Abstract
Italy is the second largest hazelnut producer worldwide and Piedmont is one of the most productive regions in the country. The changing climatic condition and fungal trunk diseases (FTD) can have a severe impact on this crop. Particularly, the considerable spread of Cytospora cankers (‘Mal dello stacco’) and dieback represent a serious concern for producers. Thus, considering the limited studies on the causal agents, different surveys were conducted in seven hazelnut orchards during 2021 and 2022. Eight fungal species were identified: Anthostoma decipiens, Botryosphaeria dothidea, Diaporthe eres, Dia. rudis, Diplodia seriata, Dip. subglobosa, Dothiorella parva and Nothophoma brennandiae. Species identification was achieved through multilocus phylogeny and morphology assessment. All the fungal species were pathogenic on healthy hazelnut plants (cultivar Tonda Gentile) and A. decipiens and Dia. eres were the most aggressive. The present study is the first report of B. dothidea and Dia. eres as causal agents of FTD on hazelnut in Italy and of Dia. rudis, Dip. subglobosa and N. brennandiae worldwide. Moreover, the study provides clarification of the fungal pathogens associated with FTD on this crop in Piedmont, thus laying the base for further studies on epidemiology, ecology and management strategies.
Similar content being viewed by others
Introduction
Hazelnut (Corylus avellana L.) is a perennial bushy plant belonging to Betulaceae native to Europe and western Asia. It represents one of the most economically important nut crops worldwide. The Mediterranean and Black sea areas are historical sites of hazelnut cultivation with Turkey covering more than 60% of the world’s production, followed by Italy, USA, Azerbaijan, Georgia, Chile, China, Iran, France and Spain (FAOSTAT 2023). In Italy, 84,526 ha are cultivated with hazelnut and 98,666 tonnes of fruit were harvested in 2022 (ISTAT 2023). Italian hazelnut production has always been present in few geographical areas that are specialized for their environmental and climatic conditions, for the developed technical knowledge and socio-economic context and for the adoption of high quality cultivars (Botta and Valentini 2018). The regions with the highest production are Piedmont (30.5%), Latium (28.7%), Campania (22.5%) and Sicily (12.8%). In Piedmont, hazelnut cultivation covers more than 27,000 ha and the majority (16,558 ha) is located in the Cuneo province (ISTAT 2023). In this region, hazelnut production is mainly based on the cultivar Tonda Gentile (synonyms: ‘Tonda Gentile delle Langhe’, ‘Tonda trilobata’). This cultivar is recognized as one of the best hazelnut cultivar worldwide for its taste and aroma appreciated for fresh consumption and, in particular, for the industry, due to the presence of major international companies specialized in confectionery production (Silvestri et al. 2021). During the last decade, a general expansion in terms of acreage (from 14,375 in 2013 to 24,701 ha in 2022) and yield (from 23,797 in 2013 to 30,180 tons in 2022) in hazelnut cultivation was observed in this region (ISTAT 2023). However, the trend in nut production is highly influenced by climatic conditions (An et al. 2020). In the last 2 years, a slight decrease was observed probably due to water deficiency, late-spring frosts and hot summer temperatures. Moreover, a considerable spread of cankers and twig dieback symptoms was observed in hazelnut orchards in Piedmont increasing the concern of the producers.
Cankers, blight, dieback and decay affecting trunk, branches, twigs and, in severe cases, the entire plant, represent a serious threat for hazelnut worldwide (Teviotdale et al. 2002). In the present study, we will include under the general name of fungal trunk diseases (FTD) the diseases caused by fungal pathogens characterized by the mentioned symptoms and that were reported in the main hazelnut growing countries across the world. In the USA, the most important hazelnut growing area is the Willamette valley in Oregon, where the eastern filbert blight (EFB) caused by Anisogramma anomala represented a severe problem (Pinkerton 1992). The adoption of resistant cultivars provided an effective strategy to control EFB disease (Johnson et al. 1996). However, the emergence of previously unknown hazelnut disease is observed and previously unassociated fungal pathogens, such as Diplodia mutila, Diaporthe eres, Dothiorella omnivora and Valsa cf. eucalypti were isolated from cankers on trunks and branches (Wiman et al. 2019). In Chile, Diplodia coryli, Dip. mutila and Diaporthe australafricana were reported as causal agent of hazelnut dieback and cankers (Guerrero and Pérez 2013a, b; Guerrero et al. 2014). Dothiorella parva was isolated from Corylus avellana in Iran and described as a new species (Abdollahzadeh et al. 2014). Studies conducted in the Guilan province, the main cultivation area of Iran, reported Botryosphaeria dothidea and Diplodia theobromae as pathogen on hazelnut plants and Dia. amygdali as causal agent of hazelnut trees decline (Mir Hosseini Moghaddam and Taherzadeh 2007; Mohammadi and Jabbari Firoozjah 2019; Ghasemi-Doodaran and Davari 2020). Recently, Cytospora sp., Phomopsis sp., Lasiodiplodia sp. and Pestalotiopsis sp. were found in association with dieback of hazelnut in the same region (Houshyarfard 2020). In Turkey, Botryosphaeria dothidea was reported as pathogen on hazelnut plants (Polat et al. 2022). Moreover, recent studies conducted on Corylus heterophylla in China found Dia. eres, Dia. corylicola, Dia. donglingensis and Dia. huairouensis as FTD causal agents (Gao et al. 2021; Bai et al. 2022). In Italy, different studies were carried out in the main hazelnut growing areas across the peninsula. The presence of Cytospora canker, caused by Cytospora corylicola and known as ‘Mal dello stacco’, was reported throughout the most relevant production regions with a significant yield decrease in Campania (Botta and Valentini 2018). Although the spread of C. corylicola, there is a lack of information about the biology and phylogeny of this species and its pathogenicity was long debated (Scortichini 2006; Lamichhane et al. 2014). In Piedmont, sporadic branch cankers were observed in hazelnut orchards and the causal agent were identified as belonging to the genera Phomopsis and Sphaeropsis (Botta and Valentini 2018). A recent study was conducted in Sardinia on twigs and branches of hazelnut trees with exudates and cankers and different fungal species were isolated form symptomatic plant materials and identified: Dip. sapinea, Dip. seriata, Dothiorella iberica, Do. omnivora, Do. parva, Do. symphoricarposicola, Diaporthella cryptica, Gnomoniopsis smithogilvyi and Anthostoma decipiens (Linaldeddu et al. 2016). The same study provided a clarification on the etiology of Cytospora canker on hazelnut caused by A. decipiens (Linaldeddu et al. 2016).
