A Review of the Ephedra genus: Distribution, Ecology, Ethnobotany, Phytochemistry and Pharmacological Properties
Abstract
:1. Introduction
2. Ecological Distribution of Ephedra Species and Insect-Ephedra Interactions
3. Ethnobotany
4. Chemistry
4.1. Natural Products
4.1.1. Alkaloids
4.1.2. Flavonoids and Phenolic Compounds
4.1.3. Amino Acid Derivatives
4.1.4. Volatile Organic Compounds in Essential Oils
4.2. Chemotaxonomy
4.3. Applications in Organic Synthesis
4.3.1. Ephedrine-Type Alkaloids Derivatives as Ligands for the Enantioselective Addition Processes
4.3.2. Marker Compounds for the Quality Control of the Manufacturing Process of Ephedrine Alkaloids-Free Ephedra Herb Extracts (EFE’s)
4.3.3. Molecularly Imprinted Co-Polymers for Recognition of (−)-Ephedrine (1)
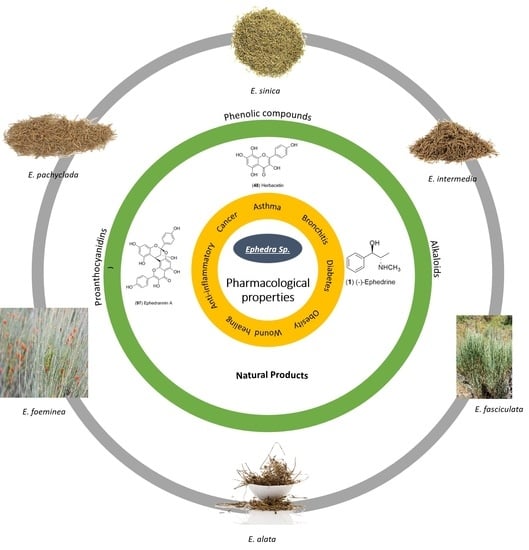
5. Pharmacological Properties
5.1. Asthma and Bronchitis Treatment
5.2. Diabetes Protective Effect
5.3. Anti-Obesity Activity
5.4. Wound Healing Effect
5.5. Anti-Inflammatory Activity
5.6. Cytotoxic and Anti-Tumor Activities
5.7. Antiviral Activity
5.8. Pharmacokinetics of Ephedrine-Type Alkaloids
6. Toxicity
7. Fungal Endophytes from Ephedra Species
7.1. Chemical Constituents of Endophytic Fungi from Ephedra Species
7.2. Biological Activities of Secondary Metabolites Produced by Fungal Endophytes from Ephedra Species
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Carrillo-Inungaray, M.L.; López, L.I.; Nevárez-Moorillón, G.V.; Aguilar, C.N. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac. J. Trop. Med. 2015, 8, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Calzada, F.; Bautista, E. Plants used for the treatment of diarrhoea from Mexican flora with amoebicidal and giadicidal activity, and their phytochemical constituents. J. Ethnopharmacol. 2020, 253, 112676. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.R.; Loza-Mejía, M.A.; Soto-Cabrera, D. Chemistry, biological activities and in silico bioprospection of sterols and triterpenes from Mexican columnar cactaceae. Molecules 2020, 25, 1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollander, J.L.; Vander Wall, S.B.; Baguley, J.G. Evolution of seed dispersal in North American Ephedra. Evol. Ecol. 2010, 24, 333–345. [Google Scholar] [CrossRef]
- Ickert-Bond, S.M.; Renner, S.S. The Gnetales: Recent insights on their morphology, reproductive biology, chromosome numbers, biogeography, and divergence times. J. Syst. Evol. 2016, 54, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Abourashed, E.A.; El-Alfy, A.T.; Khan, I.A.; Walker, L. Ephedra in perspective-a current review. Phyther. Res. 2003, 17, 703–712. [Google Scholar] [CrossRef]
- Lee, M.R. The history of Ephedra (ma-huang). J. R. Coll. Physicians Edinb. 2011, 41, 78–84. [Google Scholar] [CrossRef]
- Stohs, S.J.; Badmaev, V. A review of natural stimulant and non-stimulant thermogenic agents. Phyther. Res. 2016, 30, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Bolinder, K.; Humphreys, A.M.; Ehrlén, J.; Alexandersson, R.; Ickert-Bond, S.M.; Rydin, C. From near extinction to diversification by means of a shift in pollination mechanism in the gymnosperm relict Ephedra (Ephedraceae, Gnetales). Bot. J. Linn. Soc. 2016, 180, 461–477. [Google Scholar] [CrossRef] [Green Version]
- Loera, I.; Sosa, V.; Ickert-Bond, S.M. Diversification in North American arid lands: Niche conservatism, divergence and expansion of habitat explain speciation in the genus Ephedra. Mol. Phylogenet. Evol. 2012, 65, 437–450. [Google Scholar] [CrossRef]
- Villanueva-Almanza, L.; Fonseca, R.M. Revisión taxonómica y distribución geográfica de Ephedra (ephedraceae) en México. Acta Bot. Mex. 2011, 96, 79–116. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Price, R.A. Estimation of the age of extant Ephedra using chloroplast rbcL sequence data. Mol. Biol. Evol. 2003, 20, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Geng, B.Y.; Dilcher, D.L.; Chen, Z.D.; Lott, T.A. Morphology and affinities of an early cretaceous Ephedra (Ephedraceae) from China. Am. J. Bot. 2005, 92, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Rydin, C.; Pedersen, K.R.; Friis, E.M. On the evolutionary history of Ephedra: Cretaceous fossils and extant molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16571–16576. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zheng, S.L. Whole fossil plants of Ephedra and their implications on the morphology, ecology and evolution of Ephedraceae (Gnetales). Chin. Sci. Bull. 2010, 55, 1511–1519. [Google Scholar] [CrossRef]
- Rydin, C.; Khodabandeh, A.; Endress, P.K. The female reproductive unit of Ephedra (Gnetales): Comparative morphology and evolutionary perspectives. Bot. J. Linn. Soc. 2010, 163, 387–430. [Google Scholar] [CrossRef] [Green Version]
- Ickert-Bond, S.M.; Wojciechowski, M.F. Phylogenetic relationships in Ephedra (Gnetales): Evidence from nuclear and chloroplast DNA sequence data. Syst. Bot. 2004, 29, 834–849. [Google Scholar] [CrossRef]
- Rydin, C.; Korally, P. Evolutionary relationships in Ephedra (gnetales), with implications for seed plant phylogeny. Int. J. Plant Sci. 2009, 170, 1031–1043. [Google Scholar] [CrossRef] [Green Version]
