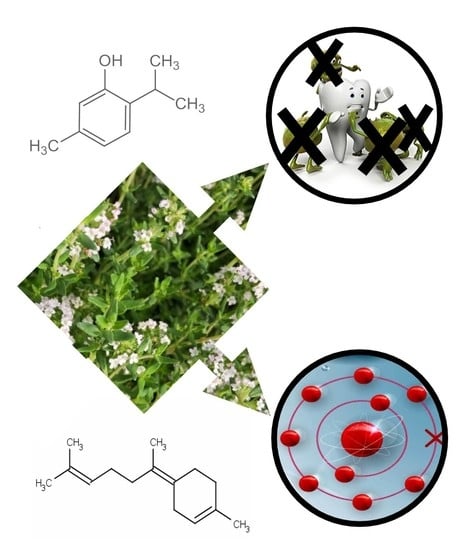
Thymus richardii subsp. nitidus (Guss.) Jalas Essential Oil: An Ally against Oral Pathogens and Mouth Health
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Essential Oils
2.2. Antimicrobial Activity of T. richardii EO (F)
2.3. Antimicrobial Activity of the Components Present in T. richardii EO (F)
2.4. Fluorescence Microscopy Analysis
2.5. Antibiofilm Activity of Essential oils (F), Thymol, and β-Bisabolene
2.6. Cytotoxic Activity of (F) and Its Principal Components
2.7. Antioxidant Activity of T. richardii EO (F)
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of Essential Oil
3.3. GC-MS Analysis
3.4. Pure Compounds
3.5. Bacterial Strains
3.6. Antimicrobial Activity Assay
3.7. Determination of Minimal Inhibitory Concentration
3.8. Fluorescence Microscopy Experiments
3.9. Antibiofilm Inhibition Tests
3.10. Eukaryotic Cell Culture
3.11. ABTS and H2O2 Scavenging Capacity Assay
3.12. Antioxidant Test on HaCat Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- POWO. Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 22 January 2023).
- Vila, R. Flavonoids and further polyphenols in the genus Thymus. In Thyme: The Genus Thymus; Stahl-Bishup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 144–176. [Google Scholar]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus spp. plants—Food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, T.; Wang, X.; Shen, M.; Yan, X.; Fan, S.; Wang, L.; Wang, X.; Xu, X.; Sui, H.; et al. Review traditional uses, chemical constituents and biological activities of plants from the Genus Thymus. Chem. Biodivers. 2019, 16, e1900254. [Google Scholar] [CrossRef] [PubMed]
- Stahl-Biskup, E.; Venskutonis, R.P. Handbook of Herbs and Spices, 2nd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2012; Volume 1, pp. 499–525. [Google Scholar]
- Alarcón, R.; Pardo-de-Santayana, M.; Priestley, C.; Morales, R.; Heinrich, M. Medicinal and local food plants in the south of Alava (Basque Country, Spain). J. Ethnopharmacol. 2015, 176, 207–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhang, G.J. A Dictionary of Compendium of Materia Medica; Shandong Science and Technology Press: Shandong, China, 2007. [Google Scholar]
- Zarshenas, M.M.; Krenn, L. A critical overview on Thymus daenensis Celak.: Phytochemical and pharmacological investigations. J. Integr. Med. 2015, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Mossa, J.S.; Al-Said, M.S.; Al-Yahya, M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia 2004, 75, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131, 33–55. [Google Scholar] [CrossRef]
- Gilca, M.; Tiplica, G.S.; Satavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Parada, M.; Carrió, E.; Bonet, M.À.; Vallè, J. Ethnobotany of the Alt Empordà region (Catalonia, Iberian Peninsula): Plants used in human traditional medicine. J. Ethnopharmacol. 2009, 124, 609–618. [Google Scholar] [CrossRef]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. J. Evid.-Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Bidak, L.M.; Kamal, S.A.; Halmy, M.W.A.; Heneidy, S.Z. Goods and services provided by native plants in desert ecosystems: Examples from the northwestern coastal desert of Egypt. J. Global Ecol. Environ. 2015, 3, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Pereira, O.R.; Peres, A.M.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Simultaneous characterization and quantification of phenolic compounds in Thymus × citriodorus using a validated HPLC-UV and ESI-MS combined method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes: Conversion of γ-terpinene to p-cymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef]
