Endophytes, a Potential Source of Bioactive Compounds to Curtail the Formation–Accumulation of Advanced Glycation End Products: A Review
Abstract
:1. Introduction
2. Endophytes, an Exceptional Source of Bioactive Compounds
3. Non-Communicable Diseases, a Global Health Problem
4. Advanced Glycation End Products
4.1. High Levels of AGEs Accumulation Are Linked to Various Diseases
4.2. Reducing AGEs Accumulation as a Potential Treatment Strategy for Some NCDs
- Blocking the carbonyl groups of reducing sugars or stabilizing the protein structure to inhibit the Maillard reaction or the formation of Schiff bases and Amadori products;
- Scavenging of free radicals and chelating metal ions. Consequently, fewer reactive carbonyl groups and fewer radical-based reactions occur;
- Blocking or breaking the AGEs cross-links to lessen the protein aggregation;
- Disrupting the AGEs–RAGE interaction, thus preventing inflammatory process and oxidative stress;
- Some indirect mechanisms may be stimulating the glyoxalase system and other dicarbonyl detoxification systems to reduce the available AGEs precursors. Inhibition of polyol pathway enzymes (aldose reductase and sorbitol dehydrogenase) to reduce fructose intake and hypoglycemic activity to reduce sugar availability, etc. [9,60].

4.3. Synthetic AntiAGEs Compounds
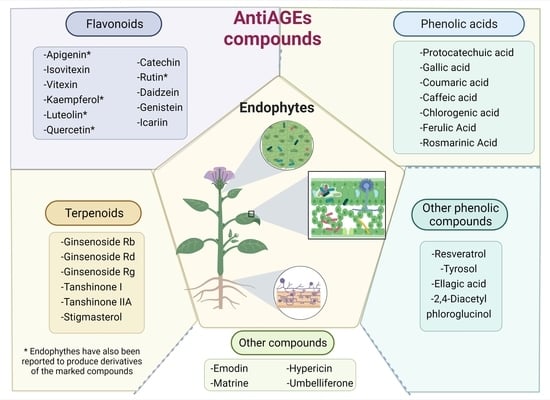
5. Plant AntiAGEs Compounds Also Are Found in Endophytes
5.1. Plant-Derived AntiAGEs Polyphenols Reported in Endophytes
5.2. AntiAGEs Terpenoids Reported as Metabolites from Endophytes
5.3. Other AntiAGEs Compounds Reported in Endophytes
6. Endophytes May Encourage the Production of antiAGEs Compounds by Plants
7. AntiAGEs Compounds Production: Endophytes vs. Plants
- Shorter production time. Microorganisms grow much faster than plants. Consequently, metabolite mass manufacturing with endophytes may be achieved in shorter periods compared to plants.
- Environmentally friendly. Culturing microorganisms does not require the use of large land areas. This averts overharvesting and reduces dependence on plant biodiversity.
- Reliable metabolite production throughout the year. Endophyte-based metabolite production does not depend on seasonal growth, in contrast to plants, nor on weather fluctuations or geographical conditions. Secondary metabolites could be produced at any time of the year with endophytes.
- More economical process. Usually, microbial sources of valued products are cheaper because they can be mass-produced; this may have an impact on the market price of the compounds of interest.
8. Challenges for the Future Use of Endophytes as Sources of AntiAGEs Compounds
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Van Staden, J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020, 39, 107462. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Non Communicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 19 May 2022).
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.D.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16R–28R. [Google Scholar] [CrossRef]
- Zeng, C.; Li, Y.; Ma, J.; Niu, L.; Tay, F.R. Clinical/translational aspects of advanced glycation end products. Trends Endocrinol. Metab. 2019, 30, 959–973. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of Advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Aldini, G.; Vistoli, G.; Stefek, M.; Chondrogianni, N.; Grune, T.; Sereikaite, J.; Sadowska-Bartosz, I.; Bartosz, G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013, 47 (Suppl. 1), 93–137. [Google Scholar] [CrossRef] [Green Version]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef]
- Chinchansure, A.A.; Korwar, A.M.; Kulkarni, M.J.; Joshi, S.P. Recent Development of plant products with anti-glycation activity: A review. RSC Adv. 2015, 5, 31113–31138. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Prevention of Protein glycation by natural compounds. Molecules 2015, 20, 3309–3334. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Sultana, R.; Lee, S.M.; Kim, T.H.; Kim, S.Y. Phytochemicals against antidiabetic complications: Targeting the advanced glycation end product signaling pathway. Arch. Pharm. Res. 2021, 44, 378–401. [Google Scholar] [CrossRef]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end products. Food Funct. 2011, 2, 289. [Google Scholar] [CrossRef] [PubMed]
- Velichkova, S.; Foubert, K.; Pieters, L. Natural products as a source of inspiration for novel inhibitors of advanced glycation end products (AGEs) formation. Planta Med. 2021, 87, 780–801. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010, 41, 1–16. [Google Scholar] [CrossRef]
- Mishra, S.; Sahu, P.K.; Agarwal, V.; Singh, N. Exploiting endophytic microbes as micro-factories for plant secondary metabolite production. Appl. Microbiol. Biotechnol. 2021, 105, 6579–6596. [Google Scholar] [CrossRef]
- Stone, J.K.; Bacon, C.W.; White, J.F. An overview of endophytic microbes: Endophytism defined. In Microbial Endophytes; Marcel Dekker: New York, NY, USA, 2000; pp. 29–33. [Google Scholar]
- Kloepper, J.W.; McInroy, J.A.; Liu, K.; Hu, C.-H. Symptoms of fern distortion syndrome resulting from inoculation with opportunistic endophytic fluorescent Pseudomonas spp. PLoS ONE 2013, 8, e58531. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.-W.; Chuang, Y.-Y.; Lee, M.-Z.; Kirschner, R. First inventory of fungi in symptomless and symptomatic Chinese mesona indicates phytopathological threat. Plant Dis. 2020, 104, 2391–2397. [Google Scholar] [CrossRef]
- Frank, A.; Saldierna Guzmán, J.; Shay, J. Transmission of bacterial endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Papik, J.; Folkmanova, M.; Polivkova-Majorova, M.; Suman, J.; Uhlik, O. The invisible life inside plants: Deciphering the riddles of endophytic bacterial diversity. Biotechnol. Adv. 2020, 44, 107614. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites (1987 to 2000). Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: Insights, avenues, and challenges. Microorganisms 2021, 9, 197. [Google Scholar] [CrossRef]
- World Health Organization; FAO (Eds.) Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a WHO-FAO Expert Consultation; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 19 July 2021).
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Providers. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 19 January 2022).
