Abstract
The pathogen Verticillium dahliae causes Verticillium wilt in a number of crops, including cotton Verticillium wilt. Chaetoviridin A, a secondary metabolite of Chaetomium globosum, significantly inhibits the growth of V. dahliae. Spore germination is a major part of the disease cycle. However, the molecular mechanism of chaetoviridin A inhibiting spore germination of V. dahliae is unknown. In this work, we found that chaetoviridin A significantly inhibited spore germination of V. dahliae. Transcriptome analysis showed that DEGs were enriched in linolenic acid metabolism, alpha-Linolenic acid metabolism, Arachidonic acid metabolism and Purine metabolism pathways at 1 h, which were related to cell membrane. At 3 h, DEGs were enriched in the pathways of galactose metabolism, diterpenoid biosynthesis, cysteine and methionine metabolism, and starch and sucrose metabolism, which were mainly related to amino acid metabolism and sugar metabolism. Several genes related to glucose metabolism were identified, mainly including Glucagon endo-1,3-alpha-glucosidase agn1, glucoamylase, maltose O-acetyltransferase, and beta-galactosidase. Stress resistance gene PAL, detoxification gene P450 2C31 and ABA receptor were also down- regulated. These genes may be related to spore germination. These results provide a theoretical basis for chaetoviridin A to control fungal diseases.
Similar content being viewed by others
Introduction
The fungus Verticillium dahliae can cause Verticillium wilt in many plants, such as tomato, pepper, strawberry, and cotton (Wei et al. 2021). Cotton Verticillium wilt can cause cotton yield and quality decline, and also result in serious economic losses in China (Shaban et al. 2018; Song et al. 2020). Moreover, the pathogen can infect more than 200 plant species, and it is difficult to be controlled (Yu et al. 2019). Biological control is one of the safest and environmentally friendly methods to control Verticillium wilt (Spurrier 1990). Many microorganisms have been proved to have biocontrol effect, and some of them have been gradually applied (Shaban et al. 2018). However, due to the changes of biotic and abiotic factors in the field, the survival and colonization of biocontrol bacteria or fungi are affected to a certain extent, and the biocontrol products that can effectively control soil borne diseases under field conditions are very limited (Mazzola and Freilich 2017).There is no effective and environmental friendly fungicide to inhibit the pathogen (Zhang et al. 2022). The biocontrol bacteria or fungi can produce a variety of secondary metabolites, which are more stable than the biocontrol bacteria or fungi themself. It is an important source of green fungicide. Finding an effective and environmentally friendly fungicide to control Cotton Verticillium wilt or is expected to solve the problem of unstable control effect in the application of biocontrol bacteria of fungi.
Fungal infection of plants is usually caused by the contact of the host with spores and then by germination (Zhou et al. 2018). The germination of Aspergillus niger spores refers to the emergence of cells from asexual spores and the formation of spore mycelia or thalli under optimal growth conditions (van Leeuwen et al. 2013). During the germination, the hydrophobicity of the spore gradually decreases, resulting in the swelling of the spore, which is called isotropic growth, followed by polarized growth characterized by germ tube formation (Dague et al. 2008). The spore germination can be considered to be one of the main steps of the disease cycle and greatly affects fungal aggressiveness and colonization (Zhou et al. 2018). Therefore, inhibiting spore germination is also very important in disease control. Some genes related to fungal spore germination have also been reported. YlyA codes a RNA polymerase-binding protein that is specifically used to regulate the expression of genes involved in spore germination (Traag et al. 2013). The genes involved in protein folding and transport were transient up-regulated during the onset of spore germination of the yeast Saccharomyces cerevisiae (Geijer et al. 2012). Deletion of the SpoVA operon from a Bacillus subtilis strain with transposon Tn1546 slightly decreased the heat and humidity resistance of spores, but the germination rate decreased significantly (Luo et al. 2021). The endochitinase VDECH from V. dahliae also inhibits spore germination (Cheng et al. 2017).
Chaetomium globosum can inhibit many plant diseases, including rape sclerotinia rot (Zhao et al. 2017), Fusarium wilt of tomato (Madbouly et al. 2017), potato late blight (Shanthiyaa et al. 2013), root rot of wheat (Yue et al. 2018), et al. Previous studies have found that C. globosum CEF-082 can inhibit cotton Verticillium wilt, and chaetoviridin A was isolated from the fermentation broth of this fungus, which can significantly inhibit the growth of V. dahliae (Zhang et al. 2021). Chaetoviridin A is an azapholone antibiotic, was first characterized from C. globosum var. flavoviride (Takahashi et al. 1990), also known to have antifungal activity, such as Botrytis cinerea, Sclerotinia sclerotiorum, Fusarium graminearum, Phytophthora capsici, and F. moniliforme (Yan et al. 2018; Tomoda et al. 1999) found that chaetoviridin A inhibited the cholesteryl ester transfer protein (CETP) in mice. Spore germination is an important step in the disease cycle. Whether chaetoviridin A can affect the spore germination of V. dahliae is unclear. To explore the molecular mechanism of chaetoviridin A inhibiting spore germination of V. dahliae has important guiding significance for using chaetoviridin A to control cotton Verticillium wilt.