- Poulose, A.J.; Croteau, R. γ-Terpinene synthetase: Akey enzyme in the biosynthesis of aromatic monoterpenes. Arch. Biochem. Biophys. 1978, 191, 400–411. [Google Scholar] [CrossRef]
- Stahl-Biskup, E. Essential oil chemistry of the genus Thymus—A global view. In Thyme: The Genus Thymus; Stahl-Bishup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 75–124. [Google Scholar]
- Stahl-Biskup, E. Thyme in Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2004; Volume 2, pp. 297–320. [Google Scholar]
- Zare, M.; Namratha, K.; Thakur, M.S.; Byrappa, K. Biocompatibility assessment and photo-catalytic activity of bio-hydrothermal synthesis of ZnO nanoparticles by Thymus vulgaris leaf extract. Mater. Res. Bull. 2019, 109, 49–59. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite films with combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef] [Green Version]
- Scaffaro, R.; Maio, A.; D’Arrigo, M.; Lopresti, F.; Marino, A.; Bruno, M.; Nostro, A. Flexible mats as promising antimicrobial systems via integration of Thymus capitatus (L.) Essential oil into PLA. Future Microbiol. 2020, 15, 1379–1392. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahjani, M.G.; Hosseini, M.; Safari, R.; Moshrefi, R.; Shiri, H.M. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid Interface Sci. 2017, 490, 444–451. [Google Scholar] [CrossRef]
- Gagliano Candela, R.; Maggi, F.; Lazzara, G.; Rosselli, S.; Bruno, M. The essential oil of Thymbra capitata and its application as a biocide on stone and derived surfaces. Plants 2019, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Casiglia, S.; Bruno, M.; Scandolera, E.; Senatore, F. Influence of harvesting time on composition of the essential oil of Thymus capitatus (L.) Hoffmanns. & Link. growing wild in northern Sicily and its activity on microorganisms affecting historical art crafts. Arabian J. Chem. 2019, 12, 2704–2712. [Google Scholar] [CrossRef] [Green Version]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential oil as natural biocides in conservation of cultural heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, G.; Badalamenti, N.; Giambra, B.; Palla, F.; Bruno, M. The application of the essential oils of Thymus vulgaris L. and Crithmum maritimum L. as biocidal on two Tholu Bommalu Indian leather puppets. Plants 2021, 10, 1508. [Google Scholar] [CrossRef]
- Bartolucci, F. Thymus L. In Flora d’Italia, 2nd ed.; Pignatti, S., Ed.; Media Business–Edagricole: Milan, Italy, 2018; Volume 3, pp. 278–290. [Google Scholar]
- Bartolucci, F.; Domina, G. The genus Thymus (Lamiaceae) in Sicily. Plant Biosyst. 2015, 149, 710–719. [Google Scholar] [CrossRef]
- Euro+Med PlantBase. Available online: https://ww2.bgbm.org/EuroPlusMed/query.asp (accessed on 16 January 2023).
