Cortinarius subsalor and C. tibeticisalor spp. nov., two new species from the section Delibuti from China
- Published
- Accepted
- Received
- Academic Editor
- Sònia Garcia
- Subject Areas
- Biodiversity, Molecular Biology, Mycology, Taxonomy
- Keywords
- Agaricales, Biogeography, ITS, Myxacium, New taxon, Phylogeny, Taxonomy
- Copyright
- © 2021 Xie et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Cortinarius subsalor and C. tibeticisalor spp. nov., two new species from the section Delibuti from China. PeerJ 9:e11982 https://doi.org/10.7717/peerj.11982
Abstract
Cortinarius subsalor and C. tibeticisalor, belonging to the section Delibuti, are described from China as new to science. Cortinarius subsalor has been found to be associated with Lithocarpus trees in subtropical China and resembling C. salor, but it differs from the later by having slender basidiomata and the narrower basidiospores. Cortinarius tibeticisalor was collected from eastern Tibetan Plateau, associated with Abies. It differs from other species within sect. Delibuti by having olive tinge of mature or dried basidiomata and bigger basidiospores. The molecular data also support C. subsalor and C. tibeticisalor as new species. The phylogenetic analyses and biogeography of sect. Delibuti are discussed and a key to the species of this section currently known in the world is provided.
Introduction
Cortinarius (Pers.) Gray is an ectomycorrhizal fungal genus, associated with a wide host range of plants, such as Betulaceae, Caesalpiniaceae, Cistaceae, Dipterocarpaceae, Fagaceae, Myrtaceae, Pinaceae, Rhamnaceae, Rosaceae, Salicaceae and some herbaceous plants (Frøslev, Brandrud & Jeppesen, 2006; Niskanen, 2008). The genus is distributed worldwide with nearly 3,000 species (Niskanen et al., 2018; Ammirati et al., 2021; Bidaud et al., 2021). Even though it is the largest genus among macrofungi, its species diversity is still unclear. Most of Cortinarius species were originally discovered from Europe and America but rarely in Asia and Africa (Horak, 1983; Garrido-Benavent et al., 2020; Xie et al., 2020). Several systems of subgenus and sections in Cortinarius are erected based on the macromorphology of geographically limited samplings, but these are not supported by phylogenetic studies (e.g. Fries, 1838; Trog, 1844; Orton, 1955; Bidaud, Moënne-Loccoz & Reumaux, 1994; Garnica, Weiß & Oberwinkler, 2003; Garnica et al., 2005; Harrower et al., 2011; Stensrud et al., 2014; Niskanen et al., 2015; Garnica et al., 2016; Soop et al., 2019). For example, Garnica et al. (2005) proposed natural classification system in Cortinarius involving the taxonomic rearrangement of the species into eight informal clades. Soop et al. (2019) presented a section-based taxonomy of Cortinarius based on four loci of a large global sampling.
Cortinarius sect. Delibuti (Fr.) Sacc. with characteristics of viscid pileus and stipe, have usually been considered as a section in subg. Myxacium (Fr.) Trog (Trog, 1844; Earle, 1902; Orton, 1955; Brandrud et al., 1989; Consiglio, Antonini & Antonini, 2003). Delibuti species can easily be distinguished by the anomaloid appearances, mild taste and subglobose basidiospores from other myxacioid species (Orton, 1955; Soop, 2014). Section Delibuti was also considered to belong to subg. Phlegmacium (Fr.) Trog (Bidaud, Moënne-Loccoz & Reumaux, 1992; Bidaud, Moënne-Loccoz & Reumaux, 1994). Recently, Soop et al. (2019) treated sect. Delibuti among anomaloid sections, not in myxacioid sections based on the shared characters of sect. Delibuti and sect. Anomali Konrad & Maubl., together with support in the phylogenetic analyses. In the past, numeral species were assigned to section Delibuti (Fries, 1838; Earle, 1902; Bidaud, Moënne-Loccoz & Reumaux, 1992; Soop, 2013; Soop, 2014); however, most species have been confirmed not to belong to this section (Orton, 1955; Consiglio, 2012; Dima et al., 2016; Soop et al., 2019). Soop et al. (2019) defined only ten species in sect. Delibuti, but the phylogenetic studies showed that the species diversity of this section is still unrevealed (Harrower et al., 2011; Garnica et al., 2016; Soop et al., 2019).
