesoffungi number: FoF 07734, Fig. 333
Scopaphoma corioli (DAOM 215577) a, b Herbarium package and specimens. c, d Appearance of dark brown conidiomata on host. e, f Vertical sections of conidiomata. g Section of peridium. h–k Conidiophores, conidiogenous cells and developing conidia. l–q Conidia. Scale bars c = 500 µm, d = 200 µm, e–f = 100 µm, g = 20 µm, h–k = 10 µm, l–q = 5 µm
Fungicolous on Polyporus sp. (Polyporaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 220–280 µm diam., 200–300 µm high, brown to dark brown, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, papillate ostioles visible in surface view, globose to subglobose, unilocular, glabrous, thick-walled. Ostiole circular, papillate, centrally located. Conidiomatal wall 20–50 µm wide, composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores formed form the innermost cells of conidiomata, hyaline, branched, septate, smooth-walled. Conidiogenous cells 10–20 × 1–3 µm, hyaline, subcylindrical to lageniform, integrated, indeterminate, smooth-walled, Conidia composed of a 2-celled, hyaline, ellipsoidal to subcylindrical, straight or slightly curved conidium body 4–14 × 1–2 µm (\( \bar{x} \) = 8 × 1.3 µm; n = 30), apical cell attenuating to flexuous, filiform, tubular, unbranched appendage 5–19 μm long (\( \bar{x} \) = 11 μm) towards apex, basal cell bearing an unbranched, tubular appendage 1–3 μm long (\( \bar{x} \) = 2 μm), separated from the conidium body by a septum.
Material examined: Canada, Ontario, Waterloo, Columbia & Erbsville Rd, on Polyporus sp. (Polyporaceae), May 1981, F. DiCosmo (DAOM 215577).
Scorias Fr., Syst. mycol. 3(2): 269, 290 (1832)
Facesoffungi number: FoF 07589, Figs. 334, 335
Scorias spongiosa (asexual morph) a Host plant. b, c Sooty moulds on the surface of host plant. d–j Immature ascomata and pycnidia. k Ostiole surrounded by hyaline hyphae. l Abundant conidia at base of pycnidium and pycnidia wall. m, n Conidia. Scale bars d = 100 μm, e, j = 40 μm, f–i = 20 μm, k–l = 10 μm, n, m = 5 μm
Scorias spongiosa (asexual morph) a Appearance of dark brown to black conidiomata on the surface of PDA. b, c Conidial mass at the apex of pycnidia. d, e Septate hyphae. f–i Immature and mature pycnidia. j Apex of immature pycnidium. k, m pycnidial wall. l Ostiole surround by hyaline hyphae. Scale bars d–e, j–m = 10 μm, f–h = 20 μm, i = 50 μm
Dothideomycetes, Dothideomycetidae, Capnodiales, Capnodiaceae
Epiphytic or saprobic on sugary exudates from insects on the surface of branches or living leaves. Thallus composed of dark brown to brown, cylindrical, branched, septate and constricted at septum, dense mycelium. Sexual morph: see Chomnunti et al. (2011). Asexual morph:Conidiomata dark brown at the base, becoming paler towards apex, pycnidial, gregarious, superficial, flask-shaped, tapering to the apex, branched at the base. Pycnidial wall composed of dark brown, thick-walled cells of helical twisting at the lower half part, becoming pale brown, thin-walled cells of textura porrecta at the upper part. Conidiophores formed from inner layers of pycnidial wall. Conidiogenous cells not observed. Conidia hyaline, ellipsoid, unicellular, smooth-walled, eguttulate.
Type species: Scorias spongiosa (Schwein.) Fr., Syst. mycol. (Lundae) 3(2): 291 (1832)
Notes: Capnodium, Conidiocarpus, Leptoxyphium, and Scorias are sooty moulds with hyaline, ellipsoid conidia (Chomnunti et al. 2011, this study). Leptoxyphium has grey-brown, superficial, synnematous conidiomata whereas they are pycnidial conidiomata in Capnodium, Conidiocarpus and Scorias. Conidiocarpus has superficial pycnidia differentiated into a basal stalk and a globose locule at the upper half, while Capnodium and Scorias have superficial, flask-shaped pycnidia tapering to the apex (Chomnunti et al. 2011). Scorias was separated from Capnodium by its sexual morph. Capnodium has 8–10-spored, bitunicate, clavate, ovoid or saccate, aparaphysate, apedicellate asci and brown, oblong or ovoid and some reniform, 3–5 trans-septate ascospores with a verrucose wall. Scorias has 8-spored, bitunicate, oblong to saccate, apedicellate asci with a long ocular chamber and brown, fusiform, 3–4 trans-septate ascospores with the upper cells slightly wider than the lower cells (Chomnunti et al. 2011).
Chomnunti et al. (2011) epitypified Scorias spongiosa based on a collection on a living leaf of Entada sp. (Fabaceae) in Thailand, and confirmed its placement in Capnodiaceae. Hongsanan et al. (2015) added S. mangiferae Hongsanan et al. from a branch of Mangifera sp. (Anacardiaceae) in Thailand. An additional eleven species are listed in Index Fungorum (2019), but they have not been studied with molecular data.
Distribution: Thailand, South Africa, USA (Chomnunti et al. 2011; Crous et al. 2011b, this study).
Septopatella Petr., Ann Mycol. 23(1/2): 128 (1925)
= Fujimyces Minter & Caine, Trans. Br. Mycol. Soc. 74(2): 434 (1980)
Facesoffungi number: FoF 07587, Fig. 336
Septopatella septata (redrawn from Dyko and Sutton 1979a) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat (e.g. Pinaceae) (Minter and Caine 1980; Dyko and Sutton 1979a). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, solitary to gregarious, superficial, initially closed, ultimately become erumpent, oval to roundish or irregular in outline, cupulate in section view, sessile, unilocular, hypostroma extending into stomata of host. Ostiole absent, dehiscence by irregular breakdown of the apical wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the basal part, becoming textura porrecta in the lateral part. Conidiophores arising from inner layer of cells of basal and lateral walls, cylindrical, branched at the base, septate, smooth-walled, invested in mucus. Conidiogenous cells hyaline, holoblastic, sympodial, cylindrical, integrated, indeterminate, smooth-walled. Conidia hyaline, filiform, with acute apex, 2–7-septate, smooth-walled, guttulate (Dyko and Sutton 1979a).
Type species: Septopatella septata (Jaap) Petr., Ann Mycol. 23(1/2): 129 (1925)
Notes: Septopatella is monotypic. The conidiomata and conidiogenous cells of S. septata have been reported as acervular, and phialidic, respectively (Morgan-Jones and Kendrick 1972; Morgan-Jones 1977). Dyko and Sutton (1979a) re-examined the type specimen and showed that the conidiomata are cupulate, sessile, and the conidiogenous cells are holoblastic, with sympodial proliferation. Septopatella resembles Fujimyces in form of conidiomata and conidia. The differences between these genera are conidiogenous cells monoblastic in Fujimyces vs sympodial proliferating in Septopatella and conidia are 3-septate in Fujimyces vs 2–7-septate in Septopatella. However, these differences could be regarded as features to distinguish each at the species level. Both are associated with Pinus, with Septopatella collected on needles of Pinus montana, P. nigra, and P. ponderosa, and Fujimyces on dead needles or cone apophyses of P. sylvestris. Therefore, Fujimyces is considered a synonym of Septopatella, as the latter has priority. Fresh collections of both types are needed to confirm this synonymy.
Distribution: Austria, Czechoslovakia, Greece, UK, USA (Minter and Caine 1980; Dyko and Sutton 1979a).
Septoria Sacc., Syll. fung. (Abellini) 3: 474 (1884)
Facesoffungi number: FoF 07588
Dothideomycetes, Dothideomycetidae, Capnodiales, Mycosphaerellaceae
Parasitic on the host plant in terrestrial habitat. Sexual morph: mycosphaerella-like (Quaedvlieg et al. 2013). Asexual morph:Conidiomata brown, pycnidial, separate or aggregated, but not confluent, immersed, globose, ostiolate. Ostiole single, circular, centrally located. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis in the outer layers, gradually merging with smaller cells in the inner layer, becoming thick-walled, darker cells around the ostiolar region. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, proliferating sympodially with unthickened scars, and/or proliferating percurrently towards the apices, ampulliform, doliiform or lageniform to short cylindrical, determinate or indeterminate, smooth-walled, formed from the inner layer of conidiomata. Conidia hyaline, filiform, multiseptate, continuous or constricted at the septa, smooth-walled (adapted from Sutton 1980; Quaedvlieg et al. 2013).
Type species: Septoria cytisi Desm., Annls Sci. Nat., Bot., sér. 3 8: 24 (1847)
Notes: Septoria species are widespread plant pathogens and commonly associated with leaf spots and stem cankers of a wide range of plant hosts (Quaedvlieg et al. 2013; Verkley et al. 2013). This genus is extremely large, with more than 2000 taxa described (Sutton 1980; Verkley and Priest 2000; Verkley et al. 2004). The number of species described in Septoria rose to this level due to the common practice of host-associated nomenclature, with a general lack of specific morphological characters (Quaedvlieg et al. 2013). Verkley et al. (2004), Feau et al. (2006) and Quaedvlieg et al. (2011) showed that Septoria is a polyphyletic genus based on molecular data. In a comprehensive phylogenetic study on Septoria and morphologically similar genera, Quaedvlieg et al. (2013) placed Septoria sensu stricto in Mycosphaerellaceae based on multi-loci of ITS, LSU, tef1, rpb2 and tub2, and introduced 14 new genera to accommodate septoria-like taxa. Furthermore, Septoria was defined by having pycnidial to acervular conidiomata, conidiogenous cells that proliferate sympodially and percurrently and hyaline, filiform, multi-septate conidia (Quaedvlieg et al. 2013). To clarify the boundary of Septoria, many new collections are needed.
Illustration see Quaedvlieg et al. (2013).
Distribution: worldwide.
Sirococcus Preuss, Linnaea 26: 716 (1855) [1853]
Facesoffungi number: FoF 06326
Sordariomycetes, Sordariomycetidae, Diaporthales, Gnomoniaceae
Saprobic or parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata brown to black, pycnidial, solitary to gregarious, immersed to erumpent, globose to subglobose, unilocular or multilocular, glabrous. Ostiole absent, dehiscence by breakdown of the upper wall. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura intricata to textura angularis. Conidiophores formed from inner layer of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, fusiform to cylindrical, 1-septate, smooth-walled, guttulate.
Type species: Sirococcus conigenus (Pers.) P.F. Cannon & Minter, Taxon 32(4): 577 (1983)
Notes: Sutton (1980) gave a full account of the taxonomy of Sirococcus and accepted two species, S. spiraea (Lebedeva) Petr. and S. strobilinus. Cannon and Minter (1983) considered Hypoderma conigenum (Pers.) DC. to be an earlier name for S. strobilinus and made the new combination, S. conigenus as the type species. This generic concept was followed by later authors (Bronson et al. 2003; Rossman et al. 2007; Sogonov et al. 2008; Crous et al. 2016a). Rossman et al. (2007) designated an epitype for S. conigenus on cones of Picea abies (Pinaceae) in Finland, and placed the genus in Gnomoniaceae. They also provided a detailed description and illustration. Crous et al. (2016a) added an additional species S. quercus Crous based on LSU and ITS sequence data. More than 40 taxa are listed in Index Fungorum (2019), but few have been studied with molecular data. A fresh collection (MFLU 15-1235) collected on P. abies from Italy clustered with the epitype (CBS 119615) and other collections of S. conigenus with high bootstrap support (100 MLBS /1 BPP) (Fig. 23). Morphologically, the fresh collection has similar form of conidiomata, conidiophores and conidia with the generic type of Sirococcus. Based on phylogeny, morphology and host, this collection is considered conspecific with Sirococcus conigenus. A detailed description and plate is provided. Sirococcus species are important pathogens causing shoot blight and tip dieback on conifers and canker on butternut (Nair et al. 1979; Konrad et al. 2007; Rossman et al. 2007). Further studies with fresh collections are needed to study the taxonomy, phylogeny and ecology of Sirococcus species.
Distribution: Austria, Canada, Finland, France, Germany, Latvia, Switzerland, UK, USA (Nair et al. 1979; Sutton 1980; Rossman et al. 2007; Crous et al. 2016a).
Sirococcus conigenus (Pers.) P.F. Cannon & Minter, Taxon 32(4): 577 (1983)
Facesoffungi number: FoF 07591, Fig. 337
Sirococcus conigenus(MFLU 15-1235) a Herbarium specimen. b–d Appearance of dark brown to black conidiomata on the host. e–f Vertical sections of conidiomata. g Section of peridium. h–l Conidiophores, conidiogenous cells and developing conidia. m–p Conidia. Scale bars b = 1000 µm, c = 500 µm, d = 200 µm, e–f = 100 µm, g = 50 µm, h–m = 10 µm, n–p = 5 µm
Saprobic or parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata 300–400 µm diam., 250–300 µm high, dark brown to black, pycnidial, solitary to gregarious, initially immersed, becoming erumpent, globose, unilocular or multilocular, convoluted, glabrous. Ostiole absent, dehiscence by breakdown of the upper wall. Conidiomatal wall 40–100 µm wide, composed of thick-walled, dark brown to brown cells of textura angularis in the exterior, becoming thin-walled, pale brown to hyaline cells of textura intricata towards hymenium. Conidiophores formed from inner layers of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells 5–18 × 2–3 µm, hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled, with a minute apical channel and collarette, periclinal thickenings at the collarette zone. Conidia 10–14 × 2–3 µm (\( \bar{x} \) = 12 × 2.8 µm; n = 50), hyaline, cylindrical to fusiform, with an obtuse and narrow apex and a slightly truncate base, 1-septate, smooth-walled, guttulate.
Material examined: Italy, Province of Forlì-Cesena, Santa Sofia, Campigna, on dead land strobilus of Picea abies (Pinaceae), 15 April 2015, Erio Camporesi, IT2447 (MFLU 15-1235); (KUN, HKAS 97485)
Sirophoma Höhn., Hedwigia 59(5): 257 (1917)
Facesoffungi number: FoF 07590, Fig. 338
Sirophoma singularis (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on the host plants in terrestrial habitat, such as Carpinus betulus (Betulaceae), Grewia villosa (Malvaceae), Nelumbo nucifera (Nelumbonaceae), Petroselinum sativum (Apiaceae), Sambucus racemose (Adoxaceae) and Viburnum opulus (Adoxaceae) (Sutton 1980; Index Fungorum 2020). Sexual morph: undetermined. Asexual morph:Conidiomata brown, pycnidial, solitary to gregarious, immersed, depressed globose, unilocular, glabrous. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the exterior, becoming paler towards hymenium. Conidiophores formed from inner wall layer of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical or doliiform, integrated or discrete, determinate, smooth-walled, with terminal or lateral apertures formed immediately below transverse septa, periclinal thickenings at the collarette zone. Conidia hyaline, globose to ellipsoid or pyriform, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Sirophoma singularis Höhn., Hedwigia 59(5): 257 (1917)
Notes: Sutton (1980) re-described and illustrated Sirophoma. An additional six species are listed in Index Fungorum (2019) but they have not been studied with molecular data. Sirophoma is similar to asexual morph of Tympanis (e.g. T. spermatiospora), Collophorina (≡ Collophora), Dendrodomus, Pyrenochaeta and Pleurophoma in having cylindrical, branched conidiophores with phialidic, acropleurogenous, branched conidiogenous cells. Collophorina (Tympanidaceae, Helotiales, Leotiomycetes) has pseudopycnidial conidiomata with textura epidermoidea wall, which differs from those in the other genera that are ostiolate pycnidia with textura angularis wall (Sutton 1980; Damm et al. 2010; Wijayawardene et al. 2017; Nasr et al. 2018). Pyrenochaeta is separated from other genera by its setose conidiomata. Sirophoma possesses hyaline, globose to ellipsoid or pyriform, aseptate conidia, whereas they are cylindrical in asexual morph of Tympanis, and ellipsoid to cylindrical in Dendrodomus and Pleurophoma. Dendrodomus differs from Pleurophoma by its paraphyses (Sutton 1980).
Distribution: Austria, Czech Republic, Finland, Pakistan, Romania (Sutton 1980; Index Fungorum 2019).
Siroplacodium Petr., in Rechinger et al., Annln naturh. Mus. Wien 50: 509 (1940)
Facesoffunginumber: FoF 07592, Fig. 339
Siroplacodium atrum (redrawn from Sutton 1980) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidioma
Ascomycota, genera incertae sedis
Saprobic on the host plants in terrestrial habitat, e.g. Campanula (Campanulaceae), Solidago virgaurea (Asteraceae), Poaceae.Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, stromatic, acervular, solitary to gregarious, immersed, applanate to lenticular, unilocular, glabrous. Ostiole absent, dehiscence by a longitudinal slit. Conidiomatal wall composed of thick-walled, dark brown to pale brown cells of textura angularis. Conidiophores arising from inner layers of basal stroma, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, integrated or discrete, determinate, smooth-walled, with a long flared collarette and marked periclinal thickening around the apertures. Conidia hyaline, cylindrical with an obtuse apex and a slightly truncate base, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Siroplacodium atrum Petr., in Rechinger, Baumgartner, Petrak & Szatala, Annln naturh. Mus. Wien 50: 509 (1940)
Notes: Siroplacodium is similar to Cyclodomus and Blennoria in having cylindrical conidia. Cyclodomus is distinguished from Siroplacodium by its pycnidial conidiomata and cylindrical to ampulliform conidiogenous cells without a long flared collarette (Sutton 1980; Nag Raj and DiCosmo 1982). Blennoria was separated from Siroplacodium by its multilocular, pycnidial conidiomata. Six species are accepted in Siroplacodium, namely S. atrum, S. caulocarpum H. Ruppr., S. cudraniae G.C. Zhao & R.L. Zhao, S. longisporum Petr., S. shastense (R. Sprague & W.B. Cooke) Petr. and S. umbelliferarum Petr. (Sutton 1980; Zhao et al. 2012; Wijayawardene et al. 2017; Index Fungorum 2019). Except for the type species, the other taxa have not been re-studied. No molecular data is available for this genus. The taxonomy of this genus warrants further investigations based on fresh collections and phylogenetic inferences.
Distribution: China, Germany, Iran, Iraq (Zhao et al. 2012; Index Fungorum 2020).
Sirozythiella Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 1532 (1909)
Facesoffungi number: FoF 07594, Fig. 340
Sirozythiella sydowiana (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Habit on leaves and culms of Phragmites communis (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary, initially immersed, becoming erumpent, globose, unilocular, glabrous. Ostiole absent, opening by irregular splits of apical wall. Conidiomatal wall composed of outer layers of thick-walled, brown to pale brown cells of textura intricata at the base, becoming thick-walled, pale brown cells of textura angularis towards hymenium and upper part. Conidiophores arising from inner layer of basal and lateral wall of conidiomata, hyaline, cylindrical to subcylindrical, branched at the base and repeatedly above, septate, smooth-walled. Conidiogenous cells hyaline, hollothallic, cylindrical, integrated, indeterminate, smooth-walled. Conidia hyaline, arthric, long doliiform, truncate at both ends, except apical conidia which are subacute, 2-septate, straight or slightly curved, smooth-walled, eguttulate, formed by disarticulation of the conidiogenous cells from the conidiophore, produced in long, branched chains with the youngest conidia at the base (Sutton 1980).
Type species: Sirozythiella sydowiana (Sacc.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 1532 (1909)
Notes: Sirozythiella remains monotypic. This genus is similar to Chondropodiella, Phacidiella and Phellostroma in having hollothallic, cylindrical conidiogenous cells, but it was separated from these genera by conidiomata and conidia form. These differences are discussed under notes of Phellostroma. No molecular data is available for Sirozythiella. Fresh collections are needed to place it in a natural group.
Distribution: Germany (Sutton 1980).
Sphaerellopsis Cooke, Grevillea 12(no. 61): 23 (1883)
Facesoffungi number: FoF 07596
Dothideomycetes, Pleosporomycetidae, Pleosporales, Leptosphaeriaceae
Parasitic on rust fungi. Sexual morph:Ascomata dark brown to black, stromatic, solitary to gregarious, immersed to semi-immersed, subglobose to irregular, unilocular, glabrous, ostiolate. Ostiole papillate, single, centrally located. Peridium composed of brown to dark brown, thin-walled cells of textura angularis at the basal and lateral part, becoming slightly thickened towards upper part. Hamathecium composed of numerous pseudoparaphyses and asci. Pseudoparaphyses cylindrical, branched, septate, frequently anastomosing, embedded in mucilaginous matrix. Asci 8-spored, bitunicate, fissitunicate, cylindrical-clavate, pedicellate, apically rounded, with an indistinct ocular chamber. Ascospores pale brown or pale honey-yellow, regularly biseriate, spindle-shaped, with narrow and obtuse ends, 2–3-septate, constricted at septa, slightly inequilateral, smooth-walled (Spegazzini 1908; Yuan et al. 1999). Asexual morph:Conidiomata dark brown to black, pycnidial, occasionally solitary, mostly gregarious or confluent, immersed to semi-immersed, unilocular or multilocular, glabrous, with locule globose or pyriform, thick-walled, ostiolate. Ostiole cylindrical, centrally located. Conidiomatal wall composed of pale brown to hyaline cells of textura angularis to textura prismatica. Conidiophores lining the cavity of the conidiomata, mostly reduced to conidiogenous cells, hyaline, cylindrical or doliiform, septate, branched, invested in mucus. Conidiogenous cells of two kinds: (a) those producing macroconidia, arising from the basal and lateral zone, hyaline, enteroblastic, ampulliform to doliiform, subcylindrical or irregular, smooth-walled, discrete or integrated, with or without percurrent proliferations; (b) those producing microconidia, restricted to the area around the ostiolar channel, hyaline, ampulliform, smooth-walled. Macroconidia hyaline, fusiform to ellipsoidal, Y-shaped or digitate, septate, smooth-walled, bearing mucoid appendages at each end or only at one end. Microconidia short-cylindrical to ellipsoidal or subglobose, unicellular, colourless, smooth-walled.
Type species: Sphaerellopsis filum (Biv.) B. Sutton, Mycol. Pap. 141: 196 (1977)
Notes: Sphaerellopsis filum was originally described by Bivona-Bernadi (1813–1816) from rust fungi on Convolvulus sepium and Populus nigra in Sicily. This species is a well-known mycoparasite associated with approximately 370 species and 30 genera of rusts, occurring in more than 50 countries (Kranz and Brandenburger 1981). Sphaerellopsis filum has been shown to colonize rust pustules and suppress rust spore production (Yuan et al. 1999). Due to this fact it has been regarded as a potential biocontrol agent (Morris et al. 1994, 1995; Kuhlman et al. 1978; Whelan et al. 1997; Pei et al. 2003; Płachecka 2005). However, little is known of the population biology and genetic diversity of S. filum.
The generic name Sphaerellopsis was reduced to a synonym of Darluca Castagne (Castagne 1851), and Ascochyta Lib. (Saccardo 1884a, b). However, Petrak (1968) regarded it as distinct genus. Sutton (1977a) showed that Sphaerellopsis is an earlier name for Darluca, and resurrected the genus, with S. filum as type species. Since then the generic concept was formally established. Nag Raj (1993) re-described and illustrated the genus and added an additional species S. anomala Nag Raj based on its Y-shaped to digitate conidia. Trakunyingcharoen et al. (2014) included molecular data of S. filum in their phylogenetic analyses and showed that it belongs to Leptosphaeriaceae (Dothideomycetes). They also designated a neotype (CBS H-21851) for S. filum, and introduced two new species S. macroconidialis Crous & Trakun. and S. paraphysata Crous & Alfenas. Crous et al. (2016a) introduced a new species S. hakeae Crous based on sequence data of LSU and ITS, together with its conidial morphology. Five species are accepted in the genus, S. anomala, S. filum, S. hakeae, S. macroconidialis and S. paraphysata.
The sexual morph of Sphaerellopsis (= Darluca) was assumed to be a well-known mycoparasite, Eudarluca Speg. (Spegazzini 1908; Keener 1951). The connection between these two morphs was confirmed via culture studies by Yuan et al. (1999) and phylogenetic sequences data by Trakunyingcharoen et al. (2014). Eudarluca is therefore reduced to a synonym of Sphaerellopsis, as the latter name has priority, is widely used in the literature, and is widely accepted by plant pathologists (Trakunyingcharoen et al. 2014). We re-examined the type specimen of S. anomala and S. filum and provide a detailed description and photo plate for both species. More collections are needed to study the boundary of Sphaerellopsis and its sexual morph.
Distribution: worldwide.
Key to species of Sphaerellopsis
1.Paraphyses present, hyaline, filiform, septate…S. paraphysata
1.Paraphyses absent…2
2.Conidia Y-shaped or digitate, triradiate…S. anomala
2.Conidia fusiform to ellipsoidal…3
3.Conidia 1–3-septate…S. macroconidialis
3. Conidia 1-septate…4
4.Conidiogenous cells annelidic, discrete to integrated, conidia 2.5–4.5 μm wide…S. filum
4. Condiogenous cells phalidic, conidia (4–)5(–6) µm wide…S. hakeae
Sphaerellopsis anomala Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 896 (1993).
Facesoffungi number: FoF 07597, Fig. 341
Sphaerellopsis anomala (DAOM 215300, holotype) a, b Herbarium specimen. c, d Appearance of dark brown to black conidiomata on the host (d note conidia mass on the top of conidioma). e, f Vertical sections of conidiomata. g Ostiole. h Section of peridium. i–n Conidiophores, conidiogenous cells and developing conidia. o–w Conidia. Scale bars: c = 500 µm, d = 200 µm, e–f, g = 50 µm, h = 20 µm, i = 5 µm, j–w = 10 µm
Parasitic on Puccinia sp. on Eupatorium sp. (Rosaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 110–210 µm diam., 140–210 µm high, dark brown to black, with yellowish conidial mass at the top of conidiomata, pycnidial, gregarious or confluent, deeply immersed, globose or pyriform, unilocular, thick-walled, glabrous, ostiolate. Ostiole 40–80 µm long, 40–90 µm wide, single, cylindrical, centrally located. Conidiomatal wall 8–30 µm wide, composed of thick-walled, pale brown to hyaline cells of textura angularis to textura prismatica at basal and lateral zone, becoming relatively thick-walled, brown to dark brown cells of textura angularis at upper zone. Conidiophores lining the cavity of the conidiomata, mostly reduced to conidiogenous cells, hyaline, invested in mucus. Conidiogenous cells of two kinds: (a) those producing macroconidia, hyaline, enteroblastic, ampulliform to doliiform, subcylindrical or irregular, smooth-walled, 10–14 × 1.5–4 μm (\( \bar{x} \) = 11 × 2.5 μm), with 1–3 percurrent proliferations; (b) those producing microconidia, hyaline, ampulliform, smooth-walled, 5–9 × 2.5–6 μm (\( \bar{x} \) = 7 × 4 μm) μm. Macroconidia composed of two kinds, (a) Y-shaped or digitate, triradiate, with main axis 7–16 × 3–5 μm (\( \bar{x} \) = 9.5 × 3.5 μm, n = 50), cylindrical to subcylindrical, narrowed and obtuse at the base, broader above, aseptate, bearing two arms 8–21 × 2–4 μm (\( \bar{x} \) = 15 × 3 μm, n = 50), obtuse or sometimes acute at both ends, 2–3-septate, with or without constrictions at the septa, smooth-walled, bearing mucoid appendages at each end or only at one end; (b) fusiform to ellipsoidal, 14–23 × 4–5 μm (\( \bar{x} \) = 19 × 4.6 μm, n = 50), with narrow and rounded base, usually 1-septate, constricted at septa, bearing mucoid, apical appendages. Microconidia 5–7 × 2–2.5 μm (\( \bar{x} \) = 6 × 2.2 μm), short-cylindrical to ellipsoidal or subglobose, unicellular, colourless, smooth-walled (measurement of microconidia from Nag Raj 1993).
Material examined: Colombia, Bogota, km 36 via Caqueza-Villavicenza, 11 October 1975, Pablo Buritica (DAOM 215300, holotype).
Sphaerellopsis filum (Biv.) B. Sutton, Mycol. Pap. 141: 196 (1977)
Facesoffungi number: FoF 07598, Fig. 342
Sphaerellopsis filum (DAOM 215317, DAOM 215318) a–d Herbarium package and specimen (c from DAOM 215317, d DAOM 215318). e, f Appearance of dark brown to black conidiomata on the host. g, h Vertical sections of conidiomatal wall. i–k Vertical sections of conidiomata. l Ostiole. m–q, w Conidiophores, conidiogenous cells and developing conidia. r–v Conidia. Scale bars: e = 200 µm, f = 100 µm, g–h = 20 µm, i–k,l = 50 µm, m–q = 10 µm, r–w = 5 µm
Foliicolous on leaves of Allium schoenoprasum and Carex sp. Sexual morph: undetermined. Asexual morph:Conidiomata 70–150 µm diam., 60–150 µm high, dark brown to black, stromatic, pycnidial, solitary to gregarious or confluent, immersed to semi-immersed, becoming erumpent, globose to subglobose, unilocular, glabrous, thick-walled, papillate, ostiolate. Ostiole 20–35 × 20–70 µm, single, circular, centrally located. Conidiomatal wall 12–32 µm wide, composed of thick-walled, pale brown to hyaline cells of textura angularis in the lower layers, gradually merging with brown to dark brown, relatively thick-walled cells in the upper and ostiolar region. Conidiophores lining the cavity of the conidiomata, hyaline, cylindrical, branched at base, invested in mucus. Macroconidiogenous cells 6–11 × 3–7 μm, hyaline, enteroblastic, annelidic, cylindrical to doliiform or lageniform, or ampulliform to conical, discrete or integrated, smooth-walled, with 1–2 percurrent proliferations (Fig. 342m, n). Macroconidia 12–17 × 2.5–4.5 μm (\( \bar{x} \) = 15 × 3.5, n = 50), fusiform to fusiform-ellipsoid or cylindrical, occasionally digitate, with a narrow and obtuse apex, and a truncate base bearing minute marginal frills, straight or slightly curved, mostly 1-septate, occasionally aseptate or 2–3-septate, smooth-walled, bearing a funnel-shaped, flared, mucoid appendage at both ends. Microconidiogenous cells hyaline, restricted to the ostiolar channel, cylindrical to ampulliform, smooth-walled. Microconidia 5–9 × 2–4 μm (\( \bar{x} \) = 7 × 3 μm, n = 20), hyaline, rounded to ellipsoidal, aseptate, smooth-walled.
Material examined: New Zealand, Auck1and, Takapuna, North Shore, on leaves of Allium schoenoprasum (Amaryllidaceae), 1 March 1982, D.W.R. Watson (comm. Gary Samuels) (DAOM 215317); Canada, Ontario, Lakeshore Village, on leaves of Carex sp. (Cyperaceae), 5 August 1973, T.R. Nag Raj (DAOM 215318).
Sphaeriothyrium Bubák, Ber. dt. bot. Ges. 34: 299 (1916)
Facesoffungi number: FoF 07593, Fig. 343
Sphaeriothyrium filicinum (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiogenous cells and developing conidium. c Conidia
Ascomycota, genera incertae sedis
Habit on petiole of Struthiopteris germanica (Onocleaceae), Pteridium aquilinum (Dennstaedtiaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, stromatic, pycnidial, solitary, immersed to semi-immersed, subglobose, unilocular, glabrous. Ostiole absent, dehiscence by breakdown of the upper wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the exterior, becoming paler towards hymenium. Conidiophores arising all around the cavity of conidioma, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, ampuliform to doliiform, determinate, smooth-walled, with a minute apical channel and collarette, periclinal thickening at the collarette zone. Conidia hyaline, ellipsoid to cylindrical, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Sphaeriothyrium filicinum Bubák, Ber. dt. bot. Ges. 34: 299 (1916)
Notes: Sphaeriothyrium is similar to asexual morph of Plenodomus (e.g. P. lingam, syn. Phoma lingam) and Phoma in having pycnidial conidiomata producing ellipsoid to cylindrical or subcylindrical conidia (Verkley et al. 2014; Ariyawansa et al. 2015). However, no molecular data is available for this genus and it is, therefore, difficult to separate them by morphology. Fresh collections are needed to place it in a natural group and link it to its sexual morph.
Distribution: Germany, Italy (Sutton 1980; Index Fungorum 2020).
Sphaerographium Sacc., Syll. fung. 3: 596 (1884)
Facesoffungi number: FoF 07595, Fig. 344
Sphaerographium squarrosum (redrawn from Verkley 2002) a Conidia. b Conidiophores, conidiogenous cells and developing conidia. c Side view of conidiomata. d Vertical sections of conidioma
Leotiomycetes, Leotiomycetidae, Chaetomellales, Chaetomellaceae
Saprobic on the host plants, such as Camellia sasanqua (Theaceae), Lonicera sp. (Caprifoliaceae) and Sorbus sp. (Rosaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, solitary or gregarious, superficial or erumpent, cylindrical to conical, unilocular, glabrous or sometimes rostrate. Ostiole single, circular. Conidiomatal wall composed of relatively thick-walled, dark brown, gelatinized cells of textura angularis in the exterior, gradually merging with pale brown cells of textura oblita, becoming thick-walled cells of textura porrecta towards the apex. Conidiophores arising from inner layers of the basal and lateral wall of conidiomata, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, determinate, smooth-walled, with terminal or lateral apertures formed immediately below transverse septa. Conidia hyaline, fusiform, falcate, 1–3-septate, smooth-walled, eguttulate (adapted from Sutton 1980; Verkley 2002).
Type species: Sphaerographium squarrosum (Riess) Sacc., Syll. fung. 3: 597 (1884)
Notes: Sphaerographium closely resembles Corniculariella (Dermateaceae, Helotiales) in having cylindrical to conical, rostrate conidiomata. However, Corniculariella has falcate or fusiform to clavate, 1–10-septate conidia, whereas Sphaerographium has fusiform, falcate, 1–3-septate conidia.
Sphaerographium was originally proposed by Saccardo (1884b) to accommodate nine species. von Höhnel (1915) lectotypified Sphaerographium with S. lonicerae (Fuckel) Sacc. as type species. Sutton (1980) gave a detailed generic description of the type species. A comprehensive revision of Sphaerographium was made by Verkley (2002), who neotypified Sphaerographium with S. squarrosum as the type species, which was listed as synonym of S. lonicerae by Sutton (1980). Verkley (2001 and Höhnel (1924) re-examined both type materials 002) accepted three species, S. petiolicola P. Karst., S. squarrosum and S. tenuirostrum Verkley, while 30 taxa were excluded or some insufficiently known taxa were discussed. Sphaerographium is placed in Chaetomellaceae (Helotiales, Leotiomycetes) based on molecular data (Decock et al. 2005; Minnis et al. 2010), but molecular data for the type species is unavailable. It is necessary to obtain fresh material and obtain DNA sequence data to confirm its placement. The sexual morph of this genus is undetermined.
Distribution: Austria, Finland, Germany, New Zealand, USA, Sweden, Switzerland (Sutton 1980; Verkley 2002).
Stagonospora (Sacc.) Sacc., Syll. fung. (Abellini) 3: 445 (1884)
Facesoffungi number: FoF 07599
Dothideomycetes, Pleosporomycetidae, Pleosporales, Massarinaceae
Saprobic on dead stem of host plant. Sexual morph: see Quaedvlieg et al. (2013). Asexual morph:Conidiomata black, pycnidial, solitary to gregarious, immersed to semi-immersed, globose to subglobose or depressed globose, unilocular, glabrous or setose, ostiolate. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the outer layers, becoming hyaline, small cells towards conidial hymenium. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, holoblastic, occasionally annellidic, doliiform or ampulliform to broadly conical, indeterminate, discrete, smooth-walled, and formed from the inner wall layer of conidiomata. Conidia hyaline, cylindrical to fusiform, with an obtuse apex and a subtruncate base, straight or slightly curved, with several transverse eusepta, smooth-walled, guttulate, with or without mucoid, apical appendage (adapted from Sutton 1980; Hyde et al. 2016).
Type species: Stagonospora paludosa (Sacc. & Speg.) Sacc., Syll. fung. (Abellini) 3: 453 (1884)
Notes: Stagonospora can be confused with Septoria, as both genera have similar forms of conidiomata and conidia. Septoria was separated from Stagonospora on the basis that Stagonospora has percurrently proliferating conidiogenous cells, whereas in Septoria they proliferate sympodially (Quaedvlieg et al. 2013). However, some Septoria species also have percurrently proliferating conidiogenous cells (Quaedvlieg et al. 2013). Thus conidiogenesis alone is not a sufficient character to define genera. Quaedvlieg et al. (2013) epitypified S. paludosa and revealed that Stagonospora sensu stricto belongs to Massarinaceae, while Septoria sensu stricto was placed in Mycosphaerellaceae. Tanaka et al. (2015) and Hyde et al. (2016) included two species (S. paspali, S. forlicesenensis), which enlarged the generic concepts of Stagonospora to include neottiosporina-like asexual morphs. Furthermore, the sequences of Neottiosporina cylindrica (LSU: MH423484, MH423486, ITS: MH423484, MH423483, MH423482) are closely related to some Stagonospora species (e.g., S. trichophoricola, S. forlicesenensis, S. victoriana, S. pseudoperfecta), therefore it was transferred to Stagonospora. Neottiosporina has been separated from Stagonospora by its conidia bearing mucoid, apical appendages. However, Crous et al. (2012b) showed that conidia appendages morphology alone is not informative at the generic level. Therefore, Neottiosporina might be congeneric with Stagonospora. To clarify the taxonomy of Neottiosporina and Stagonospora, the type species of Neottiosporina (N. apoda (Speg.) Subram.) will have to be recollected, and epitypified, so that authentic cultures and DNA sequence can confirm this hypothesis.
Distribution: worldwide.
Stagonospora cylindrica (B. Sutton & Alcorn) W.J. Li & K.D. Hyde, comb. nov.
≡Neottiosporina cylindrica B. Sutton & Alcorn, Trans. Br. Mycol. Soc. 84(3): 444 (1985)
Index Fungorum number: IF557177, Facesoffungi number: FoF 07600, Fig. 345
Parasitic on leaves of Kyllinga brevifolia (Cyperaceae). Mycelium hyaline, septate, branched. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, solitary to gregarious, immersed, subepidermal in origin, appearing as slightly elevated brown pustules with the ostioles visible in surface view, oval to ellipsoid in sectional view, unilocular, thick-walled, glabrous, ostiolate. Ostiole circular to oval, single. Conidiomata wall composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, ampulliform to broadly conical, smooth, arising from the base and part away up the side walls of the conidiomata. Conidia 13–20 × 4.4–6 µm (\( \bar{x} \) = 17 × 5 µm; n = 30), hyaline, subcylindrical to clavate, with a rounded apex and a narrow, truncate base, straight or slightly curved, 1-septate, guttulate, smooth-walled, bearing a mucoid, apical appendage (adapted from Nag Raj 1993).
Material examined: Australia, Queensland, Mt. Tamborine, on leaves Kyllinga brevifolia (Rottb.) Endl. ex Hassk. (Cyperaceae), J.L. Alcorn, 13 March 1984 (IMI 285696, holotype).
Stagonospora forlicesenensis Phukhams., Camporesi & K.D. Hyde, in Hyde et al., Fungal Diversity: [77] (2016)
Facesoffungi number: FoF02384, Fig. 346
Stagonospora forlicesenensis (MFLU 16-1337, holotype) a Herbarium specimen. b–c Appearance of dark brown conidiomata on the host. d, e Vertical sections of conidiomata. f, g Vertical sections of peridium. h Ostiole. i–o Conidiogenous cells and developing conidia. p–u Conidia (arrows show apical appendages). Scale bars b = 200 µm, c = 100 µm, d–e = 50 µm, f–h = 20 µm, i–u = 10 µm
Saprobic on dead branches of Phragmites australis (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 100–170 µm diam., 140–250 µm high, dark brown to black, pycnidial, usually solitary, deeply immersed with only the dark brown spot visible in the surface view, globose to subglobose, unilocular, setose, thick-walled, ostiolate. Ostiole circular, centrally located. Conidiomatal setae brown at the base, becoming paler towards apex, subulate, slightly curved, septate, unbranched, smooth-walled. Conidiomatal wall 15–30 µm wide, composed of thick-walled, brown cells of textura angularis in the outer layer, becoming hyaline cells in the inner layer. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 6–11 × 4–9 µm, hyaline, enteroblastic, phialidic, ampulliform, determinate, discrete, smooth-walled, arising from the inner wall layer of conidiomata. Conidia 26–34 × 7–8.5 µm (\( \bar{x} \) = 29 × 8 µm; n = 30), hyaline, cylindrical to fusiform, rounded at apex, subtruncate at base, straight, 3-septate, smooth-walled, bearing infundibuliform to campanulate-like, mucoid, apical appendages (1.2–5 µm long, 6–10 µm wide).
