Abstract
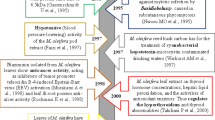
Livestock grazing in pastures that include large populations of Lolium perenne and Lolium arundinaceum were found to be suffering a ‘curious’ neurological problem in the late 1950s, which was subsequently named as the ‘ryegrass staggers’, because the sheep suffered that problem when they fed on the perennial ryegrass, L. perenne. The chemicals that trigger staggers in the livestock have been resolved: tremorgenic alkaloids, especially lolitrems of the indole-diterpene group, particularly lolitrem B, that are synthesized during the biotrophic association of Epichloë festucae var. lolii in the shoots of L. perenne and L. arundinaceum. With an agenda posted to us in Charles Sturt University, Orange Campus, New South Wales, we investigated the quality and quantity of secondary metabolites and assayed the antioxidant capacity of the phenolics using populations of L. perenne and L. arundinaceum that included determined strains of E. festucae var. lolii and E. coenophiala obtained from different localities of temperate Australia. N-acetylnorloline was detected only in E. coenophiala (strain AR542) infected L. arundinaceum, regardless of being of either the Mediterranean or the Continental cultivars. Tridecanoic, n-capric, eicosatrienoic, and linoleic acids were detected in all the tested L. arundinaceum cultivars, irrespective of either their cultivar or their endophyte status. Phenolic extracts from L. perenne infected with Epichloë ‘wild type’ scavenged DPPH (2, 2-diphenyl-1-picrylhydrazyl) at a much faster rate than that infected with AR1, AR37, and that free of Epichloë. The extracts from the Epichloë-free Mediterranean L. arundinaceum showed the maximum antioxidant capacity, followed by the Epichloë-free Continental L. arundinaceum. Lolium perenne infected with Epichloë ‘wild-type’ showed the greatest scavenging capacity, whereas the endophyte-free L. perenne showed the least. Those L. perenne populations infected with strains AR1 and AR37 showed intermediate capacities. Alkaloids synthesized in Epichloë-infected Poaceae induce toxicity in horses, cattle, and sheep. Today we know of most of the secondary metabolites produced in Epichloë-infected Poaceae. Ergot alkaloids (e.g., ergoamides) induce psychotropic effects in consumer animals, whereas vasomotor effects are less pronounced. Ergopeptides and their derivatives usually occur in Epichloë-infected Lolium-s, especially in L. arundinaceum. Ergopeptines cause vasomotor effects resulting in ‘fescue foot’, which is, invariably, aggravated by elevated atmospheric temperature. Ergovaline, another abundant Epichloë-derived alkaloid in poaceous seeds, presently known to bear the greatest vasomotor effect. Studies made, thus far, clarify that interactions with drug-metabolizing enzymes happen and these interactions have a role in the elevating the toxicity levels of the ergot-family of alkaloids.



Source: https://en.wikipedia.org/wiki/Ergovaline#/media/File:Ergovaline.svg, public domain

Source: Qawasmeh et al. 2011

Source: Qawasmeh et al. 2011

Source: Qawasmeh et al. 2012a

Source: Qawasmeh et al. 2012a

Source: Qawasmeh et al. 2012b
Similar content being viewed by others
References
Bacon, C. W., Porter, J. K., Robbins, J. D., & Luttrell, E. S. (1977). Epichloë typhina from toxic tall fescue grasses. Applied Environmental Microbiology,34, 576–581.
Bourke, C. A., Hunt, E., & Watson, R. (2009). Fescue-associated oedema of horses grazing on endophyte-inoculated tall fescue grass (Festuca arundinacea) pastures. Australian Veterinary Journal,87, 492–498.
Card, S., Johnson, L., Teasdale, S., & Caradus, J. (2016). Deciphering endophyte behaviour: the link between endophyte biology and efficacious biological control agents. FEMS Microbiology Ecology,92, 1–19.
Chase, C., Lutz, K., McKenzie, E., & Tibary, A. (2017). Blackwell’s five-minute veterinary consult: Ruminants (2nd ed., p. 1008). New York: Wiley.
Clement, S. L., Hu, J., Stewart, A. V., Wang, B., & Elberson, L. R. (2011). Detrimental and neutral effects of a wild grass-fungal endophyte symbiotum on insect preference and performance. Journal of Insect Science,11, 77. https://doi.org/10.1673/031.011.7701.