In China, over 237 Cortinairus species, including several new species, have been described from China (Wei & Yao, 2013; Xie et al., 2019; Xie et al., 2020; Xie et al., 2021; Yuan et al., 2020; Luo & Bau, 2021). Four species within sect. Delibuti, C. betulinus J. Favre from Heilongjiang, C. delibutus Fr. from Heilongjiang, Jilin, Qinghai, Sichuan and Yunnan, C. illibatus Fr. from Ningxia, and C. salor Fr. from Heilongjiang, Jilin, Liaoning and Inner Mongolia, were reported (e.g. Teng, 1963; Yuan & Sun, 1995; Shao & Xiang, 1997; Li & Azbukina, 2011; Xie, 2018; Wang et al., 2020), but the occurrence of species in China is controversial due to the lack of voucher specimens.
In this study, we have conducted taxonomic and phylogenetic studies of Cortinairus in China. Some glutinously violet Cortinarius specimens resembling C. salor were found during the intensive field work but during the identification process they turned out to be new species which we describe here based on morphological and ecological characteristics, as well as phylogenetic analyses evidences. We also discuss the phylogenetic relationship and biogeography of sect. Delibuti. A key is provided the species of sect. Delibuti.
Materials & methods
Specimens and morphological description
Specimens were collected from Zhejiang Province and Tibet Autonomous Region, respectively. The collection sites in Zhejiang are the subtropical areas with the evergreen broadleaf forests dominated by Lithocarpus brevicaudatus. Meanwhile, the collection sites in Tibet are the plateau-alpine areas with coniferous forests dominated by Abies georgei var. smithii. Fresh basidiomata were photographed in the field. Dried specimens were deposited in the Herbarium of Mycology, Jilin Agricultural University (HMJAU), Changchun, China. Macroscopic characteristics were measured and recorded for every basidiomata and color codes followed Kornerup & Wanscher (1978). Microscopic features were examined and described in 5% KOH, Congo Red or Melzer’s reagent and observed using a Zeiss AX10 light microscope. Thirty to forty mature basidiospores were measured (excluding apiculus and ornamentation) per collection. Q = variation in the L/W ratios between the specimens studied. Xav. and Qav. = average value of basidiospores of per specimen.
Phylogenetic reconstruction
DNA extraction, PCR amplifications, and sequencing methods followed Xie et al. (2019) and Guan & Zhao (2020). The primers ITS1F and ITS4 were used amplification of nrDNA ITS region (White et al., 1990; Gardes & Bruns, 1993). The newly generated ITS sequences were submitted to GenBank. The ITS sequences for the phylogenetic analyses were selected based on results of BLASTn (>90% identity) in GenBank and UNITE and followed the publication by Garnica et al. (2016) and Soop et al. (2019). Two species in section Cyanites Nespiak were chosen as outgroup followed Xie et al. (2021).
Sequences (Table 1) for the phylogenetic analyses were aligned and edited with BioEdit 7.1.3.0 and Clustal X (Thompson et al., 1997; Hall, 1999). For phylogenetic analyses, Bayesian Inference (BI), Maximum Likelihood (ML) and Maximum Parsimony (MP) methods were implemented in this study. MrModeltest 2.3 was used to calculate the best model (HKY+I+G) for BI analysis (Nylander et al., 2008). The BI analysis was performed with MrBayes 3.2.6 (Ronquist & Huelsenbeck, 2003). Four Markov chains were run for 500,000 generations until the split deviation frequency value < 0.01, and sampled every 100th generation. The posterior probability values were estimated from the samples after discarding the first 25% (1,250) generations. A 50% majority rule consensus tree of all remaining trees were calculated. RAxML v. 1.5, implemented in raxmlGUI, were used to construct a ML tree, with a rapid bootstrapping algorithm involving 1,000 replicates (Silvestro & Michalak, 2012; Stamatakis, 2014). All parameters in the ML analysis were kept as defaults except for GTRGAMMA were chose as the model. The MP analysis was conducted in MEGA X (Kumar et al., 2018). The most parsimonious tree with length = 1,012 is shown. The consistency index is (0.442350), the retention index is (0.708912), and the composite index is 0.355860 (0.313587) for all sites and parsimony-informative sites (in parentheses). The bootstrap test was performed 1,000 replicates (Felsenstein, 1985). The MP tree was obtained using the Tree-Bisection-Regrafting (TBR) algorithm (Nei & Kumar, 2000) with search level 3 in which the initial trees were obtained by the random addition of sequences (10 replicates). The phylogenetic trees were visualized in FigTree 1.4.3. The Bayesian posterior probabilities values (BPP) ≥ 0.95, ML bootstrap values (ML) ≥ 75% or MP bootstrap values (MP) ≥ 75% are shown on the branches at the nodes (BPP/ML/MP).