- Bino, R.J.; Meeuse, A.D.J. Entomophily in dioecious species of Ephedra: A preliminary report. Acta Bot. Neerl. 1981, 30, 151–153. [Google Scholar] [CrossRef]
- Stelleman, P. Reflections on the transition from wind pollination to ambophily. Acta Bot. Neerl. 1984, 33, 497–508. [Google Scholar] [CrossRef]
- Celedón-Neghme, C.; Santamaría, L.; González-Teuber, M. The role of pollination drops in animal pollination in the Mediterranean gymnosperm Ephedra fragilis (Gnetales). Plant Ecol. 2016, 217, 1545–1552. [Google Scholar] [CrossRef]
- Niklas, K.J. A biophysical perspective on the pollination biology of Ephedra nevadensis and E. trifurca. Bot. Rev. 2015, 81, 28–41. [Google Scholar] [CrossRef]
- Maher, L.J. Ephedra pollen in sediments of the Great Lakes region. Ecology 1964, 45, 391–395. [Google Scholar] [CrossRef]
- Rydin, C.; Bolinder, K. Moonlight pollination in the gymnosperm Ephedra (Gnetales). Biol. Lett. 2015, 11, 10–13. [Google Scholar] [CrossRef]
- Meeuse, A.D.J.; De Meijer, A.H.; Mohr, O.W.P.; Wellinga, S.M. Entomophily in the dioecious gymnosperm Ephedra aphylla Forsk (= E. Alte C.A.Mey.), with some notes on Ephedra campylopoda C.A.Mey. III. Further anthecological studies and relative importance of entomophily. Isr. J. Bot. 1990, 39, 113–123. [Google Scholar] [CrossRef]
- Von Aderkas, P.; Prior, N.; Gagnon, S.; Little, S.; Cross, T.; Hardie, D.; Borchers, C.; Thornburg, R.; Hou, C.; Lunny, A. Degradome and secretome of pollination drops of Ephedra. Bot. Rev. 2015, 81, 1–27. [Google Scholar] [CrossRef]
- Margot, J.L. Insufficient evidence of purported lunar effect on pollination in Ephedra. J. Biol. Rhythms 2015, 30, 454–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bino, R.I.; Dafni, A.; Meeuse, A.D.J. Entomophily in the dioecious gymnosperm Ephedra aphylla Forsk. (=E. alte C.A.Mey.), with some notes on E. campylopoda C.A.Mey.l. Aspects of the entomophilous syndrome. Proc. Kon. Nederl. Akad. Wetensch. 1984, 87, 1–13. [Google Scholar]
- Bino, R.I.; Devente, N.; Meeuse, A.D.J. Entomophily in the dioecious gymnosperm Ephedra aphylla Forsk. (=E. alte C.A.Mey.), with some notes on E. campylopoda C.A.Mey. ll. Pollination droplets, nectaries and nectarial secretion in Ephedra. Proc. Kon. Nederl. Akad. Wetensch. 1984, 87, 14–22. [Google Scholar]
- The Plant List (2013). Version 1.1. Available online: http://www.theplantlist.org/ (accessed on 15 April 2020).
- Herman, R.P.; Bynum, H.G.; Alexander, A.B. Interaction between the black yeast Aureobasidium pullulans and the gall midge Lasioptera ephedricola in gall formation on the desert shrub Ephedra trifurca. Ecography (Cop.) 1993, 16, 261–268. [Google Scholar] [CrossRef]
- Boecklen, W.J.; Hoffman, M.T. Sex-biased herbivory in Ephedra trifurca: The importance of sex-by-environment interactions. Oecologia 1993, 96, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.A.; Polhemus, J.T. Ephedrodoma, a new genus of orthotyline Miridae (Hemiptera) from western United States. Proc. Entomol. Soc. Wash. 1984, 86, 550–554. [Google Scholar]
- Askew, R.R.; Nieves-Aldrey, J.L. Eupelmidae (hymenoptera, chalcidoidea) of Iberia and the Canary Islands: An annotated checklist with descriptions of some previously unrecognised males and a new species of Calosota Curtis, 1836. Graellsia 2017, 73, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Patra, B.; Bera, S.; Molchanoff, S.; Wang, Y.; Yang, J.; Li, C. Morpho-anatomy of Xerephedromiya ustjurtensis (Diptera: Cecidomyiidae) induced galls and intersexual variation of gall density in Ephedra distachya L. (Ephedraceae) from Ukraine. Acta Bot. Hung. 2012, 54, 377–389. [Google Scholar] [CrossRef]
- Askew, R.R.; Blasco-Zumeta, J. Parasitic hymenoptera inhabiting seeds of Ephedra nebrodensis in Spain, with descriptions of a phytophagous pteromalid and four other new species of Chalcidoidea. J. Nat. Hist. 1997, 31, 965–982. [Google Scholar] [CrossRef]
- Ribera, I.; Blasco-Zumeta, J. Biogeographical links between steppe insects in the Monegros region (Aragón, NE Spain), the eastern Mediterranean, and central Asia. J. Biogeogr. 1998, 25, 969–986. [Google Scholar] [CrossRef]
- Gagné, R.J. The transition from fungus-feeding to plant-feeding in Cecidomyiidae (Diptera). Proc. Entomol. Soc. Wash. 1986, 88, 381–384. [Google Scholar]
- Chen, J.K.; Chen, T.T. Chinese Medicinal Herbology and Pharmacology; Art of Medicine Press: City of Industry, CA, USA, 2004; pp. 31–40. [Google Scholar]
- Native American Ethnobotany. Available online: http://naeb.brit.org/uses/search/?string=Ephedra (accessed on 7 May 2018).
- Russell, F. The Pima Indians. Library of Congress; Government Printing Office: Washington, DC, USA, 1908; pp. 79–84.
- Curtin, L.S.M. By the Prophet of the Earth, Ethnobotany of the Pima Indians; University of Arizona Press: Tucson, AZ, USA, 1984; pp. 47–111. ISBN 978-0816508549. [Google Scholar]
- Standley, P.C. Trees and Shrubs of Mexico/By Paul C. Standley; Government Printing Office: Washington, DC, USA, 1920; pp. 1–9.
- Martinez, M. Las Plantas Medicinales de México; Ediciones Botas: Mexico City, Mexico, 1969. [Google Scholar]
- Biblioteca Digital Medicina Tradicional Mexicana. Ephedra. Available online: http://www.medicinatradicionalmexicana.unam.mx/monografia.php?l=3&t=Popotillo&id=7751 (accessed on 7 May 2018).
