New Insights on the Integrated Management of Plant Diseases by RNA Strategies: Mycoviruses and RNA Interference
Abstract
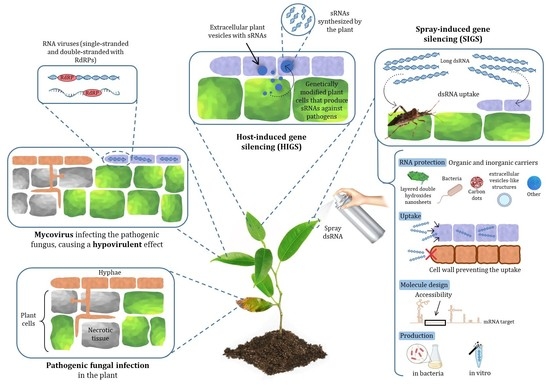
:1. Overview
2. Mycoviruses: A Natural Source of Fungal Hypovirulence
2.1. Mycoviruses at a Glance
2.2. How Mycoviruses Are Transmitted and the Obstacles They Face
2.3. Hypovirulence as a Tool: Can We Copy Nature?
| Mycovirus * | Genome | Fungal Host | Mycovirus Family | Host Plant | Fungal Disease | Reference |
|---|---|---|---|---|---|---|
| CHV-1 | +ssRNA | Cryphonectria parasitica | Hypoviridae | Castanea sativa | Chestnut blight | [84] |
| CHV-2 | +ssRNA | [85] | ||||
| CHV-3 | +ssRNA | [86] | ||||
| OnuMV | +ssRNA | Ophiostoma novo-ulmi | Narnaviridae | Ulmus spp. | Dutch elm disease | [77] |
| SsMV-1/HC025 | +ssRNA | Slerotinia sclerotiorum | Narnaviridae | Glycine max, Brassica napus, Lupinus angustifolius, Pisum sativum | White mold | [87] |
| SsHADV-1 | ssDNA | Genomoviridae | [32] | |||
| SsHV-1 | +ssRNA | Hypoviridae | [88] | |||
| SsHV-2 | +ssRNA | Hypoviridae | [89] | |||
| SmEV-1 | +ssRNA | Sclerotinia minor | Endornaviridae | Lactuca sativa, Arachis hypogaea, Brassica rapa, Brassica napus, sunflower | Sclerotinia blight | [90] |
| AaCV-1 | dsRNA | Alternaria alternata | Chrysoviridae | Herbaceous annual plants, ornamental plants, and trees (citrus, apple, etc.) | Leaf spots, rots, and blights | [91] |
| AaHV-1 | +ssRNA | Hypoviridae | [92] | |||
| FgV-ch9 | dsRNA | Fusarium graminearum | Chrysoviridae | Small-grain cereals (wheat and barley) | Fusarium head blight (FHB) | [93] |
| FgHV-2 | +ssRNA | Hypoviridae | [94] | |||
| FodV-1 | dsRNA | Fusarium oxysporum f. sp .dianthi | Chrysoviridae | Dianthus caryophyllus | Carnation disease | [95] |
| BcMV-1 | +ssRNA | Botrytis cinerea | Narnaviridae | Vegetables and small fruit crops (tomato, raspberry, grape, strawberry, blueberry, apple, and pear) | Gray mold disease | [96] |
| RnMBV-1 | dsRNA | Rosellinia necatrix | Megabirnaviridae | Fruit trees (apples, apricots, avocados, cassava, strawberries, pears, citruses, and Narcissus) | Rosellinia root rot | [97] |
| PtCV-1 | dsRNA | Pestalotiopsis theae | Chrysoviridae | Camelia sinensis | Thea blight | [74] |
| BdCV-1 | dsRNA | Botryosphaeria dothidea | Chrysoviridae | Pyrus pyrifolia | Pear ring spot | [78] |
| BdPV-1 | dsRNA | Partitiviridae | ||||
| BmBRV-1-BdEW220 | dsRNA | Botybirnaviridae | [79] |
2.4. Virocontrol: Lessons Learned and Challenges Ahead
3. RNA Interference (RNAi): The Targeted Management for Plant Disease Control
3.1. An Overview of RNAi
3.2. How Nature Works and How We Benefit from It: HIGS and SIGS
3.2.1. HIGS
3.2.2. SIGS
3.3. Implementation of SIGS in Plant Protection: Major Challenges
3.3.1. Uptake Efficiency
3.3.2. Target Choice
3.3.3. Protection Duration and dsRNA Stability
3.3.4. Technology Safety
3.3.5. Environmental Implications
3.3.6. Economic Competitiveness
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; Mcroberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Niu, D.; Hamby, R.; Niño-Sanchez, J.; Cai, Q.; Yan, Q.; Jin, H. RNAs—A New Frontier in Crop Protection. Curr. Opin. Biotechnol. 2021, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, A.G.; Walker, P.L.; Wytinck, N.; Sullivan, D.S.; Whyard, S.; Belmonte, M.F. Developing New RNA Interference Technologies to Control Fungal Pathogens. Can. J. Plant Pathol. 2018, 40, 325–335. [Google Scholar] [CrossRef]
- Raco, M.; Vainio, E.J.; Sutela, S.; Eichmeier, A.; Hakalová, E.; Jung, T.; Botella, L. Diverse virome of a native population of a tree pathogen Phytophthora castaneae revealed by high-throughput sequencing of total and small RNA. In Proceedings of the V–International Mycovirus Symposium, Gargnano, Italy, 30 May–2 June 2022. [Google Scholar]
- Ghabrial, S.A.; Suzuki, N. Viruses of Plant Pathogenic Fungi. Annu. Rev. Phytopathol. 2009, 47, 353–384. [Google Scholar] [CrossRef]
- Hollings, M. Viruses Associated with A Die-Back Disease of Cultivated Mushroom. Nature 1962, 196, 962–965. [Google Scholar] [CrossRef]
- Ali, A. Fungal Viruses: An Unlikely Ally. In Applied Plant Virology; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128186541. [Google Scholar]
- Kotta-Loizou, I. Mycoviruses and Their Role in Fungal Pathogenesis. Curr. Opin. Microbiol. 2021, 63, 10–18. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Yi, X.; Jiang, D. Extracellular Transmission of a DNA Mycovirus and Its Use as a Natural Fungicide. Proc. Natl. Acad. Sci. USA 2013, 110, 1452–1457. [Google Scholar] [CrossRef]
- Varsani, A.; Krupovic, M. Sequence-Based Taxonomic Framework for the Classification of Uncultured Single-Stranded DNA Viruses of the Family Genomoviridae. Virus Evol. 2017, 3, vew037. [Google Scholar] [CrossRef]
- Liu, S.; Xie, J.; Cheng, J.; Li, B.; Chen, T.; Fu, Y.; Li, G.; Wang, M.; Jin, H.; Wan, H.; et al. Fungal DNA Virus Infects a Mycophagous Insect and Utilizes It as a Transmission Vector. Proc. Natl. Acad. Sci. USA 2016, 113, 12803–12808. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.; Zhang, L.; Qiu, D.; Zhou, X.; Guo, L. A Tripartite SsDNA Mycovirus from a Plant Pathogenic Fungus Is Infectious as Cloned DNA and Purified Virions. Sci. Adv. 2020, 6, eaay9634. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Macdiarmid, R.M. A Mechanically Transmitted DNA Mycovirus Is Targeted by the Defence Machinery of Its Host, Botrytis cinerea. Viruses 2021, 13, 1315. [Google Scholar] [CrossRef]
- Ahlquist, P. RNA-Dependent RNA Polymerases, Viruses, and RNA Silencing. Science 2002, 296, 1270–1273. [Google Scholar] [CrossRef]
- ICTV. Available online: https://talk.ictvonline.org/ (accessed on 2 April 2022).