In Piedmont, the knowledge on FTD of hazelnut is still limited and no studies were recently conducted to investigate the etiology and epidemiology of this disease. Thus, considering the diversity of the symptoms observed in the field and the relevant economic value of this crop, this study was conducted with the aim to investigate the etiology of hazelnut FTD in this region, in detail: (i) to identify the fungal species in association with FTD of hazelnut trees using molecular tools and phylogeny, (ii) to assess the morphological features of the identified species and (iii) to test the pathogenicity of the species found and to fulfil Koch’s postulates.
Materials and methods
Field survey and fungal isolation
Field surveys were conducted from March 2021 to September 2022 in seven hazelnut orchards in Piedmont (Table 1). Samples of trunks, branches and twigs were collected from symptomatic plants of ‘Tonda Gentile’ showing Cytospora cankers (‘Mal dello stacco’) and dieback symptoms. Wood samples (5–10 mm) were surface sterilised in 1% sodium hypochlorite for 1 min, rinsed in sterile distilled water (SDW) for 1 min and dried on sterile absorbent paper. Small fragments (2–3 mm) were cut from the edge between healthy and necrotic tissues and plated on potato dextrose agar (PDA, VWR Chemicals, Leuven, Belgium) amended with 25 mg l−1 of streptomycin sulphate (PDA-S, AppliChem GmbH, Darmstadt, Germany). The plates were incubated at 25 ± 1 °C under a 12 h photoperiod. Following 48 to 72 h of incubation, mycelial plugs were taken from the margin of developing colony and placed on new PDA-S and water agar (WA, Microbiol Diagnostici, Cagliari, Italy) plates. After 4 to 5 days, pure cultures were established by transferring single hyphal tips on new PDA-S plates. A total of 35 isolates were obtained and used for characterization. Stock cultures of these isolates are kept at −80 °C in the culture collection of the University of Torino, Italy.
DNA extraction, polymerase chain reaction (PCR) amplification, and sequencing
Total genomic DNA was extracted from 0.1 g of mycelium grown on PDA-S, using the E.Z.N.A. Fungal DNA Mini Kit (Omega Bio-Tek), following the manufacturer’s instructions. Four different genomic loci were amplified and sequenced for species identification. The nuclear ribosomal internal transcribed spacer (ITS) region of each isolate was amplified using the universal primers ITS1 and ITS4 (White et al. 1990). The primers EF1-728F and EF1-986R (Carbone and Kohn 1999) were used to amplify partial region of translation elongation factor-1α (tef1) in isolates of Botryosphaeriaceae and Diaporthe species (Guarnaccia et al. 2020, 2022a; Aiello et al. 2022). The partial β-tubulin (tub2) gene was amplified with primers T1–Bt2b (Glass and Donaldson 1995; O’Donnell and Cigelnik 1997) for isolates belonging to Botryosphaeriaceae family and Diaporthe genus (Guarnaccia et al. 2020, 2022a), whilst primers Tub2fd–Tub4fd (Woudenberg et al. 2009) were used to amplify the same region in isolates of the genus Nothophoma (Chen et al. 2015). The partial RNA polymerase second largest subunit (rpb2) gene was amplified with the primers: Rpb2-5f2–Rpb2-7cr (Liu et al. 1999; Reeb et al. 2004) for isolates identified as Nothophoma sp. (Chen et al. 2015). PCR mixtures and conditions were followed as described in the above-cited references. PCR amplification was examined by electrophoresis on 1% agarose (VWR Life Science AMRESCO® biochemicals) gels stained with GelRedTM. Eurofins Genomics Service (Cologne, Germany) sequenced PCR products in both directions. The DNA sequences generated were analysed and consensus sequences were computed using the program Geneious v. 11.1.5 (Auckland, New Zealand).
Phylogenetic analyses
The sequences obtained were blasted against the NCBI’s GenBank nucleotide database to determine the closest relatives for a taxonomic framework of the studied isolates. Alignments of different genomic regions, including sequences obtained from this study and sequences downloaded from GenBank, were initially performed with the MAFFT v. 7 online server (http://mafft.cbrc.jp/alignment/server/index.html) (Katoh and Standley 2013), and then manually adjusted in MEGA v. 7 (Kumar et al. 2016). Analyses were conducted individually for each locus (data not shown) and as multilocus sequence analyses using the following loci combinations: ITS, tub2 and tef1 for members of Botryosphaeriaceae and Diaporthe spp. (Guarnaccia et al. 2020; Zhang et al. 2021) and ITS, tub2 and rpb2 for Nothophoma spp. (Chen et al. 2015). For isolates belonging to Anthostoma a single locus analysis was performed on ITS region (Linaldeddu et al. 2016). Lasiodiplodia theobromae (CBS 164.96) (Zhang et al. 2021) was used as outgroup for species belonging to Botryosphaeriaceae. Diaporthella corylina (CBS 121124) (Guarnaccia et al. 2020) was selected as outgroup for Diaporthe spp. Allophoma minor (CBS 325.82) (Chen et al. 2015) was used as outgroup for Nothophoma spp. and Cryptovalsa ampelina (STEU 8113) (Moyo et al. 2018) was selected as outgroup for Anthostoma sp. Phylogenies were based on Bayesian Inference (BI) and Maximum Parsimony (MP) analyses. Regarding BI, the best evolutionary model for each partition was determined with MrModeltest v. 2.3 (Nylander 2004) and incorporated in the analyses. MrBayes v. 3.2.5 (Ronquist et al. 2012) was used to generate phylogenetic trees under optimal criteria per partition. The Markov Chain Monte Carlo (MCMC) analysis used four chains and started from a random tree topology. The heating parameter was set with the value of 0.2 and trees were sampled every 1000 generations. Analyses stopped at the moment which the average standard deviation of split frequencies was below 0.01. The MP analyses were performed using PAUP (Phylogenetic Analysis Using Parsimony, v. 4.0b10) (Swofford 2003). Phylogenetic relationships were estimated by heuristic searches with 100 random addition sequences. Tree bisection-reconnection was used, with the branch swapping option set on ‘best trees’ only with all characters weighted equally and alignment gaps treated as fifth state. Tree length (TL), consistency index (CI), retention index (RI) and rescaled consistence index (RC) were calculated for parsimony and the bootstrap analyses (Hillis and Bull 1993) were based on 1000 replications.