| Species | Voucher | Locality | Accession No. | References |
|---|---|---|---|---|
| C. acutovelatus | F16388 (UBC) | Canada | FJ039609 | Harrower et al. (2011) |
| C. albocyaneus Epitype | CFP1177 (S) | Sweden, Jämtland | KX302206 | Dima et al. (2016) |
| C. alpinus | HMJAU44407 | China, Inner Mongolia | MW911727 | This study |
| C. anomalus Neotype | CFP1154 (S) | Sweden, Ångermanland | KX302224 | Dima et al. (2016) |
| C. basipurpureus | PERTH 04259629 | Australia | AY669607 | Garnica et al. (2005) |
| C. bolaris | TUB 0118524 | Germany | AY669596 | Garnica et al. (2005) |
| C. boreicyanites Holotype | CFP931 (S) | Sweden, Jämtland | NR130214 | Liimatainen et al. (2014) |
| C. calaisopus | 60224 (OTA) | New Zealand | MN846380 | GenBank |
| C. calaisopus Holotype | PDD 94050 | New Zealand, Dunedin | NR157880 | GenBank |
| C. camphoratus | DAVFP26155 | Canada | EU821659 | Harrower et al. (2011) |
| C. camphoratus | SMI193 | Canada | FJ039626 | Harrower et al. (2011) |
| C. carneoroseus | EN76 (CORD) | Argentina | JX983157 | GenBank |
| C. collinitus | IB 19940257 | Sweden | AY033096 | Peintner et al. (2002) |
| C. croceocoeruleus | TUB 011833 | Germany | AY669590 | Garnica et al. (2005) |
| C. cyanites Neotype | AT2005069 (UPS) | Sweden, Uppland | NR130233 | Liimatainen et al. (2014) |
| C. cypripedi Holotype | PDD 107723 | New Zealand, Otago | KT875199 | Soop (2016) |
| C. cystidiocatenatus | HO A20518A6 | Australia, Tasmania | AY669651 | Garnica et al. (2005) |
| C. delibutus | F17048 (UBC) | Canada | FJ717515 | Harrower et al. (2011) |
| C. delibutus | SAT01-301-12 | USA | FJ717513 | Harrower et al. (2011) |
| C. durifoliorum Holotype | PDD 101829 | New Zealand, Westland | KJ635210 | Soop, Wallace & Dima (2018) |
| C. eunomalus | PDD 107706 | New Zealand | KT875201 | GenBank |
| C. illibatus | HMJAU48760 | China, Heilongjiang | MW911735 | This study |
| C. illibatus | AT2004220 (UPS) | Sweden | UDB002173 | UNITE |
| C. illitus Holotype | IB 19630414 | Argentina | AF389128 | Peintner, Moncalvo & Vilgalys (2004) |
| C. illitus | MQ19-CMMF003109 | Canada, Quebec | MN751331 | GenBank |
| C. illuminus Neotype | F44877 (S) | Sweden | KP866156 | Niskanen et al. (2015) |
| C. khinganensis Holotype | HMJAU44507 | China, Inner Mongolia | MT299952 | Xie et al. (2021) |
| C. microglobisporus Holotype | IB 20110123 | Italy | NR153027 | Peintner et al. (2014) |
| C. obtusus | SAT00-298-30 | USA | FJ717550 | Harrower et al. (2011) |
| C. phlegmophorus | Typus-M3 | India | AY083186 | Peintner et al. (2003) |
| C. pluvius | HMJAU44391 | China, Inner Mongolia | MW911726 | This study |
| C. porphyroideus | 61406 (OTA) | New Zealand | JX178612 | Teasdale et al. (2013) |
| C. pseudocandelaris | F17165 OC93 (UBC) | Canada, BC | GQ159908 | Harrower et al. (2011) |
| C. psilomorphus Holotype | PDD 103885 | New Zealand | KF727393 | Soop (2016) |
| C. putorius Holotype | TN 07-411 (H) | USA | NR153038 | Ariyawansa et al. (2015) |
| C. pyrenaicus | JB-8573/15 | Spain, Gisclareny | KX239900 | Cadiñanos, Gomez & Ballarà (2016) |
| C. rattinoides Holotype | PDD 88283 | New Zealand | JX000375 | GenBank |
| C. rotundisporus | PERTH 05255074 | Australia | AY669612 | Garnica et al. (2005) |
| C. rotundisporus | G12 | Australia | AF136738 | Sawyer, Chambers & Cairney (1999) |