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Media Business–Edagricole: Milano/Bologna, Italy, 2017; Volume 4. [Google Scholar]
- Morales, R. Synopsis of the genus Thymus L. in the Mediterranean area. Lagascalia 1997, 19, 249–262. [Google Scholar]
- Gianguzzi, L.; Scuderi, L.; Pasta, S. The vascular flora of Marettimo Island (Egadian Archipelago, W Sicily): Updating and phytogeographic analysis. Webbia 2006, 61, 359–402. [Google Scholar] [CrossRef]
- Giardina, G.; Raimondo, F.M.; Spadaro, V. A catalogue of plants growing in Sicily. Bocconea 2007, 20, 5–582. [Google Scholar]
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red Listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- Gugliuzza, G.; Domina, G.; Giovino, A. Seed germination of Thymus richardii subsp. nitidus (Lamiaceae). Flora Mediterranea 2021, 31, 291–293. [Google Scholar]
- Prieto, J.M.; Bader, A.; Martini, F.; Ríos, J.L.; Morelli, I. Screening of some rare endemic Italian plants for inhibitory activity on 5-lipoxygenase. Fitoterapia 2005, 76, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential oils and health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Kouidhi, B.; Al Qurashi, Y.M.A.; Chaieb, K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microbial Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.; Denny, C.; Benso, B.; De Alencar, S.; Rosalen, P. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toscano-Garibay, J.D.; Arriaga-Alba, M.; Sánchez-Navarrete, J.; Mendoza-García, M.; Flores-Estrada, J.J.; Moreno-Eutimio, M.A.; Espinosa-Aguirre, J.J.; González-Ávila, M.; Ruiz-Pérez, N.J. Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Sci. Rep. 2017, 7, 11479. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Alam Tumpa, A.; Zehravi, M.; Sarker, T.; Yamin; Islam, R.; Harun-Or-Rashid; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An overview of antimicrobial stewardship optimization: The use of antibiotics in humans and animals to prevent resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]
- Mocanu, R.C.; Martu, M.-A.; Luchian, I.; Sufaru, I.G.; Maftei, G.A.; Ioanid, N.; Martu, S.; Tatarciuc, M. Microbiologic profiles of patients with dental prosthetic treatment and periodontitis before and after photoactivation therapy—Randomized clinical trial. Microorganisms 2021, 9, 713. [Google Scholar] [CrossRef]
- De Feo, V.; Bruno, M.; Tahiri, B.; Napolitano, F.; Senatore, F. Chemical composition and anti-bacterial activity of the essential oils of Thymus spinulosus Ten. (Lamiaceae). J. Agr. Food Chem. 2003, 51, 3849–3853. [Google Scholar] [CrossRef]
- Badalamenti, N.; Ilardi, V.; Rosselli, S.; Bruno, M.; Maggi, F.; Leporini, M.; Falco, T.; Loizzo, M.R.; Tundis, R. Ferulago nodosa subsp. geniculata (Guss.) Troia & Raimondo from Sicily (Italy): Isolation of essential oil and evaluation of its bioactivity. Molecules 2020, 25, 3249. [Google Scholar] [CrossRef]
- Badalamenti, N.; Vaglica, A.; Maggio, A.; Bruno, M. A new ferulol derivative isolated from the aerial parts of Ferulago nodosa (L.) Boiss. growing in Sicily (Italy). Nat. Prod. Res. 2022, in press. [Google Scholar] [CrossRef]
- Maresca, V.; Badalamenti, N.; Ilardi, V.; Bruno, M.; Bontempo, P.; Basile, A. Chemical composition of Thymus leucotrichus var. creticus essential oil and its protective effects on both damage and oxidative stress in Leptodictyum riparium Hedw. induced by cadmium. Plants 2022, 11, 3529. [Google Scholar] [CrossRef]