Clement, S. L., Kaiser, W. J., & Eichenseer, H. (1994). Acremonium endophytes in germplasms of major grasses and their utilization for insect resistance. In C. W. Bacon & J. F. White Jr. (Eds.), Biotechnology of endophytic fungi of grasses (pp. 185–200). Boca Raton, FL: CRC Press.
Cope-Selby, N., Cookson, A., Squance, M., Donnison, I., Flavell, R., & Farrar, K. (2017). Endophytic bacteria in Miscanthus seed: implications for germination, vertical inheritance of endophytes, plant evolution and breeding. GCB Bioenergy,9, 57–77.
Cunningham, I. J., & Hartley, W. J. (1959). Ryegrass staggers. New Zealand Veterinary Journal,7, 1–7.
Daglia, M. (2012). Polyphenols as antimicrobial agents. Current Opinion in Biotechnology,23, 174–181.
de Bary, H. A. (1863). Über die Entwicklung der Sphaeria typhina Pers. und Bail’s Mycologische Studien. Flora,46, 401–402.
de Bary, H. A. (1879). Die Erscheinung der Symbiose (p. 30). Strasbourg: Verlag von Karl J. Trübner.
Diehl, W. W. (1950). Balansia and the Balansiae in America, Washington (p. 81). DC.: United States Department of Agriculture.
Dierking, R. M., Young, C. A., & Kallenbach, R. L. (2012). Mediterranean and Continental Tall Fescue: I. Effects of endophyte status on leaf extension, proline, mono- and disaccharides, fructan, and freezing survivability. Crop Science,52, 451–459.
Elliott, M. (2009). Grass tetany in cattle — treatment and prevention. Primefacts NSW DPI,421, 1–4.
Faeth, S. H., & Fagan, W. F. (2002). Fungal endophytes: common host plant symbionts but uncommon mutualists. Integrative and Comparative Biology,42, 360–368.
Faeth, S. H., & Sullivan, T. J. (2003). Mutualistic asexual endophytes in a native grass are parasitic. The American Naturalist,161, 310–325.
Ferreira, A., Quecine, M. C., Lacava, P. T., Oda, S., Azevedo, J. L., & Araújo, W. L. (2008). Diversity of endophytic bacteria from Eucalyptus species seeds and colonization of seedlings by Pantoea agglomerans. FEMS Microbiology Letters,287, 8–14.
Fletcher, L. R., & Harvey, I. C. (1981). An association of a Lolium endophyte with ryegrass staggers. New Zealand Veterinary Journal,29, 185–186.
Gagic, M., Faville, M. J., Zhang, W., Forester, N. T., Rolston, M. P., Johnson, R. D., et al. (2018). Seed transmission of Epichloë endophytes in Lolium perenne is heavily influenced by host genetics. Frontiers in Plant Sciences,9, 1580. https://doi.org/10.3389/fpls.2018.01580.
Gallagher, R. T., Hawkes, A. D., Steyn, P. S., & Vlegaar, R. (1984). Tremorgenic neurotoxins from perennial ryegrass causing ryegrass staggers disorder of livestock: structure elucidation of lolitrem B. Journal of the Chemical Society (Chemical Communications),9, 614–616.
Gallagher, R. T., White, E. P., & Mortimer, P. H. (1981). Ryegrass staggers: isolation of potent neurotoxins Lolitrem A and Lolitrem B from staggers-producing pastures. New Zealand Veterinary Journal,29, 189–190.
Guerre, P. (2015). Ergot alkaloids produced by endophytic fungi of the genus Epichloë. Toxins,7, 773–790.
Hill, N. S. (2018). Ecological relationships of Balansiae-infected graminoids. In C. W. Bacon & J. F. White (Eds.), Biotechnology of endophytic fungi of grasses (pp. 59–71). Boca Raton, Florida: CRC Press.
Hodgson, S., de Cates, C., Hodgson, J., Morley, N. J., Sutton, B. C., & Gange, A. C. (2014). Vertical transmission of fungal endophytes is widespread in forbs. Ecology & Evolution,4, 1199–1208.
