-
Cassia angustifolia Vahl., also known as Senna, is a flowering plant species that belongs to the family Fabaceae. It is native to East Africa and the Arabian Peninsula but is now widely cultivated in many parts of the world, including India, Pakistan, and Sudan. This plant is a shrub or small tree that can grow up to 2−3 m tall, with long, narrow leaves and yellow flowers. The leaves are compound, with 4−8 pairs of leaflets 1−3 cm long and 0.2−0.5 cm wide. The flowers are arranged in clusters at the end of the branches and are about 2 cm in diameter. The botanical species referred to as 'senna' is used today and in the past as stimulating laxatives in pharmaceuticals. There are roughly 330 Cassia species; about 300 of them assigned to the genus Senna due to evolutionary relationships, while about 30 still classified as Cassia[1]. For instance, three related genera Senna Mill. (13 species), Chamaecrista Moench. (three species), and Cassia L. (seven species) were reclassified from 23 Sudanese Cassia species[2]. Regarding the Senna group and which species is a true Senna, the literature is rather ambiguous. Senna alexandrina Mill.[3] is the name of the plant that is referred to in the United States as 'senna'. This is puzzling because Senna alexandrina is still widely referred to as Cassia angustifolia, Cassia acutifolia, and Cassia senna. The European Pharmacopoeia still regards C. angustifolia and C. senna as separate species, each with unique geographic origins, morphologies, quality and grade designations, and active principle contents[4−8]. As a result, the two species will be described in this article using their Latin pharmacopeial and trade names[9−12]. Other varieties of senna that are used as medicines are not covered in this article, including American Senna (C. marilandica), which was once listed in the United States Pharmacopoeia (USP)[13−15] .
Senna, also known as Indian senna or Tinnevelly senna, has been used for medicinal purposes since ancient times and is now recognized by Indian, British, and American pharmacopeias[16−18]. The economic sections of the plant contain dianthrone glycosides (sennosides A, B, C, D, etc.), free anthraquinones (aloe-emodin, chrysophanol, rhein), and anthraquinone glycosides, as well as anthraquinone glycosides. Sennod B, present in more significant amounts, provides laxative effects via two mechanisms: enhanced intestinal fluid transport and increased intestinal motility. The sennoside content varies between leaves and pods from 1.5% to 3.5%. The variation in herbal constituents may be due to harvest seasons, plant origins, drying processes, and developmental stages[18−21]. Senna is used as a diuretic and a treatment for skin diseases, jaundice, and liver disorders. The leaves are collected when the plant is about two years old, and the pods are collected when they are ripe. After harvesting, the leaves and pods are dried in the sun and then stored in a cool, dry place until they are ready for use. It is vital to use senna as directed, as excessive use can lead to abdominal cramps, electrolyte imbalances, and dependence. In addition to its medicinal benefits, senna is also used as a natural dye and in the production of paper. The plant is a good source of tannins used for tanning leather and in the production of ink. The pods are also used as a natural dye for fabrics, producing a yellowish-brown color. The plant is also used in traditional medicine for several other purposes, such as the treatment of diabetes and cancer. Aside from its medicinal uses, senna also has some ornamental value due to its attractive yellow flowers and feathery foliage. It is occasionally planted as an ornamental shrub in gardens or used in landscaping projects[19, 22, 23].
Overall, senna is a versatile plant that has many uses, including medicinal, industrial, and ornamental purposes. Its natural laxative properties make it a popular choice for treating constipation and promoting regular bowel movements. However, it is important to use it under the guidance of a healthcare professional to avoid any adverse effects.
-
The plant (Cassia angustifolia) seems to be a native of North Africa. It grows wild as a perennial in the arid tract of Somalia, Ethiopia, Sudan, Egypt, Southern Arabia, Jordan, Iran, Qatar, Yemon, Sultanate of Oman, and the United Arab Emirates. It was initially introduced into Europe in the 12th century through the Medical School Salerno and the works of Arab Physicians[24]. As it became known in English, Senna became 'The perfect reliable purge'[25] and the most popular cleaner today[26]. Khan et al. discovered wild growing senna in the Arabian Peninsula, now known as Saudi Arabia. Senna alexandrina originated in Yemen, the Hadramaunt province of Saudi Arabia, and Somali land[22, 26, 27]. However, this species is naturalized in Gujarat (Mundra coastal tract and Aijal near Bhuj in Kutch region), India[28,29], and in Sind and North-West Pakistan[30]. Over the period, the species naturalized in India and cultivated in semi-arid parts of Tamil Nadu (Tirunelvelle, Ramanathapuram, Madurai, Selem, Tiruchirappalli), Karnataka, Rajasthan (Jodhpur, Jaisalmer, Barmer, and Jaipur) and Gujarat. Cassia acutifolia Delile is distributed in tropical North Africa in Ethiopia, Northern Sudan, Upper Nile, and Egypt[ 31].
-
Senna belongs to the genus Cassia, subtribe Cassiinae, tribe Cassieae, subfamily Caesalpinioideae, family Fabaceae, order Fabales, subclass Rosidae, class Magnoliopsida, division Magnoliophyta, and kingdom Plantae. Taxonomic classification based on morphological traits is complex, and boundary shifting has been reported[32,33]. The genus Cassia was originally treated as a large, widespread, diverse genus characterized by distinctive floral morphology, including species with enantio stylous, asymmetric flowers, and extra floral nectarines. It is a heterogeneous and sole representative of the subtribe Cassiinae.
