Analyses of the Complete Mitochondrial Genome of Paraconiothyrium sp. and Gene Rearrangement Diversity in the Pleosporales
Abstract
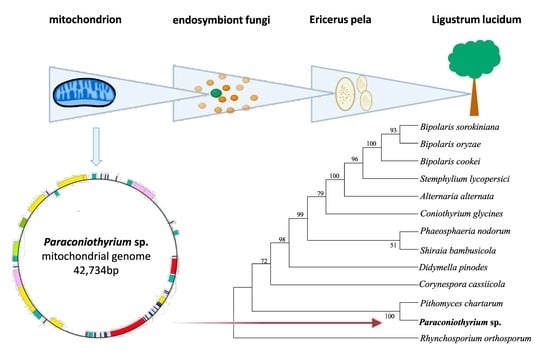
:1. Introduction
2. Methods
2.1. DNA Extraction and Sequencing
2.2. Mitochondrial Genome Assembly
2.3. Gene Annotation
2.4. Gene Component and Structure Analyses
2.5. Phylogenetic Analysis
2.6. Synteny Analysis
2.7. Gene Rearrangement Analysis
3. Results
3.1. General Genomic Features
3.2. Protein-Coding Genes and Codon Usage
3.3. rRNAs and tRNAs
3.4. Phylogenetic Analysis
3.5. Synteny Analysis
3.6. Gene Rearrangement
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Schoch, C.; Fournier, J.; Crous, P.; de Gruyter, J.; Woudenberg, J.; Hirayama, K.; Tanaka, K.; Pointing, S.; Spatafora, J.; et al. Multi-locus phylogeny of Pleosporales: A taxonomic, ecological and evolutionary re-evaluation. Stud. Mycol. 2009, 64, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Câmara, M.P.; Palm, M.E.; van Berkum, P.; O’Neill, N.R. Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia 2002, 94, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shao, S.; Liu, C.; Song, Z.; Liu, S.; Wu, S. The genus Paraconiothyrium: Species concepts, biological functions, and secondary metabolites. Crit. Rev. Microbiol. 2021, 47, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Cloete, M.; Fourie, P.H.; Damm, U.; CROUS, P.W.; MOSTERT, L. Fungi associated with die-back symptoms of apple and pear trees, a possible inoculum source of grapevine trunk disease pathogens. Phytopathol. Mediterr. 2011, 50, S176–S190. [Google Scholar]
- Ligoxigakis, E.K.; Papaioannou, I.A.; Markakis, E.A.; Typas, M.A. First report of leaf spot of Phoenix theophrasti Caused by Paraconiothyrium variabile in Greece. Plant Dis. 2013, 97, 1250. [Google Scholar] [CrossRef]
- Gordon, R.A.; Sutton, D.A.; Thompson, E.H.; Shrikanth, V.; Verkley, G.J.M.; Stielow, J.B.; Mays, R.; Oleske, D.; Morrison, L.K.; Lapolla, W.J.; et al. Cutaneous phaeohyphomycosis caused by Paraconiothyrium cyclothyrioides. J. Clin. Microbiol. 2012, 50, 3795–3798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-X.; Wang, L.; Chen, J.-F.; Guo, Z.-Y.; Tu, X.; Deng, Z.-S.; Zou, K. Paraconfuranones A-H, eight new furanone analogs from the insect-associated fungus Paraconiothyrium brasiliense MZ-1. Magn. Reason. Chem. 2015, 53, 317–322. [Google Scholar] [CrossRef]
- Ren, F.; Chen, S.; Zhang, Y.; Zhu, S.; Xiao, J.; Liu, X.; Su, R.; Che, Y. Hawaiienols A-D, highly oxygenated p-terphenyls from an insect-associated fungus, Paraconiothyrium hawaiiense. J. Nat. Prod. 2018, 81, 1752–1759. [Google Scholar] [CrossRef]
- Fu, Z.Y.; An, J.Q.; Liu, W.; Zhang, H.P.; Yang, P. Genomic Analyses of the Fungus Paraconiothyrium sp. Isolated from the Chinese White Wax Scale Insect Reveals Its Symbiotic Character. Genes 2022, 13, 338. [Google Scholar] [CrossRef]
