Abstract
Synthetic agrochemicals, including herbicides, cause diverse environmental and health effects that lead to searching for herbicide formulations with high biocompatibility, biosafety, and biodegradability. Developmental formulations of herbicides from biological sources, mainly microorganisms, are considered suitable alternative due to their high efficacy without affecting non-target organisms. This study prepared developmental formulations of fungal stains with notable herbicidal activity as biogranular and microemulsion preparation, followed by recording herbicidal activity against Amaranthus retroflexus and phytotoxic metabolite production. Screening of herbicidal activity was carried out by recording necrotic lesions, oxidative stress marker expression pattern, reactive oxygen species (ROS) generation rate, and membrane permeability changes. The respective formulation was prepared with viable fungal spores of soil isolates of Alternaria alternata, Paecilomyces sp., Fusarium oxysporum, and Aspergillus niger. Among the formulations prepared, biogranular followed by groundnut oil microemulsion preparation recorded maximum formulation efficacy and substantial herbicidal effect against the tested weed. Among the formulations of the fungal strain, Fusarium oxysporum biogranular and groundnut oil microemulsion revealed a significant herbicidal impact on the tested weed by showing a high rate of necrosis induction with significant ROS generation, membrane permeability changes, and oxidative stress marker expression. Biogranular formulation of F. oxysporum also supported highly stable, phytotoxic metabolites with high antibacterial activity against the plant pathogenic bacterial strain Pseudomonas syringae pv. syringae. Extracted metabolites recorded no toxic effect on the embryonic stages of zebrafish, revealing the high biocompatibility of metabolites. The present finding implies that the biogranular formulation of F. oxysporum can be used as an effective weed control and antimicrobial agent with high biocompatibility and biosafety.
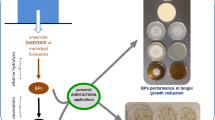
Graphical abstract


























Similar content being viewed by others
Data availability
Data will be made available on request from the authors.
References
Zargar M, Bayat M, Astarkhanova T (2020b) Study of postemergence-directed herbicides for redroot pigweed (Amaranthus retroflexus L) control in winter wheat in southern Russia. J Plant Prot Res 60
Johnson EN, Wang Z, Geddes CM, Coles K, Hamman B, Beres BL (2018) Pyroxasulfone is effective for management of Bromus spp. in winter wheat in Western Canada. Weed Technol:32. https://doi.org/10.1017/wet.2018.70
Mohammed YMM, Badawy MEI (2020) Potential of phytopathogenic fungal isolates as a biocontrol agent against some weeds. Egypt J Biol Pest Control 30. https://doi.org/10.1186/s41938-020-00295-0
Singh AK, Pandey AK (2020) Evaluation of herbicidal potential of selected mycoherbicidal strain against a noxious weed Cassia otusifolia L. Acta Sci Agricult 4(11):03–07
Singh AK, Pandey AK (2020) Evaluation of herbicidal potential of selected mycoherbicidal strain against a noxious weed Cassia otusifolia L. Acta Sci Agricult 4(11):03–07
Ibrahim N, Tawfik M (2019) Fungi as potential biocontrol agents against Convolvulus arvensis and Portulaca oleracea infesting the agroecosystems of Egypt. Egypt J Microbiol. https://doi.org/10.21608/ejm.2019.17443.1117
Geng X, Jin L, Shimada M, Kim MG, Mackey D (2014) The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta. https://doi.org/10.1007/s00425-014-2151-x
Kim W, Chen W (2019) Phytotoxic metabolites produced by legume-associated Ascochyta and its related genera in the Dothideomycetes. Toxins (Basel). https://doi.org/10.3390/toxins11110627