Based on microscopic observations, Fairbairn & Sheshtra[34] demonstrated that Alexandrian senna (Cassia senna syn. Cassia acutifolia) and Tinnevelly senna (Senna alexandrina) are two distinct species. Irwin & Barnebv[32] removed the genera Senna and Chamaecrista from Cassia. Based on anthraquinone derivatives, Zafar et al.[23] classified the genus Cassia into two groups: Group-I includes nine species: Cassia alata, C. fistula, C. grandis, C. javanica, C. nodosa, C. obovata, C. ranigera, C. siamea, and C. Sophera. The major components of these species are rhein and chrysophanol. Group II contains eight species; Cassia angustifolia, C. auriculata, C. laevigata, C. mimosoides, C. occidentalis, C. roxburghii, C. spectabilis, and C. tora. The primary ingredients of these species are emodin, aloe-emodin, and physcion. Ghareeb et al.[35] divided the genus Cassia into three sub-genera (Cassia, Chamacirista, Senna) based on seed protein banding profiles, chromosome number, and morphological character studies. This taxonomic classification is valid and accepted.
Senna alexandrina is a small, perennial shrub 60−75 cm tall. The leaves are large, compound, pinnate, 5−8 jugate; leaflets- oval-lanceolate, glabrous, bluish green to pale green color; lamina- 2.5−6 cm long, 7−8 mm wide, entire margin, apex acute. It usually flowers in 65−70 d. The pods are slightly curved, cylindrical, or more frequently flattened. They are green in color when young and turn brown on maturity. It encloses 5−7 obovate, cuneate, compressed, smooth yellowish-white color seeds. Pods dehisce through one or both sutures. Flowers are bright yellow, axillary (or sub-terminal), erect, many-flowered racemes, considerably extending to subtending leaf, bracts-membranous. Legumes-flat, 15−17 mm wide; seeds-obovate, cuneate, compressed; cotyledons-plane[36].
The leaves and pods of Cassia accutifolia are smaller in size and thinner in consistency compared to Senna alexandrina. Some taxonomists have clubbed these two species into common taxa under Cassia senna[34] (Tables 1−3).
Table 1. Some medicinally important Cassia species found in India.
Species no. Botanical name Place/origin 1. Cassia italica sub. species italica Tenkukillum Asour 2. C.italica sub. species micrantha Tenkukillum Asour 3. C.italica sub. species micrantha Tenkukillum Asour 4. C.italica sub. species micrentha Tenkukillum Asour 5. Cassia italica sub. species italica Sanguluna (Tinnvelly) 6. Cassia accidentalis Sanguluna (Tinnvelly) 7. Cassia accidentalis Sanguluna (Tinnvelly) 8. Cassia accidentalis Valliloor Tirunelvellro 9. Cassia accidentalis Ramnathpurum 10. Cassia italica sub. species italica (Creeping type) Marimadmum 11. C.italica sub. species micrentha Asoor 12. Cassia uniflora Kaamthapanai 13. Cassia italica sub. species italica Kaamthapanai 14. C.italica sub. species micrentha Kaamthapanai 15. Cassia auriculata Kaamthapanai 16. Cassia italica sub. species italica Ramnathpurum 17. Cassia italica sub. species italica Ramnathpurum 18. Cassia uniflora Kaamthapanai 19. Cassia uniflora Ramnathpurum 20. Cassia. nigrica Ramnathpurum Table 2. Some species of Cassia having anthraquinones derivatives, their origin, and chromosome numbers.
Species Place of origin 2n chromosome numbers C. alata Linn. Cosmopoliton tropical 12 24 28 C. angustifolia Africa tropical 28 C . auriculata Linn. Indian orientalis 14,16 14,16,28 28 C. fistula Asia tropical C. laevigata Willd. Cosmopoliton tropical 26 28 C. obovata Collad. Geront tropic 28 C. javanica Linn. Malaya 28 C. mimosoides Linn. Cosmopoliton tropical 16 16,32 C. nodosa Buch-Ham. ex Roxb. Asia tropical 24 24, 28 28 C. occidentalis Linn. Cosmopoliton tropical 26 26, 28 28 C. renigera Wall. ex Benth. Burma 24 28 C. roxburghii D.C. Indian orientalis, 24 28 C. siamea Lam. Indian orientalis, Malaya 26, 28 28 C. sophera Linn. Geront tropic 24 28 C. tora Linn. Cosmopoliton tropical 24 26 26, 28 26, 52 28 Table 3. Scientific classifications of Senna.