Morphology
Based on molecular characterization, representative isolates were selected to assess their morphological features. Agar plugs (5-mm-diam) were taken from the edge of actively growing cultures and transferred to the center of 9-cm-diam Petri dishes containing PDA-S. Isolates belonging to Botryosphaeriaceae were transferred onto the centre of 9 cm diam Petri dishes containing 2% water agar supplemented with sterile pine needles (PNA) (Smith et al. 1996) to induce sporulation. Isolates belonging to Diaporthe were placed both on PNA and on malt extract agar (MEA; Merck, Darmstadt, Germany) to induce sporulation. Plates were then incubated at 25 ± 1 °C under a 12 h photoperiod. Colony characters were observed after 7 days and culture colours were determined according to Rayner (1970). Cultures were examined periodically for the development of conidiomata. Conidia characteristics were examined by mounting fungal structures in SDW and the length and width of 30 conidia were measured for each isolate using an optic microscope at ×40 magnification. The average and standard deviation were calculated.
Pathogenicity
The pathogenicity of representative isolates of the identified species was determined to fulfil Koch’s postulates. Two representative isolates of each species were used to inoculate 1-year-old healthy hazelnut plants of ‘Tonda Gentile’. Seven plants per fungal isolate were inoculated. For each plant, one inoculation point was considered. The inoculation area was surface disinfected with 70% ethanol solution. A sterile scalpel was used to remove the outer bark to expose the vascular tissues. Mycelium plugs (4 mm diam.) were taken from 7-days-old cultures on PDA-S and placed with the mycelium in contact with the internal plant tissues. The same number of plants were treated with sterile PDA-S plugs as controls. The inoculation points were sealed with Parafilm®. All the plants were placed in a completely randomized design under a shade canopy for 3 months. After this period, the bark was removed, and the internal lesion length was measured. Small wood pieces (2–3 mm) of symptomatic tissue from the margin of the lesions were surface disinfected and placed on PDA-S to re-isolate the inoculated fungal species to fulfill Koch’s postulate. The identification of the pathogens was confirmed by colony characteristics. The data obtained were tested for normality, homogeneity of variances, and residual patterns. Logarithmic transformation of the data was performed. ANOVA was conducted with lesion length as dependent variable and fungal isolates as independent variable. Treatment means of lesion length were compared according to Tukey’s HSD test at α = 0.05. The data analysis was conducted using SPSS software 26 (IBM Corporate).
Results
Field survey and fungal isolation
In all the surveyed orchards (Table 1), with the exception of orchard 3, dieback symptoms, observed with a variable disease incidence from 30 to 40%, seriously compromised entire portions of the tree canopy, mainly in the higher part (Fig. 1a-b). Longitudinal streaks were observed on the bark (Fig. 1c-d) and internal dark brown vascular necrosis were found looking at transversal section (Fig. 1e) and, after bark removal, at longitudinal section (Fig. 1f-g). In four out of seven surveyed orchards (orchard 1, 2, 3, and 6), symptoms of Cytospora canker, known as ‘Mal dello stacco’, were observed with a disease incidence from 10 to 20%. Typical reddish-brown irregular spots and elongated cankers on the bark were found leading, in severe cases, to the break of branches (Fig. 1h-i). Oozing conidia in a reddish-brown matrix were observed on the outer surface of the trunk in late summer (Fig. 1j). Tissues under the bark showed necrotic dark brown vascular discoloration (Fig. 1k). Both the described symptoms were observed more frequently on mature plants (>10 year-old). Fungal isolates obtained from symptomatic plant samples were grouped as Botryosphaeriaceae, Diaporthe, Cytospora-like and Phoma-like species according to their culture characteristics. The species recovered from each orchard are reported in Table 1.
Symptoms of FTD of hazelnut trees observed in the field. a, b Dieback symptoms affecting the higher part of the tree canopy. c, d Longitudinal streaks on the bark. e–g Internal dark brown vascular necrosis. h, i Cytospora canker (‘Mal dello stacco’) symptoms causing the break of branches. j Oozing conidia in a reddish-brown matrix. k Necrotic dark brown internal vascular discoloration
Phylogenetic analyses
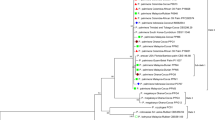
Sequences generated in this study are deposited in GenBank (Table 2). The combined-locus phylogeny of Botryosphaeriaceae consisted of 79 sequences, including the outgroup, and a total of 1444 characters (ITS: 1–549, tef1: 556–958 and tub2: 965–1444) was included. A total of 31 sequences, including the outgroup, was included in the Diaporthe phylogenetic analyses that consisted of 1690 characters (ITS: 1–612, tef1: 619–1036 and tub2: 1043–1690). The analyses conducted on the Nothophoma group consisted of 25 sequences, including the outgroup, with a total of 1437 characters (ITS: 1–493, tub2: 500–835 and rpb2: 842–1437). The single-locus phylogeny of Anthostoma based on ITS consisted of 28 taxa, including the outgroup, and it consisted of 573 characters. A maximum of 1000 equally most parsimonious trees were saved and characteristics of the combined gene partitions used for each phylogenetic analysis are reported in Table 3. Bootstrap support values from the Maximum Parsimony (MP) analysis were plotted on the Bayesian Inference (BI) phylogenies presented in Figs. 2 to 4. For the BI analyses, the models recommended by MrModeltest are reported in Table 4. Unique site patterns for each partition and all the parameters of the Bayesian analyses are reported in Table 3. In the Botryosphaeriaceae analyses, two isolates clustered with the epitype and one reference isolate of Botryosphaeria. dothidea, four isolates clustered with the epitype and four reference isolates of Diplodia seriata, two isolates clustered with the epitype and one reference isolate of Dip. subglobosa and two isolates clustered with the ex-type and two reference isolates of Dothiorella parva (Fig. 2). Regarding phylogenies of Diaporthe, two isolates clustered with five reference isolates of Dia. rudis, whilst four isolates clustered with four reference isolates of Dia. eres (Fig. 3). The final tree generated for Nothophoma showed that six isolated clustered with the ex-type and two reference isolates of N. brennandiae (Fig. 4). Thirteen isolates clustered with three representative sequences of A. decipiens (Fig. 5).