Materials and methods
V. dahliae strain culture
V. dahliae V991, the pathogen of cotton Verticillium wilt, was provided by the Institute of Cotton Research of Chinese Academy of Agricultural Sciences (Anyang, China). V. dahliae V991 was cultured on potato dextrose agar (PDA) medium and incubated in the dark at 25 °C for 7 d, then inoculated into liquid potato dextrose broth (PDB), cultured at 25 °C, 150 rpm for 7 d. The mycelia were filtered out and removed to obtain spore suspension.
Effect of chaetoviridin A on spore germination of V. dahliae
The spore suspension of V. dahliae V991 was collected into a 1.5 mL sterile centrifuge tube, centrifuged, removed PDB, and collected spores about the size of mung bean. Then, 490 µL PDB and 10 µL chaetoviridin A was added to the centrifuge tube with a final concentration of 150 µg/mL. Chaetoviridin A is an azapholone antibiotic, and was isolated from C. globosum CEF-082 (Fig. S1). Chaetoviridin A can inhibit the colony expansion of V. dahliae, and it is also likely to inhibit spore germination. Ten µL of sterile methanol instead of chaetoviridin A was added in the control group, and the experiment was repeated three times. Spore suspension was cultured at 25 °C and 200 rpm. Samples were collected after 1 and 3 h, a total of 12 samples, CK1h-1, CK1h-2, CK1h-3, Treat1h-1, Treat1h-2, Treat1h-3, CK3h-1, CK3h-2, CK3h-3, Treat3h-1, Treat3h-2, Treat3h-3, respectively. The sample was centrifuged to remove the supernatant, then was quickly frozen with liquid nitrogen and put into the − 80 °C refrigerator for storage. Before collecting samples for 3 h, the spore suspension was inoculated onto PDA plates, and each replicate was inoculated with 10 µL. The spore germination on PDA was observed at 3 h, 4 h, 5 h, 6 h, 7 h, 8 and 12 h. Five visual fields were observed for each treatment, and each visual field was not less than 50 spores. Then the germination rate was calculated.
RNA extraction
RNA was extracted from the 12 samples collected above, and the RNA of the samples was isolated and purified with Trizol (Invitrogen, CA, USA) according to the operating protocol provided by the manufacturer. The amount and purity of total RNA were then controlled with NanoDrop ND-1000 (NanoDrop, Wilmington, DE, USA). The integrity of RNA was detected by Bioanalyzer 2100 (Agilent, CA, USA) and verified by agarose electrophoresis. The RNA samples with Concentration > 50 ng/µL, RIN > 7.0, OD260/280 > 1.8, total RNA > 1 µg were sent to Hangzhou Lianchuan Biotechnology Co., Ltd. for sequencing.
RNA sequencing (RNA-seq)
Oligo (DT) magnetic beads (Dynabeads oligo (DT), Article No. 25-61005, Thermo Fisher, USA) were used to specifically capture the mRNA containing polyadenylate (polyadenylate) by two rounds of purification. The captured mRNA was subjected to high temperature conditions using a magnesium ion disruption Kit (nebnext ® Magnesium RNA fragmentation module, Article No. e6150s, USA) for 5–7 min at 94 °C. The fragmented RNA was synthesized into cDNA by reverse transcriptase (Invitrogen superscript ™ II reverse transcriptase, Article No. 1,896,649, CA, USA). Double ended sequencing of cDNA was performed according to standard operation using Illumina novaseq™ 6000 (LC Bio Technology Co., Ltd. Hangzhou, China), and the sequencing mode was PE150.
Functional annotation of differential expressed genes (DEGs)
Reads obtained from the sequencing machines were filtered by Cutadapt (https://cutadapt.readthedocs.io/en/stable/, version: cutadapt-1.9) through removing reads containing adapters, polyA and polyG, reads containing more than 5% of unknown nucleotides (N), and low quality reads containing more than 20% of low quality (Q-value ≤ 20) bases. The clean reads were spliced and aligned to the reference V. dahliae genome retrieved from the genome website (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA28529). The expression level of genes was measured by Fragments Per Kilobase of exon model per Million mapped reads (FPKM) or Reads Per Kilobase of exon model per Million mapped reads (RPKM). In this study, FPKM was used to calculate the expression abundance of known genes in different samples. Genes differential expression analysis was performed by DESeq2 software between two different groups. The difference multiple FC ≥ 2 or FC ≤ 0.5 (i.e., the absolute value of log2FC ≥ 1) and P < 0.05 were taken as the criteria, and the genes screened were considered as DEGs. The R language ade4 package (1.7.13) was used for principal coordinate analysis (PCoA) analysis. Kyoto Encylopedia of Genes and Genomes (KEGG) pathways and Gene Ontology (GO) terms were enriched by DEGs.
