Abstract
Macrospora leaf spot, caused by Stenocarpella macrospora, is an important disease of maize. However, little information is available regarding the infection process of this fungus on maize leaves. This study aimed to describe stages of the infection process of S. macrospora on maize leaves by using both light and scanning electron microscopy. The adaxial surfaces of maize leaves were inoculated with a conidial suspension of S. macrospora and leaf samples were collected from 24 to 96 h after inoculation (hai) and also at 20 days after inoculation (dai). At 24 hai, the germ tubes from the germinated conidia attempted to penetrate the leaf cuticle without any sign of positive tropism to stomata. After penetration, fungal hyphae colonized the epidermal cells as well as the underlying mesophyll cells. At 20 dai, prominent fungal growth was noticed in the phloem vessels, parenchyma cells, xylem vessels and in the bundle sheath cells as well as in the vessel elements. Fungal hyphae emerged in different developmental stages through the stomata and pycnidia were noticed in the necrotic lesions at 20 dai. These results provide new insight into the infection process of S. macrospora on maize leaves and may contribute to the development of more effective control of macrospora leaf spot.
Similar content being viewed by others
Introduction
Maize (Zea mays L.) is one of the most important cereal crops in the world and is widely cultivated in both tropical and subtropical regions (Lobell et al. 2009; CIMMYT 2012). Macrospora leaf spot (MLS), caused by the fungus Stenocarpella macrospora (Earle) Sutton (syn. Diplodia macrospora Earle), is one of the most important diseases affecting maize production especially in the humid subtropical and tropical regions (Latterell and Rossi 1983; Dai et al. 1987).
The first symptoms of MLS are small brown spots with a chlorotic halo and a water-soaked appearance on maize leaf blades (Dai et al. 1987). These lesions then expand and become irregular or elliptical; they also develop concentric rings and a reddish or yellow halo (Dai et al. 1987; Casa et al. 2006, Bradley et al. 2010). Inside the necrotic leaf tissues, subepidermal, globose or elongated pycnidia that are dark brown in color are produced (Bradley et al. 2010). The S. macrospora saprophytically survives in maize debris in the form of mycelia and pycnidia, which constitute the main source of primary inoculum (Casa et al. 2003). Conidia are often released from pycnidia in cirri and are easily dispersed by wind and rain (Flett et al. 1992; Casa et al. 1998; Casa et al. 2004). MLS is controlled mainly by the use of crop rotation and sowing of healthy seeds, which aim at reducing the primary inoculum. Little information is available regarding the chemical control of this disease and resistant cultivars are lacking (Casa et al. 2006; Bampi et al. 2012).
Fungal disease pathogens have developed different strategies and mechanisms to break the cuticle and the epidermal cell wall to colonize the host tissues (Dean 1997; Van Kan 2006). The strategy used by necrotrophic fungal pathogens to infect plants generally starts with the adhesion of conidia to the leaf surface. This step is followed by germination, penetration and sporulation (Nicholson et al. 1988; Prins et al. 2000; Laluk and Mengiste 2010). Necrotrophic pathogens may also penetrate their hosts through wounds, natural openings, and directly through the surface via the secretion of lytic enzymes and non-host selective toxins responsible for the dissolution of cell walls and the complete disintegration of cells (Kolattukudy 1985; Have et al. 2001; Cabanne and Donéche 2002; Van Kan 2006). Oxidases, cutinases and lipases are secreted by fungi to modify the plant cutin and wax layer (Movahedi and Heale 1990; Laluk and Mengiste 2010).
Considering the importance of MLS as an yield-limiting factor in maize production and that little information is available regarding the infection process of S. macrospora on maize leaves, this study aimed to provide a detailed description of the events of the infection process of S. macrospora on maize leaves by using both light and scanning electron microscopy.
Material and methods
Plant cultivation
Maize seeds from cultivar HIB 32R48H, which is highly susceptible to S. macrospora, were sown in plastic pots containing 2 kg of Tropstrato® (Vida Verde, Mogi Mirim, São Paulo, Brazil) substrate composed of a 1:1:1 mixture of pine bark, peat and expanded vermiculite. A total of 1.63 g of calcium phosphate monobasic was added to each plastic pot. A total of five seeds were sown per pot, and each pot was thinned to three seedlings 5 days after seedling emergence. Plants were kept in a greenhouse during the experiments (temperature 26 ± 2 °C during the day and 14 ± 4 °C at night, relative humidity 72 ± 5 %) and were fertilized weekly with 50 mL of a nutrient solution composed of 2.6 mM KCl, 0.6 mM K2SO4, 1.2 mM MgSO4, 1.0 mM CH4N2O, 1.2 mM NH4NO3, 0.0002 mM (NH4)6Mo7O24, 0.03 mM H3BO4, 0.04 mM ZnSO4, 0.01 mM CuSO4 and 0.03 mM MnCl2. The nutrient solution was prepared using deionized water. Plants were watered as needed.