- Di Napoli, M.; Maresca, V.; Varcamonti, M.; Bruno, M.; Badalamenti, N.; Basile, A.; Zanfardino, A. (+)-(E)-Chrysanthenyl acetate: A molecule with interesting biological properties contained in the Anthemis secundiramea (Asteraceae) flowers. Appl. Sci. 2020, 10, 6808. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Gagliano Candela, R.; Maggi, F. Chemical composition of the es-sential oil of Elaeoselinum asclepium (L.) Bertol subsp. meoides (Desf.) Fiori (Umbelliferae) collected wild in Central Sicily and its antimicrobial activity. Nat. Prod. Res. 2022, 36, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.; Maggio, A.; Badalamenti, N.; Bruno, M.; D’Angelo, G.D.; D’Anneo, A. Essential oil of Foeniculum vulgare subsp. piperitum fruits exerts an anti-tumor effect in triple-negative breast cancer cells. Mol. Med. Rep. 2022, 26, 243. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Flamini, G.; Cioni, P.L.; Morelli, I. Thymus richardii Pers. subsp. nitidus (Guss.) Jalas: A new thymus type rich in β-bisabolene. J. Essent. Oil Res. 2001, 13, 8–10. [Google Scholar] [CrossRef]
- Llorens, L.; Llorens-Molina, J.A.; Agnello, S.; Boira, H. Geographical and environment-related variations of essential oils in isolated populations of Thymus richardii Pers. in the Mediterranean basin. Biochem. System. Ecol. 2014, 56, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Hazzit, M.; Baaliouamer, A.; Veríssimo, A.R.; Faleiro, M.L.; Miguel, M.G. Chemical composition and biological activities of Algerian Thymus oils. Food Chem. 2009, 116, 714–721. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef]
- Michet, A.; Chalchat, J.-C.; Figuéredo, G.; Thebaud, G.; Billy, F.; Petel, G. Chemotypes in the volatiles of wild thyme (Thymus pulegioides L.). J. Essent. Oil Res. 2008, 20, 101–103. [Google Scholar] [CrossRef]
- Torras, J.; Grau, M.D.; López, J.F.; de las Heras, F.X.C. Analysis of essential oils from chemotypes of Thymus vulgaris in Catalonia. J. Sci. Food Agric. 2007, 87, 2327–2333. [Google Scholar] [CrossRef]
- Adzet, T.; Granger, R.; Passet, J.; San Martin, R. Le polymorphisme chimique dans le genre Thymus: Sa signification taxonomique. Biochem. Syst. Ecol. 1977, 5, 269–272. [Google Scholar] [CrossRef]
- Arsenijević, J.; Drobac, M.; Šoštarić, I.; Ražić, S.; Milenković, M.; Couladis, M.; Maksimović, Z. Bioactivity of herbal tea of Hungarian thyme based on the composition of volatiles and polyphenolics. Ind. Crops Prod. 2016, 89, 14–20. [Google Scholar] [CrossRef]
- Shin, S.; Kim, J.H. Antifungal activities of essential oils from Thymus quinquecostatus and T. magnus. Planta Med. 2004, 70, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, J.H. In vitro inhibitory activities of essential oils from two Korean Thymus species against antibiotic-resistant pathogens. Arch. Pharm. Sci. Res. 2005, 28, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shin, S. In vivo antifungal activity of the essential oil fraction from Thymus species and in vitro synergism with clotrimazole. Nat. Prod. Sci. 2007, 13, 258–262. [Google Scholar]
- Manconi, M.; Petretto, G.; D’Hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf. B 2018, 171, 115–122. [Google Scholar] [CrossRef]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Vaglica, A.; Ilardi, V.; Varcamonti, M.; Bruno, M.; Zanfardino, A. Chemical composition, antimicrobial and antioxidant activities of the essential oil of italian Prangos trifida (Mill.) Herrnst. & Heyn. Nat. Prod. Res. 2022, in press. [Google Scholar] [CrossRef]
- Tanhaieian, A.; Pourgonabadi, S.; Akbari, M.; Mohammadipour, H. The effective and safe method for preventing and treating bacteria-induced dental diseases by herbal plants and a recombinant peptide. J. Clin. Exp. Dent. 2020, 12, e523–e532. [Google Scholar] [CrossRef]