Quantitative real-time PCR (qRT-PCR) analysis
RNA was extracted from the sequenced samples and reverse transcribed into cDNA. The analyzed DEGs were designed with primers by beacon Designer (7.92) for gene expression verification (Table 1). qRT-PCR was performed via a Bio-Rad CFX96 Real-Time System, and each PCR mixture consisted of 2 µL of cDNA, 0.4 µL of each primer, 10 µL of ChamQ Universal SYBR qPCR Master Mix (Vazyme) and 7.2 µL of sterile water. Each sample involved at least three technical repeats. The PCR cycle was the same as that used by Zhang et al. (2021). The V. dahliae β-tubulin gene was used as the internal reference, and relative gene expression was calculated using the 2−ΔΔCt method (Han et al. 2015). The primer of V. dahliae β-tubulin was the same as that described by Han et al. (2015) (Table 1).
Statistical analysis
The statistical procedures for spore germination experiments were performed using the statistical software Statistix (8.1), and means were separated by Tukey’s honestly significant difference test. P value < 0.05 was considered statistically significant.
Results
Effect of chaetoviridin A on spore germination of V. dahliae
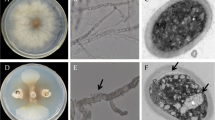
After spores of V. dahliae treated with chaetoviridin A 3 h, the spore suspension was then inoculated onto PDA. There was no difference in the spore germination rate between the control group and the treatment group after 3 and 4 h (Fig. 1A) inoculated onto PDA. While at 5 h, 6 h (Fig. 1B), 7 h, 8 (Fig. 1C) and 12 h, the germination rate of V. dahliae in the control group was significantly higher than that in the treatment group, and the spores in the control group almost completely germinated at 12 h, with a germination rate of 98.40% (Fig. 1D). The results showed that chaetoviridin A could inhibit the spore germination of V. dahliae.
Spore germination of V. dahliae after chaetoviridin A treatment. A, Spore germination of V. dahliae at 4 h; B, Spore germination of V. dahliae at 6 h; C, Spore germination of V. dahliae at 8 h. The red arrow refers to germinated spores, while the green arrow refers to non-germinated spores, Bar = 50 μm; D, The spore germination rate at 3 h, 4 h, 5 h, 6 h, 7 h, 8 and 12 h after inoculation of V. dahliae on PDA. Different letters indicate significant difference at the level of P < 0.05
Functional annotation and analysis of DEGs
At 1 and 3 h after the spores of V. dahliae treated with chaetoviridin A, there were 33 and 47 DEGs respectively (Fig. 2A). In these two periods, only one same DEGs was VDAG_08399 (o-methylsterigmatocystin oxidoreductase) and were up-regulated (Fig. 2B). Clustering heatmaps were drawn for the expression of these DEGs at 1 and 3 h (Fig. 2C). At 1 h, there were 4 up-regulated genes and 29 down-regulated genes; At 3 h after treatment, there were 3 up-regulated genes and 44 down-regulated genes, respectively. Among the DEGs at 1 and 3 h, 91.25% were down-regulated and 8.75% were up-regulated.
KEGG pathways involved in the treatment of chaetoviridin A
KEGG analysis showed that there were four signal pathways (at the level of P < 0.05) 1 h after the spores of V. dahliae treated with chaetoviridin A, namely, linoleic acid metabolism, alpha-linolenic acid metabolism, arachidonic acid metabolism and purine metabolism. Three hours after the spores of V. dahliae treated with chaetoviridin A, the signal pathways were galactose metabolism, dieterpenoid biosynthesis, cysteine and methionine metabolism, and starch and sucrose metabolism (Table 2). In the main pathways of KEGG enrichment at 1 (Fig. 3A) and 3 h (Fig. 3B), the same signal pathways were galactose metabolism, propanoate metabolism and pyruvate metabolism, but the difference was not significant.
Three hours after the spores of V. dahliae treated with chaetoviridin A, all the 11 DEGs enriched in the signal pathways were down-regulated, including a phenylalanine ammonia-lyase gene (VDAG_05831) and a beta-galactosidase gene (VDAG_04734) (Fig. 4A). The gene expression of 8 DEGs included VDAG_05831 and VDAG_04734 enriched in the signal pathways at 1 an 3 h was detected by qRT-PCR, and the results showed that the expression of DEGs in the treatment group was lower than that in the control group, which was consistent with the transcriptome data, and the 8 DEGs were down-regulated (Fig. 4B).
Expression analysis of several DEGs on the KEGG enrichment signal pathways. A, The expression patterns analysis of DEGs on the KEGG enrichment signal pathways by clustering heatmap. Gene expression was detected by RNA-seq. The coloured scale varies from blue to red. Blue represents lower expression and red represents higher expression; B, The expression analysis of DEGs on the KEGG enrichment signal pathways by qRT-PCR. a, VDAG_05831; b, VDAG_04734; c, VDAG_05832; d, VDAG_08795; e, VDAG_09834; f, VDAG_01953; g, VDAG_05835; h, VDAG_03313.