Material examined: Italy, Province of Forlı`-Cesena, Pian di Spino, Meldola, on dead stem of Phragmites australis (Poaceae), 22 December 2014, E. Camporesi, IT2306 (MFLU 16-1337, holotype).
Stamnaria Fuckel, Jb. nassau. Ver. Naturk. 23-24: 309 (1870)
= Titaeospora Bubák, Ann Mycol. 14(5): 345 (1916)
Facesoffungi number: FoF 07601, Fig. 347
Stamnaria persoonii (asexual morph, redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells, paraphyses and developing conidia
Leotiomycetes, Leotiomycetidae, Rhytismatales, Calloriaceae
Saprobic on the host plant in terrestrial habitat. Sexual morph: see Haelewaters et al. (2018). Asexual morph:Conidiomata yellowish brown, acervular, solitary to gregarious or confluent, immersed, circular to elongated, unilocular, glabrous. Ostiole absent, dehiscence by breakdown of host tissue. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis. Paraphyses hyaline, filiform, attenuated above, branched, septate. Conidiophores arising from the inner wall layer of basal stroma, hyaline, cylindrical, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical, integrated or discrete, determinate, smooth-walled. Conidia hyaline, fusiform to almost falcate, with obtuse apex and truncate base, often anastomosing in the acervulus by germination tubes produced just above the base, 1-septate, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Stamnaria persoonii (Moug.) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 309 (1870) [1869-70]
= Titaeospora detospora (Sacc.) Bubák, Ann Mycol. 14(5): 345 (1916)
Notes: Titaeospora is characterized by fusiform to falcate conidia interconnected by germination tubes produced just above the base. Titaeospora equiseti is a synonym of T. detospora (type of Titaeospora) and was considered as asexual morph of Stamnaria persoonii (type of Stamnaria) (von Arx 1970; Sutton 1980; Gruber 2006). Johnston et al. (2014) regarded them are congeneric and recommended to used older name Stamnaria. Based on the available sequence data, Stamnaria is placed in Calloriaceae (Rhytismatales, Leotiomycetes) (Ekanayaka et al. 2018). No molecular data is available for asexual morph of Stamnaria. Fresh collections are needed to confirm the connection between the two morphs.
Distribution: worldwide (Wijayawardene et al. 2017).
Staurophoma Höhn., Denkschr. Kaiserl. Akad. Wiss., Math.-Naturwiss. Kl. 83: 34 (1907)
Facesoffungi number: FoF 07602, Fig. 348
Staurophoma panici (redrawn from Morgan-Jones et al. 1972d) a Vertical section of conidiomata. b Conidiogenous cells. c Conidia. d Conidiomatal setae
Ascomycota, genera incertae sedis
Saprobic on petiole of Calamus walkeri (Arecaceae) and on leaves of Panicum sulcatum (Poaceae) (Yanna et al. 1998b). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary or gregarious, superficial, globose, unilocular, setose. Ostiole absent, dehiscence by breakdown of apical wall. Conidiomatal setae brown, concentrated on the upper surface, thick-walled, branched, aseptate, with stellate apex. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura intricata to textura angularis. Conidiophores arising from inner wall of conidioma, reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, unbranched, smooth-walled. Conidia hyaline, oval to ellipsoidal, 0–1-septate, smooth-walled, eguttulate (Morgan-Jones et al. 1972d; Yanna et al. 1998b).
Type species: Staurophoma panici Höhn., Denkschr. Kaiserl. Akad. Wiss., Math.-Naturwiss. Kl. 83: 34 (extr.) (1907)
Notes: Chaetasbolisia, Dasysticta and Staurophoma possess pycnidial, globose, unilocular, setose conidiomata and phialidic, doliiform to ampulliform conidiogenous cells and hyaline conidia (Sutton 1980). Staurophoma differs from Chaetasbolisia and Dasysticta by its short, stellate conidiomatal setae (17 × 4.5 μm). Dasysticta was separated from Chaetasbolisia by its much longer conidiomatal setae (up to 650 μm long) and cylindrical conidia often with a large central guttule. Staurophoma was re-described by Sutton (1980) and illustrated by Morgan-Jones et al. (1972d). The second species S. calami Yanna, K.D. Hyde & Goh collected on Calamus walkeri from Hong Kong was included by Yanna et al. (1998b). This species is distinguished from S. panici by its larger conidiomata and 1-septate conidia. The genus has not been studied with molecular data. Fresh collections of type species are needed to place it in a natural group.
Distribution: Brazil, China (Sutton 1980; Yanna et al. 1998b).
Stictosepta Petr., Sydowia 17: 230 (1964) [1963]
Facesoffungi number: FoF 07603, Fig. 349
Stictosepta cupularis (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c, d Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on dead branches of Fraxinus sp. (Oleaceae). Sexual morph: undetermined. Asexual morph:Conidiomata, pycnidial, immersed, globose to collabent, unilocular, convoluted, glabrous, papillate. Ostiole single, circular, centrally located. Conidiomatal wall composed of thin-walled, hyaline cells of textura intricata. Conidiophores arising from the inner wall layer of conidiomata, hyaline, irregular, branched, septate, anastomosing. Conidiogenous cells hyaline, holoblastic, sympodial or synchronous, cylindrical to irregular, integrated, indeterminate, smooth-walled, with usually two small, unthickened, apical, slightly protuberant conidiogenous loci. Conidia hyaline, cylindrical to filiform, with obtuse apex and slightly truncate base, straight or curved, multiseptate, slightly constricted at the septa, smooth-walled, guttulate (adapted from Sutton 1980).
Type species: Stictosepta cupularis Petr., Sydowia 17: 230 (1964) [1963]
Notes: Stictosepta remains monotypic. This genus is similar to Septoria in having pycnidial conidiomata producing hyaline multiseptate conidia, but it was distinguished from the latter by its sympodial conidiogenous cells and the presence of conidiophores. No molecular data is available for Stictosepta. Fresh collections are needed to investigate its taxonomy and its relationship with closely related genera.
Distribution: Czech Republic (Sutton 1980; Quaedvlieg et al. 2013).
Stilbophoma Petr., Bot. Arch. 43: 93 (1942) [1941]
Facesoffungi number: FoF 07705, Fig. 350
Stilbophoma microspora (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata blackish brown, stromatic, pycnidial, solitary, semi-immersed to erumpent, hemispherical to pulvinate, unilocular, glabrous, pushing up a flap of epidermis. Ostiole papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown to black, dome-shaped, small cells of textura angularis in the upper and lateral part, becoming thin-walled, pale brown in the base. Conidiophores arising from inner layers of basal stroma, hyaline, filiform, cylindrical or irregular, branched, with many septa delimiting small cells, aggregated into synnemata within the locule. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform, integrated, determinate, smooth-walled, with lateral apertures immediately below transverse septa. Conidia hyaline, cylindrical with an obtuse apex, and a narrow base, straight or slightly curved, unicellular, smooth-walled, eguttulate (adapted from Sutton 1980).
Type species: Stilbophoma microspora Petr., Bot. Arch. 53: 93 (1942) [1941]
Notes: Stilbophoma is characterized by pycnidial conidiomata with thick-walled, blackish brown, sclerotized upper and lateral wall, and stilboid aggregations of conidiophores, monophialidic, doliiform conidiogenous cells with solitary terminal or lateral apertures, and cylindrical conidia (Sutton and Funk 1975; Sutton 1980). Pragmopycnis shares similar form of conidiomata with Stilbophoma, but the conidiomata of Pragmopycnis are not stipitate, the conidiogenous cells are polyphialidic, and the conidia are fusiform, and/or? allantoid. Sutton (1980) revised Stilbophoma and accepted two species, S. microspore collected on Borassus sp., (Arecaceae) and S. inaequalis (Sacc. & Trotter) B. Sutton collected on Pandanus sp. Neither species has been studied with molecular data. To clarify the taxonomy of Stilbophoma and closely related genera, fresh collections of the type species are needed.
Distribution: Congo, India (Sutton 1980).
Strasseria Bres. & Sacc., in Strasser, Verh. zool.-bot. Ges. Wien 52: 436 (1902)
Facesoffungi number: FoF 07708, Fig. 351
Strasseria carpophila (redrawn from Nag Raj 1993) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, pycnidial, solitary to gregarious, immersed, globose, subglobose or irregular, unilocular or multilocular, glabrous, ostiolate. Ostiole circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the basal and lateral part, gradually merging with thin-walled, pale brown cells of textura prismatica in the middle part of locular wall, becoming thicker and darker towards ostiolar region. Conidiophores arising from inner layers of each locular wall, hyaline, cylindrical to doliiform or irregular, branched, septate, invested in mucus. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to lageniform, integrated or discrete, determinate, smooth-walled, with a minute channel and collarette. Conidia hyaline, allantoid to botuliform, with obtuse apex and truncate base, bearing a single, filiform, flexuous, unbranched, often oblique appendage at base, and a mucoid, infundibuliform appendage at apex (adapted from Sutton 1980; Nag Raj 1993).
Type species: Strasseria carpophila Bres. & Sacc., in Strasser, Verh. zool.-bot. Ges. Wien 52: 436 (1902)
Notes: In Eleutheromyces, Monodia, Nothostrasseria, Pullospora, Strasseria, Strasseriopsis, the formation of the basal, cellular appendages precede the development of the conidia body (Nag Raj 1983, 1993). The pale brown to brown, verruculose-walled conidia in Nothostrasseria separate it from other genera that have hyaline, smooth-walled conidia. Strasseria possesses both cellular and mucoid appendages, whereas Eleutheromyces, Monodia, Pullospora, and Strasseriopsis have only cellular appendages. Strasseriopsis was separated from Monodia and Pullospora on the basis of the shape of the conidia, the numbers of conidial appendage, and the structure of the conidiomata. Monodia and Pullospora are coprophilous fungi, and the differences among these genera are discussed under Monodia.
Strasseria is a monotypic genus, although Sutton (1980) estimated eight species in Strasseria. Nag Raj (1983) made a comprehensive revision of Strasseria, and accepted the single species, S. geniculata (Berk. & Broome) Höhn. Other species were transferred to Phyllosticta Pers. and Apostrasseria Nag Raj or excluded from Strasseria. Strasseria carpophila was listed as a synonym of S. geniculata by Sutton (1980) and Nag Raj (1983). However, Wijayawardene et al. (2017) considered S. carpophila to be an earlier name, and selected it as type species. This species is associated with branch and twig cankers and necrotic needles, black rot and blackened, necrotic tissue on various hosts (Nag Raj 1993). To clarify the taxonomy of Strasseria, the type species will have to be recollected, and epitypified.
Distribution: Austria, Canada, Netherlands, UK, USA (Nag Raj 1993).
Strasseriopsis B. Sutton & Tak. Kobay., Mycologia 61(6): 1068 (1970) [1969]
Facesoffungi number: FoF 07709, Fig. 352
Strasseriopsis tsugae (redrawn from Nag Raj 1993) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on stem and twig of Tsuga sieboldii (Pinaceae). Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, sclerotioid and hypocrelloid, pycnidial, solitary, seemingly superficial but erumpent, multilocular, with immersed locules arranged peripherally in the same plane, glabrous, ostiolate. Ostiole single to each locule, circular, papillate, centrally located. Conidiomatal wall composed of thick-walled, dark brown cells of textura globulosa in the exterior, gradually merging with thin-walled, paler cells towards inner part, becoming thin-walled, pale brown to hyaline cells of textura porrecta in the interlocular wall. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from inner layers of each locular wall, hyaline, holoblastic, ampulliform, lageniform or subglobose, discrete, determinate, smooth-walled. Conidia hyaline, ovoid to broadly fusiform, unicellular, smooth-walled, bearing a narrow-conic, short, apical appendage, and a filiform, tubular, unbranched, flexuous basal appendage (adapted from Nag Raj 1993).
Type species: Strasseriopsis tsugae (Tak. Kobay.) B. Sutton & Tak. Kobay., Mycologia 61(6): 1069 (1970) [1969]
Notes: The conidia of Strasseriopsis closely resemble those of Eleutheromyces, but it was distinguished from the latter by its conidiogenous cells and conidiomata structure (Nag Raj 1993; Crous et al. 2015d). Strasseriopsis has superficial, pycnidial, multilocular conidiomata, which is similar to entomopathogenic fungi (e.g., Aschersonia, Moelleriella and Samuelsia). Aschersonia and Moelleriella have fusiform conidia, Samuelsia has allantoid conidia lacking appendages, whereas Strasseriopsis has ovoid to broadly fusiform conidia bearing an appendage at each end (Chaverri et al. 2008; Tibpromma et al. 2017). In addition, Aschersonia, Moelleriella and Samuelsia have paraphyses, a feature absent in Strasseriopsis.
Strasseriopsis remains monotypic, and the sexual morph is undetermined. Strasseriopsis tsugae is associated with blight disease on Tsuga sieboldii in Japan (Sutton and Kobayashi 1969). To clarify the taxonomic treatment of Strasseriopsis, the type species will have to be recollected and epitypified.
Distribution: Japan (Sutton and Kobayashi 1969).
Strigula Fr., Syst. mycol. (Lundae) 2(2): 535 (1823)
= Discosiella Syd. & P. Syd., Leafl. of Philipp. Bot. 5: 1546 (1912)
Facesoffungi number: FoF 07706
Dothideomycetes, Dothideomycetidae, Strigulales, Strigulaceae
Lichenized on leaves or more rarely on bark and rocks (Hyde et al. 2013). Sexual morph: see Hyde et al. (2013). Asexual morph: Conidiomata dull brownish black to glistening black, pycnidial, scattered to gregarious, epidermal, immersed to erumpent, unilocular, glabrous, clypeate, ostiolate. Ostiole single, with an outer clypeus covering the hymenium. Conidiomatal wall composed of thick-walled, dark brown to black, leathery cells of textura epidermoidea to textura intricata in outer clypeus, becoming membranous, gelatinous, thin-walled, hyaline cells of textura angularis in inner layers. Conidiophores reduced to conidiogenous cells. Macroconidiogenous cells arising from palisade-like cells of the inner wall layer of conidiomata, hyaline, enteroblastic, ampulliform, lageniform, subcylindrical or conical, often with a broad inflated venter and a narrow attenuated neck, smooth-walled, with several percurrent proliferations. Macroconidia hyaline, ellipsoid to fusiform, or subcylindrical to cylindrical, 0–1-septate, smooth-walled, guttulate, bearing an unbranched, attenuated, mucoid appendage at each end. Microconidiogenous cells hyaline, lageniform to cylindrical, smooth-walled. Microconidia hyaline, ellipsoid, unicellular, smooth-walled (adapted from Nag Raj 1993).
Type species: Strigula smaragdula Fr., Linnaea 5: 550 (1830)
Notes: The members of Discosiella are lichenized mycobionts (Nag Raj 1981a). Nag Raj (1981a, 1993) linked Discosiella species to sexual morph Strigula based on both conidiomata and perithecia consistently occurring on same substrate. In later studies, Discosiella species have been often reported associated with Strigula (Aptroot et al. 1997, 2008; Lücking 2008). Jiang et al. (2016, 2017) introduced several new taxa in Strigula with an asexual morph, based on molecular data. These studies provided more evidence for sexual and asexual morph connection between Discosiella and Strigula. Therefore, Discosiella and Strigula are considered as taxonomic congeneric. Strigula is a lichenized genus containing more than 70 species (Lücking 2008; Hyde et al. 2013). Only four taxa were recognized in Discosiella (Nag Raj 1993). Because Strigula is a well known genus and it has priority, Discosiella is reduced to a synonym of Strigula. However, it should be noted that if the type species of Discosiella is found not to be congeneric with Strigula, it remains available for use.
Strigula cylindrospora (Syd. & P. Syd.) W.J. Li & K.D. Hyde, comb. nov.
≡Discosiella cylindrospora Syd. & P. Syd., Leafl. of Philipp. Bot. 5: 1546 (1912)
Index Fungorum number: IF557178, Facesoffungi number: FoF 07707, Fig. 353
Strigula cylindrospora (asexual morph, redrawn from Morgan-Jones 1974) a Vertical section of conidioma. b Enlarged view of conidiomatal wall. c Conidiophores and conidia. d Conidia
Foliicolous to lignicolous. Thallus endophloeodal when lignicolous; crustose, irregularly netlike, dull fuliginous when foliicolous (Nag Raj 1981a). Sexual morph: see Nag Raj (1981a). Asexual morph:Conidiomata dull brownish black to glistening black, pycnidial, scattered to gregarious, immersed, oval to suborbicular in outline, subglobose to scutate in section view, unilocular, glabrous, clypeate, ostiolate. Ostiole single, centrally located, with an outer clypeus covering the hymenium. Conidiomatal wall composed of leathery, thick-walled, dark brown to black cells of textura epidermoidea in outer clypeus, gradually merging with membranous, gelatinous, thin-walled, hyaline cells of textura angularis in inner layer. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from inner wall layer of conidiomata, hyaline, ampulliform to conical, with a broad inflated venter and a narrow attenuated neck, smooth-walled, with several percurrent proliferations, invested in mucus. Microconidiogenous cells hyaline, lageniform to cylindrical, smooth-walled. Conidia hyaline, subcylindrical to ellipsoidal, rounded at the both ends, 1-septate, not constricted at the septum, smooth-walled, guttulate, bearing unbranched, attenuated, mucoid appendage at each end. Microconidia hyaline, ellipsoid, unicellular, smooth-walled (adapted from Morgan-Jones 1974; Nag Raj 1981a, 1993).
Suttoniella S. Ahmad, Biologia, Lahore 6: 257 (1961) [1960]
Facesoffungi number: FoF 07710, Fig. 354
Suttoniella gaubae (redrawn from Morgan-Jones 1977) a Conidia. b Vertical section of conidioma. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown, pycnidial, solitary to gregarious or confluent, immersed, oval or circular or irregular, unilocular, glabrous. Ostiole absent, dehiscence by irregular slits in the upper wall. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis to textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from inner layers of the basal stroma, hyaline, enteroblastic, polyphialidic, cylindrical to doliiform or irregular, determinate, smooth-walled, with 1–3 separate apertures restricted to the apical region. Conidia hyaline, triradiate, Y-shaped, aseptate, with two branches connected to the main axis by narrow isthmi, tapered towards the apices, smooth-walled (adapted from Sutton 1972a, 1980).
Type species: Suttoniella gaubae (Petr.) S. Ahmad, Biologia, Lahore 6(2): 128 (1961) [1960]
Notes: Suttoniella resembles Cornutispora and Furcaspora in having hyaline, triradiate, Y-shaped conidia with aseptate main axis and arms (Nag Raj 1993; Punithalingam 2003). In Suttoniella and Furcaspora, the conidia are composed of main axes and arms which are constricted at base. However, the conidia of Cornutispora are unicellular, and bifurcate at the apex, resulting in two symmetrical arms or horn-like structures that are attenuated towards apex (Punithalingam 2003). Furcaspora was separated from Suttoniella as its arms and main axis bear an appendage at each end, and by its holoblastic, sympodial, conidiogenous cells (Nag Raj 1993; Punithalingam 2003; Crous et al. 2007).
Sutton (1972a) amended the generic diagnosis of Suttoniella and accepted two species, S. gaubae on Alyxia buxifolia (Apocynaceae) and Mangifera indica (Anacardiaceae), and S. eriobotryae (S. Ahmad) S. Ahmad. on Eriobotrya japonica (Rosaceae). Subramanian and Sudha (1980) and Hoyo and Gómez-Bolea (2004) added S. arnaudii Hoyo & Gómez-Bolea on Buxus sempervirens (Buxaceae) and S. ixorae Subram. & Sudha on Ixora parviflora (Rubiaceae). No molecular data is available for this genus. Fresh specimens are needed to clarify Suttoniella species.
Distribution: Australia, India, Pakistan, Spain (Sutton 1980; Punithalingam 2003; Hoyo and Gómez-Bolea 2004).
Tetranacrium H.J. Huds. & B. Sutton, Trans. Br. Mycol. Soc. 47(2): 202 (1964)
Facesoffungi number: FoF 07715, Fig. 355
Tetranacrium gramineum (redrawn from Morgan-Jones et al. 1972e) a Vertical section of conidioma. b, d Conidiophores, conidiogenous cells and developing conidia. c Conidia
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat or on decayed wood submerged in freshwater habitats (Hudson and Sutton 1964; Shearer and Crane 1971). Sexual morph: undetermined. Asexual morph:Conidiomata blackish brown to black, stromatic, pycnidial, solitary or gregarious, subcuticular, eventually erumpent, carbonous, subglobose in section view, unilocular, glabrous. Ostiole absent, dehiscence by longitudinal fissure of the apical wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the exterior, becoming pale brown to hyaline cells towards hymenium. Conidiophores arising from inner wall layer of conidiomata. Conidiogenous cells hyaline, holoblastic, cylindrical to doliiform, determinate, smooth-walled. Conidia hyaline, tetraradiate, composed of one vertical arm and three or four equidistant horizontal arms, septate, smooth-walled, guttulate; arms originating from a central, spherical basal cell, with a truncate base, tapered towards the apex, constricted near the point of attachment to the basal cell (adapted from Hudson and Sutton 1964; Morgan-Jones 1977; Sutton 1980).
Type species: Tetranacrium gramineum H.J. Huds. & B. Sutton, Trans. Br. Mycol. Soc. 47(2): 202 (1964)
Notes: The hyaline, septate, four or five radiate conidia in Tetranacrium is reminiscent of Crucellisporiopsis, Crucellisporium, Eriosporella, Quadricladium, Tetracrium and Tricornispora (Sutton 1980; Nag Raj 1993; Punithalingam 2003; Seifert et al. 2011). Of these genera, Quadricladium, Tetracrium and Tricornispora are recognized as hyphomycetes (Seifert et al. 2011). Crucellisporiopsis, Crucellisporium, Eriosporella have clavate to subcylindrical main axes and filiform arms, whereas Tetranacrium has a spherical, centrally located basal axis and clavate to subcylindrical arms. The differences among Crucellisporiopsis, Crucellisporium and Eriosporella are discussed under Crucellisporiopsis.
Tetranacrium was re-described and re-illustrated by Morgan-Jones et al. (1972e) and Sutton (1980). Apart from the type species, the other two species listed in Index Fungorum (2019), T. eugeniae Subhedar & V.G. Rao on Eugenia jambolana (Myrtaceae), and T. malpighiacearum J.L. Bezerra & Poroca on Malpighiaceae, have not been re-examined. Due to a lack of molecular sequence data for T. gramineum, the placement of this genus is undetermined.
Distribution: Brazil, India, Jamaica, UK (Hudson and Sutton 1964; Sutton 1980).
Thoracella Oudem., Ned. kruidk. Archf, 3 sér. 2: 267 (1901)
Facesoffungi number: FoF 07712, Fig. 356
Thoracella ledi (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidiophores, conidiogenous cells and developing conidia. c Conidia
Ascomycota, genera incertae sedis
Parasitic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata black, pycnidial, solitary to gregarious or confluent, immersed, subglobose, multilocular, glabrous. Ostiole absent, dehiscence by irregular splits in the upper wall. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis to textura intricata. Conidiophores arising from inner layers of the basal stroma, pale brown to hyaline, subcylindrical to irregular, branched, septate. Conidiogenous cells hyaline, holoblastic, sympodial or synchronous, filiform, integrated, indeterminate, smooth-walled, loci indistinct. Conidia hyaline, cylindrical to oblong or irregular, unicellular, smooth-walled, guttulate (adapted from Sutton 1980).
Type species: Thoracella ledi Oudem., Ned. kruidk. Archf, 3 sér. 2: 267 (1901)
Notes: Thoracella is monotypic, and no molecular data is available. Sutton (1980) illustrated two types of conidiogenous cells, and interpreted that the annellations might be ornamental incrustations of conidiophores, but he could not confirm if this interpretation was correct. Thoracella ledi is associated with tar spots on leaves of Ledum palustre (Sutton 1980). Fresh collections of Thoracella ledi are needed to place it in a natural group.
Distribution: Netherlands (Sutton 1980).
Thrinacospora Petr., Sydowia 2(1–6): 49 (1948)
Facesoffungi number: FoF 07711, Fig. 357
Thrinacospora insignis (redrawn from Nag Raj and DiCosmo 1982) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on dead leaves of Coriaria thymifolia (Coriariaceae). Sexual morph: undetermined. Asexual morph:Conidiomata pale brown, acervular, solitary to gregarious, semi-immersed, oval to circular or irregular, unilocular, glabrous, gelatinous. Ostiole absent, dehiscence by irregular breakdown of host tissue. Conidiomatal wall composed of thick-walled, pale brown to subhyaline cells of textura angularis. Conidiophores arising from inner layers of the basal stroma, hyaline, subcylindrical to irregular, branched, septate, invested in mucilage. Conidiogenous cells hyaline, enteroblastic, annellidic, cylindrical, integrated or discrete, indeterminate, smooth-walled. Conidia hyaline, cuneiform to cylindrical, truncate at the base, straight or bent, phragmosporous, 2-septate, apical cells with 2-4 short projections, smooth-walled, eguttulate (adapted from Morgan-Jones 1997).
Type species: Thrinacospora insignis Petr., Sydowia 2(1–6): 49 (1948)
Notes: Thrinacospora is a monotypic genus. Sutton (1980) described conidiogenous cells as holoblastic, discrete, determinate, forming a single apical conidium, while Nag Raj and DiCosmo (1982) described it as enteroblastic, annellidic, cylindrical, integrated. In this paper, the description and illustration are based on Nag Raj and DiCosmo (1982). Thrinacospora has conidia with several short protuberances at the apex, which is similar to Asteroconium and Tribolospora. However, the latter two genera have aseptate, star-like conidia, whereas Thrinacospora has 2-septate, cuneiform to cylindrical conidia (Sutton 1980; Nag Raj and DiCosmo 1982). Asteroconium possesses acervular conidiomata, holoblastic, monoblastic or sympodial conidiogenous cells with 1–2 sympodial or synchronously formed conidiogenous loci and tetrahedral to star-like conidia with much reduced lumina (Sutton 1980). While Tribolospora has pycnidial conidiomata, holoblastic conidiogenous cells without sympodial conidiogenous loci, and stellate conidia with up to 6 short protuberances (Nag Raj and DiCosmo 1982). There is no sequence data available for Thrinacospora. To clarify the taxonomy of these similar and closely related genera, fresh collections and phylogenetic inferences of types are needed.
Distribution: Ecuador (Sutton 1980).
Thyronectria Sacc., Grevillea 4(no. 29): 21 (1875)
Facesoffungi number: FoF 07713
Sordariomycetes, Hypocreomycetidae, Hypocreales, Nectriaceae
Saprobic or parasitic on the host plant in terrestrial habitat or fungicolous (Jaklitsch and Voglmayr 2014; Checa et al. 2015). Sexual morph: see Hirooka et al. (2012), Jaklitsch and Voglmayr (2014). Asexual morph:Conidiomata orange to bay, stromatic, pycnidial, erumpent through epidermis or developing in stroma with ascomata, solitary or aggregated in groups, superficial or immersed, subglobose to discoidal or irregularly in shape, unilocular or multilocular, often convoluted, glabrous, thick-walled, KOH+ slightly darker, LA+ slightly yellow. Conidiomatal wall composed of thick-walled, pale brown cells of textura angularis to textura globulosa or textura prismatica. Conidiophores arising from the innermost layer of wall cells of conidiomata, hyaline, densely branched, generally verticillately 1–3 branched, septate, smooth-walled, with acropleurogenous conidiogenous cells or sterile hyphae. Sterile hyphae occasionally present, hyaline, filiform, unbranched or branched, straight or curved, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, subcylindrical to lageniform, integrated or discrete, determinate, smooth-walled. Conidia hyaline, ellipsoidal to cylindrical, oblong to allantoid, straight or slightly curved, unicellular, smooth-walled (adapted from Hirooka et al. 2012; Jaklitsch and Voglmayr 2014).
Type species: Thyronectria rhodochlora (Mont.) Seeler, J. Arnold Arbor. 21: 455 (1940)
For illustration see Hirooka et al. (2012), Jaklitsch and Voglmayr (2014).
Notes: Thyronectria species are commonly saprobic and show a degree of host-specificity (Jaklitsch and Voglmayr 2014), some are fungicolous (Jaklitsch and Voglmayr 2014), while a few are plant pathogens (Seeler 1940a; Crandall 1942; Hudler and Oshima 1976; Crowe et al. 1982; Bedker and Wingfield 1983; Hirooka et al. 2012). Thyronectria shares a similar characters with Nectria in having light to bright-coloured, soft-textured, uniloculate perithecia, but can be distinguished by its yellowish green scurf which is absent in Nectria species, as well as the asexual morph. The asexual morph of Thyronectria has been connected to Gyrostroma (Seeler 1940b) and Zythiostroma (Rossman et al. 1999). However, Hirooka et al. (2012) re-examined the generic type of Gyrostroma, and excluded it from Hypocreales. They circumscribed the asexual morph of Thyronectria to those species which are characterized by having superficial and immersed, aggregarious or caespitose pycnidia, branched 1–3 times conidiophores, monophialidic conidiogenous cells and hyaline, ellipsoidal to cylindrical, oblong or allantoid conidia. The taxonomy, phylogeny and nomenclature of Thyronectria were studied in detail by Hirooka et al. (2012) and Jaklitsch and Voglmayr (2014). To data, 34 species are recognized in Thyronectria (Checa et al. 2015; Zeng and Zhuang 2016).
Distribution: Asia (China, Japan, Pakistan), North America (USA), Europe (Austria, Czech Republic, France, Germany, Italy, Netherlands, Russia, Spain), North Africa (Jaklitsch and Voglmayr 2014; Checa et al. 2015; Zeng et al. 2016).
Thyrsidina Höhn., Ann Mycol. 3(4): 337 (1905)
Facesoffungi number: FoF 07714, Fig. 358
Thyrsidina carneominiata (redrawn from Sutton 1980) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Caulicolous and fungicolous. Sexual morph: undetermined. Asexual morph: Conidiomata pale yellow to yellow-orange, sporodochial or spuriously acervular, solitary to gregarious, initially immersed, ultimately erumpent, pulvinate, gelatinous. Ostiole absent, dehiscence by irregular rupture of the overlapping host tissue. Subhymenium composed of thick-walled, hyaline, septate, compact hyphae of textura oblita invested in mucus at the base. Conidiophores arising from the upper cells of basal stroma, hyaline, cylindrical or irregular, branched, septate, smooth-walled, with clamp connections giving rise to lateral or terminal conidiogenous cells, invested in mucus. Conidiogenous cells hyaline, holoblastic, cylindrical or irregular, integrated, determinate, smooth-walled. Conidia hyaline, arising from fusion of several aggregated clamp connections, at maturity clavate to bulbous or irregular in shape, with narrow and truncate base, dictyoseptate, constricted at the septa, smooth-walled, guttulate (adapted from Morgan-Jones 1977; Nag Raj and DiCosmo 1984).
Type species: Thyrsidina carneominiata Höhn., Ann Mycol. 3(4): 337 (1905)
Notes: Thyrsidina carneominiata is a hyperparasitic fungus on the ascomata of Diaporthe platanoides and Melanconium sp. on Acer pseudoplatanus (Sapindaceae) (von Höhnel 1905; Nag Raj and DiCosmo 1984). A revised description and a detailed illustration were published by Morgan-Jones (1977), Nag Raj and DiCosmo (1978, 1984). Thyrsidina is characterized by having sporodochial or spuriously acervular conidiomata, hyaline, compact conidiophores with clamp connections at the septa and bulbous or irregular conidia. Nag Raj and DiCosmo (1984) considered Thyrsidina as asexual morph of an undetermined basidiomycete, but no evidence is provided for this connection. The genus has remained monotypic.
Distribution: Austria (Nag Raj 1984).
Tiarosporella Höhn., in Weese, Ber. dt. bot. Ges. 37: 159 (1919)
Facesoffungi number: FOF 00333
Dothideomycetes, Incertae sedis, Botryosphaeriales, Botryosphaeriaceae
Saprobic or endophytic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, pycnidial, solitary to gregarious or confluent, subepidermal, deeply immersed, globose to subglobose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, brown to pale brown cells of textura angularis to textura prismatica. Conidiophores arising from the innermost layers of conidiomata, hyaline, short-cylindrical or dolliform, or irregular, branched, swollen at base, septate, thick and smooth-walled. Macroconidiogenous cells hyaline, holoblastic, cylindrical to ampuliform, discrete or integrated, indeterminate, smooth-walled, with or without proliferations. Macroconidia hyaline, ellipsoidal, fusiform or long clavate, unicellular, smooth-walled, bearing 2 or 4 tentaculiform, undulate or 1 flame-shape or irregular, mucoid, apical appendages, and sometimes 1 subcylindrical or irregular basal appendage. Microconidiogenous cells hyaline, holoblastic, ampuliform, integrated or sympodial, smooth-walled. Microconidia when present, hyaline, globose to ovoid, truncate at base, unicellular, smooth-walled.
Type species: Tiarosporella paludosa (Sacc. & Fiori) Höhn., in Weese, Ber. dt. bot. Ges. 37: 159 (1919)
Notes: Many species of Tiarosporella are saprobic on members of Poaceae (Sutton and Marasas 1976; Nag Raj 1993) and woody hosts (Jami et al. 2012, 2014). Some are known as pathogens on needle of conifers and as endophytes (Sieber 1988; Karadžić 1998; Müller and Hantula 1998). The genus is characterized by dark brown to black, unilocular conidiomata with textura angularis wall cells, and hyaline, long clavate conidia bearing 3–4 tentacular appendages attached to the apex of the conidium (Sutton and Marasas 1976). Morphologically, Tiarosporella can be confused with Neottiosporina, but the significant difference between these two genera relates to the conidial appendages. The appendages of conidia in Tiarosporella are located at the apex, while they are located at the base in Neottiospora.
von Höhnel (1919) introduced Tiarosporella to accommodate N. paludosa Sacc. & Fiori and N. schizochlamys Ferd. & Winge with N. arenaria Syd. & P. Syd. as a synonym. Pirozynski and Shoemaker (1971) re-examined the type specimen of N. paludosa and N. arenaria and found these two species were conspecific. Thus they proposed T. paludosa as type species of Tiarosporella and provided a list of synonyms for T. paludosa. Since then the generic concept of Tiarosporella has been formally established. Subsequently, the revisionary accounts of the genus have been made by Nag Raj (1973a, b) and Sutton and Marasas (1976). Sutton (1980) accepted seven species and one variety in the genus, viz. T. abietis H.S. Whitne et al., T. graminis (Piroz. & Shoemaker) Nag Raj, T. graminis var. karoo B. Sutton & Marasas, T. madreeya (Subram. & K. Ramakr.) Nag Raj, T. paludosa (Sacc. & Fiori) Höhn., T. parca (Berk. & Broome) H.S. Whitney et al., T. pseudotsugae H.S. Whitney, et al., and T. tritici B. Sutton & Marasas. Crous et al. (2006a) showed Tiarosporella might be related to Botryosphaeriaceae based on DNA sequences of T. graminis var. karroo, T. madreeya and T. tritici. Crous et al. (2015b) epitypified the type species of the genus Tiarosporella, T. paludosa and confirmed it is a member of Botryosphaeriaceae. Tiarosporella was shown to be a poly- and paraphyletic genus (Crous et al. 2015b). A group of fungi morphologically similar to T. paludosa clustered to Phacidiaceae, was allocated in Darkera (Leotiomycetes). However, these species clustering in Botryosphaeriaceae, were allocated in Eutiarosporella based on E. tritici (= T. tritici), Marasasiomyces based on M. karoo (= T. graminis var. karoo), and one reinstated genus Sakireeta based on S. madreeya (= T. madreeya) (Crous et al. 2015b).
Three species are included in the genus, T. graminis, T. paludosa and an undescribed species. Tiarosporella graminis was temporarily located in Tiarosporella based on morphology in this study. Once new collection of this species have been made and subjected to DNA analysis, it will be possible to confirm it in a natural classification.
Distribution: Canada, USA.
Tiarosporella graminis (Piroz. & Shoemaker) Nag Raj, Can. J. Bot. 51(12): 2469 (1974) [1973]
Facesoffungi number: FoF 07718, Fig. 359
Tiarosporella graminis (DAOM 90210, holotype) a, b Herbarium package and specimen. c, d Appearance of brown to dark brown conidiomata on the host. e, f Vertical sections of conidiomata. g Section of peridium. h–n Conidiophores, conidiogenous cells with developing conidia. o–r Conidia. Scale bars c = 500 µm, d–f = 100 µm, g = 20 µm, h–r = 10 µm
Follicolous, occurring on leaves of Calamovilfa longifolia (Poaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 140–200 µm diam., 150–160 µm high, brown to dark brown, pycnidial, solitary to gregarious, subepidermal, deeply immersed, globose to subglobose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, circular, centrally located. Conidiomatal wall 20–40 µm wide, composed of thick-walled, brown cells of textura globosa in the lower layers at the outer part, gradually merging with pale brown to hyaline cells of textura prismatica near conidial hymenium, becoming relatively thick-walled, brown to pale brown cells of textura angularis in the lateral and ostiolar region. Conidiophores arising from the innermost layers of conidiomata, hyaline, short-cylindrical or dolliform, or irregular, branched, swollen at base, septate, often constricted at septa, thick- and smooth-walled. Macroconidiogenous cells 7–20 × 3–5 µm, hyaline, holoblastic, cylindrical to ampuliform, integrated, indeterminate, smooth-walled, without proliferations. Macroconidia 20–30 × 5–11.5 µm (\( \bar{x} \) = 25.5 × 8.6 µm; n = 50), hyaline, ellipsoidal, rounded at both ends, or sometimes narrowed and truncate at base, bulging in the middle part, unicellular, smooth-walled, bearing 2 undulate, or 1 flame-shape or irregular, mucoid, apical appendages which arise as segments of a partial mucoid sheath initially enclosing the upper part of the developing conidium, eventually flared. Microconidiogenous cells hyaline, holoblastic, ampuliform, integrated, smooth-walled. Microconidia not observed.
Material examined: USA, Wisconsin, Madison Dane County, on leaves of Calamovilfa longifolia (Poaceae), 14 November 1962, H.C. Greene (DAOM 90210, holotype).
Notes: Tiarosporella graminis was originally regarded as a variety of T. palusoda, and later was raised to species rank by Nag Raj (1973a, b). This species is similar to T. palusoda in form of conidiomata, conidiogenous cells and conidia, but it is distinct in conidial shape. In Tiarosporella graminis, conidia are usually fusiform, tapered to obtuse at apex and slightly truncate at base, and widest in the middle, whereas, in T. palusoda conidia are consistently long clavate.