- Rivas Caldas, R.; Le Gall, F.; Revert, K.; Rault, G.; Virmaux, M.; Gouriou, S.; Héry-Arnaud, G.; Barbier, G.; Boisramé, S. Pseudomonas aeruginosa and periodontal pathogens in the oral cavity and lungs of cystic fibrosis patients: A case-control study. J. Clin. Microbiol. 2015, 53, 1898–1907. [Google Scholar] [CrossRef] [Green Version]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind. Crops Prod. 2013, 50, 304–311. [Google Scholar] [CrossRef]
- Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-inflammatory and antimicrobial properties of Thyme oil and its main constituents. IJMS 2023, 24, 6936. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Barreto, A.S.; Semedo-Lemsaddek, T. Antimicrobial effects of essential oils on oral microbiota biofilms: The toothbrush in vitro model. Antibiotics 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Gömöri, C.; Vidács, A.; Kerekes, E.B.; Nacsa-Farkas, E.; Böszörményi, A.; Vágvölgyi, C.; Krisch, J. Altered antimicrobial and anti-biofilm forming effect of thyme essential oil due to changes in composition. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, A.M.A.; Brandão, M.G.L.; Oliveira, G.B.; Fortes, I.C.P.; Chartone-Souza, E. Synergistic bactericidal activity of Eremanthus erythropappus oil or β-bisabolene with ampicillin against Staphylococcus aureus. Antonie Leeuwenhoek 2007, 92, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C. Thymol: Antibacterial, antifungal and antioxidant activities. G. Ital. Ostet. Ginecol. 2005, 27, 267–272. [Google Scholar]
- Heydari, M.; Zanfardino, A.; Taleei, A.; Bushehri, A.A.S.; Hadian, J.; Maresca, V.; Sorbo, S.; Napoli, M.D.; Varcamonti, M.; Basile, A.; et al. Effect of heat stress on yield, monoterpene content and antibacterial activity of essential oils of Mentha x piperita var. mitcham and Mentha arvensis var. piperascens. Molecules 2018, 23, 1903. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three lebanese Salvia species. Ind. Crops Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kang, S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol. 2014, 165, 559–565. [Google Scholar] [CrossRef]
- Kipanga, P.N.; Demuyser, L.; Vrijdag, J.; Eskes, E.; D’hooge, P.; Matasyoh, J.; Callewaert, G.; Winderickx, J.; Van Dijck, P.; Luyten, W. Investigating the antifungal mechanism of action of polygodial by phenotypic screening in Saccharomyces cerevisiae. IJMS 2021, 22, 5756. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Antimicrobial, antibiofilm, and antioxidant properties of essential oil of Foeniculum vulgare Mill. leaves. Plants 2022, 11, 3573. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, E.; Cheng, Y.; Mahmood, T.; Ge, F.; Zhou, K.; Bao, M.; Lv, L.; Li, L.; Yi, J.; et al. Is combined medication with natural medicine a promising therapy for bacterial biofilm infection? Biomed. Pharmacother. 2020, 128, 110184. [Google Scholar] [CrossRef] [PubMed]
- Arrais, A.; Bona, E.; Todeschini, V.; Caramaschi, A.; Massa, N.; Roncoli, M.; Minervi, A.; Perin, E.; Gianotti, V. Thymus vulgaris essential oil in beta-cyclodextrin for solid-state pharmaceutical applications. Pharmaceutics 2023, 15, 914. [Google Scholar] [CrossRef] [PubMed]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The effect of ten essential oils on several cutaneous drug-resistant microorganisms and their cyto/genotoxic and antioxidant properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef] [Green Version]
- Al-Fatimi, M.; Wurster, M.; Schröder, G.; Lindequist, U. In vitro antimicrobial, cytotoxic and radical scavenging activities and chemical constituents of the endemic Thymus laevigatus (Vahl). Rec. Nat. Prod. 2010, 4, 49–63. [Google Scholar]
- Wu, S.; Wei, F.X.; Li, H.Z.; Liu, X.G.; Zhang, J.H.; Liu, J.X. Chemical composition of essential oil from Thymus citriodorus and its toxic effect on liver cancer cells. Zhongyaocai 2013, 36, 756–759. [Google Scholar]