- Reynaert, N.L.; Gopal, P.; Rutten, E.P.A.; Wouters, E.F.M.; Schalkwijk, C.G. Advanced glycation end products and their receptor in age related, non-communicable chronic inflammatory diseases; overview of clinical evidence and potential contributions to disease. Int. J. Biochem. Cell Biol. 2016, 81, 403–418. [Google Scholar] [CrossRef]
- Kuzan, A. Toxicity of advanced glycation end products (review). Biomed. Rep. 2021, 14, 46. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation end products—role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. Methylglyoxal and glucose metabolism: A historical perspective and future avenues for research. Drug Metabol. Drug Interact. 2008, 23, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Henning, C.; Glomb, M.A. Pathways of the Maillard reaction under physiological conditions. Glycoconj. J. 2016, 33, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced glycation end products (AGEs): Biochemistry, signaling, analytical methods, and epigenetic effects. Oxid. Med. Cell. Longev. 2020, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of advanced glycation end product (AGE) formation and accumulation. In Reactive Oxygen Species; Schmidt, H.H.H.W., Ghezzi, P., Cuadrado, A., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2020; Volume 264, pp. 395–423. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef] [Green Version]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. Negative consequences of glycation. Metabolism 2000, 49, 9–13. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Lu, C.-H.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. The development of Maillard reaction, and advanced glycation end product (AGE)-Receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef]
- Goh, S.-Y.; Cooper, M.E. The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groener, J.B.; Oikonomou, D.; Cheko, R.; Kender, Z.; Zemva, J.; Kihm, L.; Muckenthaler, M.; Peters, V.; Fleming, T.; Kopf, S.; et al. Methylglyoxal and advanced glycation end products in patients with diabetes—What we know so far and the missing links. Exp. Clin. Endocrinol. Diabetes 2019, 127, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Sun, L.; Lu, Y.; Zhang, Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J. Neurol. Sci. 2012, 317, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Kamil, M.; Hasan, A.; Irfan, S.; Sheikh, S.; Khatoon, A.; Nazir, A.; Mir, S.S. Advanced glycation end products (AGEs), protein aggregation and their cross talk: New insight in tumorigenesis. Glycobiology 2020, 30, 2–18. [Google Scholar] [CrossRef]
- Fukami, K.; Yamagishi, S.; Okuda, S. Role of AGEs-RAGE system in cardiovascular disease. Curr. Pharm. Des. 2014, 20, 2395–2402. [Google Scholar] [CrossRef]
- Dozio, E.; Vettoretti, S.; Lungarella, G.; Messa, P.; Corsi Romanelli, M.M. Sarcopenia in chronic kidney disease: Focus on advanced glycation end products as mediators and markers of oxidative stress. Biomedicines 2021, 9, 405. [Google Scholar] [CrossRef]
- Willett, T.L.; Pasquale, J.; Grynpas, M.D. Collagen modifications in postmenopausal osteoporosis: Advanced glycation end products may affect bone volume, structure and quality. Curr. Osteoporos. Rep. 2014, 12, 329–337. [Google Scholar] [CrossRef]
- Tarannum, A.; Zarina, A.R.I.F.; Khursheed, A.L.A.M.; Ahmad, S.; Uddin, M. Nitroxidized-albumin advanced glycation end product and rheumatoid arthritis. Arch. Rheumatol. 2019, 34, 461–475. [Google Scholar] [CrossRef] [Green Version]
- de Leeuw, K.; Graaff, R.; de Vries, R.; Dullaart, R.P.; Smit, A.J.; Kallenberg, C.G.; Bijl, M. Accumulation of advanced glycation endproducts in patients with systemic lupus erythematosus. Rheumatology 2007, 46, 1551–1556. [Google Scholar] [CrossRef] [Green Version]
- Papagrigoraki, A.; Maurelli, M.; del Giglio, M.; Gisondi, P.; Girolomoni, G. Advanced glycation end products in the pathogenesis of psoriasis. Int. J. Mol. Sci. 2017, 18, 2471. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.; Bhanumathy, K.K. AGE-RAGE axis in the pathophysiology of chronic lower limb ischemia and a novel strategy for its treatment. Int. J. Angiol. 2020, 29, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kaur, S.; Sarkar, M.; Sarin, B.C.; Changotra, H. The AGE-RAGE axis and RAGE genetics in chronic obstructive pulmonary disease. Clin. Rev. Allergy Immunol. 2021, 60, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Lindner, C.; Gonzàlez, I.; Morales, M.A. Advanced-glycation end-products axis: A contributor to the risk of severe illness from COVID-19 in diabetes patients. World J. Diabetes 2021, 12, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Jahan, H.; Choudhary, I. Glycation, carbonyl stress and AGEs inhibitors: A patent review. Expert Opin. Ther. Pat. 2015, 25, 1267–1284. [Google Scholar]
- Palimeri, S.; Diamanti-Kandarakis, E.; Palioura, E. Current perspectives on the health risks associated with the consumption of advanced glycation end products: Recommendations for dietary management. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 415. [Google Scholar] [CrossRef] [Green Version]
- FDA. FDA Approves Oral Form for the Treatment of Adults with Amyotrophic Lateral Sclerosis (ALS). 2022. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-oral-form-treatment-adults-amyotrophic-lateral-sclerosis-als (accessed on 29 May 2022).
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-derived bioactive compounds produced by endophytic fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [Green Version]
- Toghueo, R.M.; Boyom, F.F. Endophytes from ethno-pharmacological plants: Sources of novel antioxidants- a systematic review. Biocatal. Agric. Biotechnol. 2019, 22, 101430. [Google Scholar] [CrossRef]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs. metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef]
