Abstract
This article is the 14th in the Fungal Diversity Notes series, wherein we report 98 taxa distributed in two phyla, seven classes, 26 orders and 50 families which are described and illustrated. Taxa in this study were collected from Australia, Brazil, Burkina Faso, Chile, China, Cyprus, Egypt, France, French Guiana, India, Indonesia, Italy, Laos, Mexico, Russia, Sri Lanka, Thailand, and Vietnam. There are 59 new taxa, 39 new hosts and new geographical distributions with one new combination. The 59 new species comprise Angustimassarina kunmingense, Asterina lopi, Asterina brigadeirensis, Bartalinia bidenticola, Bartalinia caryotae, Buellia pruinocalcarea, Coltricia insularis, Colletotrichum flexuosum, Colletotrichum thasutense, Coniochaeta caraganae, Coniothyrium yuccicola, Dematipyriforma aquatic, Dematipyriforma globispora, Dematipyriforma nilotica, Distoseptispora bambusicola, Fulvifomes jawadhuvensis, Fulvifomes malaiyanurensis, Fulvifomes thiruvannamalaiensis, Fusarium purpurea, Gerronema atrovirens, Gerronema flavum, Gerronema keralense, Gerronema kuruvense, Grammothele taiwanensis, Hongkongmyces changchunensis, Hypoxylon inaequale, Kirschsteiniothelia acutisporum, Kirschsteiniothelia crustaceum, Kirschsteiniothelia extensum, Kirschsteiniothelia septemseptatum, Kirschsteiniothelia spatiosum, Lecanora immersocalcarea, Lepiota subthailandica, Lindgomyces guizhouensis, Marthe asmius pallidoaurantiacus, Marasmius tangerinus, Neovaginatispora mangiferae, Pararamichloridium aquisubtropicum, Pestalotiopsis piraubensis, Phacidium chinaum, Phaeoisaria goiasensis, Phaeoseptum thailandicum, Pleurothecium aquisubtropicum, Pseudocercospora vernoniae, Pyrenophora verruculosa, Rhachomyces cruralis, Rhachomyces hyperommae, Rhachomyces magrinii, Rhachomyces platyprosophi, Rhizomarasmius cunninghamietorum, Skeletocutis cangshanensis, Skeletocutis subchrysella, Sporisorium anadelphiae-leptocomae, Tetraploa dashaoensis, Tomentella exiguelata, Tomentella fuscoaraneosa, Tricholomopsis lechatii, Vaginatispora flavispora and Wetmoreana blastidiocalcarea. The new combination is Torula sundara. The 39 new records on hosts and geographical distribution comprise Apiospora guiyangensis, Aplosporella artocarpi, Ascochyta medicaginicola, Astrocystis bambusicola, Athelia rolfsii, Bambusicola bambusae, Bipolaris luttrellii, Botryosphaeria dothidea, Chlorophyllum squamulosum, Colletotrichum aeschynomenes, Colletotrichum pandanicola, Coprinopsis cinerea, Corylicola italica, Curvularia alcornii, Curvularia senegalensis, Diaporthe foeniculina, Diaporthe longicolla, Diaporthe phaseolorum, Diatrypella quercina, Fusarium brachygibbosum, Helicoma aquaticum, Lepiota metulispora, Lepiota pongduadensis, Lepiota subvenenata, Melanconiella meridionalis, Monotosporella erecta, Nodulosphaeria digitalis, Palmiascoma gregariascomum, Periconia byssoides, Periconia cortaderiae, Pleopunctum ellipsoideum, Psilocybe keralensis, Scedosporium apiospermum, Scedosporium dehoogii, Scedosporium marina, Spegazzinia deightonii, Torula fici, Wiesneriomyces laurinus and Xylaria venosula. All these taxa are supported by morphological and multigene phylogenetic analyses. This article allows the researchers to publish fungal collections which are important for future studies. An updated, accurate and timely report of fungus-host and fungus-geography is important. We also provide an updated list of fungal taxa published in the previous fungal diversity notes. In this list, erroneous taxa and synonyms are marked and corrected accordingly.
Similar content being viewed by others
Table of contents
Phylum Ascomycota R.H. Whittaker
Subphylum Pezizomycotina O.E. Erikss. & Winka
Class Dothideomycetes O.E. Erikss. & Winka
Subclass Dothideomycetidae P.M. Kirk et al.
Mycosphaerellales (Nannf.) P.F. Cannon
Mycosphaerellaceae Lindau
1512. Pseudocercospora vernoniae Archana Singh & N.K. Dubey, sp. nov. (contributed by A. Singh and N.K. Dubey)
Subclass Pleosporomycetidae C.L. Schoch et al.
Kirschsteiniotheliales Hern.-Restr. et al.
Kirschsteiniotheliaceae Boonmee & K.D. Hyde
1513. Kirschsteiniothelia acutisporum S. Wang, Q. Zhao & K.D. Hyde, sp. nov. (contributed by S. Wang and Y.R. Sun)
1514. Kirschsteiniothelia crustaceum S. Wang, Q. Zhao & K.D. Hyde, sp. nov. (contributed by S. Wang and Y.R. Sun)
1515. Kirschsteiniothelia extensum. S. Wang, Q. Zhao & K.D. Hyde, sp. nov. (contributed by S. Wang, K.D. Hyde and Y.R. Sun)
1516. Kirschsteiniothelia septemseptatum S. Wang, Q. Zhao & K.D. Hyde, sp. nov. (contributed by S. Wang and Y.R. Sun)
1517. Kirschsteiniothelia spatiosum S. Wang, Q. Zhao & K.D. Hyde, sp. nov. (contributed by S. Wang and Y.R. Sun)
Pleosporales Luttrell ex M.E. Barr
Amorosiaceae Thambug. & K.D. Hyde
1518. Angustimassarina kunmingense H.D. Yang & K.D. Hyde, sp. nov. (contributed by H. Yang, K.D. Hyde and C. Bhunjun)
Bambusicolaceae D.Q. Dai & K.D. Hyde
1519. Bambusicola bambusae D.Q. Dai & K.D. Hyde, new host/substrate record from Thailand (contributed by D.F. Bao and K.D. Hyde)
1520. Corylicola italica Wijesinghe, Camporesi, Yong Wang bis & K.D. Hyde, new host record from Italy (contributed by P. Pahoua and E. Camporesi)
1521. Palmiascoma gregariascomum Phookamsak & K.D. Hyde, new host record from Thailand (contributed by M.C. Samarakoon)
Coniothyriaceae W.B. Cooke
1522. Coniothyrium yuccicola Chaiwan, Jayaward., Bulgakov & K.D. Hyde, sp. nov. (Contributed by N. Chaiwan and T.S. Bulgakov)
Didymellaceae Gruyter, Aveskamp & Verkley
1523. Ascochyta medicaginicola Q. Chen & L. Cai, new host record from Russia (contributed by N. Chaiwan and T.S. Bulgakov)
Didymosphaeriaceae Munk
1524. Spegazzinia deightonii (S. Hughes) Subram., new host record from Thailand (contributed by D. Bundhun and B.C. Samarakoon)
Lindgomycetaceae K. Hiray. et al.
1525. Hongkongmyces changchunensis Phukhams., W.X. Su, & Y. Li, sp. nov. (contributed by C. Phukhamsakda, K.D. Hyde and W.X. Su)
1526. Lindgomyces guizhouensis J. Ma, Y.Z. Lu & K.D. Hyde, sp. nov. (contributed by J. Ma, J. Y. Zhang and Y.Z. Lu)
Lophiostomataceae Sacc
1527. Neovaginatispora mangiferae Tennakoon, M.S. Calabon, E.B.G. Jones, K.D. Hyde, sp. nov. (contributed by M. S. Calabon, K.D. Hyde and D.S Tennakoon)
1528. Vaginatispora flavispora M.S. Calabon, E.B.G. Jones, K.D. Hyde, sp. nov. (contributed by M. S. Calabon, K.D. Hyde and E.B.G. Jones)
Phaeoseptaceae S. Boonmee, Thambugala & K.D. Hyde
1529. Phaeoseptum thailandicum Samarak. & K.D. Hyde, sp. nov. (contributed by M. C. Samarakoon and K.D. Hyde)
1530. Pleopunctum ellipsoideum N.G. Liu, K.D. Hyde & J.K. Liu, new host record from Thailand (contributed by Y.R. Sun)
Phaeosphaeriaceae M.E. Barr
1531. Nodulosphaeria digitalis W.J. Li, Camporesi, Bhat & K.D. Hyde, new host record from Italy (contributed by D. Bundhun)
Pleosporaceae Nitschke
1532. Bipolaris luttrellii Alcorn, new host record from China (contributed by K.M. Thambugala and R.S. Jayawardena)
1533. Curvularia alcornii Manamgoda, L. Cai & K. D. Hyde, new host record Sri Lanka (contributed by H.S. Ferdinandez and D.S. Manamgoda)
1534. Curvularia senegalensis (Speg.) Subram., new host record from Sri Lanka (contributed by H.S. Ferdinandez and D.S. Manamgoda)
1535. Pyrenophora verruculosa Madrid & Cantillo, sp. nov. (contributed by H. Madrid and T. Cantillo)
Tetraplosphaeriaceae Kaz. Tanaka & K. Hiray
1536. Tetraploa dashaoensis C.F. Liao & Doilom, sp. nov. (contributed by C.F. Liao, K.D. Hyde and M. Doilom)
Torulaceae Corda
1537. Torula fici Crous, new host record from Taiwan and Thailand (contributed by B.C. Samarakoon, D.S. Tennakoon and P. Chomnunti)
1538. Torula sundara (Subram.) Y.R. Sun, Yong Wang bis & K.D. Hyde, comb. nov. (contributed by Y.R. Sun)
Periconiaceae (Sacc.) Nann.,
1539. Periconia byssoides Pers., new host and geographical record from India (contributed by S. Mahadevakumar, Y.S. Deepika, N. Lakshmidevi and S. S. N. Maharachchikumbura)
1540. Periconia cortaderiae Thambugala & K.D. Hyde, new host and geographical record from Russia (contributed by B.C. Samarakoon and T.S. Bulgakov)
Tubeufiales Boonmee & K.D. Hyde
Tubeufiaceae M.E. Barr
1541. Helicoma aquaticum Y.Z. Lu, J.C. Kang & K.D. Hyde, new host record from Thailand (contributed by X. Tang)
Wiesneriomycetaceae Suetrong, Rungjind., Somrith. & E.B.G. Jones
1542. Wiesneriomyces laurinus (Tassi) P.M. Kirk, new host record from China (contributed by Y. Yang and I.S. Manawasinghe)
Dothideomycetes orders incertae sedis
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss
Asterinaceae Hansf
1543. Asterina brigadeirensis A.L. Firmino & O.L. Pereira, sp. nov. (contributed by O. L. Pereira and A. L. Firmino)
1544. Asterina lopi A.L. Firmino & O.L. Pereira, sp. nov. (contributed by O. L. Pereira and A. L. Firmino)
Botryosphaeriales C.L. Schoch, Crous & Shoemaker
Aplosporellaceae Slippers, Boissin & Crous
1545. Aplosporella artocarpi Trakun., L. Lombard & Crous, new host record from Thailand (contributed by Z. H. Htet and A. Mapook)
Botryosphaeriaceae Theiss. & Syd. [as 'Botryosphaeriacae']
1546. Botryosphaeria dothidea (Moug.) Ces. & De Not., new geographical and habitat record from China (contributed by H. Yang and R.S. Jayawardena)
Class Laboulbeniomycetes Engler
Laboulbeniales Lindau
Laboulbeniaceae G. Winter
1547. Rhachomyces cruralis W. Rossi & M. Leonardi, sp. nov. (contributed by W. Rossi & M. Leonardi)
1548. Rhachomyces hyperommae W. Rossi & M. Leonardi, sp. nov. (contributed by W. Rossi & M. Leonardi)
1549. Rhachomyces magrinii W. Rossi & M. Leonardi, sp. nov. (contributed by W. Rossi & M. Leonardi)
1550. Rhachomyces platyprosophi W. Rossi & M. Leonardi, sp. nov. (contributed by W. Rossi & M. Leonardi)
Class Lecanoromycetes O.E. Erikss. & Winka
Subclass Lecanoromycetidae P.M. Kirk et al.
Caliciales Bessey
Caliciaceae Chevall
1551. Buellia pruinocalcarea Aptroot, M.F. Souza & Spielmann, sp. nov. (contributed by Aptroot, Souza and Spielmann)
Lecanorales Nannf
Lecanoraceae Körb
1552. Lecanora immersocalcarea Aptroot, M.F. Souza & Spielmann, sp. nov. (contributed by Aptroot, Souza and Spielmann)
Teloschistales D. Hawksw. & O.E. Erikss
Teloschistaceae Zahlbr
1553. Wetmoreana blastidiocalcarea Aptroot, M.F. Souza & Spielmann, sp. nov. (contributed by Aptroot, Souza and Spielmann)
Class Leotiomycetes O.E. Erikss. & Winka
Phacidiales C.E. Bessey
Phacidiaceae Fr
1554. Phacidium chinense G.C. Ren & K.D. Hyde, sp. nov. (contributed by G.C. Ren and K.D. Hyde)
Class Sordariomycetes O.E. Erikss. & Winka
Subclass Diaporthomycetidae Senan., Maharachch. & K.D. Hyde
Diaporthaceae Höhn. ex Wehm
1555. Diaporthe foeniculina (Sacc.) Udayanga & Castl., new host record from Italy (contributed by P. D. Abeywickrama and E. Camporesi)
1556. Diaporthe longicolla (Hobbs) J.M. Santos, Vrandečić & A.J.L. Phillips, new host record from India (contributed by S. Mahadevakumar, Y.S. Deepika, N. Lakshmidevi and S. S. N. Maharachchikumbura)
1557. Diaporthe phaseolorum (Cooke & Ellis) Sacc., new host record from India (contributed by S. Mahadevakumar, Y.S. Deepika, N. Lakshmidevi and S. S. N. Maharachchikumbura)
Melanconiellaceae Senan., Maharachch. & K.D. Hyde
1558. Melanconiella meridionalisVoglmayr & Jaklitsch, new host and geographical record from Italy (contributed by N. I. de Silva and E. Camporesi)
Pararamichloridiales Crous
Pararamichloridiaceae Crous
1559. Pararamichloridium aquisubtropicum J.Y. Zhang, Y.Z. Lu & K.D. Hyde, sp. nov. (contributed by J.Y. Zhang, J.Ma, Y.Z. Lu, and K.D. Hyde)
Distoseptisporales Z.L. Luo, K.D. Hyde & H.Y. Su
Distoseptisporaceae K.D. Hyde & McKenzie
1560. Distoseptispora bambusicola X. Tang, Jayaward., J.C Kang & K.D. Hyde sp. nov. (contributed by X. Tang).
Glomerellales Chadef. ex Réblová et al
Glomerellaceae Locq. ex Seifert & W. Gams
1561. Colletotrichum aeschynomenes B.S. Weir & P.R. Johnst., new host record from Thailand (contributed by D. Gomdola and R.S. Jayawardena)
1562. Colletotrichum flexuosum Damm, sp. nov. (contributed by U. Damm)
1563. Colletotrichum pandanicola Tibpromma & K.D. Hyde, new host records from India and Thailand, geographical record from India (contributed by S. Mahadevakumar, Y.S. Deepika, N. Lakshmidevi, S. S. N. Maharachchikumbura and R.S. Jayawardena)
1564. Colletotrichum thasutense Armand, K.D. Hyde, Jayaward., sp. nov. (contributed by A. Armand and R.S. Jayawardena)
Hypocreales Lindau
Nectriaceae Tul. & C. Tul
1565. Fusarium brachygibbosum Padwick, new host record from India (contributed by S. Mahadevakumar, Y.S. Deepika, N. Lakshmidevi and S.S.N. Maharahchikumbura)
1566. Fusarium purpurea S.L. Han, M. Raza, W.J. Duan & L. Cai, sp. nov. (contributed by S.L. Han and M. Raza)
Microascales Luttr
Microascaceae Luttr. ex Malloch
1567. Scedosporium apiospermum Sacc. ex Castell. & Chalm., a new host record from Thailand (contributed by A. J. Gajanayake)
1568. Scedosporium dehoogii Gilgado, new record from India (contributed by Devadatha and Sarma)
1569. Scedosporium marina Devadatha & V.V Sarma, sp. nov. (contributed by Devadatha and Sarma)
Hypocreomycetidae incertae sedis (Rhexoacrodictys and Dematipyriforma clade)
1570. Dematipyriforma aquatica Abdel-Aziz &Abdel-Wahab, sp. nov. (contributed by Abdel-Aziz and Abdel-Wahab)
1571. Dematipyriforma globispora Abdel-Aziz &Abdel-Wahab, sp. nov. (contributed by Abdel-Aziz and Abdel-Wahab)
1572. Dematipyriforma nilotica Abdel-Aziz &Abdel-Wahab, sp. nov. (contributed by Abdel-Aziz and Abdel-Wahab)
Subclass Savoryellomycetidae Hongsanan, K.D. Hyde & Maharachch
Coniochaetales Huhndorf, A.N. Mill. & F.A. Fernández
Coniochaetaceae Malloch and Cain
1573. Coniochaeta caraganae D. Pem, Bulgakov & K.D. Hyde, sp. nov. (Contributed by D. Pem, T.S. Bulgakov and M. Raza)
Pleurotheciales Réblová & Seifert
Pleurotheciaceae Réblová & Seifert
1574. Rhexoacrodictys erecta (Ellis & Everh.) W.A. Baker & Morgan-Jones, in Baker, Partridge & Morgan-Jones, Mycotaxon 82: 99 (2002) new host record from Thailand (contributed by X.G. Tian and S. Tibpromma)
1575. Phaeoisaria goiasensis H.M. Silva, A.D. Cavalcanti & J.D.P. Bezerra, sp. nov. (contributed by H.M. Silva, A.D. Cavalcanti and J.D.P. Bezerra)
1576. Pleurothecium aquisubtropicum J. Ma, Y.Z. Lu & K.D. Hyde, sp. nov (contributed by J. Ma, J.Y. Zhang and Y.Z. Lu)
Subclass Xylariomycetidae O.E. Erikss & Winka
Amphisphaeriales D Hawksw & OE Erikss
Apiosporaceae K.D. Hyde, J. Fröhl., Joanne E. Taylor & M.E. Barr
1577. Apiospora guiyangensis Samarak., Jian K. Liu & K.D. Hyde, new host record from China (contributed by D.P. Wei)
Sporocadaceae Corda
1578. Bartalinia bidenticola Htet, Mapook & K.D. Hyde, sp.nov (contributed by Z. H. Htet, K.D. Hyde and A. Mapook)
1579. Bartalinia caryotae Senan., Kular. & K.D. Hyde, sp. nov. (contributed by I.C. Senanayake and N. D. Kularathnage)
1580. Pestalotiopsis piraubensis V.P. Abreu & O.L. Pereira, sp. nov. (contributed by V.P. Abreu and O.L. Pereira)
Xylariales Nannf
Diatrypaceae Nitschke
1581. Diatrypella quercina (Pers.) Cooke, new host record from Russia (contributed by S. N. Wijesinghe and T.S. Bulgakov)
Hypoxylaceae DC
1582. Hypoxylon inaequale S.C. He & Jayaward., sp. nov (contributed by S.C. He)
Xylariaceae Tul. & C. Tul
1583. Astrocystis bambusicola R.H. Perera & K.D. Hyde, new host record from China (contributed by D.P. Wei)
1584. Xylaria venosula Speg., new geographical record from India (contributed by M. Niranjan and V. V. Sarma)
Phylum Basidiomycota R.T. Moore
Subphylum Agaricomycotina Doweld
Class Agaricomycetes Doweld
Agaricales Underw
Agaricaceae Chevall
1585. Chlorophyllum squamulosum A.K. Dutta, Soumili Bera & K. Acharya, new record from Thailand (contributed by J. Kumla and N. Suwannarach)
1586. Lepiota metulispora (Berk. & Broome) Sacc., new record from Laos (contributed by P. Sysouphanthong and N. Thongklang)
1587. Lepiota pongduadensis Sysou., new record from Laos (contributed by P. Sysouphanthong and N. Thongklang)
1588. Lepiota subthailandica Sysouph., K.D. Hyde & Thongkl., sp. nov (contributed by P. Sysouphanthong and N. Thongklang)
1589. Lepiota subvenenata Hai J. Li, Y.Z. Zhang & C.Y. Sun, new record from Laos (contributed by P. Sysouphanthong and N. Thongklang)
Atheliales Jülich
Atheliaceae Jülich
1590. Athelia rolfsii (Curzi) C.C. Tu & Kimbr., new record from India (contributed by S. Mahadevakumar, Y.S. Deepika, N. Lakshmidevi and S. S. N. Maharachchikumbura)
Hymenochaetales Oberw
Hymenochaetaceae Donk
1591. Coltricia insularis P.-A. Moreau, Bellanger, Loizides & A. Rinaldi, sp. nov. (contributed by P.-A. Moreau, Bellanger, Loizides and A. Rinaldi)
1592. Fulvifomes jawadhuvensis Kezo, K., Gunaseelan, S., & Kaliyaperumal, M., sp. nov. (contributed by K. Kezo, S. Gunaseelan, M. Kaliyaperumal and T. Luangharn)
1593. Fulvifomes malaiyanurensis Gunaseelan, S., Kezo, K. & Kaliyaperumal, M., sp. nov. (contributed by contributed by K. Kezo, S. Gunaseelan, M. Kaliyaperumal and T. Luangharn)
1594. Fulvifomes thiruvannamalaiensis Gunaseelan, S., Kezo, K. and Kaliyaperumal, M., sp. nov. (contributed by contributed by K. Kezo, S. Gunaseelan, M. Kaliyaperumal and T. Luangharn)
Hymenogastraceae Vittad
1595. Psilocybe keralensis K.A. Thomas, Manim. & Guzmán, new record from Thailand (contributed by N. Suwannarach and J. Kumla)
Marasmiaceae Roze ex Kühner
1596. Marasmius pallidoaurantiacus Wannathes, N. Suwannarach, J. Kumla & S. Lumyong, sp. nov. (contributed by N. Wannathes, N. Suwannarach, J. Kumla and S. Lumyong)
1597. Marasmius tangerinus Wannathes, N. Suwannarach, J. Kumla & Lumyong, sp. nov. (contributed by N. Wannathes, N. Suwannarach, J. Kumla and S. Lumyong)
Physalacriaceae Corner
1598. Rhizomarasmius cunninghamietorum Chun Y. Deng, J.P. Li & Gafforov, sp. nov. (contributed by Chun Y. Deng, J.P. Li and Y. Gafforov)
Polyporales Gäum
Polyporaceae Fr. ex Corda
1599. Grammothele taiwanensis C.C. Chen, sp. nov. (contributed by C.C. Chen)
Incrustoporiaceae Jülich
1600. Skeletocutis cangshanensis B.K. Cui & Shun Liu, sp. nov. (contributed by B.K. Cui and Shun Liu)
1601. Skeletocutis subchrysella B.K. Cui & Shun Liu, sp. nov. (contributed by B.K. Cui and Shun Liu)
Psathyrellaceae Vilgalys, Moncalvo & Redhead,
1602. Coprinopsis cinerea (Schaeff.) Redhead, Vilgalys & Moncalvo, new record from India (contributed by S. Mahadevakumar, Y.S. Deepika, N. Lakshmidevi and S.S.N. Maharachchikumbura)
Thelephorales Corner ex Oberw
Thelephoraceae Chevall
1603. Tomentella exiguelata Y.H. Mu & H.S. Yuan, sp. nov. (contributed Y.H. Mu, T. Cao and H.S. Yuan)
1604. Tomentella fuscoaraneosa Y.H. Mu & H.S. Yuan, sp. nov. (contributed Y.H. Mu, T. Cao and H.S. Yuan).
Agaricales genera incertae sedis
1605. Gerronema atrovirens Wannathes, N. Suwannarach, J. Kumla, Phonrob & S. Lumyong, sp. nov. (contributed by N Wannathes, N Suwannarach J Kumla and S Lumyong)
1606. Gerronema flavum Wannathes, N. Suwannarach, J. Kumla, Phonrob & S. Lumyong, sp. nov. (contributed by N Wannathes, N Suwannarach J Kumla and S Lumyong)
1607. Gerronema keralense K. P. D. Latha & Manim, new record from Thailand (contributed by N Wannathes, N Suwannarach J Kumla, S Khuna, W Phonrob and S Tabtan)
1608. Gerronema kuruvense K. P. D. Latha & Manim, new record from Thailand (contributed by N Wannathes, N Suwannarach J Kumla, S Khuna, W Phonrob and S Tabtan)
1609. Tricholomopsis lechatii Courtec., S. Dumez, S. Welti & P.-A. Moreau, sp. nov. (contributed by Courtec., S. Dumez, S. Welti and P.-A. Moreau)
Subphylum Ustilaginomycotina Doweld
Class Ustilaginomycetes R. Bauer et al.
Ustilaginales G. Winter
Ustilaginaceae Tul & C. Tul
1610. Sporisorium anadelphiae-leptocomae T. Denchev, Denchev, Kemler, M.P. Martín & Begerow, sp. nov. (contributed by T. Denchev, Denchev, Kemler, M.P. Martín and Begerow)
Introduction
Fungi play a key role in many biological processes, influencing ecosystems (Schimann et al. 2017). They are saprobes, epiphytes, endophytes, animal and plant pathogens or symbionts (Chethana et al. 2021a, b). High species diversity in fungi exhibits a huge variation in morphology, lifestyles and the mode of dispersal (Hyde et al. 2018). Fungi are also important in biotechnological applications (Hyde et al. 2019).
The current estimate of fungal diversity is highly uncertain, ranging from 1.5 to 12 million species (Wu et al. 2019; Hyde et al. 2021; Bhunjun et al. 2022). Of this massive number, only around 150,000 species have been named and classified to date. With the introduction of DNA-based techniques in species delimitation, the newly described taxa per year have dramatically increased. Whether these newly introduced taxa are novel is another challenge the mycologists face. With only 10% of fungi being named and classified, many species remain to be discovered (Hyde et al. 2021). Some species are poorly described and lack molecular data. This can be overcome if we collect, isolate, sequence and provide new data on fungi from different hosts and habitats. Identification of new taxa, recollection of already known taxa, the establishment of reference specimens and epi-typification or neo-typification of taxa with fresh material and cultures are necessary as they contribute to providing a stable taxonomy for fungi Chethana et al. (2021a) as well as for carrying out assays to identify any potential compounds that can be harnessed at the industrial level. Identification and documentation of the host and the geographical range of a fungus can be particularly important in disease management (Dugan et al. 2009).
In order to provide an outlet for the mycologists to publish their findings in mycology, different publication series such as AJOM new records and collections of fungi (Hyde et al. 2019; Chethana et al. 2021b), Fungal Diversity notes (Liu et al. 2015; Ariyawansa et al. 2015; Hyde et al. 2017, 2019, 2020; Tibpromma et al. 2018; Wanasinghe et al. 2018; Phookamsak et al. 2019; Boonmee et al. 2021), Fungal planet (Crous et al. 2015a, b, c, 2017, 2018) and Mycosphere notes (Thambugala et al. 2015; Hyde et al. 2018, 2021; Jayawardena et al. 2018; Manawasinghe et al. 2022), are now available. As a result, numerous new taxa, geographical and host records, new combinations, and reference data were introduced along with morphological and multigene analyses.
This is the 14th in the series of Fungal Diversity Notes with entries mainly collected from Australia, Brazil, Burkina Faso, Chile, China, Cyprus, Egypt, France, French Guiana, India, Indonesia, Italy, Laos, Mexico, Russia, Sri Lanka, Thailand, and Vietnam. We aim to provide new data including morphological, geographical and sequence data for a stable taxonomy and phylogeny, which become significantly important for the accurate identification of fungi as suggested by Cao et al. (2021), Chethana et al. (2021a), Manawasinghe et al. (2019), Maharachchikumbura et al. (2021), Jayawardena et al. (2021b) and Pem et al. (2021). We provide a detailed description and an updated tree for the genus or family of each entry. The ‘notes’ under each entry discuss how the new taxa are established, including the host and geographical ranges. The data compiled in this study can be used by future researchers for a better understanding of the taxonomy of each different group of fungi.
Materials and methods
Materials and methods follow the previous fungal diversity notes (Hyde et al. 2016, 2020a, b, c; Tibpromma et al. 2017; Wanasinghe et al. 2018; Phookamsak et al. 2019; Boonmee et al. 2021 and Senanayake et al. 2020). When specific details are available for material and methods they are given in the ‘notes’ section of each taxon. Taxa described in this study were collected from Australia, Brazil, Burkina Faso, Chile, China, Cyprus, Egypt, France, French Guiana, India, Indonesia, Italy, Laos, Mexico, Russia, Sri Lanka, Thailand, and Vietnam. Taxa were described and illustrated based on morphological features, coupled with phylogenetic analyses performed by maximum likelihood (ML), maximum parsimony (MP) and Bayesian posterior probability (BYPP) criteria. Colour codes followed the Methuen Handbook of Colour (Kornerup and Wanscher 1978). Phylogenetic analyses were performed based on details outlined by Dissanayake et al. (2020). Details of each analysis are given in Supplementary Table 1. The pairwise homoplasy index (PHI) test was carried out when necessary, using Split Trees as described by Quaedvlieg et al. (2014) to determine the recombination level within phylogenetically closely related species. The new taxa are justified based on the guidelines of Cao et al. (2021), Chethana et al. (2021a, b), Manawasinghe et al. (2021), Maharachchikumbura et al. (2021), Jayawardena et al. (2021b) and Pem et al. (2021).
Results
Ascomycota R.H. Whittaker
Notes: We follow the latest treatments and updated accounts of Ascomycota in Wijayawardene et al. (2020, 2022).
Subphylum Pezizomycotina O.E. Erikss. & Winka
Class Dothideomycetes O.E. Erikss. & Winka
Notes: We follow the latest treatments and updated accounts of Dothideomycetes in Hongsanan et al. (2020a, b) and Wijayawardene et al. (2020, 2022).
Subclass Dothideomycetidae P.M. Kirk, P.F. Cannon, J.C. David & Stalpers ex C.L. Schoch, Spatafora, Crous & Shoemaker
Mycosphaerellales (Nannf.) P.F. Cannon
Notes: Abollahzadeh et al. (2020) based on LSU, tef1 and rpb2 sequence data revalidated Mycosphaerellales as a separate order. Mycosphaerellales include species that are saprobes, ectophytes, plant pathogens and lichenised fungi. This order includes eight families viz. Cystocoleaceae, Dissoconiaceae, Extremaceae, Mycosphaerellaceae, Neodevriesiaceae, Phaeothecoidiellaceae, Schizothyriaceae and Teratosphaeriaceae (see Abdollahzadeh et al. 2020).
Mycosphaerellaceae Lindau, Nat. Pflanzenfamilien: 421(1897)
Notes: Mycosphaerellaceae was established by Lindau (1896) with Mycosphaerella as the type genus. This is one of the largest families including asexual morphs, asexual holomorphs or species with mycosphaerella-like sexual morphs. The majority of them are parasitic or saprobic on plants, fungi and lichens (Hyde et al. 2013). Wijayawardene et al. (2022) accepted a total of 119 genera having molecular data under Mycosphaerellaceae.
Pseudocercospora Speg., Anales del Museo Nacional de Historia Natural Buenos Aires 20 (13): 438 (1910)
Notes: Pseudocercospora was established by Spegazzini (1910) with P. vitis as the type genus. The genus is characterized by conidiophores solitary, fasciculate, synnematal or arranged in sporodochia, conidia coloured, scars unthickened or slightly thickened (Crous and Braun 2003; Crous et al. 2014). They are mostly plant pathogenic fungi associated with leaf and fruit spots and are widely distributed in a wide range of climatic conditions including cool temperate, sub-tropical and tropical regions (Crous et al. 2014).
Pseudocercospora vernoniae Archana Singh & N.K. Dubey, sp. nov
Mycobank Number: MB 834618, Facesoffungi number: FoF 07979, Figs. 1, 2
Etymology: Based to the host genus from which the taxon was isolated
Holotype: AMH:10043
Asexual morph: on leaf spots of Vernonia cineria, hypophyllous later amphiphyllous, 2–5 mm, angular, vein limited, discrete and later forming irregular larger patches, grayish brown on lower surface and dark blackish- brown on upper surface. Caespituli hypophyllous later amphiphyllous, dark brown, erumpent. Stromata substomatal, few cells to well-developed, made up of oval to round 3–5 µm wide pseudoparenchymatous cells, median to dark brown. Conidiophores fasciculate, unbranched or rarely branched, geniculate, 1–8-septate, light brown 13.6–40.3 (50) × 3.5–5.5 µm. Conidiogenous cells integrated, polyblastic, cicaterised. Conidia septate (1–7), catenate in branched chains, straight to curved, cylindrical, constricted at septa, olivaceous brown, subcylindrical, base obclavate to obconico truncate, tip subacute to obtuse 21.7–44.8 (92) × 4.5–5.5 µm. Sexual morph: Not observed.
Culture characteristics: Conidia germinating on Potato Dextrose Agar (PDA). Colonies very slow growing, velvety, greyish brown; reaching 2–5 mm diam., in 28 days at 27 °C, margin circular to irregular, reverse blackish brown raising centrally, of dense cottony mycelium and hard texture. Mycelium smooth, branched, asexual and sexual spores not formed within 60 days.
Material examined: India, Sonebhadra U.P., on living leaves of Vernonia cineria (L.) Less (Asteraceae), Dec 2017, AMH: 10043 (Holotype), culture ex type NFCCI: 4441.
GenBank numbers: MN691042 (LSU); MN691041 (ITS); MT106617 (act); MT106618 (tef1)
Notes: Pseudocercospora species are mostly host-specific (with few exceptions) related to a single host species, host genus or closely related host genera (Braun et al. 2013; Crous et al. 2013). Two species of Pseudocercospora has been reported earlier on Vernonia, Pseudocercospora cinereae (Deighton 1976) and Pseudocercospora vernoniacearum (Shukla et al. 1982). Pseudocercospora cineriae has dark brown circular, coalescing leaf spots and P. vernoniacearum has oval, effuse leaf spots whereas P. vernoniae has grayish brown, angular and vein limited leaf spots. Conidiophores are much smaller (14–40 µm) and more septate (1–8) in P. vernoniae compared to previously described species P. cinereae (1–3 septate, 40–150 × 3.5–5 µm) and P. vernoniacearum (44–133 × 3.5–5.4 µm). Conidia are simple and longer in P. cinereae (28.5–145 × 2.8–5.7 µm) and P. vernoniacearum (40–100 × 3.5–5.4 µm). The presence of catenate conidia in branched chains with smaller and variable in size 21.74– 44.76 × 4.5–5.5 µm) differentiate P. vernoniae from the previously described species. Molecular analysis based on combined gene analysis of LSU, ITS, act and tef1 (Fig. 3) reveals that P. vernoniae clusters with P. hakeae (CBS 144520) with moderate support.
Maximum likelihood tree illustrating the phylogeny of Pseudocercospora vernoniae with related species in Pseudocercospora based on LSU, ITS, act and tef1 concatenated sequences. Branches are labelled with ML and MP values ≥ 50% and BYPP ≥ 0.95 are indicated above the node respectively. The ex-types/reference strains are in bold; the new species is in blue. The tree is rooted with Pallidocercospora heimii (CPC:11716)
Subclass Pleosporomycetidae C.L. Schoch, Spatafora, Crous & Shoemaker
Kirschsteiniotheliales Hern. -Restr., R.F. Castañeda, Gené & Crous
Kirschsteiniotheliales was introduced by (Hernandez-Restrepo et al. 2017) based on phylogenetic analysis. Kirschsteiniotheliales consists with Kirschsteiniotheliaceae, and two genera incertae sedis, viz. Brachysporiella, Taeniolella (Hongsanan et al. 2020a; Wijayawardene et al. 2020)
Kirschsteiniotheliaceae Boonmee & K.D. Hyde, in Boonmee et al., Mycologia 104(3): 705 (2012)
The monotypic family, Kirschsteiniotheliaceae, was introduced by Boonmee et al. (2012) to accommodate Kirschsteiniothelia species based on morphology and phylogenetic analyses. Kirschsteiniotheliaceae species are mostly saprobes on dead wood from terrestrial and aquatic habitats in tropical and subtropical regions (Boonmee et al. 2012; Su et al. 2016; Mehrabi et al. 2017; Bao et al. 2018; Sun et al. 2021).
Kirschsteiniothelia D. Hawksw., Bot. J. Linn. Soc. 91: 182 (1985)
We follow the latest treatment and updated accounts of Kirschsteiniothelia in Sun et al. (2021)
Kirschsteiniothelia acutisporum S. Wang, Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF559759; Facesoffungi number: 1FoF1799; Fig. 4
Etymology: Named after the acute conidia
Holotype: MFLU 21-0127
Saprobic on decaying plant substrates. Sexual morph: Not observed. Asexual morph: Colonies effuse, scattered, dark-brown to black, glistening, hairy, sparse. Mycelium partly superficial, partly immersed in the substratum, composed of dark brown, septate, branched hyphae. Conidiophores macronematous, mononematous, solitary, cylindrical, straight or slightly flexuous, dark brown, slightly tapering towards the apex, 8–12 septate, truncate at the apex, 180–260 µm (\(\bar{x}\) = 230 µm, n = 10) long, 7–12.5 μm (\(\bar{x}\) = 9 µm, n = 10) wide. Conidiogenous cells integrated, terminal, monoblastic, cylindrical and brown, calyciform. Conidia acrogenous, solitary, obclavate to obspathulate, tapering to the apex, rostrate, 7–12-euseptate, mid to dark brown, becoming pale brown to pale towards the apex, truncate at the base, 75–120 µm (\(\bar{x}\) = 92 µm, n = 15) long, 10.5–19.5 μm (\(\bar{x}\) = 15 µm, n = 15) wide.
Material examined: Thailand, Chiang Mai Province, saprobic on decaying wood at the Mushroom Research Center (MRC), August 2020, Song Wang, SW231 (MFLU 21-0127, holotype).
GenBank numbers: ON980758 (LSU); ON980754 (SSU); OP120780 (ITS); OP009582 (rpb2)
Notes: Kirschsteiniothelia acutisporum shares similar characteristics with K. fluminicola in having macronematous, unbranched, cylindrical, septate, conidiophores and solitary, obclavate, septate, conidia. However, Kirschsteiniothelia acutisporum differs from K. fluminicola in having a gelatinous rounded sheath at the apex of shorter and thinner conidia (33–43 × 7.5–8.5 μm vs 47.5–86.5 × 8–10 μm). Kirschsteiniothelia acutisporum phylogenetically creates an independent branch with 100ML/100MP/1.00BYPP support (Fig. 5).
Phylogram generated from maximum likelihood analysis based on combined LSU, SSU, ITS sequence data. Forty-six taxa were included in the combined analyses, which comprised 2,104 characters (LSU = 1–788 bp, SSU = 789–1,632 bp, ITS = 1,633–2,104 bp), including alignment gaps. Among them, 1,191 characters were constant, 239 characters were singleton sites, and 674 characters were parsimony informative. The best scoring RA × ML tree is presented. Bootstrap support values for ML and MP ≥ 75% and BYPP ≥ 0.95 are given above the nodes. Pseudorobillarda eucalypti (MFLUCC 12–0422) and P. phragmitis (CBS 398.61) were used as the outgroup taxa. The newly generated sequences are indicated in red. The ex-type strains are indicated in bold
Kirschsteiniothelia crustaceum S. Wang, Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF559760; Facesoffungi number: FoF11802; Fig. 6
Etymology: Referring to the conidial ‘shell’ shape.
Holotype: MFLU 21-0129
Saprobic on decaying bamboo culms. Sexual morph: Not observed. Asexual morph: Colonies effuse, scattered, dark brown to black, glistening, hairy, sparse. Mycelium partly superficial, partly immersed in the substratum, composed of dark brown, septate, branched hyphae. Conidiophores macronematous, mononematous, solitary, cylindrical, straight or slightly flexuous, brown to dark brown, slightly tapering towards the apex, 4–8 septate, truncate at the apex, 60–170 µm (\(\bar{x}\) = 128 µm, n = 15) long, 6.5–10.5 μm (\(\bar{x}\) = 8 µm, n = 15) wide. Conidiogenous cells integrated, terminal, monoblastic, cylindrical and calyciform, brown, 9–16 µm (\(\bar{x}\) = 12 µm, n = 15) long, 5.5–8 μm (\(\bar{x}\) = 6.5 µm, n = 15) wide. Conidia acrogenous, solitary, obclavate to obspathulate, globose to the apex and hyaline to light brown, rostrate, 5–6-euseptate, mid to dark brown, becoming pale brown to pale towards the apex, truncate at the base, 45–75 µm (\(\bar{x}\) = 55 µm, n = 20) long, 10–18 μm (x̄ = 14 µm, n = 20) wide.
Material examined: Thailand, Nang Lae, Mueang Chiang Rai, Chiang Rai Province, saprobic on decaying bamboo, submerged in a freshwater stream, July 2020, Rongju Xu, MD71 (MFLU 21–0129, holotype)
GenBank numbers: MW851854 (LSU); MW851849 (ITS)
Notes: Kirschsteiniothelia crustaceum shares similar morphology with K. rostrata in having macronematous, unbranched, cylindrical, septate, conidiophores and solitary, obclavate, septate, conidia. However, conidiophores of Kirschsteiniothelia crustaceum (60–170 × 6.5–10.5 μm) are much shorter than those of K. rostrata (up to 280 μm long, 12 μm wide). Conidia of K. crustaceum (45–75 × 10–18 μm) are much shorter than those of K. rostrata (up to 115 μm long, 15 μm wide) also. The combined LSU, SSU and ITS phylogenetic analysis show that Kirschsteiniothelia crustaceum represents a sister taxon to K. rostrata with good separation (89ML/1.00BYPP) (Fig. 5).
Kirschsteiniothelia extensum. S. Wang, Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF559761; Facesoffungi number: FoF11803; Fig. 7
Etymology: Referring to the conidiophore extending characteristic
Holotype: MFLU 21-0130
Saprobic on decaying wood. Sexual morph: Not observed. Asexual morph: Colonies effuse, scattered, brown or black, hairy, glistening. Mycelium partly superficial, partly immersed in the substratum, composed of brown, septate, branched hyphae. Conidiophores macronematous, mononematous, solitary, cylindrical, straight or slightly flexuous, dark brown, unbranched, thick-walled, smooth, slightly tapering towards the apex, 4–9 septate, truncate at the apex, 80–230 µm (\(\bar{x}\) = 140 µm, n = 15) long, 6.5–9.5 μm (\(\bar{x}\) = 7.5 µm, n = 15) wide. Conidiogenous cells integrated, terminal, monoblastic, percurrent, pale brown, cylindrical, 11–19 µm (\(\bar{x}\) = 15 µm, n = 15) long, 4–7.5 μm (\(\bar{x}\) = 6 µm, n = 15) wide. Conidia acrogenous, solitary, smooth, obclavate, straight or slightly curved, tapering to the apex, 5–8-euseptate, becoming pale brown to pale towards the apex, truncate at the base, 45–120 µm (\(\bar{x}\) = 60 µm, n = 30) long, 5–12 μm (\(\bar{x}\) = 9 µm, n = 30) wide.
Material examined: Thailand, Nang Lae, Mueang Chiang Rai, Chiang Rai Province, saprobic on decaying wood, July 2020, Rongju Xu, MD73 (MFLU 21-0130, holotype).
GenBank numbers: MW851855 (LSU); MW851850 (ITS)
Notes: Kirchsteiniothelia extensum is introduced here based on both morphology and molecular data. Kirchsteiniothelia extensum forms a distinct clade within Kirschsteiniotheliaceae and is sister to K. submersa (Fig. 5). The difference between them is that conidiophores of Kirschsteiniothelia extensum (80–230 × 6.5–9.5 μm) are much shorter than those of K. submersa (220–280 × 6–7 μm)
Kirschsteiniothelia septemseptatum S. Wang, Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF559762; Facesoffungi number: FoF11800; Fig. 8
Etymology: Referring to the number of septa mostly observed in conidia
Holotype: MFLU 21–0126
Saprobic on decaying wood. Sexual morph: Not oberved. Asexual morph: Colonies on natural substrate, scattered or fascicular, effuse, hairy, dark brown to black, glistening. Mycelium partly superficial, partly immersed in the host tissue, composed of smooth, light brown, branched, septate. Conidiophores macronematous, mononematous, single to loosely fasciculate, erect, straight to slightly flexuous, branched at the apex, dark brown, multiseptate, 9–16 septate, 250–580 µm (x̄ = 415 µm, n = 20) long, 6.5–14.5 μm (x̄ = 10 µm, n = 20) wide. Conidiogenous cells mostly polytretic, sometimes monotretic, integrated, discrete, terminal and lateral, calyciform, 2 septate, 9.5–21 µm (x̄ = 16 µm, n = 20) long, 4–8 μm (x̄ = 6 µm, n = 20) wide. Conidia acrogenous, solitary, dry, olivaceous brown to brown, pale at apex, obclavate, rostrate, smooth, straight or curved, truncate at base, 5–8– euseptate, 25–55 μm (x̄ = 41 µm, n = 20) long, 6.5–12.5 µm (\(\bar{x}\) = 10.5 μm, n = 20) wide.
Material examined: Thailand, ChiangMai Province, saprobic on decaying wood at MRC, July 2020, Song Wang, SW212, (MFLU 21–0126, holotype)
GenBank numbers: ON980757 (LSU); ON980752 (SSU); OP120779 (ITS); OP009581 (rpb2)
Notes: Kirschsteiniothelia septemseptatm shares similar characteristics with K. fluminicola in having macronematous, unbranched, cylindrical, septate, conidiophores and solitary, obclavate, septate, conidia. However, K. cangshanensis differs from K. fluminicola in having a gelatinous rounded sheath at the apex of shorter and thinner conidia (33–43 × 7.5–8.5 μm vs 47.5–86.5 × 8–10 μm). In our phylogetic analyses, K. septemseptatum forms an independent branch with 91ML/95MP/1.00BYPP support (Fig. 5)
Kirschsteiniothelia spatiosum. S. Wang, Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF559763; Facesoffungi number: FoF11801; Fig. 9
Etymology: Referring to the long conidia
Holotype: MFLU 21-0128
Saprobic on decaying wood. Sexual morph: Not oberved. Asexual morph: Colonies effuse on natural substrate, scattered or fascicular, hairy, black, glistening. Mycelium partly immersed, partly superficial in the substrate, composed of pale brown, ranched hyphae. Conidiophores macronematous, mononematous, solitary or sometimes caespitose, cylindrical, wide at base, tapering towards apex, straight or slightly flexuous, smooth, light brown to dark brown, unbranched, 6–12 septate, 70–128 µm (x̄ = 100 µm, n = 15) long, 7.5–12.5 μm (x̄ = 9 µm, n = 15) wide. Conidiogenous cells holoblastic, monoblastic, integrated, terminal, determinate, cylindrical, smooth, mid to dark brown. Conidia acrogenous, solitary, dry, olivaceous brown to brown, pale at apex, obclavate, rostrate, smooth, straight or curved, truncate at base, 8–23– euseptate, sometimes with a mucilaginous sheath, 90–139 μm (x̄ = 113 µm, n = 15) long, 9.5–16.5 µm (x̄ = 14 μm, n = 15) wide.
Material examined: Thailand, Chiang Mai Province, saprobic on decaying wood at MRC, August 2020, Song Wang, SW280 (MFLU 21–0128, holotype)
GenBank numbers: OP077294 (LSU); ON980753 (SSU)
Notes: In the phylogenetic analyses our strain is closely realted with K. tectonae (Fig. 5). Kirschsteiniothelia spatiosum shares similar characteristics with Kirschsteiniothelia tectonae in having macronematous, unbranched, cylindrical, septate, conidiophores and solitary, obclavate, septate, conidia. However, K. spatiosum differs from K. tectonae in having a gelatinous rounded sheath at the apex of shorter and thinner conidia and in having shorter and thinner conidia (90–139 × 9.5–16.5 μm vs 135–150 × 16–19 μm). Kirschsteiniothelia spatiosum differs from K. tectonae in having shorter conidiophores (70–128 × 7.5–12.5 μm vs 200 × 4–8 μm).
Pleosporales Luttrell ex M.E. Barr.
Notes: We follow the latest treatments and updated accounts of Pleoporales in Hongsanan et al. (2020b) and Wijayawardene et al. (2022).
Amorosiaceae Thambug. & K.D. Hyde
Thambugala et al. (2015) introduced this family to accommodate Amorosia Mantle & D. Hawksw. and Angustimassarina Thambug., Kaz. Tanaka & K.D. Hyde. The family is characterized by immersed or semi-immersed ascomata with a short, crest-like papilla, and hyaline ascospores with a mucilaginous sheath (Thambugala et al. 2015). Wijayawardene et al. (2022) accepted five genera in this family.
Angustimassarina Thambug., Kaz. Tanaka & K.D. Hyde
Thambugala et al. (2015) introduced this genus to accommodate fungi that have ascospores resembling Massarina, while being narrowly fusiform. There are 12 species listed in the Index Fungorum (accessed on 30 August 2022). In this study, we introduce a new species from China based on molecular phylogeny and morphology.
Angustimassarina kunmingense H.D. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF559764; Facesoffungi number: FoF11804; Fig. 10
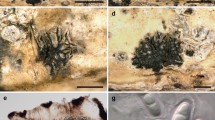
Angustimassarina kunmingense (YHD216, holotype). a, b Ascomata immersed on host surface. c Section through ascoma. d, e Peridium. f, g Mature bitunicate asci (g. asci stained with Congo red). h–k Ascospores. l Gemmating ascospores. m, n Colonies on PDA. Scale bars: c–d = 100 μm, e = 50 μm, l = 30 μm, f–g = 20 μm, i–k = 10 μm, h = 5 μm
Etymology: Referring to the collecting site, Kunming City, Yunnan, China.
Holotype: HKAS123210
Saprobic on dead aerial stem of Camellia semiserrata. Sexual morph: Ascomata (162–)190–332(–333) × (119–)142–289(–300) μm (x̅ = 261 × 221 μm, n = 5), scattered, gregarious, immersed to semi-immersed in the host tissue, black, globose to subglobose, ostiolate. Ostiole in the centre, crest-like, rounded, papillate, with a pore-like opening. Peridium 27–56 μm thick, comprised of 5–10 layers of cells of textura angularis, cells smaller at the base and the apex, and larger at the side, brown to hyaline. Hamathecium composed of 1.2–2 μm (x̅ = 1.6 μm, n = 30) wide, numerous, septate, clamped, unbranched, hyaline, pseudoparaphyses, embedded in a gelatinous matrix, longer than asci. Asci (56–) 60–74(–77) × (7.2–)7.5–8.7(–9.3) μm (x̅ = 68 × 8.1 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindric-clavate, with short pedicel at the base, rounded at the apex with a minute ocular chamber. Ascospores (18–)20–22(–23) × (3.1)3.3–3.8(–4.1) μm (x̅ = 20 × 3.5 μm, n = 30), 1–2 overlapping seriate, hyaline, fusiform, dimidiate, widest at the centre and tapering toward the ends, with 1–3 constricted septate septum, filled with 1–2 guttules per cell, smooth-walled and surrounded by a mucilaginous sheath. Asexual morph: Not observed.
Culture characteristics: Ascospores germinating on PDA within 24 h and producing germ tubes from both ends and sides. Colonies on PDA reaching 28 mm diam. after 33 days at 20 °C, nearly circular, flat, dense, radial sulcate, edge entire, smoke grey to grey-white on the surface, dark brown on the reverse and becoming grey-white at the margin.
Material examined: China, Yunnan Province, Kunming City, Panlong District, on Camellia semiserrata C.W. Chi (Theaceae), 25° 8′ 29.27″ N, 102° 44′ 16.03″, 17 Dec 2021, Hongde Yang, (HKAS123210, holotype); ex-type living culture, KUNCC22-10799.
GenBank numbers: ON352672 (ITS); ON352671 (LSU); ON352675 (SSU); ON364144 (tef1); ON791602 (act); ON791682 (tub2)
Notes: Species of Angustimassarina are broadly distributed in Belgium, Germany and Italy (Hyde et al. 2020a, b, c; Phukhamsakda et al. 2020), but, have never been reported from China. Our collections from China are morphologically and phylogenetically related to Angustimassarina. Our new species Angustimassarina kunmingense resembles other Angustimassarina species in terms of ascomata, asci and ascospores (Table 1) and the new species was isolated from similar habitat to other Angustimassarina species (Thambugala et al. 2015). However, the taxon is charactered by slender asci and ascospores. The megablast search of the ITS and tef1 sequences show the highest similarity with Angustimassarina populi (457/463, 98%) and Angustimassarina populi (827/830, 99%), respectively. In the phylogenetic analysis, Angustimassarina kunmingense formed a well-supported monophyletic clade basal to Angustimassarina species (97ML/1.00BYPP). Our phylogenetic tree was constructed using multi gene loci (ITS, SSU, LSU and tef1, Fig. 11). However, most taxa were not strongly supported. This could suggest that additional markers are required to achieve a more accurate identity, thus we also provide protein gene act and tub2 herein.
Phylogram generated from maximum likelihood analysis based on combined ITS, SSU, LSU and tef1 sequence data of Angustimassarina. Twenty strains were included in the analysis of the combined loci which comprised 2700 characters. The tree is rooted with Guttulispora crataegi (MFLUCC 13–0442) and Lophiostoma caulium (KT794). Bootstrap support values ≥ 50% in ML and BYPP ≥ 0.95 are given at the nodes. The ex-types and reference strains are in bold; the new isolate is in blue
Bambusicolaceae D.Q. Dai & K.D. Hyde, in Hyde et al., Fungal Diversity 63: 49 (2013)
Notes: Bambusicolaceae was placed in Dothideomycetes by Hyde et al. (2013) to accommodate Bambusicola (Dai et al. 2012; Liu et al. 2015; Jayasiri et al. 2019; Yang et al. 2019; Bhunjun et al. 2021; Calabon et al. 2022). Four genera viz. Bambusicola, Corylicola, Leucaenicola and Palmiascoma are accepted in this family (Wijayawardene et al. 2022). Bambusicolaceae are characterized by solitary, scattered, immersed, semi-immersed to erumpent and conical or globose to subglobose ascomata, anastomosing, branching interascal filaments, cylindrical to clavate asci with a short furcate or rounded to obtuse pedicel and slightly broad-fusiform or clavate to ellipsoidal, hyaline or yellowish to brown, single-septate ascospores with a gelatinous sheath (Dai et al. 2012; Hyde et al. 2013; Liu et al. 2015; Dai et al. 2017).
Bambusicola D.Q. Dai & K.D. Hyde, in Dai, Bhat, Liu, Chukeatirote, Zhao & Hyde, Cryptog. Mycol. 33(3): 367 (2012)
Notes: Bambusicola is a well-studied genus, established by Dai et al. (2012). There are 15 species accepted in the genus and all species have sequence data in GenBank (Dai et al. 2012, 2015, 2017; Thambugala et al. 2015; Yang et al. 2018; Dong et al. 2020; Monkai et al. 2021). Both sexual and asexual morphs of Bambusicola are reported (Dai et al. 2012, 2015, 2017; Thambugala et al. 2015; Yang et al. 2018; Dong et al. 2020; Monkai et al. 2021). The sexual morph of Bambusicola is characterized by gregarious, immersed or semi-immersed, globose to subglobose, uni- to multi- loculate, coriaceous ascomata, bitunicate, cylindrical or cylindric-clavate, short pedicellate asci with a shallow or well-developed chamber and fusiform, septate, hyaline to pale brown ascospores mostly surrounded by a gelatinous sheath. The asexual morph of Bambusicola is characterized by pycnothyrial, immersed to semi-immersed, acerose or subglobose, pyriform or irregular, uni- to multi-loculate conidiomata, holoblastic, annellidic, discrete, cylindrical conidiogenous cells and cylindrical to ellipsoidal, pale brown to brown, septate conidia (Dai et al. 2012, 2017; Thambugala et al. 2015; Dong et al. 2020; Monkai et al. 2021).
Bambusicola species have been reported from both terrestrial and freshwater habitats in China and Thailand (Dai et al. 2012, 2015, 2017; Thambugala et al. 2015; Yang et al. 2018; Dong et al. 2020). Most Bambusicola species are reported as saprobes on bamboo. In this study, we report a new record of Bambusicola bambusae on submerged decaying wood from freshwater habitats for the first time.
Bambusicola bambusae D.Q. Dai & K.D. Hyde, Cryptog. Mycol. 33(3): 372 (2012)
Index Fungorum number: IF 801046; Facesofungi number: FoF11797; Fig. 12
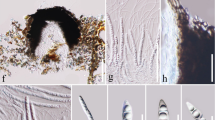
Bambusicola bambusae (MFLU 22–0080, new record). a–c ascomata on wood d section of ascoma e peridium, f, g ostiole. h pseudoparaphyses. i–k asci. l–p ascospores. q Germinating ascospore. r, s culture on PDA from surface and reverse. Scale bars: b, f = 100 μm, e = 50 μm, g = 30 μm, h–k = 20 μm, l–q = 10 μm
Saprobic on decaying wood in a freshwater stream. Ascomata 135–175 µm high × 190–245 µm diam. (\(\bar{x}\) = 155 × 216 µm, n = 10), solitary, scattered to gregarious, immersed under the host tissue, conical in section, brown to dark brown, coriaceous, subglobose, ostiolate. Ostiole crest-like, central, elongated to papillate, with a pore-like opening, plugged by hyaline, filamentous hyphae. Peridium comprising host and fungal tissues, 17–31 μm thick, composed of brown to dark brown cells of textura angularis intermingled with host cells. Hamathecium composed of numerous, filamentous, hyaline, septate, branched, 1.0–1.5 μm, pseudoparaphyses. Asci 55–75 × 7.5–9.5 μm (\(\bar{x}\) = 66.3 × 8.5 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, with a shallow apical chamber and a short furcate pedicel. Ascospores 19–21 × 4.0–4.5 μm (\(\bar{x}\) = 20 × 4.5 μm, n = 30), 2–3-seriate, 1-septate, constricted at the septum, slightly broad fusiform, tapering towards the ends, occasionally with large upper cell, with narrowly rounded ends, hyaline, guttulate, smooth-walled.
Culture characteristics: Ascospores germinating on PDA within 24 h and germ tubes produced from both ends. Colonies growing on PDA, reaching a diam. of 20–25 mm after 20 d at 25 °C, surface smooth to velvety, with entire to slightly undulate edge, greenish in the centre, white at the edge; reverse dark greenish to black in the centre, white at the edge.
Material examined: Thailand, Tao Ngoi, Sakon Nakhon, on decaying wood submerged in a river, 12 November 2017, D.F. Bao, B110 (MFLU 22–0080), living culture, MFLUCC 22–0021.
Host/Substrate: Bamboo (Poaceae) (Dai et al. 2012); decaying wood submerged in a river (this study)
Distribution: Thailand (Dai et al. 2012; this study)
GenBank numbers: ON764309 (ITS); ON764310 (LSU); ON764313(SSU); ON788004 (rpb2)
Notes: In the phylogenetic analysis, our new isolate B110 clustered with the ex-type strain of Bambusicola bambusae (MFLUCC 11–0614) with 98% ML/1.00 BYPP support (Fig. 13). The morphology of our collection is almost identical to the holotype of Bambusicola bambusae except for the size of ascomata and the sheath of the ascospores. The ascomata of our collection are smaller than the holotype (190–245 vs. 450–70 µm diam) and the holotype of B. bambusae has ascospores with a thick sheath (Dai et al. 2012), whereas, the sheath of ascospores were not observed in our collection. A comparison of the ITS and rpb2 gene regions of MFLUCC 11–0614 and B110 revealed 0 and 3 base pair differences and therefore we identified our new collection as Bambusicola bambusae as recommended by Pem et al. (2021). Bambusicola bambusae was described by Dai et al. (2012), it was collected on bamboo from terrestrial habitats in Thailand. Our collection was from freshwater habitats and this is the first time this species reported from freshwater habitats.
Phylogram generated from ML analysis, based on combined ITS, LSU, SSU, tef1 and rpb2 sequence data for Bambusicolaceae. The combined dataset comprises 37 strains with 4617 characters including gaps (LSU: 854 bp, SSU: 1016 bp, ITS: 805 bp, tef1: 950 bp, rpb2: 992 bp). The tree is rooted with Murilentithecium lonicerae (MFLUCC 18–0675) and M. clematidis (MFLUCC 14–0561). Maximum likelihood bootstrap values ≥ 75% and baysian BYPP ≥ 0.95 are displayed on the nodes, respectively. Newly introduced taxa are indicated in red. Ex-type and representative strains are in bold
Corylicola Wijesinghe, Camporesi, Yong Wang bis & K.D. Hyde, in Wijesinghe et al., Biodiversity Data Journal 8(e55957): 8 (2020)
Corylicola was introduced by Wijesinghe et al. (2020). This genus is characterized by uniseriate, fusiform to ellipsoidal, yellowish to pale brown, single-septate, echinulate ascospores, accumulating as yellowish-brown masses at the apices of ascomatal neck (Wijesinghe et al. 2020). We provide a new host record of Corylicola italica from Rubus sp. in Italy.
Corylicola italica Wijesinghe, Camporesi, Yong Wang bis & K.D. Hyde, in Wijesinghe et al., Biodiversity Data Journal 8(e55957): 8 (2020).
Index Fungorum number: IF557768; Facesofungi number: FoF08684; Fig. 14
Corylicola italica (MFLU 20–0251, new host record). a–b Appearance of ascomata on a twig of Rubus sp. c–d Section through ascomata. e Close-up of ostiole. f Pseudoparaphyses. g Peridium. h–j Asci. k–n. Ascospores. o. Culture characteristics on PDA after 20 days from above. p Culture characteristics on PDA after 20 days from below. Scale bars: c–d = 50 μm, e–g = 20 μm, h–j = 10 μm, k–n = 5 μm
Saprobic on a dead branch of Rubus sp. Sexual morph: Ascomata 109–141 high, 91.5–106 µm diam. (x̄ = 128.5 × 101.5 µm; n = 4), solitary, scattered, immersed, erumpent at maturity, raised as brown to dark spots on the substrate, globose to subglobose, coriaceous, uni-loculate with an ostiole. Ostiole 46–68 µm wide, central, papillate, lined with hyaline periphyses. Peridium composed of two layers, unequally thickened, 15–29 µm wide comprising brown, blackish to dark brown cells of textura angularis fused with host tissues, inner layer comprising hyaline cells of textura prismatica. Hamathecium comprising numerous pseudoparaphyses 1–2 µm wide (x̄ = 1.6 µm, n = 6), filamentous, cellular, with distinct septa, not constricted at the septa, branching and anastomosing above the asci. Asci 52–74 × 4–6 µm (x̄ = 61 × 5 µm, n = 5), 8-spored, bitunicate, fissitunicate, cylindrical, short distinct pedicel with furcate ends, apically rounded, well-developed ocular chamber. Ascospores 10–12 × 3–4 µm (x̄ = 10 × 3.6 µm, n = 11), overlapping, uni-seriate, fusiform to ellipsoidal, 1-septate straight, hyaline and yellowish when young, becoming pale brown at maturity. Asexual morph: Not observed.
Culture characteristics: Spore germinating on PDA within 24 h from singles pore isolation. Colonies on PDA reaching 10 mm diam. after 20 days at 20 °C, circular, submerged, crenated edge, flat with dense, brown to whitish in the middle, grey at the edges from upper and reverse brownish-black in the lower surface of the colony.
Material examined: Italy, Forlì-Cesena Province near Meldola, on dead aerial branches of Rubus sp. (Rosaceae), 4 February 2020, Erio Camporesi IT-4596C (MFLU 20–0251); living culture MFLUCC 21-0118.
Host/Substrate: Corylus avellana (Betulaceae) (Wijesinghe et al. 2020); Rubus sp. (Rosaceae) (this study)
Distribution: Italy (Wijesinghe et al. 2020; this study).
GenBank numbers: OM471788 (ITS), OM630433 (tef1).
Notes: Wijesinghe et al. (2020) reported this species from Corylus avellana. Morphologically our collection resembles the ex-type strain of this species. Based on our phylogenetic analyses, our strain MFLUCC 21-0118 clustered together with MFLU 19–0500 and MFLUCC 20–0111 (Fig. 13) with 100/ML and1.00/BYPP support. Therefore, we introduce our collection as a new host record.
Palmiascoma Phook. & K.D. Hyde, in Liu et al., Fungal Diversity: https://doi.org/10.1007/s13225-015-0324-y, [65] (2015).
Notes: Palmiascoma was introduced by Liu et al. (2015) and is typified by P. gregariascomum collected from a dead frond of a palm. Palmiascoma is similar to Didymosphaeria in having didymosporous, brown, and echinulate ascospores, but differs in phylogeny. Monkai et al. (2021) introduced the second species into the genus as P. qujingense isolated from dead twigs of Fagaceae sp. in Yunnan, China.
Palmiascoma gregariascomum Phookamsak & K.D. Hyde, in Liu et al., Fungal Diversity: https://doi.org/10.1007/s13225-015-0324-y, [65] (2015).
Index Fungorum number: IF550927; Facesoffungi number: FoF00429; Fig. 15
Palmiascoma gregariascomum (MFLU 18–0845, new host record) a-c Conidiomata on the substrate. d Vertical section of conidioma. e Peridium. f–h Conidiogenous cells and conidiogenesis. i,o Conidia (o in culture), j Top view of culture in PDA. k Reverse view of culture. l,m Conidiomata on PDA. n Peridium. Scale bars: a,l = 1000 µm, b = 500 µm, c,m = 200 µm, d = 50 µm, e = 20 µm, n = 10 µm, f-i,o = 5 µm
Saprobic on dead twigs of Rosa sp. Sexual morph: See Liu et al. (2015). Asexual morph: Conidiomata 140–200 μm high, 130–220 μm diam. (x̅ = 172 × 165 μm, n = 5), pycnidial, solitary or aggregated, immersed, erumpent neck, visible as black, uni- to multi-loculate, globose to subglobose, rarely irregular, glabrous, ostiole central, with minute papilla. Conidiomata walls 14–38 μm (x̅ = 27 μm, n = 8), wide, thick-walled, of equal thickness, composed of several layers of hyaline to dark brown, pseudoparenchymatous cells, outer layers comprising 4–5 cell layers of 6–12 × 2–5 μm (x̅ = 8.7 × 3.4 μm, n = 15), thick-walled, dark brown to black, organized in a textura angularis to textura prismatica cells, inner layers comprising 2–3 layers of 3–8 × 2–4 μm (x̅ = 5.3 × 3.2 μm, n = 15), thin-walled, hyaline, organized in a textura angularis. Conidiophores arising from basal cavity of conidiomata mostly reduced to conidiogenous cells. Conidiogenous cells 5–7 × 1–2 μm (x̅ = 5.8 × 1.6 μm, n = 25), holoblastic, phialidic, discrete, ampulliform to cylindrical, hyaline, aseptate, smooth-walled, guttulate. Conidia 3.2–4.5 × 1.7–2.4 μm (x̅ = 3.7 × 2.1 μm, n = 35), in culture Conidia 3.4–4.7 × 1.6–2.5 μm (x̅ = 4.1 × 2.1 μm, n = 35), solitary, one-celled, oblong to ellipsoidal, with rounded or obtuse ends, initially hyaline, becoming brown at maturity, smooth-walled.
Culture characteristics: Colonies on PDA fast growing, 33–37 mm diam. after 2 weeks at 25–30 °C, greenish-grey to grey, forming white tufts on surface, slightly radiating; reverse brown to dark brown at the margin, dark brown to black in the centre; medium dense, circular, flattened to slightly raised, dull to rough with entire edge, fairy fluffy to velvety, slightly radially furrowed.
Material examined: Thailand, Mueang, Chiang Rai District, Chiang Rai 57100, (20° 03′ 24.7′′ N, 99° 52′ 23.5′′ E), dead twigs of Rosa sp. (Rosaceae), 20 August 2017, MC. Samarakoon, SAMC070 (MFLU 18–0845, HKAS 102350), living culture MFLUCC 18-0505.
Hosts: on dead frond of palm (Liu et al. 2015), Rosa sp. (this study)
Distribution: Thailand (Liu et al. 2015; this study)
GenBank numbers: OM293742 (LSU), OM293753 (SSU), OM305060 (tef1), OM305066 (tub2)
Notes: Our new collection of Palmiascoma gregariascomum is described on dead twigs of Rosa species from Thailand. We found the asexual morph of the taxon with a similar range of conidiogenous cells (5–7 × 1–2 μm vs 5–12 × 2–4 μm) and conidia (3.2–4.5 × 1.7–2.4 μm, 3.4–4.7 × 1.6–2.5 μm vs 4–6 × 2–3 μm) in morphologies compared to the type species (MFLU 11-0211). In multigene phylogeny, our strain clusters with MFLU 11–0211 with high statistical (81/ML, 1.00 BYPP) support. Based on similar morphology and phylogenetic analyses, here we provide a new host record of Palmiascoma gregariascomum on Rosa sp. from Thailand (Fig. 16).
Phylogram generated from maximum likelihood analysis based on combined LSU, SSU and tef1 sequenced data for Bambusicola and allied genera Twenty-two strains are included in the combined sequence analyses, which comprise 2761 characters with gaps. Murilentithecium clematidis (MFLUCC 14–0562) is used as the outgroup taxon. Tree topology of the ML analysis was similar to the BYPP. Bootstrap support values for ML ≥ 50% and BYPP ≥ 0.95 are given above the nodes. New strain is in red. Ex-type and representative strains are in bold
Coniothyriaceae W.B. Cooke, Revta Biol., Lisb. 12: 289 (1983) [1980–1983]
Notes: Coniothyriaceae was introduced by Cooke (1983) to accommodate species of Coniothyrium. Kirk et al. (2008) synonymized Coniothyriaceae with Leptosphaeriaceae. De Gruyter et al. (2013) based on morphology and phylogenetic analyses showed that the type species C. palmarum is distinct from Leptosphaeriaceae and reinstated Coniothyriaceae in Pleosporales. Wijayawardene et al. (2022) accepted Coniothyrium, Foliophoma, Neoconiothyrium, Ochrocladosporium and Staurosphaeria in this family.
Coniothyrium Corda, Icon. fung. (Prague) 4: 38 (1840)
The genus is typified with C. palmarum Corda. In earlier studies Contiothyrium was considered as the asexual morph of Leptosphaeria, Mycosphaerella and Massarina (Sivanesan 1984). However, later studies based on molecular data transferred many species from Contiothyrium (Verkley et al. 2014; Hongsanan et al. 2020b). De Gruyter et al. (2013) reinstated Coniothyriaceae and included Coniothyrium as the family type.
Coniothyrium yuccicola Chaiwan, Jayaward., & K.D. Hyde, sp. nov.
Index Fungorum number: IF559467; Facesoffungi number: FoF08170; Fig. 17
Coniothyrium yuccicola (MFLU 17–2529, holotype) a Specimen with conidiomata. b Black acervuli. c Brown setae. d Conidiophores with basal parts of setae. e Hyaline conidiogenous cells. f Conidiomata on PDA. g Hyaline conidia. h Germinating conidium. i Appressoria. j Reverse view of the colony. k Upper view of the colony. Scale bars: a = 1000 μm, b = 500 μm, c = 20 μm, d = 15 μm, e, f = 10 μm
Etymology: Referring to the host Yucca
Holotype: MFLU 17–2529
Pathogenic on living leaves and peduncle stems of Yucca filamentosa. Asexual morph: Conidiomata 250–450 μm (x̅ = 329 μm, n = 10) diam., superficial on or immersed in the host. Conidiophores not present. Conidiogenous cells lining entire cavity, hyaline, cylindrical, 8 ± 15 (x̅ = 11.9 μm) 3 ± 8 μm (x̅ = 5.1 μm), longer in culture than on host plants. Conidiogenesis holoblastic, proliferating percurrently. Conidia cylindrical, broadly rounded at apex, initially somewhat truncate at base, produced deep within the conidiogenous cells, secession rhexolytic, outer wall of conidiogenous cell often remaining on conidium, except at base, eventually disintegrating, olivaceous brown, 3-septate, lightly punctate, (2–)6.6–10.5(–14) × (1–)2–5(–6) μm (x̅ = 7.3 × 3.6 μm, n = 40) brown, smooth-walled or verruculose, aseptate, curved, both sides gradually tapering towards the round to slightly acute apex and truncate base, guttulate. Sexual morph: Not observed.
Material examined: Russia, Donetsk People's Republic, Donetsk City, Donetsk Botanical Garden, flowerbed, on dying peduncle stem and live leaves of Yucca filamentosa L. (Asparagaceae), 20 May 2017, Timur S. Bulgakov, DNK-108 (MFLU 17–2529, holotype); ex-type living culture MFLUCC 18–0456.
GenBank numbers: OM235094 (SSU); OM235097(LSU)
Notes: Coniothyrium yuccicola is an asexual morph. Based on our phylogenetic tree this species is closely related to C. concentricum (Fig. 18). Conidia of this species are brown aseptate, bacilliform, ellipsoid and often thick-walled (Fig. 17). Three Coniothyrium species are recorded on Yucca species (Farr and Rossman 2022): Coniothryrium bartholomaei from the USA (Oregon), C. herbarum from the USA (California), and C. yuccae from Argentina. Coniothyrium bartholomaei was reported as a plant pathogen that caused leaf spots of Yucca in Oregon (USA) (Pscheidt and Ocamb 2018; Barr 1992). Coniothyrium herbarum is known from USA (California) on the leaves of several closely related plants: Dracaena indivisa, Sansevieria sp. and Yucca angustifolia (Cash 1952), however, this species is invalid. Coniothyrium yuccae was found on dead leaves of Yucca gloriosa in Argentina (Buenos-Aires) (Farr 1973). Phaeosphaeriopsis yuccae is another morphologically similar taxon described from living leaves of Yucca filamentosa from Russia, Rostov region, Botanical Garden of Southern Federal University (Tibpromma 2017).
Phylogram generated from ML analysis based on combined LSU and SSU sequence data of selected taxa. The combined dataset comprises 41 strains with 1834 characters including gaps. The tree is rooted to Aposphaeria populina (CBS543.70) and Westerdykella capitulum (CBS 337.65). Maximum Likelihood bootstrap values ≥ 65% and BYPP ≥ 0.90 are displayed on the nodes, respectively. Newly introduced taxa are indicated in red. Ex-type and representative strains are in bold
Didymellaceae Gruyter, Aveskamp & Verkley, Mycol. Res. 113(4): 516 (2009)
Members of this family have a wide host range and have different life modes: endophytic, pathogenic and saprobic (Hongsanan et al. 2020b). Forty-four genera are accepted in this family (Wijayawardene et al. 2022)
Ascochyta Lib., Pl. crypt. Arduenna, fasc. (Liège) 1(Praef.): 8 (1830)
Notes: Ascochyta was introduced by Libert (1830) with A. pisi as the type species. Species of Ascochyta are characterized by the globose locules with perithecial protuberances immersed in the stroma (Chen et al. 2015). Species are mostly endophytes, pathogens and saprobes with a wide host range and a geographical distribution (Hongsanan et al. 2020b; Farr and Rossman 2022). We provide a new host record of Ascochyta medicaginicola from Prunus cerasifera in Russia.
Ascochyta medicaginicola Q. Chen & L. Cai, in Chen et al., Stud. Mycol. 82: 187 (2015)
Index Fungorum number: IF814129; Facesoffungi number: FoF08216; Fig. 19
Pathogenic on living twigs of Prunus cerasifera, noticeable as black, circular dots on the host surface. Asexual morph: Conidiomata 165–190 μm high, 170–210 μm wide, black, scattered or gregarious, superficial to immersed, black, subglobose to globose, uniloculate. Ostiolar neck 25–50 μm long, 3–5 μm wide, covered with 1-celled, thick-walled, dark brown to almost black. Peridium 15–25 μm wide at the base, 30–80 μm wide at the sides, thick, comprising 3–4 layers, outer most layer heavily pigmented, thick-walled, comprising blackish to dark brown loosely packed cells of textura angularis, inner layer composed 3–5 layers, pale brown to hyaline, cells towards the inside lighter, flattened, thick-walled cells of textura angularis. Sexual morph: Not observed.
Material examined: Russia, Rostov region, Shakhty, near a railroad, on dead twigs of Prunus cerasifera Ehrh. (Rosaceae), 11 May 2017, Timur S. Bulgakov, T-1832 (MFLU 17–2138); living culture MFLUCC 18–0453.
Hosts: Medicago albus, Medicago sativa, Medicago sp. (Fabaceae, Hyde et al. 2020), Prunus cerasifera (Rosaceae, this study), Scabiosa sp. (Caprifoliaceae, Tibpromma et al. 2017) and Trichosanthes dioica (Cucurbitaceae, Sarkar et al. 2018-pathogenicity data are available).
Distribution: Canada, Czech Republic, France, Italy, USA (Hyde et al. 2020a, b, c), India (Sarkar et al. 2018), Thailand (this study)
GenBank number: OM235096 (ITS)
Notes: Our collection shares similar morphological characteristics with the ex-type strain of A. medicaginicola (Boerema et al. 2004; Chen et al. 2013). The multigene phylogenetic analysis shows that our specimen groups in the Ascochyta medicaginicola clade with 96/0.90 ML/BYPP support (Fig. 20). Four Ascochyta species have been recorded based on the morphological description from Rosaceae plants in Russia (Melnik 2000; Farr and Rossman 2022): Ascochyta idaei on Rubus idaeus in the Leningrad region, Kursk region, and Stavropol region; A. potentillarum on Potentilla reptans in Arkhangelsk region (Melnik 2000), Lipetsk region (Sarycheva et al. 2009), Republic of Crimea (Ovcharenko 2011) and Voronezh region (Melkumov 2015); A. pruni on Prunus padus in Leningrad region; Ascochyta sorbina on Sorbus torminalis in Stavropol region. As these species were identified based on morphology alone, correct species identification is yet to be done. Our collection provides the first host record of Ascochyta medicaginicola on Rosaceae based on both morphological and phylogenetic data.
Phylogram generated from maximum likelihood analysis based on combined, ITS, LSU and tub2 sequence data of selected taxa. Related sequences were obtained from GenBank. Forty-one strains are included in the analyses, which comprise 633 characters including gaps. The tree was rooted with Phoma herbarum (CBS 377.92 and CBS 502.91). The maximum likelihood bootstrap (ML) values > 65%) are given above the nodes. The new isolate in red bold
Didymosphaeriaceae Munk, Dansk bot. Ark. 15(no. 2): 128 (1953).
Didymosphaeriaceae represents an important family in Dothideomycetes. The family is typified by Didymosphaeria, with D. epidermidis as the type species (Hongsanan et al. 2020b). While taxa of Didymosphaeriaceae are often endophytic, pathogenic or saprobic on various plant hosts (Gonçalves et al. 2019; Hongsanan et al. 2020b), they can sometimes also be pathogenic to human beings (Hongsanan et al. 2020b). Species of Didymosphaeriaceae are mainly characterised by brown, 1–3-septate or muriform ascospores and cellular or trabeculate pseudoparaphyses in their sexual morphs while their asexual morphs are fusicladium-like or phoma-like (Hyde et al. 2013; Hongsanan et al. 2020b). After several taxonomic revisions, 32 genera have been accepted in the family (Hongsanan et al. 2020b).
Spegazzinia Sacc., Spegazzinia: [1] (1879)
Spegazzinia was introduced by Saccardo (1880), with S. ornata as the type species and it currently comprises hyphomycetous taxa. The genus was initially accommodated in Apiosporaceae (Sordariomycetes) based on morphology (Hyde et al. 1998). It was then transferred to Didymosphaeriaceae (Dothideomycetes) based on molecular evidence (Tanaka et al. 2015). Taxa in this genus are mainly characterised by a distinctive conidiophore ontogeny as well as two types of conidia (Samarakoon et al. 2020; Hongsanan et al. 2020b). The latest two taxa added to Spegazzinia are S. musae, reported as a saprobe on Musa sp., and S. camelliae, isolated as an endophyte from Camellia sinensis var. assamica (Samarakoon et al. 2020; Suwannarach et al. 2021).
Spegazzinia deightonii (S. Hughes) Subram., J. Indian bot. Soc. 35: 78 (1956).
Index Fungorum Number: IF 306062; Facesoffungi number: FoF 07238; Fig. 21
Spegazzinia deightonii (MFLU 22-0277, new host record) a Host. b Close-up of conidia on host. c Mass of conidia. d Conidiogenous cell of stellate conidia. e Stellate conidium on a conidiophore. f Stellated conidium. g Disk-liked conidium. h Disk-like conidium with attached conidiogenous cell. Scale bars: c–h = 10 μm
Saprobic on palm stem. Asexual morph: Hyphomycetous. Sporodochia powder-like, dark, dense, dry, 1–3.5 mm in diameter. Conidiophores 65–120 × 1–3 μm (x̅ = 93.5 × 2.4 μm, n = 15), macronematous, micronematous, narrow, subspherical to doliiform, flexuous or erect, unbranched, hyaline to pale brown, verruculose. Conidiogenous cells 10–20 × 2–4 μm (x̅ = 15.8 × 3 μm, n = 15), basauxic, terminal, erect, unbranched, hyaline to pale brown, verruculose, each producing a single, holoblastic conidium at the conidiophore apex. Conidia two types, disc-like and stellate; disc-like conidia 20–28 × 17–19 μm (x̅ = 25.2 × 18.1 μm, n = 20), usually 8-celled, solitary, hyaline when immature, pale to dark brown on maturity, cross-septate, slightly constricted at the septa, with short and blunt spines at the periphery, frequently accompanied by attached conidiogenous cells post splitting from the conidiophores; stellate conidia 18–27 × 16–29 μm (x̅ = 22.6 × 24.3 μm, n = 20), globose or variously shaped, frequently 4- to 6-celled, solitary, septate, deeply constricted at the septa, pale to dark brown, comprising spines 4–5 μm long. Sexual morph: Not observed.
Culture characteristics: Conidia germinating on PDA within 16–18 h. Colonies growing on PDA, reaching a diameter of 55 mm after 14 days at 25 °C, aerial, moderately dense, undulate margine, middle grey, periphery olive green at immature stage and brownish gray at maturity; reverse greyish white to white.
Material examined: Thailand, Chiang Rai, on dead stem of palm (Arecaceae), 20 December 2019, Binu C. Samarakoon, E003 MFLU 22-0277), living culture MFLUCC 22-0180.
Hosts: Spegazzinia deightonii has been reported from several hosts, including, Andropogon glomeratus (Arnold 1986), Areca catechu (Tianyu 2009; Matsushima 1980), Arundo donax (Tanaka et al. 2011), Bambusa vulgaris (Camino-Vilaro et al. 2019), Calathea makoyana (Tianyu 2009), Cocos nucifera (Tianyu 2009), Imperata cylindrical (Thaung 2008), Musa sp. (Samarakoon et al. 2018), Panicum maximum (Lu et al. 2000; Wong and Hyde 2003), Phoenix hanceana (Tianyu 2009), Quercus xalapensis (Heredia et al. 1995), Saccharum spontaneum (Mel'nik et al. 2000), Thysanolaena latifolia (Mel'nik et al. 2000) and Tillandsia sp. (Delgado-Rodriguez et al. 2002).
Distribution: China (Tianyu 2009), Cuba (Arnold 1986; Delgado-Rodriguez et al. 2002; Camino-Vilaro et al. 2019), Hong Kong (Lu et al. 2000; Wong and Hyde 2003), Japan (Tanaka et al. 2011), Mexico (Heredia et al. 1995), Myanmar (Thaung 2008), Philippines (Whitton et al. 2012), Taiwan (Matsushima 1980), Thailand (Samarakoon et al. 2020), United States (Delgado 2008), Vietnam (Mel'nik et al. 2000).
GenBank numbers: ON885254 (SSU); ON873996(LSU); ON873998(ITS); ON885741 (tef1)
Notes: Our isolate (MFLUCC 22-0180) clusters with other strains of Spegazzinia deightonii with 95% ML, statistical support in the multi-loci phylogenetic tree (Fig. 22). Pairwise comparison of DNA sequence data shows insignificant differences among our strain (MFLUCC 22-0180) and the other strains of S. deightonii, following which our isolate is considered as S. deightonii. Furthermore, the isolate in the present study shares similar features with other strains of S. deightonii, including two types of pale to dark brown conidia which are multi-cellular, constricted at the septa and comprise either long (stellate conidia) or short (disc-like conidia) spines (Ellis 1961; Tanaka et al. 2011; Samarakoon et al. 2018). Small differences in sizes may be accounted for by host variations. Spegazzinia deightonii has been reported on a palm substrate in China, Taiwan and the United States (Matsushima 1980; Delgado 2008; Tianyu 2009). We recovered the species from Thailand and thus report it as a new record on Palm from Thailand. Recently this species was also reported to occur in Thailand, in the same Province and area, on Musa sp. (Samarakoon et al. 2020).
Phylogram generated from maximum likelihood analysis based on combined LSU, SSU, ITS and tef sequence data of selected taxa in Didymosphaeriaceae. Bootstrap support values for ML ≥ 65% and BYPP ≥ 0.95 are indicated above or below the branches. Ex-type strains are in bold. The new isolate is in blue. The tree is rooted with Laburnicola zaaminensis (TASM 6152) and L. murtfirmis (MFLUCC 16-0290).
Lindgomycetaceae K. Hiray., Kaz. Tanaka & Shearer, in Hirayama et al., Mycologia 102(3): 733 (2010)
Notes: Lindgomycetaceae was introduced by Hirayama et al. (2010) with Lindgomyces (L. ingoldianus) as the generic type. Most Lindgomycetaceae members have been recorded from freshwater habitats and the freshwater isolates form a distinct lineage from other Dothideomycetes taxa based on ribosomal sequence data (Zhang et al. 2013; Hyde et al. 2013; Dong et al. 2021). There are seven genera in the family, including Hongkongmyces which is also associated with human diseases (Tsang et al. 2014; Linqiang et al. 2020). The genus forms a close relationship to Aquimassariosphaeria, Clohesyomyces, Lolia, and Trematosphaeria in the phylogenetic analyses of the ribosomal DNA and protein-coding genes such as tef (Abdel-Aziz and Abdel-Wahab 2010; Hyde et al. 2016). We, therefore, encourage further taxonomic studies to include protein-coding regions for a better resolution of taxa in Lindgomycetaceae (Fig. 24).
Hongkongmyces Tsang et al., Medical Mycol. 52(7): 740 (2014)
Hongkongmyces was introduced as a monotypic genus associated with human infections, and typified by H. pedis (Tsang et al. 2014). Six species H. aquaticus, H. brunneosporus, H. kokensis, H. pedis, H. snookiorum, and H. thailandicus are listed in Species Fungorum (2022a, b). The species are commonly associated with freshwater habitats as saprobes. Hyde et al. (2016) introduced the second species of Hongkongmyces based on sexual morph characters, which was collected on submerged wood in a stream in Thailand. Later, an asexual morph of H. snookiorum was isolated from submerged wood (Crous et al. 2018). Hongkongmyces snookiorum was reported as an opportunistic fungal infection in a transplant patient (Deng et al. 2020). Schoch et al. (2012) and Aime et al. (2021) recommend using the fungal barcode locus such as the ITS region as well as any additional secondary barcode locus such as protein-coding region for the introduction of new species. Most Hongkongmyces species lack barcodes in public databases, and therefore, delineating species in the genus is a challenging task. In this paper, we introduce a new species of Hongkongmyces which was collected in a freshwater pond in Jilin Province of China based on comprehensive morphology and phylogeny analyses (Figs. 23 and 24).
Hongkongmyces changchunensis (HMJAU 60185, holotype) a, b Appearance of conidiomata on host surface. c Vertical section through conidioma. d Ostiole canal. e Section of partial conidioma peridium. f–h Conidiogenous cells and developing conidia. i–l Developmental state of conidia. Scale bars: a = 500, b = 200 µm, c = 100 µm, d, e = 50, f, l = 20 µm, g–k = 10 µm
Hongkongmyces changchunensis Phukhams., W.X. Su, & Y. Li, sp. nov.
Index Fungorum number: IF559493; Facesoffungi number: FoF10725; Fig. 23
Etymology: The epithet reflects the locality, Changchun.
Holotype: HMJAU 60185.
Saprobic on decaying Betula twigs submerged in a freshwater stream. Sexual morph: Not observed. Asexual morph: Conidiomata 77–188 × 85–174 μm diam, pycnidia scattered, semi-immersed, globose or ellipsoidal, black, coriaceous, ostiolate. Peridium 10–28 μm thick, composed of large, irregular, and dark brown cells arranged in a textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 2.6–23.5 × 1.6–4.9 μm (x̅ = 10 × 3 μm, n = 20), enteroblastic, phialidic, determinate, cylindrical to subcylindrical, hyaline, thin-walled. Conidia 10–18 × 7–13 μm (x̅ = 13 × 10 μm, n = 43), ellipsoidal, obovoid or irregular, aseptate, guttulate, hyaline, thin-walled, lacking mucilaginous sheath, turning brown at senescence stage.
Culture Characteristics: Colonies on PDA reaching 20 mm in 7 days at 20 °C, flat with entire margin, circular, grey to black, aerial mycelium becoming grey towards the edge; reverse grey-olivaceous to black, smooth.
Material examined: China, Jilin Province, Changchun District, Jingyuetan National Scenic Areas, on the submerged twigs in a stream of Betula sp. (Betulaceae), 12 May 2021, Chayanard Phukhamsakda (SWX32), (HMJAU 60185, holotype); ex-type living culture, CCMJ5008.
GenBank numbers: OL897173 (LSU), OL891809(SSU), OL996122 (ITS), OL944603 (tef1), OL944507(rpb2).
Notes: Phylogenetic analyses of LSU, SSU, ITS and tef sequence data show that Hongkongmyces changchunensis (CCMJ5008) is related to the type species Hongkongmyces thailandicus (MFLUCC 16–0406), with good support (92% ML/0.93 BYPP; Fig. 23). Hongkongmyces changchunensis was collected from submerged substrates in a freshwater habitat in Jilin Province of China. Both species were collected from fresh water habitats, but from different country (Hyde et al. 2016). Hongkongmyces changchunensis is similar to Hongkongmyces aquaticus in having phialidic conidiogenous cells and hyaline, variable shaped conidia, but it lacks sympodial proliferations (Dong et al. 2020). Hongkongmyces changchunensis is characterized by wet, globular, mass of conidia on natural substrates after drying and smooth surfaced conidia containing multiple small bubbles. Herein, we propose a new species based on both morphology and phylogenetic analyses.
Phylogram generated from Bayesian 50% majority-rule consensus phylogram based on combined LSU, SSU, ITS and tef sequence data of Lindgomycetaceae. The topology and clade stability of the combined gene analyses was compared to the single gene analyses. The tree is rooted with members of the Aigialaceae. Bootstrap values ≥ 50% (MP and ML) and BYPP ≥ 0.90 are given at the nodes. The type-delivered strains are in bold; the new isolates are in blue
Lindgomyces K. Hiray., Kaz. Tanaka & Shearer, in Hirayama et al., Mycologia 102(3): 733 (2010)
Notes: Lindgomyces was introduced in Lindgomycetaceae by Hirayama et al. (2010) based on the type species L. ingoldianus (Shearer & K.D. Hyde) K. Hiray., Kaz. Tanaka & Shearer. The morphology of the species of Lindgomyces is similar to Massarina eburnea and Lophiostoma macrostomum, but they differ from ascomata and ascospores (Raja et al. 2013; Hirayama et al. 2010). There are 14 epithets listed in Lindgomyces in Index Fungorum (2022a, b). Keys to the species of Lindgomyces were provided by Dong et al. (2020). In this study, we describe a novel species, Lindgomyces guizhouensis based on the unique morphological features and multi–gene phylogenetic analysis of a combined LSU and ITS sequence data (Fig. 26).