- Yang, P.; Yu, S.; Hao, J.; Liu, W.; Zhao, Z.; Zhu, Z.; Sun, T.; Wang, X.; Song, Q. Genome sequence of the Chinese white wax scale insect Ericerus pela: The first draft genome for the Coccidae family of scale insects. GigaScience 2019, 8, 8. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, J.-Y.; Gong, Z.-J.; Xu, D.-L.; Chen, X.-M.; Liu, W.-W.; Lin, X.-D.; Li, Y.-F. Transcriptome analysis of the Chinese white wax scale Ericerus pela with focus on genes involved in wax biosynthesis. PLoS ONE 2012, 7, e35719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Wang, X.Q.; Zhao, Z.L.; Yu, S.H.; Yang, P.; Chen, X.M. A Lethal Fungus Infects the Chinese White Wax Scale Insect and Causes Dramatic Changes in the Host Microbiota. Sci. Rep. 2018, 8, 5324. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, X.M. Protein profiles of Chinese white wax scale, Ericerus pela, at the male pupal stage by high-throughput proteomics. Arch. Insect Biochem. Physiol. 2014, 87, 214–233. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-H.; Yang, P.; Sun, T.; Qi, Q.; Wang, X.-Q.; Xu, D.-L.; Chen, X.-M. Identification and evaluation of reference genes in the Chinese white wax scale insect Ericerus pela. Springerplus 2016, 5, 791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.A.; Okusu, A.; Normark, B.B. Molecular phylogenetics of Aspidiotini armored scale insects (Hemiptera: Diaspididae) reveals rampant paraphyly, curious species radiations, and multiple origins of association with Melissotarsus ants (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 2018, 129, 291–303. [Google Scholar] [CrossRef]
- Yang, P.; Chen, X.M.; Liu, W.W.; Feng, Y.; Sun, T. Transcriptome analysis of sexually dimorphic Chinese white wax scale insects reveals key differences in developmental programs and transcription factor expression. Sci. Rep. 2015, 5, 8141. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Crous, P.W.; Schoch, C.L.; Hyde, K.D. Pleosporales. Fungal Divers. 2012, 53, 1–221. [Google Scholar] [CrossRef] [Green Version]
- Kruys, A.; Eriksson, O.E.; Wedin, M. Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycol. Res. 2006, 110, 527–536. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lagreca, S. New bottles for old wine: Fruit body types, phylogeny, and classification. Mycol. Res. 2007, 111, 999–1000. [Google Scholar] [CrossRef]
- Liew, E.C.; Aptroot, A.; Hyde, K.D. Phylogenetic significance of the pseudoparaphyses in Loculoascomycete taxonomy. Mol. Phylogenetics Evol. 2000, 16, 392–402. [Google Scholar] [CrossRef]
- Shen, X.Y.; Li, T.; Chen, S.; Fan, L.; Gao, J.; Hou, C.L. Characterization and phylogenetic analysis of the mitochondrial genome of Shiraia bambusicola reveals special features in the order of pleosporales. PLoS ONE 2015, 10, e0116466. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, S.-H.; Zhang, H.-P.; Fu, Z.-Y.; An, J.-Q.; Zhang, J.-Y.; Yang, P. Two Cladosporium Fungi with Opposite Functions to the Chinese White Wax Scale Insect Have Different Genome Characters. J. Fungi 2022, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaser, R.; Sović, I.; Nagarajan, N.; Šikić, M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome. Res. 2017, 27, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Litter, J.; Keszthelyi, A.; Hamari, Z.; Pfeiffer, I.; Kucsera, J. Differences in mitochondrial genome organization of Cryptococcus neoformans strains. Antonie Van Leeuwenhoek 2005, 88, 249–255. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, F.; Hon, C.C.; Zhang, Y.; Leung, F.C. The mitochondrial genome of the Basidiomycete fungus Pleurotus ostreatus (oyster mushroom). FEMS Microbiol. Lett. 2008, 280, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Formighieri, E.F.; Tiburcio, R.A.; Armas, E.D.; Medrano, F.J.; Shimo, H.; Carels, N.; Góes-Neto, A.; Cotomacci, C.; Carazzolle, M.F.; Sardinha-Pinto, N.; et al. The mitochondrial genome of the phytopathogenic basidiomycete Moniliophthora perniciosa is 109 kb in size and contains a stable integrated plasmid. Mycol. Res. 2008, 112, 1136–1152. [Google Scholar] [CrossRef]
