Alien Species in the Pioneer and Ruderal Vegetation of Ukraine
Abstract
:1. Introduction
A Short History of the Development of the Territory of Ukraine
2. Materials and Methods
2.1. Study Area
2.2. The Dataset
2.3. Analysis
- anthropogenization (IAn), as the proportion between alien plant species and all species in the studied flora (coenofloras);
- archaeophytization (IArch), as the proportion of archaeophytes in all species pools of each coenoflora;
- kenophytization (IKen), as the proportion of kenophytes in all species pools of each coenoflora;
- modernization (IM), as the proportion between kenophytes and all species in the studied coenofloras;
- fluctuation (instability) (IF), as the proportion of diaphytes (ergasiophytes and ephemerophytes) in all species pools of each coenoflora.
2.4. Nomenclature
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.; Vítovcová, K.; Řehounková, K.; Müllerová, A.; Janečková, P.; Pospíšilová, P.; Prach, K. Alien species in vegetation succession: Participation, temporal trends and determining factors in various central European series. Biol. Invasions 2021, 23, 3435–3445. [Google Scholar] [CrossRef]
- Kuzmichev, A.I. Hyhrophyllous Flora of South-West of Russian Lowland and Its Genesis; Parfyonov, V.I., Ed.; Hydrometizdat: Sankt-Petersburg, Russia, 1992. [Google Scholar]
- Ilyin, M.M. Flora of littoral and desert in their connections. Sov. Bot. 1947, 5, 249–256. [Google Scholar]
- Zosimovich, V.P. Evolution of Goosefoot and the Genus Beta. Scientific Papers on Selection, Agricultural Technology, Mechanization and Crop Protection; Ahrarna Nauka: Kyiv, Ukraine, 1958; Volume 38, pp. 59–73. [Google Scholar]
- Zozulin, G.M. Historical suites of vegetation of the European part of the USSR. Bot. J. 1973, 58, 1081–1092. [Google Scholar]
- Zlobin, Y.A. Phytocoenotic Barrier. Materials on the Dynamics of the Vegetation Cover; Reports at the Interuniversity Conference in September 1968; Vladimir Printing House of the Glavpoligrafprom: Vladimir, Russia, 1968; pp. 9–10. [Google Scholar]
- Sax, D.F.; Stachowicz, J.J.; Brown, J.H.; Bruno, J.F.; Dawson, M.N.; Gaines, S.D.; Grosberg, R.K.; Hastings, A.; Holt, R.D.; Mayfield, M.M.; et al. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 2007, 22, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Burda, R.I.; Protopopova, V.V.; Shevera, M.V.; Kucher, O.O.; Koniakin, S.M. Alien Species in the Flora of Ukraine: Years and Authors. Bibliographic List/Kyiv. 2022. Available online: http://www.botany.kiev.ua/doc/bibliograf9.pdf (accessed on 1 November 2022).
- Shevera, M.V.; Protopopova, V.V.; Burda, R.I.; Zavialova, L.V.; Kucher, O.O.; Korniyenko, O.M. Historical overview of the studies of alien flora of Ukraine. Acta Horti Bot. Bucurest 2018, 45, 5–32. [Google Scholar]
- Protopopova, V.V. Synanthropic Flora of Ukraine and Ways of Its Development; Naukova Dumka: Kyiv, Ukraine, 1991. [Google Scholar]
- Dengler, J.; Jansen, F.; Glöckler, F.; Peet, R.K.; De Cáceres, M.; Chytrý, M.; Ewald, J.; Oldeland, J.; Lopez-Gonzalez, G.; Finckh, M.; et al. The Global Index of Vegetation-Plot Databases (GIVD): A new resource for vegetation science. J. Veget. Sci. 2012, 22, 582–597. [Google Scholar] [CrossRef]
- Dubyna, D.; Dziuba, T.; Iemelianova, S. Database of pioneer vegetation of Ukraine. In Proceedings of the 15th meeting of the German Working Group on Vegetation Databases, Potsdam, Germany, 2–4 March 2016; p. 42. [Google Scholar]
- Tolmachev, A.I. Introduction to Plant Geography; Leningrad Univ. Press: Sankt-Petersburg, Russia, 1974. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography, Being the Collected Papers of C. Raunkiær; Gilbert-Carter, H.; Fausbøll, A.; Tansley, A.G., Translators; Oxford University Press: Oxford, UK, 1934. [Google Scholar]
- Takhtajan, A.L. The Floristic Regions of the World; Nauka: Sankt-Petersburg, Russia, 1978. [Google Scholar]
- Kornaś, J. Geograficzno-historyczna klasyfikacja roślin synantropijnych. Mater. Zakładu Fitosocjologii Stosow. Uniw. Warsz. 1968, 25, 33–41. [Google Scholar]
- Jackowiak, B. Antropogeniczne Przemiany Flory Roślin Naczyniowych Poznania; Seria Biologia; Wydawnictwo Naukowe Uniwersytetu Adama Mickiewicza: Poznań, Poland, 1990; Volume 42. [Google Scholar]
- Didukh, Y.P. The Ecological Scales for the Species of Ukrainian Flora and Their Use in Synphytoindication; Phytosociocentre: Kyiv, Ukraine, 2011. [Google Scholar]
- Tichy, L. JUICE, software for vegetation classification. J. Veget. Sci. 2002, 13, 451–453. [Google Scholar] [CrossRef]
- Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://www.europlusmed.org (accessed on 22 November 2022).