Go terms involved after chaetoviridin A treatment
Go terms analysis showed that 1 h after the spores of V. dahliae treated with chaetoviridin A, there were 10 DEGs distributed in biological process, 15 DEGs distributed in cell composition and 8 DEGs distributed in molecular function. There were 15 terms at the level of P < 0.05 (Fig. 5A). Three hours after the spores of V. dahliae treated with chaetoviridin A, there were 9, 16 and 7 DEGs in the biological process, cell composition and molecular function, respectively. There were 29 terms at the level of P < 0.05, and 3 of the top 20 terms were the same, which were secondary metabolic biological process, small molecular metabolic process and oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen, NAD(P)H as one donor, and incorporation of one atom of oxygen (Fig. 5B). The DEGs in the two periods were the most in cell composition (Fig. 5).
Predicted genes related to spore germination of V. dahliae
Nine genes were randomly selected from the transcriptome data, and the expression levels of the control group and the treatment group were compared by qRT-PCR. The results of qRT-PCR were consistent with the expression trend of genes in the transcriptome (Fig. S2).
After obtaining functional annotation of the transcripts from gene and protein databases, we selected 20 DEGs, which may be related to spore germination of V. dahliae. 19 of these 20 genes were down-regulated. Most of the down-regulated genes are related to glucose metabolism, such as FAD binding domain-containing protein, glucagon endo-1,3-alpha-glucosidase agn1, glucoamylase, maltose O-acetyltransferase, aldehyde reductase and beta-galactosidase. Stress resistance gene phenylalanine ammonia-lyase (PAL) and cytochrome P450 2C31 were also found in the down-regulated transcripts. Abscisic acid ABA receptor and transcription factor C6 zinc finger domain-containing protein were also down-regulated (Table 3). These genes may be related to spore germination of V. dahliae.
Discussion
Studies have shown that C. globosum has control effects on a variety of plant diseases (Zhao et al. 2017), and its antagonistic mechanism is mainly to produce a variety of antifungal metabolites such as chaetomugilins D and A, chaetoglobosins A and C, and chaetosin (Darshan et al. 2020). Chaetoviridin A is a kind of azotropic substance isolated from C. globosum. It has strong antifungal activity against a variety of pathogenic fungi and has broad antigungal spectrum (Yan et al. 2018; Park et al. 2005; Awad et al. 2014). Previous studies found that chaetoviridin A can inhibit the hyphal growth of V. dahliae and inhibit the germination of microsclerotia (Zhang et al. 2021). The results of this study showed that chaetoviridin A could also significantly inhibit the spore germination of V. dahliae. Therefore, this study will provide a theoretical basis for using chaetoviridin A to control cotton Verticillium wilt.
At 1 and 3 h after the spores of V. dahliae treated with chaetoviridin A, 12 samples in these two periods were analyzed by PCoA, and the results showed that there were differences between the control groups and the treatment groups at 1 and 3 h (Fig. S3). The number of DEGs identified increased gradually from 1 to 3 h after treatment, which may be the reason for the gradual enhancement of the inhibitory effect. DEGs at 1 h were significantly enriched in linolenic acid metabolism, alpha-linolenic acid metabolism, arachidonic acid metabolism and purine metabolism pathways. The three metabolic pathways of linolenic acid metabolism, alpha-linolenic acid metabolism and arachidonic acid metabolism are related to the cell membrane. Most of arachidonic acid is bound to the cell membrane phospholipids. Linolenic acid and alpha linolenic acid are components of the cell membrane. Balotf et al. (2021) found that the DEGs during spore germination of the pathogen Spongospora subterranea also involved in fatty acid metabolism; the DEGs at 3 h were significantly enriched in galactose metabolism, dieterpenoid biosynthesis, cystaine and methionine metabolism and starch and cross metabolism pathways. The intermediate products of branched chain amino acid biosynthesis are essential for spores germination of the pathogenic fungus Metarhizium robertsii (Luo et al. 2020; Balotf et al. 2021) also found that DEGs in germinated spores were enriched in biosynthesis of amino acids, such as arginine. Transcriptome analysis of the pathogen Penicillium expansum showed that DEGs during spore germination were enriched in the pathways such as lysine biosynthesis, cystaine and methionine metabolism and biosynthesis of amino acids (Zhou et al. 2018). The results of this study are consistent with those of the above-mentioned literature. Tomoda et al. (1999) found that chaetoviridin A can inhibit the activity of cholesterol ester transfer protein, which can react with lysine and other amino acids in protein to form covalent bonds. The cysteine and methionine metabolism pathway was also involved in this study. It is possible that chaetoviridin A can also react with these two amino acids.