| C. salor | IB 19940297 | Austria | UDB001066 | Peintner et al. (2001) |
| C. salor | TUB 011838 | Germany | AY669592 | Garnica et al. (2005) |
| C. salor II | TUF106868 | Estonia | UDB011268 | UNITE |
| C. salor II | TAAM128516 | Estonia | UDB015945 | UNITE |
| C. septentrionalis | ARAN Fungi03516 | Sweden, Harjedalen | KX239915 | Cadiñanos, Gomez & Ballarà (2016) |
| C. spilomeus Neotype | TEB CFP1137 (S) | Sweden | KX302267 | Dima et al. (2016) |
| C. stillatitus | TUB 011587 | Germany | AY669589 | Garnica et al. (2005) |
| C. subsalor | HMJAU48758 | China, Zhejiang | MW911733 | This study |
| C. subsalor Holotype | HMJAU48759 | China, Zhejiang | MW911734 | This study |
| C. subsalor | MHHNU 30409 | China, Hunan | MK250915 | GenBank |
| C. suecicolor Holotype | PDD 74698 | New Zealand | JX000360 | GenBank |
| C. tabularis Epitype | CFP949 (S) | Sweden | KX302275 | Dima et al. (2016) |
| C. tasmacamphoratus | HO A20606A0 | Tasmania | AY669633 | Garnica et al. (2005) |
| C. tessiae | PDD 94054 | New Zealand, Dunedin | JQ287698 | GenBank |
| C. tessiae | PDD 72611 | New Zealand | HM060317 | GenBank |
| C. tibeticisalor | HMJAU48761 | China, Tibet | MW911731 | This study |
| C. tibeticisalor | HMJAU48762 | China, Tibet | MW911732 | This study |
| C. tibeticisalor | HMJAU48763 | China, Tibet | MW911730 | This study |
| C. tibeticisalor Holotype | HMJAU48764 | China, Tibet | MW911729 | This study |
| C. vanduzerensis | VMS28 | Canada | FJ717562 | Harrower et al. (2011) |
| C. vibratilis | IB 19970078 | USA | AF325584 | Peintner et al. (2001) |
| C. sp. | CSU CO 2476 | Colombia, Antioquia | MF599228 | GenBank |
| C. sp. | FLAS-F-60161 | USA | MF153022 | GenBank |
| C. sp. | YM714 | Japan, Hokkaido | LC175538 | GenBank |
| C. sp. | 1780 | Italy | JF907917 | Osmundson et al. (2013) |
| C. sp. | SWUBC500 | Canada | DQ481723 | Wright, Berch & Berbee (2009) |
| C. sp. | PDD 72685 | New Zealand | MH101524 | GenBank |
Note:
New species is in bold.
Nomenclature
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants, and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. In addition, new names contained in this work have been submitted to MycoBank from where they will be made available to the Global Names Index. The unique MycoBank number can be resolved and the associated information viewed through any standard web browser by appending the MycoBank number contained in this publication to the prefix “http://www.mycobank.org/MycoTaxo.aspx?Link=T&Rec=”. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central, and CLOCKSS.
Results
BLASTn results
The BLASTn against GenBank and UNITE databases taked the holotype specimens as the examples. The BLASTn results showed that these two new species distinct from other members of Cortinarius and close to sect. Delibuti. The ITS sequence of C. subsalor (MW911734, holotype) has 99% identity with C. salor (MK250915). Here we addressed it as C. subsalor. The percent identity of C. subsalor with C. salor s.l. (AY669592, UDB015945) and C. delibutus (FJ717515) are 96% and 95%, respectively. The ITS sequence of C. tibeticisalor (MW911729, holotype) has 93%, 91%, 90% identity with Cortinarius sp. (LC098750), C. delibutus (FJ717515) and C. tessiae (JQ287698), respectively.