Lindgomyces guizhouensis J. Mai, Y.Z. Lu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559506; Facesoffungi number: FoF 10676; Fig. 25
Lindgomyces guizhouensis (GZAAS 21–0383, holotype). a–c Superficial ascomata on unidentified plant substrate. d Vertical section of an ascoma. e Peridium. f Hamathecium g Asci with hamathecium h–i Asci j–n Ascospores o Germinating ascospore p–q Colony on PDA from above and below. Scale bars: d, e = 100 μm, f–o = 20 μm
Etymology: Referring to collecting site in Guizhou Province, China.
Holotypus: GZAAS 21–0383
Saprobic on decaying wood in terrestrial habitats. Asexual morph: Ascomata 396–548 μm high × 466–514 μm diam., superficial, black, smooth, scattered, solitary, erumpent, subglobose to broadly conical, with a centrally located ostiole. Peridium 44–79 μm thick, thick–walled, composed of pale brown cells of textura angularis. Hamathecium 1–2 μm wide, septate, branched, pseudoparaphyses above the asci. Asci 110–154 × 15–28 μm, bitunicate, fissitunicate, apically rounded with an indistinct ocular chamber, subsessile, broadly cylindrical to clavate. Ascospores 30–51 × 8–12 μm, (x̅ = 44.7 × 10.5 μm, n = 25), obliquely uniseriate to 2–3–seriate, fusiform, slightly curved with narrowly rounded ends, 1–septate, 2–4 guttules in each cell, wall gray while in the ascus, smooth or slightly verruculose. Sexual morph: Not observed.
Culture characteristics: Colonies on PDA reaching 43 mm in 20 days at 25 °C, flat, filiform, gray to near–black from center to edge, with moderate aerial mycelium, smooth, irregular; In reverse, gray to pale brown.
Material examined: China, Guizhou Province, Longli, on decaying wood submerged in a freshwater stream, 2 September 2020, Jian Ma, LLSB06 (GZAAS 21–0383, holotype); ex–type living culture, GZCC 21–0669.
GenBank numbers: OM339435 (ITS), OM339432 (LSU).
Notes: In a BLASTn search of NCBI GenBank, the closest match of LSU and ITS sequence data for Lindgomyces guizhouensis is 99.54% and 96.96% similar to L. pseudomadisonensis (KT 2742). The multi–loci phylogenetic analyses of the combined LSU and ITS sequence dataset confirmed the new strain obtained belonging to Lindgomyces, where it is sister to L. griseosporus with 99% ML and 1.00 BYPP support (Fig. 25). Morphologically, L. guizhouensis is also most similar to L. griseosporus in having superficial, black with roughened surface ascomata; a thick peridium; bitunicate, clavate, subsessile asci and fusiform, 1-septate, guttules ascospores. However, our new collection differs from L. griseosporus in having larger ascomata (396–548 × 466–514 μm vs 240–290 × 320–350 μm), fragile peridium and smaller asci (110–154 × 15–28 μm vs 140–180 × 24–30). Therefore, we introduce Lindgomyces guizhouensis as a new species.
Phylogram generated from maximum likelihood analysis based on combined LSU and ITS sequence data. Twenty–two taxa were included in the combined analyses, which comprised 2096 characters (LSU: 841, ITS: 1255) after alignment. Bootstrap support values for ML ≥ 50% and BYPP ≥ 0.95 are given above the nodes. Hongkongmyces aquaticus (MFLUCC 18–1150), H. pedis (HKU35) and H. thailandica (MFLUCC 16–0406) were used as the outgroup taxa. The newly generated sequence is indicated in blue. The ex–type strains are indicated in bold
Lophiostomataceae Sacc.
Lophiostomataceae, typified by Lophiostoma, was first erected by Nitschke (1869) as ″Lophiostomeae″, but it was established by Saccardo (1883) as ″Lophiostomaceae″ and placed in Pleosporales. Lophiostomataceae species are mostly characterized by slot-like ostiole on apex of a flattened neck that are usually saprobes that grow on herbaceous and woody plants from terrestrial and aquatic environments (Holm and Holm 1988; Mugambi and Huhndorf 2009; Thambugala et al. 2015; Hashimoto et al. 2018). The historical account of Lophiostomataceae and recent generic notes of Lophiostomataceae were provided by Hyde et al. (2013) and Hongsanan et al. (2020a, b), respectively. Wijayawardene et al. (2022) outlined and accepted 30 genera of Lophiostomataceae with Lophiostoma as speciose genera (ca. 100). Calabon et al. (2022) listed 20 species under 10 genera of Lophiostomataceae from freshwater environments. In this series, we introduced two new species of Lophiostomataceae from terrestrial and freshwater habitats.
Neovaginatispora A. Hashim., K. Hiray. & Kaz. Tanaka.
Notes: Neovaginatispora, typified by N. fuckelii, was introduced by Hashimoto et al. (2018) based on their phylogenetic analysis and morphological differences of peridium (i.e., thinner peridium that is uniformly thick and composed of two cell layers) compared to Vaginatispora (Thambugala et al. 2015). Recent phylogenetic analysis shows that Neovaginatispora strains form a separate subclade with Lentistoma and Vaginatispora (Bao et al. 2019; Phukhamsakda et al. 2020; Hyde et al. 2020a, b, c). Two Neovaginatispora species are accepted, N. fuckelii and N. clematidis. Neovaginatispora fuckelii has a cosmopolitan distribution that thrives on various hosts in terrestrial habitats (Wang and Lin 2004; Thambugala et al. 2015; Hyde et al. 2016; Hyde et al. 2020a, b, c) while Bao et al. (2019) reported this on freshwater habitats. The second species, N. clematidis, was introduced by Phukhamsakda et al. (2020) from the dead stems of Clematis viticella in Belgium. Neovaginatispora clematidis differs from N. fuckelii on their ascospore morphology (broad fusiform with a single eusepta). In this study, the third Neovaginatispora species is introduced based on the collection from dead stems of Mangifera indica in Taiwan.
Neovaginatispora mangiferae Tennakoon, M.S. Calabon, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF559843; Facesoffungi number: FoF12720; Fig. 27
Neovaginatispora mangiferae (MFLU 18–0069, holotype). a Appearance of ascomata in host substrate. b Vertical section of ascoma. c Peridium. d Pseudoparaphyses. e Ostiole with numerous periphysis. f, g Bitunicate asci. h–k Ascospores in different stages of maturity. Scale bars: b = 100 µm, c, d, h–k = 5 µm, e, f, g = 20 µm
Etymology: Name reflects the host Mangifera indica, from which the holotype was collected.
Holotype: MFLU 18–0069
Saprobic on decaying stem of Mangifera indica. Sexual morph: Ascomata 430–500 μm high, 320–350 μm diam., scattered, semi-immersed to immersed, papilla erumpent through host surface, coriaceous to carbonaceous, dark brown to black, globose to subglobose, ostiolate. Ostiole crest-like, variable in shape, central, periphysate, broadly papillate, with an irregular pore-like opening. Peridium 25–40 μm wide, thick, composing two layers of irregular cells arranged in a textura angularis, outer layer with darker, light brown to brown flattened cells, inner layer comprising several layers of hyaline cells. Hamathecium 1.5–3 μm wide, massive, long cylindrical cellular, anastomosed, cellular pseudoparaphyses, hyaline, septate with small guttules. Asci 60–80 × 7–9 μm (x̅ = 60.9 × 7.2 μm, n = 20), 8-spored, bitunicate, cylindrical to cylindric-clavate, short pedicellate with furcate to obtuse ends, apically rounded with an indistinct ocular chamber. Ascospores 13–17 × 3.0–5.5 μm (x̅ = 15.1 × 3.7 μm, n = 25), biseriate, overlapping, fusiform to sunbfusoid, strongly constricted at the median septum, straight or slightly curved, 1-septate at the center, enlarged near the septum at the upper cell, hyaline, guttulate, smooth-walled, mostly with 4 guttules, with a helmet-shaped to subcylindrical mucilaginous sheath at each end when immature, invisible at maturity, 2.1–5.6 μm long, 2.2–4.4 μm wide. Asexual morph: Not observed.
Culture characteristics: Conidia germinating on potato dextrose agar (PDA) within 24 h. Germ tubes produced from the apical cell of conidia. Colonies growing on PDA, reaching 25–30 mm in 2 weeks at 25 °C. Mycelia superficial, medium dense, irregular, flat, slightly raised, surface smooth with crenate edge, fluffy to velvety with smooth aspects, zonate with different sector yellowish-brown to moss brown at the margin brownish-grey at the center; from below, light moss brown at margin, dark gray at the middle, brown at the center, no pigmentation and sporulation.
Material examined: Taiwan, Chiayi, Fanlu Township area, Dahu village, dead stems of Mangifera indica (Anacardiaceae), 5 August 2017, D.S. Tennakoon, DTW 018C (18–0069, holotype), ex-type living culture, MFLUCC 17–2652.
GenBank numbers: MG931027(LSU), MG931030 (SSU), MG931033 (ITS).
Notes: The isolate MFLUCC 17–2652 has formerly identified as Neovaginatispora fuckelii by Tennakoon et al. (2018) but in our phylogenetic analysis, it clustered with Neovaginatispora sp. MFLUCC 11–0577, and forms a separate subclade with N. fuckelii and N. clematidis with 100% MP, 1.00 BYPP support (Fig. 28). The pairwise nucleotide comparison of LSU and ITS of N. mangiferae MFLUCC 17–2652 show 2 bp (0/24%, 838 bp) and 1 bp (0.23%, 442 bp) differences with Neovaginatispora sp. MFLUCC 11–0577, respectively, with no differences on SSU sequence data. For this reason, we named the unidentified Neovaginatispora species (strain MFLUCC 11–0577) as another strain of N. mangiferae. In pairwise nucleotide comparisons of Neovaginatispora mangiferae MFLUCC 17–2652 with N. fuckelii MFLUCC 17–1334, there is a nucleotide difference of 6.94% (30/432 bp) in ITS, and 2.71% (22/813 bp) in LSU genes. A pairwise nucleotide comparisons of LSU, SSU, and ITS sequence data of N. mangiferae MFLUCC 17–2652 and N. clematidis reveals 22 bp (2.72%, 810 bp), 6 bp (0.64%, 938 bp), and 27 bp (7.03%, 384 bp) differences, respectively. Neovaginatispora mangiferae differs from N. fuckelii KT 634 by having larger ascomata (430–500 × 320–350 μm vs 150–180 × 200–250 μm) and thicker peridium (25–40 μm vs 15–25 μm), and periphyses in ostiole. The asci and ascospores are similar in size but ascospores of N. mangiferae is fusiform with acute ends but N. fuckelii has a fusiform ascospores with obtuse ends (Thambugala et al. 2015; Tennakoon et al. 2018; Bao et al. 2019). Neovaginatispora mangiferae has larger ascomata (430–500 × 320–350 μm vs 145–250 × 108–160 μm), shorter asci (60–80 × 7–9 μm vs. 53–105 × 9–12 µm), and smaller ascospores (13–17 × 3.0–5.5 μm vs. 16–19 × 5–7 µm) compared to N. clematidis (Phukhamsakda et al. 2020).
Phylogram generated from maximum likelihood analysis based on combined LSU, SSU, ITS, tef1, and rpb2 sequence data representing Lophiostomataceae (Pleosporales). Eighty-one strains are included in the combined analyses which comprised 3726 characters (741 characters for LSU, 973 characters for SSU, 526 characters for ITS, 1001 characters for tef1, and 1011 characters for rpb2) after alignment. Angustimassarina acerina (MFLUCC 14–0505) and Angustimassarina populi (MFLUCC 13–0034) in Amorosiaceae (Pleosporales) were used as the outgroup taxa. Bootstrap support values for ML ≥ 75% are given above the nodes (left side) and BYPP ≥ 0.95 are given above the nodes (right side). Ex-type strains are in bold and newly generated sequences are in blue
Vaginatispora K.D. Hyde, amended
Saprobic on submerged wood, intertidal wood, dead twigs, endocarp or fallen fruit pericarp. Sexual morph: Ascomata solitary or scattered, immersed to erumpent, uniloculate, subglobose, glabrous, dark brown to black. Ostiolar neck slit-like, elongate, laterally compressed, composed of globose to elongate, brown to black cells, with a pore-like opening and hyaline periphyses. Peridium unequal in thickness, two-layered, outer layer comprising somewhat flattened cells, fusing and indistinguishable from the host tissues, inner layer comprising lightly pigmented to hyaline cells. Pseudoparaphyses numerous, cellular, hypha-like, hyaline, septate, anastomosing above the asci. Asci 8-spored, bitunicate, fissitunicate, cylindrical to clavate, with a short or long pedicel, apically round with an ocular chamber. Ascospores uni- to bi-seriate, narrowly ellipsoidal or fusiform, straight to slightly curved, hyaline when immature, becoming yellow when mature, 1-septate, occasionally producing pseudosepta, septum mostly median, upper cell slightly broader than lower cell, smooth, thin-walled, with or without bipolar appendages or entire sheath. Asexual morph: Hyphomycetous. Hyphae septate, hyaline to lightly pigmented, mostly smooth, thick-walled, moniliform. Chlamydospores numerous, mostly in chains, globose to subglobose, smooth, initially hyaline then lightly pigmented at maturity, arising from the mycelium, formed intercalarily or terminally.
Type species: Vaginatispora aquatica K.D. Hyde
Notes: Hyde (1995) introduced Vaginatispora to accommodate Vaginatispora aquatica K.D. Hyde (= Lophiostoma vaginatispora Huang Zhang & K.D. Hyde), which was previously placed in Massarinaceae. Eight species are included in this genus and members are characterized by depressed globose ascomata, immersed beneath a blackened neck, with a slot-like ostiole, numerous filamentous pseudoparaphyses, cylindrical to clavate asci and narrowly ellipsoidal, hyaline, 1-septate ascospores with a mucilaginous collar around its equator, having large guttules in each cell, and a spreading papilionaceous sheath (Thambugala et al. 2015; Hashimoto et al. 2018). We followed the treatment of Dong et al. (2020) and Hongsanan et al. (2020a, b) in this genus.
Vaginatispora lignicola M.S. Calabon, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF559844; Facesoffungi number: FoF12721; Fig. 29
Vaginatispora lignicola (MFLU 22–0116, holotype). a Host twig. b, c Appearance of erumpent ascomata in host substrate. d Vertical section of ascoma. e Slot-like ostiole with numerous periphysis. f Peridium. g Pseudoparaphyses. h Bitunicate asci. i Ocular chamber. j–n Ascospores in different stages of maturity. o Germinated ascospore. p Culture on MEA. q–s Hyphae. (t) Terminally and (u) intercalary swollen cells. v–x Moniliform hyphae with constricted septa. y–ab Multicellular bodies. Scale bars: a = 2 mm, b–d = 200 µm, e, g = 20 µm, f = 50 µm, h, j = 100 µm, k–o, y, z = 50 µm, q–x, aa = 20 µm
Etymology: Referring to this taxon dwelling on wood
Holotype: MFLU 22–0116
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: Ascomata 200–360 × 220–415 µm diam. (x̅ = 269 × 294 µm; n = 10), scattered to gregarious, immersed to semi-immersed, erumpent at maturity, coriaceous, black, subglobose, ostiolate. Ostiole slot-like, central, elongated, pore-like opening, plugged by hyaline, filamentous hyphae, periphysate. Peridium 40–65 µm wide, circular, symmetric, outermost layer heavily pigmented, comprising a blackish to dark brown amorphous layer, flattened and loosely packed cells of textura angularis, inner layer composed of hyaline cell layers, flattened, thick-walled cells of textura angularis. Hamathecium 1.3–2.5 µm wide (x̅ = 1.9 µm, n = 30) comprising numerous, filamentous, branched, septate, pseudoparaphyses. Asci 125–145 × 15–30 µm (x̅ = 137 × 23 µm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical-clavate, short-pedicellate, apex rounded with a minute ocular chamber. Ascospores 50–72 × 14–17 µm (x̅ = 62 × 16 µm, n = 30), uniseriate to bi-seriate, overlapping, straight or slightly-curved, initially hyaline, becoming yellowish at maturity, fusiform, mostly with narrow acute ends, 1–3-septate, strongly constricted at the septa, smooth-walled, with numerous small guttules and 2 distinct large guttules. Asexual morph: Hyphae 2–5 μm wide, septate, hyaline to lightly pigmented, mostly smooth, thick-walled, moniliform. Chlamydospores 6–19 × 4.5–16 (x̅ = 10.9 × 8.4, n = 50), numerous, mostly in short chains, globose to subglobose, smooth, initially hyaline then becoming light brown at maturity, arising from the mycelium, formed intercalarily or terminally.
Culture characteristics: Conidia germinating on malt extract agar (MEA) within 24 h. Germ tubes produced from the basal and apical cell of conidia. Colonies growing on MEA, reaching 20–25 mm in 2 weeks at 25 °C. Mycelia superficial, circular, with entire margin, flat, smooth, from above greyish brown to grey, from below dark grey to black.
Material examined: Thailand, Tak Province, Tha Sing Yang, Ban Mae Ja Wang on decaying wood submerged in a freshwater river, 17 October 2019, N. Padaruth, CC43 (MFLU 22–0116, holotype), ex-type living culture, MFLUCC 22–0078.
GenBank numbers: MW287233 (LSU), MW287229(SSU), MW260329(ITS), MW512605(tef1), OP251197(rpb2)
Notes: Three species of Vaginatispora are recorded in freshwater habitats: V. aquatica (Hyde 1995; Tsui et al. 2000; Zhang et al. 2014a, b), V. armatispora (Hu et al. 2010; Bao et al. 2019; Hyde et al. 2019), and V. nypae (Hyde et al. 2020a, b, c; Boonmee et al. 2021). In this series, we introduce another novel species of Vaginatispora, V. lignicola, from a freshwater habitat in Thailand. Vaginatispora lignicola (MFLUCC 22–0078) confirms with the generic ascomatal morphology (erumpent, uniloculate, glabrous, slot-like ostiole, with numerous hyaline periphyses) but differs from the known members of the genus in having ascospores without a wide papilionaceous sheath or distinct hyaline appendages at both ends. Based on the phylogenetic analysis of combined LSU, SSU, ITS, tef1, and rpb2 sequence data, V. lignicola is basal to V. aquatica with high bootstrap support (97 ML and 1.00 BYPP) (Fig. 28). The former differs from V. aquatica in the measurement (50–72 × 14–17 µm vs 36–48 × 11–16) and color of the ascospores (initially hyaline then becoming yellowish at maturity vs hyaline), and the absence of a sheath when stained in Indian ink wherein the latter have wide papilionaceous sheaths (Hyde 1995). The novel taxon differs from the freshwater strains of V. armatispora and V. nypae by having larger ascospores (50–72 × 14–17 µm vs 22–30 × 5.5–8 µm vs 26–29 × 6–7 µm) without a mucilaginous sheath and hyaline appendages at both ends (Bao et al. 2019; Hyde et al. 2019; Boonmee et al. 2021). Multi-locus phylogenetic analyses showed that V. lignicola is a distinct taxon in Vaginatispora with 49 bp (9.32%, 526 bp) and 14 bp (1.74%, 804) nucleotide differences in the ITS and LSU sequence data of the type species, V. aquatica, respectively. The present work is the first report also of the morphological characteristics of the asexual morph of Vaginatispora. A simplified key to species of Vaginatispora is provided herein.
Key to species of Vaginatispora
1 Ascospores yellow at maturity, without sheath or appendages……………………………V. lignicola
1 Ascospores hyaline, with either sheath or appendages…………………………… 2
2 Ascospores with wide papilionaceous sheath, lacking appendages……………………………V. aquatica
2 Ascospores with or without a mucilaginous sheath, with distinct hyaline appendages……………………………3
3 Ascospores < 25 μm long……………………………V. scabrispora
3 Ascospores > 25 μm long……………………………4
4 Ascomata lack slit-like ostiole…………………V. nypae
4 Ascomata with slit-like opening…………5
5 Appendages 2–8 μm long…………V. microarmatispora
5 Appendages 6–8 μm long…………6
6 Ascomata > 450 μm wide, peridium up to > 40 μm thick…………V. amygdali
6 Ascomata < 450 μm wide, peridium up to < 40 μm thick…………7
7 Ascospores up to < 40 μm long…………V. armatispora
7 Ascospores up to > 40 μm long…………8
8 Ascospores 40–45 × 10–15 μm…………V. appendiculata
8 Ascospores 23–45 × 6–9 μm…………V. palmae
Phaeoseptaceae S. Boonmee, Thambugala & K.D. Hyde, Mycosphere 9(2): 323 (2018).
Notes: Phaeoseptaceae was introduced by Hyde et al. (2018) to accommodate Phaeoseptum, Lignosphaeria and Neolophiostoma. The LSU-SSU-rpb2-tef1 multigene phylogeny in Hyde et al. (2018) showed a well-supported Phaeoseptaceae clade sister to Halotthiaceae. The species in Phaeoseptaceae share immersed or erumpent ascomata, dark brown to black outer peridium, cylindrical, branched, septate pseudoparaphyses, 8-spored, bitunicate, cylindrical-clavate, long pedicellate asci, and brown, muriform ascospores. Based on morphology and phylogeny, Thambugala et al. (2015) treated Lignosphaeria in Dothideomycetes, genera incertae sedis, and Liu et al. (2019a, b, c, d) treated Neolophiostoma in Halotthiaceae. In a recent revision of Dothideomycetes, Hongsanan et al. (2020b) accepted only Phaeoseptum and Pleopunctum in Phaeoseptaceae which was followed by Wijayawardena et al. (2020, 2022).
Phaeoseptum Ying Zhang, J. Fourn. & K.D. Hyde, in Zhang, Fournier, Phookamsak, Bahkali & Hyde, Mycologia 105(3): 606 (2013)
Notes: Zhang et al. (2013) introduced Phaeoseptum, which differs from Mauritiana in having dictyosporous ascospores. There are six Phaeoseptum species: P. aquaticum (on driftwood of Salix sp., France), P. carolshearerianum (on decaying wood of Avicennia marina, India), P. hydei (on dead twigs of Delonix regia, Thailand), P. mali (on dead stems of Malus halliana, China), P. manglicola (on decaying wood of Avicennia marina, India) and P. terricola (on dead wood, Thailand).
Phaeoseptum thailandicum Samarak. & K.D. Hyde, sp. nov
Index Fungorum number: IF559754; Facesoffungi number: FoF11798; Fig. 30
Etymology: The specific epithet reflects the name of Thailand, where the species was collected.
Holotype: MFLU 19–2136
Saprobic on dead branches in terrestrial habitats. Sexual morph: Ascomata 270–350 μm high, 160–305 μm diam. (x̅ = 309 × 230.3 µm, n = 10), scattered to gregarious, fully immersed under a small blackened pseudoclypeus, if appearing as black, elongated regions on host surface 540–915 μm (x̅ = 640 µm, n = 8) length; ascomata depressed spherical, laterally flattened. Pseudoclypeus composed of host cells with black deposits. Peridium 6–22 µm (x̅ = 640 µm, n = 8) wide, pseudoparenchymatous, of thin-walled cells, at apex comprising isodiametric angular cells that are more pigmented outwardly, at sides with flattened hyaline cells, at base of angular pigmented cells. Hamathecium comprising 1.4–2.5 μm (x̅ = 1.9 µm, n = 20), wide septate, cellular pseudoparaphyses, situated between and above the asci, embedded in a gelatinous matrix. Asci 100–155 × 20–28.5 μm (x̅ = 129 × 23.9 μm, n = 25), 8-spored, rarely 32-spored, bitunicate, fissitunicate, cylindrical-clavate, with a distinct pedicel, apically rounded with a minute ocular chamber. Ascospores 25–35 × 8–11.8 μm (x̅ = 30 × 9.9 μm, n = 30), uniseriate at base and overlapping 2–3-seriate at apex, pale to dark brown, broadly fusoid with broadly rounded ends, slightly curved, 11 (9–12-transversally septate, with a vertical septum in nearly all median cells, not constricted at the septa, the septa partly pale brown, having at maturity a thickened and heavily pigmented appearance, wall smooth, without sheath or appendages. Asexual morph: Not observed.
Material examined: Thailand, Nan, Pua District, on an unidentified dicotyledonous dead branch, 29 January 2019, MC Samarakoon (SAMC216), (MFLU 19-2136, holotype; HKAS 106993, isotype), Phrae, on an unidentified dicotyledonous dead branch, 24 January 2019, MC Samarakoon (SAMC203), (MFLU 19–2126; HKAS 106983, paratypes).
GenBank numbers: MFLU 19-2126—OM293748 (ITS), OM293743 (LSU), OM293754 (SSU), OM305061 (tef1), OM305067 (tub2)
MFLU 19-2136—OM293749 (ITS), OM293744 (LSU), OM305056 (rpb2), OM293755 (SSU), OM305062 (tef1), OM305068 (tub2)
HKAS 106993—OM293750 (ITS), OM293745 (LSU), OM305057 (rpb2), OM293756 (SSU), OM305063 (tef1), OM305069 (tub2)
Notes: Our two new collections of Phaeoseptum thailandicum share similar morphology of Phaeoseptum in having immersed ascomata under a small blackened pseudoclypeus, cylindrical-clavate asci with a distinct pedicel and broadly fusoid, brown ascospores with multi-transverse septa. Combined phylogeny shows that the novel taxon is sister to P. mali + P. manglicola clade with high statistical support (Fig. 31).
Phylogram generated from maximum likelihood analysis based on combined LSU, ITS and tef1sequence data of Phaeoseptaceae. Bootstrap support values for ML ≥ than 75% and BYPP ≥ 0.95 are given above the nodes. The ex-types are in bold; the new isolates are in blue. The tree is rooted with Amorosia littoralis (NN 6654) and Angustimassarina populi (MFLUCC 13–0034)
Pleopunctum N.G. Liu, K.D. Hyde & J.K. Liu, in Liu et al., Mycosphere 10(1): 767 (2019)
Notes: Pleopunctum was introduced to accommodate two hyphomycetous species based on phylogenetic analyses and divergence time which is the first asexual morph genus in Phaeoseptaceae (Liu et al. 2019a, b, c, d). There are five Pleopunctum species are accepted in Species Fungorum (2022a, b) with molecular data and all of them were found from China and Thailand in terrestrial habitate (Liu et al. 2019a, b, c, d; Phukhamsakda et al. 2020; Boonmee et al.2021).
Pleopunctum ellipsoideum N.G. Liu, K.D. Hyde & J.K. Liu, in Liu et al., Mycosphere 10(1): 767 (2019).
Index Fungorumnumber: IF556523; Facesoffungi number: FoF 06114; Fig. 32
Saprobic on decaying wood. Asexual morph: Hyphomycetous. Colonies on natural substrate forming sporodochial conidiomata, superficial, black, scattered, velvety, glistening, orbicular. Mycelium immersed, composed of branched, septate, subhyaline to pale brown hyphae. Conidiophores and conidiogenous cells not observed. Conidia 33–45 × 16–20 μm (\(\bar{x}\) = 38 × 18 μm, n = 35), acrogenous, solitary, oval to ellipsoidal, muriform, constricted at septa, yellowish brown to dark brown, broadly obtuse at apex, truncate at base. Basal cell 6.5–10.5 × 9.5–11.5 μm (\(\bar{x}\) = 9–10.5 μm, n = 35), hyaline, elliptical to subglobose, smooth walled. Sexual morph: Not observed.
Culture characteristics: Conidium germinated on PDA within 12 h. Colonies on PDA reaching 20 mm in 4 weeks at 26 °C. Mycelia superficial, circular, entire, flat, rough, grey brown from above, dark brown from below.
Material examined: Thailand, Chiang Mai Province, Mae Taeng District, MRC, on bamboo culms, 15 July 2020, Y.R. Sun, M10 (MFLU 21–0091); living culture, MFLUCC 21–0064.
Hosts: decaying wood (Liu et al. 2019a, b, c, d) and bamboo (this study)
Distribution: China (Liu et al. 2019a, b, c, d) and Thailand (this study)
GenBank numbers: OM250079 (ITS), OM258687 (LSU)
Notes: Pleopunctum ellipsoideum was isolated from decaying wood in China by Liu et al. (2019a, b, c, d). The morphological characters of our collection are the same as in P. ellipsoideum (MFLUCC 19-0390). Phylogenetic analysis based on a combined LSU, ITS and tef1 sequence data indicated that our isolate and P. ellipsoideum (MFLUCC 19–0390) clustered together with high support (ML 100% and 0.99 BYPP; Fig. 31). Based on both morphology and phylogeny, we identified our taxon as P. ellipsoideum. This is the first geographical and host report of P. ellipsoideum on bamboo in Thailand.
Phaeosphaeriaceae M.E. Barr, Mycologia 71(5): 948 (1979)
Phaeosphaeriaceae has been subjected to various taxonomic changes since its establishment by Barr (1987). The inception of multi-gene phylogenetic analyses coupled with morphology has greatly resolved many taxonomic inconsistencies along with the introduction of several novel taxa (Phookamsak et al. 2014, 2017; Li et al. 2016; Crous et al. 2018; Hyde et al. 2020a, b, c). However, confusion in the placement of many taxa in the family still remains uncertain and problematic (Hongsanan et al. 2020b). Phaeosphaeriaceae at present accommodates more than 80 genera, with the taxa mainly exhibiting an endophytic, pathogenic and saprobic or hyperparasitic lifestyles (Hongsanan et al. 2020b).
Nodulosphaeria Rabenh., Klotzschii Herb. Viv. Mycol., Edn Nov, Ser. Sec., Cent. 8: no. 725 (in sched.) (1858)
Nodulosphaeria, was introduced by Rabenhorst (1858) and typified by N. hirta and was accommodated in Phaeosphaeriaceae by Barr (1987). The genus comprises endophytic, saprobic and pathogenic taxa which occur on a variety of hosts (Mapook et al. 2020; Chaiwan et al. 2019; Pasouvang et al. 2021). Nodulosphaeria taxa are principally characterised by ascomata with brown setae at the ostiole and three-to multi-septate ascospores with a swollen cell and often with terminal appendages (Shoemaker 1984; Mapook et al. 2016; Chaiwan et al. 2019). Molecular data for several Nodulosphaeria species listed in MycoBank (http://www.mycobank.org/, 07/2022) and Index Fungorum (http://www.indexfungorum.org/Names/Names.asp, 07/2022) are unavailable. A reference specimen for the type species N. hirta was recently designated by Mapook et al. (2016), who also provided molecular data for the same.
Nodulosphaeria digitalis W.J. Li, Camporesi, Bhat & K.D. Hyde, in Li et al., Mycosphere 6(6): 683 (2015)
Index Fungorum number: IF551664; Faces of fungi number: FoF 01302; Fig. 33
Nodulosphaeria digitalis (MFLU 22-0278). a Ascomata on dead aerial stem of Solidago virgaurea. b Close-up of ascomata on host. c Vertical section through an ascoma. d Close-up of an ostiole. e Vertical section of ascomatal wall. f Pseudoparaphyses. g–i Immature and mature asci. j–l Immature and mature ascospores (j, l arrows indicate appendages). Scale bars: a–c = 100 µm, d, g–i = 20 µm, e, j–l = 10 µm, f = 3 µm
= Nodulosphaeria thalictri D. Pem, Camporesi & K.D. Hyde, in Hyde et al., Fungal Diversity: https://doi.org/10.1007/s13225-019-00429-2, [56] (2019)
Saprobic on stem of Solidago virgaurea. Sexual morph: Ascomata 185–200 µm high, 200–225 µm diam. (x̅ = 192.3 × 213.1 µm, n = 5), appearing as black dots on host surface, immersed, unilocular, perithecial, solitary, usually scattered, globose to subglobose, dark brown, ostiolate. Ostioles 45–55 µm wide (x̅ = 52.2 µm, n = 5), centric, comprising internal dark brown setae. Ascomatal wall 14–23 µm at the sides and base, 4–5-layered; 25–35 µm near the apex, 5–7-layered; outer layers made up of thick-walled, dark brown cells of textura angularis, innermost layer comprising thin-walled, pale brown to hyaline cells of textura angularis or flattened cells. Pseudoparaphyses 1–3 µm wide, numerous, filiform, hyaline, branched. Asci 55–90 × 8–12 µm (x̅ = 74.1 × 9.2 µm, n = 40), 8-spored, bitunicate, cylindric-clavate, slightly curved, sessile to short-pedicellate, apically rounded with a minute ocular chamber. Ascospores (17–)20–27 × 3–4 µm (x̅ = 22.6 × 3.7 µm, n = 45), overlapping 1–2-seriate, hyaline when immature, becoming yellowish-brown at maturity, long fusiform, ellipsoidal to subcylindrical, straight or slightly curved, with 4–5 transverse septa, second cell from the apex slightly swollen, constricted at second septum from the apex, thick- and smooth-walled, with rounded ends, with hyaline appendages (1–2.5 µm long, 2.5–4 µm wide) at both ends. Asexual morph: Illustrated in Li et al. (2015).
Material examined: Italy, Forlì Cesena, Valico del Tramazzo, on dead aerial stem of Solidago virgaurea L. (Asteraceae), 19 June 2021, Erio Camporesi, IT4714 (MFLU 22-0278)
Hosts: Campanula trachelium (Campanulaceae, Chaiwan et al. 2019), Dactylis sp. (Poaceae, Li et al. 2015), Solidago virgaurea (Asteraceae, this study), Thalictrum sp. (Ranunculaceae, Hyde et al. 2019)
Distribution: Italy (Li et al. 2015; Chaiwan et al. 2019; Hyde et al. 2019; this study)
GenBank numbers: ON873995 (LSU); ON873997(ITS); ON885742 (tef1)
Notes: The strain MFLU 22-0278 clustered with the strains Nodulosphaeria digitalis MFLUCC 15-2716 (type strain) and MFLUCC 17-2418, and Nodulosphaeria thalictri MFLUCC 18-1138 with a good statistical support (99% ML, 0.98 BYPP) in the SSU-LSU-ITS-tef1 phylogenetic tree. Pairwise comparison among the two strains of N. digitalis MFLUCC 15-2716 and MFLUCC 17-2418, and N. thalictri MFLUCC 18-1138 is as follows: one base pair (bp) difference out of 1000 bp (0.1%) in SSU between N. thalictri MFLUCC 18-1138 and N. digitalis MFLUCC 17-2418; no bp difference among the three strains out of 844 bp in LSU and 504 bp in ITS respectively; two bp differences out of 891 bp (0.2%) in tef1 between N. thalictri MFLUCC 18-1138 and N. digitalis MFLUCC 17-2418. The pairwise identity therefore reveals insignificant differences among the three strains (N. digitalis MFLUCC 15-2716 and MFLUCC 17-2418, and N. thalictri MFLUCC 18-1138) according to Jeewon and Hyde (2016). Therefore, N. thalictri MFLUCC 18-1138 is another strain of N. digitalis.
Morphologically, there are some differences between ‘N. thalictri’ and N. digitalis in terms of the position of ascomata (immersed or semi-immersed in ‘N. thalictri’ while superficial to semi-immersed in N. digitalis), ostiole (comprising internal brown to dark brown cells of textura globulosa in ‘N. thalictri’ while no such observation was made for N. digitalis) (Chaiwan et al. 2019, Hyde et al. 2019). Furthermore, there are some differences in size of the morphological structures between ‘N. thalictri’ and N. digitalis (Table 2) (Chaiwan et al. 2019; Hyde et al. 2019). However, all these differences may be the result of phenotypic plasticity which has arisen from the need to adapt to environmental variations and/or different hosts (Hyde et al. 2019). The differences in the morphological characters are not being reflected by any significant phylogenetic difference herein. Such occurrence has also been observed for other taxa (Mapook et al. 2016; Hyde et al. 2019; Pasouvang et al. 2021). Based on the above morpho-phylogenetic approach, ‘N. thalictri’ is considered as N. digitalis.
The strain MFLU 22-0278 in this study clusters with the three strains of N. digitalis with 99% bootstrap support and 0.98 BYPP. There is no significant difference in the pairwise comparison among strains MFLU 22-0278 and MFLUCC 15-2716, MFLUCC 17-2418 as well as MFLUCC 18-1138 with regards to the LSU, ITS and tef1 sequence data. Our strain MFLU 22-0278 resembles N. digitalis MFLUCC 17-2418 and MFLUCC 18-1138 (N. digitalis MFLUCC 15-2716 was described in its asexual morph) in having ascomatal wall made up of cells of textura angularis, sessile or short-pedicellate asci and septate ascospores with a swollen cell and terminal appendages (Chaiwan et al. 2019; Hyde et al. 2019). Our collection differs from N. digitalis MFLUCC 17-2418 and MFLUCC 18-1138 mainly in having ostioles with internal brown setae (Fig. 34). The morphological characters also vary in size (Table 2). This can be accounted for by environmental and host variations as stated above. These morpho-phylogenetic analyses therefore support MFLU 22-0278 as another strain of N. digitalis. The latter is herein reported as a new record from Solidago virgaurea in Italy.
Phylogram generated from maximum likelihood analysis based on combined SSU, LSU, ITS and tef sequence data of selected taxa in Phaeosphaeriaceae. Bootstrap support values for maximum likelihood ≥ 65% and Bayesian posterior probabilities ≥ 0.95 are indicated above or below the branches. Type strains are in bold; the new isolate is in blue. The tree is rooted with Bhagirathimyces himalayensis NFCCI 4580, Pseudoophiobolus galii MFLUCC 17–2257 and Pseudoophiobolus mathieui MFLUCC 17–1785
Pleosporaceae Nitschke, Verh. naturh. Ver. preuss. Rheinl. 26: 74 (1869)
This family consists of endophytes, pathogens and saprobes and has a worldwide distribution (Hongsanan et al. 2020b). Twenty-three taxa are accepted in this family (Wijayawardene et al. 2022)
Bipolaris Shoemaker, Can. J. Bot. 33:882(1959)
Species of this genus are pathogens, saprobes and endophytes on a wide range of hosts with a worldwide distribution (Jayawardena et al. 2019a). Bhunjun et al. (2020), provided polyphasic approaches to delineate species in Bipolaris. There are 140 records in Index Fungorum (2022a, b) for this genus, however less than 50 species have molecular data.
Bipolaris luttrellii Alcorn, Mycotaxon 39: 378 (1990)
Index Fungorum number: IF127658; Facesoffungi number: FoF01302; Fig. 35
Saprobic on Poaceae sp. Sexual morph: Not observed. Asexual morph: Conidiophores up to 200 μm long and 7–10 μm thick, arising singly or in groups of a few conidia, simple, septate, straight or flexuous, sometimes geniculate at the upper part, smooth, pale to mid-brown. Conidiogenous nodes dark brown, distinct. Conidia (75–)90–125 × 17–26(–)28) μm (\(\bar{x}\) = 107 × 21 μm, n = 30), straight or curved, broadly fusiform or obclavate fusiform, widest near centre, tapering towards rounded ends, pale to mid brown, 5–9-distoseptate, smooth-walled. Hilum slightly protuberant, single germ tubes arising from each end.
Material examined: China, Guizhou Province, Guizhou Academy of Agricultural Sciences, dead leaves of Poaceae sp., 20 July 2015, Kasun M. Thambugala CN020 (MFLU 16-2836), living culture MFLUCC 16–0281, GZCC 15–0045.
Host: Dactyloctenium aegyptium, on dead leaves of unidentified Poaceae host (Alcorn 1990; This study)
Distribution: Australia, China (Alcorn 1990; This study)
GenBank numbers: OQ154965 (ITS)
Notes: Bipolaris luttrelli is only known from the type specimen before this record. This species is morphologically similar to B. setarieae (Manamgoda et al. 2014). Bipolaris luttrelli can be distinguished from its sister taxa by having fewer conidiogenous loci on the conidiophores and its darker conidia with pale end cells (Manamgoda et al. 2014). In our phylogenetic analyses (Fig. 36), our strain clustered with the ex-type strain of B. luttrelli with a higher bootsrap support. There are 10 base pair differences between our strain and the ex-type strain in ITS. Both of these strains lack tef1 gene region. Therefore, we identified our strain as B. luttrelli and provides the first report from China.
One of the most parsimonious trees obtained in the combined analyses of ITS-gapdh-tef1. Maximum Parsimony values ≥ 70% and BYPP ≥ 0.90 are indicated above the nodes. The ex-types and reference strains are in bold; the new record is in blue. The tree is rooted with Curvularia buchloes (CBS 246.49) and C. subpapendorfii (CBS 656.74)
Curvularia Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13(1): 123 (1933)
This is a species-rich genus which comprised of numerous pathogenic, saprobic and epiphytic fungi (Manamgoda et al. 2012a, b, 2015; Jayawardena et al. 2019a). Phytopathogenic species have been recorded mostly on poaceous hosts as well as non-poaceous hosts. Several Curvularia spp. have also been reported as opportunistic human pathogens on immunocompromised patients (Madrid et al. 2014; Manamgoda et al. 2011, 2015; Danish Khan et al. 2017; Tóth et al. 2020). There are more than 200 epithets recorded in Index Fungorum (2022a, b). To date, 130 species of Curvularia have been accepted within the genus (Ferdinandez et al. 2021).
Curvularia alcornii Manamgoda, L. Cai & K. D. Hyde, Sydowia 64(2): 259 (2012)
Index Fungorum number: IF800665; Facesoffungi number: FOF10679; Fig. 37
Saprobic on dried leaf of Panicum virgatum. On CMA Hyphae 4–5 μm, septate, branched. Conidiophores up to 241 μm long, micronematous to macronematous, pale brown to dark brown, simple or branched, septate, flexuous, highly geniculate. Conidiogenous cells (8–)9–17(–19) × (4–)5–6 μm (\(\bar{x}\) = 13 × 5 μm, n = 10), hyaline to pale brown, smooth–walled, terminal or intercalary, monotretic to polytretic. Conidia (18–)20–26(–31) × (7–)8–10(–11) μm (\(\bar{x}\) = 23 × 9 μm, n = 30) apical and basal cells hyaline or pale brown, matured conidia brown, straight, rarely curved, inequilateral ellipsoidal or clavate, dark brown septa, 3–4-distoseptate, enlarged middle cells; hila 1–2 μm distinctly protuberant, darkened. Sexual morph: Not observed.
Culture characteristics: Colonies on PDA reaching 57 mm in 7 days at 25 °C, slightly convex with entire margin, brown centre, sparse aerial mycelium becoming brown to grey towards the edge, reverse black to dark brown, concentric. Colonies on CMA reaching 60 mm in 7 days at 25 °C, flat with entire margin, pale brown to dark brown, moderate aerial mycelium becoming dark brown to black towards the edge, reverse dark brown, concentric. Colonies on MEA reaching 67 mm in 7 days at 25 °C, flat with entire margin, dark green, sparse aerial mycelium becoming olivaceous green towards the edge, reverse black, concentric.
Material examined: Sri Lanka, Anuradhapura District, Thuruwila, N 8° 14′ 50.53859″, E 80° 25′ 9.24013″, on dried leaf of Panicum virgatum L. (Poaceae), 13 June 2019, D.S. Manamgoda, USJ-H-075, living culture USJCC-0088.
Hosts: Panicum spp., Pennisetum clandestinum, Oryza spp. and Zea mays (Manamgoda et al. 2012a, b; Khemmuk et al. 2016)
Distribution: Australia and Thailand (Farr and Rossman 2022)
GenBank numbers: MZ948821 (ITS), MZ971267 (gadph), MZ971253 (tef1)
Notes: According to the phylogenetic result, isolate USJCC-0088 is identified as Curvularia alcornii. This taxon was originally described in Manamgoda et al. (2012a), as a saprobe on a leaf sample of Zea mays collected in Thailand (holotype MFLU 12-0397). In this study, the fresh isolate was identified as a saprobe on a dead leaf of Panicum virgatum (Fig. 38). To our knowledge, this is a new record from Sri Lanka and a new fungus-host association.
Phylogram generated from parsimony analysis based on combined ITS, gadph and tef1 sequence data of Curvularia. Bootstrap support values of MP and ML ≥ 50% and BYPP ≥ 0.95 are indicated above the nodes. The ex-types are in bold; the new records are highlighted in greenish-blue. The tree is rooted with Bipolaris maydis (CBS137271/C5)
Curvularia senegalensis (Speg.) Subram., Journal of the Indian Botanical Society. 35(4): 467 (1956)
≡ Brachysporium senegalense Speg., Anales del Museo Nacional de Historia Natural Buenos Aires 26: 133 (1914)
Index Fungorum number: IF296254; Facesoffungi number: FoF13382; Fig. 39
Saprobic on dead panicle of Zea mays. On PDA Hyphae 3–4 μm, septate, branched. Conidiophores up to 219 μm long, micronematous to macronematous, pale brown to dark brown, simple or branched, septate, straight to flexuous, geniculate at the apex. Conidiogenous cells (8–) 9–15(–17) × 3–5 μm (\(\bar{x}\) = 12 × 4 μm, n = 10), hyaline to pale brown, smooth–walled, terminal or intercalary, monotretic to polytretic. Conidia (17–)18–27(–34) × (7–)9–12(–13) μm (\(\bar{x}\) = 22 × 11 μm, n = 30) apical and basal cells hyaline or pale brown, matured conidia brown, straight to curved, sometimes clavate, dark brown septa, 3–4-distoseptate, enlarged middle cells; hila 1–2 μm flat, darkened. Sexual morph: Not observed.
Culture characteristics: Colonies on PDA reaching 73 mm in 7 days at 25 °C, flat with entire margin, grey to olivaceous black centre, abundant aerial mycelium becoming brown towards the edge, reverse black to dark brown, concentric. Colonies on CMA reaching 69 mm in 7 days at 25 °C, flat with entire margin, grey to pale brown, reverse white to pale brown, concentric. Colonies on MEA reaching 83 mm in 7 days at 25 °C, flat with entire margin, dark brown, abundant aerial mycelium becoming grey towards the edge, reverse dark brown to pale brown, concentric.
Material examined: Sri Lanka, Matale District, Palapathwela, N 7° 33′ 22.8″, E 80° 36′ 38.2″, on panicle of Zea mays L. (Poaceae), 08 November 2018, D.S. Manamgoda, USJ-H-031, living culture USJCC-0025.
Distribution: Australia, Brazil, China, Cuba, Hawaii, India, Malaysia, Mexico, Myanmar, Nigeria, Samoa, South Africa, Sri Lanka, Tanzania, Thailand, Texas, United States, Virginia and West Indies (Farr and Rossman 2022)
Hosts: Andropogon caricosus, Archontophoenix alexandrae, Bauhinia purpurea, Carya illinoensis, Citrullus vulgaris, Cymbopogon flexuosus, Cynodon dactylon, Dichanthium caricosum, Gmelina arborea, Hevea brasiliensis, Hibiscus cannabinus, Jasminum sambac, Liquidambar macrophylla, Musa nana, Musa × paradisiaca, Oryza sativa, Ougeinia oojeinensis, Paspalum notatum, Paspalum paniculatum, Passiflora edulis, Persea Americana, Pinus caribaea, Pinus khasya, Quercus germana, Saccharum sp., Stigmaphyllon sagraeanum, Tamarindus indica, Thuja orientalis, Urena lobate, Vigna unguiculata, Zea mays (Farr and Rossman 2022)
GenBank numbers: MT410577 (ITS), MZ971268 (GADPH), MZ971254 (tef1)
Notes: Isolate USJCC-0025 is identified as Curvularia senegalensis based on morphology and phylogeny (Fig. 38). The fresh isolate was collected from a dead panicle of Zea mays. Curvularia senegalensis has so far recorded in Sri Lanka only from Hevea brasiliensis (Adikaram and Yakandawala 2020). Moreover, it has only been reported on Zea mays from Brazil, Malaysia and Nigeria. To our knowledge, this is the first record of Curvularia senegalensis on Zea mays from Sri Lanka.
Pyrenophora Fr., Summa veg. Scand., Sectio Post. (Stockholm): 397 (1849)
Pyrenophora is a species-rich genus in Pleosporales which encompasses saprobic and phytopathogenic fungi associated mainly with poaceous hosts. The sexual morphs are characterized by black, thick-walled, subglobose to pyriform ascomata with an apical ostiole and conspicuous dark brown setae. Asci are bitunicate, show a large non-amyloid ring and usually contain eight pale yellowish brown or pale brown, muriform ascospores surrounded by a mucilaginous sheath. Asexual morphs show macronematous, brown, sympodial conidiophores with tretic conidiogenous cells and dematiaceous, rather straight, distoseptate conidia with a dark, non-protruding basal scar. Conidial germination occurs from polar or intermediate cells (Sivanesan 1987). This genus is monophyletic and genetically clearly distinct from other pleosporalean genera with a superficially similar conidial apparatus, such as Bipolaris, Exserohilum and Porocercospora. Multilocus DNA sequence data is currently available for at least 26 Pyrenophora species (Marin-Felix et al. 2019).
Pyrenophora verruculosa Madrid & Cantillo, sp. nov.
Mycobank number: 844464; Facesoffungi number: FoF 10420; Fig. 40
Pyrenophora verruculosa (SGO 168420, holotype). a, c, d Conidia attached to conidiogenous cells. b Conidium passively released from a conidiogenous cell. e–h Conidia (verruculose ornamentation can be observed in e). i Hyphae with mucilaginous dark brown material. j, k Mycelia with microsclerotia. Scale bars: a–d = 40 µm, e–i = 20 µm, j–k = 45 µm
Etymology: The name refers to the verruculose conidia produced by this species
Holotype: SGO 168420
Probably saprobic or pathogenic to an unidentified member of Poaceae. Sexual morph: Not observed. Asexual morph: Hyphomycetous. Vegetative hyphae septate, branched, light olivaceous to mid olivaceous brown, thin to thick-walled, smooth, 2–6 µm wide, anastomosing, occasionally showing deposits of a mucilaginous dark brown material. Conidiophores macronematous, mononematous, solitary, septate, simple, slightly flexuous to strongly geniculate, light olivaceous brown to dark brown, often paler at the apex, smooth to verruculose, with cell walls often thicker than those of the supporting vegetative hyphae, 300–1270 × 5–9 µm with subnodulose to nodulose intercalary swellings up to 11 µm wide. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- to polytretic, proliferating sympodially, 15–28 µm long. Conidia narrowly clavate, narrowly ellipsoidal to fusiform or subcylindrical, straight to slightly curved, light olivaceous brown to dark brown, verruculose, (26–)32–63(–74) × 12–21 µm, 3–5(mostly 4)-distoseptate, often constricted at the uppermost distoseptum, with a rounded apex and an obconically truncate or rounded base, basal cell sometimes delimited by a thick, dark septum. Hilum thick and dark. Microsclerotia abundant, mostly 45–240 µm wide.
Culture characteristics: Colonies on water agar with sterilized maize leaves dark brown, hairy, with abundant clumps of microsclerotia.
Material examined: Chile, El Loa Province, Atacama Desert, near Calama, isolated from unidentified dead Poaceae, 15 October 2015, H. Madrid & L. Linaje (SGO 168420, holotype).
GenBank numbers: ON722346 (ITS), ON722346 (LSU), ON736764 (gpdh).
Notes: Due to mobility restrictions during the SARS-CoV-2 pandemic, the ex-type strain (HM 201), which had been preserved in sterile water, could not be properly maintained for several months. Recent attempts to reactivate the strain have been unsuccessful and the fungus probably died. However, the holotype was deposited at SGO and ex-type sequences of ITS, LSU and gpdh are available in GenBank. DNA sequence analyses revealed that Pyrenophora verruculosa is clearly distinct from all other members of Pyrenophora represented in GenBank. The closest hits in BLAST searches with the ITS sequence of strain HM 201 were Pyrenophora novozelandica (CBS 127934) (ex-type, GenBank MK539997, 96.55% similarity), P. fugax (CBS 509.77) (GenBank MK539985, 95.23% similarity), P. nisikadoi (CBS 190.29) (ex-type, as Bipolaris brizae, GenBank MH855213, 93.57% similarity), and P. nobleae (CBS 259.80) (GenBank MK539994, 91.33% similarity). BLAST searches with the gpdh sequence showed P. fugax (CBS 509.77) (GenBank AY004822, 95.70% similarity) and P. nobleae (CBS 966.87) (GenBank AY004824, 88.81% similarity) as the closest matches. With LSU, similar results were obtained, but with higher similarity percentages, as expected for this rather conserved locus. In the phylogenetic analysis, only ITS sequence data was used considering that LSU offers little resolution for closely related taxa in Pleosporales, and gpdh is available for a smaller number of species than ITS. In the ITS-based phylogenetic tree (Fig. 41), P. verruculosa, P. novozelandica and P. fugax formed a clade with 83% bootstrap support. These close relatives can easily be distinguished from P. verruculosa on the basis of conidial dimensions, i.e. smaller in P. novozelandica, 20.5–58 × 9.5–14 µm, and longer in P. fugax, 50–170 × 14–24 µm (Ellis 1961; Sivanesan 1987; Marin-Felix et al. 2019). Clumps of thick-walled, strongly pigmented cells superficially resembling the microsclerotia of P. verruculosa, have been reported in other Pyrenophora species, such as P. nisikadoi and P. pseudoerythrospila (Marin-Felix et al. 2019). These species, however, are phylogenetically clearly distinct from P. verruculosa (Fig. 41).
Maximum likelihood tree based on ITS sequences, showing the phylogenetic relationships of Pyrenophora species. Bootstrap support values > 70% are shown near the internodes. The tree is rooted to Bipolaris maydis. GenBank accession numbers of ITS sequences are given, in parentheses, after each strain number. Aauthentic strain, Tex-type strain, ETex-epitype strain, HTholotype material. New species is in bold
Tetraplosphaeriaceae Kaz. Tanaka & K. Hiray., Stud. Mycol. 64: 177 (2009)
Tetraplosphaeriaceae was established to accommodate five genera, viz Polyplosphaeria, Pseudotetraploa, Quadricrura, Tetraplosphaeria with Tetraploa sensu stricto asexual morphs (the type genus) and Triplosphaeria (Tanaka et al. 2011). Hyde et al. (2013) later treated Tetraplosphaeria as a synonym of Tetraploa which has been applied previously. Up to now, nine genera are accepted in Tetraplosphaeriaceae viz Aquatisphaeria, Byssolophis, Ernakulamia, Polyplosphaeria, Pseudotetraploa, Quadricrura, Shrungabeeja, Tetraploa and Triplosphaeria (Wijayawardene et al. 2022). Members of this family are mostly reported as saprobe from aquatic and terrestrial habitats (Tibpromma et al. 2018; Hongsanan et al. 2020a, b; Hyde et al. 2020a, b, c; Li et al. 2021a, b).
Tetraploa Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 5: 459 (1850) MycoBank: MB10199
= Tetraplosphaeria Kaz. Tanaka & K. Hiray., in Tanaka et al., Stud. Mycol. 64: 177 (2009)
Notes: Tetraploa was introduced by Berkeley and Broome (1850) with T. aristata as the type species. The sexual morph is characterized by small globose ascomata, narrowly fusiform ascospores and appendage-like sheath. The asexual morph belonging to Tetraploa sensu stricto has a columnar conidial body, with several prominent setose appendages at the apex (Hyde et al. 2013; Li et al. 2021a, b). Hyde et al. (2013) transferred species in Tetraplosphaeria to Tetraploa. In this study, we introduce one new species to Tetraploa based on morphology and molecular analysis with full description and illustration.
Tetraploa dashaoensis C.F. Liao & Doilom, sp. nov.
Index Fungorum number: IF559273; Facesoffungi number: FoF 10585; Fig. 42
Etymology: In reference to the location where the fungus was collected.
Holotype: KUN–HKAS 107636
Saprobic on dead stem of Saccharum arundinaceum. Sexual morph: Not observed. Asexual morph: hyphomycetous. Colonies superficial, effuse, gregarious, brown to dull green. Mycelium partly immersed in natural substratum, brunched, septate, hyaline. Conidiophores absent. Conidiogenous cells micronematous, integrated, monoblastic, intercalary, short cylindrical. Conidia 25–43 × 13–36 µm (\(\bar{x}\) = 31 × 23 µm, n = 30), solitary, straight, septate, unbranched, mostly smooth-walled at the base of immature conidia, becoming verrucose at mature conidia, composed of 1–4 columns at the base, 1–3-septate in each column, with 1–4 apical appendages. Appendage 148–258 µm long (\(\bar{x}\) = 186 µm, n = 30), 6–15 µm wide at the base with dull green, 3–8 µm wide at the apex with hyaline, usually composed of three to four appendages, rarely one or two, euseptate, 6–23-septate, smooth, straight or divergent to intertwined; immature appendages 21–81 µm long (\(\bar{x}\) = 43 µm, n = 30), with 1–5-septate.
Culture characteristics: Conidia germinating on PDA, germination tube growing from both ends. Colonies on PDA reaching 10 to 14 mm diam. in 17 days at room temperature (25 ± 2 °C), mycelium dense, floccose, surface smooth, velutinous spot centre with flat substrate, circle or irregular margin, light brown to yellow–brown, dark brown towards to white margin in above; white at centre, dark brown towards margin in reverse. No pigment production.
Material examined: China, Yunnan Province, Kunming City, from dead stem of Saccharum arundinaceum (Retz.) (Poaceae), 4 July 2020, C.F. Liao, (KUN–HKAS 107636, holotype), ex-type living culture KUMCC 21-0010.
GenBank number: OL473549 (ITS), OL473555 (LSU), OL473556 (SSU), OL505601 (tub2), OL505599 (tef1).
Notes: Phylogenetic analyses of the combined LSU, ITS, SSU, tub2 and tef1 sequence data showed that our new collection Tetraploa dashaoensis KUMCC 21-0010 is related to Tetraploa aquatica (MFLU 19-0995, MFLU 19-0996), but forms a distinct linage with 98% ML, 83% MP and 0.99 BYPP values (Fig. 43). Tetraploa dashaoensis shares some morphological characteristics with the phylogenetically closest species T. aquatica in having cylindrical conidiogenous cells, a verrucose conidial base and 1–4 apical appendages with euseptate. However, T. dashaoensis differs from T. aquatica in having a different conidial colour (dull green vs. brown to pale brown), longer conidia (25–43 µm vs. 22.5–27 µm), longer appendages (148–258 µm vs. 98–134 μm), and more septate appendages (6–23-septate vs. 6–10-septate). In addition, T. dashaoensis has intertwined appendages, which were absent in T. aquatic (Li et al. 2021a, b). Phylogenetic analyses and morphological characters support our collection to be different species. Thus, we introduce T. dashaoensis as a new species based on morphology and phylogeny.
Phylogram generated from maximum likelihood analysis of Tetraploa including related genera based on a combined LSU, ITS, SSU, tub2 and tef1 sequence data. Relevant sequences were referred from Dong et al. (2020), Hyde et al. (2020a), and Li et al. (2021a, b). The data set consisted of 3720 characters with gaps. Tree topology of the ML analysis was similar to the MP and BYPP tree topologies. Bootstrap support values of ML and MP ≥ 75% with BYPP ≥ 0.95 are given at the nodes. The ex-type cultures are indicated using ″T″; the new isolate is in blue
Torulaceae Corda, in Sturm, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 2: 71 (1829)
Notes: Sturm (1829) introduced Torulaceae with Torula as the type genus. There are six genera accepted in Torulaceae, namely Dendryphion, Neotorula, Rostriconidium, Rutola, Sporidesmioides and Torula (Hongsanan et al. 2020b). Torulaceae species are characterized by having erect, micro- or macronematous, straight or flexuous, subcylindrical conidiophores and doliiform to ellipsoid or clavate, brown, smooth to verruculose conidiogenous cells. Conidia are subcylindrical, phragmosporous, acrogenous, brown, dry, and smooth to verrucose, characteristically produced in branched chains (Hyde et al. 2016; Li et al. 2017a, b; Mapook et al. 2020). In this study, we follow Mapook et al. (2020) as the recent treatment for Torulaceae.
Torula Pers, Ann. Bot. (Usteri) 15: 25 (1795)
Notes: Persoon (1794) established Torula and was typified by T. herbarum. Torula species are characterized by having a terminal or lateral, monoblastic or polyblastic conidiogenous cells, which have a basally thickened and heavily melanized wall, with the apex thin-walled and frequently collapsing and becoming coronate (Crane and Miller 2016). There are 54 Torula species listed in Species Fungorum (2022a, b), however, only 18 taxa have molecular data.
Torula fici Crous, IMA Fungus 6: 192 (2015).
Index Fungorum number: IF816154; Facesoffungi number: FoF02712, Fig. 44
Saprobic on the dead pseudo stem parts of Musa sp. Sexual morph: Not observed. Asexual morph: Colonies effuse, black, powdery and thread-like. Mycelium slightly immersed, septate, branched, smooth, with pale brown hyphae. Conidiophores 2–3 μm long × 1–3 μm diameter (\(\bar{x}\) = 2.77 × 1.91 μm, n = 10), macronematous, mononematous, solitary, erect, thick–walled, consisted of 2 distinct cells, first cell from the bottom is pale brown to sub hyaline, wide at the base and narrow at apex, the second cell is pale brown to brown, different from the first cell by shape, cylindrical to subcylindrical at the base and globose to subglobose at apex, conidiophore arising from prostrate hypha. Conidiogenous cells 2–3 μm long × 1–2 μm diam, (\(\bar{x}\) = 2.18 × 1.33 μm, n = 10) polyblastic, and terminal, dark brown to black, verruculose, thick-walled, ellipsoid, paler or sub hyaline at apex, dark and black at the bottom. Conidia 19– 25 μm long 2–3.5 μm wide (\(\bar{x}\) = 18.56 × 2.88 μm, n = 10) solitary to catenate, acrogenous, simple, phragmosporous, dark brown, apical cell often pale brown, minutely verruculose, often 3–4 septate, rounded at both ends, composed of subglobose cells, slightly constricted at some septa. Immature conidia are sub hyaline to pale brown and arising to multi-angles from mature conidia. Conidial secession is rheixolytic.
Culture characteristics: Conidia germinating on PDA within 18 h and germ tubes produced from the tip cell. Colonies growing on PDA, reaching 5 cm in 5 days at 25 °C, mycelium partly immersed to superficial, slightly effuse, hairy, with regular edge, pink or pale brown.
Material examined: Thailand, Chiang Mai, Mushroom Research Center, on dead plant material of Musa sp. (Musaceae), 30 December 2018, Binu Samarakoon, E001 (MFLU 22-0251), living culture MFLUCC 22-0176.
Other material examined: Taiwan, Ali shan mountain, Fanlu Township area, Dahu forest, dead leaves of Ficus septica (Moraceae), 20 September 2018, D. S. Tennakoon, HAY029A (MFLU 19-2775); living culture, MFLUCC 20-0167, ibid., 10 July 2019, HAY029B (NCYU 19-0367); living culture, NCYUCC 19-0248.
Known hosts: Chromolaena odorata (Asteraceae), Ficus septica (Moraceae), Ficus religiosa (Moraceae), Garcinia sp. (Clusiaceae), Magnolia grandiflora (Magnoliaceae), Olea europaea (Oleaceae), Pandanus sp. (Pandanaceae), Musa sp. (Musaceae) (Crous et al. 2015a, b, c, Li et al. 2017a, b; Tibpromma et al. 2018; Jayasiri et al. 2019; Mapook et al. 2020; Samarakoon et al. 2021; Tennakoon et al. 2021; this study).
Known distribution: Taiwan (this study), Cuba, South Africa, Thailand (this study) (Crous et al. 2015a, b, c, Li et al. 2017a, b; Tibpromma et al. 2018; Jayasiri et al. 2019; Mapook et al. 2020; Samarakoon et al. 2021; this study).
GenBank numbers: MFLUCC 22-0176 – OP099550 (LSU), OP097673 (SSU), OP099562 (ITS), OP113821 (tef1) MFLUCC 20-0167 – MZ317501 (LSU); MZ317506 (ITS); MZ326657 (tef1); MZ326659 (rpb2) NCYUCC 19-0248 – MZ317502 (LSU); MZ317507 (ITS); MZ326658 (tef1); MZ326660 (rpb2)
Notes: In the multi-gene phylogeny, our strain (MFLUCC 22-0176) grouped with T. fici strains with moderate statistical support in ML analysis and a higher support in Baysian analysis (81% ML, 0.98 BYPP) (Fig. 45). Morphological comparisons revealed that our collection is similar to the holotype of T. fici (Crous et al. 2015a, b, c) by the distinct shape of the conidiophores and having 3–4 celled conidia. In addition, the conidiogenous cells of the type specimen are identical in shape with our collection. Therefore, based on both morphology and phylogeny evidence, we identify our specimen (MFLU 22-0251) as T. fici from Chiang Mai, Thailand. Torula fici was previously found from China on Musa sp. by Samarakoon et al. (2021). In this study, we report T. fici on Musa sp. for the first time from Thailand as a new collection. In addition, our strains (MFLU 19-2775 and NCYU 19-0367) share the size range of the conidial characters with T. fici (Crous et al. 2015a, b, c; Tibpromma et al. 2018; Mapook et al. 2020). The phylogeny also indicates that both strains were nested with other Torula fici strains in a 100% ML, 1.00 BYPP supported clade (Fig. 45). We conclude that our stains (MFLU 19-2775 and NCYU 19-0367) also belong to T. fici which was reported on Ficus septica from Taiwan as a new host record.
The best scoring RAxML tree for combined dataset of LSU, SSU, ITS, tef1 and rpb2 sequence data. The topology and clade stability of the combined gene analyses was compared to the single gene analyses. The tree is rooted with Neooccultibambusa chiangraiensis (MFLUCC 12–0559) and Occultibambusa bambusae (MFLUCC 13–0855). Ex-type strains are in bold and newly generated sequences are in red. Bootstrap support values for ML ≥ 60% and BYPP ≥ 0.90 are given above the nodes
Torula sundara (Subram.) Y.R. Sun, Yong Wang bis & K.D. Hyde, comb.nov.
≡ Dwayabeeja sundara Subram., J. Indian Bot. Soc.37: 56 (1958)
= Pseudotorula sundara (Subram.) J.L. Crane & A. Mill
Index Fungorum number: IF559464; Facesoffungi number: FoF09933; Fig. 46
Saprobic on decaying bamboo culms in terrestrial habitat. Asexual morph: Hyphomycetous. Colonies on natural substrate superficial, powdery, dark brown to black. Mycelium immersed, composed of branched, septate, pale brown to brown hyphae. Conidiophores 2.5–4 μm wide, micronematous to semi-macronematous, mononematous, solitary, erect, sample, straight or slightly flexuous, unbranched, paler brown to brown, thin-walled, septate, with ampulliform cells, arising from prostrate hypha. Conidiogenous cells polyblastic, terminal, with the apex thin-walled and freuently collapsing and becoming coronate, dark brown to black, ellipsoid to coronal. Conidia two types, short conidia and long conidia. Short conidia 41–60 × 9–15 μm (\(\bar{x}\) = 53 × 12 μm, n = 30), acrogenous, phragmosporous, single or in chains, broadly fusiform, yellow brown to dark brown, 5–10-septate, slightly constricted at some septa, verruculose. Long conidia acrogenous, phragmosporous, single, straight or flexuous, cylindrical, up to 50-septate, constricted at the septa, brown to dark brown, up to 200 μm long. Sexual morph: Not observed.
Culture characteristics: Conidium germinated on PDA within 12 h. Colonies on PDA reaching 20 mm in two weeks at 26 °C. Mycelia superficial, circular, entire, flat, white from above, pale brown from below.
Material examined: Thailand, Chiang Mai Province, Mae Taeng District, MRC, on bamboo culms, 10 September 2020, H.W. Shen, M55 (MFLU 21-0089); living culture, MFLUCC 21-0067).
GenBank numbers: OM276824 (ITS), OM287866 (LSU)
Notes: Dwayabeeja sundara as the type species in Dwayabeeja was introduced by Subramanian (1958). It has dark blackish-brown colonies and two types of conidia. Crane and Miller (2016) transferred D. sundara to Pseudotorula based on catenate phragmoconidia. It was recollected and isolated from bamboo culms in Chiang Mai Province, Thailand. Here, we provided sequences for it. Phylogenetic analyses show it is a distinct clade in Torula and sister to T. acaciae with high support (ML 100% and 1.00 BYPP). According to the similarities in morphology along with phylogenetic analyses, we propose Pseudotorula sundara as a synonym of Torula sundara.
Periconiaceae (Sacc.) Nann., Repertorio sistematico dei miceti dell'uomo e degli animali 4: 482 (1934).
Periconiaceae was accommodated as a distinct lineage in Massarineae by Tanaka et al. (2011) based on morpho molecular data. Four genera (viz. Bambusistroma, Flavomyces, Noosia and Periconia) are accepted with endophytic, saprobic and pathogenic nutritional modes (Sarkar et al. 2019; Hongsanan et al. 2020b, Samarakoon et al. 2020; Wijayawardene et al. 2022).
Periconia Tode, Fung. mecklenb. sel. (Lüneburg) 2: 2 (1791).
Periconia was introduced by Tode (1791) and typified by P. lichenoides. The asexual morph of the genus has pale to dark brown branched or unbranched conidiophores (also referred to as stipe). Conidiogenous cells of Periconia are monoblastic or polyblastic, and formed at the terminal ends or intercalary parts of the conidiophore. Periconia produces spherical, asepete, catenate or solitary conidia in pale to dark-brown. Both sexual and hypomycetous asexual morphs are recorded in this genus (Tanaka et al. 2015; Liu et al. 2017). There are 187 epithets listed in Index Fungorum (2022a, b). Periconia has been reported as a plant pathogen, endophyte and a common saprobe in terrestrial and aquatic habitats with a cosmopolitan distribution (Ellis 1961, 1976; Markovskaja and Kačergius 2014; Liu et al. 2017).
Periconia byssoides Pers., Syn. meth. fung. (Göttingen)1: 18 (1801)
Index Fungorum number: 144538; Faceoffungi number: FOF09319; Figs. 47, 48
Periconia byssoides (MD2, new host and geographical record): a–c Conidiophores terminating with heads of conidia d–e Heads of conidia showing phialides f – Basal part of conidiophores showing their distinct nature (color and septa) g Close view of the conidial head showing conidia and phialides h–j Conidia enlarged. Scale bars: a − g = 50 μm, h − j = 20 μm
Saprobic on leaves of Vigna unguiculata. Sexual morph: Not observed. Asexual morph: Conidiophores with conidial heads were observed on foliar lesions of cowpea. Conidiophores observed after 2–3 weeks, simple, micro- and semi-macronematous, unbranched and branched, initially subhyaline to brownish, verruculose, and variable in length. Conidiogenous cells discrete, determinate, terminal or lateral, subglobose, mono- and polyblastic, smooth to verruculose, pale brown producing global verrucose conidia in acropetal chains (3–4 in number), 11.5–12.5 × 15 − 17 μm diam. (x̄ = 11.9 × 15.2 μm, n = 20), conidia pale brown to brown verrucose. Conidiogenous cells were formed on an apical cell and in the collar region around the septa, sometimes on short hyaline or subhyaline branchlets. From primary, hyaline, globose conidiogenous cells numerous secondary conidiogenous cells arise, which produce short chains of spherical, commonly verruculose but sometimes verrucose, pale brown to brown conidia measured 8.5–11.4 μm diam.
Cultural characteristics: Culture on MEA reaching 35 mm after 20 days at 25 °C. Fungal colony appeared cottony with abundant white to orange-white aerial mycelium. In reverse colony dark olive to dark olivaceous-grey with concentric dark olivaceous-brown. Hyphae hyaline sometime greyish green, smooth and verruculose later becomes brown orange.
Material examined: India, Karnataka, Mysore, Doddamaragowdanahally, on foliar lesions of cowpea (Vigna unguiculata (L.) Walp.- Fabaceae) 18 May 2020, S. Mahadevakumar, Y.S. Deepika, N. Lakshmidevi (Specimen UOM-IOE 18/21), living culture (MD2).
GenBank numbers: OM811496 (ITS); OM811504 (LSU)
Notes: Detail descriptions and illustrations are presented in Mason and Ellis (1953) and Markovskaja and Kačergius (2014). Morphological inspection and measurements of conidiophores and conidia revealed that the fungal specimens described by Markovskaja and Kaergius (2014) for several Apiaceae hosts correspond well with the P. byssoides, which was based on lectotype material (Fries 1832). Phylogenetic analyses (Fig. 49) This is the first time that P. byssoides is reported from Fabaceae on Vigna unguiculata representing a new host record and geographical record (Table 3).
Maximum likelihood tree revealed by RAxML from an analysis of a concatenated SSU, LSU, ITS and tef1 sequence dataset of the species in Periconiaceae, showing the phylogenetic position of Periconia delonicis (MFLUCC 20–0235). Bootstrap supports ≥ 60% and BYPP ≥ 0.95 are given above the branches as ML/BYPP. The tree is rooted with Helminthosporium dalbergiae (MAFF 243853) and Massarina cisti (CBS 266.62). Strains generated in this study are indicated in red bold. Ex–type strains are indicated in black bold. The scale bar 0.02 represents the expected number of nucleotide substitutions per site
Periconia cortaderiae Thambugala & K.D. Hyde, in Thambugala et al., Mycosphere 8(4): 734 (2017)
Index Fungorum number: IF553165; Facesoffungi number: FoF03226; Fig. 50
Periconia cortaderiae (MFLU 18–1896, new host and geographical record). a − b Colonies on host c − f, i, j Conidiophores bearing conidia g, h, k Conidiogenesis from terminal and intercalary parts of the conidiophore l-p Conidial chains and conidia. Scale bars: a = 3 mm, b = 3.5 mm, c = 50 μm, e − j = 15 μm, d, k − p = 5 μm
Saprobic on decaying leaves of Musa basjoo. Sexual morph: Not obersved. Asexual morph: hyphomycetous. Colonies on host black, powdery, conidial masses are clearly visible on the host. Mycelium sub hyaline to pale brown or brown branched, having black conidial clusters Conidiophores 60–130 × 3 − 4 μm (x̄ = 76.5 × 3.5 μm, n = 15), macronematous, mononematous, appear as single or a cluster, erect, rough-walled, sub hyaline, brown to dark brown, septate at some points, significantly branched and flexuous. Conidiogenesis can be observed at the apices of the branches or from the middle of the conidiophores. Conidiogenous cells 3.5–4.5 μm × 2.5–3.5 μm (x̄ = 3.5 × 2.8 μm, n = 10), annellidic, monoblastic, discrete on the stipe, percurrent proliferations present as scars at the apex of the conidiophore. Conidia 4–8 × 4–7 μm (x̄ = 6.8 × 6.2 μm, n = 40), appear as chains or single, globose, one-celled, immature conidia are hyaline to pale brown, mature conidia are brown to dark brown, smooth or minutely verruculose, thick- walled.
Culture characteristics: Conidia germinating on PDA within 36–48 h. Colonies on PDA, reach 15 mm after 18 days at 25 °C, at maturity, unevenly distributed radial furrows or linear marks were observed, surface notably rough at maturity with crenulate to crenate margin, the colony is completely black and powdery at maturity, moderately dense, reverse white to black.
Material examined: Russia, Krasnodar region, Sochi, Khstinsky City District, M.V. Frunze Health Care Resort, park, on a dying leafstalk of Musa basjoo Siebold & Zucc. ex Iinuma (Musaceae), 15 October 2018, Timur S. Bulgakov, BNR-001 (MFLU 18–1896), living culture MFLUCC 22-0178.
Known hosts: Cortaderia sp. (Poaceae, Monocotyledon) (Thambugala et al. 2017); on Caragana arborescens (Fabaceae, Dicotyledon) (Phookamsak et al. 2019); on Musa sp. (Musaceae, Monocotyledon) (Samarakoon et al. 2021 and this study).
Distribution: From Russia (This study), from Thailand (Thambugala et al. 2017, Samarakoon et al. 2021; this study), from Yunnan, China (Phookamsak et al. 2019)
GenBank numbers: OP097674 (LSU), OP099358 (SSU), OP099551 (ITS), OP113822 (tef1)
Notes: Based on BLASTn search results of SSU, LSU, ITS sequence data, MFLUCC 22-0178, showed high similarity to Periconia cortaderiae (MFLUCC 18-0668) as follows; SSU = 99.76%, LSU = 99.88%, ITS = 99.80%. Our new collection is similar to the holotype of P. cortaderiae (Thambugala et al. 2017), except the length and the shape of the conidiophores. Our collection has short conidiophores with respect to the holotype (60–130 × 3–4 μm vs. 400–800 × 4–9.4 μm). The conidiophore of our strain is notably curved and branched compared to other collections. The conidiogenesis is also found as terminal and intercalary on the conidiophores in our strain. The other collections of P. cortaderiae only had terminal conidial formation with respect to our finding. There is no significant difference in the nucleotide base pair comparison of our strain with the ex-type strain. In our multigene phylogeny, MFLUCC 22-0178 grouped with P. cortaderiae (MFLUCC 15-0451, MFLUCC 18-0668, MFLUCC 20-0236) with strong statistical support (ML = 100%, BYPP = 1.00). In this study, we identify our new collection as P. cortaderiae, from Musa sp. (Monocotyledon), from Russia for the first time as a new geographical record.
Tubeufiales S. Boonmee & K.D. Hyde.
Notes: Tubeufiales includes three families viz. Bezerromycetaceae, Tubeufiaceae and Wiesneriomycetaeae with 56 genera (Wijayawardene et al. 2022).
Tubeufiaceae M.E. Barr, Mycologia 71(5): 948 (1979).
Notes: Barr (1979) defined Tubeufiaceae based exclusively on the generic type, Tubeufia, and treated it in Pleosporales. Based on unique morphology and multigene phylogenetic investigations, Boonmee et al. (2012) formed the order Tubeufiales and designated Tubeufiaceae as the typical family. Previously, 19 genera were compared in Tubeufiaceae (Boonmee et al. 2012). Following that, Lu et al. (2018) reappraised Tubeufiaceae and accepted 43 taxa to this family based on phylogenetic analyses and morphological evidence. With the expansion of fungal investigations, the family Tubeufiaceae presently contains 47 genera (Lu et al. 2018; Liu et al. 2019a, b, c, d; Wijayawardena et al. 2022).
Helicoma Corda, Icon. fung. (Prague) 1: 15 (1837)
Notes: Helicoma was introduced by Corda (1837) who treated H. muelleri as the type species. It is one of the earliest described helicosporous hyphomycete genera (Liu et al. 2019a, b, c, d). Helicoma species have been found in tropical and temperate locations (Boonmee et al. 2012, 2014; Lu et al. 2018; Brahmanage et al. 2017), and there are 59 records in the genus (Lu et al. 2018; Liu et al. 2019a, b, c, d; Barreto et al. 2021).
Helicoma aquaticum Y.Z. Lu, J.C. Kang & K.D. Hyde, in Lu et al. Fungal Diversity 92: 174 (2018).
Index Fungorum: 554836; Facesoffungi number: FoF04712; Fig. 51
Saprobic on decaying fruits of Dipterocapus sp. Asexual morph: Hyphomycetous, helicosporous. Colonies on the substratum superficial, gregarious, brown. Mycelium composed of partly immersed, partly superficial, hyaline to pale brown, septate, hyphae. Conidiophores macronematous, mononematous, cylindrical, erect, straight, unbranched, septate, 119–180 μm long, 6.5–7 μm wide, the lower part brown to dark brown, the upper part pale brown, smooth-walled. Conidiogenous cells holoblastic, mono- to polyblastic, integrated, intercalary, cylindrical, with denticles, arising laterally from the lower part of conidiophores, 10–15 × 5–9 μm (x̅ = 12 × 7 μm, n = 20), brown, smooth-walled. Conidia solitary, pleurogenous, helicoid, tapering towards apex, rounded at tip, 33–37 μm diam. and conidial filament 3.5–6 μm wide (x̅ = 5 μm, n = 20), 290–376 μm long (x̅ = 321 μm, n = 20), 20–27-septate, constricted at septa, coiled 11/2–41/4 times, becoming loosely coiled or uncoiled in water sometimes, hyaline to pale brown, smooth-walled. Sexual morph: Not observed.
Culture characteristics: Colonies growing on PDA at 25 °C, edge undulate, flat, circular, spreading, with fluffy pale brown air-mycelium, pale brown to brown mycelium. reverse dark brown, pale brown at the edge side, lobate at the center, without pigmented.
Material examined: Thailand, Chiang Mai Province, MRC, on dead fruits of Dipterocarpus sp. (Dipterocarpaceae), 15 August 2019, Xia Tang, Dip 18 (MFLU 21-0176), living culture MFLUCC 21-0141.
GenBank numbers: OM232106 (ITS), OM248446 (LSU), OM272846 (tef1)
Hosts: Unindentified submerged decaying wood (Lu et al. 2018), dead fruits of Dipterocarpus sp. (Dipterocarpaceae) (this study).
Distribution: Thailand (Lu et al. 2018, this study).
Notes: Helicoma aquaticum was described and illustrated by Lu et al. (2018) from an unidentified submerged decaying wood in Thailand. Based on the morphological characteristics, our collection shares similar morphology with the ex-type strain (Fig. 52). Comparisons of ITS, LSU and tef1 sequence data between our collection and the holotype showed that they are 2/ 549 bp (0.3%) of ITS, 9/1201 (0.7%) of LSU and 9/879 bp (1%) of tef1. We consider that our collection is the same as Helicoma aquaticum following the guidelines for species delineation proposed by Jeewon and Hyde (2016). This is the first report of Helicoma aquaticum on a Dipterocarpus sp. in Thailand.
Phylogram generated from parsimony analysis based on combined ITS, LSU and tef1 sequence data of Helicoma. ML and MP bootstrap support values ≥ 70% are indicated above the nodes, and branches with Bayesian posterior probabilities ≥ 0.95 are given above the nodes. The ex-types (reference strains) are in bold; the new isolates are in blue bold. The tree is rooted with Kamalomyces thailandicus (MFLUCC 13–0233) and Kamalomyces thailandicus (MFLUCC 11–0158)
Wiesneriomycetaceae Suetrong, Rungjind., Somrith. & E.B.G. Jones
Based on morphology and molecular phylogeny, Suetrong et al. (2014) introduced Wiesneriomycetaceae as order incertae sedis. Pratibha et al. (2015) placed Wiesneriomycetaceae in Tubeufiales. Bezerra et al. (2017) introduced Wiesneriomycetales to accommodate Wiesneriomycetaceae based on morphological characteristics and phylogenetic analyses. Based on phylogenetic inference and divergence times estimates, Liu et al. (2017) considered Wiesneriomycetales as a synonym of Tubeufiales. Hongsanan et al. (2020b) and Wijayawardena et al. (2020, 2022) accepted this family in Tubeufiales.
Wiesneriomyces Koord., Verh. K. Akad. Wet., tweede section 13(4): 246 (1907).
Notes: Wiesneriomyces species have setose, branched conidiophores, single-chain hyaline conidia, with a short isthmi separating the conidia (Suetrong et al. 2014; Hongsanan et al. 2020b). The sexual morph for this genus is not yet reported.
Wiesneriomyces laurinus (Tassi) P.M. Kirk, Trans Br Mycol Soc 82: 748 (1984)
Index Fungorum number: IF107371; Facesoffungi number: FoF09126, Fig. 53
Saprobic on dead leaves of Dimocarpus longan. Sexual morph: Not observed. Asexual morph: Colonies effuse, yellowish-brown. Conidiomata sporodochial, solitary, 3–10 setae arising from the margins of the sporodochial stalk. Setae 140–300 × 3–8 µm (x̄ = 180 × 5 μm, n = 30), subulate, apex acute, septate, thick-walled, deep brown, arising on leaf surface. Conidiophores 30–50 × 2–4 µm (x̄ = 40 × 3 μm, n = 21), emi-mucronematous, close to one another, brown to sub-hyaline at the base, hyaline towards the apex, septate, irregularly branched. Conidiogenous cells 6–9 × 2–4 µm (x̄ = 7 × 3 μm, n = 26), located at the conidiophores terminal, cylindrical, hyaline, integrated. Conidia 50–70 × 2–4 μm (x̄ = 60 × 3 μm, n = 30), solitary, clumped together into a semi-mucus, hyaline, smooth, sandy, cylindrical, taper towards both ends that are obtusely rounded, 5–6-septate, prominently constricted at septa, median cells 6–10 × 2–4 µm long, terminal cells 6–10 × 2–4 µm.
Culture characteristics: Colonies on PDA, 37–42 mm diam. after 3 weeks, colonies from above: medium dense, flat, slightly raised, rough surface with irregular edges, fluffy not smooth, leaden grey to light grey at the margin, black to ollvnceous black in the centre; reverse: grey to light grey at the margin, grey-brown to grey in the centre.
Material examined: China, Guangdong Province, Guangzhou City, Haizhu District, Zhongkai University of Agriculture Engineering, 23° 6′ 32″ N, 113° 16′ 37″ E, alt. 20 m, on the dead leaves of Dimocarpus longan Lour. (Sapindaceae), 23 July 2021, YH. Yang & CF. Liao (ZHKU 22-0008); living cultures ZHKUCC 22-0008, ZHKUCC 22-0009.
Known hosts: Carissa carandas (Apocynaceae), Clusia rosea (Clusiaceae), Hibiscus elatus (Malvaceae), Ficus ampelas (Moraceae), Gyranthera caribensis (Malvaceae), leaf litter of Caesalpinia echinata (Fabaceae), Laurus nobilis (Lauraceae), Ocotea leucoxylon (Lauraceae), Pandanus urophyllus (Pandanaceae), Psidium guajava (Myrtaceae), Roystonea regia (Arecaceae), Talipariti elatum, Theobroma cacao (Malvaceae), Thuja occidentalis (Cupressaceae), Dimocarpus longan (Sapindaceae)
Distribution: Brazil (da Silva and Grandi 2008), China (this study), Cuba (Delgado-Rodriguez et al. 2002), Hong Kong (Lu et al. 2000; Zhuang 2001), Mexico (Begerow et al. 2018), Myanmar (Thaung 2008), Russia (Melnik and Popushoi 1992), United Kingdom (Dennis 1986), Venezuela (Castaneda-Ruiz et al. 2003).
GenBank numbers: ZHKUCC 22-0008—OM780294 (LSU), OM780298 (SSU), OM780284(ITS)
ZHKUCC 22-0009—OM780295 (LSU), OM780306 (SSU), OM780286 (ITS)
Notes: Our two strains (ZHKUCC 22-008 and ZHKUCC 22-009) share similar characters with Wiesneriomyces laurinus (Heredia et al. 2000; Rajashekhar and Kaveriappa 2000; Suetrong et al. 2014; Pratibha et al. 2015; Tennakoon et al. 2021). The phylogeny also showed that our strains (ZHKUCC 22-008 and ZHKUCC 22-009) clustered with other Wiesneriomyces laurinus strains (closer to BCC18609), with moderate statistical support (Fig. 54). This is the first report of Wiesneriomyces laurinus on Dimocarpus longan.
The best scoring RAxML tree for Wiesneriomycetaceae, for combined dataset of LSU, SSU and ITS sequence data. The tree is rooted with Bezerromyces brasiliensis (URM7411) and Bezerromyces pernambucoensis (URM7412). Ex-type strains are in bold and newly generated sequences are in red. Bootstrap support values for ML ≥ 50% and BYPP ≥ 0.95 are given above the nodes
Dothideomycetes orders incertae sedis
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss.
Notes: The order was introduced by Hawksworth and Eriksson (1986), based on the type species of the order, Asterina melastomatis Léveillé, which had its DNA extracted, sequenced and studied phylogenetically for the first time by Guatimosim et al. (2015), demonstrating that the Asterinales is polyphyletic. Based on molecular data of the type species, Asterinales stricto sensu includes two families, namely: Asterinaceae and Parmulariaceae (Guatimosim et al. 2015; Giraldo et al. 2017; Phookamsak et al. 2019; Johnston and Park 2019; Hongsanan et al. 2020a; Le Renard et al. 2020; Firmino and Pereira 2021).
Asterinaceae Hansford, Mycol. Pap. 15: 188 (1946)
Notes: Asterinaceae was proposed by Hansford (1946), undergoing several modifications over time in relation to the genera belonging to the family. Asterinaceae is polyphyletic and the Asterinaceae stricto sensu comprises the species grouping with the type species, Asterina melastomatis.
Asterina Léveillé, Annls Sci. Nat., Bot., sér. 3 3: 59 (1845)
Notes: The genus was described by Léveillé (1845), having Asterina melastomatis as the type species. The genus is characterized by having circular shaped ascomata, opening by a star-shaped fissure, with absence of hypostroma, adhering to the host by superficial hyphae with lateral appressoria (hyphopodia), bitunicate asci disposed as an upright palisade layer, and 2–celled brownish ascospores.
Asterina brigadeirensis A.L. Firmino & O.L. Pereira, sp. nov.
Index Fungorum number: IF900066; Facesoffungi number: FoF13383; Fig. 55
Asterina brigadeirensis (VIC 44217, holotype) a Colony with open thyriothecia and surface mycelium. b Ascomata opened by a central star-shaped fissure. c Cross section of the ascomata. d Globose to pyriform unicelular appressoria. e Immature ascus with pseudoparaphyses. f Mature ascus. g Immature ascospores. h Brown and verruculose ascospore. Scale bars: a = 200 µm, b–c = 50 µm, d–e–f = 20 µm, d = 10 µm, g–h = 10 µm
Etymology: Name refers to the mountain range, where the fungus was collected, Serra do Brigadeiro.
Holotype: VIC 44217
Sexual morph: Colonies amphigenous, circular to irregular, single to confluent, dark brown, black, 0.1–5 mm diam. Hyphae straight to slightly flexuous, branching irregularly, brown, septate, hyphal cells cylindrical, 4.5–7.5 μm diam., smooth. Appressoria numerous, entire to lobate, sessile, lateral, alternate to unilateral, never opposed, globose to pyriform, unicellular, straight to angular, 7.5–10 × 6–9.5 μm, brown, penetration peg central on the appressorial cell. Ascomata superficial, thyriothecia, scutiform, on top of mycelial mat, circular to ellipsoid, single to confluent, fringed at margins, randomly distributed in the colony, 135–262.5 μm diam., opening by a central star-shaped fissure to an irregular fissure, dark brown to black; wall of textura radiata to irregulate on cells isodiametric to cylindrical. Pseudoparaphyses cylindrical, filiform, septate, unbranched, hyaline, up to 2.5 μm wide. Asci bitunicate in structure, fissitunicate, disposed as an upright palisade layer, ovoid to cylindrical, 8-spored, hyaline, 65–95 × 35–45 μm. Ascospores cylindrical to oblong, ends rounded, straight or slightly arched, 1-septate, constricted at the median septum, hyaline, becoming brown at maturity, verruculose, 30–35 × 12.5–16 μm. Asexual morph: Not observed.
Material examined: Brazil, Minas Gerais, Araponga, on living leaves of Miconia cinnamomifolia Naudin (Melastomataceae), 10 September 2014, A.L. Firmino (VIC 44217, holotype).