Kingdom Plantae Synonyms Clade Tracheophytes Cassia acutifolia Delile Clade Angiosperms Cassia alexandrina (Garsault) Thell. Clade Eudicots Cassia angustifolia M. Vahl Clade Rosids Cassia lanceolata Collad Order Fabales Cassia lenitiva Bisch Family Fabaceae Cassia senna L. Subfamily Caesalpinioideae Senna acutifolia (Delile) Batka Genus Senna Senna alexandrina Garsault Species S. alexandrina Senna angustifolia (Vahl) Batka Binomial name Senna alexandrina Mill. C. lanceolata Pers. is a synonym of Chamaecrista desvauxii var. mollissima C. lanceolata Link is a synonym of Senna sophera var. sophera) -
Darlington & Wylie[37] listed three basic chromosome numbers of x = 6, 7, and 8, and parenthetically a secondary basic number of x = 13. Irwin & Turner[33] suggested a basic chromosome number for the genus Cassia x = 7. The other basic chromosome numbers (x = 6, x = 8) have been derived by aneuploid either through the loss or gain of one chromosome. Cumulative worldwide cytological data revealed that Cassia is a multibasic genus with x = 6, 7, 8, 10, 11, 13, and 15. However, all Indian species so far investigated show x = 6, 7, and 8[38]. It is suggested that the species with x = 14, the most successful number in the genus, may be of polyploidy origin from x = 7. Previous studies[33, 39,40] suggested that the basic number of chromosomes is x = 14 and 2n = 28. Others[41,42] indicated that the basic number of Cassia angustifolia is x = 14.
-
Senna contains 2n = 28 chromosomes[43]. It is predominantly self-pollinated. Plants have a mechanism imposing self-fertilization - a form of chasmogamy, and pollination takes place before flowers open[44,45]. However, Gupta & Prareek reported a relatively large percentage (%) of cross-pollination carried out by beetles. The flowering duration is quite long (range). The pollen grains are triangular, smooth, 2.8 to 4 mm, and highly fertile (98%). Flowering lasts 35−40 d[14,43, 46,47]. The nature and spectrum of existing intra-specific variability are limited due to the self-pollinating nature of the crop. Pandse et al.[48] analyzed the senna collections from different parts of India, but they needed assistance identifying significant variations among them. There was a lack of intraspecific variability, possibly due to limited cross-pollination[18].
In the senna plant, high heritability has been observed for fresh and dry leaf weight, plant height, total pod production, and sennosides concentration[49,50]. According to Sankaranarayanan & Muthuswamy[51] and Singh et al.[52], the leaflet significantly contributes to dry leaf yield at the genotypic level. Sennoside content in senna has been subject to mutagenic-induced variability, as demonstrated by Khalatkar & Bhargawa's[53] investigation. South India has been cultivating the introduced Arabian genetic stocks with little variation for more than 150 years. From existing variability, Gujarat Agricultural University has developed two late flowering varieties for leaf yield through selection after several generations. Forty accessions collected from different parts of India were evaluated for genetic divergence and grouped into seven clusters[14]. Accession-wise, distinct identities were recommended for a hybridization program for recombination breeding. High sennosides leaf-yielding and widely adapted variety 'Sona' was released and commercialized by the CSIR-CIMAP, Lucknow[54] (Fig. 1a−d).
-
Senna grows well in sandy loam and laterite soils with low to moderate fertility but mainly on red loam, including gravelly coarse soils, alluvial loams, and rich clayey rice fields. The crop can be grown in soil with a pH range of 7.0−8.5. Senna requires dry weather with moderate temperatures. In Northern India, it is a 130−150 d summer crop, but in Southern India, it is a winter crop. It is also grown successfully as a perennial crop for leaf yield in the hot arid conditions of Rajasthan, India, where the temperature is around 45 °C from May to June.
Crop stand in saline soil is poor but, once established, can withstand moderate saline conditions with yield reduction. Deep sand (≥ 1 m) and dunes have been found suitable for their long taproot development and good branching[55]. The saline-alkali or water-logged soils are unsuitable for cultivation. Early Kharif sown crop produces luxuriant growth and remains in the field for 130−150 d. It allows three pickings of leaves and pods[56]. Plants shed leaves during the winter and, if not removed, ultimately die in the field.
Sowing
-
Seeds are used to grow commercial crops. Because sowing dates are specific, flowering and pod formation does not coincide with the monsoon season. In Northern Central India, the crop is grown after the winter harvest and is sown in February-March. In Western and Central India, it is sown in July-September. In Southern India, crops are grown in two seasons: rain-fed crops are sown with the onset of the northeast monsoon in September–October and irrigation-supported crops are sown in February-March.
The seed rate is about 30 kg/ha for the rain-fed crop and 15 kg/ha for the irrigation-supported crop[48, 57, 58]. Dry or presoaked seeds are generally scattered in prepared fields. It may also be drilled in rows, and the distance between rows is 30−50 cm. Seeds are placed at a 1.5−2 cm depth using a tractor-driven mechanical device. The deeper-placed seeds fail to germinate. Light irrigation is given in case of a prolonged dry spell after sowing to ensure timely and uniform germination[57]. Depending upon soil moisture, seeds germinate within 7−10 d. Since the seeds have a hard seed coat, a certain amount of rubbing of its surface by pounding the seeds lightly with coarse sand in a mortar is desirable to induce quick germination. Presoaking for 10−12 h improves germination by up to 90%[59].
Seed treatment with fungicide, optimum sowing time, seeding depth, and soil moisture are essential to obtain the desired production level. A 12−15 kg/ha seed rate with 70%−80% germination produces a population of 70−75,000 plants/ha, the desired level for economic yield[60]. The crop may be thinned after 30−35 d post-sowing to maintain the plant-to-plant distance of 30 cm. Seeds may be sown in dry conditions even in June in areas like undulated land and dunes /deserts in the arid zone. The seeds germinate after rain. In agro-climatic situations, the plant becomes perennial and gives commercial produce for 4 to 5 years. The crop hardly requires weeding and pest management, which reduces the use of hazardous agrochemicals and saves the environment. The plant can survive at low moisture, where pathogen incidences are minimal. Seed treatment with fungicide protects the seedlings from damping-off and seedling blight. The irrigated crop produces a higher yield with superior quality.