- Dubyna, D.V.; Dziuba, T.P.; Iemelianova, S.M.; Bagrikova, N.O.; Borysova, O.V.; Borsukevych, L.M.; Vynokurov, D.S.; Gapon, S.V.; Gapon, Y.V.; Davydov, D.A.; et al. Prodrome of the Vegetation of Ukraine; Naukova Dumka: Kyiv, Ukraine, 2019. [Google Scholar]
- Dubyna, D.; Iemelianova, S.; Dziuba, T.; Ustymenko, P.; Felbaba-Klushyna, L.; Davydova, A.; Davydov, D.; Tymoshenko, P.; Baranovski, B.; Borsukevych, L.; et al. Ruderal vegetation of Ukraine: Syntaxonomic diversity and territorial differentiation. Chornomors’k. Bot. Z. 2021, 17, 253–275. [Google Scholar] [CrossRef]
- Dubyna, D.V.; Dziuba, T.P.; Iemelianova, S.M.; Makhynia, L.M. Syntaxonomy and ecological differentiation of the pioneer vegetation of Ukraine. 1. Classes: Cakiletea maritimae, Ammophiletea, Crithmo-Staticetea, Crypsietea aculeatae, Therosalicornietea. Biosyst. Divers. 2020, 28, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Dubyna, D.V.; Dziuba, T.P.; Iemelianova, S.M.; Tymoshenko, P.A. Syntaxonomy and ecological differentiation of the pioneer vegetation of Ukraine. 2. Helichryso-Crucianelletea maritimae, Festucetea vaginatae, Koelerio-Corynephoretea canescentis classes. Biosyst. Divers. 2020, 28, 298–319. [Google Scholar] [CrossRef]
- Dubyna, D.V.; Dziuba, T.P.; Iemelianova, S.M.; Felbaba-Klushyna, L.M. Syntaxonomy and ecological differentiation of the pioneer vegetation of Ukraine. Classes: Isoëto-Nanojuncetea, Bidentetea. Environ. Soc.-Econ. Stud. 2021, 9, 32–52. [Google Scholar] [CrossRef]
- Dubyna, D.V.; Dvoretskyi, T.V.; Iemelyanova, S.M.; Dzyuba, T.P.; Tymoshenko, P.A. Taxonomic structure of coenofloras of the classes of pioneer vegetation of Ukraine. Ukr. Bot. J. 2017, 74, 421–430. [Google Scholar] [CrossRef]
- Protopopova, V.V.; Mosyakin, S.L.; Shevera, M.V. Plant Invasions in Ukraine as a Threat to Biodiversity: The Present Situation and Tasks for the Future; M.G. Kholodny Institute of Botany, NAS of Ukraine: Kyiv, Ukraine, 2002. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.-P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; Gavilán García, R.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar]
- Tickner, D.P.; Angold, P.G.; Gurnell, A.M.; Mountford, J.O. Riparian plant invasions: Hydrogeomorphological control and ecological impacts. Prog. Phys. Geogr. 2001, 25, 22–52. [Google Scholar] [CrossRef]
- Medvecká, J.; Jarolímek, I.; Zaliberová, M. Dynamics and distribution of neophytes in ruderal vegetation of the Horná Orava Region (Northern Slovakia). Hacquetia 2010, 8, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Verheyen, K.; Baeten, L.; De Frenne, P.; Bernhardt-Römermann, M.; Brunet, J.; Cornelis, J.; Decocq, G.; Dierschke, H.; Eriksson, O.; Hédl, R.; et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J. Ecol. 2012, 100, 352–365. [Google Scholar] [CrossRef]
- Förster, A.; Becker, T.; Gerlach, A.; Meesenburg, H.; Leuschner, C. Long-term change in understorey plant communities of conventionally managed temperate deciduous forests: Effects of nitrogen deposition and forest management. J. Veg. Sci. 2017, 28, 747–761. [Google Scholar] [CrossRef]
- Vild, O.; Šipoš, J.; Szabó, P.; Macek, M.; Chudomelová, M.; Kopecký, M.; Suchánková, S.; Houška, J.; Kotačka, M.; Hédl, R. Legacy of historical litter raking in temperate forest plant communities. J. Veg. Sci. 2018, 29, 596–606. [Google Scholar] [CrossRef]
- Adolf, C.; Tovar, C.; Kühn, N.; Behling, H.; Berrío, J.C.; Dominguez-Vázquez, G.; Figueroa-Rangel, B.; Gonzalez-Carranza, Z.; Islebe, G.A.; Hooghiemstra, H.; et al. Identifying drivers of forest resilience in long-term records from the Neotropics. Biol. Lett. 2020, 16, 20200005. [Google Scholar] [CrossRef] [PubMed]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. The effects of invasive species on the decline in species richness: A global meta-analysis. Networks of Invasion: A Synthesis of Concepts. Adv. Ecol. Res. 2017, 56, 61–83. [Google Scholar] [CrossRef]
- Pyšek, P.; Prach, K.; Šmilauer, P.; Rejmánek, M.; Wade, M. Relating Invasion Success to Plant Traits: An Analysis of the Czech Alien Plora. Plant Invasions: Gerneral Aspects and Special Problems; Pyšek, P., Prach, K., Rejmanek, M., Wade, M., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1995; pp. 39–60. [Google Scholar]
- Pfadenhauer, J.S.; Klötzli, F.A. Die Vegetation der Erde. Grundlagen, Ökologie, Verbreitung; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Axmanová, I.; Kalusová, V.; Danihelka, J.; Dengler, J.; Pergl, J.; Pyšek, P.; Večeřa, M.; Attorre, F.; Biurrun, I.; Boch, S.; et al. Neophyte invasions in European grasslands. J. Veget. Sci. 2021, 32, e12994. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
- Levin, D.A. Ecological Speciation: Lessons from Invasive Species. Syst. Bot. 2003, 28, 643–650. Available online: https://www.jstor.org/stable/25063912 (accessed on 22 November 2022).