Tiarosporella paludosa (Sacc. & Fiori) Höhn., in Weese, Ber. dt. bot. Ges. 37: 159 (1919)
Facesoffungi number: FoF 07719, Figs. 360, 361
Tiarosporella paludosa (DAOM 215285) a, b Herbarium package and specimen. c, d Appearance of brown to dark brown conidiomata on the host. e, f Vertical sections of conidiomata. g, h Sections of peridium. i–k Conidiophores, conidiogenous cells with developing conidia. l–w Conidia. Scale bars c = 500 µm, d–f = 100 µm, g–h = 20 µm, i–w = 10 µm
Tiarosporella paludosa (DAOM 215582) a, b Herbarium package and specimen. c, d Appearance of brown to dark brown conidiomata on the host. e, f Sections of peridium. g, h Vertical sections of conidiomata. i–m Conidiophores, conidiogenous cells and developing conidia n Apical appendages. o–w Conidia. (note o–q in 10% KOH, r–w in water). Scale bars c = 500 µm, d = 200 µm, e–f = 20 µm, g–h = 100 µm, i = 5 µm, j–w = 10 µm
Follicolous, occurring on leaves of sedge. Sexual morph: undetermined. Asexual morph:Conidiomata 140–160 µm diam., 140–200 µm high, brown to dark brown, pycnidial, mostly gregarious, subepidermal, deeply immersed, globose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, circular, centrally located. Conidiomatal wall 20–60 µm wide, composed of thick-walled, dark brown to brown cells of textura angularis in the lower layers, gradually merging with pale brown to hyaline cells of textura prismatica towards conidial hymenium, Conidiophores arising from the innermost layers of conidiomata, hyaline, broadly-cylindrical or dolliform, or rounded or irregular, unbranched, septate, often constricted at septa, thick- and smooth-walled. Conidiogenous cells 6–20 × 2–5 µm, hyaline, enteroblastic, ampuliform to cylindrical, discrete or integrated, indeterminate, smooth-walled, with 1–2 percurrent proliferations (Fig. 361i, j). Conidia 16–45 × 3.5–8.6 µm (\( \bar{x} \) = 34 × 5.8 µm; n = 50), hyaline, long clavate, with obtuse apex and narrowly truncate base, straight or slightly curved, unicellular, smooth-walled, bearing appendgaes at both ends or at one end; apical appendages mostly 2, occasionally 3 or 4, tentaculiform, undulate in sterile water, angle-like in shape, or irregular in 10% KOH, mucoid, resulting from eversion and subsequent longitudinal splits in the mucilaginous sheath which envelops the upper part of the young conidium; basal appendages absent or present, subcylindrical or irregular, with blunt apex and narrow base, arising from base.
Material examined: Canada, Ontario, Bruce Peninsula, Oliphant, on leaves of sedge, 3 October 1977, G. Murase & M. Brundrett (DAOM 215285); Long Point, on leaves of undetermined sedge, 25 April 1989, T.R. Nag Raj (DAOM 215582).
Notes: Since the holotype and isotype of Tiarosporella paludosa are not in good condition, we re-examined additional specimen of this species from DAOM herbarium. We found the conidia of Tiarosporella paludosa have a subcylindrical or irregular, mucoid, basal appendage, but it degrades at maturity. Except for the basal appendage, other characters, such as conidiomata, conidiogenous cells and conidia form and dimension match to holotype and epitype described by Pirozynski and Shoemaker (1971) and Crous et al. (2015b).
Towyspora Wanasinghe, E.B.G. Jones & K.D. Hyde, in Li et al., Fungal Diversity 78: [32] (2016)
Facesofungi number: FoF 01671
Dothideomycetes, Pleosporomycetidae, Pleosporales, Lentitheciaceae
Saprobic on the host plant. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, eustromatic, scattered to gregarious or confluent, semi-immersed to erumpent, unilocular or multilocular, ostiolate, glabrous. Ostiole short, circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, lageniform to subcylindrical, thick- and smooth-walled. Conidia hyaline, cylindrical, 0–1-septate, guttulate, smooth-walled.
Type species: Towyspora aestuari Wanasinghe, E.B.G. Jones & K.D. Hyde, in Li et al., Fungal Diversity 78: [35] (2016)
Notes: Towyspora is monotypic. This genus is similar to Chaetosphaeronema in having phialidic conidiogenous cells and cylindrical, 0–1-septate conidia. However, Chaetosphaeronema has pycnidial conidiomata with periphyses, whereas Towyspora has eustromatic conidiomata lacking periphyses. Phylogenetically, Towyspora was placed in Lentitheciaceae, while Chaetosphaeronema was placed in Phaeosphaeriaceae (Li et al. 2016, this study).
Distribution: UK.
Towyspora aestuari Wanasinghe, E.B.G. Jones & K.D. Hyde, in Li et al., Fungal Diversity 78: [35] (2016)
Facesoffungi number: FoF 01672, Fig. 362
Towyspora aestuari (MFLU 15–3543, holotype) a Herbarium specimen. b, c Appearance of dark brown-black conidiomata on the host. d, e Vertical section of conidiomata. f–h Sections of peridium. i–k Conidiogenous cells and developing conidia. l–o Conidia. Scale bars d = 500 µm, e = 100 µm, f–h = 20 µm, i–o = 5 µm
Saprobic on dead branches of Phragmites communis. Sexual morph: undetermined. Asexual morph:Conidiomata 250–1000 µm diam., 200–300 µm high, black, eustromatic, scattered to gregarious or confluent, unilocular or mutilocular, closed when young, erumpent at maturity, subglobose, ostiolate, glabrous, thick-walled. Ostiole short, single, circular, centrally located. Conidiomatal wall 10–30 µm thick, composed of thick-walled, dark brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–10 × 2–4 µm, hyaline, enteroblastic, phialidic, lageniform to subcylindrical, thick- and smooth-walled. Conidia 10–18 × 3–5 µm (\( \bar{x} \) = 14.5 × 4 µm; n = 30), hyaline, cylindrical, with an obtuse apex and a slightly truncate base, 0–1-septate, thick- and smooth-walled, guttulate.
Material examined: UK, Lanstephan, 8 July 2015, on dead branches of Phragmites communis (Poacaeae), E.B.G. Jones (MFLU 15–3543, holotype).
Tracylla (Sacc.) Tassi, Bulletin Labor. Orto Bot. de R. Univ. Siena 6: 62 (1904) non Tracyella Zambet-takis (nom. illegit.) in Rev. Mycol. 35: 165. (1970)
Facesoffungi number: FoF 07716, Fig. 363
Tracylla spartinae (redrawn from Nag Raj 1993) a Vertical section of conidioma. b Conidiogenous cells and developing conidia. c Conidia
Sordariomycetes, Incertae sedis, Tracyllalales, Tracyllaceae
Saprobic on the host plant in terrestrial habit. Sexual morph: undetermined. Asexual morph: Conidiomata brown to dark brown, pycnothyrial, solitary to gregarious and confluent, superficial, orbicular to oval or irregular in outline, connected to the immersed mycelium by a central supporting column of cells. Conidiomatal wall composed of thick-walled cells of textura angularis in the centeral zone, becoming thinner-walled cells of textura prismatica at the peripheral zone with radially elongated cells that are entire or invaginated at the edge. Conidiophores arising from the inner wall layer of upper pycnothyrial shield and the central column of supporting cells, reduced to conidiogenous cells, invested in mucus. Conidiogenous cells hyaline to subhyaline, enteroblastic, phialidic, lageniform to cylindrical, determinate, smooth-walled. Conidia hyaline, broadly ellipsoidal to naviculate or lunate, truncate at the base, unicellular, smooth-walled, guttulate, bearing unbranched, attenuated, flexuous appendage at one end or both ends (Nag Raj 1974, 1975, 1993).
Type species: Tracylla spartinae (Peck) Tassi, Bulletin Labor. Orto Bot. de R. Univ. Siena 6: 62 (1904)
Notes: Tracyella (Cif.) Zambett. typified with T. hydrocharidis Zambett. was considered as a nomen dubium (Nag Raj 1975). A detailed revision of Tracylla was made by Nag Raj (1975). Peck (1894) used the binomial Leptothyrium spartinae Peck for a fungus collected on dead stems of Spartina juncea (Poaceae) in the USA. Peck (1894) noted that this species was distinct from other Leptothyrium species in its shield-like conidiomata with radiate structure in the margins and larger conidia with a filiform appendage at each end. Saccardo (1895) subdivided the genus Leptothyrium into the subgenera: Eu-Leptothyrium Sacc. for species with muticate conidia and Tracylla Sacc. for those with conidia bearing an appendage at both ends. Tassi (1904) elevated Tracylla to generic rank with T. spartinae (Peck) Tassi as type species. Another species T. aristata (Cooke) Tassi was also included in Tracylla. Moesz (1914) added three additional taxa, T. andrasovszkyi Moesz, T. julia (Speg.) Moesz and T. minima (Berk. & Curt.) Moesz. Nag Raj (1975, 1993) re-described Tracylla and accepted only two taxa, T. aristata and T. spartinae. Crous et al. (2018a) introduced a new family Tracyllaceae to accommodate Tracylla and added a new species T. eucalypti. The type species of Tracylla has not been studied by molecular data. Fresh collections and cultures are needed to confirm its placement..
Tracylla has superficial flattened, pycnothyrial conidiomata with radiate upper or sometimes lower walls, features similar to those found in Abropelta, Diedickea, Peltistromella, and Poropeltis. However, these genera are separated by conidial morphology. Abropelta possesses hyaline, fusiform, irregularly curved to lunate, 2-septate conidia bearing a podiform, excentric, tubular, basal appendage (Nag Raj 1993). Diedickea possesses hyaline, oblong, aseptate conidia. Peltistromella possesses pale yellow or subhyaline, obovoid to ellipsoidal, 1-septate conidia (Nag Raj and DiCosmo 1978). Poropeltis possesses brown to dark brown oval to elliptic, aseptate conidia with a narrow lighter band in the median part, (Nag Raj and DiCosmo 1978). Tracylla possesses hyaline, broadly ellipsoidal to naviculate or lunate, aseptate conidia with or without appendage at one end or both ends (Nag Raj 1993).
Distribution: Australia, Brazil, Jamaica, New Zealand, Philippines, Sri Lanka, Thailand, USA (Nag Raj 1993; Crous et al. 2018a).
Trematophoma Petr., Ann Mycol. 22(1/2): 152 (1924)
Facesoffungi number: FoF 07717, Fig. 364
Trematophoma lignicola (redrawn from Sutton 1980) a Vertical section of conidioma. b Conidia. c Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Saprobic on dead wood lying in water. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, pycnidial, solitary, semi-immersed, globose to elongated, unilocular, glabrous. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from inner wall layers of conidiomata, hyaline, enteroblastic, phialidic, cylindrical to lageniform, indeterminate, smooth-walled, often percurrently proliferating. Conidia hyaline, broadly obovoid, rounded at the apex, truncate at the base, with one central guttule (adapted from Sutton 1980).
Type species: Trematophoma lignicola Petr., Ann Mycol. 22(1/2): 152 (1924)
Notes: Trematophoma is similar to the asexual morph of Abrothallus (= Vouauxiomyces) and Lichenoconium, but differs from the latter two genera in several features, such as the shape, colour, septa and surface structure of the conidia (Hawksworth and Dyko 1979; Pérez-Ortega et al. 2011). In Trematophoma, the conidia are hyaline, broadly obovoid, aseptate, and smooth-walled; in the asexual morph of Abrothallus, the conidia are hyaline to pale yellowish, clavate to pyriform, aseptate, almost smooth-walled, and invested in part in a mucilaginous matrix; in Lichenoconium the conidia are brown, obpyriform to obovate, 1-septate, and verrucose (Hawksworth and Dyko 1979; Nag Raj and DiCosmo 1980). Trematophoma is monotypic and no molecular data is available. Fresh collections of type are needed to place Trematophoma in a natural group.
Distribution: Austria (Sutton 1980).
Tribolospora D.A. Reid, Aust. J. Bot. 14: 31 (1966)
Facesoffungi number: FoF 07720, Fig. 365
Tribolospora sycopsidis (redrawn from Morgan-Jones 1977) a Conidiogenous cells and developing conidia. b Conidia. c Vertical section of conidiomata. d Hyphae covered with a very fine brown granular deposit
Ascomycota, genera incertae sedis
Saprobic on leaves of Sycopsis dunnii (Hamamelidaceae) (Reid 1966). Sexual morph: undetermined. Asexual morph:Conidiomata black, stromatic, pycnidial, with a small narrow stipe-like base, solitary to gregarious, superficial, orbicular, multilocular, glabrous. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, dark brown, loosely interwoven hyphae of textura intricata in the base part, becoming almost hyaline, loosely arranged hyphae densely covered with a very fine brown granular deposit in the locular wall. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the inner wall layer of locular wall, hyaline, holoblastic, cylindrical to obclavate, attenuating apically into fine hair-like sterigmata, smooth-walled. Conidia hyaline, stellate, with 3–6 short protuberances, unicellular, smooth-walled, arising singly from the apex of the conidiogenous cells (adapted from Reid 1966).
Type species: Tribolospora sycopsidis D.A. Reid, Aust. J. Bot. 14: 31 (1966)
Notes: The conidiogenous cells of Tribolospora sycopsidis were described as phialides by Sutton (1980), but the original description is “The cavity is lined by conidiophores consisting of a narrow cylindrical body or alternatively of an obclavate structure, 5–8 by 2–2.5 µm, which in either instance is prolonged apically into a fine hair-like sterigma”. Morgan-Jones (1977) described and illustrated it as holoblastic conidiogenous cells. Based on Reid (1966) and Morgan-Jones (1977), we consider conidiogenous cells of T. sycopsidis are holoblastic rather than enteroblastic. Tribolospora is a monotypic genus. The conidia of Tribolospora are close to Asteroconium, but it was separated from the latter by its pycnidial conidiomata and holoblastic conidiogenous cells. There is no molecular data available for Tribolospora. To clarify the taxonomic placement of this genus, fresh collections of the type and cultures are needed.
Distribution: Australia (Reid 1966).
Tryblidiopsis P. Karst. [as ‘Triblidiopsis’], Bidr. Känn. Finl. Nat. Folk 19: 262 (1871)
= Tryblidiopycnis Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 127(8–9): 562 (1918)
Facesoffungi number: FoF 07721, Fig. 366
Tryblidiopsis pinastri (redrawn from Nag Raj and DiCosmo 1982) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Leotiomycetes, Leotiomycetidae, Rhytismatales, Rhytismataceae
Saprobic or endophytic on the host plant in terrestrial habitat (Gremmen 1955; Sutton 1980; Livsey and Minter 1994; Barklund and Kowalski 1994). Sexual morph: see Livsey and Minter (1994). Asexual morph:Conidiomata black, stromatic, pycnidial, solitary to aggregated or confluent, semi-immersed to erumpent, circular to applanate or irregular in shape, unilocular or multilocular, glabrous, thick-walled. Ostiole absent, dehiscing by an irregular split of the upper wall. Conidiomatal wall composed of thick-walled, dark brown cells of textura angularis in the exterior, gradually merging with thicker, pale brown cells of textura oblita in the interior, becoming hyaline cells of textura angularis in the conidial hemenium. Conidiophores arising from the inner layer of conidiomata, hyaline, branched at the base, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, sympodial or synchronous, subcylindrical to lageniform, indeterminate, mostly discrete, with 1–2 apical, small, unthickened, scarcely protuberant scars. Conidia hyaline, falcate or hamate, unicellular, smooth-walled (adapted from Sutton 1980; Nag Raj and DiCosmo 1982).
Type species: Tryblidiopsis pinastri (Pers.) P. Karst., Bidr. Känn. Finl. Nat. Folk 19: 262 (1871)
≡ Tryblidiopycnis pinastri Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 127(8–9): 562 (1918)
Notes: The taxonomic position of Tryblidiopsis was revised by Livsey and Minter (1994) and Wang et al. (2014a, b). The genus is presently placed in Rhytismataceae (Rhytismatales) (Kirk et al. 2008; Gernandt et al. 2001; Lantz et al. 2011), and three species are accepted, T. pinastri, T. sichuanensis Shuang Wang et al. and T. sinensis Shuang Wang et al. (2014a, b). The asexual morph of Tryblidiopsis has been connected to Tryblidiopycnis by Gremmen (1957) with experimental proof. This connection was confirmed by Livsey and Minter (1994) and (Wang et al. 2014a, b) based on culture and phylogenetic studies, respectively. Tryblidiopycnis pinastri Höhn. type species of Tryblidiopycnis, is asexual morph of T. pinastri, type species of Tryblidiopsis (Sutton 1980). Three taxa are recognized in Tryblidiopsis, while only a single species is included in Tryblidiopycnis. Therefore, the generic name Tryblidiopsis is conserved over Tryblidiopycnis, as it has priority and is more widely used.
Distribution: worldwide (Wijayawardene et al. 2017).
Tympanis Tode, Fung. mecklenb. sel. (Lüneburg) 1: 24 (1790)
Facesoffungi number: FoF 07722
Leotiomycetes, Leotiomycetidae, Leotiales, Tympanidaceae
Saprobic or parasitic on the host plant. Sexual morph: Apothecia black, gregarious, separate or cespitose in small clusters, erumpent, sessile, glabrous. Asci 8-spored or multi-spored, cylindrical, with obtuse apex and narrow base, pedicellate. Ascospores hyaline, uniseriate, cylindrical to allantoid or ellipsoid to fusiform, 1-septate. Paraphyses hyaline, filiform, with slightly swollen apex, simple or branched, embedded in a brownish, gelatinous matrix, forming an epithecium (Groves 1952). Asexual morph: Conidiomata dark brown to black, stromatic, gregarious, separate or cespitose, immersed to erumpent, sessile, ovoid to subglobose or sometimes conical, unilocular or multilocular, glabrous. Ostiole absent, dehiscence by irregular splits of the apical wall. Conidiomatal wall composed of thick-walled, brown to dark brown cells of textura angularis. Conidiophores arising from the inner layer cells of the locular wall, hyaline, filiform, branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, integrated or discrete, determinate, smooth-walled. Conidia hyaline, cylindrical, unicellular, smooth-walled (Sutton and Funk 1975; Nag Raj 1977a; Sutton 1980).
Type species: Tympanis saligna Tode, Fung. mecklenb. sel. (Lüneburg) 1: 24 (1790)
Notes: Groves (1952) revised Tympanis and accepted 36 taxa and one variety, of which 23 were studied in culture. He established the connection between sexual morph Tympanis and asexual morph Pleurophomella Höhn. on the basis of culture study. Sutton and Funk (1975) re-studied the isotype of Sirodothis populi Clem. (type of Sirodothis) and considered Pleurophomella was congeneric with Sirodothis. Because Sirodothis has priority, Pleurophomella was reduced to a synonym (Sutton and Funk 1975; Sutton 1980, Cole and Kendrick 1981). Sirodothis populi (= S. populnea (Thüm.) B. Sutton & A. Funk) was linked to its sexual morph T. spermatiospora based on culture studies (Groves 1952). Sirodothis saligna (Höhn.) B. Sutton & A. Funk was linked to T. saligna (type of Tympanis). According to one fungus = one name initiative, Johnston et al. (2014) and Wijayawardene et al. (2017b) proposed the generic name Tympanis for conservation over Sirodothis and Pleurophomella. We provide a detailed description of asexual morph of Tympanis. Molecular studies are needed to evaluate this genus.
Distribution: worldwide (Wijayawardene et al.2017).
Tympanis spermatiospora (Nyl.) Nyl., Not. Sällsk. Fauna et Fl. Fenn. Förh., Ny Ser. 10: 70 (1868) [1869]
= Sirodothis populi Clem., Gen. fung. (Minneapolis): 176 (1909)
= Sirodothis populnea (Thüm.) B. Sutton & A. Funk, Can. J. Bot. 53(6): 524 (1975)
Facesoffungi number: FoF 07723, Fig. 367
Tympanis spermatiospora (redrawn from 1977a) a Conidia. b Vertical section of conidioma. c Conidiophores, conidiogenous cells and developing conidia
Habit on Populus tremuloides and Populus spp. (Salicaceae). Sexual morph: see Groves (1952). Asexual morph: Conidiomata dark brown to black, stromatic, caulicolous, immersed to erumpent, consisting of one to several caespitose, globose, unilocular, pycnidium-like structures arising from a common basal stroma, glabrous or pruinose. Ostiole absent, dehiscence by irregular splits of the apical wall. Conidiomatal wall composed of thick-walled, brown to dark brown cells of textura angularis, becoming darker, thicker, sclerotioid toward the margin. Conidiophores arising from the inner layer cells of the locular wall, hyaline, filiform, branched mostly at the base, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, phialidic, cylindrical to subcylindrical, integrated or discrete, determinate, smooth-walled, terminal or lateral, apertures arising immediately below transverse septa, with periclinal thickenings wall at collarette region. Conidia hyaline, cylindrical, unicellular, smooth-walled (Nag Raj 1977a; Sutton 1980).
Vestigium Piroz. & Shoemaker, Can. J. Bot. 50(6): 1163 (1972)
Facesoffungi number: FoF 07724, Fig. 368
Vestigium felicis (redrawn from Morgan-Jones et al. 1972d) a Mature conidia. b Conidiophores, conidiogenous cells and developing conidia. c Vertical section of conidioma
Leotiomycetes, Leotiomycetidae, Helotiales, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark amber, acervular, solitary, semi-immersed to erumpent or superficial, circular or elliptical in outline, blister-like, subglobose in section view, unilocular, glabrous. Ostiole absent, dehiscence by irregular splits of the overlapping host tissue. Conidiomatal wall composed of thin-walled, pale brown cells of textura angularis in the basal stroma. Conidiophores arising from the upper cells of the basal stroma, hyaline, pallisade-like, fasciculate, branched above, septate, with acropleurogenous conidia. Conidiogenous cells hyaline, holoblastic, sympodial, cylindrical, integrated, indeterminate, smooth-walled, with 1–2 sympodial proliferations at the apex. Conidia hyaline, paw-like, applanate, with a central angular cell and 3–6 ovoid, often curved, peripheral cells, smooth-walled or verrucose, guttulate (Pirozynski and Shoemaker 1972; Morgan-Jones et al. 1972d; Sutton 1980).
Type species: Vestigium felicis Piroz. & Shoemaker, Can. J. Bot. 50(6): 1163 (1972)
Notes: Pirozynski and Shoemaker (1972) proposed a new genus for a single species V. felicis collected on young twigs of Thuja plicata (Cupressaceae). This genus is characterized by acervular conidiomata, pallisade-like, filiform conidiophores, holoblastic, sympodial conidiogenous cells and toe-like, applanate conidia (Pirozynski and Shoemaker 1972; Morgan-Jones et al. 1972d; Sutton 1980). Shoemaker et al. (2013) included the second species V. trifidum Shoemaker & Malloch on Abies balsamea (Pinaceae), which was separated from the type by its superficial pycnothyrium and 3-toed conidia with verrucose wall. Molecular data of Vestigium trifidum placed it in Helotiales, genera incertae sedis (Leotiomycetes) (Shoemaker et al. 2013; Crous et al. 2014d). However, sequence data is not available for the type species. Therefore, fresh collections of type are needed to confirm its placement.
Distribution: Canada, USA (Pirozynski and Shoemaker 1972; Shoemaker et al. 2013).
Xenidiocercus Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia : 975 (1993)
Facesoffungi number: FoF 07725, Fig. 369
Xenidiocercus macarangae (redrawn from Nag Raj 1993) a Conidia. b Vertical section of conidioma. c Enlarged view of conidiomatal wall surrounding ostiolar region. d Conidiophores, conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on living leaves, forming circular to irregular, yellow-brown to dark brown lesions with a diffuse margin merging gradually into healthy green tissue (Wu and Sutton 1995). Sexual morph: undetermined. Asexual morph:Conidiomata yellow-brown to dark brown, pycnidial, solitary to gregarious, immersed, globose to subglobose, unilocular, glabrous, ostiolate. Ostiole single, circular, centrally located. Conidiomatal wall composed of thick-walled, brown to dark brown cells of textura angularis in the exterior, gradually merging with thin-walled, pale brown to hyaline cells towards inner layers, becoming thick-walled, dark brown to black cells in the ostiolar region. Conidiophores arising from the inner wall layer of conidiomata, hyaline, cylindrical, unbranched or branched, septate, smooth-walled. Conidiogenous cells hyaline, enteroblastic, annellidic, cylindrical to lageniform, integrated or discrete, indeterminate, smooth-walled, with several percurrent proliferations. Conidia hyaline, ellipsoid to oblong or clavate, or pyriform to ovoid, unicellular, smooth-walled, with a hyaline, cylindrical to subcylindrical, unbranched, cellular appendage at the base (adapted from Nag Raj 1993; Wu and Sutton 1995).
Type species: Xenidiocercus macarangae (T.S. Ramakr.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 977 (1993)
Notes: Xenidiocercus was proposed by Nag Raj (1993) for a segregate from Idiocercus, X. macarangae. Wu and Sutton (1995) re-examined type specimens of I. pirozynskii and X. macarangae and accepted Nag Raj’s concept. Two additional species, X. macrospora on leaves of Macaranga rowlandii and X. pyriformis on leaves of M. huraefolia (Euphorbiaceae) were included in Xenidiocercus. These two species differ from the type species in having wider and ellipsoidal or pyriform conidia.
Xenidiocercus shares similar form of conidia (subcylindrical to ellipsoidal, with tubular, cylindrical to subcylindrical basal appendage) with Bellulicauda B. Sutton (Sutton 1967a; Nag Raj 1993). The main difference separating these two genera are conidial colour and conidiomatal structure. Xenidiocercus has simple, unilocular conidiomata and hyaline conidia, while Bellulicauda has unilocular, but convoluted conidiomata and pale brown conidia. However, we speculate that Bellulicauda and Xenidiocercus could be congeneric because the conidial colour is not a sufficient character to delineate genera. For example, both hyaline and brown conidia can be found in Botryosphaeria (B. dothidea) (Phillips et al. 2013, this study). Concerning conidiomata, different conidiomatal structures (pycnidium, acervuli, and sporodochium) also can be recognized in the same genus (e.g., Chaetomella, Crucellisporiopsis, Nectria) (Rossman et al. 2004; Hirooka et al. 2012, this study). No molecular data is available for Bellulicauda and Xenidiocercus, and fresh collections are needed to verify this hypothesis.
Distribution: Ghana, India, Sierra Leone (Nag Raj 1993; Wu and Sutton 1995).
Yalomyces Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 988 (1993)
Facesoffungi number: FoF 07726
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata pycnidial, hypophyllous, solitary, deeply immersed in the host mesophyll with only the ostiole visible in surface view, globose to subglobose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, circular, centrally located, with subcylindrical, branched or unbranched, septate periphyses. Conidiomatal wall composed of thick-walled, brown to hyaline cells of textura angularis to textura globulosa or textura globulosa. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the inner wall layer of conidiomata, hyaline, enteroblastic, cylindrical, smooth-walled. Conidia hyaline, elongate-ellipsoid or broadly ellipsoid or obovoid, muriform, smooth-walled, enclosed in a distinct mucoid envelope, bearing tentaculiform or irregular mucoid appendages at each end.
Type species: Yalomyces pulcher Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 988 (1993)
Notes: Nag Raj (1993) introduced Yalomyces to accommodate a single species Y. pulcher, associated with leaf spots on Litsea sp. The muriform conidia of Yalomyces are similar to Camarosporidiella, Camarosporium, Neocamarosporium, and Staurosphaeria (Sutton 1980, Wanasinghe et al. 2017). However, Yalomyces has hyaline, elongate-ellipsoid or obovoid, muriform conidia bearing bipolar, mucoid appendages, while the other genera have brown to dark brown conidia lacking mucoid appendages. There is no molecular data available for Yalomyces. Fresh collections of type are needed to place it in a nature classification.
Distribution: India.
Yalomyces pulcher Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 988 (1993)
Facesoffungi number: FoF 07727, Fig. 370
Yalomyces pulcher (DAOM 215257, holotype) a, b Herbarium package and specimen. c, d Appearance of brown conidiomata on the host (removed host tissue view). e, f Vertical sections of conidiomata. g, m, x Vertical sections of conidiomatal wall. h–l Conidiogenous cells and developing conidia. n–w Conidia (note n–s in 10% KOH, t–w in sterile water). Scale bars: c–d = 200 µm, e–f = 100 µm, g, l ,n–w = 20 µm, h–k, x = 10 µm
Saprobic on leaves of Litsea sp. Sexual morph: undetermined. Asexual morph:Conidiomata 120–170 µm diam., 120–200 µm high, pycnidial, hypophyllous, solitary, deeply immersed in the host mesophyll with only the ostiole visible in surface view, globose to subglobose, unilocular, glabrous, thick-walled, ostiolate. Ostiole single, circular, centrally located, lined with periphyses in the ostiolar channel. Periphyses hyaline, subcylindrical, branched or unbranched, septate, smooth-walled. Conidiomatal wall 10–30 µm wide, composed of thick-walled, pale brown to hyaline cells of textura angularis in the outer layers, gradually merging with hyaline cells of textura prismatica in the inner layers, but becoming thicker, dark brown to pale brown cells of lenticular textura angularis or textura angularis to textura globulosa around the ostiolar region. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–9 × 2–4 μm, arising from the inner wall layer of conidiomata, hyaline, enteroblastic, cylindrical, smooth-walled, with percurrent proliferations above. Conidia 27–63 × 17–24 μm (\( \bar{x} \) = 51 × 22 μm, n = 30), hyaline, elongate-ellipsoid, broadly ellipsoid or obovoid, with rounded ends, straight or slightly curved, dictyoseptate, muriform, smooth-walled, bearing tentaculiform or irregular mucoid appendages.
Material examined: India, Coorg, Somwarpet, on dead leaf of Litsea sp. (Lauraceae), 30 June 1962, T.R. Nag Raj, (DAOM 215257, holotype).
Yoshinagaia Henn., Hedwigia 43(2): 143 (1904)
= Japonia Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 879 [67 repr.] (1909)
Facesoffungi number: FoF 00136
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: see Sivanesan and Hsieh (1995). Asexual morph:Conidiomata dark brown to black, pycnidial, epiphyllous, solitary to gregarious or confluent, superficial, with a very short stipe, globose to discoid, unilocular, glabrous, somewhat rough on the exterior, lacking ostiole. Conidiomatal wall thick-walled all around except at the base where it thins out and merges with the basal stroma; basal stroma pulvinate, of textura prismatica with thick-walled, dark brown cells in the outer layers, becoming paler towards the conidia hymenium; upper and lateral wall of textura angularis with thick-walled, dark brown, verruculose cells. Conidiophores arising from the innermost layers of basal wall, hyaline, branched, septate, smooth-walled. Conidiogenous cells hyaline, holoblastic, cylindrical, usually integrated, indeterminate, smooth-walled. Conidia hyaline, smooth-walled, guttulate, composed of conidium body and an apical appendage delimited by a septum; conidium body clavate to fusiform, 3-septate, constricted at septa; central cell longer than apical cell and basal cell; basal cell subcylindrical, with a truncate base, straight or slightly curved; apical appendage arising as a tubular extension of the conidium body and separated from it by a septum, branched, attenuated, tubular, flexuous.
Type species: Yoshinagaia quercus Henn., Hedwigia 43(2): 143 (1904)
Notes: Hennings (1904) described Yoshinagaia to accommodate Y. quercus, a fungus collected from leaves of Quercus glauca (Fagaceae). von Höhnel (1909) re-examined part of the type specimen deposited in Berlin and found another two coelomycetes on the type material. He described these two as new genera, and stated one of them, Japonia Höhn. has closely affinity with Yoshinagaia. Sivanesan and Hsieh (1995) reappraised Yoshinagaia based on a collection from Taiwan, and confirmed J. quercus (type species of Japonia) is an asexual morph of Yoshinagaia by development of both sexual and asexual morph within the same stromatic locule. Yoshinagaia was placed in the Dothioraceae based on ascomatic, hamathecial, ascus and ascospore character. This concept was followed by Barr (2001). According to International Code of Nomenclature for Algae, Fungi, and Plants (ICN; Melbourne Code), Yoshinagaia (1904, sexual morph) is the preferred earlier name over Japonia (1909, asexual morph) (Wingfield 2011). Yoshinagaia is a monotypic genus. There is no molecular data available for Yoshinagaia. Fresh collections of both sexual and asexual morph are needed to confirm this connection.
Distribution: China, Japan (Nag Raj 1993; Sivanesan and Hsieh 1995).
Yoshinagaia quercus Henn., Hedwigia 43(2): 143 (1904)
≡Japonia quercus Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 880 [69 repr.] (1909)
Facesoffungi number: FoF 00137, Fig. 371
Yoshinagaia quercus (asexual morph, FH 01142399). a, b Herbarium package and specimen. c, d Appearance of black conidiomata on the host. e, f Vertical sections of conidiomata. g, h Vertical sections of peridium. i, j, n Conidiophores, conidiogenous cells and developing conidia. k–m Conidia. Scale bars c–e = 200 µm, f = 500 µm; g–h = 50 µm. i–n = 20 µm
Saprobic on leaf of Quercus glauca (Fagaceae). Sexual morph: Ascomata 1–1.5 mm diam., up to 800 µm high, dark brown to black, superficial, epiphyllous, scattered or sometimes gregarious, discoid, with a short stipe, unilocular, glabrous, thick-walled, lacking an ostiole, but opening by an irregular rupture of upper wall. Peridium up to 100 µm thick, upper and lateral wall composed of textura angularis with relatively thick-walled, dark brown, verruculose cells and basal stroma pulvinate, of textura prismatica with thick-walled, dark brown cells in the outer layers, becoming paler towards inner layer. Hamathecium absent. Asci 150–220 × 25–30 µm, 8-spored, bitunicate, fissitunicate, cylindrical, with a short-stalk. Ascospores 22–30 × 10–13 µm, hyaline, ovoid with obtuse ends, unicellular, smooth-walled (adapted from Sivanesan and Hsieh 1995). Asexual morph:Conidiomata 800–1100 µm diam., 280–500 µm high, dark brown to black, pycnidial, epiphyllous, solitary to gregarious or confluent, superficial (Fig. 371e, f), with a very short stipe, globose to discoid, unilocular, glabrous, somewhat rough on the exterior. Ostiole absent, dehiscence by irregular splits in the apical wall. Conidiomatal wall 50–130 µm wide, composed of pulvinate, thick-walled, dark brown cells of textura prismatica in basal part, gradually merging with pale brown to hyaline cells towards the conidia hymenium, becoming thick-walled, dark brown, verruculose cells of textura angularis in upper and lateral part. Conidiophores arising from the innermost layers of basal wall, hyaline, branched, septate, smooth-walled. Conidiogenous cells 12–40 × 2–4 µm, hyaline, holoblastic, cylindrical, usually integrated, indeterminate, smooth-walled. Conidia 33–50 × 7.5–10 µm (\( \bar{x} \) = 44 × 8 µm; n = 30), hyaline, smooth-walled, guttulate, composed of conidium body and an apical appendage delimited by a septum; conidium body clavate to fusiform, 3-septate, constricted at septa; central cell longer than apical and basal cell; basal cell subcylindrical, with a truncate base, straight or slightly curved; appendage unbranched up to a length of 10–40 µm long, then branching irregularly with 2–5, attenuated, flexuous branches.
Material examined: Japan, on leaf of Quercus glauca (Fagaceae), February 1910, P. Surreya (FH 01142399).
Zelandiocoela Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 990 (1993)
Facesoffungi number: FoF 07728
Ascomycota, genera incertae sedis
Saprobic on the host plant in terrestrial habitat. Sexual morph: undetermined. Asexual morph:Conidiomata dark brown to black, stromatic, pycnidial, amphigenous, solitary to gregarious or confluent, immersed to erumpent, rounded or elongate, or sometimes concave in surface view, broadly conical in sectional view, unilocular, and locule often irregularly divided and convoluted, glabrous, thick-walled. Ostiole absent, opening in a circular to oval channel in the apical wall. Conidiomatal wall composed of thick-walled, pale brown to hyaline cells of textura angularis. Conidiophores formed from the innermost layers of conidiomata, hyaline, subcylindrical, swollen and septate at base, smooth-walled. Conidiogenous cells hyaline, enteroblastic, annelidic, cylindrical to lageniform, or dolliform, discrete or integrated, indeterminate, smooth-walled, with 1–2 percurrent proliferations. Conidia hyaline, fusiform, unicellular, thick- and smooth-walled, guttulate, bearing mucoid appendages at both ends.
Type species: Zelandiocoela ambigua (Nag Raj & W.B. Kendr.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 991 (1993)
Notes: The monotypic genus Zelandiocoela was introduced by Nag Raj (1993) to accommodate a species segregated from Allantophomopsis. He discussed the differences between Zelandiocoela and several morphologically similar genera. The significant difference between Zelandiocoela and Allantophomopsis refers to the fact that the conidium appendages in Allantophomopsis are of type C, while the appendages in Zelandiocoela are of type H (Nag Raj 1993). Zelandiocoela is a monotypic genus. There is no molecular data available for Zelandiocoela. To clarify the taxonomy of Zelandiocoela and closely related genera, fresh collections are needed.
Distribution: New Zealand.
Zelandiocoela ambigua (Nag Raj & W.B. Kendr.) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 991 (1993)
≡ Apostrasseria ambigua Nag Raj & W.B. Kendr., Can. J. Bot. 61: 15 (1983)
Facesoffungi number: FoF 07729, Fig. 372
Zelandiocoela ambigua (DAOM 215326) a, b Herbarium package and specimen. c, d Appearance of dark brown to black conidiomata on the host. e Vertical section of conidioma. f–h Vertical sections of conidiomatal wall. i–m Conidiophores, conidiogenous cells and developing conidia. n–u Conidia. Scale bars: d = 200 µm, e = 100 µm, f–h = 20 µm, i–l, n–u = 5 µm, m = 10 µm
Saprobic on leaves litter of Podocarpus hallii (Podocarpaceae). Sexual morph: undetermined. Asexual morph:Conidiomata 150–200 µm diam., 80–120 µm high, dark brown to black, stromatic, pycnidial, amphigenous, solitary to gregarious or confluent, initially immersed, eventually becoming erumpent, rounded or elongate, or sometimes concave in surface view, broadly conical in sectional view, unilocular, locule often irregularly divided and convoluted, glabrous, thick-walled. Ostiole absent, opening in a circular to oval channel in the apical wall. Conidiomatal wall 10–25 µm wide, composed of thick-walled, pale brown cells of textura angularis in the outer layers, becoming hyaline cells towards conidial hymenium. Conidiophores formed from the innermost layers of conidiomata, hyaline, subcylindrical, swollen and septate at base, smooth-walled. Conidiogenous cells 4–10 × 2–4 μm, hyaline, enteroblastic, annelidic, cylindrical to lageniform, or dolliform, discrete or integrated, indeterminate, smooth-walled, with 1–2 percurrent proliferations (Fig. 372k, l). Conidia 9–12 × 4–11 μm (\( \bar{x} \) = 10 × 5 μm, n = 30), hyaline, fusiform, apiculate at both ends, unicellular, thick- and smooth-walled, guttulate, with flame-shaped or funnel-shaped to irregular, mucoid appendages at both ends.
Material examined: New Zealand, Mt. Cook Nat. Park, Governor’s Bush Track, on leaf litter of Podocarpus hallii (Podocarpaceae), 3 April 1980, W.B. Kendrick (DAOM 215326).
Zelosatchmopsis Nag Raj, in Saikawa, Castañeda Ruiz, Kendrick & Nag Raj, Can. J. Bot. 69(3): 633 (1991)
Facesoffungi number: FoF 07731
Ascomycota, genera incertae sedis
Saprobic on fallen leaves of Guazuma ulmifolia (Sterculiaceae). Sexual morph: undetermined. Asexual morph:Conidiomata brown to dark brown, scattered to gregarious or confluent, superficial, sessile, cupulate to infundibuliform, unilocular, glabrous. Conidiomatal wall one-cell thick, composed of pale brown to brown cells of textura prismatica in most of conidiomata, becoming vertically elongated almost parallel cells in the uppermost layer. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, enteroblastic, phialidic, doliiform to lageniform, arising only from the basal wall or all around the cavity of conidiomata. Conidia hyaline, fusiform to falcate, with an acute apex and a narrowly truncate base, usually curved, 1-septate, bearing single hyaline, cellular, excentric, unbranched, attenuated, basal appendage.