Plant source beta-galactosidase (BGALs) belongs to the polygenic family and is an enzyme related to cell wall polysaccharide metabolism. β-galactosidase can degrade structural polysaccharides in plant cell walls to release free galactose in various biological processes, including cell wall expansion and degradation (Hou et al. 2021). β-galactosidase in the cell wall was involved in germination and seedling growth of chickpea (Hernández-Nistal et al. 2014). β-galactosidase gene DkGAL1 revealed that cell wall modification was related to fruit ripening in tomato (Ban et al. 2018). β-galactosidase was also detected in fungi, and most of the research was on the characteristics of β-galactosidase (Park & Oh 2009; Isobe et al. 2013). The biological function of β-galactosidase in fungi has not been reported yet.
Abscisic acid (ABA) is long known for its role in modulating plants response against both biotic and abiotic stress. The plant hormone ABA plays a crucial role in regulating seed germination and post-germination growth (Huang et al. 2017). Although the information of ABA biosynthesis in plants has been well documented, the knowledge of ABA biosynthesis in other organisms is still at an early stage. Fungi also produce and secrete ABA, ABA may play a role in enhancing or accelerating fungal virulence. Studies have shown that Botrytis cinerea (Siewers et al. 2006; Hirai et al. 1986; Siewers et al. 2004), multiple Cercospora species (Okamoto et al. 1988; Neill et al. 1982, 1987; Oritani et al. 1984; Oritani and Kiyota 2003) and rice blast pathogen Magnaporthe oryzae (Jiang et al. 2010; Spence and Bais 2015b) can all produce ABA. Fungal ABA biosynthesis and catabolic pathways are entirely different from those observed in plants. Exogenous ABA could accelerate spore germination and appressoria formation in rice blast pathogen M. orzyae (Spence and Bais 2015b), and treatment with exogenous ABA increased the colonization efficiency of fungi (Tatjana et al. 2015). Fungal ABA biosynthesis is required for infection in M. oryzae. A knockout mutant of M. oryzae with impaired ABA biosynthesis could not form lesions on rice (Spence et al. 2015a). The above research shows that fungal ABA biosynthesis is required for infection in M. oryzae, and it may have similar roles in V. dahliae. In this study, we found that after chaetoviridin A treated the spores of V. dahliae, the ABA gene was down-regulated and the spore germination rate was significantly decreased. This result is consistent with the above-mentioned literature. When ABA content increases, it promotes fungal spore germination and colonization, and conversely, it inhibits spore germination.
In plants, PAL activity has been detected in monocots, dicots, gymnosperms, ferns, and lycopods (Young et al. 2011). PAL activity was found relatively less in fungi, only in a few basidiomycetes and deuteromycetes, and in one Nectria cinnabarina (Bandoni et al. 1968). PAL enzymes in certain fungi have activity towards L-tyrosine, and this was considered to be tyrosine ammonia lyase (TAL) activity (Young et al. 2011; Camm & Towers 1973; Vance et al. 1975). The biological role of PAL in fungi is limited. palA, palB, palC, palF, palI, palH and pacC mediate the signals of fungi sensing environmental pH (Peñalva et al. 2008). BbPAL is a CaM-binding protein, and CaM negatively regulate the BbPAL activity in Beauveria bassiana. In addition, heat and cold stresses inhibited the BbPAL activity in B. bassiana (Kim et al. 2015). The function of PAL genes in V. dahliae has not been reported yet. In this study, chaetoviridin A could also inhibit the expression of PAL gene, indicating that chaetoviridin A may be a stress similar to heat and cold stress, thus affecting the subsequent germination of spores.
The cytochrome P450 (CYP) superfamily regulate various biosynthetic and detoxification pathways (Xu et al. 2015), and plays crucial roles in promoting plants growth and development and response to stresses. Several fungi P450s have been shown to play a key role in primary and secondary metabolism as well as degradation of xenobiotics (Cresnar and Petric 2011). A genome-wide deletion mutant set covering 102 P450s was constructed, and the changes of these mutants in 38 phenotypic categories, including fungal development, response to several xenobiotics and stress response, were analyzed in F. graminearum. They found that five P450 mutants (Fg03700, Fg02111, Fg00012, Fg10451 and Fg12737) were revealed to be specifically defective in virulence, and the spore production of the mutant ΔFg06068 strain in carboxymethyl cellulose medium (CMC) was significantly reduced compared with the wild-type strain. Most P450s seem to play redundant roles in the degradation of xenobiotics in F. graminearum (Shin et al. 2017). Azole antifungal agents can inhibit fungal sterol 14a-demethylase (Cyp51) (Liu et al. 2011). In this study, after treatment with chaetoviridin A, the expression of P450 gene was down-regulated, which may lead to the weakening of the detoxification effect of P450 on chaetoviridin A, so the subsequent spore germination was affected.