- Gutiérrez-García, K.; Neira-González, A.; Pérez-Gutiérrez, R.M.; Granados-Ramírez, G.; Zarraga, R.; Wrobel, K.; Barona-Gómez, F.; Flores-Cotera, L.B. Phylogenomics of 2,4-diacetylphloroglucinol-producing Pseudomonas and novel antiglycation endophytes from Piper auritum. J. Nat. Prod. 2017, 80, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- West, B.J.; Deng, S.; Uwaya, A.; Isami, F.; Abe, Y.; Yamagishi, S.; Jensen, C.J. Iridoids are natural glycation inhibitors. Glycoconj. J. 2016, 33, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Vijaykrishnaraj, M.; Wang, K. Dietary natural products as a potential inhibitor towards advanced glycation end products and hyperglycemic complications: A phytotherapy approaches. Biomed. Pharmacother. 2021, 144, 112336. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Tsai, S.-J.; Huang, C.-S.; Yin, M.-C. Antiglycative effects of protocatechuic acid in the kidneys of diabetic mice. J. Agric. Food Chem. 2011, 59, 5117–5124. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Dua, T.K.; Khanra, R.; Joardar, S.; Nandy, A.; Saha, A.; De Feo, V.; Dewanjee, S. Protocatechuic acid, a phenolic from Sansevieria roxburghiana leaves, suppresses diabetic cardiomyopathy via stimulating glucose metabolism, ameliorating oxidative stress, and inhibiting inflammation. Front. Pharmacol. 2017, 8, 251. [Google Scholar] [CrossRef]
- Eze, P.M.; Nnanna, J.C.; Okezie, U.; Buzugbe, H.S.; Abba, C.C.; Chukwunwejim, C.R.; Okoye, F.B.C.; Esimone, C.O. Screening of metabolites from endophytic fungi of some Nigerian medicinal plants for antimicrobial activities. EuroBiotech J. 2019, 3, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, M.; Abdel-Razek, A.S.; Previtali, V.; Clausen, M.H.; Gotfredsen, C.H.; Laatsch, H.; Ding, L. Sulochrins and alkaloids from a fennel endophyte Aspergillus sp. FVL2. Nat. Prod. Res. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Wu, J.-W.; Hsieh, C.-L.; Wang, H.-Y.; Chen, H.-Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Umadevi, S.; Gopi, V.; Elangovan, V. Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chem. Biol. Interact. 2014, 208, 28–36. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Su, T.-J.; Cai, S.-M.; Wu, W. Fungal endophyte-derived Fritillaria unibracteata var. Wabuensis: Diversity, antioxidant capacities in vitro and relations to phenolic, flavonoid or saponin compounds. Sci. Rep. 2017, 7, 42008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, F.-H.; Jing, T.-Z.; Wang, Z.-X.; Zhan, Y.-G. Fungal endophytes from Acer ginnala Maxim: Isolation, identification and their yield of gallic acid. Lett. Appl. Microbiol. 2009, 49, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, P.; Kumar, A.; Chadha, P.; Kaur, R.; Kaur, A. Antioxidant and in vivo genoprotective effects of phenolic compounds identified from an endophytic Cladosporium velox and their relationship with its host plant Tinospora Cordifolia. J. Ethnopharmacol. 2016, 194, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Parvandi, M.; Rezadoost, H.; Farzaneh, M. Introducing Alternaria tenuissima SBUp1, as an endophytic fungus of Ferula assa-foetida from Iran, which is a rich source of rosmarinic acid. Lett. Appl. Microbiol. 2021, 73, 569–578. [Google Scholar] [CrossRef]
- Maruf, A.A.; Lip, H.; Wong, H.; O’Brien, P.J. Protective effects of ferulic acid and related polyphenols against glyoxal- or methylglyoxal-induced cytotoxicity and oxidative stress in isolated rat hepatocytes. Chem. Biol. Interact. 2015, 234, 96–104. [Google Scholar] [CrossRef]
- Selvakumar, G.; Venu, D.; Kuttalam, I.; Lonchin, S. Inhibition of advanced glycation end product formation in rat tail tendons by polydatin and p-coumaric acid: An in vitro study. Appl. Biochem. Biotechnol. 2022, 194, 339–353. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bastos, D.H.M.; Schulze, J.; Souza, M.F.F. Caffeic and chlorogenic acids in Ilex Paraguariensis extracts are the main inhibitors of age generation by methylglyoxal in model proteins. Fitoterapia 2009, 80, 339–344. [Google Scholar] [CrossRef]
- Chao, C.; Mong, M.; Chan, K.; Yin, M. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef]
- Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Stancu, C.S.; Sima, A.V. Caffeic acid attenuates the inflammatory stress induced by glycated LDL in human endothelial cells by mechanisms involving inhibition of AGE-receptor, oxidative, and endoplasmic reticulum stress: Caffeic Acid Attenuates Inflammation in Endothelial Cells. BioFactors 2017, 43, 685–697. [Google Scholar] [CrossRef]
- Wu, C.-H.; Huang, H.-W.; Lin, J.-A.; Huang, S.-M.; Yen, G.-C. The proglycation effect of caffeic acid leads to the elevation of oxidative stress and inflammation in monocytes, macrophages and vascular endothelial cells. J. Nutr. Biochem. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Das, M.; Prakash, H.S.; Nalini, M.S. Bioactive sesquiterpene, plasticizer, and phenols from the fungal endophytes of Polygonum chinense L. Ann. Microbiol. 2018, 68, 595–609. [Google Scholar] [CrossRef]
- Wang, X.; Qin, L.; Zhou, J.; Li, Y.; Fan, X. A novel design to screen chlorogenic acid-producing microbial strains from the environment. Sci. Rep. 2018, 8, 14756. [Google Scholar] [CrossRef]
- Gagana, S.L.; Kumaraswamy, B.E.; Shivanna, M.B. Diversity, antibacterial and antioxidant activities of the fungal endophytes associated with Schleichera oleosa (Lour.) Merr. S. Afr. J. Bot. 2020, 134, 369–381. [Google Scholar] [CrossRef]
- Kaur, N.; Arora, D.S.; Kalia, N.; Kaur, M. Antibiofilm, antiproliferative, antioxidant and antimutagenic activities of an endophytic fungus Aspergillus fumigatus from Moringa oleifera. Mol. Biol. Rep. 2020, 47, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Srey, C.; Hull, G.L.J.; Connolly, L.; Elliott, C.T.; del Castillo, M.D.; Ames, J.M. Effect of inhibitor compounds on Nε-(carboxymethyl)lysine (CML) and Nε-(carboxyethyl)lysine (CEL) formation in model foods. J. Agric. Food Chem. 2010, 58, 12036–12041. [Google Scholar] [CrossRef] [Green Version]
- Silván, J.M.; Assar, S.H.; Srey, C.; Dolores del Castillo, M.; Ames, J.M. Control of the Maillard reaction by ferulic acid. Food Chem. 2011, 128, 208–213. [Google Scholar] [CrossRef]
- Liu, J.; He, Y.; Wang, S.; He, Y.; Wang, W.; Li, Q.; Cao, X. Ferulic acid inhibits advanced glycation end products (AGEs) formation and mitigates the AGEs-induced inflammatory response in HUVEC cells. J. Funct. Foods 2018, 48, 19–26. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Wang, M.; Ou, S. Effect of rosmarinic acid and carnosic acid on AGEs formation in vitro. Food Chem. 2017, 221, 1057–1061. [Google Scholar] [CrossRef]
- Shamsi, A.; Ahmed, A.; Khan, M.S.; Husain, F.M.; Bano, B. Rosmarinic acid restrains protein glycation and aggregation in human serum albumin: Multi spectroscopic and microscopic insight-possible therapeutics targeting diseases. Int. J. Biol. Macromol. 2020, 161, 187–193. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, I.-H.; Kim, C.-S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch. Pharm. Res. 2011, 34, 495–500. [Google Scholar] [CrossRef]