Consensus phylogram of 3462 trees resulting from a Bayesian analysis of the combined ITS, tef1 and tub2 sequences of Botryosphaeriaceae isolates. Bayesian posterior probability values and bootstrap support values are indicated at the nodes. The tree was rooted to Lasiodiplodia theobromae (CBS 164.96). Isolates from the current study are indicated in red
Consensus phylogram of 1922 trees resulting from a Bayesian analysis of the combined ITS, tub2 and rpb2 sequences of Nothophoma isolates. Bayesian posterior probability values and bootstrap support values are indicated at the nodes. The tree was rooted to Allophoma minor (CBS 325.82). Isolates from the current study are indicated in red
Consensus phylogram of 1002 trees resulting from a Bayesian analysis of the combined ITS, tef1 and tub2 sequences of Diaporthe isolates. Bayesian posterior probability values and bootstrap support values are indicated at the nodes. The tree was rooted to Diaporthella corylina (CBS 121124). Isolates from the current study are indicated in red
Consensus phylogram of 1732 trees resulting from a Bayesian analysis of the ITS sequences of Anthostoma isolates. Bayesian posterior probability values and bootstrap support values are indicated at the nodes. The tree was rooted to Cryptovalsa ampelina (STEU 8113). Isolates from the current study are indicated in red
Morphology
Morphological features, supported by phylogenetic inference, were observed and used to describe the eight known species (Fig. 6). Colony characters and colours were observed on plates of PDA-S. Botryosphaeriaceae isolates were characterized by the presence of abundant fast-growing aerial mycelium that covered the entire PDA-S petri dishes after 7 days. Botryosphaeria dothidea colonies were white to pale grey. Colony reverse color was pale grey. Conidia were hyaline, aseptate, thin-walled, fusiform to subclavate, with dimensions of 20.7–28.9 × 4.6–6.7 μm, mean ± SD = 24.8 ± 2 × 5.6 ± 0.6 μm. Colonies of Dip. seriata were light grey on the front side and light grey turning dark in the centre on the reverse side. Conidia were light brown, ovoid with truncated or rounded base and obtuse apex, aseptate, with dimensions of 20.2–26.8 × 8.2–11.2 μm, mean ± SD = 23 ± 1.6 × 9.5 ± 0.8 μm. Colonies of Dip. subglobosa were light grey turning dark grey in the center both on the obverse and reverse sides. Conidia were hyaline, aseptate, smooth, thick-walled, oblong to ovoid, straight, ends broadly rounded and measured 26.9–33.2 × 13.3–17.1 μm, mean ± SD = 30.2 ± 1.3 × 15.4 ± 0.9 μm. Dothiorella parva colonies were pale olivaceous grey on the obverse side and dull green- to dark olivaceous- grey on the reverse side. Conidia were ellipsoid to ovoid, brown, 1-septate, moderately thick-walled, ends rounded, often with a truncate base, with dimensions of 16.3–21.5 × 7.5–10.3 μm, mean ± SD = 20.1 ± 1 × 8.9 ± 0.7 μm. Isolates of Diaporthe showed fluffy aerial mycelium. Diaporthe eres colonies were white to pale grey on the front side and pale grey turning dark in the center on the reverse side. Alpha conidia were aseptate, hyaline, smooth, ovoid to ellipsoid, guttulate and measured 5.4–7.5 × 1.5–2.9 μm, mean ± SD = 6.3 ± 0.6 × 2.2 ± 0.3 μm. Beta conidia were hyaline, aseptate, smooth, spindle shaped, slightly curved, measuring 20.4–30.3 × 1.0–1.4 μm, mean ± SD = 25.1 ± 2.5 × 1.2 ± 0.1. Diaporthe rudis colonies were white to beige with a brownish halo around the margin on the front side and pale beige in the margin to buff honey in the center on the reverse side. Alpha conidia were hyaline, aseptate, smooth, biguttulate and ellipsoid with subtruncate bases and measured 4.2–6.9 × 1.4–2.6 μm, mean ± SD = 5.7 ± 0.7 × 1.9 ± 0.3 μm. Beta conidia were not observed. Colonies of N. brennandiae were characterized by cottony moderate aerial mycelium dark brick to sepia on the obverse side and dark brick to cinnamon on the reverse. Conidia were hyaline becoming brown, ellipsoidal to oblong, straight and measured 3.9–6.2 × 2–4.3 μm, mean ± SD = 5.1 ± 0.6 × 3.2 ± 0.5 μm. Anthostoma decipiens colonies showed cottony scarce aerial mycelium white to pale grey on the front side and honey in the margin to buff in the center on the reverse side. Conidia were hyaline, lunate and unicellular measuring 5.9–9.7 × 1.2–2.4 μm, mean ± SD = 7.4 ± 0.8 × 1.7 ± 0.3 μm.