GenBank numbers: MZ475298 (LSU)
Notes: Asterina brigadeirensis differs from the species previously reported on Melastomataceae (Léveillé 1845; Hennings 1904, 1909; Theissen 1912, 1913; Sydow and Sydow 1916; Yates 1917; Maublanc 1920; Ryan 1924, 1928; Sydow 1927, 1930; Sydow and Petrak 1929; Chardón and Toro 1930; Orejuela 1944; Petrak 1950; Hansford 1954; Yamamoto 1957; Hosagoudar and Abraham 2000). It is closest to A. venezuelana, which has smaller and ovoid to conoid appressoria, smaller and ovoid to clavate asci, and smaller and dark brown ascospores. Asterina brigadeirensis is easily separated from A. amadelpha, A. belluciae, A. centroniae, A. chrysophylli, A. confertissima, A. hypophyla, A. maublancii, A. melanotes, A. melastomatis, A. melastomatis-candidi, A. memecylonicae, A. pulla, A. schlechteriana, A. sinsuieiensis, A. uribei in having verruculose ascospores. Asterina antioquensis is distinct from the new species in having ovoid to ellipsoid appressoria, smaller asci, and smaller ascospores with an upper third septum. Asterina astroniae has narrow hyphae, smaller and ovoid appressoria, smaller and subglobose to ovoid asci, and finally smaller ascospores. Asterina denigrata differs from Asterina brigadeirensis in the hypophyllous colonies, smaller ascomata and asci with 2–6 spores, and smaller and dark brown ascospores. Asterina hughesii differs in the narrow appressoria, smaller and spatulate asci, lacking pseudoparaphyses, and smaller ascospores. Asterina melastomatacearum differs from Asterina brigadeirensis in the smaller asci, and smaller and ellipsoid-ovoidal ascospores. Asterina madikeriensis differs in the colonies epiphyllous, entire appressoria and smaller ascospores with tuberculate wall. Asterina melastomaticola differs in the narrow hyphae, lacking pseudoparaphyses, and much smaller ascospores with echinulate ornamentations. Asterina miconiae differs from Asterina brigadeirensis in the smaller and cylindrical to subglobose appressoria, smaller and elliptical-clavate asci, and smaller ascospores. Asterina miconiicola differs in the much smaller ascospores with an upper third septum. Asterina theissenii differs in having narrow and dark brown hyphae, cylindrical to hemispherical appressoria, lacking pseudoparaphyses, and much smaller asci and ascospores. Asterina transiens differs from new species in the cylindrical appressoria, smaller and elliptical asci, and much smaller ascospores. Asterina venezuelana differs in the narrow and dark brown hyphae, smaller and ovoid to clavate asci, and smaller and dark brown ascospores. Finally, Asterina lopi, described below differs from the new species in having smaller and cylindrical to globose appressoria, smaller and ovoid to subclavate asci, and much smaller ascospores. (Léveillé 1845; Hennings 1904, 1909a; Theissen 1912, 1913; Sydow and Sydow 1916; Yates 1917; Maublanc 1920; Ryan 1924, 1928; Sydow 1927, 1930; Sydow and Petrak 1929; Chardón and Toro 1930; Orejuela 1944; Petrak 1950; Hansford 1954; Yamamoto 1957; Hosagoudar and Abraham 2000; Hosagoudar 2006). Based on our phylogenetic analyses (Fig. 56) and morphological analyses, herein we introduce a new species, A. brigadeirensis. Asterina brigadeirensis is the tenth species of Asterina reported on hosts belonging to Melastomataceae in Brazil, and the twelfth on Miconia.
The phylogenetic tree was obtained by Bayesian inference methods using the sequences of the LSU region. The posterior probability values are indicated at the nodes. Strain numbers are indicated after species names. New sequence data is in bold and blue. The analyses included 33 strains including representative genera of Asterinales stricto sensu and Asterinales lato sensu. The tree is rooted with Venturia populina (CBS 256.38) and V. inaequalis (CBS 815.69) (Pleosporales) as outgroup
Asterina lopi A.L. Firmino & O.L. Pereira, sp. nov.
Index Fungorum number: IF900067; Facesoffungi number: FoF 13384; Fig. 57
Asterina lopi (VIC 44219, holotype) a Colony with open thyriothecia and surface mycelium. b Ascoma opened by a central star-shaped fissure. c Cross section of the ascoma. d Cylindrical to globose unicelular appressoria. e Immature ascus. f Mature ascus. g Immature ascospores. h Mature brown ascospores. Scale bars: a = 200 µm, b = 50 µm, c–f = 20 µm, d–e = 10 µm, g–h = 5 µm
Etymology: The name refers to the mountain range, where the fungus was collected, Serra do Lopo.
Holotype: VIC 44219
Sexual morph: Colonies epiphyllous, circular to irregular, single to confluent, black, 1–6 mm diam. Hyphae straight to flexuous, branching unilaterally or irregularly, brown, septate, hyphal cells cylindrical, 4–4.5 μm diam., smooth. Appressoria numerous, entire to irregularly lobate, sessile, lateral, alternate to unilateral, never opposed, cylindrical to globose, unicellular, straight to angular, 5–7.5 × 5–6 μm, brown, penetration peg central on the appressorial cell. Ascomata superficial, thyriothecia, scutiform, on top of mycelial mat, circular to ellipsoid, single to confluent, fringed at margins, randomly distributed in the colony, 112–212.5 μm diam., opening by a central star-shaped fissure, dark brown; wall of textura radiate to irregulata, cells isodiametric to cylindrical to irregular. Pseudoparaphyses cylindrical, filiform, septate, unbranched, hyaline, up to 2.5 μm wide. Asci bitunicate in structure, fissitunicate, disposed as an upright palisade layer, ovoid to subclavate, 8-spored, and hyaline, 35–55 × 20–25 μm. Ascospores cylindrical to oblong, ends broadly rounded, straight, 1-septate, constricted at the median septum, hyaline, becoming pale brown to brown at maturity, verruculose, 12.5–15 × 6–7 μm. Asexual morph: Not observed.
Material examined: Brazil, Minas Gerais, Extrema, on living leaves of Miconia sp. (Melastomataceae), 24 December 2014, A.L. Firmino & S.R. Pacheco (VIC 44219, holotype).
GenBank numbers: MZ475299 (LSU)
Notes: Twenty-seven species of Asterina have been reported previously in association with living leaves of melastomataceous hosts. Asterina guianensis on Miconia guianensis from Costa Rica and Miconia mirabilis from Puerto Rico and Virgin Islands, A. racemosae on Miconia racemose from Puerto Rico, and A. tetrazygiae on Tetrazygia sp., and Tetrazygia elaeagnoides from Puerto Rico and Virgin Islands, were described by Ryan (1924) and not used in the comparisons for having an ostiolar opening, a characteristic that does not belong to Asterina.
Asterina lopi differs from the species previously reported on Melastomataceae (Léveillé 1845; Hennings 1904, 1909; Theissen 1912, 1913; Sydow and Sydow 1916; Yates 1917; Maublanc 1920; Ryan 1924, 1928; Sydow 1927, 1930; Sydow and Petrak 1929; Chardón and Toro 1930; Orejuela 1944; Petrak 1950; Hansford 1954; Yamamoto 1957; Hosagoudar and Abraham 2000; Hosagoudar 2006) in morphology and on host association (Table 4). It is morphologically closer to A. melastomatacearum, which has larger and ellipsoid-ovoidal ascospores. Asterina lopi is easily separated from A. amadelpha, A. belluciae, A. centroniae, A. chrysophylli, A. confertissima, A. hypophyla, A. maublancii, A. melanotes, A. melastomatis, A. melastomatis-candidi, A. memecylonicae, A. pulla, A. schlechteriana, A. sinsuieiensis, and A. uribei in having verruculose ascospores; A. antioquensis, A. madikeriensis, A. miconiae, A. transiens and A. venezuelana in having lobate appressoria. Asterina astroniae is distinct from the new species in having ovoid appressoria, ascomata with irregular fissure, wider asci, and larger ascospores. Asterina denigrata has hypophyllous colonies, wider and dark brown hyphae, dark brown and subglobose to conoid appressoria, wider and ovoid to ellipsoid asci with 2–6 spores, and larger and dark brown ascospores. Asterina hughesii differs from Asterina lopi in the wider hyphae, larger appressoria, narrow and spatulate asci, lacking pseudoparaphyses, and larger ascospores. Asterina melastomaticola differs in the pulvinate to conoid appressoria, globose to ovoid asci, lacking pseudoparaphyses, and larger ascospores. Asterina miconiicola differs from Asterina lopi in the wider hyphae, larger appressoria, and narrow ascospores with an upper third septum. Asterina theissenii differs in having narrow and dark brown hyphae, globose to ovoid asci, lacking pseudoparaphyses, and larger ascospores (Léveillé 1845; Hennings 1904, 1909; Theissen 1912, 1913; Sydow and Sydow 1916; Yates 1917; Maublanc 1920; Ryan 1924, 1928; Sydow 1927, 1930; Sydow and Petrak 1929; Chardón and Toro 1930; Orejuela 1944; Petrak 1950; Hansford 1954; Yamamoto 1957; Hosagoudar and Abraham 2000). Based on our phylogenetic analyses (Fig. 56) and morphology, herein we introduce a new species, A. lopi. Asterina lopi is the ninth species of Asterina reported on hosts belonging to Melastomataceae in Brazil, and the eleventh on Miconia.
Botryosphaeriales C.L. Schoch, Crous & Shoemaker.
Notes: Botryosphaeriales was introduced to accommodate a single family Botryosphaeriaceae by Schoch et al. (2006). Schoch et al. (2009a) accepted its position in the Pleosporomycetidae. Later, Planistromellaceae was recognised as a distinct family within Botryosphaeriales (Minnis et al. 2012) Phyllostictaceae was reinstated for Phyllosticta (Wikee et al. 2013). Another three families were introduced by Slippers et al. (2013), namely Saccharataceae for Saccharata, Aplosporellaceae for Aplosporella and Melanopsaceae for Melanops. Wyka and Broders (2016) introduced Septorioideaceae for Septorioides. Yang et al. (2018) raised Endomelanconiopsis and Pseudofusicoccum to familial status as Endomelanconiopsidaceae and Pseudofusicoccaceae, respectively. Wijayawardene et al. (2018) accepted the above nine families in the order. However, Phillips et al. (2019) synonymised Endomelanconiopsidaceae with Botryosphaeriaceae, Pseudofusicoccaceae with Phyllostictaceae, and Septorioideaceae with Saccharataceae based on morphology, phylogeny and evolutionary divergence times. Therefore, six families, i.e. Aplosporellaceae, Botryosphaeriaceae, Melanopsaceae, Phyllostictaceae, Planistromellaceae, Saccharataceae, are phylogenetically proved in the order now (Hongsanan et al. 2020a, b; Wijayawardene et al. 2020).
Aplosporellaceae Slippers, Boissin & Crous, Stud. Mycol. 76(1): 41 (2013)
Aplosporellaceae was introduced in Botryosphaeriales with two genera Aplosporella and Bagnisiella (Slippers et al. 2013). Sharma et al. (2017) added Alanomyces as a new genus in this family based on four loci phylogeny. Two genera are accepted in Aplosporellaceae viz. Alanomyces and Aplosporella (Dissanayake et al. 2021a, b; Wijayawardene et al. 2022).
Aplosporella Speg. Anal. Soc. cient. argent 10(4): 157 (1880).
Aplosporella was treated as a type genus of Aplosporellaceae in Botryosphaeriaceae (Crous et al. 2006; Schoch et al. 2006; Damm et al. 2007; Liu et al. 2012). The genus was established by Spegazzini (1880) and typified by Aplosporella chlorostroma. Later, Aplosporellaceae was introduced as a new family by Slippers et al. (2013). Wijayawardene et al. (2014a, b, 2016, 2020, 2022) also confirmed the phylogenetic placement and accepted Aplosporellaceae in Botryosphaeriaceae. There are 342 epithets of Aplosporella listed in Index fungorum (2022a, b). Aplosporella is characterized by muti-locular conidiomata, with hyaline to brown, aseptate conidia and, filiform paraphyses (Damm et al. 2007; Ekanayaka et al. 2016; Dissanayake et al. 2021a, b; Hyde et al. 2021). Aplosporella species are not easy to identify based on only morphology because of their wide range of host and morphological similarities. Many species were introduced based on their host occurrence and suggested that species in Aplosporella are not host-specific (Damm et al. 2007; Fan et al. 2015; Ekanayaka et al. 2016; Dou et al. 2017).
Aplosporella artocarpi Trakun., L. Lombard & Crous, in Trakunyingcharoen et al., Persoonia 34: 91 (2014)
Index Fungorum number: IF810167; Facesoffungi number: FoF10747, Fig. 58
Aplosporella artocarpi (MFLU 22–0108, new host record). a, b Appearance of conidiomata on host substrate. c Section through conidoma. d Peridium. e Pseudoparaphyses. f Conidia on the conidiogenous cells. g-j Conidia. k Sporulation l–m Culture on PDA from surface and reverse. Scale bars. a = 1000 µm, b = 500 µm, c = 100 µm, d = 30 µm, e = 5 µm, f, l, m = 20 µm, g,h,I,j,k = 10 µm
Saprobic on dead stems of Chromolaena odorata. Sexual morph: Not observed. Asexual morph: Conidiomata (280–) 300–350 × 370–450 (–500) µm (x̄ = 300 × 415 µm, n = 5), solitary, immersed to semi-immersed with 2–3 locules, globose to subglobose, black. Ostiole absent. Peridium 45–50 (–60) µm wide, multi-layered, comprised of dark brown cells of Textura angularis. Hamanthecium 3–5 µm wide, numerous, hyaline, aseptate, paraphyses. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 1–2 µm wide, holoblastic, cylindrical, hyaline. Conidia 15–20 × 5–10 µm (x̄ = 17 × 7 µm, n = 15), aseptate, rough-walled, granular appearance, hyaline to dark brown, ellipsoidal, without appendages.
Culture characteristics: Conidia germinating on PDA within 24 h, reaching 85 mm after 7 days at room temperature, concentric, flat, irregular, rough surface, greenish-grey.
Material examined: Thailand, Chiang Rai Province, Doi Pui, on the dead stems of Chromolaena odorata (L.) (Asteraceae), 10 July 2020, Zin Hnin Htet, SW23 (MFLU 22–0108), living culture MFLUCC 22-0010.
Known hosts and distribution: On asymptomatic twig of Artocarpus heterophyllus (Moraceae) in Chiang Mai Province, Thailand (Trakunyingcharoen et al. 2015); on asymptomatic leaves of Stoechospermum marginatum (Dictyotaceae) and Caulerpa taxifolia (Caulerpaceae) in India (Sahoo et al. 2021); on dead branches of Mangifera indica (Anacardiaceae) in China (Yang et al. 2022); on dead stems of Chromolaena odorata (Asteraceae) in Chiang Rai Province, Thailand (this study)
GenBank numbers: ON834371(LSU), ON823183(ITS)
Notes: Aplosporella artocarpi was introduced by Trakunyingcharoen et al. (2015). The species has morphologically similar to other Aplosporella species such as A. hesperidica, A. prunicola, and A. thailandica in having dark-brown, multilocular conidiomata with aseptate, hyaline to dark-brown conidia (Damm et al. 2007; Ekanayaka et al. 2016; Dissanayake et al. 2021a, b). In our phylogenetic analysis, Aplosporella artocarpi (MFLUCC 22-0010) is closely related to Aplosporella artocarpi (CPC 22,791) with ML = 70%, BYPP = 0.54. (Fig. 59). According to BLASTn result, the closest match for the LSU sequence was Aplosporella artocarpi (CPC 22791) with 99.83% similarity. The closest match for the ITS sequence was Aplosporella prunicola (CBS 121167) with 97.81% similarity. Furthermore, comparisons of ITS region between our taxon, Aplosporella artocarpi (MFLUCC 22-0010) and ex-type strain of A. artocarpi (CPC 22791) show one base pair difference (0.18%) across 531 nucleotides. Aplosporella species can be found on a wide range of hosts such as Anacardiaceae, Asteraceae, Caulerpaceae, Cupressaceae, Dictyotaceae, Fabaceae, Gingkoaceae, Moraceae, Myrtaceae, Proteaceae, and Rosaceae (Du et al. 2017; Mapook et al. 2020; Sahoo et al. 2021; Yang et al. 2021). We collected Aplosporella artocarpi (MFLU 22-0108) from Thailand and reported here as a new host record associated with Chromolaena odorata.
Phylogram generated from maximum likelihood analysis based on combined LSU, ITS, and tef1 sequence data for the Aplosporella. The combined dataset consists of 19 taxa from Aplosporella and our taxon, Aplosporella artocarpi (MFLUCC 22–0010). Melanops tulasnei (CBS 116805 and CBS 116806) are used as outgroup. The topology of the maximum likelihood analysis is similar to Bayesian analysis. Bootstrap support value for ML ≥ 60% and BYPP ≥ 0.95 are given above the branches. The ex-type strains are bold. The newly generated sequence is indicated in bold and red
Botryosphaeriaceae Theiss. & Syd. [as 'Botryosphaeriacae'], Annls mycol. 16(1/2): 16 (1918)
Notes: Botryosphaeriaceae was introduced by Theissen and Sydow (1918) for three genera, Botryosphaeria, Phaeobotryon and Dibotryon. Initially, the family had been successively put into the orders Dothideales (Müller and von Arx 1950) and Pleosporales (Luttrell 1955), until Schoch et al. (2006) raised Botryosphaeriaceae to orderal status as Botryosphaeriales. Over decades of taxonomic revisions and updates based on morphology, the family has become increasingly complex. Kirk et al. (2008) estimated that there are 26 genera and 1517 species in the family, while Liu et al. (2012) accepted 29 genera and approximately 1485 species. Phillips et al. (2013) consider morphological characters alone as inadequate to define genera or identify species, and they detailed described 17 genera and 110 species which has molecular data. Thereafter, Dissanayake et al. (2017) introduced six new genera and 85 new species/species combinations. So far, 22 genera are accepted in Botryosphaeriaceae (Phillips et al. 2019; Hongsanan et al. 2020a).
Botryosphaeria Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 211 (1863)
Notes: Botryosphaeria was introduced by Cesati and De Notaris (1863) based on the type species B. dothidea. However, as the type material was immature, B. dothidea was epitypified by Slippers et al. (2004) based on morphology and phylogenetic data which combined ITS, tef1 and tub2 genes. The sexual morphs are characterized by brown to black, globose ascostromata, comprising a botryose aggregate, or sometimes solitary, with a central ostiole, papillate or not, bitunicate, clavate asci, with a short pedicellate and a small ocular chamber, intermixed with hyphae-like, wide, septate pseudoparaphyses, and hyaline, aseptate, fusoid to ovoid ascospores, with or without a mucilaginous sheath (Liu et al. 2012). The asexual morphs of Botryosphaeria were reported as Dichomera, Diplodia, and Fusicoccum (Crous and Palm 1999; Slippers et al. 2004; Crous et al. 2006). They are characterized by uni- to multilocular pycnidial, frequently embedded in stromatic tissue, holoblastic, hyaline, subcylindrical conidiogenous cells, with 1–2 percurrent proliferation, and hyaline, aseptate, narrowly fusiform, or irregularly fusiform conidia, rarely forming a septum before germination, smooth with granular contents (Slippers et al. 2004). So far, 286 species names are recorded in Index Fungorum (2022a, b), in which 30 species known from culture are accepted in the genus.
Botryosphaeria dothidea (Moug.) Ces. & De Not., Comm. Soc. crittog. Ital. 1(fasc. 4): 212 (1863).
See Species fungorum for synonyms.
Index Fungorum number: IF183247; Faces of fungi number:FoF03512; Fig. 60
Botryosphaeria dothidea (IFRD500–008, new geographic and habitat record) a, b Appearance of ascomata on host substrate. c Section of ascomata. d Peridium. e Pseudoparaphyses and asci. f–i Asci. j–l Ascospores. m–n Colony on PDA (m from front, n from reverse). Scale bars: a = 500 μm, b = 200 μm, c, e = 100 μm, d = 30 μm, f–i = 20 μm, j–l = 10 μm
Saprobic on submerged wood of freshwater. Sexual morph: Ascomata 300–500 μm diam., black, circular or subglobose to globose, scattered, gregarious, uni- to multi-loculate, immersed to erumpent on host tissue, with visible black dots or papilla. Ostiole circular, central, papillate. Peridium composed of two-layered locules, outer layer composed of dark brown or brown thick-walled cells of textura angularis, inner layer composed of hyaline thin-walled cells of textura angularis lining the locule. Pseudoparaphyses 2–4 μm wide, hyphae-like, septate. Asci 96–144 × 18–25 μm (\(\bar{x}\) = 116 × 23 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical to clavate, short pedicellatea, pically rounded with an ocular chamber. Ascospores 21–32 × 10–14 μm (\(\bar{x}\) = 26.3 × 11.7 μm, n = 40), biseriate, hyaline, aseptate, fusoid to ovoid, sometimes with tapered ends, spindle-shaped, thin-walled, smooth with granular contents. Asexual morph: Not observed.
Cultural characteristics: Ascospore germinating on PDA within 24 h. Colonies on PDA fast-growing, reaching 7–8 cm diameter in 50 days at 20–25 °C, with dense, hairy, black mycelium on the surface, reverse black.
Material examined: China, Yunnan Province, a small river of Puzhehei wetland, on dead submerged decaying wood of unidentified plants, 23 June 2019, Hao Yang, p27 (IFRD500–008), living culture KUMCC 20–0186.
Known hosts and distribution: broad range of hosts and wide geohraphical distribution (Farr and Rossman 2022)
GenBank numbers: MT559116 (LSU), MT559099 (ITS)
Notes: Botryosphaeria dothidea is the type species of the genus, and was reported extensively from all around the world (Fries 1823; Arx and Müller 1954). It was epitypified by Slippers et al. (2004) based on morphology and phylogeny. This taxon was collected from southwest China and its ITS sequence data of our isolate are 100% identical to the verified sequences of B. dothidea (MH992666 and MH973592). Based on morphology and phylogenetic analyses (Fig. 61), we identify the strain as B. dothidea. It is a new geographic record from China and a new habitat record from freshwater.
Phylogram generated from maximum likelihood analysis based on combined ITS, and tef1 sequence data for the Botryosphaeria. The combined dataset consists of 23 taxa from Botryosphaeria with our strain. The tree is rooted with Macrophomina phaseolina (CBS 227.33). The topology of the maximum likelihood analysis is similar to Bayesian analysis. Bootstrap support value for ML ≥ 60% and BYPP ≥ 0.90 are given above the branches. The ex-type strains are in bold. The newly generated sequence is in red
Class Laboulbeniomycetes Engler
Laboulbeniales Lindau
Notes: This order includes more than 2000 species described as obligate ectosymbionts on Arthropods. Wijayawardena et al. (2022) accepted three families viz. Ceratomycetaceae, Euceratomycetaceae and Laboulbeniaceae in this order.
Laboulbeniaceae G. Winter [as 'Laboulbenieae'], Rabenh. Krypt.-Fl., Edn 2 (Leipzig) 1.2: 918 (1886)
Notes: Goldman and Weir (2018) based on SSU rDNA sequence data identified this family to be consist of essentially terrestrial and sexually reproducing taxa with simple or compound endogenous antheridia. Santamaria and Pedersen (2021) accepted 147 genera in this family.
Rhachomyces Thaxt., Proc. Amer. Acad. Arts & Sci. 30: 468 (1895) [1894]
Notes: Rhachomyces is quite numerous: with six species described very recently, the number of accepted species in the genus is now 91 (Rossi and Christian 2020; Rossi and Leonardi 2020; Santamaria et al. 2020; Buyck et al. 2021). This genus is characterized by a series of superposed, usually short cells forming an axis that remembers a spinal column (hence the name, from Greek rachis = spine); these cells produce laterally both sterile appendages of various lengths and antheridial appendages, the latter ending with a single antheridium consisting of a simple phialide. In mature thalli the perithecia are usually found in an apical position and more frequently are single, but can be two or even more in a few species. The cells forming the outer wall of perithecia are arranged in four rows, each consisting of four unequal cells. Most of the species of Rhachomyces occur on ground beetles (Carabidae), but a few are associated with rove beetles (Staphylinidae) and two were found on small carrion beetles (Leiodidae Cholevinae). A single sequence is available for species in this genus (Goldmann and Weir 2018).
Rhachomyces cruralis W. Rossi & M. Leonardi, sp. nov.
Index Fungorum number: IF559505; Facesoffungi number: FoF13385; Fig. 62
Etymology: From Latin crus = leg, because the thalli of the new species are found only on the legs of the host insect.
Holotype: FI WR1997
Axis of the receptacle is straight or slightly curved, consisting of 10–15 brownish cells gradually increasing in size and bearing a dark brown band in the lower portion. Appendages almost straight, more numerous in the upper portion of the thallus, usually consisting of 5–6 dark brown cells separated by back septa and slight constriction, the distal one being distinctly longer and gradually paler, with the lower portion slightly inflated and the tip hyaline or almost so. Antheridial appendages very few, consisting of a short and brownish lower cell followed by a straight hyaline antheridium. Perithecium reddish-brown, subsessile, symmetrically elliptical, about twice as long as it is broad, regularly tapering to the darker, subconical tip and almost hyaline, rounded apex. Length from foot to perithecial apex 270–350 µm. Perithecium, including basal cells 125–175 × 60–80 µm. Longest appendages 100 µm.
Material examined: South Africa, E Transvaal, Mt. Sheba Nat. Res., 14–15.II.1995, S. Zoia, on posterior legs of Pachydesus rufipes (Boheman) (Carabidae, Trechini) (FI WR1997, holotype). Same data as the type, M. Zapparoli legit (FI WR1987 and WR 2000, paratypes); same data as the type, A. Vigna Taglianti legit, FI WR1998 and WR1999, paratypes).
Notes: The species is more similar to Rhachomyces cruralis and R. moreti W. Rossi et Proaño, parasitic on Trechisibus calathiformis Deuve from Ecuador. The latter fungus, however, has an oblong perithecium, the axis of the receptacle consists of 16–18 cells, and the appendages are paler and slenderer (Rossi and Proaño Castro 2009).
Rhachomyces hyperommae W. Rossi & M. Leonardi, sp. nov.
Index Fungorum number: IF559509; Facesoffungi number: FoF13386; Fig. 63
Etymology: Named after the host insect genus.
Holotype: CAMB WR2331a
Axis of the receptacle composed of 12–17 cells very different in size, shape, and color: the basal and the suprabasal are blackened, narrow, and elongate; the following 2 or 3 are relatively small, dark and isodiametric; the others gradually broader and paler from below upwards. Appendages short, stiff, one-sided, projected obliquely upwards, consisting of 4 cells, of which the lower 3 are dark brown while the upper has a paler and curved tip. Antheridial appendages similar to the sterile ones but shorter and paler, each bearing distally a brown, elongate antheridium tapering in a curved and truncate paler tip. Perithecium broadly fusiform, with the posterior side more convex, nearly hyaline or tinged with pale yellow, brownish yellow near the base in older specimens, the hyaline and conical tip ending in a blunt apex. Length from foot to perithecial apex 280–380 µm. Perithecium, including basal cells 110–145 × 35–42 µm. Antheridia 20–25 µm. Longest appendage 90 µm.
Material examined: Autralia, NSW Border Ranges N. P., Tweed Range Rd., 4.6 km SW of Bridle Ck. Rd., alt. 580 m, 4.II–9.IV.1993, M. Gray & G. Cassis, on a femur and the abdomen of Hyperomma sp. (Staphylinidae, Paederinae, Paederini, Cryptobiina), CAMB WR2331a, holotype; FI WR2331b isotype).
Notes: The new species seems to be allied to Rhachomyces arbusculus Thaxt., described on an unidentified rove-beetle from Liberia, West Africa (Thaxter 1896). The two parasites have similar appendages and are also somewhat similar in the general habitat. However, R. abusculus is distinctly more slender and more elongate, with the cells forming the receptacle more numerous (20–25) and not strongly different from each other, and with a much narrower perithecium suffused with brown at the apex.
Rhachomyces magrinii W. Rossi & M. Leonardi, sp. nov.
Index Fungorum number: IF559510; Facesoffungi number: FoF13387; Fig. 64
Etymology: Named after the entomologist Paolo Magrini, who supplied us with the material utilized for the description of the new species.
Holotype: FI WR4048
Axis of the receptacle from almost straight to distinctly curved or slightly sigmoid, consisting of (14)18–29 cells; basal and suprabasal cells slender, elongate and dark brown colored, the others gradually larger and paler. Appendages occurring in the lower part of the thallus are slender, consisting of 5–6 brownish, elongate cells; these appendages are visible only on young thalli, but are broken off in very early stages of development. The upper and more lasting appendages are thicker, generally consisting of 5 relatively short, subequal cells, the series ending with a 6th cell distinctly longer and much paler, with an almost hyaline tip. Antheridial appendages consist of a single, pale brown cell bearing apically a hyaline antheridium with a tapering and distinctly curved efferent neck. Perithecium subsessile, pale grayish brown, more or less bent as to the axis of the receptacle, symmetrical or slightly asymmetrical, about three times longer than broad, slightly more inflated below, the truncate-conical and paler tip ending in a broad and blunt apex. Length from foot to perithecial apex 525–950 µm. Perithecium 135–180(200) × 45–60(70) µm. Antheridial appendages 40–45 µm. Longest sterile appendages 150 µm.
Material examined: Mexico, Querétaro, Pinal de Amoles, Ojo de Agua, 9.III.2011, G. Trezzi, on Mexaphaenops elegans Barr (Carabidae, Trechini) (FI WR4048, holotype; FI WR4049, WR4488, paratype).
Notes: The only previously described species in Rhachomyces on a cave-dwelling Trechini from Central America is R. quetzalcoatl Balazuc, occurring on Paratrechus spp. from Mexico and Guatemala. The two species of fungi share the absence of dark pigmentation on the perithecial tip and the structure of the upper appendages, but differ greatly in other characteristics, as the much shorter receptacle of R. quetzalcoatl, consisting of dozens of cells, and its more inflated perithecium (Rossi and Cesari-Rossi 1977, Fig. 63).
Rhachomyces platyprosophi W. Rossi & M. Leonardi, sp. nov.
Index Fungorum number: IF559511; Facesoffungi number: FoF13388; Fig. 65
Etymology: Named after the host insect genus.
Holotype: FI WR3973.
Axis of the receptacle from almost straight to variably curved or sigmoid, consisting of 15–22 brownish cells gradually enlarging upwards and separated by oblique septa. Appendages spreading, nearly opaque except for the gradually paler and curved tip, longer and more numerous in the upper portion of the receptacle, reaching and sometimes exceeding the perithecial apex. Antheridial appendages relatively numerous, chestnut brown colored, consisting of a slender and elongate cell followed by a very slender antheridium with a paler and sigmoid tip. Perithecium long and slender, subsessile, oblong, light brown colored, the tip gradually tapering, hardly distinguished except for the darker color, the apex hyaline and subtruncate. Length from foot to perithecial apex 500–830 µm. Perithecium 190–290 × 50 µm. Antheridial appendages 50–65 µm. Longest sterile appendage 520 µm.
Material examined: Indonesia, Sumatra, Palembang, s. d., s. c. (from the collection of A. Fauvel in the Institut Royal des Sciences Naturelles, Bruxelles), on abdomen and legs of Platyprosopus indicus Motschulski (Staphylinidae, Staphylininae, Platyprosopini) (FI WR3973a, holotype; FI WR3973b and WR3973c isotypes).
Notes: Due to the large dimensions and the oblong perithecium, the new species can be compared with Rhachomyces carbonii W. Rossi & M. Leonardi, recently described on a rove beetle from Sierra Leone, which however bears a slender and much darker receptacle, shorter and slender appendages and has perithecium with spirally twisted wall cells with an abruptly distinguished tip (Rossi and Leonardi 2018).
Class Lecanoromycetes O.E. Erikss. & Winka
Subclass Lecanoromycetidae P.M. Kirk et al.
Caliciales Bessey.
Notes: Caliciales is an order of lichenized fungi, including mostly crustose, but also foliose forms (Lücking et al. 2016)
Caliciaceae Chevall. [as 'Calicineae'], Fl. gén. env. Paris (Paris) 1: 314 (1826)
Notes: Caliciaceae is a family of lichenized fungi, including mostly crustose, but also foliose forms (Lücking et al. 2016)
Buellia De Not., G. bot. ital. 2(1.1): 195 (1846)
Notes: Buellia is a large, heterogeneous and probably a polyphyletic genus. Some species groups are relatively well-defined and may warrant formal recognition. This includes the Buellia subalbula (Nyl.) Müll. Arg. aggregate (Bungartz et al. 2011). Below we describe a new species that belongs to this group.
Buellia pruinocalcarea Aptroot, M.F. Souza & Spielmann, sp. nov.
Index Fungorum number: IF900068; Facesoffungi number: FOF13389; Fig. 66
Saxicolous Buellia on limestone with thallus thick, white, apothecia flush with the thallus, densely white pruinose, and ascospores 12–14 × 6–7 µm.
Holotype: Aptroot 77815.
Etymology: Named after the white pruina and the calcareous habitat.
Sexual morph: Thallus crustose, covering areas up to 20 cm diam., marginal 3 mm continuous, remaining parts rather regularly cracked with areoles flat, c. 0.5 mm diam., not corticate, dull, almost pure white, very regularly c. 0.3 mm thick, not surrounded by a prothallus, but margin thinner though at least 0.1 mm thick. Isidia and soredia absent. Ascomata numerous, singly or aggregated, partly in concentrical zones (especially the outer rim of apothecia at 3 mm from the margin), immersed, flush with the thallus, rather uniformly 0.3–0.5 mm diam., sparse, solitary, disc flat, grey, margin barely raised, c. 0.05 mm thick, white, both disc and margin densely white pruinose. Excipulum uniformly dark brown in section. Epihymenium a layer of c. 15 µm high of brown paraphysal tips overlain with an c. 25 µm high epipsamma composed of many grey crystals that do not dissolve in K (calcium oxalate). Hymenium 70–90 µm high, hyaline, not inspersed, amyloid, paraphyses 1–1.5 µm wide, with dark brown upper. Hypothecium dark brown, in the central part up to 75 µm high, towards the margin tapering to c. 25 µm high. Ascospores 8/ascus, brown, 1-septate, ellipsoid, 12–14 × 6–7 µm. Asexual morph: not observed.
Chemistry: Thallus and apothecia UV + patchily salmon-orange, especially in the submarginal zone, C–, P–, K–. TLC: nil.
Ecology and distribution: On exposed limestone in Atlantic rain forest biome; only known from Brazil.
Material examined: Brazil, Mato Grosso do Sul: Serra da Bodoquena, Bodoquena, Dente de Cão, summit, alt. 450 m, 20° 47ʹ 05″ S, 56° 45ʹ 03″ W, on exposed limestone in Atlantic rain forest biome, 7 November 2018, Aptroot 77815 (holotype, CGMC).
GenBank number: MW322683 (ITS).
Notes: This species is not keyed out in Malme (1912). It shows similarities with species of the Buellia subalbula (Nyl.) Müll. Arg. aggregate (Bungartz et al. 2011) and seems most similar to Buellia amabilis de Lesd. (see Bungartz and Nash 2004), which differs by the ornamented ascospores and the apothecia that become more convex, and the absence of UV luminescence. Phylogenetically, it clusters deep inside Buellia De Not. in the current sense (Fig. 66). Sequences of other species of the B. subalbula aggregate are not available.
As part of a continuous effort to explore lichenologically relatively unidentified regions in Brazil, we investigated the microlichens on an isolated limestone outcrop in a tropical south-western inland region, viz. the Dente de Cão in the Parque Nacional da Serra da Bodoquena in the state of Mato Grosso do Sul, close to the borders with Paraguay and Bolivia. This range of hills is the only larger forested area still in existence in this state. Many microlichens were recently reported from the area (Aptroot and Spielmann 2020). This area is close to the venue of IAL9, the nearby town of Bonito.
The natural vegetation of the Serra da Bodoquena is the Atlantic rain forest, a biome that stretches all along the coast from north-eastern to south-eastern Brazil. In fact, it is the most western patch of Atlantic rainforest in existence, and as such unique. Exposed limestone outcrops occur in various places in the tropics, but their extent is often limited and they tend to be soon grown over by vegetation. The Dente de Cão is formed of white Precambrian (neoproterozoic, see Boggiani 1997) limestone known as the Bocaiana formation, and reaches 450 m alt. It weathers into karst and the name of the rock outcrop is after the sharp tooth-like rock points. Somewhat to our surprise, the exposed limestone outcrop, although only scarcely colonized by fanerogams, was not completely covered by lichens. Cyanobacteria were more abundant. The most common lichens were the species of Collemataceae and Lichinaceae (Fig. 67).
Phylogram generated from maximum likelihood analysis based on ITS. Bootstrap support values for ML ≥ 80% and Bayesian posterior probabilities ≥ 0.95 are given near nodes respectively. The tree is rooted in Diplotomma rivas-martinezii (13365 BA). Ex-type strains are in bold. The newly generated sequences are indicated in bold blue
Lecanorales Nannf.
Notes: Lecanorales is the largest order of lichenized fungi, one of the largest containing crustose lichens and the largest containing foliose lichens (Lücking et al. 2016).
Lecanoraceae Körb. [as 'Lecanoreae'], Syst. lich. germ. (Breslau): 104 (1855).
Notes: Lecanoraceae is a family of lichenized fungi, one of the largest containing crustose lichens (Lücking et al. 2016).
Lecanora Ach., in Luyken, Tent. Hist. Lich.: 90 (1809).
Notes: Lecanora is a large, heterogeneous and possibly paraphyletic genus. Some species of this group are well-defined. Below we describe a new species in the somewhat aberrant Lecanora marginata (Schaer.) Hertel & Rambold group (Rambold 1989).
Lecanora immersocalcarea Aptroot, M.F. Souza & Spielmann, sp. nov.
Index Fungorum number: IF900069; Facesoffungi:FOF13390; Fig. 68
Saxicolous Lecanora on limestone with thallus 0.5–0.8(-2.0) mm thick, very pale ochraceous white, apothecia immersed in the thallus, black, immature.
Etymology: Named for the immersed apothecia and the calcareous habitat.
Holotype: Aptroot 77822.
Thallus crustose, superficial, covering areas up to 10 cm diam., dull, cretaceous, very pale ochraceous white, regularly cracked with the areoles angular, slightly convex and c. 0.5–0.9 mm diam., c. 0.5–0.8 mm thick but in some places with lobate superficial outgrowths of up to 2 mm thick, not surrounded by a prothallus, but margin thick and raised, sharply delimited, not lobed or fissured. Isidia and soredia absent. Ascomata immature apothecia, several per areole, flush with the thallus, rounded, 0.2–0.4 mm diam.; disc black, margin thin, black. Epihymenium grey, without crystals. Excipulum without crystals. Hypothecium hyaline. Mature ascospores not observed. Pycnidia not observed.
Chemistry: Thallus UV–, C–, P + yellow, K + yellow. TLC: atranorin.
Distribution: On exposed limestone outcrop in Atlantic rain forest biome; only known from Brazil.
Material examined: Brazil. Mato Grosso do Sul: Serra da Bodoquena, Bodoquena, Dente de Cão, summit, alt. 450 m, 20° 47ʹ 05″ S, 56° 45ʹ 03″ W, on exposed limestone in an Atlantic rain forest biome, 7 November 2018, Aptroot 77822 (holotype, CGMC).
GenBank number: MW322682 (ITS)
Notes: This species is locally abundant. Fully fertile material was not found, but the type specimen was sequenced and clustered inside Lecanora Ach. in the current sense. Morphologically it would belong to the Lecanora marginata (Schaer.) Hertel & Rambold group, in which indeed calciferous species or at least specimens are known. Only one described species seems close, however, viz. Lecanora oreinodes (Körb.) Hertel & Rambold (Rambold 1989), which differs by the flatter, flush areoles and the somewhat fractured/lobate thallus margin, and which is not known to occur on pure limestone. Phylogenetically, it clusters deep inside Lecanora, where it clusters with several species with usnic acid instead of atranorin, but with low support (Fig. 69). Sequences of other species of the L. marginata group are not available. This species is described from the same locality as Buellia pruinocalcarea (for details see under Buellia pruinocalcarea).
Phylogram generated from maximum likelihood analysis based on ITS. Bootstrap support values for ML ≥ 80% and Bayesian posterior probabilities ≥ 0.95 are given near nodes respectively. The tree is rooted in Tephromela atra (L1489). Ex-type strains are in bold. The newly generated sequences are indicated in bold blue
Teloschistales D. Hawksw. & O.E. Erikss.
Notes: Teloschistales is a large order of lichenized fungi, one of the largest containing crustose lichens (Arup et al. 2013).
Teloschistaceae Zahlbr. [as 'Theloschistaceae'], in Engler, Syllabus, Edn 2 (Berlin): 45 (1898).
Notes: Teloschistaceae is a family of lichenized fungi, one of the largest containing crustose lichens. Most species are generally still treated under the genus Caloplaca Th. Fr. (Schumm and Aptroot 2019).
Wetmoreana Arup, Søchting & Frödén, in Arup, Søchting & Frödén, Nordic Jl Bot. 31(1): 66 (2013)
Notes: Wetmoreana is a recently split genus in Teloschistaceae (Arup et al. 2013). At the moment, three to five species are recognized in this genus, depending on whether or not Fulgogassparrea S.Y. Kondr., N.-H. Jeong, Kärnefelt, Elix, A. Thell & Hur (Kondratyuk et al. 2013) is recognized as a separate genus.
Wetmoreana blastidiocalcarea Aptroot, M.F. Souza & Spielmann sp. nov.
Index Fungorum number: IF900070; Facesoffungi number: FOF13391; Fig. 70
Saxicolous Wetmoreana on limestone with thallus thick, yellow-orange, radially lobate, central part of the thallus areolate, covered by c. 0.05 mm diam, semiglobose corticate bulbs of thallus color that might serve as blastidia.
Etymology: Named after the calcareous and the blastidious habitat.
Holotype: Aptroot 77806.
Thallus placodioid, up to 2 cm diam., corticate, dull, yellow-orange, white pruinose on the marginal lobes, up to 0.3 mm thick. The central part of the thallus areolate, covered by c. 0.05 mm diam, semiglobose corticate bulbs of thallus colour that might serve as (and would in former species of Fulgensia usually being called) blastidia, sometimes partly dissected into secondary lobes resembling the marginal lobes. Marginal lobes much radially divided into less than 0.1 mm wide lobuli, gradually thinning towards the margin. Isidia and soredia absent. Ascomata and pycnidia not observed.
Chemistry: Thallus and apothecia UV + red, C−, P−, K+ crimson. TLC: anthraquinones.
Distribution: On exposed limestone in Atlantic rain forest biome; only known from Brazil.
Material examined: Brazil. Mato Grosso do Sul: Serra da Bodoquena, Bodoquena, Dente de Cão, summit, alt. 450 m, 20° 47ʹ 05″ S, 56° 45ʹ 03″ W, on exposed limestone in Atlantic rain forest biome, 7 November 2018, Aptroot 77806 (holotype; CGMC).
GenBank number: MW322681 (ITS).
Notes: Morphologically, this species shows similarities with several genera in the Teloschistaceae. The corticated granules in the central part of the thallus resemble the blastidia known from several species of Fulgensia A. Massal. & De Not. In this sense, it was used for most of the past century. Sequencing of the type showed that this species belongs to Wetmoreana. None of the species in this genus are blastidiate. Phylogenetically, it clusters deep inside Wetmoreana (Fig. 71). Note that Caloplaca brouardii de Lesd. is phylogenetically also a Wetmoreana (Wilk et al. in prep). This species is described from the same locality as Buellia pruinocalcarea (for details see under that species).
Phylogram generated from maximum likelihood analysis based on ITS. Bootstrap support values for ML ≥ 80% and Bayesian posterior probabilities ≥ 0.95 are given near nodes respectively. The tree is rooted in Xanthoria parietina (pop4_26). Ex-type strains are in bold. The newly generated sequences are indicated in bold blue
Class Leotiomycetes O.E. Erikss. & Winka
Phacidiales C.E. Bessey
Phacidiales was placed in Leotiomycetes by Bessey (1907). Quijada et al. (2018) included three families in Phacidiale (viz. Helicogoniaceae, Phacidiaceae, Tympanidaceae) and one informal taxonomic lineage with 29 genera. Wijayawardene et al. (2022) accepted two families viz. Helicogoniaceae and Phacidiaceae in this order and accepted Tympanidaceae in Leotiales.
Phacidiaceae Fr. [as 'Phacidiacei'], Summa veg. Scand., Sectio Post. (Stockholm): 367 (1849).
Phacidiaceae was introduced by Fries (1849) and typified by Phacidium (Crous et al. 2014). Six genera (Allantophomopsiella, Allantophomopsis, Bulgaria, Darkera, Phacidium and Potebniamyces) were included in Phacidiaceae based on DNA sequence data (Crous et al. 2014; Li et al. 2020). Wijayawardene et al. (2022) accepted nine genera in this family.
Phacidium Fr., Observ. mycol. (Havniae) 1: 167 (1815).
The generic name Phacidium was introduced by Fries (1815) with Phacidium lacerum as the type species. Phacidium species are widely distributed throughout the globe and has been reported as pathogens on dead leaf tips and as saprobic on dead leaves of several host families (Crous et al. 2014; Li et al. 2020). Both asexual morph and sexual morph of this genus are known (Li et al. 2020). There are 43 taxa are listed in Species Fungorum (2022a, b) (http://www.speciesfungorum.org/Names/Names.asp). However, it is a poorly studied genus due to the lack of molecular data. Here we introduce a new species of Phacidium from decaying wood in terrestrial habitats in China.
Phacidium chinense G.C. Ren & K.D. Hyde sp. nov.
Index Fungorum number: IF559693; Facesoffungi number: FoF10836, Fig. 72
Phacidium chinense (KUN-HKAS 112899, holotype). a Herbarium. b Conidiomata on the host. c Vertical sections of conidioma. d Sections of peridium. e Conidiogenous cells and developing conidia. f–k Conidia. l Germinating conidium. m, n Culture on PDA. Scale bars: c = 100 µm, d = 50 µm, e, k = 10 µm, f–j = 5 µm, l = 20 µm
Etymology: The species epithet reflects the country where the species was collected.
Holotype: KUN-HKAS 112899
Saprobic on dead wood of Rosa sp. Sexual morph: Not observed. Asexual morph: Conidiomata 170–200 μm high, 150–240 μm diam. (\(\bar{x}\) = 180 × 200 μm, n = 5), black, pseudostromatic, solitary or gregarious, semi-immersed to superficial, multi-locular, with 3–10 locules embedded in the pseudostroma. Ostioles 60–85 × 45–70 (\(\bar{x}\) = 73.5 × 58.5, n = 5) μm, centrally located, circular. Conidiomata wall 15–35 µm thick, 3–5 layered, comprising brown cells of textura angularis, thick-walled at basal, thin-walled at side. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3.5–5.5 × 1.2–2.0 µm (\(\bar{x}\) = 4.8 × 1.5, n = 10), hyaline, enteroblastic, phialidic, discrete, cylindrical, smooth-walled, arising from stratum. Conidia 4.5–6 × 2.0–2.4 µm (\(\bar{x}\) = 5.2 × 2.1 µm, n = 30), hyaline, oblong, unicellular, thick- and smooth-walled.
Culture Characters: Colonies on PDA, reaching 80–90 mm diam., after four weeks at 20–25 °C, medium dense, circular, rough, fluffy, cotton, gray, with white papillate on the surface, reverse dark-gray.
Material examined: China, Yunnan Province, Diqing Autonomous Prefecture, Xianggelila (27.28′ 8° N, 99.50′ 45° E), 2958 m, on dead wood of Rosa sp. (Rosaceae), 30 August 2020, Guang-Cong Ren, W06 (KUN-HKAS 112899, holotype), ex-type living culture KUMCC 20-0168.
GenBank numbers: ON490924 (LSU), ON490925(ITS), ON506923(tef1), ON506922 (rpb2)
Notes: Phacidium chinense is introduced as a new species based on its distinct morphology, which is supported by phylogenetic analyses. In the phylogenetic analyses, P. chinense is distinct from extant species in Phacidium and formed a sister clade to Phacidium calderae, however, there is no bootstrap support (Fig. 73). Phacidium chinense is different to P. calderae in having oblong conidia and phialidic, cylindrical conidiogenous cells, while P. calderae in having subcylindrical conidia with apical mucoid appendage and proliferating with periclinal thickening conidiogenous cells (Crous et al. 2014).
Phylogram generated from maximum likelihood analysis based on combined LSU, ITS, tef1, and rpb2 sequence data. Forty-eight strains are included in the combined analyses which comprised 3378 characters (8200 characters for LSU, 546 characters for ITS, 879 characters for tef1, 1133 characters for rpb2) after alignment. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The evolutionary model SYM + G applied to ITS sequence data, while SYM + I + G applied to LSU, GTR + G tef1 and rpb2 gene regions. Bootstrap support values for ML ≥ 80% and BYPP ≥ 0.95 are given near nodes respectively. The tree is rooted in Phlyctema vincetoxici (CBS 123,726). Ex-type strains are in bold. The newly generated sequences are indicated in bold red
Class Sordariomycetes O.E. Erikss. & Winka
Subclass Diaporthomycetidae Senan., Maharachch. & K.D. Hyde
Diaporthales Nannf
Diaporthales containing numerous important endophytic, saprobic and phytopathogenic ascomycetous families. Even though, families, and genera in this order showed high distinct morphological diversity, taxonomic placements are still problematic (Senanayake et al. 2017).
Diaporthales introduced by Nannfeldt (1932), to accommodate Höhnel’s Eu-Diaportheen and Valseen taxa (Senanayake et al. 2018) and currently 30 families are in this order named: Apiosporopsidaceae, Apoharknessiaceae, Asterosporiaceae, Auratiopycnidiellaceae, Coryneaceae, Cryphonectriaceae, Cytosporaceae, Diaporthaceae, Diaporthosporellaceae, Diaporthostomataceae, Dwiroopaceae, Erythrogloeaceae, Gnomoniaceae, Harknessiaceae, Juglanconidaceae, Lamproconiaceae, Macrohilaceae, Melanconidaceae, Melanconiellaceae, Neomelanconiellaceae, Phaeoappendicosporaceae, Phaeochorellaceae, Prosopidicolaceae, Pseudomelanconidaceae, Pseudoplagiostomataceae, Schizoparmaceae, Stilbosporaceae, Sydowiellaceae, Synnemasporellaceae and Tubakiaceae (Hyde et al. 2020a, b, c; Wijayawardena et al. 2022).
Diaporthaceae Höhn. ex Wehm., Am. J. Bot. 13: 638 (1926)
Diaporthaceae was introduced and placed in Diaporthales by von Höhnel (1917). The members of Diaporthaceae are known to be endophytic, pathogenic and saprobic. Species in Diaporthaceae mostly inhabit in terrestrial hosts and rarely on aquatic hosts (Udayanga et al. 2011; Dissanayake et al. 2017; Senanayake et al. 2017). There are 15 genera accepted in Diaporthaceae, viz. Apioporthella, Apiosphaeria, Chaetoconis, Chiangraiomyces, Diaporthe, Hyaliappendispora, Leucodiaporthe, Massariothea, Mazzantia, Ophiodiaporthe, Paradiaporthe, Phaeocytostroma, Phaeodiaporthe, Pustulomyces, and Stenocarpella (Hyde et al. 2020a, b, c).
Diaporthe Nitschke, Fungi rhenani exsic., suppl., fasc. 5: no. 1988 (1867)
Diaporthe is the type genus of Diaporthaceae, and it was established by Nitschke (1867). Diaporthe species have been recorded as endophytes or saprobes on a wide range of host plants in different geographical areas (Udayanga et al. 2011; Dissanayake et al. 2017; Abeywickrama et al. 2020). Many economically significant crops are infected by pathogenic Diaporthe species leading to severe crop losses (Manawasinghe et al. 2019; Abeywickrama et al. 2020), with blights, fruit and root rots, cankers, diebacks, wilts and leaf spots (Manawasinghe et al. 2019; Abeywickrama et al. 2020). The genus contains 1152 epithets in Index Fungorum (assessed in 29.08.2022; Index Fungorum 2022a, b).
Diaporthe foeniculina (Sacc.) Udayanga & Castl., in Udayanga et al., Persoonia 32: 95 (2014).
Index Fungorum number: IF803929; Facesoffungi number: FoF02183; Fig. 74
Saprobic on dead aerial branch of Ficus carica. Sexual morph: See Udayanga et al. (2014). Asexual morph: coelomycetous. Conidiomata observed as small black dots on the host, semi-immersed to immersed, pycnidial, pyriform, scattered, ostiolate, 150–300 µm diam. Conidiomata wall consisting of 3–4 layers of pale brown, thick-walled cells of textura angularis. Conidiophores hyaline, smooth, unbranched. Alpha conidia hyaline, smooth-walled, bi- to multi-guttulate, ovate to ellipsoidal, base sub-truncate, 5–7.5 × 1.5–3 µm (n = 20). Beta conidia aseptate, hyaline, smooth, apex and base bluntly rounded, slightly curved, 15–25 × 0.5–2 µm (n = 10).
Culture characteristics: Colonies on PDA entirely white both on surface and reverse. Aerial mycelium cottony, colonies reaching 60 mm diam. after 7 days in room temperature.
Material examined: Italy, Province of Forlì-Cesena [FC], near Pianetto—Galeata, on dead and aerial branch of Ficus carica L. (Moraceae), 21 December 2018, E. Camporesi, IT 4192 (JZBH 320201), living culture JZB 320201.
Hosts: Wide host range, including Achillea, Ailanthus, Amorpha, Angelica, Arctium, Asparagus, Camellia, Castanea, Chenopodium, Citrus, Cupressus, Diospyros, Eucalyptus, Ficus carica Hemerocallis, Lunaria, Melilotus, Microcitrus, Persea, Platanus, Prunus, Rosa, Rubus, Vicia and Wisteria (Farr and Rossman 2022; this study).
Distribution: Wide geographical range, including in Chile, Greece, Iran, Italy, Malta, New Zealand, Portugal, Serbia, South Africa, Spain, Thailand, Turkey, Uruguay, US (Farr and Rossman 2022; this study).
GenBank numbers: OP002068 (ITS), OP837431(his), OP837429 (tub2)
Notes: Diaporthe foeniculina (JZB 320201) was recovered from a dead aerial branch of Ficus carica in Italy. Our strain shared similar morphology with the type strain of D. foeniculina (CBS 111553) which was introduced by Udayanga et al. (2014), with minor dimensional differences. Conidiomata of our strain (JZB 320201) are comparatively smaller than those of D. foeniculina (CBS 111553) (150–300 μm diam. vs 400– 700 µm diam.). Further we have observed smaller alpha conidia in our strain than CBS 111553 (5–7.5 × 1.5–3 µm vs 8.8 ± 0.3 × 2.4 ± 0.1 μm) (Udayanga et al. 2014). These morphological differences probably due to environmental factors and host variations. Phylogenetic analyses using combined ITS, cal, his, tef1, tub2 sequence data confirmed that our strain is D. foeniculina and it is clade with the strain MFLUCC 20-0151 with high statistical support (92/1.00) (Fig. 75). Comparisons of base pair differences for ITS, tub2 and his genes between our strain (JZB 320201) and the ex-epitype strain of D. foeniculina (CBS 111553) reveal less than 1% base pair differences in ITS and tub2 gene regions (ITS = 0.57%, tub2 = 0.97%). However, we observe 5.77% base pair difference in HIS gene (94.23% similarity). We were unable to obtain cal and tef1 sequence data for our strain, and we could not compare the base pair difference for them. Thus, based on the multi-gene phylogeny and morphology; this study presents the first report of D. foeniculina from a Ficus carica from Italy.
Phylogram generated from maximum likelihood analysis based on ITS, cal, his, tef1 and tub2 sequenced data of given Diaporthe species. Related sequences were obtained from GenBank, and 223 strains are included in the sequence analyses, with 2674 columns, 1972 distinct patterns 1439 parsimony-informative, 340 singleton sites, 894 constant sites. Diaporthella corylina (CBS121124) is used as the outgroup taxon. Bootstrap support values for ML ≥ 65%, BYPP ≥ 0.90 are given near the nodes. Type strains are in bold. Newly generated strains are in red bold
Diaporthe longicolla (Hobbs) J.M. Santos, Vrandečić & A.J.L. Phillips, in Santos, Vrandečić, Čosić, Duvnjak & Phillips, Persoonia 27: 13 (2011)
Index Fungorum number: IF164797; Faceoffungi number: FOF11682; Figs. 76, 77
Micromorphological features of D. longicolla (CPDl21, new host record) a. Cowpea stem affected by Diaporthe longicolla. b. Stereo view of infected region showing pycnidial structures. c–d. Stereo view showing cirri of spores erupted from pycnidia. e. SEM image of pycnidium. f. Conidia of D. longicolla observed in SEM. Scale bars: b = 10 mm; c– d = 2 mm; e – f = 10 µm
Pathogenic and associated with stem of Vigna unguiculata. Sexual morph: Not observed. Asexual morph: Conidiomata 80–130 µm high, 230–320 µm diam. \(\bar{x}\) = 118 × 290 μm, n = 20, pycnidial, pyriform, initially immersed, erumpent at maturity, globose to pyriform, black, elongated neck, often with light yellowish white conidial cirrus extruding from ostiole. Pycnidial wall parenchymatous consisting of 4–7 layers of pale brown, thick-walled cells of textura angularis, Pycnidia globose locules and prominent beaks, which immersed in medium, black, solitary, discoid or irregular. Conidiophores 4–7 × 4.1–7.3 μm (\(\bar{x}\) = 4.4 × 6.3 μm, n = 30), ampulliform, straight to sinuous, unbranched, hyaline, smooth. Conidiogenous cells 7.8–13.8 × 1.4–2.7 μm (\(\bar{x}\) = 10.9 × 2.1 μm, n = 30), phialidic, terminal, cylindrical, slightly tapering towards the apex. Alpha-conidia 5.1–7.5 × 1.2–3.4 μm (\(\bar{x}\) = 6.1 × 2.6 μm, n = 20), aseptate, hyaline, smooth, ovate to ellipsoidal, guttulate. Beta-conidia 5.8–7.5 × 2.5–3.5 μm (\(\bar{x}\) = 6.4 × 2.8 μm, n = 10), hyaline, filiform, hamate.
Cultural characteristics: On potato dextrose agar, the fungus initially produced white fluffy aerial hyphae, forming relatively dense concentric pattern colony, which subsequently exhibited light yellow pigmentation.
Material examined: India, Karnataka, Mysuru Doddamaragowdanahally, on infected stem of cowpea plants as pathogen. July, 2020, S. Mahadevakumar, Y.S. Deepika (UOM-IOE 20/25), living cultures CPDl21, CPDl22, MKSVu012.
Hosts: Wide host range, including Abutilon, Acer, Actinidia, Ambrosia, Arachis, Chamaesyce, Cucumis, Euphorbia, Glycine, Helianthus, Ipomoea, Kalanchoe, Phaseolus, Pisum, Pyrus, Rumex, Solanum, Trichilia, Vigna and Xanthium (Farr and Rossman 2022; this study).
Distribution: Wide geographical range, including in Argentina, Australia, Brazil, China, Croatia, Greece, India, Italy, Malaysia, Missouri, South Korea, US (Farr and Rossman 2022; this study).
GenBank Numbers: CPDl21—MW737797 (ITS), OM934823 (tub2), OM934820 (tef1)
CPDl22–MW737798 (ITS), OM934824 (tub2), OM934821(tef1)
MKSVu012– KT819767 (ITS), OM934825(tub2), OM934822 (tef1)
Notes: The symptoms were observed on stems of cowpea. Initial symptoms appeared as small lesions, more or less circular, later elongated, blackish-brown lesions, eventually pycnidia developed (Fig. 76). Stem girdling occurs and the shoot above the infected area wilts and dries up. Pathogenicity tests were conducted and proved to be pathogenic on healthy cowpea plants. Morphologically our strain shares similar morphology with the ex-type strain of D. longicolla (Fig. 77). In the multigene phylogenetic analyses, our strain clusters with D. longicolla with a high bootstrap support (Fig. 75). Previously, Phomopsis longicolla is known to be associated with cowpea seeds. However, no reports are available on the association of D. longicolla of cowpea in India. This is the first report of D. longicolla associated with cowpea from India.
Diaporthe phaseolorum (Cooke & Ellis) Sacc., Syll. Fung. (Abellini) 1: 692 (1882).
Index Fungorum number: IF164797; Faceoffungi number: FoF10638; Figs: 78, 79.
Diagnostic features of cowpea stem blight and pod blight disease caused by Diaporthe phaseolorum and D. longicolla: a–b. field view of cowpea plants affected with Diaporthe stem blight and pod blight disease. c, d, g. Stem blight disease caused by D. longicolla. e,f,h,i. pod blight disease symptoms caused by D. phaseolorum
Pathogenic and associated with stem of Vigna unguiculata. Sexual morph: Not observed. Asexual morph: Conidiomata 105–192 µm high, 165–285 µm diam. \(\bar{x}\) = 122 × 255 μm, n = 30, pycnidial, pyriform, initially immersed, erumpent at maturity, globose to pyriform, black, elongated neck, often with light yellowish white conidial cirrus extruding from ostiole. Pycnidial wall parenchymatous consisting of 3–6 layers of pale brown, thick-walled cells of textura angularis. Pycnidia globose locules and prominent beaks, which immersed in medium, black, solitary, discoid or irregular. Conidiophores 3.8–7.5 × 3.8–7.5 μm (\(\bar{x}\) = 4.2 × 5.8 μm, n = 30), ampulliform, straight to sinuous, unbranched, hyaline, smooth. Conidiogenous cells 8.2–12.8 × 1.6–2.5 μm (\(\bar{x}\) = 9.8 × 2.2 μm, n = 30), phialidic, terminal, cylindrical, slightly tapering towards the apex. Alpha-conidia 5.3–7.7 × 1.5–4.6 μm (\(\bar{x}\) = 6.5 × 2.8 μm, n = 30), aseptate, hyaline, smooth, ovate to ellipsoidal, guttulate. Beta-conidia 10.2–17.5 × 1.2–2.3 μm (\(\bar{x}\) = 12.6 × 1.2 μm, n = 30), hyaline, filiform, hamate.
Culture characteristics: On PDA, colonies with white, floccose, aerial mycelium were recorded after 7 days of incubation. Pure cultures obtained from the colonies expressed from infected pod and stem samples.
Material examined: India, Karnataka, Mysuru Doddamaragowdanahally, on infected stem of cowpea plants as pathogen, July, 2020, S. Mahadevakumar, Y.S. Deepika, N. Lakshmidevi (UOM-IOE 20/26), living cultures CPDp1, CPDp2.
Hosts: Wide host range, including Acer, Actinidia, Aeschynomene, Arctium, Aspalathus, Aster, Calopogonium, Cannabis, Caperonia, Capsicum, Centrosema, Clitoria, Cyphomandra, Desmanthus, Desmodium, Eriobotrya, Euphorbia, Glycine, Helianthus, Hylocereus, Ipomoea, Jatropha, Lablab, Lupinus, Lycopersicon, Macroptilium, Macrotyloma, Maytenus, Ocimum, Olearia, Panicum, Phalaris, Phaseolus, Pyrus, Stokesia, Vigna, Vitis and Zea (Farr and Rossman 2022; this study).
Distribution: Wide geographical range, including in Australia, Barbados, Brazil, Brunei Darussalam, Cameroon, Canada, China, Colombia, Cook Islands, Croatia, Cuba, Dominican Republic, Fiji, India, Italy, Jamaica, Korea, Maryland, Mauritius, Missouri, New Zealand, Papua New Guinea, Spain, South Africa, Switzerland, Thailand, Tonga, Trinidad and Tobago, United Kingdom, United States, Uruguay, Venezuela and West Indies (Farr and Rossman 2022; this study).
GenBank Numbers: CPDp1– MW737799 (ITS), OM934818 (tub2), OM934816 (tef1)
CPDp2– MW737800 (ITS), OM934819 (tub2), OM934817 (tef1)
Notes: Morphologically our strain is similar to the ex-type strain of D. phaseolorum (Fig. 78). In the multigene phylogenetic analyses, our strain clustered with the ex-type strain of D. phaseolorum (Fig. 75). Pathogenicity tests were conducted and proved to be pathogenic on healthy cowpea plants. Diaporthe longicolla causes stem blight of cowpea while D. phaseolorum causes pod blight of the same host (Fig. 79). This is the first report of D. phaseolorum associated with cowpea from India and worldwide.
Melanconiellaceae Senan., Maharachch. & K.D. Hyde, in Senanayake et al., Stud. Mycol. 86: 275 (2017).
Melanconiellaceae was invalidly introduced (Locquin 1984) for Melanconiella and it was validated by Senanayake et al. (2017). The type species of Melanconiella is M. spodiaea (Tul. & C. Tul.) Sacc. (Fan et al. 2018). Fan et al. (2018) accepted Melanconiella, Microascospora and Sheathospora and Senanayake et al. (2018) accepted Greeneria, Melanconiella and Microascospora in the family. Hyde et al (2020c) accepted five genera; Greeneria, Melanconiella, Microascospora, Septomelanconiella and Sheathospora in the family.
Melanconiella Sacc., Syll. fung. (Abellini) 1: 740 (1882)
Melanconiella was established by Saccardo (1882) for the type species M. spodiaea Tul. & C. Tul. and second species M. chrysostroma (Fr.) Tul. & C. Tul. Melanconiella species are found in overwintered plants as saprobes or as mild canker causing agents (Voglmayr et al. 2012; Senanayake et al. 2018). There are 40 species epithets recorded in Index Fungorum (January 2022).
Melanconiella meridionalisVoglmayr & Jaklitsch, in Voglmayr et al., Fungal Diversity 57(1):33 (2012).
Index Fungorum number: IF800123; Facesoffungi number: FoF10701, Fig. 80
Melanconiella meridionalis (MFLU 15–2604, new host and geographical record) a, b Appearance of pseudostromata on dead branch of Fagus sylvatica c Pseudostroma in transverse section d, e Pseudostroma in vertical sections f Peridium. g Paraphyses.h–j Asci. k–n Ascospores. Scale bars: d = 50 μm, e = 80 μm, f = 10 μm, h–j = 25 μm, k–n = 10 μm
Saprobic on dead twigs of Fagus sylvatica. Sexual morph: Pseudostromata indistinct, less commonly distinct and circularoutline, causing minute bumps in the bark. Ectostromatic disc flat, 2–2.3 mm long, well-defined, with circular or elliptic outline, cream, light or pale yellow. Central column dark brown. Entostroma yellowish hyphae. Ostiole central. Perithecia 200–600 μm, subglobose, immersed, coriaceous, brown to black, Perithecia wall 8–10 µm wide, comprising brown cells of textura angularis of inner layer and 15–18 µm wide, unequally thick, comprising irregular dark brown cells of textura prismatica of outer layer. Hamathecium comprising 1–1.5 µm septate, unbranched, cellular pseudoparaphyses. Asci (85–)95–100(–115) × (8–)10–12(–15) μm (\(\bar{x}\) = 97 × 11 µm, n = 20), broadly cylindrical to fusoid, 8-spored, distinct apical ring with short pedicel or sessile. Ascospores uni- or irregularly biseriate, hyaline, fusoid, constricted at the septum, (20–)23–25(–28) × (4–) 5–6(–7) μm, (\(\bar{x}\) = 24 × 5.2 µm, n = 30); ends rounded, upper cell mostly larger and some guttules. Asexual morph: Not observed.
Material examined: Italy, Province of Forlì-Cesena, Santa Sofia, on twigs of Fagus sylvatica L. (Fagaceae), 3 August 2015, E. Camporesi, IT2575 (MFLU 15-2604).
Host and distribution: Ostrya carpinifolia in China (Voglmayr et al. 2012; Fan et al. 2018), Europe and North America (Voglmayr et al. 2012), Fagus sylvatica in Italy (this study).
GenBank numbers: OM403250 (ITS), OM403249 (LSU)
Notes: The new strain shares a close phylogenetic affinity to Melanconiella meridionalis in our combined LSU, ITS, rpb2 and tef1 sequence data analyses (Fig. 81). This species was previously recorded from dead corticated twigs and branches of Ostrya carpinifolia (Betulaceae) from different localities i.e. Australia, Croatia, Greece, Italy and Slovenia (Voglmayr et al. 2012). Melanconiella meridionalis has not been reported from Fagaceae and here we provide the first association of sexual morph of species with Fagus sylvatica.
Phylogram generated from the maximum likelihood analysis based on combined LSU, ITS, rpb2 and tef1 sequence data representing family Melanconiellaceae. Related sequences are taken from Fan et al. (2018) and Senanayake et al. (2018). Melanconis stilbostoma (CFCC 50,480) and M. stilbostoma (MS) are used as the outgroup taxa. Twenty-eight strains are included in the combined gene analyses comprising 3380 characters after alignment (880 characters for LSU, 500 characters for ML ≥ 75% and BYPP ≥ 0.95 are given above the nodes. Ex-type strains are in bold and new strain is indicated in blue
Pararamichloridiales Crous
Notes: Pararamichloridiales was introduced by Crous et al. (2017) to accommodate a monotypic family including two genera, namely Pararamichloridium and Woswasia. However, Woswasia (Woswasiaceae) was treated in Diaporthomycetidae families incertae sedis by Zhang et al. (2017) based on morphological and phylogenetical analyses. This result was supported by Hyde et al. (2020a, b, c). Divergence time estimates for Pararamichloridiales are crown age of 50.08 Mya and stem age of 101.46 Mya (Hyde et al. 2020a, b, c). The data of divergence time estimates for Pararamichloridiales is line with recommendations for ranking families.
Pararamichloridiaceae Crous, in Crous et al., Persoonia 39: 357 (2017)
Notes: Pararamichloridiaceae was introduced as a monotypic family by Crous et al. (2017) for Pararamichloridium Crous. However, Zhang et al. (2017) established a new family Woswasiaceae to accommodate Cyanoannulus and Xylomelasma while Woswasia was placed in Diaporthomycetidae families incertae sedis based on its close phylogenetic affinity, and this was conferred in later studies (Hyde et al. 2020a, b, c; Wijayawardene et al. 2020). Members of Pararamichloridiaceae are pathogenic on plant leaves (Crous et al. 2017, 2018).
Pararamichloridium Crous, in Crous et al., Persoonia 39: 357 (2017)
Notes: Pararamichloridium, the type genus of Pararamichloridiaceae, was established by Crous et al. (2017) to accommodate two species, P. livistonae Crous (as the type species) and P. verrucosum (V. Rao & de Hoog) Crous. Crous et al. (2018) introduced the third species of Pararamichloridium based on blast search and phylogenetic analysis. In this study, morphological characteristics and multi-gene phylogenetic analysis of a combined LSU and ITS sequence data reveals the fourth new species of Pararamichloridium from dead wood collected in freshwater from China (Figs. 82, 83).
Phylogram generated from maximum likelihood analysis based on combined LSU and ITS sequence data. Twenty–two taxa were included in the combined analyses, which comprised 1524 characters (LSU: 885, ITS: 639) after alignment. Bootstrap support values for ML ≥ 50% and BYPP ≥ 0.95 are given above the nodes. The tree is rooted with Diaporthe passiforae (CBS 132,527) and D. obtusifoliae (CPC 32,226). The ex–type strains are indicated in bold. The newly generated sequence is indicated in blue
Pararamichloridium aquisubtropicum J.Y. Zhang, Y.Z. Lu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559507; Facesoffungi number: FoF10677; Fig. 82
Etymology: Referring to the aquatic habitat and collecting site in subtropical country, China
Holotype: GZAAS 21–0382
Saprobic on submerged decaying wood. Asexual morph: Colonies on natural substrate superficial, brown, smooth, solitary. Mycelium partly immersed, consisting of branched, smooth, brown hyphae. Conidiophores 119–202 × 2.6–4.7 μm (\(\bar{x}\) = 161.4 × 3.6 μm, n = 24), macronematous, mononematous, sucylindrical, straight, unbranched, brown, smooth, 6–9-septate. Conidiogenous cells 27.3–37 × 2.6–3.8 μm (\(\bar{x}\) = 27.6 × 3.3 μm, n = 15), holoblastic, polyblastic, integrated, terminal, subhyaline to pale brown, subcylindrical. Conidia 4–8 × 3–4.7 μm (\(\bar{x}\) = 5.2 × 3.7 μm, n = 25), acrogenous, solitary, aseptate, pale brown, globose to subglobose. Sexual morph: Not observed.
Culture Characters: Colonies on PDA reaching 33 mm in 44 days at 25 °C, flat, curler, near–round or round, gray, smooth, middle erumpent; In reverse, yellow at the center, lightly brown or gray at the margin.
Material examined: China, Guizhou Province, Xishui County, on decaying wood submerged in a freshwater stream, 16 September 2020, Jian Ma, XY2(GZAAS 21–0382, holotype); ex–type living culture, GZCC 21–0668.
GenBank numbers: OM339437 (ITS), OM339434 (LSU).
Notes: The phylogenetic analysis (Fig. 83) revealed that Pararamichloridium aquisubtropicum forms distinct lineage belonging to Pararamichloridium, where it is sister to P. caricicola with 100% BS, 1.00 BYPP, high support. Pararamichloridium caricicola is the closest species based on BLASTn result of LSU and ITS region with 99.55% and 96.11% similarity, respectively. Pararamichloridium caricicola is found in culture, while our new collection was saprobic on submerged decaying wood. Pararamichloridium aquisubtropicum shares the same morphology with P. caricicola in having brown, septate, smooth conidiophores integrated, terminal, subcylindrical conidiogenous cells and ellipsoid, pale brown aseptate, conidia. However, P. aquisubtropicum differs from P. caricicola by it darker and longer (119–202 × 2.5–4.7 μm vs 35–100 × 2.5–3 μm) conidiophores. Thus, P. aquisubtropicum is introduced here as a distinct novel species based on its distinct morphological features and phylogenetic placement.
Distoseptisporales Z.L. Luo, K.D. Hyde & H.Y. Su Fungal Diversity 99: 482 (2019).
Distoseptisporaceae was established by Su et al. (2016) with a single genus, Distoseptispora. Based on morphology and phylogenetic analysis of combined LSU, SSU, rpb2, and tef1 sequence data, Luo et al. (2019) established a new order, Distoseptisporales. Previously, the order Distoseptisporales was classified under the class Sordariomycetes, subclass Diaporthomycetidae. Aquapteridospora was described by Yang et al. (2015) and assigned to the Diaporthomycetidae genus incertae sedis. Later, Dong et al. (2021) performed a molecular phylogeny study using combined LSU, ITS, tef1, and rpb2 sequence data and established a new family, Aquapteridosporaceae K.D. Hyde & Hongsanan, to accommodate a single genus, Aquapteridospora, and placed Aquapteridosporaceae in the order Distoseptisporales. Distoseptisporales currently consists of two families (Aquapteridosporaceae and Distoseptisporaceae), with Distoseptisporaceae serving as the type family.
Distoseptisporaceae K.D. Hyde & McKenzie, Fungal Diversity 80: 402 (2016)
Su et al. (2016) identified two Sporidesmium-like taxa with distinct morphology and phylogenetic relationships. They can be distinguished from Sporidesmiaceae based on strong molecular evidence and morphological investigation. As a result, the family Distoseptisporaceae was established to include Sporidesmium-like species under the type genus Distoseptispora. In the previous study, there was no sexual morph known for this family (Su et al. 2016; Yang et al. 2018; Hyde et al. 2019, 2020a, b, c, 2021; Luo et al. 2019; Sun et al. 2020a, b). Recently, Yang et al. (2021) proposed a sexual morph of Distoseptispora and this is the first time that the sexual morph of Distoseptispora has been found.
Distoseptispora K.D. Hyde, McKenzie, Maharachch. Fungal Diversity 80:375–409 (2016)
Su et al. (2016) defined Distoseptispora as a genus with Distoseptispora fluminicola as the type species. Yang et al. (2018) updated the Distoseptispora genus description. In addition, the sexual morph of this species is unidentified in the previous study (Su et al. 2016; Yang et al. 2018; Hyde et al. 2019, 2020a, b, c; Luo et al. 2019; Sun et al. 2020a, b). There is only one species of sexual morph described in Distoseptispora (Yang et al. 2021). The sexual morph is characterized by being solitary or gregarious, immersed to semi-immersed, perithecial, subglobose to ellipsoidal, ostiolate, dark brown ascomata with a short neck and hyaline, 0–3-septate, ascospores with a mucilaginous sheath (Yang et al. 2021). The asexual morph is distinguished by hyphomycetous, macronematous conidiophores, percurrent, elongate conidiogenous cells, olivaceous, brown, yellowish, or reddish brown, euseptate or distoseptate conidia, and rarely muriform conidia. (Su et al. 2016; Xia et al. 2017; Luo et al. 2018a, b; Tibpromma et al. 2018; Yang et al. 2018; Hyde et al. 2020a, b, c). The genus now has 46 recognized species, 14 of which are from terrestrial habitats and 32 from freshwater habitats (Su et al. 2016; Hyde et al. 2016, 2019, 2020, 2021; Xia et al. 2017; Yang et al. 2015, 2018; Luo et al. 2018a, b, 2019; Monkai et al. 2020; Song et al. 2020; Sun et al. 2020a, b; Li et al. 2021a, b; Yang et al. 2021, Hyde et al. 2021, Index Fungorum 2022a, b). Distoseptispora hyalina J. Yang and K.D. Hyde is the first sexual morph reported in the genus based on molecular DNA data (Yang et al. 2021).
Distoseptispora bambusicola X. Tang, Jayaward, J.C Kang & K.D. Hyde sp. nov.
Index Fungorum number: IF558533; Facesoffungi number: FoF 09940; Fig. 84
Etymology: Named after the host bamboo from which the holotype was found.
Holotypus: GZAAS21-0379.
Saprobic on decaying stems of bamboo submerged in a freshwater stream habitat. Sexual morph: Not observed. Asexual morph: Colonies effuse, scattered, hairy, brown to dark brown. Mycelium mostly immersed, composed of branched, septate, brown, smooth hyphae. Conidiophores macronematous, mononematous, pale brown to brown, solitary, 4–7-septate, erect, straight or flexuous, unbranched, slightly constricted at septa, smooth, cylindrical, 64–116 μm × 4–7 μm (\(\bar{x}\) = 90.5 × 5.5 μm, n = 30), truncate at the apex. Conidiogenous cells holoblastic, monoblastic, integrated, terminal, determinate, brown, cylindrical. Conidia 72–193 μm × 7.5–14.5 μm (\(\bar{x}\) = 126 × 14 μm, n = 30) acrogenous, solitary, obclavate or lanceolate, rostrate, straight or slightly curved, multi-distoseptate, up to 16-distoseptate, guttulate, pale brown, tapering towards the rounded apex, truncate at the base, slightly constricted at septa, smooth-walled, rounded at apex, with a truncate base and faintly to heavily pigmented scar.
Culture characters: Colony grown on PDA in room temperature, circular, fluffy, white, dense in the center, but sparse, slightly olivaceous in the outside. In reverse, deeply olivaceous in the center, slightly yellow at the entire margin. Refer to the pigment produced on PDA and OA, purpure.
Material examined: China, Guizhou Province, Zunyi City, on decaying stems of bamboo submerged in a freshwater stream, 21 February 2021, Xia Tang, K1 (GZAAS21-0379, holotype), ex-type living culture, GZCC21-0667.
GenBank numbers: MZ474873 (ITS), MZ474872 (LSU), MZ474866 (SSU), OM272845 (tef1).
Notes: Distoseptispora bambusicola clustered with D. hydei, D. obpyriformis, and D. rostrata with a 100%ML, 99%MP and 1.00 BYPP support. According to the morphological comparisons (Fig. 85), our novel species can be distinguished from closely related species. Distoseptispora bambusicola differs from D. rostrata by its smaller conidiophores (64–116 μm × 4–7 μm vs 82–126 μm × 5–7 μm), larger conidia (72–193 μm × 7.5–14.5 μm vs 115–155 μm × 9–11 μm) and less distosepta (16-distosepta vs 23-distosepta). Distoseptispora bambusicola shares similar morphological characteristics with D. obpyriformis in the size, shape, color of conidiophores, but differ in conidial size (72–193 μm × 7.5–14.5 μm vs 53–71 μm × 12–16 μm). Distoseptispora bambusicola differs from D. obpyriformis by having obclavate or lanceolate, longer conidia. Distoseptispora bambusicola can be distinct from Distoseptispora hydei by conidal shape and size (72–193 μm × 7.5–14.5 μm vs 32–58 μm × 10–15 μm; obclavate or lanceolate vs obpyriform to fusiform). Distoseptispora hydei is characterized with a gelatinous sheath around the tip of conidia, while D. bambusicola lacks this character. According to the comparisons of our novel species with other phylogenetically related taxa based on a pairwise nucleotide comparison of ITS (Jeewon and Hyde 2016), D. bambusicola differs from D. rostrata in 13/ 517 bp (2.5%), D. hydei in 8/ 395 bp (2.0%) and differs from D. obpyriformis in 25/571 bp (4.0%). Pairwise nucleotide comparison of LSU and tef1 also showed that D. bambusicola differs from D. rostrata in 10/ 826 bp (1.2%) for LSU and 42/ 857 bp (4.9%) for tef1, differs from D. hydei in 7/ 848 bp (0.8%) for LSU, and differs from D. obpyriformis in 6/ 790 bp (0.7%) for LSU and 40/ 838 bp (4.7%) for tef1. Thus, we consider D. bambusicola as a novel species in Distoseptispora.
Phylogram generated from parsimony analysis based on combined ITS, LSU, SSU, rpb2 and tef1 sequence data of Distoseptispora. The ML and MP bootstrap support values ≥ 70% are and branches with BYPP ≥ 0.95 are given above the nodes. The ex-types (reference strains) are in bold; the new isolates are in blue bold. The tree is rooted with Aquapteridospora fusiformis (MFLU 18–1601), Aquapteridospora lignicola (MFLUCC 15–0377) and Aquapteridospora aquatica MFLUCC 17–2371)
Glomerellales Chadef. ex Réblová et al.
Réblová et al. (2011) validated Glomerellales to accommodate the families Australiascaceae, Glomerellaceae and Reticulascaceae. Maharachchikumbura et al. (2016) and Tibpromma et al. (2018) added Plectosphaerellaceae and Malaysiascaceae to this order.
Glomerellaceae Locq. ex Seifert & W. Gams, in Zhang et al., Mycologia 98(6): 1083 (2007) [2006].
The monotypic family is characterised by a Colletotrichum asexual morph and a Glomerella sexual morph (Hyde et al. 2020a, b, c).
Colletotrichum Corda, in Sturm, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 3(12): 41 (1831).
Colletotrichum comprises important plant pathogens, endophytes and saprobes as well as human and animal pathogens (Cannon et al. 2012; Jayawardena et al. 2021a; Talhinhas and Baroncelli 2021). Bhunjun et al. (2021) demonstrated that coalescent approaches and multi-locus phylogeny are vital in establishing species boundaries in Colletotrichum. Liu et al. (2022) accepted 280 species in 16 species complexes and 15 singleton species and established a genome tree comprising 94 species. Further 13 species were added by Alizadeh et al. (2022), Hassan et al. (2022) and Zheng et al. (2022).
Colletotrichum aeschynomenes B.S. Weir & P.R. Johnst., in Weir, Johnston & Damm, Stud. Mycol. 73: 135 (2012).
Index Fungorum number: IF563590; Facesofungi number: FoF 11441; Fig. 86
Colletotrichum aeschynomenes (MFLU 22–0148, new host record) a Shorea siamensis with leaf spots, which the fungus was isolated from b Front colony on MEA c Reverse colony on MEA d Orange coloured spore masses on MEA e–j Conidiogenesis k Conidia fused by conidial anastomosis tubes l–o Conidia. Scale bar. d = 1000 μm, e–o = 10 μm
Associated with leaf spots of Shorea siamensis. Sexual morph: Not observed. Asexual morph: Vegetative hyphae 1.5–4.5 µm diam. (\(\bar{x}\) = 3.4, n = 15), hyaline to pale brown, smooth-walled, septate, branched. Pycnidia forming on MEA, 500–1000 μm diam. solitary or aggregated, globose to irregular, releasing conidia in milk-orange, slimy, glistening masses. Setae not observed. Conidiophores 8.0–53.0 × 1.5–4.5 μm (\(\bar{x}\) = 25.1 × 2.8 μm, n = 20), hyaline to light brown, cylindrical to clavate, smooth-walled, septate, sometimes branched or reduced to conidiogenous cells. Conidiogenous cells 1.5–16 × 1.5–4 μm (\(\bar{x}\) = 6.1 × 3.0 μm, n = 15), hyaline to pale brown, solitary or aggregated, cylindrical, ovoid or ampulliform, smooth-walled. Conidia 17.0–24.5 × 5–7.5 μm (\(\bar{x}\) = 20.2 × 6.0 μm, n = 60), aseptate, hyaline, cylindrical, clavate or ellipsoidal, with slightly curved basal end and mostly rounded apices, smooth-walled or slightly verruculose, guttulate, forming conidial anastomosis tubes. Conidial anastomosis tubes 2–10.5 × 2–3 μm (\(\bar{x}\) = 4.6 × 2.4 μm, n = 15), hyaline to brown, smooth-walled, aseptate. Appressoria not observed.
Culture characteristics: Colonies on MEA flat or effuse with entire margin. Greyish white, reverse olivatious, grey towards the edge, reaching approximately 70 mm diam. in 5 days at 25 °C, with 20-days for sporulation. Aerial mycelium dense.
Material examined: Thailand, Chiang Mai Province, Omkoi, on leaf spots of Shorea siamensis (Dipterocarpaceae), 15 October 2019, D. Gomdola, MFLU22-0148; living culture MFLUCC 22-0086.
Known host and distribution: Aeschynomene virginica (USA), Manihot esculenta (Thailand), Myrciaria dubia (Brazil), Platostoma palustre (Taiwan), Shorea siamensis (Thailand, this study), Theobroma cacao (Brazil) (Farr and Rossman 2022).
GenBank numbers: OP278978 (ITS), OQ053325 (gapdh)
Notes: Weir et al. (2012) introduced C. aeschynomenes from a stem lesion of Aeschynomene virginica. Colletotrichum aeschynomenes is a common phytopathogen known to cause anthracnose in leaves. Listed chronologically, the pathogen has been found to infect cassava in central Thailand (Sangpueak et al. 2018), and cause anthracnose on Theobroma cacao in Brazil (Nascimento et al. 2019), Myrciaria dubia in Brazil (Matos et al. 2020) and Ixora coccinea in China (Li et al. 2021a, b). Herein, C. aeschynomenes is associated with leaf spots of Shorea siamensis. To delineate C. aeschynomenes from C. fructicola, tub2 or gapdh gene regions are required (Weir et al. 2012). Phylogenetic analyses based on the concatenated ITS and gapdh sequence data depict our isolate as C. aeschynomenes. The latter is located in the C. gloeosporioides complex and clusters with the ex-type strain (ICMP 17673) with 71% MP, 84% ML and 0.91 BYPP (Fig. 87). We report our collection as a new host of C. aeschynomenes on living leaves of Shorea siamensis.
One of two most parsimonious trees obtained with PAUP v. 4.0b10 (Swofford 2003) from a heuristic search of the combined sequence alignment of ITS, gapdh, chs-1, act and tub2 of the Colletotrichum gleosporioides species complex, rooted with C. truncatum (CBS 151.35). Bootstrap support values of MP and ML ≥ 70% and BYPP values ≥ 0.90 are shown at the nodes. Ex-type and reference strains are in bold. New sequence data are in red
Colletotrichum flexuosum Damm, sp. nov.
Index Fungorum number: IF558527; Facesoffungi number: FoF 10680; Fig. 88
Colletotrichum flexuosum (CBS 134419, ex-type living culture) a–b. Conidiomata. c, h. Tips of setae. d, i. Bases of setae. e–g, j–k. Conidiophores. m–r. Appressoria. s–t. Conidia. a, c–g, s. from Anthriscus stem. b, h–r, t. from SNA. a–b. Dissecting microscope (DM). c–t. Differential interference contrast illumination (DIC). Scale bars: a = 100 µm, e = 10 µm. Scale bar of a applies to a–b. Scale bar of e applies to c–t
Etymology: The species epithet is derived from the shape of the setae that are often flexuous.
Holotype: CBS H-21899
Associated with leaf spots of Xanthophyllum sylvestre. Asexual morph on synthetic nutrient-poor agar medium (SNA): Vegetative hyphae 1.5–8 µm diam., hyaline to pale brown, smooth-walled, septate, branched. Chlamydospores not observed. Conidiomata absent, conidiophores and setae formed directly on hyphae. Setae medium brown, smooth-walled, verrucous (warts 1–2 mm diam.) close to the tip, 80–140 µm long, 3–4-septate, base inflated, 6–10 µm diam., tip ± acute to round, often strongly bent. Conidiophores pale brown, smooth-walled, simple or septate, to 20 µm long. Conidiogenous cells pale brown, smooth-walled, cylindrical to subglobose, 9–20(–26) × 5–9 µm, opening 2–2.5 µm diam., collarette 1 µm long, periclinal thickening conspicuous. Conidia hyaline, smooth-walled, aseptate (few septate conidia observed), straight, cylindrical, with both ends rounded, (14.5–)16.5–24(–21) × (6.5–)7–8.5(–9.5) µm, mean ± SD = 18.9 ± 2.3 × 7.8 ± 0.8 µm, L/W ratio = 2.4 (n = 30). Appressoria single, pale brown, smooth-walled, with a navicular to clavate outline and a lobate to crenate margin, (9–)12.5–18(–24) × (4–)5.5–8.5(–10) µm, mean ± SD = 15.1 ± 2.8 × 7.1 ± 1.4 µm, L/W ratio = 2.1 (n = 30). Sexual morph: not observed.
Asexual morph on Anthriscus stem: Conidiomata, conidiophores and setae formed on medium brown, smooth-walled, roundish to angular cells, 5–11 µm diam. Setae medium to dark brown, smooth-walled, 100–180 µm long, 3–4-septate, straight to ± flexuous, base cylindrical or restricted, 4–8 µm diam., tip ± round to acute. Conidiophores pale to medium brown, smooth-walled, septate, branched, to 90 µm long. Conidiogenous cells pale to medium brown, smooth-walled, cylindrical to clavate, 15–32 × 5–8 µm, opening 1.5–2 µm diam., collarette 0.5–1 µm long, periclinal thickening distinct. Conidia hyaline, smooth-walled, aseptate, cylindrical, with both ends rounded, sometimes a distinct membranous appendage remains at the base, (21–)23.5–28.5(–33.5) × (5–)6–7.5(–8) µm, mean ± SD = 25.9 ± 2.6 × 6.9 ± 0.8 µm, L/W ratio = 3.8 (n = 30). Sexual morph: not observed.
Culture characteristics: Colonies on SNA flat with entire margin, hyaline to honey, agar medium, filter paper and Anthriscus stem partly covered with grey acervuli and short floccose white aerial mycelium, reverse hyaline to honey, agar medium, filter paper and Anthriscus stem partly pale grey; growth 13.5–18 mm in 7 days at 20 °C under near UV light with 12 h photoperiod (21–28 mm in 10 days). Colonies on OA flat with entire margin; buff, grey olivaceous to olivaceous grey, partly covered with short floccose white aerial mycelium and dark grey to black spots, reverse olivaceous-grey, growth 20–21.5 mm in 7 days (30.5–32 mm in 10 days). Conidia in mass whitish.
Material examined: Vietnam, Ninh Bình Province, Cúc Phương National Park, rain forest, from leaf spots of Xanthophyllum sylvestre (Polygalaceae), 6 December 2012, Ulrike Damm (CBS H-21899, holotype); ex-holotype living culture, CBS 134419. Ninh Bình Province, Cúc Phương National Park, rain forest, from leaf spots of Xanthophyllum sylvestre (Polygalaceae), 6 December 2012, Ulrike Damm, living culture CBS 137338.
GenBank numbers: CBS 134419—MZ444580 (ITS), MZ444582 (tub2), MZ444584 (gapdh), MZ444586 (act), MZ444588 (chs-1), MZ444590 (his3).
CBS 137338—MZ444581 (ITS), MZ444583 (tub2), MZ444585 (gapdh), MZ444587 (act), MZ444589 (chs-1), MZ444591 (his3).
Notes: Colletotrichum flexuosum was isolated from leaves of Xanthophyllum sylvestre, a tree species native to Laos, Thailand and Vietnam (http://www.plantsoftheworldonline.org). Few fungi were previously reported from Xanthophyllum, including an unidentified Colletotrichum species on X. octandrum in Australia (Simmonds 1966; Farr and Rossman 2022). No Colletotrichum species was previously described or reported from X. sylvestre.