Type species: Zelosatchmopsis sacciformis (R.F. Castañeda) Nag Raj & R.F. Castañeda, in Saikawa, Castañeda Ruiz, Kendrick & Nag Raj, Can. J. Bot. 69(3): 633 (1991)
Notes: Zelosatchmopsis is a monotypic genus. The cupulate to infundibuliform, glabrous conidiomata in Zelosatchmopsis closely resembles Infundibulomyces (Chaetosphaeriaceae, Chaetosphaeriales, Sordariomycetes) and Satchmopsis (Cochlearomycetaceae, Leotiales, Leotiomycetes) (Wijayawardene et al. 2017; Ekanayaka et al. 2019). However, Zelosatchmopsis possesses fusiform to falcate, 1-septate conidia bearing a basal appendage, which differs from the cylindrical, 0–1-septate conidia bearing two appendages at each end in Infundibulomyces and cylindrical or acerose, unicellular conidia in Satchmopsis (Nag Raj 1993; Plaingam et al. 2003; this study). The conidiophores of Zelosatchmopsis are reduced to conidiogenous cells, while they are present in Infundibulomyces and Satchmopsis. No molecular data is available for Zelosatchmopsis sacciformis. The taxonomy of Zelosatchmopsis needs further investigation based on fresh collections and phylogenetic inferences.
Distribution: Cuba.
Zelosatchmopsis sacciformis (R.F. Castañeda) Nag Raj & R.F. Castañeda, in Saikawa, Castañeda Ruiz, Kendrick & Nag Raj, Can. J. Bot. 69(3): 633 (1991)
Facesoffungi number: FoF 07732, Fig. 373
Zelosatchmopsis sacciformis (DAOM 211931) a, b Herbarium package and specimens. c, d Appearance of brown conidiomata on dried malt agar plate cultures derived from holotype. e–g Side view of conidiomata. h–i, m Side view of conidiomatal wall. j, k Vertical sections of conidiomata. l, p Vertical sections of conidiomatal wall. n–o Conidiogenous cells and developing conidia. q–v Conidia. Scale bars: c = 200 µm, d–g = 100 µm, h–k = 50 µm, l, n–p = 10 µm, m = 20 µm, q–v = 5 µm
Foliicolous, saprobic on fallen leaves of Guazuma ulmifolia (Malvaceae) and undetermined trees. Sexual morph: undetermined. Asexual morph:Conidiomata 100–160 µm diam., 70–100 µm high, pale brown to brown, with dark brown rims bordering the wide aperture at the apex, epiphyllous, scattered to gregarious or confluent, superficial, sessile, sacciform to cupulate in surface view, collabent when dry, mature conidia collecting in a pearl-white gloeoid mass, subglobose to cylindrical in sectional view, glabrous. Conidiomatal wall 4–7 μm wide, uppermost row of wall cells dark brown, tend to be cylindrical to subcylindrical, somewhat encrusted, bordering the rim of the conidiomata, lower wall cells pale brown, short cylindrical (Fig. 373h). Conidiogenous cells 3–5 × 3–8 µm, constituting most of the conidiomatal wall, ampulliform with a central or acentral aperture, smooth-walled. Conidia 16–25 × 1–2 μm (\( \bar{x} \) = 20 × 1.5 μm, n = 20), hyaline, fusiform to falcate, with acute apex and narrowly truncate base bearing minute marginal frill, curved, mostly 1-septate, smooth-walled, with single, cellular, unbranched, somewhat attenuated, excentric basal appendage (3 × 5 μm).
Material examined: Cuba, Aspiro, Pinar del Rio, on dried malt agar plate cultures derived from holotype in INIFAT C87/53-1, 9 August 1990, T.R. Nag Raj (DAOM 211931).
Zunura Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 1006 (1993)
Facesoffungi number: FoF 07730, Fig. 374
Zunura appendiculata (redrawn from Nag Raj 1993) a Conidia. b Vertical section of conidioma. c, d Conidiogenous cells and developing conidia
Ascomycota, genera incertae sedis
Parasitic on living leaves of Carpinus viminea (Betulaceae). Sexual morph: undetermined. Asexual morph: Conidiomata black, acervular, initially immersed, ultimately erumpent, unilocular, glabrous. Ostiole absent, dehiscence by irregular rupture of the overlapping host tissue. Conidiomatal wall composed of thick-walled, pale brown cells of textura angularis in the base part. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising from the upper cells of basal stroma, pale brown at the base, become paler above, enteroblastic, annellidic, ampulliform, discrete, indeterminate, smooth-walled. Conidia hyaline, globose to ellipsoidal, with narrow and truncate base, unicellular, smooth-walled, bearing single, tubular, unbranched apical appendage (adapted from Nag Raj 1993).
Type species: Zunura appendiculata (B. Sutton) Nag Raj, Coelomycetous Anamorphs with Appendage-bearing Conidia: 1009 (1993)
Notes: Zunura is a monotypic genus. This genus is similar to the asexual morph of Diplocarpon (= Entomosporium) and Monostichella in having immersed to erumpent, acervular conidiomata producing hyaline conidia. Monostichella was separated from the other two genera by its ellipsoid to pyriform, slightly curved conidia lacking an appendage, and enteroblastic, phialidic conidiogenous cells (Sutton 1980; Nag Raj 1993). Zunura has unicellular, globose to ellipsoidal conidia with single, tubular, unbranched apical appendage, which differs from the asexual morph of Diplocarpon in which the conidia are cruciform, 4-celled, occasionally 3–6-celled, bearing single apical appendage at each end of apical and lateral cell except basal cell (Nag Raj 1993). There is no molecular data available for Zunura. To clarify the taxonomy of Zunura, fresh collections and sequence data of the type species are needed.
Distribution: India (Nag Raj 1993).
General discussion and conclusion
The taxonomy and nomenclature of coelomycetes has undergone major changes in recent years, mainly through the application of molecular techniques. These advanced techniques have resulted in a more natural classification for coelomycetous fungi than was possible when having to rely entirely on morphological attributes. Several studies have tried to resolve the taxonomy of selected coelomycetous groups, such as camarosporium-like species, pestalotioid-like, phoma-like, pyrenochaeta-like, septoria-like and its relatives, with more or less success (Aveskamp et al. 2010; De Gruyter et al. 2010; Quaedvlieg et al. 2013; Wijayawardene et al. 2014c, 2016). Aveskamp et al. (2010), Chen et al. (2015, 2017) and Valenzuela-Lopez et al. (2018) have set the foundation for the systematics and taxonomy of Didymellaceae. To date, more than 30 genera are accepted in this family, and most of them are well circumscribed (Chen et al. 2017, Valenzuela-Lopez et al. 2018). In addition, the most useful molecular genes have been identified for species delimitation. Wanasinghe et al. (2017) studied many camarosporium-like asexual morphs and their sexual morphs and proposed two new families Camarosporidiellaceae and Neocamarosporiaceae and resurrected the family Camarosporiaceae. Valenzuela-Lopez et al. (2018) clarified the generic concept of Pyrenochaeta, and divided pyrenochaeta-like morphs into several new families Neopyrenochaetaceae, Parapyrenochaetaceae, Pseudopyrenochaetaceae and Pyrenochaetopsidaceae. Liu et al. (2019) recognized 30 pestalotioid-like genera in Sporocadaceae, and emended the generic circumscriptions of Diploceras, Disaeta, Hymenopleella, Monochaetia, Morinia, Pseudopestalotiopsis, Sarcostroma, Seimatosporium, Synnemapestaloides and Truncatella.
However, in spite of these studies, the taxonomy of many coelomycetous taxa are unresolved. To infer a natural classification system of coelomycetes, we carried out a comprehensive analysis of different groups based on various gene analyses. Eighty-six fresh collections of coelomycetous fungi are described and illustrated, including three new genera, 23 new species, 15 new combinations and six records (Colletotrichum sansevieriae, Koorchaloma bambusae, Neofusicoccum sinoeucalypti, Phacidium anomala, Phacidium subcorticalis, Pseudorobillarda magna), three new links between sexual morph and asexual morph. In addition, herbarium specimens were studied of another 67 genera and 98 species. One-hundred and fifty genera were illustrated by line drawings.
One of the notable findings of this study is the ratio of coelomycetous genera found in different classes of Ascomycota. One-hundred and sixteen are genera of Dothideomycetes, 66 genera of Sordariomycetes, 48 genera of Leotiomycetes, ten genera of Eurotiomycetes, three genera of Lecanoromycetes. Seven coelomycetous genera are also Basidiomyceta. This was not made clear in the studies of Ekanayaka et al (2017, 2018, 2019) who mainly dealt with the sexual morphs.
Generic concepts and boundary of coelomycetes
Dinemasporium and allied genera
Several coelomycetous genera, such as Brunneodinemaporium, Dendrophoma, Dinemasporium, Neopseudolachnella, Pseudodinemasporium, Pseudolachnea, and Pseudolachnella share similar characters of acervular conidiomata with numerous black setae and hyaline, conidia bearing appendages. These similar morphological characters of conidiomata and conidia have often resulted in difficulties in the intergeneric classification (Sutton 1977a, 1980; Castañeda 1987; Kirk et al. 2008). Hashimoto et al. (2015b) analyzed ITS and LSU sequence data and showed that Pseudolachnea and Pseudolachnella are two natural groups. They also concluded that conidiomatal structure was more reliable for generic circumscription between Pseudolachnea and Pseudolachnella than conidial septation. They also introduced two new genera Neopseudolachnella and Pseudodinemasporium. Neopseudolachnella was similar to Pseudolachnea and Pseudolachnella in having hyaline, cylindrical, septate conidia, but was characterized by conidiomata lacking a lateral excipulum. Pseudodinemasporium was distinguished from Dinemasporium by its conidiomata composed of a well-developed lateral excipulum. However, some Dinemasporium species such as D. pseudodecipiens and D. sasae were observed with a well-developed basal stroma and lateral excipulum (Hashimoto et al. 2015a; this study). In addition, different types of conidiomata have been found in the genus Nectria (pycnidia, sporodochia, synnemata) (Hirooka et al. 2012) and Crucellisporiopsis (sporodochium, synnemata) (Nag Raj 1993). Thus, we conclude that conidiomatal structure alone is of limited importance for generic circumscription between Dinemasporium and Pseudodinemasporium. Brunneodinemaporium included two taxa, while Dendrophoma remains monotypic. The morphological circumscriptions of these two genera along with Dinemasporium are ambiguous. Dinemasporium was expanded to accommodate diarimella-like (conidia with 1–3, unbranched or branched, bipolar appendages) and stauronema-like morphs (conidia with a single apical and basal appendage and 3–4 lateral appendages) based on LSU and ITS sequence data (Crous et al. 2012b; Hashimoto et al. 2015a, this study). Liu et al. (2019) studied pestalotioid-like asexual morphs in Sporocadaceae based on a combined multi-gene (ITS, LSU, rpb2, tub2, tef1) analysis, and concluded that the presence or absence of conidial appendages was a reliable taxonomic character for generic delineation between Seimatosporium and Sporocadus. However, the morphological distinctness between these two genera was not supported by ITS and LSU phylogeny analyses (Barber et al. 2011). In this regard, further analyses based on additional gene regions, such as tef1, rpb2, tub2, will be required to clarify the taxonomic importance of conidial appendages and the generic circumscription of Dinemasporium and its allied genera.
Tiarosporella and allied genera
Sutton (1980) accepted seven species and one variety in Tiarosporella, of which six taxa were re-examined by Nag Raj (1993). Crous et al. (2015b) made a major revision of Tiarosporella and showed that the genus is heterogeneous. Based on molecular analysis, most species accepted by Sutton (1980) and Nag Raj (1993) were assigned to different families in different classes, such as Botryosphaeriaceae (Dothideomycetes), Phacidiaceae (Leotiomycetes) (Crous et al. 2006a, 2015b; Phillips et al. 2013, this study). Of these species, Tiarosporella abietis, T. parca and T. pseudotsugae were transferred to Darkera (generic type D. parca = T. parca). Tiarosporella tritici was placed in a new genus Eutiarosporella (generic type E. tritici = T. tritici). Tiarosporella madreeya and T. graminis var. karoo were placed in the reinstated genera Sakireeta (generic type S. madreeya = T. madreeya) and Marasasiomyces (generic type M. karoo = T. graminis var. karoo), respectively. Tiarosporella paludosa, however, was retained in Tiarosporella. We re-examined the holotype of T. graminis, and found that this species is similar to T. paludosa in form of conidiomata and conidiogenous cells. Thus, we retain T. graminis in Tiarosporella until further molecular data can confirm its placement. Four collections from Italy were identified as Darkera picea (Phacidiaceae) and Mucoharknessia anthoxanthi (Botryosphaeriaceae) based on multi-locus analyses. This result agrees with that of Crous et al. (2015b) who noted that Tiarosporella is a poly- and paraphyletic genus. The generic type of Mucoharknessia, M. cortaderiae, resembles species of Harknessia (Harknessiaceae, Diaporthales), but is distinguished from that genus by its conidiomata that lack furfuraceous tissue surrounding its ostiole and conidia that have a mucoid apical appendage. The other species, M. anthoxanthi, has a tiarosporella-like asexual morph. Hence the species boundary of Mucoharknessia needs further study based on a large number of collections.
We re-examined the type of all Darkera species, except D. durmitorensis, and found that all taxa are associated with a coniferous host. In this regard, we suspect that host preference in this genus might correlate with their phylogenetic distinction, and further collections of these species are required to clarify the relationship between their evolution and host preference. Moreover, the presumed sexual morphs of D. abietis and D. parca were observed from the same host as their asexual morph. Li et al. (2016) included a sexual morph of Eutiarosporella (E. dactylidis). This sexual morph is largely distinct to D. abietis and D. parca sexual morphs in the form of ascomata, peridium, paraphyses, asci, and ascospores (Li et al. 2016, this study). Thus, we suspect that the morphologically informative characters of the sexual state would be useful to segregate Tiarosporella and its allied genera in further studies.
Clamp connections
Clamp connections are structures unique to the phylum Basidiomycota, especially Polyporales and Agaricales (Furtado 1966). The presence or absence of clamp connections has been used to classify basidiomycetes at the species level (Hesler and Smith 1963) and generic level (Singer 1962, Teixeira 1962). Due to the presence of clamp connections, Nag Raj (1993) designated four coelomycetous genera Cenangiomyces, Ellula, Fibulocoela, and Pycnovellomyces as asexual morphs of undetermined basidiomycetes. Recent DNA-based research has also linked three coelomycetous genera, Chaetospermum, Giulia and Mycotribulus to basidiomycetes (Rungjindamai et al 2008; Crous et al. 2014a; Tangthirasunun et al. 2014b; He et al. 2019). We re-examined herbarium specimens of these three genera, but were unable to find any clamp connections. This, calls into question whether clamp connections are unique characters of all basidiomycetes.
Morphology and phylogeny classification, and the way forward
Phylogenetic analyses coupled with morphological study are today generally used to classify coelomycetous taxa, and other groups of fungi, at all taxonomic levels. This approach provides valuable insights in establishing a natural classification of coelomycetous taxa, and helps to clarify morphological ambiguities, if any. However, contradictions between phenotypic and genotypic characters can be seen. As mention above, the morphological characters of Dinemasporium-like and tiarosporella-like species did not correlate with multi-loci phylogenetic results. This may be due to several factors, including incomplete sampling and insufficient DNA sequence. To resolve contradictions between phenotypic and genotypic characters, the following approaches could be applied. The first is increased taxa sampling of the major group. A large number of taxa would provide increased taxonomic data and possibly resolve the conflict. The second approach is to include more gene regions, especially protein genes i.e. rpb2, tub2 and tef1. Complete datasets can resolve the contradictions of generic delimitation.
Research advantages
This study has set the foundation for the systematics and taxonomy of coelomycetes. Many genera included in this study have been well-circumscribed, and useful molecular genes have been identified for genera or species delimitation. This study further indicates that many novel coelomycetous fungi will doubtless continue to be described, especially from previously understudied ecosystems. A number of efforts made in this study are shown below:
-
One hundred and two herbarium specimens were examined and checked as to whether their names are acceptable or synonyms, nomina dubia, or even illegitimate. A detailed description and photo plates were prepared for these fungi. This will facilitate future studies.
-
This study provides a backbone tree for hyaline-spored coelomycetes based on LSU sequence data. Many genera such as Chaetosphaeronema, Botryosphaeria Dinemasporium, Heterosphaeria, Melanops and Neofusicoccum are well-circumscribed based on multi-loci analyses.
-
The application of a phylogenetic approach has integrated asexual morphs with sexual morphs at the class level, e.g., Elongaticonidia rosae (Lecanoromycetes), family e.g., Alloneottiosporina (Phaeosphaeriaceae), Ciliosporella (Microdochiaceae), Corniculariella (Dermateaceae) and Koorchaloma (Stachybotriaceae), genus-level e.g., Dermea (= Foveostroma), Phacidium (= Ceuthospora) and species-level e.g., Quaternaria quaternata, Leptosphaeria sydowii.
-
This study adopts one name for one fungus in Dothideomycetes, Leotiomycetes and Sordariomycetes, and further it summarizes recent nomenclature changes in the coelomycetes. For example, Japonia was synonymized under Yoshinagaia and Entomosporium was synonymized under Diplocarpon.
Future perspectives
What more can we learn from the coelomycetes classification? Although 248 genera were investigated, only one half of these are known to have sequence data. Consequently, the taxonomic concept and generic delimitation of many genera remains unclear. Due to a lack of sequence data many genera or species need to be recollected and epitypified. Our current knowledge of the ecology and biology of coelomycetous taxa is just the “tip of the iceberg” and, thus, additional collections will assist in further understanding their biology. To address these short comings the following research is suggested.
-
Need to re-visit poorly known taxa
A very high proportion of coelomycetous taxa described are known from only a single collection, or on only a single host.
-
Need to continue to study the classification of coelomycetous fungi
Based on “one fungus, one name”, some new linkages between asexual and sexual morphs have been made in this study, e.g., Keissleriella quadriseptata, Quaternaria quaternata, Leptosphaeria sydowii. However, many coelomycetous taxa have been treated as Ascomycota, genera incertae sedis, and are not linked with their sexual morphs. Therefore, more work on connecting asexual morphs with their sexual morphs based on a combination of morphological data and phylogenetic analyses are needed.
-
Need to study the evolution of coelomycetous fungi related with their hosts and their classification using molecular clock methods (Hongsanan et al. 2017; Liu et al. 2017; Zeng et al. 2019).
Future works are likely to focus increasingly on understanding the relationship between coelomycetes evolution and host preference, and on using increasingly sophisticated analyses of whole genomes. It is only then that we are likely to begin to understand coelomycetous taxa in their evolutionary context, rather than as cultures in collections.
-
Need to study the biology of coelomycetous taxa
Many coelomycetous taxa have been shown to synthesize a high number of bioactive compounds, e.g., Bartalinia, Colletotrichum, Diaporthe, Morinia, Pestalotiopsis and Phoma (Collado et al. 2006; Gangadevi and Muthumary 2008; Liu et al. 2009; Aveskamp et al. 2010; Bi et al. 2011; Gomes et al. 2013). Therefore, there is potential to discover novel compounds and metabolites.
-
Need to investigate clinical samples related to coelomycetous taxa
There are many habitats where asexual coelomycetous taxa have been poorly studied. For example, little is known about their potential as human pathogens. Valenzuela-Lopez et al. (2018) studied a large set of coelomycetous taxa isolated from clinical specimens and showed that many of them were new species. Therefore, study of human (and animal) parasitic coelomycetes may be an interesting topic for future research.
References
Abbas SQ, Sutton BC, Ghaffar A (1997) Conidial appendages as taxonomic criteria in coelomycetes. Pak J Bot 29:199–205
Abbas SQ, Sutton BC, Ghaffar A (1998) Studies on nuclei and appendages in some coelomycetes. Pak J Bot 30:51–68
Abbas SQ, Sutton BC, Ghaffar A (2002) Stauronematopsis Abbas, Sutton and Ghaffar gen. nov., an addition to coelomycetes. Can J Bot 34:117–124
Abdollahzadeh J, Zare R, Phillips AJL (2013) Phylogeny and taxonomy of Botryosphaeria and Neofusicoccum species in Iran, with description of Botryosphaeria scharifii sp. nov. Mycologia 105:210–220
Abeln ECA, De Pagter MA, Verkley GJM (2000) Phylogeny of Pezicula, Dermea and Neofabraea inferred from partial sequences of the nuclear ribosomal RNA gene cluster. Mycologia 92:685–693
Acero FJ, González V, Sánchez-Ballesteros J, Rubio V, Checa J, Bills GF, Salazar O, Platas G, Pelaé F (2004) Molecular phylogenetic studies on the Diatrypaceae based on rDNa-ITS sequences. Mycologia 96:249–259
Adamčíková K, Juhásová G, Kobza M (2011) The first report of Libertella spp. on Fagaceae in Slovakia. Mycoscience 52:268–270
Adams GC, Surve-iyer RS, Iezzoni AF (2002) Ribosomal DNA sequence divergence and group I introns within the Leucostoma species L. cinctum,L. persoonii, and L. parapersoonii sp. nov., ascomycetes that cause Cytospora canker of fruit trees. Mycologia 94:947–967
Adams GC, Wingfield MJ, Common R, Roux J (2005) Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud Mycol 52:1–144
Adams GC, Roux J, Wingfield MJ (2006) Cytospora species (Ascomycota, Diaporthales, Valsaceae): introduced and native pathogens of trees in South Africa. Austral Plant Pathol 35:521–548
Ahmad S (1961) Further contributions to the fungi of West Pakistan. I. Some corrections. Biol Lahore 6:257–258
Ahmadpour SA, Mehrabi-Koushki M, Farokhinejad R (2017) Neodidymelliopsis farokhinejadii, a new fungal species from dead branches of trees in Iran. Sydowia 69:171–182
Alcorn JL, Irwin JAG (1987) Acrocalymma medicaginis gen. et sp. nov. causing root and crown rot of Medicago sativa in Australia. Trans Br Mycol Soc 88:163–167
Aldaoud R, DeAlwis S, Salib S, Cunnington JH, Doughty S (2011) First record of Colletotrichum sansevieriae on Sansevieria sp. (mother- in-law’s tongue) in Australia. Aust Plant Dis Notes 6:60–61
Allegrucci N, Elíades L, Cabello M, Arambarri A (2011) New species Koorchaloma and Ciliochorella from xeric forests in Argentina. Mycotaxon 115:175–181
Aptroot A, Diederich P, Sérusiaux E, Sipman HJM (1997) Lichens and lichenicolous fungi from New Guinea. Bibl Lichenol 64:1–220
Aptroot A, Lücking R, Sipman HJM, Umaña L, Chaves JL (2008) Pyrenocarpous lichens with bitunicate asci: a first assessment of the lichen biodiversity inventory in Costa Rica. Bibl Lichenol 97:1–162
Ariyawansa HA, Hawksworth DL, Hyde KD, Jones EBG, Maharachchikumbura SSN, Manamgoda DS, Thambugala KM, Udayanga D, Camporesi E, Daranagama A, Jayawardena R, Liu JK, McKenzie EHC, Phookamsak R, Senanayake IC, Shivas RG, Tian Q, Xu JC (2014) Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Divers 69:57–91
Ariyawansa HA, Bulgakov TS, Phukhamsakda C, Thambugala KM, Bulgakov TS, Wanasinghe DN, Perera RH, Mapook A, Camporesi E, Kang JC, Jones EBG, Bahkali AH, Jayasiri SC, Hyde KD, Liu ZY, Bhat JD (2015a) Revision and phylogeny of Leptosphaeriaceae. Fungal Divers 74:19–51
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen JJ, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBG, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel-Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal-Forno M, Dollhofer V, Fliegerová K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytövuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Lumbsch HT, Matsumura M, Moncada B, Nuankaew S, Parnmen S, de AzevedoSantiago ALCM, Sommai S, Song Y, de Souza CAF, de Souza-Motta CM, Su HY, Suetrong S, Wang Y, Wei SF, Wen TC, Yuan HS, Zhou LW, Réblová M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH, Miettinen O, Spirin V (2015b) Hernawati Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:27–274
Ariyawansa HA, Hyde KD, Liu JK, Wu SP, Liu ZY (2016) Additions to Karst fungi 1: Botryosphaeria minutispermatia sp. nov., from Guizhou Province, China. Phytotaxa 275:35–44
Arnaud G (1952) Mycologie concrète: genera. Bull Soc Mycol Fr 68:181–223
Ash GJ, Stodart B, Sakuanrungsirikul S, Anschaw E, Crump N, Hailstones D, Harper JDI (2010) Genetic characterization of a novel Phomopsis sp., a putative biocontrol agent for Carthamus lanatus. Mycologia 102:54–61
Aveskamp MM, de Gruyter J, Crous PW (2008) Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Divers 31:1–18
Aveskamp MM, Woudenberg JHC, De Gruyter J, Turco E, Groenewald JZ, Crous PW (2009) Development of taxon-specific sequence characterized amplified region (SCAR) markers based on actin sequences and DNA amplification fingerprinting (DAF): a case study in the Phoma exigua species complex. Mol Plant Pathol 10:403–414
Aveskamp MM, De Gruyter J, Woudenberg JHC, Verkley GJM, Crous PW (2010) Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Baayen RP, Bonants PJ, Verkley G, Carroll G, Van Der Aa H, De Weerdt M, Van Brouwershaven I, Schutte G, Maccheroni W Jr, De Blanco C (2002) Nonpathogenic isolates of the citrus black spot fungus, Guignardia citricarpa, identified as a cosmopolitan endophyte of woody plants, G. mangiferae (Phyllosticta capitalensis). Phytopathology 92:464–477
Bandre TR, Sasek V (1977) Antibiotic activity of pyrenomycetes under submerged conditions. Folia Microbiol 22:269–274
Barber PA, Crous PW, Groenewald JZ, Pascoe IG, Keane P (2011) Reassessing Vermisporium (Amphisphaeriaceae), a genus of foliar pathogens of eucalypts. Persoonia 27:90–118
Barghoorn ES, Linder DH (1944) Marine fungi: their taxonomy and biology. Farlowia 1:395–467
Barklund P, Kowalski T (1994) Endophytic fungi in branches of Norway spruce with particular reference to Tryblidopsis pinastri. Can J Bot 74:673–678
Barr E (1968) The Venturiaceae in North America. Can J Bot 46:799–864
Barr ME (1972) Preliminary studies on the Dothideales in temperate North America. Contributions from the University of Michigan Herbarium 9:523–638
Barr ME (1978) The Diaporthales in North America: with emphasis on Gnomonia and its segregates. Mycol Mem 7:1–232
Barr ME (1990) Melanommatales (Loculoascomycetes). N Am Flora Ser II 13:1–129
Barr ME (2001) Revisionary studies on the Dothioraceae. Harv Paper Bot 6:25–34
Batista AC, Ciferri R (1963) The sooty-molds of the family Asbolisiaceae. Quad Lab Crittogam Ist Bot Univ Pavia 31:1–229
Bedker PJ, Wingfield MJ (1983) Taxonomy of three canker-causing fungi of honey locust in the United States. Trans Br Mycol Soc 81:179–183
Berkeley MJ, Broome CE (1850) Notices of British fungi. Ann Mag Nat Hist 2:365–380
Berkeley MJ, Curtis MA (1869) Fungi Cubenses (Hymenomycetes). Biol J Linn Soc 10:280–392
Berraf-Tebbal A, Guereiro MA, Phillips AJL (2014) Phylogeny of Neofusicoccum species associated with grapevine trunk diseases in Algeria, with description of Neofusicoccum algeriense sp. nov. Phytopathol Mediterr 52:478–489
Berthet P (1964) Formes conidiennes de divers Discomycètes. Bull Soc Mycol France 80:125–149
Bessey EA (1906) Dilophospora alopecuri. J Mycol 12:57–58
Bi J, Yuan J, Pan J, Yu Y, Chen H, Zhu XD (2011) A new taxol-producing fungus (Pestalotiopsis malicola) and evidence for taxol as a transient product in the culture. Afr J Biotechnol 10(34):6647–6654
Bianchinotti MV (1997) A new species of Pseudorobillarda from a leguminous tree in Argentina. Mycol Res 101:1233–1236
Boerema GH, Bollen GJ (1975) Conidiogenesis and conidial septation ads differentiating criteria between Phoma and Ascochyta. Persoonia 8:111–144
Boerema GH, de Gruyter J, Noordeloos ME, Hamers MEC (2004) Phoma identification manual: differentiation of specific and infra-specific taxa in culture. CABI, Wallingford, p 470
Bonar L (1942) Studies on some California fungi: II. Mycologia 34:180–192
Bonar L (1962) Stegopezizella balsameae and Gloeosporium balsameae. Mycologia 54:395–399
Boom PVD, Sérusiaux E, Diederich P, Brand M, Aptroot A, Spier L (1998) A lichenlogical excursion in May 1997 near Han-sur-Lesse and Saint-Hubert, with notes on rare or critical taxa of the flora of Belgium and Luxembourg. Lejeunia (Belgium) 158:1–58
Bose T, Reynolds DR, Berbee ML (2014) Common, unsightly and until now undescribed: Fumiglobus pieridicola sp. nov., a sooty mold infesting Pieris japonica from western North America. Mycologia 106:746–756
Botella L, Diez JJ (2011a) Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis. Fungal Divers 47:9–18
Botella L, Diez JJ (2011b) Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis. Fungal Divers 47:9–18
Boudier E (1907) Histoire et classification des Discomycètes d’Europe. Paul Klincksieck, Paris
Boyette CD, Gealy D, Hoagland RE, Vaughn KC, Bowling AJ (2011) Hemp sesbania (Sesbania exaltata) control in rice (Oryza sativa) with the bioherbicidal fungus Colletotrichum gloeosporioides f. sp. aeschynomene formulated in an invert emulsion. Biocontrol Sci Technol 21:1399–1407
Braun U (2013) (2210–2232) Proposals to conserve the teleomorph- typified name Blumeria against the anamorph-typified name Oidium and twenty-two teleomorph-typified powdery mildew species names against competing anamorph-typified names (Ascomycota: Erysiphaceae. Taxon 62:1328–1331
Breton A, Faurel L (1970) Etude comparative des Monodia elegans nov. gen., nov. sp., et Pullospora tetrachaeta Faur. et Schott. Sphaeropsidales. Rev Mycol 35:22–40
Brisson JD, Pauze JF (1975) A new Coelomycete (Dwayalomella vaccinii gen. and sp. nov.) from Vaccinium angustifolium in Canada. Can J Bot 53:2866–2871
Bronson JJ, Stanosz GR, Putnam ML (2003) First report of Sirococcus conigenus on deodar cedar in Oregon. Plant Dis 87:1006
Brubacher DC, Rawla GS, Sharma R (1984) A new species of Crucellisporiopsis from India. Mycotaxon 21:449–458
Buchan A, Newell SY, Moreta JIL, Moran MA, (2002) Analysis of Internal Transcribed Spacer (ITS) Regions of rRNA Genes in Fungal Communities in a Southeastern U.S. Salt Marsh. Micro Ecol 43:329–340
Buddin W, Wakefield EM (1926) On the life-history of a fungus parasitic on Antirrhinum majus, with some remarks on the genus Heterosphaeria. Trans Br Mycol Soc 11:169–186
Burton JHM, Gwendolyn M (1943) Study of Bagnisiopsis species on the Melastomaceae. Mycologia 35:312–334
Butin H (1977) Taxonomy and morphology of Ascodichaena gen. et sp. nov. Trans Br Mycol Soc 69:249–254
Butin H, Kher R (1998) Gloeosporidina platani sp. nov., the spermatial state of the anthracnose fungus Apiognomonia veneta (Sacc., Speg.) Höhn. Eur J Plant Pathol 28:297–305
Cai L, Zhang KQ, McKenzie EHC, Hyde KD (2003) Freshwater fungi from bamboo and wood submerged in the Liput River in the Philippines. Fungal Divers 13:1–12
Cai L, Ji KF, Hyde KD (2006) Variation between freshwater and terrestrial fungal communities on decaying bamboo culms. Antonie Van Leeuwenhoek 89:293–301
Cai L, Hyde KD, Taylor PWJ, Weir B, Waller J, Abang MM, Zhang JZ, Yang YL, Phoulivong S, Liu ZY, Prihastuti H, Shivas RG, Mckenzie EHC, Johnston PR (2009) A polyphasic approach for studying Colletotrichum. Fungal Divers 39:183–204
Campbell J, Shearer C, Crane J, Fallah P (2003) A reassessment of two freshwater ascomycetes, Ceriospora caudae-suis and Submersisphaeria aquatica. Mycologia 95:41–53
Campbell R (1979) Ultrastructure of conidium ontogeny of Pseudobasidiospora caroliniana. Trans Br Mycol Soc 72:207–212
Cannon PF (1996) Systematics and diversity of the Phyllachoraceae associated with Rosaceae, with a monograph of Polystigma. Mycol Res 100:1409–1427
Cannon PF, Damm U, Johnston PR, Wei BS (2012) Colletotrichum-current status and future directions. Stud Mycol 73:181–213
Cannon PF, Kirk PM (2007) Fungal Families of the world, illustrate. CABI, Wallingford, p 456
Cannon PF, Minter DW (1983) The nomenclatural history and typification of Hypoderma and Lophodermium. Taxon 32:572–583
Cannon PF, Minter DW (1984) Rhytisma acerinum. CMI Descrip Pathog Fungi Bact 791:1–2
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Carlucci A, Raimondo ML, Cibelli F, Phillips AJL, Lops F (2013) Pleurostomophora richardsiae, Neofusicoccum parvum and Phaeoacremonium aleophilum associated with a decline of olives in southern Italy. Phytopathol Mediterr 52:517–527
Carmichael WJ (1981) Pleomorphism. In: Coke GT, Kendrick B (eds) Biology of conidial fungi, vol 1. Academic, New York, pp 135–143
Carmona M, Barreto D, Fortugno C (1996) Occurrence of halo spot in barley caused by Pseudoseptoria donacis in Argentina. EPPO Bull 26:437–439
Carris LM (1990) Cranberry black rot fungi: Allantophomopsis cytisporea and Allantophomopsis lycopodina. Can J Bot 68:2283–2291
Cash EK (1934) Godronia urceolus and Other Cenangiaceae on Ribes. Mycologia 26:266–272