Morphological characteristics of the different fungal species grown 7 days on PDA-S. a, b, c Botryosphaeria dothidea. d, e, f Diplodia seriata. g, h, i Diplodia subglobosa. j, k, l Dothiorella parva. m, n, o Diaporthe eres. p, q, r Diaporthe rudis. s, t, u Nothophoma brennandiae. v, w, x Anthostoma decipiens. Scale bar = (c, f, i, l) 20 µm. o, r, u, x 10 µm
Pathogenicity
All the tested isolates caused symptoms similar to those found in the field (Fig. 7). The plants inoculated with the fungal isolates showed external longitudinal streaks or dark brown lesions on the bark. Plants inoculated with representative isolates of A. decipiens were characterized by elongate canker development at the inoculation point. Internal necrosis and vascular discolouration were observed. Isolate CVG1374 of A. decipiens showed the highest lesion length (76.4 ± 22.6 mm), followed by A. decipiens isolate CVG1380 (lesion length = 76.4 ± 22.6 mm) and Dia. eres isolate CVG1334 (lesion length = 30.3 ± 7.3 mm). The values of mean lesion length for nine strains ranged from 13.5 ± 4.0 to 4.8 ± 1.8 mm for B. dothidea strain CVG2219 and Dia. rudis strain CVG1333, respectively, however their aggressiveness in hazelnut plants was not significantly different among them. The four remaining strains caused lesions which showed no significant differences among them, with lesion length ranging from 4.1 ± 0.7 to 2.5 ± 0.6 mm for Dip. subglobosa strain CVG1367 and Do. parva strain CVG1415 (Fig. 8). All the inoculated species were re-isolated and the morphological characteristics (color, shape, mycelium texture and conidia) were assessed to confirm their identity. Koch’s postulates were fulfilled. Weak necrosis observed on control plants (mean lesion length = 2.5 ± 0.6 mm) were restricted to the inoculation point and considered as a reaction to wounding. No fungal colonies were retrieved after re-isolation from control plants.
Internal lesion of hazelnut branches of cv. ‘Tonda Gentile’ at 3 months after inoculation with mycelial plugs of the species: a Anthostoma decipiens. b Botryosphaeria dothidea. c Diaporthe eres. d Dothiorella parva. e Diplodia seriata. f Nothophoma brennandiae. g Diaporthe rudis. h Diplodia subglobosa. i control
Disease severity (lesion length, mm) on hazelnut plants of ‘Tonda Gentile’ at 3 months after inoculation of representative fungal isolates of A. decipiens, B. dothidea, Dia. eres, Dia. rudis, Dip. seriata, Dip. subglobosa, Do. parva and N. brennandiae. Columns represent the mean data of seven replicate plants per strain. Columns with common letters do not differ significantly according to Tukey’s HSD test (P = 0.05) for lesion length. Mean comparison test was applied to log-transformed lesion length data. Horizontal lines on the columns are the standard error of the mean
Discussion
The present study represents the first investigation on the fungal species diversity in association with FTD of hazelnut in Piedmont. Currently, the spread of different FTD symptoms across this area is representing an increasing concern to hazelnut producers. The surveys, conducted in seven orchards, focused on sample collection from plants showing different symptoms, thus, to clarify the identification of the fungal species associated with the main two wood diseases reported. Isolates recovered from plants showing twig and branch dieback were grouped in Botryosphaeriaceae, Diaporthe spp. and Phoma-like spp. according to their colony morphology (Phillips et al. 2013; Udayanga et al. 2014; Chen et al. 2015). Isolates from plants showing symptoms of Cytospora canker, known also as ‘Mal dello stacco’, were grouped in Botryosphaeriaceae and Cytospora-like spp., based on their culture characteristic (Phillips et al. 2013; Lawrence et al. 2018). Sequencing of molecular loci and phylogenetic analyses allowed the identification of eight different species associated with FTD on hazelnut in Piedmont: Anthostoma decipiens, Botryosphaeria dothidea, Diaporthe eres, Dia. rudis, Diplodia seriata, Dip. subglobosa, Dothiorella parva and Nothophoma brennandiae. Particularly, Diaporthe spp., Diplodia spp., Do. parva and N. brennandiae were isolated in association with twig and branch dieback, whilst A. decipiens and B. dothidea were found in association with Cytospora canker (‘Mal dello stacco’). This study is also the first report of B. dothidea and Dia. eres as causal agents of FTD on hazelnut in Italy. Moreover, it is the first report of Dia. rudis, Dip. subglobosa and N. brennandiae causing twig and branch dieback on hazelnut trees worldwide. Species belonging to Botryosphaeriaceae family are well known as polyphagous pathogens for their wide distribution and virulence on multiple plant hosts (Batista et al. 2021). Botryosphaeria dothidea was previously reported on hazelnut trees in Iran and Turkey (Mohammadi and Jabbari Firoozjah 2019; Polat et al. 2022) and Dip. seriata was reported as wood pathogen on hazelnut in Sardinia (Linaldeddu et al. 2016). Hereby, B. dothidea was isolated in association with A. decipiens in the surveyed orchard n°6 (Monteu Roero-CN), whilst Dip. seriata was found in two different sites, orchard n°4 (Feisoglio-CN) and orchard n°7 (Monteu Roero-CN). Diplodia subglobosa was described as a new species in association with Fraxinus spp. and Lonicera nigra and it was recently reported on Fraxinus excelsior in Italy and Slovenia (Alves et al. 2014; Linaldeddu et al. 2020, 2022). In this study, this species was found in co-occurrence with A. decipiens in orchard n°2 (Diano d’Alba-CN). Dothiorella parva was isolated from Corylus avellana in Spain and reported as Dothiorella sp. (Phillips et al. 2008). This species was described as sp. nov. considering isolates from hazelnut in Iran and it was later reported as pathogen on Ostrya carpinifolia, a forest tree within the Betulaceae, in Slovenia and Italy (Abdollahzadeh et al. 2014; Pavlic-Zupanc et al. 2015). Recently, its name was proposed as a synonym of Do. sarmentorum (Zhang et al. 2021). In the present survey, it was found in co-occurrence with