- Wang, Q.; Yang, Y.; Zhao, X.; Zhu, B.; Nan, P.; Zhao, J.; Wang, L.; Chen, F.; Liu, Z.; Zhong, Y. Chemical variation in the essential oil of Ephedra sinica from Northeastern China. Food Chem. 2006, 98, 52–58. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, D.; Liu, Y. GC-MS analysis of the supercritical CO2 fluid extraction of Ephedra sinica roots and its antisudorific activity. Chem. Nat. Compd. 2009, 45, 434–436. [Google Scholar] [CrossRef]
- Elhadef, K.; Smaoui, S.; Fourati, M.; Ben Hlima, H.; Chakchouk Mtibaa, A.; Sellem, I.; Ennouri, K.; Mellouli, L. A review on worldwide Ephedra history and story: From fossils to natural products mass spectroscopy characterization and biopharmacotherapy potential. Evidence-Based Complement. Altern. Med. 2020, 2020, 1–22. [Google Scholar] [CrossRef]
- Ibragic, S.; Sofić, E. Chemical composition of various Ephedra species. Bosn. J. Basic Med. Sci. 2015, 15, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, K.; Hulst, R.; Kellogg, R.M. Thiol and disulfide derivatives of Ephedra alkaloids 2: A mechanistic study of their effect on the addition of diethyl zinc to benzaldehyde. Tetrahedron Asymmetry 1995, 6, 1861–1864. [Google Scholar] [CrossRef] [Green Version]
- Oguni, N.; Omi, T. Enantioselective addition of diethylzinc to benzaldehyde catalyzed by a small amount of chiral 2-amino-1-alcohols. Tetrahedron Lett. 1984, 25, 2823–2824. [Google Scholar] [CrossRef]
- Parrott, R.W.; Hitchcock, S.R. β-Amino alcohols derived from (1R,2S)-norephedrine and (1S,2S)-pseudonorephedrine as catalysts in the asymmetric addition of diethylzinc to aldehydes. Tetrahedron Asymmetry 2008, 19, 19–26. [Google Scholar] [CrossRef]
- Dean, M.A.; Hitchcock, S.R. A structural examination of the impact of oxygenated side chains in Ephedra compounds in the catalytic asymmetric addition of diethylzinc to aldehydes. Tetrahedron Asymmetry 2009, 20, 2351–2356. [Google Scholar] [CrossRef]
- Banerjee, S.; Groeper, J.A.; Standard, J.M.; Hitchcock, S.R. N-Pyridylmethylephedrine derivatives in the catalytic asymmetric addition of diethylzinc to aldehydes and diphenylphosphinoylimines. Tetrahedron Asymmetry 2009, 20, 2154–2161. [Google Scholar] [CrossRef]
- Banerjee, S.; Camodeca, A.J.; Griffin, G.G.; Hamaker, C.G.; Hitchcock, S.R. Aromatic motifs in the design of Ephedra ligands for application in the asymmetric addition of diethylzinc to aldehydes and diphenylphosphinoylimines. Tetrahedron Asymmetry 2010, 21, 549–557. [Google Scholar] [CrossRef]
- Pelaez, F.; Collado, J.; Arenal, F.; Basilio, A.; Cabello, A.; Diez Matas, M.T.; Garcia, J.B.; Gonzalez Del Val, A.; Gonzalez, V.; Gorrochategui, J.; et al. Endophytic fungi from plants living on gypsum soils as a source of secondary metabolites with antimicrobial activity. Mycol. Res. 1998, 102, 755–761. [Google Scholar] [CrossRef]
- Bashyal, B.P.; Kithsiri Wijeratne, E.M.; Faeth, S.H.; Gunatilaka, A.A.L. Globosumones A-C, cytotoxic orsellinic acid esters from the Sonoran desert endophytic fungus Chaetomium globosum. J. Nat. Prod. 2005, 68, 724–728. [Google Scholar] [CrossRef]
- Al-Qarawi, A.; Hashem, A.; Abd-Allah, E. Seed mycoflora of Ephedra aphylla and amino acid profile of seed-borne Aspergillus flavus. Acta Microbiol. Immunol. Hung. 2012, 59, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, W.-J.; Long, J.; Yang, A.-M.; Yang, Z.-D.; Liu, X.-F.; Hua, D.; Wang, W.-J.; Ma, J.-H. Isolation of endophytic fungi from Ephedra intermedia and research antibacterial activity of secondary metabolite produced by the fungi. Adv. Mater. Res. 2014, 881–883, 488–492. [Google Scholar] [CrossRef]
- Wijeratne, E.M.K.; Paranagama, P.A.; Gunatilaka, A.A.L. Five new isocoumarins from Sonoran desert plant-associated fungal strains Paraphaeosphaeria quadriseptata and Chaetomium chiversii. Tetrahedron 2006, 62, 8439–8446. [Google Scholar] [CrossRef]
- Hong, H.; Chen, H.B.; Yang, D.H.; Shang, M.Y.; Wang, X.; Cai, S.Q.; Mikage, M. Comparison of contents of five ephedrine alkaloids in three official origins of Ephedra Herb in China by high-performance liquid chromatography. J. Nat. Med. 2011, 65, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Tamada, M.; Endo, K.; Hikino, H.; Kabuto, C. Structure of ephedradine A, a hypotensive principle of Ephedra roots. Tetrahedron Lett. 1979, 84, 873–876. [Google Scholar] [CrossRef]
- Krizevski, R.; Bar, E.; Shalit, O.; Sitrit, Y.; Ben-Shabat, S.; Lewinsohn, E. Composition and stereochemistry of ephedrine alkaloids accumulation in Ephedra sinica Stapf. Phytochemistry 2010, 71, 895–903. [Google Scholar] [CrossRef]
- Konno, C.; Taguchi, T.; Tamada, M.; Hikino, H. Ephedroxane, anti-inflammatory principle from Ephedra herbs. Phytochemistry 1979, 18, 697–698. [Google Scholar] [CrossRef]
- Starratt, A.N.; Caveney, S. Quinoline 2-carboxylic acids from Ephedra species. Phytochemistry 1996, 42, 1477–1478. [Google Scholar] [CrossRef]
- Al-Khalil, S.; Alkofahi, A.; El-Eisawi, D.; Shibib, A. Transtorine, a new quinoline alkaloid from Ephedra transitoria. J. Nat. Prod. 1998, 61, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.A.M.; El-Sissi, H.I.; Barakat, H.H. Flavonoid constituents of Ephedra alata. Phytochemistry 1984, 23, 2937–2939. [Google Scholar] [CrossRef]
- Tamada, M.; Endo, K.; Hikino, H. Structure of ephedradine B, a hypotensive principle of Ephedra roots. Heterocylces 1979, 12, 783–786. [Google Scholar] [CrossRef]
- Konno, C.; Tamada, M.; Endo, K.; Hikino, H. Structure of ephedradine C, a hypotensive principle of Ephedra roots. Heterocylces 1980, 14, 295–298. [Google Scholar]