Caldwell CJ, Hynes RK, Boyetchko SM, Korber DR (2012) Colonisation and bioherbicidal activity on green foxtail by Pseudomonas fluorescens BRG100 in a pesta formulation. Can J Microbiol 58. https://doi.org/10.1139/W11-109
Schein D, Santos MSN, Schmaltz S, Nicola LEP, Bianchin CF, Ninaus RG, Menezes BBD, Santos RCD, Zabot GL, Tres MV, Mazutti MA (2022) Microbial prospection for bioherbicide production and evaluation of methodologies for maximizing phytotoxic activity. Processes 10:2001
Li W, Shen S, Chen H (2021) Bio-herbicidal potential of wheat rhizosphere bacteria on Avena fatua L. grass. Bioengineered 12(1):516–526
Singh AK, Pandey AK (2021) Evaluation of herbicidal activity of weed pathogenic fungi secondary metabolites as mycoherbicide for weed Sida acuta Burm f. Int J Res Eng Sci 09(10):23–29
Bo AB, Kim JD, Kim YS, Sin HT, Kim HJ, Khaitov B, Ko YK, Park KW, Choi JS (2019) Isolation, identification and characterisation of Streptomyces metabolites as a potential bioherbicide. PLoS One 14. https://doi.org/10.1371/journal.pone.0222933
Cheng L, Zhu H, Wei Y, Guo L, Weng H, Guo Q (2022) Study on herbicidal potential of two fungi in Qinghai region. OAlib 09. https://doi.org/10.4236/oalib.1108294
Daba A, Berecha G, Tadesse M, Belay A (2021) Evaluation of the herbicidal potential of some fungal species against Bidens pilosa, the coffee farming weeds. Saudi J Biol Sci 28. https://doi.org/10.1016/j.sjbs.2021.07.011
Hoagland RE, Boyette CD (2021) Effects of the fungal bioherbicide, Alternaria cassia on peroxidase, pectinolytic and proteolytic activities in sicklepod seedlings. J Fungi (Basel) 7(12):1032. https://doi.org/10.3390/jof7121032
Qi Y, Yan B, Fu G, Guan X, Du L, Li J (2017) Germination of seeds and seedling growth of Amaranthus retroflexus L. following sublethal exposure of parent plants to herbicides. Sci Rep 7. https://doi.org/10.1038/s41598-017-00153-4
Zargar M, Bayat M, Astarkhanova T (2020a) Study of postemergence-directed herbicides for redroot pigweed (Amaranthus retroflexus L.) control in winter wheat in southern Russia. J Plant Prot Res 60. https://doi.org/10.24425/jppr.2019.131272
Sarabi V, Mohassel MHR (2011) Response of redroot pigweed (Amaranthus retroflexus L.) to tank mixtures of 2,4-D plus MCPA with foramsulfuron. Aust J Crop Sci 5:605
Karthick Raja Namasivayam S, Karthika Pandian U, Vani Chava RS, Arvind Bharani M, Kavisri MM (2023) Chitosan nanocomposite as an effective carrier of potential herbicidal metabolites for noteworthy phytotoxic effect against major aquatic invasive weed water hyacinth (Eichhornia crassipes). Int J Biol Macromol 31(226):1597–1610. https://doi.org/10.1016/j.ijbiomac.2022.11.272
Namasivayam SKR, Vinodhini RK, Kavisri M, Bharani RSA, Moovendhan M (2022b) Formulation of biocontrol agents from Trichoderma viride and evaluation of viability, compatibility with metallic nanoparticles and decomposition efficacy of organic wastes. Biomass Convers Biorefin:1–9
Chakravarthi BVSK, Singh S, Kamalraj S, Gupta VK, Jayabaskaran C (2020) Evaluation of spore inoculum and confirmation of pathway genetic blueprint of T13αH and DBAT from a Taxol-producing endophytic fungus. Sci Rep 10. https://doi.org/10.1038/s41598-020-77605-x
Hatsugai N, Katagiri F (2018) Quantification of plant cell death by electrolyte leakage assay. Bio Protoc 8. https://doi.org/10.21769/bioprotoc.2758
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. https://doi.org/10.1016/j.jpha.2015.11.005
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3. https://doi.org/10.1038/nprot.2007.521
