Abstract
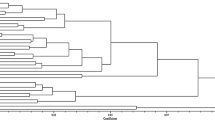
Twenty six isolates of genus Pantoea collected from leaves of rice and carrot (Experimental Farm, IARI, New Delhi), potato tuber (Hapur, Uttar Pradesh) and tomato leaves (Bengaluru, Karnataka) were characterized as Pantoea agglomerans by using morphological, physiological, biochemical methods and 16S rRNA nucleotide sequence analysis. Twenty six isolates of P. agglomerans, two reference strains of P. agglomerans (DWM-1and DWM-2) and three out group bacteria viz., Bacillus subtilis DTBS-5, B. cereus JHTBS-7, and Pseudomonas fluorescens DTPF-3 were taken to study genetic diversity by using BOX–PCR primers. The BOX primer produced 8–26 bands with mol. size 0.25 kb to 10 kb. DRP-16 and UPP-21 isolates had maximum number of fragments (15 fragments) followed by DRP-11, DRP-13 and DRP-14, UPP-18, UPP-19. Fifteen major groups at 75% similarity coefficient were obtained and high similarities were found among genetic profile of Delhi and Uttar Pradesh isolated strains, whereas the strains of Karnataka state separated in other groups. Out of 28 isolates of P. agglomerans, 12 showed antagonistic property against R. solanacearum in vitro conditions. Isolate DRP-7 showed highest inhibition zone (2.8 cm2) against pathogenic bacterium (R. solanacearum) followed by 2.5 cm2 in DRP-8 and UPP-20. Plant growth promoting activities of P. agglomerans isolates were tested in vitro situation. Majority of the isolates of P. agglomerans had good potentiality to solubilize the phosphorus, produce siderophores, indole acetic acid and ammonia. The result showed that the isolates DRP-7 and UPP-20 have both antagonistic and growth promoting ability, which is a good candidate to manage wilt disease and promote growth of tomato plants.



Similar content being viewed by others
References
Antoun H, Beauchamp CJ, Goussard N, Chabot R, Lalande R (1998) Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Plant Soil 204(1):57–67
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth-stimulation. Soil Biol Biochem 19(4):451–457
Brady SF, Wright SA, Lee JC, Sutton AE, Zumoff CH, Wodzinski RS, Beer SV, Clardy J (1999) Pantocin B, an antibiotic from Erwinia herbicola discovered by heterologous expression of cloned genes. J Am Chem Soc 121(50):11912–11913
Braun-Kiewnick A, Jacobsen BJ, Sands DC (2000) Biological control of Pseudomonas syringae pv. syringae, the causal agent of basal kernel blight of barley, by antagonistic Pantoea agglomerans. Phytopathology 90:368–375
Camlica E, Tozlu E (2019) Biological control of Alternaria solani in tomato. Fresenius Environ Bull 28(10):7092–7100
Cao Y, Hualiang P, Chandrangsu P, Yongtao L, Wang Y, Zhou H, Xiong H, John D, Helmann, Cai Y (2018) Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci Rep 8:4360. https://doi.org/10.1038/s41598-018-22782-z
Cappuccino JC, Sherman N (1992) Biochemical activities of microorganisms. In: Microbiology: a laboratory manual. Benjamin/Cummings Pub. Co., New York, pp 125–179
Dutkiewicz J, Mackiewicz B, Lemieszek MK, Marcin G, Milanowski J (2016) Pantoea agglomerans: a mysterious bacterium of evil and good. Part IV. Beneficial effects. Ann Agric Environ Med 23(2):206–222
Ewing WH, Fife MA (1972) Enterobacter agglomerans (Beijerinck) comb. nov. (the Herbicola-Lathyri bacteria). Int J Syst Bacteriol 22:4–11
Fernando WGD, Nakkeeran S, Zhang Y (2004) Ecofriendly methods in combating Sclerotinia sclerotiorum (Lib.) de Bary. Recent Res Deve Environ Biol 1:329–347
Fiske CH, Subbarow Y (1925) A colorimetric determination of phosphorus. J Biol Chem 66:375–400
Gavini F, Mergaert J, Bej A, Mielcarek LC, Daniel I, Kersters K, De Ley J (1989) Transfer of Enterobacter agglomerans (Beijerinck 1888) Ewing and Fife 1972 to Pantoea gen. nov. as Pantoea agglornerans comb. nov. and description of Pantoea dispersa sp. nov. Int J Syst Bacteriol 39(3):337–345
Gordon SA, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26(1):192
Gutiérrez-Mañero FJ, Ramos-Solano B, Probanza A, Mehouachi J, Tadeo RF, Talon M (2001) The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol Plant 111(2):206–211
Iimura K, Hosono A (1996) Biochemical characteristics of Enterobacter agglomerans and related strains found in buckwheat seeds. Inter J Food Microbiol 30(3):243–253
Ishimaru CA, Klos EJ, Brubaker RR (1988) Multiple antibiotic production by Erwinia herbicola. Phytopathology 78(6):746–750
Johnson KB, Stockwell VO, McLaughlin RJ, Sugar D, Loper JE, Roberts RG (1993) Effect of antagonistic bacteria on establishment of honey bee-dispersed Erwinia amylovora in pear blossoms and on fire blight control. Phytopathology 83:995–1002
Li JH, Wang ET, Chen WF, Chen WX (2008) Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biol Biochem 40(1):238–246
Manulis S, Gafni Y, Clark E, Zutra D, Ophir Y, Barash I (1991) Identification of a plasmid DNA probe for detection of strains of Erwinia herbicola pathogenic on Gypsophila paniculata. Phytopathology 81(1):54–57