- Simonová, D.; Lososová, Z. Which factors determine plant invasions in man-made habitats in the Czech Republic? Perspect. Plants Ecol. 2008, 10, 89–100. [Google Scholar] [CrossRef]
- Vedder, D.; Leidinger, L.; Cabral, J.S. Propagule pressure and an invasion syndrome determine invasion success in a plant community model. Ecol. Evol. 2021, 11, 17106–17116. [Google Scholar] [CrossRef]
- Dalle Fratte, M.; Bolpagni, R.; Brusa, G.; Caccianiga, M.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E.L. Alien plant species invade by occupying similar functional spaces to native species. Flora 2019, 257, 151419. [Google Scholar] [CrossRef]
- Bartelheimer, M.; Poschlod, P. Functional characterizations of Ellenberg indicatorvalues—A review on ecophysiological determinants. Funct. Ecol. 2016, 30, 506–516. [Google Scholar] [CrossRef]
- Berg, C.; Welk, E.; Jäger, E.J. Revising Ellenberg’s indicator values for continentality based on global vascular plant species distribution. App. Veg. Sci. 2017, 20, 482–493. [Google Scholar] [CrossRef]
- Chytrý, M.; Tichý, L.; Dřevojan, P.; Sádlo, J.; Zelený, D. Ellenberg-type indicator values for the Czech flora. Preslia 2018, 90, 83–103. [Google Scholar] [CrossRef] [Green Version]
- Rion, V.; Gallandat, J.D.; Gobat, J.M.; Vittoz, P. Recent changes in the plant composition of wetlands in the Jura Mountains. Appl. Veg. Sci. 2018, 21, 121–131. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Brooker, R.W.; O’Brien, D.; Genney, D. Using species records and ecological attributes of bryophytes to develop an ecosystem health indicator. Ecol. Ind. 2019, 104, 127–136. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Carlón, L.; Fernández-Pascual, E.; Acedo, C.; Alfaro-Saiz, E.; Alonso Redondo, R.; Cires, E.; del Egido Mazuelas, F.; del Río, S.; Díaz-González, T.E.; et al. Checklist of the vascular plants of the Cantabrian Mountains. Mediterr. Bot. 2021, 42, e74570. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; van Kleunen, M.; Winter, M.; et al. Global rise in emerging alien species results from increased accessibility of new source pools. Proc. Natl. Acad. Sci. USA 2018, 115, E2264–E2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protopopova, V.V.; Shevera, M.V. Participation of alien species in urban floras in different botanical and geographical zones of Ukraine: A preliminart assessment. Biodiv. Res. Conserv. 2008, 11–12, 9–16. [Google Scholar]
- Protopopova, V.V.; Shevera, M.V.; Anischenko, I.M.; Teren’teva, N.G. Analysis of the species composition of kenophytes in urban floras of different phytogeographical zones of Ukraine using mathematical statistics methods. Ukr. Bot. J. 2010, 67, 536–546. [Google Scholar]
- Scherrer, D.; Bürgi, M.; Gessler, A.; Kessler, M.; Nobis, M.P.; Wohlgemuth, T. Abundance changes of neophytes and native species indicate a thermophilisation and eutrophisation of the Swiss flora during the 20th century. Ecol. Indic. 2022, 135, 108558. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Alessandrini, A.; Arrigoni, P.V.; Assini, S.; Banfi, E.; Barni, E.; Bovio, M.; Brundu, G.; Cagiotti, M.R.; Camarda, I.; et al. Non-native flora of Italy: Species distribution and threats. Plant Biosyst. 2010, 144, 12–28. [Google Scholar] [CrossRef]
- Chytrý, M.; Maskell, L.C.; Pino, J.; Pyšek, P.; Vilà, M.; Font, X.; Smart, S.M. Habitat invasions by alien plants: A quantitative comparison among Mediterranean, subcontinental and oceanic regions of Europe. J. Appl. Ecol. 2008, 45, 448–458. [Google Scholar] [CrossRef]
- Šilc, U.; Vrbničanin, S.; Božić, D.; Čarni, A.; Dajić Stevanović, Z. Alien plant species and factors of invasiveness of anthropogenic vegetation in the Northwestern Balkans—A phytosociological approach. Cent. Eur. J. Biol. 2012, 7, 720–730. [Google Scholar] [CrossRef] [Green Version]
- Chytrý, M.; Pyšek, P.; Tichý, L.; Knollová, I.; Danihelka, J. Invasions by alien plants in the Czech Republic: A quantitative assessment across habitats. Preslia 2005, 77, 339–354. [Google Scholar]
- Pretto, F.; Celesti-Grapow, L.; Carli, E.; Brundu, G.; Blasi, C. Determinants of non-native plant species richness and composition across small Mediterranean islands. Biol. Invasions 2012, 14, 2559–2572. [Google Scholar] [CrossRef]
- Fristoe, T.S.; Chytrý, M.; Dawson, W.; Essl, F.; Heleno, R.; Kreft, H.; Maurel, N.; Pergl, J.; Pyšek, P.; Seebens, H.; et al. Dimensions of invasiveness: Links between local abundance, geographic range size, and habitat breadth in Europe’s alien and native floras. Proc. Natl. Acad. Sci. USA 2021, 118, e2021173118. [Google Scholar] [CrossRef] [PubMed]
- Protopopova, V.V.; Shevera, M.V. Invasive species in the flora of Ukraine. I. The group of highly active species. GEO&BIO 2019, 17, 116–135. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Vilà, M.; Weber, E.; Antonio, C.M. Conservation Implications of Invasion by Plant Hybridization. Biol. Invasions 2000, 2, 207–217. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.-S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef] [Green Version]
- Pejchar, L.; Liba, P.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef]
- Murphy, G.E.P.; Romanuk, T.N. A meta-analysis of declines in local species richness from human disturbances. Ecol. Evol. 2014, 4, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Chabrerie, O.; Massol, F.; Facon, B.; Thevenoux, R.; Hess, M.; Ulmer, R.; Pantel, J.H.; Braschi, J.; Amsellem, L.; Baltora-Rosset, S.; et al. Biological Invasion Theories: Merging Perspectives from Population, Community and Ecosystem Scales. Preprints 2019, 2019100327. Available online: https://hal-amu.archives-ouvertes.fr/hal-02345539 (accessed on 22 November 2022).