- Chen, X.; Sang, X.; Li, S.; Zhang, S.; Bai, L. Studies on a chlorogenic acid-producing endophytic fungi isolated from Eucommia ulmoides Oliver. J. Ind. Microbiol. Biotechnol. 2010, 37, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Bibi, N.; Jan, G.; Jan, F.G.; Hamayun, M.; Iqbal, A.; Hussain, A.; Rehman, H.; Tawab, A.; Khushdil, F. Cochliobolus sp. acts as a biochemical modulator to alleviate salinity stress in okra plants. Plant Physiol. Biochem. 2019, 139, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Kim, J.S. Constituents of the flowers of Platycodon grandiflorum with inhibitory activity on advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharm. Res. 2010, 33, 875–880. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.-W.; Gong, J.; Li, E.T.S.; Wang, M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem. Pharmacol. 2019, 166, 231–241. [Google Scholar] [CrossRef]
- Gu, C.B.; Ma, H.; Ning, W.J.; Niu, L.L.; Han, H.Y.; Yuan, X.H.; Fu, Y.J. Characterization, culture medium optimization and antioxidant activity of an endophytic vitexin-producing fungus Dichotomopilus funicola Y3 from pigeon pea [Cajanus cajan (L.) Millsp.]. J. Appl. Microbiol. 2018, 125, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Zhang, Z.; Niu, L.; Gu, C.; Zheng, W.; Cui, H.; Yuan, X. Fusarium solani G6, a novel vitexin-producing endophytic fungus: Characterization, yield improvement and osteoblastic proliferation activity. Biotechnol. Lett. 2021, 43, 1371–1383. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, J.; Zu, Y.; Fu, Y.; Liang, L.; Luo, M.; Wang, W.; Efferth, T. Antioxidant properties, superoxide dismutase and glutathione reductase activities in HepG2 cells with a fungal endophyte producing apigenin from pigeon pea [Cajanus cajan (L.) Millsp.]. Food Res. Int. 2012, 49, 147–152. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol modulates pro-inflammatory NF-ΚB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. AGE 2010, 32, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Al-Musayeib, N.; Perveen, S.; Fatima, I.; Nasir, M.; Hussain, A. Antioxidant, anti-glycation and anti-inflammatory activities of phenolic constituents from Cordia sinensis. Molecules 2011, 16, 10214–10226. [Google Scholar] [CrossRef] [Green Version]
- Veeresham, C.; Rama Rao, A.; Asres, K. Aldose reductase inhibitors of plant origin: Aldose reductase inhibitors. Phytother. Res. 2014, 28, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Malik, S.; Khan, S.; Malhotra, R.; Goyal, S.; Bhatia, J.; Ojha, S.; Arya, D. Molecular pathways involved in the amelioration of myocardial injury in diabetic rats by kaempferol. Int. J. Mol. Sci. 2017, 18, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-X.; Zhang, J.; Zhang, X.-R.; Zhang, K.; Zhang, X.; He, X.-R. Mucor fragilis as a novel source of the key pharmaceutical agents podophyllotoxin and kaempferol. Pharm. Biol. 2014, 52, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Tijith, K.G.; Joy, A.; Divya, K.; Jisha, M.S. In vitro and in silico docking studies of antibacterial compounds derived from endophytic Penicillium setosum. Microb. Pathog. 2019, 131, 87–97. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Matkowski, A. Inhibition of advanced glycation end-product formation and antioxidant activity by extracts and polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef] [Green Version]
- Ebada, S.S.; Eze, P.; Okoye, F.B.C.; Esimone, C.O.; Proksch, P. The fungal endophyte Nigrospora oryzae produces quercetin monoglycosides previously known only from plants. ChemistrySelect 2016, 1, 2767–2771. [Google Scholar] [CrossRef]
- Cervantes-Laurean, D.; Schramm, D.D.; Jacobson, E.L.; Halaweish, I.; Bruckner, G.G.; Boissonneault, G.A. Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites. J. Nutr. Biochem. 2006, 17, 531–540. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.; Li, X.; Fu, X.; Sui, Y.; Guo, T.; Xie, B.; Sun, Z. A significant inhibitory effect on advanced glycation end product formation by catechin as the major metabolite of Lotus seedpod oligomeric procyanidins. Nutrients 2014, 6, 3230–3244. [Google Scholar] [CrossRef]
- Shao, X.; Chen, H.; Zhu, Y.; Sedighi, R.; Ho, C.-T.; Sang, S. Essential structural requirements and additive effects for flavonoids to scavenge methylglyoxal. J. Agric. Food Chem. 2014, 62, 3202–3210. [Google Scholar] [CrossRef]
- Hsieh, P.-W.; Hsu, L.-C.; Lai, C.-H.; Wu, C.-C.; Hwang, T.-L.; Lin, Y.-K.; Wu, Y.-C. Evaluation of the bioactivities of extracts of endophytes isolated from Taiwanese herbal plants. World J. Microbiol. Biotechnol. 2009, 25, 1461–1469. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.-M.; Guan, S.-S.; Chiang, C.-K.; Wu, C.-T.; Liu, S.-H. The Antifibrotic and anti-inflammatory effects of icariin on the kidney in a unilateral ureteral obstruction mouse model. Phytomedicine 2019, 59, 152917. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Shi, P.; Liu, Y.; Li, T.; Liu, S.; Wang, C.; Wang, L.; Cao, Y. Icariin treatment reduces blood glucose levels in type 2 diabetic rats and protects pancreatic function. Exp. Ther. Med. 2020, 19, 2690–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2–Keap1 signaling. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2011, 1812, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Xu, Z.; Sheng, Z. Ability of resveratrol to inhibit advanced glycation end product formation and carbohydrate-hydrolyzing enzyme activity, and to conjugate methylglyoxal. Food Chem. 2017, 216, 153–160. [Google Scholar] [CrossRef]
- Shi, J.; Zeng, Q.; Liu, Y.; Pan, Z. Alternaria sp. MG1, a resveratrol-producing fungus: Isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol. 2012, 95, 369–379. [Google Scholar] [CrossRef]
- Dwibedi, V.; Saxena, S. Arcopilus aureus, a resveratrol-producing endophyte from Vitis vinifera. Appl. Biochem. Biotechnol. 2018, 186, 476–495. [Google Scholar] [CrossRef]
- Dwibedi, V.; Saxena, S. Diversity and phylogeny of resveratrol-producing culturable endophytic fungi from Vitis species in India. 3 Biotech 2019, 9, 182. [Google Scholar] [CrossRef]
- Dwibedi, V.; Saxena, S. Effect of precursor feeding, dietary supplementation, chemical elicitors and co-culturing on resveratrol production by Arcopilus aureus. Prep. Biochem. Biotechnol. 2022, 52, 404–412. [Google Scholar] [CrossRef]