Castagne L (1851) Supplément au catalogue des plantes qui croissent naturellement aux environs de Marseille. Olicot & Pardigon, Charleston
Castañeda RF (1987) Fungi cubensis II. Instituto de Investigaciones Fundamentales en Agricultura tropical “Alejandro de Humboldt.”, La Habana
Castañeda RF, Kendrick B (1991) Ninety-nine conidial fungi from Cuba and three from Canada. Univ Waterloo Biol Ser 35:1–132
Chaverri P, Liu M, Hodge KT (2008) A monograph of the entomopathogenic genera Hypocrella, Moelleriella, and Samuelsia gen. nov. (Ascomycota, Hypocreales, Clavicipitaceae), and their aschersonia-like anamorphs in the Neotropics. Stud Mycol 60:1–66
Checa J, Jaklitsch WM, Blanco MN, Moreno G, Olariaga I, Tello S, Voglmayr H (2015) Two new species of Thyronectria from Mediterranean Europe. Mycologia 107:1314–1322
Cheewangkoon R, Groenewald JZ, Summerell BA, Hyde KD, To-anun C, Crous PW (2009) Myrtaceae, a cache of fungal biodiversity. Persoonia 23:55–85
Chen XH, Wu JR, Zhang JL (1991) White vein disease: a new disease in Cinnamomum glanduliferum. J Southwest For College 11:205–207
Chen SF, Pavlic D, Roux J, Slippers B, Xie YJ, Wingfield MJ, Zhou XD (2011) Characterization of Botryosphaeriaceae from plantation-grown Eucalyptus species in South China. Plant Pathol 60:739–751
Chen Q, Jiang JR, Zhang GZ, Crous PW (2015) Resolving the Phoma enigma. Stud Mycol 82:137–217
Chen C, Verkley GJM, Sun G, Groenewald JZ, Crous PW (2016) Redefining common endophytes and plant pathogens in Neofabraea, Pezicula, and related genera. Fungal Biol 120:1291–1322
Chen Q, Hou LW, Duan WJ, Crous PW, Cai L (2017) Didymellaceae revisited. Stud Mycol 87:105–159
Chilvers MI, Rogers JD, Dugan FM, Stewart JE, Chen W, Peever TL (2009) Didymella pisi sp. nov., the teleomorph of Ascochyta pisi. Mycol Res 113:391–400
Chomnunti P, Schoch CL, Aguirre-Hudson B, Ko-Ko TW, Hongsanan S, Jones EBG, Kodsueb R, Phookamsak R, Chukeatirote E, Bahkali AH, Hyde KD (2011) Capnodiaceae. Fungal Divers 51:103–134
Chomnunti P, Hongsanan S, Aguirre-hudson B, Tian Q, PerŠoh D, Dhami MK, Alias AS, Xu JC, Liu XZ, Stadler M, Hyde KD (2014) The sooty moulds. Fungal Divers 66:1–36
Clark J, Lott T (1989) Age heterokaryon studies in Didymium iridis. Mycologia 81:636–638
Clements RE, Shear CL (1931) The Genera of Fungi, 2nd edn. New York, H.W, Wilson
Cole GT, Kendrick B (1981) Biology of Conidial Fungi, vol 1. Academic Press, INC., New York
Collado J, Platas G, Bills GF, Basilio A, Vicente F, Tormo JR, Hernández P, Díez MT, Peláez F (2006) Studies on Morinia: Recognition of Morinia longiappendiculata sp. nov. as a new endophytic fungus, and a new circumscription of Morinia pestalozzioides. Mycologia 98:616–627
Conners IL (1959) Species of Leptothyrium and Kabatia on Lonicera. Can J Bot 37:419–429
Cooke MC (1883) New American Fungi. Grevillea 12:22–33
Cooke MC, Ellis JB (1877) New Jersey fungi. Grevillea 6:1–18
Crandall BS (1942) Thyronectria disease of honey locust in the South. Plant Dis Report 26:376
Crane JL (1971) Illinois fungi. 1. Chaetosticta. Can J Bot 49:31–34
Crouch JA, Tredway LP, Clarke BB, Hillman BI (2009) Phylogenetic and population genetic divergence correspond with habitat for the pathogen Colletotrichum cereale and allied taxa across diverse grass communities. Mol Ecol 18:123–135
Crous PW (1991) Two newly reported leaf pathogens of Eucalyptus grandis in South Africa. S Afr For J 157:12–15
Crous PW (1993) New and interesting fungi. 13. Foliicolous microfungi. S Afr J Bot 59:602–610
Crous PW (2009) Taxonomy and phylogeny of the genus Mycosphaerella and its anamorphs. Fungal Divers 38:1–24
Crous PW, Groenewald JZ (2017) The Genera of Fungi-G4: Camarosporium and Dothiora. IMA Fungus 8:131–152
Crous PW, Wingfield MJ, Koch SH (1990) New and Interesting records of South Africal fungi. X. New records of Eucalyptus leaf fungi. S Afr J Bot 56:583–586
Crous PW, Mohammed C, Glen M, Verkley GJM, Groenewald JZ (2007) Eucalyptus microfungi known from culture. 3. Eucasphaeria and Sympoventuria genera nova, and new species of Furcaspora, Harknessia, Heteroconium and Phacidiella. Fungal Divers 25:19–36
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006a) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–254
Crous PW, Verkley GJM, Groenewald JZ (2006b) Eucalyptus microfungi known from culture. 1. Cladoriella and Fulvoflamma genera nova, with notes on some other poorly known taxa. Stud Mycol 55:53–63
Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hoog GS, Groenewald JZ (2009) Phylogenetic lineages in the Capnodiales. Stud Mycol 64:17–47
Crous PW, Groenewald JZ, Shivas RG, Edwards J, Seifert KA, Alfenas AC, Alfenas RF, Burgess TI, Carnegie AJ, Hardy GESTJ, Hiscock N, Hüberli D, Jung T, Louis-Seize G, Okada G, Pereira OL, Stukely MJC, Wang W, White GP, Young AJ, McTaggart AR, Pascoe IG, Porter IJ, Quaedvlieg W (2011a) Fungal planet description sheets: 69–91. Persoonia 26:108–156
Crous PW, Summerell BA, Shivas RG, Romberg M, Mel’nik VA, Verkley GJM, Groenewald JZ (2011b) Fungal Planet description sheets: 92–106. Persoonia 27:130–162
Crous PW, Summerell BA, Alfenas AC, Edwards J, Pascoe IG, Porter IJ, Groenewald JZ (2012a) Genera of diaporthalean coelomycetes associated with leaf spots of tree hosts. Persoonia 28:66–75
Crous PW, Verkley GJM, Christensen M, Castañeda-Ruiz RF, Groenewald JZ (2012b) How important are conidial appendages? Persoonia 28:126–137
Crous PW, Wingfield MJ, Guarro J, Cheewangkoon R, van der Bank M, Swart WJ, Stchige AM, Cano-Lira JF, Roux J, Madrid H, Damm U, Wood AR, Shuttleworth LA, Hodges CS, Munster M, de Jesús Yáñez-Morales M, Zúñiga-Estrada L, Cruywagen EM, de Hoog GS, Silvera C, Najafzadeh J, Davison EM, Davison PJN, Barrett MD, Barrett RL, Manamgoda DS, Minnis AM, Kleczewski NM, Flory SL, Castlebury LA, Clay K, Hyde KD, Maússe-Sitoe SND, Chen SF, Lechat C, Hairaud M, Lesage-Meessen L, Pawłowska J, Wilk M, Śliwińska-Wyrzychowska A, Mętrak M, Wrzosek M, Pavlic-Zupanc D, Maleme HM, Slippers B, Mac Cormack WP, Archuby DI, Grünwald NJ, Tellería MT, Dueñas M, Martín MP, Marincowitz S, de Beer ZW, Perez CA, Gené J, Marin-Felix Y, Groenewald JZ (2013) Fungal Planet description sheets: 154–213. Persoonia 31:188–296
Crous PW, Giraldo A, Hawksworth DL, Robert V, Kirk PM, Guarro J, Robbertse B, Schoch CL, Damm U, Trakunyingcharoen T, Groenewald JZ (2014a) The Genera of Fungi: fixing the application of type species of generic names. IMA Fungus 5:141–160
Crous PW, Quaedvlieg W, Hansen K, Hawksworth DL, Groenewald JZ (2014b) Phacidium and Ceuthospora (Phacidiaceae) are congeneric: taxonomic and nomenclatural implications. IMA Fungus 5:173–193
Crous PW, Wingfield MJ, Schumacher RK, van der Bank M, Zhang Y, Summerell BA, Guarro J, Wingfield MJ, Wood AR, Alfenas AC, Braun U, Cano-Lira JF, García D, Marin-Felix Y, Alvarado P, Andrade JP, Armengol J, Assefa A, den Breeÿen A, Camele I, Cheewangkoon R, De Souza JT, Duong TA, Esteve-Raventós F, Fournier J, Frisullo S, García-Jiménez J, Gardiennet A, Gené J, Hernández-Restrepo M, Hirooka Y, Hospenthal DR, King A, Lechat C, Lombard L, Mang SM, Marbach PAS, Marincowitz S, Marin-Felix Y, Montaño-Mata NJ, Moreno G, Perez CA, Pérez Sierra AM, Robertson JL, Roux J, Rubio E, Schumacher RK, Stchigel AM, Sutton DA, Tan YP, Thompson EH, van der Linde E, Walker AK, Walker DM, Wickes BL, Wong PTW, Groenewald JZ (2014c) Fungal Planet description sheets: 214–280. Persoonia 33:212–289
Crous PW, Wingfield MJ, Schumacher RK, van der Bank M, Zhang Y, Summerel BA, Guarro J, Wingfield MJ, Wood AR, Alfenas AC, Braun U, Cano-Lira JF, García D, Marin-Felix Y, Alvarado P, Andrade JP, Armengol J, Assefa A, den Breeÿen A, Camele I, Cheewangkoon R, De Souza JT, Duong TA, Esteve-Raventós F, Fournier J, Frisullo S, García-Jiménez J, Gardiennet A, Gené J, Hernández-Restrepo M, Hirooka Y, Hospenthal DR, King A, Lechat C, Lombard L, Mang SM, Marbach PAS, Marincowitz S, Marin-Felix Y, Montaño-Mata NJ, Moreno G, Perez CA, Pérez Sierra AM, Robertson JL, Roux J, Rubio E, Schumacher RK, Stchige AM, Sutton DA, Tan YP, Thompson EH, van der Linde E, Walker AK, Walker DM, Wickes BL, Wong PTW, Groenewald JZ (2014d) Fungal Planet description sheets: 281–319. Persoonia 32:184–306
Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernández-Restrepo M, Jaklitsch WM, Lebrun Marc-Henri, Schumacher RK, Stielow JB, Van der Linde EJ (2015a) The Genera of Fungi: fixing the application of the type species of generic names-G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia, IMA Fungus 6:163–198
Crous PW, Müller MM, Sánchez RM, Giordano L, Bianchinotti MV, Anderson FE, Groenewald JZ (2015b) Resolving Tiarosporella spp. allied to Botryosphaeriaceae and Phacidiaceae. Phatotaxa 202:73–93
Crous PW, Schumacher RK, Wingfield MJ, Lombard L, Giraldo A, Christensen M, Gardiennet A, Nakashima C, Pereira OL, Smith AJ, Groenewald JZ (2015c) Fungal systematics and evolution: FUSE 1. Sydowia 67:81–118
Crous PW, Wingfield MJ, Guarro J, Hernández-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Dueñas M, Dutta AK, Gené J, Gouliamova DE, Groenewald M, Lombard L, Morozova OV, Sarkar J, Smith MTh, Stchige AM, Wiederhold NP, Alexandrova AV, Antelmi I, Armengo J, Barnes I, Cano-Lira JF, Castañeda Ruiz RF, Contu M, PrR Courtecuisse, da Silveira AL, Decock CA, de Goes A, Edathodu J, Ercole E, Firmino AC, Fourie A, Fournier J, Furtado EL, Geering ADW, Gershenzon J, Giraldo A, Gramaje D, Hammerbacher A, He XL, Haryadi D, Khemmuk W, Kovalenko AE, Krawczynski R, Laich F, Lechat C, Lopes UP, Madrid H, Malysheva EF, Marín-Felix Y, Martín MP, Mostert L, Nigro F, Pereira OL, Picillo B, Pinho DB, Popov ES, Rodas Peláez CA, Rooney-Latham S, Sandoval-Denis M, Shivas RG, Silva V, Stoilova-Disheva MM, Telleria MT, Ullah C, Unsicker SB, van der Merwe NA, Vizzini A, Wagner HG, Wong PTW, Wood AR, Groenewald JZ (2015d) Fungal Planet description sheets: 320–370. Persoonia 34:167–266
Crous PW, Wingfield MJ, Le Roux JJ, Richardson DM, Strasberg D, Shivas RG, Alvarado P, Edwards J, Moreno G, Sharma R, Sonawane MS, Tan YP, Altés A, Barasubiye T, Barnes CW, Blanchette RA, Boertmann D, Bogo A, Carlavilla JR, Cheewangkoon R, Daniel R, de Beer ZW, de Jesús Yáñez-Morales M, Duong TA, Fernández-Vicente J, Geering ADW, Guest DI, Held BW, Heykoop M, Hubka V, Ismail AM, Kajale SC, Khemmuk W, Kolařík M, Kurli R, Lebeuf R, Lévesque CA, Lombard L, Magista D, Manjón JL, Marincowitz S, Mohedano JM, Nováková A, Oberlies NH, Otto EC, Paguigan ND, Pascoe IG, Pérez-Butrón JL, Perrone G, Rahi P, Raja HA, Rintoul T, Sanhueza RMV, Scarlett K, Shouche YS, Shuttleworth LA, Taylor PWJ, Thorn RG, Vawdrey LL, Solano-Vidal R, Voitk A, Wong PTW, Wood AR, Zamora JC, Groenewald JZ (2015e) Fungal Planet description sheets: 371–399. Persoonia 35:264–327
Crous PW, Wingfield MJ, Burgess TI, Hardy GESTJ, Crane C, Barrett S, Cano-Lira JF, Le Roux JJ, Thangave R, Guarro J, Stchige AM, Martín MP, Alfredo DS, Barber PA, Barreto RW, Baseia IG, Cano-Canals J, Cheewangkoon R, Ferreira RJ, Gené J, Lechat C, Moreno G, Roets F, Shivas RG, Sousa JO, Tan YP, Wiederhold NP, Abell SE, Accioly T, Albizu JL, Alves JL, Antoniolli ZI, Aplin N, Araújo J, Arzanlou M, Bezerra JDP, Bouchara J-P, Carlavilla JR, Castillo A, Castroagudín VL, Ceresini PC, Claridge GF, Coelho G, Coimbra VRM, Costa LA, da Cunha KC, da Silva SS, Daniel R, de Beer ZW, Dueñas M, Edwards J, Enwistle P, Fiuza PO, Fournier J, García D, Gibertoni TB, Giraud S, Guevara-Suarez M, Gusmão LFP, Haituk S, Heykoop M, Hirooka Y, Hofmann TA, Houbraken J, Hughes DP, Kautmanová I, Koppel O, Koukol O, Larsson E, Latha KPD, Lee DH, Lisboa DO, Lisboa WS, López-Villalba Á, Maciel JLN, Manimohan P, Manjón JL, Marincowitz S, Marney TS, Meijer M, Miller AN, Olariaga I, Paiva LM, Piepenbring M, Poveda-Molero JC, Raj KNA, Raja HA, Rougeron A, Salcedo I, Samadi R, Santos TAB, Scarlett K, Seifert KA, Shuttleworth LA, Silva GA, Silva M, Siqueira JPZ, Souza-Motta CM, Stephenson SL, Sutton DA, Tamakeaw N, Telleria MT, Valenzuela-Lopez N, Viljoen A, Visagie CM, Vizzini A, Wartchow F, Wingfield BD, Yurchenko E, Zamora JC, Groenewald JZ (2016a) Fungal Planet description sheets: 469–557. Persoonia 37:218
Crous PW, Wingfield MJ, Burgess TI, Le Roux JJ, Strasberg D, Edwards J, Roets F, Hubka V, Taylor PWJ, Heykoop M, Martín MP, Moreno G, Sutton DA, Wiederhold NP, Barnes CW, Carlavilla JR, Gené J, Giraldo A, Guarnaccia V, Guarro J, Hernández-Restrepo M, Kolařík M, Manjón JL, Pascoe IG, Popov ES, Sandoval-Denis M, Woudenberg JHC, Acharya K, Alexandrova AV, Alvarado P, Barbosa RN, Baseia IG, Blanchette RA, Boekhout T, Burgess TI, Cano-Lira JF, Čmoková A, Dimitrov RA, Dyakov MY, Dueñas M, Dutta AK, Esteve-Raventós F, Fedosova AG, Fournier J, Gamboa P, Gouliamova DE, Grebenc T, Groenewald M, Hanse B, Hardy GESTJ, Held BW, Jurjević Ž, Kaewgrajang T, Latha KPD, Lombard L, Luangsa-ard JJ, Lysková P, Mallátová N, Manimohan P, Miller AN, Mirabolfathy M, Morozova OV, Obodai M, Oliveira NT, Ordóñez ME, Otto EC, Paloi S, Peterson SW, Phosri C, Roux J, Salazar WA, Sánchez A, Sarria GA, Shin H-D, Silva BDB, Silva GA, Smith MTH, Souza-Motta CM, Stchigel AM, Stoilova-Disheva MM, Sulzbacher MA, Telleria MT, Toapanta C, Traba JM, Valenzuela-Lopez N, Watling R, Groenewald JZ (2016b) Fungal Planet description sheets: 400–468. Persoonia 36:316–458
Crous PW, Wingfield MJ, Burgess TI, Carnegie AJ, Hardy GESTJ, Smith D, Summerell BA, Cano-Lira JF, Guarro J, Houbraken J, Lombard L, Martín MP, Sandoval-Denis M, Alexandrova AV, Barnes CW, Baseia IG, Bezerra JDP, Guarnaccia V, May TW, Hernández-Restrepo M, Stchigel AM, Miller AN, Ordoñez ME, Abreu VP, Accioly T, Agnello C, Agustin Colmán A, Albuquerque CC, Alfredo DS, Alvarado P, Araújo-Magalhães GR, Arauzo S, Atkinson T, Barili A, Barreto RW, Bezerra JL, Cabral TS, Camello Rodríguez F, Cruz RHSF, Daniëls PP, da Silva BDB, de Almeida DAC, de Carvalho Júnior AA, Decock CA, Delgat L, Denman S, Dimitrov RA, Edwards J, Fedosova AG, Ferreira RJ, Firmino AL, Flores JA, García D, Gené J, Giraldo A, Góis JS, Gomes AAM, Gonçalves CM, Gouliamova DE, Groenewald M, Guéorguiev BV, Guevara-Suarez M, Gusmão LFP, Hosaka K, Hubka V, Huhndorf SM, Jadan M, Jurjević Ž, Kraak B, Kučera V, Kumar TKA, Kušan I, Lacerda SR, Lamlertthon S, Lisboa WS, Loizides M, Luangsa-ard JJ, Lysková P, Mac Cormack WP, Macedo DM, Machado AR, Malysheva EF, Marinho P, Matočec N, Meijer M, Mešić A, Mongkolsamrit S, Moreira KA, Morozova OV, Nair KU, Nakamura N, Noisripoom W, Olariaga I, Oliveira RJV, Paiva LM, Pawar P, Pereira OL, Peterson SW, Prieto M, Rodríguez-Andrade E, Rojo De Blas C, Roy M, Santos ES, Sharma R, Silva GA, Souza-Motta CM, Takeuchi-Kaneko Y, Tanaka C, Thakur A, Smith MTh, Tkalčec Z, Valenzuela-Lopez N, van der Kleij P, Verbeken A, Viana MG, Wang XW, Groenewald JZ (2017) Fungal Planet description sheets: 625–715. Persoonia 39:270–467
Crous PW, Wingfield MJ, Burgess TI, Hardy GESTJ, Gené J, Guarro J, Baseia IG, García D, Gusmão LFP, Souza-Motta CM, Thangavel R, Adamčík S, Barili A, Barnes CW, Bezerra JDP, Bordallo JJ, Cano-Lira JF, de Oliveira RJV, Ercole E, Hubka V, Iturrieta-González I, Kubátová A, Martín MP, Moreau P-A, Morte A, Ordoñez ME, Rodríguez A, Stchige AM, Vizzini A, Abdollahzadeh J, Abreu VP, Adamčíková K, Albuquerque GMR, Alexandrova AV, Álvarez Duarte E, Armstrong-Cho C, Banniza S, Barbosa RN, Bellanger J-M, Bezerra JL, Cabral TS, Caboň M, Caicedo E, Cantillo T, Carnegie AJ, Carmo LT, Castañeda-Ruiz RF, Clement CR, Čmoková A, Conceição LB, Cruz RHSF, Damm U, da Silva BDB, da Silva GA, da Silva RMF, de A Santiago ALCM, de Oliveira LF, de Souza CAF, Déniel F, Dima B, Dong G, Edwards J, Félix CR, Fournier J, Gibertoni TB, Hosaka K, Iturriaga T, Jadan M, Jany J-L, Jurjević Ž, Kolařík M, Kušan I, Landell MF, Leite Cordeiro TR, Lima DX, Loizides M, Luo S, Machado AR, Madrid H, Magalhães OMC, Marinho P, Matočec N, Mešić A, Miller AN, Morozova OV, Neves RP, Nonaka K, Nováková A, Oberlies NH, Oliveira-Filho JRC, Oliveira TGL, Papp V, Pereira OL, Perrone G, Peterson SW, Pham THG, Raja HA, Raudabaugh DB, Řehulka J, Rodríguez-Andrade E, Saba M, Schauflerová A, Shivas RG, Simonini G, Siqueira JPZ, Sousa JO, Stajsic V, Svetasheva T, Tan YP, Tkalčec Z, Ullah S, Valente P, Valenzuela-Lopez N, Abrinbana M, Viana Marques DA, Wong PTW, Xavier de Lima V, Groenewald JZ (2018a) Fungal Planet description sheets: 716–784. Persoonia 40:240–393
Crous PW, Wingfield MJ, Carnegie AJ, Carnegie AJ, Hernández-Restrepo M, Lombard L, Roux J, Barreto RW, Baseia IG, Cano-Lira JF, Martín MP, Morozova OV, Stchige AM, Summerel BA, Brandrud TE, Dima B, García D, Giraldo A, Guarro J, Gusmão LFP, Khamsuntorn P, Noordeloos ME, Nuankaew S, Pinruan U, Rodríguez-Andrade E, Souza-Motta CM, Thangavel R, van Iperen AL, Abreu VP, Accioly T, Alves JL, Andrade JP, Bahram M, Bara HO, Barbier E, Barnes CW, Bendiksen E, Bernard E, Bezerra JDP, Bezerra JL, Bizio E, Blair JE, Bulyonkova TM, Cabral TS, Caiafa MV, Cantillo T, Colmán AA, Conceição LB, Cruz S, Cunha AOB, Darveaux BA, da Silva AL, da Silva GA, da Silva GM, da Silva RMF, de Oliveira RJV, Oliveira RL, De Souza JT, Dueñas M, Evans HC, Epifani F, Felipe MTC, Fernández-López J, Ferreira BW, Figueiredo CN, Filippova NV, Flores JA, GenéJ Ghorbani G, Gibertoni TB, Glushakova AM, Healy R, Huhndorf SM, Iturrieta-González I, Javan-Nikkhah M, Juciano RF, Jurjević Ž, Kachalkin AV, Keochanpheng K, Krisai-Greilhuber I, Li YC, Lima AA, Machado AR, Madrid H, Magalhães OMC, Marbach PAS, Melanda GCS, Miller AN, Mongkolsamrit S, Nascimento RP, Oliveira TGL, Ordoñez ME, Orzes R, Palma MA, Pearce CJ, Pereira OL, Perrone G, Peterson SW, Pham THG, Piontelli E, Pordel A, Quijada L, Raja HA, Rosas de Paz E, Ryvarden L, Saitta A, Salcedo SS, Sandoval-Denis M, Santos TAB, Seifert KA, Silva BDB, Smith ME, Soares AM, Sommai S, Sousa JO, Suetrong S, Susca A, Tedersoo L, Telleria MT, Thanakitpipattana D, Valenzuela-Lopez N, Visagie CM, Zapata M, Groenewald JZ (2018b) Fungal Planet description sheets: 785–867. Persoonia 41:238–417
Crous PW, Schumacher RK, Akulov A, Thangavel R, Hernández-Restrepo M, Carnegie AJ, Cheewangkoon R, Wingfield MJ, Summerell BA, Quaedvlieg W, Coutinho TA, Roux J, Wood AR, Giraldo A, Groenewald JZ (2019) New and interesting fungi.2. Fungal Syst Evol 3:57–134
Crowe F, Starkey D, Lengkeek V (1982) Honey locust canker in Kansas caused by Thyronectria austro-americana. Plant Dis 66:155–158
Croxall HE (1950) Studies on British Pyrenomycetes. III. The British species of the genus Diatrypella Cesati and de Notaris. Trans Br Mycol Soc 33:45–72
Cunnell GJ (1957) On Neottiospora caricina (Desm.) Hohnel. Trans. Brit. Mycol. Soc. 40:433–442
Cunnington JH, Priest MJ, Powney RA, Cother NJ (2007) Diversity of Botryosphaeria species on horticultural plants in Victoria and New South Wales. Austral Plant Pathol 36:157–159
Da Luz Calado M, Barata M (2012) Salt marsh fungi. In: Jones EBG, Pang K-L (eds) Marine fungi: and fungal-like organisms. Walter de Gruyter, Berlin
Dai DQ, Wijayawardene NN, Bhat DJ, Chukeatirote E, Zhao RL, Wang Y, Bahkali AH, Hyde KD (2014) The Phylogenetic placement of Eriosporella bambusicola sp. nov. in Capnodiales. Cryptogam Mycol 35:41–49
Dai DQ, Phookamsak R, Wijayawardene NN, Li WJ, Bhat DJ, Xu JC, Taylor JE, Hyde KD, Chukeatirote E (2017) Bambusicolous fungi. Fungal Divers 82:1–105
Damm U, Fourie PH, Crous PW (2010) Coniochaeta (Lecythophora), Collophora gen. nov. and Phaeomoniella species associated with wood necroses of Prunus trees. Persoonia 24:60–80
Damm U, Cannon PF, Woudenberg JHC, Crous PW (2012a) The Colletotrichum acutatum species complex. Stud Mycol 73:37–113
Damm U, Cannon PF, Woudenberg JHC, Johnston PR, Weir BS, Tan YP, Shivas RG, Crous PW (2012b) The Colletotrichum boninense species complex. Stud Mycol 73:1–36
Damm U, Sato T, Alizadeh A, Groenewald JZ, Crous PW (2019) The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud Mycol 46:1–46
Darker GD (1963) A new genus of Phacidiaceae on Picea mariana. Can J Bot 41:1389–1393
Darker GD (1967) Revision of the genera of the Hypodermataceae. Can J Bot 45:1399–1444
Davidson JA, Hartley D, Priest M, Krysinska-Kaczmarek M, Herdina McKay A, Scott ES (2009) A new species of Phoma causes ascochyta blight symptoms on field peas (Pisum sativum) in South Australia. Mycologia 101:120–128
Dayarathne MC, Phookamsak R, Ariyawansa HA, Jones EBG, Camporesi E, Hyde KD (2015) Phylogenetic and morphological appraisal of Leptosphaeria italica sp. nov. (Leptosphaeriaceae, Pleosporales) from Italy. Mycosphere 6:634–642
Dayarathne M, Maharachchikumbura S, Jones EBG, Goonasekara ID, Bulgakov TS, Al-Sadi AM, Hyde KD, Lumyong S, McKenzie EHC (2017) Neophyllachora gen nov. (Phyllachorales), three new species of Phyllachora from Poaceae and resurrection of Polystigmataceae. Mycosphere 8:1598–1625
De Bivona-Bernadi A (1813–1816) Stirpium rariorum, minusque cognitarum in Sicilia sponte provenientium descriptione, nonnullis iconibus auctae Manipulus I–IV. Typis Regiis, Palermo
De Gruyter J, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009) Molecular phylogeny of Phoma and allied anamorph genera: towards a re-classification of the Phoma complex. Mycol Res 113:508–519
De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2010) Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102:1066–1081
De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2013) Redisposition of phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36
Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430
Dearness J (1928) New and noteworthy fungi-V. Mycologia 20:235–246
Decock C, Delgado Rodriguez G, Seifert K (2005) Taxonomy and phylogeny of Synchaetomella lunatospora, a new genus and species of synnematous fungi from Southeast Asia. Antonie van Leeuwenhoek 88:231–240
Denman PW, Taylor JE, Kang JC, Pascoe I, Michael J (2000) An overview of the taxonomic history of Botryosphaeria, and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Stud Mycol 45:29–140
Dennis RWG (1978) British Ascomycetes. CABI, Egham, Surrey, UK
Desmazières JBHJ (1840) Sur un genre nouveau de l’ordre des Pyrenomycetes. Ann Sci Nat Bot Sér 2(14):5–7
Desmazièrcs JBHJ (1843) Dixième notice sur quelques plantes cryptogames, la plupart inédites, récemment découvertes en France, et qui vont paraître en nature dans la collection publiée par l’auteur. Ann Sci nat Bot 2:335–373
Dettrakul S, Kittakoop P, Isaka M, Nopichai S, Suyarnsestakorn C, Tanticharoenb M, Thebtaranonth Y (2003) Antimycobacterial pimarane diterpenes from the fungus Diaporthe sp. Bioorg Med Chem Lett 7:1253–1255
Dharkar N, Hande D, Shahezad MA (2009) Ajrekarella asetosa, A new coelomycete from Vidarbha, India. Kavaka 37:3–5
Dianese JC, Tessman DJ, Furlanetto C (1994) Reinstating Oswaldina icarahyensis as the name of the anamorph of Apiosphaeria guaranitica. Sydowia 46:233–237
Dicosmo F (1978) A revision of Corniculariella. Can J Bot 56:1665–1690
DiCosmo F, Nag Raj TR, Kendrick B (1983) Prodromus for a revision of the Phacidiaceae and related anamorphs. Can J Bot 61:31–44
DiCosmo F, Nag Raj TR, Kendrick B (1984) A revision of the Phacidiaceae and related anamorphs. Mycotaxon 21:1–234
Diederich P, Pvanden Boom, Aptroot A, Diederich P (2001) Cladonhcola staurospora gen. et sp. nov., a new lichenicolous coelomycete from western Europe. Belg J Bot 134:127–130
Diederich P, Lawrey JD, Sikaroodi M, van den Boom PPG, Ertz D (2012) Briancoppinsia, a new coelomycetous genus of Arthoniaceae (Arthoniales) for the lichenicolous Phoma cytospora, with a key to this and similar taxa. Fungal Divers 52:1–12
Diedicke H (1912) Die Abteilung Hyalodidymae der Sphaerioideen. Ann Mycol 10:135–152
Diogo ELF, Santos JM, Phillips AJL (2010) Phylogeny, morphology and pathogenicity of Diaporthe and Phomopsis species on almond in Portugal. Fungal Divers 44:107–115
Dissanayake AJ, Phillips AJL, Li XH, Hyde KD (2016) Botryosphaeriaceae: current status of genera and species. Mycosphere 7:1001–1073
Dixon JR (1975) Chlorosplenium and its segregates. II. The genera Chlorociboria and Chlorencoelia. Mycotaxon 1:193–237
Doilom M, Dissanayake AJ, Wanasinghe DN, Boonmee S, Liu JK, Bhat DJ, Taylor JE, Bahkali AH, McKenzie EHC, Hyde KD (2017) Microfungi on Tectona grandis (teak) in Northern Thailand. Fungal Divers 82:107–182
Duan JX, Wu WP, Liu XZ (2007) Dinemasporium (coelomycetes). Fungal Divers 26:205–218
Dyko BJ, Sutton BC (1978) Two new genera of water-borne coelomycetes from submerged leaf litter. Nova Hedwig 29:167–175
Dyko BJ, Sutton BC (1979a) A revision of Linodochium, Pseudocenaizgiuin, Septopatella, and Siroscyphella. Can J Bot 57:370–385
Dyko BJ, Sutton BC (1979b) Two new and unusual deuteromycetes. Trans Br Mycol Soc 72:411–417
Dyko BJ, Sutton BC, Roquebert MF (1979) The genus Protostegia. Mycologia 71:918–934
Ekanayaka AH, Ariyawansa HA, Hyde KD, Jones EBG, Daranagama DA, Phillips AJL, Hongsanan S, Jayasiri SC, Zhao Q (2017) DISCOMYCETES: the apothecial representatives of the phylum Ascomycota. Fungal Divers 87:237–298
Ekanayaka AH, Hyde KD, Jones EBG, Zhao Q (2018) Taxonomy and phylogeny of operculate discomycetes: Pezizomycetes. Fungal Divers 90:161–243
Ekanayaka AH, Hyde KD, Gentekaki E, McKenzie EHC, Zhao Q, Bulgakov TS, Camporesi E (2019) Preliminary classification of Leotiomycetes. Mycosphere 10:310–489
Ellis DE, Gill LS (1945) Mycological society of a new Rhabdogloeum associated with Rhabdocline pseudotsugae in the Southwest. Mycologia 37:326–332
Ellis JB (1882) New North American fungi. Bull Torrey Bot Club 9:18–20
Ellwood SR, Kamphuis LG, Oliver RP (2006) Identification of sources of resistance to Phoma medicaginis isolates in Medicago truncatula SARDI core collection accessions, and multigene differentiation of isolates. Phytopathology 96:1330–1336
Ertz D, Diederich P, Lawrey JD, Berger F, Freebury CE, Coppins B, Gardiennet A, Hafellner J (2015) Phylogenetic insights resolve Dacampiaceae (Pleosporales) as polyphyletic: Didymocyrtis (Pleosporales, Phaeosphaeriaceae) with phoma-like anamorphs resurrected and segregated from Polycoccum (Trypetheliales, Polycoccaceae fam. nov.). Fungal Divers 74:53–89
Evans HC (1984) The genus Mycosphaerella and its anamorphs Cercoseptoria, Dothistroma and Lecanosticta on pines. Myc Papers 153:1–102
Fallah PM, Shearer CA (1998) Freshwater Ascomycetes: Phomatospora spp. from lakes in Wisconsin. Mycologia 91:145–156
Fan XL, Hyde KD, Liu M, Liang YM, Tian CM (2015a) Cytospora species associated with walnut canker disease in China, with description of a new species C. gigalocus. Fungal Biol 119:310–319
Fan XL, Hyde KD, Yang Q, Liang YM, Ma R, Tian CM (2015b) Cytospora species associated with canker disease of three anti-desertification plants in northwestern China. Phytotaxa 197:227–244
Fan XL, Bezerra JDP, Tian CM, Crous PW (2018) Families and genera of diaporthalean fungi associated with canker and dieback of tree hosts. Persoonia 40:119–134
Fan XL, Bezerra JDP, Tian CM, Crous PW (2020) Cytospora (Diaporthales) in China. Persoonia 45:1–45
Farr DF, Rossman AY (2016) Fungal databases, systematic mycology and microbiology laboratory, ARS, USDA. http://nt.ars-grin.gov/fungaldatabases/. Accessed 11 Jan 2015
Farr ML (1968) The nomenclatural status and synonymy of Phyllachora bambusina, Lateropeltis bambusarun, and their conidial stages. Mycologia 60:924–931
Farr ML, Horner HTJ (1968) Fungi on Selaginella. Nova Hedwig 15:239–283
Faurel L, Schotter G (1965) Notes mycologiques. IV. Champignons coprophiles du Sahara central et notamment de la Tefedest. Rev Mycol 30:141–165
Feau N, Hamelin RC, Bernier L (2006) Attributes and congruence of three molecular data sets: Inferring phylogenies among Septoria related species from woody perennial plants. Mol Phylogenet Evol 40:808–829
Ferreira FA, Demuner NL, Rezende DV (1992) Mancha de folha, desfolha e antracnose do Jatobá (Hymenaea spp.) causadas por Erythrogloeum hymenaeae. Fitopatol Bras 17:106–109
Ferrer C, Pérez-Santonja JJ, Rodríguez AE, Colom MF, Gené J, Alio JL, Verkley GJM, Guarro J (2009) New Pyrenochaeta species causing keratitis. J Clin Microbiol 47:1596–1598
Flakus A, Farkas E (2013) A contribution to the taxonomy of Lyromma (Lyrommataceae, lichenized Ascomycota) with a species key. Mycotaxon 124:127–141
Fonseka RN (1960) The morphology of Chaetospermum chaetosporum. Trans Br Mycol Soc 43:631–636
Fries EM (1825) Systema orbis vegetabilis, vol 1. Typographia Academica, Lund, pp 1–374
Fries EM (1828) Elenchus fungorum, vol II. E. Mauritius, Greifswald
Fries EM (1832) Systema Mycologicum. E. Moritz, Greifswald, Germany 3:261–524
Fries EM (1849) Summa Vegetabilum Scandinaviae. Typographia Academica. Holmiae & Lipsiae, Uppsala
Fuckel L (1870) Symbolae mycologicae: Beiträge zur Kenntniss der rheinischen Pilze. Jahrb Nassau Vereins Nat 23–24:1–459
Funk A (1979) Rileya, a new genus of Coelomycetes. Can J Bot 57:7–10
Furtado JS (1966) Significance of the clamp-connection in the basidiomycetes. Persoonia 4:125–144
Gabel AC (1993) Sizes of Cryptomycina pteridis (= Cryptomycella pteridis) Macroconidia. Mycologia 85:861–865
Gams W (1994) Report of the Committee for Fungi: 4. Taxon 43:265–267
Gamundí IJ, Minter DW, Romero AI, Barrera VA, Giaiotti AL, Messuti MI, Stecconi M (2004) Checklist of the Discomycetes (Fungi) of Patagonia, Tierra del Fuego and Adjacent Antarctic Areas. Darwiniana 42:63–164
Gangadevi V, Muthumary J (2008) Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J Microbiol Biot 24:717–724
Gao YH, Liu F, Duan WJ, Crous PW, Cai L (2017) Diaporthe is paraphyletic. IMA Fungus 8:153–187
Gerl C, Kalb K (1993) Die Flechtengattung Dibaeis. Eine Übersicht Ober die rosafrüchtigen Arten von Baeomyces sens. lat. nebst Anmerkungen zu Phyllobaeis gen. nov. Herzogia 9:593–645
Gernand DS, Camacho FJ, Stone JK (1997) Meria laricis, an anamorph of Rhabdocline. Mycologia 89:735–744
Gernandt DS, Platt JL, Stone JK, Spatafora JW, Holst-Jensen A, Hamelin RC, Kohn LM (2001) Phylogenetics of Helotiales and Rhytismatales based on partial small subunit nuclear ribosomal DNA sequences. Mycologsia 93:915–933
Ghazanfar MU, Sahi ST, Wakil W (2010) Effect of ascochyta blight on the agronomic characters of selected chickpea cultivars/lines. Pak J Phytopathol 22:113–119
Glass NL, Donaldson G (1995) Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Glienke C, Pereira O, Stringari D, Fabris J, Kava-Cordeiro V, Galli-Terasawa L, Cunnington J, Shivas R, Groenewald J, Crous PW (2011) Endophytic and pathogenic Phyllosticta species, with reference to those associated with citrus black spot. Persoonia 26:47–56
Golzar H, Burgess TI (2011) Neofusicoccum parvum, a causal agent associated with cankers and decline of Norfolk Island pine in Australia. Australas Plant Pathol J 40:484–489
Gomes R, Glienke C, Videira S, Lombard L, Groenewald J, Lombard L, Groenewald JZ, Crous PW (2013) Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31:1–41
Grasso FM, Marini M, Vitale A, Firrao G, Granata G (2012) Canker and dieback on Platanus acerifolia caused by Diaporthe scabra. For Pathol 42:510–513
Greene HC (1949) Notes on Wisconsin Parasitic Fungi. XIII. Am Midl Nat 41:740–758
Gremmen J (1955) New and noteworthy discomycetous fungi on coniferous hosts from Switzerland. Sydowia 9:432–437
Gremmen J (1957) Further notes on Discomycetous fungi on coniferous hosts. Sydowia Beih 1:179–182
Greville RK (1828) Scottish Cryptogamic Flora. Edinburgh: MacLachlan Stewart. 6:331–360
Grove WB (1919) Mycological notes IV. J Bot Lond 57:206–210
Grove WB (1935) British stem- and leaf-fungi, 1-2. University Press, Cambridge. 488:1937
Grove WB (1937) British stem- and leaf-fungi (coelomycetes). 1 sphaeropsidales, vol II. Cambridge University Press, Cambridge
Groves JW (1946) North American species of Dermea. Mycologia 38:351–431
Groves JW (1936) Ascocalyx abietis and Bothrodiscus pinicola. Mycologia 28:451–462
Groves JW (1940) Three Pezicula species occurring on Alnus. Mycologia 32:112–123
Groves JW (1952) The genus Tympanisi. Can J Bot 30:571–651
Groves JW (1965) The genus Godronia. Can J Bot 43:1195–1276
Groves JW (1968) Two new species of Ascocalyx. Can J Bot 46:1273–1278
Gruber E (2006) Studien über die equiseticole Gattung Stamnaria Fuckel (Ascomycota, Helotiales, Helotiaceae), einschließlich ökologischer Beobachtungen in der Steiermark (Österreich). Karl-Franzens-Universität, Graz, Austria, Thesis
Guarnaccia V, Vitale A, Cirvilleri G, Aiello D, Susca A, Epifani F, Perrone G, Polizzi G (2016) Characterisation and pathogenicity of fungal species associated with branch cankers and stem-end rot of avocado in Italy. Eur J Plant Pathol 146:963–976
Guarnaccia V, Groenewald JZ, Li H, Glienke C, Carstens E, Hattingh V, Fourie PH, Crous PW (2017) First report of Phyllosticta citricarpa and description of two new species, P. paracapitalensis and P. paracitricarpa, from citrus in Europe. Stud Mycol 87:161–185
Guerber JC, Correll JC (2001) Characterization of Glomerella acutata, the teleomorph of Colletotrichum acutatum. Mycologia 93:216–229
Guo B, Dai JR, Ng S, Huang Y, Leong C, Ong W, Carté BK (2000) Cytonic acids A and B: Novel tridepside inhibitors of hCMV protease from the endophytic fungus Cytonaema species. J Nat Prod 63:602–604
Habibi A, Banihashemi Z (2016) Mating system and role of pycnidiospores in biology of Polystigma amygdalinum, the causal agent of almond red leaf blotch. Phytopathol Mediterr 55:98–108
Habibi A, Banihashemi Z, Mostowfizadeh-Ghalamfarsa R (2015) Phylogenetic analysis of Polystigma and its relationship to Phyllachorales. Phytopathol Mediterr 54:45–54
Haelewaters D, Filippova NV, Baral HO (2018) A new species of Stamnaria (Leotiomycetes, Helotiales) from Western Siberia. MycoKeys 32:49–63
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid Symp Ser 41:95–98
Hamza S, Samir S, Rebai A, Salah R, Kahl G, Moncef H (2000) Pathotype variation of the representative genotypes of Ascochyta rabiei in the Beja region. J Plant Pathol 82:23–28