- Liu, L.; Xie, J.; Cheng, J.; Fu, Y.; Li, G.; Yi, X.; Jiang, D. Fungal Negative-Stranded RNA Virus That Is Related to Bornaviruses and Nyaviruses. Proc. Natl. Acad. Sci. USA 2014, 111, 12205–12210. [Google Scholar] [CrossRef]
- Donaire, L.; Pagán, I.; Ayllón, M.A. Characterization of Botrytis cinerea Negative-Stranded RNA Virus 1, a New Mycovirus Related to Plant Viruses, and a Reconstruction of Host Pattern Evolution in Negative-Sense SsRNA Viruses. Virology 2016, 499, 212–218. [Google Scholar] [CrossRef]
- Hao, F.; Wu, M.; Li, G. Molecular Characterization and Geographic Distribution of a Mymonavirus in the Population of Botrytis cinerea. Viruses 2018, 10, 432. [Google Scholar] [CrossRef]
- Ruiz-Padilla, A.; Rodríguez-Romero, J.; Gómez-Cid, I.; Pacifico, D.; Ayllón, M.A. Novel Mycoviruses Discovered in the Mycovirome of a Necrotrophic Fungus. MBio 2021, 12, e03705-20. [Google Scholar] [CrossRef]
- Wang, L.; He, H.; Wang, S.; Chen, X.; Qiu, D.; Kondo, H.; Guo, L. Evidence for a Novel Negative-Stranded RNA Mycovirus Isolated from the Plant Pathogenic Fungus Fusarium graminearum. Virology 2018, 518, 232–240. [Google Scholar] [CrossRef]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of Filamentous Fungi and Their Relevance to Plant Pathology. Mol. Plant Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef]
- Bao, X.; Roossinck, M.J. Multiplexed Interactions: Viruses of Endophytic Fungi. Adv. Virus Res. 2013, 86, 37–58. [Google Scholar] [CrossRef]
- Márquez, L.M.; Redman, R.S.; Rodriguez, R.J.; Roossinck, M.J. A Virus in a Fungus in a Plant: Three-Way Symbiosis Required for Thermal Tolerance. Science 2007, 315, 513–515. [Google Scholar] [CrossRef]
- Nuss, D.L. Hypovirulence: Mycoviruses at the Fungal–Plant Interface. Nat. Rev. Microbiol. 2005, 3, 632–642. [Google Scholar] [CrossRef]
- Ahn, I.P.; Lee, Y.H. A Viral Double-Stranded RNA Up Regulates the Fungal Virulence of Nectria radicicola. Mol. Plant-Microbe Interact. 2001, 14, 496–507. [Google Scholar] [CrossRef]
- Shah, U.A.; Kotta-Loizou, I.; Fitt, B.D.L.; Coutts, R.H.A. Identification, Molecular Characterization, and Biology of a Novel Quadrivirus Infecting the Phytopathogenic Fungus Leptosphaeria biglobosa. Viruses 2018, 11, 9. [Google Scholar] [CrossRef]
- Buck, K.W. Fungal Virology—An Overview. In Fungal Virology; CRC Press: Boca Raton, FL, USA, 1986; pp. 1–84. [Google Scholar] [CrossRef]
- Shah, U.A.; Kotta-Loizou, I.; Fitt, B.D.L.; Coutts, R.H.A. Mycovirus-Induced Hypervirulence of Leptosphaeria biglobosa Enhances Systemic Acquired Resistance to Leptosphaeria maculans in Brassica napus. Mol. Plant-Microbe Interact. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Flores-Pacheco, J.A.; Martínez-Álvarez, P.; Martín-García, J.; Fernández, M.; Diez, J.J. Effect of Mycoviruses on the Virulence of Fusarium circinatum and Laccase Activity. Physiol. Mol. Plant Pathol. 2016, 94, 8–15. [Google Scholar] [CrossRef]
- Van Alfen, N.K.; Jaynes, R.A.; Anagnostakis, S.L.; Day, P.R. Chestnut Blight: Biological Control by Transmissible Hypovirulence in Endothia parasitica. Science 1975, 189, 890–891. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Jiang, D.; Ghabrial, S.A.; Li, G.; Peng, Y.; Xie, J.; Cheng, J.; Huang, J.; et al. A Geminivirus-Related DNA Mycovirus That Confers Hypovirulence to a Plant Pathogenic Fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 8387–8392. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, J.; Fu, Y.; Cheng, J.; Qu, Z.; Zhao, Z.; Cheng, S.; Chen, T.; Li, B.; Wang, Q.; et al. A 2-Kb Mycovirus Converts a Pathogenic Fungus into a Beneficial Endophyte for Brassica Protection and Yield Enhancement. Mol. Plant 2020, 13, 1420–1433. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.; Chen, Q.; Zhu, M.; Zhou, E. A Novel Mycovirus Closely Related to Viruses in the Genus Alphapartitivirus Confers Hypovirulence in the Phytopathogenic Fungus Rhizoctonia solani. Virology 2014, 456–457, 220–226. [Google Scholar] [CrossRef]
- Chun, J.; Ko, Y.H.; Kim, D.H. Transcriptome Analysis of Cryphonectria parasitica Infected with Cryphonectria Hypovirus 1 (CHV1) Reveals Distinct Genes Related to Fungal Metabolites, Virulence, Antiviral RNA-Silencing, and Their Regulation. Front. Microbiol. 2020, 11, 1711. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Adams, M.J.; Benk, M.; Breitbart, M.; Brister, J.R.; Carstens, E.B.; Davison, A.J.; Delwart, E.; Gorbalenya, A.E.; Harrach, B.; et al. Virus Taxonomy in the Age of Metagenomics. Nat. Rev. Microbiol. 2017, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rumbou, A.; Vainio, E.J.; Büttner, C. Towards the Forest Virome: High-Throughput Sequencing Drastically Expands Our Understanding on Virosphere in Temperate Forest Ecosystems. Microorganisms 2021, 9, 1730. [Google Scholar] [CrossRef] [PubMed]
- Chiapello, M.; Rodríguez-Romero, J.; Ayllón, M.A.; Turina, M. Analysis of the Virome Associated to Grapevine Downy Mildew Lesions Reveals New Mycovirus Lineages. Virus Evol. 2020, 6, veaa058. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Nelson, B.D.; Ajayi-Oyetunde, O.; Bradley, C.A.; Hughes, T.J.; Hartman, G.L.; Eastburn, D.M.; Domier, L.L. Identification of Diverse Mycoviruses through Metatranscriptomics Characterization of the Viromes of Five Major Fungal Plant Pathogens. J. Virol. 2016, 90, 6846–6863. [Google Scholar] [CrossRef]
- Myers, J.M.; Bonds, A.E.; Clemons, R.A.; Thapa, N.A.; Simmons, D.R.; Carter-House, D.; Ortanez, J.; Liu, P.; Miralles-Durán, A.; Desirò, A.; et al. Survey of Early-Diverging Lineages of Fungi Reveals Abundant and Diverse Mycoviruses. mBio 2020, 11, e02027-20. [Google Scholar] [CrossRef]
- Velasco, L.; Arjona-Girona, I.; Cretazzo, E.; López-Herrera, C. Viromes in Xylariaceae Fungi Infecting Avocado in Spain. Virology 2019, 532, 11–21. [Google Scholar] [CrossRef]
- Yao, Z.; Zou, C.; Peng, N.; Zhu, Y.; Bao, Y.; Zhou, Q.; Wu, Q.; Chen, B.; Zhang, M. Virome Identification and Characterization of Fusarium sacchari and F. andiyazi: Causative Agents of Pokkah Boeng Disease in Sugarcane. Front. Microbiol. 2020, 11, 240. [Google Scholar] [CrossRef]
- Picarelli, M.A.S.C.; Forgia, M.; Rivas, E.B.; Nerva, L.; Chiapello, M.; Turina, M.; Colariccio, A. Extreme Diversity of Mycoviruses Present in Isolates of Rhizoctonia solani AG2-2 LP from Zoysia japonica from Brazil. Front. Cell. Infect. Microbiol. 2019, 9, 244. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Diez, J.J.; Fernández, M.M.; Hantula, J.; Vainio, E.J. Characterization of Small RNAs Originating from Mitoviruses Infecting the Conifer Pathogen Fusarium circinatum. Arch. Virol. 2018, 163, 1009–1018. [Google Scholar] [CrossRef]
- Vainio, E.J.; Jurvansuu, J.; Streng, J.; Rajamä, M.-L.; Hantula, J.; Valkonen, J.P.T. Diagnosis and Discovery of Fungal Viruses Using Deep Sequencing of Small RNAs. J. Gen. Virol. 2015, 96, 714–725. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, S.; Xiao, X.; Cheng, J.; Fu, Y.; Chen, T.; Jiang, D.; Xie, J. Discovery of Two Mycoviruses by High-Throughput Sequencing and Assembly of Mycovirus-Derived Small Silencing RNAs from a Hypovirulent Strain of Sclerotinia sclerotiorum. Front. Microbiol. 2019, 10, 1415. [Google Scholar] [CrossRef]
- Van De Sande, W.W.J.; Vonk, A.G. Mycovirus Therapy for Invasive Pulmonary Aspergillosis? Med. Mycol. 2019, 57, S179–S188. [Google Scholar] [CrossRef]
- Kotta-Loizou, I.; Coutts, R.H.A. Mycoviruses in Aspergilli: A Comprehensive Review. Front. Microbiol. 2017, 8, 1699. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; Debets, A.J.M.; Hoekstra, R.F. Intra- and Interspecies Virus Transfer in Aspergilli via Protoplast Fusion. Fungal Genet. Biol. 1998, 25, 171–180. [Google Scholar] [CrossRef]
- van Diepeningen, A.D.; Debets, A.J.M.; Hoekstra, R.F. Dynamics of DsRNA Mycoviruses in Black Aspergillus Populations. Fungal Genet. Biol. 2006, 43, 446–452. [Google Scholar] [CrossRef]