- Liu, Y.; Nan, L.; Liu, J.; Yan, H.; Zhang, D.; Han, X. Isolation and identification of resveratrol-producing endophytes from wine grape Cabernet Sauvignon. SpringerPlus 2016, 5, 1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandramohan, R.; Pari, L.; Rathinam, A.; Sheikh, B.A. Tyrosol, a phenolic compound, ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Chem. Biol. Interact. 2015, 229, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-I.; Jang, H.-D.; Shetty, K. Evaluation of Rhodiola crenulata and Rhodiola rosea for management of type II diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 425–432. [Google Scholar] [PubMed]
- McMullin, D.R.; Green, B.D.; Prince, N.C.; Tanney, J.B.; Miller, J.D. Natural products of Picea endophytes from the Acadian Forest. J. Nat. Prod. 2017, 80, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Borges Coutinho Gallo, M.; Coêlho Cavalcanti, B.; Washington Araújo Barros, F.; Odorico de Moraes, M.; Veras Costa-Lotufo, L.; Pessoa, C.; Kenupp Bastos, J.; Tallarico Pupo, M. Chemical constituents of Papulaspora immersa, an endophyte from Smallanthus sonchifolius (Asteraceae), and their cytotoxic activity. Chem. Biodivers. 2010, 7, 2941–2950. [Google Scholar] [CrossRef]
- Cui, J.; Guo, T.; Chao, J.; Wang, M.; Wang, J. Potential of the endophytic fungus Phialocephala fortinii Rac56 found in Rhodiola plants to produce salidroside and p-tyrosol. Molecules 2016, 21, 502. [Google Scholar] [CrossRef] [Green Version]
- Rathnayake, G.R.N.; Savitri Kumar, N.; Jayasinghe, L.; Araya, H.; Fujimoto, Y. Secondary metabolites produced by an endophytic fungus Pestalotiopsis microspora. Nat. Prod. Bioprospect. 2019, 9, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.-L.; Giner, R.; Marín, M.; Recio, M. A Pharmacological update of ellagic acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [Green Version]
- Jannat, S.; Rahman, M.M. Ginsenoside derivatives inhibit advanced glycation end-product formation and glucose–fructose mediated protein glycation in vitro via a specific structure–activity relationship. Bioorg. Chem. 2021, 111, 104844. [Google Scholar] [CrossRef]
- Jin, Z.; Gao, L.; Zhang, L.; Liu, T.; Yu, F.; Zhang, Z.; Guo, Q.; Wang, B. Antimicrobial activity of saponins produced by two novel endophytic fungi from Panax notoginseng. Nat. Prod. Res. 2017, 31, 2700–2703. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.-Y.; You, X.-L.; Li, Y.-H. Diversity of endophytic fungi from roots of Panax ginseng and their saponin yield capacities. SpringerPlus 2013, 2, 107. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Yang, H.; You, X.; Li, Y. Isolation and characterization of saponin-producing fungal endophytes from Aralia elata in northeast China. Int. J. Mol. Sci. 2012, 13, 16255–16266. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Lin, C.; Liu, Q.; He, Y.; Ruganzu, J.B.; Jin, H.; Peng, X.; Ji, S.; Ma, Y.; Yang, W. Tanshinone IIA Attenuates Neuroinflammation via Inhibiting RAGE/NF-ΚB signaling pathway in vivo and in vitro. J. Neuroinflamm. 2020, 17, 302. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Jung, K.-H.; Lee, B.-C. Protective effect of tanshinone IIA on the early stage of experimental diabetic nephropathy. Biol. Pharm. Bull. 2009, 32, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Ming, Q.; Han, T.; Li, W.; Zhang, Q.; Zhang, H.; Zheng, C.; Huang, F.; Rahman, K.; Qin, L. Tanshinone IIA and tanshinone I production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomedicine 2012, 19, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, R.; Zhan, F.; Mekawi, E.; Khalifa, I.; Liang, H.; Li, B. The noncovalent conjugations of bovine serum albumin with three structurally different phytosterols exerted antiglycation effects: A study with AGEs-inhibition, multispectral, and docking investigations. Bioorg. Chem. 2020, 94, 103478. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Liu, T.; Xin, Z. The individual lipid compositions produced by Cunninghamella sp. salicorn 5, an endophytic oleaginous fungus from Salicornia bigelovii Torr. Eur. Food Res. Technol. 2014, 238, 621–633. [Google Scholar] [CrossRef]
- Jang, D.S.; Lee, G.Y.; Kim, Y.S.; Lee, Y.M.; Kim, C.-S.; Yoo, J.L.; Kim, J.S. Anthraquinones from the seeds of Cassia tora with inhibitory activity on protein glycation and aldose reductase. Biol. Pharm. Bull. 2007, 30, 2207–2210. [Google Scholar] [CrossRef] [Green Version]
- Alhadrami, H.A.; Sayed, A.M.; El-Gendy, A.O.; Shamikh, Y.I.; Gaber, Y.; Bakeer, W.; Sheirf, N.H.; Attia, E.Z.; Shaban, G.M.; Khalifa, B.A.; et al. A metabolomic approach to target antimalarial metabolites in the Artemisia annua fungal endophytes. Sci. Rep. 2021, 11, 2770. [Google Scholar] [CrossRef]
- Xie, J.; Strobel, G.A.; Feng, T.; Ren, H.; Mends, M.T.; Zhou, Z.; Geary, B. An endophytic Coniochaeta velutina producing broad spectrum antimycotics. J. Microbiol. 2015, 53, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Kusari, S.; Zühlke, S.; Košuth, J.; Čellárová, E.; Spiteller, M. Light-independent metabolomics of endophytic Thielavia subthermophila provides insight into microbial hypericin biosynthesis. J. Nat. Prod. 2009, 72, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Park, J.J.; Islam, M.N.; Jin, S.E.; Min, B.-S.; Lee, J.-H.; Sohn, H.S.; Choi, J.S. Inhibitory activity of coumarins from Artemisia capillaris against advanced glycation endproduct formation. Arch. Pharm. Res. 2012, 35, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Koorbanally, N.A.; Islam, M.S. Umbelliferone stimulates glucose uptake; modulates gluconeogenic and nucleotide-hydrolyzing enzymes activities, and dysregulated lipid metabolic pathways in isolated psoas muscle. J. Funct. Foods 2020, 67, 103847. [Google Scholar] [CrossRef]
- Cui, L.; Cai, Y.; Cheng, W.; Liu, G.; Zhao, J.; Cao, H.; Tao, H.; Wang, Y.; Yin, M.; Liu, T.; et al. A novel, multi-target natural drug candidate, matrine, improves cognitive deficits in Alzheimer’s disease transgenic mice by inhibiting aβ aggregation and blocking the RAGE/Aβ axis. Mol. Neurobiol. 2017, 54, 1939–1952. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Optimization of submerged fermentation medium for matrine production by Aspergillus terreus, an endophytic fungus harboring seeds of Sophora flavescens, using response surface methodology. Mycobiology 2017, 45, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Do, M.H.; Kim, S.Y. Hypericin, a naphthodianthrone derivative, prevents methylglyoxal-induced human endothelial cell dysfunction. Biomol. Ther. 2017, 25, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Hu, N.; Yue, H.; Wang, H. Inhibitory activity and mechanism investigation of hypericin as a novel α-glucosidase inhibitor. Molecules 2021, 26, 4566. [Google Scholar] [CrossRef]