Hanlin RT (1997) Illustrated genera of Ascomycetes. Volume II. APS Press
Hantula J, Hallaksela AM, Kurkela T (1998) Relationship between Prosthemium betulinum and Pleomassaria siparia. Mycol Res 102:1509–1512
Hashimoto A, Sato G, Matsuda T (2015a) Molecular taxonomy of Dinemasporium and its allied genera. Mycoscience 56:86–101
Hashimoto A, Sato G, Matsuda T, Matsumura M, Hatakeyama S, Harada Y, Ikeda H, Tanaka K (2015b) Taxonomic revision of Pseudolachnea and Pseudolachnella and establishment of Neopseudolachnella and Pseudodinemasporium gen. nov. Mycologia 107:383–408
Hashimoto A, Matsumura M, Hirayama K, Fujimoto R, Tanaka K (2017) Pseudodidymellaceae fam. nov.: Phylogenetic affiliations of mycopappus-like genera in Dothideomycetes. Stud Mycol 87:187–206
Hawksworth DL (1976) New and interesting microfungi from Slapton, South Devonshire: Deuteromycotina III. Trans Br Mycol Soc 67:51–59
Hawksworth DL (1981a) A survey of the fungicolous conidial fungi. In: Coke GT, Kendrick B (eds) Biology of conidial fungi. Academic, New York, pp 171–244
Hawksworth DL (1981b) The lichenicolous Coelomycetes. Bull Br Mus Nat Hist Bot 9:1–98
Hawksworth DL (1983) The nomenclature of the Beech Bark Fungus: a solution to the complex case of Ascodichaena, Dichaena and Polymorphum. Taxon 32:212–217
Hawksworth DL (2012) Managing and coping with names of pleomorphic fungi in a period of transition. IMA Fungus 3:15–24
Hawksworth DL, Dyko BJ (1979) Lichenodiplis and Vouauxiomyces: two new genera of lichenicolous coelomycetes. Lichenologist 11:51–61
Hawksworth DL, Punithalingam E (1973) Typification and nomenclature of Dichaena Fr., Heterographa Fée, Polymorphum Chev., Psilospora Rabenh. and Psilosporina Died. Trans Br Mycol Soc 60:501–509
He MQ, Zhao RL, Hyde KD, Begerow D, Kemler M, Yurkov A, McKenzie EHC, Raspé O, Kakishima M, Sánchez-Ramıírez S, Vellinga EC, Halling R, Papp V, Zmitrovich IV, Buyck B, Ertz D, Wijayawardene NN, Cui BK, Schoutteten N, Liu XZ, Li TH, Yao YJ, Zhu XY, Liu AQ, Li GJ, Zhang MZ, Ling ZL, Cao B, Antonín V, Boekhout T, da Silva BDB, De Crop E, Decock C, Dima B, Dutta AK, Fell JW, Geml J, Ghobad-Nejhad M, Giachini AJ, Gibertoni TB, Gorjón SP, Haelewaters D, He SH, Hodkinson BP, Horak E, Hoshino T, Justo A, Lim YW, Menolli N Jr, Mešić A, Moncalvo JM, Mueller GM, Nagy LG, Nilsson RH, Noordeloos M, Nuytinck J, Orihara T, Ratchadawan C, Rajchenberg M, Silva-Filho AGS, Sulzbacher MA, Tkalčec Z, Valenzuela R, Verbeken A, Vizzini A, Wartchow F, Wei TZ, Weiß M, Zhao CL, Kirk PM (2019) Notes, outline and divergence times of Basidiomycota. Fungal Divers 99:105–367
Healy R, Pfister DH, Rossman AY, Marvanová L, Hansen K (2016) Competing sexual-asexual generic names of Pezizomycetes and recommendations for use. IMA Fungus 7:285–288
Hennings P (1902) Fungi blumenaeviensis. II. a cl. Alfr Möller lecti Hedwig 41:1–33
Hennings P (1904) Einige neue Pilze aus Japan. Hedwigia 43:140–146
Hern M, Akulov A, Carnegie AJ, Akulov A, Carnegie AJ, Cheewangkoon R, Gramaje D, Groenewald JZ, Guarnaccia V, Halleen F, Lombard L, Luangsa-ard J, Marincowitz S, Moslemi A, Mostert L, Quaedvlieg W, Schumacher RK, Spies CFJ, Thangavel R, Taylor PWJ, Wilson AM, Wingfield BD, Wood AR, Crous PW (2019) Genera of phytopathogenic fungi: GOPHY 2. Stud Mycol 92:47–133
Hesler LR, Smith AH (1963) North American species of Hygrophorus. Univ. Tenn. Press, Knoxville. USA
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192
Hirooka Y, Rossman AY, Samuels GJ, Chaverri P (2010) Taxonomy and biogeography of Nectria pseudotrichia (Nectriaceae, Hypocreales, Sordariomycetes) based on a multiple-locus phylogeny. Inoculum 61:55
Hirooka Y, Rossman AY, Chaverri P (2011) A morphological and phylogenetic revision of the Nectria cinnabarina species complex. Stud Mycol 68:35–56
Hirooka Y, Rossman AY, Samuels GJ, Lechat C, Chaverri P (2012) A monograph of Allantonectria, Nectria, and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Stud Mycol 71:1–210
Hohnel F (1909) Fragmente zur Mykologie (VII. Mitteilung, Nr. 289 bis 353). Sitzungsberichte der Kaiserliche Akademie der Wissenschaften in Wien Mathematische-Naturwissenschaftliche Klasse. Abt. 1:813–904
Holm L (1957) Études taxonomiques sur les Pléosporacées. Symb Bot Ups 14:5–188
Holst-Jensen A, Kohn LM, Schumacher T (1997) Nuclear rDNA phylogeny of the Sclerotiniaceae. Mycologia 89:885–899
Holst-Jensen A, Vralstad T, Schumacher T (2004) Kohninia linnaeicola, a new genus and species of the Sclerotiniaceae pathogenic to Linnaea borealis. Mycologia 96:135–142
Hongsanan S, Tian Q, Hyde KD, Chomnunti P (2015) Two new species of sooty moulds, Capnodium coffeicola and Conidiocarpus plumeriae in Capnodiaceae. Mycosphere 6:814–824
Hongsanan S, Maharachchikumbura SSN, Hyde KD, Samarakoon MC, Jeewon R, Zhao Q, Al-Sadi AM, Bahkali AH (2017) An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers 84:25–41
Hooker JD (1855) The botany of the Antarctic Voyage II. Fl Nov Zel 2:1–378
Hou CL, Piepenbring M (2005) Two new species of Colpoma on trees from China. For Path 35:359–364
Hou LW, Liu F, Duan WJ, Cai L (2016) Colletotrichum aracearum and C. camelliae-japonicae, two holomorphic new species from China and Japan. Mycosphere 7:1111–1123
Hoyo MP, Gómez-Bolea A (2004) Suttoniella arnaudii sp. nov. (Coelomycetes) on dead leaves of Buxus sempervirens. Mycotaxon 89:39–45
Hsieh WH (1979) The causal organism of sugarcane leaf blight. Mycologia 71:892–898
Huang WY, Cai YZ, Surveswaran S, Hyde KD, Corke H, Sun M (2009) Molecular phylogenetic identification of endophytic fungi isolated from three Artemisia species. Fungal Divers 36:69–88
Huang F, Chen GQ, Hou X, Fu YS, Cai L, Hyde KD, Li HY (2013) Colletotrichum species associated with cultivated citrus in China. Fungal Divers 61:61–74
Huang F, Udayanga D, Wang XH, Hou X, Mei XF, Fu YS, Hyde KD, Li HY (2015) Endophytic Diaporthe associated with Citrus: A phylogenetic reassessment with seven new species from China. Fungal Biol 119:331–347
Hudler GW, Oshima M (1976) The occurrence and distribution of Thyronectria austro-americana on honey locust in Colorado. Plant Dis Rep 60:200–922
Hudson HJ (1960) Pyrenomycetes of sugar cane and other grasses in Jamaica: I. Conidia of Apiospora camptospora and Leptosphaeria sacchari. Trans Br Mycol Soc 43:607–616
Hudson HJ, Sutton BC (1964) Trisulcosporium and Tetranacrium, two new genera of fungi imperfecti. Trans Br Mycol Soc 47:197–203
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Hughes SJ (1949) The perithecia and pycnidia of Leptosphaeria nigrans. Trans Br Mycol Soc 32:63–68
Hughes SJ (1976) Sooty moulds. Mycologia 68:693–820
Hyde KD (1993) Tropical Australian freshwater fungi. VI. Tiarosporella paludosa and Clohesymyces aquaticus gen. et sp. nov. (Coelomycetes). Aust Syst Bot 6:169–173
Hyde KD, Sutton BC (1992) Nypaella frondicola gen. et sp. nov., Plectophomella nypae sp. nov. and Pleurophomopsis nypae sp. nov. (Coelomycetes) from intertidal fronds of Nypa fruticans. Mycol Res 96:210–214
Hyde KD, Stanley SJ, Steinke TD (1996) Fungi associated with leaf spots of palms. Maculatifrondis aequatoriensis gen. et sp. nov., with a Cyclodomus anamorph, and Myelosperma parasitica sp. nov. Mycol Res 100:1509–1514
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake I, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yan JY, Yacharoen S, Zhang M (2013) Families of Dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AW, Dissanayake AJ, Glockling SL, Goonasekara ID, Gorczak M, Hahn M, Jayawardena RS, van Kan JAL, Laurence MH, André Lévesque C, Li XH, Liu JK, Maharachchikumbura SSN, Manamgoda DS, Martin FN, McKenzie EHC, McTaggart AR, Mortimer PE, Nair PVR, Pawłowska J, Rintoul TL, Shivas RG, Spies CFJ, Summerell BA, Taylor PWJ, Terhem RB, Udayanga D, Vaghefi N, Walther G, Wilk M, Wrzosek M, Xu JC, Yan JY, Zhou N (2014) One stop shop: backbones trees for important phytopathogenic genera: I (2014). Fungal Divers 67:21–125
Hyde KD, Hongsanan S, Jeewon R, Bhat DJ, McKenzie EHC, Jones EBG, Phookamsak R, Ariyawansa HA, Boonmee S, Zhao Q, Abdel-Aziz FA, Abdel-Wahab MA, Banmai S, Chomnunti P, Cui BK, Daranagama DA, Das K, Dayarathne MC, de Silva NI, Dissanayake AJ, Doilom M, Ehanayaka AH, Gibertoni TB, Góes-Neto A, Huang SK, Jayasiri SC, Jayawardena RS, Konta S, Lee HB, Li WJ, Lin CG, Liu JK, Lu YZ, Luo ZL, Manawasinghe IS, Manimohan P, Mapook A, Niskanen T, Norphanphoun C, Papizadeh M, Perera RH, Phukhamsakda C, Richter C, de A Santiago ALCM, Drechsler-Santos ER, Senanayake IC, Tanaka K, Tennakoon TMDS, Thambugala KM, Tian Q, Tibpromma S, Thongbai B, Vizzini A, Wanasinghe DN, Wijayawardene NN, Wu HX, Yang J, Zeng XY, Zhang H, Zhang JF, Bulgakov TS, Camporesi E, Bahkali AH, Amoozegar MA, Araujo-Neta LS, Ammirati JF, Baghela A, Bhatt RP, Bojantchev D, Buyck B, de Silva GA, de Lima CLF, de Oliveira RJV, de Souza CAF, Dai YC, Dima B, Duong TT, Ercole E, Mafalda-Freire F, Ghosh A, Hashimoto A, Kamolhan S, Kang JC, Karunarathna SC, Kirk PM, Kytövuori I, Lantieri A, Liimatainen K, Liu ZY, Liu XZ, Lücking R, Medardi G, Mortimer PE, Nguyen TTT, Promputtha I, Raj KNA, Reck MA, Lumyong S, Shahzadeh-Fazeli SA, Stadler M, Soudi MR, Su HY, Takahashi T, Tangthirasunun N, Uniyal P, Wang Y, Wen TC, Xu JC, Zhang ZK, Zhao YC, Zhou JL, Zhu L (2016) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 80:1–270
Hyde KD, Norphanphoun C, Abreu VP, Bazzicalupo A, Chethana KWT, Clericuzio M, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, He MQ, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Karunarathna A, Konta S, Kušan I, Lee H, Li J, Lin CG, Liu NG, Lu YZ, Luo ZL, Manawasinghe IS, Mapook A, Perera RH, Phookamsak R, Phukhamsakda C, Siedlecki I, Soares AM, Tennakoon DS, Tian Q, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Abdel-Aziz FA, Li WJ, Senanayake IC, Shang QJ, Daranagama DA, De Silva NI, Thambugala KM, Abdel-Wahab MA, Bahkali AH, Berbee ML, Boonmee S, Bhat DJ, Bulgakov TS, Buyck B, Camporesi E, Castañeda-Ruiz RF, Chomnunti P, Doilom M, Dovana F, Gibertoni TB, Jadan M, Jeewon R, Jones GEB, Kang JC, Karunarathna SC, Lim YW, Liu JK, Liu ZY, Plautz HL Jr, Lumyong S, Maharachchikumbura SSN, Matočec N, Mckenzie EHC, Mešić A, Miller D, Pawłowska J, Pereira OL, Promputtha I, Romero AI, Ryvarden L, Su HY, Suetrong S, Tkalčec Z, Vizzini A, Wen TC, Wisitrassameewong K, Wrzosek M, Xu JC, Zhao Q, Zhao RL, Mortimer PE (2017) Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Divers 87:1–235
Hyde KD, Chaiwan N, Norphanphoun C, Boonmee S, Camporesi E, Chethana KWT, Dayarathne MC, de Silva NI, Dissanayake AJ, Ekanayaka AH, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Jiang HB, Karunarathna A, Lin CG, Liu JK, Liu NG, Lu YZ, Luo ZL, Maharachchimbura SSN, Manawasinghe IS, Pem D, Perera RH, Phukhamsakda C, Samarakoon MC, Senwanna C, Shang QJ, Tennakoon DS, Thambugala KM, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Zhang JF, Zhang SN, Bulgakov TS, Bhat D, Cheewangkoon R, Goh TK, Jones EBG, Kang JC, Jeewon R, Liu ZY, Lumyong S, Kuo CH, McKenzie EHC, Wen TC, Yan JY, Zhao Q (2018) Mycosphere notes 169–224. Mycosphere 9:271–430
Hyde KD, Norphanphoun C, Maharachchikumbura SSN, Bao DF, Bhat DJ, Boonmee S, Bundhun D, Calabon MS, Chaiwan N, Chen YJ, Chethana KWT, Dai DQ, Dayarathne MC, Devadatha B, Dissanayake AJ, Dissanayake LS, Doilom M, Dong W, Fan XL, Goonasekara ID, Hongsanan S, Huang SK, Jayawardena RS, Jeewon R, Jones EBG, Karunarathna A, Konta S, Kumar V, Lin CG, Liu JK, Liu N, Lu YZ, Luangsa-ard J, Lumyong S, Luo ZL, Marasinghe DS, McKenzie EHC, Niego AGT, Niranjan M, Perera RH, Phukhamsakda C, Rathnayaka AR, Samarakoon MC, Samarakoon SMBC, Sarma VV, Senanayake IC, Shang QJ, Stadler M, Tibpromma S, Wanasinghe DN, Wei DP, Wijayawardene NN, Xiao YP, Xiang MM, Yang J, Zeng XY, Zhang SN (2020) Refined families of Sodariomycetes. Mycosphere (in process)
Ihlen PG (1998) The lichenicolous fungi on species of the genera Baeomyces, Dibaeis, and Icmadophila in Norway. Lichenologist 30:27–57
Illman WI, White GP (1985) A new Morrisographium-like species with appendaged conidia assigned to the genus Conicomyces. Can J Bot 63:419–422
Index fungorum (2020) http://www.indexfungorum.org/names/names.asp
Ismail SI, Batzer JC, Harrington TC, Crous PW, Lavrov DV, Li HY, Gleason ML (2016) Ancestral state reconstruction infers phytopathogenic origins of sooty blotch and flyspeck fungi on apple. Mycologia 108:292–302
Iturritxa E, Slippers B, Mesanza N, Wingfield MJ (2011) First report of Neofusicoccum parvum causing canker and die-back of Eucalyptus in Spain. Australas Plant Dis Notes 6:57–59
Jaklitsch WM, Voglmayr H (2014) Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated. Persoonia 33:182–211
Jami F, Slippers B, Wingfield MJ, Gryzenhout M (2012) Five New Species of the Botryosphaeriaceae from Acacia Karroo in South Africa. Cryptogam Mycol 33:245–266
Jami F, Slippers B, Wingfield MJ, Gryzenhout M (2014) Botryosphaeriaceae species overlap on four unrelated, native South African hosts. Fungal Biol 118:168–179
Jami F, Marincowitz S, Crous PW, Jacobsohn A, Wingfield MJ (2018a) A new Cytospora species pathogenic on Carpobrotus edulis in its native habitat. Fungal Syst Evol 2:37–43
Jami F, Marincowitz S, Slippers B, Wingfield MJ (2018b) New Botryosphaeriales on native red milkwood (Mimusops caffra). Australas Plant Pathol 47:475–484
Jayasiri SC, Hyde KD, Abd-Elsalam KA, Abdel-Wahab MA, Ariyawansa HA, Bhat J, Buyck B, Dai YC, Ertz D, Hidayat I, Jeewon R, Jones EBG, Karunarathna SC, Kirk P, Lei C, Liu JK, Maharachchikumbura SSN, McKenzie E, Ghobad-Nejhad M, Nilsson H, Pang KL, Phookamsak R, Rollins AW, Romero AI, Stephenson S, Suetrong S, Tsui CKM, Vizzini A, Wen TC, De Silva NI, Promputtha I, Kang JC (2015) The Facesoffungi database: fungal names linked with morphology, molecular and human attributes. Fungal Divers 74:3–18
Jayasiri SC, Hyde KD, Jones EBG, Jeewon R, Ariyawansa HA, Bhat JD, Camporesi E, Kang JC (2017) Taxonomy and multigene phylogenetic evaluation of novel species in Boeremia and Epicoccum with new records of Ascochyta and Didymella (Didymellaceae). Mycosphere 8:1080–1101
Jayawardena RS, McKenzie EHC, Chen YJ, Phillips AJL, Hongsanan S, Norphanphoun C, Abeywikrama PD, Maharachchikumbura SSN, Manawasinghe IS, McTaggart AR, Shivas RG, Gentekaki E, Hyde KD (2019) https://onestopshopfungi.org/, a database to enhance identification of phytopathogenic genera. AJOM 2:281–286
Jeewon R, Hyde KD (2016) Establishing species boundaries and new taxa among fungi: Recommendations to resolve taxonomic ambiguities. Mycosphere 7:1669–1677
Jiang S, Wei X, Wei J (2016) Strigula sinoaustralis sp. nov. and three Strigula spp. new to China. Mycotaxon 131:795–803
Jiang SH, Wei XL, Wei JC (2017) Two new species of Facesoffungi (lichenised Dothideomycetes, Ascomycota) from China, with a key to the Chinese foliicolous species. MycoKeys 19:31–42
Jiang N, Phillips AJL, Zhang ZX, Tian CM (2018) Morphological and molecular identification of two novel species of Melanops in China. Mycosphere 9:1187–1196
Jiang N, Fan XL, Tian CM (2019) Identification and pathogenicity of Cryphonectriaceae species associated with chestnut canker in China. Plant Pathol 68:1132–1145
Johnston PR (1986) Rhytismataceae in New Zealand 1. Some foliicolous species of Coccomyces de Notaris and Propolis (Fries) Corda. N Z J Bot 24:89–124
Johnston PR (1990) Rhytismataceae in New Zealand 3. The genus Hypoderma. N Z J Bot 28:159–183
Johnston PR (1991) Rhytismataceae in New Zealand 5. Wood- and bark-inhabiting species in the genera Colpoma and Propolomyces. N Z J Bot 29:405–410
Johnston PR, Manning MA, Meier X, Park D, Fullerton RA (2004) Cryptosporiopsis actinidiae sp. nov. Mycotaxon 89:131–136
Johnston PR, Seifert KA, Stone JK, Rossman AY, Maranová L (2014) Recommendations on generic names competing for use in Leotiomycetes (Ascomycota). IMA Fungus 5:91–120
Jordaan A, Taylor JE, Rossenkhan R (2006) Occurrence and possible role of endophytic fungi associated with seed pods of Colophospermum mopane (Fabaceae) in Botswana. S Afr J Bot 72:245–255
Kadowaki K, Sato H, Yamamoto S, Tanabe AS, Hidaka A, Toju H (2014) Detection of the horizontal spatial structure of soil fungal communities in a natural forest. Popul Ecol 56:301–310
Kalani IK (1964) Stauronema indicum sp. nov. from India. Curr Sci India 33:622–623
Kamat MN, Kalani IK (1964) Ajrekarella, a new member of the Sphaeropsidales. Mycopath. Mycol Appl 24:297–300
Kandaswamy M, Sundaram NV (1957) An interesting disease of Themeda tremula. Indian Phytopathol 9:202–204
Karadžić DM (1998) Tiarosporella durmitorensis sp. nov.: a new pathogenic fungus on needles of Abies alba. Eur J For Pathol 28:145–152
Karimi K, Arzanlou M, Babai-Ahari A, Pertot I (2016) Biological and molecular characterisation of Pilidium lythri, an emerging strawberry pathogen in Iran. Phytopathol Mediterr 52:478–489
Karsten PA (1871) Mycologia fennica. Pars prima, Discomycetes. Bid till Natur och Folk 19:1–264
Karsten PA (1890) Sphaeropsidae hucusque in Fennia observatae. Acta Soc Fauna Fl Fenn 6:19
Katoh K, Rozewicki J, Yamada KD (2017) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. https://doi.org/10.1093/bib/bbx108
Katoh K, Standley DM (2013) MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol 30:772–780
Keener PD (1951) An ascigerous stage of Darluca filum (Biv.) Castagne. Plant Dis Rep 35:86–87
Kendrick WB (1979) An appraisal of the taxonomic significance of some different modes of producing blastic conidia. In: Kendrick B (ed) The whole fungus, vol 1. National Museums of Canada, Ottawa, pp 63–80
Kendrick WB (2000) The Fifth Kingdom, 3rd edn. Focus Publishing, Newbury
Khanna PK (1966) A new species of Chaetoseptoria [C. indica] on decaying shoots of Bothriochloa pertusa. Lloydia 29:146
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Dictionary of the fungi, 10th edn. CABI, Wallingford
Kirk PM, Stalpers JA, Braun U, Crous PW, Hansen K, Hawksworth DL, Hyde KD, Lücking R, Lumbsch TH, Rossman AY, Seifert KA, Stadler M (2013) A without-prejudice list of generic names of fungi for protection under the international Code of nomenclature for algae, fungi, and plants. IMA Fungus 4:381–443
Kirschner R, Oberwinkler F (2009) Supplementary notes on Basidiopycnis hyalina (Basidiomycota, Atractiellales) and its anamorph. Mycotaxon 109:29–38
Kishino H, Hasegawa M (1989) Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. J Mol Evol 29:170–179
Kiss L, Russell JC, Szentiványi O, Xu X, Jeffries (2004) Biology and biocontrol potential of Ampelomyces mycoparasites, natural antagonists of powdery mildew fungi. Biocontrol Sci Technol 14:635–651
Klebahn H (1933) Über Bau und Konidienbildung bei einigen stromatischen Sphaeropsideen. Phytopathol 6:229–304
Kobayashi T (1964) A new species of Phellostroma associated with stem and twig blight of Tsuga sieboldii Carr. T Mycol Soc Jpn 5:6–8
Kobayashi T (1973) Notes on new or little-known fungi inhabiting woody plants in Japan V. T Mycol Soc Jpn 14:266–279
Kohlmeyer J, Volkmann-Kohlmeyer B (2001) Fungi on Juncus roemerianus. 16. more new Coelomycetes, Including Tetranacriella, gen. nov. Bot Mar 44:147–156
Koike ST, Subbarao KV, Verkley GJM, Fogle D, O’Neill TM (2006) Phoma basal rot of romaine lettuce in California caused by Phoma exigua: occurrence, characterization, and control. Plant Dis 90:1268–1275
Konrad H, Stauffer C, Kirisits T, Halmschlager E (2007) Phylogeographic variation among isolates of the Sirococcus conigenus P group. For Pathol 37:22–39
Korf RP (1962) A Synopsis of the Hemiphacidiaceae, a family of the Helotiales (Discomycetes) causing needle-blights of conifers. Mycologia 54:12–33
Kranz J, Brandenburger W (1981) An amended host list of the rust parasite Eudarluca caricis. J Plant Dis Prot 88:682–702
Kubono T (1993) Gloeosporidina cryptomeriae sp. nov. causing twig blight of Japanese cedar (Cryptomeria japonica). T Mycol Soc Jpn 34:261–265
Kubono T, Hosoya T (1994) Stromatinia cryptomeriae sp. nov., the teleomorph of Gloeosporidina cryptomeriae causing twig blight of Japanese cedar. Mycoscience 35:279–285
Kuhlman E, Matthews F, Tillerson H (1978) Efficacy of Darluca filum for biological control of Cronartium fusiforme and C. strobilinum. Phytopathology 68:507–511
Kumaran RS, Hur B (2009) Screening of species of the endophytic fungus Phomopsis for the production of the anticancer drug taxol. Biotechnol Appl Bioc 54:21–30
Kuntze O (1891) Revisio Generum Plantarum, 2. Arthur Felix, Leipzig, pp 375–1011
Kuraku S, Zmasek CM, Nishimura O, Katoh K (2013) aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res 41:W22–W28
Lantz H, Johnston PR, Park D, Minter DW (2011) Molecular phylogeny reveals a core clade of Rhytismatales. Mycologia 103:57–74
Larsson A (2014) AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30:3276–3278
Lawrence DP, Travadon R, Pouzoulet J, Rolshausen PE, Wilcoxc WF, Baumgartner K (2017) Characterization of Cytospora isolates from wood cankers of declining grapevine in North America, with the descriptions of two new Cytospora species. Plant Pathol 66:713–725
Lawrence DP, Holland LA, Nouri MT, Travadon R, Abramians A, Michailides TJ, Trouillas FP (2018) Molecular phylogeny of Cytospora species associated with canker diseases of fruit and nut crops in California, with the descriptions of ten new species and one new combination. IMA Fungus 9:333–370
Lee S, Crous PW (2003) New coelomycetes occurring on Restionaceae. Sydowia 55:115–128
Leuchtmann A (1985) Kulturversuche mit einigen Arten der Gattung Lophiostoma Ces., de Not. Sydowia 38:158–170
Leuchtmann A (1987) Species of Heterosphaeria (Discomycetes) and their anamorphs. Mycotaxon 28:261–284
Léveillé JH (1846) Description des champignons de I’Herbier du Museum de Paris. Ann Sci Nat Sér 3:249–305
Li WC, Zhou J, Guo SY, Guo LD (2007) Endophytic fungi associated with lichens in Baihua mountain of Beijing, China. Fungal Divers 25:69–80
Li WJ, Bhat JD, Hyde KD, Wang Y (2014) Towards a natural classification of Dothideomycetes 4: The genera Bryopelta, Bryorella, Bryosphaeria, Lophiosphaerella and Maireella (Dothideomycetes incertae sedis). Phytotaxa 176:28–41
Li WJ, Bhat D, Camporesi E, Tian Q, Wijayawardene NN, Dai DQ, Phookamsak R, Chomnunti P, Bahkali AH, Hyde K (2015a) New asexual morph taxa in Phaeosphaeriaceae. Mycosphere 6:681–708
Li WJ, Liu JK, Bhat DJ, Camporest E, Dai DQ, Mortimer PE, Xu JC, Hyde KD, Chomnunti P (2015b) Molecular phylogenetic analysis reveals two new species of Discosia from Italy. Phytotaxa 203:37–46
Li WJ, Maharachchikumbura SSN, Li QR, Bhat DJ, Camporesi E, Tian Q, Senanayake IC, Dai DQ, Chomnunti P, Hyde KD (2015c) Epitypification of Broomella vitalbae and introduction of a novel species of Hyalotiella. Cryptogam Mycol 36:93–108
Li GJ, Hyde KD, Zhao RL, Hongsanan S, Abdel-Aziz FA, Abdel-Wahab MA, Alvarado P, Alves-Silva G, Ammirati JF, Ariyawansa HA, Baghela A, Bahkali AH, Beug M, Bhat DJ, Bojantchev D, Boonpratuang T, Bulgakov TS, Camporesi E, Boro MC, Ceska O, Chakraborty D, Chen JJ, Chethana KWT, Chomnunti P, Consiglio G, Cui BK, Dai DQ, Dai YC, Daranagama DA, Das K, Dayarathne MC, De Crop E, De Oliveira RJV, de Souza CAF, de Souza JI, Dentinger BTM, Dissanayake AJ, Doilom M, Drechsler-Santos ER, Ghobad-Nejhad M, Gilmore SP, Góes-Neto A, Gorczak M, Haitjema CH, Hapuarachchi KK, Hashimoto A, He MQ, Henske JK, Hirayama K, Iribarren MJ, Jayasiri SC, Jayawardena RS, Jeon SJ, Jerônimo GH, Jesus AL, Jones EBG, Kang JC, Karunarathna SC, Kirk PM, Konta S, Kuhnert E, Langer E, Lee HS, Lee HB, Li WJ, Li XH, Liimatainen K, Lima DX, Lin CG, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Lücking R, Lumbsch HT, Lumyong S, Leaño EM, Marano AV, Matsumura M, McKenzie EHC, Mongkolsamrit S, Mortimer PE, Nguyen TTT, Niskanen T, Norphanphoun C, O’Malley MA, Parnmen S, Pawłowska J, Perera RH, Phookamsak R, Phukhamsakda C, Pires-Zottarelli CLA, Raspé O, Reck MA, Rocha SCO, de Santiago ALCMA, Senanayake IC, Setti L, Shang QJ, Singh SK, Sir EB, Solomon KV, Song J, Srikitikulchai P, Stadler M, Suetrong S, Takahashi H, Takahashi T, Tanaka K, Tang LP, Thambugala KM, Thanakitpipattana D, Theodorou MK, Thongbai B, Thummarukcharoen T, Tian Q, Tibpromma S, Verbeken A, Vizzini A, Vlasák J, Voigt K, Wanasinghe DN, Wang Y, Weerakoon G, Wen HA, Wen TC, Wijayawardene NN, Wongkanoun S, Wrzosek M, Xiao YP, Xu JC, Yan JY, Yang J, Da Yang S, Hu Y, Zhang JF, Zhao J, Zhou LW, Peršoh D, Phillips AJL, Maharachchikumbura SSN (2016) Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 78:1–237
Li GQ, Liu FF, Li JQ, Liu QL, Chen SF (2018) Botryosphaeriaceae from Eucalyptus plantations and adjacent plants in China. Persoonia 40:63–95
Limber DP (1955) Studies in the genus Sporonema. Mycologia 47:389–402
Lin X, Huang Y, Fang M, Wang J, Zheng Z, Su W (2005) Cytotoxic and anti- microbial metabolites from marine lignicolous fungi, Diaporthe sp. FEMS Microbiol Lett 251:53–58
Lind J (1913) Danish fungi as represented in the herbarium of E. Rostrup. Gyldendalske Boghandel-Nordisk Forlag, Copenhagen, pp 1–648
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808
Liu L, Li Y, Liu S, Zheng ZH, Chen XL, Zhang H, Guo LD, Che YS (2009) Chloropestolide A, an antitumor metabolite with an unprecedented spiroketal skeleton from Pestalotiopsis fici. Org Lett 11:2836–2839
Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, Ariyawansha H, Boonmee S, Chomnunti P, Dai DQ, Bhat JD, Romero AI, Zhuang WY, Monkai J, Jones EBG, Chukeatirote E, Ko Ko TW, Zhao YC, Wang Y, Hyde KD (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Liu JK, Phookamsak R, Dai DQ, Tanaka K, Jones EBG, Xu JC (2014) Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoella gen. nov. Roussoella and Roussoellopsis. Phytotaxa 181:1–33
Liu F, Weir BS, Damm U, Crous PW, Wang Y, Liu B, Wang M, Zhang M, Cai L (2015a) Unravelling Colletotrichum species associated with Camellia: employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia 35:63–86
Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SSN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, Dsouza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipo S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe D, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Liu XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2015b) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Liu F, Wang M, Damm U, Crous PW, Cai L (2016) Species boundaries in plant pathogenic fungi: a Colletotrichum case study. BMC Evolut Biol 16:1–14
Liu JK, Hyde KD, Jeewon R, Phillips AJL, Maharachchikumbura SSN, Ryberg M, Liu ZY, Zhao Q, Dsouza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipo S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe D, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Liu XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2017) Ranking higher taxa using divergence times: a case study in Dothideomycetes. Fungal Divers 84:75–99
Liu F, Bonthond G, Groenewald JZ, Cai L, Crous PW (2019) Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Stud Mycol 92:287–415
Livsey S, Minter DW (1994) The taxonomy and biology of Tryblidiopsis pinastri. Can J Bot 72:549–557
Lombard L, van der Merwe NA, Groenewald JZ, Crous PW (2015) Generic concepts in Nectriaceae. Stud Mycol 80:189–245
Lu YZ, Hyde KD, Bhat DJ, Xiao YP, Tian Q, Wen TC, Boonmee S, Kang JC (2016) Brunneodinemasporium jonesii and Tainosphaeria jonesii spp. nov. (Chaetosphaeriaceae, Chaetosphaeriales) from southern China. Mycosphere 7:1322–1331
Lucas MT, Webster J (1967) Conidial states of British species of Leptosphaeria. Trans Br Mycol Soc 50:85–121
Lücking R (2008) Foliicolous lichenized fungi. Flora neotropica. The New York Botanical Garden Press, Bronx. 103:1–866
Luttrell E (1940) An undescribed fungus on Japanese cherry. Mycologia 32:530–536
Ma XY, Nontachaiyapoom S, Jayawardena RS, Hyde KD, Gentekaki E, Zhou SX, Qian YX, Wen TC, Kang JC (2018) Endophytic Colletotrichum species from Dendrobium spp. in China and Northern Thailand. MycoKeys 43:23–57
Maharachchikumbura SSN, Guo LD, Cai L, Chukeatirote E, Wu WP, Sun X, Crous PW, Bhat DJ, McKenzie EHC, Bahkali AH, Hyde KD (2012) A multi-locus backbone tree for Pestalotiopsis, witha polyphasic characterization of 14 new species. Fungal Divers 56:95–129
Maharachchikumbura SSN, Guo LD, Chukeatirote E, Bahkali AH, Hyde Kevin D (2011) Pestalotiopsis: morphology, phylogeny, biochemistry and diversity. Fungal Divers 50:167–187
Maharachchikumbura SSN, Hyde KD, Groenewald JZ, Xu J, Crous PW (2014) Pestalotiopsis revisited. Stud Mycol 79:121–186
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang SK, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu JK, Liu ZY, Norphanphoun C, Pang KL, Perera RH, Senanayake IC, Shang Q, Shenoy BD, Xiao YP, Bahkali AH, Kang JC, Somrothipol S, Suetrong S, Wen TC, Xu JC (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72:199–301
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat DJ, Dayarathne MC, Huang SK, Norphanphoun C, Senanayake IC, Perera RH, Shang QJ, Xiao YP, D’souza MJ, Hongsanan S, Jayawardena RS, Daranagama DA, Konta S, Goonasekara ID, Zhuang WY, Jeewon R, Phillips AJL, Abdel-Wahab MA, Al-Sadi AM, Bahkali AH, Boonmee S, Boonyuen N, Cheewangkoon R, Dissanayake AJ, Kang JC, Li QR, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Pang KL, Phookamsak R, Promputtha I, Suetrong S, Stadler M, Wen TC, Wijayawardene NN (2016) Families of Sordariomycetes. Fungal Divers 79:1–317
Mandal NC, Dasgupta MK (1984) Five new diseases of perishable fruits in storage. Indian Phytopathol 37:673–676
Mardones M, Oster S, Elliott M, Elliott M, Urbina H, Schmitt I, Piepenbring M (2017) Phylogeny of the order Phyllachorales (Ascomycota, Sordariomycetes): among and within order relationships based on five molecular loci. Persoonia 39:74–90
Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ (2008) Microfungi occurring on Proteaceae in the Fynbos. CBS Biodivers Ser 7:1–166
Marincowitz S, Gryzenhout M, Wingfield MJ (2010) New and rare coelomycetes with appendage-bearing conidia from Pondoland, South Africa. Mycotaxon 111:309–322
Marin-Felix Y, Groenewald JZ, Cai L, Chen Q, Marincowitz S, Barnes I, Bensch K, Braun U, Camporesi E, Damm U, de Beer ZW, Dissanayake A, Edwards J, Giraldo A, Hernández-Restrepo M, Hyde KD, Jayawardena RS, Lombard L, Luangsa-ard J, McTaggart AR, Rossman AY, Sandoval-Denis M, Shen M, Shivas RG, Tan YP, van der Linde EJ, Wingfield MJ, Wood AR, Zhang JQ, Zhang Y, Crous PW (2017) Genera of phytopathogenic fungi. GOPHY 216:99–216
Marques MW, Lima NB, de Morais MA, Jr Michereff SJ, Phillips AJL, Câmara MPS (2013) Botryosphaeria, Neofusicoccum, Neoscytalidium and Pseudofusicoccum species associated with mango in Brazil. Fungal Divers 61:195–208
Massalongo AB (1855) Frammenti lichenografici. Ramanzini, Verona, pp 1–27
Masumoto S, Tojo M, Uchida M, Imura S (2014) Rhytisma polaris: Morphological and molecular characterization of a new species from Spitsbergen Island, Norway. Mycol Prog 13:181–188
Masumoto S, Tojo M, Uchida M, Imura S (2015) Morphological and molecular characterization of Rhytisma filamentosum sp. nov. from Nagano Prefecture, Japan. Mycol Prog 14:44
Matsushima T (2003) Matsushima mycological memoirs. memoirs. N°10. Matsushima T, Kobe, pp 1–214
Mehl JWM, Slippers B, Roux J, Wingfield MJ (2017) Overlap of latent pathogens in the Botryosphaeriaceae on a native and agricultural host. Fungal Biol 121:405–419
Mehrabi M, Hemmati R, Vasilyeva LN, Trouillas FP (2016)Diatrypella macrospora sp. nov. and new records of diatrypaceous fungi from Iran. Phytotaxa 252:43–55
Mejía LC, Rossman AY, Castlebury LA, White JF (2011) New species, phylogeny, host-associations and geographic distribution of genus Cryptosporella (Gnomoniaceae, Diaporthales). Mycologia 103:379–399
Mel’nik Va (1971) Taxonomy of the genus Ascochyta Lib. Mikol. i Fitopatol. 5:15–22
Ménard L, Brandeis PE, Simoneau P, Poupard P, SérandatI Detoc J, Robbes L, Bastide F, Laurent E, Gombert J, Morel E (2014) First report of umbel browning and stem necrosis caused by Diaporthe angelicae on carrot in France. Plant Dis 98:421–422
Mendes-Pereira E, Faris-Mokaiesh S, Bertrandy J, Spire D (1999) Characterisation of mycelial glycoproteins of the ‘Ascochyta pea complex’ (Ascochyta pisi Lib., Mycosphaerella pinodes (Berk. and Blox.) and Phoma medicaginis var. pinodella (Jones) Boerema. Agronomie 19:21–29
Mills PR, Hodson A, Brown AE (1992) Molecular differentiation of Colletotrichum gloeosporioides isolates infecting tropical fruits. Colletotrichum: Biology, Pathology and Control (Bailey JA, Jeger MJ). CABI, Wallingford, pp 269–288
Minnis AM, Kennedy AH, Grenier DB, Palm ME, Rossman AY (2012) Phylogeny and taxonomic revision of the Planistromellaceae including its coelomycetous anamorphs: contributions towards a monograph of the genus Kellermania. Persoonia 29:11–28
Minnis AM, Rossman AY, Farr DF, Olsen RT (2010) Fungal Planet 51. Persoonia 25:122–123
Minter DW (1997) Hypohelion scirpinum. IMI Descr Fungi Bact 1336:1–3
Minter DW, Caine TS (1980) Trichocrea Marchal, Fujimyces and Ebollia gen. nov. Trans Br Mycol Soc 74:434–437
Moesz G (1914) Kisázsiai gombák. Bot Közl 13:142–148
Moesz G (1915) Mykologiai közlemények. II. közlemény. Bot Közl 14:145–158
Mongkolsamrit S, Luangsa-ard JJ, Spatafora JW, Sung GH, Hywel-Jones NL (2009) A combined ITS rDNA and ß-tubulin phylogeny of Thai species of Hypocrella with non-fragmenting ascospores. Mycol Res 113:684–699
Monkai J, Liu JK, Boonmee S, Chomnunti P, Chukeatirote E, Jones EBG, Wang Y, Hyde KD (2013) Planistromellaceae (Botryosphaeriales). Cryptogam Mycol 34:45–77
Monod M (1983) Monographie taxonomique des Gnomoniaceae (Ascomycètes de l’ordre des Diaporthales) I. Beih Sydowia. 9:1–314