Conclusion
In conclusion, this study found that chaetoviridin A could significantly inhibit the spore germination of V. dahliae. Transcriptome analysis showed that the DEGs graduallyincreased from 33 to 47 after spores of V. dahliae treated with chaetoviridin A 1 and 3 h, and the inhibitory effectgradually increased. These DEGs were mainly enriched in linolenic acid metabolism, alpha-linolenic acid metabolism, arachidonic acid metabolism and purine metabolism pathways, galactose metabolism, diterpenoid biosynthesis, cysteine and methionine metabolism and starch and sucrose metabolism. These pathways mainly related to fatty acid metabolism, amino acid metabolism and sugar metabolism, and the process of inhibition was from cell membrane to cytoplasm. Several genes that may be related to spore germination were screened, such as PAL (VDAG_05831), cytochrome P450 2C31 (VDAG_05830) and abscisic acid ABA receptor (VDAG_02498).
Data Availability
Data will be made available on request.
Abbreviations
- DEGs:
-
Differentially expressed genes
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- qRT-PCR:
-
Quantitative real-time PCR
- RNA-seq:
-
RNA sequencing
- GO:
-
Gene ontology
- CMC:
-
Carboxymethyl cellulose medium
- TAL:
-
Tyrosine ammonia lyase
- ABA:
-
Abscisic acid
- BGALs:
-
Beta-galactosidase
- PAL:
-
Phenylalanine ammonia-lyase
- PCoA:
-
Principal coordinate analysis
- FPKM:
-
Fragments Per Kilobase of exon model per Million mapped reads
- PDA:
-
Potato dextrose agar
- CETP:
-
Cholesteryl ester transfer protein
References
Awad NE, Kassem HA, Hamed MA, El-Naggar MAA, El-Feky AMM (2014) Bioassays guided isolation of compounds from Chaetomium globosum. J Mycol Med 24:35–42
Balotf S, Tegg RS, Nichols DS, Wilson CR (2021) Spore germination of the Obligate biotroph Spongospora subterranea: transcriptome analysis reveals germination associated genes. Front Microbiol 12:691877
Ban QY, Han Y, He YH, Jin MJ, Han SK, Suo JT, Rao JP (2018) Functional characterization of persimmon β-galactosidase gene DkGAL1 in tomato reveals cell wall modification related to fruit pipening and radicle elongation. Plant Sci 274:109–120
Bandoni RJ, Moore K, Subba Rao PV, Towers GH (1968) Phenylalanine and tyrosine ammonia-lyase activity in some Basidiomycetes. Phytochemistry 7:205–207
Camm EL, Towers GHN (1973) Review article: phenylalanine ammonia lyase. Phytochemistry 12:961–973
Cheng XX, Zhao LH, Klosterman SJ, Feng HJ, Feng ZL, Wei F, Shi YQ, Li ZF, Zhu HQ (2017) The endochitinase VDECH from Verticillium dahliae inhibits spore germination and activates plant defense responses. Plant Sci 259:12–23
Cresnar B, Petric S (2011) Cytochrome P450 enzymes in the fungal kingdom. BBA-Proteins Proteom 1814:29–35
Dague E, Alsteen D, Latgé JP, Dufrêne YF (2008) High-resolution cell surface dynamics of germination aspergillus fumigatus conidia. Biophys J 94:656–660
Darshan K, Aggarwal R, Bashyal BM, Singh J, Shanmugam V, Gurjar MS, Solanke AU (2020) Transcriptome profiling provides insights into potential antagonistic mechanisms involved in Chaetomium globosum against Bipolaris sorokiniana. Front Microbiol 11:578115
Geijer C, Pirkov I, Vongsangnak W, Ericsson A, Nielsen J, Krantz M, Hohmann S (2012) Time course gene expression profiling of yeast spore germination reveals a network of transcription factors orchestrating the global response. BMC Genomics 13:554
Han Q, Wu FL, Wang XN, Qi H, Shi L, Ren A, Liu QH, Zhao MW, Tang CM (2015) The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signaling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ Microbiol 17:1166–1188
Hernández-Nistal J, Martín I, Dopico B, Labrador E (2014) Coordinated action of β-galactosidases in the cell wall of embryonic axes during chickpea germination and seedling growth. Plant Biol 16:404–410
Hirai NN, Masahiko O, Koshimiza K (1986) The 1′, 4′-trans-diol of abscisic acid, a possible precursor of abscisic acid in Botrytis cinerea. Phytochemistry 25:1865–1868
Hou FY, Du TF, Qin Z, Xu T, Li XA, Dong SX, Ma DF, Li ZY, Wang QM, Zhang LM (2021) Genome-wide in Silico identification and expression analysis of Beta-galactosidase family members in sweetpotato [Ipomoea batatas (L.) Lam]. BMC Genomics 22:140
Huang XZ, Zhang XY, Gong ZZ, Yang SH, Shi YT (2017) ABI4 represses the expression of type-a ARRs to inhibit seed germination in Arabidopsis. Plant J 89:354–365