- Hikino, H.; Shimoyama, H.; Kasahara, G.; Takanashi, M.; Konno, S. Structures of mahuannin A and B, hypotensive principle of Ephedra roots. Heterocycles 1982, 19, 1381–1384. [Google Scholar] [CrossRef]
- Hikino, H.; Ogata, K.; Konno, C.; Sato, S. Hypotensive actions of ephedradines, macrocytic spermine alkaloids of Ephedra roots. Planta Med. 1983, 48, 290–293. [Google Scholar] [CrossRef]
- Hikino, H.; Ogata, M.; Konno, C. Structure of feruloylhistamine, a hypotensive principle of Ephedra roots. Planta Med. 1983, 48, 108–110. [Google Scholar] [CrossRef]
- Hikino, H.; Kiso, Y.; Ogata, M.; Konno, C.; Aisaka, K.; Kubota, H.; Hirose, N.; Ishihara, T. Pharmacological actions of analogues of feruloylhistamine, an imidazole alkaloid of Ephedra roots. Planta Med. 1984, 50, 478–480. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Kassem, F.F.; Abdallah, R.M. Two alkaloids from Ephedra aphylla growing in Egypt. Nat. Prod. Sci. 2003, 9, 52–55. [Google Scholar]
- Schaneberg, B.T.; Crockett, S.; Bedir, E.; Khan, I.A. The role of chemical fingerprinting: Application to Ephedra. Phytochemistry 2003, 62, 911–918. [Google Scholar] [CrossRef]
- Caveney, S.; Charlet, D.A.; Freitag, H.; Maier-Stolte, M.; Starratt, A.N. New observations on the secondary chemistry of world Ephedra (Ephedraceae). Am. J. Bot. 2001, 88, 1199–1208. [Google Scholar] [CrossRef] [Green Version]
- Chumbalov, T.K.; Chekmeneva, L.N.; Polyakov, V.V. Phenolic acids of Ephedra equisetina. Chem. Nat. Compd. 1977, 13, 238–239. [Google Scholar] [CrossRef]
- Weinges, K.; Kaltenhäuser, W.; Marx, H.; Nader, E.; Nader, F.; Perner, J.; Seiler, D. Zur Kenntnis der Proanthocyanidine, X Procyanidine aus Früchten. Justus Liebigs Ann. Chem. 1968, 711, 184–204. [Google Scholar] [CrossRef]
- Jacques, D.; Haslam, E.; Bedford, G.R.; Greatbanks, D. Plant proanthocyanidins. Part II. Proanthocyanidin-A2 and its derivatives. J. Chem. Soc. Perkin Trans. 1 1974, 2663–2671. [Google Scholar] [CrossRef]
- Zakirova, B.M.; Omurkamzinova, V.B.; Erzhanova, M.S. Flavonoids of Ephedra lomatolepis. Chem. Nat. Compd. 1983, 18, 748–749. [Google Scholar] [CrossRef]
- Hussein, S.A.M.; Barakat, H.H.; Nawar, M.A.M.; Willuhn, G. Flavonoids from Ephedra aphylla. Phytochemistry 1997, 45, 1529–1532. [Google Scholar] [CrossRef]
- Zang, X.; Shang, M.; Xu, F.; Liang, J.; Wang, X.; Mikage, M.; Cai, S. A-type proanthocyanidins from the stems of Ephedra sinica (Ephedraceae) and their antimicrobial activities. Molecules 2013, 18, 5172–5189. [Google Scholar] [CrossRef] [Green Version]
- Song, K.S.; Sankawa, U.; Ebizuka, Y. A novel benzoic acid derivative from yeast-elicited Ephedra distachya cultures. Arch. Pharm. Res. 1994, 17, 54–55. [Google Scholar] [CrossRef]
- Cottiglia, F.; Bonsignore, L.; Casu, L.; Deidda, D.; Pompei, R.; Casu, M.; Floris, C. Phenolic constituents from Ephedra nebrodensis. Nat. Prod. Res. 2005, 19, 117–123. [Google Scholar] [CrossRef]
- Pullela, S.V.; Takamatsu, S.; Khan, S.I.; Khan, I.A. Isolation of lignans and biological activity studies of Ephedra viridis. Planta Med. 2005, 71, 789–791. [Google Scholar] [CrossRef]
- Tao, H.; Wang, L.; Cui, Z.; Zhao, D.; Liu, Y. Dimeric proanthocyanidins from the roots of Ephedra sinica. Planta Med. 2008, 74, 1823–1825. [Google Scholar] [CrossRef]
- Kasahara, Y.; Shimoyama, N.; Konno, C.; Hikino, H. Structure of mahuanin C, a hypotensive principle of Ephedra roots. Heterocycles 1983, 20, 1741–1744. [Google Scholar]
- Amakura, Y.; Yoshimura, M.; Yamakami, S.; Yoshida, T.; Wakana, D.; Hyuga, M.; Hyuga, S.; Hanawa, T.; Goda, Y. Characterization of phenolic constituents from Ephedra herb extract. Molecules 2013, 18, 5326–5334. [Google Scholar] [CrossRef]
- Hyuga, S.; Hyuga, M.; Yoshimura, M.; Amakura, Y.; Goda, Y.; Hanawa, T. Herbacetin, a constituent of ephedrae herba, suppresses the HGF-induced motility of human breast cancer MDA-MB-231 cells by inhibiting c-met and akt phosphorylation. Planta Med. 2013, 79, 1525–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Cui, Y.; Ding, G.; Zhou, M.; Ma, X.; Hou, Y.; Jiang, M.; Liu, D.; Bai, G. Mahuannin B an adenylate cyclase inhibitor attenuates hyperhidrosis via suppressing β2-adrenoceptor/cAMP signaling pathway. Phytomedicine 2017, 30, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Kallassy, H.; Fayyad-Kazan, M.; Makki, R.; El-Makhour, Y.; Rammal, H.; Leger, D.Y.; Sol, V.; Fayyad-Kazan, H.; Liagre, B.; Badran, B. Chemical composition and antioxidant, anti-inflammatory, and antiproliferative activities of Lebanese Ephedra campylopoda. Med. Sci. Monit. Basic Res. 2017, 23, 313–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, G.; Zhang, T.; Hou, Y.; Ding, G.; Jiang, M.; Luo, G. From quality markers to data mining and intelligence assessment: A smart quality-evaluation strategy for traditional Chinese medicine based on quality markers. Phytomedicine 2018, 44, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Oshima, N.; Maruyama, T.; Yamashita, T.; Uchiyama, N.; Amakura, Y.; Hyuga, S.; Hyuga, M.; Nakamori, S.; Takemoto, H.; Kobayashi, Y.; et al. Two flavone C-glycosides as quality control markers for the manufacturing process of ephedrine alkaloids-free Ephedra Herb extract (EFE) as a crude drug preparation. J. Nat. Med. 2018, 72, 73–79. [Google Scholar] [CrossRef]
- Song, K.Y.; Ishikawa, Y.; Kobayashi, S.; Sankawa, U.; Ebizuka, Y. N-Acylaminoacids from Ephedra distachya cultures. Phytochemistry 1992, 31, 823–826. [Google Scholar] [CrossRef]