- Habashy, N.H.; Abu Serie, M.M.; Attia, W.E.; Abdelgaleil, S.A.M. Chemical characterization, antioxidant and anti-inflammatory properties of Greek Thymus vulgaris extracts and their possible synergism with Egyptian Chlorella vulgaris. J. Funct. Foods. 2018, 40, 317–328. [Google Scholar] [CrossRef]
- Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.; Hou, J.; Xiao, C.; Liu, Y.; Jin, Z. Chemical composition and antioxidant activity of the essential oils of citral-rich chemotype Cinnamomum Camphora and Cinnamomum Bodinieri. Molecules 2022, 27, 7356. [Google Scholar] [CrossRef]
- Lu, C.; Li, H.; Li, C.; Chen, B.; Shen, Y. Chemical composition and radical scavenging activity of Amygdalus pedunculata Pall leaves’ essential oil. Food Chem. Toxicol. 2018, 119, 368–374. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2,2-diphenyl-1-picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef]
- Luo, Q.; Tian, Z.; Zheng, T.; Xu, S.; Ma, Y.; Zou, S.; Zuo, Z. Terpenoid composition and antioxidant activity of extracts from four chemotypes of Cinnamomum camphora and their main antioxidant agents. Biofuels Bioprod. Bioref. 2022, 16, 510–522. [Google Scholar] [CrossRef]
- Basile, S.; Badalamenti, N.; Riccobono, O.; Guarino, S.; Ilardi, V.; Bruno, M.; Peri, E. Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules 2022, 27, 588. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical compositions and antioxidant activities of essential oils, and their combinations, obtained from flavedo by-product of seven cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Pota, G.; Zanfardino, A.; Di Napoli, M.; Cavasso, D.; Varcamonti, M.; D’Errico, G.; Pezzella, A.; Luciani, G.; Vitiello, G. Bioinspired antibacterial PVA/melanin-TiO2 hybrid nanoparticles: The role of poly-vinyl-alcohol on their self-assembly and biocide activity. Coll. Surf. B Biointerfaces 2021, 202, 111671. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.; Melone, P.; Silvestri, B.; Pezzella, A.; Di Donato, P.; D’Errico, G.; Di Napoli, M.; Zanfardino, A.; Varcamonti, M.; Luciani, G. Titanium based complexes with melanin precursors as a tool for directing melanogenic pathways. Pure Appl. Chem. 2019, 91, 1605–1616. [Google Scholar] [CrossRef]
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’Errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver (I) N-heterocyclic carbene complexes: A winning and broad spectrum of antimicrobial properties. IJMS 2021, 22, 2497. [Google Scholar] [CrossRef]
- Di Napoli, M.; Maresca, V.; Sorbo, S.; Varcamonti, M.; Basile, A.; Zanfardino, A. proteins of the fruit pulp of Acca sellowiana have antimicrobial activity directed against the bacterial membranes. Nat. Prod. Res. 2021, 35, 2942–2946. [Google Scholar] [CrossRef]
- Vitiello, G.; Zanfardino, A.; Tammaro, O.; Di Napoli, M.; Caso, M.F.; Pezzella, A.; Varcamonti, M.; Silvestri, B.; D’Errico, G.; Costantini, A.; et al. Bioinspired hybrid eumelanin–TiO2 antimicrobial nanostructures: The key role of organo–inorganic frameworks in tuning eumelanin’s biocide action mechanism through membrane interaction. RSC Adv. 2018, 8, 28275–28283. [Google Scholar] [CrossRef] [Green Version]
- Napoli, M.D.; Luccia, B.D.; Vitiello, G.; D’Errico, G.; Carpentieri, A.; Pezzella, A.; Pizzo, E.; Notomista, E.; Varcamonti, M.; Zanfardino, A. Characterisation of EFV12 a bio-active small peptide produced by the human intestinal isolate Lactobacillus gasseri SF1109. Benef. Microbes 2020, 11, 815–824. [Google Scholar] [CrossRef]