Montagne JPFC (1848) Sixième Centurie de plantes exotiques nouvelles. Décades I et II. Ann Sci Nat Bot 10:106–136
Morelet M (1968) De aliquibus in Mycologia Novitatibus. Bull Soc Sci Nat Archéol Toulon Var 175:5–6
Morgan-Jones G (1971) An addition to the genus Discogloeum Petrak. Can J Bot 49:1461–1462
Morgan-Jones G (1974) Icones generum coelomycetum VII. Univ Waterloo Bio Ser 14:1–42
Morgan-Jones G (1977) Icones Generum Coelomycetum IX. Univ Waterloo Bio Ser 17:1–42
Morgan-Jones G, Kendrick B (1972) Icones generum coelomycetum III. Univ Waterloo Bio Ser 5:1–42
Morgan-Jones G, Nag Raj TR, Kendrick B (1972a) Genera coelomycetarum V. Alpakesa and Bartalinia. Can J Bot 50:877–882
Morgan-Jones G, Nag Raj TR, Kendrick B (1972b) Genera coelomycetarum VI. Kellermania. Can J Bot 50:1641–1648
Morgan-Jones G, Nag Raj TR, Kendrick B (1972c) Icones generum coelomycetum I. Univ Waterloo Bio Ser 3:1–42
Morgan-Jones G, Nag Raj TR, Kendrick B (1972d) Icones generum coelomycetum II. Univ Waterloo Bio Ser 4:1–40
Morgan-Jones G, Nag Raj TR, Kendrick B (1972e) Icones generum coelomycetum IV. Univ Waterloo Bio Ser 6:35–36
Morgan-Jones G, Nag Raj TR, Kendrick B (1972f) lcones generum coelomycetum V. Univ Waterloo Bio Ser 7:1 –52
Morris RAC, Royle DJ, Whelan MJ, Arnold GM (1994) Biocontrol of willow rusts with the hyperparasite Sphaerellopsis filum. Proc Brighton Crop Prot Conf Pests Dis 3:1121–1126
Morris RAC, Royle DJ, Whelan MJ (1995) Potential for biological control of willow rust with the hyperparasite Sphaerellopsis filum. In: Final report of Project ETSU B/W6/00230/REP. Energy Technology Support Group, Department of Trade and Industry, UK
Mostert L, Crous PW, Kang JC, Phillips AJL (2001) Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: morphological, cultural, molecular and pathological characterization. Mycologia 93:146–167
Müller E (1950) Die schweizerischen Arten der Gattung Leptosphaeria und ihrer verwandten. Sydowia 4:185–319
Müller E (1953) Kulturversuche mit Ascomyceten I. Sydowia 7:325–334
Müller E (1986) On the genus Diachora. J Müller Trans Bot Soc Edinb 45:69–75
Müller MM, Hantula J (1998) Diversity of Tiarosporella parca in Finland, Norway and Switzerland. Mycol Res 102:1163–1168
Murali TS, Suryanarayanan TS, Geeta R (2006) Endophytic Phomopsis species: host range and implications for diversity estimates. Can J Microbiol 52:673–680
Nag Raj TR (1973a) Genera coelomycetum. IX. Brycekendrickia gen. nov. and Vasudevella. Can J Bot 51:1137–1341
Nag Raj TR (1973b) Genera coelomycetum. X. Ellisiella, Samulkuta, and Sakireeta. Can J Bot 51:2463–2472
Nag Raj TR (1974) Icones generum coelomycetum VI. Univ Waterloo Bio Ser 13:1–41
Nag Raj TR (1975) Genera coelomycetum. XII. Tracyella and Amerodiscosiella. Can J Bot 53:2435–2442
Nag Raj TR (1977a) Icones Generum Coelomycetum VIII. Univ Waterloo Bio Ser 13:1–41
Nag Raj TR (1977b) Miscellaneous microfungi. II. Can J Bot 55:757–765
Nag Raj TR (1978) Genera coelomycetum. XVI. Fibulocoela form-gen. nov., a coelomycete with basidiomycetous affinities. Can J Bot 56:1485–1491
Nag Raj TR (1979a) Genera coelomycetum. XVII. New anamorph genera: Libartania and Plectronidiopsis. Can J Bot 57:1389–1397
Nag Raj TR (1979b) Some coelomycetous anamorphs and their teleomorphs. In: Kendrick B (ed) The whole fungus, vol 1. National Museums of Canada, Ottawa, pp 183–200
Nag Raj TR (1980) Genera coelomycetum. XVIII. Ellula anamorph-gen. nov., another coelomycete with basidiomycetous affinities. Can J Bot 58:2007–2014
Nag Raj TR (1981a) Genera coelomycetum. XIX. Discosiella, a lichenized mycobiont. Can J Bot 59:2519–2533
Nag Raj TR (1981b) Coelomycete systematics. In: Coke GT, Kendrick B (eds) Biology of conidial fungi. Academic Press, New York, pp 43–84
Nag Raj TR (1982) Genera coelomycetum. XX. Crucellisporiopsis anam. gen. nov., a discomycete analog. Can J Bot 60:2601–2607
Nag Raj TR (1983) Genera coelomycetum. XXI. Strasseria and two new anamorph genera: Apostrasseria and Nothostrasseria. Can J Bot 61:1–30
Nag Raj TR (1984) Koorchaloma, Koorchalomella, and Kananascus gen. nov. Mycotaxon 19:167–212
Nag Raj TR (1993) Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo, pp 1–1101
Nag Raj TR, DiCosmo F (1978) Icones generum coelomycetum X. Univ Waterloo Bio Ser 19:1–45
Nag Raj TR, DiCosmo F (1980) Icones generum coelomycetum XI. Univ Waterloo Bio Ser 21:1–42
Nag Raj TR, DiCosmo F (1981a) Icones generum coelomycetum XII. Univ Waterloo Bio Ser 22:1–41
Nag Raj TR, DiCosmo F (1981b) A monograph of Harknessia and Mastigosporella, with notes on associated teleomorphs. Bibl Mycol 80:1–62
Nag Raj TR, DiCosmo F (1982) Icones generum coelomycetum XIII. Univ Waterloo Bio Ser 21:1–41
Nag Raj TR, DiCosmo F (1984) Genera coelomycetum. XXII. Amphiciliella, Choanatiara anam. gen. nov., Ciferriella, Collonaemella, Cymbothyrium, Melanostroma, Phialophorophoma, and Thyrsidina. Can J Bot 62:709–721
Nag Raj TR, Kendrick B (1972) Genera coelomycetarum. III. Pestalozziella. Can J Bot 50:607–617
Nag Raj TR, Kendrick B (1978) Genera coelomycetum. XV. Belaina, Belainopsis, and Crucellisporiurn. Can J Bot 56:708–714
Nag Raj TR, Kendrick B (1988) Genera coelomycetum. XXIII. Catenophoropsis anamorph gen. nov. Can J Bot 66:898–902
Nag Raj TR, Kendrick WB (1970) Mycotribulus, a new genus of Sphaeropsidales. Can J Bot 48:2219–2221
Nag Raj TR, Morgan-Jones G (1973) Genera coelomycetum. VII. Rhabdogloeopsis Petrak. Can J Bot 51:565–569
Nag Raj TR, Morgan-Jones G, Kendrick B (1972) Genera coelomycetarum. IV. Pseudorobillarda gen. nov., a generic segregate of Robillarda Sacc. Can J Bot 50:861–867
Nag Raj TR, Morgan-Jones G, Kendrick B (1973) Pseudorobillarda Nag Raj et al., a later homonym of Pseudorobillarda Morelet. Can J Bot 51:688–689
Nag Raj TR, Holubová-Jechová V, Castañeda Ruiz RF (1989) Genera coelomycetum. XXVII. Pycnovellomyces redescribed. Can J Bot 67:3386–3390
Nair VMG, Kostichka CJ, Kuntz JE (1979) Sirococcus clavigignenti-juglandacearum: An undescribed species causing canker on butternut. Mycologia 71:641–646
Nakamura M, Ohzono M, Iwai H, Arai K (2006) Anthracnose of Sansevieria trifasciata caused by Colletotrichum sansevieriae sp. nov. J Gen Plant Pathol 72:253–256
Nannfeldt JA (1932) Studien über die Morphologie und Systematik der nicht-lichenisierten inoperculaten Discomyceten. Nova Acta Regiae Soc Sci Uppsaliensis IV 8:1–368
Nasr S, Bien S, Soudi MR, Alimadadi N, Fazeli SAS, Damm U (2018) Novel Collophorina and Coniochaeta species from Euphorbia polycaulis, an endemic plant in Iran. Mycol Prog 17:755–771
Norphanphoun C, Hongsanan S, Doilom M, Bhat DJ, Wen TC, Senanayake IC, Bulgakov TS, Hyde KD (2016) Lamproconiaceae fam. nov. to accommodate Lamproconium desmazieri. Phytotaxa 270:89–102
Norphanphoun C, Doilom M, Daranagama D, Phookamsak R, Wen TC, Bulgakov TS, Hyde KD (2017) Revisiting the genus Cytospora and allied species. Mycosphere 8:51 –97
Norphanphoun C, Raspé O, Jeewon R, Wen TC, Hyde KD (2018) Morphological and phylogenetic characterisation of novel Cytospora species associated with mangroves. MycoKeys 38:93–120
Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24:581–583
Obornik M, Stouthamer R, Meekes E, Schilthuittzen M (1999) Molecular characterization and phylogeny of the entomopathogenic fungus Aschersonia spp. Plant Prot Sci Prague 35:1–9
Okada G, Tobaki K (1987) Conidiomatal structures of the stlbellaceous and allied fungi. Sydowia 39:148–159
Oliveira RJV, Lima TEF, Cunha IB, Coimbra VRM, Silva GA, Bezerra JL, Cavalcanti MAQ (2014) Corniculariella brasiliensis, a new species of coelomycetes in the rhizosphere of Caesalpinia echinata (Fabaceae, Caesalpinioideae) in Brazil. Phytotaxa 178:197–204
Płachecka A (2005) Microscopical observations of Sphaerellopsis filum, a parasite of Puccinia recondita. Acta Agrobot 58:67–71
Palavouzis SC, Tzamos S, Paplomatas E, Thomidis T (2015) First report of Cytospora punicae isolated from pomegranate plants with symptom of collar rot in northern Greece. J Plant Pathol 97:209–220
Palm ME (1991) Taxonomy and morphology of the synanamorphs Pilidium concavum and Hainesia lythri (Coelomycetes). Mycologia 83:787–796
Palmateer AJ, Tarnowski TLB, Lopez P (2012) First report of Colletotrichum sansevieriae causing anthracnose of Sansevieria trifasciata in Florida. Plant Dis 96:293Parker AK, Reid J (1969) The genus Rhabdocline Syd. Can J Bot 47:1533–1545
Park JH, Han KS, Kim JY, Shin HD (2013) First report of anthracnose caused by Colletotrichum sansevieriae on Sansvieria in Korea. Plant Dis 97:1510
Parker AK, Reid J (1969) The genus Rhabdocline Syd. Can J Bot 47:1533–1545
Parkin J (1906) Fungi parasitic upon scale insects (Coccidae and Aleyrodidae): a general account with special reference to Ceylon forms. Ann Roy Bot Gard (Peradeniya) 3:11–82
Pärtel K, Baral HO, Tamm H, Põldmaa K (2017) Evidence for the polyphyly of Encoelia and Encoelioideae with reconsideration of respective families in Leotiomycetes. Fungal Divers 82:183–219
Patouillard MN (1888) Note sur une Tubercularée graminicole. Bull Trimest Soc Mycol Fr 4:39–40
Patouillard NT (1891) Sur l’organisation de quelques champignons exotiques. Bull Soc Mycol Fr 7:42–49
Patouillard NT, Hariot P (1900) Énumération des champignons récoltés par M.A. Chevalier au Sénégal et dans le Soudan occidental. J Bot (Morot) 14:234–246
Pavlic D, Slippers B, Coutinho TA, Wingfield MJ (2009) Molecular and phenotypic characterization of three phylogenetic species discovered within the Neofusicoccum parvum/N. ribis complex. Mycologia 101:636–647
Pavlic-Zupanc D, Wingfield MJ, Boissin E, Slippers B (2015) The distribution of genetic diversity in the Neofusicoccum parvum/N. ribis complex suggests structure correlated with level of disturbance. Fungal Ecol 13:93–102
Peck CH (1878) Report of the Botanist (1877). Annual Report on the New York State Museum of Natural History. 31:19–60
Peck C (1894) Report of the Botanist (1893). Rep NY State Mus Nat Hist 47:129–174
Peglion V (1901) Über die Nematospora coryli Pegl. Centralbl Bakteriol Parasitenk Ser 2(7):754–761
Pei M, Hunter T, Ruiz C, Bayon C, Harris J (2003) Quantitative inoculation of willow rust Melampsora larici-epitea with the mycoparasite Sphaerellopsis filum (teleomorph Eudarluca caricis). Mycol Res 107:57–63
Pem D, Hongsanan S, Doilom M, Tibpromma S, Wanasinghe DN, Dong W, Ningguo L, Phookamsak R, Phillips AJL, Jeewon R, Hyde KD (2019) https://www.dothideomycetes.org: an online taxonomic resource for the classification, identification, and nomenclature of Dothideomycetes. AJOM 2:287–297
Pérez-Ortega S, Suija A, de los Rios A (2011) The connection between Abrothallus and its anamorph state Vouauxiomyces established by denaturing gradient gel electrophoresis (DGGE). Lichenologist 43:277–279
Petch T (1921) Studies in entomogenous fungi. II. The genera Hypocrella and Aschersonia. Ann R Bot Gard Perad 7:167–278
Petch T (1925) Entomogenous fungi and their use in controlling insect pests. Bull Dep Agric Ceylon 71:1–40
Petch T (1943) British Nectrioideae and allied genera. Trans Br Mycol Soc 26:53–70
Petch T (1981) Plasia, a new genus for the macroconidial anamorph of Durella atrocyanea. Trans Br Mycol Soc 77:196–200
Petrak F (1921) Mykologische Notizen II. Ann Mycol 19:17–128
Petrak F (1924) Mykologische Notizen VII. Ann Mycol 22:1–182
Petrak F (1925) Mykologische Notizen VIII. Ann Mycol 23:1–143
Petrak F (1927a) Myk. Notizen 595. Über die Gattung Hemidothis. Syd Ann Myc 25:326–328
Petrak F (1927b) Mykologische Notizen IX. Ann Mycol 25:193–343
Petrak F (1929) Myk. Notigen, 660. Über Höhnels Auffassung der Gattung Hemidothis. Syd Ann Myc 27:374
Petrak F (1942) Hysterodiscula nov. gen., eine neue Gattung der patellaten Sphaeropsideen. Bot Arch 43:207–214
Petrak F (1944) Über die Gattungen Chaetopyrena Pass., Sclerochaeta v. Höhn, Sclerochaetella v. Höhn, Vermiculariella Oud, Chaetosphaeronema Moesz und Pseudophoma v. Höhn. Ann Mycol 42:58–71
Petrak F (1953) Erythrogloeum n. gen., eine neue Gattung der Sphaeropsideen. Sydowia 7:378–380
Petrak F (1957a) Mykologische Bemerkungen. Sydowia 11:337–353
Petrak F (1957b) Über die Gattungen Dothichiza Lib. und Chondroplea Kleb. Sydowia 10:201–235
Petrak F (1968) Ober die Gattung Sphaerellopsis Cooke. Sydowia 20:200–202
Petrak F (1970) Über Diaporthe hranicensis Petr. Sydowia 24:256–260
Petrak F, Ciferri R (1932) Fungi dominicani II. Annls mycol 30:149–353
Petrak F, Sydow H (1935) Kritisch-systematische Originaluntersuchungen über Pyrenomyzeten, Sphaeropsideen und Melanconieen VI. Ann Mycol 33:157–193
Petrini O (1986) Taxonomy of endophytic fungi of aerial plant tissues. In: Fokkema NJ, van den Heuvel J (eds) Microbiology of the Phyllosphere. Cambridge University Press, Cambridge, UK, pp 175–187
Phillips AJL (1998) Botryosphaeria dothidea and other fungi associated with excoriose and dieback of grapevines in Portugal. J Phytopathol 146:327–332
Phillips AJL, Alves A (2009) Taxonomy, phylogeny, and epitypification of Melanops tulasnei, the type species of Melanops. Fungal Divers 38:155–166
Phillips AJL, Pennycook SR (2004) Taxonomy of Botryosphaeria melanops and its anamorph, Fusicoccum advenum. Sydowia 56:68–75
Phillips AJL, Oudemans PV, Correia A, Alves A (2006) Characterisation and epitypification of Botryosphaeria corticis, the cause of blueberry cane canker. Fungal Divers 21:141–155
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley Akulov AA, Crous PW (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21:29–55
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167
Phillips AJL, Hyde KD, Alves A, Liu (Jack) JK (2019) Families in Botryosphaeriales: a phylogenetic, morphological and evolutionary perspective. Fungal Divers 94:1–22
Phookamsak R, Liu JK, McKenzie EHC, Manamgoda DS, Ariyawansa H, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68:159–238
Phookamsak R, Norphanphoun C, Tanaka K, Dai DQ, Luo ZL, Liu JK, Su HY, Bhat DJ, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2015) Towards a natural classification of Astrosphaeriella-like species; introducing Astrosphaeriellaceae and Pseudoastrosphaeriellaceae fam. nov. and Astrosphaeriellopsis, gen. nov. Fungal Divers 74:143–197
Phookamsak R, Wanasinghe DN, Hongsanan S, Phukhamsakda C, Huang SK, Tennakoon DS, Norphanphoun C, Camporesi E, Bulgakov TS, Promputtha I, Mortimer PE, Xu JC, Hyde KD (2017) Towards a natural classification of Ophiobolus and ophiobolus-like taxa; introducing three novel genera Ophiobolopsis, Paraophiobolus and Pseudoophiobolus in Phaeosphaeriaceae (Pleosporales). Fungal Divers 87:299–339
Photita W, Lumyong S, Hyde KD (2001) Endophytic fungi ofwild banana (Musa acuminata) at Doi Suthep Pui National Park, Thailand. Mycol Res 105:1508–1513
Photita W, Lumyong S, Lumyong P, McKenzie EHC, Hyde KD (2004) Are some endophytes of Musa acuminata latent pathogens? Fungal Divers 16:131–140
Photita W, Taylor PWJ, Ford R, Hyde KD, Lumyong S (2005) Morphological and molecular characterization of Colletotrichum species from herbaceous plants in Thailand. Fungal Divers 18:117–133
Pirozynski KA (1971) Note on Geastrumia polystigmatis. Mycologia 63:897–901
Pirozynski KA (1973) Three hyperparasites of ascomycetes. Mycologia 65:761–767
Pirozynski KA, Morgan-Jones G (1968) Notes on microfungi. III. Trans Br Mycol Soc 51:185–206
Pirozynski KA, Shoemaker RA (1971) Some Coelomycetes with appendaged conidia. Can J Bot 49:529–541
Pirozynski KA, Shoemaker RA (1972) Vestigizcm, a new genus of coelomycetes. Can J Bot 50:1163–1164
Plaingam N, Somrithipol S, Jones EBG (2003) Infundibulomyces: a new genus of coelomycetes from Thailand. Can J Bot 81:732–737
Plaingam N, Somrithipol S, Jones EBG (2005) Pseudorobillarda siamensis sp. nov. and notes on P. sojae and P. texana from Thailand. Nova Hedwig 80:335–348
Potlaichuk VI (1964) Duae species novae fungorum in glandibus inventae. Nov sist Niz Rast 1:218–220
Powell PE (1973) The genera Duplicaria (Rhytismataceae) and Crandallia (Leptostromataceae). Mycologia 65:1356–1370
Preuss CG (1855) Übersicht untersuchter Pilze, besonders aus der Umgegend von Hoyerswerda (Fortsetzung). Linnaea 26:705–725
Promputtha I, Hyde KD, McKenzie EHC, Peberdy JF, Lumyong S (2010) Can leaf degrading enzymes provide evidence that endophytic fungi becoming saprobes? Fungal Divers 41:89–99
Pu J, Xie Y, Zhang X, Qi Y, Zhang C, Liu X (2008) Preinfection behaviour of Phyllosticta musarum on banana leaves. Austral Plant Pathol 37:60–64
Punithalingam E (1969) New species of Monochaetiella and Septoria. Trans Br Mycol Soc 53:311–315
Punithalingam E (1980) Leptosphaeria coniothyrium. IMI Descr Fungi Bact 67:663
Punithalingam E (1984) New microfungi from Malaysia and Papua New Guinea. Nova Hedwig 39:57–74
Punithalingam E (1999) Proboscispora Punith. (1984) and Proboscispora S.-W. Wong & K.D. Hyde (1999). Kew Bull 54:234
Punithalingam E (2003) Nuclei, micronuclei and appendages in tri- and tetraradiate conidia of Cornutispora and four other coelomycete genera. Mycol Res 107:917–948
Purohit DK, Chawla GC (1989) A new species of Phacidiella Karst. from India. Geobios New Reports. 8:79–80
Qiu JZ, Su YB, Weng CS, Guan X (2011) Aschersonia conica sp. nov. (Clavicipitaceae) from Hainan Province, China. Mycotaxon 118:325–329
Quaedvlieg W, Kema GHJ, Groenewald JZ, Verkley GJM, Seifbarghi S, Razavi M, Mirzadi Gohari A, Mehrabi R, Crous PW (2011) Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia 26:57–69
Quaedvlieg W, Barreto RW, Alfenas AC, Barreto RW, Alfenas AC, Swart WJ, Groenewald JZ, Crous PW (2013) Sizing up Septoria. Stud Mycol 75:307–390
Quandt CA, Kepler RM, Gams W, Araújo JPM, Ban S, Evans HC, Hughes D, Humber R, Hywel-Jones N, Li ZZ, Luangsa-ard JJ, Rehner SA, Sanjuan T, Sato H, Shrestha B, Sung GH, Yao YJ, Zare R, Spatafora JW (2014) Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus 5:121–134
Quijada L, Tanney JB, Popov E, Johnston PR, Pfister DH (2020) Cones, needles and wood: Micraspis (Micraspidaceae, Micraspidales fam. et ord. nov.) speciation segregates by host plant tissues. Fungal Syst Evol 5:99–111
Rajagopal K, Maheswari S, Kathiravan G (2012) Diversity of endophytic fungi in some tropical medicinal plants: a report. Afr J Microbiol Res 6:2822–2827
Ramaley A (1991) Fungi of Yucca baccata 1. Piptarthron pluriloculare and its teleomorph. Planistroma yuccigena. Mycotaxon 42:63–75
Ramaley AW (1992) Fungi from Yucca baccata. 2. Planistroma obtusilunatum sp. nov., and its anamorph, Piptarthron uniloculare sp. nov. Mycotaxon 45:449–460
Ramaley AW (1993) New fungi from Yucca: Planistromella yuccifoliorum, gen. et sp. nov., its anamorph, Kellermania yuccifoliorum, sp. nov., and Planistromella uniseptata, sp. nov., the teleomorph of Kellermania yuccigena. Mycotaxon 47:259–274
Ramaley AW (1995) New species of Kellermania, Piptarthron, Planistroma, and Planistromella from members of the Agavaceae. Mycotaxon 55:255–268
Ramaley AW (1998) New teleomorphs of the anamorphic genus Kellermania. Mycotaxon 66:509–514
Rambaut A (2014) FigTree v1.4.2, A graphical viewer of phylogenetic trees. http://treebio.ed.ac.uk/software/figtree
Rannala B, Yang Z (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Rappaz F (1987) Taxonomie et nomenclature des Diatrypacées à asques octosporées (1). Mycol Helv 2:285–648
Rappaz F (1992) Phomatospora berkeleyi, P. arenaria and their Sporothrix anamorphs. Mycotaxon 45:323–330
Rashmi M, Kushveer JS, Sarma VV (2019) A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 10:798–1079
Rayner RW (1970) A mycological colour chart. British Mycological Society, Commonwealth Mycological Institute (UK)
Raza M, Zhang ZF, Hyde KD, Diao YZ, Cai L (2019) Culturable plant pathogenic fungi associated with sugarcane in southern China. Fungal Divers 99:1–104
Réblová M, Miller AN, Rossman AY, Seifert KA, Crous PW, Hawksworth DL, Abdel-Wahab MA, Cannon PF, Daranagama DA, De Beer ZW, Huang SK, Hyde KD, Jyawardena R, Jaklitsch W, Jones EBG, Ju YM, Judith C, Maharachchikumbura SSN, Pang KL, Petrini LE, Raja HA, Romero AI, Shearer C, Senanayake IC, Voglmayr H, Weir BS, Wijayawarden NN (2016) Recommendations for competing sexual-asexually typified generic names in Sordariomycetes (except Diaporthales, Hypocreales, Hypocreales, and Magnaporthales). IMA Fungus 7:131–153
Rehm H (1896) Ascomyceten: Hysteriaceen und Discomyceten. Rabenhorst, L., Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. Eduard Kummer, Leipzig, pp 1–1275
Rehm H (1912) Zur Kenntnis der Discomyceten Deutschlands, Deutsch-Öster-reichs und der Schweiz. Ber Bayer Bot Ges 13:102–206
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98
Rehner SA, Samuels GJ (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol Res 98:625–634
Rehner SA, Samuels GJ (1995) Molecular systematics of the Hypocreales: A teleomorph gene phylogeny and the status of their anamorphs. Can J Bot 73:816–823
Reid D (1966) Two new fungi from New Guinea. Aust J Bot 14:31–34
Reid J, Dowsett JA (1990) On Dicarpella, Sphaerognomonia, and Apiosporopsis. Can J Bot 68:2398–2407
Reusser F (1964) Übcr einige Arten der Gattung Guignardia Viala et Ravaz. J Phytopathol 51:205–240
Riedl H (1977) Die Gattung Dothiorina v. Hoehnel. Sydowia 29:146–154
Riley IT (1994) Dilophospora alopecuri and decline in annual Ryegrass toxicity in Western Australia. Aust J Agric Res 45:841–850
Rimpau RH (1961) Untersuchungen über die Gattung Drepanopeziza (Kleb.) v. Höhn. Phytopathology Z 43:257–306
Rivera-Orduña FN, Suarez-Sanchez RA, Flores-Bustamante ZR, Gracida-Rodriguez JN, Flores-Cotera LB (2011) Diversity of endophytic fungi of Taxus globosa (Mexican yew). Fungal Divers 47:65–74
Roberts DW, Humber RA (1981) Entomogenous fungi. In: Coke GT, Kendrick B (eds) Biology of conidial fungi. Academic, New York, pp 201–236
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rossman AY (1989) A synopsis of the Nectria cinnabarina group. Mem N Y Bot Gdn 49:253–265
Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999) Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Stud Mycol 42:1–248
Rossman AY, Aime MC, Farr DF, Peterson R, Leahy R (2004) The coelomycetous genera Chaetomella and Pilidium represent a newly discovered lineage of inoperculate discomycetes. Mycol Prog 3:275–290
Rossman AY, Castlebury LA, Farr DF, Stanosz GR (2007) Sirococcus conigenus, Sirococcus piceicola sp. nov. and Sirococcus tsugae sp. nov. on conifers: anamorphic fungi in the Gnomoniaceae. Diaporthales. For Pathol 38:47–60
Rossman AY, Seifert KA, Samuels GJ, Minnis AM, Schroers HJ, Lombard L, Crous PW, Põldmaa K, Cannon PF, Summerbell RC, Geiser DM, Zhuang WY, Hirooka Y, Herrera C, Salgado-Salazar C, Chaverri P (2013) Genera in Bionectriaceae, Hypocreaceae, and Nectriaceae (Hypocreales) proposed for acceptance or rejection. IMA Fungus 4:41–51
Rossman AY, Adams GC, Cannon PF, Castlebury LA, Crous PW, Gryzenhout M, Jaklitsch WM, Mejia LC, Stoykov D, Udayanga D, Voglmayr H, Walke DM (2015a) Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 6:145–154
Rossman AY, Crous PW, Hyde KD, Hawksworth DL, Aptroot A, Bezerra JL, Bhat JD, Boehm E, Braun U, Boonmee S, Camporesi E, Chomnunti P, Dai DQ, D’souza MJ, Dissanayake A, Jones EBG, Groenewald JZ, Hernández-Restrepo M, Hongsanan S, Jaklitsch WM, Jayawardena R, Li WJ, Kirk PM, Lawrey JD, Mapook A, McKenzie EHC, Monkai J, Phillips AJL, Phookamsak R, Raja HA, Seifert KA, Senanayake I, Slippers B, Suetrong S, Taylor JE, Thambugala KM, Tian Q, Tibpromma S, Wanasinghe DN, Wijayawardene NN, Wikee S, Woudenberg JHC, Wu HX, Yan JY, Yang T, Zhang Y (2015b) Recommended names of pleomorphic genera in Dothideomycetes. IMA Fungus 6:507–523
Rossman AY, Allen WC, Braun U, Castlebury LA, Chaverri P, Crous PW, Hawksworth DL, Hyde KD, Johnston P, Lombard L, Romberg M, Samson RA, Seifert KA, Stone JK, Udayanga D, White JF (2016a) Overlooked competing asexual and sexually typified generic names of Ascomycota with recommendations for their use or protection. IMA Fungus 7:289–308
Rossman AY, Allen WC, Castlebury LA (2016b) New combinations of plant-associated fungi resulting from the change to one name for fungi. IMA Fungus 7:1–7
Rungjindamai N, Sakayaroj J, Plaingam N, Somrithipol S, Jones EBG (2008) Putative basidiomycete teleomorphs and phylogenetic placement of the coelomycete genera: Chaetospermum, Giulia and Mycotribulus based on nu-rDNA sequences. Mycol Res 112:802–810
Rungjindamai N, Sakayaroj J, Somrithipol S, Plaingam N, Jones EBG (2012) Phylogeny of the appendaged coelomycete genera: Pseudorobillarda, Robillarda, and Xepiculopsis based on nuclear ribosomal DNA sequences. Cryptogam Mycol 33:319–332
Ruszkiewicz-Michalska M (2016) The genus Asteromella (Fungi: Ascomycota) in Poland. Monographiae Botanicae 106:1–164
Saccardo PA (1880) Conspectus generum fungorum Italiae inferiorum nempe ad Sphaeropsideas, Melanconieas et Hyphomyceteas pertinentium systemate sporologico dispositorum. Michelia 2:1–38
Saccardo PA (1882) Sylloge fungorum omnium hucusque cognitorum I. Sumptibus Auctoris, Patavii, pp 106–107
Saccardo PA (1884a) Sylloge Fungorum omnium hucusque cognitorum III. Sumptibus Auctoris Typis Seminarii, Patavii
Saccardo PA (1884b) Sylloge Fungorum: Sylloge Sphaeropsidearum et Melanconiearum. Syll Fung 3:1–840
Saccardo PA (1889) Sylloge Fungorum 8:1–114
Saccardo PA (1892) Sylloge Fungorum, vol 10. P.A. Saccardo, Padova
Saccardo PA (1893) Florula mycologica Lusitanica sistens contributionem decimam ad eandem floram nec non conspectus fungorum omnium in Lusitania hucusque observatorum. Bolm Soc Broter 11:9–70
Saccardo PA (1895) Sylloge Fungorum 11:625–630
Saccardo PA (1913) Notae mycologicae. Series XVII. Ann Mycol 11:546–568
Saccardo PA, Saccardo D (1906) Supplementum universale. Pars VII. Discomycetae-Deuteromycatae. Syll Fung 18:1–838
Saikawa M, Castañeda Ruiz RF, Kendrick B, Nag Raj TR (1991) Genera coelomycetum XXVIII. Zelosatchmopsis anam.-gen. nov. Can J Bot 69:630–633
Sakalidis ML, Slippers B, Wingfield BD, Hardy GSJ, Burgess TI (2013) The challenge of understanding the origin, pathways and extent of fungal invasions: global populations of the Neofusicoccum parvum-N. ribis species complex. Divers Distrib 19:873–883
Sanchez RM, Bianchinotti MV (2007) Dothiorina: taxonomic concepts and comments on its condiogenesis. Mycotaxon 102:395–402
Sankaran KV, Sutton BC (1991) Myriellina imperatae sp. nov. on Imperata from Papua New Guinea and Australia. Mycol Res 95:1021–1022
Santos JM, Correia VG, Phillips AJL (2010) Primers for mating-type diagnosis in Diaporthe and Phomopsis: their use in teleomorph induction in vitro and biological species definition. Fungal Biol 114:255–270
Santos LTP (2011) Micobiota foliícola de Salacia crassifolia. Micobiota foliícola de Salacia crassifolia. 2011. 116 f. Dissertação (Mestrado em Fitopatologia)-Universidade de Brasília, Brasília
Sato G, Tanaka K, Hosoya T (2008) Bambusicolous fungi in Japan (8): A new species of Pseudolachnella from Yakushima Island, southern Japan. Mycoscience 49:392–394
Schroers H, Gräfenhan T, Nirenberg HI, Seifert KA (2011) A revision of Cyanonectria and Geejayessia gen. nov., and related species with Fusarium-like anamorphs. Stud Mycol 68:115–138
Schroers HJ (2001) A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorphs. Stud Mycol 46:1–214
Seaver FJ (1945) Photographs and Descriptions of Cup-Fungi: XXXIX. The Genus Godronia and its allies. Mycologia 37:333–359
Seaver FJ, Velazquez J (1933) Dermea and Pezicula. Mycologia 25:139–149
Seeler EV (1943) Several fungicolous fungi. Farlowia 1:119–133
Seeler EV Jr (1940a) Two diseases of Gleditsia caused by a species of Thyronectria. J Arnold Arbor 21:405–427
Seeler EV Jr (1940b) A monographic study of the genus Thyronectria. J Arnold Arbor 21:429–460
Seephueak P, Phongpaichit S, Hyde KD, Petcharat V (2011) Diversity of saprobic fungi on decaying branch litter of the rubber tree (Hevea brasiliensis). Mycosphere 2:307–330
Seifert KA (1985a) A monograph of Stilbella and some allied hyphomycetes. Stud Mycol 27:1–235
Seifert KA (1985b) Notes on several apocryphal genera of synnematal hyphomycetes. Trans Br Mycol Soc 85:123–133
Seifert KA (1999) Conicomyces nassensis, a new synnematous fungus from the Nass River Valley, British Columbia. Mycotaxon 71:301–306
Seifert KA, Samuels GJ (2000) How should we look at anamorphs? Stud Mycol 45:5–18
Seifert KA, Morgan-Jones G, Gams W, Kendrick B (2011) The genera of Hyphomycetes. CBS Biodiversity Series 9. CBS-KNAW Fungal Biodiversity Centre, Utrecht
Semeniuk G (1982) Time required for alfalfa infection by Leptotrochila medicaginis. Proc S D Acad Sci 61:1–107
Senanayake IC, Al-Sadi AM, Bhat JD, Camporesi E, Dissanayake AJ, Lumyon S, Maharachchikumbura SSN, Hyde KD (2016) Phomatosporales ord. nov. and Phomatosporaceae fam. nov., to accommodate Lanspora, Phomatospora and Tenuimurus, gen. nov. Mycosphere 7:628–641
Senanayake IC, Crous PW, Groenewald JZ, Maharachchikumbura SSN, Jeewon R, Phillips AJL, Bhat JD, Perera RH, Li QR, Li WJ, Tangthirasunun N, Norphanphoun C, Karunarathna SC, Camporesi E, Manawasighe IS, Al-Sadi AM, Hyde KD (2017) Families of Diaporthales based on morphological and phylogenetic evidence. Stud Mycol 86:217–296
Senanayake IC, Jeewon R, Chomnunti P, Wanasinghe DN, Norphanphoun C, Karunarathna A, Pem D, Perera RH, Camporesi E, McKenzie EHC, Hyde KD, Karunarathna SC (2018) Taxonomic circumscription of Diaporthales based on multigene phylogeny and morphology. Fungal Divers 93:241–443
Shamoun SF, Zhao S (2005) First report of Phoma exigua as a pathogen of Salal (Gaultheria shallon) in British Columbia. Plant Dis 89:685
Shang QJ, Hyde KD, Camporesi E, Norphanphoun C, Brooks S, Liu JK (2019) Additions to the genus Cytospora with sexual morph in Cytosporaceae. Mycosphere 11:189–224
Shear CL (1907) New species of fungi. Bul Torr Bot Club 34:305–317
Shear CL, Dodge BO (1921) The life history and identity of “Patellina fragariae,” “Leptothyrium macrothecium,” and “Peziza oenotherae.”. Mycologia 13:135–170
Shearer CA, Crane JL (1971) Fungi of the Chesapeake Bay and its tributaries. I. Patuxent River. Mycologia 63:237–260
Shenoy BD, Jeewon R, Wu WP, Bhat DJ, Hyde KD (2006) Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycol Res 110:916–928
Shenoy BD, Jeewon R, Hyde KD (2007) Impact of DNA sequence-data on the taxonomy of anamorphic fungi. Fungal Divers 26:1–54
Shenoy BD, Jeewon R, Wang HK, Amandeep KH, Wellcome HH, Bhat DJ, Crous PW, Hyde KD (2010) Sequence data reveals phylogenetic affinities of fungal anamorphs Bahusutrabeeja, Diplococcium, Natarajania, Paliphora, Polyschema, Rattania and Spadicoides. Fungal Divers 44:161–169
Sherriff C, Whelan MJ, Arnold GM, Lafay JF, Brygoo Y, Bailey JA (1994) Ribosomal DNA sequence analysis reveals new species groupings in the genus Colletotrichum. Exp Mycol 18:121–138
Sherwood-Pike M, Stone JK, Carroll GC (1986) Rhabdocline parkeri, a ubiquitous foliar endophyte of Douglas-fir. Can J Bot 64:1849–1855
Shetty KG, Minnis AM, Rossman AY, Jayachandran K (2011) The Brazilian peppertree seed-borne pathogen, Neofusicoccum batangarum, a potential biocontrol agent. Biol Control 56:91–97
Shoemaker RA (1965) Pseudomassaria sepincolaeformis and Neottiospora caricina in Canada. Can J Bot 44:255–258
Shoemaker RA, Babcock CE, Irwin JAG (1991) Massarina walkeri n. sp., the teleomorph of Acrocalymma medicaginis from Medicago sativa contrasted with Leptosphaeria pratensis, L. weimeri n. sp., and L. viridella. Can J Bot 69:569–573
Shoemaker RA, Malloch D, Hambleton S, Liu M (2013) A new Vestigium on Abies balsamea. North American Fungi 8:1–6
Sieber T (1988) Endophytische Pilze in Nadeln von gesunden und geschädigten Fichten (Picea abies [L.] Karsten). Eur J Plant Pathol 18:321–342
Sigler L (1990) Occurrence of a yeastlike synanamorph in the fungicolous coelomycete Eleutheromyces subulatus. Crypt Bot 1:384–389
Sinclair RC, Eicker A, Morgan-Jones G (1983) Conicomyces, a unique synnematous hyphomycete genus from South Africa. Mycologia 75:1100–1103
Singer R (1962) The Agaricales in modern taxonomy. J. Cramer, Weinheim
Sivanesan A (1984) The bitunicate Ascomycetes and their anamorphs. J. Cramer, Vaduz, pp 1–701
Sivanesan A, Hsieh WH (1995) A re-appraisal of the systematic status of the ascomycete genus Yoshinagaia. Mycol Res 99:1295–1298
Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ (2004) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96:83–101
Slippers B, Johnson GI, Crous PW, Coutinho TA, Wingfield B, Wingfield MJ (2005) Phylogenetic and morphological re-evaluation of the Botryosphaeria species causing diseases of Mangifera indica in Australia. Mycologia 97:99–110
Slippers B, Boissin E, Phillips AJL, Groenewald JZ, Lombard L, Wingfield MJ, Postma A, Burgess T, Crous PW (2013) Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Stud Mycol 76:31–49
Slippers B, Roux J, Wingfield MJ, van der Walt FJJ, Jami F, Mehl JWM, Marais GJ (2014) Confronting the constraints of morphological taxonomy in the Botryosphaeriales. Persoonia 33:155–168
Slopekl SW, Labun TJ (1992) First report of halo spot of barley caused by Pseudoseptoria stomaticola in Alberta. Can Plant Dis Surv 72:5–8
Smerlis E (1962) Taxonomy and morphology of Potebniamyces balsamicola sp. nov. associated with a twig and branch blight of balsam fir in Quebec. Can J Bot 40:351–359
Sogonov MV, Castlebury LA, Rossman AY, White JF (2007) The type species of Apiognomonia, A. veneta, with its Discula anamorph is distinct from A. errabunda. Mycol Res 111:693–709
Sogonov MV, Castlebury LA, Rossman AY, Mejía LC, White JF (2008) Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Stud Mycol 62:1–77
Spegazzini C (1880) Fungi argentini. Pugillus secundus (Continuacion). An Soc Cient Arg 10:5–33
Spegazzini C (1908) Fungi aliquot Paulistani. Rev Mus Plata 15:7–48