Fertilizer
-
Senna is a leguminous plant, but it doesn't develop nodules that fix nitrogen from the air. Generally, it thrives on the residual nutrients of a previous cereal crop. The general recommendation of fertilizer for obtaining economic yield is 80:40:20 kg/ha N (nitrogen) P (phosphorus) K (potash). Nitrogen application should be divided into four doses and applied after sowing, thinning, and first and second picking[61]. The moderate use of organic manure is beneficial in rain-fed cultivation. However, trials conducted in different parts of India have shown that depending upon the growth, the number of pickings, residual moisture, fertility status, and growing conditions, the crop can utilize 50−100 kg of N, 20−50 kg of P2O5 and 24−30 kg of K2O/ha in a growing period of 130−150 d[62]. The use of potash can be avoided in the potash-rich soils of North-Western India. Basak and Gajbhiye[63] discovered that RP-charged compost was quite effective in boosting senna herbage yield and was comparable to Di-ammonium Phosphate (DAP) fertilizer.
The ascendancy of hydrogel doses on the growth and yield parameters on senna
-
According to Jnanesha et al.[64], hydrogel is essential for increasing soil absorption capacity and water retention, lowering water shortage, and reducing the negative consequences of drought. While there were no statistical differences in bulk density or porosity amongst hydrogel dosages, bulk density differed considerably from the control and starting values.
Irrigation and intercultural operations
-
Senna is a rain-fed crop, but 5−6 irrigations in North India increase the herb yield. An average distributed rainfall of 24−40 cm is sufficient. In the early growth of plants, two to three weedings are adequate to raise a good crop. Once the crop attains 20 to 24 cm height, it suppresses weed growth. As an irrigated crop, two weeding-hoeing is done, each after the first and second pickings[14,46,47, 65].
Crop rotation and intercropping
-
In Southern India, irrigated senna is grown after the paddy as intercrop cultivation in between rows of chilies, brinjal (Solanum melongena L.), okra (Abelmoschus spp.), and tomato (Lycopersicon esculentum Mill.). In the Northern plains of India, senna-mustard and senna-coriander rotations produced higher income, proving superior to other rotations. As a 130−150 d crop, senna fits nicely as a Kharif crop in Southern, Central, and Western India[61]. This crop has been introduced to the dense population in the agroforestry program to cover the newly reclaimed lands in Egypt[66]. The wild-growing populations in these countries are stripped of their leaves and pods. Crops harvested in September (rainy season) produces a higher yield than those harvested in April (dry season)[43]. Cassia aungustifolia can be propagated in the arid region's Silvi-herbal system. Even in extreme drought conditions, when other crops fail, farmers and residents receive constant economic returns. Silvi-herbal activities can be a part of an alternative and viable land-use strategy in dry climates[67].
Harvesting
-
Six to seven fresh leaves with elliptical, deep green broad leaflets have been shown to have a higher sennoside concentration and are hence more valued. The initial picking should take place between 70 and 90 d after planting, when the sennoside concentration is at its greatest, to obtain the appropriate biomass level. The majority of the growing tips are removed by hand picking, which promotes more leafy growth and delays flowering. While the final selection takes 130−150 d, the second picking takes 90−110 d. The last harvesting includes entire plants (leaves and pods)[61]. The produce is sold by weight. Leaves containing ≥ 2% and pods with ≥ 2.5 sennoside are acceptable to the industries. The collection of young leaves and pods does not fully compensate for the loss in weight at harvest. Hence, mature leaves are picked in the first and second pickings, whereas the third picking consists of remaining leaves and pods (of all age groups). In the case of pods, the collection at 15 to 24 d of age strikes a balance between weight and content. There is a decrease in sennoside content in the older pods due to high fiber content[43].
Since the leaf is the preferred crude drug in Europe as a herbal tea ingredient, its yield can be increased up to 75% at the expense of pod yield through defloration at the 40−60 d stage, which delays the reproductive phase and induces leaf growth[60]. Growth, dry matter yield, and sennoside content are increased by a six-weekly spraying of 50−500 IBA at the two to three-leaf stages, but this was not realized in field conditions[68]. The crop is grown as a perennial in the arid zone and harvested from 5−10 cm above the ground. The crop blooms three times a year; thus, three harvestings can be carried out. The best times for harvesting are the second fortnight of October, February, and May. October cutting should not be delayed because Senna is susceptible to cold.
Yield
-
With the exception of the seeds, almost all parts of the plant contain sennosides. Besides leaves and pods (rich in sennosides), the peduncle, rachis, and stem bark have 2.73%, 2.89% and 1.92% of total sennosides, respectively[43]. Under irrigated and appropriate management conditions, a good crop of senna can produce 15 q/ha of dry leaves and 7 q/ha of pods. Under rain-fed environments, leaf yield is about 10 q/ha, and pod yield is about 4 q/ha. The irrigated crop is considered to be of superior quality and fetches a higher market price. Senna is generally received in the market during June, July, and August.