- Napolitano, A.; Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Cicio, A.; Bruno, M.; Piacente, S.; Maresca, V.; Cianciullo, P.; Capasso, L.; et al. The chemical composition of the aerial parts of Stachys spreitzenhoferi (Lamiaceae) growing in Kythira island (Greece), and their antioxidant, antimicrobial, and antiproliferative properties. Phytochemistry 2022, 203, 113373. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Seunghee, B.; Eun-Jin, L.; Jae, H.L.; In-Chul, P.; Su-Jae, L.; Hyung, J.H.; Kyu, J.H.; Sungkwan, A.; In-Sook, A.; Hwa, J.C. Oridonin protects HaCaT keratinocytes against hydrogen peroxide-induced oxidative stress by altering microRNA expression. IJMS 2014, 33, 185–193. [Google Scholar] [CrossRef] [Green Version]










| No. | Compounds a | LRI b | LRI c | Pre-Flowering (%) | Flowering (%) |
|---|---|---|---|---|---|
| 1 | α-Pinene | 1009 | 1017 | 1.40 | 1.28 |
| 2 | 3-Thujene | 1029 | 1035 | - | 0.59 |
| 3 | β-Pinene | 1094 | 1099 | - | 0.11 |
| 4 | Sabinene | 1103 | 1115 | - | 0.13 |
| 5 | 3-Carene | 1135 | 1143 | - | 0.05 |
| 6 | 4-Carene | 1148 | 1157 | 0.50 | 1.32 |
| 7 | Limonene | 1185 | 1193 | 6.23 | 15.59 |
| 8 | Sylvestrene | 1200 | 1205 | - | 1.16 |
| 9 | γ-Terpinene | 1237 | 1255 | 1.34 | - |
| 10 | p-Cymene | 1256 | 1272 | 24.45 | 9.04 |
| 11 | α-Copaene | 1492 | 1497 | - | 0.10 |
| 12 | α-Bourbonene | 1523 | 1528 | - | 0.39 |
| 13 | Linalool | 1546 | 1553 | 0.18 | 6.70 |
| 14 | Thymol methyl ether | 1595 | 1604 | 15.90 | 8.87 |
| 15 | Dihydrocarvone | 1618 | 1624 | 5.04 | - |
| 16 | α-Amorphene | 1666 | 1675 | 0.74 | 0.48 |
| 17 | γ-Muurolene | 1700 | 1704 | 0.39 | 0.54 |
| 18 | Germacrene D | 1701 | 1706 | 3.19 | 6.14 |
| 19 | β-Bisabolene | 1739 | 1741 | 28.54 | 17.91– |
| 20 | cis-α-Bisabolene | 1748 | 1759 | 0.90 | - |
| 21 | Thymol | 2184 | 2198 | 4.56 | 16.26 |
| 22 | Carvacrol | 2231 | 2239 | 4.49 | 7.82 |
| 23 | τ-Cadinol | 2180 | 2187 | - | 2.13 |
| 24 | α-Cadinol | 2248 | 2255 | 0.28 | 1.01 |
| Monoterpene Hydrocarbons | 33.92 | 29.27 | |||
| Oxygenated Monoterpenes | 30.17 | 39.65 | |||
| Sesquiterpene Hydrocarbons | 33.76 | 25.56 | |||
| Oxygenated Sesquiterpenes | 0.28 | 3.14 | |||
| Total | 98.13 | 97.62 | |||
| Strains | MIC100 [mg/mL] |
|---|---|
| E. coli DH5α | >0.5 |
| P. aeruginosa PAO1 | >0.5 |
| S. Typhimurium ATCC14028 | >0.5 |
| S. aureus ATCC6538P | 0.5 |
| S. mutans ATCC 35668 | 0.5 |
| S. oralis CECT 8313 | 0.25 |
| Sample | IC50 of ABTS (mg/mL) | Sample | IC50 of H2O2 (mg/mL) |
|---|---|---|---|
| (F) | 0.1 | (F) | 0.025 |
| Ascorbic acid | 0.00003 | Resveratrol | 0.00005 |
| Sample | IC50 of H2O2 (mg/mL) | IC50 of ABTS (mg/mL) |
|---|---|---|
| p-Cymene | 0.27 | 0.7 |
| Thymol methyl ether | 0.45 | 0.1 |
| Limonene | 0.25 | 1 |
| Thymol | 0.15 | <0.1 |
| β-Bisabolene | 0.25 | 0.25 |
| CTRL | 0.00005 | 0.00003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagliuolo, G.; Di Napoli, M.; Vaglica, A.; Badalamenti, N.; Antonini, D.; Varcamonti, M.; Bruno, M.; Zanfardino, A.; Bazan, G. Thymus richardii subsp. nitidus (Guss.) Jalas Essential Oil: An Ally against Oral Pathogens and Mouth Health. Molecules 2023, 28, 4803. https://doi.org/10.3390/molecules28124803
Castagliuolo G, Di Napoli M, Vaglica A, Badalamenti N, Antonini D, Varcamonti M, Bruno M, Zanfardino A, Bazan G. Thymus richardii subsp. nitidus (Guss.) Jalas Essential Oil: An Ally against Oral Pathogens and Mouth Health. Molecules. 2023; 28(12):4803. https://doi.org/10.3390/molecules28124803
Chicago/Turabian StyleCastagliuolo, Giusy, Michela Di Napoli, Alessandro Vaglica, Natale Badalamenti, Dario Antonini, Mario Varcamonti, Maurizio Bruno, Anna Zanfardino, and Giuseppe Bazan. 2023. "Thymus richardii subsp. nitidus (Guss.) Jalas Essential Oil: An Ally against Oral Pathogens and Mouth Health" Molecules 28, no. 12: 4803. https://doi.org/10.3390/molecules28124803