- Yuan, X.-L.; Cao, M.; Shen, G.-M.; Zhang, H.-B.; Du, Y.-M.; Zhang, Z.-F.; Li, Q.; Gao, J.-M.; Xue, L.; Wang, Z.-P.; et al. Characterization of Nuclear and Mitochondrial Genomes of Two Tobacco Endophytic Fungi Leptosphaerulina chartarum and Curvularia trifolii and Their Contributions to Phylogenetic Implications in the Pleosporales. Int. J. Mol. Sci. 2020, 21, 2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.; Kong, W.S.; Sung, G.H. Complete mitochondrial genome of the entomopathogenic fungus Beauveria pseudobassiana (Ascomycota, Cordycipitaceae). Mitochondrial DNA. 2015, 26, 777–778. [Google Scholar] [CrossRef]
- Lu, C.; Huang, X.; Deng, J. The challenge of Coccidae (Hemiptera: Coccoidea) mitochondrial genomes: The case of Saissetia coffeae with novel truncated tRNAs and gene rearrangements. Int. J. Biol. Macromol. 2020, 158, 854–864. [Google Scholar] [CrossRef]
- Mohajeri, A.; Nobandegani, F.F. Detection and evaluation of hydrogen bond strength in nucleic acid base pairs. J. Phys. Chem. A 2008, 112, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.B.; McCorison, C.B.; Cavaletto, J.R.; Culley, D.E.; LaButti, K.; Baker, S.; Grigoriev, I. The mitochondrial genome of the ethanol-metabolizing, wine cellar mold Zasmidium cellare is the smallest for a filamentous ascomycete. Fungal Biol. 2016, 120, 961–974. [Google Scholar] [CrossRef] [Green Version]
- Stone, C.L.; Frederick, R.D.; Tooley, P.W.; Luster, D.G.; Campos, B.; Winegar, R.A.; Melcher, U.; Fletcher, J.; Blagden, T. Annotation and analysis of the mitochondrial genome of Coniothyrium glycines, causal agent of red leaf blotch of soybean, reveals an abundance of homing endonucleases. PLoS ONE 2018, 13, e0207062. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wu, Y.; Yang, C.; Gu, X.; Wilson, J.J.; Li, H.; Cai, W.; Yang, H.; Song, F. Evolution of tRNA gene rearrangement in the mitochondrial genome of ichneumonoid wasps (Hymenoptera: Ichneumonoidea). Int. J. Biol. Macromol. 2020, 164, 540–547. [Google Scholar] [CrossRef]
- Gong, L.; Shi, W.; Si, L.Z.; Kong, X.Y. Rearrangement of Mitochondrial Genome in Fishes. Dongwuxue Yanjiu 2013, 34, 666–673. [Google Scholar] [CrossRef]
- Lee, Y.P.; Kim, S.; Lim, H.; Ahn, Y.; Sung, S.K. Identification of mitochondrial genome rearrangements unique to novel cytoplasmic male sterility in radish (Raphanus sativus L.). Theor. Appl. Genet. 2009, 118, 719–728. [Google Scholar] [CrossRef]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Heitman, J.; Hom, E.F.Y.; Ianiri, G.; Jones, A.C.; et al. Fungi in the Marine Environment: Open Questions and Unsolved Problems. mBio 2019, 10, e01189-18. [Google Scholar] [CrossRef] [Green Version]
- Cao, F.; Meng, Z.H.; Wang, P.; Luo, D.Q.; Zhu, H.J. Dipleosporalones A and B, Dimeric Azaphilones from a Marine-Derived Pleosporales sp. Fungus. J. Nat. Prod. 2020, 83, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, H.; Ye, J.; Wu, X.; Wang, W.; Lin, H.; Yan, X.; Lazaro, J.; Wang, T.; Naman, C.; et al. Cytotoxic Polyketide Metabolites from a Marine Mesophotic Zone Chalinidae Sponge-Associated Fungus Pleosporales sp. NBUF144. Mar. Drugs 2021, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Prachyawarakorn, V.; Mahidol, C.; Sureram, S.; Sangpetsiripan, S.; Wiyakrutta, S.; Ruchirawat, S.; Kittakoop, P. Diketopiperazines and phthalides from a marine derived fungus of the order pleosporales. Planta Med. 2008, 74, 69–72. [Google Scholar] [CrossRef] [PubMed]