Inoculum production and plant inoculation
Plants were inoculated with a monosporic isolate of S. macrospora (UFV-DFP Sm 01). The isolate of S. macrospora was grown in Petri dishes containing oat-agar medium and incubated for 35 days in an incubator (22 °C, photoperiod of 12 h). All of the leaves of each plant were inoculated with a conidial suspension of S. macrospora (6 × 104 conidia/ml) at 30 days after emergence (growth stage V5) (Bensch et al. 1992) using a VL Airbrush atomizer (Poasche Airbrush Co). Gelatin (1 % w v −1) was added to the suspension to aid conidial adhesion on the leaf blades. Immediately after inoculation, plants were transferred to a growth chamber at 26 ± 2 °C, 85 ± 5 % relative humidity and a 12 h light and 12 h dark photoperiod for 30 h. After this period, plants were transferred to a plastic mist growth chamber (temperature of 25 ± 2 °C (day) to 20 ± 2 °C (night) and relative humidity of 90 ± 5 %, with a misting system of nozzles (model NEB-100; KGF Company) that sprayed mist every 30 min above the plant canopies) inside a greenhouse for the duration of the experiments. The relative humidity and temperature were measured with a thermo-hygrograph (TH-508, Impac). The maximum natural photon flux density at plant canopy height was approximately 925 μmol m−2 s−1.
Development of MLS symptoms
Leaves were collected at 24, 48, 72 and 96 h after inoculation (hai) and at 10 and 20 days after inoculation (dai) and photographed (×6.5) under a stereomicroscope (Stemi 2000-C; Carl Zeiss) coupled with a digital camera (PowerShot A640; Canon).
Processing of leaf fragments for light microscopy
Leaf fragments (≈ 5 mm2) were collected at 24, 48, 72 and 96 hai and at 20 dai. The fragments were placed in glass vials, fixed with 2.5 % glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) during 48 h, dehydrated in an ethanol series and embedded in methacrylate HistoResin (Leica Instruments). A total of four blocks were made for each sampling time. Each block also contained two leaf fragments. Thick cross sections (5 mm2) of each block were obtained with a rotary microtome (auto-advance model RM 2255 Leica Microsystems Inc.) and stained with toluidine blue 0.05 % (pH 4.7). A total of 25 thick-fine cuts were obtained per each block, which were then distributed into six glass slides. The images containing details of the fungal infection were acquired digitally (Axio Cam HR, Carl Zeiss) using a Carl Zeiss Axio Imager A1 microscope (Carl Zeiss) in the bright-field mode and further processed using AXION VISION v. 4.8.1 software.
Processing of leaf fragments for scanning electron microscopy
Leaf fragments (≈ 5 mm2) were collected at 24, 48, 72 and 96 hai and at 20 dai. The fragments were placed in glass vials, fixed with 2.5 % glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) and stored at 4 °C. The samples were post-fixed for 2 h at room temperature with osmium tetroxide 1 % in sodium cacodylate buffer 0.1 M (pH 7.2). The fragments were then washed four times with the same buffer, with each wash lasting 10 min. The fragments were then dehydrated in an ethanol series. After dehydration, the fragments were subjected to a critical point dryer using the unit “Critical Point Dryer” (CPD 020 Model, Bal-Tec). The fragments were mounted on metal stubs with the aid of an aluminum double-sided tape and coated with colloidal gold in a “Sputter Coater” apparatus coupled with a “Freezing Drying Unit” (FDU010 Model, Bal-Tec). The scanning electron micrographs were obtained using an LEO scanning electron microscope operating at 10 Kv, with a working distance ranging from 10 to 30 mm.
Results
Macrospora leaf spot symptoms on maize leaves
The lesions of water-soaked appearance on maize leaves were first noticed at 48 hai. As the lesions of MLS expanded, they became elliptical or irregular and brown surrounded by chlorotic or reddish haloes. At 10 dai, the lesions appeared as large brown patches of desiccated tissue with darker concentric rings. At 20 dai, pycnidia at different stages of development started to become visible in the necrotic lesions (Fig. 1).