- Flores-Bustamante, Z.R.; Rivera-Orduña, F.N.; Martínez-Cárdenas, A.; Flores-Cotera, L.B. Microbial paclitaxel: Advances and perspectives. J. Antibiot. 2010, 63, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, B.; Xing, J.; Li, C. Endophytes: The novel sources for plant terpenoid biosynthesis. Appl. Microbiol. Biotechnol. 2021, 105, 4501–4513. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J. Plants and endophytes: Equal partners in secondary metabolite production? Biotechnol. Lett. 2015, 37, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Singh, S.; Babu, C.S.V.; Shanker, K.; Srivastava, N.K.; Kalra, A. Endophytes of Opium poppy differentially modulate host plant productivity and genes for the biosynthetic pathway of benzylisoquinoline alkaloids. Planta 2016, 243, 1097–1114. [Google Scholar] [CrossRef]
- Lin, L.-C.; Lin, C.-Y.; Lin, W.-R.; Tung, Y.-T.; Wu, J.-H. Effects of ericoid mycorrhizal fungi or dark septate endophytic fungi on the secondary metabolite of Rhododendron pseudochrysanthum (R. morii) seedlings. Appl. Ecol. Environ. Res. 2021, 19, 1221–1232. [Google Scholar] [CrossRef]
- Zych, M.; Wojnar, W.; Kielanowska, M.; Folwarczna, J.; Kaczmarczyk-Sedlak, I. Effect of berberine on glycation, aldose reductase activity, and oxidative stress in the lenses of streptozotocin-induced diabetic rats in vivo—a preliminary study. Int. J. Mol. Sci. 2020, 21, 4278. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Tian, S.; Chen, L.; Yuan, J. Bioinformatics analysis of endophytic bacteria related to berberine in the Chinese medicinal plant Coptis teeta Wall. 3 Biotech 2020, 10, 96. [Google Scholar] [CrossRef]
- Zimowska, B.; Bielecka, M.; Abramczyk, B.; Nicoletti, R. Bioactive products from endophytic fungi of sages (Salvia spp.). Agriculture 2020, 10, 543. [Google Scholar] [CrossRef]
- Bier, M.C.J.; Medeiros, A.B.P.; Soccol, C.R. Biotransformation of limonene by an endophytic fungus using synthetic and orange residue-based media. Fungal Biol. 2017, 121, 137–144. [Google Scholar] [CrossRef]
- Tian, Y.; Amand, S.; Buisson, D.; Kunz, C.; Hachette, F.; Dupont, J.; Nay, B.; Prado, S. The fungal leaf endophyte Paraconiothyrium variabile specifically metabolizes the host-plant metabolome for its own benefit. Phytochemistry 2014, 108, 95–101. [Google Scholar] [CrossRef]
- Harwoko, H.; Hartmann, R.; Daletos, G.; Ancheeva, E.; Frank, M.; Liu, Z.; Proksch, P. Biotransformation of host plant flavonoids by the fungal endophyte Epicoccum nigrum. ChemistrySelect 2019, 4, 13054–13057. [Google Scholar] [CrossRef] [Green Version]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Wang, S.; Li, Y.; Zheng, M.; Xi, X.; Cao, H.; Cui, X.; Guo, H.; Han, C. Yield enhancement strategies of rare pharmaceutical metabolites from endophytes. Biotechnol. Lett. 2018, 40, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, H.; Zhao, X.; Xu, X.; Di, Y.; Jiang, C.; Shi, J.; Shao, D.; Huang, Q.; Yang, H.; et al. Production of bioproducts by endophytic fungi: Chemical ecology, biotechnological applications, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2018, 102, 6279–6298. [Google Scholar] [CrossRef]







| AntiAGEs Compound | Concentration/Study Model | Action Mechanism | Endophytic Source/Host Plant | Analytical Method of Identification |
|---|---|---|---|---|
| Protocatechuic acid | * 2–4% in powder diet of T2D rats [71] * 50–100 mg/kg p.o in T2D rats with high fat diet [72] | Reduces formation of CML, pentosidine and the expression of aldose reductase, sorbitol dehydrogenase, and RAGEs. Improves glyoxalase I expression and insulin sensibility. It has antioxidant, hypoglycemic and anti-inflammatory activity [71,72] | -NID endophytes/Newbouldia laevis and Ocimum gratissimum [73] -Aspergillus sp., FVL2/Foeniculum vulgare [74] | -HPLC-PDA a -1H, 13C, HSQC, and HMBC NMR a |
| Gallic acid | * 50–200 µg/mL in BSA-glucose system [75] * 25 mg/kg/day in rats [76] * 100 µM in BSA-glucose, BSA-ribose and BSA-MGO system [77] | Diminishes fluorescent AGEs formation and RAGEs expression. Chelates ion metals entrap carbonyl species and have antioxidant, anti-inflammatory and hypoglycemic activities [75,76,77] | -Fusarium sp./Fritillaria unibracteata [78] -Alternaria spp., Penicillium spp., Neurospora spp., Cladosporium spp., Phoma spp. Fusarium spp., Phomopsis spp. and Pleosporales spp./Acer ginnala [79] -Cladosporium velox/Tinospora cordifolia [80] -Fusarium spp./Ferula assa-foetida [81] | -HPLC-DAD b -HPLC-dual λ detector * -HPLC-DAD b -HPLC-PDA * |
| Coumaric acid | * 0.2 mM in rat hepatocytes [82] * 20 mM in rat tail tendons [83] | Decreases collagen cross-links and cytotoxicity induced by GO and MGO in hepatocytes, has antioxidant and anti-inflammatory activity [82,83] | -Cladosporium velox/Tinospora cordifolia [80] -Fusarium spp./Ferula assa-foetida [81] | -HPLC-DAD b -HPLC-PDA * |
| Caffeic acid | * 0.5–2 mM in BSA-MGO and histones-MGO system [84] * 2.5–5% in powder diet of T2D rats [85] * 10 µM in human endothelial cells system [86] * 0.1–2.5 mM BSA-MGO, HUVEC system [87] | Reduces the levels of CML, fluorescent AGEs and inflammatory hormones. Inhibits aldose reductase, sorbitol dehydrogenase activity and RAGEs expression, has antioxidant, anti-inflammatory activities [84,85,86] * There are contradictory reports about the beneficial effect of caffeic acid. Wu et al. [87] reported proglycation effect of caffeic acid, which leads to the elevation of oxidative stress and inflammation in monocytes, macrophages and vascular endothelial cells * | -Four Fusarium spp./Fritillaria unibracteata [78] -Fusarium chlamydosporum and Penicillium canescens/Polygonum chinense L. [88] -Colletotrichum acutatum S216/Camellia [89] -Arcopilus cupreus/Schleichera oleosa [90] -Aspergillus fumigatus/Moringa oleífera [91] -Cladosporium velox/Tinospora cordifolia [80] -Fusarium spp./Ferula assa-foetida [81] | -HPLC-DAD b -HPLC-ESI-MS/MS b -UPLC-MS/MS b -OHR-LC-MS (ESI and APCI) a -UHPLC-DAD b -HPLC-DAD b -HPLC-PDA * |