- Kanhayuwa, L.; Kotta-Loizou, I.; Özkan, S.; Gunning, A.P.; Coutts, R.H.A. A Novel Mycovirus from Aspergillus fumigatus Contains Four Unique DsRNAs as Its Genome and Is Infectious as DsRNA. Proc. Natl. Acad. Sci. USA 2015, 112, 9100–9105. [Google Scholar] [CrossRef]
- García-Pedrajas, M.D.; Cañizares, M.C.; Sarmiento-Villamil, J.L.; Jacquat, A.G.; Dambolena, J.S. Mycoviruses in Biological Control: From Basic Research to Field Implementation. Phytopathology 2019, 109, 1828–1839. [Google Scholar] [CrossRef]
- Biella, S.; Smith, M.L.; Aist, J.R.; Cortesi, P.; Milgroom, M.G. Programmed Cell Death Correlates with Virus Transmission in a Filamentous Fungus. Proc. R. Soc. B Boil. Sci. 2002, 269, 2269–2276. [Google Scholar] [CrossRef]
- Thapa, V.; Roossinck, M.J. Determinants of Coinfection in the Mycoviruses. Front. Cell. Infect. Microbiol. 2019, 9, 169. [Google Scholar] [CrossRef]
- Zamora, P.; González Casas, A.; Dueñas, M.; San Martin, R.; Diez, J.J. Factors Influencing Growth, Sporulation and Virus Transfer in Cryphonectria parasitica Isolates from Castilla and León (Spain). Eur. J. Plant Pathol. 2017, 148, 65–73. [Google Scholar] [CrossRef]
- Liu, Y.C.; Linder-Basso, D.; Hillman, B.I.; Kaneko, S.; Milgroom, M.G. Evidence for Interspecies Transmission of Viruses in Natural Populations of Filamentous Fungi in the Genus Cryphonectria. Mol. Ecol. 2003, 12, 1619–1628. [Google Scholar] [CrossRef]
- Cornejo, C.; Hisano, S.; Bragança, H.; Suzuki, N.; Rigling, D. A New Double-Stranded RNA Mycovirus in Cryphonectria naterciae Is Able to Cross the Species Barrier and Is Deleterious to a New Host. J. Fungi 2021, 7, 861. [Google Scholar] [CrossRef]
- Wu, M.; Jin, F.; Zhang, J.; Yang, L.; Jiang, D.; Li, G. Characterization of a Novel Bipartite Double-Stranded RNA Mycovirus Conferring Hypovirulence in the Phytopathogenic Fungus Botrytis porri. J. Virol. 2012, 86, 6605–6619. [Google Scholar] [CrossRef]
- Hamid, M.R.; Xie, J.; Wu, S.; Maria, S.K.; Zheng, D.; Hamidou, A.A.; Wang, Q.; Cheng, J.; Fu, Y.; Jiang, D. A Novel Deltaflexivirus That Infects the Plant Fungal Pathogen, Sclerotinia sclerotiorum, Can Be Transmitted among Host Vegetative Incompatible Strains. Viruses 2018, 10, 295. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D. New Insights into Mycoviruses and Exploration for the Biological Control of Crop Fungal Diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef]
- Zamora, P.; Martín, A.B.; Dueñas, M.; Martin, R.S.; Diez, J.J. Cryphonectria parasitica Isolates of the Same Vegetative Compatibility Type Display Different Rates of Transfer of CHV1 Hypovirus. Eur. J. Plant Pathol. 2015, 143, 767–777. [Google Scholar] [CrossRef]
- Brusini, J.; Robin, C. Mycovirus Transmission Revisited by in Situ Pairings of Vegetatively Incompatible Isolates of Cryphonectria parasitica. J. Virol. Methods 2013, 187, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Andika, I.B.; Pang, T.; Lian, Z.; Wei, S.; Niu, E.; Wu, Y.; Kondo, H.; Liu, X.; Sun, L. Facilitative and Synergistic Interactions between Fungal and Plant Viruses. Proc. Natl. Acad. Sci. USA 2020, 117, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Zamora, P.; Martín, A.B.; San Martín, R.; Martínez-Álvarez, P.; Diez, J.J. Control of Chestnut Blight by the Use of Hypovirulent Strains of the Fungus Cryphonectria parasitica in Northwestern Spain. Biol. Control 2014, 79, 58–66. [Google Scholar] [CrossRef]
- Sharma, M.; Guleria, S.; Singh, K.; Chauhan, A.; Kulshrestha, S. Mycovirus Associated Hypovirulence, a Potential Method for Biological Control of Fusarium Species. VirusDisease 2018, 29, 134–140. [Google Scholar] [CrossRef]
- Shapira, R.; Choi, G.H.; Nuss, D.L. Virus-like Genetic Organization and Expression Strategy for a Double-Stranded RNA Genetic Element Associated with Biological Control of Chestnut Blight. EMBO J. 1991, 10, 731–739. [Google Scholar] [CrossRef]
- Choi, G.H.; Nuss, D.L. Hypovirulence of Chestnut Blight Fungus Conferred by an Infectious Viral CDNA. Science 1992, 257, 800–803. [Google Scholar] [CrossRef]
- Fulbright, D.W.; Paul, C.P.; Garrod, S.W. Hypovirulence: A Natural Control of Chestnut Blight. Biocontrol Plant Dis. 2020, 121–139. [Google Scholar] [CrossRef]
- Hillman, B.I.; Suzuki, N. Viruses of the Chestnut Blight Fungus, Cryphonectria parasitica. Adv. Virus Res. 2004, 63, 423–472. [Google Scholar] [CrossRef]
- Milgroom, M.G.; Cortesi, P. Biological Control of Chestnut Blight with Hypovirulence: A Critical Analysis. Annu. Rev. Phytopathol. 2004, 42, 311–338. [Google Scholar] [CrossRef]
- Segers, G.C.; Van Wezel, R.; Zhang, X.; Hong, Y.; Nuss, D.L. Hypovirus Papain-like Protease P29 Suppresses RNA Silencing in the Natural Fungal Host and in a Heterologous Plant System. Eukaryot. Cell 2006, 5, 896–904. [Google Scholar] [CrossRef]
- Jacob-Wilk, D.; Turina, M.; Kazmierczak, P.; Van Alfen, N.K. Silencing of Kex2 Significantly Diminishes the Virulence of Cryphonectria parasitica. Mol. Plant-Microbe Interact. 2009, 22, 211–221. [Google Scholar] [CrossRef]
- Kazmierczak, P.; McCabe, P.; Turina, M.; Jacob-Wilk, D.; Alfen, N.K. Van The Mycovirus CHV1 Disrupts Secretion of a Developmentally Regulated Protein in Cryphonectria parasitica. J. Virol. 2012, 86, 6067–6074. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Kotta-Loizou, I.; Dong, K.; Li, S.; Ni, D.; Hong, N.; Wang, G.; Xu, W. A Mycovirus Modulates the Endophytic and Pathogenic Traits of a Plant Associated Fungus. ISME J. 2021, 15, 1893–1906. [Google Scholar] [CrossRef]
- Kamaruzzaman, M.; He, G.; Wu, M.; Zhang, J.; Yang, L.; Chen, W.; Li, G. A Novel Partitivirus in the Hypovirulent Isolate QT5-19 of the Plant Pathogenic Fungus Botrytis cinerea. Viruses 2019, 11, 24. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Guo, J.; Hu, Z.; Zhang, X.T.; Li, X.G.; Zhong, J. A Novel Partitivirus That Confer Hypovirulence to the Plant Pathogenic Fungus Colletotrichum liriopes. Front. Microbiol. 2021, 12, 653809. [Google Scholar] [CrossRef]
- Hong, Y.; Dover, S.L.; Cole, T.E.; Brasier, C.M.; Buck, K.W. Multiple Mitochondrial Viruses in an Isolate of the Dutch Elm Disease Fungus Ophiostoma Novo-Ulmi. Virology 1999, 258, 118–127. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Wang, Y.; Hong, N.; Zhang, F.; Xu, W.; Wang, G. Hypovirulence of the Phytopathogenic Fungus Botryosphaeria dothidea: Association with a Coinfecting Chrysovirus and a Partitivirus. J. Virol. 2014, 88, 7517–7527. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, M.; Zhang, M.; Hong, N.; Wang, G. Characterization of a Botybirnavirus Conferring Hypovirulence in the Phytopathogenic Fungus Botryosphaeria dothidea. Viruses 2019, 11, 266. [Google Scholar] [CrossRef]
- Hu, W.; Luo, H.; Yang, Y.; Wang, Q.; Hong, N.; Wang, G.; Wang, A.; Wang, L. Comprehensive Analysis of Full Genome Sequence and Bd-MilRNA/Target MRNAs to Discover the Mechanism of Hypovirulence in Botryosphaeria dothidea Strains on Pear Infection with BdCV1 and BdPV1. IMA Fungus 2019, 10, 3. [Google Scholar] [CrossRef]
- Özkan, S.; Mohorianu, I.; Xu, P.; Dalmay, T.; Coutts, R.H.A. Profile and Functional Analysis of Small RNAs Derived from Aspergillus fumigatus Infected with Double-Stranded RNA Mycoviruses. BMC Genom. 2017, 18, 657. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Zhang, J.; Qiu, D.; Guo, L. Generation of a High Resolution Map of SRNAs from Fusarium graminearum and Analysis of Responses to Viral Infection. Sci. Rep. 2016, 6, 26151. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Mercedes Fernández, M.; Diez, J.J. The Use of Mycoviruses in the Control of Forest Diseases. Biocontrol Sci. Technol. 2016, 26, 577–604. [Google Scholar] [CrossRef]