Hohenboken, W. D., & Blodgett, D. J. (1997). Growth and physiological responses to toxicosis in lines of mice selected for resistance or susceptibility to endophyte-infected tall fescue in the diet. Journal of Animal Science,75, 2165–2173.
Hoveland, C. S. (1993). Importance and economic significance of the Acremonium endophytes to performance of animals and grass plants. Agriculture, Ecosystems & Environment,44, 3–12.
Hoveland, C. S., Haaland, R. L., King, C. C., Anthony, W. B., Clark, E. M., McGuire, J. A., et al. (1980). Association of Epichloë typhina fungus and steer performance on tall fescue pasture. Agronomy Journal,72, 1064–1065.
Ju, Y., Sacalis, J. N., & Still, C. C. (1998). Bioactive flavonoids from endophyte-infected blue grass (Poa ampla). Journal of Agricultural and Food Chemistry,46, 3785–3788.
Keogh, M., Henry, M., & Clifton, L. (2015). The Economic Importance of Australia’s Livestock Industries and the Role of Animal Medicines and Productivity-enhancing Technologies (p. 91). Surry Hills: Australian Farm Institute.
Koh, S., Vicari, M., Ball, J. P., Rakocevic, T., Zaheer, S., & Hik, D. S. (2006). Rapid detection of fungal endophytes in grasses for large-scale studies. Functional Ecology,20, 736–742.
Leuchtmann, A., Bacon, C. W., Schardl, C. L., White, J. F., Jr., & Tadych, M. (2014). Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia,106, 202–215.
Malinowski, D. P., & Belesky, D. P. (1999). Tall fescue aluminium tolerance is affected by Neotyphodium coenophialum endophyte. Journal of Plant Nutrition,22, 1335–1349.
Markey, B. K., Leonard, F., Archambault, M., Cullinane, A., & Maguire, D. (2013). Clinical veterinary microbiology (2nd ed., p. 920). Edinburgh: Mosby Elsevier.
Martens, H., & Schewigel, M. (2000). Pathophysiology of grass tetany and other hypomagnesimias. Veterinary Clinics of North America Food Animal Practice,16, 339–368.
McCosker, T. (2000). Cell grazing — the first 10 years in Australia. Tropical Grasslands,34, 207–218.
Mortimer, P. H., & di Menna, M. E. (1983). Ryegrass staggers: further substantiation of a Lolium endophyte aetiology and the discovery of weevil resistance of ryegrass pastures infected with lolium endophyte. Proceedings of the New Zealand Grassland Association,44, 240–243.
Murray, T. D., Schroeder, B. K., Schneider, W. L., Luster, D. G., Sechler, A., Rogers, E. E., et al. (2017). Rathayibacter toxicus, other Rathayibacter species inducing bacterial head blight of grasses, and the potential for livestock poisonings. Phytopathology,107, 804–815.
Musgrave, A. S. (1964). Insect mycetomes. The Canadian Entomologist,96, 377–389.
Nash, T., III, Geiser, L., McCune, B., Triebel, D., Tomescu, A. M., & Sanders, W. (2010). Biology of lichens — symbiosis, ecology, environmental monitoring, systematics and cyber applications (p. 256). Biblioteca Lichenologica, VI, Stuttgart: J. Cramer.
Neill, J. C. (1941). The endophytes of Lolium and Festuca. New Zealand Journal of Science & Technology, Technical Report,23A, 185.
Nielsen, C. F., & Smedsgaard, J. (2003). Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography–UV–mass spectrometry methodology. Journal of Chromatography A,1002, 111–136.
Osweiler, G. D. (2019). Fescue poisoning. MSD veterinary manual. Retrieved September 18, 2019, from https://www.msdvetmanual.com/toxicology/mycotoxicoses/fescue-poisoning.
Oulhen, N., Schulz, B. J., & Carrier, T. J. (2016). English translation of Heinrich Anton de Bary’s 1878 speech, ‘Die Erscheinung der Symbiose’ (‘de la symbiose’). Symbiosis. https://doi.org/10.1007/s13199-016-0409-8.
Philipson, M. N., & Christey, M. C. (1986). The relationship of host and endophyte during flowering, seed formation, and germination of Lolium perenne. New Zealand Journal of Botany,24, 125–134.