Isobe K, Yamashita M, Chiba S, Takahashi N, Koyama T (2013) Characterization of new β-galactosidase from acidophilic fungus, Teratosphaeria acidotherma AIU BGA-1. J Biosci Bioeng 116:293–297
Jiang CJ, Shimono M, Sugano S, Kojima M, Yazawa K, Yoshida R, Inoue H, Hayashi N, Sakakibara H, Takatsuji H (2010) Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice-magnaporthe grisea interaction. Mol Plant-Microbe Interact 23:791–798
Kim J, Park H, Han JG, Oh J, Choi HK, Kim SH, Sung GH (2015) Regulation of a phenylalanine ammonia lyase (BbPAL) by calmodulin in response to environmental changes in the entomopathogenic fungus Beauveria Bassiana. Environ Microbiol 17:4484–4494
Liu X, Yu F, Schnabel G, Wu J, Wang Z, Ma Z (2011) Paralogous cyp51 genes in Fusarium graminearum mediate differential sensitivity to sterol demethylation inhibitors. Fungal Genet Biol 48:113–123
Luo FF, Zhou HX, Zhou X, Xie XY, Li Y, Hu FL, Huang B (2020) The intermediates in branched-chain amino acid biosynthesis are indispensable for conidial germination of the insect-pathogenic fungus metarhizium robertsii.Appl Environ Microb86
Luo Y, Korza G, DeMarco AM, Kuipers OP, Li YQ, Setlow P (2021) Properties of spores of Bacillus subtilis with or without a transposon that decreases spore germination and increases spore wet heat resistance. J Appl Microbiol 131:2918–2928
Madbouly AK, Abdel-Aziz MS, Abdel-Wahhab MA (2017) Biosynthesis of nanosilver using Chaetomium globosum and its, application to control Fusarium wilt of tomato in the, greenhouse. IET Nanobiotechnol 11:702–708
Mazzola M, Freilich S (2017) Prospects for biological soilborne disease control: application of indigenous versus synthetic microbiomes. Phytopathology 107:256–263
Neill SJ, Horgan R, Walton DC, Lee TS (1982) The biosynthesis of abscisic acid in Cercospora rosicola. Phytochemistry 21:61–65
Neill SJ, Horgan R, Walton DC, Mercer CAM (1987) The metabolism of a-ionylidene compounds by Cercospora rosicola. Phytochemistry 26:2515–2519
Okamoto M, Hirai N, Koshimizu K (1988) Biosynthesis of abscisic acid from a-ionylideneethanol in Cercospora pini-densiflorae. Phytochemistry 27:3465–3469
Oritani T, Ichimura M, Yamashita K (1984) A novel abscisic acid analog, (+)-(2Z,4E)-5-(l′,4′-Dihydroxy-6′,6′-dimethyl-2′-methylene-cyclohexyl)-3-methyl-2,4-pentadienoic acid, from Cercospora cruenta. Agric Biol Chem 48:1677–1678
Oritani T, Kiyota H (2003) Biosynthesis and metabolism of abscisic acid and related compounds. Nat Prod Rep 20:414–425
Park AR, Oh DK (2009) Galacto-oligosaccharide production using microbial β-galactosidase: current state and perspectives. Appl Microbiol Biot 85:1279–1286
Park JH, Choi GJ, Jang KS, Lim HK, Kim HT, Cho KY, Kim JC (2005) Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol Lett 252:309–313
Peñalva MA, Tilburn J, Bignell E, Arst HN (2008) Ambient pH gene regulation in fungi: making connections. Trends Microbiol 16:291–300
Shaban M, Miao Y, Ullah A, Khan AQ, Menghwar H, Khan AH, Ahmed MM, Tabassum MA, Zhu L (2018) Physiological and molecular mechanism of defense in cotton against Verticillium dahliae. Plant Physiol Biochem 125:193–204
Shanthiyaa V, Saravanakumar D, Rajendran L, Karthikeyan G, Prabakar K, Raguchander T (2013) Use of Chaetomium globosum for biocontrol of potato late blight disease. Crop Prot 52:33–38
Shin J, Bui DC, Lee Y, Nam H, Jung S, Fang M, Kim JC, Lee T, Kim H, Ja Choi G et al (2017) Functional characterization of cytochrome P450 monooxygenases in the cereal head blight fungus fusarium graminearum. Environ Microbiol 19:2053–2067
Siewers V, Kokkelink L, Smedsgaard J, Tudzynski P (2006) Identification of an abscisic acid gene cluster in the grey mold Botrytis cinerea. Appl Environ Microbiol 72:4619–4626
Siewers V, Smedsgaard J, Tudzynski P (2004) The P450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Appl Environ Microbiol 70:3868–3876
Song R, Li J, Xie C, Jian W, Yang X (2020) An overview of the molecular genetics of plant resistance to the Verticillium wilt pathogen verticillium dahliae. Int J Mol Sci 21:1120
Spence C, Bais H (2015b) Role of plant growth regulators as chemical signals in plant-microbe interactions: a double edged sword. Curr Opin Plant Biol 27:52–58