- Capinha, C.; Essl, F.; Seebens, H.; Moser, D.; Pereira, H.M. The dispersal of alien species redefines biogeography in the Anthropocene. Science 2015, 348, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Lowry, E.; Rollinson, E.J.; Laybourn, A.J.; Scott, T.; Aiello-Lammens, M.; Gray, S.M.; Mickley, J.; Gurevitch, J. Biological invasions: A field synopsis, systematic review and database of the literature. Ecol. Evol. 2013, 3, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Martínez, S.; Fernández-González, F.; Loidi, J.; Lousã, M.; Penas, A. Syntaxonomical checklist of vascular plant communities of Spain and Portugal to association level. Itinera Geobot. 2001, 14, 5–341. [Google Scholar]
- Bardat, J.; Bioret, F.; Botineau, M.; Boullet, V.; Delpech, R.; Genu, J.-M.; Haury, J.; Lacoste, A.; Rameau, J.-C.; Royer, J.-M.; et al. The Prodrome of French Vegetation; Muséum National d’Histoire Naturelle: Paris, France, 2004. [Google Scholar]
- Tzonev, R.T.; Dimitrov, M.A.; Roussakova, V.H. Syntaxa according to the Braun-Blanquet approach in Bulgaria. Phytol. Balc. 2009, 15, 209–233. [Google Scholar]
- Chytry, M. (Ed.) Vegetation of the Czech Republic. 3. Aquatic and Wetland Vegetation; Academia: Prague, Czech Republic, 2011. [Google Scholar]
- Biondi, E.; Blasi, C.; Allegrezza, M.; Anzellotti, I.; Azzella, M.M.; Carli, E.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Facioni, L.; et al. Plant communities of Italy: The Vegetation Prodrome. Plant Biosyst. 2014, 148, 728–814. [Google Scholar] [CrossRef] [Green Version]
- Stępień, E.; Rosadziński, S. Communities of the Bidentetea class of small coastal river valleys of the Western Pomerania (Poland). Ecol. Montenegrina 2020, 28, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Abramova, L.M.; Golovanov, Y.M. Review of higher units of synanthropic vegetation in the European part of Russia. Work. State Nikitsk. Bot. Gard. 2016, 143, 7–15. [Google Scholar]
- Borhidi, A. Plant Associations in Hungary; Akadémiai Kiadó: Budapest, Hungary, 2003. [Google Scholar]
- Šilc, U. Synanthropic Vegetation: Pattern of Various Disturbances on Life History Traits. Acta Bot. Croat. 2010, 69, 215–227. Available online: https://hrcak.srce.hr/59732 (accessed on 22 November 2022).
- Felzines, J.-C.; Loiseau, J.-E. Les groupements fluviatiles des Bidentetea de la Loire moyenne, du bas Allier et de la Dordogne moyenne. Modifications apportées à la synsystématique de la classe des Bidentetea. Bull. Société Bot. Cent. Ouest 2005, 36, 159–204. [Google Scholar]
- Marignani, M.; Lussu, M.; Murru, V.; Bacaro, G.; Cogoni, A. Effect of Invasive Alien Species on the Co-Occurrence Patterns of Bryophytes and Vascular Plant Species—The Case of a Mediterranean Disturbed Sandy Coast. Diversity 2020, 12, 160. [Google Scholar] [CrossRef] [Green Version]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Tordoni, E.; Bacaro, G.; Weigelt, P.; Cameletti, M.; Janssen, J.A.M.; Acosta, A.T.R.; Bagella, S.; Filigheddu, R.; Bergmeier, E.; Buckley, H.L.; et al. Disentangling native and alien plant diversity in coastal sand dune ecosystems worldwide. J. Veget. Sci. 2021, 32, e12861. [Google Scholar] [CrossRef]
- Gallien, L.; Mazel, F.; Lavergne, S.; Renaud, J.; Douzet, R.; Thuiller, W. Contrasting the effects of environment, dispersal and biotic interactions to explain the distribution of invasive plants in alpine communities. Biol. Invasions 2015, 17, 1407–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Dostál, P.; Dawson, W.; van Kleunen, M.; Keser, L.H.; Fischer, M. Central European plant species from more productive habitats are more invasive at a global scale. Glob. Ecol. Biogeogr. 2013, 22, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Carboni, M.; Münkemüller, T.; Lavergne, S.; Choler, P.; Borgy, B.; Violle, C.; Essl, F.; Roquet, C.; Munoz, F.; Thuiller, W. What it takes to invade grassland ecosystems: Traits, introduction history and filtering processes. Ecol. Lett. 2016, 19, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Henn, J.J.; Yelenik, S.G.; Damschen, E.I. Environmental gradients influence differences in leaf functional traits between native and non-native plants. Oecologia 2019, 191, 397–409. [Google Scholar] [CrossRef]