- Tamada, M.; Endo, K.; Hikin, H. Maokonine, hypertensive principle of Ephedra roots. Planta Med. 1978, 34, 291–293. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Kitani, Y.; Zhu, S.; Omote, T.; Tanaka, K.; Batkhuu, J.; Sanchir, C.; Fushimi, H.; Mikage, M.; Komatsu, K. Molecular analysis and chemical evaluation of Ephedra plants in Mongolia. Biol. Pharm. Bull. 2009, 32, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, M.; Minamino, Y.; Kameoka, H. Volatile components of Ephedra sinica Stapf. Flavour Fragr. J. 1997, 12, 15–17. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-H.; Kim, B.-S.; Han, K.; Kim, H. Ephedra-treated donor-derived gut microbiota transplantation ameliorates high fat diet-induced obesity in rats. Int. J. Environ. Res. Public Health 2017, 14, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawwar, M.A.M.; Barakat, H.H.; Buddrus, J.; Linscheid, M. Alkaloidal, lignan and phenolic constituents of Ephedra alata. Phytochemistry 1985, 24, 878–879. [Google Scholar] [CrossRef]
- Omurkamzinova, V.B.; Pashinina, L.T.; Erzhanova, M.S. Proanthocyanidins of Ephedra lomatolepis. Chem. Nat. Compd. 1984, 20, 500–501. [Google Scholar] [CrossRef]
- Oshima, N.; Yamashita, T.; Hyuga, S.; Hyuga, M.; Kamakura, H.; Yoshimura, M.; Maruyama, T.; Hakamatsuka, T.; Amakura, Y.; Hanawa, T.; et al. Efficiently prepared ephedrine alkaloids-free Ephedra Herb extract: A putative marker and antiproliferative effects. J. Nat. Med. 2016, 70, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Guo, Z.; Zhang, X.; Wu, X. Synthesis of molecularly imprinted co-polymers for recognition of ephedrine. Anal. Methods 2013, 5, 5179–5187. [Google Scholar] [CrossRef]
- Löwhagen, O. Diagnosis of asthma—New theories. J. Asthma 2015, 52, 538–544. [Google Scholar] [CrossRef]
- Croisant, S. Epidemiology of Asthma: Prevalence and Burden of Disease. In Heterogeneity in Asthma, Advances in Experimental Medicine and Biology; Brasier, A.R., Ed.; Humana Press: Boston, MA, USA, 2014; pp. 17–29. ISBN 978-1-4614-8603-9. [Google Scholar]
- Stewart, H.H. The use of ephedrine in asthma and whooping-cough. Br. Med. J. 1929, 1, 293–295. [Google Scholar] [CrossRef] [Green Version]
- Garbis, H. Antiasthmatic and cough medication. In Drugs during Pregnancy and Lactation: Treatment Options and Risk Assessment; Schaefer, C., Peters, P., Miller, R.K., Eds.; Elsevier: London, UK, 2007; pp. 63–77. ISBN 978-0-444-52072-2. [Google Scholar]
- Sa-ih, N.; Reakkamnuan, C.; Samerphob, N.; Cheaha, D.; Niyomdecha, S.; Kumarnsit, E. Local field potential power spectra and locomotor activity following treatment with pseudoephedrine in mice. Acta Neurobiol. Exp. 2020, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Shergis, J.L.; Wu, L.; May, B.H.; Zhang, A.L.; Guo, X.; Lu, C.; Xue, C.C. Natural products for chronic cough: Text mining the East Asian historical literature for future therapeutics. Chron. Respir. Dis. 2015, 12, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Ma, Z.Q.; Fu, Q.; Ma, S.P. Ma Huang Tang ameliorates asthma though modulation of Th1/Th2 cytokines and inhibition of Th17 cells in ovalbumin-sensitized mice. Chin. J. Nat. Med. 2014, 12, 361–366. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, June 2016. [Google Scholar]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Shamah-Levi, T.; Cuevas-Nasu, L.; Dommarco-Rivera, J.; Hernandez-Avila, M. Encuesta Nacional de Salud y Nutrición de Medio Camino 2016; Instituto Nacional de Salud Pública: Mexico City, Mexico, 2017; ISBN 978-607-511-160-5. [Google Scholar]
- Meduru, H.; Wang, Y.-T.; Tsai, J.J.P.; Chen, Y.-C. Finding a potential dipeptidyl peptidase-4 (DPP-4) inhibitor for type-2 diabetes treatment based on molecular docking, pharmacophore generation, and molecular dynamics simulation. Int. J. Mol. Sci. 2016, 17, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeda-Montes, M.J.; Ardid-Ruiz, A.; Tomás-Hernández, S.; Gimeno, A.; Cereto-Massagué, A.; Beltrán-Debón, R.; Mulero, M.; Garcia-Vallvé, S.; Pujadas, G.; Valls, C. Ephedrine as a lead compound for the development of new DPP-IV inhibitors. Future Med. Chem. 2017, 9, 2129–2146. [Google Scholar] [CrossRef]
- Wang, Q.; Long, M.; Qu, H.; Shen, R.; Zhang, R.; Xu, J.; Xiong, X.; Wang, H.; Zheng, H. DPP-4 inhibitors as treatments for type 1 diabetes mellitus: A systematic review and meta-analysis. J. Diabetes Res. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.; Lim, H.; Woo, S.; Shin, S.S.; Yoon, M. The herbal composition GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica inhibits visceral obesity and insulin resistance by upregulating visceral adipose genes involved in fatty acid oxidation. Pharm. Biol. 2015, 53, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Song, M.K.; Um, J.Y.; Jang, H.J.; Lee, B.C. Beneficial effect of dietary Ephedra sinica on obesity and glucose intolerance in high-fat diet-fed mice. Exp. Ther. Med. 2012, 3, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Xiu, L.M.; Miura, A.B.; Yamamoto, K.; Kobayashi, T.; Song, Q.H.; Kitamura, H.; Cyong, J.C. Pancreatic islet regeneration by ephedrine in mice with streptozotocin-induced diabetes. Am. J. Chin. Med. 2001, 29, 493–500. [Google Scholar] [CrossRef]
- Hwa-Won, L.; Ji-Yeon, Y.; Hoi-Seon, L. Quinoline-2-carboxylic acid isolated from Ephedra pachyclada and its structural derivatives show inhibitory effects against α-glucosidase and α-amylase. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Roman, M.C.; Gray, D.; Luo, G.; McClanahan, R.; Perez, R.; Roper, C.; Roscoe, V.; Shevchuk, C.; Suen, E.; Sullivan, D.; et al. Determination of ephedrine alkaloids in botanicals and dietary supplements by HPLC-UV: Collaborative Study. J. AOAC Int. 2004, 87, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Obesity and Overweight. World Health Organization, 2017. Available online: https://www.who.int/westernpacific/health-topics/obesity (accessed on 15 April 2020).