On the contrary, a significant part of the rain-fed crop, which is poor in appearance, is collected from February to March. The yield of dry herbs may be increased with an increase in spacing or number of plants/hills. Three plants/hill at a distance of 30 cm in rows significantly increased the yield of dry pods. A 15q/ha yield can be obtained if the crop is grown for seed[66].
Drying and storage
-
After harvesting or collecting senna leaves and pods from wild-grown plants, they are dried. It is usually preferable to dry in the sun for 4 to 6 h and then shade dry in a thin layer in well-ventilated sheds. The drying process takes 4 to 5 d[61,69]. Ovens are used for drying vast quantities of plant materials, which are arranged in many trays piled on top of each other. Electric or steam heating can be used to power drying ovens. A fan or blower circulates hot air to speed up the drying process. The oven must be kept at a temperature of less than 50 °C. Higher temperatures damage the active constituents of the herb. Improper and delayed drying leads to changes in color from green to black-brown, which reduces the quality of the drug[54]. This is mainly caused by faulty drying or absorption of moisture by the product during storage. Therefore, special care should be taken in drying the crop.
The produce is graded into two or three grades, mainly based on color and size. Usually, bold, yellowish-green leaves and pods are considered first quality and sell at a premium. The second grade is made of brownish leaves and pods. Smaller and broken leaves and pods are graded low. The dried produce is pressed, wrapped in hessian cloth, and stored in a cool, dry, and dark place[61].
Seed
-
At the dough stage, seed is collected and properly dried. Ripe seeds should be light yellow to yellow[14,54]. Crops grown for quality seed production should not be collected for crude drug manufacture (Fig. 2a−d).
-
Senna is tolerant to the most common fungi and pests. Plants are protected from infection by the hot and dry growing conditions. According to Gupta & Prareek, die-back is the fungus-caused shedding of leaves and stems that turn black. The three main diseases that affect senna are damping-off (Rhizoctonia bataticola), leaf spot (Cercospora spp.), and leaf blight (Phyllosticta ssp.).
Seed treatment with Thiram/Captan (2.5 kg/ha) and a fortnightly spray of 0.15% solution of Diathan-M-45 can control the diseases. However, chemical fungicide spray should be avoided to protect the quality of the drug. White angled sulphur (Anteos clorinde) of senna is a pest that damages senna plants' leaves and significantly impacts leaf yield (Fig. 1d).
-
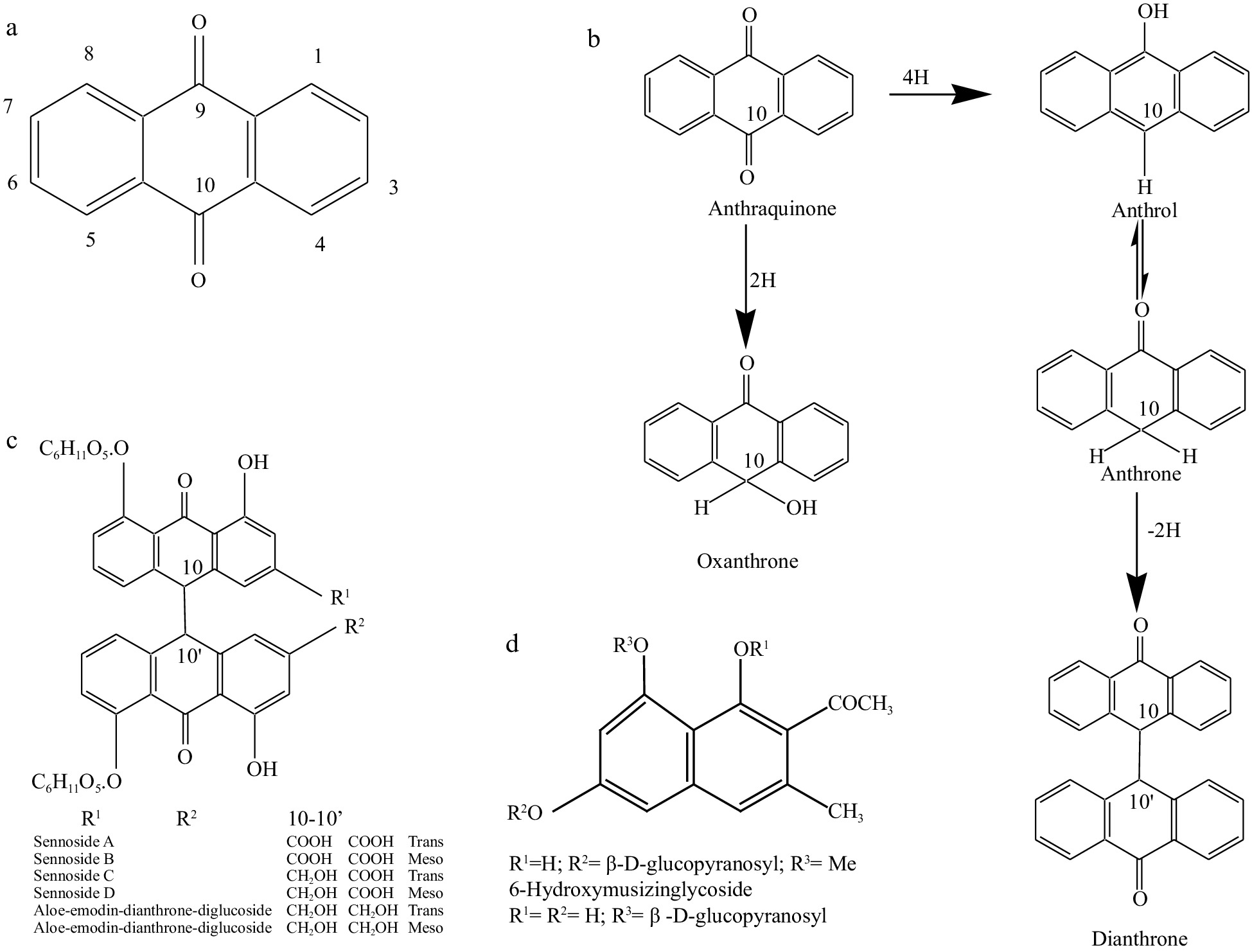
The use of senna as a laxative dates back to the 9th or 10th centuries, long before the chemistry of a chemical was known. Kirtikar & Basu listed the usefulness of the senna plant in treating liver complications, abdominal troubles, loss of appetite, spleen enlargement, dyspepsia, jaundice, anemia, fever, rheumatism, tumors, bronchitis, skin diseases, and poisoning. However, many researchers have investigated the plant's laxative properties[70−71]. The purgative drug is the chemical anthraquinone from rhubarb, aloe, senna, and cascara (Fig. 2a−d). This was identified as glycosides[71]. Additional research indicated that natural products also contain reduced anthraquinone derivatives (oxanthrones, anthrols, and anthrones) and dianthrones, compounds generated by the union of two anthrone molecules (Fig. 2a−d). Tutin was the first to identify and isolate aloe-emodin and Rhein (aglycones) from senna leaf, and the number of compounds found grew. Stoll et al.