Ponce, M. A., Bompadre, M. J., Scervino, J. M., Ocampo, J. A., Chaneton, E. J., & Godeas, A. M. (2009). Flavonoids, benzoic acids and cinnamic acids isolated from shoots and roots of Italian rye grass (Lolium multiflorum Lam.) with and without endophyte association and arbuscular mycorrhizal fungus. Biochemical Systematics and Ecology,37, 245–253.
Prestidge, R. A., Pottinger, R. P., & Barker, G. M. (1982). An association of Lolium endophyte with ryegrass resistance to Argentine stem weevil. Proceedings of the New Zealand Pest Control Conference,35, 119–122.
Qawasmeh, A., Bourke, C., Lee, S., Gray, M., Wheatley, W., Sucher, N. J., et al. (2011). GC—MS analysis of volatile secondary metabolites in ‘Mediterranean’ and ‘Continental’ Festuca arundinacea (Poaceae) infected with the fungal endophyte Neotyphodium coenophialum Strain AR542. Acta Chromatograhica,23, 621–628.
Qawasmeh, A., Obied, H. K., Raman, A., & Wheatley, W. (2012a). Influence of fungal endophyte infection on phenolic content and antioxidant activity in grasses: interaction between Lolium perenne and different strains of Neotyphodium lolii. Journal of Agricultural and Food Chemistry,60, 3381–3388.
Qawasmeh, A., Raman, A., & Wheatley, W. (2015). Volatiles in perennial ryegrass infected with strains of endophytic fungus: impact on African black beetle host selection. Journal of Applied Entomology,139, 94–104.
Qawasmeh, A., Raman, A., Wheatley, W., & Nicol, H. (2012b). Antioxidative capacity of phenolic compounds extracted from Lolium perenne and Lolium arundinaceum infected with Neotyphodium (Hypocreales: Clavicipitaceae). Acta Physiologia Plantarum,34, 827–833.
Raman, A., & Suryanarayanan, T. S. (2017). Fungus—plant interaction influences plant-feeding insects. Fungal Ecology,29, 123–132.
Raman, A., Wheatley, W., & Popay, A. (2012). Endophytic fungus—vascular plant—insect interactions. Environmental Entomology,41, 433–447.
Rasmussen, S., Parsons, A. J., Fraser, K., Xue, H., & Newman, J. A. (2008). Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiology,146, 1440–1453.
Rasmussen, S., Parsons, A. J., & Newman, J. A. (2009). Metabolomics analysis of the Lolium perenne − Neotyphodium lolii symbiosis: more than just alkaloids? Phytochemistry Reviews,8, 535–550.
Reed, K. F. M., Nie, Z. N., Walker, L. V., Mace, W. J., & Clark, S. G. (2011). Weather and pasture characteristics associated with outbreaks of perennial ryegrass toxicosis in southern Australia. Animal Production Science,51, 738–752.
Reed, K. & Ware, J.W. (2007). Perennial ryegrass toxicoses, Meat & Livestock Australia, Sydney. Retrieved August 12, 2019, from https://publications.mla.com.au/login/redirectFrame.
Riley, I. T., Schmitz, S., & de Silva, P. (2001). Anguina australis, a vector for Rathayibacter toxicus in Ehrharta longiflora. Australasian Plant Pathology,30, 171–175.
Rodriguez, R. J., White, J. F., Jr., Arnold, A. E., & Redman, R. S. (2009). Fungal endophytes: diversity and functional roles. New Phytologist,182, 314–330.
Rowan, D. D., & Latch, G. C. M. (1994). Utilization of endophyte-infected perennial ryegrasses for increased insect resistance. In C. W. Bacon & J. F. White (Eds.), Biotechnology of endophytic fungi of grasses (pp. 169–183). Boca Raton: CRC Press.
Rudgers, J. A., Afkhami, M. E., Rúa, M. A., Davitt, A. J., Hammer, S., & Huguet, V. M. (2009). A fungus among us: broad patterns of endophyte distribution in the grasses. Ecology,90, 1531–1539.
Sacchi, L., Corona, S., Grigolo, A., Laudani, U., Selmi, M. G., & Bigliardi, E. (1996). The fate of the endocytobionts of Blattella germanica (Blattaria: Blattellidae) and Periplaneta americana (Blattaria: Blattidae) during embryo development. Italian Journal of Zoology,63, 1–11.