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.; Chae, K.; Jung, Y.S.; Rho, Y.H.; Lee, J.; Ha, J.; Yoon, K.H.; Kim, G.C.; Oh, K.S.; Shin, S.S.; et al. The Korean traditional medicine Gyeongshingangjeehwan inhibits obesity through the regulation of leptin and PPARα action in OLETF rats. J. Ethnopharmacol. 2008, 119, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Park, D.; Lee, H.Y.; Hong, Y.; Choi, J.; Oh, J.; Lee, H.; Lee, H.R.; Kim, M.R.; Shen, Z.B.; et al. The herbal composition GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica reduces obesity via skeletal muscle AMPK and PPARα. Pharm. Biol. 2012, 50, 506–515. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Jung, Y.; Chae, S.; Cho, S.H.; Yoon, M.; Yang, H.; Shin, S.S.; Yoon, Y. Gangjihwan, a polyherbal composition, inhibits fat accumulation through the modulation of lipogenic transcription factors SREBP1C, PPARγ and C/EBPα. J. Ethnopharmacol. 2018, 210, 10–22. [Google Scholar] [CrossRef]
- Kang, J.W.; Nam, D.; Kim, K.H.; Huh, J.E.; Lee, J.D. Effect of gambisan on the inhibition of adipogenesis in 3T3-L1 adipocytes. Evidence-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Song, M.Y.; Kim, H. The anti-obesity effect of Ephedra sinica through modulation of gut microbiota in obese Korean women. J. Ethnopharmacol. 2014, 152, 532–539. [Google Scholar] [CrossRef]
- Al Saeed, W.; Al Dhamen, M.; Ahmad, R.; Ahmad, N.; Naqvi, A.A. Clinical uses and toxicity of Ephedra sinica: An evidence-based comprehensive retrospective review (2004–2017). Pharmacogn. J. 2019, 11, 439–444. [Google Scholar] [CrossRef]
- Nayak, B.S.; Raju, S.S.; Eversley, M.; Ramsubhag, A. Evaluation of wound healing activity of Lantana camara L.—A preclinical study. Phytother. Res. 2009, 23, 241–245. [Google Scholar] [CrossRef]
- Firdous, S.M.; Sautya, D. Medicinal plants with wound healing potential. Bangladesh J. Pharmacol. 2018, 13, 41–52. [Google Scholar] [CrossRef]
- Kittana, N.; Abu-Rass, H.; Sabra, R.; Manasra, L.; Hanany, H.; Jaradat, N.; Hussein, F.; Zaid, A.N. Topical aqueous extract of Ephedra alata can improve wound healing in an animal model. Chin. J. Traumatol. 2017, 20, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.F. Macrophages: Important physiologic and pathologic sources of polypeptide growth factors. Am. J. Respir. Cell Mol. Biol. 1990, 2, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Park, Y.J.; Yoon, S.J.; Lee, H.B. Ephedrannin A and B from roots of Ephedra sinica inhibit lipopolysaccharide-induced in flammatory mediators by suppressing nuclear factor-κB activation in RAW 264.7 macrophages. Int. Immunopharmacol. 2010, 10, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Meng, X.; Wang, Z.; Liu, J.; Kuang, H.; Wang, Q. Polysaccharide from Ephedra sinica Stapf inhibits inflammation expression by regulating Factor-β1/Smad2 signaling. Int. J. Biol. Macromol. 2018, 106, 947–954. [Google Scholar] [CrossRef]
- Wang, Q.; Shu, Z.; Xing, N.; Xu, B.; Wang, C.; Sun, G.; Sun, X.; Kuang, H. A pure polysaccharide from Ephedra sinica treating on arthritis and inhibiting cytokines expression. Int. J. Biol. Macromol. 2016, 86, 177–188. [Google Scholar] [CrossRef]
- Yeom, M.J.; Lee, H.C.; Kim, G.H.; Lee, H.J.; Shim, I.; Oh, S.K.; Kang, S.K.; Hahm, D.H. Anti-arthritic effects of Ephedra sinica STAPF herb-acupuncture: Inhibition of lipopolysaccharide-induced inflammation and adjuvant-induced polyarthritis. J. Pharmacol. Sci. 2006, 100, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Sierra, J.R.; Tsao, M.-S. c-MET as a potential therapeutic target and biomarker in cancer. Ther. Adv. Med. Oncol. 2011, 3, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Ben-Arye, E.; Mahajna, J.; Aly, R.; Ali-Shtayeh, M.S.; Bentur, Y.; Lev, E.; Deng, G.; Samuels, N. Exploring an herbal “wonder cure” for cancer: A multi- disciplinary approach. J. Cancer Res. Clin. Oncol. 2016, 142, 1499–1508. [Google Scholar] [CrossRef] [Green Version]
- Mendelovich, M.; Shoshan, M.; Fridlender, M.; Mazuz, M.; Namder, D.; Nallathambi, R.; Gopinath, S.; Kumari, P.; Ion, A.; Smadar, W.; et al. Effect of Ephedra foeminea active compounds on cell viability and actin structures in cancer cell lines. J. Med. Plants Res. 2017, 11, 690–702. [Google Scholar] [CrossRef] [Green Version]
- Ben-Arye, E.; Lavie, O.; Samuels, N.; Khamaisie, H.; Schiff, E.; Gressel-Raz, O.; Mahajna, J. Safety of herbal medicine use during chemotherapy in patients with ovarian cancer: A “bedside-to-bench” approach. Med. Oncol. 2017, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kima, S.S.; Jeonga, H.S.; Haa, J.H.; Oh, S.H.; Jeong, M.H.; Choi, W.Y.; Seo, Y.C.; Lee, H.Y. Effect of nanoparticles of Ephedra sinica stapf extracts on suppression of tumor cell growth in ICR Mice. World Acad. Sci. Eng. Technol. 2010, 65, 447–448. [Google Scholar]
- Lee, S.-A.; Hong, S.-K.; Suh, C.-I.; Oh, M.-H.; Park, J.-H.; Choi, B.-W.; Park, S.-W.; Paik, S.-Y. Anti-HIV-1 efficacy of extracts from medicinal plants. J. Microbiol. 2010, 48, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-P.; Pang, J.; Wang, X.-W.; Shen, Z.-Q.; Jin, M.; Li, J.-W. In vitro screening of traditionally used medicinal plants in China against enteroviruses. World J. Gastroenterol. 2006, 12, 4078–4081. [Google Scholar] [CrossRef]
- Soni, M.G.; Carabin, I.G.; Griffiths, J.C.; Burdock, G.A. Safety of Ephedra: Lessons learned. Toxicol. Lett. 2004, 150, 97–110. [Google Scholar] [CrossRef] [PubMed]
- White, L.M.; Gardner, S.F.; Gurley, B.J.; Marx, M.A.; Wang, P.L.; Estes, M. Pharmacokinetics and cardiovascular effects of ma-huang (Ephedra sinica) in normotensive adults. J. Clin. Pharmacol. 1997, 37, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, F.; Xing, X.; Ren, M.; Ma, Q.; Xie, Y.; Tang, Q.; Luo, J. Concurrent quantification and comparative pharmacokinetic analysis of bioactive compounds in the Herba Ephedrae-Semen Armeniacae Amarum herb pair. J. Pharm. Biomed. Anal. 2015, 109, 67–73. [Google Scholar] [CrossRef]