[70] discovered two active crystalline glycosides, sennoside A and sennoside B, which generated glucose and the aglycones sinnidin A and sinnidin B when hydrolyzed. Pods contain active elements comparable to leaves but in greater concentrations. Alexandrian Senna and Indian Senna can be distinguished by 6-hydroxymusicni and tinnevellin glucosides, respectively. Many chemicals include sennoside C and D, palmidin A, rhein- anthrone 8-glycoside, rhein 8-diglycoside, aloe-emodin 8-glycosides, aloe-emodin-anthrone-glycoside, rhein-1 glucose, emodin-8-0-glucoside were isolated[72,73]. Some of the compounds were found to be pharmacologically active. Besides sennosides and other flavonoids as previously mentioned, isorhamnetin and kaempferols as glycosides (kaempferin) are also present in senna leaves[72,73]. Leaves also contain 7% mucilage. Roots contain physcion, physcionin, chrysphain, and sennadin C. Pharmacologically, the most active constituents in senna are sennoside A and B (Fig. 2a−d), dianthrones (dimer) of the anthrone skeleton.
Senna's fresh leaves include glycosides that contain extra sugar groups. These are naturally degraded to sennoside A and B. Lamili et al.[72] identified two naphthalene glycosides from Alexandrian and Indian senna leaves, namely 6-hydroxyl musizin and tinnevellin (Fig. 2a−d). In sensitive senna plants, chysophanol is the first anthraquinone to develop. Through successive oxidation of the 3-methyl groups of chrysophanol, the ontogenic sequence was in close agreement with the biogenetic order, resulting in aloe-emodin, emodin, and eventually rhein. Thus ontogenic sequence was in close accord with the biogenetic order. Later, glycosylation occurs in the presence of light, and the glycoside is produced and translocated to various body areas. Flowers have the most considerable quantities (> 4%), followed by leaves (> 2%) and fruits (> 2%), with roots and stems having the lowest concentrations[74−76].
Sennosides do not occur in living plants. However, sennosides are formed after dehydration of leaves by rapidly warming at 60 °C (dried within a few hours) and allowed to gradually lose water at room temperature. The degree of water loss in leaves and the number of sennosides formed are strongly associated[77−79]. Aflatoxins, secondary metabolites of Aspergillus flavus Link and A. parasiticus, sometimes are associated with senna pods, which are highly toxic and carcinogenic to mammals. Müller & Basedow[80] reported that the leaves and flowers of senna were usually free from aflatoxins. Still, pods may contain 2 to 245 μg/kg against the tolerable limit of 2 μg/kg for aflatoxin B1 and 4 μg/kg for the sum of aflatoxins B1, B2, G1, and G2 (fixed by the European Union). Aflatoxin content in pods is doubled in the controlled sun drying and increased by four times in shade drying.
Sennosides A to D are the purgative sennosides among the known sennosides[81,82]. For estimating the presence of sennosides and their quantification, many isocratic HPLC methods without perfect baseline resolution have been described. A simple liquid chromatographic method was developed to determine sennosides B and A in C. angustifolia leaves. The novel method offers a dependable tool for quickly screening large numbers of C. angustifolia samples, which is important in breeding/genetic engineering and genetic mapping investigations[83]. A practical, quick, accurate, and reproducible method for separating and determining sennoside A and B and the main composition (Sennidin A and B) in sennoside metabolism was developed through linear-gradient HPLC by Sun et al.[84]. Sennoside A and B in senna leaves through a simple liquid chromatography method quantifying these constituents by gradient reversed-phase liquid chromatography and UV absorbance at 270 nm using a photodiode array detector was determined[85,86].