- Chen, B.; Nuss, D.L. Infectious CDNA Clone of Hypovirus CHV1-Euro7: A Comparative Virology Approach To Investigate Virus-Mediated Hypovirulence of the Chestnut Blight Fungus Cryphonectria parasitica. J. Virol. 1999, 73, 985–992. [Google Scholar] [CrossRef]
- Hillman, B.I.; Halpern, B.T.; Brown, M.P. A Viral DsRNA Element of the Chestnut Blight Fungus with a Distinct Genetic Organization. Virology 1994, 201, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, J.; Pault, C.P.; Fulbrightt, D.W.; Nuss, D.L. Structural Properties of Double-Stranded RNAs Associated with Biological Control of Chestnut Blight Fungus. Proc. Natl. Acad. Sci. USA 1986, 83, 9109–9113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, S.; Liu, L.; Cheng, J.; Fu, Y.; Jiang, D.; Xie, J. A Mitovirus Related to Plant Mitochondrial Gene Confers Hypovirulence on the Phytopathogenic Fungus Sclerotinia sclerotiorum. Virus Res. 2015, 197, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xiao, X.; Fu, Y.; Liu, H.; Cheng, J.; Ghabrial, S.A.; Li, G.; Jiang, D. A Novel Mycovirus Closely Related to Hypoviruses That Infects the Plant Pathogenic Fungus Sclerotinia sclerotiorum. Virology 2011, 418, 49–56. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Pearson, M.N. Characterisation of a Novel Hypovirus from Sclerotinia sclerotiorum Potentially Representing a New Genus within the Hypoviridae. Virology 2014, 464–465, 441–449. [Google Scholar] [CrossRef]
- Yang, D.; Wu, M.; Zhang, J.; Chen, W.; Li, G.; Yang, L. Sclerotinia Minor Endornavirus 1, a Novel Pathogenicity Debilitation-Associated Mycovirus with a Wide Spectrum of Horizontal Transmissibility. Viruses 2018, 10, 589. [Google Scholar] [CrossRef]
- Okada, R.; Ichinose, S.; Takeshita, K.; Urayama, S.I.; Fukuhara, T.; Komatsu, K.; Arie, T.; Ishihara, A.; Egusa, M.; Kodama, M.; et al. Molecular Characterization of a Novel Mycovirus in Alternaria alternata Manifesting Two-Sided Effects: Down-Regulation of Host Growth and up-Regulation of Host Plant Pathogenicity. Virology 2018, 519, 23–32. [Google Scholar] [CrossRef]
- Li, H.; Bian, R.; Liu, Q.; Yang, L.; Pang, T.; Salaipeth, L.; Andika, I.B.; Kondo, H.; Sun, L. Identification of a Novel Hypovirulence-Inducing Hypovirus from Alternaria alternata. Front. Microbiol. 2019, 10, 1076. [Google Scholar] [CrossRef]
- Darissa, O.; Adam, G.; Schäfer, W. A DsRNA Mycovirus Causes Hypovirulence of Fusarium graminearum to Wheat and Maize. Eur. J. Plant Pathol. 2012, 134, 181–189. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Chen, X.; Qiu, D.; Guo, L. Molecular Characterization of a Novel Hypovirus from the Plant Pathogenic Fungus Fusarium graminearum. Virology 2015, 481, 151–160. [Google Scholar] [CrossRef]
- Torres-Trenas, A.; Prieto, P.; Cañizares, M.C.; García-Pedrajas, M.D.; Pérez-Artés, E. Mycovirus Fusarium Oxysporum f. Sp. Dianthi Virus 1 Decreases the Colonizing Efficiency of Its Fungal Host. Front. Cell. Infect. Microbiol. 2019, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, L.; Li, G.; Jiang, D.; Ghabrial, S.A. Genome Characterization of a Debilitation-Associated Mitovirus Infecting the Phytopathogenic Fungus Botrytis cinerea. Virology 2010, 406, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Salaipeth, L.; Lin, Y.-H.; Sasaki, A.; Kanematsu, S.; Suzuki, N. A Novel Bipartite Double-Stranded RNA Mycovirus from the White Root Rot Fungus Rosellinia necatrix: Molecular and Biological Characterization, Taxonomic Considerations, and Potential for Biological Control. J. Virol. 2009, 83, 12801–12812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, D.; Nuss, D.L. Variations in Hypovirus Interactions with the Fungal-Host RNA-Silencing Antiviral-Defense Response. J. Virol. 2012, 86, 12933–12939. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Eusebio-Cope, A.; Kondo, H.; Hillman, B.I.; Suzuki, N. Investigation of Host Range of and Host Defense against a Mitochondrially Replicating Mitovirus. J. Virol. 2019, 93, e01503-18. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, A.; Urayama, S.I.; Suo, R.; Itoi, S.; Fuji, S.I.; Moriyama, H.; Hagiwara, D. Mycovirus-Induced Tenuazonic Acid Production in a Rice Blast Fungus Magnaporthe oryzae. Front. Microbiol. 2020, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakis, S.L. Biological Control of Chestnut Blight. Science 1982, 215, 466–471. [Google Scholar] [CrossRef]
- Double, M.L.; Jarosz, A.M.; Fulbright, D.W.; Davelos Baines, A.; MacDonald, W.L. Evaluation of Two Decades of Cryphonectria parasitica Hypovirus Introduction in an American Chestnut Stand in Wisconsin. Phytopathology 2018, 108, 702–710. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the Causal Agent of Chestnut Blight: Invasion History, Population Biology and Disease Control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef]
- Edgar, R.C.; Taylor, J.; Lin, V.; Altman, T.; Barbera, P.; Meleshko, D.; Lohr, D.; Novakovsky, G.; Buchfink, B.; Al-Shayeb, B.; et al. Petabase-Scale Sequence Alignment Catalyses Viral Discovery. Nature 2022, 602, 142–147. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent Andspecific Genetic Interferenceby Double-StrandedRNA in Caenorhabditis Elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Guo, S.; Kemphues, K.J. Par-1, a Gene Required for Establishing Polarity in C. Elegans Embryos, Encodes a Putative Ser/Thr Kinase That Is Asymmetrically Distributed. Cell 1995, 81, 611–620. [Google Scholar] [CrossRef]
- Rothstein, S.J.; DiMaio, J.; Strand, M.; Rice, D. Stable and Heritable Inhibition of the Expression of Nopaline Synthase in Tobacco Expressing Antisense RNA. Proc. Natl. Acad. Sci. USA 1987, 84, 8439–8443. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in Trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Romano, N.; Macino, G. Quelling: Transient Inactivation of Gene Expression in Neurospora crassa by Transformation with Homologous Sequences. Mol. Microbiol. 1992, 6, 3343–3353. [Google Scholar] [CrossRef]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-Directed Nuclease Mediates Post-Transcriptional Gene Silencing in Drosophila Cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-Nucleotide RNAs Mediate RNA Interference in Cultured Mammalian Cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. DsRNA Uptake in Plant Pests and Pathogens: Insights into RNAi-Based Insect and Fungal Control Technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Shabalina, S.A.; Koonin, E.V. Origins and Evolution of Eukaryotic RNA Interference. Trends Ecol. Evol. 2008, 23, 578–587. [Google Scholar] [CrossRef]
- Bramlett, M.; Plaetinck, G.; Maienfisch, P. RNA-Based Biocontrols—A New Paradigm in Crop Protection. Engineering 2020, 6, 522–527. [Google Scholar] [CrossRef]
- Wilson, R.C.; Doudna, J.A. Molecular Mechanisms of RNA Interference. Annu. Rev. Biophys. 2013, 42, 217–239. [Google Scholar] [CrossRef]
- Bartoszewski, R.; Sikorski, A.F. Editorial Focus: Understanding off-Target Effects as the Key to Successful RNAi Therapy. Cell. Mol. Biol. Lett. 2019, 24, 69. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The Current State and Future Directions of RNAi-Based Therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.-L.; Barthel, A.; et al. RNA Interference in Lepidoptera: An Overview of Successful and Unsuccessful Studies and Implications for Experimental Design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef]
- Obbard, D.J.; Gordon, K.H.J.; Buck, A.H.; Jiggins, F.M. The Evolution of RNAi as a Defence against Viruses and Transposable Elements. Philos. Trans. R. Soc. B Biol. Sci. 2008, 364, 99–115. [Google Scholar] [CrossRef]
- Mello, C.C.; Conte, D. Revealing the World of RNA Interference. Nature 2004, 431, 338–342. [Google Scholar] [CrossRef]