Symptoms of Macrospora leaf spot on maize leaves. a Small water-soaked lesions started to develop at 48 hai; b Lesions with a brown color surrounded by yellow halos became apparent at 96 hai; c Lesions expanded resulting in necrosis of the leaf tissue at 10 dai; d Dark brown sub-epidermal pycnidia on the necrotic leaf tissue at 20 dai
Conidial germination and fungus penetration
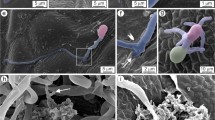
At 24 hai, conidia started to germinate on the leaves without a specific pattern. The germ tubes were formed from one of the conidial cells and germination tended to eventually become bipolar (Fig. 2a). The germ tubes grew along the leaf blade and apparently without any positive tropism to the stomata. Occasionally, the germ tube grew toward to the stomata without any evidence of appressoria formation (Fig. 2b). The dissolution of the epicuticular wax layer in the regions around the conidia and germ tubes on the leaf surface was also noticed (Fig. 2a and b). Details of the infection process of S. macrospora obtained at 24 hai were quite similar to what was seen from 48 to 96 hai (data not shown).
Scanning electron micrographs of the adaxial surface of maize leaves at 24 h after inoculation (a and b) and of fractured leaf samples with Stenocarpella macrospora at 20 days after inoculation (c–f). A. Bipolar-germinated conidium with germ tubes emerging from each cell. Germ tubes grew through the stomata without any evidence of direct penetration. Erosion of the host cuticle around the conidia and germ tubes on the adaxial leaf surface; b Conidia produced one germ tube with its tip growing in the direction of the stomata. c–e Profuse fungal hyphal colonizing the mesophyll cells; f Hyphae emerging through stomata. C conidia, Ep epidermis, Me mesophyll; * stomata, arrowhead hyphae, arrow branching hyphae. Bars: 10 μm
Fungus colonization and sporulation
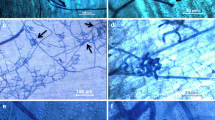
Fungal hyphae profusely colonized the leaf tissues both inter- and intracellularly at 20 dai, resulting in profound cellular destruction (Figs. 2d–e, 3g and 4). Fungal hyphae first colonized the adjacent epidermal cells as well as the underlying mesophyll cells (Figs. 2c and 3c). Prominent fungal growth was also observed in phloem vessels (Fig. 4c and e), the parenchyma cells (Fig. 4e–h), the xylem vessels, and the bundle sheath cells (Figs. 3g and h and 4d) as well as in the vessel elements (Fig. 4f–h). Fungal hyphae emerged through the stomata at 20 dai (Figs. 2f and 3a and b). Pycnidia at different stages of development were first noticed in the necrotic lesions at 20 dai (Fig. 3c–f and i and j).
Scanning electron micrographs of the adaxial surface of maize leaves at 24 h after inoculation (a–e) and of fractured leaf samples at 20 days after inoculation with Stenocarpella macrospora (f–j). A. Fungal hyphae emerging through stomata on the adaxial leaf surface; b–f Pycnidia formed on the leaf surface; g and h Profuse fungal hyphae colonizing the xylema vessels; i and j Fractured pycnidia. C conidium, arrowhead fungal hyphae, arrow conidia, Py pycnidia, Xy xylem vessels. Bars: A, E, and J = 10 μm; B, C, D, H, and I = 20 μm; F and G = 100 μm
Light micrographs of transverse (a–d) and longitudinal (e–h) sections of maize leaves at 20 days after inoculation with Stenocarpella macrospora. Sections were obtained from non-inoculated (a) and inoculated plants (b–h). c Fungal hyphae colonized the epidermal cells and the phloem vessels; d Fungal hyphae colonized the xylem vessels and the bundle sheath cells; e Fungal hyphae colonized the parenchyma cells and the phloem vessels; f–h Fungal hyphae colonized the entire vessels elements. Ep epidermis, Pa parenchymal, Ph phloem, Xy xylem, Bsc bundle sheath cells, arrowhead fungal hyphae. Bars: 10 μm
Discussion
The present study provides, to the best of our knowledge, the first microscopic details of the infection process of S. macrospora on maize leaves. For some disease-causing fungi, certain physical and chemical features of the host surface can influence conidia germination, germ tube growth and appressoria formation (Wynn 1981; Howard and Valent 1996; Dean 1997). The bicellular conidia of S. macrospora produced more than one germ tube from each cell. The germ tube attempted to penetrate the maize leaves mainly through the cuticle without appressoria formation. Kema et al. (1996) reported that Mycosphaerella graminicola also directly penetrated the wheat leaves. Occasionally, the germ tubes passed through the stomata without any evidence of penetration. For Cercospora zeae-maydis in maize (Beckman and Payne 1982), M. graminicola in wheat (Kema et al. 1996), S. macrospora in wheat (Brunelli et al. 2004) and Cercospora henningsii in cassava (Babu et al. 2009), there was no evidence of fungal penetration through the stomata.