-
Hydroxyanthracene glycosides cause the therapeutic activity of senna, especially sennoside A and B, present in leaves, flowers, and pods. These β-linked glycosides possessing the property of secretagogues enhance the net secretion of fluids and specifically influence colonic motility, improving colonic transit[87]. The upper intestinal tract does not absorb these glycosides[88−91]. This article aims to give a broad overview of senna and its products' quality assurance procedures, pharmacology, clinical application, patented and commercialized formulations, and international trade environment. Besides being highly effective in eradicating severe constipation, it also relieves severe irritable bowel syndrome[92,93]. Ewe et al.[94], Hietala et al.[95] and Godding[90] showed that sennosides significantly increase the colonic transit rate and colonic peristalsis, increasing fecal weight and dry bacterial mass.
Nijs et al.[96] have shown that rhein anthrone possesses in vitro activity which is dependent on contact with the mucosa, while their action on the reversed segment was inhibited by B.W. 755CL (a dual inhibitor of cyclo–oxygenase and lipoxygenase) by indometacin and SC 192220 (an antagonist of PGE2 and PGF2 α). They concluded that arachidonic acid metabolites, especially PGE2 and PGF2 α, are involved in the effect of rhein anthrones on the reversed segment. Rumsey et al.[97] showed that the large intestine could expel luminal contents in a caudal direction following the addition of sennosides. Yamauchi et al.[98] concluded that in addition to prostaglandins, diarrhea induced by rhein anthrone must also involve the calcium channel, which can be blocked by nifedipine, but not verapamil. Staumont et al.[99] provide evidence for an increase in propulsive activity expressed as an increased number of migrating long spike bursts in the left and human sigmoid colon. In non-specific or induced diarrhoea, this motor-specific colonic pattern appears to be prevalent. Sennoside A and B and their aglycones Sinnidin A and B are transported across CaCO2 monolayers in a concentration-dependent and linear manner[100]. The absorption from apical to basolateral was weak, and PCC values were comparable to mannitol. The transport was more significant in the secretary direction, indicating that poorly absorbed chemicals were effluxed (e.g., through efflux pumps) into the intestinal lumen. As a result, metabolites, rather than the naturally occurring dianthrone, are primarily responsible for senna's laxative effects (Table 2).
-
Long-term continuous use of sennosides may cause spasmodic gastrointestinal problems, cardiac arrhythmias, nephropathies, edema, accelerated bone deterioration, tecany, aspartyl glucosamine secretion, hypogamena globulinema, and hypokalemia caused by lesser electrolytes (especially potassium). In contradiction to an earlier belief, pregnant and lactating women should not use senna because it is a 'stimulant laxative' of choice during pregnancy and lactation[101]. Sennosides did not stimulate uterine motility in pregnant ewes[102]. Further, the absence of abnormal stool consistency in infants breastfed by a senna laxative-ingesting mother was observed[103]. In contrast, Faber & Strege-Hesse[104, 105] recommended taking non-standardized laxatives during pregnancy. Anthraquinone purgatives in excess may cause the degeneration of neurons[106−108]. Two hydroxylanthraquinones, i.e., aloe-emodin and emodin, present as minor components in senna and may be genotoxic or have a carcinogenetic risk for humans[109]. Heidemann et al.[110] showed that an extract of senna and aloe-emodin was genotoxic and examined in-vitro. However, the effect of the drug fructus sennae under in-vivo conditions has not yet been reported. The sennosides and rhein were negative under in-vitro and in-vivo conditions.
The high dose of long-term daily use of sennoside and dianthrone in the ascending and descending colons decreased maximal contraction[111]. The finding that continuous treatment with androids had no aberrant motility pattern except a residual pharmacological activity 48 h after the last high dosages were similar to sensitivity to rhein, an active metabolite of sennosides. Treatment with sennoside did not affect the serum aldosterone level and mucosal Na+-P+- ATPase activities in the small intestine and colon. Further, despite chronic diarrhea over six months, there were no signs of habituation or secondary hyperaldosteronism due to sennosides or dianthrone[112]. The higher average number of aberrant crypts per focus can predict tumor outcomes when the rats were treated with the highest dose of glycosides and the 1,2-dimethyl hydrazine (DMH) than DMH alone. This suggested that senna and cascara glycosides might be weak promoters in rat colon carcinogenesis[113]. Anthroid laxatives induced apoptosis of colonic epithelial cells in humans within 6 h after ingestion[19,114,115].
Rhein, the active metabolite of sennoside laxative, is a cytotoxic substance capable of producing apoptosis and works as a substrate for the MRP1 (Multiple drug resistance-associated protein 1) drug efflux pump. This cytotoxicity has nothing to do with topoisomerase II or DNA intercalation. The ability to extrude the anthranoid laxative may be vital for protecting the epithelium. It would be interesting to look at MRP1 levels in chronic anthranoid laxative users. MRP2 expressions are linked to a lower incidence of colon cancer in anthranoid laxative users, while MRP1 mutations or polymorphisms may also play a role[115]. Continuous usage of senna laxatives was linked to severe symptoms such as fluid and electrolyte loss and chronic diarrhoea. Severe hepatotoxicity is infrequent. It could, however, be explained by the liver being exposed to hazardous anthraquinone glycoside metabolites in high quantities[116]. According to an objective causality assessment, hepatotoxicity may have been linked to using senna laxatives. According to the experimental model, anthraquinone derivatives may accumulate in the kidneys and produce nephrotoxicity. Studies on the antimutagenic and genotoxic potentials of aqueous senna extracts (SAE) in a cell-free system have single and double-strand breaks in plasmid DNA.