Based on blastn searches and sequence comparisons on NCBI GenBank, the closest neighbour of the strains from Xanthophyllum is C. vietnamense, belonging to the C. gigasporum complex. The ITS, tub2, gapdh, act, chs-1 and his3 sequences of this species were 80, 97, 91, 98, 98 and 96% identical (105, 23, 9, 4, 4and 15 nucleotides difference) with those of the ex-holotype strain of C. vietnamense (Liu et al. 2014). No strain was more than 82% identical with its ITS sequences. The closest match with the act sequences was Colletotrichum sp. gnqczg15 (KC293585, F. Huang, unpubl. study) with two nucleotides difference. In a phylogeny inferred from concatenated ITS, tub2, gapdh, act, chs-1 and his3 sequences of the C. gigasporum species complex (Fig. 89), the two strains formed a well-supported (BS 100%, BYPP 1) sister clade to C. vietnamense and a clade formed by two recently described species, C. subvariabile and C. variabile (Liu et al. 2022). Colletotrichum flexuosum can be identified with all loci included.
One of two most parsimonious trees obtained with PAUP v. 4.0b10 (Swofford 2003) from a heuristic search of the combined sequence alignment (gene boundaries of ITS: 1–550, tub2: 551–1240, gapdh: 1241–1510, act: 1511–1756, chs-1: 1757–2007, his3: 2008–2385) of the Colletotrichum gigasporum species complex, rooted with C. gloeosporioides CBS 112999 (sequences from Damm et al. 2012; Rakotoniriana et al. 2013; Liu et al. 2014; Silva et al. 2018; Zhou et al. 2019; Liu et al. 2022). Bootstrap support values (BS) above 70% (bold) and Bayesian posterior probability (BYPP) values above 0.90 are shown at the nodes. Bootstrap support values have been calculated based on 10 000 replicates, and a Markov Chain Monte Carlo algorithm was used to generate phylogenetic trees with Bayesian probabilities using MrBayes v. 3.2.6 (Ronquist et al. 2012). Numbers of ex-type strains are in bold
The species from Xanthophyllum is morphologically different from all previously described species of the C. gigasporum complex. Its conidia are shorter than those of C. gigasporum (22–32 × 6–9 μm on PDA), C. magnisporum (av. 34.3 × 9.7 μm on SNA, av. 33.8 × 9.9 μm on Anthriscus stem), C. serranegrense (24–37 × 7–9 μm on MEA) and C. vietnamense (av. 31.2 × 9.6 μm on SNA, av. 32.3 × 9.5 μm on Anthriscus stem) and larger than those of C. jishouense (av. 10.8 × 3.7 μm on PDA). In contrast to C. arxii, C. jishouense, C. pseudomajus, C. radicis and C. vietnamense, neither curved nor clavate conidia were formed, while conidia with membranous appendages and distinctly flexuous setae as formed by C. flexuosum were previously not observed in the C. gigasporum complex (Rakotoniriana et al. 2013; Liu et al. 2014, 2022; Silva et al. 2018; Zhou et al. 2019). Conidiogenous cells with a distinct periclinal thickening are typical for species of the C. boninense complex; within the C. gigasporum complex this was previously only observed with C. zhaoqingense (Liu et al. 2022). However, in contrast to C. zhaoqingense, conidial bases of C. flexuosum sometimes end with a membranous appendage, while most species of the C. boninense and the C. dracaenophilum complexes develop a prominent scar (Damm et al. 2012, 2019; Liu et al. 2022).
Colletotrichum pandanicola Tibpromma & K.D. Hyde, in Tibpromma et al., MycoKeys 33: 47 (2018).
Index Fungorum number: IF823841; Facesoffungi number: FoF05832; Figs. 90, 91
Colletotrichum pandanicola (MFLU 18–1852, new host record) a Dead leaf of Mangifera indica. b Germinating spore. c Reverse view of the 7d old culture on PDA. d Upper view of the 7d old culture on PDA. e Setae. f Vegetative hyphae. g Conidiogenous cell. h Conidia. i–j Appressoria. Scale bars: e = 50 μm, b = 10 μm; scale bar of b applies to f–j
a–b Leaf blight/early symptoms of anthracnose disease on Alstonia scholaris. c Complete death of infected branch due to anthracnose disease. d–e anthracnose symptoms appearing at the tip of the leaves caused necrosis. f a close view of anthracnose symptoms. g pure cultures of 10 days old Colletotrichum siamense on PDA. i–k Conidiogenous cells and conidia of C. siamense observed under a compound microscope (Scale bar: 10 µm)
Saprobic on Mangifera indica and pathogenic on Alstonia scholaris. Sexual morph: Not observed. Asexual morph: On PDA vegetative hyphae greyish white, dense, cottony. Conidiomata acervuli, black, circular to oval, submerged, solitary or aggregated. Sporulation abundant. Setae scattered, straight or ± bent, dark brown up to the tip, opaque, 2- to 4-septate, 50–80 µm long, smooth-walled, base cylindrical, 2–5 µm diam., tip acute. Conidiophores hyaline to light brown, cylindrical to clavate, smooth-walled, simple, occurring in densely arranged clusters. Conidiogenous cells enteroblastic, hyaline, smooth-walled, cylindrical to slightly inflated, periclinal thickening not visible. Conidia 7–12 × 2–6 μm (\(\bar{x}\) = 7 × 4 μm, n = 40), hyaline, smooth-walled, aseptate, ovoid, cylindrical or clavate with rounded apices, guttulate. Appressoria 9–16 × 5–6 μm (\(\bar{x}\) = 13 × 5 μm, n = 10), solitary to aggregated, in small groups or short chains, medium to dark brown, smooth-walled, irregular, rarely lobed.
Culture characteristics: Colonies grown from single conidia on PDA 50–70 mm diam. in 7 days, at first white becoming dark grey, reverse pale yellow.
Material Examined: Thailand, Chiang Rai Province, on leaf of Mangifera indica L. (Anacardiaceae), 19 May 2018, Ruvishika S. Jayawardena (MFLU 18-1852), living culture MFLUCC 18-1182. India, Karnataka, Mysuru on infected leaves of Alstonia scholaris (Apocyanaceae) as pathogen, May 2019, S. Mahadevakumar (UOM-IOE 19/15), living cultures (AsCs1, AsCs4).
GenBank numbers: MFLUCC 18-1182-MK629453 (ITS), MK639363 (gapdh), MK639357 (chs-1), MK639359 (act) and MK639361 (tub2)
AsCs1–OM912803 (ITS), OM934812 (gapdh), OM934814 (tub2)
AsCs4–OM912804 (ITS), OM934813(gapdh), OM934815 (tub2)
Notes: The species of the C. gloeosporioides species complex are mainly known as plant pathogens (Weir et al. 2012; Jayawardena et al. 2016, 2018, 2021a, b; Bhunjun et al. 2021), some species are also as endophytes or as saprobes (Jayawardena et al. 2021a, b). Strain MFLUCC 18-1182 belongs to the Colletotrichum gloeosporioides species complex and clusters with the ex-type of C. pandanicola (Fig. 87). However, strain MFLUCC 18–1182 differs from C. pandanicola by forming setae and appressoria, which were not observed in the ex-type strain of C. pandanicola (Tibpromma et al. 2018). This represents the first report of C. pandanicola on Mango from Thailand.
Colletotrichum pandanicola was isolated from leaves of Alstonia scholaris, is an evergreen tropical tree and an important medicinal plant distributed throughout the Indian peninsula including Western Ghats regions of Karnataka. Colletotrichum gleosporioides has been reported as a pathogen on A. scholaris (Chandra 1974; Mathur 1979; Sarbhoy and Agarwal 1990; Sarbhoy et al. 1971). Pathogenicity tests were performed on healthy A. scholaris plants. After 10–12 days of pot inoculation, the initial necrotic spots developed on leaves were smaller and later coalesced to form larger necrotized lesions. The lesions developed on inoculated plants were fusiform and grayish brown. Phylogeny supports (Fig. 87) our strain to be C. pandanicola. This is a new report of C. pandanicola on Alstonia scholaris from India.
Colletotrichum thasutense Armand, K.D. Hyde and Jayaward., sp. nov.
Index Fungorum number: IF900127; Faceoffungi number: FoF13361; Fig. 92
Etymology: Referring to the sub-district where the specimen was collected.
Holotype: MFLU 22-0206
Associated with leaf spot on Syngonium sp. Sexual morph: not observed. Asexual morph: On host: Acervuli present, setose, creamy to greyish white, subepidermal, produced solitary. Conidiophores rarely observed, hyaline, barrel shaped, 6.5–9 × 3–4 μm; Conidiogenous cells hyaline, cylindrical; 10.5–23 × 2.5–4 μm (x̄ = 16 × 3 μm, n = 20). Conidia aseptate, hyaline, smooth-walled, cylindrical, straight to slightly curved, mostly rounded ends, rarely obtuse at one end, 13.5–17.5 × 3.5–5 μm (x̄ = 15.5 × 4.5 μm, n = 20), Appressoria not observed.
On PDA: Acervuli, Setae and Sclerotia absent.
Culture characteristics: Colonies on PDA white to pale yellow, reverse same color, reaching 70 mm diam. in 7 days at 28 °C. Colonies cottony, circular, slightly raised and depressed in the center, Aerial mycelia white and medium in dense.
Material examined: Thailand, Chiang Rai, Mueang, ThaSut, from Syngonium sp. (Araceae), associated with leaf spot; 16 October 2021, A. Armand, A2 (MFLU 22-0206, holotype), living ex-type living culture MFLUCC 22-0173.
GenBank numbers: OP821902 (ITS), OP831280 (act), OP831281 (chs-1),: OP831282 (gapdh), OP831283 (tub2).
Notes: Based on the phylogenetic analyses, Colletotrichum thasutense is closely related to C. xishuangbannaense (Fig. 87). Morphologically, C. thasutense differs from C. xishuangbannaense by producing longer and thicker conidia. Colletotrichum xishuangbannaense has 9–12 × 3–4 µm conidia (de Silva et al. 2021), whereas C. thasutense has 13.5–17.5 × 3.5–5 μm conidia. Colletotrichum thasutense can be differentiated from C. xishuangbannaense in having slightly curved conidia and obtuse at one end. However, C. xishuangbannaense produces straight conidia with rounded ends. Moreover, C. thasutense bears longer and thicker conidiogenous cells (10.5–23 × 2.5–4 μm) than C. xishuangbannaense (15–18 × 1.5–2 µm).
Hypocreales Lindau
Hypocreales includes 15 families, which are considered subtropical and tropical, namely Bionectriaceae, Calcarisporiaceae, Clavicipitaceae, Cocoonihabitaceae, Cordycipitaceae, Cylindriaceae, Flammocladiellaceae, Hypocreaceae, Myrotheciomycetaceae, Nectriaceae, Niessliaceae, Ophiocordycipitaceae, Sarocladiaceae, Stachybotryaceae, and Tilachlidiaceae, based on the basis of molecular evidence (Hyde et al. 2020a, b, c; Wijayawardene et al. 2022). These families differ in the presence of fleshy and colorful perithecial ascomata, ostiolate perithecia, varied ascospores, and pigment production. It has been estimated that Hypocreales diverged 229 million years ago (Hyde et al. 2020a, b, c).
Nectriaceae Tul. & C. Tul. [as 'Nectriei'], Select. fung. carpol. (Paris) 3: 3 (1865)
Nectriaceae was introduced by Tulasne and Tulasne (1865) and re-evaluated by Lombard et al. (2015) based on morphology and ten genes (acl1, act, cmdA, his3, ITS, LSU, rpb1, rpb2, tef1 and tub2). This family includes 74 genera that are recognized by different perithecial pigments (Wijayawardene 2022; Lombard et al. 2015; Crous et al. 2021). The family contains a wide range of species, including plant pathogens, human pathogens, and industrial and commercial species (Rossman 1996; Luo and Zhuang 2008; Chaverri et al. 2011; Lechat et al. 2015; Lombard et al. 2015).
Fusarium Link, Mag. Gesell. naturf. Freunde, Berlin 3(1–2): 10 (1809).
Fusarium is famous for its difficulty in identification and pathogenicity. It has been extensively discussed in recent decades as one of the most taxonomically confusing genus within Nectriaceae (Gräfenhan et al. 2011; O'Donnell et al. 2013; Crous et al. 2021; Geiser et al. 2021). Crous et al. (2021) and Wang et al. (2022) have updated the taxonomy of this genus to include 19 species complexes. In addition, a variety of species within this genus contain virulent crop pathogens, such as F. graminearum and F. oxysporun, two of the top ten most economically damaging fungal pathogens (Dean et al. 2012; Leslie and Summerell 2006).
Fusarium brachygibbosum Padwick, Mycological Papers 12: 11 (1945).
Index Fungorum number: IF286508; Faceoffungi number: FoF11683; Figs. 93 , 94
Pathogenic on roots of Vigna unguiculata. Sexual morph: Not observed. Asexual morph: Conidiophores 27–58 µm long, carried on aerial mycelium, unbranched or irregularly and/or sympodially branched bearing a terminal phialide. Conidiogenous cells 8–22 × 2–4 µm, polyphialide, subulate to subcylindical, smooth. Macroconidia 15.2–22 × 2–3 µm, hyaline, slightly curved with five distinct septa, wide central cells, slightly sharp apexes, basal cells with foot like shape. Microconidia rarely observed. Chlamydospores 6–24 µm diam. abundant, spherical o globose, smooth, slightly verrucose, formed terminally or intercalary in chains of two or three, wall 1–1.5 µm.
Culture characteristics: Colonies on PDA reaching 90 mm at 28 °C after 14 d in 12/12 dark, colonies appeared white to pink with abundant aerial mycelium.
Materials examined: India, Karnataka, Mysuru, Doddamaragowdanahally, diseased root of cowpea (Vigna unguiculata (L.) Walp. Fabaceae), May 2019, S. Mahadevakumar & Y.S. Deepika (UOM-IOE 19/16), living cultures CPFb1, CPFb2, CPFb3, CPFb4.
Hosts: Wide host range, including Allium, Beta, Cannabis, Citrullus, Citrus, Euphorbia, Glycine, Gossypium, Helianthus, Nerium, Nicotiana, Phoenix, Plasmopara, Prunus, Sansevieria, Sorghum, Triticum, Vigna and Zea (Farr and Rossman 2022; this study).
Distribution: Wide geographical range, including in Australia, Azerbaijan, China, India, Iran, Malaysia, Oman, Qatar, Soudi Arabia, Tunisia, Turkey and United States (Farr and Rossman 2022; this study).
GenBank numbers: CPFb1– MT804589 (ITS), OM938019 (tef1)
CPFb2– MT804590 (ITS), OM938020 (tef1)
CPFb3– MT804591 (ITS), OM938021(tef1)
CPFb4– MT804592 (ITS), OM938022 (tef1)
Notes: Fusarium brachygibbosum is known to associated with 19 host plants of which two records are represented from India (Sorghum vulgare, Plasmopara viticola) (Farr and Rossman 2022). This is the first record of F. brachygibbosum recorded on Cowpea (Fabaceae) from India (new host record) (Fig. 95).
Fusarium purpurea S.L. Han, M. Raza, W.J. Duan & L. Cai, sp. nov.
Index Fungorum number: IF 555883; Facesoffungi number: FoF10818; Fig. 96
Etymology: Refers to the pigment produced on PDA and OA, purpure.
Holotype: HMAS 351947.
Asexual morph: Hyphae 1.7–3.5 μm diam, hyaline, smooth-walled, septate, branched. Conidiophores arises on aerial mycelium, unbranched or irregularly branched, 11–29.5 × 2–4.5 μm (\(\bar{x}\) = 19.9 × 3.0 μm, n = 35). Phialides mono- and polyphialide, subulate to subcylindrical, smooth- and thin-walled, 4.8–17.4 × 1.6–3.9 μm, periclinal thickening inconspicuous or absent. Microconidia hyaline, smooth- and thin-walled, two types, clavate with truncate base conidia (aseptate): 4.5–9.5 × 1.5–3.5 μm (\(\bar{x}\) = 6.3 × 2.5 μm, n = 50); globose conidia (aseptate): 6.5–11 × 7–11.5 μm (\(\bar{x}\) = 9.7 × 9.4 μm, n = 50). Sporodochia and chlamydospores not observed. Sexual morph: Not observed.
Culture characteristics: Colonies on PDA slow growing, reaching 52–57 mm diam in 7 d after incubation at 25 °C in the dark, colony flat, medium, filamentous, felted to velvety, rhizoid; colony from above; raised, dull, wrinkled folded, surface orchid purple (14C8) in the center, white (–A1) at the margin; reverse beetroot purple (13D8) in the center, white (–A1) at the margin; odour absent, not producing pigment in PDA media. On OA reaching 41–49 mm in 7 d after incubation 25 °C in the dark; raised, felted to dusty, with abundant aerial mycelium, margin entire; surface amethyst (15C6) in the center, white (–A1) at the margin; reverse oak brown (5D6); odour absent.
Material examined: Kazakhstan, intercepted at Alashankou Port, isolated at Ningbo Customs, from seeds of Triticum aestivum imported to China, Jan. 2019, W.J. Duan & W.Z. Li (HMAS 351947, holotype), ex-type living culture, CGMCC 3.23515 = LC15871. ibid., LC15872; ibid., LC15873; ibid., LC15874.
GenBank numbers: CGMCC 3.23515 – ON365812 (CaM), ON365816 (rpb1), ON365820 (rpb2), ON365828 (tef1), ON365824 (tub2)
LC15872 – ON365813 (CaM), ON365817 (rpb1), ON365821 (rpb2), ON365829 (tef1), ON365825 (tub2)
LC15873 – ON365814 (CaM), ON365818 (rpb1), ON365822 (rpb2), ON365830 (tef1), ON365826 (tub2)
LC15874 – ON365815 (CaM), ON365819 (rpb1), ON365823 (rpb2), ON365831 (tef1), ON365827 (tub2)
Notes: Fusarium purpurea formed a well-supported sister clade to F. globosum with 100% ML and 1.00 Bayesian posterior probabilities (BYPP) support (Fig. 96). Fusarium purpurea differs by 3 bp in the CaM gene, 16 bp in the rpb2 gene, 6 bp in the tef1 gene, and 1 bp in the tub2 gene compared to F. globosum (Proctor et al. 2013; Yilmaz et al. 2021). Morphologically, F. purpurea differs in the types of microconida production and its number of septation. For example, F. purpurea produces two types of microconidia: clavate with a truncate base (aseptate) and globose (aseptate) without papilla, while three types of microconidia were found in F. globosum: clavate with a truncate base (0- to 3-septate), napiform/pyriform, and globose (0- to 1-septate) which often have a distinct papilla (Rheeder et al. 1996; Leslie and Summerell. 2006) (Fig. 97).
RAxML phylogenetic tree generated from combined CaM, rpb1, rpb2, tef1 and tub2 sequence data of Fusarium fujikuroi species complex. Maximum likelihood bootstrap support values greater than 75% (in blue) and Bayesian posterior probabilities > 0.95 (in green) are indicated on the branches. Ex-type and epi-type cultures are indicated in bold with ‘T’ and ‘ET’
Microascales Luttr. ex Benny & R.K. Benj. (1980).
Microascales composed of seven families namely Ceratocystidaceae, Chadefaudiellaceae, Gondwanamycetaceae, Graphiaceae, Halosphaeriaceae, Microascaceae and Triadelphiaceae (Maharachchikumbura et al. 2016; Hyde et al. 2020a, b, c; Wijayawardene et al. 2022). A total of 109 genera were accepted under Microascales which were distributed in seven families (Wijayawardene et al. 2022).
Microascaceae Luttr. ex Malloch, Mycologia 62(4): 734 (1970)
Microascaceae was confined by Luttrell (1951) in Microascales and authenticated by Malloch (1970) (Maharachchikumbura et al. 2016). It includes saprobic, plant and opportunistic human pathogenic fungal genera (de Hoog et al. 2000; Sandoval-Denis et al. 2016; Maharachchikumbura et al. 2016). Currently this family is composed of 23 genera (Wijayawardene et al.2022).
Scedosporium Sacc. ex Castell. & Chalm., Manual of Tropical Medicine: 1122 (1919)
Scedosporium is a ubiquitous filamentous fungus with a worldwide distribution. Pseudallescharia boydii is the sexual morph of Scedosporium apiospermum, which was first discovered in 1889 as a causative agent of human otitis (Siebenmann et al. 1899). Later Monosporium apiospermum was discovered as an anamorphic state of P. bodydii from a patient with human mycetoma in 1919 (Shear 1922).
This genus is typified by a sexual morph (Pseudallescheria boydii) characterized by closed ascomata (cleistothecia), a peridium (ascomata wall) of ‘textura epidermoidea’, asci that are broadly clavate or spherical, and ascospores that are ellipsoidal or fusiform, which are symmetrical or nearly so (von Arx et al. 1988; De Hoog et al. 2000). The asexual morph includes: Scedosporium, characterized by hyaline, cylindrical conidiogenous cells arising from undifferentiated hyphae that produce obovoidal, hyaline, sticky conidia. Synnemata are characterized by large, erect bundles of hyphae terminating in a dense aggregate of conidiogenous cells. It produces conidia from a short extension of the conidiogenous cells with annellidic development (Gueho 1991; Lackner et al. 2014; Ramirez-Garcia et al. 2018).
Scedosporium species have been reported commonly from natural substrates such as soil, water anthropogenic influenced habitats, cattle dung and sewage (De Hoog et al. 2000; Ramirez-Garcia et al. 2018). Seventeen species are accepted under Scedosporium and these include: S. americanum, S. angustum, S. apiospermum, S. aurantiacum, S. boydii, S. cereisporum, S. deficiens, S. dehoogii, S. desertorum, S. fusoideum, S. haikouense, S. hainanense, S. magalhaesii, S. minutisporum, S. multisporum, S. rarisporum and S. sclerotiale (http://www.indexfungorum.org). The disease caused by Scedosporium species is termed as Scedosporosis. The occurrence of Scedosporium apiospermum associated with mushroom cultivation and Scedosporium marina and S. dehoogii from the marine environment are reported here.
Scedosporium apiospermum Sacc. ex Castell. & Chalm., Manual of tropical medicine (London): 1122 (1919).
Index Fungorum number: IF432048; Facesoffungi number: FoF11704; Fig. 98
Scedosporium apiospermum (MFLU 22-0160, new record) a. Contaminated oyster mushroom grow substrate. b,c. Colony on PDA. d. Sporulation of the colony on PDA. e-i. Conidial attachments and conidiogenous cells. j,k. Synnema with conidia. l. Conidia. Scale bars: a = 5 cm, b,c = 30 mm, d = 200 μm, e,f,l = 4 μm, g = 20 μm, h = 35 μm, i = 25 μm, j = 65 μm, k = 40 μm
Growing on Oyster mushroom grow substrate. Sexual morph: Not observed. Asexual morph: on host, Mycelium black powdery mass. On PDA, hyphomycetous, Hyphae 1.5–3.5 μm (\(\bar{x}\) = 2.2 μm) wide, branched, septate, hyaline. Conidiophores solitary or synnematous, solitary conidiophores 75–128 × 1.8–3 μm (\(\bar{x}\) = 102.5 × 2.6 μm, n = 10) hyaline, branched, forms 1–3 conidiogenous cells at the end. synnematous conidiophores 132–352 × 8.2–17.5 μm (\(\bar{x}\) = 288.4 × 12.7 μm, n = 15) cylindrical stipe, erect, forms conidia at the end. Conidiogenous cells 4.8–25 × 1.2–2.5 μm (\(\bar{x}\) = 15.2 × 1.3 μm, n = 10) lateral or terminal formation on solitary conidiophores, hyaline, cylindrical. Conidia hyaline, guttulate, conidia arising from conidiogenous cells 6.2–8.2 × 2–4.5 μm (\(\bar{x}\) = 7.4 × 3.3 μm, n = 30) obvoid to ellipsoidal, conidia arising from synnematous conidiophores 6.4–9.2 × 1.7–3.5 μm (\(\bar{x}\) = 7.5 × 2.8 μm, n = 35) cylindrical or claviform with a truncate base, conidia from undifferentiated hyphae 6–7.5 × 5.2–6.6 μm (\(\bar{x}\) = 6.4 × 5.2 μm, n = 20) sessile, globose to subglobose.
Culture characteristics: colonies on PDA attaining 65–75 mm diameter after 14 days at 25 °C, cottony, dense, lanose, light gray to dark grey, margins fimbriate and irregular; reverse dark grey to brown, grey to white margins.
Material examined: Thailand, Phayao Province, growing on the oyster mushroom growing substrate, 06 June 2020, AJ. Gajanayake, AJ 072 (inactive dry culture, MFLU 22-0160, new host record), living culture, MFLUCC 22-0192.
GenBank numbers: ON714510 (ITS), ON714511(LSU), ON730889 (tub2).
Notes: Our isolate MFLUCC 22-0192, clusters within Scedosporium apiospermum with a 90% ML and 0.97 BYPP support (Fig. 99). Furthermore, the morphology of our isolate MFLUCC 22-0192 resembles the original description and illustrations for Scedosporium apiospermum by Saccarado in Castellani and Chalmers (1919) and the description and morphological illustrations by Gilgado et al. (2010). However, there are size differences of conidiophores, conidiogenous cells and conidia, when we compare our strain with the strains described in Castellani and Chalmers (1919) and Gilgado et al. (2010). The reason for this may be the differences in the media in which the colonies were grown.
Phylogram generated from maximum likelihood analysis based on combined ITS and tub2 sequence data representing the species of Scedosporium and related genera. Related sequences are taken from Zhang et al. (2021). Sixty-two taxa are included in the combined analyses. Microascus longirostris (CBS 196.61), Scopulariopsis brevicaulis (MUCL 40,726), Wardomyces anomalus (CBS 299.61) and Wardomyces giganteus (CBS 746.69) are used as the outgroup taxa. Bootstrap support values for ML ≥ 70% and BYPP ≥ 0.95 are given near the nodes. The newly generated sequence is in blue. The type strains are indicated in black bold
Scedosporium apiospermum has been mainly identified as an opportunistic clinical pathogen relevant to many infections which commonly occur in the bones, central nervous system, lungs, paranasal sinuses, skin and soft tissues (Shinohara and George (2009); Goldman et al. 2016). Seephueak et al. (2017) isolated 21 fungal species from spent mushroom substrate of Pleurotus sp. collected from mushroom farms in southern Thailand. Among those 21 fungal species there were, Alternaria spp., Aspergillus spp., Chaetomium sp., Cunninghamella spp., Fusarium spp., Lasiodiplodia sp., Neurospora sp., Penicillium spp., Rhizoctonia sp. and Trichoderma spp. (Seephueak et al. 2017). Suada et al. (2015) have reported Aspergillus spp., Fusarium spp., Gliocladium sp., Mucor spp., Neurospora spp., Paecilomyces sp., Penicillium spp., Pythium sp., Stachybotrys spp. and Trichoderma spp. as contaminants from oyster mushroom growing substrate. According to best our knowledge this is the first report of Scedosporium apiospermum as a contaminant of oyster mushroom growing substrate.
Scedosporium marina Devadatha & V.V Sarma, sp. nov.
Index Fungorum number: IF558432, Facesoffungi number: FoF05035; Figs. 100, 101
Scedosporium marina on a twig (AMH-9946, holotype). a. Colonies on decaying woody stem of Suaeda monoica. b − c Synnemata. d − e, h Head and conidiophores. f Apical part of synnema producing conidia. g Conidiogenous cells with annellidic conidia. i Subcylindrical conidia. k − l Claviform conidia. m − o Obovoid conidia. Scale bars: b = 50 µm, c = 100 µm d − o = 10 µm
Scedosporium marina in culture (NFCCI-4273). a Synnemata on PDA b − c Synnemata. g − h Culture on PDA after 14 days. d − e Slimy head and Conidiophores. f Conidiogenous cells with annellidic conidia. l Apical part of synnema producing conidia. m Subcylindrical conidia. n − r, s Sessile obovoid conidia o − q Claviform Conidia. Scale bars: b − c = 100 µm d = 50 µm, e − f, i − s = 10 µm
Etymology: The specific epithet is in reference to the marine environment in which the fungus was collected.
Holotype: AMH-9946.
Saprobic on decaying woody stem of the halophyte Suaeda monoica. Asexual morph: Colonies effuse, light brown. Mycelium immersed, composed of septate, branched, smooth, pale brown to hyaline hyphae. Synnemata solitary to gregarious, erect, dark brown, 170–1110 µm tall with a cylindrical stipe, 10–25 µm wide (\(\bar{x}\) = 642 × 16 µm, n = 10), dark gray, smooth-walled, terminate into a slimy head of conidia, slimy head 60–140 µm long and 60–165 µm wide (\(\bar{x}\) = 86 × 111 µm, n = 10). Hyphae interwoven at the base, unbranched in the stipe, branching at the apex to form conidiophores. Conidiophores synnematous, solitary, branched, often reduced to conidiogenous cells, growing laterally bearing a single verticil conidiogenous cell. Conidiogenous cells percurrent, terminal or lateral, hyaline, smooth-walled, cylindrical to slightly flask-shaped, 25–55 × 2–2.5 µm (\(\bar{x}\) = 35 × 2.25 µm, n = 10). Conidiogenous cells arising from undifferentiated hyphae are cylindrical to slightly flask-shaped, producing slimy heads of one-celled, smooth-walled, sub hyaline, obovoid or sub-cylindrical conidia. Three types of conidia are produced: (i) those produced on solitary conidiophores sub hyaline, smooth-walled, obovoid, or sub cylindrical 7–13 × 2.5–5.5 µm (\(\bar{x}\) = 10 × 4 µm, n = 20); (ii) those produced on synnemata predominantly cylindrical or claviform, 5–13 × 1–3 µm (\(\bar{x}\) = 9 × 2 µm, n = 20) with a wide truncate base; (iii) those developed mainly from the undifferentiated hyphae of the substrate, sessile or on short protrusions, solitary, lateral, brown, smooth, and thick-walled, mostly obovoid 2.5–10 × 2–2.5 µm (\(\bar{x}\) = 9 × 2.3 µm, n = 20). Sexual morph: Not observed.
Culture characteristics: Conidia germinating on sea water agar within 24 h. Germ tubes produced from the conidial base. Colonies on PDA attaining 30–35 mm diameter after 14 days at 25 °C, circular, raised, light grayish at center and smoke gray at margins, cottony; yellow in reverse with entire margin. The optimum growth temperature was from 25 to 37 °C, and did not grow at 45 °C. Hyphae hyaline to pale brown, branched and septate, 3–7.5 µm wide. Synnemata developed after 30 days of incubation at 25 °C; synnemata solitary to gregarious, erect, dark brown, 190–1400 µm tall with a cylindrical stipe from 10 to 25 µm wide (\(\bar{x}\) = 647 × 16.9 µm, n = 10), dark gray, smooth walled, terminated into a slimy head of conidia, 25–35 µm long and 35–50 µm wide (\(\bar{x}\) = 30 × 36 µm, n = 10). Conidiophores solitary, branched, often reduced to conidiogenous cells growing laterally bearing single verticil of conidiogenous cells. Conidiogenous cells terminal or lateral, hyaline, smooth-walled, cylindrical to slightly flask-shaped, 25–50 × 2–3 µm (\(\bar{x}\) = 35 × 16 µm, n = 10). Conidiogenous cells arising from undifferentiated hyphae, cylindrical to slightly flask-shaped, producing slimy heads of one-celled, smooth-walled, sub-hyaline, obovoid or sub-cylindrical conidia. There were three types of conidia: (i) those produced on solitary conidiophores subhyaline, smooth-walled, obovoid or subcylindrical 5–10 × 1.5–3 µm (\(\bar{x}\) = 6.8 × 2.3 µm, n = 20); (ii) those produced on synnemata predominantly cylindrical or claviform, 7.5–10 × 2–3 µm (\(\bar{x}\) = 8.7 × 2.4 µm, n = 20) with a wide truncate base; (iii) those developing mainly from the undifferentiated hyphae of the substrate, sessile or on short protrusions, solitary, lateral, brown, smooth, and thick-walled, mostly obovoid 5–10 × 2–2.5 µm (\(\bar{x}\) = 9 × 2.4 µm, n = 20). Sexual state not observed after incubation for 2 months at 25 °C.
Material examined: India, Tamil Nadu, Tiruvarur, Muthupet mangroves (10.4° N 79.5° E), on decaying woody stem of the halophyte Suaeda monoica Forssk. ex J.F.Gmel. (Amaranthaceae) 28 November 2015, B. Devadatha (AMH-9946, holotype), ex-type living culture NFCCI-4273.
GenBank numbers: MF182397 (ITS), KY863508 (LSU), MH571780 (SSU), MF687078 (tub2), MF182399 (tef1)
Notes: Our present collection of Scedosporium marina (NFCCI-4273) has been assigned to Scedosporium based on its similar morphological characteristics in having Graphium-like synnemata and scattered, poorly differentiated, percurrent conidiogenous cells (Gueho 1991). The present taxon, Scedosporium marina (NFCCI-4273) and S. aurantiacum (FMR8630) share similar morphological characteristics in producing three different types of conidia like the obovoid, or sub cylindrical, cylindrical or claviform, sessile obovoid conidia with overlapping conidial dimensions and yellow diffusible pigments on PDA. However, combined multigene phylogenetic anlayses of combined datasets of ITS and tub2 revealed that Scedosporium marina (NFCCI-4273) formed distinct lineage sharing a sister relation with Scedosporium aurantiacum with significant statistical support 100% ML, 97% MP (105). Morphologically S. marina (NFCCI-4273) is distinct from S. aurantiacum (FMR8630) in having long and wide synemmata (170–1110 × 10–25 vs 330–750 × 7.5–17.5), smaller heads (60–140 × 60–165 vs 60–70 × 140). The condiogenous cells of S. marina (NFCCI-4273) are percurrent and long in contrast to S. aurantiacum (FMR8630) (25–55 × 2–2.5 vs 10–37 × 1.5 2.5).
The optimum growth temperature of S. marina (NFCCI-4273) was from 25 to 37 °C and did not grow at 45 °C whereas S. aurantiacum (FMR8630) was from 37 to 40 °C and growth was also found at 45 °C but the fungus did not grow at 50 °C (Gilgado et al. 2005). The ITS sequence comparison revealed more than 30 nucleotide base pair differences between two taxa, which supports the establishment of a new species for our new taxon. Furthermore, S. marina is a saprobe on decaying woody stem of the halophyte Suaeda monoica from a marine habitat unlike other species of Scedosporium which are known to be human pathogens. Scedosporium aurantiacum is clearly distinguished from S. marina as an opportunistic human pathogen that is known to cause various infections in lungs, ears, respiratory sinuses and subcutaneous abscess in patients of diabetes and malignant lymphoma (Kondo et. al 2018). Hence, based on the above mentioned morphological, cultural, molecular and habitat differences we introduce a new species, S. marina, in Scedosporium.
Scedosporium dehoogii Gilgado, Cano, Gene´ Guarro in Journal of Clinical Microbiology 46: 2 (2008).
Index Fungorum number: IF538388; Facesoffungi number: FoF04829; Fig. 102, 103
Saprobic on decaying woody of Avicennia marina. Asexual morph: Colonies effuse, light brown. Mycelium mostly immersed, composed of septate, branched, smooth, pale brown to hyaline hyphae. Synnemata solitary to gregarious, erect, dark brown, 190–390 µm tall with a cylindrical stipe from 10 to 30 µm wide (\(\bar{x}\) = 242 × 16 µm, n = 10), dark gray, smooth-walled, terminated into a slimy head of conidia, slimy head 45–75 µm long, 55–80 µm wide (\(\bar{x}\) = 60 × 64 µm, n = 10). Hyphae interwoven at the base, unbranched in the stipe, branched at the apex to form conidiophores. Conidiophores synnematous, solitary, branched, often reduced to conidiogenous cells which were subhyaline. Conidiogenous cells terminal or lateral, hyaline, smooth-walled, cylindrical to slightly flask-shaped, 25–40 × 2–3 µm (\(\bar{x}\) = 31 × 2.5 µm, n = 10). Conidiogenous cells arise from undifferentiated hyphae, cylindrical to slightly flask-shaped, producing slimy heads of one-celled, smooth-walled, sub-hyaline, obovoid or sub-cylindrical conidia. Conidia: two types of conidia: (i) those produced on synnemata and solitary conidiophores were predominantly cylindrical or claviform, hyaline, 5–10 × 2–3 µm (\(\bar{x}\) = 7.3 × 2.7 µm, n = 20) with a wide truncate base; (ii) those developed mainly from the undifferentiated hyphae of the substrate were sessile or on short protrusions, solitary, lateral, brown, smooth, and thick-walled, mostly obovoid 2.5–7.5 × 2–3 µm (\(\bar{x}\) = 4.3 × 2.4 µm, n = 20). Sexual morph: Not observed.
Culture characteristics: Conidia germinating on Sea Water agar within 24 h. Germ tubes produced from the conidial base. Colonies on PDA fast growing, attaining 40–45 mm diameter after 14 days at 25 °C, circular, raised, with white to grey at centre and white to cream at margins, cottony; pale yellow in reverse with entire margin. The optimum growth temperature was from 25 to 37 °C. The fungus did not grow at 45 °C. Hyphae hyaline to pale brown, branched and septate, 1–3 µm wide. Conidiophores synemmatous; synnemata solitary to gregarious, erect, dark brown, 180–255 µm tall with a cylindrical stipe from 10–30 µm wide (\(\bar{x}\) = 223 × 17 µm, n = 10), dark gray, smooth walled, terminating into a slimy head of conidia, 45–85 µm long and 55–90 µm wide (\(\bar{x}\) = 63 × 66 µm, n = 10). Hyphae interwoven at the base, unbranched in the stipe, branching at the apex to form conidiophores. Conidiophores solitary, branched often reduced to conidiogenous cells which were sub-hyaline. Conidiogenous cells terminal or lateral, hyaline smooth-walled, cylindrical to slightly flask-shaped, 30–45 × 2–3 µm (\(\bar{x}\) = 35 × 2.4 µm, n = 10). Conidiogenous cells arising from undifferentiated hyphae, cylindrical to slightly flask-shaped, producing slimy heads of one-celled, smooth-walled, sub hyaline, obovoid or sub-cylindrical conidia. Conidia: two types: (i) those produced on synnemata and solitary conidiophores, predominantly cylindrical or claviform, hyaline, 5–15 × 2–5 µm (\(\bar{x}\) = 10 × 3 µm, n = 20) with a wide truncate base; (ii) those developed mainly from the undifferentiated hyphae of the substrate were sessile or on short protrusions, solitary, lateral, brown, smooth, and thick-walled, mostly obovoid 2.5–9 × 2–5 µm (\(\bar{x}\) = 5 × 3.5 µm, n = 20).
Material examined: India, Tamil Nadu, Tiruvarur, Muthupet mangroves (10.4° N 79.5° E), on decaying wood of Avicennia marina (Forssk.) Vierh. (Acanthaceae), 28 November 2015, B. Devadatha (AMH 9945), living culture, NFCCI- 4274.
Hosts: Agricultural areas, playgrounds, riverbanks, Soil, Human infections (Rougeron et al. 2018)
Distribution: Australia, Austria, Chile, India, Netherlands, Spain, Thailand (Rougeron et al. 2018)
GenBank numbers: MH569493 (ITS), MH569492 (LSU), MH571777 (SSU)
Notes: Multigene phylogenetic anlayses of combined datasets of ITS sequence data revealed that Scedosporium dehoogii (NFCCI-4274) clustered together with the type and other existing strains of S. dehoogii with moderate statistical 74% ML and 78% MP support (Fig. 103). Scedosporium dehoogii (NFCCI-4274) from decaying wood of Suaeda monoica and Avicennia marina and colonies on PDA also share similar morphological characteristics with S. dehoogii (CBS-117406) reported from the soil (Gilgado et al. 2008). Scedosporium dehoogii is a common environmental species occurs on locations with high human activities like soil, water, agricultural areas and not involved in human infections (Kaltseis et al. 2009). However, this is the first report of Scedosporium dehoogii (NFCCI- 4274) from marine habitats. Earlier Medicopsis romeroi has been reported from mangroves (Devadatha et al. 2020). Also, Calabon et al. (2018) reported S. aurantiacum from Sponges in mangroves. With the present two species from mangroves totally three Scedosporium spp. are recorded from marine environments (Figs. 103, 104).
Scedosporium dehoogii (NFCCI- 4274) a − b Cultures on PDA after 14 days. c − d Synnemata. e Slimy head and Conidiophores. f sessile obovoid conidia. g Conidiogenous cells with annellidic conidia. h. sub cylindrical conidia i − n Cylindrical and obovoid conidia and Scale bars: c = 50 µm d − n = 10 µm
Phylogram based on the RAxML analysis of a combined ITSrDNA and tub2 sequence dataset. Bootstrap support values for ML and MP higher than 70% values are given above each branch respectively. The new isolates are represented in blue. The tree is rooted to Parascedosporium sanyaense EM65901. Thirty-eight sequences are included in the phylogenetic analyses with 1159 characters including gaps. The maximum parsimonious dataset consisted of 855 characters were constant, 166 parsimony-informative and 138 parsimony-uninformative. The parsimony analysis of the data matrix resulted in Two hundred and seventy equally parsimonious trees with a length of 486 steps (CI = 0.759, RI = 0.888, RC = 0.674, HI = 0.241)
Hypocreomycetidae incertae sedis (Rhexoacrodictys and Dematipyriforma clade)
Notes: In the current study, Rhexoacrodictys and Dematipyriforma form a distinct clade with a high statistical support (100/100/100 for ML/MP/BYPP, respectively) that is phylogenetically related to the orders Pleurotheciales and Savoryellales and might represent a new lineage (Fig. 105). The phylogenetic placement of Rhexoacrodictys is controversial in various studies. Shi et al. (2021) placed Rhexoacrodictys in Pleurotheciales, while Boonmee et al. (2021) placed the genus in Savoryellales. Sun et al. (2017) placed Dematipyriforma in Savoryellales where it forms a basal branch to representatives of the order.
Phylogenetic relationship of Dematipyriforma with related taxa based on the nucleotide sequences of the combined SSU and LSU rDNA. The maximum likelihood (ML) tree was constructed in MEGA X (Kumar et al. 2018). The maximum parsimonious data set of the combined genes consisted of 30 taxa with three representatives of Hypocreales used as outgroup. Phylogenetic trees obtained from ML, MP and BYPP were similar in topology. Bootstrap support on the nodes represents ML and MP ≥ 50%. Branches with a BYPP of ≥ 95% are in bold. The three new Dematipyriforma species are in blue
Dematipyriforma L. Y. Sun, Hai-Yan Li, Xiang Sun & L.D. Guo.
Notes: Dematipyriforma is a monotypic genus typified by D. aquilaria L. Y. Sun, Hai-Yan Li, Xiang Sun & L.D. Guo that was isolated as an endophyte from a trunk of Aquilaria crassna in Laos (Sun et al. 2017). The genus is characterized by monoblastic, integrated, intercalary or terminal, pale brown to brown, determinate, cylindrical conidiogenous cells; solitary, pyriform conidia with transverse and often oblique or longitudinal, usually with a single small basal cell; produce variously shaped Chlamydospores in culture (Sun et al. 2017). During an ongoing study of freshwater fungi from River Nile in Egypt (e.g. Abdel-Aziz 2016a, b, c, 2020), three new species of Dematipyriforma were recorded on submerged wood and date palm rachis in the River Nile, Egypt that are described in this article based on morphology and their phylogenetic placement. Species of Dematipyriforma are phylogenetically related to Rhexoacrodictys species, both genera have rhexolytic conidial secession, however the later genus has macronematous conidiophores and percurrently extending conidiogenous cells vs. absent or micronematous conidiophores and determinate conidiogenous cells in Dematipyriforma. Boonmee et al. (2021) described Rhexoacrodictys nigrospora Boonmee, D.F. Bao & K.D. Hyde from decaying wood in Thailand with micronematous or semi-macronematous conidiophores and determinate conidiogenous cells. They did not include the sequences of Dematipyriforma aquilariain their phylogenetic analyses and in our opinion R. nigrospora can be placed in Dematipyriforma based on morphology of the conidiophores (micronematous vs. macronematous with bulbous base) and conidiogenesis (determinate vs. percurrent proliferation in Rhexoacrodictys erecta (Ellis & Everh.) W.A. Baker & Morgan-Jones) and the phylogenetic analyses (Fig. 105).
Dematipyriforma aquatica Abdel-Aziz &Abdel-Wahab sp. nov.
Index Fungorum number: IF900081; Facesoffungi number: FoF13400; Fig. 106
Etymology: Named after the aquatic habitat, where this fungus was collected.
Holotype: SUMCC H-12001 (Sohag University Microbial Culture Collection)
Saprobic on submerged wood in the River Nile. Sexual morph: Not observed. Asexual morph: Mycelium immersed and superficial, sub-hyaline to brown, septate, branched, smooth, 1.5–4 μm wide. Conidiophores absent or present, when present micronematous, mononematous, sub-hyaline to brown, flexuous, smooth, unicellular, 7–15 μm long, 1.5–4 μm wide. Conidiogenous cells holoblastic, integrated, intercalary or terminal, sub-hyaline to brown, determinate, ampulliform, clavate, subglobose, smooth, 5–8 μm long, 5–6 μm wide. Conidial secession rhexolytic. Conidia solitary or aggregated, effuse and heavily covered the surface of the wood, intercalary or terminal, smooth, pyriform or subglobose, rounded at the apex, black, with brown to dark-brown basal cell, muriform, 4–10 cells, 3–5 transverse septa and 0–2 longitudinal septa, not or slightly constricted at the septa, 27–38 × 15–26 μm (\(\bar{x}\) = 32.2 × 20.9 μm, n = 50), apex and basal cells are singles. Chlamydospores intercalary or terminal, catenated, straight, or curved, brown to dark brown, smooth, granulate, phragmoseptate.
Culture characteristics: Colonies on PDA reaching 25 mm diam after 3 weeks, at 25 °C, dark-brown to black, reverse dark-brown to black. Conidial dimensions and shapes are similar to those found on natural wood.
Material examined: Egypt, Sohag City, the River Nile, on submerged wood, 14 August 2012, F. A. Abdel-Aziz, SUMCC H-12001, holotype, ex-type living culture, SUMCC 12101.
GenBank numbers: MT522154 (SSU), MT522155 (LSU)
Notes: Combined phylogenetic analyses of SSU and LSU rDNA placed the three new species of Dematipyriforma with the type species D. aquilaria with high statistical support as distinct new taxa. The three new species differ from D. aquilaria in having black conidia vs. pale grey olivaceous to pale brown in the latter species. Dematipyriforma aquilaria was isolated as an endophyte from a trunk of Aquilaria crassna in Laos (Sun et al. 2017), while the three new species are freshwater taxa. Conidia of D. aquilaria are evenly pigmented, while the three new species have black conidia with basal cells that are lighter. Chlamydospores of D. aquilaria have thick walls with axial perforative canals, these canals are absent in the three new species. A comparison of the 590 nucleotides of the D1/D2 region of the LSU rDNA of the three new species of Dematipyriforma with D. aquilarias hows 14 base pair differences (2.37%) which justifies the erection of the three new species following the guidelines of Jeewon and Hyde (2016).
Dematipyriforma globispora Abdel-Aziz &Abdel-Wahab sp. nov.
Index Fungorum number: IF900083; Facesoffungi number: FoF13865; Fig. 107
Dematipyriforma globispora (SUMCC H-12002, holotype).a–g, i–lVariously shaped conidia at different stages of maturity. h Chlamydospores in culture. a–e Conidia from natural wood. f–g, i–l Conidia from pure culture. c–d Conidia are surrounded by network of hyphae. e, l Conidia with small buds. Scale bars: a–l = 10 µm
Etymology: Named after the shape of the globose shape of the conidia.
Holotype: SUMCC H-12002
Saprobic on submerged wood in the River Nile. Sexual morph: Not observed. Asexual morph: Mycelium immersed and superficial, sub-hyaline to brown, septate, branched, smooth, 2–4 μm wide. Conidiophores absent or present, when present micronematous, mononematous, sub-hyaline to brown, flexuous, smooth, unicellular, 6–12.5 μm long, 1.5–4.5 μm wide. Conidiogenous cells holoblastic, integrated, intercalary or terminal, sub-hyaline to brown, determinate, clavate, smooth, 4–6.5 μm long, 2.5–4.5 μm wide. Conidial secession rhexolytic. Conidia solitary or aggregated, effuse and heavily covered the surface of the wood, intercalary or terminal, smooth, globose or subglobose, rounded at the apex, black, with brown to dark-brown basal cell, muriform, with irregular transverse, longitudinal and oblique septa and form mass of cells, not constricted at the septa, sometimes surrounded by network of hyphae, 17–37 × 15–30 μm (\(\bar{x}\) = 27.7 × 20.9 μm, n = 60), smaller buds are produced from conidia that are yellow–brown to brown in color, muriform, 11–19.2 × 7.1–14.5 μm. Chlamydospores intercalary or terminal, catenated, straight, or curved, brown to black, smooth, form large, black muriform masses similar to conidia but much larger in size 37–120 × 17–30 μm.
Culture characteristics: Colonies on PDA reaching 20 mm diam after 3 weeks, at 25 °C, dark-brown to black, reverse dark-brown to black. Conidial dimensions and shapes are similar to those found on natural wood.
Material examined: Egypt, Sohag City, the River Nile, on submerged wood, 14 August 2012, F. A. Abdel-Aziz, SUMCC H-12002, holotype, ex-type living culture, SUMCC 12102.
GenBank numbers: MT522158 (SSU), MT522153 (LSU).
Notes: Dematipyriforma globispora differs from the other three species in having smaller conidia, that are mostly globose, sometimes surrounded by networks of hyphae and produce buds that are pale-brown to brown. Dematipyriforma globispora and D. aquatica are phylogenetically related, however, they differ in their morphology. Conidial cells in D. globispora are arranged irregularly and conidia are globose with small buds and surrounded by network of hyphae. Conidia in D. aquatica are pyriform or subglobose with 3 to 5 continuous transverse septa. Rhexoacrodictys nigrospora produce similar conidia with overlapping dimensions, however, conidia of D. globispora are surrounded by network of hyphae with brown buds. Mycelium in R. nigrospora are narrow (1–2 μm wide) with verruculose or finely echinulate-walled (Boonmee et al. 2021), while hyphae in D. globispora are 2–4 μm wide and smooth. A comparison of the 805 nucleotides of the LSU rDNA for D. globispora and R. nigrosporashows 11 base pair differences (1.36%) that confirm they are two different species.
Dematipyriforma nilotica Abdel-Aziz &Abdel-Wahab, sp. nov.
Index Fungorum number: IF900084; Facesoffungi number: FoF13401; Fig. 108
Dematipyriforma nilotica (SUMCC H-12003, holotype). a–l Variously shaped conidia at different stages of maturity. a–d Conidia from natural date palm rachis. e–l Conidia from pure culture. m Chlamydospores in culture. b,d Conidia surrounded by fine fibers. a–b, g–i Gradual filling of the conidial rows by black material. Scale bars: a–m = 10 µm
Etymology: Named after the River Nile where the fungus was collected.
Holotype: SUMCC H-12003
Saprobic on submerged date palm rachis in the River Nile. Sexual morph: Not observed. Asexual morph: Mycelium immersed and superficial, sub-hyaline to brown, septate, branched, smooth, 1.5–3.5 μm wide. Conidiophores absent or present, when present micronematous, mononematous, sub-hyaline to reddish-brown, flexuous, smooth, unicellular, 7–13 μm long, 2.5–4.5 μm wide. Conidiogenous cells holoblastic, integrated, intercalary or terminal, sub-hyaline to brown, determinate, ampulliform, clavate, subglobose, cylindrical, smooth, 1.5–8.5 μm long, 4.5–6.5 μm wide. Conidial secession rhexolytic. Conidia solitary or aggregated, effuse and heavily covered the surface of the wood, intercalary or terminal, smooth, or surrounded by fine fibers, pyriform, globose or subglobose, rounded at the apex, conidial cells are filled gradually with black material, black when mature, with brown to dark-brown basal cell, muriform, 6–9 cells, 3–5 transverse septa and 0–2 longitudinal or oblique septa, not or slightly constricted at the septa, 31–45 × 21–37 μm (\(\bar{x}\) = 37 × 26.3 μm, n = 50), basal cells are singles. Chlamydospores intercalary or terminal, dark-brown to black, smooth, form large, black muriform masses with irregular shapes.
Culture characteristics: Colonies on PDA reaching 30 mm diam after 3 weeks, at 25 °C, brown to reddish dark-brown, reverse brown to dark-brown. Conidial dimensions and shapes are similar to those found on natural wood.
Material examined: Egypt, Sohag City, the River Nile, on submerged date palm rachis, 14 August 2012, F. A. Abdel-Aziz, SUMCC H-12003, holotype, ex-type living culture, SUMCC 12103.
GenBank numbers: MT522157 (SSU), MT522156 (LSU).
Notes: Dematipyriforma nilotica differs from the other three species in having larger conidial dimensions that sometimes surrounded by fine fibres. Dematipyriforma nilotica is phylogenetically related with D. aquilaria, however, the first species have black and larger conidia (31–45 × 21–37 μm vs. 25–37 × 15–22 μm in D. nilotica and D. aquilaria respectively) and grow on decaying date palm rachis in freshwater habitat, while the latter species produce brown conidia and live as an endophyte. Chlamdydospores in D. aquilaria have perforative canal that is absent in D. nilotica. Both D. aquatica and D. nilotica have 3–5 continuous transverse septa with 0–2 longitudinal septa, however, conidia in the latter species are surrounded by fine fibres and are larger in size (27–38 × 15–26 μm vs. 31–45 × 21–37 μm for D. aquatica and D. nilotica respectively). A comparison of the 800 nucleotides of the LSU rDNA for D. aquatica and D. niloticashows 15 base pair differences (1.87%) that confirm they are two different species.
Key to Dematipyriforma species
1. Endophytic species, produce brown conidia…………………………… D. aquilaria
1*.Saprobic species in freshwater habitat, produce black conidia…………………………… 2
2. Conidial cells arranged in rows with continuous transverse septa…………………………… 3
2*.Conidial cells arranged irregularly, globose conidia with small buds and surrounded by network of hyphae……………………………D. globispora.
3. Conidial width less than 30 µm; smooth conidial wall……………………………D. aquatica
3*.Conidial width more than 30 µm; conidial wall with fine fibres ……….D. nilotica.
Subclass Savoryellomycetidae Hongsanan, K.D. Hyde & Maharachch.
Coniochaetales Huhndorf, A.N. Mill. & F.A. Fernández, Mycologia 96(2): 378 (2004).
Coniochaetales was introduced by Huhndorf et al. (2004) with Coniochaetaceae as the type family. Coniochaetales comprises two families namely Coniochaetaceae and Cordanaceae. Cordanaceae was previously accommodated in Cordanales. Hongsanan et al. (2017) treated Cordanales as a synonym of Coniochaetales based on molecular clock evidence. Hyde et al. (2020a, b, c) suggested the divergence time for Coniochaetales to be around 131 MYA. Coniochaetales comprises two families and five genera (Hyde et al. 2020a, b, c).
Coniochaetaceae Malloch and Cain (1971).
Coniochaetaceae was introduced by Malloch and Cain (1971) to accommodate two genera Coniochaeta and Coniochaetidium (Sacc.). Coniochaetaceae is typified by Coniochaeta and accommodates two genera Barrina A.W. Ramaley and Coniochaeta (Sacc.) Cooke (Wijayawardene et al. 2020) based on multi gene phylogeny (Maharachchikumbura et al. 2015a, b; Samarakoon et al. 2018). Species belonging to this family are saprobes and pathogenic on various decaying wood and plants or animals, respectively (Khan et al. 2013; Maharachchikumbura et al. 2015a, b, 2016; Wijayawardene et al. 2017a, b; Samarakoon et al. 2018).
Coniochaeta (Sacc.) Cooke, Grevillea 16(no. 77): 16 (1887)
Coniochaeta was introduced by (Sacc.) Cooke (1887a, b) and is typified by Coniochaeta ligniaria (Grev.) Cooke. Coniochaeta is characterized by solitary or aggregated, typically setose, dark brown to black, pyriform to globose ascomata, membranaceous to pseudoparenchymatous or coriaceous peridium, paraphysate hamathecium, unitunicate and thin-walled asci with a small non-amyloid apical ring and one-celled, usually dark brown and often laterally compressed with a germ slit ascospores (Greville 1823–1824, Cooke 1887a, b). The hyphomycetous asexual morph is characterized by phialidic conidiogenous cells, previously described in Lecythophora (Weber 2002; Khan et al. 2013). In this study, we introduce a new species C. caraganae collected on dead twigs of Caragana frutex in Russia based on morphology and phylogenetic evidence.
Coniochaeta caraganae D. Pem, Bulgakov & K.D. Hyde, sp. nov.
Index Fungorum number: IF 559528, Facesoffungi number: FoF 08686; Fig. 109
Etymology: ″caraganae″ refers to the host plant from which the fungus was isolated.
Holotype: MFLU 17-2500
Saprobic on dead branch of Caragana frutex. Sexual morph: Not observed. Asexual morph: Coelomycetous. Conidiomata 106–114 μm diam., 4–7 μm high (x̄ = 110.7 × 6.2 μm, n = 20), small, pycnidial, solitary, scattered, immersed, uniloculate, globose to subglobose, thin-walled. Peridium 26–32 μm composed of two layers. Inner layer consisting cells of textura prismatica, hyaline to subhyaline, strongly compressed. Outer layer consisting of densely packed, moderately thick-walled, brown cells of textura angularis, tending to be darker and more isodiametric towards the outside. Conidiophores 7–10 × 2–3 μm (x̄ = 6.5 × 2.3 μm, n = 20), hyaline, straight or irregularly bent, reduced to conidiogeneous cells. Conidiogenous cells 2–3 × 4–6 μm (x̄ = 3.1 × 5.1 μm, n = 20), holoblastic, annellidic, simple, determinate, hyaline, doliiform to cylindrical, smooth-walled. Conidia 3–4 × 5–6 μm (x̄ = 4.1 × 5.8 μm, n = 20) oblong to ovoid, straight, rounded at both ends, sometimes truncate at base, cylindrical, aseptate, smooth and thick-walled, eguttulate.
Culture characteristics: colonies on MEA, reaching 25–35 mm diam. after 4 weeks at 25 °C, grey whitish, dense, effuse, with white hyphal stands towards the edge, rough surface towards centre, diffuse margin; reverse dark grey with whitish edges, grayish orange at the center, radiating, effuse and zonate.
Material examined: Russia, Donetsk People's Republic, Shakhtersk district, regional landscape park «Donetsk ridge» (Rus. «Donetsky kryazh»), steppe near Leontievsky forest, on dead twigs of Caragana frutex (L.) K. Koch (Fabaceae), 19 May 2017, Timur S. Bulgakov (MFLU 17-2500, holotype); ex-type living culture MFLUCC 18-0780.
GenBank numbers: MT573224 (ITS), MT573223 (LSU).
Notes: Based on phylogenetic analyses, the new asexual species Coniochaeta caraganae is closely related with C. coluteae (Samarakoon et al. 2018; Fig. 110). The asexual morph of C. coluteae is hyphomycetous and has been obtained from culture. It is characterized by hyaline vegetative hyphae, hyphoide conidiophores, phialidic conidiogenous cells and one celled hyaline conidia. Our new species C. caraganae has been obtained from dead branch of Caragana frutex and is characterized by pycnidial conidiomata, hyaline conidiophores and oblong to ovoid conidia. In a comparison of the 565 ITS (+ 5.8S) nucleotides of these two strains C. coluteae MFLUCC 17-2299 to that of C. caraganae reveals 11 (1.9%) nucleotide differences which justifies these two isolates as two distinct taxa (Jeewon and Hyde 2016). Therefore, C. caraganae is introduced herein as a new species.
Phylogenetic tree generated from Maximum likelihood analysis (RAxML) based on combined ITS and LSU sequence data of Coniochaetaceae in the order Coniochaetales. Maximum likelihood bootstrap support values ≥ 70% (in blue) and Bayesian posterior probabilities ≥ 0.95 (in green) are indicated on the branches. The new isolate is in blue. The tree is rooted with Chaetosphaeria jonesii (MFLUCC 15–1015) and Chaetosphaeria garethjonesii (MFLUCC 15–1012)
Pleurotheciales Réblová & Seifert
Notes: Pleurotheciales was introduced by Réblová et al. (2016) with Pleurotheciaceae as the type family. Wijayawardene et al. (2020, 2022) listed Pleurotheciales as a monotypic order and new taxa have been included in it (Boonmee et al. 2021; Crous et al. 2021; Dong et al. 2021).
Pleurotheciaceae Réblová & Seifert, in Réblová, Seifert, Fournier & Štěpánek, Persoonia 37: 63 (2015) [2016]
Notes: Wijayawardene et al. (2022) listed 14 genera in Pleurotheciaceae. Recently, Saprodesmium was introduced in this family for a saprobic fungus found on decaying wood submerged in freshwater in China (Dong et al. 2021). Pleurotheciaceae was introduced by Réblová et al. (2016) with Pleurothecium recurvatum as the type species. Fourteen genera viz. Adelosphaeria, Anapleurothecium, Coleodictyospora, Dematipyriforma, Helicoascotaiwania, Melanotrigonum, Monotosporella, Neomonodictys, Phaeoisaria, Phragmocephala, Pleurotheciella, Pleurothecium, Saprodesmium, and Sterigmatobotrys are accepted in the family (Hyde et al. 2020a, b, c; Boonmee et al. 2021; Dong et al. 2021; Bao et al. 2022). Species in Pleurotheciaceae are cosmopolitan with a wide range of hosts and substrates from terrestrial and freshwater habitats (Hyde et al. 2020a, b, c; Dong et al. 2021; Boonmee et al. 2021; Bao et al. 2022).
The sexual morphs of Pleurotheciaceae share perithecial, immersed to superficial, papillate ascomata, leathery to fragile, carbonaceous peridial walls, unitunicate, cylindrical, 8-spored, asci with a distinct non-amyloid apical annulus, abundant paraphyses and ellipsoidal to fusiform, septate, hyaline or versicolorous ascospores (Réblová et al. 2016; Luo et al. 2018a, b; Hyde et al. 2020a, b, c). The asexual morphs of Pleurotheciaceae have been reported as hyphomycetes forming indeterminate synnemata or loose fascicles. Conidiophores are macronematous or semi-macronematous. Conidiogenous cells produce holoblastic conidia, with rhexolytic conidial secession on short denticles or extending polyblastically on a sympodial rachis. Conidia are hyaline to brown, varied in shape, septate or aseptate (Hyde et al. 2020a, b, c, Dong et al. 2021, Boonmee et al. 2021, Bao et al. 2022).
Rhexoacrodictys W.A. Baker & Morgan-Jones, Mycotaxon 82: 98 (2002)
Rhexoacrodictys was introduced by Baker et al. (2002) to accommodate species previously identified as Acorcdictys (i.e., A. erecta, A. fimicola, A. fuliginosa and A. queenslandica) and wherein Rhexoacrodictys erecta was designated as the type. Two additional species R. martini and R. broussonetiae were subsequently added to the genus based on morphological characteristics (Delgado 2009; Xiao et al. 2018). While R. martini and R. queenslandica were transferred to Distoseptispora and Junewangia based on phylogenetic analysis (Xia et al. 2017). Currently, four species are accepted in Rhexoacrodictys (R. broussonetiae, R. erecta, R. fimicola, and R. fuliginosa).
Rhexoacrodictys erecta (Ellis & Everh.) W.A. Baker & Morgan-Jones, in Baker, Partridge & Morgan-Jones, Mycotaxon 82: 99 (2002)
Refer to Species Fungorum (2022a, b) for synonyms.
Index Fungorum number: IF381123; Facesoffungi number: FoF13392; Fig. 111
Saprobic on dead culms of bamboo. Sexual morph: Not observed. Asexual morph: Colonies on the substratum superficial, hairy, effuse, blackish, shining. Mycelium mostly immersed, cylindrical, brown to dark brown hyphae. Conidiophores 28–57 × 4–5 μm (\(\bar{x}\) = 42.5 × 4.5 μm, n = 15), macronematous, mononematous, erect, single, straight or somewhat flexuous, cylindrical, smooth-walled, brown to dark brown, septate. Conidiogenous cells monoblastic, pale brown to brown, integrated, terminal. Conidia 19–27.5 × 15–19 μm (\(\bar{x}\) = 23.5 × 17 μm, n = 30), holoblastic, solitary, dry, broad oval to subglobose, muriform, acrogenous, transversely and longitudinally septate, dark brown to black, smooth-walled, narrowly truncate at the base.
Culture characteristics: Colonies on PDA, 30 mm diam after two weeks at 28 °C, brown to blackish at the front and reverse sides, mycelium sparse; reverse blackish.
Material examined: Thailand, Chiang Rai Province, Muang District, on dead culms of Bambusa sp. (Poaceae), 11 November 2020, X. G. Tian U–2–3 (MFLU 21–0277), living culture, MFLUCC 21–0157.
Habitat: on submerged wood, Arundo donax, Bambusa multiplex, Bambusa sp., Fagus crenata, palm tree, Saccharum officinarum, Sorghum bicolor, Sporoschisma saccardoi and Zea mays (Ellis 1961; Baker et al. 2002; Zhao et al. 2011; Xia et al. 2017; Shi et al. 2021).
Distribution: Known from China, India, Japan, Sierra Leone, South Africa, Thailand, USA and Venezuela (Ellis 1961; Baker et al. 2002; Zhao et al. 2011; Xia et al. 2017; Shi et al. 2021; This study).
GenBank numbers: OL606411 (ITS), OL606151 (LSU), OL606015 (SSU).
Notes: This species was reported as Acrodictys erecta on Arundo donax in Venezuela and on Zea mays in USA by Ellis (1961). Baker et al. (2002) examined several type specimens of synonyms of this species and erected Rhexoacrodictys based on morphological analysis. Our new isolate (MFLUCC 21-0157) clustered with the strains of Rhexoacrodictys erecta (HMAS 245615, IFRD500–016 and HMAS 245616; Fig. 112). Morphologically, our new collection is similar to those of Rhexoacrodictys erecta (Ellis 1961; Baker et al. 2002).
Combined phylogeny using ITS, LSU SSU, rpb2 and tef1 of selected members of four orders of the Hypocreomycetidae. The dataset of combined ITS, LSU, SSU, rpb2 and tef1 sequence data comprise 79 strains with 4413 characters including gaps (rpb2: 1–1051 bp, tef1: 1052–2046 bp, ITS: 2047–2623 bp, LSU: 2624–3413 bp, SSU: 3414–4413 bp). Leotia lubrica (AFTOL-ID1) and Microglossum rufum (AFTOL-ID 1292) were used as outgroup taxa. RAxML and Bayesian analyses were conducted and resulted in generally congruent topologies. Bootstrap support values for ML ≥75% and BYPP ≥0.95 are given as ML/BYPPP above the nodes. Newly obtained sequences are indicated in red and ex-type strains are in bold
Based on nucleotide comparisons of ITS, LSU and SSU, our new strain (MFLUCC 21–0157) and three of Rhexoacrodictys erecta (HMAS 245615, IFRD500–016 and HMAS 245616) show no differences; however, the new strain (MFLUCC 21–0157) is different from Monotosporella seteosa (HKUCC 3712) in 14/516 bp (2.71%) of the LSU (data contains 6 gaps). Based on both phylogeny and morphology showed that our strain (MFLUCC 21-0157) is identical to Rhexoacrodictys erecta.
Phaeoisaria Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 118: 330 (1909).
Notes: Phaeoisaria is a genus of hyphomycetes (von Höhnel 1909) having morphological features mainly characterized by ‘indeterminate synnemata with aseptate or septate ellipsoidal, obovoidal, fusiform-cylindrical to falcate, hyaline conidia’ (Boonmee et al. 2021). We follow Crous et al. (2021), Boonmee et al. (2021) and Wijayawardene et al. (2022) for the classification and updated account of Phaeoisaria species.
Phaeoisaria goiasensis H.M. Silva, A.D. Cavalcanti & J.D.P. Bezerra, sp. nov.
MycoBank number: MB840294, Facesoffungi number: FoF09975, Fig. 113
Etymology: The name refers to the Brazilian state, Goiás, where the fungus was isolated.
Holotypus: UFG 71083.
Isolated from a Petri-dish with culture medium storage in a fridge. Asexual morph: Hyphae hyaline to pale brown with age, smooth wall, septate, 1.5–2.5(–3) µm wide. Synnemata erect, brown, smooth, indeterminate, composed of compactly and parallels conidiophores and commonly with conidiogenous cells in the above half, 93–147 × 3.5–4.5 µm. Conidiophores straight or slightly curved, septate, reduced to conidiogenous cell, cylindrical, hyaline to pale brown, smooth wall, (10–)15–49(–72) × (1.5–)2–2.5(–3.5) µm. Conidiogenous cells polyblastic, integrated, terminal or intercalary, cylindrical, hyaline, smooth wall, (3–)3.5–9.5(–15.5) × (1.5–)2–3(–3.5) µm, forming conidia on denticles, 1–2 µm long, 0.5–1 µm wide, scattered or clustered in the apical region. Conidia ellipsoidal to obovoid, straight or slightly curved, rounded at the ends or occasionally tapering toward the base, hyaline, aseptate, guttulate, smooth wall, (4.5–)7.5–9(–10.5) × (2–)2.5–3(–4) µm. Chlamydospores terminal, globose, pyriform, first hyaline and becoming brown to dark brown with age, (8–)8.5–10.5(–17) × (2–)7–8(–8.5) µm. Sexual morph: Not observed.
Culture Charactersistics: Colonies grew in the dark for 7 days at 25 °C. On PDA, colonies elevated, aerial mycelium absent, irregular, greyish to dark grey with edge whitish, up to 15 mm. On MEA, colonies plane, aerial mycelium absent, greyish to dark grey with edge brown to dark brown, up to 10 mm. On oatmeal agar (OA), colonies growing up to 18 mm. At 36 °C, colonies are similar to at 25 °C, growing up to 8 mm on PDA, 9 mm on MEA, and 6 mm on AO. No growth at 10 °C.
Material examined: Brazil, Goiás state, Goiânia City, Universidade Federal de Goiás (UFG), Instituto de Patologia Tropical e Saúde Pública (IPTSP), Laboratório de Micologia (LabMicol), isolated from a Petri dish with culture medium storage in a fridge, 19 November 2019, J.D.P. Bezerra & H.M. Silva (UFG 71083, holotype); ex-type living culture URM 8387 = FCCUFG 02; ibid. living culture FCCUFG 03.
GenBank numbers: URM 8387 = FCCUFG 02 –MT210320 (ITS), MT375865 (LSU), MT384422 (tub2), MT384424 (tef1)
FCCUFG 03 – MT210321 (ITS), MT375866 (LSU), MT384423 (tub2), MT384425 (tef1)
Notes: Phaeoisaria was described by von Höhnel (1909) and has 32 records in the Index Fungorum and MycoBank databases (8 June 2022). Based on our phylogenetic analysis (Fig. 114), our isolates here are proposed for the new species P. goiasensis. Phaeoisaria goiasensis differs from P. annesophieae, which was isolated from soil in The Netherlands, by the presence of synnemata in old cultures (after 30 days) and defined conidiophores in P. goiasensis, and by the size of conidiogenous cells (12–39 × 1–3.5 µm), conidia (4.5–9 × 2–3.5 µm), and chlamydospores (9–18 × 7–9.5 µm) of P. annesophieae (Crous et al. 2017). The BLASTn searches (8 June 2022) using ITS sequences of P. goiasensis demonstrated that they are identical to sequences deposited as Phaeoisaria sp. INBio 4514E, which was isolated from substrate related to Passalidae galleries in decayed trunks (Vargas-Asensio et al. 2014), and to sequences obtained from submerged wood and deposited as Ascomycota (Brown et al. 2016). The ITS sequences from P. goiasensis also had highest similarity to P. annesophieae (strain CBS 143235, GenBank MG022180.1; Identities = 500/511 (98%), 0 gap (0%)). The LSU sequences had high identity to Phaeoisaria sp. INBio 4514E and P. annesophieae MFLU 19–0531, amongst other sequences deposited as Phaeoisaria species/isolates. The tef1 sequences had high similarity to P. filiformis MFLU 18-1462 and it was also 96.59% identical to P. sedimenticola S-908. The tub2 sequences had low identity to Sordariomycetes species.
Phylogram generated from Bayesian analysis based on combined ITS and LSU rDNA sequence data of Phaeoisaria conducted in MrBayes on XSEDE in the CIPRES science gateway. Twenty-nine strains/isolates are included in the combined analysis, which comprised a total of 1435 characters (ITS = 591 and LSU = 844), including gaps. The substitution model GTR + I + G was used for ITS and LSU alignments. The tree is rooted with Pleurothecium semifecundum CBS 131271 and Pleurothecium recurvatum CBS 138747 and the scale bar indicates the number of changes. The ex-types (reference strains) are in bold and the new isolates are in blue. Maximum likelihood bootstrap (ML-BS) support values obtained with RAxML using 1000 replicates greater than 70% and Bayesian posterior probabilities (BYPP) greater than 0.95 are indicated near nodes
Pleurothecium Höhn., Ber. dt. bot. Ges. 37: 154 (1919).
Notes: Pleurothecium was introduced by Höhnel (1919) with Pleurothecium recurvatum (Morgan) Höhn as the type species. There are 11 species listed in Index Fungorum (June, 2022), and a new species, P. aquisubtropicum, is described and illustrated here (Fig. 5).
Pleurothecium aquisubtropicum J. Ma, Y.Z. Lu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559508, Facesoffungi number: FoF08709; Fig. 115
Etymology: Referring to the aquatic habitat and collecting site in subtropical country, China.
Holotype: GZAAS 21–0384
Saprobic on decaying wood in a freshwater stream. Asexual morph: Colonies on natural substrate superficial, effuse, brown or dark brown, smooth. Mycelium immersed or superficial, smooth. Conidiophores 82–177 × 3.5–5.5 μm (\(\bar{x}\) = 121.3 × 4.3 μm, n = 20), macronematous, mononematous, sucylindrical, straight, unbranched, smooth, septate, brown, paler towards the apex. Conidiogenous cells 25–38 × 2.7–3.8 μm (\(\bar{x}\) = 29.6 × 3.2 μm, n = 15), holoblastic, polyblastic, integrated, terminal, subhyaline to pale brown, subcylindrical. Conidia 13.4–15.5 × 3.4–5.5 μm (\(\bar{x}\) = 14.4 × 4.4 μm, n = 21), acrogenous, solitary, aseptate, pale brown, straight, guttulate, hyaline or pale green, smooth. Sexual morph: Not observed.
Culture Characters: Colonies growing slowly on PDA, reaching 37 mm in 35 days at 25 °C, flat, filiform, round, gray or white, smooth; In reverse, milky at the center, brown or dark brown at the margin.
Material examined: China, Guizhou Province, Xishui County, on decaying wood submerged in a freshwater stream, 13 February 2021, Jian Ma, TL2(GZAAS 21–0384, holotype); ex–type living culture, GZCC 21–0670.
GenBank numbers: OM339436 (ITS), OM339433 (LSU)
Notes: Our new collection fits well with the generic concept of Pleurothecium in having macronematous, mononematous, brown conidiophores, polyblastic, integrated conidiogenous cells and septate, smooth allantoid or fusiformis conidia. In our phylogenetic analyses, our new collection of Pleurothecium aquisubtropicum was placed within Pleurothecium and is basal to other Pleurothecium species (Fig. 116). Pleurothecium aquisubtropicum resembles P. aquaticum in the shape of the conidiophores, and conidia (Luo et al. 2018a, b). However, Pleurothecium aquisubtropicum differs from P. aquaticum by its darker and longer conidiophores (82–177 μm vs 53–65 μm) and smaller conidia (13.5–15.5 × 3–5.5 μm vs 19–21 × 4.5–5.5 μm). Hence, based on both morphology and phylogeny, we introduce our collection as a new species of Pleurothecium aquisubtropicum.
Phylogram generated from maximum likelihood analysis based on combined LSU and ITS sequence data. Twenty–six taxa were included in the combined analyses, which comprised 1420 characters (LSU: 910, ITS: 510) after alignment. The best scoring RA × ML tree with a final likelihood value of is presented. Bootstrap support values for ML ≥ 50% and BYPP ≥ 0.95 are given above the nodes. The tree is rooted with Conioscypha lignicola CBS 335.93 and C. japonica CBS 387.84
Subclass Xylariomycetidae O.E. Erikss & Winka.
Amphisphaeriales D Hawksw & OE Erikss.
Amphisphaeriales was introduced by Eriksson and Hawksworth (1986). However, Amphisphaeriales was synonymized with Xylariales by Eriksson and Hawksworth (1987). Based on morphological and molecular data, these two orders were separated and Amphisphaeriales was resurrected (Senanayake et al. 2015). The evidence for the continuation of Amphisphaeriales and Xylariales as distinct orders in Xylariomycetidae is also provided in Maharachchikumbura et al. (2016), Samarakoon et al. (2016a, b), Hongsanan et al. (2017) and Daranagama et al. (2018). Amphisphaeriales comprises with 17 families viz Amphisphaeriaceae, Apiosporaceae, Beltraniaceae, Castanediellaceae, Clypeophysalosporaceae, Cylindriaceae, Hyponectriaceae, Iodosphaeriaceae, Melogrammataceae, Oxydothidaceae, Phlogicylindriaceae, Pseudomassariaceae, Pseudosporidesmiaceae, Pseudotruncatellaceae, Sporocadaceae, Vialaeaceae, and Xyladictyochaetaceae (Hyde et al. 2020a, b, c; Wijayawardena et al. 2020).
Sporocadaceae Corda, [as 'Sporocadeae'], Icon. fung. (Prague) 5: 34 (1842).
Sporpcadaceae was first established by Corda (1842) and typified by Sporodocadus Corda (1839). The members of Sporocadaceae can be found as saprobes, pathogens, and endophytes in a variety of habitats (Nag Raj, 2993, Tanaka et al. 2011, Hyde et al. 2020a, b, c). The family contains 32 genera viz Allelochaeta, Annellolacinia, Bartalinia, Broomella, Ciliochorella, Diploceras, Disaeta, Discosia, Distononappendiculata, Diversimediispora, Doliomyces, Heterotruncatella, Hyalotiella, Hymenopleella, Immersidiscosia, Monochaetia, Morinia, Neopestalotiopsis, Nonappendiculata, Parabartalinia, Pestalotiopsis, Pseudopestalotiopsis, Pseudosarcostroma, Robillarda, Sarcostroma, Seimatosporium, Seiridium, Sporocadus, Strickeria, Synnemapestaloides, Truncatella, Xenoseimatosporium (Hyde et al. 2020a, b, c; Tibproma et al. 2020; Wijayawardene et al. 2020).
Bartalinia Tassi, Bulletin Labor. Orto Bot. de R. Univ. Siena 3: 4 (1900)
Bartalinia was introduced by Tassi (1900) and belongs to Sporocadaceae, (Hyde et al. 2020a, b, c; Wijayawardene et al. 2020). Bartalinia is morphologically similar to Heterotruncatella, Hymenopleella, Morinia, Parabartalinia, Pestalotiopsis, Pseudosarcostroma, and Truncatella. The characteristic features of Bartalinia are pycnidial conidiomata and fusiform, 3–4-septate, appendage bearing conidia with an acute or blunt apex (Senanayake et al. 2015). There are 22 Bartalinia species in the Species Fungorum (2022a, b) and a new species B. kevinhydei was introduced by Tibpromma et al. (2021). The generic and species boundaries of Bartalinia-like taxa are complicated due to overlapping morphological characteristics. Liu et al. (2019a, b, c, d) has revised the taxa with appendage-bearing conidia in Sporocadaceae based on morphology and multigene phylogeny. A new genus Parabartalinia was introduced based on Bartalinia-like taxon.
Bartalinia bidenticola Htet, Mapook & K.D. Hyde, sp.nov.
Index Fungorum number: IF559553; Facesoffungi number: FoF10766; Fig. 117
Bartalinia bidenticola (MFLU 22–0103, holotype). a,b Appearance of conidiomata on host substrate. c Section through conidioma. d Peridium. e Conidia on the conidiogenous cells. f–i Conidia. J–k Germinating conidia. l–m Culture on MEA from surface and reverse. Scale bar a = 300 µm, b = 200 µm, c = 50 µm, d = 20 µm, e = 10 µm, f,g,h,I,j,k,l,m = 20 µm
Etymology: The name refers to the host plant from which it was collected Bidens pilosa.
Holotype: MFLU 22-0103
Saprobic on dead stems of Bidens pilosa. Sexual morph: Not observed. Asexual morph: Conidiomata 120–130 × 190–200 μm (\(\bar{x}\) = 123 × 196 μm, n = 5), uniloculate, solitary, immersed to semi-immersed, globose to subglobose. Ostiole absent. Peridium 23–30 μm wide, 3–4 layered, comprised of brown cells of textura globulosa. Conidiogenous cells 1–2 μm wide, filiform, hyaline. Conidia 23–27 × 4–6 µm, (\(\bar{x}\) ± SD = 24.9 ± 0.8 × 4.9 ± 0.3 µm), fusoid to ellipsoid, straight to slightly curved at the apex, 4-septate, constricted ate the septa, brown in three middle cells and hyaline at the basal and apical cells; basal cells 2–4 µm long, hyaline, obconic to conic with a truncate base, thin-walled; three median cells 14–18 µm long, (\(\bar{x}\) ± SD = 15.8 ± 0.8 µm), (second cell from the base pale brown, 6–7 μm long; third cell pale brown, 4–6 μm long; fourth cell pale brown, 4–7 μm long), doliiform, wall rugose, versicoloured; apical cells 3–6 µm long, hyaline, subcylindrical, smooth-walled; with 2–3 tubular appendages (mostly 2), unbranched, filliform (13–)17–24(–24) µm long, (\(\bar{x}\) ± SD = 22.7 ± 2.7 µm); basal appendage single, tubular, unbranched, centric 4–8(–10) µm long.
Culture characteristics: Spores germinating on MEA within 24 h, reaching 50 mm after 7 days at room temperature, irregular, undulate, concentric, flat, leathery surface, grey.
Material examined: Thailand, Chiang Rai Province, Doi Pui, on the dead stems of Bidens pilosa Linn var radiata (Asteraceae), 10 July 2020, Zin Hnin Htet SW35 (MFLU 22-0103, holotype), ex-type living culture (MFLUCC 22-0008).
GenBank numbers: ON715467(LSU), ON715520(ITS).
Notes: Bartalinia bidenticola (MFLUCC 22-0008) resembles Bartalinia in having subcylindrical to fusoid, pale yellow septate conidia, with an apical cell modified into branched appendages. According to the BLASTn results, the closest match for the LSU sequence of Bartalinia bidenticola (MFLUCC 22-0008) was Bartalinia kevinhydei (MFLUCC 12-0384) with 97.32% similarity and the closest match for ITS sequence was Bartalinia pondoensis (CCTU 459) with 98.85% similarity. Phylogenetic analyses of a combined ITS and LSU sequence dataset (Fig. 118) show that Bartalinia bidenticola (MFLUCC 22-0008) is phylogenetically well distinguished and branched off from all other species in Bartalinia with ML = 100% and BYPP = 1.00 support. We, therefore, identify our isolate as a new species which was found from Bidens pilosa.
Phylogram generated from maximum likelihood analysis based on combined LSU and ITS sequence data for Bartalinia. Twenty taxa were included in the combined analyses, which comprised 1479 characters (LSU = 897 bp, ITS = 582 bp) after alignment. Bootstrap support values for maximum likelihood (ML) ≥ 50% and clade credibility values ≥ 0.90 from Bayesian inference analysis are labelled at each node. Ex-type strains are in bold and the new isolate is indicated in blue bold. Hymenopleella endophytica (APBSWTPF108, EML AS5-1, EML AS5-2) were used as the outgroup taxa
Bartalinia caryotae Senan., Kular. & K.D. Hyde, sp. nov.
Index Fungorum number: IF558407; Facesoffungi number: FoF 10699; Fig. 119
Etymology: Species epithet refers to the host genus.
Holotype: HKAS115853
Associated with leaf-tip spots of Caryota sp. Sexual morph: Not observed. Asexual morph: Coelomycetous. Conidiomata 55–75 × 115–140 μm (\(\bar{x}\) = 62 × 120 μm, n = 15), pycnidial, superficial or rarely erumpent, solitary, scattered, dark brown, uniloculate, conical, glabrous, ostiolate, without a papilla. Conidiomata wall 3–8 μm wide (\(\bar{x}\) = 6 μm, n = 5), comprising several layers of brown, pseudoparenchymatous cells of textura angularis, paler towards the inner layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–7 × 1.5–4 μm (\(\bar{x}\) = 5 × 3 μm, n = 10), enteroblastic, annellidic, integrated, hyaline to subhyaline, ampulliform to subcylindrical, or obclavate, aseptate, thin- and smooth-walled. Conidia 17.5–19 × 5–7 μm (\(\bar{x}\) = 18 × 6 μm, n = 20), subcylindrical, hyaline when immature, olivaceous when mature, straight to slightly curved, 4-septate, constricted at septa, smooth, with longest cell second from base, bearing appendages at both ends; basal cell 2–4 μm long, conical, hyaline, to pale brown, paler than middle cells; second cell from base 5–8 μm long, pale brown; third cell 3–5 μm long, pale brown; fourth cell 3–5 μm long, pale brown; apical cell 2–3 μm long, conical, hyaline, smooth-walled with a tubular, flexuous, divergently 3–4, unbranched, 20–30 μm long, tubular appendages arising from the tip of apical cell, with a basal 10–25 μm long, centric to eccentrically located, unbranched, flexuous, tubular to filiform appendage.
Culture characteristics: Colonies on PDA reaching 2 cm diam. after 5 days at 16 °C, flattened, circular, smooth margin, white with off-white aerial mycelia; reverse cream.
Material examined: China, Guangdong Province, Shenzhen, Nanshan District, Mountain Yangtai Forest Park, 22° 39′ 21.26″ N 113° 57′ 18.53″ E, living leaves of Caryota sp (Arecaceae), 4 October 2019, ND Kularathnage, NDK 24, (HKAS115853, holotype), ex-type living culture, ZHKUCC 21–0093; China, Guangdong Province, Shenzhen, Nanshan District, Mountain Yangtai Forest Park, 22° 39′ 21.26″ N 113° 57′ 18.53″ E, living leaves of Caryota sp (Arecaceae), 15 September 2018, IC. Senanayake, SI 43, (ZHKU 21–0005, paratype), ex-paratype living culture, ZHKUCC 21–0094.
GenBank numbers: ZHKUCC 21–0093 – MZ520792(ITS), MZ520794 (LSU).
ZHKUCC 21–0094 –MZ520793(ITS), MZ520795 (LSU)
Notes: In the combined ITS and LSU gene analysis, Bartalinia caryotae formed a distinct clade with (MP = 100 and BYPP = 1.00) support, basal to B. kevinhydei (Fig. 118). The comparison of the DNA sequence of ITS and LSU loci of Bartalinia caryotae with B. kevinhydei revealed base pair differences of 0.3% and 0.4% respectively. However, Bartalinia caryotae is morphologically different from Bartalinia kevinhydei in having small, conical conidiomata with large, 3–4 unbranched apical appendages (Liu et al. 2019a, b, c, d). Therefore, we introduce Bartalinia caryotae as a novel taxon from leaves of Caryota species and Bartalinia species are rarely reported on monocotyledon plants. Our species forms superficial to erumpent conidiomata on leaves.
Pestalotiopsis Steyaert, Bull. Jard. bot. État Brux. 19: 300 (1949).
Notes: Steyaert (1949), based on the conidial features, divided Pestalotia into three genera, namely Pestalotia, Pestalotiopsis and Truncatella. Species with 5-celled conidia (4-septate) were grouped within Pestalotiopsis. Based on multilocus phylogenetic and morphological analyses of Pestalotiopsis-like species, Maharachchikumbura et al. (2014) divided the complex into three genera: Pestalotiopsis, Neopestalotiopsis and Pseudopestalotiopsis. Pestalotiopsis is characterized by median cells concolourous, i.e. three pale-pigmented median cells. Pestalotiopsis is a complex genus and has considerable phenotypic diversity (Maharachchikumbura et al. 2014). Thus, the identification is complemented with DNA sequence data and phylogenetic analyses based on the combination of three gene regions (ITS, tub2 and tef1) (Jeewon et al. 2003; Maharachchikumbura et al. 2011, 2012, 2013; Geng et al. 2013).
Pestalotiopsis piraubensis V.P. Abreu & O.L. Pereira, sp. nov.
Index Fungorum number: IF556023; Facesoffungi number: FoF04861; Fig. 120
Pestalotiopsis piraubensis (VIC 44,199, holotype) a disease symptom on the fruit of Psidium guajava in a commercial orchard in the city of Piraúba, state of Minas Gerais, Brazil. b Colony on MEA (Malt Extract Agar) after 7 d at 25 ºC with a photoperiod of 12 h in the dark in Petri dishes (90 × 15 mm) (COAD 2165). c Colony on PDA (Potato Dextrose Agar) after 7 d at 25 ºC with a photoperiod of 12 h in the dark in Petri dishes (90 × 15 mm) (COAD 2165). d Conidioma sporulating on PDA. e Conidial masses. f Conidia (1–3 apical appendages). Scale bars: e = 50 µm, f–h = 20 µm
Etymology: Name refers to the city of Piraúba, state of Minas Gerais, Brazil, where the fungus was collected.
Holotype: VIC 44199
Asexual morph: Culture obtained by direct isolation from diseased guava fruits (Fig. 120a). On MEA, conidiomata sporodochial, globose, solitary, semi-immersed, black, exuding globose, dark brown to black conidial masses (Fig. 120d–e). Conidiophores indistinct, reduced to conidiogenous cells. Conidiogenous cells discrete, cylindrical or spathulate, hyaline, smooth-walled, 4–10 × 1.5–3 μm. Conidia fusoid, ellipsoid, straight to slightly curved, 4-septate, 25–33.5 × 5–7.5 μm, basal cell conic, hyaline, smooth and thin-walled, 4.5–7.5 μm long; three median cells doliiform, 15.5–20.5 μm long, minutely verruculose, concolourous, pale brown, septa darker than the rest of the cell (second cell from the base 5–7.5 μm long; third cell 4.5–6.5 μm long; fourth cell 5–7 μm long); apical cell 3.5–6 μm long, hyaline, cylindrical to subcylindrical, thin- and smooth-walled; with 1–3 tubular apical appendages, arising from the apical crest, unbranched, filiform, flexuous 12–25.5 μm long; basal appendage single, tubular, unbranched, centric, 2–6.5 μm long (Fig. 120f–h). Sexual morph: Not observed.
Culture characteristics: Colonies on MEA attaining 43 mm diam after 7 days at 25 °C, with a regular edge, whitish to pale yellow-coloured, with dense aerial mycelium and dark brown to black conidial masses (Fig. 120b). Colonies on PDA attaining 68 mm diam after 7 days at 25 °C, with irregular edge, whitish to pale yellow-coloured, with sparse aerial mycelium on the central surface and dark brown to black conidial masses (Fig. 120c).
Pathogenicity test: The inoculation method used consisted of the wounding of the detached fruits and on these, the mycelium plug, with the mycelial part facing the surface of the fruit was added. Causing fruit rot disease, seven days after the inoculation of healthy ripe guava fruits.
Material examined: Brazil, Minas Gerais, Piraúba, in a commercial orchard, on fruits of Psidium guajava L. (Myrtaceae), 20 January 2014, V.P. Abreu & O.L. Pereira (VIC 44199, holotype), ex-type living culture COAD 2165.