The epicuticular wax layer in the surrounding areas of the conidia and germ tubes of S. macrospora was dramatically affected. The weakening of the leaf blades at the locations of conidia deposition and adhesion as well as in the regions around the germ tubes suggests that the action of lytic enzymes or non-host selective toxins released by S. macrospora acted to modify the leaf surface. This modification would ensure conidia and germ tubes gain full access to the host’s leaf tissue. For the barley-Erysiphe graminis and the rice-Magnaporthe grisea interactions, the erosion of the host cuticle was a direct result of enzymatic modification, possibly by the action of the cutinases and esterases released by these fungi (Kunoh et al. 1990; Howard and Valent 1996).
After fungal penetration, two patterns of tissue colonization were noticed. At the early stages of fungal infection, hyphae were less abundant in the epidermal and subepidermal cells and the integrity of the cell walls was profoundly altered, suggesting the participation of non-host selective toxins in the infection process. The mesophyll cells of sorghum and wheat leaves were profoundly affected by the infection of Colletotrichum sublineolum and M. graminicola, respectively, without the presence of mycelia of these fungi, suggesting that cell wall degrading enzymes and non-host selective toxins were of greater importance for the fungal colonization of the leaf tissue (Wharton et al. 2001).
Fungal hyphae of S. macrospora fully colonized the epidermis, parenchyma cells, phloem and xylem vessels as well as the vessels elements and bundle sheath cells. The extensive growth of the hyphae of C. graminicola in maize leaves and of Rhynchosporium secalis on barley leaves were reported by Mims and Vaillancourt (2002) and Jorgensen et al. (1993), respectively. After massive cell collapse, fungal hyphae emerged through the stomata in the necrotic leaf tissue suggesting that the initial phase of pycnidia formation took place. This finding is in agreement with previous reports of the wheat-M. graminicola (Kema et al. 1996; Palme and Skinner 2002) and macadamia-Pseudocercospora macadamiae (Miles et al. 2009) interactions.
Considering the importance of MLS to maize production worldwide and the lack of information in the literature regarding the infectious process of S. macrospora, the results of the present study provide novel information that will allow us to better understand the infection process of this fungus and help to develop more effective disease control strategies.
References
Babu AM, Philip T, Kariappa BK, Kamble CK (2009) Scanning electron microscopy of the infection process of Cercospora henningsii on cassava leaves. J Phytopathol 157:57–62
Bampi D, Casa RT, Bogo A, Sangoi L, Sachs C, Bolzan JM, Piletti G (2012) Fungicide performance on the control of macrospora leaf spot in corn. Summa Phytopathol 38:319–322
Beckman PM, Payne GA (1982) External growth, penetration, and development of Cercospora zeae-maydis in corn leaves. Phytopathology 72:810–815
Bensch MJ, Van Staden J, Rijkenberg FHJ (1992) Time and site inoculation of maize for optimum infection of ears by Stenocarpella maydis. J Phytopathol 136:265–269
Bradley CA, Pedersen DK, Zhang GR, Pataky NR (2010) Occurrences of diplodia leaf streak caused by Stenocarpella macrospora on corn (Zea mays) in Illinois. Plant Dis 94:1262
Brunelli KR, Athayde Sobrinho C, Cavalcanti LS, Ferreira PTO, Camargo LEA (2004) Germinação e penetração de Stenocarpella macrospora em folhas de milho. Fitopatol Bras 30:187–190
Cabanne C, Donéche B (2002) Polygalacturonase isoenzymes produced during infection of the grape berry by Botrytis cinerea. Vitis 41:129–132
Casa RT, Zambolim L, Reis EM (1998) Transmissão e controle de Diplodia em sementes de milho. Fitopatol Bras 23:436–441
Casa RT, Reis EM, Zambolim L (2003) Decomposição dos restos culturais do milho e sobrevivência saprofítica de Stenocarpella macrospora e Stenocarpella maydis. Fitopatol Bras 28:355–361
Casa RT, Reis EM, Zambolim L (2004) Dispersão vertical e horizontal de conídios de Stenocarpella macrospora e Stenocarpella maydis. Fitopatol Bras 29:141–147