Phylogenetic analyses
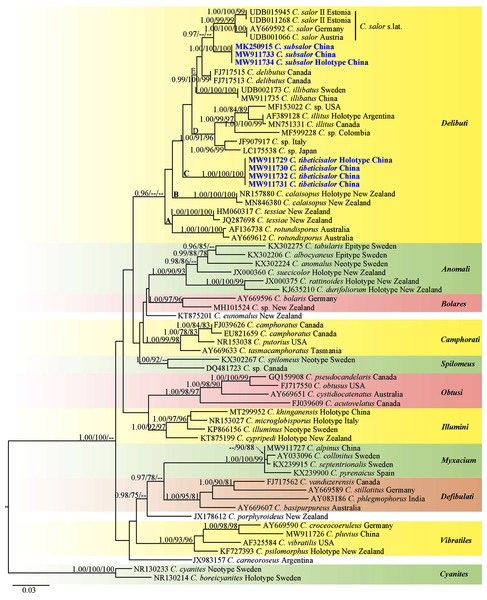
The matrix contained 66 ITS sequences with 767 nucleotide sites is available from TreeBASE under S28399 (https://www.treebase.org/treebase-web/search/study/summary.html?id=28399). The BI, ML and MP results showed similar topologies and ML tree was selected as the backbone phylogeny (Fig. 1). The phylogenetic analyses showed 11 sections including one singleton species from Argentina, and two singletons from New Zealand. Every section formed separate monophyletic lineages with strong statistical support. Section Delibuti formed a distinct clade (BPP = 0.96) separate from other sections. Section Delibuti split into five main clades based on the analyses of ITS sequences. Clade A and B consist of Australasian species. Clade C is a clade including our new species from the Tibetan Plateau. Clade D consist of the species distributed in Europe, Asia and North and South America. Clade E represents species in the Northern Hemisphere. Cortinarius subsalor (BPP/ML/MP = 1.00/100%/100%, clade E) and C. tibeticisalor (BPP/ML/MP = 1.00/100%/100%, clade C) formed a dinstinct lineages with high statistical support, respectively. Furthermore, C. subsalor formed a sister relationship with the European C. salor (BPP = 0.97, clade E).
Figure 1: ML phylogram inferred from nrDNA ITS sequence data.
The tree is rooted with sect. Cyanites. The Bayesian posterior probabilities (BPP) ≥ 0.95, ML bootstrap values (ML) ≥ 75% and MP bootstrap values (MP) ≥ 75% are shown on the branches (BPP/ML/MP). New species is marked by blue bold.Taxonomy
Cortinarius subsalor M.L. Xie, T.Z. Wei & Y. Li, sp. nov.
MycoBank No. MB839320
(Fig. 2)
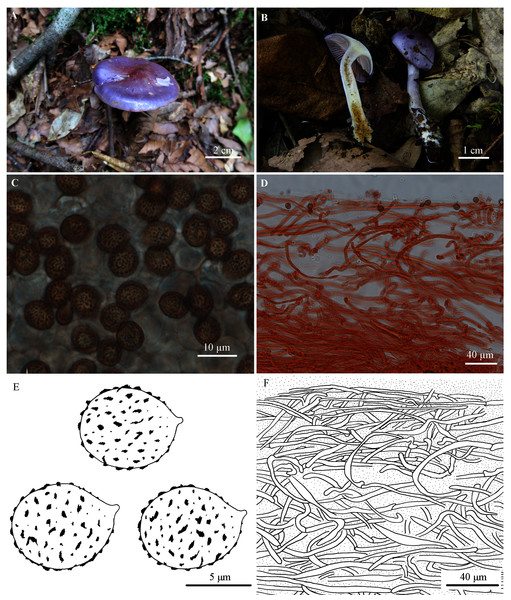
Figure 2: Cortinarius susalor.
(A) HMJAU48759 (Holotype). (B) HMJAU48758. (C, E) Basidiospores (HMJAU48759). (D, F) Pileipellis (HMJAU48759). (Photo credits: (A) Jun-Liang Chen; (B)–(F) Meng-Le Xie).Etymology. The name refers to its affinity to Cortinarius salor.
Holotype. CHINA. Zhejiang: Baishanzu Mountain, Qingyuan county, on moist soil under Lithocarpus brevicaudatus (Fagaceae) forest with scattered Theaceae and Rhododendron, 27°45′44″N, 119°11′50″E, ASL 1,510 m, 20 July 2020, Jun-Liang Chen, QY-0235(1192-1198) (HMJAU48759), GenBank: MW911734.
Diagnosis. Pileus hemispherical to plane, violet, glutinous; lamellae violet at first, then turning pale grayish violet; stipe slender, pale violet, then brown; glutinous veil violet. Basidiospores on average 8.0–8.3 × 6.9–7.0 µm, subglobose to broadly ellipsoid. Differing from other species in sect. Delituti by the violet color of basidiomata, the distribution of subtropical China and association with Lithocarpus brevicaudatus.