Krishnamurthi VR, Niyonshuti II, Chen J, Wang Y (2021) A new analysis method for evaluating bacterial growth with microplate readers. PLoS One 16. https://doi.org/10.1371/journal.pone.0245205
Al-Enazi NM, Awaad AS, Al-Othman MR, Al-Anazi NK, Alqasoumi SI (2018) Isolation, identification and anti-candidal activity of filamentous fungi from Saudi Arabia soil. Saudi Pharm J 26. https://doi.org/10.1016/j.jsps.2017.12.003
Khan MA, Khan SA, Waheed U, Raheel M, Khan Z, Alrefaei AF, Alkhamis HH (2021) Morphological and genetic characterisation of Fusarium oxysporum and its management using weed extracts in cotton. J King Saud Univ Sci 33. https://doi.org/10.1016/j.jksus.2020.101299
Khattak SU, Iqbal Z, Lutfullah G, Bacha N, Khan AA, Saeed M, Ali M (2014) Phytotoxic and herbicidal activities of Aspergillus and Penicillium species isolated from rhizosphere and soil. Pakistan J Weed Sci Res 20
Marra M, Camoni L, Visconti S, Fiorillo A, Evidente A (2021) The surprising story of fusicoccin: a wilt-inducing phytotoxin, a tool in plant physiology and a 14-3-3-targeted drug. Biomolecules. https://doi.org/10.3390/biom11091393
Namasivayam SK, Raja AA, Choudhury SH (2014) Selective isolation and screening of fungi with herbicidal potential and evaluation of herbicidal activity against Vernonia species. Afr J Biotechnol 13
Zhu H, Lin Z, Ma Y (2023) Evaluation of the biocontrol potential of the fungus Botrytis euroamericana strain HZ-011 for herbicidal activity. Egypt J Biol Pest Control 33:51. https://doi.org/10.1186/s41938-023-00695-y
Ortiz SC, Huang M, Hull CM (2019) Spore germination as a target for antifungal therapeutics. Antimicrob Agents Chemother 63. https://doi.org/10.1128/AAC.00994-19
Piyasena KGNP, Wickramarachchi WART, Kumar NS, Jayasinghe L, Fujimoto Y (2015) Two phytotoxic azaphilone derivatives from Chaetomium globosum, a fungal endophyte isolated from Amaranthus viridis leaves. Mycology 6. https://doi.org/10.1080/21501203.2015.1089332
Saad MMG, Abdelgaleil SAM, Shiono Y (2021) Antibacterial and herbicidal properties of secondary metabolites from fungi. Nat Prod Res 35. https://doi.org/10.1080/14786419.2020.1779718
Sephton-Clark PCS, Voelz K (2018) Spore germination of pathogenic filamentous fungi. In: Advances in Applied Microbiology. https://doi.org/10.1016/bs.aambs.2017.10.002
Shamly V, Kali A, Srirangaraj S, Umadevi S (2014) Comparison of microscopic morphology of fungi using lactophenol cotton blue (LPCB), iodine glycerol and congo red formaldehyde staining. J Clin Diagn Res 8:DL01
Himanen M, Prochazka P, Hänninen K, Oikari A (2012) Phytotoxicity of low-weight carboxylic acids. Chemosphere 88. https://doi.org/10.1016/j.chemosphere.2012.02.058
Huang H, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00800
Acknowledgements
We acknowledge SAIF IIT Madras for characterization studies.
Author information
Authors and Affiliations
Contributions
SKRN and MM: Conceptualization, supervision, data curation, review and editing, funding acquisition, and writing—original draft. BD and RSAB: Methodology, investigation, resources, and formal analysis. KS and MK: Conceptualization, supervision, and review and editing
Corresponding author
Ethics declarations
Ethics approval
In this study, animal experiment was not applicable.
Consent to participate
In this study, animals and human trails are not applicable.
Consent for publication
Not applicable
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
S. Karthick Raja Namasivayam, Bikramjit Deka, R. S. Arvind Bharani et al. Impact of formulation on the fungal biomass–based herbicidal activity and phytotoxic metabolite production. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04717-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04717-5