- Tordoni, E.; Petruzzellis, F.; Nardini, A.; Bacaro, G. Functional divergence drives invasibility of plant communities at the edges of a resource availability gradient. Diversity 2020, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Kalusová, V.; Cubino, J.P.; Fristoe, T.S.; Chytrý, M.; van Kleunen, M.; Dawson, W.; Essl, F.; Kreft, H.; Mucina, L.; Pergl, J.; et al. Phylogenetic structure of alien plant species pools from European donor habitats. Glob. Ecol. Biogeogr. 2021, 30, 2354–2367. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Yu, F.; Barreiro, R. EDITORIAL: Plant invasions: Mechanisms, impacts and management. Flora 2020, 267, 151603. [Google Scholar] [CrossRef]
- Ellis, E.C.; Antill, E.C.; Kreft, H. All is not loss: Plant biodiversity in the anthropocene. PLoS ONE 2012, 7, e30535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rentch, J.S.; Fortney, R.H.; Stephenson, S.L.; Adams, H.S.; Grafton, W.N.; Anderson, J.T. Vegetation-site relationships of roadside plant communities in West Virginia, USA. J. Appl. Ecol. 2005, 42, 129–138. [Google Scholar] [CrossRef]

















| Pioneer Vegetation | Ruderal Vegetation | ||||||
|---|---|---|---|---|---|---|---|
| Rank | Families | Number | Rank | Families | Number | ||
| Species | % | Species | % | ||||
| 1 | Asteraceae | 26 | 23.01 | 1 | Asteraceae | 65 | 20.00 |
| 2 | Brassicaceae | 22 | 19.47 | 2 | Brassicaceae | 38 | 11.69 |
| 3 | Poaceae | 15 | 13.27 | 3 | Poaceae | 29 | 8.92 |
| 4 | Chenopodiaceae | 6 | 5.31 | 4 | Fabaceae | 16 | 4.92 |
| 5 | Fabaceae | 5 | 4.42 | 5 | Chenopodiaceae | 15 | 4.62 |
| 6 | Lamiaceae | 4 | 3.54 | 6 | Apiaceae | 13 | 4.00 |
| 7 | Caryophillaceae | 3 | 2.65 | 7 | Lamiaceae | 10 | 3.08 |
| Cucurbitaceae | 3 | 2.65 | Boraginaceae | 10 | 3.08 | ||
| 8 | Malvaceae | 2 | 1.77 | 8 | Polygonaceae | 9 | 2.77 |
| Onagraceae | 2 | 1.77 | 9 | Amaranthaceae | 8 | 2.46 | |
| Ranunculaceae | 2 | 1.77 | Scrophulariaceae | 8 | 2.46 | ||
| Verbenaceae | 2 | 1.77 | 10 | Malvaceae | 7 | 2.15 | |
| Pioneer Vegetation | Ruderal Vegetation | ||||
|---|---|---|---|---|---|
| Rank | Generas | Number of Species | Rank | Generas | Number of Species |
| 1 | Sisymbrium | 5 | 1 | Xanthium | 9 |
| 2 | Lepidium | 4 | 2 | Amaranthus | 8 |
| 3 | Anisantha | 3 | 3 | Chenopodium | 6 |
| Atriplex | 3 | Geranium | 6 | ||
| 4 | Artemisia | 2 | Sisymbrium | 6 | |
| Bidens | 2 | Veronica | 6 | ||
| Bromus | 2 | 4 | Atriplex | 5 | |
| Camelina | 2 | Vicia | 5 | ||
| Centaurea | 2 | 5 | Anthemis | 4 | |
| Digitaria | 2 | Bromus | 4 | ||
| Eragrostis | 2 | Centaurea | 4 | ||
| Mentha | 2 | Lepidium | 4 | ||
| Oenothera | 2 | Malva | 4 | ||
| Papaver | 2 | 6 | Anchusa | 3 | |
| Senecio | 2 | Carduus | 3 | ||
| Setaria | 2 | Cuscuta | 3 | ||
| Sonchus | 2 | Euphorbia | 3 | ||
| Thlaspi | 2 | Fumaria | 3 | ||
| Verbena | 2 | Helianthus | 3 | ||
| Vicia | 2 | Hordeum | 3 | ||
| Xanthium | 2 | Lamium | 3 | ||
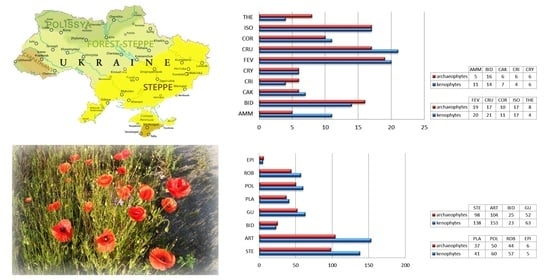
| IArch | IKen | IM | IJ | ||
|---|---|---|---|---|---|
| (a) | AMM | 4.0 | 9.0 | 68.0 | 3.0 |
| CAK | 10.0 | 11.7 | 53.8 | – | |
| CRI | 8.6 | 5.7 | 40.0 | – | |
| CRY | 7.5 | 7.5 | 50.0 | 8.3 | |
| THE | 8.4 | 4.2 | 33.3 | – | |
| BID | 17.0 | 15.3 | 46.6 | 4.3 | |
| ISO | 8.8 | 8.8 | 50.0 | 2.1 | |
| CRU | 6.0 | 7.5 | 55.3 | 5.2 | |
| FEV | 5.9 | 6.2 | 51.3 | 1.2 | |
| COR | 4.5 | 4.9 | 52.4 | 0.4 | |
| All | 6.6 | 6.8 | 50.4 | 1.5 | |
| (b) | STE | 13.1 | 18.3 | 58.3 | 5.0 |
| ART | 9.4 | 13.6 | 59.2 | 3.9 | |
| BID | 11.4 | 10.0 | 46.9 | 2.2 | |
| GU | 9.2 | 10.7 | 53.8 | 2.2 | |
| PLA | 12.6 | 14.6 | 53.8 | 2.4 | |
| POL | 16.6 | 20.3 | 55.0 | 3.4 | |
| ROB | 9.1 | 12.3 | 57.4 | 3.4 | |
| EPI | 3.2 | 2.6 | 45.5 | 1.1 | |
| All | 7.3 | 12.6 | 63.4 | 3.8 |
| Species | Syntaxa |
|---|---|
| Acer negundo (ken.) | Chelidonio-Acerion negundi |
| Acer platanoides (ken.) | Geo-Acerion platanoidis |
| Anisantha tectorum (arch.) | Festucion beckeri |
| Apera spica-venti (arch.) | Scleranthion annui |
| Artemisia absinthium (arch.) | Onopordion acanthii |
| Atriplex prostrata (arch.) | Crypsietea aculeatae, Crypsietalia aculeatae, Cypero-Spergularion salinae, Heleochloion schoenoidis, Crithmo-Staticetea |
| Bassia laniflora (ken.) | Koelerion glaucae |
| Ballota nigra (arch.) | Artemisietea vulgaris, Onopordetalia acanthii, Arction lappae |
| Bidens connata (ken.) | Bidentetea |
| Bidens frondosa (ken.) | |
| Capsella bursa-pastoris (arch.) | Stellarietea mediae |
| Carduus acanthoides (arch.) | Artemisietea vulgaris, Onopordetalia acanthii, Arction lappae |
| Carduus nutans (arch.) | Onopordion acanthii |
| Centaurea diffusa (ken.) | Koelerio-Corynephoretea |
| Conium maculatum (arch.) | Arction lappae |
| Consolida regalis (arch.) | Aperetalia spicae-venti |