Description
Pileus 20–50 mm, hemispherical at first, then convex to applanate; bluish violet (18B6-18C7) at first, purple (15B6-15C7) to purplish red (14A6-14B7) at the centre, then grayish violet (17B4-17C5), pale violet (19A3) at the margin; surface glutinous. Lamellae emarginate; moderately crowded; violet (17B6) when young, then grayish violet (17B4-17C5) to pale grayish violet (15B1-15C2); edge almost even. Stipe slender, 35–65 mm long, 3–7 mm thick, clavate at base (up to 14 mm); pale violet to grayish violet (19A3-19B5), later whitish, lightly brown to brown (7D6-7E7); surface with viscid universal veil, basal mycelium white with bluish tinge. Universal veil viscid, violet, remnants forming a girdle on the upper part of the stipe, disappearing with age. Context whitish at the pileus, slightly with yellowish tinge at the center, pale violet tinge extend outward, hygrophanous near lamellae; white with pale violet tinge at the apex of the stipe, yellow at the lower part; somewhat hollow within stipe. Odor not significant, taste mild.
Basidiospores 7.7–9.5 (10.6) × 6.2–7.7 (8.7) µm, Q = 1.10–1.29 (holotype), Xav. = 8.0–8.3 × 6.9–7.0 µm, Qav. = 1.20, subglobose to broadly ellipsoid, moderately coarsely verrucose, moderately dextrinoid. Basidia 4-spored. Lamellar edges fertile. Pileipellis: epicutis strongly gelatinous, about 180–250 µm thick, with hyphae 2–7 µm wide, yellowish to colorless in 5% KOH, some hyphae with small encrusted granules. Hypodermium present; hypodermial hyphae 4–10 µm wide, cylindrical, almost colorless in 5% KOH, smooth. Clamp connections present.
Exsiccatae. Pileus grayish violet (19B3-19C4) at the margin, light brown to dark brown (6D6-6F8) at the centre; lamellae rust brown (6E8); stipe brown (6D7-6E7), lighter downwards, yellowish white (4A2) at base.
ITS sequence. The ITS sequence of the holotype is distinct from other members of sect. Delibuti and deviating from them by at least 22 substitutions and indel positions.
Ecology and distribution. In subtropical evergreen broadleaf forests, associated with Lithocarpus brevicaudatus (Fagaceae). Known from Zhejiang and Hunan province of China.
Additional specimens examined. CHINA. Zhejiang: Baishanzu Mountain, Qingyuan county, on moist soil under Lithocarpus brevicaudatus (Fagaceae) forest with scattered Theaceae and Rhododendron, 27°45′55″N, 119°11′0″E, ASL 1500 m, 20 August 2020, Meng-Le Xie, 20xml12101 (HMJAU48758), GenBank: MW911733.
Cortinarius tibeticisalor M.L. Xie, T.Z. Wei & Y. Li, sp. nov.
MycoBank No. MB839321
(Fig. 3)
Figure 3: Cortinarius tibeticisalor.
(A) HMJAU48764 (Holotype). (B) HMJAU48762. (C, E) Basidiospores (HMJAU48764). (D, F) Pileipellis (HMJAU48764). (Photo credits: Meng-Le Xie).Etymology. The name refers to the Tibetan Plateau, the type locality, and its similarity to C. salor.
Holotype. CHINA. Tibet Autonomous Region: Sejila Mountain, Linzhi city, on moist soil in Abies forest with scattered Rhododendron, 29°35′26″N, 94°35′53″E, ASL 4120 m, 5 September 2020, Meng-Le Xie, 20xml12416 (HMJAU48764), GenBank: MW911729.
Diagnosis. Pileus hemispherical to applanate, violet, glutinous, margin wavy, somewhat olive when mature; lamellae for a long time violet, then pale grayish violet to violet gray; stipe robust, bluish gray to brown with olive tinge; veil glutinous, violet. Basidiospores on average 10.3–10.8 × 8.7–8.9 µm, subglobose to broadly ellipsoid, rarely ellipsoid. Differing from other species in sect. Delituti by the olive tinge of basidiomata and the large basidiospores.