- Katiyar-Agarwal, S.; Gao, S.; Vivian-Smith, A.; Jin, H. A Novel Class of Bacteria-Induced Small RNAs in Arabidopsis. Genes Dev. 2007, 21, 3123–3134. [Google Scholar] [CrossRef]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D.G. A Plant MiRNA Contributes to Antibacterial Resistance by Repressing Auxin Signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Y.L.; Zhao, J.H.; Wang, S.; Jin, Y.; Chen, Z.Q.; Fang, Y.Y.; Hua, C.L.; Ding, S.W.; Guo, H.S. Cotton Plants Export MicroRNAs to Inhibit Virulence Gene Expression in a Fungal Pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.P.H.J.; Huang, H.D.; Jin, H. Bidirectional Cross-Kingdom RNAi and Fungal Uptake of External RNAs Confer Plant Protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.; Palmquist, J.; Huang, S.-D.; Jin, H. Plant Send Small RNAs in Extracellular Vesicles to Fungal Pathogen to Silence Virulence Genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef]
- Wagner, E.G.H.; Romby, P. Small RNAs in Bacteria and Archaea: Who They Are, What They Do, and How They Do It. Adv. Genet. 2015, 90, 133–208. [Google Scholar] [CrossRef]
- Mayoral, J.G.; Hussain, M.; Joubert, D.A.; Iturbe-Ormaetxe, I.; O’neill, S.L.; Asgari, S. Wolbachia Small Noncoding RNAs and Their Role in Cross-Kingdom Communications. Proc. Natl. Acad. Sci. USA 2014, 111, 18721–18726. [Google Scholar] [CrossRef]
- Huang, G.; Allen, R.; Davis, E.L.; Baum, T.J.; Hussey, R.S. Engineering Broad Root-Knot Resistance in Transgenic Plants by RNAi Silencing of a Conserved and Essential Root-Knot Nematode Parasitism Gene. Proc. Natl. Acad. Sci. USA 2006, 103, 14302–14306. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Kogel, K.H.; Jin, H. Cross-Kingdom RNA Trafficking and Environmental RNAi—Nature’s Blueprint for Modern Crop Protection Strategies. Curr. Opin. Microbiol. 2018, 46, 58–64. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H. Fungal Small RNAs Suppress Plant Immunity by Hijacking Host RNA Interference Pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Le Bihan, T.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes Secreted by Nematode Parasites Transfer Small RNAs to Mammalian Cells and Modulate Innate Immunity. Nat. Commun. 2014, 5, 5488. [Google Scholar] [CrossRef]
- Garcia-Silva, M.R.; Cura Das Neves, R.F.; Cabrera-Cabrera, F.; Sanguinetti, J.; Medeiros, L.C.; Robello, C.; Naya, H.; Fernandez-Calero, T.; Souto-Padron, T.; De Souza, W.; et al. Extracellular Vesicles Shed by Trypanosoma Cruzi Are Linked to Small RNA Pathways, Life Cycle Regulation, and Susceptibility to Infection of Mammalian Cells. Parasitol. Res. 2014, 113, 285–304. [Google Scholar] [CrossRef]
- Dunker, F.; Trutzenberg, A.; Rothenpieler, J.S.; Kuhn, S.; Pröls, R.; Schreiber, T.; Tissier, A.; Kemen, A.; Kemen, E.; Hückelhoven, R.; et al. Oomycete Small RNAs Bind to the Plant RNA-Induced Silencing Complex for Virulence. eLife 2020, 9, e56096. [Google Scholar] [CrossRef]
- Hou, Y.; Zhai, Y.; Feng, L.; Karimi, H.Z.; Rutter, B.D.; Zeng, L.; Choi, D.S.; Zhang, B.; Gu, W.; Chen, X.; et al. A Phytophthora Effector Suppresses Trans-Kingdom RNAi to Promote Disease Susceptibility. Cell Host Microbe 2019, 25, 153–165.e5. [Google Scholar] [CrossRef]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-Binding Proteins Contribute to Small RNA Loading in Plant Extracellular Vesicles. Nat. Plants 2021, 7, 342–352. [Google Scholar] [CrossRef]
- Zand Karimi, H.; Baldrich, P.; Rutter, B.D.; Borniego, L.; Zajt, K.K.; Meyers, B.C.; Innes, R.W. Arabidopsis Apoplastic Fluid Contains SRNA- and Circular RNA–Protein Complexes That Are Located Outside Extracellular Vesicles. Plant Cell 2022, 34, 1863–1881. [Google Scholar] [CrossRef]
- Jin, H.; Wang, S.; He, B.; Wu, H.; Ramirez-Sánchez, O.; Goodger, C.; Birch, P. Extracellular Vesicle-Mediated Cross-Kingdom Transport of Plant MRNAs into Fungal Cells to Suppress Pathogenicity. In Proceedings of the 31st Fungal Genetics Conference, Pacific Grove, CA, USA, 15–20 March 2022. [Google Scholar]
- Nowara, D.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J.; Schweizer, P. HIGS: Host-Induced Gene Silencing in the Obligate Biotrophic Fungal Pathogen Blumeria graminis. Plant Cell 2010, 22, 3130–3141. [Google Scholar] [CrossRef]
- Koch, A.; Wassenegger, M. Host-Induced Gene Silencing—Mechanisms and Applications. New Phytol. 2021, 231, 54–59. [Google Scholar] [CrossRef]
- Nunes, C.C.; Dean, R.A. Host-Induced Gene Silencing: A Tool for Understanding Fungal Host Interaction and for Developing Novel Disease Control Strategies. Mol. Plant Pathol. 2012, 13, 519–529. [Google Scholar] [CrossRef]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a Cotton Bollworm P450 Monooxygenase Gene by Plant-Mediated RNAi Impairs Larval Tolerance of Gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of Coleopteran Insect Pests through RNA Interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Eschen-Lippold, L.; Landgraf, R.; Smolka, U.; Schulze, S.; Heilmann, M.; Heilmann, I.; Hause, G.; Rosahl, S. Activation of Defense against Phytophthora Infestans in Potato by Down-Regulation of Syntaxin Gene Expression. New Phytol. 2012, 193, 985–996. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Epstein, L.; Wroblewski, T.; Michelmore, R.W. Host-Induced Gene Silencing Inhibits the Biotrophic Pathogen Causing Downy Mildew of Lettuce. Plant Biotechnol. J. 2015, 13, 875–883. [Google Scholar] [CrossRef]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.H. Host-Induced Gene Silencing of Cytochrome P450 Lanosterol C14α-Demethylase-Encoding Genes Confers Strong Resistance to Fusarium Species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef]
- Yin, C.; Jurgenson, J.E.; Hulbert, S.H. Development of a Host-Induced RNAi System in the Wheat Stripe Rust Fungus Puccinia striiformis f. sp. Tritici. Mol. Plant-Microbe Interact. 2010, 24, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jin, Y.; Zhao, J.-H.; Gao, F.; Zhou, B.-J.; Fang, Y.-Y.; Guo, H.-S. Host-Induced Gene Silencing of the Target Gene in Fungal Cells Confers Effective Resistance to the Cotton Wilt Disease Pathogen Verticillium dahliae. Mol. Plant 2016, 9, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.N.; Åsman, A.K.M.; Corcoran, P.; Fogelqvist, J.; Vetukuri, R.R.; Dixelius, C. Plant-Mediated Gene Silencing Restricts Growth of the Potato Late Blight Pathogen Phytophthora Infestans. J. Exp. Bot. 2015, 66, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO Maize against Western Corn Rootworm and Northern Corn Rootworm: Efficacy and Resistance Management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-Based Biocontrol Products: Market Status, Regulatory Aspects, and Risk Assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef]
- ISAAA-GM Approval Database. Available online: https://www.isaaa.org/gmapprovaldatabase/default.asp (accessed on 16 March 2022).
- Christiaens, O.; Dzhambazova, T.; Kostov, K.; Arpaia, S.; Joga, M.R.; Urru, I.; Sweet, J.; Smagghe, G. Literature Review of Baseline Information on RNAi to Support the Environmental Risk Assessment of RNAi-based GM Plants. EFSA Support. Publ. 2018, 15, 1424E. [Google Scholar] [CrossRef]
- Dávalos, A.; Henriques, R.; Latasa, M.J.; Laparra, M.; Coca, M. Literature Review of Baseline Information on Non-Coding RNA (NcRNA) to Support the Risk Assessment of NcRNA-Based Genetically Modified Plants for Food and Feed. EFSA Support. Publ. 2019, 16, 1688E. [Google Scholar] [CrossRef]
- EN 32001L0018; EC. Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the Deliberate Release into the Environment of Genetically Modified Organisms and Repealing Council Directive 90/220/EEC. Commission Declaration. Official Journal of the European Union: Brussels, Belgum, 2001; Volume 106, pp. 1–39.
- EN 32003R1829; EC. Regulation (EC) No. 1829/2003 of the European Parliament and of the Council of 22 September 2003 on Genetically Modified Food and Feed. Official Journal of the European Union: Brussels, Belgum, 2003; Volume 268, pp. 1–23.