N. brennandiae in the orchard n°5 (Feisoglio-CN). All the Botryosphaeriaceae species found in the present study, B. dothidea, Dip. seriata, Dip. subglobosa and Do. parva, were pathogenic when inoculated on healthy hazelnut plants with a different virulence depending on both the tested species and isolates. Particularly, Dip. subglobosa showed minor symptoms with respect to the other tested isolates within Botryosphaeriaceae. The different virulence of the two isolates of Do. parva suggests the presence of intraspecific variability. Diaporthe spp. are included among the most globally relevant causal agents of FTD on different fruit crops (Lawrence et al. 2015; Guarnaccia et al. 2022b). Diaporthe eres was reported as wood pathogen on Corylus avellana in Oregon and Chile (Guerrero and Pérez 2013a; Wiman et al. 2019) and on Corylus heterophylla in China (Gao et al. 2021; Bai et al. 2022). Diaporthe rudis was reported on hazelnut only as causal agent of kernel defects, as well as Dia. eres (Pscheidt et al. 2019; Arciuolo et al. 2020, 2022). In this study, both species were found in co-occurrence with Botryosphaeriaceae in two out of seven orchards, particularly in orchard n°2 (Diano d’Alba-CN) and n°7 (Monteu Roero-CN). The pathogenicity test on these species showed a high virulence of one isolate of Dia. eres, whilst the other isolates caused minor lesions as both the isolates of Dia. rudis. The difference found suggests an intraspecific variability for pathogenicity in Dia. eres that was already reported for this species (Lawrence et al. 2015). Nothophoma brennandiae was originally isolated from Ulmus × hollandica in Italy and reported as N. quercina and then described as a new species isolated from garden soil in The Netherlands (Hou et al. 2020). It was found in co-occurrence with A. decipiens and Do. parva in orchard n°1 (Albaretto della Torre-CN) and orchard n°5 (Feisoglio-CN), respectively. This species caused minor symptoms when inoculated on hazelnut plants. The identification of A. decipiens represents the clarification of the causal agent of Cytospora canker (‘Mal dello stacco’) in the surveyed area and it is in line with the previous report in Sardinia (Linaldeddu et al. 2016). The doubts about the fungal species causing the Cytospora canker on hazelnut was long debated in Piedmont as well as in other Italian regions (Scortichini 2006; Botta and Valentini 2018). The cultural characteristics of A. decipiens, which have a high similarity with Cytospora spp. in terms of size and shape of conidia, have so far led the attribution of Cytospora canker to Cytospora corylicola or Cytospora spp. (Linaldeddu et al. 2016). Anthostoma decipiens was reported as pathogen on Carpinus betulus, another plant of the Betulaceae, in Italy (Saracchi et al. 2008; Rocchi et al. 2010) and Iran (Mirabolfathy et al. 2018). Moreover, it was found in association with grapevine trunk disease in Spain (Luque et al. 2012) and reported as pathogen of different plant hosts such as Alnus glutinosa, Betula pendula, Castanea sativa, Corylus avellana, Vagus sylvatica and Ostrya carpinifolia (Saracchi et al. 2015). In the present study, both isolates used in the pathogenicity test showed high virulence and caused the most severe lesions with respect to the other tested species. The ability of this pathogen to cause severe internal lesion with consequent cracks in the bark represents a serious threat to hazelnut, especially in late summer when conidia evasion occurs promoting the inoculum spread. Conidia of A. decipiens are thought to be dispersed through wind and rains as those of Botryosphaeriaceae and Diaporthe spp., representing a primary source of inoculum (Phillips et al. 2013; Lawrence et al. 2015). Pruning wounds, along with natural openings, are the main pathway for the colonization (Moral et al. 2019). The use of non-contaminated tools, the protection of wounds and the removal of pruning debris must be adopted. Proper agronomic practices contribute to avoid the inoculum spread, especially considering that several pathogens hereby found were reported in the same area on other fruit crops such as apple, grapevine and blueberry (Martino et al. 2022, 2023; Dardani et al. 2023). Additional surveys in more orchards as well as pathogenicity tests are planned, to investigate the incidence of the disease in North-western Italy as well as the infection rate caused by spore suspensions of each fungal pathogen. Moreover, the influence of inoculum density on the disease development will be assessed along with the seasonal dynamic of spore production, then to provide a correct pruning timing. Further studies on co-infection of different fungal species will be addressed to provide useful information on the complexity of FTD on hazelnut. Several fungal species found in the present study caused minor symptoms on healthy hazelnut plants, suggesting that they could act as secondary pathogens. Insights on the biology and ecology of these species are needed to establish effective disease management strategies. Moreover, understanding the interaction with abiotic stress and wood microbial community could contribute to the knowledge on FTD, promoting the high value of the cultivar Tonda Gentile produced in Piedmont to which the Protected Geographical Indication (PGI) was recognized by the European Union with the name ‘Nocciola Piemonte’ (‘Nocciola Piemonte delle Langhe’, from 2019).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
28 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s42161-024-01617-z
References
Abdollahzadeh J, Javadi A, Zare R, Phillips AJL (2014) A phylogenetic study of Dothiorella and Spencermartinsia species associated with woody plants in Iran, New Zealand, Portugal and Spain. Pers Mol Phylogeny Evol Fungi 32:1–12. https://doi.org/10.3767/003158514X678606
Aiello D, Guarnaccia V, Costanzo MB, Leonardi GR, Epifani F, Perrone G, Polizzi G (2022) Woody canker and shoot blight caused by Botryosphaeriaceae and Diaporthaceae on mango and litchi in Italy. Horticulturae 8:330