| Gene | Position (bp) | Length (bp) | Direction | Anti-/Start Codons | A + T% |
|---|---|---|---|---|---|
| trnR | 140–210 | 71 | - | TCT | 73.24 |
| trnC | 3051–3120 | 70 | + | GCA | 61.43 |
| cox1 | 5663–6543 | 881 | + | ATG | 65.72 |
| cox2 | 6872–7537 | 666 | + | ATG | 67.26 |
| trnR | 9070–9141 | 72 | + | CCT | 70.83 |
| trnL | 9775–9857 | 83 | + | TAG | 57.83 |
| trnQ | 9990–10,061 | 72 | + | TTG | 66.67 |
| trnH | 10,338–10,410 | 73 | + | GTG | 57.54 |
| trnR | 11,460–11,530 | 71 | + | TCG | 74.65 |
| nad4L | 11,581–11,850 | 270 | + | ATG | 75.18 |
| nad5 | 11,850–14,032 | 2183 | + | ATG | 69.54 |
| trnV | 15,072–15,145 | 74 | + | TTA | 62.16 |
| trnR | 15,208–15,267 | 60 | + | CCT | 73.33 |
| nad4 | 15,474–16,909 | 1436 | + | ATT | 72.63 |
| atp6 | 17,935–18,700 | 766 | + | AAC | 72.98 |
| cob | 19,966–21,031 | 1066 | + | ATG | 68.76 |
| trnC | 22,029–22,098 | 70 | + | GCA | 62.85 |
| cox3 | 22,348–23,640 | 1293 | + | ATG | 71.77 |
| nad2 | 23,766–25,535 | 1770 | + | ATG | 73.79 |
| nad3 | 25,713–26,099 | 387 | + | ATA | 74.16 |
| trnV | 27,281–27,353 | 73 | + | TAC | 63.02 |
| nad1 | 27,958–28,789 | 832 | - | ACA | 70.68 |
| trnM | 30,993–31,064 | 72 | - | CAT | 61.11 |
| trnF | 31,106–31,178 | 73 | - | GAA | 58.91 |
| trnA | 31,746–31,817 | 72 | - | TGC | 65.28 |
| trnE | 31,847–31,919 | 73 | - | TTC | 53.43 |
| trnL | 32,285–32,367 | 83 | - | TAA | 59.04 |
| trnM | 32,647–32,719 | 73 | - | CAT | 60.28 |
| trnM | 32,736–32,806 | 71 | - | CAT | 53.52 |
| trnT | 32,828–32,898 | 71 | - | TGT | 56.34 |
| rrnL | 33,303–36,429 | 3127 | - | 65.94 | |
| trnP | 36,523–36,595 | 73 | - | TGG | 53.43 |
| trnS | 36,746–36,830 | 85 | - | TGA | 63.53 |
| trnR | 37,176–37,247 | 72 | - | ACG | 59.72 |
| trnI | 37,251–37,322 | 72 | - | GAT | 61.11 |
| trnW | 37,554–37,627 | 74 | - | TCA | 67.56 |
| trnS | 37,887–37,966 | 80 | - | GCT | 61.25 |
| trnD | 38,204–38,276 | 73 | - | GTC | 53.43 |
| trnG | 38,279–38,349 | 71 | - | TCC | 54.93 |
| trnK | 38,359–38,430 | 72 | - | TTT | 66.67 |
| trnV | 38,458–38,530 | 73 | - | TAC | 61.65 |
| nad6 | 38,559–39,147 | 589 | - | ATG | 77.75 |
| trnN | 39,523–39,593 | 71 | - | GTT | 64.79 |
| trnY | 39,662–39,746 | 85 | - | GTA | 63.53 |
| trnL | 41,253–41,329 | 77 | - | CAA | 62.33 |
| rrnS | 41,351–42,716 | 1366 | - | 65.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Fan, C.; Fu, Z.; Zhang, H.; Yang, P. Analyses of the Complete Mitochondrial Genome of Paraconiothyrium sp. and Gene Rearrangement Diversity in the Pleosporales. Diversity 2022, 14, 601. https://doi.org/10.3390/d14080601
An J, Fan C, Fu Z, Zhang H, Yang P. Analyses of the Complete Mitochondrial Genome of Paraconiothyrium sp. and Gene Rearrangement Diversity in the Pleosporales. Diversity. 2022; 14(8):601. https://doi.org/10.3390/d14080601
Chicago/Turabian StyleAn, Jiaqi, Chunli Fan, Zuoyi Fu, Hongping Zhang, and Pu Yang. 2022. "Analyses of the Complete Mitochondrial Genome of Paraconiothyrium sp. and Gene Rearrangement Diversity in the Pleosporales" Diversity 14, no. 8: 601. https://doi.org/10.3390/d14080601