- Custers, R.; Bartsch, D.; Fladung, M.; Nilsson, O.; Pilate, G.; Sweet, J.; Boerjan, W. EU Regulations Impede Market Introduction of GM Forest Trees. Trends Plant Sci. 2016, 21, 283–285. [Google Scholar] [CrossRef]
- Pelai, R.; Hagerman, S.M.; Kozak, R. Biotechnologies in Agriculture and Forestry: Governance Insights from a Comparative Systematic Review of Barriers and Recommendations. For. Policy Econ. 2020, 117, 102191. [Google Scholar] [CrossRef]
- Masip, G.; Sabalza, M.; Pérez-Massot, E.; Banakar, R.; Cebrian, D.; Twyman, R.M.; Capell, T.; Albajes, R.; Christou, P. Paradoxical EU Agricultural Policies on Genetically Engineered Crops. Trends Plant Sci. 2013, 18, 312–324. [Google Scholar] [CrossRef]
- Tabara, H.; Grishok, A.; Mello, C.C. RNAi in C. elegans: Soaking in the Genome Sequence. Science 1998, 282, 430–431. [Google Scholar] [CrossRef]
- Timmons, L.; Fire, A. Specific Interference by Ingested DsRNA. Nature 1998, 395, 854. [Google Scholar] [CrossRef]
- Whangbo, J.S.; Hunter, C.P. Environmental RNA Interference. Trends Genet. 2008, 24, 297–305. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-Based Control of Fusarium graminearum Infections through Spraying of Long DsRNAs Involves a Plant Passage and Is Controlled by the Fungal Silencing Machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Koch, A.; Stein, E.; Kogel, K.H. RNA-Based Disease Control as a Complementary Measure to Fight Fusarium Fungi through Silencing of the Azole Target Cytochrome P450 Lanosterol C-14 α-Demethylase. Eur. J. Plant Pathol. 2018, 152, 1003–1010. [Google Scholar] [CrossRef]
- Koch, A.; Höfle, L.; Werner, B.T.; Imani, J.; Schmidt, A.; Jelonek, L.; Kogel, K.H. SIGS vs HIGS: A Study on the Efficacy of Two DsRNA Delivery Strategies to Silence Fusarium FgCYP51 Genes in Infected Host and Non-Host Plants. Mol. Plant Pathol. 2019, 20, 1636–1644. [Google Scholar] [CrossRef]
- McLoughlin, A.G.; Wytinck, N.; Walker, P.L.; Girard, I.J.; Rashid, K.Y.; De Kievit, T.; Fernando, W.G.D.; Whyard, S.; Belmonte, M.F. Identification and Application of Exogenous DsRNA Confers Plant Protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep. 2018, 8, 7320. [Google Scholar] [CrossRef]
- Duanis-Assaf, D.; Galsurker, O.; Davydov, O.; Maurer, D.; Feygenberg, O.; Sagi, M.; Poverenov, E.; Fluhr, R.; Alkan, N. Double-stranded RNA Targeting Fungal Ergosterol Biosynthesis Pathway Controls Botrytis cinerea and Postharvest Grey Mould. Plant Biotechnol. J. 2022, 20, 226. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Pecchia, S. Silencing of the Slt2-Type MAP Kinase Bmp3 in Botrytis cinerea by Application of Exogenous DsRNA Affects Fungal Growth and Virulence on Lactuca sativa. Int. J. Mol. Sci. 2021, 22, 5362. [Google Scholar] [CrossRef]
- Sundaresha, S.; Sharma, S.; Bairwa, A.; Tomar, M.; Kumar, R.; Bhardwaj, V.; Jeevalatha, A.; Bakade, R.; Salaria, N.; Thakur, K.; et al. Spraying of DsRNA Molecules Derived from Phytophthora Infestans, as an Effective Plant Protection Strategies for the Management of Potato Late Blight. Preprints 2021. [Google Scholar] [CrossRef]
- Cheng, D.W.; Lin, M.M.; Chu, D.M.; Xiang, M.G.; Guo, M.J.; Jiang, M.Y.; Guan, D.; He, D.S. RNAi-Based Gene Silencing of RXLR Effectors Protects Plant against the Oomycete Pathogen Phytophthora Capsici. Mol. Plant-Microbe Interact. 2022, 35, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Nerva, L.; Sandrini, M.; Gambino, G.; Chitarra, W. Double-Stranded RNAs (DsRNAs) as a Sustainable Tool against Gray Mold (Botrytis cinerea) in Grapevine: Effectiveness of Different Application Methods in an Open-Air Environment. Biomolecules 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Taning, C.N.T.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Mezzetti, B.; Sabbadini, S.; Sorteberg, H.G.; Sweet, J.; Ventura, V.; et al. RNA-Based Biocontrol Compounds: Current Status and Perspectives to Reach the Market. Pest Manag. Sci. 2020, 76, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Bachman, P.; Fischer, J.; Song, Z.; Urbanczyk-Wochniak, E.; Watson, G. Environmental Fate and Dissipation of Applied DsRNA in Soil, Aquatic Systems, and Plants. Front. Plant Sci. 2020, 11, 21. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H. Spray-Induced Gene Silencing: A Powerful Innovative Strategy for Crop Protection. Trends Microbiol. 2017, 25, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-Induced Gene Silencing for Disease Control Is Dependent on the Efficiency of Pathogen RNA Uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Höfle, L.; Biedenkopf, D.; Werner, B.T.; Shrestha, A.; Jelonek, L.; Koch, A. Study on the Efficiency of DsRNAs with Increasing Length in RNA-Based Silencing of the Fusarium CYP51 Genes. RNA Biol. 2020, 17, 463–473. [Google Scholar] [CrossRef]
- Song, X.-S.; Gu, K.-X.; Duan, X.-X.; Xiao, X.-M.; Hou, Y.-P.; Duan, Y.-B.; Wang, J.-X.; Yu, N.; Zhou, M.-G. Secondary Amplification of SiRNA Machinery Limits the Application of Spray-Induced Gene Silencing. Mol. Plant Pathol. 2018, 19, 2543–2560. [Google Scholar] [CrossRef]
- Yoo, B.C.; Kragler, F.; Varkonyi-Gasic, E.; Haywood, V.; Archer-Evans, S.; Lee, Y.M.; Lough, T.J.; Lucas, W.J. A Systemic Small RNA Signaling System in Plants. Plant Cell 2004, 16, 1979–2000. [Google Scholar] [CrossRef]
- Palauqui, J.C.; Elmayan, T.; Pollien, J.M.; Vaucheret, H. Systemic Acquired Silencing: Transgene-Specific Post-Transcriptional Silencing Is Transmitted by Grafting from Silenced Stocks to Non-Silenced Scions. EMBO J. 1997, 16, 4738–4745. [Google Scholar] [CrossRef]
- Betti, F.; Ladera-Carmona, M.J.; Weits, D.A.; Ferri, G.; Iacopino, S.; Novi, G.; Svezia, B.; Kunkowska, A.B.; Santaniello, A.; Piaggesi, A.; et al. Exogenous MiRNAs Induce Post-Transcriptional Gene Silencing in Plants. Nat. Plants 2021, 7, 1379–1388. [Google Scholar] [CrossRef]
- Rosa, C.; Kuo, Y.W.; Wuriyanghan, H.; Falk, B.W. RNA Interference Mechanisms and Applications in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef]
- Iqbal, S.; Fosu-Nyarko, J.; Jones, M.G.K. Attempt to Silence Genes of the RNAi Pathways of the Root-Knot Nematode, Meloidogyne Incognita Results in Diverse Responses Including Increase and No Change in Expression of Some Genes. Front. Plant Sci. 2020, 11, 328. [Google Scholar] [CrossRef]
- Ameres, S.L.; Martinez, J.; Schroeder, R. Molecular Basis for Target RNA Recognition and Cleavage by Human RISC. Cell 2007, 130, 101–112. [Google Scholar] [CrossRef]
- Bohula, E.A.; Salisbury, A.J.; Sohail, M.; Playford, M.P.; Riedemann, J.; Southern, E.M.; Macaulay, V.M. The Efficacy of Small Interfering RNAs Targeted to the Type 1 Insulin-like Growth Factor Receptor (IGF1R) Is Influenced by Secondary Structure in the IGF1R Transcript. J. Biol. Chem. 2003, 278, 15991–15997. [Google Scholar] [CrossRef]
- Vickers, T.A.; Koo, S.; Bennett, C.F.; Crooke, S.T.; Dean, N.M.; Baker, B.F. Efficient Reduction of Target RNAs by Small Interfering RNA and RNase H-Dependent Antisense Agents: A Comparative Analysis. J. Biol. Chem. 2003, 278, 7108–7118. [Google Scholar] [CrossRef]
- Patzel, V.; Rutz, S.; Dietrich, I.; Köberle, C.; Scheffold, A.; Kaufmann, S.H.E. Design of SiRNAs Producing Unstructured Guide-RNAs Results in Improved RNA Interference Efficiency. Nat. Biotechnol. 2005, 23, 1440–1444. [Google Scholar] [CrossRef]
- He, W.; Xu, W.; Xu, L.; Fu, K.; Guo, W.; Bock, R.; Zhang, J. Length-Dependent Accumulation of Double-Stranded RNAs in Plastids Affects RNA Interference Efficiency in the Colorado Potato Beetle. J. Exp. Bot. 2020, 71, 2670–2677. [Google Scholar] [CrossRef]
- Tafer, H.; Ameres, S.L.; Obernosterer, G.; Gebeshuber, C.A.; Schroeder, R.; Martinez, J.; Hofacker, I.L. The Impact of Target Site Accessibility on the Design of Effective SiRNAs. Nat. Biotechnol. 2008, 26, 578–583. [Google Scholar] [CrossRef]