Nonetheless, they are not cytotoxic or mutagenic to Escherichia coli strains[117]. In effect, SAE can protect Escherichia coli IC 203 (nvrAOXYR), and IC 205 (nvrAmut M) strains from H2O2-induced mutagenesis and toxicity, pointing to a new antioxidant/antimutagenic function of SAE. Senna-containing laxatives accidentally ingested by young children can cause severe diaper rash, blisters, and skin sloughing[118, 119].
Overall, senna is an important crop plant with a wide range of medicinal and other uses. While it is primarily used for its laxative properties, it also has potential for use in other areas, such as natural dyeing and paper production. As with all medicinal plants, it is important to use senna responsibly and under the guidance of a healthcare professional.
-
Senna, known since 1600 BC, is a unique drug in the animal kingdom in modern and traditional systems of medicine with little or more toxicity to humans. Its chemical constituents, structures, and pharmacological and toxicological features have all been extensively investigated. The uniqueness of the drug is due to its chemical components, mainly anthraquinones glucosides. Bacteria in the colon convert these molecules to rhein-anthrone, which has two effects. Firstly, it stimulates colon activity, which speeds up bowel movements, and secondly, it promotes colon fluid secretion, which helps to prevent persistent constipation, which is a significant human health risk. Genetic studies that serve as the foundation for understanding the genetic diversity and developing superior varieties, such as having increased sennoside content on consistent quality, have yet to receive much attention.
This work is supported by the Council of Scientific and Industrial Research, India, under CSIR- Emeritus Scientist No. 21 (1020)/16/EMR-II dated 18-11-2016. The authors thank the director of CSIR-IMAP, Lucknow, India, for providing facilities and encouragement throughout the work. CIMAP Publication No. CIMAP/Pub/2018/61.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Lal RK, Chanotiya CS, Kumar A. 2023. The prospects and potential of the horticultural and pharmacological medicinal herb senna (Cassia angustifolia Vahl.): A review. Technology in Horticulture 3:20 doi: 10.48130/TIH-2023-0020
The prospects and potential of the horticultural and pharmacological medicinal herb senna (Cassia angustifolia Vahl.): A review
- Received Date: 09 June 2023
- Accepted Date: 09 August 2023
- Published Online: 25 October 2023
Abstract: The dried leaves and pods of Cassia L., a member of the Caesalpinioideae subfamily of the Fabaceae family and the Cassiinae sub-tribe are called 'senna drug'. The drug is known by the names Senna, Indian Senna, Tinnevelly Senna, and Cassia Senna in the Indian, British, and American pharmacopeias. It appears to be native to North Africa. Sennoside A and B, anthraquinone glycosides responsible for the plant's laxative effects, are found in the leaves, pods, and seeds, which are the plant's most valuable parts. The plant grows as a perennial in the dry regions of Somalia, Ethiopia, Sudan, Egypt, Southern Arabia, and neighboring countries. Through the Medical School Salerno and the writings of Arab Physicians, it was first introduced into Europe in the 12th century. Cassia accutifolia, or senna as it became known in English, is found in tropical North Africa's Northern Sudan, Egypt's Upper Nile, and some areas of Ethiopia. Senna alexandrina was born in Yemen, the Saudi province of Hadramgunl, and some areas of Sind (Pakistan). It is, however, common along the Gujarat coast, particularly in the Mundra coastal tract and Aigal near Bhuj in the Kutch area of India. The plant must have, at some stage, been transported from its native North Africa to India. It is believed that it was originally used in Tirunelvelle, an area of Tamil Nadu, sometime around the middle of the 11th century. The plant has become a native in India throughout the years. It is grown in semi-arid regions of Gujarat, Rajasthan, and Tamil Nadu (Tinunevelle, Ramanathapuram, Madurai, Salem, and Tineichirapalli). Senna alexandrina is a tiny, obtuse-branched perennial shrub that grows to 60 to 75 cm. Large, compound, pinnate, 5−8 gugate, oval-lanceolate, plebrous, bluish-green to pale green, lamina 2.5−6 cm long, 7−8 mm broad, whole edge, acutely apical leaves. It typically blooms after 65 to 70 d. Bright yellow, axillary (or subterminal), many erect racemes with membranous bracts have bright yellow flowers. Legumes are flat, 15−17 mm wide, with compressed, obovate, and cuneate seeds. Compared to Cassia angustifolia, Cassia accutifolia has smaller, thinner-consistency leaves and pods. Some taxonomists combined these two species into a single taxon called Cassia senna. Senna is taken orally as a tea, pill formulation, or powder encapsulated to relieve intestinal constipation. Senna has been the subject of several reviews; however, we focus on the most recent facts rather than previous review papers. Senna's origin, botany and taxonomy, agriculture, genetic advancement, cytogenetics, mutation breeding, diseases and pests, pharmaceutical significance, microbiological activities, etc. were thoroughly reviewed. A brief review of available cytogenetics, genetic advancement, and agricultural technology was also addressed.
-
Key words:
- Anthraquinones /
- Chrysophanol /
- Hydrogel /
- Sennosides /
- Tinnevelly senna