Description
Pileus 50–85 mm, hemispherical at first, then convex to plane, sometimes slightly depressed, wavy at margin of mature basidiomata, violet (17C7) at first, especially at the centre, paler violet towards the margin, then grayish orange (5B5) to brown (5D6-5E7) with olive tinge, dark at the centre; surface glutinous. Lamellae emarginate, moderately crowded, persistently violet (17C7), then grayish violet (19B4-19C6) to violet gray (19B2); edge uneven, slightly serrate. Stipe 85–120 mm long, 10–15 mm thick, clavate at base (up to 23 mm); surface with viscid bluish gray (19B2) universal veil remnants, then becoming yellow to brown with olive tinge (4B6-4D7), grayish violet (19B4-19C6) at the apex; basal mycelium white. Universal veil viscid, violet, remnants forming a girdle on the upper part of the stipe, dispearing with age. Context white with marbled violet tinge at first, slightly yellowish from the center of the pileus, then yellow at the stipe, especially at the middle. Odor weak when fresh, somewhat like honey when old or dry. Taste mild.
Basidiospores 9.7–10.9 (12.6) × 7.7–9.0 (10.0) µm, Q = 1.13–1.30 (holotype), Xav. = 10.3–10.8 × 8.7–8.9 µm, Qav. = 1.20–1.23, subglobose to broadly ellipsoid, rarely ellipsoid, moderately coarsely verrucose, weakly dextrinoid. Basidia 4-spored. Lamellar edges fertile, with narrow clavate cells. Pileipellis: epicutis strongly gelatinous, about 300–410 µm thick, hyphae 3–8 µm wide, with yellowish intracellular pigment in 5% KOH, smooth. Hypodermium present, hyphae 7–15 µm wide, irregular, almost colorless in 5% KOH, smooth. Clamp connections present.
Exsiccatae. Pileus olive brown (4E6-4F7) at margin, yellowish brown (5D7-5E8) at centre; lamellae dark bluish gray (19E2-19F2); stipe bluish white at apex, light brown (6D6-6E7) to dark brown (6F4-6F8), white at base.
ITS sequence. The ITS sequence of the holotype is distinct from other members of sect. Delibuti and deviating from them by at least 40 substitutions and indel positions.
Ecology and distribution. In plateau-alpine coniferous forests, associated with Abies (Pinaceae) trees. Known from Tibetan Plateau of China.
Additional specimens examined. CHINA. Tibet Autonomous Region: Sejila Mountain, Linzhi city, on moist soil under Abies forest with scattered Rhododendron, 29°35′25″N, 94°35′55″E, ASL 4170 m, 28 August 2019, Meng-Le Xie, 19xml10976 (HMJAU48761), GenBank MW911731, 19xml10981 (HMJAU48762), GenBank MW911732; Sejila Mountain, Linzhi city, on moist soil under Abies forest with scattered Rhododendron, 29°35′26″ N, 94°35′53″E, ASL 4120 m, 5 September 2020, Meng-Le Xie, 20xml12395 (HMJAU48763), GenBank: MW911730.
Key to species of sect. Delibuti
1 Distributed in Northern Hemisphere2
- Distributed in Southern Hemiphere9
2. Pileus usually yellowish to ochraceous without blue3
- Pileus more or less violet to blue when young, sometimes partly yellow4
3. Lamellae usually blue when young, veil yellowishC. delibutus
- Lamellae pinkish ochraceous clay, veil not yellowishC. illibatus
4. Pileus frankly blue when young, stipe bluish, veil violet5
- Pileus grayish blue to olive brown, stipe pale, veil different7
5. Basidiomata usually small, lamellae violet, then grayish to brownish, stipe usually slender (< 10 mm), base white with bluish tinge, basidiospores on average 8.0–8.3 × 6.9–7.0 µm, subglobose to broadly ellipsoid, distributed in subtropical China, associated with Lithocarpus brevicaudatusC. subsalor
- Basidiomata usually bigger, lamellae persistently lilaceous or bluish, stipe usually more robust (> 10 mm thick)6
6. Pileus usually staining buff or fading from the centre, stipe base usually grayish brown, basidiospores 7–9 × 6–8 µm, globose to subglobose, distributed in Europue, associated with deciduous and coniferous treesC. salor
- Pileus usually olive brown when mature, stipe base usually white, basidiospores 10.3–10.8 × 8.7–8.9 µm, subglobose to broadly ellipsoid, rarely ellipsoid, distributed in Tibetan Plateau of China, associated with AbiesC. tibeticisalor
7. Basidiomata small, pileus yellow to olive-ochre at the centre, grayish blue towards the margin, soon fading, veil yellow, basidiospores 7.5–9.5 × 6.5–7.5 µm, subglobose, associated with Betula. C. betulinus