| Cyanus segetum (arch.) | Scleranthion annui |
| Descurainia sophia (arch.) | Stellarietea mediae, Sisymbrietalia sophiae, Chenopodio albi-Descurainion sophiae |
| Diplotaxis tenuifolia (ken.) | Medicagini falcatae-Diplotaxion tenuifoliae |
| Erigeron annuus (ken.) | Robinietea, Chelidonio-Robinietalia pseudoacaciae, Chelidonio majoris-Robinion pseudoacaciae |
| Erigeron canadensis (ken.) | Stellarietea mediae |
| Fallopia convolvulus (arch.) | Stellarietea mediae |
| Fumaria officinalis (arch.) | |
| Fumaria schleicheri (arch.) | Chenopodio albi-Descurainion sophiae |
| Heracleum mantegazzianum (ken.) | Galio-Urticetea |
| Impatiens glandulifera (ken.) | |
| Impatiens parviflora (ken.) | |
| Iva xanthiifolia (ken.) | Sisymbrietalia sophiae |
| Lamium album (arch.) | Artemisietea vulgaris |
| Lappula squarrosa (arch.) | Onopordion acanthii |
| Leonurus cardiaca (arch.) | Arction lappae |
| Lepidium campestre (arch.) | Scleranthion annui |
| Lepidium draba (ken.) | Agropyretalia intermedio-repentis, Convolvulo arvensis-Agropyrion repentis |
| Lepidium ruderale (arch.) | Stellarietea mediae, Polygono-Poetea annuae, Polygono-Coronopodion |
| Matricaria chamomilla (arch.) | Anthemido ruthenicae-Sisymbrion orientalis |
| Matricaria discoidea (ken.) | Polygono-Poetea annuae, Polygono-Coronopodion |
| Myosotis arvensis (arch.) | Aperetalia spicae-venti |
| Nigella arvensis (arch.) | Caucalidion |
| Oenothera biennis (ken.) | Koelerio-Corynephoretea |
| Oenothera rubricaulis (ken.) | Koelerion glaucae |
| Onopordum acanthium (arch.) | Onopordetalia acanthi, Onopordion acanthii |
| Oxalis stricta (ken.) | Oxalidion europaeae |
| Papaver rhoeas (arch.) | Papaveretalia rhoeadis, Galeopsion bifidae |
| Portulaca oleracea (arch.) | Eragrostietalia, Eragrostion, Saginion procumbentis |
| Raphanus raphanistrum (arch.) | Panico-Setarion |
| Rapistrum rugosum (ken.) | Sisymbrietalia sophiae |
| Reseda lutea (arch.) | Onopordion acanthii |
| Reynoutria japonica (ken.) | Galio-Urticetea |
| Reynoutria sachalinensis (ken.) | |
| Robinia pseudoacacia (ken.) | Robinietea, Chelidonio-Robinietalia pseudoacaciae, Chelidonio majoris-Robinion pseudoacaciae, Balloto nigrae-Robinion pseudoacaciae, Galio-Urticetea |
| Rubus idaeus (ken.) | Sambucetalia racemosae |
| Rudbeckia laciniata (ken.) | Galio-Urticetea |
| Scleranthus annuus (arch.) | Koelerio-Corynephoretea |
| Sclerochloa dura (arch.) | Polygono-Poetea annuae, Polygono arenastri-Poetalia annuae |
| Senecio viscosus (ken.) | Epilobion augustifolii |
| Senecio vulgaris (arch.) | Veronico-Euphorbion |
| Setaria pumila (arch.) | Atriplici-Chenopodietalia albi, Panico-Setarion |
| Setaria viridis (arch.) | |
| Sinapis arvensis (arch.) | Stellarietea mediae, Papaveretalia rhoeadis, Panico-Setarion |
| Sisymbrium altissimum (ken.) | Sisymbrion officinalis |
| Sisymbrium officinale (arch.) | |
| Sisymbrium orientale (ken.) | Anthemido ruthenicae-Sisymbrion orientalis, Sisymbrion officinalis |
| Sisymbrium loeselii (ken.) | Sisymbrion officinalis |
| Solidago canadensis (ken.) | Galio-Urticetea |
| Sonchus arvensis (arch.) | Stellarietea mediae, Papaveretalia rhoeadis |
| Sonchus asper (arch.) | Stellarietea mediae |
| Sonchus oleraceus (arch.) | |
| Stachys annua (arch.) | Panico-Setarion |
| Thlaspi arvense (arch.) | Stellarietea mediae, Atriplici-Chenopodietalia albi |
| Tribulus terrestris (ken.) | Eragrostietalia, Saginion procumbentis |
| Tripleurospermum inodorum (arch.) | Stellarietea mediae, Cakiletea maritimae, Aperetalia spicae-venti, Verbenion supinae, Polygono-Chenopodion, Sisymbrion officinalis |
| Urtica urens (arch.) | Malvion neglectae |
| Verbena supina (ken.) | Isoëto-Nanojuncetea |
| Veronica arvensis (arch.) | Koelerio-Corynephoretea |
| Veronica persica (ken.) | Veronico-Euphorbion |
| Vicia pannonica (arch.) | Caucalidion |
| Vicia villosa (arch.) | Anthemido ruthenicae-Sisymbrion orientalis |
| Viola arvensis (arch.) | Aperetalia spicae-venti |
| Xanthium orientale subsp. riparium (ken.) | Bidentetea, Bidentetalia, Chenopodion rubri |
| Xanthium pungens (ken.) | Sisymbrion officinalis |
| Xanthium strumarium (arch.) | Bidentetea, Bidentetalia, Chenopodion rubri, Thero-Atriplicetalia, Sisymbrietalia sophiae, Sisymbrion officinalis, Amarantho blitoidis-Echinochloion cruris-galli |
| Syntaxon of Classes | Diagnostic | Constant | Dominant |
|---|---|---|---|