Spence CA, Lakshmanan V, Donofrio N, Bais HP (2015a) Crucial roles of abscisic acid biogenesis in virulence of rice blast fungus Magnaporthe oryzae. Front Plant Sci 6:1082
Spurrier EC (1990) Pesticides-there will be change. Plant Dis 74:103–110
Takahashi M, Koyama K, Natori S (1990) Four new azaphilones from Chaetomium globosum var. Flavo-viridae. Chem Pharm Bull 38:625–628
Tatjana PB, Amaya VB, Teresa MM, Erich G, Michael R, Jonathan G, Thomas R (2015) Sustained exposure to abscisic acid enhances the colonization potential of the mutualist fungus Piriformospora indica on Arabidopsis thaliana roots. New Phytol 208:873–886
Tomoda H, Matsushima C, Tabata N, Namatame I, Tanaka H, Bamberger MJ, Arai H, Fukazawa M, Inoue K, Omura S (1999) Structure-specific inhibition of cholesterylester transfer protein by azaphilones. J Antibiot 52:160–170
Traag BA, Ramirez-Peralta A, Wang Erickson AF, Setlow P, Losick R (2013) A novel RNA polymerase-binding protein controlling genes involved in spore germination in Bacillus subtilis. Mol Microbiol 89:113–122
van Leeuwen MR, Krijgsheld P, Bleichrodt R, Menke H, Stam H, Stark J, Wösten HAB, Dijksterhuis J (2013) Germination of conidia of Aspergillus niger is accompanied by major change in RNA profile. Stud Mycol 74:59–70
Vance CP, Bandoni RJ, Towers GHN (1975) Further observations on phenylalanine ammonia-lyase in fungi. Phytochemistry 14:1513–1514
Wei F, Feng HJ, Zhang DZ, Feng ZL, Zhao LH, Zhang YL, Deakin G, Peng J, Zhu HQ, Xu XM (2021) Composition of rhizosphere microbial communities associated with healthy and Verticillium wilt diseased cotton plants. Front Microbiol 12:618169
Xu J, Wang XY, Guo WZ (2015) The cytochrome P450 superfamily: key players in plant development and defense. J Integr Agr 14:1673–1686
Yan W, Cao LL, Zhang YY, Zhao R, Zhao SS, Khan B, Ye YH (2018) New metabolites from endophytic fungus Chaetomium globosum CDW7. Molecules 23:2873
Young MR, Towers GH, Neish AC (2011) Taxonomic distribution of ammonia-lyases for L-phenylalanine and L-tyrosine in relation to lignification. Can J Bot 44:341–349
Yu DM, Fang YL, Tang C, Klosterman SJ, Tian CM, Wang YL (2019) Genomewide transcriptome profiles reveal how Bacillus Subtilis lipopeptides inhibit microsclerotia formation in Verticillium dahliae. Mol Plant-Microbe In 32:622–634
Yue HM, Wang M, Gong WF, Zhang LQ (2018) The screening and identification of the biological control fungi Chaetomium spp. against wheat common root rot. FEMS Microbiol Lett 365:fny242
Zhang XJ, Zhao LH, Liu SC, Zhou JL, Wu YJ, Feng ZL, Zhang YL, Zhu HQ, Wei F, Feng HJ (2022) Identification and functional analysis of a novel hydrophobic protein VdHP1 from Verticillium dahliae. Microbiol Spectr 10:E0247821
Zhang Y, Zhu HQ, Ye YH, Tang CM (2021) Antifungal activity of chaetoviridin A from Chaetomium globosum CEF-082 metabolites against Verticillium dahliae in cotton. Mol Plant-Microbe Interact 34:758–769
Zhao SS, Zhang YY, Yan W, Cao LL, Xiao Y, Ye YH (2017) Chaetomium globosum CDW7, a potential biological control strain and its antifungal metabolites. FEMS Microbiol Lett 364:fnw287
Zhou T, Wang XH, Luo J, Ye BS, Zhou YY, Zhou LW, Lai TF (2018) Identification of differentially expressed genes involved in spore germination of Penicillium expansum by comparative transcriptome and proteome approaches. MicrobiologyOpen 7:E00562-N/a.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was supported by Central Guidance of Local Science and Technology Development Special Foundation (YDZX2022077), and Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2022E06).
Author information
Authors and Affiliations
Contributions
ZY and YYX designed the experiment; ZY, YYX and WAY performed the experiment; XC analyzed the data; ZY wrote the manuscript, ZM and ZJH revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Conflict of interest
The authors declare that they have no competing interests, any financial or personal relationships that may be perceived as influencing their work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Yang, Y., Wang, A. et al. Transcriptome reveals the molecular mechanism of chaetoviridin A inhibiting the spore germination of Verticillium dahliae. J Plant Pathol 105, 767–779 (2023). https://doi.org/10.1007/s42161-023-01344-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-023-01344-x