GenBank numbers: MH627381 (ITS), MH643773 (tub2), MH643774 (tef1-α).
Notes: Pestalotiopsis spp. were previously considered opportunistic pathogens that affect stressed plants (Coyier and Roane 1987). Pirone (1978) reported different species of Pestalotiopsis causing leaf spots on a range of ornamentals. However, in recent years, there has been an increase in reports of these pathogens causing widespread damage to several economically important crops (Keith et al. 2006; Ko et al. 2007; Rodrigues et al. 2014; Rosado et al. 2015; Solarte et al. 2018). Therefore, the increase of guava planting areas has contributed to the emergence of several diseases, and there is no data on the environmental requirements of Pestalotiopsis infection on guavas in Brazil, nor any studies on field epidemiology for these diseases or post-harvest management. The topology of the concatenated tree was similar to that of individual trees, thus, only the concatenated tree is presented here (Fig. 121). Molecular data showed that Pestalotiopsis piraubensis COAD 2165 did not group with any other species reported in the literature. Morphologically, P. piraubensis differs from P. trachicarpicola for presenting larger conidia. Pestalotiopsis piraubensis has 1–3 apical appendages and the other species have 2–4 (mostly 3). Pestalotiopsis kenyana and P. biciliata differ from P. piraubensis by having conidiomata pycnidial in culture on PDA. P. photinicola presents smaller conidia (18–24 × 4–5 μm) than P. piraubensis. Although most of the morphological characteristics did not differ so much, the most striking feature of P. piraubensis was the size of the conidiogenous cells, which was much smaller (4–10 × 1.5–3 μm diam), when compared to the other species. Phylogenetic analyses and morphological comparisons support the introduction of P. piraubensis as a new species within this genus. Besides that, this study may be helpful for further studies on the management of guava diseases.
Phylogram generated from Bayesian Inference analysis based on combined ITS, tub2 and tef1 sequence data for several closely related species in Sporocadaceae. Sequence data of type cultures, ex-type or ex-epitype obtained from Maharachchikumbura et al. (2014), Chen et al. (2017a, b), Akinsanmi et al. (2017), Liu et al. (2017) were included in this study. The combined genes sequence analysis included 61 taxa, which comprise total 1994 characters (557 characters for ITS, 830 characters for tub2, 607 characters for tef1-α), and outgroup taxon Neopestalotiopsis saprophytica MFLUCC 12–0282. Bayesian posterior probability are indicated at the nodes, and values ≥ 0.95 are in bold. Isolate numbers are indicated after species names. The ex-type or ex-epitype strains are in bold and black. The newly generated sequence is indicated in bold and blue
Apiosporaceae K.D. Hyde, J. Fröhl., Joanne E. Taylor & M.E. Barr, in Hyde, Fröhlich & Taylor, Sydowia 50(1): 23 (1998).
Notes: Apiosporaceae was introduced by Hyde et al. (1998), with Apiospora as the type genus. Description of Apiosporaceae has been provided in Hyde et al. (2020a, b, c), wherein the authors listed five genera in this family, Appendicospora, Arthrinium, Dictyoarthrinium and Endocalyx, Nigrospora. However, phylogenetic analysis resulted in the transfer of Dictyoarthrinium and Spegazzinia to Didymosphaeriaceae (Pleosporales) (Tanaka et al. 2015; Samarakoon et al. 2020).
Apiospora Sacc., Atti Soc. Veneto-Trent. Sci. Nat., Padova, Sér. 4 4: 85 (1875).
Notes: Apiospora is characterized by densely arranged perithecia arranged in a longitudinal stroma, clavate to broadly cylindrical asci and apiospores in the sexual morph and Arthrinium-like asexual morph (Hyde et al. 2020a, b, c). Species of this genus commonly live as endophytes, epiphytes, saprobes on grass in Poaceae (Samarakoon et al. 2022). Apiospora and Arthrinium has no clear boundary as to the morphological characteristics. However, Apiospora and Arthrinium sensu stricto were recognized in Apiosporaceae as two independent lineages in this family (Pintos and Alvarado 2021).
Apiospora guiyangensis Samarak., Jian K. Liu & K.D. Hyde, in Samarakoonet al., Fungal Diversity 112: 19 (2022).
Index Fungorum number: IF558711; Facesoffungi number: FoF 10187; Fig. 122
Apiospora guiyangensis (KUN-HKAS 125898, new host record)a Host. b, c Appearance of ascostroma on host. d, e Section through ascomata. f Peridium. g Paraphysis. h–j Asci (j is stained in cotton blue). k–m Ascospores. n Germinal spore. o Upper and reverse view of cultures on PDA at 8 days incubation. Scale bars: d = 300 μm, e = 50 μm, f, h–j, n = 20 μm, k–m = 10 μm
Saprobic on a dead twig of Bothriochloa ischaemum. Sexual morph: Stromata visible as fusiform, black, erumpent pustules with alongitudinal slit on the top. Ascomata 123–165 × 109–185 (\(\bar{x}\) = 140 × 130, n = 10) μm, subglobose, unilocular, immersed, papillate, arranged in a linear row along with the thelongitudinal slit. Peridium 11–15 (\(\bar{x}\) = 13, n = 10) μm in width, consisting of dark brown cell of textura angularis, thin at base, becoming thicker near the ostioles, with a pseudoparenchymatous wall at the most inner layer. Paraphyses 3.6–7.6 (\(\bar{x}\) = 5.2, n = 10) μm, hyaline, cylindrical, septate, guttulate. Asci 60–84 × 9.3–13 (\(\bar{x}\) = 70 × 11, n = 20) μm, fusiform, unitunicate, 8-spored, apex lacking apical mechanism, with a short basal pedicel. Ascospores 22–28 × 4.6–6.7 (\(\bar{x}\) = 25 × 5.4, n = 30) μm, hyaline, ellipsoid to reniform, slightly curve, smooth-walled, aseptate, guttulate. Asexual morph: see description in Samarakoon et al. (2022).
Culture characteristics: Colonies on PDA rapid growing, reaching 45 mm diam. after 8 days at 20–25 °C, colonies medium dense, circular, mycelium superficial in media, cottony, round aspect, white from above and reverse.
Material examined: China, Guizhou Province, Qianxinan Buyei and Miao Autonomous Prefecture, Ceheng County, Gaofeng Village, on dead culms of Bothriochloa ischaemum (Poaceae), 8 August 2018, D. P. Wei, GFC613 (KUN-HKAS 125898), living culture KUNCC22-12539.
Known hosts and distribution: Poaceae (Guizhou, China) (Samarakoon et al. 2022).
GenBank numbers: OQ029540 (ITS), OQ029613(LSU), OQ061263(SSU), OQ186444(tef1), OQ186446 (tub2).
Notes: Apiospora guiyangensis was introduced by Samarakoon et al. (2022) from an unidentified Poaceae species in Guizhou, China. Our species colonizing on dead column of Bothriochloa ischaemum (Poaceae) was collected from the same province of type strains of A. guiyangensis. Its sexual morph fits well to the description of A. guiyangensis in the linear stromata with a slit-like opening, immersed, subglobose, gregarious, ascomata and ellipsoid to reniform ascospores. Additionally, the phylogenetic analysis of a combination of ITS-LSU-tef1 -tub2 shows our isolate sisters to A. guiyangensis with ML = 100% and BYPP = 1.00 support (Fig. 123). Thus, we determine our isolate as a new collection of A. guiyangensis and this finding indicates that A. guiyangensis probably specific to Poaceae.
Xylariales Nannf.
Notes: Xylariales belongs to subclass Xylariomycetidae and the placement is confirmed by different phylogenetic and evolutionary studies (Maharachchikumbura et al. 2016; Samarakoon et al. 2016a, b; Hongsanan et al. 2017). The continuous taxonomic studies expand the number of families and genera in Xylariales. Twenty-one families and 183 genera are listed in this order (Konta et al. 2016; Dayarathne et al. 2017; Wijayawardene et al. 2022; Hyde et al. 2020a, b, c).
Diatrypaceae Nitschke, [as 'Diatrypeae'], Verh. naturh. Ver. preuss. Rheinl. 26: 73 (1869).
Notes: Diatrypaceae is a significant family in Xylariales introduced by Nitschke (1869) with the generic type Diatrype (Augusto et al. 2016; Hyde et al. 2020a, b, c; Boonmee et al. 2021). Numerous saprobic, endopytic and pathogenic diatrypaceaous taxa are available in both terrestrial and aquatic habitats worldwide (Dissanayake et al. 2021a, b; Zhu et al. 2021; Wijayawardene et al. 2020). The family is characterized by erumpent or immersed ascostromata that contain 8-spored or polysporous, long pedicel asci with allantoid ascospores in the sexual morph and coelomycetous or hyphomycetous asexual morphs (Konta et al. 2020; Carpouron et al. 2021; Dissanayake et al. 2021a, b). The early identification of Diatrypaceae species were based only on morphology. Currently most studies are performed by using both morphological observations with ITS and tub2 sequence data analyses (Konta et al. 2020; Dissanayake et al. 2021a, b; Wijayawardene et al. 2022). However, many genera of the family are polyphyletic and further taxonomic studies still need to be performed for resolving diatrypaceous taxa (Dissanayake et al. 2021a, b). There are 22 genera in Diatrypaceae with more than 1500 species (Carpouron et al. 2021; Wijayawardene et al. 2020). In this study we discuss a novel host record of Diatrypella species collected from Russia.
Diatrypella (Ces. & De Not.) De Not., Sfer. Ital.: 29 (1863)
Diatrypella was established to constitute stromatic Sphaeriales with ovoid and multi spored asci (Croxall 1950, Carpouron et al. 2021). Diatrypella was introduced by Cesati and De Notaris (1863) and typified D. verruciformis. The genus is characterized by a libertella-like coelomycetous asexual morph. In the sexual morph, conical to truncate and discoid, cushion-like stromata are delimited by black zones on the substrates, umbilicate or sulcate ostiolar necks, long-stalked, cylindrical and polysporous asci with allantoid, hyaline or yellowish ascospores (Kirk et al. 2008; Dissanayake et al. 2020; Hyde et al. 2020a, b, c). Recently several Diatrypella species were introduced by different authors from different hosts (Dissanayake et al. 2020; Hyde et al. 2020a, b, c; Zhu et al. 2021). There are 84 records under Diatrypella in Species Fungorum (2022a, b) while, only 24 taxa have molecular data in GenBank. We follow the latest treatment for Diatrypaceae in Boonmee et al. (2021) to resolve the taxonomic placements of our strain and updated phylogenetic tree is presented in Fig. 124. In this study, we discuss a new collection of Diatrypella quercina from Russia.
Diatrypella quercina (Pers.) Cooke, J. Bot., Lond. 4: 99 (1866).
Index Fungorum number: IF 215896; Facesoffungi number: FoF 11778; Fig. 124
Diatrypella quercina (MFLU 18–1865, new host record). a-c. Appearance of ascostromata on a twig of Quercus petraea subsp. polycarpa host. d. Longitudinal section of an ascoma. e. Ostiole. f. Paraphyses. g. Peridium h–k. Asci l-n. Ascospores. a = 500 μm b-c, f = 200 μm, d-e = 100 μm, h–k = 20 μm, g = 10 μm, l-n = 5 μm
Saprobic on dead twigs on Quercus robur. Sexual morph: Stromata 1.0–1.5 mm in diam., well-developed, solitary to gregarious, immersed to semi immersed, erumpent at the maturity, globose to subglobose, black. Ascomata 610–660 μm high, 550–600 μm diam. (\(\bar{x}\) = 650 × 580 μm, n = 10), perithecial, surrounded by white entostroma, 6–8 perithecia arranged in a valsoid configuration, conical, individual ostiole with a long neck. Neck 450–490 μm long (\(\bar{x}\) = 480 μm, n = 10), cylindrical, with periphyses. Peridium 20–30 μm wide (\(\bar{x}\) = 26 μm, n = 10), composed outermost layers of brown, thick-walled cells in textura angularis, inner layers hyaline, cells forming of textura prismatica. Hamathecium comprises 1–2 μm wide (\(\bar{x}\) = 1.5 μm, n = 20) paraphyses arising from base of perithecia, hyaline, long, narrow, unbranched, septate, guttulate, narrowing and tapering towards apex. Asci 70–120 × 10–15 μm (\(\bar{x}\) = 100 × 14 μm, n = 30), polysporous, unitunicate, strongly curved, apically round, with a J-apical ring, long pedicellate (50–70 μm). Ascospores 6–9 × 1.8–2.3 μm (\(\bar{x}\) = 7.5 × 2.0 μm, n = 30), overlapping, hyaline, yellowish in mass, allantoid, aseptate, guttulate, guttules conspicuous near to apex, smooth-walled. Asexual morph: see Adamčíková et al. (2013).
Material examined: Russia, Sochi, Khostinsky City District, the territory of Subtropic Scientific Centre of Russia Academy of on a dead branch of Quercus petraea subsp. polycarpa (Schur) Soó (syn. Q. colchica Czeczott, Q. iberica Steven ex M. Bieb.) (Fagaceae), Timur S. Bulgakov, 4 August 2018, T-7330 (MFLU 18–1865).
GenBank number: ON705330 (ITS), ON713468 (tub2).
Notes: Ruhland (1900) considered Diatrypella quercina as a species in Diatrype because of strongly developed ectostromata. Later, Wehmeyer (1926) discussed the possibility of including this taxon in Diatrype. Croxall (1950) distinguished D. quercina from other Diatrypella species because of its strongly curved ascospores. Cryptovalsa and Diatrypella also have polysporous asci and cannot easily be distinguished, based on morphological comparisons (Acero et al. 2004; Vasilyeva and Stephenson 2005; Dissanayake et al. 2021a, b). A number of taxonomic studies have been done for D. quercina species based on morphological observations from different countries in the world (Farr and Rossman 2022). Adamčíková et al. (2011) reported the records of Libertella quercina based on their morphology on Castanea sativa from Slovakia. Libertella quercina was also reported on bark of Quercus in England and France (Grove 1937; Adamčíková et al. 2011). Saccardo (1906) described Cytosporina quercina (basionym Libertella quercina) on branches of Quercus and Castanea in Italy, France, and Germany and later, the taxon was identified as the asexual morph of Diatrypella quercina (Grove 1937). Popov et al. (2008) reported D. quercina on Quercus robur from Russia based on morphology while our strain MFLU 18–1865, was from Q. petraea subsp. polycarpa is also known as Georgian, or Colchician oak—the native oak species for western Caucasus. However, no genetic studies were reported from Russia for this taxon. There were only two studies providing molecular data for the species from Spain (on Quercus faginea) (Acero et al. 2004; Vu et al. 2019 taken from GenBank 2022). We provided a comprehensive taxonomic study for D. quercina based on their morpho-molecular and phylogenetic analyses. In our phylogenetic analyses our strain (MFLU 18–1865) grouped with other isolates of D. quercina (CBS 108.18 and DL30M) in Clade B, with 85% MLBS, 0.95 BYPP support. Also, our strain forms a sister lineage to CBS 108.18 and grouped with high bootstrap support (95% ML). In comparison of base pair differences between CBS 108.18 and DL30M (D. quercina), 6 bp differences (1.16%) have revealed by 513 nucleotides in ITS region while tub2 regions are not available. However, we provided tub2 sequence data for D. quercina in this study. We revealed 1 and 5 pb differences from 515 nucleotides (0.19% and 0.97%) in ITS regions when comparing our strain (MFLU 18–1865) with CBS 108.18 and DL30M respectively. Trimen et al. (1866, https://www.biodiversitylibrary.org/page/16233050) provided incomplete morphology for the taxa and these characters are matched with the morphology of our strain. Based on referred morpho-molecular data we conclude that our stain should be another collection of D. quercina from Russia. This is the first genetic study on D. quercina in Russia with detailed morphology and it is also the new host record on Q. petraea subsp. polycarpa. However, detailed morphological studies are suggested in future for this taxon (Figs. 124, 125).
Phylogram generated from maximum likelihood analysis based on combined ITS and tub2 sequence data representing Diatrypaceae in Xylariales. Related sequences are taken from Boonmee et al. (2021) and additions according to the BLAST searches in NCBI. Hundred and thirty-five strains are included in the combined analyses which comprised 912 characters (517 characters for ITS and 395 characters for tub2) after alignment. Kretzschmaria deusta (CBS 826.72) and Xylaria hypoxylon (CBS 122,620) in Xylariaceae (Xylariales) were used as the outgroup taxa. Bootstrap support values for ML ≥ 75% are given above the nodes (left side). Bayesian posterior probabilities (BYPP) ≥ 0.95 are given above the nodes (right side). Ex-type strains are in bold and newly generated sequence is in red
Hypoxylaceae DC., in Lamarck & de Candolle, Fl. franç., Edn 3 (Paris) 2: 280 (1805)
Xylariales comprises with 26 families. Xylariaceae and Hypoxylaceae are two of the most diverse families in this order. Hypoxylaceae is represented by 16 genera including which comprises sexual morphs 16 and asexual morphs 11 genera (Wijayawardene et al. 2022). Hypoxylaceae are distributed in tropical, subtropical regions (Lambert et al. 2019), known for its huge species diversity and abundant bioactive secondary metabolites (Helaly et al. 2018) and plays an important ecological role in protecting host plants from pathogens (Song et al. 2022). The family is characterised by carbonised stromatal tissue. Stromata surface is usually blackened when mature, olivaceous, with %10 KOH are purplish or orange. Ostioles that are always higher than the level of stromatal surface (Cruz et al. 2021).
Hypoxylon Bull., Hist. Champ. Fr. (Paris) 1(1): 168 (1791)
Hypoxylon was introduced by Bulliard, that contains primarily saprotrophs and endophytes of angiospermous plants. The type genus Hypoxylon is the largest genus in the Hypoxylaceae, with more than 200 species (Pourmoghaddam et al. 2020). Members of the genus have a worldwide distribution, but they display a higher diversity in the tropics and subtropics (Kuhnert et al. 2014). In the twentieth century, the generic concept of Hypoxylon was based only on morphological characteristics (Ju and Rogers 1996). Currently, morphological, phylogenetic, and chemotaxonomic evidence, has also been used to infer species limits in inter- and intra-genera in Hypoxylaceae (Sir et al. 2016). Hypoxylon is quite common in China; however, the occurrence of the species in China has not been confirmed by molecular phylogenetic analyses, and the species diversity and distribution of the genus in China are unclear (Hyde et al. 2020a, b, c). The aims of this study were to confirm the taxonomic status of the new species, explore the species diversity of Hypoxylon, and infer the evolutionary relationships of Hypoxylon.
Hypoxylon inaequale S.C. He & Jayaward., sp. nov.
Index Fungorum number: IF900071; Facesoffungi number:FoF13393; Fig. 126
Hypoxylon inaequale. (HKAS123207, holotype) a Substrate. b, c Stromata showing ostioles. d, e Vertical section through stromata. f Perithecium. g Peridium. h–k asci. l paraphyses. m–q Immature to mature ascospores. r, s Germinating ascospores. t, u Culture on PDA. Scale bars: e–f, h = 200 μm, g = 100 μm i–l = 50 μm, m–s = 5 μm
Etymology: Based on the inequilateral spore character.
Holotypus: HKAS123207.
Saprobic on dead stem plant from Itoa orientalis. Sexual morph: Stromata 40–50 × 20–30 mm (M = 45 × 25 mm, n = 15), glomerate, fawn, sessile, gregarious or solitary, with white ostioles. Perithecia 250–300 × 260–450 μm (x̄ = 275 × 365 μm, n = 15), spherical to obovoid, completely immersed in stromata. Ostioles 90–120 μm diam, umbilicate, white. Paraphyses 2.5 × 3.1 μm wide, copious, filiform, aseptate, unbranched. Asci 132 − 153 × 9–11 μm (x̄ = 142 × 10 μm, n = 10), cylindrical, 8-spored, uniseriate, stipitate, with apical apparatus colorless in Melzer’s reagent. stipe 40–112 μm (n = 10). Ascospores 9.8–11.8 × 5.0–5.9 μm (x̄ = 10.45 × 5.45 μm n = 20), pale dark brown when immature, becoming dark brown with age, ellipsoid-inequilateral with round ends, unicellular, with two round guttulae, obliquely arranged. Asexual morph: Not observed.
Culture characteristics: Culture was made from germinating ascospores and was incubated with PDA media at 25 ℃, reaching 2.2–2.4 cm in 24 days. The colony white, occasionally raised, fluffy, with dense mycelia, umbonate margin, reverse chrome yellow.
Material Examined: China, Yunnan Province, Kunming City, Kunming Institute of Botany, Chinese Academy of Sciences, on Itoa orientalis Hemsl. (Salicaceae), 30 August 2021,Shu-Cheng He, HSC20B (HKAS123207, holotype); ex-type living culture, KUNCC22-10798.
GenBank numbers: ON329812 (ITS).
Notes: Based on the multi-gene phylogenetic results, our specimen is closely related to Hypoxylon delonicis (Perera et al. 2020). Based on morphology, our strain differes from Hypoxylon delonicis by having wider asci and shorter and woder ascospores. Based on a megablast search of the NCBIs nucleotide database using the multi-gene sequence, the highest similarities ITS (GenBank KU683766; Identities = 480/541(89%), Gaps = 17/541(3%)), tub2 (GenBank AY951740; Identities = 1126/1265(89%), other species of Hypoxylon delonicis with strong bootstrap support (82/0.99, Fig. 127). We identified Hypoxylon inaequale as a new species of Hypoxylon.
Phylogram generated from maximum likelihood analysis based on combined ITS, tub2 sequence data representing Hypoxylaceae in Xylariales. Related sequences are taken from (Cedeño–Sanchez 2020) and additions according to the BLAST searches in NCBI. Seventy-two strains are included in the combined analyses which comprised 1654 characters (532 characters for ITS,1122 characters for tub2) after alignment. Biscogniauxia nummularia (MUCL 51,395) and Annulohypoxylon annulatum (CBS 140775) in Xylariales were used as the outgroup taxa. Bootstrap support values for ML ≥ 70% are given above the nodes (left side). Bayesian posterior probabilities ≥ 0.95 are given above the nodes (right side). Ex-type strains are in bold and newly generated species is in red
Xylariaceae Tul. & C. Tul. [as 'Xylariei'], Select. fung. carpol. (Paris) 2: 3 (1863).
Notes: Xylariaceae is the type and largest family of Xylariales (Hyde et al. 2020a, b, c). Number of genera in this family has been subjected to multiple revision in different articles. Kirk et al. (2001) and Eriksson (2007) estimated that there are 75 genera and about 800 species in Xylariaceae. Hyde et al. (2020a, b, c) counted 32 genera in this family and provided notes for each accepted genus. Wijayawardene et al. (2022) listed 38 genera in Xylariaceae, without giving supported annotation. Xylariaceae can live as saprobes, pathogens, or endophytes, habiting wood, leaves and fruits (Hyde et al. 2020a, b, c). Xylariaceae is characterized by perithecia embedded in erect, applanate or effuse-pulvinate, dark-coloured stromata, cylindrical asci with an amyloid apical ring, brown to black, 1–2-celled, ellipsoidal, subglobose or reniform, with with germ slits or pores (Tang et al. 2009; Maharachchikumbura et al. 2016; Hyde et al. 2020). Xylariaceae was divided into two subfamilies including Xylaroideae and Hypoxyloideae based on their respective anamorphic types, their stromatal pigments and secondary metabolites. Xylaroideae generally is considered to comprise species with Nodulisporium-type anamorph and KOH+ stromatal pigments, while it is Geniculosporium-type and KOH− in Hypoxyloideae. Multigene phylogenetic analysis based on LSU, SSU and rpb2 sequence well reflect these two subfamilies (Samarakoon et al. 2016a, b).
Astrocystis Berk. & Broome, J. Linn. Soc., Bot. 14(no. 74): 123 (1873) [1875].
Notes: Astrocystis was introduced by Berkeley and Broome (1850) and typified by Astrocystis mirabilis. Index fungorum (2022a, b) lists 28 epithets in Astrocystis, while four of them have been excluded from this genus. Species of this genus mainly exist in saprobes on monocotyledonous substrates, such as bamboo, palm and Smilax (Laessøe and Spooner 1993). Astrocystis is characterized by uni- to multi-peritheciate stromata, carbonaceous peridium, asci with a relatively short stipe and small, amyloid, stopper-shaped ascal ring (Pinnoi et al. 2010). It is widely accepted that Astrocystis bear similar morphology with Rosellinia, while the former genus can be distinguished from the latter by small and cylindrical or funnel-shaped apical ring of asci. Rosellinia has conspicous, barrel-shaped apical apparatus (Dulymamode et al. 1998).
Astrocystis bambusicola R.H. Perera & K.D. Hyde, in Hyde et al., Fungal Diversity 87: 173 (2017).
Index Fungorum: IF553799; Facesoffungi number: FoF10187; Fig. 128
Astrocystis bambusicola (KUN-HKAS 125897, new record) a Substrate. b, c. Ascomata. d Section through ascoma. e–h Asci. i Paraphysis. j Asci with J.+ apical ring. k Ascospore. l, m Upper and reverse view of cultures on PDA at 8 days incubation. n Germinating spore. Scale bars: d, e = 50 μm, f–j = 30 μm, k, n = 10 μm. (j stained in Melzer's reagent)
Saprobic on dead culms of Microstegium sp. appearing as black raised spots on the host. Sexual morph: Ascomata up to 173 μm in width and 135 μm in high, subglobose, black, perithecial, gregarious, superficial, 1–2-loculate, with flattened top and projecting papillas. Peridium 43–60 (x̄ = 50, n = 10) μm, composed of membranous inner wall and black, fragile, carbonaceous outer wall. Paraphyses 3.8–8.3 (x̄ = 6.2, n = 25) μm, septate, cylindrical, hyaline, unbranched, guttulate. Asci 84–121 × 6.8–11 (x̄ = 99 × 8.8, n = 30) μm, 8-spored, unitunicate, cylindrical, with a short pedicel and J+ apical ring. Ascospores12–15 × 5.7–8 (x̄ = 14 × 6.5, n = 50) μm, reniform, dark brown to black, uniseriate, aseptate, guttulate, smooth-walled, with a germ-slit, without a gelatinous sheath. Asexual morph: Not observed.
Culture characteristics: Culture was made from germinal ascospores that germinated on PDA within 24 h, at 23–25 °C. Colonies rapidly growing on PDA, reaching 30 mm at 8 days, white from above and reverse, cottony, circular, umbonate, edge irregular.
Material examined: China, Guizhou Province, Qianxinan Buyei and Miao Autonomous Prefecture, Ceheng County, Gaofeng Village, on dead culms of Microstegium sp. (Poaceae), 8 August 2018, D. P. Wei, GFC612 (KUN-HKAS 125898), living culture KUNCC 22-12539.
Known hosts and distribution: Bamboo (Yunnan, China; Thailand) (Hyde et al. 2017, 2020a, b, c).
Genbank numbers: OQ029540 (ITS), OQ029613 (LSU), OQ061263 (SSU), OQ186444 (tef1), OQ186446 (tub2).
Notes: Astrocystis bambusicola has been reported on bamboo column from China and Thailand (Hyde et al. 2017, 2020a, b, c). Our isolate phylogenentically groups with Astrocystis bambusicola with great support (100% ML/1.00 BYPP, Fig. 129). Morphologically our isolate bears resemblance with Astrocystis bambusicola in the subglobose, black, superficial ascomata, carbonaceous peridium, cylindrical asci with J+ apical ring and reniform, dark brown to black ascospores with a germ-slit. We introduce our isolate as a new host record species of Astrocystis bambusicola from Microstegium sp. in Guizhou Province, China.
Xylaria Hill ex Schrank, Baier. Fl. (München) 1: 200 (1786).
Notes: Xylaria is one of the largest genera in Xylariaceae and it includes more than 600 species (Hyde et al. 2020a, b, c; Boonmee et al. 2021). Its occurrence in diverse environments shows its unique role as saprobes, endophytes and as plant pathogens (Edwards et al. 2003; Ju et al. 2018; Hyde et al. 2020a, b, c). Xylaria species mostly have long stalked thread-like macro structures. A few species of this genus coexist with plants as endophytes (Chen et al.2013), which may later turn into saprobes (Promputtha et al. 2007) when plants die. They also extend their habits as coprophilous and endolichenic (Piasai and Manoch 2009; Cañón et al. 2019). Most Xylaria species also serve as economically important compound producers (Ratnaweera et al. 2014; Adeleke and Babalola 2021; Wangsawat et al. 2021; Becker and Stadler 2021), which act as antibacterial, antifungal and/or biocontrol agents. Therefore, there is a need for the discovery of novel species and new geographical records of this genus. Annually ten or more, new species are introduced to this genus. For instance, in 2020, ten new species were discovered and in the next year, 15 species were discovered (Index Fungorum 2022a, b) by morphology and molecular data.
Xylaria venosula Speg. Boletín de la Academia Nacional de Ciencias en Córdoba 11 (4): 511 (1889).
Index Fungorum number: IF 247711; Facesoffungi number: FoF09866; Fig. 130
Xylaria venosula (Herbarium AMH-10068, new record) a, d, e Stromata on decaying host, b Vertical section of stromata c Horizontal section of stromata f Paraphyses, g-i Asci j Textura intricata k Peridium, l m Germinating spore n, o Culture on MEA plates, p Hypha. Scale bars: k = 50 μm, f-i = 20 μm. j, m, n, p = 10 μm
Saprobic on decaying twig. Sexual morph: Ascostromata 1 cm long, superficial, aggregated in clusters, surface undulated, rarely with parallel cracks, with acute apices. Ascomata 340–385 × 370–450 μm (\(\bar{x}\) = 362 × 406 μm, n = 5), globose, erumpent, pulvinate, with central periphysate necks 90–120 × 60–100 μm (\(\bar{x}\) = 101 × 75 μm, n = 5). Peridium 32 μm wide, with brown to hyaline, textura porrecta cell layers. Hamathecium: paraphyses septate, branched, 2.8 μm wide, longer than asci, sparsely present. Asci 110 –132 × 5.6–8 μm (\(\bar{x}\) = 117.5 × 6.6 μm, n = 25), unitunicate, 8–spored, cylindrical, apically rounded with J + apical rings, rings 2.6–3.6 × 1.7–2.3 μm (\(\bar{x}\) = 3.2 × 2 μm, n = 25), long-pedicellate, persistent. Ascospores 12.5 –15.5 × 4.7–7 μm (\(\bar{x}\) = 13.6 × 5.7 μm, n = 25), overlapping uniseriate, hyaline to brown at maturity, oblong to navicular, with straight germ slits, uni-guttulate, obtuse ends, smooth-walled. Asexual morph: Not observed.
Distribution: Brazil, China, Ecuador, India and USA.
GenBank numbers: MZ292933 (ITS).
Material examined: India, Andaman and Nicobar Islands, South Andaman, Mount Harriet, (11° 71′ 09.8″ N 92° 73′ 30.6″ E), recorded on an unidentified decaying log, 7 December, 2017, M. Niranjan and V. V. Sarma (PUFNI 1763). Herbarium submitted in Ajrekar Mycological Herbarium-AMH (AMH-10068) and Living culture (NFCC-4520) deposited in National Fungal Culture Collection of India (NFCCI), Pune.
Notes: The references for descriptions of X. venosula could be found in Index Fungorum (https://www.biodiversitylibrary.org/page/2937143#page/534/mode/1up) and the global fungal red list (http://iucn.ekoo.se/iucn/species_view/247711). The present taxon has morphological characteristics that are similar to the type and other with slight differences. The present collection consists of paraphyses, smaller asci (109 –132 × 5.6–8 vs. 90–400 × 7–9) and oblong to navicular, smaller ascospores (12.5 –15.5 × 4.7–7 vs. 14 –18 × 6–7 μm). Geographical distribution of X. venosula in five countries mentioned in distribution (https://www.gbif.org/occurrence/search?q=Xylaria%20venosula&taxon_key=5487903) and this is the first report of X. venosula from the Andaman Islands, India and as such the present collection extends the geographical distribution and range of this taxon globally (Fig. 131).
Maximum parsimony tree generated from by using the ITS sequences belongs to Xylariaceae species. The tree includes the ML, MP and BYPP values. In the phylogenetic tree the sequence analysis of Xylaria venosula NFCC-4520 (black bold letters) is shown with other species of Xylaria and Rosellinia australiensis as out-group
Basidiomycota R.T. Moore.
We follow the latest treatments of Basidiomycota in Zhao et al. (2017), He et al. (2019) and Wijayawardene et al. (2022).
Agaricomycotina Doweld.
Agaricomycetes Doweld.
Agaricales Underw.
Agaricaceae Chevall.
Notes: Agaricaceae was erected by Chevallier (1826) based on the type genus Agaricus L. Previously, this family contained only gilled fungi. However, research-based on molecular data transferred many non-gilled fungal families such as Lycoperdaceae, Nidulariaceae and Tulostomataceae to Agaricaceae. Now, it is represented by more than 1300 species belonging to 85 genera (Kirk et al. 2008; He et al. 2019; Wijayawardene et al. 2022). Some gasteroid pufball genera included in the Agaricaceae are Arachnion Schwein., Bovista Pers., Calvatia Fr. and Lycoperdon Pers.
Chlorophyllum Massee, Bull. Misc. Inf., Kew (no. 138): 135 (1898).
Chlorophyllum (Agaricaceae, Agaricales) was introduced by Massee (1898) with C. molybdites (G. Mey.) Massee as the type species. Chlorophyllum species are widely distributed in tropical, subtropical, and temperate areas throughout the world as saprobes (Ge and Yang 2006; Kirk et al. 2008; Crous et al. 2015a, b, c; Ge et al. 2018; Dutta et al. 2020). This genus is characterized by agaricoid, secotioid or sequestrate habits, a hymenidermal pileus covering and smooth stipe, white, green or brown basidiospores either lacking a germ pore or with a germ pore that is caused by a depression in the episporium (Ge and Yang 2006; Vellinga 2002, 2003a, 2004; Crous et al. 2015a, b, c; Loizides et al. 2020). Most Chlorophyllum species are known to be poisonous (Vellinga and de Kok 2002; Leudang et al. 2017). There are 28 accepted species of Chlorophyllum in Index Fungorum (2022a, b). Chlorophyllum is divided into six infrageneric sections: Chlorophyllum Massee, Ellipsoidospororum Z.W. Ge, Endoptychorum (Czern.) Z.W. Ge, Parvispororum Z.W. Ge, Rhacodium Z.W. Ge and Sphaerospororum Z.W. Ge based on morphological and phylogenetic analyses (Ge et al. 2018). Only four Chlorophyllum species, C. globosum (Mossebo) Vellinga, C. hortense (Murrill) Vellinga, C. molybdites (G. Mey.) Massee and C. rhacodes (Vittad.) Vellinga, have been reported from Thailand (Chandrasrikul et al. 2011; Leudang et al. 2017; Ge et al. 2018; Sysouphanthong et al. 2021; Suwannarach et al. 2022).
Chlorophyllum squamulosum A.K. Dutta, Soumili Bera & K. Acharya, Phytotaxa 451: 121 (2020).
Index Fungorum number: IF835117; Facesoffungi number: FoF10684. Fig. 132
Basidiomata agaricoid, medium to large. Pileus 50–75 mm in diam., convex to broadly convex, often with a shallow central depression, sometimes with an upturned margin with age; surface white to cream, covered with squamules, entire at the disc, elsewhere disrupting in some specimens, mostly small plate-like, arranged in a concentric manner from center towards the margin, flat or curved upwards, greyish brown (8E3) to reddish-brown (8E4) or dark brown (8F5) at the center, elsewhere greyish brown (8D3) to brownish grey (7C2) or dull red (9C3). Lamellae 4–6 mm broad, adnexed, crowded with two series of lamellulae, white to cream, concolorous; edge even to slightly wavy, yellowish with KOH. Stipe 60–90 × 9–12 mm, central, cylindrical, gradually broader towards the base, at base 15–27 mm wide and bulbous to subbulbous, hollow, white (6A1), turning brown (6D6–6E7) on bruising or with KOH. Annulus double; upper portion concolorous with the stipe surface; lower portion white to cream, sometimes with greyish brown (8D3) to reddish-brown (8E4) at the margin, rarely with a brownish border; edge sometimes floccose.
Basidiospores 7.5–12 × 5–7.5 μm (n = 50), Q = 1.25–1.85, Qm = 1.5 ± 0.13, ellipsoid, smooth, hyaline, dextrinoid, with a prominent wide germ-pore, truncated, thick-walled; apiculus short, 0.5–1 μm long. Basidia 25–50 × 8–10 μm, cylindrical to clavate or subclavate, thin-walled, 4-spored, sterigmata upto 10 μm long, cylindrical. Pleurocystidia absent. Cheilocystidia 17–25 × 7.5–12 μm, clavate or spheropedunculate, hyaline, thin-walled. Annulus hyphae 3–7 μm broad, tightly arranged, hyaline, often branched, hyphal end obtuse to clavate, thin-walled. Pileipellis (pileal squamules) a tightly packed hymeniderm, with cylindrical and flexuous, or narrowly clavate terminal elements, measuring 5–12.5 μm broad, with pale brown intracellular pigments, often incrusted, sometimes branched, thin-walled. Pileus trama hyphae 7–14 μm broad, incrusted, thin-walled. Stipitipellis hyphae 5–10 μm broad, parallel to subparallel, light yellowish with KOH, sometimes branched, non-incrusted, thin-walled. Stipe trama hyphae 5–12 μm broad, incrustations present, parallel to subparallel, thin-walled. Caulocystidia absent. Clamp connections absent in all the tissues.
Material examined: Thailand, Chiang Mai Province, Muang District, Chiang Mai University, 18°48′2″N 98°57′18″E, elevation 335 m, solitary on soil in grassland, 3 August 2019, J. Kumla, SDBR-CMUNK0585, 18°48′14″N 98°57′15″E, elevation 333 m, solitary on sandy humus mixed soil, 26 July 2020, J. Kumla, SDBR-CMUNK0731.
Habitat: Solitary, on sandy humus mixed soil in the dry deciduous forests and grassland.
Distribution: Known from India and Thailand (Dutta et al. 2020; this study).
GenBank numbers: SDBR-CMUNK0585- MZ4502085 (ITS), MZ452086 (LSU)
SDBR-CMUNK0731- MZ4502084 (ITS), MZ452070 (LSU)
Notes: Chlorophyllum squamulosum belongs to the Chlorophyllum section Rhacodium based on a combination of morphological and molecular data (Fig. 133). Morphologically, C. squamulosum is similar to C. nothorhacodes, C. rhacodes, C. olivieri and C. brunneum by its brownish to reddish colouration of the stipe upon bruising, truncated basidiospores and clavate cheilocystidia. The phylogenetic tree indicated that C. squamulosum formed a sister taxon to C. nothorhacodes. However, C. nothorhacodes differs from C. squamulosum by its much larger basidiocarp (pileus of up to 280 mm in diam. and stipe up to 250 mm × 25–60 mm) (Vellinga 2003a). Furthermore, C. rhacodes differs from C. squamulosum by its broader basidiospores (9.8–11.1 × 6.3–7.7 μm), comparatively larger cheilocystidia (16–43 × 8.5–25 μm) and the presence of clamp connections in the basidia, cystidia and tramal hyphae (Vellinga 2001, 2003b). Chlorophyllum olivieri has clamped basidia and broader cheilocystidia (35–45 μm) when compared with C. squamulosum (Vellinga 2001). Chlorophyllum brunneum differs from C. squamulosum by the presence of clamp connections at the base of the basidia and cystidia (Bougher and Syme 1998; Vellinga 2002, 2003a).
Phylogenetic tree derived from maximum likelihood analysis of a combined ITS and LSU genes of 38 sequences and the aligned dataset was comprised of 1613 characters including gap. The average standard deviation of the split frequencies of the BI analysis was 0.00481. Agaricus megacystidiatus (MFLU 12–0137) and Xanthagaricus flavosquamosus (GDGM50918) were used as outgroup taxa. The numbers above branches are the bootstrap statistics percentages (left) and Bayesian posterior probabilities (right). Branches with bootstrap values ≥ 70% are shown at each branch and the bar represents 0.1 substitutions per nucleotide position. Hyphen (-) represents support values ≤ 70%/0.90. Ex-type strains are in black bold. The newly generated sequences are indicated in blue
Lepiota (Pers.) Gray, Nat. Arr. Brit. Pl. (London) 1: 601 (1821).
Lepiota belongs to Agaricaceae, and is consisted of 450 species (He et al. 2019). Vellinga (2001) accepted Lepiota for six sections based on the morphology, which are Sect. Echinatae Fay., Sect. Fuscovinaceae Bon & Candusso, Sect. Lepiota (Pers.) Gray, Sect. Lilaceae M. Bon, Sect. Ovisporae (J.E. Lange) Kühner and Section Stenosporae (J.E. Lange) Kühner. However, the genus is not monophyletic according to many molecular studies (Vellinga 2003a, b; Liang et al. 2011; Hou and Ge 2020). In this study, three species of Lepiota are recorded for the first time in Laos, and a new species is described from Thailand.
Lepiota metulispora (Berk. & Broome) Sacc., Syll. fung. (Abellini) 5: 38 (1887).
Index Fungorum number: IF461315; Facesoffungi number: FoF 09887; Figs. 134 , 135
Pileus 30–65 mm, sub umbonate to umbonate, expanding to plano-concave, with inflexed margin; greyish orange to brownish orange (6B5-8, 6C7-8) glabrous at umbo, with concolorous squamules toward margin on white fibrillose background; margin sulcate, with partial veil remnants, with concolorous squamules on surface. Lamellae free, broadly ventricose, 3–5 mm wide, white, moderately crowded, with 3 length lamellulae, with eroded edge. Stipe 60–80 × 5–4 mm, cylindrical or slightly tapering to apex, completely fibrillose or cortinate at annular zone, white, with white to greyish orange to brownish orange (6B5-8, 6C7-8) squamules under annular zone downward base, on white to orange-white (5A2) background. Annulus an annular zone, cortinate with white fibrils. Context in pileus white, up to 4 mm wide; in stipe hollow, concolorous with surface. Smell and taste unknown. Spore print white.
Basidiospores [50,2,2] 13–16.5 × 4–5 µm, avl × avw = 17.7 × 4.7 µm, Q = 2.91–3.30, Qav = 3.20, in side-view cylindrical, with attenuate or rounded apex, with straight abaxial side, with an inflexed hilar appendage, with suprahilar depression, fusiform to cylindrical in frontal view, slightly thick-walled, hyaline, dextrinoid, congophilous. Basidia 22–33 × 7–9 µm, clavate, slightly thick-walled, hyaline, 4-spored. Cheilocystidia 15–35 × 5–20 µm, clavate to broadly clavate, sometimes utriform, branched or with septate under element cell, thick-walled, hyaline. Pileus covering a trichoderm made up of two layers of element; upper layers made up of cylindrical elements with rounded or attenuate apex, 70–190 × 5–13 µm, hyaline to pale brown, slightly thick-walled, smooth-walled, with parietal pigment; under layers made up of shortly clavate to clavate elements, 35–50 × 8–14 µm, smooth or rough-walled, with hyaline to parietal pale brown pigment. Stipe covering of squamules similar to pileus covering. Clamp connections present.
Material examined: Laos, Oudomxay Province, Xay District, Houay Houm Village, N 20° 32′ 00.67″, E 101° 53′ 48. 17.16″. 917 m., 15 June 2014, P. Sysouphanthong, PS2014-1465 (HNL503136); ibidem, 20 July 2014, P. Sysouphanthong, PS2014-1480 (HNL503151).
Habitat and distribution: solitary or grow in a small cluster with few basidiomes, on dead leaves and humus soil, saprotrophic. The species was only reported from tropical regions viz. Sri Lanka (Pegler 1972), India (Kumar and Manimohan 2009), China and Hong Kong (Liang et al. 2011), Tanzania (Pegler 1977), Thailand (Sysouphanthong et al. 2012). This is the first report of L. metulispora in northern Laos.
GenBank numbers: HNL503136–MT436261 (ITS).
HNL503151–MT436262 (ITS).
Notes: Lepiota metulispora has a trichodermal pileus covering and penguin-shaped basidiospores, and it is placed in the section Lepiota (Vellinga 2001). The species is widespread in tropical countries. Lao specimens were found in the mature stage, colour of squamules on pileus is paler than Thai specimens, but the morphology is identical (Sysouphanthong et al. 2012). The type specimen of the species from Sri Lanka is closer to Lao and Thai specimens in morphology, but basidiospores are slightly larger and cheilocystidia are undetermined (Pegler 1972). Liang et al. (2011) studied the type material from Sri Lanka and compared it with the Chinese specimen, and the type specimen has a smaller basidiospore size. However, it seems that the size of basidiospores is not much different in all specimens found in China, Laos, Sri Lanka and Thailand; and the morphology and size can be minorly different in different specimens. According to the analysis of nrITS sequence data (Fig. 136), Lao specimens are identical to specimens from China with high (100%) bootstrap support.
Maximum likelihood phylogenetic tree based on nrITS sequences of Lepiota species. Bootstrap support ≥ 70% is indicated at the nodes. New sequences from this study are in blue. The GenBank accession numbers are indicated after the species name. Abbreviation L = Lepiota, M = Macrolepiota. The tree is rooted in Macrolepiota orientiexcoriata
Lepiota thrombophora from Thailand is most similar to L. metulispora in morphology, but differ in smaller basidiospores size (10–14 × 3–5 μm) (Hyde et al. 2021); and the type specimen of L. thrombophora from Sri Lanka is different in dark brown squamules on pileus and shorter elements of pileus covering (25–100 × 5–15 µm). Liang et al. (2011) described L. thrombophora from China, based on its longer elements (up to 330 µm long). The analysis of nrITS sequence data showed that Chinese and Thai specimens of L. thrombophora are identical, and related to L. metulispora (Fig. 136). The type specimen of L. attenuata from China is closer to L. metulispora in morphology but differs in much longer elements on pileus covering (231 µm long), and basidiospores are more attenuated at the apex (Liang et al. 2011). Thai specimens of L. attenuata from Thailand have longer and typically attenuate elements (300 µm long) (Hyde et al. 2021). Other similar species to L. metulispora were discussed in Sysouphanthong et al. (2012).
Lepiota pongduadensis Sysou., Hyde & Vellinga in Sysouphanthong et al., Cryptog. Mycol. 33(1): 37 (2012).
Index Fungorum number: IF519961; Facesoffungi number: FoF 09886 Figs. 137 , 138
Pileus 30–45 mm, campanulate, expanding to convex or umbonate with small umbo, applanate with low umbo, straight margin; glabrous to rough at umbo, brown to dark brown (6E5-8, 7F7-8), with light brown to brown (7D6-8, 7E7-8) glabrous around umbo, later surface broken in radial streaks from around umbo towards the margin, with concolorous tomentose to crowded squamules towards the margin, on white to yellowish-white (4A2) black ground; marginal zone broken, split, fringed, cortinate with write bibrils and light brown to brown (7D6-8, 7E7-8) partial veil remnants. Lamellae free, slightly crowded, ventricose to broadly, 3–5 mm wide, with 2 length lamellulae, white to yellowish-white (4A2), with concolorous eroded edge. Stipe 35–45 × 4–6 mm, cylindrical, fibrillose or cortinate at middle zone, then with light brown to brown (7D6-8, 7E7-8) squamules downwards base, with white to yellowish-white (4A2) black ground from middle to apex, with reddish-white (7A2) below middle zone downwards base. Annulus an annular zone, cortinate with white fibrils. Context white and up to 3 mm wide in pileus; hollow and concolorous with surface. Smell and taste unknown. Spore print white.
Basidiospores [50,2,2] 11.5–16.5 × 4–5.5 µm, avl × avw = 13.4 × 4.6 µm, Q = 2.8–3, Qav = 2.91, in side-view cylindrical amygdaliform, with attenuate apex, with straight abaxial side, with hilar appendage, with superhilar depression, in frontal view fusiform, hyaline, slightly thick-walled, dextrinoid, congophilous. Basidia 16–26 × 7–9 µm, clavate, hyaline, thick-walled, 4-spored. Cheilocystidia abundant, 27–35 × 6.5–15 µm, mostly fusiform or clavate, sometimes utriform, thick-walled, hyaline. Pileus covering a trichoderm made up of narrowly cylindrical elements, normally wider at middle and narrow to apex, with attenuate apex 70–400 × 6–13.5 µm, thick-walled, with brown parietal and intracellular pigment, smooth, sometimes incrusted at base of element and hyphae. Stipe covering of squamules a trichoderm similar to pileus covering. Clamp-connections present.
Material examined: Laos, Oudomxay Province, Xay District, Houay Houm Village, N 20° 32′ 00.67″, E 101° 53′ 48. 17.16″. 917 m., 10 July 2014, P. Sysouphanthong, PS2014-1460 (HNL503131); ibidem, 12 August 2014, P. Sysouphanthong, PS2014-1479 (HNL503150).
Habitat and distribution: Growing solitary to a small group; saprotrophic and terrestrial on humus soil; originally described from northern Thailand (Sysouphanthong et al. 2012). This is the first report of L. pongduadensis in Laos.
GenBank numbers: HNL503131–MT436259 (ITS).
HNL503150–MT436260 (ITS).
Notes: Lepiota pongduadensis is a new record from Laos; two Lao specimens were collected from Oudomxay Province of northern Laos, and they show similar morphology and nrITS sequences to the type specimen (Fig. 136). Lepiota pongduadensis was originally described from Chiang Mai and Chiang Rai Provinces of northern Thailand. The species is placed in the section Lepiota by Sysouphanthong et al. (2012) and is distinguished from other species in the section. Only a few species are similar to L. pongduadensis (Sysouphanthong et al. 2012). Lepiota attenuata, the type specimens from China, is similar to L. pongduadensis in morphology, but different in lacking a dark brown surface on umbo, lighter colour of squamules on pileus, and stipe covering (brownish-yellow to yellowish-brown), larger basidiospores (14.5–19 × 4–5.5 mm), shorter elements of pileus covering (80–231 × 3.8–13 mm) (Liang et al. 2011).
Lepiota subthailandica Sysouph., K.D. Hyde & Thongkl., sp. nov.
MycoBank no: MB839987, Facesoffungi number: FoF 09889; Figs. 139 , 140
Etymology: the morphological characteristics of this species are similar to L. thailandica.
Holotype: MFLU 10–0616
Diagnosis: similar to L. thailandica in morphology, but the difference in larger basidiomata, lacking of utriform or fusiform cheilocystidia and nrITS sequences.
Pileus 10–16 mm diam., convex to umbonate, expanding to plano-concave, with straight margin; rough or with crowded squamules at center, light brown to brown (7D6-8, 7E7-8) at center, with concolorous squamules around umbo towards margin, slightly distant at marginal zone on white to orange-white (5A2) background; margin broken, sulcate or striate, appendicular, with concolorous squamules on the surface and white fibrillose remnants. Lamellae free, broadly ventricose, 3–4 mm wide, white, moderately crowded, with 1 length lamellulae, with eroded edge. Stipe 25–30 × 3–4 mm, cylindrical, slightly wider at base; completely fibrillose, crowded at annular zone down toward base, white, with light brown to brown (7D6-8, 7E7-8) squamules at base zone, on white to orange-white (5A2) background. Annulus with an annular zone or cortinate with white fibrils. Context white in pileus, up to 1 mm wide; hollow in stipe, concolorous with surface. Taste and smell unknown. Spore print white.
Basidiospores [50,2,2] 5–6 × 3.2–4 µm, avl × avw = 5.54 × 3.64 µm, Q = 1.45–1.57, Qav = 1.52, in side-view ellipsoid ovoid, in frontal view ovoid to, slightly thick-walled, dextrinoid, congophilous, cyanophilous, not metachromatic. Basidia 15–18 × 5–7 µm, clavate, slightly thick-walled, hyaline, 4-spored. Cheilocystidia 18.5–29 × 5–10 µm, narrowly clavate to clavate, rarely broadly clavate, slightly thick-walled, hyaline. Pleurocystidia absent. Pileus covering a trichoderm made up of two layer of elements; upper layer made up of cylindrical to narrowly cylindrical elements with rounded apex, sometimes swollen at middle and tapering to base and apex, 60–140 × 9–17.5 µm, with pale brown parietal and intracellular pigment; underpayer made up of shortly clavate elements, 30–45.0 × 10.0–20 µm, with pale brown parietal and intracellular pigment. Stipe covering of squamules at base zone similar to pileus covering. Clamp connections present in all tissues.
Material examined: Thailand, Chiang Mai Province: Mae Taeng district, Pha Deng village, N 19° 07.13′, E 98° 43.52′, 905 m, 04 July 2010, P. Sysouphanthong P98 (MFLU 09–0616, holotype); ibidem, 25 July 2008, P. Sysouphanthong PS093 (MFLU 09–0166, paratype).
Habitat and distribution: solitary, saprotrophic, on decayed humus soil; found in high elevation deciduous forests of northern Thailand.
GenBank numbers: MFLU 09–0616–MT436254 (ITS).
MFLU 09–0166–MT436253 (ITS).
Note: Lepiota subthailandica has a tiny basidioma, a trichodermal structure of pileus covering and ellipsoid ovoid basidiospores; and the species is located in Lepiota sect. Ovidsporae (J.E. Lange) Kühner (Vellingar 2001). In the same section, Lepiota subthailandica is very similar to L. thailandica Sysouph., K.D. Hyde, J.C. Xu & P.E. in morphology; and they are widespread in the same location of Chiang Mai, northern Thailand. However, L. thailandica has smaller basidiomata (3–4 mm diam. in pileus), utriform and fusiform cheilocystidia, and shorter elements of pileus covering (55–95 × 5.5–22 µm) (Sysouphanthong et al. 2016). The second species, L. microcarpa Sysouph., K.D. Hyde & Vellinga, is similar in micromorphology with L. subthailandica. However, L. microcarpa has penguin-shaped basidiospores, and belongs to sect. Lepiota (Sysouphanthong et al. 2012). Based on the nrITS sequences analysis, two samples of L. subthailandica are not related to L. thailandica, and are separated from other species in the sect. Ovisporae (Fig. 136).
Lepiota subvenenata Hai J. Li, Y.Z. Zhang & C.Y. Sun.
Index Fungorum number: not found; Facesoffungi number: FoF 09888; Figs. 141 , 142
Pileus 30–50 mm diam., first subglobose, expanding to parabolic to convex or umbonate, plano-convex when mature, with straight margin; when young glabrous to granulose, completely light brown to brown (7D7-8), when mature surface breaking and leaving concolorous glabrous or granulose umbo, with concolorous squamules around umbo towards margin, on white to orange-white (5A2) background; margin with concolorous squamules and white partial veil remnants. Lamellae free, broadly ventricose, with 3 length lamellulae, up to 4 mm wide, white, crowded, with white eroded edge. Stipe 40–70 × 5–7 mm, cylindrical, with white fibrillose and light brown to brown (7D7-8) squamules at annular zone, with concolorous squamules under annular zone downwards base, on white to orange-white (5A2) background. Annulus an annular zone, with white fibrillose and concolorous squamules. Context in pileus white, 3–5 mm wide; in stipe hollow, concolorous with surface. Taste and smell unknown. Spore print white.
Basidiospores [50,1,1] 4.5–6.3 × 2.5–3.5 µm, avl × avw = 5.02 × 3 µm, Q = 1.7–1.8, Qav = 1.75, in side-view oblong ovoid, in frontal view oblong, slightly thick-walled, hyaline, dextrinoid, congophilous. Basidia 18–23 × 7–10 µm, clavate, slightly thick-walled, hyaline, 4-spored, rarely 2-spored. Cheilocystidia 13–25 × 6–10 µm, cylindrical to irregular cylindrical, narrowly clavate to clavate, sometimes fusiform or utriform, slightly thick-walled, hyaline. Pleurocystidia absent. Pileus covering a trichoderm made up of cylindrical elements with rounded or attenuate apex, 60–170 × 7–13 µm, thick-walled, with parietal pale brown and intracellular pigment. Stipe covering of squamules similar to pileus covering. Clamp connections present.
Material examined: Laos, Oudomxay Province, Xay District, Houay Houm Village, N 20° 32′ 00.67″, E 101° 53′ 48. 17.16″. 917 m., 25 July 2014, P. Sysouphanthong, PS2014-1450 (HNL503121).
Habitat and distribution: Growing in a small group, on humus soil mixed with dead leaves; known from Yunnan province of Southwest China (Zhang et al. 2019). We report the first record of L. subvenenata from Laos in this study.
GenBank numbers: HNL503121–MT436258 (ITS).
Notes: Lepiota subvenenata was described from Yunnan province of Southwest China. It is closely related to L. venenata Z. H. Chen & Zhu L. Yang in morphology. Lao specimens clustered with the type specimens in the nrITS phylogenetic analyses and share similar morphology (Fig. 136).
Atheliales Jülich.
Atheliales is an order mostly composed of corticioid fungi (Sulistyo et al. 2021). Based on phylogenetic analyses Sulistyo et al. (2021) accepted five families: Atheliaceae, Byssocorticiaceae, Lobuliciaceae, Pilodermataceae, and Tylosporaceae in this order. However, Wijayawardene et al. (2022) accepted only Atheliaceae and Lobuliciaceae in this order.
Atheliaceae Jülich, Biblthca Mycol. 85: 355 (1982) [1981].
This is the type family of Atheliales. Jülich (1982) included the genera Athelopsis, Caerulicium, Confertobasidium, Leptosporomyces, and Luellia in Atheliaceae.Wijayawardene et al. (2022) accepted 20 genera in this family. Atheliaceae mainly consists with saprotrophic taxa, with one lichenicolous species (Athelia arachnoidea) (Sulistyo et al. 2021).
Athelia Pers., Traité champ. Comest. (Paris): 57 (1818).
Athelia is a genus of corticioid fungi. Some species are facultative parasites of plants and of lichens (Sulistyo et al. 2021). A species of Athelia also engaged in a symbiotic relationship with termites (Reticulitermes), in which the fungus forms sclerotia that mimic termite eggs and worker termites handling the sclerotia as if they were eggs (Matsuura et al. 2005). Wijayawardene et al. (2022) accepted 32 species in this genus.
Athelia rolfsii (Curzi) C.C. Tu & Kimbr., Botanical Gazette Crawfordsville 139: 460 (1978).
Index Fungorum number: IF309351; Faceoffungi number FoF13394; Figs. 143, 144
Pathogenic on roots and stem of Lablab purpureus. Sexual morph: not observed. Asexual morph: numerous globoid sclerotial bodies were developed on the collar region of infected Lablab purpureus plants at the stem soil interface. Sclerotia measured 1–3 mm in diam. (n = 50), initially whitish and later turned to brownish upon maturity.
Cultural characteristics: On PDA, reaching 90 mm at 28 °C after 7 d in 12/12 h light/dark, dense white fluffy colonies developed rapidly over the culture plates and produced characteristic sclerotia near the edges of plates and on the upper lid surface. After, 12–15 days of incubation sclerotia were recorded per plate ranging to 180 – 490 (mean 358 ± 24, n = 20). Colonies on PDA reaching 90 mm at 28 °C after 14 d in 12/12 dark, colonies appeared white to pink with abundant aerial mycelium.
Material examined: India, Karnataka, Mysore, Doddamaragowdanahally, on infected plants of Lablab purpureus (L.) Sweet (Fabaceae), 17 April 2013, S. Mahadevakumar (UOM-Sr-13–4), living culture Sr-LP3.
Habitat: Wide range of hosts (Farr and Rossman 2022), Lablab purpureus (This study).
Distribution: Brazil, China, Cuba, Fiji, New Zealand, Panama, Papua New Guinea, Phillippines, South Africa, Spain, Sri Lanka, West Indies and USA (Farr and Rossman 2022), India (This study).
GenBank number: KJ002765 (ITS).
Notes: The characteristic foot-rot and leaf blight disease of Lablab purpureus are reported in the present study and the diagnostic features are presented. The disease was observed at all stages of plant growth. In the early infection stage, the collar rot affected seedlings collapsed. Initial symptoms appeared as tan water-soaked lesions usually near the stem-soil interface, with lesions that enlarged and expanded towards the shoot apex, causing rotting. Similarly, lesions expanded towards the roots causing root decay and death of the host plant. The pathogen produced numerous globoid sclerotial bodies over the surface of the host plants. The cabbage heads' rotting was recorded with sclerotia development over the head surface. The disease was most prevalent during the rainy season. In dry weather, infected tissues showed the presence of mycelial threads with very few hard, melanized sclerotial bodies. In USDA host fungal database, the record 51387 specifies a geographical record for India, which shows the sequences submitted by our group to GenBank in 2013 and referred by Paul et al. (2017), a new geographical record for Athelia rolfsii on Ipomoea batatas from Korea. Based on the morphological, and cultural characteristics and ITS-rDNA analysis, the fungal pathogen was identified as Athelia rolfsii (Fig. 145). This is the first report on the association of A. rolfsii causing root rot and leaf blight disease in L. purpureus from India and worldwide.
Hymenochaetales Oberw.
The concept of hymenochaetaceous fungi was initiated by Patouillard (1900) in Série des Igniaires, which includes xanthochoroid polypores with simple, clampless hyphae and setal elements. Later, Oberwinkler (1977) raised the order Hymenochaetales, based on the characteristics of the Hymenochaetoidae, Hymenochaetaceae (proposed earlier by Donk (1948)). Hymenochaetales comprises with homobasidiomycetes with diverse basidiomatal characters, annual to perennial, resupinate to pileate, stipitate to spathulate with smooth, poroid or hydnoid hymenophore. Microscopically, simple, clampless, mono to di or trimitic hyphal system with smooth to ornamented, thin to thick-walled, hyaline to coloured, globose to cylindrical basidiospores are characteristic features. The presence or absence of sterile elements such as cystidia, cystidioles or seate also plays a vital role in identification (Fiasson and Niemela 1984).
MycoBank recorded 19 associated families (http://www.mycobank.org) whereas 1530 taxa were submitted in GenBank (https://www.ncbi.nlm.nih.gov) (as of 17 January 2022). Wijayawardene et al. (2022) accepted six families in this order.
Hymenochaetaceae Donk, Bull. Bot. Gdns Buitenz. 17(4): 474 (1948).
Donk (1948) classified the xanthochroid Aphyllophorales under Hymenochaetaceae which comprises wood-decaying white-rot fungi and medicinal fungi with styrylpyrone pigments (which are responsible for a positive xanthochoric reaction) (Dai et al. 2007, 2009). Hymenochaetaceae are described by yellow to deep brown, resupinate to pileate or stipitate basidiomata with smooth to hydnoid hymenial surface, simple mono to dimitic hyphal system, presence or absence of setae, thin to thick-walled, hyaline or coloured basidiospores (Ryvarden 1991; Dai 2010). Sharma (1995) reported 16 genera and 91 species of Hymenochaetaceae under Indian Aphyllophorales. Hymenochaetaceae is one of the largest families in Basidiomycota (Kirk et al. 2008). Dai (2010) mentioned Phellinus sensu lato, Inonotus sensu lato and Hymenochaete as the three largest genera of Hymenochaetaceae. There are 71 associated genera documented in MycoBank (http://www.mycobank.org) and 1,103 taxa submitted in GenBank (https://www.ncbi.nlm.nih.gov) (as of 1 January 2022). Wijayawardene et al. (2022) accepted 42 genera in this family.
Coltricia Gray, Nat. Arr. Brit. Pl. (London) 1: 644 (1821).
The genus is typified by C. perennis (L.) Murrill. Species of this genus are cosmopolitan and have few species that have been found to be associated with plant roots (Tedersoo et al. 2007). Coltricia is characterized by poroid and stipitate basidiocarps, a monomitic hyphal system lacking clamp connections and coloured, ellipsoid to subglobose, smooth basidiospores (Dai 2010). Wijayawardene et al. (2022) accepted 40 species in this genus.
Coltricia insularis P.-A. Moreau, Bellanger, Loizides & A. Rinaldi, sp. nov.
Index Fungorum number IF900072; Faceoffungi number FoF13395; Fig. 146
Coltricia insularis. a-c Basidiocarps. d Basidiospores. e, f Pileipellis. g Hymenial trama, longitudinal section. h Stipitipellis. All from LIP 0,401,817 (holotype, pictures by D. Borgarino (b) and P.-A. Moreau) except a (Hal-BP-72, sequence MT594499, picture by A. Rinaldi) and c (Hal-BP-19, sequence MT594498, picture by A. Rinaldi). Scale bars = 5 mm (a-c), 10 µm (d-g)
Etymology. Insularis = of islands, a Latin adjective referring to Corsica, Cyprus and Sardinia, three Mediterranean islands in which the species was discovered.
Holotype: LIP 0401817
Pileus 15–60 mm, irregularly infundibuliform, rough, sometimes weakly lobed, subtomentose or more distinctly tomentose to subhirsute towards the centre, radially corrugated or furrowed and usually with well-defined concentric chromatic zones; colours ranging from brown, red-brown, grey-brown, purple, umber or ochraceous; margin often paler and somewhat undulating. Hymenial surface poroid, comprised of adnexed to irregularly decurrent, shallow, angular, or somewhat elongated pores, mostly 2–3 per mm, becoming poorly defined towards the margin and sometimes fusing to form sterile tomentose patches or a sterile marginal zone; warm grey to buff or ochraceous. Stipe 20–40 × 3–8 mm, usually irregularly furrowed, densely tomentose, buff or pale brown at the apex but quickly staining ochraceous or brown, darker chestnut to red-brown or orange-brown lower, deeply submerged into the substrate and often with sand and litter tightly adhered to it. Context corky, thick and indistinctly zonate in the pileus, darker in the stipe, chestnut to red-brown or umber.
Spores (6.8) 7.5–9 (10.2) × 3.2–3.9 (4.1) µm, ellipsoid to broadly ellipsoid or ovoid when immature, cylindrical to fusiform when projected, with a light supra-apicular depression, pale yellow in KOH and in Melzer’s, wall < 0.3 µm thick, smooth. Basidia 12–19 × 7–7.5 µm, 4-spored (partly 2-spored on immature specimens) with spindle-shaped straight sterigmata, shortly cylindrical, hyaline. Hymenophoral setae absent. Edges sterile, made of bunches of slender thin-walled hairs 2.3–3 µm wide, encrusted with yellowish granular deposits. Pileipellis 60–80 µm-thick, convoluted trichocutis, made of generative hyphae 3.5–6(7) µm wide, terminal elements 45–80 µm long, branching often with right-angled furcations, rounded to mucronate at the apex. Stipitipellis a trichocutis made of flexuose, mostly unbranched skeletoid hairs, 70–180 × 3.5–5.5 µm, with occasional secondary septa; wall smooth or with few hyaline mucoid deposits towards the apex, 0.8–1.2 µm thick, yellow in 5% KOH; apex rounded. Clamps absent from all observed septa. Smell weak, faintly acidic.
Habitat and Distribution: Associated with Cistaceae (Cistus spp., Halimium halimifolium) shrubs in dry, sandy places. So far collected from Cyprus, France (Corsica), Italy (Sardinia) and Spain (Andalucia), but probably widespread in xerothermic localities throughout the Mediterranean basin.
Material examined: France: Corsica (Haute-Corse), Monaccia d’Aullène, Réserve naturelle du Mucchiu Biancu, 25 Nov. 2006, D. Borgarino, L. Hugot, C. Lavoise, P.-A. Moreau & F. Richard, PAM06112616 (LIP 0401817, holotype).
Other material examined: Cyprus: Trimiklini, M. Loizides, 28 Feb. 2019, ML91292CO (LIP 0401819). Italy: Sardinia, Gonnesa, A. Rinaldi, 5 Dec. 2019, Hal-BP-135, LIP 0401740. Spain: Andalucia, Huelva, Almonaster-la-Real, under Pinus halepensis and Cistus monspeliensis in a semi-open heathland, A. Gasch Illescas & P.-A. Moreau, 28 Dec. 2019, PAM1912814 (LIP 0401740).
Notes: Coltricia insularis is a xerophilic Mediterranean species characteristic of Cistaceae shrublands, found especially among sands, where it may grow in dense clusters adhered to the bases of rockroses. Also known from the thermo-mediterranean zone in Cyprus and the supramediterranean zone in Andalucia, where a slenderer form of this species was collected on sandy ground, at the edge of a pine forest. In Sardinia, it occurs in pure Halimium stands in coastal areas. However, in places where more potential hosts are present, it might be part of the extensive ectomycorrhizal networks that are common in several Mediterranean ecological settings (Taudiere et al. 2015; Leonardi et al. 2020). From what is known about Coltricia mycorrhizal biology, members of the genus appear to establish ectomycorrhizal associations with a range of hosts (Rinaldi et al. 2008). Coltricia insularis phylogenetically belongs to the group of Coltricia perennis, which has not been the object of monographic revision since the advent of DNA studies. The phylogenetic analysis (Fig. 147) shows the unexpected diversity unravelled in this lineage, in which at least three main subclades can be identified among the available sequences in GenBank and UNITE. Most of these are devoid of Linnaean names and the need of typification of C. perennis itself (type of the genus) is obvious, considering this name has been arbitrarily applied to nearly all sequences available in the’Perennis-clade’. Coltricia montagnei Fr. (in Montagne 1836, p. 341), originally described from Northern France (Ardennes, near Sedan), has been variously interpreted and was only recently described in detail (Rivoire 2020). Coltricia confluens Keizer (1997, p. 389), from which the isotype collection Keizer 93060 was kindly provided by the author, but unfortunately, could not be sequenced. Coltricia insularis differs from all currently recognized European species by spore dimensions and shape: mature specimens display spores with an average Q of around 2.3 and a typical fusiform profile. Like C. perennis and C. confluens (but not C. montagnei), the hyphae of pileipellis are frequently T-branched, but the taxonomic importance of this feature requires further observations. Coltricia’confluens’ and a sister Mexican species (Fig. 147), represent an independent well-supported clade. Amongst the subclade 3 (Fig. 147), C. insularis has an American sister species (sequences ITS MH211968 and MK966429 from pine forests in Oregon); both sequences form a well-supported subclade, distinct from the two other subclades of Clade 3 representing apparently circumboreal North European, North American and Asian species. None of the collections available in these clades suggests a thermophilic or xerophilic Mediterranean origin. A detailed revision of collections representing each clade is required before a thorough comparison of this cluster of species with C. insularis and within the whole “Perennis-clade” can be made.
Phylogenetic analyses were conducted online at www.phylogeny.fr (Dereeper et al. 2008). Multiple sequence alignments were performed with MUSCLE v. 3.7 (Edgar 2004). Maximum likelihood (ML) phylogenetic analysis was achieved with PhyML v. 3.0 (Guindon et al. 2010), using the GTR + I + Γ model of evolution and the Shimodaira Hasegawa version of the approximate likelihood-ratio test (SH-aLRT) of branch support (Anisimova et al. 2011). Phylogram was built using TreeDyn 198.3 (Chevenet et al. 2006) and edited with Inkscape 0.91 (https://inkscape.org/fr). Newly generated sequences for this study are in bold. The tree is rooted by mid-point rooting method under PhyML (Guindon et al. 2010)
Fulvifomes Murrill, Northern Polypores (5): 49 (1914).
Fulvifomes (typified as Fulvifomes robiniae Murrill) was described by Murrill (1914) to include perennial, sessile, ungulate, woody xanthochoroid fungus with dimitic hyphal system, colored basidiospores and lacks setae. Earlier Fulvifomes was treated as a synonym of Phellinus Quél. (Ryvarden and Johansen 1980; Dai 1999) later, many validated the generic rank of Fulvifomes, by studying the macro-microscopical description, nuclear behaviour and molecular data (Fiasson and Niemelä 1984; Wagner and Fischer 2002). Fulvifomes was revaluated and extensive ranges of characteristics were added to the classical description which includes annual to perennial, resupinate to pileate, applanate to ungulate, smooth to rimose basidiomata, homogenous to duplex context and poroid hymenial layer with mono-dimitic to dimitic hyphal system, presence or absence of setae, cystidioles, smooth, thick-walled, colored, globose to ellipsoid, cyanophilic to acyanophilic and inamyloid basidiospores (Murrill 1914; Wagner and Fischer 2002; Dai 2010; Salvador-Montoya et al. 2018; Tchoumi et al. 2020). Salvador-Montoya et al. (2018) and Zheng et al. (2021) proposed keys to American and Chinese Fulvifomes spp., respectively, since few samplings were done in other parts of the world.
Approximately 331 sequences belonging to 50 taxa were submitted in NCBI (https://www.ncbi.nlm.nih.gov) and 65 associated taxa are available in MycoBank (http://www.mycobank.org) (as of 1 January 2022). Wijayawardene et al. (2022) accepted 33 species in this genus.
Fulvifomes jawadhuvensis Kezo, K., Gunaseelan, S., & Kaliyaperumal, M., sp. nov.
Index Fungorum: IF558179; Facesoffungi Number: FoF10745; Fig. 148
Etymology: The species epithet jawadhuvensis refers to the type locality of basidiomata collection.
Holotype: MUBL4011
Basidiocarps perennial, solitary, pileate, sessile, light in weight, hard when dry. Pileus dimidiate, convex to meagrely ungulate, with no distinct crust, projecting up to 5.7 cm, 9.5 cm wide and 3.4 cm thick near the base. Pilear surface velvety, light brown (6D6) to rust-brown (6E8) and meagerly warted when young, on maturity pilei becoming rough, weakly rimose, concentrically, narrowly sulcate, weakly zonate. Margin entire, round to obtuse, brown (6E4) to dark brown (6F5), velutinate when young, developing into brownish grey (6F2) to greyish brown (6E3), glabrous on maturity. Pore surface raw umber brown (5F8) to brown (6E7). Pores round to angular, regular, 4–8 per mm. Dissepiments entire, thick. Context up to 2.7 cm, homogeneous, yellowish-brown (5E8) to dark brown (6F7). Tubes yellowish-brown (5D8) to light brown (6D6), up to 0.7 cm thick, tube layers stratified, each stratum 0.2 to 0.4 cm with a thin layer of context in between.
Hyphal system strictly dimitic, Generative hyphae dominant; both skeletal and generative hyphae acyanophilous; tissue darkening with KOH without swelling. Context Generative hyphae, thin to thick-walled, hyaline to yellow, simple septate, branched, 2–6.5 μm diam.; skeletal hyphae, thick-walled with narrow lumen, unbranched, yellowish-brown, aseptate, 2–5.7 μm diam. Trama Generative hyphae, thin to thick-walled, yellow to brown, septate, rarely branched, 2–5.2 μm dia.; skeletal hyphae, thick-walled with narrow to wide lumen, yellowish-brown, aseptate, unbranched, 2–5.2 μm dia. Setae, cystidioles absent. Basidioles dominant, clavate, 7.7–14 × 3.8–7.2 μm. Basidia clavate to broadly clavate, with four sterigmata, 8.5–15 × 5–8.5 μm. Basidiospores broadly ellipsoid to subglobose, thick-walled, smooth, yellow in water, turning rust-brown in KOH, (4.8–) 5.1–6.4 (–6.9) × (4.1–) 4.4–4.9 (–5.2) μm (n = 50/2), Q = 1.1–1.3, CB ̄, IKI ̄. Chlamydospores globose to subglobose, thick-walled rust brown to reddish-brown, 5.2 – 8.5 × 4.1–7 μm, CB ̄, IKI ̄.
Specimen examined: India, Tamil Nadu, Thiruvannamalai district, Jawadhu hills, Jamunamarathur, 12.64° 54′ 19.1″ N 79° 18′ 33″ E, on living angiosperm tree (Albizia amara (Roxb.) Boiv., Fabaceae), 09 February 2018, Kezhocuyi Kezo (MUBL4011, holotype).
GenBank numbers: MW040079 (ITS), MW048886 (LSU), MW690924 (tef1).
Notes: Fulvifomes jawadhuvensis shares similarities with F. grenadensis by having dimidiate to ungulate pileus, round to obtuse margin, absences of cystidioles, but the former lacks distinct crust after velvety pileus wears off and no. of pores per mm. Larger broadly ellipsoid to subglobose spores in F. jawadhuvensis differ from smaller spores in F. grenadensis (4–6 × 3–4 µm) (Ryvarden 2004). The new Indian species share other common characteristics with F. elaeodendri, F. hainanensis, F. thailandicus, in having dimidiate to ungulate basidiomata, zonate pileus with rimose patten (except F. hainanensis), pores per mm, distinctly thick-walled, colored basidiospores, absence of setae, but significantly varies basidiospores size and absences of cystidioles (Zhou 2014; Tchoumi et al. 2020). Fulvifomes jawadhuvensis and F. centroamericanus share only dimidiate pilei and absences of cystidioles and differ in other features; the former varies entirely from F. krugiodendrii (Ji et al. 2017). Fulvifomes jawadhuvensis shows variations with F. nonggangensis and F. tubogeneratus in basidiomata characters and microscopic illustrations (Zheng et al. 2021).
Fulvifomes malaiyanurensis Gunaseelan, S., Kezo, K. & Kaliyaperumal, M., sp. nov.
MycoBank: MB 821517; Facesoffungi number: FoF 10744; Fig. 149
Etymology: The species epithet ″malaiyanurensis″ refers to the type locality of the collection site.
Holotype: CAL 1618
Basidiocarps perennial, solitary, sessile, broadly attached to substrate, hard, light when dry. Pilei dimidiate, ungulate to triquetrous, projecting up to 5 cm, 12 cm wide and 4 cm thickness near the attachment. Pilear surface velutinate towards the margin, partially, concentrically zonate, brown (6E6) to greyish brown (6F3), cracked during collection. Margin obtuse often round, velvety shining, brown (5E8) to yellowish brown (5E4). Pore surface umber-brown (5E8). Pores regular, circular to angular, 5–7 per mm. Dissepiments thick, entire. Context yellowish-brown (5C7) to dark golden brown (5C7), woody corky, up to 2 cm thick. Tube layer brown (6D7), up to 2 cm long in distinctly stratified, individual layer up to 0.5 cm in length.
Hyphal system strictly dimitic, skeletal and generative hyphae acyanophilous, tissue darkening with KOH without swelling. Context: Hyphal system subparallel, generative hyphae dominant hyaline to yellow, simple septate, thin to thick-walled, 2.2–6.4 μm in diam, rarely septate and branched, skeletal hyphae rare, yellow to brown, thick-walled, with a narrow to wide lumen, infrequently septate, unbranched, 1.8–5.2 μm in dia. Trama Hyphae interwoven, color not distinct from context; generative hyphae thin to thick-walled, frequently septate 2–5.2 μm wide, skeletal hyphae thick-walled, unbranched, aseptate with narrow to wide lumen 2.7–5.2 μm. Hymenial setae, cystidia and cystidioles absent. Basidioles clavate, 5–16 × 3–7 μm. Basidia broadly clavate, with four sterigmata, 7–16 × 5–8 μm. Basidispores broadly ellipsoid to subglobose, smooth, yellow to golden yellow in water, turning golden brown to rust-brown in KOH, thick-walled, (4.2–) 4.6–5.4 (–5.7) × (3.9–) 4.2 – 4.9 (–5.2) μm, Q = 1.09, (n = 50/2), Q = 1.05–1.25, CB ̄, IKI ̄. Chlamydospores globose to subglobose, thick-walled, rust-brown to reddish-brown, 5.7– 12.8 × 5.2–11.6 μm, CB ̄, IKI ̄.
Material examined: India, Tamil Nadu, Vizhupuram district, Malaiyanur, 37° 25′ 19.1″ N 23° 36′ 33″ W, on living angiosperm tree (Tamarindus indica L., Fabaceae), 25 October 2016, Sugantha Gunaseelan, MLCASB020, holotype.
Additional specimen examined: India, Tamil Nadu, Vizhupuram district, Malaiyanur, 37° 25′19.1″ N 23° 36′33″ W, on living angiosperm tree (Tamarindus indica, Fabaceae), 25 October 2016, Sugantha Gunaseelan, (MLCASB021, Isotype).
GenBank numbers: MLCASB020-MF155651 (ITS), MF155652 (LSU).
MLCASB021- MW048883 (LSU), MW690925 (tef1).
Notes: Fulvifomes malaiyanurensis is similar to F. thailandicus (Zhou 2015) in sharing yellowish-brown, broadly attached basidiomata, pore, dimitic hyphal and absence of cystidioles. However, F. malaiyanurensis is distinct by having ungulate to triquetrous, yellowish-brown, velutinate basidiocarp, acyanophilic subglobose basidiospores, lacking cystidioles. Fulvifomes malaiyanurensis differs from F. grenadensis (Ryvarden 2004), F. hainanensis (Zhou 2014) and F. imbricatus (Zhou 2015) in pileus character, pore per mm, shape and size of basidiospore. F. malaiyanurensis share a similar pileial character with F. robinae (Salvador-Montoya et al. 2018) but differs in hyphal system and basidiospore but shares pores/mm. Fulvifomes malaiyanurensis shares similarities with F. elaeodendri and F. yoroui in triquetrous up to ungulate basidiomata and pores per mm (Tchoumi et al. 2020; Olou et al. 2019) and differs in other characters. Macroscopically, F. malaiyanurensis may resemble African (Tchoumi et al. 2020), Asian (Zhou 2014, 2015; Liu et al. 2020; Du et al. 2021) and American (Ji et al. 2017) known species but shows variation in other taxonomic characters.
Fulvifomes thiruvannamalaiensis Gunaseelan, S., Kezo, K. and Kaliyaperumal, M., sp. nov.
Index Fungorum number: IF558486; Facesoffungi number: FoF 10726; Fig. 150
Etymology: The species epithet ″thiruvannamalaiensis″ refers to the type locality of collection site.
Holotype: MUBL4013
Basidiocarp perennial, solitary, sessile, applanate, broadly attached to the substrate by the narrow side, hard, woody when dry. Pileus dimidiate, convex to meagrely ungulate, lacks crust, projecting up to 4.8 cm in length, 10.4 cm in width and 3.4 cm thick near the attachment. Pilear surface partly covered with microalgae, glabrous, light brown (6D8, 6E7), rust-brown (6E8) to dark brown (6F7), finely cracked with small brownish grey scales (6E3, 6F2) concentric and radially sulcate, but coarse and deep sulci, scrupose zones near the attachment/older region, meagrely wavy near the margin. Margin entire, round to obtuse, velutinate to smooth, dark brown (6F7), brownish grey (6F2), often wavy. Pore surface yellowish raw umber brown (5F8), yellowish-brown (5E8) to brown (6E7, 6F8). Pores round, regular, 4–7 per mm. Dissepiments entire, thick. Context up to 1.6 cm, homogeneous, fibrous to corky, brown (6E7, 6F8) to dark brown (6F7). Tubes yellowish-brown (5E8), light brown (6D6) to brown (6E7, 6F8), up to 1.3 cm thick, tube layers stratified with thin-walled bright yellowish generative hyphae usually running between the old tubes, each stratum up to 0.4 cm.
Hyphal system strictly dimitic, skeletal and generative hyphae acyanophilous; tissue darkening with KOH without swelling. Context Generative hyphae dominant, thin to thick-walled, simple septate, branched, hyaline to brown, 1.2–4.8 μm diam.; skeletal hyphae, thick-walled with narrow to wide lumen, unbranched, aseptate, yellow to brown, 2.4–4.3 μm diam. Trama Generative hyphae, thin to thick-walled, septate, rarely branched, hyaline to brown, 1.2–4.8 μm diam.; skeletal hyphae, thick-walled with narrow to wide lumen, aseptate, unbranched, brown, 2.4–4.8 μm diam. Setae absent. Cystidioles thin-walled, hyaline, varies in shape, fusoid to ventricose with elongated apical portion, 12.9–27 × 2.8–7.2 μm. Basidioles clavate to broadly clavate, 8.5–15.5 × 3.5–6.5 μm. Basidia clavate to broadly clavate, with four sterigmata, 9.4–15.7 × 5.2–8 μm. Basidiospores broadly ellipsoid to subglobose, thick-walled, smooth, yellow in water, turning brown in KOH, (5.3–) 5.5–6.7 (–6.9) × (4.6–) 4.8–5.1(–5.5) μm (n = 30/2), Q = 1.05–1.3, CB ̄, IKI ̄.
Material examined: India, Tamil Nadu, Thiruvannamalai district, Jawadhu hills, Jamunamarathur, 12.64° 54′ 19.1″ N 79° 18′33″ E, on living angiosperm tree (Albizia amara, Fabaceae), 09 February 2018, Sugantha Gunaseelan, MUBL4013, holotype.
GenBank numbers: MZ221598 (ITS), MZ221600 (LSU).
Notes: Fulvifomes thiruvannamalaiensis, characterized by perennial, solitary, dimidiate, applanate to ungulate basidiomes, significantly cracked pilear surface with brownish-grey scales, stratified tube layer with dimitc hyphal system and thick-walled yellow to brown, acyanophilic subglobose to ellipsoid basidiospores.
African species, F. yoroui (Olou et al. 2019) shows close resemblance with F. thiruvannamalaiensis, in sharing perennial, pileate, ungulate basidiomata, dimitic hyphal system and presence of fusoid cystidioles, but the size and shape of the basidiospores (subglobose to globose basidiospores 5.5–6.5 × 4.7–5.6 μm), differ from F. thirvannamalaiensis. Fulvifomes krugiodendri (Ji et al. 2017), F. rimosus (Hattori et al. 2014) and F. thailandicus (Zhou 2015) are closely similar to F. thirvannamalaiensis morphologically by sharing dimidiate, concentrically sulcate, cracked basidiocarp, dimitic hyphal system and presence of fusoid cystidioles, yet differs in the number of pores and the size of basidiospores.
Despite few morphological resemblances between F. elaeodendri (Tchoumi et al. 2020) such as sulcate, glabrous pileus becoming cracked with age, stratified tube layer the distinct microscopical traits viz., absence of cystidioles and the size of the basidiospores are distinguishing F. thiruvannamalaiensis from F. elaeodendri. Fulvifomes centroamericanus (Ji et al. 2017), F. hainanensis (Zhou 2014) and F. imbricatus (Zhou 2015) besides having dimitic hyphal systems are significantly distinct from F. thiruvannamalaiensis in morphology and microscopic features, by having uncracked pilear surface, shape and size of basidiospores.
Fulvifomes thiruvannamalaiensis shares a few similar morphological traits with the American species, F. cedrelae, F. robinae and F. squamosus (Salvador-Montoya et al. 2018) by having perennial, applanate, ungulate, sessile basidiomata with sulcate, cracked pilei, homogenous context and stratified tubes, however, the former differs from the later in hyphal system, number of pores and size of the basidiospore. The Chinese species F. submerrillii (Liu et al. 2020), shows high variations both morphologically and microscopically from the newly described Indian F. thiruvannamalaiensis (Fig. 151).
Phylogram of Fulvifomes species, obtained from maximum likelihood (RAxML) of combined ITS, LSU, and tef1 datasets. Bootstrap values (BS) from maximum likelihood (ML, left) and Maximum parsimony (MP, middle) greater than 70% and Bayesian posterior probabilities (PP), greater than 0.95, are indicated above the nodes as ML/MP/BYPP. The tree is rooted with Phylloporia nodostipitata FLOR 51153. New species and new records are indicated in black bold
Hymenogastraceae Vittad, [as 'Hymenogastereae'], Monogr. Tuberac. (Milano): 11 (1831)
Note: Hymenogastraceae was erected by Vittadini (1893) with contained gilled and false-truffle fungi. According to the recent study by He et al. (2019), Hymenogastraceae is one of the larger families in the Agaricales and comprises 10 genera, namely Anamika K.A. Thomas, Peintner, M.M. Moser & Manim., Flammula (Fr.) P. Kumm., Galerina Earle, Gymnopilus P. Karst., Hebeloma (Fr.) P. Kumm., Hymenogaster Vittad., Naucoria (Fr.) P. Kumm., Phaeocollybia R. Heim, Psathyloma Soop, J.A. Cooper & Dima and Psilocybe (Fr.) P. Kumm. Now, it is represented by more than 1500 species in Hymenogastraceae (Matheny et al. 2006; Redhead et al. 2007; Kirk et al. 2008; He et al. 2019).
Psilocybe (Fr.) P. Kumm., Führ. Pilzk. (Zerbst): 21 (1871)
Psilocybe was first introduced by Kummer (1871) with P. semilanceata (Fr.) P. Kumm. as the type species. This genus is saprotrophic and widely distributed in both tropical and temperate areas (Singer and Smith 1958; Guzmán 1978, 1983; Redhead et al. 2007; Kirk et al. 2008), while Psilocybe sensu lato is known to include Deconica. Both Psilocybe and Deconica have been characterized by typically hygrophanous basidiomata, brown to yellow–brown pileus, lilac-brown to dark brown to dark purple-brown spore prints, ellipsoid to rhomboid to subhexagonal basidiospores with a distinct apical germ pore (Singer and Smith 1958; Guzmán 1978, 1983; Redhead et al. 2007). Most Psilocybe contains psychedelic compounds, e.g. baeocystin, psilocin and psilocybin (Stamets 1996), whereas Deconica possesses none of these compounds (Marcano et al. 1994). Traditionally, Psilocybe and Deconica belong to Strophariaceae, order Agaricales (Guzmán 1978, 1983). However, multi-phylogenetic analyses have revealed that Psilocybe formed a monophyletic genus in Hymenogastraceae, order Agaricales, which clearly separates it from Deconica (Ramírez-Cruz et al. 2013). There are more than 300 accepted species of Psilocybe in the Index Fungorum (2022a, b), however, only eight Psilocybe species have been reported in Thailand (P. cubensis (Earle) Singer, P. magnispora E. Horak, Guzmán & Desjardin, P. samuiensis Guzmán, Bandala & J.W. Allen, P. subaeruginascens Höhn, P. thaiaerugineomaculans Guzmán, Karun. & Ram.-Guill., P thaicordispora Guzmán, Ram.-Guill. & Karun., P. thaiduplicatocystidiata Guzmán, Karun. & Ram.-Guill., and P. thailandensis E. Horak, Guzmán & Desjardin and P. thaizapoteca Guzmán, Karun. & Ram.-Guill.) (Guzmán et al. 1993, 2012; Horak et al. 2009; Chandrasrikul et al. 2011).
Psilocybe keralensis K.A. Thomas, Manim. & Guzmán, Mycotaxon 83: 196, 2002.
Index Fungorum number: IF380972; Facesoffungi number: FoF10681;Fig. 152
Pileus 13–25 mm diameter, hemisphearic, subconic or campanulate, hygrophanous, brownish-orange (6C4) to greyish orange (5B6), fading to light orange (5A4) to orange-white (5A2), surface glabrous, lucidus when dry and often somewhat bluish when touched or on drying, especially at the edge, not viscid and the margin finely translucent striate when moist; context pale yellow (2A3) or yellowish-white (2A2), bruising ink blue. Lamellae adnate to slightly sinuate, close, light orange (5A4) to orange (5A6) to brownish grey (9C2) or reddish-brown (9E5), often with ink blue tinge, edges serrulate and remaining whitish. Stipe 45–80 × 1.5–3 mm, equal, sometimes flattened and becoming tapered toward the base, nearly concolorous with the pileus, darker below, often with ink blue to blackish tinge when touched or when dry, and shiny when dry; surface longitudinally striate and covered with appressed whitish fibrils or flocculose, sometimes uneven and with scrobicula and grooves; base with white mycelium and often radicating; annulus absent; context fragile, yellowish and yellowish-brown towards the surface and base, staining somewhat ink blue when bruised. Spore print dark brown.