| Stellarietea mediae | Adonis aestivalis, Amaranthus albus, A. blitoides, A. retroflexus, Ambrosia artemisiifolia, Anagallis arvensis, Anisantha sterilis, A. tectorum, Apera spica-venti, Artemisia annua, Atriplex prostrata, A. sagittata, A. tatarica, Bassia scoparia, Bromus squarrosus, Camelina microcarpa, Capsella bursa-pastoris, Lepidium draba, Carduus acanthoides, Centaurea diffusa, Chenopodiastrum hybridum, Conium maculatum, Consolida regalis, Crepis micrantha, Dasypyrum villosum, Descurainia sophia, Digitaria sanguinalis, D. ischaemum, Diplotaxis muralis, Echinochloa crus-galli, Eragrostis minor, Erigeron canadensis, Erysimum repandum, Euphorbia helioscopia, E. peplus, Fallopia convolvulus, Galinsoga parviflora, Hibiscus trionum, Hordeum murinum, H. murinum subsp. leporinum, Hyoscyamus niger, Iva xanthiifolia, Lactuca serriola, Lamium amplexicaule, L. purpureum, Lappula squarrosa, Lepidium campestre, L. perfoliatum, Malva neglecta, Matricaria chamomilla, Myosotis arvensis, Neslia paniculata, Oenothera biennis, Oxalis dillenii, O. stricta, Oxybasis rubra, Papaver rhoeas, P. dubium, Peganum harmala, Portulaca oleracea, Raphanus raphanistrum, Rapistrum rugosum, Reseda lutea, R. luteola, Scleranthus annuus, Senecio vulgaris, Setaria pumila, S. viridis, Sinapis arvensis, Sisymbrium altissimum, S. loeselii, S. officinale, S. orientale, Solanum nigrum, Sonchus arvensis, S. asper, S. oleraceus, Spergula arvensis, Stachys annua, Thlaspi arvense, Noccaea perfoliata, Tribulus terrestris, Tripleurospermum inodorum, Urtica urens, Veronica persica, Vicia sativa subsp. nigra, V. tetrasperma, V. pannonica, Viola arvensis, Xanthium strumarium, X. orientale subsp. riparium, X. spinosum | Amaranthus retroflexus, Ambrosia artemisiifolia, Anisantha sterilis, A. tectorum, Apera spica-venti, Artemisia absinthium, Atriplex prostrata, Ballota nigra, Capsella bursa-pastoris, Lepidium draba, Carduus acanthoides, Centaurea diffusa, Cichorium intybus, Consolida regalis, Cyanus segetum, Descurainia sophia, Diplotaxis muralis, Echinochloa crus-galli, Eragrostis minor, Erigeron canadensis, Erysimum repandum, Fallopia convolvulus, Hordeum murinum, Iva xanthiifolia, Lactuca serriola, Lepidium perfoliatum, L. ruderale, Oenothera biennis, Onopordum tauricum, Papaver dubium, P. rhoeas, Portulaca oleracea, Setaria pumila, S. viridis, Sisymbrium loeselii, S. officinale, Sonchus arvensis, S. oleraceus | Amaranthus albus, A. retroflexus, Ambrosia artemisiifolia, Anisantha sterilis, A. tectorum, Artemisia annua, Atriplex prostrata, A. tatarica, Bromus squarrosus, Erigeron canadensis, Descurainia sophia, Digitaria sanguinalis, D. ischaemum, Echinochloa crus-galli, Eragrostis minor, Fallopia convolvulus, Galinsoga parviflora, Geranium pusillum, Hordeum murinum, Iva xanthiifolia, Bassia scoparia, Lamium purpureum, Lepidium draba, L. perfoliatum, L. ruderale, Malva neglecta, M. pusilla, Peganum harmala, Portulaca oleracea, Reseda lutea, Scleranthus annuus, Setaria viridis, S. pumila, Sisymbrium altissimum, S. officinale, S. loeselii, Sonchus oleraceus, Tribulus terrestris |
| Artemisietea vulgaris | Anchusa officinalis, Anisantha tectorum, Artemisia absinthium, Asclepias syriaca, Atriplex prostrata, A. sagittata, A. tatarica, Ballota nigra, Bupleurum rotundifolium, Carduus acanthoides, C. nutans, Centaurea diffusa, Conium maculatum, Erigeron canadensis, Crepis micrantha, Diplotaxis tenuifolia, D. muralis, Ecballium elaterium, Galium spurium, Grindelia squarrosa, Lamium album, L. purpureum, Lathyrus tuberosus, Leonurus cardiaca, Lepidium draba, L. graminifolium, Lycium barbarum, Malva pusilla, M. sylvestris, Onopordum acanthium, Papaver rhoeas, Prunus cerasifera, Ranunculus arvensis, Reseda lutea, Sinapis arvensis, Solanum nigrum, Sonchus oleraceus, Verbena officinalis, Veronica arvensis, V. triphyllos, Vulpia ciliata, Xanthium spinosum, X. strumarium | Amaranthus retroflexus, Ambrosia artemisiifolia, Anisantha tectorum, Apera spica-venti, Artemisia absinthium, Atriplex prostrata, Ballota nigra, Bromus squarrosus, Capsella bursa-pastoris, Lepidium draba, Carduus acanthoides, Centaurea diffusa, Cichorium intybus, Conium maculatum, Consolida regalis, Erigeron canadensis, Crepis micrantha, Descurainia sophia, Diplotaxis tenuifolia, Erigeron annuus, Grindelia squarrosa, Lactuca serriola, Lamium purpureum, Leonurus cardiaca, Onopordum acanthium, Sisymbrium loeselii | Ambrosia artemisiifolia, Anisantha tectorum, Artemisia absinthium, Asclepias syriaca, Atriplex sagittata, Ballota nigra, Bromus squarrosus, Lepidium draba, Carduus acanthoides, Conium maculatum, Erigeron canadensis, Grindelia squarrosa, Lathyrus tuberosus, Leonurus cardiaca, Lepidium perfoliatum, Lycium barbarum, Onopordum acanthium, Rhamnus alaternus, Xanthium orientale subsp. riparium, X. strumarium, X. spinosum |