- Jackson, A.L.; Bartz, S.R.; Schelter, J.; Kobayashi, S.V.; Burchard, J.; Mao, M.; Li, B.; Cavet, G.; Linsley, P.S. Expression Profiling Reveals Off-Target Gene Regulation by RNAi. Nat. Biotechnol. 2003, 21, 635–637. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Y.; Zhang, H.; Wang, K.; Zhao, C.; Zhu, G.; Reddy Palli, S.; Han, Z. Off-Target Effects of RNAi Correlate with the Mismatch Rate between DsRNA and Non-Target MRNA. RNA Biol. 2021, 18, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chan, C.Y.; Lawrence, C.E. Sfold Web Server for Statistical Folding and Rational Design of Nucleic Acids. Nucleic Acids Res. 2004, 32, W135–W141. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Latek, R.; Hossbach, M.; Tuschl, T.; Lewitter, F. SiRNA Selection Server: An Automated SiRNA Oligonucleotide Prediction Server. Nucleic Acids Res. 2004, 32, W130–W134. [Google Scholar] [CrossRef] [PubMed]
- Good, R.T.; Varghese, T.; Golz, J.F.; Russell, D.A.; Papanicolaou, A.; Edwards, O.; Robin, C. OfftargetFinder: A Web Tool for Species-Specific RNAi Design. Bioinformatics 2016, 32, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yoshimura, J.; Morishita, S.; Ui-Tei, K. SiDirect 2.0: Updated Software for Designing Functional SiRNA with Reduced Seed-Dependent off-Target Effect. BMC Bioinform. 2009, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Lück, S.; Kreszies, T.; Strickert, M.; Schweizer, P.; Kuhlmann, M.; Douchkov, D. SiRNA-Finder (Si-Fi) Software for RNAi-Target Design and Off-Target Prediction. Front. Plant Sci. 2019, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, M.; Xu, Z.P. Clay Nanosheets for Topical Delivery of RNAi for Sustained Protection against Plant Viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Šečić, E.; Kogel, K.H. Requirements for Fungal Uptake of DsRNA and Gene Silencing in RNAi-Based Crop Protection Strategies. Curr. Opin. Biotechnol. 2021, 70, 136–142. [Google Scholar] [CrossRef]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous Application of RNAi-Inducing Double-Stranded RNA Inhibits Aphid-Mediated Transmission of a Plant Virus. Front. Plant Sci. 2019, 10, 265. [Google Scholar] [CrossRef]
- Jain, R.G.; Fletcher, S.J.; Manzie, N.; Robinson, K.E.; Li, P.; Lu, E.; Brosnan, C.A.; Xu, Z.P.; Mitter, N. Foliar Application of Clay-Delivered RNA Interference for Whitefly Control. Nat. Plants 2022, 8, 535–548. [Google Scholar] [CrossRef]
- Zhang, H.; Demirer, G.S.; Zhang, H.; Ye, T.; Goh, N.S.; Aditham, A.J.; Cunningham, F.J.; Fan, C.; Landry, M.P. DNA Nanostructures Coordinate Gene Silencing in Mature Plants. Proc. Natl. Acad. Sci. USA 2019, 116, 7543–7548. [Google Scholar] [CrossRef]
- Demirer, G.; Zhang, H.; Goh, N.; Pinnals, R.L.; Chang, R.; Landry, M. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon Dots for Efficient Small Interfering RNA Delivery and Gene Silencing in Plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Das, S.; Debnath, N.; Cui, Y.; Unrine, J.; Palli, S.R. Chitosan, Carbon Quantum Dot, and Silica Nanoparticle Mediated DsRNA Delivery for Gene Silencing in Aedes Aegypti: A Comparative Analysis. ACS Appl. Mater. Interfaces 2015, 7, 19530–19535. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Xu, D.; Goh, N.S.; Demirer, G.S.; Cestellos-Blanco, S.; Chen, Y.; Landry, M.P.; Yang, P. Gold-Nanocluster-Mediated Delivery of SiRNA to Intact Plant Cells for Efficient Gene Knockdown. Nano Lett. 2021, 21, 5859–5866. [Google Scholar] [CrossRef]
- Lei, W.X.; An, Z.S.; Zhang, B.H.; Wu, Q.; Gong, W.J.; Li, J.M.; Chen, W.L. Construction of Gold-SiRNA NPR1 Nanoparticles for Effective and Quick Silencing of NPR1 in Arabidopsis thaliana. RSC Adv. 2020, 10, 19300–19308. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef]
- Santana, I.; Wu, H.; Hu, P.; Giraldo, J.P. Targeted Delivery of Nanomaterials with Chemical Cargoes in Plants Enabled by a Biorecognition Motif. Nat. Commun. 2020, 11, 2045. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Hamby, R.; Quian, L.; Jin, H. The Use of Nanotechnology in Spray-Induced Gene Silencing (SIGS) Provides a Steady RNAi Effect against Botrytis cinerea Infection on Plant Material. In Proceedings of the APS Annual Meeting, Online, 10–14 August 2020. [Google Scholar]
- Podesta, J.E.; Kostarelos, K. Engineering Cationic Liposome. SiRNA Complexes for In Vitro and In Vivo Delivery. In Methods in Enzymology; Elsevier Masson SAS: Paris, France, 2009; Volume 464. [Google Scholar]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of Therapeutic Agents by Nanoparticles Made of Grapefruit-Derived Lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652. [Google Scholar] [CrossRef]
- De Schutter, K.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. Boosting DsRNA Delivery in Plant and Insect Cells with Peptide-and Polymer-Based Carriers: Case-Based Current Status and Future Perspectives. In RNAi for Plant Improvement and Protection; Mezzetti, B., Sweet, J., Burgos, L., Eds.; CABI: Wallingford, UK, 2021; pp. 102–116. ISBN 9781789248890. [Google Scholar]
- Martinez, Z.; De Schutter, K.; Van Damme, E.J.M.; Vogel, E.; Wynant, N.; Vanden Broeck, J.; Christiaens, O.; Smagghe, G. Accelerated Delivery of DsRNA in Lepidopteran Midgut Cells by a Galanthus Nivalis Lectin (GNA)-DsRNA-Binding Domain Fusion Protein. Pestic. Biochem. Physiol. 2021, 175, 104853. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Zhang, R.; Bi, S.; Li, P.; Sun, L.; Mitter, N.; Carroll, B.J.; Xu, Z.P. Sheet-like Clay Nanoparticles Deliver RNA into Developing Pollen to Efficiently Silence a Target Gene. Plant Physiol. 2021, 187, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Goh, N.S.; Wang, J.W.; Pinals, R.L.; González-Grandío, E.; Demirer, G.S.; Butrus, S.; Fakra, S.C.; Del Rio Flores, A.; Zhai, R.; et al. Nanoparticle Cellular Internalization Is Not Required for RNA Delivery to Mature Plant Leaves. Nat. Nanotechnol. 2021, 17, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Niño-Sánchez, J.; Chen, L.-H.; De Souza, J.T.; Mosquera, S.; Stergiopoulos, I. Targeted Delivery of Gene Silencing in Fungi Using Genetically Engineered Bacteria. J. Fungi 2021, 7, 125. [Google Scholar] [CrossRef]
- Ivashuta, S.I.; Petrick, J.S.; Heisel, S.E.; Zhang, Y.; Guo, L.; Reynolds, T.L.; Rice, J.F.; Allen, E.; Roberts, J.K. Endogenous Small RNAs in Grain: Semi-Quantification and Sequence Homology to Human and Animal Genes. Food Chem. Toxicol. 2009, 47, 353–360. [Google Scholar] [CrossRef]
- Yadav, J.S.; Ogwok, E.; Wagaba, H.; Patil, B.L.; Bagewadi, B.; Alicai, T.; Gaitan-Solis, E.; Taylor, N.J.; Fauquet, C.M. RNAi-Mediated Resistance to Cassava Brown Streak Uganda Virus in Transgenic Cassava. Mol. Plant Pathol. 2011, 12, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Bachman, P.M.; Huizinga, K.M.; Jensen, P.D.; Mueller, G.; Tan, J.; Uffman, J.P.; Levine, S.L. Ecological Risk Assessment for DvSnf7 RNA: A Plant-Incorporated Protectant with Targeted Activity against Western Corn Rootworm. Regul. Toxicol. Pharmacol. 2016, 81, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.B.; Petrick, J.S. Safety Considerations for Humans and Other Vertebrates Regarding Agricultural Uses of Externally Applied RNA Molecules. Front. Plant Sci. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.M.; Borrero, V.B.; van Leeuwen, D.M.; Lever, M.A.; Mateescu, B.; Sander, M. Environmental Fate of RNA Interference Pesticides: Adsorption and Degradation of Double-Stranded RNA Molecules in Agricultural Soils. Environ. Sci. Technol. 2019, 53, 3027–3036. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide Emergence of Resistance to Antifungal Drugs Challenges Human Health and Food Security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Amarzguioui, M.; Holen, T.; Babaie, E.; Prydz, H. Tolerance for Mutations and Chemical Modifications in a SiRNA. Nucleic Acids Res. 2003, 31, 589–595. [Google Scholar] [CrossRef]
- Zotti, M.; dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA Interference Technology in Crop Protection against Arthropod Pests, Pathogens and Nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- Newmark, P.A.; Reddien, P.W.; Cebrià, F.; Alvarado, A.S. Ingestion of Bacterially Expressed Double-Stranded RNA Inhibits Gene Expression in Planarians. Proc. Natl. Acad. Sci. USA 2003, 100, 11861–11865. [Google Scholar] [CrossRef]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; dos Santos, E.Á.; Smagghe, G.; Zotti, M.J. Management of Pest Insects and Plant Diseases by Non-Transformative RNAi. Front. Plant Sci. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Attasart, P.; Namramoon, O.; Kongphom, U.; Chimwai, C.; Panyim, S. Ingestion of Bacteria Expressing DsRNA Triggers Specific RNA Silencing in Shrimp. Virus Res. 2013, 171, 252–256. [Google Scholar] [CrossRef]