- Wei, P.; Huo, H.L.; Ma, Q.H.; Li, H.C.; Xing, X.F.; Tan, X.M.; Luo, J.B. Pharmacokinetic comparisons of five ephedrine alkaloids following oral administration of four different Mahuang-Guizhi herb-pair aqueous extracts ratios in rats. J. Ethnopharmacol. 2014, 155, 642–648. [Google Scholar] [CrossRef]
- Zheng, E.X.; Navarro, V.J. Liver injury from herbal, dietary, and weight loss supplements: A review. J. Clin. Transl. Hepatol. 2015, 3, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Dhar, R.; Stout, C.W.; Link, M.S.; Homoud, M.K.; Weinstock, J.; Estes, N.A.M. Cardiovascular toxicities of performance-enhancing substances in sports. Mayo Clin. Proc. 2005, 80, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Bavadekar, S.A.; Davis, Y.M.; Lalchandani, S.G.; Nagmani, R.; Schaneberg, B.T.; Khan, I.A.; Feller, D.R. Pharmacological effects of ephedrine alkaloids on human α1- and α2-adrenergic receptor subtypes. J. Pharmacol. Exp. Ther. 2007, 322, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.L.; Moawad, F.J.; Hartzell, J.D.; Iglesias, M.; Jackson, W.L. Hypertensive retinopathy associated with use of the Ephedra-free weight-loss herbal supplement Hydroxycut. Medscape Gen. Med. 2006, 8, 82. [Google Scholar]
- Persky, A.M.; Berry, N.S.; Pollack, G.M.; Brouwer, K.L.R. Modelling the cardiovascular effects of ephedrine. Br. J. Clin. Pharmacol. 2004, 57, 552–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enders, J.M.; Dobesh, P.P.; Ellison, J.N. Acute myocardial infarction induced by ephedrine alkaloids. Pharmacotherapy 2003, 23, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Sudden death of a healthy college student related to ephedrine toxicity from a Ma Huang-containing drink. J. Clin. Psychopharmacol. 1997, 17, 437–439. [Google Scholar] [CrossRef]
- Haller, C.; Kearney, T.; Bent, S.; Ko, R.; Benowitz, N.; Olson, K. Dietary supplement adverse events: Report of a one-year poison center surveillance project. J. Med. Toxicol. 2008, 4, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, N.C.; Howland, M.A.; Greller, H.A.; Hoffman, R.S.; Nelson, L.S. Ischemic stroke associated with use of an Ephedra-free dietary supplement containing synephrine. Mayo Clin. Proc. 2005, 80, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Samenuk, D.; Link, M.S.; Homoud, M.K.; Contreras, R.; Theohardes, T.C.; Wang, P.J.; Estes, N.A.M. Adverse cardiovascular events temporally associated with ma huang, an herbal source of ephedrine. Mayo Clin. Proc. 2002, 77, 12–16. [Google Scholar] [CrossRef]
- Haller, C.A.; Benowitz, N.L. Adverse cardiovascular and central nervous system events associated with dietary supplements containing Ephedra alkaloids. N. Engl. J. Med. 2000, 343, 1833–1838. [Google Scholar] [CrossRef]
- Han, H.Y.; Huh, J.I.; Han, S.R.; Kang, M.G.; Yoon, S.; Han, J.S.; Lee, B.S.; Kim, J.A.; Min, B.S. Assessing the safety of an Ephedrae Herba aqueous extract in rats: A repeat dose toxicity study. Regul. Toxicol. Pharmacol. 2018, 94, 144–151. [Google Scholar] [CrossRef]













| Ephedra Species | Common Name | Accepted | Synonym |
|---|---|---|---|
| E. antisyphilitica | Canatilla, cañatilla, popotillo, tepopete. | Ephedra antisyphilitica Berland. ex C.A.Mey. | Ephedra antisyphilitica var. brachycarpa Cory Ephedra antisyphilitica f. monstrosa Torr. ex Stapf Ephedra antisyphilitica var. pedunculata S.Watson |
| E. aspera | Canatilla, canetilla, cañutilla, hintimoreal, ítamo real, pico de pájaro, pito real, pitamoreal, popotillo, tepopote. | Ephedra aspera Engelm. ex S.Watson | Ephedra nevadensis var. aspera (Engelm. ex S.Watson) L.D.Benson Ephedra peninsularis I.M.Johnst. Ephedra reedii Cory |
| E. californica | California ephedra, California jointfir, canatilla, desert tea, Mormon tea, | Ephedra californica S.Watson | Ephedra californica var. funerea (Coville & C.V.Morton) L.D.Benson |
| E. compacta | Canutillo, comida de víbora, retama real, real popotillo, sanguinaria | Ephedra compacta Rose | No synonyms are recorded for this name |
| E. nevadensis | Mormon tea, Nevada jointfir, té de camioneros, té de varas | Ephedra nevadensis S.Watson | Ephedra nevadensis var. aspera (Engelm. ex S.Watson) L.D.Benson Ephedra nevadensis f. rosea H.C.Cutler Ephedra nevadensis var. viridis (Coville) M.E.Jones |
| E. pedunculata | Canatilla, comida de víbora, hintimoreal, itamoreal, pitamoreal, popotillo, retama, retamo real, tepopote, sanguinaria | Ephedra pedunculata Engelm. ex S.Watson | No synonyms are recorded for this name |
| E. torreyana | Mexican tea, Torrey’s jointfir | Ephedra torreyana S.Watson | Ephedra torreyana var. torreyana |
| E. trifurca | Cola de zorra, longleaf jointfir, longleaf teabush, popotillo, tea weed, tepopote | Ephedra trifurca Torr. ex S.Watson | Ephedra trifaria Parl. [Spelling variant] |
| Ephedra Species | Geographic Location | Animal Species | Interaction | Reference |
|---|---|---|---|---|
| E. aspera Engelm ex S.Watson | U.S.A | Insecta: Hemiptera | Host in Ephedra | [33] |
| Ephedrodoma multilineata | ||||
| E. aphylla Forssk. | Israel | Insecta: Diptera | Pollination | [19] |
| Syrphidae: Metasyrphus corollae (Fabr.), M. latifasciatus (Marquart), Syritta pipiens (L.). Episyrphus halteatus (De Geer), Sphaerophoria scripta (L.), Scaeva albomaculata (Marq.). Eristolodes taeniops (Wied.), Paragus sp. Chrysotoxum sp., Melanostoma sp. | ||||
| Calliphoridae: Lucilia sp., Sarcophaga sp. | ||||
| Muscidae: Musca sp. | ||||
| Insecta: Hymenoptera, Apoidae; Apis, Halictus sp. | ||||
| Israel | Insecta: Diptera, Musci sp., Calliphora sp., Lucilia caesar, Sarcophaga sp., Sarcophagidae sp., Anthomyidae, Chloropinae | Pollination | [25] | |
| Insecta: Hymenoptera, Hymenoptera sp., Formicidae sp. | ||||
| E. foeminea Forssk. (Syn. E. campylopoda) | Israel | Insecta: Hymenoptera, Apoidae; Apis mellifera | Pollination | [25] |
| E. distachya L. | Greece | Insecta: Hymenoptera, Chalcidoidea, Formicidae, Coleoptera, Dermestidae | Pollination | [9] |
| Spain | Insecta: Hymenoptera, Chalcidoidea; Eurytoma gallephedrae | Parasitic (inhabiting seeds). | [34] | |
| Ukraine | Insecta: Diptera, Cecidomyiidae; Xerephedromiya ustjurtensis | Parasitic (gall formation) | [35] | |