| Ferulic acid | * 50–200 µg/mL in BSA-glucose system [75]. * 0.2 mM in rat hepatocytes [82] * Equimolar or a 5-fold molar excess with respect to the lysine content of flour and egg white in cake [92]. * 12.95 mM in BSA-fructose or soy glycinin–fructose system [93] * 5–20 mM in BSA-glucose system; 0.1 and 0.2 mM in HUVEC system [94] | Inhibits production of CML, fluorescent AGEs, dicarbonyl compounds, CEL, and melanoidins. Decreases cytotoxicity induced by GO and MGO in hepatocytes, reduces protein cross-linking and has antioxidant, anti-inflammatory and antihyperglycemic activities [75,82,92,93,94] | -NID endophytes/Newbouldia laevis and Ocimum gratissimum [73] -Three Fusarium spp./Fritillaria unibracteata [78] -Fusarium chlamydosporum and Penicillium canescens/Polygonum chinense L. [88] -Alternaria tenuissima SBUp1, Fusarium sp./Ferula assa-foetida [81] | -HPLC-PDA a -HPLC-DAD b -HPLC-ESI-MS/MS b -HPLC-PDA * |
| Rosmarinic acid | * 6.25–400 µg/mL in BSA-glucose, BSA-GO and BSA-MGO system [95] * 10 µM in HSA–MGO system [96] | Inhibits formation of fluorescent AGEs, CML, and CEL. Reduces MGO levels, protein aggregation, and fibril formation induced by AGEs in human serum albumin [95,96] | -Two Fusarium spp./Fritillaria unibracteata [78] -Alternaria tenuissima SBUp1, Fusarium sp./Ferula assa-fotida [81] | -HPLC-DAD b -HPLC-PDA b |
| Chlorogenic acid | * 0.5–2 mM in BSA-MGO and histones-MGO system [84] * AGEs IC50 = 148.32 ± 3.13 µM in BSA-glucose system; crosslinking IC50 = 0.68 ± 0.10 mM in AGEs-BSA-rat tail tendon collagen system; carbonyl trapping IC50 = 48.26 ± 16.98 mM [97] | Inhibits production of fluorescent AGEs and alpha glycosidases. Reduces cross-linking of AGEs-BSA to collagen, entraps MGO, has antihyperglycemic and antioxidant activities [84,97] | -Sordariomycete sp./Eucommia ulmoides [98] -Cochliobolus lunatus/Mirabilis jalapa L. [99] -Brevibacillus borstelensis B14, Bacillus amyloliquefaciens B17, Bacillus badius B19, Sphingomonas yabuuchiae N21, Enterobacter tabaci N22, and Lodderomyces elongisporus P212 and Colletotrichum acutatum S216/Mentha haplocalyx (B14, B17, B19), Ipomoea batatas and Camellia [89] -Cladosporium velox/Tinospora cordifolia [80] -Fusarium sp./Ferula assa-foetida [81] | -HPLC, UPLC-PDA-QTOF-MS b -LC-ESI-MS/MS -Chromogenic method, TLC b, HPLC-UV b, UPLC-MS/MS b -HPLC-DAD b -HPLC-PDA * |
| Apigenin (A) and derivatives (V = vitexin and I = isovitexin) | * AGEs IC50 = 85.2–185.2 µM in BSA-glucose-fructose system; aldose reductase IC50 A = 2.47–6.67 µM in RLAR system [100] * Aldose reductase IC50 V = 1.47 ± 0.08 µM, IC50 I = 0.49 ± 0.08 µM, IC50 A = 0.97 ± 0.26 µM in RLAR system and IC50 V = 12.07 ± 0.03 µM, IC50 I = 0.13 ± 0.03 µM, IC50 A = 11.65 ± 0.07 µM in HRAR system; AGES IC50 V = 243.54 ± 8.86 µM, IC50 I = 175.66 ± 3.73 µM, IC50 A = 204.14 ± 9.31 µM in BSA-fructose-glucose system [101] * 10–25 µM IN AGEs-HUVECs system [102] | Inhibits aldose reductase and acetylcholinesterase activities, as well as the formation of fluorescent AGEs. Entraps MGO and reduces inflammatory cytokines and adhesion molecules, has antioxidant and anti-inflammatory activities [100,101,102] | -Dichotomopilus funicola/Cajanus cajan L. (pigeon pea) [103] -Two Fusarium spp./Fritillaria unibracteata [78] -Fusarium solani/Cajanus cajan [104] -Chaetomium globosum/Cajanus cajan [105] -Arcopilus cupreus/Schleichera oleosa [90] -Alternaria tenuissima SBUp1, Fusarium sp./Ferula assa-foetida [81] | -HPLC-ESI-MS b -HPLC-DAD b -HPLC-UV-Vis *, LC-MS-ESI b, 1H, 13C NMR a -HPLC-MS/MS a -OHR-LC-MS (ESI and APCI) a -HPLC-PDA * |
| Kaempferol and derivatives | * 2–4 mg/kg b.w/day in rats; 1–5 µM in YPEN cells [106]. * Scavenging activity IC50 = 39.5–55.5 µM [107] * IC50 = 10 µM in RLAR system [108] * 20 mg/kg/day in diabetic rats [109] | Inhibits aldose reductase and entraps dicarbonyl compounds. Reduces AGEs levels and hyperglycemia, suppressing AGEs–RAGEs axis activation. It has antioxidant and anti-inflammatory activities [106,107,108,109] | -Mucor fragilis/Sinopodophyllum hexandrum [110] -Penicillium setosum/Withania somnifera [111] -Aspergillus fumigatus/Moringa oleífera [91] | -TLC b, HPLC-UV b, 1H, 13C NMR a -HPLC-UV-Vis-Q-ToF-ESI-MS a -UHPLC-DAD b |
| Luteolin and derivatives | * AGEs IC50 = 16.5–88.9 µM in BSA-glucose-fructose system; aldose reductase IC50 A = 0.087–0.94 µM in RLAR system [100]. * 100 µg/mL in BSA-glucose-fructose system [112] | Inhibits aldose reductase, and production of pentosidine and other fluorescent AGEs. Reduces protein cross-linking [100,112] | -Nigrospora oryzae/Loranthus micranthus [113] -Fusarium sp./Fritillaria unibracteata [78] -Alternaria tenuissima SBUp1, Fusarium sp./Ferula assa-foetida [81] | -HPLC-DAD-ESI-MS a, 1H, 13C, HSQC, and HMBC NMR -HPLC-DAD b -HPLC-PDA * |
| Quercetin and derivatives | * 50–200 µg/mL in BSA-glucose system [75] * 100 µM in BSA-glucose, BSA-ribose and BSA-MGO system [77] * AGEs gral IC50 = 65 µM, Pentosidine IC50 = 18 µM and 75–300 mM in collagen-glucose system [114] * 0.5–2.5 mM in BSA-MGO and BSA-GO system [115] | Inhibits aldose reductase, and the formation of alpha dicarbonyl compounds, CML, and fluorescent AGEs. Entraps MGO and GO, and reduces cross-linking of proteins and glucose autooxidation, chelates metal ions, has antioxidant activity [75,77,108,114,115] | -Nigrospora oryzae/Loranthus micranthus [113] -Fusarium chlamydosporum and Penicillium canescens/Polygonum chinense L. [88] -Penicillium setosum/Withania somnifera [111] -Arcopilus cupreus/Schleichera oleosa [90] -Aspergillus fumigatus/Moringa oleífera [91] -Alternaria tenuissima SBUp1, Fusarium sp./Ferula assa-foetida [81] | -HPLC-DAD-ESI-MS a, 1H, 13C, HSQC, and HMBC NMR -HPLC-ESI-MS/MS b -HPLC-UV-Vis-Q-ToF-ESI-MS a -OHR-LC-MS (ESI and APCI) a -UHPLC-DAD b -HPLC-PDA * |
| Catechin | * 50–200 µg/mL in BSA-glucose system [75] * 100 µM in BSA-glucose, BSA-ribose and BSA-MGO system [77] * AGEs IC50 = 0.049 ± 0.019 mg/mL in BSA-glucose system, radical scavenging IC50 = 7.927 ± 0.007 and 5 mM for MGO scavenging | Inhibits the formation of fluorescent AGEs. Chelates metal ions, entraps dicarbonyl compounds, and has antioxidant activity [75,77,116] | -Fusarium spp./Fritillaria unibracteata [78] | -HPLC-DAD b |
| Daidzein | * 1 mM in MGO system [117] | Entraps MGO [117] | -Rahnella aquatilis/Emilia sonchifolia [118] | -ESI-MS a, 1H, 13C NMR a |
| Genistein | * 100 µM in BSA-glucose, BSA-ribose and BSA-MGO system [77] * 1 mM in MGO system [117] | Chelates metal ions, entraps MGO, has antioxidant activity [77,117] | -Rahnella aquatilis/Emilia sonchifolia [118] -Arcopilus cupreus/Schleichera oleosa [90] | -ESI-MS a, 1H, 13C NMR a -OHR-LC-MS (ESI and APCI) a |
| Icariin | * 20 mg/kg/day in diabetic rats [119] * 10 and 20 mg/kg b.w. in diabetic rats [120] | Reduces blood glucose levels in diabetic rats, has antioxidant, anti-inflammatory and antihyperglycemic activities [119,120] | -Fusarium spp./Fritillaria unibracteata [78] | -HPLC-DAD b |