Sackett, D., Holmes, P., Abbott, K., Jephcott, S., & Barber, M. (2006). Assessing the economic cost of endemic disease on the profitability of Australian beef cattle and sheep producers (p. 119). North Sydney: Meat & Livestock Australia.
Sampson, K. (1933). The systematic infection of grasses by Epichloë typhina (Pers.) Tul. Transactions of the British Mycological Society,18, 30–47.
Schiff, P. L., Jr. (2006). Ergot and its alkaloids. American Journal of Pharmaceutical Education,70, 1–10. https://doi.org/10.5688/aj700598.
Settivari, R. S., Evans, T. J., Yarru, L. P., Eichen, P. A., Sutovsky, P., Rottinghaus, G. E., et al. (2009). Effects of short-term heat stress on endophytic ergot alkaloid-induced alterations in rat hepatic gene expression. Journal of Animal Science,87, 3142–3155.
Shahzad, R., Khan, A. L., Bilal, S., Asaf, S., & Lee, I. J. (2018). What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2018.00024.
Shivanna, K. R. (1979). Recognition and rejection phenomena during pollen–pistil interactions. Proceedings of the Indian Academy of Sciences,88B, 115–141.
Spanu, P., & Kämper, J. (2010). Genomics of biotrophy in Fungi and Öomycetes—emerging patterns. Current Opinion in Plant Biology,13, 409–414.
Spiers, D. E., Evans, T. J., & Rottinghaus, G. E. (2005). Interaction between thermal stress and fescue toxicosis: animal models and new perspectives. In C. A. Roberts, C. P. West, & D. E. Spiers (Eds.), Neotyphodium in cool season grasses (pp. 243–270). Ames: Blackwell.
Suryanarayanan, T. S. (2017). Fungal endophytes: an eclectic review. Kavaka,49, 1–9.
Tadych, M., Bergen, M. S., & White, J. F., Jr. (2014). Epichloë associated with grasses: new insights on life cycles, dissemination and evolution. Mycologia,106, 181–201.
Tapper, B. A., & Latch, G. C. M. (1999). Selection against toxin production in endophyte-infected perennial ryegrass. Grassland Research & Practice,7, 107–111.
Tenberge, K. B. (2007). Morphology and cellular organisation in Botrytis interactions with plants. In Y. Elad, B. Williamson, P. Tudzynski, & N. Delen (Eds.), Botrytis: biology, pathology and control (pp. 67–84). Dordrecht: Springer.
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A., & Dufresne, A. (2015). The importance of the microbiome of the plant holobiont. New Phytologist,206, 1196–1206.
Young, C. A., Hume, D. E., & McCulley, R. E. (2013). Fungal endophytes of tall fescue and perennial ryegrass: pasture friend or foe? Journal of Animal Science,91, 2379–2394.
Zanzalari, K. P., Heitmann, R. N., McLaren, J. B., & Fribourg, H. A. (1989). Effects of endophyte-infected fescue and cimetidine on respiration rates, rectal temperatures and hepatic mixed function oxidase activity as measured by hepatic antipyrine metabolism in sheep. Journal of Animal Science,67, 3370–3378.
Zbib, N., Repussard, C., Tardieu, D., Priymenko, N., Domange, C., & Guerre, P. (2014). Ergovaline in tall fescue and its effect on health, milk quality, biochemical parameters, oxidative status, and drug metabolizing enzymes of lactating ewes. Journal of Animal Science,92, 5112–5123.
Zhang, H. W., Song, Y. C., & Tan, R. X. (2006). Biology and chemistry of endophytes. Natural Products Report,23, 753–771.
Acknowledgements
Thanks are due to Anamika Sharma (Montana State University, Conrad, USA) for help with the arrangement of figures and tables.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In celebration of the life of and the science promoted by Trichur Subramanian Suryanarayanan, who pioneered the study of endophytic fungi associated with Indian plants.
Rights and permissions
About this article
Cite this article
Raman, A. Endophytic Epichloë (Clavicipitaceae) association with Lolium perenne and Lolium arundinaceum (Poaceae) resulting in health problems for the livestock and horses in temperate Australian pastures: assay of secondary metabolites and antioxidant activity. Plant Physiol. Rep. 24, 474–486 (2019). https://doi.org/10.1007/s40502-019-00481-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-019-00481-9