Basidiospores 6–10 × 5–7 × 4–6 μm, often subrhomboid or ovoid, sometimes ellipsoid, occasionally inconspicuous subhexagonal in face view, Q = 1.1–1.7, Qm = 1.35 ± 0.13, ellipsoid or subovoid, sometimes nearly oblong inside view, Q = 1.2–1.8, Qm = 1.55 ± 0.15; yellowish-brown with a purple tinge in water, dark yellow to yellowish-brown in KOH, dark purplish-brown in deposit; wall smooth, slightly thick (0.5–1 μm), complex, with 0.8–1.5 μm wide apical germ pore. Basidia 18–28 × 5–8 μm, hyaline, long subcylindric to clavate, often constricted in middle and narrowed in the lower half, 4-spored and 2-spored, rarely 1-spored; sterigmata up to 6 μm. Pleurocystidia are relatively rare and scattered, 13–22 × 4–6.5 μm, hyaline, ventricose to sublageniform, sometime fusoid or clavate, occasionally nearly cylindric-clavate or obclavate, often with a 1–7.5 × 1–2 μm neck or rostrum, not branched, the top or apex often seems wall thickened or contain some matter. Cheilocystidia abundant, 14–35.5 × 4–8 μm, hyaline, similar to pleurocystidia, mostly with a 1.5–13 × 1–3 μm rostrum or neck, sometimes with an acuminate apex, the top or apex often seems wall thickened or contain some matter. Pileipellis an ixocutis, 15–80 μm thick, made up of creeping, interwoven, 2–6 μm wide filiform to slender tubular hyphae, hyaline and colourless, wall-smoothed and thin; subpileipellis more pigmented to dark yellow in KOH. Subhymenium subcellular, hyaline, composed of irregular vesiculose to polygonal or subglobose cells. Caulocystidia abundant, 14–49 × 3.5–12 μm, scattered, gregarious to clustered at the upper part of the stipe, hyaline, similar to cheilocystidia, hyaline, thick-walled. Clamp connections present in all tissues.
Material examined: Thailand, Ubon Ratchathani Province, Phibun Mangsahan, District, 15° 3′ 25″ N 105° 26′ 50″ E, elevation 164 m, on soil, 4 May 2019, N. Suwannarach & J. Kumla, SDBR-CMUNK0448.
Habitat: Growing solitary to scattered on dung or soil of meadows, or grassland in an open area.
Distribution: India (Thomas et al. 2002), Southwestern China (Ma et al. 2016) and Thailand (This study).
GenBank numbers: MZ452082 (ITS), MZ452083 (LSU).
Notes: Morphologically, P. keralensis is similar to P. columbiana Guzmán; however, P. columbiana differs significantly in the absence of pleurocystidia. It has been known to only be from Colombia (Guzmán 1978, 1983; Guzmán et al. 2005). Phylogenetically, P. keralensis formed a sister taxon to P. thaizapoteca (Fig. 153). However, the large pileus (20–50 mm in diameter) and small basidiospores (6–7 × 3–4.5 × 3–3.5 μm) of P. thaizapoteca clearly differ from P. keralensis (Guzmán et al. 2012).
Phylogenetic tree derived from maximum likelihood analysis of a combined ITS and LSU genes of 25 sequences and the aligned dataset was comprised of 1640 characters including gap. The average standard deviation of the split frequencies of the BI analysis was 0.00962. Panaeolus acuminatus (CBS 270.47) and Pa. subbalteatus (CBS 326.34) were used as outgroup taxa. The numbers above branches are the bootstrap statistics percentages (left) and Bayesian posterior probabilities (right). Branches with bootstrap values ≥ 70% are shown at each branch and the bar represents 0.1 substitutions per nucleotide position. Hyphen (-) represents support values ≤ 70/0.90. Ex-type strains are in black bold. The newly generated sequences are indicated in blue
Marasmiaceae Roze ex Kühner.
Note: Marasmiaceae was erected by Kühner (1980) with a combination of Marasmieae, Collybieae and Myceneae of Tricholomataceae R. Heim in Singer (1986) classification. Molecular phylogeny has revealed that Marasmiaceae is monophyletic, containing ten genera (Amyloflagellula Singer, Brunneocorticium Sheng H. Wu, Campanella Henn., Chaetocalathus Singer, Crinipellis Pat., Hymenogloea Pat., Marasmius Fr., Moniliophthora H.C. Evans, Stalpers, Samson & Benny, Neocampanella Nakasone, Hibbett & Goranova and Tetrapyrgos E. Horak) with more than 1590 species, Marasmius being the generic type (Matheny et al. 2006; Kirk et al. 2008; He et al. 2019) of this family.
Marasmius Fr., Fl. Scan.: 339 (1836).
Marasmius is a large genus of plant debris decomposers (Singer 1976a, b, 1986; Antonín and Noordeloos 2010) that is represented by approximately 600 species worldwide (He et al. 2019). The genus was first introduced by Fries (1835) and Marasmius rotula (Scop.). Fr. was later designated as the type species by Singer and Smith (1946). Marasmius is characterized by small or possibly large to robust basidiomata, convex or campanulate, striate to sulcate, white to strongly pigmented pilei, well-developed lamellar, thin and typically tough, cylindrical, often brown or darkly fuscous stipe, white spore print, hyaline, smooth and inamyloid spores and hymeniform pileipellis of smooth or diverticulate cells (Singer 1976a, b, 1986; Antonín 2007). Generic circumscription of Marasmius sensu stricto was restricted (Wilson and Desjardin 2005) and the infrageneric classification has been revised based on the results of a phylogenetic analysis of combined sequences of LSU and ITS incorporated with other notable morphological features (Oliveira et al. 2020b).
Marasmius pallidoaurantiacus Wannathes, N. Suwannarach, J. Kumla & S. Lumyong, sp. nov.
MycoBank number: MB 840,379; Facesoffungi number: FoF 10682;Fig. 154
Etymology: ‘pallio’ = pale, ‘aurantiacus’ = orange colour, refers to the pileus colour
Holotype: BKF10248
Pileus 1–2 mm diam., hemispherical, umbilicate, with a tiny dark brown papilla at center; dull, dry, glabrous, slightly striate; orange white (5A2) to pale orange (5A3) with darker disc. Context thin, white. Lamellae adnate to a small collarium, distant (10–11), broad (1 mm), orange white (5A2), non-marginate. Stipe 8–13 × 0.2 mm, cylindrical, central; glabrous, dull, dry; insititious; dark brown overall, sometime stipe arising directly from dark brown rhizomorph.
Basidiospores 7–10(–11) × 4–5(–6) µm [x = 8.20 ± 1.35 × 4.18 ± 0.58, Q = 1.75–2.57, q = 1.97 ± 0.30, n = 25] ellipsoid, fusoid, smooth, hyaline, inamyloid, thin-walled. Basidia 14–16 × 4–6 µm, clavate, with 4 sterigmata, thin-walled, inamyloid. Cheilocystidia abundant, of Siccus- type broom cells; main body 5–7 × 4–6 µm, cylindrical to clavate, hyaline, inamyloid, thin-walled; apical setulae 1–4 × 1 µm, cylindrical to conical, obtuse, hyaline to pale yellow, thin- to thick-walled. Pleurocystidia absent. Pileipellis hymeniform, mottled, composed of Siccus-type broom cells; main body 10–14 × 4–6 µm, clavate to broadly clavate, flexuose, pale yellow, inamyloid, thin- to thick-walled; apical setulae (1–) 2–6 × 1 µm, cylindrical, regular in outline, obtuse, brown, thick-walled. Pileus trama interwoven, inamyloid. Lamellar trama interwoven, hyphae 2–5 µm diam., cylindrical, inflated, smooth, hyaline, inamyloid, thin-walled, non-gelatinous. Stipitipellis subparallel, hyphae 2–4 µm diam., cylindrical, dark brown, smooth, inamyloid, thick-walled (up to 1 µm), non-gelatinous. Stipe trama subparallel, hyphae 2–5 µm diam., cylindrical, hyaline to pale yellow, smooth, dextrinoid, thin-walled, non-gelatinous. Caulocystidia absent. Clamp connections present in almost all tissues.
Material examined: Thailand, Nakhon Ratchasima Provinve, Khao Yai National Park, 33 km marker on Hyw 2090, 22 Sep 2018, collectors N Wannathes, N Suwannarach J Kumla, S Lumyong, BKF10248 (holotype), NW1108 (isotype).
Habit, habitat and known distribution: Gregarious on dicotyledonous leave, known only from Thailand.
GenBank numbers: MZ452673 (ITS).
Notes: Marasmius pallidoaurantiacus is characterized by a tiny, hemispherical with dark brown conical papilla at the center, slightly striate, plae orange pileus, collariate, distant lamellae, thin stipe, sometimes arising directly from the rhizomorph, ellipsoid to fusoid basidiospores with mean 8.2 × 4.2 µm, and the presence of Siccus-type broom cell cheilocytidia. This new species is morphologically similar to Marasmius pallenticeps Singer, which was originally described from Argentina. The latter differs by forming a pure white pileus, stipe usually arising directly from rhizomorphs and the presence of nodes (Desjardin and Ovrebo 2006). Marasmius pallidoaurantiacus pilei appear pigmented like those of M. longibasidiatus J.S. Oliveira, a species recently described from Brazil. This species differs by forming bigger (1.3–4 mm diam.), sulcate pileus, longer basidia (33–42 × 7.8–10 µm) and pileipellis composed of mixed Siccus type broom cell and non-setulost cells (Oliveira 2020). Phylogenetic analyses inferred from ITS sequence data (Fig. 155) confirmed that M. pallidoaurantiacus is a distinct species from other related morphological species and other taxa within this genus.
Phylogenetic tree derived from maximum likelihood analysis of a combined ITS gene of 65 sequences and the aligned dataset was comprised of 1116 characters including gap. The average standard deviation of the split frequencies of the BI analysis was 0.00825. Marasmius siccus KG 039 and KG 119 were used as outgroup. Numbers above branches are the bootstrap statistics percentages (left) and Bayesian posterior probabilities (right). Branches with bootstrap values ≥ 70% are shown at each branch and the bar represents 0.1 substitutions per nucleotide position. Hyphen (-) represents support values ≤ 70%/0.95. Ex-type strains are in black bold. The newly generated sequences are indicated in blue and bold type species
Marasmius tangerinus Wannathes, N. Suwannarach, J. Kumla & Lumyong, sp. nov.
MycoBank number: MB840380; Facesoffungi number: FoF10683; Fig. 156
Etymology: ‘tangerinus’ = tangerine colour, refers to the pileus colour
Holotype: BKF10249
Pileus 2–3 mm diam., hemispherical to convex, umbilicate, with a tiny dark brown papilla at center; dull, dry, glabrous, striate; orange red (8A8) overall when young, orange red (8A8) at disc, orange (6B7) to tangerine (6B8) at margin in age. Context thin, white. Lamellae adnate to a small collarium, distant (10–11), narrow (0.5 mm), pale orange (5A3), marginate with orange. Stipe 13–20 × 0.3 mm, cylindrical, central, glabrous, dull, dry, insititious, golden brown (5D7) overall, sometimes stipe arising directly from golden brown rhizomorph.
Basidiospores 8–10 × (4–)4.5–5 µm [x = 8.56 ± 0.71 × 4.92 ± 0.28, Q = 1.6–2.0, q = 1.74 ± 0.16, n = 25] ellipsoid, fusoid, smooth, hyaline, inamyloid, thin-walled. Basidia 16–18 × 6–8 µm, clavate, with 4 sterigmata, thin-walled, inamyloid. Cheilocystidia abundant, of Siccus- type broom cells; main body 10–14 × 5–7 µm, cylindrical to clavate, sometime branched, hyaline, inamyloid, thin-walled; apical setulae 1–4 × 1(–2) µm, cylindrical to conical, obtuse, pale yellow, thin- to thick-walled. Pleurocystidia absent. Pileipellis hymeniform, not mottled, composed of 2 type of cells: (a) Siccus-type broom cells; main body 18–38 × 10–16 µm, clavate to broadly clavate or turbinate, irregular in outline, hyaline to pale yellow, inamyloid, thin- to thick-walled; apical setulae 2–10 × 1–4 µm, cylindrical to conical, regular in outline, obtuse, yellow to pale brown, inamyloid, thick-walled; (b) non-setulose cell, 8–32 × 10–15 µm, clavate to broadly clavate, sometime branched, yellow to pale brown, inamyloid, thick-walled. Pileus trama interwoven, inamyloid. Lamellar trama interwoven, hyphae 4–8 µm diam., cylindrical, inflated, smooth, hyaline, inamyloid, thin-walled, non-gelatinous. Stipitipellis subparallel, hyphae 4–6 µm diam., cylindrical, pale brown, smooth, dextrinoid, thick-walled (up to 1 µm), non-gelatinous. Stipe trama subparallel, hyphae 2–4 µm diam., cylindrical, hyaline, smooth, dextrinoid, thin-walled, non-gelatinous. Caulocystidia absent. Clamp connections present in almost all tissues.
Material examined: Thailand, Nakhon Ratchasima Provinve, Khao Yai National Park, 33 km marker on Hyw 2090, 22 Sep 2018, collectors N Wannathes, N Suwannarach J Kumla, S Lumyong, BKF10249 (holotype), NW1224 (isotype).
Habit, habitat and known distribution: Gregarious on dicotyledonous wood, known only from Thailand.
GenBank numbers: MZ452087 (ITS).
Notes: Marasmius tangerinus is characterized by a tiny, hemispherical, bright orange pileus, with dark brown papilla at the center, collariate, distant lamellae, thin stipe, sometimes arising directly from the rhizomorph, ellipsoid to fusoid basidiospores with mean 8.6 × 4.9 µm, pileipellis composed of mixed Siccus type broom cell and non-setulost cells, with the presence of cheilocytidia, and ligicolous habit. Marasmius tangerinus is morphologically similar to M. trichorhizus Speg. which has been characterized from a neotropic realm. The latter species differ by forming more lamellae (12–15), longer stipe (4–71 mm long), and smaller basidiospores with mean 6.9 × 3.4 µm (Oliveira 2020). The phylogenetic analyses of the ITS sequence data (Fig. 155) indicate that M. tangerinus is a distinct species from other related morphological species and other taxa within this genus.
Physalacriaceae Corner, Beih. Nova Hedwigia 33: 10 (1970).
Notes: The family was typified by Physalacria Peck (Peck 1883), which contained a number of marasmoid fungi, including the important tree pathogen Armillaria (Fr.) Staude and edible fungi Flammulina P. Karst., this group usually have pilocystdia and non-dextrinoid tissue (Petersen and Hughes 2010). More than 28 genera included in this family by Index Fungorum (2022a, b) and are accepted by Wijayawardene et al. (2022).
Rhizomarasmius R.H. Petersen, Mycotaxon 75: 333 (2000).
Notes: Petersen (2000) accommodated two Marasmius species, M. pyrrhocephalus Berk. and M. undatus (Berk.) Fr. to form a new genus, Rhizomarasmius R.H. Petersen. This genus is characterized by convex to hemispherical pileus, distant, thick, ascending, white lamellae and central stipe apically pale and darkening downward to brown-black, smooth basidiospores, pileipellis forming a hymeniderm layer of clavate, globose to sphaeropedunculate, smooth elements and scattered, elongate pileocystidia, as well as the presence of cheilo-, pleuro- and caulocystidia (Petersen 2000; Antonín and Noordeloos 2010). Most members of marasmoid are saprotrophic or parasitic fungi (Pacioni and Lalli 1989; Filippi 1991; Ronikier and Ronikier 2011; Moreau et al. 2015). Rhizomarasmius epidryas (Kühner ex A. Ronikier) A. Ronikier and M. Ronikier is a common arctic-alpine saprotrophic fungus (Ronikier 2009, 2011), and this species was originally described by Kühner as Marasmius epidryas Kühner in 1935 (Kühner 1936). Wilson and Desjardin (2005) and Noordeloos and Antonín (2008) transferred this species into Mycetinis Earle. However, later established that the species belongs to the new genus Rhizomarasmius by Anna & Macheł Ronikier and changed the current name accordingly (Ronikier 2009, 2011). Recently Moreau et al. (2015) added two members, Rhizomarasmius oreinus (Pacioni & Lalli) Vizzini, Antonín & A. Urb. and R. setosus (Sowerby) Antonín & A. Urb. To date, there are five species described within this genus. Petersen (2000), Petersen and Hughes (2010) and Ronikier and Ronikier (2011), showed Rhizomarasmius to form a well-supported clade in phylogenetic analyses.
Rhizomarasmius cunninghamietorum Chun Y. Deng, J.P. Li & Gafforov, sp. nov.
Index Fungorum number: IF558751; Facesoffungi number: FoF 10408; Figs. 157 , 158
Etymology: the species epithet refers to the host genus Cunninghamia.
Holotype: HGASMF01-10709
Basidiomata gregarious, marasmioid, marasmielloid or collybioid. Pileus 9–36 mm in diameter, convex to plano-convex when young, expanding to applanate with age, obtuse or slightly depressed at the centre, often wrinkled, slightly sticky, sometimes hygrophanous, translucent when mosit, radially striate or sulcate from the margin up to 3/4 of the radius nearly to the centre, with transversely sulcate between each radial striate when old, involute then inflexed to reflexed at margin, with marginal zone often undulating with age, almost white overall when young, with somewhat milk white (1A2) pigment, then change to generally orange white (6A2), pale orange (6A3) or cinnamon (6D6) overall, paler towards margin, henna (7E8) to dark brown (8E8) at disc when old, darker in the groove, paler at margin. Lamellae adnate, sometimes with slightly decurrently tooth, furcate, intervenose, or anastomosing, often split, linear to ventricose, subdistant, L = 10–14, l = 1–2, white overall, often with light brown (6D4) to reddish brown (8E8) tint, up to 3 mm broad. Stipe 6–23 × 1–3 mm, central, insititious, hollow, pruinose, more or less cylindrical above, sometimes broadened in somewhere, often tapering towards base, pallid at the apex, orange white (6A2) or pale orange (6A3) to dark brown (8E8) below, black at base. Basidiospores (3.5)5–6.5 (7) × 3–4 μm (average = 5.77 × 3.42 μm, E = 1.43–2(–2.16), Q = 1.69), ellipsoid, amygdaliform, thin-walled, non-dextrinoid, hyaline, smooth. Basidia 21–35 × 4.5–6 μm, 4-spored, clavate, hyaline. Basidioles 21–35.5 × 4–6 μm, clavate, cylindrical, hyaline. Cheilocystidia sparse to abundant, 11–50 × 7–17 μm, ellipsoid, cylindrical, irregular clavate, narrowly utriform to narrowly fusiform with capitate apex, thin-walled. Pleurocystidia scattered, sparse, narrowly utriform to narrowly fusiform with capitate apex, 39–64.5 × 7–12.5 μm. Pileipellis a hymeniform layer of clavate to sphaeropedunculate smooth cells measuring 12.5–29.5 × 9–15.5 μm, possibly gelatinized. Pileiocystidia 35.5–51 × 8–16.5 μm, fusiform, narrowly lageniform to lageniform with capitate apex, thin-walled. Stipitipellis a cutis, of cylindrical, parallel, slightly thick-walled, non-dextrinoid, up to 19.5 μm wide hyphae. Caulocystidia 35–59 × 6–12 μm, cylindrical to broadly cylindrical with or without capitate apex, sometimes lobed, thick-walled. Clamp connections present in all tissue.
Material examined: China, Guizhou Province, Qiandongnan Miao Autonomous Prefecture, Liping County, Dongfeng tree farm, on the dead trunk of Cunninghamia lanceolata (Lamb.) Hook., (Cupressaceae), 17 October 2020, Ji-Peng Li (LJP563), HGASMF01-10,709 (holotype), ibid., 17 October 2020, Ji-Peng Li (LJP564), HGASMF01-10,708 (paratype).
GenBank numbers: HGASMF01-10709- MW326779 (ITS), MW332104 (LSU).
HGASMF01-10708- MW326780 (ITS), MW332105 (LSU)
Notes: Rhizomarasmius cunninghamietorum has an insititious stipe, a pileipellis as a hymeniform layer of clavate to sphaeropedunculate smooth cells, with narrowly lageniform to lageniform with capitate apex pileocystidia. These morphological characteristics of R. cunninghamietorum are consistent with the circumscription of Rhizomarasmius (Moreau et al. 2015). The phylogenetic evidence showed that R. cunninghamietorum formed a single branch and was clearly separate from the known species of Rhizomarasmius and closer to its related genera Cibaomyces and Gloiocephala (Fig. 159). However, Cibaomyces was accepted as a monotypic genus based on the subglobose to broadly ellipsoid basidiospores with conspicuous spines and Gloiocephala sensu Singer with smooth spores, subcapitate to capitate cystidia, a gelatinized trama (Singer 1976a, b, 1986) is probably polyphyletic (Moncalvo et al. 2002; Binder et al. 2006; Vizzini et al. 2012; Hao et al. 2014). Thus, Rhizomarasmius is probably polyphyletic based on the phylogenetic analysis generated here. Morphologically, among the known species of Rhizomarasmius, R. epidryas has entirely brown-black and distinctly velutinose stipe, bigger basidiospores (average 8.85 × 5.7 μm) and narrowly clavate or cylindrical pleurocystidia (Ronikier 2009); R. setosus with different habitat on leaf petioles and its microscopic features without clamps (Antonín and Noordeloos 2010); Rhizomarasmius oreinus has smooth red-brown pileus, entirely tomentose pubescent stipe and bigger basidiospores (average 12.5 × 8.8 μm, Moreau et al. 2015); R. pyrrhocephalus has pubescent to tomentose stipe, and bigger basidiospores (average 8.2 × 3.7 μm, Desjardin 1989) and R. undatus has longer (5.0–6.3 cm) and strigose stipe (Smith 1836).
Phylogenetic tree derived from maximum likelihood analysis of a combined ITS and LSU sequence data. The tree is rooted with Laccariopsis mediterranea and Hydropus mediterraneus. Branches with bootstrap values ≥ 70% are shown at each branch and the bar represents 0.1 substitutions per nucleotide position. Ex-type strains are in black bold. The newly generated sequences are indicated in blue and bold type species
Polyporales Gäum.
Notes: Polyporales is a large group of Agaricomycetes, accommodating about 2500 species (He et al. 2019). The latest treatments and updated accounts of Polyporales are followed in Justo et al. (2017), He et al. (2019), and Wijayawardene et al. (2022).
Polyporaceae Fr. ex Corda [as 'Polyporei'], Icon. fung. (Prague) 3: 49 (1839).
Notes: Polyporaceae, typified by Polyporus P. Micheli ex Adans, currently includes 90 genera, most of which are polypores, rarely corticioid species (Justo et al. 2017; He et al. 2019; Wijayawardene et al. 2022). Microscopically, the hyphal system is usually dimitic or trimitic, rarely monomitic; the generative hyphae are usually with clamp-connections; cystidia are mostly absent; basidiospores are thin- to thick-walled, smooth to ornamented and colorless to brown. All species produce a white rot (Justo et al. 2017).
Grammothele Berk. & M.A. Curtis, Journal of the Linnean Society. Botany 10: 327 (1869).
Notes: Grammothele, typified by G. lineata Berk. & M.A. Curtis, accommodates 24 species (Zhou and Dai 2012; Ryvarden 2015; Yuan 2015; Wu et al. 2016; Hyde et al. 2019). The genus is characterized by having resupinate basidiomata with shallow poroid hymenophore consisting of angular, partly sinuous, irregular or incomplete pores, dimitic or trimitic hyphal system with clamped generative hyphae and dextrinoid skeletal hyphae, usually presence of dendrohyphidia, and ellipsoid to cylindrical basidiospores that are thin-walled, smooth, colorless and not reacting both in Melzer’s reagent and Cotton Blue. Species of Grammothele commonly occur on hardwoods and monocotyledons in tropical to subtropical regions, causing a white rot (Ryvarden 1979, 2015). Previous phylogenetic studies have shown that Grammothele is polyphyletic and closely related to some other genera in the Polyporaceae, such as Porogramme (Pat.) Pat., Theleporus Fr. and Tinctoporellus Ryvarden (Zhou and Dai 2012; Yuan 2015; Wu et al. 2016; Hyde et al. 2019).
Grammothele taiwanensis C.C. Chen, sp. nov.
Index Fungorum number: IF900073; Facesoffungi number: FoF13396; Fig. 160
Grammothele taiwanensis (GC 1704–17, holotype). a, b Basidiomata. c Generative hyphae from trama. d Skeletal hyphae from trama. e Generative hyphae from context. f Skeletal hyphae from context. g Dendrohyphidia. h Hyphidia. i Cystidioles. j Basidia. k Basidiospores. Scale bars: a–b = 1 cm, b–j = 10 μm, k = 5 μm
Etymology: Referring to the type locality, Taiwan.
Holotype: GC 1704-17
Basidiomata annual, resupinate, effused, adnate, corky when dry, up to 15 cm long, 6 cm wide, and 1.3 mm thick in section, sparsely cracked, growing beneath the bark; margin sterile, cottony or slightly fimbriate, sometimes indistinct. Pore surface cream, buff-yellow or buff upon drying, not changing in KOH; pores angular, 3–5 per mm; dissepiments thin, entire, sometimes lacerate. Tubes concolorous with pore surface, corky, up to 1 mm deep. Context cream, corky, up to 0.3 mm thick, or sometimes invisible. Hyphal system dimitic in both context and trama; generative hyphae nodose-septate; tissues not changing in KOH. Context generative hyphae colorless, unbranched, 2.5–3.5 µm diam, thin-walled; skeletal hyphae, colorless, slightly flexuous, occasionally branched, 2.5–3.5 µm diam, thick-walled with a wide lumen or almost solid, weekly dextrinoid. Tramal generative hyphae colorless, flexuous, frequently branched, 2–3.5 µm diam, thin-walled; skeletal hyphae colorless, slightly flexuous, rarely branched, 3–4.5 µm diam, thick-walled with a wide lumen or subsolid, often agglutinated in bundles, weekly dextrinoid. Cystidia absent. Cystidioles fusoid, colorless, thin-walled, 14–20 × 4.5–5 µm. Dendrohyphidia colorless, thin-walled, 9–23 × 1.5–2.5 µm. Hyphidia colorless, thin-walled, 19–26 × 2–3 µm. Basidia clavate or suburniform, with four sterigmata and a basal clamp connection, colorless, thin-walled, 17–25 × 6–7 µm. Basidiospores cylindrical, colorless, thin-walled, smooth, inamyloid, nondextrinoid, acyanophilous, mostly 7.9–10.5 × 3.3–4.4 µm. (7.8–)8.4–10.5(–12.3) × (3.5–)3.7–4.4(–5.1) µm, L = 9.4 µm, W = 4.1 µm, Q = 2.23–2.43 (n = 30) (holotype). (7–)7.9–9.4(–10.6) × (3–)3.3–3.9(–4.3) µm, L = 8.6 µm, W = 3.6 µm, Q = 2.3–2.45 (n = 30) (GC 1704–16).
Material examined: China: Taiwan, Nantou County, Yuchih Township, Shuishetashan Trail, 23° 51′ N, 120° 56′ E, 980 m, on angiosperm branch, 8 April 2017, GC 1704–17 (TNM F31469, holotype).
GenBank number: MW440487 (ITS).
Additional specimen examined: China: Taiwan, Nantou County, Yuchih Township, Shuishetashan Trail, 23° 51′ N, 120° 56′ E, 980 m, on angiosperm branch, 8 April 2017, GC 1704–16 (TNM F31468).
GenBank number: MW440486 (ITS).
Notes: Grammothele taiwanensis is characterized by having resupinate, cream to buff basidiomata with larger pores (3–5 per mm), dimitic hyphal system with clamped generative hyphae and weekly dextrinoid skeletal hyphae, presence of fusoid cystidioles, dendrohyphidia and hyphidia, and cylindrical basidiospores (7.9–10.5 × 3.3–4.4 µm). Grammothele taiwanensis resembles G. pulchella (Bres.) Ryvarden in having similar pore and basidiospore sizes [4–5 per mm, 7–10 × 3–4 µm in Hjortstam and Ryvarden (1982), as G. ochracea Ryvarden] and presence of dendrohyphidia; however, G. pulchella differs from this species in having cork-colored to wood-colored pore surface and pale brown context, and absence of cystidioles and hyphidia (Hjortstam and Ryvarden 1982). Grammothele taiwanensis is also similar to G. hainanensis F. Wu & L.W. Zhou and G. hondurensis (Murrill) Ryvarden, but the latter two species have hyphal pegs and smaller basidiospores [G. hainanensis: 7–8.1 × 2.3–2.9 µm in Wu et al. (2016); G. hondurensis: 5–8 × 3–3.5 µm in Ryvarden (1985)]. Phylogenetically, two sequences of G. taiwanensis formed an isolated lineage with high support (Fig. 161).
Phylogram generated from maximum likelihood analysis based on ITS sequence data of Grammothele taiwanensis (in bold) and related species. The selection of strains and species consulted Zhou and Dai (2012), Yuan (2015), Wu et al. (2016) and Hyde et al. (2019). Funalia trogii is used as the outgroup taxa. Forty-six strains are included in the sequence analyses, which comprise 665 characters with gaps. The tree topology of the ML analysis was similar to the BI. The best scoring RAxML tree with a final likelihood value of is presented. Bootstrap support values for ML ≥ 70%, BYPP ≥ 0.9 are given above the nodes
Incrustoporiaceae Jülich, Biblthca Mycol. 85: 373 (1982) [1981].
Notes. Incrustoporiaceae was established by Jülich (1981) and typified by Incrustoporia Domański. This family was treated as a synonymy of Polyporaceae according to the 10th edition of Dictionary of the Fungi (Kirk et al. 2008). Justo et al. (2017) provided a revised family-level classification of the Polyporales based on phylogenetic analyses inferred from ITS, nLSU and rpb2 genes, and four genera were accepted in Incrustoporiaceae, viz., Incrustoporia, Piloporia Niemelä, Skeletocutis Kotl. & Pouzar and Tyromyces P. Karst.
Skeletocutis Kotlába & Pouzar, Ceská Mykologie 12 (2): 103 (1958).
Notes: Skeletocutis Kotl. & Pouzar was established in 1958 and its type species is S. amorpha (Fr.) Kotl. & Pouzar (Kotlába and Pouzar 1958). The genus has resupinate to pileate basidiocarps, encrusted generative hyphae covered by fine crystals and tiny basidiospores, these are the most important characteristics of the genus. In addition, species in Skeletocutis cause white rot disease (Niemelä 1998). Species in this genus have wide distribution in the world, but the majority of the known species so far are found in the Northern Hemisphere (Gilbertson and Ryvarden 1986; Núñez and Ryvarden 2001; Ryvarden and Melo 2014). There are more than 60 taxa recorded in Skeletocutis and 25 of them occur in China (Cui and Dai 2008; Dai 2012; Zhou and Qin 2012; Bian et al. 2016; Fan et al. 2017; Du and Dai 2020).
Recently, taxonomic and phylogenetic studies of polypore fungi from China have been extensively carried out, and many new genera or new species have been found (Zhao et al. 2015; Han et al. 2016; Chen and Cui 2017; Chen et al. 2017a, b; Song and Cui 2017; Zhou and Cui 2017; Xing et al. 2018; Cui et al. 2019; Shen et al. 2019; Wu et al. 2019; Zhu et al. 2019; Sun et al. 2020a, b; Liu et al. 2021a, b), however, only very few studies have been focused on Skeletocutis of the Incrustoporiaceae. In this study, two new species of Skeletocutis are described from China based on morphological characteristics and phylogenetic analyses inferred from ITS + nLSU sequences.
Skeletocutis cangshanensis B.K. Cui & Shun Liu, sp. nov.
Index Fungorum number: IF559465; Facesoffungi number: FoF10674; Figs. 162 a, 163
Differs from other Skeletocutis species by its white pore surface when fresh, white to buff-yellow upon drying, small and circular to angular pores (7–10 per mm), and cylindrical basidiospores (2.7–3.5 × 0.8–1.5 μm).
Etymology. Cangshanensis (Lat.): refers to the type locality (Cangshan Park) of the type specimen.
Holotype: Cui 17978.
Fruiting body. Basidiocarps annual, resupinate, not easily separated from substrate, soft leathery, without odour or taste when fresh, becoming corky upon drying, up to 8.5 cm long, 3.5 cm wide, and 1 mm thick at center. Pore surface white when fresh, becoming white to buff-yellow upon drying; pores circular to angular, 7–10 per mm; dissepiments thick, entire. Subiculum white, corky, up to 0.2 mm thick. Tubes darker than poroid surface, corky, up to 0.5 mm long.
Hyphal Structure. Hyphal system dimitic; generative hyphae with clamp connections, hyaline, thin- to slightly thick-walled, dominant at dissepiment edge; skeletal hyphae thick-walled with a wide to narrow lumen; IKI–, CB–, unchanged in KOH.
Subiculum. Generative hyphae frequent, hyaline, thin- to slightly thick-walled, rarely branched and bearing fine crystals, 1.5–2.8 μm in diameter; skeletal hyphae dominant, thick-walled with a narrow lumen, flexuous, unbranched, interwoven, 2–3.5 μm in diameter.
Tubes. Generative hyphae frequent, thin-walled, frequently branched, usually covered by fine crystals, sharply pointed encrustations, especially at dissepiment edge, 1.5–2.5 μm in diameter; skeletal hyphae dominant, thick-walled with a wide to narrow lumen, occasionally branched, subparallel along the tubes, not agglutinated, 2–3 μm in diameter. Dissepiment edge dimitic with smooth skeletal hyphae, and dominant winding, encrusted generative hyphae. Cystidia absent, cystidioles abundant, bottle-shaped, with a conical apex, 7.5–17 × 3.2–4.7 μm. Basidia clavate, with a basal clamp connection and four sterigmata, 9.6–13.5 × 3.2–4.7 μm; basidioles in shape similar to basidia, but slightly smaller.
Spores. Basidiospores cylindrical, hyaline, thin-walled, smooth, IKI–, CB–, (2.6–)2.7–3.5 × 0.8–1.5 μm, L = 3.02 μm, W = 1.02 μm, Q = 2.76–3.23(n = 90/3).
Material examined: China, Yunnan Province, Dali, Cangshan Park, on angiosperm stump, 4 November 2019, Cui 17978 (holotype, BJFC).
Additional specimens examined. China, Yunnan Province, Dali, Cangshan Park, on fallen angiosperm branch, 4 November 2019, Cui 17990, 17994 (Paratypes, BJFC).
GenBank numbers: Cui 17978—MZ327279 (ITS), MZ348535 (LSU),
Cui 17990—MZ327280 (ITS), MZ348536 (LSU).
Cui 17994—MZ327281 (ITS), MZ348537(LSU).
Notes: In the phylogenetic tree, the three specimens of Skeletocutis cangshanensis formed a highly supported lineage (Fig. 164), and grouped together with S. bambusicola L.W. Zhou & W.M. Qin. Morphologically, both S. cangshanensis and S. bambusicola have similar pores, but S. bambusicola differs by having wider basidiospores (2.5–3 × 1.5–2 μm) and growth on Bambusa (Zhou and Qin 2012). Skeletocutis lepida A. Korhonen & Miettinen, S. mopanshanensis C.L. Zhao and S. yunnanensis L.S. Bian, C.L. Zhao & F. Wu were also discovered from Yunnan Province. Skeletocutis lepida differs from the new species by having narrower basidiospores (2.9–3 × 0.5–0.6 µm; Korhonen et al. 2018); S. mopanshanensis differs by having larger pores (4–5 per mm) and basidiospores (4.7–6.6 × 3.2–4.5 µm; Wu et al. 2017); S. yunnanensis differs by having larger pores (5–6 per mm) and basidiospores (3.4–4.5 × 1–1.2 µm; Bian et al. 2016).
Maximum likelihood tree illustrating the phylogeny of Skeletocutis based on the combined sequences dataset of ITS + nLSU. Branches are labeled with maximum likelihood bootstrap higher than 50%, parsimony bootstrap proportions higher than 50% and Bayesian posterior probabilities more than 0.90 respectively. Bold names = New species
Skeletocutis subchrysella B.K. Cui & Shun Liu, sp. nov.
Index Fungorum number: IF559466; Facesoffungi number: FoF10675; Figs. 162 b, 165
Diagnosis: Differs from other Skeletocutis species by its white to cream pore surface when fresh, cream to cinnamon-buff upon drying, and allantoid basidiospores (2.7–3.2 × 0.7–1 μm).
Etymology. Subchrysella (Lat.): refers to the new species resembling Skeletocutis chrysella Niemelä in morphology.
Holotype: Cui 17748.
Fruiting body. Basidiocarps annual, resupinate, not easily separated from substrate, soft leathery, without odour or taste when fresh, becoming corky upon drying, up to 6 cm long, 1.5 cm wide, and 3 mm thick at center. Pore surface white to cream when fresh, becoming cream to cinnamon-buff upon drying; pores angular, 6–8 per mm; dissepiments slightly thick, entire to lacerate. Subiculum cream, corky, up to 0.5 mm thick. Tubes darker than poroid surface, corky, up to 2 mm long.
Hyphal Structure. Hyphal system dimitic; generative hyphae with clamp connections, hyaline, thin-walled, dominant at dissepiment edge; skeletal hyphae with a wide to narrow lumen; IKI–, CB–, unchanged in KOH.
Subiculum. Generative hyphae frequent, hyaline, thin-walled, unbranched and bearing fine crystals, 1.5–2.8 μm in diameter; skeletal hyphae dominant, thick-walled with a narrow lumen, flexuous, unbranched, interwoven, 2–4 μm in diameter.
Tubes. Generative hyphae frequent, thin-walled, unbranched, usually covered by fine crystals, sharply pointed encrustations, especially at dissepiment edge, 1.5–2.5 μm in diameter; skeletal hyphae dominant, thick-walled with a wide to narrow lumen, unbranched, subparallel along the tubes, not agglutinated, 2–3.7 μm in diameter. Dissepiment edge dimitic with smooth skeletal hyphae, and dominant winding, encrusted generative hyphae. Cystidia absent, cystidioles abundant, bottle-shaped, with a conical apex, 8–13.8 × 2–3.6 μm. Basidia clavate, with a basal clamp connection and four sterigmata, 10.3–15.6 × 3.2–4.5 μm; basidioles in shape similar to basidia, but slightly smaller.
Spores. Basidiospores allantoid, hyaline, thin-walled, smooth, IKI–, CB–, (2.6–)2.7–3.2 × (0.5–)0.7–1 μm, L = 2.93 μm, W = 0.8 μm, Q = 3.33–4.5(n = 60/2).
Material examined: China, Sichuan Province, Shimian County, Liziping National Nature Reserve, on fallen angiosperm trunk, 14 September 2019, Cui 17748 (holotype, BJFC).
Additional specimen examined: China, Yunnan Province, Baoshan, Gaoligongshan Nature Reserve, on fallen angiosperm trunk, 8 November 2019, Cui 18141 (paratype, BJFC).
GenBank numbers: Cui 17748- MZ327278 (ITS), MZ348534 (LSU).
Cui 18141- MZ327278 (ITS), MZ348538(LSU).
Notes: In the phylogenetic tree (Fig. 164), Skeletocutis subchrysella grouped with S. chrysella, S. delicata Niemelä & Miettinen, S. exilis Miettinen & Niemelä and S. kuehneri A. David. Morphologically, they all have allantoid basidiospores; but S. chrysella differs from S. subchrysella in its trimitic hyphal system and longer basidiospores (2.8–4.5 × 0.7–1 μm; Niemelä 1998); S. delicata and S. exilis differ by having larger pores (3–6 per mm in S. delicata, 3–5 per mm in S. exilis) and basidiopores (3.2–4.2 × 1.1–1.4 µm in S. delicata, 3.2–3.9 × 0.9–1.1 µm in S. exilis; Miettinen and Niemelä 2018); S. kuehneri differs by having thin and brittle basidiocarps and growth on dead wood of Picea and Pinus (David 1982). Skeletocutis bambusicola, S. lepida, S. mopanshanensis and S. yunnanensis were also discovered from Yunnan Province. Skeletocutis bambusicola differs from the new species by having smaller pores (8–11 per mm) and ellipsoid basidiospores (2.7–3.1 × 1.5–1.9 µm; Zhou and Qin 2012); S. lepida differs by having half-resupinate basidiocarps with ochraceous upper surface when dry (Korhonen et al. 2018); S. mopanshanensis differs by having larger pores (4–5 per mm) and basidiospores (4.7–6.6 × 3.2–4.5 µm; Wu et al. 2017); S. yunnanensis differs by having larger basidiospores (3.4–4.5 × 1–1.2 µm; Bian et al. 2016).
Psathyrellaceae Vilgalys, Moncalvo & Redhead, in Redhead et al., Taxon 50(1): 226 (2001).
Wächter and Melzer (2020) based on phylogenetic and morphological characteristics, introduced six new monophyletic genera to this family. Wijayawardene et al. (2022) accepted 21 genera in Psathyrellaceae.
Coprinopsis cinerea (Schaeff.) Redhead, Vilgalys & Moncalvo, in Redhead et al., Taxon 50 (1): 227 (2001).
Index Fungorum number: IF474379; Faceoffungi number: FoF11681; Figs. 166 , 167 , 168
Coprinopsis cinerea associated with root rot of cowpea: a Cowpea root showing fungal mycelium. b Close view of root showing white mycelium. c Root sample incubated at room temperature showing the formation of young basidiocarp. d Root samples showing emergence of basidiocarps on incubation at room temperature. e–f Close view of basidiocarp showing characteristic feature of Coprinopsis cinerea. g Basidium of Coprinopsis cinerea recorded. Scale bar: g = 20 µm
Saprobic on roots of Vigna unguiculata. Sexual morph: Basidiomycetous. Basidiocarp 0.3–4 cm long, formed on the roots of cowpea, mycelial strands encircled the host tissues on the root region, and basidiocarp emerged upon incubation in moist chamber. Pileus strongly convex, and/or parasol-shaped to flat or depressed, hymenium borne on gills, becoming deliquescent and inky, lamellae thin, basidia unmodified, basidiospores 7.5–12.2 × 5 − 10 μm diam., ballistosporic, blue-black, smooth with a distinct germ pore. Asexual morph: not obesrved.
Cultural characteristics: On PDA medium, colony were white and free from fruiting bodies till 5 days but later, development of basidiocarp was noticed which later enlarged and produced typical fruiting body. Upon examination of the basidiocarp from the PDA plates, microscopic examination of basidiospores revealed that they were same in morphological features.
Material examined: India, Karnataka, Mysore, Doddamaragowdanahally, on infected leaves of Vigna unguiculata (L.) Walp. (Fabaceae) placed on PDA as secondary saprophytes, 18 May 2017 Mahadevakumar (UOM-IOE-18/24), living culture (MD8).
Habitat: commonly found on dung or wood chips (Kamada 2002), roots and leaves of Vigna unguiculate (This study).
Distribution: worldwide.
GenBank number: OM812073(ITS).
Notes: Coprinopsis species are known to grow on various substrates as secondary saprophytes and are also known to occur as primary components of soil and leaf litter. However, an association of Coprinopsis species has not been reported on any crop plants. Cowpea plants affected with a characteristic white cottony mycelium profusely grown on roots were observed which were eventually wilted and dried off which caused the death of the host plants. Infected materials were collected and subjected to pathogen isolation. Few infected parts were incubated at room temperature and observed for mycelial development. To our surprise, a characteristic mushroom fruiting bodies were developed. Therefore, the spores were photographed and subjected for identification. Here, the mushrooms are developed in three parts. Infected roots collected from the field, root samples incubated at room temperature and leaves incubated in a moist chamber (in a Petri plate) showed the development of small basidiocarp. Upon microscopic examination, it was identified as Coprinopsis sp. based on fruiting bodies, basidia, basidiospores, sterigmata and other associated structures. This is the first time that C. cinerea is reported from Fabaceae, Vigna unguiculata representing a new host record (Fig. 169).
Phylogenetic tree for species of Coprinopsis generated from maximum likelihood (RAxML) based on ITS region. The Maximum likelihood bootstrap value ≥ 50% are given at the nodes. The newly generated sequences are in blue. The tree is rooted to Parasola auricoma (SZMC.NL:0087) and Parasola conopilea (LO186.02)
Thelephorales Corner ex Oberw.
Notes: The order was established by Oberwinkler (1976) based on the type family Thelephoraceae Chevall. Thelephorales is reported to incorporate two families: Thelephoraceae and Bankeraceae Donk, and ten major clades: Amaurodon, Boletopsis, Hydnellum/Sarcodon, Lenzitopsis, Odontia, Phellodon/Bankera, Pseudotomentella/Polyozellus, Sarcodon, Thelephora/Tomentella and Tomentella (Stalpers 1993; Vizzini et al. 2016). Twelve genera and about 800 described species are accommodated within the order according to Index Fungorum 2022a, b (http://www.indexfungorum.org). Wijayawardene et al. (2022) accepted 14 genera in this order.
Thelephoraceae Chevall. [as’Thelephoreae’], Fl. Gén. Env. Paris (Paris) 1: 84 (1826).
Notes: Thelephoraceae was proposed by Chevall (1826), and Thelephora Ehrh. ex Willdwas regarded as its type genus. Thelephoraceae, as a relatively large one, comprises nine genera within the Thelephorales (Wijayawardene et al. 2022). Many species in the family, as ectomycorrhiza formers, are believed to be of great ecological importance in maintaining the balance of terrestrial ecosystems (Haug et al. 2005; Jakucs and Erős-Honti 2008; Kuhar et al. 2016).
Tomentella Pers. ex Pat., Hyménomyc. Eur. (Paris): 154 (1887).
Notes: Tomentella was validated by Patouillard (1887). Species of the genus usually form cottony or spider web–likereproductive structures and grow on fallen wood, leaf litter, soil and other substrates (Larsen 1974; Tedersoo et al. 2003). Around 400 names have been recorded and about 200 species were described. The species of Tomentella are reported to be widely distributed throughout the world and have been immensely taxonomically studied from Eurasia, North America, South America, Australia, Asia and WestAfrica (Thind and Rattan 1971; Larsen 1998; Agerer et al. 2001; Yorou and Agerer 2008; Alvarez-Manjarrez et al. 2015; Kuhar et al. 2016). During the investigation of resupinate-thelephoroid fungi from the subtropical forests in China, several Tomentella specimens were collected, and two undescribed species have been identified using morphological characteristics and molecular phylogenetic analyses (Fig. 173).
Tomentella exiguelata Y.H. Mu & H.S. Yuan, sp. nov.
Index Fungorum number: IF900080; Facesoffungi number: FoF13398; Figs. 170, 171, 172
Etymology: Refers to the presence of slightly thick-walled basidia.
Holotype: IFP 019495
Basidiocarps annual, resupinate, adherent to the substrate, mucedinoid, without odour or taste when fresh, 0.5–1 mm thick, continuous. Hymenophoral surface granulose, grayish brown to light brown (6D3–6D4) and concolorous withsubiculum when dry. Sterilemargin often determinate, farinaceous, concolorous with hymenophore. Rhizomorphs absent. Subicular hyphae monomitic; generative hyphae clamped and rarely simple septate, thin- to slightly thick-walled, moderately branched, 3–5μmdiam, without encrustation, yellow in KOH, cyanophilous in sightly thick-walled hyphae, inamyloid. Subhymenial hyphae clamped and rarely simple septate, thin-to slightly thick-walled, frequently branched, 3–5 μm diam; hyphal cells more or less uniform, yellow in KOH, cyanophilousin sightly thick-walled hyphae, inamyloid. Cystidia absent. Basidia10–47 μm long and 4–8 μm diam at apex, 3–5μmat base, with a clamp connection or simple septate at base, utriform, thin-walled and rarely slightly thick-walled, not stalked, sinuous, yellow in KOH, yellow in distilled water, 4-sterigmate; sterigmata 2.5–4.5μmlong and 0.5–1 μm diam at base. Basidiospores thick-walled, (6.2–)7.1–8.1(–8.3) × (5.4–)5.8–6.9(–7.1) μm, L = 7.33 μm, W = 6.21 μm, Q = 1.18–1.21 (n = 60/2), subglobose, triangular or lobed in frontal view and subglobose to ellipsoid in lateral view, echinulate to aculeolate, yellow in KOH and in distilled water, cyanophilous, inamyloid; echinuli usually isolated, sometimes grouped in 2 or more, up to 2.5 μm long.
Material examined: China, Zhejiang Province, Kaihua County, Gutianshan National Nature Reserve, on fallen angiosperm debris, 24 July 2018, Yuan 12805 (IFP 019495, holotype); on fallen angiosperm branch, 25 July2018, Yuan 12900 (IFP 019496, paratype).
GenBank numbers: IFP 019495- MZ329771(ITS), MZ329775 (LSU).
IFP 019496- MZ329772 (ITS), MZ329776 (LSU)
Notes: Tomentella exiguelata forms a close phylogenetic relationship with T. galzinii and T. subtestacea (Fig. 173). Tomentella galzinii and T. exiguelata share the following similar morphological and anatomical characteristics: mucedinoid basidiocarps adherent to the substrate, granulose hymenophoral surface, farinaceous sterile margin, similar-wide subhymenial hyphae, and not stalked and sinuous basidia. However, T. galzinii has discontinuous and dull green to olive-brown basidiocarps, indeterminate sterile margin, and the presence of cystidia (Bourdot and Galzin 1924). Tomentella subtestacea resembles T. exiguelata in mucedinoid basidiocarps adherent to the substrate, clamped and rarely simple septate subicular hyphae, thin-to slightly thick-walled subhymenial hyphae, utriform and not stalked basidia and echinulate basidiospores of similar length. However, T. subtestacea can be distinguished by reddish-brown to grayish buff hymenophoral surface, arachnoid sterile margin, and the presence of cystidia (Svrček 1958).
Phylogram generated from maximum likelihood analysisbased on combined ITS and LSU sequence data. Related sequences were obtained from GenBank and Unite. One hundred and ten strains are included in the combined sequence analyses, which comprise 1451 characters withgaps. Single gene analyses were also performed and topology andclade stability compared from combinbed gene analyses. Odontia ferruginea (TU110988) is used as the outgroup taxon. Bootstrap support values for ML ≥ 50% are given. The newly generated sequences are in bold
Tomentella fuscoaraneosa Y.H. Mu &H.S. Yuan, sp. nov.
Index Fungorum number: IF900079; Facesoffungi number: FoF13399; Figs. 174 , 175 , 176
Etymology: Refers to the brown and arachnoid basidiocarps.
Holotype: IFP019493
Basidiocarps annual, resupinate, separable to the substrate, arachnoid, without odour or taste when fresh, 0.8–1.5 mm thick, continuous. Hymenophoral surface smooth, light brown to brown (7D8–7E8) and concolorous with subiculum when dry. Sterile margin often determinate, byssoid, concolorous with hymenophore. Rhizomorphs present in subiculum and margin, 10–35 μm diam; rhizomorphic surface more or less smooth; hyphae in rhizomorphmonomitic, undifferentiated, of type B, compactly arranged and of uniform; single hyphae with both clamps and simple septa, thick-walled, unbranched 2–3 μm diam, yellow in KOH, cyanophilous, inamyloid. Subicular hyphae monomitic; generative hyphaeclamped and rarely simple septate, thin- to slightly thick-walled, frequently branched, 2–3 μm diam, withoutencrustation, yellow in KOH, cyanophilous in sightly thick-walled hyphae, inamyloid. Subhymenial hyphae clamped and rarely simple septate, thin-walled, occasionally branched, 2.5–4 μm diam, without encrustation; hyphal cells short and not inflated, yellow in KOH, acyanophilous, inamyloid. Cystidia absent. Basidia10–65 μm long and 4–7 μm diam at apex, 2–4 μm at the base, with a clamp connection or simple septate at the base, utriform, thin-walled, stalked, sinuous, occasionally with transverse septa, yellow in KOH, yellow in distilled water, 4-sterigmate; sterigmata 2–5 μm long and 1–2 μm diam at the base. Basidiospores thick-walled, (5–)5.5–7.5(–8) × (4–)4.5–6(–7) μm, L = 6.29 μm, W = 5.32 μm, Q = 1.12–1.18 (n = 60/2), subglobose to ellipsoid in frontal view and subglobose to ellipsoid in lateral view, echinulateto aculeolate, yellow in KOH, yellow in distilled water, cyanophilous, inamyloid; echinuli usually isolated, sometimes grouped in 2 or more, up to 1.5 μm long.
Material examined: China, Zhejiang Province, KaihuaCounty, Gutianshan National Nature Reserve, on fallen angiosperm branch, 25 July 2018, Yuan 12875(IFP 019493, holotype); on fallen angiosperm branch, 25 July2018, Yuan 12910 (IFP 019494, paratype).
GenBank numbers: IFP 019493-MZ329769 (ITS), MZ329773 (LSU).
IFP 019493-MZ329770 (ITS), MZ329774 (LSU)
Notes: Tomentella fuscoaraneosa is closely related to T. aureomarginata in the phylogeny (Fig. 173). In morphology, T. fuscoaraneosa is similar to T. aureomarginata in having a determinate and byssoid sterile margin, the presence of type B rhizomorphs, monomitic generative hyphae with clamps and simple septa, and the absence of cystidia. However, T. aureomarginata differs from T. fuscoaraneosa by having pelliculose basidiocarps adherent to the substrate, golden brown to yellowish-brown hymenophoral surface turning darker when dry, not stalked basidia and wider basidiospores (6–6.5 μm vs. 4.5–6 μm in T. fuscoaraneosa) with shorter echinuli (up to 1 μm vs. up to 1.5 μm in T. fuscoaraneosa) (Yuan et al. 2020). Tomentella fuscoaraneosa shares common features with T. brunneoflava in arachnoid and continuous basidiocarps, the presenceof rhizomorphs and clamped and rarely simple septate subicular hyphae and short and not inflated subhymenial hyphae. However, T. brunneoflava differs from T. fuscoaraneosa by having brownish-yellow basidiocarps adherent to the substrate and clavate and not stalked basidia (Yuan et al. 2020).
Agaricales genera incertae sedis
Gerronema Singer, Mycologia 43(5): 599 (1951).
Gerronema is a minor genus of lignicolous agaric and is distributed worldwide (Singer 1986). There are 66 epithets (excluding synonyms) listed in the Index Fungorum (2021). Gerronema. melanomphax Singer is the specific type species. Historically, different circumscriptions of Gerronema based on pigmentations established by Singer (1964) and Bigelow (1970) have been problematic. Subsequently, Redhead (1986) and Norvell et al. (1994) restricted the delimitation of Gerromena and characterized its descriptive features as elastic to fleshy, omphalinoid to clitocyboid basidiomata, white spore print, smooth, thin-walled, inamyloid basidiospores, cutis pileipellis often with intracellular pigment, sarcodimitics trama tissue and lignicolous habit. This circumscription correlates with the molecular phylogenetic analysis of the combined nrITS and nrLSU dataset and revealed that Gerronema is monophyletic and forms a clade with Megacollybia and Trogia in Hydropoid calde (Antonín et al. 2019).
Gerronema atrovirens Wannathes, N. Suwannarach, J. Kumla, Phonrob & S. Lumyong, sp.nov.
MycoBank number: MB840183; Facesoffungi number: FoF 10685, Figs. 177 a, 178
Etymology: ‘atro’ = dark; ‘virens’ = green, refers to the dark green colour of basidiomata.
Holotype: BKF10264
Pileus 21–51 mm diam., convex with depress center when young and deeply infundibuliform in age, elastic, glabrous, translucent-striate to striate at margin, dull green (27E3) at center, dark green (27F3) at margin, grey (5E1) to brownish grey (5E2) in old specimen; Lamellar decurrent, subdistant to close (24–28) with 1–2 series of lamellulae, narrow (up to 1.5 mm), withe (27A1), non-marginate; Stipe 14–36 × 2.5–4.0 mm, cylindrical, slightly broadened at base, usually flatten, flexuose, hollow, elastic, central, pubescent, greenish grey (27F2) at apex fading paler to brownish grey (5C2) at base, basal mycelium. Context thin, elastic. Odor and taste not distinctive.
Basidiospore 7–8 × 4–5 µm [x = 7.56 ± 0.51 × 4.36 ± 0.49, Q = 1.4–2.0, q = 1.75 ± 0.21, n = 25, s = 3] ellipsoid, smooth, hyaline, inamyloid, thin-walled. Basidia 21–27 × 6–7 μm, clavate, with 4 sterigmata, sometime with 2 sterigmata, thin-walled, inamyloid. Cheilocystidia abundant, 21–51 × 5–11 µm, cylindrical with 1–2 slight constrictions, flexuose, irregular in shape, sometimes 2-celled, hyaline, inamyloid, thin-walled. Pleurocystidia absent. Lamellar trama subregular to interwoven, arranged in two directions, hyphae 4–8 µm diam., cylindrical, smooth, hyaline, inamyloid, thin-walled, gelatinous. Pileipellis composed with cutis of repent hyphae, radially arrangement, 3–10 µm, cylindrical, incrusted, greyish green in KOH, inamyloid, thin-walled, true pileocystidia absent. Pileus trama sarcodimitic, subregular, composed of 2 type of hyphae: a) sarco-hyphae, elongate fusoid cell 162–195 × 5–15 µm, hyaline, smooth, inamyloid, thick-walled (up to 1 µm); b) generative hyphae 2–6 µm wide, cylindrical, branched, hyaline, smooth, inamyloid, thin-walled. Stipitipellis cutis, hyphae 3–8 µm diam., parallel, cylindrical, greenish brown in KOH, smooth, inamyloid, thin-walled. Stipe trama sarcodimitic, subparallel, composed 2 type of hyphae: a) sarco-hyphae, elongate fusoid cell 125–200 × 3–25 µm, hyaline, smooth, inamyloid, thick-walled (up to 1 µm); b) generative hyphae 2–13 µm wide, cylindrical, branched, hyaline, smooth, inamyloid, thin-walled. Caulocystidia abundant, 19–34 × 5–9 µm, cylindrical with 1–2 constrictions, flexuose, irregular in shape, hyaline to pale green in KOH, inamyloid, thin-walled. Clamp connections present in all tissues.
Habit, habitat and known distribution: Gregarious on decayed bamboo wood, known only from Thailand.
Material examined: Thailand, Sukhothai Provinve, Si Satchanalai National Park, Natural trail, 22 Aug 2020, collector N Wannathes N Suwannarach and J Kumla, BKF10264 (holotype).
Additional material examined: Thailand, Sukhothai Province, Si Satchanalai National Park, Natural trail, 23 Aug 2020, collector N Wannathes N Suwannarach and J Kumla, BKF10265, NW1372 (isotype).
GenBank numbers: BKF10264- MZ452088(ITS), MZ452671(LSU).
BKF10265- MZ452668(ITS), MZ452672 (LSU)
Notes: Gerronema atrovirens is characterized by a medium size of omphaliod, elastic basidiomata, convex with depress center to deeply infundibuliform, translucent striate at margin, dull green pileus, decurrent, subdistant lamellae, cylindrical, central, greenish grey stipe, ellipsoid basidiospores with mean 7.6 × 4.4 µm, pileipellis composed of incrusted hyphae with greyish green colour, present of irregular cylindrical cheilocystidia and cualocystidia, sarcodimitic trama tissue, and grow on decayed bamboo wood. A new species is morphologically similar to G. cyathiforme (Berk. & M.A. Curtis) Singer, species originally described from a Neotropic, differs in forming a distinct radial stripes pileus, absent of cystidia, and mahogany red (in KOH) lamellar trama (Singer 1970). Gerronema atrovirens is also closely related G. indigoticum T. Bau & L.N. Liu, a green–blue species from subtropical China. The latter species differs in forming smaller pilei (9–16 mm wide) with green–blue, shorter (20–27 × 8–12 µm), simple clavate cheilocystidia, and simple clavate cualocystidia (Lui et al. 2019), and the phylogenetic analyses inferred from combined sequences (Fig. 179) confirmed that G. atrovirens is closely allied with G. indigoticum and it distinct species from related morphological species and other taxa in this genus.
Phylogenetic tree derived from maximum likelihood analysis of a combined ITS and LSU genes of 21 sequences and the aligned dataset was comprised of 1700 characters including gap. The average standard deviation of the split frequencies of the BI analysis was 0.00612. Togia infundibuliformis KUN HKAS56709 and T. venenata KUN HKAS56679 were used as outgroup. Numbers above branches are the bootstrap statistics percentages (left) and Bayesian posterior probabilities (right). Branches with support values ≥ 70%/0.90 are shown at each branch and the bar represents 0.1 substitutions per nucleotide position. Hyphen (-) represents support values ≤ 70%/0.95. Ex-type strains are in black bold. The newly generated sequences are indicated in blue and bold type species
Gerronema flavum Wannathes, N. Suwannarach, J. Kumla, Phonrob & S. Lumyong, sp.nov.
MycoBank number: MB 840184; Facesoffungi number: FoF 10686; Figs. 177 c, 180
Etymology: ‘flavum’ = yellow, refers to the colour of basidiomata.
Holotype: BKF10253
Pileus 4–11 mm diam., convex, umbonate when young and plano-convex with depress in center to infundibuliform in age, glabrous, radially fibrillose when young, translucent striate to striate at marging in age, dull, light yellow (4A5) overall when young, sunflower yellow (4A7) overall in age, hygrophanous, become yellowish white. Lamellar decurrent, subdistant (14–16) with 2–3 series of lamellulae, narrow, yellowish withe (4A2), non-marginate; Stipe 4–19 × 1–2 mm, tapering upward when young, cylindrical in age, elastic, hollow, central, flexuose, pubescent, yellowish withe (4A2) overall. Context thin, Odor and taste not distinctive.
Basidiospore 7–8(–9) × 4–5(–6) μm [x = 7.68 ± 0.63 × 4.68 ± 0.56, Q = 1.3–2.25, q = 1.66 ± 0.23, n = 25, s = 1] broadly ellipsoid, smooth, hyaline, inamyloid, thin-walled. Basidia 25–32 × 6–9 μm, clavate, with 4 sterigmata, thin-walled, inamyloid. Cheilocystidia abundant, 25–40 × 5–8 µm, knobby cylindrical to clavate, irregular in shape, hyaline, inamyloid, thin-walled. Pleurocystidia abundant, 24–33 × 9–11 µm, clavate to cylindrical, sometime knobbed, hyaline, inamyloid, thin-walled. Lamellar trama interwoven, hyphae 3–6 µm diam., cylindrical, smooth, hyaline, inamyloid, thin-walled. Pileipellis composed with cutis of repent hyphae, radially arrangement, 4–15 µm, cylindrical, non-incrustation, hyaline in KOH, inamyloid, thin-walled, true pileocystidia absent. Pileus trama sarcodimitic, interwoven, composed of 2 type of hyphae: a) sarco-hyphae, elongate fusoid cell 50–60 × 10–14 µm, hyaline, smooth, inamyloid, thin-walled; b) generative hyphae 3–11 µm wide, cylindrical, branched, hyaline, smooth, inamyloid, thin-walled. Stipitipellis cutis, hyphae 3–10 µm diam., parallel, cylindrical, hyaline to pale yellow in KOH, smooth, inamyloid, thin-walled. Stipe trama sarcodimitic, parallel, composed 2 type of hyphae: a) sarco-hyphae, elongate fusoid cell 87–165 × 4–15 µm, hyaline, smooth, inamyloid, thick-walled (1–4 µm); b) generative hyphae 3–12 µm wide, cylindrical, branched, hyaline, smooth, inamyloid, thin-walled. Caulocystidia abundant, 28–47 × 7–9 µm, cylindrical to clavate, irregular in shape, flexuose, hyaline KOH, inamyloid, thin-walled. Clamp connections present in all tissues.
Habit, habitat and known distribution: Gregarious on decayed wood, known only from Thailand.
Material examined: Thailand, Nakhon Ratchasima Provinve, Khao Yai National Park, trial to Pha Kluai Mai waterfall, 21 Sep 2018, collector N Wannathes, N Suwannarach J Kumla, S Lumyong, BKF10253 (holotype).
GenBank numbers: BKF10253- MZ1452142 (ITS), MZ452170 (LSU).
Notes: Gerronema flavum is characterized by a small omphaliod basidiomata that appears convex with depressed center infundibuliform, radially fibrillose, glabrous, yellow pileus, decurrent, subdistant lamellae, cylindrical, central, yellowish white stipe, broadly ellipsoid basidiospores with mean dimensions of 7.7 × 4.7 µm. The pileipellis is composed of hyaline with non-incrusted hyphae, the presence of irregular cylindrical cheilo-, pleuro- and cualocystidia, and sarcodimitic trama tissue. Gerronema kuruvense K.P.D. Latha & Manim and G. subchrysophyllum (Murrill) Singer are morphologically similar to a new species, namely Gerronema kuruvense, which was originally described from tropical India. It is distinguished by forming orange yellow pileus, bigger basidiospores with mean dimensions of 9.5 × 5.9 µm., an absence of cheilo- and pleurocystidia and the presence of diverticulate caulocystidia (Latha et al. 2018). Gerronema subchrysophyllum, a North American species, differs by having a larger basidiomata of nearly double the size (pilei 4–21 mm wide and stipe 4–32 mm long), simple clavate cheilocystidia and a lack a pleurocystidia (Singer 1970). The phylogenetic analyses inferred from combined sequences (Fig. 179) confirmed that G. flavum is a distinct species. This was further confirmed via an examination of other related morphological species and other taxa within this genus.
Gerronema keralense K. P. D. Latha & Manim Phytotaxa 364 (1): 85–88 (2018).
Index Fungorum number: IF 824928; Facesoffungi number: FoF 10687; Fig. 181
Pileus 20 mm diam., infundibuliform, appressed-fibrillose, brownish orange to light brown (5C5-5D5); Lamellar decurrent to deeply decurrent, distant (12) with 2–3 series of lamellulae, greyish yellow (4B6) up to 1.5 mm.; Stipe 21 × 0.75 mm, central, cylindrical, pruinose, slightly broadened at base, solid, greyish yellow (4B6) overall. Context thin, Odor and taste not distinctive.
Basidiospore 7–9 × (3–)4–5 μm [x = 7.76 ± 0.78 × 4.4 ± 0.65, Q = 1.4–2.3, q = 1.79 ± 0.25, n = 25, s = 1] ellipsoid, smooth, hyaline, inamyloid, thin-walled. Basidia 25–34 × 5–7 μm, clavate, with 4 sterigmata, thin-walled, inamyloid. Cheilocystidia scattered, 27–31 × 5–8 µm, clavate, flexuose, irregular in shape, hyaline, inamyloid, thin-walled. Pleurocystidia absent. Lamellar trama subregular, hyphae 4–15 µm diam., smooth, hyaline, inamyloid, thin-walled. Pileipellis cutis with pileocystidia, 3–12 µm, cylindrical, hyaline in KOH, inamyloid, inclusion cytoplasm turns to brown to light brown in Melzer's reagent, thin-walled. Pileus trama sarcodimitic, subregular, composed of 2 type of hyphae a) sarco-hyphae, elongate fusoid cell 98–196 × 10–14 µm, hyaline, smooth, inamyloid, thin-walled; b) generative hyphae 2–5 µm wide, cylindrical, branched, hyaline, smooth, inamyloid, thin-walled. Stipitipellis cutis, hyphae 3–6 µm diam., parallel, cylindrical, pale yellow in KOH, smooth, inamyloid, slightly thick-walled. Stipe trama sarcodimitic, parallel, composed 2 type of hyphae: a) sarco-hyphae, elongate fusoid cell 74–230 × 7–10 µm, hyaline, smooth, inamyloid, slightly thick-walled; b) generative hyphae 3–7 µm wide, cylindrical, branched, hyaline, smooth, inamyloid, slightly thick-walled. Caulocystidia 28–47 × 7–9 µm, agglutinated, cylindrical to clavate, hyaline in KOH, inamyloid, slightly thick-walled. Clamp connections present in all tissues.
Habit, habitat and known distribution: Solitary on decayed wood, known from topical India and Thailand.
Material examined: Thailand, Sukhothai Province, Si Satchanalai National Park, Natural trial, 15 June 2019, collector N Wannathes, N Suwannarach J Kumla, S Khuna, BKF10263.
GenBank numbers: BKF10263- MZ452107 (ITS), MZ452144 (LSU).
Notes: Gerronema keralense is characterized by a medium-sized omphaliod basidiomata, infundibuliform, brownish orange to light brown pileus, decurrent to deeply decurrent, distant, greyish yellow lamella, cylindrical, central, greyish yellow stipe, ellipsoid basidiospores with mean dimensions of 7.8 × 4.4 µm, pileipellis with pileocystidia, non-incrusted hyphae, cytoplasmic inclusion that turned from brown to light brown in Melzer's reagent, the presence of irregular cylindrical cheilo-, cualocystidia and sarcodimitic trama tissue. Our Thai description is consistent with Gerronema keralense that was originally described from India, except for the type specimen that formed an applanate with a slight central depression pileus (Latha et al. 2018). Notably, these variations may be caused by a mutuality of basidiomata. Gerronema keralense is morphologically similar to G. kuruvense K.P.D. Latha & Manim, but the latter differs by having bigger basidiospores with mean dimensions of 9.1 × 4.9 µm, a cytoplasmic inclusion of pileocystidia that never changes colour with Melzer's reagent and caulocystidia that are not agglutinated (Latha et al. 2018).
Gerronema kuruvense K. P. D. Latha & Manim. Phytotaxa 364 (1): 82–85 (2018).
Index Fungorum number: IF824927; Facesoffungi number: FoF10688; Figs. 177b, 182
Pileus 27 mm diam., convex with depress center, appressed-fibrillose, pale yellow (3A3); Lamellar subdecurrent, distant (16) with 2–3 series of lamellulae, narrow, light yellow (3A5) up to 2 mm.; Stipe 25 × 1 mm, central, cylindrical, pubescent, slightly broadened at base, hollow, yellowish white (3A2) overall. Context thin, Odor and taste not distinctive.
Basidiospore 8–10(–11) × 4–5(–6) μm [x = 9.08 ± 0.81 × 4.88 ± 0.43, Q = 1.6–2.25, q = 1.89 ± 0.25, n = 25, s = 1] ellipsoid, smooth, hyaline, inamyloid, thin-walled. Basidia 22–36 × 7–8 μm, clavate, with 2 sterigmata, sometime with 3–4 sterigmata, thin-walled, inamyloid. Cheilocystidia scattered, 21–51 × 5–11 µm, clavate, flexuose, irregular in shape, sometimes 2-celled, hyaline, inamyloid, thin-walled. Pleurocystidia absent. Lamellar trama interwoven, arranged in two directions, hyphae 3–14 µm diam., cylindrical, smooth, hyaline, inamyloid, thin-walled. Pileipellis cutis with scattered ascending pileocystidia, 4–14 µm, cylindrical, hyaline in KOH, inamyloid, thin-walled. Pileus trama sarcodimitic, subregular to interwoven, composed of 2 type of hyphae: (a) sarco-hyphae, elongate fusoid cell 94–212 × 10–14 µm, hyaline, smooth, inamyloid, thin-walled; (b) generative hyphae 3–7 µm wide, cylindrical, branched, hyaline, smooth, inamyloid, thin-walled. Stipitipellis cutis, hyphae 3–8 µm diam., parallel, cylindrical, hyaline in KOH, smooth, inamyloid, slightly thick-walled (up to 0.5 µm). Stipe trama sarcodimitic, parallel, composed 2 type of hyphae: a) sarco-hyphae, elongate fusoid cell 80–230(– over 250) × 8–15 µm, hyaline, smooth, inamyloid, thick-walled (1–2 µm); b) generative hyphae 3–6 µm wide, cylindrical, branched, hyaline, smooth, inamyloid, thin-walled. Caulocystidia abundant, 28–47 × 7–9 µm, cylindrical to clavate, irregular in shape, flexuose, hyaline in KOH, inamyloid, slightly thick-walled (up to 0.5 µm). Clamp connections present in all tissues.
Habit, habitat and known distribution: Solitary on decayed wood, known from topical India and Thailand.
Material examined: Thailand, Sukhothai Province, Si Satchanalai National Park, trial to Tad Duan waterfall, 30 Aug 2020, collector N Wannathes, N Suwannarach J Kumla, S Khuna, W Phonrob, S Tabtan, BKF10266.
GenBank numbers: BKF10266-MZ452090 (ITS), MZ452669(LSU).
Notes: Gerronema kuruvense is characterized by a medium-sized omphaliod basidiomata that is convex with a depressed center. It has a pale-yellow pileus that is subdecurrent, distant, with light yellow lamella, cylindrical, central, yellowish white stipe, ellipsoid basidiospores with mean dimensions of 9.1 × 4.9 µm. The pileipellis is composed of hyaline with pileocystidia, non-incrusted hyphae, the presence of irregular cylindrical cualocystidia and seldomly present cheilocystidia and sarcodimitic trama tissue. The Thai specimen is almost indistinguishable from Gerronema kuruvense, which was originally described from topical India. The holotype forms a smaller size basidiomata (pilei 4–11 mm wide and stipe 3–18 mm long) and lacks cheilocystidia (Latha et al. 2018). Gerronema kuruvense is morphologically similar to G. strombodes (Berk. & Mont.) Singer, but differs by forming larger basidiomata (pilei 25–80 mm wide and stipe of 30–60 × 2–6.8 mm) greyish white pileus, pileipellis lacking pileocystidia and a complete lack of caulocystidia (Singer 1970).
Tricholomopsis Singer, Schweiz. Z. Pilzk. 17: 56 (1939).
Tricholomopsis was established to accommodate a group of saprophytic tricholomatoid fungi which have a fibrillose or squamulose pileus, inamyloid smooth basidiospores, and a sterile lamella edge covered with large prominent cheilocystidia (Mao et al. 2021). There are 76 species epithets in the index fungorum for this genus.
Tricholomopsis lechatii Courtec., S. Dumez, S. Welti & P.-A. Moreau, sp. nov.
MycoBank number: MB843163, Facesoffungi number: FoF13397; Fig. 183
Etymology: The species is warmly dedicated to our friend Christian Lechat (1952–2022), who recently and suddenly died. He was an eminent specialist of the Hypocreales worldwide and a very enthusiastic and faithful member of the numerous field trips organized in the Tropics by one of us (RC). He was present when this species was collected.
Holotypus: 0202264 (LIP).
Pileus 10–14 mm diam, slightly convex or almost flat, showing a little umbilic (not deep) in the centre or slightly eccentric; shape regular, circular or almost so, sometimes slightly elliptical when seen from above. Surface remarkably dull, finely tomentose or even velvety under lens. Color very special, dirty olivaceous with ochraceous hue at center but more yellow at the margin, which is slightly enrolled or at least very obtuse and scarcely pectinate at the extreme edge. Lamellae adnate, rather crowded, with two rather regular rows of smaller gills, thin. Color rather bright and deep, mustard yellow. Edge rather thick, clearly bearing a row of small, densely crowded, aggregates of crystals. Stipe up to 15 × 2 mm, slightly excentrical, cylindraceous, more or less narrowly fistulose, dull yellow, paler than the gills but sometimes pale brownish toward mid-length, arising from a thin greyish mycelial patch of subiculum (up to 35 mm diam.), which sometimes climbs up to the stipe up to a few millimeters, thus greyish at the basis. Context yellowish in the stipe, deeper yellow in the cap. Smell none. Taste not recorded.
Basidiospores 6.2–7.5 × 4.8–5 µm, rather broad, elliptical to slightly tear-shaped, apex broader and generally clearly rounded (not elongate nor conical). Apiculus small, faintly distinct. Content with many droplets (sometimes a large one) and confuse, cloudy or punctate around them. Wall smooth, inamyloid, not cyanophilic. Basidia 20–28 × 5–8 µm, 2-spored (very few 1-spored and only one 3-spored seen) with very long and sharp sterigmata (up to 10 µm), containing large droplets, the bigger ones at the top. Clamp present at the base of all basidia. Subhymenium ramose, rather thick with short, clamped hyphae up to 1.5–2.5 µm wide, somewhat wavy with some longer hyphae parallel to the hymenium. Hymenophoral trama parallel in a wavy arrangement, weakly interwoven, made of clamped hyphae 4–8 µm wide. Edge sterile; cheilocystidia very numerous and prominent, cylindrical to slightly clavate, often regularly thickened toward apex, 35–45 × 8–12 µm, thin-walled, hyaline; base clamped, originated from simple or rarely forked hyphae. Pleurocystidia absent; facial sterile cells present as sparse slender “hyphids” cylindrical or slightly thickened at apex, up to 4 µm wide. Pileipellis a trichoderm of more or less straightly erected, unicellular or sometimes articulate elements, rather densely arranged, 30–70 × 7–15 µm, the apex mostly rounded or sometimes irregular, rarely mucronate to appendiculate or exceptionally forked; pigmentation epiparietal and vacuolar: wall yellowish, very finely incrusting (minutely punctate or finely marked with transverse zebra depending on the focus made on the cell wall) in all parts of the basidiome; vacuolar pigment abundant in subpellis, also present in broad elements of suprapellis, brown-yellow.
Habitat: Saprobic on rotten wood of unidentified Angiosperm, wet tropical forest, along a wet depression. So far only known from the type locality, French Guiana.
Material examined: French Guiana: Saül, Roche Bateau trail, 23 Aug. 2018, R. Courtecuisse & C. Lechat, RC/Guy18.018 (LIP 0202264, holotype).
GenBank numbers: LIP 0202264-OM793061 (ITS), OM793062 (LSU).
Notes: Tricholomopsis lechatii phylogenetically belongs to the T. aurea-complex, a pantropical group of collybioid species so far rather poorly documented in molecular databases (Fig. 184). Tricholomopsis aurea was originally described from DR Congo (as Marasmius aureus; Beeli, 1928), and recently transferred in the genus Tricholomopsis by Desjardin and Perry (2017) based on recent collections from São Tomé. Although the species is reported as common in many tropical regions (GBIF data: https://www.gbif.org/en/species/332554), only few sequences from the neotropics are available, and the ITS marker indicates only faint differences between African and American collections (Desjardin and Perry 2017, consider them as conspecific at this time). The new species described here differs significantly from T. aurea, by a pileipellis of trichodermioid structure, with a well-differentiated subpellis and a distinct vacuolar pigment (responsible of the grey-brown tomentum on pileus), whilst T. aurea has a thin cutis-like structure, resulting in a glabrous, golden yellow surface with ± inflate terminal elements with granular intracellular pigmentation (Pegler 1983).
Only few neotropical species of Tricholomopsis were recorded in the Neotropics, and none can be attributed to T. lechatii. Tricholomopsis tropica Dennis from Trinidad is devoid of yellow tinge in context and displays abundant hymenial gloeocystidia (Pegler 1983), what makes its classification in this genus somewhat doubtul; T. atrogrisea Pegler from Martinique, possibly more related to T. lechatii, has a gelatinized pileipellis made of adpressed hyphae; T. elegans Dennis (Dennis 1961) could be the closest relative of T. lechatii but differs at least by smaller, subglobose spores (4.5–5 µm long) and 4-spored basidia. No species described so far display either the spectacular pale grey mycelial patch observed in T. lechatii, nor its conspicuous 2-spored basidia (Fig. 183).
Phylogenetic analyses were conducted online at www.phylogeny.fr (Dereeper et al. 2008). Multiple sequence alignments were performed with MUSCLE v. 3.7 (Edgar 2004). Maximum likelihood (ML) phylogenetic analysis was achieved with PhyML v. 3.0 (Guindon et al. 2010), using the GTR + I + Γ model of evolution and the Shimodaira Hasegawa version of the approximate likelihood-ratio test (SH-aLRT) of branch support (Anisimova et al. 2011). Phylogram was built using TreeDyn 198.3 (Chevenet et al. 2006) and edited with Inkscape 0.91 (https://inkscape.org/fr). Newly generated sequences for this study are in bold. The tree is rooted by a sequence of Pluteus atromarginatus (Pluteaceae)
Ustilaginomycotina Doweld.
We follow the latest treatment and updated account of Ustilaginomycotina in Begerow and McTaggart (2018).
Ustilaginomycetes R. Bauer et al.
The classification of the orders in Ustilaginomycetes follows Begerow and McTaggart (2018).
Ustilaginales G. Winter.
There are seven families in this order: Anthracoideaceae Denchev, Clintamraceae Vánky, Geminaginaceae Vánky, Melanotaeniaceae Begerow et al., Pericladiaceae Vánky, Ustilaginaceae Tul & C. Tul., and Websdaneaceae Vánky (He et al. 2019; but excluding Cintractiellaceae Vánky, see McTaggart et al. 2020).
Ustilaginaceae Tul & C. Tul. [as 'Ustilagineae'], Annls Sci. Nat., Bot., sér. 3 7: 14 (1847).
Ustilaginaceae was introduced by Tulasne and Tulasne (1847). Twenty-eight genera are currently recognized in this family (He et al. 2019).
Sporisorium Ehrenb. ex Link, in Willdenow, Sp. pl., Edn 4 6(2): 86 (1825).
Sporisorium is a grass-infecting genus of smut fungi. It is characterized by sori formed in different parts of the inflorescence or destroying the entire inflorescence, sometimes also comprising the upper part of the stem. The sori are initially enclosed by a thick, brownish peridium that later ruptures irregularly exposing a single, stout columella surrounded by a mass of spores and sterile cells.
Until ten years ago, with 326 species Sporisorium was perceived by far the most species-rich genus of smut fungi. A new concept for Ustilago, Sporisorium, and Macalpinomyces was proposed by McTaggart et al. (2012a, b). In its modern circumscription, Sporisorium comprises 198 species (Vánky 2011, 2013; Denchev et al. 2012, 2016; McTaggart et al. 2012b; Denchev and Denchev 2013, 2016; Wang et al. 2015).
During an examination of specimens of grasses in the herbarium MA of the Real Jardín Botánico (Madrid, Spain), a smut fungus belonging to Sporisorium was found on a specimen of Anadelphia leptocoma (MA 690545) from Burkina Faso. Based on distinct morphology and phylogenetic evidence (Fig. 186), this fungus is introduced here as a novel species.
Most likely tree generated using maximum likelihood analysis (RAxML 8.2.11, Stamatakis 2014) based on concatenated MAFFT v7.450 (Katoh and Standley 2013) alignments of ITS and LSU dataset. The tree is rooted with Mycosarcoma maydis Bref. and M. mackinlayi (McTaggart & R.G. Shivas) McTaggart et al. Values at nodes indicate bootstrap values inferred by 1000 replicates; only values ≥ 60% are shown
Sporisorium anadelphiae-leptocomae T. Denchev, Denchev, Kemler, M.P. Martín & Begerow, sp. nov.
Index Fungorum number: IF558175; Facesoffungi number: FoF09652; Fig. 185.
Etymology: The specific epithet refers to the host species.
Holotype: SOMF 30250
Parasitic on Anadelphia leptocoma. Infection systemic. Sori destroying the racemes, 5–10 mm long, fusiform, all racemes of an infected plant affected; initially covered by a thick, yellow–brown peridium which later ruptures irregularly, exposing a single columella, surrounded by a powdery, dark reddish-brown mass of spores and sterile cells. Columella shorter than the sorus, flagelliform, apically slightly branched, sometimes slightly flattened or with shallow longitudinal furrows. Sori at first completely enclosed by the spatheoles, later more or less visible. Sterile cells firmly packed in irregular groups, collapsed, 9–14(–17) μm long, usually larger than the spores, hyaline to pale yellow; wall 0.6–1.0 μm thick. In SEM, smooth, sometimes partially rugulose. Spores subglobose, slightly irregular, broadly ellipsoidal, ellipsoidal or ovoid, (6.5–)7.5–9.5(–10.5) × (6–)7–8.5(–9.5) (8.6 ± 0.6 × 7.8 ± 0.6) μm (n = 300), medium reddish brown; wall evenly thickened, 0.5–0.7 μm thick, smooth. In SEM, minutely echinulate-verruculose, ornaments up to 0.2 μm high, densely punctate between the main ornaments.
Material examined: Burkina Faso, Cascades Region, Léraba Province, Sindou Department, Tourni, dam, riverbed with seeping water, fallow fields, 10°46′ N, 05°09′ W, alt. 200–500 m, on Anadelphia leptocoma (Trin.) Pilg. (Poaceae), 28 October 1997, S. Lægaard, H. Mipro & T. Soberé, no. 18421 (SOMF 30250, holotype; MA 690545, isotype).
GenBank numbers: MW599285(ITS), MW599284 (LSU).
Notes: Anadelphia Hack. is a small genus of Poaceae Barnhart, tribe Andropogoneae Dumort., subtribe Andropogoninae J. Presl (including 514 species in 25 genera) (Soreng et al. 2017). Anadelphia comprises 14 species (Clayton et al. 2015) characterized by compound inflorescence composed of racemes. Anadelphia leptocoma is distributed in Tropical Africa (from Senegal to Zambia), mainly in West Tropical Africa (Clayton et al. 2015; Poilecot et al. 2015; Ibrahim et al. 2018). It is a characteristic grass of the savannahs (Poilecot et al. 2015).
Seven smut fungi have been previously reported on hosts in Anadelphia and its closely related genera, Elymandra Stapf and Monocymbium Stapf: Anthracocystis anadelphiae (Vienn.-Bourg.) McTaggart & R.G. Shivas, Jamesdicksonia anadelphiae (Vienn.-Bourg.) Piątek, J. anadelphiae-trichaetae T. Denchev & Denchev, Macalpinomyces elymandrae (Vienn.-Bourg.) Vánky, Sporisorium anadelphiae-trichaetae T. Denchev & Denchev, S. monocymbii (Syd.) Vánky, and Tilletia elymandrae Vienn.-Bourg. (Denchev and Denchev 2016). Sporisorium anadelphiae-leptocomae can be easily distinguished from S. anadelphiae-trichaetae by having (i) sori that destroy the racemes entirely, while the sori of S. anadelphiae-trichaetae affect only the spikelets, and (ii) minutely echinulate-verruculose spores with ornaments up to 0.2 μm in height, while S. anadelphiae-trichaetae possesses moderately echinulate spores, with spinules up to 0.7 μm in height. The sori of Sporisorium monocymbii, destroying spikelets or groups of spikelets and forming a very characteristic, strongly branched body (see Figs. 45, 47, 48 in Denchev and Denchev 2016), also differ from those of S. anadelphiae-leptocomae. Additionally, Sporisorium monocymbii has minutely echinulate spores, with spinules up to 0.3(–0.4) μm in height and larger sterile cells, 9–19(–24) μm long vs 9–14(–17) μm long for S. anadelphiae-leptocomae.
The phylogenetic analysis of Sporisorium, based on combined ITS and LSU sequences resulted in a similar topology to previous analyses (McTaggart et al. 2012a). Sporisorium anadelphiae-leptocomae formed a statistically very well supported clade (100% bootstrap) together with S. queenslandicum Vánky et al., infecting Sehima nervosa (Roem. & Schult.) Stapf, and S. exsertum (McAlpine) L. Guo, infecting Themeda spp. Sister to this clade is a group containing S. rarum R.G. Shivas et al., S. ryleyi Vánky & R.G. Shivas, S. paspali (Speg.) Vánky, S. porosum (Langdon) McTaggart & R.G. Shivas, S. trachypogonicola Vánky & C. Vánky, S. vanderystii (Henn.) Langdon & Full., and S. wynaadense (Sundaram) Vánky & R.G. Shivas. All of the species from these two clades are parasitizing grasses from the tribe Andropogoneae, with the exception of S. paspali, infecting Paspalum spp., belonging to the tribe Paspaleae (Fig.*).
The smut fungi of Burkina Faso are very poorly known with only six species reported from this country: Anthracocystis ehrenbergii (J.G. Kühn) McTaggart & R.G. Shivas, A. livingstoneana (Vánky) McTaggart & R.G. Shivas, Moesziomyces penicillariae (Bref.) Vánky, Sporisorium reilianum (J.G. Kühn) Langdon & Full., S. scitamineum (Syd) M. Piepenbr. et al., and S. sorghi Ehrenb. ex Link (Vánky et al. 2011; Piepenbring et al. 2020).
Key to the smut fungi on Anadelphia, Elymandra, and Monocymbium (modified after Denchev and Denchev 2016).
1 Sori in leaves and spatheoles……………………………2
1*Sori in racemes, spikelets or ovaries……………………………3.
2 Sori form non-erumpent streaks or patches on leaves and spatheoles. Spores (9–)9.5–15.5(–18.5) μm long, spore wall 1.2–3.0(–3.8) μm thick. [On Anadelphia pumila]……………………………Jamesdicksonia anadelphiae.
2*Sori form erumpent streaks on leaves and spatheoles. Spores (11.5–)13–23.5(–26.5) μm long, spore wall (2.0–)2.5–7.0(–9.0) μm thick. [On Anadelphia trichaeta]……………………………Jamesdicksonia anadelphiae-trichaetae.
3 Sori in some ovaries of an inflorescence. [On Elymandra androphila]……………………………4.
3*Sori in racemes or spikelets……………………………5.
4 Spores 10.5–15 μm long; spore walls echinate, with 1–1.5 μm high spines……………………………Macalpinomyces elymandrae.
4* Spores 17–27 μm long; spore walls with apically flattened or rounded, densely spaced projections, 1–2.5(–3) μm high……………………………Tilletia elymandrae.
5 Spore balls present. Spores dimorphic. Outer spores (9.5–)10.5–15(–16) μm long. Sterile cells absent. [On Anadelphia pumila]……………………………Anthracocystis anadelphiae.
5* Spore balls absent. Spores not dimorphic, smaller. Sterile cells present……………………………6.
6 Sori destroying all racemes. Spores minutely echinulate-verruculose, with ornaments up to 0.2 μm in height. [On Anadelphia leptocoma]……………………………Sporisorium anadelphiae-leptocomae.
6* Sori in spikelets or groups of spikelets. Spore ornamentation echinulate, with higher ornaments……………………………7.
7 Spore wall moderately echinulate, spinules up to 0.7 μm high. Spores (8.5–)9–11.5(–12.5) (10.5 ± 0.7) μm long. [On Anadelphia trichaeta]……………………………Sporisorium anadelphiae-trichaetae.