| Rutin and derivatives | * 50–100 mg/kg body weight in diabetic rats (review) [121] | Inhibits alpha-glucosidases, alpha-amylases, aldose reductase, intestinal carbohydrate absorption, and AGEs formation. Increases glucose uptake and insulin secretion. Reduces activity of enzymes involved in gluconeogenesis and has antioxidant and anti-inflammatory activities [121] | -Fusarium spp./Fritillaria unibracteata [78] -Aspergillus fumigatus/Moringa oleífera [91] -Alternaria tenuissima SBUp1, Fusarium sp./Ferula assa-foetida [81] | -HPLC-DAD b -UHPLC-DAD b -HPLC-PDA * |
| Resveratrol | * 5 mg/kg b.w. in diabetic rats [122] * 50–300 μg/mL in BSA-fructose, BSA-MGO and arginine-MGO system; α-amylase IC50 = 3.62 μg/mL and α-glucosidase IC50 = 17.54 μg/mL [123] | Inhibits aldose reductase, alpha-glucosidase, alpha-amylase, and sorbitol dehydrogenase. Chelates metal ions and entraps dicarbonyls. Improves insulin sensitivity, glyoxalase-I activity, and adiponectin levels. Reduces AGEs levels in diabetic rats, has antioxidant and anti-inflammatory activities [122,123] | -Alternaria spp., Botryosphaeria sp., Penicillium spp., Cephalosporium spp., Aspergillus sp., Geotrichum sp., and Mucor sp./Vitis vinifera L. cv. Merlot, Vitis quinquangularis and Polygonum cuspidatum [124] -Arcopilus aureus, Penicillium spp., Lasiodiplodia spp., Nigrospora sp., Botryosphaeria spp., Fusarium spp., Xilaria sp., Aspergillus spp. and Alternaria sp./Vitis vinifera [125,126,127] -Aspergillus niger/Vitis vinifera Cabernet Sauvignon [128] | -HPLC-dual λ * -Biochemical assays, TLC b, HPLC * -Chromogenic method, TLC b, UV spectra b, LC * |
| Tyrosol | * 5–20 mg/kg b.w. in normal and diabetic rats [129] * α-glucosidase IC50 = 70.8 µg total phenolic/mL [130] | Inhibits alpha-glucosidase, relieves hyperglycemia, and has antioxidant activity [129,130] | -Rhytismataceae sp./Picea mariana [131] -Papulaspora immersa/Smallanthus sonchifolius [132] -Phialocephala fortinii/Rhodiola angusta and R. crenulata [133] -Pestalotiopsis microspore/Manilkara zapota [134] | -HPLC a, 1H, 13C NMR a -Optical rotation, IR, ID, and 2D NMR and MS data a -HPLC-UV b, UPLC/Q-ToF-MS, and 1H-NMR b −1H, 13C NMR a, and FABMS a |
| Ellagic acid | * Aldose reductase IC50 = 0.27 µM in HRAR and IC50 = 0.047 µM in RLAR system [108] | Inhibits aldose reductase and sorbitol dehydrogenase activities. Reduces production of CEL, CML, and fluorescent AGEs. Entraps dicarbonyl compounds. Enhances insulin signaling, adiponectin receptors, glucose transporters, and inflammatory mediators. Decreases blood glucose levels and has anti-inflammatory activity [108,135] | -Cladosporium velox/Tinospora cordifolia [80] -Aspergillus fumigatus/Moringa oleífera [91] | -HPLC-DAD b -UHPLC-DAD b |
| Ginsenosides (Rb, Rd, Rg) | * AGEs IC50 = 15–220 µM in BSA-fructose-glucose system [136] | Inhibits production of fructosamine, fluorescent AGEs, and CML. Reduces levels of amyloid cross-B structure, has hypoglycemic activity [136] | -Fusarium sp. and Aspergillus sp./Panax notoginseng [137] -Fusarium spp., Aspergillus spp., Verticillium spp., Penicillium spp., Nectria spp., and Plectosphaerella sp./Panax ginseng [138] -Penicillium sp., Dictyochaeta sp. and Camarosporium sp./Aralia elata [139] | -HPLC-UV, HPLC-ESI-MS b -HPLC-PAD b -HPLC b |
| Tanshinones | * 5 and 20 mg/kg/day in transgenic mice [140] *10 mg/kg/day in diabetic rats [141] | Reduces plasma glucose, AGEs levels, and RAGE expression. Suppress the activation of NF-κB signaling pathway mediated by RAGE, has anti-inflammatory activity [140,141] | -Trichoderma atroviride/Salvia miltiorrhiza [142] | -HPLC-HRMS/MS b |
| Stigmasterol | * 0.1 mg/mL in BSA-glucose system [143] | Inhibits formation of fluorescent AGEs and protein glycoxidation. Entraps carbonyl intermediates, blocks lysyl residues of BSA, and consequently reduces its binding with glucose. It has antioxidant activity [143] | -Cunninghamella sp./Salicornia bigelovii Torr [144] | -ESI-MS, 1H-NMRa |
| Emodin | * AGEs IC50 = 118 µM in BSA-fructose-glucose system, aldose reductase IC50 = 15.9 µM in RLAR system [145] | Inhibits aldose reductase activity and formation of fluorescent AGEs and CML. Entraps MGO, has antioxidant activity [145] | -Talaromyces spp. Apergillus spp. and Fusarium spp./Artemisia annua L. [146] -Coniochaeta velutina/Tsuga heterophylla [147] -Thielavia subthermophila/Hypericum perforatum [148] | -Metabolomic analysis by LC-HRMS/MS ab -LC-MS-IT-TOF and NMR data a -HPLC-HRMS * |
| Umbelliferone | * AGEs IC50 = 2.95 ± 0.02 µM in BSA-fructose-glucose system [149] * 15–240 µg/mL in psoas muscle system, α-amylase IC50 = 8.06 µg/mL | Inhibits production of alpha-glycosidase, alpha-amylase, aldose reductase, fluorescent AGEs, and alpha-dicarbonyl compounds. Improves insulin secretion and glucose uptake, has antioxidant and hypoglycemic activities [149,150] | -Cladosporium velox/Tinospora cordifolia [80] | -HPLC-DAD b |
| Matrine | * 50–100 mg/kg in transgenic mice, 10–50 µM | Inhibits RAGEs activation, has anti-inflammatory activity [151] | -Aspergillus terreus/Sophora flavescens [152] | -HPLC-PAD b |
| Hypericin | * 1–10 µM in BSA-MGO system, 0.01–0.5 µM in HUVEC-MGO system [153] * α-glucosidase IC50 = 4.66 ± 0.27 mg/L [154] | Inhibits production of α-glucosidase and fluorescent AGEs. Protects against MGO-induced apoptosis and oxidative damage [153,154] | -Thielavia subthermophila/Hypericum perforatum [148] | -HPLC-HRMS *, detection of hyp-1 gene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochín-Hernández, L.S.; Rochín-Hernández, L.J.; Flores-Cotera, L.B. Endophytes, a Potential Source of Bioactive Compounds to Curtail the Formation–Accumulation of Advanced Glycation End Products: A Review. Molecules 2022, 27, 4469. https://doi.org/10.3390/molecules27144469
Rochín-Hernández LS, Rochín-Hernández LJ, Flores-Cotera LB. Endophytes, a Potential Source of Bioactive Compounds to Curtail the Formation–Accumulation of Advanced Glycation End Products: A Review. Molecules. 2022; 27(14):4469. https://doi.org/10.3390/molecules27144469
Chicago/Turabian StyleRochín-Hernández, Lory Sthephany, Lory Jhenifer Rochín-Hernández, and Luis Bernardo Flores-Cotera. 2022. "Endophytes, a Potential Source of Bioactive Compounds to Curtail the Formation–Accumulation of Advanced Glycation End Products: A Review" Molecules 27, no. 14: 4469. https://doi.org/10.3390/molecules27144469