Alves A, Linaldeddu BT, Deidda A, Scanu B, Phillips AJL (2014) The complex of Diplodia species associated with Fraxinus and some other woody hosts in Italy and Portugal. Fungal Divers 67:143–156. https://doi.org/10.1007/s13225-014-0282-9
An N, Turp MT, Türkeş M, Kurnaz ML (2020) Mid-term impact of climate change on hazelnut yield. Agriculture 10:159. https://doi.org/10.3390/agriculture10050159
Arciuolo R, Chiusa G, Castello G, Leggieri MC, Spigolon N, Battilani P (2022) Diaporthe spp. is confirmed as the main fungus associated with defective Turkish hazelnuts. Plant Health Prog 23:440–448. https://doi.org/10.1094/PHP-01-22-0006-RS
Arciuolo R, Santos C, Soares C, Castello G, Spigolon N, Chiusa G, Lima N, Battilani P (2020) Molecular characterization of Diaporthe species associated with hazelnut defects. Front Plant Sci 11:611–655. https://doi.org/10.3389/fpls.2020.611655
Bai Y, Pan M, Gao H, Lin L, Tian C, Fan X (2022) Studies of Diaporthe species causing hazelnut canker disease in Beijing, China, with two new species described. Plant Pathol 71:1980–1991. https://doi.org/10.1111/ppa.13625
Batista E, Lopes A, Alves A (2021) What do we know about Botryosphaeriaceae? An overview of a worldwide cured dataset. Forests 12:313. https://doi.org/10.3390/f12030313
Botta R, Valentini N (2018) Il Nocciolo. Edagricole, Edizioni Agricole New Business Media srl, Milano
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556. https://doi.org/10.1080/00275514.1999.12061051
Chen Q, Jiang JR, Zhang GZ, Cai L, Crous PW (2015) Resolving the Phoma enigma. Stud Mycol 82:137–217. https://doi.org/10.1016/j.simyco.2015.10.003
Dardani G, Mugnai L, Bussotti S, Gullino ML, Guarnaccia V (2023) Grapevine dieback caused by Botryosphaeriaceae species, Paraconiothyrium brasiliense, Seimatosporium vitis-viniferae and Truncatella angustata in Piedmont: characterization and pathogenicity. Phytopathol Mediterr 62:293–316. https://doi.org/10.36253/phyto-14673
FAOSTAT (2023) https://www.fao.org/faostat/en/#data/QCL
Gao H, Pan M, Tian C, Fan X (2021) Cytospora and Diaporthe species associated with hazelnut canker and dieback in Beijing, China. Front Cell Infect Microbiol 11:664366. https://doi.org/10.3389/fcimb.2021.664366
Ghasemi-Doodaran S, Davari M (2020) Fungal diseases of hazelnut in Iran. Univ Yasouj Plant Pathol Sci 9:85–94
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Guarnaccia V, Martino I, Brondino L, Gullino ML (2022a) Paraconiothyrium fuckelii, Diaporthe eres and Neocosmospora parceramosa causing cane blight of red raspberry in Northern Italy. J Plant Pathol 104:683–698. https://doi.org/10.1007/s42161-022-01068-4
Guarnaccia V, Kraus C, Markakis E, Alves A, Armengol J, Eichmeier A, Compant S, Gramaje D (2022b) Fungal trunk diseases of fruit trees in Europe: pathogens, spread and future directions. Phytopathol Mediterr 61:563–599. https://doi.org/10.36253/phyto-14167
Guarnaccia V, Martino I, Tabone G, Brondino L, Gullino ML (2020) Fungal pathogens associated with stem blight and dieback of blueberry in northern Italy. Phytopathol Mediterr 59:229–245. https://doi.org/10.14601/Phyto-11278
Guerrero J, Pérez S (2013a) First report of Diaporthe australafricana - caused stem canker and dieback in European hazelnut (Corylus avellana L.) in Chile. Plant Dis 97:1657. https://doi.org/10.1094/PDIS-03-13-0286-PDN
Guerrero JA, Pérez SM (2013b) First report of shoot blight and canker caused by Diplodia coryli in hazelnut trees in Chile. Plant Dis 97:144. https://doi.org/10.1094/PDIS-07-12-0667-PDN
Guerrero JC, Pérez SF, Ferrada EQ, Cona LQ, Bensch ET (2014) Phytopathogens of hazelnut (Corylus avellana L.) in Southern Chile. Acta Hortic 1052:269–273. https://doi.org/10.17660/ActaHortic.2014.1052.36
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192. https://doi.org/10.1093/sysbio/42.2.182
Hou L, Hernández-Restrepo M, Groenewald JZ, Cai L, Crous PW (2020) Citizen science project reveals high diversity in Didymellaceae (Pleosporales, Dothideomycetes). MycoKeys 65:49–99. https://doi.org/10.3897/mycokeys.65.47704
Houshyarfard M (2020) Survey on etiology and distribution of dieback/decline of hazelnuts (Corylus avellana L.) in Northern Iran. J Nuts 11:245–256. https://doi.org/10.22034/jon.2020.1871078.1061
ISTAT (2023) https://www.istat.it/
Johnson KB, Mehlenbacher SA, Stone JK, Pscheidt JW, Pinkerton JN (1996) Eastern filbert blight of European hazelnut–it’s becoming a manageable disease. Plant Dis 80:1308–1316
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Lamichhane JR, Fabi A, Varvaro L (2014) Summer heat and low soil organic matter influence severity of hazelnut cytospora canker. Phytopathology 104:387–395. https://doi.org/10.1094/PHYTO-05-13-0136-R
Lawrence DP, Holland LA, Nouri MT, Travadon R, Abramians A, Michailides TJ, Trouillas FP (2018) Molecular phylogeny of Cytospora species associated with canker diseases of fruit and nut crops in California, with the descriptions of ten new species and one new combination. IMA Fungus 9:333–369. https://doi.org/10.5598/imafungus.2018.09.02.07
Lawrence DP, Travadon R, Baumgartner K (2015) Diversity of Diaporthe species associated with wood cankers of fruit and nut crops in northern California. Mycologia 107:926–940. https://doi.org/10.3852/14-353
Linaldeddu BT, Bottecchia F, Bregant C, Maddau L, Montecchio L (2020) Diplodia fraxini and Diplodia subglobosa: the main species associated with cankers and dieback of Fraxinus excelsior in North-Eastern Italy. Forests 11:883. https://doi.org/10.3390/f11080883
Linaldeddu BT, Bregant C, Montecchio L, Brglez A, Piškur B, Ogris N (2022) First report of Diplodia fraxini and Diplodia subglobosa causing canker and dieback of Fraxinus excelsior in Slovenia. Plant Dis 106:26–29. https://doi.org/10.1094/PDIS-06-21-1204-SC
Linaldeddu BT, Deidda A, Scanu B, Franceschini A, Alves A, Abdollahzadeh J, Phillips AJL (2016) Phylogeny, morphology and pathogenicity of Botryosphaeriaceae, Diatrypaceae and Gnomoniaceae associated with branch diseases of hazelnut in Sardinia (Italy). EurJ Plant Pathol 146:259–279. https://doi.org/10.1007/s10658-016-0912-z