7*Spore wall minutely echinulate, spinules up to 0.3(–0.4) μm high. Spores (7–)7.5–10.5(–11.5) (9.1 ± 0.6) μm long. [On Monocymbium ceresiiforme]……………………………Sporisorium monocymbii. (Table 5)
References
Abdel-Aziz FA (2016a) The genus Lolia from freshwater habitats in Egypt with one new species. Phytotaxa 267:279–288
Abdel-Aziz FA (2016b) Two new cheirosporous asexual taxa (Dictyosporiaceae, Pleosporales, Dothideomycetes) from freshwater habitats in Egypt. Mycosphere 7:448–457
Abdel-Aziz FA (2016c) Freshwater fungi from the River Nile. Egypt Mycosphere 7:741–756
Abdel-Aziz FA, Abdel-Wahab MA (2010) Lolia aquatica gen. et sp. nov. (Lindgomycetaceae, Pleosporales), a new coelomycete from freshwater habitats in Egypt. Mycotaxon 114:33–42
Abdel-Aziz FA, Bahkali AH, El-Gorban AM, Abdel-Wahab MA (2020) Pleurotheciella nilotica sp. nov. (Pleurotheciales, Ascomycota) from freshwater habitats in Egypt. Nova Hedwigia 110:91–98
Abdollahzadeh J, Groenewald JZ, Coetzee MP, Wingfield MJ, Crous PW (2020) Evolution of lifestyles in Capnodiales. Stud Mycol 95:381–414
Abeywickrama PD, Wanasinghe DN, Karunarathna SC, Jayawardena RS, Hyde KD, Zhang W, Li X, Yan J (2020) A new host report of Diaporthe manihotia (Diaporthales, Ascomycota) from Camellia sp. in Yunnan Province. China Asian J of Mycol 3:473–489
Abrantes RA, Refojo N, Hevia AI, Fernández J et al (2021) Scedosporium spp. from Clinical Setting in Argentina, with the Proposal of the New Pathogenic Species Scedosporium americanum. Journal of Fungi 7:160–174
Acero FJ, González V, Sánchez-Ballesteros J, Rubio V, Checa J, Bills GF, Salazar O, Platas G, Peláez F (2004) Molecular phylogenetic studies on the Diatrypaceae based on rDNA-ITS sequences. Mycologia 96:249–259
Adamčíková KA, Juhásová G, Kobza M, Ondrušková E (2013) Diversity of microfungi on branches of Castanea sativa in Slovakia. Polish Bot J 58:741–746
Adeleke BS, Babalola OO (2021) Pharmacological Potential of Fungal Endophytes Associated with Medicinal Plants: A Review. Journal of Fungi 7:147
Adikaram NKB, Yakandawala DMD (2020) A checklist of plant pathogenic fungi and Oomycota in Sri Lanka. Ceylon J Sci 49:93–123
Agerer R, Bougher NL, Bougher NL (2001) Tomentella subamyloidea sp. nov. and T. radiosa (Thelephoraceae, Hymenomycetes, Basidiomycota) from Australia. Aust Syst Bot 14:607–614
Aime MC, Miller AN, Aoki T, Bensch K, Cai L, Crous PW, Hawksworth DL, Hyde KD, Kirk PM et al (2021) How to publish a new fungal species, or name, version 3.0. IMA Fungus 12:1–5
Akinsanmi AO, Nisa S, Jeff-Ego OS, Shivas RG, Drenth A (2017) Dry flower disease of macadamia in Australia Caused by Neopestalotiopsis macadamiae sp. nov. and Pestalotiopsis macadamiae sp. nov. Plant Dis 101:45–53
Alcorn JL (1990) Additions to Bipolaris, Cochliobolus and Curvularia. Mycotaxon 39:361–392
Alizadeh A, Javan-Nikkhah M, Nourmohammadi Nazarian R, Liu F, Zare R, Fotouhifar KB, Stukenbrock EH, Damm U. (2022) New species of Colletotrichum from wild Poaceae and Cyperaceae plants in Iran. Mycologia (in press)
Alvarez-Manjarrez J, Villegas-Ríos M, Garibay-Orijel R, Contreras-Pacheco M, Kõljalg U (2015) Tomentella brunneoincrustata, the first described species of the Pisonieae associated Neotropical Tomentella clade, and phylogenetic analysis of the genus in Mexico. Mycol Prog 15:10
Amaradasa BS, Madrid H, Groenewald JZ, Crous PW, Amundsen K (2014) Porocercospora seminalis gen. et comb. nov., the causal organism of buffalo grass false smut. Mycologia 106:77–85
Anastasiou CJ (1963) Fungi from salt lakes. II. Ascomycetes and Fungi Imperfecti from Salton Sea. Nova Hedwigia 6:243–276
Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O (2011) Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Systematic Biol 60:685–699
Antonín V (2007) Monograph of Marasmius, Gloiocephala, Palaeocephala and Setulipes in Tropical Africa. Fungus Fl Trop Afr 1:1–164
Antonín V, Noordeloos ME (2010) A monograph of marasmioid and collybioid fungi in Europe. IHW Verlag, Eching, p 478
Antonín V, Borovicka J, Holec J, Piltaver A, Kolarik M (2019) Taxonomic update of Clitocybula sensu lato with a new generic. Fungal Biol 123:431–447
Aptroot A, Spielmann AA (2020) New lichen species and records from the Serra da Bodoquena, Mato Grosso do Sul, Brazil, the westernmost Atlantic rain forest. Archive for Lichenology 17:1–25
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KW, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS et al (2015a) Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:27–34
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KW, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M et al (2015b) Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:27–274
Armillaria (Fr.) (1857) Staude, Schwämme Mitteldeutschl. 28: xxviii, 130
Arnold GRW (1986). Lista de Hongos Fitopatogenos de Cuba. Ministerio de Cultura Editorial Cientifico-Tecnica, pp207.
Arup U, Søchting U, Frödén P (2013) A new taxonomy of the family Teloschistaceae. Nord J Bot 31:16–83
Arx JA Von, Müller E (1954) Die Gattungen der amerosporen Pyrenomyceten. Beitr Kryptog Fl Schweiz 11:1–434
Asgari B, Zare R, Gams W (2007) Coniochaeta ershadii, a new species from Iran, and a key to well-documented Coniochaeta species. Nova Hedwigia 84:175–187
Ashraf R, Shahid F, Ali TA (2007) Association of fungi, bacteria and actinomycetes with different composts. Pak J Bot 39:2141–2151
Baker WA, Partridge EC, Morgan-Jones G (2002) Notes on hyphomycetes LXXXVII. Rhexoacrodictys, a new segregate genus to accommodate four species previously classified in Acrodictys. Mycotaxon 82:95–113
Bao DF, Luo ZL, Liu JK, Bhat DJ, Sarunya N, Li WL, Su HY, Hyde KD (2018) Lignicolous freshwater fungi in China III: New species and record of Kirschsteiniothelia from northwestern Yunnan Province. Mycosphere 9:755–768
Bao DF, Su HY, Maharachchikumbura SSN, Liu JK, Nalumpang S, Luo ZL, Hyde KD (2019) Lignicolous freshwater fungi from China and Thailand: Multi-gene phylogeny reveals new species and new records in Lophiostomataceae. Mycosphere 10:1080–1099
Bao DF, Bhat DJ, Boonmee S, Hyde KD, Luo ZL, Nalumpang S (2022) Lignicolous freshwater ascomycetes from Thailand: Introducing Dematipyriforma muriformis sp. nov., one new combination and two new records in Pleurotheciaceae. MycoKeys 93:57–79
Barr ME (1979) A classification of Loculoascomycetes. Mycologia 71:935–957
Barr ME (1987) Prodomus to class Loculoascomycetes. Publ. by the author, Amherst
Barr ME (1992) Additions to and notes on the Phaeosphaeriaceae (Pleosporales, Loculoascomycetes). Mycotaxon 43:371–400
Barr ME (1993) Notes on the Pleomassariaceae. Mycotaxon 49:129–142
Barreto GG, Carmo LT, Gusmão LF (2021) Helicoma barretoi sp. nov. from the Brazilian Atlantic rainforest. Mycotaxon 136:79–83
Becker K, Stadler M (2021) Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J Antibiot 74:1–23
Beeli M (1928) Contribution à l’étude de la flore mycologique du Congo. V Fungi Goossensiani. Champignons récoltés par Mme Goossens et déterminés par M. Beeli Bull Soc Roy Bot Belgique 60:153–171
Begerow D, McTaggart A (2018) Ustilaginomycotina. In: Begerow D et al. (eds) Syllabus of plant families – A. Engler’s Syllabus der Pflanzenfamilien, Part 1/3: Basidiomycota and Entorrhizomycota. Schweizerbart Science Publishers, Stuttgart, pp 92–129
Berkeley MJ, Broome CE (1850). Notices of British fungi. nn. Mag. Nat. Hist. (Series 2) 5:455– 66.
Bessey CE (1907) A synopsis of plant phyla. University of Nebraska Studies 7:275–373
Bezerra JD, Oliveira RJ, Paiva LM, Silva GA, Groenewald JZ, Crous PW, Souza-Motta CM (2017) Bezerromycetales and Wiesneriomycetales ord. nov. (class Dothideomycetes), with two novel genera to accommodate endophytic fungi from Brazilian cactus. Mycol Prog 16:297–309
Bhunjun CS, Phukhamsakda C, Jayawardena RS, Jeewon R, Promputtha I, Hyde KD (2021) Investigating species boundaries in Colletotrichum. Fungal Divers 107:107–127
Bhunjun CS, Niskanen T, Suwannarach N, Wannathes N, Chen YJ et al (2022) The numbers of fungi: are the most speciose genera truly diverse? Fungal Divers 114:387–462
Bian LS, Zhao CL, Wu F (2016) A new species of Skeletocutis (Polyporales, Basidiomycota) from Yunnan of China. Phytotaxa 270:267–276
Bigelow HE (1970) Omphalina in North America. Mycologia 62:1–32
Binder M, Hibbett DS, Wang Z, Farnham WF (2006) Evolutionary relationships of Mycaureola dilseae (Agaricales), a basidiomycete pathogen of a subtidal rhodophyte. Am J Bot 93:547–556
Boerema GH, de Gruyter J, Noordeloos ME, Hamers MEC (2004) Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. CABI Publishing, Wallingford, UK.
Boggiani P (1997) Análise estratigráfica da bacia Corumbá (neoproterozóico) – Mato Grosso do Sul. Thesis, São Paulo.
Bonthond G, Sandoval-Denis M, Groenewald JZ, Crous PW (2018) Seiridium (Sporocadaceae): an important genus of plant pathogenic fungi. Persoonia 40:96–118
Boonmee S, Ko TW, Chukeatirote E, Hyde KD, Chen H, Cai L, McKenzie EH, Jones EG, Kodsueb R, Hassan BA (2012) Two new Kirschsteiniotheli a species with Dendryphiopsis anamorphs cluster in Kirschsteiniotheliaceae fam. nov. Mycologia 104:698–714
Boonmee S, Wanasinghe DN, Calabon MS, Huanraluek N, Chandrasiri SKU, Jones GEB, Rossi W, Leonardi M, Singh SK, Rana S, Singh PN, Maurya DK, Lagashetti AC, Choudhary D, Dai YC, Zhao CL, Mu YH, Yuan HS, He SH, Phookamsak R, Jiang HB, Martín MP, Dueñas M, Telleria MT, Kałucka IL, Jagodziński AM, Liimatainen K, Pereira DS, Phillips AJL, Suwannarach N, Kumla J, Khuna S, Lumyong S, Potter TB, Shivas RG, Sparks AH, Vaghefi N, Abdel-Wahab MA, Abdel-Aziz FA, Li GJ, Lin WF, Singh U, Bhatt RP, Lee HB, Nguyen TTT, Kirk PM, Dutta AK, Acharya K, Sarma VV, Niranjan M, Rajeshkumar KC, Ashtekar N, Lad S, Wijayawardene NN, Bhat DJ, Xu RJ, Wijesinghe SN, Shen HW, Luo ZL, Zhang JY, Sysouphanthong P, Thongklang N, Bao DF, Aluthmuhandiram JVS, Abdollahzadeh J, Javadi A, Dovana F, Usman M, Khalid AN, Dissanayake AJ, Telagathoti A, Probst M, Peintner U, Garrido-Benavent I, Bóna L, Merényi Z, Boros L, Zoltán B, Stielow JB, Jiang N, Tian CM, Shams E, Dehghanizadeh F, Pordel A, Javan-Nikkhah M, Denchev TT, Denchev CM, Kemler M, Begerow D, Deng CY, Harrower E, Bozorov T, Kholmuradova T, Gafforov Y, Abdurazakov A, Xu JC, Mortimer PE, Ren GC, Jeewon R, Maharachchikumbura SSN, Phukhamsakda C, Mapook A, Hyde KD (2021) Fungal diversity notes 1387–1511: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 111:1–335
Bougher NL, Syme K (1998) Fungi of Southern Australia. University of Western Australia Press, Australia
Bourdot H, Galzin A (1924) Hyménomycètes de France. X. Phylactériés. Bulletin De La Société Mycologique De France 40:105–162
Brahmanage RS, Lu YZ, Bhat DJ, Wanasinghe DN, Yan JY, Hyde KD, Boonmee S (2017) Phylogenetic investigations on freshwater fungi in Tubeufiaceae (Tubeufiales) reveals the new genus Dictyospora and new species Chlamydotubeufia aquatica and Helicosporium flavum. Mycosphere 8:917–933
Braun U, Nakashima C, Crous PW (2013) Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi. Pteridophyta and Gymnospermae IMA Fungus 4:265–345
Brown SP, Ferrer A, Dalling JW, Heath KD (2016) Don’t put all your eggs in one basket: a cost-effective and powerful method to optimize primer choice for rRNA environmental community analyses using the Fluidigm Access Array. Mol Ecol Resour 16:946–956
Bungartz F, Grube U, Elix JA, Heininger C, Mayrhofer H (2011) A taxonomic revision of the Buellia subalbula-group in the Southern Hemisphere using fluorescence microscopy. Bibl Lichenol 106:21–39
Bungartz F, Nash TH III (2004) Buellia subalbula (Nyl.) Müll. Arg. and B. amabilis de Lesd., two species from North America with one-septate ascospores: A comparison with Buellia [″Diplotomma″] venusta (Körb.) Lettau. Bibl Lichenol 88:49–66
Buyck B, Eyssartier G, Dima B, Consiglio G, Noordeloos ME, Papp V, Bera I, Ghosh A, Rossi W, Leonardi M, Das K (2021) Fungal Biodiversity Profiles 101–110. Cryptogam, Mycol 42:63–89
Cai L, Jeewon R, Hyde KD (2005) Phylogenetic evaluation and taxonomic revision of Schizothecium based on ribosomal DNA and protein coding genes. Fungal Divers 19:1–210
Cai L, Jeewon R, Hyde KD (2006) Phylogenetic investigations of Sordariaceae based on multiple gene sequences and morphology. Mycol Res 110:137–150
Cai Q, Chen ZH, He ZM, Luo H, Yang ZL (2018) Lepiota venenata, a new species related to toxic mushroom in China. J Fungal Res 16:63–69
Calabon MS, Sadaba RB, Campos WL (2018) Fungal diversity of mangrove-associated sponges from New Washington, Aklan, Philippines. Mycology 10:6–21
Calabon MS, Hyde KD, Jones EBG, Luo ZL, Dong W, Hurdeal VG, Gentekaki E, Rossi W, Leonardi M, Thiyagaraja V, Lestari AS, Shen H-W, Bao DF, Boonyuen N, Zeng M (2022) Freshwater Fungal Numbers Fungal Divers 114:3–235
Camino-Vilaro M, Castro-Hernandez L, Abreu-Herrera Y, Mena-Portales J, Cantillo-Perez T (2019) Fungi associated with invasive plant species in Cuba. Phytotaxa 419:239–267
Cannon PF, Damm U, Johnston PR, Weir B (2012) Colletotrichum – current status and future directions. Stud in Mycol 73:181–213
Cañón ER, de Albuquerque MP, Alves RP, Pereira AB, Victoria FD (2019) Morphological and molecular characterization of three endolichenic isolates of Xylaria (Xylariaceae), from Cladonia curta Ahti & Marcelli (Cladoniaceae). Plants 8:399
Cao B, Haelewaters D, Schoutteten N, Begerow D, Boekhout T, Giachini AJ, Gorjón SP, Gunde- Cimerman N, Hyde KD et al (2021) Delimiting species in Basidiomycota: a review. Fungal Divers 109:181–237
Carpouron JE, Wijesinghe SN, Shang QJ, Perera RH, Camporesi E, Hyde KD (2021) Diatrypella macrospora, a new host and geographical record from Forlì-Cesena. Italy Stud Fungi 6:273–287
Cash EK (1952) A record of the fungi named by J.B. Ellis (Part 1). U.S.D.A. Special Publ 2:1–165
Castaneda-Ruiz RF, Iturriaga T, Minter DW, Saikawa M, Vidal G, Velazquez-Noa S (2003) Microfungi from Venezuela, A new species of Brachydesmiella, a new combination, and new records. Mycotaxon 85:211–229
Castellani A and Chalmers AJ (1919) Manual of tropical medicine. Baillière, Tindall and Cox
Cesati V, De Notaris G (1863) Schema di classificazione degle sferiacei italici aschigeri piu’ o meno appartenenti al genere Sphaeria nell’antico significato attribuitoglide Persono. Comment Soc Crittogamol Ital 1:177–420
Chaisiri C, Liu X, Lin Y, Fu Y, Zhu F, Luo C (2021) Phylogenetic and haplotype network analyses of Diaporthe eres species in China based on sequences of multiple loci. Biology 10:179
Chaiwan N, Wanasinghe DN, Camporesi E, Tibpromma S, Boonmee S, Lumyong S, Hyde KD (2019) Molecular taxonomy reveals the sexual morph of Nodulosphaeria digitalis in Phaeosphaeriaceae from Campanula trachelium in Italy. Phytotaxa 400:1–3
Chandra S (1974) Some new leaf-spot diseases from Allahabad (India). Beih. Nova Hedwigia 47:35–102
Chandrasrikul A, Suwanarit P, Sangwanit U, Lumyong S, Payapanon A, Sanoamuang N, Pukahuta C, Petcharat V, Sardsud U, Duengkae K, Klinhom U, Thongkantha S, Thongklam S (2011) Mushroom (basidiomycetes) in Thailand. Office of Natural Resources and Environmental Policy and Planning, Bangkok
Chardón CE, Toro RA (1930) Mycological explorations of Colombia. Journal of the Department of Agriculture of Porto Rico 14:195–369
Chaverri P, Salgado C, Hirooka Y, Rossman AY, Samuels G (2011) Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Stud Mycol 68:57–78
Chen H, Cui BK (2017) Multi-locus phylogeny and morphology reveal five new species of Fomitiporia (Hymenochaetaceae) from China. Mycol Prog 16:687–701
Chen Q, Jiang JR, Zhang GZ, Cai L, Crous PW (2015a) Resolving the Phoma enigma. Stud Mycol 82:137–217
Chen Q, Jiang JR, Zhang GZ, Cai L, Crous PW (2015b) Resolving the Phoma Enigma Stud Mycol 82:137–217
Chen CY, Wang CL, Huang JW (2006) Two new species of Kirschsteiniothelia from Taiwan. Mycotaxon 98:153–158
Chen J, Zhang LC, Xing YM, Wang YQ, Xing XK, Zhang DW, Liang HQ, Guo SX (2013) Diversity and taxonomy of endophytic xylariaceous fungi from medicinal plants of Dendrobium (Orchidaceae). PLoS ONE 8:e58268
Chen YY, Maharachchikumbura SSN, Liu JK, Hyde KD, Nanayakkara RR, Zhu GS, Liu ZY (2017a) Fungi from Asian Karst formations I. Pestalotiopsis photinicola sp. nov., causing leaf spots of Photinia serrulata. Mycosphere 8:103–110
Chen YY, Wu F, Wang M, Cui BK (2017b) Species diversity and molecular systematics of Fibroporia (Polyporales, Basidiomycota) and its related genera. Mycol Prog 16:521–533
Chethana KW, Manawasinghe IS, Hurdeal VG, Bhunjun CS, Appadoo MA, Gentekaki E, Raspé O, Promputtha I, Hyde KD (2021a) What are fungal species and how to delineate them? Fungal Divers 109:1–25
Chethana KWT, Niranjan M, Dong W, Samarakoon MC, Bao DF, Calabon MS, Chaiwan N, Chuankid B, Dayarathne MC, De Silva NI, Devadatha B, Dissanayake AJ, Goonasekara ID, Huanraluek N, Jayawardena RS, Karunarathna A, Luo ZL, Marasinghe DS, Ma X, Norphanphoun C, Pem D, Perera RH, Rathnayaka AR, Raspé O, Samarakoon BC, Senwanna C, Sun YR, Tang X, Thiyagaraja V, Tennakoon DS, Zeng M, Zeng XY, Zhang JY, Zhang SN, Bulgakov TS, Camporesi E, Sarma VV, Wang Y, Bhat DJ, Hyde KD (2021b) AJOM new records and collections of fungi: 101–150. Asian J Mycol 4:113–260
Chevallier FF (1826) Flore Générale des Environs de Paris 1. Ferra Jeune, Paris, p 102
Chevenet F, Brun C, Bañuls AL, Jacq B, Christen R (2006) TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinform 7:439
Chew HF, Jungkind DL, Mah DY, Raber IM, Toll AD, Tokarczyk MJ, Cohen EJ (2010) Post-traumatic fungal keratitis caused by Carpoligna sp. Cornea 29:449–452
Chilvers MI, Rogers JD, Dugan FM, Stewart JE, Chen W, Peever TL (2009) Didymella pisi sp. nov., the teleomorph of Ascochyta pisi. Mycol Res 113:391–400
Chung WH, Tsukiboshi T (2005) A new species of Curvularia from Japan. Mycotaxon 91:49–54
Collado J, Platas G, Bills G (2006) Studies on Morinia: Recognition of Morinia longiappendiculata sp. nov. as a new endophytic fungus, and a new circumscription of Morinia pestalozzioides. Mycologia 98:616–627
Cooke MC (1887a) Synopsis Pyrenomycetum Grevillea 16:16–19
Cooke MC (1887b) New Australian Fungi Grevillea 16:1–6
Corda ACJ (1837) Icones fungorum hucusque cognitorum (Vol. 1). JG Calve.
Corda ACJ (1839) Icones Fungorum Hucusque Cognitorum 3:1–55
Corda ACJ (1842) Icones Fungorum Hucusque Cognitorum 5:1–92
Coyier DL, Roane MK (1987) Compendium of Rhododendron and Azalea Diseases. American Phytopathological Society, St. Paul, MN
Crane JL, Miller AN (2016) Studies in genera similar to Torula: Bahusaganda, Bahusandhika, Pseudotorula, and Simmonsiella gen. nov. IMA Fungus 7:29–45
Crous PW, Braun U (2003) Mycosphaerella and its anamorphs: 1. Names published in Cercospora and Passalora, Centraalbureau voor Schimmelcultures (CBS)
Crous PW, Palm ME (1999) Reassessment of the anamorph genera Botryodiplodia, Dothiorella and Fusicoccum. Sydowia 51:167–175
Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernández-Restrepo M, Jaklitsch WM, Lebrun MH, Schumacher RK, Stielow JB, van der Linde EJ, Vilcāne J, Voglmayr H, Wood AR (2015a) The Genera of Fungi – fixing the application of the type species of generic names - G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 6:163–198
Crous PW, Luangsa-Ard JJ, Wingfield MJ, Carnegie AJ, Hernández-Restrepo M, Lombard L, Roux J, Barreto RW, Baseia IG, Cano-Lira JF, Martín MP, Morozova OV, Stchigel AM, Summerell BA, Brandrud TE, Dima B, García D, Giraldo A, Guarro J, Gusmão LFP, Khamsuntorn P, Noordeloos ME, Nuankaew S, Pinruan U, Rodríguez-Andrade E, Souza-Motta CM, Thangavel R, van Iperen AL, Abreu VP, Accioly T, Alves JL, Andrade JP, Bahram M, Baral HO, Barbier E, Barnes CW, Bendiksen E, Bernard E, Bezerra JDP, Bezerra JL, Bizio E, Blair JE, Bulyonkova TM, Cabral TS, Caiafa MV, Cantillo T, Colmán AA, Conceição LB, Cruz S, Cunha AOB, Darveaux BA, da Silva AL, da Silva GA, da Silva GM, da Silva RMF, de Oliveira RJV, Oliveira RL, De Souza JT, Dueñas M, Evans HC, Epifani F, Felipe MTC, Fernández-López J, Ferreira BW, Figueiredo CN, Filippova NV, Flores JA, Gené J, Ghorbani G, Gibertoni TB, Glushakova AM, Healy R, Huhndorf SM, Iturrieta-González I, Javan-Nikkhah M, Juciano RF, Jurjević Ž, Kachalkin AV, Keochanpheng K, Krisai-Greilhuber I, Li YC, Lima AA, Machado AR, Madrid H, Magalhães OMC, Marbach PAS, Melanda GCS, Miller AN, Mongkolsamrit S, Nascimento RP, Oliveira TGL, Ordoñez ME, Orzes R, Palma MA, Pearce CJ, Pereira OL, Perrone G, Peterson SW, Pham THG, Piontelli E, Pordel A, Quijada L, Raja HA, Rosas de Paz E, Ryvarden L, Saitta A, Salcedo SS, Sandoval-Denis M, Santos TAB, Seifert KA, Silva BDB, Smith ME, Soares AM, Sommai S, Sousa JO, Suetrong S, Susca A, Tedersoo L, Telleria MT, Thanakitpipattana D, Valenzuela-Lopez N, Visagie CM, Zapata M, Groenewald JZ (2018) Fungal Planet description sheets: 785–867. Persoonia 41:238–417
Crous PW, Quaedvlieg W, Hansen K, Hawksworth DL, Groenewald JZ (2014) Phacidium and Ceuthospora (Phacidiaceae) are congeneric: taxonomic and nomenclatural implications. IMA Fungus 5:173–193
Crous PW, Slippers B, Wingfield MJ, Reeder J, Marasas WFO, Phillips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
Crous PW, Wingfield MJ, Burgess TI, Carnegie AJ, Hardy GESJ, Smith D, Summerell BA, Cano-Lira JF, Guarro J, Houbraken J, Lombard L, Martín MP, Sandoval-Denis M, Alexandrova AV, Barnes CW, Baseia IG, Bezerra JDP, Guarnaccia V, May TW, Hernández-Restrepo M, Stchigel AM, Miller AN, Ordoñez ME, Abreu VP, Accioly T, Agnello C, Agustin Colmán A, Albuquerque CC, Alfredo DS, Alvarado P, Araújo-Magalhães GR, Arauzo S, Atkinson T, Barili A, Barreto RW, Bezerra JL, Cabral TS, Camello Rodríguez F, Cruz RHSF, Daniëls PP, da Silva BDB, de Almeida DAC, de Carvalho Júnior AA, Decock CA, Delgat L, Denman S, Dimitrov RA, Edwards J, Fedosova AG, Ferreira RJ, Firmino AL, Flores JA, García D, Gené J, Giraldo A, Góis JS, Gomes AAM, Gonçalves CM, Gouliamova DE, Groenewald M, Guéorguiev BV, Guevara-Suarez M, Gusmão LFP, Hosaka K, Hubka V, Huhndorf SM, Jadan M, Jurjević Ž, Kraak B, Kučera V, Kumar TKA, Kušan I, Lacerda SR, Lamlertthon S, Lisboa WS, Loizides M, Luangsa-Ard JJ, Lysková P, Mac Cormack WP, Macedo DM, Machado AR, Malysheva EF, Marinho P, Matočec N, Meijer M, Mešić A, Mongkolsamrit S, Moreira KA, Morozova OV, Nair KU, Nakamura N, Noisripoom W, Olariaga I, Oliveira RJV, Paiva LM, Pawar P, Pereira OL, Peterson SW, Prieto M, Rodríguez-Andrade E, Rojo De Blas C, Roy M, Santos ES, Sharma R, Silva GA, Souza-Motta CM, Takeuchi-Kaneko Y, Tanaka C, Thakur A, Smith MT, Tkalčec Z, Valenzuela-Lopez N, van der Kleij P, Verbeken A, Viana MG, Wang XW, Groenewald JZ (2017) Fungal Planet description sheets: 625–715. Persoonia 39:270–467
Crous PW, Wingfield MJ, Burgess TI, Hardy GE, Crane C, Barrett S, Cano-Lira JF, Le Roux JJ, Thangavel R, Guarro J, Stchigel AM, Martín MP, Alfredo DS, Barber PA, Barreto RW, Baseia IG, Cano-Canals J, Cheewangkoon R, Ferreira RJ, Gené J, Lechat C, Moreno G, Roets F, Shivas RG, Sousa JO, Tan YP, Wiederhold NP, Abell SE, Accioly T, Albizu JL, Alves JL, Antoniolli ZI, Aplin N, Araújo J, Arzanlou M, Bezerra JD, Bouchara JP, Carlavilla JR, Castillo A, Castroagudín VL, Ceresini PC, Claridge GF, Coelho G, Coimbra VR, Costa LA, da Cunha KC, da Silva SS, Daniel R, de Beer ZW, Dueñas M, Edwards J, Enwistle P, Fiuza PO, Fournier J, García D, Gibertoni TB, Giraud S, Guevara-Suarez M, Gusmão LF, Haituk S, Heykoop M, Hirooka Y, Hofmann TA, Houbraken J, Hughes DP, Kautmanová I, Koppel O, Koukol O, Larsson E, Latha KP, Lee DH, Lisboa DO, Lisboa WS, López-Villalba Á, Maciel JL, Manimohan P, Manjón JL, Marincowitz S, Marney TS, Meijer M, Miller AN, Olariaga I, Paiva LM, Piepenbring M, Poveda-Molero JC, Raj KN, Raja HA, Rougeron A, Salcedo I, Samadi R, Santos TA, Scarlett K, Seifert KA, Shuttleworth LA, Silva GA, Silva M, Siqueira JP, Souza-Motta CM, Stephenson SL, Sutton DA, Tamakeaw N, Telleria MT, Valenzuela-Lopez N, Viljoen A, Visagie CM, Vizzini A, Wartchow F, Wingfield BD, Yurchenko E, Zamora JC, Groenewald JZ (2016) Fungal Planet description sheets: 469–557. Persoonia 37:218–403
Crous PW, Wingfield MJ, Le RJJ, Richardson DM, Strasberg D, Shivas RG, Alvarado P, Edwards J, Moreno G, Sharma R, Sonawane MS, Tan YP, Altés A, Barasubiye T, Barnes CW, Blanchette RA, Boertmann D, Bogo A, Carlavilla JR, Cheewangkoon R, Daniel R, de Beer ZW (2015b) Yáñez-Morales M de Jesús, Duong TA, Fernández-Vicente J, Geering ADW, Guest DI, Held BW, Heykoop M, Hubka V, Ismail AM, Kajale SC, Khemmuk W, Kolařík M, Kurli R, Lebeuf R, Lévesque CA, Lombard L, Magista D, Manjón JL, Marincowitz S, Mohedano JM, Nováková A, Oberlies NH, Otto EC, Paguigan ND, Pascoe IG, Pérez-Butrón JL, Perrone G, Rahi P, Raja HA, Rintoul T, Sanhueza RMV, Scarlett K, Shouche YS, Shuttleworth LA, Taylor PWJ, Thorn RG, Vawdrey LL, Solano-Vidal R, Voitk A, Wong PTW, Wood AR, Zamora JC, Groenewald JZ. Persoonia 35:264–327
Crous PW, Wingfield MJ, Guarro J, Hernández-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Dueñas M, Dutta AK, Gené J, Gouliamova DE, Groenewald M, Lombard L, Morozova OV, Sarkar J, Smith MTH, Stchigel AM, Wiederhold NP, Alexandrova AV, Antelmi I, Armengol J, Barnes I, Cano-Lira JF, Castañeda RRF, Contu M, PrR C, da Silveira AL, Decock CA, de Goes A, Edathodu J, Ercole E, Firmino AC, Fourie A, Fournier J, Furtado EL, Geering ADW, Gershenzon J, Giraldo A, Gramaje D, Hammerbacher A, He X-L, Haryadi D, Khemmuk W, Kovalenko AE, Krawczynski R, Laich F, Lechat C, Lopes UP, Madrid H, Malysheva EF, Marín-Felix Y, Martín MP, Mostert L, Nigro F, Pereira OL, Picillo B, Pinho DB, Popov ES, Rodas PCA, Rooney-Latham S, Sandoval-Denis M, Shivas RG, Silva V, Stoilova-Disheva MM, Telleria MT, Ullah C, Unsicker SB, van der Merwe NA, Vizzini A, Wagner H-G, Wong PTW, Wood AR, Groenewald JZ (2015c) Fungal Planet description sheets: 320–370. Persoonia 34:167–266
Crous PW, Lombard L, Sandoval-Denis M, Seifert KA, Schroers HJ, Chaverri P, Gené J, Guarro J, Hirooka Y, Bensch K, Kema GH et al (2021) Fusarium: more than a node or a foot-shaped basal cell. Stud Mycol 98:100116
Croxall HE (1950) Studies on british pyrenomycetes III. The British species of the genus Diatrypella Cesati and de Notaris. Trans Br Mycol Soc 33:45–72
Cruz KD, Lima MC, de Jesus MA, de Souza AQ, Sales-Campos C (2021) Annulohypoxylon (Hypoxylaceae, Ascomycota) from Amazonian-forest of Brazil, with a description of one new species. N Z J Bot 59:267–284
Cui BK, Dai YC (2008) Skeletocutis luteolus sp. nov. from southern and eastern China. Mycotaxon 104:97–101
Cui BK, Li H-J, Zhou J-L, Song J, Si J, Yang Z-L, Dai Y-C (2019) Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers 97:137–392
da Cunha KC, Sutton DA, Fothergill AW, Gené J, Cano J, Madrid H, de Hoog S, Crous PW, Guarro J (2013) In vitro antifungal susceptibility and molecular identity of 99 clinical isolates of the opportunistic fungal genus Curvularia. Diagn Microbiol Infect Dis 76:168–174
da Silva P, Grandi RAP (2008) Hyphomycetes on leaf litter of Caesalpinia echinata Lam. with two new records from Brazil. Hoehnea 35:477–488
Dai D, Bhat DJ, Liu J, Chukeatirote E, Zhao R, Hyde KD (2012) Bambusicola, a new genus from bamboo with asexual and sexual morphs. Cryptogam Mycol 33:363–790
Dai YC (1999) Phellinus sensu lato (Aphyllophorales, Hymenochaetaceae) in East Asia. Acta Bot Fennica 166:1–115
Dai YC (2010) Hymenochaetaceae (Basidiomycota) in China. Fungal Divers 45:131–343
Dai YC (2012) Two new polypores from tropical China, and renaming two species of Polyporus and Phellinus. Mycoscience 53:40–44
Dai YC, Cui BK, Yuan HS, Li BD (2007) Pathogenic wood-decaying fungi in China. For Pathol 37:105–120
Dai YC, Yang ZL, Cui BK, Yu CJ, Zhou LW (2009) Species diversity and utilization of medicinal mushrooms and fungi in China (Review). Int J Med Mushrooms 11:287–302
Dai DQ, Bahkali AH, Li WJ, Bhat DJ, Zhao RL, Hyde KD (2015) Bambusicola loculata sp. nov. (Bambusicolaceae) from bamboo. Phytotaxa 213:122–130
Dai DQ, Phookamsak R, Wijayawardene NN, Li WJ, Bhat DJ, Xu JC, Taylor JE, Hyde KD, Chukeatirote E (2017) Bambusicolous Fungi Fungal Divers 82:1–05
Damm U, Crous PW, Fourie PH (2007) Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora spp. nov. Mycologia 99:664–680
Damm U, Cannon PF, Woudenberg JHC, Johnston PR, Weir B, Tan YP, Shivas RG, Crous PW (2012) The Colletotrichum boninense species complex. Stud Mycol 73:1–36
Damm U, Sato T, Alizadeh A, Groenewald JZ, Crous PW (2019) The Colletotrichum draecaenophilum, C. magnum and C. orchidearum species complexes. Stud Mycol 92:1–46
Danish Khan I, Makkar A, Malik A, Khan S, Mehdi I, Arif S, Aden D, Somayaji P, Roomi K (2017) Curvularia keratomycosis after cataract surgery. J Arch Mil Med 5:e57331
Daranagama DA, Hyde KD, Sir EB, Thambugala KM et al (2018) Towards a natural classification and backbone tree for Graphostromataceae. Hypoxylaceae, Lopadostomataceae and Xylariaceae Fungal Divers 88:1–165
David A (1982) Étude monographique du genre Skeletocutis (Polyporaceae). Naturalist Can 109:235–272
Dayarathne MC, Maharachchikumbura SS, Jones EB, Goonasekara ID, Bulgakov TS, Al-Sadi AM, Hyde KD, Lumyong S, McKenzie EH (2017) Neophyllachora gen nov. (Phyllachorales), three new species of Phyllachora from Poaceae and resurrection of Polystigmataceae (Xylariales). Mycosphere 8:1598–1625
Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430
de Gruyter J, Woudenberg JH, Aveskamp MM, Verkley GJ, Groenewald JZ, Crous PW (2013) Redisposition of phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36
De Hoog GS, Guarro J, Gene J, Figueras MJ (2000) Atlas of clinical fungi, Centraalbureau voor Schimmelcultures. Utrecht, The Netherlands 276–282
Deighton FC (1976). Studies on Cercospora and allied genera. VI. Pseudocercospora Speg., Pantospora Cif. and Cercoseptoria Petr. Mycol Pap 140.
Delgado G (2008) South Florida microfungi: new records of saprophytic hyphomycetes on plant debris. Florida Scientist Jan 1:76–89.
Delgado G (2009) South Florida microfungi: Veramycella bispora, a new palmicolous anamorphic genus and species, with some new records for the continental USA. Mycotaxon 107(1):357–373
Delgado-Rodriguez G, Mena-Portales J (2004) Hifomicetos (hongos anamorficos) de la reserva ecologica ″alturas de banao″ (Cuba). Bol Soc Micol Madrid 28:115–124
Delgado-Rodríguez G, Mena-Portales J, Calduch M, Decock C (2002) Hyphomycetes (hongos mitospóricos) del área protegida Mil Cumbres, Cuba Occidental. Crypto Mycol 23:277–293
Denchev CM, Denchev TT (2013) Erratomycetaceae, fam. nov., and validation of some names of smut fungi recently described from India. Mycobiota 1:63–70
Denchev TT, Denchev CM (2016) Anthracocystis rhytachnes-rottboellioidis and A. urelytri, two new combinations of smut fungi (Ustilaginales) from Africa. Phytotaxa 253:227–231
Denchev CM, Fiaz M, Denchev TT, Ahmad H, Khalid AN (2012) Additions to the smut fungi of Pakistan. 1. Mycotaxon 121:165–170
Denchev TT, Sun H, Denchev CM, Boufford DE (2016) A new smut fungus on a new grass: Sporisorium capillipedii-alpini (Ustilaginales) sp. nov. infecting Capillipedium alpinum (Poaceae) sp. nov., from Sichuan. China Phytotaxa 252:217–227
Dennis RWG (1961) Fungi Venezuelani IV. Agaricales. Kew Bull 15:67–156
Dennis RWG (1986) Fungi of the Hebrides. Royal Botanic Gardens, Kew.
de Oliveira JJ, Moncalvo JM, Margaritescu S, Capelari M (2020a) Phylogenetic and morphological analyses of species of Marasmius sect. Marasmius from the Atlantic Rainforest. Brazil Plant Syst Evol 306:1–46
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM (2008). Phylogeny. fr: robust phylogenetic analysis for the non-specialist. Nucl Acids Res 36(suppl_2):W465–9.
de Silva NI, Maharachchikumbura SSN, Thambugala KM, Bhat DJ, Karunarathna SC, Tennakoon DS, Phookamsak R, Jayawardena RS, Lumyong S, Hyde KD (2021) Morphomolecular taxonomic studies reveal a high number of endophytic fungi from Magnolia candolli and M. garrettii in China and Thailand. Mycosphere 11:163–237
Desjardi DE, Retnowati A, Horak E (2000) Agaricales of Indonesia. 2. A preliminary monograph of Marasmius from Java and Bali. Sydowia 52:92–193
Desjardin DE (1989) The genus Marasmius from the Southern Appalachian Mountains. PhD. Thesis. University of Tennessee, Knoxville, 837 pp
Desjardin DE, Ovrebo CL (2006) New species and new records of Marasmius from Panamá. Fungal Diver 21:19–39
Desjardin DE, Perry BA (2017) The gymnopoig fungi (Basidiomycota, Agaricales) from the Republic of São Tomé and Principe, West Africa. Mycosphere 8:1317–1391
Devadatha B, Prakash PY, Jones EB, Sarma VV (2020) Do mangrove habitats serve as a reservoir for Medicopsis romeroi, a clinically important fungus. Mycol Prog 19:1267–1280
Dissanayake AJ, Camporesi E, Hyde KD, Zhang W et al (2017) Molecular phylogenetic analysis reveals seven new Diaporthe species from Italy. Mycosphere 8:853–877
Dissanayake AJ, Phillips AJL, Li XH, Hyde KD (2016) Botryosphaeriaceae: Current status of genera and species. Mycosphere 7:1001–1073
Dissanayake AJ, Bhunjun CS, Maharachchikumbura SSN, Liu JK (2020) Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere 11:2652–2676
Dissanayake LS, Wijayawardene NN, Dayarathne MC, Samarakoon MC, Dai DQ, Hyde KD, Kang JC (2021a) Paraeutypella guizhouensis gen. et sp. nov. and Diatrypella longiasca sp. nov. (Diatrypaceae) from China. Biodivers Data J 9: e63864
Dissanayake AJ, Chen YY, Cheewangkoon R, Liu JK (2021b) Occurrence and Morpho-Molecular Identification of Botryosphaeriales Species from Guizhou Province. China J Fungi 7:893
Dong W, Hyde KD, Jeewon R, Doilom M, Yu XD, Wang GN, Liu NG, Hu DM, Nalumpang S, Zhang H (2021) Towards a natural classification of annulatascaceae-like taxa II: Introducing five new genera and eighteen new species from freshwater. Mycosphere 12:1–88
Dong W, Wang B, Hyde KD, McKenzie EHC, Raja HA, Tanaka K, Abdel-Wahab MA, Abdel-Aziz FA, Doilom M, Phookamsak R, Hongsanan S, Wanasinghe DN, Yu X-D, Wang G-N, Yang H, Yang J, Thambugala KM, Tian Q, Luo Z-L, Yang J-B, Miller AN, Fournier J, Boonmee S, Hu D-M, Nalumpang S, Zhang H (2020) Freshwater Dothideomycetes Fungal Divers 105:319–575
Donk MA (1948) Notes on Malesian fungi. I. Bulletin Du Jardin Botanique De Buitenzorg 17:473–482
Dou ZP, Lu M, Wu JR, He W, Zhang Y (2017) A new species and interesting records of Aplosporella from China. Sydowia 69:1–7
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Du P, Cao TX, Wu YD, Zhou M, Liu ZB (2021) Two new species of Hymenochaetaceae on Dracaena cambodiana from tropical China. MycoKeys 80:1–17
Du R, Dai YC (2020) A new species and three new combinations of Skeletocutis (Incrustoporiaceae, Basidiomycota). Mycosystema 39:637–644
Du Z, Fan XL, Yang Q, Tian CM (2017) Host and geographic range extensions of Melanconiella, with a new species M. cornuta in China. Phytotaxa 327:252–260
Dugan FM, Glawe DA, Attanayake RN, Chen W (2009) The importance of reporting new hostfungus records for ornamental and regional crops. Plant Health Prog 10:34
Dulymamode R, Cannon PF, Peerally A (1998) Fungi from Mauritius: three Astrocystis species from Pandanus. Mycol Res 102:1325–1330
Dutta AK, Bera S, Paloi S, Rakshit S, Tarafder E, Sherpa A, Acharya K (2020) Lepiotaceous fungi of West Bengal, India: the genus Chlorophyllum. Phytotaxa 451:113–131
Edgar RC (2004) MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Edwards RL, Jonglaekha N, Anandini K, Maitland DJ, Mekkamol S, Nugent LK, Phosri C, Rodtong S, Ruchichachorn N, Sangvichien E, Sharples GP (2003) The Xylariaceae as phytopathogens. Recent Research Developments in Plant Science 1:1–9
Ekanayaka AH, Dissanayake AJ, Jayasiri SC, To-anun C, Jones EBG, Zhao Q, Hyde KD (2016) Aplosporella thailandica, a novel species revealing the sexual-asexual connection in Aplosporellaceae (Botryosphaeriales). Mycosphere 7:440–447
Ekanayaka AH, Hyde KD, Gentekaki E, McKenzie EH, Zhao Q, Bulgakov TS, Camporesi E (2019) Preliminary Classification of Leotiomycetes Mycosphere 10:310–489
Ellis MB (1961) Dematiaceous hyphomycetes. III. Mycol pap 82
Ellis MB (1971) Dematiaceous Hyphomycetes. CMI, Kew
Ellis MB (1976) More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England
Eriksson OE (2007) Outline of Ascomycota - 2007. Myconet 13:1–58
Eriksson O, Hawksworth DL (1986) An alphabetical list of the generic names of ascomycetes. CAB International Mycological Institute
Eriksson OE, Hawksworth DL (1987). Notes on ascomycete systematics Nos 225–463 Syst Ascomycetum 6:111–165
Fan LF, Ji XH, Si J (2017) A new species in the Skeletocutis subincarnata complex (Polyporales, Basidiomycota) from southwestern China. Mycosphere 8:1253–1260
Fan X, Du Z, Bezerra JD, Tian C (2018) Taxonomic circumscription of melanconis-like fungi causing canker disease in China. MycoKeys 42:89–124
Fan XL, Yang Q, Cao B, Liang YM, Tian CM (2015) New record of Aplosporella javeedii on five hosts in China based on multi-gene analysis and morphology. Mycotaxon 130:749–756
Farr DF, Rossman AY (2022) Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA. http://nt.ars-grin.gov/fungaldatabases/
Farr ML (1973) An annotated list of Spegazzini’s fungus taxa, Vol. 1. Biblioth Mycol 35:1–823
Ferdinandez HS, Manamgoda DS, Udayanga D, Deshappriya N, Munasinghe MS, Castlebury LA (2021) Molecular phylogeny and morphology reveal three novel species of Curvularia (Pleosporales, Pleosporaceae) associated with cereal crops and weedy grass hosts. Mycol Prog 20:431–451
Fiasson JL, Niemela T (1984) The Hymenochaetales: a revision of the European poroid taxa. Karstenia 24:14–28
Filippi I (1991) Osservazioni ecologiche su Hydropus mediterraneus Pacioni & Lalli. Micol Ital 20:43–46
Firmino AL, Pereira OL (2021) A simple method for the cultivation of the ″unculturable″ asterinaceous fungi (Asterinales/Dothideomycetes). J Microbiol Methods 187: article 106272
Fries EM (1815) Observationes Mycologicae, vol 2. G. Bonnier, Copenhagen
Fries EM (1823) Systema Mycologicum 2:276–620
Fries EM (1832) Systema Mycologicum 3: 1–202. Greifswald.
Fries EM (1849) Summa Vegetabilum Scandinaviae. Vol. 2. Uppsala: Typographia Academica.
Karst FP (1891) Meddn Soc. Fauna Flora Fenn 18:62
Fournier J, Lechat C, Mifsud S, Sammut C. (2021) Xylaria melitensis (Xylariaceae), a new penzigioid species from the Maltese Islands. Ascomycete.org 13:59–67
Fries E (1838) Epicrisis systematis mycologici. Typographia Academica, Upsaliæ
Gajanayake AJ, Abeywickrama PD, Jayawardena RS, Camporesi E, Bundhun D (2020) Pathogenic Diaporthe from Italy, and the first report of D. foeniculina associated with Chenopodium sp. Plant Pathol Quaran 10:172–197
Gangadevi V, Muthumary J (2008) Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J Microbiol Biotech 24:717–724
Garcia-Aroca T, Price PP, Tomaso-Peterson M, Allen TW, Wilkerson TH, Spurlock TN, Faske TR, Bluhm B, Conner K, Sikora E, Guyer R (2021) Xylaria necrophora, sp. nov., is an emerging root-associated pathogen responsible for taproot decline of soybean in the southern United States. Mycologia 113:326–347
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gardiennet A, Lechat C, Fournier J (2019) Ericboehmia, a new genus segregated from Ostreichnion in the Hysteriaceae, with the new species E. saulensis. Ascomycete.org 11:24
Ge ZW, Jacobs A, Vellinga EC, Sysouphanthong P, van der Walt R, Lavorato C, An YF, Yang ZL (2018) A multi-gene phylogeny of Chlorophyllum (Agaricaceae, Basidiomycota): new species, new combination and infrageneric classification. MycoKeys 32:65–90
Ge ZW, Yang ZL (2006) The genus Chlorophyllum (Basidiomycetes) in China. Mycotaxon 96:181–191
Geiser DM, Al-Hatmi AM, Aoki T, Arie T, Balmas V, Barnes I, Bergstrom GC, Bhattacharyya MK, Blomquist CL, Bowden RL, Brankovics B (2021) Phylogenomic analysis of a 55.1-kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani species complex. Phytopathology 111:1064–1079
Geng K, Zhang B, Song Y, Hyde KD, Kang JI, Wang Y (2013) A new species of Pestalotiopsis from leaf spots of Licuala grandis from Hainan, China. Phytotaxa 88:49–54
Gilbertson RL, Ryvarden L (1986) North American polypores 1. Fungiflora, Oslo
Gilgado F, Cano J, Gené J et al (2008) Molecular and phenotypic data supporting distinct species statuses for Scedosporium apiospermum and Pseudallescheria boydii and the proposed new species Scedosporium dehoogii. J Clin Microbiol 46:766–771
Gilgado F, Cano J, Gené J, Guarro J (2005) Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J Clin Microbiol 43:4930–4942
Gilgado F, Gené J, Cano J, Guarro J (2010) Heterothallism in Scedosporium apiospermum and description of its teleomorph Pseudallescheria apiosperma sp. nov. Med Mycol 48:122–128
Giraldo A, Crous PW, Schumacher RK, Cheewangkoon R, Ghobad-Nejhad M, Langer E (2017) The genera of Fungi—G3: Aleurocystis, Blastacervulus, Clypeophysalospora, Licrostroma, Neohendersonia and Spumatoria. Mycol Prog 16:325–348
Goldman C, Akiyama MJ, Torres J, Louie E et al (2016) Scedosporium apiospermum infections and the role of combination antifungal therapy and GM-CSF: A case report and review of the literature. Med Mycol Case Rep 11:40–43
Goldmann L, Weir A (2018) Molecular phylogeny of the Laboulbeniomycetes (Ascomycota). Fungal Biol 122:87–100
Gonçalves MF, Vicente TF, Esteves AC, Alves A (2019) Neptunomyces aureus gen. et sp. nov.(Didymosphaeriaceae, Pleosporales) isolated from algae in Ria de Aveiro, Portugal. MycoKeys 60:31
Gräfenhan T, Schroers HJ, Nirenberg HI, Seifert KA (2011) An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud Mycol 68:79–113
Greville KR (1823–1824) Scottish cryptogamic flora 2: 82–83
Grove WB (1937). British stem- and leaf-fungi (Coelomycetes). 2. Cambridge University Press, Cambridge.
Guatimosim E, Firmino AL, Bezerra JL, Pereira OL, Barreto RW, Crous PW (2015) Towards a phylogenetic reappraisal of Parmulariaceae and Asterinaceae (Dothideomycetes). Persoonia 35:230–241
Gueho E (1991) Taxonomy of the medical species of Pseudallescheria and Scedosporium. J Mycol Med 118:3–9
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Guzmán G (1978) The species of Psilocybe known from Central and South America. Mycotaxon 7:225–255
Guzmán G (1983) The genus Psilocybe. Beihefte Nova Hedwigia 74
Guzmán G, Bandala VM, Allen JW (1993) A new bluing Psilocybe from Thailand. Mycotaxon 46:155–160
Guzmán G, Jacobs JQ, Ramírez-Guillen F, Murrieta D, Gandara E (2005) The taxonomy of Psilocybe fagicola-complex. J Microbiol 43:158–165
Guzmán G, Ramírez-Guillén F, Hyde KD, Karunarathna SC (2012) The genus Psilocybe s.s. in Thailand: a review of the known species and description of four new species. Mycotaxon 119:65–81
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic acids symposium series (Vol. 41, No. 41, pp. 95–98). [London]: Information Retrieval Ltd., c1979–c2000
Han ML, Chen YY, Shen LL, Song J, Vlasák J, Dai YC, Cui BK (2016) Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers 80:343–373
Han Y-F, Zheng H, Zhang Z-Y, Chen W-H, Luo Y, Wang Y-R, Liang Z-Q (2017) Two new Scedosporium species from Guangxi and Hainan. Mycosystema 36:145–153
Hansford GC (1946) The foliicolous ascomycetes their parasites and associated fungi. Mycol Pap 15:1–139
Hansford CG (1954) Some Microthyriales and other fungi from Indonesia. Reinwardtia 3:113–144
Hao YJ, Qin J, Yang ZL (2014) Cibaomyces, a new genus of Physalacriaceae from East Asia. Phytotaxa 162:198–210
Hashimoto A, Hirayama K, Takahashi H, Matsumura M, Okada G, Chen CY, Huang JW, Kakishima M, Ono T, Tanaka K (2018) Resolving the Lophiostoma bipolare complex: Generic delimitations within Lophiostomataceae. Stud Mycol 90:161–189
Hassan O, Kim JS, Romain BBND, Chang T (2022) An account of Colletotrichum species associated with anthracnose of Atractylodes ovata in South Korea based on morphology and molecular data. Plos one 17:e0263084
Hattori T, Sakayaroj J, Jones EBG, Suetrong S, Preedanon S, Klaysuban A (2014) Three species of Fulvifomes (Basidiomycota, Hymenochaetales) associated with rots on mangrove tree Xylocarpus graniatum in Thailand. Mycoscience 55:344–354
Haug I, Weiß M, Homeier J, Oberwinkler F, Kottke I (2005) Russulaceae and Thelephoraceae form ectomycorrhizas with members of the Nyctaginaceae (Caryophyllales) in the tropical mountain rain forest of southern Ecuador. New Phytol 165:923–936
Hawksworth DL (1985a) Kirschsteiniothelia, a new genus for the Microthelia incrustans-group (Dothideales). Bot J Linn Soc 91:181–202
Hawksworth DL (1985b) A redisposition of the species referred to the ascomycete genus Microthelia. Bull Br Mus Nat 14:43–181
He M-Q, Zhao R-L, Hyde KD, Begerow D, Kemler M, Yurkov A, McKenzie EHC et al (2019) Notes, outline and divergence times of Basidiomycota. Fungal Divers 99:105–367
Helaly SE, Thongbai B, Stadler M (2018) Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat Prod Rep 35:992–1014
Hennings P (1904) Fungi Amazonici a cl. Ernesto Ule Collecti III Hedwigia 43:351–400
Hennings P (1909) Fungi S. Paulensis IV a cl. Puttemans Collecti Hedwigia 48:1–20
Heredia G, Mercado Sierra A, Mena Portales J (1995) Conidial fungi from leaf litter in a mesophilic cloud forest of Veracruz, Mexico. Mycotaxon 55:473–490
Heredia GA, Arias RM, Reyes M (2000) Leaf litter fungi. Eight setose conidial species unknown from Mexico. Rev Mex Micol 16:17–25
Hernandez-Restrepo M, Schumacher RK, Wingfield MJ, Ahmad I, Cai L, Duong TA, Edwards J, Gene J, Groenewald JZ, Jabeen S, Khalid AN et al (2016) Fungal systematics and evolution: FUSE 2. Sydowia 68:193–230
Hernandez-Restrepo M, Gene J, Castaneda-Ruiz RF, Mena-Portales J et al (2017) Phylogeny of saprobic microfungi from Southern Europe. Stud Mycol 86:53–97
Hernández-Restrepo M, Groenewald JZ, Crous PW (2015) Neocordana gen. nov., the causal organism of Cordana leaf spot on banana. Phytotaxa 205:229–238
Hernandez-Restrepo M, Madrid H, Tan YP, Da Cunha KC, Gene J, Guarro J, Crous PW (2018) Multi-locus phylogeny and taxonomy of Exserohilum. Persoonia 41:71–108
Higgins D, Thompson J, Gibson T, Thompson JD, Higgins G, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Hirayama K, Tanaka K, Raja HA, Miller AN, Shearer CA (2010) A molecular phylogenetic assessment of Massarina ingoldiana sensu lato. Mycologia 102:729–746
Hjortstam K, Ryvarden L (1982) Aphyllophorales from northern Thailand. Nord J Bot 2:273–281
Holm L, Holm K (1988) Studies in the Lophiostmataceae with emphasis on the Swedish species. Symbolae Botanicae Upsalienses 28:1–50
Hongsanan S, Tian Q, Bahkali AH, Yang JB, McKenzie EH, Chomnunti P, Hyde KD (2015) Zeloasperisporiales ord. nov., and two new species of Zeloasperisporium Cryptogam. Mycol 36:301–317
Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Boonmee S, Lücking R, Bhat DJ, Liu NG, Tennakoon DS, Pem D, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe IS, Tibpromma S, Jayasiri SC, Sandamali DS, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Phukhamsakda C, Thambugala KM, Dai DQ, Chethana KWT, Samarakoon MC, Ertz D, Bao DF, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Konta S, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, Norphanphoun C, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-González R, Aptroot A, Kashiwadani H, Harishchandra D, Sérusiaux E, Aluthmuhandiram JVS, Abeywickrama PD, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Chomnunti P, Suetrong S, Chaiwan N, Dayarathne MC, Yang J, Rathnayaka AR, Bhunjun CS, Xu JC, Zheng JS, Liu G, Feng Y, Xie N (2020a) Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 11:1553–2107
Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Lücking R, Boonmee S, Bhat DJ, Liu NG, Tennakoon DS, Pem D, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe IS, Tibpromma S, Jayasiri SC, Sandamali D, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Phukhamsakda C, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Thambugala KM, Dai DQ, Samarakoon MC, Chethana KWT, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Konta S, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, Norphanphoun C, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-González R, Aptroot A, Kashiwadani H, Harishchandra D, Sérusiaux E, Abeywickrama PD, Bao D-F, Devadatha B, Wu H-X, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Chomnunti P, Suetrong S, Chaiwan N, Bhunjun CS, Dayarathne MC, Yang J, Rathnayaka AR, Xu J, Zheng J, Liu G, Feng Y, Xie N (2020b) Refined families of Dothideomycetes: Orders and families incertae. Fungal Divers 105:17–318
Hongsanan S, Maharachchikumbura SSN, Hyde KD, Samarakoon MC, Jeewon R, Zhao Q, Al-Sadi AM, Bahkali AH (2017) An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers 84:25–41
Horak E, Guzmán G, Desjardin DE (2009) Four new species of Psilocybe from Malaysia and Thailand, with a key to the species of sect. Neocaledonicae and discussion on the distribution of the tropical and temperate species. Sydowia 61:25–37
Hosagoudar VB, Abraham TK (2000) A list of Asterina Lev. species based on the literature. J Econ and Taxon Bot 24:557–587
Hosagoudar VB, Biju H, Appaiah KA (2006) Studies on foliicolous fungi-XX. Microfungi of Coorg. Karnataka J Mycopathol Res 44:1–25
Hou YJ, Ge ZW (2020) New species of Echinoderma and Lepiota (Agaricaceae) from China. Phytotaxa 447:221–236
Hu DM, Cai L, Chen H, Bahkali AH, Hyde KD (2010) Fungal diversity on submerged wood in a tropical stream and an artificial lake. Biodivers Conserv 19:3799–3808
Huhndorf SM, Miller AN, Fernández FA (2004) Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae redefined. Mycologia 96:368–387
Hyde KD (1995) Tropical Australian freshwater fungi. IX. Vaginatispora aquatica gen. et sp. nov. Nov Hedwigia 61:233–241
Hyde KD, Jones EB, Leaño E, Pointing SB, Poonyth AD, Vrijmoed LL (1998) Role of fungi in marine ecosystems. Biodivers Conserv 7:1147–1161
Hyde KD, Chaiwan N, Norphanphoun C, Boonmee S, Camporesi E, Chethana KWT, Dayarathne MC, de Silva NI, Dissanayake AJ, Ekanayaka AH, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Jiang HB, Karunarathna A, Lin CG, Liu JK, Liu NG, Lu YZ, Luo ZL, Maharachchimbura SSN, Manawasinghe IS, Pem D, Perera RH, Phukhamsakda C, Samarakoon MC, Senwanna C, Shang QJ, Tennakoon DS, Thambugala KM, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Zhang JF, Zhang SN, Bulgakov TS, Bhat DJ, Cheewangkoon R, Goh TK, Jones EBG, Kang JC, Jeewon R, Liu ZY, Lumyong S, Kuo CH, McKenzie EHC, Wen TC, Yan JY, Zhao Q (2018) Mycosphere notes 169–224. Mycosphere 9:271–430
Hyde KD, Norphanphoun C, Maharachchikumbura SSN, Bhat DJ, Jones EBG, Bundhun D, Chen YJ, Bao DF, Boonmee S, Calabon MS, Chaiwan N, Chethana KWT, Dai DQ, Dayarathne MC, Devadatha B, Dissanayake AJ, Dissanayake LS, Doilom M, Dong W, Fan XL, Goonasekara ID, Hongsanan S, Huang SK, Jayawardena RS, Jeewon R, Karunarathna A, Konta S, Kumar V, Lin CG, Liu JK, Liu NG, Luangsa-ard J, Lumyong S, Luo ZL, Marasinghe DS, McKenzie EHC, Niego AGT, Niranjan M, Perera RH, Phukhamsakda C, Rathnayaka AR, Samarakoon MC, Samarakoon SMBC, Sarma VV, Senanayake IC, Shang QJ, Stadler M, Tibpromma S, Wanasinghe DN, Wei DP, Wijayawardene NN, Xiao YP, Yang J, Zeng XY, Zhang SN, Xiang MM (2020a) Refined Families of Sordariomycetes Mycosphere 11:305–1059
Hyde KD, de Silva NI, Jeewon R, Bhat DJ, Phookamsak R, Doilom M, Boonmee S, JayawardenaRS MSSN, Senanayake IC, Manawasinghe IS, Liu NG, Abeywickrama PD, Chaiwan N, Karunarathna A, Pem D, Lin CG, Sysouphanthong P, Luo ZL, Wei DP, WanasingheDN NC, Tennakoon DS, Samarakoon MC, Jayasiri SC, Jiang HB, Zeng XY, Li JF, Wijesinghe SN, Devadatha B, Goonasekara ID, Brahmanage RS, Yang EF, Aluthmuhandiram JVS, Dayarathne MC, Marasinghe DS, Li WJ, Dissanayake LS, Dong W, Huanraluek N, Lumyong S, Liu JK, Karunarathna SC, Jones EBG, Al-Sadi AM, Xu JC, Harishchandra D, Sarma VV (2020b) AJOM new records and collections of fungi: 1–100. Asian Journal of Mycology 3:22–294
Hyde KD, Dong Y, Phookamsak R, Jeewon R, Bhat DJ, Jones EBG, Liu N-G, Abeywickrama PD, Mapook A, Wei D, Perera RH, Manawasinghe IS, Pem D, Bundhun D, Karunarathna A, Ekanayaka AH, Bao D-F, Li J, Samarakoon MC, Chaiwan N, Lin C-G, Phutthacharoen K, Zhang S-N, Senanayake IC, Goonasekara ID, Thambugala KM, Phukhamsakda C, Tennakoon DS, Jiang H-B, Yang J, Zeng M, Huanraluek N, Liu J-K, (Jack), Wijesinghe SN, Tian Q, Tibpromma S, Brahmanage RS, Boonmee S, Huang S-K, Thiyagaraja V, Lu Y-Z, Jayawardena RS, Dong W, Yang E-F, Singh SK, Singh SM, Rana S, Lad SS, Anand G, Devadatha B, Niranjan M, Sarma VV, Liimatainen K, Aguirre-Hudson B, Niskanen T, Overall A, Alvarenga RLM, Gibertoni TB, Pfliegler WP, Horváth E, Imre A, Alves AL, da Silva Santos AC, Tiago PV, Bulgakov TS, Wanasinghe DN, Bahkali AH, Doilom M, Elgorban AM, Maharachchikumbura SSN, Rajeshkumar KC, Haelewaters D, Mortimer PE, Zhao Q, Lumyong S, Xu J, Sheng J (2020) Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 100:5–277
Hyde KD, Hongsanan S, Jeewon R, Bhat DJ, McKenzie EHC, Jones EBG, Phookamsak R, Ariyawansa HA, Boonmee S, Zhao Q, Abdel-Aziz FA, Abdel-Wahab MA, Banmai S, Chomnunti P, Cui BK, Daranagama DA, Das K, Dayarathne MC, de Silva NI, Dissanayake AJ, Doilom M, Ekanayaka AH, Gibertoni TB, Góes-Neto A, Huang SK, Jayasiri SC, Jayawardena RS, Konta S, Lee HB, Li WJ, Lin CG, Liu JK, Lu YZ, Luo ZL, Manawasinghe IS, Manimohan P, Mapook A, Niskanen T, Norphanphoun C, Papizadeh M, Perera RH, Phukhamsakda C, Richter C, André ALCM, Drechsler-Santos ER, Senanayake IC, Tanaka K, Tennakoon TMDS, Thambugala KM, Tian Q, Tibpromma S, Thongbai B, Vizzini A, Wanasinghe DN, Wijayawardene NN, Wu HX, Yang J, Zeng XY, Zhang H, Zhang JF, Bulgakov TS, Camporesi E, Bahkali AH, Amoozegar MA, Araujo-Neta LS, Ammirati JF, Baghela A, Bhatt RP, Bojantchev D, Buyck B, da Silva GA, de Lima CLF, de Oliveira RJV, de Souza CAF, Dai YC, Dima B, Duong TT, Ercole E, Mafalda-Freire F, Ghosh A, Hashimoto A, Kamolhan S, Kang JC, Karunarathna SC, Kirk PM, Kytövuori I, Lantieri A, Liimatainen K, Liu ZY, Liu XZ, Lücking R, Medardi G, Mortimer PE, Nguyen TTT, Promputtha I, Raj KNA, Reck MA, Lumyong S, Shahzadeh-Fazeli SA, Stadler M, Soudi MR, Su HY, Takahashi T, Tangthirasunun N, Uniyal P, Wang Y, Wen TC, Xu JC, Zhang ZK, Zhao YC, Zhou JL, Zhu L (2016) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 80:1–270
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Li LJD, YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, De Hoog S, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M (2013) Families of Dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Norphanphoun C, Abreu VP, Bazzicalupo A, Thilini Chethana KW, Clericuzio M, Dayarathne MC, Dissanayake AJ, Ekanayaka AH et al (2017b) Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Divers 87:1–235
Hyde KD, Norphanphoun C, Abreu V, Bazzicalupo AL, Thilini Chethana KW, Clericuzio M, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, He M, Hongsanan S, Huang S, Jayasiri SC, Jayawardena RS, Karunarathna A, Konta S, Kušan I, Lee HG, Li J, Lin C, Liu N, Lu Y, Luo Z, Manawasinghe IS, Mapook A, Perera RH, Phookamsak R, Phukhamsakda C, Siedlecki I, Soares AM, Tennakoon DS, Tian Q, Tibpromma S, Wanasinghe DN, Xiao Y, Yang J, Zeng X, Abdel-Aziz FA, Li W, Senanayake IC, Shang Q, Daranagama DA, Silva NI, Thambugala KM, Abdel-Wahab MA, Bahkali AH, Berbee ML, Boonmee S, Bhat DJ, Bulgakov TS, Buyck B, Camporesi E, Castañeda-Ruiz RF, Chomnunti P, Doilom M, Dovana F, Gibertoni TB, Jadan M, Jeewon R, Jones E, Kang J, Karunarathna SC, Lim YW, Liu J, Liu Z, Plautz HL, Lumyong S, Maharachchikumbura SS, Matočec N, McKenzie EH, Mešić A, Miller D, Pawłowska J, Pereira OL, Promputtha I, Romero AI, Ryvarden L, Su H, Suetrong S, Tkalčec Z, Vizzini A, Wen T, Wisitrassameewong K, Wrzosek M, Xu J, Zhao Q, Zhao R, Mortimer PE (2017) Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Divers 87:1–235
Hyde KD, Dong Y, Phookamsak R, Jeewon R, Bhat DJ, Jones EB, Liu NG, Abeywickrama PD, Mapook A, Wei D, Perera RH et al (2020d) Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 100:5–277
Hyde KD, Suwannarach N, Jayawardena RS, Manawasinghe IS, Liao CF, Doilom M, Cai L, Zhao P, Buyck B, Phukhamsakda C, Su WX, Fu YP, Li Y, Zhao RL, He MQ, Li JX, Tibpromma S, Lu L, Tang X, Kang JC, Ren GC, Gui H, Hofstetter V, Ryoo R, Antonín V, Hurdeal VG, Gentikaki E, Zhang JY, Lu YZ, Senanayake IC, Yu FM, Zhao Q, Bao DF (2021) Mycosphere notes 325–344 – Novel species and records of fungal taxa from around the world. Mycosphere 12:1101–1156
Hyde KD, Tennakoon DS, Jeewon R, Bhat DJ, Maharachchikumbura SSN, Rossi W, Leonardi M, Lee HB, Mun HY, Houbraken J, Nguyen TTT, Jeon SJ, Frisvad JC, Wanasinghe DN, Lücking R, Aptroot A, Cáceres MES, Karunarathna SC, Hongsanan S, Phookamsak R, de Silva NI, Thambugala KM, Jayawardena RS, Senanayake IC, Boonmee S, Chen J, Luo ZL, Phukhamsakda C, Pereira OL, Abreu VP, Rosado AWC, Bart B, Randrianjohany E, Hofstetter V, Gibertoni TB, da Soares AM, S, Plautz HL, Sotão HMP, Xavier WKS, Bezerra JDP, de Oliveira TGL, de Souza-Motta CM, Magalhães OMC, Bundhun D, Harishchandra D, Manawasinghe IS, Dong W, Zhang SN, Bao DF, Samarakoon MC, Pem D, Karunarathna A, Lin CG, Yang J, Perera RH, Kumar V, Huang SK, Dayarathne MC, Ekanayaka AH, Jayasiri SC, Xiao Y, Konta S, Niskanen T, Liimatainen K, Dai YC, Ji XH, Tian XM, Mešić A, Singh SK, Phutthacharoen K, Cai L, Sorvongxay T, Thiyagaraja V, Norphanphoun C, Chaiwan N, Lu YZ, Jiang HB, Zhang JF, Abeywickrama PD, Aluthmuhandiram JVS, Brahmanage RS, Zeng M, Chethana T, Wei D, Réblová M, Fournier J, Nekvindová J, do Nascimento Barbosa R, dos Santos JEF, de Oliveira NT, Li GJ, Ertz D, Shang QJ, Phillips AJL, Kuo CH, Camporesi E, Bulgakov TS, Lumyong S, Jones EBG, Chomnunti P, Gentekaki E, Bungartz F, Zeng XY, Fryar S, Tkalčec Z, Liang J, Li G, Wen TC, Singh PN, Gafforov Y, Promputtha I, Yasanthika E, Goonasekara ID, Zhao RL, Zhao Q, Kirk PM, Liu JK, Yan JY, Mortimer PE, Xu J, Doilom M, (2019) Fungal diversity notes 1036–1150: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 96:1–24
Hyde KD, Tennakoon DS, Jeewon R, Bhat DJ, Maharachchikumbura SS, Rossi W, Leonardi M, Lee HB, Mun HY et al (2019b) Fungal diversity notes 1036–1150: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 96:1–242
Hyde KD (1997)– Ascomycetes described on Freycinetia. Sydowia. 49:1–20
Ibrahim KM, Dube S, Peterson PM, Hosni HA (2018) Grasses of Mali. Smithsonian Institution Scholarly Press, Washington DC
Index Fungorum (2022a) Index Fungorum. http://www.indexfungorum.org/
Jaklitsch WM, Gardiennet A, Voglmayr H (2016) Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia 37:82–105
Jakucs E, Erős-Honti Z (2008) Morphological-anatomical characterization and identification of Tomentella ectomycorrhizas. Mycorrhiza 18:277–285
Jayasiri SC, Hyde KD, Jones EBG, McKenzie EHC, Jeewon R, Phillips AJL, Bhat DJ, Wanasinghe DN, Liu JK, Lu YZ, Kang JC, Xu J, Karunarathna SC (2019) Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 10:1–186
Jayawardena RS, Hyde KD, Damm U, Cai L, Liu M, Li XH, Zhang W, Zhao WS, Yan JY (2016) Notes on currently accepted species of Colletotrichum. Mycosphere 7:1192–1260
Jayawardena RS, Hyde KD, Chethana KWT, Daranagama DA, Dissanayake AJ, Goonasekara ID, Manawasinghe IS, Mapook A, Jayasiri SC et al (2018) Mycosphere Notes 102–168: Saprotrophic fungi on Vitis in China, Italy, Russia and Thailand. Mycosphere 9:1–114
Jayawardena RS, Hyde KD, Jeewon R, Ghobad-Nejhad M, Wanasinghe DN, Liu N, Phillips AJ, Oliveira-Filho JR, da Silva GA, Gibertoni TB, Abeywikrama P, Carris LM, Chethana KW, Dissanayake AJ, Hongsanan S, Jayasiri SC, McTaggart AR, Perera RH, Phutthacharoen K, Savchenko KG, Shivas RG, Thongklang N, Dong W, Wei D, Wijayawardena NN, Kang J (2019a) One stop shop II: taxonomic update with molecular phylogeny for important phytopathogenic genera: 26–50 (2019a). Fungal Divers 94:41–129
Jayawardena RS, Hyde KD, McKenzie EH, Jeewon R, Phillips AJ, Perera RH, de Silva NI, Maharachchikumburua SS, Samarakoon MC, Ekanayake AH, Tennakoon DS, Dissanayake AJ, Norphanphoun C, Lin C, Manawasinghe IS, Tian Q, Brahmanage RS, Chomnunti P, Hongsanan S, Jayasiri SC, Halleen F, Bhunjun CS, Karunarathna A, Wang Y (2019b) One stop shop III: taxonomic update with molecular phylogeny for important phytopathogenic genera: 51–75 (2019b). Fungal Divers 98:160–177
Jayawardena RS, Hyde KD, Chen Y, Papp V, Palla B, Papp D, Bhunjun CS, Hurdeal VG, Senwanna C, Manawasinghe IS, Harischandra DL, Gautam AK, Avasthi S, Chuankid B, Goonasekara ID, Hongsanan S, Zeng X, Liyanage KK, Liu N, Karunarathna A, Hapuarachchi KK, Luangharn T, Raspé O, Brahmanage RS, Doilom M, Lee HB, Mei L, Jeewon R, Huanraluek N, Chaiwan N, Stadler M, Wang Y (2020) One stop shop IV: taxonomic update with molecular phylogeny for important phytopathogenic genera: 76–100 (2020). Fungal Divers 103:87–218
Jayawardena RS, Bhunjun CS, Hyde KD, Gentekaki E, Itthayakorn P (2021a) Colletotrichum: lifestyles, biology, morpho-species, species complexes and accepted species. Mycosphere 12:519–669
Jayawardena RS, Hyde KD, de Farias AR, Bhunjun CS, Ferdinandez HS, Manamgoda DS, Udayanga D, Herath IS, Thambugala KM, Manawasinghe IS, Gajanayake AJ, Samarakoon BC, Bundhun D, Gomdola D, Huanraluek N, Sun Y, Tang X, Promputtha I, Thines M (2021b) What is a species in fungal plant pathogens? Fungal Divers 109:239–266
Jeewon R, Liew ECY, Hyde KD (2003) Phylogenetic signicance of morphological characters in the taxonomy of Pestalotiopsis species. Mol Phyl Evol 27:372–383
Jeewon R, Hyde KD (2016) Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7:1669–1677
Ji XH, Wu F, Dai YC, Vlasák J (2017) Two new species of Fulvifomes (Hymenochaetales, Basidiomycota) from America. MycoKeys 22:1–13
Johnston PR, Park D (2019) Blastacervulus metrosideri sp. nov. leaf spot on Metrosideros excelsa in New Zealand. Fungal Syst Evol 3:165–169
Jones EBG, Suetrong S, Sakayaroj J, Bahkali AH, Abdel-Wahab MA, Boekhout T, Pang KL (2015) Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers 73:1–72
Ju Y-M, Rogers JD (1996) A revision of the genus Hypoxylon. Mycologia Memoir No. 20. St. Paul: APS Press. 365 p
Ju YM, Rogers JD, Hsieh HM (2018) Xylaria species associated with fallen fruits and seeds. Mycologia 110:726–749
Jülich W (1981) Higher Taxa of Basidiomycetes Bibl Mycol 85:1–485
Justo A et al (2017) A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol 121:798–824
Kaltseis J, Rainer J, De Hoog GS (2009) Ecology of Pseudallescheria and Scedosporium species in human-dominated and natural environments and their distribution in clinical samples. Sabouraudia 47:398–405
Kamada T (2002) Molecular genetics of sexual development in the mushroom Coprinus cinereus. BioEssays 24:449–459
Katoh K, Asimenos G, Toh H (2009) Multiple Alignment of DNA Sequences with MAFFT. In: Posada, D. (ed.) Bioinformatics for DNA Sequence Analysis. Methods mol biol 537:39–64
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Keith LM, Velasquez ME, Zee FT (2006) Identification and characterization of Pestalotiopsis spp. causing scab disease of guava, Psidium guajava, in Hawaii. Plant Dis 90:16–23
Keizer PJ (1997) Coltricia confluens: A new polypore from the Netherlands. Persoonia 16:389–391
Khan Z, Gene J, Ahmad S, Cano J, Al-Sweih N, Joseph L, Guarro J (2013) Coniochaeta polymorpha, a new species from endotracheal aspirate of a preterm neonate, and transfer of Lecythophora species to Coniochaeta. Antonie Van Leeuwenhoek 104:243–252
Khemmuk W, Shivas RG, Henry RJ, Geering AD (2016) Fungi associated with foliar diseases of wild and cultivated rice (Oryza spp.) in northern Queensland. Australas Plant Pathol 45:297–308
Kirk PM, Cannon PF, David JC et al (2001) Ainsworth & Bisby’s Dictionary of the Fungi, 9th edn. CABI International University Press, Wallingford, UK
Kirk PM, Cannon PF, Minter D, Stalpers JA (2008) Dictionary of the fungi, 10th edn. CAB International, Wallingford, UK
Ko Y, Yao KS, Chen CY, Lin CH (2007) First report of gray leaf spot of mango (Mangifera indica) caused by Pestalotiopsis mangiferae in Taiwan. Plant Dis 91:1684
Kondo M, Goto H, Yamanaka K (2018) Case of Scedosporium aurantiacum infection detected in a subcutaneous abscess. Med Mycol Case Rep 20:26–27
Kondo M, Goto H, Yamanaka K (2018b) Case of Scedosporium aurantiacum infection detected in a subcutaneous abscess. Med Mycol Case Rep 20:26–27
Kondratyuk SY, Jeong M-H, Yu N-H, Kärnefelt I, Thell A, Elix JA, Kim J, Kondratyuk AS, Hur J-S (2013) Four new genera of teloschistoid lichens (Teloschistaceae, Ascomycota) based on molecular phylogeny. Acta Bot Hung 55:251–274
Konta S, Hongsanan S, Tibpromma S, Thongbai B, Maharachchikumbura SS, Bahkali AH, Hyde KD, Boonmee S (2016) An advance in the endophyte story: Oxydothidaceae fam. nov. with six new species of Oxydothis. Mycosphere 7:1425–1446
Konta S, Maharachchikumbura SSN, Senanayake IC, McKenzie EHC, Stadler M, Boonmee S, Phookamsak R, Jayawardena RS, Senwanna C, Hyde KD, Elgorban AM, Eungwanichayapant PD (2020) A new genus Allodiatrype, five new species and a new host record of diatrypaceous fungi from palms (Arecaceae). Mycosphere 11:239–268
Korhonen A, Seelan JSS, Miettinen O (2018) Cryptic species diversity in polypores: the Skeletocutis nivea species complex. Mycokeys 36:45–82
Kornerup A, Wanscher JH (1978) Methuen handbook of colour. Eyre Methuen, London, p 248
Kotlába F, Pouzar Z (1958) Polypori novi vel minus cogniti Cechoslovakiae 3. Ceská Mykologie 12:95–104
Kuhar F, Barroetaveña C, Rajchenberg M (2016) New species of Tomentella (Thelephorales) from the Patagonian Andes forests. Mycologia 108:780–790
Kühner R (1936) Nouvelles recherches sur le genre Marasmius. Ann Soc Linn Lyon 79:99–120
Kuhnert E, Fournier J, Peršoh D, Luangsa-ard JJ, Stadler M (2014) New Hypoxylon species from Martinique and new evidence on the molecular phylogeny of Hypoxylon based on ITS rDNA and β-tubulin data. Fungal Divers 64:181–203
Kumar TA, Manimohan P (2009) The genus Lepiota (Agaricales, Basidiomycota) in Kerala State, India. Mycotaxon 107:105–138
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549
Kummer P (1871) Der Führer in die Pilzkunde. Verlag von E. Luppe’s Buchhandlung, Zerbst, pp 1–146
Lackner M, de Hoog GS, Yang L, Ferreira Moreno L, Ahmed SA, Andreas F, Kaltseis J, Nagl M, Lass-Flörl C, Risslegger B, Rambach G, Speth C, Robert V, Buzina W, Chen SC, Bouchara JP, Cano-Lira JF, Guarro J, Gené J, Fernández Silva F, Haido RM, Haase G, Havlíček V, García-Hermoso D, Meis JF, Hagen F, Kirchmair M, Rainer J, Schwabenbauer K, Zoderer M, Meyer W, Gilgado F, Vicente VA, Piecková E, Regenermel M, Rath P, Steinmann J, Alencar XW, Symoens F, Tintelnot K, Ulfig K, Velegraki A, Tortorano AM, Giraud S, Mina S, Rigler-Hohenwarter K, Hernando FL, Ramirez-Garcia A, Pellón A, Kaur JP, Bergter EB, de Meirelles JV, da Silva ID, Delhaes L, Alastruey-Izquerdo A, Li R, Lu Q, Moussa TA, Almaghrabi OA, Al-Zahrani HS, Okada G, Deng S, Liao W, Zeng J, Issakainen J, Liporagi Lopes LC (2014) Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Divers 67:1–10
Laessøe T, Spooner BM (1993) Rosellinia & Astrocystis (Xylariaceae): new species and generic concepts. Kew Bulletin 1–70
Lambert C, Wendt L, Hladki AI, Stadler M, Benjamin E (2019) Hypomontagnella (Hypoxylaceae): a new genus segregated from Hypoxylon by a polyphasic taxonomic approach. Mycol Prog 18:187–201
Larena I, Salazar O, González V, Julián MC, Rubio V (1999) Design of a primer for ribosomal DNA internal transcribed spacer with enhanced specificity for ascomycetes. J Biotech 75:187–194
Larsen MJ (1968) Tomentelloid fungi of North America. State University College of Forestry at Syracuse University, Syracuse, New York, Technical Publication, p 157
Larsen MJ (1974) A contribution to the taxonomy of the genus Tomentella. Mycol Memoir 4:1–145
Larsen MJ (1998) Tomentella (Basidiomycota) and related genera in temperate Eurasia, Synopsis Fungorum 9. Mycologia 90:738–739
Latha KPD, Nanu S, Sharafudheen SA, Manimohan P (2018) Two new species of Gerronema (Agaricales, Basidiomycota) from Kerala State, India. Phytotaxa 364:81–91
Lechat C, Fournier J, Nordén B (2015) Stylonectria norvegica Nectriaceae, a new species from Norway. Ascomycete or 7:220–224
Le Renard L, Firmino AL, Pereira OL, Stockey RA, Berbee ML (2020) Character evolution of modern fly-speck fungi and implications for interpreting thyriothecial fossils. Am J Bot 107:1021–1040
Leonardi M, Marinho N, Furtado A, Comandini O et al (2020) Halimium as an ectomycorrhizal symbiont: new records and an appreciation of known fungal diversity. Mycol Prog 19:1495–1509
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell, Ames
Leudang S, Sikaphan S, Parnmen S, Nantachaiphong N, Polputpisatkul D, Ramchiun S, Teeyapant P (2017) DNA-based identification of gastrointestinal irritant mushrooms in the genus Chlorophyllum: a food poisoning case in Thailand. J Health Res 31:41–49
Léveillé JH (1845) Champignons exotiques. Annales Des Sciences Naturelles Botanique, Série 3(3):38–71
Li WJ, Bhat DJ, Camporesi E, Tian Q, Wijayawardene NN, Dai DQ, Phookamsak R, Chomnunti P, Bahkali AH, Hyde KD (2015) New asexual morph taxa in Phaeosphaeriaceae. Mycosphere 6:681–708
Li GJ, Hyde KD, Zhao RL, Hongsanan S et al (2016) Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 78:1–237
Li JF, Phookamsak R, Jeewon R, Bhat DJ, Mapook A, Camporesi E, Shang QJ, Chukeatirote E, Bahkali AH, Hyde KD (2017a) Molecular taxonomy and morphological characterization reveal new species and new host records of Torula species (Torulaceae, Pleosporales). Mycol Prog 16:447–461
Li M, Zhang S, Gao Z, Wang Y, Zhang W, Gong D, Zhao C, Hu M (2021a) First report of Colletotrichum aeschynomenes causing leaf anthracnose on Ixora coccinea in China. J Plant Pathol 1:3
Li WJ, McKenzie EHC, Liu JK, Bhat DJ, Dai DQ, Camporesi E, Tian Q, Maharachchikumbura SSN, Luo ZL, Shang QJ, Zhang JF, Tangthirasunun N, Karunarathna SC, Xu JC, Hyde KD (2020) Taxonomy and phylogeny of hyaline-spored coelomycetes. Fungal Divers 100:279–801
Li WL, Liu ZP, Zhang T, Dissanayake AJ, Luo ZL, Su HY, Liu JK (2021b) Additions to Distoseptispora (Distoseptisporaceae) associated with submerged decaying wood in China. Phytotaxa 520:75–86
Li WL, Luo ZL, Liu JK, Bhat DJ et al (2017b) Lignicolous freshwater fungi from China I : Aquadictyospora lignicola gen. et sp. nov. and new record of Pseudodictyosporium wauense from northwestern Yunnan Province. Mycosphere 8:1587–1597
Liang JF, Yang ZL (2011) Two new taxa close to Lepiota cristata from China. Mycotaxon 116:387–394
Liang JF, Yang ZL, Xu DP (2011) A new species of Lepiota from China. Mycologia 103:820–830
Liang Y, Ran SF, Bhat J, Hyde KD, Wang Y, Zhao DG (2018) Curvularia microspora sp. nov. associated with leaf diseases of Hippeastrum striatum in China. MycoKeys 29:49–61
Libert MA (1830) Mémoire concernant les plantes cryptogames qui peuent être réunies sous le nom d’ Ascoxytacei. Mem Soc Sci Agr Lille 1831:174–176
Linqiang D, Yiguo C, Heping X, Dongke C, Longhua H, Xiaomei G, Xia Z (2020) Subcutaneous phaeohyphomycosis caused by Hongkongmyces snookiorum in a kidney transplant patient: a case report. BMC Infect Dis 20:1–5
Liu F, Bonthond G, Groenewald JZ, Cai L, Crous PW (2019a) Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Stud Mycol 92:287–415
Liu F, Cai L, Crous PW, Damm U (2014) The Colletotrichum gigasporum species complex. Persoonia 33:83–97
Liu F, Hou L, Raza M, Cai L (2017) Pestalotiopsis and allied genera from Camellia, with description of 11 new species from China. Sci Rep 7: article866
Liu F, Ma ZY, Hou LW, Diao YZ, Wu WP, Damm U, Song S, Cai L (2022) Updating species diversity of Colletotrichum, with a phylogenomic overview. Stud Mycol 101:1–56
Liu F, Wang J, Li H, Wang W, Cai L (2019b) Setophoma spp. on Camellia sinensis. Fungal Sys Evol 4:43–57
Liu GH, Ji XH, Wang YP, Chen JJ (2020) A new species of Fulvifomes (Basidiomycota) from China. Phytotaxa 470:194–202
Liu JK, Chomnunti P, Cai L, Phookamsak R et al (2010) Phylogeny and morphology of Neodeightonia palmicola sp. nov. from palms. Sydowia 62:261–276
Liu JK, Hyde KD, Jones EBG, Ariyawansa HA et al (2015) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, Ariyawansha H, Boonmee S, Chomnunti P, Dai DQ, Bhat JD, Romero AI, Zhuang W-Y, Monkai J, Jones EBG, Chukeatirote E, Ko TWK, Zhao YC, Wang Y, Hyde KD (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Liu L, Li Y, Liu S (2009) Chloropestolide A, an antitumor metabolite with an unprecedented spiroketal skeleton from Pestalotiopsis fici. Org Lett 11:2836–2839
Liu LN, Mou GF, Bau T (2019c) A new Gerronema species with striking colours from China. Phytotaxa 405:074–082
Liu NG, Hyde KD, Bhat DJ, Jumpathong J, Liu JK (2019d) Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China. Mycosphere 10:757–775
Liu S, Han ML, Xu TM, Wang Y, Wu DM, Cui BK (2021a) Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from east Asia. Front Microbiol 12:644979
Liu S, Shen LL, Wang Y, Xu TM, Gates G, Cui BK (2021b) Species diversity and molecular phylogeny of Cyanosporus (Polyporales, Basidiomycota). Front Microbiol 12:631166
Locquin M (1984) General and structural mycology. General and structural mycology. Lombard L, Van der Merwe NA, Groenewald JZ, Crous PW (2015). Generic Concepts in Nectriaceae Stud Mycol 80:189–245
Loizides M, Alvarado P, Polemis E, Dimou DM, Zervakis GI, Thines M, Telle S, Konstantinou G, Gube M (2020) Multiple evolutionary origins of sequestrate species in the agaricoid genus Chlorophyllum. Mycologia 112:400–422
Lombard L, Van der Merwe NA, Groenewald JZ, Crous PW (2015) Generic concepts in Nectriaceae. Stud Mycol 80:189–245
Lu B, Hyde KD, Ho WH, Tsui KM, Taylor JE, Wong KM, Yanna Zhou D (2000). Checklist of Hong Kong Fungi. Fungal Diversity Press, Hong Kong, 207 pages.
Lu YZ, Liu JKJ, Hyde KD, Jeewon R, Kang JC, Fan C, Fan C, Boonmee S, Bhat DJ, Luo ZL, Lin CG, Eungwanichayapant PD (2018) A taxonomic reassessment of Tubeufiales based on multi-locus phylogeny and morphology. Fungal Divers 92:131–344
Lücking R, Hodkinson BP, Leavitt SD (2016) The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota – Approaching one thousand genera. The Bryologist 119:61–416
Luo J, Zhuang WY (2008) Two new species of Cosmospora (Nectriaceae, Hypocreales) from China. Fungal Divers 31:83–93
Luo Z-L, Hyde KD, Bhat DJ, Jeewon R, Maharachchikumbura SS, Bao D-F, Li W-L, Su X-J, Yang X-Y, Su H-Y (2018a) Morphological and molecular taxonomy of novel species Pleurotheciaceae from freshwater habitats in Yunnan, China. Mycol Prog 17:511–530
Luo ZL, Hyde KD, Liu JK, Bhat DJ, Bao DF, Li WL, Su HY (2018b) Lignicolous freshwater fungi from China II: Novel Distoseptispora (Distoseptisporaceae) species from northwestern Yunnan Province and a suggested unified method for studying lignicolous freshwater fungi. Mycosphere 9:444–461
Luo ZL, Hyde KD, Liu JKJ, Maharachchikumbura SS, Jeewon R, Bao DF, Bhat DJ, Lin CG, Li WL, Yang J, Liu NG, Lu YZ, Jayawardena RS, Li JF, Su HY (2019) Freshwater Sordariomycetes Fungal Divers 99:451–660
Luttrell ES (1951) Taxonomy of the Pyrenomycetes. Curators of University of Missouri 24:1–3
Luttrell ES (1955) The ascostromatic ascomycetes. Mycologia 47:511–532
Ma T, Ling XF, Hyde KD (2016) Species of Psilocybe (Hymenogastraceae) from Yunnan, southwest China. Phytotaxa 284:181–193
Madrid H, Da Cunha KC, Gené J, Dijksterhuis J, Cano J, Sutton DA, Guarro J, Crous PW (2014) Novel Curvularia species from clinical specimens. Persoonia 33:48–60
Magaña-Dueñas V, Stchigel AM, Cano-Lira JF (2020) New taxa of the family amniculicolaceae (Pleosporales, dothideomycetes, ascomycota) from freshwater habitats in Spain. Microorganisms 8:1355
Maharachchikumbura SSN, Guo LD, Chukeatirote E, Bahkali AH, Hyde KD (2011) Pestalotiopsis—morphology, phylogeny, biochemistry and diversity. Fungal Divers 50:167–187
Maharachchikumbura SSN, Guo LD, Cai L, Chukeatirote E, Wu WP, Sun X, Crous PW, Bhat DJ, McKenzie EHC, Bahkali AH, Hyde KD (2012) A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers 56:95–129
Maharachchikumbura SSN, Chukeatirote E, Hyde KD (2013) Improving the backbone tree for the genus Pestalotiopsis, addition of P. steyaertii and P. magna sp. nov. Mycol Prog 13:617–624
Maharachchikumbura SSN, Hyde KD, Groenewald JZ, Xu J, Crous PW (2014) Pestalotiopsis revisited. Stud Mycol 79:121–186
Maharachchikumbura SS, Hyde KD, Jones EG, McKenzie EH, Huang SK, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S (2015a) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72:199–301
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang SK, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu JK, Liu ZY, Norphanphoun C, Pang KL, Perera RH, Senanayake IC, Shang QJ, Shenoy BD, Xiao YP, Bahkali AH, Kang JC, Somrothipol S, Suetrong S, Wen TC, Xu JC (2015b) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72:199–301
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat JD, Dayarathne MC, Huang SK, Norphanphoun C, Senanayake IC, Perera RH, Shang QJ, Xiao YP, D’souza MJ, Hongsanan S, Jayawardena RS, Daranagama DA, Konta S, Goonasekara ID, Zhuang WY, Jeewon R, Phillips AJL, Abdel-Wahab MA, Al- Sadi AM, Bahkali AH, Boonmee S, Boonyuen N, Cheewangkoon R, Dissanayake AJ, Kang J, Li QR, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Pang KL, Phookamsak R, Promputtha I, Suetrong S, Stadler M, Wen TC, Wijayawardene NN (2016) Families of Sordariomycetes Fungal Divers 79:1–317
Maharachchikumbura SS, Chen Y, Ariyawansa HA, Hyde KD, Haelewaters D, Perera RH, Samarakoon MC, Wanasinghe DN, Bustamante DE et al (2021) Integrative approaches for species delimitation in Ascomycota. Fungal Divers 109:155–179
Malloch D (1970) New concepts in the Microascaceae illustrated by two new species. Mycologia 62:727–740
Malloch D, Cain RF (1971) New cleistothecial Sordariaceae and a new family, Coniochaetaceae. Can J Bot 49:869–880
Malme GO (1912). Xyridaceae friesianae: Beiträge zur Xyridazee-Flora Afrikas.