| E. foeminea Forssk. | Greece | Insecta: Hymenoptera | Potential pollinator visitors | [9] |
| Formicidae; Aphaenogaster sp., Camponotus sp., Cataglyphis sp. | ||||
| Diptera | ||||
| Brachycera | ||||
| Syrphidae; Paragus quadrifasciatus | ||||
| Ceratopogonidae | Parasitic | |||
| Insecta: Hymenoptera | ||||
| Chalcidoidea | ||||
| Vespoidae | ||||
| Coleoptera | ||||
| Mordellidae | ||||
| Insecta: Diptera | Other visitors | |||
| Muscidae | ||||
| Sciaridae | ||||
| Lepidoptera | ||||
| Geometridae | ||||
| Tortricidae; Cnephasia sp. | ||||
| E. fragilis Desf. | Spain | Sauropsida: Squamata | Pollination | [21] |
| Lacertidae: Podarcis lilfordi | ||||
| Insecta: Diptera, Syrphidae | ||||
| Insecta: Hymenoptera, | Parasitic (gall formation) | [34] | ||
| Chalcidoidea; Eupelmus confusus, | ||||
| Eurytoma gallephedrae, | Parasitic (inhabiting seeds). | |||
| Eupelmus gemellus | Unknown | |||
| E. major Host (Syn. E. nebrodensis) | Spain | Insecta: Hymenoptera | Parasitic (inhabiting seeds). | [36] |
| Pteromalidae; Blascoa ephedrae, Mesopolobus semenis, Mesopolobus arcanus | Phytophagous | |||
| Eupelmidae; Eupelmus sp. | ||||
| Eulophidae; Aprostocetus iutescens, Baryscapus aenescens | ||||
| Braconidae; Bracon sp. | ||||
| Spain | Insecta: Coleoptera | Phytophagy | [37] | |
| Curculionidae; Theodorinus hispanicus, Paroxyonyx imitator | ||||
| Hymenoptera | ||||
| Chalcidoidea; Eurytoma sp. Nikanoria ephedrae | Parasitic (gall formation) | |||
| Spain | Insecta: Hymenoptera Chalcidoidea; Eurytoma gallephedrae | Parasitic (gall formation) | [34] | |
| E. trifurca Torr. ex S.Watson | U.S.A | Insecta: Diptera | Phytophagy | [31,38] |
| Cecidomyiidae; Lasioptera ephedrae: galls are simple stem swellings without obvious fungal presence | ||||
| U.S.A | Insecta: Diptera: | Phytophagy | [31] | |
| Cecidomyiidae; Lasioptera ephedricola: The gall midge L. ephedricola act as vector of the black yeast Aureobasidium pullulans (Dothideomycetes: Dothideales) | ||||
| U.S.A | Insecta: Hemiptera | Host in Ephedra | [33] | |
| Ephedrodoma multilineata |
| Species | Geographic Distribution | Ephedra Alkaloids | Other Metabolites with Relative Abundance | Reference |
|---|---|---|---|---|
| E. sinica Stapf | Eurasia | * | Tetramethyl pyrazine (26) | [99] |
| Terpinen-4-ol | [46] | |||
| Linalol | [47] | |||
| α-Terpineol | [101] | |||
| 2,3-Dihydro-2-methylbenzofuran | ||||
| cis-p-Menth-2-en-7-ol | ||||
| Mahuannins B (99), D (95), E (96) and F (97) | ||||
| Ephedranin A (98) | ||||
| Herbacetin 8-methyl ether 3-glucoside (69) | ||||
| p-Vinylanisole | ||||
| Phytol | ||||
| γEudesmanol | ||||
| Eudesm-7(11)-4-en-ol | ||||
| γ-Sitosterol | ||||
| 9Z,12Z-Octadecadienoic acid | ||||
| E. alata Decne. | Eurasia | * | 6-Methoxykynurenic acid (12) | [67] |
| Nilocitin (59) | [102] | |||
| Ephedralone (14) | [6] | |||
| Herbacetin 8-methyl ether 3-O-Glucoside-7-O-rutinoside (74) | ||||
| Herbacetin-7-O-(6″-quinylglucoside) (75) | ||||
| Herbacetin 7-glucoside (63), Vicenin II (66) | ||||
| Lucenin III (71) | ||||
| Kaempferol-3-O-rhamnoside (72) | ||||
| Quercetin 3-O-rhamnoside (73) | ||||
| E. intermedia Schrenk. & C.A.Mey. | Eurasia | - | Ephedraloxane (7) | [64] |
| E. foliata Boiss. ex C.A.Mey. | Eurasia | 6-Hydroxykynurenic acid (11) | [65] | |
| cis-3,4-Methanoproline | ||||
| E. transitoria Riedl | Eurasia | Transtorine (13) | [66] | |
| E. pachyclada Boiss. | Eurasia | * | 6-Methoxykynurenic acid (12) | [102] |
| 6-Hydroxykynurenic acid (11), Kynurenic acid (10) | [65] | |||
| E. altissima Desf. | Eurasia | * | (2S,3S,4S)-2-(Carboxycyclopropyl)glycine (103) | [65] |
| E. lomatolepis Schrenk | Eurasia | Proanthocyanidins | [103] | |
| E. foeminea Forssk. | Eurasia | * | (2S,3R,4S)-2-(Carboxycyclopropyl)glycine (105) | [65] |
| 6-Hydroxykynurenic acid (11) | ||||
| cis-3,4-Methanoproline | ||||
| E. fragilis Desf. | Eurasia | * | (2S,3R,4S)-2-(Carboxycyclopropyl)glycine (105) | [76] |
| E. distachya subsp. helvetica (C.A.Mey.) Asch. & Graebn. (Syn. E. helvetica) | Eurasia | * | Catechin (45) | [82] |
| Gallocatechin (47) | [6] | |||
| E. major Host (Syn. E. nebrodensis) | Eurasia | - | Nebrodenside A (44) and B (36) | [84] |
| o-Coumaric acid glucoside (37) | ||||
| E. viridis Coville | North America | Lariciresinol (55) | [85] | |
| Isolariciresinol (56) | ||||
| 9-Acetoxylariciresinol (57) | ||||
| 9-Acetoxyisolariciresinol (58) | ||||
| E. antisyphytica S.Watson | North America | - | Apigenin (52), | [6] |
| Lucenin 1 (70), | ||||
| Lucenin 3 (71) | ||||
| E. fasciculata A.Nelson | North America | - | 4-Hydroxyquinoline-2-carboxylic acid (10) | [76] |
| E. funerea Coville & C.V.Morton | North America | - | 4-Hydroxyquinoline-2-carboxylic acid (10) | [76] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Juárez, D.E.; Escobedo-Moratilla, A.; Flores, J.; Hidalgo-Figueroa, S.; Martínez-Tagüeña, N.; Morales-Jiménez, J.; Muñiz-Ramírez, A.; Pastor-Palacios, G.; Pérez-Miranda, S.; Ramírez-Hernández, A.; et al. A Review of the Ephedra genus: Distribution, Ecology, Ethnobotany, Phytochemistry and Pharmacological Properties. Molecules 2020, 25, 3283. https://doi.org/10.3390/molecules25143283
González-Juárez DE, Escobedo-Moratilla A, Flores J, Hidalgo-Figueroa S, Martínez-Tagüeña N, Morales-Jiménez J, Muñiz-Ramírez A, Pastor-Palacios G, Pérez-Miranda S, Ramírez-Hernández A, et al. A Review of the Ephedra genus: Distribution, Ecology, Ethnobotany, Phytochemistry and Pharmacological Properties. Molecules. 2020; 25(14):3283. https://doi.org/10.3390/molecules25143283
Chicago/Turabian StyleGonzález-Juárez, Daphne E., Abraham Escobedo-Moratilla, Joel Flores, Sergio Hidalgo-Figueroa, Natalia Martínez-Tagüeña, Jesús Morales-Jiménez, Alethia Muñiz-Ramírez, Guillermo Pastor-Palacios, Sandra Pérez-Miranda, Alfredo Ramírez-Hernández, and et al. 2020. "A Review of the Ephedra genus: Distribution, Ecology, Ethnobotany, Phytochemistry and Pharmacological Properties" Molecules 25, no. 14: 3283. https://doi.org/10.3390/molecules25143283