Casa RT, Reis EM, Zambolim L (2006) Doenças do milho causadas por fungos do gênero Stenocarpella. Fitopatol Bras 31:427–439
CIMMYT (2012) Maize annual report: research program on Maize. CGIAR. p 28
Dai K, Nagai M, Sasaki H, Nakamura H, Tachechi K, Warabi M (1987) Detection of Diplodia maydis (Berkeley) Saccardo from imported corn seed. Res Bull Plant Protect Serv 23:1–6
Dean RA (1997) Signal pathways and appressorium morphogenesis. Annu Rev Phytopathol 35:211–234
Flett BC, Wehner FC, Smith MF (1992) Relationship between maize stubble placement in soil and survival of Stenocarpella maydis (Diplodia maydis). J Phytopathol 134:33–38
Have AT, Breuli WO, Wubben JP, Visser J, van Kan JAL (2001) Botrytis cinerea endopolygalacturonase genes are differentially expressed in various plant tissues. Fungal Genet Biol 33:97–105
Howard RJ, Valent B (1996) Penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu Rev Microbiol 50:491–512
Jorgensen HJL, De Neergaard E, Smedegaard-Petersen V (1993) Histological examination of the interaction between Rhynchosporium secalis and susceptible and resistant cultivars of barley. Physiol Mol Plant Pathol 42:345–358
Kema GHJ, Yu D, Rijkenberg FHJ, Shaw MW, Baayen RP (1996) Histology of the pathogenesis of Mycosphaerella graminicola in wheat. Phytopathology 86:777–786
Kolattukudy PE (1985) Enzymatic penetration of the cuticle by fungal pathogens. Annu Rev Phytopathol 23:223–250
Kunoh H, Nicholson RL, Yosioka H, Yamaoka N, Kobayashi I (1990) Preparation of the infection court by Erysiphe graminis: degradation of the host cuticle. Physiol Mol Plant Pathol 36:397–407
Laluk K, Mengiste T (2010) Necrotroph attacks on plants: wanton destruction or covert extortion? The Arabidopsis Book, pp 1–34
Latterell FM, Rossi AE (1983) Stenocarpella macrospora (=Diplodia macrospora) and S. maydis (=D. maydis) compared as pathogens of corn. Plant Dis 67:725–729
Lobell DB, Cassman KG, Field CB (2009) Crop yield gaps: their importance, magnitudes and causes. Ann Rev Environ Res 34:179–204
Miles AK, Akinsanmi OA, Sutherland PW, Aitken EAB, Drenth A (2009) Infection, colonization and sporulation by Pseudocercospora macadamiae on macadamia fruit. Australas Plant Pathol 38:36–43
Mims CW, Vaillancourt LJ (2002) Ultraestructural characterization of infection and colonization of maize leaves by Colletotrichum graminicola, and by a C. graminicola pathogenicity mutant. Phytopathology 92:803–812
Movahedi S, Heale JB (1990) The roles of aspartic proteinase and endo-pectin lyase enzymes in the primary stages of infection and pathogenesis of various host tissues by different isolates of Botrytis cinerea Pers ex. Pers. Physiol Mol Plant Pathol 36:303–324
Nicholson RL, Yoshioka H, Yamaoka N, Kunoh H (1988) Preparation of the infection court by Erysiphe gramnis. II release of esterase enzyme from conidia in response to a contact stimulus. Exp Mycol 12:336–349
Prins TW, Tudzynski P, Tiedemann AV, Tudzynski B, Have A, Hansem ME, Tenberge K, van Kan JAL (2000) Infection strategies of Botrytis cinerea and related necrotrophic pathogens. In: Kronstad JW (ed) Fungal Pathology. Kluwer Academic Publishers, The Netherlands, pp 33–64
Van Kan JAL (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11:248–253
Wharton PS, Julian AM, O’Connel RJ (2001) Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum. Phytopathology 91:149–158
Wynn WK (1981) Tropic and taxic response of pathogens to plants. Annu Rev Phytopathol 19:237–255
Acknowledgments
Prof. F. A. Rodrigues thanks the CNPq for his research fellowship. The authors thank the “Núcleo de Microscopia e Microanálise” of the Universidade Federal de Viçosa” for the use of their equipment. We thank Dr. João Américo Wordell Filho for his technical assistance. This study was supported by grants from CAPES, CNPq and FAPEMIG to Prof. F. A. Rodrigues.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Elaine A. de Souza
Rights and permissions
About this article
Cite this article
Bermudez-Cardona, M.B., Cruz, M.F.A. & Rodrigues, F.A. Microscopic study of the Stenocarpella macrospora infection process on maize leaves. Trop. plant pathol. 41, 115–122 (2016). https://doi.org/10.1007/s40858-016-0079-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-016-0079-3