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol 16:1799–1808. https://doi.org/10.1093/oxfordjournals.molbev.a026092
Luque J, Garcia-Figueres F, Legorburu FJ, Muruamendiaraz A, Trouillas FP (2012) Species of Diatrypaceae associated with grapevine trunk diseases in Eastern Spain. Phytopathol Mediterr 51:528–540
Martino I, Tabone G, Giordano R, Gullino ML, Guarnaccia V (2022) First report of Diaporthe eres causing stem blight and dieback on highbush blueberry (Vaccinium corymbosum) in Italy. Plant Dis 107:1236. https://doi.org/10.1094/PDIS-07-22-1673-PDN
Martino I, Agustí-Brisach C, Nari L, Gullino ML, Guarnaccia V (2023) Characterization and pathogenicity of fungal species associated with dieback of apple trees in Northern Italy. Plant Dis. Published online. https://doi.org/10.1094/PDIS-04-23-0645-RE
Mir Hosseini Moghaddam SA, Taherzadeh M (2007) Isolated fungi from hazelnut, their damage and economic importance in Guilan province. Ijfrpr 5:96–98
Mirabolfathy M, Javadi A, Ashnaei SP (2018) The occurrence of Anthostoma decipiens, the causal agent of ‘Carpinus betulus decline’, in Northern Iran. New Dis Rep 37:20-20. https://doi.org/10.5197/j.2044-0588.2018.037.020
Mohammadi H, Jabbari Firoozjah M (2019) Branch canker of hazelnut trees caused by Botryosphaeria dothidea in Iran. Plant Prot (Sci J Agric) 42:1–15
Moral J, Morgan D, Trapero A, Michailides TJ (2019) ecology and epidemiology of diseases of nut crops and olives caused by Botryosphaeriaceae fungi in California and Spain. Plant Dis 103:1809–1827. https://doi.org/10.1094/PDIS-03-19-0622-FE
Moyo P, Mostert L, Spies CFJ, Damm U, Halleen F (2018) Diversity of Diatrypaceae species associated with dieback of grapevines in South Africa, with the description of Eutypa cremea sp. nov. Plant Dis 102:220–230. https://doi.org/10.1094/PDIS-05-17-0738-RE
Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 7:103–116. https://doi.org/10.1006/mpev.1996.0376
Pavlic-Zupanc D, Piškur B, Slippers B, Wingfield MJ, Jurc D (2015) Molecular and morphological characterization of Dothiorella species associated with dieback of Ostrya carpinifolia in Slovenia and Italy. Phytopathol Mediterr 54:222–231
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167. https://doi.org/10.3114/sim0021
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Pers Mol Phylogeny Evol Fungi 21:29–55. https://doi.org/10.3767/003158508X340742
Pinkerton JN (1992) Distribution and characteristics of the Eastern filbert blight epidemic in Western Oregon. Plant Dis 76:1179–1182. https://doi.org/10.1094/PD-76-1179
Polat Z, Gültekin MA, Palacıoğlu G, Bayraktar H (2022) First report of Botryosphaeria dothidea causing stem canker of hazelnut in Turkey. J Plant Pathol 104:467–467. https://doi.org/10.1007/s42161-021-01002-0
Pscheidt JW, Heckert S, Wiseman M, Jones L (2019) Fungi associated with and influence of moisture on development of kernel mold of hazelnut. Plant Dis 103:922–928. https://doi.org/10.1094/PDIS-09-18-1520-RE
Rayner RW (1970) A mycological colour chart. Kew, Commonwealth Mycological Institute & British Mycological Society, Kew, pp 34
Reeb V, Lutzoni F, Roux C (2004) Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol Phylogenet Evol 32:1036–1060. https://doi.org/10.1016/j.ympev.2004.04.012
Rocchi F, Quaroni S, Sardi P, Saracchi M (2010) Studies on Anthostoma decipiens involved in Carpinus betulus decline. J Plant Pathol 92:637–644
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Saracchi M, Rocchi F, Quaroni S (2008) Further studies on the etiological agents of Carpinus betulus decline. J Plant Pathol 90:S2–S453
Saracchi M, Sardi P, Kunova A, Cortesi P (2015) Potential host range of Anthostoma decipiens and Endothiella sp., agents of hornbeam blight. J Plant Pathol 97:93–97
Scortichini M (2006) Le principali avversità del nocciolo nel Lazio. Petria 16:31–44
Silvestri C, Bacchetta L, Bellincontro A, Cristofori V (2021) Advances in cultivar choice, hazelnut orchard management, and nut storage to enhance product quality and safety: an overview. J Sci Food Agric 101:27–43. https://doi.org/10.1002/jsfa.10557
Smith H, Wingfield MJ, Coutinho TA, Crous PW (1996) Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. S Afr J Bot 62:86–88. https://doi.org/10.1016/S0254-6299(15)30596-2
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods) v. 4.0b10. Sinauer Associates, Sunderland
Teviotdale BL, Michailides TJ, Pscheidt JW (2002) Compendium of nut crop diseases in temperate zones. APS Press, St Paul, MN
Udayanga D, Castlebury LA, Rossman AY, Hyde KD (2014) Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Pers Mol Phylogeny Evol Fungi 32:83–101. https://doi.org/10.3767/003158514X679984
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications, vol 18. Academic Press, San Diego, pp 315–322
Wiman NG, Webber JB, Wiseman M, Merlet L (2019) Identity and pathogenicity of some fungi associated with hazelnut (Corylus avellana L.) trunk cankers in Oregon. PLoS One 14:e0223500. https://doi.org/10.1371/journal.pone.0223500
Woudenberg JHC, Aveskamp MM, De Gruyter J, Spiers AG, Crous PW (2009) Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Pers Mol Phylogeny Evol Fungi 22:56–62. https://doi.org/10.3767/003158509X427808
Zhang W, Groenewald JZ, Lombard L, Schumacher RK, Phillips AJL, Crous PW (2021) Evaluating species in Botryosphaeriales. Pers Mol Phylogeny Evol Fungi 46:63–115. https://doi.org/10.3767/persoonia.2021.46.03
Acknowledgements
This study was supported by the project “Nocciola di qualità”. V.G. thanks Corilanga Agricultural Cooperative for the kind support with specimen collection, and Giulia Tabone for laboratory technical support. I.M. thanks BPER Banca S.p.A. for funding her PhD project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martino, I., Monchiero, M., Gullino, M. et al. Characterization and pathogenicity of fungal species associated with hazelnut trunk diseases in North-western Italy. J Plant Pathol (2024). https://doi.org/10.1007/s42161-024-01595-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-024-01595-2