| Galio-Urticetea | Asclepias syriaca, Calepina irregularis, Chrozophora tinctoria, Echinocystis lobata, Erysimum cheiranthoides, Geranium rotundifolium, Helianthus tuberosus, Heracleum mantegazzianum, H. pubescens, Impatiens glandulifera, I. parviflora, Lamium album, Lepidium graminifolium, Medicago arabica, Ranunculus arvensis, Reynoutria japonica, R. sachalinensis, Robinia pseudoacacia, Rudbeckia laciniata, Solidago canadensis, S. gigantea, Tripleurospermum inodorum, Verbena officinalis | Ballota nigra, Erigeron annuus | Bryonia alba, Helianthus tuberosus, Heracleum mantegazzianum, H. pubescens, Impatiens glandulifera, I. parviflora, Solidago canadensis |
| Bidentetea (ruderal) | Atriplex prostrata, Bidens connata, B. frondosa, Echinochloa crus-galli, Xanthium orientale subsp. riparium, X. strumarium | Bidens connata, B. frondosa | Bidens connata, B. frondosa |
| Robinietea | Acer negundo, A. platanoides, Ailanthus altissima, Amorpha fruticosa, Ballota nigra, Caragana arborescens, Celtis occidentalis, Cotinus coggygria, Erigeron annuus, E. canadensis, Fraxinus pennsylvanica, Galinsoga parviflora, Gleditsia triacanthos, Impatiens parviflora, Lactuca serriola, Parthenocissus quinquefolia, Quercus rubra, Robinia pseudoacacia, Rubus idaeus, Solanum nigrum | Acer negundo, A. platanoides, Ballota nigra, Robinia pseudoacacia | Acer negundo, A. platanoides, Ailanthus altissima, Anisantha sterilis, A. tectorum, Ballota nigra, Celtis occidentalis, Cotinus coggygria, Gleditsia triacanthos, Impatiens parviflora, Parthenocissus quinquefolia, Robinia pseudoacacia |
| Epilobietea angustifolii | Erigeron canadensis, Oenothera biennis, Rubus idaeus, Senecio viscosus | Rubus idaeus | |
| Polygono-Poetea annuae | Anagallis arvensis, Erigeron canadensis, Eragrostis minor, Lappula squarrosa, Lepidium ruderale, Portulaca oleracea, Sclerochloa dura, Sisymbrium orientale, Tragus racemosus, Tribulus terrestris | Capsella bursa-pastoris | Eragrostis minor, Sclerochloa dura |
| Plantaginetea majoris | Juncus tenuis, Lepidium ruderale, Mentha pulegium, Ranunculus arvensis, Verbena officinalis | Erigeron canadensis, Juncus tenuis | Juncus tenuis |
| Cakiletea maritimae | Atriplex prostrata, Tripleurospermum inodorum, Xanthium strumarium s. l. | Atriplex prostrata | – |
| Festucetea vaginatae | Anisantha tectorum, Bromus squarrosus, Centaurea diffusa, Tragus racemosus, Tribulus terrestris | Ambrosia artemisiifolia, Anisantha sterilis, A. tectorum, Apera spica-venti, Bromus squarrosus, Camelina rumelica, Centaurea diffusa, Cichorium intybus, Descurainia sophia, Eragrostis minor, Erysimum repandum | Anisantha sterilis, Apera spica-venti |
| Crithmo-Staticetea | Atriplex prostrata | Anisantha tectorum, Atriplex prostrata | – |
| Crypsietea aculeatae | Atriplex prostrata | Atriplex tatarica | – |
| Therosalicornietea | – | Atriplex tatarica, A. prostrata, Bromus squarrosus | – |
| Helichryso-Crucianelletea maritimae | Anisantha tectorum | Anisantha tectorum, Bromus squarrosus, Carduus acanthoides, Bassia laniflora | – |
| Koelerio-Corynephoretea canescentis | Centaurea diffusa, Bassia laniflora, Oenothera biennis, O. rubricaulis, Veronica arvensis | Artemisia absinthium, Erigeron annuus | – |
| Isoëto-Nanojuncetea | Amaranthus retroflexus, Digitaria ischaemum, Mentha pulegium, Tripleurospermum inodorum, Verbena supina | Oxybasis rubra | – |
| Bidentetea (pion.) | Atriplex prostrata, Bidens connata, B.frondosa, Oxybasis glauca, Xanthium strumarium | Bidens connata, B. frondosa | Bidens connata, B. frondosa, Oxybasis glauca |
| Ammophiletea | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubyna, D.V.; Dziuba, T.P.; Iemelianova, S.M.; Protopopova, V.V.; Shevera, M.V. Alien Species in the Pioneer and Ruderal Vegetation of Ukraine. Diversity 2022, 14, 1085. https://doi.org/10.3390/d14121085
Dubyna DV, Dziuba TP, Iemelianova SM, Protopopova VV, Shevera MV. Alien Species in the Pioneer and Ruderal Vegetation of Ukraine. Diversity. 2022; 14(12):1085. https://doi.org/10.3390/d14121085
Chicago/Turabian StyleDubyna, Dmytro V., Tetiana P. Dziuba, Svitlana M. Iemelianova, Vira V. Protopopova, and Myroslav V. Shevera. 2022. "Alien Species in the Pioneer and Ruderal Vegetation of Ukraine" Diversity 14, no. 12: 1085. https://doi.org/10.3390/d14121085