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43(1):51–56
Miethling R, Wieland G, Backhaus H, Tebbe CC (2000) Variation of microbial rhizosphere communities in response to crop species, soil origin, and inoculation with Sinorhizobium meliloti L33. Microbial Ecol 40(1):43–56
Miller RL, Higgins VJ (1970) Association of cyanide with infection of birdfoot trefoil Stemphylium loti. Phytopathology 60:104–110
Mishra A, Mishra SK, Karmakar SK, Sarangi CR, Sahu GS (1995) Assessment of yield loss due to wilting in some popular tomato cultivars. Environ Ecol 13:287
Özaktan H, Bora T (2004) Biological control of fire blight in pear orchards with a formulation of Pantoea agglomerans strain Eh 24. Braz J Microbiol 35(3):224–229
Palazzini JM, Adriana MT, Chulze SN (2013) Biological control of Fusarium head blight of wheat: from selection to formulation. https://doi.org/10.1007/978-94-007-7091-1_12. © Springer Science+Business Media Dordrecht 2013, pp 191–2004
Pikovskaya RI (1948) Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiology 17:362–370
Pusey PL, Stockwell VO, Reardon CL, Smits TH, Duffy B (2011) Antibiosis activity of Pantoea agglomerans biocontrol strain E325 against Erwinia amylovora on apple flower stigmas. Phytopathology 101(10):1234–1241
Raafat KH, Hanan SA, Rabab AM (2015) Antibacterial activity of antagonistic bacteria and plant extract on Erwinia amylovora the pathogen of fire blight disease in Egypt. Int J Phytopathol 4:73–79
Raaska L, Viikari L, Mattila-Sandholm T (1993) Detection of siderophores in growing cultures of Pseudomonas spp. J Indu Microbiol 11(3):181–186
Rohlf FJ (2000) NTSYS-pc: numerical taxonomy and multivariate analysis system, version 2.1. Exeter Publishing Setauket, New York, p 43
Schaad NW, Jones JB, Chun W (2001) Laboratory guide for the identification of plant pathogenic bacteria, 3rd edn. American Phytopathological Society, St. Paul, p 373
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 60(1):47–56
Singh D, Sinha S, Yadav DK, Sharma JP, Srivastava DK, Lal HC, Mondal KK, Jaiswal RK (2010) Characterization of biovar/races of Ralstonia solanacearum, the incitant of bacterial wilt in solanaceous crops. Indian Phytopathol 63(3):261–265
Singh D, Yadav DK, Chaudhary G, Rana VS, Sharma RK (2016) Potential of Bacillus amyloliquefaciens for biocontrol of bacterial wilt of tomato incited by Ralstonia solanacearum. J Plant Pathol Microbiol 7:327
Sneath PHA, Sokal RP (1973) Numerical taxonomy: the principles and practice of numerical classification. WH Freeman and Company, San Francisco, p 573
Upadhyay A, Srivastava S (2010) Evaluation of multiple plant growth promoting traits of an isolate of Pseudomonas fluorescens strain Psd. Indian J Exp Biol 48(6):601–609
Vazquez P, Holguín G, Puente ME, Lopez-Cortes A, Bashan Y (2000) Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol Ferti Soils 30(5–6):460–468
Versalovic J, Koeuth T, Lupski JR (1991) Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19:6823–6831
Wattiau P, Renard ME, Ledent P, Debois V, Blackman G, Agathos S (2001) A PCR test to identify Bacillus subtilis and closely related species and its application to the monitoring of wastewater biotreatment. Appl Microbiol Biotechnol 56(5–6):816–819
Wilson M, Epton HAS, Sigee DC (1990) Biological control of fire blight of hawthorn (Crataegus monogyna) with Erwinia herbicola under protected conditions. Plant Pathol 39:301–308
Wodzinski RS, Umholtz TE, Rundle JR, Beer SV (1994) Mechanisms of inhibition of Erwinia amylovora by E. herbicola in vitro and in vivo. J Appl Bacteriol 76(1):22–29
Wright SAI, Beer SV (2005) Pantoea agglomerans, a biocontrol agent and ubiquitous microorganism—friend or foe? Biocontrol of bacterial plant diseases, 1st symposium, pp 334–338
Wright SAI, Zumoff CH, Schneider L, Beer SV (2001) Pantoea agglomerans strain EH318 produces two antibiotics that inhibit Erwinia amylovora in vitro. Appl Environ Microbiol 67(1):284–292
Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y (1996) Validation of the publication of new names and new combinations previously effectively published outside the IJBS. Int J Syst Bacteriol 46(2):625–626
Zhang L, Birch RG (1996) Biocontrol of sugarcane leaf scald disease by an isolate of Pantoea dispersa which detoxifies albicidin phytotoxins. Lett Appl Microbiol 22:132–136
Acknowledgements
The authors are grateful to Dr. Rashmi Aggarwal, Head, Division of Plant Pathology, ICAR- Indian Agricultural Research Institute, New Delhi for her continuous support and encouragement for conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, D., Yadav, D.K. & Fatima, F. Characterization and genetic diversity of Pantoea agglomerans isolates having dual potentiality to suppress growth of Ralstonia solanacearum and plant growth promoting ability. Indian Phytopathology 73, 643–653 (2020). https://doi.org/10.1007/s42360-020-00268-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-020-00268-1