- Weiss, S.; Chakraborty, T. Transfer of Eukaryotic Expression Plasmids to Mammalian Host Cells by Bacterial Carriers. Curr. Opin. Biotechnol. 2001, 12, 467–472. [Google Scholar] [CrossRef]
- Tenllado, F.; Martínez-García, B.; Vargas, M.; Ramón Díaz-Ruíz, J. Crude Extracts of Bacterially Expressed DsRNA Can Be Used to Protect Plants against Virus Infections. BMC Biotechnol. 2003, 3, 3. [Google Scholar] [CrossRef]
- Niehl, A.; Soininen, M.; Poranen, M.M.; Heinlein, M. Synthetic Biology Approach for Plant Protection Using DsRNA. Plant Biotechnol. J. 2018, 16, 1679–1687. [Google Scholar] [CrossRef]
- Sun, Z.-N.; Yin, G.-H.; Song, Y.-Z.; An, H.-L.; Zhu, C.-X.; Wen, F.-J. Bacterially Expressed Double-Stranded RNAs against Hot-Spot Sequences of Tobacco Mosaic Virus or Potato Virus Y Genome Have Different Ability to Protect Tobacco from Viral Infection. Appl. Biochem. Biotechnol. 2010, 162, 1901–1914. [Google Scholar] [CrossRef]
- Yin, G.; Sun, Z.; Liu, N.; Zhang, L.; Song, Y.; Zhu, C.; Wen, F. Production of Double-Stranded RNA for Interference with TMV Infection Utilizing a Bacterial Prokaryotic Expression System. Appl. Microbiol. Biotechnol. 2009, 84, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Verdonckt, T.-W.; Vanden Broeck, J. Methods for the Cost-Effective Production of Bacteria-Derived Double-Stranded RNA for in Vitro Knockdown Studies. Front. Physiol. 2022, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Joyce, W. RNAi Opens a New Vista for Pest Control. AgroPages’ 2020 Biologicals Special. July 2020. Available online: http://report.agropages.com/userfiles/pdf/2020biexold.pdf (accessed on 16 August 2022).
- Maxwell, B.; Boyes, D.; Tang, J.; Rodrigues, T.; Desai, S.; Cunningham, D.; Ramachandriya, K.; Abshire, J.; Cobb, C. Enabling the RNA Revolution; Cell-Free DsRNA Production and Control of Colorado Potato Beetle. 2019. Available online: http://www.globalengage.co.uk/pgc/docs/PosterMaxwell.pdf (accessed on 30 March 2022).
- Rodrigues, T.; Sridharan, K.; Manley, B.; Cunningham, D.; Narva, K. Development of dsRNA as a Sustainable Bioinsecticide: From Laboratory to Field. In Crop Protection Products for Sustainable Agriculture; American Chemical Society: Washington, DC, USA, 2021; Volume 17, pp. 65–82. [Google Scholar] [CrossRef]
| Mycovirus | Fungal Host | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome | Viral Family | Agaricomycetes | Dothideomycetes | Eurotiomycetes | Glomeromycetes | Leotiomycetes | Pezizomycetes | Pucciniomycetes | Saccharomycetes | Sordariomycetes | Tremellomycetes | Ustilaginomycetes | NA **1 |
| ssDNA(−) | Anelloviridae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 |
| dsRNA | Amalgaviridae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 |
| Chrysoviridae | 0 | 5 | 6 | 0 | 0 | 0 | 0 | 1 | 10 | 0 | 0 | 0 | |
| Curvulaviridae | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | |
| Megabirnaviridae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | |
| Partitiviridae | 18 | 3 | 6 | 0 | 6 | 0 | 1 | 0 | 16 | 0 | 0 | 0 | |
| Polymycoviridae | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | |
| Quadriviridae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Reoviridae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | |
| Totiviridae | 2 | 6 | 3 | 1 | 11 | 1 | 1 | 4 | 18 | 2 | 1 | 0 | |
| ssRNA(+) | Alphaflexiviridae | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Barnaviridae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Botourmiaviridae | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | |
| Deltaflexiviridae | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Endornaviridae | 9 | 1 | 0 | 0 | 6 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | |
| Fusariviridae | 0 | 2 | 2 | 0 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | |
| Gammaflexiviridae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hypoviridae | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | |
| Mitoviridae | 4 | 2 | 0 | 11 | 13 | 1 | 5 | 0 | 12 | 0 | 0 | 0 | |
| Narnaviridae | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | |
| Nodaviridae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Secoviridae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Tombusviridae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Virgaviridae | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| ssRNA(−) | Aspiviridae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mymonaviridae | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Phenuiviridae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| ssRNA-RT | Metaviridae | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DNA | Genomoviridae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NA **2 | NA **2 | 5 | 6 | 4 | 1 | 10 | 0 | 0 | 2 | 24 | 0 | 0 | 0 |
| Tool | Use | Source | URL | Access |
|---|---|---|---|---|
| siDESIGN Center | siRNAs design | Dharmacon | https://horizondiscovery.com/en/ordering-and-calculation-tools/sidesign-center accessed on 16 August 2022 | Free-access |
| DsiRNA | Custom design of Dicer-Substrate siRNA (DsiRNA) | IDT | https://www.eu.idtdna.com/site/order/designtool/index/DSIRNA_CUSTOM accessed on 16 August 2022 | Free-access |
| BLOCK-iT™ RNAi Designer | Design of siRNAs, shRNAs, Stealth RNAi™ siRNAs and miR RNAs | ThermoFisher | https://rnaidesigner.thermofisher.com/rnaiexpress/ accessed on 16 August 2022 | Free-access |
| Sfold web server | Prediction of RNA secondary structure | Ding et al., 2004 [190] | https://sfold.wadsworth.org/cgi-bin/index.pl accessed on 16 August 2022 | Free-access |
| siRNA at Whitehead | siRNAs design | Whitehead Institute for Biomedical Research Yuan et al., 2004 [191] | http://sirna.wi.mit.edu/ accessed on 16 August 2022 | Free-access |
| siMax siRNA design tool | siRNAs design | Eurofins | https://eurofinsgenomics.eu/en/dna-rna-oligonucleotides/oligo-tools/sirna-design-tool/ accessed on 16 August 2022 | Free-access |
| OfftargetFinder | Off-target prediction | Good et al., 2016 [192] | no longer available | Free-access |
| siDirect | siRNAs design and off-target prediction | Naito et al., 2009 [193] | http://sidirect2.rnai.jp/ accessed on 16 August 2022 | Free-access |
| si-Fi (siRNA-Finder) | RNAi design and off-target prediction | Luck et al., 2019 [194] | open-source (CC BY-SA license) desktop software | Free-access |
| RNAfold WebServer | Prediction of RNA secondary structure | Institute for Theoretical Chemistry (University of Vienna) | http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi accessed on 16 August 2022 | Free-access |
| RNAxs web server | siRNAs design | Theoretical Biochemistry Group (University of Vienna), Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences, Max Perutz Labs Vienna | http://rna.tbi.univie.ac.at/cgi-bin/RNAxs/RNAxs.cgi accessed on 16 August 2022 | Free-access |
| RNA plfold | Assess the mRNA target site accessibility | Theoretical Biochemistry Group (University of Vienna) | https://www.tbi.univie.ac.at/RNA/RNAplfold.1.html accessed on 16 August 2022 | Free-access |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocos-Asenjo, I.T.; Niño-Sánchez, J.; Ginésy, M.; Diez, J.J. New Insights on the Integrated Management of Plant Diseases by RNA Strategies: Mycoviruses and RNA Interference. Int. J. Mol. Sci. 2022, 23, 9236. https://doi.org/10.3390/ijms23169236
Bocos-Asenjo IT, Niño-Sánchez J, Ginésy M, Diez JJ. New Insights on the Integrated Management of Plant Diseases by RNA Strategies: Mycoviruses and RNA Interference. International Journal of Molecular Sciences. 2022; 23(16):9236. https://doi.org/10.3390/ijms23169236
Chicago/Turabian StyleBocos-Asenjo, Irene Teresa, Jonatan Niño-Sánchez, Mireille Ginésy, and Julio Javier Diez. 2022. "New Insights on the Integrated Management of Plant Diseases by RNA Strategies: Mycoviruses and RNA Interference" International Journal of Molecular Sciences 23, no. 16: 9236. https://doi.org/10.3390/ijms23169236