Spegazzini C (1911) Mycetes Argentinenses (Series V). An Mus Nac Hist Nat B Aires Ser 3(13):329–467
Spiers AG, Hopcroft DH (1998) Morphology of Drepanopeziza species pathogenic to poplars. Mycol Res 102:1025–1037
Sreenivasaprasad S, Brown AE, Mills PR (1992) DNA sequence variation and interrelationship among Colletotrichum species causing strawberry anthracnose. Physiol Mol Plant Pathol 41:265–281
Stadler M, Kuhnert E, Peršoh D, Fournier J (2013) The Xylariaceae as model example for a unified nomenclature following the “One fungus-one name” (1F1N) concept. Mycology 4:5–21
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Stone JK, Gernandt DS (2005) A reassessment of Hemiphacidium, Rhabdocline, and Sarcotrachila (Hemiphacidiaceae). Mycotaxon 91:115–126
Strømeng GM, Stensvand A (2011) Godronia Canker (Godronia cassandrae f. sp. vaccinii) in Highbush Blueberry. Eur J Plant Sci Biotechnol 5:35–41
Subramanian S (1980) In: Kavaka Vol 7. p 90
Subramanian CV, Ramakrishnan K (1953) On the nature of the spore appendage in Neottiospora Desm. Proc Indian Acad Sci 37:228–231
Subramanian CV, Ramakrishnan K (1954) Alpakesa, a new genus of the Sphaeropsidales. J Indian Bot Soc 33:203–205
Subramanian CV, Ramakrishnan K (1956) Ciliochorella Sydow, Plagionema Subram., Ramakr., and Shanoria gen. nov. Trans Br Mycol Soc 39:314–318
Subramanian CV, Ramakrishnan K (1957) Neottiospora Desm. and two new genera Samukuta and Sakireeta. J Indian Bot Soc 36:68–86
Sung GH, Hywel-Jones NL, Sung JM, Luangsa-ard JJ, Shrestha B, Spatafora JW (2007a) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57:5–59
Sung GH, Sung JM, Hywel-Jones NL, Spatafora JW (2007b) A multigene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Mol Phylogenet Evol 44:1204–1223
Sun JZ, Liu XZ, Mckenzie EHC, Jeewon R, Liu JK(Jack), Zhang XL, Zhao Q, Hyde KD (2019) Fungicolous fungi: terminology, diversity, distribution, evolution, and species checklist. Fungal Divers 95:337–430
Suto Y (2009) Three ascomycetes on leaves of evergreen Ilex trees from Japan: Rhytisma ilicis-integrae sp. nov., R. ilicis-latifoliae, and R. ilicis-pedunculosae sp. nov. Mycoscience 50:357–368
Sutton BC (1967a) Two new genera of the Sphaeropsidales and their relationships with Diachorella, Strasseria, and Plagiorhabdus. Can J Bot 45:1249–1263
Sutton BC (1967b) Redescription of Ajrekarella (Kamat, Kalani). Mycopath Mycol Appl 33:76–80
Sutton BC (1968) Kellermaniaand its generic segregates. Can J Bot 46:181–196
Sutton BC (1972a) Wakefieldia punctata arnaud and Blennoria buxi Fr. sensu Arnaud. Trans Br Mycol Soc 59:285–294
Sutton BC (1972b) Nomenclature of Ceuthospora, Pyrenophora and Blennoria (Fungi). Taxon 21:319–326
Sutton BC (1973a) Pucciniopsis, Mycoleptodiscus and Amerodiscosiella. Trans Br Mycol Soc 60:525–536
Sutton BC (1973b) Coelomycetes (Chap. 11). In: Ainsworth GC, Sparrow FK, Sussman AS (eds) In the fungi. IV. Academic Press, New York, pp 513–582
Sutton BC (1975) Eucalyptus microfungi: Satchmopsis gen. nov. and new species of Coniella, Coniothyrium and Harknessia. Nova Hedwig 26:1–16
Sutton BC (1977a) Coelomycetes VI. Nomenclature of generic names proposed for Coelomycetes. Mycol Pap 141:1–253
Sutton BC (1977b) Nomenclature and taxonomy of Shanoria, Placonema, and Neoplaconema (Sphaeropsidales). Kew Bull 31:461–464
Sutton BC (1980) The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew, pp 1–696
Sutton BC (1991) Notes on Deuteromycetes III. Sydowia 43(2):264–280
Sutton BC, Alcorn JL (1985) Undescribed species of Crinitospora gen. nov., Massariothea, Mycoleptodiscus and Neottiosporina from Australia. Trans Br Mycol Soc 84:437–445
Sutton BC, Davison EM (1983) Three stromatic coelomycetes from Western Australia. Trans Br Mycol Soc 81:291–301
Sutton BC, Dicosm F (1977) A revision of Monochaetiella (Deuteromycotina). Can J Bot 55:2535–2543
Sutton BC, Funk A (1975) Conidial states of some Pragmopora and Tympanis species. Can J Bot 53:521–526
Sutton BC, Gibson IAS (1977) Pezizella oenotherae. CMI Descr Pathog Fungi Bact 535:1–2
Sutton BC, Hodges CS (1990) Revision of Cercospora-like fungi on Juniperus and related Conifers. Mycologia 82:313–325
Sutton BC, Kobayashi T (1969) Strasseriopsis gen. nov., based on Phellostroma tsugae. Mycologia 61:1066–1071
Sutton BC, Marasas WFO (1976) Observations on Neottiosporina and Tiarosporella. Trans Br Mycol Soc 67:69–76
Sutton BC, Pascoe IG (1987) Some cupulate coelomycetes from native Australian plants. Trans Br Mycol Soc 88:169–180
Sutton BC, Pollack FG (1973) Gloeosporium Cercocarpi and Sphaceloma cercocarpi. Mycologia 65:1125–1134
Sutton BC, Waterston JM (1966) Leptosphaeria nodorum. CMI Descr PathogFungi Bact No. 86. Mycological Institue, CAB International, Kew
Sutton BC, Rao VG, Mhaskar DN (1976) Kendrickomyces gen. nov. and Waydora nom. nov., two unusual stromatic coelomycetes. Trans Br Mycol Soc 67:243–249
Sutton BC, Mugnai L, Sanguineti G (1996) Ciliochora calabrica sp. nov. isolated from Fagus sylvatica in Italy. Mycol Res 100:405–408
Sutton DA, Timm WD, Morgan-Jones G, Rinaldi MG (1999) Human phaeohyphomycotic osteomyelitis caused by the coelomycete Phomopsis Saccardo 1905: criteria for identification, case history, and therapy. J Clin Microbiol 37:807–811
Suzuki Y, Hatakeyama S, Harada Y, Tanaka K (2008) Polystigma fulvum, a red leaf blotch pathogen on leaves of Prunus spp., has the Polystigmina pallescens anamorph/andromorph. Mycoscience 49:395–398
Swart HJ (1988) Australian leaf-inhabiting fungi. XXVI. Some noteworthy Coelomycetes on Eucalyptus. Trans Br Mycol Soc 90:279–291
Swofford DL (2002) PAUP* phylogenetic analysis using parsimony (*and other methods) Sinauer Associates, Sunderland. Massachusetts, Version, p 4
Sydow H (1930) Fungi venezuelani. Ann Mycol 28:29–224
Sydow H (1935) Fungi venezuelani. Additamentum. Ann Mycol 33:85–100
Sydow H, Petrak F (1922) Ein Beitrag zur Kenntnis der Pilzflora Nordamerikas, insbesondere der nordwestlichen Staaten. Ann Mycol 20:178–218
Sydow H, Sydow P (1914) Fungi from northern Palawan. Philip J Sci Sect C 9:157–189
Sydow H, Sydow P (1917) Beitrag zur Kenntniss der Pilzflora der Philippinen-Inseln. Ann Mycol 15:165–268
Sydow H, Sydow P, Bulter EJ (1916) Fungi Indiae orientalis pars V. Ann Mycol 14:177–220
Tanaka K, Ooki Y, Hatakeyama S, Harada Y, Barr ME (2005) Pleosporales in Japan (5): Pleomassaria, Asteromassaria, and Splanchnonema. Mycoscience 46:248–260
Tanaka K, Mel’nik VA, Kamiyama M, Hirayama K, Shirouzu T (2010) Molecular phylogeny of two coelomycetous fungal genera with stellate conidia, Prosthemium and Asterosporium, on Fagales trees. Botany 88:1057–1071
Tanaka K, Endo M, Hirayama K, Okane I, Hosoya T, Sato T (2011) Phylogeny of Discosia and Seimatosporium, and introduction of Adisciso and Immersidiscosia genera nova. Persoonia 26:85–98
Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y, Shirouzu T, Hosoya T (2015) Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud Mycol 82:75–136
Tangthirasunun N, Silar P, Bhat DJ, Chukeatirote E, Wijayawardene DNN, Maharachchikumbura SSN, Hyde KD, Wang Y (2014a) Morphology and phylogeny of Pseudorobillarda eucalypti sp. nov., from Thailand. Phytotaxa 176:251–259
Tangthirasunun N, Silar P, Bhat DJ, Chukeatirote E, Wikee S, Maharachchikumbura SSN, Hyde KD (2014b) Morphology and phylogeny of Chaetospermum (asexual coelomycetous Basidiomycota). Phytotaxa 175:61–72
Tassi F (1904) Origine e sviluppo delle Leptostromaceae e loro rapporti con le famiglie affine. Bull Lab Orto Bot Reale Univ Siena 6:3–124
Taylor JW (1995) Making the Deuteromycota redundant: a practical integration of mitosporic and meiosporic fungi. Can J Bot 73:754–759
Tehon LR (1937) Notes on the parasitic fungi of Illinois—VI. Mycologia 29:434–446
Teixeira AR (1962) The taxonomy of the Polyporaceae. Biol Rev 37:51–81
Teng SC (1936) Additional fungi from China IV. Sinensia 7:752–823
Theissen F, Sydow H (1915) Die Dothideales. Ann Mycol 113:1 49–746
Thompson SM, Tan YP, Young AJ, Neate SM, Aitken EA, Shivas RG (2011) Stem cankers on sunflower (Helianthus annuus) in Australia reveal a complex of pathogenic Diaporthe (Phomopsis) species. Persoonia 27:80–89
Thynne E, Mcdonald MC, Evans M, Wallwork H, Neate S, Solomon PS (2015) Re-classification of the causal agent of white grain disorder on wheat as three separate species of Eutiarosporella. Australas Plant Pathol 44:527–539
Tian Q, Liu JK, Hyde KD, Wanasinghe DN, Boonmee S, Jayasiri SC, Luo ZL, Taylor JE, Phillips AJL, Bhat DJ, Li WJ, Ariyawansa H, Thambugala KM, Jones EBG, Chomnunti P, Bahkali AH, Xu JC, Camporesi E (2015) Phylogenetic relationships and morphological reappraisal of Melanommataceae (Pleosporales). Fungal Divers 74:267–324
Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SSN, Liu JK, Bhat DJ, Jones EBG, McKenzie EHC, Camporesi E, Bulgakov TS, Doilom M, de Azevedo Santiago ALCM, Das K, Manimohan P, Gibertoni TB, Lim YW, Ekanayaka AH, Thongbai B, Lee HB, Yang JB, Kirk PM, Sysouphanthong P, Singh SK, Boonmee S, Dong W, Raj KNA, Latha KPD, Phookamsak R, Phukhamsakda C, Konta S, Jayasiri SC, Norphanphoun C, Tennakoon DS, Li JF, Dayarathne MC, Perera RH, Xiao Y, Wanasinghe DN, Senanayake IC, Goonasekara ID, de Silva NI, Mapook A, Jayawardena RS, Dissanayake AJ, Manawasinghe IS, Chethana KWT, Luo ZL, Hapuarachchi KK, Baghela A, Soares AM, Vizzini A, Meiras-Ottoni A, Mesic A, Dutta AK, de Souza CAF, Richter C, Lin CG, Chakrabarty D, Daranagama DA, Lima DX, Chakraborty D, Ercole E, Wu F, Simonini G, Vasquez G, da Silva GA, Plautz HL Jr, Ariyawansa HA, Lee H, Kusan I, Song J, Sun J, Karmakar J, Hu K, Semwal KC, Thambugala KM, Voigt K, Acharya K, Rajeshkumar KC, Ryvarden L, Jadan M, Hosen MI, Mikšŕk M, Samarakoon MC, Wijayawardene NN, Kim NK, Matocec N, Singh PN, Tian Q, Bhatt RP, de Oliveira RJV, Tulloss RE, Aamir S, Kaewchai S, Svetasheva STY, Nguyen TTT, Antonın V, Li WJ, Wang Y, Indoliya Y, Tkalcec Z, Elgorban AM, Bahkali AH, Tang AMC, Su HY, Zhang H, Promputtha I, Luangsa-ard J, Xu JC, Yan J, Chuan KJ, Stadler M, Mortimer PE, Chomnunti P, Zhao Q, Phillips AJL, Nontachaiyapoom S, Wen TC, Karunarathna SC (2017) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261
Tibpromma S, Hyde KD, McKenzie EHC, Bhat DJ, Phillips AJL, Wanasinghe DN, Samarakoon MC, Jayawardena RS, Dissanayake AJ, Tennakoon DS, Doilom M, Phookamsak R, Tang AMC, Xu JC, Mortimer PE, Promputtha I, Maharachchikumbura SSN, Khan S, Karunarathna SC (2018) Fungal diversity notes 840–928: micro-fungi associated with Pandanaceae. Fungal Divers 1:1–167
Tiwari CK, Jagrati P, Verma RK (2012) A new and rare species of Phlyctaeniella from central India. Mycosphere 3:450–453
Toh YF, Yew SM, Chan CL, Na SL, Lee KW, Hoh CC, Yee WY, Ng KP, Kuan CS (2016) Genome anatomy of Pyrenochaeta unguis- hominis UM 256, a multidrug resistant strain isolated from skin scraping. PLoS ONE 11:e0162095
Tommerup I (1970) Hypnotheca graminis gen. et sp. nov., perfect state of Monochaetiella themedae. Trans Br Mycol Soc 55:463–475
Tonolo A (1956) Ricerche colturali su alcune specie della famiglia delle Massariaceae. J Phytopathol 26:131–142
Torres C, Camps R, Aguirre R, Besoain XA (2016) First report of Diaporthe rudis in Chile causing Stem-End rot on ‘Hass’ avocado fruit imported from California, USA. Plant Dis 100:19–51
Trakunyingcharoen T, Lombard L, Groenewald JZ, Cheewangkoon R, Toanun C, Alfenas AC, Crous PW (2014) Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus 5:391–414
Treigienė A (2006) New species of Kananascus and its anamorph from Lithuania. Mycotaxon 96:173–180
Tsuneda A, Murakami S, Gill WM, Maekawa N (1997) Black Spot disease of Lentinula edodes caused by the Hyphozyma Synanamorph of Eleutheromyces subulatus. Mycologia 89:867–875
Tulasne LR (1856) Note sur l’appareil reproducteur multiple des Hypoxylées (DC.) ou Pyrénomycetes (Fr.). Ann Sci Nat Bot 5:107–118
Tulasne LR, Tulasne C (1865) Selecta Fungorum Carpologia: Nectriei-Phacidiei-Pezizei. 3
Twyman ES (1947) Notes on the die-back of oak caused by Colpoma quercinum (FR.) Wallr. Trans Br Mycol Soc 29:234–241
Udayanga D, Xingzhong L, McKenzie EHC, Chukeatirote E, Bahkali AHA, Hyde KD (2011) The genus Phomopsis: biology, applications, species concepts and names of common pathogens. Fungal Divers 50:189–225
Udayanga D, Liu XZ, Mckenzie EHC, Chukeatirote E, Hyde KD (2012a) Multi-locus phylogeny reveals three new species of Diaporthe from Thailand. Cryptogam Mycol 33:295–309
Udayanga D, Xingzhong L, Crous PW, McKenzie EHC, Chukeatirote E, Hyde KD (2012b) A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis). Fungal Divers 56:157–171
Udayanga D, Castlebury LA, Rossman AY, Hyde KD (2014) Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 32:83–101
Udayanga D, Castlebury LA, Rossman AY, Chukeatirote E, Hyde KD (2015) The Diaporthe sojae species complex: phylogenetic re-assessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biol 119:383–407
Uecker FA (1988) A world list of Phomopsis names with notes on nomenclature, morphology and biology. Mycological memoirs series 13. Cramer, Berlin
Uecker FA, Kulik MM (1986) Pseudorobillarda sojae, a new pycnidial coelomycete from soybean stems. Mycologia 78:449–453
Úrbez-Torres JR, Peduto F, Vossen PM, Krueger WH, Gubler WD (2013) Olive twig and branch dieback: etiology, incidence, and distribution in California. Plant Dis 97:231–244
Vaidya G, Lohman DJ, Meier R (2011) SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27:171–180
Valenzuela-Lopez N, Cano-Lira JF, Guarro J, Sutton DA, Wiederhold N, Crous PW, Stchigel AM (2018) Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud Mycol 90:1–69
Van der Aa HA (1975) The perfect state of Sarcophoma miribelii. Persoonia 8:283–289
Van der Aa HA, van Oorschot CAN (1985) A redescription of some genera with staurospores. Persoonia 12:415–425
Van der Aa HA, Vanev S (2002) A revision of the species described in Phyllosticta. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands
Van Rensburg JCJ, Lamprecht SC, Groenewald JZ, Castlebury LA, Crous PW (2006) Characterization of Phomopsis spp. associated with die-back of rooibos (Aspalathus linearis) in South Africa. Stud Mycol 55:65–74
Vanev SG, van der Aa HA (1998) An annotated list of the published names in Asteromella. Persoonia 17:47–67
Vasilyeva LN (2011) Quaternaria carpinicola, a comb. nov. (Diatrypaceae). Mycosphere 2:515–517
Venter E, Lennox C, Meitz-Hopkins J (2017) First report of Cytospora punicae causing post-harvest fruit rot on pomegranate in South Africa. Plant Dis 101:631
Verkley GJM (1999) A monograph of the genus Pezicula and its anamorphs. Stud Mycol 44:1–180
Verkley GJM (2001) On Sphaerographium petiolicola and a new species, S. tenuirostrum, taxa from a rarely collected genus of coelomycetes. Mycologia 93:205–211
Verkley GJM (2002) A revision of the genus Sphaerographium and the taxa assigned to Rhynchophoma (anamorphic Ascomycetes). Nova Hedwig 75:433–450
Verkley GJM, Dukik K, Renfurm R, Göker M, Stielow JB (2014) Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota). Persoonia 32:25–51
Verkley GJM, Priest MJ (2000) Septoria and similar coelomycetous anamorphs of Mycosphaerella. Stud Mycol 45:123–128
Verkley GJM, Zijlstra JD, Summerbell RC, Berendse F (2003) Phylogeny and taxonomy of root-inhabiting Cryptosporiopsis species, and C. rhizophila sp. nov., a fungus inhabiting roots of several Ericaceae. Mycol Res 107:689–698
Verkley GJM, Starink-Willemse M, van Iperen A, Abeln ECA (2004) Phylogenetic analyses of Septoria species based on the ITS and LSU-D2 regions of nuclear ribosomal DNA. Mycologia 96:558–571
Verkley GJM, Quaedvlieg W, Shin HD, Crous PW (2013) A new approach to species delimitation in Septoria. Stud Mycol 75:213–305
Vijaykrishna D, Jeewon R, Hyde KD (2006) Molecular taxonomy, origins and evolution of freshwater ascomycetes. Fungal Divers 23:351–390
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Vobis G, Hawksworth DL (1981) Conidial lichen-forming fungi. In: Cole GT, Kendrick B (eds) Biology of conidial fungi, vol 1. Academic, New York, pp 245–273
Von Arx JA (1957a) Revision der zu Gloeosporium Gestellten Pilze. Verh K Ned Akad Wet Amst 51:1–153
Von Arx JA (1957b) Three new fungi from North America. Acta Bot Neerl 6:381–385
Von Arx JA (1963) Revision der zu Gloeosporium gestellten Pilze. Nachträge und Berichtigungen. Verh K Ned Akad Wet Afd Nat Tweede Sect 66:172–182
Von Arx JA (1970) A revision of the fungi classified as Gloeosporium. Bibl Mycol 24:1–203
Von Arx JA (1983) Mycosphaerella and its anamorphs. Proc K Nederl Wet C 86:15–54
Von Arx J, Müller E (1954) Die Gattungen der amerosporen Pyrenomyceten. Beitr Kryptogamenfl Schweiz 11:1–434
Von Höhnel F (1905) Mycologische Fragmente. LXXVII-XCVII. Ann Mycol 3:323–339
Von Höhnel F (1909) Fragmente zur Mykologie. Sitzungsb Kaiserl Akad Wiss Math Nat Kl 118:813–904
Von Höhnel F (1911) Fragmente zur Mykologie. XIII Mitteilung (Nr. 642 bis 718). Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Math.-naturw. Klasse Abt I 120:379–484
Von Höhnel F (1915) Fragmente zur Mykologie no. 912. Über Sphaerographium Saccardo. Sitzungsber Kaiserl Akad Wiss Wien Math 124:100–103
Von Höhnel F (1916) Fragmente zur Mykologie (XVIII. Mitteilung, Nr. 944 bis 1000). Sitzungsber Kaiserl Akad Wiss Wien Math 125:1–112
Von Höhnel F (1917) Mycologische Fragmente. Ann Mycol 15:293–383
Von Höhnel F (1918a) Fragmente zur Mykologie XXII. Sitzungsber Kaiserl Akad Wiss Wien Math 127:549–634
Von Höhnel F (1918b) Mykologische Fragmente. Ann Myc Berl 16:35–174
Von Höhnel F (1919) Fünfte vorlăufige Mitteilung mycologischer Ergebnisse (Nr. 399–500). Ber Deutsch Bot Ges 37:153–161
Von Höhnel F (1920) Fungi imperfecti. Beiträge zur Kenntnis derselben. Hedwigia 62:56–89
Von Höhnel F (1924) Über die Gattung Neottiospora Desm. Mitt Bot Inst Technol Hochsch Wien 1:78–85
Von Höhnel F (1925) Über Psilonia discoidea Berk. et Br. Mitt Bot Inst Technol Hochsch Wien 2:62–63
Vucinic Z, Latinovic J (1999) Colletotrichum gloeosporioides, a new Olive (Olea europaea L.) parasite in Yugoslavia. Acta Hortic 474:577–580
Vujanovic V, St-Arnaud M (2003) A new species of Pseudorobillarda, an endophyte from Thuja occidentalis in Canada, and a key to the species. Mycologia 95:955–958
Walker J, Sutton BC (1974) Dilophia sacc. and Dilophospora desm. Trans Br Mycol Soc 62:231–241
Wanasinghe DN, Jones EBG, Camporesi E, Boonmee S, Ariyawansa HA, Wijayawardene NN, Mortimer PE, Xu JC, Yang JB, Hyde KD (2014) An exciting novel member of Lentitheciaceae in Italy from Clematis vitalba. Cryptogam Mycol 35:323–337
Wanasinghe DN, Hyde KD, Jeewon R, Crous PW, Wijayawardene NN, Jones EBG, Bhat DJ, Phillips AJL, Groenewald JZ, Dayarathne MC, Phukhamsakda C, Thambugala KM, Bulgakov TS, Camporesi E, Gafforov YS, Mortimer PE, Karunarathna SC (2017) Phylogenetic revision of Camarosporium (Pleosporineae, Dothideomycetes) and allied genera. Stud Mycol 87:207–256
Wanasinghe DN, Phukhamsakda C, Hyde KD, Jeewon R, Lee HB, Jones EBG, Tibpromma S, Tennakoon DS, Dissanayake AJ, Jayasiri SC, Gafforov Y, Camporesi E, Bulgakov TS, Ekanayake AH, Perera RH, Samarakoon MC, Goonasekara ID, Mapook A, Li WJ, Senanayake IC, Li JF, Norphanphoun C, Doilom M, Bahkali AH, Xu JC, Mortimer PE, Tibell L, Savic ST, Karunarathna SC (2018) Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers 89:1–236
Wang Y, Guo LD, Hyde KD (2005) Taxonomic placement of sterile morphotypes of endophytic fungi from Pinus tabulaeformis (Pinaceae) in northeast China based on rDNA sequences. Fungal Divers 20:235–260
Wang MM, Jin LT, Jiang CX, Hou CL (2009) Rhytisma huangshanense sp. nov. described from morphological and molecular data. Mycotaxon 108:73–82
Wang S, Cannon P, Li ZJ, Hou CL (2014a) Multigene phylogenetic analysis detects cryptic species of Tryblidiopsis in China. Mycologia 106:5–104
Wang S, Kirschner R, Wang YW, Hou CL (2014b) Hypohelion from China. Mycol Prog 13:781–789
Wang XL, Wei JL, Huang LL, Kang ZS (2011) Re-evaluation of pathogens causing Valsa canker on apple in China. Mycologia 103:317–324
Webber HJ (1897) Sooty mould of the orange and its treatment. U S Dep Agric Div Veg Phys Pathol Bull 13:1–14
Weber R, Webster J (2002) Teaching techniques for mycology: 18. Rhytisma acerinum, cause of tar-spot disease of sycamore leaves. Mycologist 16:120–123
Webster J (1955) Graminicolous pyrenomycetes. V. Conidial states of Leptosphaeria michotii, L. microscopica, Pleospora vagans and the perfect state of Dinemasporium graminum. Trans Br Mycol Soc 38:347–365
Webster J, Hudson HJ (1957) Graminicolous pyrenomycetes: IV. Conidia of Ophiobolus herpotrichus, Leptosphaeria luctuosa, L. fuckelii, L. pontiformis and L. eustomoides. Trans Br Mycol Soc 40:509–522
Wells K, Bandoni RJ (2001) Heterobasidiomycetes. In: McLaughlin DJ, McLaughlin EG, Lemke PA (eds) The Mycota. vol. VII part B. Systematics and evolution. Springer, Berlin, pp 85–120
Weresub LK, Pirozynski KA (1979) Pleomorphism of fungi as treated in the history of mycology and nomenclature. In: Kendrick B (ed) The whole fungus. National Museums of Canada, Ottawa, pp 17–25
Weymeyer LE (1966) Development of the ascocarp in Cryptornycina pteridis. Mycologia 58:752–760
Whelan MJ, Hunter T, Parker SR, Royle DJ (1997) How effective is Sphaerellopsis filum as a biological control agent of Melampsora willow rust? Asp Appl Biol 49:143–148
Whetzel HH, White WL (1940) Mollisia tetrica, Peziza sojournei, and the genera Phaeociboria and Pycnopeziza. Mycologia 32:609–620
White W, Whetzel H (1938) Pleomorphic life cycles in a new genus of the Helotiaceae. Mycologia 30:187–203
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Whitney HS, Reid J, Pirozynski KA (1975) Some new fungi associated with needle blight of conifers. Can J Bot 53:3051–3063
Wicht B, Petrini O, Jermini M, Gessler C, Broggini GAL (2012) Molecular, proteomic and morphological characterization of the ascomycete Guignardia bidwellii, agent of grape black rot: a polyphasic approach to fungal identification. Mycologia 104:1036–1045
Wijayawardene NN, Bhat DJ, Hyde KD, Camporesi E, Chethana KWT, Tangthirasunun N, Wang Y (2014a) Camarosporium sensu stricto in Pleosporinae, Pleosporales with two new species. Phytotaxa 183:16–26
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Dai DQ, D’souza MJ, Diederich P, Dissanayake A, Doilom M, Hongsanan S, Jones EBG, Groenewald JZ, Jayawardena R, Lawrey JD, Liu JK, Lücking R, Madrid H, Manamgoda DS, Muggia L, Nelsen MP, Phookamsak R, Suetrong S, Tanaka K, Thambugala KM, Wanasinghe DN, Wikee S, Zhang Y, Aptroot A, Ariyawansa HA, Bahkali AH, Bhat DJ, Gueidan C, Homnunti P, De Hoog GS, Knudsen K, Li WJ, McKenzie EHC, Miller AN, Phillips AJL, Piątek M, Raja HA, Shivas RS, Slippers B, Taylor JE, Tian Q, Wang Y, Woudenberg JHC, Cai L, Jaklitsch WM, Hyde KD (2014b) Naming and outline of Dothideomycetes-2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55
Wijayawardene NN, McKenzie EHC, Chukeatirote E, Wang Y, Hyde KD (2012a) Coelomycetes. Cryptogam Mycol 33:215–244
Wijayawardene NN, Udayanga D, McKenzie EHC, Wang Y, Hyde KD (2012b) The future of coelomycete studies. Cryptogam Mycol 33:381–391
Wijayawardene NN, Hyde KD, Wanasinghe DN, Papizadeh M, Goonasekara ID, Camporesi E, Bhat DJ, McKenzie EHC, Phillips AJL, Diederich P, Tanaka K, Li WJ, Tangthirasunun N, Phookamsak R, Dai DQ, Dissanayake AJ, Weerakoon G, Maharachchikumbura SSN, Hashimoto A, Matsumura M, Bahkali AH, Wang Y (2016) Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers 77:1–316
Wijayawardene NN, Hyde KD, Bhat DJ, Camporesi E, Schumacher RK, Chethana KWT, Wikee S, Bahkali AH, Wang Y (2014c) Camarosporium-like species are polyphyletic in Pleosporales; introducing Paracamarosporium and Pseudocamarosporium gen. nov. in Montagnulaceae. Cryptogam Mycol 35:177–198
Wijayawardene NN, Hyde KD, Rajeshkumar KC, Hawksworth DL, Madrid H, Kirk PM, Braun U, Singh RV, Crous PW, Kukwa M, Lücking R, Kurtzman CP, Yurkov A, Haelewaters D, Aptroot A, Lumbsch HT, Timdal E, Ertz D, Etayo J, Phillips AJL, Groenewald JZ, Papizadeh M, Selbmann L, Dayarathne MC, Weerakoon G, Jones EBG, Suetrong S, Tian Q, Castañeda-Ruiz RF, Bahkali AH, Pang K-L, Tanaka K, Dai DQ, Sakayaroj J, Hujslová M, Lombard L, Shenoy BD, Suija A, Maharachchikumbura SSN, Thambugala KM, Wanasinghe DN, Sharma BO, Gaikwad S, Pandit G, Zucconi L, Onofri S, Egidi E, Raja HA, Kodsueb R, Cáceres MES, Pérez-Ortega S, Fiuza PO, Monteiro JS, Vasilyeva LN, Shivas RG, Prieto M, Wedin M, Olariaga I, Lateef AA, Agrawal Y, Fazeli SAS, Amoozegar MA, Zhao GZ, Pfliegler WP, Sharma G, Oset M, Abdel-Wahab MA, Takamatsu S, Bensch K, de Silva NI, De Kesel A, Karunarathna A, Boonmee S, Pfister DH, Lu YZ, Luo ZL, Boonyuen N, Daranagama DA, Senanayake IC, Jayasiri SC, Samarakoon MC, Zeng XY, Doilom M, Quijada L, Rampadarath S, Heredia G, Dissanayake AJ, Jayawardana RS, Perera RH, Tang LZ, Phukhamsakda C, Hernández-Restrepo M, Ma XY, Tibpromma S, Gusmao LFP, Weerahewa D, Karunarathna SC (2017a) Notes for genera: Ascomycota. Fungal Divers 86:1–594
Wijayawardene NN, Hyde KD, Tibpromma S, Wanasinghe DN, Thambugala KM, Tian Q, Wang Y, Fu L (2017b) Towards incorporating asexual fungi in a natural classification: checklist and notes 2012–2016. Mycosphere 8:1457–1555
Wikee S, Wulandari NF, McKenzie EHC, Hyde KD (2012) Phyllosticta ophiopogonis sp. nov. from Ophiopogon japonicus (Liliaceae). Saudi J Biol Sci 19:13–16
Wikee S, Lombard L, Nakashima C, Motohashi K, Chukeatirote E, Cheewangkoon R, McKenzie EHC, Hyde KD, Crous PW (2013) A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Stud Mycol 76:1–29
Wingfield MJ, De Beer ZW, Slippers B, Wingfield BD, Groenewald JZ, Lombard L, Crous PW (2012) One fungus one name promotes progressive plant pathology. Mol Plant Pathol 13:604–613
Winter G (1887) Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. Die Pilze Deutschlands, Oesterreichs und der Schweiz. Ascomyceten: Gymnoasceen und Pyrenomyceten, 2nd edn. Kummer, Leipzig
Wolf FA (1912) The perfect stage of Actinonema rosae. Bot Gaz 54:218–234
Wong SW, Hyde KD (1999) Proboscispora aquatica gen. et sp. nov., from wood submerged in freshwater. Mycol Res 103:81–87
Wong M, Crous PW, Henderson J, Groenewald JZ, Drenth A (2012) Phyllosticta species associated with freckle disease of banana. Fungal Divers 56:173–187
Wu WP, Sutton BC (1995) Additions to the genus Xenidiocercus (coelomycetes) from Ghana. Mycoscience 36:271–275
Wu SP, Liu YX, Yuan J, Wang Y, Hyde KD, Liu ZY (2014) Phyllosticta species from banana (Musa sp.) in Chongqing and Guizhou Provinces, China. Phytotaxa 188:135–144
Xu C, Wang C, Ju L, Zhang R (2015) Multiple locus genealogies and phenotypic characters reappraise the causal agents of apple ring rot in China. Fungal Divers 71:215–231
Yadav S, Bhat DJ (2009) Dimastigosporium yanense, a new coprophilous fungus from the forests of Western Ghats in Karnataka State, India. Mycotaxon 107:397–403
Yamamoto W (1961) Species of the genera of Glomerella and Guignardia with special reference to their imperfect stages. Sci Rep Hyogo Univ Agric 5:1–12
Yang YL, Liu ZY, Cai L, Hyde KD, Yu ZN, McKenzie EHC (2009) Colletotrichum anthracnose of Amaryllidaceae. Fungal Divers 39:123–146
Yang H, Ariyawansa HA, Wu HX, Hyde KD (2014) The genus Leptoxyphium (Capnodiaceae) from China. Phytotaxa 176:174–183
Yang T, Groenewald JZ, Cheewangkoon R, Jami F, Abdollahzadeh J, Lombard L, Crous PW (2017) Families, genera and species of Botryosphaeriales. Fungal Biol 121:322–346
Yang Q, Fan XL, Guarnaccia V, Tian CM (2018) High diversity of Diaporthe species associated with dieback diseases in China, with twelve new species described. MycoKeys 149:97–149
Yanna, Hyde KD, Goh TK (1998a) Koorchaloma novojournalis sp. nov., a new sporodochial fungus from Hong Kong. Fungal Divers 1:193–197
Yanna, Hyde KD, Goh TK (1998b) Staurophoma calami, a new coelomycete from Hong Kong. Sydowia 50:139–143
Yerkes Jr. WD (1956) Chaetoseptoria wellmani in Mexico. Mycologia 48:738–740
Yuan ZQ, Mohammed C (1997) Two new coelomycetes with appendaged conidia on stems of eucalypts from Australia. Mycol Res 101:1531–1534
Yuan Z, Verkley GJM (2014) Pezicula neosporulosa sp. nov. (Helotiales, Ascomycota), an endophytic fungus associated with Abies spp. in China and Europe. Mycoscience 56:205–213
Yuan ZW, Pei MH, Hunter T, Ruiz C, Royle DJ (1999) Pathogenicity to willow rust, Melampsora epitea, of the mycoparasite Sphaerellopsis filum from different sources. Mycol Res 103:509–512
Zalasky H (1974) Keissleriella emergens, a perfect state of Diplodia tumefaciens in roots of poplar. Can J Bot 52:11–13
Zeng ZQ, Zhuang WY (2015) A new species of Nectria (Nectriaceae, Hypocreales) with multiseptate ascospores. Nova Hedwig 101:327–334
Zeng ZQ, Zhuang WY (2016) Revision of the genus Thyronectria (Hypocreales) from China. Mycologia 108:1130–1140
Zeng ZQ, Zhuang WY, Yu ZH (2018) New species and new Chinese records of Nectriaceae from Tibet, China. Nova Hedwig 106:283–294
Zeng XY, Wu HX, Hongsanan S, Jeewon R, Wen TC, Maharachchikumbura SSN, Chomnunti P, Hyde KD (2019) Taxonomy and the evolutionary history of Micropeltidaceae. Fungal Divers 97:393–436
Zhang Y, Schoch CL, Fournier J, Crous PW, de Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD (2009) Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang H, Hyde KD, McKenzie EHC, Bahkali AH, Zhou DQ (2012a) Sequence data reveals phylogenetic affinities of Acrocalymma aquatica sp. nov., Aquasubmersa mircensis gen. et sp. nov. and Clohesyomyces aquaticus (freshwater coelomycetes). Cryptogam Mycol 33:333–346
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012b) Pleosporales. Fungal Divers 53:1–221
Zhang M, Lin S, He W, Zhang Y (2017) Three species of Neofusicoccum (Botryosphaeriaceae, Botryosphaeriales) associated with woody plants from southern China. Mycosphere 8:797–808
Zhang JF, Liu JK, Jeewon R, Wanasinghe DN, Liu ZY (2019) Fungi from Asian Karst formations III. Molecular and morphological characterization reveal new taxa in Phaeosphaeriaceae. Mycosphere 10:202–220
Zhao GC, Zhao RL (2012) The higher microfungi from forests of Yunnan Province. Yunnan Science and Technology Press, Kunming, pp 1–572
Zhao RL, Yang YM, Zhao GC (2004) Pseudolachnella vermospora sp. nov. from Yushania vigens in China. Fungal Divers 15:255–260
Zhaxybayeva O, Gogarten JP (2002) Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genom 3:4
Zhou YP, Zhang M, Dou ZhP, Zhang Y (2017) Botryosphaeria rosaceae sp. nov. and B. ramosa, new botryosphaeriaceous taxa from China. Mycosphere 8:162–171
Zhu HY, Tian CM, Fan XL (2018) Multigene phylogeny and morphology reveal Cytospora spiraeae sp. nov. (Diaporthales, Ascomycota) in China. Phytotaxa 338:49–62
Zhu HY, Pan M, Bonthond G, Tian CM, Fan XL (2019) Diaporthalean fungi associated with canker and dieback of trees from Mount Dongling in Beijing, China. MycoKeys 59:67–94
Acknowledgements
Kevin D. Hyde thanks the 2019 high-end foreign expert introduction plan (granted by the Ministry of Science and Technology of the People’s Republic of China, Grant Number G20190139006) and the Thailand Research Fund entitled “Impact of climate Change on fungal diversity and biogeography in the greater Mekong subregion” (RDG6130001). Wen-Jing Li, Qing Tian, Qiu-Ju Shang thank the Mushroom Research Foundation (MRF), Chiang Rai Province, Thailand for providing Postgraduate Scholarships. Wen-Jing Li would like to thank Ya-Ting Li, Li-Han Sheng for help re-drawing part of photos. Wen-Jing Li is also grateful to Assistant Prof. Huang Zhang, Dr. Putarak Chomnunti for images of Aquasubmersa mircensis, Clohesyomyces aquaticus, Neopyrenochaeta annellidica and Scorias spongiosa, and Prof. Alan J.L. Phillips, Xiao-Ya Ma, Yuan-Pin Xiao, Sheng-Nan Zhang, Jing Yang, Ming Zeng, Yong-Zhong Lu for assistance and valuable suggestions. Wen-Jing Li would like to thank the Molecular Biology Experimental Center at Kunming Institute of Botany for facilities of molecular work. We would like to thank the curators of the herbarium CUP, DAOM, FH, HAL, HHUF, IMI, K, and PREM for loaning herbarium specimens and for being very helpful in locating specimens. Without their help this work would not have been possible. The abbreviations of herbarium are those listed in Index Herbariorum (2019). We also wish to acknowledge Saranyaphat Boonmee, curator of MFLU (Mae Fah Luang University) and Liu Ende, Assistant Curator, Herbarium, Kunming Institute of Botany, Chinese Academy of Sciences (KUN), Kunming, for arranging the loan of specimens from various herbaria. We also thank the technical staff of Center of Excellence in Fungal Research, Sornram Sukpisit and Wilawan Punyaboon for their invaluable assistance. We also would like to thank Shaun Pennycook for assistance in checking the name of new taxa. We would like to thank Prof. Crous for providing culture from CBS (Westerdijk Fungal Biodiversity Institute). Erio Camporesi is grateful to Giancarlo Lombardi, Sergio Montanari and Gigi Stagioni for their help in identifying host plants of fresh collections. Dong-Qin Dai would like to thank the National Natural Science Foundation of China (No. NSFC 31760013), the Scientific Research Foundation of Yunnan Provincial Department of Education (2017ZZX186) and the Thousand Talents Plan, Youth Project of Yunnan Provinces for finance support. Samantha C. Kaunarathna thanks CAS President's International Fellowship Initiative (PIFI) for funding his postdoctoral research (No. 2018PC0006) and the National Science Foundation of China (NSFC) for funding this work under the project code 31851110759.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, WJ., McKenzie, E.H.C., Liu, JK.(. et al. Taxonomy and phylogeny of hyaline-spored coelomycetes. Fungal Diversity 100, 279–801 (2020). https://doi.org/10.1007/s13225-020-00440-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-020-00440-y