Manamgoda DS, Cai L, Bahkali AH, Chukeatirote E, Hyde KD (2011) Cochliobolus: an overview and current status of species. Fungal Divers 51:3–42
Manamgoda DS, Cai L, McKenzie EHC, Chukeatirote E, Hyde KD (2012a) Two new Curvularia species from northern Thailand. Sydowia 64:255–266
Manamgoda DS, Cai L, McKenzie EH, Crous PW, Madrid H, Chukeatirote E, Shivas RG, Tan YP, Hyde KD (2012b) A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia complex. Fungal Divers 56:131–144
Manamgoda DS, Rossman AY, Castlebury LA, Chukeatirote E, Hyde KD (2015) A taxonomic and phylogenetic re-appraisal of the genus Curvularia (Pleosporaceae): human and plant pathogens. Phytotaxa 212:175–198
Manamgoda DS, Rossman AY, Castlebury LA, Crous PW, Madrid H, Chukeatirote E, Hyde KD (2014) The genus Bipolaris. Stud Mycol 79:221–288
Manawasinghe IS, Dissanayake A, Liu M, Wanasinghe D et al. (2019) High genetic diversity and species complexity of Diaporthe associated with Grapevine Dieback in China. Front Microbiol 10: article 1936
Manawasinghe IS, Phillips AJ, Xu J, Balasuriya A, Hyde KD, Stępień Ł, Harischandra DL, Karunarathna A, Yan J, Weerasinghe J, Luo M (2021) Defining a species in fungal plant pathology: beyond the species level. Fungal Diver 109:267–282
Manawasinghe IS, Calabon MS, Jones EBG, Zhang YX, Liao CF, Xiong Y, Chaiwan N, Kularathnage ND, Liu NG et al (2022) Mycosphere notes 345–386. Mycosphere 13:454–557
Mao N, Xu YY, Fan L (2021) Three new species of Tricholomopsis (incertae sedis) from North China based on morphological and molecular data. Phytotaxa 507:155–166
Mapook A, Boonmee S, Ariyawansa HA, Tibpromma S, Campesori E, Jones EB, Bahkali AH, Hyde KD (2016) Taxonomic and phylogenetic placement of Nodulosphaeria. Mycol Prog 15:1–5
Mapook A, Hyde KD, McKenzie EH, Jones EG, Bhat DJ, Jeewon R, Stadler M, Samarakoon MC, Malaithong M, Tanunchai B, Buscot F (2020) Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (Siam weed). Fungal Divers 101:1–175
Marcano V, Morales Méndez A, Castellano F, Salazar FJ, Martínez L (1994) Occurrence of psilocybin and psilocin in Psilocybe pseudobullacea (Petch) Pegler from the Venezuelan Andes. J Ethnopharmacol 43:157–159
Marin-Felix Y, Groenewald JZ, Cai L, Chen Q, Marincowitz S, Barnes I, Bensch K, Braun U, Camporesi E, Damm U, De Beer ZW et al (2017) Genera of phytopathogenic fungi: GOPHY 1. Stud Mycol 86:99–216
Marin-Felix Y, Hernández-Restrepo M, Iturrieta-González I, García D, Gené J, Groenewald JZ, Cai L, Chen Q, Quaedvlieg W, Schumacher RK, Taylor PW et al (2019a) Genera of phytopathogenic fungi: GOPHY 3. Stud Mycol 94:1–24
Marin-Felix Y, Hernández-Restrepo M, Wingfield MJ, Akulov A, Carnegie AJ, Cheewangkoon R, Gramaje D, Groenewald JZ, Guarnaccia V et al (2019b) Genera of phytopathogenic fungi: GOPHY 2. Stud Mycol 92:47–133
Marin-Felix Y, Hernández-Restrepo M, Crous PW (2020) Multi-locus phylogeny of the genus Curvularia and description of ten new species. Mycol Prog 19:559–588
Markovskaja S, Kačergius A (2014) Morphological and molecular characterization of Periconia pseudobyssoides sp. nov. and closely related P. byssoides. Mycol Prog 13:291–302
Mason EW, Ellis MB (1953) Brithish Species of Periconia Mycol Pap 56:1–127
Massee G (1898) Fungi exotici, I. Kew Bull 1898:113–136
Matheny PB, Curtis JM, Hofstetter V, Aime MC, Moncalvo JM, Ge ZW, Yang ZL, Slot JC, Ammirati JF, Baroni TJ, Bougher (2006) Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98:982–995
Matheny PB, Kudzma LV (2019) New species of Inocybe (Agaricales) from eastern North America. J Torrey Bot Soc 146:213–235
Mathur RS (1979) The Coelomycetes of India. Bishen Singh Mahendra Pal Singh, Delhi, India, pp 460
Matos KS, Machado JF, Chagas PC, Siqueira RHS, Silva GF, Xavier Filha MS, Lima-Primo HE, Chagas EA (2020) First report of Colletotrichum aeschynomenes and C. tropicale causing anthracnose on myrciaria dubia in brazil. Plant Dis 104:2517–2517
Matsushima T (1975) Icones Microfungorum: a Matsushima lectorum. Matsushima T (published by the author), Kobe.
Matsushima T (1980) Matsushima Mycological Memoirs No. 1. Saprophytic Microfungi from Taiwan, Part 1. Hyphomycetes. Matsushima Fungus Collect., Kobe, Japan, pp 82.
Matsuura K (2005) Distribution of termite egg-mimicking fungi (“termite balls”) in Reticulitermes spp.(Isoptera: Rhinotermitidae) nests in Japan and the United States. Appl Entomol Zool 40:53–61
Maublanc A (1920) Contribution à l’étude de la flore mycologique brésilienne. Bull Trimest Soc Mycol Fr 36:33–43
McTaggart AR, Prychid CJ, Bruhl JJ, Shivas RG (2020) The PhyloCode applied to Cintractiellales, a new order of smut fungi with unresolved phylogenetic relationships in the Ustilaginomycotina. Fungal Syst Evol 6:55–64
McTaggart AR, Shivas RG, Geering ADW, Callaghan B, Vánky K, Scharaschkin T (2012a) Soral synapomorphies are significant for the systematics of the Ustilago-Sporisorium-Macalpinomyces complex (Ustilaginaceae). Persoonia 29:63–77
McTaggart AR, Shivas RG, Geering ADW, Vánky K, Scharaschkin T (2012b) Taxonomic revision of Ustilago, Sporisorium and Macalpinomyces. Persoonia 29:116–132
Mehrabi M, Hemmati R, Asgari B (2017) Kirschsteiniothelia arasbaranica sp. nov., and an emendation of the Kirschsteiniotheliaceae. Cryptogam Mycol 38:13–25
Melkumov GM (2015) Herbarium of micromycetes of the Department of Botany and Mycology of Voronezh State University. Mycol Phytopathol 49:56–62 (in Russian)
Mel'nik VA (2000) Key to the fungi of the genus Ascochyta Lib. (Coelomycetes). Parey, Berlin, pp. 1–192
Melnik VA, Popushoi IS (1992) [Imperfect fungi on tree and shrubby species: Atlas / Academy of Sciences of the Republic of Moldova. Institute of Plant Physiology, Chisinau] (Translated from Russian). Shtiintsa 368 pages.
Miettinen O, Niemelä T (2018) Two new temperate polypore species of Skeletocutis (Polyporales, Basidiomycota). Ann Bot Fenn 55:195–206
Miiller E, von Arx JA (1950) Einige Aspekte zur Systematik pseudopharialer Ascomyceten. Ber Schweiz Bot Ges 60:329–397
Miller AN, Huhndorf SM, Fournier J (2014) Phylogenetic relationships of five uncommon species of Lasiosphaeria and three new species in the Helminthosphaeriaceae (Sordariomycetes). Mycologia 106:505–524
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, Louisiana, November 2010, 1–8.
Milne I, Wright F, Rowe G, Marshal DF, Husmeier D, McGuire G (2004) TOPALi: software for automatic 496 identification of recombinant sequences within DNA multiple alignments. Bioinformatics 20:1806–1807
Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime MC, Hofstetter V, Verduin SJW, Larsson E, Baroni TJ, Thorn RG, Jacobsson S, Clémençon H, Miller JOK (2002) One hundred and seventeen clades of euagarics. Mol Phylo Evol 23:357–400
Moncalvo OJJS, Margaritescu J-M, Capelari S, M, (2020a) Phylogenetic and morphological analyses of species of Marasmius sect. Marasmius from the Atlantic Rainforest. Brazil Plant Syst Evolu 306:1–46
Moncalvo OJJS, Margaritescu J-M, Capelari S, M, (2020b) A morphological and phylogenetic evaluation of Marasmius sect. Globulares (Globulares-Sicci complex) with nine new taxa from the Neotropical Atlantic Forest. Persoonia 44:240–277
Monkai J, Boonmee S, Ren GC, Wei DP, Phookamsak R, Mortimer PE (2020) Distoseptispora hydei sp. nov. (Distoseptisporaceae), a novel lignicolous fungus on decaying bamboo in Thailand. Phytotaxa 459:93–107
Monkai J, Wanasinghe DN, Jeewon R, Promputtha I, Phookamsak R (2021) Morphological and phylogenetic characterization of fungi within Bambusicolaceae: introducing two new species from the Greater Mekong Subregion. Mycol Prog 20:721–732
Montagne JPFC (1836) Notice sur les plantes cryptogames récemment découvertes en France, contenant aussi l’indication précise des localités de quelques espèces les plus rares de la flore française. Annales Des Sciences Naturelles Botanique 5:337–348
Monteiro JS, Gusmão LFP, Castañeda-Ruiz RF (2016) Pleurothecium bicoloratum & Sporidesmiopsis pluriseptata spp. nov. from Brazil. Mycotaxon 131:145–152
Moreau PA, Vila J, Aime MC, Antonín V, Horak E, Pérez-Butrόn JS, Richard F, Urban A, Welti S, Vizzini A (2015) Cibaomyces and Cyptotrama, two new genera for Europe, and an emendation of Rhizomarasmius (Basidiomycota, Physalacriaceae). Mycol Prog 14:4
Mugambi GK, Huhndorf SM (2009) Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae recircumscribed (Plesporomycetidae, Dothideomycetes, Ascomycota). Stud Mycol 64:103–121
Murrill WA (1914) Northern polypores. Privately printed, New York
Nag Raj TR (1993) Coelomycetous anamorphs with appendage-bearing conidia Mycologue Publications, Waterloo, Ontario, pp 1–1101
Nannfeldt JA (1932) Studien über die Morphologie und Systematik der nichtlichenisierten inoperculaten Discomyceten. Nova Acta Regiae Societatis Scientiarum Upsaliensis 8:1–368
Nascimento AD, Lima MO, Feijó FM, Júnior JH, Sobrinto RR, Assunção IP, Lima GS (2019) First report of Colletotrichum aeschynomenes causing anthracnose in Cacao (Theobroma cacao) in Brazil. Plant Dis 103:3284
Nelsen MP, Lücking R, Grube M, Mbatchou JS et al (2009) Unraveling the phylogenetic relationships of lichenized fungi in Dothideomyceta. Stud Mycol 64:135–144
Nguyen TT, Hee LS, Jeong JS, Burm LH (2019) First records of rare Ascomycete fungi, Acrostalagmus luteoalbus, Bartalinia robillardoides, and Collariella carteri from Freshwater Samples in Korea. Mycobiology 47:1–11
Niemelä T (1998) The Skeletocutis subincarnata complex (Basidiomycetes), a revision. Act Bot Fenn 161:1–35
Nitschke TH (1867) Pyrenomycetes Germanici. Die Kernpilze Deutschlands, Erster Band. Trewendt: Breslau, Germany. pp. 264–265.
Noordeloos ME, Antonín V (2008) Contribution to a monograph of marasmioid and collybioid fungi in Europe. Czech Mycol 60:21–27
Norvell LL, Redhead SA, Ammirati JF (1994) Omphalina sensu lato in North America 1–2. 1: Omphalina wynniae and the genus Chrysomphalina, 2: Omphalina sensu Bigelow. Mycotaxon 50:379–407
Núñez M, Ryvarden L (2001) East Asian polypores 2. Synop Fungorum 14:165–522
Nylander JAA (2004) MrModeltest v2 Program distributed by the author. Uppsala University, Uppsala, Evolutionary Biology Centre
O’Donnell K (1992) Ribosomal DNA internal transcribed spacers are highly divergent in the phytopathogenic ascomycete Fusarium sambucinum (Gibberella pulicaris). Curr Genet 22:213–220
O’Donnell KL (1993) Fusarium and its relatives. In: Taylor JW, Reynolds DR (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, pp 225–233
O’Donnell K, Rooney AP, Proctor RH, Brown DW, McCormick SP, Ward TJ, Frandsen RJ, Lysøe E, Rehner SA, Aoki T, Robert VA (2013) Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet Biol 52:20–31
Oberwinkler F (1976) Eine agaricoide Gattung der Thelephorales. Sydowia 28:359–361
Oberwinkler F (1977) Das neue System der Basidiomyceten. In: Frey W, Hurka H, Oberwinkler F (eds) Beiträge zur Biologie der niederen Pflanzen. Gustav Fischer Verlag, Stuttgart, New York, pp 59–104
Oliveira JJ, Moncalvo JM, Margaritescu S, Capelari M (2020b) A morphological and phylogenetic evaluation of Marasmius sect. Globulares (Globulares-Sicci complex) with nine new taxa from the Neotropical Atlantic Forest. Persoonia 44:240–277
Olou BA, Ordynets A, Langer E (2019) First new species of Fulvifomes (Hymenochaetales, Basidiomycota) from tropical Africa. Mycol Progress 18:1383–2139
Orange A, James PW, White FJ (2001) Microchemical Methods for the Identification of Lichens. British Lichen Society, London
Orejuela CG (1944) New or heretofore unreported species of the higher ascomycetes from Colombia and Venezuela. Mycologia 36:429–459
Ovcharenko NS (2011) Consortative relationships of aromatic and medicinal plants with fungi. Plant Biology and Horticulture: Theory, Innovation 133:143–151 (in Russian)
Pacioni G, Lalli G (1989) Novità micologiche dei monti simbruini. Micologia e Vegetazione Meditterranea 4:29–32
Pan XY, Song ZK, Qu Z, Liu TD, Ma HX (2022) Three new Xylaria species (Xylariaceae, Xylariales) on fallen leaves from Hainan tropical rainforest national park. MycoKeys 86:47–63
Pasouvang P, Karunarathna A, Mapook A, Gafforov Y, Norimova G, Sibounavong P, Jayawardena RS (2021) Nodulosphaeria aconiti, a new host record on Artemisia sp. In Uzbekistan Plant Pathol Quar J 11:191–199
Patouillard N (1887) Les hyménomycètes d’Europe. Paris, pp 1–166
Patouillard N (1900) Essai taxonomique sur les familles et les genres des Hyménomycètes:1–184
Paul NC, Hwang EJ, Nam SS, Lee HU, Lee JS, Yu GD, Kang YG, Lee KB, Go S, Yang JW (2017) Phylogenetic placement and morphological characterization of Sclerotium rolfsii (teleomorph: Athelia rolfsii) associated with blight disease of Ipomoea batatas in Korea. Mycobiology 45:129–138
Peck CH (1882) Fungi in wrong genera. Bull Torrey Bot Club 9:1–4
Pegler DN (1983) Preliminary Agaric Flora of the Lesser Antilles. Kew Bull. Add. Ser. 9:668 pp., 27 pl
Pegler DN (1972) A revision of the genus Lepiota from Ceylon. Kew Bull 27:155–202
Pem D, Jeewon R, Chethana KW, Hongsanan S, Doilom M, Suwannarach N, Hyde KD (2021) Species concepts of Dothideomycetes: classification, phylogenetic inconsistencies and taxonomic standardization. Fungal Divers 109:283–319
Perera RH, Hyde KD, Maharachchikumbura SSN, Jones EBG, McKenzie EHC, Stadler M, Lee HB, Samarakoon MC, Ekanayaka AH, Camporesi E, Liu JK, Liu ZY (2020) Fungi on wild seeds and fruits. Mycosphere 11:2108–2480
Persoon CH (1794) Neuer Versuch einer systematischen Eintheilung der Schwaumme. N Mag Die Bot Ihrem Ganzen Umfange 1:63–128
Petersen RH (2000) Rhizomarasmius, gen. nov. (Xerulaceae, Agaricales). Mycotaxon 75:333–342
Petersen RH, Hughes KW (2010) The Xerula/Oudemansiella complex (Agaricales). Nova Hedwig Beih 137:1–625
Petrak F (1950) Beiträge zur Pilzflora von Ekuador. Sydowia 4:450–587
Phillips A, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald J, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167
Phillips AJL, Hyde KD, Alves A, Liu JK (2019) Families in Botryosphaeriales: a phylogenetic, morphological and evolutionary perspective. Fungal Divers 94:1–22
Phookamsak R, Liu JK, McKenzie EH, Manamgoda DS, Ariyawansa H, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH (2014) Revision of Phaeosphaeriaceae Fungal Divers 68:159–238
Phookamsak R, Wanasinghe DN, Hongsanan S, Phukhamsakda C, Huang SK, Tennakoon DS, Norphanphoun C, Camporesi E, Bulgakov TS, Promputtha I, Mortimer PE (2017) Towards a natural classification of Ophiobolus and ophiobolus-like taxa; introducing three novel genera Ophiobolopsis, Paraophiobolus and Pseudoophiobolus in Phaeosphaeriaceae (Pleosporales). Fungal Divers 87:299–339
Phookamsak R, Hyde KD, Jeewon R, Bhat DJ, Gareth Jones EB, Maharachchikumbura SSN, Raspé O, Karunarathna SC, Wanasinghe DN, Hongsanan S, Doilom M, Tennakoon DS, Machado AR, Firmino AL, Ghosh A, Karunarathna A, Mešić A, Dutta AK, Thongbai B, Devadatha B, Norphanphoun C, Senwanna C, Wei D, Pem D, Ackah FK, Wang GN, Jiang HB, Madrid H, Lee HB, Goonasekara ID, Manawasinghe IS, Kušan I, Cano J, Gené J, Li J, Das K, Acharya K, Raj KNA, Latha KPD, Chethana KWT, He MQ, Dueñas M, Jadan M, Martín MP, Samarakoon MC, Dayarathne MC, Raza M, Park MS, Telleria MT, Chaiwan N, Matočec N, De Silva NI, Pereira OL, Singh PN, Manimohan P, Uniya P, Shang QJ, Bhatt RP, Perera RH, Alvarenga RLM, Nogal-Prata S, Singh SK, Vadthanarat S, Oh SY, Huang SK, Rana S, Konta S, Paloi S, Jayasiri SC, Jeon SJ, Mehmood T, Gibertoni TB, Nguyen TTT, Singh U, Thiyagaraja V, Sarma VV, Dong W, Yu XD, Lu YZ, Lim YW, Chen Y, Tkalčec Z, Zhang ZF, Luo ZL, Daranagama DA, Thambugala KM, Tibpromma S, Camporesi E, Bulgakov TS, Dissanayake AJ, Senanayake IC, Dai DQ, Tang LZ, Khan S, Zhang H, Promputtha I, Cai L, Chomnunti P, Zhao RL, Lumyong S, Boonmee S, Wen TC, Mortimer PE, Xu J (2019) Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers 95:1–273
Phukhamsakda C, McKenzie EHC, Phillips AJL, Gareth Jones EB, Jayarama Bhat D, Stadler M, Bhunjun CS, Wanasinghe DN, Thongbai B, Camporesi E, Ertz D, Jayawardena RS, Perera RH, Ekanayake AH, Tibpromma S, Doilom M, Xu J, Hyde KD (2020) Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers 102:1–203
Piasai O, Manoch L (2009) Coprophilous ascomycetes from Phu Luang wildlife sanctuary and Khao Yai national park in Thailand. Agric Nat Resour 43:34–40
Piepenbring M, Maciá-Vicente JG, Codjia JEI, Glatthorn C, Kirk P, Meswaet Y, Minter D, Olou, BA, Reschke K, Schmidt M, Yorou NS (2020) Mapping mycological ignorance – checklists and diversity patterns of fungi known for West Africa. IMA Fungus 11: article 13
Pinnoi A, Phongpaichit P, Jeewon R, Tang AMC, Hyde KD, Jones EBG (2010) Phylogenetic relationships of Astrocystis eleiodoxae sp. nov.(Xylariaceae). Mycosphere 1:1–9
Pintos Á, Alvarado P (2021) Phylogenetic delimitation of Apiospora and Arthrinium. Fungal Syst Evol 7:197–221
Pirone PP (1978) Diseases and Pests of Ornamental Plants. Wiley Interscience, New York
Popov ES, Shabunin DA, Melnik VA (2008) Contributions to the Studies of Mycobiota in Novgorod and Pskov Regions. Mikol Fitopatol 42:137–151
Pourmoghaddam MJ, Lambert C, Surup F, Khodaparast SA, Krisai-Greilhuber I, Voglmayr H, Stadler M (2020). Discovery of a new species of the Hypoxylon rubiginosum complex from Iran and antagonistic activities of Hypoxylon spp. against the Ash Dieback pathogen, Hymenoscyphus fraxineus, in dual culture. MycoKeys 66:105.
Pratibha J, Nguyen HD, Mel’nik VA, Bhat DJ, White GP, Seifert KA (2015) Lectotypification, epitypification, and molecular phylogeny of the synnematous hyphomycete Pseudogliophragma indicum, the second genus in the Wiesneriomycetaceae. Mycoscience 56:387–395
Proctor RH, Van Hove F, Susca A, Stea G, Busman M, van der Lee T, Waalwijk C, Moretti A, Ward TJ (2013) Birth, death and horizontal transfer of the fumonisin biosynthetic gene cluster during the evolutionary diversification of Fusarium. Mol Microbiol 90:290–306
Promputtha I, Lumyong S, Dhanasekaran V, McKenzie EH, Hyde KD, Jeewon R (2007) A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microb Ecol 53:579–590
Quaedvlieg W, Binder M, Groenewald JZ, Summerell BA, Carnegie AJ, Burgess TI, Crous PW (2014) Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia-Mol Phylogeny Evol Fungi 33:1–40
Quijada L, Johnston PR, Cooper JA, Pfister DH (2018) Overview of Phacidiales, including Aotearoamyces gen. nov. on Nothofagus. IMA Fungus 9:371–382
Rabenhorst GL (1858) Klotzschii herbarium vivum mycologicum sistens fungorum per totam Germaniam crescentium collectionem perfectam. Editio nova. Centuria VIII. 701–800
Raja HA, Oberlies NH, El-Elimat T, Miller AN, Zelski SE, Shearer CA (2013) Lindgomyces angustiascus (Lindgomycetaceae, Pleosporales, Dothideomycetes), a new lignicolous species from freshwater habitats in the USA. Mycoscience 54:353–361
Raja HA, Paguigan ND, Fournier J, Oberlies NH (2017) Additions to Lindgomyces (Lindgomycetaceae, Pleosporales, Dothideomycetes), including two new species occurring on submerged wood from North Carolina, USA, with notes on secondary metabolite profiles. Mycol Prog 16:535–552
Raja HA, Tanaka K, Hirayama K, Miller AN, Shearer CA (2011) Freshwater ascomycetes: two new species of Lindgomyces (Lindgomycetaceae, Pleosporales, Dothideomycetes) from Japan and USA. Mycologia 103:1421–1432
Rajashekhar M, Kaveriappa KM (2000) Effects of temperature and light on growth and sporulation of aquatic hyphomycetes. Hydrobiologia 441:149–153
Rakotoniriana EF, Scauflaire J, Rabemanantsoa C, Urveg-Ratsimamanga S, Corbisier AM, Quetin-Leclercq J, Declerck S, Munaut F (2013) Colletotrichum gigasporum sp. nov., a new species of Colletotrichum producing long straight conidia. Mycol Prog 12:403–412
Rambold G (1989) A monograph of the saxicolous lecideoid lichens of Australia (excl. Tasmania). Bibl Lichenol 34:1–345
Ramírez-Cruz V, Guzmán G, Guzmán-Dávalos L (2013) Type studies of Psilocybe sensu lato (Strophariaceae, Agaricales). Sydowia 65:277–319
Ramirez-Garcia A, Pellon A, Rementeria A et al (2018) Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol 56:S102–S125
Rannala B, Yang Z (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Ratnaweera PB, Williams DE, de Silva ED, Wijesundera RL, Dalisay DS, Andersen RJ (2014) Helvolic acid, an antibacterial nortriterpenoid from a fungal endophyte, Xylaria sp. of orchid Anoectochilus setaceus endemic to Sri Lanka. Mycology 5:23–28
Réblová M, Gams W, Seifert KA (2011) Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Stud Mycol 68:163–191
Réblová M, Seifert K, Fournier J, Štěpánek V (2016) Newly recognised lineages of perithecial ascomycetes: the new orders Conioscyphales and Pleurotheciales. Persoonia 37:57
Réblová M, Seifert KA, Fournier J, Štěpánek V (2012) Phylogenetic classification of Pleurothecium and Pleurotheciella gen. nov. and its dactylaria-like anamorph (Sordariomycetes) based on nuclear ribosomal and protein-coding genes. Mycologia 104:1299–1314
Réblová M, Štěpánek V, Schumacher RK (2014) Xylochrysis lucida gen. et sp. nov., a new lignicolous ascomycete (Sordariomycetidae) with holoblastic conidiogenesis. Mycologia 106:564–572
Réblová M, Nekvindová J, Fournier J, Miller AN (2020) Delimitation, new species and teleomorph-anamorph relationships in Codinaea, Dendrophoma, Paragaeumannomyces and Striatosphaeria (Chaetosphaeriaceae). MycoKeys 74:17
Redhead SA (1986) Mycological observations: 17–20. Nomenclatural notes on some omphalioid genera in Canada: Chrysomphalina, Rickenella, Gerronema Omphalina. Acta Mycol Sin 1:297–304
Redhead SA, Moncalvo JM, Vilgalys R, Matheny PB, Guzmán-Dávalos L, Guzmán G (2007) Proposal to conserve the name Psilocybe (Basidiomycota) with a conserved type. Taxon 56:255–257
Rheeder JP, Marasas WFO, Nelson PE (1996) Fusarium globosum, a new species from corn in southern Africa. Mycologia 88:509–513
Rinaldi AC, Comandini O, Kuyper TW (2008) Ectomycorrhizal fungal genera: separating wheat from the chaff. Fungal Divers 33:1–45
Rivoire B (2020) Polypores de France et d’Europe. Mycopolydev, Orliénas: 876 p.
Rodrigues FA, Silva IT, Cruz MFA, Carré-Missio V (2014) The infection process of Pestalotiopsis longisetula leaf spot on strawberry leaves. J Phytopathol 162:690–692
Ronikier A (2009) Validation of Marasmius epidryas (Agaricomycetes), an emblematic arctic-alpine fungus. Mycol Prog 8:381
Ronikier M, Ronikier A (2011) Rhizomarasmius epidryas (Physalacriaceae): phylogenetic placement of an arctic-alpine fungus with obligate saprobic affinity to Dryas spp. Mycologia 103:1124–1132
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Rosado AWC, Machado AR, Pereira OL (2015) Postharvest stem-end on immature coconut caused by Pestalotiopsis adusta in Brazil. Plant Dis 99:1036
Rossi W, Cesari-Rossi MG (1977) Due nuove specie di Dimeromyces (Laboulbeniales). Rivista di parassitologia.
Rossi W, Cesari Rossi MG E (1977) Sulle Laboulbeniali (Ascomycetes) parassite dei Trechinae del Messico (Coleoptera, Carabidae). Accad. Naz. Lincei, Quaderno 171:373–376, Plate 1.
Rossi W, Christian E (2020) Laboulbeniales (Ascomycota) from Austria and neighbouring areas. Sydowia 72:140–161
Rossi W, Leonardi M (2018) New species and new records of Laboulbeniales (Ascomycota) from Sierra Leone. Phytotaxa 358:91–116
Rossi W, Leonardi M (2020) New Laboulbeniales from China (Ascomycota). Cryptogam Mycol 41:1–7
Rossi W, Proaño Castro AC (2009) New species of Rhachomyces from Ecuador, one of which is dimorphic. Mycologia 101:674–680
Rossman AY (1996) Morphological and molecular perspectives on systematics of the Hypocreales. Mycologia 88:1–19
Rougeron A, Giraud S, Alastruey-Izquierdo A, Cano-Lira J, Rainer J, Mouhajir A, Le Gal S, Nevez G, Meyer W, Bouchara JP (2018) Ecology of Scedosporium species: present knowledge and future research. Mycopathologia 183:185–200
Ruhland W (1900) Untersuchungen zu einer Morphologie der stromabildenden Sphaeriales. Hedwigia 39:1–79
Ruibal C, Gueidan C, Selbmann L, Gorbushina AA et al (2009) Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Stud Mycol 64:123–133
Ryan R (1928) Asterina spp. from India. Memoirs of the Department of Agriculture India 15:103–105
Ryan RW (1924) The Microthyriaceae of Porto Rico. Mycologia 16:177–196
Ryvarden L (1979) Porogramme and related genera. Trans Br Mycol Soc 73:9–19
Ryvarden L (1991) Genera of polypores: nomenclature and taxonomy. Synop Fungorum 5:1–363
Ryvarden L (2004) Neotropical polypores Part 1 – Introduction, Ganodermataceae and Hymenochaetaceae. Synop Fungorum 19:1–229
Ryvarden L (2015) Studies in Neotropical polypores 40. A note on the genus Grammothele. Synopsis Fungorum 33:36–42
Ryvarden L, Johansen I (1980) A preliminary polypore flora of East Africa. Fungifora, Oslo, pp 1–636
Ryvarden L, Melo I (2014) Poroid Fungi of Europe Synop Fungorum 31:1–455
Saccardo PA (1880) Conspectus generum fungorum italiae inferiorum nempe ad Sphaeropsideas, Melanconieas et Hyphomyceteas pertinentium, systemate sporologico dispositu. - Michelia 2:1–38
Saccardo PA (1882) SyllogeFungorum 1. Padova: published by the author
Saccardo PA (1906) Sylloge Fungorum. 18. Sumptibus P. A. Saccardo, Patavii
Sahoo S, Subban K, Chelliah J (2021) Diversity of marine macro-algicolous endophytic fungi and cytotoxic potential of Biscogniauxia petrensis metabolites against cancer cell lines. Front Microbiol 12:650177
Salvador-Montoya CA, Popoff OF, Reck M, Drechsler-Santos ER (2018) Taxonomic delimitation of Fulvifomes robiniae (Hymenochaetales, Basidiomycota) and related species in America: F. squamosus sp. nov. Plant Syst Evol 304:445–459
Samarakoon MC, Gafforov Y, Liu N, Maharachchikumbura SSN, Bhat JD, Liu J-K, Promputtha I, Hyde KD (2018) Combined multi-gene backbone tree for the genus Coniochaeta with two new species from Uzbekistan. Phytotaxa 336:43–58
Samarakoon MC, Hyde KD, Promputtha I, Ariyawansa HA, Hongsanan S (2016a) Divergence andranking of taxa across the kingdoms Animalia, Fungi and Plantae. Mycosphere 7:1678–1689
Samarakoon MC, Hyde KD, Promputtha I, Hongsanan S, Ariyawansa HA, Maharachchikumbura SSN, Daranagama DA, Stadler M, Mapook A (2016b) Evolution of Xylariomycetidae (Ascomycota: Sordariomycetes). Mycosphere 7:1746–1761
Samarakoon BC, Phookamsak R, Wanasinghe DN, Chomnunti P, Hyde KD, McKenzie EH, Promputtha I, Xu JC, Li YJ (2020) Taxonomy and phylogenetic appraisal of Spegazzinia musae sp. nov. and S. deightonii (Didymosphaeriaceae, Pleosporales) on Musaceae from Thailand. MycoKeys 70:19.
Samarakoon BC, Phookamsak R, Karunarathna SC, Jeewon R, Chomnunti P, Xu JC, Li YJ (2021) New host and geographic records of five pleosporalean hyphomycetes associated with Musa spp. (Banana). Stud Fungi 6:92–115.
Samarakoon MC, Hyde KD, Maharachchikumbura SS, Stadler M, Gareth Jones EB, Promputtha I, Suwannarach N, Camporesi E, Bulgakov TS, Liu JK (2022) Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers 112:1–88
Sangpueak R, Phansak P, Buensanteai N (2018) Morphological and molecular identification of Colletotrichum species associated with cassava anthracnose in Thailand. J Phytopathol 166:129–142
Sandoval-Denis M, Guarro J, Cano-Lira JF, Sutton DA, Wiederhold NP, De Hoog GS, Abbott SP, Decock C, Sigler L, Gené J (2016) Phylogeny and taxonomic revision of Microascaceae with emphasis on synnematous fungi. Stud Mycol 83:193–233
Santamaria S, Pedersen J (2021) Laboulbeniomycetes (Fungi, Ascomycota) of Denmark. Eur J Taxon 781:1–425
Santamaria S, Cuesta-Segura AM, Guardia L (2020) New and remarkable species of Laboulbeniales (Ascomycota) from Spain. Nova Hedwigia 110:347–367
Sarbhoy Ak, Agarwal DK (1990) Descriptions of tropical plant pathogenic fungi. Set 1. Malhotra Publ. House, New Delhi
Sarbhoy AK, Lal G, Varshney JL (1971) Fungi of India (1967-71) Navyug Traders, New Delhi, pp 148
Sarkar T, Chakraborty P, Karmakar A, Saha A, Saha D (2018) First report of Ascochyta medicaginicola causing leaf blight disease of pointed gourd in India. Plant Dis 102:2657
Sarkar T, Chakraborty P, Karmakar A, Saha A, Saha D (2019) First report of Periconia macrospinosa causing leaf necrosis of pointed gourd in India. J Plant Pathol 101:1281
Sarycheva LA, Svetasheva TYu, Bulgakov TS, Popov ES, Malysheva VF (2009) Mycobiota of Lipetsk Region. Publishing House of Voronezh State University, Voronezh, pp. 1–287 (in Russian)
Schimann H, Bach C, Lengelle J, Louisanna E, Barantal S, Murat C, Buée M (2017) Diversity and structure of fungal communities in neotropical rainforest soils: the effect of host recurrence. Microb Ecol 73:310–320
Schoch C, Crous PW, Groenewald JZ, Boehm E, Burgess TI, De Gruyter J, De Hoog GS, Dixon L, Grube M, Gueidan C (2009) A class-wide phylogenetic assessment of Dothideomycetes. Stud Mycol 64:1–15
Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98:1041–1052
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W et al (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci 109:6241–6246
Schumm F, Aptroot A (2019) Images of the lichen genus Caloplaca. Vols 1–4. Norderstedt, 2733 pp.
Seephueak P, Preecha C, Seephueak W (2017) Isolation and screening of cellulolytic fungi from spent mushroom substrates. Int J Agric Technol 13:729–739
Seephueak P, Preecha C, Seephueak W (2017b) Isolation and screening of cellulolytic fungi from spent mushroom substrates. Int J Agric Technol 13:729–739
Senanayake IC, Maharachchikumbura SSN, Hyde KD, Bhat JD, Gareth Jones EB, McKenzie EHC, Dai DQ, Daranagama DA, Dayarathne MC, Goonasekara ID, Konta S, Li WJ, Shang QJ, Stadler M, Wijayawardene NN, Xiao YP, Norphanphoun C, Li Q, Liu XZ, Bahkali AH, Kang JC, Wang Y, Wen TC, Wendt L, Xu JC, Camporesi E (2015) Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers 73:73–144
Senanayake IC, Crous PW, Groenewald JZ, Maharachchikumbura SS, Jeewon R, Phillips AJ, Bhat JD, Perera RH, Li QR, Li WJ, Tangthirasunun N (2017) Families of Diaporthales based on morphological and phylogenetic evidence. Stud Mycol 86:217–296
Senanayake IC, Jeewon R, Chomnunti P, Wanasinghe DN, Norphanphoun C, Karunarathna A, Pem D, Perera RH, Camporesi E, McKenzie EH, Hyde KD (2018) Taxonomic circumscription of Diaporthales based on multigene phylogeny and morphology. Fungal Divers 93:241–443
Senanayake IC, Rathnayaka AR, Marasinghe DS, Calabon MS, Gentekaki E, Lee HB, Hurdeal VG, Pem D, Dissanayake LS et al (2020) Morphological approaches in studying fungi: collection, examination, isolation, sporulation and preservation. Mycosphere 11:2678–2754
Sharma JR (1995) Hymenochaetaceae of India. Botanical survey of India 31. Calcutta: Stephen House: 219 pp
Sharma R, Kulkarni G, Sonawane MS (2017) Alanomyces, a new genus of Aplosporellaceae based on four loci phylogeny. Phytotaxa 297:168–175
Shear CL (1922) Life history of an undescribed ascomycete isolated from a granular mycetoma of man. Mycologia 14:239–243
Shearer CA, Raja HA, Miller AN, Nelson P et al (2009) The molecular phylogeny of freshwater Dothideomycetes. Stud Mycol 64:145–153
Shearer CA (1993) A new species of Kirschsteiniothelia (Pleosporales) with an unusual fissitunicate ascus. Mycologia 85:963–969
Shen LL, Wang M, Zhou JL, Xing JH, Cui BK, Dai YC (2019) Taxonomy and phylogeny of Postia. Multi-gene phylogeny and taxonomy of the brown-rot fungi: Postia (Polyporales, Basidiomycota) and related genera. Persoonia 42:101–126
Shi L, Yang H, Hyde KD, Wijayawardene NN, Wang G-N, Yu X-D, Zhang H (2021) Freshwater Sordariomycetes: new species and new records in Pleurotheciaceae, Pleurotheciales. Phytotaxa 518:143–166
Shinohara MM, George E (2009) Scedosporium apiospermum: an emerging opportunistic pathogen that must be distinguished from Aspergillus and other hyalohyphomycetes. J Cutan Pathol 36:39–41
Shoemaker RA (1984) Canadian and some extralimital Nodulosphaeria and Entodesmium species. Canad J Bot 62:2730–2753
Shukla DN, Singh AK, Kumar P, Kamal (1982) Fungi Gorakhpur-XV. Indian Phytopath 35:86–91
Siebenmann. F, Die. Schimmelmykosen, des. menschlichen, et al (1899) The Mycoses of the Human Ear. Wiesbaden, Germany: Bergmann.
Silva MD, Cruz ES, Veloso TGR, Miranda L, Pereira OL, Bocayuva MF, Kasuya MCM (2018) Colletorichum serranegrense sp. nov., a new endophytic species from the roots of the endangered Brazilian epiphytic orchid Cattleya jongheana. Phytotaxa 351:163–170
Simmonds JH (1966) Host index of plant diseases in Queensland. Queensland Department of Primary Industries, Brisbane, 111 pp.
Singer R (1964) Die Gattung Gerronema Nova Hedwigia 7:53–92
Singer R (1970) Omphalinae (Clitocybeae, Tricholomataceae, Basidiomycetes). Flora Neotropica 3:1–84
Singer R (1976a) Flora Neotropica Monograph No. 17: Marasmieae (Basidiomycetes-Tricholomataceae). The New York Botanical Garden Press, Bronx, p 347
Singer R (1976b) Marasmiaceae (Basidiomycetes Tricholomataceae). Flora Neotropica Monograph 17:1–347
Singer R, Smith AH (1946) Proposals concerning the nomenclature of gill fungi including list of proposed lectotype and genera conservada. Mycologia 38:240–299
Singer R (1986) The Agaricales in modern taxonomy (4th revised edition). Koeltz Scientific Books, Koenigstein, p 982
Singer R, Smith AH (1958) Taxonomic monograph of Psilocybe sect. Caerulescentes Mycologia 50:262–303
Sir EB, Kuhnert E, Lambert C, Hladki AI, Romero AI, Stadler M (2016) New species and reports of Hypoxylon from Argentina recognized by a polyphasic approach. Mycol Prog 15:1–42
Sivanesan A (1984) The bitunicate ascomycetes and their anamorphs. J. Cramer.
Sivanesan A (1987) Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. CAB International.
Slippers B, Boissin E, Phillips AJL, Groenewald JZ et al (2013) Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Stud Mycol 76:31–49
Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ (2004) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96:83–101
Smith JE (1836) The English Flora. Volume 5: part2. Longman, Hurst, Rees, Orme, Brown, and Green, London, 450pp.
Solarte F, Muñoz CG, Maharachchikumbura SSN, Álvarez E (2018) Diversity of Neopestalotiopsis and Pestalotiopsis spp., causal agents of guava scab in Colombia. Plant Dis 102:49–59
Song J, Cui BK (2017) Phylogeny, divergence time and historical biogeography of Laetiporus (Basidiomycota, Polyporales). BMC Evol Biol 17:102
Song HY, Sheikha AF, Zhai ZJ, Zhou JP, Chen MH, Huo GH, Huang XG, Hu DM (2020) Distoseptispora longispora sp. nov. from freshwater habitats in China. Mycotaxon 135:513–523
Song ZK, Zhu AH, Liu ZD, Qu Z, Li Y, Ma HX (2022) Three New Species of Hypoxylon (Xylariales, Ascomycota) on a Multigene Phylogeny from Medog in Southwest China. J Fungi 8:500
Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Teisher JK, Clark LG, Barberá P, Gillespie LJ, Zuloaga FO (2017) A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications. J Syst Evol 55:259–290
Species Fungorum (2022b). http://www.speciesfungorum.org/
Spegazzini, C 1880. Fungi argentini. Pugillus primus. In Anales de la Sociedad Científica Argentina (Vol. 9, No. 4, pp. 158–192)
Stalpers JA (1993) The aphyllophoralous fungi 1. Key to the species of the Thelephorales. Stud Mycol 35:1–168
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771
Stamets P (1996) Psilocybin mushrooms of the world. Ten Speed Press, Berkeley
Steyaert RL (1949) Contribution à l’étude monographique de Pestalotia De Not. et Monochaetia Sacc. (Truncatella gen. nov. et Pestalotiopsis gen. nov.). Bull Jard Bot Natl Belg 19:285–354
Storey M (2002) BioImages. The Virtual Field-Guide (UK).
Sturm J (1829) Deutschlands Flora. Abt III Die Pilze Deutschlands 2:1–136
Sturm J. Deutschlands Flora (1829), Abt. III. Die Pilze Deutschlands 2:1–136
Su HY, Hyde KD, Maharachchikumbura SSN, Ariyawansa HA, Luo ZL, Promputtha I, Tian Q, Lin CG, Shang QJ, Zhao YC, Chai HM, Liu XY, Bahkali AH, Bhat JD, Zhou MEHC, DQ, (2016) The families Distoseptisporaceae fam. nov., Kirschsteiniotheliaceae, Sporormiaceae and Torulaceae, with new species from freshwater in Yunnan Province. China Fungal Divers 80:375–409
Su HY, Udayanga D, Luo ZL, Manamgoda DS et al (2015) Hyphomycetes from aquatic habitats in Southern China: Species of Curvularia (Pleosporaceae) and Phragmocephala (Melanommataceae). Phytotaxa 226:201–216
Suada IK, Sudarma I, Kim BS, Cha JY et al (2015) Fungal contaminant threaten Oyster mushroom (Pleurotus ostreatus (Jacq. ex Fr.) Kummer) cultivation in Bali. J Fac Agri, Kyushu Uni 60:309–313
Subramanian CV (1958) Hyphomycetes v J Ind Bot Soc 37:47–64
Suetrong S, Rungjindamai N, Sommai S, Rung-Areerate P, Sommrithipol S, Jones EG (2014) Wiesneriomyces a new lineage of Dothideomycetes (Ascomycota) basal to Tubeufiales. Phytotaxa 176:283–297
Sulistyo BP, Larsson KH, Haelewaters D, Ryberg M (2021) Multigene phylogeny and taxonomic revision of Atheliales sl: Reinstatement of three families and one new family. Lobuliciaceae Fam Nov Fungal Biol 125:239–255
Sun LY, Li HY, Sun X, Guo LD, Guo LD (2017) Dematipyriforma aquilaria gen. et sp. nov., a new hyphomycetous taxon from Aquilaria crassna. Cryptogam, Mycol 38:341–351
Sun YF, Costa-Rezende DH, Xing JH, Zhou JL, Zhang B, Gibertoni TB, Gates G, Glen M, Dai YC, Cui BK (2020a) Multi-gene phylogeny and taxonomy of Amauroderma s.lat. (Ganodermataceae). Persoonia 44:206–239
Sun YR, Goonasekara ID, Thambugala KM, Jayawardena RS, Wang Y, Hyde KD (2020b) Distoseptispora bambusae sp. nov. (Distoseptisporaceae) on bamboo from China and Thailand. Biodivers Data J 8: e53678
Sun YR, Jayawardena RS, Hyde KD, Wang Y (2021) Kirschsteiniothelia thailandica sp. nov. (Kirschsteiniotheliaceae) from Thailand. Phytotaxa 490:172–182
Sung GH, Poinar GO Jr, Spatafora JW (2008) The oldest fossil evidence of animal parasitism by fungi supports a Cretaceous diversification of fungal–arthropod symbioses. Mol Phyl Evol 49:495–502
Suwannarach N, Kumla J, Lumyong S (2021) Spegazzinia camelliae sp. nov. (Didymosphaeriaceae, Pleosprales), a new endophytic fungus from northern Thailand. Phytotaxa 483:117–128
Suwannarach N, Kumla J, Khuna S, Wannathes N, Thongklang N, Sysouphanthong P, Luangharn T, Wongkanoun S, Karunarathna SC et al (2022) History of Thai Mycology and Resolution of Taxonomy for Thai Macrofungi Confused with Europe and American Names. Chiang Mai J Sci 49:654–683
Svrček M (1958) Contribution to the taxonomy of the resupinate thelephoraceous fungi. Ceská Mykologie 12:66–77
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, Massachusetts.
Sydow H (1927) Fungi in itinere Costaricensi collecti. Pars Tertia Ann Mycol 25:1–166
Sydow H (1930) Fungi Venezuelani Ann Mycol 28:29–224
Sydow H, Petrak F (1929) Fungi costaricensis a cl. Prof Alberto m Brenes Collecti Ann Mycol 27:1–86
Sydow H, Sydow P (1916) Fungi amazonici a cl. E Ule Lecti Ann Mycol 14:65–97
Sysouphanthong P, Guo J, Hyde KD, Xu J, Mortimer PE (2016) Lepiota thailandica (Agaricaceae), a new species from Thailand. Phytotaxa 245:262–270
Sysouphanthong P, Hyde KD, Chukeatirote E, Bahkali AH, Vellinga EC (2012) Lepiota (Agaricales) in northern Thailand–2 Lepiota section Lepiota. Cryptogam Mycol 33:25–42
Sysouphanthong P, Thongklang N, Liu JK, Vellinga EC (2021). Description of lepiotaceous fungal species of the genera Chlorophyllum, Clarkeinda, Macrolepiota, Pseudolepiota, and Xanthagaricus, from Laos and Thailand. Diversity 13:666
Talhinhas P, Baroncelli R (2021) Colletotrichum species and complexes: geographic distribution, host range and conservation status. 110:109–198
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729
Tan YP, Crous PW, Shivas RG (2018) Cryptic species of Curvularia in the culture collection of the Queensland Plant Pathology Herbarium. MycoKeys 35:1–25
Tan YP, Madrid H, Crous PW, Shivas RG (2014) Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australas Plant Pathol 43:589–603
Tanaka K, Endo M, Hirayama K, Okane I, Hosoya T, Sato T (2011) Phylogeny of Discosia and Seimatosporium, and introduction of Adisciso and Immersidiscosia genera nova. Persoonia 26:85–98
Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y, Shirouzu T (2015) Revision of the massarineae (Pleosporales, Dothideomycetes). Stud Mycol 82:75–136
Tang AMC, Jeewon R, Hyde KD (2009) A re-evaluation of the evolutionary relationships within the Xylariaceae based on ribosomal and protein-coding gene sequences. Fungal Divers 34:127–155
Tassi, F. (1900) Bartalinia Fl. Tassi. Nuovo genere di Sphaeropsidaceae. Bullettino del Laboratorio ed Orto Botanico della R[eale]. Università di Sien 3:3–5
Taudiere A, Munoz F, Lesne A et al (2015) Beyond ectomycorrhizal bipartite networks: projected networks demonstrate contrasted patterns between early- and late-successional plants in Corsica. Front Plant Sci 6:881
Tchoumi JMT, Coetzee MPA, Rajchenberg M, Roux J (2020) Poroid Hymenochaetaceae associated with trees showing wood-rot symptoms in the Garden Route National Park of South Africa. Mycologia 112:722–741
Tedersoo L, Suvi T, Beaver K, Saar I (2007) Ectomycorrhizas of Coltricia and Coltriciella (Hymenochaetales, Basidiomycota) on Caesalpiniaceae, Dipterocarpaceae and Myrtaceae in Seychelles. Mycol Prog 6:101–107
Tennakoon DS, Kuo CH, Jeewon R, Thambugala KM, Hyde KD (2018) Saprobic Lophiostomataceae (Dothideomycetes): Pseudolophiostoma mangiferae sp. nov. and Neovaginatispora fuckelii, a new record from Mangifera indica. Phytotaxa 364:157–171
Tennakoon DS, Kuo CH, Maharachchikumbura SS, Thambugala KM, Gentekaki E, Phillips AJ, Bhat DJ, Wanasinghe DN, de Silva NI, Promputtha I, Hyde KD (2021) Taxonomic and phylogenetic contributions to Celtis formosana, Ficus ampelas, F. septica, Macaranga tanarius and Morus australis leaf litter inhabiting microfungi. Fungal Divers 108:1–215
Thambugala KM, Hyde KD, Tanaka K, Tian Q, Wanasinghe DN, Ariyawansa HA, Jayasiri SC, Boonmee S, Camporesi E, Hashimoto A, Hirayama K, Schumacher RK, Promputtha I, Liu ZY (2015) Towards a natural classification and backbone tree for Lophiostomataceae, Floricolaceae, and Amorosiaceae fam. nov. Fungal Divers 74:199–266
Thambugala KM, Wanasinghe DN, Phillips AJ, Camporesi E, Bulgakov TS, Phukhamsakda C, Ariyawansa HA, Goonasekara ID, Phookamsak R, Dissanayake A, Tennakoon DS et al (2017) Mycosphere notes 1–50: grass (Poaceae) inhabiting Dothideomycetes. Mycosphere 8:697–796
Thaung MM (2008) A list of hypomycetes (and agonomycetes) in Burma. Australas Mycol 27:149–172
Thaxter R (1896) Contribution towards a monograph of the Laboulbeniaceae. Mem Amer Acad Arts Sci 12:187–429
Theissen F (1912) Fragmenta brasilica V. nebst Besprechung einiger palaeotropischer Microthyriaceen. Ann Mycol 10:159–204
Theissen F (1913) Die Gattung Asterina in systematischer Darstellung. Abhandlungen Der Zoologisch-Botanischen Gesellschaft Wien 7:1–130
Theissen F (1916) Mykologische Abhandlungen. Verh. der Kaiserlich– Koniglichen Zoologisch-Botanischen Gesell. Wien 66:296–400
Theissen F, Sydow H (1918) Vorentwurfe Zu Den Pseudosphaeriales. Ann Mycol 16:1–34
Thind KS, Rattan SS (1971) Thelephoraceae of India IV the Genus Tomentella. Indian Phytopathol 24:32–42
Thomas KA, Manimohan P, Guzmán G, Tapia F, Ramírez-Guillén F (2002) The genus Psilocybe in Kerala State, India. Mycotaxon 83:195–207
Thompson SM, Tan YP, Young AJ, Neate SM et al (2011) Stem cankers on sunflower (Helianthus annuus) in Australia reveal a complex of pathogenic Diaporthe (Phomopsis) species. Persoonia 27:80
Tianyu Z (2009). Flora Fungorum Sincorum Vol. 31: 26 Genera of Dematiaceous Dictyosporous Hyphomycetes Excluding Alternaria. Science Press, Beijing 31: 231.
Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SS, Liu JK, Bhat DJ, Jones EB, McKenzie EH, Camporesi E et al (2017b) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261
Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SS, Liu JK, Bhat DJ, Jones EB, McKenzie EH, Camporesi E, Bulgakov TS, Doilom M et al (2017c) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261
Tibpromma S, Hyde KD, Bhat JD, Mortimer PE, Xu J, Promputtha I, Doilom M, Yang JB, Tang AM, Karunarathna SC (2018) Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. MycoKeys 33:25–67
Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SSN, Liu JK, Bhat DJ, Jones EBG, McKenzie EHC, Camporesi E, Bulgakov TS, Doilom M, de Azevedo SALCM, Das K, Manimohan P, Gibertoni TB, Lim YW, Ekanayaka AH, Thongbai B, Lee HB, Yang JB, Kirk PM, Sysouphanthong P, Singh SK, Boonmee S, Dong W, Raj KNA, Latha KPD, Phookamsak R, Phukhamsakda C, Konta S, Jayasiri SC, Norphanphoun C, Tennakoon DS, Li JF, Dayarathne MC, Perera RH, Xiao Y, Wanasinghe DN, Senanayake IC, Goonasekara ID, de Silva NI, Mapook A, Jayawardena RS, Dissanayake AJ, Manawasinghe IS, Chethana KWT, Luo ZL, Hapuarachchi KK, Baghela A, Soares AM, Vizzini A, Meiras-Ottoni A, Mešić A, Dutta AK, de Souza CAF, Richter C, Lin CG, Chakrabarty D, Daranagama DA, Lima DX, Chakraborty D, Ercole E, Wu F, Simonini G, Vasquez G, da Silva GA, Plautz HL Jr, Ariyawansa HA, Lee H, Kušan I, Song J, Sun J, Karmakar J, Hu K, Semwal KC, Thambugala KM, Voigt K, Acharya K, Rajeshkumar KC, Ryvarden L, Jadan M, Hosen MI, Mikšík M, Samarakoon MC, Wijayawardene NN, Kim NK, Matocˇec N, Singh PN, Tian Q, Bhatt RP, de Oliveira RJV, Tulloss RE, Aamir S, Kaewchai S, Svetasheva STY, Nguyen TTT et al (2017) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261
Tibpromma S, Karunarathna SC, Mortimer PE, Xu JC, Doilom M, Lumyong S (2021) Bartalinia kevinhydei (Ascomycota), a new leaf-spot causing fungus on teak (Tectona grandis) from Northern Thailand. Phytotaxa 474:027–039
Tode HJ (1791) Fungi Mecklenburgenses selecti. Fasc. II, Generum novorum appendicem, Lüneburg.
Tóth EJ, Varga M, Takó M, Homa M, Jáger O, Hermesz E, Orvos H, Nagy G, Vágvölgyi C, Papp T (2020) Response of human neutrophil granulocytes to the hyphae of the emerging fungal pathogen Curvularia lunata. Pathogens 9:235
Trakunyingcharoen T, Lombard L, Groenewald JZ, Cheewangkoon R, To-Anun C, Crous PW (2015) Caulicolous Botryosphaeriales from Thailand. Persoonia 34:87–99
Tsang CC, Chan JF, Trendell-Smith NJ, Ngan AH, Ling IW, Lau SK, Woo PC (2014) Subcutaneous phaeohyphomycosis in a patient with IgG4-related sclerosing disease caused by a novel ascomycete, Hongkongmyces pedis gen. et sp. nov.: first report of human infection associated with the family Lindgomycetaceae. Medical Mycol 52:736–747
Tsui CKM, Hyde KD, Hodgkiss IJ (2000) Biodiversity of fungi on submerged wood in Hong Kong streams. Aquat Microb Ecol 21:289–298
Tulasne L-R, Tulasne C (1847) Mémoire sur les Ustilaginées comparées aux Urédinées. Ann. Sci. Nat. Bot., Sér. 3, 7:12–127 + Pls. 2–7.
Tulasne LR, Tulasne C (1865) Selecta Fungorum Carpologia. Vol. 3. (English translation). Clarendon Press, Oxford
Udayanga D, Castlebury LA, Rossman AY, Hyde KD. (2014) Species limits in Diaporthe: molecular reassessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia:32:83
Udayanga D, Liu X, McKenzie EH, Chukeatirote E et al (2011) The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Divers 50:189
van der Aa HA, Boerema GH, De Gruyter J (2001) Contributions towards a monograph of Phoma (Coelomycetes) VI—1. Section Phyllostictoides: Characteristics and nomenclature of its type species Phoma exigua. Persoonia-Mol Phylo Evol of Fungi 17:435–456
Vánky K (2011) [‘2012’] Smut fungi of the world. APS Press, St. Paul, Minnesota, USA
Vánky K (2013) Sporisorium emariae sp. nov. (Ustilaginomycetes) on Sclerachne punctata (Poaceae). Mycobiota 1:57–61
Vánky K, Vánky C, Denchev CM (2011) Smut fungi in Africa – a checklist. Mycol Balcan 8:1–77
Vargas-Asensio G, Pinto-Tomas A, Rivera B, Hernandez M, Hernandez C, Soto-Montero S, Murillo C, Sherman DH, Tamayo-Castillo G (2014) Uncovering the cultivable microbial diversity of Costa Rican beetles and its ability to break down plant cell wall components. PLoS ONE 9:e113303
Vasilyeva LN, Stephenson SL (2005) Pyrenomycetes of the Great Smoky Mountains National Park. II. Cryptovalsa Ces. et De Not. and Diatrypella (Ces. et De Not.) Nitschke (Diatrypaceae). Fungal Divers 19:189–200
Vellinga EC (2002) New combinations in Chlorophyllum. Mycotaxon 83:415–417
Vellinga EC (2003a) Chlorophyllum and Macrolepiota (Agaricaceae) in Australia. Aust Syst Bot 16:361–370
Vellinga EC (2003b) Type studies in Agaricaceae-Chlorophyllum rachodes and allies. Mycotaxon 85:259–270
Vellinga EC (2004) Ecology and distribution of lepiotaceous fungi (Agaricaceae) – A review. Nova Hedwigia 78:273–299
Vellinga EC, de Kok RPJ (2002) Proposal to conserve the name Chlorophyllum Massee against Endoptychum Czern. (Agaricaceae). Taxon 51:563–564
Vellinga EC (2001) Lepiota (Pers.: Fr.) S.F. Gray. In: Noordeloos ME, Kuyper ThW, Vellinga EC (eds) Flora Agaricina Neerlandica 5. A.A. Balkema Publishers, Lisse/Abingdon/Exton (PA)/Tokyo, pp 109–151
Verkley GJ, Dukik K, Renfurm R, Göker M, Stielow JB (2014) Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota). Persoonia 32:25–51
Vijaykrishna D, Jeewon R, Hyde KD (2006) Molecular taxonomy, origins and evolution of freshwater ascomycetes. Fungal Divers 23:351–390
Vizzini A, Angelini C, Losi C, Ercole E (2016) Thelephora dominicana (Basidiomycota, Thelephorales), a new species from the Dominican Republic, and preliminary notes on thelephoroid genera. Phytotaxa 265:27–38
Vizzini A, Ercole E, Voyron S (2012) Laccariopsis, a new genus for Hydropus mediterraneus (Basidiomycota, Agaricales). Mycotaxon 121:393–403
Vllinga EC (2001) Chlorophyllum. In: Noordeloos ME, Kuyper TW, Vellinga EC (eds) Flora Agaricina Neerlandica, Balkema publishers, Tokyo, vol5, pp 74–75
Voglmayr H, Rossman AY, Castlebury LA, Jaklitsch WM (2012) Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Divers 57:1–44
von Arx JA, Figueras MJ, Guarro J (1988) Sordariaceous ascomycetes without ascospore ejaculation
von Höhnel F (1909) Fragmente zur Mykologie VIII. Sitzungsberichten der kaiserlichen Kaiserl. Akad Wiss Math-Naturwiss Cl Abt1 118:1157–1246
Von Höhnel FXR (1917) Über die Benennung Stellung und Nebenfruchtformen von Sphaerella Fries Berichte der Deutschen Botanischen Gesellschaft 35:627–631
Vrijmoed LLP, Hodgkiss IJ, Thrower LB (1982) Seasonal patterns of primary colonization by lignicolous marine fungi in Hong Kong. Hydrobiologia 89:253–262
Vu D, Groenewald M, De Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T (2018) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol 91:23–36
Wächter D, Melzer A (2020) Proposal for a subdivision of the family Psathyrellaceae based on a taxon-rich phylogenetic analysis with iterative multigene guide tree. Mycol Prog 19:1151–1265
Wagner T, Fischer M (2002) Proceedings towards a natural classification of the worldwide taxa Phellinus s.l. and Inonotus s.l., and phylogenetic relationships of allied genera. Mycologia 94:998–1016
Wanasinghe DN, Hyde KD, Jeewon R, Crous PW, Wijayawardene NN, Jones EB, Bhat DJ, Phillips AJ, Groenewald JZ, Dayarathne MC, Phukhamsakda C (2017) Phylogenetic revision of Camarosporium (Pleosporineae, Dothideomycetes) and allied genera. Stud Mycol 87:207–256
Wanasinghe DN, Phukhamsakda C, Hyde KD, Jeewon R, Lee HB, Gareth Jones EB, Tibpromma S, Tennakoon DS, Dissanayake AJ et al (2018a) Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers 89:1–236
Wanasinghe DN, Phukhamsakda C, Hyde KD, Jeewon R, Lee HB, Gareth Jones EB, Tibpromma S, Tennakoon DS, Dissanayake AJ, Jayasiri SC, Gafforov Y et al (2018b) Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers 89:1–236
Wang CL, Lin CC (2004) Five new records of Ascomycetes in Taiwan. Fung Sci 19:21–29
Wang Q-M, Begerow D, Groenewald M, Liu X-Z, Theelen B, Bai F-Y, Boekhout T (2015) Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina. Stud Mycol 81:55–83
Wang YZ, Aptroot A, Hyde KD (2004) – Revision of the Ascomycete genus Amphisphaeria. Fungal Divers Res Ser 13:1–168
Wang MM, Crous PW, Sandoval-Denis M, Han SL, Liu F, Liang JM, Duan WJ, Cai L (2022) Fusarium and allied genera from China: species diversity and distribution. Persoonia 48:1–53
Wangsawat N, Ju YM, Phosri C, Whalley AJ, Suwannasai N (2021) Twelve New Taxa of Xylaria Associated with Termite Nests and Soil from Northeast Thailand. Biology 10:575
Weber E (2002) The Lecythophora-Coniochaeta complex: I. Morphological studies on Lecythophora species isolated from Picea abies. Nova Hedwigia 1:159–185
Weber E, Görke C, Begerow D (2002) The Lecythophora-Coniochaeta complex: II. Molecular studies based on sequences of the large subunit of ribosomal DNA. Nova Hedwigia 74:187–200
Wehmeyer LE (1926) A biologic and phylogenetic study of the stromatic sphaeriales. Amer J Bot 13:574–645
Wei DP, Wanasinghe DN, Gentekaki E, Thiyagaraja V, Lumyong S, Hyde KD (2021) Morphological and phylogenetic appraisal of novel and extant taxa of Stictidaceae from Northern Thailand. J Fungi 7:880
Weir BS, Johnston PR, Damm U (2012) The Colletotrichum gloeosporioides species complex. Stud Mycol 73:115–180
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Shinsky J, White T (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Whitton SR, McKenzie EH, Hyde KD (2012) Anamorphic fungi associated with Pandanaceae. Fungi associated with Pandanaceae. Springer, Dordrecht, pp 125–353
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL et al (2014a) –Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55
Wijayawardene NN, Hyde KD, Al-Ani LKT, Tedersoo L, Rajeshkumar KC, Zhao RL, Aptroot A, Leontyev DV, Saxena RK, Tokarev YS, Dai DQ, Letcher PM, Stephenson SL, Ertz D, Lumbsch HT, Haelewaters D, Bensch K, Madrid H, Kukwa M, Issi IV, Lateef AA, Phillips AJL, Selbmann L, Pfliegler WP, Horváth E, Pereira OL, Kirk PM, Kolaříková Z, Raja HA, Radek R, Papp V, Dima B, Ma J, Malosso E, Takamatsu S, Rambold G, Gannibal PB, Triebel D, Gautam AK, Avasthi S, Suetrong S, Timdal E, Fryar SC, Delgado G, Réblová M, Doilom M, Dolatabadi S, Pawłowska J, Humber RA, Kodsueb R, Sánchez–Castro I, Goto BT, Silva DKA, de Souza FA, Oehl F, da Silva GA, Silva IR, Błaszkowski J, Jobim K, Maia LC, Barbosa FR, Fiuza PO, Divakar PK, Shenoy BD, Castañeda–Ruiz RF, Somrithipo S, Karunarathna SC, Tibpromma S, Mortimer PE, Wanasinghe DN, Phookamsak R, Xu JC, Wang Y, Fenghua T, Alvarado P, Li DW, Kušan I, Matočec N, Maharachchikumbura SSN, Papizadeh M, Heredia G, Wartchow F, Bakhshi M, Boehm E, Youssef N, Hustad VP, Lawrey JD, Santiago ALCMA Bezerra JDP, Souza-Motta CM, Firmino AL, Tian Q, Houbraken J, Hongsanan S, Tanaka K, Dissanayake AJ, Monteiro JS, Grossart HP, Suija A, Weerakoon G, Etayo J, Tsurykau A, Kuhnert E, Vázquez V, Mungai P, Damm U, Li QR, Zhang H, Boonmee S, Lu YZ, Becerra AG, Kendrick B, Brearley FQ, Motiejűnaitë J, Sharma B, Khare R, Gaikwad S, Wijesundara DSA, Tang LZ, He MQ, Flakus A, Rodriguez-Flakus P, Zhurbenko MP, McKenzie EHC, Stadler M, Bhat DJ, Liu JK (Jack), Raza M, Jeewon R, Nassonova ES, Prieto M, Jayalal RGU, Yurkov A, Schnittler M, Shchepin ON, Novozhilov YK, Liu P, Cavender JC, Kang YQ, Mohammad S, Zhang LF, Xu RF, Li YM, Dayarathne MC, Ekanayaka AH, Wen TC, Deng CY, Navathe S (2020). Outline of Fungi (including fossil fungi) and fungi-like taxa Mycosphere 11:1060–1456
Wijayawardene NN, Hyde KD, Dai DQ, Sánchez-García M, Goto BT, Saxena RK, Erdoğdu M, Selçuk F, Rajeshkumar KC, Aptroot A, Błaszkowski J, Boonyuen N, da Silva GA, de Souza FA, Dong W4, Ertz D, Haelewaters D, Jones EBG, Karunarathna SC, Kirk PM, Kukwa M, Kumla J, Leontyev DV, Lumbsch HT, Maharachchikumbura SSN, Marguno F, Martínez-Rodríguez P, Mešić A, Monteiro JS, Oehl F, Pawłowska J, Pem D, Pfliegler WP, Phillips AJL, Pošta A, He MQ, Li JX, Raza M, Sruthi OP, Suetrong S, Suwannarach N, Tedersoo L, Thiyagaraja V, Tibpromma S, Tkalčec Z, Tokarev YS, Wanasinghe DN, Wijesundara DSA, Wimalaseana SDMK, Madrid H, Zhang GQ, Gao Y, Sánchez-Castro I, Tang LZ, Stadler M, Yurkov A, Thines M, (2022) Outline of Fungi and fungus-like taxa – 2021. Mycosphere 13:53–453
Wijayawardene NN, Hyde KD, Lumbsch T, Liu JK et al (2018) – Outline of Ascomycota – 2017. Fungal Divers 88:167–263
Wijayawardene NN, Hyde KD, Rajeshkumar KC, Hawksworth DL, Madrid H, Kirk PM, Braun U, Singh RV, Crous PW, Kukwa M, Lu¨cking R, Kurtzman CP, Yurkov A, Haelewaters D, Aptroot A, Lumbsch HT, Timdal E, Ertz D, Etayo J, Phillips AJL, Groenewald JZ, Papizadeh M, Selbmann L, Dayarathne MC, Weerakoon G, Jones EBG, Suetrong S, Tian Q, Castaneda-Ruiz RF, Bahkali AH, Pang K-L, Tanaka K, Dai DQ, Sakayaroj J, Hujslova´ M, Lombard L, Shenoy BD, Suija A, Maharachchikumbura SSN, Thambugala KM, Wanasinghe DN, Sharma BO, Gaikwad S, Pandit G, Zucconi L, Onofri S, Egidi E, Raja HA, Kodsueb R, Caceres MES, Pe´rez-Ortega S, Fiuza PO, Monteiro JS, Vasilyeva LN, Shivas RG, Prieto M, Wedin M, Olariaga I, Lateef AA, Agrawal Y, Fazeli SAS, Amoozegar MA, Zhao GZ, Pfliegler WP, Sharma G, Oset M, Abdel-Wahab MA, Takamatsu S, Bensch K, de Silva NI, De Kese A, Karunarathna A, Boonmee S, Pfister DH, Lu Y-Z, Luo Z-L, Boonyuen N, Daranagama DA, Senanayake IC, Jayasiri SC, Samarakoon MC, Zeng X-Y, Doilom M, Quijada L, Rampadarath S, Heredia G, Dissanayake AJ, Jayawardana RS, Perera RH, Tang LZ, Phukhamsakda C, Herna´ndez-Restrepo M, Ma X, Tibpromma S, Gusmao LFP, Weerahewa D, Karunarathna SC, (2017a) Notes for genera-Ascomycota. Fungal Divers 86:1–594
Wijayawardene NN, Hyde KD, Tibpromma S, Wanasinghe DN et al (2017b) Towards incorporating asexual fungi in a natural classification: checklist and notes 2012–2016. Mycosphere 8:1457–1554
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Dai DQ, D’souza MJ, Diederich P, Dissanayake A, Doilom M (2014b) Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55
Wijayawardene NN, Hyde KD, Wanasinghe DN, Papizadeh M, Goonasekara ID, Camporesi E, Bhat DJ, McKenzie EH, Phillips AJ, Diederich P, Tanaka K (2016) Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers 77:1–316
Wijesinghe SN, Wang Y, Camporesi E, Wanasinghe DN, Boonmee S, Hyde KD (2020) A new genus of Bambusicolaceae (Pleosporales) on Corylus avellana (Fagales) from Italy. Biodivers Data J 8:e55957
Wikee S, Lombard L, Nakashima C, Motohashi K, Chukeatirote E, Cheewangkoon R, McKenzie EH, Hyde KD, Crous P (2013) A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Stud Mycol 76:1–29
Wilson AW, Desjardin DE (2005) Phylogenetic relationships in the gymnopoid and marasmioid fungi (Basidiomycetes, euagarics clade). Mycologia 97:667–679
Wong MKM, Jones EB, Abdel-Wahab MA, Au DW, Vrijmoed LL (2003) Ultrastructure of conidiogenesis and appendage ontogeny in the coelomycete Bartalinia robillardoides. Canad J Bot 81:1083–1090
Wu F, Zhou LI, Ji XH, Tian XU, He SH (2016) Grammothele hainanensis sp. nov. (Polyporales, Basidiomycota) and related species from Hainan, southern China. Phytotaxa 255:160–166
Wu F, Dai SJ, Vlasák J, Spirin V, Dai YC (2019) Phylogeny and global diversity of Porodaedalea, a genus of gymnosperm pathogens in the Hymenochaetales. Mycologia 111:40–53
Wu N, Dissanayake AJ, Manawasinghe IS, Rathnayaka AR, Liu JK, Phillips AJL, Promputtha I, Hyde KD (2021) https://botryosphaeriales.org/, an up-to-date classification and account of taxa of Botryosphaeriales. Database (in press)
Wu ZQ, Wang ZH, Luo KY, Shi ZW, Wu F, Zhao CL (2017) Skeletocutis mopanshanensis sp. nov. (Polyporales, Basidiomycota) evidenced by morphological characters and phylogenetic analysis. Nova Hedwigia 107:167–177
Wyka SA, Broders KD (2016) The new family Septorioideaceae, within the Botryosphaeriales and Septorioides strobi as a new species associated with needle defoliation of Pinus strobus in the United States. Fungal Biol 120:1030–1040
Xia JW, Ma YR, Li Z, Zhang XG (2017) Acrodictys-like wood decay fungi from southern China, with two new families Acrodictyaceae and Junewangiaceae. Sci Rep 7:1–21
Xiao ZJ, Li XX, Wang HD, Song PY, Tang L (2018) Rhexoacrodictys broussonetiae sp. nov. from Guizhou, China. Mycotaxon 133(1): 149–152.
Xing JH, Sun YF, Han YL, Cui BK, Dai YC (2018) Morphological and molecular identification of two new Ganoderma species on Casuarina equisetifolia from China. MycoKeys 34:93–108
Yamamoto W (1957) The Formosan species of the. Microthyriaceae - II. Science Reports of the Hyogo University of Agriculture 3:23–31
Yang J, Liu LL, Jones EB, Li WL, Hyde KD, Liu ZY (2021) Morphological variety in Distoseptispora and introduction of six novel species. J Fungi 7:945
Yang J, Maharachchikumbura SSN, Bhat DJ, McKenzie EHC, Bahkali AH, Liu JEBG, ZY, (2015) Aquapteridospora lignicola gen. et sp. nov., a new hyphomycetous taxon (Sordariomycetes) from wood submerged in a freshwater stream. Cryptogam Mycol 36:469–478
Yang J, Maharachchikumbura SSN, Liu JK, Hyde KD, Jones EBG, Al-Sadi AM, Liu ZY (2018) Pseudostanjehughesia aquitropica gen. et sp. nov. and Sporidesmium sensu lato species from freshwater habitats. Mycol Prog 17:591–616
Yang EF, Karunarathna SC, Dai DQ, Stephenson SL, Elgorban AM, Al-Rejaie S, Xiong YR, Promputtha I, Samarakoon MC, Tibpromma S (2022) Taxonomy and Phylogeny of Fungi Associated with Mangifera indica from Yunnan. China J Fungi 8:1249
Yates HS (1917) Some recently collected Philippine fungi. Philipp J Sci Section C Bot 12:361–385
Yilmaz N, Sandoval-Denis M, Lombard L, Visagie CM, Wingfield BD, Crous PW (2021) Redefining species limits in the Fusarium fujikuroi species complex. Persoonia 46:129–162
Yorou SN, Agerer R (2008) Tomentella africana, a new species from Benin (West Africa) identified by morphological and molecular data. Mycologia 100:68–80
Yuan HS (2015) Molecular and morphological evidences reveal two new species in Grammothele and Theleporus Basidiomycota from southern China. Phytotaxa 213:46–56
Yuan HS, Lu X, Dai YC, Hyde KD, Kan YH, Kusan I, He SH, Liu NG, Sarma VV, Zhao CL, Cui BK, Yousaf N, Sun GY, Liu SY, Wu F, Lin CG, Dayarathne MC, Gibertoni TB, Conceicao LB, Garibay-Orijel R, Villegas-Rios M, Salas-Lizana R, Wei TZ, Qiu JZ, Yu ZF, Phookamsak RT, Zeng M, Paloi S, Bao DF, Abeywickrama PD, Wei DP, Yang J, Manawasinghe IS, Harishchandra D, Brahmanage RS, de Silva NI, Tennakoon DS, Karunarathna A, Gafforov Y, Pem D, Zhang SN, Santiago ALCMD, Bezerra JDP, Dima B, Acharya K, Alvarez-Manjarrez J, Bahkali AH, Bhatt VK, Brandrud TE, Bulgakov TS, Camporesi E, Cao T, Chen YX, Chen YY, Devadatha B, Elgorban AM, Fan LF, Du X, Gao L, Goncalves CM, Gusmao LFP, Huanraluek N, Jadan M, Jayawardena RS, Khalid AN, Langer E, Lima DX, de Lima-Junior NC, de Lira CRS, Liu JK, Liu S, Lumyong S, Luo ZL, Matocec N, Niranjan M, Oliveira JRC, Papp V, Perez-Pazos E, Phillips AJL, Qiu PL, Ren YH, Ruiz RFC, Semwal KC, Soop K, de Souza CAF, Souza-Motta CM, Sun LH, Xie ML, Yao YJ, Zhao Q, Zhou LW (2020) Fungal diversity notes 1277–1386: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 104:1–266
Zhang M, Zhang T (2004) Taxonomic studies of Curvularia from China I. A new species and a new Chinese record on Gramineae. Mycosystema 23:328–330
Zhang Y, Fournier J (2015) Kirschsteiniothelia thujina Peck D. Hawksw Kirschsteiniotheliaceae, reported from Europe for the first time. Ascomycete or 7:31–37
Zhang M, Wu H, Pei Z, Zhang T (2007) A new species and a new variety of Curvularia in China. Southwest China J Agri Sci 20:1144–1145
Zhang Y, Fournier J, Phookamsak R, Bahkali AH, Hyde KD (2013) Halotthiaceae fam. nov. (Pleosporales) accommodates the new genus Phaeoseptum and several other aquatic genera. Mycologia 105:603–609
Zhang H, Hyde KD, Zhao YC, McKenzie EHC, Zhou D (2014a) Freshwater ascomycetes: Lophiostoma vaginatispora comb. nov. (Dothideomycetes, Pleosporales, Lophiostomaceae) based on morphological and molecular data. Phytotaxa 176:184–191
Zhang Y, Zhang X, Fournier J, Chen J, Hyde KD (2014b) Lindgomyces griseosporus, a new aquatic ascomycete from Europe including new records. Mycoscience 55:43–48
Zhang H, Dong W, Hyde KD, Maharachchikumbura SS, Hongsanan S, Bhat DJ, Al-Sadi AM, Zhang D (2017) Towards a natural classification of Annulatascaceae-like taxa: introducing Atractosporales ord. nov. and six new families. Fungal Divers 85:75–110
Zhang YZ, Zhang KP, Zhang HS, Sun J, Yin Y, Li HJ et al (2019) Lepiota subvenenata (Agaricaceae, Basidiomycota), a new poisonous species from southwestern China. Phytotaxa 400:265–272
Zhang Q, Yang ZF, Cheng W, Wijayawardene NN, Hyde KD, Chen Z, Wang Y (2020) Diseases of Cymbopogon citratus (Poaceae) in China: Curvularia nanningensis sp. nov. MycoKeys 63:49–67
Zhang ZY, Shao QY, Li X, Chen WH et al (2021) Culturable fungi from urban soils in China I: description of 10 new taxa. Microbiol Spectr 9:e00867-e921
Zhao G, Cao A, Zhang T, Liu X (2011) Acrodictys (Hyphomycetes) and related genera from China. Mycol Prog 10:67–83
Zhao CL, Chen H, Song J, Cui BK (2015) Phylogeny and taxonomy of the genus Abundisporus (Polyporales, Basidiomycota). Mycol Prog 14:38
Zhao X, Leavitt S, Zhao Z, Zhang L, Arup U, Grube M, Pérez-Ortega S, Printzen C, Śliwa L, Kraichak E, Divakar P, Crespo A, Lumbsch HT (2016) Towards a revised generic classification of lecanoroid lichens (Lecanoraceae, Ascomycota) based on molecular, morphological and chemical evidence. Fungal Divers 78:293–304
Zhao RL, Li GJ, Sánchez-Ramírez S, Stata M, Yang ZL, Wu G, Dai YC, He SH, Cui BK, Zhou JL, Wu F, He MQ, Moncalvo JM, Hyde KD (2017) A six-gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Divers 84:43–74
Zheng HF, Huang FC, Liu B, Shao YY, Qin PS (2021) Fulvifomes nonggangensis and F. tubogeneratus (Hymenochaetales, Basidiomycota) Two new species from Southern China based on morphological and molecular evidences. Mycobiology 49:213–222
Zheng H, Yu Z, Jiang X, Fang L, Qiao M (2022) Endophytic Colletotrichum Species from Aquatic Plants in Southwest China. J Fungi 8:87
Zhou LW (2014) Fulvifomes hainanensis sp. nov. and F. indicus comb. nov. (Hymenochaetales, Basidiomycota) evidenced by a combination of morphology and phylogeny. Mycoscience 55:70–77
Zhou LW (2015) Fulvifomes imbricatus and F. thailandicus (Hymenochaetales, Basidiomycota): two new species from Thailand based on morphological and molecular evidence. Mycol Prog14:89
Zhou LW, Qin WM (2012) A new species of Skeletocutis on bamboo (Polyporaceae) in tropical China. Mycotaxon 119:345–350
Zhou JL, Cui BK (2017) Phylogeny and taxonomy of Favolus (Basidiomycota). Mycologia 109:766–779
Zhou S, Qiao L, Jayawardena RS, Hyde KD, Ma X, Wen T, Kang J (2019) Two new endophytic Colletotrichum species from Nothapodytes pittosporoides in China. MycoKeys 49:1–14
Zhu L, Song J, Zhou JL, Si J, Cui BK (2019) Species diversity, phylogeny, divergence time and biogeography of the genus Sanghuangporus (Basidiomycota). Front Microbiol 10:812
Zhu H, Pan M, Wijayawardene NN, Jiang N, Ma R, Dai D, Tian C, Fan X (2021) The hidden diversity of diatrypaceous fungi in China. Front Microbiol 12:646262
Zhuang W (Ed.) (2001) Higher Fungi of Tropical China. Mycotaxon, Ltd., Ithaca, NY, 485 pages
Acknowledgements
We thank Prof. Shaun Pennycook for revising the Latin names. Ruvishika S. Jayawardena thanks the National Research Council of Thailand (NRCT) grant “Biodiversity, taxonomy, phylogeny and evolution of Colletotrichum in northern Thailand” (grant no. NRCT-TRG010), Thailand Science Research and Innovation (TSRI) grant “Biodiversity, taxonomy, phylogeny and evolution of Colletotrichum on Avacado, Citrus, Durian and Mango in northern Thailand” (grant no. 652A01003) and Mae Fah Luang University grant “Identification of fungicolous fungi in northern Thailand” (grant no. 651B01010) grants for funding this research. Kevin D. Hyde thanks the National Research Council of Thailand (NRCT) grant “Total fungal diversity in a given forest area with implications towards species numbers, chemical diversity and biotechnology” (grant no. N42A650547), Thailand Science Research and Innovation (TSRI) grant “Macrofungi diversity research from the Lancang-Mekong Watershed and Surrounding areas” (grant no. DBG6280009) and the Basic Research Fund (Grant No. 652A01001), entitled “Studies of fungi associated with Asteraceae and the discovery of biological properties”. K.D. Hyde also thanks the visiting professorship offered by Chiang Mai University. Nopparat Wannathes, Wiphawanee Phonrob, Nakarin Suwannarach and Jaturong Kumla thank the Plant Genetic Conservation Project under the Royal initiative of Her Royal Highness Princess Maha Chakri Sirindhorn, Pibulsongkram Rajabhat University, Thailand. The field collection in Khao Yai National Park is under permit number 0907.4/13702 and in Si Satchanalai Nationa Park is under permit numbers 0907.4/13696 and 0907.4/15381. André Aptroot, Maria F. Souza, Lidiane A. dos Santos and Adriano A. Spielmann thank the colleagues at the Botanical Laboratory of the UFMS for organizing the excursion, Sandro Pereira for guidance in the field, Wellington Fava for extracting DNA and PCR, and Edna Maria Facincani for information about geology. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 who provided a visiting professorship to the first author. Many thanks to Wellington Fava for generating the sequences. Jadson D.P. Bezerra and colleagues thank the CNPq, CAPES (finance code 001) and FACEPE for financial support and scholarship. The study of Cvetomir M. Denchev and Teodor T. Denchev received support from the SYNTHESYS Project http://www.synthesys.info/, financed by European Community Research Infrastructure Action under the FP7 “Capacities” Program at the Royal Botanic Garden, Madrid (Grant no. ES-TAF-6618). The research of C.C. Chen is supported by the Ministry of Science and Technology of ROC (Taiwan) (Grant No. MOST 107-2621-B178-002-MY3). Chun Y. Deng acknowledges the Science and Technology Support Project of Guizhou Province (Project no. 20192451-2) for research support. Yusufjon Gafforov acknowledges the Ministry of Innovative Development of the Republic of Uzbekistan (nos. AL 2021090820, MUK-2021-46) and the State Scientific and Technical Program of Uzbekistan for research support. Sugantha Gunaseelan and Kezhocuyi Kezo thank DST, SERB-EMR (EMR/2016/003078) for the financial assistance; Kezhocuyi Kezo acknowledges SEED-DST (SP/YO/007/2008) grant. Mark S. Calabon is grateful to the Mushroom Research Foundation and Department of Science and Technology-Science Education Institute (Philippines). E.B. Gareth Jones is supported under the Distinguished Scientist Fellowship Program (DSFP), King Saud University, Kingdom of Saudi Arabia. André L. Firmino, Vanessa P. Abreu and Olinto L. Pereira thank the CNPq, FAPEMIG and CAPES (Finance Code 001) for the financial support the guava producer of the city of Piraúba, for supplying fundamental materials for the accomplishment of this work. W. Rossi and M. Leonardi wish to thank the Italian entomologists who supplied them with the insects bearing the new species of Laboulbeniales: A. Bordoni, P. Magrini and the late A. Vigna Taglianti; they also wish to thank S. Santamaria for the photographs with DIC optics. V.V. Sarma would like to thank the Ministry of Earth Sciences (MOES), Govt. of India (MOES/36/OOIS/Extra/40/2014/PC-IV) for funding this work. He also would like to thank the Tamil Nadu Forest Department and District Forest Office, Tiruvarur, Tamil Nadu for providing permission to collect samples. Department of Biotechnology, Pondicherry University is thanked for providing the facilities. B. Devadatha would like to thank the Ministry of Earth Sciences, Govt. of India for providing a fellowship. V.V. Sarma would like to thank SERB, Department of Science and Technology, Government of India, for funding a project (SERB/SB/SO/PS/18/2014 dt.19.5.2015) the Department of Biotechnology, Pondicherry University for facilities; forest departments of Andaman and Nicobar Islands and Tamil Nadu, India are thanked for providing permission to collect samples. M Niranjan thanks SERB, Govt. of India for a fellowship. Archana Singh is thankful to the University Grants Commission (UGC) (grant no. F.15-1/2016-17/PDFWM-2015-17-UTT-32206 (SA-II), New Delhi, India for financial support. The author also thanks the Head, CAS in Botany, Banaras Hindu University, Varanasi for providing laboratory facilities; Central Instrumental facility (CIF), Indian Institute of Technology (IIT), Banaras Hindu University, Varanasi for SEM analysis and Curator, NFCCI, Agharkar Research Institute, Pune for herbarium and culture deposition and providing accession number. Thatsanee Luangharn thanks Thailand Science Research and Innovation (TSRI) for the Fundamental Fund “Taxonomy, phylogeny, screening of biologically active secondary metabolite and cultivation of Ganoderma species” (Grant No. 652A16009) and Mae Fah Luang University grant “Testing for optimal condition and cultivation Phallus indusiatus in northern, Thailand” (Grant No. 651A16028). Naritsada Thongklang thanks Thailand Science Research and Innovation (TSRI) grant entitled “Value-added products from wild Thai Auricularia to use as a new nutraceutical” (Grant No. 652A01007). Ausana Mapook thanks the Mae Fah Luang University Fund (Grant No. 651A16029), entitled “Taxonomy, phylogeny, risk assessment, and potential impact of fungi on Siam weed in northern Thailand”.The work of Timur S. Bulgakov was done within the framework of the State Task of the Federal State Budgetary Institution “Federal Research Center” “Subtropical Scientific Center of the Russian Academy of Sciences”, the research theme No FGRW-2022-0006. Putarak Chomnunti thanks the National Research Council of Thailand (NRCT) grant “A polyphasic taxonomy and bioactive compound of fungi on Musa spp.” (Grant No. N41A640165) for funding this research and thanks Reinventing University 2021 for supporting the research assistant. Hai-Sheng Yuan thanks the National Natural Science Foundation of China (Grant Nos. 31970017 and U2102220) for financial support. Saisamorn Lumyong also thanks Chiang Mai University, Thailand for financial and facility support. Authors thank Mae Fah Luang University grant entitled “Taxonomy, phylogeny of micro and macro fungi in Mae Fah Luang University premises” (Grant No. 6431600). Huang Zhang would like to thank the National Natural Science Foundation of China (Project ID: NSF 31500017).
Funding
Funding was provided by the National Research Council of Thailand (Grant Nos. NRCT-TRG010, N42A650547, N41A640165), Ministry of Science and Technology of Thailand (Grant Nos. 652A01003, DBG6280009, 652A01001, 652A16009, 662A01014), Mae Fah Luang University (Grant Nos. 651B01010, 651A16029, 651A16028, 6431600), Ministry of Higher Education, Science, Research and Innovation, Thailand, Reinventing University System Project (652A16049), Thailand Research Grant (DBG6180015), European Community Research Infrastructure Action (Grant No. ES-TAF-6618), Ministry of Science and Technology, Taiwan (Grant No. MOST 107-2621-B178- 002-MY3), Science and Technology Program of Guizhou Province (Grant No. 20192451-2), Ministry of Innovative Development of the Republic of Uzbekistan (Grant Nos. AL 2021090820, MUK-2021-46), Ministry of Earth Sciences (Grant No. MOES/36/OOIS/Extra/40/2014/ PC-IV), University Grants Commission (Grant No. F.15-1/2016-17/PDFWM-2015-17-UTT-32206), Ministry of Higher Education, Science, Research and Innovation, Thailand (Grant No. 652A16049), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES - Finance Code 001), SYNTHESYS Project http://www.synthesys.info/, financed by European Community Research Infrastructure Action under the FP7 “Capacities” Program at the Royal Botanic Garden, Madrid (Grant no. ES-TAF-6618), DST, SERB-EMR (EMR/2016/003078), SEED-DST (SP/YO/007/2008), Ministry of Earth Sciences (MOES), Govt. of India (MOES/36/OOIS/Extra/40/2014/PC-IV) Department of Science and Technology, Government of India (SERB/SB/SO/PS/18/2014 dt.19.5.2015), University Grants Commission (UGC) (grant no. F.15-1/2016-17/PDFWM-2015-17-UTT-32206 (SA-II), Scientific Center of the Russian Academy of Sciences", the research theme No FGRW-2022-0006, Key Laboratory of Green Prevention and Control on Fruits and Vegetables in South China, Guangdong (KA21031C502), Zhongkai University of Agriculture and Engineering, Guangzhou, Guangdong, China (KA22016B746), National Natural Science Foundation of China (grant nos. 31970017, 31500017 and U2102220).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Handling Editor: Jian-Kui Liu.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jayawardena, R.S., Hyde, K.D., Wang, S. et al. Fungal diversity notes 1512–1610: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity 117, 1–272 (2022). https://doi.org/10.1007/s13225-022-00513-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-022-00513-0