- Basidiomata robust, associated with coniferous forests8
8. Pileus usually olive brown with a violet margin, veil olive brown, basidiospores 8–10 × 7–8 µm, globose, associated with PiceaC. transiens
- Pileus not olive brown, but prefer orange tinge, basidiospores 7.5–9.5×6.5–7.5 µm, subglobose, usually associated with Abies, rarely occur in Picea forests C. largodelibutus
9. Associated with Nothofagus10
- Associated with Myrtaceae trees11
10. Pileus viscid, blue-green to aerugineous, stipe blue green, basidiospores 6.5–8.5 × 6–7 µm, subglobose, destributed in AustralasiaC. tessiae
- Pileus glutinous, greyish yellow to greyish orange, stipe violet, then becoming white to pale brownish, basidiospores ellipsoid, destributed in North and South AmericaC. illitus
11. Basidiomata distinctly viscid to glutinous, mainly greyish blue-green, basidiospores 7–9 × 7–8 µm, globose to subgloboseC. rotundisporus
- Basidiomata weakly viscid, stipe often dry, mainly yellow-green to olive, Veil orange to ochraceous, basidiospores 6–7.5 × 5.5–6.5 µm, subgloboseC. calaisopus
Discussion
Cortinarius subsalor is similar to C. betulinus, C. salor and C. transiens (Melot) Soop due to the bluish tinge of the basiodiomata. However, C. betulinus is usually grayish blue at the margin of the pileus and soon fading, the stipe is often pale and the veil usually is yellow (Kibby, 2005; Niskanen et al., 2008; Soop, 2014). The pileus of C. transiens has a violet tone towards the margin, while the centre is more olive gray to yellowish brown even in young specimens, the stipe is pale, and the gelatinous veil is olive brown (Soop, 1990, 2014). In China, sometimes some bluish myxacioid species have been misidentified as C. salor (MHHNU30409, GenBank: MK250915), collected from Hunan Province. Our phylogenetic analyses showed that this sequence belong to the new species C. subsalor. Cortinarius salor has persistently lilaceous lamellae, the stipe is more robust (>10 mm thick) and the base is more grayish brown, the basidiospores are rounder (7–9 × 6–8 µm), and it occurs in European woodlands (Orton, 1955; Consiglio, Antonini & Antonini, 2003; Soop, 2014). Based on these features, C. salor can be distinguished from the Asian C. subsalor.
Cortinarius tibeticisalor is characterized by the basidiomata usually violet when young, then grayish orange to brown with an olive tinge, larger basidiospores and a restricted distribution in the Tibetan Plateau. Cortinarius tibeticisalor is similar to C. salor in young stage, however, the basidiospores (7–9 × 6–8 µm) of C. salor are significantly smaller and rounder, and the basidiomata never have olive tinge (Orton, 1955; Consiglio, Antonini & Antonini, 2003; Soop, 2014).
According to our phylogenetic analyses, sect. Delibuti demonstrates a widely distributed lineage of Cortinarius, in both the Northern and Southern Hemispheres. This bihemispherical distribution is also seen in several other lineages in Cortinarius, such as Anomali, Bolares, Camphorati, Defibulati, Illumini, and Vibratiles, this is concordant with other studies (e.g. Harrower et al., 2015; Garnica et al., 2016; Soop et al., 2019). The nrDNA ITS region is not suitable to draw conclusions for comprehensive phylogenetic evaluation, however, there are some interesting patterns indicated in sect. Delibuti to be further discussed. The basal lineages (clade A and B) of Delibuti are solely distributed in the Australasia showing a presumable origin of this section in Australasia. Interestingly, clade D contains species from multiple continents in the Northern and Southern Hemispheres. Some species are distributed in Asia (Cortinarius sp., LC175538), in Europe (Cortinarius sp., JF907917), and South America, like Cortinarius sp. (MF599228) from Colombia and C. illitus Moser & Horak (1975) originally described from Argentina, but also found in North America (according to the sequences in GenBank).These patterns could explain that the evolution of sect. Delibuti is limited to the ectomycorrhizal host specificity, as well as geographic barriers (Wang & Qiu, 2006; Brandrud, 1996; Wilson, Hosaka & Mueller, 2017; Feng et al., 2016). The evolution and origin of sect. Delibuti, including the genus Cortinarius will be a subject for future research.